Abstract
Galectins, a family of soluble β-galactoside-binding proteins, serve as mediators of fundamental biological processes, such as cell growth, differentiation, adhesion, migration, survival, and death. The purpose of this review is to summarize the current knowledge regarding the ways in which the expression of individual galectins differs in normal and transformed human cells exposed to various stimuli mimicking physiological and pathological microenvironmental stress conditions. A conceptual point is being made and grounded that the modulation of galectin expression profiles is a key aspect of cellular stress responses. Moreover, this modulation might be precisely regulated at transcriptional and post-transcriptional levels in the context of non-overlapping transcription factors and miRNAs specific to galectins.
Keywords: Galectins, Cellular stress responses, Hypoxia, Cancer, Apoptosis, Cell survival, Transcription factors, miRNA
Introduction
Animal and human cells respond to exogenous stressors of a chemical and physical nature through a number of specific adaptive stress response pathways that attempt to mitigate damage and maintain or re-establish homeostasis [1]. These pathways are highly conserved in most metazoans, including mammals, highlighting the central and obligatory roles played by such pathways in organisms’ responses to environmental insults. At the molecular level, cells use stress-specific sensors that signal through individual transcriptional factors such as Nrf2 (oxidative stress), HSF-1 (heat shock response), p53 (DNA damage), HIF-1 (hypoxia), MTF-1 (metal stress), NFAT5 (osmotic stress), and NF-κB (inflammation stress). These factors may crosstalk with XBP-1/ATF6/ATF4, controlling unfolded protein response (UPR) due to the accumulation of damaged, aggregated, or misfolded proteins [2]. Although different stress stimuli engage on default own primary sensors, a global remodeling of stressed cells might include a common molecular signature due to the ultimate selection between only two choices: cell death or cell survival. Recent findings indicate that specific glycosylation patterns of cellular proteins, as well as changes in the expression of glycan-binding proteins (lectins), may accompany the stress responses, suggesting the glycobiological mechanisms of such regulation. Animal lectins are central to these mechanisms and consist of at least 15 diverse families of proteins, each with characteristic structural motifs represented by one or several carbohydrate-recognition domains (CRDs) specific to different glycans [3].
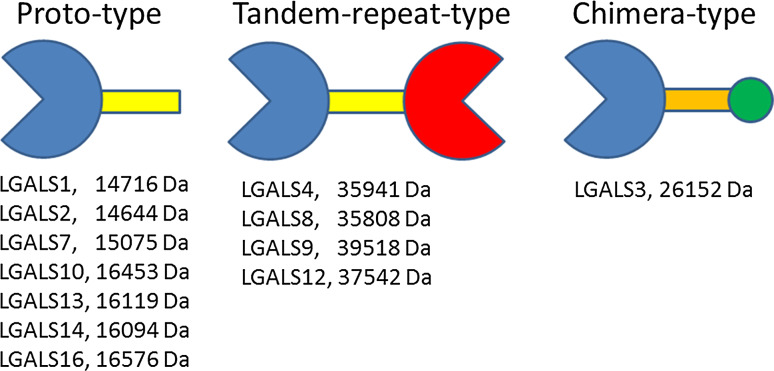
Galectins, a family of soluble β-galactoside-binding proteins, have attracted special attention over the last decade due to the role they play in the regulation of fundamental biological processes, such as cell growth, differentiation, adhesion, migration, survival, and death [4, 5]. The conventional classification of galectins was originally proposed by Hirabayashi and Kasai [6]. This classification considers the structural features of these proteins, distinguishing three subfamilies: proto-type galectins (galectin-1, -2, -5, -7, -10,-11, -13, -14, -15, and -16) with one carbohydrate-recognizing domain (CRD), tandem-repeat galectins (galectin-4, -6, -8, -9, and -12) with two homologous CRDs, and a chimeric galectin-3 with one CRD linked to a non-lectin N-terminal domain (Fig. 1). Human cells are known to express 12 of these galectins, missing murine galectin-5 and -6 and ruminant galectin-11 and -15. Proto-type galectins are smaller proteins (14–16 kDa) than chimeric (26 kDa) and tandem-repeat galectins (~40 kDa). However, they can form homodimers and multimers and cross-link structures that contain the sugar galactose on the cell surface and within the extracellular matrix, resulting in a variety of specific cellular responses, which regulate cell survival and programmed cell death (apoptosis). For instance, extracellular galectins can stimulate signaling systems, leading to the generation of reactive oxygen species (ROS), the mobilization of intracellular calcium, and the secretion of vascular endothelial growth factors (VEGFs) [7–9]. Galectins can also function intracellularly, controlling apoptosis and mRNA splicing processes in a glycan-independent manner [10, 11]. Most galectins possess multiple cellular stress-related functions, which are associated with stimulatory and inhibitory response mechanisms depending on the cell type and galectin localization (Table 1) [3, 12–15]. Of interest, apoptotic sensitivity of immune cells to tumor-derived galectins has been proposed as a potential mechanism assisting tumor cells to survive and escape from immune surveillance in the body [12].
Fig. 1.
Basic structure and molecular characteristics of human galectins. Carbohydrate-recognizing domains (CRDs) are schematically presented as pacman-like symbols (purple and red) linked to non-lectin domains (showed as yellow/orange bars). Proto-type galectins can form noncovalent homodimers (not shown), while tandem-repeat galectins contain two covalently linked homologous CRDs. CRD of chimera-type galectin-3 is linked to a collagen-α-like sequence (orange) followed by a small N-terminal end (green circle) mediating the formation of oligomers. Sizes of human galectin molecules were derived from the GeneCards Database at www.genecards.org
Table 1.
Selected cellular stress-related responses and biological functions, which are regulated by galectins (based on reviews [3, 12–15])
| Human galectin | Stimulatory effects | Inhibitory effects |
|---|---|---|
| LGALS1 |
Apoptosis of activated T cells Survival of naïve T cells Tumor cells apoptosis (extracellular mechanism) Muscle repair and cell differentiation Tumor cell growth and migration Proliferation of neural stem cells Regeneration of axons Respiratory burst in neutrophils Plasma cell survival and differentiation Angiogenesis Mitogenesis of spleen or lymph node cells, vascular cells, and Hepatic stellate cells |
T cell viability B cell proliferation Acute inflammation Nitric oxide release from macrophages Growth of neuroblastoma and stromal bone marrow cells |
| LGALS2 | T cell apoptosis |
T cell viability Pro-inflammatory cytokine secretion |
| LGALS3 |
T cell and monocyte apoptosis (extracellular mechanism) Tumor growth Re-epithelization of wounded corneas Growth and differentiation of lymphocytes Respiratory burst in macrophages and neutrophils Eosinophil death Angiogenesis Inflammation |
T cell apoptosis (intracellular mechanism) T cell viability Inflammation Survival of activated B cells Apoptosis in B cell lymphoma |
| LGALS4 |
T cell apoptosis Axon growth |
Intestinal inflammation |
| LGALS7 |
Tumor cell apoptosis (intracellular mechanism) Keratinocyte differentiation p53-mediated apoptosis of keratinocyte |
Cell growth Cell proliferation |
| LGALS8 |
Apoptosis of lung carcinoma and synovial fluid cells Respiratory burst of neutrophils Plasma cell differentiation Angiogenesis Autophagy Cell growth arrest |
Autoimmune inflammation |
| LGALS9 |
Apoptosis of Th1 cells, T cells, thymocytes and NK cells Dendritic cell maturation Tumor cells apoptosis (extracellular mechanism) Respiratory burst in must cells |
T cell viability |
| LGALS10 (CLC) |
CD4+ T cells apoptosis Differentiation of promyelocytic cells |
Proliferation of T regulatory cells |
| LGALS12 |
Tumor cell apoptosis (intracellular mechanism) Adipocyte apoptosis and differentiation |
Tumor cell growth |
| LGALS13 |
T cell apoptosis Apoptosis promotion in U-937 macrophage cell line |
nd |
| LGALS14 | T cell apoptosis | nd |
| LGALS16 | T cell apoptosis | nd |
nd no data
Galectin expression profiles differ in normal and tumorous tissues [16, 17] and between different cell lines [18–20]; they can undergo a variety of changes under the stress conditions encountered in tumor microenvironments or those associated with inflammation, fibrosis, or cardiovascular and other diseases [21]. For instance, a specific and readily detectable 5- to 30-fold increase in the circulating levels of several galectins has been reported in the bloodstream of patients with various types of cancers, including breast [22], head and neck [23], bladder [24], melanoma [25], pancreatic [26, 27], and colorectal [28–30] carcinomas. These observations have prompted the development of galectin-targeting drugs, some of which have been tested in clinical trials [31]. In fact, other studies have demonstrated that stress stimuli can induce non-uniform changes in the expression of various galectin genes, which can be tentatively classified as upregulated, downregulated, and constitutive galectins [32]. For instance, the monocytic differentiation of HL-60 cells was accompanied by the upregulation of galectin-3 and the down-regulation of galectin-9 mRNA expression, while no changes or expression were detected with galectins -1, -2, -4, -7, and -8 [33]. This conceptual point has also readily been demonstrated in studies with prostate carcinoma tissues, showing that, as the cancer progressed toward more aggressive stages, the level of galectin-1 increased, the levels of galectins -3, -4, -9, and -12 gradually decreased, and galectin-8 remained stably expressed [34]. As such, many fundamental questions about the cell stress biology of galectin proteins remain insufficiently answered, leaving a number of unresolved issues and limiting the practical application of galectin-based therapies. First, we do not know why cells express as many as 16 different galectins and how/whether these galectins interact with each other. Second, we do not know how a global network of galectins is remodeled in cells under stress conditions and whether these changes can provide a biomarker code or molecular signature of cellular stress responses. Third, we do not know what signaling mechanisms regulate the differential expression of galectin genes in cells and whether a collaboration between common and diverse stress-sensitive signaling pathways is required. Considering recent comprehensive reviews and books on galectins [3, 5, 12–17, 35–37], the main goal of this short review is to focus specifically on the stress-induced changes in the expression of individual galectins in human cells including cancer cell lines and to point out some potential regulatory mechanisms likely deserving of attention in elucidating the crosstalk between various members of the galectin network at the transcriptional and post-transcriptional levels.
Proto-type galectins
The expression of many human proto-type galectins (-1, -2, -7, -10, and -13), with the exception of the relatively less studied galectins 14 and 16, has been reported to be readily sensitive to a variety of stress stimuli, including hypoxia, redox stress, ER stress, and DNA damage, as well as to stimuli inducing cell differentiation.
