Abstract
Ovulation in Caenorhabditis elegans requires inositol 1,4,5-triphosphate (IP3) signaling activated by the epidermal growth factor (EGF)-receptor homolog LET-23. We generated a deletion mutant of a type I 5-phosphatase, ipp-5, and found a novel ovulation phenotype whereby the spermatheca hyperextends to engulf two oocytes per ovulation cycle. The temporal and spatial expression of IPP-5 is consistent with its proposed inhibition of IP3 signaling in the adult spermatheca. ipp-5 acts downstream of let-23, and interacts with let-23–mediated IP3 signaling pathway genes. We infer that IPP-5 negatively regulates IP3 signaling to ensure proper spermathecal contraction.
INTRODUCTION
A crucial aspect of signal transduction is understanding how regulatory mechanisms ensure a precise biological response to pathway activation. Activation of receptor tyrosine kinases (RTK) stimulates phospholipase C to hydrolyze phosphatidyl 4,5-bisphosphate to inositol 1,4,5 triphosphate (IP3), which binds the tetrameric IP3 receptor to mobilize intracellular calcium (Majerus, 1992; Berridge, 1993). IP3 signaling mediates many cellular processes (Berridge and Irvine, 1989; Berridge, 1993). Mechanisms for attenuating and terminating signaling, such as provided by proteins that metabolize IP3, are critical in maintaining fine control of the physiological responses dependent on IP3-mediated calcium release. Thus, it is important to understand negative regulation of IP3 signaling.
Previous biochemical studies have shown how IP3 is produced (Berridge and Irvine, 1984), how it is metabolized (Majerus, 1992), and how it releases intracellular calcium (Berridge, 1995; Bootman and Berridge, 1995; Clapham, 1995); however, they have not directly addressed IP3 function in an intact metazoan. Two enzymes have been biochemically identified that participate in IP3 metabolism and potentially regulate signaling output: IP3 kinase (IP3K) and inositol polyphosphatase 5-phosphatase (Majerus, 1992; Drayer et al., 1996). 5-Phosphatases lower IP3 levels by dephosphorylating the 5′-phosphate, and they vary in substrate specificity (Mitchell et al., 1996; Erneux et al., 1998). Type I 5-phosphatases are the most active in hydrolyzing IP3 and IP4 (Verjans et al., 1992; Laxminaryayan et al., 1993). Thus, they likely play a larger role in regulating cellular levels of IP3 than do the type II phosphatases, which additionally hydrolyze the 5′-phosphoinositols, phosphatidyl inositol 4,5-bisphosphate and phosphatidyl inositol 3,4,5-trisphosphate (Mitchell et al., 1996). The distinct functional roles of various type II 5-phosphatase family members have been demonstrated by examining targeted deletions of mammalian type II 5-phosphatases in mice (Helgason et al., 1998; Janne et al., 1998; Cremona et al., 1999) and targeted disruption of type II 5-phosphatases in yeast (Stolz et al., 1996). The functional consequence of removing type I 5-phosphatase activity in vivo is unknown.
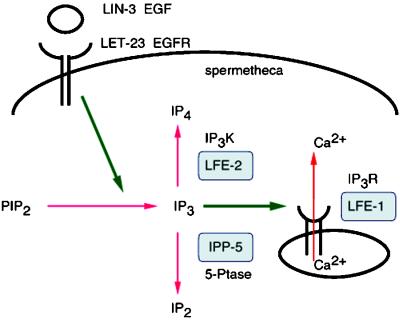
Ovulation in Caenorhabditis elegans hermaphrodites provides a genetic system to study the regulation of IP3 signaling in vivo. C. elegans mutants defective in IP3K, known as lfe-2, have no obvious ovulation defect, suggesting that an alternate pathway to metabolize IP3 and inhibit signaling exists. Here, we report the characterization of the gene, ipp-5, that encodes the C. elegans type I 5-phosphatase. We demonstrate that it acts downstream of the LET-23 RTK, based on epistasis analyses. We present, for the first time, in vivo characterization of a type I 5-phosphatase in an intact animal by describing ovulation defects of a deletion mutant ipp-5(sy605) in C. elegans, and we place the negative regulatory function of a type I 5-phosphatase in a behavioral context.
MATERIALS AND METHODS
Genetic Strains
Standard methods for maintaining C. elegans at 20°C were used. EMS (50 mM) was used as a mutagen (Brenner, 1974). Bristol strain N2 was used as the wild type. The following alleles were used: LG1, unc-57(ad592), lfe-2(sy326); LGII, unc-4(e120), let-23(sy10), let-23(sa62); LGIV, unc24(e138), lin-3(n1058), lfe-1/itr-1(sy290), dec-4(sa73); LGX, lon-2(e678), unc-6(e78), ipp-5(sy605). szT1[lon-2(e678)] is a reciprocal I;X translocation balancer. mnC1[dpy-10(e128) unc-52(e444)] is a rearrangement balancer chromosome on LGII. DnT1[nT1[unc(n745dm)let] is a reciprocal IV;V translocation balancer (strains are from Brenner, 1974; Ferguson and Horvitz, 1985; Fodor and Deak, 1985; Aroian et al., 1991; Iwasaki et al., 1995; Katz et al., 1996; Clandinin et al., 1998).
Brood Assay
L4 larvae hermaphrodites were serially transferred to fresh plates every 12 h for 4 d at 20°C, and progeny were counted 2 d after eggs hatched.
Polymerase Chain Reaction (PCR)
A PCR-based strategy was used to screen a library of 245, 000 EMS mutagenized N2 haploid genomes for a targeted deletion in ipp-5 (using the method of G. Moulder and R. Barstead, personal communication; http://pcmc41.ouhsc.edu/Knockout/). The deletion removes 242 base pairs (bp) upstream of the ATG through 25 bp of exon 3. The following primers were used to detect the deletion in lysates: Round I, JC43 (5′ TGCCTTGCACAAGATTATCG) and JC46 (5′ CTCTCCTTCTTCCACCAA); Round II, JC30 (5′CAGCCCATGAGTCACTACTTCC) and JC68 (5′ CTAGGAGGTTTTGAATTTTGACCTG). Wild-type animals amplify a 1300-bp product, whereas ipp-5 deletion mutants amplify a 480-bp product. The deletion mutant was backcrossed seven times to lon-2 unc-6. The presence of the deletion in double mutants was verified by PCR using primer pairs JC68 and BY4 (5′ CGTTTTCCTTTGACGAAAGCTCGG) in Round II to distinguish homozygotes (700-bp band) from heterozygotes (700 bp, 1600-bp band). The mutants were scored by Nomarski video recordings of ovulation.
Microscopy and Image Processing
Worms were anesthetized for 30 min in a solution of M9 with 0.1% tricaine and 0.01% tetramisole (Sigma, St. Louis, Missouri) before recording (McCarter et al., 1999). Animals were mounted on 5% agarose pads with 20 μl of anesthetic, covered with an 18-mm glass coverslip, and the edges of the coverslip were sealed with Vaseline. Observations were made at 20°–23°C. Animals were mounted on an Axioscope (Zeiss, Thornwood, New York) and recorded under Nomarski optics (Plan 100 objective) for no more than 4 h. The microscope was connected to a CCD72 DAGE-MTI (Michigan City, Indiana) charged-coupled device video camera module and VCR. Images were recorded on VHS tape in real time. While under anesthetic, oocyte maturation, and sheath and spermathecal activity at ovulation proceed, whereas pharyngeal pumping and egg laying cease (McCarter et al., 1999). Spermathecal extension distance was calculated by direct measurement of length (starting from the side of the proximal oocyte nearest the spermatheca to the point at which the spermathecal valve closes to envelop the oocytes) on the monitor during video production. Distance was calibrated on each set-up to convert centimeters on the monitor to true micrometer values using a stage micrometer. For ovulation movies, VHS video recordings were dubbed onto DVCAM digital tapes, captured as computer DV stream files via fire wire, and then the frame speed increased by 900% using the software program Final Cut Pro (Apple Computer, Cupertino, California). Video compression for Internet playback was performed using the Cleaner 5 Software Application (Autodesk/Discreet Logic Inc., Montreal, Quebec). Still-frame images (720 × 480 pixels) were grabbed from the ovulation computer-captured DV stream file (Digital Media Center, Caltech, Pasadena, California).
