Abstract
Human potassium channels are widely inhibited by peptide toxins from venomous animals. However, no human endogenous peptide inhibitor has been discovered so far. In this study, we demonstrate for the first time using electrophysiological techniques, that endogenous human β–defensin 2 (hBD2) is able to selectively and dose-dependently inhibit the human voltage-gated Kv1.3 channel at picomolar peptide concentration. The co-immunoprecipitation assays further supported the selective binding of hBD2 to Kv1.3 channel. Using mutagenesis experiments, we found that the outer pore domain of Kv1.3 channel was the binding site of hBD2, which is similar to the interacting site of Kv1.3 channel recognized by animal toxin inhibitors. The hBD2 was able to suppress IL-2 production through inhibition of Kv1.3 channel currents in human Jurkat cells, which was further confirmed by the lack of hBD2 activity on IL-2 production after Kv1.3 knockdown in these cells. More interestingly, hBD2 was also found to efficiently inhibit Kv1.3 channel currents and suppress IL-2 production in both human primary CD3+ T cells and peripheral mononuclear cells from either healthy donors or psoriasis patients. Our findings not only evidenced hBD2 as the first characterized endogenous peptide inhibitor of human potassium channels, but also paved a promising avenue to investigate newly discovered function of hBD2 as Kv1.3 channel inhibitor in the immune system and other fields.
Electronic supplementary material
The online version of this article (doi:10.1007/s00018-014-1715-z) contains supplementary material, which is available to authorized users.
Keywords: Antibacterial and antiviral peptide, Endogenous inhibitors, Lymphocyte, Interaction mechanism, Cytokine secretion, Adaptive immune modulation
Introduction
With about 100 members, human potassium channels serve a variety of physiological and pathological functions [1]. These channels are widely blocked or inhibited by peptide toxins from venomous animals, such as scorpions, snakes, spiders, bees, sea anemones and marine cone snails [2]. However, whether there is human endogenous peptide inhibitor of potassium channels remains a question so far. Among the potassium channels, Kv1.3 channel shows an unusually broad sensitivity to peptide toxins from the venomous animals [2]. Many toxins could block Kv1.3 channel currents in the nanomolar to picomolar range of potency, such as scorpion toxins charybodotoxin (ChTX), OSK1, BmKTX and their analogs [3–5], and sea anemone toxin ShK and its derivatives [6]. Recently, scorpion Kunitz-type toxins were also found to block Kv1.3 channel [7]. These toxin binding interfaces with diverse structural features suggest that human Kv1.3 channel might be targeted by own endogenous peptides.
Human β-defensin 2 (hBD2) is an antimicrobial peptide which protects hosts from microbial infection by killing bacteria, fungi and viruses [8], and recruits memory T cells through interacting with CCR6 [9]. Recently, hBD2 was predicted to be an inhibitor of rat Kv1.2 channel by computational docking [10], and found to be a novel opener via interacting with human β1 subunit coexpressed with mouse α subunit of large conductance Ca2+-activated potassium channel [11]. In this work, the in-depth structural analysis indicated that hBD2 and different Kv1.3 channel-blocking toxins are basic peptides while they are also structurally constrained by several disulfide bridges [2, 12]. These similar basic features suggested that hBD2 might be a toxin-like inhibitor of Kv1.3 channel. Through extensive experiments, hBD2 was found to be a specific inhibitor for Kv1.3 channels via interaction with the channel vestibule, and could further suppress IL-2 production for immune modulation. Together, these findings paved a promising avenue to study hBD2 functions as an endogenous Kv1.3 channel inhibitor in the immune system and other fields.
Results
Similar basic features between hBD2 and Kv1.3 channel-blocking toxins
The vestibule of human Kv1.3 channel is responsible for the recognition of animal toxins. As shown in Supplementary Fig. S1a, there are 20 acidic residues on the binding interface of Kv1.3 channel which is composed of four identical channel chains. Through the dominant electrostatic interactions, this outer entrance of Kv1.3 channel is recognized by different basic peptide toxins with diverse structural motifs, such as the classical scorpion toxin ChTX using an antiparallel β-sheet motif as binding interface (Supplementary Fig. S1c) [13], sea anemone toxin ShK adopting the second helix as binding interface (Supplementary Fig. S1d) [14], and ‘novel’ scorpion Kunitz-type toxins [7]. In comparison with Kv1.3 channel-blocking toxins, hBD2 is also a basic peptide with many positively-charged residues (Supplementary Fig. S1b). These similar features between hBD2 and toxins implied that hBD2 might interact with human Kv1.3 channel.
hBD2 is an endogenous inhibitor of human potassium channels
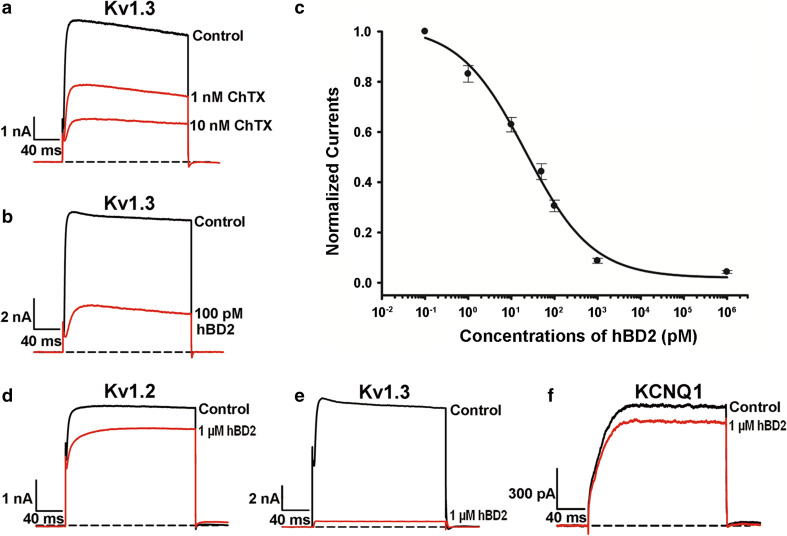
Before verifying our hypothesis, the blockade of human Kv1.3 channel transfected in HEK293 cells was successfully tested by the classical scorpion toxin ChTX. As shown in Fig. 1a, both 1 and 10 nM ChTX could significantly inhibit Kv1.3 channel currents. Next, we investigated the potential inhibition of hBD2 on Kv1.3 channel. As expected, it was found that hBD2 was a potent Kv1.3 inhibitor, and 100 pM hBD2 inhibited 69.4 ± 2.3 % of Kv1.3 channel currents (Fig. 1b). Noticeably, such hBD2 binding was difficult to be washed off from Kv1.3 channel (Supplementary Fig. S2). Concentration-dependent experiments further showed that hBD2 inhibited Kv1.3 channel currents with an IC50 value of 22.0 ± 7.1 pM (Fig. 1c). These data demonstrate that hBD2 is an endogenous and potent inhibitor of human Kv1.3 channel.
