Abstract
Earlier we demonstrated that activation of a ceramide-activated protein phosphatase (CAPP) conferred normal growth and secretion to yeast lacking their complement of exocytic v-SNAREs (Snc1,2) or bearing a temperature-sensitive mutation in an exocytic t-SNARE (Sso2). CAPP activation led to Sso dephosphorylation and enhanced the assembly of t-SNAREs into functional complexes. Thus, exocytosis in yeast is modulated by t-SNARE phosphorylation. Here, we show that endocytic defects in cells lacking the v- and t-SNAREs involved in endocytosis are also rescued by CAPP activation. Yeast lacking the Tlg1 or Tlg2 t-SNAREs, the Snc v-SNAREs, or both, undergo endocytosis after phosphatase activation. CAPP activation correlated with restored uptake of FM4-64 to the vacuole, the uptake and degradation of the Ste2 receptor after mating factor treatment, and the dephosphorylation and assembly of Tlg1,2 into SNARE complexes. Activation of the phosphatase by treatment with C2-ceramide, VBM/ELO gene inactivation, or by the overexpression of SIT4 was sufficient to confer rescue. Finally, we found that mutation of single PKA sites in Tlg1 (Ser31 to Ala31) or Tlg2 (Ser90 to Ala90) was sufficient to restore endocytosis, but not exocytosis, to snc cells. These results suggest that endocytosis is also modulated by t-SNARE phosphorylation in vivo.
INTRODUCTION
Intracellular protein and lipid trafficking involves the selective transfer of cargo molecules from one compartment to the next. This is accomplished by the fusion of membranes derived from the donor compartment with those belonging to a specific target. A number of proteins are involved in the tethering, docking, and fusion steps that confer bilayer fusion and subsequent protein transport. Among those, SNAREs comprise three evolutionarily conserved families (e.g., the syntaxin, synaptobrevin/VAMP, and SNAP-25 families) of membrane-associated proteins that are required for the docking and fusion steps (Ferro-Novick and Jahn, 1994; Rothman and Warren, 1994). On encroachment to the target compartment, SNAREs from the donor or vesicular membranes (v-SNAREs) form complexes in trans with cognate SNARE partners from the opposing target membrane (t-SNAREs). The SNARE complex usually involves three or four SNARE molecules, each contributing one or two α-helical cores to the formation of a four-helix coiled-coil bundle (Sutton et al., 1998; Katz et al., 1998). Formation of this complex is necessary and sufficient for membrane fusion in vitro but necessitates interactions between specific and precisely arrayed SNARE partners (Weber et al., 1998; McNew et al., 2000).
Because SNAREs play a pivotal role in the fusion process, it is likely that they receive input from the cellular environment to either enhance or impede membrane trafficking events. In particular, kinases involved in growth control are likely to modulate SNARE functions, presumably by the posttranslational modification of residues that participate in SNARE partnering or the binding of SNARE regulatory proteins (reviewed in Gerst, 1999; Turner et al., 1999; Klenchin and Martin, 2000). Numerous studies have outlined the possible involvement of kinases in the regulation of SNARE protein interactions (Hirling and Scheller, 1996; Shimazaki et al., 1996; Foster et al., 1998; Shuang et al., 1998; Risinger and Bennett, 1999; Chung et al., 2000) as well as the availability of SNAREs to participate in membrane trafficking events (Cabaniols et al., 1999; Foletti et al., 2000; Kataoka et al., 2000). Therefore, a role for signal-induced protein kinases in regulating SNARE complex assembly is likely and offers a potential mechanism for coordinating the dynamics of cell growth with cell cycle control.
In yeast, the Snc v-SNAREs (Gerst et al., 1992; Protopopov et al., 1993) assemble into fusion complexes with the Sso and Sec9 t-SNAREs to form the exocytic SNARE complex (Brennwald et al., 1994; Couve and Gerst, 1994; Rossi et al., 1997). Deletion of the SNC genes results in the intracellular accumulation of secretory vesicles, in an inhibition in protein secretion, and in various conditional lethal phenotypes (Protopopov et al., 1993; David et al., 1998). Mutations in either of two genes that encode homologous ER-localized proteins (Vbm1,2/Elo2,3), which are involved in long-chain fatty acid (LCFA) elongation, result in the intracellular accumulation of phytosphingosine and rescue snc cells (David et al., 1998). This occurs via activation of a sphingoid base/ceramide-activated phosphatase (CAPP), Sit4, and the subsequent dephosphorylation of discrete PKA sites in the Sso t-SNAREs (Marash and Gerst, 2001). Thus, two signal transduction pathways in yeast converge upon the exocytic t-SNAREs and have opposing effects on their ability to form SNARE complexes. The PKA pathway, which is involved in growth control, phosphorylates Sso proteins and inhibits their assembly into SNARE complexes in vitro and in vivo. In contrast, a CAPP signaling pathway, which mediates cellular stress responses, activates Sit4 and dephosphorylates the Sso t-SNAREs. Dephosphorylation of the t-SNAREs enhances SNARE complex assembly and led to restored secretion in cells bearing mutations in the Snc v-SNAREs or Sso t-SNAREs (Marash and Gerst, 2001).
The Snc exocytic v-SNAREs also mediate endocytosis (Gurunathan et al., 2000). In snc null cells or cells possessing a temperature-sensitive allele of SNC1 (snc1ala43) that were shifted to restrictive temperatures, uptake of the soluble dye FM4-64 and the Ste2 mating factor receptor to the vacuole is abolished. Thus, Snc v-SNAREs confer both anterograde and retrograde transport events between the Golgi and the plasma membrane. Appropriately, their endocytic functions are likely to be dependent on two different t-SNAREs, Tlg1 and Tlg2, which are trans-Golgi and endosomal t-SNAREs involved in endosomal protein sorting (Abeliovich et al., 1998; Holthuis et al., 1998a; Seron et al., 1998; Coe et al., 1999; Abeliovich et al., 1999). This is because Snc proteins coimmunoprecipitate with the Tlg t-SNAREs (Abeliovich et al., 1998; Holthuis et al., 1998a); Snc1ala43 is nonfunctional in their absence (Gurunathan et al., 2000); and snc tlg1 and snc tlg2 strains show synthetically enhanced phenotypes (Gurunathan et al., 2000). Moreover, Tlg1 and 2 have been recently shown to bind Snc v-SNAREs and to mediate an in vitro fusion event similar to endocytosis (Paumet et al., 2001).
Here we show that CAPP activation also confers normal endocytic functioning to cells lacking the Snc v-SNAREs. Rescue occurs by either the exogenous treatment of cells with active ceramide analogs, by VBM gene inactivation, or by overexpression of SIT4 itself. Interestingly, endocytic defects in cells lacking either Tlg1 or Tlg2 alone or in combination with deletions in the SNC genes are also rescued by CAPP activation. In all cases, rescue correlated with the dephosphorylation of Tlg1 and Tlg2 and their assembly into complexes both in vivo and in vitro. Finally, we demonstrate that the mutation of single PKA sites in the putative NH2-terminal auto-inhibitory domains of Tlg1 and Tlg2 results in constitutively activated SNAREs that confer endocytosis in the absence of CAPP activation. Thus, both the PKA and CAPP signaling pathways modulate endocytosis by regulating phosphorylation of the Tlg t-SNAREs and their ability to assemble into SNARE complexes.
MATERIALS AND METHODS
Media, DNA, and Genetic Manipulations
Yeast were grown in standard growth media containing either 2% glucose or 3.5% galactose. Synthetic complete (SC) and dropout media similar to that described (Rose et al., 1990) were prepared. Standard methods were used for the introduction of DNA into yeast and the preparation of genomic DNA (Rose et al., 1990).
Growth Tests
Yeast were grown on synthetic and rich growth media (Rose et al., 1990). In experiments involving ceramide, N-acetyl-d-erythro-sphingosine (C2-ceramide; Sigma, St. Louis, MO) was dissolved in ethanol (at 15 mM or 1 mg/ml) and added to the media to a final concentration of 10 μM. For growth tests on plates, yeast were counted using a hemocytometer, diluted serially, and plated by drops onto solid medium preincubated at different temperatures. For growth of cells in liquid culture, cells were cultured overnight in medium containing C2-ceramide.
For growth tests involving snc and snc tlg mutants, which carry a galactose-inducible form of SNC1, cells were grown to log phase on galactose-containing synthetic medium. Next, cells were shifted to glucose-containing medium (36 h) to induce the snc phenotype. Cells were then seeded at an optical density (OD600) of 0.05 into fresh glucose-containing medium at 26°C with or without the addition of 10 μM C2-ceramide. Cells were monitored for growth (OD measured at 600 nm) every 3 h up to 36 h. Saturation of the culture (with or without ceramide) was reached usually by 27 h. OD600 values were plotted on graphs versus time to show the kinetics of saturation as well as on logarithmic graphs to calculate cell division times for the different strains.
For growth tests involving snc1ala43 and snc1ala43 tlg cells, cells were grown to log phase on glucose-containing medium at 26°C. Cells were then seeded at an OD600 of 0.05 into prewarmed glucose-containing medium either containing or lacking C2-ceramide (10 μM) and grown at 37°C. Cells were monitored for growth as described above.
Yeast Strains
Yeast strains used are listed in Table 1. To create snc tlg vbm1 yeast, the snc vbm1 strains DD1 and MM1 were cured of their GAL-SNC1-containing plasmids and transformed with linearized DNA fragments isolated from the enzymatic digestion of pTLG1L and pTLG2T (Gurunathan et al., 2000). Transformants were selected and the disruptions verified by PCR analysis. To create snc1ala43 tlg yeast, snc tlg cells (JG9-TLG1 or JG9-TLG2) were transformed with either plasmid pLADH-SNC1ala43 or pTADH-SNC1ala43. Transformants were cured of their GAL-SNC1–containing plasmids on synthetic medium at 26°C. For cells expressing STE2-GFP constructs that bear the kanr resistance marker, strains were transformed with YCpKSTE2-GFP and selected for on synthetic medium containing 100 μg/ml geneticin.
