Abstract
Oncogenic transformation involves reprogramming of cell metabolism, whereby steady-state levels of intracellular NAD+ and NADH can undergo dramatic changes while ATP concentration is generally well maintained. Altered expression of nicotinamide phosphoribosyltransferase (NAMPT), the rate-limiting enzyme of NAD+-salvage, accompanies the changes in NAD(H) during tumorigenesis. Here, we show by genetic and pharmacological inhibition of NAMPT in glioma cells that fluctuation in intracellular [NAD(H)] differentially affects cell growth and morphodynamics, with motility/invasion capacity showing the highest sensitivity to [NAD(H)] decrease. Extracellular supplementation of NAD+ or re-expression of NAMPT abolished the effects. The effects of NAD(H) decrease on cell motility appeared parallel coupled with diminished pyruvate-lactate conversion by lactate dehydrogenase (LDH) and with changes in intracellular and extracellular pH. The addition of lactic acid rescued and knockdown of LDH-A replicated the effects of [NAD(H)] on motility. Combined, our observations demonstrate that [NAD(H)] is an important metabolic component of cancer cell motility. Nutrient or drug-mediated modulation of NAD(H) levels may therefore represent a new option for blocking the invasive behavior of tumors.
Electronic supplementary material
The online version of this article (doi:10.1007/s00018-012-1249-1) contains supplementary material, which is available to authorized users.
Keywords: NAD(H), Cancer metabolism, Migration, Invasion, Lactic acid
Introduction
Invasive tumor growth involves a complex program in which altered cell proliferation and survival combines with cell–cell dissociation, matrix degradation and migration through individual, or collective cell movement [1, 2]. To sustain their proliferation, cancer cells rewire their metabolic pathways in an ensemble organization that is called “the Warburg phenotype”, characterized by intense glycolysis even in the presence of oxygen [3, 4]. Besides changes in use of metabolic enzymes, cancer cells are typified by the altered steady-state concentration of small molecular key compounds that serve as direct substrate(s), allosteric modifiers, or co-factors. One of the best-known examples is the higher cytosolic NAD+/NADH ratio and level that is needed to sustain the increased glycolytic flux. Likewise, higher levels of co-enzyme NADPH are needed for promotion of fatty acid synthesis and for coping with the increased oxidative stress [5]. Unfortunately, details on how subcellular control over NAD+, NADH, NADP+ and NADPH levels is involved in regulation of cell fate and behavior are still unclear [6, 7]. A related unsolved issue is whether NAD+ metabolism—given its complex interlinked nature—could stand out as a target for the exploration of new cancer therapies based on metabolic interference.
We [8] and others [9] recently found strongly increased NAD(H) levels in early Ras-transformed tumor cells, accompanied by a strong upregulation of nicotinamide phosphoribosyltransferase (NAMPT). NAMPT (also known as PBEF/Visfatin) catalyzes the formation of nicotinamide mononucleotide (NMN) by transfer of a 5-phosphoribosyl-1-pyrophosphate to nicotinamide (Supplementary Fig. 1, Garten et al. [9]), the first and rate-limiting step in the main pathway for NAD+ salvage synthesis in mammals. Not all aspects of NAMPT’s function in growth and differentiation are well understood, but its role in spatiotemporal aspects of NAD+-biosynthesis has been established [10]. By determining the (local) availability of NAD+, NAMPT is a critical regulator of the activity of NAD+-consuming enzymes like ADP-ribosyltransferases, sirtuins [11], and NAD+ glycohydrolases [7]. Thereby, next to energy state, NAD+ levels regulate nuclear gene expression, nitrogen metabolism in mitochondria, and protein-acetylation, principles which all differentially determine (differentiation) fate and behavior of normal and cancer cells. Recently, a role of NAMPT in lipogenesis in tumor cells was found [12].
A selective inhibitor of NAMPT, FK866, is already being tested in phase II clinical trials for several cancer types [13], but for further progress in disease therapy, many questions regarding effects of NAD(P)(H) on cell viability and behavior still have to be solved. Specifically the relationship of NAD(H) levels and cell morphodynamics is a topic that has been poorly addressed in existing literature.
In the present study, we used genetic and pharmacological NAMPT inhibition to gradually modulate NAD(H) levels in glioma cells and studied how this differentially affects proliferation, motility, and invasion. Surprisingly, we found that the capacity for motility and invasion of these cells was more sensitive to lowering of NAD+ levels than growth capacity. Effects on motility/invasion were associated with lowering of internal pH and dominantly determined by lactate dehydrogenase-A (LDH-A) dependent pyruvate-lactate conversion. Our data provide evidence for a controlling role of NAD(H) in the motile behavior of malignant cancer cells.
