Abstract
This is the first report showing that an epitope-specific ex vivo modulation of an allogeneic hematopoietic stem cell graft by the anti-human CD4 antibody MAX.16H5 IgG1 simultaneously facilitates the anti-tumor capacity of the graft (Graft-versus-leukemia effect, GvL) and the long-term suppression of the deleterious side effect Graft-versus-host-disease (GvHD). To distinguish and consolidate GvL from GvHD, the anti-human CD4 antibody MAX16.H5 IgG1 was tested in murine GvHD and tumor models. The survival rate was significantly increased in recipients receiving a MAX.16H5 IgG1 short-term (2 h) pre-incubated graft even when tumor cells were co-transplanted or when recipient mice were treated by MAX.16H5 IgG1 before transplantation. After engraftment, regulatory T-cells are generated only supporting the GvL effect. It was also possible to transfer the immune tolerance from GvHD-free recipient chimeras into third party recipient mice without the need of reapplication of MAX.16H5 IgG1 anti-human CD4 antibodies. These findings are also benefical for patients with leukemia when no matched related or unrelated donor is available and provides a safer allogeneic HSCT, which is more effective against leukemia. It also facilitates allogeneic (stem) cell transplantations for other indications (e.g., autoimmune-disorders).
Electronic supplementary material
The online version of this article (doi:10.1007/s00018-013-1476-0) contains supplementary material, which is available to authorized users.
Keywords: GvHD, GvL, Allogeneic hematopoetic stem cell transplantation, Triple transgenic mice, Anti-CD4 antibodies
Introduction
Graft-versus-host-disease (GvHD) is a life-threatening disease after allogeneic HSCT [1]. GvHD is initiated by donor immune T-cells recognizing and attacking host antigens of the recipient, which results in the induction of cytokines leading to the release of pro-inflammatory factors. Irradiation and chemotherapy lead to organ damage with the release of pro-inflammatory cytokines (TNF-α) and the activation of APCs. After donor T-cell activation and release of Il-12 by APCs, TH1-cells activate CD8+ T-cells by the release of INF-γ and Il-2 leading to apoptosis of target host cells [2, 3]. Therapy options include high-dose prednisone [4] and immunosuppressive strategies against key elements of T-cell activation [5]. However, these strategies are associated with less long-term success and increased toxicity, infectious complications, and relapses of the underlying hematological malignancy, as well as a general suppression of all T-cell activity. Only 50 % of GvHD-affected individuals respond to the current therapy. The balance between GvHD and the beneficial GvL-effect (anti-cancer capacity of donor immune cells) are crucial. Strategies for GvHD-prophylaxis and therapy could not distinguish between different T-cell clones and therefore not between GvHD and the GvL-effect. GvHD and GvL are maintained by different T-cell clones [6]. Therefore, GvHD is initiated by CD4+ T-cell clones, which activate CD8+ T-cells. These CD8+ T-cells are using the Fas-FasL pathway instead of the perforin-granzyme B pathway, which is used by CD8+ T-cells mainly responsible for the GvL effect. In contrast to what has been observed in some murine GvHD models, GvHD and GvL are not always mediated by different T-cell clones in humans. GvHD is usually associated with the GvL effect [7].
Modulation of CD4+ T-cells by anti-human CD4 antibodies should lead to a time-dependent, targeted modulation of the immune system. CD4+ molecules bind directly to constant regions of HLA molecules on APCs to allow a complete T-cell activation. Non-depleting monoclonal antibodies inhibit this activation by a total steric blockage, by shortening cell–cell contact between APCs and T-cells [8], by the induction of negative signals through inhibition of protein tyrosine phosphorylation [9], or by the induction of T-cell anergy [10, 11].
Previously, the murine anti-human CD4 monoclonal antibody MAX.16H5 IgG1 was used in patients with auto-immune diseases or as a protective therapy against transplant rejection [12–14]. In human kidney transplantation, MAX.16H5 IgG1 effectively reduce graft rejection [15–17]. The use of MAX.16H5 IgG1 not only resulted in the suppression of immune activity but also in the induction of tolerance against tetanus toxoid in a triple transgenic mouse model [18–20]. CD4/DR3 mice expressing human CD4 and HLA-DR17, a split antigen of HLA-DR3, on a cd4-deficient background, were initially bred at the Institute for Laboratory Animal Science, Medical School, Hanover, Germany [21]. Using these mice, anti-human CD4 antibodies can be used directly and host and donor hematopoiesis could be distinguished by means of human and murine cd4 molecules [22–24]. In this paper, we will show the prevention of GvHD with preserved GvL effect by ex vivo and in vivo modulation of CD4+ T-cells by MAX.16H5 IgG1 anti-CD4 antibodies.
Materials and methods
Animals
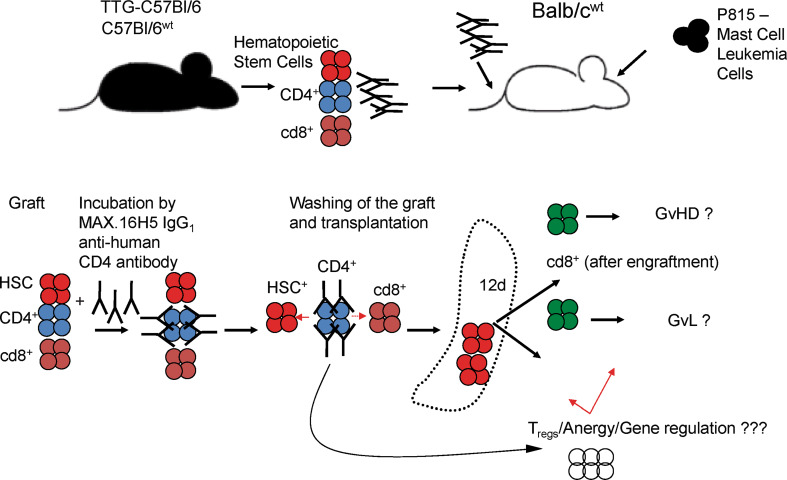
Donor triple transgenic mice (human CD4+/+, murine cd4−/−, HLA-DR3+/+) TTG-C57Bl/6 were bred at the Universität Leipzig and donor C57Bl/6wt mice and recipient Balb/cwt mice were purchased from Charles River (Sulzfeld, Germany) [22, 23, 25]. All mice were housed, treated, or handled in accordance with the guidelines of the Universität Leipzig Animal Care Committee and the Regional Board of Animal Care for Leipzig (animal experiment registration number 28/08 and 55/11). Several recipient mice were treated with MAX.16H5 IgG1 (15 μg/g body weight) at day 0. Survival, engraftment, and hematological (full blood cell count) and immunological reconstitution (murine cd4, human CD4, murine cd8, HLA-DR3, and H2Kb) were measured weekly (Fig. 1).
Fig. 1.
The principle of allogeneic hematopoietic stem cell transplantation after short-term pre-incubation of the graft by anti-human CD4 antibodies (MAX.16H5 IgG1) or treatment of recipient Balb/cwt mice at day of transplantation (day 0) is shown. Allogeneic hematopoietic stem cell grafts were incubated with MAX.16H5 IgG1 2 h before transplantation into Balb/cwt mice or recipient mice were treated with 15 μg/g body weight at day 0. Engraftment and hematological (WBC subsets) and immunological reconstitution (murine cd4, human CD4, murine cd8, HLA-DR3, and H2Kb) were measured weekly (four independent transplantation experiments, one experiment for direct antibody application, at least four animals per group in the transplantation experiments and three animals per time point in the antibody application experiments)
Statistical analysis
All data are presented as mean ± standard deviation. Statistical analysis and graphic presentations were made using SigmaPlot 10.0/SigmaStat 3.5 software (SYSTAT, Erkrath, Germany). p values were calculated using t tests. Kaplan–Meier survival curves were analyzed using the Logrank test and pair-wise multiple comparisons of means (Holm–Sidak method).
Irradiation protocol of Balb/c recipients and antibody injection in TTG-C57Bl/6 and Balb/cwt mice
For the irradiation of mice, the X-Ray apparatus (D3225, Orthovoltage; Gulmay Medical, Camberley, UK) was adjusted for animal irradiation to reach an irradiation dose of 8 Gy [23]. Antibody concentration of 15 and 1.5 μg antibody/g for intra-venous injection of MAX.16H5 IgG1 in TTG-C57Bl/6 or Balb/cwt mice was calculated according to the observations of Laub et al. [19].
