Abstract
Background
Clostridioides difficile is the main pathogen of antimicrobial-associated diarrhoea and health care facility-associated infectious diarrhoea. This study aimed to investigate the prevalence, toxin genotypes, and antibiotic resistance of C. difficile among hospitalized patients in Xi’an, China.
Results
We isolated and cultured 156 strains of C. difficile, representing 12.67% of the 1231 inpatient stool samples collected. Among the isolates, tcdA + B + strains were predominant, accounting for 78.2% (122/156), followed by 27 tcdA-B + strains (27/156, 17.3%) and 6 binary toxin gene-positive strains. The positive rates of three regulatory genes, tcdC, tcdR, and tcdE, were 89.1% (139/156), 96.8% (151/156), and 100%, respectively. All isolates were sensitive to metronidazole, and the resistance rates to clindamycin and cephalosporins were also high. Six strains were found to be resistant to vancomycin.
Conclusion
Currently, the prevalence rate of C. difficile infection (CDI) in Xi’an is 12.67% (156/1231), with the major toxin genotype of the isolates being tcdA + tcdB + cdtA-/B-. Metronidazole and vancomycin were still effective drugs for the treatment of CDI, but we should pay attention to antibiotic management and epidemiological surveillance of CDI.
Keywords: Clostridioides difficile, Toxin genes, Toxin gene expression, Antibiotic resistance
Background
Clostridioides difficile(C. difficile), previously known as Clostridium difficile, is a Gram-positive, anaerobic, spore-forming bacillus that colonizes the gastrointestinal tract of humans and animals. It is recognized as the leading cause of antimicrobial-associated diarrhoea and health care facility-associated infectious diarrhoea [1]. The spectrum of C. difficile infection (CDI) ranges from asymptomatic colonization and diarrhoea of varying severities to pseudomembranous colitis, toxic megacolon, and even death [2].
CDI is primarily mediated by toxins A (enterotoxin) and B (cytotoxin), which are encoded by the tcdA and tcdB genes, located in the pathogenicity locus (PaLoc) of the bacterial chromosome [3]. PaLoc also contains two genes encoding regulatory factors (tcdR and tcdC) and a holin-like gene (tcdE) that is involved in toxin release, although the function of tcdC remains controversial [4, 5]. Some C. difficile strains can also produce a binary toxin (CDT) encoded by the cdtA and cdtB genes, which can enhance the toxicity of TcdA and TcdB. Studies have reported that approximately 1.6-10% of C. difficile isolates carry binary toxin genes [6, 7]. The antibiotic resistance of C. difficile varies among countries and regions, but it remains sensitive to metronidazole and vancomycin [8]. Therefore, vancomycin and metronidazole are still recommended drugs for the treatment of CDI [9].
Due to the widespread and irresponsible use of broad-spectrum antibacterial drugs, the incidence of CDI has increased significantly. In the last decade, research on C. difficile in China has also been on the rise. Multiple-drug-resistant strains of C. difficile have been detected clinically, and hypervirulent strains have been identified in Beijing and other areas [10]. Wu Yuan et al. [11] showed that common clinical isolates and drug-resistance characteristics of C. difficile in China were generally consistent with international reports. However, the distribution of the genotypes, antibiotic resistance, and toxin genes varies in space and time.
There have been many epidemiological studies on C. difficile in eastern China, while research on the topic is relatively scarce in northwest China. Xi ‘an, as the economic and medical hub of northwest China, is regionally representative. Therefore, this study was conducted to investigate the current prevalence of CDI, toxin genotypes, and antibiotic resistance among inpatients in the region, by analyzing the status of patients in three tertiary hospitals in Xi’an.
Result
C. Difficile isolation and characteristics of the patients
A total of 1231 stool samples were collected in this study, and 156 strains of C. difficile were isolated and cultured, for a positive rate of 12.67%. There was a significant difference in the positive rate of C. difficile culture between the three hospitals, while there was no significant difference between the sexes. Table 1 shows that the highest positive rate of C. difficile culture was observed in the age group 61–70 years old (17.31%, 27/156), followed by 3–10 years old (14.55%, 8/55), and the lowest was 20–30 years old (2/44, 4.54%), but the differences were not statistically significant.
Table 1.
Differences in positive C. difficile culture rates among 1231 inpatients by hospital, gender and age
| Patient characteristics | C. difficile culture | Positive rate (%) | χ2 | P value | |
|---|---|---|---|---|---|
| - | + | ||||
| Hospital | 6.051 | 0.049* | |||
| A | 408 | 70 | 14.64 | ||
| B | 165 | 15 | 7.67 | ||
| C | 477 | 71 | 12.72 | ||
| Gender | 2.992 | 0.084 | |||
|
Female male male |
550 | 80 | 11.27 | ||
| Male | 379 | 76 | 14.59 | ||
| Age (year) | 6.707 | 0.569 | |||
| 0–3 | 454 | 64 | 12.36 | ||
| 4–10 | 47 | 8 | 14.55 | ||
| 11–20 | 13 | 2 | 13.33 | ||
| 21–30 | 42 | 2 | 4.54 | ||
| 31–40 | 71 | 8 | 10.13 | ||
| 41–50 | 83 | 13 | 13.68 | ||
| 51–60 | 115 | 16 | 12.21 | ||
| 61–70 | 129 | 27 | 17.31 | ||
| ≥ 71 | 121 | 16 | 11.68 | ||
Hospital A: The First Affiliated Hospital of Xi’an Jiaotong University, B: Xi’an High-tech Hospital, C: Xi’an Children’s Hospital
*Significant difference
A total of 156 patients with positive C. difficile cultures were hospitalized for 10.3 ± 11.8 days and were aged 31.7 ± 30.1 years. The most common departments for these hospitalized patients was the infectious diseases department (29.5%, 46/156), followed by digestive medicine (25%, 39/156), and the ICU (13.5%, 21/156). Other departments had only a few patients.
