Abstract
The identification of numerous deregulated signaling pathways on cancer cells and supportive stromal cells has revealed several molecular targets whose downregulation can elicit significant benefits for cancer treatment. In this respect, gene downregulation can be efficiently achieved by exploiting the RNA interference mechanism, particularly by the delivery of chemical synthesized small-interfering RNAs (siRNAs), which have the ability to mediate, in a specific manner, the degradation of any mRNA with complementary nucleotide sequence. However, several concerns regarding off-target effects and immune stimulation have been raised. Depending on their sequence, siRNAs can trigger an innate immune response, which might mediate undesirable side effects that ultimately compromise their clinical utility. This is a very relevant effect that will be discussed in the present manuscript. Moreover, the major drawback in the translation of siRNAs into the clinical practice is undoubtedly their inability to accumulate in tumor sites, particularly in organs other than the liver. In fact, upon systemic administration, owing to siRNAs physico-chemical features, they are rapidly cleared from the blood stream. Therefore, the development of a proper drug delivery system is of utmost importance. In this review, some of the latest advances on different nanotechnological platforms for siRNA delivery under clinical evaluation will be discussed. Along with this, targeting approaches towards cancer and/or endothelial cells will also be addressed, as these are some of the most promising strategies to enhance specific tumor accumulation while avoiding healthy tissues. Finally, clinical information on ongoing studies in patients with advanced solid tumors will be also provided.
Keywords: siRNA, Immunostimulation, Systemic delivery, Nanotechnology, Cancer, Clinical trials
Introduction
Cancer is still one of the most deadly diseases in the Western world, despite its mortality rate has decreased in the last decades [1]. Moreover, the adverse effects and limited effectiveness of conventional treatment strategies (chemotherapy, surgery, or radiotherapy) have generated considerable interest on the development of novel anticancer agents, with improved target specificity at the molecular level.
The increasing knowledge on oncobiology over the last decades has revealed cancer as a disease that involves several irreversible genetic and epigenetic alterations, which occur progressively over time. The transformation of a normal cell into a malignant cell is the result of a multi-step process mediated by the accumulation of successive mutations that lead to the activation of oncogenes and/or loss of function of tumor suppressor genes. These alterations, along with mutations in other genes that control normal cell functions, trigger the deregulation of numerous signaling pathways, enabling cancer cells to acquire specific biological capabilities to adapt to internal and environmental changes [2, 3]. This deeper understanding has revealed numerous molecular targets, both on cancer and supportive stroma cells (e.g., endothelial cells from tumor vasculature), whose downregulation can elicit significant benefits for cancer treatment. To achieve such a goal, one promising strategy is undoubtedly the RNA interference (RNAi) mechanism, which is mediated by small RNAs including, among others, the endogenous microRNAs (miRNAs) [4] and the exogenous chemically synthesized short-hairpins RNAs (shRNAs) [5], and small-interfering RNAs (siRNAs) [6], which are focused on in this review.
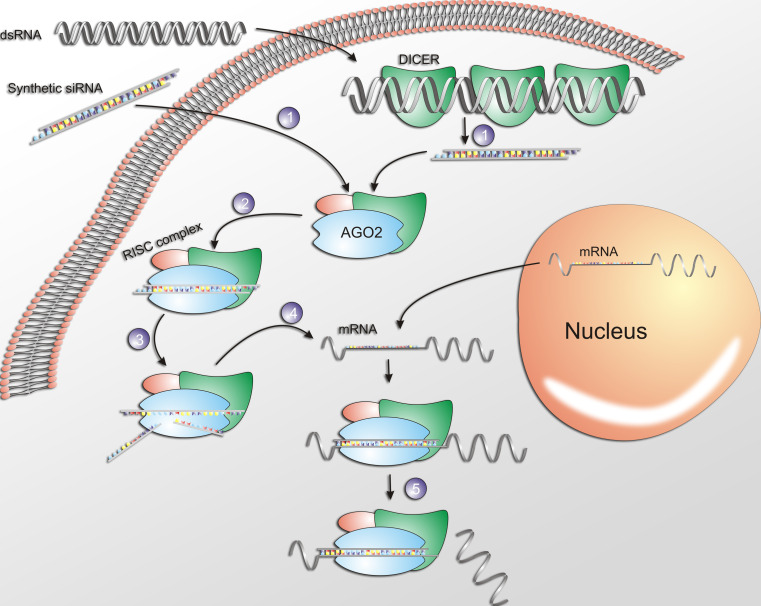
Small-interfering RNAs are 21–23 nucleotides-long double-stranded (ds) RNA which, upon cellular internalization, are incorporated into a multi-protein complex, the RNA induced silencing complex (RISC). The sense strand (non-guiding strand) is then cleaved by an endonuclease of the RISC, the Argonaute 2 (AGO 2), whereas the antisense strand guides RISC towards the perfectly complementary target mRNA, which is further cleaved by AGO 2 into two mRNA fragments. The cleavage takes place between nucleotides 10 and 11 relative to the 5′end of the siRNA guiding strand, leading to the subsequent degradation of the mRNA fragments by cellular exonucleases. Furthermore, the antisense strand-RISC complex has the ability to be recycled thus, leading to the degradation of additional target mRNA molecules and further propagation of the gene silencing activity [7–9] (Fig. 1).
Fig. 1.
The RNAi mechanism mediated by siRNAs. 1 SiRNA is internalized into the cell cytoplasm then, 2 incorporated into the RISC, and 3 the sense (passenger) strand of RNA is cleaved by AGO2. 4 The activated RISC complexed with the antisense strand is guided towards the complementary mRNA 5 mediating its cleavage and therefore, silencing of the target gene
Hence, siRNAs have an enormous potential to become a novel class of pharmaceutical drugs within different fields of medicine, since they can inhibit the expression of any pathological protein. Oncology is one of the medical areas that can benefit the most with this novel therapeutic strategy, as it allows modulating the expression of any gene involved in tumor initiation, growth, and metastasis formation.
siRNA as a novel therapeutic strategy: pros and cons
siRNAs have some important advantages over traditional pharmaceutical drugs, like small molecules or proteins and derivatives, as they can be designed to silence any target gene in the body, thus, constituting a class of drugs with a broader therapeutic potential. Moreover, new siRNAs sequences aiming at targeting a specific gene can be rapidly and rationally designed when the mRNA sequence is known, being the manufacturing process rapid and scalable. Cross-reactivity between species is often possible with siRNAs, while maintaining their specificity and potency. Additionally, their high specificity makes them less toxic than traditional drugs [10].
Despite all the therapeutic potential of siRNA-based technology, numerous limitations have to be overcome in order to reach the clinical setting. It is now widely accepted that siRNAs induce various undesirable effects, as they can also interfere with the translation of others mRNAs besides the target one, and induce an immune response as well.
Off-target effects
The effects obtained due to silencing of genes other than the intended one are known as off-target effects and can compromise the siRNA utility to study gene function [11]. The off-target effects result from the interference of the siRNA with mRNA sequences to which the former has only partial complementarity, by a miRNA-like mechanism, or by the incorporation of the sense strand into the RISC. This interference results in silencing of a variety of genes [12] that, in some cases, regardless of the target gene, can induce cell death [13].
Furthermore, it has been observed that even scrambled sequences, which theoretically do not target any mRNA, can have a moderate to high impact on the cell viability, depending on the cell line and siRNA concentration [14]. The mechanism of such toxicity is not yet fully understood. It is unlikely to be the consequence of a single gene knockdown but rather the accumulation of interference with several genes that results in cellular stress [13]. Interestingly, even an siRNA against an exogenous protein such as Green Fluorescence Protein, can deregulate a wide range of genes in different human cell lines [12].
Innate immune system stimulation
The presence of specific motifs in siRNAs sequences, as 5′-GUCCUUCAA-3′, induces immunostimulatory activity both in vitro and in vivo [15]. Interestingly, siRNA-mediated immune stimulation was not detected in Toll-like receptor 7 (TLR7) knockout mice, suggesting that TLR7 was involved in the recognition of such motif. In fact, in immune-competent cells, TLRs act as sensors for the recognition of external nucleic acids (e.g., viral) that are internalized via endocytosis, triggering an interferon response and production of inflammatory cytokines as a defense mechanism. Most TLRs are expressed in the plasma membrane, although TLR7 and TLR8, both single-stranded RNA sensors, and TLR9, a CpG-DNA sensor, are mainly expressed in the endosomal compartment whereas TLR3, a dsRNA sensor, is expressed in both cell and endosomal membranes [16].
Judge et al. [17] also reported that different siRNAs sequences with high content of GU motifs, namely 5′-UGUGU-3′ or 5′-UGU-3′, and delivered by cationic lipid-based nanocarriers, led to the production of interferon α (IFN-α) and inflammatory cytokines such as interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α), both in mouse models and in human peripheral blood mononuclear cells (PBMC). Strikingly, immune stimulation was not detected when the delivery vehicle was not applied. This suggested that active endocytosis of siRNA was required for immune stimulation.
In the work of Sioud [18, 19] it was demonstrated that IFN-α response and inflammatory cytokine production in human PBMC cells were not restricted to the siRNA duplex but also achievable with the individualized sense or antisense single strands, the latter being much more immunostimulatory than the siRNA duplex. In accordance with other reports [15, 17, 20, 21], immune activation, either by double- or single-strand siRNAs, was only triggered when the delivery was mediated by complexation of the nucleic acid with a cationic lipid. Indeed, siRNA delivery by electroporation did not lead to immune activation, evidencing the involvement of the endosomal compartment in the siRNA immunogenic activity. It is expected that in endosomes, the acidic environment unwound the double siRNA into its single strands, which are likely to be recognized by the TLR7/8 receptors present in the endosomal compartment [22].
Another interesting work was reported by Kleinman et al. [23], who exhaustively demonstrated that angiogenesis inhibition is a common unspecific effect of 21 nucleotides or longer siRNAs, independently of their sequence and target. Indeed, local injection into the vitreous humor of free siRNAs targeting non-mammalian genes, non-expressed genes, non-genomic sequences, RNAi-incompetent siRNAs or 2′-O-methyl modified siRNAs, all suppressed blinding choroidal neovascularization in mouse models. This effect took place at an extent comparable to siRNAs specifically designed to target the pro-angiogenic vascular endothelial growth factor (VEGF) pathway.
Since cells do not internalize free siRNAs, its recognition by a cell surface TLR was suggested. In fact, inhibition of angiogenesis has not taken place either in TLR3 knockout mice or in the presence of TLR3 antibodies, suggesting the cell surface TLR3 on mouse and human choroidal endothelial cells as the mediator of the anti-angiogenic effect of siRNA [23].
The previous results were further confirmed in other mouse models of neovascularization (cornea suture injury and hind limb ischemia), where it was demonstrated that besides angiogenesis, lymphangiogenesis was also inhibited by activation of TLR3 expressed on the surface of lymphatic endothelial cells [24]. The anti-angiogenic mechanism upon TLR3 activation is not yet well known. However, it was recently demonstrated that in normoxia conditions, TLR3 activation in aggressive prostate cancer cells, increased expression of hypoxia inducible factor 1, leading to reduced apoptosis and increased secretion of VEGF [25].
Overall, these reports strongly demonstrated that siRNAs are potent activators of the innate immune system, both in vitro and in vivo. This unspecific effect can have important implications in the interpretation of gene-silencing effects mediated by siRNA. From a therapeutic perspective, although such immune stimulation can contribute to control some pathological processes, for instance, viral replication, tumor growth, and angiogenesis, it can also lead to undesirable side effects due to cytokine-associated toxicity.
Overcoming siRNA immune stimulation
In order to circumvent siRNA immunostimulatory activity, it is highly recommended to avoid poly-U- and GU-rich regions [15] and the two immunostimulatory motifs already identified, 5′-GUCCUUCAA- 3′ and 5′-UGUGU-3′ [17]. However, this approach significantly limits the number of novel siRNA sequences that can be designed against a given gene target [26] thus, requiring alternative strategies.
One of the most promising strategies relies on the insertion of chemical modifications without compromising the siRNA silencing potency. Indeed, the explored chemical modifications at the ribose, phosphodiester or base levels, aiming at increasing serum stability of different types of nucleic acids [27, 28], have recently demonstrated to abrogate off-target effects [29] and immunostimulation as well [26].
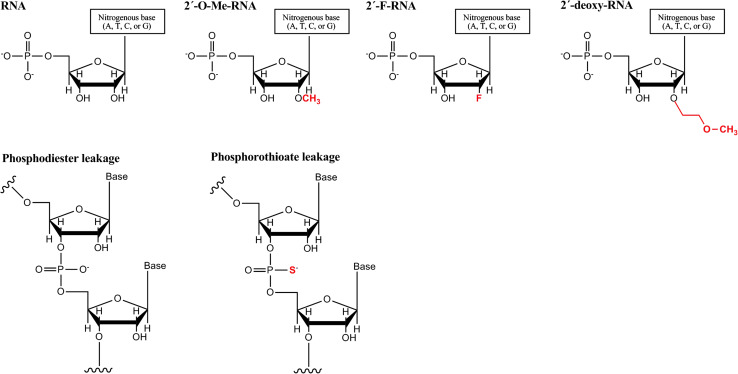
Most of the chemical modifications have been carried out in the ribose moiety, especially in the 2′ position, as it was demonstrated that the 2′OH group was not necessary for the siRNA gene silencing activity [30] (Fig. 2).
Fig. 2.
The most common chemical modifications applied to siRNAs. a The hydroxyl group on position 2 of ribose has been replaced by 2′-O-methyl, 2′-fluoro or 2′-O-methoxyethyl groups and b the phosphodiester leakage can be modified as a phosphorothioate bond, which retains the negative charge of the phosphate group. Adapted from [30]
Morrissey et al. [31, 32] verified total abrogation of siRNA-mediated immunostimulation upon incorporation of different types of chemical modifications (2′-O-methyl (2′-OMe), 2′-fluoro (2′-F), 2′-O-methoxyethyl (2′-MOE), phosphorothioate linkages and terminus capping chemistries) in more than 90 % of the nucleotides of a siRNA sequence against hepatitis B virus (HBV). After systemic administration of unmodified anti-HBV siRNA, encapsulated in lipid-based nanocarriers (stabilized nucleic acid lipid particles (SNALP)), high levels of IFN-α, inflammatory cytokines (IL-6, TNF-α) were detected. This immunostimulation was correlated with liver inflammation (elevated levels of transaminases) and transient lymphopenia and thrombocytopenia. In contrast, mice treated with the 2′-modified counterpart did not exhibit any immune response, which was translated with total absence of toxicity. Surprisingly, reduction in HBV serum titers was achieved with both unmodified and 2′-modified siRNAs, which was likely the result of different mechanisms (innate immune stimulation and RNAi, respectively).
