Abstract
Dual TCR T cells are a common and natural product of TCR gene rearrangement and thymocyte development. As much as one third of the T cell population may have the capability to express two different TCR specificities on the cell surface. This discovery provoked a reconsideration of the classic model of thymic selection. Many potential roles for dual TCR T cells have since been hypothesized, including posing an autoimmune hazard, dominating alloreactive T cell responses, inducing allergy, and expanding the TCR repertoire to improve protective immunity. Yet, since the initial wave of publications following the discovery of dual TCR T cells, research in the area has slowed. In this study, we aim to provide a brief but comprehensive history of dual TCR T cell research, re-evaluate past observations in the context of current knowledge of the immune system, and identify key issues for future study.
The identity of a T lymphocyte is largely determined by its TCR. The TCR dictates the specificity and influences T cell fate decisions. This central importance to T cell identity led early immunologists to assume T cells express a single TCR specificity to avoid identity confusion, an extension of Burnet’s clonal selection theory of Ab production (1). This assumption shaped our understanding of T cell immunology until 1988 when T cells expressing two in-frame–rearranged TCRβ alleles were cloned from mice (2, 3). Shortly thereafter, T cell clones expressing two different TCR Vα segments were discovered (4, 5). Further investigation indicated that as many as one third of murine T cells express two in-frame–rearranged TCRα transcripts, suggesting that TCRα transcriptional allelic exclusion was virtually absent (6, 7). In 1993, human T cells expressing two different TCR Vα segments on the cell surface were identified, proving the existence of dual TCR–specificity T cells (8). Subsequent studies have estimated that ~10% of αβ T cells express dual surface TCR α-chains (9-11), whereas ~1% express dual surface TCR β-chains (12-16). Despite the abundance of dual TCR T cells and their multiple postulated effects on immunity, modern immunology textbooks provide little consideration of them. In this study, we explore our current understanding of dual TCR T cell biology and examine the consequences of dual TCR expression on immunity.
Allelic exclusion
TCRβ-chain.
Allelic exclusion is the process by which one allele of a gene is expressed whereas the other is silenced. The discrepancy in prevalence between dual TCRα and dual TCRβ cells can be explained by differences in mechanisms of allelic exclusion. TCR β-chain allelic exclusion is stringent and multifaceted. TCR β-chain rearrangement initiates on one allele during the CD4− CD8− double negative stage in an ordered fashion (Dβ-Jβ, then Vβ-DJβ). Several mechanisms combine to prevent simultaneous rearrangement of both alleles, including nuclear localization, chromatin conformation, and accessibility of RAG 1 and 2 (15). Successful rearrangement of an in-frame TCR β-chain results in signaling that halts further rearrangement by inducing RAG protein degradation and initiating the formation of dense chromatin at the TCRβ allele (7, 15, 17, 18). Collectively, these mechanisms severely limit the number of thymocytes expressing two functionally rearranged TCR β-chains.
TCR α-chain.
TCR α-chain rearrangement occurs during the CD4+ CD8+ double positive (DP) stage. In contrast to the sequential rearrangement of the TCRβ locus, TCRα (Vα-Jα) rearrangement occurs on both alleles simultaneously (19, 20). Additionally, the organization of the TCRα locus allows multiple, processive rearrangements of the same allele: proximal Vα and Jα regions pair and distal Vα regions pair to distal Jα regions (21). Therefore, TCRα rearrangement does not have to stop at the formation of an in-frame TCR α-chain, but rather continues until the thymocyte has rearranged a selectable TCR or dies from neglect (20, 22). This lack of transcriptional allelic exclusion is predicted to result in one third of T cells expressing two TCR α-chains, because two thirds of randomly generated reading frames would be abortive (6). Indeed, transcriptional analyses estimate that ~30% of T cells express two functionally rearranged TCR α-chain mRNAs (6, 9, 23-28). The discordance between the fraction of cells expressing two TCR α-chain mRNAs (~30%) and the fraction that express two TCR α-chain proteins on the cell surface (~10%) indicates that posttranslational allelic exclusion mechanisms prevent surface expression of both TCR α-chains on some cells. Two models have been put forth to explain phenotypic allelic exclusion: the competition model and the selective retention model (9, 24, 29-33) (Fig. 1).
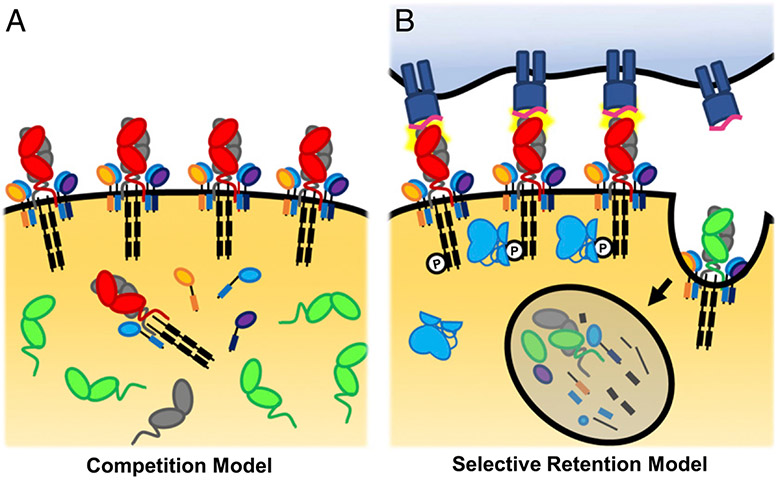
FIGURE 1.
Competition model versus selective retention model. (A) The competition model of phenotypic allelic exclusion proposes that the two rearranged TCR α-chains (red and green) compete for either the pairing TCR β-chain (gray) or CD3 chains (blue, orange, purple, and black). The TCR α-chain with the highest binding affinity for TCRβ and/or the CD3 chains will preferentially form complete TCRs and gain access to the cell surface, whereas the lower-affinity binding TCR α-chain would be excluded from the cell surface. (B) The selective retention model is based on the observation that TCRs that recognize self-peptide–MHC are retained on the cell surface because of signaling-mediated protection from the TCR internalization machinery. TCRαβ pairs that do not recognize self-peptide–MHC are endocytosed and degraded and are, therefore, excluded from the cell surface.
The competition model posits that the TCR α-chains compete for pairing with TCR β- or CD3 chains, with the more favorable pairing dominating the cell surface (30, 32, 34) (Fig. 1A). A transgenic T cell hybridoma line expressing a single TCR β-chain and two different TCR α-chains demonstrated preferential TCRβ pairing and surface expression of one TCR α-chain over the other (34). Similar competition between transgenic and endogenous TCR subchains was reported in TCR transgenic mouse models (32). There is also evidence that interaction between the Vα and Vβ CDR3s can influence pairing; specifically, the HY-specific TCR C6 β-chain pairs efficiently with the C6 α-chain but poorly to other TCR α-chains because of steric incompatibility at CDR3 (35). However, multiple interactions between TCRα, TCRβ, and the CD3 subunits also occur in the conserved TCRα C region (36-38); thus, whereas cases of CDR3 steric incompatibility may exist, most TCR α- and β-chains may pair adequately. Furthermore, artificially increasing TCRβ or CD3ζ expression in an attempt to overcome the effect of competitive binding did not eliminate TCRα phenotypic allelic exclusion, indicating that alternative mechanisms of TCRα surface-expression exclusion exist (9).