Detection of toxin genes and toxins A and B
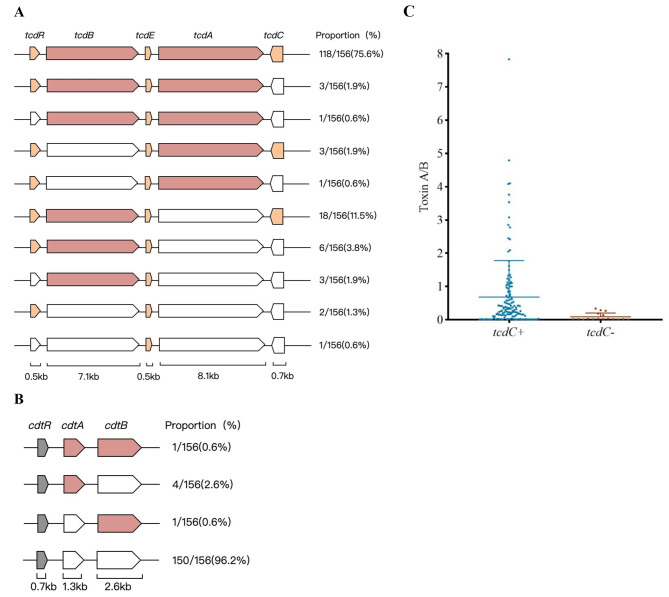
The toxin gene was detected in C. difficile isolates, of which 98.08% (153/156) were toxigenic strains, and 1.92% (3/156) were non-toxic strains. Toxin genotyping of the strains for tcdA and tcdB showed that the toxin profiles of tcd A + B+, tcd A + B-, and tcd A-B + accounted for 78.2% (122/156), 2.6% (4/156), and 17.3% (27/156) of the strains, respectively. A total of 3.85% (6/156) of the C. difficile strains were binary toxin positive, and only one sample presented the two genes that encode the binary toxin. For all isolates that were binary toxin positive, the toxin gene profiles of tcdA and tcdB were tcdA + B+. As for the accessory genes, the positive rates for tcdC, tcdR, and tcdE in the isolates were 89.1% (139/156), 96.8% (151/156), and 100%, respectively. The distribution of toxin genes is shown in Fig. 1.
Fig. 1.
Detections of toxin genes and toxin A/B. (A) The PaLoc encodes the toxin A and toxin B, and three accessory proteins. The colored ones indicate that the detected isolates are positive for this gene, the colorless ones indicate a negative result, and the stripe indicates undetected. Pink is the main toxin gene, and the light orange and grey represent the accessory gene. (B) The CdtLoc encodes the two binary toxin genes and one accessory gene. The representation of color is the same as A. (C) Differences in toxin A/B secretion of different tcdC genes, with error bars indicating the standard deviation from the mean
Among the 156 C. difficile isolates, 61 (39.1%, 61/156) were hand a positive toxin A/B test, and 95 (60.9%, 95/156) were negative/suspicious for toxin A/B. Further comparing the difference in toxin A/B secretion between tcdC + and tcdC- strains, the results showed that the A/B toxin value secreted by tcdC + strains (0.68 ± 1.10) was higher than that of tcdC- strains (0.09 ± 0.11) (P < 0.001), as shown in Fig. 1.
Antimicrobial susceptibility of isolates
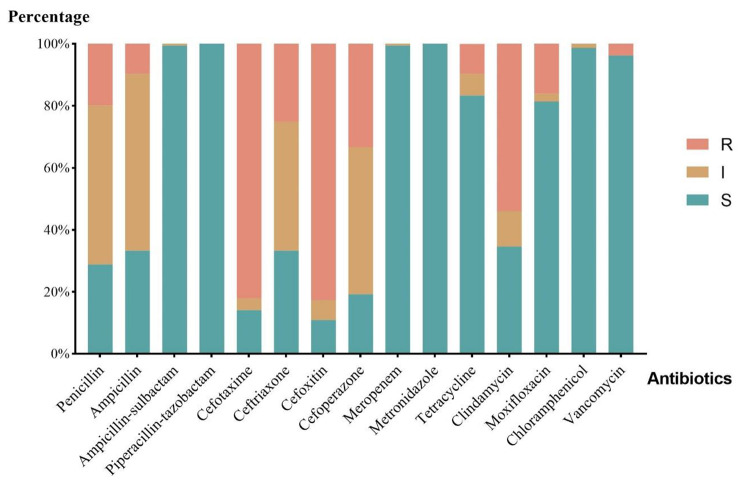
Figure 2 shows the results of the antibiotic susceptibility test. All isolates were susceptible to metronidazole and piperacillin-tazobactam and were not resistant to ampicillin/sulbactam, meropenem, or chloramphenicol. Six (3.8%, 6/156) strains were resistant to vancomycin. The antibiotic resistance rates of C. difficile to ampicillin (9.6%, 15/156), tetracycline (9.6%, 15/156), moxifloxacin (16.0%, 25/156), penicillin (19.9%, 31/156), and ceftriaxone (25.0%, 39/156) were low. Cefoxitin (82.7%, 129/156) was the most resisted antibiotic in the study, followed by cefotaxime (82.1%, 128/156), clindamycin (53.8%, 84/156), and cefoperazone (33.3%, 52/156).
Fig. 2.
The resistance characteristics of isolated C. difficile strains. The name of 15 tested antibiotics is in the horizontal axis. For the vertical line, the percentages refer to the proportion of strains involved in R (Resistant), I (intermediate), and S (susceptible)
Differences in antimicrobial resistance according to toxin genotyping and the level of toxins produced
Table 2 shows the results of the antibiotic susceptibility testing and correlation analysis. The results showed that the resistance of strains varieds between hospitals. For cefotaxime, cefoxitin, tetracycline, and clindamycin the strains isolated from Xi’an Children’s Hospital had higher resistance rates than those from The First Affiliated Hospital of Xi’an Jiaotong University. There were differences in resistance between strains with different levels of toxin production. For ceftriaxone, cefoperazone, and tetracycline, the strains that tested positive for A/B toxin had significantly lower resistance rates than those that tested negative/suspicious. In addition, between different toxin genotypes, there were significant differences in the resistance rates to tetracycline, with tcdA-B + strains having higher resistance rates than tcdA + B + strains.
