Abstract
Rapid nerve conduction requires the coating of axons by a tightly packed multilayered myelin membrane. In the central nervous system, myelin is formed from cellular processes that extend from oligodendrocytes and wrap in a spiral fashion around an axon, resulting in the close apposition of adjacent myelin membrane bilayers. In this review, we discuss the physical principles underlying the zippering of the plasma membrane of oligodendrocytes at the cytoplasmic and extracellular leaflet. We propose that the interaction of the myelin basic protein with the cytoplasmic leaflet of the myelin bilayer triggers its polymerization into a fibrous network that drives membrane zippering and protein extrusion. In contrast, the adhesion of the extracellular surfaces of myelin requires the down-regulation of repulsive components of the glycocalyx, in order to uncover weak and unspecific attractive forces that bring the extracellular surfaces into close contact. Unveiling the mechanisms of myelin membrane assembly at the cytoplasmic and extracelluar sites may help to understand how the myelin bilayers are disrupted and destabilized in the different demyelinating diseases.
Keywords: Myelin, Oligodendrocytes, Myelin basic protein, Proteolipid protein
Introduction
Myelin evolved several times independently during evolution [1]. Irrespective of its origin, myelin in the different taxa shares a remarkably uniform multilamellar spiral structure forming the physical basis for the fast saltatory conduction of the current. The key ultrastructural features of vertebrate myelin, both in the peripheral and central nervous system, include its tightly wrapped membranes that are regularly spaced, giving rise to alternating major dense and intraperiod lines [2, 3]. The major dense line is made up of closely condensed cytoplasmic myelin membranes, and the intraperiod line represents the tightly apposed outer membranes [2, 4]. Whereas simple mixtures of lipids can spontaneously form densely stacked multilamellar membrane, such structures are rarely found in vivo. Exceptions are multivesicular bodies that are generated in the endosomal–lysosomal system after protein degradation or in pathological conditions such as lysosomal storage diseases [5, 6]. In most cases, plasma membranes are covered by a carbohydrate-rich extracellular glycocalyx and a filamentous protein-rich cytocortex that prevent the self-organization of a lipid-bilayer into membrane whorls [7, 8]. In this review, we discuss the physical principles that bring about myelin membrane stacking at the cytoplasmic and extracellular sides as the membrane spirals around axons. Here, we will focus on myelin compaction in the central nervous system and on the myelin basic protein (MBP), a protein that is absolutely essential for the myelin membrane assembly. In the later parts of the review, we will discuss the role of generic forces in myelin membrane compaction at the extracellular interface.
Lateral self-assembly of myelin basic proteins on membrane surfaces
A key feature of MBP is its intrinsically unstructured polypeptide chain [9–11]. Due to the low content of hydrophobic and aromatic amino acid residues and the higher proportions of polar and charged amino acids, intrinsically unstructured proteins (IUPs) are unable to bury their hydrophobic core sufficiently and thus cannot spontaneously fold into a well-organized globular structure. Instead these proteins have an extended conformation with little secondary structure, but often fold upon binding to their biological targets [12–16]. A number of different approaches including NMR spectroscopy, circular dichroism, Fourier transform infrared spectroscopy and X-ray scattering have shown that MBP exists as a flexible coil with some minor elements of secondary structure in aqueous solution, but adopts both α-helical and β-sheet structures upon association with membranes [10]. Whereas structural knowledge on MBP in solution and bound to various ligands continuously progresses [10, 17–19], information on MBP in a cellular context, when bound to a membrane, is sparse. Only recently we are beginning to understand how structural conformation of MBP affects its physicochemical properties, which directly influence its biological function in myelin.
From the natural mouse mutant shiverer, which lacks functional MBP, it is known that MBP is required for the compaction and biogenesis of myelin [20, 21]. However, the biophysical principles how MBP carries out this essential function are not well understood. A simplified answer to this question is that MBP binds to the two opposing cytoplasmic leaflet of the myelin bilayer and brings them closely together. However, when looking at electron microscope images of myelin there is a striking uniformity in the organization of the tightly opposed cytoplasmic surfaces giving rise to one homogenous, so-called, major dense line, raising the question of the underlying mechanisms of its assembly. If the binding to two negatively charged surfaces would be the sole task of MBP, a large number of other proteins should intermix within the compacted myelin.
One important clue to how MBP performs its function comes from studies comparing the physicochemical properties of MBP in solution and when bound to a membrane. In solution MBP is self-repulsive as there is little secondary structure and the positively charged amino acids are freely exposed. However, when MBP interacts with a membrane, its charge is neutralized and self-association is triggered [10, 15, 16, 22–24]. Thus, membrane binding appears to switch the properties of MBP to allow its polymerization into a protein meshwork at the interface of two cytoplasmic membrane surfaces. This sharp transition from a soluble to a polymerized pool of molecules is reminiscent to phase transitions that are often observed in non-living systems, where in most cases temperature transforms one state of matter into another.
Whereas most biochemists and cell biologists are familiar with phase transitions of lipids in membranes, a number of recent findings indicate that proteins can also change their physical and functional properties by phase transitions [25–27]. For example, it was not known how RNA/protein bodies form spherical assemblies in the cytoplasm or nucleoplasm in cells without a surrounding membrane [28, 29]. From studies on the germ (P) granules in Caenorhabditis elegans it now becomes evident that RNA granules have liquid-like properties that rapidly dissolve and condense [28]. How do proteins drive such large-scale segregations?. Recently, weak multivalent protein interactions were shown to be critical. Proteins were engineered to contain up to five repeats of Src homology 3 (SH3) domains or its proline-rich motif (PRM) ligand. When the proteins with more than three repeats were mixed, rapid unmixing from the bulk solution was observed resulting in the formation of numerous spherical droplets with liquid-like properties demonstrating that multivalency is one key factor that defines such phase transitions [30]. In another study, Kato et al. [29] investigated the cell-free formation of RNA granules and found that low complexity sequence domains can undergo a phase transition into a hydrogel-like state. Within these low complexity sequences they identified tripeptide sequences with [G/S]Y[G/S] repeats that were required for hydrogel formation [29]. Previous work on the organization of the nuclear pore complex also revealed that phenylalanine-mediated inter-repeat interactions of nucleoporins are involved in the formation of similar hydrogels [31].
The self-assembly of MBP has been studied in various in vitro systems in the past [11, 24, 32–34]. Some factors that promoted or modulated self-assembly include charge reduction of MBP by deimination and divalent cations. Interestingly, phenylalanine residues were also shown to be critical for self-assembly of MBP molecules in solution [22]. When a pH shift was used as a strategy to mimic the in vivo charge neutralization by the negatively charged membranes, purified MBP formed μm-sized droplets as described above for the SH3 and PRM proteins. Mutant MBP (F → S mutant) in which these phenylalanines were replaced by serines did not phase segregate suggesting that the process is critically dependent on hydrophobic phenylalanine mediated interactions. When the MBP F → S mutant was analyzed in cells, membrane binding was still observed, but the self-association of MBP into a polymerized protein network was abolished [22]. An important question is how these phenylalanines mediate protein polymerization. One possibility is that they are directly involved in protein–protein interactions as described for the FG-repeats in nucleoporins [31]. Another possibility is that the phenylalanines mediate these effects indirectly. In fact, when considering the localization of one of the critical phenylalanines patches within the MBP sequence, it overlaps with a highly conserved segment, which has been shown to form a membrane-associating amphipathic α-helix (Fig. 1) [35]. Interestingly, using site-directed spin labeling in combination with electron paramagnetic resonance spectroscopy it was shown that Phe86–Phe87 pair penetrated at a depth of up to 1.2 nm into the lipid bilayer [36]. The upstream Phe–Phe pair could also be part of such structure, but this has not been proven yet [9, 37]. It could be that the interaction of the amphipathic helix with the membrane together induces a conformational change in MBP, which is required for the self-association. Indeed using FTIR it was found that there were fundamental differences in the structure of MBP wild-type and F → S mutant when interacting with a membrane. Whereas both the amount of α-helices and β-sheets increases for wild-type MBP, there is only an increase in α-helical structure when the F → S mutant is added to a synthetic lipid bilayer [22].
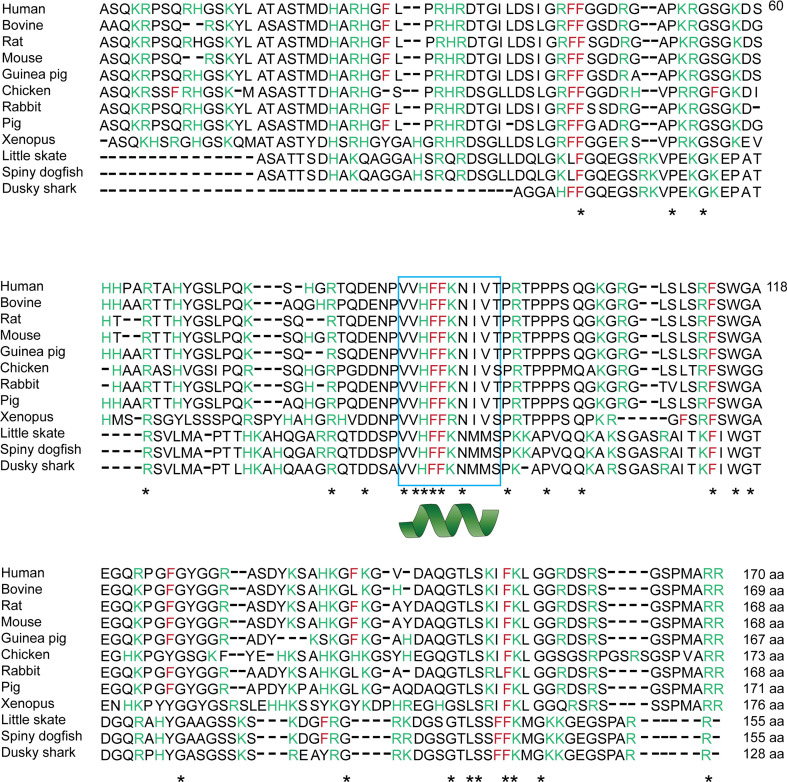
Fig. 1.
