Abstract
Several different cell types constitute the intestinal wall and interact in different manners to maintain tissue homeostasis. Elegant reports have explored these physiological cellular interactions revealing that glial cells and neurons not only modulate peristalsis and mechanical stimulus in the intestines but also control epithelial proliferation and sub-epithelial angiogenesis. Although colon carcinoma arises from epithelial cells, different sub-epithelial cell phenotypes are known to support the manifestation and development of tumors from their early steps on. Therefore, new perspectives in cancer research have been proposed, in which neurons and glial cells not only lead to higher cancer cell proliferation at the tumor invasion front but also further enhance angiogenesis and neurogenesis in tumors. Transformation of physiological neural activity into a pro-cancer event is thus discussed for colon carcinogenesis herein.
Keywords: Colon cancer, Enteric neurons, Glial cells, Endothelial cells, Stem cells
Introduction
Colon cancer is one of the most common malignancies worldwide [1–3]. Over the past decades, it has been suggested that colon tumors arise from mutations in epithelial progenitor cells [2, 4]. This process has been named either carcinogenesis or tumorigenesis, in which genetic mutations in epithelial cells are the starting event towards malignancy [1, 4–7]. Detailed analysis revealed several steps required for such malignant transformation of epithelial cells, representing the best model to investigate colon carcinogenesis [8]. However, homeostasis of epithelial stem cell niches depends on complex interactions with non-neural and neural cell phenotypes at the colonic microenvironment [9, 10]. Indeed, understanding the role of tumor microenvironment has become an active and exciting area in cancer research [7].
Here, we revisit basic functions of the enteric nervous system (ENS) in colonic physiological and cancer-related conditions. This leads to the discussion of whether malignant changes in enteric neurons and microvessels might support the development of malignant mutations in epithelia giving rise to colon tumors and cancer.
Colon carcinogenesis
The adenoma–adenocarcinoma sequence model
It is well known that sequential somatic mutations are crucial events for epithelial cancer development. Fearon and Vogelstein [1] proposed a unifying hypothesis linking the adenoma–carcinoma sequence with the initiation–promotion model of chemical carcinogenesis. It is now accepted that mutations in one or two gatekeeper genes would disrupt the regular cell turnover and homeostasis within the epithelial stem cell niche, as hypothesized as initiation [11]. Lgr5-EGFP−IRES−creERT2 /Apc flox/flox mice, engineered by Clever’s research group, carry a specific stem cell knocking reporter for tamoxifen-inducible loss of the adenomatous polyposis coli (APC) gene sequence. Deleting APC, they have shown that β-catenin accumulates in the nuclei of epithelial stem cells leading to their transformation within days [2]. We have shown that hyperplastic polyps and adenomas develop through a proliferation-related mechanism at the stem cell compartment [4, 5]. Furthermore, the p53 tumor suppressor gene can be inactivated through mutations in its sequence, named TP53 [1, 7]. In a murine model for specific p53 deletion in intestinal epithelial cells, this tumor suppressor was found to act as a double-edged sword leading to survival and control of DNA damage during cancer initiation steps, whereas its loss enabled tumor progression through the induction of a reactive microenvironment and epithelial–mesenchymal transition [7]. These events provide tumor initiating cells with growth advantages for the development of lesions, which is enhanced through additional genetic hits giving rise to a variety of malignant subclones among daughter cells [1, 11]. This means that promotion is headed by hyperproliferation which allows the accumulation and fixation of mutations in epithelial cells [1, 2, 11].
Although boundaries between promotion and progression still remain unclear, it is accepted that the selective manifestation of genetic and epigenetic mutations leads to higher proliferation of mutant clones through changes on molecular signaling-driven cell cycle regulatory proteins and gatekeeper genes (i.e.: p21, p27, p53, Rb, and cyclins). This in turn reduces apoptosis, as well as cell–cell contact and signaling [1, 2, 4, 7, 11]. Furthermore, understanding progression becomes even more puzzling considering that growth factors, pro-inflammatory chemokines, adipokines, and changes of molecular signaling within the microenvironment also promote tumor growth [1, 11–14].
