Abstract
The innate immune system recognizes deviation from homeostasis caused by infectious or non-infectious assaults. The threshold for its activation seems to be established by a calibration process that includes sensing of microbial molecular patterns from commensal bacteria and of endogenous signals. It is becoming increasingly clear that adaptive features, a hallmark of the adaptive immune system, can also be identified in the innate immune system. Such adaptations can result in the manifestation of a primed state of immune and tissue cells with a decreased activation threshold. This keeps the system poised to react quickly. Moreover, the fact that the innate immune system recognizes a wide variety of danger signals via pattern recognition receptors that often activate the same signaling pathways allows for heterologous innate immune stimulation. This implies that, for example, the innate immune response to an infection can be modified by co-infections or other innate stimuli. This “design feature” of the innate immune system has many implications for our understanding of individual susceptibility to diseases or responsiveness to therapies and vaccinations. In this article, adaptive features of the innate immune system as well as heterologous innate immunity and their implications are discussed.
Keywords: Heterologous immunity, Innate immunity, Adjuvant, T cell, Inflammation, Contact dermatitis
Introduction
The innate immune system responds immediately to infections, mechanical or chemical assaults which disturb tissue homeostasis. It recognizes a large variety of microbe- (MAMPs) or pathogen-associated molecular patterns (PAMPs) as well as exogenous and endogenous non-microbial danger signals such as damage-associated molecular patterns (DAMPs). These danger signals trigger germline-encoded pattern recognition receptors (PRRs) such as the Toll-like receptors (TLRs) [1–5]. PRR triggering leads to an innate inflammatory response that aims at the restoration of homeostasis and repair of tissue damage. Moreover, this response is a prerequisite for efficient activation of the adaptive immune system, e.g., the priming of naïve T cells by activated dendritic cells (DCs) [6].
Adaptive features as illustrated by affinity maturation, receptor editing and formation of memory cells were always attributed exclusively to the T- and B lymphocytes of the adaptive immune system [7–11]. However, in recent years evidence for adaptive features of the innate immune system is accumulating. These manifest themselves, for example, in a heightened reactivity of innate immune cells following a primary immune response (Fig. 1). Another interesting feature of the innate immune system is the fact that different members of a given PRR family feed into the same or similar signaling pathways. This enables heterologous innate immune stimulation [12] leading, for example, to the amplification of innate signaling by a pathogen through additive or synergistic activation of the same pathways via different PRRs due to co-infection with different pathogens (Figs. 2, 3).
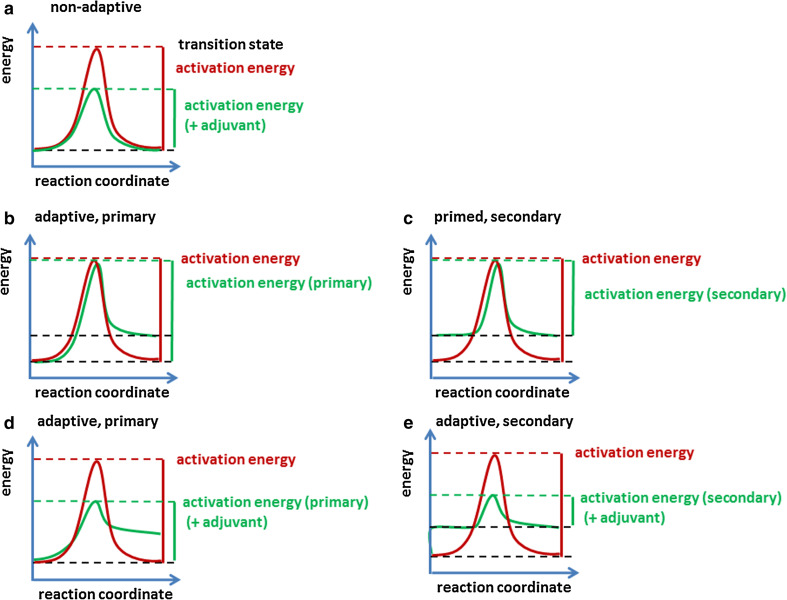
Fig. 1.
Impact of heterologous innate immune stimulation on activation thresholds. An immune reaction can be compared with a chemical reaction. The activation threshold is determined by the activation energy, i.e., the energy difference between the basal energy level and the energy of the transition state. a In the case of a non-adaptive response, the immune system becomes activated and returns to the former, homeostatic energy level that has been established in a calibration process (red line). Heterologous stimuli (e.g., adjuvants) may act like catalysts and lower the energy level of the transition state thereby lowering the activation threshold of the primary response (green line). b If the system is primed by an immune response, it returns to a higher basal energy level in a primary response. c This lowers the activation energy required for the secondary response (green line). d Heterologous stimulation (e.g., by adjuvants) can lower the activation threshold of the primary response and the system can be primed in addition (green line). e The result is an even lower activation energy required for the secondary response (green line)
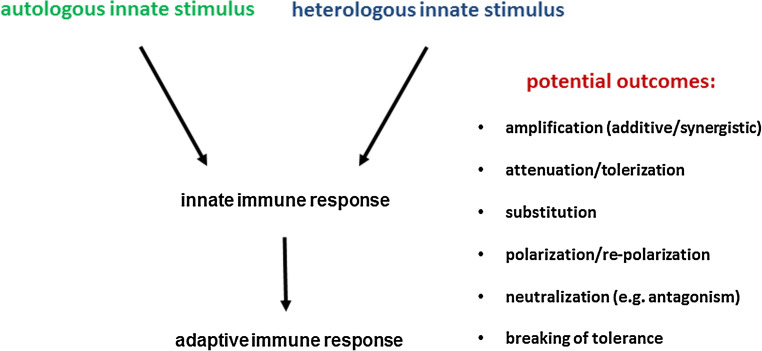
Fig. 2.
Principle of autologous and heterologous innate immune stimulation and potential outcomes of heterologous innate immune stimulation. As an example, upon infection autologous stimuli are associated with the pathogen trigger PRRs such as TLRs (e.g., LPS via TLR4). Co-infection or tissue damage can provide heterologous stimuli acting via the same or different TLRs (e.g., DNA via TLR9). This can lead to different potential outcomes
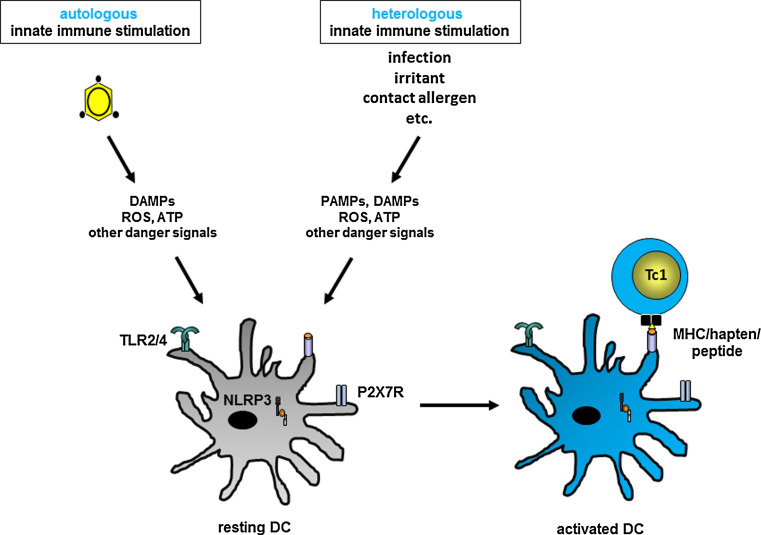
Fig. 3.
Heterologous innate immune stimulation in allergic contact dermatitis. Contact allergens either directly or indirectly trigger PRRs to cause production/release of other danger signals including ROS and ATP. Combinations of contact allergens, addition of irritants or co-infections can provide heterologous innate immune stimulation leading, for example, to additive or synergistic amplification of the innate inflammatory response
Adaptive immune features are also found in bacteria and invertebrates. Bacteria use the clustered regularly interspaced short palindromic repeats (CRISPR)-Cas system to induce adaptive immunity [13]. They incorporate sequences from foreign genetic elements such as viruses and plasmids as spacers (CRISPR spacers) into an array of endogenous repeat sequences (CRISPR repeats). CRISPR loci are flanked by CRISPR-associated (Cas) genes. Transcription of these loci results in the production of small interfering RNAs. These bind to complementary sequences, which are then targeted and degraded by Cas endonucleases. Moreover, invertebrates use RNA interference to generate immunity to infection [14, 15].
Innate adaptation resulting in “trained” innate immunity [16] and heterologous innate immunity are important in our understanding of individual disease susceptibility, responsiveness to therapies or vaccinations. Most importantly, these features open up many possibilities to modulate innate and, subsequently, adaptive immune responses in the treatment of disease and in vaccination strategies. This may be achieved, for example, by altering activation thresholds and using positive or negative stimulatory adjuvants as heterologous innate immune stimuli to promote immunity or tolerance.
This article gives a point-of-view on the “design” and function of the innate immune system with a focus on adaptive features and heterologous innate immunity.
Calibration of the adaptive immune system
The adaptive immune system consists of T- and B cells with highly diverse clonotypic antigen-specific T cell (TCR) and B cell receptors (BCR), respectively. Activation thresholds for T cells are being defined during thymic selection. T cells, which receive survival signals by low-affinity interactions of their TCRs with self-peptide/MHC complexes (pMHC) are positively selected [17]. In the periphery tonic signaling triggered by self-pMHC ensures T cell survival and increased antigen reactivity [18, 19] as well as maintenance of functional memory T cells [20]. The BCR is capable of ligand-independent tonic signaling that regulates B cell survival and differentiation [21, 22]. One of the hallmarks of adaptive immunity is the generation of long-lasting antigen-specific memory. This enables a fast recall response to repeated challenges with previously encountered antigens by recirculating and tissue-resident high-affinity T- and B cells [9, 23–25]. Due to the selection of high-affinity receptors and the independence of co-stimulatory signals, the threshold for the activation of memory T cells is significantly lower.
Calibration of the innate immune system
As in the adaptive immune system, activation thresholds are established for innate immune responses (Fig. 1). This is achieved by a calibration process that involves continuous exposure to homeostatic microbial signals from commensal bacteria at barriers such as skin, lung or gut [26, 27]. In addition, continuous exposure to endogenous danger signals generated during normal cell and extracellular matrix (ECM) turnover might contribute to this calibration process [28–30]. A further calibration system is based on the sensing of endogenous viruses integrated as retroelements into the genome [31]. Cyotosolic DNA sensors can be activated by cDNA intermediates of such retroelements. The sensitivity of this system has to be adjusted such that autoreactivity is prevented without compromising immune responses to DNA viruses and retroviruses such as HIV. Relevant cytosolic DNA sensors are IFI6 or the cyclic GMP-AMP synthase cGAS [32]. These eventually trigger the production of type I interferon production via the ER transmembrane protein STING. Autoreactivity of the innate immune response to endogenous retroelements is prevented in part by enzymes that metabolize cDNA intermediates. Mutations in such enzymes, however, leads to their accumulation and can cause disease. One example is the mutation of the exonuclease Trex1 as found in Aicardi–Goutières syndrome or some forms of systemic lupus erythematosus (SLE) [31]. In this case, accumulation of endogenous DNA triggers the cGAS-STING axis and activation of interferon-stimulated genes. This was prevented in cGAS knockout mice [33]. Moreover, derepression of endogenous retroelements by UV light may cause inflammation.