The selective retention model posits that in thymocytes with two rearranged TCR α-chains one TCR α-chain transduces signals that promote its persistence on the cell surface, whereas the other TCR α-chain does not. This model is based on observations that TCR signaling protects the TCR from surface downmodulation during auditioning for positive selection (9, 30, 33, 39) (Fig. 1B). However, the mechanism by which this occurs is not entirely understood. Based on available evidence, we propose a model whereby weak TCR–pMHC engagement initiates conformational changes to the TCR complex that prevent internalization.
TCRs on DP thymocytes are constitutively internalized and degraded. ZAP70 and Src-like adaptor protein (SLAP) serve as adaptor proteins that bring the ubiquitin ligase c-Cbl in proximity to CD3ζ, targeting the TCR complex to the lysosome for degradation (9, 40-44). This process ensures the low level of surface TCR expression characteristic of auditioning thymocytes. During auditioning for positive selection, TCR signaling protects the TCR from internalization, but only for that receptor, not a second receptor that is not positively selected. Thus, in the selective retention model, phenotypic allelic exclusion would be established via preferential internalization of the excluded TCR. Indeed, a dual transgenic TCR mouse model demonstrated that proteasomal inhibition did not improve surface expression of the excluded TCR in DP thymocytes; instead, the excluded TCRs were found in internalized vesicles (39).
How might TCR signal strength alter constitutive internalization during positive selection? Following TCR engagement, conformational changes release the CD3 ζ-chains from the lipid bilayer exposing their ITAMs (45). CD3 subchain ITAMs are then phosphorylated in an ordered fashion directly related to the strength of TCR–pMHC interaction: lower-affinity interactions induce low-level CD3ζ ITAM phosphorylation, whereas stronger ones induce robust ITAM phosphorylation on CD3ζ and the other CD3 subchains (46, 47). The popular model predicts that TCR–pMHC engagement brings CD4/8–associated Lck into proximity of the CD3ζ ITAMs to phosphorylate two tyrosine residues that serve as a docking site for inactive ZAP70 (48). Constitutive docking of inactive ZAP70 on CD3ζ has been observed in developing thymocytes, indicating low tonic TCR signaling induced by self-peptide–MHC is common (47, 49, 50). When Boyd et al. (33) analyzed prepositive selection thymocytes expressing transgenic Vα2-containing and endogenous Vα11-containing chains, they found that cross-linking with anti-Vα2 Ab resulted in the retention of Vα2 on the cell surface and the corresponding loss of Vα11. Conversely, cross-linking the transgenic Vβ8.1 chain or CD3ε resulted in downregulation of both Vα2 and Vα11 (33). These findings suggest that TCRα and its unique associated CD3 chains (CD3ζ and δ) protect the TCR complex from internalization (33). Taken together with the ITAM phosphorylation order, it seems likely that CD3ζ ITAM phosphorylation (and perhaps the docking of inactive ZAP70) resulting from weak TCR stimulation could prevent TCR internalization, whereas both unengaged TCRs and high-affinity TCRs would be internalized and degraded (Fig. 2). We posit that in thymocytes with two functionally rearranged TCR α-chains, the net result of these differences in TCR signaling is selective retention of low-affinity TCRs on the cell surface and phenotypic allelic exclusion of both unengaged and high-affinity TCRs. This model is consistent with the capacity for dual TCRα expression to promote positive selection of cells with otherwise unselectable (unengaged) TCRs and also to allow cells with high-affinity TCRs to escape clonal deletion as discussed later.
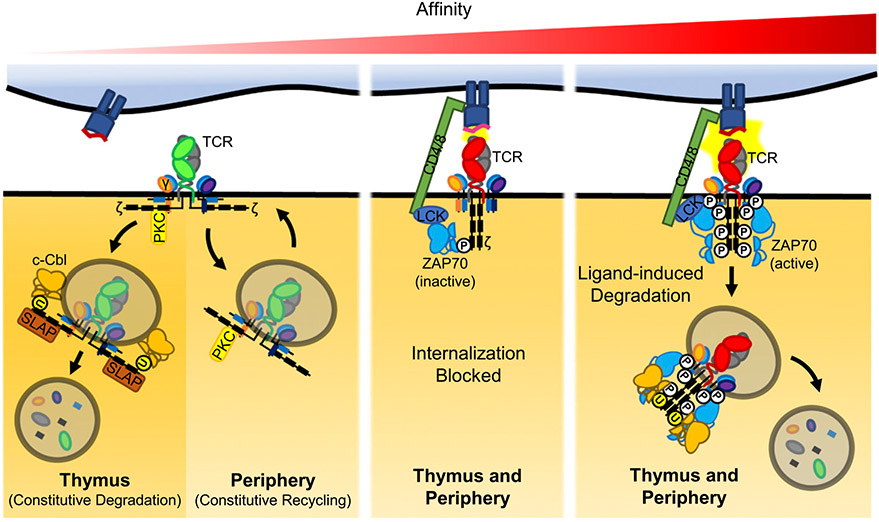
FIGURE 2.
TCR ligand affinity dictates surface expression. TCR are constitutively internalized in developing thymocytes and mature T cells. In DP thymocytes, internalized TCR are constitutively degraded. ZAP70 or SLAP serve as adaptors to mediate the ubiquitination of the CD3 ζ-chain by c-Cbl, which directs the cell to the lysosome for degradation. In contrast, internalized TCR are routinely recycled to the cell surface via the CD3γ–PKC pathway in mature T cells. During positive selection, TCRs engaged with self-peptide–MHC are retained on the cell surface. In dual TCR T cells, this method could result in preferential surface expression of one TCR, whereas the other is constitutively degraded, thus establishing phenotypic allelic exclusion. We predict that similar low-level self-peptide–MHC recognition in the periphery maintains phenotypic allelic exclusion. Conformational changes to CD3 ζ-chain following TCR engagement free the CD3ζ ITAMs from the lipid bilayer to be phosphorylated and serve as docking sites for inactive ZAP70. We suspect this interferes with the constitutive recycling pathway machinery to maintain surface expression of the engaged TCR. Higher-affinity TCR interactions result in Lck-mediated activation of ZAP70, which leads to the spread of CD3 chain ITAM phosphorylation. Strongly activated TCR is then internalized and degraded through a distinct mechanism. Selective downmodulation of TCR following high-affinity interactions also have the potential to alter TCR composition on dual TCR T cells, indicating dual TCR surface composition is a likely dynamic.
Whether the TCRα phenotypic allelic exclusion established in thymocytes is maintained in mature T cells is not known, but the similar proportion of dual-surface TCR T cells among postselection thymocytes and peripheral T cells implies some level of preservation. The mechanisms that maintain TCRα allelic exclusion in mature T cells have not been described, but it is logical to surmise the mechanism that establishes TCRα allelic exclusion also maintains it, with the notable exceptions that mature T cells exhibit low SLAP expression and express Cbl-b rather than its homologue c-Cbl (43, 51). Consequently, in mature T cells, unengaged/internalized TCRs are frequently recycled to the cell surface rather than constitutively degraded. Therefore, phenotypic allelic exclusion could be maintained if the selected TCR continues to receive low-level stimulation, thus preventing its internalization. Indeed, mature T cells frequently encounter self-peptide–MHC capable of minimum TCR stimulation (52). In contrast, high-affinity TCR–pMHC interactions in mature T cells cause widespread phosphorylation of the CD3 chains. Once internalized, these highly phosphorylated TCRs are directed to lysosomes and degraded, rather than recycled, leading to TCR downmodulation; this would also alter surface TCR composition in dual TCR T cells (41, 53, 54) (Fig. 2). Altogether, we propose that both unengaged and strongly engaged TCRs are frequently internalized. In mature T cells, unengaged TCRs persist in endosomes, which can be recycled back to the cell surface, whereas strongly engaged TCRs are degraded in lysosomes. Conversely, low-affinity TCR interactions prevent internalization, thereby maintaining TCR surface expression and phenotypic allelic exclusion.