The alignment of protein sequence of the major isoform of MBP shows a high similarity among different spices (http://www.uniprot.org/). The position of the hydrophobic phenylalanine residues (F) in MBP is shown, which are required for its polymerization into a cohesive protein meshwork [22]. One of the critical phenylalanines patches within the MBP sequence overlaps with a highly, conserved segment, which has been shown to form a membrane-associating amphipathic α-helix [19, 35, 37]. Positively charged amino acids are highlighted in green
It is possible that these β-sheets mediate self-assembly of MBP by amyloid-like fibrillogenesis. Several fluorescent dyes which stain amyloids, have been shown to consistently label the white matter of wild-type, but not shiverer mice [22, 38]. However, unlike classical pathological amyloid fibrils, which form extremely stable and rigid structures, amyloid-like interactions by MBP give rise to weaker and reversible connections [22, 39, 40]. While classical amyloid is insensitive to the solubilizing effects of SDS [41], this is not the case for MBP [42]. Thus, there appears to be a growing number of proteins which form dynamic amyloid-like interactions. These have been described for certain nucleoporins within the permeability barrier of the nuclear pore and for low complexity sequence domain proteins in RNA granules [29, 43]. A particular interesting example is the self-association of hormones in reversible amyloid-like aggregates. Here, the low pH inside the secretory vesicles triggers the reversible functional amyloid assembly of the hormones, which are rapidly solubilized upon secretion when the pH is neutralized. This process reflects a classical phase transition in which the pH drives the reaction [44]. From these examples, it becomes clear that phase transitions of proteins from a soluble pool can give to both physiological assemblies and pathological aggregates [39]. This may also explain why many intrinsically unstructured proteins are associated with human diseases, in which pathological amyloid aggregates are formed [45]. Pathological amyloid generation may represent an erroneous phase transition into a solid, irreversible aggregate [40].
In this context, it is surprising that MBP aggregation has not been associated with any diseases in human. It is likely that a number of control mechanisms operate in oligodendrocytes to prevent the uncontrolled assembly of MBP. One of these mechanisms is the regulation of MBP transcription and translation [46–49].
MBP mRNA is synthesized in the cell perikaryon and transported as mRNA granules along processes to myelin [50]. Oligodendrocytes may have developed this mechanism of local synthesis of MBP at the plasma membrane to ensure that MBP exerts its adhesive action at the appropriate place in the cell [51]. This process has been described to be regulated by the signaling events resulted from axon–glia interaction. Axonal signals such as laminins, L1-CAM and action potentials induce MBP mRNA translation through β1-integrin, F3/contactin and glutamate receptor signaling pathways, respectively [50, 52, 53]. These signaling pathways engage different molecules such as tumor overexpressed gene (TOG) and hnRNPs to transport mRNA granules inside oligodendrocytes [54–56]. For instance, integrin-mediated neuronal signals recruit hnRNP-K to transport MBP mRNA to the axon–glia contact site [52]. Non-receptor tyrosine kinase Fyn is also involved in the hnRNPs-mediated MBP mRNA granules translocation. Phosphorylation of hnRNP-A2 and hnRNP-F by Fyn regulates the movement of MBP mRNA into the growing myelin sheath [53, 56]. Moreover non-coding RNAs such as sncRNA715 have been recently revealed to regulate MBP translation [57].
Another mechanism to regulate the physicochemical properties of MBP is post-translational modification [9]. A number of post-translational modifications have been identified in MBP including deimination, methylation, phosphorylation, and N-terminal acylation [10]. The different modifications give rise to multiple charge variants denoted as C1–C8, when separated biochemically [58, 59]. The C1 component is the least modified with a net charge of +19 at neutral pH, whereas the C8 variant has the least charge [9, 10, 60]. It is likely that these various modifications regulate the interaction of MBP to the membrane and, thus, provide a system to antagonize the self-assembly of MBP outside of compacted myelin membrane. For example, deimination has already been shown to alter MBP assembly on lipid monolayers [11, 33]. An example of how post-translational modification regulates membrane affinity is provided by the “electrostatic switch” of the MARCKS and Src proteins [61, 62]. These proteins interact by a basic patch and a myristoyl lipid-anchor with the membrane. Phosphorylation of serine residues within the basic patch neutralizes the charge and promotes dissociation of the proteins from the membrane [62]. Interestingly, within the MBP sequence adjacent to the region that forms the amphipathic α-helix (P82-I90) a highly-conserved central segment consists of a proline-rich region that can be post-translationally modified through phosphorylation by mitogen-activated protein (MAP) kinases. Phosphorylation at the MAP-kinase sites alters the energy landscape of the central segment by disfavoring α-helical formation, which could inhibit MBP-membrane association [19]. Phosphorylation also appears to play a direct role in MBP-membrane interaction by altering both the tilt angle and the penetration depth of the helix into the membrane [19]. Such a mechanism might represent a molecular switch regulating MBP function by phosphorylation/dephosphorylation [17, 19, 63, 64].
Association with other proteins such as cytoskeletal components, SH3 domain-containing proteins, and Ca2+-calmodulin may also regulate MBP structure and function [10, 65]. Another way could be the association with chaperones, such as heat shock proteins that are expressed in relatively high amounts in oligodendrocytes [66]. The heat shock protein-70 has been found to be required for optimal expression of MBP during oligodendrocyte differentiation and a direct physical interaction has been described in brains of MS patients [67, 68].
The binding of MBP to divalent cations, such as zinc and copper, are also known to modulate the lateral self-assembly of MBP [32, 69]. Zinc is of particular importance, because its concentration in myelin is higher than of any other trace element. In fact, human white matter contains about 0.5 pmol of zinc per gram dry weight [70]. Submillimolar concentrations of zinc ions stabilize the association of MBP with purified myelin membranes and thus appear to play a key role in maintaining the integrity of the myelin sheath [69, 71–76].
In contrast, an increase in Ca2+ has a deleterious effects on myelin [77], possibly in part by effecting the binding between MBP and the headgroup of phosphoinositol 4,5-bisphosphate in the lipid bilayer [78–80] Moreover, increase in calcium levels activates peptidylarginine deiminase which triggers MBP deimination [81]. Interestingly, MBP acts as a calcium influx regulator [82], suggesting a reciprocal controlling mechanism between MBP and Ca2+ ions.
Protein extrusion and diffusion barriers
The transition of MBP from a soluble pool of molecules to a fibrous network after contacting the two opposing cytoplasmic leaflet of the myelin bilayer is likely to have important consequences for myelin membrane biogenesis. One possibility is that the force exerted by the polymerization of MBP is used to push the myelin membrane forward, in a similar fashion as actin polymerization advances the leading edge in migratory cells. However, when MBP assembly is visualized in oligodendrocytes in culture it is clear that sheet biogenesis and MBP assembly are uncoupled [22, 83]. In fact, myelin membrane sheet biogenesis occurs before MBP appears in most cells in culture [22, 83]. The conclusion that sheet formation and MBP assembly are independent events is supported by the analysis of oligodendrocytes derived from shiverer mice, which in most cases still form membrane sheets [83]. Whereas MBP might not be required for the formation of sheets per se, MBP has a major impact on their organization. In oligodendrocytes lacking functional MBP there is a complete loss of membrane polarization. For example, CNPase and MAG, which are confined to the cytoplasmic channels and the cell body in wild-type cells, are homogenously distributed throughout the membrane sheets in oligodendrocytes from shiverer mice [83]. In addition, MBP appears to play an important role in regulating the organization and stability of the wild-type oligodendrocyte cytoskeleton [84, 85]. Interestingly, cytoplasmic proteins and organelles are also absent from membrane sheets of cells lacking MBP [83], indicating that other factors then MBP must regulate the polarization of the cytosol. When visualizing how MBP is expressed during oligodendrocyte differentiation in culture, a strong local accumulation of MBP is observed at specific sites of the developing membrane sheets before being distributed within the entire sheet [22]. Thus, we propose a nucleation—zipping model to explain MBP assembly in the sheets. According to this hypothesis, MBP attaches first to two opposing cytoplasmic leaflets to form specific nucleation sites. These domains may serve as recruitment sites for the assembly and growth of MBP in the sheets. The MBP that attaches to these nucleation sites may act as a molecular zipper, which closes the adjacent plasma membrane bilayers to generate compacted myelin membrane sheets (Fig. 2). We speculate that analogue to amyloid fibrillation it is the nucleation that is rate-limiting step in the zippering of the membrane. Where and how these nucleation sites are formed is not known, but it can be speculated that specific lipids, such as phosphoinositol 4,5-bisphosphate are required. Interestingly, binding of MBP to phosphoinositides have shown to induce shape changes in artificial membranes [86]. Another possibility is that the distance of the opposing cytoplasmic leaflets of the myelin bilayer defines the initial sites of MBP assembly. For example sequestration of phosphoinositides into domains of high local concentration, in regions of myelin where the membrane is greatly curved could facilitate nucleation of MBP. Once nucleation sites are formed newly synthesized MBP is likely to attach to these sites by diffusional trapping thereby extending the fibrous network. We hypothesize that the forces exerted by the polymerization of the meshwork of MBP are used for protein extrusion and myelin membrane zippering. In addition, such a cohesive structure is likely to provide the necessary amount of stability to myelin to allow its extension of several wraps of membrane around an axon.