Table 1.
Strains used in this study
| Name | Genotype | Source |
|---|---|---|
| JG8 | MATa can1 his3 his4 leu2 trp1 snc1∷URA3 snc2∷ADE8 pTGAL-SNC1 or pLGAL-SNC1 | J. Gerst |
| DD1 | MATa can1 his3 his4 leu2 trp1 snc1∷URA3 snc2∷ADE8 vbm1∷TRP1 pLGAL-SNC1 | J. Gerst |
| MM1 | MATa can1 his3 his4 leu2 trp1 snc1∷URA3 snc2∷ADE8 vbm1∷LEU2 pTGAL-SNC1 | This study |
| JG8 SNC1A43L | MATa can1 his3 his4 leu2 trp1 snc1∷URA3 snc2∷ADE8 pLADH-SNC1ala43 | J. Gerst |
| JG8 SNC1A43T | MATa can1 his3 his4 leu2 trp1 snc1∷URA3 snc2∷ADE8 pTADH-SNC1ala43 | J. Gerst |
| JG9-TLG2 | MATa can1 his3 his4 trp1 snc1∷URA3 snc2∷ADE8 tlg2∷LEU2 pTGAL-SNC1 | J. Gerst |
| JG9-TLG2,VBM1 | MATa can1 his3 his4 trp1 snc1∷URA3 snc2∷ADE8 tlg2∷LEU2 vbm1∷TRP1 | This study |
| JG9-TLG2,SNC1A43 | MATa can1 his3 his4 trp1 snc1∷URA3 snc2∷ADE8 tlg2∷LEU2 pTADH-SNC1ala43 | J. Gerst |
| JG9-TLG1 | MATa can1 his3 his4 leu2 snc1∷URA3 snc2∷ADE8 tlg1∷TRP1 pLGAL-SNC1 | J. Gerst |
| JG9-TLG1,VBM1 | MATa can1 his3 his4 leu2 snc1∷URA3 snc2∷ADE8 tlg1∷TRP1 vbm1∷LEU2 | This study |
| JG9-TLG1,SNC1A43 | MATa can1 his3 his4 leu2 snc1∷URA3 snc2∷ADE8 tlg1∷TRP1 pLADH-SNC1ala43 | J. Gerst |
| SP1 | MATa can1 his3 leu2 trp1 ura3 ade8 | M. Wigler |
| NY778 | MATα ura3-52 leu2-3,112 sec6-4 | P. Novick |
Plasmids
Previously described SNC expression plasmids included the following: pADH-SNC1 (Gerst et al., 1992); pADH-HASNC1 and pTGAL-SNC1 (Protopopov et al., 1993); and pAHGAL-SNC2 (David et al., 1998). Plasmids pRS314STE2-GFP and pRS314ste2Δtail-GFP, which express the STE2-GFP or STE2-tailGFP gene fusions (Stefan and Blumer, 1999), respectively, were provided by K. Blumer (Washington University School of Medicine). snc1ala43 expression constructs included: pADH-SNC1ala43; pTADH-SNC1ala43; and pLADH-SNC1ala43 (Gurunathan et al., 2000). TLG1,2 disruption constructs, pTLG1L and pTLG2T, respectively, were described previously, as was a CEN plasmid that expresses STE2HA, pLADH-STE2HA (Gurunathan et al., 2000). Plasmids for the expression of SIT4 (YEpSIT4) in yeast and TPK1 (pGEX-TPK1) in bacteria were described previously (Marash and Gerst, 2001). Plasmids created for this study are listed in Table 2.
Table 2.
Plasmids created for this study
| Plasmid name | Gene expressed | Vector | Sites of cloning | Oligo namea |
|---|---|---|---|---|
| pGST-SIT4 | SIT4 | pGEX4T-3 | EcoRI and XhoI | Sit4-f, Sit4-r |
| pGST-TLG11–206 | TLG11–206 | pGEX4T-3 | EcoRI and SalI | Tlg1-f, Tlg1-r |
| pGST-TLG21–317 | TLG21–317 | pGEX4T-3 | EcoRI and SalI | Tlg2-f, Tlg2-r |
| pADH-mycTLG1 | myc-TLG1 | pAD6 | SalI and SacI | Tlg1-f, Tlg1-r |
| pADH-mycTLG2 | myc-TLG2 | pAD6 | SalI and SacI | Tlg2-f, Tlg2-r |
| pADH-mycTLG1ala31 | myc-TLG1ala31 | pAD6 | SalI and SacI | Tlg1A31-f, Tlg1A31-r |
| pADH-mycTLG2ala6 | myc-TLG2ala6 | pAD6 | SalI and SacI | Tlg2A6-f, TlgA6-r |
| pADH-mycTLG2ala15 | myc-TLG2ala15 | pAD6 | SalI and SacI | Tlg2A15-f, TlgA15-r |
| pADH-mycTLG2ala54 | myc-TLG2ala54 | pAD6 | SalI and SacI | Tlg2A54-f, TlgA54-r |
| pADH-mycTLG2ala90 | myc-TLG2ala90 | pAD6 | SalI and SacI | Tlg2A90-f, TlgA90-r |
| pADH-mycTLG2ala101 | myc-TLG2ala101 | pAD6 | SalI and SacI | Tlg2A101-f, TlgA101-r |
| pADH-mycTLG2ala138 | myc-TLG2ala138 | pAD6 | SalI and SacI | Tlg2A138-f, TlgA138-r |
| pGEM-SIT4 | SIT4 | pGEM-T | SalI | Sit4-2-f, Sit4-2-r |
| YEpTSIT4b | SIT4 | pRS424 | SalI | |
| pTADH-mycTLG1c | myc-TLG1 | pRS424 | BamHI | |
| YCpKSTE2-GFPd | STE2-GFP | pRS314STE2-GFP | EcoRI | |
| pKADH-SNC1e | SNC1 | pADH-SNC1 | ClaI |
Oligonucleotide sequences will be provided upon request.
This plasmid was created by subcloning a SalI fragment from pGEM-SIT4 into pRS424.
This plasmid was created by subcloning a BamHI fragment from pADH-mycTLG1 into pRS424.
This plasmid was created by subcloning a blunt-ended EcoRV and SalI fragment encoding kanr into pRS314STE2-GFP.
This plasmid was created by subcloning a blunt-ended EcoRV and SalI fragment encoding kanr into pADH-SNC1.
Microscopy
Cells were labeled with FM4-64 and monitored for fluorescence, as described (Gurunathan et al., 2000). GFP fluorescence was visualized via the FITC channel in strains expressing the appropriate GFP fusion proteins. Thin sectioning and electron microscopy was performed essentially as described by Zelicof et al. (1996). Quantification of the vesicles per unit area was calculated by counting the number of vesicles in photos of thin-sectioned cells. Next, the total area counted was determined by weighing cutouts of the cells and dividing them by the weight of a cutout equivalent to 1 μm2. Dividing the former by the latter yields the number of vesicles per μm2. At least 30 cells per strain were used in the measurements.
Metabolic Labeling
Pulse-chase studies for Ste2 uptake, using [35S]methionine (Amersham, Amersham, United Kingdom), were performed essentially as described previously (Hicke and Riezman, 1996). snc cells grown on galactose-containing medium at 26°C were shifted to glucose-containing medium for 30 h before pulse-labeling (30 min) with [35S]methionine (0.1 mCi/OD600 unit). After a 30-min chase in medium containing 5 mM methionine and cysteine, cells were treated with α-factor (1 μM) for up to 60 min. Cell extracts were prepared, and Ste2HA was immunoprecipitated using anti-HA antibodies. Precipitated Ste2HA was solubilized in SDS-containing sample buffer, heated for 15min at 37°C, and resolved on 10% SDS-PAGE gels. For experiments involving snc1ala43 cells at 37°C, strains were shifted to 37°C 1 h before labeling.
SNARE Complex Measurement from Cell Lysates
SNARE complexes present in cell lysates were monitored by the immunoprecipitation (IP) of SNAREs from cell extracts, as described in Couve and Gerst (1994). However, the following additions to the lysis and IP buffers were made: 0.5% NP-40 (instead of Triton X-100); MG132 (100 μM), ATPγS (20 μM), EDTA (2 mM), and n-ethylmaleimide (1 mM), to inhibit SNARE complex dissociation and degradation. Anti-Tlg1 and anti-Tlg2 antibodies (gifts of H. Pelham and H. Abeliovich) were used for IP (1 μl per reaction) and detection (1:2000). The amount of Tlg1 present in heteromeric complexes with Tlg2 was determined using the anti-Tlg2 antiserum for IP and detection with the anti-Tlg1 antiserum. The presence of Tlg1 or Tlg2 in homomeric complexes were determined by expressing myc-tagged Tlg1 or Tlg2 in snc tlg2 and snc tlg1 cells, respectively. IP was performed using anti-myc antibodies (Santa Cruz Biotechnology, Santa Cruz, CA), and subsequent detection of both tagged and untagged proteins was performed using anti-Tlg1 or anti-Tlg2 antibodies. Samples of TCLs, and immunoprecipitates were resolved by electrophoresis and detected by Western blotting. Detection was performed using chemiluminescence (ECL).
