Abstract
The tissue inhibitors of metalloproteinases (TIMPs) are well recognized for their role in extracellular matrix remodeling by controlling the activity of matrix metalloproteinases (MMPs). Independent of MMP inhibition, TIMPs act as signaling molecules with cytokine-like activities thereby influencing various biological processes including cell growth, apoptosis, differentiation, angiogenesis, and oncogenesis. Recent studies on TIMP-1’s cytokine functions have identified complex regulatory networks involving a specific surface receptor and subsequent signaling pathways including miRNA-mediated posttranscriptional regulation of gene expression that ultimately control the fate and behavior of the cells. The present review summarizes the current knowledge on TIMP-1 as a cytokine modulator of cell functions, outlines recent progress in defining molecular pathways that transmit TIMP-1 signals from the cell periphery into the nucleus, and discusses TIMP-1’s role as a cytokine in the pathophysiology of cancer and other human diseases.
Keywords: Tissue inhibitor of metalloproteinases, TIMP-1 signaling pathway, CD63, Let-7f miRNA, Cell growth, Apoptosis, Cell differentiation, Cancer
Introduction
Tissue inhibitor of metalloproteinases (TIMP-1) was discovered in 1975 in form of a collagenase inhibitor present in human serum being released from skin fibroblasts [1, 2]. In 1985, a protein described to stimulate the growth of erythroid progenitor cells was cloned and found to be identical to TIMP-1 [3–5]. Between 1985 and 1996, the other three members of the TIMP family were identified by molecular cloning and characterization at the protein level [6–8]. The four human TIMPs are the natural endogenous inhibitors of the matrix metalloproteinases (MMPs), a family of endopeptidases that regulate the turnover of extracellular matrix (ECM) and cleave various bioactive molecules including cytokines, chemokines and growth factors [9, 10]. Changes in TIMP expression levels were shown to correlate with the occurrence and progression of various diseases including cancer and heart failure [11–14].
Over a long period of time, TIMPs have been almost exclusively examined in their function as MMP inhibitors. Accumulating evidence from the past decade however clearly indicates that TIMPs have additional biological activities by acting as signaling molecules in their own right. This occurs independent of metalloproteinase inhibition by direct binding to specific surface receptors to induce cellular responses. These cytokine-like activities of TIMPs include the modulation of cell proliferation, apoptosis, differentiation, and angiogenesis [15]. The development of specific TIMP mutants devoid of MMP-inhibitory activity has facilitated a clear distinction of direct cytokine-like effects from indirect influence through TIMP inhibition of protease activity. By this means, substantial information has accumulated for cytokine functions of TIMP-1 as well as TIMP-2 [16]. In contrast, less data are available on TIMP-3 and TIMP-4 in terms of cellular effects induced by direct signaling. The present review summarizes recent progress in the understanding of TIMP-1’s activity as a cytokine with a focus on signaling pathways mediating its multiple effects in cells. Increased comprehension of the pleiotropic activities of TIMPs in tissue homeostasis and increased knowledge of the molecular mechanisms that control these functions would help to develop novel strategies in the treatment of cancer and other chronic diseases.
The TIMP family
The human genome has four genes encoding TIMPs designated as TIMP-1, TIMP-2, TIMP-3, and TIMP-4 [17]. They are 22–28 kDa proteins depending on the presence and degree of glycosylation. Structure, function and evolution of TIMPs have been recently reviewed in detail by Brew and Nagase [18]. TIMPs are expressed by a variety of cell types and present in most human tissues and body fluids. All four TIMPs act as potent regulators of the proteolytic activity of MMPs and, in some instances, of the disintegrin metalloproteinases (ADAMS) and the disintegrin metalloproteinases with thrombospondin motifs (ADAMTS) by forming non-covalent 1:1 stoichiometric complexes with their target proteases [18]. TIMPs comprise two distinct domains that are each stabilized by three disulfide bonds: an N-terminal domain of about 125 amino acids and a C-terminal domain of about 65 residues. Both domains have the ability to function independently; the N-terminal domains are fully active as inhibitors of MMPs, and the C-terminal domain is important in complex formation with pro-enzymes (proMMPs) [19]. Although TIMPs are about 40 % identical in amino acid sequence to each other, they differ in many aspects including solubility, interaction with proMMPs and regulation of expression. TIMP-1, for instance, is present in a soluble form while TIMP-3 is tightly bound to the ECM. In terms of MMP inhibition, TIMP-1 specifically interacts with proMMP-9 while TIMP-2 preferentially binds to proMMP-2. Moreover, TIMP-1 expression in cells is significantly regulated by growth factors and cytokines, whereas TIMP-2 biosynthesis is constitutive and less sensitive to external stimuli [15]. The characteristic features of the four human TIMPs are summarized in Table 1.
Table 1.
| Property | TIMP-1 | TIMP-2 | TIMP-3 | TIMP-4 |
|---|---|---|---|---|
| Mature protein (kDa) | 28 | 22 | 22 or 27 | 22 |
| N-glycosylation sites | 2 | 0 | 1 | 0 |
| Amino acid residues | 184 | 194 | 188 | 194 |
| pI | 8.5 | 6.5 | 9.1 | 7.2 |
| Protein expression | Inducible | Constitutive | Inducible | Inducible |
| proMMP binding | proMMP-9 | proMMP-2 | proMMP-2/-9 | proMMP-2 |
| MMP inhibition | All | All | All | Most |
| MT-MMP inhibition | Weak | Yes | Yes | Yes |
| ADAM inhibition | ADAM10 | ADAM12 | ADAM10/12/17/19/33; ADAMTS-1/-2/-4/-5 | ADAM17/28 |
| Protein localization | Soluble, cell surface | Soluble, cell surface | ECM bound, cell surface | Soluble, cell surface |
| Cell surface localization | Yes | Yes | Yes | Yes |
| Surface binding partners | CD63, LRP-1/MMP-9 | α3β1 integrin, LRP-1 | VEGFR2, Angiotensin-IIR, EFEMP1 | |
| Effect on proliferation | Positive and negative | Positive and negative | Positive | Positive and negative |
| Effect on apoptosis | Negative | Positive and negative | Positive | Negative |
| Effect on differentiation | Positive and negative | Positive | Positive and negative | |
| Tumor angiogenesis | Positive and negative | Negative | Negative | |
| Angiogenesis in 3D collagen or fibrin gels | No effect | Negative | Negative | Negative |
| Chromosomal location | Xp11.23-11.4 | 17q23, 17q25 | 22q12.1, 22q13.2 | 3p25 |
| mRNA (kb) | 0.9 | 1.0 and 3.5 | 5.0 | 1.4 |
| Nested synapsin gene | SYN1 | n/a | SYN3 | SYN2 |
| Genetic disorder | Idiopathic scoliosis | Sorsby fundus dystrophy | Kawasaki disease |
ADAM a disintegrin and metalloproteinase domain protease, ADAMTS a disintegrin and metalloproteinase domain protease with thrombospondin motifs, ECM extracellular matrix, EFEMP1 EGF-containing fibulin-like extracellular matrix protein-1, LRP-1 low density lipoprotein receptor-related protein-1, VEGFR2 vascular endothelial growth factor receptor 2
Orthologs of TIMPs have been identified in various animal species ranging from molluscs, worms and insects to vertebrates such as fish and birds, but they are absent in plants. The four human TIMPs appear to be expressed in all mammalian species [18]. However, their orthologs are not present in all vertebrates and TIMPs from invertebrates show increased variability in sequence and structure [18]. Several studies have demonstrated developmental defects in TIMP-deficient organisms in both mammalian and non-mammalian systems, indicating the importance of these proteins during embryonic development [20, 21].
TIMP regulation of cell functions: metalloproteinase-dependent and metalloproteinase-independent mechanisms
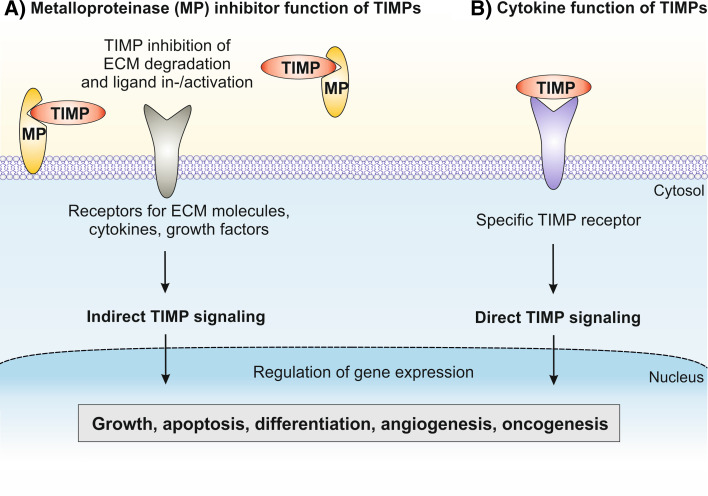
The four TIMPs inhibit the activity of all 23 MMPs found in humans. MMPs are secreted as soluble proteins or expressed on the cell surface regulating cellular interactions with the ECM [9]. The ECM is a dynamic network of macromolecules such as collagens, fibronectin, laminin and proteoglycans that represents an environment influencing the fate and behavior of cells. After disruption from their association with ECM, cells lose their differentiated phenotype and undergo anoikis (apoptotic cell death induced by loss of cell adhesion) [22, 23]. MMPs are key regulators of ECM turnover during normal and pathological processes including development, tissue remodeling and cell growth as well as tumor cell invasion and metastasis [24, 25]. The activities of MMPs that include collagenases, gelatinases, stromelysins, matrilysins and membrane-type MMPs are under tight control through inhibition by TIMPs. A fine-tuned balance between levels of MMPs and TIMPs controls the extent of local ECM degradation in the periphery of cells and thereby influences cellular processes such as migration, proliferation and survival. Moreover, MMPs as well as the membrane-anchored ADAMs affect the behavior of cells also by cleavage of cell surface-bound molecules including cytokines, chemokines, receptors and adhesion factors involved in cell growth and survival [10, 26]. As a matter of fact, a certain proportion of the previously reported TIMP activities on cell function can be explained by metalloproteinase inhibition (Fig. 1a).
Fig. 1.