Microenvironment and colon tumors
It is currently accepted that inflammation induces mutations in crypt stem cell niches [14]. Inflammation is associated with oxidative stress, which in turn causes DNA damage. Damaged DNA activates the p53-dependent G1 checkpoint, and the damage may be repaired. If p53 function is exhausted by an overload of damage, non-repaired DNA damage may remain and manifest as gene mutations, as well as genomic instability within cryptal stem cell niches; all of these alterations would support the development of colon tumors [15]. Indeed, the p53 gene sequence was suggested to undergo mutations, such as allelic loss, as early as dysplastic lesions develop in the colon [16, 17]. It has been further reported that mutations in the p53 gene sequence in epithelial cells induce the formation of an inflammatory microenvironment through activation of nuclear factor kappa B (NF-κB) in the sub-epithelial cellular compartment during progression of colon carcinogenesis [7]. This concept is reinforced by the observation that inflammatory conditions enable sub-epithelial cells to transform epithelial progenitor cells towards malignancy [7, 13–15].
An elegant report has specifically shown that epithelial tumors develop after disruption of transforming growth factor-β (TGFβ) signaling within the sub-epithelia compartment in forestomachs [18]. TGFβ receptor 2 (TGFβr2) was knocked out from stromal fibroblasts (Tgfbr2 Fsp1KO mice), which in turn induced the loss of a DNA-fragment encoding the cyclin-dependent kinase inhibitors 2A and 2B at chromosome 4 within the epithelial compartment of the stomach, which resulted in the development of gastric epithelial tumors [18]. It had been previously reported that TGFβ signaling from fibroblasts showed tumor-promoting potential on epithelia [19]. These insights have been applied in colon cancer research bringing up striking evidence that sub-epithelial cells are a key element for the development of epithelial tumors [7, 13].
Epithelia, angiogenesis, and neurons
The colon epithelia
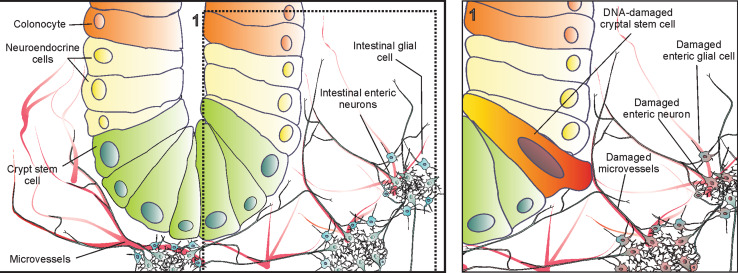
One of the most elegant strategies to understand the control of the intestinal epithelial stem cell niches has been achieved by engineering transgenic mice harboring multicolor-lineage tracing reporters in one transgene encoding a stem cell-specific promoter region. In brief, Cre recombinase stochastically excises and inverts the loxP sites allowing Rosa26 to drive the expression of multiple combinations of fluorescent proteins for cryptal stem cells and their progeny [20]. This strategy allowed the identification of intestinal stem cells replenishing their niche and randomly differentiating into sub-progenies which repopulate the crypts [20]. It thus supports the idea of self-organized and maintained stem cell niche and crypt structure [21]. Other reports suggested that an epithelia-like stem cell population at the cryptal base has close connections with the sub-epithelial layer and receives a large amount of signals from which proliferation and homeostasis are controlled (Fig. 1) [7, 12, 14]. Therefore, intestinal microenvironment and crypt stem cell niches might have profound interactions under physiological conditions that become even more complex during carcinogenic events [7, 18].
Fig. 1.
Structure of a colon crypt and its neurovascular microenvironment. Crypt stem cells and colonocytes are in close connection with microvessels, enteric neurons, and glial cells. Inset 1 shows a hypothetical condition where not only a crypt stem cell is damaged genetically, but rather all cells within crypt microenvironment, namely enteric neurons, glial cells, and microvessels (endothelial cells), undergo DNA damage
Intestinal angiogenesis
Angiogenesis means de novo microvessel development from endothelial progenitor cells which differentiate into endothelial cells. In this process, these cells first migrate towards the target spot and then proliferate. In sequence, these newly raised endothelial cell niches then align and give origin to tube-like structures that branch and perform anastomosis [22–26]. Intestinal angiogenesis is a complex matter which requires special attention in physiological or pathological conditions [22–24]. For example, it was reported that postnatal intestinal angiogenesis requires a bacteria-driven epithelial cell signaling. In this case, microbes colonizing the intestinal mucosa activate Paneth cells and assign to them the regulatory responsibility for intestinal angiogenesis [24]. Moreover, endothelial progenitor cells were characterized as mesenchymal-like stem cells [25]. Based on this, microvessel-isolated CD34-positive/CD31-negative cells were intramuscularly injected in CD1 Foxn1nu/nu mice, where they induced de novo vascularization of ischemic limbs [26].