Table 2.
Antibiotic resistance rates of C. difficile according to hospital, toxin production and toxin genotyping
| Antimicrobial agent (number of resistant strains) | Hospital, n (%) | Detection of Toxin A/B, n (%) | Toxin genotyping, n (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| A (n = 70) |
B (n = 15) |
C (n = 71) |
P value | Negative/ Suspicious (n = 95) |
Positive (n = 61) |
P value | tcdA + B+ (n = 122) |
tcdA-B+ (n = 27) |
P value | |
| Penicillin | 12(17.1) | 3(20.0) | 16(22.5) | 0.735 | 23(24.2) | 8(13.1) | 0.090 | 22(18) | 9(33.3) | 0.076 |
| Ampicillin | 9(12.9) | 2(13.3) | 4(5.6) | 0.327 | 9(9.5) | 6(9.8) | 0.940 | 11(9.0) | 4(14.8) | 0.476 |
| Cefotaxime | 46(65.7) a | 13(86.7) a, b | 69(97.2) b | <0.001* | 78(82.1) | 50(82.0) | 0.983 | 101(82.8) | 24(88.9) | 0.570 |
| Ceftriaxone | 11(15.7) a | 7(46.7) b | 21(29.6) a, b | 0.021* | 30(31.6) | 9(14.8) | 0.018* | 32(26.2) | 6(22.2) | 0.666 |
| Cefoxitin | 48(68.6) a | 14(93.3) a, b | 67(94.4) b | <0.001* | 80(84.2) | 49(80.3) | 0.532 | 102(83.6) | 23(85.2) | 1.000 |
| Cefoperazone | 15(21.4) a | 9(60.0) b | 28(39.4) a, b | 0.005* | 39(41.4) | 13(21.3) | 0.011* | 38(31.1) | 11(40.7) | 0.337 |
| Tetracycline | 2(2.9) a | 2(13.3) a, b | 11(15.5) b | 0.031* | 14(14.7) | 1(1.6) | 0.007* | 9(7.4) | 6(22.2) | 0.032* |
| Clindamycin | 30(42.9) a | 9(60.0) a, b | 45(63.4) b | 0.044* | 55(57.9) | 29(47.5) | 0.206 | 63(51.6) | 18(66.7) | 0.156 |
| Moxifloxacin | 20(28.6) a | 2(13.3) a, b | 3(4.2) b | 0.001* | 13(13.7) | 12(19.7) | 0.320 | 19(15.6) | 5(18.5) | 0.773 |
| Vancomycin | 5(7.1) | 0 | 1(1.4) | 0.187 | 5(5.3) | 1(1.6) | 0.405 | 3(2.5) | 1(3.7) | 0.555 |
*Significant difference
Hospital A: The First Affiliated Hospital of Xi’an Jiaotong University, B: Xi’an High-tech Hospital, C: Xi’an Children’s Hospital
a, b: If identical letters exist, they are not significant difference
Correlation between clinical manifestations and patient characteristics
We divided the CDI patients into asymptomatic and overt infections based on clinical symptoms and analysed the correlation between them and patients’ clinical characteristics, as shown in Table 3. The results showed that patients aged 0–10 years presented with symptoms more frequently than those aged 11–50 years or > 50 years, and more frequently than the total population, all statistically significant differences. There was no statistically significant difference in the frequency of symptoms between patients aged 11–50 and patients aged > 50 years. Patients who had used proton pump inhibitors (PPIs) were significantly less likely to have symptoms than patients who had not used PPIs.
Table 3.
Differences in clinical symptoms by age, gender, PPI and antibiotic use in C. difficile infection
| Patient characteristics | Clinical symptoms | Positive rate (%) | χ2 | P value | ||||
|---|---|---|---|---|---|---|---|---|
| - | + | |||||||
| Total | 51 | 105 | 67.3 | |||||
| Age(year) | 36.57 | <0.001* | ||||||
| 0-10a | 6 | 66 | 91.7 | 15.59c | <0.001*, c | |||
| 11-50b | 12 | 13 | 52.0 | 2.23c | 0.175c | |||
| >50b | 33 | 26 | 44.1 | 9.71c | 0.002*, c | |||
| Gender | 0.95 | 0.331 | ||||||
| Female | 22 | 54 | 71.1 | |||||
| Male | 29 | 51 | 63.8 | |||||
| PPI | 5.15 | 0.023* | ||||||
| Yes | 20 | 23 | 53.5 | 2.81c | 0.107c | |||
| No | 31 | 82 | 72.6 | 0.86c | 0.421c | |||
| Antibiotics | 0.52 | 0.469 | ||||||
| Yes | 36 | 68 | 65.4 | |||||
| No | 15 | 37 | 71.2 | |||||
*Significant difference
a, b: If identical letters exist, they are not significant difference
c: Compared with total (156)
PPI: Proton pump inhibitors
Discussion
CDI is a significant public health concern worldwide. In China, research about C. difficile has grown significantly in the last decade, and a meta-analysis of the literature showed that up to 14% of patients with diarrhoea tested positive for C. difficile [12]. In our study, we cultured 156 strains of C. difficile (12.67%) from faecal specimens of 1231 hospitalized patients. The positivity rate varied significantly across the three participating hospitals. We did not find a significant difference in the positivity rate between sexes or age groups. The incidence rate of CDI in our study was lower than the reported overseas (20-32%) [13, 14], and similar to the incidence rate in eastern regions of China, such as Zhejiang, Shanghai, and Shandong (10-14%) [15–17]. Differences in CDI may be related to geographical distribution, infection control policies, and antibiotic use regulations [18]. Although the incidence rate of CDI was higher among elderly patients in our study, the difference in incidence rate between other age groups was not statistically significant. This result contradicts the traditional belief that older age is a risk factor for CDI, which may be due to the widespread use of broad-spectrum antibiotics in China, leading to the continuous rejuvenation of CDI risk factors [15, 16, 19].
