Abstract
Background:
This study aimed to investigate the thickness changes of the heel fat pad and the plantar fascia associated with loading and unloading in healthy individuals and patients with heel pain and reveal the differences between them.
Methods:
The study included adult male participants with (n = 9) and without (n = 26) heel pain. The participants placed their right foot on an evaluation apparatus with a polymethylpentene resin board (PMP), while their left foot was positioned on a weighing scale used to adjust the loading weight. The heel fat pad was differentiated into superficial Microchamber and deep Macrochamber layers. These layers and plantar fascia thickness were measured using an ultrasonographic imaging device at loading phase ranging from 0% to 100% of their body weight and unloading phase from 100% to 0%. Additionally, the study examined the thickness change ratios of the superficial and deep heel fat pad layers when the load increased from 0% (unload) to 100% (full load).
Results:
In healthy individuals and patients with heel pain, no significant thickness changes were observed in the Microchamber layer of the heel fat pad or the plantar fascia during loading and unloading evaluations. However, significant thickness changes were observed in the Macrochamber layer of the heel fat pad, and the pattern of change differed between the loading and unloading phases. Additionally, patients with heel pain showed differences in the thickness change and thickness change ratios of the microchamber and macrochamber layers of the heel fat pad during both loading and unloading phases. The thickness of the plantar fascia did not show significant differences between both groups.
Conclusion:
Compared with healthy individuals, in our relatively small study, patients with heel pain had greater deep fat pad compression in loading and less recovery after load removal. This finding suggests that these patients have different intrinsic fat pad function and related morphology than those without heel pain.
Level of Evidence:
Level III, retrospective cohort study.
Keywords: heel fat pad, microchamber layer, macrochamber layer, ultrasound
Introduction
The heel is the initial point of contact (heel strike) in activities such as walking, and it undergoes repeated phases of weightbearing and unloading. Compressive stress on the heel during ground contact can be a contributing factor in heel pain.15,20
Heel pain is a collective term for pain originating from the plantar aspect of the heel. Clinically, it is characterized by symptoms such as tenderness in the same region and morning pain when walking. Although the primary source of pain is the plantar fascia, the fat pad present in the heel (referred to as the heel fat pad) may also be a contributing factor. The heel fat pad primarily absorbs shock and distribution during walking and running. Prolonged exposure to compressive loads may lead to degeneration and breakdown in this area, potentially increasing the risk of heel pain.12,14,25,31 Therefore, understanding the differences between healthy individuals and those with heel fat pad conditions is crucial for understanding the pathogenesis of heel pain and developing preventive measures.
As the function of tissues depends on their structure, many studies have focused on evaluating the function of the heel fat pad based on its morphology. However, some studies suggest an increase in thickness during loading and unloading, and others indicate no change in thickness.11,23,26,27,29 Thus, there is no consensus on the relationship between the morphology of the heel fat pad and heel pain. Additionally, reports on in vitro testing of heel fat pad function have not provided a clear consensus, and assessing the function of dynamic soft tissues with static measures may have limitations. 30 Therefore, evaluating changes in the load on the heel fat pad in a living organism is considered important. Hsu et al 8 have emphasized the importance of assessing dynamic changes to understand soft tissue function and have reported that tissue behavior can suggest the presence of diseases.
Previous studies have investigated changes in the heel fat pad using radiographs, computed tomography scans, magnetic resonance imaging, and ultrasound imaging.2,3,5,6,22,24,25,28,32 However, obtaining a clear and detailed depiction of the internal structure has been challenging. Furthermore, the heel fat pad can be divided into 2 layers: the superficial part, also known as microchambers (MICs), and the deep part, referred to as macrochambers (MACs), separated by fibrous septa (fibrous partitions). MICs are primarily composed of elastic fibers, whereas MACs consist of collagen and elastic fibers, resulting in different tissue morphologies between the 2 layers.4,7,10,13,30 It is hypothesized that they exhibit different dynamic changes under loading. However, research that focuses on the differences between the 2 layers is limited and has various limitations, including weight restrictions of 20 kg; thus, investigating changes from unloading to full loading and vice versa is challenging. To address these issues, we developed an evaluation tool using polymethylpentene resin boards (PMP).7,19 This device allows measurement of changes in the morphology of the heel fat pad during full loading. The resin board used in this device is close to the acoustic impedance of the human body, which offers the potential for precise and detailed measurements of internal structures. 18
This study aimed to use this PMP evaluation tool to investigate changes in heel fat pad and plantar fascia thickness during loading (from unloading to full loading) and unloading (from full loading to unloading) in healthy individuals and patients with heel pain.
Materials and Methods
Participants
A total of 26 healthy adult males (control: CON) and 9 male patients with plantar heel pain (Table 1) were recruited for the study. The healthy adult males who met any of the following criteria were excluded from the study: (1) engaged in vigorous physical activity in the past 48 hours; (2) had a history of foot or ankle surgery or significant trauma; (3) had orthopedic injuries related to the foot or ankle, such as heel pain or ligament damage; (4) had rheumatic diseases like osteoarthritis, gout, or rheumatoid arthritis; and (5) had systemic diseases such as diabetes or connective tissue disorders.
