Abstract
Cerebellar Purkinje cells (PC) physiologically reveal an age-dependent expression of progesterone with high endogenous concentrations during the neonatal period. Even if progesterone has been previously shown to induce spinogenesis, dendritogenesis and synaptogenesis in immature PC, data about the effects of progesterone on mature PC are missing, even though they could be of significant therapeutic interest. The current study demonstrates for the first time a progesterone effect, depending on the developmental age of PC. Comparable with the physiological course of the progesterone concentration, experimental treatment with progesterone for 24 h achieves the highest effects on the dendritic tree during the early neonate, inducing an highly significant increase in dendritic length, spine number and spine area, while spine density in mature PC could not be further stimulated by progesterone incubation. Observed progesterone effects are certainly mediated by classical progesterone receptors, as spine area and number were comparable to controls when progesterone incubation was combined with mifepristone (incubation for 24 h), an antagonist of progesterone receptors A and B (PR-A/PR-B). In contrast, an increase in the spine number and area of both immature and mature PC was detected when slice cultures were incubated with mifepristone for more than 72 h (mifepristone long-time incubation, MLTI). By including time-lapse microscopy, electron microscopic techniques, PCR, western blot, and MALDI IMS receptor analysis, as well as specific antagonists like trilostane and AG 205, we were able to detect the underlying mechanism of this diverging mifepristone effect. Thus, our results provide new insights into the function and signaling mechanisms of the recently described progesterone receptor membrane component 1 (PGRMC1) in PC. It is highly suitable that progesterone does not just induce effects by the well-known genomic mechanisms of the classical progesterone receptors but also acts through PGRMC1 mediated non-genomic mechanisms. Thus, our results provide first proofs for a previously discussed progesterone-dependent induction of neurosteroidogenesis in PC by interaction with PGRMC1. But while genomic progesterone effects mediated through classical PR-A and PR-B seem to be restricted to the neonatal period of PC, PGRMC1 also transmits signals by non-genomic mechanisms like regulation of the neurosteroidogenesis in mature PC. Thus, PGRMC1 might be an interesting target for future clinical studies and therapeutic interventions.
Electronic supplementary material
The online version of this article (doi:10.1007/s00018-013-1448-4) contains supplementary material, which is available to authorized users.
Keywords: Confocal laser scanning microscopy, Electron microscopy, Neuronal development, Microinjection, MALDI IMS, Organotypic slice cultures, PGRMC1, AG 205, Trilostane
Introduction
Glial and neuronal cells of vertebrates, including Purkinje cells (PC), have been shown to express several kinds of steroidogenic enzymes [1–5]. Indeed, PC possess the key enzymes of progesterone synthesis, cytochrome P450 side-chain cleavage enzyme (P450scc) as well as 3β-hydroxysteroid dehydrogenase (3β-HSD), and are able to synthesize progesterone de novo from cholesterol [6, 7]. Interestingly, pregnenolone as the initial substrate for all neurosteroids is also permanently traceable in high concentration in matured PC, while progesterone reveals an age-dependent expression pattern with high concentrations only during the neonatal period of PC [8].
To gain deeper insights into the role of progesterone for the development of the cerebellar tissue, the present studies were done using organotypic cerebellar slice cultures. PC, as the principal neurons of the cerebellar cortex, are physiologically subjected to a complex maturation process, which starts before birth and proceeds into the neonate. During the developmental period, the morphology of PC changes dramatically from unpolarized neurons with abundant perisomatic processes and varying dendritic terminations to polarized neuronal cells with the characteristic appearance of highly branched nearly two-dimensional dendritic arbors covered with matured dendritic spines. After this initial 30-day period, only quantitative changes of the dendritic arbors are detectable [9, 10]. Unfortunately, most of the mechanisms of PC development and differentiation still remain uncertain.
Thus, the description of de novo synthesis of progesterone and other steroid hormones from cholesterol by glial and neuronal cells has become of increasing interest in the last two decades [1, 5]. And, indeed, the temporal correlation between physiologically high concentrations of intracellular progesterone during the neonate and dramatic changes of the cerebellar circuit in this period indicate an involvement of progesterone in the development of PC and the organization of the cerebellar circuit [5, 10]. This hypothesis is supported by the present and other studies, which revealed a progesterone-dependent induction of dendritogenesis, spinogenesis, and synaptogenesis in developing PC [11, 12]. Thus, it is most likely that progesterone is indeed involved in the physiological maturation process of PC. But, for clinical reasons, these findings raise the question about the progesterone sensitivity of adult PC. It is of significant therapeutic interest whether the progesterone-dependent inducibility of PC growth and maturation continues beyond the time progesterone is physiologically synthesized in PC. One aim of the current study was thus to investigate whether spinogenesis and dendritogenesis in mature PC are also inducible by progesterone. Furthermore, the molecular basis of these progesterone effects is not yet fully understood. Admittedly, some of these progesterone-induced effects have been attributed to the progesterone receptors A (PR-A) and B (PR-B), which are often referred to as classical progesterone receptors [11–14]. But recently, the presence of two other different types of progesterone-binding transmembrane proteins, called seven transmembrane domain progesterone receptor β (7TMPR β) and progesterone receptor membrane component 1 (PGRMC1), have been discovered in PC [14–18]. A detailed description of function and signaling mechanisms of these newly described progesterone binding proteins in PC is still missing. Thus, the second aim of our study was to investigate the molecular mechanisms of the different progesterone receptors in PC by employing incubation and blocking studies combined with morphometric and molecular analyses. In these analyses, we had a special focus on PR-A, PR-B and PGRMC1.
Materials and methods
Cell cultures
Primary cerebellar slice cultures were obtained from Wistar rats of male and female pups (P9–P10) according to the roller-tube technique [19]. In different neuronal tissues, sex differences of progesterone effects were detectable, but in the cerebellum there are no hints for intersexual differences. Furthermore, different studies have proved that progesterone receptor expression of both classical progesterone receptors and PGRMC1 do not show sex differences in the expression within the cerebellum, either in neonatal or in adult PC [13, 17]. In addition, cerebellar slices were obtained from neonatal rats and all results were obtained before rats reached sexual maturity [7]. Thus, slices of female and male pups were used equally in this study. Altogether more than 250 pups were used. After decapitation, cerebella were prepared out of the rat cranium and stripped out from the meninges and blood vessels under visual control with a binocular microscope. Afterwards, cerebella were aseptically cut into 200- to 275-μm-thick parasagittal slices on a McIlwain tissue chopper. Then, slices were attached to collagen-faced (C7661; Sigma-Aldrich) glass coverslips (32 mm; Kindler, Freiburg, Germany) fixed by a plasma clot (P3266; Sigma) coagulated with thrombin (605157; Calbiochem). Together with nutrient medium, slice cultures were maintained in roller-tubes, which slightly rotate in a roller-drum incubator at 37 °C in an atmosphere of humidified 95 % air and 5 % CO2. The roller-tube nutrient medium was composed of basal medium eagle (BME, B1522; Sigma-Aldrich) enriched with 25 % fetal horse serum (S9135; Biochrom), 25 % Hanks, 6.5 mg/ml glucose (Sigma), 1 % l-glutamine (G7513; Sigma-Aldrich), 1 % penicillin (Sigma-Aldrich) and 25 ng/ml nerve growth factor (NGF-7S, N0513; Sigma). After 3 days in vitro (div), a mitosis inhibitor was added to the medium for 24 h. Afterwards, slices were maintained with roller-tube medium, which possesses a reduced concentration of fetal horse serum (15 %). The roller-tube medium was replaced twice a week for a period of up to 30 div.
In vitro treatment
The effects of progesterone on PC morphology and mediating receptor mechanisms were investigated by in vitro treatment of the cerebellar slice cultures with progesterone (10 nM, P8783; Sigma-Aldrich), mifepristone (1 μM, M8046; Sigma) trilostane (1 and 10 μM, S1404; Selleck) and AG 205 (5 nM, ST050150; TimTec, USA) alone or in varying combinations for 24 h up to 27 days. These concentrations are in accordance with previous studies [11, 12, 20].
Microinjection
Plasmid injections into single PC of cerebellar slice cultures were performed by microinjection [21]. For this study, several vectors like pEYFP-actin (6902-1; BD Biosciences), pLife-Act Tag RFP (60102; Ibidi), pEYFP-Tub vector (6118-1; Clontech) and GFP-NF-M [22] were used. For microinjection, plasmids were dissolved in distilled water and back-filled in sterile glass capillaries (∅ 0.2–0.5 μm, Femtotips; Eppendorf, Germany). The glass capillaries filled with 2 μl plasmid solution were fitted to an inverted microscope with phase contrast optics (Zeiss, Germany) and a pressure injection device (Eppendorf). The pressure injection tool was set to inject with up to 90–100 hPa for 0.5 s. The constant pressure was defined as 70 hPa [continued pressure (pc) = 70–80 hPa, injection pressure (pi) = 80–100 hPa, injection time (ti) = 0.5 s]. During microinjection, cultures were kept at 37 °C. After microinjection, cell cultures were rinsed thoroughly in fresh medium and post-incubated for at least 1 day. Besides applying single vectors, simultaneous injections of two plasmids were also performed.
Immunohistochemistry
For immunhistochemistry, cerebellar slice cultures were fixed in 4 % paraformaldehyde (PFA) in phosphate-buffered saline (PBS) for 20 min followed by permeabilisation with 1 % Triton-X-100 (T8532; Sigma-Aldrich) in PBS for 15–20 min. Thereafter, cultures were rinsed with PBS and incubated with the primary antibody dissolved in PBS at 4 °C overnight. PC were specifically labeled by monoclonal mouse antibodies against calbindin-D28k (C9848; Sigma-Aldrich). Functional synapses were identified by a rabbit polyclonal antibodies against PSD95 (250839; Abbiotec) and axonal neurofilaments were labeled by rabbit polyclonal antibodies against neurofilament M (AB1987; Chemicon). After intensive washing with PBS and blocking non-specific binding components with 10 % goat serum (G9023; Sigma-Aldrich) for 30 min, samples were incubated with secondary antibodies like anti-rabbit IgG FITC (F6005; Sigma-Aldrich), anti-rabbit IgG TRITC (T5268; Sigma-Aldrich), anti- mouse IgG FITC (F0257; Sigma-Aldrich), and anti-mouse IgG TRITC (T5393; Sigma-Aldrich) at room temperature for 2 h. Nuclear staining was done by incubation with bisBenzimide H 33342 trihydrochloride (DAPI, B2261; Sigma-Aldrich) for 20 min. Finally, cell cultures were rinsed in PBS and cover-slipped in fluorescence mounting medium (S3023; Dako).
Experimental groups
The experiments were performed with three groups of cerebellar slice cultures to investigate effects of steroids depending on the different stages of PC maturation. Slices were cultured for 7 days in vitro (div, group 1), 15 div (group 2), and 30 div (group 3) and afterwards fixed in 4 % PFA and subjected to immunohistochemistry. For live-cell imaging slices of group 2 were used for experimental purposes.