Materials and methods
Cell culture
U251MG, U87MG glioblastoma (gift from Dr. J. van Schalkwijk, Department of Dermatology, NCMLS, Nijmegen) and 293FT cells (Invitrogen, Carlsbad, CA, USA) were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Invitrogen) containing 25 mM glucose and supplemented with 10 % fetal calf serum (FCS), 2 mM sodium pyruvate, and 4 mM glutamine. To manipulate metabolites in culture medium, nutrient-deficient DMEM powder (Sigma-Aldrich) was dissolved and complemented with 10 % dialyzed FCS, 4 mM glutamine, 0.37 % sodium bicarbonate, glucose (1 or 25 mM) and pyruvate (2 mM).
Antibodies and reagents
Anti-NamPT (Bethyl Laboratories, Montgomery, TX, USA), anti-LDH-A (Cell Signaling Technologies, Boston, MA, USA) and anti-tubulin (DSHB, University of Iowa) primary antibodies and IRDye (Rockland) and Alexa (Invitrogen)-conjugated secondary antibodies were used. Alexa-conjugated phalloidin was purchased from Invitrogen. FK866 was from RTI International, BB94 was from British Biotech and other reagents were purchased from Sigma-Aldrich.
ShRNA, lentiviral infection, mutagenesis and complementation, RNA isolation, and Northern blotting
ShRNA-mediated knockdown, lentiviral transduction, site-directed mutagenesis, overexpression of NAMPT and RNA isolation, and Northern blotting are described in the Supplementary Materials and methods.
Western blotting
SDS-PAGE and Western blotting were performed using standard procedures. Signals were detected and quantified and corrected for protein loading using the Odyssey Imaging System (LI-COR Biosciences, Lincoln, NE, USA).
NAD+, NADH, NADP+, and NADPH measurements
For determination of cellular NAD+, NADH, NADP+, and NADPH we modified the protocol of [49]. An amount of 2 × 106 glioma cells were trypsinized, washed with 2 ml 0.9 % NaCl, and split into two equal fractions (for reduced and oxidized pyridines) and placed on ice. To determine NADH and NADPH, cell pellets were resuspended in 200 μl 0.02 M NaOH/0.5 mM l-cysteine, incubated at 60 °C for 10 min and neutralized with 60 μl 0.5 M Gly-Gly buffer (pH 7.6), mixed and stored on ice. To determine NAD+ and NADP+, cell pellets were resuspended in 200 μl ice-cold PCA, incubated on ice for 15 min and neutralized with 80 μl 2 M KOH/0.2 M KxPO4 (pH 7.5). Neutralization was checked by pH indicator strips and samples were placed on ice. Both samples were centrifuged for 3 min at 13,000 rpm at 4 °C and supernatants were snap-frozen in liquid nitrogen and stored at −80 °C. Pyridine concentrations were measured using enzymatic MTT cycling reactions. The reaction for NADH and NADPH contained 50 mM Tris (pH 8.0), 1.8 mM MTT, 70 μM 1-methoxy-5-methyl-phenzinium methyl sulfate, 20 mM succinate, 5 mM glucose-6-phosphate and 0.45 IU glucose-6-phosphate dehydrogenase. The reaction for NAD+ and NADP+ contained 64 mM Gly-Gly buffer (pH 7.6), 1.8 mM MTT, 70 μM 1-methoxy-5-methyl-phenzinium methyl sulfate, 20 mM succinate, 64 mM nicotinamide, 0.32 M ethanol, and 20 IU alcohol dehydrogenase. To start the cycling reaction, 140 μl of reaction buffer was added to 10 μl cell extract or NAD+/NADP+ standards, incubated for 30–60 min at room temperature in the dark, and the absorbance was measured at 510 nm. Samples were measured in triplicate and blanks, without NAD+/NADP+, were used for background correction.
Lactic acid measurements
Lactic acid levels in cell culture medium and cellular lysates were determined using the Amplex Red glucose/glucose oxidase kit (Molecular Probes), with lactate oxidase (Sigma) in the reaction instead of glucose oxidase. Lactic acid levels were normalized to cell numbers and repeated three times.
