Abstract
In cells, the levels of sterol vary greatly among organelles. This uneven distribution depends largely on non-vesicular routes of transfer, which are mediated by soluble carriers called lipid-transfer proteins (LTPs). These proteins have a domain with a hydrophobic cavity that accommodates one sterol molecule. However, a demonstration of their role in sterol transport in cells remains difficult. Numerous LTPs also contain membrane-binding elements, but it is not clear how these LTPs couple their ability to target organelles with lipid transport activity. This issue appears critical, since many sterol transporters are thought to act at contact sites between two membrane-bound compartments. Here, we emphasize that biochemical and structural studies provide precious insights into the mode of action of sterol-binding proteins. Recent studies on START, Osh/ORP and NPC proteins suggest models on how these proteins could transport sterol between organelles and, thereby, influence cellular functions.
Keywords: Sterol transport, Lipid transfer protein, Osh/ORP, START
Introduction
In cells, despite constant material exchanges, differences in lipid composition among organelles are kept steady thanks to the coordination between lipid metabolism and lipid transfer. Notably, a gradient of sterol exists throughout the endomembrane system. In mammalian cells, cholesterol is rare in the endoplasmic reticulum (ER, 5 mol % of lipids) but far more concentrated (up to 30 mol %) in the trans-Golgi network (TGN), the endosomal recycling compartment (ERC) and the plasma membrane (PM) [1, 2]. Strikingly, the PM holds nearly 60 % of the cellular sterol, while the ER accounts for only 5 % [3]. The existence of a cholesterol gradient from the cis to the trans-Golgi has also been suggested [4]. In the budding yeast Saccharomyces cerevisiae, a similar distribution is seen for ergosterol. The yeast PM contains ~108 ergosterol molecules, which corresponds to ~40 mol % of lipids at the PM and about 70 % of total cellular ergosterol [5]. Instead, the ER displays only a low concentration of ergosterol [6].
Enrichment of sterol in certain compartments is directly related to the atypical, rigid chemical structure of this lipid. Sterol is known to alter membrane physicochemical properties [7]. Due to its intrinsic rigidity, it reduces the flexibility of neighboring phospholipid acyl-chains, thereby increasing membrane thickness and impermeability to small molecules. In addition, sterol makes preferential contacts with lipids bearing saturated fatty acyl-chains and large polar heads, typically sphingolipids [8, 9]. These intermolecular contacts might drive the formation of well-ordered lipid domains, ensuring membrane stiffness. It is generally accepted that high levels of sterol and sphingolipids make the PM a rigid and thick divider between intracellular and external media. In contrast, the scarcity of sterol in the ER, which is also characterized by a low degree of phospholipid saturation, is thought to keep the membrane fluid for processes such as folding of integral proteins whose insertion between lipids must be facilitated.
The unequal repartition of sterol among organelles requires transport mechanisms. Sterol is largely insoluble in aqueous phase (<100 nM), and its spontaneous desorption and free diffusion between membranes occur with half-times as low as 1–2 h [10]. In cells, two types of strategies coexist to achieve fast sterol transport between organelles. First, sterols in the membrane of transport vesicles are conveyed en bloc from one compartment to another. However, this mode of transport cannot concern organelles that are unconnected to vesicular trafficking routes, such as mitochondria [11]. In addition, vesicular trafficking might not be a major route for sterol, even along the secretory pathway. In yeast, ergosterol moves from the ER to the PM mostly via nonvesicular routes. Indeed, a conditional defect of Sec18p/NSF, an essential ATPase that primes transport vesicles for fusion through the secretory pathway, has no consequence on sterol distribution. Thus, lipid transfer proteins (LTPs) must exist to ensure non vesicular transport [12]. As detailed in this review, a central issue is to define the identity of these sterol carriers.
A second and related question is to understand how LTPs convey sterol among cell membranes, notably whether they could help creating a sterol gradient between the ER and the PM. A common statement is that LTPs speed up chemical equilibration of sterol between membranes. The availability of sterol for a chemical or a physical process is given by its chemical activity, a, which depends on its concentration (c) and its chemical activity coefficient (γ), according to a = γ × c. Sterols have a reduced off rate from membranes rich in sphingolipids and phospholipids with saturated chains, that is, a weak ability to participate in “collisionnal” transfer with a second membrane or soluble acceptors [13, 14]. Sterol in such membranes has a low γ due to its ability to make condensed complex with saturated lipids [13, 15]. Because saturated acyl-chains are more abundant in the PM than in the ER, γ should be lower in the PM than in the ER [16–19]. Consequently, equilibration of the chemical activity of sterol in both membranes should result to high and low amounts of this lipid in the PM and the ER, respectively. The role of LTPs would be to accelerate a process mainly driven by an enhanced stabilization and sequestration of sterol by neighboring lipids in the PM.
Eukaryotic cells are known to encode for proteins gifted with a capacity to trap sterol as well as to target membranes identified by specific markers. Some of these proteins have been suggested to transport sterol in a harmonized manner with other LTPs at organelles contact sites. We will show how recent studies, based on biochemical and structural data, provide valuable information on the mode of action of LTPs in sterol transport. We will argue that some LTPs are likely not simple sterol equilibrators but proteins with an ability to detect membrane identities and to contribute to vectorial routes between organelles.
Sterol sensing through sterol transport between PM and ER
In humans, 15 proteins of a conserved family contain a StAR-related lipid transfer (START) domain of ~210 amino-acids with distinct lipid-binding specificities [20]. Its founding member, STARD1 (or StAR), has been studied for years [21]. In steroidogenic cells, STARD1 delivers sterol into mitochondria for steroid hormone synthesis. Many START proteins have additional domains that control their cellular localization or achieve another function [22]. Five START proteins are selective for sterol (STARD1, STARD3-6), three others are phospholipid transfer proteins (STARD2/PCTP, STARD7 and STARD10), whereas STARD11, also called CERT, is a ceramide transporter [23]. Ligand specificity for the other family members has not yet been determined.
The STARD4 subgroup includes STARD4, STARD5, and STARD6: these are soluble proteins that solely consist in a START-domain specific for sterol. STARD4 and STARD5 share 30 % amino acid identity and are widely expressed [24], while STARD6 expression is restricted to a few tissues. STARD4 has been shown to regulate intracellular free cholesterol. STARD5 and STARD6 function is less documented, but it is noteworthy that STARD5 mRNA expression is promoted up to sixfold by ER stress [25], which can be induced by high levels of intracellular free cholesterol [26]. Despite several studies on STARD5, its exact role in sterol transport and metabolism remains elusive [27].
Much is known about STARD4, which seems to be directly involved in cholesterol homeostasis regulation. One of the key systems that regulate the levels of unesterified cholesterol in cells is mediated by the sterol regulatory element-binding protein-2 (SREBP-2) [28]. When the ER is poor in sterol (<5 mol %), the membrane protein SREBP-2 is escorted from the ER to the Golgi by the cholesterol-sensing protein SCAP (SREBP cleavage-activating protein). SREBP-2 is then cleaved into a cytosolic fragment, which translocates into the nucleus and activates the expression of genes involved in sterol synthesis, uptake, and metabolism. Above the 5 mol % threshold, cholesterol-bound SCAP and SREBP-2 remain in the ER, preventing SREBP-2 processing. How sterol sensed in the ER by the SCAP/SREBP-2 machinery reflects cellular sterol levels is a long standing issue. Interestingly, STARD4 has been identified as a gene whose expression is regulated by cholesterol levels [24]. On high cholesterol diet, hepatic STARD4 mRNA level decreases two-threefold in a SREBP-2-dependent manner [25]. Remarkably, STARD4 is the only gene encoding a sterol carrier among all genes for which transcription is regulated by SREBP-2 [29]. STARD4 is mostly cytosolic, but can, under certain circumstances, bind to the ER [22, 30]. In addition, overexpressing the protein enhances cholesteryl-ester formation by the ER-resident enzyme acyl-CoA:cholesterol acyl-transferase (ACAT) [31]. Recently, by imaging cells labeled with dehydroergosterol (DHE), a naturally fluorescent sterol and perfect cholesterol mimetic, Maxfield and coworkers [32] observed that STARD4 increases the rate of cholesterol transport to the ER. They noted that overexpressing STARD4 accelerates the incorporation of DHE into lipid droplets, a process that can be blocked by the ACAT inhibitor 58035 [32]. Other clues suggest that STARD4 acts as a sterol transporter. In vitro, one STARD4 protein can transfer 7 sterol molecules per minute between liposomes [32]. In cells, a high level of STARD4 prevents the ER-to-Golgi trafficking of SCAP, presumably as a consequence of higher ER sterol levels. Conversely, reduction of STARD4 expression by siRNA results in an increase in free cholesterol, which is likely related to a reduction in the SREBP-2 response and a reduction in ACAT-dependant cholesterol esterification. These data suggest desensitization of the cholesterol homeostatic machinery when STARD4 cellular levels are reduced. Note that excessive amounts of STARD4 may also lead to a desensitization (Fig. 1a). In this case, the ER-resident homeostatic machinery would be oversupplied in sterol, and could not perceive mild variations of cellular sterol level. Therefore, fine-tuning of STARD4 abundance seems to be required for optimal sterol sensing and this likely explains why its expression must be controlled by SREBP-2. In conclusion, it seems that STARD4 ensures rapid cholesterol exchange, allowing the cholesterol level sensed in the ER to reflect the cholesterol abundance in other organelles, in particular the PM [32]. Hence, STARD4, as a transporter, would be a key component of the cholesterol homeostasis regulatory machinery. However, unexpected results came from STARD4−/− mice recently created [33]. Although smaller than wild-type mice, their survival indicates that STARD4 is not essential. A functional redundancy might occur among START domain proteins or with other families of transporters.
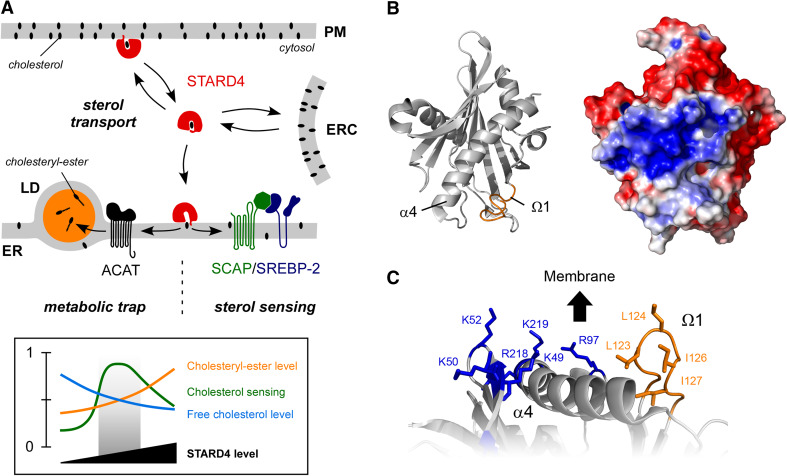
Fig. 1.
Intracellular cholesterol transport by STARD4 and its structural properties. a The top panel illustrates a model for cholesterol transport by STARD4 and its consequences on sterol metabolism. The two ER-based major regulatory mechanisms by which levels of unesterified cholesterol are controlled are represented. ACAT rapidly removes cellular free cholesterol by converting it into cholesteryl-esters, which are stored in lipid droplets (LD). This metabolic trap is thought to influence sterol delivery to the ER. STARD4-mediated sterol transport to the ER allows the SCAP/SREBP-2 homeostatic machinery to be informed about the free cholesterol levels in the cell. The bottom panel illustrates variations in free and esterified cholesterol according to the relative STARD4 abundance in the cell. Then, levels of STARD4 must be fine-tuned to optimize sterol sensing. b Left, ribbon representation of STARD4 structure featuring the Ω1 loop (orange), which presumably operates as a lid. Right, surface representation of STARD4 colored according to electrostatic potential. A close-up view of the possible membrane-interacting region of STARD4 is represented in (c). Several positively charged amino-acids (blue) are exposed near the non-polar Ω1 loop
How does STARD4 deliver sterol into membrane? Structural data suggest helpful prospects. To date, structures for the mammalian START domains of STARD1-5, STARD11/CERT, STARD13, and STARD14 have been solved [34]. START domains are characterized by an α/β “helix-grip” fold, in which a 9-stranded antiparallel β-sheet is bordered by long N-terminal and C-terminal helices (α1 and α4). Two Ω loops are inserted between strands β5 and β6 (Ω1) and strands β7 and β8 (Ω2). The concave face of the β-sheet and the C-terminal α-helix form a hydrophobic cavity (~850 Å3 in the case of sterol-binding START domains), which could accommodate one lipid molecule (the volume of cholesterol is 741 Å3) [35]. Unfortunately, none of the sterol-binding START domains has been crystallized with its ligand. However, computational simulations provide reasonable models for how sterol interacts with the hydrophobic cavity. It has been suggested that the hydroxyl function of cholesterol is oriented toward the bottom of the pocket [36, 37], leading to specific interactions between the ligand and exposed amino-acids of the cavity [22, 38]. More difficult is to predict how the START domain can pick up or release sterol.
