Abstract
Mammalian myeloid cells are crucial effectors of host innate immune defense. Normal and pathological responses of these cells require adaptation to signaling stress through the hypoxia-inducible factor 1 (HIF-1) transcription complex. Adapted cells activate the mammalian target of rapamycin (mTOR), via S2448 phosphorylation, which induces de novo translation of vital signaling proteins. However, the molecular mechanisms underlying this signaling dogma remain unclear. Here, we demonstrate for the first time that inactivation of HIF-1, by silencing its inducible alpha subunit, significantly decreases mTOR S2448 phosphorylation caused by ligand-dependent activation of human myeloid leukemia cells. This shows that HIF-1 is essential for the activation of mTOR and serves at a crucial juncture of myeloid cell function in both in vitro and in vivo systems.
Electronic supplementary material
The online version of this article (doi:10.1007/s00018-013-1421-2) contains supplementary material, which is available to authorized users.
Keywords: Myeloid cells, Inflammation, HIF-1 transcription complex, mTOR (mammalian target of rapamycin)
Introduction
Mammalian myeloid cells are a hematopoietic lineage of key effectors of both host innate immune defense and hypersensitivity reactions [1]. Growth and differentiation of premature myeloid cells is controlled by stem cell factor (SCF), a hematopoietic and melanogenic cytokine, acting through the Kit receptor, also known as CD117 [1, 2]. This receptor is expressed in different types of non-differentiated hematopoietic cells including myeloblasts and pro-monoblasts. Importantly, human myeloid leukemia cells express the Kit receptor and their proliferation is crucially controlled by SCF [1–3]. Myeloid cells also express Toll-like receptors (TLRs), key regulators of immune responses to microorganisms and non-cellular pathogens (viruses). TLRs can be associated with either plasma membranes of myeloid cells or located inside the cells, signaling using acidic endosome [4–6]. Both SCF/Kit-dependent [7] and ligand-induced TLR-mediated responses activate the mammalian target of rapamycin (mTOR) [8]. The mTOR protein is a highly conserved serine/threonine kinase, which is a central regulator of cell growth, metabolism, and aging. Ligand-activated TLRs or Kit stimulate mTOR via phosphatidylinositol 3-kinase (PI3K) in an Akt-dependent manner. Akt inactivates the TSC1/TSC2 complex, which is a recognized physiological inhibitor of mTOR, blocking its phosphorylation in the S2448 position [9]. S2448-phosphorylated mTOR leads to the activation of S6K1 and eukaryotic initiation factor 4E (eIF4E), thus inducing translation of crucial signaling proteins including hypoxia-inducible factor-1 alpha (HIF-1α), an inducible subunit of the HIF-1 transcription complex [9]. HIF-1 directly influences the expression of over 40 target genes that control glycolysis, angiogenesis, and cell adhesion [10]. During TLR- and SCF/Kit-dependent signaling, cells become more dependent on glycolysis [7, 11]. Further decreases in ATP are likely to lead to increased accumulation of AMP due to the activation of adenylate kinase (ADK), which generates one ATP and one AMP molecule from two ADP molecules [12]. AMP acts as an allosteric modulator of AMP-dependent kinase (AMPK), which phosphorylates mTOR in the T2446 position [13, 14]. This prevents S2448 phosphorylation and therefore terminates mTOR activation. However, to date, there is no evidence regarding direct or indirect involvement of HIF-1 in the control of mTOR signaling activity. Understanding this signaling and biochemical link would substantially widen our conceptual knowledge concerning the physiological functions of HIF-1. Moreover, it could considerably change the existing strategies for targeting mTOR activation when treating malignant and inflammatory disorders, the majority of which are serious medical burdens in both industrialized and developing nations.
To elucidate this novel pathway, we aimed to investigate a direct link between HIF-1 and regulation of mTOR signaling activity. Here we show for the very first time that silencing HIF-1α in human myeloid leukemia cells leads to a significant decrease in mTOR S2448 phosphorylation induced by both endosomal TLR7/8 and plasma membrane-associated TLR2 ligands as well as for SCF/Kit-induced processes. Pharmacological inhibition of either ADK or AMPK attenuated the down-regulatory effects of HIF-1α silencing on S2448 phosphorylation of mTOR during both TLR and SCF/Kit-dependent signaling. Importantly, these results were confirmed using in vivo studies with mice where the HIF-1 transcriptional inhibitor chetomin attenuated peptidoglycan (TLR2 ligand)-induced mTOR S2448 phosphorylation and increased ADK activity in blood cells.
Materials and methods
Materials
RPMI-1640 medium, fetal calf serum and supplements, DOTAP transfection reagent, and an ATP luminometric detection kit were purchased from Sigma (Suffolk, UK). Maxisorp microtiter plates were obtained from Nunc (Roskilde, Denmark). Mouse monoclonal antibodies to HIF-1α, mTOR and β-actin as well as rabbit polyclonal antibodies against phospho-S2448 mTOR and phospho-T2446 mTOR were from Abcam (Cambridge, UK). Goat anti-mouse and goat anti-rabbit fluorescence dye-labeled antibodies were obtained from Li-Cor (Lincoln, NE, USA). ELISA-based assay kits for the detection of VEGF were purchased from R&D Systems (Abingdon, UK). Human recombinant SCF was obtained from Invitrogen (Paisley, UK). All other chemicals were of the highest grade of purity and commercially available.
THP-1 human myeloid cells
THP-1 human leukemia monocytic macrophages were purchased from the European Collection of Cell Cultures (ECACC) (Salisbury, UK). Cells were grown in RPMI-1640 media supplemented with 10 % fetal calf serum, penicillin (50 IU/ml) and streptomycin sulphate (50 μg/ml).
Transfer of HIF-1α siRNA into THP-1 cells
We employed a HIF-1α-specific siRNA target sequence (ugu gag uuc gca ucu uga u dtdt) localized at position 146 bases downstream of the HIF-1α start codon [15]. As a control, we used corresponding random siRNA (uac acc guu agc aga cac c dtdt). In addition, we also used an alternative HIF-1α-specific siRNA (ccu acu gca ggg uga aga a dtdt), targeting position 2,433 bases downstream of the start codon for human HIF-1α complementary DNA (cDNA) [16]. Both are recognized HIF-1α-specific siRNA sequences that demonstrated high specificity to HIF-1α mRNA [15, 16]. Transfection into THP-1 cells was performed using DOTAP reagent according to the manufacturer’s protocol. Random siRNA did not impact the investigated processes (see supplementary Figs. 1, 2), confirming that the effects observed were caused by knocking down specific HIF-1α when respective specific siRNAs were used [16]. The efficiency of HIF-1α knockdown was 45 ± 8 %.