A similar approach was used by Zimmerman et al. [33] in the design of a siRNA against apolipoprotein B (APOB), upon systemic delivery by SNALP into cynomolgus monkeys. Incorporation of several 2′-OMe nucleotides and phosphorothioate linkages (Fig. 2) in the siRNA sequence has enabled total abrogation of unmodified siRNA-mediated immune stimulation, without altering the original gene silencing activity.
Later, Judge et al. [34] demonstrated that the incorporation of only a few 2′-OMe uridines or guanosines (less than 20 % of the nucleotides) in the sense strand of the duplex encapsulated in SNALP was enough to totally abrogate the immunostimulatory siRNA activity (both in human PBMC and in mice), without compromising gene silencing activity. Interestingly, even siRNAs containing the immunostimulatory motifs described above had their immunostimulation ability inhibited when as low as two nucleotides were methylated. Indeed, other reports have correlated high content of uridines with IFN-α response, which could be abrogated through the replacement of uridines by adenosines [19, 35] or by 2′-OH substitutions (2′-F, 2′-OMe, 2′-MOE) [19, 34]. Sioud [19] demonstrated that 2′-OH substitutions only in uridines were sufficient to evade immune recognition of either single-stranded or dsRNA, even when complexed with the cationic lipid 1,2-dioleoyl-3-trimethylammonium-propane (DOTAP). In addition, 2′-OMe modification was identified as the one with higher immunosuppressive effect when compared with 2′-F or 2′-MOE modifications [36]. Further, the effectiveness of 2′-OMe modifications was confirmed in vivo by Robbins et al. [37]. In this work, it was shown that 2′-OMe modifications in uridines, guanosines, or adenines, independently of their position, abrogate immune activation but not 2′-OMe cytosines. Hamm et al. [38] suggested alternated 2′-OMe nucleotides in the sense strand as a general approach to generate non-immunostimulatory siRNAs without affecting the siRNA gene silencing activity.
Surprisingly, the immune activity of unmodified siRNA was abrogated by the simultaneous administration of 2′-OMe siRNAs, an effect likely related with the higher affinity of 2′-OMe siRNAs towards TLR7 than the unmodified siRNAs counterpart [36, 38]. However, more studies are needed to fully understand the interaction of 2′-OMe and unmodified siRNAs with TLRs.
Overall, it seems that by some means, 2′-OMe-modified siRNAs do not activate the TLRs downstream intracellular pathways, thus circumventing immune stimulation. Nevertheless, general guidelines allowing a rational design of 2′-OMe siRNAs are still necessary in order to avoid screening of numerous siRNA sequences.
Adjuvant effect of immunostimulatory siRNAs
Several publications have reported significant therapeutic outcomes upon treatment with unmodified siRNAs. However, most of them have not accessed properly immunostimulation, raising the question of whether such a therapeutic effect was actually mediated by RNAi or rather the result of a combined effect of RNAi and immune stimulation. In fact, in the last decade, several immunostimulatory siRNAs have been used without the fully perception of their aptitude to elicit non-specific therapeutic effects in a wide range of diseases, as viral infections, cancer, or inflammation.
Robbins et al. [39] demonstrated that the viral titer reduction reported by others [40, 41], upon treatment with anti-influenza siRNAs, complexed with cationic liposomes, was mainly due to immune stimulation rather than being RNAi-mediated. Indeed, treatment with 2′-OMe siRNAs completed abrogated immunostimulation and no measurable effect on the viral load was observed.
Despite the well-established fact that unmodified siRNAs are potentially immunostimulatory, they are still being used in the majority of in vivo studies. Therefore, caution in the interpretation of several published results reporting therapeutic RNAi should be taken.
Nevertheless, one can actually take advantage from an immunostimulatory effect. Unmodified siRNAs that combine immunostimulatory properties with gene silencing activity against immunosuppressive factors or cancer-related genes (bifunctional siRNAs) may hold great potential as an adjuvant immune therapy for cancer treatment [42]. This strategy has been pursued in the development of anti-cancer vaccines based on dendritic cells (DCs). Delivery, upon complexation with DOTAP, of a bifunctional siRNA against IL-10, an immunosuppressive cytokine overexpressed on the tumor microenvironment, to immature DCs resulted in effective protein downregulation with further DC maturation and upregulation of different players of the immune system: cytokines (TNF-α, IL-6, and IL-12), cell surface receptors that mediated T cell activation (CD80, CD86), major histocompatibility complex molecules, and the chemokine receptor CCR7, which induces DC migration to lymph nodes [42]. In addition, these anti-IL-10 siRNA-transfected cells were capable of enhancing the proliferation of T lymphocyte [42]. Anti-IL-10 siRNA-transfected DCs loaded with leukemia cells (acute myeloid leukemia, AML) antigens were tested as vaccines in rat models of AML. This vaccination induced proliferation of cytotoxic T cells, which was correlated by a decrease in metastasis formation and an increase in the overall mean survival [43].
Recently, in mouse models of cervical cancer, systemic administration of a bifunctional siRNA against the human papilloma virus (HPV)-type E6/E7 oncogene, encapsulated in liposomes, resulted in inhibition of tumor growth. Additionally, incorporation of 2′-OMe modifications in the anti-HPV siRNA strongly impaired the antitumor effect, whereas treatment with a scrambled immunostimulatory siRNA still exhibited moderate antitumor activity. These observations clearly indicated that combining silencing of a cancer-related gene with immune stimulation, mediated by the same siRNA sequence, may bring additional therapeutic benefits [44].
Systemic delivery of siRNA
Notwithstanding some of the challenges previously addressed, site-specific delivery has been the major bottleneck in the therapeutic application of siRNAs. The route of administration is highly dependent on the accessibility of the target organ or tissue within the body. Local administration of siRNAs has shown promising results either by intraocular, intranasal, intra-tumor, or direct administration in the nervous system, as reviewed elsewhere [45]. However, such an approach is not feasible in the treatment of advanced solid tumors with distant metastasis or hematologic tumors. In this respect, systemic administration is the most suited strategy but also the most challenging. Upon intravenous (iv) administration, naked siRNAs are cleared from the blood stream in a few minutes owing to rapid renal elimination, unspecific uptake by the mononuclear phagocytic system and degradation by serum nucleases. At the cell level, the physical–chemical properties of siRNA (size around 13 kDa, negative charge and hydrophilicity) strongly impair their cellular internalization [8, 27, 46–48].
An alternative strategy to achieve RNAi in mammalian cells is the artificial transcription of shRNAs, long structures characterized by a stem and a loop region, which are processed by Dicer into small duplexes like miRNAs. The delivery of shRNAs in cells is carried out by viral vectors (such as lentiviral vectors), which upon genetic integration induce a stable and long-term knockdown. This is in contrast to the transient effect, from dilution over successive cellular divisions, associated with siRNA [49]. However, some concerns arise from the absence of cell-specificity, difficulties with large-scale manufacture, as well as high risk of immunogenicity and mutagenesis, which ultimately limit their use in a clinical setting [50].
On the other hand, non-viral strategies, such as lipid- or polymeric-based nanoparticles, are alternative strategies generally perceived as safer than virus. Therefore, strong efforts have been directed to the design of effective and safe delivery platforms able to modulate the siRNAs pharmacokinetic and biodistribution properties, which will allow their translational into the clinical.
The use of the hydrophilic polymer poly(ethylene glycol) (PEG) has revolutionized the drug-delivery field upon enabling the development of nanocarriers with prolonged blood circulation times upon iv injection, which is critical to target extra-hepatic tumors. In fact, nearly all the nanocarriers developed for systemic administration have their surface modified by the presence of PEG [51]. Nevertheless, it is important to point out that some limitations have been associated wit PEG, such as impaired cellular uptake or efficient escape from the endocytic pathway, as well as activation of an immune response upon repeated administrations. This subject has been extensively described elsewhere [48, 51].
In the present section, nanocarriers for the systemic administration of siRNAs, and under clinical evaluation in oncological patients, will be discussed.
Stabilized nucleic acid lipid particles (SNALP)
Encapsulation of siRNAs into PEGylated lipid-based nanoparticles is one of the most explored strategies for the systemic delivery of siRNAs, being the SNALP technology developed by Tekmira Pharmaceuticals Corporation one of the most promising.
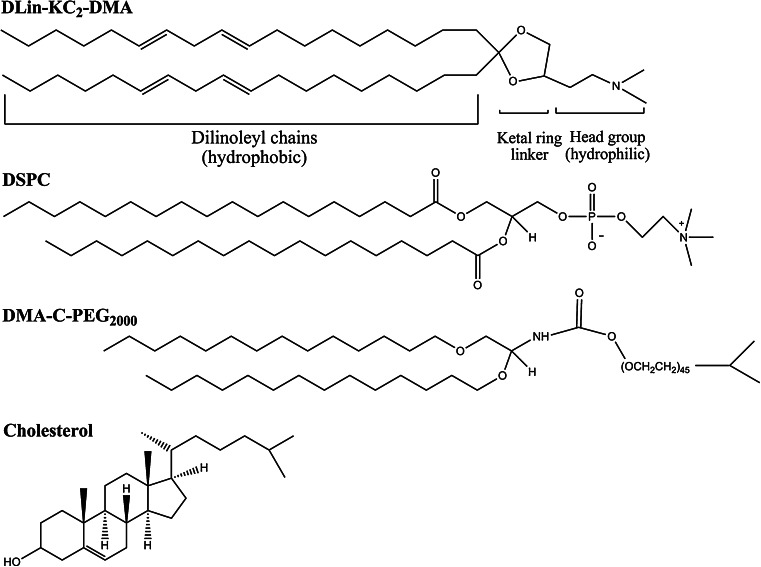
Typically, SNALP are formulated with four lipids: (1) an ionizable cationic lipid (such as, 2,2-dilinoleyl-4-(2-dimethylaminoethyl)-[1, 3]-dioxolane (DLin-KC2-DMA)); (2) cholesterol; (3) a neutral helper lipid with a high transition temperature [such as, 1,2-distearoyl-sn-glycero-3-phosphocoline (DSPC)] and; (4) an exchangeable PEG-derivatized lipid [such as PEGylated diacylglycerols (PEG-DAGs)] (Fig. 3).
Fig. 3.
Chemical structure of SNALP-forming lipids
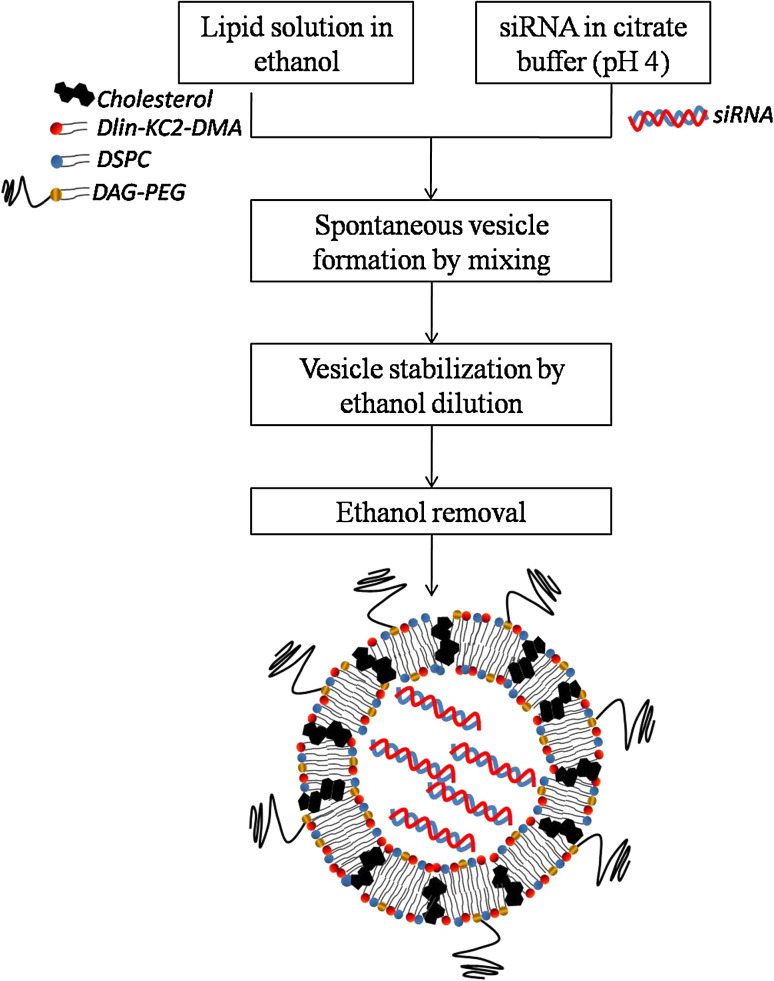
SNALP are spontaneously formed by rapidly mixing the above-mentioned lipids, dissolved in ethanol, with an aqueous solution of siRNA, at a low pH, in a highly controlled stepwise manner (Fig. 4). At acidic pH, the ionizable lipid is positively charged thus enabling the negatively charged siRNA encapsulation. However, after siRNA encapsulation, the adjustment of the external pH to a physiological value resulted in neutral liposomes, a critical feature aiming at reducing the extent of opsonization following intravenous administration [52, 53].
Fig. 4.
Ethanol dilution method of siRNA encapsulation. Liposomes are spontaneously formed upon mixing the lipids dissolved in ethanol with the siRNA dissolved in an aqueous buffer at acidic pH. The dilution of ethanol below the lipid solubility favors vesicle stabilization, which has the major advantage of avoiding an additional extrusion step to obtain small and homogenous liposomes [54]. The final liposomes have a surface charge close to neutrality, a mean size around 80 nm (or even smaller), and siRNA encapsulation efficiencies higher than 90 % [33, 55]
Since the first reports related to SNALP [56, 57], several improvements have been achieved owing to the rational design of new cationic lipids. In the work of Semple et al. [53], it was demonstrated that the transfection efficiency of a cationic lipid is inversely correlated to the number of double bounds per hydrocarbon chain, two bounds being the optimal number. From this work, 1,2-dilinoleyloxy-N,N-dimethyl-3-aminopropane (DLinDMA) has emerged as the leader compound [52], which was further improved by the incorporation of: (1) a ketal ring linker that enhances chemical stability and; (2) a methylene group into the headgroup (tertiary amine with pKa ~ 7). The latter contributes to maintain the neutrality of SNALP at physiologic pH whereas at acidic conditions, it is rapidly protonated adopting an inverted hexagonal phase, thus leading to the liposomal membrane destabilization and the consequent delivery of the payload [53]. The outcome of these optimizations was the 2,2-dilinoleyl-4-(2-dimethylaminoethyl)-[1, 3]-dioxane (DLin-KC2-DMA) lipid (Fig. 3), which has enabled a drastic reduction of the required siRNA doses in mice to ~0.01 mg/kg [53].