Morphometric analysis of dendritic length, soma dimension, and total cell area
To investigate the effects of progesterone on dendritic length, soma dimension, and total cell area within the same neuron pEYFP-actin microinjected PC of group 2 were used. Morphometric analysis was done with aid of confocal laser scanning microscopy (CLSM, LSM 510; Zeiss) in combination with Zeiss ×40 (LD-C Apochromat NA 1.4) aqua immersions lenses using a “rose chamber” system. Three conditions were investigated. At least 24 h after microinjection, cerebellar slices were imaged by the CLSM for the first time. Subsequent to this native imaging of pEYFP-actin expressing PC, the slice cultures were incubated with progesterone, mifepristone, or progesterone + mifepristone for 24 h (n = 10 PC in each condition from ten slice cultures of five different pups). After 1 day, PC were imaged again with identical microscope parameters. Dendritic length, soma dimension, and total cell area of the very same PC were measured with the aid of the Zeiss physiology kit before and after treatment. Changes in the rate of each parameter are quoted in percentage. Altogether, statistical analysis of the effects on PC morphology was performed with 40 PC.
Morphometric analysis of dendritic spine density
To study the influence of progesterone, mifepristone, AG205, and trilostane on dendritic spine density at different stages of cerebellar maturation calbindin-labeled PC were morphometrically analyzed. Therefore, eight conditions were compared to controls. For the first three conditions, slices after 6, 14 and 29 div were incubated with progesterone (condition 1), mifepristone (condition 2), and progesterone + mifepristone (condition 3) for 24 h. In addition, long-term incubation of slice cultures was performed with progesterone (data not shown), mifepristone (condition 4), progesterone + mifepristone (condition 5), mifepristone + trilostane (condition 6), AG205 (condition 7), and mifepristone + AG 205 (condition 8). The treatment always started after 3 div and lasted 4 days (group 1), 12 days (group 2), and 27 days (group 3). Afterwards, PC were immunohistochemically labeled with calbindin and analyzed by CLSM in combination with Zeiss ×63 (Plan-Neofluar NA 1.4) oil immersion lenses. For the analysis, the numbers of dendritic spines were counted in a region of interest along 40 μm dendritic arbor, on the distal tip of tertiary dendrites, within 30 cells in each condition and group. Here, six PC were measured in different slices of the same pup. Thus, in each condition and group, spine density was counted in PC of at least five different pups. For statistical analysis of spine number under different conditions, altogether the spine numbers in 810 PC of 135 pups were counted (control 7 div/15 div/30 div, n = 90; 1 day progesterone 7 div/15 div/30 div, n = 90; 1 day mifepristone 7 div/15 div/30 div, n = 90; 1 day progesterone plus mifepristone 7 div/15 div/30 div, n = 90; 4 days mifepristone, n = 30; 4 days progesterone plus mifepristone: n = 30; 4 days mifepristone plus trilostane, n = 30; 4 days AG205, n = 30; 4 days mifepristone plus AG204, n = 30; 12 days mifepristone, n = 30; 12 days progesterone plus mifepristone, n = 30; 12 days mifepristone plus trilostane, n = 30; 12 days AG205 n = 30; 12 days AG205 plus mifepristone n = 30; 27 days mifepristone, n = 30; 27 days progesterone plus mifepristone, n = 30; 27 days mifepristone plus trilostane, n = 30; 27 days AG205 n = 30; 27 days AG205 plus mifepristone, n = 30). The statistical significance was evaluated by use of the software Statistica (release 10; StatSoft, USA). We performed one-way analysis of variance (ANOVA) with additional post hoc analysis (Scheffe Test), thereby comparing the PC parameters in treated and untreated cultures.
Live-cell imaging and fluorescence recovery after photo bleaching (FRAP)
Subsequent microinjection time-lapse imaging was performed by the aid of confocal laser scanning microscopy (CLSM; Zeiss LSM 510) and 40-LD-Apochromate lense (Plan-Neuofluar NA 1.1). To maintain the incubation settings at 37 °C a Tempcontrol 37-2 (Zeiss) was used. To determine FRAP of YFP-actin, dendrites were imaged at low magnification (zoom level 1, 1024 × 1024 pixels), and laser power of 0.2 % (pre-bleach image). Afterwards, the laser was zoomed to a region of interest on a single dendritic spine, the power increased to 100 %, and the area scanned 100 times, until fluorescence was removed. The laser was zoomed out again, laser power was reduced to 0.2 %, and spines were captured for approximately 1 h at intervals of 30 s. Quantification of FRAP was done with aid of a Zeiss LSM physiology kit.
Electron microscopy
Cerebellar slice cultures for transmission electron microscopy (TEM) were fixed in 2.5 % glutaraldehyde in PB, postfixed in OsO4, dehydrated, and embedded in Epon. Sections were collected on Formvar-coated grids and contrasted with uranyl acetate. Immunogold labeling of RFP-actin was done pre-embedding with mouse anti-RFP antibodies (MAB3580; Milipore). Rapid-freeze deep-etch electron microscopy was performed as previously reported [23]. Briefly, slice cultures were frozen using a Balzers’ Cryojet (Balzers, Liechtenstein) apparatus, and then fractured and etched in a Balzers’ freeze-etching device. Platinum was applied at an angle of 35° to the rotating specimens. The platinum–carbon replicas were cleaned with household bleach, washed in distilled water, and placed unsupported on mesh grids. Specimens were recorded on a Philips EM 410 (Philips, Holland) transmission electron microscope equipped with a digital CCD camera (Model 792 BioScan; Gatan, USA) at ×29,000 magnification using a voltage of 80 kV. Morphometric analysis of spine area was done with aid of Image J 1.47v (National Institute of Health, USA).
Semi-quantitative reverse transcription-PCR
Total RNA was isolated from control and experimental PC using TRIzol (Invitrogen) reagent extraction method. Then, 1 μg of total RNA was reverse transcribed using QuantiTect Reverse Transcription Kit (Qiagen) to prepare first strand cDNA. For PCR, an aliquot of the cDNA solution corresponding to 50 ng of the total RNA was used as the template in a 20-μl reaction mixture. The PCR was carried out using Taq DNA Polymerase kit (Qiagen). The primers used and the details are listed below. The experiments were repeated at least three times to confirm the results.
| Primers | Sequence | References | Amplicon (bp) |
|---|---|---|---|
| PGRMC1 | |||
| Forward | ′5-CTCTCAACCTGCTGCTCCTT-3′ | Glaser et al. (2008) | 443 |
| Reverse | ′5-CGCTCCTTCAAGCAGTTTTC-3′ | ||
| PR-A + B | |||
| Forward | ′5- CCCACAGGAGTTTGTCAAGCTC-3′ | Park and Mayo (1991); Park-Sarge and Mayo (1994) | 326 |
| Reverse | ′5- TAACTTCAGACATCATTTCCGG-3′ | ||
| PR-B | |||
| Forward | ′5- ACTGAGCTGCAGGCAAAG-3′ | Park and Mayo (1991); Park-Sarge and Mayo (1994) | 244 |
| Reverse | ′5- CGGACAGCGACTGCTGA-3′ | ||
| Rat β-actin | |||
| Forward | ′5- GAGACCTTCAACACCCCAGC-3′ | Sakamoto et al. [11]; Nudel et al. 1983 | 645 |
| Reverse | ′5- CACAGAGTACTTGCGCTCAG -3′ | ||
Western blot
The protein extracts were mixed with 0.25 vol of 5× SDS gel-loading buffer, and 20 μL of the samples were subjected to 15 % (w⁄v) SDS-PAGE, followed by protein transfer onto a poly (vinylidene difluoride) membrane using the PerfectBlue™ Semi-Dry-Electroblotter (Peqlab, Erlangen, Germany) and 48 mM Tris, 39 mM glycine, 0.037 % (w/v) SDS and 20 % (v/v) methanol as transfer buffer. Blotting membranes were blocked with 3 % (w/v) low-fat milk powder dissolved in Tris-buffered saline supplemented with 0.05 % (v/v) Tween-20. Immuno detection of anti-PGRMC1 or anti-β-Actin antibodies were done using the ECL detection reagent (Amersham Biosciences). Primary antibodies used were anti-PGRMC1 (AB 48012; Abcam, Germany) and anti-Actin (A-2066; Sigma). The experiments were repeated at least three times to confirm the results.
Matrix-assisted laser desorption/ionization imaging mass spectrometry (MALDI IMS)
Sample preparation
Tissue sectioning: Cryosections from adult rats were prepared for mass spectrometry (MS) analysis and staining. The 10-μm-thin cerebellum tissue sections was mounted on a special conductive glass slide (Bruker Daltonics, Bremen, Germany). Prior to MS, the tissue on slide was first washed in 70 % ethanol (high performance liquid chromatography grade) two times and additionally in 100 % ethanol for 1 min each, and the tissue on slide was dried for 1 h in a vacuum concentrator.
Tissue digestion: The proteins on the surface of the tissue were digested by using 200 μl trypsin (100 ng/μl, #V5111; Promega, Mannheim, Germany) solution (50 mM NH4HCO3, pH 7.5–8.5). The trypsin was deposited by the ImagePrep™ (Bruker Daltonics) machine using the appropriate ImagePrep™ method. This method repeatedly sprays small amounts of trypsin solution onto the tissue. Afterwards, the tissue was incubated at 37 °C overnight in a humid environment.
Matrix application: Matrix (2.5-dihydroxybenzoic acid (DHB) 30 mg/ml # 85707; Sigma-Aldrich, Steinheim, Germany) was solved in 50 % Methanol and 1 % Trifluoroacetic acid (TFA) in order to dissolve the peptides of the specimen and incorporate them into the matrix crystal lattice. For matrix application, the ImagePrep™ spraying device (Bruker) was used to obtain a homogeneous matrix layer by using the appropriate method for DHB deposition. To define and circumcise the cerebellum to be imaged, slides were scanned prior to measurement using a flatbed scanner (Mirage II; Umax, Dallas, USA) with a resolution of 1,200 dpi.
Data processing, visualization: The imaging mass spectrometry experiment was performed using the Ultraflextreme MALDI TOF mass spectrometer (Bruker Daltonics) by using the FlexControl 3.3, Fleximaging 3.0, and Flexanalysis 3.3 software (Bruker Daltonics). The spectra acquisition was performed in the reflector mode using a MALDI imaging method acquiring data in the range of 900 to 4,500 Da. The area to be analyzed was marked in the fleximaging software and a measurement spot grid of 50-μm center-to-center spacing was set for spectra acquisition. At each measuring point, 500 sufficient laser shots were summed up. An external calibration was performed by using a peptide calibration standard (Bruker Daltonics). After IMS, the slide was washed using 70 % EtOH to remove the matrix and stained by conventional hematoxylin and eosin (H&E) staining. Afterwards, the slide was scanned with a Hamamatsu NanoZoomer 2.0-HT slide scanner (Hamamatsu Photonics Germany, Hechendorf, Germany) and co-registered with the unstained slide image used for teaching purposes in the mass spectrometer.
Data analysis: The theoretical peptides resulting out of a tryptic digest of PGRMC1 were obtained by using the protein prospector (prospector.ucsf.edu, v.5.10.4) theoretical digest option. The mass distribution and intensities of those masses on the tissue were visualized by fleximaging. The bin width for spectra analysis was 0.25 Da. Masses of the theoretical digest are shown in supplementary table 1.