Galectin-1
Since the seminal findings by Le et al. [38], galectin-1 has been recognized as a strong biomarker of hypoxia-induced cellular stress responses with respect to several cell lines (FaDu, SCC4, SQB20, Panc1, and V2P3) associated with head and neck squamous cell carcinoma (HNSCC). It was shown that galectin-1 was upregulated by hypoxia (0.2 and 2 % O2), which mimicked the local microenvironmental conditions of tumorous tissues, at both transcriptional and post-translational levels, although global mRNA accumulation surprisingly lagged behind the protein accumulation/secretion [38]. In line with this finding, the level of circulating galectin-1 was found to be elevated in tumor-bearing mice, whereas the expression of galectin-1 in HNSCC tissues was inversely correlated with the T cell marker CD3. Since galectin-1 was originally shown to promote T cell apoptosis [39], as well as to inhibit various aspects of T cell effector functions [40, 41], it was suggested that the hypoxia-induced upregulation of galectin-1 is essential for tumor cells to escape from cellular immune surveillance [38]. Subsequent testing of other cancer cell lines associated with human and mouse melanoma (A375 and B16-F0), mouse breast carcinoma (4T1), and human prostate carcinoma (LNCaP) confirmed the hypoxia-induced expression of galectin-1 at both mRNA and protein levels [42]. In renal cell carcinoma cell line CAK-1, the mimicking of hypoxia stress with CoCl2, which inhibits prolyl hydroxylase and HIF-1α ubiquitination, also resulted in an almost 14-fold, dose-dependent increase in galectin-1 protein expression [43]. Interestingly, the transcriptional control of galectin-1 upregulation under hypoxic conditions seems to be specific to the type of responsive cells and not always dependent on classical hypoxia-induced transcription factors. For example, galectin-1 expression was found to be controlled by HIF-1α in colorectal carcinoma [44], by C/EBPa in acute myeloid leukemia [45], by AP-1 in classical Hodgkin lymphoma [46], and by ROS and NF-kB in Kaposi’s sarcoma [42] cells. Other lines of evidence supporting the hypoxia-induced upregulation of galectin-1 have recently been reported in a model of acute myocardial infarction considering the hypoxic microenvironment in infarcted hearts [47]. In particular, the exposure of HL-1 cardiomyocytes in a cell culture to hypoxia (1 % O2, 18 h) or pro-inflammatory cytokines (IL-17, TNF-α, IFN-γ) increased the respective levels of galectin-1 in total cell lysates or cell culture media. Moreover, exogenous and endogenous galectin-1 did not affect the viability of cardiomyocytes, eliminating an apoptotic aspect of its activity. Since galectin-1 is a part of the contractile apparatus of cardiac striated muscles colocalizing with sarcomeric actin on I bands [48], a positive outcome of galectin-1 activity under hypoxic stress has been suggested and considered as a possible therapeutic mode for preventing heart failure [47].
In addition to hypoxia, the elevation of galectin-1 levels was reported under metabolic stress (glutamine deprivation or ammonia accumulation) in the serum-free cell culture medium of CHO cells [49]. At the organismal level, a rapid (within 1 h) increase of galectin-1 was observed in the serum of rats under restrain stress, which coincided with the increase of corticosterone and was controlled by the sympathetic nervous system [50]. Immunoblot analysis revealed a strong increase of galectin-1 protein in the human glioma cell lines A172 and U118 after a 4-h treatment with a single dose of ~6 Gy ionizing radiation [51]. It is likely that galectin-1 protects glioma cells through its direct role in the UPR, as the siRNA-mediated knockdown of galectin-1 coincided with diminished IRE1 expression and ultimately impaired the ability of human hs683 glioblastoma cells to respond to ER stress [52]. Moreover, decreased galectin-1 expression has been associated with the decreased mRNA level of the hypoxia-related genes implicated in angiogenesis, which confirms a galectin-1-integrated relationship between ER stress and hypoxia [52].
Galectin-2
A tandem-repeat galectin-2 is a paralog of galectin-1 with a wide range of biological activity. It performs an anti-inflammatory function in the intestine, inducing the apoptosis of specific populations of T cells [53, 54]. This lectin has been shown to move to the nucleus of fibroblastic cells exposed to physical (UV light), chemical (mitomycin C, serum withdrawal), or cell biological (coculture with stromal cells) treatment modalities [55]. These results suggest that changes in the compartmentalization and localization of galectin-2 in cells might be important for regulating stress-induced cellular responses—a mechanism that can also be considered for other galectins. Similar to galectin-4 and galectin-8, the circulating levels of galectin-2 increased in the serum of patients with colon and breast cancer [29], correlating with the ability of these galectins to induce the secretion of cancer-promoting cytokines (G-CSF, IL-6, MCP-1/CCL2, and GROα/CXCL1) from the vascular endothelium both in vitro and in mice [56]. In gastric cancer, however, an inverse correlation was noticed between the tissue level of galectin-2 and lymph node metastasis [57].
Galectin-7
Galectin-7 is a proto-type galectin, the expression of which is readily activated by p53 in an association with the apoptotic process, as was initially shown in a model of colorectal cancer cell line DLD-1 [58]. There are several lines of evidence confirming the pro-apoptotic activity of galectin-7 and its role in genotoxic and oxidative stress responses. For instance, UVB radiation has been found to induce galectin-7 expression in human epidermal keratinocytes, particularly in sunburned apoptotic cells [59] and NHEK neonatal foreskin cells [60]. More specifically, galectin-7 was found to be paired with the anti-apoptotic Bcl-2 protein in mitochondria, but this interaction was disrupted by UVB radiation, which sensitized the apoptotic response of cells [61]. Furthermore, galectin-7 transfectants of HeLa and DLD-1 cells showed enhanced sensitivity to apoptosis induced by UV radiation, actinomycin D, TNF-α plus cycloheximide, etoposide, or camptothecin [62]. The expression of galectin-7 is sensitive to the availability of an antioxidant enzyme Cu/Zn-containing extracellular superoxide dismutase (EC-SOD). In particular, both skin cells from EC-SOD transgenic mice and EC-SOD-transfected keratinocyte cell line HaCaT exhibited a significant upregulation of galectin-7 expression based on the western blotting analysis [63]. Interestingly, Lee et al. [63] claimed that the upregulation of galectin-7 expression occurs in a PGE2-dependent manner as a result of the EC-SOD-mediated activation of COX-2, which leads ultimately to the accumulation of pro-apoptotic molecules, such as caspase-3, caspase-9, Bax, and Bcl-Xs. As to the pathological stress conditions, including cancer, it has been suggested that galectin-7 plays a dual role as a result of its ability to mediate apoptosis and cancer suppression via a p53-dependent pathway and to promote cancer progression via NF-κB-dependent pathway [64]. Since both p53 and NF-κB belong to stress-induced transcription factors, the corresponding upregulation of galectin-7 should be considered in the context of specific cellular stress responses with differential outcomes, which fits perfectly with the conceptual paradigm of stress-induced selection between cell death or cell survival. Indeed, the biological role of galectin-7 cannot be solely related to its pro-apoptotic functions due to the crosstalk between both p53 and NF-κB, as recently demonstrated in MCF7 breast cancer cells [65]. In addition, recent studies have revealed that the upregulation of galectin-7 in breast cancer cell lines MCF7 and MDA-MB-231 is driven by C/EBPβ-2, which can explain the paradox of concomitant galectin-7 overexpression in cancer cells and p53 mutation [66]. It should be noted that the role of C/EBP transcription factors in cellular responses to stresses, including inflammatory and ER stresses, is well known [67]. As such, the possibility of regulating the galectin-7 gene via the signaling pathway under stress is a very attractive proposition.
Galectin-10
Galectin-10 belongs to the subfamily of proto-type galectins and has been recognized as a main protein component of the Charcot–Leyden crystals in human eosinophils [68–70]. The overexpression of this protein has also been detected in regulatory T cells [71] and in differentiated HL-60 cells (a human promyelocytic cell line) [33]. Galectin-10 binds not only β-galactoside sugars [68] but also mannose [69], a feature not found in other galectins. The upregulation of galectin-10 has been reported in several models of physiological and pathological stresses. Bronchial and nasal inflammation is accompanied by the activation and recruitment of eosinophils, and a corresponding accumulation of galectin-10 in peripheral blood [72], samples of sputum [73], and nasal lavage [74]. Elevated levels of galectin-10 have also been reported in gut biopsies of patients with celiac disease, an autoimmune disorder of the intestine caused by an allergy to gluten [75]. Lastly, a drastic time-dependent increase in the expression of galectin-10 at the mRNA and protein levels was reported in the process of the myeloid differentiation of HL-60 cells into neutrophilic or eosinophilic lineages, as induced by DMSO or sodium butyrate, respectively [33]. Since no expression of galectin-10 has been observed in undifferentiated HL-60 cells, this galectin deserves attention as a potential biomarker of cellular stress responses in other models. In particular, the presence of binding sites for redox-sensitive transcription factors Sp1 and Oct1 in the promoter region of the galectin-10 gene [76] may explain its high expression in ROS-producing granulocytes.
Galectin-13
Galectin-13 (placenta tissue protein 13, PP13) is a proto-type galectin, which forms stable homodimers through disulfide bonds [77–79]. These dimers were not observed in a Laemmli solution containing 10 % 2-mercaptoethanol, and the galactoside-binding activity and haemagglutination were impaired in the presence of 1 mM dithiothreitol [79]. These properties of galectin-13 might be important for the redox regulation of its biological activity. What is interesting in this context is that the 48-h treatment of a choriocarcinoma cell line BeWo with vitamin C, a reducing agent, was found to increase the PP13 protein expression at protein level in a dose-dependent manner [80]. The available information about galectin-13 expression has been limited largely to placental tissue and more specifically to syncytiotrophoblasts, although initial findings have detected the protein in a few other normal (spleen, fetal kidney, and adult bladder) and tumorous tissues [81]. In terms of reproductive biology, galectin-13/PP13 is specifically known and recognized to be one of biomarkers of preeclampsia [82, 83], as it shows different expression dynamics compared to control subjects [84, 85].
Galectin-14 and galectin-16
Human galectin-14 and galectin-16 genes are expressed predominantly in placental tissues, together with galectin-13 in the Chr19 cluster. These three galectins have been proposed to contribute to immunosuppression at the maternal–fetal interface mostly by inducing T cell apoptosis [86]. Ovine galectin-14 has been studied in more detail and detected primarily in eosinophils [87]. Eosinophils likely serve as a source of secreted galectin-14, which has been detected in bronchoalveolar lavage fluid, mammary gland lavage, and gastrointestinal tract mucus following allergen or parasite challenge [88, 89], indicating a relationship to inflammatory stress response.
Chimeric type galectin-3
The overexpression of galectin-3 has been well documented in different cancer cell models and tumorous tissues under hypoxic conditions. Indeed, the promoter region of chimeric type galectin-3 gene contains binding sites for the transcription factor HIF-1, which drives hypoxia-induced galectin-3 mRNA and protein expression, as observed in HeLa cells and mouse embryonic fibroblasts from HIF-1α wild-type, but not HIF-1α null mice [90]. The expression of galectin-3 gene was also strongly upregulated by hypoxia in the murine melanoma cell line BF-F10 [91]; however, this effect could be cell-specific because subsequent studies revealed no significant changes in five out of six human melanoma cell lines [92]. It is interesting to note that the combined hypoxia-induced upregulation of galectin-3 and epidermal growth factor receptor has been proposed to enhance the invasive potential of tumor cells that are exposed to stressed microenvironmental conditions [93]. Proteomic analysis also revealed the upregulation of galectin-3 in human placental cell line BeWo, which was associated with trophoblast syncytialization [94]. This species- or cell-specific variability needs further examination considering complex galectin networks in cells. Nevertheless, the weak but significant hypoxia-induced upregulation of galectin-3 gene in the WM278 human melanoma cell line [92] and the strong immunostaining for galectin-3 in hypoxic regions of cancer tissue biopsies from patients diagnosed with breast DCIS [95] suggest a potential application for this molecule with anti-apoptotic activity [96] in protecting cancer cells from hypoxia stress. The accumulation of cancer cells with high levels of galectin-3 protein has also been confirmed in a xenotransplant model of glioblastoma multiformes in specific hypoxic areas, which histochemically appeared as so-called pseudopalisades representing hypercellular zones around the necrotic tissues [97]. In cell culture, the NG97ht hybrid glioblastoma cells showed a very strong upregulation of galectin-3 in a HIF-1α and NF-κB-dependent manner under conditions mimicking the tumor’s microenvironment, i.e., combined hypoxia and nutrient deprivation. This galectin-3 upregulation was essential for cell survival because the siRNA-mediated galectin-3 knockdown sensitized transfected cells to the cell death [97]. However, the role of galectin-3 in hypoxia-mediated responses can be more complex and tissue-specific, because it can serve as a multifunctional inflammatory mediator. For instance, in a mice neonate model, hypoxia–ischemia treatment led to the upregulation of galectin-3 in microglia/macrophages associated with specific brain injuries in the deep gray matter areas [98]. As such, the ability of galectin-3 to contribute to various inflammatory responses (chemotaxis, phagocytosis, stimulation of cytokines, and ROS) [99–103] may explain the extent of tissue damage.