Measurement of Tlg Phosphorylation and SNARE Assembly In Vitro
Recombinant GST fusions of Tlg1 and 2, Tpk1, and Sit4 were expressed separately in BL21 Escherichia coli and purified over glutathione-Sepharose beads, using standard procedures. Affinity-purified GST-Tlg11–206 and GST-Tlg21–317 were phosphorylated in vitro by Tpk1, using the procedure described previously for GST-Sso11–265 (Marash and Gerst, 2001). Labeled proteins were detected in SDS-PAGE gels using autoradiography or by protein-dye staining using Coomassie blue (Bio-Rad Laboratories, Richmond, CA). To measure dephosphorylation, recombinant GST-Sit4 (2 μg) or GST-Sit4 (2 μg) with C2-ceramide (0.05 mM) was added. To measure the association of Tlg1 and Tlg2, affinity-purified in vitro phosphorylated or nonphosphorylated GST-Tlg11–206 and GST-Tlg21–317 proteins were mixed together at a ratio of 1:1 (5 μg each) in buffer containing 0.5% Triton X-100 in PBS and allowed to incubate overnight at 4°C. After which, the proteins were IP'd with anti-Tlg1 (1 μl per IP reaction), resuspended in sample buffer, and resolved by SDS-PAGE. Detection and quantification of the proteins using either anti-Tlg1 or -Tlg2 antibodies (1:2000 dilution) was performed using ECL. ECL signals were measured over a linear range of activity, as determined using known quantities of recombinant GST-Tlg proteins as standards in Western blots.
RESULTS
vbm Mutation or the Exogenous Addition of C2-ceramide Rescue Growth Defects in snc, snc tlg, and Temperature-shifted snc1ala43 and snc1ala43 tlg Cells
Previous work demonstrated that defects in exocytosis in snc null cells or cells bearing a temperature-sensitive mutation in the Sso2 t-SNARE can be rescued by the exogenous addition of active sphingoid bases and ceramide analogs to the growth medium. These molecules activate CAPP and mimic the effects seen upon vbm gene mutation in snc yeast, leading to dephosphorylation of the Sso t-SNAREs. Because the Snc v-SNAREs are also involved in endocytosis, we determined whether the exogenous addition of C2-ceramide, an active form of ceramide, or vbm gene mutation can restore normal endocytic trafficking in snc cells. We also wanted to test its effects on snc tlg1 and snc tlg2 yeast, which lack one of the TLG genes and are more growth deficient than snc cells. Because the snc and tlg mutations each block endocytic functioning, it is likely that the synthetic phenotypes observed in the triple mutants result from an additional role for Tlg1 and 2 in anterograde transport (Gurunathan et al., 2000).
First, it was important to determine the effects of ceramide addition or vbm mutation on the growth of snc tlg cells to show whether cells lacking both v- and t-SNAREs are rescued (Table 3). Cells were grown at 26°C in liquid culture in glucose-containing medium in order to shut off SNC1 expression from under a galactose-inducible promoter. In the absence of SNC1 expression, snc and snc tlg cells grew slowly and had doubling times of ∼4 h, whereas snc cells constitutively expressing SNC1 double every 2 h. In contrast, snc and snc tlg cells treated with ceramide had shorter doubling times of 2.4 h and ∼3 h, respectively. Introduction of a vbm1/elo3 mutation in snc tlg yeast had a similar effect and lowered the doubling time to ∼2 h. Both snc tlg vbm1 cells and ceramide-treated snc tlg cells reached the same level of saturation as snc cells constitutively expressing SNC1 (our unpublished results). Finally, we found that snc tlg cells treated with ceramide grew much better at other temperatures (i.e., 15°C, 30°C, 35°C, and 37°C), including snc tlg1 cells, which normally do not grow at 15°C and at temperatures > 26°C (Gurunathan et al., 2000; our unpublished results). Thus, both ceramide treatment and VBM gene disruption, which activate CAPP, restore growth in snc tlg cells, as shown previously for snc cells. We note that the growth of cells bearing the tlg1 mutation was slightly slower than that of snc tlg2 cells, even in the presence of ceramide or after the disruption of VBM1. This corresponds with earlier results that indicate TLG1 disruption is more severe than that of TLG2 (Gurunathan et al., 2000).
Table 3.
Cell division times
| Cell type | Time (h) − C2-ceramide | Time (h) + C2-ceramide |
|---|---|---|
| SNC1 | 2.0 | 2.1 |
| snc | 4.0 | 2.4 |
| snc tlg1 | 4.4 | 3.1 |
| snc tlg2 | 4.1 | 3.0 |
| snc vbm1 | 2.0 | N.D.a |
| snc tlg1 vbm1 | 2.2 | N.D. |
| snc tlg2 vbm1 | 2.0 | N.D. |
| SNC1 tlg1 | 2.2 | 2.1 |
| SNC1 tlg2 | 2.2 | 2.1 |
| SNC1 (37°C) | 2.0 | 2.1 |
| snc1ala43(37°C) | N.A.b | 3.0 |
| snc1ala43 tlg1 (37°C) | N.A. | 3.2 |
| snc1ala43 tlg2 (37°C) | N.A. | 3.1 |
Not determined.
Not applicable, fails to grow at 37°C.
We also tested whether the growth of tlg1 and tlg2 deletion mutants at 26°C is improved upon ceramide treatment or the overexpression of SIT4. We found that tlg cells have a doubling time of ∼2.2 h, whereas after ceramide treatment (Table 3) or the overexpression of SIT4 (our unpublished results) this value remained basically the same (2.1 h for both tlg1 and tlg2 yeast). Because the growth defects in tlg yeast are mild, it is likely that CAPP activation has only little effect.
The snc1ala43 allele encodes a thermosensitive v-SNARE (Gurunathan et al., 2000) that is defective specifically in its retrieval and recycling from the plasma membrane (Grote et al., 2000; Gurunathan et al., 2000; Lewis et al., 2000). When expressed from a single-copy plasmid, snc1ala43 confers the growth of snc cells up to 35°C, although the growth rate is slower than that conferred by native SNC1 (Gurunathan et al., 2000). Interestingly, the expression of snc1ala43 in snc tlg cells does not rescue their growth defects, suggesting that the TLG gene products are essential for Snc1ala43 function and/or recycling (Gurunathan et al., 2000). We next examined the effects of ceramide addition on snc1ala43 and snc1ala43 tlg cells that were shifted to the restrictive temperature (37°C; Table 3). In the absence of ceramide, we found that snc1ala43 and snc1ala43 tlg cells were unable to grow altogether, as shown previously (Gurunathan et al., 2000). However, after ceramide treatment all strains grew robustly at 37°C and had doubling times of ∼3 h. Treated snc1ala43 tlg yeast grew slightly slower than their snc1ala43 counterparts (Table 3), but reached a similar final density (our unpublished results). Thus, ceramide treatment appears to restore the functionality of Snc1ala43.
Ceramide Treatment Inhibits Vesicle Accumulation in snc tlg Cells
Secretory vesicles (SVs) are hardly seen in thin-sectioned wild-type yeast but are common in snc cells growing at permissive temperatures (Protopopov et al., 1993; David et al., 1998). We next examined the accumulation of 100–120 nm SVs in ceramide-treated and untreated snc and snc tlg cells by electron microscopy. We counted the number of SVs present in thin-sectioned snc tlg1 and snc tlg2 cells and found they had 24 ± 1 and 19.0 ± 0.5 vesicles per μm2, respectively, which was similar to control snc cells (17.0 + 0.7 vesicles per μm2). However, upon treatment with ceramide, the number of vesicles per μm2 declined by >60%; snc tlg1 and snc tlg2 cells had 9.0 ± 0.7 and 7.0 ± 0.6 vesicles per μm2, respectively. Control snc cells were found to have 5.0 ± 0.9 vesicles per μm2 after treatment, as shown previously (Marash and Gerst, 2001). Thus, although snc tlg cells accumulate more SVs than snc cells, their numbers are also greatly reduced upon ceramide treatment.
Delivery of the Soluble Dye FM4-64 to the Vacuole Is Restored in snc, snc tlg, and Temperature-shifted snc1ala43 and snc1ala43 tlg Cells upon Ceramide Treatment or vbm Mutation
Because ceramide treatment has pronounced effects on exocytosis (i.e., cell growth and SV accumulation) in both snc and snc tlg yeast, we determined whether endocytosis is restored in these cells by the exogenous addition of C2-ceramide. Previously, we have shown that these strains are defective in the uptake of the vacuolar staining dye, FM4-64 (Gurunathan et al., 2000). Labeling of the cytoplasm and small vacuolar bodies was shown to occur in glucose-shifted snc cells and temperature-shifted snc1ala43 cells as a function of time. snc tlg cells also are labeled in a similar manner, even when expressing SNC1, due to the loss in Tlg functioning (Gurunathan et al., 2000).
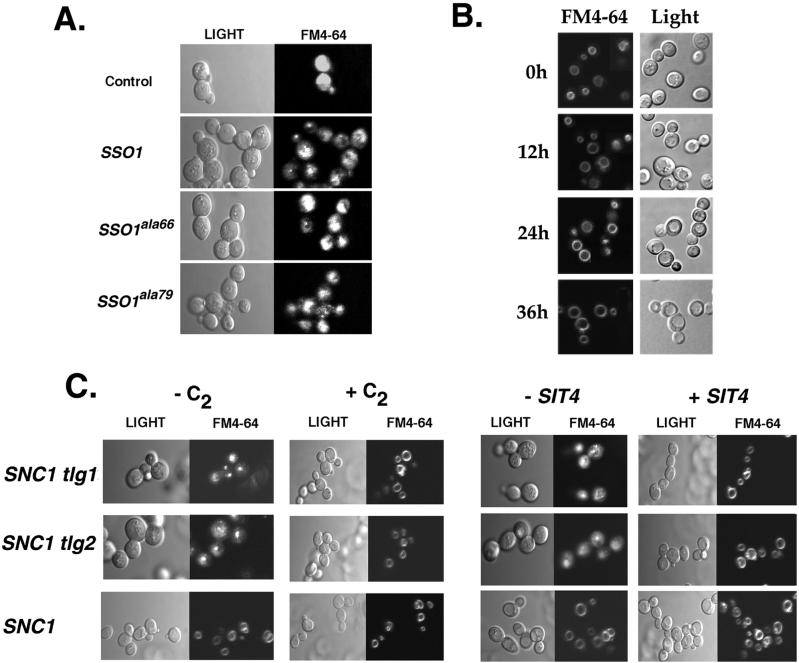
snc and snc tlg strains were shifted to glucose-containing medium for 24 h to induce the snc phenotype. Cells were incubated with FM4-64 for short periods and examined in vivo using fluorescence and visual (Nomarski) microscopy (Figure 1A). As expected, untreated snc cells had a hazy staining of the cytoplasm and small, fragmented, vacuolar bodies. Likewise, snc tlg1 and snc tlg2 cells had an identical staining pattern, unlike control snc cells expressing SNC1. Ceramide treatment restored FM4-64 staining of the vacuole not only in snc cells, but also in snc tlg cells. Moreover, large round vacuoles were apparent in the treated cells, as observed using Nomarski optics. These results suggest that ceramide treatment restores dye trafficking and vacuole biogenesis to snc cells, even in the presence of tlg mutations.
