Abstract
Dmrt genes encode a large family of transcription factors characterized by the presence of a DM domain, an unusual zinc finger DNA binding domain. While Dmrt genes are well known for their important role in sexual development in arthropodes, nematodes and vertebrates, several new findings indicate emerging functions of this gene family in other developmental processes. Here, we provide an overview of the evolution, structure and mechanisms of action of Dmrt genes. We summarize recent findings on their function in sexual regulation and discuss more extensively the role played by these proteins in somitogenesis and neural development.
Electronic supplementary material
The online version of this article (doi:10.1007/s00018-013-1288-2) contains supplementary material, which is available to authorized users.
Keywords: Dmrt, Sexual differentiation, Somitogenesis, Neurogenesis, Olfactory placode, Telencephalon
Introduction
The Dmrt (doublesex and mab3-related-transcription factor) family derives its name from two founding members, doublesex (dsx) in Drosophila melanogaster and male abnormal (mab-3) in Caenorhabditis elegans. It is defined by the presence of the DM domain DNA binding motif, an unusual cysteine-rich zinc DNA binding motif constituted of two intertwined zinc fingers [1, 2]. Dmrt genes are well known to play a conserved role in sex determination, sexual dimorphism or other aspects of sexual reproduction [3, 4], but a growing body of evidence indicates that they are also conserved regulators of other developmental processes [5]. In this review, we provide an updated overview of the evolution, structure and mechanisms of action of DM genes. We summarize recent findings on their function in sexual development and discuss more extensively recent functional studies demonstrating their important function in somitogenesis and neural development. In particular, we highlight the important role of a subgroup of them including Dmrt3, Dmrt4 and Dmrt5, characterized by the presence of an additional highly conserved domain designated DMA, in neurogenesis and patterning of the developing nervous system.
Taxonomic distribution and architecture of Dmrt proteins
Although DM domain genes have been extensively studied in model organisms, little is known about the evolution of this gene family. DM domain genes have been detected in many bilaterian species, including in the amphioxus [6], the oyster Crassostrea gigas [7], and in two cnidarian species, the coral Acropora millepora [8] and the sea anemone Nematostella vectensis [9]. Nevertheless, the evolutionary origin of Dmrt genes in animals has not been further investigated due to a lack of data from basal metazoans. Using available whole-genome sequences from several basal metazoans, we identified novel DM genes in the cnidarian Acropora digitifera, in the placozoan Trichoplax adhaerens, in the ctenophore Mnemiopsis leidyi and in the sponges Sycon ciliatum and Oscarella carmella (Fig. 1a; Suppl. Table 1). However, DM domain genes were not detected in the genome of the sponge Amphimedon queenslandica, and in several non-metazoan holozoans including choanoflagellates, the closest relatives to metazoans. Thus, the DM domain gene family probably arose during early metazoan evolution after the divergence with choanoflagellates, and expanded in the metazoan lineage.
Fig. 1.
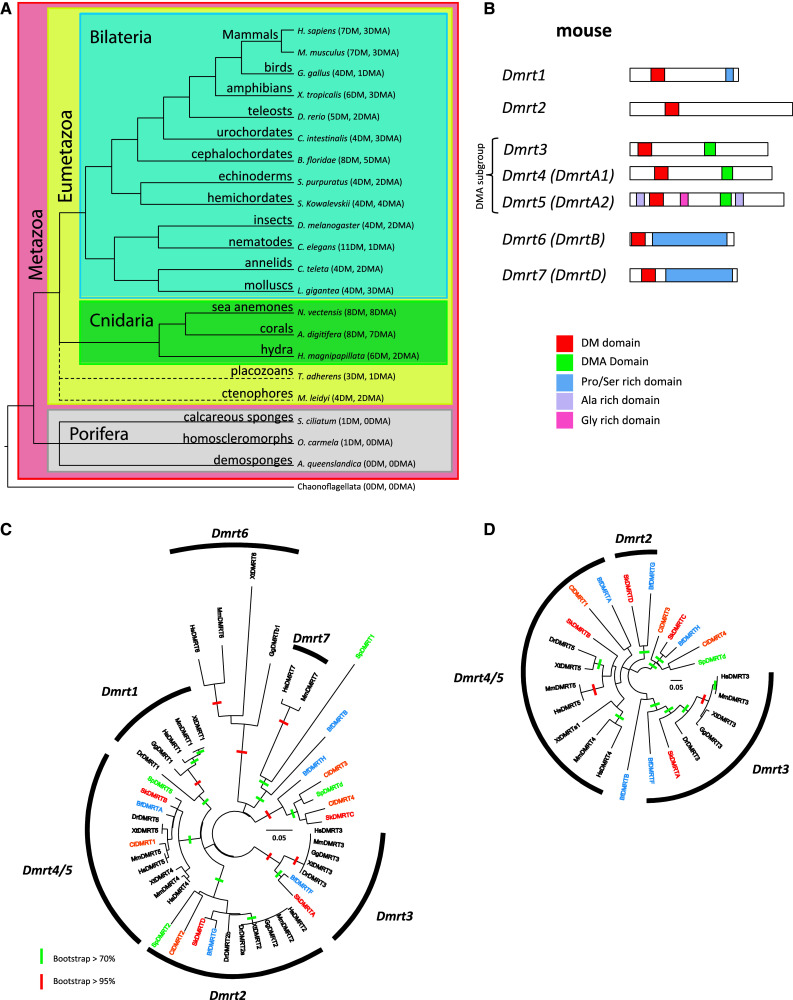
Taxonomic distribution and phylogeny of the Dmrt genes and domain architecture of the proteins. a Distribution of Dmrt genes in representatives of metazoans. For each species, the number of Dmrt paralogs, with or without a DMA domain, is reported in parentheses. Accession numbers of the sequences included in this analysis are provided in Suppl. Table S1. Metazoa are highlighted in red, Eumetazoa in yellow, Bilateria in blue, Cnidaria in green and Porifera in gray. The phylogenetic relationships of placozoans and ctenophores with the other metazoan groups are still controversial and are therefore represented by dashed lines. In some phylogenetic studies [12], ctenophores together with cnidarians constitute the sister group to bilaterians, while in other studies [11], ctenophores are found as the sister group to all other metazoans including sponges. Similarly, placozoans have been reported either as belonging to eumetozoans [12], or as the sister group to all other metazoans [102]. b Domain architecture of the mouse Dmrt paralogs. For each Dmrt protein, the additional names are provided. Dmrt proteins share highly conserved protein motifs, including the DM domain, and are subdivided into a few subgroups based on sequence similarity. Dmrt3, Dmrt4, and Dmrt5 are categorized as a subgroup because their products share a highly conserved DMA domain at their C-termini. Pro/Ser-, Ala- and Gly-rich domains are also indicated. c, d Phylogenetic relationships between deuterostomian Dmrts inferred by neighbor-joining (NJ) analyses of the DM (c) and DMA (d) domains. Sequences included belong to the major groups of deuterostomes: hemichordates (sequence names in red), echinoderms (green), cephalochordates (blue), urochordates (orange) and vertebrates (black); Bf, Branchiostoma floridae; Dr, Danio rerio; Hs, Homo sapiens; Mm, Mus musculus; Gg, Gallus gallus; Sk, Saccoglossus kowalevskii; Sp, Strongylocentrotus purpuratus; Ci, Ciona intestinalis; Xt, Xenopus tropicalis. See Table S1 for information about sequences. For each genomes, all identified sequences have been included except the very divergent B. floridae DmrtC, DmrtD and DmrtE. Branches crossed by a green or red bar indicate bootstrap value ≥70 and ≥95 %, respectively (1,000 NJ bootstrap replicates)
Vertebrates have multiple Dmrt genes (e.g., Dmrt1 to Dmrt8 in mice and humans). Dmrt proteins have been classified into several subfamilies, based on the presence of additional conserved protein domains and on the exon–intron structure of the corresponding genes. Dmrt4 (Dmrta1), Dmrt5 (Dmrta2) and Dmrt3 (Dmrta3) constitute one such subfamily characterized by the presence of a conserved DMA domain at their C-termini (Fig. 1b). Each gene of this subfamily has two coding exons, with the DM domain encoded by the first coding exon and the DMA domain encoded by the second one [10]. In N. vectensis, a DMA domain is present in all eight Dmrt genes, suggesting that its presence may represent the ancestral condition for cnidarians and bilaterians [9]. We observed the presence of a DMA domain in some or all the Dmrt genes in most metazoan species we looked at. This includes non-bilaterians such as the placozoan Trichoplax and the ctenophore Mnemiopsis (Fig. 1a; Suppl. Table 1), indicating that Dmrt genes with both DM and DMA domains were already present during early metazoan evolution. Uncertainties about the phylogenetic relationships of non-bilaterian animals [11, 12] and the relative paucity of genomic data from non-bilaterian species do not allow the drawing of firm conclusions about whether a DM + DMA architecture may represent the ancestral state of Dmrt genes in metazoans. Further studies are also required to define whether the last common ancestor of bilaterians and that of bilaterians and cnidarians only owned Dmrt genes with DM and DMA domains, as is observed in some present-day species (such as Nematostella and Saccoglossus), or whether both genes with and without DMA domain coexisted in these remote ancestors, as found in many extant species (such as vertebrates).
Phylogeny of the Dmrt genes
We performed a phylogenetic analysis of the Dmrt sequences to study the evolution of individual Dmrt gene members. The phylogenetic relationship among Dmrt proteins is not obvious, as there is little sequence similarity outside the short DM and DMA domains. Here, we limited our study to deuterostomes including sequences from various chordates, echinoderms and hemichordates. A thorough phylogenetic analysis is needed to draw conclusions about the origin and the evolution of the Dmrt family at the level of the metazoans. Phylogenetic analyses of both DM and DMA domains (Fig. 1c, d) suggest that the Dmrt2, Dmrt3 and Dmrt4/5 vertebrate subgroup is monophyletic and that orthologs of each of these genes were already present in the ancestor of deuterostomes and contained both DM and DMA domains. Comparison between DM and DMA domain phylogenies suggest that vertebrate Dmrt2 have lost their DMA domain since related sequences in cephalochordates and hemichordates contain this domain. Vertebrate Dmrt1, Dmrt6 and Dmrt7 also appear to be monophyletic. They could not be related unambiguously to non-vertebrate deuterostome sequences or other Dmrt vertebrate subgroups due to weak phylogenetic signal. The question of their origin remains unsolved. Dmrt8 (Dmrtc1) show strong similarity over their entire sequence to Dmrt7 (Dmrtc2) but lack the DM domain [13]. Both have been found in mammals but not in other vertebrate species. In Dmrt8 from human, chimp and orangutan, the open reading frame is disrupted by a stop codon 5′ of the DM domain. Therefore, primate Dmrt8 genes do not have the potential to encode a DM domain [14].
Mechanisms of action of Dmrt proteins
Despite the importance of Dmrt proteins, how they regulate the expression of specific target genes remains elusive. Dmrt3 and Dmrt5 have been shown to encode nuclear proteins [9, 15]. Sequence-specific DNA binding via the DM domain has been determined in vitro for most vertebrate Dmrt proteins [9, 15, 16]. They all bind very similar DNA sequences resembling those bound by DSX [17, 18] and MAB-3 [19]. The consensus consists in a palindromic sequence (G/A)NNAC(A/T)A(A/T)GTNN(C/T) composed of two half-sites around a central (A/T) base pair. As predicted from the symmetric nature of this site, Dmrt proteins bind DNA as homodimers or heterodimers with other DM domain proteins [16, 17].
