Abstract
The vast majority of mammalian testes are located outside the body cavity for proper thermoregulation. Heat has an adverse effect on mammalian spermatogenesis and eventually leads to sub- or infertility. Recent studies have provided insights into the molecular response of male germ cells to high temperatures. Here, we review the effects of heat on male germ cells and discuss the mechanisms underlying germ cell loss and impairment. We also discuss the role of translational control in male germ cells as a potential protective mechanism against heat-induced germ cell apoptosis.
Keywords: Hyperthermia, Spermatogenesis, Infertility, Heat stress, Germ cell apoptosis, DNA damage, Stress granules
Introduction
Spermatogenesis is a highly coordinated process entailing cell division and differentiation for the production of haploid male germ cells from diploid progenitor cells. Mammalian spermatogenesis can be divided into pre-meiotic, meiotic, and post-meiotic phases. In the pre-meiotic phase, diploid spermatogonia proliferate mitotically and differentiate into spermatocytes. A primary spermatocyte produces four haploid round spermatids through two meiotic divisions. In the post-meiotic phase of spermiogenesis, round spermatids undergo morphological changes leading to elongated spermatids and mature spermatozoa [1, 2]. This process takes ~35 days in mice and ~75 days in humans and continues throughout life, ensuring a continuous supply of sperm in males [3].
In most mammals, the testes where spermatogenesis takes place are located in the scrotum, outside the body cavity. If the testis fails to descend into the scrotum during development, it is exposed to an elevated temperature and loses germ cells [4–6]. The scrotum is generally 2–7 °C cooler than the core body temperature, and the temperature of the testis is regulated by a heat-exchange system [7–12]. Although the testes of some mammals, including elephants, edentates, and cetaceans, are located within the abdomen, the testes in these animals appear to be cooled by special blood vessels, at least in dolphins and seals [13–16]. Thus, tight thermoregulation of the testis is essential for spermatogenesis, but it remains unclear why most mammals have evolved to maintain their testes at low temperatures [17, 18].
A number of studies conducted over the past several decades have documented the adverse effects of heat on spermatogenesis in diverse mammal species [19–28]. Recent studies have provided new insights into the underlying molecular and cellular mechanisms. In this review, we focus on the effect of heat on male germ cells and the molecular mechanisms involved in this process. We further highlight recent advances in our understanding of the heat stress response of male germ cells. Experimental techniques and physiological outcomes of heat stress on mammalian testes have been thoroughly reviewed elsewhere [17, 29].
Effect of heat stress on mammalian male germ cells
Germ cell apoptosis
The most significant consequence of the heat stress on the testis is the loss of germ cells via apoptosis. Experimental cryptorchidism, in which one or both testes are surgically exposed to abdominal temperatures, induces DNA fragmentation in germ cells [30–32]. A short exposure of the testes to heat by immersing the scrotum in a hot water bath at 43 °C for 15–20 min also results in germ cell apoptosis [33, 34]. In addition, isolated male germ cells from immature rats undergo apoptosis when maintained at 43 °C for 1 h [35]. The tumor suppressor p53 is a potential inducer of germ cell apoptosis in response to heat. Testicular p53 level is elevated after heat stress and is associated with germ cell loss [36–38]. When cryptorchidism was induced in p53 knockout mice, male germ cell death was delayed but still occurred, suggesting that germ cell apoptosis is mediated by both p53-dependent and p53-independent pathways [39]. Fas may be responsible for the p53-independent phase of germ cell apoptosis [40]. However, subsequent studies have shown that apoptosis is not blocked in gld (Fas ligand-defective) and lpr cg (Fas-defective) mice, suggesting that the Fas signaling system is not necessary for heat-induced germ cell apoptosis [41–43].
Recent studies have revealed molecular details of germ cell apoptosis triggered by heat stress. A single instance of scrotal heat stress induces a number of pro-apoptotic processes in male germ cells, such as: relocation of pro-apoptotic Bax (Bcl-2-associated x protein) into nuclear or perinuclear regions; upregulation and phosphorylation of anti-apoptotic Bcl-2 (B cell leukemia/lymphoma 2); cytosolic translocation of cytochrome c and DIABLO (direct inhibitor of apoptosis-binding protein with low pI); activation of the initiator caspase 9 and the executioner caspases 3, 6 and 7; and cleavage of PARP (poly(ADP-ribose) polymerase) [41–45]. Q-VD-OPH and minocycline, inhibitors of caspases and cytochrome c release, respectively, attenuate heat-induced germ cell apoptosis [46, 47]. Nitric oxide synthase (NOS), p38 mitogen-activated protein kinase (MAPK), and caspase 2 were identified as potential upstream signals of this apoptotic pathway. Studies employing genetic ablation and overexpression of NOS as well as treatment with a NOS inhibitor revealed that NOS is involved in male germ cell apoptosis under heat stress [48–50]. Furthermore, p38 MAPK appears to act upstream of NOS because inhibition of p38 MAPK suppresses NOS induction and cytochrome c release and eventually confers resistance to germ cell apoptosis in heat stress conditions [45]. Caspase 2 activation is considered to be upstream of the p38 MAPK pathway because a specific inhibitor of caspase 2 prevents heat-induced activation of p38 and NOS [51]. Taken together, these results suggest that the mitochondria-dependent apoptotic pathway, triggered by activation of upstream signals such as p38 MAPK, is critical for heat-induced germ cell death.
Germ cell apoptosis in response to heat stress occurs in a developmental stage-specific manner. A spatio-temporal rhythm of mammalian spermatogenesis creates a cycle of seminiferous tubules in which certain types of male germ cells are associated with specific developmental stages [52]. In adult rats, for example, there are 14 tubular stages, and germ cell apoptosis is prominent in early (I–IV) and late (XII–XIV) stages after heat treatment at 43 °C for 15 min. The most affected germ cell types are the pachytene and diplotene spermatocytes and the early round spermatids [33]. Similar results have been observed in experimental cryptorchidism of mice and rats [30, 32, 53]. Thus, it is generally accepted that pachytene and diplotene spermatocytes and early spermatids are the most susceptible to heat [17]. It is not known why only certain types of male germ cells are selectively affected by heat. It has been proposed that intratesticular testosterone protects germ cells at the relevant stages (VII–VIII) against heat-induced germ cell apoptosis [33]. A recent study provided evidence for another possibility: stress granules are formed in spermatocytes and spermatogonia after heat stress and confer resistance to apoptosis by suppressing the p38 MAPK pathway [54].
The intensity of heat stress determines the timing of germ cell apoptosis. In mice, for example, the number of apoptotic germ cells begins to increase 6–7 days after experimental cryptorchidism (i.e., exposure to abdominal temperatures) [32], whereas apoptotic germ cells are first observed 8 h after exposure at 43 °C for 20 min [34]. In contrast, a short exposure of the testes to lower temperatures of 39–40 °C has no obvious effect on germ cell death [34]. Thus, male germ cells seem to have a threshold of time–temperature dosage for apoptosis [18, 55]. Consistently, no apoptotic germ cells appear after a 10-min exposure of rat testes at 43 °C, but apoptotic germ cells appear within a few days after a 15-min exposure [20]. Apoptosis is further intensified by increasing the exposure time up to 30 min [20]. Fertility has been adversely affected by repeated exposure of testes to heat, albeit normal after a single exposure [56], suggesting the requirement of a time–temperature dosage for germ cell apoptosis.
Sperm DNA damage
Heat-induced sub- or infertility in males is largely attributed to a reduction in the sperm count via germ cell apoptosis, but there is a strong line of evidence that the sperm DNA integrity is also affected by heat. Sperm DNA damage, as determined by comet or sperm chromatin structure assays, is detected in mature spermatozoa a few days as well as weeks after the heat stress [57–60]. This result indicates that the chromatin integrity of male germ cells at the epididymal sperm stage and of developing spermatocytes is affected by heat. Indeed, heat stress induces defects in DNA synapsis and DNA strand breaks in pachytene spermatocytes [60]. A failure in the X and Y chromosome pairing has also been noted in primary spermatocytes [61, 62]. These damaged spermatocytes may avoid heat-induced apoptosis and develop to mature spermatozoa with defects in their chromatin integrity.
It is well known that oxidative stress, which is caused by the excessive generation of reactive oxygen species (ROS), can also induce DNA damage in spermatozoa [63–66]. Heat stress is implicated in the induction of oxidative stress within the testis [35, 67–69]. Interestingly, several antioxidants have attenuated heat-induced germ cell apoptosis [35, 70]. Furthermore, male germ cells from mice lacking a superoxide dismutase are highly sensitive to heat stress [71]. These studies have not directly evaluated sperm DNA damage, but the treatments would be expected to influence the levels of DNA damage in germ cells. It is also considered that sperm DNA damage can be caused by impaired DNA repair system in spermatocytes [60, 72]. A microarray-based study has shown that some genes for DNA repair and cellular antioxidants are down-regulated in the heat-stressed testis [34]. Taken together, these results suggest that heat stress induces DNA damage in germ cells by increasing ROS and suppressing gene expression associated with defense mechanisms against DNA damage.
It has long been noted that fertilization and normal embryonic development are affected by paternal heat stress. When embryos are sired by heated males, their mortality rate increases [25, 34, 73–75] and their growth is frequently retarded [60, 76–79]. When in vitro fertilization is performed with the sperm of males exposed to heat, the sperm penetration rate, fertilization rate, and pronuclear formation rate decrease [76, 79–81], and defects in early embryonic development are observed [60, 82]. These defects may reflect harmful effects of sperm DNA damage on diverse aspects of fertility, though these studies did not explicitly show a link between DNA damage and fertility outcomes. Future studies should be conducted to determine whether sperm DNA damage is responsible for these deleterious phenotypes.
Other effects
The male germ cell is not the only cell type affected by heat; testicular somatic cells, specifically, Sertoli and Leydig cells, also respond to heat stress. Sertoli and Leydig cells, whose main functions are to support germ cells and regulate steroidogenesis, respectively, provide germ cells with the proper environment for development. Any misregulation of their functions might influence male germ cell development. A decrease in the production of testicular androgen binding protein after experimental cryptorchidism suggests that heat has an adverse effect on Sertoli cells [83, 84]. Recent work has revealed that the expression of some molecules involved in Sertoli cell function is affected by hyperthermic conditions [85–89]. In contrast, it was believed that Leydig cells are not affected by heat [29]. However, degenerative morphology of Leydig cells was observed after six consecutive daily exposures of rat testes to 43 °C for 30 min [90, 91]. In addition, there is evidence that HIF1A (hypoxia-inducible factor 1 alpha) and HMOX1 (heme oxygenase 1) are up-regulated in Leydig cells, suggesting that heat induces hypoxia and oxidative stress in these cells [69, 92]. Therefore, it is possible that heat may indirectly harm germ cells by modifying somatic cell functions.
Elevated temperature has also been reported to increase the rate of spermatocyte differentiation and the progression of spermatogenesis [93, 94]. The reduced cycle of spermatogenesis may lead to the disruption of spermatogenic cells. However, it is still unknown how heat modulates the timing of the spermatogenic cycle.
Testis is known to recover from the heat damage with time. However, the scar can be too deep to be fully recovered. Testis loses its weight after a single heat exposure and then gains its weight about 40 days later, but the weight does not return to normal level even 60 or more days later [95, 96]. In a study with an extended observation, testis weight was recovered up to 70 % of control at 97 days after heating, but there was a second fall to 50 % at 182 days later [97]. The initial decline in testis weight after heat stress is largely attributed to the apoptosis of heat-susceptible germ cells discussed above. However, the existence of a second fall in testis weight after heating suggests the possibility that heat also affects the differentiation and development of spermatogonia by unknown mechanisms [97, 98].
