Abstract
Hypoxic/ischemic injury remains the most dreaded cause of neurological disability and mortality. Despite the humbling experiences due to lack of promising therapy, our understanding of the complex cascades underlying the neuronal insult has led to advances in basic science research. One of the most noteworthy has been the effect of opioid receptors, especially the delta-opioid receptor (DOR), on hypoxic/ischemic neurons. Our recent studies, and those of others worldwide, present strong evidence that sheds light on DOR-mediated neuroprotection in the brain, especially in the cortex. The mechanisms of DOR neuroprotection are broadly categorized as: (1) stabilization of the ionic homeostasis, (2) inhibition of excitatory transmitter release, (3) attenuation of disrupted neuronal transmission, (4) increase in antioxidant capacity, (5) regulation of intracellular pathways—inhibition of apoptotic signals and activation of pro-survival signaling, (6) regulation of specific gene and protein expression, and (7) up-regulation of endogenous opioid release and/or DOR expression. Depending upon the severity and duration of hypoxic/ischemic insult, the release of endogenous opioids and DOR expression are regulated in response to the stress, and DOR signaling acts at multiple levels to confer neuronal tolerance to harmful insult. The phenomenon of DOR neuroprotection offers a potential clue for a promising target that may have significant clinical implications in our quest for neurotherapeutics.
Keywords: Delta-opioid receptor, Hypoxia, Ischemia, Opioids, Neurons, Neuroprotection
Introduction
Hypoxic/ischemic injury to the brain is essentially an umbrella term that includes a multitude of pathophysiological processes causing neuronal dysfunction and consequential cell death due to an insufficient supply of oxygen and/or blood flow to the brain. Furthermore, it also characterizes specific molecular pathways of ischemic damage in the brain.
Owing to their high oxygen demand along with limited glucose storage capacity, mammalian neurons are highly susceptible to hypoxic/ischemic injury. Survival of neurons, astrocytes, oligodendrocytes, and endothelial cells is threatened during the energy deprivation and again during the reperfusion phase [1–3].
Stroke, a common result of hypoxic/ischemic insult, is the leading cause of morbidity and mortality worldwide, second only to cancer and cardiovascular causes. It is estimated that nearly 795,000 people, amounting to 1 person in every 40, suffer a new or recurrent stroke in the United States [4]. Unfortunately, despite being an extensively researched and studied neuropathological event, there is no promising therapy for neuroprotection against hypoxic/ischemic stress and repair available at large and we are still in pursuit of one. This is an age-old conundrum that has baffled both neuroscientists and clinicians despite the extensive research conducted in the last few decades that has greatly enhanced our understanding of the complex domino effect associated with hypoxia/ischemia.
The hypoxic/ischemic insult to the brain involves myriad cellular and molecular events. Recent research has led to novel explorations of neural tolerance and acclimatization to hypoxia and ischemia at cellular and molecular levels. Furthermore, we can see a transition from the previous thought of characterizing ischemic penumbra—the region of intense inflammatory response, restoration and active angiogenesis—as a mere diagnostic tool to a biochemical target with applications in improving brain plasticity, neuroprotection and repair. Research in this area has identified a myriad apoptotic and signal pathways resulting in disruption of ionic homeostasis, reactive oxygen species (ROS) activation, mitochondrial swelling, DNA damage, and caspase activation that trigger cell injury and eventually cell death [5–13]. Ongoing and delayed neuronal damage from apoptotic pathways occur in the region surrounding the ischemic/necrotic core, called the penumbra, following exposure to hypoxic/ischemic insult and during reperfusion [14–16]. The understanding of these mechanisms have made available new molecular targets in the region of penumbra from the neurons, astrocytes, microglia, and bone marrow-derived cells, as well as other progenitor cells for directing neuroprotective and neurorestorative therapies as opposed to the current concepts of targeting the ischemic core [5, 9, 13, 17].
There is abundant evidence suggesting the excess activation of NMDA receptors as the culprit behind the molecular events including increased Ca2+ influx, ionic imbalance, activation of cytotoxic proteins, and eventual neuronal death. Therefore, NMDA receptors have emerged as a potential target for directing therapy for neuroprotection against hypoxic/ischemic injury [7, 12, 18, 19]. In addition, free radical scavenging/antioxidation is another route of neuroprotection that has been extensively studied. A recent neurotherapeutic wonder drug that has caught attention is NEU2000, which is proposed to have a dual action on both NMDA receptors as well as reactive oxygen species (ROS) [9]. Moreover, some studies have implicated new targets like Neuregulin-1, an epidermal growth factor that potentially modulates the activation of Akt and Bcl-2 after cerebral ischemia and enhances cellular tolerance to hypoxic/ischemic insults [20], and mitochondrial connexin43 protein and permeability transition pore complex present novel molecular targets owing to their potential counteraction of Ca2+-induced mitochondrial demise and neuronal injury [21, 22].
Basic science literature is rife with discovery of new potential therapeutic targets such as Bcl-2 associated proteins, granulocyte colony stimulating factor, citicholine, caspase inhibitors, poly (ADP-ribose) polymerase inhibitor, and anti-inflammatory interventions via post stroke immune modulation of astrocyte and microglia cells [5, 12, 22–24]. The recent advances in the stroke research have re-conceptualized the idea of neuroprotection with the emergence and adoption of a combined approach of neuroprotection, repair, and restoration (recovery-enhancement during reperfusion) as evidenced by granulocyte colony stimulating factor and citicholine studies [23, 25]. Of late, many breakthrough interventional therapies have been spawned, such as vascular interventions like recanalization, thrombectomy and embolectomy, clot busters and collateral enhancement [26]. In addition, the role of stem cells in neuroprotection is increasingly being embraced and offers great promise. Currently, phase I/II clinical trials are underway involving use of embryonic and fetal stem cells along with mesenchymal and monocellular stem cells [27]. Stem cell neuroprotection involves enhancing brain plasticity and axonal remodeling during the post-stroke recovery period or during chronic stroke [28]. These new advances and observations may have a potentially far-reaching impact not only on the neuroprotective and repair therapy but also on the prevention against reperfusion injury during the post-stroke period and hypoxic encephalopathy.
However, the world of neuroscience has witnessed a humbling experience with the failure of the translation of successfully evaluated neuroprotective therapies in animal models to stroke patients. This has further clouded the already enigmatic stroke research as well as fomented basic research towards the unmet challenges in neuroprotection. In fact, owing to the failure to reiterate the promise of experimental therapies in actual patients, t-PA remains (with a reported 4 % limitation) the sole FDA-approved pharmacological agent with a proven benefit in stroke patients [5]. Many neuroscientists/investigators have closely examined the issue of neuroprotection from cerebral ischemia covering all angles to analyze the possible causes of these shortcomings [24, 29, 30]. The general consensus on these drawbacks narrows it down to suboptimal experimental or clinical models and designs, or an inadequate dosing in humans. This dearth in neurotherapeutics has brought the prevalent research methodology under question and has given room for evolution and acceptance of newer perspectives like targeting axonal regrowth for post-stroke improvement, or revisiting the less explored therapeutic targets in the immune system, purine nucleosides, and inflammatory responses [17, 24, 31]. On this road, we and colleagues worldwide have observed that one interesting and potentially promising discoveries in the past decade has been the delta-opioid receptor (DOR)-mediated neuroprotection against hypoxic/ischemic insult.
Opioids and their receptors: recapitulating perspectives
Opioids (or opiates—from opium poppy) are one of the oldest drugs known to mankind, and both therapeutic and non-therapeutic effects have been harnessed since ancient times [32]. In the early 1800s, the isolation of morphine, with an alkaloid [32] as the active ingredient, limited the use of the term opiate to all natural alkaloids of opium. Acheson coined the term “opioids” for all synthetic and semi-synthetic morphine derivatives with similar activity yet distinct chemical structure [33]. Opioids have a long history as potent analgesics and ecstasy inducers in medical and non-medical settings. By the 1960s, extensive research had been conducted on understanding the mechanisms of morphine/opioid-induced analgesia and pain modulation from which were spawned many important concepts for the first time, including the existence of opioid receptors and neurological association with opioid activity. Also around the same time, the existence of endogenous opioids was introduced [34]. This marked the beginning of later discoveries of various types of endogenous opioids—a family of chemically distinct endogenous compounds with properties like morphine and including enkephalins, endorphins, and dynorphins [34]. Studies delineated the specificity of opioid activity to certain areas of the brain that was blocked by opioid antagonists [35, 36]. All these observations could be best explained by the existence of different types of opiate binding sites and their non-uniform distribution in the central nervous system [32]. Martin et al. [37] provided the first evidence of opioid receptors in vivo and postulated the occurrence of mu and kappa-receptors. Presently, it is known that the endogenous opioids and opioid receptors are widely distributed in both peripheral and central nervous systems [35–43]. The opioid system is essentially an inhibitory neurotransmitter system and consists of three major subtypes of opioid receptors: mu, kappa, and delta opioid receptors (MOR, KOR, and DOR) [39, 42–44]. Pharmacological studies suggest that each type of opioid receptor consists of 2–3 subtypes and are members of the rhodopsin subfamily in the superfamily of seven-transmembrane nucleotide binding regulatory G-protein coupled receptors (GPCRs) [43, 45–50]. Although homologies exist, these receptors recognize structurally diverse exogenous/endogenous peptide and non-peptide ligands. These receptors are present not only in the nervous system but also inhabit other organs such as the heart, lungs, liver, and gastrointestinal and reproductive tracts [39, 51]. There exists a differential distribution of DOR and MOR in different parts of the brain, with a higher density of DOR in the cortical regions, especially the outer and inner as opposed to the middle layers, than in the subcortical regions of the brain [38, 40]. A multitude of reviews in the published literature have detailed the history, properties, structure, and G-protein coupling signal transduction, and early works on opioid receptor function [39, 43, 46–50, 52].
Understanding of the opioid system interplay in the neuronal responses to hypoxic/ischemic stress is quite fuzzy. This ignorance was further aggravated by the existing controversies in the available literature dating from before our extensive work. Since then, documented research has shown conflicting results on the effects of opioid receptor activation and inhibition during neuronal hypoxia. Until our serial studies from 1999 were published, little was known about the specific role of DOR in neuroprotection and its underlying mechanisms. Below, we will succinctly walk through the past, present, and potential future in opioid research under hypoxic/ischemic stress.