C. difficile produces TcdA, TcdB, and binary toxins (CDT), which are encoded by the tcdA, tcdB, and cdtA/B genes, respectively [20]. In this study, the toxin-gene-carrying rate of the isolated C. difficile strains was 98.08% (153/156), which is higher than the 82-90% isolation rate of poison-producing strains found in eastern China [15–17], and higher than the 63-94% isolation rate found in South Korea, Japan and other East Asian countries [17–19]. The main toxin genotype was found to be tcdA + tcdB + cdtA-/B-, accounting for 74.3% (116/156), which is consistent with the 69-77% ratio in eastern China [15–17]. This ratio is lower than the proportion of 77-94% in East Asian countries such as South Korea [21–23], and higher than the proportion of document coverage in Greece, Iran, Saudi Arabia, and Thailand (27-65%) [18, 24–26], indicating that the distribution of toxin genes varies greatly in between countries and regions. In this study, we isolated 27 tcdA-B + strains (17.3%, 27/156), and according to relevant literature, they may have higher resistance rates to a variety of antibiotics [27]. At the same time, six strains carrying the binary toxin genes were detected in this study (3.85%, 6/156), while no binary toxin genes were detected in studies conducted in Shanghai in 2007–2008, Zhejiang in 2014–2016 or Beijing in 2018 [15, 16, 28]. The detection rate in Asian countries such as South Korea and Thailand is also relatively low [21, 26], whereas the detection rates have been higher in Europe, such as 51% in Greece and 34% in the Czech Republic [22, 29]. The expression of binary toxin genes is believed to be associated with higher toxicity of TcdA and TcdB, higher spore production rates, and more severe diseases [30, 31]. One sample of a cdtA + B + strain from an elderly woman who was admitted to the infection unit for 25 days with a diagnosis of cirrhosis of the liver with ascites, was isolated in this study. In recent years, strains carrying CDT have been detected in China [32, 33], showing the need for attention and monitoring. In this study, the regulatory genes tcdC, tcdR, and tcdE on PaLoc were detected at rates of 89.1% (139/156), 96.8% (151/156), and 100%, respectively. Although the function of TcdR as a positive regulator has been determined, the roles of TcdC and TcdE are still under investigation [5]. We found that the secretion of toxin A/B in tcdC positive strains was significantly higher than that in tcdC negative strains, indicating that tcdC may play a positive regulatory role in the secretion of toxin A/B. The results of B Dupuy [34] and Kate E Dingle [35] showed that tcdC negatively regulates the expression of tcdA and tcdB genes, while the results of Prerna Vohra [36] and Michelle Merrigan [37] showed that tcdC gene regulates toxin secretion but is not strictly inhibitory. In addition, the study by Cartman ST [38] did not observe a relationship between toxin gene expression and tcdC genotypes. Therefore, further research on the function of the tcdC gene is needed.
According to the updated 2017 guidelines in the United States [9], vancomycin is the first-line clinical treatment recommended for CDI, while metronidazole is recommended for use when vancomycin cannot be obtained. The results of drug susceptibility testing in this study show that all isolates were sensitive to metronidazole, consistent with some domestic and foreign reports [16, 21, 25, 26, 35, 36]. For vancomycin, we detected six resistant strains (3.8%, 6/156), with five strains coming from patients aged 60–70 years old, who had underlying diseases such as rectal cancer, renal cancer, lymphoma, and intestinal tuberculosis. Since the Clinical and Laboratory Standards Institute (CLSI) standards in the United States have not given a recommended resistance breakpoint for vancomycin, this study used the resistance breakpoint proposed by the European Committee on Antimicrobial Susceptibility Testing (EUCAST), which defines the critical value of drug sensitivity minimum inhibitory concentration (MIC). Strains resistant to vancomycin and metronidazole have also been detected at home and abroad. The longitudinal monitoring of antibiotic resistance in 22 European countries conducted by J. Freeman et al. [39] showed that 0.87% (8/918) of strains were resistant to vancomycin and 0.11% (1/916) were resistant to metronidazole. T. Dilnessa et al. [40] included 15 cross-sectional studies from Iran, China, the United States, Poland, and other countries in a meta-analysis of antibiotic resistance rates, which showed that the resistance rates for vancomycin and metronidazole were 3% (83/2755) and 5% (138/2753), respectively. Although both antibiotics can still be used for the treatment of CDI in the region, continuous monitoring of drug resistance and strict management of antibiotic use are needed to reduce the emergence of drug-resistant strains. We found that all isolates were not resistant to piperacillin/tazobactam, ampicillin/sulbactam, and meropenem. Due to these drugs’ distribution in the intestine and their impact on the normal intestinal flora, they may increase the risk of CDI and even worsen the condition [16]., so, these antibiotics are not routinely recommended for the treatment of CDI. Meanwhile, the resistance rates to traditional antibiotics such as chloramphenicol, tetracycline, ampicillin, and penicillin were relatively low, possibly due to the decreasing use of these traditional antibiotics in tertiary hospitals in recent years in China. In this study, the isolates had high resistance rates to third-generation cephalosporin antibiotics and clindamycin, which are high-risk drugs for CDI [40, 41]. This suggests that, in the region, the use of these drugs may be a risk factor for CDI and that the use of clindamycin and cephalosporins should be limited [9]. The relative risk associated with the use of specific antibiotics and their correlation with CDI depends on the prevalence of highly resistant strains to specific antibiotics in the local area [41]. Although fluoroquinolones are also considered high-risk drugs for CDI [42], the resistance rate of the isolates to moxifloxacin in this study was 16.0% (25/156), which is lower than the reported resistance rates of 25-93% in other domestic and foreign reports [32, 39, 43]. As a result, the use of moxifloxacin may not be the main risk factor for CDI in the region though further research is needed to verify this.