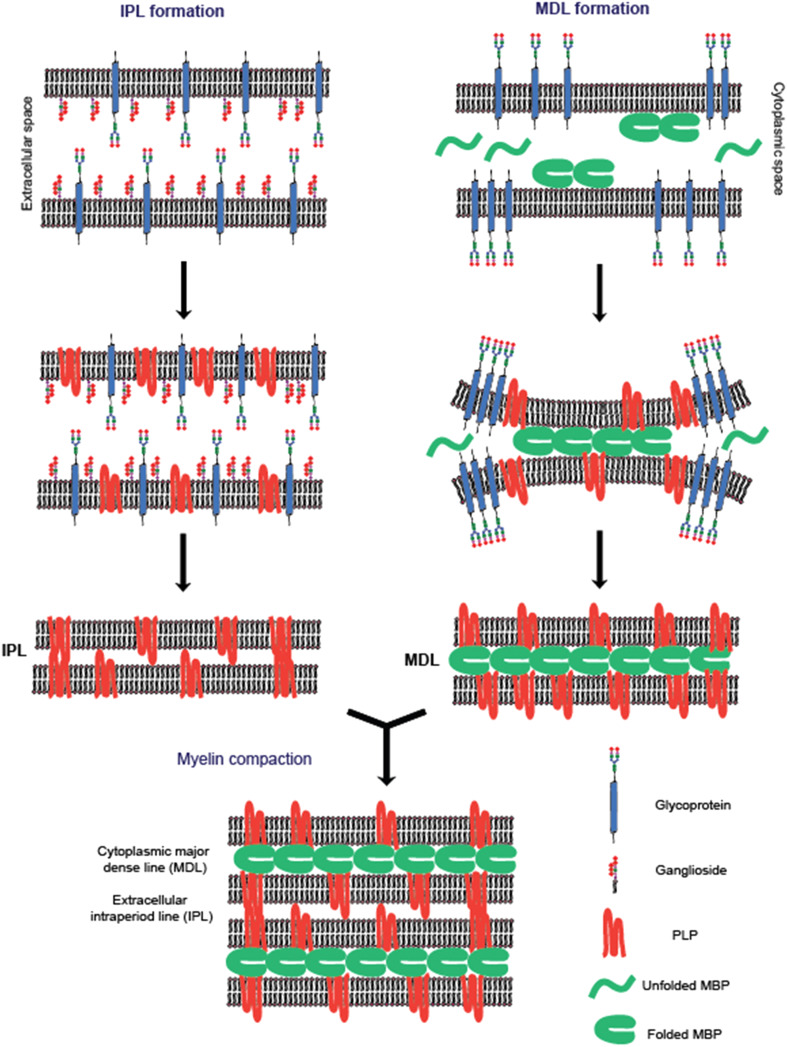
Fig. 2.
Schematic model of how the myelin bilayer may interact and compact at the extracellular and cytoplasmic leaflets. The formation of the extracellular intraperiod line (IPL) is thought to result from the removal of large and charged glycocalyx components along with the expression of PLP on the membrane. At the cytosolic surface of the membrane the self-assembly of MBP into a cohesive protein meshwork extrudes proteins with bulky cytoplasmic domains from the inter-membrane space and prevents the entry of other bulky proteins into this area. This leads to formation of cytosolic major dense line (MDL) of myelin. The independent formations of IPL and MDL bring the myelin layers into close contact and orchestrate myelin membrane assembly [22, 83, 120]
Another function of the fibrous network of MBP molecules is to form a molecular sieve that restricts the diffusion of proteins with large cytoplasmic domains into compacted myelin [83, 87]. Serial cytoplasmic truncations of Neurofascin-155, Tmem10 and MAG revealed that proteins with cytosolic domain of more than 20–30 amino acids do not enter the sheets. This size cut-off does not only seem to be defined by the distance between the two opposing bilayers that are brought together by MBP, but also by MBP itself [83]. However, the size of a protein might not be the only determinant, since proteins with large cytoplasmic domains were found to enter myelin sheets when bound to MBP [83]. Such specific interactions with MBP may enable a protein to overcome the barrier. In this context it is interesting to compare the barrier formed by MBP with the permeability barrier of the nuclear pore. Whereas small proteins diffuse freely through the pores of the barrier, larger proteins require the interaction with nuclear transport receptors to overcome the size limit of the sieve by a competitive disruption of inter-repeat contacts, which transiently opens adjoining meshes [88].
Since MBP appear to cover large areas of the sheet, it is likely also to be intimately intertwined in the organization of the lipids in the myelin bilayer. One important consequence of MBP accumulation within myelin occurs on the ratio of protein to lipids in the membrane. Whereas protein-to-lipid ratios ranges from 1.0 to 4.0 (wt/wt) in most plasma membranes, the ratio is extremely low in myelin (0.25) [89]. The most likely explanation is that most membrane proteins are not able to cross the barrier generated by MBP [83].
In addition, MBP may also influence the lipid composition in myelin [90]. Indeed, when the myelin was purified from wild-type and shiverer mice and subjected to mass spectrometry analysis, a lower content of hexosylceramide, cholesterol and plasmalogens were observed [91]. Nonetheless, the differences could be attributed to impurities in the isolated myelin from shiverer mice. An alternative possibility is that the formation of the condensed fluid phase of MBP between the cytoplasmic leaflets drives a phase separation of lipids in the myelin bilayer [22]. By these means, a phase transition of proteins in the cytosol could be coupled to a reorganization of lipid in the myelin bilayer. Whereas the role of MBP in promoting membrane adhesion by interacting with lipids in apposing surfaces is well known, little is known about how MBP influences the lipid organization in a lateral dimension in vivo. However, a number of studies have been carried out in artificial membranes. In simple lipid mixtures containing only neutral and acidic lipids, MBP induces a phase separation by sequestering the negatively charged lipids [92]. In fact, MBP is able to bind to around 20 molecules of acidic lipids per molecules [93]. In more complex lipid mixtures (phosphatidylserine, sphingomyelin, phoshatidylcholine, and cholesterol), MBP induces macroscopic phase segregation in a cholesterol-dependent manner. The dependence of cholesterol for the segregation suggests that the driving force is not only electrostatic, but also hydrophobic [94–98].
Using lipid monolayers with the cytosolic membrane composition of myelin, MBP has been shown not only to induce domain segregation but also fusion [99]. In these experiments, MBP partially inserted into the lipid monolayer, followed by the attraction and the aggregation of the lipid domains. The authors attribute domain fusion to a minimization of electrostatic repulsion and reduction of line tension in the membrane.
Taking these studies to consideration, a model emerges indicating that MBP induces a significant rearrangement of membranes by a combination of electrostatic and hydrophobic interactions. Whereas the electrostatic interactions are known to occur with phosphatidylserine and phosphoinositol 4,5-bisphosphate, the hydrophobic interactions of MBP are less defined. In liposome aggregation and binding assays, it appears that cholesterol is involved as it enhances membrane binding and vesicle aggregation by MBP [94–98]. On one hand, MBP seems to bind to cholesterol-rich domains, but on the other hand, it preferentially associated with liquid-disordered membrane domains in monolayer experiments [80, 100, 101]. Since it is difficult to extrapolate all of these studies to the behavior of MBP in myelin, it is critical to study this in vivo.
Non-specific, generic forces are the major organizers of intraperiod line formation
Although the organization of major dense line has been mainly attributed to the function of MBP, the mechanisms that mediate the close interaction of myelin lamellae at their external surfaces are poorly understood. One reason is that no single mutant induces a significant destruction of the intraperiod line structure in the CNS. Therefore it is possible that the formation of this domain depends on several mechanisms. In general, cell–cell or cell–tissue interaction is mediated by lateral segregation of adhesion molecules in special domains where they form adhesion sites between two membranes in a specific lock-and-key fashion [102, 103]. One example is the interaction between immune cells where the adhesion between T cells and antigen-presenting cells is mediated by the formation of immune synapses [104, 105]. In this type of adhesion the repulsive molecules are excluded from the contact area. This can be mediated by lateral movement of repellers in the membrane or their internalization into the cell by endocytosis. Therefore, cells can modulate their adhesion by dynamic recruitment of adhesion and repeller molecules to the contact area [102]. In myelin, adhesion between lamellae occurs over a large area and thus is considered as a bilayer-to-bilayer interaction, which is in general prevented by repulsive forces generated by thermal undulation and glycocalyx components [106, 107]. To organize an intermembrane space around 2–3 nm between adjacent layers, molecules with large and charged extracellular domains must be excluded from the myelin membrane. Most of these molecules are glycoproteins, which contain large and negatively charged polysaccharide chains and would, thus, induce steric and electrostatic repulsion between the myelin layers [108, 109]. As a result, the adhesion of myelin appears to be regulated by competition between van der Waals attractive forces and electrostatic repulsion, generated from charged glycoproteins and glycolipids.
Instead of dynamic internalization from myelin membrane, repellers are likely to be synthesized in lower amounts as oligodendrocytes differentiate. Indeed, transcriptome analysis of oligodendrocytes has revealed a striking reduction in the expression levels of many enzymes involved in the glycoproteins and glycolipids synthesis during cell differentiation [110]. Proliferating oligodendrocyte precursors contain many glycocalyx components such as chondroitin sulfate proteoglycans 4 (NG2), polysialic acid-neural cell adhesion molecule (PSA-NCAM) and CD44, which are abolished from mature cells [111–116].
Heparan sulfate proteoglycans, such as syndecan, perlecan, and glypican, are also developmentally regulated during oligodendrocyte-lineage progression. A significant degree of these heparan sulfate proteoglycans are shed from the cell surface by proteolytic cleavage by matrix metalloproteinases [117]. Enzymatic degradation of sugar residues is another possible mechanism for glycocalyx removal from myelin. The main evidence is provided by the finding of intrinsic sialidase activity of myelin which is elevated during the period of active myelination [118, 119]. The barrier function of MBP is also likely to contribute to the reduction of glycoprotein from compacted myelin [83]. By reducing glycoprotein content, the formation of the major dense line by MBP could indirectly be coupled to the adhesion of the extracellular leaflets of the myelin bilayers. The reduction of glycocalyx components is likely to constitute a major mechanism to eliminate the possible electrostatic and steric repulsive forces in order to uncover non-specific, weak generic interactions that bring the myelin lamellae into close contact at their extracellular surfaces [120].