Principal ways by which TIMPs can regulate cell functions. a By means of metalloproteinase-dependent mechanisms, TIMPs interact with soluble or membrane-bound MMPs or other metalloproteinases in the cellular periphery and thereby modulate the cleavage of ECM proteins including collagens, laminin and fibronectin or bioactive molecules such as cytokines, chemokines, and growth factors which activate or suppress receptor-mediated signaling in the cells. b By means of metalloproteinase-independent mechanisms, TIMPs similar to cytokines directly interact with specific surface-receptors that initiate intracellular signaling cascades resulting in altered gene expression and changes in cell behavior
However, an increasing number of TIMP-ascribed effects on cell proliferation, apoptosis, differentiation, and angiogenesis have been clearly demonstrated to be independent of MMP inhibition. This became possible by utilization of TIMP mutants that do not inhibit MMPs but still exhibited similar activity on cell function. Stetler-Stevenson and colleagues [27] were the first to describe the preparation of a TIMP variant devoid of MMP inhibitory activity nevertheless retaining its other biological activities. Basically, these TIMP mutants are engineered by appending one or two amino acid residues to the amino-terminal cysteine in position 1, which is conserved in all four human TIMPs. This modification results in a loss of inhibitory activity by disrupting the cysteine-1 interaction with the active zinc atom in the catalytic domain of metalloproteinases. These TIMP variants have become most helpful tools for investigators to assess whether TIMP effects are unique biological activities of this protein or dependent on MMP inhibition [28]. In fact, recent work by several laboratories has identified a general mechanism for members of the TIMP family to induce cellular response involving specific cell surface binding partners and downstream signaling pathways, similar to cytokine-mediated effects in cells (Fig. 1b).
TIMP-1 influence on cell growth
When the TIMP-1 cDNA was first cloned and sequenced [4], it was found to be identical to a protein that exhibits erythroid potentiating activity (EPA) [5]. EPA was identified as a T lymphoblastic factor present in serum, which promotes the growth of early erythroid progenitor cells by a mechanism involving direct cell surface binding [29]. EPA potentiates erythropoietin-stimulated colony formation by erythroid precursor cells. Beyond that activity, TIMP-1 was shown to have cell growth promoting properties in a wide array of cell types including keratinocytes, fibroblasts, chondrocytes, epithelial cells, breast carcinoma cells and various leukemic cell lines [30–32]. Interestingly, TIMP-2 had no influence on cell growth in these experiments, suggesting that these effects are TIMP-1 specific. Later studies revealed TIMP-2 to also have growth-promoting activity in erythroid precursors and other cell types [32–34]. TIMP-1’s stimulatory impact on cell proliferation is independent of its ability to inhibit MMPs. This has been demonstrated by use of TIMP-1 mutants that lack MMP inhibitory activity but fully retained erythroid potentiating activity, which was not the case by application of synthetic MMP inhibitors [35]. Stimulation of cell division was shown to require free TIMP-1 because its growth promoting activity was abolished by complex formation with either proMMPs or active MMPs [31]. In addition, former binding studies in keratinocytes indicated the presence of high affinity cell receptors with KD values in the low nanomolar range for TIMP-1 [30]. These findings strongly suggest that the effects of TIMP-1 on cell growth are mediated by direct binding to the cell surface through a cell receptor mechanism that remains to be identified. Binding of TIMP-1 to the cell surface is not competed by TIMP-2 suggesting that TIMP-1 and TIMP-2 have their own specific receptors [34].
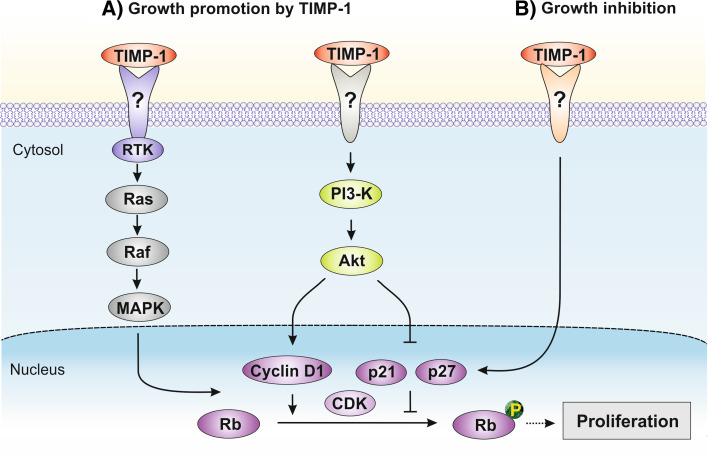
Several distinct signaling pathways have been implicated in TIMP’s growth promoting activity. Both TIMP-1 and TIMP-2 were shown to activate the G protein Ras by increasing Ras-GTP but utilizing different signaling pathways; TIMP-1 engages the classical mitogen activated protein kinase (MAPK) pathway involving receptor tyrosine kinase (RTK) activity and phosphorylation of Raf, whereas TIMP-2 signaling is mediated via protein kinase A activation and Ras/PI3-K complex formation [36]. These findings demonstrate that the mitogenic activities of TIMP-1 and TIMP-2 require different receptors and signaling pathways explaining the contextual functions of TIMPs in the promotion of cell growth. TIMP-1 but not a synthetic MMP inhibitor stimulates proliferation of aortic smooth muscle cells in association with activation of Ras, increased phosphorylation of extracellular signal-regulated kinase (ERK) and enhanced cyclin D1 expression involving the phosphoinositide 3-kinase (PI3-K) pathway [37], indicating these effects to be independent of MMP-inhibition. In fibroblasts, TIMP-1 increases proliferation through activation of PI3-K and phosphorylation of Akt thereby upregulating cyclin D1 and downregulating the cyclin D kinase (CDK) inhibitors p21 and p27, which promotes cell cycle progression [38] (Fig. 2a). Consistent with this model, TIMP-1 stimulation of growth in hepatic stellate cells correlates with Akt phosphorylation [39]. Moreover, TIMP-1 deficiency in hematopoietic stem cells isolated from TIMP-1-null mice display a dysregulated cell cycle distribution at the G1 phase accompanied with increased levels of p21, p53 and p57 [40].
Fig. 2.
Signaling pathways involved in TIMP-1-mediated promotion (a) and inhibition (b) of cell proliferation independent of MMP-inhibition
Recent findings indicate that TIMP-1 is important in the regulation of neural cell function involving metalloproteinase-dependent pathways because TIMP-1 interaction with MMPs was shown to modulate the outgrowth of cortical neurons and proliferation of astrocytes [41, 42].
Beside TIMP-1’s growth-promoting effects, there is evidence that TIMP-1 can also act as an inhibitor of cell proliferation. In mice deficient for TIMP-1 expression, mammary epithelial cell proliferation is upregulated and can be restored to basal level by the introduction of exogenous TIMP-1 into the animals [43]. In vitro studies in human MCF10A breast epithelial cells demonstrated that TIMP-1 downregulates the growth rate by inducing cell cycle arrest at G1 associated with a decrease in cyclin D1 and a simultaneous increase in the CDK inhibitor p27 [44]. This inhibits phosphorylation of the retinoblastoma (Rb) protein, which is important for cell cycle progression (Fig. 2b).
Together, these studies show that TIMP-1 regulates cell division by direct engagement of signaling pathways that affect the expression and activity of nuclear factors such as cyclin D1, p21, and p27. Further investigations on participating TIMP-1 surface receptors and intracellular signaling factors would greatly improve the understanding of positive and negative mechanisms by which TIMP-1 modulates cell proliferation.
TIMP-1 influence on apoptosis
In addition to its effects on cell growth, TIMP-1 is reported to suppress the process of programmed cell death. Burkitt’s lymphoma cell lines that express TIMP-1 are resistant to extrinsic and intrinsic (cold-shock, serum deprivation and γ-irradiation) apoptosis, whereas TIMP-1-negative cells are not [45]. In these studies, addition of recombinant TIMP-1 and transgenic expression of TIMP-1 in a TIMP-1 negative cell line reduced the susceptibility to induction of apoptosis through CD95-dependent and CD95-independent pathways involving diminishment of caspase-3 activity. This anti-apoptotic effect is not observed with TIMP-2 or application of a synthetic MMP inhibitor, indicating that the mechanism is receptor-specific and independent of MMP inhibition [45]. Furthermore, TIMP-1 suppression of apoptosis in lymphoma cells correlates with increased expression of the pro-survival protein B-cell lymphoma-extra large (Bcl-xL) and inhibitor of nuclear factor kappa B alpha (IκBα) protein as well as decreased nuclear factor kappa B (NFκB) activity [45], suggesting that these effects are mediated by a specific anti-apoptotic signaling pathway (Fig. 3). The survival-promoting activity of TIMP-1 is not restricted to tumor cells. In rat pancreatic islets, the expression of TIMP-1 but not TIMP-2 prevents cytokine-mediated apoptosis involving inhibition of NFκB [46]. In germinal center B cells, TIMP-1 upregulates the expression of survival factors including interleukin 10 that contributes to cell survival [47]. Besides that, TIMP-1 has anti-apoptotic activity in normal human granulocytes and endothelial cells although these effects were not shown to be MMP-independent [48, 49].
Fig. 3.
Signaling pathways involved in pro-survival activities of TIMP-1 independent of MMP-inhibition
For a long time, a putative receptor responsible for TIMP-1 binding on the cell surface and subsequent intracellular signaling was not characterized. In 2006, Jung and colleagues [50] reported that TIMP-1’s anti-apoptotic activity in MCF10A human breast epithelial cells is mediated through its binding to CD63, a member of the tetraspanin family. CD63 interacts with the β1 subunit of integrins and the TIMP-1-CD63-integrin β1 complex thus constitutively turns on survival signals via activation of focal adhesion kinase (FAK), PI3-K and ERK pathways [51, 52]. This TIMP-1-initiated mechanism protects the cells from intrinsic and extrinsic cell death in a fashion that is independent of its metalloproteinase inhibitory activity [51, 52]. Recently, TIMP-1 complex formation with CD63 and β1 integrin was reported to increase adhesion, migration and resistance to apoptosis in CD34+ hematopoietic stem/progenitor cells [53]. CD63 is a ubiquitous protein that is localized not only at the cell surface but also within the endosomal system after endocytosis via AP-2 and clathrin-coated pits [54]. This enables regulation of TIMP-1/CD63-mediated effects also through sequestration mechanisms.
