Abstract
Wnt signaling is required for neurogenesis, the fate of neural progenitors, the formation of neuronal circuits during development, neuron positioning and polarization, axon and dendrite development and finally for synaptogenesis. This signaling pathway is also implicated in the generation and differentiation of glial cells. In this review, we describe the mechanisms of action of Wnt signaling pathways and their implication in the development and correct functioning of the nervous system. We also illustrate how a dysregulated Wnt pathway could lead to psychiatric, neurodegenerative and demyelinating pathologies. Lithium, used for the treatment of bipolar disease, inhibits GSK3β, a central enzyme of the Wnt/β-catenin pathway. Thus, lithium could, to some extent, mimic Wnt pathway. We highlight the possible dialogue between lithium therapy and modulation of Wnt pathway in the treatment of the diseases of the nervous system.
Keywords: Neuroprotection, Myelination, Wnt/beta-catenin pathway, Nuclear receptors, Lithium, GSK3β
Introduction
Wnt/β-catenin pathway plays a crucial role in neural development [1–3]: it is required for neural induction and anterior-posterior axis formation [4, 5], neural stem cell proliferation, differentiation and migration [6], axonal outgrowth, guidance and branching [1], dendritogenesis [7], synapse formation and connection [8], and even for remodeling and plasticity in the mature central nervous system (CNS) [9, 10]. Furthermore, the role of Wnt/β-catenin in myelin formation has been addressed [11–14].
Wnt/β-catenin pathway is also implicated in several psychiatric, neurodegenerative [15], and demyelinating pathologies as well as in traumatic brain injuries [16]. In this review, we will first describe the Wnt pathways. Then, we will show how a dysregulated Wnt pathway could lead to severe pathologies of the nervous system. Finally, we will describe the possible relation between lithium therapy and modulation of Wnt pathway.
Overview of the molecular pathways modulated by Wnt signaling
Wnts are secreted factors that mediate paracrine signaling and influence cell behavior by activating several signaling pathways. During development, they can act as morphogens by forming a concentration gradient across a developing tissue [17]. Wnt proteins can be classified in two distinct categories, canonical Wnt proteins defined by their ability to transform or induce a secondary axis, and noncanonical, non-transforming Wnts, unable to induce ectopic axis [18]. They bind to a combination of cell surface receptor (the low-density lipoprotein receptor-related proteins (LRP) 5/6), and members of the Frizzled receptor family, to induce different cellular programs (reviewed in [19])
the canonical Wnt pathway where β-catenin protein is stabilized and influence -Wnt-target gene expression [20],
the Wnt/Ca2 + pathway which stimulates the intracellular release of Ca2 + to activate calcium-dependent mediators [21],
and the noncanonical Wnt signaling or planar cell polarity (PCP) pathways implicated in the establishment of tissue polarity of many epithelia (reviewed in [22]).
Canonical Wnt signaling pathway
It acts through the stabilization of β-catenin that forms a complex with the transcription factor TCF/LEF (T cell lymphoid enhancer factor) to activate target genes expression. TCF/LEF family comprises four members in vertebrates (TCF1, LEF1, TCF3, and TCF4), expressed in a tissue-dependent manner. They function both as transcriptional activators or repressors (reviewed in [23]): TCF1 and LEF1 are activators while TCF3 acts as a repressor, replaced by TCF1 after Wnt stimulation.
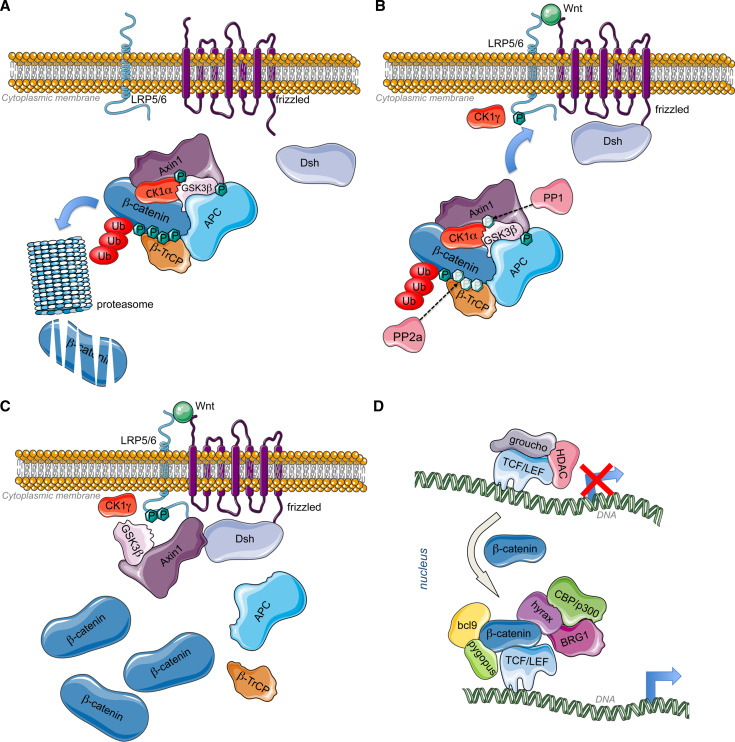
Destabilization of β-catenin protein: In the absence of Wnt ligands, β-catenin is targeted to degradation by a multiproteic complex composed of the scaffolding proteins Axis inhibition protein-1 (Axin1) [24], adenomatous polyposis coli (APC), and the serine/threonine kinases, Casein kinase 1alpha (CK1α) and glycogen synthase kinase 3β (GSK3β). Axin is phosphorylated by CK1α to provide binding site for GSK3β [25]. GSK3β phosphorylates APC. Axin and APC form a scaffold for phosphorylation of β-catenin through CK1α and GSK3β. Those phosphorylations provide binding site for an E3 ubiquitin ligase subunit, the β-transductin-repeat-containing protein (β-TrCP) that targets the ubiquitination of β-catenin and its degradation in the proteasome [26] (Fig. 1a). At the nuclear level in the absence of β-catenin, TCFs bind to transcriptional corepressors, such as the C-terminal binding protein CtBP and Groucho that recruit histone deacetylases (HDAC) and suppress target gene expression by chromatin compaction (reviewed in [27]) (Fig. 1d, upper panel).
Fig. 1.
a Inactive Wnt pathway: Without Wnt ligands, CK1α phosphorylates axin for the binding of GSK3β that in turn phosphorylates APC. The scaffold formed by axin and APC allow the phosphorylation of β-catenin by Axin-bound CK1α and GSK3β [25, 285, 286]. CK1α phosphorylates β-catenin on Ser45, while Ser33, Ser37, and Thr41 are phosphorylated by GSK3β. Those modifications allow the recruitment of β-TrCP that will target ubiquitination and proteasomal degradation of β-catenin [26], leading to constitutive low amount of β-catenin. b Active Wnt pathway 1: When a canonical Wnt ligand binds to Fz receptors at the level of their cystein rich domains (CRD) and to LRP6 coreceptors, the serine/threonine protein phosphatases PP1 and PP2A dephosphorylate Axin and β-catenin, respectively [28]. Concomitantly, Fz recruits Dishevelled (Dsh) at the plasma membrane through its PDZ domain (post synaptic density protein (PSD95), Drosophila disc large tumor suppressor (Dlg1), and zonula occludens-1 protein (Zo-1)). Axin-GSKβ complex is then recruited by LRP5/6 coreceptor via the DIX domain of Dsh, responsible of Dsh polymerization. The plasma membrane-associated GSK3β along with CK1 gamma (CK1γ) phosphorylate LRP5/6 at conserved PPSP motifs (Pro-Pro-Ser-Pro) [29, 287]. This process also requires PFTK and CyclinY [288]. Dsh is also involved in the regulation of GSK3β and Axin internalization by inducing their sequestration in multivesicular bodies [289]. c Active Wnt pathway 2: Following Axin-GSK3β recruitment to the plasma membrane, β-catenin is stabilized by a decrease in GSK3β -mediated phosphorylation through PP2A. Moreover, Dsh can recruit PtdIns 4-kinase type II (PI4KIIa) and PtdIns-4-phosphate 5-kinase type I (PIP5KI) to stimulate the formation of phosphatidylinositol 4,5-bisphosphate (PtdIns(4,5)P2) [290]. Both PDZ and DEP (Dishevelled, Egl-10 and Pleckstrin) domains of Dsh interact with PI4KII, whereas its DIX (DIshevelled and aXin) domain binds to and activates PIP5KI. The resulting ternary complex allows PtdIns(4,5)P2 formation, required for clustering and phosphorylation of LRP6. Finally, the trimeric GTP-binding protein Gαo [291] and the βγ-subunits of G protein also participate to Dsh functioning. While βγ-subunits of G protein bind and relocalize Dsh to the plasma membrane, the α-subunit of G protein interacts with and recruits Axin to the plasma membrane, consequently leading to β-catenin stabilization [292, 293]. Gβγ may also participate to the negative feed-back regulation of Dsh activity via the activation of the lysosomal degradation of Dsh in mammalian cells. As a consequence, β-catenin accumulates in the cytoplasm and translocates to the nucleus to bind DNA-bound TCFs and induce transcriptional activation. d Genomic action of β-catenin: TCF are transcription factors that bind to specific response elements, the TCFE (TCF response elements) located at the level of Wnt-responsive promoters. They interact with DNA through their high mobility group (HMG) domain and recruit β-catenin via their catenin-binding domain (CBD). β-catenin bound to TCF recruits Bcl9 and pygopus (pygo) at the level of its N-terminal transactivation domain (NTD) [33, 294, 295]. In addition, Hyrax (Hyx)/Parafibromin binds to the C-terminal transactivation domain (CTD) of β-catenin for activation of target gene expression [34]. Dsh has also been found to participate to transcriptional activation of Wnt target genes via its interaction with β-catenin (and c-jun) [296]. Subsequently, chromatin remodeling enzymes are recruited such as histone acetyltransferases (HATs) CBP/p300 and SET1, and brahma-related gene 1 (BRG1) [35, 297, 298]
Stabilization of β-catenin: Wnt ligand binding to the CRD of Frizzled (Fz) receptors and to LRP6 coreceptors, triggers the dephosphorylation of Axin and β-catenin by PP1 and PP2A, respectively [28]. Concomitantly, Disheveled (Dsh) is recruited at the level of plasma membrane by Frizzled (Fz) while Axin-GSKβ complex is targeted at the cytoplasmic tail of the LRP5/6 coreceptor phosphorylated by CK1γ [29] (Fig. 1B). Axin-GSK3ß clustering at the plasma membrane triggers the dissociation of the destruction complex, leading to β-catenin stabilization and accumulation (Fig. 1C).
β-catenin nuclear translocation: β-catenin is known to dynamically shuttle between the cytoplasm and nucleus. However, the protein does not contain any nuclear localization signal (NLS) or nuclear export signal (NES) and its nuclear import occurs in an importin-karyopterin independent mechanism [30]. β-catenin would directly interact with different nuclear pore complex components [31] to pass through the nuclear pores. This process requires the phosphorylation of the Tyr654, located in the last ARM repeats of β-catenin [31]. Another phosphorylation site has been implicated in β-catenin nuclear import, via Rac-activated JNK2 that phosphorylates β-catenin at serine residues 191 and 605 to promote β-catenin nuclear localization [32].
Stimulation of Wnt-responsive genes transcription: The binding of β-catenin by TCF allows the recruitment of Bcl9 and pygopus (pygo) [33]. In addition, Hyrax (Hyx)/Parafibromin binds to β-catenin for activation of target gene expression [34]. Subsequently, histone acetyltransferases (HATs), such as CBP/p300, and chromatin remodeling enzyme brahma-related gene 1 (BRG1) are recruited to create a permissive environment for target gene expression [35]. β-catenin/TCF complex can also recruit the histone methylase SET8 to mediate histone monomethylation of some Wnt target genes and induce their expression [36] (Fig. 1D, right panel).
Wnt/Ca2 + pathway
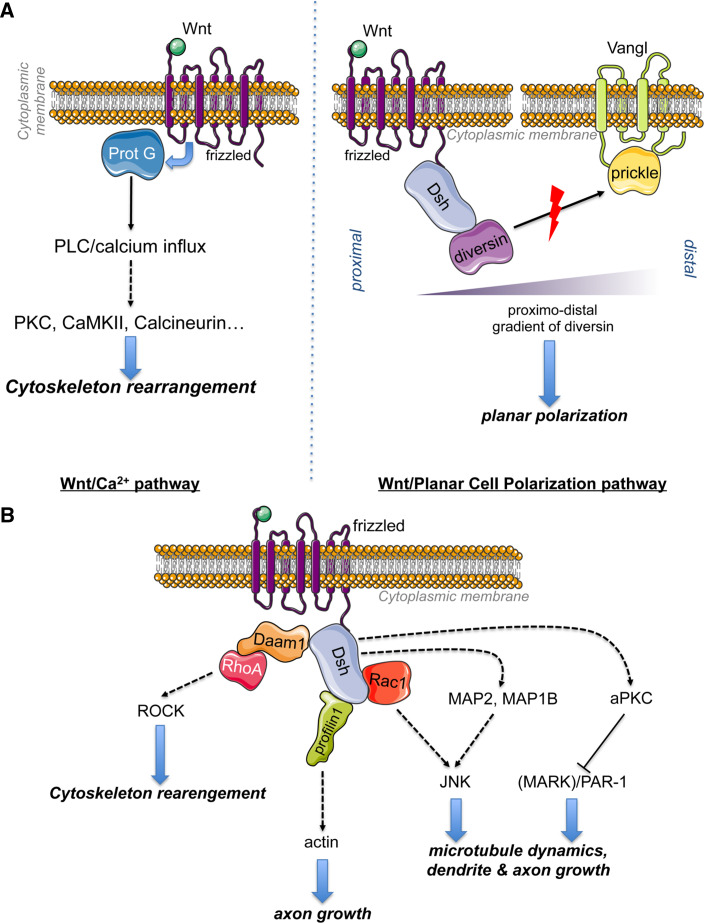
Wnt/Ca2 + signaling involves the action of Wnt proteins and Fz receptors to activate G proteins that activate phospholipase C, generating diacylglycerol and IP3 to allow intracellular calcium fluxes [37]. This sudden release Ca2 + activates Ca2 + -dependent modulators, such as protein kinase C (PKC), calcium calmodulin mediated kinase II (CAMKII), and the calcium-sensitive phosphatase calcineurin [38]. The Wnt/Ca2 + -pathway leads to cytoskeleton rearrangement and has been implicated in cancer via the induction of epithelial to mesenchymal transition (EMT) [39] (Fig. 2A, lower panel).
Fig. 2.
a Wnt/Ca2 + pathway: binding of Wnt proteins on Fz receptors that signal through G proteins to activate phospholipase C leading to diacylglycerol and Inositol-triphosphate (IP3) generation. The resulting transient release of calcium from intracellular stores activates Ca2 + -dependent modulators, such as protein kinase C (PKC), calcium calmodulin mediated kinase II (CAMKII) and calcineurin to remodel the cytoskeleton. Wnt/PCP pathway: Fz–Dsh complex localized distally and Vangl-prickle localized proximally compete for Diversin recruitment. Upon Wnt stimulation, Diversin is recruited by Dsh to promote its activation to inhibit Prickle activity. b alternative pathways: Daam-1 (Dsh associated activator of morphogenesis 1) is a cytoplasmic protein that exists at an autoinhibited state [299]. Daam-1 is used by Dsh to interact with the small GTPase RhoA. Wnt stimulation leads to the disruption of the intramolecular inhibition of Daam1 [46]. The resulting activation of Daam-1 leads to the recruitment of the RhoA to activate ROCK kinase for cytoskeleton remodeling [47]. Daam-1 can also act through the actin-binding protein Profilin1 to modulate actin stability for axon growth [48]. Dsh can also activate Rac1 to stimulate c-JunN-terminal kinase (JNK) implicated in dendritic growth [7]. Alternatively, Dsh activates JNK1 through the phosphorylation of MAP2 and MAP1B for the modulation of microtubule dynamics. Dsh regulates atypical PKC (aPKC) activity [51] to inhibit Par-1 [52] or microtubule affinity regulating kinase 2 [51], inducing microtubule assembly and axon formation
Planar cell polarity pathway
The PCP (planar cell polarity) signaling is mediated by Fz and Dsh independently of β-catenin [40]. It is transmitted locally from cell to cell to polarize cells in an epithelium. The initiation of PCP signaling requires two complexes relocated at opposing sides of each cell: the Fz–Dsh–Diversin complex localized distally and the Vangl-prickle complex localized proximally, leading to planar polarization (reviewed by [40]). Diversin promotes Dsh activation that in turn inhibits Prickle by competing its binding to diversin [41]. Prickle can no longer prevent Dsh membrane location to antagonize its activity [42] (Fig. 2A, right panel). Moreover, Dsh directly modulate Prickle stability by recruiting E3 ubiquitin ligase Smurfs to the Fz–Dsh complex with Par6, leading to a Smurf- and Dsh2-dependent degradation of Prickle [43].
Dsh at the crossroads of various Wnt signaling
The molecular hub of Wnt signaling can modulate diverse protein kinases activity by either direct or indirect action, leading to stabilization of actin fibers [44] and microtubules [45] (Fig. 2B)
Dsh interact with the small GTPase RhoA through Daam1 recruitment [46]. Following recruitment of the Rho guanine nucleotide exchange factor WGEF, the Rho-GTP complex activates ROCK kinase and remodel cytoskeleton [47]. Daam-1 can also recruit the actin-binding protein Profilin1 for the control of axon growth [48].
Dsh can activate JNK1 through another small GTPase of the Rho family, Rac1, for the modulation of dendritic growth [7]. This kinase is also regulated by Dsh-dependant phosphorylation of MAP2 and MAP1B in a process that also requires GSK3β inhibition [49] for the regulation of microtubule dynamics [50].
Dsh directly regulates atypical PKC (aPKC) activity to induce microtubule assembly and axon formation [51]. Dsh activates aPKC by increasing the amount of phospho-aPKC, that subsequently impairs the kinase activity of Par-1 [52] or microtubule affinity regulating kinase 2 [51].
The following section will focus on Wnt importance in nervous system development and functioning.
Wnt: a critical pathway in the nervous system
Wnt signaling is essential for patterning, cell fate specification and stem cell regulation during the development of many tissues including the mammalian brain [1]. Many constituents of Wnt signaling pathways are expressed in the developing and mature nervous systems. Wnt signaling controls initial formation of the neural plate and many patterning decisions in the embryonic nervous system, including formation of the neural crest [53]. But Wnt signaling continues to be equally important at later embryonic and postnatal stages [54]. Besides, Wnts have been shown to regulate the neuronal cytoskeleton [55] and the differentiation of synapses in the cerebellum [56]. In the adult brain, Wnt signaling also regulates numerous critical processes such as cell migration, cell polarization, neurite outgrowth, axon remodeling, synapse formation and plasticity, and neurogenesis [3, 9].
Wnt signaling during development of the nervous system
During development of the vertebrate central nervous system, Wnt signaling regulates several crucial processes such as patterning and cell fate specification, proliferation and neuronal morphology.
Patterning: In the early vertebrate embryo, Wnt signaling promotes posterior development and suppresses anterior development of the neural tube. At later stages, Wnt signaling is also essential for dorsal/ventral patterning of the neural tube and for the specification of the neural crest [1].
Proliferation: Wnt signaling also regulates proliferation of neural precursor cells throughout CNS development. Wnt/β-catenin signaling promotes progenitor proliferation in the developing neural tube as well as the midbrain and hippocampus.
Axon growth, guidance and remodeling: In addition to regulating patterning and cell proliferation, Wnts are involved in axon guidance and remodeling. GSK3β inhibitors promote neurite outgrowth and axon formation, and increase axon size and branching in many cell types, including cerebellar granular cells, dorsal root ganglion neurons and hippocampal neurons [57]. The stabilization of β-catenin may play a role by indirectly affecting cadherin-mediated adhesion [58]. Furthermore, Wnts and Dsh influence microtubule stability, hence changing the dynamic of cytoskeleton. As this occurs via the canonical pathway through inhibition of GSK3β, it indicates a potential role for the canonical pathway in axon remodeling [1].
Wnt induces a loss of the directionality of microtubule growth resulting in the formation of looped microtubules. These changes in microtubule behavior are also associated with decreased growth cone translocation but increased growth cone size and branching [56]. These effects are the signals that regulate the terminal remodeling of axons before synapse formation.
Wnts can function as both chemoattractive and chemorepulsive cues in axon guidance, depending on the receptors. Wnts act as attractants to regulate commissural axon guidance after crossing the midline in the developing spinal cord in a PI3 K and APC-dependent manner but independently of LRP6 [59].