Preparation of BM cells, splenocytes, mast cell leukemia cells, and antibody incubation
As isotype control, the antibody from LEAF™ (Low Endotoxin, Azide-Free)™ Purified Mouse IgG1(κ isotype control antibody; BioLegend, San Diego, CA, USA) was used. MAX.16H5 IgG1 antibodies were used as described by Emmrich et al. [13]. MAX.16H5 IgG1 anti-human CD4 antibodies recognize an epitope, which has been described by three-dimensional mapping of overlapping peptides and which is located in the first CD4 domain (F. Emmrich, unpublished data). Anti-human CD4 antibody clone RPA-T4 IgG1 was purchased from AbSerotec (Cat: MCA127EL). RPA-T4 IgG1 recognise an epitope within domain 1 of the extracellular region of the CD4 molecule (©2013, BioRad Laboratories, according to the data sheet). BM cells and splenocytes were prepared by methods already published in the literature [22, 24, 25]. Mast cell leukemia cells (P815-cells) were handled according to a protocol from Demehri et al. [26]. Labeling of P815 cells was done with Amaxa® Cell Line Nucleofector® Kit T as described by the manufacturer (Lonza Cologne, Cologne, Germany). For antibody incubation, the calculated amount of antibodies was diluted before use to a final concentration of 1 mg/ml in Dulbecco’s Modified Eagle Medium without FCS. BM (1.4 × 108) and splenocytes (1.4 × 108) from donors were incubated with 800 μg MAX.16H5 IgG1 or 800 μg RPA-T4 IgG1 in 15 ml Dulbecco’s Modified Eagle Medium without FCS for 2 h at room temperature in the dark. As control, BM and splenocytes from the donors without antibody pre-incubation were prepared under the same conditions. After 2 h of incubation, cells were centrifuged at 300 × g for 10 min to pellet them and washed once in PBS (1×) at 300 × g for 10 min to remove unbound antibodies.
Cell transplantation
For co-transplantation experiments, 2 × 107 pre-incubated BM cells of donor TTG-C57Bl/6 or C57Bl/6wt mice were added to 2 × 107 MAX.16H5 IgG1 pre-incubated splenocytes. The cell concentration was adjusted to a final volume of 150 μl sterile 0.9 % NaCl. For GvHD induction, 2 ×107 BM cells of donor TTG-C57Bl/6 or C57Bl/6wt mice were added to 2 × 107 splenocytes. Subsequently, the allogeneic grafts were transplanted in the lateral tail vain of recipient Balb/cwt mice in a volume of 150 μl [22, 23, 25]. The GvHD score after transplantation was calculated according to Cooke et al. [27].
Flow cytometry
FACSCanto BD™ II was set up by unstained and single stained cells as controls. For compensation of fluorochromes and calibration, compensation beads (CompBeads; BD Biosciences, Heidelberg, Germany) were used according to the manufacturer’s recommendations.
Donor TTG-C57Bl/6 or C57Bl/6wt and recipient Balb/cwt mice were analyzed by flow cytometry. For cytometric analysis, cells were prepared and incubated with anti-murine cd4-PECy7, MHC-I (H2Kb)-PE, anti-murine cd8-PerCP (BD Biosciences), anti-murine cd3-FITC, anti-human CD4-APC (Beckman Coulter, Brea, USA), and anti-human HLA-DR3-FITC (Immunotools, Friesoythe, Germany) according to the MiFlow-Cyt standards [25]. For cytometric analysis of murine FoxP3, the murine Treg Detection Kit (Miltenyi Biotec, Bergisch Gladbach, Germany) was used according to the manufacturer’s protocol. The Forward Scatter and Side Scatter gate was used to define the lymphocytes. Forward Scatter-cd3 gate was applied to the lymphocytes to discriminate T-cells. The gates for murine cd4/cd3, human CD4/cd3 were applied on the cd3+ T-cells to determine the engraftment (analysis of chimerism) and for discrimination of human and murine CD4+ cells. The gate for cd8/cd3 was applied on the cd3+ T-cells to investigate the cytotoxic T-cells and the gate for Forward Scatter-H2Kb was used for analyzing the chimerism after transplantation. Gates for human CD4–cd25 and murine cd4–cd25 were applied on the lymphocyte gate to determine how many CD4 cells are positive for CD25. Gates for cd25-FoxP3 were applied on human CD4–cd25 and murine cd4–cd25 gates for the investigation of donor and host CD4+CD25+FoxP3+ regulatory T-cells. The gate for human CD4-HLA-DR was applied on lymphocyte gate for detection of CD4 and HLA-DR-expressing cells after allogeneic transplantation. GFP-labeled P815 cells were detected in the FITC channel. Data were acquired on a BD FACSCantoIITM Flow Cytometer and analyzed using BD FACSDIVATM software (both BD Biosciences).
Histology of recipient mice, RNA isolation, FoxP3-PCR
Histology was done as described previously [24]. The TUNEL assay for detection of apoptosis in skin samples of GvHD mice was done according a protocol described by Jerome et al. [28]. Total RNA was isolated from tissue samples of murine livers and spleens from recipient mice and reverse transcribed into cDNA [23]. Real-time PCR was performed on a Roche LightCycler® (Roche, Grenzach-Wyhlen, Germany) using the QuantiTect SYBR Green PCR Kit (Qiagen) in a 20-μl reaction mix containing 100 ng of the cDNA (2 μl), 2 μl primer mix (each 20 pmol), 6 μl water, and QuantiTect SYBR Green Master Mix (with HotStarTaq DNA Polymerase, PCR Buffer, deoxynucleotide triphosphate mix, SYBR Green I, ROX passive reference dye, and 5 mM MgCl2). Amplification was performed by real-time PCR: 15 min activation and denaturation at 95 °C, followed by repetitive cycles of denaturation at 94 °C for 15 s, annealing at primer-specific annealing temperature for 20 s [FoxP3 5′-cag ctg cct aca gtg ccc cta g-3′ 5′-cat ttg cca gca gtg ggt ag-3′ [29] (annealing temperature 65 °C), Glyceraldehyde 3-phosphate dehydrogenase 5′-ccc act aac atc aaa tgg gg-3′ 5-cct tcc aca atg cca aag tt-3′ [30] (annealing temperature 56 °C)], and polymerization at 72 °C (20 s). A melting curve was obtained by heating at 70 °C for 20 s, then increasing to 99 °C at a rate of 0.1 °C/s while recording SYBR Green fluorescence. Relative gene expression was performed with the delta–delta method using the Relative Expression Software Tool© [31].
Results
Anti-human CD4 antibody administration to TTG-C57Bl/6 mice
For the evaluation of the effects to human CD4+/murine cd3+ cells in the TTG-C57Bl/6 donor mice, we investigated the development of human CD4+/murine cd3+ cells in the blood after intravenous injection of MAX.16H5 IgG1 (1.5 and 15 μg antibody/g body weight) by flow cytometry. Blood was investigated within 70 h after injection with regard to human CD4/murine cd3-expressing cells. After injection of 1.5 μg of the antibodies/g body weight, the human CD4+/murine cd3+ cells were not detectable for at least 6 h and again detectable after 9 h. After injection of 15 μg antibodies/g body weight (Fig. 2), the cells were again detectable after 24 h. After antibody administration, the human CD4 molecules were masked and the duration was dose-dependent.
Fig. 2.