Cholesterol and DSPC (Fig. 3) are other important lipids for the preparation of SNALP. Together, they help to form an organized and rigid lipid bilayer, with the necessary stability for an iv administration [56]. The PEG-derivatized lipid is a critical component of SNALP as it avoids aggregation during the preparation process, besides improving pharmacokinetics upon reducing the extent of opsonization [56, 57]. Exchangeable PEG-derivatized lipids, as PEG-DAGs, have the ability to diffuse out from the lipid bilayer, at a rate that is controlled by the length of the acyl chain. This effect restores the ability of liposomes to contact with cell membranes thus favoring cell internalization and the subsequent siRNA endosomal escape, notwithstanding decreases the nanocarriers’ blood residence time to a great extent [58]. Therefore, the PEG-derivatized lipid has to be carefully selected in order to achieve the best commitment between the blood circulation time and the transfection efficacy. Typically, when the liver is the primary disease site, exchangeable PEG-derivatized lipids with short acyl chains [such as 14 carbons (C)], and hence with a short blood residence time, are preferred. In contrast, targeting extra-hepatic solid tumors requires PEG-derivatized lipids with longer acyl chains (such as 18 C). In fact, prolonged blood residence of the nanocarriers is one critical requisite to achieve a significant tumor accumulation [58–60].
SNALP, as occurs for most of the nanocarriers for systemic administration, predominantly accumulate in the liver, which is in part explained by the liver distinct anatomy (good perfusion and fenestrated endothelium) as well as by the capacity of the mononuclear phagocytic system in removing foreign bodies [61]. Moreover, the efficient uptake of SNALP by the hepatocytes seems to be mediated by the opsonization of these nanoparticles with endogenous apolipoprotein E, which is recognized and internalized by low-density lipoprotein receptors expressed on hepatocytes [62]. Therefore, most of the achievements with SNALP were attained in liver-associated diseases such as hepatitis B [31, 32], hypercholesterolemia [33], Ebola [55, 63], and liver cancer [59] (http://www.Tekmira.com). Alnylam Pharmaceuticals is another biotech company that is also exploiting the SNALP technology for liver-associated diseases such as transthyretin amyloidosis, hemophilia, porphyria, hemoglobinopathies, and alpha-1 antitrypsin-associated liver disease (http://www.alnylam.com).
At last, it is important to point out that all the siRNA sequences of Tekmira and Alnylam are 2′-modified in order to avoid the immunostimulatory effects previously mentioned.
Hepatic tumor penetration of SNALP-mediated siRNA delivery
From a retrospective analysis of a variety of siRNA lipid-based delivery platforms, SNALP has emerged as the most promising nanoparticle for hepatic delivery of siRNAs in mice. However, analysis of gene silencing spatial distribution in tumor sections revealed that only 10–15 % of cancer cells were effectively transfected [64]. These cells were located in areas with increased accessibility as they were adjacent to functional tumor blood vessels (normoxic regions) [65].
Indeed, SNALP containing a DY647-labeled anti-RAN GTPase siRNA injected into mice bearing highly vascularized HepG2 orthotopic hepatic tumors demonstrated effective siRNA delivery crosswise tumor sections, in contrast with the observation in poorly vascularized HCT116 orthotopic hepatic tumors. This was likely the reason why, despite that HCT116 cells were more sensitive than HepG2 to in vitro RAN GTPase silencing, tumor growth inhibition was more marked in the HepG2 model than in the HCT116 model [65–68]. These results suggested that the effectiveness of a transfection-competent delivery platform (such as SNALP) is highly dependent on the vascularization network of each tumor, as it can influence the accessibility of the nanoparticles across the tumor mass and consequently, the final therapeutic outcome [65].
Moreover, the vascular network within the same tumor can be highly heterogeneous being the central region often characterized by poor perfusion, which impairs the access of the therapeutic agents to the aggressive cancer cells usually found in hypoxia regions [69]. This vascular dysfunction in the central part of solid tumors, together with the lack of functional lymphatic vessels, results in increased interstitial pressure at this level, which along with a dense extracellular matrix, impairs the penetration of the nanoparticles through the interstitial space, thus compromising a uniform distribution throughout the tumor [67, 70].
Targeting SNALP to non-hepatic solid tumors
An extended blood circulation is mandatory for achieving significant levels of accumulation in non-hepatic solid tumors. As mentioned before, extended blood residence can be achieved by incorporating PEG-derivatized lipids with longer alkyl chain, which have a slower exchangeable rate from the SNALP surface, thus providing an improved shielding in the vascular compartment.
In the work of Judge et al. [59], the therapeutic potential of SNALP encapsulating an siRNA against Polo-like kinase 1 (PLK1) was evaluated. PLK1 is an important mitotic regulator, often found overexpressed in cancer cells of tumors with diverse histological origin [71]. It was shown that two iv administrations per week, during 3 weeks, of anti-PLK1 siRNA-containing SNALP (2 mg siRNA/kg) induced an anti-tumor effect both in orthotopic as well as in subcutaneous (sc) mouse models of hepatic (Hep3B) cancer. However, in order to reach the non-hepatic tumor (sc model), formulation with PEG-S-DSG (18 C) was demanded, instead of the highly exchangeable PEG-S-DMG (14 C) used in the formulation of SNALP targeted to hepatic tumors. The former PEG-derived lipid enables SNALP to take advantage of the enhanced permeability and retention (EPR) effect [72].
The second generation of SNALP: the association of cationic-lipid-like materials–lipidoids
Alnylam Pharmaceuticals, together with researchers from the Massachusetts Institute of Technology, developed extensive libraries of cationic lipid-like materials, termed lipidoids, which can be incorporated into lipid-based nanoparticles such as SNALP. A library of combinatorial epoxide-derived oligoamine-containing lipidoids has recently been developed by Love et al. [73]. These lipidoids were formulated together with cholesterol, DSPC, and a PEG-derived lipid in a similar manner as SNALP. In vivo studies revealed that the C12-200 (Fig. 5) lipidoid-based SNALP was the most potent. C12-200 contains multiple tertiary amines and five alkyl chains with a length of 12 C.
Fig. 5.
Chemical structure of the lipidoid C12-200
Remarkably, a single iv injection in mice of C12-200 lipidoid-based SNALP containing an anti-Factor VII siRNA, at a dose as low as 0.01 mg/kg, induced complete Factor VII downregulation in hepatocytes. It took 20 days for protein to recover to baseline levels. Moreover, after a single injection, specific and simultaneous inhibition of five genes in the liver was also achieved with the C12-200 lipidoid-based formulation, containing a pool of the corresponding five siRNAs, at 1 mg∕kg (total siRNA dose). The potential of this lipidoid was further validated in cynomolgus monkeys, upon targeting the transthyretin gene in liver [73].
The efficiency revealed by the C12-200 lipidoid-based nanoparticles regarding siRNA delivery likely relies on the main mechanism of internalization, macropinocytosis, which avoids lysosomal degradation, a common problem encountered with nanocarriers that enter cells through receptor-mediated endocytosis [48]. Taken together, C12-200 lipidoid and the previously mentioned DLin-KC2-DMA lipid, represent some of the most advanced cationic materials for siRNA delivery. Moreover, these reports highlighted important criteria for the designing of cationic transfection reagents: (1) one or more tertiary amines with a pKa ~7, thus enabling a pH-dependent surface charge and; (2) two or more alkyl chains with a length that may be compromised between 12 and 18 C [74].
RNAi/oligonucleotide nanoparticle delivery (RONDEL)
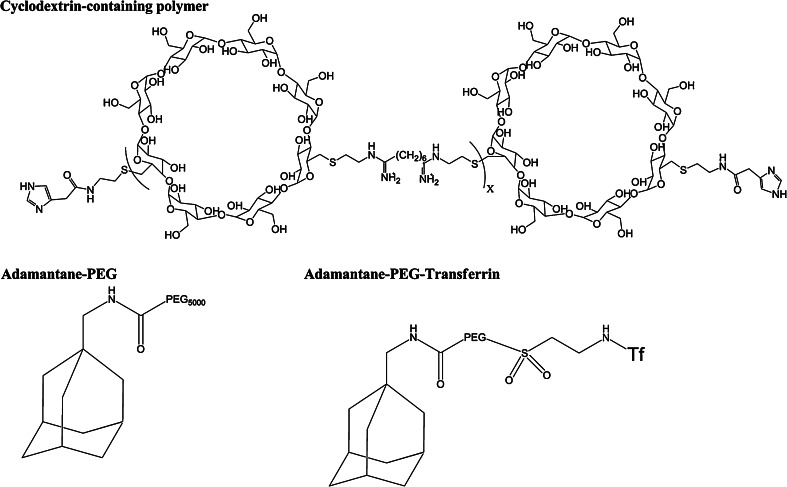
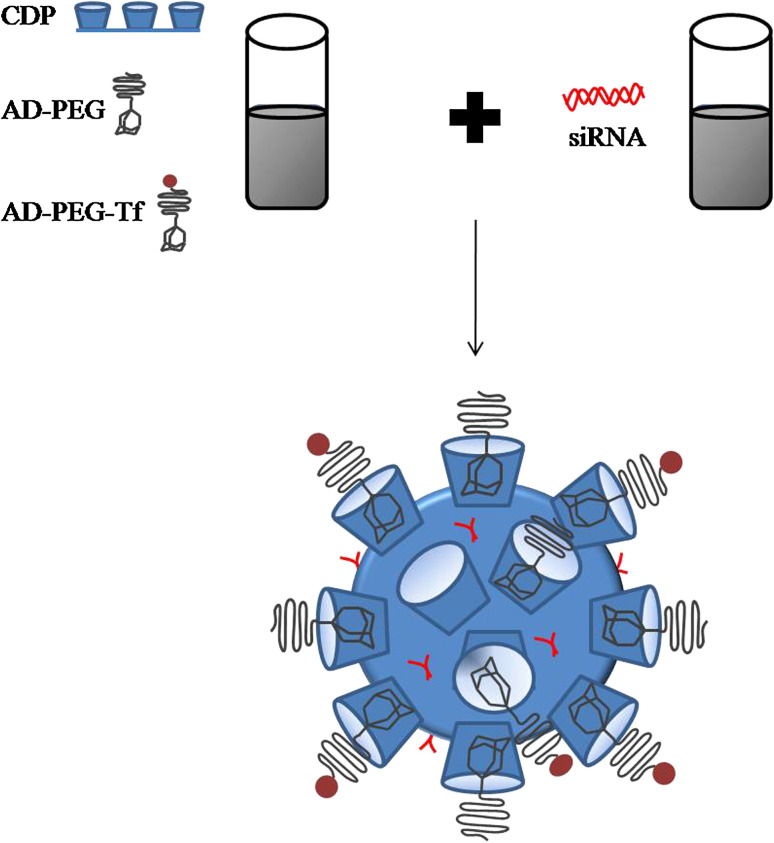
RONDEL nanoparticles are formulated with: (1) cyclodextrin-containing polymer (CDP); (2) PEGylated adamantane (AD-PEG); (3) human transferrin (Tf) as a targeting ligand covalently attached to the extremity of PEG (AD-PEG-Tf) and; (4) a non-modified siRNA (Fig. 6).
Fig. 6.
Chemical structure of RONDEL components
CPD is a linear and water-soluble polymer that contains several β-cyclodextrins (cyclic oligosaccharides). Owing to its positive charge, it self-assembles with the siRNA into nanoparticles, fully protecting the nucleic acid from degradation by serum nucleases [75, 76]. Moreover, CDP contains organic groups that are protonated at pH ~ 6, which, together with the imidazole end groups, mediates an efficient siRNA delivery into the cell cytoplasm [75, 76] (Fig. 6). Adamantane forms an inclusion complex (non-covalent interaction) with the hydrophobic core of β-cyclodextrins whereas PEG is maintained on the nanoparticle surface (Fig. 7). Finally, the presence of Tf at the PEG extremity enhances the internalization by Tf receptor-overexpressed cancer cells (Fig. 7).
Fig. 7.
Components and formulation of RONDEL. The formulation consists of two vials, one with the siRNA and the other with the delivery components (CDP, AD-PEG, and AD-PEG-Tf). When the content from the two vials is mixed, the components self-assemble into RONDEL nanoparticles of approximately 70 nm, where the siRNA is fully encapsulated within the CDP polymer
RONDEL encapsulating a potent siRNA against the ribonucleotide reductase subunit 2 (RRM2) [77], when systemically administered during three consecutive days to mice bearing sc Neuro2A tumors, at a siRNA dose of 2.5 mg/kg, significantly slowed tumor growth. In contrast, the non-targeted counterpart had only minimal effects [78].
In 2008, these Tf-targeted CPD-based nanoparticles containing the anti-RRM2 siRNA entered clinical trials under the designation of CALAA-01 (Table 1). Recently, it was demonstrated that CALAA-01 was also effective in a mouse xenograft sc model of head and neck squamous cell carcinoma derived from Tu212 cells. Systemic administrations of CALAA-01 (at 5 or 10 mg siRNA/kg) on four consecutive days significantly reduced tumor progression by suppressing cell proliferation and inducing apoptosis. At the highest siRNA dose, tumor growth was reduced by an average of 2.9-fold when compared to the control siRNA. Moreover, a significant reduction of RRM2 on the tumors was reported [79].
Table 1.