Hyperthermic and hypothermic conditions were found to have opposite effects, stimulation vs inhibition, on the expression of galectin-3 in the microglial cells of hippocampal brain tissues from gerbils following experimental ischemia [104, 105]. Interestingly, the increased levels of galectin-3 at a high temperature (39 °C) were associated with less severe apoptotic damage in brain tissues, suggesting that galectin-3 plays a protective role. In a different context, interaction between galectin-3 and IL-10 was required to protect human breast carcinoma BT549 cells against liver ischemia–reperfusion-induced cytotoxicity [106]. Oxidative stress induced by ozone exposure was also associated with the rapid (within 3 h) and prolonged (up to 72 h) accumulation of galectin-3 in the bronchiolar epithelium and alveolar macrophages of rats [107, 108]. Since galectin-3 has been found to have a positive effect on the re-epithelialization of wounds [109], it is anticipated that this effect may be involved in both the protection against the oxidative damage of lungs and wound repair [107]. Galectin-3 upregulation has also been found to be a feature of different types of acute and chronic inflammatory responses associated with microbial infection, asthma, liver injuries, and fibrosis [110]. The increased level of circulating galectin-3 has also been recognized as one of the biomarkers of chronic and acute heart failure [111–113]. Since heart failure as a clinical syndrome deals largely with oxidative stress [114], a related mechanism could be responsible for the upregulation of galectin-3.
Silencing galectin-3 in HeLa cells has resulted in the increased resistance of transfected cells to DNA-damaging agents, such as ionizing radiation (10–40 Gy), etoposide, carboplatin, and mitomycin C [115]. Galectin-3 protein levels in glioblastoma cells increased in response to UV-C radiation and treatments with alkylating reagents, and required the involvement of such transcription factors as NF-κB and Jun [116]. However, it should be noted that under certain stress conditions, e.g., immobilization stress in mice, the levels of galectin-3 in alveolar macrophages and spleen and liver tissues decreased. The same trend was observed in human glioblastoma A1235 cells under hyperthermic conditions, the mechanisms of which are still uncertain [117]. Such non-uniform changes in the expression of galectin-3 under stress conditions underscore the need for a more comprehensive analysis of galectin protein networks in cells and the interactions between different galectin members.
Tandem-repeat type galectins
Human tandem-repeat galectins (4-, -8, -9, and -12) have been relatively less studied with respect to their participation in cellular stress responses. However, the alterations in the expression of these galectins have been reported in relation to inflammation and cancer.
Galectin-4
Tandem-repeat galectin-4 is expressed chiefly in the intestine tissue and contributes substantially to the regulation of inflammation, the activation of immune cells, the expansion of memory T cells in mucosal tissue [118–121], and the stabilization of lipid rafts in cells [122, 123]. Although no data are available on the effects of environmental stress on the expression of galectin-4 in mammalian cells, the level of this galectin is known to be drastically elevated (11–25 folds) in the sera of patients with colorectal and breast cancer in comparison with healthy subjects. This elevation occurs concurrently with galectin-2, galectin-3 and galectin-8 [29], all enhancing the production of cytokines and chemokines by endothelial cells, which are involved in processes of angiogenesis and metastasis [56]. The upstream regulatory elements of the galectin-4 gene include the binding sites for HNF-4, MyoD, c-Rel, HNF-3β, C/EBP, and HFH-2 [124], which might be indirectly involved in a variety of cellular stress responses.
Galectin-8
Galectin-8 is a tandem-repeat galectin with one of the longest 3′UTR regions among mRNA transcripts, which indicates the complex mechanisms of its expression regulation [125]. Therefore, it is not surprising that, despite the massive amount of information regarding the expression of galectin-8 in tumors and its up- and down-regulation compared to healthy tissues [126], the details of galectin-8′s transcriptional and translational machinery remain unexplored and in need of in-depth study based on stress-induced cellular models. The ability of galectin-8 to induce strong superoxide production in human neutrophils [127] indicates the potential significance of oxidative stress in this context. In addition, the fact that several splicing variants of galectin-8 with different biological activity exist [128, 129] provides an exciting direction of research in the context of the stress-dependent regulation of alternative pre-mRNA splicing in cells [130].
Galectin-9
Galectin-9 is a tandem-repeat galectin that serves as a distinctive regulator of adaptive and innate immunity, which is able to weaken the immune system in hyper-immune conditions (autoimmune disease, asthma, infection, allograft rejection) and enhance it in immune-compromised conditions (e.g., cancer) [131]. These immunomodulatory effects of galectin-9 result from the elimination or activation of specific subpopulations of immune cells, which shifts the immune response in the required direction. It is not surprising that, with respect to pathological stress conditions, tumors and especially metastatic sites have mostly shown lower levels of galectin-9 than normal tissues [132]. At the same time, the exposure of host cells to bacterial or viral infections has been found to induce galectin-9 expression [131]. Moreover, individual factors mimicking or associated with inflammatory stress, such as LPS, IFN-γ, TNF, and IL-1β, are powerful stimuli for galectin-9 expression in a variety of cells, including vascular endothelial cells [133, 134], monocytes [135], macrophages [136], fibroblasts [137], multipotent mesenchymal stromal cells [138], and astrocytes [139, 140]. A variety of transcription factors and related signaling pathways have been reported to be essential for the upregulation of galectin-9, including a redox-sensitive JNK/c-Jun signaling pathway in astrocytes [140], the phosphorylation of STAT-1 in HUVEC cells [141], and Smad3 in regulatory T cells [142]. Although it remains unclear how galectin-9 collaborates with other galectins, the administration of the recombinant galectin-9 seems to be a very promising strategy for treating immune and cancer diseases [131].
Galectin-12
Galectin-12 is a tandem-repeat galectin that is expressed variably in different tissues, but relatively strongly in peripheral blood leukocytes, myeloid cell lines [143], and adipocytes [144]. The intracellular level of galectin-12 mRNA is upregulated by reagents that synchronize cells at the G1 phase (theophylline plus dibutyryl-cAMP) or G1/S boundary (hydroxyurea or thymidine) of the cell cycle [143]. The time-dependent increase of the galectin-12 mRNA transcripts over a 7-day period was found to accompany the differentiation of preadipocyte mouse 3T3-L1 cells into mature adipocytes [145]. A detectable amount of galectin-12 has also been associated with lipid droplets in cells and involved in the regulation of lipid metabolism and energy homeostasis [146]—an interesting aspect with important consequences for cell stress biology.
The complexity of the transcriptional and post-transcriptional regulation of galectin networks, and suggestions for future studies
Galectins represent a complex family of glycan-binding proteins with defining specificity to β-galactoside sugars due to the quite similar structural and dynamic properties of carbohydrate-recognizing domains [147]. By comparison, the promoter regions of the genes encoding galectins and the 3′ untranslated regions of galectin mRNAs are very different, explaining the variety of patterns of galectin expression in cells treated with stress. Indeed, a robust bioinformatics analysis of human galectin genes demonstrates very few overlaps between tentative transcription factors and between miRNAs targeting mRNA transcripts (Table 2). A detailed systems biology approach is required to establish galectin regulatory networks that integrate stimulatory and inhibitory pathways. The role of different transcription factors has been addressed in many studies, as highlighted in the previous sections. However, the details of how signaling mechanisms control the expression of stress-sensitive galectins still need elaboration. Furthermore, the role of galectin-specific miRNAs cannot be overlooked because of the very well-known upregulation of non-protein coding genes in cells under stress [148]. The current experimental findings related to the miRNA-mediated regulation of galectin expression have been limited to few studies. In particular, the transfection of a renal carcinoma cell line CAK-1 miR-22 was found to be very efficient in inhibiting both galectin-1 and HIF-1α [43]. A sequence called miR-322 was claimed to recognize human galectin-3 3′UTR, and the silencing of miR-322 with antisense oligo was found to upregulate galectin-3 mRNA transcripts in several cancer cell lines [149]. The list of potential galectin-specific miRNAs (Table 1), which has been retrieved using a DIANA microT algorithm for microRNA target prediction [150, 151], makes it evident that this regulation can vary dramatically between the various galectins, utilizing multiple miRNAs (galectins -3, -8, -9, -12, -13), very few (galectins -1, -4, -7, -10), or none (galectin-2). Remarkably, galectin-8 stands out against other galectins by overwhelming number of potential target miRNAs (~120), which is due to the longest 3′-UTR among all galectins. The biological significance of this diversity remains obscure and awaits further studies with different cell stress models.
Table 2.