Gene expression changes in male germ cells after heat stress
Hyperthermic effects on male germ cells lead to changes in gene expression, posttranslational modification, and protein localization. Several studies on such changes have focused on identifying the underlying molecular mechanisms of thermal effects on spermatogenesis. In this section, we summarize the molecular changes so far examined, which have been confirmed in male germ cells with defined regimes of heat stress. Although DNA microarray [34, 68] and proteomic analysis [99, 100] of heat-treated testes can, in an unbiased manner, provide valuable information for our understanding of how male germ cells respond to elevated temperature, we do not address these data in our discussion to avoid confounding effects originating from the testicular somatic cells.
Genes implicated in apoptosis
Apoptosis is a crucial cellular response for the maintenance and homeostasis of organisms, and, as such, its pathway is highly conserved in a metazoan lineage [101]. As we have discussed, many conserved factors of the intrinsic death pathway are involved in heat-induced germ cell apoptosis. Accordingly, heat stress triggers molecular changes that reflect the ongoing process of apoptosis in male germ cells (Table 1). Many factors that positively induce apoptosis are up-regulated [37, 41, 44, 45, 48, 51, 102–108], while some are down-regulated [37, 102]. Other molecular changes include protein translocation [41, 43–45, 47, 51, 105], modifications such as acetylation and phosphorylation [45, 51, 102, 105, 107, 109], and cleavage [41, 43, 47, 51, 69], all of which are prominent features of apoptosis.
Table 1.
Molecular changes in male germ cells after heat stress
| Gene | Name | Function | Change | Level | Heat stress | References |
|---|---|---|---|---|---|---|
| Apoptosis-related genes | ||||||
| Fas | TNF receptor superfamily member 6 | Induction of apoptosis | Up | mRNA/protein | EC, LH | [40, 104] |
| Noa | Protein | LH | [41, 105] | |||
| Htra2 | HtrA serine peptidase 2 | Proteolysis | Up | mRNA/protein | EC | [103] |
| Nos2 | Nitric oxide synthase 2 | Oxidation–reduction process | Up | Protein | LH | [45, 48, 51, 106] |
| p21 | Cyclin-dependent kinase inhibitor 1A | DNA damage response | Up | mRNA/protein | EC, IH | [102, 107] |
| p53 | Transformation related protein 53 | DNA damage response | Up | mRNA/protein | EC, IH, LH | [37, 102, 107] |
| Acetylation | Protein | IH | [107] | |||
| Phlda1 | Pleckstrin homology-like domain, family A, member 1 | Induction of apoptosis, FasL biosynthetic process | Up | mRNA/protein | EC, LH | [104, 108] |
| Bcl-2 | B cell leukemia/lymphoma 2 | Anti-apoptosis | Up | Protein | LH | [41, 44, 105] |
| Phosphorylation | Protein | LH | [45, 105] | |||
| Nr2c1 | Nuclear receptor subfamily 2, group C, member 1 | Regulation of transcription | Down | mRNA | EC | [102] |
| Noa | mRNA | LH | [37] | |||
| Nr2c2 | Nuclear receptor subfamily 2, group C, member 2 | Regulation of transcription | Down | mRNA | EC, LH | [37, 102] |
| Nr4a1 | Nuclear receptor subfamily 4, group A, member 1 | Regulation of transcription | Down | mRNA | LH | [37] |
| Noa | mRNA | EC | [102] | |||
| Bax | Bcl-2-associated X protein | Apoptotic mitochondrial changes | Translocation | Protein | LH | [41, 43, 44, 105] |
| Cyc | Cytochrome c | Apoptotic process, electron carrier activity | Translocation | Protein | LH | [41, 43, 45, 51, 105] |
| Diablo | Direct inhibitor of apoptosis-binding protein with low pI | Induction of apoptosis | Translocation | Protein | LH | [43, 47, 105] |
| Erk | Mitogen-activated protein kinase 1/3 | Stress-activated MAPK cascade | Phosphorylation | Protein | LH | [45, 105] |
| p38 | Mitogen-activated protein kinase 14 | Stress-activated MAPK cascade | Phosphorylation | Protein | LH | [45, 51, 109] |
| Rb | Retinoblastoma 1 | Negative regulation of cell cycle | Hypo-phosphorylation | Protein | EC | [102] |
| Casp2 | Caspase 2 | Induction of apoptosis | Cleavage | Protein | LH | [51] |
| Casp3 | Caspase 3 | Induction of apoptosis | Cleavage | Protein | LH | [41, 43, 47, 51, 69] |
| Casp9 | Caspase 9 | Induction of apoptosis | Cleavage | Protein | LH | [41, 43, 47, 51] |
| Parp1 | Poly (ADP-ribose) polymerase family, member 1 | DNA repair | Cleavage | Protein | LH | [41, 47] |
| Heat shock protein-related genes | ||||||
| Hsp60 (Hspd1) | Heat shock protein 1 (chaperonin) | Response to unfolded protein | Up | mRNA/protein | LH | [117] |
| Hsp70-1 (Hspa1b) | Heat shock protein 1B | Response to heat, anti-apoptosis | Upb | mRNA/protein | LH | [34] |
| Hsp70-3 (Hspa1a) | Heat shock protein 1A | Response to heat, DNA repair | Up | mRNA/protein | IH, LH | [34, 114, 115, 118] |
| Hsp105 (Hsph1) | Heat shock 105 kDa/110 kDa protein 1 | Response to unfolded protein | Upb | Protein | IH | [116] |
| Translocation | Protein | EC | [116] | |||
| Downa | mRNA/protein | LH | [117] | |||
| Hsp70-2 (Hspa2) | Heat shock protein 2 | Male meiosis, response to stress | Down | mRNA/protein | EC, LH | [27, 118, 178] |
| Noa | mRNA/protein | IH, LH | [120, 179] | |||
| Hsc70t (Hspa1 l) | Heat shock protein 1-like | Response to stress | Down | mRNA | LH | [118] |
| Hsf1 | Heat shock factor 1 | Response to heat, regulation of transcription | Down | mRNA/protein | EC | [108] |
| DNA binding activity | Protein | IH, LH, EC | [115, 119, 120] | |||
| Hsf2 | Heat shock factor 2 | Regulation of transcription | Down | mRNA/protein | EC | [108] |
| Other genes | ||||||
| Capn2 | Calpain 2 | Proteolysis | Up | mRNA | LH | [109] |
| Ptgs2 | Prostaglandin-endoperoxide synthase 2 | Oxidation–reduction process | Up | mRNA/protein | EC | [125] |
| Ucp2 | Uncoupling protein 2 | Response to superoxide | Up | Protein | LH | [124] |
| Capn11 | Calpain 11 | Proteolysis | Down | mRNA | LH | [109] |
| Ccnb1 | Cyclin B1 | Regulation of cell cycle | Down | Protein | EC | [127] |
| Cdk1 | Cyclin-dependent kinase 1 | Regulation of cell cycle | Down | Protein | EC, LH | [127, 129] |
| Cirp | Cold inducible RNA binding protein | Stress response, translational regulation | Down | mRNA/protein | IH, LH, EC | [59, 180] |
| Csda | Cold shock domain protein A | Regulation of transcription | Down | mRNA/protein | IH, LH, EC | [181] |
| Lig3 | DNA ligase III | DNA repair | Down | Protein | EC | [128] |
| Mta1 | Metastasis associated 1 | Regulation of transcription | Down | mRNA/protein | IH, LH, EC | [107, 182] |
| Pde4a | Phosphodiesterase 4A, cAMP specific | Metabolic process | Down | Protein | EC | [183] |
| Polb | DNA polymerase beta | DNA repair | Down | mRNA | EC | [126] |
| Prm2 | Protamine 2 | Chromosome condensation | Down | mRNA | EC, LH | [181] |
| Eif2s1 | Eukaryotic translation initiation factor 2, subunit 1 alpha | Regulation of translation | Phosphorylation | Protein | LH | [54] |
Heat shock protein genes
In response to heat stress, heat shock proteins (HSPs) play protective roles in preventing the nonspecific aggregation and thermal denaturation of cellular proteins. Protective mechanisms of HSPs, such as molecular chaperones, are conserved among all living cells [110]. Due to their vital function in heat-protective mechanisms, HSPs have attracted much attention in the field of male germ cell research. Two types of HSPs are identified in male germ cells: constitutively expressed HSPs and heat-inducible HSPs [111]. The first group of HSPs, which includes Hsp70-2, is not heat-inducible and seems to be developmentally regulated to function in normal spermatogenesis [112, 113]. The latter group includes Hsp70-1 and Hsp70-3, whose expression is induced in response to various thermal conditions [34, 114–118] (Table 1).
In general, heat-inducible HSPs are activated by heat shock transcription factors (HSFs). HSF1 undergoes protein modification and is thus activated in heat-stressed spermatocytes [115]. Considering the central role of HSPs in cellular thermotolerance, one would expect that the heat-inducible HSPs triggered by HSF1 protect against the adverse effects of heat. Currently, however, there is no direct evidence to support this view. Rather, studies have shown that HSF1 promotes germ cell death independent of the activation of HSP genes [104, 119, 120] and that constitutive expression of the heat-inducible Hsp70-1 in spermatocytes protects against neither heat-induced nor HSF1-induced apoptosis [121, 122]. Although the opposite role of HSF1 as a cell survival factor in spermatogonia has been suggested, this is also independent of the activation of HSP genes [120]. A recent study has identified numerous meiotic genes to be regulated by HSF1 in oocyte maturation [123]. Thus, it is possible that the HSF1–HSPs pathway is differently regulated in somatic and male germ cells, but this issue remains to be further investigated.
Other genes
The expression of other genes in male germ cells also appears to be affected by heat stress. A few genes that are possibly related to apoptotic processes are up-regulated [109, 124, 125], whereas genes for DNA repair and cell cycle regulation are down-regulated after heat stress [126–129]. It is possible that the down-regulation of DNA repair genes impairs the chromatin integrity of sperm in heat stress conditions. However, in most cases, it is not clear whether the down-regulation of gene expression contributes to germ cell death or survival or whether the decreased expression merely reflects impaired function due to cellular damage.