In various LTPs, lipid exchange is controlled by a molecular lid that covers the entrance of the lipid-binding pocket. For START proteins, it has been initially suggested that sterol release depends on partial unfolding of the protein into a “molten-globule” state. For STARD1, this process could occur as the protein approaches the acidic environment of the mitochondrial outer membrane (supposed to be pH ~4.5) [39]. A second model proposes that the opening of the binding pocket results solely from the unfolding of the C-terminal α4 helix [38]. However, because α4 is part of the wall of the hydrophobic cavity, such a drastic conformational change seems unlikely, as it would leave the cavity wide open to the solvent. Moreover, molecular dynamics simulations suggest that the STARD1 structure remains stable, even at pH 4 [40]. Interestingly, conformation transitions of the Ω1 loop were remarkably frequent during simulations. Along with this, in the crystal structure of the apo-form of the CERT START domain, the B-factors (indicative of local motions) were found to be high for the Ω1 loop but not for the α4 helix [41]. Other simulations suggest that the structural changes involved in cholesterol release are restricted to the Ω1 loop, which adopts an open conformation as cholesterol leaves the cavity and then closes once the lipid is away [36].
It is likely that the START domain can recognize donor and acceptor membranes for sterol exchange. For many LTPs, a membrane-binding site exists near the entry of the lipid-binding cavity. In the phosphatidylinositol transfer protein α (PI-TPα), a loop between two β-strands (called lipid exchange loop) acts as a lid but also contributes to anchor the protein to membranes [42]. By homology to PI-TPα, the hydrophobic Ω1 loop of some START proteins has been suggested to promote membrane binding. In the case of CERT, mutating a hydrophobic residue in the Ω1 loop affects its ceramide-tranfert activity [41]. For STARD4, a structural analysis suggests another membrane-binding site: a patch made of basic residues exposed near the Ω1 loop (Fig. 1b) [32, 35]. In vitro, the rate by which STARD4 transfers sterol between liposomes is enhanced by up to tenfold when liposomes are enriched with anionic lipids such as phosphatidylserine (PS). It thus appears that electrostatic interactions drive the association of STARD4 with membranes, thereby facilitating sterol exchange. In line with this, neutralizing the basic patch by mutagenesis decreases STARD4 efficiency. Interestingly, an even higher transfer rate (up to threefold) was observed when the acceptor but not the donor liposomes were enriched with unsaturated lipids [32]. It is possible that, akin to other START proteins, the STARD4 Ω1 loop participates in membrane binding; its insertion would be facilitated by loose lipid packing induced by lipid unsaturation. Because the PM cytosolic leaflet is strongly enriched in anionic lipids [43], and the ER contains more unsaturated lipids than any other organelle, the in vitro experiments support a role of STARD4 in PM-to-ER sterol transfer.
STARD4 has been found to deliver sterol to the ERC [32], and also to mitochondria, although its expression is not activated, like STARD1, during steroidogenesis [25]. Thus, one can wonder to which extent STARD4 can specifically target given compartments. To examine this, intracellular sterol transport mediated by STARD4 has been compared with that of the simple sterol carrier methyl-β-cyclodextrin (MCD). Interestingly, like STARD4 overexpression, microinjecting MCD increases the rate of sterol delivery to the ER, and can even restore normal levels in free cholesterol and sterol esterification in cells lacking STARD4 [32]. The fact that a simple and “blind” sterol equilibrator can replace STARD4 in a key regulatory mechanism of cholesterol homeostasis suggests that the ER, through the activity of ACAT and generation of cholesteryl-ester, behaves as a sink for free cholesterol. Sterol transport mediated by STARD4 would be driven by the difference in cholesterol chemical activity. Nevertheless, in vitro experiments strongly suggest that STARD4, owing to its structural features, is kinetically more efficient than MCD to rectify free sterol imbalance between PM and ER membranes. By doing so, STARD4 will be the perfect partner of the ER-based sterol regulatory machinery, allowing rapid responses to slight changes in sterol level. More work is needed to determine the exact contribution of STARD4 structural features, in particular the Ω1 loop, in membrane targeting.
Sterol transport to mitochondria: a worthy suicide
In steroidogenic cells, STARD1 supplies mitochondria with cholesterol for steroid hormone synthesis [21]. STARD1 has a mitochondrial import signal, which allows this protein to enter into mitochondrial matrix; but, from a mechanistic point of view, the presence of this sequence has long been a mystery. Initially, STARD1 was supposed to transit sterol from the outer to the inner mitochondrial membranes [44]. However, Miller and coworkers [45] reported later that a cytosolic form of STARD1, lacking its mitochontrial presequence, was fully active and supplied mitochondria with sterol. It appears now that a burst of STARD1 production, in response to physiological needs, is followed by the import of the protein into mitochondria [46]. This protein “suicide” strategy would generate a single wave of cholesterol to the mitochondria, leading to rapid induction and termination of steroid hormone synthesis. This clever mechanism would prevent cell toxicity caused by hormone overproduction [45]. Pathological effects of STARD1 in lipid metabolism disorders have been reviewed elsewhere [46, 47].
Cholesterol efflux from late endosome and lysosome: a struggle for leaving the nest
In mammalian cells, less that 5 % of cellular sterol is synthesized; the rest is brought by receptor-mediated endocytosis of low-density lipoproteins (LDLs), which convey cholesterol esters through the bloodstream [48]. After LDL delivery to late endosomes and lysosomes (LE/LY), cholesteryl-esters are converted into cholesterol, which escapes rapidly from these compartments to reach other membranes, including PM and ER. A large effort has been made to apprehend the mechanism by which cholesterol exits from LE/LY, as any deficiency in this process leads to the Niemann-Pick C (NPC) disease, a rare and fatal hereditary lysosomal storage disorder [49]. Cholesterol efflux is impaired when genes encoding NPC1 (Niemann-Pick C-1 protein) or NPC2 are mutated. Consequently, LE/LY become anomalous entities with internal whorls of membrane accumulating not only LDL-derived cholesterol but also cholesterol coming from other organelles [50]. A general depletion in free cellular cholesterol takes place and affects the trafficking of proteins and other lipids, such as sphingolipids, thereby leading to pathogenesis [51]. NPC1 and NPC2 are not structurally related but both contain a module that binds cholesterol with a 1:1 stoechiometry (Fig. 2a). NPC1 is a large polytopic protein that resides in the LE/LY-limiting membrane (Fig. 2b). It exhibits a luminal N-terminal domain of 240 amino acids called NPC1(NTD) that binds sterol with a nanomolar affinity [52, 53]. NPC2 is soluble, localized in the lumen of LE/LY, and also has a sterol-binding ability. By which mechanism do NPC1 and NPC2 drive sterol out of LE/LY?
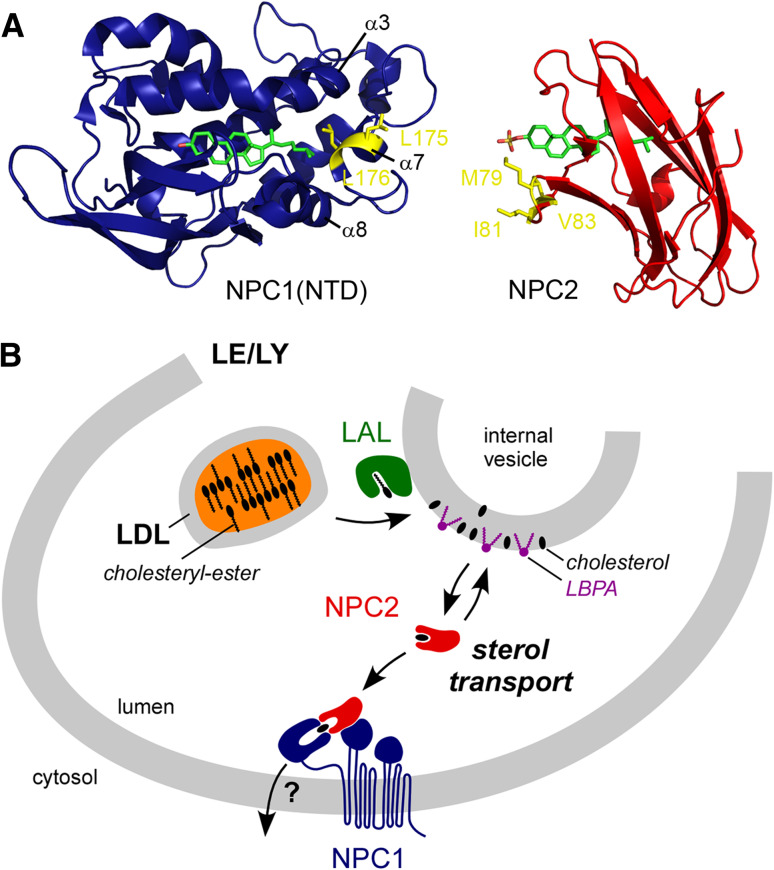
Fig. 2.
Cholesterol efflux from late endosomes and lysosomes mediated by NPC proteins. a Ribbon representation of the structures of NPC1(NTD) bound to cholesterol and NPC2 bound to cholesterol sulfate. The three short helices (α3, α7 and α8) of NPC1(NTD) must be displaced, presumably by NPC2, to let the sterol access the hydrophobic tunnel. Amino acids that are crucial for cholesterol transfer between NPC2 and NPC1(NTD) are colored in yellow on both structures. b Model for the sequential action of lysosomal acid lipase (LAL), NPC2 and NPC1 for cholesterol egress from LE/LY. Cholesteryl-esters are hydrolyzed by LAL to produce free cholesterol and fatty acid. Then, sterol from membrane is transferred to NPC2 by a mechanism that is accelerated by LBPA, which comprises 15 % of late endosomes total phospholipids [135]. In fact, it is currently unknown if LAL transfers cholesterol to internal vesicle membranes or directly to NPC2. The unusual orientation that sterol adopts in NPC2 could be problematic considering an extraction from lipid membrane, but may be facilitated by direct protein–protein sterol transfer between LAL and NPC2 [52, 136]. Finally, NPC2 interacts with NPC1 and transfers sterol to its NTD. How NPC1 operates to excise cholesterol from LE/LY is still obscure
NPC2 has the simplest structure possible, merely a small sterol-binding pocket closed by a hydrophobic “aperture”, which opens when sterol goes through [54, 55]. NPC2 extracts cholesterol from liposomes with rates that increase by two orders of magnitude in the presence of lysobisphosphatidic acid (LBPA), a LE/LY-specific lipid, but not with other anionic lipids such as PS [56]. Because LBPA has no effect on sterol transfer by MCD [57], LBPA seems to mediate specific membrane recognition by NPC2, rather than modulating the sterol chemical activity coefficient (γ). Although several clusters of positively charged residues are exposed on the NPC2 surface, the specificity for LBPA is not well understood [58].
NPC2 is able to transfer sterol not only between liposomes, but also, and interestingly, from liposomes to the NPC1 domain NPC1(NTD) [59]. A soluble version of NPC1(NTD) achieves sterol extraction and delivery to membranes, two steps found to be accelerated by NPC2. Thus, it has been proposed that NPC2 transfers cholesterol to and/or from NPC1 in the LE/LY lumen [59]. The structure of NPC1(NTD) solved recently indicates that the entry/exit of sterol must rely on the rearrangement of three short helices (α3, α7 and α8) and/or of a loop between helices α8 and α7, which defines the entrance of the hydrophobic tunnel (Fig. 2a) [52]. Interestingly, sterol transfer from NPC2 to NPC1(NTD) is strongly impaired by mutations of hydrophobic residues near the tunnel entrance of each protein [60]. This suggests that NPC2 interacts with NPC1(NTD) to open its binding pocket, and cholesterol would then shuttle between the two NPCs. Efforts to detect a NPC2/NPC1(NTD) complex have so far been unsuccessful. A distinct study provides evidences for a direct (and cholesterol-dependent) interaction between NPC2 and the luminal domain 2 of NPC1 [61]. This interaction may stabilize NPC2 near NPC1(NTD) to facilitate sterol transfer. How NPC1 proceeds to carry cholesterol outside from LE/LY is still unknown, but it is noteworthy that the predicted 13 transmembrane helices of NPC1 encompass a putative sterol sensing domain (SSD), related to SSDs present in other proteins regulating sterol metabolism, such as SCAP and HMG-CoA reductase [62]. Although the SSD appears to be involved in the sterol export function of NPC1 [63], its exact mode of action has not yet been elucidated.
