Abstract
The transcription factor NF-κB plays a key role in numerous physiological processes such as inflammation, immunity, cell proliferation or control of cell death. Its activation is tightly controlled by a kinase complex, IκB kinase (IKK), composed of three core proteins: IKK1/IKKα, IKK2/IKKβ and NEMO/IKKγ. The first two are structurally related kinases whereas the third one is a regulatory subunit exhibiting affinity for upstream activators modified by polyubiquitin chains. Over the years, several inherited diseases caused by mutations of each of the three subunits of IKK have been identified in humans together with diseases caused by mutations of several of its substrates. They are associated with very specific and complex phenotypes involving a broad range of abnormalities such as impaired innate and acquired immune response, perturbed skin development and defects of the central nervous system. Here, we summarize the diverse clinical, cellular and molecular manifestations of IKK-related genetic diseases and show that studying patient-related mutations affecting the IKK subunits and some of their substrates offers the opportunity to understand the various functions of NF-κB in humans, complementing studies performed with mouse models. This analysis also provides glimpses about putative functions of IKK subunits that may be NF-κB-independent.
Keywords: NF-κB, IKK, Genetic diseases, Immunodeficiencies, Skin development
The standard procedure for dissecting signaling pathways through combined biochemical/structural analysis of their components and interrogation of their function(s) through genetic manipulations in model organisms such as mouse, zebrafish or drosophila has recently benefited from the development of powerful technics that allow high-rate DNA sequencing of patients suffering from a wide range of inherited diseases. In several instances, this has provided a deeper view of the physiological role played by key proteins in these pathways and suggested strategies to correct their deregulated functions in humans. The case of the NF-κB pathway, which plays a critical role in immunity, inflammation and cell death and can be a direct cause or an amplification element of various pathological conditions, illustrates the interest of this combined approach.
The NF-κB signaling pathway
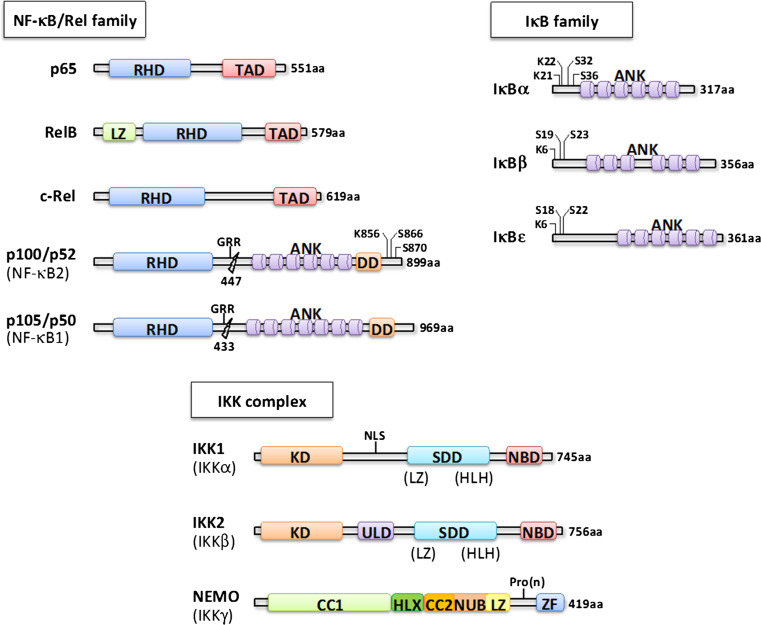
NF-κB represents an evolutionarily conserved family of inducible transcription factors that controls a large set of physiological processes ranging from basic inflammatory responses and innate and acquired immunity to the regulation of cell death such as apoptosis, autophagy and senescence. In addition, NF-κB coordinates the expression of specific genes that mediates proliferation, cell adhesion and differentiation [1, 2]. The NF-κB family consists of NFΚB1 (p50), NFΚB2 (p52), RelA (p65), RelB and c-Rel proteins, which associate to form homo- or heterodimers (Fig. 1). The subunits p50 and p52 are synthesized as a p105 and p100 precursor protein, respectively. They undergo—constitutively or in a stimulus-dependent manner—a proteasome-mediated limited processing to yield the active form.
Fig. 1.
Components of the NF-κB signaling pathway. Alternative nomenclatures appear in brackets. The respective domains of each protein family are indicated with RHD REL homology domain, TAD transactivation domain, LZ leucine zipper, GRR glycine-rich region, ANK ankyrin repeat, DD death domain, KD kinase domain, NLS nuclear localization sequence, SDD α-helical scaffold/dimerization domain, HLH helix–loop–helix, CC coiled coil, HLX helix, NUB NEMO ubiquitin binding, Pro(n) proline rich region, ZF zinc finger. Residues modified by phosphorylation or ubiquitination are also shown
In resting cells, NF-κB is sequestered in the cytoplasm in an inactive state through the interaction with IκB inhibitors (IκBα, IκBβ and IκBε) (Fig. 1). Following activation, IκBs are phosphorylated at conserved serine residues, then ubiquitinated by the E3 ubiquitin ligase SCF-βTrCP and finally degraded by the proteasome, which in turn allows the nuclear translocation of NF-κB and thereby activation of its target genes. The kinase complex responsible for the phosphorylation of IκBs has remained elusive for many years until the biochemical purification of a cytoplasmic high-molecular weight complex containing two serine/threonine kinases, IKK1 (IKKα or CHUK) and IKK2 (IKKβ) [3] (Fig. 1). A third non-catalytic component called NEMO (IKKγ) was subsequently identified by complementation of an NF-κB-unresponsive cell line and by sequencing of IKK-associated polypeptides [3] (Fig. 1). Studies based on cell transfections and mouse gene ablation revealed that NF-κB can actually be activated by two alternative signaling pathways. The canonical pathway is induced by a large variety of stimuli such as pro-inflammatory cytokines (IL-1β, TNF-α), bacterial products (LPS, peptidoglycan), viral proteins (Tax, LMP-1) or diverse forms of stress (UV, oxidative stress) and is strictly dependent on NEMO, which ensures the signal transmission from the receptor to the catalytic IKK2 subunit [2]. In most situations, IKK1 only mildly contribute to the overall kinase activity of IKK [4] but in the absence of IKK2 might help preserving low levels of NF-κB activation (see below). In contrast, the non-canonical pathway of NF-κB activation is turned on by a restricted group of stimuli such as lymphotoxin-α/β, CD40L or BAFF and is independent on NEMO and IKK2 but specifically requires IKK1. In this case, the signaling complexes stabilize kinase NIK that phosphorylates IKK1, which in turn phosphorylates and marks p100 for C-terminal processing by the proteasome to generate p52-relB dimers. This non-canonical pathway appears to play a critical role in the development of lymphoid organs and mammary glands [5]. In addition, IKK1 is able to regulate NF-κB-dependent and -independent transcription through its nuclear localisation and ability to phosphorylate Ser10 of Histone H3 [6, 7].
This way of presenting the two distinct modes of NF-κB activation does not take into account the functional interplays that exist between them and their integration in a single module [8]. Indeed, it has been shown that several receptors activating the non-canonical pathway can also activate the canonical one. Moreover, the expression or activity of several components of one pathway is controlled by the other. For instance, canonical NF-κB dimers regulate the transcription of RELB whose product is the main partner of p52, whereas IκBδ, which is derived from p100, provides a negative feedback on the canonical NF-κB signaling.
