Abstract
Tissue injury initiates extracellular matrix molecule expression, including fibronectin production by local cells and fibronectin leakage from plasma. To benefit tissue regeneration, fibronectin promotes opsonization of tissue debris, migration, proliferation, and contraction of cells involved in the healing process, as well as angiogenesis. When regeneration proceeds, the fibronectin matrix is fully degraded. However, in a diseased environment, fibronectin clearance is often disturbed, allowing structural variants to persist and contribute to disease progression and failure of regeneration. Here, we discuss first how fibronectin helps tissue regeneration, with a focus on normal cutaneous wound healing as an example of complete tissue recovery. Then, we continue to argue that, although the fibronectin matrix generated following cartilage and central nervous system white matter (myelin) injury initially benefits regeneration, fibronectin clearance is incomplete in chronic wounds (skin), osteoarthritis (cartilage), and multiple sclerosis (myelin). Fibronectin fragments or aggregates persist, which impair tissue regeneration. The similarities in fibronectin-mediated mechanisms of frustrated regeneration indicate that complete fibronectin clearance is a prerequisite for recovery in any tissue. Also, they provide common targets for developing therapeutic strategies in regenerative medicine.
Keywords: Fibronectin, Wound healing, Osteoarthritis, Multiple sclerosis, Tissue regeneration
Fibronectin: elements of the scaffold
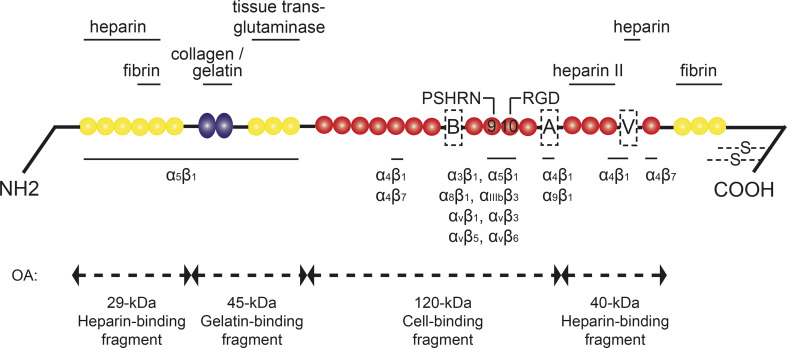
Fibronectin (Fn) is a high molecular weight glycoprotein that consists of three types of repeating amino acid units, named type I, type II, and type III repeats (Fig. 1). The structure of Fn depends on whether it is secreted in plasma or synthesized by resident cells. Plasma Fn (pFn) is produced by hepatocytes, and present in human blood at a concentration of 300 μg/ml [1, 2]. Cellular Fn (cFn) contains the alternatively spliced extra domain A (EDA) and/or extra domain B (EDB) (nomenclature for humans; for rodents: EIIIA and EIIIB). In addition, a third alternatively spliced domain, the IIICS domain (for rodents: the V-region), can be included, but regulations for its inclusion have not been fully discovered yet. pFn and cFn are secreted as a dimer, in which both subunits do not have to contain the same alternatively spliced variants. Physiological Fn monomers and dimers will hereafter be referred to as “native Fn”. The main Fn receptors comprise a variety of integrin receptors (Fig. 1) [3]. In addition, Fn binds other extracellular matrix (ECM) molecules, including heparin, collagen, and fibrin, and together these protein networks form the ECM [4].
Fig. 1.
Structure of fibronectin and major fibronectin fragments that are generated in osteoarthritis. The yellow circles represent type I, the blue ellipses type II, and the red circles type III repeats. The alternative splice variants are referred to as “A” for the EDA/EIIIA domain, “B” for the EDB/EIIIB domain, and V for the IIICS/V-region. Protein interaction sites are depicted above the linear structure, integrin binding sites below. PSHRN and RGD refer to these specific fibronectin domains. Four main fibronectin fragments with catabolic potential in osteoarthritis (OA) are shown under the double-headed arrows. The arrows correspond to the cleaving sites of these fragments. Adapted from [4, 48, 149]
The main function of Fn is to serve as a scaffold for cell adhesion and migration, thereby also regulating cell proliferation and differentiation [4, 5]. These functions are supported by a variety of small proteins, such as growth factors, when they accumulate in the Fn network, increasing their concentration locally. Hence, these small molecules can be regarded as “the builders” on a scaffold of ECM including Fn, although Fn itself also stimulates tissue regeneration. The Fn matrix is essential for normal embryonic development [6]. In healthy adult tissue, Fn is expressed at low levels. Transient Fn (re)-expression by plasma leakage and synthesis from resident cells is a common “default” response of tissue injury, ranging from skin wounds to joint inflammation [7] and myelin degradation (demyelination). Here, we discuss first how this temporary Fn matrix facilitates tissue regeneration, with a focus on normal cutaneous wound healing as an example of complete tissue regeneration. Next, we review how in osteoarthritis (OA) and multiple sclerosis (MS), clearance of the Fn matrix is disturbed, and contributes to failure of tissue regeneration via distinct mechanisms.
The fibronectin scaffold helps to rebuild tissue: focus on cutaneous wound healing
Wounds are defined as disruption of the normal anatomical structure and function of tissue. The wound-healing process is the physiological response to wounding in any tissue. Therefore, when we discuss how Fn benefits cutaneous wound healing, these functions equally apply to other tissues, although detailed features vary between tissue types [8]. In cutaneous wounds, regeneration involves (a) hemostasis and inflammation to provide temporary closure of the defect, (b) migration and proliferation of epithelial cells to replace the temporary seal, and (c) maturation and remodeling of the new epithelium and angiogenesis (reviewed, among many others, in [8–10]). Fn is involved in each of these steps to a greater or lesser extent (extensively reviewed in [11–13]).
On skin injury, a temporary Fn matrix (“the Fn scaffold”) originates from plasma leakage and cellular expression. First, whole blood containing pFn leaks from the disrupted vessels and pFn is a major component of the subsequently formed hemostatic clot, although pFn is not essential for normal hemostasis [14]. Hemostatic thrombi are, however, more stable with pFn than without [15]. The hemostatic clot provides the basis of a provisional matrix, which also contains fibrin and other plasma proteins. The provisional matrix secures additional hemostasis, and assists migration and proliferation of epithelial cells. The presence of pFn in this matrix is not essential for normal wound healing [14], which is explained by compensatory actions of cFn. For as soon as a few hours after wounding [14], cells start to deposit Fn in the provisional matrix. Initially, mainly platelets secrete cFn [14], followed by macrophages, then fibroblasts [16], and possibly endothelial cells [17]. In addition, neutrophils express Fn mRNA at 24 h [17], but are negative again at 2 days after skin wounding [16]. Therefore, neutrophils also contribute to initial cFn expression early after wounding. Analogous to cutaneous wounds, a temporary Fn matrix is generated on cartilage damage [18] and myelin damage (demyelination) in the central nervous system (CNS) [19–23]. In these injuries, Fn leaks from plasma [19, 20] and is secreted by resident chondrocytes in cartilage [24, 25], and resident astrocytes, microglia, and endothelial cells in the CNS [23]. Therefore, the generation of a temporary Fn scaffold with pFn and cFn is a common response to tissue injury.
Functions attributed to the transient Fn matrix include stimulation of: (a) coating and ingestion (opsonization) from tissue debris by inflammatory cells, (b) migration and proliferation of regenerating cells via chemo- and/or haptotaxis, and (c) angiogenesis (for cutaneous wounds reviewed in: [9, 11, 13, 26]). Fn functions are similar among different tissue injuries, but mechanisms are best described in cutaneous wound healing. Fibroblast migration in wound healing requires functional RGD, heparin II, and cFn IIICS domains [27] (Fig. 1), and is promoted by EIIIA from cFn via β-catenin [28] and integrin α9β1 for keratinocytes [29]. Further, myofibroblast differentiation is stimulated by EIIIA [30] via integrin α4β7 [31]. In fact, EIIIA is essential for normal wound contraction in mice as shown via EIIIA knockout [32], although in mice from a different genetic background, EIIIA knockout did not impair wound healing [33]. Without EIIIB, mouse rib fractures heal normally, but more specific experiments are required to confirm its redundancy in cutaneous wound healing, especially since fibroblast proliferation and Fn matrix assembly in vitro are slightly reduced on EIIIB knockout [34].
Before tissue regeneration is completed, the Fn matrix is cleared. However, if Fn persists, this correlates to chronic failure of regeneration. In cutaneous wounds, analysis of wound fluid from human chronic venous ulcers showed persistence of Fn-degradation products, possibly as a result of increased matrix metalloproteinase 9 (MMP-9) activity [35, 36]. Certainly, failure of tissue regeneration is mediated by many factors, including changes in expression of growth factors, cytokines, and matrix proteins as well as receptor expression patterns, and tissue oxygen levels [37, 38]. Therefore, a structurally altered Fn matrix will only contribute to failure of regeneration in a complex interplay with changes in other factors, but nonetheless mediates tissue damage. Fn-degradation products in chronic venous ulcers, for example, likely stimulate neutrophil degranulation [39], and EIIIA activates Toll-like receptor 4 (TLR4) on inflammatory cells. This TLR4 stimulation may help opsonization of tissue debris at first, but eventually results in chronic inflammation [40, 41]. Interestingly, Fn fragments have also been implicated in OA disease progression, which will be discussed next.