DM domain proteins are able to act as transcriptional activators or repressors. Reporter gene transcription assays and deletion analysis have indicated that their regulatory domains are located at their C-termini [9, 20, 21]. To date, transcriptional regulation by Dmrt proteins has only been investigated at a large scale in the case of Dmrt1 in mouse sexual differentiation (see below). In cell cultures, Dmrt1 activates or represses transcription depending on cell type and promoter structure. In vivo, in the developing mouse male and female gonads, Dmrt1 acts simultaneously at multiple sites across the genome, activating some genes and repressing others. Thus, Dmrt1 functions as a bifunctional transcriptional regulator. It also binds its own promoter as well as one of the other Dmrt genes, suggesting auto- and cross-regulation of these genes. Chromatin immunoprecipitation (ChIP) analysis using conditional mutant testes showed that DNA binding and transcriptional regulation of individual target genes can differ between germ cells and Sertoli cells, and that some genes exhibit Dmrt1 binding only in one cell type. Differential response of genes to loss of Dmrt1 appears to correspond to differences in the binding motif, suggesting that other transactivating factors modulate its activity [22–25]. Whether other DM proteins function like Dmrt1 as bifunctional transcriptional regulators is unclear. MAB-3 represses transcription of yolk gene (vitellogenin) in the intestine and blocks transcription of the antineural bHLH gene ref-1, a repressor of the proneural protein lin-32 in male ray precursor cells [19, 26]. dsx is alternatively spliced into sex-specific isoforms that encode proteins that share the DM domain but have distinct C-termini. DSXM in males blocks, whereas DSXF in females activates, transcription of genes such as the female-specific yolk protein genes or the bric a brac 1 (bab1) and bab2 genes which control pigmentation [27–29]. Recently, novel DSX targets during genital development have been identified, some being activated and others being repressed by DSX [30]. However, it is not known whether these novel downstream genes are direct or indirect targets. Thus, so far, only Dmrt1 has been shown to be bifunctional.
How do Dmrt proteins achieve transcription repression or activation in a cell-specific manner? In contrast to other zinc finger proteins, DM proteins interact with DNA in the minor groove rather than the major groove [2, 31]. This enables them to bind to DNA on sites overlapping those of major groove binding proteins and to physically interfere with their binding or to cooperate with them. Such a mechanism has been observed in the case of DSX and MAB-3 [26, 28, 29, 32, 33]. Whether this mechanism is also used by Dmrt proteins in vertebrate is not known. Whether they recruit coactivators or corepressors and associate with chromatin-modifying enzymes also remains to be investigated.
Dmrts are conserved regulators of sexual development
DM domain-containing genes are conserved genetic components involved in sex differentiation in all animals that have been studied. In flies, dsx act downstream in the sex-determination pathway. dsx is expressed in somatic gonadal primordium and its function in the somatic gonad is required for sex-specific germline development. Later in development, dsx is widely expressed in a subset of non-gonadal cells where it acts cell autonomously and non-cell autonomously, via interactions with conserved Hox genes and signaling pathways, to integrate sex-specific, spatial and temporal cues and induce localized sex-specific differentiation. Three other DM domain genes are present in flies, but only dsx has been shown to regulate sexual dimorphism.
Caernorhabditis elegans has 11 DM domain genes. Among them, 3 are known to regulate sexual development: mab-3, mab-23 and DM domain 3 (dmd-3). They are dispensable to gonad development but are involved in the sexual differentiation of somatic tissues including copulatory structures such as sensory rays, spicules and mating muscles. As in flies, DM domain genes in nematodes integrate sexual and various positional and temporal cues to initiate a sex-specific developmental program (reviewed in [3, 4]).
In Daphnia magna, a freshwater branchiopod crustacean which parthenogenetically produces males in response to environmental cues, a DM domain gene, DapmaDsx1, has been identified. DapmaDsx1 shows higher expression in male-specific structures. Knockdown of DapmaDsx1 in male embryos directs the production of female traits whereas ectopic expression of DapmaDsx1 in female results in the development of male-like phenotypes [34].
In Acropora millepora, transcripts of the DM domain gene AmD1 are present at higher levels during sexual differentiation [8], suggesting that the implication of Dmrt genes in sexual regulation may have predated the divergence between cnidarians and bilaterians.
In vertebrates, all Dmrt family members are expressed in the indifferent gonad and in most cases they are subsequently maintained at higher levels in male as opposed to female gonads (Table 1). Among the eight DM domain genes in vertebrates, three of them have been established to have roles in gonad and/or germ line development, namely Dmrt1, Dmrt4 and Dmrt7 (Table 2). In the following section, we briefly highlight the functions of two of them, Dmrt1 and Dmrt7, and show that Dmrt1 or a close paralog has recently moved up the regulatory hierarchy from downstream positions in gonad and germ line development to the top level of sex determination during evolution in fish, birds and frogs. The role of Dmrt4 in gonadal and non-gonadal tissues is discussed in the next section. Recent excellent reviews have more extensively discussed the role of Dmrts in the development and evolution of sex dimorphism [3, 4, 35, 36].
Table 1.
An outline of reported Dmrt factor embryonic expression in vertebrates
| Factor | Species | Embryonic expression in non-gonadal tissue (in situ) | Expression in gonads | References |
|---|---|---|---|---|
| Dmrt1 | Mouse | nd | Genital ridge prior to sexual differentiation. Expression becomes XY specific during gonadogenesis. Both in Sertoli and germ cells (mitotic spermatogonia). | [49, 55, 103] |
| Chicken | nr | Genital ridge, higher in male. Becomes testis-specific after the onset of sexual differentiation. | [49, 103] | |
| Xenopus | nr | First detected in primordial gonad in both ZW and ZZ tadpoles in cells surrounding the PGCs. Expression is maintained and becomes higher in testis than ovary after metamorphosis. | [20, 71, 104] | |
| (dm-w) | Xenopus | nr | Expression exclusively in primordial gonads of ZW tadpoles in cells surrounding the PGCs. Expression is not maintained in the ZW and ZZ gonads. | [71, 72] |
| Platyfish | nd | Spermatogonia and Sertoli cells in adult testis. | [61, 105] | |
| Zebrafish | nr | Testis and ovary, higher in testis than ovary. Germ cells. | [106] | |
| Medaka | nr | No expression in gonads in embryos before stage 20 of development. Expressed in spermatogonium-supporting cells after testicular differentiations (20 days after hatching). | [62, 67, 93] | |
| (Dmy) | Medaka | nr | Expressed during development from neurula stage in somatic cells surrounding germ cells exclusively in XY gonads. Expression maintained in adult testes. | [62, 63, 67] |
| Dmrt2 | Mouse | Presomitic mesoderm and dermomyotome of somites. | Barely detectable expression, higher in testis than ovary during embryonic development. | [80, 83, 85, 86, 107] |
| Chicken | Transient asymmetric expression in the chick Hensen’s node, anterior presomitic mesoderm and dorsal compartment of somites. | nr | [84] | |
| Medaka | Somites. | Sertoli cells in adult testis. Early stage oocytes in adult ovaries. | [93] | |
| Zebrafish | Kupffer’s vesicle in 3-somite stage embryos; presomitic mesoderm and newly formed somites in bud stage embryos (Dmrt2a). Somites and branchial (Dmrt2b). | Adult testis (Dmrt2b). | [80–83] | |
| Platyfish | Somites; branchial arches (Dmrt2a). | nd | [105] | |
| Dmrt3 | Mouse | Nasal placode, telencephalon, spinal cord interneurons. | Expression initially similar in testis and ovary; higher in testis after E13.5. Interstitial cells. | [87, 107] |
| Chicken | Nasal placode, telencephalon, dorsal spinal cord interneurons, somites, müllerian ducts (higher in females than males). | nd | [87] | |
| Zebrafish | Nasal placode, anterior neural tube, dorsal spinal cord interneurons. | Undifferentiated gonads from 17 dpf. Adult testis (spermatogonia and spermatocytes) and ovary (oocytes). | [15] | |
| Medaka | Dorsal spinal cord interneurons. | Differentiating gonads (12–20 dph) and adult testis. | [93] | |
| Dmrt4 | Mouse | Ubiquitous, high expression in olfactory tissues. | Similar levels in testis and ovary (from E11.5). | [89, 107] |
| Xenopus | Nasal placode, telencephalon, foregut, gall bladder. | Adult testis. | [88] | |
| Medaka | Olfactory system, telencephalon. | Differentiating gonads, adult testis and ovary. | [93, 108] | |
| Platifish | Olfactory placode, forebrain, branchial arches. | nd | [105] | |
| Dmrt5 | Mouse | Nasal placode, dorsal telencephalon, ventral forebrain/midbrain border at E9.5 extending later to the entire ventral midbrain, optic stalk, lateral head ectoderm, maxillary and mandibular processes, eyes, hypophysis. | Higher levels in ovary versus testis (E12.5 to E15.5). | [90, 91, 107] |
| Xenopus | Nasal placode, dorsal telencephalon, ventral diencephalon. | nr | [9] | |
| Zebrafish | Nasal placode, dorsal and ventral telencephalon, ventral diencephalon. | Germ cells in adult testis and ovary. | [95, 109] | |
| Platifish | Forebrain, olfactory placode, midbrain, lens. | nd | [105] | |
| Dmrt6 | Mouse | nr | nd | [107] |
| Dmrt7 | Mouse | nd | Higher level in ovary versus testis in embryonic and adult gonads. Postnatally, expression is male specific and restricted to spermatocytes and sperm. | [75, 76, 107] |
| Dmrt8 | Mouse | nr | Higher level in testis than in ovary in embryonic gonads. Testis-specific expression restricted to Sertoli cells in the adult. | [14] |
nr not reported, nd not detected
Table 2.
An overview of the phenotypes associated with loss or gain of function of chordate Dmrt genes
| Factor | Species | Loss-of-function/gain-of-function phenotypes | References | |
|---|---|---|---|---|
| Dmrt1 | Mouse | KO | Defects in germ radial migration, reactivation of mitosis and survival. Failure of Sertoli cell differentiation. Teratoma formation in fetal germ cells. Failure to control the mitosis versus meiosis decision in male germ cells. Female reprogramming in the postnatal testis. Reduction of primordial follicles in the juvenile ovary. | [22–24, 50, 51, 56] |
| Medaka | M | Male-to-female sex reversal after sex determination. | [70] | |
| (Dmy) | Medaka | M | Female development in genetic males. | [62, 65] |
| GOF | Male development in genetic females. Inhibition of primordial germ cell proliferation. | [66, 110] | ||
| KD | Loss of proliferation inhibition in primordial germ cells. | [110] | ||
| (dm-w) | Xenopus | GOF | Ovarian cavities in gonads of ZZ tadpoles overexpressing dm-w. | [71, 72] |
| KD | Male development in ZW individuals. | [72] | ||
| Chicken | KD | Feminization of gonads of embryonic males. | [74] | |
| Dmrt2 | Mouse | KO | Embryonic somite patterning and myogenic defects. | [83, 85, 111] |
| GOF | Increased myogenesis. | [86] | ||
| (Dmrt2a/Terra) | Zebrafish | GOF | Increased apoptosis. | [80] |
| KD | Randomization of left-sides specific genes and desynchronization of the segmentation clock. | [84] | ||
| Dmrt2b | Zebrafish | KD | Defects in somitogenesis and hedgehog signaling. Neural tube patterning defects. Randomization of left-side specific genes. Impairment of slow muscle development. | [81] |
| Dmrt3 | Mouse | KO |
Male sexual development abnormalities. Dental malocclusions. |
[112] |
| KO | Defects in spinal circuits involved in locomotion | [21] | ||
| Horse | M | Defects in pattern locomotion in horses | [21] | |
| Xenopus | GOF | mDmrt3 promotes neurogenesis in caps. | [9] | |
| Dmrt4 | Mouse | KO | Females with polyovular follicles and male sexual development abnormalities. | [89] |
| Xenopus | KD | Impaired neurogenesis in the olfactory placode. | [88] | |
| GOF | Promotes neurogenesis in caps. | [88] | ||
| Dmrt5 | Mouse | KO | Reduced development of the caudomedial cerebral cortex. | [91, 92] |
| GOF | Promotes dopamine neurons in ES cells. | [90] | ||
| KD | Inhibition of ES cells differentiation towards a ventral medial mesencephalic cell fate. | [90] | ||
| Xenopus | KD | Impaired neurogenesis in the olfactory placode. | [9] | |
| GOF | Promotes neurogenesis in caps. | [9] | ||
| Zebrafish | M, KD | Defects in telencephalic neurogenesis. | [95] | |
| (Dmrt1) | Ciona | M | Defects in anterior neural plate derivatives. | [100] |
| KD | Impaired Six1/2, Six3/6, Meis and ZicL in the developing brain and FoxC in palps. | [99] | ||
| Dmrt7 | Mouse | KO | Infertility with spermatogenic arrest in pachytene stage and sex chromatin defects. | [75, 76] |
GOF gain of function, KD knockdown, KO knockout, M mutation
Dmrt1 triggers male-specific and represses female-specific differentiation
In humans, Dmrt1 is localized in a region on the short arm of chromosome 9 (9p24.3) including three Dmrt genes (Dmrt1–3). Deletions of this region are associated with testicular dysgenesis and, in some cases, cause sex reversal of the XY embryonic gonad into ovarian tissue [37–40]. Among the three genes, Dmrt1 is the strongest candidate for XY gonadal dysgenesis. It is indeed expressed in the human embryonic genital ridges of human male, but not female embryos [41]. Deletions or mutations of Dmrt1 can cause XY gonadal dysgenesis [42–45]. Therefore, Dmrt1 was thought to have an important function in sex determination. Feminization associated with the loss of Dmrt1 is, however, more likely due to a failure of male gonadal differentiation or to the reprogramming of Sertoli cells into granulosa-like cells, as recently discovered in mice (see below). Dmrt1 is also associated with testicular germ cell cancer [46–48], which is consistent with a role of Dmrt1 as a tumor suppressor in 129v mice [22].
