Abstract
Astrocytic activation is a cellular response to disturbances of the central nervous system (CNS). Recent advances in cellular and molecular biology have demonstrated the remarkable changes in molecular signaling, morphology, and metabolism that occur during astrocyte activation. Based on these studies, it has become clear that the astrocyte activation process is regulated by a variety of signaling pathways, which result in metabolic support, wound healing and scar formation. While normal astrocyte activation pathways drive homeostasis and/or repair in the CNS, dysregulation of these pathways can lead to astrocyte abnormalities, including glioma formation with similar phenotypes as reactive astrocytes. We review the principle pathways responsible for astrocytic activation, as well as their potential contribution to tumor formation in the CNS.
Keywords: Glia, Astrocytoma, Gliosis, Brain tumor, Tumorigenesis, Central nervous system
Introduction
Astrocytes are the most abundant non-neural cells in the central nervous system (CNS). They form elaborate connections with surrounding neurons, other nervous system cells, and blood vessels [1]. Astrocytes are electrically unexcitable and have been considered supportive structures of the CNS. However, recent advances of cellular and molecular biology have revealed numerous important biologic functions of astrocytes [2]. These functions include (1) maintaining a metabolic homeostasis in the CNS through release of key metabolites such as lactate and glutamine [3, 4], (2) reuptake of toxic substances in extracellular milieu, such as glutamate and glycine [5], (3) stabilization and regulation of vascular structures [6], and (4) modulation of synaptic transmission through the release of glial transmitters [5, 6].
Aside from their established homeostatic and metabolic functions, astrocytes can also exhibit reactive and proliferative phenotypes when exposed to CNS pathology. Based on their response and appearance, Fromann and colleagues [1] first described astrocytes in a multiple sclerosis specimen as “reactive astrocytes”. Since that time, reactive changes in astrocytes have been identified in virtually every type of CNS pathology. Consequently, these astrocytic changes have generally been considered a universal, non-specific response to stimuli in the CNS. However, recent evidence indicates that reactive astrocytic changes are the result of precise molecular and cellular changes regulated by key signaling molecules [7–9].
We are now beginning to understand that the proliferation, migration, and morphogenesis of reactive astrocytes provide remarkable beneficial effects and assists in the functional recovery of the CNS [10, 11]. Here, we review the critical molecular mechanisms that initiate normal astrocytic activation. We also discuss the dysregulation of these signaling pathways in the context of CNS neoplasia.
Morphologic characteristics astrocytes
Normal astrocytes
“Astrocyte” derives from the Greek word “astron”, which is descriptive of the cell’s star-shaped appearance. Since the late 19th century, astrocytes have been categorized into two subtypes, reflective of their morphology and anatomic location. These include protoplasmic astrocytes and fibrous astrocytes. Protoplasmic astrocytes are the most common astrocytes in the CNS. They typically exhibit a radial shape with 5–10 primary branches and several smaller processes or “end-feet” [12]. Fibrous astrocytes are less complicated in morphology and are mainly located in white matter fiber tracts. The morphology and function of astrocytes varies in location as well as the microenvironment.
The stellate morphology of astrocytes is commonly illustrated by glial fibrillary acidic protein (GFAP) staining [13]. A better understanding of astrocytic morphology has been provided by recent advancements that demonstrate the complex detail of the fine astrocytic processes. Structural changes of astrocytic end-feet can occur within minutes in response to a variety of stimuli such as changes in neuronal activity, ischemic damage, and traumatic injury [1]. Changes in end-feet can include gliding of lamellipodia-like end-feet and process extension/retraction [14].
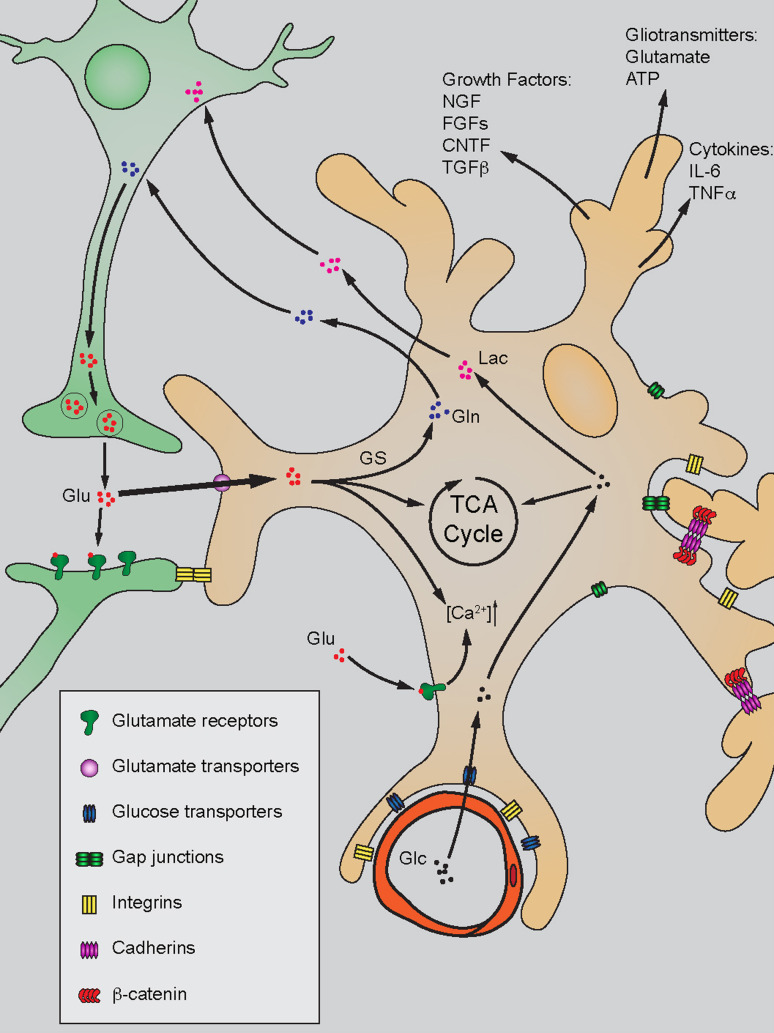
Astrocyte end-feet form critical contacts with adjacent blood vessels and other CNS cellular components (Fig. 1). Peri-vascular astrocytic end-feet are crucial components for the blood–brain barrier, which modulate vessel dilation/contraction through the release of cytokines such as PGE2 [15, 16]. Several recent discoveries have shown that astrocytes establish distinct boundaries with one another resulting in little overlap in the spatial domains of neighboring processes [17]. This organizational pattern suggests that astrocytes organize brain activity in focal units composed of neurons, astrocytes, and blood vessels by way of metabolic support and the release of cytokines, growth factors, and gliotransmitters [5].
Fig. 1.
Primary functions of astrocytes in the quiescent stage. Astrocytes establish contact inhibition with other brain cells by gap junction, integrin, and cadherin-mediated mechanisms. They modulate CNS homeostasis by releasing growth factors, gliotransmitters, and cytokines. Astrocytes efficiently uptake glutamate (Glu) released from pre-synaptic neurons through glutamate transporters on plasma membrane. Internalized Glu is mainly involved in glutamine (Gln) production catalyzed by glutamine synthetase (GS), and released for neuronal use. Glu can also be utilized in the TCA cycle as well as induction of intracellular calcium signaling ([Ca2+]). Additionally, astrocytes take up glucose (Glc) from the blood and transport its metabolite lactate (Lac) to the neuron
Immunomodulation is another essential biologic function of astrocyte. Astrocytes express several essential cell surface proteins such as major histocompatibility complex (MHC) antigen, adhesion molecule ICAM-1 and possible costimulatory molecules such as B7 and CD40 [18]. The presence of these important molecules indicates that astrocytes participate in immunomodulation of the CNS, likely as antigen presenting cells. Indeed, it has been shown that astrocytes can mediate T helper cell responses in the CNS [19]. Additionally, astrocytes modulate immune response in the CNS through the secretion of cytokine/chemokines such as interleukins, TNF-α, TGF-β, GM-CSF, M-CSF, and G-CSF, in order to regulate the extent and intensity of the immune response [20]. Although the role of astrocytes in CNS immunomodulation has not yet been fully elucidated, it is likely that astrocytes modulate the immune response when the blood brain barrier (BBB) is compromised in cases like traumatic brain injury.
Reactive astrocytes
Cellular activation is a common phenomenon by which astrocytes become reactive as a result of various CNS disturbances. The activation process is controlled by a variety of signaling pathways (Fig. 2). During cellular activation to the injury, astrocytes exhibit remarkable morphologic changes, including increases in the number and length of their primary radial branches. Upregulation of interfillament expression (i.e., GFAP, nestin and vimentin) contributes to cellular hypertrophy and morphologic changes of reactive astrocytes. The extent and duration of astrocyte activation depends on the type and intensity of CNS stimuli, ranging from mild, transient morphologic changes to astrogliosis and scar formation [1, 7]. Minimal astrocytic activation does not interfere with the established astrocytic territories or their metabolic functions, physiologic functions of astrocytes can be largely preserved during activation after mild injury [21]. The involvement of reactive astrocytes in CNS diseases has been summarized in several major reviews [22]. In this paper, we specifically focus on the potential relationship between reactive astrocytes and tumor formation.