Figure 1.
Delivery of FM4-64 to the vacuole is restored in snc, snc tlg, and temperature-shifted snc1ala43cells by CAPP activation. (A) Delivery of FM4-64 to the vacuole is restored in snc tlg cells. SNC1, snc (JG8), snc tlg1 (JG9-TLG1), and snc tlg2 (JG9-TLG2) cells were grown to log phase on galactose-containing medium, before being shifted to glucose-containing medium at 26°C for 36 h, with (+C2) or without (−C2) C2-ceramide. Cells were incubated with FM4-64 (1 μg/ml) and processed for fluorescence and light (Nomarski) microscopy. (B) Delivery of FM4-64 to the vacuole is restored in snc vbm1 (MM1), snc tlg1 vbm1 (JG9-TLG1, VBM1), and snc tlg2 vbm1 (JG9-TLG2, VBM1) cells. Cells were grown on glucose-containing medium for 24 h at 26°C, incubated with FM4-64, and processed for microscopy. (C) Delivery of FM4-64 to the vacuole is restored in ceramide-treated snc1ala43 cells shifted to restrictive temperatures. snc cells expressing snc1ala43 (JG8-SNC1A43L) were grown in glucose-containing medium at 26°C and either maintained at 26°C during labeling or shifted for 0.5 h to 37°C, before labeling with FM4-64 at the restrictive temperature. Some cells were treated with C2-ceramide (+C2) during growth at 26°C.
Similarly, we found that the mutation of VBM1 in snc tlg cells also restored FM4-64 staining of the vacuole as well as conferring normal vacuole biogenesis (Figure 1B). Thus, both ceramide treatment and vbm mutation have identical effects. Finally, we tested whether ceramide treatment could restore vacuolar staining in temperature-shifted snc1ala43 cells (Figure 1C). Indeed, we found that staining of vacuoles was normal in snc1ala43 cells shifted to 37°C for 2 h. Therefore, defects that arise from inactivating mutations in the v- and t-SNAREs involved in endocytosis are corrected upon CAPP activation.
Because ceramide treatment or vbm mutation result in the dephosphorylation of Sso and rescue snc and sso2–1 cells (Marash and Gerst, 2001), it was possible that the enhanced rate of exocytosis alone might confer an improvement in endocytosis. To test this, we expressed a constitutively activated form of Sso1 (Sso1ala79; Marash and Gerst, 2001), which rescues snc cells in the absence of CAPP activation, in snc yeast and examined the uptake of FM4-64. In contrast to ceramide-treated cells, FM4-64 was unable to label vacuoles or vacuolar bodies in snc yeast expressing Sso1ala79, and the labeling was identical to that seen in control snc cells (Figure 2A). Thus, the effects of ceramide or vbm mutation on endocytosis are direct and not a result of enhanced exocytosis.
Figure 2.
SIT4, but not SSO1ala79, overexpression restores the delivery of FM4-64 to the vacuole in snc cells. (A) Expression of SSO1ala79 does not restore FM4-64 labeling of the vacuole. snc yeast (JG8) transformed with a plasmid expressing SSO1ala79 were grown on galactose-containing medium at 26°C, before being shifted to glucose-containing medium for 36 h to deplete Snc1. Cells were incubated with FM4-64 at 26°C and processed for microscopy. (B) SIT4 overexpression restores FM4-64 labeling of the vacuole. snc cells (JG8) transformed with a plasmid expressing SIT4 were grown and labeled with FM4-64 as described above. (C) Ceramine treatment and SIT4 overexpression restore FM4-64 labeling of the vacuole in tlg yeast. tlg1 and tlg2 yeast treated with (+C2) or without (−C2) ceramide, or overexpressing SIT4, were labeled with FM4-64 and processed for microscopy.
Overproduction of Sit4 Confers FM4-64 Labeling of the Vacuole in snc and tlg Null Cells
Rescue of growth and exocytosis in snc cells by either ceramide treatment or vbm mutation necessitates the activation of the catalytic subunit of CAPP, Sit4 (Nickels and Broach, 1996), whose overexpression can also rescue snc yeast (Marash and Gerst, 2001). Therefore, we next examined whether SIT4 overexpression alone could confer the normal uptake of FM4-64 to the vacuole.
We found that snc cells transformed a plasmid expressing SIT4 gave normal staining of the vacuole by FM4-64, after being shifted from galactose-containing medium to glucose-containing medium (Figure 2B). This rescue was apparent up to 36 h after the shift. However, at later time points it also became apparent that this restoration was temporal, because hazy staining of the cytoplasm and vacuolar fragmentation became more obvious (our unpublished results). This time-dependent decrease in the ability of SIT4 overexpression to restore FM4-64 trafficking could be reversed upon the addition of ceramide into the medium (our unpublished results).
Finally, we examined whether ceramide addition or SIT4 overexpression could confer normal FM4-64 staining of the vacuole in tlg1 and tlg2 mutant cells (Figure 2C). As shown above for snc and snc tlg cells, defects in FM4-64 labeling of the vacuole in tlg cells were abolished upon ceramide addition or SIT4 overexpression.
Ligand-mediated Delivery of the Ste2 Mating Factor Receptor to the Vacuole Is Restored in snc, snc tlg, and Temperature-shifted snc1ala43 Cells by Ceramide Addition or vbm Mutation
Because defects in FM4-64 delivery to the vacuole in snc and tlg mutant yeast are restored independently by CAPP activation, we examined whether ligand-mediated uptake of a cell surface receptor is also rescued. Previously, we demonstrated that uptake of a Ste2-GFP fusion protein (Stefan and Blumer, 1999) to the vacuole (after α-factor treatment) was blocked in snc and temperature-shifted snc1ala43 cells (Gurunathan et al., 2000).
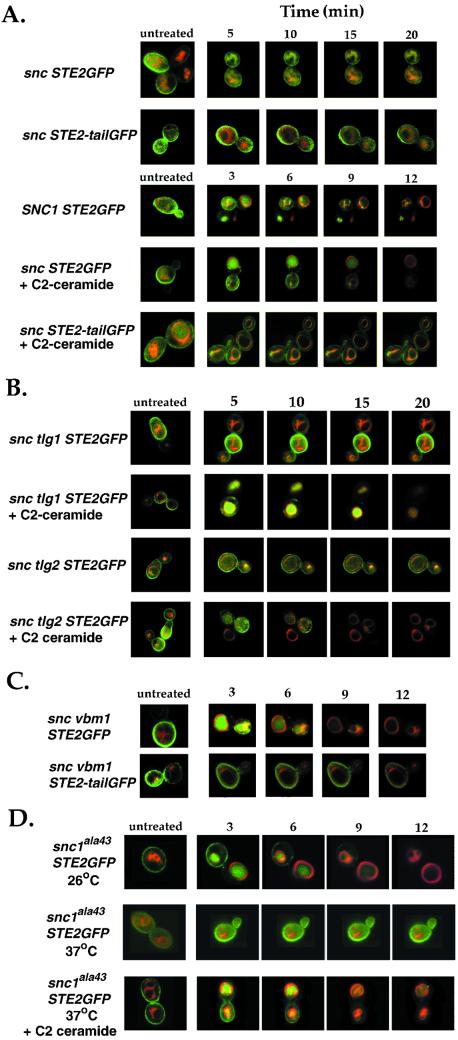
In snc cells (JG8) shifted to glucose-containing medium for 36 h, we found both Ste2-GFP and Ste2-tailGFP present primarily on the plasma membrane in both untreated and α-factor–treated cells (Figure 3A), as described previously. The Ste2-tail GFP protein is deficient in its ability to be endocytosed (Stefan and Blumer, 1999) and served as a control. In contrast to the snc control cells, snc cells expressing SNC1 showed a time-dependent uptake of Ste2-GFP upon α-factor treatment, as described (Gurunathan et al., 2000). We then checked whether ceramide addition had an effect on Ste2 uptake in α-factor–treated snc yeast. Addition of ceramide restored the time-dependent uptake of Ste2-GFP, but not of Ste2-tailGFP, to the vacuole (Figure 3A). The disappearance of Ste2-GFP in ceramide-treated snc cells was similar to that seen for snc cells expressing SNC1, upon α-factor addition. Thus, ceramide treatment restored endocytic uptake of a membrane protein in snc cells.
Figure 3.