For fluorescent microscopy, animals were viewed with ×100 objective under a green fluorescent protein (GFP) filter. Images were collected using a digital camera (C4742–95; Hamamatsu, Middlesex, New Jersey), transferred to a computer (G3 Macintosh; Apple Computer, Cupertino, California) running Open Lab Imaging 1.7.8r3 Software (Improvision, Coventry, England, and assembled in Photoshop (Adobe Systems, Mountain View, California).
ipp-5 cDNA Sequence Analysis
A full-length cDNA, yk341d7, kindly provided by Yuji Kohara (expressed sequence tag partial sequence GenBank accession C44206), was sequenced on both strands and compared with the wild-type ipp-5 genomic sequence from the Sequencing Consortium (The C. elegans Sequencing Consortium, 1998) to obtain the splicing pattern. Genefinder predicts a slightly different cDNA (WormBase WS51) than the full-length cDNA we sequenced (GenBank accession AF411588). One base pair was found missing in exon 2 in the cDNA clone yk341d7, creating a frameshift premature stop codon. Genomic DNA amplified from N2 worms was sequenced on both strands and matched the sequence provided by the Consortium, indicating the mutation in the cDNA was an artifact. The mutation was repaired using a QuikChange Site-Directed Mutagenesis kit (Stratagene, La Jolla, California), yielding mut2IP5. Sequencing confirmed the mutation was corrected and that no other artifactual mutations occurred in the cDNA.
Construction of Transgenes
Standard molecular biology techniques were used (Sambrook et al., 1989). To examine the ipp-5 expression pattern, a 2-kb sequence upstream of the ATG, including exon 1, was amplified from genomic N2 DNA (using high-fidelity LA Takara Taq) and cloned into the pGEM TA cloning vector and then subcloned into the GFP expression vector pPD95.77 (A. Fire) with MluNI and Kpn sites, yielding pYB10. The entire ipp-5 locus was amplified from N2 genomic DNA and was subcloned into pPD49.83 (A. Fire) at the Kpn/EcoRV site, yielding pYB8. The entire ipp-5 genomic region was sequenced, and no extraneous mutations were found. A 2-kb sequence upstream of the start ATG codon was subcloned in place of the HS promoter at the MluNI and Kpn sites of pYB8, yielding pYB11 to test if the native promoter driving expression of the genomic ipp-5 locus rescues the sy605 phenotype.
Three constructs using heterologous promoters driving the ipp-5 cDNA were generated. A 1.0-kb region upstream of ZK370.3 was amplified from pCeh (kindly provided by A. Parker) using primers SphpCeh (5′ CCCGCATGCCTGCAGTTCTCCTCTCTG CC) and KpnpCeh (5′ CCCCGGTACCAAAAAAATTAATTTTTTTGGGGGC) and was cloned into pPD49.83 (A. Fire) at the MluN and Kpn site. A BamHI/Asp718 fragment containing the pCeh promoter was then subcloned into vector mut2IP5 with BamHI and EcoRI/blunted sites, yielding pYB13. The promoter was sequenced to verify that no additional mutations occurred during amplification. We examined dpy-5(e905) hIs29[pCeh361; pCeh363] (kindly provided by A. Parker), which carries the pCeh::GFP transgene, and we confirmed that the pCeh promoter shows expression in the adult spermatheca and not the sheath (A. Parker, personal communication). For pYB14, the entire cDNA from mut2IP5 was subcloned into the heat shock vector pPD49.83 (A. Fire) at the Nhe/Kpn site. For pYB15, a 3-kb nlp-8 promoter (Nathoo et al., 2001), which expresses in the sheath (Anne Hart, personal communication), was amplified using primers AN46 (5′ GAAGCTTCTGACTCATGTCGC) and BamHIAN49 (5′ CCGCGGATCCTGCATGCATTACTGTATTCAAAATTACGGTG) from genomic DNA and was subcloned into mut2IP5 using BamHI and EcoRI/blunted sites. We examined the strain him-5; lin-15(n795) rtEx22[nlp-8::GFP, lin-15(+)] (kindly provided by A. Hart) and confirmed expression in the proximal sheath cells and not in the spermatheca (Anne Hart, personal communication).
Construction of Transgenic Strains
Transgenic strains were generated as described by Mello et al. (1991). To assess whether the genomic ipp-5 locus, including the 2-kb 5′ sequence upstream of the ATG, rescues the phenotype in sy605 animals, we injected pYB11 along with myo-2::gfp as a transformation marker. Rescue of ipp-5(sy605) was determined by scoring the ovulation phenotype of three lines under Nomarski optics and recording ovulations of transgenic ipp-5(sy605) syEx[pYB11; myo-2::gfp] worms. We observed one ovulation event in each gonad arm per animal. Moreover, sy605 transgenics containing the heat shock transgene pYB14 and myo-2::gfp also showed rescue upon induction with a heat shock pulse of 33°C for 40 min in the young adult stage. The cosmids encompassing ipp-5 did not show the expected restriction pattern, and thus were not used to assay for rescue. We built the transgenic strain sy605 syEx[pYB13; myo-2::gfp] to test rescue with a heterologous spermathecal promoter. Additionally, we built the transgenic strain sy605 syEx[pYB15; myo-2::gfp] to test rescue with a heterologous sheath promoter. The ipp-5::GFP fusion was injected with pRF4 [rol-6(su1006)] as a transformation marker in sy605 animals or N2 animals to examine the expression pattern. Both transgenic strains show identical expression.