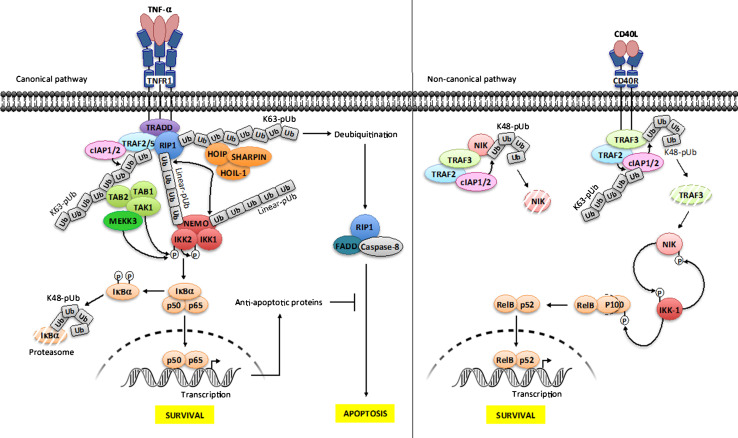
Although many signaling pathways can induce NF-κB activation, there is a sequence of common molecular events that leads to the activation of IKK. This sequence involves the modification of specific residues of NEMO by lys63- or linear-linked polyubiquitin chains [9] (see Fig. 2 for the specific case of the TNF-α signaling pathway). In contrast to lys48-linked polyubiquitination, these linkages do not lead to proteasomal degradation but serve as binding sites for other molecules required for the activation of IKK. In addition, NEMO can also recognize lys63 and linear ubiquitin chains from a multitude of modified proteins [9]. This dual ability allows polyubiquitinated activators to be put in proximity to IKKs or to induce conformational change in the IKK complex. The non-canonical pathway of NF-κB activation is also regulated by complex ubiquitination processes which result in stabilization of labile NIK, allowing activation of IKK1 and processing of p100 (see Fig. 2 for the specific case of the CD40 signaling pathway).
Fig. 2.
Ubiquitination and phosphorylation processes in NF-κB signaling. Canonical NF-κB pathway/TNF-R1 receptor (left panel). The engagement of TNF-α on its cognate receptor induces the recruitment of the adapter TRADD, the E3 ligases cIAP1/2 and TRAF2 and the kinase RIP1. cIAP1/2 catalyze the formation of lys63-linked polyubiquitin (K63-pUb) chains on TRAF2/TRAF5 and RIP1. K63-pUb recruits on one hand the TAB 1–TAB 2–TAK1 kinase complex and on the other hand the LUBAC ligase complex (HOIP, HOIL-1 and SHARPIN), which catalyzes the formation of linear-linked polyubiquitin (linear-pUb) chains on RIP1. Linear-pUb is recognized by NEMO, allowing the recruitment of the IKK complex. TAK1 mediates the activation of IKK-2 by phosphorylation, allowing its auto-phosphorylation, which in turn enables IKK2 to phosphorylate IκB proteins, inducing their degradation by the proteasome and the release of active NF-κB. Another kinase, MEKK3 also participates in this process although its function is less understood. The ubiquitination of NEMO by LUBAC through linear-pUb might amplify NF-κB activation by recruiting more IKK complex. Shortly after signal transmission deubiquitination of RIP1 also results in formation of a FADD-RIP1-Caspase-8 complex, which provokes apoptosis. This process is negatively regulated by a set of anti-apoptotic proteins whose transcription is under NF-κB control. Non-canonical NF-κB pathway/CD40 receptor (right panel). In basal condition, cIAP1/2 catalyzes the formation of lys48-linked polyubiquitin chains (K48-pUb) on NIK, leading to its constitutive degradation. Upon activation, TRAF2-TRAF3-cIAP1/2 complex is recruited to the receptor, allowing TRAF2 to ubiquitinate cIAP1/2 through K63-pUb. This modification on cIAP1/2 induces a switch on its K48 ubiquitin ligase activation from NIK to TRAF3. The consecutive degradation of TRAF3 allows the accumulation of newly synthesized NIK, which in turn phosphorylates IKK-1. Then, IKK-1 phosphorylates and marks p100 for processing through degradation, releasing relB-p52 that translocates to the nucleus for gene activation. As a negative feedback loop, IKK-1 phosphorylates NIK for turning off its activity
The physiological roles of the various IKK components have been analyzed using a collection of KO mice. Mice deficient for IKK2 die during embryogenesis at E12.5–13.5 as a result of massive hepatic apoptosis [10] which emphasizes the essential role of IKK2 in the canonical activation of NF-κB. Upon crossing with Tnfr1 KO mice, survival is observed, indicating that TNF-α is the inducer of liver apoptosis. Using conditional targeting of Ikk2 in the mouse allowed to demonstrate the key function of IKK2 in immunity [11]. The same kind of lethality by hepatic apoptosis is observed at E12.5-E13 in male mice invalidated for the X-linked Nemo gene [12]. In contrast, Nemo +/− females survive and develop a dermatosis which mimics what is observed in humans (see below) [13, 14]. Mice lacking IKK1 survive until birth and do not present any signs of liver apoptosis. However, the mice have severe defects in epidermal differentiation and skeleton development [4, 15], which shows that besides NF-κB signaling pathways, IKK1 has diverse functions. The NF-κB family genes have also been knocked out. While RelA deficient mice are embryonic lethal at E15, again due to excessive liver apoptosis [16], mice lacking one of the other NF-κB subunits, such as p50, p52 or c-rel survive but present various immune defects due to abnormal T or B cell response [17].
NF-κB-related genetic diseases
NEMO-related genetic diseases
As the regulatory subunit of IKK, NEMO is absolutely required for proper activation of the canonical NF-κB pathway in response to a wide range of stimuli, as outlined above. Another key feature of this protein is the location of its encoding gene on the X-chromosome. The intricate consequences of these combined properties, a broad involvement of NEMO in many NF-κB-inducing pathways and an X-linkage, became apparent when patients displaying mutations of NEMO were identified. Here, we will only summarize the main characteristics of these diseases since recent reviews describing them in details can be found elsewhere [18, 19].
Incontinentia pigmenti
Incontinentia pigmenti (IP, [MIM # 308300]), also known as Bloch–Sulzberger syndrome, is an X-linked dominant genodermatosis that is lethal for males before the second trimester of gestation [20]. In females, the inflammatory outbreak, which occurs within 2 weeks after birth, is the first phase of the disease with large dyskeratotic cells and intraepidermal spongiosis (vesicular stage). This stage is followed by hyperproliferation of keratinocytes in the epidermis with acanthosis and papillomatosis (verrucous stage). The third phase is characterized by an accumulation of melanocytes that produce pathologically high amounts of melanin (pigmented stage). The process is terminated by regression of the cutaneous lesions (atrophic stage), leaving behind hypopigmented areas and lack of eccrine glands. This complete process takes weeks or months but can restart following an infection during the rest of patient’s life. It appears to be caused by cells expressing the mutated X-chromosome along the so-called Blaschko lines that are selectively eliminated around the time of birth.
Frequently this genodermatosis is accompanied by unilateral ophthalmological problems (foveal hypoplasia, retinal ischemia and strabismus) that are often highly debilitating. Indeed, ocular involvement persists during all the patient’s life, in contrary to dermalogical symptoms that are attenuated over the years. These abnormalities are often associated with neurological involvement, which are present in 30 % of cases and characterized by psychomotor retardation, cerebellar ataxia, microcephaly, hemiplegia, epilepsy and intellectual impairment. A correlation between the severity of the ophthalmologic problem and the neurological phenotype might be due to vascular insufficiency caused by necrosis and ischemia.
With a prevalence of 33 %, hair changes are reported in IP patients like clairsement during infancy and mostly alopecia on the vertex or dull and brittleness hair in adulthood. Hyperplasia or absence of eyebrows and eyelashes may be observed. Finally, IP is a congenital factor of malocclusion and oligodontia. Patients may present hemifacial hypoplasia and delayed tooth eruption with conical teeth in 80 % of the IP cases. Dental features are quite similar in another disease called EDA-ID caused by NEMO mutations (see below).