In mouse embryos, Dmrt1 is expressed in the genital ridge of both sexes before any overt signs of sex differentiation. Later, Dmrt1 expression declines in the ovary but is maintained in the testis, where it is restricted to pre-meiotic germ cells and Sertoli cells [49]. In mice, Dmrt1 is not required for primary sex determination as XY Dmrt1 mutants are born as males. It is, however, involved in multiple aspects of the male gonad differentiation. Indeed, in those mutants, Sertoli cells overproliferate, lose expression of the male-specific Sox9 protein and acquire expression of female specific Forkhead box L2 (Foxl2) and other granulosa markers. Germ cells fail to undergo radial migration, to reactivate mitosis, to enter meiosis and to survive beyond P10 [50]. In order to identify the function of Dmrt1 in Sertoli and germ cells, Kim et al. have used conditional gene targeting. This approach revealed that Dmrt1 is required in Sertoli cells for their postnatal differentiation, for germ line maintenance and for meiotic progression. In germ cells, Dmrt1 is required for radial migration, for mitotic reactivation just after birth, and for survival beyond the first postnatal week. Thus, in mice, Dmrt1 is required autonomously in both cell lineages. Dmrt1 activity in Sertoli cells is also required non-cell autonomously to maintain the germ line [51].
A recent study has addressed the function of Dmrt1 in Sertoli cells in the postnatal testis. In mammals, sex is determined in the fetal gonad by the presence or absence of the Y chromosome gene Sry, which controls whether bipotential precursor cells differentiate into testicular Sertoli cells or ovarian granulosa cells. This pivotal decision in a single gonadal cell type ultimately controls sexual differentiation throughout the body. Sex determination can be viewed as a battle for primacy in the fetal gonad between a male genetic network in which Sry activates Sox9 and a female network involving Wnt signaling and Foxl2, a female-specific transcription factor expressed in granulosa and theca cells. Loss of Dmrt1, even in adult Sertoli cells, activates ovary-specific genes such as Foxl2 and causes the loss of male-promoting genes such as Sox9, and reprograms Sertoli cells into granulosa cells. In this environment, theca cells form, oestrogen is produced, and germ cells appear feminized [24, 25]. Conversely, loss of Foxl2 in adult granulosa cells causes ectopic expression of Dmrt1 and their reprogrammation into Sertoli cells [52]. Thus, Dmrt1 is required to prevent female reprogramming in the postnatal mammalian testis, and the sexual fate of the somatic gonad is postnatally controlled by the opposed activity of Dmrt1 and Foxl2.
Another function of Dmrt1 in male germ cells was revealed when Dmrt1 was deleted in postnatal undifferentiated spermatogonia. In mammals, meiosis begins at puberty, and sperm is produced throughout life. Spermatogenesis occurs in three phases: a mitotic proliferative phase involving spermatogonia stem/progenitor cells, two reductive divisions of meiosis, and then a postmeiotic phase of spermiogenesis. The switch from mitosis to meiosis requires retinoic acid (RA), which activates meiotic inducers, including Stra8 [53, 54]. Dmrt1 is detected in all mitotic spermatogonia, and its expression decreases with the onset of spermatogonial differentiation and disappears at the initiation of meiosis. Loss of Dmrt1 in undifferentiated spermatogonia causes them to precociously exit the spermatogonial program and enter meiosis. Dmrt1 appears to act in spermatogonia by suppressing RA via transcriptional repression of Stra8, and by promoting the production of the spermatogonial differentiation basic helix-loop-helix transcription factor Sohlh1 [55]. These data indicate that Dmrt1 is a transcriptional gatekeeper that controls the mitosis versus the meiosis decision in male germ cells.
The role of Dmrt1 in the mammalian fetal ovary has also been investigated. In females, meiosis begins in the fetus, and germ cells remain arrested in the diplotene stage of prophase I until after puberty. As in males, meiotic activation occurs under the influence of RA and the downstream meiosis inducer Stra8 [54, 56]. Dmrt1 mutant germ cells were found to have severely reduced Stra8 expression and to undergo abnormal meiotic prophase [23]. mRNA profiling and ChIP suggest that transcriptional activation of Stra8 is the main function of Dmrt1 in the fetal ovary, and that this regulation is likely to be direct. Thus, Dmrt1 controls Stra8 sex-specifically, activating it in the fetal ovary and repressing it in the adult testis.
Studies on the function of Dmrt1 in sexual development have also been conducted in non-mammalian vertebrates. In species with temperature-dependent sex determination such as turtles, lizards and alligators, Dmrt1 expression was found to be higher in developing male gonads than in female ones, suggesting that it is involved in this process [57–59].
In the medaka, Oryzias latipes, which like mammals uses the XX/XY sex determination system, Dmrt1 has undergone duplication. Gene duplication generating Dmy recently occured during evolution of the genus Oryzias as the gene is absent in other fishes, including other Oryzias species [60, 61]. One of the newly derived paralogs, called Dm domain on Y, also known as Dmrt1bY or Dmy, is located on the sex-determining region of the Y chromosome. Dmy is expressed exclusively in somatic cells of XY gonads in the early gonadal primordium before morphological sexual dimorphism is observed. Dmy is a master regulator of sex determination as it is both required and sufficient for male development [62–66]. The other paralog, Dmrt1, is expressed in spermatogonium-supporting cells, which is the same lineage of cells expressing Dmy, but after testis differentiation [67]. High temperature or steroid treatment induces Dmrt1 expression in XX embryos and leads to XX sex-reversed testis [68, 69]. Conversely, in a Dmrt1 mutant line, XY individuals developed into normal egg-laying females. The XY mutant gonads first developed into the normal testis type, but by day 10 after hatching, the gonads transdifferentiate into the ovary type [70]. These data suggest that Dmrt1 in medaka is essential to maintain testis differentiation after Dmy-triggered male differentiation pathway.
Xenopus laevis uses the female heterogametic ZZ/ZW-type sex determining system. Similarly to medaka, a duplicated variant of Dmrt1 residing on the female-specific W chromosome has been isolated (termed dm-w). While Dmrt1 is expressed continuously in both ZZ and ZW developing gonads, dm-w is expressed exclusively in female ZW primordial gonads at sex determination. dm-w is an ovary-determining gene in X. laevis as exogenous dm-w causes developing ovotestes in ZZ tadpoles and dm-w knockdown in ZW individuals induces male development [71]. Dm-w appears to direct female sex by antagonizing the autosomal Dmrt1 gene to determine a testis fate. Dm-w encodes a truncated Dmrt1 protein which has a DM domain but lacks more carboxy-terminal sequences. It is proposed that dm-w blocks Dmrt1 by dimerizing and antagonizing Dmrt1 transcriptional activity [72]. As in medaka, this sex-determining role is a recent innovation. Indeed, a closely related species, Silurana tropicalis, lacks the dm-w gene [73].
Birds also use a female heterogametic sex ZZ/ZW system. Sex is supposed to be determined by the higher Z chromosome dosage in males, by the presence of a W chromosome in females, or possibly by a combination of both [35]. In all birds, Dmrt1 is located on the Z chromosome. In embryos, it is expressed in the early bipotential gonad and shows higher expression levels in ZZ versus ZW embryos [49]. If Dmrt1 activity is experimentally reduced, the gonads of genetically male (ZZ) embryos are feminized [74], demonstrating that Dmrt1 is required for testicular differentiation. Thus, in birds, Dmrt1 plays an important role in sex determination; the higher expression level in male (ZZ) embryos triggers the testis specification pathway whereas only a lower dose, as found in females, is compatible with ovarian development. Whether elevated Dmrt1 expression is sufficient in ZW genital ridge to induce male development remains however to be tested.
Dmrt7 is required for male meiosis
Dmrt7 is present in placental mammals and marsupials but no ortholog has been reported in non-mammalian vertebrates. In mouse, Dmrt7 is expressed in both male and female fetal gonads. In the ovary, Dmrt7 expression is independent of the germ line, as, in XX c-kit mutants which lack germ cells, the level of its expression remains similar to that observed in wild-types. In adults, Dmrt7 expression is male specific. It is predominantly detected in mid- to late-pachytene spermatocytes and the protein preferentially localizes to the XY body, a densely stained chromatin domain harboring sex chromosomes, essential for male meiotic progression. Consistent with this expression pattern, mice deficient in Dmrt7 are infertile and most mutant cells show spermatogenic arrest in pachytene stage and abnormal cellular organization of Sertoli cells [75, 76]. The germ cell defects of Dmrt7 mutants are not caused by aberrant Sertoli cell organization since animals with deletion of Dmrt7 just in Sertoli cells have normal testis and spermatogenesis. Dmrt7 mutant cells establish a normal XY body in mid-pachynema, but then have multiple epigenetic defects in the sex chromatin transition from pachynema to diplonema. This suggests that Dmrt7 plays a role in the control of the transition from meiotic sex chromosome inactivation to postmeiotic sex chromatin in males [76].
Dmrts are important during embryogenesis in non-gonadal tissues
Following their initial expression in the developing gonads, a subset of the Dmrt family members, including Dmrt2, Dmrt3, Dmrt4 and Dmrt5, show differential expression in a limited number of non-gonadal tissues and organs. In most species, Dmrt genes have been detected in tissues such as the central nervous system, nasal placode or somites. Their expression pattern is often conserved across species, but there are also differences. For example, while Dmrt3 is expressed in the neural tube and in the presomitic mesoderm in chick, it is only detected in the nervous system in fish and mouse suggesting its function has shifted during evolution (Table 1). Those four DM domain genes have been recently the subject of functional analysis in mouse, fish or Xenopus. Table 2 summarizes some of these gain- and loss-of-function studies and the resulting phenotypes. Here, we discuss these recent studies which show that Dmrts, like DSX, direct a variety of cell differentiation events and often function in the specification of progenitor cells. They also indicate that some of these Dmrt factors, as DSX in flies [33, 77–79], act by modulating signaling pathways, suggesting that this may be a common theme for DM domain proteins across species.