Measuring intracellular pH (pHi)
pHi was measured ratiometrically using video imaging techniques and the fluorescent pH indicator BCECF (Invitrogen-Molecular Probes, Eugene, OR, USA). Cells were plated onto uncoated glass-bottom dishes (Ø 35.0 mm, Greiner BIO-ONE GmbH, Frickenhausen, Germany) at ~20 % confluence. Then, 24 h after seeding, cells were treated with FK866 (10 nM). After another 24 h, the HCO3 −-buffered DMEM was replaced by a HEPES-buffered DMEM (10 mM HEPES, pH 7.4). Cells were allowed to adapt to these conditions in a 37 °C heating cabinet for at least 1 h. Cells were then exposed to HEPES-buffered DMEM containing 5 μg/ml BCECF-AM for 2–5 min. The glass-bottom dishes were placed on the stage of an inverted microscope (Axiovert 200, Carl Zeiss) and the cells were continuously superfused with prewarmed (37 °C) Ringer solution of pH 7.4 (adjusted by adding 1 M NaOH), containing (in millimolar) 122.5 NaCl, 5.4 KCl, 0.8 MgCl2, 1.2 CaCl2, 1.0 NaH2PO4·2H2O, 5.0 glucose, and 10.0 HEPES. The excitation wavelength alternated between 440 and 490 nm while the emitted fluorescence intensities were monitored at 500 nm using a Photometrics camera (CoolSnapfx, Visitron Systems, Puchheim, Germany). The different wavelengths were generated by a high-speed polychromator system (Visichrome, Visitron Systems). Polychromator and data acquisition were controlled by Metafluor software (Visitron Systems). Fluorescence intensities were measured at 37 °C at 35-s intervals and corrected for background fluorescence by subtracting intensities from adjacent, extracellular regions. Exposure times were 100 and 250 ms for the 490- and the 440-nm images, respectively, and the camera gain used was 2. A ratio was calculated from the intensities measured at 440 and 490 nm. At the end of each experiment, the pH measurements were calibrated by successively superfusing the cells with modified, high-K+ Ringer solutions of pH 7.5, 7.0, and 6.5 containing (in millimolar) 125 KCl, 1 MgCl2, 1 CaCl2, 20 HEPES, and 10 μM nigericin (Sigma, Taufkirchen, Germany) [50].
Measuring pH at the cell surface (pHe)
pH in the glycocalyx was measured as described previously [51, 52]. Briefly, the different U251 cells were prepared and measured as described above for the pHi measurements except that after the 1-h adaptation to the HEPES-buffered DMEM, cells were incubated with 12.5 μg/ml of the fluorescein-conjugated wheat germ agglutinin (WGA, Invitrogen-Molecular Probes) instead of being treated with BCECF. The calibration of the pH measurements followed the aforementioned protocol.
Cell proliferation assays
Cell proliferation was measured using sulforhodamine B (SRB) total protein stain [53]. In brief, cells were washed with PBS, fixed with 10 % trichloroacetic acid for 1 h at 4 °C, and washed with water. Cells were stained with 0.5 % SRB for 15 min, washed with 1 % acetic acid, dried, and protein-bound SRB was dissolved in 10 mM Tris (pH 10.5). Absorbance was measured at 510 nm. Cell viability was measured using MTT mitochondrial activity assays. Briefly, cells were grown in the presence of 1 mg/ml MTT for 3 h, medium was removed, and MTT dissolved in DMSO for 30 min at room temperature. Plates were shaken and absorbance was measured at 510 nm. Samples were measured in triplicate and blanks, without cells, were used for background correction.
Cell migration and invasion assays
Cell migration assays, including 2D barrier and gap closure assays, 3D motility assays and cell invasion assays, matrix degradation, spheroid outgrowth, and inverted invasion assays are described in the Supplementary Materials and methods.
Time-lapse microscopy
For gap closure, barrier migration, and spheroid outgrowth assays, a Nikon DiaPhot microscope equipped with a Microscope Stage Incubator (Oko-Lab, Ottaviano, Italy) and Hamamatsu C8484-05G digital camera was used. Images were taken every 10 min using TimeLapse Software (Oko-Lab), version 2.7 with a 10× objective. Analysis of 3D cell movements in collagen lattices was performed on Leica DMIL microscopes equipped with CCD cameras taking an image every 4 min.
LDH isoenzyme assay
LDH isoenzyme assay was performed using the LDH isoenzyme Kit (Helena Biosciences, Gateshead, Tyne and Wear, UK) according to the manufacturers’ protocol.
Statistical analysis
Data are shown as averaged ± SEM and were analyzed in pairs by Student’s t test and one-sample t test for relative values and considered statistically different when p < 0.05.