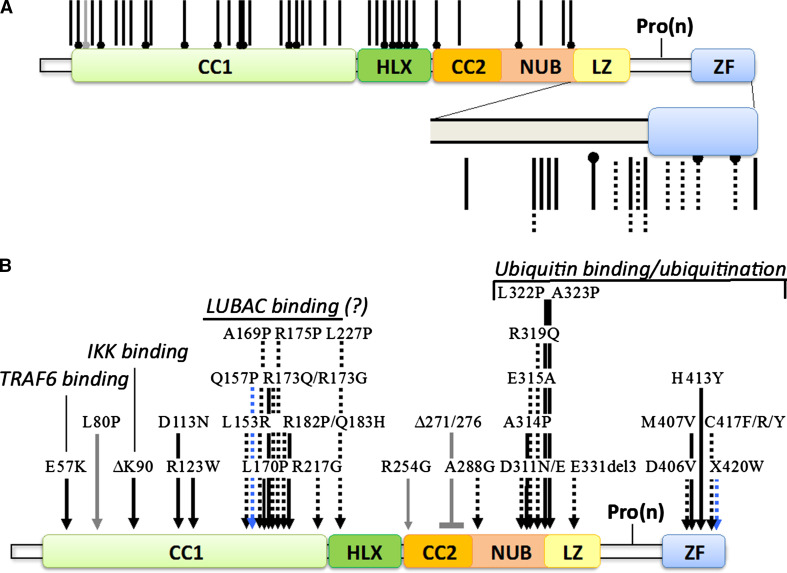
Approximately 70 % of IP patients share the same genomic rearrangement that eliminates exons 4–10 of the NEMO gene [21]. This rearrangement involves a NEMO pseudogene that is located nearby and contains sequences highly similar to those of exons 4–10 of NEMO. In addition to this frequent recurrent rearrangement, the NEMO locus of IP patients can also present other kinds of rearrangements [22] and non-sense or frameshift mutations that severely truncate the protein or even rare missense mutations [19] (Fig. 3).
Fig. 3.
NEMO mutations found in human diseases. a Truncating mutations. Non-sense mutations generating IP or EDA-ID are indicated by dumbbell-shaped black or dotted bars, respectively. Frameshift mutations generating IP or EDA-ID are indicated by black or dumbbell-shaped bars, respectively. An EDA-ID-linked N-terminal deletion resulting from a translational reinitiation is indicated by a gray dumbbell-shaped bar. b Missense mutations and short deletions. IP-associated mutations are indicated by black arrows, EDA-ID-associated ones by dotted arrows, OL-EDA-ID-associated ones by blue arrows and ID-associated ones by gray arrows. The defined or putative molecular dysfunctions are also indicated. Mutations are compiled from [18, 21]
The lack of function of truncated and unstable NEMO protein in IP results in higher sensitivity of cells to TNF-α and increased apoptosis. This may explain why male patients do not survive during development if liver apoptosis occurs like in Nemo KO mice [12–14], something not formally demonstrated yet. In female, this clearly contributes to the dermatosis process and its resolution. Indeed, it has been proposed that the mosaic status of the neonatal skin/epidermis of IP females, composed of wild-type cells expressing NEMO and mutant cells lacking NEMO, is the trigger for the whole process [13]. Mutant cells, most likely keratinocytes, would start to overexpress pro-inflammatory cytokines such as IL-1β after birth, the reason why remaining uncertain [necrosis of some cells? Dysregulated adaptation to the (bacterial) environment at birth?…]. Production of IL-1β by mutant cells would induce TNF-α synthesis by neighboring wild-type cells, and TNF-α would in turn act back on mutant cells, inducing their death and clearance. This sequence of events may explain the final atrophic stage of IP dermatosis, linked to lesion disappearance. This model is based on what has been observed in mice exhibiting impaired NF-κB signaling in the skin, for instance by invalidating Nemo [23] or Ikk2 [24] specifically in the epidermis. In this case, mice develop abnormalities similar to the ones observed in IP, with extensive inflammation and cell death, which can be fully corrected by only inhibiting TNFR-1 signaling. Besides regulating cell death TNF-α may also have an additional pro-inflammatory function in the process. Indeed, it has been shown that, upon TNF-α exposure, upregulation of IL-19/24 in NF-κB-deficient keratinocytes results in STAT3 activation and cytokines/chemokines production [25].
X-linked anhidrotic ectodermal dysplasia with immunodeficiency
Anhidrotic ectodermal dysplasia with immunodeficiency [EDA-ID, (MIM # 300291)] is a rare syndrome affecting only males. It is characterized by impaired development of skin appendages (teeth, hair and sweat glands) and recurrent bacterial and viral infections [26–29].
On the EDA side, the development of tooth buds in EDA-ID patients frequently gives absent teeth and/or pointed teeth with enamel potentially defective whereas abnormalities of the hair follicles result in excessively thin and sparse hair that may grow very slowly or sporadically. In addition, patients have often fragile and lightly pigmented skin and cannot perspire because of sweat glands dysfunctions or inactive proteins at this level. Therefore, the body cannot regulate temperature properly.
Combined to this EDA is a severe life-threatening immunodeficiency characterized by weak cell responses to IL-1β, IL-18, CD40 or lipopolysaccharide (LPS). The production of specific antibodies is defective, with decreased IgG and increased IgA synthesis, accompanied by hyper-IgM syndrome. These B cell defects may be linked to impaired co-stimulation by CD40 and are associated with T cell impairment and problem of NK cell activation, despite normal cell count and proper development [30]. Moreover, there is a defective interferon (IFN) synthesis resulting in reduced response to virus exposure [31].
EDA-ID is caused by hypomorphic NEMO mutations that allow male survival and cause negligible effects (very weak IP signs) in females [26–29]. In most cases, short truncations of the protein occur due to non-sense or frameshift mutation but missense mutations are also observed (Fig. 3). They often affect domains or sequences required for recognition of ubiquitin by NEMO but the activity of the protein is not completely impaired since this recognition involves two distinct portions of NEMO located at the C-terminus of NEMO (NEMO zinc finger) and in the middle of it (NEMO ubiquitin binding (NUB) domain), respectively [32].
The identification of NEMO mutations and associated impaired activation of NF-κB, as a cause of EDA has allowed identification of a new component playing a role of effector in the eda/EDAR pathway that had been previously identified through the genetic study of patients exhibiting pure EDA. In these studies, a ligand (ectodysplasin) working through its specific receptor (EDAR) and adaptor (EDARADD) had been demonstrated to be absolutely required for skin appendage development [33].
Another related pathology to EDA-ID, caused by the destabilizing addition of 27aa at the C-terminal of NEMO (mutation X420 W), has been reported [27]. It is called Anhidrotic Ectodermal Dysplasia with Immunodeficiency, Osteopetrosis and Lymphedema [OL-EDA-ID, (MIM # 300301)]. The immunodeficiency syndrome presents similarities to the EDA-ID-related one but patients also display increased bone mass due to defective osteoclast function and lymphedema caused by impaired function of lymphatic vessels. This phenotype supports the idea that NF-κB plays an important role in skeletal development and demonstrated a new role for this transcription factor in lymphatic vessel physiology.
Immunodeficiencies
Several cases of primary immunodeficiencies without associated EDA were also shown to be caused by missense mutations or short truncations of NEMO (NEMO ID) [34–37]. These male patients suffer from recurring infections, often Mycobacteria related, and some specific impaired cell responses are observed, such as reduced IL-12 synthesis in response to CD40. Upon close examination, very mild tooth abnormalities can also be detected in some patients. How those NEMO mutations, despite their similarities to the ones causing EDA-ID, generate a phenotype that does not include skin appendage abnormalities remains unclear. The simplest possibility would be that mild mutations of NEMO unable to affect the skin eda/EDAR signaling pathway are still able to perturb the few immune-related pathways that are the most sensitive to slightly reduced NF-κB activity and the eda/EDAR pathway responsible for tooth development.
IKK1-related genetic disease (Cocoon syndrome)
IKK1 is one of the two catalytic subunits of IKK but in contrast to NEMO, which only play a very narrow range of functions outside of the NF-κB pathway, performs additional NF-κB-independent functions [38]. In addition, it has been shown very early on to be dispensable for full IKK catalytic activity, being an intrinsically weaker kinase than IKK-2. In contrast, it plays a unique role in the non-canonical NF-κB activation pathway (see above).
Two cases of fetal encasement malformations occurring in a consanguineous finnish family were recently reported to present some striking similarities with the phenotype of Ikk1 KO mice [39]. Among the numerous abnormalities detected in the fetuses at 12–13 weeks of gestation were defective face, with an abnormal cyst in the cranial region and a large defect in the craniofacial area, an omphalocele and lobulation defect in the lungs. Poorly developed skeletal muscles were observed as well as seemingly absent limbs, which were bound to the trunk and encased under the skin.
