Abstract
Severe plasma ADAMTS13 deficiency results in the clinical disorder thrombotic thrombocytopenic purpura. However, other potential pathophysiological roles of ADAMTS13 in endothelial cell biology remain unexplored. The goals of this study were to understand the angiogenic pathways ADAMTS13 activates and to identify the important structural components of ADAMTS13 that stimulate angiogenesis. Incubation of human umbilical vein endothelial cells (HUVEC) with 150 ng/mL (1 nM) of recombinant human ADAMTS13 induced VEGF expression by 53 % and increased VEGF mRNA by over sixfold, both within 10 min; the measured VEGF levels steadily decreased over 2 h, as shown by Western blot and ELISA. Phosphorylation of VEGFR2 was significantly enhanced in HUVEC after incubation with ADAMTS13 (1 nM). Structure–function analysis showed that an ADAMTS13 variant containing thrombospondin type 1 (TSP1) 2–8 repeats (TSP1 2–8), TSP1 2–8 plus CUB domains (TSP1 2–8 plus CUB), or TSP1 5–8 repeats plus CUB domains (TSP1 5–8 plus CUB) increased HUVEC proliferation by 41–54 % as compared to the EBM-2 controls. Chemotaxis assays further demonstrated that the TSP1 domains of ADAMTS13 increased HUVEC migration by 2.65-fold. Incubation of HUVEC with both ADAMTS13 variants containing TSP1 repeats and anti-VEGF IgG abrogated the enhanced effect of ADAMTS13 on proliferation, migration, and VEGFR2 phosphorylation. In conclusion, ADAMTS13-induced endothelial cell angiogenesis occurs via the upregulation of VEGF and phosphorylation of VEGFR2. This angiogenic activity depends on the C-terminal TSP1 repeats of ADAMTS13.
Keywords: ADAMTS13, VEGF, Angiogenesis, HUVEC, Proliferation, Migration
Introduction
A disintegrin and metalloproteinase with thrombospondin type 1 motifs, 13(ADAMTS13) cleaves ultra-large (UL) von Willebrand factor (vWF) multimers at the Tyr1605 and Met1606 bond under shear stress [1, 2]. During vascular injury, vWF promotes platelet adhesion to the vascular wall under high shear rates [3, 4], and ADAMTS13 regulates platelet aggregation and microthrombi formation. Patients with severe deficiency of plasma ADAMTS13 may develop a clinical disorder, thrombotic thrombocytopenic purpura (TTP), characterized by the spontaneous formation of platelet thrombi in microvasculature [5].
Among the 19 members of the ADAMTS family, ADAMTS13 is unique as it contains a signal peptide, prodomain, metalloproteinase domain, disintegrin-like domain, first thrombospondin type 1 (TSP1) motif, cysteine-rich domain, and spacer domain. In addition, the distal C terminus contains additional 7 TSP1 repeats and two CUB (C1r/C1s, urchin endothelial growth factor-like protein, and bone morphogenetic protein domains). Productive cleavage of UL-VWF appears to require the binding of zinc and calcium ions at the metalloproteinase domain and the binding of C-terminal TSP1 repeats and the CUB domain to UL-VWF [2, 6]. However, recent studies have shown that C-terminal TSP1 2–8 and CUB may not be required for function in vitro and in vivo [7].
ADAMTS13 is primarily synthesized in hepatic stellate cells [8], but endothelial cells and megakaryoctes also secrete small amounts of active ADAMTS13 [9–11]. The functionality of ADAMTS13 produced in endothelial cells is unclear, however, it is possible that endothelial cells (EC) may be a secondary source of plasma ADAMTS13. Several truncated ADAMTS13 variants that occur as the result of alternative splicing are also identified in placenta [12], skeletal muscle [12], hepatic stellate cells [13], and even in human lung cancer cells [13].
While the majority of the ADAMTS family members (i.e. ADAMTS1 [14], ADAMTS2 [15], ADAMTS4 [16], ADAMTS5 [17], ADAMTS9 [18], and ADAMTS12 [19]) have been reported to be anti-angiogenic, ADAMTS1 [20] has been found to be both pro-angiogenic and anti-angiogenic. We have previously reported that ADAMTS13 may modulate angiogenesis [21]. Treatment of human umbilical vein endothelial cells (HUVECs) with recombinant ADAMTS13 (in the absence of VEGF) significantly promoted angiogenesis in tube formation, proliferation, and migration assays. Additionally, ADAMTS13 partially inhibited VEGF-induced angiogenesis. Herein, we extend our previous observations to investigate the mechanistic pathways and the structure–function aspects of ADAMTS13 to regulate angiogenesis.
Materials and methods
Reagents
Full-length ADAMTS13 (FL-ADAMTS13) was purchased from R&D Systems (Minneapolis, MN, USA) and used without any further purification. Truncated ADAMTS13 variants (tr-ADAMTS13) were expressed in HEK293 or CHO-s cells [7] and carefully purified prior to experimental use. Vascular endothelial growth factor-165 (VEGF165), GAPDH antibody, and normal rabbit IgG antibody were purchased from R&D Systems. Human VEGF165 antibody, total VEGFR2 antibody, and 0.25 % trypsin/EDTA were purchased from Life Technologies (Grand Island, NY, USA). Trypsin solution was diluted to 0.05 % in PBS for cell dissociation. Phospho-VEGF receptor-2 (phospho-Tyr1054/Tyr1059) antibody and Microcon centrifugal filters were purchased from Millipore (Billerica, MA, USA). EBM-2 basal medium was supplied by Lonza (Walkersville, MD, USA). Medium 199, HEPES, and VEGF ELISA kit were obtained from Sigma-Aldrich (St. Louis, MO, USA). Fetal bovine serum (FBS) was purchased from Atlanta Biologicals (Lawrenceville, GA, USA). Phospho-VEGFR2 ELISA kit, BrdU cell proliferation assay kit, and LumiGlo chemiluminescent substrate were supplied by Cell Signaling Technology (Danvers, MA, USA).