The list of tentative transcription factors and miRNAs that may regulate the expression of galectins in human cells
| Human galectin | Transcription factors | hsa-miRNAsa |
|---|---|---|
| LGALS1 | AP-1, c-Jun, p53, SEF-1(1), ATF-2, MyoD, YY1, HEN1, E2F, E2F-1 | 4635, 22-3p, 4717-5p |
| LGALS2 | AP-1, PPAR-γ1, PPAR-γ2, c-Fos, c-Jun, HNF-4α1, HNF-4α2, COUP, COUP-TF, COUP-TF1, ATF-2, ATF6 | No predictions |
| LGALS3 | NF-κB, NF-κB1, AML1a, AP-1, c-Jun, Sp1, HNF-4α1, HNF-4α2 | 612, 548at-5p, 3187-5p, 5189, 1285-3p, 3190-5p, 3622b-5p, 4253, 24-3p |
| LGALS4 | CBF-A, NF-Y, NF-YA, c/EBPα, TBP, TFIID, RelA, PPAR-γ1, PPAR-γ2, CREB, AML1a, AP-1, ATF-2, δCREB, c-Jun | 4688, 27a-5p, 185-3p, 4278 |
| LGALS7 | P53, CREB, NF-1, HSF2, δCREB, HSF1 (long), Arnt | 3194-5p, 3972 |
| LGALS8 | NF-E2, NF-E2 p45, Nkx5-1, RORα2, Bach2, CUTL1, ER-α | 3065-3p, 388-5p, 300, 1913, 4742-3p, 607, 381, 196a-5p, 3662, 431-5p, 196b-5p, 5096, 148a-3p, 573, 3671, 152, 889, 545-5p, 3616-5p, 148b-3p, 29c-3p, 5003-3p, 29b-3p, 2054, 890, 29a-3p, 5008-3p, 3922-3p, 522-3p, 3672, 4775, 105-5p, 3129-3p, 507, 1910, 664-5p, 4778-5p, 29b-2-5p, 892b, 651, 4794, 557, 506-5p, 205-3p, 324-3p, 4666a-5p, 499a-5p, 4533, 3646, 5583-5p, 548c-3p, 4307, 5701, 1272, 4291, 3680-3p, 4670-3p, 27b-3p, 4263, 3152-5p, 2681-5p, 130a-5p, 10a-3p, 562, 3129-5p, 27a-3p, 3613-3p, 4729, 4711-3p, 9-5p, 7-5p, 876-3p, 4802-3p, 2052, 224-3p, 508-5p, 5692a, 33a-3p, 5589-3p, 606, 5700, 579, 3163, 302c-5p, 512-3p, 4747-5p, 320e, 3617, 551b-5p, 3611, 2355-5p, 335-3p, 3159, 4276, 1285-5p, 18b-3p, 1301, 644b-3p, 320c, 5047, 3140-3p, 320b, 3185, 320a, 4282, 582-3p, 5585-5p, 4693-5p, 524-5p, 4708-5p, 136-5p, 5480-3p, 320d, 374b-3p, 4760-3p, 3134, 499a-3p, 19b-1-5p, 1277-5p, 4694-3p, 520d-5p, 4429 |
| LGALS9 | NRSF form 1/form 2, STAT1, STAT1α, STAT1β, POU2F2 (Oct-2.1), Oct-B1, Oct-B3, Oct-B2, CUTL1, POU2F2C, POU2F1, POU2F1a, POU2F2B | 764, 3190-3p, 1197, 3202, 541-3p, 3934, 654-5p, 4657, 486-3p, 4477b, 4459, 505-5p, 548an, 4646-5p, 5090, 665, 4447, 4787-5p, 4314, 4736, 4793-3p, 4472 |
| LGALS10 (CLC) | STAT3, p53, FOXI1, HFH-3, HFH-1, FOXL1 | 573, 3616-5p |
| LGALS12 | SREBP-1a, SREBP-1b, SREBP-1c, c/EBPα, c/EPBβ, PPAR-γ1, PPAR-γ2 | 5692a, 5590-3p, 1291, 765, 3928, 4719, 4650-5p, 484 |
| LGALS13 | NRSF form 1, NRSF form 2, Bach2 | 4314, 204-3p, 4646-5p, 4778-5p, 3192, 374b-3p, 4690-5p, 3927, 657, 4650-3p |
| LGALS14 | No predictions | 4778-5p, 4779, 3927, 204-3p, 4646-5p, 4650-3p, 330-5p, 4314, 4320, 326 |
| LGALS16 | No predictions | 4779, 4778-5p, 5196-5p, 3927, 3155a, 3155b, 4689, 4650-3p, 4652-3p |
The results represent the search for the specific information on the following websites: GeneCards (http://www.genecards.org/), SABioscience (most relevant regulatory transcription binding sites, http://www.sabiosciences.com), and miRNA target prediction software microT-CDS at Diana Tools (http://diana.imis.athena-innovation.gr/DianaTools/)
aThe predicted miRNAs are listed in order of the overall miRNA target gene (miTG) score decreasing. The miTG score is supposed to correlate with fold changes in protein expression [146] and its threshold was set up to 0.7 as per default option of the microT-CDS search
The network of galectins in cells represents a well-balanced system that is sensitive to a variety of stress stimuli mimicking, for instance, the physiological cues for cell differentiation or the pathological microenvironment of tumorous tissues. The alteration of galectin expression profiles seems to be a very delicate mechanism that contributes to cell survival or cell death in the context of cellular stress responses. It is evident that the exposure of cells to stress remodels the galectin expression profiles, including the up- and down-regulation of certain galectins [27]. However, we are still far from gaining an ultimate understanding of the biological significance of galectin networks in cells, especially since some galectins, e.g., galectins -1, -3, and -8, are abundantly expressed in a variety of cell lines, while others are either tissue-specific or silent [18, 152]. Although the activation of stress-specific transcription factors can explain some aspects of the remodeling of galectin networks, additional global molecular mechanisms must be taken into account, including the epigenetic regulation of galectin gene expression and the destabilizing effects of miRNAs targeting galectin mRNAs. For instance, it has been reported that, in cancerous tissues, the promoter regions of all galectin genes contain multiple CpG sites available for the methylation and DNA methylation/demethylation of galectin genes [153]. Accordingly, an efficient way to activate the expression of silent galectins is by inhibiting DNA methylation. This approach has been demonstrated to be efficient in the case of galectin-1 [154] and was recently confirmed in the case of the low expressing galectin-7 in various cell lines, using a specific DNA methylation inhibitor 5-aza-2′-deoxycytidine [155]. The application of this strategy to examining cellular stress responses deserves more attention in the context of galectin expression in cells and tissues with different DNA methylation statuses.
In sum, the differential expression of galectins in tissues and individual cell lines requires the thorough examination of galectin expression profiles and galectin networking in the context of cellular stress responses. Knowledge of the galectin signatures of stressed cells can provide a platform for understanding the functional differences between upregulated and downregulated galectins and the potential value of these galectins as biomarkers or new molecular targets for stress-associated cellular disorders. As such, a comprehensive profiling of galectin expression and subsequent combined inhibition of multiple stress-inducible galectins rather than individual galectins might be a promising strategy for developing new anti-cancer therapies.
Acknowledgments
This work was supported by the Western Strategic Support for NSERC Success Seed Grant (Western University, London, Ontario).
References
- 1.Fulda S, Gorman AM, Hori O, Samali A. Cellular stress responses: cell survival and cell death. Int J Cell Biol. 2010;2010:214074. doi: 10.1155/2010/214074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simmons SO, Fan CY, Ramabhadran R. Cellular stress response pathway system as a sentinel ensemble in toxicological screening. Toxicol Sci. 2009;111:202–225. doi: 10.1093/toxsci/kfp140. [DOI] [PubMed] [Google Scholar]
- 3.Gupta GS. Animal lectins: form, function and clinical applications. Wien: Springer-Verlag; 2012. [Google Scholar]
- 4.Perillo NL, Marcus ME, Baum LG. Galectins: versatile modulators of cell adhesion, cell proliferation, and cell death. J Mol Med (Berl) 1998;76:402–412. doi: 10.1007/s001090050232. [DOI] [PubMed] [Google Scholar]
- 5.Klyosov AA, Witczak ZJ, Platt D, editors. Galectins. Hoboken: Wiley; 2008. [Google Scholar]
- 6.Hirabayashi J, Kasai K. The family of metazoan metal-independent β-galactoside-binding lectins: structure, function and molecular evolution. Glycobiology. 1993;3:297–304. doi: 10.1093/glycob/3.4.297. [DOI] [PubMed] [Google Scholar]
- 7.Timoshenko AV, André S, Kaltner H, Dong X, Gabius HJ. Generation of H2O2 by human neutrophils and changes of cytosolic Ca2+ and pH of rat thymocytes in response to galactoside-binding proteins (lectins or immunoglobulins) Biosci Rep. 1997;17:219–230. doi: 10.1023/A:1027389614391. [DOI] [PubMed] [Google Scholar]
- 8.Timoshenko AV, Gorudko IV, Maslakova OV, André S, Kuwabara I, Liu FT, Kaltner H, Gabius HJ. Analysis of selected blood and immune cell responses to carbohydrate-dependent surface binding of proto- and chimera-type galectins. Mol Cell Biochem. 2003;250:139–149. doi: 10.1023/A:1024952727159. [DOI] [PubMed] [Google Scholar]
- 9.Timoshenko AV, Kaltner H, André S, Gabius HJ, Lala PK. Differential stimulation of VEGF-C production by adhesion/growth-regulatory galectins and plant lectins in human breast cancer cells. Anticancer Res. 2010;30:4829–4833. [PubMed] [Google Scholar]
- 10.Hsu DK, Yang RY, Liu FT. Galectins in apoptosis. Methods Enzymol. 2006;417:256–273. doi: 10.1016/S0076-6879(06)17018-4. [DOI] [PubMed] [Google Scholar]
- 11.Liu FT, Patterson RJ, Wang JL. Intracellular functions of galectins. Biochim Biophys Acta. 2002;1572:263–273. doi: 10.1016/S0304-4165(02)00313-6. [DOI] [PubMed] [Google Scholar]
- 12.Liu FT, Rabinovich GA. Galectins as modulators of tumour progression. Nat Rev Cancer. 2005;5:29–41. doi: 10.1038/nrc1527. [DOI] [PubMed] [Google Scholar]
- 13.Yang RY, Rabinovich GA, Liu FT. Galectins: structure, function and therapeutic potential. Expert Rev Mol Med. 2008;10:e17. doi: 10.1017/S1462399408000719. [DOI] [PubMed] [Google Scholar]
- 14.Cumming R, Liu FT, et al. Galectins. In: Varki A, et al., editors. Essentials of glycobiology. 2. New York: Cold Spring Harbor Laboratory Press; 2009. pp. 475–487. [PubMed] [Google Scholar]
- 15.Arthur CM, Baruffi MD, Cummings RD, Stowell SR. Evolving mechanistic insights into galectin functions. Methods Mol Biol. 2015;1207:1–35. doi: 10.1007/978-1-4939-1396-1_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laderach DJ, Gentilini L, Jaworski FM, Compagno D. Galectins as new prognostic markers and potential therapeutic targets for advanced prostate cancers. Prostate Cancer. 2013;2013:519436. doi: 10.1155/2013/519436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thijssen VL, Heusschen R, Caers J, Griffioen AW. Galectin expression in cancer diagnosis and prognosis: a systematic review. Biochim Biophys Acta. 2015;1855:235–247. doi: 10.1016/j.bbcan.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 18.Lahm H, André S, Hoeflich A, Fischer JR, Sordat B, Kaltner H, Wolf E, Gabius HJ. Comprehensive galectin fingerprinting in a panel of 61 human tumor cell lines by RT-PCR and its implications for diagnostic and therapeutic procedures. J Cancer Res Clin Oncol. 2001;127:375–386. doi: 10.1007/s004320000207. [DOI] [PubMed] [Google Scholar]