In addition to complex formation with CD63, TIMP-1 binds with high affinity to proMMP-9. Both interactions are mediated by the C-terminal domain of TIMP-1 [18]. Thus, CD63 expressed on the cell surface and proMMP-9 present in the cell periphery compete for binding to the C-terminal domain of TIMP-1. As a consequence, elevated concentrations of proMMP-9 act as a sink by reducing free TIMP-1 limiting its interaction with CD63 that results in an attenuation of TIMP-1-mediated signaling and gene expression in these cells. This mechanism represents an important method in the control of TIMP-1’s cytokine activities.
Recent studies indicate that TIMP-1 suppression of apoptosis in UT7 erythroid cells is mediated through binding of TIMP-1 to proMMP-9 in complex with CD44 on the cell surface and involves signaling through the Janus kinase 2 (JAK2)/PI3-K/Akt/Bcl-2-associated death promoter (Bad) pathway [55–57]. TIMP-1 engagement of this pathway is independent on MMP-9 inhibition but requires proMMP-9 as an adaptor for binding to CD44 and subsequent intracellular signaling [56]. The authors demonstrate that sequential activation of Lyn, JAK2 and PI3-K plays a crucial role in the initial steps of this pathway resulting in the phosphorylation of Akt and Bad that eventually increases the release of anti-apoptotic Bcl-xL in the cells [55, 58] (Fig. 3). Consistently, upregulation of Bcl-xL was found to be associated with TIMP-1 protection from apoptosis in lymphoma cells [45]. Another anti-apoptotic protein of the Bcl-family, Bcl-2, increases TIMP-1 expression in breast epithelial cells, and TIMP-1 then promotes survival in these cells [59]. TIMP-1 protection of neurons from apoptosis induced by staurosporine and human immunodeficiency virus-1 is associated with stabilization of Bcl-xL and Bcl-2 protein in these cells and shown to be MMP-independent [60]. Treatment of bone marrow stromal cells with TIMP-1 preserves from intrinsic apoptosis via activation of PI3-K and c-Jun N-terminal kinase (JNK) pathways resulting in an upregulation of Bcl-2 and decrease of the pro-apoptotic Bcl-2-associated X (Bax) protein [61]. Similar to PI3 K/Akt, the ERK and JNK pathways are reported to phosphorylate Bad thereby preventing Bad association with anti-apoptotic Bcl-xL and Bcl-2 at the mitochondrial membrane [62, 63]. Together these findings indicate a central role of Bad and its interaction with Bcl-xL/Bcl-2 in the final steps of TIMP-1-promoted cell survival irrespective of the underlying upstream signaling pathways (Fig. 3).
A pro-apoptotic function of TIMP-1 is not reported. However, TIMP-3, which is unique amongst the TIMP family in that it specifically interacts with the ECM, was shown to promote Fas-dependent cell death in various cell types through inhibition of metalloproteinases [64, 65]. In contrast, TIMP-1 prevents this process independent of MMP-inhibition [52]. This indicates opposite roles and different mechanisms for TIMP-1 and TIMP-3 in the regulation of extrinsic apoptosis. The effect of TIMP-2 on programmed cell death has not been well characterized with conflicting reports in the literature and hitherto no clearly defined signaling pathways [66, 67].
Cell survival in general is significantly influenced by interactions of the cells with the ECM. Interruption of cell-matrix interaction in anchorage-dependent cells results in apoptotic cell death [22, 23]. Consistently, mammary epithelial cells overexpressing MMP-3 were shown to undergo programmed cell death, which was rescued by simultaneous production of TIMP-1 in these cells [68, 69]. TIMP-1 attenuation of apoptosis in activated hepatic stellate cells is dependent on MMP inhibition as demonstrated by utilization of non-MMP-inhibitory TIMP-1 mutants [70].
Taken together, TIMP-1 can regulate cell survival through direct engagement of receptor-mediated signaling and by indirect mechanisms based on the inhibition of metalloproteinases. These TIMP-1 pathways co-exist and their individual activities depend on the cellular and molecular context. This includes the ECM composition in the cellular environment, the presence of apoptotic stimuli, the availability of TIMP-1 receptors on the cell surface, and the levels of (pro)MMPs that bind to TIMP-1 and thereby limit the interaction of free TIMP-1 with its cell surface receptors. In fact, the TIMP-1/proMMP-9 complex binds to low density lipoprotein (LRP-1) on the surface of mouse embryonic fibroblasts and is subsequently internalized [71], representing a mechanism that modulates the amount of free TIMP-1 available for receptor binding and direct signaling. Further studies are required to evaluate whether cell type-specific differences may account for qualitative and quantitative variations in cellular response to TIMP-1.
TIMP-1 influence on cell differentiation
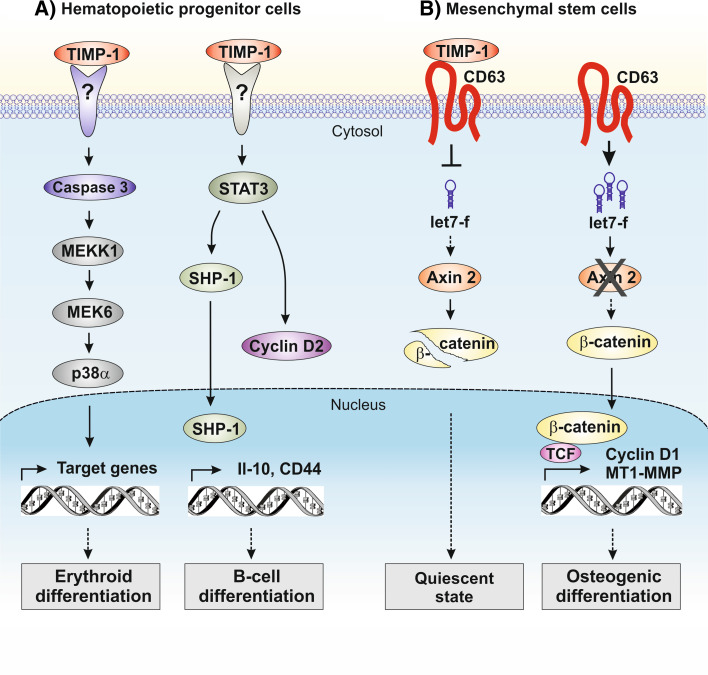
The inhibitory functions of TIMP-1 on cell growth and apoptosis are in close relationship to TIMP-1’s role as a promotor of cell differentiation. Early studies in erythroleukemic cells showed that TIMP-1 in addition to its growth stimulatory activity also influences the maturation of these cells [72]. More recent data reported by Petitfrere and colleagues [73, 74] demonstrate TIMP-1 to induce the differentiation of erythroid UT-7 cells, myeloid 32D cells and normal erythroid progenitors implicating p38 MAPK activity. In this cell model, TIMP-1 leads to the activation of caspase-3 that cleaves and thereby activates MEKK1, which phosphorylates MEK6 enabling its complex formation with p38α [74] (Fig. 4a). Remarkably, the TIMP-1-mediated caspase-3 activation in hematopoietic cells does not increase apoptotic cell death, which is thought to be a consequence of TIMP-1’s survival promoting effects in these cells [55].
Fig. 4.
Signaling pathways involved in TIMP-1-modulated differentiation of hematopoietic progenitor cells (a) and mesenchymal stem cells (b) independent of MMP-inhibition
Several studies reported by Stetler-Stevenson’s group [75, 76] demonstrate that TIMP-1 promotes survival and affects differentiation in normal and neoplastic B-cells. Because TIMP-1 is secreted from hematopoietic and stromal cells in the bone marrow, TIMP-1 can function as a regulator of hematopoiesis in both autocrine and paracrine fashion. TIMP-1 controls the differentiation of germinal center B-cells to plasma cells involving upregulation of Il-10 in these cells [47]. The TIMP-1-promoted plasmacytic/plasmablastic differentiation of Burkitt lymphoma cells is accompanied by activation of signal transducer and activation of transcription 3 (STAT-3) and switch to cyclin D2 expression without altering proliferation in these cells [76, 77]. During late stages of TIMP-1-induced differentiation of Burkitt lymphoma cells, CD44 surface expression is upregulated and the activity and nuclear localization of protein-tyrosine phosphatase 1 (SHP-1) is increased in these cells [78] (Fig. 4a). This is consistent with findings in hematopoietic stem cells showing that TIMP-1-deficiency reduces CD44 expression and cell cycle arrest at the G1 phase [40].
Besides hematopoietic stem cells, mesenchymal stem cells (MSCs) represent another stem/progenitor cell population residing in bone marrow. MSCs are multipotent stromal cells that can differentiate into a variety of cell types including osteoblasts (bone cells), chondrocytes (cartilage cells) and adipocytes (fat cells), in response to stimulation by various environmental factors [79]. Our own previous results demonstrated that human MSCs (hMSCs) constitutively release high amounts of TIMP-1 [80], which led to the assumption that TIMP-1 might act as an endogenous growth factor in these cells. Hence, Egea and colleagues [81] introduced a specific knockdown of TIMP-1 expression in hMSCs by RNA interference and observed an increase in proliferation, metabolic activity and potential for osteogenic differentiation. This is in line with the observation that overexpression of TIMP-1 decreases osteoblastic proliferation and differentiation in vivo [82, 83]. Further studies on the underlying mechanisms in hMSCs elucidated that endogenous and exogenous TIMP-1 affects the activity of the Wnt/β-catenin signaling pathway in a MMP-independent manner as shown by use of a non-inhibitory TIMP-1 mutant [81]. The Wnt/β-catenin pathway is closely associated with growth and development of stem cells [84] and also regulates proliferation and migration of hMSCs [85]. High levels of TIMP-1 expression in hMSCs contribute to a low stability of cytosolic β-catenin, the key effector of this pathway [81]. Low levels of TIMP-1 expression in hMSCs increase β-catenin stability and nuclear translocation. This upregulates the transcription of the Wnt/β-catenin target genes cyclin D1 and MT1-MMP in these cells [81]. Moreover, CD63 was established as a TIMP-1 receptor on the surface of hMSCs involved in TIMP-1 attenuation of β-catenin activity. Additional investigations of the mechanisms by which TIMP-1 mediates the suppression of β-catenin activity in hMSCs revealed the involvement of microRNAs in this process. This group of RNA molecules represses or cleaves target mRNAs and thereby controls the biosynthesis of the respective proteins [86]. Blockage of TIMP-1 expression in hMSCs alters the levels of several miRNAs within cells, with let-7f showing the strongest increase in expression [81]. Inhibition of let-7f suppresses the increase in β-catenin activity normally observed in TIMP-1-depleted hMSCs, indicating let-7f to impact Wnt/β-catenin signaling in these cells. Both in silico analysis and in vitro studies identified the mRNA of axin 2 as a true target of let-7f [81]. Because axin 2 is a component of the complex responsible for degrading β-catenin, down-regulation of axin 2 by increased let-7f can explain the enhanced β-catenin activity observed in TIMP-1-depleted cells (Fig. 4b). In fact, modulation of intracellular let-7f levels in hMSCs enhanced or diminished the osteogenic differentiation of these cells [81]. These findings indicate that TIMP-1 interaction with CD63 attenuates Wnt/β-catenin pathway activity in hMSCs by means of posttranscriptional and posttranslational regulatory mechanisms lately promoting a quiescent state in these cells (Fig. 4b). Interestingly, TIMP-1 also preserves quiescence in hematopoietic stem cells [40], underlining its important role in the function of stem cells derived from bone marrow.