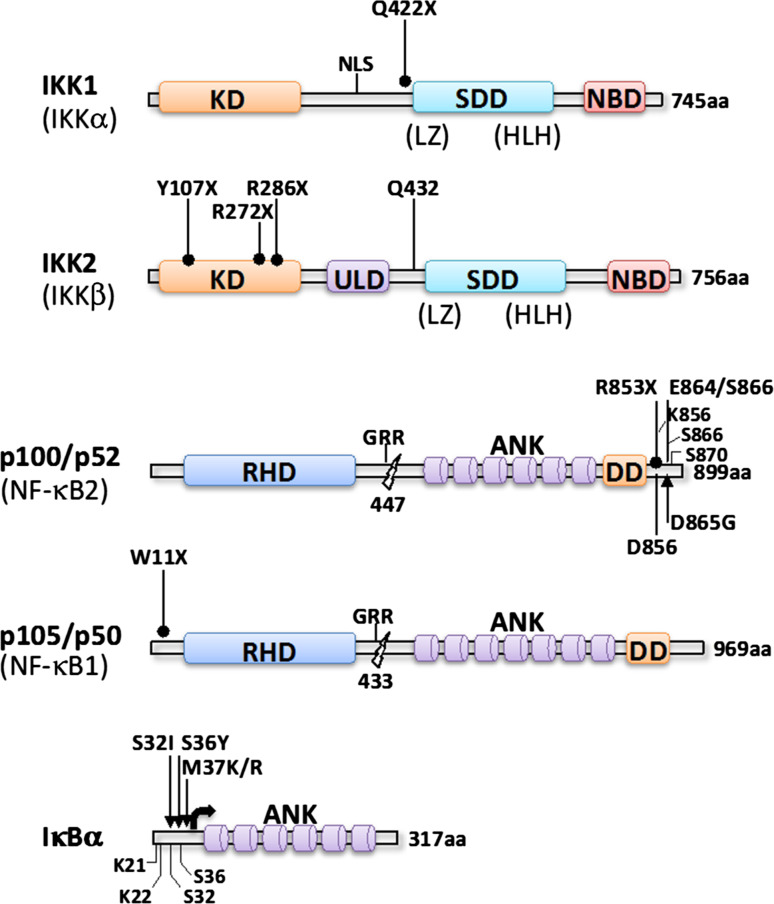
This limb encasement being reminiscent of the phenotype observed after targeting IKK1 in the mouse convinced the authors to sequence the IKBKA gene in the two fetuses. An homozygous missense mutation, c.1264C—T, was detected in exon 12, generating a large truncation of IKK1 deleting its catalytic domain (aa 422–745) (Fig. 4). Actually, the overall expression of this deleted protein appeared strongly decreased, demonstrating its instability. Since a similar case was previously reported in the literature [40], but not genetically characterized, and qualified as “Cocoon fetus” the term “Cocoon syndrome” has been proposed by Lahtela et al. [39] to be used for this IKK1-related inherited disease.
Fig. 4.
IKK- and IKK substrates-related mutations found in human diseases. Non-sense mutations are indicated by dumbbell-shaped black bars, frameshift mutations by black bars and missense mutations by black arrows. An N-terminal deletion of IκBα resulting from a translational reinitiation at Met37 is indicated by a bracketed arrow
The molecular basis of the Cocoon syndrome remains poorly defined. In particular, it remains to be examined if the proposed effect of IKK1 as a repressor of FGF members in skeletal and craniofacial development [41] also operates in humans. Analyzing the transcriptome of F1 and F2, Lahtela et al. [39] demonstrated a severe reduction in numerous mRNAs. Among them, one coding for matrix metallopeptidase 14 (MMP-14) was present at only 5 % of the control. In contrast, the opposite was observed when studying the expression level of MMP-14. Since this enzyme plays an important role in skeletal development such aberrant regulation might also be involved in craniofacial and skeletal abnormalities.
How may this and other described abnormalities observed in the Cocoon syndrome relate to NF-κB? As previously demonstrated by Hu et al. [41], the peculiar skin phenotype resulting from IKK-1 lack of function is unlikely to directly result from a canonical IKK/NF-κB deregulation. Besides being very different from the one observed in incontinentia pigmenti, clearly caused by impaired NF-κB activation in the epidermis, it is also characterized by a lack of keratinocyte differentiation which can not be corrected by activating NF-κB. Actually, as a whole, the Cocoon syndrome displays several similarities with a limb pterygium syndrome (LPS) called lethal-type popliteal pterygium syndrome (LPPS)/Bartsocas–Papas syndrome (BPS) (MIM # 263650) [42]. Interestingly, this specific syndrome has been demonstrated to be caused by mutations of RIPK4 [43, 44], which codes for a member of the RIP kinase family originally proposed to be an NF-κB activator in PKCβ-dependent pathways [45, 46]. Nevertheless, the original data were obtained by performing overexpression experiments, which can often generate artefacts. Besides this putatively weak link between the main signs observed in Ikk1 KO mice/Cocoon syndrome and the NF-κB signaling pathway other developmental pathways may be affected, involving both IKK1 and RIPK4, sequentially or in parallel. Among them is the p63 pathway, which appears deregulated in BPS [43], and the β-Catenin pathway which was recently shown to be regulated by RIPK4 [47]. Even in the case of a p63 involvement, an indirect link with NF-κB can not be formally excluded as isoforms of p63 have been shown to be activators of IKK1 in the epidermis and to interact with c-rel and relA, two members of the NF-κB family of transcription factors [48]. It remains, therefore, quite difficult to estimate which parts of Cocoon syndrome anomalies, if any, are NF-κB related, something complicated by the severity of the disease and its paucity. Nevertheless, Ikk1 KO mice being available a back and forth analysis between data issued from human samples and mice might be envisioned.
IKK2-related genetic disease (IKK2 SCID)
As the kinase displaying the strongest catalytic activity within IKK, IKK2 is considered to be a key enzymatic element of this protein complex. Nevertheless, it has been shown that its absence in numerous cellular settings is not as detrimental in the canonical NF-κB activation process as the absence of NEMO. Indeed, residual IKK activation can still occur due to IKK1 being able to compensate to some extent the lack of IKK2 activity [13, 49].
A severe combined immunodeficiency (SCID) characterized by recurrent infections and hypogammaglobulinemia/agammaglobulinemia but normal T cell counts were recently identified in several individuals [50–53]. The first ones and the most extensively characterized presented a common genetic origin (Northern Cree ancestry), but no consanguinity [50]. Homozygosity mapping revealed a candidate 11.6 Mbp region on chromosome 8. After sequencing the 40 genes located at this locus only one was shown to exhibit in four distinct cases an homozygous duplication (c.1292dupG) in exon 13 of the gene encoding IKK2. This frameshift mutation was shown to produce a very unstable truncated IKK2 protein (Fig. 4), as revealed by Western blotting. Unexpectedly, the expression level of other IKK components, IKK1 and NEMO, appeared reduced also.
Several signaling pathways were shown to be affected by the lack of IKK2 when patient-derived cells were stimulated. Among the most perturbed pathways in fibroblasts were the ones responding to TNF-α, LPS (TLR4), flagellin (TLR5) or PMA. In contrast, the IL-1 signaling pathway appeared less impaired. As a consequence, target genes of NF-κB were differentially affected depending on the stimulus used and more or less sensitive to a lack of IKK2 activity.
Expression of activation markers such as CD25 and CD69 was reduced upon exposure of IKK2 SCID T cells to CD3/CD28 but proliferation of the cells was only mildly impaired. Indeed, if little response was observed to soluble or plate-bound anti-CD3 antibodies, Phytohemagglutinin mitogenic responses were moderately reduced as well as proliferation in response to CD3/CD28. One may, therefore, consider the overall activation capacity of T cells to be reduced to some extent but not abolished.
When patient-derived B cells were stimulated with CD40L and IL-21, which are usually provided by follicular helper T cell, they did not proliferate and differentiate into plasmablasts. In addition, they did not produce immunoglobulins (Igs). In contrast, proliferation was still observed when induced by the B cell receptor or the cytosine–guanine dinucleotide polymer CpG, through TLR9, but Ig production was impaired.
