Abstract
Inflammatory bowel disease (IBD) is a chronic inflammatory disease with increasing incidence, such as Crohn's disease and ulcerative colitis. The accurate etiology and pathogenesis of IBD remain unclear, and it is generally believed that it is related to genetic susceptibility, gut microbiota, environmental factors, immunological abnormalities, and potentially other factors. Currently, the mainstream therapeutic drugs are amino salicylic acid agents, corticosteroids, immunomodulators, and biological agents, but the remission rates do not surpass 30–60% of patients in a real-life setting. As a consequence, there are many studies focusing on emerging drugs and bioactive ingredients that have higher efficacy and long-term safety for achieving complete deep healing. This article begins with a review of the latest, systematic, and credible summaries of the pathogenesis of IBD. In addition, we provide a summary of the current treatments and drugs for IBD. Finally, we focus on the therapeutic effects of emerging drugs such as microRNAs and lncRNAs, nanoparticles-mediated drugs and natural products on IBD and their mechanisms of action.
Keywords: Inflammatory bowel disease, pathogenesis, therapeutics
Introduction
Inflammatory bowel disease (IBD) is a chronic and relapsing inflammatory gastrointestinal (GI) disorder, encompassing ulcerative colitis (UC) and Crohn's disease (CD). IBD, characterized by abdominal pain, diarrhea, bloody stools, and weight loss, has emerged as a global disease with a significant burden, seriously impacting patients’ quality of life.1,2 In addition, IBD can extend beyond the GI tract and affect other organs such as joints, skin, eyes, liver, lungs, and pancreas. 3
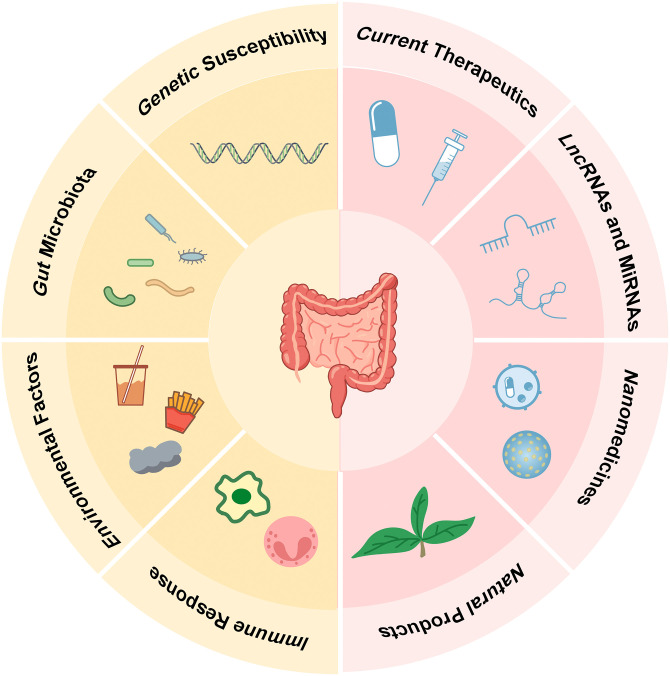
The pathogenesis of IBD is recognized as a multifactorial disease influenced by genetic susceptibility, gut microbiota, environmental factors, and immunological abnormalities. 4 Genome-wide association studies (GWAS) have identified over 200 genetic risk loci associated with IBD. 5 Proline-rich tyrosine kinase 2B (PTK2B) is a newly associated IBD susceptibility loci, involved in monocyte migration and neutrophil degranulation. 5 The abundance of Clostridium boltae, Erysipelatoclostridium ramosum, and Ruminococcus gnavus and the levels of imidazole propionate, long-chain polyunsaturated fatty acids, and primary bile acids increased in patients with IBD, which suggests a potential influence of diet and lifestyle. 6 Significant compositional changes across immune and stromal cell subsets have been revealed in CD, and the role of interleukin-33 (IL-33) and interferon-γ (IFN-γ) is important in inducing the accumulation of active eosinophils in IBD.7,8 Additionally, environmental factors such as lifestyle and diet, play a significant role in the development of IBD, with certain dietary components, like mannose, showing potential for ameliorating colitis.9–11 IBD pathogenesis is associated with the complex interactions between genetic predispositions, gut microbiome perturbations, environmental factors, and immune response dysregulation.
Current therapies have not sufficiently addressed the clinical requirements for IBD, with high failure rates of drugs and over 10% of patients ultimately necessitating surgical intervention. 12 In 2021, the International Organization for the Study of IBD updated the STRIDE II, emphasizing the ultimate goal of achieving complete deep healing which encompasses clinical remission, complete endoscopic, histological healing, and transmural healing. 13 Despite the availability of various therapeutic agents and treatments for IBD, such as aminosalicylic acid agents, corticosteroids, immunomodulators, and biological agents, their therapeutic effects remain unsatisfactory. Some patients still require surgery for medically refractory disease. Although total proctocolectomy would potentially cure the colon inflammation, postoperative complications are frequent, notably pouchitis and fecal incontinence. 12 Consequently, there is an urgent need to develop more cost-effective, safer, and highly efficient drugs. Recent research suggests that emerging treatment modalities, such as microRNAs and lncRNAs, natural products, and nanoparticles (NPs)-mediated drugs, hold great potential in the treatment of IBD. In this review, we provide a comprehensive summary of the pathogenesis of IBD and focus on the latest research in IBD treatment, highlighting the therapeutic advantages, and mechanisms of these emerging drugs.
Disease pathogenesis
Genetic susceptibility
More than 240 susceptibility loci have been identified to be involved in IBD, 45 of which are statistically conclusive causal variants.14,15 Nucleotide binding oligomerization domain containing 2 (NOD2) mutations are the strongest genetic risk locus in IBD, and the mutated NOD2 protein is unable to sense muramyl dipeptide, which leads to excessive production of pro-inflammatory cytokines and type I IFNs and the impairment of monocytes phenotypic switch.16,17 Protein tyrosine phosphatase nonreceptor type 22 (PTPN22) could protect from IBD via affecting the composition of the intestinal microbiota, the activation of nucleotide-binding and oligomerization domain-like receptor family pyrin domain containing 3 (NLRP3) inflammasome, and PTPN22 deficiency could impact the antimicrobial function of granulocytes.18–20 Tumor necrosis factor receptor super family 25 (TNFRSF25) encoding death domain receptor 3 deficiency could increase intestinal permeability and inhibit tissue repair after dextran sulfate sodium (DSS)-induced injury. 15
Among IBD risk genes, caspase activation and recruitment domain 9 (CARD9) is unique in both risk and protective effects. 21 Previous studies have shown that CARD9-deficient mice can shape an altered microbiota and impair their tryptophan metabolism, leading to hypersusceptibility for colitis. 22 CARD9 expressed in neutrophils is involved in the protective role against colitis, the deficiency of which induces mitochondrial dysfunction with increasing production of reactive oxygen species (ROS), leading to neutrophil apoptosis and fungal containment impairment. 23 A polysaccharide-enriched diet could counteract the defective pathogen-specific antibody response in CARD9-deficient mice, which suggests genetic susceptibility to gut pathogens can be overcome with dietary interventions that restore humoral immunity and a competing microbiota. 24
In addition, the majority of these polymorphisms identified by GWAS are located within noncoding elements. 25 Colitis associated interferon regulatory factor 1 (IRF1) antisense regulator of intestinal homeostasis (CARINH), a lncRNA-C5orf56 from a noncoding region, protects against IBD. 26 RNA transcript from CARINH interacts with E1A-associated protein p300/cyclic-AMP response element binding protein to promote the deposition of activating histone modifications H3K27ac at the IRF1 locus, thus increasing the expression of IRF1 for upregulating expression of interleukin 18 binding protein. 26 A study of Russian CD patients reveals that rs9858542 of bassoon presynaptic cytomatrix protein (BSN), rs3816769 of signal transducer and activator of transcription 3 (STAT3) and rs1793004 of neural epidermal growth factor-like like 1 (NELL1) are associated with a higher risk of CD, which shows a negative correlation with Bacteroidetes phylum, phylum Bacteroidetes and family Ruminococcaceae. 27 Thus, genetic susceptibility to IBD may alter the composition of gut microbiota, and environmental factors may affect gene susceptibility-related inflammatory, which indicates the interactions between them play a great role in IBD pathogenesis.
Gut microbiota
It is generally recognized that dysbiosis plays a significant role in IBD. In UC or CD patients, the abundance of the phylum Proteobacteria, the genus Escherichia, the species Escherichia coli, and fungi such as Candida, Malassezia, and Epicoccum nigrum are higher, and the abundance of Saccharomyces and Akkermansia muciniphila are lower.28–30 However, Reiss et al. show that their retroauricular crease and lumbar region skin microbiota diversity is increased, including Delftia, Corynebacterium, and Pseudomonas. 31 In addition, fungi, increased in activate IBD patients and mice, activate dectin-1-Syk-NF-κB to enhance the glutaminolysis in CD4+T cells, which contributes to the progression of IBD. 32 A. muciniphila with decreased abundance in IBD patients and IL-10 deficient colitis mice presumably be a consequence of mucin depletion especially Mucin-2.30,33 A. muciniphila and its membrane protein Amuc_1100 enhance intestinal cyclic adenosine monophosphate-responsive element-binding protein H expression, which improves gut barrier permeability by upregulating expression of protective gut tight junctions (TJs) and promotes intestinal wound healing by increasing the expression of microRNA-143/145. 30 The enrichment of adherent-invasive E. coli (AIEC) exacerbates intestinal inflammation in mice, which may enhance intestinal fibrosis in CD patients, via the inhibition of intestinal epithelial cell (IECs)-derived exosomal let-7b.34,35
Microbial metabolites such as bile acids metabolites, short-chain fatty acids (SCFAs), and tryptophan metabolites are key mediators of host–microbial interactions. SCFAs such as butyrate have a protective effect against IBD by regulating Th17/Treg balance. 36 Deoxycholic acid aggravates intestinal inflammation via promoting the expression of IL-1β and the proportion of CD3+ and CD4+T cells and reducing the number of tuft cells. 37 A consortium of Clostridium AP sp000509125, Bacteroides ovatus and Eubacterium limosum could produce secondary bile acids ursodeoxycholic acid and lithocholic acid to improve gut-barrier integrity in colitis mice. 38 Metabolite of tryptophan indole-3-propionic acid, which is decreased in UC and CD patients fecal, has the potential to restrain inflammation and fibrosis. 39 Skatole, another tryptophan-derived gut microbiota metabolite, increases TNF-α expression by activating p38 and c-Jun N-terminal kinase for contributing to the progression of IBD. 40 Palmitoleic acid, an anti-inflammatory metabolite, decreased in IBD patients with failures or compromised effects of anti-TNF-α therapy, shows a potential therapeutic function of shaping the magnitude and diversity of gut microbiota. 41 Although alterations in the composition of the gut microbiota and its metabolite are likely to be involved in IBD pathogenesis, there is insufficient evidence to support a direct causal relationship between gut microbes and IBD.
Environmental factors
Some prospective cohort studies underline the associations between IBD risks and environmental factors such as lifestyle, smoking, drinking, and diets.9,42,43 Considering declined smoking rates and increased incidence and prevalence of IBD, Wetwittayakhlang et al. 42 support that smoking has become less important in CD development. Long-term exposure to manganese, mercury, selenium, sulfur tetroxide, chlorine, and nitrate nitrogen from drinking water is associated with a higher risk of IBD, while drinking water containing zinc and fluorine is associated with a lower risk of IBD. 43 And 30% sucrose solution or 20% fructose solution, like sugar-sweetened beverages, can aggravate high-fat-diets-induced or restraint stress-induced colitis by altering the composition of the gut microbiota.44,45 Mice with cold stress induced by cold water exhibit colitis-like phenotypes such as gut barrier disruption and low bacterial diversity. 46
There has been increased attention to the role of dietary patterns in the treatment of IBD in recent years. Active CD patients tend to adopt unbalanced nutritional patterns, while healthy eating patterns are associated with a lower risk of older-onset.47,48 Mediterranean diets, rich in polyphenols and fiber, are considered a beneficial dietary regimen for IBD. 10 Low dietary fiber promotes mucin-degrading bacteria eroding healthy colon mucus, leading to lethal colitis in IL-10-deficient mice. 49 Western dietary patterns, characterized by high consumption of fats and sugars and a low fiber intake, contribute to the development of IBD, because of the decreased abundance of SCFA-producing bacteria and the increased abundance of pathogens such as Helicobacter trogontum. 50 Although saturated fat is highly recognized a risk factor for IBD, O'Mahony et al. 51 report that a lard-based high-fat diet demonstrates the potential to reduce DSS-induced colitis via inducing high level of the secondary bile acid lithocholic acid and activating the vitamin D pathway. Nevertheless, Khademi et al. 52 support that there is no significant link between pro-inflammatory diets containing french fries, pizza, snacks, and red and processed meats with UC risk. There is currently no dietary approach that can be successfully applied to all patients with IBD. 53 Further clinical trials are needed to develop personalized dietary patterns that are more appropriate for IBD patients.
Many dietary contents have protective potential in IBD treatments, such as Spermidine, Chlorophyllin in green vegetables, steamed or raw broccoli sprouts with high levels of sulforaphane and fruits like avocado.54–59 Spermidine, with high levels in soybeans, wheat germ, and aged cheese, has strong anti-inflammatory potential via promoting an M2-like macrophage differentiation, while it is ineffective in the macrophages of IBD patients carrying PTPN2 SNP rs1893217. 54 Mannose, exerts a potential therapeutic effect on both DSS-induced and IL-10-deficient colitis mouse models by limiting cathepsin B release from lysosomes which prevents mitochondrial dysfunction and TJs disruption. 60 Wheat germ supplementation reduces the intestinal inflammation by IL-22 signaling and phosphorylated STAT3-mediated production of Th17 proinflammatory cytokines in IL-10 knock-out mice with atherogenic diet. 61 Microalga Lobosphaera incisa (strain P127) in diets, accumulates a rare omega-6 long-chain polyunsaturated fatty acid (LC-PUFA) dihomo-ɣ-linolenic acid under nitrogen starvation and ameliorates 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced gut inflammation. 62 Nervonic acid, an omega-9 LC-PUFA, alleviates intestinal inflammation by inhibiting NF-κB signal pathway. 63
In addition, environmental factors such as mycotoxin contamination, food-grade titanium dioxide, antibiotic exposure, and some pollutants may be related to IBD pathogenesis.64–66 A nontoxic dose of Deoxynivalenol, which can contaminate food and feed, exacerbates DSS-induced colitis via the Janus kinase 2 (JAK2)/STAT3 signaling pathway in mice. 64 Titanium dioxide NPs can counteract the protective effect of PTPN22 variant on IBD by inducing cytotoxic CD8 T-cells in the lamina propria of PTPN22-R619 W mice. 65 A population-based cohort study supports that antibiotic exposure could increase the risk of IBD accumulatively especially in individuals aged 40 years and older. 66 Sanmarco et al. 67 reveal that herbicide propyzamide promotes intestinal inflammation by interfering with aryl hydrocarbon receptor inhibition of activating NF-κB-CCAAT/enhancer binding protein β signaling in T cells and dendritic cells. Eosinophils in the colon of UC patients could be activated by exposure to house dust mites. 68 Identifying environmental factors that are associated with IBD offers a promising approach to elucidate the pathogenesis and find possible therapeutic targets.