Other proteins, such as STARD3, might function downstream (or in parallel) from NPC1 to facilitate cholesterol egress from LE/LY. STARD3 (aka MLN64) is inserted in the LE-limiting membrane through a multispanning transmembrane N-terminal domain, and has a C-terminal sterol-binding START domain facing the cytosol [64]. A recent study suggests that STARD3 transfers sterol from LE to mitochondria [65]. In NPC1-deficient cells, a build-up in mitochondrial cholesterol was observed, which was attenuated when STARD3 was silenced. Measurements of pregnenolone production indicate that STARD3 knockdown also impairs cholesterol transport (by 30–40 %) to mitochondria in normal cells. However, previous studies showed that STARD3 has a weak steroidogenic activity compared to STARD1 [66]. Moreover, mice lacking functional STARD3 appear normal, with no defect in steroidogenesis and only minor sterol metabolism alterations [67]. The LE-to-mitochondria sterol flux mediated by STARD3 might be a minor pathway and/or might be superseded by other LTPs [67]. Nevertheless, it is worth noting that STARD3 is ubiquitously expressed; therefore, it might be involved in some facets of sterol metabolism concerning both LE/LY and mitochondria, such as oxysterol production stimulated by LDL-derived cholesterol [68]. In this regard, STARD3 could play a significant role in tissues devoid of STARD1; however, a clear understanding of its function in cholesterol trafficking is still lacking. Recent data indicate that STARD3 overexpression prevents increase in cholesterol esterification in response to acetylated LDL and enhances cholesterol efflux to apolipoprotein A1 [69]. These findings corroborate other data showing that STARD3 deficiency in mice leads to a significant increase in hepatic cholesterol ester upon high-fat diet feeding [67]. These data suggest that STARD3 prevents the delivery of LDL-derived free cholesterol to the ER and its subsequent esterification by ACAT. Alternatively, STARD3 could participate in ER-to-LE sterol transfer. More work will be needed to unveil the exact function of STARD3 in these processes.
Sterol/PI(4)P counterexchange: new hypothesis for Osh/ORP proteins function
Osh/ORP proteins
The Osh/ORP family, which exists in all eukaryotes, seems to play a key role in sterol transport. Its founding member, OSBP, is characterized by a C-terminal oxysterol-binding domain. By definition, all Osh/ORP proteins contain an OSBP-related domain (ORD) whose hallmark is the EQVSHHPP sequence motif [70]. Mammals, including humans, have 12 ORP encoding genes, which give, through alternative translation or splicing, 16 main ORPs classified into six subgroups [71]. The yeast S. cerevisiae genome encodes seven ORP homologues (Osh protein) which are divided into four subgroups (Osh1p/Osh2p; Osh3p; Osh4p/Osh5p; Osh6p/Osh7p) [71, 72]. Most of the Osh/ORP proteins contain not only an ORD at the C-terminus but also a N-terminal PH domain to interact with phosphoinositides (PIPs) present on some subcellular membranes and an internal FFAT motif [two phenylalanines (FF) in an acidic tract] recognized by the ER receptors VAP-A and VAP-B (Scs2p/Scs22p in yeast). Osh1-2p and ORP1L also contain ankyrin repeats whereas Osh3p has a GOLD (Golgi dynamics) domain. ORP5 and ORP6 do not have a FFAT motif but a TM domain downstream of the ORD. Osh4p and Osh5p share 70 % of similarity and are the shortest Osh, consisting only of an ORD domain. Osh6p and Osh7p are slightly longer with an additional segment of ~40 amino-acids upstream of the ORD. Some ORP proteins exist in shorter versions with only an ORD (ORP1S, ORP4S) or a FFAT motif and an ORD (ORP2S, ORP9S).
A central question is to know whether Osh/ORP proteins act as sterol transporters. Yeast is a valuable model to address this issue, because it does not encode for START proteins. In pioneer works, Rine and coworkers [72, 73] found that the absence of the entire Osh family is lethal. In yeast strains lacking all but one Osh genes, turning off the last remaining Osh gene causes accumulation of sterol in intracellular structures at the expense of PM. Any of the seven Osh, expressed alone, was found to restore cell viability and a correct sterol distribution. Thus, Osh proteins have been proposed to share an overlapping function: to transfer newly synthesized sterol from ER to PM [5, 74]. However, Prinz and coworkers [75] suggested that Osh proteins transport sterol in the opposite direction, from PM to ER. Sterol accumulation in the ER and its esterification by ACAT was slowed down ~7 times when all Osh were missing. Osh4p and other Osh have been shown to transport sterol between liposomes in vitro [75]. The fact that Osh proteins can transfer sterol between the ER and the PM in both directions suggests that they simply act as sterol equilibrators [74]. Ikonen and coworkers also provided the first evidence that ORPs have the ability to transfer cholesterol in live cells [76]. However, Menon and coworkers [77] reported more recently, notably by imaging DHE in yeast, that sterol transfer from PM to ER does not rely on Osh proteins. Surprisingly, they also concluded that, in contrast to previous results [5, 73, 74], the Osh proteins are not essential for the transport in the opposite direction (ER to PM). Overall, these reports did not clarify whether or not Osh/ORP proteins are authentic LTPs in cells. Any conclusion was actually difficult to draw because of intriguing data on Osh4p: several studies indicated that Osh4p is involved at the trans-Golgi in PI(4)P metabolism, and, as a repressor of exocytosis, two functions hard to relate to an ability to convey sterol between compartments [74].
PI(4)P is a master regulator lipid at the TGN, driving, together with membrane-associated Arf and Rab, small G proteins, the genesis of various coated and noncoated transport vesicles [78]. PI(4)P and activated Arf1 recruit the clathrin adaptor AP-1 or GGA (Golgi-localized, γ-ear-containing Arf-binding protein), which couples the gathering of protein cargo to the formation of the clathrin coat. Once generated, clathrin-coated vesicles transport specific cargo to the endosomal system. Other vesicles derive from the TGN to ensure polarized exocytosis, a major secretory pathway that supplies PM with lipids and proteins during asymmetric cell division [78]. These vesicles fuse with the PM to promote the expansion of a bud that evolves into a daughter cell. In this pathway, PI(4)P and the Rab Ypt32p-GTP recruit Sec2p at the vesicle surface, which in turn activates the Rab protein Sec4p [79]. Myo2p, a type V myosin motor that conveys vesicles along actin cables towards the cell bud is also first recruited by PI(4)P and Ypt32p-GTP, and then by Sec4p-GTP [80]. Last, Sec4p-GTP recruits Sec15p, a subunit of the exocyst complex that eventually tethers vesicles to the PM and promotes membrane fusion [79].
In yeast, PI(4)P is synthesized by two PI-4 kinases: Pik1p produces PI(4)P at the TGN whereas Stt4p does it at the PM [81]. Inactivating Pik1p stops exocytosis from the TGN [82, 83]. Two other proteins participate in regulating PI(4)P: first, the PI/PC transfer protein Sec14p maintains proper PI levels in the TGN, thereby promoting a PI(4)P production by Pik1p [84]. Second, the PI(4)P phosphatase Sac1p, which resides mainly at the ER, converts PI(4)P into PI [85, 86]. Osh4p has strong genetic interactions with Pik1p, Sac1p and Sec14p [87–89]. Notably, Osh4p is the only Osh whose expression is lethal in Sec14p-deficient yeast [88, 89]. Lack of Sec14p results in a drop of PI(4)P level, which becomes limiting. In this context, it has been proposed that all remaining PI(4)P molecules are monopolized by Osh4p, whose endogenous expression is high [87, 90]. Interestingly, Sac1p deletion counterbalances this lethal effect [89]. More intriguingly, overexpressing Osh4p reduces the cellular level of PI(4)P [87]. Altogether, these data suggested a strong link between Osh4p and PI(4)P metabolism but was difficult to integrate with the idea that Osh4p was a genuine sterol transporter.
Osh4p is a sterol/PI(4)P exchanger
Key insights into Osh proteins function came from the structural and biochemical analysis of Osh4p. In 2005, Hurley and coworkers [91] described the structure of Osh4p both in the presence and in the absence of sterols. Its structure consists in a 19-strand β-barrel forming a cavity to accommodate one molecule of cholesterol, ergosterol or oxysterol. Upon sterol-binding, the N-terminal segment (29 amino acids) forms a lid that blocks the sterol molecule. (Fig. 3a). The sterol 3-hydroxyl moiety makes direct and water-mediated contacts with polar residues at the bottom of the pocket. When Osh4p is empty, the N-terminal segment is unfolded and leaves the pocket accessible. These results suggested that Osh proteins adopt two distinct conformations: an empty form that might target a donor membrane to pick up sterol and a sterol-bound form that recognizes an acceptor membrane to supply it with sterol. Interestingly, we found that the lid could alternatively fold into an ALPS motif that senses lipid-packing defect, suggesting an ability for Osh4p to extract sterol from curved membranes [92].
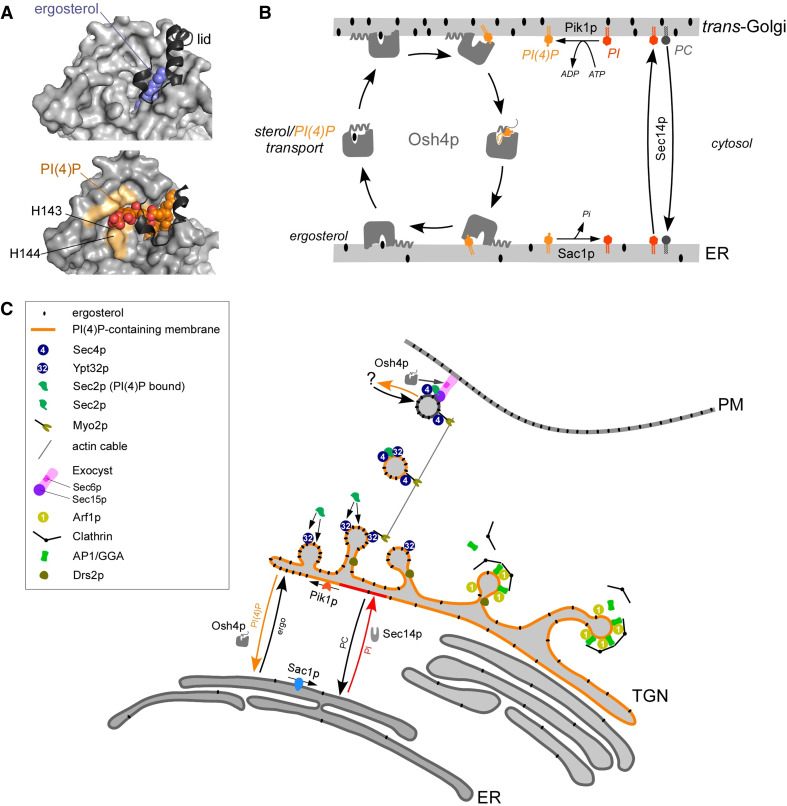
Fig. 3.
