Abstract
Directional cell migration is required for proper embryogenesis, immunity, and healing, and its underpinning regulatory mechanisms are often hijacked during diseases such as chronic inflammations and cancer metastasis. Studies on migratory epithelial tissues have revealed that cells can move as a collective group with shared responsibilities. First thought to be restricted to proper epithelial cell types able to maintain stable cell–cell junctions, the field of collective cell migration is now widening to include cooperative behavior of mesenchymal cells. In this review, we give an overview of the mechanisms driving collective cell migration in epithelial tissues and discuss how mesenchymal cells can cooperate to behave as a collective in the absence of bona fide cell–cell adhesions.
Keywords: Collective cell migration, Epithelium-to-mesenchyme transition, Contact-inhibition of locomotion, Chemotaxis, Mechanosensing, Mesoderm, Neural crest cells, Cancer
Introduction
Collective cell migration is the coordinated migration of a group of cells during which cells are influenced by interactions with their neighbors [1–3]. Collective movements have been observed during development, wound healing, and invasion of cancer cells [2–4].
What kind of interactions are required or sufficient to promote collectiveness remains open for debate, as some authors argue that maintenance of stable physical links between cells is a pre-requisite for collective migration [3]. Others, on the other hand, have suggested a broader definition including all large cohorts of cells migrating within the same space during a given period of time [2]. The latter definition potentially opens the field of collective migration to all migratory cells that are not solitary whereas the former only includes epithelial-like cells. None of these definitions are fully satisfactory, since cooperation and collectiveness have been experimentally demonstrated in some mesenchymal cells [5–7], suggesting that collectiveness cannot be solely assessed by looking at the stability of cell–cell junction or at which density cells travel together.
Therefore, it is important to note that the simple observation of a large number of mesenchymal cells actively migrating in a given region does not imply collectiveness, as cells may simply ignore each other. Therefore, for collectiveness to be assessed, a detailed analysis of cell behavior has to be performed. The movement of the whole cell population should be compared with the behavior of a cell within the group and that of isolated cells regardless of the dynamics of cell–cell interactions that are taking place. Parameters such as velocity, persistence, and cell polarity should be used to make the comparison (discussed in [1]).
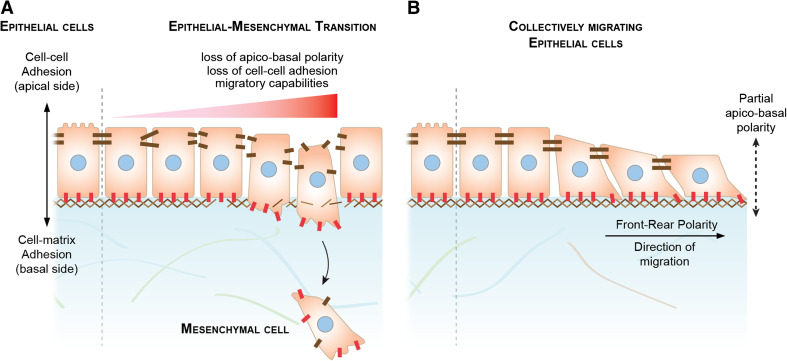
Mesenchymal cells are produced by an epithelial-mesenchymal transition (EMT) (Fig. 1a). During EMT, stable cell–cell junctions are disassembled, apico-basal polarity is lost, and migratory capabilities are enhanced [8]. Since mesenchymal cells do not maintain long-lasting cell–cell adhesion, they were considered to have a solitary behavior by default. An archetypal mesenchymal cell would be self-propelled and its guidance would only depend on external cues present in its direct environment such as attractants, repellents, and availability of extracellular matrix.
Fig. 1.
Epithelial-mesenchymal transition and epithelial collective migration. a Epithelial-mesenchymal transition (EMT). EMT is a complex, non-linear multi-step process that, when completed, converts epithelial cells into archetypal mesenchymal cells. Epithelial cells have a clear apico-basal polarity with cell–matrix adhesion at their basal side and cell–cell adhesion at their apical side. EMT includes: loss of apico-basal polarity; loss of cell–cell adhesion, and acquisition of cell motility. Each of these aspects is controlled by an array of transcription factors and can, to some extent, be regulated independently. Despite having no cell–cell adhesions, mesenchymal cells often express cell–cell adhesion molecules at their cell membrane. Cell–matrix and cell–cell adhesion molecules are shown in red and brown, respectively. b Collective migration of epithelial cells. When epithelial cells undergo collective migration, they maintain part of their epithelial characteristics. For instance, they maintain stable cell–cell junctions throughout migration and part of their apico-basal polarity. Such an intermediate phenotype is often called metastable
Epithelial-mesenchymal transition (EMT) is often seen as a linear process that starts with a loss of stable junctions allowing motility to emerge. However, it is now clear that EMT is a complex, reversible, non-linear, multiple-step operation that needs not to be systematically completed [4, 8–10]. For instance, cells can acquire motile properties while maintaining cell–cell adhesion and a remnant of apico-basal polarity, adopting a metastable state as seen during wound healing, vascular remodeling, or mammary gland branching [8, 11–14] (Fig. 1). In addition, cells that eventually complete EMT do not necessarily disassemble their cell–cell junctions at the onset of migration. Some cells rupture their cell body leaving pieces behind and most mesenchymal cells maintain cell–cell adhesion molecules at their surface throughout migration long after they have separated from their neighbors [6, 15–23].
Thus, it is clear that there is a gradation of phenotypes between a pure epithelial cell type, with its full array of cell–cell adhesions [adherens junctions (AJs), tight junctions (TJs), desmosomes, gap junctions] and a typical solitary mesenchymal cell type with no cell–cell junctions (Fig. 1a). Consequently, mesenchymal cells have the potential to interact with each other, even though transiently, and such interactions may be sufficient for cooperation and collective behavior to emerge.
In this review, we will first present the mechanisms controlling collective movement in epithelial tissues where collectiveness depends on stable cell–cell adhesion and is thus more readily observable. We will then address the specific conditions under which collectiveness can arise in mesenchymal cells.
Collective cell migration in epithelial tissues
Migratory epithelial cells maintain stable cell–cell junctions throughout migration (Figs. 1b, 2a). Although some cell types such as branching mammary gland cells or sprouting vascular cells do keep TJs during migration [8], the common structure observed in all examples of collectively migrating epithelial cells described so far is the cadherin-based AJ. Formation of AJs has been thoroughly documented and reviewed [24–27]. Briefly, when two epithelial cells make contact, they probe each other using Rac1-driven lamellipodia. These protrusions bring cadherins from the two adjacent cells in contact to form primitive AJs. The lamellipodia quickly collapse at their original touching point and progress sideways to enlarge the area of interaction [28, 29]. Small GTPases activities have to be finely regulated to keep contractility at intermediate levels; too low and the contact will quickly disassemble, and too strong and it will be disrupted [29–32].
Fig. 2.
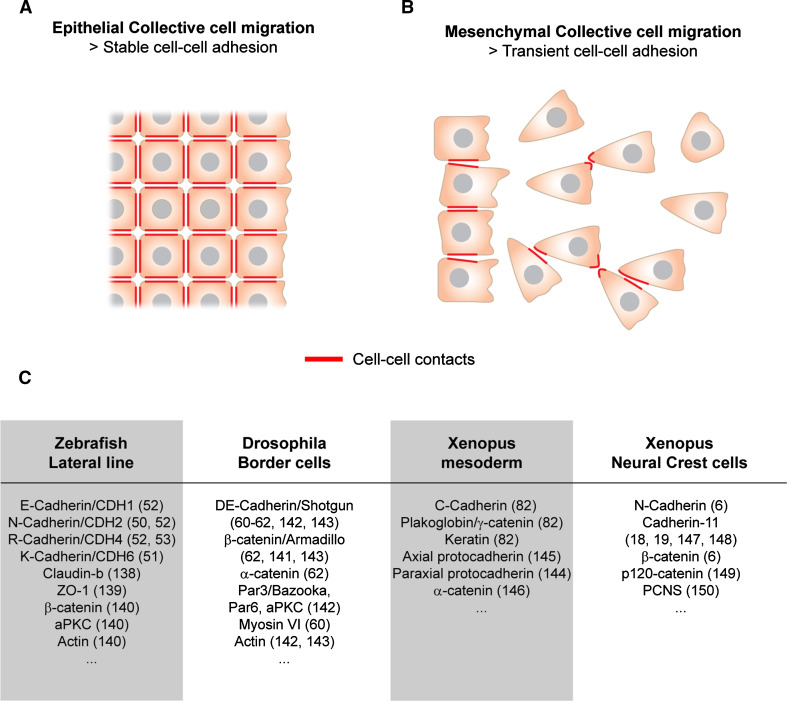
Epithelial versus mesenchymal collective cell migration. a Epithelial collective cell migration showing stable cell–cell contact (red lines). b Mesenchymal collective cell migration showing transient cell–cell contact (red lines), which are sufficient to polarize the cells. c Some examples of cell–cell adhesion molecules expressed during epithelial or mesenchymal collective cell migration. References: Zebrafish lateral line [50–53, 138–140]; Drosophila border cells [60–62, 141–143]; Xenopus mesoderm [82, 144–146]; Xenopus NC cells [6, 18, 19, 147–150]
Cadherins interact with a wide range of cytoplasmic proteins, which link them to the actin cytoskeleton and the microtubules [30]. Of particular interest is α-catenin, which binds to cadherins via β-catenin and recruits additional actin-binding factors such as vinculin and afadin to link the AJ to actin fibers [33, 34]. In addition, cytoplasmic α-catenin can interact with actin monomers to compete with the Arp2/3 complex and prevent excessive actin branching at the site of contact [35–37]. Also, cytoplasmic p120-catenin can act as a negative RhoA regulator and a positive Rac1/Cdc42 regulator [38, 39]. Thus, p120 recruitment to the cell–cell adhesion complex might alleviate its effect on these small GTPases favoring a local increase of RhoA activity. Moreover, E and N-cadherin can directly affect focal adhesion turnover [40, 41]. This indicates that AJs can influence cell polarity in at least two ways: by inhibiting protrusive activity at the site of contact, but also favor protrusive activity on the opposite side of the cell.