Role of proteolipid protein in CNS myelin compaction
One important question is whether removal of repulsive structures from myelin surface is co-ordinated with the expression of specific molecules with adhesive properties on the membrane. The main focus has been on proteolipid protein (PLP), which together with its splice isoform DM20 is the most abundant transmembrane protein in CNS myelin [121]. PLP is a highly hydrophobic molecule and has been proposed to be responsible for tight opposition of membrane sheaths via its hydrophilic extracellular domains [122]. However, mice lacking PLP and DM20 generate relatively normal myelin, but develop axonal degeneration and swelling upon aging [123–125]. Although the myelin from these mice is compacted in vivo it is physically unstable during processing for electron microscopic analysis [123, 124, 126, 127], suggesting a possible function of PLP in myelin adhesion and stability. Liposome preparation from myelin-derived lipids has revealed a reduction in vesicle aggregation in the absence of PLP. Atomic force microscopy analysis has also demonstrated weaker adhesion forces between two membranes upon deletion of PLP from the myelin [120]. In addition reconstitution of PLP into liposomes has shown that PLP induces rolling, but not fusion of lipid bilayer to create myelin-like structures in vitro [128]. Altogether, these studies suggest a function of PLP in the adhesion and stabilizing of myelin, but how these interactions occur is not yet established. The interaction of the extracellular domains of PLP molecules in trans is one scenario. Another possibility is the interaction of PLP/DM20 with myelin lipids at the opposing membrane. The evidence comes from the double knockout mice for PLP and galactosylcerebroside (GalC), which exhibit a large number of unmyelinated axons [129]. However, the adhesive properties of PLP must be weak as mice lacking PLP have only minor ultrastructural abnormalities [124]. We therefore suggest that loss of repulsive forces that uncovers weak generic interactions is the driving force in the assembly of the extracellular surface of myelin lamellae over long distances (Fig. 2) [120]. The reason why oligodendrocytes appear to use weak forces for the alignment of extracellular membrane surfaces might lie in the way myelin is generated. It is likely that the newly synthesized myelin membrane have to glide along each other, which requires dynamic and weak connections.
Another protein, which might be involved in myelin adhesion, is OSP/claudin 11 [130]. Claudin 11 forms the radial component, a junctional complex that is believed to stabilize the apposition of myelin membranes in the internode [130]. The radial component is formed late in myelin biogenesis and is likely to provide additional structural support once myelin generation is terminated [131, 132]. Interestingly, mice lacking both PLP and OSP/claudin 11 develop severe structural abnormalities, demonstrating that weak generic forces are not sufficient in maintaining myelin ultrastructure over longer time spans [133].
In addition, the specific interactions of lipid headgroups on the opposing extracellular leaflets in myelin are likely to play a role as well. During oligodendrocyte differentiation, there is a significant change in myelin lipid synthesis with an upregulation of glycosphingolipids, GalC and its derivative sulfatide synthesis [134, 135]. The carbohydrate–carbohydrate trans-interaction between these two lipids from opposing membranes mediated by calcium cations has been reported [136–138]. Analysis of artificial bilayers has also revealed stronger attractive forces between opposing galactosylceramides bilayers as compared to the forces between two phosphatidylcholine bilayers [139]. Furthermore, the synthesis of negatively charged and repulsive gangliosides is reduced at the onset of myelination [140]. This will likely result in an increase in the adhesive properties of myelin as the introduction of exogenous gangliosides to differentiated oligodendrocyte has been shown to weaken the interaction of the extracellular leaflets of the myelin membranes with each other [120] Thus, the rearrangement of myelin membrane lipid composition and the down-regulation of glycocalyx components are essential in providing myelin its adhesive properties.
Myelin disorganization in pathological conditions
The destabilization of myelin membrane integrity contributes to several pathological conditions such as multiple sclerosis, stroke, neurodegenerative disease and psychiatric disorders [141–145]. One possible mechanism to explain how myelin is broken down in diseases is by changes in the adhesion forces between myelin layers. For instance, during the chronic phase of secondary degeneration after rat optic nerve transection, sustained decompaction of myelin membrane along with swelling of myelinated axons leads to progressive loss of visual function [146]. In addition, an inflammatory attack on myelin as for example seen in MS, results in a splitting of the myelin lamellae followed by a vacuolization of the myelin sheath [147].
It is possible that changes local ionic strength and pH reduce the adhesion of the myelin lamellae and decrease myelin stability. For example, affecting the adhesive properties of MBP may trigger myelin decompaction and instability. Local ionic strength and pH are factors that are known to influence the membrane interaction of MBP [97, 148–150]. Thus, alteration in proton and ion concentration may regulate protein-bilayer interaction by affecting membrane surface charges and MBP ionization. Another possible mechanism of MBP dysfunction is abnormal posttranslational modifications [9, 11, 33, 151]. The prominent form of MBP in healthy myelin has no or only few posttranslational modifications, while a higher degree of modifications have been observed in multiple sclerosis [58]. In multiple sclerosis, the increase in deimination of MBP may reduce its interaction with the lipid bilayer [33, 152]. Interestingly, the severity of the disease correlates with the degree of MBP deimination [153]. Changes in post-translational modification might also interfere with the ability of MBP to self-assemble and polymerize. Alteration in the lipid composition of myelin is another possible mechanism of how the function of MBP could be affected. For example, in ceramide synthase 2 knockout mice, which do not produce glycosphingolipids with long chain fatty acids, myelin development is accompanied by loss of myelin stability and a reduction of MBP levels [154, 155].
Conclusions
Here, we propose several mechanisms of how the myelin bilayer is brought into close contact at the extracellular and cytoplasmic leaflet to generate a tightly packed membrane stack. The interaction of MBP with the cytoplasmic leaflet results in charge neutralization and may trigger its polymerization into a fibrous network that drives membrane zippering and protein extrusion. The interaction of MBP with the membrane is likely to be complex and not simply mediated by electrostatic, but also by hydrophobic lipid interactions. One major consequence of the generation of a network of MBP at the cytocortex, is the depletion of most peripheral and membrane proteins from compacted myelin. This appears to occur by two different mechanisms: protein extrusion and the generation of a diffusion barrier. A protein-poor membrane, lacking major glycoproteins at the extracellular leaflet, is likely to uncover weak generic forces that promote the association of the bilayer over longer distances, thereby driving membrane compaction at the extracellular site. Another mechanism that contributes to the depletion of the glycocalyx is a reduced synthesis as oligodendrocytes differentiate.
We have discussed the mechanism of myelin assembly by focusing on the components that reside within myelin itself; however, it is clear that each of these molecules is under the control of different signaling machineries. A challenging task is therefore to link the mechanisms that govern the organization of proteins and lipids to the various signaling pathways that have been implicated in myelination. Another challenge is to understand myelination with more temporal and spatial details. We still do not know where and when myelin membrane zippering takes place as the membrane spreads and wraps around an axon. In addition, recent experiments have provided evidence that myelin is not as stable as previously thought, but undergoes considerable remodeling under environmental stimuli [156–160]. How these epigenetic and genetic programs feed into the myelin membrane synthesis machinery is another open area of research that will bring us closer to a system-level understanding of myelination.
Acknowledgments
We thank Natalia Manrique-Hoyos for editing the manuscript. M.S. is supported by an ERC Strating Grant and by Grants from the German Research Foundation (SI 746/9-1; SI 746/10-1; TRR43), a GIF grant, the Tschira Stiftung and from the BMBF (E-rare).
Contributor Information
Mostafa Bakhti, Phone: +49-551-3899533, FAX: +49-551-3899201, Email: mostafa.bakhti@helmholtz-muenchen.de.
Mikael Simons, Phone: +49-551-3899533, FAX: +49-551-3899201, Email: msimons@gwdg.de.