When compared to TIMP-1, which is well described for positive and negative effects on differentiation depending on the cellular context, less is known about TIMP-2’s influence on this process. Results obtained in neural cell models indicate that TIMP-2 promotes neuronal differentiation through TIMP-2 interaction with surface α3β1-integrins and enhanced ERK pathway activity accompanied by cell cycle arrest resulting from decreased expression of cyclins B/D and increased production of p21 [87, 88]. Recent studies show that TIMP-3 affects proliferation, differentiation and trafficking of hematopoietic stem cells [89], and suppresses differentiation of pre-adipocytes by an autocrine mechanism [90]. This suggests that TIMP-3 similar to TIMP-1 is an important regulator in hematopoietic and stromal stem/progenitor cell function.
TIMP implications in angiogenesis
The biological process of vasculogenesis (de novo development of an organized vascular system from endothelial stem/progenitor cells) is functionally distinct from angiogenesis (formation of new vessels from existing vasculature). Angiogenesis especially requires local endothelial cell proliferation and migration through existing ECM structures. The newly formed microvessels are then stabilized by association with pericytes and subsequent inhibition of endothelial proliferation and basement membrane formation [91]. Because several MMPs are involved in endothelial cell migration and capillary formation, the inhibition of MMPs by TIMPs can prevent angiogenesis [92]. TIMP-1 was first described to decrease angiogenesis by use of a chick yolk-sac experimental model system [93]. Inhibition of MMP activity by TIMP-1 and a synthetic MMP inhibitor has anti-angiogenic effects in the cornea and in a murine hemangioma model in vivo, respectively [94, 95]. Pre-adipocytes that overexpress TIMP-1 contribute to a decreased de novo blood vessel formation in adipose tissue in vivo [96]. Moreover, TIMP-1 overexpression in pancreatic cancer cells attenuates angiogenesis [97].
Besides that, studies on molecular mechanisms of TIMP-1 suppression of angiogenesis revealed that TIMP-1 blocks the migration of microvascular endothelial cells independent of MMP inhibition through upregulation of phosphatase and tensin homolog (PTEN) and an increase in downstream FAK activity [98].
In the case of TIMP-2 and TIMP-3, several reports describe their roles as cytokines in angiogenesis as previously reviewed [99].
TIMP-1 in cancer and other diseases
Numerous clinical studies demonstrated significantly increased TIMP-1 concentrations in tumor patients and showed the utility of TIMP-1 as a biomarker or independent prognostic factor in several types of cancer such as breast cancer [13, 100], colorectal cancer [14], prostate cancer [101], lung cancer [102], gastric cancer [103], glioblastoma [104], melanoma [105] and multiple myeloma [106, 107]. The TIMP-1 correlation with poor survival in cancer patients may be at least partially explained by TIMP-1’s growth-promoting and anti-apoptotic impact in tumor cells. Hayakawa and colleagues [31] were the first to report that TIMP-1 present in blood serum acts as a growth factor to support proliferation of several tumor cell lines. Furthermore, TIMP-1 is a suppressor of apoptosis in a variety of cell types including Burkitt lymphoma cells [45, 47, 77] and breast cancer cells [44, 50–52], although these effects appear to be cell type-specific. The clinical observations that TIMP-1 is frequently overexpressed in tumor tissue [100] and FAK activity is often upregulated in tumor cells [108] supports the hypothesis that the TIMP-1/CD63/FAK/Akt signaling pathway is involved in the suppression of tumor cell apoptosis in breast cancer. Recent findings indicate that the elevated expression of nitric oxide synthase (NOS2) in breast tumor tissue enhances TIMP-1-mediated pro-survival signaling via increased Akt phosphorylation after interaction of nitric oxide with TIMP-1/CD63 on the cell surface [109]. This mechanism is supposed to be one explanation for the poor clinical outcome in breast cancer patients expressing high levels of NOS2 and TIMP-1.
In addition to promoting growth and survival in tumor cells, TIMP-1 has additional oncogenic activities. In non-malignant kidney epithelial MDCK cells, overexpression of TIMP-1 triggers epithelial-mesenchymal transition (EMT) by increased production of EMT transcription factors and facilitates cell invasion by upregulation of MMP expression, effects that are independent of TIMP-1’s MMP-inhibitory domain [110]. Consistently, overexpression of TIMP-1 in MDA-MB-231 breast cancer cells alters expression of numerous tumor associated genes including MMPs [111].
Inhibition of metalloproteinases by TIMP-1 increases hepatocyte growth factor (HGF) signaling in stromal cells through blocking the activity of ADAM10, a sheddase of the HGF receptor cMet, resulting in enhanced liver metastasis by lymphoma cells [112, 113]. The authors also show that HGF/cMet signaling is dependent on TIMP-1 upregulation of hypoxia-inducible factor 1α (HIF-1α) [114]. These mechanisms make the liver more susceptible to metastasis and provide one explanation for the association of elevated TIMP-1 levels with poor clinical outcomes in cancer patients. Studies in breast cancer cells revealed that TIMP-1 confers resistance to anti-tumor drug induced apoptosis by promoting the degradation of cyclin B1 [115].
Linkage of recombinant TIMP-1 to a glycosylphosphatidylinositol (GPI) anchor (GPI-TIMP-1) and addition to cells allows efficient insertion of the fusion protein into the plasma membrane, which then displays differential effects on cell proliferation and migration [116]. The exogenous application of GPI-TIMP-1 to renal cell carcinoma cells, melanoma cells and colon carcinoma cells inhibits growth and increases susceptibility to FAS-induced apoptosis involving regulation of Bcl-2 family proteins [117–119]. This suggests that the recombinant GPI-TIMP-1 fusion protein may represent a potential therapeutical agent to limit tumor regrowth after surgery. The cellular effects of GPI-TIMP-1, however, are in contrast to the growth-promoting and anti-apoptotic activities of endogenous TIMP-1 indicating different regulatory mechanisms for the membrane-inserted GPI-TIMP-1 and the natural soluble form of TIMP-1. The effects of GPI-TIMP-1 may be mediated in part through the biology of transforming growth factor (TGF)-β1. Proteases including MMP-2 and MMP-9 activate TGF-β1 through proteolytic degradation of the latent TGF-β1 complex. TIMP-1-GPI treatment appears to result in an effective blockade of MMP-2 and MMP-9 activity at the cell surface leading to a reduction in TGF-β1 processing with corresponding effects on paracrine and autocrine TGF-β1 signaling [120].
In addition to its role in cancer, TIMP-1 is a potential plasmatic biomarker for prognosis in various other pathophysiological conditions including psoriasis [121], liver fibrosis [122], hypertension [12], heart failure [11] and myocardial infarction [123]. Cytokine functions of TIMPs have been implicated in the physiological and pathological remodeling of the myocard induced by physical stress, ischemia and infection (for review see [124]). Myocardial remodeling is the result of highly regulated interactions between fibroblasts, smooth muscle cells, endothelial cells, cardiomyocytes, and infiltrating leukocytes, which are modulated by TIMP-1. As demonstrated in a cell therapeutical study, TIMP-1 released from embryonic stem cells implanted for the treatment of myocardial infarction inhibits apoptosis in cardiomyocytes and thus improves cardiac remodeling following myocardial infarction [125, 126].
Conclusions and future prospects
Research of the last two decades significantly improved the understanding of TIMP’s action as cytokines in the regulation of cell growth, apoptosis, differentiation and angiogenesis. It is clear that the pleiotropic activities of TIMPs are complex and depend upon subtle interactions with other extracellular components especially MMPs, as well as direct interactions with binding partners on the cell surface. Important progress has been made through identification of TIMP receptors, particularly CD63 for TIMP-1 [50], α3β1 integrin for TIMP-2 [127] and VEGF receptor 2 for TIMP-3 [128]. LRP-mediated endocytosis of TIMPs and TIMP/MMP complexes add further complexity to TIMP-mediated regulation of cell behavior. The molecular dissection of signaling events associated with the cytokine functions of TIMP-1 revealed diverse signaling molecules and pathways to be activated dependent on cell type and biological context. TIMP-1/CD63-mediated growth-promoting and anti-apoptotic effects involve PI3-K/Akt signaling in many different cell types including cancer cells. Distinct signaling pathways comprising miRNA-mediated post-transcriptional mechanisms participate in TIMP-1 regulation of cell differentiation; in mesenchymal stem cells, TIMP-1/CD63-induced signaling promotes quiescence by attenuating let-7f-controlled Wnt/β-catenin activity that is reversed upon TIMP-1 deficiency facilitating the transition of these cells into an active cell state with increased capacity for differentiation. This indicates the importance of post-transcriptional and post-translational mechanisms in TIMP-1 regulation of cell functions. Furthermore, the TIMP-1 interaction with cell surface receptors and subsequent gene regulation is markedly modulated by pericellular levels of proMMP-9, the major target protease of TIMP-1. The bifacial property of TIMP-1 acting as cytokine and metalloproteinase inhibitor underlines its crucial role in the communication between intracellular signaling networks and the ECM. Further studies on the molecular mechanisms of TIMP’s cytokine activities in different cell types would improve the understanding of TIMP-controlled cellular processes under physiological and pathological conditions. This information may be helpful in the development of rational, mechanism-based therapies in the treatment of cancer and other chronic diseases.
Acknowledgments
Work in C.R.’s laboratory is funded by grants from the Institute of Cardiovascular Prevention, Ludwig-Maximilians-University of Munich and by contract from the German Federal Ministry of Defense research project M/SABX/8A002.