In line with the severe phenotype of the patients and the signal transduction abnormalities described above, many immune defects were identified in situ. Although T and B cell development was not impaired many signs of their perturbed differentiation were observed. For instance, γδ T cells were absent and most CD4 and CD8 cells expressed naive antigens such as CD45RA or CD27 despite the exposure of patients to various germs. Absence of CD25highFOXP3+CD4+Tregs was also noted in two patients. B cells were almost exclusively naive, with a normal proportion of CD38+IGM+ transitional cells and a lack of CD27+ IGD− class-switched memory cells and CD38+ CD20− plasmablasts. In addition, activation of NK cells appeared deficient.
Surprisingly, no EDA was observed in these IKK2 SCID patients whereas this is a feature of patients displaying hypomorphic NEMO mutations (see above) and autosomal dominant IΚBΑ mutations (see below). This suggests that IKK2 is dispensable for skin appendage development. Most likely, the catalytic activity of IKK1 is sufficient to compensate for the absence of IKK2 catalytic activity in this setting.
Most of the abnormalities observed in patients carrying an homozygous nucleotide duplication in IKBKB were also recently found in other patients displaying mutations in the same locus [51–53]. An homozygous non-sense mutation in IKBKB (R286X) resulting in truncation of IKK2 (Fig. 4) induced a lack of its expression [51]. The level of NEMO was also decreased confirming the observation made by Pannicke et al. [50]. Defective activation of T, B and NK cells was detected, associated with the absence of memory B cells, accumulation of CD45RA+ T cells and low number of Tregs. A similar clinical and cellular picture was reported for other non-sense mutations (Y107X and R272X) leading to IKK2 truncation (Fig. 4) [52, 53]. In all these cases, no obvious signs of EDA were observed confirming the negligible role of IKK2 in skin adnexal development. The only major discrepancy reported by these different studies concerns the reduction in NEMO amount, which was observed by Pannicke et al. [50] and Mousallem et al. [51], and in IKK1 amount reported by Pannicke et al. [50], but were not confirmed by Nielsen et al. [53]. The reason for these differences is puzzling since all four reported mutations result in the same lack of IKK2 expression.
Genetic diseases caused by mutations of IKK substrates
IκBα-related genetic disease (autosomal-dependent EDA-ID)
As shown above, EDA-ID can be caused by hypomorphic mutations of NEMO. Another component of the NF-κB signaling pathway that can generate EDA-ID when mutated is IκBα. In several instances, heterozygous mutations of IκBα impairing its degradation by IKK have been reported to cause a syndrome that shares many clinical similarities with NEMO-linked EDA-ID [54–61] [autosomal-dominant (AD) EDA-ID, (MIM # 612132)]. So far, all these mutations affect the Ser residues (Ser32, Ser36) that are phosphorylated by IKK upon cell stimulation [54–56], the residues nearby the DSGLDS phosphorylation motif, such as Met37 [57, 58], or delete the N-terminus of IκBα (Q9X, W11X, E14X), reinitiation of translation occurring at Met37 and thus eliminating the phospho-acceptor sites [59–61] (Fig. 4). Because of this lack of phosphorylation, IκB can not be degraded and acts as a dominant negative protein by accumulating and sequestering NF-κB species such as relA, c-rel or p50 in the cytoplasm. This explains why an heterozygous mode of genetic transmission is sufficient enough to trigger the disease.
Mutations of both NEMO and IκBα as a cause of EDA-ID in humans confirm in vivo the participation of these two components in the same signaling pathways. Nevertheless, how NF-κB signaling is impaired in each case is biochemically quite distinct. In NEMO-mutated patients generating EDA-ID the overall catalytic activity of IKK is reduced but not abolished. Therefore, the various substrates of IKK, among them the members of the IκB family of inhibitors, are putatively similarly affected. In the case of mutations affecting IκBα, degradation of the other IκB inhibitors is still properly controlled. The similitude between NEMO- and IκBα-related EDA-IDs indicates that IκBα is indeed a prime target of IKK in many distinct tissues, among them the hematopoietic compartment and the skin appendages. It also confirms that its function in controlling the activity of specific NF-κB subunits can not be overcome by other properly regulated IκB/NF-κB complexes.
Because of the severity of EDA-ID, caused by either IΚBΑ or NEMO mutations, treating those patients with allogenic hematopoietic stem cell transplantation (HSCT) represents a therapeutic option. In several cases, caused by IΚBΑ mutations, HSCT was shown to allow a full recovery of immune functions [62]. In a more severe case caused by a NEMO mutation, this approach was unfortunately not successful [63].
NF-κB1-related genetic disease
It has been recently reported the description of a patient exhibiting bone and joints defects, ectodermal dysplasia, hypergammaglobulinemia and sterile inflammation [64]. Upon DNA sequencing, an heterozygous mutation was detected in NFΚB1, the gene encoding p105, the precursor of the NF-κB p50 subunit (Fig. 4). Since this mutation introduces a stop codon (W11E) at the beginning of the coding sequence of p105, a 50 % reduced expression of this protein can be predicted. How such reduction in p105 expression may generate such complex-associated phenotype remains uncertain and will require the characterization of additional patients.
NF-κB2-related genetic disease (NF-κB2-CVID)
Common variable immunodeficiency [CVID, (MIM # 607594)], which occurs in approximately 1:10,000–1:50,000 people, is a clinically and genetically heterogeneous disorder characterized by hypogammaglobulinemia with poor response to antigens [65]. It can be caused by mutations affecting many genes coding for proteins regulating peripherical or terminal B cell development. For instance, 8–10 % of CVID patients have been identified with homozygous and heterozygous variants in TNFRSF13B and 1–2 % of individuals harbor variants in ICOS.
Recently, four cases (out of 37) from two distinct families were shown to exhibit after exome sequencing heterozygous mutations of NFΚB2, the gene encoding p100 [66] (Fig. 4). In each situation, the mutations generated short C-terminal truncations of p100 at aa 852 or 854, respectively. Importantly, this short truncation was large enough to eliminate not only Serine residues S866 and S870, but also Lysine residue K856 which are all essentials for processing of p100 to p52. Indeed, S866 and S870 are phosphorylation sites for IKK1 which are then required to trigger ubiquitination at K856 and recognition by the proteasome [5]. A lack of p100 processing was demonstrated using EBV-transformed B lymphocytes derived from three NFΚB2 mutated patients. In all cases, a shorter form of p100 was detected below the full-length p100, as expected from their heterozygous status, and production of p52 appeared reduced by half. As a consequence, nuclear translocation of p52 was impaired.
Like other CVID patients, NFKB2-mutated patients exhibited markedly reduced levels of serum immunoglobulins, including low IgG levels, and poor response to antigens but normal levels of circulating B cells. They suffered from recurrent infections (viral respiratory infections, pneumonia, sinusitis, etc.) and asthma. Interestingly, they also suffered of alopecia areata and central adrenal insufficiency (ACTH and cortisol deficiency). Since this latter abnormality is an unusual feature of CVID its intriguing link to p100/NF-κB will require further investigation. Chen et al. [66] have proposed that alterations in thymic AIRE expression may result in aberrant T cell-mediated self-tolerance of endocrine-related organs.
More recently, other patients displaying heterozygous NFKB2 mutations impairing p52 production were identified [67–69], allowing a more comprehensive view of this subtype of CVID. Besides confirming a recurrent specific impairment of B cell functions and auto-immunity (alopecia areata and/or central adrenal insufficiency) they also suggested a putative phenotype heterogeneity which might be due to an indirect effect of specific NFKB2 mutations on the canonical pathway of NF-κB activation in addition to the non-canonical one. For instance, it has been proposed that full-length proteins exhibiting missense mutations, such as D865G, may behave like IκB-like molecules, also acting on the canonical pathway of NF-κB activation and impairing B cell development in addition to B cell function [69]. Therefore, a complex genetic basis mixing haploinsufficiency and autosomal dominant effects is at the origin of NFKB2-related CVIDs.