Actually, angiogenesis is crucial for colon carcinogenesis [12, 23, 27, 28]. It was suggested that DNA damage would first occur among endothelial cells rather than in epithelial stem cell niches [22]. Moreover, colon tumors show a high microvessel density due to increased capillary nodules and anastomoses [23]. Endothelial tumor cells show an aberrant phenotype with wide and irregular intercellular gap junctions, allowing the invasion of tumor cells into the circulation towards metastasis [29]. In addition, endothelial cells were shown to release signals towards neural stem cells controlling neurogenesis through the activation of Notch and Hes 1 [30].
Intestinal enteric neurons
A branch of the autonomic nervous system named ENS is responsible for most of the gastrointestinal functions. It is known that ENS has about one hundred million neurons if the sub-epithelial (Meissner’s) and myenteric (Auerbach’s) plexuses are taken together, as well as four hundred million glial cells [31, 32]. The sub-epithelial plexus controls microvessels and epithelial cell functions, whereas myenteric neurons are responsible for peristalsis spreading throughout the inner circular and outer longitudinal layers of muscularis propria [33, 34]. This nervous system embedded within the gastrointestinal layers is known for its autonomic functions, and to operate independently from the brain and spinal cord instructions [31]. This independence comes from its composition of efferent, afferent, and inter-neurons enabling the generation, transmission, and integration of a broad amount of neural signals, either towards or backwards from the central nervous system [31–36].
Laranjeira et al. [36] have engineered Sox10 ::iCreERT2;R26ReYFP transgenic mice, which express fluorescent proteins after activation of a tamoxifen-inducible CreERT2 reporter under control of the SRY box-containing gene 10 (Sox10) regulatory sequence. In these mice, it was observed that Sox10-expressing stem cells present aging-associated loss of neurogenic capacity for differentiation into glial cell phenotype. The authors then engineered hGFAP ::CreERT2;R26ReYFP mice (express fluorescent proteins under control of the glial fibrillary acidic protein regulatory sequence), and found that enteric glial cells differentiated into neurons when exposed to chemical injury in vivo.
Gershon et al. have further suggested that enteric neural progenitors are clustered in cell niches outside the enteric ganglia and give rise to differentiated enteric-like neural stem cells after a slow migration-related proliferation into the neuronal ganglia [32]. A dependent mouse 5-HT4 receptor knockout model was applied for in vivo and in vitro mechanistic experiments demonstrating that these serotoninergic receptors are directly associated with enteric intestinal neurogenesis. Thus, 5-HT4 receptors were suggested to activate protein kinase A (PKA), which turns to phosphorylate cAMP response element-binding protein (pCREB) promoting the proliferation and survival of extra-ganglionic neural stem cells [32].
On the other hand, the activity of the autonomic nervous system in cancer has been investigated. In prostate carcinogenesis, β2- and β3-adrenergic receptors were found to control the development of early tumor phases, while autonomic neural fibers were infiltrated within tumors regulating their growth and metastasis [37]. We have previously shown that chemical denervation reduced the development of tumors in stomach and colon tissues [38, 39]. Moreover, it has been suggested that besides the cancer-related neoangiogenesis, tumor cells induce neoneurogenesis which results in both neurite outgrowth and axonogenesis within certain tumors, such as prostate cancer [37].
Building basic connections
Enteric nervous system controls cryptal stem cell niches
There is growing evidence for an important role of ENS in the modulation of cryptal stem cells. Enteric glial cells were shown to modulate proliferation of epithelial cells through TGFβ signaling in intestines [40]. Cholera toxin was reported to stimulate neurotransmitter release from the enteric neural system enhancing the upward epithelial cell migration within intestinal crypts [41]. Lundgren et al. [34] suggested that maintenance of the intestinal epithelial barrier requires enteric neural signaling, since male Sprague–Dawley rats perfused with 1.6 mM capsaicin showed increased incorporation of thymidine kinase and BrdU into DNA of cryptal cells, what was not observed in denervated intestines. In fact, knockout mice for capsaicin receptor presented a 1.2-fold decrease of nuclear thymidine and BrdU incorporation in cryptal bottom cells.
5-HT-related signaling from intestinal epithelial cells was reported to activate enteric afferent neurons [42]. The intestinal epithelial cell turnover was investigated in mechanistic experiments with SERT −/−, TPH1 −/−, and TPH2 −/− mice (knockout for serotonin transporter, and tryptophan hydroxylase 1 and 2, respectively) [43]. In these mice, epithelial proliferation was modulated by neural rather than epithelial serotonergic signaling, since 5-HT2A receptors were activated in cholinergic stromal neurons, which in turn stimulated epithelial effectors through muscarinic innervation [43].