References
- 1.Woolley DE, Roberts DR, Evanson JM. Inhibition of human collagenase activity by a small molecular weight serum protein. Biochem Biophys Res Commun. 1975;66(2):747–754. doi: 10.1016/0006-291x(75)90573-2. [DOI] [PubMed] [Google Scholar]
- 2.Bauer EA, Stricklin GP, Jeffrey JJ, Eisen AZ. Collagenase production by human skin fibroblasts. Biochem Biophys Res Commun. 1975;64(1):232–240. doi: 10.1016/0006-291x(75)90243-0. [DOI] [PubMed] [Google Scholar]
- 3.Westbrook CA, Gasson JC, Gerber SE, Selsted ME, Golde DW. Purification and characterization of human T-lymphocyte-derived erythroid-potentiating activity. J Biol Chem. 1984;259(16):9992–9996. [PubMed] [Google Scholar]
- 4.Docherty AJ, Lyons A, Smith BJ, Wright EM, Stephens PE, Harris TJ, Murphy G, Reynolds JJ. Sequence of human tissue inhibitor of metalloproteinases and its identity to erythroid-potentiating activity. Nature. 1985;318:66–69. doi: 10.1038/318066a0. [DOI] [PubMed] [Google Scholar]
- 5.Gasson JC, Golde DW, Kaufman SE, Westbrook CA, Hewick RM, Kaufman RJ, Wong GG, Temple PA, Leary AC, Brown EL, et al. Molecular characterization and expression of the gene encoding human erythroid-potentiating activity. Nature. 1985;315:768–771. doi: 10.1038/315768a0. [DOI] [PubMed] [Google Scholar]
- 6.Stetler Stevenson WG, Krutzsch HC, Liotta LA. Tissue inhibitor of metalloproteinase (TIMP-2). A new member of the metalloproteinase inhibitor family. J Biol Chem. 1989;264:17374–17378. [PubMed] [Google Scholar]
- 7.Pavloff N, Staskus PW, Kishnani NS, Hawkes SP. A new inhibitor of metalloproteinases from chicken: ChIMP-3. A third member of the TIMP family. J Biol Chem. 1992;267(24):17321–17326. [PubMed] [Google Scholar]
- 8.Greene J, Wang M, Liu YE, Raymond LA, Rosen C, Shi YE. Molecular cloning and characterization of human tissue inhibitor of metalloproteinase 4. J Biol Chem. 1996;271(48):30375–30380. doi: 10.1074/jbc.271.48.30375. [DOI] [PubMed] [Google Scholar]
- 9.Murphy G, Nagase H. Progress in matrix metalloproteinase research. Mol Aspects Med. 2008;29(5):290–308. doi: 10.1016/j.mam.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2(3):161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 11.Kramer F, Milting H. Novel biomarkers in human terminal heart failure and under mechanical circulatory support. Biomarkers. 2011;16(Suppl 1):S31–S41. doi: 10.3109/1354750X.2011.561498. [DOI] [PubMed] [Google Scholar]
- 12.Marchesi C, Dentali F, Nicolini E, Maresca AM, Tayebjee MH, Franz M, Guasti L, Venco A, Schiffrin EL, Lip GY, Grandi AM. Plasma levels of matrix metalloproteinases and their inhibitors in hypertension: a systematic review and meta-analysis. J Hypertens. 2012;30(1):3–16. doi: 10.1097/HJH.0b013e32834d249a. [DOI] [PubMed] [Google Scholar]
- 13.Wurtz SO, Schrohl AS, Mouridsen H, Brunner N. TIMP-1 as a tumor marker in breast cancer—an update. Acta Oncol. 2008;47(4):580–590. doi: 10.1080/02841860802022976. [DOI] [PubMed] [Google Scholar]
- 14.Lee JH, Choi JW, Kim YS. Plasma or serum TIMP-1 is a predictor of survival outcomes in colorectal cancer: a meta-analysis. J Gastrointestin Liver Dis. 2011;20(3):287–291. [PubMed] [Google Scholar]
- 15.Lambert E, Dasse E, Haye B, Petitfrere E. TIMPs as multifacial proteins. Crit Rev Oncol Hematol. 2004;49(3):187–198. doi: 10.1016/j.critrevonc.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 16.Stetler-Stevenson WG. Tissue inhibitors of metalloproteinases in cell signaling: metalloproteinase-independent biological activities. Sci Signal. 2008;1(27):re6. doi: 10.1126/scisignal.127re6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murphy G. Tissue inhibitors of metalloproteinases. Genome Biol. 2011;12(11):233. doi: 10.1186/gb-2011-12-11-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brew K, Nagase H. The tissue inhibitors of metalloproteinases (TIMPs): an ancient family with structural and functional diversity. Biochim Biophys Acta. 2010;1803(1):55–71. doi: 10.1016/j.bbamcr.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murphy G, Houbrechts A, Cockett MI, Williamson RA, O’Shea M, Docherty AJ. The N-terminal domain of tissue inhibitor of metalloproteinases retains metalloproteinase inhibitory activity. Biochemistry. 1991;30:8097–8102. doi: 10.1021/bi00247a001. [DOI] [PubMed] [Google Scholar]
- 20.Zhang J, Bai S, Tanase C, Nagase H, Sarras MP., Jr The expression of tissue inhibitor of metalloproteinase 2 (TIMP-2) is required for normal development of zebrafish embryos. Dev Genes Evol. 2003;213(8):382–389. doi: 10.1007/s00427-003-0333-9. [DOI] [PubMed] [Google Scholar]
- 21.Jaworski DM, Soloway P, Caterina J, Falls WA. Tissue inhibitor of metalloproteinase-2(TIMP-2)-deficient mice display motor deficits. J Neurobiol. 2006;66(1):82–94. doi: 10.1002/neu.20205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frisch SM, Francis H. Disruption of epithelial cell-matrix interactions induces apoptosis. J Cell Biol. 1994;124(4):619–626. doi: 10.1083/jcb.124.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boudreau N, Werb Z, Bissell MJ. Suppression of apoptosis by basement membrane requires three-dimensional tissue organization and withdrawal from the cell cycle. Proc Natl Acad Sci USA. 1996;93(8):3509–3513. doi: 10.1073/pnas.93.8.3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141(1):52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol. 2007;8(3):221–233. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Edwards DR, Handsley MM, Pennington CJ. The ADAM metalloproteinases. Mol Aspects Med. 2008;29(5):258–289. doi: 10.1016/j.mam.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wingfield PT, Sax JK, Stahl SJ, Kaufman J, Palmer I, Chung V, Corcoran ML, Kleiner DE, Stetler-Stevenson WG. Biophysical and functional characterization of full-length, recombinant human tissue inhibitor of metalloproteinases-2 (TIMP-2) produced in Escherichia coli. Comparison of wild type and amino-terminal alanine appended variant with implications for the mechanism of TIMP functions. J Biol Chem. 1999;274(30):21362–21368. doi: 10.1074/jbc.274.30.21362. [DOI] [PubMed] [Google Scholar]
- 28.Stetler-Stevenson WG. The tumor microenvironment: regulation by MMP-independent effects of tissue inhibitor of metalloproteinases-2. Cancer Metastasis Rev. 2008;27(1):57–66. doi: 10.1007/s10555-007-9105-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gasson JC, Bersch N, Golde DW. Characterization of purified human erythroid-potentiating activity. Prog Clin Biol Res. 1985;184:95–104. [PubMed] [Google Scholar]
- 30.Bertaux B, Hornebeck W, Eisen AZ, Dubertret L. Growth stimulation of human keratinocytes by tissue inhibitor of metalloproteinases. J Invest Dermatol. 1991;97(4):679–685. doi: 10.1111/1523-1747.ep12483956. [DOI] [PubMed] [Google Scholar]
- 31.Hayakawa T, Yamashita K, Tanzawa K, Uchijima E, Iwata K. Growth-promoting activity of tissue inhibitor of metalloproteinases-1 (TIMP-1) for a wide range of cells. A possible new growth factor in serum. FEBS Lett. 1992;298:29–32. doi: 10.1016/0014-5793(92)80015-9. [DOI] [PubMed] [Google Scholar]
- 32.Saika S, Kawashima Y, Okada Y, Tanaka SI, Yamanaka O, Ohnishi Y, Ooshima A. Recombinant TIMP-1 and -2 enhance the proliferation of rabbit corneal epithelial cells in vitro and the spreading of rabbit corneal epithelium in situ. Curr Eye Res. 1998;17(1):47–52. doi: 10.1076/ceyr.17.1.47.5247. [DOI] [PubMed] [Google Scholar]
- 33.Stetler Stevenson WG, Bersch N, Golde DW. Tissue inhibitor of metalloproteinase-2 (TIMP-2) has erythroid- potentiating activity. FEBS Lett. 1992;296:231–234. doi: 10.1016/0014-5793(92)80386-u. [DOI] [PubMed] [Google Scholar]
- 34.Hayakawa T, Yamashita K, Ohuchi E, Shinagawa A. Cell growth-promoting activity of tissue inhibitor of metalloproteinases-2 (TIMP-2) J Cell Sci. 1994;107(Pt 9):2373–2379. doi: 10.1242/jcs.107.9.2373. [DOI] [PubMed] [Google Scholar]
- 35.Chesler L, Golde DW, Bersch N, Johnson MD. Metalloproteinase inhibition and erythroid potentiation are independent activities of tissue inhibitor of metalloproteinases-1. Blood. 1995;86(12):4506–4515. [PubMed] [Google Scholar]
- 36.Wang T, Yamashita K, Iwata K, Hayakawa T. Both tissue inhibitors of metalloproteinases-1 (TIMP-1) and TIMP-2 activate Ras but through different pathways. Biochem Biophys Res Commun. 2002;296(1):201–205. doi: 10.1016/s0006-291x(02)00741-6. [DOI] [PubMed] [Google Scholar]
- 37.Akahane T, Akahane M, Shah A, Thorgeirsson UP. TIMP-1 stimulates proliferation of human aortic smooth muscle cells and Ras effector pathways. Biochem Biophys Res Commun. 2004;324(1):440–445. doi: 10.1016/j.bbrc.2004.09.063. [DOI] [PubMed] [Google Scholar]
