Abstract
Zinc transporters, the Zrt-, Irt-like protein (ZIP) family and the Zn transporter (ZnT) family transporters, are found in all aspects of life. Increasing evidence has clarified the molecular mechanism, in which both transporters play critical roles in cellular and physiological functions via mobilizing zinc across the cellular membrane. In the last decade, mutations in ZIP and ZnT transporter genes have been shown to be implicated in a number of inherited human diseases. Moreover, dysregulation of expression and activity of both transporters has been suggested to be involved in the pathogenesis and progression of chronic diseases including cancer, immunological impairment, and neurodegenerative diseases, although comprehensive understanding is far from complete. The diverse phenotypes of diseases related to ZIP and ZnT transporters reflect the multifarious biological functions of both transporters. The present review summarizes the current understanding of ZIP and ZnT transporter functions from the standpoint of human health and diseases. The study of zinc transporters is currently of great clinical interest.
Keywords: Zinc transporter, Zrt- Irt-like protein (ZIP), Zn transporter (ZnT), Health, Diseases
Introduction: brief overview of zinc physiology
Zinc is the second most abundant trace element in humans. The human body contains 2–3 g of zinc, and about 60 % is in skeletal muscle, 30 % is in bone, and 5 % is in liver and skin [1] (Fig. 1). Only about 0.1 % of the body zinc is replenished daily through the diet [2]. Zinc contributes to unique and extensive functions in numerous biological processes including cell division, growth and differentiation, as a structural, catalytic and regulatory component within proteins, such as transcription factors, enzymes, transporters, and receptors [3–5]. Thus, zinc is indispensable, and alterations in zinc homeostasis, in particular its deficiency, have been suggested to be linked to the development of numerous diseases [6–9].
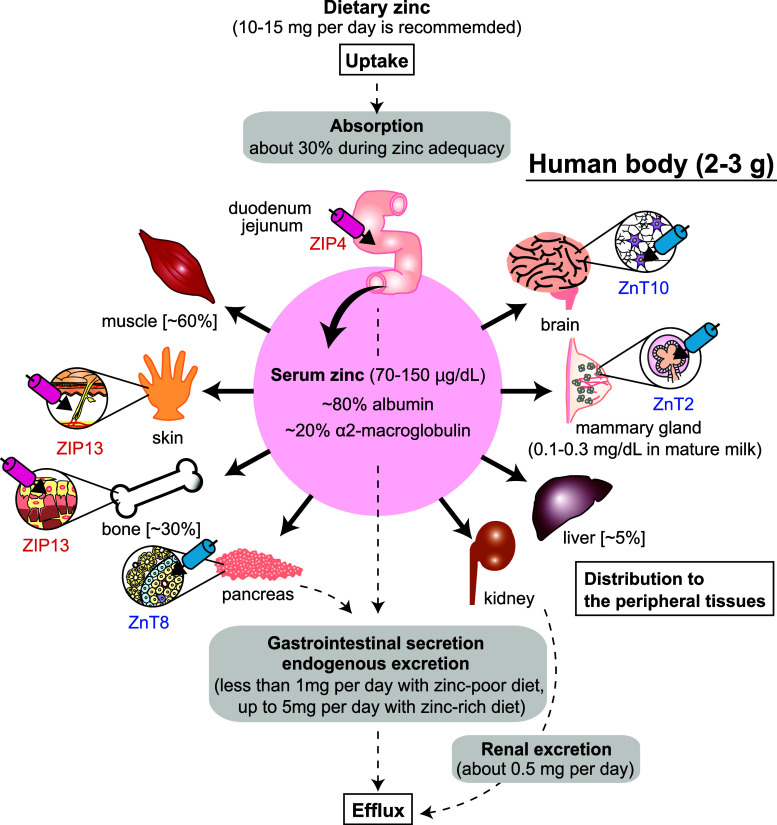
Fig. 1.
Zinc distribution in the body. About 30 % of dietary zinc is absorbed mainly by duodenal and jejunal enterocytes in normal conditions [178]. Approximately 60 % of zinc is stored in skeletal muscle, about 30 % is in bones, and about 5 % is in liver and skin [1]. The remainder is distributed to other tissues such as brain, mammary gland, pancreas, and kidney. Only 0.1 % of total zinc circulates in the plasma bound to albumin and α2-macroglobulin. Zinc is egested primarily via gastrointestinal secretion and excretion, which depends on the zinc status [179]. Compared with gastrointestinal secretion and excretion, the urinary loss of zinc is minor [14]. Integument, another minor route of zinc excretion, is not shown. Zinc distribution in the body is elaborately controlled in the cellular and systemic levels through coordinated regulation, where ZIP and ZnT transporters play important roles. ZIP and ZnT transporters, whose mutations and SNPs are involved in inherited diseases or the increased risk of diseases, are shown in red and blue, respectively
Zinc homeostasis is tightly controlled by the coordinated regulation of the uptake, efflux, distribution, and storage of zinc (reviewed in [3, 10–13]). Zinc is taken up from the diet by intestinal epithelial cells. When zinc is limited in the diet, zinc absorption increases in the small intestine, and release from the pancreas and small intestine decreases. Meanwhile, excess zinc is believed to be released primarily through gastrointestinal secretion, sloughing of mucosal cells, and integument [10, 14, 15]. The absorbed zinc is mostly bound to albumin (loosely, exchangeable pool) and α2-macroglobulin (tightly, non-exchangeable) in the blood and delivered to peripheral tissues [16, 17]. The former binds about 80 % of the serum zinc, and the latter binds 20 %. Once inside the cell, zinc is distributed to the cytoplasm (50 %), nucleus (30–40 %), and membrane (10 %) [18, 19]. In the cytosol, a portion of the zinc pool is bound by metallothioneins, of which there are known to be about a dozen in humans [20]. The zinc proteome estimates that about 10 % of the human genome encodes zinc proteins [4, 21], more than half of which are thought to be transcription factors and enzymes. Importantly, these estimates are based on the intramolecular zinc-binding sites; however, the number of zinc-binding motifs would increase if intermolecular zinc-binding sites, like the one found in the meiotic recombination 11 (Mre11) complex [22], were considered.
Zinc in human health: general aspects
Zinc deficiency in humans was discovered about 50 years ago by Prasad et al. [23]. Since the finding, a broad range of zinc-deficiency symptoms such as growth retardation, diarrhea, skin lesions, alopecia, hypogonadism, immune system dysfunction, and neurological disorders have been described (reviewed in [6–8, 24]), although it had been “much ignored as a global health problem” [25, 26]. Zinc deficiency has also been shown to be associated with sepsis [27], and may be a risk factor for developing asthma [9]. Mild zinc deficiency is commonly found in healthy elderly subjects, resulting in impaired cell-mediated immune responses [28]. Clinical risk factors and pathologies associated with zinc deficiency have been extensively described by Mocchegiani et al. [29].
Numerous reports have described the efficacy of zinc supplementation in human health. Zinc supplementation has shown the potential to improve immune functions and to decrease the incidence of infections in elderly people [30, 31]. Zinc supplementation decreases the incidence of blindness and the risk of developing it in patients with age-related macular degeneration and vision loss [32]. The efficacy has been shown in liner growth and body weight gain in children in the meta-analysis [33]. Zinc supplementation has been reported to show beneficial effects, and thereby a WHO and UNICEF report recommends the inclusion of zinc in the oral rehydration solution to reduce diarrhea in infants and children in developing countries [34]. In these countries, zinc deficiency is responsible for 4 % of the global morbidity and mortality of young children, and for 176,000 diarrhea-related and 406,000 pneumonia-related deaths of children <5 years of age [35]. These facts indicate that zinc is one of the key factors for good health.
Overview of ZIP and ZnT zinc transport proteins
The sophisticated control of systemic and cellular zinc homeostasis is essential for human health. Therefore, a number of proteins such as various zinc permeable and transport membrane proteins and metallothioneins are employed in the body for this control. Among them, zinc transporters belonging to Zrt, Irt-like protein [ZIP, Solute Carrier family 39A (SLC39A)] and Zn transporter (ZnT, SLC30A) play crucial roles.
In humans, 14 members of the ZIP family and ten members of the ZnT family have been identified, although ZnT9 has not been shown to be involved in zinc transport [5, 36, 37]. In general, ZIP proteins function in the uptake of zinc into the cytosol of the cell from the extracellular space or intracellular compartments, while ZnT proteins function in the efflux of zinc from the cytosol of the cell to the extracellular space or intracellular compartments. It has been speculated that ZIP proteins have eight transmembrane domains (TMDs). In contrast to the high conservation in the TMDs, the cytoplasmic histidine-rich intracellular loop between TMDs III and IV or the extracellular amino-terminal portion is highly variable in sequence and length. ZIP proteins are thought to form homodimers to transport zinc across the cellular membrane [38]. The conserved hydrophilic residue in the CHEXPHEXGD motif in TMD V may determine metal specificity [39, 40], because ZIP8 and ZIP14, which possess glutamic acid instead of the conserved histidine, can efficiently transport iron, manganese, and cadmium in addition to zinc [41–43].
Compared with ZIP transporters, the molecular characterization of ZnT transporters has progressed. ZnT proteins, except for ZnT5 and ZnT6, which form heterodimers [44], form homodimers [40, 45, 46], which has been confirmed in the X-ray crystal structure of the Escherichia coli homolog of ZnTs [47, 48]. Most ZnT proteins, except for ZnT5, are predicted to have six TMDs with an intramembranous zinc-binding site that consists of four conserved hydrophilic residues (two histidines and two aspartic acids) in TMDs II and V [40, 45]. Coordination of zinc at the intramembranous zinc-binding site is thought to be allosterically modulated by binding of zinc to a cytosolic binuclear zinc-sensing site formed in the carboxyl-terminal region [45, 47, 48], although this regulatory role of the carboxyl-terminal region was not supported by a recent electron crystallography study [49]. The characteristic cytoplasmic histidine-rich loop between TMDs IV and V may function as a determinant of zinc selectivity, a sensor of the cytosolic zinc levels, and a modulator of zinc transport activity [50, 51]. However, its precise functions are unknown, in part because information about its structure is limited [47, 48]. Generally, zinc mobilization by ZnTs is thought to be dependent on the proton electrochemical gradient [52]. Thus, ZnT proteins transport zinc in a Zn2+/H+ exchange manner, which could be explained by the alternating access mechanism. This mechanism has been proposed by a recent elegant study based on the 3D structure of both electron crystallography and X-ray crystallography of bacterial ZnT homologs [49]. New findings have begun to shed light on the molecular mechanism of zinc transport by both transporters, but further clarification of this mechanism is still required in future studies. In particular, the zinc selectivity determinant should be re-evaluated, because ZnT10 has been shown to be involved in manganese transport [53, 54].
Accumulating evidence has revealed that the coordinated zinc mobilization by ZIP and ZnT transporters is indispensable for maintaining zinc homeostasis. Thus, it plays critical physiological functions and profoundly affects health positively or negatively, as it is involved in a wide variety of diseases [5, 36, 37, 40]. Mutations in ZIP and ZnT transporter genes that impair their biological functions result in genetic disorders. Therefore, the study of ZIP and ZnT transporters is currently receiving great clinical interest and attention.
Human genetic disorders caused by mutations and single-nucleotide polymorphisms in zinc transporter genes
A number of genetic disorders are caused by mutations in the genes encoding ZIP and ZnT transporters [26, 36] (Table 1). Here, the current understanding of these zinc transporters is briefly reviewed. Specifically, ZIP4 in acrodermatitis enteropathica (AE), ZIP13 in the spondylocheiro dysplastic form of Ehlers–Danlos syndrome (SCD-EDS), and ZnT2 in transient neonatal zinc deficiency (TNZD) are reviewed along with their protein functions. ZnT8, in which a single-nucleotide polymorphism (SNP) has been shown to be associated with increased risk of type 2 diabetes mellitus (T2DM), is also reviewed together with its contribution to type 1 diabetes mellitus (T1DM). Moreover, mutations of ZnT10 are discussed, which have been shown to result in Parkinsonism and dystonia by disturbing manganese homeostasis, not zinc. Because of space limitations, information on phenotypes of knockout (KO) mice of ZIPs and ZnTs is kept to a minimum, and is described in the case of relating human diseases. Information on KO animal models and their contribution to understanding the pathophysiological basis of ZIPs and ZnTs can be found in the literature [5, 36, 37, 55, 56].
Table 1.