Tissue culture
Human umbilical vein endothelial cells were isolated from human umbilical veins by collagenase digestion, seeded on gelatin-coated plates, and cultured in Medium 199 supplemented with 20 % FBS, penicillin, streptomycin, and amphotericin (Fungizone™). Second or third-passage HUVECs were used in all angiogenesis assays.
Purification and concentration of ADAMTS13
Full-length ADAMTS13 (affinity-purified) was purchased commercially and used without any further purification. All five tr-ADAMTS13 proteases were purified to homogeneity by Q-fast ion exchange and Ni-chelating affinity chromatography. To determine the optimal concentration of ADAMTS13 and time of experiment, we performed dose–response and time-course studies in each assay prior to our studies. The concentrations reported here represent the optimal dose of ADAMTS13 that induced the most significant response in each assay.
Expression of VEGF (time-course study)
Human umbilical vein endothelial cells were cultured in 10 cm petri dishes until 70–80 % confluence. The cells were starved in serum-free EBM-2 media supplemented with 0.1 % BSA for 24 h. 1 nM full-length ADAMTS13 was added to the cells. HUVEC treated with serum-free EBM-2 and EBM-2 supplemented with 260 pM VEGF165 were used as controls. The cells were incubated for 10, 30, 60, and 120 min, and afterwards, they were washed twice with PBS equilibrated at 37 °C. To prepare the cell lysate, 250 µL of Mammalian Protein Extraction Reagent (Thermo Scientific, Rockford, IL, USA) was added to the petri dishes. The cells were scraped off the dish and transferred to a micro-centrifuge tube. The micro-centrifuge tube was placed on ice for 5 min and vortexed for 10 s every minute. Afterwards, the cells were centrifuged for 5 min at 3,000 rpm and the supernatants were collected and stored at −70 °C until analysis.
Western blot analysis
Cell lysates (40 µL) were applied to Bis–Tris polyacrylamide gels and transferred to a PVDF membrane. The membranes were incubated with VEGF anti-human polyclonal antibody and visualized with horseradish peroxidase-conjugated secondary antibody against rabbit IgG using the LumiGlo chemiluminescent substrate.
ELISA
Cell lysates collected from procedures as described above were used. The expression of VEGF over a time-course of 2 h was studied using a VEGF ELISA kit.
Real time-PCR assay
Cells were cultured as mentioned above and serum starved in serum-free EMB-2 supplemented with 0.1 % BSA for 24 h. After 24 h, 1 nM ADAMTS13 or 260 pM VEGF165 (positive control) was added to the cells and returned to the 37 °C incubator for 10 min. Afterwards, cells were washed with PBS (equilibrated to 37 °C) three times before cell lysis and RNA extraction. Total RNA was extracted by utilizing the RNA easy mini prep kit (Qiagen, Valencia, CA, USA). 2 µg of total RNA was reverse transcribed using the SuperScript®III First-Strand cDNA synthesis kit (Invitrogen). Primers used for total VEGF were forward 5′-ATCTTCAAGCCATCCTGTGTGC and reverse 5′-GCTCACCGCCTCGGCTTGT. Primers for the detection of the reference gene GAPDH were forward 5′-TGCACCACCAACTGCTTAG and reverse 5′-TAGAGGCAGGGATGATGTTC. Primer efficiencies were determined by cDNA serial dilutions to be between 1.95 and 2.05. RT-PCR was carried out on a Bio-Rad C1000 Touch™ Thermal Cycler. Relative mRNA expression was carried out utilizing the Pfaffl method as previously reported [22].
Phosphorylation of VEGFR2
Human umbilical vein endothelial cells were cultured in 10 cm petri dishes until ~90 % confluence. 12 h prior to the experiment, serum-free EBM-2 was added to the cells. The medium was aspirated and EBM-2 with ADAMTS13 or its variants (1 nM) was added, and cells were incubated in the CO2 incubator for 10 min. The cells were washed once with ice-cold PBS with 1 mM sodium orthovanadate to inhibit all tyrosine phosphatases. Cell lysis buffer (Cell Signaling) supplemented with 1 mM AEBSF was then added and the cells were incubated for another 3 min on ice. The cells were then dissociated from the plate using a scraper, and the lysate/cell debris was collected and sonicated over ice for 3 min. After 10 min of centrifugation, all cell lysates were transferred to Eppendorf tubes and stored in a –70 °C freezer until assayed.
Western blot analysis
Cell lysates were concentrated using Microcon centrifugal filters. 40 µL cell lysates was loaded on a Bis–Tris polyacrylamide gel and transferred to a PVDF membrane. The assessment of VEGR2 phosphorylation was performed using a polyclonal phospho-VEGF receptor-2 (phospho-Tyr1054/Tyr1059) antibody and visualized with horseradish peroxidase-conjugated secondary antibody against rabbit IgG using the Immun-Star WesternC substrate (Bio-Rad, Hercules, CA).
ELISA
The phosphorylation of VEGFR2 induced by FL-ADAMTS13 and tr-ADAMTS13 was measured using an ELISA kit. The procedures were performed following the manufacturer’s protocol.