Dmrt2 is involved in establishing left–right asymmetry and somitogenesis
The first Dmrt gene suggested to have a role unrelated to sexual development was Dmrt2. It was first identified in zebrafish through a systematic search for genes with tissue-specific expression and was selected because of its somite and presomitic mesoderm-specific expression pattern. The identified gene, originally called terra, was found to play a role in somitogenesis based on the observation that its overexpression induces rapid apoptosis in the mesoderm [80]. A duplicated copy of the Dmrt2 gene was later described in the zebrafish genome, and Dmrt2a/terra and Dmrt2b have been designated to distinguish them. Both genes are expressed during somitogenesis, but the fish-specific Dmrt2b is also expressed in branchial arches [81, 82]. In addition to the developing somites, Dmrt2a is transiently asymmetrically expressed in the zebrafish Kupffer’s vesicle and in the equivalent structure in the chick, the Hensen’s node, which suggest a left–right patterning function during development [83, 84]. Indeed, in zebrafish, using a morpholino-based approach, Dmrt2a was found to be required for left–right synchronization of the segmentation clock. It is also required for left–right patterning in the lateral plate mesoderm and thus the correct positioning of the internal organs on each side of the midline. As such, Dmrt2a is a key factor linking left–right patterning with bilateral synchronization of the segmentation clock in the mesoderm [84]. Dmrt2b morphants also display defects in heart and visceral organ asymmetry, and some lateral plate mesoderm markers expressed in the left side are randomized. Dmrt2b knockdown also leads to notable defects in somitogenesis and reduces target gene expression of Hedgehog signaling, which results in significant impairment in slow muscle development. Dmrt2a cannot compensate for the loss of Dmrt2b and vice versa. These data indicate that functional divergence has occurred between the two duplicated genes, with Dmrt2b maintaining the common function for left–right establishment and contributing to a divergent function in somitogenesis through Hedgehog signaling [81].
In the mouse, Dmrt2 is expressed in the presomitic mesoderm and is then confined to the dermomyotome, the dorsal epithelial domain of the developing somites containing muscle stem cells, but is absent from the node [83]. As in zebrafish, its targeted disruption leads to severe somite patterning defects, first visible at day E10.5. Both the dermomyotome and myotome fail to adopt a normal epithelial morphology. Accompanying these morphological defects, alterations in the expression of dermomyotomal and myotomal transcription factors such as Pax3, Paraxis, Myf5, Myogenin, Mrf4 and MyoD were observed [85]. In agreement with the absence of its expression from the mouse node, Dmrt2 homozygous mutants do not show left–right desynchronization of somite formation or defects in left–right asymmetric organ positioning. Thus, the role of Dmrt2 in symmetric somite formation and in the regulation of the laterality pathway is not conserved during zebrafish and mouse embryonic development [83]. Whether this loss of Dmrt2 function in left–right patterning in the mouse arise from mutations occurring in the enhancer responsible for the node expression or from the loss of a protein(s) necessary to activate specifically the node enhancer remains unknown. In a recent report, Dmrt2 has been identified as a target of Pax3, a critical regulator of skeletal muscle stem cells. Furthermore, Dmrt2 was found to directly regulate early activation of the myogenic determination gene Myf5, required for the formation of the first skeletal muscle in the somites. Conditional overexpression of Dmrt2 in Pax3-expressing cells in the somite confirms the role of this factor in the activation of Myf5 [86]. Thus, a genetic network comprising Pax3/Dmrt2/Myf5 operates in the muscle stem cells of the dermomyotome in the mouse embryo to orchestrate the onset of myogenesis.
Dmrt3, Dmrt4 and Dmrt5 play key roles in neurogenesis
Several studies have shown that members of the Dmrt3–5 subfamily of DM genes are expressed in a restricted manner in the developing nervous system. All three genes are expressed in the developing telencephalon and olfactory placode in the mouse (Fig. 2), and this expression pattern in the developing CNS is conserved in most vertebrate embryos studied, including chick, mouse and zebrafish embryos (Fig. 3a–d) [87–92]. In addition, Dmrt3 is also strongly expressed in the spinal cord in dorsal interneurons [15, 87, 93] and Dmrt5 in the ventral medial mesencephalon (Fig. 3a) [90, 91]. The functional relevance of these expression patterns has recently been investigated for Dmrt4 and Dmrt5 in Xenopus olfactory placode and zebrafish telencephalon neurogenesis and for Dmrt3 in mouse spinal cord neuronal specification (Fig. 4).
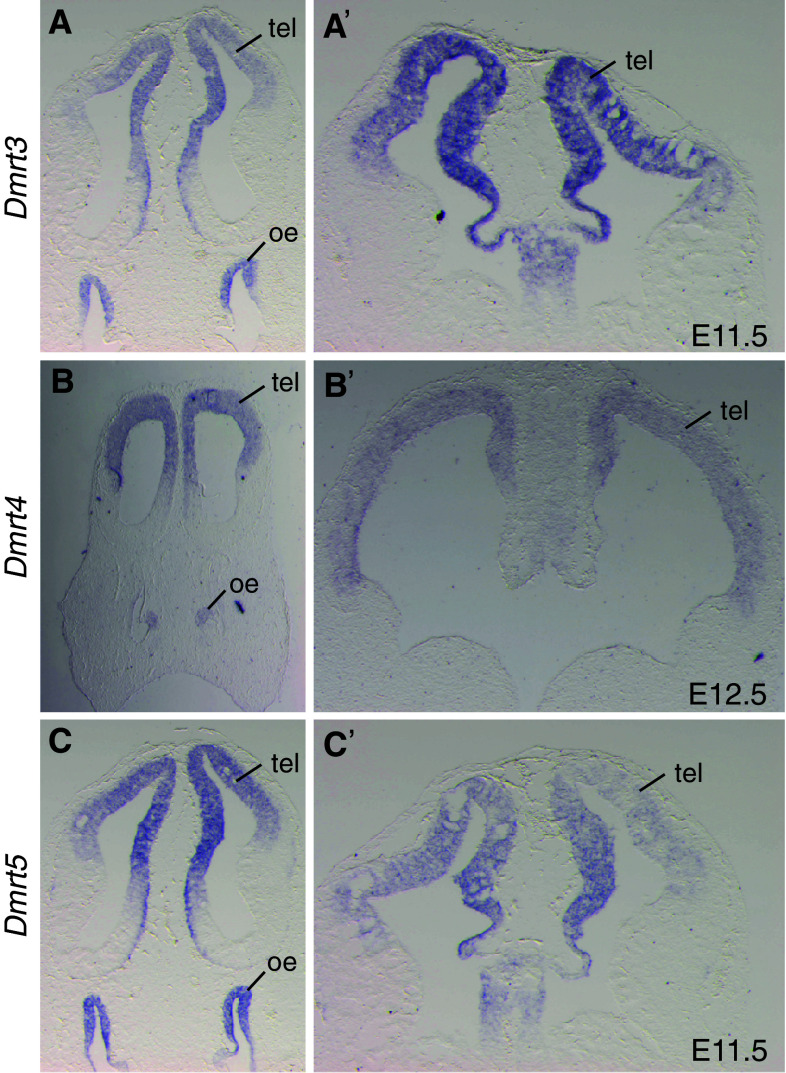
Fig. 2.
In situ analysis of Dmrt3, Dmrt4 and Dmrt5 expression in the head of mouse embryos. Coronal sections at the indicated stages in the nasal region (a–c) and at the level of the anterior telencephalon (a′–c′) are shown. All three genes are expressed in a graded manner in the developing telencephalon and in the olfactory epithelium. Oe olfactory epithelium, tel telencephalon
Fig. 3.
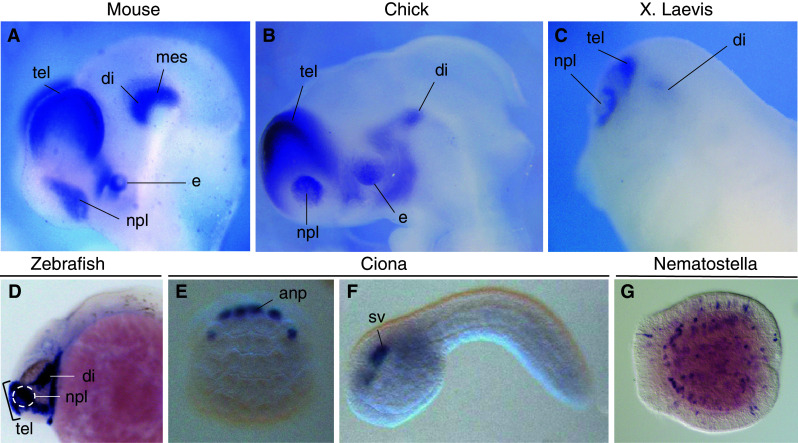
Whole-mount in situ analysis of Dmrt5 expression in the mouse, chick, frog and fish embryos and of related genes in ascidian and cnidarian. a–d In mouse (stage E10.5), chick (stage 18), Xenopus laevis (stage 28) and zebrafish (24 hpf) embryos, Dmrt5 is strongly expressed in the nasal placode and dorsal telencephalon. It is also highly transcribed in the ventral diencephalon in chick, frog and zebrafish embryos and in the mouse in the ventral diencephalon and mesencephalon. e, f In Ciona 64-cell stage embryos (e), Dmrt1 that is related to Dmrt4/5, is expressed in progenitors of the anterior neural plate and later at tailbud stage (f) is restricted to the anterior brain. (Images from Ghost database, http://ghost.zool.kyoto-u.ac.jp/SearchGenomekh.html). g In Nematostella vectensis, NvDmrtB that is related to Dmrt4/5 is expressed in scattered cells in both the ectodermal and endodermal layers in late planula embryos. The strongest staining is most prominent at that stage in the endoderm, correlating with the onset of neurogenesis. In (a–d), lateral views of the head of the embryos are shown with anterior to the left. In (e), the anterior side of the embryos is to the top. In (f), lateral view of a tailbud stage embryo is shown. In (g), the blastopore is to the right. Anp anterior neural plate progenitors, Di diencephalon, e eye, mes mesencephalon, npl nasal placode, sv sensory vesicle, tel telencephalon
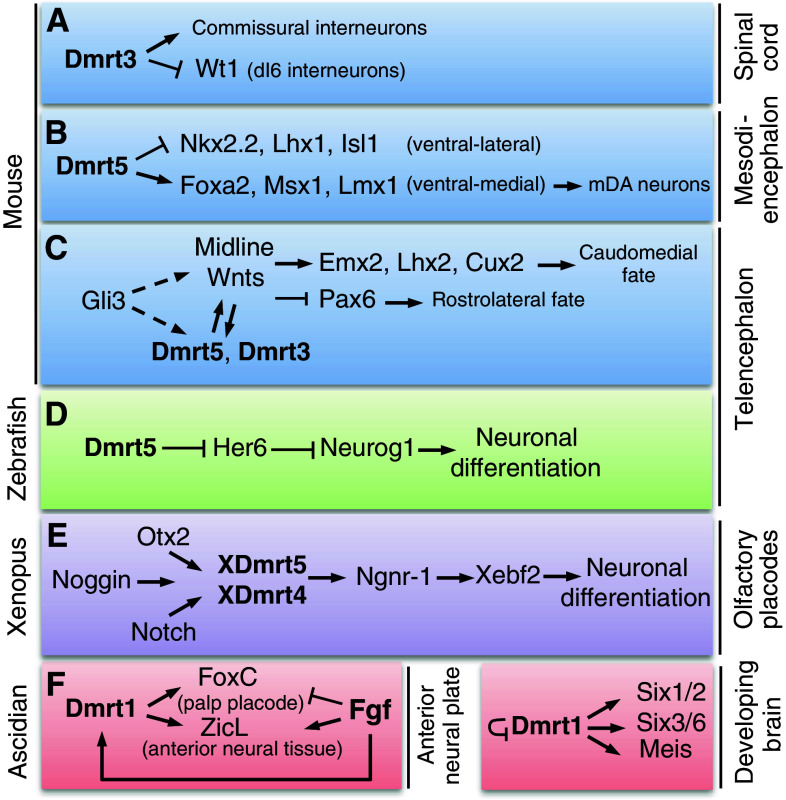
Fig. 4.