- 19.Satelli A, Rao PS, Gupta PK, Lockman PR, Srivenugopal KS, Rao US. Varied expression and localization of multiple galectins in different cancer cell lines. Oncol Rep. 2008;19:587–594. [PubMed] [Google Scholar]
- 20.Katzenmaier EM, André S, Kopitz J, Gabius HJ. Impact of sodium butyrate on the network of adhesion/growth-regulatory galectins in human colon cancer in vitro. Anticancer Res. 2014;34:5429–5438. [PubMed] [Google Scholar]
- 21.Klyosov AA, Traber PG. Galectins in disease and potential therapeutic approaches. ACS Symp Ser. 2012;1151:3–43. [Google Scholar]
- 22.Iurisci I, Tinari N, Natoli C, Angelucci D, Cianchetti E, Iacobelli S. Concentrations of galectin-3 in the sera of normal controls and cancer patients. Clin Cancer Res. 2000;6:1389–1393. [PubMed] [Google Scholar]
- 23.Saussez S, Lorfevre F, Lequeux T, Laurent G, Chantrain G, Vertongen F, Toubeau G, Decaestecker C, Kiss R. The determination of the levels of circulating galectin-1 and -3 in HNSCC patients could be used to monitor tumor progression and/or responses to therapy. Oral Oncol. 2008;44:86–93. doi: 10.1016/j.oraloncology.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 24.Sakaki M, Oka N, Nakanishi R, Yamaguchi K, Fukumori T, Kanayama HO. Serum level of galectin-3 in human bladder cancer. J Med Invest. 2008;55:127–132. doi: 10.2152/jmi.55.127. [DOI] [PubMed] [Google Scholar]
- 25.Vereecken P, Awada A, Suciu S, Castro G, Morandini R, Litynska A, Lienard D, Ezzedine K, Ghanem G, Heenen M. Evaluation of the prognostic significance of serum galectin-3 in American Joint Committee on Cancer stage III and stage IV melanoma patients. Melanoma Res. 2009;19:316–320. doi: 10.1097/CMR.0b013e32832ec001. [DOI] [PubMed] [Google Scholar]
- 26.Senapati S, Chaturvedi P, Chaney WG, Chakraborty S, Gnanapragassam VS, Sasson AR, Batra SK. Novel interaction of MUC4 and galectin: potential pathobiological implications for metastasis in lethal pancreatic cancer. Clin Cancer Res. 2011;17:267–274. doi: 10.1158/1078-0432.CCR-10-1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xie L, Ni WK, Chen XD, Xiao MB, Chen BY, He S, Lu CH, Li XY, Jiang F, Ni RZ. The expressions and clinical significances of tissue and serum galectin-3 in pancreatic carcinoma. J Cancer Res Clin Oncol. 2012;138:1035–1043. doi: 10.1007/s00432-012-1178-2. [DOI] [PubMed] [Google Scholar]
- 28.Watanabe M, Takemasa I, Kaneko N, Yokoyama Y, Matsuo E, Iwasa S, Mori M, Matsuura N, Monden M, Nishimura O. Clinical significance of circulating galectins as colorectal cancer markers. Oncol Rep. 2011;25:1217–1226. doi: 10.3892/or.2011.1198. [DOI] [PubMed] [Google Scholar]
- 29.Barrow H, Guo X, Wandall HH, Pedersen JW, Fu B, Zhao Q, Chen C, Rhodes JM, Yu LG. Serum galectin-2, -4, and -8 are greatly increased in colon and breast cancer patients and promote cancer cell adhesion to blood vascular endothelium. Clin Cancer Res. 2011;17:7035–7046. doi: 10.1158/1078-0432.CCR-11-1462. [DOI] [PubMed] [Google Scholar]
- 30.Barrow H, Rhodes JM, Yu LG. Simultaneous determination of serum galectin-3 and -4 levels detects metastases in colorectal cancer patients. Cell Oncol (Dordr) 2013;36:9–13. doi: 10.1007/s13402-012-0109-1. [DOI] [PubMed] [Google Scholar]
- 31.Klyosov AA, Traber PG (eds) (2012) Galectins and disease implications for targeted therapeutics, vol 1151. ACS Symposium Series, pp 1–443
- 32.Kozak K, Lanteigne J, Timoshenko AV (2014) Glycobiological aspects of cellular stress responses. Mol Biol Cell 25, Abstract No. P1500 (The Annual Meeting of the American Society for Cell Biology, December 6–10, 2014, Philadelphia)
- 33.Abedin MJ, Kashio Y, Seki M, Nakamura K, Hirashima M. Potential roles of galectins in myeloid differentiation into three different lineages. J Leukoc Biol. 2003;73:650–656. doi: 10.1189/jlb.0402163. [DOI] [PubMed] [Google Scholar]
- 34.Laderach DJ, Gentilini LD, Giribaldi L, Delgado VC, Nugnes L, Croci DO, Al Nakouzi N, Sacca P, Casas G, Mazza O, Shipp MA, Vazquez E, Chauchereau A, Kutok JL, Rodig SJ, Elola MT, Compagno D, Rabinovich GA. A unique galectin signature in human prostate cancer progression suggests galectin-1 as a key target for treatment of advanced disease. Cancer Res. 2013;73:86–96. doi: 10.1158/0008-5472.CAN-12-1260. [DOI] [PubMed] [Google Scholar]
- 35.Stowell SR, Cummings RD (eds) (2015) Galectins: methods and protocols. Meth Mol Biol 1207: 1–488
- 36.Compagno D, Jaworski FM, Gentilini L, Contrufo G, González Pérez I, Elola MT, Pregi N, Rabinovich GA, Laderach DJ. Galectins: major signaling modulators inside and outside the cell. Curr Mol Med. 2014;14:630–636. doi: 10.2174/1566524014666140603101953. [DOI] [PubMed] [Google Scholar]
- 37.Vladoiu MC, Labrie M, St-Pierre Y. Intracellular galectins in cancer cells: potential new targets for therapy (Review) Int J Oncol. 2014;44:1001–1014. doi: 10.3892/ijo.2014.2267. [DOI] [PubMed] [Google Scholar]
- 38.Le QT, Shi G, Cao H, Nelson DW, Wang Y, Chen EY, Zhao S, Kong C, Richardson D, O’Byrne KJ, Giaccia AJ, Koong AC. Galectin-1: a link between tumor hypoxia and tumor immune privilege. J Clin Oncol. 2005;23:8932–8941. doi: 10.1200/JCO.2005.02.0206. [DOI] [PubMed] [Google Scholar]
- 39.Perillo NL, Pace KE, Seilhamer JJ, Baum LG. Apoptosis of T cells mediated by galectin-1. Nature. 1995;378:736–739. doi: 10.1038/378736a0. [DOI] [PubMed] [Google Scholar]
- 40.Chung CD, Patel VP, Moran M, Lewis LA, Miceli MC. Galectin-1 induces partial TCR ζ-chain phosphorylation and antagonizes processive TCR signal transduction. J Immunol. 2000;165:3722–3729. doi: 10.4049/jimmunol.165.7.3722. [DOI] [PubMed] [Google Scholar]
- 41.Rabinovich GA, Ariel A, Hershkoviz R, Hirabayashi J, Kasai KI, Lider O. Specific inhibition of T-cell adhesion to extracellular matrix and proinflammatory cytokine secretion by human recombinant galectin-1. Immunology. 1999;97:100–106. doi: 10.1046/j.1365-2567.1999.00746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Croci DO, Salatino M, Rubinstein N, Cerliani JP, Cavallin LE, Leung HJ, Ouyang J, Ilarregui JM, Toscano MA, Domaica CI, Croci MC, Shipp MA, Mesri EA, Albini A, Rabinovich GA. Disrupting galectin-1 interactions with N-glycans suppresses hypoxia-driven angiogenesis and tumorigenesis in Kaposi’s sarcoma. J Exp Med. 2012;209:1985–2000. doi: 10.1084/jem.20111665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.White NM, Masui O, Newsted D, Scorilas A, Romaschin AD, Bjarnason GA, Siu KW, Yousef GM. Galectin-1 has potential prognostic significance and is implicated in clear cell renal cell carcinoma progression through the HIF/mTOR signaling axis. Br J Cancer. 2014;110:1250–1259. doi: 10.1038/bjc.2013.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao XY, Chen TT, Xia L, Guo M, Xu Y, Yue F, Jiang Y, Chen GQ, Zhao KW. Hypoxia inducible factor-1 mediates expression of galectin-1: the potential role in migration/invasion of colorectal cancer cells. Carcinogenesis. 2010;31:1367–1375. doi: 10.1093/carcin/bgq116. [DOI] [PubMed] [Google Scholar]
- 45.Zhao XY, Zhao KW, Jiang Y, Zhao M, Chen GQ. Synergistic induction of galectin-1 by CCAAT/enhancer binding protein and hypoxia-inducible factor 1 and its role in differentiation of acute myeloid leukemic cells. J Biol Chem. 2011;286:36808–36819. doi: 10.1074/jbc.M111.247262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Juszczynski P, Ouyang J, Monti S, Rodig SJ, Takeyama K, Abramson J, Chen W, Kutok JL, Rabinovich GA, Shipp MA. The AP1-dependent secretion of galectin-1 by Reed Sternberg cells fosters immune privilege in classical Hodgkin lymphoma. Proc Natl Acad Sci USA. 2007;104:13134–13139. doi: 10.1073/pnas.0706017104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Seropian IM, Cerliani JP, Toldo S, Van Tassell BW, Ilarregui JM, González GE, Matoso M, Salloum FN, Melchior R, Gelpi RJ, Stupirski JC, Benatar A, Gómez KA, Morales C, Abbate A, Rabinovich GA. Galectin-1 controls cardiac inflammation and ventricular remodeling during acute myocardial infarction. Am J Pathol. 2013;182:29–40. doi: 10.1016/j.ajpath.2012.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dias-Baruffi M, Stowell SR, Song SC, Arthur CM, Cho M, Rodrigues LC, Montes MA, Rossi MA, James JA, McEver RP, Cummings RD. Differential expression of immunomodulatory galectin-1 in peripheral leukocytes and adult tissues and its cytosolic organization in striated muscle. Glycobiology. 2010;20:507–520. doi: 10.1093/glycob/cwp203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Woolley JF, Al-Rubeai M. The isolation and identification of a secreted biomarker associated with cell stress in serum-free CHO cell culture. Biotechnol Bioeng. 2009;104:590–600. doi: 10.1002/bit.22408. [DOI] [PubMed] [Google Scholar]
- 50.Iwamoto M, Taguchi C, Sasaguri K, Kubo KY, Horie H, Yamamoto T, Onozuka M, Sato S, Kadoya T. The galectin-1 level in serum as a novel marker for stress. Glycoconj J. 2010;27:419–425. doi: 10.1007/s10719-010-9288-z. [DOI] [PubMed] [Google Scholar]
- 51.Strik HM, Schmidt K, Lingor P, Tönges L, Kugler W, Nitsche M, Rabinovich GA, Bähr M. Galectin-1 expression in human glioma cells: modulation by ionizing radiation and effects on tumor cell proliferation and migration. Oncol Rep. 2007;18:483–488. [PubMed] [Google Scholar]