Thus, the innate immune system must maintain a delicate balance that prevents “self-reactivity” to commensals, endogenous danger signals such as DAMPs or endogenous retroelements under homeostatic conditions and at the same time allows to react to infection or tissue damage. Similar to tonic TCR signaling which preserves antigen reactivity in the naïve T cell pool, tonic innate immune receptor signaling may keep the innate immune system in a more reactive, alert state.
Commensal bacteria are very important in the calibration of the innate immune system. Apart from defects in the development and maturation of the immune system in germ-free mice, the overall fitness of the innate immune system is compromised in their absence [27]. Antibiotic-treated mice have disrupted commensal bacterial communities. Innate immunity at barrier surfaces is impaired as shown, for example, by reduced innate antiviral responses to influenza infection. The production of pro-inflammatory cytokines and chemokines including inflammasome-dependent IL-1β production as well as the type I interferon (IFN) response is compromised [26, 34]. At the cellular level, it has been shown that innate-like lymphocytes such as marginal zone B cells recognize circulating bacterial products with adjuvant properties. They express polyreactive BCRs as well as TLRs that recognize diverse microbial patterns. They are in a pre-activated, primed state and rapidly respond to blood-borne antigens. This primed state seems to depend on circulating microbial products, since germ-free mice have impaired natural antibody responses [35]. Similar priming by commensal-derived signals has been shown for mononuclear phagocytes [36]. Germ-free mice have strongly impaired expression of inflammatory response genes including type I IFNs in these cells from non-lymphoid organs. This is due to the lack of activating histone marks in the cytokine gene promoters preventing binding of the translocated transcription factors NF-κB and IRF3. As a consequence, NK cells are not appropriately primed and antiviral immune responses are compromised. These findings indicate that systemic effects on innate immune fitness are mediated via circulating bacterial products with adjuvant properties.
In summary, the innate immune system is calibrated by commensal microbiota and, most likely, endogenous signals to ensure tissue homeostasis, tissue repair and responsiveness to infections and other, non-infectious challenges. Such challenges can cause priming of innate immune cells, which lowers their activation threshold and keeps the innate immune system poised for more rapid and more efficient responses (Fig. 1).
Other means to lower activation thresholds are the depletion or functional impairment of immunoregulatory cell populations such as regulatory T cells (Treg), invariant NKT cells (iNKT) or myeloid-derived suppressor cells (MDSC) [37–40]. Moreover, genetic defects in pathways that prevent inflammation decrease activation thresholds. An example is Nrf2-deficient mice with a deficient antioxidant phase 2 response. Due to their heightened pro-inflammatory state, chemical-induced skin irritation or allergic contact dermatitis (ACD) are more easily triggered [41].
Mechanisms of adaptation in the innate immune system
Adaptation of the innate immune system to assaults can be due to transient or stable changes in chromatin conformation and microRNA (miRNA) profiles leading to altered gene expression patterns. Thereby, previous immunologic experience can be memorized over a longer period of time. Plants lack an adaptive immune system, but they also recognize PAMPs/MAMPs using PPRs including TLR- and NLR-like receptors [42, 43]. Recognition of PAMPs/MAMPs and other forms of biotic and abiotic stress results in defense priming in plants. This is characterized by a heightened state of resistance and faster and stronger responses to stress including infection. The local response can be transmitted systemically due to systemic acquired resistance (SAR) mediated by plant hormones such as salicylic acid [44]. This innate plant defense system thus exerts adaptive features. The underlying mechanisms include epigenetic modifications that can be heritable [45–47].
Interestingly, short-term innate memory has also been described for immune cells [48]. Histone modifications, some forms of transient DNA methylation and changes in the expression of miRNAs have been identified as potential mechanisms. Epigenetic modifications in macrophages induced by TLR signaling have been reported in the mouse system of LPS tolerance [49]. Here, both silencing of pro-inflammatory and priming of antimicrobial genes was detected identifying an innate immune response with features of adaptive immunity. Such mechanisms may also contribute to the adaptive features of other innate immune cells like NK cells [50]. For NK cells’ memory, T cell-like functions have been identified in antiviral immunity and chemical-induced ACD [51–57]. Monocytes may also display memory-like features [58]. It was shown that T/B cell-deficient mice were protected against reinfection with Candida albicans. This was dependent on monocytes, which exhibited increased cytokine production. Epigenetic changes were suggested to be responsible for this “trained” innate immune response [16, 59]. Interestingly, similar epigenetic modifications in monocytes were observed following Bacillus Calmette Guérin (BCG) vaccination against tuberculosis [60]. This may explain in part the non-specific protective effects of the BCG vaccine on unrelated infections [61, 62] and provides an example for heterologous innate immunity.
Memories of the past: does the tissue memorize previous immune responses?
It is conceivable that the different tissues and organs of an organism can adapt to different immune stimuli. Barrier tissues such as skin, lung or gut adapt to the commensal microbiota. This adaptation process is essential for the establishment of immune homeostasis [27, 63–65]. Disturbance of the microbiota can result in the induction of disease as a consequence of dysbiosis [66–68]. Restoration of a healthy microbiota achieved, for example, by fecal microbiota transplantation (FMT) can ameliorate disease states [69]. In addition, acute and chronic infections may leave traces in the tissue in the form of transient or long-term adaptation processes that may be imprinted by epigenetic modifications such as histone and DNA modifications and altered miRNA expression profiles. Infections may thus shape immune responses to heterologous infections and other challenges at different levels. Eventually, the tissue may be more responsive, poised to react more quickly due to a heightened inflammatory state and, thereby, lowered activation thresholds. Consequently, the quality of the immune response to new, heterologous challenges may be altered. In the case of chronic infections, subsequent immune responses are often impaired [70]. This may indicate an adaptation process that serves to prevent immunopathology by downregulation of immunity. Moreover, the type of immune response may be altered due to changes in the cytokine milieu. As a consequence, the polarization of DC cytokine and co-stimulatory/co-inhibitory receptor expression profiles can be altered affecting the polarization of T cell responses induced by these DCs in local draining lymph nodes, and the reactivity of tissue-resident memory T cells [11] and of other resident or infiltrating immune cells. Moreover, alterations in chemokine profiles may impact the type of immune cells that can be recruited into the tissue. In addition, the existence of tissue-resident memory T cells may be explained in part by sustained chemokine production by tissue cells as part of innate memory-like features [71–73]. The sustained production of CCL27 by keratinocytes is one example. Thus, retention of CCR10 expressing skin homing T cells may underlie the phenomenon of recall contact dermatitis or flare-up reactions at the site of the first contact allergen antigen encounter upon re-challenge with the same contact allergen at a different site [74–76].
Adaptation and susceptibility to allergies
Infections influence the susceptibility to allergy and atopy. This was formulated in the hygiene hypothesis [77]. Today, it is clear that besides genetic predisposition that correlates in part with polymorphisms in innate immune receptor genes such as TLR- and NLR genes [78, 79], exposure to infectious agents and other environmental factors is a critical determinant for susceptibility. Epidemiological evidence shows a correlation of increased hygiene, i.e., reduced microbial exposure, and the rise of allergic and autoimmune diseases [80], but many controversial issues remain to be investigated further [81, 82]. Exposure to infections shapes the innate immune system and subsequently polarizes the T cell response [83]. Pre-natal microbial farm exposure as well as early life exposure and consumption of unprocessed cow’s milk reduces the risk of asthma development [84]. These exposures correlated with an upregulation of TLRs and a bias of the T cell response towards a Th1 rather than a Th2 type [85, 86]. This adaptation and polarization of the pre-natal and perinatal immune system was reflected in epigenetic modifications in asthma candidate genes [87]. Studies in mice have revealed that specific bacteria such as Acinetobacter lwofii or Staphylococcus sciuri W620 as contained in stable dusts in part explain the effects and drive epigenetic modifications, for example, in Th1/Th2-relevant cytokine genes [88–91]. In these studies, a protection of the offspring from airway hyperreactivity was observed when pregnant female mice were exposed to such bacteria. The effect was dependent on maternal expression of Toll-like receptors (TLR) [91, 92]. In addition, intestinal microbiota contributes to the protective effects [93]. Thus, antibiotic-treated mice or germ-free mice revealed higher IgE levels and higher levels of circulating basophils suggesting a role for the commensal microbiota [94]. Interestingly, mice with a B cell-specific defect of MyD88 had a similar phenotype arguing for a contribution of TLR signaling in B cells in shaping the immune system and determining susceptibility to allergies. These findings from mouse and human studies contribute to our understanding of gene–environment interactions and the influence of hygiene in influencing the susceptibility for atopic diseases and asthma [95, 96] by impacting the adaptation and polarization of the innate and, consequently, adaptive immune response.
In general, immune responses are shaped individually owing to the fact that our immune system is not naïve and is modulated transiently or stably by previous or current experience due to adaptation [97]. The innate immune system does not only adapt to its exposures by calibration and heightened reactivity, but also by polarization that translates into the type of T cell response due to T cell subset differentiation. Therefore, individually different immune history and experience and the resulting conditioned tissue or systemic immune microenvironments may in part explain the differences in disease susceptibility and responsiveness to therapies as well as overall immune reactivity. It remains to be determined whether these parameters are also influenced by the great inter-individual variability regarding commensal communities that impact basal calibration and maybe also polarization of the immune system [27, 98].
In summary, the adaptation of the tissue microenvironment to immune challenges, including pre-natal and perinatal innate immune stimulation, by microbial exposure can significantly shape immune responses to future challenges.
Heterologous innate immunity
The innate immune system is typically activated via PRRs. The most prominent PRR families are the TLRs, NOD-like receptors (NLRs), C-type-lectin receptors (CLRs) and RIG-I-like receptors (RLRs) [5, 99–102] and a variety of DNA sensors [32, 103, 104]. These PRRs recognize a diverse array of microbial and non-microbial lipids, proteins, nucleic acids or carbohydrates as danger signals. This broad specificity of PRRs allows the innate immune system to mount similar responses to a diverse array of pathogens, thereby ensuring appropriate immune defense as well as the restoration of tissue homeostasis.
The disadvantage of the restriction of clonotypic TCR- and BCR expression to single cell lineages and of their exquisite antigen specificity is the low cell number specific for a given antigen in primary immune responses. As a consequence, after an infection the necessity for priming, differentiation and expansion of the few naïve T- or B cells with distinct antigen specificity delays an efficient adaptive immune response by days. This time gap is bridged by the immediately activated innate immune system. The advantage of the innate PRR system is that the same PRRs can be found on many different cell types such as innate immune cells and also on non-hematopoietic tissue cells such as epithelial and endothelial cells as well as on T- [105] and B lymphocytes [106]. Different cell types can thus be simultaneously activated by the same array of PAMPs, MAMPs or DAMPs allowing for the generation of a strong and immediate innate immune response. Due to the triggering of the same or similar signaling pathways by different PRRs such as the 10 human and 13 murine functional TLRs, additive or synergistic triggering of several TLRs by ligands that may derive from different sources allows for amplification of innate immune responses (Fig. 4). This make-up of the innate immune system provides the basis for heterologous innate immunity.
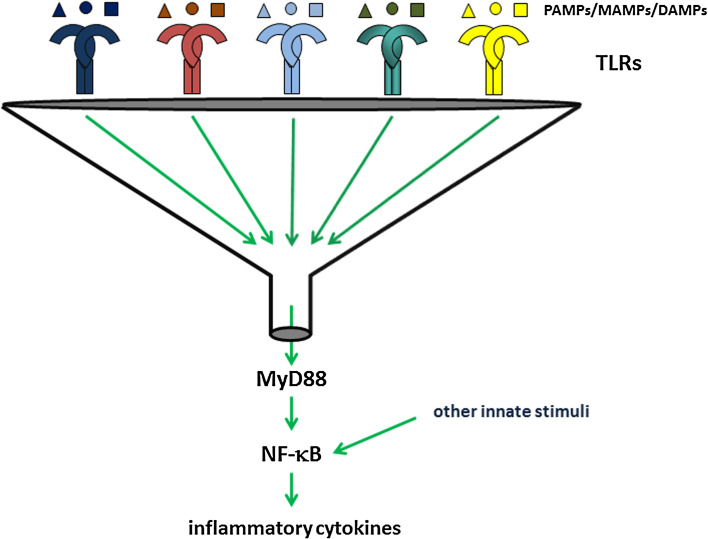
Fig. 4.