Effect of dual TCRα expression on T cell signaling
As discussed above, phenotypic allelic exclusion in T cells with two functionally recombined TCRs either arises through competition for the pairing chains or through selective retention of the positively selected TCR α-chain on the cell surface. For T cells in which selective retention determines phenotypic allelic exclusion, the excluded TCRα reaches the cell surface but is preferentially internalized. It, therefore, follows that both TCR α-chains are likely expressed on the cell surface, albeit with one of them typically below the limit of detection. Likewise, depending on their relative affinities for the pairing TCR β-chain, occasional surface expression of the typically excluded TCR α-chain may also occur in the competitive exclusion model. Because engaging a small number of TCRs is sufficient for activation (55, 56), one might question whether TCRα phenotypic allelic exclusion results in functional monospecificity or if signaling through the secondary excluded TCRα could activate the cell. In a TCR transgenic model, dual TCRα T cells with disproportionate TCRα expression levels were stimulated with the cognate Ag of either the minor TCR or the dominant TCR, and proliferation was measured (39). Stimulation of the dominant TCR drove robust proliferation, whereas stimulation of the minor TCR resulted in minimal proliferation over background (39). These data seem to indicate that functional monospecificity can exist in dual TCRα T cells if one of the TCRs is expressed on the cell surface at a level insufficient to activate the cell. However, in this transgenic system, the minor TCR α-chain had fewer transcripts (39). In contrast, normal dual TCRα T cells might be expected to have similar amounts of each TCRα transcript because TCRα allelic exclusion primarily occurs posttranslationally (4, 29). Therefore, the observed monospecificity could have been due to peculiarities of the transgenic system (39). Conversely, other models have demonstrated that TCRs incapable of promoting positive selection when solely expressed could survive selection and respond to Ag in the periphery when a second selectable TCR was coexpressed (57, 58). The unselected TCR specificity was rarely detected by flow cytometric analysis in naive mice but became common following immunization. This outcome could result from robust clonal expansion of a very small number of dual TCR T cells with equivalent initial surface expression of both TCR α-chains. However, the same outcome could result from signaling-induced changes in TCR surface composition such that the unselected TCR α-chain becomes detectable on the cell surface (57) (Fig. 2B). Further investigation is required to understand the plasticity of TCR α-chain surface composition in dual TCRα T cells.
In contrast to cells with disproportionate TCRα surface expression, cells commonly considered to be dual TCRα T cells have detectable and similar levels of both surface TCRs. These cells have repeatedly been shown to respond to both epitopes equivalently to T cells solely expressing either TCR specificity [i.e., they are functionally dual Ag specific (59-66)]. However, the outcome of the response was dictated by the original stimulation. That is, if the initial TCR stimulation resulted in a memory or regulatory T (Treg) cell response, then that response was maintained following subsequent stimulation of the second TCR (63-66).
Can engagement of one TCR affect the function of the second TCR? This question is relevant to understanding how dual TCR expression affects tolerance to both self- and foreign antigens. Different model systems have led to disparate conclusions regarding the degree to which one TCR influences the other in dual TCR T cells (56, 59-62, 67, 68). In one TCR transgenic model, inhibition of one TCR with a TCR-specific antagonist resulted in some level of inhibition of the second TCR not directly bound by the antagonist, although the effect on the second TCR was 10- to 20-fold less pronounced (59). Conversely, another study concluded that the two TCRs can function independently, without cross-regulation. In this in vivo system, dual TCRα T cells tolerized through a TCR recognizing a self-tumor Ag were fully capable of proliferating in response to stimulation of the secondary, foreign Ag-specific TCR (62). Furthermore, the proliferation induced by activating the secondary TCR broke tolerance of the self-specific TCR (62). In a TCR transgenic model expressing both OT-I and P14 TCRs, stimulation of P14 led to downregulation of P14 surface expression and a slight increase in OT-I expression; in contrast, stimulation of OT-I downregulated both TCRs, although the effect on P14 was less pronounced (56). These various effects between model systems have been attributed to differences between CD4 and CD8 T cell biology, the assays used to measure TCR signaling inhibition, the surface proportion of the two TCRs, affinity for peptide–MHC, and the method of tolerance induction, underscoring the complex and context-dependent nature of T cell inhibition (59, 62, 69, 70). Furthermore, since the publication of these studies, numerous T cell–inhibitory pathways have been elucidated (CTLA-4, programmed cell death protein 1 [PD-1], lymphocyte-activation gene 3 [LAG-3], and T cell Ig and mucin domain protein 2 [TIM-2]). As we learn more about these and other T cell–inhibitory pathways, it will be important to evaluate how each pathway influences TCR signaling and tolerance in dual TCR T cells.
Impact of dual TCRα expression on thymic selection
Positive selection.
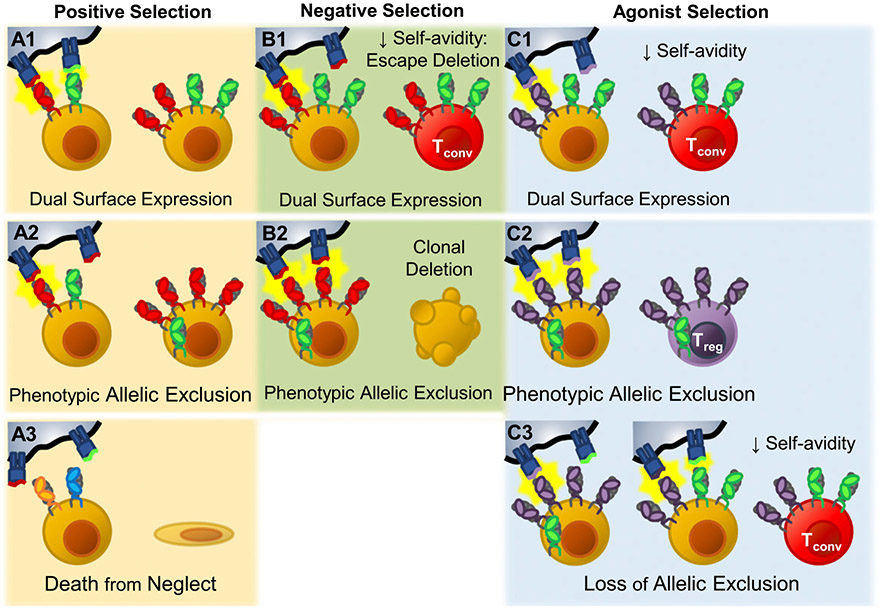
During positive selection a dual TCRα thymocyte has three possible outcomes (Fig. 3A1-3). 1) Both TCRs are retained on the cell surface (Fig. 3A1). Because only 10–15% of all TCRs are capable of positive selection, the chance that a given cell would produce two selectable TCRs is calculated to be 1–2.25%; this scenario might only account for some of the dual surface TCRα-expressing T cells (71, 72). 2) One TCR is retrained on the cell surface, whereas the other is excluded (Fig. 3A2). The cumulative avidity would determine the cell’s fate. In those that survive, phenotypic allelic exclusion may not be absolute, and these cells would have the potential to express both TCRs on their surface in response to cognate Ag. This would explain observations from TCR transgenic mice in which foreign-reactive (57) or autoreactive (58) TCRs are not selected unless a second, selectable TCR is coexpressed. These first two mechanisms underlie the ability of dual TCR expression to expand the TCR repertoire. 3) The final possible outcome is that neither TCR is preferentially retained. If neither TCRα allele rearranges a TCR capable of signaling, the cell dies by neglect (Fig. 3A3).