Table 1.
Physical Characteristics of Participants. a
| Variable | Control | Plantar Heel Pain | P Value | 95% CI |
|---|---|---|---|---|
| n | 26 | 9 | ||
| Age, y | 21.8 (3.4) | 22.1 (2.9) | 0.807 | −2.266 to 2.888 |
| Height, cm | 173.9 (5.6) | 172.0 (3.4) | 0.340 | −5.993 to 2.131 |
| Body mass, kg | 81.0 (17.6) | 78.2 (12.6) | 0.462 | −17.857 to 8.302 |
| BMI | 26.6 (4.7) | 25.8 (4.5) | 0.670 | −4.479 to 2.917 |
| Foot length, cm | 27.5 (1.5) | 27.3 (1.3) | 0.693 | −1.360 to 0.915 |
Abbreviation: BMI, body mass index.
Data are shown as mean (SD).
The patients with plantar heel pain were university student-athletes actively participating in sports competitions and sought treatment at the clinic affiliated with the university. The athletes represented various sports, including track and field (3), basketball (2), American football (2), and judo (2). They all reported localized plantar tenderness at the most prominent part of the heel bone and experienced induced pain on applying body weight after a period of rest. Additionally, all patients had a duration of symptoms lasting at least 6 months, and their visual analog scale (VAS) scores during weightbearing at the time of the clinic visit averaged 35.6 ± 6.4 mm. At the time of the athlete’s examination, in addition to tenderness at the medial attachment of the plantar aponeurosis, evaluation using the Doppler function of an ultrasound imaging system revealed that there was a noticeable increase in blood flow within the heel fat pad. Additionally, there were no findings suggestive of tarsal tunnel syndrome, and magnetic resonance imaging examination revealed no bony abnormalities such as calcaneal stress fractures or osteomyelitis. All were examined by the same doctor.
Before the study, all participants were provided with explanations regarding the purpose of the research, measurement methods, and ethical considerations and were required to provide informed consent. This study was conducted with the approval of the Ethical Review Committee for Human Research (approval number 2020-228).
Protocol
Load-measuring instruments
The load-measuring instruments consisted of two 4-legged iron frames, each measuring 400 mm in height and 350 mm on all sides. One of these frames supported an evaluation platform with a 20-mm-thick polycarbonate resin top, whereas the other held a weighing scale for measuring the load. Given that the average sagittal plane foot axis angle in adult males during walking is approximately 7 degrees, the 2 evaluation platforms were positioned to open at a 7-degree angle to the sagittal plane (Figure 1). 21 The top surface of the platform featured cross-shaped holes, allowing the ultrasound probe to image the heel fat pad from below. 19
Figure 1.
Load-measuring instrument. (A and B) Load-measuring instrument and tank: Lines are drawn on the weightbearing device to indicate where to place your feet. A water tank was placed along the drawn line, and the subjects were instructed to place the second metatarsal bone and calcaneus bone on the line. A fixture was attached to the measurement surface to prevent the water tank from moving during unloading operations. (C) Position of the foot on the load device: The right foot was immersed in water, and observations were made using an ultrasonographic imaging diagnostic device through the cross-shaped hole created in the heel area.
In a study by Matsumoto et al, 19 ultrasound gel was used to measure changes in the morphology of the heel fat pad under loading. However, preliminary experiments revealed that observing the changes in the heel fat pad's morphology during unloading using ultrasonography was challenging because of the deformation of the ultrasound gel under loading. Therefore, an original tank made from PMP was created, allowing continuous measurement of loading and unloading. A tank made of 5-mm-thick PMP plates, measuring 90 mm in height, 350 mm in length, and 155 mm in width, was fixed to the cross-shaped holes in the platform top. Water was added to the tank, filling it up to a height of 10 mm.