Cell proliferation assays
Human umbilical vein endothelial cells were cultured in 10 cm petri dishes until ~70 % confluence. The cells were dissociated from the plate using 0.05 % trypsin and re-suspended in EBM-2 supplemented with 10 mM HEPES and pen/Strep/Fungizone™. Using a 96-well plate, 7,000 cells were seeded in each well and placed inside a CO2 incubator overnight for cell synchronization. After washing the cells three times with PBS pre-equilibrated at 37 °C, EBM2 with 0.1 % BSA and supplemented with full-length or truncated ADAMTS13 (5.2 nM) was added to the cells. After 48 h of incubation, the cells were washed three times with PBS, fixed with 3.7 % paraformaldehyde, and stained with 0.2 % crystal violet. HUVECs were imaged using an Olympus IX70 microscope coupled with an Olympus DP71 charge-coupled device camera (20× magnification) with DP Manager software (v. 3.1.1.208). The number of cells was counted visually and electronically using ImageJ v. 1.43u software (National Institutes of Health, USA).
Alternatively, HUVECs were cultured using the same method as described above in the manual counting assay. After 48 h of incubation, BrdU solution was added and the cells were incubated for an additional 24 h. Following the manufacturer’s suggested protocol, the rate of proliferation of HUVECs was measured by monitoring the optical density at 450 nm using Softmax Pro v. 5.2 software (Molecular Devices; Sunnyvale, CA, USA).
Cell migration assay
Second or third-passage HUVECs were cultured in M-199 basal medium supplemented with 20 % FBS and pen/Strep/Fungizone™ until ~70 % confluence. The medium was replaced by serum-free EBM-2 media 12 h prior to assay. The cells were dissociated from the culture dish using 0.05 % trypsin–EDTA and re-suspended in EBM-2 supplemented with 0.1 % BSA at a density of 160,000 cells/mL.
Using a modified chemotaxis chamber (Neuro Probes, Inc.; Gaithersburg, MD, USA), chemoattractants (controls and 65.1 pM ADAMTS13) were carefully loaded into the lower chambers. A polycarbonate membrane with 8 µm pore size that had been coated with gelatin was then inserted between the lower and upper chambers. 50 µL of HUVEC (8,000 cells) was then loaded into the upper chamber and incubated in the CO2 incubator for 3 h.
Following the manufacturer’s protocol, the membrane was carefully removed. After washing three times with PBS, the cells that had migrated to the bottom layer of the membrane were stained with 0.2 % crystal violet for 25 min. The membrane was then transferred to a Lovins micro-slide field finder (Electron Microscopy Sciences; Hatfield, PA, USA) and the number of cells on the membrane was visually counted using a Nikon bright-field microscope (20× magnification).
Inhibition of VEGFR2 phosphorylation, proliferation, and migration by anti-VEGF antibody
In the VEGFR2 phosphorylation, proliferation, and migration assays, HUVECs were co-incubated with ADAMTS13 (full length or truncated) and a polyclonal antibody against human VEGF165 (R&D Systems) or a control IgG (2 µg/mL, 13.3 nM) under the same conditions as described above.
Results
Effect of ADAMTS13 on expression of VEGF
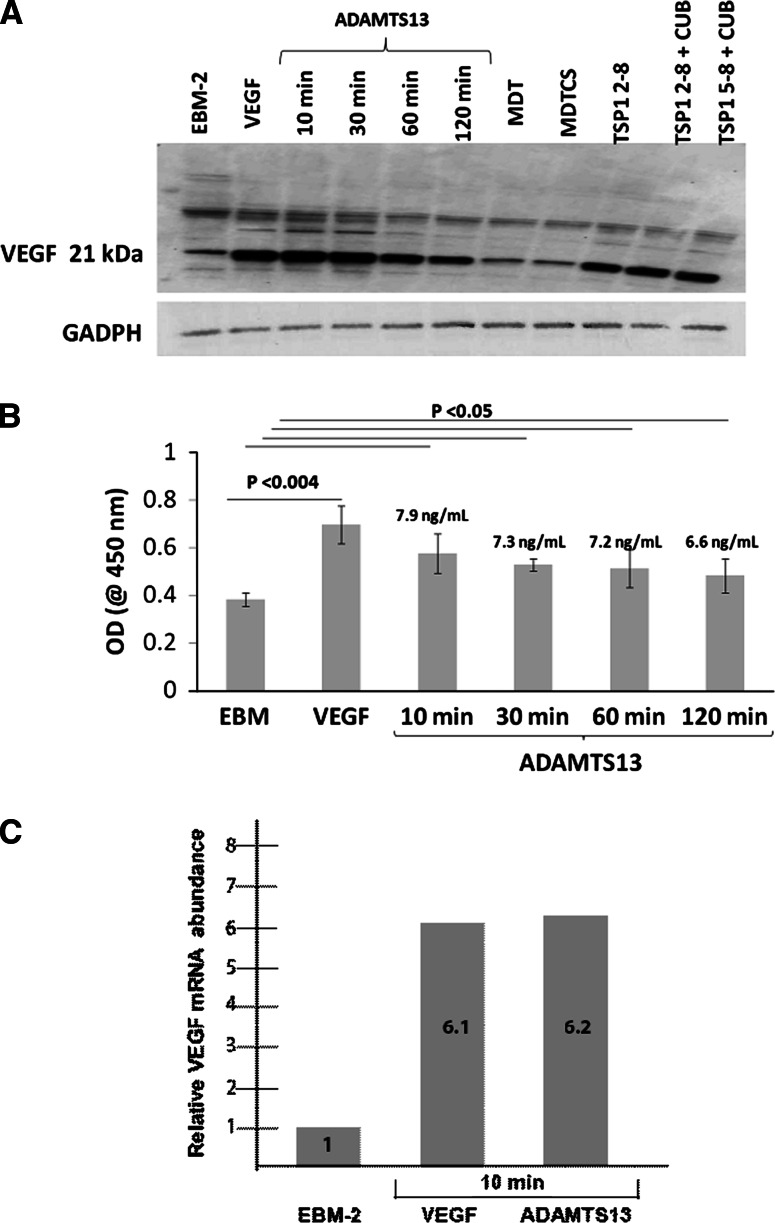
Expression of VEGF in HUVEC lysates was upregulated when HUVEC were incubated with 1 nM recombinant full-length ADAMTS13 (Fig. 1). VEGF levels were increased in HUVEC lysates 10 min after incubation of HUVEC with FL-ADAMTS13, and the level of VEGF gradually decreased over a time-course of 2 h, as shown by Western blot and ELISAs (Fig. 1a). After 10 and 120 min of incubation, VEGF levels in the cell lysate were reduced from 7.9 to 6.6 ng/mL (Fig. 1b). Among the five tr-ADAMTS13 tested, ADAMTS13 TSP1 2–8, ADAMTS13 TSP1 2–8 plus CUB, and ADAMTS13 5–8 plus CUB induced VEGF expression similar to FL-ADAMTS13 over a time course of 10 min. A RT-PCR VEGF mRNA assay confirmed the Western blot and ELISA results. 1 nM recombinant full-length ADAMTS13 increased VEGF mRNA 6.2-fold over the EBM-2 serum-starved cells after 10 min of incubation (Fig. 1c).