Epithelial cells can undergo collective migration in various ways. They can migrate as a separate group like the border cells in the Drosophila egg chamber [42] or the posterior lateral line (pLL) primordium in zebrafish [43]. Epithelial cells can move as wide cell sheet as seen during wound healing and dorsal closure of Drosophila embryos [2, 3, 44, 45]. They can form strands stretching out of a tissue such as those observed during invasive carcinomas or fibrosarcomas [3, 46]. Finally, epithelial cells can form hollow tubes outgrowing from a previous network of epithelial tubules or vessels, which has been described during blood vessels sprouting or mammary gland formation [14, 47–49]. All these examples rely on stable cell–cell adhesion to perform collective migration and impairing cadherins’ function and/or expression in these cells has dramatic consequences on their ability to migrate as a group, for instance, the fish pLL expresses E-cadherin (CDH1), N-cadherin (CDH2), R-cadherin (CDH4), and K-cadherin (CDH6) [50–53] (Fig. 2c). Inhibition of cadherins 2, 4, and 6 impairs pLL migration [50, 51, 53]. Interestingly, while migrating, the pLL normally deposits epithelial rosettes called neuromasts that later develop into mechanosensing organs. These epithelial rosettes detach from the rear of the pLL and their separation from the rest of the population owing to a local loss of E- and N-cadherin at the presumptive region of separation between pro-neuromasts and the rest of the pLL [54]. Interfering with these changes in cadherin distribution affects the separation of pro-neuromasts from the pLL, indicating that cohesion is primarily cadherin-dependent in these cells and that cadherin have to be downregulated to allow cells to leave the group. Importantly, directional migration of the pLL depends on stromal cell-derived factor 1 (Sdf1) a well-studied chemoattractant [55–59]. The pLL is thought to be polarized because of a differential distribution of Sdf1 receptors with Cxcr4 at the front and Cxcr7, acting as decoy receptor, at the rear [56]. The fact that targeting cadherins is sufficient to disturb pLL migration as a whole suggests that the chemotactic abilities of the individual cells are rather poor. If this is the case, stable cell–cell junctions could be primarily needed to maintain weakly responding cells together with better responding ones. Alternatively, the ability to chemotax could be downstream of the cell–cell junctions such that membrane localization of CXCR4/7 may be depending on cell polarity induced by AJs formation. These ideas remain to be tested in this system.
Drosophila border cells migrate as a small cluster of cells that delaminate from one end of the egg chamber and move in between other cells to reach the oocyte. Cell–cell adhesion between the border cells and between border cells and their surrounding tissues involve E-cadherin [60–62] (Fig. 2c). The group adopts a radial polarity with all cells forming protrusions outward. The cluster is then further polarized by the action of PDGF-VEGF-related factor (PVF) and epidermal growth factor (EGF) [42, 63–66]. Cells with the higher level of PVF/EGF signaling become leader cells and exhibit more stable cell protrusions, hence creating a front-rear polarity at the group level. Interestingly, E-cadherin is primarily involved in migration rather than cell adhesion since its inhibition blocks migration but does not promote dissociation of the border cell cluster [61]. The lack of cell protrusions after E-cadherin inhibition has been interpreted as an effect on cell-substrate adhesion. However, since E-cadherin massively accumulates at the interface between border cells it is possible that cells exhibit shorter protrusions due to a reduced cell polarity.
A key role for E-cadherin has been described in epithelial cell sheets undergoing wound closure [44, 67–69]. Such epithelial cell sheets undergo a transient and partial EMT and are known to migrate under an intermediate metastable phenotype, which allows for a quick reversal to complete epithelial features once the wound has closed (Fig. 1b). In this system, cells are connected via E-cadherin-dependent AJs and inhibiting cell–cell adhesion reduces cohesion and the overall directionality of the cells [69]. Importantly, AJs are essential to transmit stresses across the cell sheet. Cells experience multiple stresses: normal stress (tension or compression) that is oriented perpendicularly to the cell–cell contact; and shear stress that is parallel to it [70–73]. Cells progressively align along the regions of minimal shear stress [74–76]. The original break of symmetry provided by the appearance of a wound is sufficient to polarize the population by generating an explorative leading edge. Then, local stress transmitted via cell–cell adhesion generates an overall polarity at the tissue level without the need to create additional gaps.
Overall, collective migration in epithelial tissues primarily relies on mechanical coupling via cell–cell junctions. These junctions are important for controlling cell polarity at the single cell and the tissue level but also to average out differences among cells in terms of motility or chemotactic ability.
Collective cell migration in mesenchymal tissues
Mesenchymal cells do not maintain stable cell–cell junctions (Figs. 1a, 2b). Nonetheless several mesenchymal migratory populations show stable spatial relationships over time.
One of the most studied examples of mesenchymal tissue is the mesoderm. Mesodermal cells are induced at early stages of development at the interface between ectoderm and endoderm. They undergo an EMT and migrate together with endodermal cells to settle in an internal position in a process known as gastrulation [77]. Despite mesoderm being a relatively loose tissue, mesodermal cells interact with each other via cadherin-dependent junctions. In fish for instance, E-cadherin is strongly expressed in mesodermal cells during migration [78]. Inhibiting E-cadherin dramatically disturbs migration and gastrulation fails. However, a single mesodermal cell experimentally isolated from the group is still able to migrate efficiently, in a manner relatively similar to that of the whole group [79, 80]. This suggests that all cells are equally capable of following the signals controlling gastrulation that are present in the environment. However, if these cells are at high cell density but prevented to interact via E-cadherin-dependent AJs they fail to cooperate. This indicates that cell–cell interactions are somehow required to organize the mesoderm in order to proceed with gastrulation, even if each individual cell is capable of migrating properly. When cells are at high density cells, front cells can shield or consume guidance cues such that followers have little left to guide them. Therefore, transient physical coupling through AJs may be used to even out differences across the group, allow cell cooperation or simply allow non-responding cells to be carried passively by their neighbors.
Studies on mesoderm migration in Xenopus have shown some interesting similarities with the work on epithelial cell sheets described above. Xenopus mesodermal cells express C-cadherin [81] and use this cadherin to polarize [82]. When two cells make contact, tension is transmitted across the C-cadherin junction and this is sufficient to promote the formation of a cell protrusion on the opposite side. Interestingly, in these cells tension downstream of AJs is primarily passed on by intermediate filaments rather than actin.
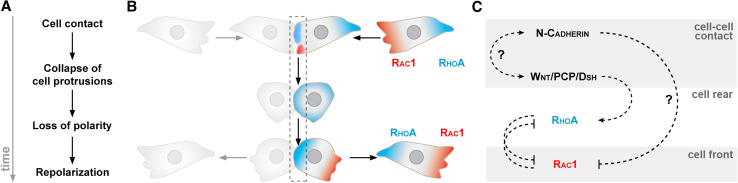
This phenomenon of polarization upon cell–cell contact is reminiscent of contact-inhibition of locomotion (CIL, Fig. 3) [83–86]. Upon collision with other cells, cells exhibiting CIL collapse their cell protrusions (lamellipodia, filopodia), stop migrating, and repolarize in the opposite direction (Fig. 3a, b). Originally identified by observing colliding fibroblasts in culture, CIL is now known to be relevant for in vivo cell migration [6, 85]. CIL is also deemed an important player in cancer metastasis, where a loss of CIL between tumor cells and their surrounding tissues is thought to promote invasiveness [86, 87]. Studies using neural crest (NC) cells as a model system have highlighted the link between contact-dependent polarity mediated by CIL and collective cell migration. NC cells are induced at the interface between the neural epithelium and the prospective epidermis [88–90]. They separate from neural and epidermal tissues via a delamination process involving a partial or complete EMT [1, 4, 91–94]. NC cells subsequently undergo a dramatic migration throughout the whole embryo.