Immune response
Immune dysregulation in IBD involves innate and adaptive immune responses, including immune cells such as macrophages, neutrophils, eosinophils, T cells, and B cells. The number, distribution, and proportion of immune cells are related to the diagnosis and severity of IBD. A high proportion of peripheral CD3+human leukocyte antigen D-related+T cells, may be associated with poor clinical outcomes in UC patients. 69 Higher CD8+lymphocyte infiltration and a higher ratio of CD8+lymphocytes / CD4+lymphocytes may be effective in distinguishing immune-related adverse event colitis from other forms of colitis. 70 The application of artificial intelligence in IBD reveals that CD and UC with histologically active disease have a significant difference in the number and distribution of mucosal CD3 and γδ T cells. 71
New factors in macrophage differentiation and maturation are involved in the pathogenesis of IBD. High level of mesencephalic astrocyte-derived neurotrophic factor in CX3CR1 intermediate positive proinflammatory macrophages inhibits the C/EBP homologous protein-basic leucine zipper ATF-like transcription factor 2 signaling pathway, for ameliorating colitis. 72 Macrophage differentiation also could be inhibited by NOD2-dependent bacterial sensing in monocytes. 16 Macrophages with monocyte chemotactic protein-1-induced protein 1 deficiency are characterized by increased migratory capacity and M1 macrophage polarization, which arrests macrophage maturation and exacerbates intestinal inflammation. 73
Increased infiltration of eosinophils and neutrophils is often associated with more severe inflammation in IBD.8,74 For instance, accumulation of active eosinophils in the inflamed colon worsens inflammation and results in the hyperactivation of CD4+T cells during acute colitis. 8 Excessive infiltration of polymorphonuclear neutrophils drives colonic inflammation through elevated lipid droplets and forkhead box O3 deficiency. 75 And deficiency of suppressors of cytokine signaling 3 in myeloid cells and colonization of Clostridioides difficile result in accumulation of neutrophils.76,77 PTK2B, upregulated in inflamed mucosa of UC patients, could enhance neutrophil migration and reduce the generation of ROS, myeloperoxidase, and antimicrobial peptides (S100A8/A9) in neutrophils. 78 MUC5AC deficiency increases the surface area of the colonic barrier in direct contact with bacteria leading to greater neutrophil frequency and severe DSS-induced colitis. 79
Innate lymphoid cells (ILCs) are a highly heterogenous family of lymphocytes, including group 1 ILCs (ILC1s), group 2 ILCs (ILC2s), group 3 ILCs (ILC3s), and regulatory ILCs. 80 ILC3s, one of the most extensively studied classes of ILCs in IBD, can be divided into natural cytotoxicity receptor (NCR)+ILC3s and NCR−ILC3s. 81 It is reported that NCR−ILC3s induced the activation of neutrophils by producing granulocyte-macrophage colony-stimulating factor, leading to the progression of IBD. 82 Increasing the level of NCR+ILC3 levels contributes to the secretion of IL-22 and the balance of NCR−ILC3/NCR+ILC3 for alleviating UC. 81 Besides, ILC3s protect the intestinal epithelium from TNF-induced cell death, which is independent of IL-22. 83 Musashi2 deficiency in ILC3s can attenuate DSS-induced colonic inflammation by altering the diversity of the intestinal microbiota. 84
During IBD, B-cell perturbations have been observed, which may be related to the release of immunoglobulin M (IgM), IgG, IgA, IgE, and IgD.85,86 There is a few study addresses that IgG plasma cells with enhanced oxidative phosphorylation leads to mucosal hypoxia in UC. 87 Although anticommensal IgG exacerbates UC, serum natural IgG does not aggravate the pathology of UC. 88 Enhanced IgA was produced in IBD patients against the microbiota. 89 Bos et al. 89 believe that Fcα receptor-1-stimulated monocytes may promote IgA B-cell differentiation with more IgA production.
In conclusion, the important role of immune dysregulation in IBD has been confirmed. Crosstalk between the microbiota and immune cells or immune cell and immune cells underlie several chronic inflammatory conditions.90,91 More details about the functions of a variety of immune cell subsets and the communications among immune cells, nonimmune cells, and inflammatory microenvironment need to be further explored for a comprehensive understanding of the pathogenesis of IBD.
IBD treatments: Based on experiments
Current therapeutics
Conventional drugs for IBD such as corticosteroids, aminosalicylates, and immunomodulators could improve the clinical symptoms but do not alter the overall disease course in IBD including mucosal healing, and along with potential adverse effects as abdominal pain, osteoporosis, and infections.92,93 Considering the potential side effects of traditional therapeutic drugs which are not suitable for long-term use, there is a spate of emerging drugs containing biologics such as TNF antagonists, integrin inhibitors and anticytokine molecules, and small molecule drugs such as JAK inhibitors, SMAD7 antisense oligonucleotides and sphingosine-1-phosphate (S1P) receptor modulators. 92
Anti-TNF agents including infliximab, adalimumab, golimumab, certolizumab pegol, anti-integrin agents including natalizumab and vedolizumab and anticytokine molecules including ustekinumab, risankizumab and mirikizumab have been approved for the treatment of patients with moderate to severe IBD who have had an inadequate response to conventional therapies.92,94 Approximately two-thirds of patients with IBD are primary or secondary nonresponders of TNF-α monoclonal antibody therapy, which may be attributed to the proteolytic cleavage of anti-TNF-α agents by mucosal metalloproteinases and antihinge autoantibodies.92,95 New anti-integrin agents such as etrolizumab, a gut-targeted anti-β7 monoclonal antibody has a disappointing result in the phase 3 trial, which may be related to the fact that etrolizumab blocks macrophage-dependent wound healing in the gut. 96 Anti-integrin oral small molecules PTG-100 and AJM300 with proof-of-concept efficacy and high safety show a promising therapeutic potential for IBD, and AJM300 has been approved in Japan.97–99
The therapeutic efficacy of oral small molecule drugs for IBD remains unsatisfactory and its long-term effectiveness and safety need more data. Pan-JAK inhibitor tofacitinib is associated with an increased risk of adverse cardiovascular events, and the risk of herpes zoster and malignancies increased with age in older UC patients treated with tofacitinib.100–102 In DSS-induced colitis mice, delayed and limited administration of tofacitinib alleviated clinical symptoms of colitis and inhibited the expression of pro-inflammatory cytokines such as IFN-γ. 103 Thus, exploring the dosing regimen of tofacitinib may be beneficial for IBD treatment. Selective JAK1 inhibitor upadacitinib is effective in the treatment of IBD patients with anti-TNF-α inadequate responders. 104 A phase 3 study about mongersen, an antisense oligodeoxynucleotide to SMAD7 was prematurely discontinued because mongersen is not effective in CD patients. 105 Nonselective S1P agonist fingolimod has serious cardiotoxicity, while etrasimod selectively activate S1P1,4,5 and ozanimod selectively activates S1P1,5, showing safe and efficacious therapeutic effects on moderate-to-severe active UC patients. 106
When traditional therapies are not effective, stem cell including hematopoietic stem cells (HSCs) and mesenchymal stem cells (MSCs) transplantation and fecal microbiota transplantation (FMT) may be used to improve clinical symptoms in patients with IBD as alternative therapies. Eleven pediatric and young adult IBD patients with inborn errors of immunity who received allogeneic hematopoietic stem cell transplantation (alloHSCT) got GI symptom remission. 107 However, monogenic IBD with syntaxin-binding protein 2 mutations is not alleviated by HSCs transplantation. 108 MSCs could promote IBD recovery via modulating gut microbiota and metabolic disorders and upregulating the expression of Mucin 1 to inhibit ferroptosis. 109 In addition, modified MSCs transplantation exhibits a precise and efficient role in the IBD treatment. For instance, hypoxia-inducible factor 1α-overexpressing MSC transplantation promotes M2-like macrophages polarization through PI3K-γ pathway with a strong therapeutic potential for IBD. 110 Peyer's patch-derived Nestin+MSCs have a superior therapeutic potency in the treatment of murine IBD. 111 Human umbilical cord-derived MSCs promote the proliferation of type 1 regulatory T cells in an indoleamine-2,3-dioxygenase-dependent manner to alleviate TNBS-induced colitis. 112 FMT is a promising treatment for IBD, by which the gut microbiota is transplanted from healthy donors to patients, and the efficacy may rely on the interplay of gut microbiota between donors and recipients. 113 Capsulized FMT not only could induce the UC remission via increasing the abundance of Alipipes sp. strain 3BBH6, Odoribacter splanchnicus to accelerate the metabolism of tryptophan and the production of indole lactic acid, but also could alter gut fungi in patients with active UC.114,115 Fecal microbiota transplantation combined with an anti-inflammatory diet could restore crypt-associated microbiota and recuperate the lost gut crypt-associated microbiota-mucosa-associated microbial interactions in UC. 116 Liu et al. 117 indicate that viable bacteria could improve colon injury and maintain intestinal homeostasis, while dead microbiota often fail to play a role. Besides, repeated FMT treatment or whole intestinal microbiota transplantation may improve efficacy, while a single FMT is unable to modulate the microbiota in the longer term.118,119
lncRNAs and MicroRNAs
lncRNAs
Long noncoding RNAs (lncRNAs) play an important role in IBD by interacting with nucleic acids and proteins.120,121 And the levels of serum lncRNA THRIL and LUCAT1, as a potential biomarker for diagnosis and prognosis of IBD, are upregulated in patients with IBD, which is associated with disease severity.122,123 In addition, high expression of lncRNA-UCL mediated by IL-22 could alleviate DSS-induced colitis symptoms. 124 Human umbilical cord MSCs-derived exosome increases lnc78583 expression, which alleviates lipopolysaccharide-induced colorectal mucosa cells (FHCs) inflammation via lnc78583/miR3202/homeobox B13 pathway. 125 The pathogenic and therapeutic roles of lncRNAs in IBD are summarized in Table 1.
Table 1.
Roles of lncRNAs in the pathogenesis and treatment of IBD.
| LncRNAs | Expression | Mechanism | Results | References |
|---|---|---|---|---|
| FBXL19-AS1 | Upregulated | FBXL19-AS1 sponges miR-339-3p and increases the expression of its downstream gene rat sarcoma homologous protein B | Ameliorate DSS-induced colitis injury in mice | Zhao et al. 126 |
| lncRNA-H19 | Upregulated | lncRNA-H19 is bound to miR-331-3p to promote the transcription of its target gene tumor necrosis factor receptor-associated factor 4 | Aggravate intestinal injury in mice | Yin et al. 127 |
| NORAD | Upregulated | NORAD as a sponge of miR-552-3p enhances the expression of MyD88 | Induce FHCs apoptosis, inflammation and oxidative stress | Lei et al. 128 |
| PCSK6-AS1 | Upregulated | PCSK6-AS1 promotes Th1 differentiation via promoting the homeodomain interacting protein kinase 2-STAT1 signaling | Aggravate the chronic colitis-related mucosal barrier damage and tissue inflammation | Han et al. 129 |
| NEAT1 | Upregulated | NEAT1 inhibits miR-493-5p expression and increases the downstream target Rab27A expression | Promote the apoptosis and inflammation | Wang et al. 130 |
| NEAT1 inhibits miR-410-3p expression and increases the downstream target lactate dehydrogenase A expression | Lead to IECs dysfunction by promoting glycolysis | Ni et al. 131 | ||
| GATA6-AS1 | Downregulated | GATA6-AS1 lower expression is associated with higher transglutaminase 2 expression | Mitochondrial dysfunction | Sosnovski et al. 132 |
| Gm31629 | Downregulated | Gm31629 regulates E2F pathways by stabilizing Y-box protein 1 | Exacerbate intestinal injury and delay epithelial regeneration induced by DSS | Feng et al. 121 |
| T-UCRs uc.230 | Downregulated | uc.230 increased CUGBP1 expression by inhibiting miR-503 binding to Cugbp1 mRNA | Increase IECs apoptosis | Yu et al. 133 |
CUGBP1: CUG-binding protein 1; DSS: dextran sulfate sodium; FBXL19-AS1: FBXL19 antisense RNA 1; IBD: inflammatory bowel disease; IEC: intestinal epithelial cell; mRNA: messenger RNS; MyD88: myeloid differentiation primary response gene 88; NEAT1: nuclear paraspeckle assembly transcript 1; NORAD: long noncoding RNA activated by DNA damage; T-UCRs: transcribed from ultraconserved regions.
MicroRNAs (miRNAs)
miRNAs are not only regulated by lncRNAs to participate in the development of IBD (as mentioned above), but also participate in a variety of signaling pathways. miRNAs such as miR-31, miR-200b, miR-200c, and miRNA-16 may be biomarkers and potential therapeutic targets for IBD.134,135 The pathogenic and therapeutic roles of miRNAs in IBD are summarized in Table 2.
Table 2.
Roles of miRNAs in the pathogenesis and treatment of IBD.
| miRNAs | Expression | Mechanism | Results | References |
|---|---|---|---|---|
| miR-375-3p | Downregulated | Promote the transcription of solute carrier family 11 member 2 | Promote the colonic epithelial cells ferroptosis and UC progression | Chen et al. 136 |
| miRNA-9a-5p | Downregulated | miRNA-9a-5p agomir inhibits ROS production by targeting NOX4 | Prevent intestinal inflammatory damage | Li et al. 137 |
| miR-141-3p | Downregulated | miR-141-3p targets SUGT1 and inhibits its expression to inhibit colonic epithelial cells pyroptosis | Alleviate ulcerative colitis | Yan et al. 138 |
| let-7b | Downregulated | let-7b targets TGFBR1 and inhibits its expression and inhibits the profibrotic polarization of macrophages | Ameliorate intestinal fibrosis | Xu et al. 35 |
| miR-222-3p | Upregulated | miR-222-3p suppression can promote the downstream expression of Brahma-related gene 1, activate Nrf2/HO-1 signaling and reduce oxidative stress | Downregulated miR- miR-222-3p play a protective role IBD | Wang et al. 139 |
| miR-155 | Upregulated | Nogo-B deficiency inhibits miR-155 maturation in macrophages | MiR-155 inhibition reduces DSS-induced colitis in mice | Zheng et al. 140 |
| miR-142-5p | Upregulated | miR-142-5p was downregulated by overexpressed circRNA CCND1, which increases its downstream nuclear receptor coactivator-3 expression | Downregulated miR-142-5p play a protective role in IBD | Xiang et al. 141 |
| miR-7 | / | miR-7 silencing could activate epidermal growth factor receptor pathway | Alleviate the pathology of DSS-induced colitis | Zhao et al. 142 |
| miR-464 | / | miR-464 upregulated by Obefazimod | Decrease pro-inflammatory factors such as IL-6 and IL-17 | Apolit et al. 143 |
| miR-124-3p | / | miR-124-3p targets RBP4 and reduces the binding of RBP4 to NLRP3 and inhibit NLRP3-mediated pyroptosis | Alleviate UC | Yuan et al. 144 |
“/” indicates that the reference is not mentioned or is unclear. DSS: dextran sulfate sodium; IBD: inflammatory bowel disease; IL: interleukin; miRNA: micro RNA; NOX4: nicotinamide adenine dinucleotide phosphate (NADPH) oxidase 4; RBP4: retinol-binding protein 4; ROS: reactive oxygen species; SUGT1: SGT1 Homolog, MIS12 Kinetochore Complex Assembly cochaperone; TGFBR1: transforming growth factor beta receptor 1; UC: ulcerative colitis.
miRNAs in exosomes play an important role in IBD. 145 For example, macrophage-derived exosomal miR-223 induces intestinal barrier dysfunction via inhibiting transmembrane and immunoglobulin domain containing 1. 146 Several studies indicate that miRNAs extend the potential therapeutic effects on IBD through exosomes-mediated intercellular delivery.147–149 Under hypoxia, miR-216a-5p in adipose-derived stem cells is transferred to macrophages by exosomes, and promotes macrophage M2 polarization to attenuate colitis by high-mobility group box 2/Toll-like receptor 1 (TLR1)/NF-κB signaling pathway. 147 Exosomes of human umbilical cord MSCs could facilitate mucosal healing by accelerating intestinal stem cells and intestinal epithelium regeneration via transferring key miRNAs such as has-miR-21-5p and has-miR-143-3p. 148 MiR-129-5p released from human umbilical cord MSCs-derived exosomes inhibits lipid peroxidation and ferroptosis by targeting acyl-CoA synthetase long-chain family member 4. 149 With TNF-α treatment, the expression of miR-24-3p in small extracellular vesicles derived from human menstrual blood-derived MSCs is upregulated, which decreases IRF1 level and then promotes the M2 macrophages polarization to reduce the damage caused by hyperinflammation. 145
Although endoscopy is considered the gold standard for diagnosing and assessing disease activity and severity in IBD, it entails significant economic costs, patient discomfort, and in severe cases, the potential for surgical complications. Conversely, detection of serum or fecal biomarkers may offer indications of higher patient compliance. Multiple circulating lncRNAs and miRNAs such as miR-149-3p and miR-149-5p, and lncRNA H19 showed strong associations with endoscopic activity scores or disease activity of CD and UC.150,151 Hence, the inclusion of lncRNAs and miRNAs biomarkers in the diagnostic protocol has the potential to enhance precision, particularly when integrated with existing diagnostic modalities, thereby mitigating the need for exclusive reliance on endoscopic examinations for ascertaining disease activity. Notably, miR-223 in fecal samples exhibits superior accuracy and specificity in evaluating disease activity compared to C-reactive protein (CRP), fecal calprotectin (FC), and miR-223 in serum or tissue. 152 Like several endoscopic scoring systems, FC, serving as an indicator of localized intestinal inflammation rather than systemic inflammation, significantly correlated with IBD endoscopic scores, holds diagnostic value in evaluating the disease activity of UC. 153 However, there is limited research on the comparison of lncRNAs and miRNAs, potential biomarkers for IBD, with FC in assessing disease activity. Further studies are recommended to directly compare these emerging potential biomarkers with established noninvasive biomarkers such as erythrocyte sedimentation rate, CRP, FC, or endoscopic activity scores to determine their specificity and sensitivity. Besides, there is a notable variance in the concentration of FC in samples collected within a single day. 154 Hence, the utilization of FC for clinical purposes may necessitate the implementation of standardized detection techniques to mitigate the inherent day-to-day variability of fecal specimens. Studies have confirmed the robust stability of miRNA in frozen human peripheral blood. 155 Nevertheless, the presence of circulating biomarkers may reflect systemic inflammation rather than specific intestinal inflammation, particularly in individuals with mild or absent clinical manifestations. Trials with large sample sizes or incorporating multiple biomarkers for assessment and diagnosis might improve precision.