Translational control in male germ cells after heat stress
The regulation of translation plays a pivotal role in cellular response to unfavorable, stressful conditions. Translational regulation allows the stressed cells to respond immediately and selectively via rapid changes in protein levels [130]. Several lines of investigation have revealed that male germ cells exposed to heat stress undergo changes in gene expression at the level of translation. It has been reported that heat reduces the incorporation of amino acids into proteins and polysome formation [131–133]. The decline of protein synthesis is linked to a limited formation of the translation initiation complex [134]. A recent study provides a possible explanation of the molecular and cellular mechanisms responsible for these translational changes: a short exposure of mouse testes at 37 °C for 20 min triggers an immediate phosphorylation of eIF2α, the α subunit of eukaryotic translation initiation factor 2, and the stage-specific formation of stress granules (SGs) in male germ cells [54]. The implications of this finding will be discussed with respect to germ cell survival and a lowered set point for heat stress response.
eIF2α phosphorylation
Various stresses (e.g., nutritional deprivation, viral infection, UV irradiation, hypoxia, oxidative stress, and heat) are known to trigger eIF2α phosphorylation at residue Ser-51 in eukaryotic cells. Phosphorylation of eIF2α inhibits the catalytic activity of eIF2B, which converts a GDP-bound eIF2 into an active GTP-bound eIF2 and thereby reduces the formation of the eIF2-GTP-Met-tRNAi ternary complex, leading to the global attenuation of translation initiation [135–137]. Paradoxically, reduction of the ternary complex formation by eIF2α phosphorylation can also elicit selective translation of a few mRNAs involved in stress response. Such selective translation under stress is well understood for two transcriptional activators, GCN4 (general control non-derepressible 4, an activator of amino acid biosynthetic genes) [138] and ATF4 (activating transcription factor 4, an activator of unfolded protein response genes) [139] (for the regulatory mechanisms, see [130, 137]). Taken together, these mechanisms are thought to promote adaptive stress response for cell survival against adverse circumstances. However, some evidence also implicates eIF2α phosphorylation in apoptosis under stress conditions such as proteasome inhibition and hypertonicity [140, 141]. Thus, the effects of eIF2α phosphorylation on cells appear to be dependent on stress conditions. A recent study of eIF2α phosphorylation in heat-stressed testes [54] suggests that male germ cells can be controlled by similar molecular mechanisms. Heat induces eIF2α phosphorylation in male germ cells, and this effect, in turn, might attenuate general translation by reducing the availability of the ternary complex; this effect might also enhance the translation of selective mRNAs implicated in the adaptive stress response. This scenario could explain the previous observations of decreased incorporation of amino acids, reduction of polysomes, and slower formation of the translation initiation complex in heat-stressed spermatogenic cells [131–134]. Consequently, eIF2α phosphorylation may serve as a trigger of cellular survival pathways in male germ cells under heat stress, although its contribution to germ cell apoptosis cannot be excluded.
In the mouse testis, eIF2α can even be phosphorylated at 37 °C, which is similar to the core body temperature, and it has been shown that germ cells are highly responsive to its phosphorylation [54]. This result is interesting because a higher temperature, usually 43–44 °C, is required to induce eIF2α phosphorylation in mammalian somatic cells. A similar, lowered temperature threshold for molecular changes in male germ cells has been noticed in HSF1 activation. It has been shown that HSF1 is even activated at 35–38 °C in male germ cells but is activated at 42 °C in somatic cells [115, 142]; the activity of HSF1 is facilitated by posttranscriptional modifications, such as phosphorylation, at many residues, although how HSF1 senses heat stress is poorly understood [143]. Therefore, to understand how male germ cells have adapted to have a lowered temperature threshold, it would be useful to find the kinases that regulate phosphorylation in response to mild heat stress. In mammalian cells, the phosphorylation of eIF2α is mediated by four different stress-sensing kinases: (1) HRI (heme-regulated inhibitor), which is activated by heme deprivation as well as oxidative, osmotic, and heat stress in erythrocytes [144, 145]; (2) PKR (double-stranded RNA-dependent protein kinase), which is activated by viral infection [146]; (3) PERK (PKR-like ER kinase), which is activated by unfolded proteins in the endoplasmic reticulum (ER stress) [147]; and (4) GCN2 (general control non-derepressible 2), which is responsive to amino acid deficiency and UV irradiation [148, 149]. With a conserved catalytic domain, all four kinases specifically phosphorylate eIF2α on Ser-51 [137]. Thus, it is possible that one or more kinases also control eIF2α phosphorylation in heat-stressed male germ cells. One such candidate may be HRI: it has been shown that HRI is activated in heat-shocked erythroid cells [145], and Hri2p, a HRI-related enzyme, is required for eIF2α phosphorylation by heat shock in the fission yeast, Schizosaccharomyces pombe [150]. Alternatively, there may be a novel, testicular version of an eIF2α kinase in male germ cells.
SG formation
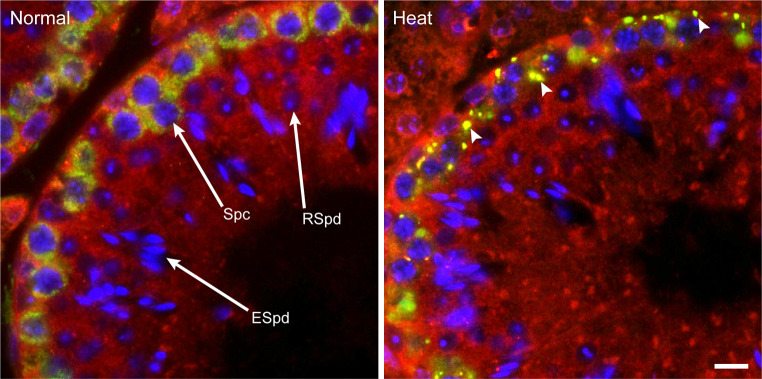
In addition to eIF2α phosphorylation, heat stress induces the transient formation of SGs in mouse male germ cells (Fig. 1). SGs, non-membranous cytoplasmic particles that contain untranslated messenger ribonucleoproteins (mRNPs), are transiently accumulated in response to adverse environmental stress [151]. Consistently, the core components of SGs are the stalled pre-initiation 48S complexes including polyadenylated mRNAs, 40S ribosomal subunits, and translation initiation factors such as eIF3, eIF4E, and eIF4G [152, 153]. In addition, proteins that regulate RNA metabolism, such as RNA-binding proteins, RNA helicases and mRNA-editing enzymes, microRNAs, and some signaling molecules have been shown to accumulate in SGs [154]. Because the mRNPs of SGs are in dynamic equilibrium with the polysomes [155], the assembly and disassembly of SGs are predominantly regulated by the status of the translational machinery: stress induces the phosphorylation of eIF2α, which in turn triggers SG assembly by preventing translation initiation, while recovery after non-lethal stress induces rapid disassembly of SGs [152]. SGs are thought to function in the regulation of mRNAs, such as their storage, degradation, or translation reinitiation during stress and recovery conditions [156]. SGs have also been thought to regulate cell survival by recruiting signaling molecules into SGs [157, 158]. In this regard, SGs of male germ cells are likely to play roles in cell survival against heat stress by protecting or regulating translationally dormant mRNAs and by sequestering proteins that regulate signaling pathways involved in apoptosis.
Fig. 1.
Formation of stress granules (SGs) in male germ cells. Adult mouse testes of control (Normal) and heated at 42 °C for 20 min (Heat) were costained for two robust markers of SGs, DAZL (green) and eIF3 (red). DNA was stained with DAPI (blue). The images were taken from the seminiferous epithelial stage IV. Arrowheads indicate SGs. Note that SGs were predominantly formed in pachytene spermatocytes. Spc spermatocyte; RSpd round spermatid; ESpd elongated spermatid. Scale bar 10 μm
How are SGs assembled in male germ cells? Using a systematic RNAi screen, several factors involved in SG assembly have been identified in cultured cells [159]. In male germ cells, however, we so far have only one such example. It has been shown that DAZL (deleted in azoospermia-like), a germ cell-specific translational regulator, localizes to SGs upon heat stress (Fig. 1) and is required for SG formation in male germ cells. DAZL is thought to act downstream of eIF2α phosphorylation for SG assembly because eIF2α was phosphorylated at a similar level upon heat stress even in the absence of DAZL [54]. Dazl belongs to the evolutionarily conserved DAZ gene family: in humans, for example, there are four copies of DAZ genes on the canonical Y chromosome that are expressed in the testis [160, 161] and two autosomal homologues, DAZL and BOULE [162, 163]. Deficiency of the DAZ gene family members has been shown to lead to infertility in many animals [164–168], indicating their conserved functions in germ cell development. Recent work has also revealed the functional roles of DAZ family genes in meiotic initiation and progression and early embryonic development [169–171]. The molecular function of the RNA-binding protein DAZL has been suggested to activate translation and facilitate transport of specific mRNAs [171–175]. Therefore, DAZL seems to contribute to germ cell development by translational regulation of its bound mRNAs in normal conditions as well as by SG formation and, possibly, accompanied translational regulation in heat stress conditions. It is still unknown whether the SG formation activity of DAZL is shared by other DAZ family proteins. This is of interest because BOULE, for example, is highly expressed in later stages of germ cell development than those showing DAZL expression [163, 176]. This issue warrants further investigation.
In male germ cells, SG formation occurs only in selective germ cell types (Fig. 1). For example, DAZL-positive SGs have been found in spermatogonia, preleptotene, and early pachytene spermatocytes, but not in leptotene, zygotene, and late pachytene spermatocytes or in spermatids of all stages [54]. Although the reason SGs are selectively induced in certain cell types remains elusive, this finding suggests a possible link between SG formation and heat-induced germ cell apoptosis with respect to their cell type specificity. SG formation has been shown to inhibit apoptosis by suppressing the p38 MAPK signaling pathway in cultured cells [158], and p38 MAPK is involved in germ cell apoptosis after heat stress [45]. As we have already discussed, heat-induced germ cell apoptosis is stage and cell type specific. Several lines of evidence support the hypothesis that SG formation inhibits germ cell apoptosis in a male germ-line: there is a negative correlation between the cell types that form SGs and those that undergo apoptosis after heat stress, in which SG formation occurs predominantly in early pachytene spermatocytes and apoptosis in late pachytene spermatocytes; a higher rate of apoptosis is induced in the Dazl knockout testes that have shown impaired SG formation; RACK1 (receptor for activated protein kinase C), an activator of the stress-responsive p38 MAPK pathway, is sequestered into SGs in wild-type germ cells but not in the Dazl knockout cells; and p38 MAPK activation is up-regulated in germ cells that contain SGs [54]. Thus, SGs are likely to prevent the male germ cells that harbor them from undergoing apoptosis after heat stress. To better understand the cell type specificity of SGs, more SG markers should be identified and tested for in the male germ cells that contain SGs. Furthermore, studies that find novel factors involved in SG formation and mutants showing abortive SG formation in their germ cells will help to reveal the relationship between SG formation and germ cell apoptosis.
Conclusion and future studies
Testes contain the spermatogenic cells at all stages of maturation, as well as somatic cells, both of which can affect the overall fertility of an animal exposed to heat stress (Fig. 2). The heat-susceptible germ cells undergo either apoptosis through the mitochondrial death pathway or DNA damage even if they have escaped apoptosis. The sperm in the epididymis is also affected by heat, which leads to sperm DNA damage that might result in subfertility of the affected male. Groups of the heat-resistant germ cells often form SGs and are subjected to translational control in response to heat stress; this control at the translation level is likely to inhibit apoptosis and protect the germ cells transiently from the unfavorable condition. Heat stress on the somatic compartment of the testis induces changes in gene expression that might impair normal spermatogenesis.
Fig. 2.
Model for heat stress response in male germ cells. Heat stress affects germ cells as well as somatic cells, leading to disruption of normal spermatogenesis. The cellular responses differ between different types of germ cells. Spc spermatocytes; SG stress granule
Although there has been much progress in understanding the heat stress response of male germ cells, some issues still remain unresolved. Why are some male germ cells susceptible to heat stress, while others often form SGs and are tolerant? Because these germ cells are in specific phases of the meiotic cell cycle, it is possible that heat susceptibility or SG formation is a cell cycle-dependent event. Such a cell cycle dependency has been found in SG formation of cultured cells after UV irradiation [177]. It will be interesting to determine whether both apoptosis and SG formation upon heat stress are dependent on the cell cycle. Another interesting issue is whether the molecular changes in male germ cells after heat stress are causes or effects of the modulation of cellular behaviors. Most of the up-regulated genes seem to participate in germ cell death, or cell survival, while it is still unclear whether the down-regulation of genes is functional or simply lowered due to germ cell loss. Genetic studies for these molecules, in conjunction with further efforts to find new genes implicated in the heat stress response, will provide insight into how complex genetic networks regulate the behavior of male germ cells under heat stress conditions. However, although we increasingly understand the mechanisms behind the heat stress response of the testis, the evolutionary reason behind this sensitivity remains a mystery; female gametogenesis happens happily at 37 °C.