References
- 1.Hartline DK, Colman DR. Rapid conduction and the evolution of giant axons and myelinated fibers. Curr Biol. 2007;17:R29–R35. doi: 10.1016/j.cub.2006.11.042. [DOI] [PubMed] [Google Scholar]
- 2.Sherman DL, Brophy PJ. Mechanisms of axon ensheathment and myelin growth. Nat Rev Neurosci. 2005;6:683–690. doi: 10.1038/nrn1743. [DOI] [PubMed] [Google Scholar]
- 3.Baumann N, Pham-Dinh D. Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol Rev. 2001;81:871–927. doi: 10.1152/physrev.2001.81.2.871. [DOI] [PubMed] [Google Scholar]
- 4.Aggarwal S, Yurlova L, Simons M. Central nervous system myelin: structure, synthesis and assembly. Trends Cell Biol. 2011;21:585–593. doi: 10.1016/j.tcb.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 5.Piper RC, Katzmann DJ. Biogenesis and function of multivesicular bodies. Annu Rev Cell Dev Biol. 2007;23:519–547. doi: 10.1146/annurev.cellbio.23.090506.123319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Platt FM, Boland B, Van Der Spoel AC. The cell biology of disease: lysosomal storage disorders: the cellular impact of lysosomal dysfunction. J Cell Biol. 2012;199:723–734. doi: 10.1083/jcb.201208152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmitz G, Müller G. Structure and function of lamellar bodies, lipid–protein complexes involved in storage and secretion of cellular lipids. J Lipid Res. 1991;32:1539–1570. [PubMed] [Google Scholar]
- 8.Weinbaum S, Tarbell JM, Damiano ER. The structure and function of the endothelial glycocalyx layer. Annu Rev Biomed Eng. 2007;9:121–167. doi: 10.1146/annurev.bioeng.9.060906.151959. [DOI] [PubMed] [Google Scholar]
- 9.Harauz G, Ladizhansky V, Boggs JM. Structural polymorphism and multifunctionality of myelin basic protein. Biochemistry. 2009;48:8094–8104. doi: 10.1021/bi901005f. [DOI] [PubMed] [Google Scholar]
- 10.Harauz G, Ishiyama N, Hill CMD, et al. Myelin basic protein-diverse conformational states of an intrinsically unstructured protein and its roles in myelin assembly and multiple sclerosis. Micron. 2004;35:503–542. doi: 10.1016/j.micron.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 11.Hill CM, Bates IR, White GF, et al. Effects of the osmolyte trimethylamine-N-oxide on conformation, self-association, and two-dimensional crystallization of myelin basic protein. J Struct Biol. 2002;139:13–26. doi: 10.1016/s1047-8477(02)00513-0. [DOI] [PubMed] [Google Scholar]
- 12.Tompa P. Intrinsically unstructured proteins. Trends Biochem Sci. 2002;27:527–533. doi: 10.1016/s0968-0004(02)02169-2. [DOI] [PubMed] [Google Scholar]
- 13.Tompa P (2009) Structure and function of intrinsically disordered proteins. Matrix 331
- 14.Dyson HJ, Wright PE. Intrinsically unstructured proteins and their functions. Nat Rev Mol Cell Biol. 2005;6:197–208. doi: 10.1038/nrm1589. [DOI] [PubMed] [Google Scholar]
- 15.Tompa P, Bánki P, Bokor M, et al. Protein–water and protein–buffer interactions in the aqueous solution of an intrinsically unstructured plant dehydrin: NMR Intensity and DSC aspects. Biophys J. 2006;91:2243–2249. doi: 10.1529/biophysj.106.084723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mouillon J-M, Eriksson SK, Harryson P. Mimicking the plant cell interior under water stress by macromolecular crowding: disordered dehydrin proteins are highly resistant to structural collapse. Plant Physiol. 2008;148:1925–1937. doi: 10.1104/pp.108.124099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Polverini E, Coll EP, Tieleman DP, Harauz G. Conformational choreography of a molecular switch region in myelin basic protein–molecular dynamics shows induced folding and secondary structure type conversion upon threonyl phosphorylation in both aqueous and membrane-associated environments. Biochim Biophys Acta. 2011;1808:674–683. doi: 10.1016/j.bbamem.2010.11.030. [DOI] [PubMed] [Google Scholar]
- 18.Smith GST, Chen L, Bamm VV, et al. The interaction of zinc with membrane-associated 18.5 kDa myelin basic protein: an attenuated total reflectance-Fourier transform infrared spectroscopic study. Amino Acids. 2010;39:739–750. doi: 10.1007/s00726-010-0513-7. [DOI] [PubMed] [Google Scholar]
- 19.Vassall KA, Bessonov K, De Avila M, et al. The effects of threonine phosphorylation on the stability and dynamics of the central molecular switch region of 18.5-kDa myelin basic protein. PloS one. 2013;8:e68175. doi: 10.1371/journal.pone.0068175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chernoff GF. Shiverer: an autosomal recessive mutant mouse with myelin deficiency. J Hered. 1981;72:128. doi: 10.1093/oxfordjournals.jhered.a109442. [DOI] [PubMed] [Google Scholar]
- 21.Roach A, Boylan K, Horvath S, et al. Characterization of cloned cDNA representing rat myelin basic protein: absence of expression in brain of shiverer mutant mice. Cell. 1983;34:799–806. doi: 10.1016/0092-8674(83)90536-6. [DOI] [PubMed] [Google Scholar]
- 22.Aggarwal S, Snaidero N, Pähler G, et al. Myelin membrane assembly is driven by a phase transition of myelin basic proteins into a cohesive protein meshwork. PLoS Biol. 2013;11:e1001577. doi: 10.1371/journal.pbio.1001577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith R. The basic protein of CNS myelin: its structure and ligand binding. J Neurochem. 1992;59:1589–1608. doi: 10.1111/j.1471-4159.1992.tb10989.x. [DOI] [PubMed] [Google Scholar]
- 24.Kattnig DR, Bund T, Boggs JM et al (2012) Lateral self-assembly of 18.5-kDa myelin basic protein (MBP) charge component-C1 on membranes. Biochim Biophys Acta, pp 1–12 [DOI] [PubMed]
- 25.Hyman AA, Brangwynne CP. Beyond stereospecificity: liquids and mesoscale organization of cytoplasm. Dev Cell. 2011;21:14–16. doi: 10.1016/j.devcel.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 26.Weber SC, Brangwynne CP. Getting RNA and protein in phase. Cell. 2012;149:1188–1191. doi: 10.1016/j.cell.2012.05.022. [DOI] [PubMed] [Google Scholar]
- 27.Hyman AA, Simons K. Beyond oil and water—phase transitions in cells. Science. 2012;337:1047–1049. doi: 10.1126/science.1223728. [DOI] [PubMed] [Google Scholar]
- 28.Brangwynne CP, Eckmann CR, Courson DS, et al. Germline P granules are liquid droplets that localize by controlled dissolution/condensation. Science. 2009;324:1729–1732. doi: 10.1126/science.1172046. [DOI] [PubMed] [Google Scholar]
- 29.Kato M, Han TW, Xie S, et al. Cell-free formation of RNA granules: low complexity sequence domains form dynamic fibers within hydrogels. Cell. 2012;149:753–767. doi: 10.1016/j.cell.2012.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li P, Banjade S, Cheng H-C, et al. Phase transitions in the assembly of multivalent signalling proteins. Nature. 2012;483:336–340. doi: 10.1038/nature10879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frey S, Richter RP, Görlich D. FG-rich repeats of nuclear pore proteins form a three-dimensional meshwork with hydrogel-like properties. Science. 2006;314:815–817. doi: 10.1126/science.1132516. [DOI] [PubMed] [Google Scholar]
- 32.Bund T, Boggs JM, Harauz G, et al. Copper uptake induces self-assembly of 18.5 kDa myelin basic protein (MBP) Biochem J. 2010;99:3020–3028. doi: 10.1016/j.bpj.2010.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ishiyama N, Bates IR, Hill CM, et al. The effects of deimination of myelin basic protein on structures formed by its interaction with phosphoinositide-containing lipid monolayers. J Struct Biol. 2001;136:30–45. doi: 10.1006/jsbi.2001.4421. [DOI] [PubMed] [Google Scholar]
- 34.Smith R. The encephalitogenic protein of myelin forms hexamers in which the polypeptides have a pleated-sheet structure. FEBS Lett. 1985;183:331–334. doi: 10.1016/0014-5793(85)80804-8. [DOI] [PubMed] [Google Scholar]
- 35.Harauz G, Libich DS. The classic basic protein of myelin–conserved structural motifs and the dynamic molecular barcode involved in membrane adhesion and protein–protein interactions. Curr Protein Pept Sci. 2009;10:196–215. doi: 10.2174/138920309788452218. [DOI] [PubMed] [Google Scholar]
- 36.Bates IR, Boggs JM, Feix JBHG. Membrane-anchoring and charge effects in the interaction of myelin basic protein with lipid bilayers studied by site-directed spin labeling. J Biol Chem. 2003;278:29041–29047. doi: 10.1074/jbc.M302766200. [DOI] [PubMed] [Google Scholar]
- 37.Bates IR, Feix JB, Boggs JM, Harauz G. An immunodominant epitope of myelin basic protein is an amphipathic alpha-helix. J Biol Chem. 2004;279:5757–5764. doi: 10.1074/jbc.M311504200. [DOI] [PubMed] [Google Scholar]
- 38.Stankoff B, Freeman L, Aigrot M-S, et al. Imaging central nervous system myelin by positron emission tomography in multiple sclerosis using [methyl-11C]-2-(4′-methylaminophenyl)-6-hydroxybenzothiazole. Ann Neurol. 2011;69:673–680. doi: 10.1002/ana.22320. [DOI] [PubMed] [Google Scholar]
- 39.Fowler DM, Koulov AV, Balch WE, Kelly JW. Functional amyloid—from bacteria to humans. Trends Biochem Sci. 2007;32:217–224. doi: 10.1016/j.tibs.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 40.Chiti F, Dobson CM. Protein misfolding, functional amyloid, and human disease. Annu Rev Biochem. 2006;75:333–366. doi: 10.1146/annurev.biochem.75.101304.123901. [DOI] [PubMed] [Google Scholar]
- 41.Alberti S, Halfmann R, King O, et al. A systematic survey identifies prions and illuminates sequence features of prionogenic proteins. Cell. 2009;137:146–158. doi: 10.1016/j.cell.2009.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith R. Self-association of myelin basic protein: enhancement by detergents and lipids. Biochemistry. 1982;21:2697–2701. doi: 10.1021/bi00540a019. [DOI] [PubMed] [Google Scholar]