Measurement of heel fat pad and plantar fascia thickness using ultrasonography
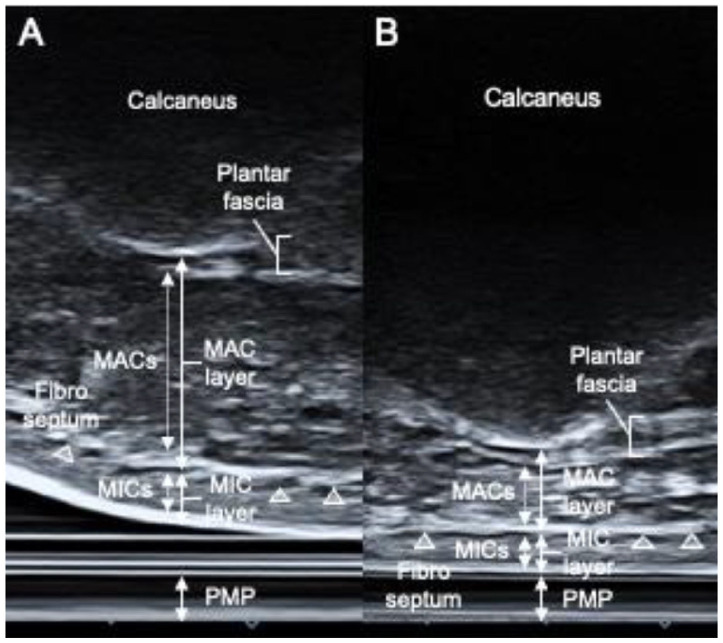
In this study, measurements were taken by individuals with more than 8 years of experience in observing the heel fat pad and plantar fascia. An ultrasound imaging diagnostic device (ApplioαVerifia, Canon, JPN) with a 10-MHz high-frequency linear probe was used, and the screen was flipped vertically for measurements. Following previous reports, the area from the lower edge of the calcaneal prominence to the bottom of the fibrous septum was designated as the MAC layer, whereas the area from the bottom of the fibrous septum to the skin was designated as the MIC layer.17 -19 The MAC layer included the plantar fascia, MACs, and the fibrous septum, whereas the MIC layer included the MICs and the skin. The plantar fascia was measured by drawing a line connecting the calcaneal prominence to the base of the second metatarsal bone and measuring its thickness at 0.5 cm proximal to the distal end.
Measurements of the heel fat pad under nonloading conditions (0% load) (Figure 2A) were taken by drawing a perpendicular line from the most prominent point of the calcaneal prominence to the transducer’s contact surface. Measurements of the heel fat pad under loading conditions (10% to 100% load) (Figure 2B) designated the area from the most prominent point of the calcaneal prominence to the surface of the PMP as the whole layer, whereas measurements of the MAC layer were taken using the same method as under nonloading conditions, and measurements of the MIC layer were taken from the bottom of the fibrous septum to the surface of the PMP.
Figure 2.
Macroscopic anatomy and loading-induced morphologic changes of the heel fat pad and plantar fascia. (A and B) Ultrasonographic images of the longitudinal section of the heel fat pad and plantar fascia: panel A represents the ultrasonographic image under nonloading conditions, whereas panel B shows the ultrasonographic image under loading conditions. Similar structures to macroscopic anatomy were observed. The MAC layer includes MACs, the plantar fascia, and the fibrous septum. The MIC layer consists of MICs and subcutaneous tissues.
Images were captured in sets of 3 at each loading point, and the average of these 3 images was used in this study.18,19
Evaluation of heel fat pad (MIC layer and MAC layer) and plantar fascia under loading and unloading conditions
In this study, all participants used their right foot for measurements, as all plantar heel pain patients had symptoms in their right foot. To ensure a uniform measurement approach, healthy control participants also used their right foot. We positioned the right foot in the water tank by detecting the longitudinal axis of the foot from the sole to the lower edge of the calcaneus through the PMP, ensuring that the second metatarsal and calcaneal prominence align with the straight line drawn on the load-measuring instrument with the PMP.
Following a previous study, we defined the evaluation of loading as starting from a state where the heel fat pad does not contact the PMP (unload) and progressing to a state with a load equivalent to 100% of the body weight (end load). The evaluation of unloading starts from a state with 100% load (end load) and continues until the heel fat pad separates completely from the PMP (fully relaxed).
The amount of loading on the right foot was adjusted based on the values measured on the left foot’s weighing scale. We measured every 20% of body weight (in total, 5 points) from 0% load to 100% load, which we designated as the loading phase. Similarly, we measured from 100% to 0% load (in total, 5 points) in the unloading phase. The measurements were conducted by one examiner using ultrasonographic imaging, whereas another examiner monitored the loading and gave instructions to the participants when they reached the designated load point to maintain that load for 5 seconds. At each measurement point, 3 ultrasonographic images were captured, and we used the average of these 3 images for analysis.
To prevent changes in posture during measurements, we provided a wall for participants to lean on. The participants were positioned in a forward-leaning posture to avoid raising their heels, and we marked their eye level in the standing position. They were instructed to maintain their eye level with the mark throughout the measurement. Furthermore, we ensured that the calcaneus did not invert or evert during loading, and we confirmed that the load axis remained aligned by observing from the rear. The measurements started with the toes in contact with the straight line drawn on the polycarbonate resin top surface, with the heel maintained in its initial position.
With these considerations in mind, our study aimed to evaluate the thickness of the MIC layer, the MAC layer, and the plantar fascia under various loading and unloading conditions. Following the methodology of a previous study, we calculated the thickness change (1) and thickness change rate (2) for the MIC and MAC layers, as well as the deformation proportion (3) as a percentage of the overall thickness change for the MIC and MAC layers. The formulas for these calculations are in the Table 2.
Table 2.