Fig. 1.
Time-dependent expression of VEGF when HUVECs were incubated with 1.0 nM ADAMTS13. a Immunoblot of VEGF and GAPDH (internal control) under reduced condition. b Time-course studies using an ELISA showed that ADAMTS13-induced VEGF production appeared at 10 min, and the expressed level gradually decreased over a course of 2 h. The concentrations of VEGF measured were quantified by comparing the measured OD value with the VEGF standard calibration plot. Data shown are mean ± SD of three experiments. c RT-PCR experiment demonstrating that ADAMTS13 induces VEGF mRNA expression within a 10 min time period. Total VEGF mRNA was compared to the reference gene GAPDH and the relative amounts are given. Data shown are the average of three independent experiments
ADAMTS13 enhances VEGFR2 phosphorylation
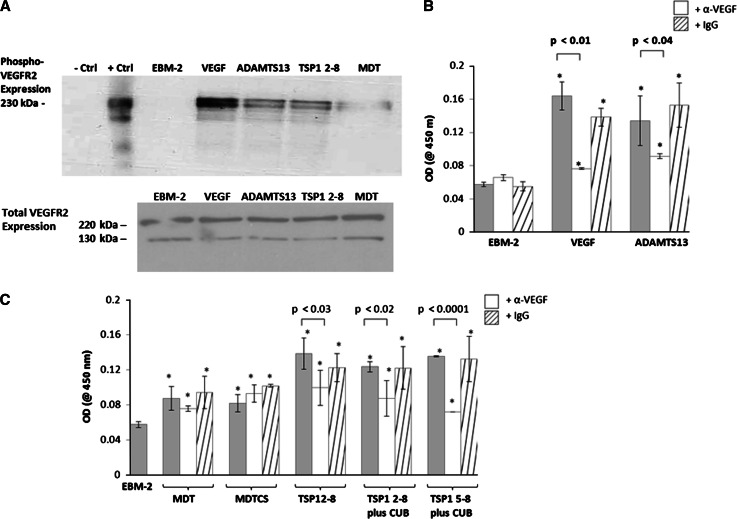
To understand the mechanism of ADAMTS13-mediated pro-angiogenic effect, we measured the phosphorylation of VEGFR2 in HUVEC in the absence or in the presence of ADAMTS13. Phosphorylation of VEGFR2 was detected in HUVEC lysates after incubation with ADAMTS13 for 10 min as shown by Western blot (Fig. 2a) and ELISA (Fig. 2b–c). In the presence of VEGF165 and ADAMTS13, VEGF increased VEGFR2 phosphorylation 3.8-fold, whereas ADAMTS13-induced phosphorylation increased by 1.9-fold when compared to the EBM-2 control (measured by ELISA) (Fig. 2b). Co-incubation of either VEGF or ADAMTS13 with anti-VEGF IgG abrogated the phosphorylation effects of VEGF or ADAMTS13 (Fig. 2b).
Fig. 2.