RESULTS
ipp-5 Encodes a Type I 5-Phosphatase Homolog
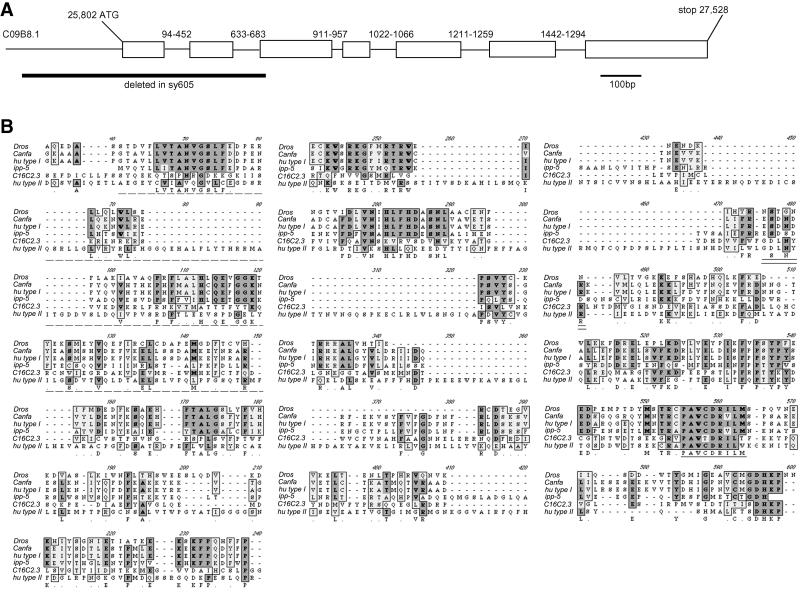
To better understand the regulation of IP3 signaling, we sought to identify additional downstream genes involved in the LET-23 RTK-mediated IP3 signaling pathway for ovulation in C. elegans (Clandinin et al., 1998). Clandinin et al. (1998) identified lfe-1, an IP3 receptor (IP3R) homolog, and lfe-2, an IP3K homolog, as suppressors of lin-3(rf) sterile ovulation defective mutants, indicating that ovulation is dependent on an evolutionary conserved IP3 signaling pathway (Figure 1). Video analysis of lfe-2 mutants shows no obvious ovulation defect (see Video 1), suggesting the inositol polyphosphate 5-phosphatase, which also metabolizes IP3 in other systems, may play a critical role inhibiting signaling in C. elegans ovulation. The C. elegans genome sequence predicts an ortholog of the human type I inositol polyphosphate 5-phosphatase (CO9B8.1), which we designate ipp-5 (The C. elegans Sequencing Consortium, 1998). We sequenced a full-length ipp-5 cDNA (kindly provided by Y. Kohara) corresponding to this region, and found 11 nucleotides of an SL1 trans-spliced leader and a single open reading frame (ORF), comprising 7 exons that encode a 400-amino acid protein 42% identical to its human counterpart (Figure 2). The C. elegans IPP-5 lacks the well-conserved motif (GDLNYRL) present in all members of the type II 5-phosphatases, as seen in the C. elegans homolog, C16C2.3 (Figure 2B). However, IPP-5 contains the conserved active site motif (PAWC/TDRV/ILM) essential for enzymatic activity of all type I and type II 5-phosphatases (Communi et al., 1996; Jefferson and Majerus, 1996; Majerus, 1996; Erneux et al., 1998). On this basis, we classify IPP-5 as a type I phosphatase. IPP-5 lacks the C-terminal isoprenylation site CCVVQ present in members of the 5-phosphatase type I family, suggesting that it relies on another mechanism for targeting to its correct intracellular location.
Figure 1.
IP3-mediated ovulation pathway. Ovulation is dependent on an IP3-mediated pathway that is activated by LIN-3 EGF and LET-23 EGFR, which likely stimulates hydrolysis of PIP2 into IP3. LFE-1 encodes an IP3R homolog, which plays a known role in releasing intracellular calcium. LFE-2 encodes an IP3K that phosphorylates IP3 into IP4 (Clandinin et al., 1998). IPP-5 encodes a 5-phosphatase (5-Ptase), which likely dephosphorylates IP3 into IP2.
Figure 2.
Molecular characterization of ipp-5. (A) Genomic structure of ipp-5. CO9B8.1 corresponds to the predicted ORF, determined by the Genefinder Program, to encode a C. elegans homolog of the inositol polyphosphate 5- phosphatase. Exons are shown as white boxes. The sequence is inferred from the cDNA (generously provided by Y. Kohara). The numbers above each intron indicate the base pair position relative to itself. The indicated region deleted in sy605 spans 240 bp upstream of the start ATG codon through exon 3. (B) Protein sequence alignment of the Drosophila, dog, human, and C. elegans homologs of the type I inositol 5-phosphatase and the C. elegans and human homologues of the type II 5-phosphatase. A Drosophila homolog has not been identified genetically, but Genie has predicted an ORF (GenBank accession AAF56383) in the sequenced genome. Identical residues are darkly shaded, and conserved substitutions are lightly shaded. The signature motif present in all 5-phosphatase family members is underlined in black. The motif well conserved among all type II phosphatases is double underlined. The residues deleted in ipp-5 are indicated by a dashed underline.
ipp-5 Affects Ovulation and Fertility
To study ipp-5 function, we isolated a deletion mutant, ipp-5(sy605), in which 240 bp upstream of the start codon is deleted through 28 bp of exon 3. We examined ipp-5(sy605) mutant animals for defective ovulation. There are no other obvious visible defects.
In a wild-type hermaphrodite, oocytes align on the proximal–distal axis of the gonadal sheath and mature in an assembly-line manner as they proceed proximally toward the spermatheca. Typically, during ovulation, the sheath contracts and pulls the dilated spermatheca over the most proximal mature oocyte in the proximal gonad to release it from the oviduct into the spermatheca (McCarter et al., 1999). Animals with defects in ovulation have a reduced brood size. For example, analysis of Nomarski ovulation videos indicate that let-23(sa62) RTK gain-of-function (gf) and dec-4/itr-1 IP3R reduction-of-function (rf) mutants show mechanical defects in ovulation [dec-4(sa73), n = 20/20 defective ovulations; let-23(sa62), n = 12/12 defective ovulations; see Videos 3 and 4] and have lower broods than wild type (mean brood 121 ± 9 [SD], see Figure 4B, [Dal Santo et al., 1999]; let-23(sa62) mean brood 173, range 45–276, n = 24, [Katz et al., 1996]). In sa62 mutants, the spermatheca constricts abnormally and tears the ovulated oocyte, leaving the nucleus behind in the gonad. The defect in sa73 mutants is more pronounced; the basal sheath contraction rate in sa73 mutants appears reduced relative to wild type, and the spermatheca dilates and constricts continually during ovulation before it finally pinches shut, tearing the oocyte and leaving the nucleus behind. Furthermore, lin-3(rf) or let-23(rf) mutants are sterile (Aroian et al., 1991) because they fail to ovulate (Clandinin et al., 1998; McCarter et al., 1999).
Figure 4.
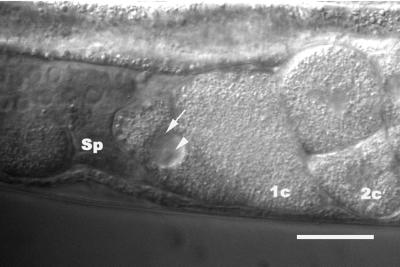
Fate of secondary oocyte in ipp-5(sy605) animals. In sy605 mutants, the second oocyte is ovulated along with the proximal oocyte and is passed into the uterus. The secondary oocyte has not undergone maturation, as the nucleolus (arrowhead) and the nuclear envelope (arrow) are still present. The proximal oocyte that was ovulated is at the one-cell (1c) stage. A two-cell embryo is also shown (2c). Scale bar, 20 μm.