By comparing their rates of resistance to different antibiotics, we showed that for some antibiotics, the resistance rate of strains isolated from Xi’an Children’s Hospital was higher than that of strains from The First Affiliated Hospital of Xi’an Jiaotong University. This may be due to the different antibiotic use habits and the antibiotics used for different age groups in the two hospitals. We also found that tcdA-B + strains showed significantly higher resistance to tetracycline than tcdA + B + strains, suggesting that tcdA-B + strains have higher resistance to some antibiotics. These findings are consistent with previous reports [15, 25, 26], indicating that toxin gene typing may be valuable in handling antibiotic use in CDI patients. In addition, the isolates that tested positive for A/B toxin were resistant to ceftriaxone, cefoperazone, and tetracycline significantly less often than the negative/suspicious isolates, suggesting that stronger strains may be less resistant to some antibiotics. The results from a study by T. Saber [25] showed that toxigenic strains had higher resistance to most antibiotics than non-toxigenic strains, indicating that further institutional research is needed to clarify the relationship between C. difficile toxin production and drug resistance phenotypes.
Our correlation analysis of the clinical characteristics and infectious symptoms of patients with CDI showed that clinical symptoms most often appeared in patients aged 0–10 years. However, it has been traditionally believed that CDI in infants and young children is mostly non-pathogenic due to the protection provided by breastfeeding and the lack of toxin receptors [44]. Previous studies have reported a high prevalence of coinfection with C. difficile and other gastrointestinal pathogens in children with diarrhoea (23.2 − 67%) [45]. As we did not test for other pathogens in our study, it is possible that coinfection with other pathogens contributed to the development of clinical symptoms in our CDI patients. Our findings also revealed that the use of PPIs was associated with a lower probability of symptoms compared to the non-use of PPIs. Although previous studies have shown that the use of PPIs significantly increases the risk of CDI, most of them have been observational studies that could not establish a causal relationship [46]. Moreover, the 2017 updated guidelines in the USA stated that there was not enough evidence to suggest that stopping PPI use is an effective measure to prevent CDI [9]. Therefore, the relationship between PPIs and the clinical symptoms of CDI patients is unclear.
This study enhances our understanding of the prevalence of CDI in the Xi’an region, but it had some limitations. First, the C. difficile isolates used in this study were collected over a period of time, and the epidemiology of CDI is dynamic. Therefore, the distribution of toxin genotypes and antibiotic resistance may have slightly biased our data analysis. Second, fidaxomicin, as one of the preferred antibacterial drugs for the treatment of CDI, was not evaluated due to the unavailability of the test substance. Finally, since there was no continuous monitoring of patient disease progress, the clinical data collected only reflected the symptoms of patients at a certain point in time, and there may be biases in the correlation analysis of clinical symptoms. Future investigations should be designed to avoid these shortcomings.
Conclusions
This study contributes to the understanding of the prevalence of CDI, toxin genotyping, and antibiotic resistance in clinical isolates of C. difficile in Xi’an, China. In summary, the CDI incidence rate in the study area was 12.67% (156/1231), with tcdA + tcdB + cdtA-/B- as the main toxin genotype. A total of 27 tcdA-B + strains and 6 strains positive for binary toxin genes were isolated. Vancomycin and metronidazole were still effective in treating CDI in the region, but antibiotic management, especially with clindamycin and cephalosporins, should be given full attention to reduce the incidence of CDI and prevent the emergence of new resistant strains. More, more in-depth studies are recommended to explore the function of the tcdC gene, clarify the relationship between toxin production and the resistance phenotype of C. difficile, evaluate the relationship between PPI use and CDI clinical symptoms, and monitor the rapidly changing CDI epidemiology.
Methods
Study design and sample collection
This cross-sectional study was carried out in the First Affiliated Hospital of Xi’an Jiaotong University, Xi’an High-tech Hospital, and Xi’an Children’s Hospital from July to October 2020. We collected 2–3 ml of fresh unformed stool samples from hospitalized patients with suspected antibiotic-associated diarrhoea and stored them at − 20 °C. Isolation, culture, and identification of C. difficile were performed within one week. We used the electronic medical record systems of the hospitals to collect relevant clinical information about patients, including sex, age, underlying illness, clinical symptoms drug use, length of stay, etc.
Culture and identification of C. Difficile
All faecal samples from patients were cultured on CHROMagar chromogenic plates for Clostridium difficile (B.N.: 20,210,127, Shanghai Xinzhong Bioengineering Co., Ltd), incubated in anaerobic bags, and placed in a 35 °C thermostatic incubator for 24–48 h [47]. C. difficile colonies show typical grey to black, irregular, and rough colonies. We selected suspicious colonies by plating on chromogenic medium and used matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS French Bio-Merieux) for identification [48]. The identification result had to be C. difficile with a confidence level of 99.9% and a green box mark.
Detection of C. Difficile toxin genes
DNA was extracted from the isolates using a bacterial DNA extraction kit (Omega D3350, China). Using the extracted DNA as a template, polymerase chain reaction (PCR) was performed for the identification of the tcdA, tcdB, tcdC, tcdE, tcdR, cdtA, and cdtB genes with, the primer sequences in Table 4 [49]. The 50µL reaction system contained 25 µL of 2×Taq Master Mix, 2.5µL of upstream primer and downstream primer, 5 µL of DNA template, and 15 µL of ddH2O. The PCR parameters were as follows: 5 min at 94 °C for initial denaturation; 35 cycles of 94 °C for 1 min, 57 °C for 5 min and 72 °C for 50 s; and a final extension cycle of 5 min at 72 °C. Two microlitres of amplification product was placed in a 2% agarose gel, and electrophoresed for 45 min under 150 V voltage, and the results were observed using the gel imaging system.
Table 4.