Calculation Formula for Evaluating the Function of the Heel Fat Pad.
| Amount of change (MIC layer) | = | 0% loading (MIC layer) | - | 100% loading (MIC layer) | |
| OR | |||||
| Amount of change (MAC layer) | = | 0% loading (MAC layer) | - | 100% loading (MAC layer) | (1) |
| Rate of change (MIC layer) | = | Amount of change (MIC layer) | × | 100 | |
| 0% loading (MIC layer) | |||||
| OR | |||||
| Rate of change (MAC layer) | = | Amount of change (MAC layer) | × | 100 | (2) |
| 0% loading (MAC layer) | |||||
| Deformation proportion×100 (MIC layer) | = | Amount of change (MIC layer) | × | 100 | |
| Amount of change (MIC layer)+ Amount of change (MAC layer) |
|||||
| OR | |||||
| Deformation proportion×100 (MAC layer) | = | Amount of change (MAC layer) | × | 100 | (3) |
| Amount of change (MIC layer)+ Amount of change (MAC layer) | |||||
Abbreviations: MAC, macrochamber; MIC, microchamber.
Statistical analysis
The statistical analysis was conducted using SPSS Statistics 28 software by IBM (USA).
Before comparing the MIC layer, the MAC layer, and plantar fascia under different conditions (unload, end load, fully relaxed) for healthy participants and those with plantar heel pain, we conducted the Shapiro-Wilk test to assess normality. Because normality was assumed, we performed a 2-way repeated measures analysis of variance (ANOVA). In cases where the test results indicated a significant difference in measurements, we conducted post hoc tests using the Bonferroni method.
Similarly, before comparing measurements at 20% increments for the MIC and MAC layers’ thickness change, thickness change rate, and the deformation proportion within each layer under loading and unloading conditions, we conducted the Shapiro-Wilk test to check for normality. Given that the data were normally distributed, we performed paired t tests to compare the measurements. In cases where the test results indicated a significant difference, we conducted post hoc tests using the Bonferroni method.
For comparisons of thickness change, thickness change rate, and deformation proportion between the MIC and MAC layers as well as between loading and unloading conditions, we conducted the Shapiro-Wilk test to check for normality. After confirming that the data were normally distributed, we conducted a 2-way repeated measures analysis of variance (ANOVA). In cases where the test results indicated a significant difference in measurements, we conducted post hoc tests using the Bonferroni method.
Results
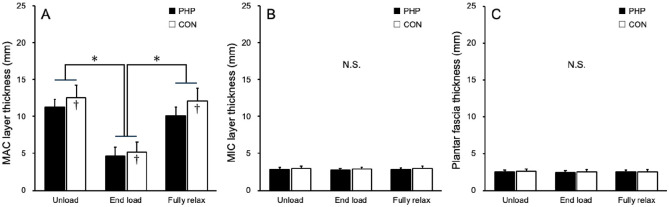
Both groups showed a statistically significant difference in MAC layer thickness between the unload and end load conditions and between the end load and fully relaxed conditions. No statistically significant differences were observed in the MIC layer or the plantar fascia thickness among the 3 conditions. However, in the MAC layer, statistically significant differences were observed between the groups at all measurement points (Figure 3).
Figure 3.
MIC layer, MAC layer, and plantar fascia thickness of unload, end load, fully relaxed. (A) MAC layer thickness. (B) MIC layer thickness. (C) Plantar fascia thickness. (MAC, macrochamber; MIC, microchamber; N.S., nonsignificant.)