a HUVECs were incubated with VEGF (260 pM) or ADAMTS13 (1 nM) for 10 min, and the cell lysates were analyzed by Western blot. Top immunoblot shows the phosphorylation of VEGFR2. ADAMTS13 (full-length or TSP1 2–8 variant) upregulates VEGFR2 phosphorylation level, as compared to the EBM-2 control. ADAMTS13 MDT variant induced a weaker VEGFR2 phosphorylation response than the TSP1 2–8 variant. The same lysates were re-used on a different Bis–Tris polyacrylamide gel in the control experiment. As shown in the bottom immunoblot, the expression of total VEGFR2 in each group was similar. EBM-2 (negative control), VEGF (positive control), and ADAMTS13 (full-length or truncated proteins) induced VEGFR2 expression at 220 kDa (mature form) and 130 kDa. b ADAMTS13-induced VEGFR2 phosphorylation was inhibited by co-incubating HUVEC with FL-ADAMTS13 and anti-VEGF IgG antibody (13.3 nM). c The effects of ADAMTS13 variants (1 nM) on VEGFR2 phosphorylation. While all five ADAMTS13 variants can phosphorylate VEGFR2, the TSP1 repeat domain variants induced more VEGFR2 phosphorylation. These activities were reversed when cells were co-incubated with an anti-VEGF IgG antibody (13.3 nM). Asterisk indicates values that were statistically different than the EBM-2 control. Data shown are mean ± SD of three experiments
To further assess the structural components of ADAMTS13 required for angiogenic effect, we measured VEGFR2 phosphorylation after incubation with variants of ADAMTS13 including MDT, MDTCS, TSP1 2–8, TSP1 2–8 plus CUB, and TSP1 5–8 plus CUB variants. All five variants were shown to enhance VEGFR2 phosphorylation. The constructs containing TSP1 repeats were among the highest inducers of phosphorylation, which were comparable to full-length ADAMTS13 (Fig. 2c). Co-incubation of ADAMTS13 constructs containing TSP1 variants with anti-VEGF IgG resulted in significantly reduced VEGFR2 phosphorylation (28–47 % reduction). These results indicate the possible involvement of VEGF-VEGFR2 pathway in ADAMTS13-induced angiogenesis. No reduction in VEGFR2 phosphorylation was observed when only the metalloproteinase domain was co-incubated with ADAMTS13 and anti-VEGF IgG. In all experiments, a control IgG did not affect the phosphorylation levels. Each experiment was repeated three times with the mean ± SD shown.
Anti-VEGF IgG abrogates ADAMTS13-induced HUVEC proliferation
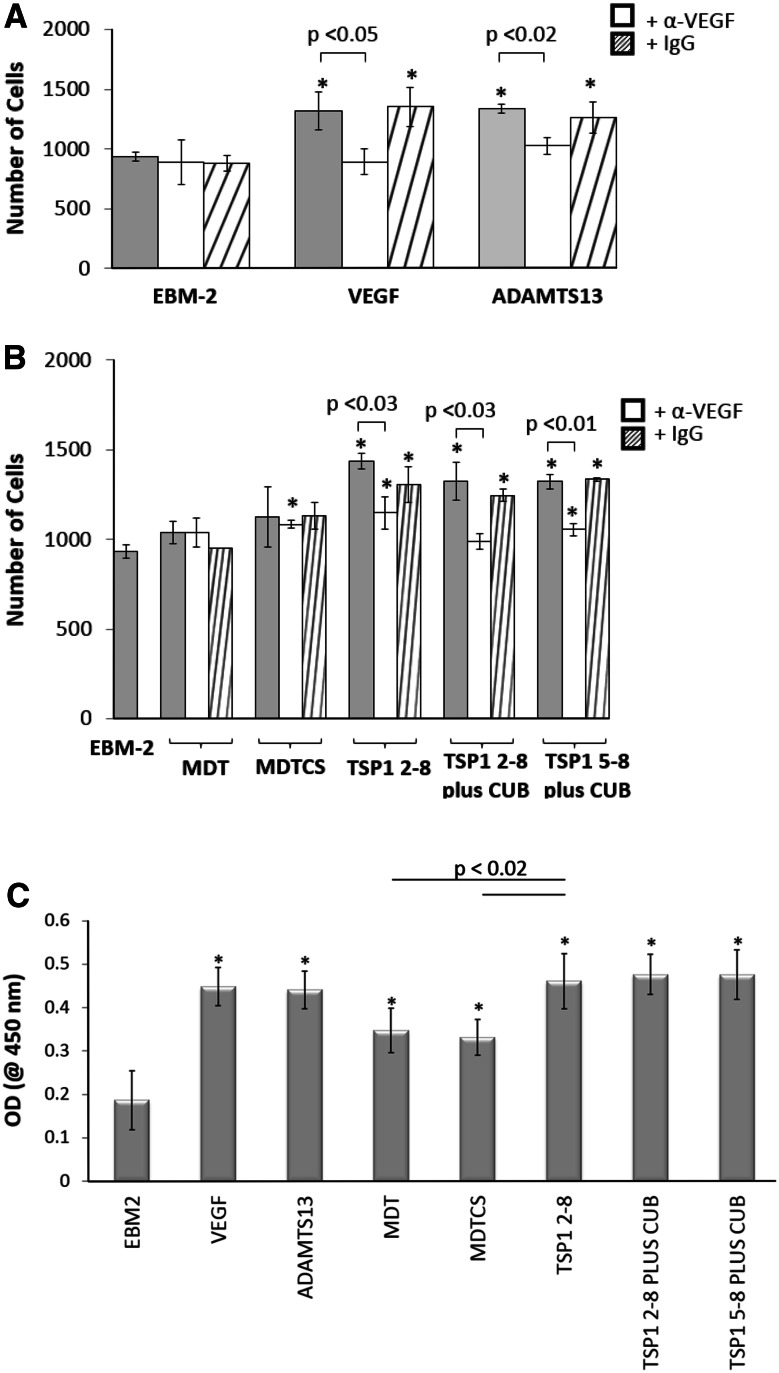
We analyzed the mitogenic activity of full-length ADAMTS13 and five truncated ADAMTS13 variants (MDT, MDTCS, TSP1 2–8, TSP1 2–8 plus CUB, and TSP1 5–8 plus CUB). In the manual counting and BrdU proliferation assays, three truncated ADAMTS13 variants containing C-terminal TSP1 repeats (TSP1 2–8, TSP1 2–8 plus CUB, and TSP1 5–8 plus CUB) induced HUVEC proliferation significantly higher than the EBM-2 control. The efficacy of these variants was comparable to that of full-length ADAMTS13 (Fig. 3a–c). Interestingly, the metalloprotease domain could also induce HUVEC proliferation, but was less potent than the constructs containing TSP1 repeats.