Funnel function of TLRs for innate signaling and heterologous innate immunity. Almost all TLRs signal by assembling a signaling complex containing the adaptor protein MyD88. As a result activation of NF-κB and induction of its target genes including the inflammatory cytokines IL-6, IL-12 and TNF-α occurs. MAP kinases and interferon regulatory factors (IRFs) can also be induced. Other, TLR-independent innate stimuli may also trigger the NF-κB pathway
Heterologous immunity was originally defined as cross-reactivity of memory T cells specific for one infectious agent to a new infectious agent or to alloantigens thereby impacting the outcome of heterologous infections, transplantation tolerance and autoimmunity [107–109]. It was shown that cross-reactive T cells can determine the individually different outcomes of a heterologous infection. As an example, mice infected with influenza virus and then later on with lymphocytic choriomeningitis virus (LCMV) developed pneumonitis of various degrees. The severity of the disease directly correlated with the frequency of T cells cross-reactively recognizing epitopes from influenza and LCMV [110].
Heterologous innate immune stimulation can be defined as the triggering of similar or identical signaling pathways, for example, via the same or different PRRs, by danger signals not derived from the original pathogen or agent that induces innate and often adaptive immunity (Figs. 2, 3). In the case of co-infections, heterologous innate stimuli can occur in addition to or instead of the autologous stimuli. Potential outcomes can be additive or synergistic amplification or attenuation/tolerization, substitution, neutralization (e.g., by antagonism), re-polarization of immune responses or breaking of tolerance (Figs. 2, 3) [111, 112]. Another outcome due to a longer-lasting adaptation of the innate immune system to vaccination can be an improved ability of the immune system to handle heterologous infections [61]. These features of the innate immune system can be beneficial or detrimental.
Heterologous innate immunity may explain the impact of previous or current acute or of chronic co-infections on the outcome of innate and adaptive immune responses to a distinct infection [70, 113] or other assaults. Examples are the cross talk of Streptococcus pneumoniae, Staphylococcus aureus and influenza virus in lung infections [114, 115]. A recent study demonstrated synergistic lethal effects of an established influenza infection of mice upon co-infection with Legionella pneumophila [116]. In this study, co-infection caused severe damage to the lung tissue. This was associated with the downregulation of genes involved in tissue protection and repair specifically in the co-infected animals. Interestingly, in mice lacking TNF, caspase-1, TLR2/4, MyD88 or nitric oxide synthase (Nos)2 or after depletion of Gr-1+ or NK1.1+ cells the lethality was not significantly altered. These data demonstrate a synergistic heterologous effect involving infection-induced tissue stress and damage responses. Lethality was due to an irreversible loss of tolerance to tissue damage upon co-infection. Most interestingly, these effects were independent of pathogen burden and the level of innate inflammation, at least for the parameters measured.
Specificity of the innate immune response and heterologous innate immunity
The exquisite antigen specificity of clonotypic TCRs on T cells is illustrated by the ability of human T cells to differentiate between the contact allergens 2,4-dinitro- (DNCB) and 2,4,6-trinitrochlorbenzol (TNCB) [117]. Penicillin-specific human T cells are able to recognize small differences in the side chains of derivatives of this β-lactam antibiotic [118]. Clonotypic TCRs also recognize differences in the amino acid sequence of short antigenic peptides and amino acid modifications.
Despite the notion that a broad spectrum of molecular patterns is recognized by PRRs, they also exhibit ligand specificity. The variety of PRRs recognizes a multitude of different ligands. Individual PRRs, however, exert specificity up to the level of recognizing small molecular differences in ligands, which impact signaling and the resulting response as revealed by the recognition of lipid A variants by the TLR4–MD2 complex [119–121] or the differentiation between fucose and mannose by DC-SIGN [122]. Nevertheless, many different PAMPs and DAMPs are assigned as activators to the same TLR. Different ligand-binding mechanisms have been identified for different TLRs [123]. This may also hold for individual TLRs and explain the diversity of activating ligands for a single TLR.
One very important notion is that similar activation and polarization of DCs with respect to their T cell subset polarizing properties can be achieved by the autologous or heterologous stimulation of different PRRs. This is the key to heterologous innate immune stimulation and allows the innate immune system to flexibly mount similar immune responses to various challenges. In addition, the simultaneous interplay of different TLRs enables fine tuning of the response [124, 125].
The “immunologists dirty little secret” [1] describes the use of heterologous innate immune stimulation in the form of adjuvants for vaccination. The efficient induction of memory responses is the goal of vaccination strategies. In this context, the innate immune system is of great importance for the design of efficient vaccines [126] due to its crucial impact on the activation and polarization of adaptive immune responses [6, 127]. Few adjuvants are licensed for clinical use [128]. They include monophosphoryl lipid A (MPLA) or alum and target TLRs and the NLRP3 inflammasome. Other adjuvants that target RLRs and other nucleic acid sensors are in development [126, 129]. Interestingly, the PAMPs used in vaccines and even more so in experimental studies are often not derived from the target pathogens of the vaccination. This demonstrates the practical relevance of heterologous innate immune stimulation [130, 131]. Attenuated vaccines, however, use the pathogens’ own PAMPs for innate immune receptor signaling addressing various innate immune receptor families [126]. In addition, live vaccines contain so-called vita-PAMPs such as bacterial mRNA, which makes them more efficient than dead vaccines [132]. It will be interesting to study the impact of the composition of heterologous innate immune stimuli on the outcome of vaccinations. Including vita-PAMPs as adjuvants may increase their efficiency.
In addition to triggering T- and B cell responses directed against the target pathogen, unspecific immune stimulation has been shown for vaccines such as the tuberculosis vaccine BCG, measles vaccine and vaccinia virus vaccine [61]. These positive effects are due to adaptation as a consequence of heterologous immunity including both cross-reactive T cells and so-called trained innate immunity [133].
In summary, the rather broad specificity of the innate immune response regarding the diversity of stimuli that can trigger similar outcomes explains why heterologous innate immune stimulation is an important issue to be considered in our understanding of diseases, of therapeutic immune interventions and of vaccination strategies.
Role of PRRs in heterologous innate immunity
The broad specificity of the innate immune system is well illustrated by the diverse array of microbial or endogenous ligands triggering the different mouse and human TLRs. These include proteins, lipids, nucleic acids and carbohydrates. The immune response resulting from the triggering of different TLRs is very similar due to the fact that all TLRs except for TLR3 signal via the adaptor protein MyD88 to activate NF-κB and MAP kinase signaling. TLR4 and TLR3 also signal via the adaptor Trif to induce type I IFNs [5, 28]. Likewise, the great variety of activators for the NLRP3 inflammasome triggers caspase-1 activation and processing of immature pro-IL-1β and pro-IL-18 to their bioactive and secreted forms [134, 135]. RIG-I and MDA5, cytosolic RLRs recognizing RNA from many different sources, signal via the adaptor MAVS to activate NF-κB and IRF3 signaling pathways [136] and the NLRs NOD1 and NOD2 engage the adaptor proteins RIP2 and CARD9 to signal via NF-κB and MAPK and AP1, respectively [137, 138]. Thus, the huge variety of PAMPs and DAMPs elicits similar innate immune responses due to a funnel function of the PRR families that focus the perception of exogenous and endogenous danger signals on a limited number of signaling pathways driving the innate inflammatory response (Fig. 4). For this reason, different combinations of PAMPs and PRRs can result in similar innate inflammatory responses. With respect to the activation and polarization of T cell responses by DCs, this also implies that different TLRs on a given DC can be triggered to yield the same polarized T cell response. The make-up of the innate immune system, therefore, allows for a complete uncoupling of innate and adaptive specificities. This means that the DC that primes and polarizes an antiviral or antibacterial T cell response does not necessarily have to be activated by danger signals represented by PAMPs derived from the pathogen that the T cell response is directed to. Heterologous innate immune stimulation may substitute for the autologous PAMPs (Fig. 4). This notion has a number of implications including additive or synergistic effects of the simultaneous triggering of several TLRs [124]. This may allow overcoming activation thresholds not reached by weak stimulation of a single TLR. In addition, TLR-independent heterologous stimuli can play an important role by triggering the same or other pro-inflammatory innate signaling pathways (Fig. 4).
Heterologous innate immune stimulation and contact dermatitis
Heterologous innate immune stimulation has many implications regarding its potential outcomes (Figs. 2, 3) including the substitution of missing autologous innate stimuli or the amplification of autologous stimuli as exemplified for ACD [111, 112, 139], a sterile inflammatory response induced by protein-reactive metal ions and organic chemicals, but they should be of general importance for immune reactions. ACD is a hypersensitivity reaction of the skin to chemicals. The main effector cells are T cells of the CD4+ Th1/Th17 and CD8+ Tc1/Tc17 subsets. Contact allergens differ in their allergenic potency as determined, for example, in the local lymph node assay (LLNA) by their ability to induce cell proliferation in skin draining lymph nodes following repeated epicutaneous application [140]. The potency seems to correlate with chemical reactivity and the strength of the innate inflammatory response that is induced and translated into the vigor of the T cell response and the allergic reaction [141]. Contact allergens activate the innate immune system directly or indirectly. Direct activation of human TLR4 was shown for nickel and cobalt. These metal ions bind to histidine residues that are present in human but not mouse TLR4 leading to TLR4 dimerization that is essential for signaling, which occurs in the absence of LPS [142, 143]. Palladium also seems to activate human TLR4 [144]. As shown in the contact hypersensitivity (CHS) mouse model, indirect PRR activation by organic chemicals such as TNCB and oxazolone depends on the generation of endogenous danger signals. This was shown for the triggering of TLR2 and TLR4 by fragments of the ECM component HA [145, 146] and for the NLRP3 inflammasome via extracellular ATP and signaling via the purinergic receptor P2X7R [147–149]. ROS are also induced by contact allergens and are required for CHS [146]. Similar pathways are triggered by acetaminophen in drug-induced liver injury (DILI) [111, 150].
Activation thresholds for the innate immune system are often not reached by potential contact allergens and sensitization, i.e., the priming of contact allergen-specific T cells does not occur. In order to exceed the threshold and to induce sensitization to such allergens repeated or chronic exposure may result in cumulative inflammatory stimuli. In addition, heterologous innate immunity is of great importance (Fig. 1) [12, 113]. Thus, additive or synergistic effects by the combination of different contact allergens or allergens and irritants may result in (facilitated) sensitization. Examples are the amplification of proliferative responses to sub-sensitizing concentrations of the strong contact allergen DNCB by sodium dodecyl sulfate (SDS) [151], amplification of sub-threshold inflammation of suboptimal, non-sensitizing doses of TNCB by oxazolone [152] or the observed amplification effects in human ACD due to combinations of weak contact allergens such as fragrances or of contact allergens and irritants [153–155]. Toxicological hazard identification and risk assessment are mostly performed with single substances. However, heterologous innate immune stimulation must be considered whenever substances are combined. Consumer products such as cosmetics are usually complex mixtures and formulations. Similar aspects apply to some drugs. In addition, consumers and patients often simultaneously use several products and drugs, respectively. Due to the fact that the contact allergen that induces sensitization does not necessarily have to provide the autologous innate stimuli that appropriately activate and polarize the antigen-presenting DCs, heterologous stimuli can amplify insufficient or substitute for absent contact allergen-dependent innate immune stimulation. Thus, even non-allergens such as irritants or infections may provide such stimuli (Fig. 3). Examples are the conversion of nickel, a non-allergen in mice, to an allergen by providing exogenous danger signals such as PAMPs like LPS (Fig. 5a) or the irritant SDS [156, 157] or the conversion of the weak contact allergen/tolerogen 2,4-dinitrothiocyanobenzene (DNTB) into an allergen by addition of SDS [158]. Interestingly, the combination of the antibiotic amoxicillin with TLR ligands results in a significant increase in DC maturation, IL-12 production and T cell proliferation in vitro, mainly with cells from patients with DTH responses to the drug [159]. This suggests that drug-induced maculopapular exanthema reactions may be exacerbated by heterologous innate stimuli.