Mice
Six-week-old CD1 male mice (25 ± 2.5 g) were used for the experiments following approval by the Institutional Animal Committee and handled in accordance with the Declaration of Helsinki protocols. Blood serum and cellular fractions (peripheral blood mononuclear cells) were isolated as described before. Each group of animals received two intraperitoneal injections: (1) Two independent injections of 0.9 % NaCl (control); (2) One injection of 1 mg/kg PGN and another of 0.9 % NaCl; (3) One injection of 1 mg/kg chetomin and another of 0.9 % NaCl; (4) One injection of 1 mg/kg PGN and another of 1 mg/kg chetomin. After 4 h, the animals were euthanized and blood was collected.
Western-blot analysis
HIF-1α, mTOR, phospho-T2446, and phospho-S2448 mTOR expressions were determined by Western-blot analysis and compared to β-actin to assess equal loading, as we previously described [7]. Li-Cor (Lincoln, NE, USA) goat secondary antibodies, conjugated with fluorescent dyes, were employed according to the manufacturer’s protocol in order to visualize the proteins of interest using a Li-Cor Odyssey imaging system. Western-blot data were subjected to quantitative analysis using Odyssey software and values were normalized against respective β-actin bands.
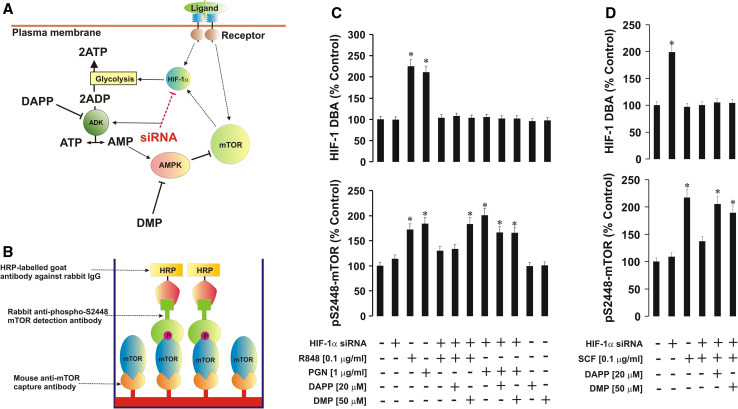
Determination of HIF-1 DNA-binding activity
HIF-1 DNA-binding activity was measured by the method described recently [7]. Briefly, a 96-well Maxisorp microtiter plate was coated with streptavidin and blocked with BSA and 2 pmol/well biotinylated 2HRE-containing oligonucleotide was immobilized by 1-h incubation at room temperature. The plate was then washed five times with TBST buffer (10 mM Tris–HCl, pH 8.0, 150 mM NaCl, 0.05 % Tween-20) followed by 1-h incubation with 20 μl/well of cell lysate at room temperature. The plate was then washed five times with TBST buffer followed by the addition of mouse anti-HIF-1α antibody (1:1,000 in TBS plus 2 % BSA). After 1-h incubation at room temperature, the plate was washed five times with TBST buffer and incubated with 1:1,000 HRP-labeled rabbit anti-mouse IgG in TBST buffer and, after extensive washing with TBST, bound secondary antibodies were detected by the peroxidase reaction (ortho-phenylenediamine/H2O2). Reactions were quenched after 10 min with an equal volume of 1 M H2SO4 and color development was measured in a microplate reader (absorbance at 492 nm).
Detection of phospho-S2448 and phospho-T2446 mTOR in cell lysates by ELISA
We also used an ELISA assay to analyze S2448 or T2446 phosphorylation of mTOR. Plates were coated with mouse anti-mTOR antibody (a surface-friendly antibody recognizing the immunogen situated between amino acid residues 1–350 located far from the studied phosphorylation sites and able to detect human, mouse, and rat mTOR in its native conformation) and blocked with 1 % BSA. Cell lysates were then added to the wells and kept at room temperature for at least 2 h (constant agitation applied). After extensive washing with TBST buffer, anti-phospho-S2448 (or anti-phospho-T2446) mTOR antibody was added and incubated for 2 h at room temperature (with constant agitation; both antibodies are recognized as suitable for ELISA and react with human, mouse and rat mTOR). The plate was then washed five times with TBST buffer and incubated with 1:1,000 HRP-labeled goat anti-rabbit IgG in TBST buffer and, after extensive washing with TBST, bound secondary antibodies were detected by the peroxidase reaction (ortho-phenylenediamine/H2O2). Reactions were quenched after 10 min with an equal volume of 1 M H2SO4 and color development was measured in a microplate reader (absorbance at 492 nm; for illustration see Fig. 4b).
Fig. 4.
The HIF-1 transcription complex is crucially involved in the control of ADK/AMPK-dependent inactivation of the mTOR signaling pathway. DAPP (ADK inhibitor) and DMP (AMPK inhibitor) were used to block the respective enzymes during TLR and SCF/Kit-dependent responses of normal and HIF-1α knockdown THP-1 cells (a). ELISA assays were used for quantitative analysis of phospho-S2448 mTOR (b). Normal and HIF-1α knockdown THP-1 cells were exposed to the indicated concentrations of R848, PGN (c) or SCF (d) for 4 h with or without 30-min pre-treatment with DAPP or DMP (concentrations are outlined in the figure). Phospho-S2448 mTOR was analyzed by ELISA. HIF-1α knockdown was controlled by measuring HIF-1 DBA. Data are mean values ± SD of at least three individual experiments. *p < 0.01 vs. control
Analysis of phospho-S2448 and phospho-T2446 mTOR in cell lysates by in-cell Western assay
We employed Li-Cor in-cell Western assay to analyze S2448 or T2446 phosphorylation of mTOR directly in THP-1 cells. A standard Li-Cor in-cell Western protocol was used.
Detection of ADK activity, intracellular ATP, AMP, glucose levels, xanthine oxidase activity, as well as characterization of glycolysis intensity
ADK activity was analyzed as described before [17]. ATP was detected using a luminometric kit (Sigma) according to the manufacturer’s protocol. Intracellular glucose concentration was analyzed in the cell lysates using a glucose oxidase assay as described before [18]. The intensity of glycolysis was characterized based on the ability of cell lysates, used as a multienzyme preparation, to convert glucose into lactate under anaerobic conditions, which was achieved by employing an anaerobic chamber. Briefly, cell lysates were incubated for 1 h at 37 °C with 1 % glucose solution within the anaerobic chamber. Proteins were then precipitated using 2 % trichloroacetic acid solution followed by carbohydrate precipitation using saturated CuSO4 solution in combination with Ca(OH)2 (60 mg/ml final concentration). Lactate was then converted into acetic aldehyde using concentrated H2SO4 at 95 °C. Acetaldehyde was detected using the veratrole (1,2-dimethoxybenzene) test [19]. AMP analysis was performed using a Promega assay kit according to the manufacturer’s protocol. Xanthine oxidase activity was measured as described before [20].