Based on a large number of in vitro experiments and animal models, lncRNAs and miRNAs are being recognized as promising candidates for therapeutic intervention in IBD and may hold potential for clinical implementation. Targeting specific lncRNAs and miRNAs for silencing or overexpression provides therapeutic benefits, such as the inhibition of miR-222-3p for relieving DSS-induced damage of the colon. 139 Another approach is to utilize lncRNAs and miRNAs as potential therapeutic agents to target the relevant pathogenic factors. A noteworthy example of this is theaflavin 3-gallate, a natural compound mimicking lncRNA Gm31629. 121 The biggest challenge in the development of nucleic acid drugs is how to keep them in the body for a sufficient amount of time and precisely reach the targeted cells to carry out therapeutic function, while preventing unintentional harm to normal cells. Chemical modifications such as phosphorothioate linkages and effective and safe delivery systems, like lipid nanoparticles and exosomes, can enable nucleic acid drugs to be precisely targeted to targeted cells or sites, improve uptake efficiency and prolong retention time. 156 Some miRNA-based drugs end clinical trials due to safety concerns. A clinical trial of liposomal miR-34a mimic was closed early due to unexpectedly severe immune-mediated toxicities, which resulted in four patient deaths in expansion cohorts. 157 To enhance safety, comprehensive pharmacodynamic and pharmacokinetic studies are essential for determining the circulating half-life of RNA therapeutics, choosing the appropriate targeting method, drug dosage and selecting the optimal delivery system. With the advantages of rapid target screening, less resistance, a broader therapeutic window, and long-term efficacy in RNA therapeutics, we firmly believe that lncRNAs and miRNAs are promising in translating these findings from bench to bedside.
Nanomedicines
Despite the advances of current therapeutics in IBD, the remission rate of no more than 30–60% fails to meet patients’ expectations.12,158,159 The application of nanotechnology in IBD treatment has the advantage of reducing side effects and precise targeting. 160 Nanomedicines can improve the therapeutic effect of traditional drugs for IBD. For example, budesonide-loaded nanosized micelles and Cortisone-loaded 6-o-stearoyl ascorbic acid nanostructured lipid carriers effectively attenuate DSS-induced colitis.161,162 DSS-coated hollow mesoporous manganese dioxide NPs as carriers to deliver budesonide, reduce the side effects of budesonide and improve the anti-inflammatory effect. 163 Polymer polyethyleneimine (PEI) mucoadhesive NPs loading dexamethasone derivatives (DDs) could enhance DDs enrichment and retention in the colitis site. 164
Considering that excessive ROS leads to intestinal epithelial injury, various nanomaterial-mediated antioxidant nanotherapeutics such as gold core and cerium oxide shell NPs (Au/CeO2) and macrophage membrane-coated mouse cathelicidin-related antimicrobial peptide-modified polydopamine NPs have emerged.165,166 Bacterial-flagella-inspired polydiiododiacetylene nanofibers retain for a longer time in the damaged mucosa and scavenge ROS, which not only enables the targeted CT visualization of IBD, but also alleviates the inflammation. 167 An oral polyphenol-armored nanomedicine with high ROS-scavenging ability, can resist the extreme environment of the stomach and modulate gut microbial homeostasis in IBD mice. 168 Aurozyme, comprised of gold NPs and glycyrrhizin, effectively scavenges ROS/reactive nitrogen species (RNS), attenuates the M1 polarization of macrophage, and increases the abundance and diversity of beneficial probiotics. 169 A folic acid-functionalized NP with ROS-responsive release and ROS-scavenging properties, can deliver Pterostilbene efficiently to achieve the therapeutic effects on UC. 170 Polycatechol-derived mesoporous polydopamine NPs extend a super potential in IBD treatment as a synergistic therapy by ROS scavenging and siRNA-mediated TNF-α gene interference. 171
Maintaining gut flora homeostasis is another approach of nanomedicines to alleviate IBD. Orally administered bilirubin-attached low-molecular-weight, water-soluble chitosan NPs with a longer retainment in the GI tract leads to recovery of dysregulated mucosal immunity and gut microbiome homeostasis, exerting robust therapeutic efficacy. 172 Therapeutic and protective effects of Bi2Se3 nanodiscs on IBD depend on regulating intestinal flora with increasing the proportion of Firmicutes to Bacteroidetes and inhibiting Proteobacteria bacteria, scavenging ROS and RNS and inhibiting proinflammatory cytokines. 160 A copolymer-modified two-dimensional H-silicene nanomaterial could improve the low-efficiency intestinal colonization of naked probiotics, which exposes the probiotic bacteria in response to the neutral/weakly alkaline intestinal environment. 173
In addition, pH-responsive nanomedicines can accurately target the gut for IBD treatment, such as methotrexate-loaded HA-CS/Eudragit® S100/Polylactic acid-glycolic acid copolymer NPs and Achillea wilhelmsii C. Koch-loaded Eudragit S100-coated chitosan NPs.174,175 Iron-based nano-biocomposite impregnated alginate formulation and ferulic acid-derived lignin nanoparticle disintegrate at colon pH, which achieves the delivery of targeting colon.176,177 Curcumin, decorated with a prussian blue analog can be released in inflammatory tissue (acidic environment), which promotes the polarization of M2 macrophages and the clearance of ROS. 178 A mesoporous polydopamine-derived nanomedicine loading polyacrylic acid releases Sulfasalazine at the inflammatory site of colon to scavenge excessive ROS. 179 Core–shell NPs with Eudragit® EPO and L100, two pH-sensitive materials coated on nano-sized curcumin, precisely target to inflamed sites. 180 Super carbonate apatite nanoparticle, a pH-sensitive delivery system for miRNA and siRNA, could activate the TGF-β/Smad signaling pathway against IBD by delivering miR-497a-5p. 181 Yang et al. 182 developed a pH-sensitive molybdenum-based polyoxometalate nanocluster, which alleviates DSS-induced UC via mitigating ferroptosis and reducing macrophages infiltration.
Even though the inorganic substances that would cause diseases are orders of magnitude larger than NPs in experiments, clinical trials and applications, this raises concerns about the long-term accumulation, aggregation and excretion of NPs in organisms. A drug-free biodegradable nanoparticle coupling PEI with antioxidant diselenide-bridged mesoporous organosilicon NPs ameliorates IBD by scavenging pro-inflammatory cell-free DNA and ROS. 183 This nanoparticle targets and accumulates in the inflamed colon, demonstrating anti-inflammatory activity at a lower dosing frequency (every 3 days) and flexible routes of administration (oral, intraperitoneal, and rectal administration). 183 Consequently, plant-derived or some biodegradable nanoparticles may be new therapeutic strategies in new medication developments with diverse drug delivery systems. In the future, Nanomedicines should be continuously explored to combine with other small molecule drugs or probiotics, or modify other chemical materials through nanotechnology, to achieve a more precise and efficient treatment of IBD.
Natural products
At present, most of the drugs on IBD treatment have side effects or the safety remains unknown, so it is of great significance to develop novel medicines with excellent efficacy and more safety. Natural products may be a source of new drugs with therapeutic effects of IBD. And many animal-based experiments on IBD have confirmed the therapeutic effects of natural products.
NF-κB signaling pathway inhibition
NF-κB, an important transcription factor, plays a vital role in promoting the progress of IBD, which is usually activated in IBD and further increases the generation of inflammatory cytokines. 184 Therefore, inhibiting NF-κB pathway by natural products may be a promising approach to treating IBD, details in Table 3.
Table 3.
Therapeutic effects of natural products on IBD via inhibiting NF-κB signaling.
| Name | Origin | Mechanism | References |
|---|---|---|---|
| Angelica sinensis aboveground part polysaccharide | Angelica sinensis | Inhibit TLR4/MyD88/NF-κB signaling pathway and change the intestinal microbiota profile and metabolic spectrum | Zou et al. 184 |
| Allium tenuissimum L. flower polysaccharide | Allium tenuissimum L. flowers | Inhibit TLR4/MyD88/NF-κB signaling pathway and promote the abundance of SCFAs-producing bacteria | Zhang et al. 185 |
| HEP10 | Hericium erinaceus | Inhibit NLRP3 inflammasome activation, and phosphorylation of NF-κB/protein kinase B/mitogen-activated protein kinase, and promote functional shifts in gut microbiota | Ren et al. 186 |
| Cis-Nerolidol | Essential oils from plants with a floral odor | Inhibit mitogen-activated protein kinase, NF-κB signaling pathways and enhance expression of key TJs | Raj et al. 187 |
| Shikimic acid | Anise | Inhibit NF-κB and mitogen-activated protein kinase signaling pathways, improve the abnormal immune response and regulate intestinal flora | Li et al. 188 |
| Sericic acid | Rosa odorata | Inhibit NF-κB signaling pathways and activate Nrf2/HO-1 pathway | Lifei et al. 189 |
| Loganic acid | Plant secondary metabolite | Inhibit TLR4/NF-κB pathway and active Sirtuin 1/Nrf2 pathways | Prakash et al. 190 |
| Gallic acid | Tannin-producing plants | Inhibit NLRP3 inflammasome activation and p65-NF-κB phosphorylation | Yu et al. 191 |
| Thyme polyphenols | Thymus vulgaris L. | Inhibit TLR4/NLRP3-NF-κB inflammasome pathway and increase the abundance of SCFAs-producing bacteria | Zhou et al. 192 |
| Eicosapentaenoic acid | Fish oil | Inhibit PI3K/AKT/NF-κB pathway | El Mahdy et al. 193 |
| Nuciferine | Lotus plant | Inhibit mitogen-activated protein kinase/NF-κB/NLRP3 pathways and oxidative stress-mediated ROS generation | Kulhari et al. 194 |
| Higenamine | Aconitum | Inhibit Galectin-3/TLR4/NF-κB pathways and improves colon epithelial cell viability | Shao et al. 195 |
| Oxymatrine | Sophora flavescens | Inhibit TLR/NF-κB signaling pathway and correct the interaction between gut microflora and inflammatory dendritic cells | Liu et al. 196 |
| Sinomenine | Sinomenium | Promote 14-3-3θ protein and inhibit NF-κB signaling | Zhou et al. 197 |
| Cinnamaldehyde | Cinnamomum cassia | Inhibit TLR4/NF-κB signaling pathway and NLRP3 inflammasome activation | Tan et al. 198 |
| Phillygenin | Forsythiae Fructus | Inhibit the activation of tyrosine kinase Src mediated by TLR4, and then reduce the phosphorylation of downstream proteins p38, c-Jun N-terminal kinase, and NF-κB | Xue et al. 199 |
| Isosteviol | Stevioside | Inhibit pyruvate dehydrogenase kinase 1/AKT/NF-κB signaling pathway | Yao et al. 200 |
| Isovitexin | Asian rice | Activate aryl hydrocarbon receptor and inhibit NF-κB activation | Mu et al. 201 |
| Albiflorin | Paeonia lactiflora Pallas | Inhibit NF-κB and mitogen-activated protein kinase pathways to reduce inflammation and oxidative stress | Wang et al. 202 |
| 7-Hydroxyl-1-methylindole-3-acetonitrile | Brassicaceae | Inhibit NF-κB and STAT3 activation and microsomal prostaglandin E synthase-1 expression | Chung et al. 203 |
| Indole-3-carboxaldehyde | Tryptophan metabolite | Balance amino acid metabolism and inhibit the activation of the TLR4/NF-kB/p38 signaling pathway | Liu et al. 204 |
| 4-Geranyloxy-2,6-dihydroxybenzophenonel | Hypericum sampsonii | Activate cyclic adenosine monophosphate/protein kinase A/cyclic-AMP response element binding protein and inhibit the NF-κB pathway | Wang et al. 205 |
| Peoniflorin-6′-O-benzene sulfonate | Paeonia | Inhibit ERK1/2-NF-κB activation | Jiang et al. 206 |
| 20(S)-Protopanaxadiol saponins | Panax notoginseng | Block the binding of high-mobility group box 1 to TLR4, which interfering with the activation of TLR4/NF-κB/NLRP3 inflammasome pathway | Chen et al. 207 |
| Sauchinone | Saururus chinensis | Reverse the activation of the NF-κB pathway and upregulate NAD(P)H dehydrogenase [quinone] 1 expression | Wu et al. 208 |
| Masticadienonic acid | Chios mastic gum | Inhibit MAPK/NF-κB pathway and active Nrf2 pathway, and upregulate TJs | Cui et al. 209 |
IBD: inflammatory bowel disease; NF-κB: Nuclear factor kappa-l B cell; NLRP3: nucleotide-binding and oligomerization domain-like receptor family pyrin domain containing 3; PI3K: phosphatidylinositol-3-kinase; ROS: reactive oxygen species; SCFA: short-chain fatty acid; STAT3: signal transducer and activator of transcription 3; TJs: tight junctions; TLR: toll like receptor 1.
Nuclear factor erythroid 2-related factor 2(Nrf2) signaling pathway activation
Nrf2 is considered to be a key regulator of inflammatory diseases, which protects different types of cells and tissues from inflammation and oxidative stress. 210 Many natural products play a protective role in IBD by targeting Nrf2, such as the sericic acid. 189 Corynoline promotes Nrf2 nuclear migration and heme oxygenase-1(HO-1) expression in the colonic tissues of UC mice and inhibits NF-κB phosphorylation and nuclear translocation. 211 Venkataraman et al. 212 described the potential therapeutic effect of 1,8-cineole in IBD, which increases the translocation of Nrf2 into the nucleus and inhibits the production of pro-inflammatory chemokines as a novel peroxisome proliferator-activated receptor γ agonist. Previous studies have revealed that Crocin has antioxidant activity through inhibiting NF-κB pathway or activating Nrf2/HO-1 pathway, while a new study shows that Crocin has anti-inflammatory and anti-apoptotic effects against IBD. 213
Gut microbiota and intestinal barrier improvement
Gut microbiota plays an important role in the pathogenesis of IBD, and intestinal barrier dysfunction is one of the characteristics of IBD. 214 Therefore, many natural products with promising therapeutic potential for IBD treatment by regulating gut microbiota and improving intestinal barrier, such as Geniposidic acid. 215 Details are in Table 4.