*Significantly different from maximal load and initial contact, unloading (P < .05).
†Significant difference between microchamber and microchamber layers (P < .05).
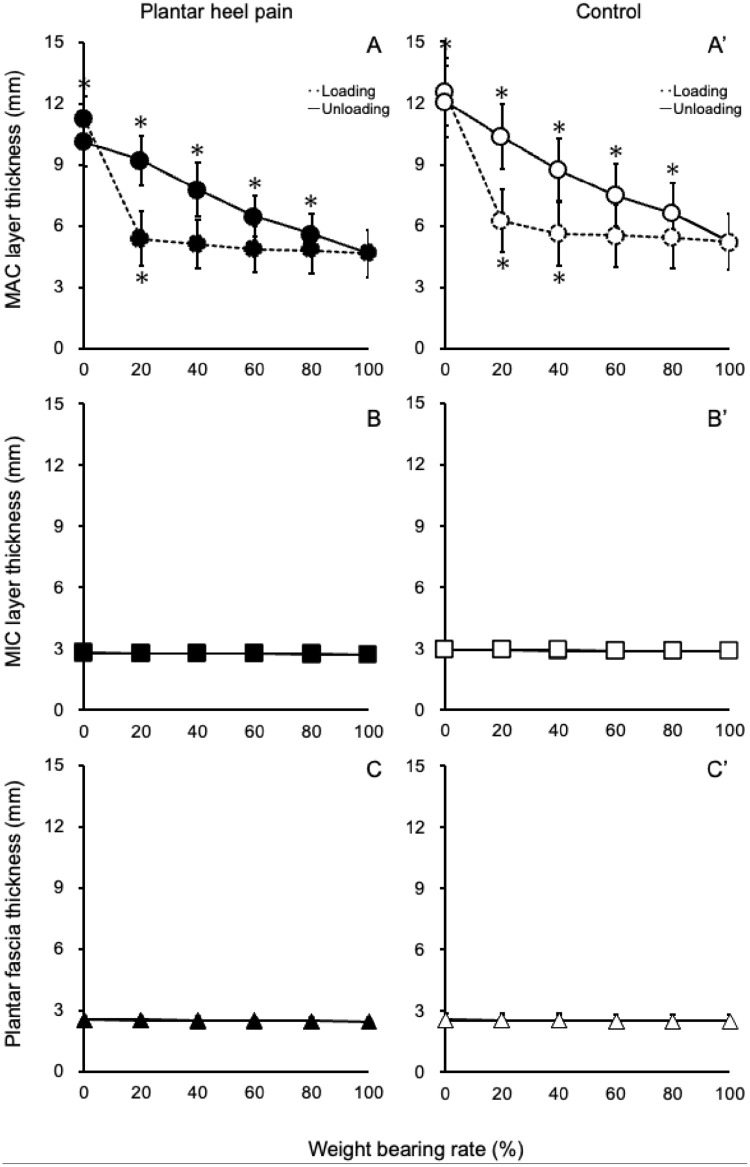
When comparing thickness measurements at 20% increments under load and unload conditions, the MAC layer of healthy participants showed statistically significant changes up to 40% of loading and significant changes at all stages during unloading. In contrast, for participants with plantar heel pain, the MAC layer exhibited statistically significant changes up to 20% during loading and statistically significant changes at all stages during unloading. However, no statistically significant changes were observed in the MIC layer and the plantar fascia for both groups during load and unload conditions (Figure 4).
Figure 4.
Change processes in the MAC layer, the MIC layer, and the plantar fascia through loading and unloading in healthy participants and those with plantar heel pain. In the graphs labeled A, B, and C (representing healthy participants), the asterisk (*) indicates a statistically significant difference compared to measurements taken at 20% before. The same applies to graphs A', B', and C' (representing participants with plantar heel pain). These figures show how the thickness of these structures changes as load increases and decreases. (MAC, macrochamber; MIC, microchamber.)
Regarding thickness change, thickness change rate, and the deformation proportion of the MIC and MAC layers, statistically significant differences were observed between the MIC and MAC layers during both loading and unloading conditions. These differences were consistent in healthy participants and those with plantar heel pain. Furthermore, in the participants with plantar heel pain, statistically significant differences were revealed in MAC layer thickness change, thickness change rate, and MIC layer thickness change rate between load and unload conditions (Table 3).
Table 3.
MIC Layer and MAC Layer Tissue Properties.
| MAC Layer | MIC Layer | P Value | |||
|---|---|---|---|---|---|
| Mean (SD) | 95% CI | Mean (SD) | 95% CI | ||
| Control | |||||
| Amount of change | |||||
| Loading | 7.3 (1.3) | 4.8 to 9.9 | 0.1 (0.1) | 0.0 to 0.3 | .001 |
| Unloading | 6.9 (1.5) | 4.0 to 9.8 | 0.1 (0.1) | 0.0 to 0.2 | .001 |
| Rate of change | |||||
| Loading | 58.7 (8.7) | 41.5 to 75.8 | 3.6 (2.4) | −1.2 to 8.3 | .001 |
| Unloading | 56.9 (9.4) | 38.4 to 75.4 | 3.4 (2.4) | −1.3 to 8.0 | .001 |
| Deformation proportion × 100 | |||||
| Loading | 98.6 (1.0) | 97.6 to 99.6 | 1.4 (1.3) | 0.1 to 2.7 | .001 |
| Unloading | 98.9 (1.0) | 97.9 to 99.9 | 1.1 (1.0) | 0.1 to 2.1 | .001 |
| PHP | |||||
| Amount of change | |||||
| Loading | 6.6* (0.8) | 5.1 to 8.2 | 0.1 (0.1) | −0.1 to 0.3 | .001 |
| Unloading | 5.5 (1.0) | 3.6 to 7.4 | 0.1 (0.1) | −0.1 to 0.2 | .001 |
| Rate of change | |||||
| Loading | 59.2* (7.8) | 43.8 to 74.5 | 3.4* (3.1) | −2.6 to 9.4 | .001 |
| Unloading | 54.4 (8.8) | 37.2 to 71.6 | 2.2 (2.5) | −2.8 to 7.2 | .001 |
| Deformation proportion × 100 | |||||
| Loading | 98.6 (1.0) | 97.6 to 99.6 | 1.4 (1.0) | 0.4 to 2.4 | .001 |
| Unloading | 98.6 (1.0) | 97.6 to 99.6 | 1.4 (1.1) | 0.3 to 2.5 | .001 |
Abbreviations: MAC, macrochamber; MIC, microchamber; PHP, plantar heel pain.