Osh4p is a sterol/PI(4)P exchanger. a Close-up view of the surface of Osh4p in complex with ergosterol (blue, top panel, PDB 1ZHZ) or PI(4)P (in orange with oxygen in red, bottom panel, PDB 3SPW). The residues involved in PI(4)P polar head recognition are colored in light orange. The N-terminal lid is represented in a dark gray ribbon. b Speculative model of the function of Osh4p (see details in the main text). c At the TGN, the genesis of exocytotic vesicles destined to the PM depends on the recruitment of Sec2p and Myo2p by PI(4)P and Ypt32p. Sec2p activates Sec4p whereas Myo2p moves vesicles along actin cables toward the PM. By exchanging sterol for PI(4)P at the ER/trans-Golgi interface, Osh4p might synchronize the recruitment of PI(4)P-binding effectors with the presence of sterol in the TGN to generate sterol-rich exocytotic vesicles. Osh4p could also extract PI(4)P from exocytotic vesicles at a late stage to trigger a conformational change in Sec2p. Sec2p would activate Sec4p, Sec4-GTP would recruit Sec15p and Sec15p would help retain Sec2p on vesicles. High levels of Sec4-GTP and Sec15p will allow vesicles to dock and fuse with the PM
Beside this, Osh4p has been assumed to bind PI(4)P or other PIP to target cell regions. This was puzzling from a structural point of view because Osh4p did not have any known PIP-binding domain [91]. In addition, in vitro binding assays failed to prove that Osh4p could target a PI(4)P-containing membrane [89, 93, 94]. However, it was proposed that Osh4p could, by detecting organelle identity, transport sterol in a vectorial manner [75]. Unexpectedly, by using novel DHE-based assays, we found that PI(4)P specifically inhibits sterol extraction because PI(4)P is itself extracted by the protein. We solved the structure of the Osh4p:PI(4)P complex and revealed how sterol and PI(4)P molecules, whose chemistry is unrelated, compete with each other [95]. Competition is explained by the fact that the two acyl-chains of PI(4)P occupy the sterol-binding pocket. The PI(4)P head lies in an adjacent shallow pocket: the phosphate groups at position 1 and 4 of the inositol ring contact exposed residues, notably the H143/H144 pair (Fig. 3a). Compared to the sterol-bound form, the lid adopts a different conformation to accommodate the PI(4)P molecule. Importantly, the H143/H144 pair belongs to the SEQVSHHPP sequence signature of all Osh/ORP. Other residues interacting with the PI(4)P head are also phylogenetically conserved [91]. Therefore, PI(4)P extraction might be a general function of Osh/ORP proteins.
Not only do these data unveil the missing link between Osh4p, sterol and PI(4)P but they also suggest a new role for the protein between the ER and TGN. The ER is almost neutral and devoid of PI(4)P [6, 96] whereas the TGN surface is pinpointed by PI(4)P and rich in anionic lipids [6, 97]. Osh4p seems designed to ensure a one-way sterol transport from the first to the second compartment. First, Osh4p solubilizes efficiently sterol in vitro from neutral, loosely packed liposomes comparable to the ER. We found that the lid could impede any return of sterol to such membranes: DHE extraction by Osh4p is fast but back delivery is ~20-fold slower. This would make extraction from the ER almost irreversible. Second, sterol is fully released by Osh4p into PI(4)P-containing membrane mimicking the TGN because of sterol/PI(4)P exchange. Thus, the PI(4)P-binding site also contributes to transport vectoriality. Eventually, Osh4p was found to transport efficiently sterol in vitro in an asymmetric system; that is, from ER-like to Golgi-like membranes. Most importantly, we reported that Osh4p, by sterol/PI(4)P exchange, transports the two lipids along opposite routes between membranes [95].
From these observations, we propose a new model in which Osh4p promotes sterol enrichment of the late secretory pathway at the expense of ER under the control of PI(4)P metabolism [95]. Osh4p would transport sterol from the ER to the TGN and PI(4)P backward. Then, PI(4)P would be converted into PI by Sac1p. Lastly, Sec14p would promote the delivery of PI at the trans-Golgi and its phosphorylation into PI(4)P Golgi by Pik1p. This could explain how Sac1p and Pik1p regulate the PI(4)P pool despite their different locations. The lethality induced by Osh4p in Sec14p-deficient yeast [89] would not be due to Osh4p interaction with all residual PI(4)P molecules as formerly proposed, but because Osh4p extracts PI(4)P physically from the Golgi membrane and transports it to Sac1p. Moreover, we noted that the consumption of ATP by Pik1p to make PI(4)P would provide energy to ensure net transfer of sterol to the late secretory compartments (Fig. 3b).
Regulation of sterol and PI(4)P balance at the TGN by Osh4p
Recent cellular studies emphasized that Osh proteins are likely modulators of PI(4)P signaling rather than sterol transporters. We suggest that these two proposed functions, at least for Osh4p, are not mutually exclusive. Alfaro and coworkers [98] clearly depicted that Osh4p regulates PI(4)P levels during polarized exocytosis, but this is strongly modulated by its ability to bind to sterol. Mousley et al. [99] also demonstrated that Osh4p acts as a sterol-dependant antagonist of PI(4)P signaling and membrane trafficking at TGN/endosomal level, and observed that this impacts nutrient sensing through modulation of amino-acid and NH4 + permease activities at the PM and downstream TOR-dependant signaling pathways. Lastly, Menon and coworkers [77] suggested that Osh proteins do not transfer sterol in yeast but rather impact membrane sterol organization in the late secretory compartments. We make the assumption that these observations and the sterol-dependant regulation of PI(4)P at the TGN level by Osh4p might directly rely on its sterol/PI(4)P transport activity. More precisely, we hypothesize that Osh4p by lipid exchange impacts the sterol/PI(4)P balance at the TGN and could even help to operate and coordinate changes in composition/organization of membranes with remodeling processes. This is likely key for the genesis of exocytotic vesicles with proper features from the TGN.
For conceptual reasons and likely technical constraints, Osh transport activity has been analyzed mainly between the ER and the PM [100]. The role of Osh4p in lipid transport at the Golgi level has not been addressed [75, 77], despite the fact that this organelle is one of the major hub by which sterol transits and where Osh4p seems to operate. It is well known that the membrane organization of the trans side undergoes massive changes: a maturation of the loosely packed membrane deriving from the cis-Golgi into a rigid and asymmetric membrane is driven by sphingolipid synthesis, flippase activity, and sterol supply [6, 101, 102]. The biogenesis and the final features of exocytic vesicles are believed to depend on the lipid composition and organization of TGN membranes, which ultimately impact on the PM. Interestingly, Menon and coworkers [77] found that inactivating Osh proteins, or even only Osh4p, alter the PM organization, notably the distribution of sterol in the external leaflet of the PM. One can suggest that Osh4p could play an important upstream role by tuning lipid composition of the vesicles that fuse with the PM. In this scenario, Osh4p does not ensure massive equilibration between compartments, but helps local TGN membrane remodeling by exchanging PI(4)P for ergosterol at the ER/TGN interface.
The budding of exocytotic vesicles does not seem to result from the mechanical action of a coat and remains poorly understood. Sphingolipids and sterols have been proposed to segregate into liquid-ordered microdomains acting as sorting platforms for integral PM proteins that must travel to the PM. These domains are believed to coalesce into larger ones that finally bud to alleviate the energy penalty due to their presence in a surrounding membrane of higher fluidity [102]. Still in debate, this model is supported by the observation that exocytotic vesicles are highly enriched in sterol and sphingolipids, compared to the TGN [18]. Moreover, a fine tuning of the level of these two lipids at the TGN seems required to sort proteins. Indeed, it has been shown that inactivating genes coding for ergosterol (Erg4, Erg6) or sphingolipids synthesis (Elo3, Sur2) arrests the transit of Pma1p, a major PM H+-ATPase, or FusMidp-GFP, a model lipid raft-associated PM cargo, from the TGN to the PM [103].
Inactivating Osh4p seems to have little impact on the transport mediated by coated vesicles toward endosomes and vacuoles [73]. In contrast, Osh inactivation blocks the exocytosis of FusMidp [103]. Strikingly, the resulting phenotype is similar to that seen when no ergosterol is synthesized. This suggests that Osh4p delivers ergosterol to TGN in PI(4)P-flagged regions and, thereby, governs the lipid-based budding of exocytotic vesicles. Interestingly, in the Alfaro et al. study, an Osh4p mutant defective in sterol-binding was shown to block exocytosis [98]. It is conceivable that PI(4)P extraction by this Osh4p mutant is not counterbalanced by sterol at the TGN through the competition mechanism described previously. Consequently, without PI(4)P, Sec2p and Myo2p are not recruited and no vesicle generated. Interestingly, stopping ergosterol production by inactivating Erg9 gene allows wild-type Osh4p to phenocopy its sterol-binding-deficient version. Here, Osh4p-mediated depletion of PI(4)P would be higher because there is no competing sterol around. This again suggests that Osh4p exchanges sterol and PI(4)P at the TGN level and that any alteration of this process perturbs exocytosis by changing the level of one or the other lipid at the TGN.
We hypothesize that Osh4p, by promoting sterol/PI(4)P exchange, could synchronize the recruitment of PI(4)P-binding effectors with the presence of sterol in the TGN to generate sterol-rich vesicles. Low amounts of sterol would promote robust PI(4)P extraction by Osh4p. This would delay vesicle formation but initiate the cycle of sterol/PI(4)P exchange at the ER/TGN interface. Once sterol at the TGN increases, PI(4)P extraction would be slowed down and TGN PI(4)P would reach levels allowing effectors to be recruited (Fig. 3c). Interestingly, Osh4p has been shown to inhibit Drs2p [104, 105], a flippase whose activity is stimulated by PI(4)P [106]. It is assumed that the generation of lipid asymmetry by the displacement of PS and PE (phosphatidylethanolamine) from the lumenal to the cytosolic leaflet of the TGN induces curvature [107] and helps membrane budding. Osh4p by modulating PI(4)P level and thereby controlling Drs2p activity could also coordinate the creation of a lipid asymmetry with sterol import. More work is needed to assess how Osh4p activity is coupled to other lipid regulatory mechanisms.
Docking regulation by Osh4p
Osh4p also seems important for a late stage of exocytosis. A decrease in PI(4)P at the surface of exocytotic vesicles triggers a conformational switch in Sec2p, which gains the ability to bind to Sec15p, an exocyst subunit [108]. A positive feedback loop likely takes place: Sec15p, recruited by activated Sec4p on vesicles, retains Sec2p, which in turn activates Sec4p. High levels of activated Sec4p and Sec15p would allow the vesicles to dock and fuse with the PM [108]. It is not known how PI(4)P level decreases in vesicles. Interestingly, Alfaro et al. [98] observed, in Osh-deficient yeast, a cytosolic accumulation of vesicles decorated by Sec4p-GFP unable to dock with the PM. Osh4p restored a viable phenotype. Intriguingly, in vitro assays show that Osh4p binds to Sec6p, an exocyst subunit. It is plausible that Osh4p triggers, by extracting PI(4)P, the conformational switch of Sec2p (Fig. 3c). Segregation of sterol with sphingolipids in vesicles could help to program this protein. Tightly associated with sphingolipids, sterol would be less prone to compete with PI(4)P for extraction by Osh4p. Consequently, PI(4)P would be easily removed. Moreover, because Pik1p is likely absent from vesicles, PI(4)P could not be regenerated.
Sterol transport at membrane contact-sites: an advanced mechanism
In our model, Osh4p works indirectly with a second transporter, Sec14p, to promote lipid exchanges between ER and TGN [95]. This model is based on long-distance transport by free diffusional carriers. However, it can be seen as a prototype version of more sophisticated systems wherein several LTPs are sandwiched between two close membranes and work in a coordinated manner. A dual interaction with opposite membranes might prevent carrier dispersion in the cytosol and improve lipid transport. Membrane contact sites (MCS) between the ER and other organelles have been observed for years, but the molecular machineries that govern their assembly and function are just beginning to emerge (see reviews [109, 110]). In higher eukaryotes, MCS between ER and TGN have been revealed by electron tomography. In such regions, the intermembrane distance can be lower than 20 nm [111, 112]. LTPs from the START, Osh/ORP and (PI/PC)-transfer proteins families might coordinate important lipid metabolic pathways in these specialized zones [109, 113, 114].
In mammalian cells, there is evidence that STARD11/CERT and OSBP work together at ER/TGN MCS to tune the sphingomyelin (SM)/sterol balance in late compartments. This hypothesis derives in part from structural analysis. These proteins share a similar architecture: in addition to a specific C-terminal lipid-binding domain, they both contain a N-terminal plekstrin homology (PH) domain and an internal FFAT motif. Both PH domains are recruited at the TGN surface by PI(4)P [23, 115]. In the case of OSBP PH, the membrane-attached small G protein Arf1 is a second anchoring determinant [116]. The FFAT motif is recognized by the ER-resident VAP receptors [117, 118]. Thus, OSBP and CERT seem well designed to bind the ER and TGN by dual-membrane determinants, thereby creating MCS or populating pre-existing ones. ER/TGN MCS could be crucial for the cells, since lack of VAP receptors leads to a dramatic change in Golgi lipid composition and morphology [119].