Ligand-mediated delivery of Ste2 to the vacuole is restored in snc, snc tlg, and temperature-shifted snc1ala43 cells by CAPP activation. (A) Delivery of Ste2-GFP, but not Ste2-tailGFP, to the vacuole is restored in ceramide-treated snc cells. snc cells (JG8) expressing either Ste2-GFP (snc STE2GFP) or a truncated form of Ste2-GFP (snc STE2-tailGFP), which does not undergo endocytosis, were grown to log phase in galactose-containing medium at 26°C, before being shifted to glucose-containing medium with (+C2) or without (−C2) added C2-ceramide (10 μM) for 36 h, and before labeling with FM4-64. After labeling, cultures were split, whereby half was mounted in soft agar containing no additions, whereas the other half was mounted in agar containing α-factor (1 μM) and processed immediately for microscopy. snc cells expressing SNC1 constitutively (SNC1 STE2GFP) were used as control. Cells were monitored by confocal microscopy as a function of time. Separate untreated cells (untreated) were used as controls. (B) Delivery of Ste2-GFP to the vacuole is restored in ceramide-treated snc tlg cells. snc tlg1 (JG9-TLG1) and snc tlg2 cells (JG9-TLG2) expressing Ste2-GFP (snc tlg1 STE2GFP or snc tlg2 STE2GFP) from the kanr plasmid were grown in galactose-containing medium at 26°C, before being shifted to glucose-containing medium with (+C2) or without (−C2) C2-ceramide (10 μM). After 24 h, cells were labeled with FM4-64, treated with α-factor and processed for microscopy. (C) Ligand-mediated delivery of Ste2-GFP to the vacuole is restored in snc cells bearing a mutation in VBM1. snc vbm1 cells (MM1) expressing either Ste2-GFP (snc vbm1 STE2GFP) or the truncated form of Ste2-GFP (snc vbm1 STE2-tailGFP) were grown to log phase at 26°C in glucose-containing medium, before labeling with FM4-64, treatment with α-factor, and processing for microscopy. (D) Ligand-mediated delivery of Ste2-GFP to the vacuole occurs in temperature-shifted snc1ala43 cells upon ceramide treatment. snc1ala43 cells (JG8-SNC1A43L) expressing Ste2-GFP were grown at 26°C in glucose-containing medium with (+C2) or without (−C2) C2-ceramide (10 μM). Half of each culture was shifted to prewarmed medium (37°C), for 1 h before FM4-64 labeling and treatment with α-factor at the restrictive temperature.
Because ceramide treatment restored FM4-64 labeling of the vacuole in snc tlg cells, we examined the uptake of Ste2-GFP in these strains (Figure 3B). As can be seen, ceramide-treated snc tlg1 and snc tlg2 cells responded to α-factor addition and Ste2-GFP was delivered to the vacuole in a time-dependent manner. In contrast, no change in Ste2-GFP localization was observed in untreated cells, after α-factor addition. Thus, ceramide treatment restores Ste2 uptake in cells lacking one of the TLG genes as well as in cells lacking both SNC genes.
Next, we examined whether Ste2-GFP uptake in snc cells is restored by vbm mutation. We measured Ste2-GFP uptake after α-factor addition to snc vbm1 cells (Figure 3C). As expected Ste2-GFP, but not Ste2-tailGFP, was delivered to the vacuole as shown above for ceramide-treated snc cells or snc cells expressing SNC1 (Figure 3, A and B).
Finally, we examined the uptake of Ste2-GFP in snc1ala43 cells that were shifted to the restrictive temperature (37°C), before the addition of α-factor (Figure 3D). We found that Ste2-GFP was delivered to the vacuole in ceramide-treated, but not untreated, snc1ala43 cells at 37°C. In contrast, Ste2-GFP was taken up in the absence of ceramide at 26°C. Thus, ceramide treatment can confer the uptake and delivery of Ste2 to the vacuole in snc, snc tlg, and in temperature-shifted snc1ala43 cells.
Ste2 Is Degraded in Ceramide-treated snc and Temperature-shifted snc1ala43 Cells
In yeast, some membrane proteins undergo ubiquitin-dependent endocytosis that delivers the internalized protein to the vacuole for degradation (Hicke, 1999). To verify that Ste2 is degraded in ceramide-treated snc and snc1ala43cells, as predicted by the experiments shown in Figure 3, we used an HA-tagged form Ste2 (Ste2HA) in pulse-chase labeling experiments. Previously, we demonstrated that Ste2HA is stable for extended periods in its higher molecular weight, presumably ubiquitinated/phosphorylated form, in α-factor–treated snc and temperature-shifted snc1ala43 cells (Gurunathan et al., 2000). We next tested whether ceramide treatment could restore the normal degradation of Ste2HA in these cells after α-factor treatment (Figure 4).
Figure 4.
Ceramide treatment restores the degradation of Ste2 in snc and temperature-shifted snc1ala43 cells. snc (JG8) and snc1ala43 (JG8-SNC1A43) cells expressing Ste2HA were grown to log phase in glucose-containing medium at 26°C with (+C2) or without (−C2) C2-ceramide. snc cells expressing SNC1 constitutively (SNC1) were grown without ceramide and were used as control. Cells were pulse-labeled with [35S]methionine and chased for 30 min in medium containing 5 mM methionine/cysteine, before treatment with α-factor (1 μM). For snc1ala43 cells, half of the cultures were shifted to prewarmed medium (37°C) for 1 h before labeling. At various times after treatment with α-factor (2–60 min), samples were removed, extracts were prepared, and Ste2HA was IP'd using anti-HA antibodies. Ste2HA was detected by autoradiography.
We found that Ste2HA is stable for up to 60 min in α-factor–treated snc and temperature-shifted snc1ala43 cells that were not treated with ceramide (Figure 4), as was shown previously. Both the lower (47 kDa) and higher (54 kDa) molecular weight forms were apparent, the lower form being present before α-factor addition. In contrast, C2-ceramide treatment restored the degradation of Ste2 in both cell types (Figure 4). The higher molecular weight form apparent after 2 min was found to disappear between 20 and 40 min after the addition of α-factor. The rate of degradation was similar to that seen in snc yeast expressing SNC1. Thus, as suggested by the GFP fluorescence data (Figure 3), ceramide treatment restores Ste2 degradation in snc and temperature-shifted snc1ala43 cells upon α-factor addition.
Ceramide Treatment Enhances Tlg Assembly into Complexes in snc and snc tlg Cells
Our results show that CAPP activation normalizes both the exocytic (Marash and Gerst, 2001) and endocytic pathways (this study). Previously, we demonstrated that rescue of the exocytic path depends on dephosphorylation of the Sso1,2 t-SNAREs, which assemble into t-t-SNARE complexes with Sec9 to, presumably, form the functional trans complex (Marash and Gerst, 2001). The question arises as to which t-SNAREs mediate the endocytosis of materials from the cell surface and whether they also undergo Sit4-dependent dephosphorylation.
Physical and genetic studies show that the Tlg t-SNAREs are involved in endocytosis and interact with the Snc v-SNAREs (Abeliovich et al., 1998; Holthuis et al., 1998a; Seron et al., 1998; Grote and Novick, 1999; Gurunathan et al., 2000). Thus, it is likely that Tlg2, a syntaxin-like t-SNARE, and Tlg1, a SNARE light-chain, form complexes with Snc1,2 at endosomes or the trans-Golgi (TGN). Although Tlg2 has been proposed to act at early endosomes (Abeliovich et al., 1998; Seron et al., 1998), it overlaps with Tlg1, whose placement is broader and encompasses the TGN (Holthuis et al., 1998a, 1998b; Coe et al., 1999). Thus, we conjectured that Tlg1 and 2 could be partners, along with Snc1 or Snc2, in SNARE complexes relevant to endocytic functioning. Although not the focus of this work, a fourth helix is probably contributed to the complex by other SNAREs involved in endosomal functioning, i.e., Vti1.
Both Tlg1 and Tlg2 are phosphorylated in vivo (our unpublished results); thus, we examined whether these t-SNAREs are present in complexes in snc cells and whether the amounts of these complexes increase upon ceramide treatment. By performing coimmunoprecipitation (coIP) experiments, we found that Tlg1 precipitates from snc cell lysates with anti-Tlg2 antibodies and that the amount of Tlg1 in a complex with Tlg2 goes up by substantially after ceramide treatment (Figure 5A). Thus, the assembly of Tlg1 and 2 into SNARE complexes is modulated by CAPP activation. Next, we performed a complimentary experiment whereby we examined whether PKA overexpression affects the assembly of Tlg1 and Tlg2 into complexes in wild-type cells. We found that TPK1 overexpression lowered the amount of Tlg1 and Tlg2 present in complexes by half (Figure 5B). Thus, both the CAPP and PKA signaling paths modulate Tlg SNARE assembly in vivo.
Figure 5.