Schematic representation of the involvement of Dmrts in neural development. a In the mouse spinal cord, loss of Dmrt3 increases the number of Wt1+ neurons and results in fewer commissural interneurons. b In mouse ES cells, Dmrt5 induces ventral-medial midbrain progenitor markers and inhibits ventral-lateral ones, suggesting that in vivo, in the ventral-medial mesencephalic neuroepithelium, it enhances the acquisition of a mDA neuronal fate. c In the mouse telencephalon, Dmrt5 and Dmrt3 are targets for Wnt signaling and are dependent on Gli3. Direct or indirect action of Gli3 on Wnts and on Dmrt5 and Dmrt3, through regulation of the Wnt signaling pathway, is indicated by dashed lines. Dmrt5 in turn is required for Wnt and Bmp expression in the dorsal midline signaling center and the expression of their downstream targets, that specify a caudomedial fate. Pax6 is upregulated in Dmrt5 mutants, presumably through its negative regulation by Wnts and Emx2. d In zebrafish, Dmrt5 is required for neurogenesis in the telencephalon, possibly by repressing Her6. e In the frog, Dmrt5 and Dmrt4 act downstream of neural induction, Otx2 and Notch and upstream of Neurogenin to control olfactory placode neurogenesis. f In Ciona, Fgf signals play a crucial role in nervous system development, activating several neural genes including Dmrt1. It also controls cell fate choice between the palp placodes and anterior central nervous system, which express FoxC and ZicL, respectively. FoxC and ZicL expressing cells derive from common progenitors that express Dmrt1 and both genes require Dmrt1 for their activation. Dmrt1 plays also a role in the promotion of Six1/2, Six3/6 and Meis in the developing brain and negatively regulates its expression
In Xenopus, Dmrt4 and Dmrt5 are coexpressed early in the anterior neural ridge (ANR) and then become progressively restricted to the dorsal telencephalon and the olfactory epithelium. Both genes are positively regulated by neural inducers and negatively by proneural factors. They are also activated by the combined action of the transcription factor Otx2, broadly transcribed in the head ectoderm, and of Notch signaling, activated in the ANR. Knockdown of Dmrt4 or Dmrt5 impairs neurogenesis in the embryonic olfactory system and in neuralized animal caps. Conversely, their overexpression promotes neuronal differentiation in animal caps as visualized using markers such as the bHLH transcription factors Ngnr-1, Ebf2 or Ath5, a property that requires the C-terminal DMA domain and downstream sequences. In animal caps, the Noggin mediated induction of Ngnr-1 and Ebf2 that is affected by the depletion of Dmrt5 can be rescued by Dmrt4 overexpression. Conversely, the inhibition of Ngnr-1 and Ebf2 in the context of Dmrt4 depleted explants can be rescued by Dmrt5 overexpression [9, 88]. Together, these data indicate that Dmrt4 and Dmrt5 have overlapping functions upstream of proneural factors during olfactory placode neurogenesis.
In the mouse, Dmrt3, Dmrt4 and Dmrt5 are strongly expressed in the developing olfactory epithelium but it is not known whether they play a role in its formation [87, 89, 91]. Dmrt4-deficient mice have been generated. Those mice are viable and fertile but have polyovular follicles, suggesting a role in folliculogenesis. Interestingly, 25 % of the mutant males exhibited copulatory behavior towards other males. As olfaction and sexual behavior are strongly linked in mice, this suggest possible olfactory function defects [94]. Nevertheless, Dmrt4-deficient mice have a histologically normal olfactory epithelium and general olfaction [89]. In contrast, the olfactory epithelium is reduced in Dmrt3 and Dmrt5 knockout (KO) mice and is almost completely absent in Dmrt3:Dmrt5 double KO (Saulnier et al., unpublished data). The lack of phenotype in Dmrt4 mutants is thus likely to be due to the presence of Dmrt3 and Dmrt5. Together, these observations indicate that Dmrt3-5 may have overlapping function in vertebrate olfactory placode development, which remains to be further investigated.
In zebrafish, a Dmrt5 mutant was isolated that shows defects in telencephalic neurogenesis. Expression of Neurog1 and other telencephalic marker genes such as Foxg1 and Emx3 were downregulated, while in contrast, Her6, a Hes-related gene that encodes a negative regulator of Neurog1, was expanded. Knockdown of Her6 rescues Neurog1 expression in the Dmrt5 −/− telencephalon, suggesting that Dmrt5 regulates Neurog1 expression by repressing Her6 [95]. Such a mechanism has been previously reported for MAB-3 in the specification of sex-specific neurons in C. elegans [26]. So, Dmrt5 regulates neurogenesis in the zebrafish posterior-dorsal telencephalon. Whether the other Dmrt genes expressed in the developing telencephalon also play a role in neurogenesis, and whether this function in neurogenesis is conserved among vertebrates, remains to be investigated.
A Dmrt4/5-related gene has been recently identified in the sea anemone N. vectensis (designated NvDmrtB), a model system from the sister group of bilaterians, the cnidarians. In Nematostella, NvDmrtB is expressed in scattered neural cells in both the ectodermal and endodermal layers (Fig. 3g). In Xenopus, its overexpression induces neurogenesis in animal cap explants. Conversely, knockdown of NvDmrtB in Nematostella reduces the number of Elav-1 positive neurons [9]. These results suggest that Dmrt’s ability to control neurogenesis derives from an ancestral function already present in the last common ancestor of cnidarians and bilaterians. Further analyses of Dmrt genes in basally branching animals, including sponges, will be required to define the ancestral functions of Dmrt genes.
A very recent study has shown that a Dmrt3 nonsense mutation has a major effect on the pattern of locomotion in horses. The phenotype indicates that Dmrt3+ spinal cord neurons have a critical role for left–right coordination and for coordinating the movement of the fore- and hind-legs. Dmrt3 is expressed in cells originating from dI6 progenitors that develop into inhibitory interneurons projecting ipsi- and contralaterally. Examination of wild-type and Dmrt3 null mice demonstrated that it takes part in neuronal specification within this subdivision, and that it is critical for spinal circuit function. The mutation leads to a truncated Dmrt3 protein retaining the DM and DMA domains but lacking the downstream sequences, which highlight their importance for its activity [21].
Dmrt5 is required for anterior neural tissue development
In the mouse, three recent studies, two using KO mice and the third using embryonic stem (ES) cells, have provided insights into the function of Dmrt5 in the development of the telencephalon and mesencephalon, respectively (Fig. 4).
Regionalization of the telencephalon is initiated by morphogens secreted from localized inductive centers. The two major patterning centers that are the most directly implicated in telencephalon patterning are the ANR located at the rostral pole of the telencephalon and the roof plate and cortical hem (CH) region at the dorsal caudal midline and immediate adjacent territories. The ANR secretes FGFs and the CH produces a variety of Wnt and Bmp ligands critical for hippocampus development. These signals establish graded expression of genes encoding transcription factors that are crucial for the regionalization of the telencephalon and subsequent arealization of the cerebral cortex in cortical progenitors. Among them are Emx2, promoting a caudomedial fate, and Pax6, promoting a rostrolateral identity [96, 97]. In the mouse, similarly to Emx2, Dmrt5 is detected in cortical progenitors in a high caudomedial to low rostrolateral gradient and is dependent on Wnt signaling [91, 92, 98]. With respect to the other transcription factors playing a crucial role in cortical development and patterning, it has been shown that Dmrt5 is dependent on the early zinc finger transcription factor Gli3. In contrast, Emx2 is not required for Dmrt5 expression. Pax6, expressed in a complementary manner to Dmrt5, may antagonize its expression. In Dmrt5 null mutants, the caudomedial cortex, including the hippocampus, is strongly reduced. Wnt and Bmp signaling in the embryonic dorsomedial telencephalon and the downstream-dependent transcription factors such as Emx2 are reduced. Pax6, which is inhibited by midline signals, is upregulated [91, 92]. In contrast, conditional ablation of Dmrt5 at neurogenic stages using the Nestin-Cre transgenic line only causes a slight reduction in telencephalon size [92]. Thus, Dmrt5 is a novel Wnt-dependent “early master” regulator of initial regional patterning of the cerebral cortex. It is likely to function, at least in part, by promoting dorsal midline signaling center formation and thereby helping to establish the graded expression of the other transcription regulators of cortical identity. Whether Dmrt3 and Dmrt4 functions overlap with that of Dmrt5 in cortical patterning, and whether they directly or indirectly regulate Wnt and Emx gene expression, are two important questions still to be addressed.
Ascidian belongs to the urochordates, which represent the closest living relatives of the vertebrates. A gene related to Dmrt4/5 with a DMA domain has been identified in the ascidian Ciona savigny (designated Dmrt1). In ascidians, the CNS develops via neurulation of cells of the neural plate, forming a simple brain called the sensory vesicle and a caudal nerve cord. The anterior neural plate produces placodal derivatives, such as the adhesive palps and stomodeum, and the anterior portion of the brain, called the sensory vesicle. Dmrt1 is expressed from the 64-cell stage in progenitors of the anterior neural plate and is later restricted to the anterior part of the sensory vesicle (Fig. 3e, f). A null mutation in the Dmrt1 gene as well as knockdown experiments have shown that Dmrt1 is required for the development of the palps and oral siphon (mouth) that derive from the stomodeum. It also leads to extensive disruption of sensory structures, such as light-sensitive ocellus, in the sensory vesicle [99–101]. Furthermore, knockdown experiments have shown that Dmrt1 is activated by FGF signals and is required for the expression of FoxC and ZicL that marks the palp placodes and anterior neural tissue, respectively. They also show that Dmrt1 promotes Six1/2, Six3/6 and meis in the developing brain and negatively regulates its own expression [99]. In Ciona, as in vertebrates, Dmrt genes thus mark anterior neural regions, including placodes and anterior neural tissue, and are required for their development (Fig. 4).
The ventral-medial caudal diencephalon and mesencephalon contain dopaminergic neurons that are essential for the control of voluntary movements and the regulation of emotion, and are severely affected in neurodegenerative diseases such as Parkinson’s disease. In response to local inductive signals (Shh, Fgf8, Wnt1), transcription factors are expressed at specific dorsal and ventral positions in the mesodiencephalon inducing distinct cell fates, including in the ventral-most progenitors a midbrain dopaminergic cell fate. As in the developing telencephalon, expression of Dmrt5 in the ventral-medial mesencephalon is restricted to progenitors that give rise to dopamine neurons. In embryonic stem (ES) cells, overexpression of Dmrt5 induces a ventral-medial progenitor phenotype and inhibits ventral-lateral mesencephalic markers. Conversely, knockdown compromises ES cell differentiation toward a ventral-medial cell fate [90]. Thus, Dmrt5 promotes midbrain dopaminergic (mDA) identity in ES cells by enforcing a ventral progenitor fate. Whether Dmrt5 controls in vivo ventral-medial mesencephalic cell fate remains to be demonstrated.
Conclusion
Besides their role in sexual development, Dmrt factors have clearly emerged as important regulators of vertebrate development. Recent findings indicate that members of the group A function as critical regulators of the development of the nervous system, functioning both in neurogenesis and patterning of the developing neural tissue, and that this ability to control neural development is an ancestral function. A better understanding of when and where these Dmrt factors binds to the genome is needed to provide insight into the molecular mechanisms employed by these factors. Elucidating the role of the conserved DMA domain will also be central to understand how Dmrt factors coordinate developmental processes, functioning as activators or repressors depending on the cellular context or locus. Future studies should also determine whether they play any cell-autonomous roles in sexual differentiation of non-gonadal tissues and whether their deregulation is associated with some human neural diseases.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
We are grateful to Dr. Yutaka Kikuchi (Hiroshima, Japan) for the image of Dmrt5 expression in zebrafish, to Dr. Fabian Rentzsch (Bergen, Norway) for the image of NvDmrtB expression in N. vectensis and to Maja Adamska (Bergen, Norway) for allowing the use of unpublished genomic sequences from the sponge S. ciliatum. We also thank Dr. Yasuo Hitoyoshi (Villefranche-sur-mer, France) for helpful discussions. This work was supported by grants from the Belgian Fonds de la Recherche Scientifique (FRFC 2.4544.12) and the Belgian Queen Elisabeth Medical Foundation (to E.B.) and the Institut Universitaire de France (to M.V.). S.D. is a postdoctoral fellow from the Belgian Fonds de la Recherche Scientifique (FNRS). M.K. is a doctoral fellow from the Belgian Fonds pour la formation à la Recherche dans l’Industrie et dans l’Agriculture (FRIA).