Zinc transporters whose mutations and SNP cause inherited diseases
| Genes | Diseases | Chromosomal localization | Phenotype MIM no. | Clinical features |
|---|---|---|---|---|
| ZIP4 | Acrodermatitis enteropathica (AE) | 8q24.3 | 201100 | Eczematous dermatitis on the perioral, perianal, and acral areas, alopecia, diarrhea, growth retardation. Ameliorated with zinc supplementation (1–3 mg/kg/day) |
| ZIP13 | Spondylocheiro dysplastic Ehlers–Danlos syndrome (SCD-EDS) | 11p11.2 | 612350 | Postnatal growth retardation, skeletal and connective tissue abnormalities, finger contractures, joint hypermobility, protruding eyes with bluish sclera, decreased hydroxyl collagen levels |
| ZnT2 | Transient neonatal zinc deficiency (TNZD) | 1p36.11 | 608118 | Erosive dermatitis around the mouth, genital region, neck, and fingers, diarrhea, hair loss, alopecia. Ameliorated with zinc supplementation during nursing |
| ZnT8 | Type II diabetes (T2DM) | 8q24.11 | 125853 | Non-synonymous SNP R325 allele variant implicated as a 14 % increased risk for T2DM. Autoantibodies of ZnT8 are present in 60–80 % of T1DM onset |
| ZnT10 | Syndrome of hepatic cirrhosis, dystonia, polycythemia, and hypermanganesemia | 1q41 | 613280 | Dysarthria, hypertonia, fine tremor, bradykinesia, spastic paraparesis. Blood manganese levels and clinical symptoms are improved by metal chelation therapy |
ZIP4 (SLC39A4) and acrodermatitis enteropathica
AE is a rare autosomal recessive disorder caused by defective intestinal zinc absorption, especially in the duodenum and jejunum, which are essential sites for zinc absorption. AE typically occurs in early infancy and its frequency is estimated at about 1 in 500,000. AE is characterized by eczematous dermatitis, alopecia, and diarrhea [57, 58], and these symptoms disappear if AE is properly diagnosed and a daily oral zinc supplementation (1–3 mg/kg/day of elemental zinc) is administered. Since the finding that the cause of AE is defects in intestinal zinc absorption [59], the molecular mechanism of dietary zinc absorption in the intestine had been mostly unknown, but in 2002, mutations in the SLC39A4/ZIP4 were shown to result in AE [60, 61]. At present, a myriad of mutations have been identified in the ZIP4 gene, namely missense (Fig. 2a), nonsense, deletion, insertion, or splice-site mutations [58, 62, 63]. The mechanism of dermatitis in AE patients and severe zinc deficiency had been unknown until the recent findings by Kawamura et al. [64]; the severe zinc deficiency in dermatitis causes a loss of Langerhans cells, which play a protective role against ATP-mediated inflammatory signals, and thus results in irritant contact dermatitis, not in allergic contact dermatitis [64].
Fig. 2.
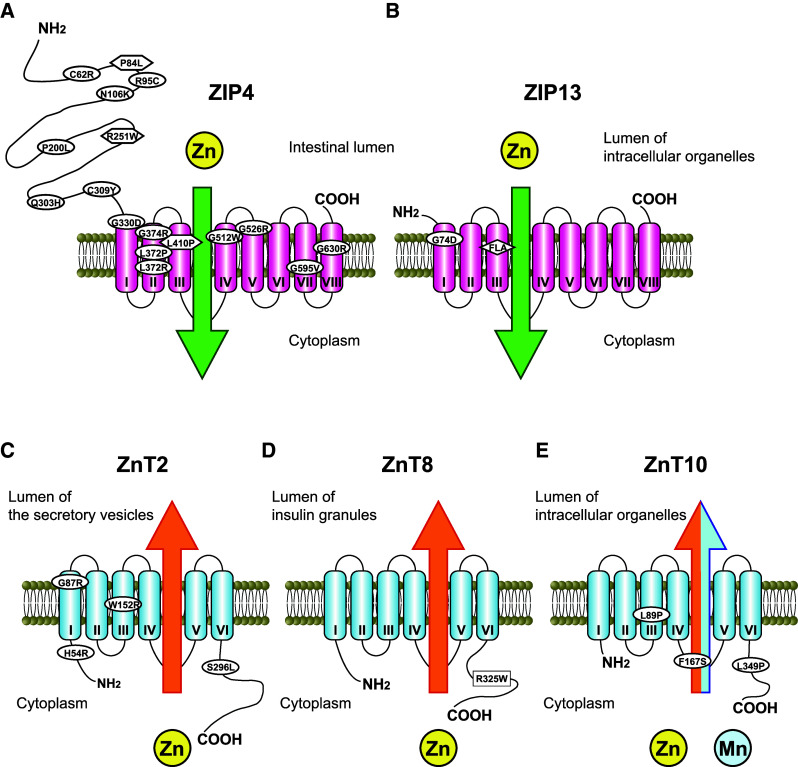
Illustrations of the predicted membrane topology of ZIP and ZnT transporters and the position of mutations and SNP related to inherited diseases. a ZIP4 is localized to the apical membrane of enterocytes only during zinc deficiency (see text). The positions of missense mutations causing AE are indicated by ovals, and those assigned to unclassified variants are indicated by hexagons [58]. b ZIP13 is localized to the Golgi apparatus [84] and intracellular vesicles [85]. The position of a missense mutation and a three-amino-acid (FLA) deletion in TMD III causing SCD-EDS is indicated by an oval and argyle. c ZnT2 is localized to the secretory vesicle, which is thought to contribute to zinc supply into breast milk. The positions of TNZD missense mutations are indicated by ovals. d ZnT8 is localized to the insulin granule membrane in pancreatic β cells. The position of R325W, which is a non-synonymous SNP, rs13266634, is indicated by a square. e The subcellular localization of ZnT10 has not been extensively investigated from the standpoint of its function, although immunostaining has revealed that ZnT10 is localized to the early/recycling endosomes [180] or Golgi apparatus [181]. The positions of missense mutations of ZnT10 leading to Parkinsonism are indicated by ovals
Studies of Zip4-KO mice have confirmed that ZIP4 protein is essential in dietary zinc absorption in mammals [65, 66], and that it has pivotal roles in intestinal integrity [66]. Moreover, the molecular basis of ZIP4 expression regulation and functions has been revealed in a number of studies using mice and transfected cells in culture [67–75]. Expression of mouse Zip4 is dynamically regulated by multiple posttranscriptional mechanisms; during zinc deficiency, Zip4 mRNA is stabilized, and Zip4 protein accumulates on the apical membrane by escaping from endocytosis and degradation, while the protein expression on the apical membrane is drastically down-regulated in response to excess zinc [72]. Some AE-causing mutations in mouse Zip4 have been shown to result in trafficking defects to the plasma membrane, probably because of misfolding and/or mislocalization in the secretory pathway. In other mutants, in which Zip4 can accumulate on the apical membrane, the zinc uptake activity has been shown to be decreased because of diminished V max of the uptake [68]. Zinc-regulated expression has been shown in human ZIP4, which accumulates on the plasma membrane in zinc-deficient conditions, but is drastically degraded via the proteasomal and lysosomal pathway in response to very high zinc concentrations [71]. The extracellular amino-terminal half of Zip4 protein is removed by proteolytic cleavage (processing) during a prolonged zinc deficiency [73]. The processing is inhibited in some mouse Zip4 mutants corresponding to AE-causing mutations, which suggests the importance of the processing in the zinc absorption process. Thus, understanding the molecular basis of ZIP4 protein expression is indispensable for unraveling the AE pathogenesis. However, patients who are not linked to the chromosomal region 8q24.3, containing the ZIP4 gene by homozygous mapping, suggest the presence of a second AE locus [76]. Therefore, another ZIP transporter may be involved in the occurrence of AE.
Recently, a number of studies have indicated that ectopic overexpression or expression dysregulation of ZIP4 are involved in cancer development including pancreatic cancer, hepatocellular carcinomas and glioma [77–80]. The tight control of ZIP4 expression may be indispensable for preventing cancer pathogenesis.
ZIP13 (SLC39A13) and spondylocheiro dysplastic form of Ehlers–Danlos syndrome
Ehlers–Danlos syndrome (EDS) is a group of genetic disorders of connective tissue development characterized by joint hypermobility, skin elasticity, and tissue fragility [81]. Based on clinical features, inheritance pattern, and molecular basis, EDS is broken into six different types, which are generally caused by mutations in collagen genes or genes involved in posttranslational modifications and assembly of collagens [81, 82]. The SLC39A13/ZIP13 gene has been identified as a gene responsible for another type of EDS, which was named the spondylocheiro dysplastic form of EDS (SCD-EDS) [83, 84]. The SCD-EDS is caused by homozygous loss-of-function mutations of the ZIP13 gene, which include a deletion of three amino acids (phenylalanine-leucine-alanine residues) within TMD III [83], or a missense substitution of G74D in TMD II (Fig. 2b) [84]. SCD-EDS patients display a variety of symptoms, such as postnatal growth retardation, hyperelastic and easily bruised skin, slender and tapering fingers, wrinkled palms, thenar atrophy, protruding eyes with bluish sclera, platyspondyly, osteopenia of the axial skeleton, short and wide femoral, joint hypermobility, and broadened metaphyseal regions of the elbows, wrists, knees, and interphalangeal joints. SCD-EDS patients show normal collagen synthesis activity, but their hydroxyl collagen levels are significantly decreased [83, 84]. These symptoms of SCD-ESD are not ameliorated by zinc supplementation, contrary to the case of AE or TNZD [26] (see Sects. “ZIP4 (SLC39A4) and acrodermatitis enteropathica” and “ZnT2 (SLC30A2) and transient neonatal zinc deficiency”).
The molecular mechanism by which ZIP13 mutations result in SCD-EDS has been molecularly investigated. Zip13-KO mice show similar phenotypes to SCD-EDS patients, such as delayed growth and abnormalities in hard and connective tissues’ development [84]. A study on Zip13-KO mice has revealed that ZIP13 is important for bone morphogenic protein (BMP)/transforming growth factor β (TGF-β)-mediated Smad translocation into the nucleus [84]. The zinc level in the Golgi apparatus is increased in primary dermal fibroblasts prepared from a Zip13-KO mouse [84]. Moreover, the fibroblasts established from an SCD-ESD patient and ZIP13-knockdown cells have revealed increased zinc in intracellular vesicles [85]. In this study, ZIP13 has been shown to operate in controlling the secretory pathway homeostasis [85]. These studies have demonstrated the involvement of ZIP13 in zinc release from intracellular compartments, including the Golgi and vesicles, and its essential functions in the regulation of cellular zinc distribution. Moreover, the SLC39A13 genetic locus has been reported to be implicated in fasting glucose homeostasis and in T2DM risk [86]. The extracellular N-terminus of ZIP13 is featured by the cysteine-rich sequence [87], which has been shown to bind zinc and potentially other cations. This sequence may be important for physiological functions of ZIP13 by regulating zinc release from intracellular compartments.
ZnT2 (SLC30A2) and transient neonatal zinc deficiency
In breast-fed full-term infants, symptomatic zinc deficiency has been rarely reported [26, 88]. Infant patients’ symptoms include erythematous and erosive dermatitis, persistent diarrhea, hair loss, alopecia, and transient growth retardation. In some cases, such zinc deficiency is caused by AE, because of reduced intestinal zinc absorption as described above. In other cases, zinc deficiency is caused by low zinc concentrations in the breast milk [89–93], which is featured by symptoms developed only during breast-feeding without reoccurrence of zinc deficiency, hence, it is termed TNZD. Symptoms of TNZD are alleviated with zinc supplementation to the infant, but not to the mother.
Recent genetic analyses have indicated that mutations within the SLC30A2/ZnT2 gene cause such conditions. Thus far, four mutations have been identified as causing impairment of ZnT2 functions through dominant negative or compound heterozygous mechanisms (Fig. 2c) [90, 92–94]. Moreover, investigation of SNPs in the ZnT2 gene has revealed the possibility that some of them may be involved in TNZD, because they may compromise mammary gland function [95, 96]. Japanese domestic journals in pediatrics and dermatology have reported TNZD almost once a year since 1981, which may indicate that the Japanese people have SNPs related to TNZD in the ZnT2 gene. Therefore, some countries may have an increased risk for neonates to suffer from TNZD. Interestingly, a similar low zinc-secretion phenotype has been found in the lethal milk (lm) mutant mice (OMIM602095) [97]. Pups suckling from lm/lm dams die before weaning, because the dams produce zinc-deficient milk caused by a nonsense mutation in the Slc30a4/Znt4 gene [98]. However, there have been no reports indicating mutations in the human SLC30A4/ZnT4 gene in women whose milk causes TNZD [88]. Moreover, it has been reported that a significant decrease in ZnT5 and ZnT6 mRNA expression was found in fibroblasts and lymphoblasts of two mothers secreting zinc-deficient milk [88], which may suggest that other ZnT proteins are involved in the etiology of TNZD.