Results
A cell model with gradually decreasing cellular NAD(H) levels
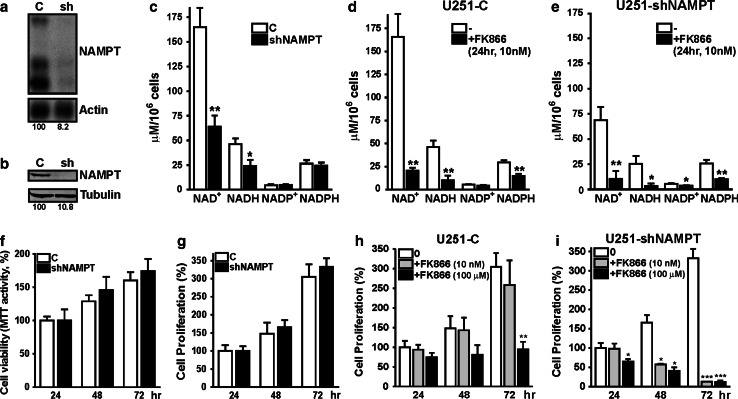
NAD+ is produced from different precursors via de novo and salvage synthesis pathways, whereby NAMPT constitutes the rate-limiting step in nicotinamide-dependent salvage (Supplementary Fig. 1 [9]. Earlier we reported that in transformed tumor cells, NAMPT expression and NAD(H) levels were increased [8]. Here we used genetic and pharmacological NAMPT inhibition as tools to generate cells with graded reductions in NAD(P)(H) level. U251 glioma cells were chosen as a model system for easy modulation of global NAD(H) concentration, as in these cells the other major route for salvage synthesis of NAD+, via conversion of nicotinic acid, is absent because the responsible enzyme, nicotinic acid phosphoribosyltransferase (NAPRT1), is not expressed [14]). Permanent knockdown by shRNA resulted in cells with stably reduced (to approximately 10 %) expression of NAMPT mRNA and protein (Fig. 1a, b). Correspondingly, NAD(H) levels decreased to approximately 40 % of controls (U251-C; Fig. 1c). Notably, the steady-state concentration of NADP(H) appeared unaffected under these conditions. Upon treatment of U251-C and U251-shNAMPT cells with the selective NAMPT inhibitor FK866 (10 nM), NAD(H) levels decreased to 14 and 6 % of control values, respectively (Fig. 1d, e). FK866 inhibition also led to a lesser but still significant decrease of NADP(H) levels. A time course experiment (Supplementary Fig. 2) confirmed that NAD(H) and NADP(H) levels are differentially sensitive towards NAMPT inhibition. Our findings suggest that NADP(H) is under more robust homeostatic control than NAD(H) confirming recent published data [14].
Fig. 1.
Partial NAMPT inhibition generated cells with graded NAD(H) levels. a Northern blot, showing that downregulation of NAMPT by shRNA in U251 glioma cells results in 90 % knockdown of mRNA transcripts. b Western blotting, confirming that shRNA knockdown also results in 90 % decrease of NAMPT protein levels. c NAD(H) and NADP(H) levels in U251-C and U251-shNAMPT cells. NAD(H) levels in shNAMPT cells are decreased to 40 % of that in controls, while NADP(H) levels remain unchanged. d Pharmacological inhibition of NAMPT by FK866 in U251-C cells results in a stronger inhibitory effect [NAD(H)] decreased to 14 %, and [NADP] decreased to 50 % of the concentration in untreated control cells. e Combined inhibition of NAMPT, by treatment of U251-shNAMPT cells with FK866 resulted in very low [NAD(H)]: 6 % of the level in U251-C controls cells. Also NADP(H) levels appear affected under these conditions. f Cell viability, measured by MTT mitochondrial activity, remains at normal level upon NAMPT knockdown. g Cell proliferation, determined as increase in total protein mass over time, does not differ between U251-C and shNAMPT cells. h Treatment of U251-C cells with FK866 (10 nM) causes no obvious differences in proliferation (grey bars), while high concentration (100 μM) resulted in a stop of cell proliferation (black bars). i The sensitivity of U251-shNAMPT cells for FK866 is increased at least 10,000-fold. At low concentration (grey bars) the viability of U251-shNAMPT cells appeared already compromised, but more pronounced cell death was observed at high concentration of FK866. All experiments were repeated three times and shown as average ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001
Cell proliferation is not affected when NAD(H) levels are moderately decreased
Measurement of mitochondrial activity or total cell protein in growing cell populations revealed that U251-shNAMPT and control cells have similar proliferative capacities (Fig. 1f, g). Thus, cells with only 40 % of NAD(H) proliferate normally and even when NAD(H) levels were further decreased by treatment with 10 nM FK866, proliferative capacity remained unchanged (Fig. 1h, grey bars). Similarly, use of 10 nM FK866 in another glioma cell line (U87) gave reduction of NAD(H) to approximately 10 % of control level, while proliferation remained normal (Supplementary Fig. 3a, b). However, further increase of the FK866 concentration to up to 100 μM halted growth of U251-C cells while U251-shNAMPT cells already started dying after 48 h of treatment with 10 nM (Fig. 1i). From these observations we conclude that knockdown of NAMPT renders U251 cells at least 10,000-fold more sensitive to FK866.