Osteoarthritis: fibronectin fragments contribute to cartilage damage
OA is characterized by articular cartilage damage, resulting in joint destruction. The pathophysiology of OA is not fully understood. The current pathogenesis concept is based on increased cytokine and chemokine activation as a result of many factors, including ageing and chronic wear and tear on cartilage. In this concept, cytokines and chemokines contribute to protease production by chondrocytes, and inhibition of cartilage synthesis. This damages articular cartilage, and eventually also joint synovium, ligaments, tendons, and muscles [42, 43], causing pain and impairment of motility. The Fn matrix that is generated on the initial cartilage injury is not completely degraded in OA. As a result, Fn fragments contribute to (a) persistent local inflammation via innate immune system activation, and (b) direct cartilage damage.
Local joint inflammation results from Fn persistence in several ways. First, after Fn is degraded into multiple fragments by proteases (Fig. 1), Fn binds the C1q component of the complement system [44, 45], likely resulting in chronic stimulation of leukocytes. Secondly, the fragment containing EIIIA stimulates TLR4, as discussed above in the context of complicated wound healing [40, 46]. However, although the roles of EIIIA–TLR4 interactions in chronic wounds remain hypotheses based on in vitro studies, there is more evidence for the significance of this binding for joint damage. Injecting EIIIA-containing fragments into joints of mice results in joint swelling through the release of pro-inflammatory cytokines from mast cells [41]. Therefore, although innate immune system activation by Fn initially facilitates tissue debris clearance on cartilage damage, non-degraded Fn fragments contribute to chronic synovial inflammation in OA.
Cartilage damage in OA (and in rheumatoid arthritis) is also mediated by Fn fragments via suppression of sulfated proteoglycans, and via stimulation of chondrocytes and synovial fibroblasts to secrete catabolic cytokines and MMPs. The four major characterized Fn fragments include a 29-kDa heparin-binding fragment, a gelatin-binding fragment, a cell-binding fragment, and a 40-kDa heparin-binding fragment [47, 48] (Fig. 1). These Fn fragments are present at high levels in synovial fluid from OA patients [49], and in human osteoarthritic cartilage [50]. Fn fragments result from degradation of the Fn matrix, mediated by MMP-1, -3, -8, and -13 and by the aggrecanases ADAMTS-4 and -5 [48, 51, 52]. Native Fn has no adverse effects on cartilage, and low concentrations of Fn fragments show anabolic effects [53]. However, at higher concentrations, Fn fragments stimulate cartilage chondrolysis in vitro [54, 55] via different effector molecules and different pathways.
First, Fn fragments contribute to release of pro-inflammatory cytokines. These include IL-1, TNF-α, and IL-6 in cultured human cartilage for the 29-kDa heparin-binding fragment [56], and IL-6, IL-8 [57], and IL-7 [58] in human articular chondrocytes for the cell-binding fragment. These cytokines subsequently stimulate MMP expression from chondrocytes, including MMP-1, -2, -3, -9, and -13 [59–62], which enhance cartilage degradation. For example, chondrocytes release MMP-3 on stimulation with the 29-kDa heparin-binding fragment, but blocking antibodies to TNF-α, IL-1, and IL-6 suppress this release [56]. Activation of some of these cytokines and MMPs is mediated by the nuclear factor-κB (NF-κB) transcription pathway [57], whereas activation of others involves mitogen-activated protein kinase (MAPK) pathway activation (reviewed in [47]).
Secondly, Fn fragments induce MMP expression directly via cell surface receptors. For example, the 40-kDa heparin-binding fragment stimulates MMP release from chondrocytes via upregulation of NF-κB through the phosphoinositide-3-OH kinase (PI3K)/Akt pathway and the CD44 hyaluronan receptor [61, 63]. Interestingly, the same heparin-binding fragment also binds TLR4 to initiate aggrecanase release from chondrocytes [64]. The cell-binding Fn fragment binds integrin α5β1 on chondrocytes and fibroblasts, which induces the secretion of MMP-13 and degradation of cartilage [59, 65, 66]. This interaction requires reactive oxygen species as second messengers [67]. Although native Fn also binds to integrin receptors, native Fn stimulation does not cause a catabolic response from chondrocytes. This contrast can be explained by the hypothesis of “Fn-integrin imbalance”. According to this hypothesis, Fn fragments alter normal Fn signals in chondrocytes by binding to distinct integrin receptors, but at the same time not binding to others. Therefore, chondrocytes perceive signals from altered clusters of integrins, and this initiates a catabolic response in OA [59, 68, 69]. Further, Fn fragments expose cryptic binding sites, which also explains the altered signaling compared to native Fn.
Thirdly, besides stimulation of cytokine and subsequent proteinase (MMP) production, Fn fragments, such as the 29-kDa heparin-binding fragment, damage cartilage via suppression of cartilage matrix synthesis, including sulfated proteoglycans [53, 70]. Also, the heparin-binding Fn fragment spanning the COOH-terminal induces an enhanced release of the free radical nitric oxide (NO) [71]. Although most of the data discussed are generated in vitro, additional in vivo studies show that injection of Fn fragments into rabbit knee joints results in cartilage destruction and joint swelling, resembling OA in humans [70, 72].
In order to reverse Fn fragment-mediated cartilage destruction in OA, and perhaps also rheumatoid arthritis, the following designs may be considered: (a) prevention of Fn fragmentation, (b) clearance of Fn fragments, and (c) by-pass of harmful Fn fragment signals. Attempts at by-passing Fn fragment signals is, to our knowledge, the only approach to have been tested so far. Anti-oxidants, including N-acetylcysteine, glutathione, and allopurinol, increase proteoglycan levels on Fn fragments as a result of a reduction of the catabolic cytokines TNF-α, IL-1, and IL-6 in vitro [73, 74]. Glucosamine and chondroitin sulfate mixtures also increase proteoglycan levels after Fn fragment administration to cultured cartilage [75]. Despite these modest, favorable effects on the damage caused by Fn fragments, none of these agents are currently used in clinical treatments of OA. Another agent, hyaluronan, is in clinical use for OA, mainly because patients” pain and joint function can improve on this drug [43]. One of the underlying mechanisms for its benefit could comprise preventing the catabolic effects of Fn fragments, because on Fn fragment injection into rabbit joints, hyaluronan upregulates proteoglycan levels and improves histological disease characteristics [72]. In addition, after treatment with Fn fragments, hyaluronan promotes proteoglycan levels, and decreases NO levels in cultured human articular cartilage from OA patients [76, 77]. Whether hyaluronan contributes to structural cartilage improvement in OA needs yet to be established in human patients.
Multiple sclerosis: fibronectin aggregates inhibit regeneration of myelin
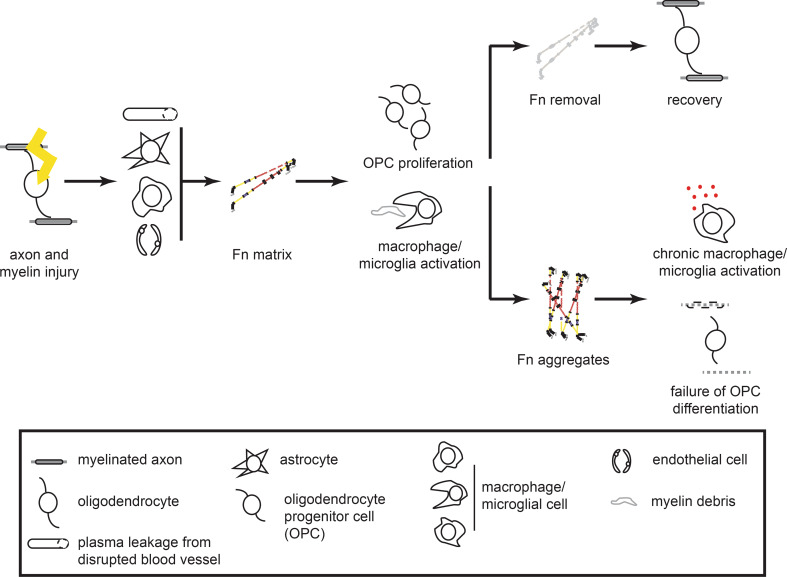
MS is a chronic disease of the CNS. Although the pathogenesis is unknown, many factors are recognized to play a role in MS onset, including genetics and environmental factors, such as cigarette smoking, Epstein–Barr virus infection and vitamin D levels [78]. Pathological hallmarks are CNS inflammation, myelin degeneration (demyelination) and axonal loss, which clinically reflect as neurological disability. Progression of MS occurs in distinct patterns, ranging from rapid accumulation of disability in primary and secondary progressive MS to episodes of fulminant inflammation and recovery in relapsing-remitting MS. On demyelination, regeneration of myelin (remyelination) is attempted by oligodendrocyte progenitor cells (OPCs), which can differentiate into myelin-forming oligodendrocytes. However, remyelination ultimately fails in MS, despite the presence of OPCs [79, 80], leaving axons unprotected by myelin sheaths, and therefore vulnerable to further degeneration [81–83]. Temporary, dimeric Fn expression occurs on demyelination, but Fn aggregates in MS lesions [23]. Persistence of Fn, likely in the form of Fn aggregates, is involved in the pathology of chronic MS via (a) (chronic) stimulation of inflammation, and (b) direct inhibition of OPC maturation to oligodendrocytes (Fig. 2).
Fig. 2.