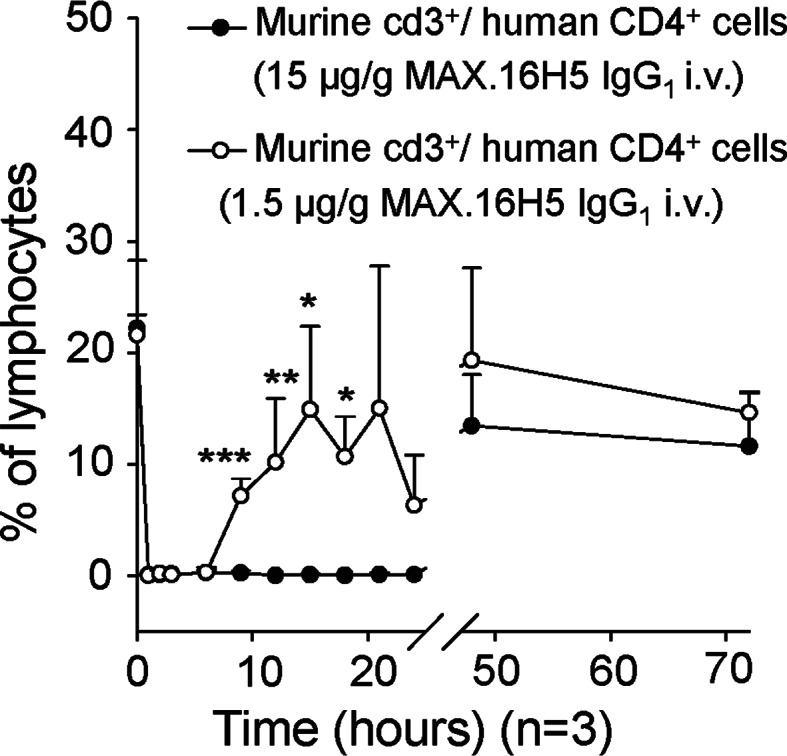
Evaluation of the distribution of CD4+ T-cells after intravenous application of MAX.16H5 IgG1 (1.5–15 μg/per g body weight) in TTG-C57Bl/6 mice (one experiment, n = 3 per time point). Human CD4+ T-cells from peripheral blood were not detectable for at least 6 h and again detectable after 9 h. The application of 15 μg/g body weight led to a prolongation of human CD4+ T-cell disappearance in peripheral blood to 24 h
Induction of GvHD by transplantation of BM cells and splenocytes from C57Bl/6wt or TTG-C57Bl/6 into Balb/cwt mice
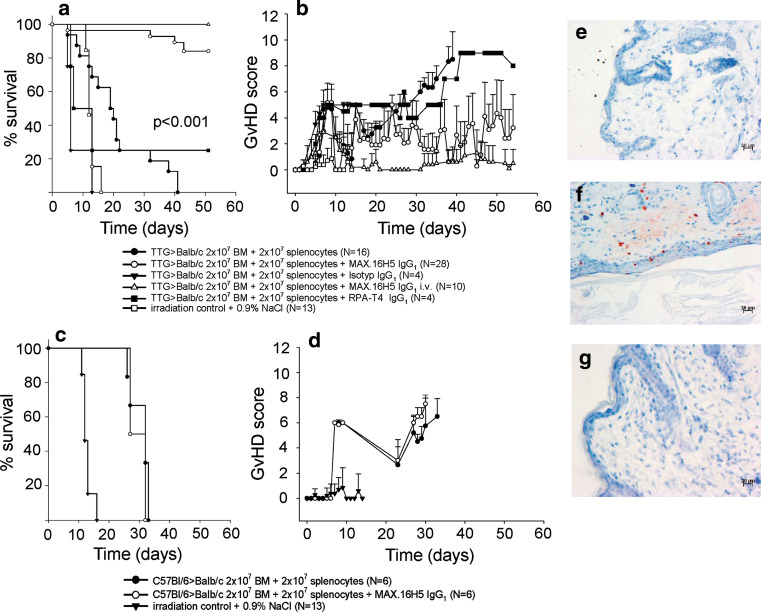
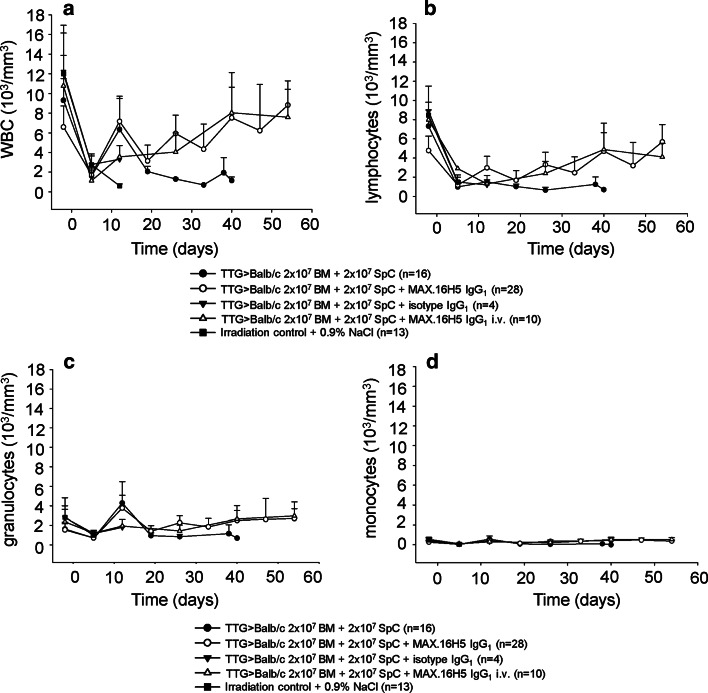
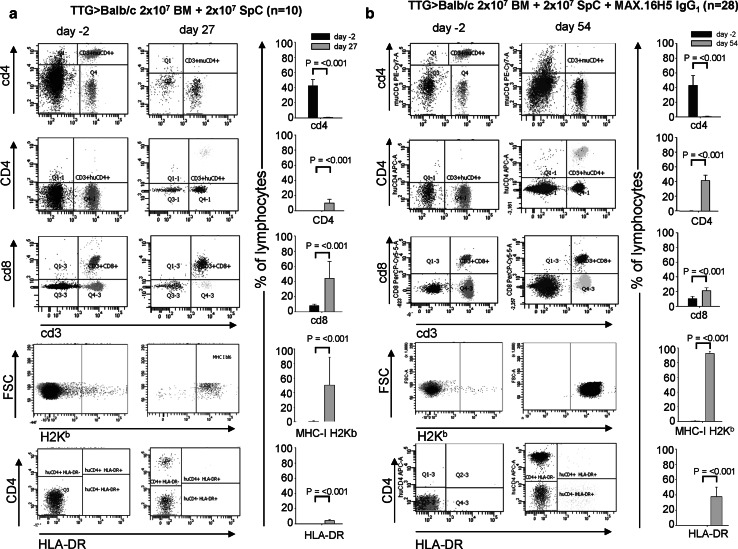
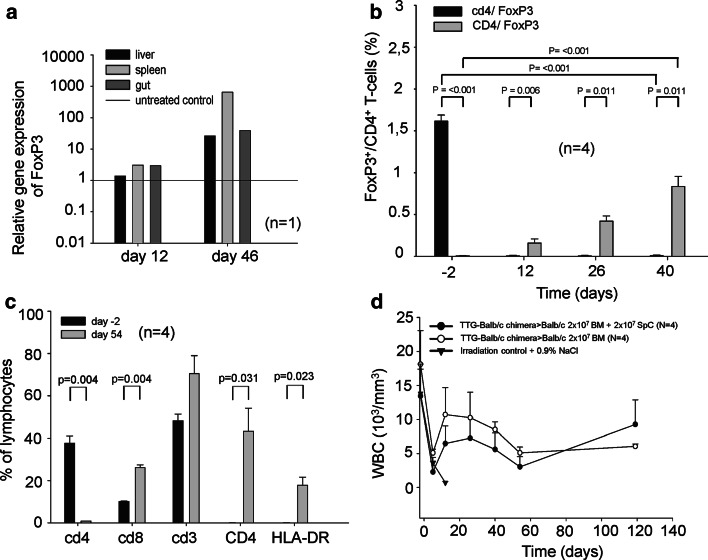
The transplantation of BM cells and splenocytes from transgenic human CD4+, murine cd4−/−, and HLA-DR+ mice in a full MHC allogeneic mismatch transplantation model (H2Kb → H2Kd) induces GvHD. Balb/cwt recipient mice transplanted with 2 × 107 BM cells and 2 × 107 splenocytes from TTG mice develop a severe GvHD within 40 days after transplantation (0 % survival; Fig. 3a). The GvHD score increases significantly from 0 at day of transplantation to 4.8 ± 1.87 at day 8 (Fig. 3b; p < 0.001). Leukocytes (WBC) from Balb/cwt recipient mice decreased from 9.31 ± 2.21 × 103/mm3 (day−2 = 2 days before transplantation) to 1.15 ± 0.35 × 103/mm3 (day 40) and showed no recovery to the initial levels (p < 0.001; Fig. 4a). Also, the WBC subsets (lymphocytes, granulocytes, and monocytes) did not reach the initial levels (Fig. 4b–d). In GvHD-mice, flow cytometry for human CD4, HLA-DR, murine cd4, murine cd8, and donor MHC-H2Kb after transplantation showed engraftment of TTG donor cells (Fig. 5a). Figure 5a shows that the expression of human CD4 (only expressed on donor T-cells) significantly increased in GvHD-animals from 0.04 ± 0.03 % (day −2) to 10.49 ± 4.9 % (day 27). The expression of HLA-DR (only expressed on donor APCs) significantly increased in GvHD-animals from 0.06 ± 0.09 % (day −2) to 4.51 ± 1.03 % (day 27), respectively. The loss of murine cd4-expressing cells also indicated the engraftment of donor cells. Murine MHC-H2Kb was expressed after transplantation in GvHD-animals (50.54 ± 38.31 %, p < 0.001, day 27 compared to day −2). The cd3+/cd8+ cells increased from 8.8 ± 2.19 % (day −2) to 44.23 ± 22.56 % (day 27, p < 0.001). Repeating the experiments by using C57Bl/6wt mice as donors instead of TTG-C57Bl/6 mice showed that all recipient Balb/cwt mice developed a severe GvHD and died within 35 days even when the grafts were pre-incubated with MAX.16H5 IgG1 anti-human CD4 antibodies (Fig. 3c, d). Histological staining of skin samples by a TUNEL assay showed apoptosis in GvHD-mice in comparison to recipients receiving a MAX.16H5 IgG1 pre-incubated graft or to recipients after treatment with MAX.16H5 IgG1 before transplantation (Fig. 3e–g).