- 38.Lu Y, Liu S, Zhang S, Cai G, Jiang H, Su H, Li X, Hong Q, Zhang X, Chen X. Tissue inhibitor of metalloproteinase-1 promotes NIH3T3 fibroblast proliferation by activating p-Akt and cell cycle progression. Mol Cells. 2011;31(3):225–230. doi: 10.1007/s10059-011-0023-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fowell AJ, Collins JE, Duncombe DR, Pickering JA, Rosenberg WM, Benyon RC. Silencing tissue inhibitors of metalloproteinases (TIMPs) with short interfering RNA reveals a role for TIMP-1 in hepatic stellate cell proliferation. Biochem Biophys Res Commun. 2011;407(2):277–282. doi: 10.1016/j.bbrc.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 40.Rossi L, Ergen AV, Goodell MA. TIMP-1 deficiency subverts cell-cycle dynamics in murine long-term HSCs. Blood. 2011;117(24):6479–6488. doi: 10.1182/blood-2009-10-248955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ould-yahoui A, Tremblay E, Sbai O, Ferhat L, Bernard A, Charrat E, Gueye Y, Lim NH, Brew K, Risso JJ, Dive V, Khrestchatisky M, Rivera S. A new role for TIMP-1 in modulating neurite outgrowth and morphology of cortical neurons. PLoS One. 2009;4(12):e8289. doi: 10.1371/journal.pone.0008289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hernandez-Guillamon M, Delgado P, Ortega L, Pares M, Rosell A, Garcia-Bonilla L, Fernandez-Cadenas I, Borrell-Pages M, Boada M, Montaner J. Neuronal TIMP-1 release accompanies astrocytic MMP-9 secretion and enhances astrocyte proliferation induced by beta-amyloid 25–35 fragment. J Neurosci Res. 2009;87(9):2115–2125. doi: 10.1002/jnr.22034. [DOI] [PubMed] [Google Scholar]
- 43.Fata JE, Leco KJ, Moorehead RA, Martin DC, Khokha R. Timp-1 is important for epithelial proliferation and branching morphogenesis during mouse mammary development. Dev Biol. 1999;211(2):238–254. doi: 10.1006/dbio.1999.9313. [DOI] [PubMed] [Google Scholar]
- 44.Taube ME, Liu XW, Fridman R, Kim HR. TIMP-1 regulation of cell cycle in human breast epithelial cells via stabilization of p27(KIP1) protein. Oncogene. 2006;25(21):3041–3048. doi: 10.1038/sj.onc.1209336. [DOI] [PubMed] [Google Scholar]
- 45.Guedez L, Stetler-Stevenson WG, Wolff L, Wang J, Fukushima P, Mansoor A, Stetler-Stevenson M. In vitro suppression of programmed cell death of B cells by tissue inhibitor of metalloproteinases-1. J Clin Invest. 1998;102(11):2002–2010. doi: 10.1172/JCI2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Han X, Sun Y, Scott S, Bleich D. Tissue inhibitor of metalloproteinase-1 prevents cytokine-mediated dysfunction and cytotoxicity in pancreatic islets and beta-cells. Diabetes. 2001;50(5):1047–1055. doi: 10.2337/diabetes.50.5.1047. [DOI] [PubMed] [Google Scholar]
- 47.Guedez L, Mansoor A, Birkedal-Hansen B, Lim MS, Fukushima P, Venzon D, Stetler-Stevenson WG, Stetler-Stevenson M. Tissue inhibitor of metalloproteinases 1 regulation of interleukin-10 in B-cell differentiation and lymphomagenesis. Blood. 2001;97(6):1796–1802. doi: 10.1182/blood.v97.6.1796. [DOI] [PubMed] [Google Scholar]
- 48.Vorotnikova E, Tries M, Braunhut S. Retinoids and TIMP1 prevent radiation-induced apoptosis of capillary endothelial cells. Radiat Res. 2004;161(2):174–184. doi: 10.1667/rr3107. [DOI] [PubMed] [Google Scholar]
- 49.Chromek M, Tullus K, Lundahl J, Brauner A. Tissue inhibitor of metalloproteinase 1 activates normal human granulocytes, protects them from apoptosis, and blocks their transmigration during inflammation. Infect Immun. 2004;72(1):82–88. doi: 10.1128/IAI.72.1.82-88.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jung KK, Liu XW, Chirco R, Fridman R, Kim HR. Identification of CD63 as a tissue inhibitor of metalloproteinase-1 interacting cell surface protein. EMBO J. 2006;25(17):3934–3942. doi: 10.1038/sj.emboj.7601281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu XW, Bernardo MM, Fridman R, Kim HR. Tissue inhibitor of metalloproteinase-1 protects human breast epithelial cells against intrinsic apoptotic cell death via the focal adhesion kinase/phosphatidylinositol 3-kinase and MAPK signaling pathway. J Biol Chem. 2003;278(41):40364–40372. doi: 10.1074/jbc.M302999200. [DOI] [PubMed] [Google Scholar]
- 52.Liu XW, Taube ME, Jung KK, Dong Z, Lee YJ, Roshy S, Sloane BF, Fridman R, Kim HR. Tissue inhibitor of metalloproteinase-1 protects human breast epithelial cells from extrinsic cell death: a potential oncogenic activity of tissue inhibitor of metalloproteinase-1. Cancer Res. 2005;65(3):898–906. [PubMed] [Google Scholar]
- 53.Wilk CM, Schildberg FA, Lauterbach MA, Cadeddu RP, Frobel J, Westphal V, Tolba RH, Hell SW, Czibere A, Bruns I, Haas R (2013) The tissue inhibitor of metalloproteinases-1 improves migration and adhesion of hematopoietic stem and progenitor cells. Exp Hematol. doi:10.1016/j.exphem.2013.04.010 [DOI] [PubMed]
- 54.Pols MS, Klumperman J. Trafficking and function of the tetraspanin CD63. Exp Cell Res. 2009;315(9):1584–1592. doi: 10.1016/j.yexcr.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 55.Lambert E, Boudot C, Kadri Z, Soula-Rothhut M, Sowa ML, Mayeux P, Hornebeck W, Haye B, Petitfrere E. Tissue inhibitor of metalloproteinases-1 signalling pathway leading to erythroid cell survival. Biochem J. 2003;372(Pt 3):767–774. doi: 10.1042/BJ20030187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lambert E, Bridoux L, Devy J, Dasse E, Sowa ML, Duca L, Hornebeck W, Martiny L, Petitfrere-Charpentier E. TIMP-1 binding to proMMP-9/CD44 complex localized at the cell surface promotes erythroid cell survival. Int J Biochem Cell Biol. 2009;41(5):1102–1115. doi: 10.1016/j.biocel.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 57.Yu Q, Stamenkovic I. Localization of matrix metalloproteinase 9 to the cell surface provides a mechanism for CD44-mediated tumor invasion. Genes Dev. 1999;13:35–48. doi: 10.1101/gad.13.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bridoux L, Etique N, Lambert E, Thevenard J, Sowa ML, Belloy N, Dauchez M, Martiny L, Charpentier E. A crucial role for Lyn in TIMP-1 erythroid cell survival signalling pathway. FEBS Lett. 2013;587(10):1524–1528. doi: 10.1016/j.febslet.2013.03.032. [DOI] [PubMed] [Google Scholar]
- 59.Li G, Fridman R, Kim HR. Tissue inhibitor of metalloproteinase-1 inhibits apoptosis of human breast epithelial cells. Cancer Res. 1999;59(24):6267–6275. [PubMed] [Google Scholar]
- 60.Ashutosh, Chao C, Borgmann K, Brew K, Ghorpade A (2012) Tissue inhibitor of metalloproteinases-1 protects human neurons from staurosporine and HIV-1-induced apoptosis: mechanisms and relevance to HIV-1-associated dementia. Cell Death Dis 3:e332 [DOI] [PMC free article] [PubMed]
- 61.Guo LJ, Luo XH, Xie H, Zhou HD, Yuan LQ, Wang M, Liao EY. Tissue inhibitor of matrix metalloproteinase-1 suppresses apoptosis of mouse bone marrow stromal cell line MBA-1. Calcif Tissue Int. 2006;78(5):285–292. doi: 10.1007/s00223-005-0092-x. [DOI] [PubMed] [Google Scholar]
- 62.Scheid MP, Schubert KM, Duronio V. Regulation of bad phosphorylation and association with Bcl-x(L) by the MAPK/Erk kinase. J Biol Chem. 1999;274(43):31108–31113. doi: 10.1074/jbc.274.43.31108. [DOI] [PubMed] [Google Scholar]
- 63.Yu C, Minemoto Y, Zhang J, Liu J, Tang F, Bui TN, Xiang J, Lin A. JNK suppresses apoptosis via phosphorylation of the proapoptotic Bcl-2 family protein BAD. Mol Cell. 2004;13(3):329–340. doi: 10.1016/s1097-2765(04)00028-0. [DOI] [PubMed] [Google Scholar]
- 64.Ahonen M, Poukkula M, Baker AH, Kashiwagi M, Nagase H, Eriksson JE, Kahari VM. Tissue inhibitor of metalloproteinases-3 induces apoptosis in melanoma cells by stabilization of death receptors. Oncogene. 2003;22(14):2121–2134. doi: 10.1038/sj.onc.1206292. [DOI] [PubMed] [Google Scholar]
- 65.Baker AH, Zaltsman AB, George SJ, Newby AC. Divergent effects of tissue inhibitor of metalloproteinase-1, -2, or -3 overexpression on rat vascular smooth muscle cell invasion, proliferation, and death in vitro. TIMP-3 promotes apoptosis. J Clin Invest. 1998;101(6):1478–1487. doi: 10.1172/JCI1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lim MS, Guedez L, Stetler-Stevenson WG, Stetler-Stevenson M. Tissue inhibitor of metalloproteinase-2 induces apoptosis in human T lymphocytes. Ann N Y Acad Sci. 1999;878:522–523. doi: 10.1111/j.1749-6632.1999.tb07715.x. [DOI] [PubMed] [Google Scholar]