Fig. 3.
Contact-inhibition of locomotion. a CIL consists of a series of events. First, cells make a physical contact. This contact triggers the collapse of cell protrusions. The colliding cells quickly lose their polarity and repolarize in the opposite direction. This repolarization often makes the cells move away from each other. b Diagram representing two colliding cells undergoing CIL. c Molecular pathways involved in CIL in Xenopus NC cells
Studies on cephalic Xenopus and zebrafish NC cells revealed that these cells experience CIL when colliding with each other and that CIL is essential for coordinated cell migration [6, 85]. In these cells, CIL requires N-cadherin-dependent contact leading to the activation of the non-canonical Wnt/planar cell polarity (PCP) pathway [6, 85] (Fig. 3c). The cell–cell interactions trigger a local increase of RhoA and a reduction of Rac1 activity at the site of contact. The interaction between N-cadherin, Wnt/PCP, and the local modulation of small GTPases in the repolarization of NC cells after contact is not yet fully understood. Interestingly, cadherins and Dishevelled (Dsh, downstream effector of Wnt signaling) can both modulate microtubules dynamics [35, 95] and data from Drosophila hemocytes and chick fibroblasts showed that microtubules are important players in CIL. In these cells, microtubules are required for cells to sense each other [96] and to repolarize after contact [97], respectively. Thus, microtubules might be important downstream effectors of the Wnt/PCP and small GTPases during CIL. At early stages of Xenopus NC migration, cells are still relatively tightly linked to each other and CIL is mostly used to prevent cells from forming protrusions on neighboring cells. Inhibition of Wnt/PCP or N-cadherin is sufficient to disorganize the whole cell population. While migration proceeds, NC cells progressively dissociate and their migration turns into cell streaming. Despite having only tip-like transient contacts (Fig. 2b), NC cells still exhibit a Wnt/PCP-N-cadherin-dependent CIL [6, 85].
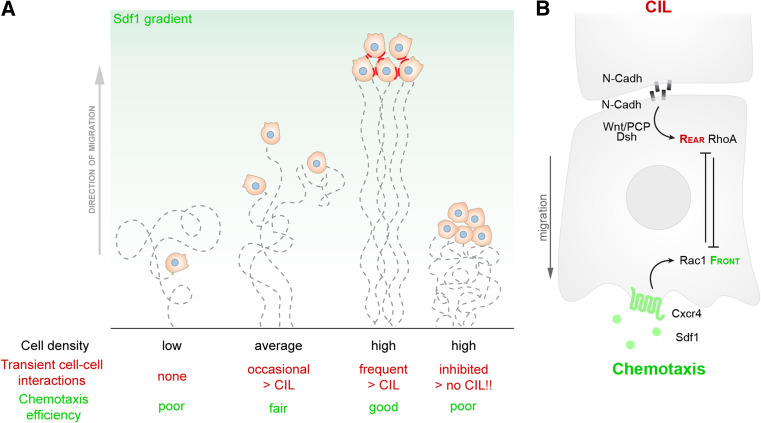
Directional migration of Xenopus NC cells is controlled by Sdf1 [6]. Importantly, single cells barely respond to the chemoattractant when prevented to interact with other cells (Fig. 4a). On the contrary, single cells that are allowed to collide with one another, or cells in a group, are highly responsive (Fig. 4a). Impairing cell–cell interactions by blocking CIL, via N-cadherin or Wnt/PCP, dramatically reduces chemotaxis (Fig. 4a). Thus, cell–cell contacts mediated by CIL are essential for the interpretation of guidance cues in NC cells. The molecular mechanism that explains this collective chemotaxis is based on the observation that a N-cadherin/PCP-dependent contact polarizes cells by inhibiting RhoA activity at the cell contact, which defines the rear of the cells, and increasing Rac1 activity at the front (Fig. 4b) [6]. In addition, the chemoattractant Sdf1 is able to increase Rac1 activity at the cell front, but only in cells that are already polarized [6]; consequently, cells polarized by CIL can respond more efficiently to Sdf1 (Fig. 4b). These results indicate that physical interactions within the migratory population allow the emergence of a collective guidance mechanism where cells at high density are better responding than cells at low density. While cell–cell interactions help chemotaxis by pre-establishing a cell polarity that can be further modulated by an external attractant, cells that are completely surrounded might be unable to read a local gradient of guidance cue. The signals may have been used by cells at the leading edge or because a cell located well inside the migratory population has no clear polarity and as such is poorly responding to the guidance cue (see above for a discussion on the effect of high cell density during mesoderm migration).
Fig. 4.
Contact-inhibition of locomotion promotes collective NC guidance. a NC cells exhibit different chemotactic abilities depending on cell density/cell–cell contacts. From left to right: isolated cells have no interactions with other cells and unstable cell polarity. Under these conditions, the chemoattractant Sdf1 is unable to impose a clear front-rear polarity and cells chemotax poorly. When cell density increases, cells can interact with each other. Each collision establishes a new front-rear polarity owing to CIL. Well-polarized cells have their cell protrusions further stabilized by the external chemoattractant, which leads to better chemotaxis. When cell–cell interactions are inhibited, chemotaxis efficiency is reduced. Cell paths are shown as dotted lines. Cell–cell contacts are shown in red. b Sdf1-Cxcr4 chemotaxis reinforces CIL-dependent cell polarity. CIL imposes a rear identity via RhoA activation. Sdf1/Cxcr4 increases Rac1, stabilizing cell protrusions at the front
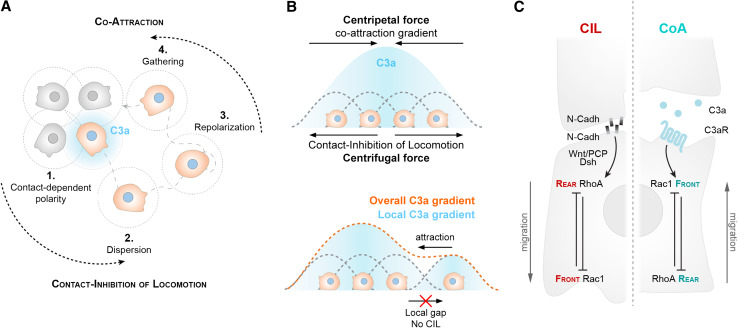
By repolarizing cells upon contact, CIL leads to dispersion and eventually reduces cell density. In vivo, several inhibitors are lining the pathways of migration, which helps to restrict the impact of CIL on cell density [4]. On top of that, NC cells are actively attracting each other through mutual attraction [or co-attraction (CoA), Fig. 5] [5]. Each NC cell secretes C3a, a well-known attractant in the immune system [98], and expresses its cognate receptor C3aR at their cell surface. Since each cell produces C3a, C3a concentration is higher in areas where the density of NC cells is high. Consequently, when an NC cell leaves the group, it can migrate back to the main population following the local gradient of C3a (Fig. 5b). C3a/C3aR activates Rac1, which leads to a repolarization of the escaping cell towards the main group [5]. Thus, by activating Rac1, CoA represents an opposite force to the dispersive activity promoted by CIL, which inhibits Rac1 (Fig. 5c). A continues cycle of CIL and CoA is assumed to maintain the collective migration of NC cells (Fig. 5a).
Fig. 5.
Integration of contact-inhibition of locomotion and co-attraction (CoA) promotes collective NC cell migration. a Co-attraction (CoA). When part of a group, cells display a radial polarity with cell protrusions pointing outward owing to CIL (1). Since cells are migratory, over time the CIL-dependent outward polarity favors cell dispersion (2). When a cell detaches from the group, the polarity imposed by CIL is rapidly lost. Each cell is secreting C3a and expresses its cognate receptor C3aR. A local gradient of C3a repolarizes the wandering cell (3), thus promoting gathering (4). b Cell clusters are under the influence of two major driving forces: a centripetal force owing to the local C3a gradient, and a centrifugal force owing to CIL. C3a is shown as shades of blue. Local and overall gradients of C3a are shown as dotted lines. When physical contact between a cell and the rest of the group is disrupted, the influence of CIL diminishes, which in turn favors CoA. c Molecular pathways involved in CIL and CoA compete to impose a front–rear cell polarity. CIL favors outward migration back by establishing a rear identity at the site of contact via RhoA activation. CoA promotes inward migration by inducing Rac1 activity. Since CIL and CoA counterbalance each other, collective cell migration is possible even in the absence of stable cell–cell adhesion
Importantly, similar observations have been made in mouse and chick embryos. NC cells in these species also migrate as a loose tissue undergoing cell streaming and forming chains with transient tip-like contacts [4, 93, 99–102]. Chick NC cells have been shown to exhibit CIL-like behavior when they collide with one another [99, 103]. In addition, in both mouse and chick embryos, NC cells migrate more efficiently when at high cell density. For instance, mouse cephalic NC cell migration is partially guided by semaphorin signals present within the surrounding tissues and response to these signals requires cell–cell interactions involving N-cadherin and gap junctions [104–106]. Furthermore, Wnt/PCP is important for the migration of trunk NC cells in chick and zebrafish embryos [107–111]. Finally, CoA-like behavior has been observed in chick cephalic NC cells [103, 112] and the pLL [113] and complement factor C3 is expressed in cephalic NC cells in chick (Mayor and Bronner, unpublished) and mouse (Mayor and Lambris, unpublished). All these observations suggest that contact-mediated cooperation leading to collective guidance and cell gathering strategies such as CoA may represent general cooperative mechanisms during collective cell migration.