Fig. 1.
The hBD2 interaction with human potassium channels transfected in HEK293 cells. a Inhibition of Kv1.3 channel currents by scorpion toxin ChTX. 48.6 ± 2.2 and 69.9 ± 1.2 % blockage by 1 nM and 10 nM ChTX, respectively. b Inhibition of Kv1.3 channel currents by hBD2. 69.4 ± 2.3 % block by 100 pM hBD2. c Concentration-dependent inhibition of Kv1.3 channel currents by hBD2. The IC50 was calculated to be 22.0 ± 7.1 pM. d–f 1 μM hBD2 inhibited potassium currents of 16.5 ± 0.2 % for hKv1.2 (d), 95.7 ± 0.6 % for Kv1.3 (e), 12.5 ± 0.9 % for KCNQ1 (f), respectively. The black and red lines represent the control and measured currents in the absence and presence of hBD2, respectively. Each channel was tested at least three times. Results were shown as the mean ± SE
Besides Kv1.3 channel, we further investigated the possible interactions of hBD2 with additional human potassium channels, which were also transfected and expressed in HEK293 cells. As shown in Fig. 1d–f, 1 μM hBD2 was able to inhibit nearly the whole Kv1.3 channel currents, whereas it could hardly inhibit other potassium channel currents. These data indicate that hBD2 behaves as a selective inhibitor of human Kv1.3 channel. Together, the marked potency and selectivity of hBD2 demonstrate that it is actually a specific inhibitor of human Kv1.3 channel.
Kv1.3 channel vestibule is the binding site of hBD2
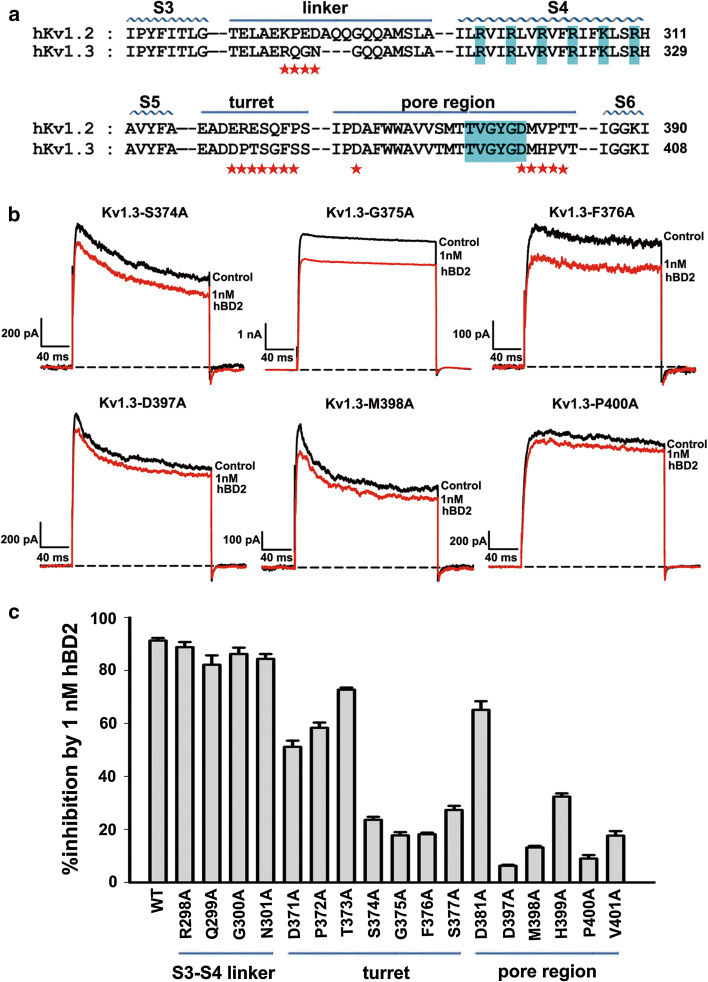
As for the interactions between peptide toxins and voltage-gated potassium channels, there are two known binding interfaces in potassium channels: one is the transmembrane helix S3–S4 linker recognized by spider toxins as gating modifiers [15, 16], the other is channel vestibule recognized by the scorpion toxins and sea anemone toxins as inhibitors (Supplementary Fig. S1a). To investigate the binding mechanism of hBD2 towards human Kv1.3 channel in HEK293 cells, the different amino acid residues of S3-S4 linkers between insensitive Kv1.2 and sensitive Kv1.3 channels were first investigated by alanine-scanning mutagenesis. As shown in Fig. 2a, Arg298, Gln299, Gly300 and Asn301 residues, different from those of Kv1.2 channel, were selected. The mutagenesis experiments indicated that their substitutions by alanine did not significantly affect hBD2 affinity (Fig. 2c). These poor effects on hBD2 inhibition indicate that it does not bind to S3–S4 linker of Kv1.3 channel.
Fig. 2.