Conclusion
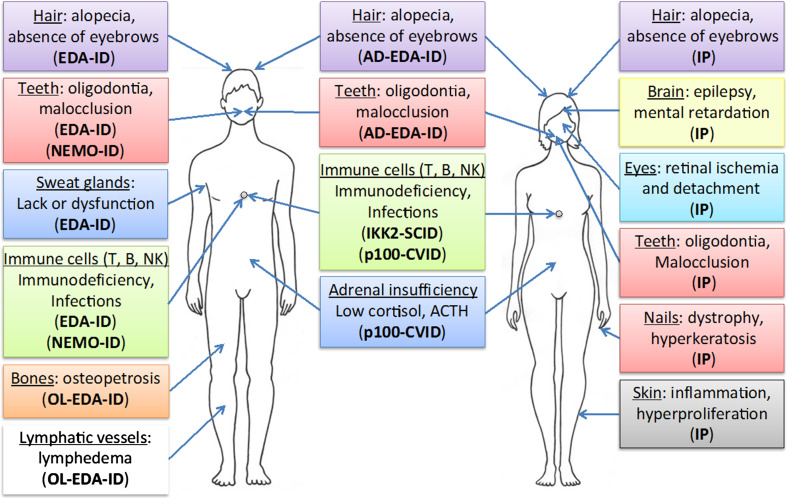
Many lessons can be drawn from the NF-κB data originating from human genetics (Fig. 5). First of all, experts in the NF-κB field will recognize their favorite pathway and its main associated features. Among them are numerous immune defects resulting from NF-κB impairment and impact on the inflammatory process, even if not necessary in the expected sense. In addition, the respective role of each IKK subunit appears to be confirmed with NEMO playing a critical role in the IKK/NF-κB activation process, IKK2 performing a key function in the canonical pathways and IKK1 having its own social life, NF-κB-dependent and independent. Even at the level of IκBs and IκB-like molecules predicted features, mostly immune related, are observed. Finally, the phenotypes caused by NF-κB-related diseases in humans are grossly similar to the ones observed with KO mice.
Fig. 5.
Main clinical features of IKK-related genetic diseases Various abnormalities found in males vs females are listed with their associated disease (see text for details). The Cocoon syndrome caused by IKK-1 mutation is not presented given its severity leading to early fetus death. In addition, the anomalies of a putatively complex syndrome resulting from an NFKB1 mutation found in only one patient are not represented
The clinical investigation of these diseases has nevertheless provided additional info regarding the NF-κB pathway, sometimes unpredicted ones. As said above, the X-linkage of NEMO, which was not originally considered when studying this protein in individual cells, has a critical impact on NEMO-related diseases and explain their complexity. This provides a unique opportunity to see how a mutated cell interacts with its wild-type environment, something especially striking for the IP dermatosis but which might also apply to the CNS abnormalities found in a fraction of IP patients. Moreover, the identification in EDA-ID of an unpredicted ectodermal anhidrotic dysplasia, associated with a much more predictable immunodeficiency, has provided the first demonstration that NF-κB was also playing a key role in skin appendage development. Only, when Traf6 KO mice were subsequently re-analyzed, such connection with NF-κB was confirmed [70]. Discovery of a severe form of EDA-ID, OL-EDA-ID, has revealed for the first time that NF-κB participates in lymphatic vessels function. More recently, the unexpected occurrence of central adrenal insufficiency in CVID patients exhibiting NFKB2 mutations has also demonstrated how informative the identification and study of NF-κB-deficient patients can be. Interestingly, this kind of abnormality has also been reported for one patient exhibiting an IΚBΑ mutation [58], demonstrating that comparing the clinical features of distinct NF-κB-related genetic diseases may also be useful.
In addition to allowing discovery of new functions of NF-κB, the study of NF-κB-related genetic diseases is also informative for assessing the degree of similarity that exists in the NF-κB signaling pathway between human and mouse, the usual organismal system used to dissect this pathway. Although it appears quite high, as noted above, differences exist and are worth noting. For instance, it is clearly established that a lack of NF-κB activity during mouse development results in liver apoptosis at E12-E14 and that TNF-α is the triggering factor for cell death [16]. This explains the disappearance of males Nemo KO and Ikk2 KO embryos during embryogenesis. The situation appears different in humans. So far, no explanation has been provided for the early death of males fetuses suffering from IP before the second trimester, leaving the question open. More surprisingly, IKK2 SCID patients develop normally despite a demonstrated lack of IKK2 protein. Whether this reflects a simple difference in sensitivity of the hepatocytes to TNF-α during mouse versus human development or a true difference in functions of NF-κB during embryogenesis between these two organisms remains to be established.
Another example of distinct phenotypic consequence of impaired NF-κB signaling between humans and mice has been noticed by Pannicke et al. [50] and concerns γδ T cells which were shown to be absent in IKK2 SCID patients.
In summary, the discovery and analysis of genetic diseases caused by mutation of NF-κB components nicely complements the study of this pathway done using standard biochemical and genetical analysis. Given the importance of the NF-κB pathway in numerous physiological processes and its frequent deregulation in pathology, refinement of its characterization in the human context is likely to have an important impact on basic knowledge of NF-κB functions but also repercussions in the clinic.
Acknowledgments
A.S is supported by a CEA/IRTELIS grant. We thank C. Gautheron for expert artwork and Incontinentia Pigmenti France for support.
Footnotes
A. Senegas and J. Gautheron contributed equally to this study.
Patients exhibiting NIK mutations have been identified by Willmann et al. (Nat Commun 5:5360, 2014. doi:10.1038/ncomms6360). They suffer from an immunodeficiency presenting similarities with the one observed in NFKBK2 mutated patients but with more severe signs.
References
- 1.Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF-κB activity. Annu Rev Immunol. 2000;18:621–663. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- 2.Hayden MS, Ghosh S. Shared principles in NF-κB signaling. Cell. 2008;132(3):344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 3.Hinz M, Scheidereit C. The IκB kinase complex in NF-κB regulation and beyond. EMBO Rep. 2014;15(1):46–61. doi: 10.1002/embr.201337983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hu Y, Baud V, Delhase M, et al. Abnormal morphogenesis but intact IKK activation in mice lacking the IKKα subunit of IκB kinase. Science. 1999;284(5412):316–320. doi: 10.1126/science.284.5412.316. [DOI] [PubMed] [Google Scholar]
- 5.Sun SC. Non-canonical NF-κB signaling pathway. Cell Res. 2011;21(1):71–85. doi: 10.1038/cr.2010.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamamoto Y, Verma UN, Prajapati S, et al. Histone H3 phosphorylation by IKK-α is critical for cytokine-induced gene expression. Nature. 2003;423:655–659. doi: 10.1038/nature01576. [DOI] [PubMed] [Google Scholar]
- 7.Anest V, Hanson JL, Cogswell PC, et al. A nucleosomal function for IκB kinase-α in NF-κB-dependent gene expression. Nature. 2003;423:659–663. doi: 10.1038/nature01648. [DOI] [PubMed] [Google Scholar]
- 8.Shih VF, Tsui R, Caldwell A, et al. A single NFκB system for both canonical and non-canonical signaling. Cell Res. 2011;21:86–102. doi: 10.1038/cr.2010.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iwai K. Diverse roles of the ubiquitin system in NF-κB activation. Biochim Biophys Acta. 2014;1843(1):129–136. doi: 10.1016/j.bbamcr.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 10.Li Q, Van Antwerp D, Mercurio F, et al. Severe liver degeneration in mice lacking the IκB kinase 2 gene. Science. 1999;284(5412):321–325. doi: 10.1126/science.284.5412.321. [DOI] [PubMed] [Google Scholar]