Zinc in breast milk is essential for the growth and health of neonates [99]. Consistent with this fact, milk zinc concentrations are considerably higher than those in the serum [100]. A recent report has indicated that infantile zinc deficiency is associated with autism spectrum disorders [101]. Thus, future study to elucidate the molecular mechanism of ZnT2 expression regulation in mammary epithelial cells is of importance both to protect breast-fed infants against zinc deficiency, and to aid in their optimal growth and development.
ZnT8 (SLC30A8) and diabetes mellitus
Diabetes is a chronic disease that damages the heart, blood vessels, eyes, kidneys, and nerves. It is a major cause of mortality and morbidity worldwide, and affects >340 million people in the world [102]. Ninety percent of people with diabetes are diagnosed as T2DM, which is a progressive and debilitating disorder characterized by loss of glycemic control and metabolic homeostasis through deterioration of pancreatic β cell function, and insulin resistance. Hereditary factors play an important role in determining T2DM risk, together with life style and behavioral factors. Thus, intense efforts to identify genetic risk factors have been made, and a number of risk factors have been identified by genome-wide association studies (GWAS) [103, 104]. In 2007, GWAS identified a non-synonymous SNP in the SLC30A8/ZnT8 gene as a risk factor for T2DM (Fig. 2d) [105–108]. More specifically, the SLC30A8 rs13266634 risk C allele frequency was higher in T2DM patients than in healthy controls, which was estimated with a 14 % increased risk in a recent meta-analysis [109]. The risk allele encodes an arginine residue at amino acid 325 instead of tryptophan at the dimer interface of the cytoplasmic carboxyl-terminal region, and the R325 variant shows lower apparent zinc transport activity than the W325 variant [110]. The risk allele (R325) variant is thought to impair pancreatic β cell functions, because this variant has been shown to be associated with reduced first-phase insulin release following an intravenous glucose load in the offspring of T2DM patients [111], and to impair conversion of proinsulin into insulin [103, 112]. Recently, Tamaki et al. [113] have reported an important finding that explains how the risk allele variant relates to the increased risk of T2DM. They have clearly shown that zinc secreted in concert with insulin form β cells can block insulin clearance by hepatocytes, using β cell-specific Znt8-KO mice, and that humans carrying the risk allele (R325) exhibit increased insulin clearance, as assessed by the C-peptide/insulin ratio [113]. ZnT8 represents an exciting therapeutic target for intervention in T2DM.
ZnT8 has also received a lot of attention as an autoimmune marker of T1DM, because the autoantibodies against ZnT8 are present in approximately 60–80 % of patients at the onset of clinical diseases such as childhood T1DM and adult-onset autoimmune diabetes [114, 115]. Autoantibodies against ZnT8 occur with a similar prevalence to those against the standard T1DM autoantigens such as proinsulin, the 65-kDa form of glutamic acid decarboxylase (GAD65), and insulinoma-associated protein 2 (IA-2) [116]. Because autoantibodies against ZnT8 overlap with these three biomarkers, but are independent of them, measuring the antibodies against ZnT8 together with other major targets significantly enhances the detection rate of diabetes-related autoimmunity [116, 117]. A recent study has revealed that ZnT8 is actually a T cell target in T1DM [116]. The rs13266634 SNP, described above, has been shown to be a key determinant in humoral autoreactivity to ZnT8 [118, 119].
In pancreatic β cells, zinc plays a critical role in insulin crystal formation. Moreover, zinc released from β cells along with insulin is known to have substantial paracrine and/or autocrine effects [113, 120]. In these processes, massive amounts of zinc must be specifically transported into secretory vesicles containing insulin (insulin granules), the majority of which is conducted only by ZnT8. ZnT5 is highly expressed in pancreatic β cells [121], but its contribution to insulin crystallization is minor [122]. Thus far, several lines of Znt8-KO mice have been established [110, 113, 123–125]. In these KO mice, the zinc contents in β cells is significantly decreased and the dense core of zinc-insulin crystals is lost or immature with pale insulin pro-granules, revealing that ZnT8 is essential for formation of zinc-insulin crystals. Meanwhile, phenotypes regarding glucose tolerance, insulin processing/secretion, and body weight vary among these mice. Recently, the physiological effects in Znt8-KO mice have been shown to be influenced by gender and genetic background [126]. A study using β-cell- and α-cell-specific Znt8-KO mice has revealed that ZnT8 contributes to the risk of developing T2DM through β-cell- and non-β-cell-specific effects [113, 127]. ZnT8 may be engaged in the pathogenesis of T2DM in multiple ways, and further molecular research is needed to better understand how the risk allele variant increases the risk of T2DM.
ZnT10 (SLC30A10) and Parkinsonism, dystonia
ZnT transporters are thought to specifically transport zinc [45, 128], which has been speculated based on the conserved sequence homology [129]. However, recent findings have revealed that ZnT10 is involved in manganese transport, because hepatic cirrhosis, polycythemia, dystonia, and hypermanganesemia (which is Parkinsonism) have been shown to be caused by homozygous mutations of the SLC30A10/ZnT10 gene including frameshift, nonsense, and missense mutations [53, 54] (Fig. 2e). SLC30A10 is highly expressed in the liver and brain including the basal ganglia, which is consistent with the high manganese accumulation in these tissues in patients. The symptoms in patients caused by ZnT10 gene mutations have been shown to be improved by a combination of chelation therapy and iron supplementation, a competitive inhibitor of intestinal manganese uptake. These findings indicate that ZnT10 is involved in manganese export out of the cells in these tissues. ZnT10 has also been shown to regulate zinc homeostasis, localized in the Golgi apparatus or early/recycling endosomes. The precise preference of transport metal of ZnT10 has not been investigated to date. A recent report has shown that ZnT10 mRNA expression significantly decreased in the frontal cortex of patients with Alzheimer’s disease [130], suggesting the importance of ZnT10 in the brain metal homeostasis.
ZnT10 has several unique features compared with other ZnT proteins. First, the conserved histidine residue in TMD II is substituted with an asparagine residue. The position of this residue has been shown to be important for determining the transport metal specificity [45]. Second, the histidine-rich cytoplasmic loop between TMDs IV and V in other ZnT proteins is replaced with the lysine/arginine-rich loop. Elucidating whether these characteristics are involved in the metal preference of ZnT10 may help understand the molecular mechanisms of transporting zinc by ZnTs.
Potential involvement of zinc transporters in disease pathogenesis and progression
In “Human genetic disorders caused by mutations and single-nucleotide polymorphisms in zinc transporter genes”, genetic disorders caused by mutations in zinc transporter genes are reviewed. Here, ZIP and ZnT transporters, dysregulation of which may contribute to human diseases, are briefly described.
Involvement of zinc transporters in human genetic disease
Epidermodysplasia verruciformis (EV), a rare autosomal recessive skin disease (OMIM 226400) associated with a high risk of developing skin carcinoma, stems from a selective susceptibility to related human papillomaviruses [131]. Two EVER proteins, EVER1 and EVER2, have been identified as being responsible for EV, and ZnT1 has been identified as their cellular partner protein [132]. ZnT1 is a zinc transporter that is primarily localized to the plasma membrane and functions in the export of cytosolic zinc into the extracellular space [133], but when ZnT1 forms complexes with EVER1 and EVER2 in keratinocytes, it changes its localization to the ER. The complexes between ZnT1, EVER1, and EVER2 have been shown to regulate cellular signaling by controlling the intracellular zinc distribution.
Potential involvement of zinc transporters in human diseases
There are many reports suggesting that dysregulation of expression and activity of ZIP and ZnT transporters are involved in the pathogenesis and progression of chronic diseases. However, their molecular relationships with the pathogenesis of these diseases are less clear, and need further investigation. In this section, the involvement of ZIP and ZnT transporters in cancer progression and metastasis, immunological impairment, and neurodegenerative diseases are briefly overviewed, with emphasis on these points.
ZIP transporters have been shown to be associated with cancer progression and its invasive activity [134–142]. The decreased expression of a number of ZIPs in prostate cancer tissues and malignant cell lines is well known [143–145]. Their decreased expression likely causes decreases in cellular zinc, and thus releasing mitochondrial aconitase activity from zinc inhibition, which may decrease citrate production and secretion into the prostatic fluid [134–137]. On another front, aberrant overexpression of ZIPs very likely contributes to cancer pathogenesis and progression, as described in the case of ZIP4 in “ZIP4 (SLC39A4) and acrodermatitis enteropathica.” The involvement of ZIPs in epithelial–mesenchymal transition, anoikis resistance, and invasive behavior [77, 79, 138–140] makes a good case for this point. In these cases, aberrant expression of ZIPs increases cytosolic zinc levels, which may prolong zinc-mediated growth factor signaling and lead to aberrant activation of cellular signaling pathways [141, 142]. Thus, ZIP transporters may serve as a potential diagnostic and prognostic marker for certain types of cancer. Compared with ZIPs, there have been less reports on the implication of ZnT transporters in cancers, but interesting discussion is possible. Various secretory and membrane-bound zinc-requiring enzymes such as matrix metalloproteinases are known to be involved in cancer metastasis [146], and are thought to capture zinc in the early secretory pathway. Therefore, ZnT transporters such as ZnT5, ZnT6, and ZnT7 operating to load zinc on zinc-requiring enzymes in the early secretory pathway may be involved in their activation [147, 148]. From this aspect, their increased mRNA expression in carcinomas [80, 149] is interesting, although direct evidence has not been verified [122].
Important roles of zinc in immune cell functions have been well established and this subject has been reviewed in much greater detail than can be presented herein [150–155]. ZIP and ZnT transporters play pivotal roles in the dynamic regulation of cytosolic zinc concentration and distribution, contributing to the fine-tuning of cellular signaling pathways in immune cells (this type of signaling function of zinc is called “zinc signaling” [5, 19, 153–155]). Specifically, ZIPs localized to the plasma membrane contribute by influxing zinc from the extracellular space [156–158], while intracellularly localized ZIPs contribute by regulating cytosolic zinc levels through releasing zinc from the subcellular compartments [159–161]. Hence, dysfunction or dysregulation of ZIP and ZnT transporters would likely result in a decline and abnormality in immune functions. Moreover, in addition to the canonical roles of zinc in immune cells, novel functions of zinc in nutritional immunity have recently started to be clarified [162, 163], where ZIP and ZnT transporters have been shown to play a role at the interface of pathogen and host on the human side [164, 165].
Alterations in zinc homeostasis have been suggested to be closely related to the development of some neurodegenerative diseases [166–169]. The zinc content in the cerebrospinal fluid is significantly increased in the brain of patients with Parkinson’s disease, Alzheimer’s disease, and amyotrophic lateral sclerosis [170]. Since alterations in expression of ZIPs and ZnTs have been reported in the brains of these patients [136, 169, 171, 172], clarifying their involvement in the pathogenesis of these diseases is a long-sought goal. Needless to say, investigation of the roles of ZnT3, a critical transporter of zinc into synaptic vesicles [173], in brain functions should be elucidated from the pathophysiology of neurodegenerative diseases. The expression level of ZnT3 in the cortex has been shown to decline in people with Alzheimer disease and in healthy humans with age [174, 175], and a rare copy number variant of the ZnT3 gene may be involved in monogenic determination of autosomal dominant early onset Alzheimer disease [176], strongly suggesting that reduced activity of ZnT3 is related to Alzheimer’s disease pathology. However, for comprehensive understanding further investigation is needed.
Conclusions
Over 20 zinc transporters operate in the body to control the homeostasis of 2–3 g of zinc, and four mutations and an SNP in the ZIP and ZnT transporter genes result in genetic disorders or increased risk of disease pathogenesis. Our understanding of the contribution of ZIP and ZnT transporters in disease pathogenesis has been steadily improving, but is still far from complete. Many questions remain to be resolved. How ZIPs and ZnTs recognize zinc as a substrate metal and transport it across the cellular membrane, and how their transport activities are regulated should be elucidated in future studies. Furthermore, how zinc mobilized across the cellular membrane by both transporters is spatial-temporally controlled, and how zinc concentration and distribution regulated by them specifically works in multifarious cellular processes, are critical questions that should also be solved. Moreover, how ZIPs and ZnTs coordinately supply zinc to target molecules and modulate the kinetics of zinc metabolism in the process should ultimately be resolved to understand their pathophysiological functions [177]. Clarifying these issues would lead to therapeutic innovation, and therefore, great attention should be paid to ZIP and ZnT transporters.
Acknowledgments
This work was supported by grants-in-aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan and by the Takeda Science Foundation (to T.K.).