- 52.Le Mercier M, Mathieu V, Haibe-Kains B, Bontempi G, Mijatovic T, Decaestecker C, Kiss R, Lefranc F. Knocking down galectin 1 in human hs683 glioblastoma cells impairs both angiogenesis and endoplasmic reticulum stress responses. J Neuropathol Exp Neurol. 2008;67:456–469. doi: 10.1097/NEN.0b013e318170f892. [DOI] [PubMed] [Google Scholar]
- 53.Paclik D, Berndt U, Guzy C, Dankof A, Danese S, Holzloehner P, Rosewicz S, Wiedenmann B, Wittig BM, Dignass AU, Sturm A. Galectin-2 induces apoptosis of lamina propria T lymphocytes and ameliorates acute and chronic experimental colitis in mice. J Mol Med. 2008;86:1395–1406. doi: 10.1007/s00109-007-0290-2. [DOI] [PubMed] [Google Scholar]
- 54.Loser K, Sturm A, Voskort M, Kupas V, Balkow S, Auriemma M, Sternemann C, Dignass AU, Luger TA, Beissert S. Galectin-2 suppresses contact allergy by inducing apoptosis in activated CD8+ T cells. J Immunol. 2009;182:5419–5429. doi: 10.4049/jimmunol.0802308. [DOI] [PubMed] [Google Scholar]
- 55.Dvoránková B, Lacina L, Smetana K, Jr, Lensch M, Manning JC, André S, Gabius HJ. Human galectin-2: nuclear presence in vitro and its modulation by quiescence/stress factors. Histol Histopathol. 2008;23:167–178. doi: 10.14670/HH-23.167. [DOI] [PubMed] [Google Scholar]
- 56.Chen C, Duckworth CA, Fu B, Pritchard DM, Rhodes JM, Yu LG. Circulating galectins -2, -4 and -8 in cancer patients make important contributions to the increased circulation of several cytokines and chemokines that promote angiogenesis and metastasis. Br J Cancer. 2014;110:741–752. doi: 10.1038/bjc.2013.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jung JH, Kim HJ, Yeom J, Yoo C, Shin J, Yoo J, Kang CS, Lee C. Lowered expression of galectin-2 is associated with lymph node metastasis in gastric cancer. J Gastroenterol. 2012;47:37–48. doi: 10.1007/s00535-011-0463-1. [DOI] [PubMed] [Google Scholar]
- 58.Polyak K, Xia Y, Zweier JL, Kinzler KW, Vogelstein B. A model for p53-induced apoptosis. Nature. 1997;389:300–305. doi: 10.1038/38525. [DOI] [PubMed] [Google Scholar]
- 59.Bernerd F, Sarasin A, Magnaldo T. Galectin-7 overexpression is associated with the apoptotic process in UVB-induced sunburn keratinocytes. Proc Natl Acad Sci USA. 1999;96:11329–11334. doi: 10.1073/pnas.96.20.11329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yamaguchi T, Hiromasa K, Kabashima-Kubo R, Yoshioka M, Nakamura M. Galectin-7, induced by cis-urocanic acid and ultraviolet B irradiation, down-modulates cytokine production by T lymphocytes. Exp Dermatol. 2013;22:840–842. doi: 10.1111/exd.12268. [DOI] [PubMed] [Google Scholar]
- 61.Villeneuve C, Baricault L, Canelle L, Barboule N, Racca C, Monsarrat B, Magnaldo T, Larminat F. Mitochondrial proteomic approach reveals galectin-7 as a novel BCL-2 binding protein in human cells. Mol Biol Cell. 2011;22:999–1013. doi: 10.1091/mbc.E10-06-0534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kuwabara I, Kuwabara Y, Yang RY, Schuler M, Green DR, Zuraw BL, Hsu DK, Liu FT. Galectin-7 (PIG1) exhibits pro-apoptotic function through JNK activation and mitochondrial cytochrome c release. J Biol Chem. 2002;277:3487–3497. doi: 10.1074/jbc.M109360200. [DOI] [PubMed] [Google Scholar]
- 63.Lee JS, Ys Lee, Jeon B, Yj Jeon, Yoo H, Kim TY. EC-SOD induces apoptosis through COX-2 and galectin-7 in the epidermis. J Dermatol Sci. 2012;65:126–133. doi: 10.1016/j.jdermsci.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 64.St-Pierre Y, Campion CG, Grosset AA. A distinctive role for galectin-7 in cancer? Front Biosci (Landmark Ed) 2012;17:438–450. doi: 10.2741/3937. [DOI] [PubMed] [Google Scholar]
- 65.Campion CG, Labrie M, Lavoie G, St-Pierre Y. Expression of galectin-7 is induced in breast cancer cells by mutant p53. PLoS One. 2013;8:e72468. doi: 10.1371/journal.pone.0072468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Campion CG, Labrie M, Grosset AA, St-Pierre Y. The CCAAT/enhancer-binding protein beta-2 isoform (CEBPβ-2) upregulates galectin-7 expression in human breast cancer cells. PLoS One. 2014;9:e95087. doi: 10.1371/journal.pone.0095087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.van der Krieken SE, Popeijus HE, Mensink RP, Plat J. CCAAT/enhancer binding protein β in relation to ER stress, inflammation, and metabolic disturbances. Biomed Res Int. 2015;2015:324815. doi: 10.1155/2015/324815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dyer KD, Rosenberg HF. Eosinophil Charcot–Leyden crystal protein binds to β-galactoside sugars. Life Sci. 1996;58:2073–2082. doi: 10.1016/0024-3205(96)00201-9. [DOI] [PubMed] [Google Scholar]
- 69.Swaminathan GJ, Leonidas DD, Savage MP, Ackerman SJ, Acharya KR. Selective recognition of mannose by the human eosinophil Charcot–Leyden crystal protein (galectin-10): a crystallographic study at 1.8 A resolution. Biochemistry. 1999;38:13837–13843. doi: 10.1021/bi990756e. [DOI] [PubMed] [Google Scholar]
- 70.Ackerman SJ, Liu L, Kwatia MA, Savage MP, Leonidas DD, Swaminathan GJ, Acharya KR. Charcot–Leyden crystal protein (galectin-10) is not a dual function galectin with lysophospholipase activity but binds a lysophospholipase inhibitor in a novel structural fashion. J Biol Chem. 2002;277:14859–14868. doi: 10.1074/jbc.M200221200. [DOI] [PubMed] [Google Scholar]
- 71.Kubach J, Lutter P, Bopp T, Stoll S, Becker C, Huter E, Richter C, Weingarten P, Warger T, Knop J, Müllner S, Wijdenes J, Schild H, Schmitt E, Jonuleit H. Human CD4+ CD25+ regulatory T cells: proteome analysis identifies galectin-10 as a novel marker essential for their anergy and suppressive function. Blood. 2007;110:1550–1558. doi: 10.1182/blood-2007-01-069229. [DOI] [PubMed] [Google Scholar]
- 72.Devouassoux G, Pachot A, Laforest L, Diasparra J, Freymond N, Van Ganse E, Mougin B, Pacheco Y. Galectin-10 mRNA is overexpressed in peripheral blood of aspirin-induced asthma. Allergy. 2008;63:125–131. doi: 10.1111/j.1398-9995.2007.01558.x. [DOI] [PubMed] [Google Scholar]
- 73.Chua JC, Douglass JA, Gillman A, O’Hehir RE, Meeusen EN. Galectin-10, a potential biomarker of eosinophilic airway inflammation. PLoS One. 2012;7:e42549. doi: 10.1371/journal.pone.0042549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Negrete-Garcia MC, Jiménez-Torres CY, Alvarado-Vásquez N, Montes-Vizuet AR, Velázquez-Rodriguez JR, Jimenez-Martinez MC, Teran-Juárez LM. Galectin-10 is released in the nasal lavage fluid of patients with aspirin-sensitive respiratory disease. Scientific World J. 2012;2012:474020. doi: 10.1100/2012/474020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.De Re V, Simula MP, Cannizzaro R, Pavan A, De Zorzi MA, Toffoli G, Canzonieri V. Galectin-10, eosinophils, and celiac disease. Ann N Y Acad Sci. 2009;1173:357–364. doi: 10.1111/j.1749-6632.2009.04627.x. [DOI] [PubMed] [Google Scholar]
- 76.Dyer KD, Rosenberg HF. Transcriptional regulation of galectin-10 (eosinophil Charcot–Leyden crystal protein): a GC box (−44 to −50) controls butyric acid induction of gene expression. Life Sci. 2001;69:201–212. doi: 10.1016/S0024-3205(01)01104-3. [DOI] [PubMed] [Google Scholar]
- 77.Bohn H, Kraus W, Winckler W. Purification and characterization of two new soluble placental tissue proteins (PP13 and PP17) Oncodev Biol Med. 1983;4:343–350. [PubMed] [Google Scholar]
- 78.Visegrády B, Than NG, Kilár F, Sümegi B, Than GN, Bohn H. Homology modelling and molecular dynamics studies of human placental tissue protein 13 (galectin-13) Protein Eng. 2001;14:875–880. doi: 10.1093/protein/14.11.875. [DOI] [PubMed] [Google Scholar]
- 79.Than NG, Pick E, Bellyei S, Szigeti A, Burger O, Berente Z, Janaky T, Boronkai A, Kliman H, Meiri H, Bohn H, Than GN, Sumegi B. Functional analyses of placental protein 13/galectin-13. Eur J Biochem. 2004;271:1065–1078. doi: 10.1111/j.1432-1033.2004.04004.x. [DOI] [PubMed] [Google Scholar]
- 80.Orendi K, Gauster M, Moser G, Meiri H, Huppertz B. Effects of vitamins C and E, acetylsalicylic acid and heparin on fusion, beta-hCG and PP13 expression in BeWo cells. Placenta. 2010;31:431–438. doi: 10.1016/j.placenta.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 81.Than NG, Sumegi B, Than GN, Berente Z, Bohn H. Isolation and sequence analysis of a cDNA encoding human placental tissue protein 13 (PP13), a new lysophospholipase, homologue of human eosinophil Charcot–Leyden Crystal protein. Placenta. 1999;20:703–710. doi: 10.1053/plac.1999.0436. [DOI] [PubMed] [Google Scholar]
- 82.Masoura S, Kalogiannidis IA, Gitas G, Goutsioulis A, Koiou E, Athanasiadis A, Vavatsi N. Biomarkers in pre-eclampsia: a novel approach to early detection of the disease. J Obstet Gynaecol. 2012;32:609–616. doi: 10.3109/01443615.2012.709290. [DOI] [PubMed] [Google Scholar]
- 83.Petla LT, Chikkala R, Ratnakar KS, Kodati V, Sritharan V. Biomarkers for the management of pre-eclampsia in pregnant women. Indian J Med Res. 2013;138:60–67. [PMC free article] [PubMed] [Google Scholar]
- 84.Nicolaides KH, Bindra R, Turan OM, Chefetz I, Sammar M, Meiri H, Tal J, Cuckle HS. A novel approach to first-trimester screening for early pre-eclampsia combining serum PP-13 and Doppler ultrasound. Ultrasound Obstet Gynecol. 2006;27:13–17. doi: 10.1002/uog.2686. [DOI] [PubMed] [Google Scholar]