FIGURE 3.
Effects of dual TCR expression on thymic selection. During positive selection, dual TCR–expressing thymocytes have three potential outcomes. (A1) Both TCRs are retained on the cell surface (Dual TCR T cell). (A2) Only one of the two TCRs is retained. The excluded TCR is preferentially internalized and degraded, leaving the other TCR to populate the cell surface, establishing phenotypic allelic exclusion. Phenotypic allelic exclusion is likely plastic; thus, these cells retain the potential to recognize both specificities. (A3) Neither TCR induces positive selection, and the cell dies from neglect. (B1) During negative selection, thymocytes that express two surface TCRs may experience decreased overall avidity to self-peptide–MHC relative to sole expression of the self-reactive TCR. In this situation dual TCR expression has the potential to allow the strongly self-reactive TCRs to escape clonal deletion. (B2) If phenotypic allelic exclusion has been established and the self-reactive TCR dominates the cell surface, avidity would likely be similar to sole expression of the self-reactive TCR, and the cell would undergo clonal deletion normally. Agonist selection of Treg cells takes place in the medulla where thymocytes are tested against new self-peptide–MHC combinations. (C1) If the thymocyte enters the medulla expressing two TCR specificities on its cell surface, it is possible that the resulting decrease in avidity could limit agonist-mediated commitment to the Treg cell lineage. (C2) If the phenotypic allelic exclusion established in the cortex is maintained in the medulla, agonist selection would be expected to function normally, and Treg cell–biasing TCRs would drive Treg cell commitment. (C3) However, if encountering new self-peptide–MHC combinations alters phenotypic allelic exclusion, it is possible that self-avidity could be reduced, effectively rerouting Treg cell–biasing TCRs into conventional T cell lineages.
Negative selection.
Many have hypothesized that dual TCR expression can decrease negative selection efficiency (8, 26, 62, 67, 73-75). If a dual TCR thymocyte retains equivalent surface expression of both TCR specificities (only one of which has high affinity for self-peptide–MHC), the overall avidity could be diminished, allowing the cell to avoid clonal deletion (Fig. 3B1). However, phenotypic allelic exclusion likely prevents equivalent surface expression of both TCR specificities in most of these cells. The biased surface expression would be expected to limit the influence the second TCR has on overall avidity, resulting in appropriate deletion of the majority of strongly self-reactive dual TCRα thymocytes (Fig. 3B2). The effect of dual TCR expression on negative selection and its downstream impacts remain an area of debate that will likely continue until better tools to detect dual TCR T cells are created.
Agonist selection.
Recently we demonstrated that dual TCR expression might also limit agonist selection of thymic Treg cells (76). Thymocytes that survive positive and negative selection in the cortex migrate to the medulla as single positive (SP) thymocytes where they encounter a new assemblage of self-peptide–MHC. Here, strongly self-reactive CD4+ T cells may be clonally deleted or undergo agonist selection to become Treg cells (77). In NOD mice lacking dual TCRα expression (TCRα+/−), we observed an increased ratio of Treg cells to SP cells in the thymus and less apoptosis among the signaled CD4SP population, indicating increased Treg commitment and decreased clonal deletion (76). This suggests that dual TCRα-expressing Treg cells should be rare, yet human thymic and peripheral T cells expressing both Vα12 and Vα2 TCRs were three times as frequent in the CD25+ Treg cell–enriched population versus the CD25− population, leading to the interpretation that dual TCRα expression may be common in Treg cells (78). However, both Vα12 and Vα2 were individually enriched in the CD25+ thymic and peripheral populations, which could imply that these Vα regions favor Treg commitment; this effect would be compounded in cells expressing both V α-chains, resulting in an even higher rate of Treg commitment (78).
Although the exact mechanisms that determine thymic Treg cell commitment are not fully elucidated, transient strong interactions with self-peptide–MHC are required (77, 79-83). It is reasonable that surface expression of a secondary TCR could reduce overall avidity, thereby limiting TCR signal strength and Treg cell lineage commitment (Fig. 3C1). Although many dual TCRα T cells would be expected to have downregulated the unselected TCR and display phenotypic allelic exclusion at the SP stage (24, 29) (Fig. 3C2), it is worth noting that thymocytes encounter previously unseen self-peptide:MHC combinations in the medulla. Therefore, it is likely that medullary thymocytes perceive different TCR signal strength than they experienced in the cortex. This change in self-peptide–MHC exposure theoretically could alter TCR surface composition (Fig. 3C3). Presently, how TCR signaling affects surface TCR expression during agonist selection of Treg cells is unknown. Whether dual TCR expression impacts conversion and homeostasis of peripheral Treg cells has not been studied. Elegant studies of Treg cell lineage commitment in the context of single TCR expression are helping to inform new hypotheses regarding how dual TCR expression might impact agonist selection (82, 84).
Implications in disease
Autoimmunity.
Many have speculated that dual TCRα expression might allow self-reactive thymocytes to escape clonal deletion because of reduced surface expression of the self-reactive TCR, effectively decreasing avidity and allowing the self-reactive TCRs to stow away (8, 26, 62, 67, 73-75, 85). Several TCR transgenic mouse models have demonstrated this effect (73, 75, 85). However, most of these models have premature TCR expression, an unnaturally high number of dual TCRα T cells, and a heavily skewed TCR repertoire. TCR transgenic systems thus describe a “can-happen” scenario by which dual TCRα expression might allow a self-reactive thymocyte to avoid clonal deletion. In contrast, a meaningful impact of dual TCRα expression on negative selection in the context of a normal (nontransgenic) TCR repertoire has not been described (26, 86, 87). Therefore, in the normal state, phenotypic allelic exclusion appears competent to maintain high-fidelity clonal deletion of the majority of potentially hazardous self-reactive dual TCRα thymocytes (85). The seemingly contradictory observations regarding the impact of dual TCRα expression on agonist selection of Treg cells further confounds our understanding of how dual TCRα T cells contribute to autoimmunity and emphasizes the need for more direct evidence demonstrating how dual TCRα expression affects Treg cell lineage commitment (76, 78).
Allergy.
Two studies have demonstrated that pathogen recognition by one TCR can result in cross-activation of the other allergen-specific TCR, triggering immune responses to an otherwise innocuous Ag (66, 88). As with the autoimmune hazard theory, these conclusions derive from TCR transgenic systems; how common this phenomenon is and whether it contributes to allergic disease in organisms with normal immune systems remains unknown.
Alloreactivity.
The alloreactive T cell population has been shown to contain a very high proportion of dual TCRα T cells (89-92). Furthermore, alloreactive responses were measurably decreased in mouse models lacking dual TCRα T cells (TCRα+/−) (72-75). More information on dual TCR expression in alloreactivity and graft-versus-host disease can be found in recent reviews (93, 94).
Evolutionary pressure
The question of whether dual TCR expression is subject to evolutionary pressures is complex. Dual TCR expression has several hypothesized negative risks, including alloreactivity, allergy, and autoimmunity. Outside of pregnancy, alloreactivity would not be expected to exert any natural evolutionary selective pressure. Severe forms of allergy and autoimmunity that impair reproductive fitness have been selected against over evolutionary time. Yet virtually all humans have dual TCRα T cells, suggesting that dual TCRα expression is either not strongly selected against or that the risk of disease predilection is balanced by the benefit of immune protection.