There was a significant difference between loading and unloading.
Discussion
Previous studies have also examined changes in MICs and MACs under pseudo-loading or partial-loading conditions in healthy individuals.1,9,12,16,35 These studies reported that the structural changes in MACs were more significant than those in MICs. Our study focused on changes in MICs and MACs from nonloading to full loading in healthy individuals and in individuals with heel pain for the first time.
Regarding the differences between MIC and MAC layers during loading and unloading, the changes in MAC layers accounted for approximately 98% of the overall thickness changes in the heel fat body, whereas MIC layers accounted for approximately 2%. This pattern of change was consistent in healthy individuals and patients with heel pain. However, the rate of change itself differed. In healthy individuals, the maximum change occurred at 40% of self-weight loading, with no further changes as loading increased. In contrast, patients with heel pain reached their maximum change point at 20% loading, earlier than healthy individuals. Moreover, the changes in the deep part of the heel fat body during loading and unloading were different. Superficial layers showed no significant changes, and no significant changes were observed in the transition from 0% loading. However, a significant difference was observed in the rate of change and thickness of the MAC and MIC layers in patients with heel pain during unloading.
The heel fat body undergoes degenerative changes with age and diseases like diabetes, resulting in thinning (degenerates and becomes thinner) and losing (go bankrupt and decrease) healthy fat tissue. Heel pain is more common in individuals aged 40-60 years, and it has been observed that MACs thin only after this age. 17 This suggests that changes in MACs might lead to reduced shock absorption and increased compressive stress, possibly contributing to heel pain. This, in turn, suggests the importance of distinguishing between the 2 layers in the heel fat body rather than examining them collectively.
Plantar heel pain is primarily thought to result from traction stress on the plantar fascia, thus, stretching exercises are one of the treatment methods. However, the treatment effectiveness is limited, and there are cases where relief is not easily achieved. The pain source may not solely be the plantar fascia, and the mechanical loads acting on the heel region may have been neglected. In our patients with heel pain, no abnormal thickness of the plantar fascia was found; however, MAC changes were observed. This suggests that the heel fat pad may affect heel pain. Compressive loads and bone spur development on the heel bone could potentially damage the plantar fascia, indicating the importance of the cushioning function of the heel fat pad underneath the calcaneus to mitigate such compressive loads. 15
This study confirmed that PMP can thoroughly and clearly evaluate the internal structure of the heel fat pad during loading. This can be applied to various motion measurements, especially for understanding everyday activities’ biomechanical behavior, such as walking and running, which is crucial for understanding a variety of movements in the future. Additionally, although this study targeted healthy individuals and patients with heel pain, the values obtained here could serve as a basis for measuring changes in the heel fat pad during loading and unloading due to degenerative changes or pathologies. This could potentially lead to a better understanding of the relationship between heel pain and the heel fat pad, as well as the mechanisms underlying the development of heel pain. Therefore, it is necessary to investigate further based on this study’s findings.
Limitations
Owing to the absence of plantar pressure measurements during loading and unloading assessments, we cannot definitively confirm whether the entire load was applied to the right foot, which was the measured foot. The limited number of participants in this study suggests the need for a larger sample size to obtain more reliable results.
Conclusions
Throughout full loading, the possibility of differential functionality between MICs and MACs within the heel fat body was suggested. Compared with healthy individuals, patients with heel pain experienced less load to reach the maximum change in MACs. Additionally, the changes during unloading were smaller compared to the changes during loading, indicating that the original morphology was not fully restored.
This study suggests that heel fat body changes may be related to heel pain.
Supplemental Material
Supplemental material, sj-pdf-1-fao-10.1177_24730114241247824 for Functional Morphologic Changes of the Heel Fat Pad and Plantar Fascia in Patients With Heel Pain During Weightbearing and Nonweightbearing by Toshihiro Maemichi, Masatomo Matsumoto, Toshiharu Tsutsui, Shota Ichikawa, Takumi Okunuki, Hirofumi Tanaka and Tsukasa Kumai in Foot & Ankle Orthopaedics
Acknowledgments
The authors would like to thank all participants who participated in the measurements.
Footnotes
Ethical Approval: This study was approved by the Ethics Committee Graduated from Waseda University School of Sports Science and conducted according to the principles of the Declaration of Helsinki (approval no. 2020-228). All participants received a detailed explanation of the experimental procedures and risks of the research before the recordings were taken. Written informed consent was obtained from all participants and their guardians.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. Disclosure forms for all authors are available online.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Public Interest Incorporated Foundation Yamaha Motor Foundation for Sports.