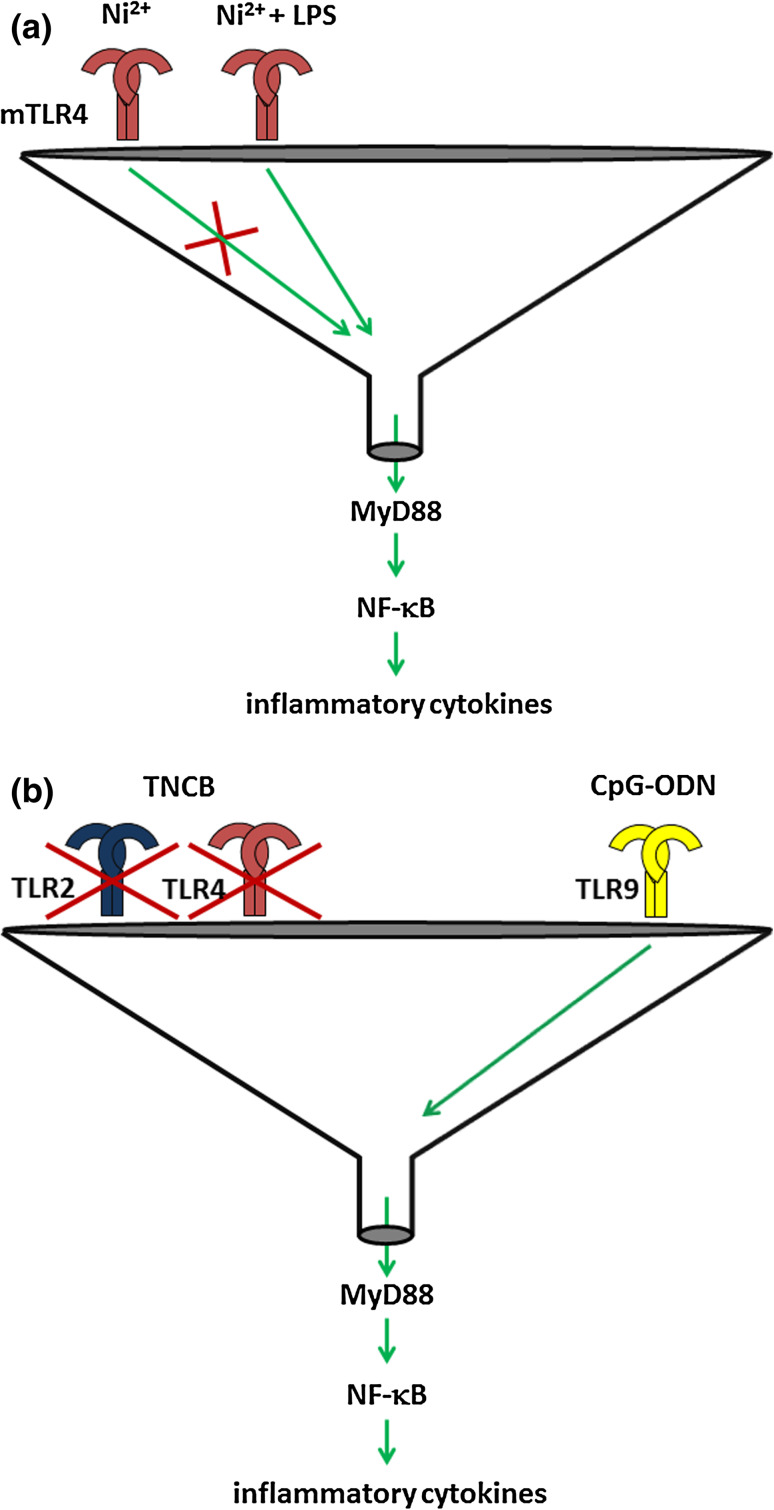
Fig. 5.
Loss of resistance to CHS due to heterologous innate immune stimulation. a Nickel ions cannot bind to murine TLR4 (mTLR4) and mice are therefore resistant to CHS. Addition of LPS, however, can trigger mTLR4 and provide a heterologous stimulus that renders mice susceptible to Ni2+-induced CHS. b Mice lacking TLR2 and TLR4 are resistant to TNCB-induced CHS. Heterologous triggering of TLR9 by CpG-ODN renders these mice susceptible to CHS
The findings in the CHS model demonstrate that heterologous innate stimuli can substitute for missing autologous innate stimuli, thereby leading to innate immune responses or the breaking of immune tolerance, or amplify autologous innate stimuli leading to stronger immune responses.
In the CHS model, sensitization can be restored in CHS-resistant TLR4/IL-12Rβ2 mice by heterologous innate stimuli. Their DCs lack sensitizing capacity when injected into the skin of wild-type mice [145]. However, heterologous innate stimulation of TLR4/IL-12Rβ2 DCs by in vitro pre-treatment with the TLR9 ligand CpG-oligodeoxynucleotide (CpG-ODN) or by direct skin injection of CpG-ODN into TLR4/IL-12Rβ2 deficient mice prior to epicutaneous sensitization with TNCB (our unpublished data) restored sensitization and CHS. Thus, lack of TLR2/4 signaling or TLR4/IL-12Rβ2 signaling can be compensated by heterologous innate immune stimulation via TLR9 (Fig. 5b). These data illustrate that heterologous innate immune stimulation can overcome genetic resistance to ACD.
The importance of appropriate and sufficient innate immune stimulation is obvious. Activation and full maturation of DCs crucially depends on innate immune stimuli. In the absence of such stimuli or in the presence of insufficient stimulation, DCs may remain immature or semi-mature. If they then present antigens to T cells anergy or Treg can be induced [160–162]. This knowledge is used for the targeting of antigen to immature DCs in the absence of innate stimulation for the induction of tolerance [163, 164]. In the presence of TLR ligands immunity is efficiently induced or enhanced [165]. In the case of contact allergens, the use of sub-sensitizing doses results in low zone tolerance, i.e., antigen presentation to T cells on tolerogenic DCs most likely due to absent or insufficient innate immune stimulation [166]. In this case, heterologous innate immune stimulation might result in the breaking of tolerance (Fig. 2).
These findings also indicate how heterologous innate immune stimulation can be used to manipulate immune responses. Thus, adjuvants can break tolerance while the lack of appropriate innate immune stimulation can result in tolerance. This opens the possibility to use stimulatory or tolerogenic adjuvants or to interfere with innate immune stimulation and, in addition, to exploit adjuvants that impact the T cell polarizing potential of DCs. For example, certain ligands for TLRs, NLRs or CLRs in fact drive DC to induce Th2 responses [167]. Moreover, the strength of the innate stimulus may be varied to achieve tolerization rather than immunity or to control the magnitude of the immune response.
A recent dramatic example for the relevance of heterologous innate immune stimulation was provided for LPS-mediated sepsis. A TLR4-independent, lipid A-mediated non-canonical pathway for caspase-11-dependent activation of the NLRP3 inflammasome and pyroptosis was identified. Intracellular sensing of LPS resulted in pyroptosis. This required priming of the NLRP3 inflammasome via TLR4. In a mouse model, TLR4-deficient mice were resistant to LPS-induced sepsis. However, when priming was performed by heterologous innate immune stimulation using the TLR3 ligand poly I:C, the mice succumbed to TLR4-independent LPS-induced sepsis due to NLRP3-mediated pyroptosis [168, 169]. Thus, co-infections or trauma that produces PAMPs or DAMPs, which trigger other TLRs, can turn resistance into susceptibility. These findings impressively demonstrate the relevance of heterologous innate immune stimulation. They also have potential clinical relevance with respect to therapeutic approaches that solely target TLR4 in septic shock.
As for delayed-type hypersensitivity (type IV allergy) such as ACD, heterologous innate immune stimulation should also be relevant for IgE-mediated immediate-type hypersensitivity (type I allergy) such as rhinitis and asthma. Pollen from many different plant species can cause type I allergies. The reactivity of B cells and T cells is highly specific for distinct epitopes from pollen proteins. As for type IV allergies, innate immune system activation is required for the allergic response. In recent years, it has become clear that pollens are not only allergen carriers but contain lipid mediators, enzymes such as NADPH oxidases and other compounds such as adenosine that can trigger, promote and polarize innate inflammatory responses [170]. Again here, the co-occurrence of different pollen species or pollen in the context of other heterologous innate immune stimulants may lead to the amplification of innate immune responses or the conversion of non-allergenic pollen into allergenic pollen due to heterologous innate immune stimulation based on the uncoupled specificities of the innate and adaptive immune response.
Conclusion
The innate immune system is able to adapt in response to commensals, infection, xenobiotic chemicals and other assaults. Its activation threshold is established in a calibration process that integrates signals from commensal bacteria and most likely from endogenous danger signals. Upon its activation, both innate immune cells and tissue cells may acquire a state of heightened reactivity due to adaptation processes that may be imprinted by epigenetic modifications and lead to trained innate immunity. This (pre-)conditioning of the tissue microenvironment and the context in which a pathogen or a chemical is acting on the immune system crucially influences the quality and quantity of the resulting immune response. Due to individually different immune histories, the thresholds for induction of innate inflammatory and adaptive immune responses may vary individually and over time as a consequence of potentially dynamic calibration and adaptation processes [16, 27]. In addition, individually different tissue- and organ-specific thresholds may be established. These may explain, for example, different organ involvements in patients with adverse reactions to the same drugs. These complexities make predictions of individual risks very difficult unless reliable biomarkers that reflect the current immune status can be identified.
The rather broad specificity of the PRR families and their signaling via common pathways enables heterologous innate immune stimulation. This can significantly impact the outcome of innate and, subsequently, adaptive immune responses in a beneficial or detrimental manner. These design features of the innate immune system help to understand how individual immune experience can shape the immune system and impact future immune responses with regards to quality and quantity, individual immune fitness and susceptibility to diseases.
Possible manipulations of the innate immune system may include the lowering or heightening of activation thresholds to increase responsiveness or prevent hypersensitivity, respectively. Heterologous innate immune stimulation can be used to optimize vaccinations, to amplify or attenuate immune responses, to change the polarization of immune responses and to induce tolerance. Therefore, a more detailed understanding of the mechanisms underlying calibration, trained immunity and heterologous innate immunity is needed. This can be exploited for the prevention and therapy of diseases and the improvement of vaccination strategies.
Acknowledgments
I am grateful to Dr. Philipp R. Esser for helpful discussions and careful reading of the manuscript.