Ceramide treatment promotes Tlg assembly into Tlg–Tlg SNARE complexes in vivo, whereas Tlg phosphorylation by Tpk1 inhibits assembly in vitro and in vivo. (A) C2-ceramide treatment promotes Tlg1–Tlg2 complex assembly in snc cells. snc cells (JG8) were grown to log phase with (+C2) or without C2-ceramide (−C2; 10 μM). Cell extracts were prepared and Tlg1–Tlg2 SNARE complexes were IP'd using purified anti-Tlg2 antibodies (abs) and detected using either anti-Tlg1 or anti-Tlg2 abs. TCL, total cell lysate (100 μg protein/lane). A histogram of the IP data, after normalization for the expression of TLG1 and TLG2, and subtraction of the background, is shown. Density is given in arbitrary units. (B) TPK1 overexpression inhibits the assembly of Tlg1 and Tlg2 in wild-type cells. Lysates were prepared from SP1 cells that either expressed TPK1 (+TPK1) or not (−TPK1) and had been treated either with (+C2) or without C2-ceramide (−C2). Tlg1–Tlg2 complexes were IP'd using anti-Tlg2 abs, and Tlg1 was detected in immunoblots using anti-Tlg1 abs. Data are represented in histograms, as described in A. (C) Ceramide treatment promotes formation of a Tlg1–Tlg1 complex in snc tlg2 cells. snc tlg2 cells expressing myc-Tlg1 were grown to log phase with (+C2) or without C2-ceramide (−C2; 10 μM). Cell extracts were prepared and myc-Tlg1 was IP'd using anti-myc abs and detected using anti-Tlg1 abs. TCL, total cell lysate (100 μg protein/lane); IP, immunoprecipitate. Data are represented in histograms, as described in A. (D) Ceramide treatment promotes formation of a Tlg2–Tlg2 complex in snc tlg1 cells. snc tlg1 cells expressing myc-Tlg2 were grown to log phase with (+C2) or without C2-ceramide (−C2; 10 μM). Cell extracts were prepared and myc-Tlg2 was IP'd using anti-myc abs and detected using anti-Tlg2 abs. TCL, total cell lysate (100 μg protein/lane); IP, immunoprecipitate. Data are represented in histograms, as described in A. (E) In vitro phosphorylation of GST-Tlg11–206 and GST-Tlg21–317 by Tpk1 and dephosphorylation by Sit4. GST-Tlg11–206 and GST-Tlg21–317 were phosphorylated in vitro using Tpk1 and radiolabeled ATP for 1 h at 30°C with or without added Sit4 or Sit4 and C2-ceramide. Proteins were resolved on SDS-PAGE gels and detected by autoradiography or by staining with Coomassie blue. A histogram of the autoradiography data is shown. (F) Tlg–Tlg assembly is inhibited by phosphorylation. Recombinant GST-Tlg11–206 and GST-Tlg21–317 were phosphorylated in vitro using recombinant Tpk1 and ATP. After phosphorylation, the proteins were mixed and IP'd using anti-Tlg1 abs. In parallel, unphosphorylated GST-Tlg11–206 and GST-Tlg21–317 were also mixed and IP'd using anti-Tlg1 abs. GST-Tlg21–317 that coIP'd with GST-Tlg11–206 was detected using anti-Tlg2 abs. Data are represented in a histogram, as described in A.
Interestingly, CAPP activation did not block the effect of TPK1 overexpression in wild-type cells (Figure 5B) and did not increase Tlg assembly in snc cells expressing SNC1 (our unpublished results). This suggests that the A kinase signaling pathway has a dominant effect in the wild-type background. We also examined whether expression of the activated form of Sso1 (Sso1ala79) affects Tlg1–Tlg2 assembly in snc cells; however, we found no increase in the level of complex formation (our unpublished results). Thus, an enhancement in exocytosis, as mediated by Sso1ala79, does not influence Tlg SNARE assembly (our unpublished results) or endocytosis per se (Figure 2A).
Because CAPP activation confers endocytosis to snc tlg and tlg cells, which lack one of the Tlg proteins, we examined whether ceramide treatment increases assembly of the remaining Tlg into multimeric complexes. This phenomenon was observed previously with the Sso t-SNAREs after ceramide treatment (Marash and Gerst, 2001). We found that ceramide treatment increased the coIP of native Tlg1 with myc-Tlg1 by about twofold in lysates prepared from snc tlg2 cells that express a myc epitope-tagged form of Tlg1 (Figure 5C). Likewise, ceramide treatment increased the coIP of native Tlg2 with myc-Tlg2 by twofold in lysates prepared from snc tlg1 cells expressing myc-Tlg2 (Figure 5D). Thus, individual Tlg t-SNAREs multimerize in response to CAPP activation and, presumably, dephosphorylation.
Tlg Phosphorylation In Vitro by Tpk1 Inhibits Assembly into Complexes
To examine whether Tlg phosphorylation also inhibits assembly in vitro, we expressed recombinant Tlg1 and Tlg2 (lacking their membrane-spanning domains) as GST-fusion proteins in bacteria. Affinity-purified proteins were phosphorylated in vitro with Tpk1 (Marash and Gerst, 2001) and used in subsequent binding reactions. Both recombinant Tlg1 and Tlg2 underwent Tpk1-dependent phosphorylation as well as Sit4-dependent dephosphorylation (Figure 5E). The latter was markedly enhanced by the addition of C2-ceramide into the assay mixture. Binding assays using either phosphorylated or unphosphorylated proteins revealed that the unphosphorylated Tlg t-SNAREs formed two- or threefold more heteromeric complexes than the phosphorylated proteins (Figure 5F). Thus, these endosomal t-SNAREs appear to undergo the same types of regulation shown for the Sso exocytic t-SNAREs, both in vivo and in vitro (Marash and Gerst, 2001).
Mutations in Putative PKA Sites in Tlg1 and 2 Result in Activated t-SNAREs that Confer Endocytosis, but not Exocytosis in snc Cells
Because the restoration of endocytosis by CAPP activation correlates with Tlg dephosphorylation and assembly, we examined potential sites for phosphorylation in Tlg1 and Tlg2. As shown previously (Marash and Gerst, 2001), mutation of a PKA site (ser-79) in the autoinhibitory domain (Habc) of Sso confers full exocytic functioning in the absence of CAPP activation. Therefore, we mutated putative PKA sites in the same region of Tlg1 (e.g., thr-31) and Tlg2 (e.g., thr-6, -15, -54, -138, and ser-90, -101) by site-directed mutagenesis. We tested these mutants for their ability to confer the uptake of FM4-64 in snc yeast in the absence of CAPP activation (Figure 6A and our unpublished results). We found that alanine substitutions at position 31 of Tlg1 and position 90 of Tlg2 restored FM4-64 uptake to the vacuole in snc cells (Figure 6A). In contrast, the other alanine substitutions in Tlg2 were unable to do so (our unpublished results).
Figure 6.
Mutation of thr-31 in Tlg1 and ser-90 in Tlg2 is sufficient to restore endocytosis, but not exocytosis, to snc yeast. (A) Mutation of thr-31 in Tlg1 and ser-90 in Tlg2 restores endocytosis to snc yeast. snc cells (JG8) expressing a control plasmid (snc) or overexpressing SNC1, TLG1, TLG2, TLG1ala31, or TLG2ala90 were grown, shifted to glucose-containing medium for 24 h, and labeled with FM4-64. Cells were visualized using Nomarski optics (Light) or via the rhodamine channel (FM4-64). (B) Mutation of thr-31 in Tlg1 and ser-90 in Tlg2 does not restore growth to snc yeast. snc cells (JG8) expressing a control plasmid (snc) or overexpressing SNC1, TLG1ala31, TLG2ala90, SSO1ala79, or both SSO1ala79and TLG1ala31 or SSO1ala79 and TLG2ala90 were grown, shifted to glucose-containing medium for 24 h, diluted serially and plated onto galactose-containing medium (SC+GAL) at 26°C, glucose-containing medium (SC+GLU) at 26, 35.5, or 37°C, or amino acid-rich medium (YPD) at 26°C. Cells were grown for 48 h.
When tested for conferring growth to snc cells, we found that expression of these mutant Tlg proteins had no significant effect alone, but did enhance the rescue mediated by Sso1ala79 (Figure 6B). Thus, the presence of Sso1ala79 and Tlg1ala31 or Sso1ala79 and Tlg2ala90 restored normal growth (exocytosis) and endocytosis in the absence of CAPP activation. Moreover, their growth appeared more robust than that of snc cells expressing SNC1. Thus, abolishment of these PKA sites leads to dominant effects on the exo- and endocytic pathways in snc yeast.
DISCUSSION
Earlier we demonstrated that yeast lacking the SNC genes (snc, snc tlg1, and snc tlg2 cells) or bearing a temperature-sensitive allele of SNC1 (snc1ala43, snc1ala4tlg1, and snc1ala4 tlg2 cells) are deficient in the endocytic uptake of components from the cell surface (Gurunathan et al., 2000). Thus, the Snc exocytic v-SNAREs are actively involved in endocytosis. More recent work suggests that CAPP activation allows snc and sso2–1 cells to overcome blocks in exocytosis, via dephosphorylation of the Sso t-SNAREs and their subsequent assembly into SNARE complexes with Sec9 (Marash and Gerst, 2001). Thus, we examined whether the endocytic defects present in snc and snc tlg cells are also ameliorated upon CAPP activation. Our results show that ceramide treatment, VBM inactivation, or SIT4 overexpression (all of which lead to CAPP activation) confer normal endocytic functioning to yeast lacking the endocytic v-SNAREs (snc cells), endocytic t-SNAREs (tlg1 and tlg2 cells), or both (snc tlg1 and snc tlg2 cells; Figures 1–3). We had expected snc tlg cells to be even more inhibited in endocytic functions than snc cells, because of the loss of an additional component of the endocytic fusion complex. Nevertheless, CAPP activation also rescues these triple mutants. Thus, CAPP has a prominent role in the control of endocytic functions as well as exocytic functions, as described previously (Marash and Gerst, 2001).
The restoration of endocytosis also involves t-SNARE dephosphorylation and assembly, as ceramide-treated snc cells showed a large increase in Tlg1–Tlg2 complex formation (Figure 5A), whereas snc tlg1 and snc tlg2 cells showed an increase in the formation of Tlg2–Tlg2 or Tlg1–Tlg1 complexes, respectively (Figures 5, C and D). Correspondingly, recombinant Tlg t-SNAREs phosphorylated in vitro showed a reduced ability to assemble into complexes (Figure 5F). Thus, phosphorylation appears to modulate Tlg SNARE assembly, as shown previously for the Sso t-SNAREs (Marash and Gerst, 2001). Interestingly, endocytosis could be restored completely in snc cells expressing mutant Tlg proteins that bear alanine substitutions in specific PKA phosphorylation sites (Figure 6A). Thus, the Tlg endosomal t-SNAREs are likely receivers of regulatory signals arising from the PKA and CAPP pathways, as shown previously for the Sso1,2 exocytic t-SNAREs (Marash and Gerst, 2001).