Primes used for the detection of C. difficile toxin genes
| Target gene | Prime | Oligonucleotide sequences (5’ – 3’) | Product length (bp) |
|---|---|---|---|
| tcdA a |
tcdA-F tcdA-R |
AGATTCCTATATTTACATGACAATAT GTATCAGGCATAAAGTAATATACTTT |
369 |
| tcdB a |
tcdB-F tcdB-R |
TGATGAAGATACAGCAGAAGC TGATTCTCCCTCAAAATTCTC |
688 |
| tcdC a |
tcdC-F tcdC-R |
AAAAGGGAGATTGTATTATGTTTTC CAATAACTTGAATAACCTTACCTTCA |
479 |
| tcdR b |
tcdR-F tcdR-R |
AAAAGCGATGCTATTATAGTCAAA CCTTATTAACAGCTTGTCTAGAT |
300 |
| tcdE b |
tcdE-F tcdE-R |
GTTTAAGTGCAATAAAAAGTCGTA GGTAATCCACATAAGCACATATT |
262 |
| cdtA a |
cdtA-F cdtA-R |
GGGAAGCACTATATTAAAGCAGAAGC CTGGGTTAGGATTATTTACTGGACCA | 200 |
| cdtB a |
cdtB-F cdtB-R |
TTGACCCAAAGTTGATGTCTGATTG CGGATCTCTTGCTTCAGTCTTTATAG |
260 |
a: The primers used in the study were designed by Primer-BLAST
b: The primers used in the study were from Reference 48
Fluorescence immunoassay of toxins A and B
The C. difficile toxins A and B of C. difficile were isolated by enzyme-linked fluorescence immunoassay (ELFA) and VIDAS automated instrument (B.N.: 1,009,414,570, French Bio-Merieux). Solidphase tubes were coated with rabbit-derived polyclonal antitoxin A antibody and mouse-derived monoclonal antitoxin B antibody to C. difficile. The reagent strip contained: 0.05 mol/L TRIS buffer, conjugate (biotin-labelled mouse-derived monoclonal anti-C. difficile toxin A antibody and biotin-labelled mouse-derived monoclonal anti-C. difficile toxin B antibody), tracer (alkaline phosphatase-labelled streptavidin), and substrate (4-methylumbelliferyl phosphate). The test was performed according to the manufacturers’ specifications. Fluorescence intensities for A/B toxin of < 0.13, ≥ 0.13 to < 0.37, and ≥ 0.37 were considered negative, equivocal, and positive, respectively [47].
Antimicrobial resistance testing
The MIC of 15 common clinical antibacterial drugs was determined by anaerobic biochemical drug sensitivity test card TDR ANA-96 (B.N.:20,211,226, Hunan Mindray Medical Technology Co., Ltd.). Antimicrobial drugs include penicillin, ampicillin, ampicillin-sulbactam, piperacillin-tazobactam, cefotaxime, ceftriaxone, cefoxitin, cefoperazone, meropenem, metronidazole, tetracycline, clindamycin, moxifloxacin, chloramphenicol, and vancomycin. The MIC breakpoints of vancomycin were based on the EUCAST recommendation [50], and the CLSI recommendation [51].
Data analysis
The statistical software used in this study was SPSS 20.0. The measurement data are expressed in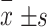 (mean ± standard deviation) and were compared using the test; The enumeration data are expressed in cases or percentages, and were compared using the chi-square test or Fisher’s exact test. Bonferroni correction was done for pairwise comparisons after multiple comparisons of the chi-square test; p < 0.05 was considered statistically significant.
(mean ± standard deviation) and were compared using the test; The enumeration data are expressed in cases or percentages, and were compared using the chi-square test or Fisher’s exact test. Bonferroni correction was done for pairwise comparisons after multiple comparisons of the chi-square test; p < 0.05 was considered statistically significant.
Acknowledgements
Not applicable.
Abbreviations
- C. difficile
Clostridioides difficile
- CDI
C. difficile infection
- PaLoc
pathogenicity locus
- PPI
proton pump inhibitor
- PCR
polymerase chain reaction
- ELFA
enzyme-linked fluorescence immunoassay
- MIC
minimum inhibitory concentration
- EUCAST
European Committee on Antimicrobial Susceptibility Testing
- CLSI
Clinical and Laboratory Standards Institute
Author contributions
Chen Ma, Wang Jing, Jing Yuan, Airong Wu and Kai Jia preparMaterial preparation and data collection were performed by. Analysis were performed by Sukai Zhan. The first draft of the manuscript was written by Sukai Zhang, Haiyue Zhang, Congcong Zhao, Ruibing Guo , Jiahao Liu and Chen Ma. The final review was done by Jin’e Lei and Yanjiong Chen. All authors read and approved the final manuscript.All authors contributed to the study conception, design and experiment.
Funding
This study was supported by the Clinical Research Award of the First Affiliated Hospital of Xi’an Jiaotong University, China (No.XJT1AF-CRF-2019-015) and Key Research and Development Program of Shaanxi (ProgramNo.2017SF-198).
Data availability
The data generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study was settled and performed according to the Declaration of Helsinki and obtained approval from the Research Ethics Committee (REC) of The First Affiliated Hospital of Xi’an Jiaotong University (No: XJTU1AF2017LSK-83). All patients provided written informed consent. The REC has the assignment to protect the dignity, rights, safety, and well-being of subjects who participate in biomedical research and to offer public accountability through the publication of their decisions. All institutes involved in this research consented to participate,
Consent for publication
Not applicable.