Synapse formation: Wnts and β-catenin also promote dendritic growth and branching, and synaptic formation [9]. There is evidence of a role for Wnt ligands in synaptic transmission and plasticity as well.
Wnts promote the assembly of central synapses by stimulating the recruitment of pre and postsynaptic components. Different responses could be triggered by the presence of distinct Wnt receptors in dendrites and axons. Moreover, several studies provide evidence for a link between neuronal activity and Wnt signaling. A role for Wnt signaling in synaptic plasticity has recently emerged [9]. Several studies now indicate that Wnts mediate changes in synaptic number and plasticity elicited by neuronal activity.
The canonical pathway has been involved in a variety of genetic regulation driving specific effects within the neural tissue:
Repression of Nkx2.2 expression in the ventral part of the neural tube is mediated by Wnt pathway in a TCF4 dependant manner leading to dorsalization of the embryo [60].
Expression of the homeobox gene Gbx2 is directly activated by Wnt/β-catenin pathway to induce neural crest specification [61].
Expression of lrx3/six3 and Gbx2 are modulated by Wnt pathway to sharpen forebrain architecture. Wnt first induces the expression of lrx3 and represses the expression of Six3 to delineate anterior and posterior forebrain. In later stages, continuous Wnt activity will drive Gbx2 expression to maintain posteriorization of the frorebrain [62].
Otx2 expression is directly regulated by Wnt1 together with lmx1 to orchestrate the morphogenesis of the midbrain [63].
expression of the transcriptional repressor sp5 is induced by Wnt/β-catenin pathway in the telencephalon leading to repression of sp1 target genes in this tissue [64].
neurogenin expression is induced in cortical neurons by Wnt/β-catenin pathway to drive the differentiation of adult neuronal population [65].
BDNF production is induced by β-catenin stabilization within spinal cord-derived neural progenitor cells (NPC) leading to enhanced neuronal differentiation [66].
the expression of the pro-neurogenic factor NeuroD1 is positively regulated by Wnt in adult hippocampal NPC to mediate neurogenesis [67].
Cav3.1 calcium channel gene expression is induced by LEF1/β-catenin complex to modulate thalamic neuronal activity [68].
Myelin protein zero (MPZ) and proteolipid protein 22kD (PMP22) expression are directly regulated by Wnt/β-catenin pathway within Schwann cells. By modulating myelin genes expression, our team demonstrated that Wnt/β-catenin pathway is a direct driver of peripheral re/myelination processes [12].
Wnt signaling in adult neurogenesis
Although significant progress has already been made in deciphering the roles of Wnt signaling during neural development, little is known about the roles of Wnts in the adult nervous system. Nevertheless, the persistent expression of Wnt ligands and Wnt signaling components in the mature mammalian CNS suggests that this pathway might also play a part in synaptic maintenance and function and protecting neuronal connections throughout the entire lifespan. Wnt have a crucial role in synaptic physiology, as they modulate the synaptic vesicle cycle, the trafficking of neurotransmitter receptors with scaffold proteins in postsynaptic regions [10].
It is now establish that Wnt pathways modulate fundamental aspects of the adult CNS, such as adult neurogenesis and synaptic stability and plasticity in some brain region (reviewed in [10]). Recent studies also provide evidence that Wnt signaling enhances proliferation of neural stem cells derived from the adult CNS and promotes proliferation of neural progenitor cells and hippocampal neurogenesis in vivo. These emerging roles of Wnt pathways in adult suggest that targeting Wnt could offer therapeutic benefits. Moreover, the ability of Wnt to dialogue with other signaling pathways highlights the possibility to subtly modulate pathological tissular/cellular processes. Drugs capable of modulating Wnt signaling thus appear as potential tools for regenerative or neuroprotective medicine.
Interplay between Wnt and nuclear receptor pathways in the nervous system
Glucocorticoid receptor and Wnt
Glucocorticoids (GCs) play a major role in the nervous system. They regulate energy metabolism, cell proliferation, as well as immune responses, and they also promote transcription of genes expressed in neurons and glial cells. GCs enhance myelin formation in both the central and peripheral nervous system, and they also stimulate the proliferation of Schwann cells. GCs are implicated in stress. High levels of glucocorticoids have devastating impact on neurogenesis.
In most cases, GCs actions are mediated by their cognate nuclear receptor, the GCs receptor (GR). Binding of GCs to GR provokes the interaction with glucocorticoid response elements (GREs) in the promoter region of target genes and the recruitment of specific coactivators, such as p160 family members. The GR–p160 complex recruits secondary coactivators, cAMP-response element binding protein (CREB)- binding protein (CBP) or its close homologue p300.
We have shown that in Schwann cells neither CBP nor p300 were involved in GR transcriptional activation. Unexpectedly, p300, considered as a coactivator of the GR, acted as a corepressor. In Schwann cells, β-catenin replaced CBP in the GR transcriptional complex. Furthermore activation of the GR enhanced the amount of β-catenin in Schwann cells, which is recruited by both TCF/LEF transcription factors and GR. This observation shows a positive interplay between Wnt/β-catenin pathway and GR [69].
But this synergistic cross-talk cannot be generalized to other tissues. It is well known that stress inhibits neurogenesis. This deleterious effect is mediated by high levels of glucocorticoid hormones secreted during stressful conditions. In the hippocampus, GCs stimulate the expression of Wnt inhibitor Dickkopt-1 (Dkk1) to induce stress damages. In this case, GCs have an inhibitory effect on canonical Wnt pathway involved in neurogenesis [70]. Furthermore, GCs decreases the levels of β-catenin in the hippocampus by increasing its phosphorylation by GSK3β. To the opposite, Leptin, an adipocyte-derived hormone with antidepressant-like properties, promotes baseline neurogenesis in the adult hippocampus by increasing total level and nuclear translocation of β-catenin [71].
Thyroid hormone receptor and Wnt
Thyroid hormones (THs) [thyroxine (T4) and 3,5,3′-tri-iodothyronine (T3)] play a critical role in development and function of the nervous system [72, 73]. Severe hypothyroidism during the rat neonatal period leads to structural alterations of the CNS, which include hypomyelination and defects of cell migration and differentiation, with long-lasting, irreversible effects on behavior and performance. THs are also essential in fetal brain development [74]. In adult humans, THs deficiency or excess, causes reversible neurologic and psychiatric symptoms [75]. THs seem also to be essential for peripheral nerve repair [76] beyond their role in correct development and maturation of the PNS. T3 allows remyelination in adult mouse brain following chronic demyelination and appears to be an inducer of oligodendrogenesis in vivo through the expression of Sonic Hedgehog and Oligo2 transcription factors [77]. Liganded thyroid hormone receptor alpha (TRα) is critical for neurogenesis in the adult mouse brain [78]. THs signaling acts as a neurogenic switch by transcriptional repression of Sox2 in the adult stem cell niche [79].
T3, which is the predominant ligand for nuclear Thyroid Hormone Receptors (TRs), is considered to be the active form of TH. Fractional TR occupancy in the brain is determined by intracellular T3 concentration regulated by multiple factors including THs production and secretion by thyroid gland, the activity of membrane transporters and local TH metabolism catalyzed by D2 and D3 [75, 80]. In the nervous system, D2, which activates T4 into T3, and D3, which degrades T4, and T3, are regulated by multiple pathways [81, 82].
THs and their receptors TRs, which control cell proliferation or cell differentiation depending on tissue context, have been described in different systems to function in synergy and/or antagonism with Wnt signaling [83, 84]. TH-TRα1 contributes to the process of intestinal maturation and its homeostatic control via the complex modulation of signaling pathways including direct transcriptional control of β-catenin and sFRP2 [83, 84]. Activation of TRβ1 by T3 suppresses the β-catenin/TCF1-mediated induction of cyclin D1 expression in human embryonic kidney and colon carcinoma cell lines [85]. Moreover, both TRα1 and TRβ1 can interact with β-catenin to form a complex and the Wnt pathway is then activated by TRα1 and blocked by TRβ1 in the intestinal epithelium and in thyrocytes, respectively [86, 87]. TH-mediated growth and differentiation of chondrocytes involves IGF-1 modulation of β-catenin signaling [88]. Although Wnt signaling is inhibited by T3 in osteoblastic cells, Wnt signaling is activated in vitro and in vivo in osteoblastic cells from mice with a dominant-negative mutation in TRβ gene demonstrating the interaction between TH and canonical Wnt signaling pathways during bone development [89]. TH-TR induces a secreted antagonist of Wnt/β-catenin pathway, Dkk-4, which suppresses cell invasion in human hepatoma cells [90]. Finally, β-catenin down-regulates D2 expression and induces D3 expression in colon cancer cells and subsequently down-regulates intracellular T3 concentration, thus favoring the proliferation of these cells [91].
Up to date, few data indicate a possible dialog between TH-TRs and Wnt pathway in nervous system. Rat pituitary cells that express TRα1, TRβ1 and TRβ2 and are induced to proliferate by T3, respond to T3 by a decreased β-catenin expression and stability, and a decreased TCF mediated transactivation [92]. In hippocampus, postnatal TH deficiency produces a decrease in neurogenesis and Wnt3a expression [93]. T3 which blocks the proliferation of oligodendrocyte progenitor cells and induces their differentiation into oligodendrocytes [94], has been recently shown to accelerate, in presence of the mitogen PDGF, the expression of SFRP1 and Sox17, which antagonize the Wnt/β-catenin [95].
Liver X receptor and Wnt
Oxysterols are natural compounds originating from the enzymatic oxidation of cholesterol. There are different oxysterols, in particular 24(S)–OH, 25–OH and 27–OH, which are, respectively synthesized by means of the cholesterol hydroxylase CYP46A1, CH25H and CYP27A1α [96]. They are implicated in cholesterol turnover, inflammation and in neurodegenerative diseases such as alzheimer’s disease [97] and multiple sclerosis [98, 99]. Glial cells are also targets for the oxysterol action: they inhibit astrocyte proliferation after brain injury [100] and cause oligodendrocyte apoptosis [101]. Oxysterols are natural ligands for LXR [102], which has two isoforms: LXRα and LXRβ. LXRs regulate gene expression by binding to responsive elements (LXRE). In the absence of ligands, LXRs bind to corepressors [103]. In response to oxysterols binding, they interact with coactivators to transactivate [104].
Studies with transgenic mice revealed important roles of LXRs within the nervous system [105]. LXRβ-/- or LXRα/β-/- transgenic mice show several defects like axonal atrophy, neuronal loss, astrogliosis and lipid accumulation in specific brain regions. We have demonstrated that the knockout of LXR in mice results in thinner myelin sheaths surrounding the axons of sciatic nerves [106]. Furthermore, oxysterols, via LXR, inhibit peripheral myelin genes expression (MPZ, PMP22) in Schwann cells. Oxysterols repress myelin genes via two mechanisms: by binding of LXRs to myelin gene promoters and by inhibiting the Wnt/β-catenin pathway. The Wnt signaling components (Dsh, TCF/LEF, β-catenin) are strongly repressed by oxysterols. Furthermore, the recruitment of β-catenin at the levels of the MPZ and PMP22 promoters is decreased. Interestingly, we observed a differential regulation of Wnt/β-catenin signaling by LXR in Schwann cells and oligodendrocytes [107]. Indeed, the transcripts of Wnt components (Dsh, TCF3, β-catenin) are activated in oligodendrocytes. Furthermore, after incubation with natural oxysterol, β-catenin is re-localized on the level of the Golgi apparatus of Schwann cells, but not of oligodendrocytes. Our findings reveal a complex cross-talk between LXR and Wnt/beta-catenin pathway in myelinating glial cells. LXR and Wnt pathways have been already shown to interfere in other tissues. LXR agonist suppresses the oncogenic activity of β-catenin to decrease the proliferation in human colon cancer cells [108]. The reduction of β-catenin was explained by direct interaction between LXRs and β-catenin, through its Armadillo repeats [109].
While natural Wnt ligands exhibit pleiotropic roles in cellular and tissular physiology, the possibility to modulate GSK3β activity through a single ion (lithium) allows envisaging GSK3β-inhibition based therapies that at least in part involve Wnt signaling modulation.
Mechanisms of action of lithium
Lithium salts have been extensively used as first-line drugs to treat psychiatric diseases, bipolar disorder in particular. Although the beneficial effects of lithium therapy have been known for decades, the mechanism of Li + action remains poorly understood. Several hypotheses have been put forward which, taken together, imply that lithium may exert its therapeutic effect via diverse and multifaceted pathways.
Li + - Na + competition in the cytosol
Clinical studies on patients with mental disorders have detected elevated levels of intracellular Na + in neurons. This has been attributed to abnormalities of Na,K-ATPase expression which decreases its activity and leads to Na + retention [110]. The hypothesis postulates that lithium enters the cell via sodium channels or transporters and accumulates in the cytosol thus competing with sodium. Increased levels of lithium decrease the intracellular levels of sodium, which in turn reduce the levels of cytosolic calcium. Indeed, prolonged lithium treatment of bipolar patients has been observed to increase the cellular concentration of Li + and increase the activity of Na,K-ATPase with simultaneous decrease of sodium and calcium levels in the cell [111]. Lowering the concentrations of intracellular sodium and calcium decreases the cell excitability and eventually normalizes the neuron activity in bipolar patients [110, 111].
Li + affects neurotransmitter signaling
Lithium may interact with specific sites/receptors that regulate the synthesis, release, turnover and reuptake of neurotransmitters such as dopamine and serotonin. For example, it has been demonstrated (by both in vitro and in vivo experiments) that lithium can bind to different synaptic serotonin receptors and stimulate an increased serotonin release [112]. Thus, it is possible that the curative effect of lithium is related, at least partly, to its ability to regulate/modify neurotransmitter signaling in the central nervous system [113].
Li+ −Mg2+ competition for protein binding sites
It has been hypothesized that the “alien” Li+ competes with the native Mg2+ for protein binding sites and, by replacing the native co-factor, inhibits key enzymes involved in specific neurotransmission pathways in the brain. These metalloproteins, expressed at elevated levels in bipolar patients, include G-proteins, GSK3β, inositol monophosphatase, inositol polyphosphate 1-phosphatase, fructose 1,6-biphosphatase, bisphosphate nucleotidase and phosphoglucomutase. Thus, Mg2+ → Li+ exchange in these systems affects a variety of processes in the CNS such as neuronal division, apoptosis, neuronal excitability and responsiveness, gene expression, cellular structure and resilience, synaptic plasticity, and circadian cycle [114].(see below). Particularly, lithium can mimic Wnt activation by inhibiting GSK3β to stabilize β-catenin. It acts directly via competition with magnesium and indirectly by increasing inhibitory serine-phosphorylation of GSK3β.
Note that lithium exhibits strong selectivity toward those particular proteins implicated in mental disorders, while other magnesium proteins, essential for the cell normal functioning and survival (e.g. ATPases and nucleases), remain functional and unaffected by lithium treatment.
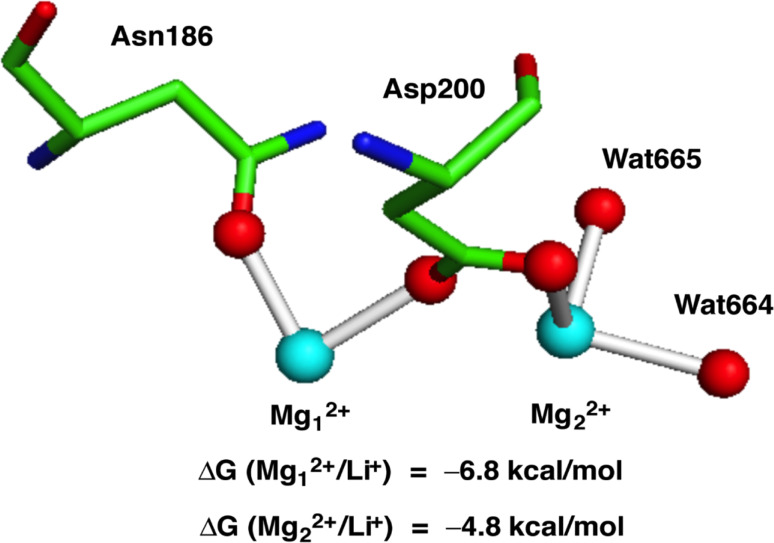
Several experimental and theoretical studies lend support to this hypothesis. In vitro experiments have demonstrated that Li+ can displace Mg2+ and act as a potent inhibitor for G-proteins [115], GSK3β [116, 117] and inositol monophosphatase [118, 119]. Computational studies (DFT calculations and molecular dynamics simulations) provide further insights into the mechanism and factors that govern the Mg2+ → Li+ substitution in these systems [120, 121]. The calculations shed light on the fascinating question of why the Mg2+ → Li+ replacement takes place only in certain enzymes related to bipolar disorder, but not in other magnesium enzymes essential for cells. Enzymes where Li+ can replace Mg2+, such as GSK3β (Fig. 3) and inositol monophosphatase, possess magnesium binding sites that are solvent exposed and characterized with high positive charge density. On the other hand, essential magnesium enzymes are known to have functional magnesium binding sites that are deeply/partially buried and possess high negative charge density.
Fig. 3.
Protein data bank (PDB) structure of metal binding site (PDB code 1PYX) and free energies of Mg2+ → Li+ exchange in GSK3β. Asn asparagine, Asp aspartic acid, Wat water
Neuroprotection by Wnt pathway and lithium
Many CNS disorders can be correlated with dysregulation of Wnt/β-catenin pathways. Furthermore, numerous studies report neuroprotective effects for lithium and involvement of Wnt/β-catenin signaling in the prevention of neurodegenerative diseases.
Mechanisms of Wnt and Lithium’s neuroprotective action
Role in neural developement and adult brain functioning
Wnt/β-catenin signaling mediates critical neuronal developmental processes. It provides early cues within the embryo to guide anterio-posterior axis specification of the neural plate, and it is involved in neural progenitor proliferation and differenciation during mammalian brain development, axon and dendritic growth, and synapse formation. Wnt/β-catenin also regulates adult brain functions (reviewed in [2, 3]), and induce remodeling and plasticity in the mature CNS [9]. Given the multiple actions of this pathway in the CNS, the neuroprotective effects of lithium can be easily considered.
Role in cytoskeleton modulation
One of the neuroprotective hypotheses involves β-catenin, able to regulate gene transcription, as well as anchorage of the actin cytoskeleton via cadherins and α-catenin interactions. Consequently, β-catenin is able to induce cytoskeleton modulation and precise changes in cellular morphology. Indeed, it has been shown that β-catenin regulates dendritic arborization, neuritogenesis and synaptic plasticity [122, 123], providing an explanation for lithium neuroprotective effects.
Disruption of the dopamine receptor complex
Lithium has been shown to inhibit GSK3β in a direct manner, by acting as a competitive inhibitor of Mg2+ ,a cofactor for GSK3β [117]; but it also can interfere indirectly with the regulation of GSK3β by activating Akt. Indeed, lithium can inhibit the behavioral action of monoamine such as dopamine, by dissociating the interaction of Akt with β-arrestin2 and PP2A, a dopamine D2 receptor-mediated complex that inactivates Akt. Furthermore, the Akt/GSK3 signaling is potently regulated by changes in monoamine homeostasis in vivo, and deregulation in this pathway are frequent in various CNS disease (reviewed in [124]). Downstream targets of Akt and GSK3 need to be identified.
Role in glutamatergic regulation
Surprisingly, a recent study suggested a β-catenin independent pathway to support some beneficial behavioral effect of lithium. Therefore, genetic stabilization of β-catenin in dopaminergic D2 neurons did not mimic antipsychotic action of lithium in behavioral tests [125]. The authors proposed other downstream targets of D2R-mediated GSK3 signaling, such as regulation of glutamate receptors [126]. Indeed, glutamate related excitotoxicity is involved in several neurodegenerative mechanisms, and numerous studies described the role of GSK3β inhibition in protecting neurons from glutamate excitotoxicity. This protective mechanism by lithium involves the regulation of NMDA receptors expression and the modulation of their activity [127]. Glutamatergic regulation mediated by lithium is associated with LTP preservation [128], enhanced BDNF expression [129] and MAP kinase pathway regulation via CREB phosphorylation [130].
Role in cholinergic regulation
Other possible mechanisms of action could involve cholinergic receptors, shown crucial in multiple anatomical, electrophysiological, biochemical, and behavioral studies. The cholinergic system appears essential for cortical development, synapse formation as well as complex brain functions including sleep and memory [131]. Furthermore, many studies describe the role of cholinergic receptor in depressive disorders [132, 133] and their activation has been shown to improve symptoms in schizophrenic patient [134]. Interestingly, Wnt-7a, by dissociating APC from β-catenin complex, is able to induce the interaction of APC with a7-nAChRs in hippocampal neurons, leading to cholinergic receptors expression and relocalization to presynaptic sites [135]. Finally, recent studies highlighted the involvement of Wnt/β-catenin in synapses organization and control of the localization of nicotinic receptor synapses, reinforcing the idea of Wnt/β-catenin importance in cholinergic synaptic plasticity [136].