One appealing suggestion is that OSBP tethers Golgi and ER membranes to facilitate the positioning of CERT and other LTPs with similar dual-membrane targeting elements. SM synthesis occurs in the Golgi and requires ceramide, which is produced in the ER and supplied to the Golgi by CERT [23]. The CERT activity is activated upon binding of OSBP to 25-hydroxycholesterol (25-OH), an oxysterol [120] whose rise in concentration might reflect an increase in intracellular free cholesterol [121]. It was thus proposed that sterol sensing by OSBP acts in turn on CERT and activates SM synthesis downstream. Remarkably, Ridgway and coworkers [122–124] revealed that OSBP, initially cytosolic, relocates to the Golgi in the presence of 25-OH. Importantly, activating OSBP also triggers the recruitment of CERT onto the TGN [120]. As no direct interaction between the two proteins exists, OSBP is proposed to play a scaffolding role by bridging locally ER and TGN in response to 25-OH in order to create, by moving membranes closer, a context suitable for CERT (Fig. 4). In line with this, deleting one of the two membrane-targeting sites of OSBP impedes CERT relocalization in response to 25-OH [120]. The coincident detection of PI(4)P and Arf1-GTP by the OSBP PH domain could offer a way to precisely localize OSBP on the TGN and initiate the MCS scaffolding at the right place. However, the sterol sensing model for OSBP remains speculative as the real nature of its ligand is still disputed. Indeed, OSBP transfers cholesterol in vitro between liposomes [125]; therefore, one can suspect that an assay showing a better affinity of OSBP for 25-OH is biased by the fact that oxysterol is more water-soluble than cholesterol. Alternatively, because cholesterol is more abundant in cells than 25-OH, it could be the natural ligand of OSBP despite lower affinity.
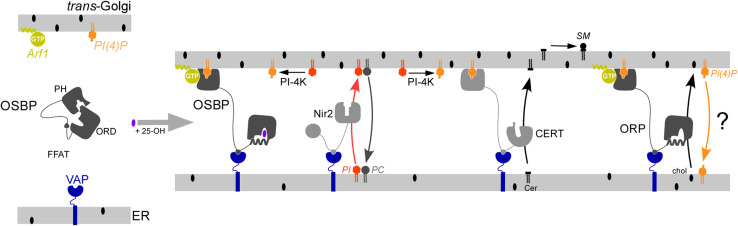
Fig. 4.
Model of the role of OSBP in creating MCS. Upon binding to 25-OH OSBP might undergo a conformational change and interact via its PH domain with the lipid PI(4)P and the small G protein Arf1 (in a GTP bound state) and, through its FFAT motif, with the ER-resident VAP receptor. As such, OSBP could bridge the ER and the trans-Golgi membrane and facilitate the recruitment of CERT at this transit zone by PI(4)P and VAP receptor. CERT would supply ceramide (Cer) to the trans-Golgi and thereby promote the synthesis of sphingomyelin (SM). The maintenance of MCS could depend on the activity of Nir2. This FFAT-containing LTP would convey phosphatidylinositol (PI) to Golgi by PI/PC exchange, promote the formation of PI(4)P by PI-4-kinase (PI-4-K) and allow in turn a stable recruitment of OSBP and other PH-containing LTPs. It is plausible that certain multidomain Osh/ORP proteins downregulate PI(4)P levels at TGN by sterol/PI(4)P exchange or by activating the ER-resident Sac1p
Active regulation of PI(4)P likely takes place at MCS to govern their maintenance through the recruitment of OSBP and CERT PH domains. Surprisingly, inactivating either PI4KIIα or PI4KIIIβ, the two major PI-4-kinases of the trans side of the Golgi in mammals, reduces PI(4)P level without preventing the 25-OH dependent recruitment of OSBP [126]. However, the absence of PI4KIIα impairs the translocation of CERT. It has also been proposed that OSBP-mediated sterol delivery to the Golgi activates PI4KIIα. In fact, this could result from lipid-modification of PI4KIIα by the activation of sterol-responsive palmitoyltransferases, thereby promoting the recruitment of the kinase at the Golgi surface [127]. An increased production of PI(4)P would in turn recruit CERT at MCS and promote SM synthesis. This amplification system may also involve Nir2, a Sec14p-like PI/PC transfer protein [128]. Nir2, owing to a FFAT motif, could locate at MCS and sustains PI(4)P formation by PI-4-kinase by supplying TGN with PI (Fig. 4) [119]. Interestingly, recent studies suggest that OSBP could have a second and antagonist pivotal role in PI(4)P regulation. Stefen and coworkers [129] have suggested that the creation of MCS between PM and cortical ER in yeast by Osh3p (which displays an OSBP-like architecture), helps the ER-located Sac1p to work on the PM in trans and reduce PM PI(4)P level. Alternatively, we suggest that Osh4p could directly transport PI(4)P to Sac1p [95]. Recently, it was reported that OSBP could extract PI(4)P in vitro [130]. Taken together, these observations suggest a working model in which OSBP or other multidomain Osh/ORP could create MCS between ER and late secretory compartments to combine sterol transfer with that of other lipids. Because Osh/ORP proteins might control PI(4)P level directly or indirectly by sterol delivery, they could organize feedback control system to govern the fate of MCS. Lastly, an additional layer of regulation might come from Arf1, whose activation promotes directly the recruitment of the PH domain of OSBP, or, indirectly, by promoting the formation of PI(4)P by PI4KIIIβ [131].
The exact mode of action of OSBP and others LTPs at MCS remains to be determined. For example, the ability of LTPs to bridge membranes has not yet been demonstrated. Likewise, how is lipid sensing coupled to lipid transfer? What is even less clear is how OSBP translates sterol-binding into a specific cell behavior. Deleting the sterol-binding domain makes OSBP constitutively bound to the Golgi, whereas a mutation within the lid of the sterol-binding domain confines OSBP with VAP receptors [120]. These puzzling data suggest an interplay between OSBP domains. Notably, an inhibitory interaction of the PH domain with the ORD has been suggested [122, 123]. A recurring hypothesis is that OSBP undergoes a conformational change upon binding to sterol, which would unmask the PH domain and target OSBP to the Golgi. In addition, the Golgi localization of OSBP seems regulated by phosphorylation of a site proximal to the PH domain [132]. More recently, it has been indicated that phosphorylation of a site between the FFAT motif and the ORD modulates the affinity of OSBP for sterol [130]. Similarly, phosphorylation has been found to mediate an autoinhibitory interaction between the PH and START domains of CERT [133].
Future perspectives
Sterol-binding proteins are often postulated to promote equilibration between membranes by shuttling sterol in a blind manner, as does MCD. This model considers that sterol extraction from a membrane by a LTP is mostly proportional to γ [100]. This proportionality has been verified experimentally for MCD, a simple and non-selective sterol carrier molecule [134], but this has not been validated for LTPs. The influence of specific lipids, not necessary modulating γ, on sterol extraction and delivery by these proteins has to be tested, notably lipids controlling the ability of LTPs to target distinct cell compartments. With Osh4p, we described a new mechanism in which a phosphoinositide is more than just an identity flag at organelle surface, but an extractable lipid that is exchanged for sterol. The ability of Osh4p to bind two different lipids is reminiscent of what is found for other LTPs. Exchanging two distinct lipids between membranes is likely an efficient strategy to ensure fast lipid transfer between organelles, and this appears valid for sterol. In addition, the model proposed for Osh4p suggests that phosphorylation of PI into PI(4)P provides energy for ensuring an active delivery of sterol to late compartments at the expense of the ER. Creating lipid “pipelines” between organelles is likely the primary advantage of MCSs. In these spatially defined regions, Osh/ORP proteins could coordinate sterol transport with PI(4)P and sphingolipid metabolism together with lipid-modifying enzymes and other lipid transporters. Because various Osh/ORP proteins might be sterol/PI(4)P exchangers, using γ as the sole index to predict sterol extraction or delivery has to be reconsidered. For example, a high level of PI(4)P could prevent sterol extraction by Osh/ORPs. Conversely, if sterol is less prone to be extracted, because of its association with sphingolipids, this should result in higher PI(4)P extraction. Therefore, it would be interesting to examine how the balance between these three lipids influences the activity of Osh proteins both in reconstituted and in cellular contexts.
An interesting question is to understand why the sterol-binding START domain and Osh/ORPs ORD are structurally different. Osh4p and maybe other Osh/ORPs have the ability to exchange sterol with PI(4)P whereas the sterol-binding START proteins seem to have sterol as sole ligand. It is plausible, as the structural versatility among START domains is high, that some of them have evolved to bind sterol and a second lipid, but this has to be determined. We also noted that it has been possible to solve the crystal structure of the sterol-bound form of Osh4p but not that of START proteins. For Osh4p, numerous data indicate that the sterol molecule is stabilized by the N-terminal lid, a key structural device that might be important for vectorial transport. Our current vision is that START proteins could be more “leaky” than Osh/ORP proteins in the sense that they may pick up and release sterol more easily, independently of a second lipid, thereby promoting fast equilibration processes in cells. Therefore, controlling their relative cellular abundance could be a suitable way to regulate sterol transport rates. Indeed, regulation at the transcription level and fast turnover seem to be the hallmark of START proteins. A better understanding of the structural aspect of LTPs in sterol transport is required to figure out the behavior of these proteins in cells.
Because the majority of LTPs have dual-membrane targeting elements in addition of a sterol-binding domain, it appears critical to know whether they work at MCS and how. Notably, a central issue is to define precisely if certain Osh/ORP proteins have gained a sophisticated design to bridge organelles and transport lipids between apposed membranes. It is assumed that a wide distance between organelles represents a major constraint against robust lipid transport. Thus, one may speculate that a spatial restriction of LTPs such as Osh/ORP proteins between two adjacent membranes may increase lipid transfer efficiency. Recently, Ikonen and coworkers [76] examined the impact of ORPs membrane-targeting domains on their ability to transport sterol in living cells. What was clearly visible was a transfer of sterol from PM to ER by short ORPs devoid of PH, likely able to freely shuttle between compartments. For other ORPs with dual-membrane targeting elements, possibly confined at MCS, no transport activity was seen. Still in infancy, such analyses raise the question whether or not the current tools are sufficiently powerful to follow sterol transport between close membranes. Attempts have also been made in vitro to understand how intermembrane distances impact sterol transport, but on Osh4p, which solely consists in a sterol-binding domain. Osh4p, by attaching opposite membranes, transports sterol faster [94]. The relevance of this mechanism proposed for Osh4p in cells remains unclear and does not explain why most of Osh/ORP proteins have developed additional molecular devices to perform this task. In the future, biochemical and structural approaches will be of first importance to get a solid understanding about the mode of action of multidomain Osh/ORP proteins.
Acknowledgments
We thank Christine Doucet for critical comments on the manuscript. Our work is financed by the ERC (Advanced Grant 268888), the CNRS and the ANR.