- 11.Pasparakis M, Luedde T, Schmidt-Supprian M. Dissection of the NF-κB signalling cascade in transgenic and knockout mice. Cell Death Differ. 2006;13(5):861–872. doi: 10.1038/sj.cdd.4401870. [DOI] [PubMed] [Google Scholar]
- 12.Rudolph D, Yeh WC, Wakeham A, et al. Severe liver degeneration and lack of NF-κB activation in NEMO/IKKγ-deficient mice. Genes Dev. 2000;14:854–862. [PMC free article] [PubMed] [Google Scholar]
- 13.Makris C, Godfrey VL, Krähn-Senftleben G, et al. Female mice heterozygous for IKKγ/NEMO deficiencies develop a dermatopathy similar to the human X-linked disorder incontinentia pigmenti. Mol Cell. 2000;5(6):969–979. doi: 10.1016/S1097-2765(00)80262-2. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt-Supprian M, Bloch W, Courtois G, et al. NEMO/IKK γ-deficient mice model incontinentia pigmenti. Mol Cell. 2000;5(6):981–992. doi: 10.1016/S1097-2765(00)80263-4. [DOI] [PubMed] [Google Scholar]
- 15.Takeda K, Takeuchi O, Tsujimura T, et al. Limb and skin abnormalities in mice lacking IKKα. Science. 1999;284(5412):313–316. doi: 10.1126/science.284.5412.313. [DOI] [PubMed] [Google Scholar]
- 16.Beg AA, Baltimore D. An essential role for NF-κB in preventing TNF-α-induced cell death. Science. 1996;274(5288):782–784. doi: 10.1126/science.274.5288.782. [DOI] [PubMed] [Google Scholar]
- 17.Gerondakis S, Grumont R, Gugasyan R, et al. Unravelling the complexities of the NF-κB signalling pathway using mouse knockout and transgenic models. Oncogene. 2006;25(51):6781–6799. doi: 10.1038/sj.onc.1209944. [DOI] [PubMed] [Google Scholar]
- 18.Sebban H, Courtois G. NF-κB and inflammation in genetic disease. Biochem Pharmacol. 2006;72(9):1153–1160. doi: 10.1016/j.bcp.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 19.Courtois G, Israël A. IKK regulation and human genetics. Curr Top Microbiol Immunol. 2011;349:73–95. doi: 10.1007/82_2010_98. [DOI] [PubMed] [Google Scholar]
- 20.Landy SJ, Donnai D. Incontinentia pigmenti (Bloch-Sulzberger syndrome) J Med Genet. 1993;30(1):53–59. doi: 10.1136/jmg.30.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smahi A, Courtois G, Vabres P, et al. Genomic rearrangement in NEMO impairs NF-κB activation and is a cause of incontinentia pigmenti. Nature. 2000;405(6785):466–472. doi: 10.1038/35013114. [DOI] [PubMed] [Google Scholar]
- 22.Conte MI, Pescatore A, Paciolla M, et al. Insight into IKBKG/NEMO locus: report of new mutations and complex genomic rearrangements leading to incontinentia pigmenti disease. Hum Mutat. 2014;35:165–177. doi: 10.1002/humu.22483. [DOI] [PubMed] [Google Scholar]
- 23.Nenci A, Huth M, Funteh A, et al. Skin lesion development in a mouse model of incontinentia pigmenti is triggered by NEMO deficiency in epidermal keratinocytes and requires TNF signaling. Hum Mol Genet. 2006;15:531–542. doi: 10.1093/hmg/ddi470. [DOI] [PubMed] [Google Scholar]
- 24.Pasparakis M, Courtois G, Hafner M, et al. TNF-mediated inflammatory skin disease in mice with epidermis-specific deletion of IKK2. Nature. 2002;417:861–866. doi: 10.1038/nature00820. [DOI] [PubMed] [Google Scholar]
- 25.Kumari S, Bonnet MC, Ulvmar MH, et al. Tumor necrosis factor receptor signaling in keratinocytes triggers interleukin-24-dependent psoriasis-like skin inflammation in mice. Immunity. 2013;39:899–911. doi: 10.1016/j.immuni.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 26.Zonana J, Elder ME, Schneider LC, et al. A novel X-linked disorder of immune deficiency and hypohidrotic ectodermal dysplasia is allelic to incontinentia pigmenti and due to mutations in IKK-γ (NEMO) Am J Hum Genet. 2000;67(6):1555–1562. doi: 10.1086/316914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Döffinger R, Smahi A, Bessia C, et al. X-linked anhidrotic ectodermal dysplasia with immunodeficiency is caused by impaired NF-kappaB signaling. Nat Genet. 2001;27(3):277–285. doi: 10.1038/85837. [DOI] [PubMed] [Google Scholar]
- 28.Jain A, Ma CA, Liu S, et al. Specific missense mutations in NEMO result in hyper-IgM syndrome with hypohydrotic ectodermal dysplasia. Nat Immunol. 2001;2(3):223–228. doi: 10.1038/85277. [DOI] [PubMed] [Google Scholar]
- 29.Aradhya S, Courtois G, Rajkovic A, et al. Atypical forms of incontinentia pigmenti in male individuals result from mutations of a cytosine tract in exon 10 of NEMO (IKK-γ) Am J Hum Genet. 2001;68(3):765–771. doi: 10.1086/318806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Orange JS, Brodeur SR, Jain A, et al. Deficient natural killer cell cytotoxicity in patients with IKK-γ/NEMO mutations. J Clin Invest. 2002;109(11):1501–1509. doi: 10.1172/JCI0214858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao T, Yang L, Sun Q, et al. The NEMO adaptor bridges the nuclear factor-κB and interferon regulatory factor signaling pathways. Nat Immunol. 2007;8:592–600. doi: 10.1038/ni1465. [DOI] [PubMed] [Google Scholar]
- 32.Gautheron J, Courtois G. “Without Ub I am nothing”: NEMO as a multifunctional player in ubiquitin-mediated control of NF-κB activation. Cell Mol Life Sci. 2010;67(18):3101–3113. doi: 10.1007/s00018-010-0404-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mikkola ML. Molecular aspects of hypohidrotic ectodermal dysplasia. Am J Med Genet A. 2009;149A(9):2031–2036. doi: 10.1002/ajmg.a.32855. [DOI] [PubMed] [Google Scholar]
- 34.Niehues T, Reichenbach J, Neubert J, et al. Nuclear factor κB essential modulator-deficient child with immunodeficiency yet without anhidrotic ectodermal dysplasia. J Allergy Clin Immunol. 2004;114:1456–1462. doi: 10.1016/j.jaci.2004.08.047. [DOI] [PubMed] [Google Scholar]
- 35.Orange JS, Levy O, Brodeur SR, et al. Human nuclear factor κB essential modulator mutation can result in immunodeficiency without ectodermal dysplasia. J Allergy Clin Immunol. 2004;114:650–656. doi: 10.1016/j.jaci.2004.06.052. [DOI] [PubMed] [Google Scholar]
- 36.Mooster JL, Cancrini C, Simonetti A, et al. Immune deficiency caused by impaired expression of nuclear factor-κB essential modifier (NEMO) because of a mutation in the 5’ untranslated region of the NEMO gene. J Allergy Clin Immunol. 2010;126:127–132. doi: 10.1016/j.jaci.2010.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Filipe-Santos O, Bustamante J, Haverkamp MH, et al. X-linked susceptibility to mycobacteria is caused by mutations in NEMO impairing CD40-dependent IL-12 production. J Exp Med. 2006;203:1745–1759. doi: 10.1084/jem.20060085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang WC, Hung MC. Beyond NF-κB activation: nuclear functions of IκB kinase α. J Biomed Sci. 2013;20:3. doi: 10.1186/1423-0127-20-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lahtela J, Nousiainen HO, Stefanovic V, et al. Mutant CHUK and severe fetal encasement malformation. N Engl J Med. 2010;363(17):1631–1637. doi: 10.1056/NEJMoa0911698. [DOI] [PubMed] [Google Scholar]
- 40.Stevenson RE, Saul RA, Collins J, et al. Cocoon fetus—fetal encasement secondary to ectodermal dysplasia. Proc Greenwood Genet Center. 1987;6:10–15. [Google Scholar]
- 41.Hu Y, Baud V, Oga T, et al. IKKα controls formation of the epidermis independently of NF-κB. Nature. 2001;410(6829):710–714. doi: 10.1038/35070605. [DOI] [PubMed] [Google Scholar]