Anti-apoptotic and pro-survival effects
The control of apoptosis by Wnt/β-catenin signaling and lithium is a key event in the regulation of pathophysiological mechanisms that can also be considered. Indeed, inhibition of GSK3β reduces proapoptotic mediator expression and induces anti-apoptotic protein expression [137, 138]. It also stimulates association of p53 with Bcl2 and Bax oligomerization [139]. Survival signaling pathways such as PI3 K/Akt and MEK/ERK pathways can also be modulated by Lithium [140].
Lithium effects: an endocrine disruptor?
Some effects of lithium in nervous system might be relevant of its modulation of TH secretion, metabolism and signaling pathways. In about 20 % of patients treated with lithium for mood disorders, lithium is able to promote hypothyroidism principally by blocking TH secretion [141]. This hypothyroidism is currently treated with T4 without to stop lithium therapy. Lithium therapy also affects hypothalamic-pituitary axis with exaggerated TSH response to TRH in at least 50 % of patients [141]. Lithium effects changes in T3 content and T4 metabolism in rat anterior pituitary tissue [142]. D2 activity inhibition by lithium is noted in the anterior pituitary gland of thyroidectomized rats injected 3-24 h earlier with lithium as also in cultured GH3 pituitary cells and neuroblastoma cells [142]. Rats treatment for 14 days with lithium at dosages used in affective disorders, has been described to increase T4 and T3 serum concentrations and to affect TH metabolism by decreasing D2 and D3 activities in the frontal cortex and other brain regions [143]. Higher dosages of lithium decrease T4 and T3 serum concentrations and affect TH metabolism by increasing D2 activity and decreasing D3 activity in different brain regions [143]. Tissue or nuclear T3 concentration in any explored brain area are not affected [144, 145]. Administration of lithium for 14 days increases T3 levels in synaptosomes of the rat amygdala [145]. Finally, lithium also affects gene expression of TR in rat brain and rat pituitary cells [146, 147].
Involvement of Wnt and lithium in psychiatric, neurodegenerative and demyelinating diseases
In the next section we will summarize the implication of Wnt and Lithium in some psychiatric, neurodegenerative and demyelinating diseases (Table 1).
Table 1.
Role of lithium and Wnt signaling in neurodegenerative and demyelinating diseases as well as CNS injuries
| CNS disease | Studied molecule | Molecular function | Effects in the nervous sytem | References |
|---|---|---|---|---|
| Mood disorder | GSK3β gene variant or copy number | Inhibition of β catenin | Increases the risk of bipolar disorder | [148, 149] |
| GSK3β activation | Inhibition of β catenin | Mania | [155, 156] | |
| GSK3β inhibition | Activation of β catenin | Antidepressant. anti-maniac | [124, 152, 153] | |
| Lithtum | Dissociates the interaction of βamecstin2 and PP2A | Inhibits the behavioral action of dopamine | [124] | |
| FZD3, GSK-3IV and DKK4 | Involved m Wnt pathway | Correlated with risk of schizophrenia | [163–165] | |
| APC | Negative regulator of Wnt signaling | Associated with schizophrenia | [106–168] | |
| Lithium | Inhibition of GSK36 | Clinical effect in patients | [169] | |
| DJSC1 and Dixdc1 | Involved neuronal progenitor proliferation and regulate Wnt pathway | Associated with schizophrenia and bipolar disorder symptoms | [172, 176–179] | |
| Wn12 | Involved miR16 expression | Anti-apoptotic | [181, 182] | |
| Alzheimer’s | GSK3β activity | Reduces βcatenin level | Increased in AD patients | [183, 184] |
| Disease | Wni3A and Lithium | Inhibition of GSK3β | Decreases Aβ production and in vitro toxicity | [185–189] |
| Lithium | Inhibition of GSK3β | Regulates tau degradation | [190–195, 198–200] | |
| Lithium | Inhibition of GSK3β | Improves behavioral deficit in mouse model for AD | [186, 196, 197] | |
| FZD1 | Receptor for Wnt | Binds t0 Aβ; reduces Aβ neurotoxicity | [202, 203] | |
| Dkk1 | Wnt antagonist | Participates in Aβ toxicity. increased in AD patients | [204–206] | |
| M1 mAChR | Muscarinic receptor able to inhibits GSK3β | Activates Wnt pathway. and reverses Aβ toxixity | [209] | |
| IBU-PO, IL1β blocking | Inhibit inflammatory effects of GSK3 < i | Protects against Aβ in vitro and ameliorates AD mouse symptoms | [211, 212] | |
| Lithium | Inhibition of GSK3β | Clinical use in AD | [214–219] | |
| Huntington disease | Lithium | Inhibition of GSK3β | Reduces apoptosis and huntingtin degradationcaipain inhibitioninduction of autophagy | [222–225][226][227] |
| βcatenin | Transducer of the Wnt pathway | Stabilized by huntingtinimproves motor and behavioral performance and exerts neuroprotective effects in animal models of HO | [228][229–233] | |
| Parkinson’s disease | Lithium | Inhibition of GSK3β | Neuroprotective effects in vitroneuroprotective effects in animal model of PDprotection against oxidative stress activates autophagic pathway | [235–237][238–241][242, 243][244, 245] |
| Dkk 1 | Wnt antagonist | Induces neurotoxicity | [247] | |
| Traumatic brain injury | Lithium | Inhibition of GSK3β | Prevent depression behavioranti-inflammatory. anti-edematous effects, memory improvementneuroprotection, anti-inflammatory motor recuperation, reduced anxiety | [256][257][258–261] |
| Demyelinating diseases | Lithium | Inhibition of GSK3D | Reduces demyeimation inflammation and progression of the disease in models of multipte sclerosis | [263, 264] |
| Axin2 | Negative regulator ol Wnt signaling | Associated with multiple sclerosis lesions, promotes remyelination | [265] | |
| ARA-014418. lithium indirubin, and 1803-mt | GSK3β inhibitors | Stimulate oligodendrocyte regeneration and promote remyelination | [266] | |
| GSK3β variant | Inhibition of GSK3β | Associates with multiple sclerosis | [267] | |
| Lithium | Inhibition of GSK3β | Stimulates peripheral myelin gene expression and promotes nerve remyelination | [14] | |
| Lithium chloride and citrate | Decrease N-acetyl aspartate | Counteracts neurodegeneration and dysmyelination in Canavan disease | [269–272] |
Wnt/β-catenin signaling and lithium in neuropsychiatric disorders
Bipolar disease
Bipolar disorder, one of the major causes of disability in the world, is characterized by extreme states of mania and depression. Alterations in Wnt/β-catenin signaling are associated with a variety of mood disorders and since more than fifty years, lithium is clinically used in the treatment of bipolar patients. Interestingly, GSK3β genetic variants are associated with the risk of bipolar disorder [148], and GSK3β gene copy number is increased in bipolar patients [149]. Greater gray matter density and reduced loss of N-acetyl aspartate, a marker for neuronal integrity and viability, were found in chronically lithium-treated patients [150, 151]. Genetic inactivation of GSK3β or pharmacological inhibition by lithium induced antidepressant and anti-maniac effects [124, 152, 153] that are similar to those observed after β-catenin overexpression [154]. Conversely, overexpression of GSK3β, or blocking of GSK3β phosphorylation induces mania-like behaviour and vulnerability to mood disturbance [155, 156].
Altogether these data support the idea that the molecular target of lithium in the treatment of psychotic disorder requires the inhibition of GSK3β. However, GSK3β has multiple downstream targets and its involvement in the psychotropic action of lithium needs to be clarified. We already mentioned that lithium can inhibit the behavioural action of monoamine such as dopamine, a key element in psychiatric disorder, by dissociating the interaction of Akt with β-arrestin2 and PP2A, a complex that inactivates Akt [124]. Other downstream effect could be attributed to KCNQ2 K + , a voltage-gated channel involved in repolarization by counteracting the Na + influx. Indeed, this channel, which is associated with psychiatric disorder [157], can be phosphorylated and activated by GSK3β, and inhibited by lithium [158]. In addition, since a correlation between circadian phases and depression has been postulated, and since lithium and GSK3β can modulate expression of genes and proteins required in circadian clock [159], regulation of the circadian rhythm could be another hypothesis to account for effect of lithium on mood disorder [160]. Indeed, recently, a second generation of GSK3β inhibitor was identified, and this compound was able to attenuate locomotor hyperactivity in Clock mutant mice [161]. Finally, in 2009, some predicted effectors of several microRNA were found to be both genetic risk candidates for bipolar disorder and involved in Wnt/β-catenin signaling [162], suggesting that they are targets for the action of psychotherapeutic drugs like lithium. Involvement of miRNA regulation is promising, and further investigations on this growing research area are needed.
Schizophrenia
Schizophrenia is another psychiatric disease, and emerging evidences suggest a neurodevelopmental origin with an alteration in synaptic connectivity. Those processes are, at least in part, controlled by Wnt/β-catenin signaling. Several genes in the Wnt pathway, such as FZD3, GSK3β, and DKK4 have been correlated with schizophrenia [163–165]. Interestingly, the level of APC mRNA, a key negative regulator of the Wnt signaling, is significantly increased in peripheral blood leukocytes of patients with schizophrenia. Single nucleus polymorphisms in the APC gene were identified to be associated with schizophrenia [166, 167]. However, in patients and in animal model of this mental disorder, expression of protein and transcripts of GSK3β are reduced [168]. In addition, even if lithium combined with neuroleptics was clinically used as medication for schizophrenia and showed beneficial effects [169], more recent data were not conclusive [170].
Many studies investigated the role of Wnt/β-catenin in mood disorder. DISC1 (Disrupted in Schizophrenia 1), a protein involved in neuronal progenitor proliferation and synaptic integration [171], has emerged as a potential link. It has been shown that a translocation in DISC1 gene cosegregates with schizophrenia and bipolar disorder [172]. Furthermore, a crosstalk between the GABAergic system, which seems involved in psychiatric disorder [173], and DISC1 has recently been described [174, 175]. Interestingly, DISC1 has been shown to interact with GSK3β to regulate its activity. DISC1 hippocampal overexpression induces Wnt/β-catenin-dependant neural progenitor proliferation, by inhibiting β-catenin degradation and promoting TCF/LEF activation [176]. Furthermore, it has been observed that the DIX domain-containing protein Dixdc1, functionally interacts with DISC1 to regulate neural progenitor proliferation by co-modulating Wnt-GSK3β/β-catenin signaling [177]. Kivimäe et al. recently described schizophrenia-like symptoms in mutant mice for Dixdc1 [178]. Furthermore, Ccd1, the human homologue of Dixdc1, stimulates Wnt signaling by interacting with Dsh [179]. Lately, a study showed that DISC1 regulates synaptic vesicle transport via a lithium-sensitive pathway [180].
Depression
Wnt2 and Wnt3a have an antidepressant activity. Serotonin reuptake inhibitor (SRI) antidepressants such as fluoxetine, promote hippocampal neurogenesis. They also increase the levels of the bcl-2 protein, whose overexpression in transgenic mice enhances adult hippocampal neurogenesis. However, the mechanisms underlying SRI-mediated neurogenesis are unclear. Recently, the microRNA miR-16 was identified as an important effector of SRI antidepressant action in serotonergic raphe and noradrenergic locus coeruleus (LC) [181]. Fluoxetine-exposed serotonergic neurons secrete brain-derived neurotrophic factor, Wnt2 and 15-Deoxy-delta12,14-prostaglandin J2 that act synergistically to regulate miR-16 at the hippocampus [182]. These signaling molecules are increased in the cerebrospinal fluid of depressed patients upon fluoxetine treatment.
Wnt/β-catenin signaling and lithium in neurodegenerative diseases
Alzheimer’s disease (AD)
AD is the most common cause of dementia, and is characterized by progressive personality changes with disorientation, memory loss and aphasia. Abnormal accumulation of Aβ peptide and tau hyper-phosphorylation are the hallmarks of the disease.
Clinical evidences described increased GSK3β activity in AD brains [183], and reduced β-catenin levels in patients with Presenilin mutation [184]. Experimental studies showed that, Wnt3a and lithium are able to decrease Aβ neurotoxicity [185, 186], and Aβ production in vitro [187–189]. Lithium treatment also demonstrated neuroprotective effects in in vivo model of AD, by inhibiting GSK3β-mediated tau phosphorylation [190, 191] and aggregation, [192], by promoting tau ubiquitination [193], by down-regulating tau transcription [194] and by reducing the development of the neurofibrillary pathology [195]. Lithium treatment can also improve memory and spatial learning deficits in transgenic mice model for AD, correlated with increased β-catenin and reduced GSK3β activity [186, 196, 197].
How Wnt signaling and lithium could lead to neuroprotective effects in AD ? Some studies suggest that lithium and other GSK3β inhibitors modulate tau phosphorylation and antagonize neuronal apoptosis by stabilizing β-catenin [198, 199]. Other data show the role of PP2A, a phosphatase involved in tau dephosphorylation, in lithium’s action [200, 201]. Frizzled-1 is also involved in the neuroprotective effect against Aβ neurotoxicity [202, 203], and the secreted Wnt antagonist Dkk1 participates in the pathological mechanism triggers by Aβ and phosphorylated tau [204–206]. M1 muscarinic receptor (mAChR) has also been shown to be involved in AD [207], and a cross talk between the muscarinic signaling and Wnt components was proposed [208, 209]. Indeed, M1 mAChR stimulation inhibits GSK-3β, stabilizes β-catenin, and therefore reverses the switch-off of the Wnt pathway caused by Aβ-toxicity. Inflammation plays a key role in AD, and microglia and inflammatory mediators are upregulated in the disease. The inhibition of the pro-inflammatory properties of GSK3β shows beneficial effects in AD [210, 211], and more recently, the blocking of the inflammatory cytokine IL1β, was able to rescue cognition, attenuated tau pathology, and restored neuronal β-catenin pathway function in an AD mouse model [212]. Lastly, it has been reported that miR-34a, a microRNA up-regulated in a double transgenic mouse model of AD, can inhibit bcl2 translation [213]. Interestingly, this same miRNA has also emerged as target for lithium [162]. Recently, genome-wide analysis of a Wnt1-regulated transcriptional network demonstrated that Wnt effectors were tightly clustered with Presenilin 1 that causes dominantly inherited forms of Alzheimer’s disease [15]. These recent findings provide new insights about the mechanisms of action of Wnt and lithium in AD neuroprotection.
It should be noted that clinical use of lithium for AD therapy remains controversial. Indeed, even if lithium treatment of bipolar patients improves memory score [214], and reduces prevalence of dementia [215–217], others studies showed no beneficial effect on AD symptoms [218, 219]. Larger trials, with different dosages and duration of lithium treatment should be tested, to examine whether lithium could be effective for the prevention of AD. In addition, trials on subjects with high AD risk may be more appropriate than patients with fully manifest dementia [220].
All these findings provide a therapeutic potential for the activation of Wnt/β-catenin signaling and the use of lithium in the treatment of AD. Inhibitors of GSK3β would also provide a novel avenue for therapeutic approaches in this devastating disorder [221].
Huntington disease (HD)
HD is a progressive neurodegenerative disorder caused by a CAG trinucleotide expansion in the gene encoding huntingtin. Numerous data suggest that lithium is also effective in Huntington disease via GSK3β inhibition. Protective effects are associated with anti-apoptotic properties (Bcl2 up-regulation and caspase-3 inhibition), decreased huntingtin aggregation [222–225], striatal cell proliferation [224], calpain inhibition [226], and induction of autophagy [227]. An original pathological mechanism by which mutant huntingtin specifically interferes with the degradation of β-catenin leading to its toxic stabilization, was also reported in a Drosophila model of Huntington disease [228]. Motor performance improvement by lithium administration in a mouse model of the disease was already demonstrated with rotarod test [229]; and recently, independent studies also showed neuroprotective and neurotrophic effects of lithium. Lithium combined with valproate, another GSK3 inhibitor, produces multiple beneficial effects in vitro and in vivo, including better motor functions, reduced anxiety behavior, and increased striatal and cortical BDNF level [230, 231]. A low-dose lithium formulation is able to ameliorate the motor and neuropathological phenotypes in a mouse model of Huntington disease, and is accompanied by improvements in multiple biochemical endpoints associated with the pathogenesis [232]. Also, the transplant efficiency of adult neural progenitor in a rat model of Huntington disease is improved when the cells are in vitro primed with lithium. Beneficial effects include induction of neural projections and acceleration of sensorimotor function outcome [233]. Since 1970, multiple clinical studies describing lithium administration, alone or combined with neuroleptics in Huntington patients, report better outcomes, including reduced chorea and amelioration in motor function [140]. Despite these plentiful positive data, others trial showed no beneficial effect or even worsened motor and cognitive performances.
Parkinson’s disease (PD)
PD is a progressive, complex disorder characterised by motor symptoms (bradykinesia, tremor, rigidity) associated with personality disorder. Many studies report deregulation of Wnt signaling in Parkinson’s disease pathogenesis and also the importance of this pathway in neuroprotection [234]. By using neurotoxins to induce neurochemical changes similar to those observed in Parkinson’s disease, multiple evidences showed beneficial effects of lithium administration both in vitro [235–237] and in vivo [238–241]. Several data suggested that lithium mechanism of action involves regulation of apoptosis mediated by GSK3β inhibition, while other data support evidence for lithium protection against oxidative stress [242, 243]. Recent studies also show that lithium activates autophagic pathway, suggesting an improvement in the mutant protein alpha synuclein degradation [244, 245]. It should be note that a recent study showed that lithium fails to protect dopaminergic neurons in a mouse model of Parkinson’s disease, but this discrepancy with other report can be due to the different mode of administration [246]. Very recently, it was described that Dkk1 is induced in neurotoxin treated-cells. It aggravates apoptosis and contributes to the cellular neurotoxicity by inhibition of Wnt signaling [247]. In human, therapeutic use of lithium demonstrated contradictory effects on involuntary movements [248].
Wnt/β-catenin signaling and lithium in traumatic brain injury
Traumatic brain injury (TBI) is a prevalent debilitating health issue that mainly affects young adults in industrialized countries with a substantial social and economic burden. The TBI causes direct damage to the brain by mechanical forces of shearing, tearing or stretching, and it results in cerebral edema, hemorrhage, contusions, focal and diffuse axonal injury, as well as psychological, neurobehavioral, locomotor, sensory and memory impairments. In response to the primary lesion due to the impact, secondary pathophysiological cascades are triggered, such as neuroinflammation, which is believed to worsen the brain injury from hours to days following the insult. Although the data from the preclinical studies have shown that the secondary pathophysiological cascades provide a large therapeutic window for pharmacological intervention, clinical trials have failed to approve the preclinical neuroprotective treatments [249]. Given the multifactorial consequences of brain injury, it is likely that simultaneous targeting of several targets with multipotential drugs may be the most promising therapeutic approach to improve outcome following TBI [249–255]. One of these potential drugs is lithium.
Several preclinical studies have addressed the potential therapeutic benefits of GSK3β inhibitors on histopathological, biochemical and functional/neurobehavioral outcomes in rodent models of TBI. Initially, Shapira and collaborators [256] have reported that pre-treatment with GSK3β inhibitors prevented a depression behavior in a model of mild TBI (mTBI). At the cellular level, mTBI resulted in increased level of phosphorylated form of GSK3β as well as in accumulation of its downstream target β-catenin in the hippocampus. TBI-induced inhibition of GSK3β, as expressed as an increase of its phosphorylated form, may therefore be part of a rehabilitation scheme, but not necessary sufficient per se, in response to the brain injury induced by mTBI. Therefore, the use of selective GSK3β inhibitors may have therapeutic benefits in the context of TBI-induced brain injury. Additionally, a chronic pre-treatment with lithium resulted in robust neuroprotection accompanied by anti-inflammatory and anti-edematous effects. In fact, the authors described a significant decrease of cerebral edema, interleukin-1β levels, lesion volume, neurodegeneration and spatial learning and memory impairment after TBI [257]. However, since this study is based on a pre-treatment protocol, its clinical relevance is questionable. The report of Yu and collaborators [258] was the first to attempt a post-insult treatment protocol with lithium after TBI. Lithium therapy resulted in reduction of lesion volume with a therapeutic window of at least 3 h post-injury. Besides, TBI-induced neuronal death, microglial activation and cyclooxygenase-2 induction were all attenuated by lithium. In terms of functional recovery, the same treatment preserved the motor coordination and reduced the anxiety-like behavior after TBI. Furthermore, lithium increased the phosphorylation of GSK3β, therefore resulting in its inhibition after injury, suggesting the underlying mechanism of lithium-induced neuroprotection following TBI.