- 85.Than NG, Abdul Rahman O, Magenheim R, Nagy B, Fule T, Hargitai B, Sammar M, Hupuczi P, Tarca AL, Szabo G, Kovalszky I, Meiri H, Sziller I, Rigo J, Jr, Romero R, Papp Z. Placental protein 13 (galectin-13) has decreased placental expression but increased shedding and maternal serum concentrations in patients presenting with preterm pre-eclampsia and HELLP syndrome. Virchows Arch. 2008;453:387–400. doi: 10.1007/s00428-008-0658-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Than NG, Romero R, Goodman M, Weckle A, Xing J, Dong Z, Xu Y, Tarquini F, Szilagyi A, Gal P, Hou Z, Tarca AL, Kim CJ, Kim JS, Haidarian S, Uddin M, Bohn H, Benirschke K, Santolaya-Forgas J, Grossman LI, Erez O, Hassan SS, Zavodszky P, Papp Z, Wildman DE. A primate subfamily of galectins expressed at the maternal-fetal interface that promote immune cell death. Proc Natl Acad Sci USA. 2009;106:9731–9736. doi: 10.1073/pnas.0903568106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dunphy JL, Barcham GJ, Bischof RJ, Young AR, Nash A, Meeusen EN. Isolation and characterization of a novel eosinophil-specific galectin released into the lungs in response to allergen challenge. J Biol Chem. 2002;277:14916–14924. doi: 10.1074/jbc.M200214200. [DOI] [PubMed] [Google Scholar]
- 88.Young AR, Barcham GJ, Kemp JM, Dunphy JL, Nash A, Meeusen EN. Functional characterization of an eosinophil-specific galectin, ovine galectin-14. Glycoconj J. 2009;26:423–432. doi: 10.1007/s10719-008-9190-0. [DOI] [PubMed] [Google Scholar]
- 89.Hoorens P, Rinaldi M, Mihi B, Dreesen L, Grit G, Meeusen E, Li RW, Geldhof P. Galectin-11 induction in the gastrointestinal tract of cattle following nematode and protozoan infections. Parasite Immunol. 2011;33:669–678. doi: 10.1111/j.1365-3024.2011.01336.x. [DOI] [PubMed] [Google Scholar]
- 90.Zeng Y, Danielson KG, Albert TJ, Shapiro IM, Risbud MV. HIF-1 alpha is a regulator of galectin-3 expression in the intervertebral disc. J Bone Miner Res. 2007;22:1851–1861. doi: 10.1359/jbmr.070620. [DOI] [PubMed] [Google Scholar]
- 91.Olbryt M, Jarzab M, Jazowiecka-Rakus J, Simek K, Szala S, Sochanik A. Gene expression profile of B 16(F10) murine melanoma cells exposed to hypoxic conditions in vitro. Gene Expr. 2006;13:191–203. doi: 10.3727/000000006783991818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Olbryt M, Habryka A, Tyszkiewicz T, Rusin A, Cichoń T, Jarząb M, Krawczyk Z. Melanoma-associated genes, MXI1, FN1, and NME1, are hypoxia responsive in murine and human melanoma cells. Melanoma Res. 2011;21:417–425. doi: 10.1097/CMR.0b013e328348db2f. [DOI] [PubMed] [Google Scholar]
- 93.de Oliveira JT, Gartner F. Dynamic tuning of galectins and their binding sites during mammary tumor progression and metastasis. ACS Symp Ser. 2012;1151:181–194. doi: 10.1021/bk-2012-1115.ch011. [DOI] [Google Scholar]
- 94.Hu R, Jin H, Zhou S, Yang P, Li X. Proteomic analysis of hypoxia-induced responses in the syncytialization of human placental cell line BeWo. Placenta. 2007;28:399–407. doi: 10.1016/j.placenta.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 95.Rêgo MJ, Vieira de Mello GS, da Silva Santos CA, Chammas R, Beltrão EI. Implications on glycobiological aspects of tumor hypoxia in breast ductal carcinoma in situ. Med Mol Morphol. 2013;46:92–96. doi: 10.1007/s00795-013-0013-4. [DOI] [PubMed] [Google Scholar]
- 96.Akahani S, Nangia-Makker P, Inohara H, Kim HR, Raz A. Galectin-3: a novel antiapoptotic molecule with a functional BH1 (NWGR) domain of Bcl-2 family. Cancer Res. 1997;57:5272–5276. [PubMed] [Google Scholar]
- 97.Ikemori RY, Machado CM, Furuzawa KM, Nonogaki S, Osinaga E, Umezawa K, de Carvalho MA, Verinaud L, Chammas R. Galectin-3 up-regulation in hypoxic and nutrient deprived microenvironments promotes cell survival. PLoS One. 2014;9:e111592. doi: 10.1371/journal.pone.0111592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Doverhag C, Hedtjärn M, Poirier F, Mallard C, Hagberg H, Karlsson A, Sävman K. Galectin-3 contributes to neonatal hypoxic-ischemic brain injury. Neurobiol Dis. 2010;38:36–46. doi: 10.1016/j.nbd.2009.12.024. [DOI] [PubMed] [Google Scholar]
- 99.Karlsson A, Follin P, Leffler H, Dahlgren C. Galectin-3 activates the NADPH oxidase in exudated but not peripheral blood neutrophils. Blood. 1998;91:3430–3438. [PubMed] [Google Scholar]
- 100.Hsu DK, Yang RY, Pan Z, Yu L, Salomon DR, Fung-Leung WP, Liu FT. Targeted disruption of the galectin-3 gene results in attenuated peritoneal inflammatory responses. Am J Pathol. 2000;156:1073–1083. doi: 10.1016/S0002-9440(10)64975-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sano H, Hsu DK, Yu L, Apgar JR, Kuwabara I, Yamanaka T, Hirashima M, Liu FT. Human galectin-3 is a novel chemoattractant for monocytes and macrophages. J Immunol. 2000;165:2156–2164. doi: 10.4049/jimmunol.165.4.2156. [DOI] [PubMed] [Google Scholar]
- 102.Fernandez GC, Ilarregui JM, Rubel CJ, Toscano MA, Gomez SA, Beigier Bompadre M, Isturiz MA, Rabinovich GA, Palermo MS. Galectin-3 and soluble fibrinogen act in concert to modulate neutrophil activation and survival: involvement of alternative MAPK pathways. Glycobiology. 2005;15:519–527. doi: 10.1093/glycob/cwi026. [DOI] [PubMed] [Google Scholar]
- 103.Jeng KC, Frigeri LG, Liu FT. An endogenous lectin, galectin-3 (εBP/Mac-2), potentiates IL-1 production by human monocytes. Immunol Lett. 1994;42:113–116. doi: 10.1016/0165-2478(94)90072-8. [DOI] [PubMed] [Google Scholar]
- 104.Satoh K, Niwa M, Binh NH, Nakashima M, Kobayashi K, Takamatsu M, Hara A. Increase of galectin-3 expression in microglia by hyperthermia in delayed neuronal death of hippocampal CA1 following transient forebrain ischemia. Neurosci Lett. 2011;504:199–203. doi: 10.1016/j.neulet.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 105.Satoh K, Niwa M, Goda W, Binh NH, Nakashima M, Takamatsu M, Hara A. Galectin-3 expression in delayed neuronal death of hippocampal CA1 following transient forebrain ischemia, and its inhibition by hypothermia. Brain Res. 2011;1382:266–274. doi: 10.1016/j.brainres.2011.01.049. [DOI] [PubMed] [Google Scholar]
- 106.Lee YJ, Song YK. Cooperative interaction between interleukin 10 and galectin-3 against liver ischemia-reperfusion injury. Clin Cancer Res. 2002;8:217–220. [PubMed] [Google Scholar]
- 107.Sunil VR, Patel-Vayas K, Shen J, Laskin JD, Laskin DL. Classical and alternative macrophage activation in the lung following ozone-induced oxidative stress. Toxicol Appl Pharmacol. 2012;263:195–202. doi: 10.1016/j.taap.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sunil VR, Vayas KN, Massa CB, Gow AJ, Laskin JD, Laskin DL. Ozone-induced injury and oxidative stress in bronchiolar epithelium are associated with altered pulmonary mechanics. Toxicol Sci. 2013;133:309–319. doi: 10.1093/toxsci/kft071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cao Z, Said N, Amin S, Wu HK, Bruce A, Garate M, Hsu DK, Kuwabara I, Liu FT, Panjwani N. Galectins-3 and -7, but not galectin-1, play a role in re-epithelialization of wounds. J Biol Chem. 2002;277:42299–42305. doi: 10.1074/jbc.M200981200. [DOI] [PubMed] [Google Scholar]
- 110.Henderson NC, Sethi T. The regulation of inflammation by galectin-3. Immunol Rev. 2009;230:160–171. doi: 10.1111/j.1600-065X.2009.00794.x. [DOI] [PubMed] [Google Scholar]
- 111.Shah RV, Chen-Tournoux AA, Picard MH, van Kimmenade RR, Januzzi JL. Galectin-3, cardiac structure and function, and long-term mortality in patients with acutely decompensated heart failure. Eur J Heart Fail. 2010;12:826–832. doi: 10.1093/eurjhf/hfq091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lok DJ, Lok SI, Bruggink-André de la Porte PW, Badings E, Lipsic E, van Wijngaarden J, de Boer RA, van Veldhuisen DJ, van der Meer P. Galectin-3 is an independent marker for ventricular remodeling and mortality in patients with chronic heart failure. Clin Res Cardiol. 2013;102:103–110. doi: 10.1007/s00392-012-0500-y. [DOI] [PubMed] [Google Scholar]
- 113.Srivatsan V, George M, Shanmugam E. Utility of galectin-3 as a prognostic biomarker in heart failure: where do we stand? Eur J Prev Cardiol. 2014 doi: 10.1177/2047487314552797. [DOI] [PubMed] [Google Scholar]
- 114.Seddon M, Looi YH, Shah AM. Oxidative stress and redox signalling in cardiac hypertrophy and heart failure. Heart. 2007;93:903–907. doi: 10.1136/hrt.2005.068270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Carvalho RS, Fernandes VC, Nepomuceno TC, Rodrigues DC, Woods NT, Suarez-Kurtz G, Chammas R, Monteiro AN, Carvalho MA. Characterization of LGALS3 (galectin-3) as a player in DNA damage response. Cancer Biol Ther. 2014;15:840–850. doi: 10.4161/cbt.28873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dumic J, Lauc G, Flögel M. Expression of galectin-3 in cells exposed to stress-roles of jun and NF-κB. Cell Physiol Biochem. 2000;10:149–158. doi: 10.1159/000016345. [DOI] [PubMed] [Google Scholar]
- 117.Dumić J, Barisić K, Flögel M, Lauc G. Galectin-3 decreases in mice exposed to immobilization stress. Stress. 2000;3:241–246. doi: 10.3109/10253890009001128. [DOI] [PubMed] [Google Scholar]
- 118.Hokama A, Mizoguchi E, Sugimoto K, Shimomura Y, Tanaka Y, Yoshida M, Rietdijk ST, de Jong YP, Snapper SB, Terhorst C, Blumberg RS, Mizoguchi A. Induced reactivity of intestinal CD4(+) T cells with an epithelial cell lectin, galectin-4, contributes to exacerbation of intestinal inflammation. Immunity. 2004;20:681–693. doi: 10.1016/j.immuni.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 119.Nishida A, Nagahama K, Imaeda H, Ogawa A, Lau CW, Kobayashi T, Hisamatsu T, Preffer FI, Mizoguchi E, Ikeuchi H, Hibi T, Fukuda M, Andoh A, Blumberg RS, Mizoguchi A. Inducible colitis associated glycome capable of stimulating the proliferation of memory CD4+ T cells. J Exp Med. 2012;209:2383–2394. doi: 10.1084/jem.20112631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Paclik D, Lohse K, Wiedenmann B, Dignass AU, Sturm A. Galectin-2 and -4, but not galectin-1, promote intestinal epithelial wound healing in vitro through a TGF-β-independent mechanism. Inflamm Bowel Dis. 2008;14:1366–1372. doi: 10.1002/ibd.20499. [DOI] [PubMed] [Google Scholar]