Abbreviations
- DHE
Dehydroergosterol
- ER
Endoplasmic reticulum
- ERC
Endosomal recycling compartment
- FFAT
Two phenylalanines in an acidic tract
- LTP
Lipid tranfer protein
- LBPA
Lysobisphosphatidic acid
- LE/LY
Late endosome/lysosome
- MCD
Methyl-β-cyclodextrin
- NPC
Niemann-Pick C
- ORP
Oxysterol-binding protein-related protein
- ORD
OSBP-related domain
- OSBP
Oxysterol-binding protein
- Osh
Oxysterol-binding homology protein
- PH
Pleckstrin homology
- PI
Phosphatidylinositol
- PI(4)P
Phosphatidylinositol 4-phosphate
- PIP
Phosphatidylinositol phosphate
- PM
Plasma membrane
- SM
Sphingomyelin
- StAR
Steroidogenic acute regulatory protein
- START
StAR-related lipid transfer
- TGN
trans-Golgi network
References
- 1.Radhakrishnan A, Goldstein JL, McDonald JG, Brown MS. Switch-like control of SREBP-2 transport triggered by small changes in ER cholesterol: a delicate balance. Cell Metab. 2008;8:512–521. doi: 10.1016/j.cmet.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maxfield FR, Menon AK. Intracellular sterol transport and distribution. Curr Opin Cell Biol. 2006;18:379–385. doi: 10.1016/j.ceb.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 3.Liscum L, Munn NJ. Intracellular cholesterol transport. Biochim Biophys Acta. 1999;1438:19–37. doi: 10.1016/s1388-1981(99)00043-8. [DOI] [PubMed] [Google Scholar]
- 4.Blanchette-Mackie EJ. Intracellular cholesterol trafficking: role of the NPC1 protein. Biochim Biophys Acta. 2000;1486:171–183. doi: 10.1016/s1388-1981(00)00055-x. [DOI] [PubMed] [Google Scholar]
- 5.Sullivan DP, Ohvo-Rekila H, Baumann NA, Beh CT, Menon AK. Sterol trafficking between the endoplasmic reticulum and plasma membrane in yeast. Biochem Soc Trans. 2006;34:356–358. doi: 10.1042/BST0340356. [DOI] [PubMed] [Google Scholar]
- 6.van Meer G, Voelker DR, Feigenson GW. Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol. 2008;9:112–124. doi: 10.1038/nrm2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maxfield FR, van Meer G. Cholesterol, the central lipid of mammalian cells. Curr Opin Cell Biol. 2010;22:422–429. doi: 10.1016/j.ceb.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mesmin B, Maxfield FR. Intracellular sterol dynamics. Biochim Biophys Acta. 2009;1791:636–645. doi: 10.1016/j.bbalip.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lange Y, Steck TL. Cholesterol homeostasis and the escape tendency (activity) of plasma membrane cholesterol. Prog Lipid Res. 2008;47:319–332. doi: 10.1016/j.plipres.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Phillips MC, Johnson WJ, Rothblat GH. Mechanisms and consequences of cellular cholesterol exchange and transfer. Biochim Biophys Acta. 1987;906:223–276. doi: 10.1016/0304-4157(87)90013-x. [DOI] [PubMed] [Google Scholar]
- 11.Prinz WA. Lipid trafficking sans vesicles: where, why, how? Cell. 2010;143:870–874. doi: 10.1016/j.cell.2010.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baumann NA, Sullivan DP, Ohvo-Rekila H, Simonot C, Pottekat A, Klaassen Z, Beh CT, Menon AK. Transport of newly synthesized sterol to the sterol-enriched plasma membrane occurs via nonvesicular equilibration. Biochemistry. 2005;44:5816–5826. doi: 10.1021/bi048296z. [DOI] [PubMed] [Google Scholar]
- 13.Ohvo-Rekila H, Ramstedt B, Leppimaki P, Slotte JP. Cholesterol interactions with phospholipids in membranes. Prog Lipid Res. 2002;41:66–97. doi: 10.1016/s0163-7827(01)00020-0. [DOI] [PubMed] [Google Scholar]
- 14.Leventis R, Silvius JR. Use of cyclodextrins to monitor transbilayer movement and differential lipid affinities of cholesterol. Biophys J. 2001;81:2257–2267. doi: 10.1016/S0006-3495(01)75873-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Radhakrishnan A, McConnell HM. Condensed complexes of cholesterol and phospholipids. Biophys J. 1999;77:1507–1517. doi: 10.1016/S0006-3495(99)76998-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schneiter R, Brugger B, Sandhoff R, Zellnig G, Leber A, Lampl M, Athenstaedt K, Hrastnik C, Eder S, Daum G, Paltauf F, Wieland FT, Kohlwein SD. Electrospray ionization tandem mass spectrometry (ESI-MS/MS) analysis of the lipid molecular species composition of yeast subcellular membranes reveals acyl chain-based sorting/remodeling of distinct molecular species en route to the plasma membrane. J Cell Biol. 1999;146:741–754. doi: 10.1083/jcb.146.4.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zinser E, Sperka-Gottlieb CD, Fasch EV, Kohlwein SD, Paltauf F, Daum G. Phospholipid synthesis and lipid composition of subcellular membranes in the unicellular eukaryote Saccharomyces cerevisiae . J Bacteriol. 1991;173:2026–2034. doi: 10.1128/jb.173.6.2026-2034.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klemm RW, Ejsing CS, Surma MA, Kaiser HJ, Gerl MJ, Sampaio JL, de Robillard Q, Ferguson C, Proszynski TJ, Shevchenko A, Simons K. Segregation of sphingolipids and sterols during formation of secretory vesicles at the trans-Golgi network. J Cell Biol. 2009;185:601–612. doi: 10.1083/jcb.200901145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pichler H, Gaigg B, Hrastnik C, Achleitner G, Kohlwein SD, Zellnig G, Perktold A, Daum G. A subfraction of the yeast endoplasmic reticulum associates with the plasma membrane and has a high capacity to synthesize lipids. Eur J Biochem. 2001;268:2351–2361. doi: 10.1046/j.1432-1327.2001.02116.x. [DOI] [PubMed] [Google Scholar]
- 20.Soccio RE, Breslow JL. StAR-related lipid transfer (START) proteins: mediators of intracellular lipid metabolism. J Biol Chem. 2003;278:22183–22186. doi: 10.1074/jbc.R300003200. [DOI] [PubMed] [Google Scholar]
- 21.Miller WL. Steroidogenic acute regulatory protein (StAR), a novel mitochondrial cholesterol transporter. Biochim Biophys Acta. 2007;1771:663–676. doi: 10.1016/j.bbalip.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 22.Alpy F, Tomasetto C. Give lipids a START: the StAR-related lipid transfer (START) domain in mammals. J Cell Sci. 2005;118:2791–2801. doi: 10.1242/jcs.02485. [DOI] [PubMed] [Google Scholar]
- 23.Hanada K, Kumagai K, Yasuda S, Miura Y, Kawano M, Fukasawa M, Nishijima M. Molecular machinery for non-vesicular trafficking of ceramide. Nature. 2003;426:803–809. doi: 10.1038/nature02188. [DOI] [PubMed] [Google Scholar]
- 24.Soccio RE, Adams RM, Romanowski MJ, Sehayek E, Burley SK, Breslow JL. The cholesterol-regulated StarD4 gene encodes a StAR-related lipid transfer protein with two closely related homologues, StarD5 and StarD6. Proc Natl Acad Sci USA. 2002;99:6943–6948. doi: 10.1073/pnas.052143799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soccio RE, Adams RM, Maxwell KN, Breslow JL. Differential gene regulation of StarD4 and StarD5 cholesterol transfer proteins. Activation of StarD4 by sterol regulatory element-binding protein-2 and StarD5 by endoplasmic reticulum stress. J Biol Chem. 2005;280:19410–19418. doi: 10.1074/jbc.M501778200. [DOI] [PubMed] [Google Scholar]
- 26.Tabas I. The role of endoplasmic reticulum stress in the progression of atherosclerosis. Circ Res. 2010;107:839–850. doi: 10.1161/CIRCRESAHA.110.224766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rodriguez-Agudo D, Ren S, Hylemon PB, Montanez R, Redford K, Natarajan R, Medina MA, Gil G, Pandak WM. Localization of StarD5 cholesterol binding protein. J Lipid Res. 2006;47:1168–1175. doi: 10.1194/jlr.M500447-JLR200. [DOI] [PubMed] [Google Scholar]
- 28.Brown MS, Goldstein JL. Cholesterol feedback: from Schoenheimer’s bottle to Scap’s MELADL. J Lipid Res. 2009;50(Suppl):S15–S27. doi: 10.1194/jlr.R800054-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Horton JD, Shah NA, Warrington JA, Anderson NN, Park SW, Brown MS, Goldstein JL. Combined analysis of oligonucleotide microarray data from transgenic and knockout mice identifies direct SREBP target genes. Proc Natl Acad Sci USA. 2003;100:12027–12032. doi: 10.1073/pnas.1534923100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodriguez-Agudo D, Calderon-Dominguez M, Ren S, Marques D, Redford K, Medina-Torres MA, Hylemon P, Gil G, Pandak WM. Subcellular localization and regulation of StarD4 protein in macrophages and fibroblasts. Biochim Biophys Acta. 2011;1811:597–606. doi: 10.1016/j.bbalip.2011.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rodriguez-Agudo D, Ren S, Wong E, Marques D, Redford K, Gil G, Hylemon P, Pandak WM. Intracellular cholesterol transporter StarD4 binds free cholesterol and increases cholesteryl ester formation. J Lipid Res. 2008;49:1409–1419. doi: 10.1194/jlr.M700537-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mesmin B, Pipalia NH, Lund FW, Ramlall TF, Sokolov A, Eliezer D, Maxfield FR. STARD4 abundance regulates sterol transport and sensing. Mol Biol Cell. 2011;22:4004–4015. doi: 10.1091/mbc.E11-04-0372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Riegelhaupt JJ, Waase MP, Garbarino J, Cruz DE, Breslow JL. Targeted disruption of steroidogenic acute regulatory protein D4 leads to modest weight reduction and minor alterations in lipid metabolism. J Lipid Res. 2010;51:1134–1143. doi: 10.1194/jlr.M003095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thorsell AG, Lee WH, Persson C, Siponen MI, Nilsson M, Busam RD, Kotenyova T, Schuler H, Lehtio L. Comparative structural analysis of lipid binding START domains. PLoS ONE. 2011;6:e19521. doi: 10.1371/journal.pone.0019521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Romanowski MJ, Soccio RE, Breslow JL, Burley SK. Crystal structure of the Mus musculus cholesterol-regulated START protein 4 (StarD4) containing a StAR-related lipid transfer domain. Proc Natl Acad Sci USA. 2002;99:6949–6954. doi: 10.1073/pnas.052140699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murcia M, Faraldo-Gomez JD, Maxfield FR, Roux B. Modeling the structure of the StART domains of MLN64 and StAR proteins in complex with cholesterol. J Lipid Res. 2006;47:2614–2630. doi: 10.1194/jlr.M600232-JLR200. [DOI] [PubMed] [Google Scholar]
- 37.Tsujishita Y, Hurley JH. Structure and lipid transport mechanism of a StAR-related domain. Nat Struct Biol. 2000;7:408–414. doi: 10.1038/75192. [DOI] [PubMed] [Google Scholar]
- 38.Lavigne P, Najmanivich R, Lehoux JG. Mammalian StAR-related lipid transfer (START) domains with specificity for cholesterol: structural conservation and mechanism of reversible binding. Subcell Biochem. 2010;51:425–437. doi: 10.1007/978-90-481-8622-8_15. [DOI] [PubMed] [Google Scholar]
- 39.Bose HS, Whittal RM, Baldwin MA, Miller WL. The active form of the steroidogenic acute regulatory protein, StAR, appears to be a molten globule. Proc Natl Acad Sci USA. 1999;96:7250–7255. doi: 10.1073/pnas.96.13.7250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baker BY, Yaworsky DC, Miller WL. A pH-dependent molten globule transition is required for activity of the steroidogenic acute regulatory protein, StAR. J Biol Chem. 2005;280:41753–41760. doi: 10.1074/jbc.M510241200. [DOI] [PubMed] [Google Scholar]
- 41.Kudo N, Kumagai K, Tomishige N, Yamaji T, Wakatsuki S, Nishijima M, Hanada K, Kato R. Structural basis for specific lipid recognition by CERT responsible for nonvesicular trafficking of ceramide. Proc Natl Acad Sci USA. 2008;105:488–493. doi: 10.1073/pnas.0709191105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Tiel CM, Schouten A, Snoek GT, Gros P, Wirtz KW. The structure of phosphatidylinositol transfer protein alpha reveals sites for phospholipid binding and membrane association with major implications for its function. FEBS Lett. 2002;531:69–73. doi: 10.1016/s0014-5793(02)03403-8. [DOI] [PubMed] [Google Scholar]
- 43.Leventis PA, Grinstein S. The distribution and function of phosphatidylserine in cellular membranes. Annu Rev Biophys. 2010;39:407–427. doi: 10.1146/annurev.biophys.093008.131234. [DOI] [PubMed] [Google Scholar]
- 44.Clark BJ, Wells J, King SR, Stocco DM. The purification, cloning, and expression of a novel luteinizing hormone-induced mitochondrial protein in MA-10 mouse Leydig tumor cells. Characterization of the steroidogenic acute regulatory protein (StAR) J Biol Chem. 1994;269:28314–28322. [PubMed] [Google Scholar]