Dual TCR expression has been hypothesized to expand the repertoire to include TCR specificities that would otherwise not survive selection. This expansion may provide evolutionary benefit by improving protective immunity (57, 95). If true, dual TCR expression would only offer an evolutionary benefit if both TCRs can signal from the cell surface. However, many T cells have two rearranged TCR α-chains but because of phenotypic allelic exclusion are presumed to be monospecific, negating the proposed evolutionary benefit. It is the opinion of the authors that the dynamic nature of TCR surface expression makes it likely that dual TCR T cells are functionally dual specific. In support of this view, it has been suggested that TCRs recycled from the cell surface can serve as an intracellular store of functional TCR that can be rapidly directed to the immune synapse after ligand engagement of surface TCRs (96). In T cells with two rearranged TCR α-chains, this intracellular store contains surface-excluded TCR α-chains that could be mobilized, overcoming phenotypic allelic exclusion and allowing the cell to be activated in response to this second specificity (Fig. 2B). Thus, the fraction of T cells with two different surface TCR specificities is likely larger than one in ten, perhaps as high as one in three (6).
An alternative view is that dual TCR expression exists as a byproduct of the processive nature of TCR α-chain rearrangement. To optimize the chances that a positively selected TCR is generated, both TCRα alleles undergo multiple processive rearrangements simultaneously (21). This greatly increases the size of the TCR repertoire screened during positive selection. Because both processive rearrangement and dual TCR expression require continued availability of the rearrangement machinery, it is plausible that the evolutionary benefit is caused solely by the repertoire expansion provided by processive rearrangement. In this scenario, dual TCRα expression occurs as a byproduct but does not provide any evolutionary benefit per se. In contrast, it is theoretically possible to achieve the similar-sized repertoire of TCR α-chains if processive rearrangement were to occur sequentially rather than simultaneously, if a mechanism were to exist that specifically prevented dual TCRα expression while allowing processive rearrangement. Such a mechanism would limit dual TCRα expression like dual TCRβ expression but might reduce the efficiency of TCRα rearrangement relative to simultaneous recombination of both alleles by increasing the average time required to form a selectable TCR. Indeed, thymocytes with one functional TCRα allele have decreased selection efficiency relative to thymocytes with two functional alleles and are outcompeted in bone marrow chimera models as a result (87, 90). However, animals with only one TCRα allele are still capable of forming a seemingly normal immune system free of any obvious deleterious phenotype, indicating that simultaneous TCRα rearrangement improves thymocyte development efficiency but is not necessary for normal immune function (87, 90). Viewed this way, an evolutionary benefit of simultaneous rearrangement of the TCRα alleles is that it improves thymocyte selection efficiency (i.e., fewer thymocytes are wasted, resulting in net energy conservation for the organism).
In striking contrast to the TCRα loci, multiple mechanisms limit simultaneous recombination of the TCRβ loci, consistent with strong selective pressure against dual TCRβ expression. Why TCRβ recombination has evolved to limit simultaneous recombination is not known but could relate to the need to maintain appropriate γδ T cell numbers. The TCR β-, TCR γ-, and TCR δ-chains all recombine during the double negative stage of thymocyte development and vie for the fate of the cell. If a functional γδ TCR forms before a TCR β-chain, then the cell will develop into a γδ T cell and vice versa. Simultaneous TCRβ recombination would favor αβ T cell lineage commitment and reduce γδ T cell numbers (87); this effect could negatively impact γδ T cell–mediated barrier immunity (97, 98). If so, simultaneous TCRβ recombination would be expected to be selected against. Several other selective pressures might also participate in driving the dichotomy between TCRα and TCRβ allelic exclusion.
Conclusions
The initial discovery of dual TCR T cells was unexpected (2, 3). That as much as 10% of the peripheral T cell population expresses two detectable surface TCR specificities was even more striking. Given that one third of T cells have two productively rearranged TCR α-chains and the apparent plastic nature of TCR surface composition, we speculate that any T cell expressing two rearranged TCR α-chains in the cytosol has the potential to be functionally dual specific.
Whereas posttranslational allelic exclusion describes how surface TCRα composition is established during positive selection, little is known about how dual TCRα surface expression is maintained. Because TCRs constitutively recycle, phenotypic allelic exclusion must be actively maintained in the periphery. The simplest explanation is that the mechanisms that establish phenotypic allelic exclusion also maintain it. We propose that the selected TCR continues to phosphorylate CD3ζ through frequent low-level interaction with self-peptide–MHC in the periphery, consistent with findings of tonic CD3ζ phosphorylation (48-50). Constitutive TCR recycling would then preferentially endocytose the unselected TCR, effectively maintaining phenotypic allelic exclusion.
Surface TCR expression is central to the hypothesized impact of dual TCR expression on cell fate, signaling, and immunity. Because T cell identity depends on TCR specificity and signal strength, it is natural to question whether expression of a second TCR specificity would confuse fate decisions and cell responses. Does dual TCR expression create an identity crisis within the T cell, resulting in inappropriate responses to stimuli (i.e., allergy or autoimmunity), or is it an efficient means to build multitasking T cells able to recognize multiple Ags, thereby improving protective immunity? The major limitation to answering this and other questions regarding dual TCR T cell biology in the context of normal immunity is the inability to detect all dual TCR T cells, so it remains unclear how often these scenarios occur, how they are regulated, and how they impact immune function. To advance this field, new tools must be generated to improve our ability to detect dual TCR expression in organisms with normal T cell repertoires.
Acknowledgments
We thank Drs. Kristin Hogquist and Marc Jenkins for helpful suggestions.
This work was supported by a University of Minnesota Department of Pediatrics grant.