Measurement of HIF-1α mRNA by quantitative real-time reverse transcription PCR (qRT-PCR)
Total RNA was isolated using a GenElute mammalian total RNA miniprep kit, followed by a HIF-1α mRNA reverse transcriptase–polymerase chain reaction (RT-PCR) performed in accordance with the manufacturer’s protocol. Quantitative real-time PCR was then performed. Primer selection was as follows: HIF-1α, 5′-CTCAAAGTCGGACAGCCTCA-3′, 5′-CCCTGCAGTAGGTTTCTGCT-3′ actin, 5′-TGACGGGGTCAC-CCACACTGTGCCCATCTA-3′, 5′-CTAGAAGCATTTGCGGTCGACGA-TGGAGGG-3′ [21]. Reactions were performed using a LightCycler 480 real-time PCR system and respective SYBR Green I Master kit (Roche, Burgess Hill, UK). Analysis was performed according to the manufacturer’s protocol. Values representing HIF-1α mRNA levels were normalized against those of actin.
Measurement of VEGF production
VEGF concentrations in the samples were analyzed by ELISA (R&D Systems assay kit) according to the manufacturer’s protocol.
Statistical analysis
Each experiment was performed at least three times and statistical analysis was conducted using a two-tailed Student’s t test. Statistical probabilities (p) were expressed as *, where p < 0.01.
Results
HIF-1α protein is crucially involved in mTOR S2448 phosphorylation induced in a TLR-dependent manner
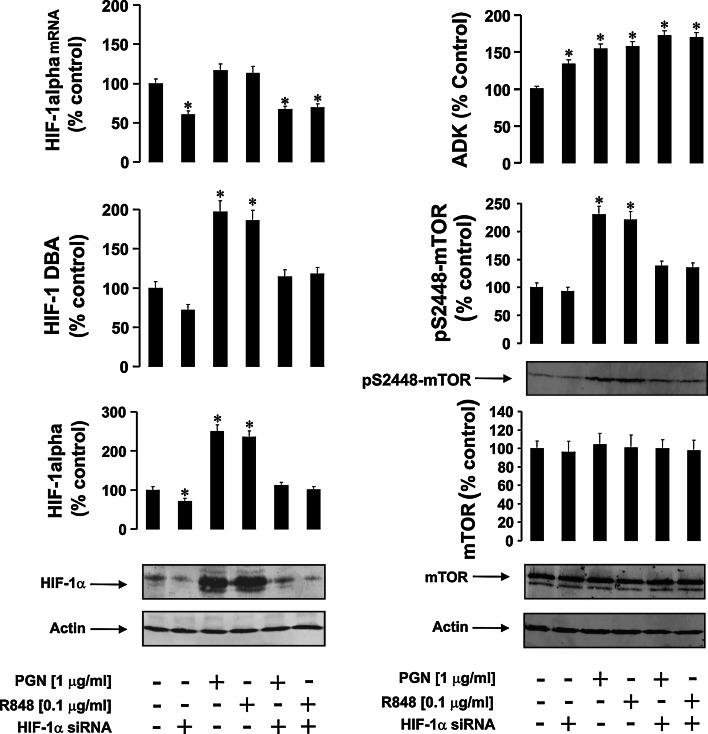
We first analyzed mTOR S2448 phosphorylation in normal and HIF-1α knockdown THP-1 human myeloid cells exposed to different TLR ligands. We treated both cell types for 4 h with 1 μg/ml peptidoglycan (PGN, ligand of monomeric TLR2) or 0.1 μg/ml R848 (a synthetic ligand of endosomal TLRs 7 and 8, which normally recognizes viral single-stranded RNA). Successful silencing of HIF-1α was controlled by Western-blot analysis, quantitative real-time PCR and measurement of HIF-1 DNA-binding activity (DBA) (Fig. 1).
Fig. 1.
The effects of PGN and R848 on HIF-1 and ADK activities as well as on mTOR S2448 phosphorylation in normal and HIF-1α knockdown THP-1 cells. Normal (non-transfected) and HIF-1α knockdown THP-1 (transfected with HIF-1α-specific siRNA) cells were exposed to the indicated concentrations of PGN and R848 for 4 h. HIF-1α protein accumulation and mRNA, HIF-1 DNA-binding activity (DBA), ADK activity, mTOR accumulation, and S2448 phosphorylation were analyzed as outlined in the "Materials and methods" section. Western-blot data show one representative experiment of six that gave similar results and were quantitatively analyzed. Other quantitative data are mean values ± SD of at least three individual experiments; *p < 0.01 vs. control
Both PGN and R848 induced HIF-1 activation (but did not significantly upregulate HIF-1 mRNA levels due to the short 4-h treatment), which confirms previous findings [21, 22]. Since TLR ligands are known to affect intracellular energy metabolism, causing time-dependent depletion of ATP resources [21–24], this is likely to upregulate ADK activity, leading to ATP as well as AMP generation. We therefore analyzed ADK activity and observed a significant upregulation (Fig. 1). ADK was also upregulated when THP-1 cells were exposed to pro-inflammatory stimuli (Fig. 1). This was likely to occur due to increased ATP consumption, which is known to take place during inflammatory responses [24]. Such an effect leads to increased intracellular ADP levels, thus allowing for enhanced ADK activity due to increased substrate (ADP) concentrations. mTOR S2448 phosphorylation was increased by both PGN and R848 but was reduced to basal levels in HIF-1α knockdown THP-1 cells (Fig. 1). Importantly, basic mTOR S2448 phosphorylation levels were very similar to each other in non-treated normal and HIF-1α knockdown THP-1 cells (Fig. 1). To confirm the effects of silencing HIF-1α expression, we also used an alternative siRNA sequence to reproduce our observations and compared this with a negative control, which included random siRNA. Similar effects were observed with both siRNA sequences and no silencing effects took place in experiments containing the negative control sequence (see "Materials and methods" and Supplementary figs. 1, 2 for further details).