- 43.Ader C, Frey S, Maas W, et al. Amyloid-like interactions within nucleoporin FG hydrogels. Proc Natl Acad Sci USA. 2010;107:6281–6285. doi: 10.1073/pnas.0910163107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maji SK, Perrin MH, Sawaya MR, et al. Functional amyloids as natural storage of peptide hormones in pituitary secretory granules. Science. 2009;325:328–332. doi: 10.1126/science.1173155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Uversky VN, Oldfield CJ, Dunker AK. Intrinsically disordered proteins in human diseases: introducing the D2 concept. Annu Rev Biophys. 2008;37:215–246. doi: 10.1146/annurev.biophys.37.032807.125924. [DOI] [PubMed] [Google Scholar]
- 46.Chivet M, Hemming F, Pernet-Gallay K, et al. Emerging role of neuronal exosomes in the central nervous system. Frontiers Physiol. 2012;3:145. doi: 10.3389/fphys.2012.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Emery B, Agalliu D, Cahoy JD, et al. Myelin gene regulatory factor is a critical transcriptional regulator required for CNS myelination. Cell. 2009;138:172–185. doi: 10.1016/j.cell.2009.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bujalka H, Koenning M, Jackson S, et al. MYRF is a membrane-associated transcription factor that autoproteolytically cleaves to directly activate myelin genes. PLoS Biol. 2013;11:e1001625. doi: 10.1371/journal.pbio.1001625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li Z, Park Y, Marcotte EM. A bacteriophage tailspike domain promotes self-cleavage of a human membrane-bound transcription factor, the myelin regulatory factor MYRF. PLoS Biol. 2013;11:e1001624. doi: 10.1371/journal.pbio.1001624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wake H, Lee PR, Fields RD. Control of local protein synthesis and initial events in myelination by action potentials. Science. 2011;333:1647–1651. doi: 10.1126/science.1206998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lyons DA, Naylor SG, Scholze A, Talbot WS. Kif1b is essential for mRNA localization in oligodendrocytes and development of myelinated axons. Nat Genet. 2009;41:854–858. doi: 10.1038/ng.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Laursen LS, Chan CW, Ffrench-Constant C. Translation of myelin basic protein mRNA in oligodendrocytes is regulated by integrin activation and hnRNP-K. J Cell Biol. 2011;192:797–811. doi: 10.1083/jcb.201007014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.White R, Gonsior C, Krämer-Albers E-M, et al. Activation of oligodendroglial Fyn kinase enhances translation of mRNAs transported in hnRNP A2-dependent RNA granules. J Cell Biol. 2008;181:579–586. doi: 10.1083/jcb.200706164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kosturko LD, Maggipinto MJ, D’Sa C, et al. The microtubule-associated protein tumor overexpressed gene binds to the RNA trafficking protein heterogeneous nuclear ribonucleoprotein A2. Mol Biol Cell. 2005;16:1938–1947. doi: 10.1091/mbc.E04-08-0709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kosturko LD, Maggipinto MJ, Korza G, et al. Heterogeneous nuclear ribonucleoprotein (hnRNP) E1 binds to hnRNP A2 and inhibits translation of A2 response element mRNAs. Mol Biol Cell. 2006;17:3521–3533. doi: 10.1091/mbc.E05-10-0946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.White R, Gonsior C, Bauer NM, et al. HnRNP F is a novel component of oligodendroglial RNA transport granules contributing to the regulation of MBP protein synthesis. J Biol Chem. 2011;287:1742–1754. doi: 10.1074/jbc.M111.235010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bauer NM, Moos C, van Horssen J, et al. Myelin basic protein synthesis is regulated by small non-coding RNA 715. EMBO Rep. 2012;13:827–834. doi: 10.1038/embor.2012.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim JK, Mastronardi FG, Wood DD, et al. Multiple sclerosis: an important role for post-translational modifications of myelin basic protein in pathogenesis. Mol Cell Proteomics. 2003;2:453–462. doi: 10.1074/mcp.M200050-MCP200. [DOI] [PubMed] [Google Scholar]
- 59.Zhang C, Walker AK, Zand R, et al. Myelin basic protein undergoes a broader range of modifications in mammals than in lower vertebrates. J Proteome Res. 2012;11:4791–4802. doi: 10.1021/pr201196e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Boggs JM. Myelin basic protein: a multifunctional protein. Cell Mol Life Sci. 2006;63:1945–1961. doi: 10.1007/s00018-006-6094-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ozkirimli E, Yadav SS, Miller WT, Post CB. An electrostatic network and long-range regulation of Src kinases. Protein Sci. 2008;17:1871–1880. doi: 10.1110/ps.037457.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McLaughlin S, Aderem A. The myristoyl-electrostatic switch: a modulator of reversible protein-membrane interactions. Trends Biochem Sci. 1995;20:272–276. doi: 10.1016/s0968-0004(00)89042-8. [DOI] [PubMed] [Google Scholar]
- 63.Ahmed MAM, De Avila M, Polverini E, et al. Solution NMR structure and molecular dynamics simulations of murine 18.5-kDa myelin basic protein segment (S72-S107) in association with dodecylphosphocholine micelles. Biochemistry. 2012 doi: 10.1021/bi300998x. [DOI] [PubMed] [Google Scholar]
- 64.Homchaudhuri L, De Avila M, Nilsson SB, et al. Secondary structure and solvent accessibility of a calmodulin-binding C-terminal segment of membrane-associated myelin basic protein. Biochemistry. 2010;49:8955–8966. doi: 10.1021/bi100988p. [DOI] [PubMed] [Google Scholar]
- 65.Bamm VV, De Avila M, Smith GST, et al. Structured functional domains of myelin basic protein: cross talk between actin polymerization and Ca(2+)-dependent calmodulin interaction. Biophys J. 2011;101:1248–1256. doi: 10.1016/j.bpj.2011.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Goldbaum O, Richter-Landsberg C. Stress proteins in oligodendrocytes: differential effects of heat shock and oxidative stress. J Neurochem. 2001;78:1233–1242. doi: 10.1046/j.1471-4159.2001.00507.x. [DOI] [PubMed] [Google Scholar]
- 67.Aquino DA, Peng D, Lopez C, Farooq M. The constitutive heat shock protein-70 is required for optimal expression of myelin basic protein during differentiation of oligodendrocytes. Neurochem Res. 1998;23:413–420. doi: 10.1023/a:1022473904335. [DOI] [PubMed] [Google Scholar]
- 68.Cwiklinska H, Mycko MP, Luvsannorov O, et al. Heat shock protein 70 associations with myelin basic protein and proteolipid protein in multiple sclerosis brains. Int Immunol. 2003;15:241–249. doi: 10.1093/intimm/dxg022. [DOI] [PubMed] [Google Scholar]
- 69.Riccio P, Giovannelli S, Bobba A, et al. Specificity of zinc binding to myelin basic protein. Neurochem Res. 1995;20:1107–1113. doi: 10.1007/BF00995566. [DOI] [PubMed] [Google Scholar]
- 70.Smeyers-Verbeke J, Defrise-Gussenhoven E, Ebinger G, Löwenthal AMD. Distribution of Cu and Zn in human brain tissue. Clin Chim Acta. 1974;51:309–314. doi: 10.1016/0009-8981(74)90317-9. [DOI] [PubMed] [Google Scholar]
- 71.Earl C, Chantry A, Mohammad N, Glynn P. Zinc ions stabilise the association of basic protein with brain myelin membranes. J Neurochem. 1988;51:718–724. doi: 10.1111/j.1471-4159.1988.tb01803.x. [DOI] [PubMed] [Google Scholar]
- 72.Liuzzi GM, Ventola A, Rizzo T, et al. Zinc as an inhibitor of myelin basic protein proteolytic breakdown in the central nervous system. Acta Neurol. 1991;13:153–161. [PubMed] [Google Scholar]
- 73.Berlet HH, Bischoff H, Weinhardt F. Divalent metals of myelin and their differential binding by myelin basic protein of bovine central nervous system. Neurosci Lett. 1994;179:75–78. doi: 10.1016/0304-3940(94)90938-5. [DOI] [PubMed] [Google Scholar]
- 74.Cavatorta P, Giovanelli S, Bobba A, et al. Myelin basic protein interaction with zinc and phosphate: fluorescence studies on the water-soluble form of the protein. Biophys J. 1994;66:1174–1179. doi: 10.1016/S0006-3495(94)80899-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tsang D, Tsang YS, Ho WK, Wong RN. Myelin basic protein is a zinc-binding protein in brain: possible role in myelin compaction. Neurochem Res. 1997;22:811–819. doi: 10.1023/a:1022031825923. [DOI] [PubMed] [Google Scholar]
- 76.Nuzzo S, Meneghini C, Mobilioo S, et al. An X-ray absorption spectroscopy study of the zinc environment in Langmuir-Blodgett phospholipid multilayers. Biophys J. 2002;83:3507–3512. doi: 10.1016/S0006-3495(02)75350-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tsutsui S, Stys PK. Metabolic injury to axons and myelin. Exp Neurol. 2012 doi: 10.1016/j.expneurol.2012.04.016. [DOI] [PubMed] [Google Scholar]
- 78.Nawaz S, Kippert A, Saab AS, et al. Phosphatidylinositol 4,5-bisphosphate-dependent interaction of myelin basic protein with the plasma membrane in oligodendroglial cells and its rapid perturbation by elevated calcium. J Neurosci. 2009;29:4794–4807. doi: 10.1523/JNEUROSCI.3955-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Musse AA, Gao W, Homchaudhuri L, et al. Myelin basic protein as a “PI(4,5)P2-modulin”: a new biological function for a major central nervous system protein. Biochemistry. 2008;47:10372–10382. doi: 10.1021/bi801302b. [DOI] [PubMed] [Google Scholar]
- 80.Musse AA, Gao W, Rangaraj G, et al. Myelin basic protein co-distributes with other PI(4,5)P2-sequestering proteins in Triton X-100 detergent-resistant membrane microdomains. Neurosci Lett. 2009;450:32–36. doi: 10.1016/j.neulet.2008.11.022. [DOI] [PubMed] [Google Scholar]