Kv1.3 channel outer vestibule responsible for hBD2 binding. a The amino acid sequence alignment for toxin-interacting regions of human Kv1.2 and Kv1.3 channels. Asterisks indicate amino acid residues of Kv1.3 channel that were changed in later functional studies. b Representative current traces of Kv1.3 channel mutants from the pore region in response to 1 nM hBD2. 1 nM hBD2 inhibited potassium currents of 23.6 ± 1.2 % for Kv1.3-S374A channel, 17.8 ± 1.2 % for Kv1.3-G375A channel, 18.2 ± 0.6 % for Kv1.3-F376A channel, 6.3 ± 0.4 % for Kv1.3-D397A channel, 13.2 ± 0.6 % for Kv1.3-M398A channel and 9.0 ± 1.3 % for Kv1.3-P400A channel, respectively. The black and red lines represent control and measured currents in the absence and presence of hBD2, respectively. c Averaged inhibition of wild-type and mutant Kv1.3 channel currents by 1 nM hBD2. The control currents amplitude in each experiment was fixed as 1 for the normalized currents and inhibition rates were compared. Each channel was tested at least three times. Results are shown as the mean ± SE
Next, the outer vestibule of Kv1.3 channel was examined for hBD2 binding. As shown in Fig. 2b, the serial alanine-scanning mutagenesis experiments indicated that the negatively-charged vestibule of Kv1.3 channel was responsible for recognition of basic hBD2 peptide. In comparison with ca. 90 % inhibition of wild-type Kv1.3 channel currents by 1 nM hBD2, each mutation of the four Ser374, Gly375, Phe376 and Ser377 residues in Kv1.3 channel turret substantially lowered hBD2 affinity (Fig. 2b, c), and mutation of other turret residues (Asp371, Pro372, Thr373 and Asp381) to alanine moderately decreased hBD2 affinity (Fig. 2c). These reduced hBD2 affinities indicate that Kv1.3 channel turret plays an essential role in hBD2 binding. Besides the importance of Kv1.3 channel turret, these amino acid residues near the selectivity filter were also found to be critical for hBD2 binding, which was shown by more significant decrease of hBD2 affinity when Asp397, Met398, Pro400 and Val401 residues were mutated into alanine (Fig. 2b, c). These results clearly revealed that hBD2, like basic animal toxins ChTX and ShK, was able to recognize the channel vestibule for inhibition of Kv1.3 channel currents (Supplementary Fig. S1).
hBD2-potassium channel interactions evidenced by co-immunoprecipitation
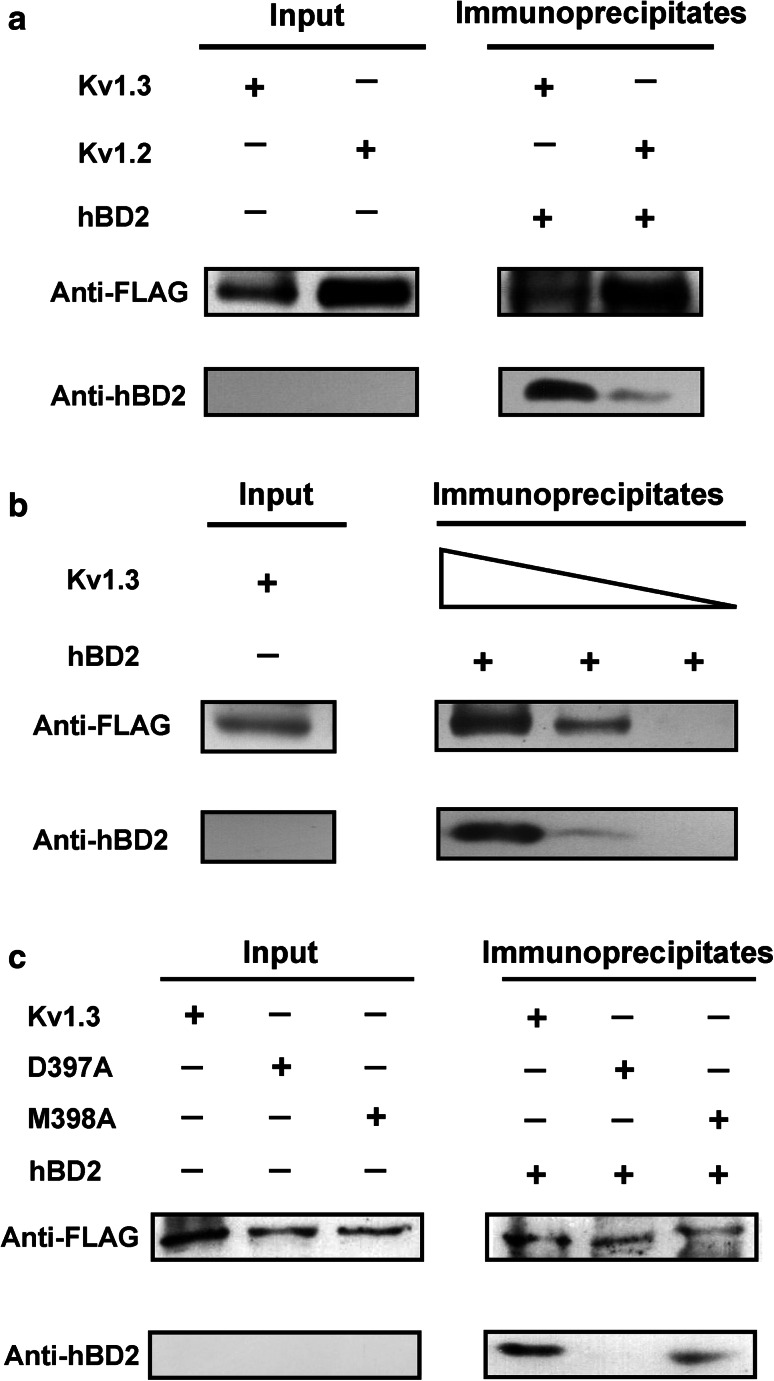
Our pharmacological experiments highlighted clear interactions between hBD2 and potassium channels. To further assess their direct binding, we immobilized FLAG-tagged Kv1.3 channel on protein G-Sepharose beads and detected whether it could retain hBD2. Subsequently, hBD2 was incubated with Kv1.3 channel-coupled Sepharose beads, which was then washed extensively (30 min) before elution of the retained proteins. The elution medium was subjected to western blotting to detect hBD2. Our results demonstrated that, as expected, Kv1.3 channel-coupled Sepharose beads retained hBD2 (Fig. 3a). Due to the weak interaction between hBD2 and Kv1.2 channel (Fig. 1d), the Kv1.2 channel-coupled Sepharose beads were found to retain much less hBD2 (Fig. 3a). It was also noted that the amount of retained hBD2 decreased when lesser amount of FLAG-tagged Kv1.3 channel was immobilized on Sepharose beads (Fig. 3b). In line with the decreased hBD2 affinity towards two mutant Kv1.3-D397A and Kv1.3–M398A channels from the previous electrophysiological experiments (Fig. 2b, c), we found that the substitution of Asp397 by alanine abolished binding, and replacement of Met398 by alanine significantly impaired binding (Fig. 3c). These biochemical results support hBD2-Kv1.3 channel interactions from the previous pharmacological experiments (Figs. 1, 2).