It has also been described the benefits of post-insult lithium therapy in decreasing Aβ load and improvement of spatial learning and memory after TBI [259, 260]. Treatment with SB-216763 (selective inhibitor of GSK3) improved motor function, but offered only a partial improvement in terms of memory function with no overt neuroprotection following TBI. Treatment with lithium, as a broad spectrum inhibitor of GSK3, offers neuroprotection and robust cognitive improvement, supporting its clinical testing for the head-injured patients. However, the caution should be taken since the data from clinical studies highlighted the fact that lithium therapy for the management of neurobehavioral consequences (such as agitation, aggression,) of TBI has not been found necessary beneficial. In some cases, lithium therapy had adverse side effects in some of head-injured patients (reviewed in [261]). Therefore, appropriate investigations should be carried out for the clinical use of lithium for head-injured patients.
Wnt/β-catenin signaling and lithium in demyelinating diseases
Involvement of Wnt/β-catenin pathway in myelination process
Wnt/β-catenin is tightly implicated in myelination process. It was shown that the blockade of some components of Wnt pathway jeopardizes the maturation of oligodendrocyte progenitor cells into oligodendrocytes. Studies have addressed the potential role of Wnt signaling in oligodendrocyte development. Constitutive activation of β-catenin or inhibition of APC expression, resulting in the stabilization of β-catenin, delayed oligodendrocyte differentiation [11, 262]. Likewise, the constitutive stabilization and nuclear translocation of β-catenin in cells of the oligodendrocyte lineage had a negative influence on oligodendrocyte maturation. However, the targeted disruption of TCF7L2 in mice led to severe defects in oligodendrocyte differentiation but not specification [13]. Based on these findings, the consensus has emerged that canonical Wnt signaling may exert a repressive effect on oligodendrocyte differentiation and on myelination.
Conversely we have demonstrated that the ultimate step of myelination process, that is myelin gene expression, is stimulated by Wnt pathway [106]. In fact the selective inhibition of Wnt components by siRNA or dominant negative forms blocks the expression of myelin protein zero (MPZ) and peripheral myelin protein 22 (PMP22) in mouse Schwann cells and proteolipid protein (PLP) in mouse oligodendrocytes. Moreover, the activation of Wnt signaling by recombinant ligands increases by three-fold the transcription of myelin genes and enhances the binding of β-catenin to TCF/LEF transcription factors present in the vicinity of the MPZ and PMP22 promoters. Most importantly, loss-of-function analyses in zebrafish embryos show, in vivo, a key role for Wnt/β-catenin signaling in the expression of myelin genes and in myelin sheath compaction, both in the peripheral and central nervous systems. Inhibition of Wnt/β-catenin signaling resulted in hypomyelination, without affecting Schwann cells and oligodendrocytes generation or axonal integrity. Therefore, to achieve remyelination, Wnt pathway must be inhibited during the differentiation and maturation of progenitors cells into oligodendrocytes and activated during myelin gene expression by oligodendrocytes or Schwann cells. This therapeutic strategy is difficult to achieve when we are not able to precisely identify during the pathology the chronology of proliferation, differentiation and maturation of glial cells.
Given the pleiotropic, and sometimes opposite, effects of Wnt signaling on myelination process progression, it appears fundamental to seek for either detrimental or beneficial influence of Wnt modulation on demyelinating diseases treatment.
We will present the therapeutic attempts to modulate Wnt pathway in several demyelinating pathologies.
Multiple sclerosis
GSK3β exerts pro-inflammatory properties and, as we have described, Wnt/β-catenin signaling is involved in myelination process. Therefore, the effect of lithium in multiple sclerosis, an inflammatory demyelinating disease of the CNS, was investigated. Twenty years ago, it was shown that intraperitoneal injections of lithium in a rat model of multiple sclerosis were able to inhibit the development of the disease. However, high doses of lithium were used and the authors supported a toxic effect to explain the immunosuppressive action of lithium [263]. Unfortunately, this conclusion leaded to discourage further studies. In 2008, the work of De Sarno et al. [264] reported that pretreatment of a mouse model of multiple sclerosis with lithium is able to reduce clinical symptoms, demyelination, microglia activation and leukocyte infiltration. Treating the mouse after the disease onset also ameliorates recovery. More recently, it was shown that Axin2, a negative regulator of Wnt signaling that induces β-catenin degradation, was found in active multiple sclerosis lesions in adults and could promote remyelination [265].
In addition, Azim and Butt [266] showed that a range of GSK3β inhibitors, including lithium, regulates oligodendrocyte differentiation and promote remyelination following in vivo chemically induced demyelination. These effects are mediated by canonical Wnt pathway by stimulating nuclear translocation of β-catenin. Moreover, recent genetic analysis described an increased frequency of a GSK3β variant in patients with multiple sclerosis, suggesting that this GSK3β genetic variability could be a susceptibility factor for this disease [267].
Nerve injury
Recently, our group investigated for the first time the effect of lithium on remyelination process in the PNS [14]. We showed that the treatment of adult mice with lithium after facial nerve crush injury stimulated the expression of myelin genes, restored the myelin structure, and accelerated the motor recovery of whisker movements. Lithium treatment also promoted remyelination of the sciatic nerve after injury. We also demonstrated that peripheral myelin gene is stimulated by GSK3β inhibitors. LiCl exerts its action in Schwann cells by increasing the amount of β-catenin, provoking its nuclear localization, and driving it to TCF/LEF identified in myelin genes. Taken together, our findings open perspectives in the treatment of nerve demyelination by administering GSK3β inhibitors such as lithium.
Canavan disease
Canavan disease is another fatal brain dysmyelinating disorder, characterized by mental retardation, hypotonia progressing to hypertonia, macrocephaly and blindness [268]. Mutations in aspartoacylase gene, by inducing accumulation of N-acetyl aspartic acid (NAA) in the brain, lead to progressive neurodegeneration and dysmyelination. A study using a rat model for this disease, reported that in vivo lithium injection reduced N-acetyl aspartate brain level [269]. After a first promising clinical administration of lithium in a child with canavan disease [270]; other studies with more patients, demonstrated decreased NAA brain level and a mild improvement in myelination in the frontal white matter [271, 272]. It should be mentioned however that these treatments used lithium citrate and not lithium chloride.
Bipolar disorder and Myelin
Interestingly, several studies reported white matter abnormality in bipolar disorder and the protective effect of lithium on myelin structure on these patients [273]. It has also been recently reported that lithium and GSK3β promoter gene variants could counteract the detrimental influences of bipolar disorder on white matter microstructure [274]. Particularly, authors described that a less active GSK3β gene-promoter variant and a long-term administration of lithium are associated with increases of DTI measures of axial diffusivity in several white matter fiber tracts of brain bipolar patients, reflecting a better integrity of axons and myelin sheaths.
This multiple line of evidence supports the finding that lithium and Wnt/β-catenin can modulate myelination processes and promote myelin repair after demyelination. Paradoxically, but also comforting the link between lithium and myelination process in human, clinical data on lithium administration reported persistent sequelae in the CNS, named Syndrome of irreversible lithium-effectuated neurotoxicity (SILENT) [275]. The most commonly sequelae is a persistent cerebellar dysfunction, and the authors hypothesized that the putative cause of SILENT is demyelination caused by lithium at multiple sites in the nervous system, including the cerebellum. This hypothesis needs to be elucidated. Whatever, the potential use of these compounds as novel therapeutic agents for CNS disorders by modulating myelination requires further investigations to gain better insight into disease pathophysiological mechanisms and treatment.
Conclusions
Wnt/β-catenin signaling promotes CNS development and also adult neurogenesis. It could also account for a large part in lithium’s neuroprotective and beneficial effects in psychiatric, neurodegenerative, and demyelinating diseases. Lithium administration is able to induce neural progenitor proliferation in vitro [71, 276, 277] and in vivo in normal and pathological brain [278–281]. Finally, innovative mechanisms of action arose recently. First, as we already mentioned, emerging data support the finding that specific microRNA are both targets of lithium and involved in the pathophysiological processes of brain diseases [213, 282]. A better understanding of this novel regulatory mechanism for lithium’s beneficial effect may provide new knowledge of disease etiology and would lead to more efficient therapy [93, 283]. Second, in view of the multiple beneficial effects induced by lithium and Wnt/β-catenin pathway modulators in a wide spectrum of neurologic diseases, a new concept allowing the explanation of such various shared mechanisms could be proposed [284]. As evoked above, lithium and Wnt/β-catenin signaling can be considered as regulators of myelination. Therefore, a myelin-centered model can help to explain and integrate the set of pleiotropic neuroprotective effects observed in the nervous system. Non-invasive technologies, via imaging and genetic approaches, would permit to clinically test this hypothesis.
Footnotes
D. Meffre and J. Grenier contributed equally to this work
References
- 1.Ciani L, Salinas PC. WNTs in the vertebrate nervous system: from patterning to neuronal connectivity. Nat Rev Neurosci. 2005;6(5):351–362. doi: 10.1038/nrn1665. [DOI] [PubMed] [Google Scholar]
- 2.Mulligan KA, Cheyette BN. Wnt signaling in vertebrate neural development and function. J Neuroimmune Pharmacol. 2012;7(4):774–787. doi: 10.1007/s11481-012-9404-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Valvezan AJ, Klein PS. GSK-3 and Wnt signaling in neurogenesis and bipolar disorder. Frontiers mol neurosci. 2012;5:1. doi: 10.3389/fnmol.2012.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carter M, Chen X, Slowinska B, Minnerath S, Glickstein S, Shi L, Campagne F, Weinstein H, Ross ME. Crooked tail (Cd) model of human folate-responsive neural tube defects is mutated in Wnt coreceptor lipoprotein receptor-related protein 6. Proc Natl Acad Sci USA. 2005;102(36):12843–12848. doi: 10.1073/pnas.0501963102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kiecker C, Niehrs C. A morphogen gradient of Wnt/beta-catenin signalling regulates anteroposterior neural patterning in xenopus. Development. 2001;128(21):4189–4201. doi: 10.1242/dev.128.21.4189. [DOI] [PubMed] [Google Scholar]
- 6.Megason SG, McMahon AP. A mitogen gradient of dorsal midline Wnts organizes growth in the CNS. Development. 2002;129(9):2087–2098. doi: 10.1242/dev.129.9.2087. [DOI] [PubMed] [Google Scholar]
- 7.Rosso SB, Sussman D, Wynshaw-Boris A, Salinas PC. Wnt signaling through Dishevelled, Rac and JNK regulates dendritic development. Nat Neurosci. 2005;8(1):34–42. doi: 10.1038/nn1374. [DOI] [PubMed] [Google Scholar]
- 8.Cerpa W, Gambrill A, Inestrosa NC, Barria A. Regulation of NMDA-receptor synaptic transmission by Wnt signaling. J neurosci. 2011;31(26):9466–9471. doi: 10.1523/JNEUROSCI.6311-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Budnik V, Salinas PC. Wnt signaling during synaptic development and plasticity. Curr Opin Neurobiol. 2011;21(1):151–159. doi: 10.1016/j.conb.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inestrosa NC, Arenas E. Emerging roles of Wnts in the adult nervous system. Nat Rev Neurosci. 2010;11(2):77–86. doi: 10.1038/nrn2755. [DOI] [PubMed] [Google Scholar]
- 11.Fancy SP, Baranzini SE, Zhao C, Yuk DI, Irvine KA, Kaing S, Sanai N, Franklin RJ, Rowitch DH. Dysregulation of the Wnt pathway inhibits timely myelination and remyelination in the mammalian CNS. Genes Dev. 2009;23(13):1571–1585. doi: 10.1101/gad.1806309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tawk M, Makoukji J, Belle M, Fonte C, Trousson A, Hawkins T, Li H, Ghandour S, Schumacher M, Massaad C. Wnt/beta-catenin signaling is an essential and direct driver of myelin gene expression and myelinogenesis. J neurosci. 2011;31(10):3729–3742. doi: 10.1523/JNEUROSCI.4270-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ye F, Chen Y, Hoang T, Montgomery RL, Zhao XH, Bu H, Hu T, Taketo MM, van Es JH, Clevers H, Hsieh J, Bassel-Duby R, Olson EN, Lu QR. HDAC1 and HDAC2 regulate oligodendrocyte differentiation by disrupting the beta-catenin-TCF interaction. Nat Neurosci. 2009;12(7):829–838. doi: 10.1038/nn.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Makoukji J, Belle M, Meffre D, Stassart R, Grenier J, Shackleford G, Fledrich R, Fonte C, Branchu J, Goulard M, de Waele C, Charbonnier F, Sereda MW, Baulieu EE, Schumacher M, Bernard S, Massaad C. Lithium enhances remyelination of peripheral nerves. Proc Natl Acad Sci USA. 2012;109(10):3973–3978. doi: 10.1073/pnas.1121367109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wexler EM, Rosen E, Lu D, Osborn GE, Martin E, Raybould H, Geschwind DH. Genome-wide analysis of a Wnt1-regulated transcriptional network implicates neurodegenerative pathways. Sci signal. 2011;4(193):ra65. doi: 10.1126/scisignal.2002282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chuang DM, Wang Z, Chiu CT. GSK-3 as a Target for Lithium-Induced Neuroprotection Against Excitotoxicity in Neuronal Cultures and Animal Models of Ischemic Stroke. Frontiers mol neurosci. 2011;4:15. doi: 10.3389/fnmol.2011.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neumann C, Cohen S. Morphogens and pattern formation. BioEssays. 1997;19(8):721–729. doi: 10.1002/bies.950190813. [DOI] [PubMed] [Google Scholar]
- 18.Du SJ, Purcell SM, Christian JL, McGrew LL, Moon RT. Identification of distinct classes and functional domains of Wnts through expression of wild-type and chimeric proteins in xenopus embryos. Mol Cell Biol. 1995;15(5):2625–2634. doi: 10.1128/mcb.15.5.2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kikuchi A, Yamamoto H, Sato A. Selective activation mechanisms of Wnt signaling pathways. Trends Cell Biol. 2009;19(3):119–129. doi: 10.1016/j.tcb.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 20.Angers S, Moon RT. Proximal events in Wnt signal transduction. Nat Rev Mol Cell Biol. 2009;10(7):468–477. doi: 10.1038/nrm2717. [DOI] [PubMed] [Google Scholar]
- 21.Kohn AD, Moon RT. Wnt and calcium signaling: beta-catenin-independent pathways. Cell Calcium. 2005;38(3–4):439–446. doi: 10.1016/j.ceca.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 22.Wu J, Mlodzik M. A quest for the mechanism regulating global planar cell polarity of tissues. Trends Cell Biol. 2009;19(7):295–305. doi: 10.1016/j.tcb.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoppler S, Kavanagh CL. Wnt signalling: variety at the core. J Cell Sci. 2007;120(Pt 3):385–393. doi: 10.1242/jcs.03363. [DOI] [PubMed] [Google Scholar]
- 24.Willert K, Logan CY, Arora A, Fish M, Nusse R. A drosophila axin homolog, daxin, inhibits Wnt signaling. Development. 1999;126(18):4165–4173. doi: 10.1242/dev.126.18.4165. [DOI] [PubMed] [Google Scholar]
- 25.Liu C, Li Y, Semenov M, Han C, Baeg GH, Tan Y, Zhang Z, Lin X, He X. Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell. 2002;108(6):837–847. doi: 10.1016/s0092-8674(02)00685-2. [DOI] [PubMed] [Google Scholar]
- 26.Aberle H, Bauer A, Stappert J, Kispert A, Kemler R. Beta-catenin is a target for the ubiquitin-proteasome pathway. EMBO J. 1997;16(13):3797–3804. doi: 10.1093/emboj/16.13.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stadeli R, Hoffmans R, Basler K. Transcription under the control of nuclear Arm/beta-catenin. Curr Biol. 2006;16(10):R378–R385. doi: 10.1016/j.cub.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 28.Luo W, Peterson A, Garcia BA, Coombs G, Kofahl B, Heinrich R, Shabanowitz J, Hunt DF, Yost HJ, Virshup DM. Protein phosphatase 1 regulates assembly and function of the beta-catenin degradation complex. EMBO J. 2007;26(6):1511–1521. doi: 10.1038/sj.emboj.7601607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davidson G, Wu W, Shen J, Bilic J, Fenger U, Stannek P, Glinka A, Niehrs C. Casein kinase 1 gamma couples Wnt receptor activation to cytoplasmic signal transduction. Nature. 2005;438(7069):867–872. doi: 10.1038/nature04170. [DOI] [PubMed] [Google Scholar]
- 30.Yokoya F, Imamoto N, Tachibana T, Yoneda Y. Beta-catenin can be transported into the nucleus in a Ran-unassisted manner. Mol Biol Cell. 1999;10(4):1119–1131. doi: 10.1091/mbc.10.4.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sharma M, Jamieson C, Johnson M, Molloy MP, Henderson BR. Specific armadillo repeat sequences facilitate beta-catenin nuclear transport in live cells via direct binding to nucleoporins Nup62, Nup153, and RanBP2/Nup358. J Biol Chem. 2012;287(2):819–831. doi: 10.1074/jbc.M111.299099. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 32.Wu X, Tu X, Joeng KS, Hilton MJ, Williams DA, Long F. Rac1 activation controls nuclear localization of beta-catenin during canonical Wnt signaling. Cell. 2008;133(2):340–353. doi: 10.1016/j.cell.2008.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Belenkaya TY, Han C, Standley HJ, Lin X, Houston DW, Heasman J, Lin X. Pygopus encodes a nuclear protein essential for wingless/Wnt signaling. Development. 2002;129(17):4089–4101. doi: 10.1242/dev.129.17.4089. [DOI] [PubMed] [Google Scholar]