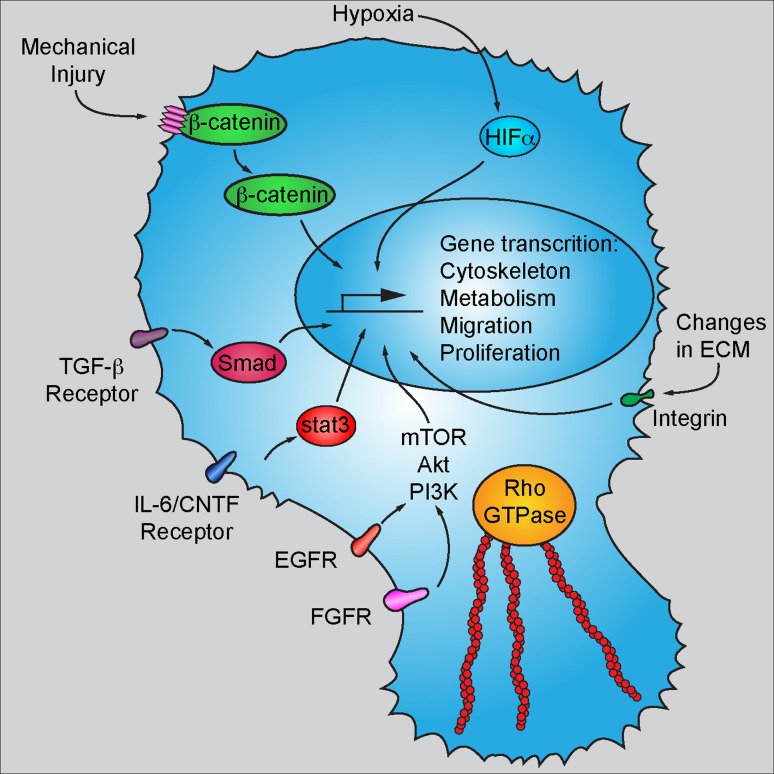
Fig. 2.
Molecular mechanisms involved in astrocytic activation. Abnormalities in existing contact inhibition such as disruption of the cadherin/β-catenin complex results in stabilization and activation of β-catenin as a transcription factor. Astrocytes express many cytokine/growth factor receptors on the plasma membrane during activation, such as TGF-β receptor, IL-6/CNTF receptor, EGFR and FGFR. Ligand binding of these receptors triggers different signaling transduction and eventually affects gene transcription in the nucleus. Elevated Rho GTPase activity in reactive astrocytes results in robust mobilization cytoskeleton remodeling and therefore leads to cell migration and tissue penetration. Finally, hypoxic insult leads to the stabilization and activation of HIFα, which when translocated into the nucleus mediates gene transcription related to metabolism, migration, and proliferation
Astrocytomas
Astrocytomas are the most common primary neoplasms of the CNS. The neoplastic cells in these tumors share many similar biologic and morphologic features with reactive astrocytes, including growth factor receptor expression, and remarkable migration and cell proliferation. Moreover, astrocytomas share the same signaling pathways that drive astrocyte activation. The molecular mechanisms for astrocytic activation may not only initiate tumorigenesis of gliomas but also involve malignant phenotypes such as enhanced proliferation and migration.
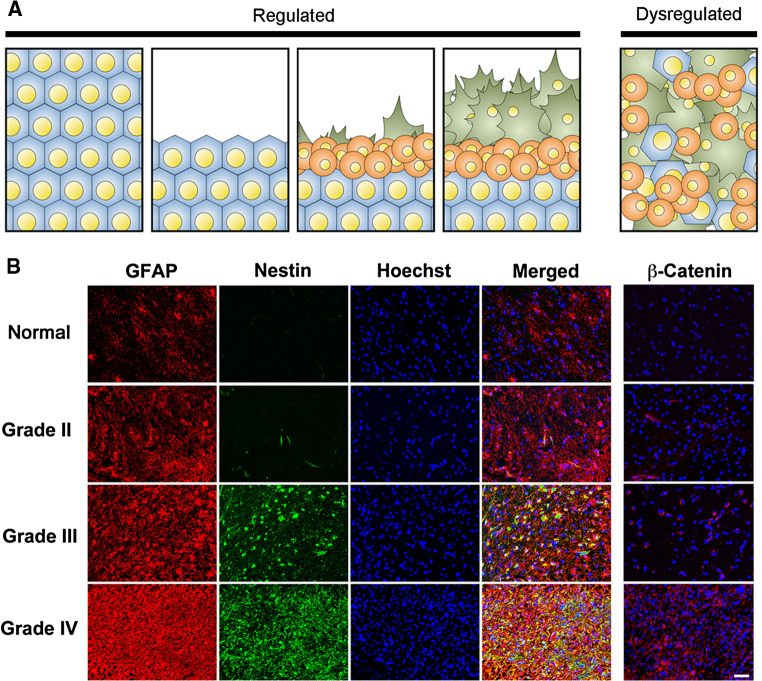
The cellular origin of astrocytomas has long been debated. Recent discoveries have elucidated that a glioma stem cell population is important for tumorigenesis and chemoresistance [23–25]. Additionally, neural stem cells and progenitor cells are also likely involved in tumor formation [26]. However, it has long been overlooked that normal astrocytes withhold intrinsic capacities to regain proliferative status after development. Reactive astrocytes exhibit enhanced migration through proteolysis of extracellular matrix, which is similar to the migratory and penetrative phenotypes of high-grade gliomas [27]. Furthermore, cellular dedifferentiation is commonly seen during astrocytic activation, which is characterized by expression of important stem cell markers such as nestin, vimentin, and sox2. Cellular activation also gives astrocytes the capacity of anchorage-independent growth, an important characteristic seen in the tumor-initiating cells of high-grade gliomas (Fig. 3) [28, 29].
Fig. 3.
Regulation and dysregulation of astrocytic activation. a Astrocytic activation is a tightly regulated cellular response. Cellular migration and proliferation are commonly seen during activation. Dysregulated activation is commonly seen in various types of CNS neoplasms. b Aggressive phenotype of cell activation in astrocytomas. Tumor stromal cells in astrocytomas exhibit similar phenotypes as reactive astrocytes, including mobilization of GFAP/Nestin, cell migration, tissue penetration and proliferation. Molecular mechanisms of cellular activation such as β-catenin are commonly dysregulated in these tumor cells. Adapted from Yang et al. (2012) [28]
Mechanisms of activation and tumorigenesis
Disruption of contact inhibition
Contact inhibition is a natural process that arrests the growth and expansion of cells when they come into contact with each other. Contact inhibition between astrocytes limits their process extension and migration and, consequently, establishes a spatial territory that excludes other astrocytes [17]. A key molecular mechanism for establishing contact inhibition between astrocytes is adherent junctions that are composed of cadherin and β-catenin [30].
Cadherins are a family of transmembrane proteins that play a critical role in calcium-dependent cell-cell adhesion. While the extracellular domains of cadherins from adjacent cells form physical interactions, their intracellular domains assemble with α/β-catenin and interact with the intracellular cytoskeleton [31]. This multi-protein complex not only serves as a physically stable connection between cells, but also arrests β-catenin on the plasma membrane and quenches its activity as a transcription factor. As a transcription factor, β-catenin initiates a subset of genes that mediate cell proliferation and differentiation. While the activity of β-catenin is crucial for establishing dorsal/ventral and anterior/posterior axes during embryogenesis, β-catenin is stabilized on the plasma membrane and rarely translocates into the nucleus in adulthood [32].
During cell activation, cadherin and β-catenin complexes serve as an important signal for loss of contact inhibition. Abnormalities in the CNS result in changes to the astrocytic plasma membrane, which result in the disruption of the integrity of cadherin/catenin complex. β-catenin is rapidly released from the plasma membrane, resulting in its accumulation in the cytoplasm and nucleus [28]. Cellular injury also concomitantly induces Cdc42-mediated activation of Par6-PKCζ. This results in inactivation of GSK-3β via Ser9 phosphorylation, which also contributes to enhanced β-catenin activation by reducing its degradation [33].
Several key components of β-catenin signaling such as Wnt and Fzd-1 are upregulated during CNS injury. These proteins initiate the canonical β-catenin signaling pathway and further inhibit GSK3β. This results in the accumulation and transcriptional activation of β-catenin [34]. β-catenin translocated to the nucleus recruits its cofactors LEF or TCF and initiates gene transcription for migration, proliferation, and differentiation of reactive astrocytes (the distinction of these pathways has yet to be elicited). β-catenin initiates a phenotype of reactive astrocytes by mediating transcription of key regulatory genes, which includes EGFR, MMPs, Sox2, cyclin D1, etc. [28].