Fig. 3.
a, b GvHD induction after transplantation of cells from human CD4+ C57Bl/6-TTG mice into Balb/cwt mice with or without short-term pre-incubation of MAX.16H5 IgG1. a Survival of Balb/cwt recipient mice after allogeneic transplantation of 2 × 107 BM cells and 2 × 107 splenocytes from human CD4+ C57Bl/6-TTG mice. Balb/cwt recipient animals, treated with the antibodies or receiving a pre-incubated graft showed a significantly higher survival rate compared to control mice (four independent experiments for transplantation of cells from human CD4+ C57Bl/6-TTG mice into Balb/cwt mice with or without short-term pre-incubation of MAX.16H5 IgG1 (800 μg per 1.4 × 108 BM cells and splenocytes), n = 16 and 28, p < 0.001, one experiment for isotype control IgG1 (800 μg per 1.4 × 108 BM cells and splenocytes, n = 4), two experiments for transplantation of cells from human CD4+ C57Bl/6-TTG mice into Balb/cwt mice with antibody treatment of recipient mice, n = 10). Without antibodies, recipient mice died within 45 days. Additionally, recipient Balb/cwt mice transplanted with cells from human CD4+ C57Bl/6-TTG mice and short-term pre-incubation (800 μg per 1.4 × 108 BM cells and splenocytes) with anti-CD4 antibodies RPA-T4 IgG1 are shown (one experiment, n = 4). b The GvHD scoring system showed a significantly lower GvHD score in Balb/cwt recipient animals that were treated with MAX.16H5 IgG1 or receiving a pre-incubated graft. c, d Transplantation of cells from C57Bl/6wt into Balb/cwt mice with or without short-term pre-incubation with MAX.16H5 IgG1. c Survival of Balb/cwt recipient mice after allogeneic transplantation of 2 × 107 BM cells and 2 × 107 splenocytes from C57Bl/6wt mice (one experiment for transplantation of cells from C57Bl/6wt mice into Balb/cwt mice with or without short-term pre-incubation of MAX.16H5 IgG1 (800 μg per 1.4 × 108 BM cells and splenocytes), n = 6, one experiment for isotype control IgG1, n = 6). b The GvHD scoring system showed a high GvHD score in all transplanted animals. Total body irradiation of recipients led to a survival of 0 % (three independent experiments, n = 13). e–g All recipient animals that developed a GvHD showed apoptosis in histological analysis (shown for skin). e Balb/cwt mice without transplantation. f Skin of Balb/c chimera with GvHD after transplantation of cells from TTG-C57Bl/6 mice without MAX.16H5 IgG1 showed apoptosis. g Skin of Balb/c chimera without GvHD after transplantation of cells from TTG-C57Bl/6 mice with MAX.16H5 IgG1 showed no apoptosis
Fig. 4.
Immune reconstitution of cells from TTG-C57Bl/6 mice transplanted into Balb/cwt mice with or without short-term pre-incubation with MAX.16H5 IgG1. a Leukocytes from mice transplanted with 2 × 107 BM+ 2 × 107 splenocytes+ MAX.16H5 IgG1 reach the initial WBC levels within 60 days after transplantation. Control mice receiving an untreated graft showed a decrease of WBC levels and did not reach the initial levels. b–d Full blood cell count after allogeneic transplantation. Reconstitution of monocytes, granulocytes, and lymphocytes from mice transplanted with 2 × 107 BM+ 2 × 107 splenocytes+ MAX.16H5 IgG1 reached the initial levels within 60 days after transplantation compared to the control mice (four independent experiments for transplantation of cells from human CD4+ C57Bl/6-TTG mice into Balb/cwt mice with or without short-term pre-incubation of MAX.16H5 IgG1, n = 16 and 28, one experiment for isotype control IgG1 n = 4, two experiments for transplantation of cells from human CD4+ C57Bl/6-TTG mice into Balb/cwt mice with antibody treatment of recipient mice, n = 10)
Fig. 5.
Flow cytometric analysis of murine cd4, human CD4, murine cd8, H2Kb, and HLA-DR on day −2 (2 days before transplantation) and day 27 (a), and day −2 and 54 (b) after transplantation. A decrease of murine cd4-expressing T-cells, an increase of human CD4-expressing T-cells, and HLA-DR-expressing cells indicates engraftment of transplanted cells in Balb/cwt recipient mice after allogeneic transplantation of 2 × 107 BM cells and 2 × 107 splenocytes from human CD4+ C57Bl/6-TTG mice with (b) or without (a) pre-incubation of MAX.16H5 IgG1 (four independent experiments for transplantation of cells from human CD4+ C57Bl/6-TTG mice into Balb/cwt mice with or without short-term pre-incubation of MAX.16H5 IgG1, n = 10 and 28)
Prevention of GvHD by short-term pre-incubation of BM cells and splenocytes coated with MAX.16H5 IgG1 anti-CD4 antibodies
The pre-incubation of BM cells and splenocytes from transgenic human CD4+, murine cd4−/−, and HLA-DR+ mice with MAX.16H5 IgG1 anti-human CD4 antibodies and subsequent transplantation in a full MHC allogeneic mismatch transplantation model prevents the induction of GvHD. BM cells (2 × 107) and splenocytes (2 × 107) from TTG mice were incubated with MAX.16H5 IgG1 anti-human CD4 antibodies before transplantation into Balb/cwt recipient mice. These mice did not develop GvHD within 60 days after transplantation compared to the GvHD controls (86 % survival; Fig. 3a; p < 0.001). The GvHD score was significantly lower at day 25 until the end of the experiment (Fig. 3b; p < 0.001) in comparison to GvHD-controls. WBC from recipient mice increased from 6.58 ± 2.15 × 103/mm3 (day −2) to 8.82 ± 2.46 × 103/mm3 (day 54) and showed recovery to the initial and normal levels (Fig. 4a). Also, the WBC subsets (lymphocytes, granulocytes, and monocytes) reached normal levels (Fig. 4c, d). Flow cytometric analysis 60 days after transplantation showed engraftment of TTG donor cells (Fig. 5b). Human CD4 expression (only expressed on donor T-cells) significantly increased in GvHD-free animals from 0.07 ± 0.05 % (day −2) to 41.4 ± 7.65 % (day 54). HLA-DR expression (only expressed on donor APCs) significantly increased in GvHD-free animals from 0.02 ± 0.03 % (day −2) to 37.69 ± 12.44 % (day 54). The loss of murine cd4-expressing cells also indicated the engraftment of the donor cells. Murine MHC-H2Kb was expressed after transplantation in GvHD-free animals (92.3 ± 3.27 %, p < 0.001). The cd3+/cd8+ cells increased from 10.18 ± 3.34 % (day −2) to 20.91 ± 4.38 % (day 54, p < 0.001).
In GvHD-free recipients, the relative FoxP3 expression showed an increase after allogeneic transplantation (up to more than 100-fold) in recipient Balb/cwt mice 46 days after transplantation in liver, spleen, and gut (Fig. 6a). Survival after transplantation of cells from TTG mice with short-term pre-incubation of RPA-T4 IgG1 antibodies (800 μg in 15 ml Dulbecco’s Modified Eagle Medium without FCS for 2 h) was significantly lower than using MAX.16H5 IgG1 antibodies. This indicated an epitope-specific effect (Fig. 3a). The GvHD scores in this group were significantly higher compared to the controls (Fig. 3b).