On central nervous system injury in multiple sclerosis, improper fibronectin degradation is related to failure of remyelination and disease progression. Myelin injury initiates fibronectin (Fn) expression from plasma leakage, and secretion by astrocytes, macrophages/microglia, and endothelial cells (depicted from top to bottom, respectively). This Fn matrix promotes myelin regeneration via different mechanisms, including stimulation of oligodendrocyte progenitor cell (OPC) migration and proliferation. Complete removal of the Fn matrix corresponds to remyelination, whereas Fn aggregates mediate failure of remyelination by impairing OPC differentiation, and possibly via continuous macrophage/microglia activation
Inflammation in MS involves, among others, the entry of immune cells to the brain, and the pathological activation of CNS-resident microglia. Leukocyte invasion to the brain in relapsing-remitting MS requires migration across the blood–brain barrier. The blood–brain barrier contains endothelial cells and astrocytes [84], expressing Fn [20, 85]. In order to cross the blood–brain barrier, leukocytes express integrin α4β1, which mainly binds to vascular cell adhesion molecule 1 (VCAM1) [86], but also to the CS1-peptide, which is a domain of Fn (a site within the IIICS-region) (Fig. 1) on endothelial cells, or can be expressed independently of Fn on astrocytes [87, 88]. The α4β1-Fn CS1 interaction is blocked by natalizumab, an approved drug for relapsing-remitting MS that was designed to prevent leukocytes from binding to VCAM1 [85]. Also, interferon β-1b, another drug for relapsing-remitting MS, inhibits the ability of T-lymphocytes to migrate via Fn on endothelial cells [89]. Therefore, Fn on endothelial cells contributes to leukocyte invasion in relapsing-remitting MS. Although cell migration and proliferation on Fn usually benefit tissue regeneration, these examples demonstrate that such physiological functions contribute to pathology under inappropriate circumstances. Of note, stimulation of cell proliferation by Fn also becomes pathological in cancer metastases, when cancer cells invade new tissue and proliferate there [90–92]. Further, Fn contributes to MS inflammation by instructing the CNS resident microglia and, possibly, invaded macrophages. Fn interacts with integrin α5β1 on microglia to enhance MMP-9 secretion [93]. Also, Fn may bind to TLR4 on microglia, as has been suggested for wound healing and OA before. Expression of TLR3 and -4 is upregulated in human MS lesions [94], with TLR4 primarily localized to microglia [94, 95]. In vitro, pFn activates microglial cells to secrete pro-inflammatory cytokines, including IL-1β [96], TNF-α, CXCL1, CCL3, and CCL5, and enhances phagocytosis by microglia [97]. Because these effects depend on the presence of MyD88, they likely result from TLR4 stimulation. Interestingly, in these studies, pFn was examined, suggesting that pFn contributes to TLR4-stimulation as well as EIIIA-containing Fn fragments [40]. Immune activated microglia likely facilitate remyelination in MS. For example, phagocytosis of myelin debris is necessary for complete remyelination [98] and moderate inflammatory activity enhances remyelination [99, 100]. Also, macrophage/microglia activation by pFn is neuroprotective in traumatic brain injury [101]. However, TLR4 stimulation by LPS induces indirect loss of OPCs and oligodendrocytes and neurodegeneration in vivo [95, 102]. Therefore, the overall effects of Fn–microglia interactions in MS remain to be established, as well as the effects of Fn aggregates.
Fn also directly affects OPCs. OPCs express a variety of integrin receptors during the (re)myelination process, including the Fn receptors αvβ1, αvβ3, αvβ5, and αvβ8 [103–105]. In vitro, coatings of pFn stimulate integrin αvβ1 to enhance OPC migration [106, 107]. In addition, αvβ3 integrin stimulation by physiological levels of platelet-derived growth factor A (PDGF-A) and Fn enhances OPC proliferation [108]. Further, integrin αvβ5 signaling is important for OPC differentiation [104]. These studies indicate that Fn promotes recruitment of OPCs in the demyelinated area via αvβ1, αvβ3, and αvβ5 integrins, benefiting remyelination. However, in vitro myelin formation of OPCs is impaired on pFn, mediated by β1-integrin signaling and mislocalized MMP-9 activity [109–111]. This impairment of OPC maturation initially plays a useful role in remyelination because it allows for precise timing of the remyelination process [82]. However, as soon as OPC recruitment has been completed, αv integrin expression decreases [112] and the Fn matrix should be degraded, allowing OPCs to proceed to form myelin. Indeed, downregulation of Fn precedes remyelination on toxin-induced demyelination [22, 23, 105]. In contrast, in chronic relapsing experimental autoimmune encephalomyelitis (cr-EAE), an animal model for relapsing-remitting MS, Fn aggregates in the lesion areas. Fn aggregates also persist in chronic demyelinated MS lesions, and inhibit CNS remyelination on toxin-induced demyelination in vivo [23]. The mechanism for remyelination impairment needs yet to be established, but could comprise the perturbation of oligodendrocyte process outgrowth, myelin-membrane-directed vesicular transport, and membrane microdomain formation, as has been shown for pFn [110, 111, 113, 114].
How Fn aggregates are formed in MS warrants further investigation. Organization of Fn into fibrils (fibrillogenesis) and, ultimately, assembly into a three-dimensional matrix is a well-balanced process during tissue development and regeneration (extensively reviewed in [115, 116]). Fn aggregation, as defined by deoxycholate (DOC)-insolubility, is likely the result of strong, noncovalent protein–protein interactions [117, 118] (our unpublished observations), and participation of other extracellular proteins in this matrix [118]. Fn aggregation may be appropriate during initial stages of tissue regeneration [115, 116, 119]. However, excessive Fn deposition and inappropriate remodeling contribute to scarring and fibrosis, and frustrate complete tissue regeneration [116, 120]. Under physiological circumstances, maintenance of the Fn matrix requires continuous Fn synthesis by cells [121], but in MS, Fn mRNA levels were undetectable in chronic demyelinated lesions, where Fn aggregates nonetheless persisted [23]. This suggests that inappropriate remodeling, rather than continuous Fn deposition, is crucial for Fn aggregation in MS lesions. Fn remodeling into aggregates is likely mediated by self-assembly, interaction with binding sites on other proteins as well as with cellular receptors (mainly integrin receptors), and local enzyme activity [115, 122, 123]. Because transglutaminase activity is proposed to be required for Fn aggregation [124, 125], and transglutaminase interactions with Fn are active in MS [126], this enzyme is one of the factors that may contribute to Fn aggregation in MS, but this requires further investigation.
Concluding remarks: timely removal of the fibronectin scaffold is necessary to complete the build
In the development of therapeutic strategies for the promotion of tissue regeneration, there is much focus on the initiating mechanisms of specific diseases, for example via the identification of gene expression patterns relevant to disease onset. The rationale behind this approach is that to unravel the disease pathogenesis will likely provide targets for stopping tissue degeneration by its cause. Alternatively, a more pragmatic strategy is to tackle persistent factors in the injury environment that hamper regeneration. Fn is such a factor. This review illustrates similarities among the responses to injury in different tissues in the creation of a Fn matrix. Fn initially facilitates regeneration of skin, cartilage, and myelin, mainly via stimulating the recruitment of inflammatory and regenerative cells. However, whereas Fn is totally removed before complete regeneration takes place, persistent structural variants contribute to failing regeneration in OA and MS. Mechanisms by which persistent Fn mediates regeneration failure differ between specific tissue types, but showed similarities, especially in their interaction with the immune system. Therefore, adequate clearance of the Fn matrix benefits regeneration, and incomplete degradation contributes to failure of tissue regeneration (Fig. 2).
The contribution of a residual Fn matrix in regeneration failure is not limited to the tissues that have been discussed in this review. For example, in myocardial infarction, the Fn matrix is necessary for myofibroblast recruitment and differentiation [127], but on incomplete clearance of the matrix, the EIIIA-containing Fn mediates adverse cardiac remodeling [128]. Also, accumulation of Fn fragments occurs during intervertebral disc degeneration [129], and further enhances disc degradation via stimulation of MMP expression [130, 131]. These examples further emphasize the benefit of complete degradation from the Fn matrix for tissue regeneration. This also underlines the importance of tightly regulated dynamic ECM expression in general [132], especially because collagen fragments and persistence of tenascin-C also contribute to OA pathology [133, 134]. Similarly, the high molecular weight variant of hyaluronan, present in MS lesions, inhibits remyelination [135].