Contribution of HIF-1α to SCF/Kit-induced mTOR activation
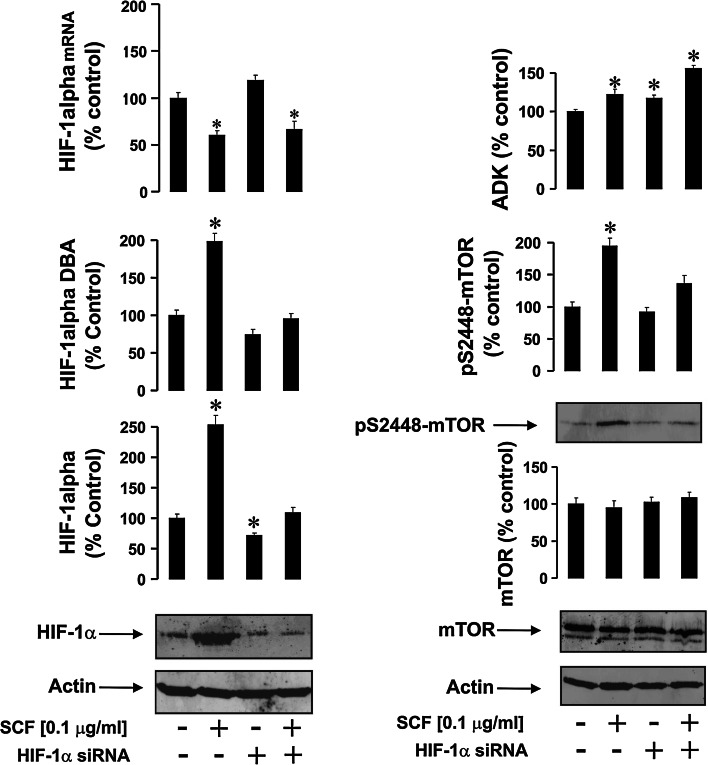
SCF/Kit signaling employs mTOR for translational control of cell differentiation and proliferation processes [7]. Exposure of THP-1 cells to 100 ng/ml SCF significantly increased HIF-1α accumulation, which was consistent with an upregulation of HIF-1 DBA (Fig. 2). The results with mRNA were also similar to those obtained with PGN/R848 (Fig. 2). Observed upregulation were abrogated in HIF-1α knockdown THP-1 cells (Fig. 2). As with TLRs, all the exposures led to a significant upregulation of ADK activity (Fig. 2). SCF treatment significantly upregulated mTOR S2448 phosphorylation, which was attenuated in HIF-1α knockdown cells (Fig. 2). These results clearly demonstrate that both TLR and SCF-mediated processes lead to the activation of the HIF-1 transcription complex, which is essentially involved in S2448 phosphorylation of mTOR kinase and induced during all types of myeloid cell responses investigated.
Fig. 2.
The effects of SCF on HIF-1 and ADK activities as well as on mTOR S2448 phosphorylation in normal and HIF-1α knockdown THP-1 cells. Normal and HIF-1α knockdown THP-1 cells were exposed to 100 ng/ml SCF for 4 h. HIF-1α accumulation, HIF-1 DBA, ADK activity, mTOR accumulation, and S2448 phosphorylation were analyzed as outlined in the "Materials and methods" section. Western-blot data show one representative experiment of six that gave similar results and were quantitatively analyzed. Other quantitative data are mean values ± SD of at least three individual experiments; *p < 0.01 vs. control
The HIF-1 transcription complex is crucially involved in the control of ADK/AMPK-dependent inactivation of mTOR
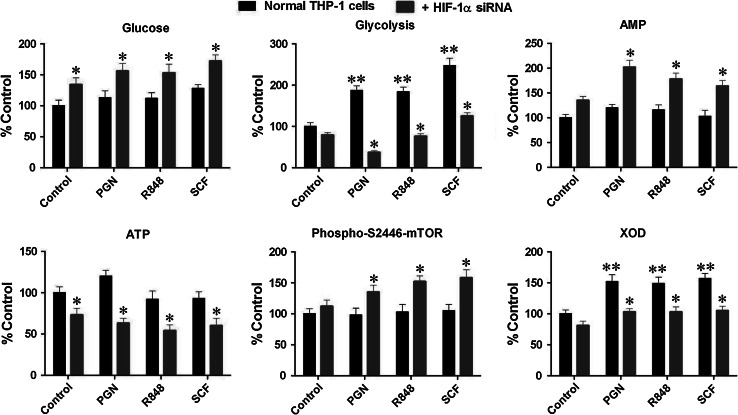
HIF-1 upregulates glycolysis, thereby allowing myeloid cells to utilize glycolytic ATP [24]. This mechanism therefore allows cells to adapt to TLR-mediated or SCF/Kit-induced signaling stress by protecting themselves against mitochondrial dysfunction, which would otherwise be followed by apoptotic death. Silencing HIF-1α attenuates upregulation of glycolysis thus leading to the accumulation of ADP, which is formed during utilization of ATP as a source of energy [12]. This was also confirmed in our experiments. Our results clearly demonstrated that silencing HIF-1α increases intracellular glucose concentrations in both non-stimulated and PGN/R848/SCF-treated cells (Fig. 3). Treatment of normal THP-1 cells with PGN/R848/SCF increased glycolysis but this effect was significantly downregulated in HIF-1α knockdown cells (Fig. 3).
Fig. 3.
Silencing HIF-1α affects intracellular glucose concentrations, decreases glucose degradation and intracellular ATP levels, as well as upregulates T2446 phosphorylation of mTOR. Normal and HIF-1α knockdown THP-1 cells were exposed to 1 μg/ml PGN, 0.1 μg/ml R848 and 0.1 μg/ml SCF for 4 h. Intracellular glucose concentrations, intensity of glycolysis, intracellular ATP levels, and phospho-T2446 mTOR were detected as outlined in the "Materials and methods" section. Quantitative data are mean values ± SD of at least three individual experiments. *p < 0.01 vs. THP-1 cells treated with respective stimuli in the absence of siRNA, **p < 0.01 vs. control
Consistent with these observations, we also found that intracellular ATP levels in HIF-1α knockdown THP-1 cells were significantly decreased compared to both non-stimulated and PGN/R848/SCF-treated normal THP-1 cells. AMP levels were upregulated respectively (Fig. 3). This increases the availability of ADK substrate and therefore causes increased intracellular AMP levels. AMP is a recognized AMPK allosteric modulator. We found that AMPK-dependent phosphorylation of T2446 of mTOR was significantly increased in HIF-1α knockdown THP-1 cells, suggesting that silencing HIF-1α causes an increase in AMPK activity (Fig. 3). The observations with mTOR phosphorylation were confirmed using in-cell Western analysis and were in line with the results obtained using Western-blot and ELISA assays (Supplementary figure 3). Interestingly, activity of xanthine oxidase (XOD), an enzyme responsible for the degradation of purine nucleotides, including AMP, was increased by all stimuli, which is in agreement with previous observations [20]. Expression of this enzyme is, in most cases, HIF-1-dependent [20] and it was therefore not surprising that XOD activity was reduced in HIF-1α knockdown THP-1 cells (Fig. 3).