- 81.Lamensa JW, Moscarello MA. Deimination of human myelin basic protein by a peptidylarginine deiminase from bovine brain. J Neurochem. 1993;61:987–996. doi: 10.1111/j.1471-4159.1993.tb03612.x. [DOI] [PubMed] [Google Scholar]
- 82.Smith GST, Paez PM, Spreuer V, et al. Classical 18.5-and 21.5-kDa isoforms of myelin basic protein inhibit calcium influx into oligodendroglial cells, in contrast to golli isoforms. J Neurosci Res. 2011;89:467–480. doi: 10.1002/jnr.22570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Aggarwal S, Yurlova L, Snaidero N, et al. A size barrier limits protein diffusion at the cell surface to generate lipid-rich myelin-membrane sheets. Dev Cell. 2011;21:445–456. doi: 10.1016/j.devcel.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 84.Dyer CA, Philibotte TM, Billings-Gagliardi S, Wolf MK. Cytoskeleton in myelin-basic-protein-deficient shiverer oligodendrocytes. Dev Neurosci. 1995;17:53–62. doi: 10.1159/000111273. [DOI] [PubMed] [Google Scholar]
- 85.Dyer CA, Phillbotte T, Wolf MK, Billings-Gagliardi S. Regulation of cytoskeleton by myelin components: studies on shiverer oligodendrocytes carrying an Mbp transgene. Dev Neurosci. 1997;19:395–409. doi: 10.1159/000111237. [DOI] [PubMed] [Google Scholar]
- 86.Ishiyama N, Hill CM, Bates IR, Harauz G. The formation of helical tubular vesicles by binary monolayers containing a nickel-chelating lipid and phosphoinositides in the presence of basic polypeptides. Chem Phys Lipids. 2006;114:103–111. doi: 10.1016/s0009-3084(02)00002-6. [DOI] [PubMed] [Google Scholar]
- 87.Zuchero JB, Barres B. Between the sheets: a molecular sieve makes myelin membranes. Dev Cell. 2011;21:385–386. doi: 10.1016/j.devcel.2011.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hülsmann BB, Labokha AA, Görlich D. The permeability of reconstituted nuclear pores provides direct evidence for the selective phase model. Cell. 2012;150:738–751. doi: 10.1016/j.cell.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 89.Singer SJ, Nicolson GL. The fluid mosaic model of the structure of cell membranes. Science. 1972;175:720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- 90.Fitzner D, Schneider A, Kippert A, et al. Myelin basic protein-dependent plasma membrane reorganization in the formation of myelin. EMBO J. 2006;25:5037–5048. doi: 10.1038/sj.emboj.7601376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yurlova L, Kahya N, Aggarwal S, et al. Self-segregation of myelin membrane lipids in model membranes. Biophys J. 2011;101:2713–2720. doi: 10.1016/j.bpj.2011.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Boggs JM, Moscarello M, Papahadjopoulos D. Phase separation of acidic and neutral phospholipids induced by human myelin basic protein. Biochemistry. 1977;16:5420–5426. doi: 10.1021/bi00644a003. [DOI] [PubMed] [Google Scholar]
- 93.Min Y, Kristiansen K, Boggs JM, et al. Interaction forces and adhesion of supported myelin lipid bilayers modulated by myelin basic protein. Proc Natl Acad Sci USA. 2009;106:3154–3159. doi: 10.1073/pnas.0813110106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rosetti CM, Maggio B, Wilke N. Micron-scale phase segregation in lipid monolayers induced by myelin basic protein in the presence of a cholesterol analog. Biochim Biophys Acta. 2010;1798:498–505. doi: 10.1016/j.bbamem.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 95.Rosetti CM, Maggio B, Oliveira RG. The self-organization of lipids and proteins of myelin at the membrane interface. Molecular factors underlying the microheterogeneity of domain segregation. Biochim Biophys Acta. 2008;1778:1665–1675. doi: 10.1016/j.bbamem.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 96.Ter Beest MB, Hoekstra D. Interaction of myelin basic protein with artificial membranes. Parameters governing binding, aggregation and dissociation. Eur J Biochem. 1993;211:689–696. doi: 10.1111/j.1432-1033.1993.tb17597.x. [DOI] [PubMed] [Google Scholar]
- 97.Jo E, Boggs JM. Aggregation of acidic lipid vesicles by myelin basic protein: dependence on potassium concentration. Biochemistry. 1995;34:13705–13716. doi: 10.1021/bi00041a053. [DOI] [PubMed] [Google Scholar]
- 98.Mac Millan SV, Ishiyama N, White GF, et al. Myelin basic protein component C1 in increasing concentrations can elicit fusion, aggregation, and fragmentation of myelin-like membranes. Eur J Cell Biol. 2000;79:327–335. doi: 10.1078/S0171-9335(04)70036-9. [DOI] [PubMed] [Google Scholar]
- 99.Hu Y, Israelachvili J. Lateral reorganization of myelin lipid domains by myelin basic protein studied at the air–water interface. Colloids Surf B Biointerface. 2008;62:22–30. doi: 10.1016/j.colsurfb.2007.09.028. [DOI] [PubMed] [Google Scholar]
- 100.Debruin LS, Harauz G. White matter rafting–membrane microdomains in myelin. Neurochem Res. 2007;32:213–228. doi: 10.1007/s11064-006-9137-4. [DOI] [PubMed] [Google Scholar]
- 101.DeBruin LS, Haines JD, Wellhauser LA, et al. Developmental partitioning of myelin basic protein into membrane microdomains. J Neurosci Res. 2005;80:211–225. doi: 10.1002/jnr.20452. [DOI] [PubMed] [Google Scholar]
- 102.Sackmann E, Goennenwein S. Cell adhesion as dynamic interplay of lock-and-key, generic and elastic forces. Prog Theor Phys Supp. 2006;165:78–99. [Google Scholar]
- 103.Sackmann E, Bruinsma RF. Cell adhesion as wetting transition? ChemPhysChem. 2002;3:262–269. doi: 10.1002/1439-7641(20020315)3:3<262::AID-CPHC262>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 104.Rodríguez-Fernández JL, Riol-Blanco L, Delgado-Martín C. What is an immunological synapse? Microbes Infect. 2010;12:438–445. doi: 10.1016/j.micinf.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 105.Reichardt P, Dornbach B, Gunzer M. APC, T cells, and the immune synapse. Curr Top Microbiol Immunol. 2010;340:229–249. doi: 10.1007/978-3-642-03858-7_12. [DOI] [PubMed] [Google Scholar]
- 106.Foa C, Soler M, Benoliel A-M, Bongrand P. Steric stabilization and cell adhesion. J Mater Sci Mater Med. 1996;7:141–148. [Google Scholar]
- 107.Bell GI, Dembo M, Bongrand P. Cell adhesion. Competition between nonspecific repulsion and specific bonding. Biophys J. 1984;45:1051–1064. doi: 10.1016/S0006-3495(84)84252-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sabri S, Soler M, Foa C, et al. Glycocalyx modulation is a physiological means of regulating cell adhesion. J Cell Sci. 2000;113:1589–1600. doi: 10.1242/jcs.113.9.1589. [DOI] [PubMed] [Google Scholar]
- 109.Morris JE. Proteoglycans and the modulation of cell adhesion by steric exclusion. Dev Dyn. 1993;196:246–251. doi: 10.1002/aja.1001960405. [DOI] [PubMed] [Google Scholar]
- 110.Dugas JC, Tai YC, Speed TP, et al. Functional genomic analysis of oligodendrocyte differentiation. J Neurosci. 2006;26:10967–10983. doi: 10.1523/JNEUROSCI.2572-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nishiyama A, Komitova M, Suzuki R, Zhu X. Polydendrocytes (NG2 cells): multifunctional cells with lineage plasticity. Nat Rev Neurosci. 2009;10:9–22. doi: 10.1038/nrn2495. [DOI] [PubMed] [Google Scholar]
- 112.Bouvier-Labit C, Liprandi A, Monti G, et al. CD44H is expressed by cells of the oligodendrocyte lineage and by oligodendrogliomas in humans. J Neurooncol. 2002;60:127–134. doi: 10.1023/a:1020630732625. [DOI] [PubMed] [Google Scholar]
- 113.Tuohy TMF, Wallingford N, Liu Y, et al. CD44 overexpression by oligodendrocytes: a novel mouse model of inflammation-independent demyelination and dysmyelination. Glia. 2004;47:335–345. doi: 10.1002/glia.20042. [DOI] [PubMed] [Google Scholar]
- 114.Nait Oumesmar B, Vignais L, Duhamel-Clérin E, et al. Expression of the highly polysialylated neural cell adhesion molecule during postnatal myelination and following chemically induced demyelination of the adult mouse spinal cord. Eur J Neurosci. 1995;7:480–491. doi: 10.1111/j.1460-9568.1995.tb00344.x. [DOI] [PubMed] [Google Scholar]
- 115.Stoykova LI, Beesley JS, Grinspan JB, Glick MC. ST8Sia IV mRNA corresponds with the biosynthesis of alpha2,8 sialyl polymers but not oligomers in rat oligodendrocytes. J Neurosci Res. 2001;66:497–505. doi: 10.1002/jnr.10002. [DOI] [PubMed] [Google Scholar]
- 116.Fewou SN, Ramakrishnan H, Büssow H, et al. Down-regulation of polysialic acid is required for efficient myelin formation. J Biol Chem. 2007;282:16700–16711. doi: 10.1074/jbc.M610797200. [DOI] [PubMed] [Google Scholar]
- 117.Winkler S, Stahl RC, Carey DJ, Bansal R. Syndecan-3 and perlecan are differentially expressed by progenitors and mature oligodendrocytes and accumulate in the extracellular matrix. J Neurosci Res. 2002;69:477–487. doi: 10.1002/jnr.10311. [DOI] [PubMed] [Google Scholar]
- 118.Saito M, Yu RK. Further characterization of a myelin-associated neuraminidase: properties and substrate specificity. J Neurochem. 1986;47:632–641. doi: 10.1111/j.1471-4159.1986.tb04547.x. [DOI] [PubMed] [Google Scholar]
- 119.Saito M, Yu RK. Possible role of myelin-associated neuraminidase in membrane adhesion. J Neurosci Res. 1993;36:127–132. doi: 10.1002/jnr.490360203. [DOI] [PubMed] [Google Scholar]
- 120.Bakhti M, Snaidero N, Schneider D, et al. Loss of electrostatic cell-surface repulsion mediates myelin membrane adhesion and compaction in the central nervous system. Proc Natl Acad Sci USA. 2013;110:3143–3148. doi: 10.1073/pnas.1220104110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Möbius W, Patzig J, Nave K-A, Werner HB. Phylogeny of proteolipid proteins: divergence, constraints, and the evolution of novel functions in myelination and neuroprotection. Neuron Glia Biol. 2008;4:111–127. doi: 10.1017/S1740925X0900009X. [DOI] [PubMed] [Google Scholar]