Clinical trials of siRNA-containing nanoparticles targeting solid tumors following systemic administration
| Name | Nanoparticle | Company | Molecular target(s) | Therapeutic indication | Route of administration | Status | Clinical trial identifier |
|---|---|---|---|---|---|---|---|
| CALAA-01 | Tf-targeting PEGylated cyclodextrin-based NP | Calando Pharmaceuticals | RRM2 (cell proliferation) | Advanced solid tumors | Multiple 30 min iv infusion |
Phase I Ongoing |
NCT00689065 |
| Atu027 | PEGylated lipoplex-siRNA | Silence therapeutics | PKN3 (angiogenesis and metastasis) | Advanced solid tumors | Multiple 4 h iv infusion |
Phase I Completed |
NCT00938574 |
| Atu027 + Gemcitabine | Advanced pancreatic cancer |
Phase I Recruiting |
NCT01808638 | ||||
| SNALP-PLK1 | SNALP | Tekmira Pharmaceuticals Corporation | PLK1 (cell proliferation) | Primary and secondary liver metastasis | Multiple hepatic intra-arterial infusion |
Phase I Completed |
NCT01437007 |
| Solid tumor or lymphomas | Multiple iv infusion | Phase I Recruiting | NCT01262235 | ||||
| ALN-VSP02 | SNALP | Alnylam Pharmaceuticals | KSP (cell proliferation) and VEGF (angiogenesis) | Advanced solid tumor with liver metastasis |
Multiple 15 min iv infusion |
Phase I Completed |
NCT00882180 |
| Patients who have responded to ALN-VS02 in the first clinical trial |
Phase I Completed |
NCT01158079 | |||||
| siRNA-EphA2-DOPC | Non-PEGylated liposomes | M.D. Anderson Cancer Center | EPHA2 (cell proliferation) | Advanced solid tumors |
Multiple 30 min iv-infusion |
Phase I Not yet recruiting |
NCT01591356 |
NP nanoparticle, RRM2 M2 subunit of ribonucleotide reductase, PKN3 protein kinase N3, PLK1 Polo-like kinase 1, KSP kinesin family member 11, VEGF vascular endothelial growth factor, EPHA2 ephrin type-A receptor 2
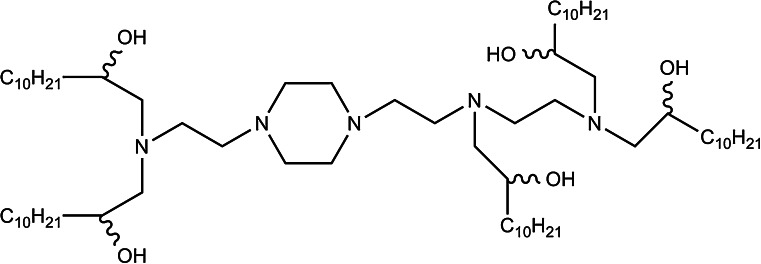
AtuPLEX/Atu027
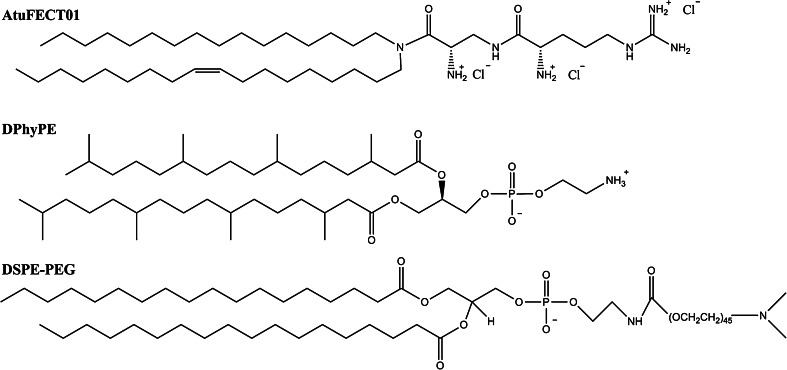
AtuPLEX is a lipid-based siRNA delivery platform, also known as siRNA-lipoplex, for the systemic delivery of siRNAs to endothelial cells. It is formulated with: (1) a biodegradable cationic lipid (β-l-arginyl-2,3-l-diaminopropionic acid-N-palmityl-N-oleyl-amide trihydrochloride, also known as AtuFECT01); (2) a PEGylated lipid [1,2-distearoyl-sn-glycero-3-phospatidylethanolamine-N-PEG (DSPE-PEG)]; (3) a fusogenic lipid (1,2-diphytanoyl-sn-glycero-3-phosphoethanolamine, DPhyPE), and; (4) a 2′-OMe modified siRNA [80] (Fig. 8).
Fig. 8.
Chemical structure of AtuPLEX-forming lipids
In vivo studies demonstrated that AtuPLEX is significantly internalized by the vascular endothelium of liver, heart, and lung [80] as well as by the tumor endothelium of different tumor xenograft models [81]. AtuPLEX complexed with an siRNA targeting the protein kinase N3 (PKN3), a validated anti-angiogenic molecular target, is already in clinical trials with the codename of Atu027. With primary vascular (HUVEC) and lymphatic (HMVEC-LLy) endothelial cells, Atu027 mediated an effective PKN3 silencing, leading to reduced cell migration and impaired tube formation without interfering with the cells’ viability. Multiple iv administrations of Atu027 in mice, rats, and non-human primates, induced strong PKN3 silencing in the lungs and liver tissues and had a moderate effect at the tumor level, without any apparent effect in heart and spleen. The level of PKN3 silencing reflected the vascularization index of the mentioned organs [82].
Interestingly, PKN3 silencing at the endothelium level had a significant impact on the regression of metastases formation in different mouse models. Treatment of a metastatic orthotopic PC3 prostate cancer mouse model with eight iv administrations of Atu027 (2.8 mg siRNA/kg), restrained tumor growth and, more importantly, inhibited the formation of lymph node metastases [82]. Moreover, in two experimental lung metastasis models (established by iv injection of Lewis lung carcinoma cells or melanoma-derived B16V cells) as well as in mouse orthotopic models of breast cancer (MDA-MB-435 and MDA-MB-231) that spontaneously metastasized, it was demonstrated that multiple administrations of Atu027 also prevented the formation of macro and micrometastasis in the lung [83]. Analysis of tumor sections did not reveal any differences neither in the microvascular density nor in the tumor vessel structure [82, 83]. On the other hand, PKN3 silencing was followed, both in vitro and in vivo, by an upregulation of VE-cadherin, which is an adherence junction protein, on the endothelial cell’s membrane. Therefore, it was hypothesized that VE-cadherin, by enhancing cell–cell adhesion, might reduce the vascular leakage and hence the intra and extravasation of metastatic tumor cells, which ultimately prevents the formation of metastasis [83, 84].
Finally, it is also important to mention that treatments with Atu027 did not induce a significant elevation of liver enzymes [aspartate aminotransferase (AST) and alanine aminotransferase (ALT)] or cytokine (IFN-α [81], IL-6, IL-10, and TNF-α) [82], thus indicating that they were not immunostimulatory or hepatotoxic.
siRNA-EphA2-DOPC
Liposomes formulated with the neutral lipid 1,2-dioleoylsn-glycero-3-phosphatidylcholine (DOPC) (Fig. 9) can be used to encapsulate nucleic acids such as oligonucleotides [85] or siRNAs [86] with an encapsulation efficiency ~65 %, which is considerably lower than the one obtained when cationic lipids/polymers are applied.
Fig. 9.
Chemical structure of DOPC
These non-PEGylated liposomes, encapsulating an siRNA against the oncogene ephrin type-A receptor 2 (EphA2), a validated molecular target for ovarian cancer, were successfully tested in orthotopic mouse models of ovarian cancer (HeyA8 or SKOV3), upon iv injection [86]. Surprisingly, they were detected at the tumor level for a time point as short as 1 h, after a single iv administration. Nevertheless, and as expected, a marked accumulation was also found in the liver, spleen, and lungs.
In order to evaluate the therapeutic potential of such strategy, anti-EphA2 siRNA-containing liposomes (0.15 mg siRNA/kg) were intravenously administered twice a week, during 3 weeks. This treatment significantly reduced ovarian tumor growth, although the best results were attained when the administration of anti-EphA2 siRNA-containing liposomes was combined with one intraperitoneal injection of paclitaxel, which mediated a tumor growth inhibition of 67 or 82 % when compared with a control siRNA or paclitaxel alone, respectively [86]. It was further demonstrated that treatment efficacy was similar when intraperitoneal injections were performed [87]. Phase I clinical trials of anti-EphA2 siRNA-containing liposomes are expected to start shortly (Table 1). In the meantime, the successful delivery of two siRNAs (against EPHA2 and focal adhesion kinase, FAK) with the lipid-based nanoparticle herein presented has opened a wide range of therapeutic opportunities [88].
Nanotoxicology
Ideally, any nanocarrier (e.g., liposomes, nanoparticles, etc.) must be non-toxic and non-immunogenic. Their components should be biodegradable and cleared from the body in order to avoid undesirable accumulation that ultimately could lead to adverse effects. When considering an iv administration, several aspects must be taken into account such as the hematocompatibility (e.g., red blood cell lysis, platelet aggregation, cell aggregation), the integrity of the nanoparticle and its payload (e.g., drug leakage and degradation, etc.), and the stimulation of the immune system (e.g., complement activation, cytokines, and antibody production) [89].
Lipid-based nanoparticles for the systemic delivery of siRNA are often formulated with cationic lipids and PEG-derivatized lipids. However, nowadays it is well known that cationic lipids can activate the complement system, which mediates a rapid clearance by the mononuclear phagocytic system and induces the release of pro-inflammatory cytokines and IFN-α [90]. The latter can induce adverse side effects, for instance, hypersensitivity and anaphylaxis reactions [90].
Numerous studies have demonstrated that in a multiple administration schedule, PEGylated liposomes lose their prolonged blood circulation property, while the extent of hepatic accumulation increases [91, 92]. This phenomenon, designated as accelerated blood clearance (ABC), is mediated by the production of anti-PEG IgM in the spleen, following the first administration. In a subsequent administration, PEGylated nanoparticles are opsonized by anti-PEG IgM and C3 protein of the complement system, which favors an unspecific internalization of the liposomes by Kupffer cells [93–96]. The ABC phenomenon can be extensively potentiated when the encapsulated siRNA is also immunostimulatory [97–99]. In addition, PEG is a non-biodegradable polymer, which also limits its safety. When it is used at high doses and/or in chronic administration, it can accumulate within the lysosomes, thus impairing the normal activity of some catabolic lysosomal enzymes. Therefore, it has been suggested that PEG should be used at the lowest molecular weight possible in order to enable renal elimination [89].
Taken together, it is extremely important that all novel nanocarriers undergo an exhaustive characterization of their toxicity profile in relevant animal models, before clinical evaluation. As both the nanocarriers and the nucleic acids can trigger an innate immune response, this is an issue that must be carefully addressed, notwithstanding that the real contribution of each component (siRNA payload versus the nanocarrier) is difficult to evaluate. Indeed, the same nanoparticle with different siRNA sequences might have different immune profiles. Overall, this is a highly relevant topic that has been addressed for some of the lipid-based nanoparticles in clinical trials, as SNALP and RONDEL.
Safety and toxicity of SNALP
Single iv administration of SNALP in mice, encapsulating chemically modified anti-HBV (at 5 mg siRNA/kg) [31, 32] or anti-PLK1 (at 2 mg siRNA/kg) [59] siRNAs, was well tolerated, without the detection of inflammatory cytokines, IFN-α or elevation of liver transaminases (ALT and AST). Similarly, in non-human primate (cynomolgus monkeys), iv administration of SNALP encapsulating a modified anti-APOB siRNA (at 2.5 mg siRNA/kg) presented a similar pattern, notwithstanding a transient elevation of liver transaminases that was fully resolved within 6 days [33].
Extensive studies with SNALP encapsulating two siRNAs, targeting VEGF or KSP genes (ALN-VSP02), showed that rats were more sensitive to SNALP than mice, the maximum tolerated dose being 3 mg/kg and 12 mg siRNA/kg, respectively, upon eight administrations over 2 weeks. In rats, at doses ≥3 mg siRNA/kg, elevation of ALT, AST, and bilirubin were detected as well as liver vacuolation, inflammation, fibrosis, hemorrhage, and necrosis, which altogether indicated strong hepatic injury. Moreover, lymphoid atrophy and necrosis were observed in the spleen, whereas renal injury was revealed by the detection of hematuria and tubular degeneration [100]. The dose that was free of any adverse effect (no observable adverse effect level, NOAEL) was 1 mg siRNA/kg. Similar studies in non-human primates (cynomolgus monkeys) also revealed NOAEL to be 1 mg/kg. At doses ≥3 mg/kg, liver and spleen toxicity was also observed. In addition, it was detected elevation of IL-6 and complement products, which indicated a moderated immunostimulation [100].
Altogether, these reports demonstrated that in respect to SNALP toxicity, the liver is the most sensitive organ, followed by the spleen and kidneys, which also reflects the high levels of SNALP accumulation in those organs.
Safety and toxicity of RONDEL
Following a single administration of Tf-targeted CDP-based nanoparticles containing anti-EWS-FL11 siRNA, at 2.5 mg/kg, in a mouse model of metastatic Ewing’s sarcoma, the levels of AST, ALT, blood urea nitrogen, and creatinine remained unchanged, as determined 2 or 24 h post-injection, thus indicating the absence of hepatic and renal toxicity. At the same time, IL-12 and INF-α were also found unchanged [101]. Surprisingly, even the use of an siRNA sequence containing an immunostimulatory motif has not elicited any immunostimulation [101]. However, it should be pointed out that the chosen time points were not the most suited for cytokines quantification, as they typically peak between 6 and 8 h [39].
In non-human primates (cynomolgus monkeys), RONDEL containing the anti-RRM2 siRNA (CALAA-01), was sequentially administrated at 3, 9, and 27 mg siRNA/kg, with 2–3 days between each dose. Until 9 mg siRNA/kg, CALAA-01 was well tolerated without signs of hepatic or renal toxicity (without elevation of AST, ALT, creatinine or blood urea nitrogen) or immunostimulation (without detection of IL-12, IFN-γ, IL-10, IL-4, IL-6, TNF-α after 6 h of CALAA-01 administration). Moreover, hematological and coagulation parameters (prothrombin time, activated partial thromboplastin time and fibrinogen) remained unchanged [77]. These results were in contrast to the ones obtained following the administration of 27 mg siRNA/kg, which is a dose that is exceptionally high. Interestingly, low titer of anti-Tf antibodies was detected for all the concentrations whereas anti-PEG antibodies were not detected. The absence of anti-PEG antibodies was in accordance with the lack of the ABC phenomenon evaluated upon the last administration at 3 mg siRNA/kg [77].
Taken together, these results demonstrated that Tf-targeted CDP-based nanoparticles encapsulating a non-chemically modified siRNA, even in multi-administration regimens, were well tolerated in mice and non-human primates.
Targeted siRNA delivery to cancer cells and the tumor microenvironment
One promising strategy to enhancing the likelihood of reaching solid tumors localized in organs other than the liver, aiming at improving efficacy while decreasing the side effects as the previously addressed, relies on the attachment at the surface of the nanocarriers of internalizing ligands, specifically targeting antigens or receptors overexpressed on the surface of cancer cells and/or other cells of the tumor microenvironment, such as the endothelial cells from angiogenic blood vessels. Different targeting ligands such as antibodies or fragments thereof, proteins, and peptides, have been explored.
Targeting cancer cells
The use of peptides as the targeting moiety is attractive because of their small size, low immunogenicity, high stability, and ease of manufacturing [102]. Moreover, the screening of random peptide phage-display libraries has enabled the identification of numerous peptides that bind specifically to receptors overexpressed on cancer cells as well as on other cells of the tumor microenvironment. In the work of Santos et al. [103], the antagonist G peptide (NH2-Arg-d-Trp-NmePhe-d-Trp-Leu-Met-CONH2) was coupled to the surface of SNALP-derived liposomes, containing an anti-BCL2 siRNA. The antagonist G peptide targets the large family of G-protein-coupled receptors, which are crucial players in tumor growth and metastization, being found overexpressed in different cancers [104]. In vitro studies showed that the extent of internalization of antagonist G-targeted liposomes, by lung cancer cell lines (A549, H69 and SW2), was fourfold to tenfold higher than the one attained with the non-targeted liposomes. However, this improved cellular internalization has not enabled BCL2 silencing, which was probably explained by the high content of PEG (10 mol % relative to total lipid) [103] that is known to significantly impair siRNA endosomal escape [105].