- 34.Mosimann C, Hausmann G, Basler K. Parafibromin/hyrax activates Wnt/Wg target gene transcription by direct association with beta-catenin/armadillo. Cell. 2006;125(2):327–341. doi: 10.1016/j.cell.2006.01.053. [DOI] [PubMed] [Google Scholar]
- 35.Griffin CT, Curtis CD, Davis RB, Muthukumar V, Magnuson T. The chromatin-remodeling enzyme BRG1 modulates vascular Wnt signaling at two levels. Proc Natl Acad Sci USA. 2011;108(6):2282–2287. doi: 10.1073/pnas.1013751108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Z, Nie F, Wang S, Li L. Histone H4 Lys 20 monomethylation by histone methylase SET8 mediates Wnt target gene activation. Proc Natl Acad Sci USA. 2011;108(8):3116–3123. doi: 10.1073/pnas.1009353108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sheldahl LC, Slusarski DC, Pandur P, Miller JR, Kuhl M, Moon RT. Dishevelled activates Ca2 + flux, PKC, and CamKII in vertebrate embryos. J cell Biol. 2003;161(4):769–777. doi: 10.1083/jcb.200211094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Semenov MV, Habas R, Macdonald BT, He X. Snapshot: noncanonical Wnt signaling pathways. Cell. 2007;131(7):1378. doi: 10.1016/j.cell.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 39.Dissanayake SK, Wade M, Johnson CE, O’Connell MP, Leotlela PD, French AD, Shah KV, Hewitt KJ, Rosenthal DT, Indig FE, Jiang Y, Nickoloff BJ, Taub DD, Trent JM, Moon RT, Bittner M, Weeraratna AT. The Wnt5A/protein kinase C pathway mediates motility in melanoma cells via the inhibition of metastasis suppressors and initiation of an epithelial to mesenchymal transition. J Biol Chem. 2007;282(23):17259–17271. doi: 10.1074/jbc.M700075200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Veeman MT, Axelrod JD, Moon RT. A second canon. Functions and mechanisms of beta-catenin-independent Wnt signaling. Dev Cell. 2003;5(3):367–377. doi: 10.1016/s1534-5807(03)00266-1. [DOI] [PubMed] [Google Scholar]
- 41.Moeller H, Jenny A, Schaeffer HJ, Schwarz-Romond T, Mlodzik M, Hammerschmidt M, Birchmeier W. Diversin regulates heart formation and gastrulation movements in development. Proc Natl Acad Sci USA. 2006;103(43):15900–15905. doi: 10.1073/pnas.0603808103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tree DR, Shulman JM, Rousset R, Scott MP, Gubb D, Axelrod JD. Prickle mediates feedback amplification to generate asymmetric planar cell polarity signaling. Cell. 2002;109(3):371–381. doi: 10.1016/s0092-8674(02)00715-8. [DOI] [PubMed] [Google Scholar]
- 43.Narimatsu M, Bose R, Pye M, Zhang L, Miller B, Ching P, Sakuma R, Luga V, Roncari L, Attisano L, Wrana JL. Regulation of planar cell polarity by Smurf ubiquitin ligases. Cell. 2009;137(2):295–307. doi: 10.1016/j.cell.2009.02.025. [DOI] [PubMed] [Google Scholar]
- 44.Capelluto DG, Kutateladze TG, Habas R, Finkielstein CV, He X, Overduin M. The DIX domain targets dishevelled to actin stress fibres and vesicular membranes. Nature. 2002;419(6908):726–729. doi: 10.1038/nature01056. [DOI] [PubMed] [Google Scholar]
- 45.Krylova O, Messenger MJ, Salinas PC. Dishevelled-1 regulates microtubule stability: a new function mediated by glycogen synthase kinase-3beta. J cell Biol. 2000;151(1):83–94. doi: 10.1083/jcb.151.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu W, Sato A, Khadka D, Bharti R, Diaz H, Runnels LW, Habas R. Mechanism of activation of the Formin protein Daam1. Proc Natl Acad Sci USA. 2008;105(1):210–215. doi: 10.1073/pnas.0707277105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Habas R, Kato Y, He X. Wnt/Frizzled activation of Rho regulates vertebrate gastrulation and requires a novel Formin homology protein Daam1. Cell. 2001;107(7):843–854. doi: 10.1016/s0092-8674(01)00614-6. [DOI] [PubMed] [Google Scholar]
- 48.Matusek T, Gombos R, Szecsenyi A, Sanchez-Soriano N, Czibula A, Pataki C, Gedai A, Prokop A, Rasko I, Mihaly J. Formin proteins of the DAAM subfamily play a role during axon growth. J Neurosci. 2008;28(49):13310–13319. doi: 10.1523/JNEUROSCI.2727-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chang L, Jones Y, Ellisman MH, Goldstein LS, Karin M. JNK1 is required for maintenance of neuronal microtubules and controls phosphorylation of microtubule-associated proteins. Dev Cell. 2003;4(4):521–533. doi: 10.1016/s1534-5807(03)00094-7. [DOI] [PubMed] [Google Scholar]
- 50.Ciani L, Salinas PC. c-Jun N-terminal kinase (JNK) cooperates with Gsk3beta to regulate Dishevelled-mediated microtubule stability. BMC Cell Biol. 2007;8:27. doi: 10.1186/1471-2121-8-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang X, Zhu J, Yang GY, Wang QJ, Qian L, Chen YM, Chen F, Tao Y, Hu HS, Wang T, Luo ZG. Dishevelled promotes axon differentiation by regulating atypical protein kinase C. Nat Cell Biol. 2007;9(7):743–754. doi: 10.1038/ncb1603. [DOI] [PubMed] [Google Scholar]
- 52.Suzuki A, Hirata M, Kamimura K, Maniwa R, Yamanaka T, Mizuno K, Kishikawa M, Hirose H, Amano Y, Izumi N, Miwa Y, Ohno S. aPKC acts upstream of PAR-1b in both the establishment and maintenance of mammalian epithelial polarity. Curr Biol. 2004;14(16):1425–1435. doi: 10.1016/j.cub.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 53.Freese JL, Pino D, Pleasure SJ. Wnt signaling in development and disease. Neurobiol Dis. 2010;38(2):148–153. doi: 10.1016/j.nbd.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Salcedo-Tello P, Ortiz-Matamoros A, Arias C. GSK3 Function in the Brain during Development, Neuronal Plasticity, and Neurodegeneration. Int J Alzheimer’s Dis. 2011;2011:189728. doi: 10.4061/2011/189728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Salinas PC. Modulation of the microtubule cytoskeleton: a role for a divergent canonical Wnt pathway. Trends Cell Biol. 2007;17(7):333–342. doi: 10.1016/j.tcb.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 56.Speese SD, Budnik V. Wnts: up-and-coming at the synapse. Trends Neurosci. 2007;30(6):268–275. doi: 10.1016/j.tins.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dill J, Wang H, Zhou F, Li S. Inactivation of glycogen synthase kinase 3 promotes axonal growth and recovery in the CNS. J Neurosci. 2008;28(36):8914–8928. doi: 10.1523/JNEUROSCI.1178-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zou Y. Wnt signaling in axon guidance. Trends Neurosci. 2004;27(9):528–532. doi: 10.1016/j.tins.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 59.Wolf AM, Lyuksyutova AI, Fenstermaker AG, Shafer B, Lo CG, Zou Y. Phosphatidylinositol-3-kinase-atypical protein kinase C signaling is required for Wnt attraction and anterior-posterior axon guidance. J Neurosci. 2008;28(13):3456–3467. doi: 10.1523/JNEUROSCI.0029-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lei Q, Jeong Y, Misra K, Li S, Zelman AK, Epstein DJ, Matise MP. Wnt signaling inhibitors regulate the transcriptional response to morphogenetic Shh-Gli signaling in the neural tube. Dev Cell. 2006;11(3):325–337. doi: 10.1016/j.devcel.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 61.Li B, Kuriyama S, Moreno M, Mayor R. The posteriorizing gene Gbx2 is a direct target of Wnt signalling and the earliest factor in neural crest induction. Development. 2009;136(19):3267–3278. doi: 10.1242/dev.036954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Braun MM, Etheridge A, Bernard A, Robertson CP, Roelink H. Wnt signaling is required at distinct stages of development for the induction of the posterior forebrain. Development. 2003;130(23):5579–5587. doi: 10.1242/dev.00685. [DOI] [PubMed] [Google Scholar]
- 63.Chung S, Leung A, Han BS, Chang MY, Moon JI, Kim CH, Hong S, Pruszak J, Isacson O, Kim KS. Wnt1-lmx1a forms a novel autoregulatory loop and controls midbrain dopaminergic differentiation synergistically with the SHH-FoxA2 pathway. Cell Stem Cell. 2009;5(6):646–658. doi: 10.1016/j.stem.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fujimura N, Vacik T, Machon O, Vlcek C, Scalabrin S, Speth M, Diep D, Krauss S, Kozmik Z. Wnt-mediated down-regulation of Sp1 target genes by a transcriptional repressor Sp5. J Biol Chem. 2007;282(2):1225–1237. doi: 10.1074/jbc.M605851200. [DOI] [PubMed] [Google Scholar]
- 65.Hirabayashi Y, Itoh Y, Tabata H, Nakajima K, Akiyama T, Masuyama N, Gotoh Y. The Wnt/beta-catenin pathway directs neuronal differentiation of cortical neural precursor cells. Development. 2004;131(12):2791–2801. doi: 10.1242/dev.01165. [DOI] [PubMed] [Google Scholar]
- 66.Su H, Zhang W, Guo J, Guo A, Yuan Q, Wu W. Lithium enhances the neuronal differentiation of neural progenitor cells in vitro and after transplantation into the avulsed ventral horn of adult rats through the secretion of brain-derived neurotrophic factor. J Neurochem. 2009;108(6):1385–1398. doi: 10.1111/j.1471-4159.2009.05902.x. [DOI] [PubMed] [Google Scholar]
- 67.Kuwabara T, Hsieh J, Muotri A, Yeo G, Warashina M, Lie DC, Moore L, Nakashima K, Asashima M, Gage FH. Wnt-mediated activation of NeuroD1 and retro-elements during adult neurogenesis. Nat Neurosci. 2009;12(9):1097–1105. doi: 10.1038/nn.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wisniewska MB, Misztal K, Michowski W, Szczot M, Purta E, Lesniak W, Klejman ME, Dabrowski M, Filipkowski RK, Nagalski A, Mozrzymas JW, Kuznicki J. LEF1/beta-catenin complex regulates transcription of the Cav3.1 calcium channel gene (Cacna1 g) in thalamic neurons of the adult brain. Neuroscience. 2010;30(14):4957–4969. doi: 10.1523/JNEUROSCI.1425-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fonte C, Grenier J, Trousson A, Chauchereau A, Lahuna O, Baulieu EE, Schumacher M, Massaad C. Involvement of {beta}-catenin and unusual behavior of CBP and p300 in glucocorticosteroid signaling in Schwann cells. Proc Natl Acad Sci USA. 2005;102(40):14260–14265. doi: 10.1073/pnas.0506930102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Matrisciano F, Busceti CL, Bucci D, Orlando R, Caruso A, Molinaro G, Cappuccio I, Riozzi B, Gradini R, Motolese M, Caraci F, Copani A, Scaccianoce S, Melchiorri D, Bruno V, Battaglia G, Nicoletti F. Induction of the Wnt antagonist Dickkopf-1 is involved in stress-induced hippocampal damage. PLoS ONE. 2011;6(1):e16447. doi: 10.1371/journal.pone.0016447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Boku S, Nakagawa S, Masuda T, Nishikawa H, Kato A, Kitaichi Y, Inoue T, Koyama T. Glucocorticoids and lithium reciprocally regulate the proliferation of adult dentate gyrus-derived neural precursor cells through GSK-3beta and beta-catenin/TCF pathway. Neuropsychopharmacology. 2009;34(3):805–815. doi: 10.1038/npp.2008.198. [DOI] [PubMed] [Google Scholar]
- 72.Legrand J. Thyroid hormones and maturation of the nervous system. J de physiologie. 1982;78(7):603–652. [PubMed] [Google Scholar]
- 73.Bernal J. Thyroid hormone receptors in brain development and function. Nat Clin Pract Endocrinol Metab. 2007;3(3):249–259. doi: 10.1038/ncpendmet0424. [DOI] [PubMed] [Google Scholar]
- 74.Berbel P, Navarro D, Auso E, Varea E, Rodriguez AE, Ballesta JJ, Salinas M, Flores E, Faura CC, de Escobar GM. Role of late maternal thyroid hormones in cerebral cortex development: an experimental model for human prematurity. Cereb Cortex. 2010;20(6):1462–1475. doi: 10.1093/cercor/bhp212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bauer M, Goetz T, Glenn T, Whybrow PC. The thyroid-brain interaction in thyroid disorders and mood disorders. J Neuroendocrinol. 2008;20(10):1101–1114. doi: 10.1111/j.1365-2826.2008.01774.x. [DOI] [PubMed] [Google Scholar]
- 76.Barakat-Walter I. Role of thyroid hormones and their receptors in peripheral nerve regeneration. J Neurobiol. 1999;40(4):541–559. doi: 10.1002/(sici)1097-4695(19990915)40:4<541::aid-neu10>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 77.Harsan LA, Steibel J, Zaremba A, Agin A, Sapin R, Poulet P, Guignard B, Parizel N, Grucker D, Boehm N, Miller RH, Ghandour MS. Recovery from chronic demyelination by thyroid hormone therapy: myelinogenesis induction and assessment by diffusion tensor magnetic resonance imaging. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28(52):14189–14201. doi: 10.1523/JNEUROSCI.4453-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lemkine GF, Raj A, Alfama G, Turque N, Hassani Z, Alegria-Prevot O, Samarut J, Levi G, Demeneix BA. Adult neural stem cell cycling in vivo requires thyroid hormone and its alpha receptor. FASEB J. 2005;19(7):863–865. doi: 10.1096/fj.04-2916fje. [DOI] [PubMed] [Google Scholar]
- 79.Lopez-Juarez A, Remaud S, Hassani Z, Jolivet P, Pierre Simons J, Sontag T, Yoshikawa K, Price J, Morvan-Dubois G, Demeneix BA. Thyroid hormone signaling acts as a neurogenic switch by repressing Sox2 in the adult neural stem cell niche. Cell Stem Cell. 2012;10(5):531–543. doi: 10.1016/j.stem.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 80.Bernal J, Morte B. Thyroid hormone receptor activity in the absence of ligand: physiological and developmental implications. Biochim Biophys Acta. 2012 doi: 10.1016/j.bbagen.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 81.Courtin F, Zrouri H, Lamirand A, Li WW, Mercier G, Schumacher M, Goascogne CL, Pierre M. Thyroid hormone deiodinases in the central and peripheral nervous system. Thyroid. 2005;15(8):931–942. doi: 10.1089/thy.2005.15.931. [DOI] [PubMed] [Google Scholar]
- 82.Mohacsik P, Zeold A, Bianco AC, Gereben B. Thyroid hormone and the neuroglia: both source and target. J Thyroid Res. 2011;2011:215718. doi: 10.4061/2011/215718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kress E, Samarut J, Plateroti M. Thyroid hormones and the control of cell proliferation or cell differentiation: paradox or duality? Mol Cell Endocrinol. 2009;313(1–2):36–49. doi: 10.1016/j.mce.2009.08.028. [DOI] [PubMed] [Google Scholar]
- 84.Sirakov M, Skah S, Nadjar J, Plateroti M. Thyroid hormone’s action on progenitor/stem cell biology: new challenge for a classic hormone? Biochim Biophys Acta. 2012 doi: 10.1016/j.bbagen.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 85.Natsume H, Sasaki S, Kitagawa M, Kashiwabara Y, Matsushita A, Nakano K, Nishiyama K, Nagayama K, Misawa H, Masuda H, Nakamura H. Beta-catenin/Tcf-1-mediated transactivation of cyclin D1 promoter is negatively regulated by thyroid hormone. Biochem Biophys Res Commun. 2003;309(2):408–413. doi: 10.1016/j.bbrc.2003.08.019. [DOI] [PubMed] [Google Scholar]
- 86.Guigon CJ, Zhao L, Lu C, Willingham MC, Cheng SY. Regulation of beta-catenin by a novel nongenomic action of thyroid hormone beta receptor. Mol Cell Biol. 2008;28(14):4598–4608. doi: 10.1128/MCB.02192-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sirakov M, Skah S, Lone IN, Nadjar J, Angelov D, Plateroti M. Multi-level interactions between the nuclear receptor TRalpha1 and the WNT effectors beta-catenin/Tcf4 in the intestinal epithelium. PLoS One. 2012;7(4):e34162. doi: 10.1371/journal.pone.0034162. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 88.Wang L, Shao YY, Ballock RT. Thyroid hormone-mediated growth and differentiation of growth plate chondrocytes involves IGF-1 modulation of beta-catenin signaling. J Bone Miner Res. 2010;25(5):1138–1146. doi: 10.1002/jbmr.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.O’Shea PJ, Kim DW, Logan JG, Davis S, Walker RL, Meltzer PS, Cheng SY, Williams GR. Advanced bone formation in mice with a dominant-negative mutation in the thyroid hormone receptor beta gene due to activation of Wnt/beta-catenin protein signaling. J Biol Chem. 2012;287(21):17812–17822. doi: 10.1074/jbc.M111.311464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liao CH, Yeh CT, Huang YH, Wu SM, Chi HC, Tsai MM, Tsai CY, Liao CJ, Tseng YH, Lin YH, Chen CY, Chung IH, Cheng WL, Chen WJ, Lin KH. Dickkopf 4 positively regulated by the thyroid hormone receptor suppresses cell invasion in human hepatoma cells. Hepatology. 2012;55(3):910–920. doi: 10.1002/hep.24740. [DOI] [PubMed] [Google Scholar]
- 91.Dentice M, Luongo C, Ambrosio R, Sibilio A, Casillo A, Iaccarino A, Troncone G, Fenzi G, Larsen PR, Salvatore D. Beta-catenin regulates deiodinase levels and thyroid hormone signaling in colon cancer cells. Gastroenterology. 2012;143(4):1037–1047. doi: 10.1053/j.gastro.2012.06.042. [DOI] [PubMed] [Google Scholar]
- 92.Miller LD, Park KS, Guo QM, Alkharouf NW, Malek RL, Lee NH, Liu ET, Cheng SY. Silencing of Wnt signaling and activation of multiple metabolic pathways in response to thyroid hormone-stimulated cell proliferation. Mol Cell Biol. 2001;21(19):6626–6639. doi: 10.1128/MCB.21.19.6626-6639.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang C. Novel functions for small RNA molecules. Curr Opin Mol Ther. 2009;11(6):641–651. [PMC free article] [PubMed] [Google Scholar]
- 94.Baas D, Bourbeau D, Sarlieve LL, Ittel ME, Dussault JH, Puymirat J. Oligodendrocyte maturation and progenitor cell proliferation are independently regulated by thyroid hormone. Glia. 1997;19(4):324–332. doi: 10.1002/(sici)1098-1136(199704)19:4<324::aid-glia5>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 95.Chew LJ, Shen W, Ming X, Senatorov VV, Jr, Chen HL, Cheng Y, Hong E, Knoblach S, Gallo V. SRY-box containing gene 17 regulates the Wnt/beta-catenin signaling pathway in oligodendrocyte progenitor cells. J NeuroSci. 2011;31(39):13921–13935. doi: 10.1523/JNEUROSCI.3343-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Russell DW. Oxysterol biosynthetic enzymes. Biochim Biophys Acta. 2000;1529(1–3):126–135. doi: 10.1016/s1388-1981(00)00142-6. [DOI] [PubMed] [Google Scholar]
- 97.Papassotiropoulos A, Lutjohann D, Bagli M, Locatelli S, Jessen F, Buschfort R, Ptok U, Bjorkhem I, von Bergmann K, Heun R. 24S-hydroxycholesterol in cerebrospinal fluid is elevated in early stages of dementia. J Psychiatr Res. 2002;36(1):27–32. doi: 10.1016/s0022-3956(01)00050-4. [DOI] [PubMed] [Google Scholar]
- 98.Leoni V, Masterman T, Diczfalusy U, De Luca G, Hillert J, Bjorkhem I. Changes in human plasma levels of the brain specific oxysterol 24S-hydroxycholesterol during progression of multiple sclerosis. Neurosci Lett. 2002;331(3):163–166. doi: 10.1016/s0304-3940(02)00887-x. [DOI] [PubMed] [Google Scholar]
- 99.Teunissen CE, Dijkstra CD, Polman CH, Hoogervorst EL, von Bergmann K, Lutjohann D. Decreased levels of the brain specific 24S-hydroxycholesterol and cholesterol precursors in serum of multiple sclerosis patients. Neurosci Lett. 2003;347(3):159–162. doi: 10.1016/s0304-3940(03)00667-0. [DOI] [PubMed] [Google Scholar]
- 100.Bochelen D, Mersel M, Behr P, Lutz P, Kupferberg A. Effect of oxysterol treatment on cholesterol biosynthesis and reactive astrocyte proliferation in injured rat brain cortex. J Neurochem. 1995;65(5):2194–2200. doi: 10.1046/j.1471-4159.1995.65052194.x. [DOI] [PubMed] [Google Scholar]