Opioid receptors and neuroprotection: evolution of the concept
With the identification of a wide array of pharmacological properties of opioids and their varying interactions through different opioid receptors, the distributional heterogeneity of opioid receptor subtypes was well established. Opioid receptors have long been known to be widely distributed across the central nervous system along with the peripheral and autonomic nervous systems. Pharmacological and physiological studies showed alterations in endogenous opioid activity in hypoxia/ischemia [40, 51, 53–57], although the exact role of these changes in terms of neuroprotection versus neuronal injury long baffled the neuroscientists.
Historical controversies
The previous ambiguity spawned from many conflicting views on the role of opioid receptors in hypoxic/ischemic stress. Some early researchers reported a noticeable increase in animal survival under hypoxic conditions after intravenous injection of opioid receptor agonists [58–61]. For example, enadoline, a kappa opioid receptor agonist, reduced brain damage after ischemia in rats [59]. The authors therefore believed that increased opioid receptor activation was a protective mechanism and rescued the brain from neuronal damage under hypoxia [57]. However, an increased animal survival time following systemic administration of opioid ligands does not necessarily indicate “neuroprotection”. Since an intravenous ligand may elicit systemic effects through many complex and varied mechanisms, it is difficult to reach a conclusion in terms of the specificity of action and associate it with neurons per se. The extended survival may result from the diverse effect of opioid agonists on various organs and organ systems. Although these early reports supported the belief that opioids induced neuroprotection, it is difficult to derive such conclusions from the methodology adopted in the studies.
In fact, there were other studies that reported opposite results. Some in vivo studies [62–67] showed that opioid receptor inhibition with opioid antagonists, like naloxone that binds non-specifically to DOR, MOR and KOR, induced neuroprotection, i.e. in other words implying that opioid activation caused neuronal damage. Hosobuchi et al. [65] studied an experimental gerbil model of surgically induced ischemia by occlusion of the right common carotid artery and noticed naloxone could reverse hemiparesis caused by cerebral ischemia, and a continuous administration could reduce the associated mortality. On a similar note, Adams et al. [62] performed a clinical trial on the dose-dependent effect of naloxone on 27 patients with acute or progressive stroke for assessment of a safe and optimal dose to treat cerebral ischemia. He reported a transient or sustained improvement in 13 patients with a worsening in 3 patients within 3 h of the discontinuation of the naloxone. All these data contradicted the conclusion of “opioid-induced neuroprotection”.
On the other hand, in the in vitro studies, one report showed that morphine or MOR agonists had no appreciable protection on hippocampal neurons [68]. In contrast, another study suggested that MOR agonists may lead to “non-opioid” effects because high doses (up to 3 mM) of MOR agonists like morphine could protect the cortical neurons from neuroexcitotoxic injury [69]. These contradictory observations further amplified the incomprehension of the complex role of opioids in hypoxic/ischemic neuronal injury.
Apparently, early research had complicated the issue rather than clearing the role of opioids during hypoxia/ischemic stress. To clarify this issue, our laboratory has adopted diverse current approaches and has consistently shown that DORs play a specific role in neuroprotection against hypoxic/ischemic stress, particularly in the cerebral cortex.
Our observations: DOR-induced neuroprotection
Working on the premise of enhancing neural tolerance against hypoxia/ischemia, we took inspiration from our earlier works on the turtle brain that showed much higher DOR density [40] and greater tolerance to hypoxic/ischemic insult [70] than the rat brain. Over the past decade, we have demonstrated the neuroprotective role of DOR against hypoxic/ischemic insult with ample evidence. Since the cortex is rich in DOR expression as compared to hippocampus and other subcortical regions [38, 40], we especially cultured cortical neurons for our initial studies with multiple reliable assessment approaches like “same-field morphology” [18], LDH measurements, DNA defragmentation analysis, florescent staining techniques for live and dead neurons, etc. To clarify the action of various opioid receptors, especially DOR in neuronal responses to excitotoxicity, we added glutamate to the cultures to induce neuroexcitotoxicity and directly applied various opioid agonists and/or antagonists to cultured neurons to determine their effects on the glutamate-induced neuroexcitotoxicity. We found that induction of DOR activation with DADLE, a DOR agonist, reduced glutamate-induced injury by almost half, and naltrindole, a DOR antagonist, completely blocked this protective effect. In addition, administration of MOR and KOR agonists (DAMGO and U50, 488H, respectively) and MOR or KOR antagonists had no such effect on glutamate-induced injury [18, 71, 72]. These experiments demonstrated that activation of DOR, but not MOR and KOR, induced neuroprotection against glutamate excitotoxicity in neocortical neurons [18]. We observed the similar results in the cortical neurons exposed to hypoxia [73]. More recently, our serial studies with molecular, transgenic and electrophysiological techniques all support the finding that DOR is neuroprotective in the brain, especially in the cortical neurons [18, 44, 54, 73–83]. DOR is an oxygen-sensitive protein [79] and actively participates in the control of neuronal survival under both normoxic and hypoxic/ischemic conditions [43, 84].
The protective effect of ischemic or hypoxic preconditioning has recently gained much attention and is being explored as a potential strategy for stroke and myocardial ischemic injury [54, 85–89]. We found that both rapid and delayed neuronal preconditioning-induced neuroprotection involves the DOR mechanisms [54, 79]. Short-term exposure to hypoxia/ischemia may precondition neurons by up-regulating DOR signaling, thus increasing neuronal resistance to subsequent stress with prolonged or severe hypoxic/ischemic insults [54, 79, 90].
It is noteworthy to indicate that, in the past few years, many independent laboratories across USA, Canada, Japan, and China have well documented that DOR is neuroprotective against hypoxic/ischemic stress [51, 52, 90–109], as, for instance, DOR mediates neuroprotection induced by morphine preconditioning in ischemia–reperfusion conditions [51]. Moreover, in our mechanistic exploration, we showed the role of PKC-ERK pathway in DOR protection [79], which was demonstrated by Narita et al. [102]. Furthermore, Pamenter and Buck [100] utilized electrophysiological approach and demonstrated that DOR indeed plays an important role in hypoxia tolerance of the turtle brain.
Reconciling with the past
All the evidence from our and other laboratories provides compelling sources to disregard the previous controversies that existed in the early literature.
First of all, a significant methodological flaw lies in the fact that the ligands that were used intravenously had limited receptor selectivity and specificity thereby resulting in diverse responses secondary to their simultaneous action at multiple receptors. Moreover, a high dose of opioid ligands underscores their ability to cross-react with more than one type of opioid receptor and increases the likelihood of inducing non-specific effects. In addition, intravenous administration of opioid ligands, used in the majority of these earlier studies, can induce multitude of systemic effects affecting various organs including the heart, blood vessels, etc. [110–115]. Such diverse effects make it a herculean effort to correctly delegate the observed effect to a specific receptor action or non-opioid action.
Another limitation of these early studies was the inappropriateness of the outcome measure employed. Some authors interpreted that DOR was neuroprotective based on extended animal survival during severe hypoxic exposure. In view of this complex interplay of mechanisms and the multiple effects induced, such interpretations are likely to have been a faux pas. Since the increase in animal survival could be a result of other associated protective/beneficial effects such as cardioprotection, to report it as a result of neuroprotective effect seems an inaccurate assumption.
In the in vivo studies, the lack of neuroprotective response to MOR ligands observed in the hippocampus by Iwai et al. [68] could be explained based on the pattern of opioid receptor distribution in various regions of the brain. Our autoradiographic studies have clearly shown a low density of DOR binding sites in the hippocampus, including the dentate gyrus although the MOR binding sites are present in abundance [40, 70]. A low concentration of MOR agonists applied to the hippocampus can activate MOR but does not induce much effect of DOR in this region. Therefore, MOR ligands did not demonstrate any neuroprotection in the hippocampus. Indeed, this notion reaffirmed that MOR plays little part in neuroprotection [18, 43, 77]. Since there is a higher density of DOR in the cortex, a high dose of MOR ligand may activate DOR along with MOR, and thus induce neuroprotection, which might be the reason behind the observation that high doses of MOR agonists protect the cortical neurons against neuroexcitotoxic injury [69] because many MOR ligands are not purely specific and may bind to DOR at a high concatenation.
Mechanisms of DOR neuroprotection: getting to the nuts and bolts!
Establishing the role of DOR in neuroprotection against hypoxic/ischemic neuronal insult has traveled a long way from a time laden with controversies to a well-recognized fact today, although the concepts of exact mechanisms that underlie this process are still somewhat murky. With recent advances in DOR research, DOR neuroprotection against hypoxic/ischemic insult has been delineated in a greater detail and improved our understanding of the mechanisms involved in DOR action.
The effects of hypoxic/ischemic insult on neurons mainly depend upon the duration and severity of injury [11, 116]. Acute insult triggers immediate responses affecting neuronal function by disrupting ionic imbalance causing anoxic neuronal depolarization, depleting the ATP levels, disruption of normal neural transmission, excessive neurotransmitter release resulting in excitotoxicity, and production of ROS leading to oxidative injury, etc. Prolonged hypoxia induces a delayed response to hypoxic/ischemic insult and can potentially cause changes in the mRNA/protein expression and bring about morphological changes in both cytoskeletal structure and DNA damage along with cellular death signaling [31]. Therefore, DOR action on the neurons is also affected by the extent of cell damage after exposure to hypoxic/ischemic stress.
Varied as they may seem, yet there are certain cellular processes that emerge as the final common pathways involved in all of these postulations that have populated the recent DOR literature. Furthermore, recent data from our laboratory and those of others have presented some ground-breaking mechanisms involved in DOR neuroprotection against both acute and prolonged hypoxic/ischemic injuries that have been increasingly accepted in the neuroscientific community.