The emergence of β-catenin activity in reactive astrocytes in adulthood suggests that cell proliferation and differentiation capacities are maintained in multi-functional proteins. They serve as molecular sensors to abnormalities of the CNS and signal damage to the nucleus and other cellular compartments to initiate activation. Dysregulation of the β-catenin pathway in astrocytes inevitably affects cell growth, proliferation, and possibly tumor transformation. For example, mutations in the gene that encodes adenomatous polyposis coli (APC), an important component in Axin/GSK3β/APC complex for β-catenin degradation, results in Turcot syndrome.
Turcot syndrome is characterized by the development of adenomatous polyposis, colorectal cancer, glioblastoma, and medulloblastoma. β-catenin escapes from degradation and results in loss of cell-cell contact inhibition. This results in increased cellular proliferation [35]. Upregulation of the key molecules in the Wnt/β-catenin pathway has been identified in astrocytomas in different pathologic grades [36–38]. Furthermore, glioma-derived tumor stem cells possess robust β-catenin signaling, which further confirms the importance of β-catenin in glioma tumorigenesis [28, 39].
Modulation of integrin-extracellular matrix (ECM) interactions
In addition to the cadherin/β-catenin complex, the integrin family also contributes to the establishment of contact inhibition in the CNS [30]. Integrins are transmembrane, non-covalently linked αβ heterodimers. They are located on the cell surface and interact with a variety of ECM components, including fibronectin, laminin, collagen, tenascin, vitronectin, and thrombospondin [40, 41]. Integrins have been found to be important in forming transmembrane connections between the ECM and cytoskeleton, as well as initiating intracellular signals when processing additional kinase activity. Integrins are essential for vasculogenesis in the CNS during development [42]. In adulthood, integrins are expressed at the astrocyte–vascular interface and maintain a permeable barrier in cerebral microvasculature [43–45].
Rearrangements of the integrin network are commonly seen during CNS disturbances. The expression and assembly of integrins are immediately altered during astrocytic activation. For example, cerebral ischemic injury results in decreased integrin expression. Integrins in astrocytic end-feet are withdrawn during ischemia, which results in increased vascular permeability [46]. Genetic silencing of integrin β1 in astrocytes elicits partial cell activation, suggesting integrins not only passively form cell contact but also are capable of inducing cellular activation during CNS injury [47].
In contrast to their role of forming structural connections in the quiescent stage, integrins exhibit transmembrane receptor activity during activation, mediating Cdc42-dependent cell polarity, and migration via PKCζ, which is the initial step of cell migration and wound healing [48]. Nevertheless, ligand binding to integrin activates talin-mediated oligomerization of focal adhesion kinase (FAK). Autophosphorylation of FAK results in a subset of secondary signal transduction pathways such as PI3K and Akt, and eventually modulates focal adhesion, cell migration, and survival [49]. These pathways are presumably related to the initiation of cellular activation and re-establishment of cell-cell contact during recovery [50–52].
Integrins are up-regulated in gliomas and expression levels correlate with the pathologic grade of the tumor [53]. These findings suggest that integrins exert an oncogenic effect in brain tumor formation. Exploited integrin signaling is related to tumor formation by inducing reactive phenotypes such as cellular proliferation and migration [53, 54]. Moreover, integrins indirectly contribute to tumorigenesis by activating other oncogene/mitogens such as hypoxia-inducible factors (HIFs) by way of focal adhesion kinase [55]. Dysfunction of integrin signaling not only affects tumor cell proliferation and survival but also contributes to the establishment of a tumor microenvironment through inducing angiogenesis and recruitment of pericytes, myeloid cells, and other precursor cells [56, 57].
Basement membrane invasion precedes meningeal dissemination and metastasis of glioma cells. Paulus and Tonn [40] identified that integrins are necessary in basement membrane invasion of glioma cells, based on the interaction of integrin and laminin [58]. These findings underscore the role that integrins play in the penetrative growth of glioma cells. Recent studies have identified the co-expression of integrin α6 with conventional cancer stem cell (CSC) markers in the perivascular region of glioblastoma multiforme (GBM). Tumor-derived integrin α6 was also found to be essential for the self-renewal, proliferation, and tumorigenesis of glioma stem cells [59]. Taken together, integrin signaling appears to have significant importance in brain tumor formation, establishment of the tumor microenvironment and metastasis.
Gap junctions establish astrocyte networks and synchronous activation
Gap junctions connect the cytoplasm of adjacent cells. Both neurons and astrocytes express hexameric connexin proteins, which dimerize to form transmembrane pores with neighboring cells of their own type. These connections establish electrically and metabolically connected networks of cells [7]. Hemichannels also exist in which hexameric connexins or pannexins are not coupled to a pair but instead are exposed to the extracellular environment. Though these channels are non-selective, permeable and potentially harmful, their role in ischemic injury is not clear. For example, dying cells of the ischemic core may propagate necrotic or apoptotic signals through these channels, which are beneficial for their own survival but detrimental for the receiving healthy cells. Conversely, gap junctions allow healthy cells to contribute ions and pro-survival signals [60, 61]. Studies antagonizing gap junctions have resulted in mixed responses, however, connexin antagonists are notably nonspecific [62].
Connexin Cx43 has been shown to induce contact inhibition of cells without affecting their log phase growth rates [63]. Glioma cells transfected with Cx43 demonstrated cell growth suppression with a concomitant increase in MFG-E8 (milk fat globule epidermal growth factor 8), a protein involved in integrin binding. Interestingly, MFG-E8 is a ligand for αvβ5 integrin receptors. Inhibition of this receptor has been the topic of a randomized phase II study for patients with GBM recurrence [64].
Cell surface receptors initiate activation via secondary signal transduction
In addition to the molecular mechanisms of cell–cell contact disruption, cytokines and chemokines are also capable of initiating astrocytic reactivation. While these factors are commonly absent in the quiescent CNS, they are robustly expressed and released during astrocytic activation [65]. Signaling by these molecules either initiates or maintains the activation and differentiation of astrocytes.
Epidermal growth factor receptor (EGFR) is a transmembrane receptor that exhibits intrinsic tyrosine kinase activity. While this potent mitogen receptor is normally undetectable in the mature CNS, it is substantially enhanced during mechanical injury or ischemia [66]. Ligand binding to EGFR causes receptor phosphorylation and activation of several important signaling pathways such as PI3K, Akt, and mTOR [67]. These molecular signals subsequently change cell morphology and motility, as well as re-organization of global gene expression [68]. Dysregulation of EGFR signaling is a very common phenomenon in gliomas. The gene encoding EGFR is frequently mutated, amplified, and/or rearranged in malignant astrocytomas, leading to the activation of downstream kinases in a similar manner as astrocyte activation. Alternatively, loss of endogenous inhibitory mechanisms (such as microRNA-7) leads to dysregulation of the EGFR pathway, which eventually causes failure of growth control and tumor manifestation. Nevertheless, EGF is an essential factor to maintain the self-renewal of glioma stem cells in vitro, suggesting EGFR signals are important in the stem cell population of GBMs.
Signal transducer and activator of transcription 3 (Stat3) is a common downstream transcription factor for various growth factors and cytokine receptors, including interleukin-6 receptor, CNTF receptor, and leukemia inhibitory factor. Phosphorylation of Stat3 is significantly increased in astrocytes shortly after CNS injury, indicating that Stat3-related signaling contributes to the activation of astrocytes. This has been confirmed by loss-of-function studies, showing that silencing STAT3 in astrocytes abolishes astrocyte reactivation in spinal cord injury. This leads to failure of wound healing and reduced neuronal functions [69]. Genetic investigations have shown that Stat3 directly binds to the promoter region of GFAP and mediates cytoskeleton mobilization and cell activation [70]. Widespread inflammation has also been identified in Stat3-deficient mice. This is likely due to failure of reactive astrocytes to establish a permeability barrier without Stat3 that leads to infiltration of myeloid components from cerebral vasculature [44]. Constitutive activation of Stat3 is related to tumor formation, possibly through initiating reactive phenotypes such as enhanced cell migration and cytoskeleton expression. IL-6 and Stat3 activation are commonly detected in malignant gliomas, unlike normal brain tissue [71]. The importance of Stat3 signaling in tumorigenesis has been shown in Mut3 mice (GFAP-cre; Nf1loxP/+; Trp53−/+), which spontaneously develop gliomas. Inoculation of these mice with cytomegalovirus (CMV), an activator of the PDGF-B/Stat3 signaling pathway induces spontaneous tumor development [72]. Our previous findings also indicate that CNTF receptor α, an activator of Stat3 signaling, is uniquely expressed in high-grade gliomas and their CSCs [73]. It is helpful for the survival of CSC, triggering cellular differentiation and likely related to tumor heterogeneity in malignant glioma.
Mechanical disruption or ischemic damage of astrocytes results in the release of adenosine triphosphate (ATP). This leads to autocrine and paracrine signaling through the P2X and P2Y purine family of receptors [74]. Downstream effects of this pathway include astrocyte–astrocyte increases in intracellular [Ca2+], microglial chemotaxis, as well as pro-inflammatory and anti-apoptotic gene upregulation. The activation of the purine receptors results in astrocyte proliferation. Specifically, GFAP, inflammatory markers (IL-1β, IL-6, IL-8, TNFα) and anti-apoptotic genes (Bcl-2, Bcl-XL) have increased expression levels upon exposure to extracellular ATP. This mitogenic response is mediated by an extracellular signal-regulated protein kinase (ERK) pathway [75, 76]. When cultured on a deformable SILASTIC membrane and exposed to reversible stretch injury, ERK activation is observed and correlates with the magnitude of injury [77]. This calcium-dependent signaling pathway secondary to extracellular ATP and purinergic receptor activation may be a key inciting event in astrocyte activation following injury.