Benzamide derivates have also been used to target cancer cells, and particularly anisamide, which targets sigma receptor-overexpressing tumors. Li et al. [106] developed a liposome-polycation-DNA (LPD) complex that contains a non-modified siRNA, mixed with a complex of DNA (calf thymus DNA) and protamine, which forms a condensed core that is further coated with cationic liposomes formulated with DOTAP/CHOL and DSPE-PEG conjugated to anisamide.
In a mouse model of non-small cell lung cancer (NCI-H460), a marked tumor accumulation for a time point as short as 4 h was observed. Three injections of LPD complexed with an siRNA against epidermal growth factor receptor (EGFR) (1.2 mg siRNA/kg) showed significant inhibition of tumor growth. Interestingly, in a lung metastatic model of melanoma (B16F10), the same targeted-LPD incorporating a mixture of three siRNAs against validated anti-tumor targets (MDM2, C-MYC, and VEGF), induced specific downregulation of the three oncogenes at the metastatic nodules. This effect was correlated with 70–80 % lung metastasis reduction, while free siRNAs or the non-targeted counterpart had a residual effect [107]. Moreover, upon changing the targeting moiety, LPD could be used to target other tumors such as breast cancer [108] and more recently, hepatocellular carcinoma [109].
Natural ligands, such as folate and transferrin, have been broadly explored in liposomes [14, 110–115] and other nanocarriers [101, 116, 117], owing to the overexpression of their receptors on the surface of cancer cells. Nonetheless, the use of these ligands has some limitations such as: (1) their receptors are also expressed on normal cells, which can mediate an unspecific accumulation in healthy tissues and; (2) the natural occurrence of these ligands in the blood may compete with the targeted nanocarriers for the specific receptors [118, 119]. Despite these limitations, different successful reports have been published.
Folate-targeted SNALP-derived liposomes containing an anti-MYCN siRNA, administrated in five consecutive days to LA-N-5-derived sc tumor-bearing mice, resulted in 50 % of MYCN mRNA downregulation at the tumor level [114]. In a bone marrow and bone metastasis xenograft mouse model of LA-N-5 neuroblastoma cells, it was demonstrated that 8 h after iv administration, the anti-MYCN siRNA delivered by folate-targeted nanoparticles, also co-localized with the bone metastasis. Treatment during five consecutive days, at 3 mg/kg of anti-MYCN siRNA, resulted in approximately 50 % of MYCN downregulation at the metastasis level, which was correlated with a significant increase of LA-N-5 apoptotic cells [120].
Application of Tf-targeted SNALP-derived liposomes developed by Mendonca et al. [14, 110], in two leukemia cells lines (K562 and LAMA-84), resulted in enhanced cellular internalization. Although these liposomes contained 8 mol% of PEG (relative to total lipid), the improved cellular internalization (and in contrast with the work of Santos et al. [103]) has mediated an effective silencing of the BCR-ABL oncogene, which was further correlated with a strong decrease in the cell viability. Indeed, this work demonstrated that Tf not only favors cellular internalization but also contributed to the siRNA endosomal escape, owing to its fusogenic properties [121].
Strikingly, Bartlett et al. [122] demonstrated in an sc mouse model of Neuro2A-Luc cells that both Tf-targeted and non-targeted CDP-based nanoparticles (RONDEL), upon iv injection, exhibited similar biodistribution and tumor accumulation, for a 24 h time point. Nonetheless, Tf-targeted nanoparticles containing anti-Luciferase siRNA were twofold more effective at reducing luciferase expression at the tumor level than the non-targeted counterpart. These results demonstrated that tumor accumulation of both targeted and non-targeted nanoparticles was highly dependent on the EPR effect [72, 123, 124], which is highly influenced by the tumor pathophysiological properties rather than by the presence of a moiety targeting cancer cells. However, at the tumor level, the presence of transferrin was crucial to enabling the improved gene silencing observed with the ligand-targeted nanoparticles.
Targeting angiogenesis
As endothelial cells from tumor blood vessels are more accessible than cancer cells to any nanoparticle injected in the vascular compartment, and being aware of the importance of angiogenesis for tumor growth and metastasis formation, several anti-cancer therapies targeting angiogenesis have been proposed. Additional advantages are found in targeting the tumor vasculature: (1) selectivity of the treatment against proliferative tumor endothelial cells; (2) minimal toxicity, as angiogenesis in the adult is limited to wound healing, ovulation and pregnancy; (4) low mutation rate within the vasculature; (5) independence of cancer-cell resistance mechanisms and; (6) different solid tumors and leukemia are dependent on angiogenesis for their survival [125, 126].
Different molecules overexpressed on endothelial cells have been explored for targeted drug delivery including integrins, cadherins, selectins, aminopeptidases, glycoproteins, angiotensin-converting enzyme, growth factor receptors (such as VEGR, FGFR, PDGFR), among others. This issue has been detailed elsewhere [127–129].
RGD (cyclic Arg–Gly–Asp) and NGR (Asp–Gly–Arg) peptides are some of the most widely used ligands to target the tumor endothelium. The first is specifically recognized and internalized by αvβ3-integrins while the latter targets aminopeptidase N (CD13). LPD-derived nanoparticles modified with the RGD peptide and containing a siRNA against the VEGF receptor 2 (VEGFR2) were internalized by HUVEC and H5V endothelial cells leading to VEGFR2 silencing. Nevertheless, this study lacks in vivo studies to validate the real anti-angiogenic activity of these RGD-targeted LPD nanoparticles.
Chen et al. [130] have also used LPD nanoparticles for the systemic delivery of siRNA to solid tumors by taking advantage of the targeting properties of the NGR peptide. In vitro studies demonstrated that this NGR-targeted LPD, containing an anti-CMYC siRNA, was efficiently internalized by human fibrosarcoma cells (HT-1080) and endothelial cells (HUVEC), leading to an efficient CMYC silencing. In vivo studies with the HT-1080 sc mouse model showed that 4 h after iv administration, the siRNA was already localized in the cancer cell’s cytoplasm although the tumor distribution was heterogeneous. One injection per day, during three consecutive days, of anti-CMYC siRNA (1.2 mg/kg) delivered by the NGR-LPD almost totally silenced CMYC expression, which was correlated with apoptosis and partial tumor growth inhibition. Significant improvements were further achieved upon co-encapsulation of anti-CMYC siRNA and doxorubicin, in the same NGR-targeted LPD nanoparticles [130]. Treatment with free doxorubicin, at a dose of 0.3 mg/kg, had no therapeutic effect, whereas the simultaneous administration of free doxorubicin and NGR-targeted LPD containing the anti-CMYC siRNA resulted in a similar tumor growth inhibition as the single treatment with NGR-targeted LPD containing the anti-CMYC siRNA. The most pronounced tumor growth inhibition was observed when doxorubicin and anti-CMYC siRNA were co-formulated in the same NGR-targeted nanoparticle [130]. However, an additional control group treated with the nanoparticle containing only doxorubicin would have been important to clarify the real benefit of co-encapsulating anti-CMYC siRNA and doxorubicin in the same NGR-targeted nanoparticle.
Dual targeting: cancer and endothelial cells
Our group has been working with the F3 peptide (Lys–Asp–Glu–Pro–Gln–Arg–Arg–Ser–Ala–Arg–Leu–Ser–Ala–Lys–Pro–Ala–Pro–Pro–Lys–Pro–Glu–Pro–Lys–Pro–Lys–Lys–Ala–Pro–Ala–Lys–Lys), which has the major advantage of simultaneously targeting two cell sub-populations of the tumor microenvironment: cancer cells and endothelial cells [131]. This peptide is internalized by nucleolin, a receptor overexpressed on the above-mentioned tumor cells’ populations [132]. We have developed two novel formulations of PEGylated F3-targeted liposomes, either non-pH-sensitive [133] or pH-sensitive [134], which presented a high extent of internalization by both cancer cells (MDA-MB-435S, MDA-MB-231, PC3) and endothelial cells from angiogenic blood vessels (HMEC-1), but not by non-transformed cells (BJ fibroblasts). In contrast, the non-targeted counterpart was not internalized by the mentioned cells. It was demonstrated that both F3-targeted liposomes, either non-pH- or pH-sensitive, induced gene silencing. The pH-sensitive liposomes were much more effective than the non-pH-sensitive counterpart, which clearly demonstrated the importance of an efficient siRNA escape from the endocytic pathway [133, 134]. Moreover, the F3-targeted pH-sensitive liposomes were biocompatible (did not induce red blood cell lysis) and stable in blood (siRNA leakage lower than 10 % over 24 h) and did not cause toxicity or an immunogenic response upon two iv administrations per week, during 3 weeks (at 2.5 mg siRNA/kg) [134]. Finally, F3-targeted pH-sensitive liposomes containing anti-PLK1 siRNA significantly reduced cell viability of both cancer cells and endothelial cells and enhanced the sensitivity of cancer cells towards paclitaxel [135]. With the aim of assessing the real potential of the developed F3-targeted strategy as an effective siRNA delivery platform for cancer treatment, several in vivo studies (biodistribution and gene silencing activity) will be performed. A rapid and specific tumor accumulation is expected. In fact, the utility of the F3 peptide, as a ligand, to target and enhanced tumor accumulation of doxorubicin-containing liposomes was already confirmed by our group [136]. Moura et al. [136] demonstrated that these liposomes were detected, in an MDA-MB-435S-derived tumor implanted in the mammary gland, for a time point as short as 4 h. This active tumor accumulation was correlated with a significant reduction of the vessel density and viable rim area (along with extensive necrosis at this level) and accompanied by a lack of tumor invasion into adjacent healthy tissues. In contrast, with the other tested control samples, including the non-targeted counterpart or targeted non-pH-sensitive liposomes, tumor invasion was evident [136]. This work also highlighted that improved tumor accumulation can be attained when nanocarriers are endowed with a vascular targeting component, which is in contrast to those strategies targeting cancer cells exclusively. In the latter, tumor accumulation is mostly dependent on the EPR effect.
Clinical trials
Despite all the obstacles found during the development of novel nanocarriers for the systemic delivery of siRNAs, there are examples of proven therapeutic efficacy (and safety) in different models of murine cancer, as mentioned in previous sections. However, the number of siRNA-containing nanocarriers that have reached clinical trials is still limited. On the US National Institute’s of Health website (http://www.clinicaltrials.gov), it is possible to identify four siRNA-containing PEGylated nanoparticles (CALAA-01, Atu027, SNALP-PLK1, and ALN-VSP02) and one non-PEGylated nanoparticle (siRNA-EphA2-DOPC) for the systemic administration of siRNA: all the listed examples are phase I studies (Table 1).
These clinical trials are open-label and dose-escalation studies designed to study the safety and toxicity, with the determination of dose-limiting toxicities and maximum tolerated dose, of single or repeated administrations in patients with advanced solid tumors. Additional aims include characterization of the pharmacokinetics, preliminary evidence of efficacy by evaluating tumor response, and finding a recommended dose for future clinical studies. The results available will be then briefly described.
CALAA-01
The results obtained with CALAA-01, in what respects tumor localization and RNAi-mediated effect, in three patients with solid cancers that were refractory to standard therapies, were recently published [116]. CALAA-01 was administrated on days 1, 3, 8, and 10 of a 21-day cycle, via a 30-min iv infusion, at an siRNA dose of 18, 24, or 30 mg/m2. The results revealed a dose-dependent and heterogeneous accumulation in the tumor tissue (but not in the adjacent epidermis), which was further correlated with RRM2 silencing, both at mRNA and protein levels. Moreover, the predicted mRNA fragments were also detected in the patient receiving the highest dose, evidencing the RNAi mechanism in humans for the first time [116]. In general, CALAA-01 was well tolerated for all the tested doses. The most common adverse effects were grade 1–2 fatigue, fever/chills, and gastrointestinal symptoms. At the highest tested dose, a transient increase in IL-6 and TNF-α was detected [100]. This clinical trial is still ongoing (Table 1).
Atu027
Atu027 was recently tested in 24 patients with advanced solid tumors. Patients were submitted to a 4 h iv infusion of Atu027, at different doses up to 0.18 mg/kg. After 3 weeks, Atu027 was re-administered, twice per week, over four additional weeks. In general, Atu027 was well tolerated. The main adverse effects were: grade 1 fatigue (seven patients) across all the tested doses, grade 1 hair loss (two patients), grade 2 sweating (one patient), grade 2 abdominal pain (one patient), grade 3 diarrhea (one patient) and grade 3 lipase elevation (one patient). No cytokine (TNF-α, IL-1β, IFN-γ, IL-6) elevation was detected. One patient with neuroendocrine cancer had disease stabilization for 9 months and another with the same type of cancer presented partial regression of lung metastasis [100, 137]. Based on the results from the phase I study, Silence Therapeutics is now recruiting patients with locally advanced or metastatic pancreatic adenocarcinoma to evaluate the combination of Atu027 with gemcitabine, which is a chemotherapeutic drug highly used in the treatment of pancreatic cancer (Table 1).
SNALP-PLK1
In December 2010, Tekmira initiated a phase I clinical trial with SNALP-PLK1. In this study, SNALP-PLK1 was administered by hepatic artery infusion in 21 patients with primary liver cancer or liver metastasis, the results being available at http://www.tekmirapharm.com. SNALP-PLK1 was administered once a week for 3 weeks at doses ranging from 0.15 to 0.90 mg/kg. One patient reached stable disease following 18 additional doses at 0.6 mg/kg over 6 months. Another patient presented partial response, having received 15 additional doses at 0.6 mg/kg over 5 months. The results showed that SNALP-PLK1 was generally well tolerated.
A new phase I clinical trial is planned where SNALP-PLK1 will be administered by iv infusion (Table 1).
ALN-VSP02
ALN-VSP02 was tested in 41 patients with advanced solid tumors with at least one liver lesion. Treatments were performed twice per week at doses ranging from 0.1 to 1.5 mg/kg. In general, treatments were well tolerated, without neither hepatic toxicity nor elevation of complement proteins. Elevation of IL-1, IL-10, IL-6, and G-CSF was detected for the highest doses, but it was resolved within 24 h following administration [100].
siRNA-EphA2-DOPC
M.D. Anderson Cancer Center will initiate a phase I study with siRNA-EphA2-DOPC, which will be administered by iv infusion twice a week during 3 weeks, at an initial dose of 0.45 mg/m2, in patients with advanced and recurrent solid tumors. Data from this study will expand our knowledge on the use of non-PEGylated liposomes for the systemic delivery of siRNAs in humans.
Altogether, these studies have demonstrated that the developed siRNA nanocarriers that are now in clinical trials are tolerable in humans, even upon multiple administrations, and have evidenced anti-tumor effects.