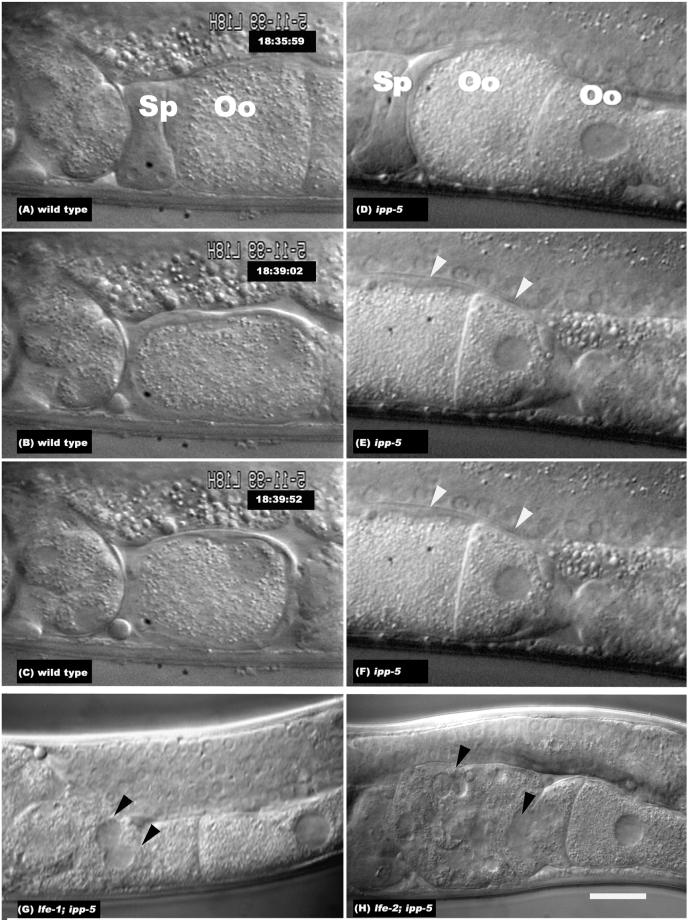
Figure 3 shows a sequential image series of a wild-type and a sy605 hermaphrodite during ovulation (see Videos 2 and 5). The deletion mutant shows a novel ovulation phenotype whereby more than one oocyte is ovulated per cycle. In these double ovulations, the spermatheca dilates and extends beyond the proximal oocyte to precociously envelop the second and in some cases the third oocyte. In sy605, the spermatheca extends on average 49.1 ± 11.9 μm (n = 18) compared with 33.9 ± 4.7 μm in the wild type (n = 30; p < 0.0001, Fisher's Exact test). This observation indicates that IPP-5 is required to prevent excessive spermathecal dilation and extension during ovulation.
Figure 3.
ipp-5(sy 605) effects on ovulation. Still-frame series of Nomarski photomicrographs of a hermaphrodite (A–C) wild-type and (D–F) ipp-5 gonad during ovulation. The wild-type images have been flipped horizontally so the orientation matches that of the ipp-5 images. The uterus is toward the left and the proximal gonad is toward the right. The ipp-5 phenotype shows two oocytes, indicated by white arrowheads, being ovulated in the spermatheca per cycle; see Supplementary Information Videos 2 and 5. Nomarski photomicrographs of (G) lfe-1/itr-1; ipp-5 and (H) lfe-2; ipp-5. Double mutants between ipp-5 and lfe-X cause the endomitotic oocyte nuclei (Emo) phenotype, indicated by black arrowheads. “Sp” denotes the position of the spermatheca, and “Oo” denotes the oocyte. Animals were photographed using a ×100 objective and differential interference contrast optics. Scale bar, 20 μm.
Prior work by McCarter et al. (1999) suggests that ovulation is coupled to oocyte meiotic maturation. In sy605 mutant animals, along with the proximal oocyte, the secondary oocyte is ovulated precociously, before the hallmarks of maturation are observed (McCarter et al., 1999). The presence of the nucleolus and nuclear envelope in these precocious oocytes indicates they have not undergone maturation (Figure 4). This mutant phenotype raises the possibility that there is no absolute requirement for an oocyte to have undergone maturation to be ovulated. In ceh-18 mutants, immature oocytes are ovulated but do not appear to form zygotes (Rose et al., 1997). In sy605, the distal oocytes ovulated before maturation are apparently not fertilized because we have seen oocytes interdigitating with fertilized multicellular eggs in the uterus (n = 9). Video analysis of the fate of the secondary oocyte (ovulated precociously) indicates fertilization and embryogenesis does not ensue, as observed in the accompanied fertilized primary oocyte (n = 11/11). Taken together, these results suggest that although meiotic maturation is not required for ovulation, it may be required for fertilization.
sy605 homozygotes have a reduced brood of 144 ± 20 (n = 25) relative to the wild-type brood of 337 ± 33 (n = 20, p < 0.001; Table 1). Animals heterozygous for the deletion have a slightly reduced brood size (314 ± 36; n = 20) relative to wild type (p = 0.0437; Table 1). Heterozygotes ovulate one oocyte/cycle (n = 54) as do wild type, indicating the double ovulation phenotype is recessive.
Table 1.
ipp-5(sy605) suppresses the sterility of lin-3(rf) and let-23(rf) and synergizes with other genes in the let-23–mediated fertility pathway
| Genotype
| ||||
|---|---|---|---|---|
| other | ipp-5 | Fertile | n | Brood |
| + | +/+ | 100% | 20 | 337 ± 33 |
| let-23(rf) | +/+ | 0% | 40 | 0 |
| lin-3(rf) | +/+ | 1.3% | 76 | 0.2 ± 1.8 |
| + | sy605/sy605 | 100% | 25 | 144 ± 20 |
| + | sy605/+ | 100% | 20 | 314 ± 36 |
| let-23(rf) | sy605/sy605 | 100% | 21 | n.d.a |
| lin-3(rf) | sy605/sy605 | 98.7% | 319 | 28 ± 9 (n = 47) |
| lfe-1(gf) | +/+ | 100% | 26 | 207 ± 35 |
| lfe-2(lf) | +/+ | 100% | 23 | 213 ± 34 |
| lfe-1(gf) | sy605/sy605 | 24% | 55 | 6.0 ± 12 |
| lfe-2(lf) | sy605/sy605 | 0% | 86 | 0 |
Fertile is defined as having greater than five offspring. Alleles used are lin-3(n1058), let-23(sy10), lfe-1(sy290) and lfe-2(sy326). unc-4(e120) was used as a marker and mnC1[dpy-10(e128) unc-52(e444)] as a balancer for let-23, and unc-24(e138) was used as a marker and DnT1[nT1[unc(n745dm)let] as a balancer for lin-3. lfe-1 was marked with unc-24(e138) and lfe-2 was marked with unc-57(ad592).
sy10 animals display a partially penetrant larval lethal phenotype associated with let-23(rf) decreases in RAS signaling, thus broods were not determined. n.d., not determined.
To confirm that the deletion in the ipp-5 locus causes the observed ovulation defect, we tested whether a transgene containing the full-length wild-type genomic ipp-5 locus, including the 2-kb sequence upstream of the start ATG codon, could complement the defect in sy605 transgenic animals. In sy605 animals, the ovulation defect is fully penetrant on a per animal basis (n = 15/15 animals double ovulate); however, looking at successive ovulations in animals, we observe that every ovulation event is not mutant (49% of gonad arms ovulate one oocyte/cycle, n = 70; Table 2B). Transgenic sy605 animals bearing the ipp-5 genomic locus as an extrachromosomal array showed rescue in all three lines examined (Table 2A). Thus, the ovulation defect is likely the result of the deletion, which removes ipp-5 function, because the phenotype can be rescued by adding back wild-type copies of the ipp-5 gene. It is conceivable that the next in-frame methionine downstream of the deletion, but upstream of the catalytic domain, initiates protein synthesis producing a protein. This protein, which lacks the N-terminal region, might result in altered protein activity, and thus the deletion might result in a gf phenotype. However, we think this possibility is inconsistent with our rescue data and our genetic epistasis and interaction data in which ipp-5(sy605) behaves as a stronger version of lfe-2, which is a loss-of-function (see below). Thus, the simplest interpretation of the data is that ipp-5(sy605) is a loss-of-function mutation. Based on our mutant phenotype, we infer that IPP-5 likely regulates IP3 signaling, which modulates spermathecal dilation/contraction.
Table 2.