Angiogenesis and enteric neurons show a positive feedback loop
Intestinal epithelial cells act on sub-epithelial microvessels through an enteric-related neural stimulation [44]. Recent evidence suggests that both epithelial and muscle-activated responses convey signals towards myenteric plexus which turn innervates sub-epithelial vasodilator neurons, thus acting on intestinal microvasculature [45]. A new three-dimensional neurohistology approach has shown that extrinsic sympathetic nerves encircle the arterioles while the intrinsic nerves ascend from the myenteric domain towards epithelial surface in intestines [33]. Thus, enteric neurons take the intestinal microvessel functions under control receiving and decoding multiple signals from different sources (Fig. 1). This enables these cells to induce the proper effect on the right target [46]. Experimental data have shown that capsaicin and P substance counteract the pre-constrictive effects of prostaglandin F2-α, whereas the effects of the vasodilator capsaicin were inhibited upon denervation of extrinsic sensory afferent fibers [47]. Since denervation of extrinsic sympathetic and sensory fibers results in a lack of vasodilation by arterioles, it was found that myenteric neurons giving rise to non-cholinergic innervation of microvessels promotes de novo vasodilation [48]. Although these results show potential effects of extrinsic sympathetic nerves on microvessel-related activities, they also argue that intrinsic intestinal neural reflexes are undoubtedly important in tissue responses to stressor agents under pathological conditions [46]. This actually supports the idea that polysynaptic pathways act on sub-epithelial vasodilator neurons, and might coordinate microvessel activities [46–48].
Inflammation and the neurovascular system during early carcinogenesis steps
Intestinal nervous system and endothelial cells modulate colonic inflammation
Inflammatory bowel diseases (IBD), such as colitis, are one of the major risks for colon cancer [49]. IBD was therefore investigated in transgenic NSE-noggin and Hand2 +/− mice, which have greater or fewer enteric neural innervation than wild-type mice, respectively. After exposure to dextran sulfate sodium, Hand2 +/− mice showed much lower inflammatory signals than wild-type littermates, whereas NSE-noggin had increased intestinal inflammation [50]. Glial-derived neurotrophic factor (GDNF) was found to be upregulated during IBD inhibiting apoptosis in colonocytes [51]. Data from TPH1 −/− mice, which have a serotonin synthesis knockout from epithelial cells, argue that this serotonergic source enhances intestinal inflammation [52]. Knocking out tryptophan hydroxylase-2 (TPH2) impairs serotonin production by enteric neurons. The fact that TPH2 −/− mice suffered from an increased inflammatory process in comparison with their wild-type littermates supports the idea of enteric serotonin being requested to counteract colonic inflammation [52, 53]. Furthermore, deleting the serotonin reuptake transporter (SERT) in epithelial cells enhances the intensity of colitis [54]. Moreover, a wide range of molecular signaling-based mechanisms that are required by enteric neurons to modulate the vascular system become impaired under inflammation, which enhances even more the inflammatory-related damage in intestines [15, 46]. Actually, neurons can be altered by damage-related inflammation in endothelial cells, inducing them to express pro-apoptotic effectors towards glial cells [55]. Thus, enteric neurogenesis and serotonergic-related signaling may be considered to be involved in the severity of intestinal inflammation instead of individual enteric neural signals or cell phenotypes acting alone in the pathogenesis of IBD [50, 52–54].
Intestinal enteric nervous system and angiogenesis are possibly required for carcinogenesis from the early steps of colon cancer onwards
A growing body of evidence (Fig. 1, inset 1) suggests that specific mutations in epithelial progenitor cells are not the only specific events responsible for colon carcinogenesis [7, 12, 13, 18, 19, 27, 28, 56]. This means that colon carcinogenesis is a multi-step sequence of changes [1], with inflammation as one of the earliest steps [49]. This, besides inducing mutations in epithelial cells, builds a pro-malignant sub-epithelial microenvironment which supports the manifestation, development, and spread of mutations in the colon [7, 23, 49]. It has been hypothesized that the intestinal microenvironment could enhance the number of mutations required for the development of tumors in the epithelia cover [7, 12, 13, 18, 19, 27, 28, 56], since specific changes in molecular signaling among colonic sub-epithelial cells are required for progression of colon carcinogenesis [7, 13].
Angiogenesis is early enhanced during the development of preneoplastic lesions into tumors, but it remains unknown whether mutations in endothelial stem cell niches are related to high microvessel density in colon tumors [23, 27, 28, 49]. Here, it must be considered that endothelial cells are known to control apoptosis in intestinal crypts and they may suffer DNA damage before epithelial cells [22]. Besides, normalizing the tumor vasculature has been suggested as a therapeutical strategy in colon cancer [29]. Considering that mutations were found to alter the function of endothelial progenitor cells inducing inflammation [57], which represents the angiogenic stem cell niche [58], we suggest that genetic damage might happen in endothelial progenitor cells inducing a de novo malignant angiogenesis increasing microvessel density in colon tumors.