Fig. 3.
The binding of hBD2 to human potassium channels as evidenced by co-immunoprecipitation assay. a Co-immunoprecipitation of Kv1.3 and Kv1.2 channels with hBD2. The complexes formed by hBD2 and potassium channels from HEK293 cell lysates were immunoprecipitated with the anti-FLAG antibody before western blotting analysis using anti-FLAG or anti-hBD2 antibody to detect potassium channel or hBD2 protein, respectively. Complexes were observed with both Kv1.2 and Kv1.3 channels, but Kv1.2 channel retained much less hBD2 than Kv1.3 channel. b The binding of hBD2 to Kv1.3 channel is dose-dependent. The Kv1.3 channel was analyzed for hBD2 binding as described in (a) using protein G-Sepharose beads with decreasing substitution (wedges depict twofold concentration range). c Mutant Kv1.3-D397A and Kv1.3-M398A channels bound less avidly to hBD2. Purified wild-type (Kv1.3) and mutant (Kv1.3-D397A and Kv1.3-M398A) proteins were analyzed for immunoprecipitation with hBD2 as described in (a). Mutant Kv1.3-D397A and Kv1.3-M398A channels abolished and impaired hBD2 binding, respectively
hBD2-induced inhibition of Kv1.3 channel currents and IL-2 production in Jurkat cells
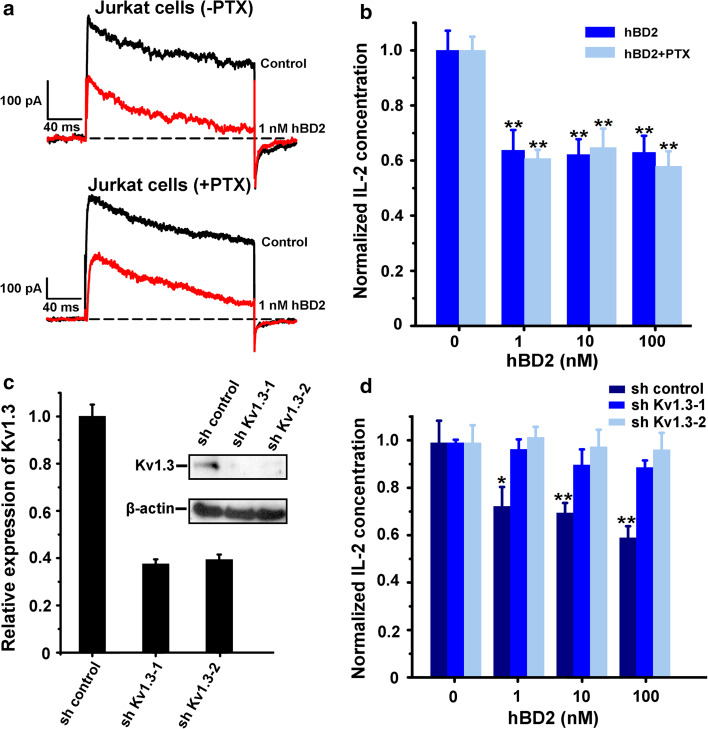
So far, only pertussis toxin (PTX)-sensitive GPCRs were found to be related to hBD2 function in human T cells. PTX could block the migration of HEK293 cells expressing CCR6 and counteract the hBD2-induced stimulation of some cytokine secretion in CD3/28-stimulated T cells [9, 17]. To investigate the functional role of hBD2 in Jurkat cells, we also used PTX to eliminate the effect of GPCR-dependent pathway. As shown in Fig. 4a, 1 nM hBD2 was able to potently inhibit Kv1.3 channel currents in the absence and presence of PTX in Jurkat cells. The PTX-independent blockage of Kv1.3 channels could further suppress IL-2 production, which was also not counteracted by 100 ng/ml PTX (Fig. 4b). Since IL-2 plays a dual role in maintaining tolerance and contributing to immunity, these results indicate that hBD2 is effective in inhibiting Kv1.3 channel currents and regulating the immune response in Jurkat cells with a GPCR-independent pathway.
Fig. 4.