Fig. 6.
a Relative FoxP3 expression 46 days after transplantation of 2 × 107 MAX.16H5 IgG1 pre-incubated BM cells and 2 × 107 splenocytes from TTG-C57Bl/6 mice into Balb/cwt mice (one experiment, n = 1). The relative FoxP3 expression showed an increase after allogeneic transplantation 46 days after transplantation in liver, spleen, and gut of a transplanted animal. b–d Tolerance transfer by transplantation of 2 × 107 BM cells and 2 × 107 splenocytes from GvHD-free chimeric TTG-Balb/c mice into third party Balb/cwt recipient mice. BM cells and splenocytes (2 × 107 BM+ 2 × 107 splenocytes) were transplanted into third party Balb/cwt mice without pre-incubation with MAX.16H5 IgG1 (n = 4, one experiment). b The relative FoxP3 expression showed a continuous increase of donor derived (human CD4+/FoxP3+) T-cells after allogeneic transplantation from Balb/c-TTG chimeras in third party Balb/cwt recipients compared to the controls (n = 4, one experiment). c Flow cytometric analysis 60 days after transplantation showed engraftment of TTG donor cells. The expression of human CD4+, HLA-DR+, and cd8+ cells significantly increased in these animals. The loss of murine cd4-expressing cells also indicated the engraftment of the donor cells (n = 4, one experiment). d Leukocytes (WBC) from surviving Balb/c recipient mice showed recovery to the initial levels (n = 4, one experiment) after 120 days. Total body irradiation from control mice led to a survival of 0 % (one experiment, n = 3)
Prevention of GvHD by in vivo application of MAX.16H5 IgG1 at day of transplantation
The direct administration of MAX.16H5 IgG1 anti-human CD4 antibodies into Balb/cwt recipient mice before transplantating BM cells and splenocytes from transgenic human CD4+, murine cd4−/−, and HLA-DR+ mice also prevented the induction of GvHD. Balb/cwt mice received 15 μg/g MAX.16H5 IgG1 anti-human CD4 antibodies intravenously before receiving 2 × 107 BM cells and 2 × 107 splenocytes from TTG mice. These mice did not develop aGvHD within 60 days after transplantation compared to the GvHD controls (100 % survival; Fig. 3a, b; n = 10, p < 0.001). Immune reconstitution was comparable to recipient Balb/cwt mice receiving a pre-incubated graft (data not shown).
Transfer of immune tolerance and determination of regulatory T-cells in recipient Balb/cwt mice
The possibility to transfer immune tolerance from transplanted GvHD-free Balb/c chimeras (Balb/c mice with TTG hematopoiesis) into third-party Balb/cwt mice without new pre-incubation of the graft with MAX.16H5 IgG1 anti-human CD4 antibodies was hypothesized. Thus, after 40 days of initial transplantation, BM cells and splenocytes were attained and transferred into new Balb/cwt mice. Engrafted recipient mice did not develop aGvHD within 60 days after transplantation even when cell numbers of BM cells and splenocytes were increased to 8 × 107 BM cells and 1 × 108 splenocytes (Supplementary Fig. 1). Fifty percent of the recipient Balb/cwt mice survived without GvHD development. Dying animals showed no engraftment and no signs of GvHD (hematopoietic failure). The relative FoxP3 expression showed a continuous increase of donor-derived human CD4+/FoxP3+ T-cells after allogeneic transplantation from Balb/c-TTG chimeras into third-party Balb/cwt recipients (Fig. 6b). Flow cytometry analysis 60 days after transplantation showed engraftment of TTG donor cells (Fig. 6c). Figure 6c shows that the expression of human CD4 significantly increased in these animals until day 54. The expression of HLA-DR significantly increased in these animals from 0.02 ± 0.02 % (day −2) to 17.8 ± 3.8 % (day 54). The loss of murine cd4-expressing cells also indicated the engraftment of the donor cells. The cd3+/cd8+ cells significantly increased from 10.17 ± 1.17 % (day −2) to 23.94 ± 1.63 %. The expression of cd3 at day 54 was 70.5 ± 8.5 % (Fig. 6c, day 54). Leukocytes (WBC) recovery reached nearly the initial levels 120 days after transplantation (Fig. 6d).
Influence of the antibody therapy to the GvL effect
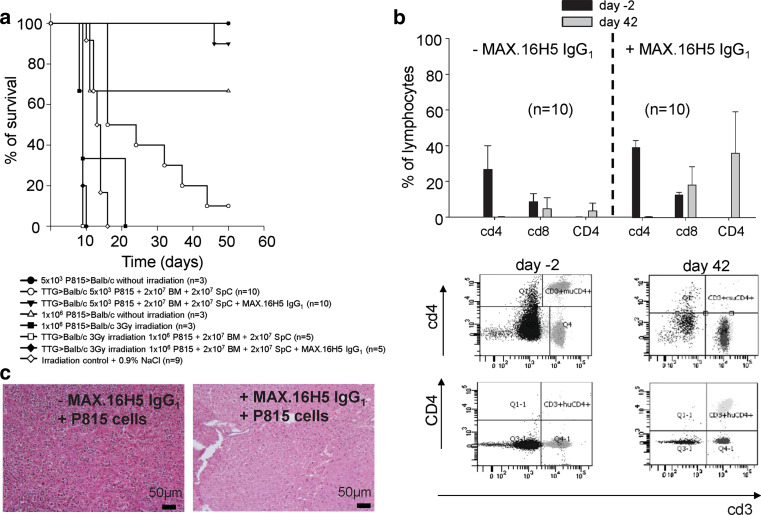
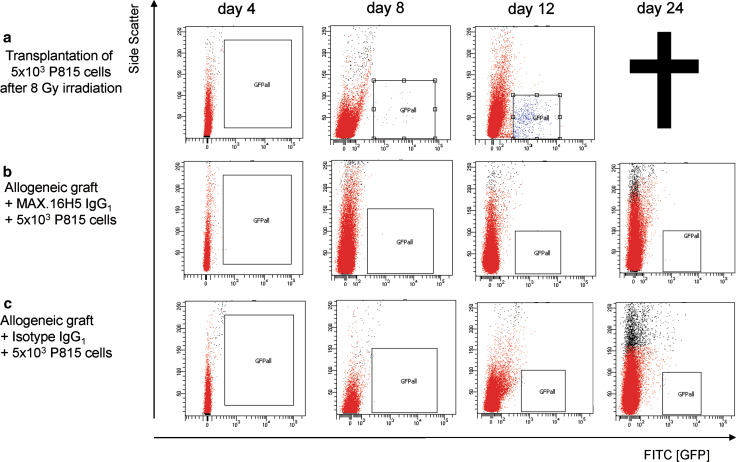
The pre-incubation of CD4+ T-cells should not have a suppressive effect on developing CD8+ T-cell clones responsible for the GvL effect. Therefore, Balb/cwt mice received different cell concentrations of P815-mast-cell leukemia cells intravenously before transplantation of 2 × 107 BM cells and 2 × 107 splenocytes from TTG mice incubated with or without MAX.16H5 IgG1 anti-human CD4 antibodies. Transplantating high concentrations of P815-cells (1 × 106 per 150 μl) led to a survival of 0 % of all recipients. Interestingly, after titration of P815-cells to 5 × 103 per 150 μl, survival in recipients receiving a pre-incubated graft was significantly higher compared to recipients receiving no MAX.16H5 IgG1 anti-human CD4 antibodies (90 vs. 10 %, p < 0.001, n = 10; Fig. 7a). Immune re-constitution was comparable to recipient Balb/cwt mice receiving a pre-incubated graft (Fig. 7b) except for human CD4, which was markedly increased at day 42 in recipients of the antibody. P815-cells could be detected in different organs by histological analysis, e.g., liver (Fig. 7c). In Balb/cwt recipients receiving an allogeneic graft (after irradiation with 8 Gy and pre-incubation with MAX.16H5 IgG1 or the isotype control IgG1) and P815GFP cells, no P815 cells were detectable after transplantation at different time points by flow cytometry indicating a GvL effect. In recipients only receiving P815GFP cells after irradiation with 8 Gy and without transplantation, P815 cells were detectable (Fig. 8a–c, shown for liver cells).
Fig. 7.
a, b Transplantation of cells from human CD4+ cells from C57Bl/6-TTG mice into Balb/cwt mice with or without short-term pre-incubation with MAX.16H5 IgG1 and with or without co-transplantation of P815 mastocytoma cells. a Transplantation of high concentrations of P815-cells (1 × 106 per 150 μl) with 3 Gy total body irradiation led to a survival of 0 % in all recipients (one experiment, n = 5). Interestingly, after titration of P815-cells to 5 × 103 per 150 μl, the survival in recipients receiving a pre-incubated graft was significantly higher compared to recipients receiving no MAX.16H5 IgG1 (90 vs. 10 %, p < 0.001, two independent experiments, n = 10) and the infiltration of P815-cells into different organs was lower (c, shown for liver). b Immune re-constitution was comparable to Balb/cwt mice receiving a pre-incubated graft without co-transplantation of P815-cells except for human CD4, which was markedly increased at day 42 in recipients of the antibody (two independent experiments, n = 10). Transplantation of 1 × 106 P815-cells without irradiation lead to a survival of 66 % (one experiment, n = 3). Total body irradiation led to a survival of 0 % (three independent experiments, n = 9), transplantation of 5 × 103 P815 cells without irradiation to a survival of 100 % (one experiment, n = 3)
Fig. 8.
a–c Transplantation of cells from human CD4+ cells from C57Bl/6-TTG mice into Balb/cwt mice with or without short-term pre-incubation with MAX.16H5 IgG1 and with co-transplantation of P815GFP mastocytoma cells. a After transplantation of P815GFP-cells (5 × 103 per 150 μl) without an allogeneic graft and 8 Gy total body irradiation, the P815GFP cells could be detected until death of the recipients (one experiment, n = 6). b, c After transplantation of P815GFP cells (5 × 103 per 150 μl) with an allogeneic graft pre-incubated with MAX.16H5 IgG1 or the isotype control IgG1, P815GFP cells were cleared from the circulation indicating the Graft-versus-leukemia effect of the graft (two independent experiments, n = 6). All dot plots and flow cytometric analysis are shown for liver cells
Discussion
We investigated whether MAX.16H5 IgG1 monoclonal anti-human CD4 antibodies can prevent the development of GvHD with preserved GvL effect when allogeneic grafts are short-term pre-incubated and unbound antibodies are removed.