Abbreviations used in this article:
- DP
double positive
- SLAP
Src-like adaptor protein
- SP
single positive
- Treg
regulatory T
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Burnet FM 1959. The Clonal Selection Theory of Acquired Immunity. Vanderbilt University Press, Nashville. [Google Scholar]
- 2.Matis LA, Ezquerra A, and Coligan JE. 1988. Expression of two distinct T cell receptor alpha/beta heterodimers by an antigen-specific T cell clone. J. Exp. Med 168: 2379–2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Triebel F, Breathnach R, Graziani M, Hercend T, and Debre P. 1988. Evidence for expression of two distinct T cell receptor beta-chain transcripts in a human diphtheria toxoid-specific T cell clone. J. Immunol 140: 300–304. [PubMed] [Google Scholar]
- 4.Furutani M, Yanagi Y, Fujisawa I, Nakayama T, Kishimoto H, Kuida K, Asano Y, and Tada T. 1989. Post-transcriptional allelic exclusion of two functionally rearranged T cell receptor alpha genes. Int. Immunol 1: 281–288. [DOI] [PubMed] [Google Scholar]
- 5.Kuida K, Furutani-Seiki M, Saito T, Kishimoto H, Sano K, and Tada T. 1991. Post-translational attainment of allelic exclusion of the T cell receptor alpha chain in a T cell clone. Int. Immunol 3: 75–82. [DOI] [PubMed] [Google Scholar]
- 6.Casanova JL, Romero P, Widmann C, Kourilsky P, and Maryanski JL. 1991. T cell receptor genes in a series of class I major histocompatibility complex-restricted cytotoxic T lymphocyte clones specific for a Plasmodium berghei nonapeptide: implications for T cell allelic exclusion and antigen-specific repertoire. J. Exp. Med 174: 1371–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malissen M, Trucy J, Jouvin-Marche E, Cazenave PA, Scollay R, and Malissen B. 1992. Regulation of TCR alpha and beta gene allelic exclusion during T-cell development. Immunol. Today 13: 315–322. [DOI] [PubMed] [Google Scholar]
- 8.Padovan E, Casorati G, Dellabona P, Meyer S, Brockhaus M, and Lanzavecchia A. 1993. Expression of two T cell receptor alpha chains: dual receptor T cells. Science 262: 422–424. [DOI] [PubMed] [Google Scholar]
- 9.Niederberger N, Holmberg K, Alam SM, Sakati W, Naramura M, Gu H, and Gascoigne NR. 2003. Allelic exclusion of the TCR alpha-chain is an active process requiring TCR-mediated signaling and c-Cbl. J. Immunol 170: 4557–4563. [DOI] [PubMed] [Google Scholar]
- 10.von Boehmer H, and Melchers F. 2010. Checkpoints in lymphocyte development and autoimmune disease. Nat. Immunol 11: 14–20. [DOI] [PubMed] [Google Scholar]
- 11.Heath WR, Carbone FR, Bertolino P, Kelly J, Cose S, and Miller JF. 1995. Expression of two T cell receptor alpha chains on the surface of normal murine T cells. Eur. J. Immunol 25: 1617–1623. [DOI] [PubMed] [Google Scholar]
- 12.Balomenos D, Balderas RS, Mulvany KP, Kaye J, Kono DH, and Theofilopoulos AN. 1995. Incomplete T cell receptor V beta allelic exclusion and dual V beta-expressing cells. J. Immunol 155: 3308–3312. [PubMed] [Google Scholar]
- 13.Steinel NC, Brady BL, Carpenter AC, Yang-Iott KS, and Bassing CH. 2010. Posttranscriptional silencing of VbetaDJbetaCbeta genes contributes to TCRbeta allelic exclusion in mammalian lymphocytes. J. Immunol 185: 1055–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sieh P, and Chen J. 2001. Distinct control of the frequency and allelic exclusion of the V beta gene rearrangement at the TCR beta locus. J. Immunol 167: 2121–2129. [DOI] [PubMed] [Google Scholar]
- 15.Brady BL, Steinel NC, and Bassing CH. 2010. Antigen receptor allelic exclusion: an update and reappraisal. J. Immunol 185: 3801–3808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davodeau F, Peyrat MA, Romagné F, Necker A, Hallet MM, Vié H, and Bonneville M. 1995. Dual T cell receptor beta chain expression on human T lymphocytes. J. Exp. Med 181: 1391–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jackson A, Kondilis HD, Khor B, Sleckman BP, and Krangel MS. 2005. Regulation of T cell receptor beta allelic exclusion at a level beyond accessibility. Nat. Immunol 6: 189–197. [DOI] [PubMed] [Google Scholar]
- 18.Aifantis I, Buer J, von Boehmer H, and Azogui O. 1997. Essential role of the pre-T cell receptor in allelic exclusion of the T cell receptor beta locus. Immunity 7: 601–607. [DOI] [PubMed] [Google Scholar]
- 19.von Boehmer H. 1990. Developmental biology of T cells in T cell-receptor transgenic mice. Annu. Rev. Immunol 8: 531–556. [DOI] [PubMed] [Google Scholar]
- 20.Borgulya P, Kishi H, Uematsu Y, and von Boehmer H. 1992. Exclusion and inclusion of alpha and beta T cell receptor alleles. Cell 69: 529–537. [DOI] [PubMed] [Google Scholar]
- 21.Carico ZM, Roy Choudhury K, Zhang B, Zhuang Y, and Krangel MS. 2017. Tcrd rearrangement redirects a processive tcra recombination program to expand the tcra repertoire. Cell Rep. 19: 2157–2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fehling HJ, and von Boehmer H. 1997. Early alpha beta T cell development in the thymus of normal and genetically altered mice. Curr. Opin. Immunol 9: 263–275. [DOI] [PubMed] [Google Scholar]
- 23.Dash P, McClaren JL, Oguin TH III, Rothwell W, Todd B, Morris MY, Becksfort J, Reynolds C, Brown SA, Doherty PC, and Thomas PG. 2011. Paired analysis of TCRα and TCRβ chains at the single-cell level in mice. J. Clin. Invest 121: 288–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alam SM, and Gascoigne NR. 1998. Posttranslational regulation of TCR Valpha allelic exclusion during T cell differentiation. J. Immunol 160: 3883–3890. [PubMed] [Google Scholar]
- 25.Howie B, Sherwood AM, Berkebile AD, Berka J, Emerson RO, Williamson DW, Kirsch I, Vignali M, Rieder MJ, Carlson CS, and Robins HS. 2015. High-throughput pairing of T cell receptor α and β sequences. [Published erratum appears in 2015 Sci. Transl. Med. 7: 309er7.] Sci. Transl. Med 7: 301ra131. [DOI] [PubMed] [Google Scholar]
- 26.Corthay A, Nandakumar KS, and Holmdahl R. 2001. Evaluation of the percentage of peripheral T cells with two different T cell receptor alpha-chains and of their potential role in autoimmunity. J. Autoimmun 16: 423–429. [DOI] [PubMed] [Google Scholar]
- 27.Stubbington MJT, Lönnberg T, Proserpio V, Clare S, Speak AO, Dougan G, and Teichmann SA. 2016. T cell fate and clonality inference from single-cell transcriptomes. Nat. Methods 13: 329–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Redmond D, Poran A, and Elemento O. 2016. Single-cell TCRseq: paired recovery of entire T-cell alpha and beta chain transcripts in T-cell receptors from single-cell RNAseq. Genome Med. 8: 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gascoigne NR, and Alam SM. 1999. Allelic exclusion of the T cell receptor alpha-chain: developmental regulation of a post-translational event. Semin. Immunol 11: 337–347. [DOI] [PubMed] [Google Scholar]
- 30.Sant’Angelo DB, Cresswell P, Janeway CA Jr., and Denzin LK. 2001. Maintenance of TCR clonality in T cells expressing genes for two TCR heterodimers. Proc. Natl. Acad. Sci. USA 98: 6824–6829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elliott JI 1998. Selection of dual Valpha T cells. Eur. J. Immunol 28: 2115–2123. [DOI] [PubMed] [Google Scholar]
- 32.Heemskerk MH, Hagedoorn RS, van der Hoorn MA, van der Veken LT, Hoogeboom M, Kester MG, Willemze R, and Falkenburg JH. 2007. Efficiency of T-cell receptor expression in dual-specific T cells is controlled by the intrinsic qualities of the TCR chains within the TCR-CD3 complex. Blood 109: 235–243. [DOI] [PubMed] [Google Scholar]