Fig. 3.
a VEGF165 (5.2 nM) and FL-ADAMTS13 (5.2 nM) induced HUVEC proliferation in a manual counting assay. Cells were fixed and stained with crystal violet and counted using Image J software. Co-incubation of FL-ADAMTS13 (5.2 nM) with anti-VEGF IgG antibody (13.3 nM) abrogates ADAMTS13-induced proliferation. Control IgG did not affect HUVEC proliferation. b Five ADAMTS13 variants (MDT, MDTCS, TSP1 2–8, TSP1 2–8 plus CUB, and TSP1 5–8 plus CUB) (5.2 nM) in EBM-2 (supplemented with 0.1 % BSA) were added to HUVEC cultures. ADAMTS13 variants containing the TSP1 repeat domain induced HUVEC proliferation statistically greater than the EBM-2 control in a manual cell counting assay, and the observed proliferation was reversed in the presence of anti-VEGF IgG antibody (13.3 nM). c Using a BrdU cell proliferation assay, we observed similar HUVEC proliferation as compared to the manual counting assay when HUVEC were incubated with 5.2 nM ADAMTS13. Asterisk indicates that the values were significantly different than the EBM-2 control (p < 0.05). Data shown are mean ± SD of three experiments
Incubation of HUVECs with VEGF or FL-ADAMTS13 and anti-VEGF antibody resulted in inhibition of cell proliferation induced by VEGF or FL-ADAMTS13, similar to the effect of the EBM-2 control (Fig. 3a). Replacement of anti-VEGF antibody with a control IgG antibody had no inhibitory effect on the proliferation induced by VEGF or ADAMTS13. Among the five ADAMTS13 variants tested, co-incubation of MDT and MDTCS with the anti-VEGF antibody did not change the proliferation rate. However, when the constructs containing the C-terminal TSP1 repeat variants were used, anti-VEGF antibody abrogated the proliferating effect induced (Fig. 3b), suggesting that the C-terminal TSP1 repeats induce HUVEC proliferation via the VEGF/VEGFR pathway.
Anti-VEGF IgG abrogates ADAMTS13-induced HUVEC migration
Using a modified Boyden chamber assay, we measured the effect of ADAMTS13 and its variants on HUVEC migration. We observed that all five truncated ADAMTS13 variants increased migration of HUVEC through the membrane. TSP1 2–8 repeats showed the strongest chemotactic effect and increased HUVEC migration by 2.4-fold, as compared with the EBM-2 control (Fig. 4). The metalloprotease domain alone exhibited a weaker chemotactic effect (1.8-fold).
Fig. 4.
a Migration of HUVEC towards FL-ADAMTS13 (65.1 pM) in a modified Boyden chamber. Co-incubation of HUVEC with ADAMTS13 and an anti-VEGF IgG antibody abrogated ADAMTS13-induced migration. b 5 ADAMTS13 variants were tested in the migration assay. The data indicated that all five tr-ADAMTS13 variants induced HUVEC migration, with the variants containing the TSP1 repeat domain exhibiting a higher chemotactic signal. Anti-VEGF IgG (13.3 nM) can reverse ADAMTS13-induced migration in the TSP1 2–8, TSP1 2–8 plus CUB, and TSP1 5–8 plus CUB groups. Asterisk indicates values that were statistically different than the EBM-2 control. Data shown are mean ± SD of three experiments
The number of HUVECs migrating across a gelatinized polycarbonate membrane towards VEGF, ADAMTS13, and its variants in the presence of anti-VEGF antibody were determined. Anti-VEGF IgG effectively reduced both VEGF and ADAMTS13-induced HUVEC migration by 60.7 and 54.4 %, respectively (Fig. 4a). The same inhibitory pattern was also observed when cells were incubated with TSP1 2–8, TSP1 2–8 plus CUB, and TSP1 5–8 plus CUB (Fig. 4b). The reduction in HUVEC migration ranged from 38.9 to 56.6 %. However, negligible effects on cell migration were observed when anti-VEGF IgG was added to the culture in the presence of MDT and MDTCS. Control IgG had no effect on FL-ADAMTS13 or variant-induced HUVEC migration.
Discussion
ADAMTS13 is known as the vWF-cleaving protease. We investigated the potential roles of ADAMTS13 that may be unrelated to vWF cleavage and pathogenesis of TTP. Previously, we reported that HUVEC treated with exogenous recombinant ADAMTS13 increases HUVEC tube formation, proliferation, and migration [21]. Our current study focuses on the identification of (a) ADAMTS13-induced angiogenic pathway, and (b) the structural components of ADAMTS13 required for the induced angiogenesis in vitro.
Incubation of HUVEC with full-length ADAMTS13 significantly increased VEGF mRNA (6.2-fold) and VEGF protein levels (7.9 ng/mL) in 10 min. Previously, we reported that HUVEC can synthesize and release ADAMTS13 [9]. The measured ADAMTS13 concentration in subconfluent HUVEC lysate is <150 pg/mL (1.0 pM), which is 1,000 times lower than the ADAMTS13 concentration used in the VEGF expression experiment [9]. The combined data suggest that ADAMTS13 may be involved in the regulation of VEGF expression in HUVEC.