Receptor for advanced glycation end products (RAGE) is a multi-ligand receptor of the immunoglobulin superfamily involved in inflammation, nephropathy, neurodegeneration, and cancer. RAGE mediates a variety of signaling pathways, including ERK1/2, Akt, p38 MAPK, and JNK. Additionally, RAGE has recently been found to regulate cell motility via RhoA/ROCK, cdc42, and Src kinase pathways [78–80]. Several recent studies revealed a possible role of RAGE in astrocytic activation, via high-mobility group box 1 (HMGB-1). Ischemic injury results in release of HMGB-1 from the neuronal nucleus, which consequently induces astrocytic expression of TNF-α and MMP9 [81–83]. Additionally, S100B also affects astrocytic function via a RAGE pathway, which induces glial activation [84].
RAGE signaling mediates transcription of transcription factors such as NF-κB, which are key regulatory elements that control cell proliferation, survival, migration, and invasion. Additionally, RAGE can be activated under stress conditions like hypoxia [85]. This strongly suggests that a dysregulation in RAGE signaling will lead to neoplastic transformation. Indeed, several studies have shown that RAGE signaling is related to colon, prostate, pancreatic, and lung cancers. Inhibition of RAGE signaling by ligand or receptor targeting effectively limits tumor growth and metastasis [86, 87]. Additionally, RAGE results in modulation of the cancer microenvironment by affecting leukocyte, endothelial cell, and fibroblast [88]. Although the potential role of RAGE in glioma is still not fully understood, its involvement in establishing an aggressive cellular phenotype suggests that RAGE signaling plays a role in glioma formation. Further investigation is necessary to understand RAGE signaling biology and potential therapeutic targets.
Cytoskeleton mobilization
During cell activation, reactive astrocytes undergo remarkable morphologic changes dependent on the severity of the injury. These include hypertrophy, hyperplasia, migration, and/or changes in cellular processes. Cytoskeleton mobilization plays an important role in the establishment of these morphologic changes in astrocytes. Specifically, loss-of-function studies have shown that mice lacking GFAP and vimentin expression exhibit failure in cellular activation and worse outcomes after CNS injury, which confirms the importance of mobilization of cytoskeletons during cell activation [89].
Jak-stat signaling is a key regulatory mechanism in cytoskeleton mobilization in reactive astrocytes. While astrocyte-specific knock out of STAT3 results in minimal changes in brain development, it hinders hypertrophy and scar formation after spinal cord injury and impairs the wound healing process and functional recovery [90]. It may be the case that Jak-stat and Smad translocate into the nucleus and recruit cofactor p300/CBP, which initiates gene expression (i.e., GFAP) for astrocytic activation [91]. Moreover, the small GTPases of Rho subfamily and Rho kinase (ROCK) are related to the regulation of cellular polarity and migration in reactive astrocytes, possibly through regulation of F-actin and focal adhesion complexes [92].
Abnormalities in the regulation of cytoskeleton have been identified in various types of tumors. For example, Rho family members have been identified in astrocytomas and melanomas. Activation of Rho or Rac is associated with the proliferative and migratory phenotype of glioma cells. Dysregulation of rac destabilizes focal adhesions and leads to the migratory phenotype of glioma cells. This is likely a result of lamellipodia formation and membrane ruffles in tumor cells. Abnormalities of cytoskeleton regulators such as Rho and ROCK enhance glioma cell migratory phenotype, which results in the local spread of high-grade gliomas [93]. The importance of cytoskeleton regulation has been underscored by inhibition of Rho kinase, which results in down-regulation of VEGF and MMPs expression by glioma cells, and reduces the migration of adjacent endothelial cells and angiogenesis. Thus, Rho kinase may play a key role in establishing a tumor microenvironment [94].
Ischemic injury induces astrocytic activation
The CNS is the most sensitive organ to tissue oxygen concentration. Ischemic insults result in rapid and irreversible neuronal damage. In contrast to neurons, astrocytes are more resistant to hypoxia and are protective in the early stages of ischemia [95, 96]. A widespread astrocytic activation has been identified shortly after ischemic injury, which may limit the damage caused by ischemia and reactive oxygen species [97, 98]. Mice lacking GFAP and vimentin expression are incapable of astrocytic activation. These mice are extremely vulnerable to ischemic damage and are unable to limit the region of infarction, which underscores the beneficial effect of astrocyte activation in ischemia.
Several molecular mechanisms can explain the protective function of reactive astrocytes. For example, activated astrocytes express additional glutamate transporters such as GLT-1, which efficiently take up extracellular glutamate and reduce glutamate-associated excitotoxicity during ischemia [99]. Endothelin-3 reduces the conductance of gap junctions in astrocytes, which further reduces the extent of ischemic injury. Silencing cyclin D1 results in a loss of proliferative astrocytes during ischemia, which exacerbates hypoxic damage of neurons, further confirming the protective role of reactive astrocytes in ischemia. Additionally, reactive astrocytes express cytokines such as Epo, IL-1α, TNFα, and IL-6, which have been shown to reduce the ischemic impact to brain.
HIFs are transcriptional factors that initiate gene expression during the adaptive response to low ambient oxygen (O2). Structurally, HIFs are composed of α and β subunits. HIFβ is constitutively expressed, whereas HIF-α subunit is activated when oxygen concentration changes. HIF-associated signaling is important in astrocytic reactivation during ischemic injury in the CNS [100]. Heterodimerization of HIF-α and β subunits exerts gene transcriptions related to cellular metabolism, proliferation, and survival, which have neuroprotective effects. HIFs induce robust gene expression of EPO, VEGF, and GLUT-1 during ischemic injury [101–103].
The paracrine signaling of EPO has been found to be critical for neuronal protection in vivo and in vitro, through activation of subsequent JAK2, PI3K, and NF-κB pathways [101, 104, 105]. Additionally, reactive astrocytes stabilize microvascular endothelial cells through VEGF, implicating HIF activation in reactive astrocytes as a regulator of cerebral vessel structure and microenvironment during ischemic injury [106]. However, HIF-associated iNOS expression is detrimental to neurons both during ischemia and post-ischemia, possibly through the release of nitric oxide and peroxynitrite [107, 108]. Pharmacologic targeting of these damaging agents could be of great importance to antagonize ischemic damage.
HIFs also mediate potent gene transcription related to tumorigenesis [109]. Stabilization and activation of HIF signaling establishes a pseudohypoxic state and leads to a proliferative phenotype similar to reactive astrocytes. Hypoxia upregulates important stem cell factors such as OCT4, NANOG, and c-Myc. Additionally, HIFs contribute to the modulation of the tumor microenvironment, possibly through secretion of growth factors such as EPO and VEGF as described above [110, 111]. Recent discoveries demonstrate that HIFs signaling has been found crucial for the self-renewal, proliferation, and survival of the stem cell population in gliomas [112, 113]. Finally, mutations of IDH1/2 genes are related to the pathogenesis of gliomas. These mutations reduce the availability of α-ketoglutarate, restricting HIF-1α hydroxylation and activating glycolysis, which leads to a Warburg effect and carcinogenesis [114–116].
Outlook and perspectives
Increasing evidence suggests that several key molecular pathways tightly control cellular activation of astrocytes. These molecular mechanisms not only contribute to the transition from the quiescent to the activated stage but also determine the duration and extent of cellular response, gliosis, and scar formation. The activation of astrocytes is beneficial to the repair of CNS injury and rearrangement of cellular connections during wound healing. Nevertheless, many of these same molecular pathways have been identified to be constitutively active in gliomas, suggesting that dysfunction of the same cellular activation pathways may contribute to the formation of brain tumors. Moreover, glioma cells exhibit many of the phenotypes that have been seen in activated astrocytes, including morphologic changes, cell proliferation, and penetrative migration. Better understanding and modulation of these normal and dysfunctional astrocyte activation pathways could be of great importance in limiting and/or treating gliomas.
Acknowledgments
This research was supported by the intramural research program in the National Institute of Neurological Disorders and Stroke at the National Institutes of Health.
Contributor Information
Chunzhang Yang, Email: yangc2@ninds.nih.gov.
Zhengping Zhuang, Phone: +1-30143-58445, FAX: +1-30148-04590, Email: zhuangp@ninds.nih.gov.