The hBD2 inhibited Kv1.3 channel currents and IL-2 production in Jurkat cells. a 50.1 ± 1.4 and 49.0 ± 3.9 % of Kv1.3 channel currents were inhibited by 1 nM hBD2 in Jurkat cells which were untreated (upper) and pretreated (lower) by 100 ng/ml PTX for 2 h. b Suppression of IL-2 production by hBD2 in Jurkat cells which were untreated or pretreated by 100 ng/ml PTX for 2 h, and activated with PHA (5 μg/ml) and PMA (50 ng/ml). In the absence of hBD2, the amounts of IL-2 were 1,382.5 ± 98.7 pg/ml and 982.2 ± 48.7 pg/ml without and with PTX addition, respectively. c Kv1.3 channel expression in Kv1.3 channel knockdown and control Jurkat cells. Jurkat cells were infected with lentivirus expressing a shRNA specific for Kv1.3 channel (sh Kv1.3-1 and sh Kv1.3-2) or control vector (sh control). Kv1.3 channel knockdown was measured by quantitative RT-PCR (left panel) and western blotting analysis (right panel). d hBD2 could not suppress IL-2 production in Kv1.3 channel knockdown cells. Different effects of hBD2 on IL-2 production between sh Kv1.3 and sh control cells, which were activated with PHA (5 μg/ml) and PMA (50 ng/ml). The data shown are the mean ± SD of three independent experiments; statistically significant inhibitions as compared to lack of drug treatment are determined using Student’s t test. (*p < 0.05,**p < 0.01)
To confirm whether hBD2 suppressed IL-2 production through interacting with Kv1.3 channels in Jurkat cells, the strategy of Kv1.3 channel knockdown was used in this work. By using the recombinant lentiviral particles containing Kv1.3 shRNAs, Kv1.3 channel knockdown cell lines were established, and their differential expression were shown at the mRNA and protein levels (Fig. 4c). Such differential expression of Kv1.3 channels played important roles in IL-2 secretions. In the absence of hBD2, the amounts of IL-2 in sh control were 1270.4 ± 120.0 pg/ml and it reduced to 793.9 ± 59.7 and 879.2 ± 11.6 pg/ml in sh Kv1.3-1 and sh Kv1.3-2 cells, respectively (Fig. 4d). In line with our expectation, IL-2 secretion was still sensitive to hBD2 in the control Jurkat cell line due to higher expression of Kv1.3 channel. However, IL-2 secretion was not suppressed by hBD2 after Kv1.3 channel knockdown in Jurkat cells (Fig. 4d). These differential effects further revealed that hBD2 was able to inhibit IL-2 production through binding to Kv1.3 channels in Jurkat cells.
hBD2-induced inhibition of Kv1.3 channel currents and IL-2 production in T cells and PBMCs from healthy donors and psoriasis patients in vitro
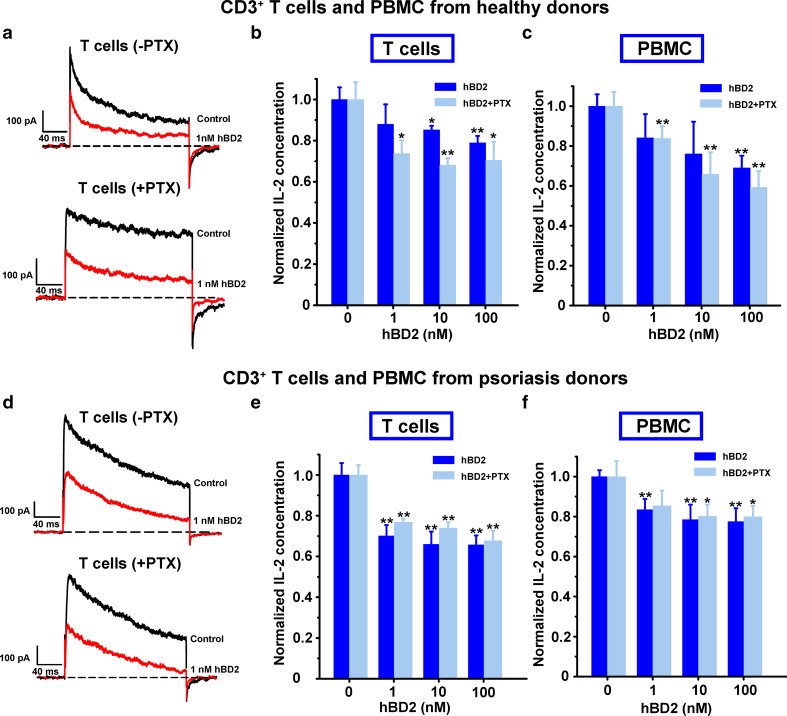
To further evaluate the effect of hBD2 on primary cells, we investigated the inhibitory potential of hBD2 on Kv1.3 channels in T cells from healthy donors. As shown in Fig. 5a, hBD2 was able to inhibit Kv1.3 channel currents in T cells, and such pharmacological effect was not counteracted by PTX. Similar to the effect of hBD2 on Jurkat cells, the blockage of Kv1.3 channels further reduced IL-2 production in T cells stimulated with anti-CD3/28 beads, and the suppression of IL-2 secretion was also regulated in a GPCR-independent pathway (Fig. 5b). Moreover, hBD2 could also reduce IL-2 secretion in PBMCs stimulated with anti-CD3/28 beads (Fig. 5c). The suppression of both Kv1.3 channel currents and IL-2 secretion indicated the immune modulatory role of hBD2 through recognition of Kv1.3 channels in T cells from healthy donors.
Fig. 5.
hBD2 inhibited Kv1.3 channel currents and IL-2 production in T cells and PBMCs from healthy donors and psoriasis patients. a 44.3 ± 3.0 and 43.4 ± 3.0 % of Kv1.3 channel currents were inhibited by 1 nM hBD2 in T cells from healthy donors which were untreated (upper) and pretreated (lower) by 100 ng/ml PTX for 2 h. b hBD2 suppressing IL-2 production in T cells from healthy donors. In the absence of hBD2, the amounts of IL-2 were 1,407.0 ± 82.7 pg/ml and 1,180.1 ± 98.4 pg/ml without and with PTX addition, respectively. c hBD2 suppressing IL-2 production in PBMCs from healthy donors. In the absence of hBD2, the amounts of IL-2 were 600.4 ± 35.5 and 383.5 ± 27.3 pg/ml without and with PTX addition, respectively. d 53.3 ± 0.5 and 49.8 ± 0.4 % of Kv1.3 channel currents were inhibited by 1 nM hBD2 in T cells from psoriasis patients which were untreated (upper) and pretreated (lower) by 100 ng/ml PTX for 2 h. e hBD2 suppressing IL-2 production in T cells from psoriasis patients. In the absence of hBD2, the amounts of IL-2 were 1,422.5 ± 45.2 and 1,294.1 ± 100.1 pg/ml without and with PTX addition, respectively. f hBD2 suppressing IL-2 production in PBMCs from psoriasis patients. In the absence of hBD2, amounts of IL-2 were 466.5 ± 27.1 and 383.1 ± 18.6 pg/ml without and with PTX addition, respectively. The data shown are the mean ± SD from three independent experiments; statistically significant inhibitions compared to the lack of drug treatment are determined using Student’s t test. (*p < 0.05, **p < 0.01)
Next, we investigated the functional role of hBD2 in serious psoriasis patients with PASI score over 20. As shown in Fig. 5d, Kv1.3 channel currents were also inhibited by hBD2 with a GPCR-independent mechanism. Cytokine secretion assay indicated that IL-2 production could be suppressed by hBD2 in anti-CD3/28 antibody-simulated T cells and PBMCs of psoriasis patients, which was not affected by PTX (Fig. 5e, f). This observation was consistent with the phenomenon observed in T cells and PBMCs from healthy donors. These results further revealed that hBD2 modulated the immune response through its interaction with Kv1.3 channels in T cells from psoriasis patients.