Abbreviations
- ZIP
Zrt, Irt-like protein
- ZnT
Zinc transporter
- SLC
Solute carrier
- TMD
Transmembrane domain
- AE
Acrodermatitis enteropathica
- EDS
Ehlers–Danlos syndrome
- SCD-EDS
Spondylocheiro dysplastic form of EDS
- TNZD
Transient neonatal zinc deficiency
- T1DM
Type 1 diabetes
- T2DM
Type 2 diabetes
- SNP
Single-nucleotide polymorphism
- EV
Epidermodysplasia verruciformis
References
- 1.Jackson MJ. Physiology of zinc: general aspects. In: Mills CF, editor. Zinc in human biology. Berlin Heidelberg New York: Springer; 1989. pp. 1–14. [Google Scholar]
- 2.Maret W, Sandstead HH. Zinc requirements and the risks and benefits of zinc supplementation. J Trace Elem Med Biol. 2006;20:3–18. doi: 10.1016/j.jtemb.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 3.Vallee BL, Falchuk KH. The biochemical basis of zinc physiology. Physiol Rev. 1993;73:79–118. doi: 10.1152/physrev.1993.73.1.79. [DOI] [PubMed] [Google Scholar]
- 4.Maret W, Li Y. Coordination dynamics of zinc in proteins. Chem Rev. 2009;109:4682–4707. doi: 10.1021/cr800556u. [DOI] [PubMed] [Google Scholar]
- 5.Fukada T, Kambe T. Molecular and genetic features of zinc transporters in physiology and pathogenesis. Metallomics. 2011;3:662–674. doi: 10.1039/c1mt00011j. [DOI] [PubMed] [Google Scholar]
- 6.Prasad AS. Discovery of human zinc deficiency and studies in an experimental human model. Am J Clin Nutr. 1991;53:403–412. doi: 10.1093/ajcn/53.2.403. [DOI] [PubMed] [Google Scholar]
- 7.Hambidge M. Human zinc deficiency. J Nutr. 2000;130(5S Suppl):1344S–1349S. doi: 10.1093/jn/130.5.1344S. [DOI] [PubMed] [Google Scholar]
- 8.Fraker PJ, King LE. Reprogramming of the immune system during zinc deficiency. Annu Rev Nutr. 2004;24:277–298. doi: 10.1146/annurev.nutr.24.012003.132454. [DOI] [PubMed] [Google Scholar]
- 9.Devirgiliis C, Zalewski PD, Perozzi G, Murgia C. Zinc fluxes and zinc transporter genes in chronic diseases. Mutat Res. 2007;622:84–93. doi: 10.1016/j.mrfmmm.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 10.Krebs NF. Overview of zinc absorption and excretion in the human gastrointestinal tract. J Nutr. 2000;130(5S Suppl):1374S–1377S. doi: 10.1093/jn/130.5.1374S. [DOI] [PubMed] [Google Scholar]
- 11.Liuzzi JP, Bobo JA, Lichten LA, Samuelson DA, Cousins RJ. Responsive transporter genes within the murine intestinal-pancreatic axis form a basis of zinc homeostasis. Proc Natl Acad Sci USA. 2004;101:14355–14360. doi: 10.1073/pnas.0406216101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kambe T, Yamaguchi-Iwai Y, Sasaki R, Nagao M. Overview of mammalian zinc transporters. Cell Mol Life Sci. 2004;61:49–68. doi: 10.1007/s00018-003-3148-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soybel DI, Kohler JE. Zinc and the gastrointestinal tract. In: Rink L, editor. Zinc in human health. Amsterdam: IOS Press; 2011. pp. 448–472. [Google Scholar]
- 14.Hambidge M, Krebs NF. Interrelationships of key variables of human zinc homeostasis: relevance to dietary zinc requirements. Annu Rev Nutr. 2001;21:429–452. doi: 10.1146/annurev.nutr.21.1.429. [DOI] [PubMed] [Google Scholar]
- 15.King JC, Shames DM, Woodhouse LR. Zinc homeostasis in humans. J Nutr. 2000;130:1360S–1366S. doi: 10.1093/jn/130.5.1360S. [DOI] [PubMed] [Google Scholar]
- 16.Reyes JG. Zinc transport in mammalian cells. Am J Physiol. 1996;270:C401–C410. doi: 10.1152/ajpcell.1996.270.2.C401. [DOI] [PubMed] [Google Scholar]
- 17.Barnett JP, Blindauer CA, Kassaar O, Khazaipoul S, Martin EM, Sadler PJ, Stewart AJ. Allosteric modulation of zinc speciation by fatty acids. Biochim Biophys Acta. 2013;1830:5456–5464. doi: 10.1016/j.bbagen.2013.05.028. [DOI] [PubMed] [Google Scholar]
- 18.Thiers RE, Vallee BL. Distribution of metals in subcellular fractions of rat liver. J Biol Chem. 1957;226:911–920. [PubMed] [Google Scholar]
- 19.Haase H, Rink L. Zinc signals and immune function. Biofactors. 2014;40:27–40. doi: 10.1002/biof.1114. [DOI] [PubMed] [Google Scholar]
- 20.Coyle P, Philcox JC, Carey LC, Rofe AM. Metallothionein: the multipurpose protein. Cell Mol Life Sci. 2002;59:627–647. doi: 10.1007/s00018-002-8454-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andreini C, Bertini I, Cavallaro G. Minimal functional sites allow a classification of zinc sites in proteins. PLoS ONE. 2011;6:e26325. doi: 10.1371/journal.pone.0026325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hopfner KP, Craig L, Moncalian G, Zinkel RA, Usui T, Owen BA, Karcher A, Henderson B, Bodmer JL, McMurray CT, Carney JP, Petrini JH, Tainer JA. The Rad50 zinc-hook is a structure joining Mre11 complexes in DNA recombination and repair. Nature. 2002;418:562–566. doi: 10.1038/nature00922. [DOI] [PubMed] [Google Scholar]
- 23.Prasad AS, Halsted JA, Nadimi M. Syndrome of iron deficiency anemia, hepatosplenomegaly, hypogonadism, dwarfism and geophagia. Am J Med. 1961;31:532–546. doi: 10.1016/0002-9343(61)90137-1. [DOI] [PubMed] [Google Scholar]
- 24.Prasad AS. Clinical manifestations of zinc deficiency. Annu Rev Nutr. 1985;5:341–363. doi: 10.1146/annurev.nu.05.070185.002013. [DOI] [PubMed] [Google Scholar]
- 25.Prasad AS. Zinc deficiency. BMJ. 2003;326:409–410. doi: 10.1136/bmj.326.7386.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weaver BP, Andrews GK. Zinc transporter mutations and human growth. In: Preedy VR, editor. Handbook of growth and growth monitoring in health and disease. Berlin: Springer Science + Business Media, LLC; 2012. pp. 2319–2336. [Google Scholar]
- 27.Besecker BY, Exline MC, Hollyfield J, Phillips G, Disilvestro RA, Wewers MD, Knoell DL. A comparison of zinc metabolism, inflammation, and disease severity in critically ill infected and noninfected adults early after intensive care unit admission. Am J Clin Nutr. 2011;93:1356–1364. doi: 10.3945/ajcn.110.008417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Uciechowski P, Kahmann L, Plumakers B, Malavolta M, Mocchegiani E, Dedoussis G, Herbein G, Jajte J, Fulop T, Rink L. TH1 and TH2 cell polarization increases with aging and is modulated by zinc supplementation. Exp Gerontol. 2008;43:493–498. doi: 10.1016/j.exger.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 29.Mocchegiani E, Romeo J, Malavolta M, Costarelli L, Giacconi R, Diaz LE, Marcos A. Zinc: dietary intake and impact of supplementation on immune function in elderly. Age (Dordr) 2013;35:839–860. doi: 10.1007/s11357-011-9377-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haase H, Mocchegiani E, Rink L. Correlation between zinc status and immune function in the elderly. Biogerontology. 2006;7:421–428. doi: 10.1007/s10522-006-9057-3. [DOI] [PubMed] [Google Scholar]
- 31.Prasad AS, Beck FW, Bao B, Fitzgerald JT, Snell DC, Steinberg JD, Cardozo LJ. Zinc supplementation decreases incidence of infections in the elderly: effect of zinc on generation of cytokines and oxidative stress. Am J Clin Nutr. 2007;85:837–844. doi: 10.1093/ajcn/85.3.837. [DOI] [PubMed] [Google Scholar]
- 32.A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8 (2001) Arch Ophthalmol 119:1417–1436 [DOI] [PMC free article] [PubMed]
- 33.Brown KH, Peerson JM, Rivera J, Allen LH. Effect of supplemental zinc on the growth and serum zinc concentrations of prepubertal children: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2002;75:1062–1071. doi: 10.1093/ajcn/75.6.1062. [DOI] [PubMed] [Google Scholar]
- 34.Wardlaw T, Salama P, Brocklehurst C, Chopra M, Mason E. Diarrhoea: why children are still dying and what can be done. Lancet. 2010;375:870–872. doi: 10.1016/S0140-6736(09)61798-0. [DOI] [PubMed] [Google Scholar]
- 35.Penny ME. Zinc supplementation in public health. Ann Nutr Metab. 2013;62(Suppl 1):31–42. doi: 10.1159/000348263. [DOI] [PubMed] [Google Scholar]
- 36.Kambe T, Weaver BP, Andrews GK. The genetics of essential metal homeostasis during development. Genesis. 2008;46:214–228. doi: 10.1002/dvg.20382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lichten LA, Cousins RJ. Mammalian zinc transporters: nutritional and physiologic regulation. Annu Rev Nutr. 2009;29:153–176. doi: 10.1146/annurev-nutr-033009-083312. [DOI] [PubMed] [Google Scholar]
- 38.Bin BH, Fukada T, Hosaka T, Yamasaki S, Ohashi W, Hojyo S, Miyai T, Nishida K, Yokoyama S, Hirano T. Biochemical characterization of human ZIP13 protein: a homo-dimerized zinc transporter involved in the spondylocheiro dysplastic Ehlers–Danlos syndrome. J Biol Chem. 2011;286:40255–40265. doi: 10.1074/jbc.M111.256784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taylor KM, Morgan HE, Smart K, Zahari NM, Pumford S, Ellis IO, Robertson JF, Nicholson RI. The emerging role of the LIV-1 subfamily of zinc transporters in breast cancer. Mol Med. 2007;13:396–406. doi: 10.2119/2007-00040.Taylor. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kambe T. Regulation of zinc transport. In: Culotta V, Scott RA, editors. Encyclopedia of inorganic and bioinorganic chemistry-metals in cells. Chichester: Wiley; 2013. pp. 301–309. [Google Scholar]
- 41.Girijashanker K, He L, Soleimani M, Reed JM, Li H, Liu Z, Wang B, Dalton TP, Nebert DW. Slc39a14 gene encodes ZIP14, a metal/bicarbonate symporter: similarities to the ZIP8 transporter. Mol Pharmacol. 2008;73:1413–1423. doi: 10.1124/mol.107.043588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fujishiro H, Yano Y, Takada Y, Tanihara M, Himeno S. Roles of ZIP8, ZIP14, and DMT1 in transport of cadmium and manganese in mouse kidney proximal tubule cells. Metallomics. 2012;4:700–708. doi: 10.1039/c2mt20024d. [DOI] [PubMed] [Google Scholar]
- 43.Liuzzi JP, Aydemir F, Nam H, Knutson MD, Cousins RJ. Zip14 (Slc39a14) mediates non-transferrin-bound iron uptake into cells. Proc Natl Acad Sci USA. 2006;103:13612–13617. doi: 10.1073/pnas.0606424103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fukunaka A, Suzuki T, Kurokawa Y, Yamazaki T, Fujiwara N, Ishihara K, Migaki H, Okumura K, Masuda S, Yamaguchi-Iwai Y, Nagao M, Kambe T. Demonstration and characterization of the heterodimerization of ZnT5 and ZnT6 in the early secretory pathway. J Biol Chem. 2009;284:30798–30806. doi: 10.1074/jbc.M109.026435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kambe T. Molecular architecture and function of ZnT transporters. Curr Top Membr. 2012;69:199–220. doi: 10.1016/B978-0-12-394390-3.00008-2. [DOI] [PubMed] [Google Scholar]
- 46.Suzuki T, Ishihara K, Migaki H, Nagao M, Yamaguchi-Iwai Y, Kambe T. Two different zinc transport complexes of cation diffusion facilitator proteins localized in the secretory pathway operate to activate alkaline phosphatases in vertebrate cells. J Biol Chem. 2005;280:30956–30962. doi: 10.1074/jbc.M506902200. [DOI] [PubMed] [Google Scholar]
- 47.Lu M, Fu D. Structure of the zinc transporter YiiP. Science. 2007;317:1746–1748. doi: 10.1126/science.1143748. [DOI] [PubMed] [Google Scholar]