References
- 1.Sun D, Jakobs TC. Structural remodeling of astrocytes in the injured CNS. Neuroscientist. 2012;18(6):567–588. doi: 10.1177/1073858411423441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seifert G, Schilling K, Steinhauser C. Astrocyte dysfunction in neurological disorders: a molecular perspective. Nat Rev Neurosci. 2006;7(3):194–206. doi: 10.1038/nrn1870. [DOI] [PubMed] [Google Scholar]
- 3.Chih CP, Roberts EL., Jr Energy substrates for neurons during neural activity: a critical review of the astrocyte-neuron lactate shuttle hypothesis. J Cereb Blood Flow Metab. 2003;23(11):1263–1281. doi: 10.1097/01.WCB.0000081369.51727.6F. [DOI] [PubMed] [Google Scholar]
- 4.Yu AC, Schousboe A, Hertz L. Metabolic fate of 14C-labeled glutamate in astrocytes in primary cultures. J Neurochem. 1982;39(4):954–960. doi: 10.1111/j.1471-4159.1982.tb11482.x. [DOI] [PubMed] [Google Scholar]
- 5.Yang CZ, Zhao R, Dong Y, Chen XQ, Yu AC. Astrocyte and neuron intone through glutamate. Neurochem Res. 2008;33(12):2480–2486. doi: 10.1007/s11064-008-9758-x. [DOI] [PubMed] [Google Scholar]
- 6.West H, Richardson WD, Fruttiger M. Stabilization of the retinal vascular network by reciprocal feedback between blood vessels and astrocytes. Development. 2005;132(8):1855–1862. doi: 10.1242/dev.01732. [DOI] [PubMed] [Google Scholar]
- 7.Sofroniew MV. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 2009;32(12):638–647. doi: 10.1016/j.tins.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pekny M, Nilsson M. Astrocyte activation and reactive gliosis. Glia. 2005;50(4):427–434. doi: 10.1002/glia.20207. [DOI] [PubMed] [Google Scholar]
- 9.Buffo A, Rolando C, Ceruti S. Astrocytes in the damaged brain: molecular and cellular insights into their reactive response and healing potential. Biochem Pharmacol. 2010;79(2):77–89. doi: 10.1016/j.bcp.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 10.Myer DJ, Gurkoff GG, Lee SM, Hovda DA, Sofroniew MV. Essential protective roles of reactive astrocytes in traumatic brain injury. Brain. 2006;129(Pt 10):2761–2772. doi: 10.1093/brain/awl165. [DOI] [PubMed] [Google Scholar]
- 11.Zhu Z, Zhang Q, Yu Z, Zhang L, Tian D, Zhu S, Bu B, Xie M, Wang W. Inhibiting cell cycle progression reduces reactive astrogliosis initiated by scratch injury in vitro and by cerebral ischemia in vivo. Glia. 2007;55(5):546–558. doi: 10.1002/glia.20476. [DOI] [PubMed] [Google Scholar]
- 12.Bushong EA, Martone ME, Jones YZ, Ellisman MH. Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. J Neurosci. 2002;22(1):183–192. doi: 10.1523/JNEUROSCI.22-01-00183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eng LF, Ghirnikar RS. GFAP and astrogliosis. Brain Pathol. 1994;4(3):229–237. doi: 10.1111/j.1750-3639.1994.tb00838.x. [DOI] [PubMed] [Google Scholar]
- 14.Hirrlinger J, Hulsmann S, Kirchhoff F. Astroglial processes show spontaneous motility at active synaptic terminals in situ. Eur J Neurosci. 2004;20(8):2235–2239. doi: 10.1111/j.1460-9568.2004.03689.x. [DOI] [PubMed] [Google Scholar]
- 15.Nedergaard M, Ransom B, Goldman SA. New roles for astrocytes: redefining the functional architecture of the brain. Trends Neurosci. 2003;26(10):523–530. doi: 10.1016/j.tins.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 16.Petzold GC, Murthy VN. Role of astrocytes in neurovascular coupling. Neuron. 2011;71(5):782–797. doi: 10.1016/j.neuron.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 17.Ogata K, Kosaka T. Structural and quantitative analysis of astrocytes in the mouse hippocampus. Neuroscience. 2002;113(1):221–233. doi: 10.1016/s0306-4522(02)00041-6. [DOI] [PubMed] [Google Scholar]
- 18.Lee SJ, Benveniste EN. Adhesion molecule expression and regulation on cells of the central nervous system. J Neuroimmunol. 1999;98(2):77–88. doi: 10.1016/s0165-5728(99)00084-3. [DOI] [PubMed] [Google Scholar]
- 19.Lee YJ, Benveniste EN. Stat1 alpha expression is involved in IFN-gamma induction of the class II transactivator and class II MHC genes. J Immunol. 1996;157(4):1559–1568. [PubMed] [Google Scholar]
- 20.Dong Y, Benveniste EN. Immune function of astrocytes. Glia. 2001;36(2):180–190. doi: 10.1002/glia.1107. [DOI] [PubMed] [Google Scholar]
- 21.Wilhelmsson U, Bushong EA, Price DL, Smarr BL, Phung V, Terada M, Ellisman MH, Pekny M. Redefining the concept of reactive astrocytes as cells that remain within their unique domains upon reaction to injury. Proc Natl Acad Sci U S A. 2006;103(46):17513–17518. doi: 10.1073/pnas.0602841103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119(1):7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ignatova TN, Kukekov VG, Laywell ED, Suslov ON, Vrionis FD, Steindler DA. Human cortical glial tumors contain neural stem-like cells expressing astroglial and neuronal markers in vitro. Glia. 2002;39(3):193–206. doi: 10.1002/glia.10094. [DOI] [PubMed] [Google Scholar]
- 24.Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumour initiating cells. Nature. 2004;432(7015):396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 25.Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444(7120):756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 26.Chen J, McKay RM, Parada LF. Malignant glioma: lessons from genomics, mouse models, and stem cells. Cell. 2012;149(1):36–47. doi: 10.1016/j.cell.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tezel G, Hernandez MR, Wax MB. In vitro evaluation of reactive astrocyte migration, a component of tissue remodeling in glaucomatous optic nerve head. Glia. 2001;34(3):178–189. doi: 10.1002/glia.1052. [DOI] [PubMed] [Google Scholar]
- 28.Yang C, Iyer RR, Yu AC, Yong RL, Park DM, Weil RJ, Ikejiri B, Brady RO, Lonser RR, Zhuang Z. beta-Catenin signaling initiates the activation of astrocytes and its dysregulation contributes to the pathogenesis of astrocytomas. Proc Natl Acad Sci U S A. 2012;109(18):6963–6968. doi: 10.1073/pnas.1118754109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang H, Cheng XP, Li JW, Yao Q, Ju G. De-differentiation response of cultured astrocytes to injury induced by scratch or conditioned culture medium of scratch-insulted astrocytes. Cell Mol Neurobiol. 2009;29(4):455–473. doi: 10.1007/s10571-008-9337-3. [DOI] [PubMed] [Google Scholar]
- 30.Huttenlocher A, Lakonishok M, Kinder M, Wu S, Truong T, Knudsen KA, Horwitz AF. Integrin and cadherin synergy regulates contact inhibition of migration and motile activity. J Cell Biol. 1998;141(2):515–526. doi: 10.1083/jcb.141.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pokutta S, Weis WI. Structure and mechanism of cadherins and catenins in cell–cell contacts. Annu Rev Cell Dev Biol. 2007;23:237–261. doi: 10.1146/annurev.cellbio.22.010305.104241. [DOI] [PubMed] [Google Scholar]
- 32.Behrens J, Jerchow BA, Wurtele M, Grimm J, Asbrand C, Wirtz R, Kuhl M, Wedlich D, Birchmeier W. Functional interaction of an axin homolog, conductin, with beta-catenin, APC, and GSK3beta. Science. 1998;280(5363):596–599. doi: 10.1126/science.280.5363.596. [DOI] [PubMed] [Google Scholar]
- 33.Akiyama T, Kawasaki Y. Wnt signalling and the actin cytoskeleton. Oncogene. 2006;25(57):7538–7544. doi: 10.1038/sj.onc.1210063. [DOI] [PubMed] [Google Scholar]
- 34.L’Episcopo F, Tirolo C, Testa N, Caniglia S, Morale MC, Cossetti C, D’Adamo P, Zardini E, Andreoni L, Ihekwaba AE, Serra PA, Franciotta D, Martino G, Pluchino S, Marchetti B. Reactive astrocytes and Wnt/beta-catenin signaling link nigrostriatal injury to repair in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson’s disease. Neurobiol Dis. 2011;41(2):508–527. doi: 10.1016/j.nbd.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hamilton SR, Liu B, Parsons RE, Papadopoulos N, Jen J, Powell SM, Krush AJ, Berk T, Cohen Z, Tetu B, et al. The molecular basis of Turcot’s syndrome. N Engl J Med. 1995;332(13):839–847. doi: 10.1056/NEJM199503303321302. [DOI] [PubMed] [Google Scholar]