Several-associated activities have been observed between microvessels and ENS [51, 55]. For instance, GDNF has been shown as effector of the autocrine anti-apoptotic loop between intestinal neurons and microvessels under inflammatory conditions [51]. Glial cells are not only enteric progenitor cells giving rise to enteric neurons [32, 35, 36], they also control proliferation of colonic epithelial cells [40]. Glial were yet shown to suffer changes during intestinal inflammation, while enteric neurons have their numbers enhanced in such condition [50]. Actually, the density of enteric neurons was found increased within sub-epithelial and myenteric domains from lymphoid hyperplasia to adenocarcinomas in the colon of carcinogen-exposed rats [59]. Moreover, we reported that, in carcinogen-exposed rats, a chemical denervation of the myenteric plexus (63 % of loss in neural cell numbers; 2 mM benzalkonium chloride) prevented the development of colon tumors [38]. Our previous reports indeed revealed that the density of intestinal neurons and microvessels is related to the development of colon cancer from its early steps on [27, 28, 38, 56, 60].
A growing body of evidence suggests that the serotonergic system acts distinctively different from epithelial and neural serotonin sources, which can either enhance damage or promote protection, when intestines undergo inflammation [50, 52–54]. We therefore observed that the serotonergic system modulates the manifestation of preneoplastic lesions in colon tissue. This specifically means that different dietary conditions suppressed 5-HT levels enhancing the development of preneoplastic lesions and microvessels surrounding such lesions [28, 60], whereas endogenously upregulating the 5-HT levels reduced such lesions and preneoplasia-related angiogenesis in colon tissue [27, 56].
Conclusion and perspectives
Taken together, it may not be ruled out that endothelial and glial cells suffer carcinogenesis-related changes giving rise to cancer-related angiogenesis and neurogenesis. This means that, besides causing mutations in epithelial cells, carcinogenic compounds could induce the colonic microenvironment (fibroblasts, enteric neurons, and glial and endothelial cells) to enhance proliferation and spread of such mutated epithelial cells. It is plausible that preneoplastic lesions developing into tumors may depend on a positive feedback loop between initiated colonic epithelial cells and altered/mutated neuronal, glial and endothelial cells driving a malignant phenotype that would give rise to tumors [7, 13–15, 22, 37, 61]. Although this idea is not widely discussed, there are several observations supporting the concept that epithelial and other types of progenitor cells undergo mutational processes during carcinogenesis, since their progeny show phenotypical and structural changes. It seems reasonable to suggest that without carcinogenic changes in the microenvironment, tumors would not fully develop. This idea highlights enteric neurons, and glial and endothelial cells as promising targets for research in colon carcinogenesis.
Conflict of interest
The authors have no conflicts of interest to disclosure.
References
- 1.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–767. doi: 10.1016/0092-8674(90)90186-I. [DOI] [PubMed] [Google Scholar]
- 2.Barker N, Ridgway RA, van Es JH, et al. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature. 2009;457:608–611. doi: 10.1038/nature07602. [DOI] [PubMed] [Google Scholar]
- 3.Zeki SS, Graham TA, Wright NA. Stem cells and their implications for colorectal cancer. Nat Rev Gastroenterol Hepatol. 2011;8:90–100. doi: 10.1038/nrgastro.2010.211. [DOI] [PubMed] [Google Scholar]
- 4.Wong WM, Mandir N, Goodlad RA, et al. Histogenesis of human colorectal adenomas and hyperplastic polyps: the role of cell proliferation and crypt fission. Gut. 2002;50:212–217. doi: 10.1136/gut.50.2.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wong WM, Garcia SB, Wright NA. Origins and morphogenesis of colorectal neoplasms. APMIS. 1999;107:535–544. doi: 10.1111/j.1699-0463.1999.tb01590.x. [DOI] [PubMed] [Google Scholar]