References
- 1.Erdman SE, Burtis KC. The Drosophila doublesex proteins share a novel zinc finger related DNA binding domain. EMBO J. 1993;12(2):527–535. doi: 10.1002/j.1460-2075.1993.tb05684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu L, Wilken J, Phillips NB, Narendra U, Chan G, Stratton SM, Kent SB, Weiss MA. Sexual dimorphism in diverse metazoans is regulated by a novel class of intertwined zinc fingers. Genes Dev. 2000;14(14):1750–1764. [PMC free article] [PubMed] [Google Scholar]
- 3.Kopp A. Dmrt genes in the development and evolution of sexual dimorphism. Trends Genet. 2012;28(4):175–184. doi: 10.1016/j.tig.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matson CK, Zarkower D. Sex and the singular DM domain: insights into sexual regulation, evolution and plasticity. Nat Rev Genet. 2012;13(3):163–174. doi: 10.1038/nrg3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hong CS, Park BY, Saint-Jeannet JP. The function of Dmrt genes in vertebrate development: it is not just about sex. Dev Biol. 2007;310(1):1–9. doi: 10.1016/j.ydbio.2007.07.035. [DOI] [PubMed] [Google Scholar]
- 6.Wang F, Yu Y, Ji D, Li H. The DMRT gene family in amphioxus. J Biomol Struct Dyn. 2012;30(2):191–200. doi: 10.1080/07391102.2012.677770. [DOI] [PubMed] [Google Scholar]
- 7.Naimi A, Martinez AS, Specq ML, Mrac A, Diss B, Mathieu M, Sourdaine P. Identification and expression of a factor of the DM family in the oyster Crassostrea gigas . Comp Biochem Physiol A. 2009;152(2):189–196. doi: 10.1016/j.cbpa.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 8.Miller SW, Hayward DC, Bunch TA, Miller DJ, Ball EE, Bardwell VJ, Zarkower D, Brower DL. A DM domain protein from a coral, Acropora millepora, homologous to proteins important for sex determination. Evol Dev. 2003;5(3):251–258. doi: 10.1046/j.1525-142x.2003.03032.x. [DOI] [PubMed] [Google Scholar]
- 9.Parlier D, Moers V, Van Campenhout C, Preillon J, Leclere L, Saulnier A, Sirakov M, Busengdal H, Kricha S, Marine JC, Rentzsch F, Bellefroid EJ. The Xenopus doublesex-related gene Dmrt5 is required for olfactory placode neurogenesis. Dev Biol. 2013;373(1):39–52. doi: 10.1016/j.ydbio.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 10.Ottolenghi C, Fellous M, Barbieri M, McElreavey K. Novel paralogy relations among human chromosomes support a link between the phylogeny of doublesex-related genes and the evolution of sex determination. Genomics. 2002;79(3):333–343. doi: 10.1006/geno.2002.6711. [DOI] [PubMed] [Google Scholar]
- 11.Dunn CW, Hejnol A, Matus DQ, Pang K, Browne WE, Smith SA, Seaver E, Rouse GW, Obst M, Edgecombe GD, Sorensen MV, Haddock SH, Schmidt-Rhaesa A, Okusu A, Kristensen RM, Wheeler WC, Martindale MQ, Giribet G. Broad phylogenomic sampling improves resolution of the animal tree of life. Nature. 2008;452(7188):745–749. doi: 10.1038/nature06614. [DOI] [PubMed] [Google Scholar]
- 12.Philippe H, Derelle R, Lopez P, Pick K, Borchiellini C, Boury-Esnault N, Vacelet J, Renard E, Houliston E, Queinnec E, Da Silva C, Wincker P, Le Guyader H, Leys S, Jackson DJ, Schreiber F, Erpenbeck D, Morgenstern B, Worheide G, Manuel M. Phylogenomics revives traditional views on deep animal relationships. Curr Biol. 2009;19(8):706–712. doi: 10.1016/j.cub.2009.02.052. [DOI] [PubMed] [Google Scholar]
- 13.Volff JN, Zarkower D, Bardwell VJ, Schartl M. Evolutionary dynamics of the DM domain gene family in metazoans. J Mol Evol. 2003;57(Suppl 1):S241–S249. doi: 10.1007/s00239-003-0033-0. [DOI] [PubMed] [Google Scholar]
- 14.Veith AM, Klattig J, Dettai A, Schmidt C, Englert C, Volff JN. Male-biased expression of X-chromosomal DM domain-less Dmrt8 genes in the mouse. Genomics. 2006;88(2):185–195. doi: 10.1016/j.ygeno.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 15.Li Q, Zhou X, Guo Y, Shang X, Chen H, Lu H, Cheng H, Zhou R. Nuclear localization, DNA binding and restricted expression in neural and germ cells of zebrafish Dmrt3. Biol Cell. 2008;100(8):453–463. doi: 10.1042/BC20070114. [DOI] [PubMed] [Google Scholar]
- 16.Murphy MW, Zarkower D, Bardwell VJ. Vertebrate DM domain proteins bind similar DNA sequences and can heterodimerize on DNA. BMC Mol Biol. 2007;8:58. doi: 10.1186/1471-2199-8-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Erdman SE, Chen HJ, Burtis KC. Functional and genetic characterization of the oligomerization and DNA binding properties of the Drosophila doublesex proteins. Genetics. 1996;144(4):1639–1652. doi: 10.1093/genetics/144.4.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luo SD, Shi GW, Baker BS. Direct targets of the D. melanogaster DSXF protein and the evolution of sexual development. Development. 2011;138(13):2761–2771. doi: 10.1242/dev.065227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yi W, Zarkower D. Similarity of DNA binding and transcriptional regulation by Caenorhabditis elegans MAB-3 and Drosophila melanogaster DSX suggests conservation of sex determining mechanisms. Development. 1999;126(5):873–881. doi: 10.1242/dev.126.5.873. [DOI] [PubMed] [Google Scholar]
- 20.Yoshimoto S, Okada E, Oishi T, Numagami R, Umemoto H, Tamura K, Kanda H, Shiba T, Takamatsu N, Ito M. Expression and promoter analysis of Xenopus DMRT1 and functional characterization of the transactivation property of its protein. Dev Growth Differ. 2006;48(9):597–603. doi: 10.1111/j.1440-169X.2006.00894.x. [DOI] [PubMed] [Google Scholar]
- 21.Andersson LS, Larhammar M, Memic F, Wootz H, Schwochow D, Rubin CJ, Patra K, Arnason T, Wellbring L, Hjalm G, Imsland F, Petersen JL, McCue ME, Mickelson JR, Cothran G, Ahituv N, Roepstorff L, Mikko S, Vallstedt A, Lindgren G, Andersson L, Kullander K. Mutations in DMRT3 affect locomotion in horses and spinal circuit function in mice. Nature. 2012;488(7413):642–646. doi: 10.1038/nature11399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krentz AD, Murphy MW, Kim S, Cook MS, Capel B, Zhu R, Matin A, Sarver AL, Parker KL, Griswold MD, Looijenga LH, Bardwell VJ, Zarkower D. The DM domain protein DMRT1 is a dose-sensitive regulator of fetal germ cell proliferation and pluripotency. Proc Natl Acad Sci USA. 2009;106(52):22323–22328. doi: 10.1073/pnas.0905431106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krentz AD, Murphy MW, Sarver AL, Griswold MD, Bardwell VJ, Zarkower D. DMRT1 promotes oogenesis by transcriptional activation of Stra8 in the mammalian fetal ovary. Dev Biol. 2011;356(1):63–70. doi: 10.1016/j.ydbio.2011.05.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matson CK, Murphy MW, Sarver AL, Griswold MD, Bardwell VJ, Zarkower D. DMRT1 prevents female reprogramming in the postnatal mammalian testis. Nature. 2011;476(7358):101–104. doi: 10.1038/nature10239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murphy MW, Sarver AL, Rice D, Hatzi K, Ye K, Melnick A, Heckert LL, Zarkower D, Bardwell VJ. Genome-wide analysis of DNA binding and transcriptional regulation by the mammalian doublesex homolog DMRT1 in the juvenile testis. Proc Natl Acad Sci USA. 2010;107(30):13360–13365. doi: 10.1073/pnas.1006243107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ross JM, Kalis AK, Murphy MW, Zarkower D. The DM domain protein MAB-3 promotes sex-specific neurogenesis in C. elegans by regulating bHLH proteins. Dev Cell. 2005;8(6):881–892. doi: 10.1016/j.devcel.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 27.Coschigano KT, Wensink PC. Sex-specific transcriptional regulation by the male and female doublesex proteins of Drosophila . Genes Dev. 1993;7(1):42–54. doi: 10.1101/gad.7.1.42. [DOI] [PubMed] [Google Scholar]
- 28.Kopp A, Duncan I, Godt D, Carroll SB. Genetic control and evolution of sexually dimorphic characters in Drosophila . Nature. 2000;408(6812):553–559. doi: 10.1038/35046017. [DOI] [PubMed] [Google Scholar]
- 29.Williams TM, Selegue JE, Werner T, Gompel N, Kopp A, Carroll SB. The regulation and evolution of a genetic switch controlling sexually dimorphic traits in Drosophila . Cell. 2008;134(4):610–623. doi: 10.1016/j.cell.2008.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chatterjee SS, Uppendahl LD, Chowdhury MA, Ip PL, Siegal ML. The female-specific doublesex isoform regulates pleiotropic transcription factors to pattern genital development in Drosophila . Development. 2011;138(6):1099–1109. doi: 10.1242/dev.055731. [DOI] [PubMed] [Google Scholar]
- 31.Narendra U, Zhu L, Li B, Wilken J, Weiss MA. Sex-specific gene regulation. The doublesex DM motif is a bipartite DNA-binding domain. J Biol Chem. 2002;277(45):43463–43473. doi: 10.1074/jbc.M204616200. [DOI] [PubMed] [Google Scholar]
- 32.Lints R, Emmons SW. Regulation of sex-specific differentiation and mating behavior in C. elegans by a new member of the DM domain transcription factor family. Genes Dev. 2002;16(18):2390–2402. doi: 10.1101/gad.1012602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang W, Kidd BJ, Carroll SB, Yoder JH. Sexually dimorphic regulation of the Wingless morphogen controls sex-specific segment number in Drosophila . Proc Natl Acad Sci USA. 2011;108(27):11139–11144. doi: 10.1073/pnas.1108431108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kato Y, Kobayashi K, Watanabe H, Iguchi T. Environmental sex determination in the branchiopod crustacean Daphnia magna: deep conservation of a Doublesex gene in the sex-determining pathway. PLoS Genet. 2011;7(3):e1001345. doi: 10.1371/journal.pgen.1001345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chue J, Smith CA. Sex determination and sexual differentiation in the avian model. FEBS J. 2011;278(7):1027–1034. doi: 10.1111/j.1742-4658.2011.08032.x. [DOI] [PubMed] [Google Scholar]
- 36.Herpin A, Schartl M. Dmrt1 genes at the crossroads: a widespread and central class of sexual development factors in fish. FEBS J. 2011;278(7):1010–1019. doi: 10.1111/j.1742-4658.2011.08030.x. [DOI] [PubMed] [Google Scholar]
- 37.Veitia R, Nunes M, Brauner R, Doco-Fenzy M, Joanny-Flinois O, Jaubert F, Lortat-Jacob S, Fellous M, McElreavey K. Deletions of distal 9p associated with 46, XY male to female sex reversal: definition of the breakpoints at 9p23.3–p24.1. Genomics. 1997;41(2):271–274. doi: 10.1006/geno.1997.4648. [DOI] [PubMed] [Google Scholar]
- 38.Muroya K, Okuyama T, Goishi K, Ogiso Y, Fukuda S, Kameyama J, Sato H, Suzuki Y, Terasaki H, Gomyo H, Wakui K, Fukushima Y, Ogata T. Sex-determining gene(s) on distal 9p: clinical and molecular studies in six cases. J Clin Endocrinol Metab. 2000;85(9):3094–3100. doi: 10.1210/jcem.85.9.6771. [DOI] [PubMed] [Google Scholar]