The fact that transient and stable contacts can equally mediate cooperation indicates that collectiveness cannot be defined solely by the observation of stable physical coupling between migratory cells. As described above, mesenchymal cells that cooperate via transient contacts and manage to maintain the high cell density required for physical interactions can also undergo collective cell migration. Importantly, having CIL and migrating at high cell density does not necessarily imply collectiveness. Enteric NC cells that colonize the gut have their migration influenced by physical contact with one another and local cell density [114–116]. However, the movement of individual enteric NC cells does not correlate with the overall migration of the group. In addition, the spatial relationships between cells during migration are extremely poor such that enteric NC cells that start migrating next to each other may end up very far apart. Similarly, melanocyte precursors show contact-dependent behavior and have their overall migration primarily depending on cell density. For instance, a reduction of the total number of melanocytes leads to non-pigmented patches on the skin because the cells have not migrated extensively [92, 117–121]. However, in most cases, melanocytes broadly disperse in all directions with no correlation between individual movement and the average migration of the cell population, although phases of coordinated directional movement have been described [120, 122].
Collective cell migration in epithelial and mesenchymal cancers
Tumors of epithelia (carcinoma) and connective tissues (sarcoma) can become invasive and undergo collective cell migration. Melanoma (skin cancer), ductal carcinoma (i.e., breast cancer), rhabdomyosarcoma (striated muscle), and fibrosarcoma (fibroblasts) all show signs of cooperative behavior while migrating [123–125].
Carcinomas start off as epithelial structures migrating as large multicellular masses, progressively turning into 3D strands. Cell proliferation plays an important part in cancer progression and motion in large invasive epithelial tumors is partially influenced by the rate of growth, which generates a pushing effect [126]. The surrounding tissues can be deformed and damaged as a consequence of tumor growth. This increases the probability of cells stretching out of the tumor into the local environment without the need to actively separate from the original tumor. Progression into the surrounding areas is further facilitated by proteolytic activity of leading cells [127, 128]. This local remodeling of extracellular matrix favors migration of the following cells by making room for larger groups or simply by revealing cryptic sites for cell–matrix adhesion [129, 130]. Interestingly, cell cooperation in tumors is not restricted to cancer cells. Invasive cells are known to recruit other cell types from their local environment [8, 123, 125, 131]. For instance, tumor-associated fibroblasts can be coerced into leading a strand of cancer cells into the local matrix by generating tracks that other cells will then follow [132–135].
Strands or chains of epithelial cancer cells can further disperse by undergoing EMT [8]. Information about cell cooperation during cell streaming in cancer migration is scarce, but the works described above in embryonic cells strongly support the idea that collectiveness is not abolished by the acquisition of a mesenchymal phenotype. Thus, cancer cells undergoing EMT are not to be considered as solitary by default. This is also supported by the fact that collective cell migration, as 3D strand, 2D sheets or streams, is also observed in tumors derived from soft tissues, which directly start with a mesenchymal phenotype [3, 124]. Mesenchymal cancer cells still express various cell–cell adhesion molecules, such as cadherins [15, 16, 136], and are thus equipped to interact with each other or neighboring cells. In addition, EMT is often partial (not all tumor cells turn into mesenchymal cells) and incomplete (not all cells starting EMT actually complete it). Consequently, tumors from epithelial tissues are often heterogeneous structures containing epithelial and mesenchymal cells.
Interestingly, some experiments have hinted that epithelial and mesenchymal cancer cells can cooperate during metastasis [137]. Grafts containing both epithelial and mesenchymal tumor cells lead to an entry of tumor cells into the bloodstream and formation of secondary tumors. When grafted alone, mesenchymal tumor cells were able to enter the bloodstream but not to settle in remote organs. Grafted epithelial tumor cells were not able to pass into the vessels but, when injected directly into the bloodstream, they were capable of forming secondary tumors [137]. The cooperative mechanisms behind such an effect are still elusive, but suggest that cooperation is likely to take place at migration and dispersion levels but also that tumor heterogeneity may lead to emerging properties due to interactions between mesenchymal and epithelial cells within the tumor.
Overall, tumors are complex organs recapitulating some aspects of developmental or regenerative processes in a non-controlled manner. Therefore, all information about cell cooperation and collective cell migration gathered from studies on physiological systems will give great insights into cancer progression.
Conclusions
Studies on collective cell migration in epithelial and mesenchymal cells show that cells can cooperate in various ways. Epithelial cells make use of stable cell–cell adhesions whereas mesenchymal cells rely on more transient and dynamic cell–cell interaction and autocrine/paracrine signaling. The different strategies of collective migration are likely to be related to physiological differences between cell types and influenced by the environment through which cells have to migrate. For instance, epithelial cells that need to travel, while assuming their function as tight barriers between compartments, must at all costs keep stable cell–cell contacts. Cells that have to separate from a given tissue to relocate elsewhere have to, at least transiently, downregulate cell–cell adhesion in order to migrate. Other cells may simply adopt a mesenchymal phenotype to cross regions where the extracellular matrix is particularly tight, or, in the case of metastatic cancer cells, to enter a blood or lymphatic vessel. Despite a broad range of phenotypes and behaviors, both epithelial and mesenchymal cells are able to cooperate to migrate collectively. Our understanding of the mechanisms that render cooperation in epithelial and mesenchymal tissues possible remains sketchy. However, it is essential that we try to decipher and understand these mechanisms if we want to comprehend the dynamics of collective cell migration during morphogenesis, healing processes, or cancer invasion.
Acknowledgments
This investigation was supported by grants from MRC, BBSRC, and Wellcome Trust to RM and the Wellcome Trust Value in People award to ET.