- 6.Schwitalla S, Fingerle AA, Cammareri P, et al. Intestinal tumorigenesis initiated by dedifferentiation and acquisition of stem-cell-like properties. Cell. 2013;152:25–38. doi: 10.1016/j.cell.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 7.Schwitalla S, Ziegler PK, Horst D, et al. Loss of p53 in enterocytes generates an inflammatory microenvironment enabling invasion and lymph node metastasis of carcinogen-induced colorectal tumors. Cancer Cell. 2013;23:93–106. doi: 10.1016/j.ccr.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 8.Humphries A, Wright NA. Colonic crypt organization and tumorigenesis. Nat Rev Cancer. 2008;8:415–424. doi: 10.1038/nrc2392. [DOI] [PubMed] [Google Scholar]
- 9.Seton-Rogers S. Microenvironment: making connections. Nat Rev Cancer. 2013;13:222–223. doi: 10.1038/nrc3492. [DOI] [PubMed] [Google Scholar]
- 10.Neunlist M, Van Landeghem L, Mahe MM, et al. The digestive neuronal-glial-epithelial unit: a new actor in gut health and disease. Nat Rev Gastroenterol Hepatol. 2013;10:90–100. doi: 10.1038/nrgastro.2012.221. [DOI] [PubMed] [Google Scholar]
- 11.Luebeck EG, Hazelton WD. Multistage carcinogenesis and radiation. J Radiol Prot. 2002;22:43–49. doi: 10.1088/0952-4746/22/3A/308. [DOI] [PubMed] [Google Scholar]
- 12.Kannen V, Garcia SB, Stopper H, et al. Glucagon-like peptide 2 in colon carcinogenesis: possible target for anti-cancer therapy? Pharmacol Ther. 2013;139:87–94. doi: 10.1016/j.pharmthera.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 13.Quante M, Varga J, Wang TC, et al. The gastrointestinal tumor microenvironment. Gastroenterology. 2013 doi: 10.1053/j.gastro.2013.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee G, Goretsky T, Managlia E, et al. Phosphoinositide 3-kinase signaling mediates beta-catenin activation in intestinal epithelial stem and progenitor cells in colitis. Gastroenterology. 2010;139:869, e861–881, 869. doi: 10.1053/j.gastro.2010.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okayasu I. Development of ulcerative colitis and its associated colorectal neoplasia as a model of the organ-specific chronic inflammation-carcinoma sequence. Pathol Int. 2012;62:368–380. doi: 10.1111/j.1440-1827.2012.02807.x. [DOI] [PubMed] [Google Scholar]
- 16.Brentnall TA, Crispin DA, Rabinovitch PS, et al. Mutations in the p53 gene: an early marker of neoplastic progression in ulcerative colitis. Gastroenterology. 1994;107:369–378. doi: 10.1016/0016-5085(94)90161-9. [DOI] [PubMed] [Google Scholar]
- 17.Burmer GC, Rabinovitch PS, Haggitt RC, et al. Neoplastic progression in ulcerative colitis: histology, DNA content, and loss of a p53 allele. Gastroenterology. 1992;103:1602–1610. doi: 10.1016/0016-5085(92)91184-6. [DOI] [PubMed] [Google Scholar]
- 18.Achyut BR, Bader DA, Robles AI, et al. Inflammation-mediated genetic and epigenetic alterations drive cancer development in the neighboring epithelium upon stromal abrogation of TGF-beta signaling. PLoS Genet. 2013;9:e1003251. doi: 10.1371/journal.pgen.1003251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhowmick NA, Chytil A, Plieth D, et al. TGF-beta signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science. 2004;303:848–851. doi: 10.1126/science.1090922. [DOI] [PubMed] [Google Scholar]
- 20.Snippert HJ, van der Flier LG, Sato T, et al. Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell. 2010;143:134–144. doi: 10.1016/j.cell.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 21.Sato T, Vries RG, Snippert HJ, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 22.Paris F, Fuks Z, Kang A, et al. Endothelial apoptosis as the primary lesion initiating intestinal radiation damage in mice. Science. 2001;293:293–297. doi: 10.1126/science.1060191. [DOI] [PubMed] [Google Scholar]
- 23.Skinner SA, Frydman GM, O’Brien PE. Microvascular structure of benign and malignant tumors of the colon in humans. Dig Dis Sci. 1995;40:373–384. doi: 10.1007/BF02065424. [DOI] [PubMed] [Google Scholar]
- 24.Stappenbeck TS, Hooper LV, Gordon JI. Developmental regulation of intestinal angiogenesis by indigenous microbes via Paneth cells. Proc Natl Acad Sci USA. 2002;99:15451–15455. doi: 10.1073/pnas.202604299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crisan M, Chen CW, Corselli M, et al. Perivascular multipotent progenitor cells in human organs. Ann NY Acad Sci. 2009;1176:118–123. doi: 10.1111/j.1749-6632.2009.04967.x. [DOI] [PubMed] [Google Scholar]