Abbreviations
- ACD
Allergic contact dermatitis
- BCR
B cell receptor
- CHS
Contact hypersensitivity
- CLR
C-type lectin receptor
- CRISPR
Clustered regularly interspaced short palindromic repeats
- DAMP
Damage-associated molecular pattern
- DC
Dendritic cell
- DNP
2,4-Dinitrophenyl
- DTH
Delayed type hypersensitivity
- DNTB
2,4-Dinitrothiocyanobenzene
- ECM
Extracellular matrix
- MAMP
Microbe-associated molecular pattern
- MDSC
Myeloid-derived suppressor cell
- NK
Natural killer
- NLR
NOD-like receptor
- PAMP
Pathogen-associated molecular pattern
- pMHC
Peptide/MHC complex
- PRR
Pattern recognition receptor
- RLR
RIG-I like receptor
- SAR
Systemic acquired resistance
- TCR
T cell receptor
- TLR
Toll-like receptor
- Treg
Regulatory T cell
- TNCB
2,4,6-Trinitrochlorobenzene
- TNP
2,4,6-Trinitrophenyl
References
- 1.Janeway CA., Jr Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb Symp Quant Biol. 1989;54(Pt 1):1–13. doi: 10.1101/sqb.1989.054.01.003. [DOI] [PubMed] [Google Scholar]
- 2.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 3.Matzinger P. Friendly and dangerous signals: is the tissue in control? Nat Immunol. 2007;8(1):11–13. doi: 10.1038/ni0107-11. [DOI] [PubMed] [Google Scholar]
- 4.Medzhitov R. Approaching the asymptote: 20 years later. Immunity. 2009;30(6):766–775. doi: 10.1016/j.immuni.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 5.Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34(5):637–650. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 6.Schenten D, Medzhitov R. The control of adaptive immune responses by the innate immune system. Adv Immunol. 2011;109:87–124. doi: 10.1016/B978-0-12-387664-5.00003-0. [DOI] [PubMed] [Google Scholar]
- 7.Victora GD, Nussenzweig MC. Germinal centers. Annu Rev Immunol. 2012;30:429–457. doi: 10.1146/annurev-immunol-020711-075032. [DOI] [PubMed] [Google Scholar]
- 8.Pieper K, Grimbacher B, Eibel H. B-cell biology and development. J Allergy Clin Immunol. 2013;131(4):959–971. doi: 10.1016/j.jaci.2013.01.046. [DOI] [PubMed] [Google Scholar]
- 9.Shlomchik MJ, Weisel F. Germinal center selection and the development of memory B and plasma cells. Immunol Rev. 2012;247(1):52–63. doi: 10.1111/j.1600-065X.2012.01124.x. [DOI] [PubMed] [Google Scholar]
- 10.Hale JS, Fink PJ. T-cell receptor revision: friend or foe? Immunology. 2010;129(4):467–473. doi: 10.1111/j.1365-2567.2010.03250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mueller SN, Gebhardt T, Carbone FR, Heath WR. Memory T cell subsets, migration patterns, and tissue residence. Annu Rev Immunol. 2013;31:137–161. doi: 10.1146/annurev-immunol-032712-095954. [DOI] [PubMed] [Google Scholar]
- 12.Page KR, Scott AL, Manabe YC. The expanding realm of heterologous immunity: friend or foe? Cell Microbiol. 2006;8(2):185–196. doi: 10.1111/j.1462-5822.2005.00653.x. [DOI] [PubMed] [Google Scholar]
- 13.Barrangou R, Marraffini LA. CRISPR-cas systems: prokaryotes upgrade to adaptive immunity. Mol Cell. 2014;54(2):234–244. doi: 10.1016/j.molcel.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bronkhorst AW, van Rij RP. The long and short of antiviral defense: small RNA-based immunity in insects. Curr Opin Virol. 2014;7C:19–28. doi: 10.1016/j.coviro.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 15.Ermolaeva MA, Schumacher B (2014) Insights from the worm: The C. elegans model for innate immunity. Seminar Immunol doi:10.1016/j.smim.2014.04.005 [DOI] [PMC free article] [PubMed]
- 16.Netea MG. Training innate immunity: the changing concept of immunological memory in innate host defence. Eur J Clin Invest. 2013;43(8):881–884. doi: 10.1111/eci.12132. [DOI] [PubMed] [Google Scholar]
- 17.Morris GP, Allen PM. How the TCR balances sensitivity and specificity for the recognition of self and pathogens. Nat Immunol. 2012;13(2):121–128. doi: 10.1038/ni.2190. [DOI] [PubMed] [Google Scholar]
- 18.Garbi N, Hammerling GJ, Probst HC, van den Broek M. Tonic T cell signalling and T cell tolerance as opposite effects of self-recognition on dendritic cells. Curr Opin Immunol. 2010;22(5):601–608. doi: 10.1016/j.coi.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 19.Garbi N, Kreutzberg T. Dendritic cells enhance the antigen sensitivity of T cells. Front Immunol. 2012;3:389. doi: 10.3389/fimmu.2012.00389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kassiotis G, Garcia S, Simpson E, Stockinger B. Impairment of immunological memory in the absence of MHC despite survival of memory T cells. Nat Immunol. 2002;3(3):244–250. doi: 10.1038/ni766. [DOI] [PubMed] [Google Scholar]
- 21.Lam KP, Kuhn R, Rajewsky K. In vivo ablation of surface immunoglobulin on mature B cells by inducible gene targeting results in rapid cell death. Cell. 1997;90(6):1073–1083. doi: 10.1016/s0092-8674(00)80373-6. [DOI] [PubMed] [Google Scholar]
- 22.Monroe JG. ITAM-mediated tonic signalling through pre-BCR and BCR complexes. Nat Rev Immunol. 2006;6(4):283–294. doi: 10.1038/nri1808. [DOI] [PubMed] [Google Scholar]
- 23.Shin H, Iwasaki A. Tissue-resident memory T cells. Immunol Rev. 2013;255(1):165–181. doi: 10.1111/imr.12087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gebhardt T, Mueller SN, Heath WR, Carbone FR. Peripheral tissue surveillance and residency by memory T cells. Trends Immunol. 2013;34(1):27–32. doi: 10.1016/j.it.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 25.Weill JC, Le Gallou S, Hao Y, Reynaud CA. Multiple players in mouse B cell memory. Curr Opin Immunol. 2013;25(3):334–338. doi: 10.1016/j.coi.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 26.Abt MC, Osborne LC, Monticelli LA, Doering TA, Alenghat T, Sonnenberg GF, Paley MA, Antenus M, Williams KL, Erikson J, Wherry EJ, Artis D. Commensal bacteria calibrate the activation threshold of innate antiviral immunity. Immunity. 2012;37(1):158–170. doi: 10.1016/j.immuni.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abt MC, Artis D. The dynamic influence of commensal bacteria on the immune response to pathogens. Curr Opin Microbiol. 2013;16(1):4–9. doi: 10.1016/j.mib.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11(5):373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 29.Chen GY, Nunez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol. 2010;10(12):826–837. doi: 10.1038/nri2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sorokin L. The impact of the extracellular matrix on inflammation. Nat Rev Immunol. 2010;10(10):712–723. doi: 10.1038/nri2852. [DOI] [PubMed] [Google Scholar]
- 31.Volkman HE, Stetson DB. The enemy within: endogenous retroelements and autoimmune disease. Nat Immunol. 2014;15(5):415–422. doi: 10.1038/ni.2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bhat N, Fitzgerald KA. Recognition of cytosolic DNA by cGAS and other STING-dependent sensors. Eur J Immunol. 2014;44(3):634–640. doi: 10.1002/eji.201344127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ablasser A, Hemmerling I, Schmid-Burgk JL, Behrendt R, Roers A, Hornung V. TREX1 deficiency triggers cell-autonomous immunity in a cGAS-dependent manner. J Immunol. 2014;192(12):5993–5997. doi: 10.4049/jimmunol.1400737. [DOI] [PubMed] [Google Scholar]
- 34.Ichinohe T, Pang IK, Kumamoto Y, Peaper DR, Ho JH, Murray TS, Iwasaki A. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc Natl Acad Sci USA. 2011;108(13):5354–5359. doi: 10.1073/pnas.1019378108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cerutti A, Cols M, Puga I. Marginal zone B cells: virtues of innate-like antibody-producing lymphocytes. Nat Rev Immunol. 2013;13(2):118–132. doi: 10.1038/nri3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ganal SC, Sanos SL, Kallfass C, Oberle K, Johner C, Kirschning C, Lienenklaus S, Weiss S, Staeheli P, Aichele P, Diefenbach A. Priming of natural killer cells by nonmucosal mononuclear phagocytes requires instructive signals from commensal microbiota. Immunity. 2012;37(1):171–186. doi: 10.1016/j.immuni.2012.05.020. [DOI] [PubMed] [Google Scholar]
- 37.Izhak L, Berzofsky JA, Terabe M. Balance is a key for happiness. Oncoimmunology. 2013;2(5):e24211. doi: 10.4161/onci.24211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ohkura N, Kitagawa Y, Sakaguchi S. Development and maintenance of regulatory T cells. Immunity. 2013;38(3):414–423. doi: 10.1016/j.immuni.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 39.Lynch L, Nowak M, Varghese B, Clark J, Hogan AE, Toxavidis V, Balk SP, O’Shea D, O’Farrelly C, Exley MA. Adipose tissue invariant NKT cells protect against diet-induced obesity and metabolic disorder through regulatory cytokine production. Immunity. 2012;37(3):574–587. doi: 10.1016/j.immuni.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goubier A, Vocanson M, Macari C, Poyet G, Herbelin A, Nicolas JF, Dubois B, Kaiserlian D. Invariant NKT cells suppress CD8(+) T-cell-mediated allergic contact dermatitis independently of regulatory CD4(+) T cells. J Invest Dermatol. 2013;133(4):980–987. doi: 10.1038/jid.2012.404. [DOI] [PubMed] [Google Scholar]
- 41.El Ali Z, Gerbeix C, Hemon P, Esser PR, Martin SF, Pallardy M, Kerdine-Romer S. Allergic skin inflammation induced by chemical sensitizers is controlled by the transcription factor nrf2. Toxicol Sci. 2013;134(1):39–48. doi: 10.1093/toxsci/kft084. [DOI] [PubMed] [Google Scholar]
- 42.Ronald PC, Beutler B. Plant and animal sensors of conserved microbial signatures. Science. 2010;330(6007):1061–1064. doi: 10.1126/science.1189468. [DOI] [PubMed] [Google Scholar]
- 43.Schwessinger B, Ronald PC. Plant innate immunity: perception of conserved microbial signatures. Annu Rev Plant Biol. 2012;63:451–482. doi: 10.1146/annurev-arplant-042811-105518. [DOI] [PubMed] [Google Scholar]
- 44.Fu ZQ, Dong X. Systemic acquired resistance: turning local infection into global defense. Annu Rev Plant Biol. 2013;64:839–863. doi: 10.1146/annurev-arplant-042811-105606. [DOI] [PubMed] [Google Scholar]
- 45.van den Burg HA, Takken FL. Does chromatin remodeling mark systemic acquired resistance? Trends Plant Sci. 2009;14(5):286–294. doi: 10.1016/j.tplants.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 46.Conrath U. Molecular aspects of defence priming. Trends Plant Sci. 2011;16(10):524–531. doi: 10.1016/j.tplants.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 47.Holeski LM, Jander G, Agrawal AA. Transgenerational defense induction and epigenetic inheritance in plants. Trends Ecol Evol. 2012;27(11):618–626. doi: 10.1016/j.tree.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 48.Monticelli S, Natoli G. Short-term memory of danger signals and environmental stimuli in immune cells. Nat Immunol. 2013;14(8):777–784. doi: 10.1038/ni.2636. [DOI] [PubMed] [Google Scholar]