In designing therapies to improve tissue regeneration, both the good and the bad sides of Fn can be taken into account. Taking advantage of the pro-regenerative properties of Fn, it can be attempted to speed up regeneration by exogenous administration of Fn. In designing such therapies, it is essential to consider the concept of “dynamic reciprocity”, which refers to the importance of well-balanced receptor and ligand signaling in time. In this concept, Fn administration can only add to healing if its (integrin) receptors are still upregulated [38]. Despite a potential mismatch between Fn and its receptors in chronic wounds, a modest additional benefit has been demonstrated for Fn-based therapies here. For example, after wounding the skin from obese diabetic mice [136], and also on rat peritoneal injury [137], the PHSRN fragment from the 9th type III domain accelerates wound healing. This acceleration benefits from the physiological properties of Fn, including an increased fibroblast and keratinocyte adhesion and migration, wound contraction [136], and angiogenesis via integrin α5β1 on epidermal and endothelial cells [138, 139]. Similarly, pFn slightly accelerates wound healing in rats when topically applied onto skin wounds [140, 141], and when injected after incisional wounding in a dose-dependent manner [142]. In patients with persistent corneal epithelial defects, topical application of pFn shows modest beneficial effects on healing [143]. Wound dressings, including Fn-based therapies, benefit healing in carefully selected wounds, and only in a subset of wounded patients [144]. Therefore, the therapeutic potential of exogenous Fn application could further be enhanced by selecting specific patient groups, such as wounded patients with diabetes mellitus [141]. In addition, these therapies may be more effective when Fn domains are coupled to (a) other supportive proteins, such as hyaluronan [145], (b) growth factors, such as PDGF [140] and hepatocyte growth factor [146], or (c) glycoprotein hormones, such as erythropoietin [31, 147]. Finally, in another elegant approach, a fibrin/Fn matrix was designed, that could bind the growth factors PDGF, vascular endothelial growth factor (VEGF), and bone morphogenetic protein (BMP) to enhance healing of skin wounds of diabetic mice [148].
To overcome the detrimental properties of persistent Fn, multiple strategies are possible: (a) ensuring proper Fn clearance, (b) eliminating Fn structural variants once they emerge, and (c) by-pass of harmful Fn signals. We briefly discussed therapeutic strategies in OA and MS that, among their other actions, by-pass Fn signals. By-passing strategies included hyaluronic acid (OA), natalizumab, and interferon β-1b (MS), although these effects occur secondary to how the drugs were designed. These and the other approaches warrant further investigation. To accelerate our understanding, a multidisciplinary approach will be helpful, comparing good and bad sides of Fn and Fn therapies between tissues. This will expand our insight into how improper Fn clearance is mediated, and could be overcome. These insights will likely benefit therapeutic strategies that promote tissue regeneration.
Acknowledgments
Work in the Baron Laboratory is supported by grants from the Netherlands Foundation for the Support of MS Research (Stichting MS Research), and the Netherlands Organization of Scientific Research NWO (VIDI and Aspasia).
References
- 1.Nishinarita S, Yamamoto M, Takizawa T, Hayakawa J, Karasaki M, Sawada S. Increased plasma fibronectin in patients with systemic lupus erythematosus. Clin Rheumatol. 1990;9:214–219. doi: 10.1007/BF02031971. [DOI] [PubMed] [Google Scholar]
- 2.Goos M, Lange P, Hanisch UK, Prinz M, Scheffel J, Bergmann R, Ebert S, Nau R. Fibronectin is elevated in the cerebrospinal fluid of patients suffering from bacterial meningitis and enhances inflammation caused by bacterial products in primary mouse microglial cell cultures. J Neurochem. 2007;102:2049–2060. doi: 10.1111/j.1471-4159.2007.04683.x. [DOI] [PubMed] [Google Scholar]
- 3.Plow EF, Haas TA, Zhang L, Loftus J, Smith JW. Ligand binding to integrins. J Biol Chem. 2000;275:21785–21788. doi: 10.1074/jbc.R000003200. [DOI] [PubMed] [Google Scholar]
- 4.Pankov R, Yamada KM. Fibronectin at a glance. J Cell Sci. 2002;115:3861–3863. doi: 10.1242/jcs.00059. [DOI] [PubMed] [Google Scholar]
- 5.Mosher DF. Fibronectin. San Diego: Academic Press, Inc.; 1988. [Google Scholar]
- 6.George EL, Georges-Labouesse EN, Patel-King RS, Rayburn H, Hynes RO. Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Development. 1993;119:1079–1091. doi: 10.1242/dev.119.4.1079. [DOI] [PubMed] [Google Scholar]
- 7.Scanzello CR, Plaas A, Crow MK. Innate immune system activation in osteoarthritis: is osteoarthritis a chronic wound? Curr Opin Rheumatol. 2008;20:565–572. doi: 10.1097/BOR.0b013e32830aba34. [DOI] [PubMed] [Google Scholar]
- 8.Velnar T, Bailey T, Smrkolj V. The wound healing process: an overview of the cellular and molecular mechanisms. J Int Med Res. 2009;37:1528–1542. doi: 10.1177/147323000903700531. [DOI] [PubMed] [Google Scholar]
- 9.Tonnesen MG, Feng X, Clark RA. Angiogenesis in wound healing. J Investig Dermatol Symp Proc. 2000;5:40–46. doi: 10.1046/j.1087-0024.2000.00014.x. [DOI] [PubMed] [Google Scholar]
- 10.Baum CL, Arpey CJ. Normal cutaneous wound healing: clinical correlation with cellular and molecular events. Dermatol Surg. 2005;31:674–686. doi: 10.1111/j.1524-4725.2005.31612. [DOI] [PubMed] [Google Scholar]
- 11.Clark RA. Potential roles of fibronectin in cutaneous wound repair. Arch Dermatol. 1988;124:201–206. [PubMed] [Google Scholar]
- 12.Colvin RB. In: Fibronectin in wound healing. Mosher DF, editor. San Diego: Academic Press, Inc.; 1989. pp. 213–254. [Google Scholar]
- 13.Midwood KS, Mao Y, Hsia HC, Valenick LV, Schwarzbauer JE. Modulation of cell-fibronectin matrix interactions during tissue repair. J Investig Dermatol Symp Proc. 2006;11:73–78. doi: 10.1038/sj.jidsymp.5650005. [DOI] [PubMed] [Google Scholar]
- 14.Sakai T, Johnson KJ, Murozono M, Sakai K, Magnuson MA, Wieloch T, Cronberg T, Isshiki A, Erickson HP, Fassler R. Plasma fibronectin supports neuronal survival and reduces brain injury following transient focal cerebral ischemia but is not essential for skin-wound healing and hemostasis. Nat Med. 2001;7:324–330. doi: 10.1038/85471. [DOI] [PubMed] [Google Scholar]
- 15.Ni H, Yuen PS, Papalia JM, Trevithick JE, Sakai T, Fassler R, Hynes RO, Wagner DD. Plasma fibronectin promotes thrombus growth and stability in injured arterioles. Proc Natl Acad Sci USA. 2003;100:2415–2419. doi: 10.1073/pnas.2628067100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown LF, Dubin D, Lavigne L, Logan B, Dvorak HF, Van de Water L. Macrophages and fibroblasts express embryonic fibronectins during cutaneous wound healing. Am J Pathol. 1993;142:793–801. [PMC free article] [PubMed] [Google Scholar]
- 17.Takamiya M, Kumagai R, Nakayashiki N, Aoki Y. A study on mRNA expressions of fibronectin in dermal and cerebral wound healing for wound age estimation. Leg Med (Tokyo) 2006;8:214–219. doi: 10.1016/j.legalmed.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 18.Miller DR, Mankin HJ, Shoji H, D’Ambrosia RD. Identification of fibronectin in preparations of osteoarthritic human cartilage. Connect Tissue Res. 1984;12:267–275. doi: 10.3109/03008208409013689. [DOI] [PubMed] [Google Scholar]
- 19.Sobel RA, Mitchell ME. Fibronectin in multiple sclerosis lesions. Am J Pathol. 1989;135:161–168. [PMC free article] [PubMed] [Google Scholar]
- 20.van Horssen J, Bo L, Vos CM, Virtanen I, de Vries HE. Basement membrane proteins in multiple sclerosis-associated inflammatory cuffs: potential role in influx and transport of leukocytes. J Neuropathol Exp Neurol. 2005;64:722–729. doi: 10.1097/01.jnen.0000173894.09553.13. [DOI] [PubMed] [Google Scholar]
- 21.Satoh JI, Tabunoki H, Yamamura T. Molecular network of the comprehensive multiple sclerosis brain-lesion proteome. Mult Scler. 2009;15:531–541. doi: 10.1177/1352458508101943. [DOI] [PubMed] [Google Scholar]
- 22.Hibbits N, Yoshino J, Le TQ, Armstrong RC. Astrogliosis during acute and chronic cuprizone demyelination and implications for remyelination. ASN Neuro. 2012;4(6):393–408. doi: 10.1042/AN20120062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stoffels JM, de Jonge JC, Stancic M, Nomden A, van Strien ME, Ma D, Siskova Z, Maier O, ffrench-Constant C, Franklin RJ, Hoekstra D, Zhao C, Baron W. Fibronectin aggregation in multiple sclerosis lesions impairs remyelination. Brain. 2013;136:116–131. doi: 10.1093/brain/aws313. [DOI] [PubMed] [Google Scholar]