The observed effects are schematically illustrated in Fig. 4a.
Given the above observations, we next asked whether specific pharmacological inhibition of ADK or AMPK in HIF-1α knockdown THP-1 cells would allow TLR-dependent or SCF/Kit-induced upregulation of mTOR S2448 phosphorylation. We therefore exposed normal and HIF-1α knockdown THP-1 cells for 4 h to 0.1 μg/ml R848, 1 μg/ml PGN or 0.1 μg/ml SCF with or without 30-min pre-treatment with 20 μM diadenosine pentaphosphate (DAPP, ADK inhibitor) or 50 μM dorsomorphin (DMP, AMPK inhibitor). Successful HIF-1α knockdown in THP-1 cells was monitored by detection of HIF-1 DBA. Phosphorylation of mTOR at S2448 was analyzed by ELISA, where intracellular mTOR was captured and its S2448 phosphorylation was analyzed (Fig. 4b, for further details see "Materials and methods").
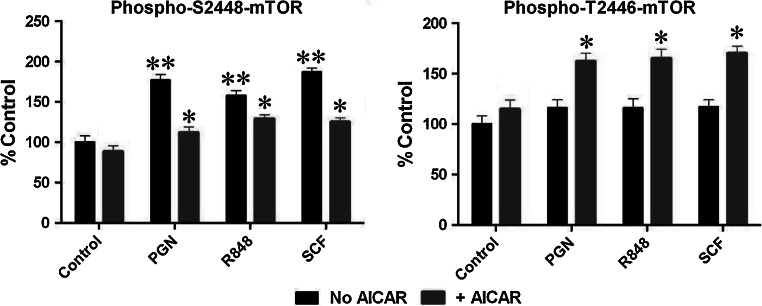
TLR ligands and SCF-induced HIF-1 DBA and mTOR S2448 phosphorylation were at levels similar to those observed in Western-blot experiments in normal THP-1 cells without HIF-1α knockdown (Fig. 4c, d). Both DAPP and DMP upregulated mTOR S2448 phosphorylation in HIF-1α knockdown THP-1 cells when exposed to PGN, R848, or SCF (Fig. 4c, d). Stimulation of non-treated THP-1 cells with DAPP or DMP did not change constitutive mTOR S2448 phosphorylation levels (Fig. 4c). To confirm the ability of AMPK to downregulate mTOR by phosphorylation of its T2446 we induced AMP kinase activity in THP-1 cells by 16-h exposure to 0.25 mM 5-aminoimidazole-4-carboxamide ribotide (AICAR) [25] followed by 4-h stimulation with either 1 μg/ml PGN, 100 ng/ml R848, or 100 ng/ml SCF. Other cells were exposed to the same stimuli in the same way but without pre-treatment with AICAR. We observed that AICAR significantly induced T2446 phosphorylation of mTOR, thus down-regulating PGN/R848/SCF-mediated S2448 phosphorylation of this protein (as measured by ELISA (see "Materials and methods"), Fig. 5).
Fig. 5.
AICAR-induced AMPK activation abolishes PGN/R848/SCF-induced mTOR S2448 phosphorylation and increases its T2446 phosphorylation. AMP kinase activity in THP-1 cells was induced by 16-h exposure to 0.25 mM AICAR followed by 4-h stimulation with 1 μg/ml PGN, 0.1 μg/ml R848, or 0.1 μg/ml SCF. An ELISA assay was used for quantitative analysis of phospho-S2448/phospho-T2446 mTOR. Data are mean values ± SD of at least three individual experiments. *p < 0.01 vs. THP-1 cells treated with respective stimulus in the absence of AICAR, **p < 0.01 vs. control
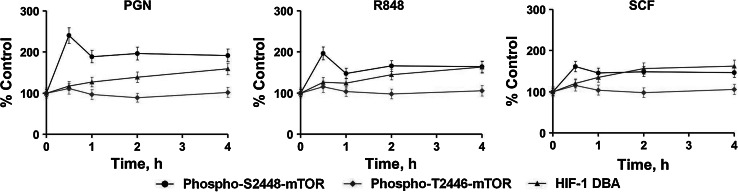
These data suggest that inhibition of ADK-catalyzed AMP generation (AMPK activator) or AMPK activity itself abrogates prevention of TLR- or SCF/Kit-mediated mTOR S2448 phosphorylation caused by silencing HIF-1α expression. Importantly, we have also confirmed that there is a temporal order for S2448 phosphorylation of mTOR and HIF1 activation that does not allow upregulation of the AMPK-dependent T2446 phosphorylation of mTOR in THP-1 cells exposed to PGN, R848, or SCF (Fig. 6). S2448 phosphorylation of mTOR was significantly increased during exposure to PGN/R848/SCF, clearly peaking within 30 min in the cases of PGN and R848, but not SCF. This leads to HIF-1 activation in a time-dependent manner, thus preventing upregulation of T2446 phosphorylation of mTOR for all the studied conditions (Fig. 6).
Fig. 6.