- 49.Foster SL, Hargreaves DC, Medzhitov R. Gene-specific control of inflammation by TLR-induced chromatin modifications. Nature. 2007;447(7147):972–978. doi: 10.1038/nature05836. [DOI] [PubMed] [Google Scholar]
- 50.Min-Oo G, Kamimura Y, Hendricks DW, Nabekura T, Lanier LL. Natural killer cells: walking three paths down memory lane. Trends Immunol. 2013;34(6):251–258. doi: 10.1016/j.it.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cooper MA, Elliott JM, Keyel PA, Yang L, Carrero JA, Yokoyama WM. Cytokine-induced memory-like natural killer cells. Proc Natl Acad Sci USA. 2009;106(6):1915–1919. doi: 10.1073/pnas.0813192106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nature. 2009;457(7229):557–561. doi: 10.1038/nature07665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sun JC, Lopez-Verges S, Kim CC, DeRisi JL, Lanier LL. NK cells and immune “memory”. J Immunol. 2011;186(4):1891–1897. doi: 10.4049/jimmunol.1003035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.O’Leary JG, Goodarzi M, Drayton DL, von Andrian UH. T cell- and B cell-independent adaptive immunity mediated by natural killer cells. Nat Immunol. 2006;7(5):507–516. doi: 10.1038/ni1332. [DOI] [PubMed] [Google Scholar]
- 55.Paust S, Gill HS, Wang BZ, Flynn MP, Moseman EA, Senman B, Szczepanik M, Telenti A, Askenase PW, Compans RW, von Andrian UH. Critical role for the chemokine receptor CXCR6 in NK cell-mediated antigen-specific memory of haptens and viruses. Nat Immunol. 2010;11(12):1127–1135. doi: 10.1038/ni.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Paust S, von Andrian UH. Natural killer cell memory. Nat Immunol. 2011;12(6):500–508. doi: 10.1038/ni.2032. [DOI] [PubMed] [Google Scholar]
- 57.Peng H, Jiang X, Chen Y, Sojka DK, Wei H, Gao X, Sun R, Yokoyama WM, Tian Z. Liver-resident NK cells confer adaptive immunity in skin-contact inflammation. J Clin Investig. 2013;123(4):1444–1456. doi: 10.1172/JCI66381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Locati M, Mantovani A, Sica A. Macrophage activation and polarization as an adaptive component of innate immunity. Adv Immunol. 2013;120:163–184. doi: 10.1016/B978-0-12-417028-5.00006-5. [DOI] [PubMed] [Google Scholar]
- 59.Quintin J, Saeed S, Martens JH, Giamarellos-Bourboulis EJ, Ifrim DC, Logie C, Jacobs L, Jansen T, Kullberg BJ, Wijmenga C, Joosten LA, Xavier RJ, van der Meer JW, Stunnenberg HG, Netea MG. Candida albicans infection affords protection against reinfection via functional reprogramming of monocytes. Cell Host Microb. 2012;12(2):223–232. doi: 10.1016/j.chom.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kleinnijenhuis J, Quintin J, Preijers F, Joosten LA, Ifrim DC, Saeed S, Jacobs C, van Loenhout J, de Jong D, Stunnenberg HG, Xavier RJ, van der Meer JW, van Crevel R, Netea MG. Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc Natl Acad Sci USA. 2012;109(43):17537–17542. doi: 10.1073/pnas.1202870109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Benn CS, Netea MG, Selin LK, Aaby P. A small jab—a big effect: nonspecific immunomodulation by vaccines. Trends Immunol. 2013;34(9):431–439. doi: 10.1016/j.it.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 62.Aaby P, Benn CS. Saving lives by training innate immunity with bacille Calmette-Guerin vaccine. Proc Natl Acad Sci USA. 2012;109(43):17317–17318. doi: 10.1073/pnas.1215761109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science. 2012;336(6086):1268–1273. doi: 10.1126/science.1223490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Honda K, Littman DR. The microbiome in infectious disease and inflammation. Annu Rev Immunol. 2012;30:759–795. doi: 10.1146/annurev-immunol-020711-074937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Garn H, Neves JF, Blumberg RS, Renz H. Effect of barrier microbes on organ-based inflammation. J Allergy Clin Immunol. 2013;131(6):1465–1478. doi: 10.1016/j.jaci.2013.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Manichanh C, Borruel N, Casellas F, Guarner F. The gut microbiota in IBD. Nat Rev Gastroenterol Hepatol. 2012;9(10):599–608. doi: 10.1038/nrgastro.2012.152. [DOI] [PubMed] [Google Scholar]
- 67.Goldszmid RS, Trinchieri G. The price of immunity. Nat Immunol. 2012;13(10):932–938. doi: 10.1038/ni.2422. [DOI] [PubMed] [Google Scholar]
- 68.Stecher B, Maier L, Hardt WD. ‘Blooming’ in the gut: how dysbiosis might contribute to pathogen evolution. Nat Rev Microbiol. 2013;11(4):277–284. doi: 10.1038/nrmicro2989. [DOI] [PubMed] [Google Scholar]
- 69.Borody TJ, Khoruts A. Fecal microbiota transplantation and emerging applications. Nat Rev Gastroenterol Hepatol. 2012;9(2):88–96. doi: 10.1038/nrgastro.2011.244. [DOI] [PubMed] [Google Scholar]
- 70.Stelekati E, Wherry EJ. Chronic bystander infections and immunity to unrelated antigens. Cell Host Microb. 2012;12(4):458–469. doi: 10.1016/j.chom.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Clark RA. Skin-resident T cells: the ups and downs of on site immunity. J Invest Dermatol. 2010;130(2):362–370. doi: 10.1038/jid.2009.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bevan MJ. Memory T cells as an occupying force. Eur J Immunol. 2011;41(5):1192–1195. doi: 10.1002/eji.201041377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Carbone FR, Mackay LK, Heath WR, Gebhardt T. Distinct resident and recirculating memory T cell subsets in non-lymphoid tissues. Curr Opin Immunol. 2013;25(3):329–333. doi: 10.1016/j.coi.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 74.Scheper RJ, von Blomberg M, Boerrigter GH, Bruynzeel D, van Dinther A, Vos A. Induction of immunological memory in the skin. Role of local T cell retention. Clin Exp Immunol. 1983;51(1):141–148. [PMC free article] [PubMed] [Google Scholar]
- 75.Rustemeyer T, de Groot J, von Blomberg BM, Bruynzeel DP, Frosch PJ, Scheper RJ. Assessment of contact allergen cross-reactivity by retesting. Exp Dermatol. 2002;11(3):257–265. doi: 10.1034/j.1600-0625.2001.110309.x. [DOI] [PubMed] [Google Scholar]
- 76.Moed H, Boorsma DM, Tensen CP, Flier J, Jonker MJ, Stoof TJ, von Blomberg BM, Bruynzeel DP, Scheper RJ, Rustemeyer T, Gibbs S. Increased CCL27-CCR10 expression in allergic contact dermatitis: implications for local skin memory. J Pathol. 2004;204(1):39–46. doi: 10.1002/path.1619. [DOI] [PubMed] [Google Scholar]
- 77.Strachan DP. Hay fever, hygiene, and household size. BMJ. 1989;299(6710):1259–1260. doi: 10.1136/bmj.299.6710.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Netea MG, Wijmenga C, O’Neill LA. Genetic variation in Toll-like receptors and disease susceptibility. Nat Immunol. 2012;13(6):535–542. doi: 10.1038/ni.2284. [DOI] [PubMed] [Google Scholar]
- 79.Lamkanfi M, Kanneganti TD. The inflammasome: a remote control for metabolic syndrome. Cell Res. 2012;22(7):1095–1098. doi: 10.1038/cr.2012.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bach JF. The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med. 2002;347(12):911–920. doi: 10.1056/NEJMra020100. [DOI] [PubMed] [Google Scholar]
- 81.Matricardi PM. 99th Dahlem conference on infection, inflammation and chronic inflammatory disorders: controversial aspects of the ‘hygiene hypothesis’. Clin Exp Immunol. 2010;160(1):98–105. doi: 10.1111/j.1365-2249.2010.04130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Brooks C, Pearce N, Douwes J. The hygiene hypothesis in allergy and asthma: an update. Curr Opin Allergy Clin Immunol. 2013;13(1):70–77. doi: 10.1097/ACI.0b013e32835ad0d2. [DOI] [PubMed] [Google Scholar]
- 83.von Mutius E, Vercelli D. Farm living: effects on childhood asthma and allergy. Nat Rev Immunol. 2010;10(12):861–868. doi: 10.1038/nri2871. [DOI] [PubMed] [Google Scholar]
- 84.Ege MJ, Mayer M, Schwaiger K, Mattes J, Pershagen G, van Hage M, Scheynius A, Bauer J, von Mutius E. Environmental bacteria and childhood asthma. Allergy. 2012;67(12):1565–1571. doi: 10.1111/all.12028. [DOI] [PubMed] [Google Scholar]
- 85.Ege MJ, Bieli C, Frei R, van Strien RT, Riedler J, Ublagger E, Schram-Bijkerk D, Brunekreef B, van Hage M, Scheynius A, Pershagen G, Benz MR, Lauener R, von Mutius E, Braun-Fahrlander C, Parsifal Study t Prenatal farm exposure is related to the expression of receptors of the innate immunity and to atopic sensitization in school-age children. J Allergy Clin Immunol. 2006;117(4):817–823. doi: 10.1016/j.jaci.2005.12.1307. [DOI] [PubMed] [Google Scholar]
- 86.Ege MJ, Herzum I, Buchele G, Krauss-Etschmann S, Lauener RP, Roponen M, Hyvarinen A, Vuitton DA, Riedler J, Brunekreef B, Dalphin JC, Braun-Fahrlander C, Pekkanen J, Renz H, von Mutius E, Protection Against Allergy Study in Rural Environments Study g (2008) Prenatal exposure to a farm environment modifies atopic sensitization at birth. J Allergy Clin Immunol 122(2):407–412, 412 e401–404. doi:10.1016/j.jaci.2008.06.011 [DOI] [PubMed]
- 87.Michel S, Busato F, Genuneit J, Pekkanen J, Dalphin JC, Riedler J, Mazaleyrat N, Weber J, Karvonen AM, Hirvonen MR, Braun-Fahrlander C, Lauener R, von Mutius E, Kabesch M, Tost J, Group Ps Farm exposure and time trends in early childhood may influence DNA methylation in genes related to asthma and allergy. Allergy. 2013;68(3):355–364. doi: 10.1111/all.12097. [DOI] [PubMed] [Google Scholar]
- 88.Debarry J, Hanuszkiewicz A, Stein K, Holst O, Heine H. The allergy-protective properties of Acinetobacter lwoffii F78 are imparted by its lipopolysaccharide. Allergy. 2010;65(6):690–697. doi: 10.1111/j.1398-9995.2009.02253.x. [DOI] [PubMed] [Google Scholar]
- 89.Brand S, Teich R, Dicke T, Harb H, Yildirim AO, Tost J, Schneider-Stock R, Waterland RA, Bauer UM, von Mutius E, Garn H, Pfefferle PI, Renz H (2011) Epigenetic regulation in murine offspring as a novel mechanism for transmaternal asthma protection induced by microbes. J Allergy Clin Immunol 128(3):618–625, e611–617. doi:10.1016/j.jaci.2011.04.035 [DOI] [PubMed]
- 90.Brand S, Kesper DA, Teich R, Kilic-Niebergall E, Pinkenburg O, Bothur E, Lohoff M, Garn H, Pfefferle PI, Renz H (2012) DNA methylation of TH1/TH2 cytokine genes affects sensitization and progress of experimental asthma. J Allergy Clin Immunol 129(6):1602–1610, e1606. doi:10.1016/j.jaci.2011.12.963 [DOI] [PubMed]