Conclusions
In the last decades, several attempts have been made to establish siRNA as a therapeutic strategy owing to its ability to interfere with the genetic basis of human diseases associated with the overproduction of proteins, such as cancer. The major drawback in the translation of these molecules into the clinic is undoubtedly the lack of a safe and efficient nanocarrier with the ability to deliver the encapsulated siRNAs at a therapeutic concentration into the cytoplasm of the target cells while avoiding healthy tissues. Nevertheless, remarkable achievements have been attained in the last years by the design of novel cationic lipids/polymers with high transfection ability. Such improvements gave rise to a few nanoparticles that might enable the clinical use of siRNAs, at least in liver-associated tumors.
From ongoing clinical trials, encouraging signs regarding the safety of siRNA-containing nanoparticles have emerged, even when repeatedly used. However, relevant and consistent information on the real therapeutic potential of those strategies in a cancer patient is still to be provided. It does not sound very plausible that cancer can be cured solely with an siRNA-based medicine. However, strong expectations rely on the rationale that specific tumor accumulation of siRNAs targeting the molecular and cellular specificities of a cancer will significantly contribute to increase life expectancy of cancer patients/survivors.
Acknowledgments
The authors would like to acknowledge Nuno Fonseca for his help with Fig. 1. The work performed by the authors was supported by the Portugal–Spain capacitation program in Nanoscience and Nanotechnology (ref.: NANO/NMed-AT/0042/2007) and grant PEst-C/SAU/LA0001/2011.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics. CA Cancer J Clin. 2012;62(1):10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 3.Luo J, Solimini NL, Elledge SJ. Principles of cancer therapy: oncogene and non-oncogene addiction. Cell. 2009;136(5):823–837. doi: 10.1016/j.cell.2009.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farazi TA, Spitzer JI, Morozov P, Tuschl T. miRNAs in human cancer. J Pathol. 2011;223(2):102–115. doi: 10.1002/path.2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siolas D, Lerner C, Burchard J, Ge W, Linsley PS, Paddison PJ, Hannon GJ, Cleary MA. Synthetic shRNAs as potent RNAi triggers. Nat Biotechnol. 2005;23(2):227–231. doi: 10.1038/nbt1052. [DOI] [PubMed] [Google Scholar]
- 6.Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411(6836):494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- 7.Aagaard L, Rossi JJ. RNAi therapeutics: principles, prospects and challenges. Adv Drug Deliv Rev. 2007;59(2–3):75–86. doi: 10.1016/j.addr.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Fougerolles A, Vornlocher HP, Maraganore J, Lieberman J. Interfering with disease: a progress report on siRNA-based therapeutics. Nat Rev Drug Discov. 2007;6(6):443–453. doi: 10.1038/nrd2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhindi R, Fahmy RG, Lowe HC, Chesterman CN, Dass CR, Cairns MJ, Saravolac EG, Sun LQ, Khachigian LM. Brothers in arms: DNA enzymes, short interfering RNA, and the emerging wave of small-molecule nucleic acid-based gene-silencing strategies. Am J Pathol. 2007;171(4):1079–1088. doi: 10.2353/ajpath.2007.070120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vaishnaw AK, Gollob J, Gamba-Vitalo C, Hutabarat R, Sah D, Meyers R, de Fougerolles T, Maraganore J. A status report on RNAi therapeutics. Silence. 2010;1(1):14. doi: 10.1186/1758-907X-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jackson AL, Bartz SR, Schelter J, Kobayashi SV, Burchard J, Mao M, Li B, Cavet G, Linsley PS. Expression profiling reveals off-target gene regulation by RNAi. Nat Biotechnol. 2003;21(6):635–637. doi: 10.1038/nbt831. [DOI] [PubMed] [Google Scholar]
- 12.Tschuch C, Schulz A, Pscherer A, Werft W, Benner A, Hotz-Wagenblatt A, Barrionuevo LS, Lichter P, Mertens D. Off-target effects of siRNA specific for GFP. BMC Mol Biol. 2008;9:60. doi: 10.1186/1471-2199-9-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fedorov Y, Anderson EM, Birmingham A, Reynolds A, Karpilow J, Robinson K, Leake D, Marshall WS, Khvorova A. Off-target effects by siRNA can induce toxic phenotype. RNA. 2006;12(7):1188–1196. doi: 10.1261/rna.28106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mendonca LS, Firmino F, Moreira JN, Pedroso de Lima MC, Simoes S. Transferrin receptor-targeted liposomes encapsulating anti-BCR-ABL siRNA or asODN for chronic myeloid leukemia treatment. Bioconjug Chem. 2010;21(1):157–168. doi: 10.1021/bc9004365. [DOI] [PubMed] [Google Scholar]
- 15.Hornung V, Guenthner-Biller M, Bourquin C, Ablasser A, Schlee M, Uematsu S, Noronha A, Manoharan M, Akira S, de Fougerolles A, Endres S, Hartmann G. Sequence-specific potent induction of IFN-alpha by short interfering RNA in plasmacytoid dendritic cells through TLR7. Nat Med. 2005;11(3):263–270. doi: 10.1038/nm1191. [DOI] [PubMed] [Google Scholar]
- 16.Sioud M. Deciphering the code of innate immunity recognition of siRNAs. Methods Mol Biol. 2009;487:41–59. doi: 10.1007/978-1-60327-547-7_2. [DOI] [PubMed] [Google Scholar]
- 17.Judge AD, Sood V, Shaw JR, Fang D, McClintock K, MacLachlan I. Sequence-dependent stimulation of the mammalian innate immune response by synthetic siRNA. Nat Biotechnol. 2005;23(4):457–462. doi: 10.1038/nbt1081. [DOI] [PubMed] [Google Scholar]
- 18.Sioud M. Induction of inflammatory cytokines and interferon responses by double-stranded and single-stranded siRNAs is sequence-dependent and requires endosomal localization. J Mol Biol. 2005;348(5):1079–1090. doi: 10.1016/j.jmb.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 19.Sioud M. Single-stranded small interfering RNA are more immunostimulatory than their double-stranded counterparts: a central role for 2′-hydroxyl uridines in immune responses. Eur J Immunol. 2006;36(5):1222–1230. doi: 10.1002/eji.200535708. [DOI] [PubMed] [Google Scholar]
- 20.Sledz CA, Holko M, de Veer MJ, Silverman RH, Williams BR. Activation of the interferon system by short-interfering RNAs. Nat Cell Biol. 2003;5(9):834–839. doi: 10.1038/ncb1038. [DOI] [PubMed] [Google Scholar]
- 21.Bridge AJ, Pebernard S, Ducraux A, Nicoulaz AL, Iggo R. Induction of an interferon response by RNAi vectors in mammalian cells. Nat Genet. 2003;34(3):263–264. doi: 10.1038/ng1173. [DOI] [PubMed] [Google Scholar]
- 22.Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S, Lipford G, Wagner H, Bauer S. Species-specific recognition of single-stranded RNA via Toll-like receptor 7 and 8. Science. 2004;303(5663):1526–1529. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- 23.Kleinman ME, Yamada K, Takeda A, Chandrasekaran V, Nozaki M, Baffi JZ, Albuquerque RJ, Yamasaki S, Itaya M, Pan Y, Appukuttan B, Gibbs D, Yang Z, Kariko K, Ambati BK, Wilgus TA, DiPietro LA, Sakurai E, Zhang K, Smith JR, Taylor EW, Ambati J. Sequence- and target-independent angiogenesis suppression by siRNA via TLR3. Nature. 2008;452(7187):591–597. doi: 10.1038/nature06765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cho WG, Albuquerque RJ, Kleinman ME, Tarallo V, Greco A, Nozaki M, Green MG, Baffi JZ, Ambati BK, De Falco M, Alexander JS, Brunetti A, De Falco S, Ambati J. Small interfering RNA-induced TLR3 activation inhibits blood and lymphatic vessel growth. Proc Natl Acad Sci USA. 2009;106(17):7137–7142. doi: 10.1073/pnas.0812317106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paone A, Galli R, Gabellini C, Lukashev D, Starace D, Gorlach A, De Cesaris P, Ziparo E, Del Bufalo D, Sitkovsky MV, Filippini A, Riccioli A. Toll-like receptor 3 regulates angiogenesis and apoptosis in prostate cancer cell lines through hypoxia-inducible factor 1 alpha. Neoplasia. 2010;12(7):539–549. doi: 10.1593/neo.92106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Judge A, MacLachlan I. Overcoming the innate immune response to small interfering RNA. Hum Gene Ther. 2008;19(2):111–124. doi: 10.1089/hum.2007.179. [DOI] [PubMed] [Google Scholar]
- 27.Moreira JN, Santos A, Moura V, Pedroso de Lima MC, Simoes S. Non-viral lipid-based nanoparticles for targeted cancer systemic gene silencing. J Nanosci Nanotechnol. 2008;8(5):2187–2204. doi: 10.1166/jnn.2008.319. [DOI] [PubMed] [Google Scholar]
- 28.Watts JK, Deleavey GF, Damha MJ. Chemically modified siRNA: tools and applications. Drug Discov Today. 2008;13(19–20):842–855. doi: 10.1016/j.drudis.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 29.Jackson AL, Burchard J, Leake D, Reynolds A, Schelter J, Guo J, Johnson JM, Lim L, Karpilow J, Nichols K, Marshall W, Khvorova A, Linsley PS. Position-specific chemical modification of siRNAs reduces “off-target” transcript silencing. RNA. 2006;12(7):1197–1205. doi: 10.1261/rna.30706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chiu YL, Rana TM. siRNA function in RNAi: a chemical modification analysis. RNA. 2003;9(9):1034–1048. doi: 10.1261/rna.5103703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morrissey DV, Lockridge JA, Shaw L, Blanchard K, Jensen K, Breen W, Hartsough K, Machemer L, Radka S, Jadhav V, Vaish N, Zinnen S, Vargeese C, Bowman K, Shaffer CS, Jeffs LB, Judge A, MacLachlan I, Polisky B. Potent and persistent in vivo anti-HBV activity of chemically modified siRNAs. Nat Biotechnol. 2005;23(8):1002–1007. doi: 10.1038/nbt1122. [DOI] [PubMed] [Google Scholar]
- 32.Morrissey DV, Blanchard K, Shaw L, Jensen K, Lockridge JA, Dickinson B, McSwiggen JA, Vargeese C, Bowman K, Shaffer CS, Polisky BA, Zinnen S. Activity of stabilized short interfering RNA in a mouse model of hepatitis B virus replication. Hepatology. 2005;41(6):1349–1356. doi: 10.1002/hep.20702. [DOI] [PubMed] [Google Scholar]
- 33.Zimmermann TS, Lee AC, Akinc A, Bramlage B, Bumcrot D, Fedoruk MN, Harborth J, Heyes JA, Jeffs LB, John M, Judge AD, Lam K, McClintock K, Nechev LV, Palmer LR, Racie T, Rohl I, Seiffert S, Shanmugam S, Sood V, Soutschek J, Toudjarska I, Wheat AJ, Yaworski E, Zedalis W, Koteliansky V, Manoharan M, Vornlocher HP, MacLachlan I. RNAi-mediated gene silencing in non-human primates. Nature. 2006;441(7089):111–114. doi: 10.1038/nature04688. [DOI] [PubMed] [Google Scholar]
- 34.Judge AD, Bola G, Lee AC, MacLachlan I. Design of noninflammatory synthetic siRNA mediating potent gene silencing in vivo. Mol Ther. 2006;13(3):494–505. doi: 10.1016/j.ymthe.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 35.Diebold SS, Massacrier C, Akira S, Paturel C, Morel Y, Reis e Sousa C. Nucleic acid agonists for Toll-like receptor 7 are defined by the presence of uridine ribonucleotides. Eur J Immunol. 2006;36(12):3256–3267. doi: 10.1002/eji.200636617. [DOI] [PubMed] [Google Scholar]
- 36.Sioud M, Furset G, Cekaite L. Suppression of immunostimulatory siRNA-driven innate immune activation by 2′-modified RNAs. Biochem Biophys Res Commun. 2007;361(1):122–126. doi: 10.1016/j.bbrc.2007.06.177. [DOI] [PubMed] [Google Scholar]
- 37.Robbins M, Judge A, Liang L, McClintock K, Yaworski E, MacLachlan I. 2′-O-methyl-modified RNAs act as TLR7 antagonists. Mol Ther. 2007;15(9):1663–1669. doi: 10.1038/sj.mt.6300240. [DOI] [PubMed] [Google Scholar]
- 38.Hamm S, Latz E, Hangel D, Muller T, Yu P, Golenbock D, Sparwasser T, Wagner H, Bauer S. Alternating 2′-O-ribose methylation is a universal approach for generating non-stimulatory siRNA by acting as TLR7 antagonist. Immunobiology. 2010;215(7):559–569. doi: 10.1016/j.imbio.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 39.Robbins M, Judge A, Ambegia E, Choi C, Yaworski E, Palmer L, McClintock K, MacLachlan I. Misinterpreting the therapeutic effects of small interfering RNA caused by immune stimulation. Hum Gene Ther. 2008;19(10):991–999. doi: 10.1089/hum.2008.131. [DOI] [PubMed] [Google Scholar]
- 40.Ge Q, Filip L, Bai A, Nguyen T, Eisen HN, Chen J. Inhibition of influenza virus production in virus-infected mice by RNA interference. Proc Natl Acad Sci USA. 2004;101(23):8676–8681. doi: 10.1073/pnas.0402486101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tompkins SM, Lo CY, Tumpey TM, Epstein SL. Protection against lethal influenza virus challenge by RNA interference in vivo. Proc Natl Acad Sci USA. 2004;101(23):8682–8686. doi: 10.1073/pnas.0402630101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Furset G, Sioud M. Design of bifunctional siRNAs: combining immunostimulation and gene-silencing in one single siRNA molecule. Biochem Biophys Res Commun. 2007;352(3):642–649. doi: 10.1016/j.bbrc.2006.11.059. [DOI] [PubMed] [Google Scholar]
- 43.Iversen PO, Semaeva E, Sorensen DR, Wiig H, Sioud M. Dendritic cells loaded with tumor antigens and a dual immunostimulatory and anti-interleukin 10-specific small interference RNA prime t lymphocytes against leukemic cells. Transl Oncol. 2009;2(4):242–246. doi: 10.1593/tlo.09154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khairuddin N, Gantier MP, Blake SJ, Wu SY, Behlke MA, Williams BR, McMillan NA. siRNA-induced immunostimulation through TLR7 promotes antitumoral activity against HPV-driven tumors in vivo. Immunol Cell Biol. 2011 doi: 10.1038/icb.2011.19. [DOI] [PubMed] [Google Scholar]