- 101.Trousson A, Bernard S, Petit PX, Liere P, Pianos A, El Hadri K, Lobaccaro JM, Ghandour MS, Raymondjean M, Schumacher M, Massaad C. 25-hydroxycholesterol provokes oligodendrocyte cell line apoptosis and stimulates the secreted phospholipase A2 type IIA via LXR beta and PXR. J Neurochem. 2009;109(4):945–958. doi: 10.1111/j.1471-4159.2009.06009.x. [DOI] [PubMed] [Google Scholar]
- 102.Janowski BA, Willy PJ, Devi TR, Falck JR, Mangelsdorf DJ. An oxysterol signalling pathway mediated by the nuclear receptor LXR alpha. Nature. 1996;383(6602):728–731. doi: 10.1038/383728a0. [DOI] [PubMed] [Google Scholar]
- 103.Chen JD, Evans RM. A transcriptional co-repressor that interacts with nuclear hormone receptors. Nature. 1995;377(6548):454–457. doi: 10.1038/377454a0. [DOI] [PubMed] [Google Scholar]
- 104.Huuskonen J, Fielding PE, Fielding CJ. Role of p160 coactivator complex in the activation of liver X receptor. Arterioscler Thromb Vasc Biol. 2004;24(4):703–708. doi: 10.1161/01.ATV.0000121202.72593.da. [DOI] [PubMed] [Google Scholar]
- 105.Andersson S, Gustafsson N, Warner M, Gustafsson JA. Inactivation of liver X receptor beta leads to adult-onset motor neuron degeneration in male mice. Proc Natl Acad Sci USA. 2005;102(10):3857–3862. doi: 10.1073/pnas.0500634102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Makoukji J, Shackleford G, Meffre D, Grenier J, Liere P, Lobaccaro JM, Schumacher M, Massaad C. Interplay between LXR and Wnt/beta-catenin signaling in the negative regulation of peripheral myelin genes by oxysterols. J Neurosci. 2011;31(26):9620–9629. doi: 10.1523/JNEUROSCI.0761-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shackleford G, Makoukji J, Grenier J, Liere P, Meffre D, Massaad C. Differential regulation of Wnt/beta-catenin signaling by Liver X Receptors in Schwann cells and oligodendrocytes. Biochem Pharmacol. 2013 doi: 10.1016/j.bcp.2013.02.036. [DOI] [PubMed] [Google Scholar]
- 108.Chuu CP. Modulation of liver X receptor signaling as a prevention and therapy for colon cancer. Med Hypotheses. 2011;76(5):697–699. doi: 10.1016/j.mehy.2011.01.037. [DOI] [PubMed] [Google Scholar]
- 109.Uno S, Endo K, Jeong Y, Kawana K, Miyachi H, Hashimoto Y, Makishima M. Suppression of beta-catenin signaling by liver X receptor ligands. Biochem Pharmacol. 2009;77(2):186–195. doi: 10.1016/j.bcp.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 110.El-Mallakh RS. Ion homeostasis and the mechanism of action of lithium. Clin Neurosci Res. 2004;4:227–231. [Google Scholar]
- 111.Huang X, Lei Z, El-Mallakh RS. Lithium normalizes elevated intracellular sodium. Bipolar Disord. 2007;9(3):298–300. doi: 10.1111/j.1399-5618.2007.00429.x. [DOI] [PubMed] [Google Scholar]
- 112.Baumann P, Nil R, Souche A, Montaldi S, Baettig D, Lambert S, Uehlinger C, Kasas A, Amey M, Jonzier-Perey M. A double-blind, placebo-controlled study of citalopram with and without lithium in the treatment of therapy-resistant depressive patients: a clinical, pharmacokinetic, and pharmacogenetic investigation. J Clin Psychopharmacol. 1996;16(4):307–314. doi: 10.1097/00004714-199608000-00006. [DOI] [PubMed] [Google Scholar]
- 113.Chenu F, Bourin M. Potentiation of antidepressant-like activity with lithium: mechanism involved. Curr Drug Targets. 2006;7(2):159–163. doi: 10.2174/138945006775515392. [DOI] [PubMed] [Google Scholar]
- 114.Quiroz JA, Machado-Vieira R, Zarate CA, Jr, Manji HK. Novel insights into lithium’s mechanism of action: neurotrophic and neuroprotective effects. Neuropsychobiology. 2010;62(1):50–60. doi: 10.1159/000314310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.de Freitas DM, Castro MMCA, Geraldes CF. Is competitive between Li + and Mg2 + the underlying theme in the proposed mechanisms for the pharmacological action of lithium salts in bipolar disorder? Acc Chem Res. 2006;39:283–291. doi: 10.1021/ar030197a. [DOI] [PubMed] [Google Scholar]
- 116.Jope RS. Lithium and GSK-3: one inhibitor, two inhibitory actions, multiple outcomes. Trends Pharmacol Sci. 2003;24(9):441–443. doi: 10.1016/S0165-6147(03)00206-2. [DOI] [PubMed] [Google Scholar]
- 117.Ryves WJ, Harwood AJ. Lithium inhibits glycogen synthase kinase-3 by competition for magnesium. Biochem Biophys Res Commun. 2001;280(3):720–725. doi: 10.1006/bbrc.2000.4169. [DOI] [PubMed] [Google Scholar]
- 118.Haimovich A, Eliav U, Goldbourt A. Determination of the lithium binding site in inositol monophosphatase, the putative target for lithium therapy, by magic-angle-spinning solid-state NMR. J Am Chem Soc. 2012;134(12):5647–5651. doi: 10.1021/ja211794x. [DOI] [PubMed] [Google Scholar]
- 119.Leech AP, Baker GR, Shute JK, Cohen MA, Gani D. Chemical and kinetic mechanism of the inositol monophosphatase reaction and its inhibition by Li+ Eur J Biochem/FEBS. 1993;212(3):693–704. doi: 10.1111/j.1432-1033.1993.tb17707.x. [DOI] [PubMed] [Google Scholar]
- 120.Dudev T, Lim C. Competition between Li + and Mg2 + in metalloproteins. Implications for lithium therapy. J Am Chem Soc. 2011;133(24):9506–9515. doi: 10.1021/ja201985s. [DOI] [PubMed] [Google Scholar]
- 121.Lu SY, Jiang YJ, Zou JW, Wu TX. Dissection of the difference between the group I metal ions in inhibiting GSK3beta: a computational study. Phys Chem Chem Phys. 2011;13(15):7014–7023. doi: 10.1039/c0cp02498h. [DOI] [PubMed] [Google Scholar]
- 122.Ciani L, Boyle KA, Dickins E, Sahores M, Anane D, Lopes DM, Gibb AJ, Salinas PC. Wnt7a signaling promotes dendritic spine growth and synaptic strength through Ca(2)(+)/Calmodulin-dependent protein kinase II. Proc Natl Acad Sci USA. 2011;108(26):10732–10737. doi: 10.1073/pnas.1018132108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yu X, Malenka RC. Beta-catenin is critical for dendritic morphogenesis. Nat Neurosci. 2003;6(11):1169–1177. doi: 10.1038/nn1132. [DOI] [PubMed] [Google Scholar]
- 124.Beaulieu JM, Gainetdinov RR, Caron MG. Akt/GSK3 signaling in the action of psychotropic drugs. Annu Rev Pharmacol Toxicol. 2009;49:327–347. doi: 10.1146/annurev.pharmtox.011008.145634. [DOI] [PubMed] [Google Scholar]
- 125.Urs NM, Snyder JC, Jacobsen JP, Peterson SM, Caron MG. Deletion of GSK3beta in D2R-expressing neurons reveals distinct roles for beta-arrestin signaling in antipsychotic and lithium action. Proc Natl Acad Sci USA. 2012;109(50):20732–20737. doi: 10.1073/pnas.1215489109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Du J, Wei Y, Liu L, Wang Y, Khairova R, Blumenthal R, Tragon T, Hunsberger JG, Machado-Vieira R, Drevets W, Wang YT, Manji HK. A kinesin signaling complex mediates the ability of GSK-3beta to affect mood-associated behaviors. Proc Natl Acad Sci USA. 2010;107(25):11573–11578. doi: 10.1073/pnas.0913138107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Caldero J, Brunet N, Tarabal O, Piedrafita L, Hereu M, Ayala V, Esquerda JE. Lithium prevents excitotoxic cell death of motoneurons in organotypic slice cultures of spinal cord. Neuroscience. 2010;165(4):1353–1369. doi: 10.1016/j.neuroscience.2009.11.034. [DOI] [PubMed] [Google Scholar]
- 128.Zhu LQ, Wang SH, Liu D, Yin YY, Tian Q, Wang XC, Wang Q, Chen JG, Wang JZ. Activation of glycogen synthase kinase-3 inhibits long-term potentiation with synapse-associated impairments. J Neurosci. 2007;27(45):12211–12220. doi: 10.1523/JNEUROSCI.3321-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hashimoto R, Takei N, Shimazu K, Christ L, Lu B, Chuang DM. Lithium induces brain-derived neurotrophic factor and activates TrkB in rodent cortical neurons: an essential step for neuroprotection against glutamate excitotoxicity. Neuropharmacology. 2002;43(7):1173–1179. doi: 10.1016/s0028-3908(02)00217-4. [DOI] [PubMed] [Google Scholar]
- 130.Kopnisky KL, Chalecka-Franaszek E, Gonzalez-Zulueta M, Chuang DM. Chronic lithium treatment antagonizes glutamate-induced decrease of phosphorylated CREB in neurons via reducing protein phosphatase 1 and increasing MEK activities. Neuroscience. 2003;116(2):425–435. doi: 10.1016/s0306-4522(02)00573-0. [DOI] [PubMed] [Google Scholar]
- 131.Berger-Sweeney J. The cholinergic basal forebrain system during development and its influence on cognitive processes: important questions and potential answers. Neurosci Biobehav Rev. 2003;27(4):401–411. doi: 10.1016/s0149-7634(03)00070-8. [DOI] [PubMed] [Google Scholar]
- 132.AhnAllen CG. The role of the alpha7 nicotinic receptor in cognitive processing of persons with schizophrenia. Curr opin Psychiatry. 2012;25(2):103–108. doi: 10.1097/YCO.0b013e3283503637. [DOI] [PubMed] [Google Scholar]
- 133.Thomsen MS, Weyn A, Mikkelsen JD. Hippocampal alpha7 nicotinic acetylcholine receptor levels in patients with schizophrenia, bipolar disorder, or major depressive disorder. Bipolar Disord. 2011;13(7–8):701–707. doi: 10.1111/j.1399-5618.2011.00961.x. [DOI] [PubMed] [Google Scholar]
- 134.Langmead CJ, Watson J, Reavill C. Muscarinic acetylcholine receptors as CNS drug targets. Pharmacol Ther. 2008;117(2):232–243. doi: 10.1016/j.pharmthera.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 135.Farias GG, Valles AS, Colombres M, Godoy JA, Toledo EM, Lukas RJ, Barrantes FJ, Inestrosa NC. Wnt-7a induces presynaptic colocalization of alpha 7-nicotinic acetylcholine receptors and adenomatous polyposis coli in hippocampal neurons. J Neurosci. 2007;27(20):5313–5325. doi: 10.1523/JNEUROSCI.3934-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Jensen M, Hoerndli FJ, Brockie PJ, Wang R, Johnson E, Maxfield D, Francis MM, Madsen DM, Maricq AV. Wnt signaling regulates acetylcholine receptor translocation and synaptic plasticity in the adult nervous system. Cell. 2012;149(1):173–187. doi: 10.1016/j.cell.2011.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Beurel E, Jope RS. The paradoxical pro- and anti-apoptotic actions of GSK3 in the intrinsic and extrinsic apoptosis signaling pathways. Prog Neurobiol. 2006;79(4):173–189. doi: 10.1016/j.pneurobio.2006.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bielecka AM, Obuchowicz E. Antiapoptotic action of lithium and valproate. Pharmacol Rep. 2008;60(6):771–782. [PubMed] [Google Scholar]
- 139.Ngok-Ngam P, Watcharasit P, Thiantanawat A, Satayavivad J. Pharmacological inhibition of GSK3 attenuates DNA damage-induced apoptosis via reduction of p53 mitochondrial translocation and Bax oligomerization in neuroblastoma SH-SY5Y CELLS. Cell Mol Biol Lett. 2013;18(1):58–74. doi: 10.2478/s11658-012-0039-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chiu CT, Chuang DM. Molecular actions and therapeutic potential of lithium in preclinical and clinical studies of CNS disorders. Pharmacol Ther. 2010;128(2):281–304. doi: 10.1016/j.pharmthera.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lazarus JH. Lithium and thyroid. Best Pract Res Clin Endocrinol Metab. 2009;23(6):723–733. doi: 10.1016/j.beem.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 142.St Germain DL. Regulatory effect of lithium on thyroxine metabolism in murine neural and anterior pituitary tissue. Endocrinology. 1987;120(4):1430–1438. doi: 10.1210/endo-120-4-1430. [DOI] [PubMed] [Google Scholar]
- 143.Baumgartner A, Pinna G, Hiedra L, Gaio U, Hessenius C, Campos-Barros A, Eravci M, Prengel H, Thoma R, Meinhold H. Effects of lithium and carbamazepine on thyroid hormone metabolism in rat brain. Neuropsychopharmacology. 1997;16(1):25–41. doi: 10.1016/S0893-133X(96)00144-3. [DOI] [PubMed] [Google Scholar]
- 144.Eravci M, Pinna G, Meinhold H, Baumgartner A. Effects of pharmacological and nonpharmacological treatments on thyroid hormone metabolism and concentrations in rat brain. Endocrinology. 2000;141(3):1027–1040. doi: 10.1210/endo.141.3.7358. [DOI] [PubMed] [Google Scholar]
- 145.Pinna G, Broedel O, Eravci M, Stoltenburg-Didinger G, Plueckhan H, Fuxius S, Meinhold H, Baumgartner A. Thyroid hormones in the rat amygdala as common targets for antidepressant drugs, mood stabilizers, and sleep deprivation. Biol Psychiatry. 2003;54(10):1049–1059. doi: 10.1016/s0006-3223(03)00414-1. [DOI] [PubMed] [Google Scholar]
- 146.Hahn CG, Pawlyk AC, Whybrow PC, Gyulai L, Tejani-Butt SM. Lithium administration affects gene expression of thyroid hormone receptors in rat brain. Life Sci. 1999;64(20):1793–1802. doi: 10.1016/s0024-3205(99)00121-6. [DOI] [PubMed] [Google Scholar]
- 147.Hahn CG, Pawlyk AC, Whybrow PC, Tejani-Butt SM. Differential expression of thyroid hormone receptor isoforms by thyroid hormone and lithium in rat GH3 and B103 cells. Biol Psychiatry. 1999;45(8):1004–1012. doi: 10.1016/s0006-3223(98)00164-4. [DOI] [PubMed] [Google Scholar]
- 148.Lin YF, Huang MC, Liu HC. Glycogen synthase kinase 3beta gene polymorphisms may be associated with bipolar I disorder and the therapeutic response to lithium. J Affect Disord. 2012 doi: 10.1016/j.jad.2012.08.025. [DOI] [PubMed] [Google Scholar]
- 149.Lachman HM, Pedrosa E, Petruolo OA, Cockerham M, Papolos A, Novak T, Papolos DF, Stopkova P. Increase in GSK3beta gene copy number variation in bipolar disorder. Am J Med Genet Part B, Neuropsychiatric Genet. 2007;144B(3):259–265. doi: 10.1002/ajmg.b.30498. [DOI] [PubMed] [Google Scholar]
- 150.Bearden CE, Thompson PM, Dalwani M, Hayashi KM, Lee AD, Nicoletti M, Trakhtenbroit M, Glahn DC, Brambilla P, Sassi RB, Mallinger AG, Frank E, Kupfer DJ, Soares JC. Greater cortical gray matter density in lithium-treated patients with bipolar disorder. Biol Psychiatry. 2007;62(1):7–16. doi: 10.1016/j.biopsych.2006.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chuang DM, Manji HK. In search of the Holy Grail for the treatment of neurodegenerative disorders: has a simple cation been overlooked? Biol Psychiatry. 2007;62(1):4–6. doi: 10.1016/j.biopsych.2007.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.O’Brien WT, Harper AD, Jove F, Woodgett JR, Maretto S, Piccolo S, Klein PS. Glycogen synthase kinase-3beta haploinsufficiency mimics the behavioral and molecular effects of lithium. J Neurosci. 2004;24(30):6791–6798. doi: 10.1523/JNEUROSCI.4753-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Omata N, Chiu CT, Moya PR, Leng Y, Wang Z, Hunsberger JG, Leeds P, Chuang DM. Lentivirally mediated GSK-3beta silencing in the hippocampal dentate gyrus induces antidepressant-like effects in stressed mice. The international journal of neuropsychopharmacology/official scientific journal of the Collegium Internationale Neuropsychopharmacologicum. 2011;14(5):711–717. doi: 10.1017/S1461145710000726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Gould TD, Einat H, O’Donnell KC, Picchini AM, Schloesser RJ, Manji HK. Beta-catenin overexpression in the mouse brain phenocopies lithium-sensitive behaviors. Neuropsychopharmacology. 2007;32(10):2173–2183. doi: 10.1038/sj.npp.1301338. [DOI] [PubMed] [Google Scholar]
- 155.Polter A, Beurel E, Yang S, Garner R, Song L, Miller CA, Sweatt JD, McMahon L, Bartolucci AA, Li X, Jope RS. Deficiency in the inhibitory serine-phosphorylation of glycogen synthase kinase-3 increases sensitivity to mood disturbances. Neuropsychopharmacology. 2010;35(8):1761–1774. doi: 10.1038/npp.2010.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Prickaerts J, Moechars D, Cryns K, Lenaerts I, van Craenendonck H, Goris I, Daneels G, Bouwknecht JA, Steckler T. Transgenic mice overexpressing glycogen synthase kinase 3beta: a putative model of hyperactivity and mania. J Neuroscience. 2006;26(35):9022–9029. doi: 10.1523/JNEUROSCI.5216-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Fedorenko O, Strutz-Seebohm N, Henrion U, Ureche ON, Lang F, Seebohm G, Lang UE. A schizophrenia-linked mutation in PIP5K2A fails to activate neuronal M channels. Psychopharmacology. 2008;199(1):47–54. doi: 10.1007/s00213-008-1095-x. [DOI] [PubMed] [Google Scholar]
- 158.Wildburger NC, Laezza F. Control of neuronal ion channel function by glycogen synthase kinase-3: new prospective for an old kinase. Frontiers Mol Neurosci. 2012;5:80. doi: 10.3389/fnmol.2012.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Yin L, Wang J, Klein PS, Lazar MA. Nuclear receptor Rev-erbalpha is a critical lithium-sensitive component of the circadian clock. Science. 2006;311(5763):1002–1005. doi: 10.1126/science.1121613. [DOI] [PubMed] [Google Scholar]
- 160.Padiath QS, Paranjpe D, Jain S, Sharma VK. Glycogen synthase kinase 3beta as a likely target for the action of lithium on circadian clocks. Chronobiol Int. 2004;21(1):43–55. doi: 10.1081/cbi-120027981. [DOI] [PubMed] [Google Scholar]
- 161.Kozikowski AP, Gunosewoyo H, Guo S, Gaisina IN, Walter RL, Ketcherside A, McClung CA, Mesecar AD, Caldarone B. Identification of a glycogen synthase kinase-3beta inhibitor that attenuates hyperactivity in CLOCK mutant mice. Chem Med Chem. 2011;6(9):1593–1602. doi: 10.1002/cmdc.201100188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zhou R, Yuan P, Wang Y, Hunsberger JG, Elkahloun A, Wei Y, Damschroder-Williams P, Du J, Chen G, Manji HK. Evidence for selective microRNAs and their effectors as common long-term targets for the actions of mood stabilizers. Neuropsychopharmacology. 2009;34(6):1395–1405. doi: 10.1038/npp.2008.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kishimoto M, Ujike H, Okahisa Y, Kotaka T, Takaki M, Kodama M, Inada T, Yamada M, Uchimura N, Iwata N, Sora I, Iyo M, Ozaki N, Kuroda S. The Frizzled 3 gene is associated with methamphetamine psychosis in the Japanese population. Behav Brain Funct. 2008;4:37. doi: 10.1186/1744-9081-4-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lovestone S, Killick R, Di Forti M, Murray R. Schizophrenia as a GSK-3 dysregulation disorder. Trends Neurosci. 2007;30(4):142–149. doi: 10.1016/j.tins.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 165.Proitsi P, Li T, Hamilton G, Di Forti M, Collier D, Killick R, Chen R, Sham P, Murray R, Powell J, Lovestone S. Positional pathway screen of wnt signaling genes in schizophrenia: association with DKK4. Biol Psychiatry. 2008;63(1):13–16. doi: 10.1016/j.biopsych.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 166.Cui DH, Jiang KD, Jiang SD, Xu YF, Yao H. The tumor suppressor adenomatous polyposis coli gene is associated with susceptibility to schizophrenia. Mol Psychiatry. 2005;10(7):669–677. doi: 10.1038/sj.mp.4001653. [DOI] [PubMed] [Google Scholar]