- 48.Lu M, Chai J, Fu D. Structural basis for autoregulation of the zinc transporter YiiP. Nat Struct Mol Biol. 2009;16:1063–1067. doi: 10.1038/nsmb.1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Coudray N, Valvo S, Hu M, Lasala R, Kim C, Vink M, Zhou M, Provasi D, Filizola M, Tao J, Fang J, Penczek PA, Ubarretxena-Belandia I, Stokes DL. Inward-facing conformation of the zinc transporter YiiP revealed by cryoelectron microscopy. Proc Natl Acad Sci USA. 2013;110:2140–2145. doi: 10.1073/pnas.1215455110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Podar D, Scherer J, Noordally Z, Herzyk P, Nies D, Sanders D. Metal selectivity determinants in a family of transition metal transporters. J Biol Chem. 2012;287:3185–3196. doi: 10.1074/jbc.M111.305649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tanaka N, Kawachi M, Fujiwara T, Maeshima M. Zinc-binding and structural properties of the histidine-rich loop of Arabidopsis thaliana vacuolar membrane zinc transporter MTP1. FEBS Open Bio. 2013;3:218–224. doi: 10.1016/j.fob.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ohana E, Hoch E, Keasar C, Kambe T, Yifrach O, Hershfinkel M, Sekler I. Identification of the Zn2+ binding site and mode of operation of a mammalian Zn2+ transporter. J Biol Chem. 2009;284:17677–17686. doi: 10.1074/jbc.M109.007203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Quadri M, Federico A, Zhao T, Breedveld GJ, Battisti C, Delnooz C, Severijnen LA, Di Toro Mammarella L, Mignarri A, Monti L, Sanna A, Lu P, Punzo F, Cossu G, Willemsen R, Rasi F, Oostra BA, van de Warrenburg BP, Bonifati V. Mutations in SLC30A10 cause Parkinsonism and dystonia with hypermanganesemia, polycythemia, and chronic liver disease. Am J Hum Genet. 2012;90:467–477. doi: 10.1016/j.ajhg.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tuschl K, Clayton PT, Gospe SM, Jr, Gulab S, Ibrahim S, Singhi P, Aulakh R, Ribeiro RT, Barsottini OG, Zaki MS, Del Rosario ML, Dyack S, Price V, Rideout A, Gordon K, Wevers RA, Chong WK, Mills PB. Syndrome of hepatic cirrhosis, dystonia, polycythemia, and hypermanganesemia caused by mutations in SLC30A10, a manganese transporter in man. Am J Hum Genet. 2012;90:457–466. doi: 10.1016/j.ajhg.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jeong J, Eide DJ. The SLC39 family of zinc transporters. Mol Aspects Med. 2013;34:612–619. doi: 10.1016/j.mam.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huang L, Tepaamorndech S. The SLC30 family of zinc transporters—a review of current understanding of their biological and pathophysiological roles. Mol Aspects Med. 2013;34:548–560. doi: 10.1016/j.mam.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 57.Maverakis E, Fung MA, Lynch PJ, Draznin M, Michael DJ, Ruben B, Fazel N. Acrodermatitis enteropathica and an overview of zinc metabolism. J Am Acad Dermatol. 2007;56:116–124. doi: 10.1016/j.jaad.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 58.Schmitt S, Kury S, Giraud M, Dreno B, Kharfi M, Bezieau S. An update on mutations of the SLC39A4 gene in acrodermatitis enteropathica. Hum Mutat. 2009;30:926–933. doi: 10.1002/humu.20988. [DOI] [PubMed] [Google Scholar]
- 59.Lombeck T, Schnippering HG, Ritzl F, Feinendegen LE, Bremer HJ. Letter: absorption of zinc in acrodermatitis enteropathica. Lancet. 1975;1:855. doi: 10.1016/s0140-6736(75)93025-1. [DOI] [PubMed] [Google Scholar]
- 60.Kury S, Dreno B, Bezieau S, Giraudet S, Kharfi M, Kamoun R, Moisan JP. Identification of SLC39A4, a gene involved in acrodermatitis enteropathica. Nat Genet. 2002;31:239–240. doi: 10.1038/ng913. [DOI] [PubMed] [Google Scholar]
- 61.Wang K, Zhou B, Kuo YM, Zemansky J, Gitschier J. A novel member of a zinc transporter family is defective in acrodermatitis enteropathica. Am J Hum Genet. 2002;71:66–73. doi: 10.1086/341125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Andrews GK. Regulation and function of Zip4, the acrodermatitis enteropathica gene. Biochem Soc Trans. 2008;36:1242–1246. doi: 10.1042/BST0361242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li CR, Yan SM, Shen DB, Li Q, Shao JP, Xue CY, Cao YH. One novel homozygous mutation of SLC39A4 gene in a Chinese patient with acrodermatitis enteropathica. Arch Dermatol Res. 2010;302:315–317. doi: 10.1007/s00403-010-1047-2. [DOI] [PubMed] [Google Scholar]
- 64.Kawamura T, Ogawa Y, Nakamura Y, Nakamizo S, Ohta Y, Nakano H, Kabashima K, Katayama I, Koizumi S, Kodama T, Nakao A, Shimada S. Severe dermatitis with loss of epidermal Langerhans cells in human and mouse zinc deficiency. J Clin Investig. 2012;122:722–732. doi: 10.1172/JCI58618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dufner-Beattie J, Weaver BP, Geiser J, Bilgen M, Larson M, Xu W, Andrews GK. The mouse acrodermatitis enteropathica gene Slc39a4 (Zip4) is essential for early development and heterozygosity causes hypersensitivity to zinc deficiency. Hum Mol Genet. 2007;16:1391–1399. doi: 10.1093/hmg/ddm088. [DOI] [PubMed] [Google Scholar]
- 66.Geiser J, Venken KJ, De Lisle RC, Andrews GK. A mouse model of acrodermatitis enteropathica: loss of intestine zinc transporter ZIP4 (Slc39a4) disrupts the stem cell niche and intestine integrity. PLoS Genet. 2012;8:e1002766. doi: 10.1371/journal.pgen.1002766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dufner-Beattie J, Wang F, Kuo YM, Gitschier J, Eide D, Andrews GK. The acrodermatitis enteropathica gene ZIP4 encodes a tissue-specific, zinc-regulated zinc transporter in mice. J Biol Chem. 2003;278:33474–33481. doi: 10.1074/jbc.M305000200. [DOI] [PubMed] [Google Scholar]
- 68.Wang F, Kim BE, Dufner-Beattie J, Petris MJ, Andrews G, Eide DJ. Acrodermatitis enteropathica mutations affect transport activity, localization and zinc-responsive trafficking of the mouse ZIP4 zinc transporter. Hum Mol Genet. 2004;13:563–571. doi: 10.1093/hmg/ddh049. [DOI] [PubMed] [Google Scholar]
- 69.Dufner-Beattie J, Kuo YM, Gitschier J, Andrews GK. The adaptive response to dietary zinc in mice involves the differential cellular localization and zinc regulation of the zinc transporters ZIP4 and ZIP5. J Biol Chem. 2004;279:49082–49090. doi: 10.1074/jbc.M409962200. [DOI] [PubMed] [Google Scholar]
- 70.Kim BE, Wang F, Dufner-Beattie J, Andrews GK, Eide DJ, Petris MJ. Zn2+-stimulated endocytosis of the mZIP4 zinc transporter regulates its location at the plasma membrane. J Biol Chem. 2004;279:4523–4530. doi: 10.1074/jbc.M310799200. [DOI] [PubMed] [Google Scholar]
- 71.Mao X, Kim BE, Wang F, Eide DJ, Petris MJ. A histidine-rich cluster mediates the ubiquitination and degradation of the human zinc transporter, hZIP4, and protects against zinc cytotoxicity. J Biol Chem. 2007;282:6992–7000. doi: 10.1074/jbc.M610552200. [DOI] [PubMed] [Google Scholar]
- 72.Weaver BP, Dufner-Beattie J, Kambe T, Andrews GK. Novel zinc-responsive post-transcriptional mechanisms reciprocally regulate expression of the mouse Slc39a4 and Slc39a5 zinc transporters (Zip4 and Zip5) Biol Chem. 2007;388:1301–1312. doi: 10.1515/BC.2007.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kambe T, Andrews GK. Novel proteolytic processing of the ectodomain of the zinc transporter ZIP4 (SLC39A4) during zinc deficiency is inhibited by acrodermatitis enteropathica mutations. Mol Cell Biol. 2009;29:129–139. doi: 10.1128/MCB.00963-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liuzzi JP, Guo L, Chang SM, Cousins RJ. Kruppel-like factor 4 regulates adaptive expression of the zinc transporter Zip4 in mouse small intestine. Am J Physiol Gastrointest Liver Physiol. 2009;296:G517–G523. doi: 10.1152/ajpgi.90568.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Antala S, Dempski RE. The human ZIP4 transporter has two distinct binding affinities and mediates transport of multiple transition metals. Biochemistry. 2012;51:963–973. doi: 10.1021/bi201553p. [DOI] [PubMed] [Google Scholar]
- 76.Wang K, Pugh EW, Griffen S, Doheny KF, Mostafa WZ, al-Aboosi MM, el-Shanti H, Gitschier J. Homozygosity mapping places the acrodermatitis enteropathica gene on chromosomal region 8q24.3. Am J Hum Genet. 2001;68:1055–1060. doi: 10.1086/319514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li M, Zhang Y, Liu Z, Bharadwaj U, Wang H, Wang X, Zhang S, Liuzzi JP, Chang SM, Cousins RJ, Fisher WE, Brunicardi FC, Logsdon CD, Chen C, Yao Q. Aberrant expression of zinc transporter ZIP4 (SLC39A4) significantly contributes to human pancreatic cancer pathogenesis and progression. Proc Natl Acad Sci USA. 2007;104:18636–18641. doi: 10.1073/pnas.0709307104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang Y, Chen C, Yao Q, Li M. ZIP4 upregulates the expression of neuropilin-1, vascular endothelial growth factor, and matrix metalloproteases in pancreatic cancer cell lines and xenografts. Cancer Biol Ther. 2010;9:236–242. doi: 10.4161/cbt.9.3.10749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Weaver BP, Zhang Y, Hiscox S, Guo GL, Apte U, Taylor KM, Sheline CT, Wang L, Andrews GK. Zip4 (Slc39a4) expression is activated in hepatocellular carcinomas and functions to repress apoptosis, enhance cell cycle and increase migration. PLoS ONE. 2010;5:e13158. doi: 10.1371/journal.pone.0013158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lin Y, Chen Y, Wang Y, Yang J, Zhu VF, Liu Y, Cui X, Chen L, Yan W, Jiang T, Hergenroeder GW, Fletcher SA, Levine JM, Kim DH, Tandon N, Zhu JJ, Li M. ZIP4 is a novel molecular marker for glioma. Neuro Oncol. 2013;15:1008–1016. doi: 10.1093/neuonc/not042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Beighton P, De Paepe A, Steinmann B, Tsipouras P, Wenstrup RJ. Ehlers–Danlos syndromes: revised nosology, Villefranche, 1997. Ehlers–Danlos National Foundation (USA) and Ehlers–Danlos Support Group (UK) Am J Med Genet. 1998;77:31–37. doi: 10.1002/(sici)1096-8628(19980428)77:1<31::aid-ajmg8>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 82.Parapia LA, Jackson C. Ehlers–Danlos syndrome—a historical review. Br J Haematol. 2008;141:32–35. doi: 10.1111/j.1365-2141.2008.06994.x. [DOI] [PubMed] [Google Scholar]