Results
Cerebellar roller-tube slice cultures are highly suitable to study progesterone effects on PC
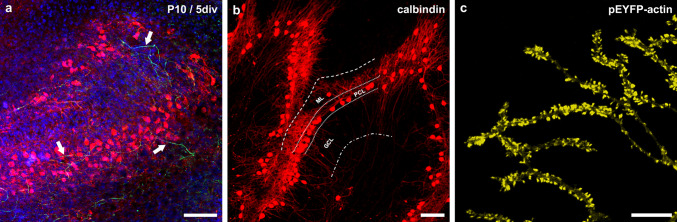
To study the effects of progesterone on mature and immature PC, cerebellar slice cultures were cultured for up to 5 weeks. To this purpose, we first tested if the characteristic organisation of the cerebellum is still retained in cerebellar slice cultures. As demonstrated by immunohistochemistry and CLSM, the cerebellar slice cultures are typically characterized by three layers, the molecular layer (ML), a distinct line-shaped arrangement of PC within the Purkinje cell layer (PCL), and a granule cell layer (GCL) (Fig. 1a, b). Dendritic arborisation arises from stem-dendrites with two or three main branches, from which multiple smaller dendrites with numerous dendritic spines diverge (Fig. 1c). Thus, cerebellar roller-tube slices represent a functional, organotypic neuronal tissue, which could be cultivated for several weeks without loss of the organotypic cerebellar morphology. Hence, this tissue is highly suitable to study developmental mechanisms of progesterone effects in a slice culture model to get more insights into developmental mechanisms, depending on the PC maturation level, and enabled us to perform long-term incubation studies for up to 30 days.
Fig. 1.
Cerebellar cortex structure in organotypic slice cultures. a Calbindin-positive PC (red), neurofilaments (green), and nuclei (blue) display the three-layered cortex structure, with organotypic organization of neuronal input and output. b Dendritic trees of PC extend into the molecular layer, while a line-shaped arrangement of PC-somata characterizes the Purkinje cell layer. Axons transit the granule cell layer. c PC-dendrites are covered with numerous dendritic spines, visualized by microinjection of pEYFP-tagged actin in single PC. Scale bars (a, b) 100 μm, (c) 10 μm
Progesterone increases dendritic length, cell area and soma size
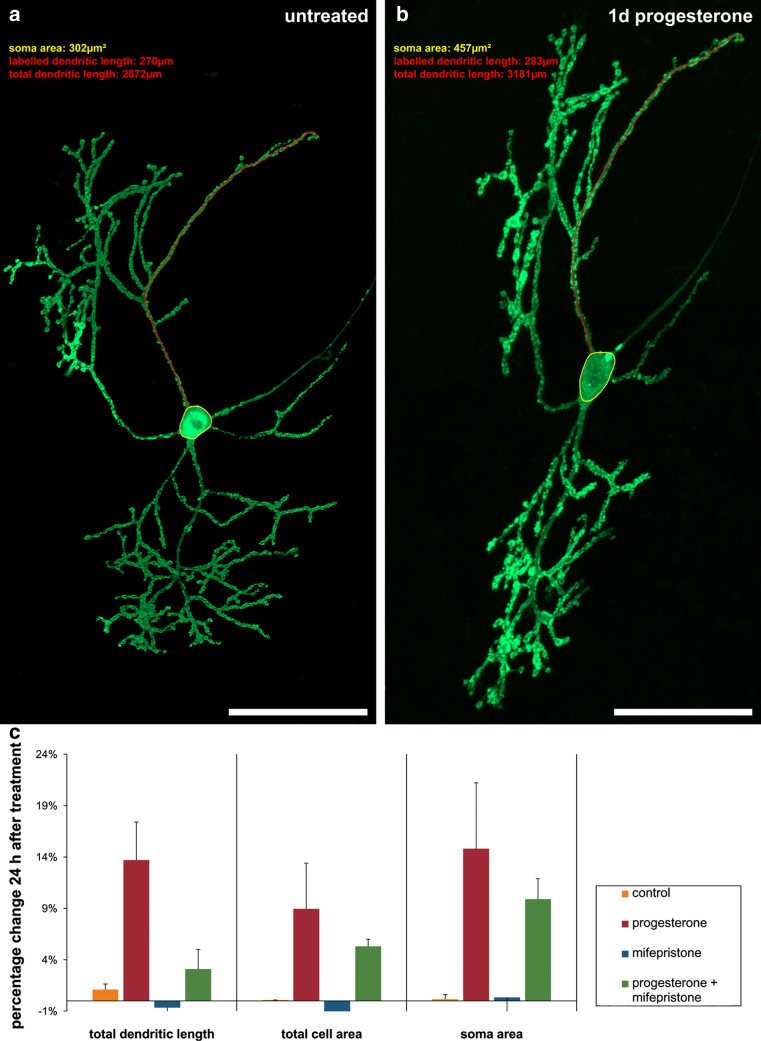
In order to visualize effects of progesterone on developing PC (15 div, group 2), we microinjected individual PC with pEYFP-actin. After taking pictures of single, untreated YFP-actin-transfected PC with the aid of CLSM (Fig. 2a), slice cultures were incubated with progesterone, mifepristone, or progesterone + mifepristone for 24 h. Thereafter, the very same PC were imaged again with identical recording parameters, to quantify the total dendritic length (red), soma size (yellow), and total cell area of each PC (Fig. 2b). Progesterone exposure induced a significant increase in total dendritic length of 13.7 %, in soma size of 14.8 %, and in the total cell area of 8.94 % (Fig. 2c). Incubation with mifepristone, a specific PR-A and PR-B antagonist, did not lead to any significant changes in these three parameters compared to controls (Fig. 2c). Incubation with a mixture of progesterone + mifepristone increased the dendritic length, soma size, and cell area compared to controls; however, all three parameters were diminished compared to progesterone-treated slices (Fig. 2c). Thus, progesterone is capable to enhance dendritogenesis in developing PC, probably through the interaction with PR-A and PR-B, as the progesterone effects are reduced by mifepristone. It is remarkable that mifepristone did not completely abolish the progesterone-induced effects on the PC morphology.
Fig. 2.
Morphometric analysis of progesterone effects on PC soma and dendrites. Morphometric analysis of total dendritic length (one dendrite exemplary labeled, red), total cell area and soma area (yellow) of transfected PC were done twice, before and after drug incubation for 24 h with the aid of a Zeiss LSM physiology kit. a Untreated PC (15 div) 24 h after transfection with YFP-actin (green). b The same PC after progesterone treatment for 1 day. c Changes of morphometric parameters are given in percent; error bars SD. All scale bars 50 μm
Progesterone increases spinogenesis and synaptogenesis in neonatal but not in mature PC
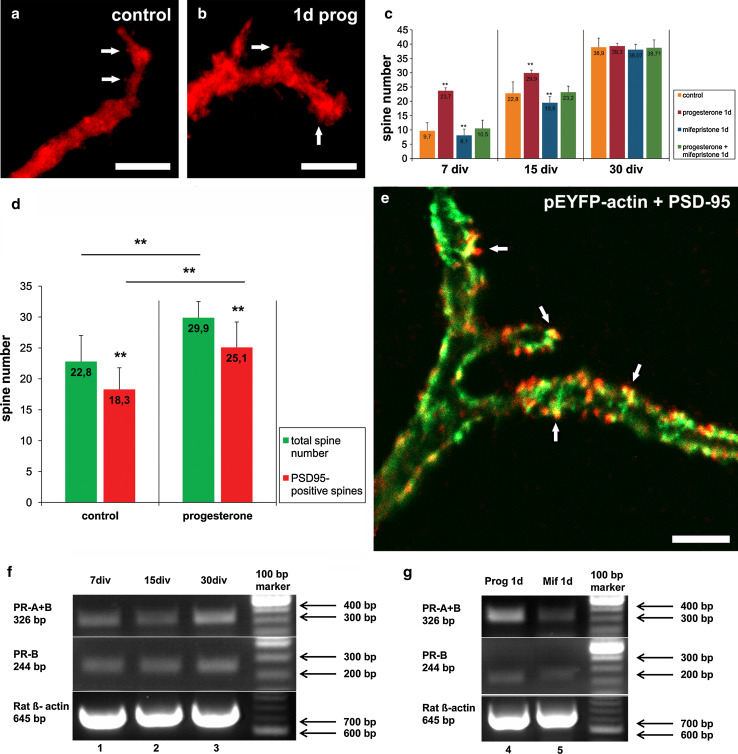
To investigate if progesterone also has an effect on the spine density in dependency to the development of the PC, we next analyzed cerebellar slices of different age (7 div, group 1; 15 div, group 2; 30 div, group 3). These different groups were treated with progesterone, mifepristone, or progesterone + mifepristone for 24 h, and thereafter spine numbers of PC (n = 360) were analyzed. Here, progesterone significantly increased the number of dendritic spines in group 1 (p < 0.001, n = 30) and group 2 (p < 0.001, n = 30) compared to controls (n = 30) (Fig. 3a–c), with a slightly higher increase in group 1 (Fig. 3c). In contrast, progesterone treatment in older slice cultures (group 3, n = 30) did not induce any significant differences in spine density compared with controls (p = 0.922, n = 30) (Fig. 3c). Thus, progesterone is capable of increasing spinogenesis in immature and young PC but not in mature PC. In addition, this mifepristone incubation for 24 h significantly decreased spine density in group 1 (n = 30) and group 2 (p < 0.001, n = 30) compared to controls (n = 30), while mifepristone had no significant effect on the spine density in group 3 (p = 0.219, n = 30) (Fig. 3c). Treatment with progesterone + mifepristone (n = 30) led to a significantly reduced spine number compared with the progesterone-treated PC in group 1 (p < 0.001, n = 30) and group 2 (p < 0.001, n = 30) (Fig. 3c), whereas spine density in group 3 was not affected (p = 0.889, n = 30) (Fig. 3c). Thus, mifepristone seems to abolish the age-related positive effects of progesterone on spinogenesis in PC.
Fig. 3.