- 45.Bose H, Lingappa VR, Miller WL. Rapid regulation of steroidogenesis by mitochondrial protein import. Nature. 2002;417:87–91. doi: 10.1038/417087a. [DOI] [PubMed] [Google Scholar]
- 46.Miller WL, Bose HS. Early steps in steroidogenesis: intracellular cholesterol trafficking. J Lipid Res. 2011;52:2111–2135. doi: 10.1194/jlr.R016675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Clark BJ. The mammalian START domain protein family in lipid transport in health and disease. J Endocrinol. 2012;212:257–275. doi: 10.1530/JOE-11-0313. [DOI] [PubMed] [Google Scholar]
- 48.Brown MS, Goldstein JL. Receptor-mediated endocytosis: insights from the lipoprotein receptor system. Proc Natl Acad Sci USA. 1979;76:3330–3337. doi: 10.1073/pnas.76.7.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pentchev PG. Niemann–Pick C research from mouse to gene. Biochim Biophys Acta. 2004;1685:3–7. doi: 10.1016/j.bbalip.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 50.Pipalia NH, Hao M, Mukherjee S, Maxfield FR. Sterol, protein and lipid trafficking in Chinese hamster ovary cells with Niemann–Pick type C1 defect. Traffic. 2007;8:130–141. doi: 10.1111/j.1600-0854.2006.00513.x. [DOI] [PubMed] [Google Scholar]
- 51.Maxfield FR, Tabas I. Role of cholesterol and lipid organization in disease. Nature. 2005;438:612–621. doi: 10.1038/nature04399. [DOI] [PubMed] [Google Scholar]
- 52.Kwon HJ, Abi-Mosleh L, Wang ML, Deisenhofer J, Goldstein JL, Brown MS, Infante RE. Structure of N-terminal domain of NPC1 reveals distinct subdomains for binding and transfer of cholesterol. Cell. 2009;137:1213–1224. doi: 10.1016/j.cell.2009.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Infante RE, Radhakrishnan A, Abi-Mosleh L, Kinch LN, Wang ML, Grishin NV, Goldstein JL, Brown MS. Purified NPC1 protein: II. Localization of sterol binding to a 240-amino acid soluble luminal loop. J Biol Chem. 2008;283:1064–1075. doi: 10.1074/jbc.M707944200. [DOI] [PubMed] [Google Scholar]
- 54.Friedland N, Liou HL, Lobel P, Stock AM. Structure of a cholesterol-binding protein deficient in Niemann–Pick type C2 disease. Proc Natl Acad Sci USA. 2003;100:2512–2517. doi: 10.1073/pnas.0437840100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xu S, Benoff B, Liou HL, Lobel P, Stock AM. Structural basis of sterol binding by NPC2, a lysosomal protein deficient in Niemann–Pick type C2 disease. J Biol Chem. 2007;282:23525–23531. doi: 10.1074/jbc.M703848200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu Z, Farver W, Kodukula S, Storch J. Regulation of sterol transport between membranes and NPC2. Biochemistry. 2008;47:11134–11143. doi: 10.1021/bi801328u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McCauliff LA, Xu Z, Storch J. Sterol transfer between cyclodextrin and membranes: similar but not identical mechanism to NPC2-mediated cholesterol transfer. Biochemistry. 2011;50:7341–7349. doi: 10.1021/bi200574f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Storch J, Xu Z. Niemann–Pick C2 (NPC2) and intracellular cholesterol trafficking. Biochim Biophys Acta. 2009;1791:671–678. doi: 10.1016/j.bbalip.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Infante RE, Wang ML, Radhakrishnan A, Kwon HJ, Brown MS, Goldstein JL. NPC2 facilitates bidirectional transfer of cholesterol between NPC1 and lipid bilayers, a step in cholesterol egress from lysosomes. Proc Natl Acad Sci USA. 2008;105:15287–15292. doi: 10.1073/pnas.0807328105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang ML, Motamed M, Infante RE, Abi-Mosleh L, Kwon HJ, Brown MS, Goldstein JL. Identification of surface residues on Niemann–Pick C2 essential for hydrophobic handoff of cholesterol to NPC1 in lysosomes. Cell Metab. 2010;12:166–173. doi: 10.1016/j.cmet.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Deffieu MS, Pfeffer SR. Niemann–Pick type C 1 function requires lumenal domain residues that mediate cholesterol-dependent NPC2 binding. Proc Natl Acad Sci USA. 2011;108:18932–18936. doi: 10.1073/pnas.1110439108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Davies JP, Ioannou YA. Topological analysis of Niemann–Pick C1 protein reveals that the membrane orientation of the putative sterol-sensing domain is identical to those of 3-hydroxy-3-methylglutaryl-CoA reductase and sterol regulatory element binding protein cleavage-activating protein. J Biol Chem. 2000;275:24367–24374. doi: 10.1074/jbc.M002184200. [DOI] [PubMed] [Google Scholar]
- 63.Millard EE, Gale SE, Dudley N, Zhang J, Schaffer JE, Ory DS. The sterol-sensing domain of the Niemann–Pick C1 (NPC1) protein regulates trafficking of low density lipoprotein cholesterol. J Biol Chem. 2005;280:28581–28590. doi: 10.1074/jbc.M414024200. [DOI] [PubMed] [Google Scholar]
- 64.Alpy F, Stoeckel ME, Dierich A, Escola JM, Wendling C, Chenard MP, Vanier MT, Gruenberg J, Tomasetto C, Rio MC. The steroidogenic acute regulatory protein homolog MLN64, a late endosomal cholesterol-binding protein. J Biol Chem. 2001;276:4261–4269. doi: 10.1074/jbc.M006279200. [DOI] [PubMed] [Google Scholar]
- 65.Charman M, Kennedy BE, Osborne N, Karten B. MLN64 mediates egress of cholesterol from endosomes to mitochondria in the absence of functional Niemann–Pick Type C1 protein. J Lipid Res. 2010;51:1023–1034. doi: 10.1194/jlr.M002345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Watari H, Arakane F, Moog-Lutz C, Kallen CB, Tomasetto C, Gerton GL, Rio MC, Baker ME, Strauss JF., 3rd MLN64 contains a domain with homology to the steroidogenic acute regulatory protein (StAR) that stimulates steroidogenesis. Proc Natl Acad Sci USA. 1997;94:8462–8467. doi: 10.1073/pnas.94.16.8462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kishida T, Kostetskii I, Zhang Z, Martinez F, Liu P, Walkley SU, Dwyer NK, Blanchette-Mackie EJ, Radice GL, Strauss JF., 3rd Targeted mutation of the MLN64 START domain causes only modest alterations in cellular sterol metabolism. J Biol Chem. 2004;279:19276–19285. doi: 10.1074/jbc.M400717200. [DOI] [PubMed] [Google Scholar]
- 68.Gill S, Chow R, Brown AJ. Sterol regulators of cholesterol homeostasis and beyond: the oxysterol hypothesis revisited and revised. Prog Lipid Res. 2008;47:391–404. doi: 10.1016/j.plipres.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 69.Borthwick F, Allen AM, Taylor JM, Graham A. Overexpression of STARD3 in human monocyte/macrophages induces an anti-atherogenic lipid phenotype. Clin Sci (Lond) 2010;119:265–272. doi: 10.1042/CS20100266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jaworski CJ, Moreira E, Li A, Lee R, Rodriguez IR. A family of 12 human genes containing oxysterol-binding domains. Genomics. 2001;78:185–196. doi: 10.1006/geno.2001.6663. [DOI] [PubMed] [Google Scholar]
- 71.Raychaudhuri S, Prinz WA. The diverse functions of oxysterol-binding proteins. Annu Rev Cell Dev Biol. 2010;26:157–177. doi: 10.1146/annurev.cellbio.042308.113334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Beh CT, Cool L, Phillips J, Rine J. Overlapping functions of the yeast oxysterol-binding protein homologues. Genetics. 2001;157:1117–1140. doi: 10.1093/genetics/157.3.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Beh CT, Rine J. A role for yeast oxysterol-binding protein homologs in endocytosis and in the maintenance of intracellular sterol-lipid distribution. J Cell Sci. 2004;117:2983–2996. doi: 10.1242/jcs.01157. [DOI] [PubMed] [Google Scholar]
- 74.Beh CT, Alfaro G, Duamel G, Sullivan DP, Kersting MC, Dighe S, Kozminski KG, Menon AK. Yeast oxysterol-binding proteins: sterol transporters or regulators of cell polarization? Mol Cell Biochem. 2009;326:9–13. doi: 10.1007/s11010-008-9999-7. [DOI] [PubMed] [Google Scholar]
- 75.Raychaudhuri S, Im YJ, Hurley JH, Prinz WA. Nonvesicular sterol movement from plasma membrane to ER requires oxysterol-binding protein-related proteins and phosphoinositides. J Cell Biol. 2006;173:107–119. doi: 10.1083/jcb.200510084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jansen M, Ohsaki Y, Rita Rega L, Bittman R, Olkkonen VM, Ikonen E. Role of ORPs in sterol transport from plasma membrane to ER and lipid droplets in mammalian cells. Traffic. 2011;12:218–231. doi: 10.1111/j.1600-0854.2010.01142.x. [DOI] [PubMed] [Google Scholar]
- 77.Georgiev AG, Sullivan DP, Kersting MC, Dittman JS, Beh CT, Menon AK. Osh proteins regulate membrane sterol organization but are not required for sterol movement between the ER and PM. Traffic. 2011;12:1341–1355. doi: 10.1111/j.1600-0854.2011.01234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Santiago-Tirado FH, Bretscher A. Membrane-trafficking sorting hubs: cooperation between PI4P and small GTPases at the trans-Golgi network. Trends Cell Biol. 2011;21:515–525. doi: 10.1016/j.tcb.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ortiz D, Medkova M, Walch-Solimena C, Novick P. Ypt32 recruits the Sec4p guanine nucleotide exchange factor, Sec2p, to secretory vesicles; evidence for a Rab cascade in yeast. J Cell Biol. 2002;157:1005–1015. doi: 10.1083/jcb.200201003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Santiago-Tirado FH, Legesse-Miller A, Schott D, Bretscher A. PI4P and Rab inputs collaborate in myosin-V-dependent transport of secretory compartments in yeast. Dev Cell. 2011;20:47–59. doi: 10.1016/j.devcel.2010.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Strahl T, Thorner J. Synthesis and function of membrane phosphoinositides in budding yeast, Saccharomyces cerevisiae. Biochim Biophys Acta. 2007;1771:353–404. doi: 10.1016/j.bbalip.2007.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Walch-Solimena C, Novick P. The yeast phosphatidylinositol-4-OH kinase pik1 regulates secretion at the Golgi. Nat Cell Biol. 1999;1:523–525. doi: 10.1038/70319. [DOI] [PubMed] [Google Scholar]
- 83.Audhya A, Foti M, Emr SD. Distinct roles for the yeast phosphatidylinositol 4-kinases, Stt4p and Pik1p, in secretion, cell growth, and organelle membrane dynamics. Mol Biol Cell. 2000;11:2673–2689. doi: 10.1091/mbc.11.8.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bankaitis VA, Phillips S, Yanagisawa L, Li X, Routt S, Xie Z. Phosphatidylinositol transfer protein function in the yeast Saccharomyces cerevisiae. Adv Enzyme Regul. 2005;45:155–170. doi: 10.1016/j.advenzreg.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 85.Foti M, Audhya A, Emr SD. Sac1 lipid phosphatase and Stt4 phosphatidylinositol 4-kinase regulate a pool of phosphatidylinositol 4-phosphate that functions in the control of the actin cytoskeleton and vacuole morphology. Mol Biol Cell. 2001;12:2396–2411. doi: 10.1091/mbc.12.8.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Manford A, Xia T, Saxena AK, Stefan C, Hu F, Emr SD, Mao Y. Crystal structure of the yeast Sac1: implications for its phosphoinositide phosphatase function. EMBO J. 2010;29:1489–1498. doi: 10.1038/emboj.2010.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fairn GD, Curwin AJ, Stefan CJ, McMaster CR. The oxysterol binding protein Kes1p regulates Golgi apparatus phosphatidylinositol-4-phosphate function. Proc Natl Acad Sci USA. 2007;104:15352–15357. doi: 10.1073/pnas.0705571104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fang M, Kearns BG, Gedvilaite A, Kagiwada S, Kearns M, Fung MK, Bankaitis VA. Kes1p shares homology with human oxysterol binding protein and participates in a novel regulatory pathway for yeast Golgi-derived transport vesicle biogenesis. EMBO J. 1996;15:6447–6459. [PMC free article] [PubMed] [Google Scholar]
- 89.Li X, Rivas MP, Fang M, Marchena J, Mehrotra B, Chaudhary A, Feng L, Prestwich GD, Bankaitis VA. Analysis of oxysterol binding protein homologue Kes1p function in regulation of Sec14p-dependent protein transport from the yeast Golgi complex. J Cell Biol. 2002;157:63–77. doi: 10.1083/jcb.200201037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.LeBlanc MA, McMaster CR. Lipid binding requirements for oxysterol-binding protein Kes1 inhibition of autophagy and endosome-trans-Golgi trafficking pathways. J Biol Chem. 2010;285:33875–33884. doi: 10.1074/jbc.M110.147264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Im YJ, Raychaudhuri S, Prinz WA, Hurley JH. Structural mechanism for sterol sensing and transport by OSBP-related proteins. Nature. 2005;437:154–158. doi: 10.1038/nature03923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Drin G, Casella JF, Gautier R, Boehmer T, Schwartz TU, Antonny B. A general amphipathic alpha-helical motif for sensing membrane curvature. Nat Struct Mol Biol. 2007;14:138–146. doi: 10.1038/nsmb1194. [DOI] [PubMed] [Google Scholar]