- 83.Giunta C, Elcioglu NH, Albrecht B, Eich G, Chambaz C, Janecke AR, Yeowell H, Weis M, Eyre DR, Kraenzlin M, Steinmann B. Spondylocheiro dysplastic form of the Ehlers–Danlos syndrome—an autosomal-recessive entity caused by mutations in the zinc transporter gene SLC39A13. Am J Hum Genet. 2008;82:1290–1305. doi: 10.1016/j.ajhg.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fukada T, Civic N, Furuichi T, Shimoda S, Mishima K, Higashiyama H, Idaira Y, Asada Y, Kitamura H, Yamasaki S, Hojyo S, Nakayama M, Ohara O, Koseki H, Dos Santos HG, Bonafe L, Ha-Vinh R, Zankl A, Unger S, Kraenzlin ME, Beckmann JS, Saito I, Rivolta C, Ikegawa S, Superti-Furga A, Hirano T. The zinc transporter SLC39A13/ZIP13 is required for connective tissue development; its involvement in BMP/TGF-beta signaling pathways. PLoS ONE. 2008;3:e3642. doi: 10.1371/journal.pone.0003642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jeong J, Walker JM, Wang F, Park JG, Palmer AE, Giunta C, Rohrbach M, Steinmann B, Eide DJ. Promotion of vesicular zinc efflux by ZIP13 and its implications for spondylocheiro dysplastic Ehlers–Danlos syndrome. Proc Natl Acad Sci USA. 2012;109:E3530–E3538. doi: 10.1073/pnas.1211775110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dupuis J, Langenberg C, Prokopenko I, Saxena R, Soranzo N, Jackson AU, Wheeler E, Glazer NL, Bouatia-Naji N, Gloyn AL, Lindgren CM, Magi R, Morris AP, Randall J, Johnson T, Elliott P, Rybin D, Thorleifsson G, Steinthorsdottir V, Henneman P, Grallert H, Dehghan A, Hottenga JJ, Franklin CS, Navarro P, Song K, Goel A, Perry JR, Egan JM, Lajunen T, Grarup N, Sparso T, Doney A, Voight BF, Stringham HM, Li M, Kanoni S, Shrader P, Cavalcanti-Proenca C, Kumari M, Qi L, Timpson NJ, Gieger C, Zabena C, Rocheleau G, Ingelsson E, An P, O’Connell J, Luan J, Elliott A, McCarroll SA, Payne F, Roccasecca RM, Pattou F, Sethupathy P, Ardlie K, Ariyurek Y, Balkau B, Barter P, Beilby JP, Ben-Shlomo Y, Benediktsson R, Bennett AJ, Bergmann S, Bochud M, Boerwinkle E, Bonnefond A, Bonnycastle LL, Borch-Johnsen K, Bottcher Y, Brunner E, Bumpstead SJ, Charpentier G, Chen YD, Chines P, Clarke R, Coin LJ, Cooper MN, Cornelis M, Crawford G, Crisponi L, Day IN, de Geus EJ, Delplanque J, Dina C, Erdos MR, Fedson AC, Fischer-Rosinsky A, Forouhi NG, Fox CS, Frants R, Franzosi MG, Galan P, Goodarzi MO, Graessler J, Groves CJ, Grundy S, Gwilliam R, Gyllensten U, Hadjadj S, Hallmans G, Hammond N, Han X, Hartikainen AL, Hassanali N, Hayward C, Heath SC, Hercberg S, Herder C, Hicks AA, Hillman DR, Hingorani AD, Hofman A, Hui J, Hung J, Isomaa B, Johnson PR, Jorgensen T, Jula A, Kaakinen M, Kaprio J, Kesaniemi YA, Kivimaki M, Knight B, Koskinen S, Kovacs P, Kyvik KO, Lathrop GM, Lawlor DA, Le Bacquer O, Lecoeur C, Li Y, Lyssenko V, Mahley R, Mangino M, Manning AK, Martinez-Larrad MT, McAteer JB, McCulloch LJ, McPherson R, Meisinger C, Melzer D, Meyre D, Mitchell BD, Morken MA, Mukherjee S, Naitza S, Narisu N, Neville MJ, Oostra BA, Orru M, Pakyz R, Palmer CN, Paolisso G, Pattaro C, Pearson D, Peden JF, Pedersen NL, Perola M, Pfeiffer AF, Pichler I, Polasek O, Posthuma D, Potter SC, Pouta A, Province MA, Psaty BM, Rathmann W, Rayner NW, Rice K, Ripatti S, Rivadeneira F, Roden M, Rolandsson O, Sandbaek A, Sandhu M, Sanna S, Sayer AA, Scheet P, Scott LJ, Seedorf U, Sharp SJ, Shields B, Sigurethsson G, Sijbrands EJ, Silveira A, Simpson L, Singleton A, Smith NL, Sovio U, Swift A, Syddall H, Syvanen AC, Tanaka T, Thorand B, Tichet J, Tonjes A, Tuomi T, Uitterlinden AG, van Dijk KW, van Hoek M, Varma D, Visvikis-Siest S, Vitart V, Vogelzangs N, Waeber G, Wagner PJ, Walley A, Walters GB, Ward KL, Watkins H, Weedon MN, Wild SH, Willemsen G, Witteman JC, Yarnell JW, Zeggini E, Zelenika D, Zethelius B, Zhai G, Zhao JH, Zillikens MC, Borecki IB, Loos RJ, Meneton P, Magnusson PK, Nathan DM, Williams GH, Hattersley AT, Silander K, Salomaa V, Smith GD, Bornstein SR, Schwarz P, Spranger J, Karpe F, Shuldiner AR, Cooper C, Dedoussis GV, Serrano-Rios M, Morris AD, Lind L, Palmer LJ, Hu FB, Franks PW, Ebrahim S, Marmot M, Kao WH, Pankow JS, Sampson MJ, Kuusisto J, Laakso M, Hansen T, Pedersen O, Pramstaller PP, Wichmann HE, Illig T, Rudan I, Wright AF, Stumvoll M, Campbell H, Wilson JF, Bergman RN, Buchanan TA, Collins FS, Mohlke KL, Tuomilehto J, Valle TT, Altshuler D, Rotter JI, Siscovick DS, Penninx BW, Boomsma DI, Deloukas P, Spector TD, Frayling TM, Ferrucci L, Kong A, Thorsteinsdottir U, Stefansson K, van Duijn CM, Aulchenko YS, Cao A, Scuteri A, Schlessinger D, Uda M, Ruokonen A, Jarvelin MR, Waterworth DM, Vollenweider P, Peltonen L, Mooser V, Abecasis GR, Wareham NJ, Sladek R, Froguel P, Watanabe RM, Meigs JB, Groop L, Boehnke M, McCarthy MI, Florez JC, Barroso I. New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat Genet. 2010;42:105–116. doi: 10.1038/ng.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Potocki S, Rowinska-Zyrek M, Valensin D, Krzywoszynska K, Witkowska D, Luczkowski M, Kozlowski H. Metal binding ability of cysteine-rich peptide domain of ZIP13 Zn2+ ions transporter. Inorg Chem. 2011;50:6135–6145. doi: 10.1021/ic200270p. [DOI] [PubMed] [Google Scholar]
- 88.Ackland ML, Michalczyk A. Zinc deficiency and its inherited disorders-a review. Genes Nutr. 2006;1:41–49. doi: 10.1007/BF02829935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Roberts LJ, Shadwick CF, Bergstresser PR. Zinc deficiency in two full-term breast-fed infants. J Am Acad Dermatol. 1987;16:301–304. doi: 10.1016/s0190-9622(87)70039-5. [DOI] [PubMed] [Google Scholar]
- 90.Chowanadisai W, Lonnerdal B, Kelleher SL. Identification of a mutation in SLC30A2 (ZnT-2) in women with low milk zinc concentration that results in transient neonatal zinc deficiency. J Biol Chem. 2006;281:39699–39707. doi: 10.1074/jbc.M605821200. [DOI] [PubMed] [Google Scholar]
- 91.Murthy SC, Udagani MM, Badakali AV, Yelameli BC. Symptomatic zinc deficiency in a full-term breast-fed infant. Dermatol Online J. 2010;16:3. [PubMed] [Google Scholar]
- 92.Lasry I, Seo YA, Ityel H, Shalva N, Pode-Shakked B, Glaser F, Berman B, Berezovsky I, Goncearenco A, Klar A, Levy J, Anikster Y, Kelleher SL, Assaraf YG. A dominant negative heterozygous G87R mutation in the zinc transporter, ZnT-2 (SLC30A2), results in transient neonatal zinc deficiency. J Biol Chem. 2012;287:29348–29361. doi: 10.1074/jbc.M112.368159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Itsumura N, Inamo Y, Okazaki F, Teranishi F, Narita H, Kambe T, Kodama H. Compound heterozygous mutations in SLC30A2/ZnT2 results in low milk zinc concentrations: a novel mechanism for zinc deficiency in a breast-fed infant. PLoS ONE. 2013;8:e64045. doi: 10.1371/journal.pone.0064045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Miletta MC, Bieri A, Kernland K, Schoni MH, Petkovic V, Fluck CE, Eble A, Mullis PE. Transient neonatal zinc deficiency caused by a heterozygous G87R mutation in the zinc transporter ZnT-2 (SLC30A2) gene in the mother highlighting the importance of Zn (2+) for Normal growth and development. Int J Endocrinol. 2013;2013:259189. doi: 10.1155/2013/259189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Qian L, Wang B, Tang N, Zhang W, Cai W. Polymorphisms of SLC30A2 and selected perinatal factors associated with low milk zinc in Chinese breastfeeding women. Early Hum Dev. 2012;88:663–668. doi: 10.1016/j.earlhumdev.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 96.Seo YA, Kelleher SL. Functional analysis of two single-nucleotide polymorphisms in SLC30A2 (ZnT2): implications for mammary gland function and breast disease in women. Physiol Genomics. 2010;42A:219–227. doi: 10.1152/physiolgenomics.00137.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Piletz JE, Ganschow RE. Zinc deficiency in murine milk underlies expression of the lethal milk (lm) mutation. Science. 1978;199:181–183. doi: 10.1126/science.619449. [DOI] [PubMed] [Google Scholar]
- 98.Huang L, Gitschier J. A novel gene involved in zinc transport is deficient in the lethal milk mouse. Nat Genet. 1997;17:292–297. doi: 10.1038/ng1197-292. [DOI] [PubMed] [Google Scholar]
- 99.Lonnerdal B. Trace element transport in the mammary gland. Annu Rev Nutr. 2007;27:165–177. doi: 10.1146/annurev.nutr.27.061406.093809. [DOI] [PubMed] [Google Scholar]
- 100.Yamawaki N, Yamada M, Kan-no T, Kojima T, Kaneko T, Yonekubo A. Macronutrient, mineral and trace element composition of breast milk from Japanese women. J Trace Elem Med Biol. 2005;19:171–181. doi: 10.1016/j.jtemb.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 101.Yasuda H, Yoshida K, Yasuda Y, Tsutsui T. Infantile zinc deficiency: association with autism spectrum disorders. Sci Rep. 2011;1:129. doi: 10.1038/srep00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Danaei G, Finucane MM, Lu Y, Singh GM, Cowan MJ, Paciorek CJ, Lin JK, Farzadfar F, Khang YH, Stevens GA, Rao M, Ali MK, Riley LM, Robinson CA, Ezzati M. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet. 2011;378:31–40. doi: 10.1016/S0140-6736(11)60679-X. [DOI] [PubMed] [Google Scholar]
- 103.Stancakova A, Kuulasmaa T, Paananen J, Jackson AU, Bonnycastle LL, Collins FS, Boehnke M, Kuusisto J, Laakso M. Association of 18 confirmed susceptibility loci for type 2 diabetes with indices of insulin release, proinsulin conversion, and insulin sensitivity in 5,327 nondiabetic Finnish men. Diabetes. 2009;58:2129–2136. doi: 10.2337/db09-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fu J, Festen EA, Wijmenga C. Multi-ethnic studies in complex traits. Hum Mol Genet. 2011;20:R206–R213. doi: 10.1093/hmg/ddr386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sladek R, Rocheleau G, Rung J, Dina C, Shen L, Serre D, Boutin P, Vincent D, Belisle A, Hadjadj S, Balkau B, Heude B, Charpentier G, Hudson TJ, Montpetit A, Pshezhetsky AV, Prentki M, Posner BI, Balding DJ, Meyre D, Polychronakos C, Froguel P. A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature. 2007;445:881–885. doi: 10.1038/nature05616. [DOI] [PubMed] [Google Scholar]
- 106.Saxena R, Voight BF, Lyssenko V, Burtt NP, Bakker PI, Chen H, Roix JJ, Kathiresan S, Hirschhorn JN, Daly MJ, Hughes TE, Groop L, Altshuler D, Almgren P, Florez JC, Meyer J, Ardlie K, Bengtsson Bostrom K, Isomaa B, Lettre G, Lindblad U, Lyon HN, Melander O, Newton-Cheh C, Nilsson P, Orho-Melander M, Rastam L, Speliotes EK, Taskinen MR, Tuomi T, Guiducci C, Berglund A, Carlson J, Gianniny L, Hackett R, Hall L, Holmkvist J, Laurila E, Sjogren M, Sterner M, Surti A, Svensson M, Tewhey R, Blumenstiel B, Parkin M, Defelice M, Barry R, Brodeur W, Camarata J, Chia N, Fava M, Gibbons J, Handsaker B, Healy C, Nguyen K, Gates C, Sougnez C, Gage D, Nizzari M, Gabriel SB, Chirn GW, Ma Q, Parikh H, Richardson D, Ricke D, Purcell S. Science. 2007;316:1331–1336. [Google Scholar]