After in vivo administration of MAX.16H5 IgG1 anti-human CD4 antibodies in TTG-C57Bl/6 mice, the antibodies covered the CD4 molecules on murine T-cells expressing the human CD4 up to 24 h depending on the dose administered. In a clinical study, patients treated with MAX.16H5 IgG1 antibodies undergo a rapid modulation of the CD4+ T-cells, the degree of which varies from patient to patient and appears to be dependent on the amount of MAX.16H5 IgG1 antibodies. Modulation of the target antigen by MAX.16H5 IgG1 was demonstrated in one study, through high levels of soluble CD4 and a decreased CD4 antigen density on T-helper cells, down to 30 % of the original level [32]. This effect occured within a few hours after the infusion started. Circulating CD4-expressing lymphocytes become coated with the antibodies, and in vivo infusions demonstrated that a maximum coating of 11 ± 7 % is reached by the end of infusion, which declines to 0–5 % by 12 h post-infusion [33].
Using TTG mice as donors, Balb/cwt mice develop a severe GvHD within 45 days, which was comparable to when C57Bl/6wt mice instead of TTG mice were used as donors. Engraftment of cells and GvHD development was first observed after 12 days. GvHD was classified according to a GvHD score [27] and by histological analysis [25]. Because the GvHD score is a clinical score considering weight, texture of the fur, and mobility, the control animals were also positively scored at different time points especially as a consequence of irradiation. However, the GvHD score in GvHD animals was significantly higher after engraftment compared to recipients receiving MAX.16H5 IgG1. Interestingly, direct application of the antibodies led to a lower GvHD score in recipient animals compared to animals receiving a pre-incubated graft. There are other GvHD models specifically using C57Bl/6wt mice as donors and Balb/cwt mice as recipients (www.stembook.org), but the originality of our model is the possibility for direct testing of anti-human CD4 antibodies in mice. All animal models of GvHD and GvL are simply animal models. The results should be confirmed using non-obese diabetic/severe combined immunodeficient mice as recipients for human immune cell grafts. In these models, the contribution of CD8+ T-cells or other cells responsible for innate or adaptive immune responses should be investigated as well as the GvL effect against human leukemia cell lines.
The reconstitution of WBC was observed in GvHD-free mice until the end of the experiment. In GvHD mice, lymphocytes and monocytes did not reach the initial levels indicating the immunosuppressive effect of GvH reactions also observed by hyposplenism in recipient animals. For analysis of hematopoietic chimerism, we examined the presence of murine and human CD4 molecules, HLA-DR, and H2Kb after transplantation. After transplantation, human CD4+, HLA-DR+, and H2Kb+ cells representing donor hematopoiesis were stably expressed from day 12.
We found that transplantating short-term (2 h) pre-incubated and washed allogeneic hematopoietic stem cell grafts with MAX.16H5 IgG1 prevents aGvHD. It is not known whether the time of incubation is crucial for the observed effect. It would be interesting to test different antibody incubation times before transplantation in recipient mice with regard to GvHD development. Furthermore, GvHD could be prevented when recipient Balb/cwt mice were treated by MAX.16H5 IgG1 anti-human CD4 antibodies before transplantation. In comparison to the ex vivo treatment of the graft, the antibody doses are probably higher when the antibodies are directly administered to the recipients. These effects should be investigated in clinical trials. However, it is encouraging that after direct administration of the antibodies to recipient mice, a normal reconstitution of all lymphocyte subsets without GvHD development occurred. Further experiments will investigate whether different administration time points have different effects to the GvHD development.
Allografts included BM cells and splenocytes. BM cells were transplanted for reconstitution of a hematopoietic system and splenocytes for induction of GvHD by CD4+ T-cells. For transplantation in human beings, peripheral mobilized stem cells and umbilical cord blood grafts are also used. These un-manipulated grafts also contain other mononuclear cells and T-cells that initiate GvHD. Especially in transplantation with reduced intense conditioning, the T-cells have an impact to facilitate the GvL effect [34].
Interestingly, CD4 and its natural ligand HLA-DR were co-transferred. Up to date, it is not clear whether this association is responsible for GvHD development in Balb/cwt mice in this transplantation model. Probably, host antigens are presented by HLA-DR-expressing cells after transplantation and GvHD T-cell clones are induced after engraftment of hematopoietic stem cells. On the other hand, human CD4+ T-cells from TTG mice can probably recognize the murine H2Kd complexes and initiate the GvHD. Experiments in which HLA-DR-expressing cells are separated prior to transplantation should be performed. After transplantation, the expression of HLA-DR significantly increased. In this model, HLA-DR is not specific for dendritic cells. HLA-DR is also expressed on B-cells that develop after T-cell reconstitution and on activated T-cells. In GvHD-free and in GvHD animals, a stable engraftment of donor cells could be observed. In GvHD-free animals, the immune reconstitution of the cells nearly reached the initial levels compared to the GvHD recipients.
A short-term contact of anti-CD4 antibodies can induce tolerance to foreign proteins and transplanted allografts [35, 36]. We hypothesize that the pre-incubation of CD4+ T-cells with MAX.16H5 IgG1 suppresses the clonal expansion of GvHD-specific T-cell clones through induction of Tregs. MAX.16H5 IgG1 modulates the activation of CD4+ T-cells through down-regulation of IL-2 mRNA levels and by inhibition of lymphocyte-specific protein tyrosine kinase transmission [37, 38]. It has been shown that anti-CD4 antibody therapy effectively induces tolerance in rats [39] and mouse strains [40]. Tregs can also be induced by CD4 ligands, which is a central mechanism in tolerance induction by monoclonal anti-CD4 antibodies. In this study, recipient animals receiving a MAX.16H5 IgG1 pre-incubated graft showed a higher Fox-P3 gene expression after transplantation in liver, spleen, and gut 46 days after transplantation.
The murine depleting anti-CD4 antibody (GK 1.5) induces tolerance against a rat anti-mouse Il-6 receptor antibody with a lower appearance of nephritis in (New Zealand Black × New Zealand White) F1 mice [41]. The induction of a donor-specific tolerance due to the combination of donor-specific T-cell infusion and anti-CD4 YTS177.9 antibody therapy was also observed [40]. However, depletion of CD4+ T-cells could be associated with higher risk for infectious diseases after transplantation. It is well known that GvHD development in murine transplantation models depends on the T-cell content in the graft. A T-cell depleted graft will not induce any GvHD development in our model. Prevention of GvHD was only achieved by pre-incubation or direct application of MAX.16H5 IgG1 indicating an epitope-specific effect. By using RPA-T4 anti-human CD4 antibodies, the observed effect could not be repeated. However, further anti-human CD4 antibodies or anti-CD4 beads binding to other epitopes of the CD4 molecule than MAX.16H5 IgG1 should be investigated.
In P815-Balb/c leukemic mice, the survival rate was significantly higher compared to controls when these animals received a MAX.16H5 IgG1 pre-incubated graft. This demonstrated that different T-cell clones at different time-points are responsible for GvHD and the GvL effect. The GvL effect in our GvHD model using TTG mice as donors and Balb/cwt mice as recipients has not so far been investigated to discover whether the GvHD and GvL effect are antigen-specific. Allo-reactive donor C57Bl/6 T-cells expressing human CD4 that recognize allo-antigens restricted by human HLA-DR would not be expected to directly recognize peptides expressed on murine H2Kd by Balb/c cells. On the other hand, the triple transgenic donor cells have more allo-reactivity than wild-type donor cells. Therefore, recipients die faster from GvHD when receiving TTG donor cells. This suggests a difference in overall allo-reactivity between transgenic and wild-type donor T-cells. Probably, human CD4+ T-cells from TTG mice recognize antigens on murine H2Kd in situ as well as on co-transferred HLA-DR, which strengthen the allo-reactivity. The overall allo-reactivity of the transgenic human CD4 T-cells may not be directly comparable to wild-type donor T-cells. Because all the murine donor CD4+ T-cells express human CD4, the effect of the anti-CD4 antibody can be an effect of a general CD4 modulation that is antigen non-specific. The GvL effect after transplantation of C57Bl/6 cells into Balb/c recipients is predominately mediated by CD8+ T-cells [42, 43]. Also, the P815 cell line is derived from Dilute Brown Non-Agouti Mouse mice (DBA/2, H2d) so that there are differences in minor antigens between it and Balb/cwt recipient mice. This can explain the relative strengths of GvL and GvHD in this model. However, MAX.16H5 IgG1 did not influence the GvL effect negatively in this model. Balb/c recipients are resistant to low doses of P815 cells, presumably through the recognition of the minor antigens. The use of a DBA/2 × Balb/c F1 as a transplant recipient will be further investigated to eliminate intrinsic resistance of the recipients to the tumor cells.