- 121.Troncoso MF, Elola MT, Croci DO, Rabinovich GA. Integrating structure and function of ‘tandem-repeat’ galectins. Front Biosci (Schol Ed) 2012;4:864–887. doi: 10.2741/S305. [DOI] [PubMed] [Google Scholar]
- 122.Braccia A, Villani M, Immerdal L, Niels-Christiansen LL, Nystrøm BT, Hansen GH, Danielsen EM. Microvillar membrane microdomains exist at physiological temperature. Role of galectin-4 as lipid raft stabilizer revealed by “superrafts”. J Biol Chem. 2003;278:15679–15684. doi: 10.1074/jbc.M211228200. [DOI] [PubMed] [Google Scholar]
- 123.Danielsen EM, Hansen GH. Lipid raft organization and function in the small intestinal brush border. J Physiol Biochem. 2008;64:377–382. doi: 10.1007/BF03174093. [DOI] [PubMed] [Google Scholar]
- 124.Huflejt ME, Leffler H. Galectin-4 in normal tissues and cancer. Glycoconj J. 2004;20:247–255. doi: 10.1023/B:GLYC.0000025819.54723.a0. [DOI] [PubMed] [Google Scholar]
- 125.Gopalkrishnan RV, Roberts T, Tuli S, Kang D, Christiansen KA, Fisher PB. Molecular characterization of prostate carcinoma tumor antigen-1, PCTA-1, a human galectin-8 related gene. Oncogene. 2000;19:4405–4416. doi: 10.1038/sj.onc.1203767. [DOI] [PubMed] [Google Scholar]
- 126.Elola MT, Ferragut F, Cárdenas Delgado VM, Nugnes LG, Gentilini L, Laderach D, Troncoso MF, Compagno D, Wolfenstein-Todel C, Rabinovich GA. Expression, localization and function of galectin-8, a tandem-repeat lectin, in human tumors. Histol Histopathol. 2014;29:1093–1105. doi: 10.14670/HH-29.1093. [DOI] [PubMed] [Google Scholar]
- 127.Nishi N, Shoji H, Seki M, Itoh A, Miyanaka H, Yuube K, Hirashima M, Nakamura T. Galectin-8 modulates neutrophil function via interaction with integrin αM. Glycobiology. 2003;13:755–763. doi: 10.1093/glycob/cwg102. [DOI] [PubMed] [Google Scholar]
- 128.Bidon-Wagner N, Le Pennec JP. Human galectin-8 isoforms and cancer. Glycoconj J. 2004;19:557–563. doi: 10.1023/B:GLYC.0000014086.38343.98. [DOI] [PubMed] [Google Scholar]
- 129.Bidon N, Brichory F, Bourguet P, Le Pennec JP, Dazord L. Galectin-8: a complex sub-family of galectins (review) Int J Mol Med. 2008;8:245–250. doi: 10.3892/ijmm.8.3.245. [DOI] [PubMed] [Google Scholar]
- 130.Thomas MP, Lieberman J. Live or let die: posttranscriptional gene regulation in cell stress and cell death. Immunol Rev. 2013;253:237–252. doi: 10.1111/imr.12052. [DOI] [PubMed] [Google Scholar]
- 131.Wiersma VR, de Bruyn M, Helfrich W, Bremer E. Therapeutic potential of galectin-9 in human disease. Med Res Rev. 2013;33(Suppl 1):E102–E126. doi: 10.1002/med.20249. [DOI] [PubMed] [Google Scholar]
- 132.Heusschen R, Griffioen AW, Thijssen VL. Galectin-9 in tumor biology: a jack of multiple trades. Biochim Biophys Acta. 2013;1836:177–185. doi: 10.1016/j.bbcan.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 133.Imaizumi T, Kumagai M, Sasaki N, Kurotaki H, Mori F, Seki M, Nishi N, Fujimoto K, Tanji K, Shibata T, Tamo W, Matsumiya T, Yoshida H, Cui XF, Takanashi S, Hanada K, Okumura K, Yagihashi S, Wakabayashi K, Nakamura T, Hirashima M, Satoh K. Interferon-gamma stimulates the expression of galectin-9 in cultured human endothelial cells. J Leukoc Biol. 2002;72:486–491. [PubMed] [Google Scholar]
- 134.Imaizumi T, Yoshida H, Nishi N, Sashinami H, Nakamura T, Hirashima M, Ohyama C, Itoh K, Satoh K. Double-stranded RNA induces galectin-9 in vascular endothelial cells: involvement of TLR3, PI3 K, and IRF3 pathway. Glycobiology. 2007;17:12C–15C. doi: 10.1093/glycob/cwm045. [DOI] [PubMed] [Google Scholar]
- 135.Matsuura A, Tsukada J, Mizobe T, Higashi T, Mouri F, Tanikawa R, Yamauchi A, Hirashima M, Tanaka Y. Intracellular galectin-9 activates inflammatory cytokines in monocytes. Genes Cells. 2009;14:511–521. doi: 10.1111/j.1365-2443.2009.01287.x. [DOI] [PubMed] [Google Scholar]
- 136.Mengshol JA, Golden-Mason L, Arikawa T, Smith M, Niki T, McWilliams R, Randall JA, McMahan R, Zimmerman MA, RangachariM Dobrinskikh E, Busson P, Polyak SJ, HirashimaM Rosen HR. A crucial role for Kupffer cell-derived galectin-9 in regulation of T cell immunity in hepatitis C infection. PLoS One. 2010;5:e9504. doi: 10.1371/journal.pone.0009504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Asakura H, Kashio Y, Nakamura K, Seki M, Dai S, Shirato Y, Abedin MJ, Yoshida N, Nishi N, Imaizumi T, Saita N, Toyama Y, Takashima H, Nakamura T, Ohkawa M, Hirashima M. Selective eosinophil adhesion to fibroblast via IFN-gamma-induced galectin-9. J Immunol. 2002;169:5912–5918. doi: 10.4049/jimmunol.169.10.5912. [DOI] [PubMed] [Google Scholar]
- 138.Gieseke F, Kruchen A, Tzaribachev N, Bentzien F, Dominici M, Müller I. Proinflammatory stimuli induce galectin-9 in human mesenchymal stromal cells to suppress T-cell proliferation. Eur J Immunol. 2013;43:2741–2749. doi: 10.1002/eji.201343335. [DOI] [PubMed] [Google Scholar]
- 139.Yoshida H, Imaizumi T, Kumagai M, Kimura K, Satoh C, Hanada N, Fujimoto K, Nishi N, Tanji K, Matsumiya T, Mori F, Cui XF, Tamo W, Shibata T, Takanashi S, Okumura K, Nakamura T, Wakabayashi K, Hirashima M, Sato Y, Satoh K. Interleukin-1β stimulates galectin-9 expression in human astrocytes. NeuroReport. 2001;12:3755–3758. doi: 10.1097/00001756-200112040-00030. [DOI] [PubMed] [Google Scholar]
- 140.Steelman AJ, Smith R, 3rd, Welsh CJ, Li J. Galectin-9 protein is up-regulated in astrocytes by tumor necrosis factor and promotes encephalitogenic T-cell apoptosis. J Biol Chem. 2013;288:23776–23787. doi: 10.1074/jbc.M113.451658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Imaizumi T, Kumagai M, Nishi N, Hirashima M, Hatakeyama M, Tamo W, Yoshida H, Nakamura T, Okumura K, Satoh K. 15-deoxy-Δ(12,14)-prostaglandin J2 inhibits IFN-γ-induced galectin-9 expression in cultured human umbilical vein endothelial cells. Int Arch Allergy Immunol. 2003;131:57–561. doi: 10.1159/000070436. [DOI] [PubMed] [Google Scholar]
- 142.Wu C, Thalhamer T, Franca RF, Xiao S, Wang C, Hotta C, Zhu C, Hirashima M, Anderson AC, Kuchroo VK. Galectin-9-CD44 interaction enhances stability and function of adaptive regulatory T cells. Immunity. 2014;41:270–282. doi: 10.1016/j.immuni.2014.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yang RY, Hsu DK, Yu L, Ni J, Liu FT. Cell cycle regulation by galectin-12, a new member of the galectin superfamily. J Biol Chem. 2001;276:20252–20260. doi: 10.1074/jbc.M010914200. [DOI] [PubMed] [Google Scholar]
- 144.Hotta K, Funahashi T, Matsukawa Y, Takahashi M, Nishizawa H, Kishida K, Matsuda M, Kuriyama H, Kihara S, Nakamura T, Tochino Y, Bodkin NL, Hansen BC, Matsuzawa Y. Galectin-12, an adipose-expressed galectin-like molecule possessing apoptosis-inducing activity. J Biol Chem. 2001;276:34089–34097. doi: 10.1074/jbc.M105097200. [DOI] [PubMed] [Google Scholar]
- 145.Yang RY, Hsu DK, Yu L, Chen HY, Liu FT. Galectin-12 is required for adipogenic signaling and adipocyte differentiation. J Biol Chem. 2004;279:29761–29766. doi: 10.1074/jbc.M401303200. [DOI] [PubMed] [Google Scholar]
- 146.Yang RY, Havel PJ, Liu FT. Galectin-12: a protein associated with lipid droplets that regulates lipid metabolism and energy balance. Adipocyte. 2012;1:96–100. doi: 10.4161/adip.19465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Guardia CM, Gauto DF, Di Lella S, Rabinovich GA, Martí MA, Estrin DA. An integrated computational analysis of the structure, dynamics, and ligand binding interactions of the human galectin network. J Chem Inf Model. 2011;51:1918–1930. doi: 10.1021/ci200180h. [DOI] [PubMed] [Google Scholar]
- 148.Leung AK, Sharp PA. MicroRNA functions in stress responses. Mol Cell. 2010;40:205–215. doi: 10.1016/j.molcel.2010.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ramasamy S, Duraisamy S, Barbashov S, Kawano T, Kharbanda S, Kufe D. The MUC1 and galectin-3 oncoproteins function in a microRNA-dependent regulatory loop. Mol Cell. 2007;27:992–1004. doi: 10.1016/j.molcel.2007.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Maragkakis M, Alexiou P, Papadopoulos GL, Reczko M, Dalamagas T, Giannopoulos G, Goumas G, Koukis E, Kourtis K, Simossis VA, Sethupathy P, Vergoulis T, Koziris N, Sellis T, Tsanakas P, Hatzigeorgiou AG. Accurate microRNA target prediction correlates with protein repression levels. BMC Bioinformatics. 2009;10:295. doi: 10.1186/1471-2105-10-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Paraskevopoulou MD, Georgakilas G, Kostoulas N, Vlachos IS, Vergoulis T, Reczko M, Filippidis C, Dalamagas T, Hatzigeorgiou AG. DIANA-microT web server v5.0: service integration into miRNA functional analysis workflows. Nucleic Acids Res. 2013;41(Web Server issue):W169–W173. doi: 10.1093/nar/gkt393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lahm H, André S, Hoeflich A, Kaltner H, Siebert HC, Sordat B, von der Lieth CW, Wolf E, Gabius HJ. Tumor galectinology: insights into the complex network of a family of endogenous lectins. Glycoconj J. 2004;20:227–238. doi: 10.1023/B:GLYC.0000025817.24297.17. [DOI] [PubMed] [Google Scholar]
- 153.Chiariotti L, Salvatore P, Frunzio R, Bruni CB. Galectin genes: regulation of expression. Glycoconj J. 2004;19:441–449. doi: 10.1023/B:GLYC.0000014073.23096.3a. [DOI] [PubMed] [Google Scholar]
- 154.Benvenuto G, Carpentieri ML, Salvatore P, Cindolo L, Bruni CB, Chiariotti L. Cell-specific transcriptional regulation and reactivation of galectin-1 gene expression are controlled by DNA methylation of the promoter region. Mol Cell Biol. 1996;16:2736–2743. doi: 10.1128/MCB.16.6.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kim SJ, Hwang JA, Ro JY, Lee YS, Chun KH. Galectin-7 is epigenetically-regulated tumor suppressor in gastric cancer. Oncotarget. 2013;4:1461–1471. doi: 10.18632/oncotarget.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]