- 24.Chevalier X, Claudepierre P, Groult N, Zardi L, Hornebeck W. Presence of ED-A containing fibronectin in human articular cartilage from patients with osteoarthritis and rheumatoid arthritis. J Rheumatol. 1996;23:1022–1030. [PubMed] [Google Scholar]
- 25.Chevalier X, Groult N, Hornebeck W. Increased expression of the Ed-B-containing fibronectin (an embryonic isoform of fibronectin) in human osteoarthritic cartilage. Br J Rheumatol. 1996;35:407–415. doi: 10.1093/rheumatology/35.5.407. [DOI] [PubMed] [Google Scholar]
- 26.Clark RA. Fibrin and wound healing. Ann NY Acad Sci. 2001;936:355–367. doi: 10.1111/j.1749-6632.2001.tb03522.x. [DOI] [PubMed] [Google Scholar]
- 27.Clark RA, An JQ, Greiling D, Khan A, Schwarzbauer JE. Fibroblast migration on fibronectin requires three distinct functional domains. J Invest Dermatol. 2003;121:695–705. doi: 10.1046/j.1523-1747.2003.12484.x. [DOI] [PubMed] [Google Scholar]
- 28.Bielefeld KA, Amini-Nik S, Whetstone H, Poon R, Youn A, Wang J, Alman BA. Fibronectin and beta-catenin act in a regulatory loop in dermal fibroblasts to modulate cutaneous healing. J Biol Chem. 2011;286:27687–27697. doi: 10.1074/jbc.M111.261677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh P, Reimer CL, Peters JH, Stepp MA, Hynes RO, Van De Water L. The spatial and temporal expression patterns of integrin alpha9beta1 and one of its ligands, the EIIIA segment of fibronectin, in cutaneous wound healing. J Invest Dermatol. 2004;123:1176–1181. doi: 10.1111/j.0022-202X.2004.23485.x. [DOI] [PubMed] [Google Scholar]
- 30.Serini G, Bochaton-Piallat ML, Ropraz P, Geinoz A, Borsi L, Zardi L, Gabbiani G. The fibronectin domain ED-A is crucial for myofibroblastic phenotype induction by transforming growth factor-beta1. J Cell Biol. 1998;142:873–881. doi: 10.1083/jcb.142.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kohan M, Muro AF, White ES, Berkman N. EDA-containing cellular fibronectin induces fibroblast differentiation through binding to alpha4beta7 integrin receptor and MAPK/Erk 1/2-dependent signaling. FASEB J. 2010;24:4503–4512. doi: 10.1096/fj.10-154435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muro AF, Chauhan AK, Gajovic S, Iaconcig A, Porro F, Stanta G, Baralle FE. Regulated splicing of the fibronectin EDA exon is essential for proper skin wound healing and normal lifespan. J Cell Biol. 2003;162:149–160. doi: 10.1083/jcb.200212079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tan MH, Sun Z, Opitz SL, Schmidt TE, Peters JH, George EL. Deletion of the alternatively spliced fibronectin EIIIA domain in mice reduces atherosclerosis. Blood. 2004;104:11–18. doi: 10.1182/blood-2003-09-3363. [DOI] [PubMed] [Google Scholar]
- 34.Fukuda T, Yoshida N, Kataoka Y, Manabe R, Mizuno-Horikawa Y, Sato M, Kuriyama K, Yasui N, Sekiguchi K. Mice lacking the EDB segment of fibronectin develop normally but exhibit reduced cell growth and fibronectin matrix assembly in vitro. Cancer Res. 2002;62:5603–5610. [PubMed] [Google Scholar]
- 35.Grinnell F, Ho CH, Wysocki A. Degradation of fibronectin and vitronectin in chronic wound fluid: analysis by cell blotting, immunoblotting, and cell adhesion assays. J Invest Dermatol. 1992;98:410–416. doi: 10.1111/1523-1747.ep12499839. [DOI] [PubMed] [Google Scholar]
- 36.Moor AN, Vachon DJ, Gould LJ. Proteolytic activity in wound fluids and tissues derived from chronic venous leg ulcers. Wound Repair Regen. 2009;17:832–839. doi: 10.1111/j.1524-475X.2009.00547.x. [DOI] [PubMed] [Google Scholar]
- 37.Falanga V. The chronic wound: impaired healing and solutions in the context of wound bed preparation. Blood Cells Mol Dis. 2004;32:88–94. doi: 10.1016/j.bcmd.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 38.Widgerow AD. Chronic wounds - is cellular “reception” at fault? Examining integrins and intracellular signalling. Int Wound J. 2013;10:185–192. doi: 10.1111/j.1742-481X.2012.00967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wachtfogel YT, Abrams W, Kucich U, Weinbaum G, Schapira M, Colman RW. Fibronectin degradation products containing the cytoadhesive tetrapeptide stimulate human neutrophil degranulation. J Clin Invest. 1988;81:1310–1316. doi: 10.1172/JCI113456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Okamura Y, Watari M, Jerud ES, Young DW, Ishizaka ST, Rose J, Chow JC, Strauss JF., 3rd The extra domain A of fibronectin activates Toll-like receptor 4. J Biol Chem. 2001;276:10229–10233. doi: 10.1074/jbc.M100099200. [DOI] [PubMed] [Google Scholar]
- 41.Gondokaryono SP, Ushio H, Niyonsaba F, Hara M, Takenaka H, Jayawardana ST, Ikeda S, Okumura K, Ogawa H. The extra domain A of fibronectin stimulates murine mast cells via Toll-like receptor 4. J Leukoc Biol. 2007;82:657–665. doi: 10.1189/jlb.1206730. [DOI] [PubMed] [Google Scholar]
- 42.Loeser RF. Molecular mechanisms of cartilage destruction in osteoarthritis. J Musculoskelet Neuronal Interact. 2008;8:303–306. [PubMed] [Google Scholar]
- 43.Edmonds S. Therapeutic targets for osteoarthritis. Maturitas. 2009;63:191–194. doi: 10.1016/j.maturitas.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 44.Bing DH, Almeda S, Isliker H, Lahav J, Hynes RO. Fibronectin binds to the C1q component of complement. Proc Natl Acad Sci USA. 1982;79:4198–4201. doi: 10.1073/pnas.79.13.4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carsons SE, Schwartzman S, Diamond HS, Berkowitz E. Interaction between fibronectin and C1q in rheumatoid synovial fluid and normal plasma. Clin Exp Immunol. 1988;72:37–42. [PMC free article] [PubMed] [Google Scholar]
- 46.Lasarte JJ, Casares N, Gorraiz M, Hervas-Stubbs S, Arribillaga L, Mansilla C, Durantez M, Llopiz D, Sarobe P, Borras-Cuesta F, Prieto J, Leclerc C. The extra domain A from fibronectin targets antigens to TLR4-expressing cells and induces cytotoxic T cell responses in vivo. J Immunol. 2007;178:748–756. doi: 10.4049/jimmunol.178.2.748. [DOI] [PubMed] [Google Scholar]
- 47.Yasuda T. Cartilage destruction by matrix degradation products. Mod Rheumatol. 2006;16:197–205. doi: 10.1007/s10165-006-0490-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sofat N. Analysing the role of endogenous matrix molecules in the development of osteoarthritis. Int J Exp Pathol. 2009;90:463–479. doi: 10.1111/j.1365-2613.2009.00676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xie DL, Meyers R, Homandberg GA. Fibronectin fragments in osteoarthritic synovial fluid. J Rheumatol. 1992;19:1448–1452. [PubMed] [Google Scholar]
- 50.Zack MD, Arner EC, Anglin CP, Alston JT, Malfait AM, Tortorella MD. Identification of fibronectin neoepitopes present in human osteoarthritic cartilage. Arthr Rheum. 2006;54:2912–2922. doi: 10.1002/art.22045. [DOI] [PubMed] [Google Scholar]
- 51.Gendron C, Kashiwagi M, Lim NH, Enghild JJ, Thogersen IB, Hughes C, Caterson B, Nagase H. Proteolytic activities of human ADAMTS-5: comparative studies with ADAMTS-4. J Biol Chem. 2007;282:18294–18306. doi: 10.1074/jbc.M701523200. [DOI] [PubMed] [Google Scholar]
- 52.Zack MD, Malfait AM, Skepner AP, Yates MP, Griggs DW, Hall T, Hills RL, Alston JT, Nemirovskiy OV, Radabaugh MR, Leone JW, Arner EC, Tortorella MD. ADAM-8 isolated from human osteoarthritic chondrocytes cleaves fibronectin at Ala (271) Arthr Rheum. 2009;60:2704–2713. doi: 10.1002/art.24753. [DOI] [PubMed] [Google Scholar]
- 53.Homandberg GA, Hui F. High concentrations of fibronectin fragments cause short-term catabolic effects in cartilage tissue while lower concentrations cause continuous anabolic effects. Arch Biochem Biophys. 1994;311:213–218. doi: 10.1006/abbi.1994.1229. [DOI] [PubMed] [Google Scholar]
- 54.Homandberg GA, Meyers R, Xie DL. Fibronectin fragments cause chondrolysis of bovine articular cartilage slices in culture. J Biol Chem. 1992;267:3597–3604. [PubMed] [Google Scholar]