- 26.Campagnolo P, Cesselli D, Al Haj Zen A, et al. Human adult vena saphena contains perivascular progenitor cells endowed with clonogenic and proangiogenic potential. Circulation. 2010;121:1735–1745. doi: 10.1161/CIRCULATIONAHA.109.899252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kannen V, Marini T, Turatti A, et al. Fluoxetine induces preventive and complex effects against colon cancer development in epithelial and stromal areas in rats. Toxicol Lett. 2011;204:134–140. doi: 10.1016/j.toxlet.2011.04.024. [DOI] [PubMed] [Google Scholar]
- 28.Kannen V, Zanette DL, Fernandes CR, et al. High-fat diet causes an imbalance in the colonic serotonergic system promoting adipose tissue enlargement and dysplasia in rats. Toxicol Lett. 2012;213:135–141. doi: 10.1016/j.toxlet.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 29.Goel S, Duda DG, Xu L, et al. Normalization of the vasculature for treatment of cancer and other diseases. Physiol Rev. 2011;91:1071–1121. doi: 10.1152/physrev.00038.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shen Q, Goderie SK, Jin L, et al. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304:1338–1340. doi: 10.1126/science.1095505. [DOI] [PubMed] [Google Scholar]
- 31.Gershon MD. Developmental determinants of the independence and complexity of the enteric nervous system. Trends Neurosci. 2010;33:446–456. doi: 10.1016/j.tins.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 32.Liu MT, Kuan YH, Wang J, et al. 5-HT4 receptor-mediated neuroprotection and neurogenesis in the enteric nervous system of adult mice. J Neurosci. 2009;29:9683–9699. doi: 10.1523/JNEUROSCI.1145-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fu YY, Peng SJ, Lin HY, et al. 3-D imaging and illustration of mouse intestinal neurovascular complex. Am J Physiol Gastrointest Liver Physiol. 2013;304:1–11. doi: 10.1152/ajpgi.00209.2012. [DOI] [PubMed] [Google Scholar]
- 34.Lundgren O, Jodal M, Jansson M, et al. Intestinal epithelial stem/progenitor cells are controlled by mucosal afferent nerves. PLoS One. 2011;6:e16295. doi: 10.1371/journal.pone.0016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gershon MD. Behind an enteric neuron there may lie a glial cell. J Clin Invest. 2011;121:3386–3389. doi: 10.1172/JCI59573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laranjeira C, Sandgren K, Kessaris N, et al. Glial cells in the mouse enteric nervous system can undergo neurogenesis in response to injury. J Clin Invest. 2011;121:3412–3424. doi: 10.1172/JCI58200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Magnon C, Hall SJ, Lin J, et al. Autonomic nerve development contributes to prostate cancer progression. Science. 2013;341:1236361. doi: 10.1126/science.1236361. [DOI] [PubMed] [Google Scholar]
- 38.Garcia SB, Oliveira JS, Pinto LZ, et al. The relationship between megacolon and carcinoma of the colon: an experimental approach. Carcinogenesis. 1996;17:1777–1779. doi: 10.1093/carcin/17.8.1777. [DOI] [PubMed] [Google Scholar]
- 39.Polli-Lopes AC, Zucoloto S, de Queiros Cunha F, et al. Myenteric denervation reduces the incidence of gastric tumors in rats. Cancer Lett. 2003;190:45–50. doi: 10.1016/S0304-3835(02)00584-0. [DOI] [PubMed] [Google Scholar]
- 40.Neunlist M, Aubert P, Bonnaud S, et al. Enteric glia inhibit intestinal epithelial cell proliferation partly through a TGF-beta1-dependent pathway. Am J Physiol Gastrointest Liver Physiol. 2007;292:231–241. doi: 10.1152/ajpgi.00276.2005. [DOI] [PubMed] [Google Scholar]
- 41.Bustamante SA, Forshult M, Lundgren O. Evidence for an intramural nervous control of epithelial cell migration in the small intestine of the rat. Acta Physiol Scand. 1989;135:469–475. doi: 10.1111/j.1748-1716.1989.tb08605.x. [DOI] [PubMed] [Google Scholar]