Acknowledgments
We thank Dr. Howard J. Cooke and our lab members, especially Won Yul Jang and Miseon Lee, for critical reading of the manuscript. This study was supported by grants from the BioImaging Research Center at GIST; the Basic Research Program [grant number 3344-20100052]; the Science Research Center Program [grant number R11-2005-009-030050] of the Ministry of Education, Science and Technology; and the second stage of the Brain Korea 21 Project in 2009 (to B.K.) and 2011 (to K.P.).
References
- 1.Sharpe RM. Regulation of spermatogenesis. In: Knobil E, Neil JD, editors. The physiology of reproduction. 2. New York: Raven; 1994. pp. 1363–1434. [Google Scholar]
- 2.Cooke HJ, Saunders PT. Mouse models of male infertility. Nat Rev Genet. 2002;3:790–801. doi: 10.1038/nrg911. [DOI] [PubMed] [Google Scholar]
- 3.Clermont Y. The cycle of the seminiferous epithelium in man. Am J Anat. 1963;112:35–51. doi: 10.1002/aja.1001120103. [DOI] [PubMed] [Google Scholar]
- 4.Fukui N. Action of body temperature on the testicle. Jpn Med World. 1923;3:160–163. [Google Scholar]
- 5.Frankenhuis MT, Wensing CJ. Induction of spermatogenesis in the naturally cryptorchid pig. Fertil Steril. 1979;31:428–433. doi: 10.1016/s0015-0282(16)43942-7. [DOI] [PubMed] [Google Scholar]
- 6.Hutson JM, Hasthorpe S, Heyns CF. Anatomical and functional aspects of testicular descent and cryptorchidism. Endocr Rev. 1997;18:259–280. doi: 10.1210/edrv.18.2.0298. [DOI] [PubMed] [Google Scholar]
- 7.Moore CR. Properties of the gonads as controllers of somatic and psychical characteristics. VI. Testicular reactions in experimental cryptorchidism. Am J Anat. 1924;34:269–316. [Google Scholar]
- 8.Moore CR, Quick WJ. The scrotum as a temperature regulator for the testis. Am J Physiol. 1924;68:70–79. [Google Scholar]
- 9.Harrison RG, Weiner JS. Abdomino-testicular temperature gradients. J Physiol. 1948;107:48–49. [Google Scholar]
- 10.Waites GM, Moule GR. Relation of vascular heat exchange to temperature regulation in the testis of the ram. J Reprod Fertil. 1961;2:213–224. doi: 10.1530/jrf.0.0020213. [DOI] [PubMed] [Google Scholar]
- 11.Zorgniotti AA. Temperature and environmental effects on the testis. New York: Plenum; 1991. [Google Scholar]
- 12.Brito LF, Silva AE, Barbosa RT, Kastelic JP. Testicular thermoregulation in Bos indicus, crossbred and Bos taurus bulls: relationship with scrotal, testicular vascular cone and testicular morphology, and effects on semen quality and sperm production. Theriogenology. 2004;61:511–528. doi: 10.1016/s0093-691x(03)00231-0. [DOI] [PubMed] [Google Scholar]
- 13.Rommel SA, Pabst DA, McLellan WA, Mead JG, Potter CW. Anatomical evidence for a countercurrent heat exchanger associated with dolphin testes. Anat Rec. 1992;232:150–156. doi: 10.1002/ar.1092320117. [DOI] [PubMed] [Google Scholar]
- 14.Rommel SA, Pabst DA, McLellan WA, Willimas TM, Freidl WA. Temperature regulation of the testes of the bottlenose dolphin (Tursiops truncatus): evidence from colonic temperatures. J Comp Physiol B. 1994;164:130–134. doi: 10.1007/BF00301654. [DOI] [PubMed] [Google Scholar]
- 15.Rommel SA, Early GA, Matassa KA, Pabst DA, McLellan WA. Venous structures associated with thermoregulation of phocid seal reproductive organs. Anat Rec. 1995;243:390–402. doi: 10.1002/ar.1092430314. [DOI] [PubMed] [Google Scholar]
- 16.Pabst DA, Rommel SA, McLellan WA, Williams TM, Rowles TK. Thermoregulation of the intra-abdominal testes of the bottlenose dolphin (Tursiops truncatus) during exercise. J Exp Biol. 1995;198:221–226. doi: 10.1242/jeb.198.1.221. [DOI] [PubMed] [Google Scholar]
- 17.Setchell BP. The Parkes lecture. Heat and the testis. J Reprod Fertil. 1998;114:179–194. doi: 10.1530/jrf.0.1140179. [DOI] [PubMed] [Google Scholar]
- 18.Morgentaler A, Stahl BC, Yin Y. Testis and temperature: an historical, clinical and research perspective. J Androl. 1999;20:189–195. [PubMed] [Google Scholar]
- 19.Casady RB, Myers RM, Legates JE. The effect of exposure to high ambient temperature on spermatogenesis in the dairy bull. J Dairy Sci. 1953;36:14–19. [Google Scholar]
- 20.Collins P, Lacy D. Studies on the structure and function of the mammalian testis. II. Cytological and histochemical observations on the testis of the rat after a single exposure to heat applied for different lengths of time. Proc R Soc Lond B. 1969;172:17–38. doi: 10.1098/rspb.1969.0009. [DOI] [PubMed] [Google Scholar]
- 21.French DJ, Leeb CS, Fahrion SL, Law OT, Jecht EW. Self-induced scrotal hyperthermia in man followed by decrease in sperm output. A preliminary report. Andrologie. 1973;5:311–316. doi: 10.1111/j.1439-0272.1973.tb00496.x. [DOI] [PubMed] [Google Scholar]
- 22.Wettemann RP, Wells ME, Omtvedt IT, Pope CE, Turman EJ. Influence of elevated ambient temperature on reproductive performance of boars. J Anim Sci. 1976;42:664–669. doi: 10.2527/jas1976.423664x. [DOI] [PubMed] [Google Scholar]
- 23.Hand JW, Walker H, Hornsey S, Field SB. Effects of hyperthermia on the mouse testis and its response to X-rays, as assayed by weight loss. Int J Radiat Biol Relat Stud Phys Chem Med. 1979;35:521–528. doi: 10.1080/09553007914550631. [DOI] [PubMed] [Google Scholar]
- 24.Freidman R, Scott M, Heath SE, Hughes JP, Daels PF, Tran TQ. The effects of increased testicular temperature on spermatogenesis in the stallion. J Reprod Fertil. 1991;44:127–134. [PubMed] [Google Scholar]
- 25.Mieusset R, Quintana Casares P, Sanchez Partida LG, Sowerbutts SF, Zupp JL, Setchell BP. Effects of heating the testes and epididymides of rams by scrotal insulation on fertility and embryonic mortality in ewes inseminated with frozen semen. J Reprod Fertil. 1992;94:337–343. doi: 10.1530/jrf.0.0940337. [DOI] [PubMed] [Google Scholar]
- 26.Mieusset R, Bujan L. Testicular heating and its possible contributions to male infertility: a review. Int J Androl. 1995;18:169–184. doi: 10.1111/j.1365-2605.1995.tb00408.x. [DOI] [PubMed] [Google Scholar]
- 27.Zhou XC, Han XB, Hu ZY, Zhou RJ, Liu YX. Expression of Hsp70-2 in unilateral cryptorchid testis of rhesus monkey during germ cell apoptosis. Endocrine. 2001;16:89–95. doi: 10.1385/ENDO:16:2:089. [DOI] [PubMed] [Google Scholar]
- 28.Schwalm A, Gauly M, Erhardt G, Bergmann M. Changes in testicular histology and sperm quality in llamas (Lama glama), following exposure to high ambient temperature. Theriogenology. 2007;67:1316–1323. doi: 10.1016/j.theriogenology.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 29.Setchell BP. The effect of heat on the testes of mammals. Anim Reprod. 2006;3:81–91. [Google Scholar]
- 30.Shikone T, Billig H, Hsueh AJ. Experimentally induced cryptorchidism increases apoptosis in rat testis. Biol Reprod. 1994;51:865–872. doi: 10.1095/biolreprod51.5.865. [DOI] [PubMed] [Google Scholar]
- 31.Ohta Y, Nishikawa A, Fukazawa Y, Urushitani H, Matsuzawa A, Nishina Y, Iguchi T. Apoptosis in adult mouse testis induced by experimental cryptorchidism. Acta Anat. 1996;157:195–204. doi: 10.1159/000147881. [DOI] [PubMed] [Google Scholar]
- 32.Yin Y, Hawkins KL, DeWolf WC, Morgentaler A. Heat stress causes testicular germ cell apoptosis in adult mice. J Androl. 1997;18:159–165. [PubMed] [Google Scholar]
- 33.Lue YH, Hikim AP, Swerdloff RS, Im P, Taing KS, Bui T, Leung A, Wang C. Single exposure to heat induces stage-specific germ cell apoptosis in rats: role of intratesticular testosterone on stage specificity. Endocrinology. 1999;140:1709–1717. doi: 10.1210/endo.140.4.6629. [DOI] [PubMed] [Google Scholar]
- 34.Rockett JC, Mapp FL, Garges JB, Luft JC, Mori C, Dix DJ. Effects of hyperthermia on spermatogenesis, apoptosis, gene expression, and fertility in adult male mice. Biol Reprod. 2001;65:229–239. doi: 10.1095/biolreprod65.1.229. [DOI] [PubMed] [Google Scholar]
- 35.Ikeda M, Kodama H, Fukuda J, Shimizu Y, Murata M, Kumagai J, Tanaka T. Role of radical oxygen species in rat testicular germ cell apoptosis induced by heat stress. Biol Reprod. 1999;61:393–399. doi: 10.1095/biolreprod61.2.393. [DOI] [PubMed] [Google Scholar]
- 36.Socher SA, Yin Y, Dewolf WC, Morgentaler A. Temperature-mediated germ cell loss in the testis is associated with altered expression of the cell-cycle regulator p53. J Urol. 1997;157:1986–1989. [PubMed] [Google Scholar]
- 37.Zhang XS, Yuan JX, Liu T, Lue YH, Jin X, Tao SX, Hu ZY, Hikim AP, Swerdloff RS, Wang C, Liu YX. Expression of orphan receptors TR2, TR3, TR4, and p53 in heat-treated testis of cynomolgus monkeys (Macaca fascicularis) J Androl. 2006;27:405–413. doi: 10.2164/jandrol.05165. [DOI] [PubMed] [Google Scholar]
- 38.Absalan F, Movahedin M, Mowla SJ. Germ cell apoptosis induced by experimental cryptorchidism is mediated by molecular pathways in mouse testis. Andrologia. 2010;42:5–12. doi: 10.1111/j.1439-0272.2009.00947.x. [DOI] [PubMed] [Google Scholar]
- 39.Yin Y, DeWolf WC, Morgentaler A. Experimental cryptorchidism induces testicular germ cell apoptosis by p53-dependent and -independent pathways in mice. Biol Reprod. 1998;58:492–496. doi: 10.1095/biolreprod58.2.492. [DOI] [PubMed] [Google Scholar]
- 40.Yin Y, Stahl BC, DeWolf WC, Morgentaler A. p53 and Fas are sequential mechanisms of testicular germ cell apoptosis. J Androl. 2002;23:64–70. doi: 10.1002/jand.2002.23.1.64. [DOI] [PubMed] [Google Scholar]