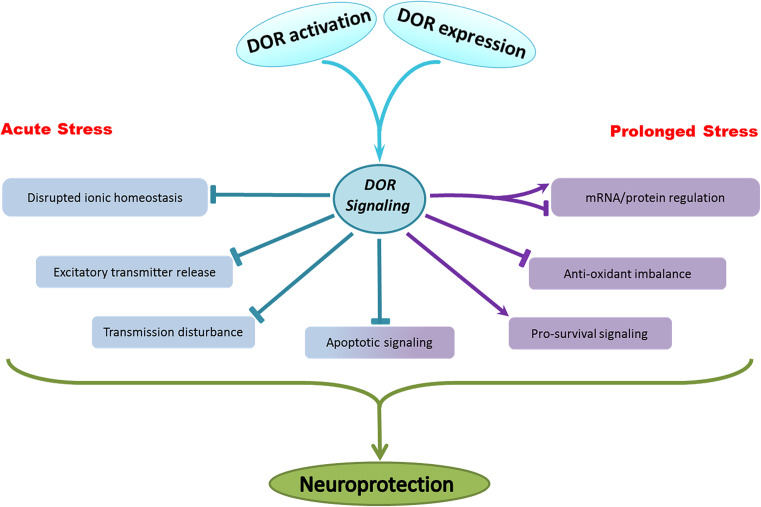
All these observations lead us to paint a picture depicting the workings of DOR that can present a helpful aid for understanding and narrowing potential therapeutic targets. Broadly speaking, the mechanisms of DOR neuroprotection that can be mainly grouped as: (1) stabilization of the ionic homeostasis, (2) inhibition of excitatory transmitter release, (3) attenuation of disrupted neuronal transmission, (4) increase in antioxidant capacity, (5) regulation of intracellular pathways—inhibition of apoptotic signals and activation of pro-survival signaling, (6) regulation of specific gene and protein expression, and (7) up-regulation of endogenous opioid release and/or DOR expression. Of these, some occur immediately in response to acute hypoxic stress while other changes appear gradually in response to a prolonged hypoxic/ischemic insult (Fig. 1).
Fig. 1.
Overview of the mechanisms underlying DOR neuroprotection
DOR-mediated membrane stabilization during short-term hypoxic/ischemic stress
Hypoxia/ischemia induces a cascade of ionic events that eventually result in neuronal injury/death [7, 43]. Ionic homeostasis is maintained as a dynamic balance under normal conditions, which is essential for the functioning of neurons. It chiefly depends on two factors: selective permeability of membrane to ions and active transport of ions. The loss of ionic homeostasis is an inherent response of the membrane proteins to the acute ischemic/hypoxic stress. It is greatly affected by the duration and severity of insults. In a severe insult, the absence of ATP and inactivation of Na+/K+/ATPase leads to a serious disruption of the ionic homeostasis, which is characterized by a large Na+ influx and K+ efflux.
It has been established that the ionic imbalance takes place in 2 phases [43]. Phase 1 involves a predominant K+ efflux and gradual rise in extracellular K+ concentrations to the so-called ceiling levels, and is then followed by phase 2 featured by an abrupt increase of extracellular K+, Na+, and Ca2+ [10, 82], as well as increased Cl− influx secondary to Na+ entry that further potentiates neuronal depolarization and results in free radical production [43, 74–78, 81]. All these form the basis of excitotoxic injury (Fig. 2).
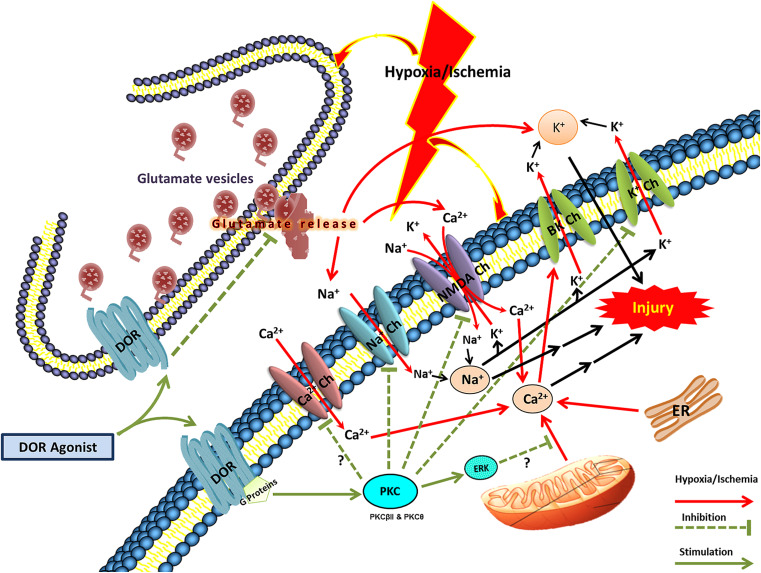
Fig. 2.
DOR effect on ionic homeostasis in hypoxic/ischemic stress. BK Ch Calcium-activated potassium channels. DOR delta-opioid receptor, TRK tyrosine kinase, Na + Ch sodium channels, Ca 2+ Ch calcium channels, NMDA Ch NMDA channels, K + Ch potassium channels. Arrows in red indicate the effect of hypoxic/ischemic effects, dashed lines indicate inhibition, and green arrows/lines indicate the survival or neuroprotective mechanisms or changes
Blockade of K+ efflux has been shown to attenuate hypoxia/ischemia-induced neuronal death [117, 118]. Our results show that activation of DOR, but not MOR, markedly reduces K+ leakage, suggesting that DOR signals membrane stabilization [74–78], which is apparently neuroprotective.
The blockade of K+ efflux is partially aided by inhibition of Ca2+ influx [76, 77], because DOR activation with DOR agonists causes a decrease in Ca2+-dependent K+ efflux via BK channels [77]. In addition, electrophysiological studies also illustrated a significant dampening of both spontaneous and stimulus-evoked excitatory impulses in the cortical neurons following DOR activation [119, 120]. This decrease in membrane excitability results in a reduced K+ leakage thereby attenuating the ionic imbalance brought about by anoxic action potential generation.
Since Na+ concentration plays a significant role in neuronal depolarization and conduction of action potential, a reduction in intracellular concentration and influx of both Na+ and Ca2+ ions can significantly prevent K+ efflux [43, 121]. In this context, we found that DOR activations results in the blockage of voltage-gated Na+ channels bringing about a large decrease in Na+ influx and attenuating the hypoxia/ischemia-induced ionic disruption including K+ leakage [43, 74, 75, 81]. Further investigations revealed that the major mechanism underlying DOR neuroprotection against anoxic disruption of K+ homeostasis in the cortex is the inhibition of Na+ entry through voltage-gated channels and NMDA receptor channels because this effect disappeared after low Na+ perfusion (with impermeable N-methyl-d-glucamine or permeable Li+ substitution), Na+ channel blocker TTX, and NMDA receptor channel blocker MK 801 [74, 75]. This observation was substantiated when we demonstrated an attenuation of anoxic Na+ influx after addition of naltrindole to the cortical slices [72, 81].
With a reduction in glutamate release along with direct action on NMDA receptors, DOR activation further reduces NMDA receptor-mediated influx of Na+ and Ca2+, thus lowering intracellular concentrations of Na+ and Ca2+ [43, 74].
Interestingly, the ability of DOR to attenuate Na+ influx via regulation of Na+ channels in the cortex is specific to hypoxic/ischemic injury and does not encompass other related conditions like hydrogen sulfide (H2S) neurotoxicity. In this respect, our recent work showed that, similar to hypoxia/ischemia, H2S induces a disruption of Na+ homeostasis mainly mediated by Na+ channels and NMDA receptors. However, unlike the attenuation of hypoxic/ischemic Na+ influx by DOR activation, H2S-induced massive Na+ influx is resistant to DOR regulation [122].
Besides postsynaptic attenuation of Na+ influx via Na+ channels and NMDA receptors [74], DOR activation also leads to presynaptic inhibition of glutamate release [43, 52, 97, 119, 120]. Immunocytochemical studies demonstrate the presence of DORs at the presynaptic terminals in mammalian cortical neurons [123, 124]. Electrophysiological evidence suggests that DOR activation can prevent the release of glutamate from presynaptic vesicles [119, 120], which may prevent K+ efflux and Na+ influx due to a reduction in NMDA receptor-mediated Na+ influx [43, 119]. Recently, another association was highlighted as a potential aspect of DOR neuroprotection at the presynaptic level, i.e., DOR activation down-regulates Na–K-2Cl co-transporter (NKCC) expression that is associated with neuronal injury secondary to severe hypoxic/ischemic insult [3, 91]. DOR activation inhibits glutamate release that in turn decreases NKCC activity thereby reducing cerebral edema and in the process protecting against neuronal damage [91].
Since DOR is a G-protein-coupled receptor, the cytoplasmic signal transduction pathways associated with G-protein/kinases are likely to mediate the DOR stabilization of ionic homeostasis during hypoxic/ischemic insult. In fact, our recent studies indicate that DOR attenuation of hypoxic/ischemic disruption of ionic homeostasis is protein kinase C-dependent and protein kinase A-independent [43, 84, 125]. Some specific PKC isoforms such as PKCβII and PKCθ are involved in DOR effects [126].
Our pioneering work in studying the mechanism of DOR neuroprotection against hypoxic disruption of ionic homeostasis has cleared most of the doubts that previously loomed over this phenomenon. These results show that DOR activation acts at multiple levels to prevent the ionic imbalance, thereby offering neuroprotection via presynaptic, postsynaptic and intracellular pathways, which have been schematically illustrated in Fig. 2.
DOR-mediated cellular and molecular regulation in prolonged hypoxia/ischemia
While acute hypoxic stress induces functional changes, prolonged stress can potentially alter cell structure, macromolecules and gene expression, and signal transduction [43, 54, 79, 90, 98, 127–131]. Thus, DOR-mediated neuronal protection is likely to regulate the complex neuronal cascade at multiple levels through complex mechanisms.
First of all, the expression of DOR and the release of endogenous opioids like enkephalins acting at DOR may be regulated in response to the stress. Studies suggest a role of endogenous opioids released during hypoxia/ischemia in protection against hypoxic/ischemic injury, although the mechanisms are not completely understood, but DOR activation has been implicated [43, 52, 67, 84, 87, 91, 101, 132, 133].
On the other hand, an up-regulation of DOR expression could be a protective mechanism adopted by neurons to overcome environmental stress. The turtle brain has a higher density of DOR than the rat brain [40], which may be one of the factors that render the turtle more tolerant to hypoxic/ischemic stress than the rat [70]. Indeed, we found that DOR is sensitive to hypoxic/ischemic stress and a short-term hypoxic stress may up-regulate DOR expression [54, 79]. An over-expression of DOR in transgenic mice induces neuroprotection against anoxic disruption of K+ homeostasis [78].