Rescue of NAD(H) levels by NAD+ addition or NAMPT complementation restores cell proliferation in FK866-treated U251-shNAMPT cells
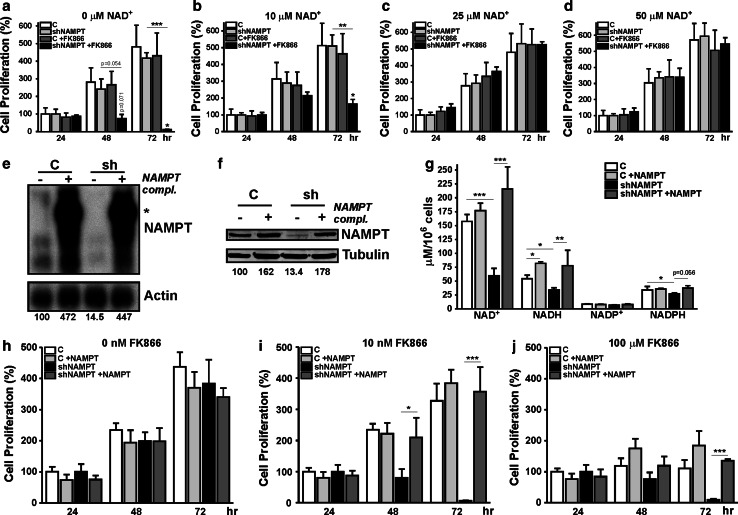
Next, to prove that FK866-induced cell death in U251-shNAMPT cells was solely due to low NAD(H), we studied the effect of NAD+ supplementation to the medium. Recently, it was demonstrated that addition of exogenous NADH results in a traceable increase of cellular concentration [15]. Indeed, by repeating FK866 treatment in the presence of extracellular NAD+ we observed rescue of proliferation in a concentration range of 10–50 μM (Fig. 2a–d). The addition of 10 μM NAD+ partially restored proliferation, higher NAD+ concentrations gave complete rescue (Fig. 2b–d, black bars). Direct measurement (Supplementary Fig. 4) confirmed that intracellular NAD(H) levels significantly increased upon extracellular addition, suggesting that uptake of intact NAD+ or breakdown and re-synthesis must be involved in the reversal of effects. Whatever the mechanism of uptake, we consider it remarkable that rebalancing to subnormal levels was already sufficient to rescue proliferative capacity completely (Fig. 2c, d; Supplementary Fig. 4).
Fig. 2.
Effects of NAMPT inhibition can be rescued by addition of NAD+ or re-expression of NAMPT. Cell proliferation assays of U251-C and U251-shNAMPT cells treated with FK866 in the absence (a) or presence (b–d) of exogenous NAD+; 10 μM extracellular NAD+ elicits a partial rescue of the proliferation of treated U251-shNAMPT cells. Higher concentrations of NAD+ resulted in complete rescue (black bars). e Northern-blot analysis, showing efficacy of re-expression of NAMPT mRNAs. Complementation with an expression vector with NAMPT cDNA insert resulted in 4–5 overexpression of NAMPT transcripts in both control and shNAMPT cells. Asterisk points to a CMV-NAMPT fusion transcript from the re-expression plasmid (not transcribed). f Western-blot analysis, showing that complementation with NAMPT resulted in protein expression in U251-C and U251-shNAMPT that is 1.5–2 times higher than that of endogenous NAMPT in U251 control cells. g Comparison of NAD(H) and NADP(H) levels between complemented and non-complemented shNAMPT U251 cells. Re-expression of NAMPT completely rescued the drop in NAD(H) levels seen upon NAMPT knockdown but overexpression to higher than endogenous NAMPT levels did not result in higher NAD(H) levels. h–j Comparison of cell proliferation capacity between complemented and non-complemented cells in the absence (h) and presence of low (10 nM, i) and high (100 μM, j) concentrations of FK866. Note, that in the absence of FK866, no differences in proliferative capacity are observed (i) and that the high sensitivity for FK866 in U251-shNAMPT cells is completely relieved when NAMPT expression is restored. All experiments were repeated three times and shown as average ± SEM, *p < 0.05, **p < 0.01, ***p < 0.001
Re-expression of NAMPT from a shRNA-resistant cDNA in U251-shNAMPT cells confirmed the direct linkage between NAD(H) salvage activity and proliferative capacity. Complementation, resulting in four to five and one to twofold higher-than-normal levels of NAMPT mRNA and protein, respectively, led to complete restoration but not to increase of the NAD(H) levels (Fig. 2e–g). Overexpression of NAMPT in U251-C yielded comparable results. As anticipated, forced (re)expression of NAMPT alleviated the high sensitivity of U251-shNAMPT cells to FK866 (10 nM), but did not have any stimulating effect on cell proliferation (Fig. 2h, i). At very high concentrations of FK866 (100 μM) all cells with normal or higher than normal NAMPT expression stopped proliferating, but U251-shNAMPT with no compensatory expression already started dying after 48 h (Fig. 2j). Collectively, these results indicate that proliferation capacity is not sensitive to alterations of steady-state [NAD(H)] when above 15 % of normal level, but that cells become vulnerable when [NAD(H)] falls below 10 % of normal. That the correlation between viability and cellular [NAD(H)] has this apparently low threshold is supported by the finding that both exogenous NAD+ addition and re-expression of NAMPT normalized U251 growth completely.