In vivo transgenic assay for rescue of ipp-5(sy605) ovulation defect
 |
(A) During ovulation in wild-type animals, the spermatheca envelops one oocyte. In ipp-5(sy605) animals, the spermatheca ovulates two and sometimes three oocytes at a time. A 2-kb sequence upstream of the ATG along with the entire genomic ipp-5 locus rescues the phenotype when injected as a transgene in sy605 animals (3/3 lines rescued). (B) A heterologous spermatheca promoter, pCeh (provided by Alex Parker), fused to the ipp-5 cDNA also rescues the defect in sy605 transgenic animals (2/2 lines rescued), whereas the nlp-8 sheath–specific promoter (Anne Hart, personal communication) does not (0/4 lines rescued). (C) A heat shock promoter driving expression of ipp-5 cDNA in adults rescues the ovulation defect (2/2 lines rescued). HS, heat shock.
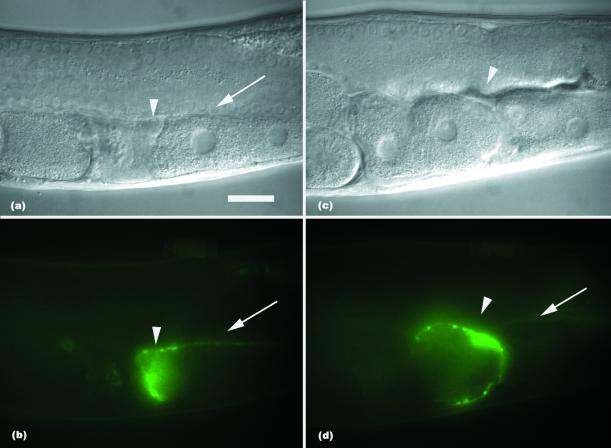
IPP-5 Acts in the Adult Spermatheca
To investigate where ipp-5 might function, we examined the expression of a ipp-5::GFP transcriptional reporter. A 2-kb fusion of 5′ sequence directed expression in the adult distal spermatheca and weakly in the proximal sheath in transgenic animals (Figure 5). This same promoter sequence driving the genomic ipp-5 locus is sufficient to rescue the defect in sy605 transgenic animals (Table 2A). To test whether expression in the spermatheca is sufficient for rescuing the ovulation defect, we used a heterologous spermathecal promoter (kindly provided by A. Parker) to drive expression of the ipp-5 cDNA. We observed rescue of the ovulation defect in two transgenic sy605 lines observed. In sy605 animals, 51% of the gonad arms examined were mutant where more than one oocyte was ovulated per cycle, whereas 33% mutant gonad arms were observed in sy605 animals bearing the transgene as an extrachromosomal array (p < 0.0246). In a second line, 20% of the gonad arms were mutant (p = 0.0002; Table 2B). Thus, expression of the transgene led to significantly more animals with normal ovulation, indicating that expression of ipp-5 in the spermatheca is sufficient to rescue the ovulation defect. Although ipp-5::GFP expression is also detected in the vulva and isthmus of the pharynx throughout larval development and adulthood, we think it unlikely that expression in these tissues affects the spermathecal contraction behavior. Transgenic sy605 worms injected with a transgene containing a sheath promoter (kindly provided by A. Hart) driving the ipp-5 cDNA did not show rescue of the ovulation defect in four lines observed (Table 2B). Together with the expression data, we infer ipp-5 likely functions within the adult spermatheca to regulate ovulation.
Figure 5.
Expression of ipp-5. Nomarski photomicrographs of a transgenic worm and their corresponding GFP fluorescence photomicrographs. The white arrowhead indicates position of the distal spermatheca, and the white arrow indicates the sheath. (A and B) A worm before ovulation. (C and D) A transgenic sy605 worm during ovulation. The arrowhead indicates the distal spermatheca enclosing two oocytes in a sy605 transgenic animal. Strong expression is detected in the distal spermatheca, whereas faint expression is detected in the sheath. Fertilized embryos are to the left, and oocytes lining up in the proximal gonad are to the right. Scale bar, 20 μm.
We did not observe expression of the ipp-5::GFP reporter transgene before adulthood in the spermatheca, suggesting the function of ipp-5 is needed in the adult spermatheca for ovulation rather than for the development or specification of the sheath and spermatheca lineages, which occurs in the L4 larval stage (Kimble and Hirsh, 1979). We observed L4 stage sy605 hermaphrodites under Nomarski optics and found that they had normal spermathecae and proximal sheath: The numbers of spermathecal cells were normal, and there was no obvious abnormality in spermathecal morphogenesis or in the proximal ovarian sheath (n = 13). To determine if expression of ipp-5 in adult stage is sufficient for its function in ovulation, we examined whether using a heat shock promoter driving the ipp-5 cDNA to induce expression in sy605 adults is sufficient to rescue the ovulation defect. In two lines observed, induced expression of ipp-5 function in adults was sufficient to rescue the double ovulation phenotype in transgenic sy605 worms: line 1, 22% mutant gonad arms after heat shock vs. 53% mutant gonad arms in control transgenic animals with no heat shock, p = 0.0005; line 2, 26% mutant gonad arms after heat shock vs. 49% mutant gonad arms in control transgenic animals with no heat shock, p = 0.0123 (Table 2C). We conclude that IPP-5 activity is not required before the L4 larval stage for its function in ovulation. The temporal and spatial regulation of ipp-5 is consistent with its regulatory function during adult ovulation rather than in developmental processes that secondarily affect dilation.
ipp-5 Suppresses the Sterility of lin-3 and let-23
LIN-3 has been proposed to activate LET-23 and an IP3 signaling pathway (Clandinin et al., 1998) that regulates ovulation. This pathway comprises lin-3, let-23, lfe-1, and lfe-2 encoding an epidermal growth factor (EGF)-like growth factor, EGFR, IP3R, and IP3K, respectively (Figure 1). lin-3(rf) and let-23(rf) mutants are sterile, producing no progeny because they fail to ovulate. These mutants have an Emo phenotype in which the oocytes become trapped in the gonad arm and undergo multiple rounds of DNA synthesis (Clandinin et al., 1998; McCarter et al., 1999). A gf mutation in lfe-1 IP3R or a loss-of-function mutation in lfe-2 IP3K can bypass the requirement for wild-type levels of LET-23 activity: lin-3(rf); lfe or let-23(rf); lfe double mutants are fertile (Clandinin et al., 1998). We tested whether the C. elegans IPP-5 functions downstream of LET-23 RTK and can suppress the sterility of either lin-3(rf) or let-23(rf). lin-3(n1058), which affects vulva induction and fertility, has an average brood size of 0.2 ± 1.8 (n = 76). In contrast, lin-3(n1058); ipp-5(sy605) double mutants are 98.7% fertile (n = 319), having an average brood size of 28 ± 9, n = 47 (Table 1); an allele of lin-3, n378, which affects only vulva induction (causing a vulvaless phenotype where the animals are unable to lay eggs), has an average brood size of 72 ± 11, n = 17 (Clandinin et al., 1998). Similarly, ipp-5 suppresses let-23(rf), as 100% of let-23(sy10); ipp-5(sy605) double mutants are fertile (n = 21; Table 1). Because sy605 can suppress the sterile defect of lin-3 as well as let-23, we conclude that ipp-5 functions downstream of lin-3 and let-23 to mediate ovulation.
ipp-5 Synergizes with Other Genes in the let-23–mediated Fertility Pathway
We next examined genetic interactions of ipp-5 with known components of the fertility pathway. lfe-1/itr-1(gf) or lfe-2(lf) single mutants have a slightly reduced brood size and ovulate normally (Clandinin et al., 1998). ipp-5 synergizes with either lfe-1(gf) or lfe-2(lf) to produce a synthetic sterile Emo phenotype (Figure 3) similar to that observed in lfe-1(gf); lfe-2(lf) double mutants (Clandinin et al., 1998) where the spermatheca fails to dilate sufficiently. lfe-1(sy290); ipp-5 double mutants have an average brood of 6 ± 12 (n = 55) compared with lfe-1(sy290), which has an average of 207 ± 35(n = 26). lfe-2(sy326) has a brood of 213 ± 34 (n = 23), whereas lfe-2(sy326); ipp-5 double mutants are sterile, producing no progeny (n = 86; Table 1). The data indicate that ipp-5 interacts with both lfe-1/itr-1 and lfe-2 and that it likely functions in the adult spermatheca to regulate the fertility pathway during ovulation. Consistent with this hypothesis, all three proteins are expressed in the adult spermatheca (Clandinin et al., 1998; Dal Santo et al., 1999; Gower et al., 2001).