Therefore, an important mechanism of neuronal preconditioning is likely related to the regulation of DOR expression besides an increase in the release of endogenous opioids. We found that hypoxic preconditioning (HPC) induces neuroprotection against excitatory or hypoxic injury [54, 79]. Rapid HPC increased the DOR protein expression with no appreciable increase in DOR mRNA [54]. Delayed preconditioning with exposure to mild hypoxia for 6–9 h followed by 24 h normoxia increased both mRNA and protein levels of DOR in cortical neurons [79]. In essence, by increasing DOR expression, rapid HPC of cortical neurons renders them more tolerant to subsequent glutamate excitotoxicity [54] and delayed HPC attenuated neuronal injury caused by severe hypoxia [79]. Moreover, the HPC effects were largely blocked by DOR antagonist, further demonstrating a role of DOR in HPC-induced neuroprotection. These results highlight the importance of DOR expression in inducing neuronal tolerance to hypoxia/ischemic injury.
However, a prolonged stress may impair DOR expression as we observed that prolonged hypoxia reduces DOR density in both in vivo brain [134] and in vitro cortical neurons [79]. Mayfield et al. [57] found that, when compared to MOR and KOR, DOR is more sensitive to the stress. Although 1- to 3-day hypoxia did not induce changes in DOR expression in the whole brain homogenate, prolonged hypoxia (7 days) induced a selective decrease in DOR expression in the exposed mouse brain but induced no effect on MOR or KOR expression [57]. This decrease in DOR density could be a reason behind the brain injury under hypoxic/ischemic stress.
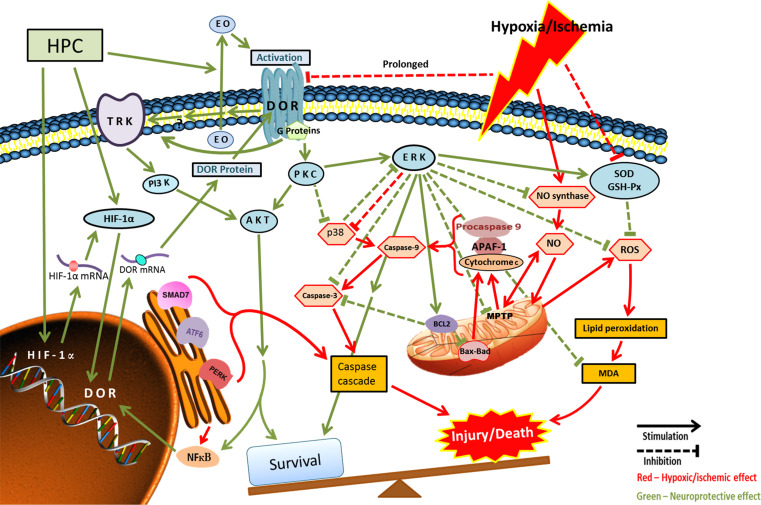
The mechanisms underlying the DOR neuroprotection involve the intricate interplay of various recognized neuroprotective signaling cascades. We found that the activation of DORs stimulates protein kinase C (PKC) and mitogen-activated protein kinase (MAPK)-ERK1/2 that eventually prevents phosphorylation of p38, thereby preventing cell death [79, 84, 90]. Other researchers [102, 135] corroborated our findings as thereby signifying the pivotal role of the PKC/MAPK/ERK signaling pathway in DOR-mediated neuroprotection. In sync with these observations, Peng et al. [90] demonstrated an up-regulation of hypoxia inducible transcription factor following hypoxic preconditioning, which in turn resulted in increased DOR expression that induced neuroprotection through ERK signaling pathway [90].
Phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt) is likely involved in the PKC/ERK pathway of DOR signaling [136, 137]. A reversal of neuroprotective effects was reported upon blocking the ERK or Akt or application of ionophores in calcium-depleted medium thereby proving the close involvement of these pathways [7]. Recently, Wang et al. [138] demonstrated that post-ischemic treatment with a DOR agonist, d-Ala2, d-Leu5-encephalin (DADLE), at low doses in a transient forebrain ischemia model induced neuroprotection against ischemic/reperfusion injury through the PI3K/Akt pathway.
Hypoxic/ischemic insult induces the production of NO and ROS leading to oxidative injury through caspase cascade [10, 79, 139]. We demonstrated an increase in antioxidant capacity following DOR activation in an ischemic model of rat brain following middle cerebral artery occlusion. Both superoxide dismutase and glutathione peroxidase activities increased and free radical and nitric oxide levels decreased following DOR activation that resulted in reduced caspase 3 expression in the ischemic regions and thereby prevented cell death [140]. In agreement with this observation, PD98059—an inhibitor of ERK kinase—accelerated death in animals thereby reaffirming the role of ERK as a mediator of DOR signaling pathways. The dogmatic role of this pathway in DOR function in hypoxic/ischemic injury was recently reasserted through an offshoot, yet strongly associated, study that demonstrated attenuation of mitochondrial injury by DOR activation by diminishing membrane depolarization, caspase 3 release, and reactive oxygen species generation in rat cortical neurons [95]. Therefore, the end result of this DOR-induced cascade is the suppression of cytochrome c via enhanced Bcl-2 activity that results in anti-apoptotic signaling [79].
Some researchers have proposed the involvement of tyrosine kinase-dependent Trk-PI3K-Akt/CaMKII pathway in DOR signaling [102, 137, 138] and DOR-induced blockade of Bax-related apoptosis [141]. In addition, a possible connection of adenosine in DOR-neuroprotection mechanisms is also worth a thought, especially in view of their common effect on protection against glutamate excitotoxicity induced during ischemic injury [142]. A role of adenosine in neuroprotection against damage inflicted by toxins, reactive oxygen species or ischemia/reperfusion has also been documented [143, 144]. Extrapolating observations on the interactions between spinal opioid and adenosine receptors in remote cardiac preconditioning [142] endorses the necessity to further investigate this important issue.
The potential mechanisms underlying DOR protection in prolonged hypoxia/ischemia is schematically demonstrated in Fig. 3.
Fig. 3.
DOR-mediated cellular and molecular regulation in prolonged hypoxia/ischemia. ROS reactive oxygen species or oxygen free radicals, HPC hypoxic pre-conditioning, HIF hypoxia inducible factor, EO endogenous opioids, SOD superoxide dismutase, MPTP mitochondrial permeability transition pore. Arrows in red indicate effect of hypoxic/ischemic effects; dashed lines indicate inhibition; and green arrows/lines indicate the survival or neuroprotective mechanisms or changes. Blue ovals represent components involved in the neuroprotective pathways while red hexagons represent components playing role in the hypoxic/ischemic cell injury
Conclusions and perspectives
We have provided a brief overview on the problem of hypoxic/ischemic injury encompassing the general public health aspect to the neurological and molecular end of the spectrum. Insights into the advances in research were briefed with an in-depth discussion on one that has come across as a paramount discovery of recent times, namely DOR neuroprotection. Research investigations from our laboratory as well as from others have explored this realm in a myriad ways and have presented some ground-breaking observations that have the potential for significant clinical implications in limiting neurological and cardiovascular consequences of hypoxic/ischemic insult. We have tried to clear the confusions and discrepancies of earlier researchers and to elucidate the mechanisms that underlie DOR neuroprotection both under acute and prolonged hypoxia/ischemia. The recent mechanistic studies on DOR neuroprotection reveals that the effect of DOR activation and signaling is largely influenced by the duration of hypoxic stress and involves different modes of action. Under acute stress, DOR activation mainly stabilizes the ionic disruption induced by hypoxic/ischemic insult, although this may not be the only mechanism involved, which necessitates a further exploration of this issue. While under prolonged stress, DOR may target serial cascades at multiple levels through complex mechanisms. While it may not be the sole pathway, but PKC/ERK-dependent mechanism of DOR signaling is a pivotal dogma that links DOR activation to prevention of neuronal injury/death in both acute and prolonged stress.
DOR neuroprotection has far-reaching clinical implications in view of the national and global health burden associated with the high morbidity and mortality linked to hypoxic/ischemic injury. The implications of these findings are not limited to stroke and other related neurodegenerative disorders, but the effects of DOR-induced neuroprotection encompass cytotoxic, oxidative and ethanol/drug-induced stress [54, 73, 79, 87, 92–94, 100–109, 145, 146]. A better understanding of the DOR mechanisms may be enabling researchers to explore untrodden paths and new concepts for better treatment of stroke. The horizons of DOR neuroprotective research have broadened in recent times to also encompass integrative medicine. For instance, electroacupuncture has been demonstrated to be neuroprotective against acute ischemic injury by inducing an up-regulation of DOR activity in animal studies [106, 147, 148].
However, many questions still remain unanswered. More importantly, translation of our knowledge into clinical benefits in terms of the development of effective neurotherapeutics is still lagging. Since up-regulation of DOR expression offers obvious therapeutic promise, more physiological mechanisms that can better induce DOR expression should be further explored. We are still in need of more research in this area to overcome these lags in understanding and translation.
Acknowledgments
This work was supported by grants of NIH (HD-034852, AT-004422), Vivian L. Smith Neurologic Foundation, NSFC (31071046), CSDP (CS20102010) and CHB (ZD201007).
Footnotes
Xiaozhou He and Harleen K. Sandhu contributed equally.