- 122.Popot JL, Pham Dinh D, Dautigny A. Major myelin proteolipid: the 4-alpha-helix topology. J Membr Biol. 1991;123:278. doi: 10.1007/BF01870411. [DOI] [PubMed] [Google Scholar]
- 123.Boison D, Büssow H, D’Urso D, et al. Adhesive properties of proteolipid protein are responsible for the compaction of CNS myelin sheaths. J Neurosci. 1995;15:5502–5513. doi: 10.1523/JNEUROSCI.15-08-05502.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Klugmann M, Schwab MH, Pühlhofer A, et al. Assembly of CNS myelin in the absence of proteolipid protein. Neuron. 1997;18:59–70. doi: 10.1016/s0896-6273(01)80046-5. [DOI] [PubMed] [Google Scholar]
- 125.Griffiths I, Klugmann M, Anderson T, et al. Axonal swellings and degeneration in mice lacking the major proteolipid of myelin. Science. 1998;280:1610–1613. doi: 10.1126/science.280.5369.1610. [DOI] [PubMed] [Google Scholar]
- 126.Rosenbluth J, Schiff R, Lam P. Effects of osmolality on PLP-null myelin structure: implications re axon damage. Brain Res. 2009;1253:191–197. doi: 10.1016/j.brainres.2008.11.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rosenbluth J, Nave K-A, Mierzwa A, Schiff R. Subtle myelin defects in PLP-null mice. Glia. 2006;54:172–182. doi: 10.1002/glia.20370. [DOI] [PubMed] [Google Scholar]
- 128.Palaniyar N, Semotok JL, Wood DD, et al. Human proteolipid protein (PLP) mediates winding and adhesion of phospholipid membranes but prevents their fusion. Biochim Biophys Acta. 1998;1415:85–100. doi: 10.1016/s0005-2736(98)00180-1. [DOI] [PubMed] [Google Scholar]
- 129.Coetzee T, Suzuki K, Nave KA, Popko B. Myelination in the absence of galactolipids and proteolipid proteins. Mol Cell Neurosci. 1999;14:41–51. doi: 10.1006/mcne.1999.0768. [DOI] [PubMed] [Google Scholar]
- 130.Gow A, Southwood CM, Li JS, et al. CNS myelin and sertoli cell tight junction strands are absent in Osp/claudin-11 null mice. Cell. 1999;99:649–659. doi: 10.1016/s0092-8674(00)81553-6. [DOI] [PubMed] [Google Scholar]
- 131.Kosaras B, Kirschner DA. Radial component of CNS myelin: junctional subunit structure and supramolecular assembly. J Neurocytol. 1990;19:187–199. doi: 10.1007/BF01217297. [DOI] [PubMed] [Google Scholar]
- 132.Karthigasan J, Kosaras B, Nguyen J, Kirschner DA. Protein and lipid composition of radial component-enriched CNS myelin. J Neurochem. 1994;62:1203–1213. doi: 10.1046/j.1471-4159.1994.62031203.x. [DOI] [PubMed] [Google Scholar]
- 133.Chow E, Mottahedeh J, Prins M, et al. Disrupted compaction of CNS myelin in an OSP/Claudin-11 and PLP/DM20 double knockout mouse. Mol Cell Neurosci. 2005;29:405–413. doi: 10.1016/j.mcn.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 134.Jackman N, Ishii A, Bansal R. Oligodendrocyte development and myelin biogenesis: parsing out the roles of glycosphingolipids. Physiology. 2009;24:290–297. doi: 10.1152/physiol.00016.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chrast R, Saher G, Nave K-A, Verheijen MHG. Lipid metabolism in myelinating glial cells: lessons from human inherited disorders and mouse models. J Lipid Res. 2011;52:419–434. doi: 10.1194/jlr.R009761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Boggs JM, Gao W, Zhao J, et al. Participation of galactosylceramide and sulfatide in glycosynapses between oligodendrocyte or myelin membranes. FEBS Lett. 2010;584:1771–1778. doi: 10.1016/j.febslet.2009.11.074. [DOI] [PubMed] [Google Scholar]
- 137.Boggs JM, Menikh A, Rangaraj G. Trans interactions between galactosylceramide and cerebroside sulfate across apposed bilayers. Biophys J. 2000;78:874–885. doi: 10.1016/S0006-3495(00)76645-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Boggs JM, Gao W, Hirahara Y. Myelin glycosphingolipids, galactosylceramide and sulfatide, participate in carbohydrate–carbohydrate interactions between apposed membranes and may form glycosynapses between oligodendrocyte and/or myelin membranes. Biochim Biophys Acta. 2008;1780:445–455. doi: 10.1016/j.bbagen.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 139.Kulkarni K, Snyder DS, McIntosh TJ. Adhesion between cerebroside bilayers. Biochemistry. 1999;38:15264–15271. doi: 10.1021/bi991725m. [DOI] [PubMed] [Google Scholar]
- 140.Cochran FB, Yu RK, Ledeen RW. Myelin gangliosides in vertebrates. J Neurochem. 1982;39:773–779. doi: 10.1111/j.1471-4159.1982.tb07959.x. [DOI] [PubMed] [Google Scholar]
- 141.Vital C, Brechenmacher C, Reiffers J, et al. Uncompacted myelin lamellae in two cases of peripheral neuropathy. Acta Neuropathol. 1983;60:252–256. doi: 10.1007/BF00691873. [DOI] [PubMed] [Google Scholar]
- 142.Vallat JM, Leboutet MJ, Jauberteau MO, et al. Widenings of the myelin lamellae in a typical Guillain-Barré syndrome. Muscle Nerve. 1994;17:378–380. doi: 10.1002/mus.880170403. [DOI] [PubMed] [Google Scholar]
- 143.Roy K, Murtie JC, El-Khodor BF, et al. Loss of erbB signaling in oligodendrocytes alters myelin and dopaminergic function, a potential mechanism for neuropsychiatric disorders. Proc Natl Acad Sci USA. 2007;104:8131–8136. doi: 10.1073/pnas.0702157104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Pollock M, Calder C, Allpress S. Peripheral nerve abnormality in multiple sclerosis. Ann Neurol. 1993;2:1–5. doi: 10.1002/ana.410020107. [DOI] [PubMed] [Google Scholar]
- 145.Sospedra M, Martin R. Immunology of multiple sclerosis. Annu Rev Immunol. 2005;23:683–747. doi: 10.1146/annurev.immunol.23.021704.115707. [DOI] [PubMed] [Google Scholar]
- 146.Payne SC, Bartlett C, Harvey AR, et al. Myelin sheath decompaction, axon swelling, and functional loss during chronic secondary degeneration in rat optic nerve. Invest Ophthalmol Vis Sci. 2012;53:6093–6101. doi: 10.1167/iovs.12-10080. [DOI] [PubMed] [Google Scholar]
- 147.Raine CS, Cannella B, Hauser SL, Genain CP. Demyelination in primate autoimmune encephalomyelitis and acute multiple sclerosis lesions: a case for antigen-specific antibody mediation. Ann Neurol. 1999;46:144–160. doi: 10.1002/1531-8249(199908)46:2<144::aid-ana3>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 148.Kettenmann H, Sonnhof U, Schachner M. Exclusive potassium dependence of the membrane potential in cultured mouse oligodendrocytes. J Neurosci. 1983;3:500–505. doi: 10.1523/JNEUROSCI.03-03-00500.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ro H, Carson JH. pH microdomains in oligodendrocytes. J Biol Chem. 2004;279:37115–37123. doi: 10.1074/jbc.M403099200. [DOI] [PubMed] [Google Scholar]
- 150.Boggs JM, Yip PM, Rangaraj G, Jo E. Effect of posttranslational modifications to myelin basic protein on its ability to aggregate acidic lipid vesicles. Biochemistry. 1997;36:5065–5071. doi: 10.1021/bi962649f. [DOI] [PubMed] [Google Scholar]
- 151.Beniac DR, Wood DD, Palaniyar N, et al. Cryoelectron microscopy of protein-lipid complexes of human myelin basic protein charge isomers differing in degree of citrullination. J Struct Biol. 2000;84:80–95. doi: 10.1006/jsbi.1999.4200. [DOI] [PubMed] [Google Scholar]
- 152.Musse AA, Boggs JM, Harauz G. Deimination of membrane-bound myelin basic protein in multiple sclerosis exposes an immunodominant epitope. Proc Natl Acad Sci USA. 2006;103:4422–4427. doi: 10.1073/pnas.0509158103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Moscarello MA, Pritzker L, Mastronardi FG, Wood DD. Peptidylarginine deiminase: a candidate factor in demyelinating disease. J Neurochem. 2002;81:335–343. doi: 10.1046/j.1471-4159.2002.00834.x. [DOI] [PubMed] [Google Scholar]
- 154.Imgrund S, Hartmann D, Farwanah H, et al. Adult ceramide synthase 2 (CERS2)-deficient mice exhibit myelin sheath defects, cerebellar degeneration, and hepatocarcinomas. J Biol Chem. 2009;284:33549–33560. doi: 10.1074/jbc.M109.031971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ben-David O, Pewzner-Jung Y, Brenner O, Laviad EL, Kogot-Levin A, Weissberg I, Biton IE, Pienik R, Wang E, Kelly S, Alroy J, Raas-Rothschild A, Friedman A, Brügger B, Merrill AH, Futerman AH. Encephalopathy caused by ablation of very long acyl chain ceramide synthesis may be largely due to reduced galactosylceramide levels. J Biol Chem. 2011;286(34):30022–30033. doi: 10.1074/jbc.M111.261206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Young KM, Psachoulia K, Tripathi RB, et al. Oligodendrocyte dynamics in the healthy adult CNS: evidence for myelin remodeling. Neuron. 2013;77:873–885. doi: 10.1016/j.neuron.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Czopka T, Ffrench-Constant C, Lyons DA. Individual oligodendrocytes have only a few hours in which to generate new myelin sheaths in vivo. Dev Cell. 2013;25:599–609. doi: 10.1016/j.devcel.2013.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Mangin J-M, Li P, Scafidi J, Gallo V. Experience-dependent regulation of NG2 progenitors in the developing barrel cortex. Nat Neurosci. 2012;15:1192–1194. doi: 10.1038/nn.3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Makinodan M, Rosen KM, Ito S, Corfas G. A critical period for social experience-dependent oligodendrocyte maturation and myelination. Science. 2012;337:1357–1360. doi: 10.1126/science.1220845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Liu J, Dietz K, DeLoyht JM, et al. Impaired adult myelination in the prefrontal cortex of socially isolated mice. Nat Neurosci. 2012;15:1621–1623. doi: 10.1038/nn.3263. [DOI] [PMC free article] [PubMed] [Google Scholar]