- 55.Yasuda T, Poole AR. A fibronectin fragment induces type II collagen degradation by collagenase through an interleukin-1-mediated pathway. Arthr Rheum. 2002;46:138–148. doi: 10.1002/1529-0131(200201)46:1<138::AID-ART10051>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 56.Homandberg GA, Hui F, Wen C, Purple C, Bewsey K, Koepp H, Huch K, Harris A. Fibronectin-fragment-induced cartilage chondrolysis is associated with release of catabolic cytokines. Biochem J. 1997;321(Pt 3):751–757. doi: 10.1042/bj3210751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pulai JI, Chen H, Im HJ, Kumar S, Hanning C, Hegde PS, Loeser RF. NF-kappa B mediates the stimulation of cytokine and chemokine expression by human articular chondrocytes in response to fibronectin fragments. J Immunol. 2005;174:5781–5788. doi: 10.4049/jimmunol.174.9.5781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Long D, Blake S, Song XY, Lark M, Loeser RF. Human articular chondrocytes produce IL-7 and respond to IL-7 with increased production of matrix metalloproteinase-13. Arthr Res Ther. 2008;10:R23. doi: 10.1186/ar2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Werb Z, Tremble PM, Behrendtsen O, Crowley E, Damsky CH. Signal transduction through the fibronectin receptor induces collagenase and stromelysin gene expression. J Cell Biol. 1989;109:877–889. doi: 10.1083/jcb.109.2.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xie DL, Hui F, Meyers R, Homandberg GA. Cartilage chondrolysis by fibronectin fragments is associated with release of several proteinases: stromelysin plays a major role in chondrolysis. Arch Biochem Biophys. 1994;311:205–212. doi: 10.1006/abbi.1994.1228. [DOI] [PubMed] [Google Scholar]
- 61.Yasuda T, Poole AR, Shimizu M, Nakagawa T, Julovi SM, Tamamura H, Fujii N, Nakamura T. Involvement of CD44 in induction of matrix metalloproteinases by a COOH-terminal heparin-binding fragment of fibronectin in human articular cartilage in culture. Arthr Rheum. 2003;48:1271–1280. doi: 10.1002/art.10951. [DOI] [PubMed] [Google Scholar]
- 62.Stanton H, Ung L, Fosang AJ. The 45-kDa collagen-binding fragment of fibronectin induces matrix metalloproteinase-13 synthesis by chondrocytes and aggrecan degradation by aggrecanases. Biochem J. 2002;364:181–190. doi: 10.1042/bj3640181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yasuda T. Activation of Akt leading to NF-kappaB up-regulation in chondrocytes stimulated with fibronectin fragment. Biomed Res. 2011;32:209–215. doi: 10.2220/biomedres.32.209. [DOI] [PubMed] [Google Scholar]
- 64.Sofat N, Robertson SD, Wait R. Fibronectin III 13–14 domains induce joint damage via Toll-like receptor 4 activation and synergize with interleukin-1 and tumour necrosis factor. J Innate Immun. 2012;4:69–79. doi: 10.1159/000329632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Forsyth CB, Pulai J, Loeser RF. Fibronectin fragments and blocking antibodies to alpha2beta1 and alpha5beta1 integrins stimulate mitogen-activated protein kinase signaling and increase collagenase 3 (matrix metalloproteinase 13) production by human articular chondrocytes. Arthr Rheum. 2002;46:2368–2376. doi: 10.1002/art.10502. [DOI] [PubMed] [Google Scholar]
- 66.Homandberg GA, Costa V, Wen C. Fibronectin fragments active in chondrocytic chondrolysis can be chemically cross-linked to the alpha5 integrin receptor subunit. Osteoarthr Cartil. 2002;10:938–949. doi: 10.1053/joca.2002.0854. [DOI] [PubMed] [Google Scholar]
- 67.Del Carlo M, Schwartz D, Erickson EA, Loeser RF. Endogenous production of reactive oxygen species is required for stimulation of human articular chondrocyte matrix metalloproteinase production by fibronectin fragments. Free Radic Biol Med. 2007;42:1350–1358. doi: 10.1016/j.freeradbiomed.2007.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huhtala P, Humphries MJ, McCarthy JB, Tremble PM, Werb Z, Damsky CH. Cooperative signaling by alpha 5 beta 1 and alpha 4 beta 1 integrins regulates metalloproteinase gene expression in fibroblasts adhering to fibronectin. J Cell Biol. 1995;129:867–879. doi: 10.1083/jcb.129.3.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Peters JH, Loredo GA, Benton HP. Is osteoarthritis a “fibronectin-integrin imbalance disorder”? Osteoarthr Cartil. 2002;10:831–835. doi: 10.1053/joca.2002.0845. [DOI] [PubMed] [Google Scholar]
- 70.Homandberg GA, Meyers R, Williams JM. Intraarticular injection of fibronectin fragments causes severe depletion of cartilage proteoglycans in vivo. J Rheumatol. 1993;20:1378–1382. [PubMed] [Google Scholar]
- 71.Yasuda T, Kakinuma T, Julovi SM, Yoshida M, Hiramitsu T, Akiyoshi M, Nakamura T. COOH-terminal heparin-binding fibronectin fragment induces nitric oxide production in rheumatoid cartilage through CD44. Rheumatology (Oxford) 2004;43:1116–1120. doi: 10.1093/rheumatology/keh274. [DOI] [PubMed] [Google Scholar]
- 72.Williams JM, Zhang J, Kang H, Ummadi V, Homandberg GA. The effects of hyaluronic acid on fibronectin fragment mediated cartilage chondrolysis in skeletally mature rabbits. Osteoarthr Cartil. 2003;11:44–49. doi: 10.1053/joca.2002.0864. [DOI] [PubMed] [Google Scholar]
- 73.Homandberg GA, Hui F, Wen C. Fibronectin fragment mediated cartilage chondrolysis. I. Suppression by anti-oxidants. Biochim Biophys Acta. 1996;1317:134–142. doi: 10.1016/s0925-4439(96)00046-4. [DOI] [PubMed] [Google Scholar]
- 74.Homandberg GA, Hui F, Wen C. Fibronectin fragment mediated cartilage chondrolysis. II. Reparative effects of anti-oxidants. Biochim Biophys Acta. 1996;1317:143–148. doi: 10.1016/s0925-4439(96)00045-2. [DOI] [PubMed] [Google Scholar]
- 75.Homandberg GA, Guo D, Ray LM, Ding L. Mixtures of glucosamine and chondroitin sulfate reverse fibronectin fragment mediated damage to cartilage more effectively than either agent alone. Osteoarthr Cartil. 2006;14:793–806. doi: 10.1016/j.joca.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 76.Kang Y, Eger W, Koepp H, Williams JM, Kuettner KE, Homandberg GA. Hyaluronan suppresses fibronectin fragment-mediated damage to human cartilage explant cultures by enhancing proteoglycan synthesis. J Orthop Res. 1999;17:858–869. doi: 10.1002/jor.1100170611. [DOI] [PubMed] [Google Scholar]
- 77.Yasuda T. Comparison of hyaluronan effects among normal, osteoarthritis, and rheumatoid arthritis cartilages stimulated with fibronectin fragment. Biomed Res. 2010;31:63–69. doi: 10.2220/biomedres.31.63. [DOI] [PubMed] [Google Scholar]
- 78.Ascherio A, Munger KL, Lunemann JD. The initiation and prevention of multiple sclerosis. Nat Rev Neurol. 2012;8:602–612. doi: 10.1038/nrneurol.2012.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wolswijk G. Chronic stage multiple sclerosis lesions contain a relatively quiescent population of oligodendrocyte precursor cells. J Neurosci. 1998;18:601–609. doi: 10.1523/JNEUROSCI.18-02-00601.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wolswijk G. Oligodendrocyte precursor cells in the demyelinated multiple sclerosis spinal cord. Brain. 2002;125:338–349. doi: 10.1093/brain/awf031. [DOI] [PubMed] [Google Scholar]
- 81.Trapp BD, Nave KA. Multiple sclerosis: an immune or neurodegenerative disorder? Annu Rev Neurosci. 2008;31:247–269. doi: 10.1146/annurev.neuro.30.051606.094313. [DOI] [PubMed] [Google Scholar]
- 82.Franklin RJ, ffrench-Constant C. Remyelination in the CNS: from biology to therapy. Nat Rev Neurosci. 2008;9:839–855. doi: 10.1038/nrn2480. [DOI] [PubMed] [Google Scholar]
- 83.Franklin RJ, ffrench-Constant C, Edgar JM, Smith KJ. Neuroprotection and repair in multiple sclerosis. Nat Rev Neurol. 2012;8:624–634. doi: 10.1038/nrneurol.2012.200. [DOI] [PubMed] [Google Scholar]
- 84.Ballabh P, Braun A, Nedergaard M. The blood–brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis. 2004;16:1–13. doi: 10.1016/j.nbd.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 85.Man S, Tucky B, Bagheri N, Li X, Kochar R, Ransohoff RM. Alpha4 Integrin/FN-CS1 mediated leukocyte adhesion to brain microvascular endothelial cells under flow conditions. J Neuroimmunol. 2009;210:92–99. doi: 10.1016/j.jneuroim.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rudick R, Polman C, Clifford D, Miller D, Steinman L. Natalizumab: bench to bedside and beyond. JAMA Neurol. 2013;70:172–182. doi: 10.1001/jamaneurol.2013.598. [DOI] [PubMed] [Google Scholar]
- 87.van der Laan LJ, De Groot CJ, Elices MJ, Dijkstra CD. Extracellular matrix proteins expressed by human adult astrocytes in vivo and in vitro: an astrocyte surface protein containing the CS1 domain contributes to binding of lymphoblasts. J Neurosci Res. 1997;50:539–548. doi: 10.1002/(SICI)1097-4547(19971115)50:4<539::AID-JNR5>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 88.van der Laan LJ, van der Goes A, Wauben MH, Ruuls SR, Dopp EA, De Groot CJ, Kuijpers TW, Elices MJ, Dijkstra CD. Beneficial effect of modified peptide inhibitor of alpha4 integrins on experimental allergic encephalomyelitis in Lewis rats. J Neurosci Res. 2002;67:191–199. doi: 10.1002/jnr.10095. [DOI] [PubMed] [Google Scholar]