- 91.Hagner S, Harb H, Zhao M, Stein K, Holst O, Ege MJ, Mayer M, Matthes J, Bauer J, von Mutius E, Renz H, Heine H, Pfefferle PI, Garn H. Farm-derived Gram-positive bacterium Staphylococcus sciuri W620 prevents asthma phenotype in HDM- and OVA-exposed mice. Allergy. 2013;68(3):322–329. doi: 10.1111/all.12094. [DOI] [PubMed] [Google Scholar]
- 92.Conrad ML, Ferstl R, Teich R, Brand S, Blumer N, Yildirim AO, Patrascan CC, Hanuszkiewicz A, Akira S, Wagner H, Holst O, von Mutius E, Pfefferle PI, Kirschning CJ, Garn H, Renz H. Maternal TLR signaling is required for prenatal asthma protection by the nonpathogenic microbe Acinetobacter lwoffii F78. J Exp Med. 2009;206(13):2869–2877. doi: 10.1084/jem.20090845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Khosravi A, Mazmanian SK. Breathe easy: microbes protect from allergies. Nat Med. 2012;18(4):492–494. doi: 10.1038/nm.2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hill DA, Siracusa MC, Abt MC, Kim BS, Kobuley D, Kubo M, Kambayashi T, Larosa DF, Renner ED, Orange JS, Bushman FD, Artis D. Commensal bacteria-derived signals regulate basophil hematopoiesis and allergic inflammation. Nat Med. 2012;18(4):538–546. doi: 10.1038/nm.2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vercelli D. Gene-environment interactions: the road less traveled by in asthma genetics. J Allergy Clin Immunol. 2009;123(1):26–27. doi: 10.1016/j.jaci.2008.11.031. [DOI] [PubMed] [Google Scholar]
- 96.Fishbein AB, Fuleihan RL. The hygiene hypothesis revisited: does exposure to infectious agents protect us from allergy? Curr Opin Pediatr. 2012;24(1):98–102. doi: 10.1097/MOP.0b013e32834ee57c. [DOI] [PubMed] [Google Scholar]
- 97.Welsh RM, Selin LK. No one is naive: the significance of heterologous T-cell immunity. Nat Rev Immunol. 2002;2(6):417–426. doi: 10.1038/nri820. [DOI] [PubMed] [Google Scholar]
- 98.Littman DR, Pamer EG. Role of the commensal microbiota in normal and pathogenic host immune responses. Cell Host Microb. 2011;10(4):311–323. doi: 10.1016/j.chom.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lamkanfi M, Dixit VM. Inflammasomes and their roles in health and disease. Annu Rev Cell Dev Biol. 2012;28:137–161. doi: 10.1146/annurev-cellbio-101011-155745. [DOI] [PubMed] [Google Scholar]
- 100.Sancho D, Reis e Sousa C. Signaling by myeloid C-type lectin receptors in immunity and homeostasis. Annu Rev Immunol. 2012;30:491–529. doi: 10.1146/annurev-immunol-031210-101352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bauernfeind F, Hornung V. Of inflammasomes and pathogens–sensing of microbes by the inflammasome. EMBO Mol Med. 2013;5(6):814–826. doi: 10.1002/emmm.201201771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dixit E, Kagan JC. Intracellular pathogen detection by RIG-I-like receptors. Adv Immunol. 2013;117:99–125. doi: 10.1016/B978-0-12-410524-9.00004-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ablasser A, Hertrich C, Wassermann R, Hornung V. Nucleic acid driven sterile inflammation. Clin Immunol. 2013;147(3):207–215. doi: 10.1016/j.clim.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 104.Wu J, Chen ZJ. Innate immune sensing and signaling of cytosolic nucleic acids. Annu Rev Immunol. 2014;32:461–488. doi: 10.1146/annurev-immunol-032713-120156. [DOI] [PubMed] [Google Scholar]
- 105.Reynolds JM, Dong C. Toll-like receptor regulation of effector T lymphocyte function. Trends Immunol. 2013;34(10):511–519. doi: 10.1016/j.it.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 106.Rawlings DJ, Schwartz MA, Jackson SW, Meyer-Bahlburg A. Integration of B cell responses through Toll-like receptors and antigen receptors. Nat Rev Immunol. 2012;12(4):282–294. doi: 10.1038/nri3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Selin LK, Brehm MA, Naumov YN, Cornberg M, Kim SK, Clute SC, Welsh RM. Memory of mice and men: CD8+ T-cell cross-reactivity and heterologous immunity. Immunol Rev. 2006;211:164–181. doi: 10.1111/j.0105-2896.2006.00394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Selin LK, Brehm MA. Frontiers in nephrology: heterologous immunity, T cell cross-reactivity, and alloreactivity. JASN. 2007;18(8):2268–2277. doi: 10.1681/ASN.2007030295. [DOI] [PubMed] [Google Scholar]
- 109.Selin LK, Wlodarczyk MF, Kraft AR, Nie S, Kenney LL, Puzone R, Celada F. Heterologous immunity: immunopathology, autoimmunity and protection during viral infections. Autoimmunity. 2011;44(4):328–347. doi: 10.3109/08916934.2011.523277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wlodarczyk MF, Kraft AR, Chen HD, Kenney LL, Selin LK. Anti-IFN-gamma and peptide-tolerization therapies inhibit acute lung injury induced by cross-reactive influenza A-specific memory T cells. J Immunol. 2013;190(6):2736–2746. doi: 10.4049/jimmunol.1201936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Martin SF. Allergic contact dermatitis: xenoinflammation of the skin. Curr Opin Immunol. 2012;24(6):720–729. doi: 10.1016/j.coi.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 112.Martin SF. Contact dermatitis: from pathomechanisms to immunotoxicology. Exp Dermatol. 2012;21(5):382–389. doi: 10.1111/j.1600-0625.2012.01471.x. [DOI] [PubMed] [Google Scholar]
- 113.Wissinger E, Goulding J, Hussell T. Immune homeostasis in the respiratory tract and its impact on heterologous infection. Semin Immunol. 2009;21(3):147–155. doi: 10.1016/j.smim.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 114.Lijek RS, Weiser JN. Co-infection subverts mucosal immunity in the upper respiratory tract. Curr Opin Immunol. 2012;24(4):417–423. doi: 10.1016/j.coi.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Metzger DW, Sun K. Immune dysfunction and bacterial coinfections following influenza. J Immunol. 2013;191(5):2047–2052. doi: 10.4049/jimmunol.1301152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jamieson AM, Pasman L, Yu S, Gamradt P, Homer RJ, Decker T, Medzhitov R. Role of tissue protection in lethal respiratory viral-bacterial coinfection. Science. 2013;340(6137):1230–1234. doi: 10.1126/science.1233632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dietz L, Esser PR, Schmucker SS, Goette I, Richter A, Schnolzer M, Martin SF, Thierse HJ. Tracking human contact allergens: from mass spectrometric identification of peptide-bound reactive small chemicals to chemical-specific naive human T-cell priming. Toxicol Sci. 2010;117(2):336–347. doi: 10.1093/toxsci/kfq209. [DOI] [PubMed] [Google Scholar]
- 118.Padovan E, Mauri-Hellweg D, Pichler WJ, Weltzien HU. T cell recognition of penicillin G: structural features determining antigenic specificity. Eur J Immunol. 1996;26(1):42–48. doi: 10.1002/eji.1830260107. [DOI] [PubMed] [Google Scholar]
- 119.Kang JY, Lee JO. Structural biology of the Toll-like receptor family. Annu Rev Biochem. 2011;80:917–941. doi: 10.1146/annurev-biochem-052909-141507. [DOI] [PubMed] [Google Scholar]
- 120.Ohto U, Fukase K, Miyake K, Shimizu T. Structural basis of species-specific endotoxin sensing by innate immune receptor TLR4/MD-2. Proc Natl Acad Sci USA. 2012;109(19):7421–7426. doi: 10.1073/pnas.1201193109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Maeshima N, Fernandez RC. Recognition of lipid A variants by the TLR4-MD-2 receptor complex. Front Cell Infect Microbiol. 2013;3:3. doi: 10.3389/fcimb.2013.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.van Liempt E, Bank CM, Mehta P, Garcia-Vallejo JJ, Kawar ZS, Geyer R, Alvarez RA, Cummings RD, Kooyk Y, van Die I. Specificity of DC-SIGN for mannose- and fucose-containing glycans. FEBS Lett. 2006;580(26):6123–6131. doi: 10.1016/j.febslet.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 123.Song DH, Lee JO. Sensing of microbial molecular patterns by Toll-like receptors. Immunol Rev. 2012;250(1):216–229. doi: 10.1111/j.1600-065X.2012.01167.x. [DOI] [PubMed] [Google Scholar]
- 124.Napolitani G, Rinaldi A, Bertoni F, Sallusto F, Lanzavecchia A. Selected Toll-like receptor agonist combinations synergistically trigger a T helper type 1-polarizing program in dendritic cells. Nat Immunol. 2005;6(8):769–776. doi: 10.1038/ni1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kasturi SP, Skountzou I, Albrecht RA, Koutsonanos D, Hua T, Nakaya HI, Ravindran R, Stewart S, Alam M, Kwissa M, Villinger F, Murthy N, Steel J, Jacob J, Hogan RJ, Garcia-Sastre A, Compans R, Pulendran B. Programming the magnitude and persistence of antibody responses with innate immunity. Nature. 2011;470(7335):543–547. doi: 10.1038/nature09737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pulendran B, Ahmed R. Immunological mechanisms of vaccination. Nat Immunol. 2011;12(6):509–517. doi: 10.1038/ni.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Iwasaki A, Medzhitov R. Regulation of adaptive immunity by the innate immune system. Science. 2010;327(5963):291–295. doi: 10.1126/science.1183021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Coffman RL, Sher A, Seder RA. Vaccine adjuvants: putting innate immunity to work. Immunity. 2010;33(4):492–503. doi: 10.1016/j.immuni.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Desmet CJ, Ishii KJ. Nucleic acid sensing at the interface between innate and adaptive immunity in vaccination. Nat Rev Immunol. 2012;12(7):479–491. doi: 10.1038/nri3247. [DOI] [PubMed] [Google Scholar]
- 130.Mbow ML, De Gregorio E, Valiante NM, Rappuoli R. New adjuvants for human vaccines. Curr Opin Immunol. 2010;22(3):411–416. doi: 10.1016/j.coi.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 131.Alving CR, Peachman KK, Rao M, Reed SG. Adjuvants for human vaccines. Curr Opin Immunol. 2012;24(3):310–315. doi: 10.1016/j.coi.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sander LE, Davis MJ, Boekschoten MV, Amsen D, Dascher CC, Ryffel B, Swanson JA, Muller M, Blander JM. Detection of prokaryotic mRNA signifies microbial viability and promotes immunity. Nature. 2011;474(7351):385–389. doi: 10.1038/nature10072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Netea MG, Quintin J, van der Meer JW. Trained immunity: a memory for innate host defense. Cell Host Microb. 2011;9(5):355–361. doi: 10.1016/j.chom.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 134.Haneklaus M, O’Neill LA, Coll RC. Modulatory mechanisms controlling the NLRP3 inflammasome in inflammation: recent developments. Curr Opin Immunol. 2013;25(1):40–45. doi: 10.1016/j.coi.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 135.Latz E, Xiao TS, Stutz A. Activation and regulation of the inflammasomes. Nat Rev Immunol. 2013;13(6):397–411. doi: 10.1038/nri3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ramos HJ, Gale M., Jr RIG-I like receptors and their signaling crosstalk in the regulation of antiviral immunity. Curr Opin Virol. 2011;1(3):167–176. doi: 10.1016/j.coviro.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Coll RC, O’Neill LA. New insights into the regulation of signalling by Toll-like receptors and nod-like receptors. J Innate Immun. 2010;2(5):406–421. doi: 10.1159/000315469. [DOI] [PubMed] [Google Scholar]