ipp-5(sy605) exhibits a semidominant synergistic effect in a sensitized background. Analysis of ovulation videos show that although sy605/+ heterozygotes and lfe-1 or lfe-2 single mutants do not double ovulate, sy605 heterozygotes that are also homozygous for lfe-2 double ovulate and are fertile (n = 17). Similarly, sy605 heterozygotes that are also homozygous for lfe-1 are fertile and double ovulate (n = 10). sy605 synergizes with lfe-1 and lfe-2 mutations in a dose-sensitive manner, because removing two copies of ipp-5 has more severe affects than removing a single copy. The Emo phenotype observed in the lin-3(rf) or let-23 (rf) single mutant and double mutant ipp-5; lfe-1 or ipp-5; lfe-2 suggests that IP3 signaling levels are critical for normal hermaphrodite ovulation and fertility. From our study on ipp-5 mutant phenotypes, we infer that IPP-5 inhibits IP3 signaling after activation to ensure that ovulation occurs properly.
To further examine the affects of varying IP3 signaling on ovulation, we examined double mutants of ipp-5 with a gf allele of let-23(sa62) RTK (Katz et al., 1996). sa62 animals have a reduced brood, which may in part be attributed to the sickness of the homozygotes (average brood 173, range 24–276 progeny, n = 24; Katz et al., 1996), and an ovulation defect where the spermathecal valve contracts prematurely, closing and tearing the oocyte as it enters (n = 12; see Video 4). ipp-5 also synergizes with sa62, causing a further reduction in brood size (average brood 7; range 0–27 progeny; n = 31). In these double mutants, the spermatheca extends beyond the proximal oocyte to the second oocyte, but it then retracts and has problems ovulating the proximal oocyte, eventually causing an Emo phenotype (n = 8). Precise control of IP3 levels appears to be crucial in vivo for proper spermathecal dilation. Thus, both insufficient and excessive IP3 signaling cause defective ovulation.
IPP-5 and LFE-2 Have Different Roles in Regulating IP3 Signaling
Both the 5-phosphatase and 3-kinase metabolize IP3; however, their respective contributions in negatively regulating IP3 signaling are unclear. Clandinin et al. (1998) showed that lfe-2(sy326) mutation disrupts kinase activity because it failed to phosphorylate IP3 in an in vitro kinase assay. The point mutation in lfe-2 and the deletion in ipp-5 are probably loss-of-function mutations. We examined the ovulation phenotype of both these alleles by Nomarski video analysis (see Videos 1 and 5). Although lfe-2(lf) mutants display a reduced brood size, video analysis shows no obvious defects in ovulation (n = 22), unlike ipp-5(sy605). Strikingly, these genes, both of which likely regulate IP3 levels, have qualitatively different loss-of-function phenotypes. We infer that IPP-5 and LFE-2 are both critical for ovulation, but act differently to regulate it. We observe no effect with misexpression of IPP-5 in wild-type worms (unpublished observations); however, misexpression of LFE-2 using a heat shock promoter causes the spermatheca to relax inappropriately (Clandinin et al., 1998). The existence of multiple proteins that differentially inhibit IP3 signaling highlight the importance of maintaining fine control of IP3 signaling in ovulation.
DISCUSSION
In an attempt to better understand how IP3 signaling downstream of the LET-23 RTK pathway affects ovulation and fertility, we have generated and characterized a deletion mutant of the C. elegans type I inositol polyphosphate 5-phosphatase. ipp-5(sy605) homozygous mutants have reduced fertility and a novel ovulation phenotype whereby the spermatheca dilates and extends abnormally. Epistasis analyses place ipp-5 downstream of let-23. Two mutant loci have previously been identified that can bypass LET-23 RTK function, let-23 fertility effectors, lfe-1(gf)/itr-1, an IP3R homolog, a positive effector, and lfe-2(lf), an IP3K, a negative effector (Clandinin et al., 1998). ipp-5(sy605) enhances lfe-1(gf) and lfe-2(lf) mutants, causing sterility. Our transgenic rescue data (Table 2) and reporter GFP expression data (Figure 5) are consistent with IPP-5 directly controlling contraction and dilation behavior in the adult spermatheca (Figure 1).
Contributions of the 5-Phosphatase and IP3K and Negative Regulation of IP3 Signaling
We observed that the C. elegans 5-phosphatase deletion mutant, ipp-5(sy605), and the IP3K mutant, lfe-2(lf), have qualitatively different ovulation phenotypes. The double ovulation phenotype of ipp-5 probably results from increased IP3 signaling upon removing IPP-5 function. Consistent with this, cell lines that stably underexpress a type I 5-phosphatase have a sustained 2.6-fold elevation in IP3, leading to enhanced intracellular calcium oscillations and cellular transformation (Speed et al., 1996, 1999; Speed and Mitchell, 2000). By contrast, lfe-2(lf) shows no noticeable ovulation defect in C. elegans. We infer that eliminating activity of either gene allows IP3 to accumulate to different levels, resulting in the two distinct phenotypes and suggesting that they have distinct negative regulatory roles within the context of ovulation. Studies of IP3 metabolism in Xenopus oocytes indicate that at low [IP3] and high [Ca2+], IP3 is metabolized predominantly by IP3K, whereas as [IP3] increases, the 5-phosphatase degrades progressively more IP3, irrespective of the [Ca2+] (Sims et al., 1996). Multiple enzymes that metabolize IP3 imply that tight regulation of IP3 is essential for ensuring proper spermathecal dilation/relaxation during ovulation. In C. elegans, elevated IP3 signaling affects spermathecal dilation, and IPP-5 is critical for inhibiting signaling for contraction.
Ovulation Is Regulated by IP3 Signaling Levels
Cell culture studies have shown that the mammalian 5-phosphatase and IP3K metabolize IP3 into either IP2 or IP4, respectively (Irvine et al., 1986; Berridge and Irvine, 1989). Simultaneously removing ipp-5 activity and lfe-2 activity should create a situation where IP3 accumulates and exerts negative feedback inhibition, which blocks further ovulation.
We interpret the effects on spermathecal dilation in the lfe-2; ipp-5 mutant as being mediated through either too low or excessively high levels of IP3 signaling. Moreover, a double mutant with ipp-5 and sa62, a gf allele of let-23 RTK (Katz et al., 1996) that has hyperactive signaling, also shows the same ovulation defect, further demonstrating the inhibitory effects of excessive IP3 signaling. Cell culture studies have shown that EGF stimulates IP3 production and a rise in intracellular calcium (Hepler et al., 1987). An activating mutation in LET-23 RTK likely results in higher levels of second messenger IP3 production. We presume that the cooperative effect of removing IPP-5 in the gf LET-23 mutant dramatically increases IP3 signaling, which prevents ovulation causing the Emo phenotype.