- 89.Stuve O, Dooley NP, Uhm JH, Antel JP, Francis GS, Williams G, Yong VW. Interferon beta-1b decreases the migration of T lymphocytes in vitro: effects on matrix metalloproteinase-9. Ann Neurol. 1996;40:853–863. doi: 10.1002/ana.410400607. [DOI] [PubMed] [Google Scholar]
- 90.Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, MacDonald DD, Jin DK, Shido K, Kerns SA, Zhu Z, Hicklin D, Wu Y, Port JL, Altorki N, Port ER, Ruggero D, Shmelkov SV, Jensen KK, Rafii S, Lyden D. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820–827. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Malik G, Knowles LM, Dhir R, Xu S, Yang S, Ruoslahti E, Pilch J. Plasma fibronectin promotes lung metastasis by contributions to fibrin clots and tumor cell invasion. Cancer Res. 2010;70:4327–4334. doi: 10.1158/0008-5472.CAN-09-3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Reticker-Flynn NE, Malta DF, Winslow MM, Lamar JM, Xu MJ, Underhill GH, Hynes RO, Jacks TE, Bhatia SN. A combinatorial extracellular matrix platform identifies cell-extracellular matrix interactions that correlate with metastasis. Nat Commun. 2012;3:1122. doi: 10.1038/ncomms2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Milner R, Crocker SJ, Hung S, Wang X, Frausto RF, del Zoppo GJ. Fibronectin- and vitronectin-induced microglial activation and matrix metalloproteinase-9 expression is mediated by integrins alpha5beta1 and alphavbeta5. J Immunol. 2007;178:8158–8167. doi: 10.4049/jimmunol.178.12.8158. [DOI] [PubMed] [Google Scholar]
- 94.Bsibsi M, Ravid R, Gveric D, van Noort JM. Broad expression of Toll-like receptors in the human central nervous system. J Neuropathol Exp Neurol. 2002;61:1013–1021. doi: 10.1093/jnen/61.11.1013. [DOI] [PubMed] [Google Scholar]
- 95.Lehnardt S, Lachance C, Patrizi S, Lefebvre S, Follett PL, Jensen FE, Rosenberg PA, Volpe JJ, Vartanian T. The toll-like receptor TLR4 is necessary for lipopolysaccharide-induced oligodendrocyte injury in the CNS. J Neurosci. 2002;22:2478–2486. doi: 10.1523/JNEUROSCI.22-07-02478.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Summers L, Kielty C, Pinteaux E. Adhesion to fibronectin regulates interleukin-1 beta expression in microglial cells. Mol Cell Neurosci. 2009;41:148–155. doi: 10.1016/j.mcn.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 97.Ribes S, Ebert S, Regen T, Czesnik D, Scheffel J, Zeug A, Bunkowski S, Eiffert H, Hanisch UK, Hammerschmidt S, Nau R. Fibronectin stimulates Escherichia coli phagocytosis by microglial cells. Glia. 2010;58:367–376. doi: 10.1002/glia.20929. [DOI] [PubMed] [Google Scholar]
- 98.Kotter MR, Li WW, Zhao C, Franklin RJ. Myelin impairs CNS remyelination by inhibiting oligodendrocyte precursor cell differentiation. J Neurosci. 2006;26:328–332. doi: 10.1523/JNEUROSCI.2615-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li WW, Setzu A, Zhao C, Franklin RJ. Minocycline-mediated inhibition of microglia activation impairs oligodendrocyte progenitor cell responses and remyelination in a non-immune model of demyelination. J Neuroimmunol. 2005;158:58–66. doi: 10.1016/j.jneuroim.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 100.Setzu A, Lathia JD, Zhao C, Wells K, Rao MS, ffrench-Constant C, Franklin RJ. Inflammation stimulates myelination by transplanted oligodendrocyte precursor cells. Glia. 2006;54:297–303. doi: 10.1002/glia.20371. [DOI] [PubMed] [Google Scholar]
- 101.Tate CC, Garcia AJ, LaPlaca MC. Plasma fibronectin is neuroprotective following traumatic brain injury. Exp Neurol. 2007;207:13–22. doi: 10.1016/j.expneurol.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 102.Lehnardt S, Massillon L, Follett P, Jensen FE, Ratan R, Rosenberg PA, Volpe JJ, Vartanian T. Activation of innate immunity in the CNS triggers neurodegeneration through a Toll-like receptor 4-dependent pathway. Proc Natl Acad Sci USA. 2003;100:8514–8519. doi: 10.1073/pnas.1432609100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Milner R, ffrench-Constant C. A developmental analysis of oligodendroglial integrins in primary cells: changes in alpha v-associated beta subunits during differentiation. Development. 1994;120:3497–3506. doi: 10.1242/dev.120.12.3497. [DOI] [PubMed] [Google Scholar]
- 104.Blaschuk KL, Frost EE, ffrench-Constant C. The regulation of proliferation and differentiation in oligodendrocyte progenitor cells by alphaV integrins. Development. 2000;127:1961–1969. doi: 10.1242/dev.127.9.1961. [DOI] [PubMed] [Google Scholar]
- 105.Zhao C, Fancy SP, Franklin RJ, ffrench-Constant C. Up-regulation of oligodendrocyte precursor cell alphaV integrin and its extracellular ligands during central nervous system remyelination. J Neurosci Res. 2009;87:3447–3455. doi: 10.1002/jnr.22231. [DOI] [PubMed] [Google Scholar]
- 106.Frost E, Kiernan BW, Faissner A, ffrench-Constant C. Regulation of oligodendrocyte precursor migration by extracellular matrix: evidence for substrate-specific inhibition of migration by tenascin-C. Dev Neurosci. 1996;18:266–273. doi: 10.1159/000111416. [DOI] [PubMed] [Google Scholar]
- 107.Milner R, Edwards G, Streuli C, ffrench-Constant C. A role in migration for the alpha V beta 1 integrin expressed on oligodendrocyte precursors. J Neurosci. 1996;16:7240–7252. doi: 10.1523/JNEUROSCI.16-22-07240.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Baron W, Shattil SJ, ffrench-Constant C. The oligodendrocyte precursor mitogen PDGF stimulates proliferation by activation of alpha(v)beta3 integrins. EMBO J. 2002;21:1957–1966. doi: 10.1093/emboj/21.8.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Buttery PC, ffrench-Constant C. Laminin-2/integrin interactions enhance myelin membrane formation by oligodendrocytes. Mol Cell Neurosci. 1999;14:199–212. doi: 10.1006/mcne.1999.0781. [DOI] [PubMed] [Google Scholar]
- 110.Siskova Z, Baron W, de Vries H, Hoekstra D. Fibronectin impedes “myelin” sheet-directed flow in oligodendrocytes: a role for a beta 1 integrin-mediated PKC signaling pathway in vesicular trafficking. Mol Cell Neurosci. 2006;33:150–159. doi: 10.1016/j.mcn.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 111.Siskova Z, Yong VW, Nomden A, van Strien M, Hoekstra D, Baron W. Fibronectin attenuates process outgrowth in oligodendrocytes by mislocalizing MMP-9 activity. Mol Cell Neurosci. 2009;42:234–242. doi: 10.1016/j.mcn.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 112.Zhao C, Fancy SP, Franklin RJ, ffrench-Constant C. Up-regulation of oligodendrocyte precursor cell alphaV integrin and its extracellular ligands during central nervous system remyelination. J Neurosci Res. 2009;87:3447–3455. doi: 10.1002/jnr.22231. [DOI] [PubMed] [Google Scholar]
- 113.Baron W, Decker L, Colognato H, ffrench-Constant C. Regulation of integrin growth factor interactions in oligodendrocytes by lipid raft microdomains. Curr Biol. 2003;13:151–155. doi: 10.1016/s0960-9822(02)01437-9. [DOI] [PubMed] [Google Scholar]
- 114.Maier O, van der Heide T, van Dam AM, Baron W, de Vries H, Hoekstra D. Alteration of the extracellular matrix interferes with raft association of neurofascin in oligodendrocytes. Potential significance for multiple sclerosis? Mol Cell Neurosci. 2005;28:390–401. doi: 10.1016/j.mcn.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 115.Singh P, Carraher C, Schwarzbauer JE. Assembly of fibronectin extracellular matrix. Annu Rev Cell Dev Biol. 2010;26:397–419. doi: 10.1146/annurev-cellbio-100109-104020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.To WS, Midwood KS. Plasma and cellular fibronectin: distinct and independent functions during tissue repair. Fibrogenesis Tissue Repair. 2011;4:21. doi: 10.1186/1755-1536-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chen H, Mosher DF. Formation of sodium dodecyl sulfate-stable fibronectin multimers. Failure to detect products of thiol-disulfide exchange in cyanogen bromide or limited acid digests of stabilized matrix fibronectin. J Biol Chem. 1996;271:9084–9089. doi: 10.1074/jbc.271.15.9084. [DOI] [PubMed] [Google Scholar]