- 107.Scott LJ, Mohlke KL, Bonnycastle LL, Willer CJ, Li Y, Duren WL, Erdos MR, Stringham HM, Chines PS, Jackson AU, Prokunina-Olsson L, Ding CJ, Swift AJ, Narisu N, Hu T, Pruim R, Xiao R, Li XY, Conneely KN, Riebow NL, Sprau AG, Tong M, White PP, Hetrick KN, Barnhart MW, Bark CW, Goldstein JL, Watkins L, Xiang F, Saramies J, Buchanan TA, Watanabe RM, Valle TT, Kinnunen L, Abecasis GR, Pugh EW, Doheny KF, Bergman RN, Tuomilehto J, Collins FS, Boehnke M. A genome-wide association study of type 2 diabetes in Finns detects multiple susceptibility variants. Science. 2007;316:1341–1345. doi: 10.1126/science.1142382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zeggini E, Weedon MN, Lindgren CM, Frayling TM, Elliott KS, Lango H, Timpson NJ, Perry JR, Rayner NW, Freathy RM, Barrett JC, Shields B, Morris AP, Ellard S, Groves CJ, Harries LW, Marchini JL, Owen KR, Knight B, Cardon LR, Walker M, Hitman GA, Morris AD, Doney AS, McCarthy MI, Hattersley AT. Replication of genome-wide association signals in UK samples reveals risk loci for type 2 diabetes. Science. 2007;316:1336–1341. doi: 10.1126/science.1142364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cauchi S, Del Guerra S, Choquet H, D’Aleo V, Groves CJ, Lupi R, McCarthy MI, Froguel P, Marchetti P. Meta-analysis and functional effects of the SLC30A8 rs13266634 polymorphism on isolated human pancreatic islets. Mol Genet Metab. 2010;100:77–82. doi: 10.1016/j.ymgme.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 110.Nicolson TJ, Bellomo EA, Wijesekara N, Loder MK, Baldwin JM, Gyulkhandanyan AV, Koshkin V, Tarasov AI, Carzaniga R, Kronenberger K, Taneja TK, da Silva Xavier G, Libert S, Froguel P, Scharfmann R, Stetsyuk V, Ravassard P, Parker H, Gribble FM, Reimann F, Sladek R, Hughes SJ, Johnson PR, Masseboeuf M, Burcelin R, Baldwin SA, Liu M, Lara-Lemus R, Arvan P, Schuit FC, Wheeler MB, Chimienti F, Rutter GA. Insulin storage and glucose homeostasis in mice null for the granule zinc transporter ZnT8 and studies of the type 2 diabetes-associated variants. Diabetes. 2009;58:2070–2083. doi: 10.2337/db09-0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Boesgaard TW, Zilinskaite J, Vanttinen M, Laakso M, Jansson PA, Hammarstedt A, Smith U, Stefan N, Fritsche A, Haring H, Hribal M, Sesti G, Zobel DP, Pedersen O, Hansen T. The common SLC30A8 Arg325Trp variant is associated with reduced first-phase insulin release in 846 non-diabetic offspring of type 2 diabetes patients–the EUGENE2 study. Diabetologia. 2008;51:816–820. doi: 10.1007/s00125-008-0955-6. [DOI] [PubMed] [Google Scholar]
- 112.Kirchhoff K, Machicao F, Haupt A, Schafer SA, Tschritter O, Staiger H, Stefan N, Haring HU, Fritsche A. Polymorphisms in the TCF7L2, CDKAL1 and SLC30A8 genes are associated with impaired proinsulin conversion. Diabetologia. 2008;51:597–601. doi: 10.1007/s00125-008-0926-y. [DOI] [PubMed] [Google Scholar]
- 113.Tamaki M, Fujitani Y, Hara A, Uchida T, Tamura Y, Takeno K, Kawaguchi M, Watanabe T, Ogihara T, Fukunaka A, Shimizu T, Mita T, Kanazawa A, Imaizumi MO, Abe T, Kiyonari H, Hojyo S, Fukada T, Kawauchi T, Nagamatsu S, Hirano T, Kawamori R, Watada H. The diabetes-susceptible gene SLC30A8/ZnT8 regulates hepatic insulin clearance. J Clin Investig. 2013;123:4513–4524. doi: 10.1172/JCI68807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wenzlau JM, Juhl K, Yu L, Moua O, Sarkar SA, Gottlieb P, Rewers M, Eisenbarth GS, Jensen J, Davidson HW, Hutton JC. The cation efflux transporter ZnT8 (Slc30A8) is a major autoantigen in human type 1 diabetes. Proc Natl Acad Sci USA. 2007;104:17040–17045. doi: 10.1073/pnas.0705894104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Delli AJ, Vaziri-Sani F, Lindblad B, Elding-Larsson H, Carlsson A, Forsander G, Ivarsson SA, Ludvigsson J, Kockum I, Marcus C, Samuelsson U, Ortqvist E, Groop L, Bondinas GP, Papadopoulos GK, Lernmark A. Zinc transporter 8 autoantibodies and their association with SLC30A8 and HLA-DQ genes differ between immigrant and Swedish patients with newly diagnosed type 1 diabetes in the Better Diabetes Diagnosis study. Diabetes. 2012;61:2556–2564. doi: 10.2337/db11-1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dang M, Rockell J, Wagner R, Wenzlau JM, Yu L, Hutton JC, Gottlieb PA, Davidson HW. Human type 1 diabetes is associated with T cell autoimmunity to zinc transporter 8. J Immunol. 2011;186:6056–6063. doi: 10.4049/jimmunol.1003815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lampasona V, Petrone A, Tiberti C, Capizzi M, Spoletini M, di Pietro S, Songini M, Bonicchio S, Giorgino F, Bonifacio E, Bosi E, Buzzetti R. Zinc transporter 8 antibodies complement GAD and IA-2 antibodies in the identification and characterization of adult-onset autoimmune diabetes: Non Insulin Requiring Autoimmune Diabetes (NIRAD) 4. Diabetes Care. 2010;33:104–108. doi: 10.2337/dc08-2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wenzlau JM, Liu Y, Yu L, Moua O, Fowler KT, Rangasamy S, Walters J, Eisenbarth GS, Davidson HW, Hutton JC. A common nonsynonymous single-nucleotide polymorphism in the SLC30A8 gene determines ZnT8 autoantibody specificity in type 1 diabetes. Diabetes. 2008;57:2693–2697. doi: 10.2337/db08-0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kawasaki E, Uga M, Nakamura K, Kuriya G, Satoh T, Fujishima K, Ozaki M, Abiru N, Yamasaki H, Wenzlau JM, Davidson HW, Hutton JC, Eguchi K. Association between anti-ZnT8 autoantibody specificities and SLC30A8 Arg325Trp variant in Japanese patients with type 1 diabetes. Diabetologia. 2008;51:2299–2302. doi: 10.1007/s00125-008-1165-y. [DOI] [PubMed] [Google Scholar]
- 120.Rungby J. Zinc, zinc transporters and diabetes. Diabetologia. 2010;53:1549–1551. doi: 10.1007/s00125-010-1793-x. [DOI] [PubMed] [Google Scholar]
- 121.Kambe T, Narita H, Yamaguchi-Iwai Y, Hirose J, Amano T, Sugiura N, Sasaki R, Mori K, Iwanaga T, Nagao M. Cloning and characterization of a novel mammalian zinc transporter, zinc transporter 5, abundantly expressed in pancreatic beta cells. J Biol Chem. 2002;277:19049–19055. doi: 10.1074/jbc.M200910200. [DOI] [PubMed] [Google Scholar]
- 122.Kambe T. An overview of a wide range of functions of ZnT and Zip zinc transporters in the secretory pathway. Biosci Biotechnol Biochem. 2011;75:1036–1043. doi: 10.1271/bbb.110056. [DOI] [PubMed] [Google Scholar]
- 123.Pound LD, Sarkar SA, Benninger RK, Wang Y, Suwanichkul A, Shadoan MK, Printz RL, Oeser JK, Lee CE, Piston DW, McGuinness OP, Hutton JC, Powell DR, O’Brien RM. Deletion of the mouse Slc30a8 gene encoding zinc transporter-8 results in impaired insulin secretion. Biochem J. 2009;421:371–376. doi: 10.1042/BJ20090530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lemaire K, Ravier MA, Schraenen A, Creemers JW, Van de Plas R, Granvik M, Van Lommel L, Waelkens E, Chimienti F, Rutter GA, Gilon P, in’t Veld PA, Schuit FC. Insulin crystallization depends on zinc transporter ZnT8 expression, but is not required for normal glucose homeostasis in mice. Proc Natl Acad Sci USA. 2009;106:14872–14877. doi: 10.1073/pnas.0906587106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wijesekara N, Dai FF, Hardy AB, Giglou PR, Bhattacharjee A, Koshkin V, Chimienti F, Gaisano HY, Rutter GA, Wheeler MB. Beta cell-specific Znt8 deletion in mice causes marked defects in insulin processing, crystallisation and secretion. Diabetologia. 2010;53:1656–1668. doi: 10.1007/s00125-010-1733-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pound LD, Sarkar SA, Ustione A, Dadi PK, Shadoan MK, Lee CE, Walters JA, Shiota M, McGuinness OP, Jacobson DA, Piston DW, Hutton JC, Powell DR, O’Brien RM. The physiological effects of deleting the mouse SLC30A8 gene encoding zinc transporter-8 are influenced by gender and genetic background. PLoS ONE. 2012;7:e40972. doi: 10.1371/journal.pone.0040972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hardy A, Wijesekara N, Genkin I, Prentice KJ, Bhattacharjee A, Kong D, Chimienti F, Wheeler M. Effects of high fat diet feeding on zinc transporter 8 (Znt8) null mice: differences between beta cell and global knockout of Znt8. Am J Physiol Endocrinol Metab. 2012;302:E1084–E1096. doi: 10.1152/ajpendo.00448.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hoch E, Lin W, Chai J, Hershfinkel M, Fu D, Sekler I. Histidine pairing at the metal transport site of mammalian ZnT transporters controls Zn2+ over Cd2+ selectivity. Proc Natl Acad Sci USA. 2012;109:7202–7207. doi: 10.1073/pnas.1200362109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Montanini B, Blaudez D, Jeandroz S, Sanders D, Chalot M. Phylogenetic and functional analysis of the cation diffusion facilitator (CDF) family: improved signature and prediction of substrate specificity. BMC Genomics. 2007;8:107. doi: 10.1186/1471-2164-8-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bosomworth HJ, Adlard PA, Ford D, Valentine RA. Altered expression of ZnT10 in Alzheimer’s disease brain. PLoS ONE. 2013;8:e65475. doi: 10.1371/journal.pone.0065475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lazarczyk M, Cassonnet P, Pons C, Jacob Y, Favre M. The EVER proteins as a natural barrier against papillomaviruses: a new insight into the pathogenesis of human papillomavirus infections. Microbiol Mol Biol Rev. 2009;73:348–370. doi: 10.1128/MMBR.00033-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lazarczyk M, Pons C, Mendoza JA, Cassonnet P, Jacob Y, Favre M. Regulation of cellular zinc balance as a potential mechanism of EVER-mediated protection against pathogenesis by cutaneous oncogenic human papillomaviruses. J Exp Med. 2008;205:35–42. doi: 10.1084/jem.20071311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Palmiter RD, Findley SD. Cloning and functional characterization of a mammalian zinc transporter that confers resistance to zinc. EMBO J. 1995;14:639–649. doi: 10.1002/j.1460-2075.1995.tb07042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Costello LC, Franklin RB. Zinc is decreased in prostate cancer: an established relationship of prostate cancer! J Biol Inorg Chem. 2011;16:3–8. doi: 10.1007/s00775-010-0736-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kolenko V, Teper E, Kutikov A, Uzzo R. Zinc and zinc transporters in prostate carcinogenesis. Nat Rev Urol. 2013;10:219–226. doi: 10.1038/nrurol.2013.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hennigar SR, Kelleher SL. Zinc networks: the cell-specific compartmentalization of zinc for specialized functions. Biol Chem. 2012;393:565–578. doi: 10.1515/hsz-2012-0128. [DOI] [PubMed] [Google Scholar]
- 137.Gumulec J, Masarik M, Krizkova S, Adam V, Hubalek J, Hrabeta J, Eckschlager T, Stiborova M, Kizek R. Insight to physiology and pathology of zinc(II) ions and their actions in breast and prostate carcinoma. Curr Med Chem. 2011;18:5041–5051. doi: 10.2174/092986711797636126. [DOI] [PubMed] [Google Scholar]
- 138.Kagara N, Tanaka N, Noguchi S, Hirano T. Zinc and its transporter ZIP10 are involved in invasive behavior of breast cancer cells. Cancer Sci. 2007;98:692–697. doi: 10.1111/j.1349-7006.2007.00446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Shen R, Xie F, Shen H, liu Q, Zheng T, Kou X, Wang D, Yang J. Negative correlation of LIV-1 and E-cadherin expression in hepatocellular carcinoma cells. PLoS ONE. 2013;8:e56542. doi: 10.1371/journal.pone.0056542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hogstrand C, Kille P, Ackland ML, Hiscox S, Taylor KM. A mechanism for epithelial-mesenchymal transition and anoikis resistance in breast cancer triggered by zinc channel ZIP6 and STAT3 (signal transducer and activator of transcription 3) Biochem J. 2013;455:229–237. doi: 10.1042/BJ20130483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Taylor KM, Vichova P, Jordan N, Hiscox S, Hendley R, Nicholson RI. ZIP7-mediated intracellular zinc transport contributes to aberrant growth factor signaling in antihormone-resistant breast cancer Cells. Endocrinology. 2008;149:4912–4920. doi: 10.1210/en.2008-0351. [DOI] [PubMed] [Google Scholar]