Incubations for 1 day (1d). a, b Spine number in calbindin-labeled PC (red, 7 div) in controls (a) and after progesterone incubation for 1 day (b). c Quantitative analysis of PC spine number in cell cultures treated with various drugs for 1 day at different developmental ages; error bars SD. d Total spine number and spines number of PSD95-positive functional spines counted on the 40-μm dendritic tree in PC 15 after 15 div; error bars SD. e YFP-actin labeled dendritic spines (green) and PSD95-labeled functional synapses (red) are also co-localized after progesterone incubation. f Semi-quantitative reverse transcription-PCR analysis of the physiological PR-A and PR-B mRNA distribution in untreated cerebellar slices after 7 (1), 15 (2), and 30 (3) div. g Semi-quantitative reverse transcription-PCR of slice cultures treated with progesterone (4) and mifepristone (5) for 1 day (1d). White arrows indicate dendritic spines, black arrows the postsynaptic density. Scale bars: (a, b) 5 μm (e) 5 μm
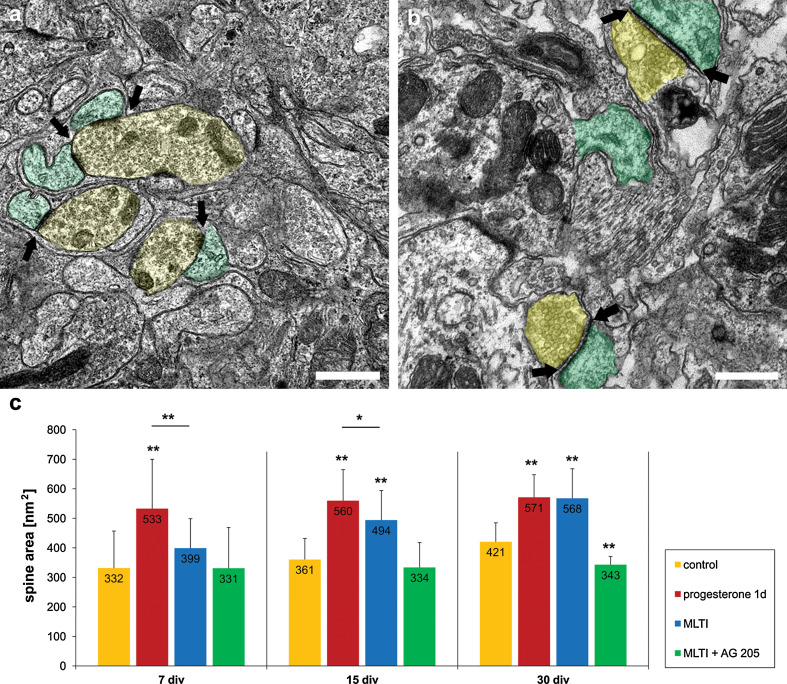
Next, it was investigated if progesterone-induced spinogenesis is accompanied by the formation of functional synapses. Indeed, using CLSM techniques, PSD-95 staining and pEYFP-actin were detectable, both co-localized within spines, indicating functional synapses (Fig. 3e). The total number of dendritic spines as well as the number of PSD-95-positive spines on the 40-μm dendritic tree were counted in progesterone-treated PC and controls (n = 10 in both groups) after 15 div. Interestingly, the amount of PSD-positive spines (85 %) did not change even after progesterone incubation (Fig. 3d, e). We confirmed theses data by electron microscopy, demonstrating numerous chemical synapses at the end of the spines after subsequent progesterone treatment for 1 day, indicating matured spines (Fig. 4a, b). Furthermore, a quantification of spine area before and after progesterone treatment was carried out (Fig. 4c). Here, a highly significant increase in the area of dendritic spines was detected in all three groups after incubation with progesterone compared to controls (p < 0.001, n = 21 in each group). Hence, these data clearly indicate that progesterone induces spinogenesis as well as synaptogenesis in developing PC; however, dendritic filopodia, which represent immature spines, could also be affected by progesterone.
Fig. 4.
Electron microscopic analysis of spine area. a, b Electron microscopic analysis of the dendritic spines revealed numerous chemical synapses, with synaptic vesicles inside. Presynaptic component with synaptic vesicles is shaded in yellow, postsynaptic component is shaded in green; arrows indicate the postsynaptic density. a Dendritic spines and synapses in controls after 15 days in vitro. b Spines and synapses after 24 h progesterone treatment in PC after 15 div. The area of dendritic spines and postsynaptic density are increased compared to controls. c Size of the dendritic spines measured in PC after 7, 15, and 30 div in controls and after progesterone incubation for 24 h, (mifepristone long-time incubation, MLTI) and MLTI plus AG 205; error bars SD. Scale bars (a, b) 1 μm
Expression of classical progesterone receptors in PC
By semi-quantitative PCR analysis the expression of classical progesterone receptor mRNA was studied in slice cultures after 7 (lane 1), 15 (lane 2), and 30 div (lane 3) (Fig. 3f). In accordance with further studies, PR-A and PR-B are expressed in all three groups [13] (Fig. 3f). Furthermore, the influence of progesterone and mifepristone incubation on the progesterone receptor mRNA concentration was analyzed (Fig. 3g). The results suggest that progesterone incubation (lane 4) especially increased PR-A expression compared to control (lane 1), while PR-B did not seem to be affected by progesterone incubation. In addition, PR-A and PR-B mRNA expression seemed not to be influenced by short-term (lane 5) treatment with mifepristone (Fig. 3g).
Does progesterone affects the cytoskeleton of PC?
Next we investigated if the classical progesterone receptors mediate some of the observed changes in the PC morphology by an interaction with the cytoskeleton. For this purpose, single PC of cerebellar slice cultures were microinjected with pEYFP-Tub vector in combination with pLife-Act Tag RFP (supplementary Fig. 1a) as well as GFP-NFM in combination with pLife-Act Tag RFP (supplementary Fig. 1b). CLSM as well as electron microscopy of rapid-freeze deep-etch specimens (supplementary Fig. 1c) revealed an accumulation of microtubules and neurofilamentes within the dendritic shaft, while RFP-labeled actin was located within dendritic spines. Furthermore, the localization of RFP-tagged actin-filaments within dendritic spines was verified by immunogold-staining (supplementary Fig. 1d). Gold-grains were localized at the actin filaments in the dendritic spine (green) close to the zone of postsynaptic density.
In order to demonstrate the role of actin filaments for spine-motility in regard to progesterone treatment, we performed time-lapse imaging. Indeed a rapid forward, backward, and sideward motion of dendritic spines in pEYFP-actin-labeled PC could be detected (supplementary Fig 2a), which is in line with earlier investigations [21]. To investigate if actin turnover is affected by progesterone, time-lapse imaging in combination with FRAP was performed with pEYFP-actin transfected PC 24 h after microinjection (supplementary Fig. 2b, c). Fluorescence recovery was monitored immediately after bleaching and quantified with aid of a Zeiss LSM physiology kit. In 20 different PC, a recovery of the pEYFP-fluorescence occurred within 4 min. Here, no transport of pEYFP-labeled actin could be observed, but recovery of fluorescence occurred within the spines after 4 min. The repetition of FRAP experiments in PC treated with progesterone or mifepristone for 24 h showed exactly the same FRAP pattern. The fluorescence recovery of YFP-actin remained unchanged compared to the untreated controls, with a fast recovery within 4 min. Taken together, progesterone seemed not to affect the actin turnover in PC, but nevertheless regulation of actin filaments by actin-binding proteins might be possible mechanisms of progesterone-induced spinogenesis and synaptogenesis.
Mifepristone long-term incubation (MLTI) increases spine density in immature and mature PC
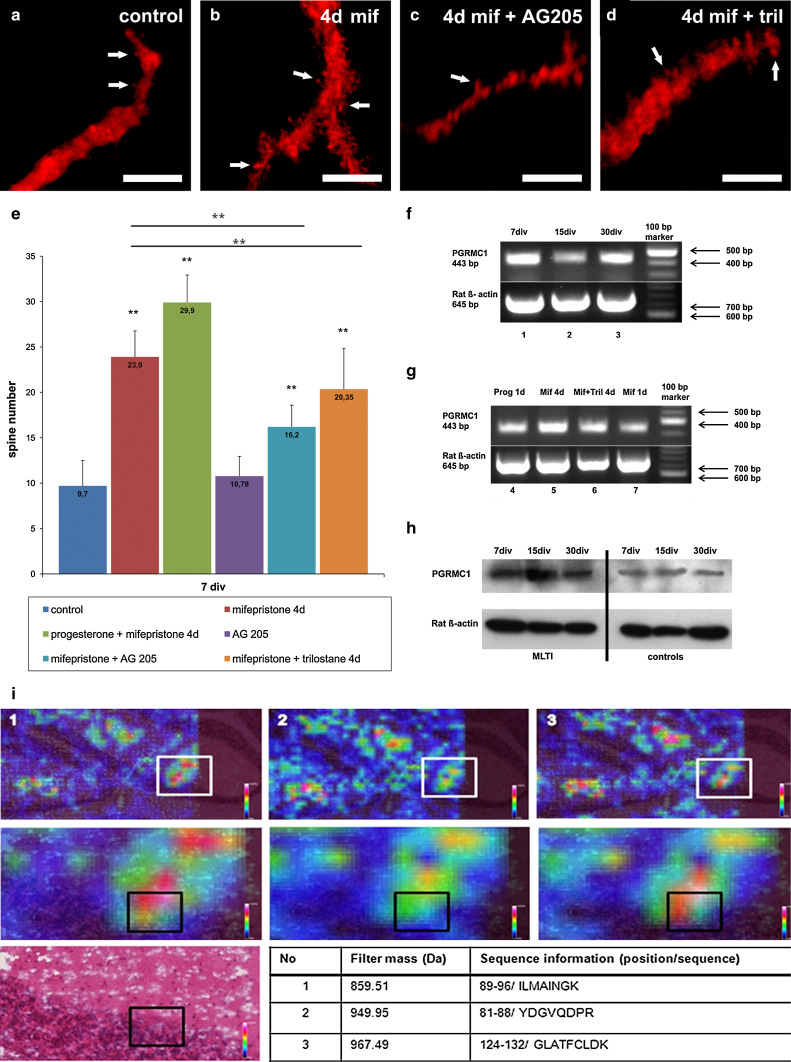
To verify if the observed effects after short-time incubation are gradable, roller-tube slices were incubated with progesterone, mifepristone, or progesterone + mifepristone for more than 72 h, starting with day 3 in vitro. Thereafter, spine density was analyzed in group 1 (treatment for 4 days, n = 30), group 2 (treatment for 12 days, n = 30), and group 3 (treatment for 27 days, n = 30) (Fig. 5a, b, e). The morphometric analysis revealed that long-time incubation (4 days, n = 30; 12 days, n = 30; 27 days, n = 30) with progesterone did not lead to any significant changes in the spine density compared to 1-day incubation with progesterone (data not shown). Surprisingly, MLTI led to a highly significant increase (p < 0.001) of spine density in all three groups (Fig. 5b, e). It is remarkable that MLTI spine density also increased in mature PC, which were not affected by progesterone (7 div: control 9.7 spines/40 μm, MLTI 23.9 spines/40 μm; 15 div: control 22.8 spines/40 μm; MLTI 29.2 spines/40 μm; 30 div: control 38.9 spines/40 μm; MLTI 42.7 spines/40 μm). Furthermore, spine morphology with a focus on spine area was electron microscopically analyzed after MLTI. Here, a highly significant increase in the spine area was detected in spines after 15 and 30 div after MLTI compared to controls (p < 0.001, n = 21 in each group) (Fig. 4c), while spine area after 7 div was not yet increased (p > 0.05, n = 21) (Fig. 4c). Spine area after progesterone and MLTI did not significantly differ in mature PC (p > 0.05), while spine area after progesterone was significantly higher in immature and young PC incubations compared to MLTI (7 div: p < 0.001, n = 21; 15 div p < 0.05, n = 21) (Fig. 4c). Additionally, effects of MLTI on spine number could be increased by combined treatment of progesterone + mifepristone in group 1 (n = 30, p < 0.001) and group 2 (n = 30, p < 0.001), while in group 3 no significant change in spine density could be detected (n = 30, p = 0.701), always compared to MLTI (Fig. 5e).