Table 4.
Therapeutic effects of natural products on IBD via regulating gut microbiota and improving intestinal barrier.
| Name | Origin | Mechanism | References |
|---|---|---|---|
| Panax quinquefolius polysaccharide | American ginseng | Increase the relative abundance of Bacteroidetes, Rikenellaceae, Shigella and Oscillospira, the content of faecal SCFAs and the expression of TJs | Ren et al. 214 |
| Glycyrrhiza polysaccharide | Glycyrrhiza | Promote the growth and reproduction of beneficial bacteria such as Lactobacillus, Bacteroides, and SCFA-producing bacteria and maintain TJs | Wei et al. 216 |
| Rehmannia glutinosa polysaccharide | Rehmannia glutinosa | Maintain the species diversity of intestinal microbes and increase the content of SCFAs | Lv et al. 217 |
| Euphorbia humifusa-derived polysaccharide | Euphorbia humifusa | Promote the production of acetic, propionic, and valeric acids and increasing the relative abundance of Bifidobacteria | Xiang et al. 218 |
| Dendrobium fimbriatum Hook polysaccharide | Dendrobium fimbriatum Hook | Promote ISCs regeneration to protect intestinal mucosa integrity by lamina propria lymphocytes-secreted IL-22 | Wang et al. 219 |
| Cordyceps sinensis polysaccharide | Cordyceps sinensis | Decrease the relative abundance of Bilophila and increase the relative abundance of Dehalobacterium, Coprococcus, Oscillospira, and Desulfovibrio, and increase the content of SCFAs | Chen et al. 220 |
| Fructooligosaccharide | Polygonatum cyrtonema Hua | Downregulate matrix metallopeptidase 13 expression and selectively enhance the growth of probiotics, including Bifidobacterium, Alloprevofella, and Alistipes | Xu et al. 221 |
| Alginate oligosaccharides | Brown algae | Reverse intestinal microbiota changes and recover SCFAs production | Wu et al. 222 |
| R. glutinosa oligosaccharides | R. glutinosa | Alleviate the oxidative stress and increase the abundance of Firmicutes, Lactobacillus and Akkermansia, and promote the production of SCFAs | Li et al. 223 |
| Sodium houttuyfonate | Houttuynia cordata Thunb | Increase the abundance of SCFAs-producing bacteria (Lachnospiraceae_NK4A136_group, Intestinimonas) | Cheng et al. 224 |
| Caesaldekarin e | Caesalpinia bonduc | Increase the abundance of Lactobacillus and decrease the abundance of Bacteroides, and reduce the level of kynurenine and kynurenic acid | Liu et al. 225 |
| Paeoniflorin | Paeonia lactiflora | Promote ISCs renewal and differentiation to accelerate epithelial regeneration and repair via PI3K-AKT-mTOR pathway activation | Ma et al. 226 |
| Humic acids | Plant and microbial residues through humification | Increase the contents of acetic acid, isovaleric acid, and isobutyric acid with an increase in the abundance of Firmicutes and a decrease in the abundance of Proteobacteria | Huang et al. 227 |
| Prodigiosin | Microorganism secondary metabolite | Increase the abundance of Bifidobacterium, Allobaculum, and Akkermansia, and accelerate the expression of intestinal TJs | Nie et al. 228 |
IBD: inflammatory bowel disease; IL: interleukin; ISC: intestinal stem cell; SCFA: short-chain fatty acid; TJs: tight junctions.
Immune dysregulation improvement
Immune response involves innate and adaptive immune responses, and is associated with the pathogenesis of IBD, as mentioned above, is one of the targets for IBD treatment. Chlorogenic acid, abundant in potatoes, apples, and blueberries, could improve defective immune systems in IL-10 knockout IBD mice. 229 Isochlorogenic acid A, abundant in Lonicerae japonicae Flos and chicory, could reduce neutrophils and macrophages infiltration and inhibit STAT3/NF-кB pathway. 230 Hederacoside C, isolated from Pulsatilla chinensis Regel, could reduce the recruitment and activation of neutrophils and decrease S100A9 leading to restoring impaired intestinal barrier in TNBS-induced colitis. 231 Both Pinus yunnanensis pollen polysaccharides and sulfated polysaccharides can alleviate DSS-induced colitis. 232 Among them, the former tends to inhibit pro-inflammatory cytokines and restore intestinal flora diversity, while the latter mainly promotes anti-inflammatory cytokines and improves intestinal immunity. 232 Salidroside effectively restricts experimental colitis via inhibiting macrophage pyroptosis and maintaining Th17/Treg balance. 233 Cod skin collagen peptide powder, made from lyophilized high-quality cod skin using bio-enzymatic technology, upregulates the expression of mitogen-activated protein kinase phosphatase-1 and promotes M2 macrophages polarization for preventing and treating UC. 234
Target programmed cell death
There is increasing evidence that IECs death is highly associated with intestinal damage and intestinal barrier dysfunction in IBD. 235 There are many natural compounds targeting cell death for the treatment of IBD. Daphnetin, a natural coumarin compound, inhibits oxidative stress and cell apoptosis by downregulating REG3A and then activating JAK2/STAT3 signaling pathway. 236 Genistein exerts its protective potential for IBD via enhancing antioxidant activity and increasing mitochondrial biogenesis, thereby reducing cell apoptosis. 237 The combination of Genistein and Sulfasalazine has an augmented and remarkable effect against oxidative stress and cell apoptosis, over either drug alone. 238 Polysaccharides from eggs of Strongylocentrotus nudus could inhibit the PI3K/protein kinase B(AKT)/mTOR pathway to mediate cytoprotective autophagy by acting on CD36 for alleviating IBD. 239 Hydroxysafflor yellow A, a chalcone glycoside isolated from safflower has been found to ameliorate DSS-induced colitis mice via suppressing pyroptosis by blocking hexokinase 1/NLRP3/gasdermin D pathway and regulating intestinal microbiota. 240 Ginsenoside Rg3 could interfere with NLRP3 inflammasome activation and inhibit pyroptosis, leading to the restoration of barrier function. 241 Kumatakenin, the main components of cloves and Alpinia purpurata upregulate the expression of enolase by hydrogen bonding with the amino acid residues Thr208, Val206, and Pro203, leading to the degradation of IRP1 mRNA, which inhibits epithelial ferroptosis to relieve IBD. 242 Protocatechuic acid, existing in many edible vegetables, fruits, and nuts, protect against DSS-induced UC through regulating gut microbiota and inhibiting ferroptosis. 243
More and more studies are pointing to the potential benefits of natural products in the treatment of IBD. Nevertheless, these active compounds’ low oral absorption, low solubility, and low bioavailability make it challenging to get them approved as medications in clinical settings. Effective routes of administration, and appropriate chemical modifications of natural products with safe, efficient and specific delivery systems may be novel strategies for IBD treatment. Elucidating the mechanisms and targets of natural products at the molecular level could facilitate the application of natural products in biotechnology and pharmaceuticals. At present, there is no definitive evidence regarding the optimal therapeutic dosage of natural products for patients with IBD. Further investigation is required to ascertain their safety, therapeutic dosage, and metabolic processes.
Patient-centered IBD management
As mentioned above, the pathogenesis of IBD is multifactorial, with heterogeneity in patients’ clinical manifestations and response to treatment. In addition to intestinal inflammation, patients suffer from other symptoms including obesity, malnutrition, pain, fatigue, and psychological health problems, which affect the course of the disease and the patient's quality of life.244–246 However, these factors are usually ignored or not well addressed by clinical IBD practitioners.
The comprehensive assessment of potential health issues in patients with IBD is crucial for disease management. Obesity and malnutrition represent contrasting nutritional statuses in IBD patients, with malnutrition often serving as an indicator of negative response to medication. 244 Massironi et al. 244 proposed that adherence to a balanced and healthy diet is paramount for people with IBD. This does not imply that malnourished IBD patients should be prescribed a high-calorie diet to overcome potentially inadequate food intake. 244 IBD patients with obesity should be encouraged to reduce their bodyweight during remission in different weightloss programs. Sarcopenia, which can occur in both malnutrition and obesity, and strategies focused on maintaining or enhancing muscle health such as physical exercise and nutritional interventions may help reduce postoperative complications and promote recovery. 245 While certain dietary interventions have shown therapeutic effects on IBD, some factors may hinder the effectiveness of dietary interventions in patients with IBD. For instance, restricting specific foods and food groups such as exclusionary whole food diets as part of dietary interventions may lead to taste fatigue, and patient compliance may also be a great challenge to adhere to nutritional intervention. And increasing exercise levels tends to improve social functioning and fatigue levels in IBD patients. 247 As a result, personalized interventions including physical exercise and dietary interventions as part of their routine clinical care combined with medication may be a great approach to improve IBD patients’ symptoms. Psychosocial factors are important parts of IBD management, which may be accompanied by fatigue, sleep disorders and emotional disturbances. 246 Among various mind–body practices and psychological interventions, yoga, and cognitive behavioral therapy are believed to be helpful in improving mental health disorders. 247 Accordingly, IBD patient-centered holistic care needs a close multidisciplinary collaboration between clinicians, nurses, psychologists, dieticians, and physiotherapist. 247 Building a sense of purpose, which requires not only alleviating inflammation, but also comprehensive assessment, intervention, and management of other health problems including obesity, malnutrition, fatigue, and mental health issues. However, there are still no established quality standards of care for patients with IBD that are applicable to all conditions and all countries. 248
Future perspectives
There is no exact and definitive consensus on the pathogenesis of IBD. Delving into the interaction between these factors such as genetics, intestinal flora, environmental factors, immune system, may be a new way to gain insight into the pathogenesis of IBD. The mechanism of disturbances in the balance between the immune system and gut microbiota (including their metabolites) and the cause-and-effect relationships among them have not been elucidated. And exploring the alterations in microbiota composition and metabolites following different forms of dietary interventions, and the resulting clinical improvement, can be beneficial.
A considerable fraction of patients do not respond to conventional therapies or lose response or worry about side effects, which calls for new therapeutic strategies. lncRNAs and miRNAs as potential diagnostic biomarkers in IBD from more accessible and stable samples such as blood may be more beneficial for translating these findings from bench to bedside. Emerging therapies including lncRNAs, miRNAs, nanomedicines, and natural products show great therapeutic potential for IBD, with less frequency of administration and longer retention period at the site of inflammation. In addition, the active ingredients and structures of natural products are complex and the optimal therapeutic dose of various natural products for IBD patients still need more research to validate data from in vitro studies and animal models, minimize the risk and optimize the treatment outcomes with long-term data. The potential differences in the pathogenesis and therapies of IBD patients with different ages or different physiological conditions are not discussed in depth due to the limited relevant research studies. Consequently, there is a lack of sufficient high-quality evidence to prove the efficacy and safety of emerging therapies. More research with long-term data is needed to elucidate the pathogenic mechanism of IBD and develop safer and more effective drugs and individualized treatment strategies.
In summary, this article provides a holistic view of IBD with cutting-edge treatments and mechanisms. IBD treatment strategies should take genetic, environmental, microbial, and other factors into consideration comprehensively, following STRIDE II recommendations, to develop more effective, safer and personalized treatments for patients with IBD (Figure 1).
Figure 1.
Graphical summary of the pathogenesis and treatment of inflammatory bowel disease.
Conclusion
In this review, we provide a comprehensive view of the etiology and emerging treatment strategies for IBD. The most convincing hypotheses for the etiology of IBD are based on interactions between genetic susceptibility, environmental and dietary factors, gut microbiome dysbiosis, and dysregulated immune responses. However, the interactions and causal relationships among these factors have yet to be fully elucidated. More research is needed to clarify the mechanism, corroborate data, and narrow knowledge gaps. Emerging therapies such as nanomedicines, natural products, lncRNAs, and miRNAs, have shown encouraging effects in IBD treatment. As many studies are based on animal models, more research is needed on these emerging treatment options to minimize the risk and optimize the treatment outcomes with long-term data.
Author biographies
Junyi Bai is a student from the First Affiliated Hospital of Zhejiang Chinese Medical University, is currently focused on investigating the role of intestinal mucosal barrier function in pathogenesis of inflammatory bowel disease and irritable bowel syndrome.
Ying Wang is a student from the First Affiliated Hospital of Zhejiang Chinese Medical University. Her current interest is the immune mechanism of irritable bowel syndrome.
Fuhao Li is a Gastroenterology PhD from Zhejiang Chinese Medical University, is currently focused on researching the pathogenesis and therapy of gastric mucosal intestinal metaplasia.
Yueyao Wu is a Gastroenterology master in Zhejiang Chinese Medical University. Her current interest is pathogenesis and treatment of inflammatory bowel disease.
Jun Chen is a Cardiology PhD in Zhejiang Chinese Medical University. His current interest is pathogenesis and treatment of viral myocarditis.
Meng Li is a Gastroenterology PhD in Zhejiang Chinese Medical University. His current interest is pathogenesis and treatment of inflammatory bowel disease.
Xi Wang is an excellent researcher in the First Affiliated Hospital of Zhejiang Chinese Medicine, is dedicated to investigating the pathogenesis of functional gastrointestinal disorders, mechanisms and therapeutic strategies of cisplatin resistance in gastric cancer.
Bin Lv is a professor of gastroenterology at Zhejiang Chinese Medical University, is currently focused on the role of intestinal flora and immune cells in the pathogenesis of inflammatory bowel disease and irritable bowel syndrome. Additionally, he is investigating precancerous lesions of gastric cancer.
Footnotes
Authors’ contributions: JB, YW, and FL contributed to the drafting of the manuscript; JC, YW, and ML reviewed it critically for important intellectual content; BL and XW conceptualized and supervised the work and supported funding acquisition. All authors were involved in the writing, reviewing, and editing of the manuscript. All the authors have read and agreed to the published version of the manuscript.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Natural Science Foundation of China (grant number 81970470) and the Zhejiang Provincial Natural Science Foundation of China (grant number LY21H030002).