References
- 1.Theveneau E, Mayor R. Can mesenchymal cells undergo collective cell migration? The case of the neural crest. Cell Adh Migr. 2011;5:490–498. doi: 10.4161/cam.5.6.18623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rorth P. Collective cell migration. Annu Rev Cell Dev Biol. 2009;25:407–429. doi: 10.1146/annurev.cellbio.042308.113231. [DOI] [PubMed] [Google Scholar]
- 3.Friedl P, Gilmour D. Collective cell migration in morphogenesis, regeneration and cancer. Nat Rev Mol Cell Biol. 2009;10:445–457. doi: 10.1038/nrm2720. [DOI] [PubMed] [Google Scholar]
- 4.Theveneau E, Mayor R. Neural crest delamination and migration: from epithelium-to-mesenchyme transition to collective cell migration. Dev Biol. 2012;366(1):34–54. doi: 10.1016/j.ydbio.2011.12.041. [DOI] [PubMed] [Google Scholar]
- 5.Carmona-Fontaine C, Theveneau E, Tzekou A, Tada M, Woods M, Page KM, Parsons M, Lambris JD, Mayor R. Complement fragment C3a controls mutual cell attraction during collective cell migration. Dev Cell. 2011;21:1026–1037. doi: 10.1016/j.devcel.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Theveneau E, Marchant L, Kuriyama S, Gull M, Moepps B, Parsons M, Mayor R. Collective chemotaxis requires contact-dependent cell polarity. Dev Cell. 2010;19:39–53. doi: 10.1016/j.devcel.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parrinello S, Napoli I, Ribeiro S, Digby PW, Fedorova M, Parkinson DB, Doddrell RD, Nakayama M, Adams RH, Lloyd AC. EphB signaling directs peripheral nerve regeneration through Sox2-dependent Schwann cell sorting. Cell. 2010;143:145–155. doi: 10.1016/j.cell.2010.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 9.Duband JL. Diversity in the molecular and cellular strategies of epithelium-to-mesenchyme transitions: insights from the neural crest. Cell Adh Migr. 2010;4:458–482. doi: 10.4161/cam.4.3.12501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 11.Uchino M, Kojima H, Wada K, Imada M, Onoda F, Satofuka H, Utsugi T, Murakami Y. Nuclear beta-catenin and CD44 upregulation characterize invasive cell populations in non-aggressive MCF-7 breast cancer cells. BMC Cancer. 2010;10:414. doi: 10.1186/1471-2407-10-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee JM, Dedhar S, Kalluri R, Thompson EW. The epithelial-mesenchymal transition: new insights in signaling, development, and disease. J Cell Biol. 2006;172:973–981. doi: 10.1083/jcb.200601018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klymkowsky MW, Savagner P. Epithelial-mesenchymal transition: a cancer researcher’s conceptual friend and foe. Am J Pathol. 2009;174:1588–1593. doi: 10.2353/ajpath.2009.080545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ewald AJ, Brenot A, Duong M, Chan BS, Werb Z. Collective epithelial migration and cell rearrangements drive mammary branching morphogenesis. Dev Cell. 2008;14:570–581. doi: 10.1016/j.devcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wheelock MJ, Shintani Y, Maeda M, Fukumoto Y, Johnson KR. Cadherin switching. J Cell Sci. 2008;121:727–735. doi: 10.1242/jcs.000455. [DOI] [PubMed] [Google Scholar]
- 16.Hazan RB, Qiao R, Keren R, Badano I, Suyama K. Cadherin switch in tumor progression. Ann NY Acad Sci. 2004;1014:155–163. doi: 10.1196/annals.1294.016. [DOI] [PubMed] [Google Scholar]
- 17.Piloto S, Schilling TF. Ovo1 links Wnt signaling with N-cadherin localization during neural crest migration. Development. 2010;137:1981–1990. doi: 10.1242/dev.048439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kashef J, Kohler A, Kuriyama S, Alfandari D, Mayor R, Wedlich D. Cadherin-11 regulates protrusive activity in Xenopus cranial neural crest cells upstream of trio and the small GTPases. Genes Dev. 2009;23:1393–1398. doi: 10.1101/gad.519409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vallin J, Girault JM, Thiery JP, Broders F. Xenopus cadherin-11 is expressed in different populations of migrating neural crest cells. Mech Dev. 1998;75:171–174. doi: 10.1016/S0925-4773(98)00099-9. [DOI] [PubMed] [Google Scholar]
- 20.Chalpe AJ, Prasad M, Henke AJ, Paulson AF. Regulation of cadherin expression in the chicken neural crest by the Wnt/beta-catenin signaling pathway. Cell Adh Migr. 2010;4(3):431–438. doi: 10.4161/cam.4.3.12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakagawa S, Takeichi M. Neural crest emigration from the neural tube depends on regulated cadherin expression. Development. 1998;125:2963–2971. doi: 10.1242/dev.125.15.2963. [DOI] [PubMed] [Google Scholar]
- 22.Rieger S, Senghaas N, Walch A, Koster RW. Cadherin-2 controls directional chain migration of cerebellar granule neurons. PLoS Biol. 2009;7:e1000240. doi: 10.1371/journal.pbio.1000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahlstrom JD, Erickson CA. The neural crest epithelial-mesenchymal transition in 4D: a;tail’ of multiple non-obligatory cellular mechanisms. Development. 2009;136:1801–1812. doi: 10.1242/dev.034785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baum B, Georgiou M. Dynamics of adherens junctions in epithelial establishment, maintenance, and remodelling. J Cell Biol. 2011;192:907–917. doi: 10.1083/jcb.201009141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nishimura T, Takeichi M. Remodelling of the adherens junctions during morphogenesis. Curr Top Dev Biol. 2009;89:33–54. doi: 10.1016/S0070-2153(09)89002-9. [DOI] [PubMed] [Google Scholar]
- 26.Meng W, Takeichi M. Adherens junction: molecular architecture and regulation. Cold Spring Harb Perspect Biol. 2009;1:a002899. doi: 10.1101/cshperspect.a002899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cavey M, Lecuit T. Molecular bases of cell–cell junctions stability and dynamics. Cold Spring Harb Perspect Biol. 2009;1:a002998. doi: 10.1101/cshperspect.a002998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kitt KN, Nelson WJ. Rapid suppression of activated Rac1 by cadherins and Nectins during de novo cell–cell adhesion. PLoS ONE. 2011;6:e17841. doi: 10.1371/journal.pone.0017841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamada S, Nelson WJ. Localized zones of Rho and Rac activities drive initiation and expansion of epithelial cell–cell adhesion. J Cell Biol. 2007;178:517–527. doi: 10.1083/jcb.200701058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yonemura S. Cadherin-actin interactions at adherens junctions. Curr Opin Cell Biol. 2011;23:515–522. doi: 10.1016/j.ceb.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 31.Miyake Y, Inoue N, Nishimura K, Kinoshita N, Hosoya H, Yonemura S. Actomyosin tension is required for correct recruitment of adherens junction components and zonula occludens formation. Exp Cell Res. 2006;312:1637–1650. doi: 10.1016/j.yexcr.2006.01.031. [DOI] [PubMed] [Google Scholar]
- 32.Hidalgo-Carcedo C, Hooper S, Chaudhry SI, Williamson P, Harrington K, Leitinger B, Sahai E. Collective cell migration requires suppression of actomyosin at cell–cell contacts mediated by DDR1 and the cell polarity regulators Par3 and Par6. Nat Cell Biol. 2010;13:49–58. doi: 10.1038/ncb2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weiss EE, Kroemker M, Rudiger AH, Jockusch BM, Rudiger M. Vinculin is part of the cadherin-catenin junctional complex: complex formation between alpha-catenin and vinculin. J Cell Biol. 1998;141:755–764. doi: 10.1083/jcb.141.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pokutta S, Weis WI. Structure and mechanism of cadherins and catenins in cell–cell contacts. Annu Rev Cell Dev Biol. 2007;23:237–261. doi: 10.1146/annurev.cellbio.22.010305.104241. [DOI] [PubMed] [Google Scholar]
- 35.Harris TJ, Tepass U. Adherens junctions: from molecules to morphogenesis. Nat Rev Mol Cell Biol. 2010;11:502–514. doi: 10.1038/nrm2927. [DOI] [PubMed] [Google Scholar]
- 36.Benjamin JM, Kwiatkowski AV, Yang C, Korobova F, Pokutta S, Svitkina T, Weis WI, Nelson WJ. AlphaE-catenin regulates actin dynamics independently of cadherin-mediated cell–cell adhesion. J Cell Biol. 2010;189:339–352. doi: 10.1083/jcb.200910041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Drees F, Pokutta S, Yamada S, Nelson WJ, Weis WI. Alpha-catenin is a molecular switch that binds E-cadherin-beta-catenin and regulates actin-filament assembly. Cell. 2005;123:903–915. doi: 10.1016/j.cell.2005.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Noren NK, Liu BP, Burridge K, Kreft B. p120 catenin regulates the actin cytoskeleton via Rho family GTPases. J Cell Biol. 2000;150:567–580. doi: 10.1083/jcb.150.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anastasiadis PZ, Moon SY, Thoreson MA, Mariner DJ, Crawford HC, Zheng Y, Reynolds AB. Inhibition of RhoA by p120 catenin. Nat Cell Biol. 2000;2:637–644. doi: 10.1038/35023588. [DOI] [PubMed] [Google Scholar]