- 39.Vialard F, Ottolenghi C, Gonzales M, Choiset A, Girard S, Siffroi JP, McElreavey K, Vibert-Guigue C, Sebaoun M, Joye N, Portnoi MF, Jaubert F, Fellous M. Deletion of 9p associated with gonadal dysfunction in 46, XY but not in 46 XX human fetuses. J Med Genet. 2002;39(7):514–518. doi: 10.1136/jmg.39.7.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tannour-Louet M, Han S, Corbett ST, Louet JF, Yatsenko S, Meyers L, Shaw CA, Kang SH, Cheung SW, Lamb DJ. Identification of de novo copy number variants associated with human disorders of sexual development. PLoS ONE. 2010;5(10):e15392. doi: 10.1371/journal.pone.0015392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moniot B, Berta P, Scherer G, Sudbeck P, Poulat F. Male specific expression suggests role of DMRT1 in human sex determination. Mech Dev. 2000;91(1–2):323–325. doi: 10.1016/s0925-4773(99)00267-1. [DOI] [PubMed] [Google Scholar]
- 42.Raymond CS, Parker ED, Kettlewell JR, Brown LG, Page DC, Kusz K, Jaruzelska J, Reinberg Y, Flejter WL, Bardwell VJ, Hirsch B, Zarkower D. A region of human chromosome 9p required for testis development contains two genes related to known sexual regulators. Hum Mol Genet. 1999;8(6):989–996. doi: 10.1093/hmg/8.6.989. [DOI] [PubMed] [Google Scholar]
- 43.Ledig S, Hiort O, Scherer G, Hoffmann M, Wolff G, Morlot S, Kuechler A, Wieacker P. Array-CGH analysis in patients with syndromic and non-syndromic XY gonadal dysgenesis: evaluation of array CGH as diagnostic tool and search for new candidate loci. Hum Reprod. 2010;25(10):2637–2646. doi: 10.1093/humrep/deq167. [DOI] [PubMed] [Google Scholar]
- 44.Mello MP, Coeli FB, Assumpcao JG, Castro TM, Maciel-Guerra AT, Marques-de-Faria AP, Baptista MT, Guerra-Junior G. Novel DMRT1 3′UTR + 11insT mutation associated to XY partial gonadal dysgenesis. Arq Bras Endocrinol Metabol. 2010;54(8):749–753. doi: 10.1590/s0004-27302010000800015. [DOI] [PubMed] [Google Scholar]
- 45.Ledig S, Hiort O, Wunsch L, Wieacker P. Partial deletion of DMRT1 causes 46, XY ovotesticular disorder of sexual development. Eur J Endocrinol. 2012;167(1):119–124. doi: 10.1530/EJE-12-0136. [DOI] [PubMed] [Google Scholar]
- 46.Turnbull C, Rapley EA, Seal S, Pernet D, Renwick A, Hughes D, Ricketts M, Linger R, Nsengimana J, Deloukas P, Huddart RA, Bishop DT, Easton DF, Stratton MR, Rahman N. Variants near DMRT1, TERT and ATF7IP are associated with testicular germ cell cancer. Nat Genet. 2010;42(7):604–607. doi: 10.1038/ng.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kanetsky PA, Mitra N, Vardhanabhuti S, Vaughn DJ, Li M, Ciosek SL, Letrero R, D’Andrea K, Vaddi M, Doody DR, Weaver J, Chen C, Starr JR, Hakonarson H, Rader DJ, Godwin AK, Reilly MP, Schwartz SM, Nathanson KL. A second independent locus within DMRT1 is associated with testicular germ cell tumor susceptibility. Hum Mol Genet. 2011;20(15):3109–3117. doi: 10.1093/hmg/ddr207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kratz CP, Han SS, Rosenberg PS, Berndt SI, Burdett L, Yeager M, Korde LA, Mai PL, Pfeiffer R, Greene MH. Variants in or near KITLG, BAK1, DMRT1, and TERT-CLPTM1L predispose to familial testicular germ cell tumour. J Med Genet. 2011;48(7):473–476. doi: 10.1136/jmedgenet-2011-100001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Raymond CS, Kettlewell JR, Hirsch B, Bardwell VJ, Zarkower D. Expression of Dmrt1 in the genital ridge of mouse and chicken embryos suggests a role in vertebrate sexual development. Dev Biol. 1999;215(2):208–220. doi: 10.1006/dbio.1999.9461. [DOI] [PubMed] [Google Scholar]
- 50.Raymond CS, Murphy MW, O’Sullivan MG, Bardwell VJ, Zarkower D. Dmrt1, a gene related to worm and fly sexual regulators, is required for mammalian testis differentiation. Genes Dev. 2000;14(20):2587–2595. doi: 10.1101/gad.834100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim S, Bardwell VJ, Zarkower D. Cell type-autonomous and non-autonomous requirements for Dmrt1 in postnatal testis differentiation. Dev Biol. 2007;307(2):314–327. doi: 10.1016/j.ydbio.2007.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Uhlenhaut NH, Jakob S, Anlag K, Eisenberger T, Sekido R, Kress J, Treier AC, Klugmann C, Klasen C, Holter NI, Riethmacher D, Schutz G, Cooney AJ, Lovell-Badge R, Treier M. Somatic sex reprogramming of adult ovaries to testes by FOXL2 ablation. Cell. 2009;139(6):1130–1142. doi: 10.1016/j.cell.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 53.Mark M, Jacobs H, Oulad-Abdelghani M, Dennefeld C, Feret B, Vernet N, Codreanu CA, Chambon P, Ghyselinck NB. STRA8-deficient spermatocytes initiate, but fail to complete, meiosis and undergo premature chromosome condensation. J Cell Sci. 2008;121(Pt 19):3233–3242. doi: 10.1242/jcs.035071. [DOI] [PubMed] [Google Scholar]
- 54.Anderson EL, Baltus AE, Roepers-Gajadien HL, Hassold TJ, de Rooij DG, van Pelt AM, Page DC. Stra8 and its inducer, retinoic acid, regulate meiotic initiation in both spermatogenesis and oogenesis in mice. Proc Natl Acad Sci USA. 2008;105(39):14976–14980. doi: 10.1073/pnas.0807297105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Matson CK, Murphy MW, Griswold MD, Yoshida S, Bardwell VJ, Zarkower D. The mammalian doublesex homolog DMRT1 is a transcriptional gatekeeper that controls the mitosis versus meiosis decision in male germ cells. Dev Cell. 2010;19(4):612–624. doi: 10.1016/j.devcel.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Baltus AE, Menke DB, Hu YC, Goodheart ML, Carpenter AE, de Rooij DG, Page DC. In germ cells of mouse embryonic ovaries, the decision to enter meiosis precedes premeiotic DNA replication. Nat Genet. 2006;38(12):1430–1434. doi: 10.1038/ng1919. [DOI] [PubMed] [Google Scholar]
- 57.Kettlewell JR, Raymond CS, Zarkower D. Temperature-dependent expression of turtle Dmrt1 prior to sexual differentiation. Genesis. 2000;26(3):174–178. [PubMed] [Google Scholar]
- 58.Sreenivasulu K, Ganesh S, Raman R. Evolutionarily conserved, DMRT1, encodes alternatively spliced transcripts and shows dimorphic expression during gonadal differentiation in the lizard Calotes versicolor . Gene Expr Patterns. 2002;2(1–2):51–60. doi: 10.1016/s0925-4773(02)00357-x. [DOI] [PubMed] [Google Scholar]
- 59.Shoemaker C, Ramsey M, Queen J, Crews D. Expression of Sox9, Mis, and Dmrt1 in the gonad of a species with temperature-dependent sex determination. Dev Dyn. 2007;236(4):1055–1063. doi: 10.1002/dvdy.21096. [DOI] [PubMed] [Google Scholar]
- 60.Kondo M, Nanda I, Hornung U, Asakawa S, Shimizu N, Mitani H, Schmid M, Shima A, Schartl M. Absence of the candidate male sex-determining gene dmrt1b(Y) of medaka from other fish species. Curr Biol. 2003;13(5):416–420. doi: 10.1016/s0960-9822(03)00125-8. [DOI] [PubMed] [Google Scholar]
- 61.Veith AM, Froschauer A, Korting C, Nanda I, Hanel R, Schmid M, Schartl M, Volff JN. Cloning of the dmrt1 gene of Xiphophorus maculatus: dmY/dmrt1Y is not the master sex-determining gene in the platyfish. Gene. 2003;317(1–2):59–66. doi: 10.1016/s0378-1119(03)00664-4. [DOI] [PubMed] [Google Scholar]
- 62.Matsuda M, Nagahama Y, Shinomiya A, Sato T, Matsuda C, Kobayashi T, Morrey CE, Shibata N, Asakawa S, Shimizu N, Hori H, Hamaguchi S, Sakaizumi M. DMY is a Y-specific DM-domain gene required for male development in the medaka fish. Nature. 2002;417(6888):559–563. doi: 10.1038/nature751. [DOI] [PubMed] [Google Scholar]
- 63.Nanda I, Kondo M, Hornung U, Asakawa S, Winkler C, Shimizu A, Shan Z, Haaf T, Shimizu N, Shima A, Schmid M, Schartl M. A duplicated copy of DMRT1 in the sex-determining region of the Y chromosome of the medaka, Oryzias latipes . Proc Natl Acad Sci USA. 2002;99(18):11778–11783. doi: 10.1073/pnas.182314699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kondo M, Hornung U, Nanda I, Imai S, Sasaki T, Shimizu A, Asakawa S, Hori H, Schmid M, Shimizu N, Schartl M. Genomic organization of the sex-determining and adjacent regions of the sex chromosomes of medaka. Genome Res. 2006;16(7):815–826. doi: 10.1101/gr.5016106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Otake H, Shinomiya A, Matsuda M, Hamaguchi S, Sakaizumi M. Wild-derived XY sex-reversal mutants in the Medaka, Oryzias latipes . Genetics. 2006;173(4):2083–2090. doi: 10.1534/genetics.106.058941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Matsuda M, Shinomiya A, Kinoshita M, Suzuki A, Kobayashi T, Paul-Prasanth B, Lau EL, Hamaguchi S, Sakaizumi M, Nagahama Y. DMY gene induces male development in genetically female (XX) medaka fish. Proc Natl Acad Sci USA. 2007;104(10):3865–3870. doi: 10.1073/pnas.0611707104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kobayashi T, Matsuda M, Kajiura-Kobayashi H, Suzuki A, Saito N, Nakamoto M, Shibata N, Nagahama Y. Two DM domain genes, DMY and DMRT1, involved in testicular differentiation and development in the medaka, Oryzias latipes . Dev Dyn. 2004;231(3):518–526. doi: 10.1002/dvdy.20158. [DOI] [PubMed] [Google Scholar]
- 68.Sato T, Endo T, Yamahira K, Hamaguchi S, Sakaizumi M. Induction of female-to-male sex reversal by high temperature treatment in Medaka, Oryzias latipes . Zool Sci. 2005;22(9):985–988. doi: 10.2108/zsj.22.985. [DOI] [PubMed] [Google Scholar]
- 69.Hattori RS, Gould RJ, Fujioka T, Saito T, Kurita J, Strussmann CA, Yokota M, Watanabe S. Temperature-dependent sex determination in Hd-rR medaka Oryzias latipes: gender sensitivity, thermal threshold, critical period, and DMRT1 expression profile. Sex Dev. 2007;1(2):138–146. doi: 10.1159/000100035. [DOI] [PubMed] [Google Scholar]
- 70.Masuyama H, Yamada M, Kamei Y, Fujiwara-Ishikawa T, Todo T, Nagahama Y, Matsuda M. Dmrt1 mutation causes a male-to-female sex reversal after the sex determination by Dmy in the medaka. Chromosome Res. 2012;20(1):163–176. doi: 10.1007/s10577-011-9264-x. [DOI] [PubMed] [Google Scholar]
- 71.Yoshimoto S, Okada E, Umemoto H, Tamura K, Uno Y, Nishida-Umehara C, Matsuda Y, Takamatsu N, Shiba T, Ito M. A W-linked DM-domain gene, DM-W, participates in primary ovary development in Xenopus laevis . Proc Natl Acad Sci USA. 2008;105(7):2469–2474. doi: 10.1073/pnas.0712244105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yoshimoto S, Ikeda N, Izutsu Y, Shiba T, Takamatsu N, Ito M. Opposite roles of DMRT1 and its W-linked paralogue, DM-W, in sexual dimorphism of Xenopus laevis: implications of a ZZ/ZW-type sex-determining system. Development. 2010;137(15):2519–2526. doi: 10.1242/dev.048751. [DOI] [PubMed] [Google Scholar]