Discussion
Except animal toxins as well-known inhibitors of human potassium channels, whether there is a human endogenous peptide inhibitor remains an open question so far. In order to discover a putative human endogenous peptide inhibitor, we focused on hBD2 mainly due to its two remarkable features: one relies on the close contact between human T cells and hBD2 for the immune reaction in the inflamed or infected skin, gastrointestinal and respiratory tracts [8, 9, 17]. Furthermore, hBD2 was also found to be a serum biomarker for disease activity in psoriasis patients [18]. The other feature is the similar basic characteristics between hBD2 and Kv1.3 channel-blocking toxins (Supplementary Fig. S1). Through extensive experiments, we demonstrated a hBD2-Kv1.3 channel interaction, which further resulted in suppression of IL-2 production in Jurkat cells, T cells and PBMCs from healthy donors and psoriasis patients.
First, the interaction between hBD2 and Kv1.3 channel was well-illustrated by pharmacology, co-immunoprecipitation and knockdown experiments. Same as the classical channel pore-blocking toxin inhibitors [13], hBD2 was able to effectively bind to Kv1.3 channel through interacting with the outer channel vestibule instead of S3–S4 linker (Figs. 1, 2, 3). In Kv1.3 channel knockdown cells, hBD2 could not suppress IL-2 secretion, which was different from the inhibitory effect of hBD2 through its interaction with Kv1.3 channels in Jurkat cells (Fig. 4). All these data strongly support the interaction between hBD2 and human Kv1.3 channel.
Second, hBD2 was able to suppress IL-2 secretion for the adaptive immune modulation through interaction with Kv1.3 channels in T cells. Previous work indicated that CCR6 was responsible for the recruitment of memory T cells by hBD2 [9], and unknown PTX-sensitive GPCR(s) affected the secretions of IFN-γ, TNF-α, IL-10, IL-1β, IL-6, IL-22 and IL-17 in anti-CD3/28-stimulated T cells [17]. Here, Kv1.3 channels, as the newly discovered receptor of hBD2, were able to be substantially inhibited by hBD2 in Jurkat cells and T cells from both healthy donors and psoriasis patients, and such pharmacological effect was not counteracted by PTX (Figs. 4, 5). Furthermore, the blockage of Kv1.3 channel currents was able to reduce IL-2 production in PHA/PMA-activated Jurkat cells, as well as in anti-CD3/28-stimulated T cells and PBMCs from both healthy donors and psoriasis patients (Figs. 4, 5). Distinct from the previously tested cytokines [17], IL-2 secretion was not counteracted by PTX during hBD2 inhibition of Kv1.3 channel currents. These results indicate that IL-2 secretion is suppressed upon hBD2 interaction with Kv1.3 channels in T cells, which is in line with the function of toxins blocking Kv1.3 channels [19–21]. This indicates a new immune modulatory function of hBD2 in the immune system.
Third, the pharmacological profile of hBD2 suggests its potential role(s) in pathological conditions. It is known that hBD2 can distribute in the inflamed or infected tissues [8, 9, 17], and even works as a serum biomarker for disease activity in psoriasis patients [18]. The distribution of human Kv1.3 channel is not only limited to human T cells, B cells and dendritic cells [22–24], but can also be observed in other human cell types, such as vascular smooth muscle cells [25] and platelets [26]. Therefore, finding of hBD2 as a selective inhibitor of Kv1.3 channel should accelerate the discovery of ‘novel’ hBD2 functions in the immune system and other fields.
Materials and methods
Ethics statement
The peripheral venous blood obtained from health donors and psoriasis patients were approved by the Ethics Committee of the College of Life Sciences in Wuhan University and Institute of Dermatology in the Chinese Academy of Medical Sciences, respectively.
Electrophysiological recordings
Electrophysiological experiments were carried out using the patch-clamp whole-cell recording mode, at room temperature. The currents through Kv channels [27] and KCNQ1 channels [28] were recorded according to previously published references. Detailed information is provided in the Supplementary materials and methods. HEK293 cells were transfected with appropriate cDNA plasmids using Sofast™ Transfection Reagent (Sunma). Potassium currents were recorded 1–3 days after transfection and cell culture and positive cells were selected based on the presence of GFP fluorescence. Jurkat cells and isolated T cells from PBMCs were patched immediately and directly. Using IGOR software (WaveMetrics, Lake Oswego, OR, USA), concentration vs response relationships were fitted according to the modified Hill equation: Itoxin/Icontrol = 1/1 + ([toxin]/IC50), where I is the peak current and [toxin] is the concentration of toxin. The parameters to be fitted were concentration at half maximal effect (IC50). Results were shown as the mean ± SE of at least three experiments.