- 42.Wade PR, Chen J, Jaffe B, et al. Localization and function of a 5-HT transporter in crypt epithelia of the gastrointestinal tract. J Neurosci. 1996;16:2352–2364. doi: 10.1523/JNEUROSCI.16-07-02352.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gross ER, Gershon MD, Margolis KG, et al. Neuronal serotonin regulates growth of the intestinal mucosa in mice. Gastroenterology. 2012;143:408–417, e402. doi: 10.1053/j.gastro.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guan X, Karpen HE, Stephens J, et al. GLP-2 receptor localizes to enteric neurons and endocrine cells expressing vasoactive peptides and mediates increased blood flow. Gastroenterology. 2006;130:150–164. doi: 10.1053/j.gastro.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 45.Reed DE, Vanner SJ. Long vasodilator reflexes projecting through the myenteric plexus in guinea-pig ileum. J Physiol. 2003;553:911–924. doi: 10.1113/jphysiol.2003.053140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vanner S, Surprenant A. Neural reflexes controlling intestinal microcirculation. Am J Physiol. 1996;271:223–230. doi: 10.1152/ajpgi.1996.271.2.G223. [DOI] [PubMed] [Google Scholar]
- 47.Vanner S. Mechanism of action of capsaicin on submucosal arterioles in the guinea pig ileum. Am J Physiol. 1993;265:51–55. doi: 10.1152/ajpgi.1993.265.1.G51. [DOI] [PubMed] [Google Scholar]
- 48.Jiang MM, Surprenant A. Re-innervation of submucosal arterioles by myenteric neurones following extrinsic denervation. J Auton Nerv Syst. 1992;37:145–154. doi: 10.1016/0165-1838(92)90243-A. [DOI] [PubMed] [Google Scholar]
- 49.Waldner MJ, Wirtz S, Jefremow A, et al. VEGF receptor signaling links inflammation and tumorigenesis in colitis-associated cancer. J Exp Med. 2010;207:2855–2868. doi: 10.1084/jem.20100438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Margolis KG, Stevanovic K, Karamooz N, et al. Enteric neuronal density contributes to the severity of intestinal inflammation. Gastroenterology. 2011;141:588, e581–598, e582. doi: 10.1053/j.gastro.2011.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Steinkamp M, Schulte N, Spaniol U, et al. Brain derived neurotrophic factor inhibits apoptosis in enteric glia during gut inflammation. Med Sci Monit. 2012;18:117–122. doi: 10.12659/MSM.882612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ghia JE, Li N, Wang H, et al. Serotonin has a key role in pathogenesis of experimental colitis. Gastroenterology. 2009;137:1649–1660. doi: 10.1053/j.gastro.2009.08.041. [DOI] [PubMed] [Google Scholar]
- 53.Gershon MD. Serotonin is a sword and a shield of the bowel: serotonin plays offense and defense. Trans Am Clin Climatol Assoc. 2012;123:268–280. [PMC free article] [PubMed] [Google Scholar]
- 54.Bischoff SC, Mailer R, Pabst O, et al. Role of serotonin in intestinal inflammation: knockout of serotonin reuptake transporter exacerbates 2,4,6-trinitrobenzene sulfonic acid colitis in mice. Am J Physiol Gastrointest Liver Physiol. 2009;296:685–695. doi: 10.1152/ajpgi.90685.2008. [DOI] [PubMed] [Google Scholar]
- 55.Takahashi K, Funata N, Ikuta F, et al. Neuronal apoptosis and inflammatory responses in the central nervous system of a rabbit treated with Shiga toxin-2. J Neuroinflammation. 2008;5:11. doi: 10.1186/1742-2094-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kannen V, Hintzsche H, Zanette DL, et al. Antiproliferative effects of fluoxetine on colon cancer cells and in a colonic carcinogen mouse model. PLoS One. 2012;7:e50043. doi: 10.1371/journal.pone.0050043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lasater EA, Li F, Bessler WK, et al. Genetic and cellular evidence of vascular inflammation in neurofibromin-deficient mice and humans. J Clin Invest. 2010;120:859–870. doi: 10.1172/JCI41443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Passman JN, Dong XR, Wu SP, et al. A sonic hedgehog signaling domain in the arterial adventitia supports resident Sca1 + smooth muscle progenitor cells. Proc Natl Acad Sci USA. 2008;105:9349–9354. doi: 10.1073/pnas.0711382105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sitohy B, El-Salhy M. Changes in the colonic enteric nervous system in rats with chemically induced colon dysplasia and carcinoma. Acta Oncol. 2002;41:543–549. doi: 10.1080/02841860214957. [DOI] [PubMed] [Google Scholar]
- 60.Kannen V, Fernandes CR, Stopper H, et al. Colon preneoplasia after carcinogen exposure is enhanced and colonic serotonergic system is suppressed by food deprivation. Toxicology. 2013 doi: 10.1016/j.tox.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 61.Liebl F, Demir IE, Rosenberg R, et al. The severity of neural invasion is associated with shortened survival in colon cancer. Clin Cancer Res. 2013;19:50–61. doi: 10.1158/1078-0432.CCR-12-2392. [DOI] [PubMed] [Google Scholar]