ORCID iD: Junyi Bai https://orcid.org/0009-0004-1627-7933
References
- 1.Nakase H, Uchino M, Shinzaki S, et al. Evidence-based clinical practice guidelines for inflammatory bowel disease 2020. J Gastroenterol 2021; 56: 489–526. 2021/04/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet 2017; 390: 2769–2778. 2017/10/21. [DOI] [PubMed] [Google Scholar]
- 3.Rogler G, Singh A, Kavanaugh A, et al. Extraintestinal manifestations of inflammatory bowel disease: current concepts, treatment, and implications for disease management. Gastroenterology 2021; 161: 1118–1132. 2021/08/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guan Q. A comprehensive review and update on the pathogenesis of inflammatory bowel disease. J Immunol Res 2019; 2019: 7247238. 2019/12/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu JZ, van Sommeren S, Huang H, et al. Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nat Genet 2015; 47: 979–986. 2015/07/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vila A V, Hu S, Andreu-Sanchez S, et al. Faecal metabolome and its determinants in inflammatory bowel disease. Gut 2023; 72: 1472–1485. 2023/03/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kong L, Pokatayev V, Lefkovith A, et al. The landscape of immune dysregulation in Crohn's disease revealed through single-cell transcriptomic profiling in the ileum and colon. Immunity 2023; 56: 444–458 e445. 2023/02/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gurtner A, Borrelli C, Gonzalez-Perez I, et al. Active eosinophils regulate host defence and immune responses in colitis. Nature 2023; 615: 151–157. 2022/12/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun Y, Yuan S, Chen X, et al. The contribution of genetic risk and lifestyle factors in the development of adult-onset inflammatory bowel disease: a prospective cohort study. Am J Gastroenterol 2023; 118: 511–522. 2023/01/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Celik K, Guveli H, Erzin Y, et al. The effect of adherence to Mediterranean diet on disease activity in patients with inflammatory bowel disease. Turk J Gastroenterol 2023; 34: 714–719. 2023/05/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xiao P, Hu Z, Lang J, et al. Mannose metabolism normalizes gut homeostasis by blocking the TNF-alpha-mediated proinflammatory circuit. Cell Mol Immunol 2023; 20: 119–130. 2022/12/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Le Berre C, Honap S, Peyrin-Biroulet L. Ulcerative colitis. Lancet 2023; 402: 571–584. 2023/08/13. [DOI] [PubMed] [Google Scholar]
- 13.Turner D, Ricciuto A, Lewis A, et al. STRIDE-II: an update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) initiative of the International Organization for the Study of IBD (IOIBD): determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology 2021; 160: 1570–1583. 2020/12/29. [DOI] [PubMed] [Google Scholar]
- 14.Mirkov MU, Verstockt B, Cleynen I. Genetics of inflammatory bowel disease: beyond NOD2. Lancet Gastroenterol Hepatol 2017; 2: 224–234. 2017/04/14. [DOI] [PubMed] [Google Scholar]
- 15.Shimodaira Y, More SK, Hamade H, et al. DR3 regulates intestinal epithelial homeostasis and regeneration after intestinal barrier injury. Cell Mol Gastroenterol Hepatol 2023; 16: 83–105. 2023/04/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chauvin C, Alvarez-Simon D, Radulovic K, et al. NOD2 in monocytes negatively regulates macrophage development through TNFalpha. Front Immunol 2023; 14: 1181823. 2023/07/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Masuta Y, Minaga K, Kurimoto M, et al. Activation of nucleotide-binding oligomerization domain 2 by muramyl dipeptide negatively regulates Toll-like receptor 9-mediated colonic inflammation through the induction of deubiquitinating enzyme A expression. Int Immunol 2023; 35: 79–94. 2022/09/29. [DOI] [PubMed] [Google Scholar]
- 18.Spalinger MR, Lang S, Gottier C, et al. PTPN22 regulates NLRP3-mediated IL1B secretion in an autophagy-dependent manner. Autophagy 2017; 13: 1590–1601. 2017/08/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spalinger MR, Schmidt TS, Schwarzfischer M, et al. Protein tyrosine phosphatase non-receptor type 22 modulates colitis in a microbiota-dependent manner. J Clin Invest 2019; 129: 2527–2541. 2019/05/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spalinger MR, Schwarzfischer M, Niechcial A, et al. Loss of PTPN22 promotes intestinal inflammation by compromising granulocyte-mediated antibacterial defence. J Crohns Colitis 2021; 15: 2118–2130. 2021/06/06. [DOI] [PubMed] [Google Scholar]
- 21.Luo P, Yang Z, Chen B, et al. The multifaceted role of CARD9 in inflammatory bowel disease. J Cell Mol Med 2020; 24: 34–39. 2019/11/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lamas B, Richard ML, Leducq V, et al. CARD9 impacts colitis by altering gut microbiota metabolism of tryptophan into aryl hydrocarbon receptor ligands. Nat Med 2016; 22: 598–605. 2016/05/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Danne C, Michaudel C, Skerniskyte J, et al. CARD9 in neutrophils protects from colitis and controls mitochondrial metabolism and cell survival. Gut 2023; 72: 1081–1092. 2022/09/28. [DOI] [PubMed] [Google Scholar]
- 24.Lamas B, Michel ML, Waldschmitt N, et al. Card9 mediates susceptibility to intestinal pathogens through microbiota modulation and control of bacterial virulence. Gut 2018; 67: 1836–1844. 2017/08/10. [DOI] [PubMed] [Google Scholar]
- 25.Soderquest K, Hertweck A, Giambartolomei C, et al. Genetic variants alter T-bet binding and gene expression in mucosal inflammatory disease. PLoS Genet 2017; 13: e1006587. 2017/02/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma H, Hu T, Tao W, et al. A lncRNA from an inflammatory bowel disease risk locus maintains intestinal host-commensal homeostasis. Cell Res 2023; 33: 372–388. 2023/04/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Markelova M, Senina A, Khusnutdinova D, et al. Association between taxonomic composition of gut microbiota and host single nucleotide polymorphisms in Crohn's disease patients from Russia. Int J Mol Sci 2023; 24: 7998, 2023/05/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shin SY, Kim Y, Kim WS, et al. Compositional changes in fecal microbiota associated with clinical phenotypes and prognosis in Korean patients with inflammatory bowel disease. Intest Res 2023; 21: 148–160. 2022/06/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krawczyk A, Salamon D, Kowalska-Duplaga K, et al. Changes in the gut mycobiome in pediatric patients in relation to the clinical activity of Crohn's disease. World J Gastroenterol 2023; 29: 2172–2187. 2023/05/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wade H, Pan K, Duan Q, et al. Akkermansia muciniphila and its membrane protein ameliorates intestinal inflammatory stress and promotes epithelial wound healing via CREBH and miR-143/145. J Biomed Sci 2023; 30: 38. 2023/06/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reiss Z, Rob F, Kolar M, et al. Skin microbiota signature distinguishes IBD patients and reflects skin adverse events during anti-TNF therapy. Front Cell Infect Microbiol 2022; 12: 1064537. 2023/01/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu M, Ding H, Gong S, et al. Fungal dysbiosis facilitates inflammatory bowel disease by enhancing CD4+ T cell glutaminolysis. Front Cell Infect Microbiol 2023; 13: 1140757. 2023/05/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lopez-Cauce B, Puerto M, Garcia JJ, et al. Akkermansia deficiency and mucin depletion are implicated in intestinal barrier dysfunction as earlier event in the development of inflammation in interleukin-10-deficient mice. Front Microbiol 2022; 13: 1083884. 2023/01/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kittana H, Gomes-Neto JC, Heck K, et al. Evidence for a causal role for Escherichia coli strains identified as adherent-invasive (AIEC) in intestinal inflammation. mSphere 2023; 8: e0047822. 2023/03/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu Y, Qian W, Huang L, et al. Crohn's disease-associated AIEC inhibiting intestinal epithelial cell-derived exosomal let-7b expression regulates macrophage polarization to exacerbate intestinal fibrosis. Gut Microbes 2023; 15: 2193115. 2023/03/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang S, Deng W, Li F, et al. Treatment with butyrate alleviates dextran sulfate sodium and Clostridium difficile-induced colitis by preventing activity of Th17 cells via regulation of SIRT1/mTOR in mice. J Nutr Biochem 2023; 111: 109155. 2022/09/27. [DOI] [PubMed] [Google Scholar]
- 37.Ju J, Zhang C, Yang J, et al. Deoxycholic acid exacerbates intestinal inflammation by modulating interleukin-1beta expression and tuft cell proportion in dextran sulfate sodium-induced murine colitis. PeerJ 2023; 11: e14842. 2023/02/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou C, Wang Y, Li C, et al. Amelioration of colitis by a gut bacterial consortium producing anti-inflammatory secondary bile acids. Microbiol Spectr 2023; 11: e0333022. 2023/03/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Flannigan KL, Nieves KM, Szczepanski HE, et al. The pregnane X receptor and indole-3-propionic acid shape the intestinal mesenchyme to restrain inflammation and fibrosis. Cell Mol Gastroenterol Hepatol 2023; 15: 765–795. 2022/10/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kurata K, Ishii K, Koto Y, et al. Skatole-induced p38 and JNK activation coordinately upregulates, whereas AhR activation partially attenuates TNFalpha expression in intestinal epithelial cells. Biosci Biotechnol Biochem 2023; 87: 611–619. 2023/03/21. [DOI] [PubMed] [Google Scholar]
- 41.Chen Y, Mai Q, Chen Z, et al. Dietary palmitoleic acid reprograms gut microbiota and improves biological therapy against colitis. Gut Microbes 2023; 15: 2211501. 2023/05/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wetwittayakhlang P, Gonczi L, Golovics PA, et al. Time trends of environmental and socioeconomic risk factors in patients with inflammatory bowel disease over 40 years: a population-based inception cohort 1977-2020. J Clin Med 2023; 12: 3026. 2023/04/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou S, Chai P, Dong X, et al. Drinking water quality and inflammatory bowel disease: a prospective cohort study. Environ Sci Pollut Res Int 2023; 30: 71171–71183. 2023/05/10. [DOI] [PubMed] [Google Scholar]
- 44.Shon WJ, Jung MH, Kim Y, et al. Sugar-sweetened beverages exacerbate high-fat diet-induced inflammatory bowel disease by altering the gut microbiome. J Nutr Biochem 2023; 113: 109254. 2022/12/27. [DOI] [PubMed] [Google Scholar]
- 45.Yu J, Liu T, Guo Q, et al. Disruption of the intestinal mucosal barrier induced by high fructose and restraint stress is regulated by the intestinal microbiota and microbiota metabolites. Microbiol Spectr 2023; 11: e0469822. 2023/02/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sun L, Wang X, Zou Y, et al. Cold stress induces colitis-like phenotypes in mice by altering gut microbiota and metabolites. Front Microbiol 2023; 14: 1134246. 2023/04/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rizzello F, Gionchetti P, Spisni E, et al. Dietary habits and nutrient deficiencies in a cohort of european Crohn's disease adult patients. Int J Mol Sci 2023; 24: 1494, 2023/01/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Khalili H, Hakansson N, Casey K, et al. Diet quality and risk of older-onset Crohn's disease and ulcerative colitis. J Crohns Colitis 2023; 17: 746–753. 2022/12/16. [DOI] [PubMed] [Google Scholar]
- 49.Martens E, Pereira G, Boudaud M, et al. Unravelling specific diet and gut microbial contributions to inflammatory bowel disease. Res Sq 2023; 13: rs.3.rs-2518251. 2023/03/31. [Google Scholar]
- 50.Panah FM, Nielsen KD, Simpson GL, et al. A westernized diet changed the colonic bacterial composition and metabolite concentration in a dextran sulfate sodium pig model for ulcerative colitis. Front Microbiol 2023; 14: 1018242. 2023/05/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.O'Mahony C, Clooney A, Clarke SF, et al. Dietary-induced bacterial metabolites reduce inflammation and inflammation-associated cancer via vitamin D pathway. Int J Mol Sci 2023; 24: 1864. 2023/02/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Khademi Z, Saneei P, Hassanzadeh-Keshteli A, et al. Association between food-based dietary inflammatory potential and ulcerative colitis: a case-control study. Sci Rep 2023; 13: 8464. 2023/05/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nitescu M, Istratescu D, Preda CM, et al. Role of an exclusion diet (reduced disaccharides, saturated fats, emulsifiers, red and ultraprocessed meats) in maintaining the remission of chronic inflammatory bowel diseases in adults. Medicina (Kaunas) 2023; 59: 329. 2023/02/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Niechcial A, Schwarzfischer M, Wawrzyniak M, et al. Spermidine ameliorates colitis via induction of anti-inflammatory macrophages and prevention of intestinal dysbiosis. J Crohns Colitis 2023; 17: 1489–1503. 2023/03/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.de Oliveira ECS, Dalmau LM, de Almeida Costa CAR, et al. Dietary intervention with avocado (Persea americana Mill.) ameliorates intestinal inflammation induced by TNBS in rats. Inflammopharmacology 2023; 31: 485–498. 2023/01/01. [DOI] [PubMed] [Google Scholar]
- 56.Zhang T, Zhang R, Zhao G, et al. Plant green pigment of chlorophyllin attenuates inflammatory bowel diseases by suppressing autophagy activation in mice. Am J Physiol Gastrointest Liver Physiol 2022; 323: G102–G113. 2022/06/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Holman JM, Colucci L, Baudewyns D, et al. Steamed broccoli sprouts alleviate DSS-induced inflammation and retain gut microbial biogeography in mice. bioRxiv 2023 2023/06/09. doi: 10.1101/2023.01.27.522641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang T, Holman J, McKinstry D, et al. A steamed broccoli sprout diet preparation that reduces colitis via the gut microbiota. J Nutr Biochem 2023; 112: 109215. 2022/11/13. [DOI] [PubMed] [Google Scholar]
- 59.Holcomb L, Holman JM, Hurd M, et al. Early life exposure to broccoli sprouts confers stronger protection against enterocolitis development in an immunological mouse model of inflammatory bowel disease. bioRxiv 2023. 2023/02/08. doi: 10.1101/2023.01.27.525953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dong L, Xie J, Wang Y, et al. Mannose ameliorates experimental colitis by protecting intestinal barrier integrity. Nat Commun 2022; 13: 4804. 2022/08/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Alake SE, Lightfoot S, Wozniak K, et al. Wheat germ supplementation reduces inflammation and gut epithelial barrier dysfunction in female interleukin-10 knockout mice fed a pro-atherogenic diet. J Nutr 2023; 153: 870–879. 2023/02/23. [DOI] [PubMed] [Google Scholar]
- 62.Novichkova E, Nayak S, Boussiba S, et al. Dietary application of the microalga Lobosphaera incisa P127 reduces severity of intestinal inflammation, modulates gut-associated gene expression, and microbiome in the zebrafish model of IBD. Mol Nutr Food Res 2023; 67: e2200253. 2023/01/24. [DOI] [PubMed] [Google Scholar]
- 63.Yuan SN, Wang MX, Han JL, et al. Improved colonic inflammation by nervonic acid via inhibition of NF-kappaB signaling pathway of DSS-induced colitis mice. Phytomedicine 2023; 112: 154702. 2023/02/11. [DOI] [PubMed] [Google Scholar]