Fig. 5.
Long-time incubations. Spine number in calbindin-labeled PC (red, 7 div) in controls (a), after MLTI (b), MLTI combined with AG205 (c), and MLTI plus trilostane (d); 4d 4 days. e Quantitative analysis of PC spine number in cell cultures treated with mifepristone in combination with progesterone, AG205, and trilostane at different developmental ages. Relevant significances were added to the diagram; error bars SD. f Semi-quantitative reverse transcription-PCR analysis of the physiological PGRMC1-mRNA distribution in untreated cerebellar slices after 7 (1), 15 (2), and 30 (3) div. g Semi-quantitative reverse transcription-PCR of slice cultures treated with progesterone for 1 day (1d, 4), mifepristone for 4 days (4d, 5), mifepristone and trilostane for 4 days (4d, 6) and mifepristone for 1 day (1d, 7). h Western blot of slices after 7, 15, and 30 div after long-time incubation with mifepristone (MLTI) compared to controls. i Localization of PGRMC1 within PC, checked by MALDI imaging. The tissue was digested using trypsin and peptides of PGRMC1 were analyzed concerning their colocalization with PC. The peptide intensity in different areas of the cerebellum is visualized by colors according to the color bars depicted on each slide. The MALDI image is colocalized with the appropriate H&E stain. White rectangles enclose zoomed areas. 1, 2, and 3 correspond to three different peptide masses described in the table by mass, location, and sequence information (1-letter symbol for each amino acid). Black rectangles in the zoomed images and the H&E image: localization of a single PC. White arrows indicate dendritic spines. Scale bars (a–d) 5 μm
Inhibition of PGRMC1 prevents effects of MLTI
To examine whether PGRMC1 is involved in the effects observed after MLTI, slice cultures of each group were incubated with mifepristone and AG205, which functions as a specific blocker of PGRMC1. Thereafter, spine number was counted in group 1 (treatment for 4 days, n = 30), group 2 (treatment for 12 days, n = 30), and group 3 (treatment for 27 days, n = 30). MLTI plus AG205 resulted in a significant reduced spine density compared to single MLTI (p < 0.001) (Fig. 5c, e). Nevertheless, spine number was still significantly higher than in controls (p < 0.001) (Fig. 5e). These results were confirmed by electron microscopic analysis of spine area after MLTI combined with AG 205 (Fig. 4c). Here, in PC after 7 and 15 div, no significant differences could be detected compared to controls (p > 0.05, n = 21), while in mature PC, a highly significant decrease in spine area was measured compared to controls (p < 0.001, n = 21) (Fig. 4c). Thus, AG205 seems to prevent mifepristone-induced increase in spine density.
Furthermore, the effect of single AG205 incubation was also studied in all three groups [group 1 (treatment for 4 days, n = 30), group 2 (treatment for 12 days, n = 30) and group 3 (treatment for 27 days, n = 30)]. Here, no significant changes in spine number were detectable compared to controls (p > 0.05) (Fig. 5e).
Effects of MLTI are diminished when 3β-HSD is antagonized
As PGRMC1 was previously discussed to influence neurosteroidogenesis, a specific antagonist of the 3β-HSD, trilostane, was applied to cultures of all three groups in combination with mifepristone. Thereafter, spine number was counted in group 1 (treatment for 4 days, n = 30), group 2 (treatment for 12 days, n = 30), and group 3 (treatment for 27 days, n = 30). Analysis of the spine number after treatment with mifepristone plus trilostane revealed a significantly decreased spine density in all three groups compared to MLTI (p < 0.001) (Fig. 5d, e). Nevertheless, the spine number was still significantly higher than in controls (p < 0.001).
MLTI seems to enhance PGRMC1 expression
By semi-quantitative PCR analysis, the expression of PGRMC1-mRNA was studied in PC after 7, 15, and 30 div (Fig. 5f). In addition to previous studies [13, 17], PGRMC1-mRNA could be shown to be expressed in all three groups. Furthermore, the mRNA expression of PGRMC1 was additionally studied after incubation with progesterone for 1 day (lane 4), MLTI (lane 5), MLTI in combination with trilostane (lane 6), and mifepristone incubation for 1 day (lane 7) in cerebellar slice cultures after 7 div (Fig. 5g). The semi-quantitative PCR analysis indicates an increase in the PGRMC1-mRNA amount after MLTI compared to a single incubation with progesterone or mifepristone for 1 day, while PGRMC1 mRNA expression does not seem to be influenced by single incubation with progesterone or mifepristone for 1 day (Fig. 5g). Western blot analysis after MLTI also indicates an increase in PGRMC1 amount on protein level compared to PGRMC1 protein level in untreated controls (Fig. 5h). To confirm the PRGMC1 expression in mature PC, we performed matrix-assisted laser desorption/ionization imaging mass spectrometry (MALDI IMS) (Fig. 5i). For this purpose, we measured a 10-μm cryosection from a mature rat cerebellum. We compared the theoretical masses of PRGMC1 with the distribution of those masses within the measured tissue (supplementary table 1). The spectra were acquired in a spot grid of 50-μm center-to-center spacing and a Hematoxylin–Eosin-stained image of the same slide was co-registered after measurement. The intensity of PRGMC1 was high in the PC-layer as well as in the ML, in which the dendrites of PC arise (Fig. 5i).
Discussion
Although progesterone and its impact on neuronal tissues are of increasing scientific and therapeutic interest, our knowledge of progesterone effects in the central nervous system (CNS) is still incomplete. As the influence of progesterone on neonatal cerebellar PC has already been the subject of different studies, an involvement of progesterone in the physiological maturation process of the cerebellum was anticipated [11, 12]. But the impact of progesterone on adult PC has not yet been analyzed; nevertheless, different studies have demonstrated neuroprotective effects of progesterone in the matured CNS, for example after traumatic brain injury [24–26]. Furthermore, our understanding of underlying signaling cascades and involved progesterone receptors is still deficient. In our current study, the effects of progesterone on immature as well as on mature PC and changes in the progesterone receptor distribution were analyzed in organotypic cerebellar roller-tube slice cultures.
By labeling single PC in these organotypic cerebellar slice cultures by intracellular microinjection, it was possible to measure soma size, total cell area, and dendritic length before and after progesterone administration in the very same neuron. This procedure allowed us a direct quantification of progesterone-induced changes on PC morphology. Indeed, the increase in all three parameters indicates a direct stimulating effect of progesterone on dendritogenesis in developing PC. Our data are in accordance with an in vivo and in vitro study of Sakamoto and colleagues, who compared progesterone-treated PC to untreated controls [11]. As, to our knowledge, no study has analyzed exogenous progesterone effects on mature PC up to now, spine numbers in immature as well as in mature PC were counted following progesterone incubation. With regard to other studies, in immature PC spine density increased after incubation with progesterone for 1 day, as described by Sakamoto and colleagues, who directly proved an increase in synapse number in developing PC after progesterone incubation [11]; furthermore, synaptogenesis is increased by progesterone. These results were confirmed by our results, which demonstrated an equal amount of PSD-95-positive spines in PC of controls and progesterone-incubated cerebellar slices. With the aid of electron microscopy, we additionally demonstrated that dendritic spines are also completely integrated into the functional cerebellar network after progesterone treatment. Moreover, the current study was first able to detect a direct stimulating influence of progesterone on spine area. Thus, it can be assumed that progesterone increases not only dendritic length and spine number but also spine area in developing PC. From a therapeutic point of view, however, the influence of progesterone on mature PC would be of significant interest. The presented data on mature PC demonstrated for the first time that the effects of progesterone seem to depend on the grade of maturation of PC at the beginning of progesterone treatment. In the present study, spine density in mature PC was not affected by exogenous progesterone. Thus, in mature PC, which are physiologically exposed to very low concentrations of progesterone, even a high concentration of exogenous-admitted progesterone seems not to be able to induce any further significant effects on spine density. In consequence, it is likely that progesterone sensitivity of PC is age-dependent, with the highest response to progesterone in the neonate. These results are in accord with the physiological expression of progesterone in PC, as several studies have demonstrated an age-dependent expression of certain progesterone-synthesis catalyzing proteins in PC [4, 6, 7, 27]. Nevertheless, neurosteroidogenesis within the CNS seems to be comparable to the steroidogenesis in peripheral organs (Fig. 6a). The expression of cytochrome P450 enzyme is age-independent, without any significant changes during the neonatal period and adulthood [6]. Thus, pregnenolone as the initial substrate for all neurosteroids is expressed in constantly high concentrations from the beginning of cerebellar differentiation to adulthood [6, 28]. In contrast, the 3β-HSD mRNA reveals an age-dependent expression with maximal amounts during development and low expression levels in adulthood. In agreement with the enzyme activity, progesterone concentration in PC reaches its maximum during the developmental period [7]. Thus, the ability to induce PC growth and maturation by exogenous progesterone directly correlates with the physiological expression of progesterone and seems not to be prolonged into adulthood.
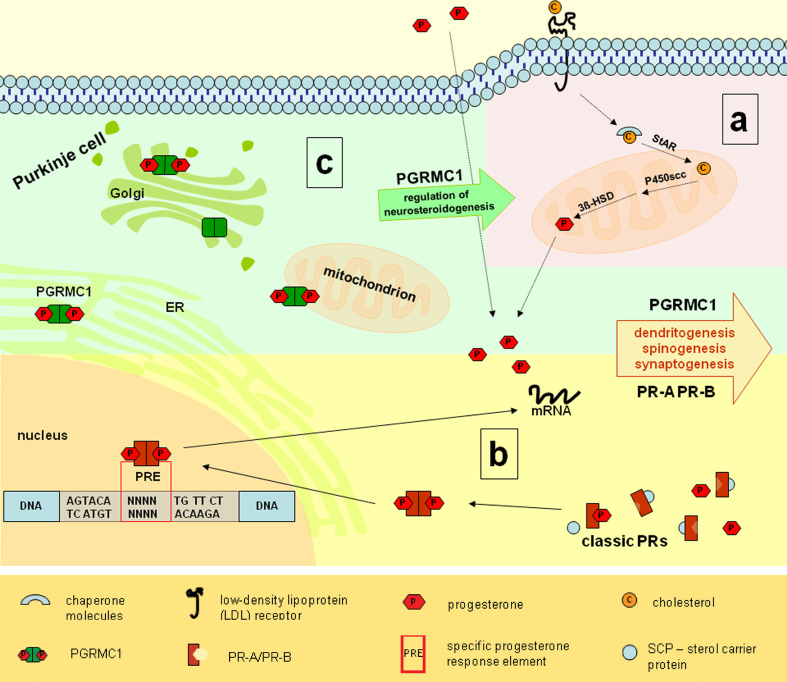
Fig. 6.