Cell motility is controlled by NAD(H) level, when in the subnormal range
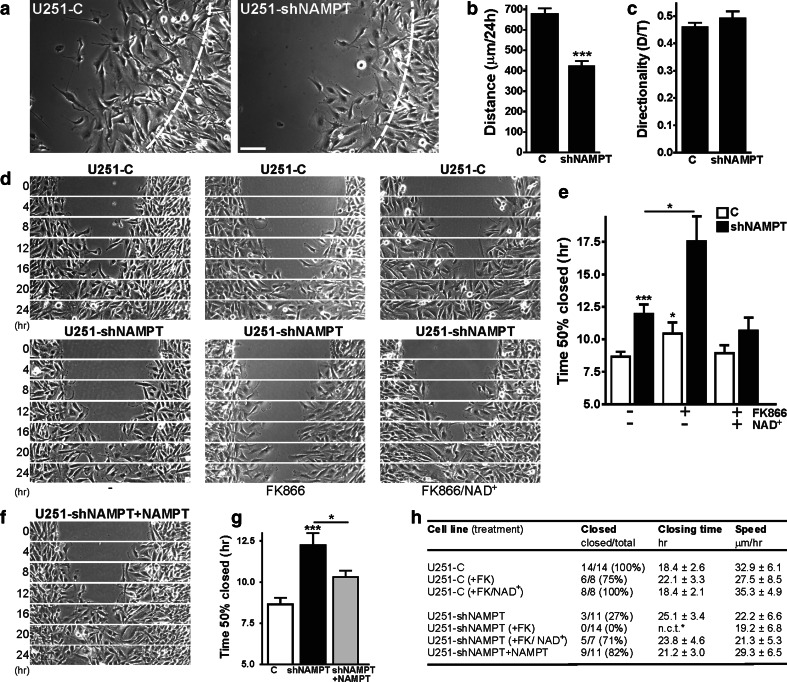
To determine whether a similar critical threshold exists for coupling between NAD(H) and morphodynamic behavior we compared U251-C and U251-shNAMPT motility in barrier migration assays [16]. Remarkably, shNAMPT cells, which still have 40 % of normal [NAD(H)], migrated more slowly than control cells (Fig. 3a, Supplementary Movie 1). Migration distance was reduced while the directionality of cell movement was comparable (Fig. 3b, c). Also in gap closure assays (without wounding the monolayer), shNAMPT cells displayed a lower migration capacity than U251-C cells. FK866 treatment inhibited migration in both control and shNAMPT cells. Repression of cell motility, again with no effects on proliferation capacity, was also seen in FK866-treated U87 cells with over tenfold lower NAD(H) levels (Supplementary Fig. 3). For quantification of gap closure performance, we calculated the time needed to close 50 % of the initial gap. Gap-closure periods increased upon NAMPT inhibition, either by shRNA only or in combination with FK866 (Fig. 3e). Qualitatively most pronounced differences were observed between the U251-shNAMPT and U251-C populations of cells. FK866-treated U251-shNAMPT cells did not manage to close the gaps at all and cannot be reliably considered in the comparison because of effects on cell proliferation at later time points (Fig. 3d, Supplementary Movies 2 and 3). Importantly, extracellular addition of NAD+ had a normalizing effect on gap closure (Fig. 3e). To confirm the specificity of this effect, we also tested supplementation with NMN, the direct product of the NAMPT reaction. As extracellular added NAD+ is thought to be degraded into NMN (and further to nicotinamide riboside [10]) before it can be taken up by the cells, NMN and NAD+ addition should show comparable effects. Indeed, by combining NMN addition with FK866 treatment in the gap closure assay, we recapitulated the rescue seen with NAD+ (Supplementary Fig. 5, Supplementary Movie 4). Also, re-expression of NAMPT partially rescued the difference in gap closure ability between U251-shNAMPT and U251-C cells (Fig. 3f, g; Supplementary Movie 5). When combined, these findings (summarized in Fig. 3h) indicate that changes in NAD(H) levels can have a strong impact on cell motility.