ORCID iDs: Toshihiro Maemichi, PhD,  https://orcid.org/0000-0001-8683-2059
https://orcid.org/0000-0001-8683-2059
Tsukasa Kumai, MD, PhD,  https://orcid.org/0000-0003-3738-9171
https://orcid.org/0000-0003-3738-9171
References
- 1. Buschmann WR, Jahss MH, Kummer F, Desai P, Gee RO, Ricci JL. Histology and histomorphometric analysis of the normal and atrophic heel fat pad. Foot Ankle Int. 1995;16(5):254-258. doi: 10.1177/107110079501600502 [DOI] [PubMed] [Google Scholar]
- 2. Campanelli V, Fantini M, Faccioli N, Cangemi A, Pozzo A, Sbarbati A. Three-dimensional morphology of heel fat pad: an in vivo computed tomography study. J Anat. 2011;219(5):622-631. doi: 10.1111/j.1469-7580.2011.01420.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen H, Ho HM, Ying M, Fu SN. Association between plantar fascia vascularity and morphology and foot dysfunction in individuals with chronic plantar fasciitis. J Orthop Sports Phys Ther. 2013;43(10):727-734. doi: 10.2519/jospt.2013.4774 [DOI] [PubMed] [Google Scholar]
- 4. De Clercq D, Aerts P, Kunnen M. The mechanical characteristics of the human heel pad during foot strike in running: an in vivo cineradiographic study. J Biomech. 1994;27(10):1213-1222. doi: 10.1016/0021-9290(94)90275-5 [DOI] [PubMed] [Google Scholar]
- 5. Falsetti P, Frediani B, Acciai C, et al. Ultrasonography and magnetic resonance imaging of heel fat pad inflammatory-oedematous lesions in rheumatoid arthritis. Scand J Rheumatol. 2006;35(6):454-458. doi: 10.1080/03009740600905398 [DOI] [PubMed] [Google Scholar]
- 6. Hsu CC, Tsai WC, Hsiao TY, et al. Diabetic effects on microchambers and macrochambers tissue properties in human heel pads. Clin Biomech (Bristol Avon). 2009;24(8):682-686. doi: 10.1016/j.clinbiomech.2009.06.005 [DOI] [PubMed] [Google Scholar]
- 7. Hsu CC, Tsai WC, Wang CL, Pao SH, Shau YW, Chuan YS. Microchambers and macrochambers in heel pads: are they functionally different? J Appl Physiol (1985). 2007;102(6):2227-2231. doi: 10.1152/japplphysiol.01137.2006 [DOI] [PubMed] [Google Scholar]
- 8. Hsu TC, Lee YS, Shau YW. Biomechanics of the heel pad for type 2 diabetic patients. Clin Biomech (Bristol Avon). 2002;17(4):291-296. doi: 10.1016/S0268-0033(02)00018-9 [DOI] [PubMed] [Google Scholar]
- 9. Jahss MH, Kummer F, Michelson JD. Investigations into the fat pads of the sole of the foot: heel pressure studies. Foot Ankle. 1992;13(5):227-232. doi: 10.1177/107110079201300501 [DOI] [PubMed] [Google Scholar]
- 10. Jørgensen U, Bojsen-Møller F. Shock absorbency of factors in the shoe/heel interaction—with special focus on role of the heel pad. Foot Ankle. 1989;9(6):294-299. doi: 10.1177/107110078900900607 [DOI] [PubMed] [Google Scholar]
- 11. Kanatli U, Yetkin H, Simsek A, Besli K, Ozturk A. The relationship of the heel pad compressibility and plantar pressure distribution. Foot Ankle Int. 2001;22(8):662-665. doi: 10.1177/107110070102200808 [DOI] [PubMed] [Google Scholar]
- 12. Ker RF, Bennett MB, Alexander RM, Kester RC. Foot strike and the properties of the human heel pad. Proc Inst Mech Eng H. 1989;203(4):191-196. doi: 10.1243/PIME_PROC_1989_203_038_01 [DOI] [PubMed] [Google Scholar]
- 13. Kimani JK. The structural and functional organization of the connective tissue in the human foot with reference to the histomorphology of the elastic fibre system. Acta Morphol Neerl Scand. 1984;22(4):313-323. [PubMed] [Google Scholar]
- 14. Kuhns JG. Changes in elastic adipose tissue. J Bone Joint Surg Am. 1949;31(3):541-547. doi: 10.2106/00004623-194931030-00010 [DOI] [PubMed] [Google Scholar]
- 15. Kumai T, Benjamin M. Heel spur formation and the subcalcaneal enthesis of the plantar fascia. J Rheumatol. 2002;29(9):1957-1964. [PubMed] [Google Scholar]
- 16. Lapidus PW, Guidotti FP. Painful heel: report of 323 patients with 364 painful heels. Clin Orthop Relat Res. 1965;39:178-186. doi: 10.1097/00003086-196500390-00016 [DOI] [PubMed] [Google Scholar]
- 17. Maemichi T, Tsutsui T, Matsumoto M, Iizuka S, Torii S, Kumai T. The relationship of heel fat pad thickness with age and physiques in Japanese. Clin Biomech (Bristol Avon). 2020;80:105110. doi: 10.1016/j.clinbiomech.2020.105110 [DOI] [PubMed] [Google Scholar]