Neurosteroidogenesis and progesterone receptor mechanisms in PC. a Endogen progesterone synthesis shaded in red. Cholesterol is incorporated into PC with aid of LDL-receptors, linked to sterol carrier proteins and transported through the outer mitochondrial layer by StAR protein. P450scc promotes the synthesis of pregnenolone. Thereafter, biosynthesis of progesterone is completed by dehydrogenation and isomerization of pregnenolone, catalyzed by 3β–HSD [4, 6, 7]. b Classical progesterone receptor mechanisms shaded in yellow. Classical progesterone receptors (PR-A and PR-B) are localized in the cytoplasm and linked to chaperone molecules. Upon progesterone binding, receptors dissociate from chaperones, dimerize, and translocate to the nucleus. Within the nucleus, the PR-complex is known to modulate the transcription through interaction with specific progesterone-response elements (PRE) [14, 58]. Through this genomic mechanisms PR-A and PR-B are linked to dendritogenesis, spinogenesis, and synaptogenesis. c PGRMC1 localization and mechanisms shaded in green. PGRMC1 is associated to membranes of the Golgi-apparatus, the endoplasmatic reticulum, and mitochondria [17, 37, 38]. PGRMC1 is likely to be involved in the regulation of neurosteroidogenesis, the cerebellar maturation process, and neuroprotective mechanisms [18, 37]. Our results indicate that PGRMC1 is involved in the maturation of cerebellar PC by increasing the endogeneous progesterone synthesis through a stimulated activation of neurosteroidogenesis
It is of significant interest how these progesterone effects are mediated within PC. Basically, the classical progesterone receptors have been discussed in context with developmental processes of the cerebellum [11, 12, 18, 29]. In accordance with previous studies, progesterone-induced effects on spine density in young PC were not detectable when progesterone treatment was combined with mifepristone [11, 12]. Therefore, it is likely that mifepristone completely antagonizes the observed progesterone effects through inhibition of classical progesterone receptors. These data indicate that, even if mifepristone has a combined anti-progesterone and anti-glucocorticosteroid activity, here anti-progesterone functions of mifepristone overbalance the inhibition of glucocorticosteroid receptors [30–32]. Nevertheless, it was shown that both glucocorticoid (GR) and mineralocorticoid (MR) receptors are expressed in developing as well as in adult PC [33, 34]. But it was also demonstrated that mifepristone-induced inhibition of GR is dose-dependent, thus GR are probably not affected by the single dose used of 2 μM mifepristone [32]. Therefore, it can be assumed that progesterone induces spinogenesis and dendritogenesis via the classical progesterone receptors. This is supported by a semi-quantitative PCR analysis which indicates an increase in PR-mRNA amount subsequent to progesterone treatment. It is conceivable that cognate progesterone receptors, which are classically defined as ligand-activated transcription factors, promote dendritic growth, spinogenesis, and synaptogenesis in developing PC through genomic mechanisms (Fig. 6b). But up to now, there is only sparse information about the relationships of PR activation and PC growth and differentiation, which are likely to be mediated by cytoskeletal proteins. As demonstrated by CLSM and electron microscopy, microtubules and neurofilaments are the elemental structures of the soma and dendrites of PC, while actin filaments are mainly localized within the dendritic spines. In previous studies, it was shown that actin polymerization in dendritic spines is essential for their shape, function, and motility [21]. Furthermore, actin dynamics have been discussed to control dendritic spine development and remodeling; therefore, it is most reasonable that progesterone-induced effects are mediated by changes in the configuration of the actin cytoskeleton [35]. As regulation of the local protein biosynthesis in dendritic spines would enable PC to respond very quickly to internal and external stimuli, we supposed that progesterone, as such a stimulator, might affect the rate of local protein biosynthesis in developing PC. However, the results of our FRAP experiments do not verify this hypothesis, as FRAP in progesterone-treated PC remained unchanged in comparison to FRAP in controls. But, conceivably, progesterone does modulate the local protein biosynthesis in PC anyway. Possibly, our FRAP experiments were not able to detect progesterone-induced changes, for example because progesterone just increases the amount of actin monomers without affecting the measured rate of translation. Hence, further experiments are necessary to clarify this issue. Above these possible genomic mechanisms of progesterone, non-genomic mechanisms mediated by progesterone receptors have been demonstrated in other tissues. Here, a direct interaction of progesterone receptors with the actin cytoskeleton in neuronal tissues was demonstrated due to interactions with different enzyme cascades activating actin-binding proteins [35]. Thus, progesterone certainly interacts with the cytoskeleton of PC though whether by genomic or by non-genomic mechanisms needs to be studied in further experiments.
Non-genomic progesterone-induced mechanisms might even be the basis of the detected paradoxical increase in spine density after MLTI. As our data indicate that the enhancing effect of MLTI also affects matured PC, it could be of great therapeutic interest to find the underlying molecular basis. Since classical progesterone receptors are antagonized by mifepristone, it is likely that another progesterone receptor transmits the observed increase in spinogenesis, one which is not directly affected by mifepristone. Sakamoto and co-workers indeed described another progesterone-binding protein which is expressed in PC [17, 18]. This progesterone-binding protein was first described as 25-Dx in the rat liver, while it is today better known as PGRMC1 [36, 37]. PGRMC1 is part of a multi-protein complex, associated with the membranous structures of the endoplasmatic reticulum and the Golgi apparatus in PC [18]. In HeLa cells, an association with mitochondria has also been previously shown [38] (Fig. 6c). However PGRMC1 seems to bind to progesterone and other steroids, it is not thought to be a typical progesterone receptor, firstly because it does not have a structural homology with steroid receptors, and secondly because purified PGRMC1 does not bind to progesterone [37, 39–41]. Because PGRMC1 does not have structural homologies to the classical progesterone receptors, mifepristone is not able to directly affect this protein. PGRMC1 seems thus to be the most likely mediator of the observed paradoxical mifepristone effects. Indeed, we could prove the expression of PGRMC1 in the PC of our cerebellar slice cultures. Furthermore, our data assume that PGRMC1 is involved in the promotion of spinogenesis induced by MLTI, as this effect is significantly decreased when mifepristone treatment is combined with a PGRMC1 antagonist. Thus, it is likely that PGRMC1 is involved in the mediation of the observed increase in spine density after MLTI.
A progesterone-induced up-regulation of PGRMC1-mRNA has been demonstrated after traumatic injury in the rat spinal cord [42], and, in the hypothalamus of knock-out mice lacking the classical progesterone receptors, an increased PGRMC1 concentration was observed [43]. Furthermore, a cyclic progesterone exposure has been shown to increase PGRMC1 in hippocampus [44]. We thus hypothesize that PGRMC1 and PR-A and PR-B physiologically compete for progesterone, which typically binds to the classical progesterone receptors. If PR-A and PR-B are antagonized by MLTI, progesterone might increasingly bind to PGRMC1 and, in consequence, lead to an up-regulation of this membranous progesterone-binding protein. Due to this, the observed effect of MLTI might be caused by an up-regulation of PGRMC1 induced by endogenous progesterone. Indeed both PCR and western blot analyses indicate an increase in PGRMC1-mRNA and -protein amounts after MLTI compared to progesterone or mifepristone incubation for 1 day. To further support this hypothesis, spine density and spine area were decreased when mifepristone treatment was combined with the PGRMC1-receptor antagonist, AG205. Thus, progesterone probably does not just mediate effects through the classical progesterone receptors but may also activate PGRMC1. Taken together, the presented data indicate that the effects observed after MLTI treatments are due to a progesterone-induced up-regulation of PGRMC1. It is remarkable that the effects do not seem to be restricted to immature PC, as we also measured a significantly increased spine-density after MLTI in mature PC. For this reason, the expression of PGRMC1 in mature rat cerebella was analyzed by MALDI-IMS. Also in mature PC, a clear expression of PGRMC1 was detectable within the somata and the dendritic arbors.
With regard to these results, the questions arise how PGRMC1 acts on PC and what function does it have for them. In other neuronal tissues, PGRMC1 is described to be involved in female reproductive behavior, in axonal pathfinding, or in maintaining the water homeostasis after traumatic brain injury, but less is known about its function in the cerebellum [43, 45, 46]. As in other organs and brain regions, PGRMC1 homologues were shown to interact with cytochrome P450 enzymes, and an involvement in the regulation of neurosteroidogenesis might also be conceivable [37, 47, 48]. Laird et al. [49] demonstrated that the steroid synthesis was inhibited when monoclonal antibodies against PGRMC1 were applied to rat adrenals. Additionally, an enhancement of the 21-hydroxylase was demonstrated, when Cyp21 and PGRMC1 were coexistent [40]. In PC, this might imply that a progesterone-induced up-regulation of PGRMC1 increases the activity of the cytochrome P450scc and 3β-HSD, which in turn raises the internal progesterone concentration. To verify this hypothesis, we employed trilostane as a blocker of 3β-HSD. Previous studies have revealed a decreased spine number after trilostane incubation due to the inhibition of the internal progesterone synthesis [12]. Indeed, the incubation of PC with mifepristone plus trilostane led to a decreased number of dendritic spines compared to MLTI. Therefore, the increased spine number after MLTI might be explained by an increased progesterone synthesis due to an up-regulation of PGRMC1. These results are underscored by semi-quantitative PCR analysis, which indicates decreased mRNA levels of PGRMC1 after MLTI combined with trilostane. Thus, we anticipate that MLTI induces spinogenesis by a PGRMC1-associated increase of endogenous progesterone through an activation of 3β-HSD activity. This hypothesis is in accordance with different other studies. In 2003, it was demonstrated that mifepristone protects neonatal PC from the developmental apoptotic process, independent of the classical progesterone receptors [50]. Also, a protective effect of mifepristone in CA1 hippocampal neurons after traumatic brain injury has been shown [51]. But, coincidentally, further studies suggest that a PGRMC1-associated induction of the neurosteroidogenesis seems not to be the only mechanism employed by PGRMC1. Thus, a progesterone-induced proliferation of adult neuronal progenitor cells in the hippocampus has been shown to be related to PGRMC1, probably through neudesin-dependent stimulation of MAPK and Akt [52, 53]. In addition, Singh described a neuroprotective effect of progesterone in various insults by phosphorylation of Akt and ERK, which could not be blocked by mifepristone [54]. Here, especially a non-genomic modulation of the actin cytoskeleton might be conceivable. Our data also indicate additional non-genomic mechanisms. On the one hand, the observed effects after MLTI could not be completely inhibited by AG205, and on the other hand, the spine number was still significantly higher after incubation with mifepristone plus trilostane compared to controls. As a possible explanation, either additional mechanisms or a PGRMC1-induced increase in other neurosteroids might be conceivable. Thus, PGRMC1 probably does not only increase progesterone synthesis but also the activity of other steroidogenic enzymes, which are not affected by trilostane. As shown in previous studies, estrogen also induces spinogenesis in developing PC and might be responsible for the higher spine density in PC after trilostane and mifepristone treatment [13, 55].
Furthermore, it has to be noted that, in addition to the PGRMC1, another progesterone-binding protein, 7TMPRβ, has also been shown to be expressed in cerebellar PC and might also be involved in some of the above-described effects [14]. Further experiments will thus be necessary to clarify whether 7TMPRβ is also involved in the mediation of progesterone effects on spinogenesis.