- 40.Camand E, Peglion F, Osmani N, Sanson M, Etienne-Manneville S. N-cadherin expression level modulates integrin-mediated polarity and strongly impacts on the speed and directionality of glial cell migration. J Cell Sci. 2012;125:844–857. doi: 10.1242/jcs.087668. [DOI] [PubMed] [Google Scholar]
- 41.Borghi N, Lowndes M, Maruthamuthu V, Gardel ML, Nelson WJ. Regulation of cell motile behavior by crosstalk between cadherin- and integrin-mediated adhesions. Proc Natl Acad Sci USA. 2010;107:13324–13329. doi: 10.1073/pnas.1002662107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Montell DJ. The social lives of migrating cells in Drosophila. Curr Opin Genet Dev. 2006;16:374–383. doi: 10.1016/j.gde.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 43.Aman A, Piotrowski T. Cell–cell signaling interactions coordinate multiple cell behaviors that drive morphogenesis of the lateral line. Cell Adh Migr. 2012;5:499–508. doi: 10.4161/cam.5.6.19113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Danjo Y, Gipson IK. Actin ‘purse string’ filaments are anchored by E-cadherin-mediated adherens junctions at the leading edge of the epithelial wound, providing coordinated cell movement. J Cell Sci. 1998;111(Pt 22):3323–3332. doi: 10.1242/jcs.111.22.3323. [DOI] [PubMed] [Google Scholar]
- 45.Fredberg JJ, Tambe DT, Hardin CC, Angelini TE, Rajendran K, Park CY, Serra-Picamal X, Zhou EHH, Zaman MH, Butler JP, et al. Collective cell guidance by cooperative intercellular forces. Nat Mater. 2011;10:469–475. doi: 10.1038/nmat3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wolf K, Wu YI, Liu Y, Geiger J, Tam E, Overall C, Stack MS, Friedl P. Multi-step pericellular proteolysis controls the transition from individual to collective cancer cell invasion. Nat Cell Biol. 2007;9:893–904. doi: 10.1038/ncb1616. [DOI] [PubMed] [Google Scholar]
- 47.Wacker A, Gerhardt H. Endothelial development taking shape. Curr Opin Cell Biol. 2011;23:676–685. doi: 10.1016/j.ceb.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 48.Gray RS, Cheung KJ, Ewald AJ. Cellular mechanisms regulating epithelial morphogenesis and cancer invasion. Curr Opin Cell Biol. 2010;22:640–650. doi: 10.1016/j.ceb.2010.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lu P, Sternlicht MD, Werb Z. Comparative mechanisms of branching morphogenesis in diverse systems. J Mammary Gland Biol Neoplasia. 2006;11:213–228. doi: 10.1007/s10911-006-9027-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kerstetter AE, Azodi E, Marrs JA, Liu Q. Cadherin-2 function in the cranial ganglia and lateral line system of developing Zebrafish. Dev Dyn. 2004;230:137–143. doi: 10.1002/dvdy.20021. [DOI] [PubMed] [Google Scholar]
- 51.Liu Q, Dalman MR, Sarmah S, Chen S, Chen Y, Hurlbut AK, Spencer MA, Pancoe L, Marrs JA. Cell adhesion molecule cadherin-6 function in Zebrafish cranial and lateral line ganglia development. Dev Dyn. 2011;240:1716–1726. doi: 10.1002/dvdy.22665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu Q, Ensign RD, Azodi E. Cadherin-1, -2 and -4 expression in the cranial ganglia and lateral line system of developing Zebrafish. Gene Expr Patterns. 2003;3:653–658. doi: 10.1016/S1567-133X(03)00109-1. [DOI] [PubMed] [Google Scholar]
- 53.Wilson AL, Shen YC, Babb-Clendenon SG, Rostedt J, Liu B, Barald KF, Marrs JA, Liu Q. Cadherin-4 plays a role in the development of Zebrafish cranial ganglia and lateral line system. Dev Dyn. 2007;236:893–902. doi: 10.1002/dvdy.21085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Matsuda M, Chitnis AB. Atoh1a expression must be restricted by Notch signaling for effective morphogenesis of the posterior lateral line primordium in Zebrafish. Development. 2010;137:3477–3487. doi: 10.1242/dev.052761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aman A, Piotrowski T. Wnt/beta-catenin and Fgf signaling control collective cell migration by restricting chemokine receptor expression. Dev Cell. 2008;15:749–761. doi: 10.1016/j.devcel.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 56.Dambly-Chaudiere C, Cubedo N, Ghysen A. Control of cell migration in the development of the posterior lateral line: antagonistic interactions between the chemokine receptors CXCR4 and CXCR7/RDC1. BMC Dev Biol. 2007;7:23. doi: 10.1186/1471-213X-7-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.David NB, Sapede D, Saint-Etienne L, Thisse C, Thisse B, Dambly-Chaudiere C, Rosa FM, Ghysen A. Molecular basis of cell migration in the fish lateral line: role of the chemokine receptor CXCR4 and of its ligand, SDF1. Proc Natl Acad Sci USA. 2002;99:16297–16302. doi: 10.1073/pnas.252339399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Haas P, Gilmour D. Chemokine signaling mediates self-organizing tissue migration in the Zebrafish lateral line. Dev Cell. 2006;10:673–680. doi: 10.1016/j.devcel.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 59.Sapede D, Rossel M, Dambly-Chaudiere C, Ghysen A. Role of SDF1 chemokine in the development of lateral line efferent and facial motor neurons. Proc Natl Acad Sci USA. 2005;102:1714–1718. doi: 10.1073/pnas.0406382102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Geisbrecht ER, Montell DJ. Myosin VI is required for E-cadherin-mediated border cell migration. Nat Cell Biol. 2002;4:616–620. doi: 10.1038/ncb830. [DOI] [PubMed] [Google Scholar]
- 61.Niewiadomska P, Godt D, Tepass U. DE-cadherin is required for intercellular motility during Drosophila oogenesis. J Cell Biol. 1999;144:533–547. doi: 10.1083/jcb.144.3.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pacquelet A, Rorth P. Regulatory mechanisms required for DE-cadherin function in cell migration and other types of adhesion. J Cell Biol. 2005;170:803–812. doi: 10.1083/jcb.200506131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Duchek P, Somogyi K, Jekely G, Beccari S, Rorth P. Guidance of cell migration by the Drosophila PDGF/VEGF receptor. Cell. 2001;107:17–26. doi: 10.1016/S0092-8674(01)00502-5. [DOI] [PubMed] [Google Scholar]
- 64.Janssens K, Sung HH, Rorth P. Direct detection of guidance receptor activity during border cell migration. Proc Natl Acad Sci USA. 2010;107:7323–7328. doi: 10.1073/pnas.0915075107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McDonald JA, Pinheiro EM, Kadlec L, Schupbach T, Montell DJ. Multiple EGFR ligands participate in guiding migrating border cells. Dev Biol. 2006;296:94–103. doi: 10.1016/j.ydbio.2006.04.438. [DOI] [PubMed] [Google Scholar]
- 66.McDonald JA, Pinheiro EM, Montell DJ. PVF1, a PDGF/VEGF homolog, is sufficient to guide border cells and interacts genetically with Taiman. Development. 2003;130:3469–3478. doi: 10.1242/dev.00574. [DOI] [PubMed] [Google Scholar]
- 67.Nguyen TN, Uemura A, Shih W, Yamada S. Zyxin-mediated actin assembly is required for efficient wound closure. J Biol Chem. 2010;285:35439–35445. doi: 10.1074/jbc.M110.119487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Desai RA, Gao L, Raghavan S, Liu WF, Chen CS. Cell polarity triggered by cell–cell adhesion via E-cadherin. J Cell Sci. 2009;122:905–911. doi: 10.1242/jcs.028183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li L, Hartley R, Reiss B, Sun Y, Pu J, Wu D, Lin F, Hoang T, Yamada S, Jiang J, et al. E-cadherin plays an essential role in collective directional migration of large epithelial sheets. Cell Mol Life Sci. 2012;69:2779–2789. doi: 10.1007/s00018-012-0951-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Leckband DE, le Duc Q, Wang N, de Rooij J. Mechanotransduction at cadherin-mediated adhesions. Curr Opin Cell Biol. 2011;23:523–530. doi: 10.1016/j.ceb.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 71.Lecuit T, Lenne PF. Cell surface mechanics and the control of cell shape, tissue patterns and morphogenesis. Nat Rev Mol Cell Biol. 2007;8:633–644. doi: 10.1038/nrm2222. [DOI] [PubMed] [Google Scholar]
- 72.Baum B, Settleman J, Quinlan MP. Transitions between epithelial and mesenchymal states in development and disease. Semin Cell Dev Biol. 2008;19:294–308. doi: 10.1016/j.semcdb.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 73.Saez A, Anon E, Ghibaudo M, du Roure O, Di Meglio JM, Hersen P, Silberzan P, Buguin A, Ladoux B. Traction forces exerted by epithelial cell sheets. J Phys Condens Matter. 2011;22:194119. doi: 10.1088/0953-8984/22/19/194119. [DOI] [PubMed] [Google Scholar]