- 36.Liu X, Wang L, Zhao S, Ji X, Luo Y, Ling F. beta-Catenin overexpression in malignant glioma and its role in proliferation and apoptosis in glioblastoma cells. Med Oncol. 2011;28(2):608–614. doi: 10.1007/s12032-010-9476-5. [DOI] [PubMed] [Google Scholar]
- 37.Pu P, Zhang Z, Kang C, Jiang R, Jia Z, Wang G, Jiang H. Downregulation of Wnt2 and beta-catenin by siRNA suppresses malignant glioma cell growth. Cancer Gene Ther. 2009;16(4):351–361. doi: 10.1038/cgt.2008.78. [DOI] [PubMed] [Google Scholar]
- 38.Zhang N, Wei P, Gong A, Chiu WT, Lee HT, Colman H, Huang H, Xue J, Liu M, Wang Y, Sawaya R, Xie K, Yung WK, Medema RH, He X, Huang S. FoxM1 promotes beta-catenin nuclear localization and controls Wnt target-gene expression and glioma tumorigenesis. Cancer Cell. 2011;20(4):427–442. doi: 10.1016/j.ccr.2011.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8(10):755–768. doi: 10.1038/nrc2499. [DOI] [PubMed] [Google Scholar]
- 40.Paulus W, Tonn JC. Basement membrane invasion of glioma cells mediated by integrin receptors. J Neurosurg. 1994;80(3):515–519. doi: 10.3171/jns.1994.80.3.0515. [DOI] [PubMed] [Google Scholar]
- 41.Cambier S, Gline S, Mu D, Collins R, Araya J, Dolganov G, Einheber S, Boudreau N, Nishimura SL. Integrin alpha(v)beta8-mediated activation of transforming growth factor-beta by perivascular astrocytes: an angiogenic control switch. Am J Pathol. 2005;166(6):1883–1894. doi: 10.1016/s0002-9440(10)62497-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hirota S, Liu Q, Lee HS, Hossain MG, Lacy-Hulbert A, McCarty JH. The astrocyte-expressed integrin alphavbeta8 governs blood vessel sprouting in the developing retina. Development. 2011;138(23):5157–5166. doi: 10.1242/dev.069153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.del Zoppo GJ, Milner R. Integrin-matrix interactions in the cerebral microvasculature. Arterioscler Thromb Vasc Biol. 2006;26(9):1966–1975. doi: 10.1161/01.ATV.0000232525.65682.a2. [DOI] [PubMed] [Google Scholar]
- 44.Voskuhl RR, Peterson RS, Song B, Ao Y, Morales LB, Tiwari-Woodruff S, Sofroniew MV. Reactive astrocytes form scar-like perivascular barriers to leukocytes during adaptive immune inflammation of the CNS. J Neurosci. 2009;29(37):11511–11522. doi: 10.1523/JNEUROSCI.1514-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Uhm JH, Gladson CL, Rao JS. The role of integrins in the malignant phenotype of gliomas. Front Biosci. 1999;4:D188–D199. doi: 10.2741/uhm. [DOI] [PubMed] [Google Scholar]
- 46.Wagner S, Tagaya M, Koziol JA, Quaranta V, del Zoppo GJ. Rapid disruption of an astrocyte interaction with the extracellular matrix mediated by integrin alpha 6 beta 4 during focal cerebral ischemia/reperfusion. Stroke. 1997;28(4):858–865. doi: 10.1161/01.str.28.4.858. [DOI] [PubMed] [Google Scholar]
- 47.Robel S, Mori T, Zoubaa S, Schlegel J, Sirko S, Faissner A, Goebbels S, Dimou L, Gotz M. Conditional deletion of beta1-integrin in astroglia causes partial reactive gliosis. Glia. 2009;57(15):1630–1647. doi: 10.1002/glia.20876. [DOI] [PubMed] [Google Scholar]
- 48.Etienne-Manneville S, Hall A. Integrin-mediated activation of Cdc42 controls cell polarity in migrating astrocytes through PKCzeta. Cell. 2001;106(4):489–498. doi: 10.1016/s0092-8674(01)00471-8. [DOI] [PubMed] [Google Scholar]
- 49.Natarajan M, Stewart JE, Golemis EA, Pugacheva EN, Alexandropoulos K, Cox BD, Wang W, Grammer JR, Gladson CL. HEF1 is a necessary and specific downstream effector of FAK that promotes the migration of glioblastoma cells. Oncogene. 2006;25(12):1721–1732. doi: 10.1038/sj.onc.1209199. [DOI] [PubMed] [Google Scholar]
- 50.Summers L, Kangwantas K, Nguyen L, Kielty C, Pinteaux E. Adhesion to the extracellular matrix is required for interleukin-1 beta actions leading to reactive phenotype in rat astrocytes. Mol Cell Neurosci. 2010;44(3):272–281. doi: 10.1016/j.mcn.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Milner R, Huang X, Wu J, Nishimura S, Pytela R, Sheppard D, ffrench-Constant C. Distinct roles for astrocyte alphavbeta5 and alphavbeta8 integrins in adhesion and migration. J Cell Sci. 1999;112(Pt 23):4271–4279. doi: 10.1242/jcs.112.23.4271. [DOI] [PubMed] [Google Scholar]
- 52.Hecker TP, Ding Q, Rege TA, Hanks SK, Gladson CL. Overexpression of FAK promotes Ras activity through the formation of a FAK/p120RasGAP complex in malignant astrocytoma cells. Oncogene. 2004;23(22):3962–3971. doi: 10.1038/sj.onc.1207541. [DOI] [PubMed] [Google Scholar]
- 53.Schnell O, Krebs B, Wagner E, Romagna A, Beer AJ, Grau SJ, Thon N, Goetz C, Kretzschmar HA, Tonn JC, Goldbrunner RH. Expression of integrin alphavbeta3 in gliomas correlates with tumor grade and is not restricted to tumor vasculature. Brain Pathol. 2008;18(3):378–386. doi: 10.1111/j.1750-3639.2008.00137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Farber K, Synowitz M, Zahn G, Vossmeyer D, Stragies R, van Rooijen N, Kettenmann H. An alpha5beta1 integrin inhibitor attenuates glioma growth. Mol Cell Neurosci. 2008;39(4):579–585. doi: 10.1016/j.mcn.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 55.Skuli N, Monferran S, Delmas C, Favre G, Bonnet J, Toulas C, Cohen-Jonathan Moyal E. Alphavbeta3/alphavbeta5 integrins-FAK-RhoB: a novel pathway for hypoxia regulation in glioblastoma. Cancer Res. 2009;69(8):3308–3316. doi: 10.1158/0008-5472.CAN-08-2158. [DOI] [PubMed] [Google Scholar]
- 56.Bello L, Francolini M, Marthyn P, Zhang J, Carroll RS, Nikas DC, Strasser JF, Villani R, Cheresh DA, Black PM. Alpha(v)beta3 and alpha(v)beta5 integrin expression in glioma periphery. Neurosurgery. 2001;49(2):380–389. doi: 10.1097/00006123-200108000-00022. [DOI] [PubMed] [Google Scholar]
- 57.Tchaicha JH, Mobley AK, Hossain MG, Aldape KD, McCarty JH. A mosaic mouse model of astrocytoma identifies alphavbeta8 integrin as a negative regulator of tumor angiogenesis. Oncog. 2010;29(31):4460–4472. doi: 10.1038/onc.2010.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fukushima Y, Ohnishi T, Arita N, Hayakawa T, Sekiguchi K. Integrin alpha3beta1-mediated interaction with laminin-5 stimulates adhesion, migration and invasion of malignant glioma cells. Int J Cancer. 1998;76(1):63–72. doi: 10.1002/(sici)1097-0215(19980330)76:1<63::aid-ijc11>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 59.Lathia JD, Gallagher J, Heddleston JM, Wang J, Eyler CE, Macswords J, Wu Q, Vasanji A, McLendon RE, Hjelmeland AB, Rich JN. Integrin alpha 6 regulates glioblastoma stem cells. Cell Stem Cell. 2010;6(5):421–432. doi: 10.1016/j.stem.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lin JH, Weigel H, Cotrina ML, Liu S, Bueno E, Hansen AJ, Hansen TW, Goldman S, Nedergaard M. Gap-junction-mediated propagation and amplification of cell injury. Nat Neurosci. 1998;1(6):494–500. doi: 10.1038/2210. [DOI] [PubMed] [Google Scholar]
- 61.Rawanduzy A, Hansen A, Hansen TW, Nedergaard M. Effective reduction of infarct volume by gap junction blockade in a rodent model of stroke. J Neurosurg. 1997;87(6):916–920. doi: 10.3171/jns.1997.87.6.0916. [DOI] [PubMed] [Google Scholar]
- 62.Contreras JE, Sanchez HA, Veliz LP, Bukauskas FF, Bennett MV, Saez JC. Role of connexin-based gap junction channels and hemichannels in ischemia-induced cell death in nervous tissue. Brain Res Brain Res Rev. 2004;47(1–3):290–303. doi: 10.1016/j.brainresrev.2004.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Goldberg GS, Bechberger JF, Tajima Y, Merritt M, Omori Y, Gawinowicz MA, Narayanan R, Tan Y, Sanai Y, Yamasaki H, Naus CC, Tsuda H, Nicholson BJ. Connexin43 suppresses MFG-E8 while inducing contact growth inhibition of glioma cells. Cancer Res. 2000;60(21):6018–6026. [PubMed] [Google Scholar]