Temporal order for mTOR S2448 phosphorylation, HIF1 activation, and mTOR T2446 phosphorylation (reflecting AMPK activity) in THP-1 cells exposed to PGN, R848, or SCF. Cells were treated with 1 μg/ml PGN, 0.1 μg/ml R848 or 0.1 μg/ml SCF for the indicated periods of time and phospho-S2448/T2446 mTOR as well as HIF-1 DNA-binding activity were measured as outlined in the "Materials and methods" section. Data are mean values ± SD of at least three individual experiments
The HIF-1 transcription complex is crucially involved in TLR2-mediated mTOR S2448 phosphorylation in mouse blood cells in vivo
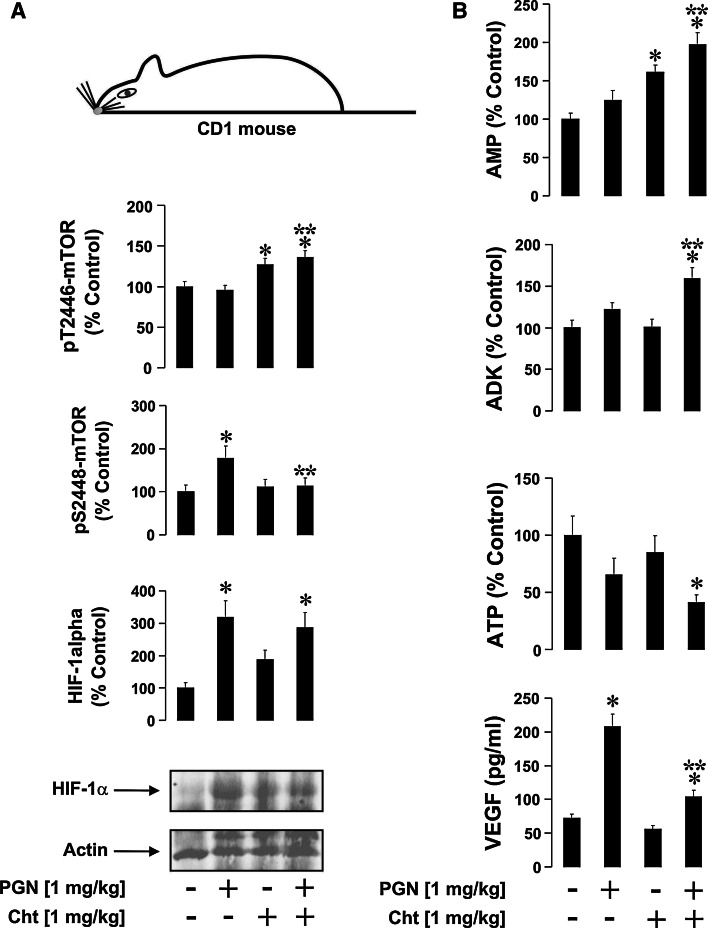
In vivo experiments were conducted on 6-week-old male mice (CD1, 25 ± 5 g). The first group (control, n = 5) received two simultaneous contralateral intra-peritoneal injections (i.p.) of 0.9 % NaCl (0.1 ml per injection). The second group (n = 5) was administered with one injection of 0.1 ml 0.9 % NaCl and another one – of 1 mg/kg PGN, both i.p.; the third group (n = 5), received one injection of 0.1 ml 0.9 % NaCl and another one of 1 mg/kg chetomin (HIF-1 transcriptional inhibitor) [23]. The last group (n = 5) received one injection of 1 mg/kg PGN and another one of 1 mg/kg chetomin i.p.. Blood was collected 4 h after injection (to investigate immediate non-chronic inflammatory reactions).
The mechanism of action of chetomin involves binding to the p300 transcriptional co-activator, which therefore serves as an optimal HIF-1 inhibitor for in vivo experiments. p300 is also used mostly by G-protein/cAMP-dependent pathways, which are not directly involved in PGN-induced TLR2 downstream pathways. Cellular fractions and plasma were separated from each other and cells were lysed and subjected to analysis of HIF-1α accumulation/HIF-1 DBA, mTOR S2448/T2446 phosphorylation, intracellular ATP levels, and ADK activity. VEGF (encoded by the HIF-1 downstream gene [9]) concentrations were measured in the blood plasma. It was found that PGN caused accumulation of HIF-1α, which was not affected by chetomin (Fig. 7a). PGN also induced upregulation of VEGF levels in blood plasma, which was abrogated by chetomin (Fig. 7b). S2448 phosphorylation of mTOR was also upregulated by PGN and this upregulation was similarly attenuated by chetomin (Fig. 7a). This was consistent with chetomin-dependent upregulation of T2446 mTOR phosphorylation (Fig. 7a).
Fig. 7.
HIF-1 is involved in PGN-induced mTOR S2448 phosphorylation in vivo. Twenty (four groups of five animals) 6-week-old male CD1 mice were used in the experiment as outlined in the "Materials and methods" section. All Western-blot data are from one experiment representative of five that gave similar results. Quantitative data are mean values ± SD (n = 5). *p < 0.05 vs. control, **p < 0.05 vs. PGN-injected mice
Intracellular ATP levels were decreased in PGN-injected animals as well as those co-injected with PGN/chetomin. However, a significant increase in ADK activity was observed in the blood cells of PGN/chetomin co-injected animals. This was in line with an increase in AMP levels (Fig. 7b). Importantly, in both cases the levels were significantly higher compared to those detected in control and PGN-injected animals. This suggests that a lack of ATP is compensated for by an increase in ADK activity, which generates both ATP and AMP out of ADP. Accumulated AMP is known to activate AMPK, which prevents S2448 mTOR phosphorylation. Consistent with these findings, we also observed similar effects (downregulation of mTOR S2448 phosphorylation) in THP-1 cells upon exposure to PGN with or without 2-h pre-treatment with 200 nM chetomin. This also led to increased ADK activity, intracellular AMP level, and pT2446 mTOR, while ATP levels were decreased (Supplementary figure 4).
Discussion
mTOR is a vital controller of ribosomal translation of signaling proteins playing a crucial role in the maturation, differentiation, and biological responses of mammalian hematopoietic cells of myeloid lineage [9]. These processes are normally associated with activation of the HIF-1 transcription complex that controls the expression of proteins that support glycolysis, angiogenesis, and cell adhesion [24]. HIF-1 is thereby responsible for adaptation of myeloid cells to signaling stress associated with their maturation, proliferation, or inflammatory reactions [24]. Recent evidence demonstrated the primary role of the PI3K/Akt/mTOR/S6K1 pathway in non-hypoxic HIF-1 activation [26–28]. Generally, successful translation processes can occur only in cells that have been adapted to signaling stress, although the molecular mechanisms underlying this dogma remained unclear. We therefore studied the biochemical/signaling links between HIF-1 and regulation of mTOR activation during TLR-mediated inflammatory reactions and SCF-dependent responses of myeloid cells.
For in vitro studies, we used normal and HIF-1α knockdown THP-1 human myeloid leukemia cells, which display most of the properties of primary myeloid innate immune cells and, at the same time (as leukemia cells), express the Kit receptor [7].