- 74.Trepat X, Fredberg JJ. Plithotaxis and emergent dynamics in collective cellular migration. Trends Cell Biol. 2011;21:638–646. doi: 10.1016/j.tcb.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tambe DT, Hardin CC, Angelini TE, Rajendran K, Park CY, Serra-Picamal X, Zhou EH, Zaman MH, Butler JP, Weitz DA, et al. Collective cell guidance by cooperative intercellular forces. Nat Mater. 2011;10:469–475. doi: 10.1038/nmat3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Angelini TE, Hannezo E, Trepat X, Marquez M, Fredberg JJ, Weitz DA. Glass-like dynamics of collective cell migration. Proc Natl Acad Sci USA. 2011;108:4714–4719. doi: 10.1073/pnas.1010059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stern CD. Gastrulation : from cells to embryo. NY: Cold Spring Harbor Laboratory Press; 2004. [Google Scholar]
- 78.Solnica-Krezel L. Gastrulation in Zebrafish—all just about adhesion? Curr Opin Genet Dev. 2006;16:433–441. doi: 10.1016/j.gde.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 79.Arboleda-Estudillo Y, Krieg M, Stuhmer J, Licata NA, Muller DJ, Heisenberg CP. Movement directionality in collective migration of germ layer progenitors. Curr Biol. 2010;20:161–169. doi: 10.1016/j.cub.2009.11.036. [DOI] [PubMed] [Google Scholar]
- 80.Ulrich F, Krieg M, Schotz EM, Link V, Castanon I, Schnabel V, Taubenberger A, Mueller D, Puech PH, Heisenberg CP. Wnt11 functions in gastrulation by controlling cell cohesion through Rab5c and E-cadherin. Dev Cell. 2005;9:555–564. doi: 10.1016/j.devcel.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 81.Winklbauer R. Cadherin function during Xenopus gastrulation. Subcell Biochem. 2012;60:301–320. doi: 10.1007/978-94-007-4186-7_13. [DOI] [PubMed] [Google Scholar]
- 82.Weber GF, Bjerke MA, Desimone DW. A mechanoresponsive cadherin–Keratin complex directs polarized protrusive behavior and collective cell migration. Dev Cell. 2011;22(1):104–115. doi: 10.1016/j.devcel.2011.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Abercrombie M, Heaysman JE. Observations on the social behaviour of cells in tissue culture. I. Speed of movement of chick heart fibroblasts in relation to their mutual contacts. Exp Cell Res. 1953;5:111–131. doi: 10.1016/0014-4827(53)90098-6. [DOI] [PubMed] [Google Scholar]
- 84.Abercrombie M, Dunn GA. Adhesions of fibroblasts to substratum during contact inhibition observed by interference reflection microscopy. Exp Cell Res. 1975;92:57–62. doi: 10.1016/0014-4827(75)90636-9. [DOI] [PubMed] [Google Scholar]
- 85.Carmona-Fontaine C, Matthews HK, Kuriyama S, Moreno M, Dunn GA, Parsons M, Stern CD, Mayor R. Contact inhibition of locomotion in vivo controls neural crest directional migration. Nature. 2008;456:957–961. doi: 10.1038/nature07441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mayor R, Carmona-Fontaine C. Keeping in touch with contact inhibition of locomotion. Trends Cell Biol. 2010;20:319–328. doi: 10.1016/j.tcb.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Astin JW, Batson J, Kadir S, Charlet J, Persad RA, Gillatt D, Oxley JD, Nobes CD. Competition amongst Eph receptors regulates contact inhibition of locomotion and invasiveness in prostate cancer cells. Nat Cell Biol. 2010;12:1194–1204. doi: 10.1038/ncb2122. [DOI] [PubMed] [Google Scholar]
- 88.Le Douarin N, Kalcheim C. The neural crest. 2. UK: Cambridge University Press; 1999. [Google Scholar]
- 89.Hall B. The neural crest and neural crest cells in vertebrate development and evolution. 2. New York: Springer; 2008. [Google Scholar]
- 90.Milet C, Monsoro-Burq AH. Neural crest induction at the neural plate border in vertebrates. Dev Biol. 2012;366:22–33. doi: 10.1016/j.ydbio.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 91.Theveneau E, Mayor R. Collective cell migration of the cephalic neural crest: the art of integrating information. Genesis. 2011;49:164–176. doi: 10.1002/dvg.20700. [DOI] [PubMed] [Google Scholar]
- 92.Kuo BR, Erickson CA. Regional differences in neural crest morphogenesis. Cell Adh Migr. 2010;4:567–585. doi: 10.4161/cam.4.4.12890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kulesa PM, Bailey CM, Kasemeier-Kulesa JC, McLennan R. Cranial neural crest migration: new rules for an old road. Dev Biol. 2010;344:543–554. doi: 10.1016/j.ydbio.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gammill LS, Roffers-Agarwal J. Division of labor during trunk neural crest development. Dev Biol. 2010;344:555–565. doi: 10.1016/j.ydbio.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gao C, Chen YG. Dishevelled: the hub of Wnt signaling. Cell Signal. 2010;22:717–727. doi: 10.1016/j.cellsig.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 96.Stramer B, Moreira S, Millard T, Evans I, Huang CY, Sabet O, Milner M, Dunn G, Martin P, Wood W. Clasp-mediated microtubule bundling regulates persistent motility and contact repulsion in Drosophila macrophages in vivo. J Cell Biol. 2010;189:681–689. doi: 10.1083/jcb.200912134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kadir S, Astin JW, Tahtamouni L, Martin P, Nobes CD. Microtubule remodelling is required for the front-rear polarity switch during contact inhibition of locomotion. J Cell Sci. 2011;124:2642–2653. doi: 10.1242/jcs.087965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010;11:785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Teddy JM, Kulesa PM. In vivo evidence for short- and long-range cell communication in cranial neural crest cells. Development. 2004;131:6141–6151. doi: 10.1242/dev.01534. [DOI] [PubMed] [Google Scholar]
- 100.Erickson CA, Weston JA. An SEM analysis of neural crest migration in the mouse. J Embryol Exp Morphol. 1983;74:97–118. [PubMed] [Google Scholar]
- 101.Serbedzija GN, Bronner-Fraser M, Fraser SE. Vital dye analysis of cranial neural crest cell migration in the mouse embryo. Development. 1992;116:297–307. doi: 10.1242/dev.116.2.297. [DOI] [PubMed] [Google Scholar]
- 102.Serbedzija GN, Fraser SE, Bronner-Fraser M. Pathways of trunk neural crest cell migration in the mouse embryo as revealed by vital dye labelling. Development. 1990;108:605–612. doi: 10.1242/dev.108.4.605. [DOI] [PubMed] [Google Scholar]
- 103.Kulesa PM, Fraser SE. In ovo time-lapse analysis of chick hindbrain neural crest cell migration shows cell interactions during migration to the branchial arches. Development. 2000;127:1161–1172. doi: 10.1242/dev.127.6.1161. [DOI] [PubMed] [Google Scholar]
- 104.Xu X, Francis R, Wei CJ, Linask KL, Lo CW. Connexin 43-mediated modulation of polarized cell movement and the directional migration of cardiac neural crest cells. Development. 2006;133:3629–3639. doi: 10.1242/dev.02543. [DOI] [PubMed] [Google Scholar]
- 105.Xu X, Li WE, Huang GY, Meyer R, Chen T, Luo Y, Thomas MP, Radice GL, Lo CW. Modulation of mouse neural crest cell motility by N-cadherin and connexin 43 gap junctions. J Cell Biol. 2001;154:217–230. doi: 10.1083/jcb.200105047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Xu X, Li WE, Huang GY, Meyer R, Chen T, Luo Y, Thomas MP, Radice GL, Lo CW. N-cadherin and Cx43alpha1 gap junctions modulates mouse neural crest cell motility via distinct pathways. Cell Commun Adhes. 2001;8:321–324. doi: 10.3109/15419060109080746. [DOI] [PubMed] [Google Scholar]
- 107.Carmona-Fontaine C, Matthews H, Mayor R. Directional cell migration in vivo: Wnt at the crest. Cell Adh Migr. 2008;2:240–242. doi: 10.4161/cam.2.4.6747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.De Calisto J, Araya C, Marchant L, Riaz CF, Mayor R. Essential role of non-canonical Wnt signalling in neural crest migration. Development. 2005;132:2587–2597. doi: 10.1242/dev.01857. [DOI] [PubMed] [Google Scholar]
- 109.Matthews HK, Marchant L, Carmona-Fontaine C, Kuriyama S, Larrain J, Holt MR, Parsons M, Mayor R. Directional migration of neural crest cells in vivo is regulated by Syndecan-4/Rac1 and non-canonical Wnt signaling/RhoA. Development. 2008;135:1771–1780. doi: 10.1242/dev.017350. [DOI] [PubMed] [Google Scholar]
- 110.Banerjee S, Gordon L, Donn TM, Berti C, Moens CB, Burden SJ, Granato M. A novel role for MuSK and non-canonical Wnt signaling during segmental neural crest cell migration. Development. 2011;138:3287–3296. doi: 10.1242/dev.067306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rios AC, Serralbo O, Salgado D, Marcelle C. Neural crest regulates myogenesis through the transient activation of NOTCH. Nature. 2011;473(7348):532–535. doi: 10.1038/nature09970. [DOI] [PubMed] [Google Scholar]
- 112.Kulesa P, Ellies DL, Trainor PA. Comparative analysis of neural crest cell death, migration, and function during vertebrate embryogenesis. Dev Dyn. 2004;229:14–29. doi: 10.1002/dvdy.10485. [DOI] [PubMed] [Google Scholar]