Due to irradiation, host APCs can easily activate CD4+ T-cells within the graft. The blockage of the CD4 molecule by MAX.16H5 IgG1 leads to T-cell anergy. After HSCT, Tregs are generated that permanently suppress GvHD-specific T-cell clones. By transplantation of an allogeneic graft from a GvHD-free recipient without pre-incubation of MAX.16H5 IgG1 into a third-party recipient, no GvHD was inducible even when higher T-cell concentrations are used. This indicates the presence of a (Treg)-cell population maintaining the effect. Recipients do not die of GvHD and the time for impact of the GvL effect was increased. An application of MAX.16H5 IgG1 antibodies after engraftment of the hematopoietic stem cells showed no success because MAX.16H5 IgG1 antibodies are cleared within 48 h from the circulation or the activation of GvHD-specific T-cell clones cannot be prevented. In comparison to GvHD, GvL can also be mediated by natural killer cells or cytotoxic CD8+ T-cells. CD8+ T-cells can be activated directly through antigen-presenting cells with high expression of B7 molecules without the need of CD4+ T-helper cells. Developing T-cell clones are still able to attack residual tumor cells.
Prevention of GvHD by preserving the beneficial immunological functions (e.g., anti-tumor capacity) is one of the major goals for cell transplantation. This is the first report showing that the anti-human CD4 antibody MAX.16H5 IgG1 was effective in long-term suppression of GvHD while preserving the anti-tumor capacity when monoclonal antibodies were used ex vivo. The separation of GvL from GvHD was CD4 epitope-specific.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1. a–d Tolerance transfer by transplantation of 8 × 107 BM and 8 × 107 BM cells + 1 × 108 splenocytes from GvHD-free chimeric TTG-Balb/c mice into third party Balb/cwt recipient mice. BM cells (8 × 107) and BM cells + splenocytes (8 × 107 BM + 1 × 108 splenocytes) were transplanted into third party Balb/cwt mice without pre-incubation with MAX.16H5 IgG1 (n = 1, one experiment). Engrafted recipient mice of both groups showed a survival of 100 % (a), did not develop aGvHD (b), put on weight (c), and showed a stable leukocyte recovery (d).
Acknowledgments
We thank Mrs. A. Braun for proof reading the manuscript. The authors have no conflicting financial interests. We thank the colleagues from the Translational Centre for Regenerative Medicine, Universität Leipzig, for providing and breeding the triple transgenic (TTG) mice, Mrs. Ramona Blaschke. Mrs. Martina Fügenschuh and Mrs. Nadja Rudolph for preparing the histological experiments, Mrs. Jutta Jahns for preparing the irradiation of recipient mice, Mrs. Ellen Svanidze and Mrs. Manuela Ackermann for preparing the flow cytometric analysis. The work presented in this paper was funded by the German Federal Ministry of Education and Research (BMBF 0313452, PtJ-Bio, 0313909).
Abbreviations
- APCs
Antigen-presenting cells
- BM
Bone marrow
- FoxP3
Forkhead box P3
- GvHD
Graft-versus-host-disease
- GvL
Graft-versus-leukemia
- HLA
Human leukocyte antigen
- HSCT
Hematopoietic stem cell transplantation
- IgG
Immunoglobulin G
- MHC
Major histocompatibility complex
- PCR
Polymerase chain reaction
- SpC
Splenocytes
- TTG mice
Triple transgenic mice
- WBC
White blood cell count
References
- 1.Jacobsohn DA, Vogelsang GB. Acute graft versus host disease. Orphan J Rare Dis. 2007;2:35. doi: 10.1186/1750-1172-2-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferrara JL, Levine JE, Reddy P, Holler E. Graft-versus-host disease. Lancet. 2009;373(9674):1550–1561. doi: 10.1016/S0140-6736(09)60237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hill GR, Krenger W, Ferrara JL. The role of cytokines in acute graft-versus-host disease. Cyto Cell Mol Ther. 1997;3(4):257–266. [PubMed] [Google Scholar]
- 4.Paczesny S, Choi SW, Ferrara JL. Acute graft-versus-host disease: new treatment strategies. Curr Opin Hematol. 2009;16(6):427–436. doi: 10.1097/MOH.0b013e3283319a6f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.von Bonin M, Stolzel F, Goedecke A, Richter K, Wuschek N, Holig K, Illmer T, Platzbecker U, Schaich M, Schetelig J, Kiani A, Ordemann R, Ehninger G, Schmitz M, Bornhauser M. Treatment of refractory acute GVHD with third-party MSC expanded in platelet lysate-containing medium. B Mar Transpl. 2009;43(3):245–251. doi: 10.1038/bmt.2008.316. [DOI] [PubMed] [Google Scholar]
- 6.Trapani JA, Smyth MJ. Functional significance of the perforin/granzyme cell death pathway. Nat Rev Immunol. 2002;2(10):735–747. doi: 10.1038/nri911. [DOI] [PubMed] [Google Scholar]
- 7.Michalek J, Collins RH, Durrani HP, Vaclavkova P, Ruff LE, Douek DC, Vitetta ES. Definitive separation of graft-versus-leukemia- and graft-versus-host-specific CD4 + T cells by virtue of their receptor beta loci sequences. Proc Natl Acad Sci USA. 2003;100(3):1180–1184. doi: 10.1073/pnas.0337543100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fehervari Z, Cooke A, Brett S, Turner J. Perturbation of naive TCR transgenic T cell functional responses and upstream activation events by anti-CD4 monoclonal antibodies. Eur J Immunol. 2002;32(2):333–340. doi: 10.1002/1521-4141(200202)32:2<333::AID-IMMU333>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 9.Harding S, Lipp P, Alexander DR. A therapeutic CD4 monoclonal antibody inhibits TCR-zeta chain phosphorylation, zeta-associated protein of 70-kDa Tyr319 phosphorylation, and TCR internalization in primary human T cells. J Immunol. 2002;169(1):230–238. doi: 10.4049/jimmunol.169.1.230. [DOI] [PubMed] [Google Scholar]
- 10.Madrenas J, Schwartz RH, Germain RN. Interleukin 2 production, not the pattern of early T-cell antigen receptor-dependent tyrosine phosphorylation, controls anergy induction by both agonists and partial agonists. Proc Natl Acad Sci USA. 1996;93(18):9736–9741. doi: 10.1073/pnas.93.18.9736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Madrenas J, Germain RN. Variant TCR ligands: new insights into the molecular basis of antigen-dependent signal transduction and T-cell activation. Semin Immunol. 1996;8(2):83–101. doi: 10.1006/smim.1996.0011. [DOI] [PubMed] [Google Scholar]
- 12.Chatenoud L, Waldmann H, Emmrich F. Tolerance induction in the adult: ‘danger’ at Le Bischenberg. Immunol Today. 1995;16(3):121–123. doi: 10.1016/0167-5699(95)80126-X. [DOI] [PubMed] [Google Scholar]
- 13.Emmrich F, Horneff G, Becker W, Luke W, Potocnik A, Kanzy U, Kalden JR, Burmester G. An anti-CD4 antibody for treatment of chronic inflammatory arthritis. Agen Act Suppl. 1991;32:165–170. doi: 10.1007/978-3-0348-7405-2_22. [DOI] [PubMed] [Google Scholar]
- 14.Emmrich J, Seyfarth M, Fleig WE, Emmrich F. Treatment of inflammatory bowel disease with anti-CD4 monoclonal antibody. Lancet. 1991;338(8766):570–571. doi: 10.1016/0140-6736(91)91133-F. [DOI] [PubMed] [Google Scholar]
- 15.Reinke P, Volk HD, Miller H, Neuhaus K, Fietze E, Herberger J, Herberger D, von Baehr R, Emmrich F. Anti-CD4 therapy of acute rejection in long-term renal allograft recipients. Lancet. 1991;338(8768):702–703. doi: 10.1016/0140-6736(91)91285-3. [DOI] [PubMed] [Google Scholar]