- 64.Reardon DA, Fink KL, Mikkelsen T, Cloughesy TF, O’Neill A, Plotkin S, Glantz M, Ravin P, Raizer JJ, Rich KM, Schiff D, Shapiro WR, Burdette-Radoux S, Dropcho EJ, Wittemer SM, Nippgen J, Picard M, Nabors LB. Randomized phase II study of cilengitide, an integrin-targeting arginine-glycine-aspartic acid peptide, in recurrent glioblastoma multiforme. J Clin Oncol. 2008;26(34):5610–5617. doi: 10.1200/JCO.2008.16.7510. [DOI] [PubMed] [Google Scholar]
- 65.Yu AC, Lau LT. Expression of interleukin-1 alpha, tumor necrosis factor alpha and interleukin-6 genes in astrocytes under ischemic injury. Neurochem Int. 2000;36(4–5):369–377. doi: 10.1016/s0197-0186(99)00145-x. [DOI] [PubMed] [Google Scholar]
- 66.Nieto-Sampedro M, Gomez-Pinilla F, Knauer DJ, Broderick JT. Epidermal growth factor receptor immunoreactivity in rat brain astrocytes. Response to injury. Neurosci Lett. 1988;91(3):276–282. doi: 10.1016/0304-3940(88)90693-3. [DOI] [PubMed] [Google Scholar]
- 67.Codeluppi S, Svensson CI, Hefferan MP, Valencia F, Silldorff MD, Oshiro M, Marsala M, Pasquale EB. The Rheb-mTOR pathway is upregulated in reactive astrocytes of the injured spinal cord. J Neurosci. 2009;29(4):1093–1104. doi: 10.1523/JNEUROSCI.4103-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu B, Chen H, Johns TG, Neufeld AH. Epidermal growth factor receptor activation: an upstream signal for transition of quiescent astrocytes into reactive astrocytes after neural injury. J Neurosci. 2006;26(28):7532–7540. doi: 10.1523/JNEUROSCI.1004-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Okada S, Nakamura M, Katoh H, Miyao T, Shimazaki T, Ishii K, Yamane J, Yoshimura A, Iwamoto Y, Toyama Y, Okano H. Conditional ablation of Stat3 or Socs3 discloses a dual role for reactive astrocytes after spinal cord injury. Nat Med. 2006;12(7):829–834. doi: 10.1038/nm1425. [DOI] [PubMed] [Google Scholar]
- 70.Sriram K, Benkovic SA, Hebert MA, Miller DB, O’Callaghan JP. Induction of gp130-related cytokines and activation of JAK2/STAT3 pathway in astrocytes precedes up-regulation of glial fibrillary acidic protein in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of neurodegeneration: key signaling pathway for astrogliosis in vivo? J Biol Chem. 2004;279(19):19936–19947. doi: 10.1074/jbc.M309304200. [DOI] [PubMed] [Google Scholar]
- 71.Weissenberger J, Loeffler S, Kappeler A, Kopf M, Lukes A, Afanasieva TA, Aguzzi A, Weis J. IL-6 is required for glioma development in a mouse model. Oncog. 2004;23(19):3308–3316. doi: 10.1038/sj.onc.1207455. [DOI] [PubMed] [Google Scholar]
- 72.Price RL, Song J, Bingmer K, Yi J, Rivera A, Ogelsbee M, Cook C, Kwon CH, Chiocca EA (2012) Cytomegalovirus enhances glioblastoma via PDGF-B/STAT3 pathway activation. Proceedings of the 103rd Annual Meeting of the American Association for Cancer Research 72 (8 Suppl):Abstract nr 4815. doi:1538-7445.AM2012-4815
- 73.Lu J, Ksendzovsky A, Yang C, Mehta GU, Yong RL, Weil RJ, Park DM, Mushlin HM, Fang X, Balgley BM, Lee DH, Lee CS, Lonser RR, Zhuang Z. CNTF receptor subunit α as a marker for glioma tumor-initiating cells and tumor grade. J Neurosurg. 2012;117:1022–1031. doi: 10.3171/2012.9.JNS1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Neary JT, Zimmermann H. Trophic functions of nucleotides in the central nervous system. Trends Neurosci. 2009;32(4):189–198. doi: 10.1016/j.tins.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 75.Abbracchio MP, Ceruti S. Roles of P2 receptors in glial cells: focus on astrocytes. Purinergic Signal. 2006;2(4):595–604. doi: 10.1007/s11302-006-9016-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Neary JT, McCarthy M, Kang Y, Zuniga S. Mitogenic signaling from P1 and P2 purinergic receptors to mitogen-activated protein kinase in human fetal astrocyte cultures. Neurosci Lett. 1998;242(3):159–162. doi: 10.1016/s0304-3940(98)00067-6. [DOI] [PubMed] [Google Scholar]
- 77.Neary JT, Kang Y, Willoughby KA, Ellis EF. Activation of extracellular signal-regulated kinase by stretch-induced injury in astrocytes involves extracellular ATP and P2 purinergic receptors. J Neurosci. 2003;23(6):2348–2356. doi: 10.1523/JNEUROSCI.23-06-02348.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sorci G, Riuzzi F, Giambanco I. Donato R (2013) RAGE in tissue homeostasis, repair and regeneration. Biochim Biophys Acta. 1833;1:101–109. doi: 10.1016/j.bbamcr.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 79.Hudson BI, Kalea AZ, Del Arriero Mar M, Harja E, Boulanger E, D’Agati V, Schmidt AM. Interaction of the RAGE cytoplasmic domain with diaphanous-1 is required for ligand-stimulated cellular migration through activation of Rac1 and Cdc42. J Biol Chem. 2008;283(49):34457–34468. doi: 10.1074/jbc.M801465200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Brozzi F, Arcuri C, Giambanco I, Donato R. S100B Protein Regulates Astrocyte Shape and Migration via Interaction with Src Kinase: Implications for astrocyte development, activation and tumor growth. J Biol Chem. 2009;284(13):8797–8811. doi: 10.1074/jbc.M805897200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Qiu J, Nishimura M, Wang Y, Sims JR, Qiu S, Savitz SI, Salomone S, Moskowitz MA. Early release of HMGB-1 from neurons after the onset of brain ischemia. J Cereb Blood Flow Metab. 2008;28(5):927–938. doi: 10.1038/sj.jcbfm.9600582. [DOI] [PubMed] [Google Scholar]
- 82.Qiu J, Xu J, Zheng Y, Wei Y, Zhu X, Lo EH, Moskowitz MA, Sims JR. High-mobility group box 1 promotes metalloproteinase-9 upregulation through Toll-like receptor 4 after cerebral ischemia. Stroke. 2010;41(9):2077–2082. doi: 10.1161/STROKEAHA.110.590463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hayakawa K, Miyamoto N, Seo JH, Pham LD, Kim KW, Lo EH, Arai K (2012) High-mobility group box 1 from reactive astrocytes enhances the accumulation of endothelial progenitor cells in damaged white matter. J Neurochem. doi:10.1111/jnc.12120 [DOI] [PMC free article] [PubMed]
- 84.Ponath G, Schettler C, Kaestner F, Voigt B, Wentker D, Arolt V, Rothermundt M. Autocrine S100B effects on astrocytes are mediated via RAGE. J Neuroimmunol. 2007;184(1–2):214–222. doi: 10.1016/j.jneuroim.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 85.Tafani M, Schito L, Pellegrini L, Villanova L, Marfe G, Anwar T, Rosa R, Indelicato M, Fini M, Pucci B, Russo MA. Hypoxia-increased RAGE and P2X7R expression regulates tumor cell invasion through phosphorylation of Erk1/2 and Akt and nuclear translocation of NF-{kappa}B. Carcinogenes. 2011;32(8):1167–1175. doi: 10.1093/carcin/bgr101. [DOI] [PubMed] [Google Scholar]
- 86.Arumugam T, Ramachandran V, Logsdon CD. Effect of cromolyn on S100P interactions with RAGE and pancreatic cancer growth and invasion in mouse models. J Natl Cancer Inst. 2006;98(24):1806–1818. doi: 10.1093/jnci/djj498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Taguchi A, Blood DC, del Toro G, Canet A, Lee DC, Qu W, Tanji N, Lu Y, Lalla E, Fu C, Hofmann MA, Kislinger T, Ingram M, Lu A, Tanaka H, Hori O, Ogawa S, Stern DM, Schmidt AM. Blockade of RAGE-amphoterin signalling suppresses tumour growth and metastases. Nature. 2000;405(6784):354–360. doi: 10.1038/35012626. [DOI] [PubMed] [Google Scholar]
- 88.Rojas A, Figueroa H, Morales E. Fueling inflammation at tumor microenvironment: the role of multiligand/RAGE axis. Carcinogenes. 2010;31(3):334–341. doi: 10.1093/carcin/bgp322. [DOI] [PubMed] [Google Scholar]