- 118.Ohashi T, Erickson HP. Revisiting the mystery of fibronectin multimers: the fibronectin matrix is composed of fibronectin dimers cross-linked by non-covalent bonds. Matrix Biol. 2009;28:170–175. doi: 10.1016/j.matbio.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.McKeown-Longo PJ, Mosher DF. Binding of plasma fibronectin to cell layers of human skin fibroblasts. J Cell Biol. 1983;97:466–472. doi: 10.1083/jcb.97.2.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Midwood KS, Williams LV, Schwarzbauer JE. Tissue repair and the dynamics of the extracellular matrix. Int J Biochem Cell Biol. 2004;36:1031–1037. doi: 10.1016/j.biocel.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 121.Sottile J, Hocking DC. Fibronectin polymerization regulates the composition and stability of extracellular matrix fibrils and cell-matrix adhesions. Mol Biol Cell. 2002;13:3546–3559. doi: 10.1091/mbc.E02-01-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.To WS, Midwood KS. Identification of novel and distinct binding sites within tenascin-C for soluble and fibrillar fibronectin. J Biol Chem. 2011;286:14881–14891. doi: 10.1074/jbc.M110.189019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ohashi T, Erickson HP. Fibronectin aggregation and assembly: the unfolding of the second fibronectin type III domain. J Biol Chem. 2011;286:39188–39199. doi: 10.1074/jbc.M111.262337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Geiger B, Bershadsky A, Pankov R, Yamada KM. Transmembrane crosstalk between the extracellular matrix–cytoskeleton crosstalk. Nat Rev Mol Cell Biol. 2001;2:793–805. doi: 10.1038/35099066. [DOI] [PubMed] [Google Scholar]
- 125.Nelea V, Nakano Y, Kaartinen MT. Size distribution and molecular associations of plasma fibronectin and fibronectin crosslinked by transglutaminase 2. Protein J. 2008;27:223–233. doi: 10.1007/s10930-008-9128-1. [DOI] [PubMed] [Google Scholar]
- 126.van Strien ME, Breve JJ, Fratantoni S, Schreurs MW, Bol JG, Jongenelen CA, Drukarch B, van Dam AM. Astrocyte-derived tissue transglutaminase interacts with fibronectin: a role in astrocyte adhesion and migration? PLoS ONE. 2011;6:e25037. doi: 10.1371/journal.pone.0025037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Dobaczewski M, Gonzalez-Quesada C, Frangogiannis NG. The extracellular matrix as a modulator of the inflammatory and reparative response following myocardial infarction. J Mol Cell Cardiol. 2010;48:504–511. doi: 10.1016/j.yjmcc.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Arslan F, Smeets MB, Riem Vis PW, Karper JC, Quax PH, Bongartz LG, Peters JH, Hoefer IE, Doevendans PA, Pasterkamp G, de Kleijn DP. Lack of fibronectin-EDA promotes survival and prevents adverse remodeling and heart function deterioration after myocardial infarction. Circ Res. 2011;108:582–592. doi: 10.1161/CIRCRESAHA.110.224428. [DOI] [PubMed] [Google Scholar]
- 129.Oegema TR, Jr, Johnson SL, Aguiar DJ, Ogilvie JW. Fibronectin and its fragments increase with degeneration in the human intervertebral disc. Spine (Phila Pa 1976) 2000;25:2742–2747. doi: 10.1097/00007632-200011010-00005. [DOI] [PubMed] [Google Scholar]
- 130.Greg Anderson D, Li X, Tannoury T, Beck G, Balian G. A fibronectin fragment stimulates intervertebral disc degeneration in vivo. Spine (Phila Pa 1976) 2003;28:2338–2345. doi: 10.1097/01.BRS.0000096943.27853.BC. [DOI] [PubMed] [Google Scholar]
- 131.Xia M, Zhu Y. Fibronectin fragment activation of ERK increasing integrin alpha and beta subunit expression to degenerate nucleus pulposus cells. J Orthop Res. 2011;29:556–561. doi: 10.1002/jor.21273. [DOI] [PubMed] [Google Scholar]
- 132.Lu P, Takai K, Weaver VM and Werb Z (2011) Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb Perspect Biol 3. doi: 10.1101/cshperspect.a005058 [DOI] [PMC free article] [PubMed]
- 133.Chowdhury TT, Schulz RM, Rai SS, Thuemmler CB, Wuestneck N, Bader A, Homandberg GA. Biomechanical modulation of collagen fragment-induced anabolic and catabolic activities in chondrocyte/agarose constructs. Arthr Res Ther. 2010;12:R82. doi: 10.1186/ar3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Goh FG, Piccinini AM, Krausgruber T, Udalova IA, Midwood KS. Transcriptional regulation of the endogenous danger signal tenascin-C: a novel autocrine loop in inflammation. J Immunol. 2010;184:2655–2662. doi: 10.4049/jimmunol.0903359. [DOI] [PubMed] [Google Scholar]
- 135.Back SA, Tuohy TM, Chen H, Wallingford N, Craig A, Struve J, Luo NL, Banine F, Liu Y, Chang A, Trapp BD, Bebo BF, Jr, Rao MS, Sherman LS. Hyaluronan accumulates in demyelinated lesions and inhibits oligodendrocyte progenitor maturation. Nat Med. 2005;11:966–972. doi: 10.1038/nm1279. [DOI] [PubMed] [Google Scholar]
- 136.Livant DL, Brabec RK, Kurachi K, Allen DL, Wu Y, Haaseth R, Andrews P, Ethier SP, Markwart S. The PHSRN sequence induces extracellular matrix invasion and accelerates wound healing in obese diabetic mice. J Clin Invest. 2000;105:1537–1545. doi: 10.1172/JCI8527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Miyamoto T, Tamura M, Kabashima N, Serino R, Shibata T, Furuno Y, Miyazaki M, Baba R, Sato N, Doi Y, Okazaki M, Otsuji Y. An integrin-activating peptide, PHSRN, ameliorates inhibitory effects of conventional peritoneal dialysis fluids on peritoneal wound healing. Nephrol Dial Transplant. 2010;25:1109–1119. doi: 10.1093/ndt/gfp601. [DOI] [PubMed] [Google Scholar]
- 138.Feng Y, Mrksich M. The synergy peptide PHSRN and the adhesion peptide RGD mediate cell adhesion through a common mechanism. Biochemistry. 2004;43:15811–15821. doi: 10.1021/bi049174+. [DOI] [PubMed] [Google Scholar]
- 139.Zeng ZZ, Yao H, Staszewski ED, Rockwood KF, Markwart SM, Fay KS, Spalding AC, Livant DL. Alpha(5)beta(1) integrin ligand PHSRN induces invasion and alpha(5) mRNA in endothelial cells to stimulate angiogenesis. Transl Oncol. 2009;2:8–20. doi: 10.1593/tlo.08187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lariviere B, Rouleau M, Picard S, Beaulieu AD. Human plasma fibronectin potentiates the mitogenic activity of platelet-derived growth factor and complements its wound healing effects. Wound Repair Regen. 2003;11:79–89. doi: 10.1046/j.1524-475x.2003.11112.x. [DOI] [PubMed] [Google Scholar]
- 141.Qiu Z, Kwon AH, Kamiyama Y. Effects of plasma fibronectin on the healing of full-thickness skin wounds in streptozotocin-induced diabetic rats. J Surg Res. 2007;138:64–70. doi: 10.1016/j.jss.2006.06.034. [DOI] [PubMed] [Google Scholar]
- 142.Kwon AH, Qiu Z, Hiraon Y. Effect of plasma fibronectin on the incisional wound healing in rats. Surgery. 2007;141:254–261. doi: 10.1016/j.surg.2006.06.036. [DOI] [PubMed] [Google Scholar]
- 143.McCulley JP, Horowitz B, Husseini ZM, Horowitz M. Topical fibronectin therapy of persistent corneal epithelial defects. Fibronectin Study Group. Trans Am Ophthalmol Soc. 1993;91:367–386. [PMC free article] [PubMed] [Google Scholar]
- 144.Boateng JS, Matthews KH, Stevens HN, Eccleston GM. Wound healing dressings and drug delivery systems: a review. J Pharm Sci. 2008;97:2892–2923. doi: 10.1002/jps.21210. [DOI] [PubMed] [Google Scholar]
- 145.Ghosh K, Ren XD, Shu XZ, Prestwich GD, Clark RA. Fibronectin functional domains coupled to hyaluronan stimulate adult human dermal fibroblast responses critical for wound healing. Tissue Eng. 2006;12:601–613. doi: 10.1089/ten.2006.12.601. [DOI] [PubMed] [Google Scholar]
- 146.Okiyama N, Kitajima T, Ito Y, Yokozeki H, Miyasaka N, Kohsaka H. Addition of the collagen binding domain of fibronectin potentiates the biochemical availability of hepatocyte growth factor for cutaneous wound healing. J Dermatol Sci. 2011;61:215–217. doi: 10.1016/j.jdermsci.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 147.Hamed S, Ullmann Y, Egozi D, Daod E, Hellou E, Ashkar M, Gilhar A, Teot L. Fibronectin potentiates topical erythropoietin-induced wound repair in diabetic mice. J Invest Dermatol. 2011;131:1365–1374. doi: 10.1038/jid.2011.15. [DOI] [PubMed] [Google Scholar]
- 148.Martino MM, Tortelli F, Mochizuki M, Traub S, Ben-David D, Kuhn GA, Muller R, Livne E, Eming SA, Hubbell JA. Engineering the growth factor microenvironment with fibronectin domains to promote wound and bone tissue healing. Sci Transl Med. 2011;3:100ra89. doi: 10.1126/scitranslmed.3002614. [DOI] [PubMed] [Google Scholar]
- 149.Schwarzbauer JE and DeSimone DW (2011) Fibronectins, their fibrillogenesis, and in vivo functions. Cold Spring Harb Perspect Biol 3. doi: 10.1101/cshperspect.a005041 [DOI] [PMC free article] [PubMed]