- 67.Valente P, Fassina G, Melchiori A, Masiello L, Cilli M, Vacca A, Onisto M, Santi L, Stetler-Stevenson WG, Albini A. TIMP-2 over-expression reduces invasion and angiogenesis and protects B16F10 melanoma cells from apoptosis. Int J Cancer. 1998;75(2):246–253. doi: 10.1002/(sici)1097-0215(19980119)75:2<246::aid-ijc13>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 68.Boudreau N, Sympson CJ, Werb Z, Bissell MJ. Suppression of ICE and apoptosis in mammary epithelial cells by extracellular matrix. Science. 1995;267(5199):891–893. doi: 10.1126/science.7531366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Alexander CM, Howard EW, Bissell MJ, Werb Z. Rescue of mammary epithelial cell apoptosis and entactin degradation by a tissue inhibitor of metalloproteinases-1 transgene. J Cell Biol. 1996;135(6 Pt 1):1669–1677. doi: 10.1083/jcb.135.6.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Murphy FR, Issa R, Zhou X, Ratnarajah S, Nagase H, Arthur MJ, Benyon C, Iredale JP. Inhibition of apoptosis of activated hepatic stellate cells by tissue inhibitor of metalloproteinase-1 is mediated via effects on matrix metalloproteinase inhibition: implications for reversibility of liver fibrosis. J Biol Chem. 2002;277(13):11069–11076. doi: 10.1074/jbc.M111490200. [DOI] [PubMed] [Google Scholar]
- 71.Hahn-Dantona E, Ruiz JF, Bornstein P, Strickland DK. The low density lipoprotein receptor-related protein modulates levels of matrix metalloproteinase 9 (MMP-9) by mediating its cellular catabolism. J Biol Chem. 2001;276(18):15498–15503. doi: 10.1074/jbc.M100121200. [DOI] [PubMed] [Google Scholar]
- 72.Murate T, Yamashita K, Ohashi H, Kagami Y, Tsushita K, Kinoshita T, Hotta T, Saito H, Yoshida S, Mori KJ, et al. Erythroid potentiating activity of tissue inhibitor of metalloproteinases on the differentiation of erythropoietin-responsive mouse erythroleukemia cell line, ELM-I-1-3, is closely related to its cell growth potentiating activity. Exp Hematol. 1993;21(1):169–176. [PubMed] [Google Scholar]
- 73.Petitfrere E, Kadri Z, Boudot C, Sowa ML, Mayeux P, Haye B, Billat C. Involvement of the p38 mitogen-activated protein kinase pathway in tissue inhibitor of metalloproteinases-1-induced erythroid differentiation. FEBS Lett. 2000;485(2–3):117–121. doi: 10.1016/s0014-5793(00)02210-9. [DOI] [PubMed] [Google Scholar]
- 74.Dasse E, Bridoux L, Baranek T, Lambert E, Salesse S, Sowa ML, Martiny L, Trentesaux C, Petitfrere E. Tissue inhibitor of metalloproteinase-1 promotes hematopoietic differentiation via caspase-3 upstream the MEKK1/MEK6/p38alpha pathway. Leukemia. 2007;21(4):595–603. doi: 10.1038/sj.leu.2404540. [DOI] [PubMed] [Google Scholar]
- 75.Stetler-Stevenson M, Mansoor A, Lim M, Fukushima P, Kehrl J, Marti G, Ptaszynski K, Wang J, Stetler-Stevenson WG. Expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in reactive and neoplastic lymphoid cells. Blood. 1997;89(5):1708–1715. [PubMed] [Google Scholar]
- 76.Guedez L, Courtemanch L, Stetler-Stevenson M. Tissue inhibitor of metalloproteinase (TIMP)-1 induces differentiation and an antiapoptotic phenotype in germinal center B cells. Blood. 1998;92(4):1342–1349. [PubMed] [Google Scholar]
- 77.Guedez L, Martinez A, Zhao S, Vivero A, Pittaluga S, Stetler-Stevenson M, Raffeld M, Stetler-Stevenson WG. Tissue inhibitor of metalloproteinase 1 (TIMP-1) promotes plasmablastic differentiation of a Burkitt lymphoma cell line: implications in the pathogenesis of plasmacytic/plasmablastic tumors. Blood. 2005;105(4):1660–1668. doi: 10.1182/blood-2004-04-1385. [DOI] [PubMed] [Google Scholar]
- 78.Kim YS, Seo DW, Kong SK, Lee JH, Lee ES, Stetler-Stevenson M, Stetler-Stevenson WG. TIMP1 induces CD44 expression and the activation and nuclear translocation of SHP1 during the late centrocyte/post-germinal center B cell differentiation. Cancer Lett. 2008;269(1):37–45. doi: 10.1016/j.canlet.2008.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 80.Ries C, Egea V, Karow M, Kolb H, Jochum M, Neth P. MMP-2, MT1-MMP, and TIMP-2 are essential for the invasive capacity of human mesenchymal stem cells: differential regulation by inflammatory cytokines. Blood. 2007;109(9):4055–4063. doi: 10.1182/blood-2006-10-051060. [DOI] [PubMed] [Google Scholar]
- 81.Egea V, Zahler S, Rieth N, Neth P, Popp T, Kehe K, Jochum M, Ries C. Tissue inhibitor of metalloproteinase-1 (TIMP-1) regulates mesenchymal stem cells through let-7f microRNA and Wnt/beta-catenin signaling. Proc Natl Acad Sci USA. 2012;109(6):E309–E316. doi: 10.1073/pnas.1115083109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schiltz C, Marty C, de Vernejoul MC, Geoffroy V. Inhibition of osteoblastic metalloproteinases in mice prevents bone loss induced by oestrogen deficiency. J Cell Biochem. 2008;104(5):1803–1817. doi: 10.1002/jcb.21747. [DOI] [PubMed] [Google Scholar]
- 83.Schiltz C, Prouillet C, Marty C, Merciris D, Collet C, de Vernejoul MC, Geoffroy V. Bone loss induced by Runx2 over-expression in mice is blunted by osteoblastic over-expression of TIMP-1. J Cell Physiol. 2010;222(1):219–229. doi: 10.1002/jcp.21941. [DOI] [PubMed] [Google Scholar]
- 84.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127(3):469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 85.Neth P, Ciccarella M, Egea V, Hoelters J, Jochum M, Ries C. Wnt signaling regulates the invasion capacity of human mesenchymal stem cells. Stem Cells. 2006;24(8):1892–1903. doi: 10.1634/stemcells.2005-0503. [DOI] [PubMed] [Google Scholar]
- 86.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136(2):215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Perez-Martinez L, Jaworski DM. Tissue inhibitor of metalloproteinase-2 promotes neuronal differentiation by acting as an anti-mitogenic signal. J Neurosci. 2005;25(20):4917–4929. doi: 10.1523/JNEUROSCI.5066-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jaworski DM, Perez-Martinez L. Tissue inhibitor of metalloproteinase-2 (TIMP-2) expression is regulated by multiple neural differentiation signals. J Neurochem. 2006;98(1):234–247. doi: 10.1111/j.1471-4159.2006.03855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shen Y, Winkler IG, Barbier V, Sims NA, Hendy J, Levesque JP. Tissue inhibitor of metalloproteinase-3 (TIMP-3) regulates hematopoiesis and bone formation in vivo. PLoS One. 2010;5(9):e13086. doi: 10.1371/journal.pone.0013086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bernot D, Barruet E, Poggi M, Bonardo B, Alessi MC, Peiretti F. Down-regulation of tissue inhibitor of metalloproteinase-3 (TIMP-3) expression is necessary for adipocyte differentiation. J Biol Chem. 2010;285(9):6508–6514. doi: 10.1074/jbc.M109.078444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Risau W. Mechanisms of angiogenesis. Nature. 1997;386(6626):671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- 92.Handsley MM, Edwards DR. Metalloproteinases and their inhibitors in tumor angiogenesis. Int J Cancer. 2005;115(6):849–860. doi: 10.1002/ijc.20945. [DOI] [PubMed] [Google Scholar]
- 93.Takigawa M, Nishida Y, Suzuki F, Kishi J, Yamashita K, Hayakawa T. Induction of angiogenesis in chick yolk-sac membrane by polyamines and its inhibition by tissue inhibitors of metalloproteinases (TIMP and TIMP-2) Biochem Biophys Res Commun. 1990;171(3):1264–1271. doi: 10.1016/0006-291x(90)90822-5. [DOI] [PubMed] [Google Scholar]
- 94.Lee S, Zheng M, Kim B, Rouse BT. Role of matrix metalloproteinase-9 in angiogenesis caused by ocular infection with herpes simplex virus. J Clin Invest. 2002;110(8):1105–1111. doi: 10.1172/JCI15755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Taraboletti G, Garofalo A, Belotti D, Drudis T, Borsotti P, Scanziani E, Brown PD, Giavazzi R. Inhibition of angiogenesis and murine hemangioma growth by batimastat, a synthetic inhibitor of matrix metalloproteinases. J Natl Cancer Inst. 1995;87(4):293–298. doi: 10.1093/jnci/87.4.293. [DOI] [PubMed] [Google Scholar]
- 96.Scroyen I, Jacobs F, Cosemans L, De Geest B, Lijnen HR. Blood vessel density in de novo formed adipose tissue is decreased upon overexpression of TIMP-1. Obesity (Silver Spring) 2010;18(3):638–640. doi: 10.1038/oby.2009.279. [DOI] [PubMed] [Google Scholar]
- 97.Bloomston M, Shafii A, Zervos EE, Rosemurgy AS. TIMP-1 overexpression in pancreatic cancer attenuates tumor growth, decreases implantation and metastasis, and inhibits angiogenesis. J Surg Res. 2002;102(1):39–44. doi: 10.1006/jsre.2001.6318. [DOI] [PubMed] [Google Scholar]
- 98.Akahane T, Akahane M, Shah A, Connor CM, Thorgeirsson UP. TIMP-1 inhibits microvascular endothelial cell migration by MMP-dependent and MMP-independent mechanisms. Exp Cell Res. 2004;301(2):158–167. doi: 10.1016/j.yexcr.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 99.Stetler-Stevenson WG, Seo DW. TIMP-2: an endogenous inhibitor of angiogenesis. Trends Mol Med. 2005;11(3):97–103. doi: 10.1016/j.molmed.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 100.Neri A, Megha T, Bettarini F, Tacchini D, Mastrogiulio MG, Marrelli D, Pinto E, Tosi P. Is tissue inhibitor of metalloproteinase-1 a new prognosticator for breast cancer? An analysis of 266 cases. Hum Pathol. 2012;43(8):1184–1191. doi: 10.1016/j.humpath.2011.09.018. [DOI] [PubMed] [Google Scholar]