Examining the spermatheca in these mutants allows us to see a more direct physiological effect of perturbing IP3 signaling in vivo. There appears to be a biphasic phenotypic effect on the extent of spermathecal dilation and extension with increasing levels of IP3 signaling in C. elegans. In lin-3(rf) and let-23(rf) mutants with reduced EGF signaling, IP3 signaling is not sufficient for the spermatheca to dilate. In ipp-5 mutants where IP3 signaling is higher, the spermatheca dilates and extends farther than in the wild type to ovulate the proximal mature oocyte along with the secondary distal oocyte, which has not undergone meiotic maturation. One explanation for this observation is that in sy605, the proximal oocyte, which has undergone meiotic maturation, triggers the spermatheca to dilate. The secondary distal oocyte is passively ovulated precociously, as a consequence of the spermatheca extension beyond the proximal oocyte due to increased IP3 signaling.
Further elevations in IP3 signaling in various double mutants prevent spermatheca dilation. IP3 positively effects gating of IP3R, but has also been shown along with calcium to exert negative feedback, which may explain this phenotype (Berridge, 1993; Ehrlich and Watras, 1988; Besprozvanny et al., 1991). Multiple layers of regulating intracellular calcium release allows fine control of many cellular processes.
LET-23 RTK-induced activation of IP3 signaling, which promotes spermatheca dilation likely through its effect on calcium release, is reminiscent of arterial smooth muscle relaxation and arterial dilation by calcium sparks (Nelson et al., 1995). The structural architecture of the myoepithelial sheath and spermatheca resemble smooth muscle. Longitudinal interdigitated thick and thin filaments make up the sheath. Actin stains the spermatheca, revealing circumferentially arranged fibers (Strome, 1986) that may undergo peristaltic vasoconstrictive and dilatory behavior like that of epithelial smooth muscle. The tension from the contracting sheath pulls the dilated spermatheca over the proximal oocyte during ovulation (McCarter et al., 1999). Decreases in IP3 signaling may trigger contraction and closure of the distal spermatheca valve so that only one oocyte is enveloped. A peristaltic wave of contraction may carry the oocyte from the distal spermatheca valve through the proximal spermathecal valve into the uterus.
Ovulation is a regulated behavior requiring coordination of the epithelial smooth muscle-like spermatheca and sheath. Animals with defective ovulation have reduced fertility, thus proper regulation of ovulation is important for normal fertility. During ovulation, we propose IPP-5 is necessary to prevent spermathecal hyperextension by negatively regulating IP3 signaling downstream of RTK, thereby ensuring proper spermathecal dilation and contraction behavior.
Supplementary Material
ACKNOWLEDGMENTS
We thank Y. Hadju-Cronin, J. Copeland, and B. Bingol for help isolating sy605; Y. Kohara for cDNA; J. Thomas for sa62 and sa73; A. Fire for GFP and HS vectors; A. Parker for the pCeh promoter and strain KR3738; A. Hart for the nlp-8 promoter and strain PT4; L.R. Garcia, E. Schwarz, other members of our laboratory, and an anonymous reviewer for valuable discussion and comments on the manuscript; and L. Maxfield (Caltech Digital Media Center) for help making web movies. The Caenorhabditis Genetics Center provided some strains. This project was supported by the Howard Hughes Medical Institute, with which P.W.S is an investigator. Y.K.B. is a National Institutes of Health trainee supported by National Institutes of Health grant 5T32GM07737.
Abbreviations used:
- EGF
epidermal growth factor
- gf
gain-of-function
- GFP
green fluorescent protein
- IP3
inositol 1,4,5 triphosphate
- IP3K
IP3 3-kinase
- IP3R
IP3 receptor
- ORF
open reading frame
- PCR
polymerase chain reaction
- rf
reduction-of-function
- RTK
receptor tyrosine kinases
Footnotes
Online version of this article contains video material. Online version available at www.molbiolcell.org.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.02–01–0008. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.02–01–0008.
REFERENCES
- Aroian R, Sternberg P. Multiple functions of let-23, a Caenorhabditis elegansreceptor tyrosine kinase gene product required for vulva induction. Genetics. 1991;128:251–267. doi: 10.1093/genetics/128.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge MJ. Inositol triphosphate and calcium signaling. Nature. 1993;361:315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. Calcium signaling and cell proliferation. Bioessays. 1995;17:491–500. doi: 10.1002/bies.950170605. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Irvine RE. Inositol triphosphate, a novel second messenger in cellular signal transduction. Nature. 1984;31:315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Irvine RE. Inositol phosphates and cell signaling. Nature. 1989;341:197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Besprozvanny I, Waras J, Ehrlich BE. Bell-shaped calcium response curves on Ins(1,4,5)P3and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature. 1991;351:751–754. doi: 10.1038/351751a0. [DOI] [PubMed] [Google Scholar]
- Bootman MD, Berridge JM. The elemental principles of calcium signaling. Cell. 1995;83:675–678. doi: 10.1016/0092-8674(95)90179-5. [DOI] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The C. elegans Sequencing Consortium. Genome sequence of the nematode C. elegans:A platform for investigating biology. Science. 1998;282:2012–2018. doi: 10.1126/science.282.5396.2012. [DOI] [PubMed] [Google Scholar]
- Clandinin TR, DeModena JA, Sternberg PW. Inositol triphosphate mediates a ras-independent response to LET-23 receptor tyrosine kinase activation in C. elegans. Cell. 1998;92:523–533. doi: 10.1016/s0092-8674(00)80945-9. [DOI] [PubMed] [Google Scholar]
- Clapham DE. Calcium signaling. Cell. 1995;80:259–268. doi: 10.1016/0092-8674(95)90408-5. [DOI] [PubMed] [Google Scholar]
- Communi D, Lecocq R, Erneux C. Arginine 343 and 350 are two active residues involved in substrate binding by human type I D-myo-inositol 1,4,5,-trisphosphate 5-phosphatase. J Biol Chem. 1996;271:11676–11683. doi: 10.1074/jbc.271.20.11676. [DOI] [PubMed] [Google Scholar]
- Cremona O, Di Paolo G, Wenk MR, Luthi A, Kim WT, Takei K, Daniell L, Nemoto Y, Shears SB, Flavell RA, McCormick DA, De Camilli P. Essential role of phosphoinositide metabolism in synaptic vesicle recycling. Cell. 1999;99:179–188. doi: 10.1016/s0092-8674(00)81649-9. [DOI] [PubMed] [Google Scholar]
- Dal Santo P, Logan MA, Chisholm AD, Jorgensen EM. The inositol triphosphate receptor regulates a 50-second behavioral rhythm in C. elegans. Cell. 1999;98:757–767. doi: 10.1016/s0092-8674(00)81510-x. [DOI] [PubMed] [Google Scholar]
- Drayer AL, Pesesse X, De Smedt F, Communi D, Moreau C, Erneux C. The family of inositol and phosphatidyl polyphosphate 5-phosphatasaes. Biochem Soc Trans. 1996;24:1001–1005. doi: 10.1042/bst0241001. [DOI] [PubMed] [Google Scholar]
- Ehrlich BE, Watras J. Inositol 1,4,5-trisphosphate activates a channel from smooth muscle sarcoplasmic reticulum. Nature. 1988;336:583–586. doi: 10.1038/336583a0. [DOI] [PubMed] [Google Scholar]