- 42.Bartsocas CS, Papas CV. Popliteal pterygium syndrome. Evidence for a severe autosomal recessive form. Med Genet. 1972;9:222–226. doi: 10.1136/jmg.9.2.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mitchell K, O’Sullivan J, Missero C, et al. Exome sequence identifies RIPK4 as the Bartsocas-Papas syndrome locus. Am J Hum Genet. 2012;90(1):69–75. doi: 10.1016/j.ajhg.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kalay E, Sezgin O, Chellappa V, et al. Mutations in RIPK4 cause the autosomal-recessive form of popliteal pterygium syndrome. Am J Hum Genet. 2012;90(1):76–85. doi: 10.1016/j.ajhg.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meylan E, Martinon F, Thome M, et al. RIP4 (DIK/PKK), a novel member of the RIP kinase family, activates NF-κB and is processed during apoptosis. EMBO Rep. 2002;3(12):1201–1208. doi: 10.1093/embo-reports/kvf236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cariappa A, Chen L, Haider K, et al. A catalytically inactive form of protein kinase C-associated kinase/receptor interacting protein 4, a protein kinase C beta-associated kinase that mediates NF-κB activation, interferes with early B cell development. J Immunol. 2003;171:1875–1880. doi: 10.4049/jimmunol.171.4.1875. [DOI] [PubMed] [Google Scholar]
- 47.Huang X, McGann JC, Liu BY, et al. Phosphorylation of Dishevelled by protein kinase RIPK4 regulates Wnt signaling. Science. 2013;339(6126):1441–1445. doi: 10.1126/science.1232253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang X, Lu H, Yan B, Romano RA, et al. ΔNp63 versatilely regulates a broad NF-κB gene program and promotes squamous epithelial proliferation, migration, and inflammation. Cancer Res. 2011;71(10):3688–3700. doi: 10.1158/0008-5472.CAN-10-3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schmidt-Supprian M, Courtois G, Tian J, et al. Mature T cells depend on signaling through the IKK complex. Immunity. 2003;19:377–389. doi: 10.1016/S1074-7613(03)00237-1. [DOI] [PubMed] [Google Scholar]
- 50.Pannicke U, Baumann B, Fuchs S, et al. Deficiency of innate and acquired immunity caused by an IKBKB mutation. N Engl J Med. 2013;369(26):2504–2514. doi: 10.1056/NEJMoa1309199. [DOI] [PubMed] [Google Scholar]
- 51.Mousallem T, Yang J, Urban TJ, et al. A nonsense mutation in IKBKB causes combined immunodeficiency. Blood. 2014;124:2046–2050. doi: 10.1182/blood-2014-04-571265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Burns SO, Plagnol V, Gutierrez BM, et al. Immunodeficiency and disseminated mycobacterial infection associated with homozygous nonsense mutation of IKKβ. J Allergy Clin Immunol. 2014;134:215–218. doi: 10.1016/j.jaci.2013.12.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nielsen C, Jakobsen MA, Larsen MJ et al (2014) Immunodeficiency associated with a nonsense mutation of IKBKB. J Clin Immunol (Epub ahead of print) [DOI] [PubMed]
- 54.Courtois G, Smahi A, Reichenbach J, et al. A hypermorphic IκBα mutation is associated with autosomal dominant anhidrotic ectodermal dysplasia and T cell immunodeficiency. J Clin Invest. 2003;112(7):1108–1115. doi: 10.1172/JCI18714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Janssen R, van Wengen A, Hoeve MA, et al. The same IκBα mutation in two related individuals leads to completely different clinical syndromes. J Exp Med. 2004;200(5):559–568. doi: 10.1084/jem.20040773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yoshioka T, Nishikomori R, Hara J, et al. Autosomal dominant anhidrotic ectodermal dysplasia with immunodeficiency caused by a novel NFKBIA mutation, p.Ser36Tyr, presents with mild ectodermal dysplasia and non-infectious systemic inflammation. J Clin Immunol. 2013;33:1165–1174. doi: 10.1007/s10875-013-9924-z. [DOI] [PubMed] [Google Scholar]
- 57.Giancane G, Ferrari S, Carsetti R, et al. Anhidrotic ectodermal dysplasia: a new mutation. J Allergy Clin Immunol. 2013;132(6):1451–1453. doi: 10.1016/j.jaci.2013.05.034. [DOI] [PubMed] [Google Scholar]
- 58.Schimke LF, Rieber N, Rylaarsdam S, et al. A novel gain-of-function IKBA mutation underlies ectodermal dysplasia with immunodeficiency and polyendocrinopathy. J Clin Immunol. 2013;33(6):1088–1099. doi: 10.1007/s10875-013-9906-1. [DOI] [PubMed] [Google Scholar]
- 59.Ohnishi H, Miyata R, Suzuki T, Nose T, et al. A rapid screening method to detect autosomal-dominant ectodermal dysplasia with immune deficiency syndrome. J Allergy Clin Immunol. 2012;129:578–580. doi: 10.1016/j.jaci.2011.09.042. [DOI] [PubMed] [Google Scholar]
- 60.McDonald DR, Mooster JL, Reddy M, et al. Heterozygous N-terminal deletion of IκBα results in functional nuclear factor κB haploinsufficiency, ectodermal dysplasia, and immune deficiency. J Allergy Clin Immunol. 2007;120(4):900–907. doi: 10.1016/j.jaci.2007.08.035. [DOI] [PubMed] [Google Scholar]
- 61.Lopez-Granados E, Keenan JE, Kinney MC, et al. A novel mutation in NFKBIA/IKBA results in a degradation-resistant N-truncated protein and is associated with ectodermal dysplasia with immunodeficiency. Hum Mutat. 2008;29:861–868. doi: 10.1002/humu.20740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kawai T, Nishikomori R, Heike T. Diagnosis and treatment in anhidrotic ectodermal dysplasia with immunodeficiency. Allergol Int. 2012;61:207–217. doi: 10.2332/allergolint.12-RAI-0446. [DOI] [PubMed] [Google Scholar]
- 63.Dupuis-Girod S, Corradini N, Hadj-Rabia S, et al. Osteopetrosis, lymphedema, anhidrotic ectodermal dysplasia, and immunodeficiency in a boy and incontinentia pigmenti in his mother. Pediatrics. 2002;109(6):e97. doi: 10.1542/peds.109.6.e97. [DOI] [PubMed] [Google Scholar]
- 64.Oberle EJ, Verbsky JW, Routes J, et al. A172: metaphyseal chondrodysplasia, ectodermal dysplasia, short stature, hypergammaglobulinemia, and spontaneous inflammation without infections in an extended family due to mutation in NFKB1A. Arthritis Rheumatol. 2014;66(Suppl 11):S224–S225. doi: 10.1002/art.38598. [DOI] [Google Scholar]
- 65.Cunningham-Rundles C, Bodian C. Common variable immunodeficiency: clinical and immunological features of 248 patients. Clin Immunol. 1999;92(1):34–48. doi: 10.1006/clim.1999.4725. [DOI] [PubMed] [Google Scholar]
- 66.Chen K, Coonrod EM, Kumánovics A, et al. Germline mutations in NFKB2 implicate the non-canonical NF-κB pathway in the pathogenesis of common variable immunodeficiency. Am J Hum Genet. 2013;93(5):812–824. doi: 10.1016/j.ajhg.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu Y, Hanson S, Gurugama P, et al. Novel NFKB2 Mutation in early-onset CVID. J Clin Immunol. 2014;34:686–690. doi: 10.1007/s10875-014-0064-x. [DOI] [PubMed] [Google Scholar]
- 68.Lindsley AW, Qian Y, Valencia CA et al (2014) Combined immune deficiency in a patient with a novel NFKB2 mutation. J Clin Immunol (Epub ahead of print) [DOI] [PMC free article] [PubMed]
- 69.Lee CE, Fulcher DA, Whittle B et al (2014) Autosomal dominant B cell deficiency with alopecia due to a mutation in NFKB2 that results in non-processible p100. Blood. pii: blood-2014-06-578542 [DOI] [PMC free article] [PubMed]
- 70.Naito A, Yoshida H, Nishioka E, et al. TRAF6-deficient mice display hypohidrotic ectodermal dysplasia. Proc Natl Acad Sci USA. 2002;99(13):8766–8771. doi: 10.1073/pnas.132636999. [DOI] [PMC free article] [PubMed] [Google Scholar]