- 41.Sinha Hikim AP, Lue Y, Yamamoto CM, Vera Y, Rodriguez S, Yen PH, Soeng K, Wang C, Swerdloff RS. Key apoptotic pathways for heat-induced programmed germ cell death in the testis. Endocrinology. 2003;144:3167–3175. doi: 10.1210/en.2003-0175. [DOI] [PubMed] [Google Scholar]
- 42.Sinha Hikim AP, Lue Y, Diaz-Romero M, Yen PH, Wang C, Swerdloff RS. Deciphering the pathways of germ cell apoptosis in the testis. J Steroid Biochem Mol Biol. 2003;85:175–182. doi: 10.1016/s0960-0760(03)00193-6. [DOI] [PubMed] [Google Scholar]
- 43.Vera Y, Diaz-Romero M, Rodriguez S, Lue Y, Wang C, Swerdloff RS, Sinha Hikim AP. Mitochondria-dependent pathway is involved in heat-induced male germ cell death: lessons from mutant mice. Biol Reprod. 2004;70:1534–1540. doi: 10.1095/biolreprod.103.024661. [DOI] [PubMed] [Google Scholar]
- 44.Yamamoto CM, Sinha Hikim AP, Huynh PN, Shapiro B, Lue Y, Salameh WA, Wang C, Swerdloff RS. Redistribution of Bax is an early step in an apoptotic pathway leading to germ cell death in rats, triggered by mild testicular hyperthermia. Biol Reprod. 2000;63:1683–1690. doi: 10.1095/biolreprod63.6.1683. [DOI] [PubMed] [Google Scholar]
- 45.Jia Y, Castellanos J, Wang C, Sinha-Hikim I, Lue Y, Swerdloff RS, Sinha-Hikim AP. Mitogen-activated protein kinase signaling in male germ cell apoptosis in the rat. Biol Reprod. 2009;80:771–780. doi: 10.1095/biolreprod.108.072843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matsuki S, Iuchi Y, Ikeda Y, Sasagawa I, Tomita Y, Fujii J. Suppression of cytochrome c release and apoptosis in testes with heat stress by minocycline. Biochem Biophys Res Commun. 2003;312:843–849. doi: 10.1016/j.bbrc.2003.10.191. [DOI] [PubMed] [Google Scholar]
- 47.Vera Y, Rodriguez S, Castanares M, Lue Y, Atienza V, Wang C, Swerdloff RS, Sinha Hikim AP. Functional role of caspases in heat-induced testicular germ cell apoptosis. Biol Reprod. 2005;72:516–522. doi: 10.1095/biolreprod.104.034520. [DOI] [PubMed] [Google Scholar]
- 48.Lue Y, Sinha Hikim AP, Wang C, Leung A, Swerdloff RS. Functional role of inducible nitric oxide synthase in the induction of male germ cell apoptosis, regulation of sperm number, and determination of testes size: evidence from null mutant mice. Endocrinology. 2003;144:3092–3100. doi: 10.1210/en.2002-0142. [DOI] [PubMed] [Google Scholar]
- 49.DeFoor WR, Kuan CY, Pinkerton M, Sheldon CA, Lewis AG. Modulation of germ cell apoptosis with a nitric oxide synthase inhibitor in a murine model of congenital cryptorchidism. J Urol. 2004;172:1731–1735. doi: 10.1097/01.ju.0000138846.56399.de. [DOI] [PubMed] [Google Scholar]
- 50.Ishikawa T, Kondo Y, Goda K, Fujisawa M. Overexpression of endothelial nitric oxide synthase in transgenic mice accelerates testicular germ cell apoptosis induced by experimental cryptorchidism. J Androl. 2005;26:281–288. doi: 10.1002/j.1939-4640.2005.tb01096.x. [DOI] [PubMed] [Google Scholar]
- 51.Johnson C, Jia Y, Wang C, Lue YH, Swerdloff RS, Zhang XS, Hu ZY, Li YC, Liu YX, Hikim AP. Role of caspase 2 in apoptotic signaling in primate and murine germ cells. Biol Reprod. 2008;79:806–814. doi: 10.1095/biolreprod.108.068833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Russell LD, Ettlin RA, Sinha-Hikim AP, Clegg ED. Histological and histopathological evaluation of the testis. Clearwater: Cache River; 1990. [Google Scholar]
- 53.Henriksén K, Hakovirta H, Parvinen M. In situ quantification of stage-specific apoptosis in the rat seminiferous epithelium: effects of short-term experimental cryptorchidism. Int J Androl. 1995;18:256–262. [PubMed] [Google Scholar]
- 54.Kim B, Cooke HJ, Rhee K. DAZL is essential for stress granule formation implicated in germ cell survival upon heat stress. Development. 2012;139:568–578. doi: 10.1242/dev.075846. [DOI] [PubMed] [Google Scholar]
- 55.Steinberger E, Dixon WJ. Some observations on the effect of heat on the testicular germinal epithelium. Fert Steril. 1959;10:578–595. [Google Scholar]
- 56.Loughlin KR, Manson K, Foreman R, Schwartz B, Heuttner P. The effect of intermittent scrotal hyperthermia on the Sprague-Dawley rat testicle. Adv Exp Med Biol. 1991;286:183–185. doi: 10.1007/978-1-4684-5913-5_17. [DOI] [PubMed] [Google Scholar]
- 57.Karabinus DS, Vogler CJ, Saacke RG, Evenson DP. Chromatin structural changes in sperm after scrotal insulation of Holstein bulls. J Androl. 1997;18:549–555. [PubMed] [Google Scholar]
- 58.Sailer BL, Sarkar LJ, Bjordahl JA, Jost LK, Evenson DP. Effects of heat stress on mouse testicular cells and sperm chromatin structure. J Androl. 1997;18:294–301. [PubMed] [Google Scholar]
- 59.Banks S, King SA, Irvine DS, Saunders PT. Impact of a mild scrotal heat stress on DNA integrity in murine spermatozoa. Reproduction. 2005;129:505–514. doi: 10.1530/rep.1.00531. [DOI] [PubMed] [Google Scholar]
- 60.Paul C, Murray AA, Spears N, Saunders PT. A single, mild, transient scrotal heat stress causes DNA damage, subfertility and impairs formation of blastocysts in mice. Reproduction. 2008;136:73–84. doi: 10.1530/REP-08-0036. [DOI] [PubMed] [Google Scholar]
- 61.Garriott ML, Chrisman CL. Hyperthermia induced dissociation of the X-Y bivalent in mice. Environ Mutagen. 1980;2:465–471. doi: 10.1002/em.2860020405. [DOI] [PubMed] [Google Scholar]
- 62.van Zelst SJ, Zupp JL, Hayman DL, Setchell BP. X-Y chromosome dissociation in mice and rats exposed to increased testicular or environmental temperatures. Reprod Fertil Dev. 1995;7:1117–1121. doi: 10.1071/rd9951117. [DOI] [PubMed] [Google Scholar]
- 63.Aitken RJ, Gordon E, Harkiss D, Twigg JP, Milne P, Jennings Z, Irvine DS. Relative impact of oxidative stress on the functional competence and genomic integrity of human spermatozoa. Biol Reprod. 1998;59:1037–1046. doi: 10.1095/biolreprod59.5.1037. [DOI] [PubMed] [Google Scholar]
- 64.Twigg J, Fulton N, Gomez E, Irvine DS, Aitken RJ. Analysis of the impact of intracellular reactive oxygen species generation on the structural and functional integrity of human spermatozoa: lipid peroxidation, DNA fragmentation and effectiveness of antioxidants. Hum Reprod. 1998;13:1429–1436. doi: 10.1093/humrep/13.6.1429. [DOI] [PubMed] [Google Scholar]
- 65.Aitken RJ, Krausz C. Oxidative stress, DNA damage and the Y chromosome. Reproduction. 2001;122:497–506. doi: 10.1530/rep.0.1220497. [DOI] [PubMed] [Google Scholar]
- 66.Aitken RJ, Baker MA. Oxidative stress, sperm survival and fertility control. Mol Cell Endocrinol. 2006;250:66–69. doi: 10.1016/j.mce.2005.12.026. [DOI] [PubMed] [Google Scholar]
- 67.Ahotupa M, Huhtaniemi I. Impaired detoxification of reactive oxygen and consequent oxidative stress in experimentally cryptorchid rat testis. Biol Reprod. 1992;46:1114–1118. doi: 10.1095/biolreprod46.6.1114. [DOI] [PubMed] [Google Scholar]
- 68.Li YC, Hu XQ, Xiao LJ, Hu ZY, Guo J, Zhang KY, Song XX, Liu YX. An oligonucleotide microarray study on gene expression profile in mouse testis of experimental cryptorchidism. Front Biosci. 2006;11:2465–2482. doi: 10.2741/1983. [DOI] [PubMed] [Google Scholar]
- 69.Paul C, Teng S, Saunders PT. A single, mild, transient scrotal heat stress causes hypoxia and oxidative stress in mouse testes, which induces germ cell death. Biol Reprod. 2009;80:913–919. doi: 10.1095/biolreprod.108.071779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kumagai A, Kodama H, Kumagai J, Fukuda J, Kawamura K, Tanikawa H, Sato N, Tanaka T. Xanthine oxidase inhibitors suppress testicular germ cell apoptosis induced by experimental cryptorchidism. Mol Hum Reprod. 2002;8:118–123. doi: 10.1093/molehr/8.2.118. [DOI] [PubMed] [Google Scholar]
- 71.Ishii T, Matsuki S, Iuchi Y, Okada F, Toyosaki S, Tomita Y, Ikeda Y, Fujii J. Accelerated impairment of spermatogenic cells in SOD1-knockout mice under heat stress. Free Radic Res. 2005;39:697–705. doi: 10.1080/10715760500130517. [DOI] [PubMed] [Google Scholar]
- 72.Paul C, Melton DW, Saunders PT. Do heat stress and deficits in DNA repair pathways have a negative impact on male fertility? Mol Hum Reprod. 2008;14:1–8. doi: 10.1093/molehr/gam089. [DOI] [PubMed] [Google Scholar]
- 73.Bellvé AR. Viability and survival of mouse embryos following parental exposure to high temperature. J Reprod Fertil. 1972;30:71–81. doi: 10.1530/jrf.0.0300071. [DOI] [PubMed] [Google Scholar]
- 74.Bellvé AR. Development of mouse embryos with abnormalities induced by parental heat stress. J Reprod Fertil. 1973;35:393–403. doi: 10.1530/jrf.0.0350393. [DOI] [PubMed] [Google Scholar]
- 75.Setchell BP, D’Occhio MJ, Hall MJ, Laurie MS, Tucker MJ, Zupp JL. Is embryonic mortality increased in normal female rats mated to subfertile males? J Reprod Fertil. 1988;82:567–574. doi: 10.1530/jrf.0.0820567. [DOI] [PubMed] [Google Scholar]
- 76.Jannes P, Spiessens C, Van der Auwera I, D’Hooghe T, Verhoeven G, Vanderschueren D. Male subfertility induced by acute scrotal heating affects embryo quality in normal female mice. Hum Reprod. 1998;13:372–375. doi: 10.1093/humrep/13.2.372. [DOI] [PubMed] [Google Scholar]
- 77.Setchell BP, Ekpe G, Zupp JL, Surani MA. Transient retardation in embryo growth in normal female mice made pregnant by males whose testes had been heated. Hum Reprod. 1998;13:342–347. doi: 10.1093/humrep/13.2.342. [DOI] [PubMed] [Google Scholar]
- 78.Zhu BK, Setchell BP. Effects of paternal heat stress on the in vivo development of preimplantation embryos in the mouse. Reprod Nutr Dev. 2004;44:617–629. doi: 10.1051/rnd:2004064. [DOI] [PubMed] [Google Scholar]