- 73.Bewick AJ, Anderson DW, Evans BJ. Evolution of the closely related, sex-related genes DM-W and DMRT1 in African clawed frogs (Xenopus) Evolution. 2011;65(3):698–712. doi: 10.1111/j.1558-5646.2010.01163.x. [DOI] [PubMed] [Google Scholar]
- 74.Smith CA, Roeszler KN, Ohnesorg T, Cummins DM, Farlie PG, Doran TJ, Sinclair AH. The avian Z-linked gene DMRT1 is required for male sex determination in the chicken. Nature. 2009;461(7261):267–271. doi: 10.1038/nature08298. [DOI] [PubMed] [Google Scholar]
- 75.Kawamata M, Nishimori K. Mice deficient in Dmrt7 show infertility with spermatogenic arrest at pachytene stage. FEBS Lett. 2006;580(27):6442–6446. doi: 10.1016/j.febslet.2006.10.066. [DOI] [PubMed] [Google Scholar]
- 76.Kim S, Namekawa SH, Niswander LM, Ward JO, Lee JT, Bardwell VJ, Zarkower D. A mammal-specific doublesex homolog associates with male sex chromatin and is required for male meiosis. PLoS Genet. 2007;3(4):e62. doi: 10.1371/journal.pgen.0030062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Keisman EL, Baker BS. The Drosophila sex determination hierarchy modulates wingless and decapentaplegic signaling to deploy dachshund sex-specifically in the genital imaginal disc. Development. 2001;128(9):1643–1656. doi: 10.1242/dev.128.9.1643. [DOI] [PubMed] [Google Scholar]
- 78.Sanchez L, Gorfinkiel N, Guerrero I. Sex determination genes control the development of the Drosophila genital disc, modulating the response to Hedgehog, Wingless and Decapentaplegic signals. Development. 2001;128(7):1033–1043. doi: 10.1242/dev.128.7.1033. [DOI] [PubMed] [Google Scholar]
- 79.Ahmad SM, Baker BS. Sex-specific deployment of FGF signaling in Drosophila recruits mesodermal cells into the male genital imaginal disc. Cell. 2002;109(5):651–661. doi: 10.1016/s0092-8674(02)00744-4. [DOI] [PubMed] [Google Scholar]
- 80.Meng A, Moore B, Tang H, Yuan B, Lin S. A Drosophila doublesex-related gene, terra, is involved in somitogenesis in vertebrates. Development. 1999;126(6):1259–1268. doi: 10.1242/dev.126.6.1259. [DOI] [PubMed] [Google Scholar]
- 81.Liu S, Li Z, Gui JF. Fish-specific duplicated dmrt2b contributes to a divergent function through Hedgehog pathway and maintains left-right asymmetry establishment function. PLoS ONE. 2009;4(9):e7261. doi: 10.1371/journal.pone.0007261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhou X, Li Q, Lu H, Chen H, Guo Y, Cheng H, Zhou R. Fish specific duplication of Dmrt2: characterization of zebrafish Dmrt2b. Biochimie. 2008;90(6):878–887. doi: 10.1016/j.biochi.2008.02.021. [DOI] [PubMed] [Google Scholar]
- 83.Lourenco R, Lopes SS, Saude L. Left-right function of dmrt2 genes is not conserved between zebrafish and mouse. PLoS ONE. 2010;5(12):e14438. doi: 10.1371/journal.pone.0014438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Saude L, Lourenco R, Goncalves A, Palmeirim I. terra is a left-right asymmetry gene required for left-right synchronization of the segmentation clock. Nat Cell Biol. 2005;7(9):918–920. doi: 10.1038/ncb1294. [DOI] [PubMed] [Google Scholar]
- 85.Seo KW, Wang Y, Kokubo H, Kettlewell JR, Zarkower DA, Johnson RL. Targeted disruption of the DM domain containing transcription factor Dmrt2 reveals an essential role in somite patterning. Dev Biol. 2006;290(1):200–210. doi: 10.1016/j.ydbio.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 86.Sato T, Rocancourt D, Marques L, Thorsteinsdottir S, Buckingham M. A Pax3/Dmrt2/Myf5 regulatory cascade functions at the onset of myogenesis. PLoS Genet. 2010;6(4):e1000897. doi: 10.1371/journal.pgen.1000897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Smith CA, Hurley TM, McClive PJ, Sinclair AH. Restricted expression of DMRT3 in chicken and mouse embryos. Gene Expr Patterns. 2002;2(1–2):69–72. doi: 10.1016/s0925-4773(02)00360-x. [DOI] [PubMed] [Google Scholar]
- 88.Huang X, Hong CS, O’Donnell M, Saint-Jeannet JP. The doublesex-related gene, XDmrt4, is required for neurogenesis in the olfactory system. Proc Natl Acad Sci USA. 2005;102(32):11349–11354. doi: 10.1073/pnas.0505106102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Balciuniene J, Bardwell VJ, Zarkower D. Mice mutant in the DM domain gene Dmrt4 are viable and fertile but have polyovular follicles. Mol Cell Biol. 2006;26(23):8984–8991. doi: 10.1128/MCB.00959-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gennet N, Gale E, Nan X, Farley E, Takacs K, Oberwallner B, Chambers D, Li M. Doublesex and mab-3-related transcription factor 5 promotes midbrain dopaminergic identity in pluripotent stem cells by enforcing a ventral-medial progenitor fate. Proc Natl Acad Sci USA. 2011;108(22):9131–9136. doi: 10.1073/pnas.1016679108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Saulnier A, Keruzore M, De Clercq S, Bar I, Moers V, Magnani D, Walcher T, Filippis C, Kricha S, Parlier D, Viviani L, Matson CK, Nakagawa Y, Theil T, Gotz M, Mallamaci A, Marine JC, Zarkower D, Bellefroid EJ. The doublesex homolog Dmrt5 is required for the development of the caudomedial cerebral cortex in mammals. Cereb Cortex. 2012 doi: 10.1093/cercor/bhs234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Konno D, Iwashita M, Satoh Y, Momiyama A, Abe T, Kiyonari H, Matsuzaki F. The mammalian DM domain transcription factor Dmrta2 is required for early embryonic development of the cerebral cortex. PLoS ONE. 2012;7(10):e46577. doi: 10.1371/journal.pone.0046577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Winkler C, Hornung U, Kondo M, Neuner C, Duschl J, Shima A, Schartl M. Developmentally regulated and non-sex-specific expression of autosomal dmrt genes in embryos of the Medaka fish (Oryzias latipes) Mech Dev. 2004;121(7–8):997–1005. doi: 10.1016/j.mod.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 94.Keverne EB. Importance of olfactory and vomeronasal systems for male sexual function. Physiol Behav. 2004;83(2):177–187. doi: 10.1016/j.physbeh.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 95.Yoshizawa A, Nakahara Y, Izawa T, Ishitani T, Tsutsumi M, Kuroiwa A, Itoh M, Kikuchi Y. Zebrafish Dmrta2 regulates neurogenesis in the telencephalon. Genes Cells. 2011;16(11):1097–1109. doi: 10.1111/j.1365-2443.2011.01555.x. [DOI] [PubMed] [Google Scholar]
- 96.O’Leary DD, Sahara S. Genetic regulation of arealization of the neocortex. Curr Opin Neurobiol. 2008;18(1):90–100. doi: 10.1016/j.conb.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mallamaci A. Molecular bases of cortico-cerebral regionalization. Prog Brain Res. 2011;189:37–64. doi: 10.1016/B978-0-444-53884-0.00017-8. [DOI] [PubMed] [Google Scholar]
- 98.Hasenpusch-Theil K, Magnani D, Amaniti EM, Han L, Armstrong D, Theil T. Transcriptional analysis of Gli3 mutants identifies Wnt target genes in the developing hippocampus. Cereb Cortex. 2012 doi: 10.1093/cercor/bhr365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Imai KS, Levine M, Satoh N, Satou Y. Regulatory blueprint for a chordate embryo. Science. 2006;312(5777):1183–1187. doi: 10.1126/science.1123404. [DOI] [PubMed] [Google Scholar]
- 100.Tresser J, Chiba S, Veeman M, El-Nachef D, Newman-Smith E, Horie T, Tsuda M, Smith WC. Doublesex/mab3 related-1 (dmrt1) is essential for development of anterior neural plate derivatives in Ciona. Development. 2010;137(13):2197–2203. doi: 10.1242/dev.045302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wagner E, Levine M. FGF signaling establishes the anterior border of the Ciona neural tube. Development. 2012;139(13):2351–2359. doi: 10.1242/dev.078485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Schierwater B, Kolokotronis SO, Eitel M, DeSalle R The diploblast-bilateria sister hypothesis: parallel revolution of a nervous systems may have been a simple step. Commun Integr Biol. 2009;2(5):403–405. doi: 10.4161/cib.2.5.8763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Smith CA, McClive PJ, Western PS, Reed KJ, Sinclair AH. Conservation of a sex-determining gene. Nature. 1999;402(6762):601–602. doi: 10.1038/45130. [DOI] [PubMed] [Google Scholar]
- 104.Osawa N, Oshima Y, Nakamura M. Molecular cloning of Dmrt1 and its expression in the gonad of Xenopus . Zool Sci. 2005;22(6):681–687. doi: 10.2108/zsj.22.681. [DOI] [PubMed] [Google Scholar]
- 105.Veith AM, Schafer M, Kluver N, Schmidt C, Schultheis C, Schartl M, Winkler C, Volff JN. Tissue-specific expression of dmrt genes in embryos and adults of the platyfish Xiphophorus maculatus . Zebrafish. 2006;3(3):325–337. doi: 10.1089/zeb.2006.3.325. [DOI] [PubMed] [Google Scholar]
- 106.Guo Y, Cheng H, Huang X, Gao S, Yu H, Zhou R. Gene structure, multiple alternative splicing, and expression in gonads of zebrafish Dmrt1. Biochem Biophys Res Commun. 2005;330(3):950–957. doi: 10.1016/j.bbrc.2005.03.066. [DOI] [PubMed] [Google Scholar]
- 107.Kim S, Kettlewell JR, Anderson RC, Bardwell VJ, Zarkower D. Sexually dimorphic expression of multiple doublesex-related genes in the embryonic mouse gonad. Gene Expr Patterns. 2003;3(1):77–82. doi: 10.1016/s1567-133x(02)00071-6. [DOI] [PubMed] [Google Scholar]
- 108.Kondo M, Froschauer A, Kitano A, Nanda I, Hornung U, Volff JN, Asakawa S, Mitani H, Naruse K, Tanaka M, Schmid M, Shimizu N, Schartl M, Shima A. Molecular cloning and characterization of DMRT genes from the medaka Oryzias latipes and the platyfish Xiphophorus maculatus . Gene. 2002;295(2):213–222. doi: 10.1016/s0378-1119(02)00692-3. [DOI] [PubMed] [Google Scholar]
- 109.Guo Y, Li Q, Gao S, Zhou X, He Y, Shang X, Cheng H, Zhou R. Molecular cloning, characterization, and expression in brain and gonad of Dmrt5 of zebrafish. Biochem Biophys Res Commun. 2004;324(2):569–575. doi: 10.1016/j.bbrc.2004.09.085. [DOI] [PubMed] [Google Scholar]
- 110.Herpin A, Schindler D, Kraiss A, Hornung U, Winkler C, Schartl M. Inhibition of primordial germ cell proliferation by the medaka male determining gene Dmrt I bY. BMC Dev Biol. 2007;7:99. doi: 10.1186/1471-213X-7-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Seo KW. Dmrt2 and Pax3 double-knockout mice show severe defects in embryonic myogenesis. Comp Med. 2007;57(5):460–468. [PubMed] [Google Scholar]
- 112.Ahituv N, Zhu Y, Visel A, Holt A, Afzal V, Pennacchio LA, Rubin EM. Deletion of ultraconserved elements yields viable mice. PLoS Biol. 2007;5(9):e234. doi: 10.1371/journal.pbio.0050234. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.