- 167.Glatt SJ, Everall IP, Kremen WS, Corbeil J, Sasik R, Khanlou N, Han M, Liew CC, Tsuang MT. Comparative gene expression analysis of blood and brain provides concurrent validation of SELENBP1 up-regulation in schizophrenia. Proc Natl Acad Sci USA. 2005;102(43):15533–15538. doi: 10.1073/pnas.0507666102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kozlovsky N, Nadri C, Agam G. Low GSK-3beta in schizophrenia as a consequence of neurodevelopmental insult. Eur Neuropsychopharmacol. 2005;15(1):1–11. doi: 10.1016/j.euroneuro.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 169.Carman JS, Bigelow LB, Wyatt RJ. Lithium combined with neuroleptics in chronic schizophrenic and schizoaffective patients. J Clin Psychiatry. 1981;42(3):124–128. [PubMed] [Google Scholar]
- 170.Wolff-Menzler C, Hasan A, Malchow B, Falkai P, Wobrock T. Combination therapy in the treatment of schizophrenia. Pharmacopsychiatry. 2010;43(4):122–129. doi: 10.1055/s-0030-1249097. [DOI] [PubMed] [Google Scholar]
- 171.Porteous DJ, Millar JK, Brandon NJ, Sawa A. DISC1 at 10: connecting psychiatric genetics and neuroscience. Trends Mol Med. 2011;17(12):699–706. doi: 10.1016/j.molmed.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Blackwood DH, Fordyce A, Walker MT, St Clair DM, Porteous DJ, Muir WJ. Schizophrenia and affective disorders–cosegregation with a translocation at chromosome 1q42 that directly disrupts brain-expressed genes: clinical and P300 findings in a family. Am J Hum Genet. 2001;69(2):428–433. doi: 10.1086/321969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Marin O. Interneuron dysfunction in psychiatric disorders. Nat Rev Neurosci. 2012;13(2):107–120. doi: 10.1038/nrn3155. [DOI] [PubMed] [Google Scholar]
- 174.Kim JY, Liu CY, Zhang F, Duan X, Wen Z, Song J, Feighery E, Lu B, Rujescu D, St Clair D, Christian K, Callicott JH, Weinberger DR, Song H, Ming GL. Interplay between DISC1 and GABA signaling regulates neurogenesis in mice and risk for schizophrenia. Cell. 2012;148(5):1051–1064. doi: 10.1016/j.cell.2011.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Steinecke A, Gampe C, Valkova C, Kaether C, Bolz J. Disrupted-in-Schizophrenia 1 (DISC1) is necessary for the correct migration of cortical interneurons. J Neurosci. 2012;32(2):738–745. doi: 10.1523/JNEUROSCI.5036-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Mao Y, Ge X, Frank CL, Madison JM, Koehler AN, Doud MK, Tassa C, Berry EM, Soda T, Singh KK, Biechele T, Petryshen TL, Moon RT, Haggarty SJ, Tsai LH. Disrupted in schizophrenia 1 regulates neuronal progenitor proliferation via modulation of GSK3beta/beta-catenin signaling. Cell. 2009;136(6):1017–1031. doi: 10.1016/j.cell.2008.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Singh KK, Ge X, Mao Y, Drane L, Meletis K, Samuels BA, Tsai LH. Dixdc1 is a critical regulator of DISC1 and embryonic cortical development. Neuron. 2010;67(1):33–48. doi: 10.1016/j.neuron.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kivimae S, Martin PM, Kapfhamer D, Ruan Y, Heberlein U, Rubenstein JL, Cheyette BN. Abnormal behavior in mice mutant for the Disc1 binding partner, Dixdc1. Transl psychiatry. 2011;1:e43. doi: 10.1038/tp.2011.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Liu YT, Dan QJ, Wang J, Feng Y, Chen L, Liang J, Li Q, Lin SC, Wang ZX, Wu JW. Molecular basis of Wnt activation via the DIX domain protein Ccd1. J Biol Chem. 2011;286(10):8597–8608. doi: 10.1074/jbc.M110.186742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Flores R, 3rd, Hirota Y, Armstrong B, Sawa A, Tomoda T. DISC1 regulates synaptic vesicle transport via a lithium-sensitive pathway. Neurosci Res. 2011;71(1):71–77. doi: 10.1016/j.neures.2011.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Baudry A, Mouillet-Richard S, Schneider B, Launay JM, Kellermann O. miR-16 targets the serotonin transporter: a new facet for adaptive responses to antidepressants. Science. 2010;329(5998):1537–1541. doi: 10.1126/science.1193692. [DOI] [PubMed] [Google Scholar]
- 182.Launay JM, Mouillet-Richard S, Baudry A, Pietri M, Kellermann O. Raphe-mediated signals control the hippocampal response to SRI antidepressants via miR-16. Transl psychiatry. 2011;1:e56. doi: 10.1038/tp.2011.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Pei JJ, Braak E, Braak H, Grundke-Iqbal I, Iqbal K, Winblad B, Cowburn RF. Distribution of active glycogen synthase kinase 3beta (GSK-3beta) in brains staged for Alzheimer disease neurofibrillary changes. J Neuropathol Exp Neurol. 1999;58(9):1010–1019. doi: 10.1097/00005072-199909000-00011. [DOI] [PubMed] [Google Scholar]
- 184.Zhang Z, Hartmann H, Do VM, Abramowski D, Sturchler-Pierrat C, Staufenbiel M, Sommer B, van de Wetering M, Clevers H, Saftig P, De Strooper B, He X, Yankner BA. Destabilization of beta-catenin by mutations in presenilin-1 potentiates neuronal apoptosis. Nature. 1998;395(6703):698–702. doi: 10.1038/27208. [DOI] [PubMed] [Google Scholar]
- 185.Alvarez AR, Godoy JA, Mullendorff K, Olivares GH, Bronfman M, Inestrosa NC. Wnt-3a overcomes beta-amyloid toxicity in rat hippocampal neurons. Exp Cell Res. 2004;297(1):186–196. doi: 10.1016/j.yexcr.2004.02.028. [DOI] [PubMed] [Google Scholar]
- 186.De Ferrari GV, Chacon MA, Barria MI, Garrido JL, Godoy JA, Olivares G, Reyes AE, Alvarez A, Bronfman M, Inestrosa NC. Activation of Wnt signaling rescues neurodegeneration and behavioral impairments induced by beta-amyloid fibrils. Mol psychiatry. 2003;8(2):195–208. doi: 10.1038/sj.mp.4001208. [DOI] [PubMed] [Google Scholar]
- 187.Phiel CJ, Wilson CA, Lee VM, Klein PS. GSK-3alpha regulates production of Alzheimer’s disease amyloid-beta peptides. Nature. 2003;423(6938):435–439. doi: 10.1038/nature01640. [DOI] [PubMed] [Google Scholar]
- 188.Sofola O, Kerr F, Rogers I, Killick R, Augustin H, Gandy C, Allen MJ, Hardy J, Lovestone S, Partridge L. Inhibition of GSK-3 ameliorates Abeta pathology in an adult-onset Drosophila model of Alzheimer’s disease. PLoS Genet. 2010;6(9):e10011087. doi: 10.1371/journal.pgen.1001087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Sun X, Sato S, Murayama O, Murayama M, Park JM, Yamaguchi H, Takashima A. Lithium inhibits amyloid secretion in COS7 cells transfected with amyloid precursor protein C100. Neurosci Lett. 2002;321(1–2):61–64. doi: 10.1016/s0304-3940(01)02583-6. [DOI] [PubMed] [Google Scholar]
- 190.Medina M, Garrido JJ, Wandosell FG. Modulation of GSK-3 as a therapeutic strategy on tau pathologies. Frontiers Mol Neurosci. 2011;4:24. doi: 10.3389/fnmol.2011.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Noble W, Planel E, Zehr C, Olm V, Meyerson J, Suleman F, Gaynor K, Wang L, LaFrancois J, Feinstein B, Burns M, Krishnamurthy P, Wen Y, Bhat R, Lewis J, Dickson D, Duff K. Inhibition of glycogen synthase kinase-3 by lithium correlates with reduced tauopathy and degeneration in vivo. Proc Natl Acad Sci USA. 2005;102(19):6990–6995. doi: 10.1073/pnas.0500466102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Perez M, Hernandez F, Lim F, Diaz-Nido J, Avila J. Chronic lithium treatment decreases mutant tau protein aggregation in a transgenic mouse model. J Alzheimer’s Dis. 2003;5(4):301–308. doi: 10.3233/jad-2003-5405. [DOI] [PubMed] [Google Scholar]
- 193.Nakashima H, Ishihara T, Suguimoto P, Yokota O, Oshima E, Kugo A, Terada S, Hamamura T, Trojanowski JQ, Lee VM, Kuroda S. Chronic lithium treatment decreases tau lesions by promoting ubiquitination in a mouse model of tauopathies. Acta Neuropathol. 2005;110(6):547–556. doi: 10.1007/s00401-005-1087-4. [DOI] [PubMed] [Google Scholar]
- 194.Rametti A, Esclaire F, Yardin C, Cogne N, Terro F. Lithium down-regulates tau in cultured cortical neurons: a possible mechanism of neuroprotection. Neurosci Lett. 2008;434(1):93–98. doi: 10.1016/j.neulet.2008.01.034. [DOI] [PubMed] [Google Scholar]
- 195.Leroy K, Ando K, Heraud C, Yilmaz Z, Authelet M, Boeynaems JM, Buee L, De Decker R, Brion JP. Lithium treatment arrests the development of neurofibrillary tangles in mutant tau transgenic mice with advanced neurofibrillary pathology. J Alzheimer’s Dis. 2010;19(2):705–719. doi: 10.3233/JAD-2010-1276. [DOI] [PubMed] [Google Scholar]
- 196.Rockenstein E, Torrance M, Adame A, Mante M, Bar-on P, Rose JB, Crews L, Masliah E. Neuroprotective effects of regulators of the glycogen synthase kinase-3beta signaling pathway in a transgenic model of alzheimer’s disease are associated with reduced amyloid precursor protein phosphorylation. J Neurosci. 2007;27(8):1981–1991. doi: 10.1523/JNEUROSCI.4321-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Toledo EM, Inestrosa NC. Activation of Wnt signaling by lithium and rosiglitazone reduced spatial memory impairment and neurodegeneration in brains of an APPswe/PSEN1DeltaE9 mouse model of alzheimer’s disease. Mol Psychiatry. 2010;15(3):272–285. doi: 10.1038/mp.2009.72. [DOI] [PubMed] [Google Scholar]
- 198.Li HL, Wang HH, Liu SJ, Deng YQ, Zhang YJ, Tian Q, Wang XC, Chen XQ, Yang Y, Zhang JY, Wang Q, Xu H, Liao FF, Wang JZ. Phosphorylation of tau antagonizes apoptosis by stabilizing beta-catenin, a mechanism involved in Alzheimer’s neurodegeneration. Proc Natl Acad Sci USA. 2007;104(9):3591–3596. doi: 10.1073/pnas.0609303104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Martin L, Page G, Terro F. Tau phosphorylation and neuronal apoptosis induced by the blockade of PP2A preferentially involve GSK3beta. Neurochem Int. 2011;59(2):235–250. doi: 10.1016/j.neuint.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 200.Tsuji S, Morinobu S, Tanaka K, Kawano K, Yamawaki S. Lithium, but not valproate, induces the serine/threonine phosphatase activity of protein phosphatase 2A in the rat brain, without affecting its expression. J Neural Transm. 2003;110(4):413–425. doi: 10.1007/s00702-002-0798-0. [DOI] [PubMed] [Google Scholar]
- 201.Martin L, Magnaudeix A, Esclaire F, Yardin C, Terro F. Inhibition of glycogen synthase kinase-3beta downregulates total tau proteins in cultured neurons and its reversal by the blockade of protein phosphatase-2A. Brain Res. 2009;1252:66–75. doi: 10.1016/j.brainres.2008.11.057. [DOI] [PubMed] [Google Scholar]
- 202.Chacon MA, Varela-Nallar L, Inestrosa NC. Frizzled-1 is involved in the neuroprotective effect of Wnt3a against Abeta oligomers. J Cell Physiol. 2008;217(1):215–227. doi: 10.1002/jcp.21497. [DOI] [PubMed] [Google Scholar]
- 203.Magdesian MH, Carvalho MM, Mendes FA, Saraiva LM, Juliano MA, Juliano L, Garcia-Abreu J, Ferreira ST. Amyloid-beta binds to the extracellular cysteine-rich domain of Frizzled and inhibits Wnt/beta-catenin signaling. J Bio Chem. 2008;283(14):9359–9368. doi: 10.1074/jbc.M707108200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Caricasole A, Copani A, Caraci F, Aronica E, Rozemuller AJ, Caruso A, Storto M, Gaviraghi G, Terstappen GC, Nicoletti F. Induction of Dickkopf-1, a negative modulator of the Wnt pathway, is associated with neuronal degeneration in alzheimer’s brain. J Neurosci. 2004;24(26):6021–6027. doi: 10.1523/JNEUROSCI.1381-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Scali C, Caraci F, Gianfriddo M, Diodato E, Roncarati R, Pollio G, Gaviraghi G, Copani A, Nicoletti F, Terstappen GC, Caricasole A. Inhibition of Wnt signaling, modulation of tau phosphorylation and induction of neuronal cell death by DKK1. Neurobiol Dis. 2006;24(2):254–265. doi: 10.1016/j.nbd.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 206.Purro SA, Dickins EM, Salinas PC. The secreted Wnt antagonist Dickkopf-1 is required for amyloid beta-mediated synaptic loss. J Neurosci. 2012;32(10):3492–3498. doi: 10.1523/JNEUROSCI.4562-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Caccamo A, Oddo S, Billings LM, Green KN, Martinez-Coria H, Fisher A, LaFerla FM. M1 receptors play a central role in modulating AD-like pathology in transgenic mice. Neuron. 2006;49(5):671–682. doi: 10.1016/j.neuron.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 208.Wang CY, Zheng W, Wang T, Xie JW, Wang SL, Zhao BL, Teng WP, Wang ZY. Huperzine A activates Wnt/beta-catenin signaling and enhances the nonamyloidogenic pathway in an alzheimer transgenic mouse model. Neuropsychopharmacology. 2011;36(5):1073–1089. doi: 10.1038/npp.2010.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Farias GG, Godoy JA, Hernandez F, Avila J, Fisher A, Inestrosa NC. M1 muscarinic receptor activation protects neurons from beta-amyloid toxicity. A role for Wnt signaling pathway. Neurobio Dis. 2004;17(2):337–348. doi: 10.1016/j.nbd.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 210.Jope RS, Yuskaitis CJ, Beurel E. Glycogen synthase kinase-3 (GSK3): inflammation, diseases, and therapeutics. Neurochem Res. 2007;32(4–5):577–595. doi: 10.1007/s11064-006-9128-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Farias GG, Godoy JA, Vazquez MC, Adani R, Meshulam H, Avila J, Amitai G, Inestrosa NC. The anti-inflammatory and cholinesterase inhibitor bifunctional compound IBU-PO protects from beta-amyloid neurotoxicity by acting on Wnt signaling components. Neurobiol Dis. 2005;18(1):176–183. doi: 10.1016/j.nbd.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 212.Kitazawa M, Cheng D, Tsukamoto MR, Koike MA, Wes PD, Vasilevko V, Cribbs DH, LaFerla FM. Blocking IL-1 signaling rescues cognition, attenuates tau pathology, and restores neuronal beta-catenin pathway function in an Alzheimer’s disease model. J Immunol. 2011;187(12):6539–6549. doi: 10.4049/jimmunol.1100620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Wang X, Liu P, Zhu H, Xu Y, Ma C, Dai X, Huang L, Liu Y, Zhang L, Qin C. miR-34a, a microRNA up-regulated in a double transgenic mouse model of alzheimer’s disease, inhibits bcl2 translation. Brain Res Bull. 2009;80(4–5):268–273. doi: 10.1016/j.brainresbull.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 214.Terao T, Nakano H, Inoue Y, Okamoto T, Nakamura J, Iwata N. Lithium and dementia: a preliminary study. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30(6):1125–1128. doi: 10.1016/j.pnpbp.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 215.Nunes PV, Forlenza OV, Gattaz WF. Lithium and risk for alzheimer’s disease in elderly patients with bipolar disorder. Br J Psychiatry : J Mental Sci. 2007;190:359–360. doi: 10.1192/bjp.bp.106.029868. [DOI] [PubMed] [Google Scholar]
- 216.Forlenza OV, Diniz BS, Radanovic M, Santos FS, Talib LL, Gattaz WF. Disease-modifying properties of long-term lithium treatment for amnestic mild cognitive impairment: randomised controlled trial. Br J Psychiatry: J Mental Sci. 2011;198(5):351–356. doi: 10.1192/bjp.bp.110.080044. [DOI] [PubMed] [Google Scholar]
- 217.Kessing LV, Sondergard L, Forman JL, Andersen PK. Lithium treatment and risk of dementia. Arch Gen Psychiatry. 2008;65(11):1331–1335. doi: 10.1001/archpsyc.65.11.1331. [DOI] [PubMed] [Google Scholar]
- 218.Leyhe T, Eschweiler GW, Stransky E, Gasser T, Annas P, Basun H, Laske C. Increase of BDNF serum concentration in lithium treated patients with early Alzheimer’s disease. J Alzheimer’s Dis. 2009;16(3):649–656. doi: 10.3233/JAD-2009-1004. [DOI] [PubMed] [Google Scholar]
- 219.Macdonald A, Briggs K, Poppe M, Higgins A, Velayudhan L, Lovestone S. A feasibility and tolerability study of lithium in Alzheimer’s disease. Int J Geriatr Psychiatry. 2008;23(7):704–711. doi: 10.1002/gps.1964. [DOI] [PubMed] [Google Scholar]
- 220.Young AH. More good news about the magic ion: lithium may prevent dementia. Br J Psychiatry J Ment Sci. 2011;198(5):336–337. doi: 10.1192/bjp.bp.110.082875. [DOI] [PubMed] [Google Scholar]
- 221.Hooper C, Killick R, Lovestone S. The GSK3 hypothesis of alzheimer’s disease. J Neurochem. 2008;104(6):1433–1439. doi: 10.1111/j.1471-4159.2007.05194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Berger Z, Ttofi EK, Michel CH, Pasco MY, Tenant S, Rubinsztein DC, O’Kane CJ. Lithium rescues toxicity of aggregate-prone proteins in drosophila by perturbing Wnt pathway. Hum Mol Genet. 2005;14(20):3003–3011. doi: 10.1093/hmg/ddi331. [DOI] [PubMed] [Google Scholar]
- 223.Carmichael J, Sugars KL, Bao YP, Rubinsztein DC. Glycogen synthase kinase-3beta inhibitors prevent cellular polyglutamine toxicity caused by the huntington’s disease mutation. J Biol Chem. 2002;277(37):33791–33798. doi: 10.1074/jbc.M204861200. [DOI] [PubMed] [Google Scholar]
- 224.Senatorov VV, Ren M, Kanai H, Wei H, Chuang DM. Short-term lithium treatment promotes neuronal survival and proliferation in rat striatum infused with quinolinic acid, an excitotoxic model of huntington’s disease. Mol Psychiatry. 2004;9(4):371–385. doi: 10.1038/sj.mp.4001463. [DOI] [PubMed] [Google Scholar]
- 225.Wei H, Qin ZH, Senatorov VV, Wei W, Wang Y, Qian Y, Chuang DM. Lithium suppresses excitotoxicity-induced striatal lesions in a rat model of huntington’s disease. Neuroscience. 2001;106(3):603–612. doi: 10.1016/s0306-4522(01)00311-6. [DOI] [PubMed] [Google Scholar]
- 226.Crespo-Biel N, Camins A, Pallas M, Canudas AM. Evidence of calpain/cdk5 pathway inhibition by lithium in 3-nitropropionic acid toxicity in vivo and in vitro. Neuropharmacology. 2009;56(2):422–428. doi: 10.1016/j.neuropharm.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 227.Sarkar S, Krishna G, Imarisio S, Saiki S, O’Kane CJ, Rubinsztein DC. A rational mechanism for combination treatment of Huntington’s disease using lithium and rapamycin. Hum Mol Genet. 2008;17(2):170–178. doi: 10.1093/hmg/ddm294. [DOI] [PubMed] [Google Scholar]
- 228.Godin JD, Poizat G, Hickey MA, Maschat F, Humbert S. Mutant huntingtin-impaired degradation of beta-catenin causes neurotoxicity in Huntington’s disease. EMBO J. 2010;29(14):2433–2445. doi: 10.1038/emboj.2010.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Wood NI, Morton AJ. Chronic lithium chloride treatment has variable effects on motor behaviour and survival of mice transgenic for the Huntington’s disease mutation. Brain Res Bull. 2003;61(4):375–383. doi: 10.1016/s0361-9230(03)00141-2. [DOI] [PubMed] [Google Scholar]
- 230.Chiu CT, Liu G, Leeds P, Chuang DM. Combined treatment with the mood stabilizers lithium and valproate produces multiple beneficial effects in transgenic mouse models of Huntington’s disease. Neuropsychopharmacology. 2011;36(12):2406–2421. doi: 10.1038/npp.2011.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Leng Y, Liang MH, Ren M, Marinova Z, Leeds P, Chuang DM. Synergistic neuroprotective effects of lithium and valproic acid or other histone deacetylase inhibitors in neurons: roles of glycogen synthase kinase-3 inhibition. J Neurosci. 2008;28(10):2576–2588. doi: 10.1523/JNEUROSCI.5467-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Pouladi MA, Brillaud E, Xie Y, Conforti P, Graham RK, Ehrnhoefer DE, Franciosi S, Zhang W, Poucheret P, Compte E, Maurel JC, Zuccato C, Cattaneo E, Neri C, Hayden MR. NP03, a novel low-dose lithium formulation, is neuroprotective in the YAC128 mouse model of Huntington disease. Neurobiol Dis. 2012;48(3):282–289. doi: 10.1016/j.nbd.2012.06.026. [DOI] [PubMed] [Google Scholar]