We found that ligand-activated plasma membrane-associated TLR2 and endosomal TLR7/8 trigger S2448 phosphorylation of mTOR and HIF-1 activation. HIF-1α knockdown in THP-1 cells failed to S2448 phosphorylate mTOR in response to stimulation with both TLR2 and TLR7/8 ligands. At the same time, they display significantly increased ADK activity, which could lead to increased AMP accumulation provided that sufficient amounts of substrate are available (Fig. 1). ADK activation by stimuli in normal THP-1 cells, however, did not attenuate mTOR S2448 phosphorylation, which could also be considered as an HIF-1-dependent effect. Degradation of purine nucleotides in myeloid cells into uric acid by XOD is also an HIF-1-dependent process [20] and thus the absence of HIF-1 does not allow AMP degradation, which results in its accumulation (Figs. 1, 3). Similar effects were observed with SCF-dependent responses (Fig. 2).
HIF-1α knockdown THP-1 cells normally lack ATP during inflammatory or SCF-dependent responses and the resulting accumulation of ADP leads to a saturation of ADK with the substrate. Accumulated AMP is a recognized allosteric modulator of AMPK, the enzyme which upon activation phosphorylates mTOR in the T2446 position [13]. This does not allow S2448 phosphorylation and blocks mTOR kinase and further signaling activity. Activation of AMPK is known to inhibit mTOR activity by suppressing S2448-phosphorylation [13, 14, 29].
Because of the above, we therefore considered whether specific pharmacological inhibition of ADK or AMPK in HIF-1α knockdown THP-1 cells could allow them to increase the levels of S2448-phosphorylated mTOR in a TLR or Kit-dependent manner. We discovered that inhibition of ADK by DAPP or AMPK by DMP indeed led to a significant increase in mTOR S2448 phosphorylation in HIF-1α knockdown THP-1 cells after 4 h of exposure to TLR2/7/8 ligands or SCF. Importantly, exposure of non-stimulated THP-1 cells to these inhibitors did not cause any changes in mTOR S2448 phosphorylation. Exposure of HIF-1α knockdown THP-1 cells to DAPP and DMP also did not cause any significant changes in the amounts of phospho-S2448 mTOR (data not shown).
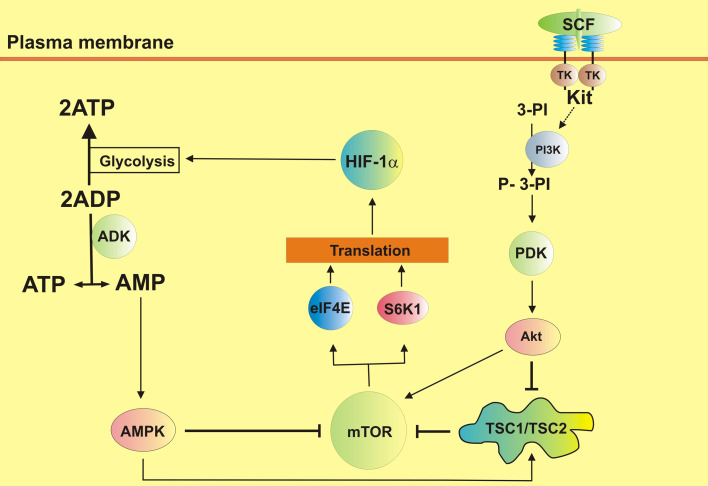
These results underline that upon stimulation of myeloid cells with TLR ligands or SCF, they become dependent on HIF-1 and, in the absence of this stress adaptor, these cells do not generate sufficient amounts of cytosolic ATP since they cannot upregulate glycolysis. Cells therefore activate the “second wind” enzyme ADK, which allows for the generation of ATP simply from accumulated ADP molecules [12]. However, this leads to a production of equal AMP amounts thus activating AMPK, which switches off mTOR S2448 phosphorylation and thus negatively impacts the translation of a number of signaling proteins. AMPK could directly phosphorylate mTOR at T2446 [13, 14] and also activate (by phosphorylation) TSC1/TSC2 complex, a negative mTOR regulator [29]. The pathways leading to mTOR activation during TLR-dependent and SCF/Kit signaling events and the role of HIF-1 in tracking mTOR activity are summarized in Fig. 8.
Fig. 8.
Crucial involvement of HIF-1 in tracking mTOR activity during the biological responses of mammalian myeloid cells (SCF is used as an example). The pathways leading to mTOR activation during TLR-dependent and SCF/Kit signaling events and the role of HIF-1 in tracking mTOR activity are summarized. The full biochemistry of these processes is presented in supplementary Fig. 5
Importantly, we also obtained in vivo confirmation of our findings using a mouse model showing that HIF-1 is crucially involved in PGN-induced mTOR S2448 phosphorylation in mouse blood cells. This process is also associated with control of ADK activation. When HIF-1 activity is blocked by chetomin, blood cells of PGN-injected mice responded by significantly increasing ADK activity. This allowed them to maintain threshold intracellular ATP levels and led to a downregulation of mTOR activation via S2448 phosphorylation.
These results unravel an important biochemical mechanism relating to the involvement of HIF-1 in the biochemical control of mTOR-dependent protein translation during the biological responses of myeloid cells.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Footnotes