References
- 1.Hertz L. Bioenergetics of cerebral ischemia: a cellular perspective. Neuropharmacology. 2008;55:289–309. doi: 10.1016/j.neuropharm.2008.05.023. [DOI] [PubMed] [Google Scholar]
- 2.Yamagata K. Pathological alterations of astrocytes in stroke-prone spontaneously hypertensive rats under ischemic conditions. Neurochem Int. 2012;60:91–98. doi: 10.1016/j.neuint.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Kahle KT, Simard JM, Staley KJ, Nahed BV, Jones PS, Sun D. Molecular mechanisms of ischemic cerebral edema: role of electroneutral ion transport. Physiology. 2009;24:257–265. doi: 10.1152/physiol.00015.2009. [DOI] [PubMed] [Google Scholar]
- 4.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Stafford R, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics–2010 update: a report from the American Heart Association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 5.Stankowski JN, Gupta R. Therapeutic targets for neuroprotection in acute ischemic stroke: lost in translation? Antioxid Redox Signal. 2011;14:1841–1851. doi: 10.1089/ars.2010.3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diarra A, Sheldon C, Brett CL, Baimbridge KG, Church J. Anoxia-evoked intracellular pH and Ca2+ concentration changes in cultured postnatal rat hippocampal neurons. Neuroscience. 1999;93:1003–1016. doi: 10.1016/S0306-4522(99)00230-4. [DOI] [PubMed] [Google Scholar]
- 7.Bickler PE. Clinical perspectives: neuroprotection lessons from hypoxia-tolerant organisms. J Exp Biol. 2004;207:3243–3249. doi: 10.1242/jeb.00977. [DOI] [PubMed] [Google Scholar]
- 8.Banasiak KJ, Burenkova O, Haddad GG. Activation of voltage-sensitive sodium channels during oxygen deprivation leads to apoptotic neuronal death. Neuroscience. 2004;126:31–44. doi: 10.1016/S0306-4522(03)00425-1. [DOI] [PubMed] [Google Scholar]
- 9.Cho SI, Park UJ, Chung J-M, Gwag BJ. Neu 2000, an NR2B-selective, moderate NMDA receptor antagonist and potent spin trapping molecule for stroke. Drug News Perspect. 2010;23:549–556. doi: 10.1358/dnp.2010.23.9.1513493. [DOI] [PubMed] [Google Scholar]
- 10.Sung J-H, Chao D, Xia Y. Neuronal responses to hypoxia. New frontiers in neurological research. Res Signpost. 2008;37:73–153. [Google Scholar]
- 11.Busl KM, Greer DM. Hypoxic-ischemic brain injury: pathophysiology, neuropathology and mechanisms. NeuroRehabilitation. 2010;26:5–13. doi: 10.3233/NRE-2010-0531. [DOI] [PubMed] [Google Scholar]
- 12.Moskowitz MA, Lo EH, Ladecola C. The science of stroke: mechanisms in search of treatments. Neuron. 2010;67:181–198. doi: 10.1016/j.neuron.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liman TG, Endres M. New vessels after stroke: postischemic neovascularization and regeneration. Cerebrovasc Dis. 2012;33:492–499. doi: 10.1159/000337155. [DOI] [PubMed] [Google Scholar]
- 14.Honig LS, Rosenberg RN. Apoptosis and neurologic disease. Am J Med. 2000;108:317–330. doi: 10.1016/S0002-9343(00)00291-6. [DOI] [PubMed] [Google Scholar]
- 15.Lo EH. A new penumbra: transitioning from injury into repair after stroke. Nat Med. 2008;14:497–500. doi: 10.1038/nm1735. [DOI] [PubMed] [Google Scholar]
- 16.Schaller B, Graf R. Cerebral ischemia and reperfusion: the pathophysiologic concept as a basis for clinical therapy. J Cereb Blood Flow Metab. 2004;24:351–371. doi: 10.1097/00004647-200404000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Benowitz LI, Carmichael ST. Promoting axonal rewiring to improve outcome after stroke. Neurobiol Dis. 2010;37:259–266. doi: 10.1016/j.nbd.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang JJ, Haddad GG, Xia Y. Delta, but not mu and kappa, opioid receptor activation protects neocortical neurons from glutamate-induced excitotoxic injury. Brain Res. 2000;885:143–153. doi: 10.1016/S0006-8993(00)02906-1. [DOI] [PubMed] [Google Scholar]
- 19.Bickler PE. Reduction of NMDA receptor activity in cerebrocortex of turtles (Chrysemys picta) during 6 week of anoxia. Am J Physiol. 1998;275:R86–R91. doi: 10.1152/ajpregu.1998.275.1.R86. [DOI] [PubMed] [Google Scholar]
- 20.Guo W-P, Fu X-G, Jiang S-M, Wu J-Z. Neuregulin-1 regulates the expression of Akt, Bcl-2, and Bad signaling after focal cerebral ischemia in rats. Biochem Cell Biol. 2010;88:649–654. doi: 10.1139/O09-189. [DOI] [PubMed] [Google Scholar]
- 21.Azarashvili T, Baburina Y, Grachev D, Krestinina O, Evtodienko Y, Stricker R, Reiser G. Calcium-induced permeability transition in rat brain mitochondria is promoted by carbenoxolone through targeting connexin43. Am J Physiol Cell Physiol. 2011;300:C707–C720. doi: 10.1152/ajpcell.00061.2010. [DOI] [PubMed] [Google Scholar]
- 22.Boltze J, Kranz A, Wagner D-C, Reymann K, Reiser G, Hess DC. Recent advances in basic and translational stroke research. Expert Rev Neurother. 2011;11:199–202. doi: 10.1586/ern.10.202. [DOI] [PubMed] [Google Scholar]
- 23.Chamorro A, Román GC. Proceedings of recent developments and future directions in stroke management and prevention symposium: preface. Stroke. 2011;42:S1–S2. doi: 10.1161/STROKEAHA.110.608802. [DOI] [PubMed] [Google Scholar]
- 24.Macrez R, Ali C, Toutirais O, Le Mauff B, Defer G, Dirnagl U, Vivien D, Mauff BL. Stroke and the immune system: from pathophysiology to new therapeutic strategies. Lancet Neurol. 2011;10:471–480. doi: 10.1016/S1474-4422(11)70066-7. [DOI] [PubMed] [Google Scholar]
- 25.Fisher M. New approaches to neuroprotective drug development. Stroke. 2011;42:S24–S27. doi: 10.1161/STROKEAHA.110.592394. [DOI] [PubMed] [Google Scholar]
- 26.Molina CA. Reperfusion therapies for acute ischemic stroke: current pharmacological and mechanical approaches. Stroke. 2011;42:S16–S19. doi: 10.1161/STROKEAHA.110.598763. [DOI] [PubMed] [Google Scholar]
- 27.Planas AM, Traystman RJ. Advances in translational medicine 2010. Stroke. 2011;42:283–284. doi: 10.1161/STROKEAHA.110.605055. [DOI] [PubMed] [Google Scholar]
- 28.Piao C-S, Gonzalez-Toledo ME, Xue Y-Q, Duan W-M, Terao S, Granger DN, Kelley RE, Zhao L-R. The role of stem cell factor and granulocyte-colony stimulating factor in brain repair during chronic stroke. J Cereb Blood Flow Metab. 2009;29:759–770. doi: 10.1038/jcbfm.2008.168. [DOI] [PubMed] [Google Scholar]
- 29.Ginsberg MD. Current status of neuroprotection for cerebral ischemia synoptic overview. Stroke. 2009;40:S111–S114. doi: 10.1161/STROKEAHA.108.528877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Young AR, Ali C, Duretête A, Vivien D, Durete A. Neuroprotection and stroke: time for a compromise. J Neurochem. 2007;103:1302–1309. doi: 10.1111/j.1471-4159.2007.04866.x. [DOI] [PubMed] [Google Scholar]
- 31.Thauerer B, Nedden zur S, Baier-Bitterlich G, Zur Nedden S. Hypoxia in brain purine nucleosides in hypoxia. J Neurochem. 2012;121:329–342. doi: 10.1111/j.1471-4159.2012.07692.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brownstein MJ. A brief history of opiates, opioid peptides, and opioid receptors. Proc Natl Acad Sci USA. 1993;90:5391–5393. doi: 10.1073/pnas.90.12.5391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martin WR. Opioid antagonists. Pharmacol Rev. 1967;19:463–521. [PubMed] [Google Scholar]
- 34.Hughes J, Smith TW, Kosterlitz HW, Fothergill LA, Morgan BA, Morris HR. Identification of two related pentapeptides from the brain with potent opiate agonist activity. Nature. 1975;258:577–580. doi: 10.1038/258577a0. [DOI] [PubMed] [Google Scholar]
- 35.Pert A, Yaksh T. Localization of the antinociceptive action of morphine in primate brain. Pharmacol Biochem Behav. 1975;3:133–138. doi: 10.1016/0091-3057(75)90092-1. [DOI] [PubMed] [Google Scholar]
- 36.Snyder SH, Matthysse S. Opiate receptor mechanisms. Neurosci Res Program Bull. 1975;13:1–166. [PubMed] [Google Scholar]
- 37.Martin WR, Eades CG, Thompson JA, Huppler RE, Gilbert PE. The effects of morphine and nalorphine like drugs in the nondependent and morphine-dependent chronic spinal dog. J Pharmacol Exp Ther. 1976;197:517–532. [PubMed] [Google Scholar]
- 38.Xia Y, Haddad GG. Ontogeny and distribution of opioid receptors in the rat brainstem. Brain Res. 1991;549:181–193. doi: 10.1016/0006-8993(91)90457-7. [DOI] [PubMed] [Google Scholar]
- 39.Feng Y, He X, Yang Y, Chao D, Lazarus LH, Xia Y. Current research on opioid receptor function. Curr Drug Targets. 2012;13:230–246. doi: 10.2174/138945012799201612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xia Y, Haddad GG. Major difference in the expression of delta and mu-opioid receptors between turtle and rat brain. J Comp Neurol. 2001;436:202–210. doi: 10.1002/cne.1061. [DOI] [PubMed] [Google Scholar]
- 41.Hiller JM, Fan LQ. Laminar distribution of the multiple opioid receptors in the human cerebral cortex. Neurochem Res. 1996;21:1333–1345. doi: 10.1007/BF02532374. [DOI] [PubMed] [Google Scholar]