- 233.Vazey EM, Connor B. Differential fate and functional outcome of lithium chloride primed adult neural progenitor cell transplants in a rat model of Huntington disease. Stem cell Res Ther. 2010;1(5):41. doi: 10.1186/scrt41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Berwick DC, Harvey K. The importance of Wnt signalling for neurodegeneration in Parkinson’s disease. Biochem Soc Trans. 2012;40(5):1123–1128. doi: 10.1042/BST20120122. [DOI] [PubMed] [Google Scholar]
- 235.Chen G, Bower KA, Ma C, Fang S, Thiele CJ, Luo J. Glycogen synthase kinase 3beta (GSK3beta) mediates 6-hydroxydopamine-induced neuronal death. FASEB J. 2004;18(10):1162–1164. doi: 10.1096/fj.04-1551fje. [DOI] [PubMed] [Google Scholar]
- 236.Duka T, Duka V, Joyce JN, Sidhu A. Alpha-synuclein contributes to GSK-3beta-catalyzed Tau phosphorylation in Parkinson’s disease models. FASEB J. 2009;23(9):2820–2830. doi: 10.1096/fj.08-120410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.King TD, Bijur GN, Jope RS. Caspase-3 activation induced by inhibition of mitochondrial complex I is facilitated by glycogen synthase kinase-3beta and attenuated by lithium. Brain Res. 2001;919(1):106–114. doi: 10.1016/s0006-8993(01)03005-0. [DOI] [PubMed] [Google Scholar]
- 238.Castro AA, Ghisoni K, Latini A, Quevedo J, Tasca CI, Prediger RD. Lithium and valproate prevent olfactory discrimination and short-term memory impairments in the intranasal 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) rat model of Parkinson’s disease. Behav Brain Res. 2012;229(1):208–215. doi: 10.1016/j.bbr.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 239.Koh SH, Song C, Noh MY, Kim HY, Lee KY, Lee YJ, Kim J, Kim SH, Kim HT. Inhibition of glycogen synthase kinase-3 reduces L-DOPA-induced neurotoxicity. Toxicology. 2008;247(2–3):112–118. doi: 10.1016/j.tox.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 240.L’Episcopo F, Tirolo C, Testa N, Caniglia S, Morale MC, Deleidi M, Serapide MF, Pluchino S, Marchetti B. Plasticity of subventricular zone neuroprogenitors in MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) mouse model of Parkinson’s disease involves cross talk between inflammatory and Wnt/beta-catenin signaling pathways: functional consequences for neuroprotection and repair. J Neurosci. 2012;32(6):2062–2085. doi: 10.1523/JNEUROSCI.5259-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Youdim MB, Arraf Z. Prevention of MPTP (N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) dopaminergic neurotoxicity in mice by chronic lithium: involvements of Bcl-2 and Bax. Neuropharmacology. 2004;46(8):1130–1140. doi: 10.1016/j.neuropharm.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 242.Arraf Z, Amit T, Youdim MB, Farah R. Lithium and oxidative stress lessons from the MPTP model of Parkinson’s disease. Neurosci Lett. 2012;516(1):57–61. doi: 10.1016/j.neulet.2012.03.055. [DOI] [PubMed] [Google Scholar]
- 243.Kim YH, Rane A, Lussier S, Andersen JK. Lithium protects against oxidative stress-mediated cell death in alpha-synuclein-overexpressing in vitro and in vivo models of Parkinson’s disease. J Neurosci Res. 2011;89(10):1666–1675. doi: 10.1002/jnr.22700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Li XZ, Chen XP, Zhao K, Bai LM, Zhang H, Zhou XP. Therapeutic effects of valproate combined with lithium carbonate on MPTP-induced Parkinsonism in mice: possible mediation through enhanced autophagy. Int J Neurosc. 2013;123(2):73–79. doi: 10.3109/00207454.2012.729234. [DOI] [PubMed] [Google Scholar]
- 245.Xiong N, Jia M, Chen C, Xiong J, Zhang Z, Huang J, Hou L, Yang H, Cao X, Liang Z, Sun S, Lin Z, Wang T. Potential autophagy enhancers attenuate rotenone-induced toxicity in SH-SY5Y. Neuroscience. 2011;199:292–302. doi: 10.1016/j.neuroscience.2011.10.031. [DOI] [PubMed] [Google Scholar]
- 246.Yong Y, Ding H, Fan Z, Luo J, Ke ZJ. Lithium fails to protect dopaminergic neurons in the 6-OHDA model of Parkinson’s disease. Neurochem Res. 2011;36(3):367–374. doi: 10.1007/s11064-010-0368-z. [DOI] [PubMed] [Google Scholar]
- 247.Dun Y, Yang Y, Xiong Z, Feng M, Zhang Y, Wang M, Xiang J, Li G, Ma R. Induction of Dickkopf-1 contributes to the neurotoxicity of MPP(+) in PC12 cells via inhibition of the canonical Wnt signaling pathway. Neuropharmacology. 2012;67C:168–175. doi: 10.1016/j.neuropharm.2012.10.031. [DOI] [PubMed] [Google Scholar]
- 248.Holroyd S, Smith D. Disabling parkinsonism due to lithium: a case report. J Geriatr Psychiatry Neurol. 1995;8(2):118–119. doi: 10.1177/089198879500800208. [DOI] [PubMed] [Google Scholar]
- 249.Loane DJ, Faden AI. Neuroprotection for traumatic brain injury: translational challenges and emerging therapeutic strategies. Trends Pharmacol Sci. 2010;31(12):596–604. doi: 10.1016/j.tips.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Homsi S, Piaggio T, Croci N, Noble F, Plotkine M, Marchand-Leroux C, Jafarian-Tehrani M. Blockade of acute microglial activation by minocycline promotes neuroprotection and reduces locomotor hyperactivity after closed head injury in mice: a twelve-week follow-up study. J Neurotrauma. 2010;27(5):911–921. doi: 10.1089/neu.2009.1223. [DOI] [PubMed] [Google Scholar]
- 251.Siopi E, Calabria S, Plotkine M, Marchand-Leroux C, Jafarian-Tehrani M. Minocycline restores olfactory bulb volume and olfactory behavior after traumatic brain injury in mice. J Neurotrauma. 2012;29(2):354–361. doi: 10.1089/neu.2011.2055. [DOI] [PubMed] [Google Scholar]
- 252.Siopi E, Cho AH, Homsi S, Croci N, Plotkine M, Marchand-Leroux C, Jafarian-Tehrani M. Minocycline restores sAPPalpha levels and reduces the late histopathological consequences of traumatic brain injury in mice. J Neurotrauma. 2011;28(10):2135–2143. doi: 10.1089/neu.2010.1738. [DOI] [PubMed] [Google Scholar]
- 253.Siopi E, Llufriu-Daben G, Cho AH, Vidal-Lletjos S, Plotkine M, Marchand-Leroux C, Jafarian-Tehrani M. Etazolate, an alpha-secretase activator, reduces neuroinflammation and offers persistent neuroprotection following traumatic brain injury in mice. Neuropharmacology. 2012;67C:183–192. doi: 10.1016/j.neuropharm.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 254.Siopi E, Llufriu-Daben G, Fanucchi F, Plotkine M, Marchand-Leroux C, Jafarian-Tehrani M. Evaluation of late cognitive impairment and anxiety states following traumatic brain injury in mice: the effect of minocycline. Neurosci Lett. 2012;511(2):110–115. doi: 10.1016/j.neulet.2012.01.051. [DOI] [PubMed] [Google Scholar]
- 255.Vink R, Nimmo AJ. Multifunctional drugs for head injury. Neurotherapeutics. 2009;6(1):28–42. doi: 10.1016/j.nurt.2008.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Shapira M, Licht A, Milman A, Pick CG, Shohami E, Eldar-Finkelman H. Role of glycogen synthase kinase-3beta in early depressive behavior induced by mild traumatic brain injury. Mol Cell Neurosci. 2007;34(4):571–577. doi: 10.1016/j.mcn.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 257.Zhu ZF, Wang QG, Han BJ, William CP. Neuroprotective effect and cognitive outcome of chronic lithium on traumatic brain injury in mice. Brain Res Bull. 2010;83(5):272–277. doi: 10.1016/j.brainresbull.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 258.Yu F, Wang Z, Tchantchou F, Chiu CT, Zhang Y, Chuang DM. Lithium ameliorates neurodegeneration, suppresses neuroinflammation, and improves behavioral performance in a mouse model of traumatic brain injury. J Neurotrauma. 2012;29(2):362–374. doi: 10.1089/neu.2011.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Yu F, Zhang Y, Chuang DM. Lithium reduces BACE1 overexpression, beta amyloid accumulation, and spatial learning deficits in mice with traumatic brain injury. J Neurotrauma. 2012;29(13):2342–2351. doi: 10.1089/neu.2012.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Dash PK, Johnson D, Clark J, Orsi SA, Zhang M, Zhao J, Grill RJ, Moore AN, Pati S. Involvement of the glycogen synthase kinase-3 signaling pathway in TBI pathology and neurocognitive outcome. PLoS One. 2011;6(9):e24648. doi: 10.1371/journal.pone.0024648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Deb S, Crownshaw T. The role of pharmacotherapy in the management of behaviour disorders in traumatic brain injury patients. Brain Injury. 2004;18(1):1–31. doi: 10.1080/0269905031000110463. [DOI] [PubMed] [Google Scholar]
- 262.Feigenson K, Reid M, See J, Crenshaw EB, 3rd, Grinspan JB. Wnt signaling is sufficient to perturb oligodendrocyte maturation. Mol Cell Neurosci. 2009;42(3):255–265. doi: 10.1016/j.mcn.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 263.Levine S, Saltzman A. Inhibition of experimental allergic encephalomyelitis by lithium chloride: specific effect or nonspecific stress? Immunopharmacology. 1991;22(3):207–213. doi: 10.1016/0162-3109(91)90045-z. [DOI] [PubMed] [Google Scholar]
- 264.De Sarno P, Axtell RC, Raman C, Roth KA, Alessi DR, Jope RS. Lithium prevents and ameliorates experimental autoimmune encephalomyelitis. J Immunol. 2008;181(1):338–345. doi: 10.4049/jimmunol.181.1.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Fancy SP, Harrington EP, Yuen TJ, Silbereis JC, Zhao C, Baranzini SE, Bruce CC, Otero JJ, Huang EJ, Nusse R, Franklin RJ, Rowitch DH. Axin2 as regulatory and therapeutic target in newborn brain injury and remyelination. Nat Neurosci. 2011;14(8):1009–1016. doi: 10.1038/nn.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Azim K, Butt AM. GSK3beta negatively regulates oligodendrocyte differentiation and myelination in vivo. Glia. 2011;59(4):540–553. doi: 10.1002/glia.21122. [DOI] [PubMed] [Google Scholar]
- 267.Galimberti D, Macmurray J, Scalabrini D, Fenoglio C, De Riz M, Comi C, Comings D, Cortini F, Villa C, Serpente M, Cantoni C, Ridolfi E, Fardipoor MH, Leone M, Monaco F, Bresolin N, Scarpini E. GSK3beta genetic variability in patients with Multiple Sclerosis. Neurosci Lett. 2011;497(1):46–48. doi: 10.1016/j.neulet.2011.04.024. [DOI] [PubMed] [Google Scholar]
- 268.Kumar S, Mattan NS, de Vellis J. Canavan disease: a white matter disorder. Ment Retard Dev Disabil Res Rev. 2006;12(2):157–165. doi: 10.1002/mrdd.20108. [DOI] [PubMed] [Google Scholar]
- 269.Baslow MH, Kitada K, Suckow RF, Hungund BL, Serikawa T. The effects of lithium chloride and other substances on levels of brain N-acetyl-l-aspartic acid in Canavan disease-like rats. Neurochem Res. 2002;27(5):403–406. doi: 10.1023/a:1015504031229. [DOI] [PubMed] [Google Scholar]
- 270.Assadi M, Janson C, Wang DJ, Goldfarb O, Suri N, Bilaniuk L, Leone P. Lithium citrate reduces excessive intra-cerebral N-acetyl aspartate in Canavan disease. Eur J Paediatr Neurol. 2010;14(4):354–359. doi: 10.1016/j.ejpn.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 271.Janson CG, Assadi M, Francis J, Bilaniuk L, Shera D, Leone P. Lithium citrate for Canavan disease. Pediatr Neurol. 2005;33(4):235–243. doi: 10.1016/j.pediatrneurol.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 272.Solsona MD, Fernandez LL, Boquet EM, Andres JL. Lithium citrate as treatment of Canavan disease. Clin Neuropharmacol. 2012;35(3):150–151. doi: 10.1097/WNF.0b013e3182515c9d. [DOI] [PubMed] [Google Scholar]
- 273.Benedetti F, Absinta M, Rocca MA, Radaelli D, Poletti S, Bernasconi A, Dallaspezia S, Pagani E, Falini A, Copetti M, Colombo C, Comi G, Smeraldi E, Filippi M. Tract-specific white matter structural disruption in patients with bipolar disorder. Bipolar Disord. 2011;13(4):414–424. doi: 10.1111/j.1399-5618.2011.00938.x. [DOI] [PubMed] [Google Scholar]
- 274.Benedetti F, Bollettini I, Barberi I, Radaelli D, Poletti S, Locatelli C, Pirovano A, Lorenzi C, Falini A, Colombo C, Smeraldi E. Lithium and GSK3-beta promoter gene variants influence white matter microstructure in bipolar disorder. Neuropsychopharmacology. 2013;38(2):313–327. doi: 10.1038/npp.2012.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Adityanjee Munshi KR, Thampy A. The syndrome of irreversible lithium-effectuated neurotoxicity. Clin Neuropharmacol. 2005;28(1):38–49. doi: 10.1097/01.wnf.0000150871.52253.b7. [DOI] [PubMed] [Google Scholar]
- 276.Hashimoto R, Senatorov V, Kanai H, Leeds P, Chuang DM. Lithium stimulates progenitor proliferation in cultured brain neurons. Neuroscience. 2003;117(1):55–61. doi: 10.1016/s0306-4522(02)00577-8. [DOI] [PubMed] [Google Scholar]
- 277.Wexler EM, Geschwind DH, Palmer TD. Lithium regulates adult hippocampal progenitor development through canonical Wnt pathway activation. Mol psychiatry. 2008;13(3):285–292. doi: 10.1038/sj.mp.4002093. [DOI] [PubMed] [Google Scholar]
- 278.Bianchi P, Ciani E, Contestabile A, Guidi S, Bartesaghi R. Lithium restores neurogenesis in the subventricular zone of the Ts65Dn mouse, a model for Down syndrome. Brain Pathol. 2010;20(1):106–118. doi: 10.1111/j.1750-3639.2008.00246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Chen G, Rajkowska G, Du F, Seraji-Bozorgzad N, Manji HK. Enhancement of hippocampal neurogenesis by lithium. J Neurochem. 2000;75(4):1729–1734. doi: 10.1046/j.1471-4159.2000.0751729.x. [DOI] [PubMed] [Google Scholar]
- 280.Son H, Yu IT, Hwang SJ, Kim JS, Lee SH, Lee YS, Kaang BK. Lithium enhances long-term potentiation independently of hippocampal neurogenesis in the rat dentate gyrus. J Neurochem. 2003;85(4):872–881. doi: 10.1046/j.1471-4159.2003.01725.x. [DOI] [PubMed] [Google Scholar]
- 281.Yan XB, Hou HL, Wu LM, Liu J, Zhou JN. Lithium regulates hippocampal neurogenesis by ERK pathway and facilitates recovery of spatial learning and memory in rats after transient global cerebral ischemia. Neuropharmacology. 2007;53(4):487–495. doi: 10.1016/j.neuropharm.2007.06.020. [DOI] [PubMed] [Google Scholar]
- 282.Rong H, Liu TB, Yang KJ, Yang HC, Wu DH, Liao CP, Hong F, Yang HZ, Wan F, Ye XY, Xu D, Zhang X, Chao CA, Shen QJ. MicroRNA-134 plasma levels before and after treatment for bipolar mania. J Psychiatr Res. 2011;45(1):92–95. doi: 10.1016/j.jpsychires.2010.04.028. [DOI] [PubMed] [Google Scholar]
- 283.Saito Y, Saito H. MicroRNAs in cancers and neurodegenerative disorders. Frontiers Genet. 2012;3:194. doi: 10.3389/fgene.2012.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Bartzokis G. Neuroglialpharmacology: myelination as a shared mechanism of action of psychotropic treatments. Neuropharmacology. 2012;62(7):2137–2153. doi: 10.1016/j.neuropharm.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Amit S, Hatzubai A, Birman Y, Andersen JS, Ben-Shushan E, Mann M, Ben-Neriah Y, Alkalay I. Axin-mediated CKI phosphorylation of beta-catenin at Ser 45: a molecular switch for the Wnt pathway. Genes Dev. 2002;16(9):1066–1076. doi: 10.1101/gad.230302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Yanagawa S, Matsuda Y, Lee JS, Matsubayashi H, Sese S, Kadowaki T, Ishimoto A. Casein kinase I phosphorylates the Armadillo protein and induces its degradation in Drosophila. EMBO J. 2002;21(7):1733–1742. doi: 10.1093/emboj/21.7.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Zeng X, Tamai K, Doble B, Li S, Huang H, Habas R, Okamura H, Woodgett J, He X. A dual-kinase mechanism for Wnt co-receptor phosphorylation and activation. Nature. 2005;438(7069):873–877. doi: 10.1038/nature04185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Davidson G, Shen J, Huang YL, Su Y, Karaulanov E, Bartscherer K, Hassler C, Stannek P, Boutros M, Niehrs C. Cell cycle control of wnt receptor activation. Dev Cell. 2009;17(6):788–799. doi: 10.1016/j.devcel.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 289.Taelman VF, Dobrowolski R, Plouhinec JL, Fuentealba LC, Vorwald PP, Gumper I, Sabatini DD, De Robertis EM. Wnt signaling requires sequestration of glycogen synthase kinase 3 inside multivesicular endosomes. Cell. 2010;143(7):1136–1148. doi: 10.1016/j.cell.2010.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Qin Y, Li L, Pan W, Wu D. Regulation of phosphatidylinositol kinases and metabolism by Wnt3a and Dvl. J Biol Chem. 2009;284(34):22544–22548. doi: 10.1074/jbc.M109.014399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Feigin ME, Malbon CC. RGS19 regulates Wnt-beta-catenin signaling through inactivation of Galpha(o) J Cell Sci. 2007;120(Pt 19):3404–3414. doi: 10.1242/jcs.011254. [DOI] [PubMed] [Google Scholar]
- 292.Egger-Adam D, Katanaev VL. The trimeric G protein Go inflicts a double impact on axin in the Wnt/frizzled signaling pathway. Dev Dyn. 2010;239(1):168–183. doi: 10.1002/dvdy.22060. [DOI] [PubMed] [Google Scholar]
- 293.Jung H, Kim HJ, Lee SK, Kim R, Kopachik W, Han JK, Jho EH. Negative feedback regulation of Wnt signaling by Gbetagamma-mediated reduction of Dishevelled. Exp Mol Med. 2009;41(10):695–706. doi: 10.3858/emm.2009.41.10.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Kramps T, Peter O, Brunner E, Nellen D, Froesch B, Chatterjee S, Murone M, Zullig S, Basler K. Wnt/wingless signaling requires BCL9/legless-mediated recruitment of pygopus to the nuclear beta-catenin-TCF complex. Cell. 2002;109(1):47–60. doi: 10.1016/s0092-8674(02)00679-7. [DOI] [PubMed] [Google Scholar]
- 295.Parker DS, Jemison J, Cadigan KM. Pygopus, a nuclear PHD-finger protein required for Wingless signaling in Drosophila. Development. 2002;129(11):2565–2576. doi: 10.1242/dev.129.11.2565. [DOI] [PubMed] [Google Scholar]
- 296.Gan XQ, Wang JY, Xi Y, Wu ZL, Li YP, Li L. Nuclear Dvl, c-Jun, beta-catenin, and TCF form a complex leading to stabilization of beta-catenin-TCF interaction. J Cell Biol. 2008;180(6):1087–1100. doi: 10.1083/jcb.200710050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Parker DS, Ni YY, Chang JL, Li J, Cadigan KM. Wingless signaling induces widespread chromatin remodeling of target loci. Mol Cell Biol. 2008;28(5):1815–1828. doi: 10.1128/MCB.01230-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Sierra J, Yoshida T, Joazeiro CA, Jones KA. The APC tumor suppressor counteracts beta-catenin activation and H3K4 methylation at Wnt target genes. Genes Dev. 2006;20(5):586–600. doi: 10.1101/gad.1385806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Alberts AS. Diaphanous-related Formin homology proteins. Curr Biol. 2002;12(23):R796. doi: 10.1016/s0960-9822(02)01309-x. [DOI] [PubMed] [Google Scholar]