Fig. 3.
Cell motility is inhibited by low NAD(H) levels. a–c Comparison of 2D cell motility in barrier migration assays. Quantification of migratory parameters shows that U251-shNAMPT cells migrate over smaller distances than control cells (b), but that the directionality of cell movement was comparable (c). Images in a are stills at 24 h from migration movies (Supplementary Movie 1); dotted lines indicate cell front at t = 0 h, bar, 100 μm. d, e Gap closure assays of U251-C (upper row) and U251-shNAMPT cells (lower row) that were left untreated, cultivated in presence of FK866 (10 nM) or co-treated with NAD+ (50 μM). Results confirm the slower migration capacity of shNAMPT cells and show that FK866 further inhibits cell motility. The effect of FK866 was rescued by NAD+. Images are stills from migration movies (Supplementary Movie 2, 3). Quantification of migration in gap closure assays (e). Bars in the diagram indicate the time it takes to close 50 % of the initial gap. f, g Images and quantification of gap closure of complemented U251-shNAMPT cells. Image in f is derived from migration movies (Supplementary Movie 4) and should be compared to d. Bars in the diagram (g) indicate that gap-closing ability is compromised by knockdown of NAMPT expression, but restored by complementation. h Listing of migratory parameters. Migration is compromised by lower NAD+ levels, even when salvage synthesis is only moderately affected as in U251-shNAMPT cells. *n.c.t. no closing time. All experiments were repeated at least three times and values given are averages ± SEM, *p < 0.05, ***p < 0.001
Matrix degradation by invadopodia is controlled by cellular NAD(H) levels
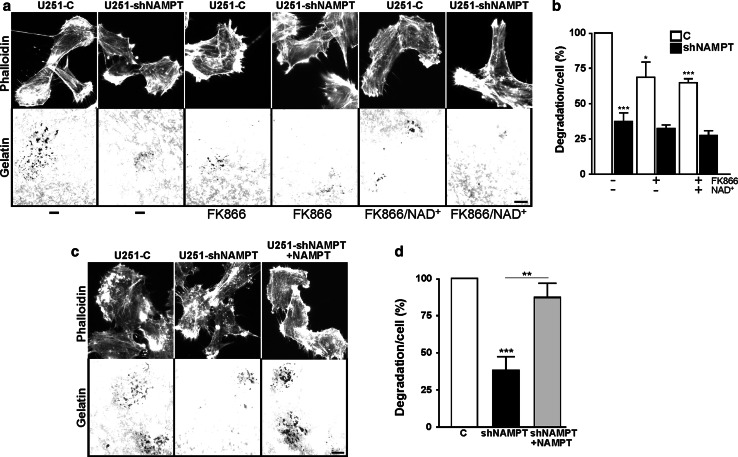
Next, we also analyzed the role of NAD(H) in other aspects of invasive growth. The formation of invadopodia by tumor cells results in focal matrix degradation and is regarded as a consistent and quantitative measure of invasive cell behavior [17]. Analysis of matrix degradation revealed that U251-shNAMPT cells had much lower invadopodia formation capacity than U251-C cells (Fig. 4a, b). Comparison in the presence of FK866 showed that invadopodia formation was inhibited in control cells, but that the already low activity of shNAMPT cells was not inhibited further. Also in U87 cells, FK866 treatment repressed invadopodia formation capacity (Supplementary Fig. 3d). Remarkably, the inhibitory effects of FK866 were not overcome by NAD+ addition (50 μM; Fig. 4b) but complete reversal was observed when NAMPT expression in U251-shNAMPT cells was restored (Fig. 4c, d). These findings can be best explained by assuming that intracellular NAD(H) levels after extracellular addition are not sufficiently restored to normalize [NAD(H)] in all subcellular locations and that invadopodia have special local demand.
Fig. 4.