- 79.Yaeram J, Setchell BP, Maddocks S. Effect of heat stress on the fertility of male mice in vivo and in vitro. Reprod Fertil Dev. 2006;18:647–653. doi: 10.1071/rd05022. [DOI] [PubMed] [Google Scholar]
- 80.Walters AH, Eyestone WE, Saacke RG, Pearson RE, Gwazdauskas FC. Bovine embryo development after IVF with spermatozoa having abnormal morphology. Theriogenology. 2005;63:1925–1937. doi: 10.1016/j.theriogenology.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 81.Walters AH, Saacke RG, Pearson RE, Gwazdauskas FC. Assessment of pronuclear formation following in vitro fertilization with bovine spermatozoa obtained after thermal insulation of the testis. Theriogenology. 2006;65:1016–1028. doi: 10.1016/j.theriogenology.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 82.Zhu B, Walker SK, Oakey H, Setchell BP, Maddocks S. Effect of paternal heat stress on the development in vitro of preimplantation embryos in the mouse. Andrologia. 2004;36:384–394. doi: 10.1111/j.1439-0272.2004.00635.x. [DOI] [PubMed] [Google Scholar]
- 83.Hagenas L, Ritzen EM. Impaired Sertoli cell function in experimental cryptorchidism in the rat. Mol Cell Endocrinol. 1975;4:25–34. doi: 10.1016/0303-7207(76)90004-6. [DOI] [PubMed] [Google Scholar]
- 84.Karpe B, Plöen L, Hagenäs L, Ritzén EM. Recovery of testicular functions after surgical treatment of experimental cryptorchidism in the rat. Int J Androl. 1981;4:145–160. doi: 10.1111/j.1365-2605.1981.tb00699.x. [DOI] [PubMed] [Google Scholar]
- 85.Zhang ZH, Hu ZY, Song XX, Xiao LJ, Zou RJ, Han CS, Liu YX. Disrupted expression of intermediate filaments in the testis of rhesus monkey after experimental cryptorchidism. Int J Androl. 2004;27:234–239. doi: 10.1111/j.1365-2605.2004.00477.x. [DOI] [PubMed] [Google Scholar]
- 86.Zhang XS, Zhang ZH, Jin X, Wei P, Hu XQ, Chen M, Lu CL, Lue YH, Hu ZY, Sinha Hikim AP, Swerdloff RS, Wang C, Liu YX. Dedifferentiation of adult monkey Sertoli cells through activation of extracellularly regulated kinase 1/2 induced by heat treatment. Endocrinology. 2006;147:1237–1245. doi: 10.1210/en.2005-0981. [DOI] [PubMed] [Google Scholar]
- 87.Guo J, Tao SX, Chen M, Shi YQ, Zhang ZQ, Li YC, Zhang XS, Hu ZY, Liu YX. Heat treatment induces liver receptor homolog-1 expression in monkey and rat Sertoli cells. Endocrinology. 2007;148:1255–1265. doi: 10.1210/en.2006-1004. [DOI] [PubMed] [Google Scholar]
- 88.Chen M, Cai H, Yang JL, Lu CL, Liu T, Yang W, Guo J, Hu XQ, Fan CH, Hu ZY, Gao F, Liu YX. Effect of heat stress on expression of junction-associated molecules and upstream factors androgen receptor and Wilms’ tumor 1 in monkey Sertoli cells. Endocrinology. 2008;149:4871–4882. doi: 10.1210/en.2007-1093. [DOI] [PubMed] [Google Scholar]
- 89.Cai H, Ren Y, Li XX, Yang JL, Zhang CP, Chen M, Fan CH, Hu XQ, Hu ZY, Gao F, Liu YX. Scrotal heat stress causes a transient alteration in tight junctions and induction of TGF-β expression. Int J Androl. 2011;34:352–362. doi: 10.1111/j.1365-2605.2010.01089.x. [DOI] [PubMed] [Google Scholar]
- 90.Aktas C, Kanter M. A morphological study on Leydig cells of scrotal hyperthermia applied rats in short-term. J Mol Histol. 2009;40:31–39. doi: 10.1007/s10735-009-9210-9. [DOI] [PubMed] [Google Scholar]
- 91.Kanter M, Aktas C. Effects of scrotal hyperthermia on Leydig cells in long-term: a histological, immunohistochemical and ultrastructural study in rats. J Mol Histol. 2009;40:123–130. doi: 10.1007/s10735-009-9222-5. [DOI] [PubMed] [Google Scholar]
- 92.Maines MD, Ewing JF. Stress response of the rat testis: in situ hydridization and immunohistochemical analysis of heme oxygenase-1 (HSP32) induction by hyperthermia. Biol Reprod. 1996;54:1070–1079. doi: 10.1095/biolreprod54.5.1070. [DOI] [PubMed] [Google Scholar]
- 93.Meistrich ML, Eng VW, Loir M. Temperature effects on the kinetics of spermatogenesis in the mouse. Cell Tissue Kinet. 1973;6:379–393. doi: 10.1111/j.1365-2184.1973.tb01626.x. [DOI] [PubMed] [Google Scholar]
- 94.Parvinen M, Pelto-Huikko M, Söder O, Schultz R, Kaipia A, Mali P, Toppari J, Hakovirta H, Lönnerberg P, Ritzén EM, Ebendal T, Olson L, Hökfelt T, Persson H. Expression of beta-nerve growth factor and its receptor in rat seminiferous epithelium: specific function at the onset of meiosis. J Cell Biol. 1992;117:629–641. doi: 10.1083/jcb.117.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Setchell BP, Waites GM. The effects of local heating of the testis on the flow and composition of rete testis fluid in the rat, with some observations on the effects of age and unilateral castration. J Reprod Fertil. 1972;30:225–233. doi: 10.1530/jrf.0.0300225. [DOI] [PubMed] [Google Scholar]
- 96.Setchell BP, Tao L, Zupp JL. The penetration of chromium-EDTA from blood plasma into various compartments of rat testes as an indicator of function of the blood-testis barrier after exposure of the testes to heat. J Reprod Fertil. 1996;106:125–133. doi: 10.1530/jrf.0.1060125. [DOI] [PubMed] [Google Scholar]
- 97.Setchell BP, Plöen L, Ritzen EM. Reduction of long-term effects of local heating of the testis by treatment of rats with a GnRH agonist and an anti-androgen. Reproduction. 2001;122:255–263. doi: 10.1530/rep.0.1220255. [DOI] [PubMed] [Google Scholar]
- 98.Setchell BP, Plöen L, Ritzen EM. Effect of local heating of rat testes after suppression of spermatogenesis by pretreatment with a GnRH agonist and an anti-androgen. Reproduction. 2002;124:133–140. doi: 10.1530/rep.0.1240133. [DOI] [PubMed] [Google Scholar]
- 99.Zhu YF, Cui YG, Guo XJ, Wang L, Bi Y, Hu YQ, Zhao X, Liu Q, Huo R, Lin M, Zhou ZM, Sha JH. Proteomic analysis of effect of hyperthermia on spermatogenesis in adult male mice. J Proteome Res. 2006;5:2217–2225. doi: 10.1021/pr0600733. [DOI] [PubMed] [Google Scholar]
- 100.Zhu H, Cui Y, Xie J, Chen L, Chen X, Guo X, Zhu Y, Wang X, Tong J, Zhou Z, Jia Y, Lue YH, Hikim AS, Wang C, Swerdloff RS, Sha J. Proteomic analysis of testis biopsies in men treated with transient scrotal hyperthermia reveals the potential targets for contraceptive development. Proteomics. 2010;10:3480–3493. doi: 10.1002/pmic.201000281. [DOI] [PubMed] [Google Scholar]
- 101.Riedl SJ, Shi Y. Molecular mechanisms of caspase regulation during apoptosis. Nat Rev Mol Cell Biol. 2004;5:897–907. doi: 10.1038/nrm1496. [DOI] [PubMed] [Google Scholar]
- 102.Mu X, Liu Y, Collins LL, Kim E, Chang C. The p53/retinoblastoma-mediated repression of testicular orphan receptor-2 in the rhesus monkey with cryptorchidism. J Biol Chem. 2000;275:23877–23883. doi: 10.1074/jbc.M910158199. [DOI] [PubMed] [Google Scholar]
- 103.Hayashi T, Yoshida S, Yoshinaga A, Ohno R, Ishii N, Yamada T. HtrA2 is up-regulated in the rat testis after experimental cryptorchidism. Int J Urol. 2006;13:157–164. doi: 10.1111/j.1442-2042.2006.01250.x. [DOI] [PubMed] [Google Scholar]
- 104.Hayashida N, Inouye S, Fujimoto M, Tanaka Y, Izu H, Takaki E, Ichikawa H, Rho J, Nakai A. A novel HSF1-mediated death pathway that is suppressed by heat shock proteins. EMBO J. 2006;25:4773–4783. doi: 10.1038/sj.emboj.7601370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jia Y, Hikim AP, Lue YH, Swerdloff RS, Vera Y, Zhang XS, Hu ZY, Li YC, Liu YX, Wang C. Signaling pathways for germ cell death in adult cynomolgus monkeys (Macaca fascicularis) induced by mild testicular hyperthermia and exogenous testosterone treatment. Biol Reprod. 2007;77:83–92. doi: 10.1095/biolreprod.106.058594. [DOI] [PubMed] [Google Scholar]
- 106.Guo J, Jia Y, Tao SX, Li YC, Zhang XS, Hu ZY, Chiang N, Lue YH, Hikim AP, Swerdloff RS, Wang C, Liu YX. Expression of nitric oxide synthase during germ cell apoptosis in testis of cynomolgus monkey after testosterone and heat treatment. J Androl. 2009;30:190–199. doi: 10.2164/jandrol.108.005538. [DOI] [PubMed] [Google Scholar]
- 107.Li W, Wu ZQ, Zhao J, Guo SJ, Li Z, Feng X, Ma L, Zhang JS, Liu XP, Zhang YQ. Transient protection from heat-stress induced apoptotic stimulation by metastasis-associated protein 1 in pachytene spermatocytes. PLoS ONE. 2011;6:e26013. doi: 10.1371/journal.pone.0026013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Liu F, Xu ZL, Qian XJ, Qiu WY, Huang H. Expression of Hsf1, Hsf2, and Phlda1 in cells undergoing cryptorchid-induced apoptosis in rat testes. Mol Reprod Dev. 2011;78:283–291. doi: 10.1002/mrd.21304. [DOI] [PubMed] [Google Scholar]
- 109.Lizama C, Lagos CF, Lagos-Cabré R, Cantuarias L, Rivera F, Huenchuñir P, Pérez-Acle T, Carrión F, Moreno RD. Calpain inhibitors prevent p38 MAPK activation and germ cell apoptosis after heat stress in pubertal rat testes. J Cell Physiol. 2009;221:296–305. doi: 10.1002/jcp.21868. [DOI] [PubMed] [Google Scholar]
- 110.Richter K, Haslbeck M, Buchner J. The heat shock response: life on the verge of death. Mol Cell. 2010;40:253–266. doi: 10.1016/j.molcel.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 111.Sarge KD, Cullen KE. Regulation of hsp expression during rodent spermatogenesis. Cell Mol Life Sci. 1997;53:191–197. doi: 10.1007/PL00000591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rosario MO, Perkins SL, O’Brien DA, Allen RL, Eddy EM. Identification of the gene for the developmentally expressed 70 kDa heat-shock protein (P70) of mouse spermatogenic cells. Dev Biol. 1992;150:1–11. doi: 10.1016/0012-1606(92)90002-x. [DOI] [PubMed] [Google Scholar]