- 45.Lu PY, Woodle MC. Delivering small interfering RNA for novel therapeutics. Methods Mol Biol. 2008;437:93–107. doi: 10.1007/978-1-59745-210-6_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Castanotto D, Rossi JJ. The promises and pitfalls of RNA-interference-based therapeutics. Nature. 2009;457(7228):426–433. doi: 10.1038/nature07758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bumcrot D, Manoharan M, Koteliansky V, Sah DW. RNAi therapeutics: a potential new class of pharmaceutical drugs. Nat Chem Biol. 2006;2(12):711–719. doi: 10.1038/nchembio839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gomes-da-Silva LC, Fonseca NA, Moura V, Pedroso de Lima MC, Simoes S, Moreira JN. Lipid-based nanoparticles for siRNA delivery in cancer therapy: paradigms and challenges. Acc Chem Res. 2012;45(7):1163–1171. doi: 10.1021/ar300048p. [DOI] [PubMed] [Google Scholar]
- 49.Rossi JJ. Expression strategies for short hairpin RNA interference triggers. Hum Gene Ther. 2008;19(4):313–317. doi: 10.1089/hum.2008.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim DH, Rossi JJ. Strategies for silencing human disease using RNA interference. Nat Rev Genet. 2007;8(3):173–184. doi: 10.1038/nrg2006. [DOI] [PubMed] [Google Scholar]
- 51.Knop K, Hoogenboom R, Fischer D, Schubert US. Poly(ethylene glycol) in drug delivery: pros and cons as well as potential alternatives. Angew Chem Int Ed Engl. 2010;49(36):6288–6308. doi: 10.1002/anie.200902672. [DOI] [PubMed] [Google Scholar]
- 52.Heyes J, Palmer L, Bremner K, MacLachlan I. Cationic lipid saturation influences intracellular delivery of encapsulated nucleic acids. J Control Release. 2005;107(2):276–287. doi: 10.1016/j.jconrel.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 53.Semple SC, Akinc A, Chen J, Sandhu AP, Mui BL, Cho CK, Sah DW, Stebbing D, Crosley EJ, Yaworski E, Hafez IM, Dorkin JR, Qin J, Lam K, Rajeev KG, Wong KF, Jeffs LB, Nechev L, Eisenhardt ML, Jayaraman M, Kazem M, Maier MA, Srinivasulu M, Weinstein MJ, Chen Q, Alvarez R, Barros SA, De S, Klimuk SK, Borland T, Kosovrasti V, Cantley WL, Tam YK, Manoharan M, Ciufolini MA, Tracy MA, de Fougerolles A, MacLachlan I, Cullis PR, Madden TD, Hope MJ. Rational design of cationic lipids for siRNA delivery. Nat Biotechnol. 2010;28(2):172–176. doi: 10.1038/nbt.1602. [DOI] [PubMed] [Google Scholar]
- 54.Jeffs LB, Palmer LR, Ambegia EG, Giesbrecht C, Ewanick S, MacLachlan I. A scalable, extrusion-free method for efficient liposomal encapsulation of plasmid DNA. Pharm Res. 2005;22(3):362–372. doi: 10.1007/s11095-004-1873-z. [DOI] [PubMed] [Google Scholar]
- 55.Geisbert TW, Hensley LE, Kagan E, Yu EZ, Geisbert JB, Daddario-DiCaprio K, Fritz EA, Jahrling PB, McClintock K, Phelps JR, Lee AC, Judge A, Jeffs LB, MacLachlan I. Postexposure protection of guinea pigs against a lethal Ebola virus challenge is conferred by RNA interference. J Infect Dis. 2006;193(12):1650–1657. doi: 10.1086/504267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Semple SC, Klimuk SK, Harasym TO, Dos Santos N, Ansell SM, Wong KF, Maurer N, Stark H, Cullis PR, Hope MJ, Scherrer P. Efficient encapsulation of antisense oligonucleotides in lipid vesicles using ionizable aminolipids: formation of novel small multilamellar vesicle structures. Biochim Biophys Acta. 2001;1510(1–2):152–166. doi: 10.1016/s0005-2736(00)00343-6. [DOI] [PubMed] [Google Scholar]
- 57.Maurer N, Wong KF, Stark H, Louie L, McIntosh D, Wong T, Scherrer P, Semple SC, Cullis PR. Spontaneous entrapment of polynucleotides upon electrostatic interaction with ethanol-destabilized cationic liposomes. Biophys J . 2001;80(5):2310–2326. doi: 10.1016/S0006-3495(01)76202-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ambegia E, Ansell S, Cullis P, Heyes J, Palmer L, MacLachlan I. Stabilized plasmid-lipid particles containing PEG-diacylglycerols exhibit extended circulation lifetimes and tumor selective gene expression. Biochim Biophys Acta. 2005;1669(2):155–163. doi: 10.1016/j.bbamem.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 59.Judge AD, Robbins M, Tavakoli I, Levi J, Hu L, Fronda A, Ambegia E, McClintock K, MacLachlan I. Confirming the RNAi-mediated mechanism of action of siRNA-based cancer therapeutics in mice. J Clin Invest. 2009;119(3):661–673. doi: 10.1172/JCI37515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wheeler JJ, Palmer L, Ossanlou M, MacLachlan I, Graham RW, Zhang YP, Hope MJ, Scherrer P, Cullis PR. Stabilized plasmid-lipid particles: construction and characterization. Gene Ther. 1999;6(2):271–281. doi: 10.1038/sj.gt.3300821. [DOI] [PubMed] [Google Scholar]
- 61.Reddy LH, Couvreur P. Nanotechnology for therapy and imaging of liver diseases. J Hepatol. 2011;55(6):1461–1466. doi: 10.1016/j.jhep.2011.05.039. [DOI] [PubMed] [Google Scholar]
- 62.Akinc A, Querbes W, De S, Qin J, Frank-Kamenetsky M, Jayaprakash KN, Jayaraman M, Rajeev KG, Cantley WL, Dorkin JR, Butler JS, Qin L, Racie T, Sprague A, Fava E, Zeigerer A, Hope MJ, Zerial M, Sah DW, Fitzgerald K, Tracy MA, Manoharan M, Koteliansky V, Fougerolles A, Maier MA. Targeted delivery of RNAi therapeutics with endogenous and exogenous ligand-based mechanisms. Mol Ther. 2010;18(7):1357–1364. doi: 10.1038/mt.2010.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Geisbert TW, Lee AC, Robbins M, Geisbert JB, Honko AN, Sood V, Johnson JC, de Jong S, Tavakoli I, Judge A, Hensley LE, Maclachlan I. Postexposure protection of non-human primates against a lethal Ebola virus challenge with RNA interference: a proof-of-concept study. Lancet. 2010;375(9729):1896–1905. doi: 10.1016/S0140-6736(10)60357-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lin X, Li L, Wang R, Wilcox D, Zhao X, Song J, Huang X, Hansen TM, Dande P, Wada C, Hubbard RD, Kohlbrenner WM, Fesik SW, Shen Y. A robust in vivo positive-readout system for monitoring siRNA delivery to xenograft tumors. RNA. 2011;17(4):603–612. doi: 10.1261/rna.2546011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li L, Wang R, Wilcox D, Zhao X, Song J, Lin X, Kohlbrenner WM, Fesik SW, Shen Y. Tumor vasculature is a key determinant for the efficiency of nanoparticle-mediated siRNA delivery. Gene Ther. 2011 doi: 10.1038/gt.2011.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jain RK. Barriers to drug delivery in solid tumors. Sci Am. 1994;271(1):58–65. doi: 10.1038/scientificamerican0794-58. [DOI] [PubMed] [Google Scholar]
- 67.Jain RK, Stylianopoulos T. Delivering nanomedicine to solid tumors. Nat Rev Clin Oncol. 2010;7(11):653–664. doi: 10.1038/nrclinonc.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jang SH, Wientjes MG, Lu D, Au JL. Drug delivery and transport to solid tumors. Pharm Res. 2003;20(9):1337–1350. doi: 10.1023/a:1025785505977. [DOI] [PubMed] [Google Scholar]
- 69.Heddleston JM, Li Z, Lathia JD, Bao S, Hjelmeland AB, Rich JN. Hypoxia inducible factors in cancer stem cells. Br J Cancer. 2010;102(5):789–795. doi: 10.1038/sj.bjc.6605551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Carmeliet P, Jain RK. Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat Rev Drug Discov. 2011;10(6):417–427. doi: 10.1038/nrd3455. [DOI] [PubMed] [Google Scholar]
- 71.Gomes CP, Gomes-da-Silva LC, Ramalho JS, Lima MC, Simoes S, Moreira JN. Impact Of Plk-1 Silencing On Endothelial Cells And Cancer Cells Of Diverse Histological Origin. Curr Gene Ther. 2013;13(3):189–201. doi: 10.2174/1566523211313030004. [DOI] [PubMed] [Google Scholar]
- 72.Fang J, Nakamura H, Maeda H. The EPR effect: unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv Drug Deliv Rev. 2011;63(3):136–151. doi: 10.1016/j.addr.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 73.Love KT, Mahon KP, Levins CG, Whitehead KA, Querbes W, Dorkin JR, Qin J, Cantley W, Qin LL, Racie T, Frank-Kamenetsky M, Yip KN, Alvarez R, Sah DW, de Fougerolles A, Fitzgerald K, Koteliansky V, Akinc A, Langer R, Anderson DG. Lipid-like materials for low-dose, in vivo gene silencing. Proc Natl Acad Sci USA. 2010;107(5):1864–1869. doi: 10.1073/pnas.0910603106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu Y, Huang L. Designer lipids advance systemic siRNA delivery. Mol Ther. 2010;18(4):669–670. doi: 10.1038/mt.2010.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bellocq NC, Pun SH, Jensen GS, Davis ME. Transferrin-containing, cyclodextrin polymer-based particles for tumor-targeted gene delivery. Bioconjug Chem. 2003;14(6):1122–1132. doi: 10.1021/bc034125f. [DOI] [PubMed] [Google Scholar]
- 76.Davis ME. The first targeted delivery of siRNA in humans via a self-assembling, cyclodextrin polymer-based nanoparticle: from concept to clinic. Mol Pharm. 2009;6(3):659–668. doi: 10.1021/mp900015y. [DOI] [PubMed] [Google Scholar]
- 77.Heidel JD, Liu JY, Yen Y, Zhou B, Heale BS, Rossi JJ, Bartlett DW, Davis ME. Potent siRNA inhibitors of ribonucleotide reductase subunit RRM2 reduce cell proliferation in vitro and in vivo. Clin Cancer Res. 2007;13(7):2207–2215. doi: 10.1158/1078-0432.CCR-06-2218. [DOI] [PubMed] [Google Scholar]
- 78.Bartlett DW, Davis ME. Impact of tumor-specific targeting and dosing schedule on tumor growth inhibition after intravenous administration of siRNA-containing nanoparticles. Biotechnol Bioeng. 2008;99(4):975–985. doi: 10.1002/bit.21668. [DOI] [PubMed] [Google Scholar]
- 79.Rahman MA, Amin AR, Wang X, Zuckerman JE, Choi CH, Zhou B, Wang D, Nannapaneni S, Koenig L, Chen Z, Chen ZG, Yen Y, Davis ME, Shin DM. Systemic delivery of siRNA nanoparticles targeting RRM2 suppresses head and neck tumor growth. J Control Release. 2012;159(3):384–392. doi: 10.1016/j.jconrel.2012.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Santel A, Aleku M, Keil O, Endruschat J, Esche V, Fisch G, Dames S, Loffler K, Fechtner M, Arnold W, Giese K, Klippel A, Kaufmann J. A novel siRNA-lipoplex technology for RNA interference in the mouse vascular endothelium. Gene Ther. 2006;13(16):1222–1234. doi: 10.1038/sj.gt.3302777. [DOI] [PubMed] [Google Scholar]
- 81.Santel A, Aleku M, Keil O, Endruschat J, Esche V, Durieux B, Loffler K, Fechtner M, Rohl T, Fisch G, Dames S, Arnold W, Giese K, Klippel A, Kaufmann J. RNA interference in the mouse vascular endothelium by systemic administration of siRNA-lipoplexes for cancer therapy. Gene Ther. 2006;13(18):1360–1370. doi: 10.1038/sj.gt.3302778. [DOI] [PubMed] [Google Scholar]
- 82.Aleku M, Schulz P, Keil O, Santel A, Schaeper U, Dieckhoff B, Janke O, Endruschat J, Durieux B, Roder N, Loffler K, Lange C, Fechtner M, Mopert K, Fisch G, Dames S, Arnold W, Jochims K, Giese K, Wiedenmann B, Scholz A, Kaufmann J. Atu027, a liposomal small interfering RNA formulation targeting protein kinase N3, inhibits cancer progression. Cancer Res. 2008;68(23):9788–9798. doi: 10.1158/0008-5472.CAN-08-2428. [DOI] [PubMed] [Google Scholar]
- 83.Santel A, Aleku M, Roder N, Mopert K, Durieux B, Janke O, Keil O, Endruschat J, Dames S, Lange C, Eisermann M, Loffler K, Fechtner M, Fisch G, Vank C, Schaeper U, Giese K, Kaufmann J. Atu027 prevents pulmonary metastasis in experimental and spontaneous mouse metastasis models. Clin Cancer Res. 2010;16(22):5469–5480. doi: 10.1158/1078-0432.CCR-10-1994. [DOI] [PubMed] [Google Scholar]
- 84.Mopert K, Loffler K, Roder N, Kaufmann J, Santel A. Depletion of protein kinase N3 (PKN3) impairs actin and adherens junctions dynamics and attenuates endothelial cell activation. Eur J Cell Biol. 2012;91(9):694–705. doi: 10.1016/j.ejcb.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 85.Gutierrez-Puente Y, Tari AM, Stephens C, Rosenblum M, Guerra RT, Lopez-Berestein G. Safety, pharmacokinetics, and tissue distribution of liposomal P-ethoxy antisense oligonucleotides targeted to Bcl-2. J Pharmacol Exp Ther. 1999;291(2):865–869. [PubMed] [Google Scholar]
- 86.Landen CN, Jr, Chavez-Reyes A, Bucana C, Schmandt R, Deavers MT, Lopez-Berestein G, Sood AK. Therapeutic EphA2 gene targeting in vivo using neutral liposomal small interfering RNA delivery. Cancer Res. 2005;65(15):6910–6918. doi: 10.1158/0008-5472.CAN-05-0530. [DOI] [PubMed] [Google Scholar]
- 87.Landen CN, Merritt WM, Mangala LS, Sanguino AM, Bucana C, Lu C, Lin YG, Han LY, Kamat AA, Schmandt R, Coleman RL, Gershenson DM, Lopez-Berestein G, Sood AK. Intraperitoneal delivery of liposomal siRNA for therapy of advanced ovarian cancer. Cancer Biol Ther. 2006;5(12):1708–1713. doi: 10.4161/cbt.5.12.3468. [DOI] [PubMed] [Google Scholar]