Even though our results strongly suggest that the observed effects after MLTI are due to an increased neurosteroidogenesis induced by PGRMC1, an involvement of GR might also be possible, which is also antagonized by mifepristone. As previous studies have revealed that repeated treatment of newborn mice with glucocorticoids resulted in an increased apoptosis of neuronal progenitor cells of the external granule layer, and in a lower cell number in the matured internal granule layer, a protective effect of MLTI for neuronal progenitor cells in cerebellum might also be possible [56]. But in our experimental set-up, mifepristone incubation started at a timepoint at which the external granule layer had already disappeared in the slice cultures. Furthermore, PC number did not seem to be affected by postnatal glucocorticoid administration [57]. Nevertheless, an involvement of GR in the observed effects should be tested in further studies.
In conclusion, we show that PC seem to have an age-dependent sensitivity for progesterone, with the highest progesterone response during neonatal development. In addition, we have gained new insights into the mechanisms of the progesterone receptors in PC. While progesterone effects that are mediated by genomic mechanisms of PR-A and PR-B seemed to be restricted to immature PC, PGRMC1-attributed non-genomic effects could also be measured in mature PC. Our data strongly indicate that progesterone mediates any effects through non-genomic mechanisms by activation of PGRMC1, and that an increase in neurosteroidogenesis is likely to be involved in the mediation of these effects.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(Supplementary Fig. 1 Distribution of the cytoskeletal proteins in PC (a, b) Microtubules (green) (a) and neurofilaments (green) (b) are localized in the dendrites and the soma, while the red-signal of actin-filaments is especially visualized in spines in the dendritic periphery. The white boxes indicate a region of interest in a higher magnification. c Deep-etch freeze-fracture of an organotypic slice culture along a dendrite, showing microtubules (blue) and mitochondria (red). d Within the dendritic spine (green) actin-filaments are in close contact to the postsynaptic density as shown by anti-RFP- immunogold-staining. White arrows indicate 5 nm gold-grains. Scale bars (a, b) 50 μm (c, d) 150 nm. TIFF 4301 kb)
(Supplementary Fig. 2 Time-lapse microscopy and FRAP in PC. a Spine motility is visualized in pEYFP-labeled PC 24 h after transfection, with backward and sideward motions of dendritic spines (white arrows). b YFP-labeled PC-dendrite 24 h after transfection. Representative images of FRAP along the dendrite (ROI) at 30-s intervals. c Fluorescence intensity along the bleached region (ROI 1, red) and in a non-bleached region (ROI 2, green) is measured in progesterone-treated and untreated PC with the aid of the Zeiss LSM physiology kit. Scale bars 5 μm. TIFF 1866 kb)
Acknowledgments
The authors gratefully thank C. Grzelak, A. Lodwig, H.-T. Nguyen and J. Elm for excellent technical assistance, as well as A. Lenz for secretarial work. C.Henkel was supported by PURE (Protein-research Unit Ruhr within Europe).
References
- 1.Baulieu EE, Robel P. Neurosteroids: a new brain function? J Steroid Biochem Mol Biol. 1990;37(3):395–403. doi: 10.1016/0960-0760(90)90490-C. [DOI] [PubMed] [Google Scholar]
- 2.Mellon SH, Deschepper CF. Neurosteroid biosynthesis: genes for adrenal steroidogenic enzymes are expressed in the brain. Brain Res. 1993;629(2):283–292. doi: 10.1016/0006-8993(93)91332-M. [DOI] [PubMed] [Google Scholar]
- 3.Compagnone NA, Bulfone A, Rubenstein JL, Mellon SH. Steroidogenic enzyme P450c17 is expressed in the embryonic central nervous system. Endocrinology. 1995;136(11):5212–5223. doi: 10.1210/endo.136.11.7588260. [DOI] [PubMed] [Google Scholar]
- 4.Furukawa A, Miyatake A, Ohnishi T, Ichikawa Y. Steroidogenic acute regulatory protein (StAR) transcripts constitutively expressed in the adult rat central nervous system: colocalization of StAR, cytochrome P-450SCC (CYP XIA1), and 3beta-hydroxysteroid dehydrogenase in the rat brain. J Neurochem. 1998;71(6):2231–2238. doi: 10.1046/j.1471-4159.1998.71062231.x. [DOI] [PubMed] [Google Scholar]
- 5.Tsutsui K, Ukena K, Usui M, Sakamoto H, Takase M. Novel brain function: biosynthesis and actions of neurosteroids in neurons. Neurosci Res. 2000;36(4):261–273. doi: 10.1016/S0168-0102(99)00132-7. [DOI] [PubMed] [Google Scholar]
- 6.Ukena K, Usui M, Kohchi C, Tsutsui K. Cytochrome P450 side-chain cleavage enzyme in the cerebellar Purkinje neuron and its neonatal change in rats. Endocrinology. 1998;139(1):137–147. doi: 10.1210/endo.139.1.5672. [DOI] [PubMed] [Google Scholar]
- 7.Ukena K, Kohchi C, Tsutsui K. Expression and activity of 3beta-hydroxysteroid dehydrogenase/delta5-delta4-isomerase in the rat Purkinje neuron during neonatal life. Endocrinology. 1999;140(2):805–813. doi: 10.1210/endo.140.2.6516. [DOI] [PubMed] [Google Scholar]
- 8.Tsutsui K. Biosynthesis and organizing action of neurosteroids in the developing Purkinje cell. Cerebellum. 2006;5(2):89–96. doi: 10.1080/14734220600697211. [DOI] [PubMed] [Google Scholar]
- 9.Berry M, Bradley P. The growth of the dendritic trees of Purkinje cells in the cerebellum of the rat. Brain Res. 1976;112(1):1–35. doi: 10.1016/0006-8993(76)90331-0. [DOI] [PubMed] [Google Scholar]
- 10.Altman J. Postnatal development of the cerebellar cortex in the rat. II. Phases in the maturation of Purkinje cells and of the molecular layer. J Comp Neurol. 1972;145(4):399–463. doi: 10.1002/cne.901450402. [DOI] [PubMed] [Google Scholar]
- 11.Sakamoto H, Ukena K, Tsutsui K. Effects of progesterone synthesized de novo in the developing Purkinje cell on its dendritic growth and synaptogenesis. J Neurosci. 2001;21(16):6221–6232. doi: 10.1523/JNEUROSCI.21-16-06221.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sakamoto H, Ukena K, Tsutsui K. Dendritic spine formation in response to progesterone synthesized de novo in the developing Purkinje cell in rats. Neurosci Lett. 2002;322(2):111–115. doi: 10.1016/S0304-3940(02)00077-0. [DOI] [PubMed] [Google Scholar]
- 13.Sakamoto H, Shikimi H, Ukena K, Tsutsui K. Neonatal expression of progesterone receptor isoforms in the cerebellar Purkinje cell in rats. Neurosci Lett. 2003;343(3):163–166. doi: 10.1016/S0304-3940(03)00362-8. [DOI] [PubMed] [Google Scholar]
- 14.Brinton RD, Thompson RF, Foy MR, Baudry M, Wang J, Finch CE, Morgan TE, Pike CJ, Mack WJ, Stanczyk FZ, Nilsen J. Progesterone receptors: form and function in brain. Front Neuroendocrinol. 2008;29(2):313–339. doi: 10.1016/j.yfrne.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu Y, Bond J, Thomas P. Identification, classification, and partial characterization of genes in humans and other vertebrates homologous to a fish membrane progestin receptor. Proc Natl Acad Sci USA. 2003;100(5):2237–2242. doi: 10.1073/pnas.0436133100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu Y, Rice CD, Pang Y, Pace M, Thomas P. Cloning, expression, and characterization of a membrane progestin receptor and evidence it is an intermediary in meiotic maturation of fish oocytes. Proc Natl Acad Sci USA. 2003;100(5):2231–2236. doi: 10.1073/pnas.0336132100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sakamoto H, Ukena K, Takemori H, Okamoto M, Kawata M, Tsutsui K. Expression and localization of 25-Dx, a membrane-associated putative progesterone-binding protein, in the developing Purkinje cell. Neuroscience. 2004;126(2):325–334. doi: 10.1016/j.neuroscience.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 18.Sakamoto H, Ukena K, Kawata M, Tsutsui K. Expression, localization and possible actions of 25-Dx, a membraneassociated putative progesterone-binding protein, in the developing Purkinje cell of the cerebellum: a new insight into the biosynthesis, metabolism and multiple actions of progesterone as a neurosteroid. Cerebellum. 2008;7(1):18–25. doi: 10.1007/s12311-008-0007-2. [DOI] [PubMed] [Google Scholar]
- 19.Meller K, Krah K, Theiss C. Dye coupling in Purkinje cells of organotypic slice cultures. Dev Brain Res. 2005;160:101–105. doi: 10.1016/j.devbrainres.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 20.Bashour NM, Wray S. Progesterone directly and rapidly inhibits GnRH neuronal activity via progesterone receptor membrane component 1. Endocrinology. 2012;153(9):4457–4469. doi: 10.1210/en.2012-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Theiss C, Meller K (2010) Microscopic techniques to study cytoskeletal dynamics in neurons. In: Méndez-Vilas A, Díaz J (eds) Microscopy: science, technology, applications and education. Formatex Research Center, Badajoz, pp 1091–1099
- 22.Theiss C, Napirei M, Meller K. Impairment of anterograde and retrograde neurofilament transport after anti-kinesin antibody microinjection in chicken dorsal root ganglia. Eur J Cell Biol. 2005;84(1):29–43. doi: 10.1016/j.ejcb.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 23.Meller K. Chromatolysis of dorsal root ganglion cells studied by cryofixation. Cell Tissue Res. 1989;256(2):283–292. doi: 10.1007/BF00218885. [DOI] [PubMed] [Google Scholar]
- 24.Grossman KJ, Goss CW, Stein DG. Effects of progesterone on the inflammatory response to brain injury in the rat. Brain Res. 2004;1008(1):29–39. doi: 10.1016/j.brainres.2004.02.022. [DOI] [PubMed] [Google Scholar]
- 25.Shear DA, Galani R, Hoffman SW, Stein DG. Progesterone protects against necrotic damage and behavioral abnormalities caused by traumatic brain injury. Exp Neurol. 2002;178(1):59–67. doi: 10.1006/exnr.2002.8020. [DOI] [PubMed] [Google Scholar]
- 26.Xiao G, Wei J, Yan W, Wang W, Lu Z. Improved outcomes from the administration of progesterone for patients with acute severe traumatic brain injury: a randomized controlled trial. Crit Care. 2008;12(2):R61. doi: 10.1186/cc6887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Do Rego JL, Seong JY, Burel D, Leprince J, Luu-The V, Tsutsui K, Tonon MC, Pelletier G, Vaudry H. Neurosteroid biosynthesis: enzymatic pathways and neuroendocrine regulation by neurotransmitters and neuropeptides. Front Neuroendocrinol. 2009;30(3):259–301. doi: 10.1016/j.yfrne.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 28.Robel P, Baulieu EE. Neuro-steroids: 3β-hydroxy-∆5-derivatives in the rodent brain. Neurochem Int. 1985;7(6):953–958. doi: 10.1016/0197-0186(85)90143-3. [DOI] [PubMed] [Google Scholar]