It is clear from our ongoing work that t-SNARE phosphorylation plays a prominent role in controlling SNARE assembly, particularly in cells that have transport defects. Signaling via the Sit4 catalytic subunit of CAPP relieves environmental stresses placed on the cell (Hannun and Luberto, 2000) as well as blocks in protein trafficking and cell growth (Marash and Gerst, 2001 and this study). These blocks can be at various points in the endo- and exocytic pathways and are modulated by PKA, which confers growth signals based on nutrient availability. Thus, signaling paths that inhibit the cell cycle in wild-type cells, such as the CAPP pathway, and those that stimulate entry, such as the RAS-adenylyl cyclase-PKA pathway, meet at the level of vesicle trafficking. The interactions between these pathways, clearly evident in yeast secretion mutants, are also likely to be operative in wild-type cells although the physiological basis remains undefined. At this point we do not believe that t-SNAREs undergo phosphorylation and dephosphorylation during each round of SNARE assembly, because alanine substitutions (which prevent phosphorylation) in the relevant PKA sites of Sso and Tlg actually enhance protein trafficking (Figure 6 and Marash and Gerst, 2001). It is more likely that phosphorylation regulates the availability of t-SNAREs to participate in trafficking events, although additional work is required to understand this important mechanism.
Other groups have shown that sphingoid bases are involved in endocytosis. Zanolari et al. (2000) demonstrated that sphingoid base synthesis is required for internalization in Saccharomyces cerevisiae, as a lcb1 mutant (involved in serine palmitoyltranferase activity) is defective in endocytic uptake. Moreover, Grote et al. (2000) identified a phosphosphingosine lyase (Dlp1) as a multicopy suppressor of the snc phenotype. Overexpression of Dlp1 also rescued endocytic defects resulting from the Snc1ala43 protein at restrictive temperatures. From the mechanistic point of view, it would appear that Dlp1 elevates the intracellular levels of sphingosine, which we have shown to activate CAPP and lead to the dephosphorylation of Sso (Marash and Gerst, 2001) and Tlg (this study). Interestingly, Friant et al. (2000) demonstrated that the inactivation of other PP2A catalytic subunits (including Pph21, Pph22, and Pph3, but not Sit4) abrogates the sphingoid base requirement in endocytosis. However, these other PP2A subunits had no effect on blocks in exocytosis and could not rescue snc cells (Marash and Gerst, 2001). Thus, Sit4 alone regulates both endo- and exocytosis, whereas the other PP2A catalytic subunits act more specifically (and, apparently, in a contrary manner) at the endocytic level. Because Sit4 dephosphorylates t-SNAREs that have been phosphorylated by PKA, it is likely that the other PP2A activities modulate endocytosis in a different manner.
Ceramides also act as regulators of intracellular trafficking events in mammalian cells (Hannun and Luberto, 2000). For example, the production of ceramide by sphingomyelinase elicits phagocytosis (Grassme et al., 1997; Hinkovska-Galcheva et al., 1998; Zha et al., 1998). Likewise, ceramide treatment has been shown to induce the formation of endocytic vesicles (Li et al., 1999). Thus, it is possible that mammalian CAPP acts on protein trafficking in a similar manner as shown for yeast.
Another finding to come from this work is that ceramide treatment bypasses the deletion of either TLG gene and confers endocytosis. Thus, the rescue mechanism by which CAPP acts is operative in the absence of the endosomal v- or t-SNAREs alone, or in combination. Deletion of either TLG gene is known to affect intracellular protein trafficking and endocytic functions to a degree and in some cell types leads to lethality (Coe et al., 1999), although conditional lethal strains bearing deletions in both genes have been reported (Holthuis et al., 1998a). In our strain background, TLG deletions have prominent effects on endocytosis, but only small effects on cell growth (Gurunathan et al., 2000). Based on the work shown here, it is likely that the deletion of individual TLG genes is tolerated in snc cells, perhaps because of the ability of Tlg proteins to form homomeric complexes (Figure 5, C and D). Although this, by itself, is insufficient to confer endocytosis, CAPP activation by ceramide treatment, VBM/ELO inactivation, or SIT4 overexpression, clearly overcomes any inhibition placed on the existing Tlg t-SNARE. This inhibition is likely to result from Tlg phosphorylation by PKA, based on the in vitro and in vivo experiments performed here (Figures 5 and 6). Thus, as shown earlier for the Sso t-SNAREs (Marash and Gerst, 2001), phosphorylation of the Tlg t-SNAREs modulates their ability to confer endocytic trafficking by inhibiting SNARE assembly. Although the constituents of the endocytic SNARE complex have not been completely resolved, combinations involving Tlg1 and Tlg2 with the Snc v-SNAREs as well as other endosomal SNAREs (i.e., Vti1) are likely to occur. Very recently, two studies demonstrated that Tlg1 and 2 assemble into complexes along with Vti1 to mediate the fusion of trans-Golgi membranes and liposomes in vitro (Brickner et al., 2001; Paumet et al., 2001). Moreover, liposome fusion was dependent on the presence of a Snc v-SNARE, suggesting that a Tlg1–Tlg2–Vti1–Snc complex mediates endosomal trafficking (Paumet et al., 2001). Whether this complex forms in wild-type yeast in vivo has yet to be determined. Moreover, the constituents of the SNARE complexes formed in the two snc tlg strains (which become competent for endocytosis upon CAPP activation) are unknown. Perhaps, a functional t-t-SNARE complex, like that proposed to exist at the exocytic level (Marash and Gerst, 2001), can also confer endocytosis in the absence of the Snc v-SNAREs.
ACKNOWLEDGMENTS
The authors thank Hagai Abeliovich, Kendall Blumer, Hugh Pelham, and Michael Wigler for the generous gifts of antibodies and reagents and Vera Shindler for electron microscopy. This work was generously supported by a grant from the Minerva Foundation, Germany. J.E.G. holds the Henry Kaplan Chair in Cancer Research.
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.01–11–0541. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.01–11–0541.
REFERENCES
- Abeliovich H, Grote E, Novick P, Ferro-Novick S. Tlg2p, a yeast syntaxin homolog that resides on the golgi and endocytic structures. J Biol Chem. 1998;273:11719–11727. doi: 10.1074/jbc.273.19.11719. [DOI] [PubMed] [Google Scholar]
- Abeliovich H, Darsow T, Emr SD. Cytoplasm to vacuole trafficking of aminopeptidase I requires a t-SNARE-Sec1p complex composed of Tlg2p and Vps45p. EMBO J. 1999;18:6005–6016. doi: 10.1093/emboj/18.21.6005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennwald P, Kearns B, Champion K, Keranen S, Bankaitis V, Novick P. Sec9 is a SNAP-25-like component of a yeast SNARE complex that may be the effector of Sec4 function in exocytosis. Cell. 1994;79:245–258. doi: 10.1016/0092-8674(94)90194-5. [DOI] [PubMed] [Google Scholar]
- Brickner JH, Blanchette JM, Sipos G, Fuller RS. The Tlg SNARE complex is required for TGN homotypic fusion. J Cell Biol. 2001;155:969–978. doi: 10.1083/jcb.200104093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabaniols J-P, Ravichandran V, Roche PA. Phosphorylation of SNAP-23 by the novel kinase SNAK regulates t-SNARE complex assembly. Mol Biol Cell. 1999;10:4033–4041. doi: 10.1091/mbc.10.12.4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung SH, Polgar J, Reed GL. Protein kinase C phosphorylation of syntaxin 4 in thrombin-activated human platelets. J Biol Chem. 2000;275:25286–25291. doi: 10.1074/jbc.M004204200. [DOI] [PubMed] [Google Scholar]
- Coe JG, Lim AC, Xu J, Hong W. A role for Tlg1p in the transport of proteins within the golgi apparatus of Saccharomyces cerevisiae. Mol Biol Cell. 1999;10:2407–2423. doi: 10.1091/mbc.10.7.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couve A, Gerst JE. Yeast Snc proteins complex with Sec9. Functional interactions between putative SNARE proteins. J Biol Chem. 1994;269:23391–23394. [PubMed] [Google Scholar]
- David D, Sundarababu S, Gerst JE. Involvement of long chain fatty acid elongation in the trafficking of secretory vesicles in yeast. J Cell Biol. 1998;143:1167–1182. doi: 10.1083/jcb.143.5.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferro-Novick S, Jahn R. Vesicle fusion from yeast to man. Nature. 1994;370:191–193. doi: 10.1038/370191a0. [DOI] [PubMed] [Google Scholar]
- Foletti DL, Lin R, Finley MA, Scheller RH. Phosphorylated syntaxin 1 is localized to discrete domains along a subset of axons. J Neurosci. 2000;20:4535–4544. doi: 10.1523/JNEUROSCI.20-12-04535.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster LJ, Yeung B, Mohtashami M, Ross K, Trimble WS, Klip A. Binary interactions of the SNARE proteins syntaxin-4, SNAP23, and VAMP-2 and their regulation by phosphorylation. Biochemistry. 1998;37:11089–11096. doi: 10.1021/bi980253t. [DOI] [PubMed] [Google Scholar]