- 42.Xia Y, Cao H, Zhang J, Chen N, Siegel K, Agulnik M, Haddad G (2001) Effect of δ-opioid receptor activation on Na+ channel expression in cortical neurons subjected to prolonged hypoxia in culture. Society for neuroscience online: SfN Abstract, program no. 740.6
- 43.Chao D, Xia Y. Ionic storm in hypoxic/ischemic stress: can opioid receptors subside it? Prog Neurobiol. 2010;90:439–470. doi: 10.1016/j.pneurobio.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Feng Y, He X, Yang Y, Chen J, Yin K, Xia Y. Effect of delta-opioid receptor over-expression on cortical expression of GABAA receptor alpha1-subunit in hypoxia. Chin J Physiol. 2011;54:118–123. doi: 10.4077/cjp.2011.amm047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Audet N, Galés C, Archer-Lahlou E, Vallières M, Schiller PW, Bouvier M, Pineyro G. Bioluminescence resonance energy transfer assays reveal ligand-specific conformational changes within preformed signaling complexes containing delta-opioid receptors and heterotrimeric G proteins. J Biol Chem. 2008;283:15078–15088. doi: 10.1074/jbc.M707941200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ballet S, Pietsch M, Abell AD. Multiple ligands in opioid research. Protein Pept Lett. 2008;15:668–682. doi: 10.2174/092986608785133672. [DOI] [PubMed] [Google Scholar]
- 47.Barry U, Zuo Z. Opioids: old drugs for potential new applications. Curr Pharm Des. 2005;11:1343–1350. doi: 10.2174/1381612053507459. [DOI] [PubMed] [Google Scholar]
- 48.Law PY, Loh HH. Regulation of opioid receptor activities. J Pharmacol Exp Ther. 1999;289:607–624. [PubMed] [Google Scholar]
- 49.Pasternak GW. Multiple opiate receptors: déjà vu all over again. Neuropharmacology. 2004;47(Suppl 1):312–323. doi: 10.1016/j.neuropharm.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 50.Waldhoer M, Bartlett SE, Whistler JL. Opioid receptors. Annu Rev Biochem. 2004;73:953–990. doi: 10.1146/annurev.biochem.73.011303.073940. [DOI] [PubMed] [Google Scholar]
- 51.Lim YJ, Zheng S, Zuo Z. Morphine preconditions Purkinje cells against cell death under in vitro simulated ischemia-reperfusion conditions. Anesthesiology. 2004;100:562–568. doi: 10.1097/00000542-200403000-00015. [DOI] [PubMed] [Google Scholar]
- 52.Johnson SM, Turner SMF. Protecting motor networks during perinatal ischemia: the case for delta-opioid receptors. Ann N Y Acad Sci. 2010;1198:260–270. doi: 10.1111/j.1749-6632.2010.05434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ammon-Treiber S, Stolze D, Schröder H, Loh H, Höllt V. Effects of opioid antagonists and morphine in a hippocampal hypoxia/hypoglycemia model. Neuropharmacology. 2005;49:1160–1169. doi: 10.1016/j.neuropharm.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 54.Zhang J, Qian H, Zhao P, Hong S–S, Xia Y. Rapid hypoxia preconditioning protects cortical neurons from glutamate toxicity through delta-opioid receptor. Stroke. 2006;37:1094–1099. doi: 10.1161/01.STR.0000206444.29930.18. [DOI] [PubMed] [Google Scholar]
- 55.Armstead WM. Opioids and nitric oxide contribute to hypoxia-induced pial arterial vasodilation in newborn pigs. Am J Physiol. 1995;268:H226–H232. doi: 10.1152/ajpheart.1995.268.1.H226. [DOI] [PubMed] [Google Scholar]
- 56.Wilderman MJ, Armstead WM. Relationship between nitric oxide and opioids in hypoxia-induced pial artery vasodilation. Am J Physiol. 1996;270:H869–H874. doi: 10.1152/ajpheart.1996.270.3.H869. [DOI] [PubMed] [Google Scholar]
- 57.Mayfield KP, Kozak W, Malvin GM, Porreca F. Hypoxia decreases opioid delta receptor expression in mouse brain. Neuroscience. 1996;72:785–789. doi: 10.1016/0306-4522(95)00585-4. [DOI] [PubMed] [Google Scholar]
- 58.Endoh H, Taga K, Yamakura T, Sato K, Watanabe I, Fukuda S, Shimoji K. Effects of naloxone and morphine on acute hypoxic survival in mice. Crit Care Med. 1999;27:1929–1933. doi: 10.1097/00003246-199909000-00035. [DOI] [PubMed] [Google Scholar]
- 59.Hayward NJ, McKnight AT, Woodruff GN. Neuroprotective effect of the kappa-agonist enadoline (CI-977) in rat models of focal cerebral ischaemia. Eur J Neurosci. 1993;5:961–967. doi: 10.1111/j.1460-9568.1993.tb00947.x. [DOI] [PubMed] [Google Scholar]
- 60.Mayfield KP, D’Alecy LG. Delta-1 opioid agonist acutely increases hypoxic tolerance. J Pharmacol Exp Ther. 1994;268:683–688. [PubMed] [Google Scholar]
- 61.Summers RL, Li Z, Hildebrandt D. Effect of a delta receptor agonist on duration of survival during hemorrhagic shock. Acad Emerg Med. 2003;10:587–593. doi: 10.1111/j.1553-2712.2003.tb00040.x. [DOI] [PubMed] [Google Scholar]
- 62.Adams HP, Olinger CP, Barsan WG, Butler MJ, Graff-Radford NR, Brott TG, Biller J, Damasio H, Tomsick T, Goldberg M. A dose-escalation study of large doses of naloxone for treatment of patients with acute cerebral ischemia. Stroke. 1986;17:404–409. doi: 10.1161/01.STR.17.3.404. [DOI] [PubMed] [Google Scholar]
- 63.Olinger CP, Adams HP, Brott TG, Biller J, Barsan WG, Toffol GJ, Eberle RW, Marler JR. High-dose intravenous naloxone for the treatment of acute ischemic stroke. Stroke. 1990;21:721–725. doi: 10.1161/01.STR.21.5.721. [DOI] [PubMed] [Google Scholar]
- 64.Chen CJ, Liao SL, Chen WY, Hong JS, Kuo JS. Cerebral ischemia/reperfusion injury in rat brain: effects of naloxone. NeuroReport. 2001;12:1245–1249. doi: 10.1097/00001756-200105080-00038. [DOI] [PubMed] [Google Scholar]
- 65.Hosobuchi Y, Baskin DS, Woo SK. Reversal of induced ischemic neurologic deficit in gerbils by the opiate antagonist naloxone. Science. 1982;215:69–71. doi: 10.1126/science.6274019. [DOI] [PubMed] [Google Scholar]
- 66.Chen CJ, Cheng FC, Liao SL, Chen WY, Lin NN, Kuo JS. Effects of naloxone on lactate, pyruvate metabolism and antioxidant enzyme activity in rat cerebral ischemia/reperfusion. Neurosci Lett. 2000;287:113–116. doi: 10.1016/S0304-3940(00)01151-4. [DOI] [PubMed] [Google Scholar]
- 67.Skarphedinsson JO, Thorén P. Endorphin mechanisms are responsible for the beneficial effects of opioid antagonists on cerebral function during relative cerebral ischaemia in rats. Acta Physiol Scand. 1988;132:281–288. doi: 10.1111/j.1748-1716.1988.tb08331.x. [DOI] [PubMed] [Google Scholar]
- 68.Iwai T, Niwa M, Nakashima M, Kambara T, Yamada H, Tsurumi K, Nozaki M. Effect of opioids on delayed neuronal death in the gerbil hippocampus. Life Sci. 1992;50:PL239–PL244. doi: 10.1016/0024-3205(92)90580-I. [DOI] [PubMed] [Google Scholar]
- 69.Choi DW, Viseskul V. Opioids and non-opioid enantiomers selectively attenuate N-methyl-d-aspartate neurotoxicity on cortical neurons. Eur J Pharmacol. 1988;155:27–35. doi: 10.1016/0014-2999(88)90399-8. [DOI] [PubMed] [Google Scholar]
- 70.Xia Y, Jiang C, Haddad GG. Oxidative and glycolytic pathways in rat (newborn and adult) and turtle brain: role during anoxia. Am J Physiol. 1992;262:R595–R603. doi: 10.1152/ajpregu.1992.262.4.R595. [DOI] [PubMed] [Google Scholar]
- 71.Zhang JH, Gibney GT, Xia Y. Effect of prolonged hypoxia on Na+ channel mRNA subtypes in the developing rat cortex. Brain Res Mol Brain Res. 2001;91:154–158. doi: 10.1016/S0169-328X(01)00114-0. [DOI] [PubMed] [Google Scholar]
- 72.Tian X-S, Zhou F, Yang R, Xia Y, Wu G-C, Guo J-C. Effects of intracerebroventricular injection of delta-opioid receptor agonist TAN-67 or antagonist naltrindole on acute cerebral ischemia in rat. Acta Physiol Sin. 2008;60:475–484. [PubMed] [Google Scholar]
- 73.Zhang J, Gibney GT, Zhao P, Xia Y. Neuroprotective role of delta-opioid receptors in cortical neurons. Am J Physiol Cell Physiol. 2002;282:C1225–C1234. doi: 10.1152/ajpcell.00226.2001. [DOI] [PubMed] [Google Scholar]
- 74.Chao D, Balboni G, Lazarus LH, Salvadori S, Xia Y. Na+ mechanism of d-opioid receptor induced protection from anoxic K+ leakage in the cortex. Cell Mol Life Sci. 2009;66:1–11. doi: 10.1007/s00018-009-8759-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chao D, Bazzy-Asaad A, Balboni G, Salvadori S, Xia Y. Activation of DOR attenuates anoxic K+ derangement via inhibition of Na+ entry in mouse cortex. Cereb Cortex. 2008;18:2217–2227. doi: 10.1093/cercor/bhm247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chao D, Donnelly DF, Feng Y, Bazzy-Asaad A, Xia Y. Cortical delta-opioid receptors potentiate K+ homeostasis during anoxia and oxygen-glucose deprivation. J Cereb Blood Flow Metab. 2007;27:356–368. doi: 10.1038/sj.jcbfm.9600352. [DOI] [PubMed] [Google Scholar]
- 77.Chao D, Bazzy-Asaad A, Balboni G, Xia Y. Delta, but not mu, opioid receptor stabilizes K+ homeostasis by reducing Ca2+ influx in the cortex during acute hypoxia. J Cell Physiol. 2007;212:60–67. doi: 10.1002/jcp.21000. [DOI] [PubMed] [Google Scholar]
- 78.Chao D, Qian H, Ghassemi F, Chen J, Xia Y (2006) Transgenic overexpression of δ-opioid receptors protects the cortex from anoxic disruption of ionic homeostasis. Soc Neurosci Abstr MM68