- 142.Hogstrand C, Kille P, Nicholson RI, Taylor KM. Zinc transporters and cancer: a potential role for ZIP7 as a hub for tyrosine kinase activation. Trends Mol Med. 2009;15:101–111. doi: 10.1016/j.molmed.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 143.Franklin RB, Feng P, Milon B, Desouki MM, Singh KK, Kajdacsy-Balla A, Bagasra O, Costello LC. hZIP1 zinc uptake transporter down regulation and zinc depletion in prostate cancer. Mol Cancer. 2005;4:32. doi: 10.1186/1476-4598-4-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Desouki MM, Geradts J, Milon B, Franklin RB, Costello LC. hZip2 and hZip3 zinc transporters are down regulated in human prostate adenocarcinomatous glands. Mol Cancer. 2007;6:37. doi: 10.1186/1476-4598-6-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chen QG, Zhang Z, Yang Q, Shan GY, Yu XY, Kong CZ. The role of zinc transporter ZIP4 in prostate carcinoma. Urol Oncol. 2012;30:906–911. doi: 10.1016/j.urolonc.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 146.Overall CM, Lopez-Otin C. Strategies for MMP inhibition in cancer: innovations for the post-trial era. Nat Rev Cancer. 2002;2:657–672. doi: 10.1038/nrc884. [DOI] [PubMed] [Google Scholar]
- 147.Suzuki T, Ishihara K, Migaki H, Matsuura W, Kohda A, Okumura K, Nagao M, Yamaguchi-Iwai Y, Kambe T. Zinc transporters, ZnT5 and ZnT7, are required for the activation of alkaline phosphatases, zinc-requiring enzymes that are glycosylphosphatidylinositol-anchored to the cytoplasmic membrane. J Biol Chem. 2005;280:637–643. doi: 10.1074/jbc.M411247200. [DOI] [PubMed] [Google Scholar]
- 148.Fukunaka A, Kurokawa Y, Teranishi F, Sekler I, Oda K, Ackland ML, Faundez V, Hiromura M, Masuda S, Nagao M, Enomoto S, Kambe T. Tissue nonspecific alkaline phosphatase is activated via a two-step mechanism by zinc transport complexes in the early secretory pathway. J Biol Chem. 2011;286:16363–16373. doi: 10.1074/jbc.M111.227173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Franz MC, Anderle P, Burzle M, Suzuki Y, Freeman MR, Hediger MA, Kovacs G. Zinc transporters in prostate cancer. Mol Aspects Med. 2013;34:735–741. doi: 10.1016/j.mam.2012.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sandstead HH, Henriksen LK, Greger JL, Prasad AS, Good RA. Zinc nutriture in the elderly in relation to taste acuity, immune response, and wound healing. Am J Clin Nutr. 1982;36:1046–1059. doi: 10.1093/ajcn/36.5.1046. [DOI] [PubMed] [Google Scholar]
- 151.Prasad AS. Clinical and biochemical manifestations of zinc deficiency in human subjects. J Am Coll Nutr. 1985;4:65–72. doi: 10.1080/07315724.1985.10720067. [DOI] [PubMed] [Google Scholar]
- 152.Prasad AS. Zinc: an overview. Nutrition. 1995;11(1 Suppl):93–99. [PubMed] [Google Scholar]
- 153.Hirano T, Murakami M, Fukada T, Nishida K, Yamasaki S, Suzuki T. Roles of zinc and zinc signaling in immunity: zinc as an intracellular signaling molecule. Adv Immunol. 2008;97:149–176. doi: 10.1016/S0065-2776(08)00003-5. [DOI] [PubMed] [Google Scholar]
- 154.Murakami M, Hirano T. Intracellular zinc homeostasis and zinc signaling. Cancer Sci. 2008;99:1515–1522. doi: 10.1111/j.1349-7006.2008.00854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Haase H, Rink L. Functional significance of zinc-related signaling pathways in immune cells. Annu Rev Nutr. 2009;29:133–152. doi: 10.1146/annurev-nutr-080508-141119. [DOI] [PubMed] [Google Scholar]
- 156.Liu MJ, Bao S, Galvez-Peralta M, Pyle CJ, Rudawsky AC, Pavlovicz RE, Killilea DW, Li C, Nebert DW, Wewers MD, Knoell DL. ZIP8 regulates host defense through zinc-mediated inhibition of NF-kappaB. Cell Rep. 2013;3:386–400. doi: 10.1016/j.celrep.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yu M, Lee WW, Tomar D, Pryshchep S, Czesnikiewicz-Guzik M, Lamar DL, Li G, Singh K, Tian L, Weyand CM, Goronzy JJ. Regulation of T cell receptor signaling by activation-induced zinc influx. J Exp Med. 2011;208:775–785. doi: 10.1084/jem.20100031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kitamura H, Morikawa H, Kamon H, Iguchi M, Hojyo S, Fukada T, Yamashita S, Kaisho T, Akira S, Murakami M, Hirano T. Toll-like receptor-mediated regulation of zinc homeostasis influences dendritic cell function. Nat Immunol. 2006;7:971–977. doi: 10.1038/ni1373. [DOI] [PubMed] [Google Scholar]
- 159.Aydemir TB, Liuzzi JP, McClellan S, Cousins RJ. Zinc transporter ZIP8 (SLC39A8) and zinc influence IFN-gamma expression in activated human T cells. J Leukoc Biol. 2009;86:337–348. doi: 10.1189/jlb.1208759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Matsuura W, Yamazaki T, Yamaguchi-Iwai Y, Masuda S, Nagao M, Andrews GK, Kambe T. SLC39A9 (ZIP9) regulates zinc homeostasis in the secretory pathway: characterization of the ZIP subfamily I protein in vertebrate cells. Biosci Biotechnol Biochem. 2009;73:1142–1148. doi: 10.1271/bbb.80910. [DOI] [PubMed] [Google Scholar]
- 161.Taniguchi M, Fukunaka A, Hagihara M, Watanabe K, Kamino S, Kambe T, Enomoto S, Hiromura M. Essential role of the zinc transporter ZIP9/SLC39A9 in regulating the activations of Akt and Erk in B-cell receptor signaling pathway in DT40 cells. PLoS ONE. 2013;8:e58022. doi: 10.1371/journal.pone.0058022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hood MI, Skaar EP. Nutritional immunity: transition metals at the pathogen-host interface. Nat Rev Microbiol. 2012;10:525–537. doi: 10.1038/nrmicro2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Corbin BD, Seeley EH, Raab A, Feldmann J, Miller MR, Torres VJ, Anderson KL, Dattilo BM, Dunman PM, Gerads R, Caprioli RM, Nacken W, Chazin WJ, Skaar EP. Metal chelation and inhibition of bacterial growth in tissue abscesses. Science. 2008;319:962–965. doi: 10.1126/science.1152449. [DOI] [PubMed] [Google Scholar]
- 164.Subramanian Vignesh K, Landero Figueroa JA, Porollo A, Caruso JA, Deepe GS,, Jr Granulocyte macrophage-colony stimulating factor induced Zn sequestration enhances macrophage superoxide and limits intracellular pathogen survival. Immunity. 2013;39:697–710. doi: 10.1016/j.immuni.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Vignesh KS, Landero Figueroa JA, Porollo A, Caruso JA, Deepe GS,, Jr Zinc sequestration: arming phagocyte defense against fungal attack. PLoS Pathog. 2013;9:e1003815. doi: 10.1371/journal.ppat.1003815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sensi SL, Paoletti P, Bush AI, Sekler I. Zinc in the physiology and pathology of the CNS. Nat Rev Neurosci. 2009;10:780–791. doi: 10.1038/nrn2734. [DOI] [PubMed] [Google Scholar]
- 167.Sensi SL, Paoletti P, Koh JY, Aizenman E, Bush AI, Hershfinkel M. The neurophysiology and pathology of brain zinc. J Neurosci. 2011;31:16076–16085. doi: 10.1523/JNEUROSCI.3454-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Takeda A, Nakamura M, Fujii H, Tamano H. Synaptic Zn(2+) homeostasis and its significance. Metallomics. 2013;5:417–423. doi: 10.1039/c3mt20269k. [DOI] [PubMed] [Google Scholar]
- 169.Szewczyk B. Zinc homeostasis and neurodegenerative disorders. Front Aging Neurosci. 2013;5:33. doi: 10.3389/fnagi.2013.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hozumi I, Hasegawa T, Honda A, Ozawa K, Hayashi Y, Hashimoto K, Yamada M, Koumura A, Sakurai T, Kimura A, Tanaka Y, Satoh M, Inuzuka T. Patterns of levels of biological metals in CSF differ among neurodegenerative diseases. J Neurol Sci. 2011;303:95–99. doi: 10.1016/j.jns.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 171.Beyer N, Coulson DT, Heggarty S, Ravid R, Hellemans J, Irvine GB, Johnston JA. Zinc transporter mRNA levels in Alzheimer’s disease postmortem brain. J Alzheimers Dis. 2012;29:863–873. doi: 10.3233/JAD-2012-112105. [DOI] [PubMed] [Google Scholar]
- 172.Smith JL, Xiong S, Markesbery WR, Lovell MA. Altered expression of zinc transporters-4 and -6 in mild cognitive impairment, early and late Alzheimer’s disease brain. Neuroscience. 2006;140:879–888. doi: 10.1016/j.neuroscience.2006.02.049. [DOI] [PubMed] [Google Scholar]
- 173.Palmiter RD, Cole TB, Quaife CJ, Findley SD. ZnT-3, a putative transporter of zinc into synaptic vesicles. Proc Natl Acad Sci USA. 1996;93:14934–14939. doi: 10.1073/pnas.93.25.14934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Beyer N, Coulson DT, Heggarty S, Ravid R, Irvine GB, Hellemans J, Johnston JA. ZnT3 mRNA levels are reduced in Alzheimer’s disease post-mortem brain. Mol Neurodegener. 2009;4:53. doi: 10.1186/1750-1326-4-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Adlard PA, Parncutt JM, Finkelstein DI, Bush AI. Cognitive loss in zinc transporter-3 knock-out mice: a phenocopy for the synaptic and memory deficits of Alzheimer’s disease? J Neurosci. 2010;30:1631–1636. doi: 10.1523/JNEUROSCI.5255-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Rovelet-Lecrux A, Legallic S, Wallon D, Flaman JM, Martinaud O, Bombois S, Rollin-Sillaire A, Michon A, Le Ber I, Pariente J, Puel M, Paquet C, Croisile B, Thomas-Anterion C, Vercelletto M, Levy R, Frebourg T, Hannequin D, Campion D. A genome-wide study reveals rare CNVs exclusive to extreme phenotypes of Alzheimer disease. Eur J Hum Genet. 2012;20:613–617. doi: 10.1038/ejhg.2011.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Fujimoto S, Itsumura N, Tsuji T, Anan Y, Tsuji N, Ogra Y, Kimura T, Miyamae Y, Masuda S, Nagao M, Kambe T. Cooperative functions of ZnT1, metallothionein and ZnT4 in the cytoplasm are required for full activation of TNAP in the early secretory pathway. PLoS ONE. 2013;8:e77445. doi: 10.1371/journal.pone.0077445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Gallaher DD, Johnson PE, Hunt JR, Lykken GI, Marchello MJ. Bioavailability in humans of zinc from beef: intrinsic vs extrinsic labels. Am J Clin Nutr. 1988;48:350–354. doi: 10.1093/ajcn/48.2.350. [DOI] [PubMed] [Google Scholar]
- 179.Faa G, Nurchi VM, Ravarino A, Fanni D, Nemolato S, Gerosa C, Eyken PV, Geboes K. Zinc in gastrointestinal and liver disease. Coord Chem Rev. 2008;252:1257–1269. [Google Scholar]
- 180.Patrushev N, Seidel-Rogol B, Salazar G. Angiotensin II requires zinc and downregulation of the zinc transporters ZnT3 and ZnT10 to induce senescence of vascular smooth muscle cells. PLoS ONE. 2012;7:e33211. doi: 10.1371/journal.pone.0033211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Bosomworth HJ, Thornton JK, Coneyworth LJ, Ford D, Valentine RA. Efflux function, tissue-specific expression and intracellular trafficking of the Zn transporter ZnT10 indicate roles in adult Zn homeostasis. Metallomics. 2012;4:771–779. doi: 10.1039/c2mt20088k. [DOI] [PubMed] [Google Scholar]