I. M. Yasinska and B. F. Gibbs contributed equally to this study.
References
- 1.Broudy VC. Stem cell factor and hematopoiesis. Blood. 1997;90:1345–1364. [PubMed] [Google Scholar]
- 2.Lee SJ, Yoon JH, Song KS. Chrysin inhibited stem cell factor (SCF)/c-Kit complex-induced cell proliferation in human myeloid leukemia cells. Biochem Pharmacol. 2007;74:215–225. doi: 10.1016/j.bcp.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 3.Welker P, Grabbe J, Henz BM. Differential expression of mast cell characteristics in human myeloid cell lines. Exp Dermatol. 2004;13:535–542. doi: 10.1111/j.0906-6705.2004.00193.x. [DOI] [PubMed] [Google Scholar]
- 4.Medzhitov R, Janeway C., Jr Innate immunity. N Engl J Med. 2000;343:338–344. doi: 10.1056/NEJM200008033430506. [DOI] [PubMed] [Google Scholar]
- 5.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 6.Kagan JC, Medzhitov R. Phosphoinositide-mediated adaptor recruitment controls Toll-like receptor signalling. Cell. 2006;125:943–955. doi: 10.1016/j.cell.2006.03.047. [DOI] [PubMed] [Google Scholar]
- 7.Gibbs BF, Yasinska IM, Oniku AE, Sumbayev VV. Effects of stem cell factor on hypoxia-inducible factor 1 alpha accumulation in human acute myeloid leukaemia and LAD2 mast cells. PLoS One. 2011;6:e22502. doi: 10.1371/journal.pone.0022502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lopez-Pelaez M, Soria-Castro I, Bosca L, Fernandez M, Alemany S. Cot/tpl2 activity is required for TLR-induced activation of the Akt p70 S6k pathway in macrophages: implications for NO synthase 2 expression. Eur J Immunol. 2011;41:1733–1741. doi: 10.1002/eji.201041101. [DOI] [PubMed] [Google Scholar]
- 9.Dazert E, Hall MN. mTOR signalling in disease. Curr Opin Cell Biol. 2011;23:744–755. doi: 10.1016/j.ceb.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 10.Walmsley SR, Cadwallader KA, Chilvers ER. The role of HIF-1alpha in myeloid cell inflammation. Trends Immunol. 2005;26:434–439. doi: 10.1016/j.it.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 11.Krawczyk CM, Holowka T, Sun J, Blagih J, Amiel E, De Berardinis RJ, Cross JR, Jung E, Thompson CB, Jones RG, Pearce EJ. Toll-like receptor-induced changes in glycolytic metabolism regulate dendritic cell activation. Blood. 2010;115:4742–4749. doi: 10.1182/blood-2009-10-249540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dzeja P, Terzic A. Adenylate kinase and AMP signalling networks: metabolic monitoring, signal communication and body energy sensing. Int J Mol Sci. 2009;10:1729–1772. doi: 10.3390/ijms10041729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng SW, Fryer LG, Carling D, Shepherd PR. Thr2446 is a novel mammalian target of rapamycin (mTOR) phosphorylation site regulated by nutrient status. J Biol Chem. 2004;279:15719–15722. doi: 10.1074/jbc.C300534200. [DOI] [PubMed] [Google Scholar]
- 14.Grimaldi C, Chiarini F, Tabellini G, Ricci F, Tazzari PL, Battistelli M, Falcieri E, Bortul R, Melchionda F, Iacobucci I. AMP-dependent kinase/mammalian target of rapamycin complex 1 signalling in T cell acute lymphoblastic leukemia: therapeutic implications. Leukemia. 2012;26:91–100. doi: 10.1038/leu.2011.269. [DOI] [PubMed] [Google Scholar]
- 15.Hanze J, Eul BG, Savai R, Krick S, Goyal P, Grimminger F, Seeger W, Rose F. RNA interference for HIF-1alpha inhibits its downstream signalling and affects cellular proliferation. Biochem Biophys Res Commun. 2003;312:571–577. doi: 10.1016/j.bbrc.2003.10.153. [DOI] [PubMed] [Google Scholar]
- 16.Yu EZ, Li YY, Liu XH, Kagan E, McCarron RM. Antiapoptotic action of hypoxia-inducible factor-1alpha in human endothelial cells. Lab Investig. 2004;84:553–561. doi: 10.1038/labinvest.3700071. [DOI] [PubMed] [Google Scholar]
- 17.Olsson T, Gulliksson H, Palmeborn M, Bergstrom K, Thore A. Leakage of adenylate kinase from stored blood cells. J Appl Biochem. 1983;5:437–445. [PubMed] [Google Scholar]
- 18.Kennedy JF, Kay IM. Effects of titanium compounds on a d-glucose-d-glucose oxidase assay system. Carbohydr Res. 1975;44:291–300. doi: 10.1016/S0008-6215(00)84173-7. [DOI] [PubMed] [Google Scholar]
- 19.Georgescu P, Paunescu E (1960) Metode biochimie de diagnostic si cercetare. Medicala, pp 415
- 20.Nicholas SA, Bubnov VV, Yasinska IM, Sumbayev VV. Involvement of xanthine oxidase and hypoxia-inducible factor 1 in Toll-like receptor 7/8-mediated activation of caspase 1 and interleukin-1beta. Cell Mol Life Sci. 2011;68:151–158. doi: 10.1007/s00018-010-0450-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sumbayev VV, Yasinska IM, Oniku AE, Streatfield CL, Gibbs BF. Involvement of hypoxia-inducible factor-1 in the inflammatory responses of human LAD2 mast cells and basophils. PLoS One. 2012;7:e34259. doi: 10.1371/journal.pone.0034259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nicholas SA, Sumbayev VV. The involvement of hypoxia-inducible factor 1 alpha in Toll-like receptor 7/8-mediated inflammatory response. Cell Res. 2009;19:973–983. doi: 10.1038/cr.2009.44. [DOI] [PubMed] [Google Scholar]
- 23.Spirig R, Djafarzadeh S, Regueira T, Shaw SG, von Garnier C, Takala J, Jakob SM, Rieben R, Lepper PM. Effects of TLR agonists on the hypoxia-regulated transcription factor HIF-1alpha and dendritic cell maturation under normoxic conditions. PLoS One. 2010;5:e0010983. doi: 10.1371/journal.pone.0010983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sumbayev VV, Nicholas SA. Hypoxia-inducible factor 1 as one of the “signalling drivers” of Toll-like receptor-dependent and allergic inflammation. Arch Immunol Ther Exp (Warsz) 2010;58:287–294. doi: 10.1007/s00005-010-0083-0. [DOI] [PubMed] [Google Scholar]
- 25.Gaidhu MP, Fediuc S, Anthony NM, So M, Mirpourian M, Perry RLS, Ceddia RB. Prolonged AICAR-induced AMP-kinase activation promotes energy dissipation in white adipocytes: novel mechanisms integrating HSL and ATGL. J Lipid Res. 2009;50:704–715. doi: 10.1194/jlr.M800480-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Agani F, Jiang BH. Oxygen-independent regulation of HIF-1: novel involvement of PI3K/AKT/mTOR pathway in cancer. Curr Cancer Drug Targets. 2013;13:245–251. doi: 10.2174/1568009611313030003. [DOI] [PubMed] [Google Scholar]
- 27.Liu Z, Yuan Q, Zhang X, Xiong C, Xue P, Ruan J. RY10-4, a novel anti-tumor compound, exhibited its anti-angiogenesis activity by down-regulation of the HIF-1alpha and inhibition phosphorylation of AKT and mTOR. Cancer Chemother Pharmacol. 2012;69:1633–1640. doi: 10.1007/s00280-012-1873-3. [DOI] [PubMed] [Google Scholar]
- 28.Finlay DK, Rosenzweig E, Sinclair LV, Feijoo-Carnero C, Hukelmann JL, Rolf J, Panteleyev AA, Okkenhaug K, Cantrell DA. PDK1 regulation of mTOR and hypoxia-inducible factor 1 integrate metabolism and migration of CD8+ T cells. J Exp Med. 2012;209:2441–2453. doi: 10.1084/jem.20112607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery M, Vasquez DS, Turk BE, Shaw RJ. AMPK phosphorylation of Raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.