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Leffler DA, Lamont JT. Clostridium difficile infection. N Engl J Med. 2015 doi: 10.1056/NEJMra1403772. [DOI] [PubMed] [Google Scholar]
- 2.Czepiel J, Dróżdż M, Pituch H, Kuijper EJ, Perucki W, Mielimonka A, Goldman S, Wultańska D, Garlicki A, Biesiada G. Clostridium difficile infection: review. Eur J Clin Microbiol Infect Dis. 2019 doi: 10.1007/s10096-019-03539-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartlett JG. Narrative review: the new epidemic of Clostridium difficile-associated enteric disease. Ann Intern Med. 2006 doi: 10.7326/0003-4819-145-10-200611210-00008. [DOI] [PubMed] [Google Scholar]
- 4.Elliott B, Androga GO, Knight DR, Riley TV. Clostridium difficile infection: evolution, phylogeny and molecular epidemiology. Infect Genet Evol. 2017 doi: 10.1016/j.meegid.2016.12.018. [DOI] [PubMed] [Google Scholar]
- 5.Majumdar A, Govind R. Regulation of Clostridioides difficile toxin production. Curr Opin Microbiol. 2022 doi: 10.1016/j.mib.2021.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gerding DN, Johnson S, Rupnik M, Aktories K. Clostridium difficile binary toxin CDT: mechanism, epidemiology, and potential clinical importance. Gut Microbes. 2014 doi: 10.4161/gmic.26854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.To KB, Napolitano LM. Clostridium difficile infection: update on diagnosis, epidemiology, and treatment strategies. Surg Infect (Larchmt) 2014 doi: 10.1089/sur.2013.186. [DOI] [PubMed] [Google Scholar]
- 8.Banawas SS. Clostridium difficile infections: A Global Overview of Drug Sensitivity and Resistance mechanisms. Biomed Res Int. 2018 doi: 10.1155/2018/8414257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McDonald LC, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA) Clin Infect Dis. 2018 doi: 10.1093/cid/cix1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng JW, Xiao M, Kudinha T, et al. The First Two Clostridium difficile Ribotype 027/ST1 isolates identified in Beijing, China-an emerging problem or a neglected threat? Sci Rep. 2016 doi: 10.1038/srep18834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu Yuan L, Wenge J, Xiaoxi W, Yuanyuan Zhang Wenzhu.Epidemiological characteristics and research progress of Clostridioides difficile in China. Disease Surveillance. 2021;36(04):319–23. [Google Scholar]
- 12.Tang C, Cui L, Xu Y, et al. The incidence and drug resistance of Clostridium difficile infection in Mainland China: a systematic review and meta-analysis. Sci Rep. 2016 doi: 10.1038/srep37865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nasiri MJ, Goudarzi M, Hajikhani B, Ghazi M, Goudarzi H, Pouriran R. Clostridioides (Clostridium) difficile infection in hospitalized patients with antibiotic-associated diarrhea: a systematic review and meta-analysis. Anaerobe. 2018 doi: 10.1016/j.anaerobe.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 14.Lopes Cançado GG, Silveira Silva RO, Rupnik M, et al. Clinical epidemiology of Clostridium difficile infection among hospitalized patients with antibiotic-associated diarrhea in a university hospital of Brazil. Anaerobe. 2018 doi: 10.1016/j.anaerobe.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 15.Huang H, Wu S, Wang M, et al. Clostridium difficile infections in a Shanghai hospital: antimicrobial resistance, toxin profiles and ribotypes. Int J Antimicrob Agents. 2009 doi: 10.1016/j.ijantimicag.2008.09.022. [DOI] [PubMed] [Google Scholar]
- 16.Jin D, Luo Y, Huang C, et al. Molecular Epidemiology of Clostridium difficile infection in hospitalized patients in Eastern China. J Clin Microbiol. 2017 doi: 10.1128/JCM.01898-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ying L. The detection, genotyping and antimicrobial resistance study of Clostridium difficile, Dissertation, Qingdao university. 2019.
- 18.Azimirad M, Naderi Noukabadi F, Lahmi F, Yadegar A. Prevalence of binary-toxin genes (cdtA and cdtB) among clinical strains of Clostridium difficile isolated from diarrheal patients in Iran. Gastroenterol Hepatol Bed Bench. 2018;11(Suppl 1):59–65. [PMC free article] [PubMed] [Google Scholar]
- 19.Hawkey PM, Marriott C, Liu WE, et al. Molecular epidemiology of Clostridium difficile infection in a major Chinese hospital: an underrecognized problem in Asia? J Clin Microbiol. 2013 doi: 10.1128/JCM.00587-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martínez-Meléndez A, Cruz-López F, Morfin-Otero R, Maldonado-Garza HJ, Garza-González E. An update on Clostridioides Difficile Binary Toxin. Toxins (Basel) 2022 doi: 10.3390/toxins14050305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Byun JH, Kim H, Kim JL, et al. A nationwide study of molecular epidemiology and antimicrobial susceptibility of Clostridioides difficile in South Korea. Anaerobe. 2019 doi: 10.1016/j.anaerobe.2019.102106. [DOI] [PubMed] [Google Scholar]
- 22.Kim H, Jeong SH, Roh KH, et al. Investigation of toxin gene diversity, molecular epidemiology, and antimicrobial resistance of Clostridium difficile isolated from 12 hospitals in South Korea. Korean J Lab Med. 2010 doi: 10.3343/kjlm.2010.30.5.491. [DOI] [PubMed] [Google Scholar]
- 23.Okada Y, Kaku N, Kosai K, et al. Molecular epidemiology of Clostridioides difficile and risk factors for the detection of toxin gene-positive strains. J Infect Chemother. 2019 doi: 10.1016/j.jiac.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 24.Kachrimanidou M, Metallidis S, Tsachouridou O, et al. Predominance of Clostridioides difficile PCR ribotype 181 in northern Greece, 2016–2019. Anaerobe. 2022 doi: 10.1016/j.anaerobe.2022.102601. [DOI] [PubMed] [Google Scholar]
- 25.Saber T, Hawash YA, Ismail KA, et al. Prevalence, toxin gene profile, genotypes and antibiotic susceptibility of Clostridium difficile in a tertiary care hospital in Taif, Saudi Arabia. Indian J Med Microbiol. 2020 doi: 10.4103/ijmm.IJMM_20_300. [DOI] [PubMed] [Google Scholar]
- 26.Imwattana K, Wangroongsarb P, Riley TV. High prevalence and diversity of tcda-negative and tcdB-positive, and non-toxigenic, Clostridium difficile in Thailand. Anaerobe. 2019 doi: 10.1016/j.anaerobe.2019.03.008. [DOI] [PubMed] [Google Scholar]
- 27.King AM, Mackin KE, Lyras D. Emergence of toxin A-negative, toxin B-positive Clostridium difficile strains: epidemiological and clinical considerations. Future Microbiol. 2015 doi: 10.2217/fmb.14.115. [DOI] [PubMed] [Google Scholar]