- Erneux C, Govaerts C, Communi D, Pesesse X. The diversity and possible functions of the inositol polyphosphate 5-phosphatases. Biochim Biophys Acta. 1998;1436:185–199. doi: 10.1016/s0005-2760(98)00132-5. [DOI] [PubMed] [Google Scholar]
- Ferguson EL, Horvitz HR. Identification and characterization of 22 genes that affect the vulval cell lineages of the nematode C. elegans. Genetics. 1985;110:17–72. doi: 10.1093/genetics/110.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fodor A, Deak P. The isolation and genetic analysis of a C. elegans translocation (szT1) strain bearing an X-chromosome balancer. J Genet. 1985;64:143–157. [Google Scholar]
- Gower NJD, Temple GR, Schein JE, Marra MA, Walker DS, Baylis HA. Dissection of the promoter region of the inositol 1,4,5-triphosphate receptor gene, itr-1, in C. elegans: A molecular basis for cell-specific expression of IP3R isoforms. J Mol Biol. 2001;306:145–157. doi: 10.1006/jmbi.2000.4388. [DOI] [PubMed] [Google Scholar]
- Helgason CD, Damen JE, Rosten P, Grewal R, Sorensen P, Chappel SM, Borowski A, Jirik F, Krystal G, Humphries RK. Targeted disruption of SHIP leads to hemopoietic perturbations, lung pathology, and a shortened life span. Genes Dev. 1998;12:1610–1620. doi: 10.1101/gad.12.11.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepler JR, Nakahata N, Lovenberg TW, DiGuiseppi J, Herman B, Earp HS, Harden TK. Epidermal growth factor stimulates the rapid accumulation of inositol (1,4,5) triphosphate and a rise in cytosolic calcium mobilized from intracellular stores in A431 cells. J Biol Chem. 1987;262:2951–2956. [PubMed] [Google Scholar]
- Irvine RF, Letcher AJ, Heslop JP, Berridge MJ. The inositol tris/tetrakisphosphate pathway: demonstration of Ins (1,4,5) P33-kinase activity in animal tissues. Nature. 1986;320:631–634. doi: 10.1038/320631a0. [DOI] [PubMed] [Google Scholar]
- Iwasaki K, Liu DWC, Thomas JH. Genes that control a temperature-compensated ultradian clock in Caenorhabditis elegans. Proc Natl Acad Sci USA. 1995;92:10317–10321. doi: 10.1073/pnas.92.22.10317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janne PA, Suchy SF, Bernard D, MacDonald M, Crawley J, Grinberg A, Wynshaw-Boris A, Westphal H, Nussbaum RL. Functional overlap between murine Inpp5b and Ocrl1 may explain why deficiency of the murine ortholog for O.C.R.L1 does not cause Lowe syndrome in mice. J Clin Invest. 1998;101:2042–2053. doi: 10.1172/JCI2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson AB, Majerus PW. Mutation of the conserved domains of two inositol polyphosphate 5-phosphatases. Biochem J. 1996;35:7890–7894. doi: 10.1021/bi9602627. [DOI] [PubMed] [Google Scholar]
- Katz WS, Lesa GM, Yannoukakos D, Clandinin TR, Schlessinger J, Sternberg PW. A point mutation in the extracellular domain activates LET-23, the Caenorhabditis elegansepidermal growth factor receptor homolog. Mol Cell Biol. 1996;16:529–537. doi: 10.1128/mcb.16.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimble J, Hirsh D. Postembryonic cell lineages of the hermaphrodite and male gonads in Caenorhabditis elegans. Dev Biol. 1979;70:396–417. doi: 10.1016/0012-1606(79)90035-6. [DOI] [PubMed] [Google Scholar]
- Laxminaryayan KM, Matzaris M, Speed CJ, Mitchell CA. Purification and characterization of a 43-kDa membrane-associated inositol polyphosphate 5-phosphatase from human placenta. J Biol Chem. 1993;268:4968–4974. [PubMed] [Google Scholar]
- Majerus PW. Inositol phosphate biochemistry. Annu Rev Biochem. 1992;61:225–250. doi: 10.1146/annurev.bi.61.070192.001301. [DOI] [PubMed] [Google Scholar]
- Majerus PW. Inositols do it all. Genes Dev. 1996;10:1051–1053. doi: 10.1101/gad.10.9.1051. [DOI] [PubMed] [Google Scholar]
- McCarter J, Bartlett B, Dang T, Schedl T. On the control of oocyte meiotic maturation and ovulation in Caenorhabditis elegans. Dev Biol. 1999;205:111–128. doi: 10.1006/dbio.1998.9109. [DOI] [PubMed] [Google Scholar]
- Mello CC, Kramer JM, Stinchocomb F, Ambros V. Efficient gene transfer in C. elegansextrachromosomal maintenance and integration of transforming sequences. EMBO J. 1991;10:3959–3970. doi: 10.1002/j.1460-2075.1991.tb04966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell CA, Brown S, Campell JK, Munday AD, Speed CJ. Regulation of second messengers by the inositol polyphosphate 5-phosphatases. Biochem Soc Trans. 1996;24:994–1000. doi: 10.1042/bst0240994. [DOI] [PubMed] [Google Scholar]
- Nathoo AN, Moeller RA, Westlund BA, Hart AC. Identification of neuropeptide-like protein gene families in Caenorhabditis elegansand other species. Proc Natl Acad Sci USA. 2001;98(24):14000–14005. doi: 10.1073/pnas.241231298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson MT, Cheng H, Rubart M, Santana LF, Bonev AD, Knot HJ, Lederer WJ. Relaxation of arterial smooth muscle by calcium sparks. Science. 1995;270:633–637. doi: 10.1126/science.270.5236.633. [DOI] [PubMed] [Google Scholar]
- Rose KL, Winfrey VP, Hoffman LH, Hall DH, Furuta T, Greenstein D. The POU gene ceh-18 promotes gonadal sheath cell differentiation and function required for meiotic maturation and ovulation in Caenorhabditis elegans. Dev Biol. 1997;192(1):59–77. doi: 10.1006/dbio.1997.8728. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fristch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd ed. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Sims CE, Allbritton NL. Metabolism of inositol 1,4,5-triphosphate and inositol 1,3,4,5-tetrakisphosphate by the oocytes of Xenopus laevis. J Biol Chem. 1998;273:4052–4058. doi: 10.1074/jbc.273.7.4052. [DOI] [PubMed] [Google Scholar]
- Speed CJ, Little PJ, Hayman JA, Mitchell CA. Underexpression of the 43-kDa inositol polyphosphate 5-phosphatase is associated with cellular transformation. EMBO J. 1996;15:4852–4861. [PMC free article] [PubMed] [Google Scholar]
- Speed CJ, Mitchell CA. Sustained elevation in inositol 1,4,5-triphosphate results in inhibition of phosphatidylinositol transfer protein activity and chronic depletion of the agonist sensitive phosphoinositide pool. J Cell Sci. 2000;113:2631–2636. doi: 10.1242/jcs.113.14.2631. [DOI] [PubMed] [Google Scholar]
- Speed CJ, Naylon CB, Little PJ, Mitchell CA. Underexpression of the 43-kDa inositol polyphosphate 5-phosphatase is associated with spontaneous calcium oscillations and enhanced responses following endothelin-1 stimulation. J Cell Sci. 1999;112:669–679. doi: 10.1242/jcs.112.5.669. [DOI] [PubMed] [Google Scholar]
- Stolz LE, Huynh CV, Thorner J, York JD. Identification and characterization of an essential family of inositol polyphosphate 5-phosphatases (INP51, INP52, and INP53 gene products) in the yeast Saccharomyces cerevisiae. Genetics. 1998;148:1715–1729. doi: 10.1093/genetics/148.4.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strome S. Fluorescence visualization of the distribution of microfilaments in gonads and early embryos of the nematode C. elegans. J Cell Biol. 1986;103:2241–2252. doi: 10.1083/jcb.103.6.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verjans B, Lecocq R, Moreau C, Erneux C. Purification of bovine brain inositol-1,4,5-trisphosphate 5-phosphatase. Eur J Biochem. 1992;204:1083–1087. doi: 10.1111/j.1432-1033.1992.tb16732.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.