- 16.Reinke P, Fietze E, Docke WD, Kern F, Ewert R, Volk HD. Late acute rejection in long-term renal allograft recipients. Diagnostic and predictive value of circulating activated T cells. Transplantation. 1994;58(1):35–41. doi: 10.1097/00007890-199407000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Reinke P, Kern F, Fietze E, Docke WD, Ewert R, Emmrich F, Volk HD. Anti-CD4 monoclonal antibody therapy of late acute rejection in renal allograft recipients––CD4+ T cells play an essential role in the rejection process. Transpl Proc. 1995;27(1):859–862. [PubMed] [Google Scholar]
- 18.Laub R, Dorsch M, Meyer D, Ermann J, Hedrich HJ, Emmrich F. A multiple transgenic mouse model with a partially humanized activation pathway for helper T cell responses. J Immunol Meth. 2000;246(1–2):37–50. doi: 10.1016/S0022-1759(00)00288-X. [DOI] [PubMed] [Google Scholar]
- 19.Laub R, Dorsch M, Wenk K, Emmrich F. Induction of immunologic tolerance to tetanus toxoid by anti-human CD4 in HLA-DR3(+)/human CD4(+)/murine CD4(−) multiple transgenic mice. Transpl Proc. 2001;33(3):2182–2183. doi: 10.1016/S0041-1345(01)01934-0. [DOI] [PubMed] [Google Scholar]
- 20.Laub R, Brecht R, Dorsch M, Valey U, Wenk K, Emmrich F. Anti-human CD4 induces peripheral tolerance in a human CD4+, murine CD4−, HLA-DR+ advanced transgenic mouse model. J Immunol. 2002;169(6):2947–2955. doi: 10.4049/jimmunol.169.6.2947. [DOI] [PubMed] [Google Scholar]
- 21.Strauss G, Vignali DA, Schonrich G, Hammerling GJ. Negative and positive selection by HLA-DR3(DRw17) molecules in transgenic mice. Immunogenetics. 1994;40(2):104–108. [PubMed] [Google Scholar]
- 22.Fricke S, Ackermann M, Stolzing A, Schimmelpfennig C, Hilger N, Jahns J, Hildebrandt G, Emmrich F, Ruschpler P, Posel C, Kamprad M, Sack U. Allogeneic non-adherent bone marrow cells facilitate hematopoietic recovery but do not lead to allogeneic engraftment. PLoS ONE. 2009;4(7):e6157. doi: 10.1371/journal.pone.0006157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fricke S, Fricke C, Oelkrug C, Hilger N, Schonfelder U, Kamprad M, Lehmann J, Boltze J, Emmrich F, Sack U. Characterization of murine non-adherent bone marrow cells leading to recovery of endogenous hematopoiesis. Cell Mol Life Sci. 2010;67(23):4095–4106. doi: 10.1007/s00018-010-0427-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fricke S. Measurement and illustration of immune interaction after stem cell transplantation. Meth Mol Biol. 2011;690:315–332. doi: 10.1007/978-1-60761-962-8_21. [DOI] [PubMed] [Google Scholar]
- 25.Fricke S, Rothe K, Hilger N, Ackermann M, Oelkrug C, Fricke C, Schonfelder U, Niederwieser D, Emmrich F, Sack U. Allogeneic bone marrow grafts with high levels of CD4(+) CD25(+) FoxP3(+) T cells can lead to engraftment failure. Cytom A. 2012;81(6):476–488. doi: 10.1002/cyto.a.22061. [DOI] [PubMed] [Google Scholar]
- 26.Demehri S, Corbin A, Loriaux M, Druker BJ, Deininger MW. Establishment of a murine model of aggressive systemic mastocytosis/mast cell leukemia. Exp Hematol. 2006;34(3):284–288. doi: 10.1016/j.exphem.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 27.Cooke KR, Hill GR, Crawford JM, Bungard D, Brinson YS, Delmonte J, Jr, Ferrara JL. Tumor necrosis factor- alpha production to lipopolysaccharide stimulation by donor cells predicts the severity of experimental acute graft-versus-host disease. J Clin Inv. 1998;102(10):1882–1891. doi: 10.1172/JCI4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jerome KR, Vallan C, Jaggi R. The tunnel assay in the diagnosis of graft-versus-host disease: caveats for interpretation. Pathology. 2000;32(3):186–190. [PubMed] [Google Scholar]
- 29.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299(5609):1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 30.Lange F, Hartl S, Ungethuem U, Kuban RJ, Hammerschmidt S, Faber S, Morawietz L, Wirtz H, Emmrich F, Krenn V, Sack U. Anti-TNF effects on destructive fibroblasts depend on mechanical stress. Scand J Immunol. 2006;64(5):544–553. doi: 10.1111/j.1365-3083.2006.01840.x. [DOI] [PubMed] [Google Scholar]
- 31.Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucl Acids Res. 2002;30(9):e36. doi: 10.1093/nar/30.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Horneff G, Sack U, Kalden JR, Emmrich F, Burmester GR. Reduction of monocyte-macrophage activation markers upon anti-CD4 treatment. Decreased levels of IL-1, IL-6, neopterin and soluble CD14 in patients with rheumatoid arthritis. Clin Exp Immunol. 1993;91(2):207–213. doi: 10.1111/j.1365-2249.1993.tb05884.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hiepe F, Volk HD, Apostoloff E, von Baehr R, Emmrich F. Treatment of severe systemic lupus erythematosus with anti-CD4 monoclonal antibody. Lancet. 1991;338(8781):1529–1530. doi: 10.1016/0140-6736(91)92353-4. [DOI] [PubMed] [Google Scholar]
- 34.Niederwieser D, Lange T, Cross M, Basara N, Al-Ali H. Reduced intensity conditioning (RIC) haematopoietic cell transplants in elderly patients with AML. Best Prac Res Clin Haematol. 2006;19(4):825–838. doi: 10.1016/j.beha.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 35.Becker C, Bopp T, Jonuleit H. Boosting regulatory T cell function by CD4 stimulation enters the clinic. Front Immunol. 2012;3:164. doi: 10.3389/fimmu.2012.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cobbold SP. T cell tolerance induced by therapeutic antibodies. Philos Trans R Soc Lond B. 2005;360(1461):1695–1705. doi: 10.1098/rstb.2005.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Broker BM, Tsygankov AY, Fickenscher H, Chitaev NA, Muller-Fleckenstein I, Fleckenstein B, Bolen JB, Emmrich F, Schulze-Koops H. Engagement of the CD4 receptor inhibits the interleukin-2-dependent proliferation of human T cells transformed by Herpesvirus saimiri. Eur J Immunol. 1994;24(4):843–850. doi: 10.1002/eji.1830240411. [DOI] [PubMed] [Google Scholar]
- 38.Horneff G, Guse AH, Schulze-Koops H, Kalden JR, Burmester GR, Emmrich F. Human CD4 modulation in vivo induced by antibody treatment. Clin Immunol Immunopathol. 1993;66(1):80–90. doi: 10.1006/clin.1993.1011. [DOI] [PubMed] [Google Scholar]
- 39.Sawitzki B, Kieselbach B, Fisser M, Meisel C, Vogt K, Gaestel M, Lehmann M, Risch K, Grutz G, Volk HD. IFN-gamma regulation in anti-CD4 antibody-induced T cell unresponsiveness. J Am Soc Nephrol. 2004;15(3):695–703. doi: 10.1097/01.ASN.0000115523.50962.C0. [DOI] [PubMed] [Google Scholar]
- 40.Nagahama K, Fehervari Z, Oida T, Yamaguchi T, Ogawa O, Sakaguchi S. Differential control of allo-antigen-specific regulatory T cells and effector T cells by anti-CD4 and other agents in establishing transplantation tolerance. Int Immunol. 2009;21(4):379–391. doi: 10.1093/intimm/dxp005. [DOI] [PubMed] [Google Scholar]
- 41.Yoshida H, Hashizume M, Suzuki M, Mihara M. Induction of high-dose tolerance to the rat anti-mouse IL-6 receptor antibody in NZB/NZW F1 mice. Rheumatol Int. 2011;31(11):1445–1449. doi: 10.1007/s00296-010-1500-8. [DOI] [PubMed] [Google Scholar]
- 42.de La Selle V, Riche N, Dorothe G, Bruley-Rosset M. CD8+ cytotoxic T cell repertoire implicated in grafts-versus-leukemia effect in a murine bone marrow transplantation model. B Mar Transpl. 1999;23(9):951–958. doi: 10.1038/sj.bmt.1701750. [DOI] [PubMed] [Google Scholar]
- 43.Teshima T, Hill GR, Pan L, Brinson YS, van den Brink MR, Cooke KR, Ferrara JL. IL-11 separates graft-versus-leukemia effects from graft-versus-host disease after bone marrow transplantation. J Clin Inv. 1999;104(3):317–325. doi: 10.1172/JCI7111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.