- 18. Matsumoto M, Maemichi T, Wada M, et al. Ultrasonic evaluation of the heel fat pad under loading conditions using a polymethylpentene resin plate: part 2. Reliability and agreement study. Ultrasound Med Biol. 2023;49(2):460-472. doi: 10.1016/j.ultrasmedbio.2022.09.015 [DOI] [PubMed] [Google Scholar]
- 19. Matsumoto M, Maemichi T, Wada M, et al. Ultrasonic evaluation of the heel fat pad under weight-bearing conditions using a polymethylpentene resin plate: part 1. Ultrasound Med Biol. 2022;48(2):358-372. doi: 10.1016/j.ultrasmedbio.2021.10.012 [DOI] [PubMed] [Google Scholar]
- 20. McCarthy DJ, Gorecki GE. The anatomical basis of inferior calcaneal lesions. A cryomicrotomy study. J Am Podiatry Assoc. 1979;69(9):527-536. doi: 10.7547/87507315-69-9-527 [DOI] [PubMed] [Google Scholar]
- 21. Murray MP, Drought AB, Kory RC. Walking patterns of normal men. J Bone Joint Surg Am. 1964;46:335-360. doi: 10.2106/00004623-196446020-00009 [DOI] [PubMed] [Google Scholar]
- 22. Ng TKW, Zheng YP, Kwan RLC, Cheing GLY. An innovative ultrasound foot scanner system for measuring the change in biomechanical properties of plantar tissue from sitting to standing. Int J Rehabil Res. 2015;38(1):68-73. doi: 10.1097/MRR.0000000000000097 [DOI] [PubMed] [Google Scholar]
- 23. Ophir J, Garra B, Kallel F, et al. Elastographic imaging. Ultrasound Med Biol. 2000;26:S23-S29. doi: 10.1016/S0301-5629(00)00156-3 [DOI] [PubMed] [Google Scholar]
- 24. Perhamre S, Lundin F, Klässbo M, Norlin R. A heel cup improves the function of the heel pad in sever’s injury: effects on heel pad thickness, peak pressure and pain. Scand J Med Sci Sports. 2012;22(4):516-522. doi: 10.1111/j.1600-0838.2010.01266.x [DOI] [PubMed] [Google Scholar]
- 25. Prichasuk S. The heel pad in plantar heel pain. J Bone Joint Surg Br. 1994;76(1):140-142. [PubMed] [Google Scholar]
- 26. Rome K, Campbell R, Flint A, Haslock I. Heel pad thickness—a contributing factor associated with plantar heel pain in young adults. Foot Ankle Int. 2002;23(2):142-147. doi: 10.1177/107110070202300211 [DOI] [PubMed] [Google Scholar]
- 27. Turgut A, Göktürk E, Köse N, Seber S, Hazer B, Günal Î. The relationship of heel pad elasticity and plantar heel pain. Clin Orthop Relat Res. 1999;360:191-196. [DOI] [PubMed] [Google Scholar]
- 28. Uzel M, Cetinus E, Ekerbicer HC, Karaoguz A. Heel pad thickness and athletic activity in healthy young adults: a sonographic study. J Clin Ultrasound. 2006;34(5):231-236. doi: 10.1002/jcu.20230 [DOI] [PubMed] [Google Scholar]
- 29. Wearing SC, Smeathers JE, Urry SR, Sullivan PM, Yates B, Dubois P. Plantar enthesopathy: thickening of the enthesis is correlated with energy dissipation of the plantar fat pad during walking. Am J Sports Med. 2010;38(12):2522-2527. doi: 10.1177/0363546510377405 [DOI] [PubMed] [Google Scholar]
- 30. Wearing SC, Smeathers JE, Yates B, Urry SR, Dubois P. Bulk compressive properties of the heel fat pad during walking: a pilot investigation in plantar heel pain. Clin Biomech (Bristol Avon). 2009;24(4):397-402. doi: 10.1016/j.clinbiomech.2009.01.002 [DOI] [PubMed] [Google Scholar]
- 31. Williams PL, Smibert JG, Cox R, Mitchell R, Klenerman L. Imaging study of the painful heel syndrome. Foot Ankle. 1987;7(6):345-349. doi: 10.1177/107110078700700607 [DOI] [PubMed] [Google Scholar]
- 32. Zheng YP, Huang YP, Zhu YP, Wong M, He JF, Huang ZM. Development of a foot scanner for assessing the mechanical properties of plantar soft tissues under different bodyweight loading in standing. Med Eng Phys. 2012;34(4):506-511. doi: 10.1016/j.medengphy.2011.11.005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-fao-10.1177_24730114241247824 for Functional Morphologic Changes of the Heel Fat Pad and Plantar Fascia in Patients With Heel Pain During Weightbearing and Nonweightbearing by Toshihiro Maemichi, Masatomo Matsumoto, Toshiharu Tsutsui, Shota Ichikawa, Takumi Okunuki, Hirofumi Tanaka and Tsukasa Kumai in Foot & Ankle Orthopaedics