- 33.Boyd R, Kozieradzki I, Chidgey A, Mittrücker HW, Bouchard D, Timms E, Kishihara K, Ong CJ, Chui D, Marth JD, et al. 1998. Receptor-specific allelic exclusion of TCRV alpha-chains during development. J. Immunol 161: 1718–1727. [PubMed] [Google Scholar]
- 34.Saito T, Sussman JL, Ashwell JD, and Germain RN. 1989. Marked differences in the efficiency of expression of distinct alpha beta T cell receptor heterodimers. J. Immunol 143: 3379–3384. [PubMed] [Google Scholar]
- 35.Bartok I, Holland SJ, Kessels HW, Silk JD, Alkhinji M, and Dyson J. 2010. T cell receptor CDR3 loops influence alphabeta pairing. Mol. Immunol 47: 1613–1618. [DOI] [PubMed] [Google Scholar]
- 36.Krshnan L, Park S, Im W, Call MJ, and Call ME. 2016. A conserved αβ transmembrane interface forms the core of a compact T-cell receptor-CD3 structure within the membrane. Proc. Natl. Acad. Sci. USA 113: E6649–E6658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuhns MS, Davis MM, and Garcia KC. 2006. Deconstructing the form and function of the TCR/CD3 complex. Immunity 24: 133–139. [DOI] [PubMed] [Google Scholar]
- 38.Birnbaum ME, Berry R, Hsiao YS, Chen Z, Shingu-Vazquez MA, Yu X, Waghray D, Fischer S, McCluskey J, Rossjohn J, et al. 2014. Molecular architecture of the αβ T cell receptor-CD3 complex. Proc. Natl. Acad. Sci. USA 111: 17576–17581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lacorazza HD, and Nikolich-Zugich J. 2004. Exclusion and inclusion of TCR alpha proteins during T cell development in TCR-transgenic and normal mice. J. Immunol 173: 5591–5600. [DOI] [PubMed] [Google Scholar]
- 40.Wang H, Holst J, Woo SR, Guy C, Bettini M, Wang Y, Shafer A, Naramura M, Mingueneau M, Dragone LL, et al. 2010. Tonic ubiquitylation controls T-cell receptor:CD3 complex expression during T-cell development. EMBO J. 29: 1285–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu H, Rhodes M, Wiest DL, and Vignali DA. 2000. On the dynamics of TCR:CD3 complex cell surface expression and downmodulation. Immunity 13: 665–675. [DOI] [PubMed] [Google Scholar]
- 42.Wang HY, Altman Y, Fang D, Elly C, Dai Y, Shao Y, and Liu YC. 2001. Cbl promotes ubiquitination of the T cell receptor zeta through an adaptor function of Zap-70. J. Biol. Chem 276: 26004–26011. [DOI] [PubMed] [Google Scholar]
- 43.Sosinowski T, Killeen N, and Weiss A. 2001. The Src-like adaptor protein downregulates the T cell receptor on CD4+CD8+ thymocytes and regulates positive selection. Immunity 15: 457–466. [DOI] [PubMed] [Google Scholar]
- 44.Myers MD, Dragone LL, and Weiss A. 2005. Src-like adaptor protein downregulates T cell receptor (TCR)-CD3 expression by targeting TCRzeta for degradation. J. Cell Biol 170: 285–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wucherpfennig KW, Gagnon E, Call MJ, Huseby ES, and Call ME. 2010. Structural biology of the T-cell receptor: insights into receptor assembly, ligand recognition, and initiation of signaling. Cold Spring Harb. Perspect. Biol 2: a005140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sloan-Lancaster J, Shaw AS, Rothbard JB, and Allen PM. 1994. Partial T cell signaling: altered phospho-zeta and lack of zap70 recruitment in APL-induced T cell anergy. Cell 79: 913–922. [DOI] [PubMed] [Google Scholar]
- 47.Kersh EN, Shaw AS, and Allen PM. 1998. Fidelity of T cell activation through multistep T cell receptor zeta phosphorylation. Science 281: 572–575. [DOI] [PubMed] [Google Scholar]
- 48.Goodfellow HS, Frushicheva MP, Ji Q, Cheng DA, Kadlecek TA, Cantor AJ, Kuriyan J, Chakraborty AK, Salomon A, and Weiss A. 2015. The catalytic activity of the kinase ZAP-70 mediates basal signaling and negative feedback of the T cell receptor pathway. Sci. Signal 8: ra49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van Oers NS, Killeen N, and Weiss A. 1994. ZAP-70 is constitutively associated with tyrosine-phosphorylated TCR zeta in murine thymocytes and lymph node T cells. Immunity 1: 675–685. [DOI] [PubMed] [Google Scholar]
- 50.Thill PA, Weiss A, and Chakraborty AK. 2016. Phosphorylation of a tyrosine residue on zap70 by Lck and its subsequent binding via an SH2 domain may Be a key gatekeeper of T cell receptor signaling in vivo. Mol. Cell. Biol 36: 2396–2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu YC, and Gu H. 2002. Cbl and Cbl-b in T-cell regulation. Trends Immunol. 23: 140–143. [DOI] [PubMed] [Google Scholar]
- 52.Jameson SC 2002. Maintaining the norm: T-cell homeostasis. Nat. Rev. Immunol 2: 547–556. [DOI] [PubMed] [Google Scholar]
- 53.Valitutti S, Müller S, Salio M, and Lanzavecchia A. 1997. Degradation of T cell receptor (TCR)-CD3-zeta complexes after antigenic stimulation. J. Exp. Med 185: 1859–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.von Essen M, Bonefeld CM, Siersma V, Rasmussen AB, Lauritsen JP, Nielsen BL, and Geisler C. 2004. Constitutive and ligand-induced TCR degradation. J. Immunol 173: 384–393. [DOI] [PubMed] [Google Scholar]
- 55.Labrecque N, Whitfield LS, Obst R, Waltzinger C, Benoist C, and Mathis D. 2001. How much TCR does a T cell need? Immunity 15: 71–82. [DOI] [PubMed] [Google Scholar]
- 56.Gladow M, Uckert W, and Blankenstein T. 2004. Dual T cell receptor T cells with two defined specificities mediate tumor suppression via both receptors. Eur. J. Immunol 34: 1882–1891. [DOI] [PubMed] [Google Scholar]
- 57.He X, Janeway CA Jr., Levine M, Robinson E, Preston-Hurlburt P, Viret C, and Bottomly K. 2002. Dual receptor T cells extend the immune repertoire for foreign antigens. Nat. Immunol 3: 127–134. [DOI] [PubMed] [Google Scholar]
- 58.Auger JL, Haasken S, Steinert EM, and Binstadt BA. 2012. Incomplete TCR-β allelic exclusion accelerates spontaneous autoimmune arthritis in K/BxN TCR transgenic mice. Eur. J. Immunol 42: 2354–2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang W, and Grey HM. 2003. Study of the mechanism of TCR antagonism using dual-TCR-expressing T cells. J. Immunol 170: 4532–4538. [DOI] [PubMed] [Google Scholar]
- 60.Ji Q, Perchellet A, and Goverman JM. 2010. Viral infection triggers central nervous system autoimmunity via activation of CD8+ T cells expressing dual TCRs. Nat. Immunol 11: 628–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hah C, Kim M, and Kim K. 2005. Induction of peripheral tolerance in dual TCR T cells: an evidence for non-dominant signaling by one TCR. J. Biochem. Mol. Biol 38: 334–342. [DOI] [PubMed] [Google Scholar]
- 62.Teague RM, Greenberg PD, Fowler C, Huang MZ, Tan X, Morimoto J, Dossett ML, Huseby ES, and Ohlén C. 2008. Peripheral CD8+ T cell tolerance to self-proteins is regulated proximally at the T cell receptor. Immunity 28: 662–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee WT, Shiledar-Baxi V, Winslow GM, Mix D, and Murphy DB. 1998. Self-restricted dual receptor memory T cells. J. Immunol 161: 4513–4519. [PubMed] [Google Scholar]
- 64.Saparov A, Kraus LA, Cong Y, Marwill J, Xu XY, Elson CO, and Weaver AT. 1999. Memory/effector T cells in TCR transgenic mice develop via recognition of enteric antigens by a second, endogenous TCR. Int. Immunol 11: 1253–1264. [DOI] [PubMed] [Google Scholar]
- 65.Zhou P, Borojevic R, Streutker C, Snider D, Liang H, and Croitoru K. 2004. Expression of dual TCR on DO11.10 T cells allows for ovalbumin-induced oral tolerance to prevent T cell-mediated colitis directed against unrelated enteric bacterial antigens. J. Immunol 172: 1515–1523. [DOI] [PubMed] [Google Scholar]