- 138.Roth S, Ruland J. Caspase recruitment domain-containing protein 9 signaling in innate immunity and inflammation. Trends Immunol. 2013;34(6):243–250. doi: 10.1016/j.it.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 139.Peiser M, Tralau T, Heidler J, Api AM, Arts JH, Basketter DA, English J, Diepgen TL, Fuhlbrigge RC, Gaspari AA, Johansen JD, Karlberg AT, Kimber I, Lepoittevin JP, Liebsch M, Maibach HI, Martin SF, Merk HF, Platzek T, Rustemeyer T, Schnuch A, Vandebriel RJ, White IR, Luch A. Allergic contact dermatitis: epidemiology, molecular mechanisms, in vitro methods and regulatory aspects. Current knowledge assembled at an international workshop at BfR, Germany. Cell Mol Life Sci. 2012;69(5):763–781. doi: 10.1007/s00018-011-0846-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Loveless SE, Api AM, Crevel RW, Debruyne E, Gamer A, Jowsey IR, Kern P, Kimber I, Lea L, Lloyd P, Mehmood Z, Steiling W, Veenstra G, Woolhiser M, Hennes C. Potency values from the local lymph node assay: application to classification, labelling and risk assessment. RTP. 2010;56(1):54–66. doi: 10.1016/j.yrtph.2009.08.016. [DOI] [PubMed] [Google Scholar]
- 141.Esser PR, Kimber I, Martin SF. Correlation of contact sensitizer potency with T cell frequency and TCR repertoire diversity. Exs. 2014;104:101–114. doi: 10.1007/978-3-0348-0726-5_8. [DOI] [PubMed] [Google Scholar]
- 142.Schmidt M, Hupe M, Endres N, Raghavan B, Kavuri S, Geserick P, Goebeler M, Leverkus M. The contact allergen nickel sensitizes primary human endothelial cells and keratinocytes to TRAIL-mediated apoptosis. J Cell Mol Med. 2010;14(6B):1760–1776. doi: 10.1111/j.1582-4934.2009.00823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Raghavan B, Martin SF, Esser PR, Goebeler M, Schmidt M. Metal allergens nickel and cobalt facilitate TLR4 homodimerization independently of MD2. EMBO Rep. 2012;13(12):1109–1115. doi: 10.1038/embor.2012.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Rachmawati D, Bontkes HJ, Verstege MI, Muris J, von Blomberg BM, Scheper RJ, van Hoogstraten IM. Transition metal sensing by Toll-like receptor-4: next to nickel, cobalt and palladium are potent human dendritic cell stimulators. Contact Dermat. 2013;68(6):331–338. doi: 10.1111/cod.12042. [DOI] [PubMed] [Google Scholar]
- 145.Martin SF, Dudda JC, Bachtanian E, Lembo A, Liller S, Durr C, Heimesaat MM, Bereswill S, Fejer G, Vassileva R, Jakob T, Freudenberg N, Termeer CC, Johner C, Galanos C, Freudenberg MA. Toll-like receptor and IL-12 signaling control susceptibility to contact hypersensitivity. J Exp Med. 2008;205(9):2151–2162. doi: 10.1084/jem.20070509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Esser PR, Wolfle U, Durr C, von Loewenich FD, Schempp CM, Freudenberg MA, Jakob T, Martin SF. Contact sensitizers induce skin inflammation via ROS production and hyaluronic acid degradation. PLoS One. 2012;7(7):e41340. doi: 10.1371/journal.pone.0041340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sutterwala FS, Ogura Y, Szczepanik M, Lara-Tejero M, Lichtenberger GS, Grant EP, Bertin J, Coyle AJ, Galan JE, Askenase PW, Flavell RA. Critical role for NALP3/CIAS1/Cryopyrin in innate and adaptive immunity through its regulation of caspase-1. Immunity. 2006;24(3):317–327. doi: 10.1016/j.immuni.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 148.Watanabe H, Gaide O, Petrilli V, Martinon F, Contassot E, Roques S, Kummer JA, Tschopp J, French LE. Activation of the IL-1beta-processing inflammasome is involved in contact hypersensitivity. J Invest Dermatol. 2007;127(8):1956–1963. doi: 10.1038/sj.jid.5700819. [DOI] [PubMed] [Google Scholar]
- 149.Weber FC, Esser PR, Muller T, Ganesan J, Pellegatti P, Simon MM, Zeiser R, Idzko M, Jakob T, Martin SF. Lack of the purinergic receptor P2X(7) results in resistance to contact hypersensitivity. J Exp Med. 2010;207(12):2609–2619. doi: 10.1084/jem.20092489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hoque R, Farooq A, Mehal WZ. Sterile inflammation in the liver and pancreas. J Gastroenterol Hepatol. 2013;28(Suppl 1):61–67. doi: 10.1111/jgh.12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Cumberbatch M, Scott RC, Basketter DA, Scholes EW, Hilton J, Dearman RJ, Kimber I. Influence of sodium lauryl sulphate on 2,4-dinitrochlorobenzene-induced lymph node activation. Toxicology. 1993;77(1–2):181–191. doi: 10.1016/0300-483x(93)90148-l. [DOI] [PubMed] [Google Scholar]
- 152.Grabbe S, Steinert M, Mahnke K, Schwartz A, Luger TA, Schwarz T. Dissection of antigenic and irritative effects of epicutaneously applied haptens in mice. Evidence that not the antigenic component but nonspecific proinflammatory effects of haptens determine the concentration-dependent elicitation of allergic contact dermatitis. J Clin Investig. 1996;98(5):1158–1164. doi: 10.1172/JCI118899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Agner T, Johansen JD, Overgaard L, Volund A, Basketter D, Menne T. Combined effects of irritants and allergens. Synergistic effects of nickel and sodium lauryl sulfate in nickel- sensitized individuals. Contact Dermat. 2002;47(1):21–26. doi: 10.1034/j.1600-0536.2002.470105.x. [DOI] [PubMed] [Google Scholar]
- 154.Pedersen LK, Johansen JD, Held E, Agner T. Augmentation of skin response by exposure to a combination of allergens and irritants: a review. Contact Dermat. 2004;50(5):265–273. doi: 10.1111/j.0105-1873.2004.00342.x. [DOI] [PubMed] [Google Scholar]
- 155.Bonefeld CM, Nielsen MM, Rubin IM, Vennegaard MT, Dabelsteen S, Gimenez-Arnau E, Lepoittevin JP, Geisler C, Johansen JD. Enhanced sensitization and elicitation responses caused by mixtures of common fragrance allergens. Contact Dermat. 2011;65(6):336–342. doi: 10.1111/j.1600-0536.2011.01945.x. [DOI] [PubMed] [Google Scholar]
- 156.Artik S, von Vultee C, Gleichmann E, Schwarz T, Griem P. Nickel allergy in mice: enhanced sensitization capacity of nickel at higher oxidation states. J Immunol. 1999;163(3):1143–1152. [PubMed] [Google Scholar]
- 157.Takahashi H, Kinbara M, Sato N, Sasaki K, Sugawara S, Endo Y. Nickel allergy-promoting effects of microbial or inflammatory substances at the sensitization step in mice. Int Immunopharmacol. 2011;11(10):1534–1540. doi: 10.1016/j.intimp.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 158.Watanabe H, Gehrke S, Contassot E, Roques S, Tschopp J, Friedmann PS, French LE, Gaide O. Danger signaling through the inflammasome acts as a master switch between tolerance and sensitization. J Immunol. 2008;180(9):5826–5832. doi: 10.4049/jimmunol.180.9.5826. [DOI] [PubMed] [Google Scholar]
- 159.Sanchez-Quintero MJ, Torres MJ, Blazquez AB, Gomez E, Fernandez TD, Dona I, Ariza A, Andreu I, Melendez L, Blanca M, Mayorga C. Synergistic effect between amoxicillin and TLR ligands on dendritic cells from amoxicillin-delayed allergic patients. PLoS One. 2013;8(9):e74198. doi: 10.1371/journal.pone.0074198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Steinman RM, Hawiger D, Liu K, Bonifaz L, Bonnyay D, Mahnke K, Iyoda T, Ravetch J, Dhodapkar M, Inaba K, Nussenzweig M. Dendritic cell function in vivo during the steady state: a role in peripheral tolerance. Ann N Y Acad Sci. 2003;987:15–25. doi: 10.1111/j.1749-6632.2003.tb06029.x. [DOI] [PubMed] [Google Scholar]
- 161.Lutz MB. Therapeutic potential of semi-mature dendritic cells for tolerance induction. Front Immunol. 2012;3:123. doi: 10.3389/fimmu.2012.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hubo M, Trinschek B, Kryczanowsky F, Tuettenberg A, Steinbrink K, Jonuleit H. Costimulatory molecules on immunogenic versus tolerogenic human dendritic cells. Front Immunol. 2013;4:82. doi: 10.3389/fimmu.2013.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Bonifaz L, Bonnyay D, Mahnke K, Rivera M, Nussenzweig MC, Steinman RM. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J Exp Med. 2002;196(12):1627–1638. doi: 10.1084/jem.20021598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Idoyaga J, Fiorese C, Zbytnuik L, Lubkin A, Miller J, Malissen B, Mucida D, Merad M, Steinman RM. Specialized role of migratory dendritic cells in peripheral tolerance induction. J Clin Investig. 2013;123(2):844–854. doi: 10.1172/JCI65260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Grossmann C, Tenbusch M, Nchinda G, Temchura V, Nabi G, Stone GW, Kornbluth RS, Uberla K. Enhancement of the priming efficacy of DNA vaccines encoding dendritic cell-targeted antigens by synergistic Toll-like receptor ligands. BMC Immunol. 2009;10:43. doi: 10.1186/1471-2172-10-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Luckey U, Schmidt T, Pfender N, Romer M, Lorenz N, Martin SF, Bopp T, Schmitt E, Nikolaev A, Yogev N, Waisman A, Jakob T, Steinbrink K (2012) Crosstalk of regulatory T cells and tolerogenic dendritic cells prevents contact allergy in subjects with low zone tolerance. J Allergy Clin Immunol 130(3):781–797, e711. doi:10.1016/j.jaci.2012.06.022 [DOI] [PubMed]
- 167.Pulendran B, Artis D. New paradigms in type 2 immunity. Science. 2012;337(6093):431–435. doi: 10.1126/science.1221064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kayagaki N, Wong MT, Stowe IB, Ramani SR, Gonzalez LC, Akashi-Takamura S, Miyake K, Zhang J, Lee WP, Muszynski A, Forsberg LS, Carlson RW, Dixit VM. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science. 2013;341(6151):1246–1249. doi: 10.1126/science.1240248. [DOI] [PubMed] [Google Scholar]
- 169.Hagar JA, Powell DA, Aachoui Y, Ernst RK, Miao EA. Cytoplasmic LPS activates caspase-11: implications in TLR4-independent endotoxic shock. Science. 2013;341(6151):1250–1253. doi: 10.1126/science.1240988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Gilles S, Behrendt H, Ring J, Traidl-Hoffmann C. The pollen enigma: modulation of the allergic immune response by non-allergenic, pollen-derived compounds. Curr Pharm Des. 2012;18(16):2314–2319. doi: 10.2174/138161212800166040. [DOI] [PubMed] [Google Scholar]