- 93.Fairn GD, McMaster CR. Identification and assessment of the role of a nominal phospholipid binding region of ORP1S (oxysterol-binding-protein-related protein 1 short) in the regulation of vesicular transport. Biochem J. 2005;387:889–896. doi: 10.1042/BJ20041915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schulz TA, Choi MG, Raychaudhuri S, Mears JA, Ghirlando R, Hinshaw JE, Prinz WA. Lipid-regulated sterol transfer between closely apposed membranes by oxysterol-binding protein homologues. J Cell Biol. 2009;187:889–903. doi: 10.1083/jcb.200905007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.de Saint-Jean M, Delfosse V, Douguet D, Chicanne G, Payrastre B, Bourguet W, Antonny B, Drin G. Osh4p exchanges sterols for phosphatidylinositol 4-phosphate between lipid bilayers. J Cell Biol. 2011;195:965–978. doi: 10.1083/jcb.201104062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fairn GD, Schieber NL, Ariotti N, Murphy S, Kuerschner L, Webb RI, Grinstein S, Parton RG. High-resolution mapping reveals topologically distinct cellular pools of phosphatidylserine. J Cell Biol. 2011;194:257–275. doi: 10.1083/jcb.201012028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Behnia R, Munro S. Organelle identity and the signposts for membrane traffic. Nature. 2005;438:597–604. doi: 10.1038/nature04397. [DOI] [PubMed] [Google Scholar]
- 98.Alfaro G, Johansen J, Dighe SA, Duamel G, Kozminski KG, Beh CT. The sterol-binding protein Kes1/Osh4p is a regulator of polarized exocytosis. Traffic. 2011;12:1521–1536. doi: 10.1111/j.1600-0854.2011.01265.x. [DOI] [PubMed] [Google Scholar]
- 99.Mousley CJ, Yuan P, Gaur NA, Trettin KD, Nile AH, Deminoff SJ, Dewar BJ, Wolpert M, Macdonald JM, Herman PK, Hinnebusch AG, Bankaitis VA. A sterol-binding protein integrates endosomal lipid metabolism with TOR signaling and nitrogen sensing. Cell. 2012;148:702–715. doi: 10.1016/j.cell.2011.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Beh CT, McMaster CR, Kozminski KG, Menon AK. A detour for yeast oxysterol binding proteins. J Biol Chem. 2012;287:11481–11488. doi: 10.1074/jbc.R111.338400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sebastian TT, Baldridge RD, Xu P, Graham TR. Phospholipid flippases: building asymmetric membranes and transport vesicles. Biochim Biophys Acta. 2012;1821:1068–1077. doi: 10.1016/j.bbalip.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Surma MA, Klose C, Simons K. Lipid-dependent protein sorting at the trans-Golgi network. Biochim Biophys Acta. 2012;1821:1059–1067. doi: 10.1016/j.bbalip.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 103.Proszynski TJ, Klemm RW, Gravert M, Hsu PP, Gloor Y, Wagner J, Kozak K, Grabner H, Walzer K, Bagnat M, Simons K, Walch-Solimena C. A genome-wide visual screen reveals a role for sphingolipids and ergosterol in cell surface delivery in yeast. Proc Natl Acad Sci USA. 2005;102:17981–17986. doi: 10.1073/pnas.0509107102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Muthusamy BP, Raychaudhuri S, Natarajan P, Abe F, Liu K, Prinz WA, Graham TR. Control of protein and sterol trafficking by antagonistic activities of a type IV P-type ATPase and oxysterol binding protein homologue. Mol Biol Cell. 2009;20:2920–2931. doi: 10.1091/mbc.E08-10-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Natarajan P, Wang J, Hua Z, Graham TR. Drs2p-coupled aminophospholipid translocase activity in yeast Golgi membranes and relationship to in vivo function. Proc Natl Acad Sci USA. 2004;101:10614–10619. doi: 10.1073/pnas.0404146101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Natarajan P, Liu K, Patil DV, Sciorra VA, Jackson CL, Graham TR. Regulation of a Golgi flippase by phosphoinositides and an ArfGEF. Nat Cell Biol. 2009;11:1421–1426. doi: 10.1038/ncb1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Graham TR. Flippases and vesicle-mediated protein transport. Trends Cell Biol. 2004;14:670–677. doi: 10.1016/j.tcb.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 108.Mizuno-Yamasaki E, Medkova M, Coleman J, Novick P. Phosphatidylinositol 4-phosphate controls both membrane recruitment and a regulatory switch of the Rab GEF Sec2p. Dev Cell. 2010;18:828–840. doi: 10.1016/j.devcel.2010.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Levine T, Loewen C. Inter-organelle membrane contact sites: through a glass, darkly. Curr Opin Cell Biol. 2006;18:371–378. doi: 10.1016/j.ceb.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 110.Toulmay A, Prinz WA. Lipid transfer and signaling at organelle contact sites: the tip of the iceberg. Curr Opin Cell Biol. 2011;23:458–463. doi: 10.1016/j.ceb.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ladinsky MS, Mastronarde DN, McIntosh JR, Howell KE, Staehelin LA. Golgi structure in three dimensions: functional insights from the normal rat kidney cell. J Cell Biol. 1999;144:1135–1149. doi: 10.1083/jcb.144.6.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mogelsvang S, Marsh BJ, Ladinsky MS, Howell KE. Predicting function from structure: 3D structure studies of the mammalian Golgi complex. Traffic. 2004;5:338–345. doi: 10.1111/j.1398-9219.2004.00186.x. [DOI] [PubMed] [Google Scholar]
- 113.De Matteis MA, Di Campli A, D’Angelo G. Lipid-transfer proteins in membrane trafficking at the Golgi complex. Biochim Biophys Acta. 2007;1771:761–768. doi: 10.1016/j.bbalip.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 114.Hanada K, Kumagai K, Tomishige N, Yamaji T. CERT-mediated trafficking of ceramide. Biochim Biophys Acta. 2009;1791:684–691. doi: 10.1016/j.bbalip.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 115.Levine TP, Munro S. The pleckstrin homology domain of oxysterol-binding protein recognises a determinant specific to Golgi membranes. Curr Biol. 1998;8:729–739. doi: 10.1016/s0960-9822(98)70296-9. [DOI] [PubMed] [Google Scholar]
- 116.Levine TP, Munro S. Targeting of Golgi-specific pleckstrin homology domains involves both PtdIns 4-kinase-dependent and -independent components. Curr Biol. 2002;12:695–704. doi: 10.1016/s0960-9822(02)00779-0. [DOI] [PubMed] [Google Scholar]
- 117.Kawano M, Kumagai K, Nishijima M, Hanada K. Efficient trafficking of ceramide from the endoplasmic reticulum to the Golgi apparatus requires a VAMP-associated protein-interacting FFAT motif of CERT. J Biol Chem. 2006;281:30279–30288. doi: 10.1074/jbc.M605032200. [DOI] [PubMed] [Google Scholar]
- 118.Loewen CJ, Roy A, Levine TP. A conserved ER targeting motif in three families of lipid binding proteins and in Opi1p binds VAP. EMBO J. 2003;22:2025–2035. doi: 10.1093/emboj/cdg201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Peretti D, Dahan N, Shimoni E, Hirschberg K, Lev S. Coordinated lipid transfer between the endoplasmic reticulum and the Golgi complex requires the VAP proteins and is essential for Golgi-mediated transport. Mol Biol Cell. 2008;19:3871–3884. doi: 10.1091/mbc.E08-05-0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Perry RJ, Ridgway ND. Oxysterol-binding protein and vesicle-associated membrane protein-associated protein are required for sterol-dependent activation of the ceramide transport protein. Mol Biol Cell. 2006;17:2604–2616. doi: 10.1091/mbc.E06-01-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Radhakrishnan A, Ikeda Y, Kwon HJ, Brown MS, Goldstein JL. Sterol-regulated transport of SREBPs from endoplasmic reticulum to Golgi: oxysterols block transport by binding to Insig. Proc Natl Acad Sci USA. 2007;104:6511–6518. doi: 10.1073/pnas.0700899104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mohammadi A, Perry RJ, Storey MK, Cook HW, Byers DM, Ridgway ND. Golgi localization and phosphorylation of oxysterol binding protein in Niemann–Pick C and U18666A-treated cells. J Lipid Res. 2001;42:1062–1071. [PubMed] [Google Scholar]
- 123.Ridgway ND, Dawson PA, Ho YK, Brown MS, Goldstein JL. Translocation of oxysterol binding protein to Golgi apparatus triggered by ligand binding. J Cell Biol. 1992;116:307–319. doi: 10.1083/jcb.116.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wyles JP, McMaster CR, Ridgway ND. Vesicle-associated membrane protein-associated protein-A (VAP-A) interacts with the oxysterol-binding protein to modify export from the endoplasmic reticulum. J Biol Chem. 2002;277:29908–29918. doi: 10.1074/jbc.M201191200. [DOI] [PubMed] [Google Scholar]
- 125.Ngo M, Ridgway ND. Oxysterol binding protein-related Protein 9 (ORP9) is a cholesterol transfer protein that regulates Golgi structure and function. Mol Biol Cell. 2009;20:1388–1399. doi: 10.1091/mbc.E08-09-0905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Banerji S, Ngo M, Lane CF, Robinson CA, Minogue S, Ridgway ND. Oxysterol binding protein-dependent activation of sphingomyelin synthesis in the Golgi apparatus requires phosphatidylinositol 4-kinase IIalpha. Mol Biol Cell. 2010;21:4141–4150. doi: 10.1091/mbc.E10-05-0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lu D, Sun HQ, Wang H, Barylko B, Fukata Y, Fukata M, Albanesi JP, Yin HL. Phosphatidylinositol 4-kinase IIalpha is palmitoylated by Golgi-localized palmitoyltransferases in cholesterol-dependent manner. J Biol Chem. 2012;287:21856–21865. doi: 10.1074/jbc.M112.348094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lev S. Non-vesicular lipid transport by lipid-transfer proteins and beyond. Nat Rev Mol Cell Biol. 2010;11:739–750. doi: 10.1038/nrm2971. [DOI] [PubMed] [Google Scholar]
- 129.Stefan CJ, Manford AG, Baird D, Yamada-Hanff J, Mao Y, Emr SD. Osh proteins regulate phosphoinositide metabolism at ER-plasma membrane contact sites. Cell. 2011;144:389–401. doi: 10.1016/j.cell.2010.12.034. [DOI] [PubMed] [Google Scholar]
- 130.Goto A, Liu X, Robinson CA, Ridgway ND. Multi-site phosphorylation of oxysterol binding protein (OSBP) regulates sterol binding and activation of sphingomyelin synthesis. Mol Biol Cell. 2012;23:3624–3635. doi: 10.1091/mbc.E12-04-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Godi A, Pertile P, Meyers R, Marra P, Di Tullio G, Iurisci C, Luini A, Corda D, De Matteis MA. ARF mediates recruitment of PtdIns-4-OH kinase-beta and stimulates synthesis of PtdIns(4,5)P2 on the Golgi complex. Nat Cell Biol. 1999;1:280–287. doi: 10.1038/12993. [DOI] [PubMed] [Google Scholar]
- 132.Nhek S, Ngo M, Yang X, Ng MM, Field SJ, Asara JM, Ridgway ND, Toker A. Regulation of oxysterol-binding protein Golgi localization through protein kinase D-mediated phosphorylation. Mol Biol Cell. 2010;21:2327–2337. doi: 10.1091/mbc.E10-02-0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kumagai K, Kawano M, Shinkai-Ouchi F, Nishijima M, Hanada K. Interorganelle trafficking of ceramide is regulated by phosphorylation-dependent cooperativity between the PH and START domains of CERT. J Biol Chem. 2007;282:17758–17766. doi: 10.1074/jbc.M702291200. [DOI] [PubMed] [Google Scholar]
- 134.Lange Y, Ye J, Steck TL. How cholesterol homeostasis is regulated by plasma membrane cholesterol in excess of phospholipids. Proc Natl Acad Sci USA. 2004;101:11664–11667. doi: 10.1073/pnas.0404766101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kobayashi T, Beuchat MH, Chevallier J, Makino A, Mayran N, Escola JM, Lebrand C, Cosson P, Gruenberg J. Separation and characterization of late endosomal membrane domains. J Biol Chem. 2002;277:32157–32164. doi: 10.1074/jbc.M202838200. [DOI] [PubMed] [Google Scholar]
- 136.Rosenbaum AI, Maxfield FR. Niemann–Pick type C disease: molecular mechanisms and potential therapeutic approaches. J Neurochem. 2011;116:789–795. doi: 10.1111/j.1471-4159.2010.06976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]