- 66.Lemaire MM, Dumoutier L, Warmer G, Uyttenhove C, Van Snick J, de Heusch M, Stevens M, and Renauld JC. 2011. Dual TCR expression biases lung inflammation in DO11.10 transgenic mice and promotes neutrophilia via microbiota-induced Th17 differentiation. J. Immunol 187: 3530–3537. [DOI] [PubMed] [Google Scholar]
- 67.Blichfeldt E, Munthe LA, Røtnes JS, and Bogen B. 1996. Dual T cell receptor T cells have a decreased sensitivity to physiological ligands due to reduced density of each T cell receptor. Eur. J. Immunol 26: 2876–2884. [DOI] [PubMed] [Google Scholar]
- 68.Dittel BN, Stefanova I, Germain RN, and Janeway CA Jr. 1999. Cross-antagonism of a T cell clone expressing two distinct T cell receptors. Immunity 11: 289–298. [DOI] [PubMed] [Google Scholar]
- 69.Edwards LJ, and Evavold BD. 2010. A unique unresponsive CD4+ T cell phenotype post TCR antagonism. Cell. Immunol 261: 64–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Edwards LJ, and Evavold BD. 2011. T cell recognition of weak ligands: roles of signaling, receptor number, and affinity. Immunol. Res 50: 39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McDonald BD, Bunker JJ, Erickson SA, Oh-Hora M, and Bendelac A. 2015. Crossreactive αβ T cell receptors are the predominant targets of thymocyte negative selection. Immunity 43: 859–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hernandez JB, Newton RH, and Walsh CM. 2010. Life and death in the thymus--cell death signaling during T cell development. Curr. Opin. Cell Biol 22: 865–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Paterson RK, Bluethmann H, Tseng P, Dunlap A, and Finkel TH. 1999. Development and function of autospecific dual TCR+ T lymphocytes. Int. Immunol 11: 113–119. [DOI] [PubMed] [Google Scholar]
- 74.Smiley ST, and Grusby MJ. 1997. Dual-receptor T cells expressing one self-restricted TCR. Scand. J. Immunol 45: 726–730. [DOI] [PubMed] [Google Scholar]
- 75.Sarukhan A, Garcia C, Lanoue A, and von Boehmer H. 1998. Allelic inclusion of T cell receptor alpha genes poses an autoimmune hazard due to low-level expression of autospecific receptors. Immunity 8: 563–570. [DOI] [PubMed] [Google Scholar]
- 76.Schuldt NJ, Auger JL, Spanier JA, Martinov T, Breed ER, Fife BT, Hogquist KA, and Binstadt BA. 2017. Cutting edge: dual TCRα expression poses an autoimmune hazard by limiting regulatory T cell generation. J. Immunol 199: 33–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stritesky GL, Jameson SC, and Hogquist KA. 2012. Selection of self-reactive T cells in the thymus. Annu. Rev. Immunol 30: 95–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tuovinen H, Salminen JT, and Arstila TP. 2006. Most human thymic and peripheral-blood CD4+ CD25+ regulatory T cells express 2 T-cell receptors. Blood 108: 4063–4070. [DOI] [PubMed] [Google Scholar]
- 79.Malchow S, Leventhal DS, Lee V, Nishi S, Socci ND, and Savage PA. 2016. Aire enforces immune tolerance by directing autoreactive T cells into the regulatory T cell lineage. Immunity 44: 1102–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Malchow S, Leventhal DS, Nishi S, Fischer BI, Shen L, Paner GP, Amit AS, Kang C, Geddes JE, Allison JP, et al. 2013. Aire-dependent thymic development of tumor-associated regulatory T cells. Science 339: 1219–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Malhotra D, Linehan JL, Dileepan T, Lee YJ, Purtha WE, Lu JV, Nelson RW, Fife BT, Orr HT, Anderson MS, et al. 2016. Tolerance is established in polyclonal CD4(+) T cells by distinct mechanisms, according to self-peptide expression patterns. Nat. Immunol 17: 187–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kieback E, Hilgenberg E, Stervbo U, Lampropoulou V, Shen P, Bunse M, Jaimes Y, Boudinot P, Radbruch A, Klemm U, et al. 2016. Thymus-derived regulatory T cells are positively selected on natural self-antigen through cognate interactions of high functional avidity. Immunity 44: 1114–1126. [DOI] [PubMed] [Google Scholar]
- 83.Li MO, and Rudensky AY. 2016. T cell receptor signalling in the control of regulatory T cell differentiation and function. Nat. Rev. Immunol 16: 220–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lio CW, and Hsieh CS. 2008. A two-step process for thymic regulatory T cell development. Immunity 28: 100–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hardardottir F, Baron JL, and Janeway CA Jr. 1995. T cells with two functional antigen-specific receptors. Proc. Natl. Acad. Sci. USA 92: 354–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Elliott JI, and Altmann DM. 1996. Non-obese diabetic mice hemizygous at the T cell receptor alpha locus are susceptible to diabetes and sialitis. Eur. J. Immunol 26: 953–956. [DOI] [PubMed] [Google Scholar]
- 87.Schuldt NJ, Auger JL, Hogquist KA, and Binstadt BA. 2015. Bi-allelic TCRα or β recombination enhances T cell development but is dispensable for antigen responses and experimental autoimmune encephalomyelitis. PLoS One 10: e0145762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Aronica MA, Swaidani S, Zhang YH, Mitchell D, Mora AL, McCarthy S, O’Neal J, Topham D, Sheller JR, and Boothby M. 2004. Susceptibility to allergic lung disease regulated by recall responses of dual-receptor memory T cells. J. Allergy Clin. Immunol 114: 1441–1448. [DOI] [PubMed] [Google Scholar]
- 89.Morris GP, and Allen PM. 2009. Cutting edge: highly alloreactive dual TCR T cells play a dominant role in graft-versus-host disease. J. Immunol 182: 6639–6643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ni PP, Solomon B, Hsieh CS, Allen PM, and Morris GP. 2014. The ability to rearrange dual TCRs enhances positive selection, leading to increased Allo- and Autoreactive T cell repertoires. J. Immunol 193: 1778–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Morris GP, Uy GL, Donermeyer D, Dipersio JF, and Allen PM. 2013. Dual receptor T cells mediate pathologic alloreactivity in patients with acute graft-versus-host disease. Sci. Transl. Med 5: 188ra74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Balakrishnan A, Gloude N, Sasik R, Ball ED, and Morris GP. 2017. Proinflammatory dual receptor T cells in chronic graft-versus-host disease. Biol. Blood Marrow Transplant 23: 1852–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Balakrishnan A, and Morris GP. 2016. The highly alloreactive nature of dual TCR T cells. Curr. Opin. Organ Transplant 21: 22–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Vincent BG, and Serody JS. 2013. One is better than two: TCR pairing and GVHD. Sci. Transl. Med 5: 188fs21. [DOI] [PubMed] [Google Scholar]
- 95.Gavin MA, and Rudensky AY. 2002. Dual TCR T cells: gaining entry into the periphery. Nat. Immunol 3: 109–110. [DOI] [PubMed] [Google Scholar]
- 96.Onnis A, Finetti F, and Baldari CT. 2016. Vesicular trafficking to the immune synapse: how to assemble receptor-tailored pathways from a basic building set. Front. Immunol 7: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fujihashi K, McGhee JR, Kweon MN, Cooper MD, Tonegawa S, Takahashi I, Hiroi T, Mestecky J, and Kiyono H. 1996. gamma/delta T cell-deficient mice have impaired mucosal immunoglobulin A responses. J. Exp. Med 183: 1929–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nielsen MM, Witherden DA, and Havran WL. 2017. γδ T cells in homeostasis and host defence of epithelial barrier tissues. Nat. Rev. Immunol 17: 733–745. [DOI] [PMC free article] [PubMed] [Google Scholar]