- 113.Breau MA, Wilson D, Wilkinson DG, Xu Q. Chemokine and Fgf signalling act as opposing guidance cues in formation of the lateral line primordium. Development. 2012;139:2246–2253. doi: 10.1242/dev.080275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Simpson MJ, Zhang DC, Mariani M, Landman KA, Newgreen DF. Cell proliferation drives neural crest cell invasion of the intestine. Dev Biol. 2007;302:553–568. doi: 10.1016/j.ydbio.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 115.Hearn CJ, Murphy M, Newgreen D. GDNF and ET-3 differentially modulate the numbers of avian enteric neural crest cells and enteric neurons in vitro. Dev Biol. 1998;197:93–105. doi: 10.1006/dbio.1998.8876. [DOI] [PubMed] [Google Scholar]
- 116.Young HM, Bergner AJ, Anderson RB, Enomoto H, Milbrandt J, Newgreen DF, Whitington PM. Dynamics of neural crest-derived cell migration in the embryonic mouse gut. Dev Biol. 2004;270:455–473. doi: 10.1016/j.ydbio.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 117.Thomas LA, Yamada KM. Contact stimulation of cell migration. J Cell Sci. 1992;103(Pt 4):1211–1214. doi: 10.1242/jcs.103.4.1211. [DOI] [PubMed] [Google Scholar]
- 118.Kelsh RN, Harris ML, Colanesi S, Erickson CA. Stripes and belly-spots—a review of pigment cell morphogenesis in vertebrates. Semin Cell Dev Biol. 2009;20:90–104. doi: 10.1016/j.semcdb.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Spieth J, Keller RE. Neural crest cell behavior in white and dark larvae of Ambystoma mexicanum: differences in cell morphology, arrangement, and extracellular matrix as related to migration. J Exp Zool. 1984;229:91–107. doi: 10.1002/jez.1402290112. [DOI] [PubMed] [Google Scholar]
- 120.Keller RE, Spieth J. Neural crest cell behavior in white and dark larvae of Ambystoma mexicanum: time-lapse cinemicrographic analysis of pigment cell movement in vivo and in culture. J Exp Zool. 1984;229:109–126. doi: 10.1002/jez.1402290113. [DOI] [PubMed] [Google Scholar]
- 121.Erickson CA. Control of neural crest cell dispersion in the trunk of the avian embryo. Dev Biol. 1985;111:138–157. doi: 10.1016/0012-1606(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 122.Belmadani A, Jung H, Ren D, Miller RJ. The chemokine SDF-1/CXCL12 regulates the migration of melanocyte progenitors in mouse hair follicles. Differentiation. 2009;77:395–411. doi: 10.1016/j.diff.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Deisboeck TS, Couzin ID. Collective behavior in cancer cell populations. Bioessays. 2009;31:190–197. doi: 10.1002/bies.200800084. [DOI] [PubMed] [Google Scholar]
- 124.Friedl P, Alexander S. Cancer invasion and the microenvironment: plasticity and reciprocity. Cell. 2011;147:992–1009. doi: 10.1016/j.cell.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 125.Nguyen DX, Bos PD, Massague J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer. 2009;9:274–284. doi: 10.1038/nrc2622. [DOI] [PubMed] [Google Scholar]
- 126.Khalil AA, Friedl P. Determinants of leader cells in collective cell migration. Integr Biol (Camb) 2010;2:568–574. doi: 10.1039/c0ib00052c. [DOI] [PubMed] [Google Scholar]
- 127.Hotary K, Li XY, Allen E, Stevens SL, Weiss SJ. A cancer cell metalloprotease triad regulates the basement membrane transmigration program. Genes Dev. 2006;20:2673–2686. doi: 10.1101/gad.1451806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wolf K, Friedl P. Extracellular matrix determinants of proteolytic and non-proteolytic cell migration. Trends Cell Biol. 2011;21:736–744. doi: 10.1016/j.tcb.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 129.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 130.Murphy G. The ADAMs: signalling scissors in the tumour microenvironment. Nat Rev Cancer. 2008;8:929–941. doi: 10.1038/nrc2459. [DOI] [PubMed] [Google Scholar]
- 131.Bidard FC, Pierga JY, Vincent-Salomon A, Poupon MF. A “class action” against the microenvironment: do cancer cells cooperate in metastasis? Cancer Metastasis Rev. 2008;27:5–10. doi: 10.1007/s10555-007-9103-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.De Wever O, Mareel M. Role of tissue stroma in cancer cell invasion. J Pathol. 2003;200:429–447. doi: 10.1002/path.1398. [DOI] [PubMed] [Google Scholar]
- 133.Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression. Nature. 2004;432:332–337. doi: 10.1038/nature03096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sanz-Moreno V, Gaggioli C, Yeo M, Albrengues J, Wallberg F, Viros A, Hooper S, Mitter R, Feral CC, Cook M, et al. ROCK and JAK1 signaling cooperate to control actomyosin contractility in tumor cells and stroma. Cancer Cell. 2011;20:229–245. doi: 10.1016/j.ccr.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 135.Gaggioli C, Hooper S, Hidalgo-Carcedo C, Grosse R, Marshall JF, Harrington K, Sahai E. Fibroblast-led collective invasion of carcinoma cells with differing roles for RhoGTPases in leading and following cells. Nat Cell Biol. 2007;9:1392–1400. doi: 10.1038/ncb1658. [DOI] [PubMed] [Google Scholar]
- 136.Maeda M, Johnson KR, Wheelock MJ. Cadherin switching: essential for behavioral but not morphological changes during an epithelium-to-mesenchyme transition. J Cell Sci. 2005;118:873–887. doi: 10.1242/jcs.01634. [DOI] [PubMed] [Google Scholar]
- 137.Tsuji T, Ibaragi S, Hu GF. Epithelial-mesenchymal transition and cell cooperativity in metastasis. Cancer Res. 2009;69:7135–7139. doi: 10.1158/0008-5472.CAN-09-1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lopez-Schier H, Starr CJ, Kappler JA, Kollmar R, Hudspeth AJ. Directional cell migration establishes the axes of planar polarity in the posterior lateral-line organ of the Zebrafish. Dev Cell. 2004;7:401–412. doi: 10.1016/j.devcel.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 139.Lecaudey V, Cakan-Akdogan G, Norton WH, Gilmour D. Dynamic Fgf signaling couples morphogenesis and migration in the Zebrafish lateral line primordium. Development. 2008;135:2695–2705. doi: 10.1242/dev.025981. [DOI] [PubMed] [Google Scholar]
- 140.Hava D, Forster U, Matsuda M, Cui S, Link BA, Eichhorst J, Wiesner B, Chitnis A, Abdelilah-Seyfried S. Apical membrane maturation and cellular rosette formation during morphogenesis of the Zebrafish lateral line. J Cell Sci. 2009;122:687–695. doi: 10.1242/jcs.032102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Loureiro JJ, Akong K, Cayirlioglu P, Baltus AE, DiAntonio A, Peifer M. Activated armadillo/beta-catenin does not play a general role in cell migration and process extension in Drosophila. Dev Biol. 2001;235:33–44. doi: 10.1006/dbio.2001.0292. [DOI] [PubMed] [Google Scholar]
- 142.Pinheiro EM, Montell DJ. Requirement for Par-6 and Bazooka in Drosophila border cell migration. Development. 2004;131:5243–5251. doi: 10.1242/dev.01412. [DOI] [PubMed] [Google Scholar]
- 143.Bastock R, Strutt D. The planar polarity pathway promotes coordinated cell migration during Drosophila oogenesis. Development. 2007;134:3055–3064. doi: 10.1242/dev.010447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kim SH, Yamamoto A, Bouwmeester T, Agius E, Robertis EM. The role of paraxial protocadherin in selective adhesion and cell movements of the mesoderm during Xenopus gastrulation. Development. 1998;125:4681–4690. doi: 10.1242/dev.125.23.4681. [DOI] [PubMed] [Google Scholar]
- 145.Yoder MD, Gumbiner BM. Axial protocadherin (AXPC) regulates cell fate during notochordal morphogenesis. Dev Dyn. 2011;240:2495–2504. doi: 10.1002/dvdy.22754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Schneider S, Herrenknecht K, Butz S, Kemler R, Hausen P. Catenins in Xenopus embryogenesis and their relation to the cadherin-mediated cell–cell adhesion system. Development. 1993;118:629–640. doi: 10.1242/dev.118.2.629. [DOI] [PubMed] [Google Scholar]
- 147.Borchers A, David R, Wedlich D. Xenopus cadherin-11 restrains cranial neural crest migration and influences neural crest specification. Development. 2001;128:3049–3060. doi: 10.1242/dev.128.16.3049. [DOI] [PubMed] [Google Scholar]
- 148.McCusker C, Cousin H, Neuner R, Alfandari D. Extracellular cleavage of cadherin-11 by ADAM metalloproteases is essential for Xenopus cranial neural crest cell migration. Mol Biol Cell. 2009;20:78–89. doi: 10.1091/mbc.E08-05-0535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ciesiolka M, Delvaeye M, Van Imschoot G, Verschuere V, McCrea P, van Roy F, Vleminckx K. p120 catenin is required for morphogenetic movements involved in the formation of the eyes and the craniofacial skeleton in Xenopus . J Cell Sci. 2004;117:4325–4339. doi: 10.1242/jcs.01298. [DOI] [PubMed] [Google Scholar]
- 150.Rangarajan J, Luo T, Sargent TD. PCNS: a novel protocadherin required for cranial neural crest migration and somite morphogenesis in Xenopus . Dev Biol. 2006;295:206–218. doi: 10.1016/j.ydbio.2006.03.025. [DOI] [PubMed] [Google Scholar]