- 113.Dix DJ, Allen JW, Collins BW, Mori C, Nakamura N, Poorman-Allen P, Goulding EH, Eddy EM. Targeted gene disruption of Hsp70-2 results in failed meiosis, germ cell apoptosis, and male infertility. Proc Natl Acad Sci USA. 1996;93:3264–3268. doi: 10.1073/pnas.93.8.3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zakeri ZF, Welch WJ, Wolgemuth DJ. Characterization and inducibility of hsp 70 proteins in the male mouse germ line. J Cell Biol. 1990;111:1785–1792. doi: 10.1083/jcb.111.5.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sarge KD. Male germ cell-specific alteration in temperature set point of the cellular stress response. J Biol Chem. 1995;270:18745–18748. doi: 10.1074/jbc.270.32.18745. [DOI] [PubMed] [Google Scholar]
- 116.Kumagai J, Fukuda J, Kodama H, Murata M, Kawamura K, Itoh H, Tanaka T. Germ cell-specific heat shock protein 105 binds to p53 in a temperature-sensitive manner in rat testis. Eur J Biochem. 2000;267:3073–3078. doi: 10.1046/j.1432-1033.2000.01336.x. [DOI] [PubMed] [Google Scholar]
- 117.Zhang XS, Lue YH, Guo SH, Yuan JX, Hu ZY, Han CS, Hikim AP, Swerdloff RS, Wang C, Liu YX. Expression of HSP105 and HSP60 during germ cell apoptosis in the heat-treated testes of adult cynomolgus monkeys (Macaca fascicularis) Front Biosci. 2005;10:3110–3121. doi: 10.2741/1767. [DOI] [PubMed] [Google Scholar]
- 118.Widlak W, Vydra N, Malusecka E, Dudaladava V, Winiarski B, Scieglińska D, Widlak P. Heat shock transcription factor 1 down-regulates spermatocyte-specific 70 kDa heat shock protein expression prior to the induction of apoptosis in mouse testes. Genes Cells. 2007;12:487–499. doi: 10.1111/j.1365-2443.2007.01069.x. [DOI] [PubMed] [Google Scholar]
- 119.Nakai A, Suzuki M, Tanabe M. Arrest of spermatogenesis in mice expressing an active heat shock transcription factor 1. EMBO J. 2000;19:1545–1554. doi: 10.1093/emboj/19.7.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Izu H, Inouye S, Fujimoto M, Shiraishi K, Naito K, Nakai A. Heat shock transcription factor 1 is involved in quality-control mechanisms in male germ cells. Biol Reprod. 2004;70:18–24. doi: 10.1095/biolreprod.103.020065. [DOI] [PubMed] [Google Scholar]
- 121.Vydra N, Malusecka E, Jarzab M, Lisowska K, Glowala-Kosinska M, Benedyk K, Widlak P, Krawczyk Z, Widlak W. Spermatocyte-specific expression of constitutively active heat shock factor 1 induces HSP70i-resistant apoptosis in male germ cells. Cell Death Differ. 2006;13:212–222. doi: 10.1038/sj.cdd.4401758. [DOI] [PubMed] [Google Scholar]
- 122.Widlak W, Winiarski B, Krawczyk A, Vydra N, Malusecka E, Krawczyk Z. Inducible 70 kDa heat shock protein does not protect spermatogenic cells from damage induced by cryptorchidism. Int J Androl. 2007;30:80–87. doi: 10.1111/j.1365-2605.2006.00713.x. [DOI] [PubMed] [Google Scholar]
- 123.Le Masson F, Razak Z, Kaigo M, Audouard C, Charry C, Cooke H, Westwood JT, Christians ES. Identification of heat shock factor 1 molecular and cellular targets during embryonic and adult female meiosis. Mol Cell Biol. 2011;31:3410–3423. doi: 10.1128/MCB.05237-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhang K, Shang Y, Liao S, Zhang W, Nian H, Liu Y, Chen Q, Han C. Uncoupling protein 2 protects testicular germ cells from hyperthermia-induced apoptosis. Biochem Biophys Res Commun. 2007;360:327–332. doi: 10.1016/j.bbrc.2007.06.071. [DOI] [PubMed] [Google Scholar]
- 125.Kubota H, Sasaki S, Kubota Y, Umemoto Y, Yanai Y, Tozawa K, Hayashi Y, Kohri K. Cyclooxygenase-2 protects germ cells against spermatogenesis disturbance in experimental cryptorchidism model mice. J Androl. 2011;32:77–85. doi: 10.2164/jandrol.109.008888. [DOI] [PubMed] [Google Scholar]
- 126.Fujisawa M, Matsumoto O, Kamidono S, Hirose F, Kojima K, Yoshida S. Changes of enzymes involved in DNA synthesis in the testes of cryptorchid rats. J Reprod Fertil. 1988;84:123–130. doi: 10.1530/jrf.0.0840123. [DOI] [PubMed] [Google Scholar]
- 127.Kong WH, Zheng G, LU JN, Tso JK. Temperature dependent expression of cdc2 and cyclin B1 in spermatogenic cells during spermatogenesis. Cell Res. 2000;10:289–302. doi: 10.1038/sj.cr.7290056. [DOI] [PubMed] [Google Scholar]
- 128.Tramontano F, Malanga M, Farina B, Jones R, Quesada P. Heat stress reduces poly(ADPR)polymerase expression in rat testis. Mol Hum Reprod. 2000;6:575–581. doi: 10.1093/molehr/6.7.575. [DOI] [PubMed] [Google Scholar]
- 129.Zhang Y, Yang X, Cao H, Chen Z, Du Y, Kong W. Heat stress induces Cdc2 protein decrease prior to mouse spermatogenic cell apoptosis. Acta Histochem. 2008;110:276–284. doi: 10.1016/j.acthis.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 130.Holcik M, Sonenberg N. Translational control in stress and apoptosis. Nat Rev Mol Cell Biol. 2005;6:318–327. doi: 10.1038/nrm1618. [DOI] [PubMed] [Google Scholar]
- 131.Nakamura M, Hall PF. The influence of temperature upon polysomes of spermatids of rat testes. Biochem Biophys Res Commun. 1978;85:756–761. doi: 10.1016/0006-291x(78)91225-1. [DOI] [PubMed] [Google Scholar]
- 132.Nakamura M, Romrell LJ, Hall PF. The effects of temperature and glucose on protein biosynthesis by immature (round) spermatids from rat testes. J Cell Biol. 1978;79:1–9. doi: 10.1083/jcb.79.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cataldo L, Mastrangelo MA, Kleene KC. Differential effects of heat shock on translation of normal mRNAs in primary spermatocytes, elongated spermatids, and Sertoli cells in seminiferous tubule culture. Exp Cell Res. 1997;231:206–213. doi: 10.1006/excr.1996.3447. [DOI] [PubMed] [Google Scholar]
- 134.Nakamura M, Hall PF. The mechanism by which body temperature inhibits protein biosynthesis in spermatids of rat testes. J Biol Chem. 1980;255:2907–2913. [PubMed] [Google Scholar]
- 135.Rowlands AG, Panniers R, Henshaw EC. The catalytic mechanism of guanine nucleotide exchange factor action and competitive inhibition by phosphorylated eukaryotic initiation factor 2. J Biol Chem. 1988;263:5526–5533. [PubMed] [Google Scholar]
- 136.Krishnamoorthy T, Pavitt GD, Zhang F, Dever TE, Hinnebusch AG. Tight binding of the phosphorylated alpha subunit of initiation factor 2 (eIF2alpha) to the regulatory subunits of guanine nucleotide exchange factor eIF2B is required for inhibition of translation initiation. Mol Cell Biol. 2001;21:5018–5030. doi: 10.1128/MCB.21.15.5018-5030.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Dever TE. Gene-specific regulation by general translation factors. Cell. 2002;108:545–556. doi: 10.1016/s0092-8674(02)00642-6. [DOI] [PubMed] [Google Scholar]
- 138.Dever TE, Feng L, Wek RC, Cigan AM, Donahue TF, Hinnebusch AG. Phosphorylation of initiation factor 2 alpha by protein kinase GCN2 mediates gene-specific translational control of GCN4 in yeast. Cell. 1992;68:585–596. doi: 10.1016/0092-8674(92)90193-g. [DOI] [PubMed] [Google Scholar]
- 139.Lu PD, Harding HP, Ron D. Translation reinitiation at alternative open reading frames regulates gene expression in an integrated stress response. J Cell Biol. 2004;167:27–33. doi: 10.1083/jcb.200408003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Jiang HY, Wek RC. Phosphorylation of the alpha-subunit of the eukaryotic initiation factor-2 (eIF2alpha) reduces protein synthesis and enhances apoptosis in response to proteasome inhibition. J Biol Chem. 2005;280:14189–14202. doi: 10.1074/jbc.M413660200. [DOI] [PubMed] [Google Scholar]
- 141.Bevilacqua E, Wang X, Majumder M, Gaccioli F, Yuan CL, Wang C, Zhu X, Jordan LE, Scheuner D, Kaufman RJ, Koromilas AE, Snider MD, Holcik M, Hatzoglou M. eIF2alpha phosphorylation tips the balance to apoptosis during osmotic stress. J Biol Chem. 2010;285:17098–17111. doi: 10.1074/jbc.M110.109439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sarge KD, Bray AE, Goodson ML. Altered stress response in testis. Nature. 1995;374:126. doi: 10.1038/374126a0. [DOI] [PubMed] [Google Scholar]
- 143.Anckar J, Sistonen L. Regulation of HSF1 function in the heat stress response: implications in aging and disease. Annu Rev Biochem. 2011;80:1089–1115. doi: 10.1146/annurev-biochem-060809-095203. [DOI] [PubMed] [Google Scholar]
- 144.Han AP, Yu C, Lu L, Fujiwara Y, Browne C, Chin G, Fleming M, Leboulch P, Orkin SH, Chen JJ. Heme-regulated eIF2alpha kinase (HRI) is required for translational regulation and survival of erythroid precursors in iron deficiency. EMBO J. 2001;20:6909–6918. doi: 10.1093/emboj/20.23.6909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lu L, Han AP, Chen JJ. Translation initiation control by heme-regulated eukaryotic initiation factor 2alpha kinase in erythroid cells under cytoplasmic stresses. Mol Cell Biol. 2001;21:7971–7980. doi: 10.1128/MCB.21.23.7971-7980.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Barber GN. The dsRNA-dependent protein kinase, PKR and cell death. Cell Death Differ. 2005;12:563–570. doi: 10.1038/sj.cdd.4401643. [DOI] [PubMed] [Google Scholar]
- 147.Patil C, Walter P. Intracellular signaling from the endoplasmic reticulum to the nucleus: the unfolded protein response in yeast and mammals. Curr Opin Cell Biol. 2001;13:349–355. doi: 10.1016/s0955-0674(00)00219-2. [DOI] [PubMed] [Google Scholar]
- 148.Kimball SR. Regulation of translation initiation by amino acids in eukaryotic cells. Prog Mol Subcell Biol. 2001;26:155–184. doi: 10.1007/978-3-642-56688-2_6. [DOI] [PubMed] [Google Scholar]