- 64.Gan F, Lin Z, Tang J, et al. Deoxynivalenol at no-observed adverse-effect levels aggravates DSS-induced colitis through the JAK2/STAT3 signaling pathway in mice. J Agric Food Chem 2023; 71: 4144–4152. 2023/02/28. [DOI] [PubMed] [Google Scholar]
- 65.Schwarzfischer M, Niechcial A, Handler K, et al. Tio(2) nanoparticles abrogate the protective effect of the Crohn's disease-associated variation within the PTPN22 gene locus. Gut 2023; 72: 1101–1114. 2022/10/04. [DOI] [PubMed] [Google Scholar]
- 66.Faye AS, Allin KH, Iversen AT, et al. Antibiotic use as a risk factor for inflammatory bowel disease across the ages: a population-based cohort study. Gut 2023; 72: 663–670. 2023/01/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sanmarco LM, Chao CC, Wang YC, et al. Identification of environmental factors that promote intestinal inflammation. Nature 2022; 611: 801–809. 2022/10/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Peng SW, Sheng JM, Feng BS, et al. Identification of mite-specific eosinophils in the colon of patients with ulcerative colitis. Autoimmunity 2022; 55: 549–558. 2022/09/06. [DOI] [PubMed] [Google Scholar]
- 69.Geng B, Ding X, Li X, et al. Peripheral blood T-lymphocyte subsets are potential biomarkers of disease severity and clinical outcomes in patients with ulcerative colitis: a retrospective study. BMC Gastroenterol 2023; 23: 136. 2023/04/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Takahashi Y, Nagaya T, Iwaya Y, et al. CD8(+) lymphocyte infiltration is a specific feature of colitis induced by immune checkpoint inhibitors. Dig Dis Sci 2023; 68: 451–459. 2022/06/25. [DOI] [PubMed] [Google Scholar]
- 71.Royset ES, Sahlin Pettersen HP, Xu W, et al. Deep learning-based image analysis reveals significant differences in the number and distribution of mucosal CD3 and gammadelta T cells between Crohn's disease and ulcerative colitis. J Pathol Clin Res 2023; 9: 18–31. 2022/11/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang L, Shen WW, Shao W, et al. MANF ameliorates DSS-induced mouse colitis via restricting Ly6C(hi)CX3CR1(int) macrophage transformation and suppressing CHOP-BATF2 signaling pathway. Acta Pharmacol Sin 2023; 44: 1175–1190. 2023/01/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lu H, Zhang C, Wu W, et al. MCPIP1 restrains mucosal inflammation by orchestrating the intestinal monocyte to macrophage maturation via an ATF3-AP1S2 axis. Gut 2023; 72: 882–895. 2023/04/05. [DOI] [PubMed] [Google Scholar]
- 74.Zhang C, Zhang J, Zhang Y, et al. Identifying neutrophil-associated subtypes in ulcerative colitis and confirming neutrophils promote colitis-associated colorectal cancer. Front Immunol 2023; 14: 1095098. 2023/02/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ghimire J, Iftikhar R, Penrose HM, et al. FOXO3 deficiency in neutrophils drives colonic inflammation and tumorigenesis. Int J Mol Sci 2023; 24: 9730. 2023/06/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhou L, Yan Z, Yang W, et al. Socs3 expression in myeloid cells modulates the pathogenesis of dextran sulfate sodium (DSS)-induced colitis. Front Immunol 2023; 14: 1163987. 2023/06/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dong D, Su T, Chen W, et al. Clostridioides difficile aggravates dextran sulfate solution (DSS)-induced colitis by shaping the gut microbiota and promoting neutrophil recruitment. Gut Microbes 2023; 15: 2192478. 2023/03/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhou G, Zhu F, Zhang H, et al. PTK2B regulates immune responses of neutrophils and protects mucosal inflammation in ulcerative colitis. FASEB J 2023; 37: e22967. 2023/06/03. [DOI] [PubMed] [Google Scholar]
- 79.Olli KE, Rapp C, O'Connell L, et al. Muc5ac expression protects the colonic barrier in experimental colitis. Inflamm Bowel Dis 2020; 26: 1353–1367. 2020/05/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schroeder JH, Roberts LB, Meissl K, et al. Sustained post-developmental T-bet expression is critical for the maintenance of type one innate lymphoid cells in vivo. Front Immunol 2021; 12: 760198. 2021/11/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xie X, Zhao M, Huang S, et al. Luteolin alleviates ulcerative colitis by restoring the balance of NCR(-)ILC3/NCR(+)ILC3 to repairing impaired intestinal barrier. Int Immunopharmacol 2022; 112: 109251. 2022/10/02. [DOI] [PubMed] [Google Scholar]
- 82.Chang Y, Kim JW, Yang S, et al. Increased GM-CSF-producing NCR(-) ILC3s and neutrophils in the intestinal mucosa exacerbate inflammatory bowel disease. Clin Transl Immunology 2021; 10: e1311. 2021/07/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhou L, Zhou W, Joseph AM, et al. Group 3 innate lymphoid cells produce the growth factor HB-EGF to protect the intestine from TNF-mediated inflammation. Nat Immunol 2022; 23: 251–261. 2022/02/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li N, Xu S, Zhang S, et al. MSI2 deficiency in ILC3s attenuates DSS-induced colitis by affecting the intestinal microbiota. Front Immunol 2022; 13: 963379. 2023/01/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Uzzan M, Martin JC, Mesin L, et al. Ulcerative colitis is characterized by a plasmablast-skewed humoral response associated with disease activity. Nat Med 2022; 28: 766–779. 2022/02/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shan M, Carrillo J, Yeste A, et al. Secreted IgD amplifies humoral T helper 2 cell responses by binding basophils via galectin-9 and CD44. Immunity 2018; 49: 709–724 e708. 2018/10/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nagase Y, Kodama M, Abe K, et al. Enhanced oxidative phosphorylation of IgG plasma cells can contribute to hypoxia in the mucosa of active ulcerative colitis. Histochem Cell Biol 2022; 158: 335–344. 2022/06/19. [DOI] [PubMed] [Google Scholar]
- 88.Liu J, Xu Y, Chen YQ, et al. PTIP deficiency in B lymphocytes ameliorates dextran sulfate sodium-induced ulcerative colitis in mice. Discov Med 2023; 35: 343–352. 2023/06/05. [DOI] [PubMed] [Google Scholar]
- 89.Bos AV, van Gool MMJ, Breedveld AC, et al. Fcalpha receptor-1-activated monocytes promote B lymphocyte migration and IgA isotype switching. Int J Mol Sci 2022; 23: 11132. 2022/10/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Eshleman EM, Shao TY, Woo V, et al. Intestinal epithelial HDAC3 and MHC class II coordinate microbiota-specific immunity. J Clin Invest 2023; 133: e162190. 2023/01/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Duong HG, Choi EJ, Hsu P, et al. Identification of CD8 + T-cell-immune cell communications in ileal Crohn's disease. Clin Transl Gastroenterol 2023; 14: e00576. 2023/03/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Na SY, Moon W. Perspectives on current and novel treatments for inflammatory bowel disease. Gut Liver 2019; 13: 604–616. 2019/06/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.de Mattos BR, Garcia MP, Nogueira JB, et al. Inflammatory bowel disease: an overview of immune mechanisms and biological treatments. Mediators Inflamm 2015; 2015: 493012. 2015/09/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Keam SJ. Mirikizumab: first approval. Drugs 2023; 83: 1045–1052. 2023/06/30. [DOI] [PubMed] [Google Scholar]
- 95.Mitoma H, Horiuchi T, Tsukamoto H, et al. Molecular mechanisms of action of anti-TNF-alpha agents—comparison among therapeutic TNF-alpha antagonists. Cytokine 2018; 101: 56–63. 2016/08/29. [DOI] [PubMed] [Google Scholar]
- 96.Sommer K, Heidbreder K, Kreiss L, et al. Anti-beta7 integrin treatment impedes the recruitment on non-classical monocytes to the gut and delays macrophage-mediated intestinal wound healing. Clin Transl Med 2023; 13: e1233. 2023/04/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sandborn WJ, Mattheakis LC, Modi NB, et al. PTG-100, an oral alpha4beta7 antagonist peptide: preclinical development and phase 1 and 2a studies in ulcerative colitis. Gastroenterology 2021; 161: 1853–1864 e1810. 2021/09/03. [DOI] [PubMed] [Google Scholar]
- 98.Matsuoka K, Watanabe M, Ohmori T, et al. AJM300 (carotegrast methyl), an oral antagonist of alpha4-integrin, as induction therapy for patients with moderately active ulcerative colitis: a multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Gastroenterol Hepatol 2022; 7: 648–657. 2022/04/03. [DOI] [PubMed] [Google Scholar]
- 99.Dhillon S. Carotegrast methyl: first approval. Drugs 2022; 82: 1011–1016. 2022/06/21. [DOI] [PubMed] [Google Scholar]
- 100.Nielsen OH, Boye TL, Gubatan J, et al. Selective JAK1 inhibitors for the treatment of inflammatory bowel disease. Pharmacol Ther 2023; 245: 108402. 2023/04/03. [DOI] [PubMed] [Google Scholar]
- 101.Winthrop KL, Vermeire S, Long MD, et al. Long-term risk of herpes zoster infection in patients with ulcerative colitis receiving tofacitinib. Inflamm Bowel Dis 2023; 29: 85–96. 2022/06/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lichtenstein GR, Bressler B, Francisconi C, et al. Assessment of safety and efficacy of tofacitinib, stratified by age, in patients from the ulcerative colitis clinical program. Inflamm Bowel Dis 2023; 29: 27–41. 2022/11/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Seal R, Schwab LSU, Chiarolla CM, et al. Delayed and limited administration of the JAKinib tofacitinib mitigates chronic DSS-induced colitis. Front Immunol 2023; 14: 1179311. 2023/06/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang J, Macoritto M, Guay H, et al. The clinical response of upadacitinib and risankizumab is associated with reduced inflammatory bowel disease anti-TNF-alpha inadequate response mechanisms. Inflamm Bowel Dis 2023; 29: 771–782. 2022/12/15. [DOI] [PubMed] [Google Scholar]
- 105.Arrico L, Stolfi C, Marafini I, et al. Inhomogeneous diastereomeric composition of mongersen antisense phosphorothioate oligonucleotide preparations and related pharmacological activity impairment. Nucleic Acid Ther 2022; 32: 312–320. 2022/03/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Verstockt B, Vetrano S, Salas A, et al. Sphingosine 1-phosphate modulation and immune cell trafficking in inflammatory bowel disease. Nat Rev Gastroenterol Hepatol 2022; 19: 351–366. 2022/02/16. [DOI] [PubMed] [Google Scholar]
- 107.Moser LM, Fekadu J, Willasch A, et al. Treatment of inborn errors of immunity patients with inflammatory bowel disease phenotype by allogeneic stem cell transplantation. Br J Haematol 2023; 200: 595–607. 2022/10/11. [DOI] [PubMed] [Google Scholar]
- 108.Fujikawa H, Shimizu H, Nambu R, et al. Monogenic inflammatory bowel disease with STXBP2 mutations is not resolved by hematopoietic stem cell transplantation but can be alleviated via immunosuppressive drug therapy. Clin Immunol 2023; 246: 109203. 2022/12/13. [DOI] [PubMed] [Google Scholar]
- 109.Wang H, Sun Y, Xiao FJ, et al. Mesenchymal stem cells ameliorate DSS-induced experimental colitis by modulating the gut microbiota and MUC-1 pathway. J Inflamm Res 2023; 16: 2023–2039. 2023/05/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhu W, Chen Q, Li Y, et al. HIF-1alpha-overexpressing mesenchymal stem cells attenuate colitis by regulating M1-like macrophages polarization toward M2-like macrophages. Biomedicines 2023; 11: 825. 2023/03/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chen J, Huang J, Shi J, et al. Nestin+ Peyer's patch resident MSCs enhance healing of inflammatory bowel disease through IL-22-mediated intestinal epithelial repair. Cell Prolif 2023; 56: e13363. 2022/11/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Qi L, Wu J, Zhu S, et al. Mesenchymal stem cells alleviate inflammatory bowel disease via Tr1 cells. Stem Cell Rev Rep 2022; 18: 2444–2457. 2022/03/12. [DOI] [PubMed] [Google Scholar]
- 113.He R, Li P, Wang J, et al. The interplay of gut microbiota between donors and recipients determines the efficacy of fecal microbiota transplantation. Gut Microbes 2022; 14: 2100197. 2022/07/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chen Q, Fan Y, Zhang B, et al. Capsulized fecal microbiota transplantation induces remission in patients with ulcerative colitis by gut microbial colonization and metabolite regulation. Microbiol Spectr 2023; 11: e0415222. 2023/04/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chen Q, Fan Y, Zhang B, et al. Specific fungi associated with response to capsulized fecal microbiota transplantation in patients with active ulcerative colitis. Front Cell Infect Microbiol 2022; 12: 1086885. 2023/01/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Markandey M, Bajaj A, Verma M, et al. Fecal microbiota transplantation refurbishes the crypt-associated microbiota in ulcerative colitis. iScience 2023; 26: 106738. 2023/05/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Liu J, Lin H, Cao M, et al. Shifts and importance of viable bacteria in treatment of DSS-induced ulcerative colitis mice with FMT. Front Cell Infect Microbiol 2023; 13: 1124256. 2023/02/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lahtinen P, Jalanka J, Mattila E, et al. Fecal microbiota transplantation for the maintenance of remission in patients with ulcerative colitis: a randomized controlled trial. World J Gastroenterol 2023; 29: 2666–2678. 2023/05/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yang Y, He J, Wang Y, et al. Whole intestinal microbiota transplantation is more effective than fecal microbiota transplantation in reducing the susceptibility of DSS-induced germ-free mice colitis. Front Immunol 2023; 14: 1143526. 2023/05/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Statello L, Guo CJ, Chen LL, et al. Gene regulation by long non-coding RNAs and its biological functions. Nat Rev Mol Cell Biol 2021; 22: 96–118. 2020/12/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Feng X, Xiao Y, He J, et al. Long noncoding RNA Gm31629 protects against mucosal damage in experimental colitis via YB-1/E2F pathway. JCI Insight 2022; 7: e150091. 2022/02/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Vierbuchen T, Agarwal S, Johnson JL, et al. The lncRNA LUCAT1 is elevated in inflammatory disease and restrains inflammation by regulating the splicing and stability of NR4A2. Proc Natl Acad Sci USA 2023; 120: e2213715120. 2022/12/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Elamir A, Shaker O, Kamal M, et al. Expression profile of serum LncRNA THRIL and MiR-125b in inflammatory bowel disease. PLoS ONE 2022; 17: e0275267. 2022/10/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.He C, Chen Z, Huang J, et al. Interleukin-22 ameliorates dextran sulfate sodium-induced colitis through the upregulation of lncRNA-UCL to accelerate claudin-1 expression via sequestering miR-568 in mice. Oxid Med Cell Longev 2022; 2022: 8543720. 2022/09/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Xu Y, Zhang L, Ocansey DKW, et al. HucMSC-Ex alleviates inflammatory bowel disease via the lnc78583-mediated miR3202/HOXB13 pathway. J Zhejiang Univ Sci B 2022; 23: 423–431. 2022/05/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhao X, Cui DJ, Yang LC, et al. Long noncoding RNA FBXL19-AS1-mediated ulcerative colitis-associated intestinal epithelial barrier defect. Tissue Eng Regen Med 2022; 19: 1077–1088. 2022/09/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yin L, Yan J, Chen W. Mechanism of lncRNA-H19 in intestinal injury of mice with ulcerative colitis. Int Arch Allergy Immunol 2022; 183: 985–996. 2022/04/29. [DOI] [PubMed] [Google Scholar]