Co-immunoprecipitation assay
Cells transfected with FLAG-tagged Kv1.3 and Kv1.2 channels were washed with ice-cold PBS, extracted using Transmembrane protein extraction kit according to the manufacturer’s instructions (Merck). Precleared protein was obtained by incubating cell supernatants with 40 μl protein G-Sepharose bead slurry (GE Healthcare) for 1 h at 4 °C to reduce non-specific binding of proteins to Sepharose beads. After centrifugation, adequate soluble hBD2 was added to the supernatant and incubated under rotation for 4 h at a temperature of 4 °C. Subsequently, 50 μl of protein G-Sepharose beads and 5 μl of anti-FLAG antibody (Sigma) were added, and rotation was continued overnight. After overnight incubation, the beads were washed three times with ice-cold modified RIPA buffer, followed by resuspension of the Sepharose beads in 2 × SDS sample buffer. The samples were separated by SDS-PAGE and then transferred onto Immobilon-P membranes (Millipore) for western analysis.
IL-2 secretion assay
For IL-2 secretion assay, 1 × 105/well of freshly isolated CD3+ T cells were activated using anti-CD3/28 dynabeads (Invitrogen) at a T cell:bead ratio of 1:1 in 200 μl of RPMI medium in 96-well plates. For Jurkat cells, the activating agents were 5 μg/ml PHA and 50 ng/ml PMA. The hBD2 diluted at different concentrations in PBS was added 1–2 h prior to bead stimulation. All assays were done in triplicate. After 16 h activation, cells were counted and supernatants were analyzed for IL-2 concentration by ELISA according to the manufacturer’s instructions (eBiosciences).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This work was supported by grants from the National Basic Research Program of China (No. 2010CB529800), the National Natural Sciences Foundation of China (Nos. 31170789 and 31200557), and Wuhan City Science and Technology Foundation of China (No. 2013070204020046).
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Weishan Yang and Jing Feng are contributed equally to the work.
Contributor Information
Zongyun Chen, Phone: ++86-(0)-27-68752831, Email: chenzy2005@126.com.
Yingliang Wu, Phone: ++86-(0)-27-68752831, Email: ylwu@whu.edu.cn.
References
- 1.Ashcroft FM. From molecule to malady. Nature. 2006;440(7083):440–447. doi: 10.1038/nature04707. [DOI] [PubMed] [Google Scholar]
- 2.Mouhat S, Andreotti N, Jouirou B, Sabatier JM. Animal toxins acting on voltage-gated potassium channels. Curr Pharm Des. 2008;14(24):2503–2518. doi: 10.2174/138161208785777441. [DOI] [PubMed] [Google Scholar]
- 3.Ohno-Shosaku T, Kim I, Sawada S, Yamamoto C. Presence of the voltage-gated potassium channels sensitive to charybdotoxin in inhibitory presynaptic terminals of cultured rat hippocampal neurons. Neurosci Lett. 1996;207(3):195–198. doi: 10.1016/0304-3940(96)12518-0. [DOI] [PubMed] [Google Scholar]
- 4.Han S, Yi H, Yin SJ, Chen ZY, Liu H, Cao ZJ, Wu YL, Li WX. Structural basis of a potent peptide inhibitor designed for Kv1.3 channel, a therapeutic target of autoimmune disease. J Biol Chem. 2008;283(27):19058–19065. doi: 10.1074/jbc.M802054200. [DOI] [PubMed] [Google Scholar]
- 5.Mouhat S, Teodorescu G, Homerick D, Visan V, Wulff H, Wu Y, Grissmer S, Darbon H, De Waard M, Sabatier JM. Pharmacological profiling of Orthochirus scrobiculosus toxin 1 analogs with a trimmed N-terminal domain. Mol Pharmacol. 2006;69(1):354–362. doi: 10.1124/mol.105.017210. [DOI] [PubMed] [Google Scholar]
- 6.Lanigan MD, Pennington MW, Lefievre Y, Rauer H, Norton RS. Designed peptide analogues of the potassium channel blocker ShK toxin. Biochemistry. 2001;40(51):15528–15537. doi: 10.1021/bi011300b. [DOI] [PubMed] [Google Scholar]
- 7.Chen ZY, Hu YT, Yang WS, He YW, Feng J, Wang B, Zhao RM, Ding JP, Cao ZJ, Li WX, Wu YL. Hg1, novel peptide inhibitor specific for Kv1.3 channels from first scorpion Kunitz-type potassium channel toxin family. J Biol Chem. 2012;287(17):13813–13821. doi: 10.1074/jbc.M112.343996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pazgier M, Hoover DM, Yang D, Lu W, Lubkowski J. Human beta-defensins. Cell Mol Life Sci. 2006;63(11):1294–1313. doi: 10.1007/s00018-005-5540-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang D, Chertov O, Bykovskaia SN, Chen Q, Buffo MJ, Shogan J, Anderson M, Schroder JM, Wang JM, Howard OM, Oppenheim JJ. Beta-defensins: linking innate and adaptive immunity through dendritic and T cell CCR6. Science. 1999;286(5439):525–528. doi: 10.1126/science.286.5439.525. [DOI] [PubMed] [Google Scholar]
- 10.Yount NY, Kupferwasser D, Spisni A, Dutz SM, Ramjan ZH, Sharma S, Waring AJ, Yeaman MR. Selective reciprocity in antimicrobial activity versus cytotoxicity of hBD-2 and crotamine. Proc Natl Acad Sci USA. 2009;106(35):14972–14977. doi: 10.1073/pnas.0904465106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu R, Zhang Z, Liu H, Hou P, Lang J, Wang S, Yan H, Li P, Huang Z, Wu H, Rong M, Huang J, Wang H, Lv L, Qiu M, Ding J, Lai R. Human beta-defensin 2 is a novel opener of Ca2+-activated potassium channels and induces vasodilation and hypotension in monkeys. Hypertension. 2013;62(2):415–425. doi: 10.1161/HYPERTENSIONAHA.111.01076. [DOI] [PubMed] [Google Scholar]