- 149.Deng J, Harding HP, Raught B, Gingras AC, Berlanga JJ, Scheuner D, Kaufman RJ, Ron D, Sonenberg N. Activation of GCN2 in UV-irradiated cells inhibits translation. Curr Biol. 2002;12:1279–1286. doi: 10.1016/s0960-9822(02)01037-0. [DOI] [PubMed] [Google Scholar]
- 150.Zhan K, Vattem KM, Bauer BN, Dever TE, Chen JJ, Wek RC. Phosphorylation of eukaryotic initiation factor 2 by heme-regulated inhibitor kinase-related protein kinases in Schizosaccharomyces pombe is important for resistance to environmental stresses. Mol Cell Biol. 2002;22:7134–7146. doi: 10.1128/MCB.22.20.7134-7146.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Anderson P, Kedersha N. Stressful initiations. J Cell Sci. 2002;115:3227–3234. doi: 10.1242/jcs.115.16.3227. [DOI] [PubMed] [Google Scholar]
- 152.Kedersha NL, Gupta M, Li W, Miller I, Anderson P. RNA-binding proteins TIA-1 and TIAR link the phosphorylation of eIF-2 alpha to the assembly of mammalian stress granules. J Cell Biol. 1999;147:1431–1442. doi: 10.1083/jcb.147.7.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kedersha N, Chen S, Gilks N, Li W, Miller IJ, Stahl J, Anderson P. Evidence that ternary complex (eIF2-GTP-tRNAiMet)-deficient preinitiation complexes are core constituents of mammalian stress granules. Mol Biol Cell. 2002;13:195–210. doi: 10.1091/mbc.01-05-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Buchan JR, Parker R. Eukaryotic stress granules: the ins and outs of translation. Mol Cell. 2009;36:932–941. doi: 10.1016/j.molcel.2009.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kedersha N, Cho MR, Li W, Yacono PW, Chen S, Gilks N, Golan DE, Anderson P. Dynamic shuttling of TIA-1 accompanies the recruitment of mRNA to mammalian stress granules. J Cell Biol. 2000;151:1257–1268. doi: 10.1083/jcb.151.6.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kedersha N, Stoecklin G, Ayodele M, Yacono P, Lykke-Andersen J, Fritzler MJ, Scheuner D, Kaufman RJ, Golan DE, Anderson P. Stress granules and processing bodies are dynamically linked sites of mRNP remodeling. J Cell Biol. 2005;169:871–884. doi: 10.1083/jcb.200502088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kim WJ, Back SH, Kim V, Ryu I, Jang SK. Sequestration of TRAF2 into stress granules interrupts tumor necrosis factor signaling under stress conditions. Mol Cell Biol. 2005;25:2450–2462. doi: 10.1128/MCB.25.6.2450-2462.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Arimoto K, Fukuda H, Imajoh-Ohmi S, Saito H, Takekawa M. Formation of stress granules inhibits apoptosis by suppressing stress-responsive MAPK pathways. Nat Cell Biol. 2008;10:1324–1332. doi: 10.1038/ncb1791. [DOI] [PubMed] [Google Scholar]
- 159.Ohn T, Kedersha N, Hickman T, Tisdale S, Anderson P. A functional RNAi screen links O-GlcNAc modification of ribosomal proteins to stress granule and processing body assembly. Nat Cell Biol. 2008;10:1224–1231. doi: 10.1038/ncb1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Saxena R, de Vries JW, Repping S, Alagappan RK, Skaletsky H, Brown LG, Ma P, Chen E, Hoovers JM, Page DC. Four DAZ genes in two clusters found in the AZFc region of the human Y chromosome. Genomics. 2000;67:256–267. doi: 10.1006/geno.2000.6260. [DOI] [PubMed] [Google Scholar]
- 161.Kim B, Lee Y, Kim Y, Lee KH, Chun S, Rhee K, Seo JT, Kim SW, Paick JS. Polymorphic expression of DAZ proteins in the human testis. Hum Reprod. 2009;24:1507–1515. doi: 10.1093/humrep/dep032. [DOI] [PubMed] [Google Scholar]
- 162.Yen PH, Chai NN, Salido EC. The human autosomal gene DAZLA: testis specificity and a candidate for male infertility. Hum Mol Genet. 1996;5:2013–2017. doi: 10.1093/hmg/5.12.2013. [DOI] [PubMed] [Google Scholar]
- 163.Xu EY, Moore FL, Pera RA. A gene family required for human germ cell development evolved from an ancient meiotic gene conserved in metazoans. Proc Natl Acad Sci USA. 2001;98:7414–7419. doi: 10.1073/pnas.131090498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Eberhart CG, Maines JZ, Wasserman SA. Meiotic cell cycle requirement for a fly homologue of human Deleted in Azoospermia . Nature. 1996;381:783–785. doi: 10.1038/381783a0. [DOI] [PubMed] [Google Scholar]
- 165.Ruggiu M, Speed R, Taggart M, KcKay SJ, Kilanowski F, Saunders P, Dorin J, Cooke HJ. The mouse Dazla gene encodes a cytoplasmic protein essential for gametogenesis. Nature. 1997;389:73–77. doi: 10.1038/37987. [DOI] [PubMed] [Google Scholar]
- 166.Houston DW, King ML. A critical role for Xdazl, a germ plasm-localized RNA, in the differentiation of primordial germ cells in Xenopus . Development. 2000;127:447–456. doi: 10.1242/dev.127.3.447. [DOI] [PubMed] [Google Scholar]
- 167.Karashima T, Sugimoto A, Yamamoto M. Caenorhabditis elegans homologue of the human azoospermia factor DAZ is required for oogenesis but not for spermatogenesis. Development. 2000;127:1069–1079. doi: 10.1242/dev.127.5.1069. [DOI] [PubMed] [Google Scholar]
- 168.Shah C, Vangompel MJ, Naeem V, Chen Y, Lee T, Angeloni N, Wang Y, Xu EY. Widespread presence of human BOULE homologs among animals and conservation of their ancient reproductive function. PLoS Genet. 2010;6:e1001022. doi: 10.1371/journal.pgen.1001022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Lin Y, Gill ME, Koubova J, Page DC. Germ cell-intrinsic and -extrinsic factors govern meiotic initiation in mouse embryos. Science. 2008;322:1685–1687. doi: 10.1126/science.1166340. [DOI] [PubMed] [Google Scholar]
- 170.Kee K, Angeles VT, Flores M, Nguyen HN, Reijo Pera RA. Human DAZL, DAZ and BOULE genes modulate primordial germ-cell and haploid gamete formation. Nature. 2009;462:222–225. doi: 10.1038/nature08562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Chen J, Melton C, Suh N, Oh JS, Horner K, Xie F, Sette C, Blelloch R, Conti M. Genome-wide analysis of translation reveals a critical role for deleted in azoospermia-like (Dazl) at the oocyte-to-zygote transition. Genes Dev. 2011;25:755–766. doi: 10.1101/gad.2028911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Collier B, Gorgoni B, Loveridge C, Cooke HJ, Gray NK. The DAZL family proteins are PABP-binding proteins that regulate translation in germ cells. EMBO J. 2005;24:2656–2666. doi: 10.1038/sj.emboj.7600738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Reynolds N, Collier B, Maratou K, Bingham V, Speed RM, Taggart M, Semple CA, Gray NK, Cooke HJ. Dazl binds in vivo to specific transcripts and can regulate the pre-meiotic translation of Mvh in germ cells. Hum Mol Genet. 2005;14:3899–3909. doi: 10.1093/hmg/ddi414. [DOI] [PubMed] [Google Scholar]
- 174.Reynolds N, Collier B, Bingham V, Gray NK, Cooke HJ. Translation of the synaptonemal complex component Sycp3 is enhanced in vivo by the germ cell specific regulator Dazl. RNA. 2007;13:974–981. doi: 10.1261/rna.465507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lee KH, Lee S, Kim B, Chang S, Kim SW, Paick JS, Rhee K. Dazl can bind to dynein motor complex and may play a role in transport of specific mRNAs. EMBO J. 2006;25:4263–4270. doi: 10.1038/sj.emboj.7601304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.VanGompel MJ, Xu EY. A novel requirement in mammalian spermatid differentiation for the DAZ-family protein Boule. Hum Mol Genet. 2010;19:2360–2369. doi: 10.1093/hmg/ddq109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Pothof J, Verkaik NS, van IJcken W, Wiemer EA, Ta VT, van der Horst GT, Jaspers NG, van Gent DC, Hoeijmakers JH, Persengiev SP. MicroRNA-mediated gene silencing modulates the UV-induced DNA-damage response. EMBO J. 2009;28:2090–2099. doi: 10.1038/emboj.2009.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kon Y, Endoh D. Heat-shock resistance in experimental cryptorchid testis of mice. Mol Reprod Dev. 2001;58:216–222. doi: 10.1002/1098-2795(200102)58:2<216::AID-MRD11>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 179.Allen RL, O’Brien DA, Jones CC, Rockett DL, Eddy EM. Expression of heat shock proteins by isolated mouse spermatogenic cells. Mol Cell Biol. 1988;8:3260–3266. doi: 10.1128/mcb.8.8.3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Nishiyama H, Danno S, Kaneko Y, Itoh K, Yokoi H, Fukumoto M, Okuno H, Millán JL, Matsuda T, Yoshida O, Fujita J. Decreased expression of cold-inducible RNA-binding protein (CIRP) in male germ cells at elevated temperature. Am J Pathol. 1998;152:289–296. [PMC free article] [PubMed] [Google Scholar]
- 181.Iuchi Y, Kaneko T, Matsuki S, Sasagawa I, Fujii J. Concerted changes in the YB2/RYB-a protein and protamine 2 messenger RNA in the mouse testis under heat stress. Biol Reprod. 2003;68:129–135. doi: 10.1095/biolreprod.102.005124. [DOI] [PubMed] [Google Scholar]
- 182.Li W, Bao W, Ma J, Liu X, Xu R, Wang RA, Zhang Y. Metastasis tumor antigen 1 is involved in the resistance to heat stress-induced testicular apoptosis. FEBS Lett. 2008;582:869–873. doi: 10.1016/j.febslet.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 183.Farooqui SM, Al-Bagdadi F, Houslay MD, Bolger GB, Stout R, Specian RD, Cherry JA, Conti M, O’Donnell JM. Surgically induced cryptorchidism-related degenerative changes in spermatogonia are associated with loss of cyclic adenosine monophosphate-dependent phosphodiesterases type 4 in abdominal testes of rats. Biol Reprod. 2001;64:1583–1589. doi: 10.1095/biolreprod64.6.1583. [DOI] [PubMed] [Google Scholar]