- 79.Ma M-C, Qian H, Ghassemi F, Zhao P, Xia Y. Oxygen-sensitive delta-opioid receptor-regulated survival and death signals: novel insights into neuronal preconditioning and protection. J Biol Chem. 2005;280:16208–16218. doi: 10.1074/jbc.M408055200. [DOI] [PubMed] [Google Scholar]
- 80.Zhang JH, Xia Y, Haddad GG. Activation of δ-opioid receptors protects cortical neurons from glutamate excitotoxic injury. Soc Neurosci Abstr. 1999;25:736. [Google Scholar]
- 81.Kang X, Chao D, Gu Q, Ding G, Wang Y, Balboni G, Lazarus LH, Xia Y. Delta-opioid receptors protect from anoxic disruption of Na+ homeostasis via Na+ channel regulation. Cell Mol Life Sci. 2009;66:3505–3516. doi: 10.1007/s00018-009-0136-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kang X, Gu Q, Ding G, Chao D, Wang Y, Balboni G, Lazarus L, Xia Y. Delta-opioid receptor activation and sodium channel inhibition in Xenopus oocytes. Acta Physiol Sin. 2008;60:124. [Google Scholar]
- 83.Zhao P, Ma M-C, Qian H, Xia Y. Down-regulation of delta-opioid receptors in Na+/H+ exchanger 1 null mutant mouse brain with epilepsy. Neurosci Res. 2005;53:442–446. doi: 10.1016/j.neures.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 84.Feng Y, Chao D, He X, Yang Y, Kang X, Lazarus LH, Xia Y. A novel insight into neuroprotection against hypoxic/ischemic stress. Acta Physiol Sin. 2009;61:585–592. [PMC free article] [PubMed] [Google Scholar]
- 85.Bhuiyan MIH, Kim YJ. Mechanisms and prospects of ischemic tolerance induced by cerebral preconditioning. Int Neurourol J. 2010;14:203–212. doi: 10.5213/inj.2010.14.4.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Morris KC, Lin HW, Thompson JW, Perez-Pinzon MA. Pathways for ischemic cytoprotection: role of sirtuins in caloric restriction, resveratrol, and ischemic preconditioning. J Cereb Blood Flow Metab. 2011;31:1003–1019. doi: 10.1038/jcbfm.2010.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhao P, Huang Y, Zuo Z. Opioid preconditioning induces opioid receptor-dependent delayed neuroprotection against ischemia in rats. J Neuropathol Exp Neurol. 2006;65:945–952. doi: 10.1097/01.jnen.0000235123.05677.4b. [DOI] [PubMed] [Google Scholar]
- 88.Zhao H. The protective effects of ischemic postconditioning against stroke: from rapid to delayed and remote postconditioning. Open Drug Discov J. 2011;5:138–147. doi: 10.2174/1877381801002010138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhao H, Ren C, Chen X, Shen J. From rapid to delayed and remote postconditioning: the evolving concept of ischemic postconditioning in brain ischemia. Curr Drug Targets. 2012;13:173–187. doi: 10.2174/138945012799201621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Peng P-HH, Huang H-SS, Lee Y-JJ, Chen Y-SS, Ma M-C. Novel role for the delta-opioid receptor in hypoxic preconditioning in rat retinas. J Neurochem. 2009;108:741–754. doi: 10.1111/j.1471-4159.2008.05807.x. [DOI] [PubMed] [Google Scholar]
- 91.Yang L, Wang H, Shah K, Karamyan VT, Abbruscato TJ. Opioid receptor agonists reduce brain edema in stroke. Brain Res. 2011;1383:307–316. doi: 10.1016/j.brainres.2011.01.083. [DOI] [PubMed] [Google Scholar]
- 92.Borlongan CV, Wang Y, Su T-P. Delta opioid peptide (d-Ala 2, d-Leu 5) enkephalin: linking hibernation and neuroprotection. Front Biosci. 2004;9:3392–3398. doi: 10.2741/1490. [DOI] [PubMed] [Google Scholar]
- 93.Borlongan CV, Hayashi T, Oeltgen PR, Su T-P, Wang Y. Hibernation-like state induced by an opioid peptide protects against experimental stroke. BMC Biol. 2009;7:31. doi: 10.1186/1741-7007-7-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhu M, Li M, Tian X, Ou X, Zhu C, Guo J. Neuroprotective role of delta-opioid receptors against mitochondrial respiratory chain injury. Brain Res. 2009;1252:183–191. doi: 10.1016/j.brainres.2008.11.030. [DOI] [PubMed] [Google Scholar]
- 95.Zhu M, Li M, Yang F, Ou X, Ren Q, Gao H, Zhu C, Guo J. Mitochondrial ERK plays a key role in delta-opioid receptor neuroprotection against acute mitochondrial dysfunction. Neurochem Int. 2011;59:739–748. doi: 10.1016/j.neuint.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 96.Duan YL, Wang SY, Zeng QW, Su DS, Li W, Wang XR, Zhao Z. Astroglial reaction to delta opioid peptide [D-Ala2, D-Leu5] enkephalin confers neuroprotection against global ischemia in the adult rat hippocampus. Neuroscience. 2011;192:81–90. doi: 10.1016/j.neuroscience.2011.06.067. [DOI] [PubMed] [Google Scholar]
- 97.Turner SMF, Johnson SM. Delta-opioid receptor activation prolongs respiratory motor output during oxygen-glucose deprivation in neonatal rat spinal cord in vitro. Neuroscience. 2011;187:70–83. doi: 10.1016/j.neuroscience.2011.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nandhu MS, Naijil G, Smijin S, Jayanarayanan S, Paulose CS. Opioid system functional regulation in neurological disease management. J Neurosci Res. 2010;88:3215–3221. doi: 10.1002/jnr.22463. [DOI] [PubMed] [Google Scholar]
- 99.Gao Y, Liang W, Hu X, Zhang W, Stetler RA, Vosler P, Cao G, Chen J. Neuroprotection against hypoxic-ischemic brain injury by inhibiting the apoptotic protease activating factor-1 pathway. Stroke. 2010;41:166–172. doi: 10.1161/STROKEAHA.109.561852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pamenter ME, Buck LT. Delta-opioid receptor antagonism induces NMDA receptor-dependent excitotoxicity in anoxic turtle cortex. J Exp Biol. 2008;211:3512–3517. doi: 10.1242/jeb.021949. [DOI] [PubMed] [Google Scholar]
- 101.Charron C, Messier C, Plamondon H. Neuroprotection and functional recovery conferred by administration of kappa and delta 1-opioid agonists in a rat model of global ischemia. Physiol Behav. 2008;93:502–511. doi: 10.1016/j.physbeh.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 102.Narita M, Kuzumaki N, Miyatake M, Sato F, Wachi H, Seyama Y, Suzuki T. Role of delta-opioid receptor function in neurogenesis and neuroprotection. J Neurochem. 2006;97:1494–1505. doi: 10.1111/j.1471-4159.2006.03849.x. [DOI] [PubMed] [Google Scholar]
- 103.Su D, Wang Z, Zheng Y, Zhao Y, Wang X. Dose-dependent neuroprotection of delta opioid peptide [d-Ala2, d-Leu5] enkephalin in neuronal death and retarded behavior induced by forebrain ischemia in rats. Neurosci Lett. 2007;423:113–117. doi: 10.1016/j.neulet.2007.06.044. [DOI] [PubMed] [Google Scholar]
- 104.Govindaswami M, Brown SA, Yu J, Zhu H, Bishop PD, Kindy MS, Oeltgen PR. Delta 2-specific opioid receptor agonist and hibernating woodchuck plasma fraction provide ischemic neuroprotection. Acad Emerg Med. 2008;15:250–257. doi: 10.1111/j.1553-2712.2008.00048.x. [DOI] [PubMed] [Google Scholar]
- 105.Xiong Z-G, Chu X-P, Simon RP. Acid sensing ion channels–novel therapeutic targets for ischemic brain injury. Front Biosci. 2007;12:1376–1386. doi: 10.2741/2154. [DOI] [PubMed] [Google Scholar]
- 106.Xiong L, Yang J, Wang Q, Lu Z. Involvement of delta and mu-opioid receptors in the delayed cerebral ischemic tolerance induced by repeated electroacupuncture preconditioning in rats. Chin Med J. 2007;120:394–399. [PubMed] [Google Scholar]
- 107.Iwata M, Inoue S, Kawaguchi M, Nakamura M, Konishi N, Furuya H. Effects of delta-opioid receptor stimulation and inhibition on hippocampal survival in a rat model of forebrain ischaemia. Br J Anaesth. 2007;99:538–546. doi: 10.1093/bja/aem220. [DOI] [PubMed] [Google Scholar]
- 108.Horiuchi T, Kawaguchi M, Kurita N, Inoue S, Sakamoto T, Nakamura M, Konishi N, Furuya H. Effects of delta-opioid agonist SNC80 on white matter injury following spinal cord ischemia in normothermic and mildly hypothermic rats. J Anesth. 2008;22:32–37. doi: 10.1007/s00540-007-0576-0. [DOI] [PubMed] [Google Scholar]
- 109.Horiuchi T, Kawaguchi M, Sakamoto T, Kurita N, Inoue S, Nakamura M, Konishi N, Furuya H. The effects of the delta-opioid agonist SNC80 on hind-limb motor function and neuronal injury after spinal cord ischemia in rats. Anesth Analg. 2004;99:235–240. doi: 10.1213/01.ANE.0000130389.77859.1C. [DOI] [PubMed] [Google Scholar]
- 110.Bofetiado DM, Mayfield KP, D’Alecy LG. Alkaloid delta agonist BW373U86 increases hypoxic tolerance. Anesth Analg. 1996;82:1237–1241. doi: 10.1097/00000539-199606000-00023. [DOI] [PubMed] [Google Scholar]
- 111.Peart JN, Gross ER, Gross GJ. Opioid-induced preconditioning: recent advances and future perspectives. Vascul Pharmacol. 2005;42:211–218. doi: 10.1016/j.vph.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 112.el Schultz JJ, Hsu AK, Nagase H, Gross GJ. TAN-67, a delta 1-opioid receptor agonist, reduces infarct size via activation of Gi/o proteins and KATP channels. Am J Physiol. 1998;274:H909–H914. doi: 10.1152/ajpheart.1998.274.3.H909. [DOI] [PubMed] [Google Scholar]
- 113.Sun FY, Zhang AZ, Xia Y. Mechanism of dynorphin inhibition on vasoconstriction in vitro. Acta Physiol Sin. 1989;41:354–360. [PubMed] [Google Scholar]