We also observed that ADAMTS13 induced phosphorylation of VEGFR2 in HUVEC. This is supported by the data that co-incubation of HUVEC with ADAMTS13 and anti-VEGF IgG abrogates ADAMTS13-induced VEGFR2 phosphorylation, proliferation, and migration. The changes of ADAMTS13 concentration after incubation with HUVEC were monitored (data not shown). In an in vitro assay, we compared the amount of ADAMTS13 added to the cell media and the amount recovered from the supernatant after the experiment. We did not observe any changes in the ADAMTS13 concentration during a time-course of 6 h, suggesting that ADAMTS13 is not substantially bound to the HUVEC cell surface. It is possible that ADAMTS13 interacts with an unidentified receptor on HUVEC to alter VEGFR2 phosphorylation and VEGF expression, and the binding can be displaced by other growth factors. One hypothetical mechanism of ADAMTS13 involvement in EC angiogenesis is the competition between ADAMTS13 and the angiogenesis inhibitor thrombospondin-1 [23, 24]. While thrombospondin-1 binds to thrombospondin-1 receptor CD47 and subsequently regulates VEGFR2 in a dose-dependent manner [25], perhaps ADAMTS13 functions as a competitor to inhibit the roles of endogenous thrombospondin-1.
The finding that ADAMTS13 increases VEGF levels in HUVEC was somewhat unexpected. In our previous report, ADAMTS13 was found to be both a pro-angiogenic and an anti-angiogenic factor. Also, ADAMTS13 bound VEGF and abrogated VEGF-induced angiogenesis [21]. Since ADAMTS13 mRNA and protein are detected in endothelial cells [9], central nervous system cells [26], etc., we hypothesize that ADAMTS13 may play a critical role in regulation of VEGF expression in cells: (a) endothelial cell-secreted ADAMTS13 may bind VEGFR2, resulting in VEGFR2 phosphorylation, thereby inducing expression of VEGF to induce angiogenesis; and (b) ADAMTS13 may function as an angiogenic inhibitor when VEGF is abundant or overexpressed as shown in our previous study [21]. It is important to note that when HUVECs were co-incubated with ADAMTS13 and an anti-VEGF IgG antibody, only partial inhibition of VEGFR2 phosphorylation, HUVEC proliferation and migration were observed (Figs. 2b, 3a, 4a). These results suggest that ADAMTS13 may be involved in additional angiogenic pathways. Nonetheless, the data obtained suggest that ADAMTS13 secreted from endothelial cells or from plasma may regulate VEGF expression. ADAMTS13, perhaps through a similar mechanism for ADAMTS1 and thrombospondin-1, modulates angiogenesis. Interestingly, thrombospondin-1, a potent anti-angiogenic factor, also demonstrates an opposite proangiogenic activity under different conditions [27]. It was reported that the heparin-binding portion (25 kDa) of thrombspondin-1 promotes angiogenesis by inducing matrix metalloproteinase MMP-9 expression in bovine aortic endothelial cells, whereas the other fragment (140 kDa) suppresses fibroblast growth factor-induced angiogenesis [27]. Interestingly, vWF, the only known substrate for ADAMTS13, has recently been shown to exhibit anti-angiogenic activities [28]. Increased vascularization was observed in vWF-deficient mice. However, no mechanistic understanding of how ADAMTS13 and vWF modulate angiogenesis is available.
We conclude that our results demonstrate that ADAMTS13 and three different ADAMTS13 variants that contain TSP1 repeats have significant effects on VEGFR2 phosphorylation, HUVEC proliferation and migration of HUVEC. These effects are abolished by the co-incubation with anti-VEGFR2 IgG, suggesting that ADAMTS13 and various fragments of ADAMTS13 containing TSP1 repeats promote angiogenesis through the VEGF–VEGFR2 signaling pathway.
Acknowledgments
We thank Mr. Jonathan Baza and Miss Courtney Hoyt for their help in culturing HUVEC. This study is partially supported by grants from American Heart Association-EIA 0940100 N, National Institutes of Health HL074124-Project 3 and National Institutes of Health HL115187001A1 to X.L.Z., and by the ARUP Laboratories Institute for Research and Development to G.R. The authors do not have any conflicts of interests to disclose.