- 29.Sakamoto H, Mezaki Y, Shikimi H, Ukena K, Tsutsui K. Dendritic growth and spine formation in response to estrogen in the developing Purkinje cell. Endocrinology. 2003;144(10):4466–4477. doi: 10.1210/en.2003-0307. [DOI] [PubMed] [Google Scholar]
- 30.Gaillard RC, Riondel A, Muller AF, Herrmann W, Baulieu EE. RU 486: a steroid with antiglucocorticosteroid activity that only disinhibits the human pituitary-adrenal system at a specific time of day. Proc Natl Acad Sci USA. 1984;81(12):3879–3882. doi: 10.1073/pnas.81.12.3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bertagna X, Bertagna C, Luton JP, Husson JM, Girard F. The new steroid analog RU 486 inhibits glucocorticoid action in man. J Clin Endocrinol Metab. 1984;59(1):25–28. doi: 10.1210/jcem-59-1-25. [DOI] [PubMed] [Google Scholar]
- 32.Cadepond F, Ulmann A, Baulieu EE. RU486 (mifepristone): mechanisms of action and clinical uses. Annu Rev Med. 1997;48:129–156. doi: 10.1146/annurev.med.48.1.129. [DOI] [PubMed] [Google Scholar]
- 33.Ahima RS, Garcia MM, Harlan RE. Intracellular localization of corticosteroid receptors in brain: potential interactions with signal transduction pathways. Proc Soc Exp Biol Med. 1992;201(3):244–253. doi: 10.3181/00379727-201-43503B. [DOI] [PubMed] [Google Scholar]
- 34.Lawson A, Ahima RS, Krozowski Z, Harlan RE. Postnatal development of corticosteroid receptor immunoreactivity in the rat cerebellum and brain stem. Neuroendocrinology. 1992;55(6):695–707. doi: 10.1159/000126189. [DOI] [PubMed] [Google Scholar]
- 35.Ethell IM, Pasquale EB. Molecular mechanisms of dendritic spine development and remodeling. Prog Neurobiol. 2005;75(3):161–205. doi: 10.1016/j.pneurobio.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 36.Selmin O, Lucier GW, Clark GC, Tritscher AM, Vanden Heuvel JP, Gastel JA, Walker NJ, Sutter TR, Bell DA. Isolation and characterization of a novel gene induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin in rat liver. Carcinogenesi. 1996;17(12):2609–2615. doi: 10.1093/carcin/17.12.2609. [DOI] [PubMed] [Google Scholar]
- 37.Rohe HJ, Ahmed IS, Twist KE, Craven RJ. PGRMC1 (progesterone receptor membrane component 1): a targetable protein with multiple functions in steroid signaling, P450 activation and drug binding. Pharmacol Ther. 2009;121(1):14–19. doi: 10.1016/j.pharmthera.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu J, Zeng C, Chu W, Pan F, Rothfuss JM, Zhang F, Tu Z, Zhou D, Zeng D, Vangveravong S, Johnston F, Spitzer D, Chang KC, Hotchkiss RS, Hawkins WG, Wheeler KT, Mach RH. Identification of the PGRMC1 protein complex as the putative sigma-2 receptor binding site. Nat Commun. 2011;2:380. doi: 10.1038/ncomms1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mifsud W, Bateman A. Membrane-bound progesterone receptors contain a cytochrome b5-like ligand-binding domain. Genome Biol. 2002;3(12):RESEARCH0068. doi: 10.1186/gb-2002-3-12-research0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Min L, Strushkevich NV, Harnastai IN, Iwamoto H, Gilep AA, Takemori H, Usanov SA, Nonaka Y, Hori H, Vinson GP, Okamoto M. Molecular identification of adrenal inner zone antigen as a heme-binding protein. FEBS J. 2005;272(22):5832–5843. doi: 10.1111/j.1742-4658.2005.04977.x. [DOI] [PubMed] [Google Scholar]
- 41.Cahill MA. Progesterone receptor membrane component 1: an integrative review. J Steroid Biochem Mol Biol. 2007;105(1–5):16–36. doi: 10.1016/j.jsbmb.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 42.Labombarda F, Gonzalez SL, Deniselle MC, Vinson GP, Schumacher M, De Nicola AF, Guennoun R. Effects of injury and progesterone treatment on progesterone receptor and progesterone binding protein 25-Dx expression in the rat spinal cord. J Neurochem. 2003;87(4):902–913. doi: 10.1046/j.1471-4159.2003.02055.x. [DOI] [PubMed] [Google Scholar]
- 43.Krebs CJ, Jarvis ED, Chan J, Lydon JP, Ogawa S, Pfaff DW. A membrane-associated progesterone-binding protein, 25-Dx, is regulated by progesterone in brain regions involved in female reproductive behaviors. Proc Natl Acad Sci USA. 2000;97(23):12816–12821. doi: 10.1073/pnas.97.23.12816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao L, Morgan TE, Mao Z, Lin S, Cadenas E, Finch CE, Pike CJ, Mack WJ, Brinton RD. Continuous versus cyclic progesterone exposure differentially regulates hippocampal gene expression and functional profiles. PLoS ONE. 2012;7(2):e31267. doi: 10.1371/journal.pone.0031267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Runko E, Kaprielian Z. Expression of Vema in the developing mouse spinal cord and optic chiasm. J Comp Neurol. 2002;451(3):289–299. doi: 10.1002/cne.10356. [DOI] [PubMed] [Google Scholar]
- 46.Meffre D, Delespierre B, Gouézou M, Leclerc P, Vinson GP, Schumacher M, Stein DG, Guennoun R. The membrane-associated progesterone-binding protein 25-Dx is expressed in brain regions involved in water homeostasis and is up-regulated after traumatic brain injury. J Neurochem. 2005;93(5):1314–1326. doi: 10.1111/j.1471-4159.2005.03127.x. [DOI] [PubMed] [Google Scholar]
- 47.Craven RJ, Mallory JC, Hand RA. Regulation of iron homeostasis mediated by the heme-binding protein Dap1 (damage resistance protein 1) via the P450 protein Erg11/Cyp51. J Biol Chem. 2007;282(50):36543–36551. doi: 10.1074/jbc.M706770200. [DOI] [PubMed] [Google Scholar]
- 48.Hughes AL, Powell DW, Bard M, Eckstein J, Barbuch R, Link AJ, Espenshade PJ. Dap1/PGRMC1 binds and regulates cytochrome P450 enzymes. Cell Metab. 2007;5(2):143–149. doi: 10.1016/j.cmet.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 49.Laird SM, Vinson GP, Whitehouse BJ. Monoclonal antibodies against rat adrenocortical cell antigens. Acta Endocrinol (Copenh) 1988;119(3):420–426. doi: 10.1530/acta.0.1190420. [DOI] [PubMed] [Google Scholar]
- 50.Ghoumari AM, Dusart I, El-Etr M, Tronche F, Sotelo C, Schumacher M, Baulieu EE. Mifepristone (RU486) protects Purkinje cells from cell death in organotypic slice cultures of postnatal rat and mouse cerebellum. Proc Natl Acad Sci USA. 2003;100(13):7953–7958. doi: 10.1073/pnas.1332667100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McCullers DL, Sullivan PG, Scheff SW, Herman JP. Mifepristone protects CA1 hippocampal neurons following traumatic brain injury in rat. Neuroscience. 2002;109(2):219–230. doi: 10.1016/S0306-4522(01)00477-8. [DOI] [PubMed] [Google Scholar]
- 52.Kimura I, Nakayama Y, Yamauchi H, Konishi M, Miyake A, Mori M, Ohta M, Itoh N, Fujimoto M. Neurotrophic activity of neudesin, a novel extracellular heme-binding protein, is dependent on the binding of heme to its cytochrome b5-like heme/steroid-binding domain. J Biol Chem. 2008;283(7):4323–4331. doi: 10.1074/jbc.M706679200. [DOI] [PubMed] [Google Scholar]
- 53.Liu L, Wang J, Zhao L, Nilsen J, McClure K, Wong K, Brinton RD. Progesterone increases rat neural progenitor cell cycle gene expression and proliferation via extracellularly regulated kinase and progesterone receptor membrane components 1 and 2. Endocrinology. 2009;150(7):3186–3196. doi: 10.1210/en.2008-1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Singh M. Ovarian hormones elicit phosphorylation of Akt and extracellular-signal regulated kinase in explants of the cerebral cortex. Endocrine. 2001;14(3):407–415. doi: 10.1385/ENDO:14:3:407. [DOI] [PubMed] [Google Scholar]
- 55.Sasahara K, Shikimi H, Haraguchi S, Sakamoto H, Honda S, Harada N, Tsutsui K. Mode of action and functional significance of estrogen-inducing dendritic growth, spinogenesis, and synaptogenesis in the developing Purkinje cell. J Neurosci. 2007;27(28):7408–7417. doi: 10.1523/JNEUROSCI.0710-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Noguchi KK, Walls KC, Wozniak DF, Olney JW, Roth KA, Farber NB. Acute neonatal glucocorticoid exposure produces selective and rapid cerebellar neural progenitor cell apoptotic death. Cell Death Differ. 2008;15(10):1582–1592. doi: 10.1038/cdd.2008.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maloney SE, Noguchi KK, Wozniak DF, Fowler SC, Farber NB. Long-term Effects of Multiple Glucocorticoid Exposures in Neonatal Mice. Behav Sci (Basel) 2011;1(1):4–30. doi: 10.3390/behavsci1010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Truss M, Beato M. Steroid hormone receptors: interaction with deoxyribonucleic acid and transcription factors. Endocr Rev. 1993;14(4):459–479. doi: 10.1210/edrv-14-4-459. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(Supplementary Fig. 1 Distribution of the cytoskeletal proteins in PC (a, b) Microtubules (green) (a) and neurofilaments (green) (b) are localized in the dendrites and the soma, while the red-signal of actin-filaments is especially visualized in spines in the dendritic periphery. The white boxes indicate a region of interest in a higher magnification. c Deep-etch freeze-fracture of an organotypic slice culture along a dendrite, showing microtubules (blue) and mitochondria (red). d Within the dendritic spine (green) actin-filaments are in close contact to the postsynaptic density as shown by anti-RFP- immunogold-staining. White arrows indicate 5 nm gold-grains. Scale bars (a, b) 50 μm (c, d) 150 nm. TIFF 4301 kb)
(Supplementary Fig. 2 Time-lapse microscopy and FRAP in PC. a Spine motility is visualized in pEYFP-labeled PC 24 h after transfection, with backward and sideward motions of dendritic spines (white arrows). b YFP-labeled PC-dendrite 24 h after transfection. Representative images of FRAP along the dendrite (ROI) at 30-s intervals. c Fluorescence intensity along the bleached region (ROI 1, red) and in a non-bleached region (ROI 2, green) is measured in progesterone-treated and untreated PC with the aid of the Zeiss LSM physiology kit. Scale bars 5 μm. TIFF 1866 kb)