- 114.Tubbs RJ, Porcaro WA, Lee WJ, Blehar DJ, Carraway RE, Przyklenk K, Dickson EW. Delta opiates increase ischemic tolerance in isolated rabbit jejunum. Acad Emerg Med. 2002;9:555–560. doi: 10.1111/j.1553-2712.2002.tb02291.x. [DOI] [PubMed] [Google Scholar]
- 115.Yamanouchi K, Yanaga K, Okudaira S, Eguchi S, Furui J, Kanematsu T. [d-Ala2, d-Leu5] enkephalin (DADLE) protects liver against ischemia-reperfusion injury in the rat. J Surg Res. 2003;114:72–77. doi: 10.1016/S0022-4804(03)00196-3. [DOI] [PubMed] [Google Scholar]
- 116.O’Reilly JP, Jiang C, Haddad GG. Major differences in response to graded hypoxia between hypoglossal and neocortical neurons. Brain Res. 1995;683:179–186. doi: 10.1016/0006-8993(95)00373-X. [DOI] [PubMed] [Google Scholar]
- 117.Liu D, Slevin JR, Lu C, Chan SL, Hansson M, Elmér E, Mattson MP. Involvement of mitochondrial K+ release and cellular efflux in ischemic and apoptotic neuronal death. J Neurochem. 2003;86:966–979. doi: 10.1046/j.1471-4159.2003.01913.x. [DOI] [PubMed] [Google Scholar]
- 118.Wei L, Yu SP, Gottron F, Snider BJ, Zipfel GJ, Choi DW. Potassium channel blockers attenuate hypoxia and ischemia-induced neuronal death in vitro and in vivo. Stroke. 2003;34:1281–1286. doi: 10.1161/01.STR.0000065828.18661.FE. [DOI] [PubMed] [Google Scholar]
- 119.Ostermeier AM, Schlösser B, Schwender D, Sutor B. Activation of mu and delta-opioid receptors causes presynaptic inhibition of glutamatergic excitation in neocortical neurons. Anesthesiology. 2000;93:1053–1063. doi: 10.1097/00000542-200010000-00029. [DOI] [PubMed] [Google Scholar]
- 120.Tanaka E, North RA. Opioid actions on rat anterior cingulate cortex neurons in vitro. J Neurosci. 1994;14:1106–1113. doi: 10.1523/JNEUROSCI.14-03-01106.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Friedman JE, Haddad GG. Anoxia induces an increase in intracellular sodium in rat central neurons in vitro. Brain Res. 1994;663:329–334. doi: 10.1016/0006-8993(94)91281-5. [DOI] [PubMed] [Google Scholar]
- 122.Chao D, He X, Yang Y, Balboni G, Salvadori S, Dong KH, Xia Y. Hydrogen sulfide induced disruption of Na+ homeostasis in the cortex. Toxicol Sci. 2012;128:198–208. doi: 10.1093/toxsci/kfs125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bausch SB, Patterson TA, Appleyard SM, Chavkin C. Immunocytochemical localization of delta opioid receptors in mouse brain. J Chem Neuroanat. 1995;8:175–189. doi: 10.1016/0891-0618(94)00044-T. [DOI] [PubMed] [Google Scholar]
- 124.Svingos AL, Cheng PY, Clarke CL, Pickel VM. Ultrastructural localization of delta-opioid receptor and Met5-enkephalin immunoreactivity in rat insular cortex. Brain Res. 1995;700:25–39. doi: 10.1016/0006-8993(95)00977-X. [DOI] [PubMed] [Google Scholar]
- 125.Chen Y, Yu FH, Surmeier DJ, Scheuer T, Catterall WA. Neuromodulation of Na+ channel slow inactivation via cAMP-dependent protein kinase and protein kinase C. Neuron. 2006;49:409–420. doi: 10.1016/j.neuron.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 126.Chao D, He X, Yang Y, Bazzy-Asaad A, Lazarus LH, Balboni G, Kim DH, Xia Y. DOR activation inhibits anoxic/ischemic Na+ influx through Na+ channels via PKC mechanisms in the cortex. Exp Neurol. 2012;236:228–239. doi: 10.1016/j.expneurol.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Brouwer M, Larkin P, Brown-Peterson N, King C, Manning S, Denslow N. Effects of hypoxia on gene and protein expression in the blue crab, Callinectes sapidus. Mar Environ Res. 2004;58:787–792. doi: 10.1016/j.marenvres.2004.03.094. [DOI] [PubMed] [Google Scholar]
- 128.Bindra RS, Schaffer PJ, Meng A, Woo J, Måseide K, Roth ME, Lizardi P, Hedley DW, Bristow RG, Glazer PM. Alterations in DNA repair gene expression under hypoxia: elucidating the mechanisms of hypoxia-induced genetic instability. Ann N Y Acad Sci. 2005;1059:184–195. doi: 10.1196/annals.1339.049. [DOI] [PubMed] [Google Scholar]
- 129.Appenzeller O, Minko T, Qualls C, Pozharov V, Gamboa J, Gamboa A, Wang Y. Gene expression, autonomic function and chronic hypoxia: lessons from the Andes. Clin Auton Res. 2006;16:217–222. doi: 10.1007/s10286-006-0338-3. [DOI] [PubMed] [Google Scholar]
- 130.Storey KB. Adventures in oxygen metabolism. Comp Biochem Physiol B Biochem Mol Biol. 2004;139:359–369. doi: 10.1016/j.cbpc.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 131.Lin HW, Thompson JW, Morris KC, Perez-Pinzon MA. Signal transducers and activators of transcription: STATs-mediated mitochondrial neuroprotection. Antioxid Redox Signal. 2011;14:1853–1861. doi: 10.1089/ars.2010.3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bolling SF, Tramontini NL, Kilgore KS, Su TP, Oeltgen PR, Harlow HH. Use of “natural” hibernation induction triggers for myocardial protection. Ann Thorac Surg. 1997;64:623–627. doi: 10.1016/S0003-4975(97)00631-0. [DOI] [PubMed] [Google Scholar]
- 133.Chen T-Y, Goyagi T, Toung TJK, Kirsch JR, Hurn PD, Koehler RC, Bhardwaj A. Prolonged opportunity for ischemic neuroprotection with selective kappa-opioid receptor agonist in rats. Stroke. 2004;35:1180–1185. doi: 10.1161/01.STR.0000125011.93188.c6. [DOI] [PubMed] [Google Scholar]
- 134.Haddad GG, Zhang JM, Bazzy AR, Xia Y. Chronic hypoxia differentially regulates δ-opioid receptor expression in adult and immature brains. Soc. Neurosci Abstr. 1999;25:579. [Google Scholar]
- 135.Sun K, Su DS, Wang XR. Delta opioid agonist [d-Ala2, d-Leu5] enkephalin (DADLE) reduced oxygen-glucose deprivation caused neuronal injury through the MAPK pathway. Brain Res. 2009;1292:100–106. doi: 10.1016/j.brainres.2009.06.104. [DOI] [PubMed] [Google Scholar]
- 136.Chen YL, Law P-Y, Loh HH. Nuclear factor kappaB signaling in opioid functions and receptor gene expression. J Neuroimmune Pharmacol. 2006;1:270–279. doi: 10.1007/s11481-006-9028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chen YL, Law P-Y, Loh HH. NGF/PI3K signaling-mediated epigenetic regulation of delta opioid receptor gene expression. Biochem Biophys Res Commun. 2008;368:755–760. doi: 10.1016/j.bbrc.2008.01.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wang S, Duan Y, Su D, Li W, Tan J, Yang D, Wang W, Zhao Z, Wang X. Delta opioid peptide [d-Ala2, d-Leu5] enkephalin (DADLE) triggers postconditioning against transient forebrain ischemia. Eur J Pharmacol. 2011;658:140–144. doi: 10.1016/j.ejphar.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 139.Chen S-D, Yang D-I, Lin T-K, Shaw F-Z, Liou C-W, Chuang Y-C. Roles of oxidative stress, apoptosis, PGC-1α and mitochondrial biogenesis in cerebral ischemia. Int J Mol Sci. 2011;12:7199–7215. doi: 10.3390/ijms12107199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yang Y, Xia X, Zhang Y, Wang Q, Li L, Luo G, Xia Y. δ-Opioid receptor activation attenuates oxidative injury in the ischemic rat brain. BMC Biol. 2009;7:55. doi: 10.1186/1741-7007-7-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tsao LI, Su TP. Hibernation-induction peptide and cell death: [d-Ala2, d-Leu5] enkephalin blocks Bax-related apoptotic processes. Eur J Pharmacol. 2001;428:149–151. doi: 10.1016/S0014-2999(01)01346-2. [DOI] [PubMed] [Google Scholar]
- 142.Yao L, Wong GTC, Xia Z, Irwin MG, Tin G, Wong C. Interaction between spinal opioid and adenosine receptors in remote cardiac preconditioning: effect of intrathecal morphine. J Cardiothorac Vasc Anesth. 2011;25:444–448. doi: 10.1053/j.jvca.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 143.Stone TW. Adenosine, neurodegeneration and neuroprotection. Neurol Res. 2005;27:161–168. doi: 10.1179/016164105X21896. [DOI] [PubMed] [Google Scholar]
- 144.Pedata F, Melani A, Pugliese AM, Coppi E, Cipriani S, Traini C. The role of ATP and adenosine in the brain under normoxic and ischemic conditions. Purinergic Signal. 2007;3:299–310. doi: 10.1007/s11302-007-9085-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Margolis EB, Fields HL, Hjelmstad GO, Mitchell JM. Delta-opioid receptor expression in the ventral tegmental area protects against elevated alcohol consumption. J Neurosci. 2008;28:12672–12681. doi: 10.1523/JNEUROSCI.4569-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Gwak M-SS, Li L, Zuo Z. Morphine preconditioning reduces lipopolysaccharide and interferon-gamma-induced mouse microglial cell injury via delta 1 opioid receptor activation. Neuroscience. 2010;167:256–260. doi: 10.1016/j.neuroscience.2010.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Tian X, Zhou F, Yang R, Xia Y, Wu G, Guo J. Electroacupuncture protects the brain against acute ischemic injury via up-regulation of delta-opioid receptor in rats. J Chin Integr Med. 2008;6:632–638. doi: 10.3736/jcim20080617. [DOI] [PubMed] [Google Scholar]
- 148.Zhao P, Guo J, Hong S, Bazzy-Asaad A, Cheng J, Xia Y (2002) Electro- acupuncture and brain protection from cerebral ischemia: The role of delta-opioid receptor. In society for neuroscience online: SfN Abstract. Program no. 490.13