- 12.Mouhat S, Jouirou B, Mosbah A, De Waard M, Sabatier JM. Diversity of folds in animal toxins acting on ion channels. Biochem J. 2004;378(Pt 3):717–726. doi: 10.1042/BJ20031860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Banerjee A, Lee A, Campbell E, Mackinnon R. Structure of a pore-blocking toxin in complex with a eukaryotic voltage-dependent K+ channel. Elife. 2013;2:e00594. doi: 10.7554/eLife.00594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rauer H, Pennington M, Cahalan M, Chandy KG. Structural conservation of the pores of calcium-activated and voltage-gated potassium channels determined by a sea anemone toxin. J Biol Chem. 1999;274(31):21885–21892. doi: 10.1074/jbc.274.31.21885. [DOI] [PubMed] [Google Scholar]
- 15.Lee HC, Wang JM, Swartz KJ. Interaction between extracellular Hanatoxin and the resting conformation of the voltage-sensor paddle in Kv channels. Neuron. 2003;40(3):527–536. doi: 10.1016/S0896-6273(03)00636-6. [DOI] [PubMed] [Google Scholar]
- 16.Swartz KJ, MacKinnon R. Mapping the receptor site for hanatoxin, a gating modifier of voltage-dependent K+ channels. Neuron. 1997;18(4):675–682. doi: 10.1016/S0896-6273(00)80307-4. [DOI] [PubMed] [Google Scholar]
- 17.Kanda N, Kamata M, Tada Y, Ishikawa T, Sato S, Watanabe S. Human beta-defensin 2 enhances IFN-gamma and IL-10 production and suppresses IL-17 production in T cells. J Leukoc Biol. 2011;89(6):935–944. doi: 10.1189/jlb.0111004. [DOI] [PubMed] [Google Scholar]
- 18.Jansen PA, Rodijk-Olthuis D, Hollox EJ, Kamsteeg M, Tjabringa GS, de Jongh GJ, van Vlijmen-Willems IM, Bergboer JG, van Rossum MM, de Jong EM, den Heijer M, Evers AW, Bergers M, Armour JA, Zeeuwen PL, Schalkwijk J. Beta-defensin 2 protein is a serum biomarker for disease activity in psoriasis and reaches biologically relevant concentrations in lesional skin. PLoS One. 2009;4(3):e4725. doi: 10.1371/journal.pone.0004725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beeton C, Wulff H, Standifer NE, Azam P, Mullen KM, Pennington MW, Kolski-Andreaco A, Wei E, Grino A, Counts, Wang PH, LeeHealey CJ, SA B, Sankaranarayanan A, Homerick D, Roeck WW, Tehranzadeh J, Stanhope KL, Zimin P, Havel PJ, Griffey S, Knaus HG, Nepom GT, Gutman GA, Calabresi PA, Chandy KG. Kv1.3 channels are a therapeutic target for T cell-mediated autoimmune diseases. Proc Natl Acad Sci USA. 2006;103(46):17414–17419. doi: 10.1073/pnas.0605136103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu L, Pennington M, Jiang Q, Whartenby KA, Calabresi PA. Characterization of the functional properties of the voltage-gated potassium channel Kv1.3 in human CD4+ T lymphocytes. J Immunol. 2007;179(7):4563–4570. doi: 10.4049/jimmunol.179.7.4563. [DOI] [PubMed] [Google Scholar]
- 21.Takacs Z, Toups M, Kollewe A, Johnson E, Cuello LG, Driessens G, Biancalana M, Koide A, Ponte CG, Perozo E, Gajewski TF, Suarez-Kurtz G, Koide S, Goldstein SA. A designer ligand specific for Kv1.3 channels from a scorpion neurotoxin-based library. Proc Natl Acad Sci USA. 2009;106(52):22211–22216. doi: 10.1073/pnas.0910123106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cahalan MD, Chandy KG, DeCoursey TE, Gupta S. A voltage-gated potassium channel in human T lymphocytes. J Physiol. 1985;358:197–237. doi: 10.1113/jphysiol.1985.sp015548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Partiseti M, Choquet D, Diu A, Korn H. Differential regulation of voltage- and calcium-activated potassium channels in human B lymphocytes. J Immunol. 1992;148(11):3361–3368. [PubMed] [Google Scholar]
- 24.Zsiros E, Kis-Toth K, Hajdu P, Gaspar R, Bielanska J, Felipe A, Rajnavolgyi E, Panyi G. Developmental switch of the expression of ion channels in human dendritic cells. J Immunol. 2009;183(7):4483–4492. doi: 10.4049/jimmunol.0803003. [DOI] [PubMed] [Google Scholar]
- 25.Cheong A, Li J, Sukumar P, Kumar B, Zeng F, Riches K, Munsch C, Wood IC, Porter KE, Beech DJ. Potent suppression of vascular smooth muscle cell migration and human neointimal hyperplasia by Kv1.3 channel blockers. Cardiovasc Res. 2011;89(2):282–289. doi: 10.1093/cvr/cvq305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCloskey C, Jones S, Amisten S, Snowden RT, Kaczmarek LK, Erlinge D, Goodall AH, Forsythe ID, Mahaut-Smith MP. Kv1.3 is the exclusive voltage-gated K+ channel of platelets and megakaryocytes: roles in membrane potential, Ca2+ signalling and platelet count. J Physiol. 2010;588(Pt 9):1399–1406. doi: 10.1113/jphysiol.2010.188136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yin SJ, Jiang L, Yi H, Han S, Yang DW, Liu ML, Liu H, Cao ZJ, Wu YL, Li WX. Different residues in channel turret determining the selectivity of ADWX-1 inhibitor peptide between Kv1.1 and Kv1.3 channels. J Proteome Res. 2008;7(11):4890–4897. doi: 10.1021/pr800494a. [DOI] [PubMed] [Google Scholar]
- 28.Chen ZY, Zeng DY, Hu YT, He YW, Pan N, Ding JP, Cao ZJ, Liu ML, Li WX, Yi H, Jiang L, Wu YL. Structural and functional diversity of acidic scorpion potassium channel toxins. PLoS One. 2012;7(4):e35154. doi: 10.1371/journal.pone.0035154. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.