- 89.Pekny M, Johansson CB, Eliasson C, Stakeberg J, Wallen A, Perlmann T, Lendahl U, Betsholtz C, Berthold CH, Frisen J. Abnormal reaction to central nervous system injury in mice lacking glial fibrillary acidic protein and vimentin. J Cell Biol. 1999;145(3):503–514. doi: 10.1083/jcb.145.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Herrmann JE, Imura T, Song B, Qi J, Ao Y, Nguyen TK, Korsak RA, Takeda K, Akira S, Sofroniew MV. STAT3 is a critical regulator of astrogliosis and scar formation after spinal cord injury. J Neurosci. 2008;28(28):7231–7243. doi: 10.1523/JNEUROSCI.1709-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Herrera F, Chen Q, Schubert D. Synergistic effect of retinoic acid and cytokines on the regulation of glial fibrillary acidic protein expression. J Biol Chem. 2010;285(50):38915–38922. doi: 10.1074/jbc.M110.170274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.John GR, Chen L, Rivieccio MA, Melendez-Vasquez CV, Hartley A, Brosnan CF. Interleukin-1beta induces a reactive astroglial phenotype via deactivation of the Rho GTPase-Rock axis. J Neurosci. 2004;24(11):2837–2845. doi: 10.1523/JNEUROSCI.4789-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zohrabian VM, Forzani B, Chau Z, Murali R, Jhanwar-Uniyal M. Rho/ROCK and MAPK signaling pathways are involved in glioblastoma cell migration and proliferation. Anticancer Res. 2009;29(1):119–123. [PubMed] [Google Scholar]
- 94.Nakabayashi H, Shimizu K. HA1077, a Rho kinase inhibitor, suppresses glioma-induced angiogenesis by targeting the Rho-ROCK and the mitogen-activated protein kinase kinase/extracellular signal-regulated kinase (MEK/ERK) signal pathways. Cancer Sci. 2011;102(2):393–399. doi: 10.1111/j.1349-7006.2010.01794.x. [DOI] [PubMed] [Google Scholar]
- 95.Yu AC, Gregory GA, Chan PH. Hypoxia-induced dysfunctions and injury of astrocytes in primary cell cultures. J Cereb Blood Flow Metab. 1989;9(1):20–28. doi: 10.1038/jcbfm.1989.3. [DOI] [PubMed] [Google Scholar]
- 96.Li L, Lundkvist A, Andersson D, Wilhelmsson U, Nagai N, Pardo AC, Nodin C, Stahlberg A, Aprico K, Larsson K, Yabe T, Moons L, Fotheringham A, Davies I, Carmeliet P, Schwartz JP, Pekna M, Kubista M, Blomstrand F, Maragakis N, Nilsson M, Pekny M. Protective role of reactive astrocytes in brain ischemia. J Cereb Blood Flow Metab. 2008;28(3):468–481. doi: 10.1038/sj.jcbfm.9600546. [DOI] [PubMed] [Google Scholar]
- 97.Duggal N, Schmidt-Kastner R, Hakim AM. Nestin expression in reactive astrocytes following focal cerebral ischemia in rats. Brain Res. 1997;768(1–2):1–9. doi: 10.1016/s0006-8993(97)00588-x. [DOI] [PubMed] [Google Scholar]
- 98.Rossi DJ, Brady JD, Mohr C. Astrocyte metabolism and signaling during brain ischemia. Nat Neurosci. 2007;10(11):1377–1386. doi: 10.1038/nn2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Swanson RA. Astrocyte glutamate uptake during chemical hypoxia in vitro. Neurosci Lett. 1992;147(2):143–146. doi: 10.1016/0304-3940(92)90580-z. [DOI] [PubMed] [Google Scholar]
- 100.Chavez JC, Agani F, Pichiule P, LaManna JC. Expression of hypoxia-inducible factor-1alpha in the brain of rats during chronic hypoxia. J Appl Physiol. 2000;89(5):1937–1942. doi: 10.1152/jappl.2000.89.5.1937. [DOI] [PubMed] [Google Scholar]
- 101.Chavez JC, Baranova O, Lin J, Pichiule P. The transcriptional activator hypoxia inducible factor 2 (HIF-2/EPAS-1) regulates the oxygen-dependent expression of erythropoietin in cortical astrocytes. J Neurosci. 2006;26(37):9471–9481. doi: 10.1523/JNEUROSCI.2838-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hossain MA, Bouton CM, Pevsner J, Laterra J. Induction of vascular endothelial growth factor in human astrocytes by lead. Involvement of a protein kinase C/activator protein-1 complex-dependent and hypoxia-inducible factor 1-independent signaling pathway. J Biol Chem. 2000;275(36):27874–27882. doi: 10.1074/jbc.M002185200. [DOI] [PubMed] [Google Scholar]
- 103.Bernaudin M, Bellail A, Marti HH, Yvon A, Vivien D, Duchatelle I, Mackenzie ET, Petit E. Neurons and astrocytes express EPO mRNA: oxygen-sensing mechanisms that involve the redox-state of the brain. Glia. 2000;30(3):271–278. [PubMed] [Google Scholar]
- 104.Digicaylioglu M, Lipton SA. Erythropoietin-mediated neuroprotection involves cross-talk between Jak2 and NF-kappaB signalling cascades. Nature. 2001;412(6847):641–647. doi: 10.1038/35088074. [DOI] [PubMed] [Google Scholar]
- 105.Ruscher K, Freyer D, Karsch M, Isaev N, Megow D, Sawitzki B, Priller J, Dirnagl U, Meisel A. Erythropoietin is a paracrine mediator of ischemic tolerance in the brain: evidence from an in vitro model. J Neurosci. 2002;22(23):10291–10301. doi: 10.1523/JNEUROSCI.22-23-10291.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chow J, Ogunshola O, Fan SY, Li Y, Ment LR, Madri JA. Astrocyte-derived VEGF mediates survival and tube stabilization of hypoxic brain microvascular endothelial cells in vitro. Brain Res Dev Brain Res. 2001;130(1):123–132. doi: 10.1016/s0165-3806(01)00220-6. [DOI] [PubMed] [Google Scholar]
- 107.Endoh M, Maiese K, Wagner J. Expression of the inducible form of nitric oxide synthase by reactive astrocytes after transient global ischemia. Brain Res. 1994;651(1–2):92–100. doi: 10.1016/0006-8993(94)90683-1. [DOI] [PubMed] [Google Scholar]
- 108.Vangeison G, Carr D, Federoff HJ, Rempe DA. The good, the bad, and the cell type-specific roles of hypoxia inducible factor-1 alpha in neurons and astrocytes. J Neurosci. 2008;28(8):1988–1993. doi: 10.1523/JNEUROSCI.5323-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhuang Z, Yang C, Lorenzo F, Merino M, Fojo T, Kebebew E, Popovic V, Stratakis CA, Prchal JT, Pacak K. Somatic HIF2A Gain-of-Function Mutations in Paraganglioma with Polycythemia. N Engl J Med. 2012;367(10):922–930. doi: 10.1056/NEJMoa1205119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Damert A, Machein M, Breier G, Fujita MQ, Hanahan D, Risau W, Plate KH. Up-regulation of vascular endothelial growth factor expression in a rat glioma is conferred by two distinct hypoxia-driven mechanisms. Cancer Res. 1997;57(17):3860–3864. [PubMed] [Google Scholar]
- 111.Heddleston JM, Li Z, McLendon RE, Hjelmeland AB, Rich JN. The hypoxic microenvironment maintains glioblastoma stem cells and promotes reprogramming towards a cancer stem cell phenotype. Cell Cycle. 2009;8(20):3274–3284. doi: 10.4161/cc.8.20.9701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Soeda A, Park M, Lee D, Mintz A, Androutsellis-Theotokis A, McKay RD, Engh J, Iwama T, Kunisada T, Kassam AB, Pollack IF, Park DM. Hypoxia promotes expansion of the CD133-positive glioma stem cells through activation of HIF-1alpha. Oncog. 2009;28(45):3949–3959. doi: 10.1038/onc.2009.252. [DOI] [PubMed] [Google Scholar]
- 113.Li Z, Bao S, Wu Q, Wang H, Eyler C, Sathornsumetee S, Shi Q, Cao Y, Lathia J, McLendon RE, Hjelmeland AB, Rich JN. Hypoxia-inducible factors regulate tumorigenic capacity of glioma stem cells. Cancer Cell. 2009;15(6):501–513. doi: 10.1016/j.ccr.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lu H, Forbes RA, Verma A. Hypoxia-inducible factor 1 activation by aerobic glycolysis implicates the Warburg effect in carcinogenesis. J Biol Chem. 2002;277(26):23111–23115. doi: 10.1074/jbc.M202487200. [DOI] [PubMed] [Google Scholar]
- 115.Dang L, Jin S, Su SM. IDH mutations in glioma and acute myeloid leukemia. Trends Mol Med. 2010;16(9):387–397. doi: 10.1016/j.molmed.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 116.Zhao S, Lin Y, Xu W, Jiang W, Zha Z, Wang P, Yu W, Li Z, Gong L, Peng Y, Ding J, Lei Q, Guan KL, Xiong Y. Glioma-derived mutations in IDH1 dominantly inhibit IDH1 catalytic activity and induce HIF-1alpha. Sci. 2009;324(5924):261–265. doi: 10.1126/science.1170944. [DOI] [PMC free article] [PubMed] [Google Scholar]