- 101.Oh WK, Vargas R, Jacobus S, Leitzel K, Regan MM, Hamer P, Pierce K, Brown-Shimer S, Carney W, Ali SM, Kantoff PW, Lipton A. Elevated plasma tissue inhibitor of metalloproteinase-1 levels predict decreased survival in castration-resistant prostate cancer patients. Cancer. 2011;117(3):517–525. doi: 10.1002/cncr.25394. [DOI] [PubMed] [Google Scholar]
- 102.Gouyer V, Conti M, Devos P, Zerimech F, Copin MC, Creme E, Wurtz A, Porte H, Huet G. Tissue inhibitor of metalloproteinase 1 is an independent predictor of prognosis in patients with nonsmall cell lung carcinoma who undergo resection with curative intent. Cancer. 2005;103(8):1676–1684. doi: 10.1002/cncr.20965. [DOI] [PubMed] [Google Scholar]
- 103.Wang CS, Wu TL, Tsao KC, Sun CF. Serum TIMP-1 in gastric cancer patients: a potential prognostic biomarker. Ann Clin Lab Sci. 2006;36(1):23–30. [PubMed] [Google Scholar]
- 104.Aaberg-Jessen C, Christensen K, Offenberg H, Bartels A, Dreehsen T, Hansen S, Schroder HD, Brunner N, Kristensen BW. Low expression of tissue inhibitor of metalloproteinases-1 (TIMP-1) in glioblastoma predicts longer patient survival. J Neurooncol. 2009;95(1):117–128. doi: 10.1007/s11060-009-9910-8. [DOI] [PubMed] [Google Scholar]
- 105.Kluger HM, Hoyt K, Bacchiocchi A, Mayer T, Kirsch J, Kluger Y, Sznol M, Ariyan S, Molinaro A, Halaban R. Plasma markers for identifying patients with metastatic melanoma. Clin Cancer Res. 2011;17(8):2417–2425. doi: 10.1158/1078-0432.CCR-10-2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Guedez L, Stetler-Stevenson WG. The prognostic value of TIMP-1 in multiple myeloma. Leuk Res. 2010;34(5):576–577. doi: 10.1016/j.leukres.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Terpos E, Dimopoulos MA, Shrivastava V, Leitzel K, Christoulas D, Migkou M, Gavriatopoulou M, Anargyrou K, Hamer P, Kastritis E, Carney W, Lipton A. High levels of serum TIMP-1 correlate with advanced disease and predict for poor survival in patients with multiple myeloma treated with novel agents. Leuk Res. 2010;34(3):399–402. doi: 10.1016/j.leukres.2009.08.035. [DOI] [PubMed] [Google Scholar]
- 108.Zhao J, Guan JL. Signal transduction by focal adhesion kinase in cancer. Cancer Metastasis Rev. 2009;28(1–2):35–49. doi: 10.1007/s10555-008-9165-4. [DOI] [PubMed] [Google Scholar]
- 109.Ridnour LA, Barasch KM, Windhausen AN, Dorsey TH, Lizardo MM, Yfantis HG, Lee DH, Switzer CH, Cheng RY, Heinecke JL, Brueggemann E, Hines HB, Khanna C, Glynn SA, Ambs S, Wink DA. Nitric oxide synthase and breast cancer: role of TIMP-1 in NO-mediated Akt activation. PLoS One. 2012;7(9):e44081. doi: 10.1371/journal.pone.0044081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jung YS, Liu XW, Chirco R, Warner RB, Fridman R, Kim HR. TIMP-1 induces an EMT-like phenotypic conversion in MDCK cells independent of its MMP-inhibitory domain. PLoS One. 2012;7(6):e38773. doi: 10.1371/journal.pone.0038773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bigelow RL, Williams BJ, Carroll JL, Daves LK, Cardelli JA. TIMP-1 overexpression promotes tumorigenesis of MDA-MB-231 breast cancer cells and alters expression of a subset of cancer promoting genes in vivo distinct from those observed in vitro. Breast Cancer Res Treat. 2009;117(1):31–44. doi: 10.1007/s10549-008-0170-7. [DOI] [PubMed] [Google Scholar]
- 112.Kopitz C, Gerg M, Bandapalli OR, Ister D, Pennington CJ, Hauser S, Flechsig C, Krell HW, Antolovic D, Brew K, Nagase H, Stangl M, von Weyhern CW, Brucher BL, Brand K, Coussens LM, Edwards DR, Kruger A. Tissue inhibitor of metalloproteinases-1 promotes liver metastasis by induction of hepatocyte growth factor signaling. Cancer Res. 2007;67(18):8615–8623. doi: 10.1158/0008-5472.CAN-07-0232. [DOI] [PubMed] [Google Scholar]
- 113.Schelter F, Grandl M, Seubert B, Schaten S, Hauser S, Gerg M, Boccaccio C, Comoglio P, Kruger A. Tumor cell-derived Timp-1 is necessary for maintaining metastasis-promoting Met-signaling via inhibition of Adam-10. Clin Exp Metastasis. 2011;28(8):793–802. doi: 10.1007/s10585-011-9410-z. [DOI] [PubMed] [Google Scholar]
- 114.Schelter F, Halbgewachs B, Baumler P, Neu C, Gorlach A, Schrotzlmair F, Kruger A. Tissue inhibitor of metalloproteinases-1-induced scattered liver metastasis is mediated by hypoxia-inducible factor-1alpha. Clin Exp Metastasis. 2011;28(2):91–99. doi: 10.1007/s10585-010-9360-x. [DOI] [PubMed] [Google Scholar]
- 115.Wang T, Lv JH, Zhang XF, Li CJ, Han X, Sun YJ. Tissue inhibitor of metalloproteinase-1 protects MCF-7 breast cancer cells from paclitaxel-induced apoptosis by decreasing the stability of cyclin B1. Int J Cancer. 2010;126(2):362–370. doi: 10.1002/ijc.24753. [DOI] [PubMed] [Google Scholar]
- 116.Djafarzadeh R, Mojaat A, Vicente AB, von Luttichau I, Nelson PJ. Exogenously added GPI-anchored tissue inhibitor of matrix metalloproteinase-1 (TIMP-1) displays enhanced and novel biological activities. Biol Chem. 2004;385(7):655–663. doi: 10.1515/BC.2004.081. [DOI] [PubMed] [Google Scholar]
- 117.Djafarzadeh R, Milani V, Rieth N, von Luettichau I, Skrablin PS, Hofstetter M, Noessner E, Nelson PJ. TIMP-1-GPI in combination with hyperthermic treatment of melanoma increases sensitivity to FAS-mediated apoptosis. Cancer Immunol Immunother. 2009;58(3):361–371. doi: 10.1007/s00262-008-0559-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Raggi MC, Djafarzadeh R, Muenchmeier N, Hofstetter M, Jahn B, Rieth N, Nelson PJ. Peritumoral administration of GPI-anchored TIMP-1 inhibits colon carcinoma growth in Rag-2 gamma chain-deficient mice. Biol Chem. 2009;390(9):893–897. doi: 10.1515/BC.2009.098. [DOI] [PubMed] [Google Scholar]
- 119.Djafarzadeh R, Noessner E, Engelmann H, Schendel DJ, Notohamiprodjo M, von Luettichau I, Nelson PJ. GPI-anchored TIMP-1 treatment renders renal cell carcinoma sensitive to FAS-meditated killing. Oncogene. 2006;25(10):1496–1508. doi: 10.1038/sj.onc.1209188. [DOI] [PubMed] [Google Scholar]
- 120.Notohamiprodjo S, Djafarzadeh R, Rieth N, Hofstetter M, Jaeckel C, Nelson PJ. Cell surface engineering of renal cell carcinoma with glycosylphosphatidylinositol-anchored TIMP-1 blocks TGF- beta 1 activation and reduces regulatory ID gene expression. Biol Chem. 2012;393(12):1463–1470. doi: 10.1515/hsz-2012-0188. [DOI] [PubMed] [Google Scholar]
- 121.Flisiak I, Zaniewski P, Chodynicka B. Plasma TGF-beta1, TIMP-1, MMP-1 and IL-18 as a combined biomarker of psoriasis activity. Biomarkers. 2008;13(5):549–556. doi: 10.1080/13547500802033300. [DOI] [PubMed] [Google Scholar]
- 122.Mannello F, Jung K. Blood sampling affects circulating TIMP-1 concentration, a useful biomarker in estimating liver fibrosis stages. Hepatology. 2008;48(2):688–689. doi: 10.1002/hep.22360. [DOI] [PubMed] [Google Scholar]
- 123.Kelly D, Squire IB, Khan SQ, Dhillon O, Narayan H, Ng KH, Quinn P, Davies JE, Ng LL. Usefulness of plasma tissue inhibitors of metalloproteinases as markers of prognosis after acute myocardial infarction. Am J Cardiol. 2010;106(4):477–482. doi: 10.1016/j.amjcard.2010.03.060. [DOI] [PubMed] [Google Scholar]
- 124.Vanhoutte D, Heymans S. TIMPs and cardiac remodeling: ‘embracing the MMP-independent-side of the family’. J Mol Cell Cardiol. 2010;48(3):445–453. doi: 10.1016/j.yjmcc.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 125.Singla DK, McDonald DE. Factors released from embryonic stem cells inhibit apoptosis of H9c2 cells. Am J Physiol Heart Circ Physiol. 2007;293(3):H1590–H1595. doi: 10.1152/ajpheart.00431.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Glass C, Singla DK. Overexpression of TIMP-1 in embryonic stem cells attenuates adverse cardiac remodeling following myocardial infarction. Cell Transpl. 2012;21(9):1931–1944. doi: 10.3727/096368911X627561. [DOI] [PubMed] [Google Scholar]
- 127.Seo DW, Li H, Guedez L, Wingfield PT, Diaz T, Salloum R, Wei BY, Stetler-Stevenson WG. TIMP-2 mediated inhibition of angiogenesis: an MMP-independent mechanism. Cell. 2003;114(2):171–180. doi: 10.1016/s0092-8674(03)00551-8. [DOI] [PubMed] [Google Scholar]
- 128.Qi JH, Ebrahem Q, Moore N, Murphy G, Claesson-Welsh L, Bond M, Baker A, Anand-Apte B. A novel function for tissue inhibitor of metalloproteinases-3 (TIMP3): inhibition of angiogenesis by blockage of VEGF binding to VEGF receptor-2. Nat Med. 2003;9(4):407–415. doi: 10.1038/nm846. [DOI] [PubMed] [Google Scholar]