- 128.Lei N, Kong P, Chen S, et al. Upregulated NORAD is implicated in apoptosis, inflammation, and oxidative stress in ulcerative colitis through the nuclear factor-kappaappaB signaling. Eur J Gastroenterol Hepatol 2022; 34: 630–639. 2022/04/13. [DOI] [PubMed] [Google Scholar]
- 129.Han C, Sheng Y, Wang J, et al. LncRNA PSCK6-AS1-HIPK2 promotes Th1 differentiation via STAT1 phosphorylation to regulate colitis-related mucosal barrier damage. Int Immunopharmacol 2023; 117: 109992. 2023/04/05. [DOI] [PubMed] [Google Scholar]
- 130.Wang H, Teng J, Wang M, et al. Expression and significant roles of the lncRNA NEAT1/miR-493-5p/Rab27A axis in ulcerative colitis. Immun Inflamm Dis 2023; 11: e814. 2023/05/30. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 131.Ni S, Liu Y, Zhong J, et al. Inhibition of LncRNA-NEAT1 alleviates intestinal epithelial cells (IECs) dysfunction in ulcerative colitis by maintaining the homeostasis of the glucose metabolism through the miR-410-3p-LDHA axis. Bioengineered 2022; 13: 8961–8971. 2022/06/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sosnovski KE, Braun T, Amir A, et al. GATA6-AS1 regulates intestinal epithelial mitochondrial functions, and its reduced expression is linked to intestinal inflammation and less favourable disease course in ulcerative colitis. J Crohns Colitis 2023; 17: 960–971. 2023/01/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yu TX, Kalakonda S, Liu X, et al. Long noncoding RNA uc.230/CUG-binding protein 1 axis sustains intestinal epithelial homeostasis and response to tissue injury. JCI Insight 2022; 7: e156612. 2022/10/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tocia C, Dumitru A, Mateescu B, et al. Tissue and circulating MicroRNA-31, MicroRNA-200b, and MicroRNA-200c reflects disease activity in Crohn's disease patients: results from the BIOMIR study. J Gastrointestin Liver Dis 2023; 32: 30–38. 2023/04/03. [DOI] [PubMed] [Google Scholar]
- 135.Atanassova A, Georgieva A. Circulating miRNA-16 in inflammatory bowel disease and some clinical correlations—a cohort study in Bulgarian patients. Eur Rev Med Pharmacol Sci 2022; 26: 6310–6315. 2022/09/17. [DOI] [PubMed] [Google Scholar]
- 136.Chen Z, Gu Q, Chen R. Promotive role of IRF7 in ferroptosis of colonic epithelial cells in ulcerative colitis by the miR-375-3p/SLC11A2 axis. Biomol Biomed 2023; 23: 437–449. 2022/11/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Li W, Sheng Y, Wang J, et al. MicroRNA-9a-5p-NOX4 inhibits intestinal inflammatory injury by regulating the M1 polarization of intestinal macrophages. J Biochem Mol Toxicol 2023; 37: e23245. 2022/10/26. [DOI] [PubMed] [Google Scholar]
- 138.Yan R, Liang X, Hu J. miR-141-3p alleviates ulcerative colitis by targeting SUGT1 to inhibit colonic epithelial cell pyroptosis. Autoimmunity 2023; 56: 2220988. 2023/06/15. [DOI] [PubMed] [Google Scholar]
- 139.Wang XJ, Zhang D, Yang YT, et al. Suppression of microRNA-222-3p ameliorates ulcerative colitis and colitis-associated colorectal cancer to protect against oxidative stress via targeting BRG1 to activate Nrf2/HO-1 signaling pathway. Front Immunol 2023; 14: 1089809. 2023/02/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zheng J, Wang S, Zhang T, et al. Nogo-B inhibition restricts ulcerative colitis via inhibiting p68/miR-155 signaling pathway. Int Immunopharmacol 2023; 120: 110378. 2023/05/28. [DOI] [PubMed] [Google Scholar]
- 141.Xiang P, Ge T, Zhou J, et al. Protective role of circRNA CCND1 in ulcerative colitis via miR-142-5p/NCOA3 axis. BMC Gastroenterol 2023; 23: 18. 2023/01/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zhao J, Guo M, Yan Y, et al. The miR-7/EGFR axis controls the epithelial cell immunomodulation and regeneration and orchestrates the pathology in inflammatory bowel disease. J Adv Res 2024; 57: 119–134. 2023/04/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Apolit C, Campos N, Vautrin A, et al. ABX464 (obefazimod) upregulates miR-124 to reduce proinflammatory markers in inflammatory bowel diseases. Clin Transl Gastroenterol 2023; 14: e00560. 2022/12/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yuan W, Tian Y, Lin C, et al. Pectic polysaccharides derived from Hainan Rauwolfia ameliorate NLR family pyrin domain-containing 3-mediated colonic epithelial cell pyroptosis in ulcerative colitis. Physiol Genomics 2023; 55: 27–40. 2022/11/29. [DOI] [PubMed] [Google Scholar]
- 145.Xu H, Fu J, Chen L, et al. TNF-alpha enhances the therapeutic effects of MenSC-derived small extracellular vesicles on inflammatory bowel disease through macrophage polarization by miR-24-3p. Stem Cells Int 2023; 2023: 2988907. 2023/03/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Chang X, Song YH, Xia T, et al. Macrophage-derived exosomes promote intestinal mucosal barrier dysfunction in inflammatory bowel disease by regulating TMIGD1 via mircroRNA-223. Int Immunopharmacol 2023; 121: 110447. 2023/06/11. [DOI] [PubMed] [Google Scholar]
- 147.Qian W, Huang L, Xu Y, et al. Hypoxic ASCs-derived exosomes attenuate colitis by regulating macrophage polarization via miR-216a-5p/HMGB1 axis. Inflamm Bowel Dis 2023; 29: 602–619. 2022/10/27. [DOI] [PubMed] [Google Scholar]
- 148.Liang X, Li C, Song J, et al. HucMSC-Exo promote mucosal healing in experimental colitis by accelerating intestinal stem cells and epithelium regeneration via Wnt signaling pathway. Int J Nanomedicine 2023; 18: 2799–2818. 2023/05/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wei Z, Hang S, Wiredu Ocansey DK, et al. Human umbilical cord mesenchymal stem cells derived exosome shuttling mir-129-5p attenuates inflammatory bowel disease by inhibiting ferroptosis. J Nanobiotechnology 2023; 21: 188. 2023/06/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Luo S, Chen XH. Tissue and serum miR-149-3p/5p in hospitalized patients with inflammatory bowel disease: correlation with disease severity and inflammatory markers. Kaohsiung J Med Sci 2024; 40: 131–138. 2023/11/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Heydari R, Fayazzadeh S, Shahrokh S, et al. Plasma extracellular vesicle LncRNA H19 as a potential diagnostic biomarker for inflammatory bowel diseases. Inflamm Bowel Dis 2023; 30: 795–807. 2023/10/19. [DOI] [PubMed] [Google Scholar]
- 152.Zhang J, Guo Z, Wang Z, et al. Fecal miR-223 is a noninvasive biomarker for estimating Crohn's disease activity. Immun Inflamm Dis 2023; 11: e1131. 2024/01/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Acharya K, Bhardwaj V, Chuahan I, et al. Comparison of fecal calprotectin with different endoscopic scores in the assessment of ulcerative colitis (UC) activity and its utility in differentiating IBS from IBD. Euroasian J Hepatogastroenterol 2023; 13: 120–123. 2024/01/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lasson A, Stotzer PO, Ohman L, et al. The intra-individual variability of faecal calprotectin: a prospective study in patients with active ulcerative colitis. J Crohns Colitis 2015; 9: 26–32. 2014/07/11. [DOI] [PubMed] [Google Scholar]
- 155.Balzano F, Deiana M, Dei Giudici S, et al. miRNA stability in frozen plasma samples. Molecules 2015; 20: 19030–19040. 2015/10/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Anwar S, Mir F, Yokota T. Enhancing the effectiveness of oligonucleotide therapeutics using cell-penetrating peptide conjugation, chemical modification, and carrier-based delivery strategies. Pharmaceutics 2023; 15: 1130. 2023/04/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hong DS, Kang YK, Borad M, et al. Phase 1 study of MRX34, a liposomal miR-34a mimic, in patients with advanced solid tumours. Br J Cancer 2020; 122: 1630–1637. 2020/04/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Onali S, Pugliese D, Caprioli FA, et al. An objective comparison of vedolizumab and ustekinumab effectiveness in Crohn's disease patients’ failure to TNF-alpha inhibitors. Am J Gastroenterol 2022; 117: 1279–1287. 2022/04/26. [DOI] [PubMed] [Google Scholar]
- 159.Castano-Milla C, Chaparro M, Saro C, et al. Effectiveness of adalimumab in perianal fistulas in Crohn's disease patients naive to anti-TNF therapy. J Clin Gastroenterol 2015; 49: 34–40. 2014/12/09. [DOI] [PubMed] [Google Scholar]
- 160.Zhang C, Li Q, Shan J, et al. Multifunctional two-dimensional Bi(2)Se(3) nanodiscs for anti-inflammatory therapy of inflammatory bowel diseases. Acta Biomater 2023; 160: 252–264. 2023/02/23. [DOI] [PubMed] [Google Scholar]
- 161.Mishra RK, Ahmad A, Kanika XX, et al. Caffeic acid-conjugated budesonide-loaded nanomicelle attenuates inflammation in experimental colitis. Mol Pharm 2023; 20: 172–182. 2022/12/07. [DOI] [PubMed] [Google Scholar]
- 162.Mishra RK, Ahmad A, Kumar A, et al. Cortisone-loaded stearoyl ascorbic acid based nanostructured lipid carriers alleviate inflammatory changes in DSS-induced colitis. Biomater Adv 2023; 148: 213383. 2023/03/24. [DOI] [PubMed] [Google Scholar]
- 163.Qiu H, Gong H, Bao Y, et al. Reactive oxygen species-scavenging hollow MnO(2) nanozymes as carriers to deliver budesonide for synergistic inflammatory bowel disease therapy. Biomater Sci 2022; 10: 457–466. 2021/12/10. [DOI] [PubMed] [Google Scholar]
- 164.Dong K, Deng SJ, He BY, et al. Mucoadhesive nanoparticles enhance the therapeutic effect of dexamethasone on experimental ulcerative colitis by the local administration as an enema. Drug Des Devel Ther 2023; 17: 191–207. 2023/02/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Li M, Liu J, Shi L, et al. Gold nanoparticles-embedded ceria with enhanced antioxidant activities for treating inflammatory bowel disease. Bioact Mater 2023; 25: 95–106. 2023/02/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Bao M, Wang K, Li J, et al. ROS scavenging and inflammation-directed polydopamine nanoparticles regulate gut immunity and flora therapy in inflammatory bowel disease. Acta Biomater 2023; 161: 250–264. 2023/03/03. [DOI] [PubMed] [Google Scholar]
- 167.Yin M, Chen Y, Liu X, et al. Targeted computed tomography visualization and healing of inflammatory bowel disease by orally delivered bacterial-flagella-inspired polydiiododiacetylene nanofibers. ACS Nano 2023; 17: 3873–3888. 2023/02/16. [DOI] [PubMed] [Google Scholar]
- 168.He H, Qin Q, Xu F, et al. Oral polyphenol-armored nanomedicine for targeted modulation of gut microbiota-brain interactions in colitis. Sci Adv 2023; 9: eadf3887. 2023/05/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kim HS, Lee S, Lee DY. Aurozyme: a revolutionary nanozyme in colitis, switching peroxidase-like to catalase-like activity. Small 2023; 19: e2302331. 2023/05/29. doi: 10.1002/smll.202302331. [DOI] [PubMed] [Google Scholar]
- 170.Yan X, Meng L, Zhang X, et al. Reactive oxygen species-responsive nanocarrier ameliorates murine colitis by intervening colonic innate and adaptive immune responses. Mol Ther 2023; 31: 1383–1401. 2023/03/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wang L, Wang Z, Pan Y, et al. Polycatechol-derived mesoporous polydopamine nanoparticles for combined ROS scavenging and gene interference therapy in inflammatory bowel disease. ACS Appl Mater Interfaces 2022; 14: 19975–19987. 2022/04/21. [DOI] [PubMed] [Google Scholar]
- 172.Rahman AT, Shin J, Whang CH, et al. Bilirubin nanomedicine rescues intestinal barrier destruction and restores mucosal immunity in colitis. ACS Nano 2023; 17: 10996–11013. 2023/05/25. [DOI] [PubMed] [Google Scholar]
- 173.Zhu YX, You Y, Chen Z, et al. Inorganic nanosheet-shielded probiotics: a self-adaptable oral delivery system for intestinal disease treatment. Nano Lett 2023; 23: 4683–4692. 2023/03/14. [DOI] [PubMed] [Google Scholar]
- 174.Lv Y, Ren M, Yao M, et al. Colon-specific delivery of methotrexate using hyaluronic acid modified pH-responsive nanocarrier for the therapy of colitis in mice. Int J Pharm 2023; 635: 122741. 2023/02/23. [DOI] [PubMed] [Google Scholar]
- 175.Maleki H, Doostan M, Farzaei MH, et al. Achillea wilhelmsii-incorporated Chitosan@Eudragit nanoparticles intended for enhanced ulcerative colitis treatment. AAPS PharmSciTech 2023; 24: 112. 2023/04/29. [DOI] [PubMed] [Google Scholar]
- 176.Varghese S, Chaudhary JP, Thareja P, et al. Newly developed nano-biocomposite embedded hydrogel to enhance drug loading and modulated release of anti-inflammatory drug. Pharm Dev Technol 2023; 28: 299–308. 2023/03/21. [DOI] [PubMed] [Google Scholar]
- 177.Zhao C, Yang J, Chen M, et al. Synthetic lignin-derived therapeutic nano reagent as intestinal pH-sensitive drug carriers capable of bypassing the gastric acid environment for colitis treatment. ACS Nano 2023; 17: 811–824. 2022/12/16. [DOI] [PubMed] [Google Scholar]
- 178.Yao H, Wang F, Chong H, et al. A curcumin-modified coordination polymers with ROS scavenging and macrophage phenotype regulating properties for efficient ulcerative colitis treatment. Adv Sci (Weinh) 2023; 10: e2300601. 2023/05/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Guan H, Xu Z, Du G, et al. A mesoporous polydopamine-derived nanomedicine for targeted and synergistic treatment of inflammatory bowel disease by pH-responsive drug release and ROS scavenging. Mater Today Bio 2023; 19: 100610. 2023/04/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Zhang G, Han W, Zhao P, et al. Programmed pH-responsive core-shell nanoparticles for precisely targeted therapy of ulcerative colitis. Nanoscale 2023; 15: 1937–1946. 2023/01/11. [DOI] [PubMed] [Google Scholar]
- 181.Tsujimura N, Ogino T, Hiraki M, et al. Super carbonate apatite-miR-497a-5p complex is a promising therapeutic option against inflammatory bowel disease. Pharmaceuticals (Basel) 2023; 16: 618. 2023/04/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Yang F, Chen Y, Xiao Y, et al. pH-sensitive molybdenum (Mo)-based polyoxometalate nanoclusters have therapeutic efficacy in inflammatory bowel disease by counteracting ferroptosis. Pharmacol Res 2023; 188: 106645. 2023/01/08. [DOI] [PubMed] [Google Scholar]
- 183.Shi C, Dawulieti J, Shi F, et al. A nanoparticulate dual scavenger for targeted therapy of inflammatory bowel disease. Sci Adv 2022; 8: eabj2372. 2022/01/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Zou YF, Li CY, Fu YP, et al. Angelica sinensis aboveground part polysaccharide and its metabolite 5-MT ameliorate colitis via modulating gut microbiota and TLR4/MyD88/NF-kappaB pathway. Int J Biol Macromol 2023; 242: 124689. 2023/05/07. [DOI] [PubMed] [Google Scholar]
- 185.Zhang Y, Yu S, Liu J, et al. A polysaccharide from Allium tenuissimum L. flowers relieves ulcerative colitis by regulating the inflammatory signaling pathway and gut microbiota. Food Funct 2023; 14: 6582–6595. 2023/07/03. [DOI] [PubMed] [Google Scholar]
- 186.Ren Y, Sun Q, Gao R, et al. Low weight polysaccharide of Hericium erinaceus ameliorates colitis via inhibiting the NLRP3 inflammasome activation in association with gut microbiota modulation. Nutrients 2023; 15: 739. 2023/02/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Raj V, Venkataraman B, Ojha SK, et al. Cis-nerolidol inhibits MAP kinase and NF-kappaB signaling pathways and prevents epithelial tight junction dysfunction in colon inflammation: in vivo and in vitro studies. Molecules 2023; 28: 2982. 2023/04/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Li X, Mo K, Tian G, et al. Shikimic acid regulates the NF-kappaB/MAPK signaling pathway and gut microbiota to ameliorate DSS-induced ulcerative colitis. J Agric Food Chem 2023; 71: 8906–8914. 2023/05/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Lifei L, Zhang J, Li X, et al. Sericic acid ameliorates DSS-induced ulcerative colitis in mice by modulating the NF-kappaB and Nrf2 pathways. Curr Mol Pharmacol 2023; 16: 759–770. 2022/09/30. [DOI] [PubMed] [Google Scholar]