- 28.Wang R, Suo L, Chen HX, Song LJ, Shen YY, Luo YP. Molecular epidemiology and antimicrobial susceptibility of Clostridium difficile isolated from the Chinese People’s Liberation Army General Hospital in China. Int J Infect Dis. 2018 doi: 10.1016/j.ijid.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 29.Krutova M, Nyc O, Matejkova J, Allerberger F, Wilcox MH, Kuijper EJ. Molecular characterisation of Czech Clostridium difficile isolates collected in 2013–2015. Int J Med Microbiol. 2016 doi: 10.1016/j.ijmm.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 30.Crobach MJT, Vernon JJ, Loo VG, et al. Understanding Clostridium difficile colonization. Clin Microbiol Rev. 2018 doi: 10.1128/CMR.00021-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hung YP, Huang IH, Lin HJ, et al. Predominance of Clostridium difficile Ribotypes 017 and 078 among Toxigenic Clinical isolates in Southern Taiwan. PLoS ONE. 2016 doi: 10.1371/journal.pone.0166159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheng JW, Xiao M, Kudinha T, et al. Molecular Epidemiology and Antimicrobial susceptibility of Clostridium difficile isolates from a University Teaching Hospital in China. Front Microbiol. 2016 doi: 10.3389/fmicb.2016.01621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luan Yang MP. Molecular epidemiological characteristics and antibiotic resistance of Clostridioides difficile in a tertiary hospital in Shaanxi Province. Chin J Microbiol Immunol. 2022;42(09):676–82. [Google Scholar]
- 34.Dupuy B, Govind R, Antunes A, Matamouros S. Clostridium difficile toxin synthesis is negatively regulated by TcdC. J Med Microbiol. 2008 doi: 10.1099/jmm.0.47775-0. [DOI] [PubMed] [Google Scholar]
- 35.Dingle KE, Elliott B, Robinson E, et al. Evolutionary history of the Clostridium difficile pathogenicity locus. Genome Biol Evol. 2014 doi: 10.1093/gbe/evt204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vohra P, Poxton IR. Comparison of toxin and spore production in clinically relevant strains of Clostridium difficile. Microbiol (Reading) 2011 doi: 10.1099/mic.0.046243-0. [DOI] [PubMed] [Google Scholar]
- 37.Merrigan M, Venugopal A, Mallozzi M, et al. Human hypervirulent Clostridium difficile strains exhibit increased sporulation as well as robust toxin production. J Bacteriol. 2010 doi: 10.1128/JB.00445-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cartman ST, Kelly ML, Heeg D, Heap JT, Minton NP. Precise manipulation of the Clostridium difficile chromosome reveals a lack of association between the tcdC genotype and toxin production. Appl Environ Microbiol. 2012 doi: 10.1128/AEM.00249-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Freeman J, Vernon J, Morris K, et al. Pan-european longitudinal surveillance of antibiotic resistance among prevalent Clostridium difficile ribotypes. Clin Microbiol Infect. 2015 doi: 10.1016/j.cmi.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 40.Dilnessa T, Getaneh A, Hailu W, Moges F, Gelaw B. Prevalence and antimicrobial resistance pattern of Clostridium difficile among hospitalized diarrheal patients: a systematic review and meta-analysis. PLoS ONE. 2022 doi: 10.1371/journal.pone.0262597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johnson S, Samore MH, Farrow KA, et al. Epidemics of diarrhea caused by a clindamycin-resistant strain of Clostridium difficile in four hospitals. N Engl J Med. 1999 doi: 10.1056/NEJM199911253412203. [DOI] [PubMed] [Google Scholar]
- 42.Pépin J, Saheb N, Coulombe MA, et al. Emergence of fluoroquinolones as the predominant risk factor for Clostridium difficile-associated diarrhea: a cohort study during an epidemic in Quebec. Clin Infect Dis. 2005 doi: 10.1086/496986. [DOI] [PubMed] [Google Scholar]
- 43.Aguilar-Zamora E, Weimer BC, Torres RC, et al. Molecular Epidemiology and Antimicrobial Resistance of Clostridioides difficile in hospitalized patients from Mexico. Front Microbiol. 2022 doi: 10.3389/fmicb.2021.787451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lees EA, Miyajima F, Pirmohamed M, Carrol ED. The role of Clostridium difficile in the paediatric and neonatal gut - a narrative review. Eur J Clin Microbiol Infect Dis. 2016 doi: 10.1007/s10096-016-2639-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de Graaf H, Pai S, Burns DA, Karas JA, Enoch DA, Faust SN. Co-infection as a confounder for the role of Clostridium difficile infection in children with diarrhoea: a summary of the literature. Eur J Clin Microbiol Infect Dis. 2015 doi: 10.1007/s10096-015-2367-0. [DOI] [PubMed] [Google Scholar]
- 46.Trifan A, Stanciu C, Girleanu I, et al. Proton pump inhibitors therapy and risk of Clostridium difficile infection: systematic review and meta-analysis. World J Gastroenterol. 2017 doi: 10.3748/wjg.v23.i35.6500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hong G, Park KS, Ki CS, Lee NY. Evaluation of the illumigene C. Difficile assay for toxigenic Clostridium difficile detection: a prospective study of 302 consecutive clinical fecal samples. Diagn Microbiol Infect Dis. 2014 doi: 10.1016/j.diagmicrobio. [DOI] [PubMed] [Google Scholar]
- 48.Kiyosuke M, Kibe Y, Oho M, Kusaba K, Shimono N, Hotta T, Kang D, Shoubuike T, Miyamoto H. Comparison of two types of matrix-assisted laser desorption/ionization time-of-flight mass spectrometer for the identification and typing of Clostridium difficile. J Med Microbiol. 2015 doi: 10.1099/jmm.0.000136. [DOI] [PubMed] [Google Scholar]
- 49.Liu XS, Li WG, Zhang WZ, Wu Y, Lu JX. Molecular characterization of Clostridium difficile isolates in China from 2010 to 2015. Front Microbiol. 2018 doi: 10.3389/fmicb.2018.00845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.The European Committee on Antimicrobial Susceptibility Testing (EUCAST). Breakpoint tables for interpretation of MICs and zone diameters. Version 13.0. 2023. http://www.eucast.org.
- 51.CLSI. Performance Standards for Antimicrobial Susceptibility Testing. 32nd ed. CLSI supplement M100. Clinical and Laboratory Standards Institute; 2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data generated and/or analyzed during the current study are available from the corresponding author on reasonable request.