- Friant S, Zanolari B, Riezmann H. Increased protein kinase or decreased PP2A activity bypass sphingoid base requirement in endocytosis. EMBO J. 2000;19:2834–2844. doi: 10.1093/emboj/19.12.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerst JE, Rodgers L, Riggs M, Wigler M. SNC1, a yeast homolog of the synaptic vesicle-associated membrane protein/synaptobrevin gene family: genetic interactions with the RAS and CAP genes. Proc Natl Acad Sci USA. 1992;89:4338–4342. doi: 10.1073/pnas.89.10.4338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerst JE. SNAREs and SNARE regulators in membrane fusion and exocytosis. Cell Mol Life Sci. 1999;55:707–734. doi: 10.1007/s000180050328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassme H, Gulbins E, Brenner B, Ferlin K, Sandhoff K, Harzer K, Lang F, Meyer TF. Acidic sphingomyelinase mediates entry of N. gonorrhoeae into nonphagocytic cells. Cell. 1997;91:605–615. doi: 10.1016/s0092-8674(00)80448-1. [DOI] [PubMed] [Google Scholar]
- Grote E, Novick PJ. Promiscuity in Rab-SNARE interactions. Mol Biol Cell. 1999;10:4149–4161. doi: 10.1091/mbc.10.12.4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grote E, Vlacich G, Pypaert M, Novick PJ. A snc1 endocytosis mutant. Phenotypic analysis and suppression by overproduction of dihydrosphingosine phosphate lyase. Mol Biol Cell. 2000;11:4051–4065. doi: 10.1091/mbc.11.12.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurunathan S, Chapman- Shimshoni D, Trajkovic S, Gerst JE. Yeast exocytic v-SNAREs confer endocytosis. Mol Biol Cell. 2000;11:3629–3643. doi: 10.1091/mbc.11.10.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannun YA, Luberto C. Ceramide in the eukaryotic stress response. Trends Cell Biol. 2000;10:73–80. doi: 10.1016/s0962-8924(99)01694-3. [DOI] [PubMed] [Google Scholar]
- Hicke L, Riezman H. Ubiquitination of a yeast plasma membrane receptor signals its ligand-stimulated endocytosis. Cell. 1996;84:277–287. doi: 10.1016/s0092-8674(00)80982-4. [DOI] [PubMed] [Google Scholar]
- Hicke L. Getting down with ubiquitin: turning of cell-surface receptors, transporters and channels. Trends Cell Biol. 1999;1:1–39. doi: 10.1016/s0962-8924(98)01491-3. [DOI] [PubMed] [Google Scholar]
- Hinkovska-Galcheva V, Kjeldsen L, Mansfield PJ, Boxer LA, Shaymann JA, Suchard SJ. Activation of a plasma membrane-associated neutral sphingomylinase and concomitant ceramide accumulation during IgG-dependent phagocytosis in human polymorphonuclear leukocytes. Blood. 1998;91:4761–4769. [PubMed] [Google Scholar]
- Hirling H, Scheller RH. Phosphorylation of synaptic vesicle proteins: modulation of the α-SNAP interaction with the core complex. Proc Natl Acad Sci USA. 1996;93:11945–11949. doi: 10.1073/pnas.93.21.11945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holthuis JC, Nichols BJ, Dhruvakumar S, Pelham HR. Two syntaxin homologues in the TGN/endosomal system of yeast. EMBO J. 1998a;17:113–126. doi: 10.1093/emboj/17.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holthuis JC, Nichols BJ, Pelham HR. The syntaxin Tlg1p mediates trafficking of chitin synthase III to polarized growth sites in yeast. Mol Biol Cell. 1998b;9:3383–3397. doi: 10.1091/mbc.9.12.3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz L, Hanson PI, Heuser JE, Brennwald P. Genetic and morphological analyses reveal a critical interaction between the C-termini of two SNARE proteins and a parallel four helical arrangement for the exocytic SNARE complex. EMBO J. 1998;17:6200–6209. doi: 10.1093/emboj/17.21.6200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka M, Kuwahara R, Iwasaki S, Shoji-Kasai Y, Takahashi M. Nerve growth factor-induced phosphorylation of SNAP-25 in PC12 cells. a possible involvement in the regulation of SNAP-25 localization. J Neurochem. 2000;74:2058–2066. doi: 10.1046/j.1471-4159.2000.0742058.x. [DOI] [PubMed] [Google Scholar]
- Klenchin VA, Martin TE. Priming in exocytosis. attaining fusion-competence after vesicle docking. Biochimie. 2000;82:399–407. doi: 10.1016/s0300-9084(00)00208-x. [DOI] [PubMed] [Google Scholar]
- Lewis MJ, Nichols BJ, Prescianotto-Baschong C, Riezman H, Pelham HR. Specific retrieval of the exocytic SNARE Snc1p from early yeast endosomes. Mol Biol Cell. 2000;11:23–38. doi: 10.1091/mbc.11.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Blanchette-Mackie J, Ladisch S. Induction of endocytic vesicles by exogenous C6-ceramide. J Biol Chem. 1999;274:21121–21127. doi: 10.1074/jbc.274.30.21121. [DOI] [PubMed] [Google Scholar]
- Marash M, Gerst JE. t-SNARE dephosphorylation promotes SNARE assembly, and exocytosis in yeast. EMBO J. 2001;20:411–421. doi: 10.1093/emboj/20.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNew JA, Parlati F, Fukuda R, Johnston RJ, Paz K, Paumet F, Sollner TH, Rothman JE. Compartmental specificity of cellular membrane fusion encoded in SNARE proteins. Nature. 2000;404:153–159. doi: 10.1038/35025000. [DOI] [PubMed] [Google Scholar]
- Nickels JT, Broach JR. A ceramide-activated protein phosphatase mediates ceramide-induced G1 arrest of Saccharomyces cerevisiae. Genes Dev. 1996;10:382–394. doi: 10.1101/gad.10.4.382. [DOI] [PubMed] [Google Scholar]
- Paumet F, Brugger B, Parlati F, McNew JA, Sollner TH, Rothman JE. A t-SNARE of the endocytic pathway must be activated for fusion. J Cell Biol. 2001;155:961–968. doi: 10.1083/jcb.200104092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protopopov V, Govindan B, Novick P, Gerst JE. Homologs of the synaptobrevin/VAMP family of synaptic vesicle proteins function on the late secretory pathway in S. cerevisiae. Cell. 1993;74:855–861. doi: 10.1016/0092-8674(93)90465-3. [DOI] [PubMed] [Google Scholar]
- Risinger C, Bennett MK. Differential phosphorylation of syntaxin and synaptosome-associated protein of 25 kDa (SNAP-25) isoforms. J Neurochem. 1999;72:614–624. doi: 10.1046/j.1471-4159.1999.0720614.x. [DOI] [PubMed] [Google Scholar]
- Rose MD, Winston F, Hieter P. Methods in yeast genetics. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1990. [Google Scholar]
- Rossi G, Salminen A, Rice LM, Brunger AT, Brennwald P. Analysis of a yeast SNARE complex reveals remarkable similarity to the neuronal SNARE complex and a novel function for the C terminus of the SNAP-25 homolog, Sec9. J Biol Chem. 1997;272:16610–16617. doi: 10.1074/jbc.272.26.16610. [DOI] [PubMed] [Google Scholar]
- Rothman JE, Warren G. Implications of the SNARE hypothesis for intracellular membrane topology and dynamics. Curr Biol. 1994;4:220–233. doi: 10.1016/s0960-9822(00)00051-8. [DOI] [PubMed] [Google Scholar]
- Seron K, Tieaho V, Prescianotto-Bachong C, Aust T, Blondel M-O, Guillaud P, Devilliers G, Rossanese OW, Glick BS, Riezman H, Keranen S, Haguenauer-Tsapis R. A yeast t-SNARE involved in endocytosis. Mol Biol Cell. 1998;9:2873–2889. doi: 10.1091/mbc.9.10.2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazaki Y, Nishiki T, Omori A, Sekiguchi M, Kamata Y, Kozaki S, Takahashi M. Phosphorylation of 25-kDa synaptosome-associated protein. Possible involvement in protein kinase C-mediated regulation of neurotransmitter release. J Biol Chem. 1996;271:14548–14553. doi: 10.1074/jbc.271.24.14548. [DOI] [PubMed] [Google Scholar]
- Shuang R, Zhang L, Fletcher A, Groblewski GE, Pevsner J, Stuenkel EL. Regulation of Munc-18/syntaxin 1A interaction by cyclin-dependent kinase 5 in nerve endings. J Biol Chem. 1998;273:4957–4966. doi: 10.1074/jbc.273.9.4957. [DOI] [PubMed] [Google Scholar]
- Stefan CJ, Blumer KJ. A syntaxin homolog encoded by VAM3 mediates down-regulation of a yeast G protein-coupled receptor. J Biol Chem. 1999;274:1835–1841. doi: 10.1074/jbc.274.3.1835. [DOI] [PubMed] [Google Scholar]
- Sutton RB, Fasshauer D, Jahn R, Brunger AT. Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4A resolution. Nature. 1998;395:347–353. doi: 10.1038/26412. [DOI] [PubMed] [Google Scholar]
- Turner KM, Burgoyne RD, Morgan A. Protein phosphorylation and the regulation of synaptic membrane traffic. Trends Neurosci. 1999;22:459–464. doi: 10.1016/s0166-2236(99)01436-8. [DOI] [PubMed] [Google Scholar]
- Weber T, Zemelman BV, McNew JA, Westermann B, Gmachl M, Parlati F, Sollner TH, Rothman JE. SNAREpins: minimal machinery for membrane fusion. Cell. 1998;92:759–772. doi: 10.1016/s0092-8674(00)81404-x. [DOI] [PubMed] [Google Scholar]
- Zanolari B, Friant S, Funato K, Sutterlin C, Stevenson BJ, Riezman H. Sphingoid base synthesis requirement for endocytosis in Saccharomyces cerevisiae. EMBO J. 2000;19:2824–2833. doi: 10.1093/emboj/19.12.2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelicof A, Protopopov V, David D, Lin XY, Lustgarten V, Gerst JE. Two separate functions are encoded by the carboxyl-terminal domains of the yeast cyclase-associated protein and its mammalian homologs. Dimerization and actin binding. J Biol Chem. 1996;271:18243–18252. doi: 10.1074/jbc.271.30.18243. [DOI] [PubMed] [Google Scholar]
- Zha X, Pierini LM, Leopold PL, Skipa PJ, Tabas I, Maxfield FR. Sphingomyelinase treatment induces ATP-independent endocytosis. J Cell Biol. 1998;140:39–47. doi: 10.1083/jcb.140.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]