- 88.Shahzad MM, Lu C, Lee JW, Stone RL, Mitra R, Mangala LS, Lu Y, Baggerly KA, Danes CG, Nick AM, Halder J, Kim HS, Vivas-Mejia P, Landen CN, Lopez-Berestein G, Coleman RL, Sood AK. Dual targeting of EphA2 and FAK in ovarian carcinoma. Cancer Biol Ther. 2009;8(11):1027–1034. doi: 10.4161/cbt.8.11.8523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gaspar R, Duncan R. Polymeric carriers: preclinical safety and the regulatory implications for design and development of polymer therapeutics. Adv Drug Deliv Rev. 2009;61(13):1220–1231. doi: 10.1016/j.addr.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 90.Karmali PP, Chaudhuri A. Cationic liposomes as non-viral carriers of gene medicines: resolved issues, open questions, and future promises. Med Res Rev. 2007;27(5):696–722. doi: 10.1002/med.20090. [DOI] [PubMed] [Google Scholar]
- 91.Dams ET, Laverman P, Oyen WJ, Storm G, Scherphof GL, van Der Meer JW, Corstens FH, Boerman OC. Accelerated blood clearance and altered biodistribution of repeated injections of sterically stabilized liposomes. J Pharmacol Exp Ther. 2000;292(3):1071–1079. [PubMed] [Google Scholar]
- 92.Ishida T, Harada M, Wang XY, Ichihara M, Irimura K, Kiwada H. Accelerated blood clearance of PEGylated liposomes following preceding liposome injection: effects of lipid dose and PEG surface-density and chain length of the first-dose liposomes. J Control Release. 2005;105(3):305–317. doi: 10.1016/j.jconrel.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 93.Ishida T, Ichihara M, Wang X, Kiwada H. Spleen plays an important role in the induction of accelerated blood clearance of PEGylated liposomes. J Control Release. 2006;115(3):243–250. doi: 10.1016/j.jconrel.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 94.Ishida T, Ichihara M, Wang X, Yamamoto K, Kimura J, Majima E, Kiwada H. Injection of PEGylated liposomes in rats elicits PEG-specific IgM, which is responsible for rapid elimination of a second dose of PEGylated liposomes. J Control Release. 2006;112(1):15–25. doi: 10.1016/j.jconrel.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 95.Koide H, Asai T, Hatanaka K, Akai S, Ishii T, Kenjo E, Ishida T, Kiwada H, Tsukada H, Oku N. T cell-independent B cell response is responsible for ABC phenomenon induced by repeated injection of PEGylated liposomes. Int J Pharm. 2010;392(1–2):218–223. doi: 10.1016/j.ijpharm.2010.03.022. [DOI] [PubMed] [Google Scholar]
- 96.Judge A, McClintock K, Phelps JR, Maclachlan I. Hypersensitivity and loss of disease site targeting caused by antibody responses to PEGylated liposomes. Mol Ther. 2006;13(2):328–337. doi: 10.1016/j.ymthe.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 97.Semple SC, Harasym TO, Clow KA, Ansell SM, Klimuk SK, Hope MJ. Immunogenicity and rapid blood clearance of liposomes containing polyethylene glycol-lipid conjugates and nucleic Acid. J Pharmacol Exp Ther. 2005;312(3):1020–1026. doi: 10.1124/jpet.104.078113. [DOI] [PubMed] [Google Scholar]
- 98.Tagami T, Nakamura K, Shimizu T, Ishida T, Kiwada H. Effect of siRNA in PEG-coated siRNA-lipoplex on anti-PEG IgM production. J Control Release. 2009;137(3):234–240. doi: 10.1016/j.jconrel.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 99.Tagami T, Uehara Y, Moriyoshi N, Ishida T, Kiwada H. Anti-PEG IgM production by siRNA encapsulated in a PEGylated lipid nanocarrier is dependent on the sequence of the siRNA. J Control Release. 2011;151(2):149–154. doi: 10.1016/j.jconrel.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 100.Barros SA, Gollob JA. Safety profile of RNAi nanomedicines. Adv Drug Deliv Rev. 2012;64(15):1730–1737. doi: 10.1016/j.addr.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 101.Hu-Lieskovan S, Heidel JD, Bartlett DW, Davis ME, Triche TJ. Sequence-specific knockdown of EWS-FLI1 by targeted, nonviral delivery of small interfering RNA inhibits tumor growth in a murine model of metastatic Ewing’s sarcoma. Cancer Res. 2005;65(19):8984–8992. doi: 10.1158/0008-5472.CAN-05-0565. [DOI] [PubMed] [Google Scholar]
- 102.Allen TM. Ligand-targeted therapeutics in anticancer therapy. Nat Rev Cancer. 2002;2(10):750–763. doi: 10.1038/nrc903. [DOI] [PubMed] [Google Scholar]
- 103.Santos AO, da Silva LC, Bimbo LM, de Lima MC, Simoes S, Moreira JN. Design of peptide-targeted liposomes containing nucleic acids. Biochim Biophys Acta. 2010;1798(3):433–441. doi: 10.1016/j.bbamem.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 104.Woll PJ, Rozengurt E. A neuropeptide antagonist that inhibits the growth of small cell lung cancer in vitro. Cancer Res. 1990;50(13):3968–3973. [PubMed] [Google Scholar]
- 105.Mishra S, Webster P, Davis ME. PEGylation significantly affects cellular uptake and intracellular trafficking of non-viral gene delivery particles. Eur J Cell Biol. 2004;83(3):97–111. doi: 10.1078/0171-9335-00363. [DOI] [PubMed] [Google Scholar]
- 106.Li SD, Chen YC, Hackett MJ, Huang L. Tumor-targeted delivery of siRNA by self-assembled nanoparticles. Mol Ther. 2008;16(1):163–169. doi: 10.1038/sj.mt.6300323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Li SD, Chono S, Huang L. Efficient oncogene silencing and metastasis inhibition via systemic delivery of siRNA. Mol Ther. 2008;16(5):942–946. doi: 10.1038/mt.2008.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gao J, Liu W, Xia Y, Li W, Sun J, Chen H, Li B, Zhang D, Qian W, Meng Y, Deng L, Wang H, Chen J, Guo Y. The promotion of siRNA delivery to breast cancer overexpressing epidermal growth factor receptor through anti-EGFR antibody conjugation by immunoliposomes. Biomaterials. 2011;32(13):3459–3470. doi: 10.1016/j.biomaterials.2011.01.034. [DOI] [PubMed] [Google Scholar]
- 109.Gao J, Yu Y, Zhang Y, Song J, Chen H, Li W, Qian W, Deng L, Kou G, Chen J, Guo Y. EGFR-specific PEGylated immunoliposomes for active siRNA delivery in hepatocellular carcinoma. Biomaterials. 2012;33(1):270–282. doi: 10.1016/j.biomaterials.2011.09.035. [DOI] [PubMed] [Google Scholar]
- 110.Mendonca LS, Moreira JN, de Lima MC, Simoes S. Co-encapsulation of anti-BCR-ABL siRNA and imatinib mesylate in transferrin receptor-targeted sterically stabilized liposomes for chronic myeloid leukemia treatment. Biotechnol Bioeng. 2010;107(5):884–893. doi: 10.1002/bit.22858. [DOI] [PubMed] [Google Scholar]
- 111.Chiu SJ, Liu S, Perrotti D, Marcucci G, Lee RJ. Efficient delivery of a Bcl-2-specific antisense oligodeoxyribonucleotide (G3139) via transferrin receptor-targeted liposomes. J Control Release. 2006;112(2):199–207. doi: 10.1016/j.jconrel.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 112.Chiu SJ, Marcucci G, Lee RJ. Efficient delivery of an antisense oligodeoxyribonucleotide formulated in folate receptor-targeted liposomes. Anticancer Res. 2006;26(2A):1049–1056. [PubMed] [Google Scholar]
- 113.Yang L, Li J, Zhou W, Yuan X, Li S. Targeted delivery of antisense oligodeoxynucleotides to folate receptor-overexpressing tumor cells. J Control Release. 2004;95(2):321–331. doi: 10.1016/j.jconrel.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 114.Feng C, Wang T, Tang R, Wang J, Long H, Gao X, Tang S. Silencing of the MYCN gene by siRNA delivered by folate receptor-targeted liposomes in LA-N-5 cells. Pediatr Surg Int. 2010;26(12):1185–1191. doi: 10.1007/s00383-010-2703-5. [DOI] [PubMed] [Google Scholar]
- 115.Cardoso AL, Simoes S, de Almeida LP, Pelisek J, Culmsee C, Wagner E, Pedroso de Lima MC. siRNA delivery by a transferrin-associated lipid-based vector: a non-viral strategy to mediate gene silencing. J Gene Med. 2007;9(3):170–183. doi: 10.1002/jgm.1006. [DOI] [PubMed] [Google Scholar]
- 116.Davis ME, Zuckerman JE, Choi CH, Seligson D, Tolcher A, Alabi CA, Yen Y, Heidel JD, Ribas A. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature. 2010;464(7291):1067–1070. doi: 10.1038/nature08956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lu W, Zhang G, Zhang R, Flores LG, 2nd, Huang Q, Gelovani JG, Li C. Tumor site-specific silencing of NF-kappaB p65 by targeted hollow gold nanosphere-mediated photothermal transfection. Cancer Res. 2010;70(8):3177–3188. doi: 10.1158/0008-5472.CAN-09-3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sapra P, Allen TM. Ligand-targeted liposomal anticancer drugs. Prog Lipid Res. 2003;42(5):439–462. doi: 10.1016/s0163-7827(03)00032-8. [DOI] [PubMed] [Google Scholar]
- 119.Sapra P, Tyagi P, Allen TM. Ligand-targeted liposomes for cancer treatment. Curr Drug Deliv. 2005;2(4):369–381. doi: 10.2174/156720105774370159. [DOI] [PubMed] [Google Scholar]
- 120.Zhu Q, Feng C, Liao W, Zhang Y, Tang S. Target delivery of MYCN siRNA by folate-nanoliposomes delivery system in a metastatic neuroblastoma model. Cancer Cell Int. 2013;13(1):65. doi: 10.1186/1475-2867-13-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.da Cruz MT, Simoes S, Pires PP, Nir S, de Lima MC. Kinetic analysis of the initial steps involved in lipoplex–cell interactions: effect of various factors that influence transfection activity. Biochim Biophys Acta. 2001;1510(1–2):136–151. doi: 10.1016/s0005-2736(00)00342-4. [DOI] [PubMed] [Google Scholar]
- 122.Bartlett DW, Su H, Hildebrandt IJ, Weber WA, Davis ME. Impact of tumor-specific targeting on the biodistribution and efficacy of siRNA nanoparticles measured by multimodality in vivo imaging. Proc Natl Acad Sci USA. 2007;104(39):15549–15554. doi: 10.1073/pnas.0707461104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Iyer AK, Khaled G, Fang J, Maeda H. Exploiting the enhanced permeability and retention effect for tumor targeting. Drug Discov Today. 2006;11(17–18):812–818. doi: 10.1016/j.drudis.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 124.Li SD, Huang L. Pharmacokinetics and biodistribution of nanoparticles. Mol Pharm. 2008;5(4):496–504. doi: 10.1021/mp800049w. [DOI] [PubMed] [Google Scholar]
- 125.Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol. 2002;29(6 Suppl 16):15–18. doi: 10.1053/sonc.2002.37263. [DOI] [PubMed] [Google Scholar]
- 126.Abdollahi A, Folkman J. Evading tumor evasion: current concepts and perspectives of anti-angiogenic cancer therapy. Drug Resist Updat. 2010;13(1–2):16–28. doi: 10.1016/j.drup.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 127.Simone E, Ding BS, Muzykantov V. Targeted delivery of therapeutics to endothelium. Cell Tissue Res. 2009;335(1):283–300. doi: 10.1007/s00441-008-0676-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ding BS, Dziubla T, Shuvaev VV, Muro S, Muzykantov VR. Advanced drug delivery systems that target the vascular endothelium. Mol Interv. 2006;6(2):98–112. doi: 10.1124/mi.6.2.7. [DOI] [PubMed] [Google Scholar]
- 129.Hajitou A, Pasqualini R, Arap W. Vascular targeting: recent advances and therapeutic perspectives. Trends Cardiovasc Med. 2006;16(3):80–88. doi: 10.1016/j.tcm.2006.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chen Y, Wu JJ, Huang L. Nanoparticles targeted with NGR motif deliver c-myc siRNA and doxorubicin for anticancer therapy. Mol Ther. 2010;18(4):828–834. doi: 10.1038/mt.2009.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Porkka K, Laakkonen P, Hoffman JA, Bernasconi M, Ruoslahti E. A fragment of the HMGN2 protein homes to the nuclei of tumor cells and tumor endothelial cells in vivo. Proc Natl Acad Sci USA. 2002;99(11):7444–7449. doi: 10.1073/pnas.062189599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Christian S, Pilch J, Akerman ME, Porkka K, Laakkonen P, Ruoslahti E. Nucleolin expressed at the cell surface is a marker of endothelial cells in angiogenic blood vessels. J Cell Biol. 2003;163(4):871–878. doi: 10.1083/jcb.200304132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gomes-da-Silva LC, Santos AO, Bimbo LM, Moura V, Ramalho JS, Pedroso de Lima MC, Simoes S, Moreira JN. Toward a siRNA-containing nanoparticle targeted to breast cancer cells and the tumor microenvironment. Int J Pharm. 2012;434(1–2):9–19. doi: 10.1016/j.ijpharm.2012.05.018. [DOI] [PubMed] [Google Scholar]
- 134.Gomes-da-Silva LC, Fernandez Y, Abasolo I, Schwartz S, Ramalho JS, Pedroso de Lima MC, Simoes S, Moreira JN. Efficient intracellular delivery of siRNA with a safe multitargeted lipid-based nanoplatform. Nanomedicine (Lond) 2013 doi: 10.2217/nnm.12.174. [DOI] [PubMed] [Google Scholar]
- 135.Gomes-da-Silva LC, Ramalho JS, Pedroso de Lima MC, Simoes S, Moreira JN. Impact of anti-PLK1 siRNA-containing F3-targeted liposomes on the viability of both cancer and endothelial cells. Eur J Pharm Biopharm. 2013 doi: 10.1016/j.ejpb.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 136.Moura V, Lacerda M, Figueiredo P, Corvo ML, Cruz ME, Soares R, de Lima MC, Simoes S, Moreira JN. Targeted and intracellular triggered delivery of therapeutics to cancer cells and the tumor microenvironment: impact on the treatment of breast cancer. Breast Cancer Res Treat. 2012;133(1):61–73. doi: 10.1007/s10549-011-1688-7. [DOI] [PubMed] [Google Scholar]
- 137.Strumberg D, Schultheis B, Traugott U, Vank C, Santel A, Keil O, Giese K, Kaufmann J, Drevs J. Phase I clinical development of Atu027, a siRNA formulation targeting PKN3 in patients with advanced solid tumors. Int J Clin Pharmacol Ther. 2012;50(1):76–78. doi: 10.5414/cpp50076. [DOI] [PubMed] [Google Scholar]