- 190.Prakash AN, Prasad N, Puppala ER, et al. Loganic acid protects against ulcerative colitis by inhibiting TLR4/NF-kappaB mediated inflammation and activating the SIRT1/Nrf2 anti-oxidant responses in-vitro and in-vivo. Int Immunopharmacol 2023; 122: 110585. 2023/07/09. [DOI] [PubMed] [Google Scholar]
- 191.Yu TY, Feng YM, Kong WS, et al. Gallic acid ameliorates dextran sulfate sodium-induced ulcerative colitis in mice via inhibiting NLRP3 inflammasome. Front Pharmacol 2023; 14: 1095721. 2023/02/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Zhou Z, He W, Tian H, et al. Thyme (Thymus vulgaris L.) polyphenols ameliorate DSS-induced ulcerative colitis of mice by mitigating intestinal barrier damage, regulating gut microbiota, and suppressing TLR4/NF-kappaB-NLRP3 inflammasome pathways. Food Funct 2023; 14: 1113–1132. 2023/01/04. [DOI] [PubMed] [Google Scholar]
- 193.Mahdy RN E, Nader MA, Helal MG, et al. Eicosapentaenoic acid mitigates ulcerative colitis-induced by acetic acid through modulation of NF-kappaB and TGF-beta/ EGFR signaling pathways. Life Sci 2023; 327: 121820. 2023/06/02. [DOI] [PubMed] [Google Scholar]
- 194.Kulhari U, Kundu S, Mugale MN, et al. Nuciferine alleviates intestinal inflammation by inhibiting MAPK/NF-kappaB and NLRP3/caspase 1 pathways in vivo and in vitro. Int Immunopharmacol 2023; 115: 109613. 2022/12/29. [DOI] [PubMed] [Google Scholar]
- 195.Shao XX, Xu Y, Xiao HY, et al. Higenamine improves DSS-induced ulcerative colitis in mice through the galectin-3/TLR4/NF-kappaB pathway. Tissue Cell 2023; 82: 102111. 2023/05/21. [DOI] [PubMed] [Google Scholar]
- 196.Liu M, Liu F, Pan Y, et al. Oxymatrine ameliorated experimental colitis via mechanisms involving inflammatory DCs, gut microbiota and TLR/NF-kappaB pathway. Int Immunopharmacol 2023; 115: 109612. 2022/12/31. [DOI] [PubMed] [Google Scholar]
- 197.Zhou Y, Chen S, Dai Y, et al. Sinomenine attenuated dextran sulfate sodium-induced inflammatory responses by promoting 14-3-3theta protein and inhibiting NF-kappaB signaling. J Ethnopharmacol 2023; 303: 116037. 2022/12/17. [DOI] [PubMed] [Google Scholar]
- 198.Tan X, Wen Y, Han Z, et al. Cinnamaldehyde ameliorates dextran sulfate sodium-induced colitis in mice by modulating TLR4/NF-kappaB signaling pathway and NLRP3 inflammasome activation. Chem Biodivers 2023; 20: e202200089. 2023/01/19. [DOI] [PubMed] [Google Scholar]
- 199.Xue HH, Li JJ, Li SF, et al. Phillygenin attenuated colon inflammation and improved intestinal mucosal barrier in DSS-induced colitis mice via TLR4/Src mediated MAPK and NF-kappaB signaling pathways. Int J Mol Sci 2023; 24: 2238. 2023/02/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Yao L, Chen X, Shen M, et al. Isosteviol attenuates DSS-induced colitis by maintaining intestinal barrier function through PDK1/AKT/NF-kappaB signaling pathway. Int Immunopharmacol 2023; 114: 109532. 2022/12/13. [DOI] [PubMed] [Google Scholar]
- 201.Mu J, Song J, Li R, et al. Isovitexin prevents DSS-induced colitis through inhibiting inflammation and preserving intestinal barrier integrity through activating AhR. Chem Biol Interact 2023; 382: 110583. 2023/06/02. [DOI] [PubMed] [Google Scholar]
- 202.Wang X, Su L, Tan J, et al. Albiflorin alleviates DSS-induced ulcerative colitis in mice by reducing inflammation and oxidative stress. Iran J Basic Med Sci 2023; 26: 48–56. 2023/01/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Chung KS, Park SE, Lee JH, et al. Protective effect of 7-hydroxyl-1-methylindole-3-acetonitrile on the intestinal mucosal damage response to inflammation in mice with DSS-induced colitis. Chem Biol Interact 2023; 370: 110316. 2022/12/22. [DOI] [PubMed] [Google Scholar]
- 204.Liu M, Wang Y, Xiang H, et al. The tryptophan metabolite indole-3-carboxaldehyde alleviates mice with DSS-induced ulcerative colitis by balancing amino acid metabolism, inhibiting intestinal inflammation, and improving intestinal barrier function. Molecules 2023; 28: 3704. 2023/05/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Wang M, Li Y, Su J, et al. Protective effects of 4-geranyloxy-2,6-dihydroxybenzophenonel on DSS-induced ulcerative colitis in mice via regulation of cAMP/PKA/CREB and NF-kappaB signaling pathways. Phytother Res 2023; 37: 1330–1345. 2022/11/27. [DOI] [PubMed] [Google Scholar]
- 206.Jiang M, Tang C, Yang M, et al. Paeoniflorin-6'-O-benzene sulfonate protected the intestinal epithelial barrier by restoring the inhibitory effect of GRK2 and beta-arrestin 2 on ERK1/2-NF-kappaB. Phytother Res 2023; 37: 743–758. 2022/10/13. [DOI] [PubMed] [Google Scholar]
- 207.Chen J, Lu P, Liu J, et al. 20(S)-protopanaxadiol saponins isolated from Panax notoginseng target the binding of HMGB1 to TLR4 against inflammation in experimental ulcerative colitis. Phytother Res 2023; 37: 4690–4705. 2023/07/10. [DOI] [PubMed] [Google Scholar]
- 208.Wu K, Liu X, Meng X, et al. Sauchinone alleviates dextran sulfate sodium-induced ulcerative colitis via NAD(P)H dehydrogenase [quinone] 1/NF-kB pathway and gut microbiota. Front Microbiol 2022; 13: 1084257. 2023/01/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Cui H, Li X, An XR, et al. Masticadienonic acid from Chios mastic gum mitigates colitis in mice via modulating inflammatory response, gut barrier integrity and microbiota. Phytomedicine 2023; 108: 154518. 2022/11/21. [DOI] [PubMed] [Google Scholar]
- 210.Kong J, Xiang Q, Shi G, et al. Licorice protects against ulcerative colitis via the Nrf2/PINK1-mediated mitochondrial autophagy. Immun Inflamm Dis 2023; 11: e757. 2023/01/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Zhang H, Lang W, Li S, et al. Corynoline ameliorates dextran sulfate sodium-induced colitis in mice by modulating Nrf2/NF-kappaB pathway. Immunopharmacol Immunotoxicol 2023; 45: 26–34. 2022/08/19. [DOI] [PubMed] [Google Scholar]
- 212.Venkataraman B, Almarzooqi S, Raj V, et al. Molecular docking identifies 1,8-cineole (eucalyptol) as a novel PPARgamma agonist that alleviates colon inflammation. Int J Mol Sci 2023; 24: 6160. 2023/04/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Albalawi GA, Albalawi MZ, Alsubaie KT, et al. Curative effects of Crocin in ulcerative colitis via modulating apoptosis and inflammation. Int Immunopharmacol 2023; 118: 110138. 2023/04/09. [DOI] [PubMed] [Google Scholar]
- 214.Ren DD, Chen KC, Li SS, et al. Panax quinquefolius polysaccharides ameliorate ulcerative colitis in mice induced by dextran sulfate sodium. Front Immunol 2023; 14: 1161625. 2023/07/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Jiang P, Zhang Y, Li X, et al. Geniposidic acid attenuates DSS-induced colitis through inhibiting inflammation and regulating gut microbiota. Phytother Res 2023; 37: 3453–3466. 2023/04/26. [DOI] [PubMed] [Google Scholar]
- 216.Wei X, Li N, Wu X, et al. The preventive effect of Glycyrrhiza polysaccharide on lipopolysaccharide-induced acute colitis in mice by modulating gut microbial communities. Int J Biol Macromol 2023; 239: 124199. 2023/03/28. [DOI] [PubMed] [Google Scholar]
- 217.Lv H, Jia H, Cai W, et al. Rehmannia glutinosa polysaccharides attenuates colitis via reshaping gut microbiota and short-chain fatty acid production. J Sci Food Agric 2023; 103: 3926–3938. 2022/11/09. [DOI] [PubMed] [Google Scholar]
- 218.Xiang N, Zhao J, Chang S, et al. In vitro fecal fermentation of euphorbia humifusa-derived polysaccharides and their protective effect against ulcerative colitis in mice. Foods 2023; 12: 751. 2023/02/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Wang YJ, Wang HY, Li QM, et al. Dendrobium fimbriatum polysaccharide ameliorates DSS-induced intestinal mucosal injury by IL-22-regulated intestinal stem cell regeneration. Int J Biol Macromol 2023; 230: 123199. 2023/01/13. [DOI] [PubMed] [Google Scholar]
- 220.Chen S, Wang J, Dong N, et al. Polysaccharides from natural Cordyceps sinensis attenuated dextran sodium sulfate-induced colitis in C57BL/6J mice. Food Funct 2023; 14: 720–733. 2023/01/05. [DOI] [PubMed] [Google Scholar]
- 221.Xu J, Tang C, Din AU, et al. Oligosaccharides of Polygonatum cyrtonema Hua ameliorates dextran sulfate sodium-induced colitis and regulates the gut microbiota. Biomed Pharmacother 2023; 161: 114562. 2023/03/20. [DOI] [PubMed] [Google Scholar]
- 222.Wu A, Gao Y, Kan R, et al. Alginate oligosaccharides prevent dextran-sulfate-sodium-induced ulcerative colitis via enhancing intestinal barrier function and modulating gut microbiota. Foods 2023; 12: 220. 2023/01/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Li X, Gui R, Wang X, et al. Oligosaccharides isolated from Rehmannia glutinosa protect LPS-induced intestinal inflammation and barrier injury in mice. Front Nutr 2023; 10: 1139006. 2023/03/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Cheng T, Xu C, Wu D, et al. Sodium houttuyfonate derived from Houttuynia cordata Thunb improves intestinal malfunction via maintaining gut microflora stability in Candida albicans overgrowth aggravated ulcerative colitis. Food Funct 2023; 14: 1072–1086. 2023/01/04. [DOI] [PubMed] [Google Scholar]
- 225.Liu T, Ning Z, Liu P, et al. Cassane diterpenoid ameliorates dextran sulfate sodium-induced experimental colitis by regulating gut microbiota and suppressing tryptophan metabolism. Front Immunol 2022; 13: 1045901. 2023/02/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Ma Y, Lang X, Yang Q, et al. Paeoniflorin promotes intestinal stem cell-mediated epithelial regeneration and repair via PI3K-AKT-mTOR signalling in ulcerative colitis. Int Immunopharmacol 2023; 119: 110247. 2023/05/09. [DOI] [PubMed] [Google Scholar]
- 227.Huang J, Xu P, Shao M, et al. Humic acids alleviate dextran sulfate sodium-induced colitis by positively modulating gut microbiota. Front Microbiol 2023; 14: 1147110. 2023/05/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Nie H, Li Y, Lu XL, et al. Prodigiosin derived from chromium-resistant Serratia sp. prevents inflammation and modulates gut microbiota homeostasis in DSS-induced colitis mice. Int Immunopharmacol 2023; 116: 109800. 2023/02/14. [DOI] [PubMed] [Google Scholar]
- 229.Lee YM, Shin DW, Lim BO. Chlorogenic acid improves symptoms of inflammatory bowel disease in interleukin-10 knockout mice. J Med Food 2020; 23: 1043–1053. 2020/10/16. [DOI] [PubMed] [Google Scholar]
- 230.Tang S, Zhong W, Li T, et al. Isochlorogenic acid A alleviates dextran sulfate sodium-induced ulcerative colitis in mice through STAT3/NF-small ka, CyrillicB pathway. Int Immunopharmacol 2023; 118: 109989. 2023/03/24. [DOI] [PubMed] [Google Scholar]
- 231.Zha ZX, Lin Y, Wang KX, et al. Hederacoside C ameliorates colitis via restoring impaired intestinal barrier through moderating S100A9/MAPK and neutrophil recruitment inactivation. Acta Pharmacol Sin 2023; 44: 105–119. 2022/06/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Wang Y, Song X, Wang Z, et al. Effects of pine pollen polysaccharides and sulfated polysaccharides on ulcerative colitis and gut flora in mice. Polymers (Basel) 2023; 15: 1414. 2023/03/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Liu X, Zhou M, Dai Z, et al. Salidroside alleviates ulcerative colitis via inhibiting macrophage pyroptosis and repairing the dysbacteriosis-associated Th17/Treg imbalance. Phytother Res 2023; 37: 367–382. 2022/11/05. [DOI] [PubMed] [Google Scholar]
- 234.Guo X, Li X, Dong Y, et al. Cod (Gadus) skin collagen peptide powder reduces inflammation, restores mucosal barrier function, and inhibits fibrosis in dextran sodium sulfate-induced colitis in mice. J Ethnopharmacol 2023; 316: 116728. 2023/06/06. [DOI] [PubMed] [Google Scholar]
- 235.Wan Y, Yang L, Jiang S, et al. Excessive apoptosis in ulcerative colitis: crosstalk between apoptosis, ROS, ER stress, and intestinal homeostasis. Inflamm Bowel Dis 2022; 28: 639–648. 2021/12/07. [DOI] [PubMed] [Google Scholar]
- 236.He Z, Liu J, Liu Y. Daphnetin attenuates intestinal inflammation, oxidative stress, and apoptosis in ulcerative colitis via inhibiting REG3A-dependent JAK2/STAT3 signaling pathway. Environ Toxicol 2023; 38: 2132–2142. 2023/05/20. [DOI] [PubMed] [Google Scholar]
- 237.Alharbi TS, Alshammari ZS, Alanzi ZN, et al. Therapeutic effects of genistein in experimentally induced ulcerative colitis in rats via affecting mitochondrial biogenesis. Mol Cell Biochem 2024; 479: 431–444. 2023/04/21. [DOI] [PubMed] [Google Scholar]
- 238.Elhefnawy EA, Zaki HF, El Maraghy NN, et al. Genistein and/or sulfasalazine ameliorate acetic acid-induced ulcerative colitis in rats via modulating INF-gamma/JAK1/STAT1/IRF-1, TLR-4/NF-kappaB/IL-6, and JAK2/STAT3/COX-2 crosstalk. Biochem Pharmacol 2023; 214: 115673. 2023/07/07. [DOI] [PubMed] [Google Scholar]
- 239.Zhou M, Zhi J, Zhi J, et al. Polysaccharide from Strongylocentrotus nudus eggs regulates intestinal epithelial autophagy through CD36/PI3K-Akt pathway to ameliorate inflammatory bowel disease. Int J Biol Macromol 2023; 244: 125373. 2023/06/17. [DOI] [PubMed] [Google Scholar]
- 240.Chen J, Pan M, Wang J, et al. Hydroxysafflor yellow A protects against colitis in mice by suppressing pyroptosis via inhibiting HK1/NLRP3/GSDMD and modulating gut microbiota. Toxicol Appl Pharmacol 2023; 467: 116494. 2023/04/01. [DOI] [PubMed] [Google Scholar]
- 241.Liu D, Tian Q, Liu K, et al. Ginsenoside Rg3 ameliorates DSS-induced colitis by inhibiting NLRP3 inflammasome activation and regulating microbial homeostasis. J Agric Food Chem 2023; 71: 3472–3483. 2023/02/09. [DOI] [PubMed] [Google Scholar]
- 242.Arenbaoligao GX, Xiong J, et al. Kumatakenin inhibited iron-ferroptosis in epithelial cells from colitis mice by regulating the Eno3-IRP1-axis. Front Pharmacol 2023; 14: 1127931. 2023/04/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Yang X, Sun X, Zhou F, et al. Protocatechuic acid alleviates dextran-sulfate-sodium-induced ulcerative colitis in mice via the regulation of intestinal flora and ferroptosis. Molecules 2023; 28: 3775. 2023/05/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Massironi S, Vigano C, Palermo A, et al. Inflammation and malnutrition in inflammatory bowel disease. Lancet Gastroenterol Hepatol 2023; 8: 579–590. 2023/03/19. [DOI] [PubMed] [Google Scholar]
- 245.Massironi S, Sileri P, Danese S. Get Fit: muscle health for crohn's disease surgical outcome optimization. Inflamm Bowel Dis 2023; 20: izad235. 2023/10/20. [DOI] [PubMed] [Google Scholar]
- 246.Amiot A, Chaibi S, Bouhnik Y, et al. Prevalence and determinants of fatigue in patients with IBD: a cross-sectional survey from the GETAID. J Crohns Colitis 2023; 17: 1418–1425. 2023/03/30. [DOI] [PubMed] [Google Scholar]
- 247.Sudhakar P, Wellens J, Verstockt B, et al. Holistic healthcare in inflammatory bowel disease: time for patient-centric approaches? Gut 2023; 72: 192–204. 2022/09/29. [DOI] [PubMed] [Google Scholar]
- 248.Fiorino G, Lytras T, Younge L, et al. Quality of care standards in inflammatory bowel diseases: a European Crohn's and colitis organisation [ECCO] position paper. J Crohns Colitis 2020; 14: 1037–1048. 2020/02/08. [DOI] [PubMed] [Google Scholar]