References
- 1.Furlan M, Robles R, Lammle B. Partial purification and characterization of a protease from human plasma cleaving von Willebrand factor to fragments produced by in vivo proteolysis. Blood. 1996;87:4223–4234. [PubMed] [Google Scholar]
- 2.Tsai H-M. Physiologic cleavage of von Willebrand factor by a plasma protease is dependent on its conformation and requires calcium ion. Blood. 1996;87:4235–4244. [PubMed] [Google Scholar]
- 3.Green D, Muller H. Platelet-binding of the von Willebrand factor. Thromb Haemost. 1978;39:689–694. [PubMed] [Google Scholar]
- 4.Senogles S, Nelsestuen G. Von Willebrand factor. A protein which binds at the cell surface interface between platelets. J Biol Chem. 1983;258:12327–12333. [PubMed] [Google Scholar]
- 5.Sadler J. Von Willebrand factor, ADAMTS13, and thrombotic thrombocytopenic purpura. Blood. 2008;112:11–18. doi: 10.1182/blood-2008-02-078170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tao Z, Want Y, Choi H, et al. Cleavage of ultralarge multimers of von Willebrand factor by C-terminus-truncated mutants of ADAMTS-13 under flow. Blood. 2005;106:141–143. doi: 10.1182/blood-2004-11-4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xiao J, Jin S, Xue J, et al. Essential domains of a disintegrin and metalloprotease with thrombospondin type 1 repeats-13 metalloprotease required for modulation of arterial thrombosis. Arterioscler Thromb Vasc Biol. 2011;31:2261–2269. doi: 10.1161/ATVBAHA.111.229609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou W, Inada M, Lee T, et al. ADAMTS13 is expressed in hepatic stellate cells. Lab Invest. 2005;85:780–788. doi: 10.1038/labinvest.3700275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kling S, Judd CA, Warner KB, Rodgers GM. Regulation of ADAMTS13 expression in proliferating human umbilical vein endothelial cells. Pathophysiol Haemost Thromb. 2008;36:233–240. doi: 10.1159/000252818. [DOI] [PubMed] [Google Scholar]
- 10.Tati R, Kristoffersson A, Ståhl A, et al. Phenotypic expression of ADAMTS13 in glomerular endothelial cells. PLoS ONE. 2011;6:e21587. doi: 10.1371/journal.pone.0021587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turner N, Nolasco L, Tao Z, et al. Human endothelial cells synthesize and release ADAMTS-13. J Thromb Haemost. 2006;4:1396–1404. doi: 10.1111/j.1538-7836.2006.01959.x. [DOI] [PubMed] [Google Scholar]
- 12.Zheng X, Chung D, Takayama T, et al. Structure of von Willebrand factor-cleaving protease (ADAMTS13), a metalloprotease involved in thrombotic thrombocytopenic purpura. J Biol Chem. 2001;276:41059–41063. doi: 10.1074/jbc.C100515200. [DOI] [PubMed] [Google Scholar]
- 13.Shomron N, Hamasaki-Katagirl N, Hunt R, et al. A splice variant of ADAMTS13 is expressed in human hepatic stellate cells and cancerous tissues. Thromb Haemost. 2010;104:531–535. doi: 10.1160/TH09-12-0860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luque A, Carpizo D, Iruela-Arispe M, et al. ADAMTS1/METH1 inhibits endothelial cell proliferation by direct binding and sequestration of VEGF165. J Biol Chem. 2003;278:23656–23665. doi: 10.1074/jbc.M212964200. [DOI] [PubMed] [Google Scholar]
- 15.Dubail J, Kesteloot F, Deroanne C, et al. ADAMTS-2 functions as anti-angiogenic and anti-tumoral molecule independently of its catalytic activity. Cell Mol Life Sci. 2010;67:4213–4232. doi: 10.1007/s00018-010-0431-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsu Y, Staton C, Cross N, et al. Anti-angiogenic properties of ADAMTS-4 in vitro. Int J Exp Pathol. 2012;93:70–77. doi: 10.1111/j.1365-2613.2011.00802.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumar S, Sharghi-Namini S, Rao N, et al. ADAMTS5 functions as an anti-angiogenic and anti-tumorigenic protein independent of its proteoglycanase activity. Am J Pathol. 2012;181:1056–1068. doi: 10.1016/j.ajpath.2012.05.022. [DOI] [PubMed] [Google Scholar]
- 18.Koo B, Coe D, Dixon L, et al. ADAMTS9 is a cell-autonomously acting, anti-angiogenic metalloprotease expressed by microvascular endothelial cells. Am J Pathol. 2010;176:1494–1504. doi: 10.2353/ajpath.2010.090655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.El Hour M, Moncada-Pazos A, Blacher S, et al. Higher sensitivity of Adamts12-deficient mice to tumor growth and angiogenesis. Oncogene. 2010;29:3025–3032. doi: 10.1038/onc.2010.49. [DOI] [PubMed] [Google Scholar]
- 20.Liu Y, Xu Y, Yu Q. Full-length ADAMTS-1 and the ADAMTS-1 fragments display pro- and antimetastatic activity, respectively. Oncogene. 2006;25:2452–2467. doi: 10.1038/sj.onc.1209287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee M, Rodansky E, Smith J, et al. ADAMTS13 promotes angiogenesis and modulates VEGF-induced angiogenesis. Microvasc Res. 2012;84:109–115. doi: 10.1016/j.mvr.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 22.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:E45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bonnefoy A, Daenens K, Feys HB, et al. Thrombospondin-1 controls vascular platelet recruitment and thombus adherence in mice by protecting (sub)endothelial VWF from cleavage by ADAMTS13. Blood. 2006;107:955–964. doi: 10.1182/blood-2004-12-4856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang A, Liu F, Dong N, et al. Thrombospondin-1 and ADAMTS13 competitively bind to VWF A2 and A3 domains in vitro. Thromb Res. 2010;126:e260–e265. doi: 10.1016/j.thromres.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 25.Kaur S, Martin-Manso G, Pendrak ML, et al. Thrombospondin-1 inhibits VEGF receptor-2 signaling by disrupting its association with CD47. J Biol Chem. 2010;285:38923–38932. doi: 10.1074/jbc.M110.172304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tauch R, Imagama S, Ohgomori T, et al. ADAMTS-13 is produced by glial cells and upregulated after spinal cord injury. Neurosci Lett. 2012;517:1–6. doi: 10.1016/j.neulet.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 27.Taraboletti G, Morbidelli L, Donnini S, et al. The heparin binding 25 kDa fragment of thrombospondin-1 promotes angiogenesis and modulates gelatinase and TIMP-2 production in endothelial cells. FASEB J. 2000;14:1674–1676. doi: 10.1096/fj.99-0931fje. [DOI] [PubMed] [Google Scholar]
- 28.Starke R, Ferraro F, Paschalaki KE, et al. Endothelial von Willebrand factor regulates angiogenesis. Blood. 2011;117:1071–1080. doi: 10.1182/blood-2010-01-264507. [DOI] [PMC free article] [PubMed] [Google Scholar]