Matrix degradation by invadopodia is regulated by NAD+-levels. a, c Representative images of fixed cells in invadopodia assays stained with phalloidin. a U251-C and shNAMPT cells were left untreated, treated with FK866 or co-treated with NAD+ for 24 h. c Similar images of shNAMPT and complemented shNAMPT cells. Cells were allowed to degrade the matrix for 1 h. Bar 10 μm. b, d Quantification of matrix degradation. The extent of degradation was determined and compared relative to controls. The strongest inhibition of invadopodia formation is seen in shNAMPT cells. The effect of FK866 on invadopod formation was milder, though still significant, but could not be rescued by addition of NAD+ (b). In contrast, complete rescue of matrix degrading ability was achieved by re-expression of the NAMPT protein in U251-shNAMPT cells (d). Experiments were repeated at least four times and data are mean ± SD, *p < 0.05, **p < 0.01, ***p < 0.001
Cell invasion is inhibited when NAD(H) levels become subnormal
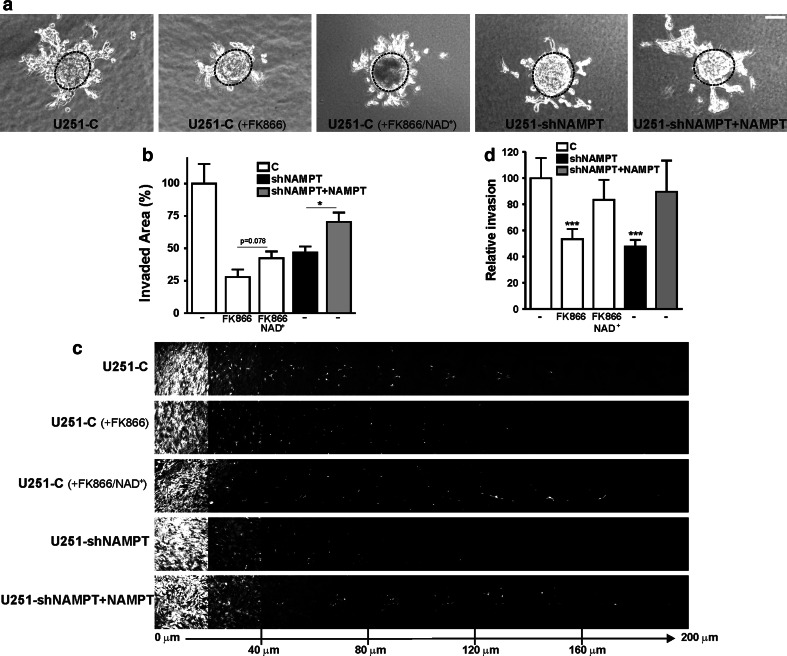
To further define the role of NAD(H) in tumor cell invasion, we performed 3D assays. Using single-cell invasion in 3D collagen matrices we observed that U251-shNAMPT cells migrated at approximately half of the speed of U251-C cells (Supplementary Fig. 6). Likewise, for outgrowth of multicellular spheroids embedded in Matrigel/agarose matrices, we saw clear effects of NAMPT knockdown, which—in part—could be rescued by restoration of NAMPT expression (Fig. 5a, b). Strikingly, not only the outgrowth capacity of spheroids but also the mode of migration of U251-shNAMPT cells appeared altered, as they had a stronger tendency to migrate as single cells when NAMPT activity was low (Supplementary Movie 6). Again, we also found that FK866 strongly inhibited invasive outgrowth of U251-C spheroids, whereby partial reversal of the effects was achieved by NAD+ addition (Fig. 5a, b). Finally, in inverted invasion assays, NAMPT knockdown or FK866 inhibition lowered the ability of cells to invade the Matrigel matrix against gravity. Effects were normalized when NAMPT was re-expressed or extracellular NAD+ was added, respectively (Fig. 5c, d). For the 3D invasion assays, we did not test additive effects of NAMPT inhibition and knockdown, as U251-shNAMPT cells in presence of FK866 lost viability within the time course of experiments, which was 72 h. Collectively, the data of different assays are congruent in that they show that subnormal NAD(H) levels are consistently associated with impaired motility and reduced invasive capacity, both in 2D and 3D environments.
Fig. 5.
Low NAD+-levels in U251 interferes with invasive growth. a Time-lapse microscopy of U251 spheroids embedded in Matrigel/agarose lattices for 72 h. Images of spheroid outgrowth are stills at t = 72 h from Supplementary Movie 5. Invasive growth is strongly inhibited in both FK866 (10 nM)-treated U251-C and U251-shNAMPT cells. Black dotted lines indicate spheroid dimensions at t = 0 h. Bar 100 μm. b Quantification of invasive outgrowth of spheroids. FK866 treatment or knockdown of NAMPT lowered the invasive potential of U251 cells. Rescue of outgrowth capacity by NAD+ addition or complementation by NAMPT re-expression was partial, but significant. c Inverted invasion of U251 cells against gravity. Images were taken as serial optical sections of the Matrigel-matrix-invading U251 cells. A sequence is given of images at 20 μm spacing at increasing invasion depths from left to right, as indicated at the bottom. d Quantification of inverted invasion. Both the inhibition by FK866 in control cells and in shNAMPT cells was rescued by addition of NAD+ and re-expression of NAMPT, respectively. Experiments were repeated at least three times, *p < 0.05, ***p < 0.001
Reversible pyruvate-to-lactate conversion catalyzed by LDH is involved in the motility phenotype of shNAMPT cells
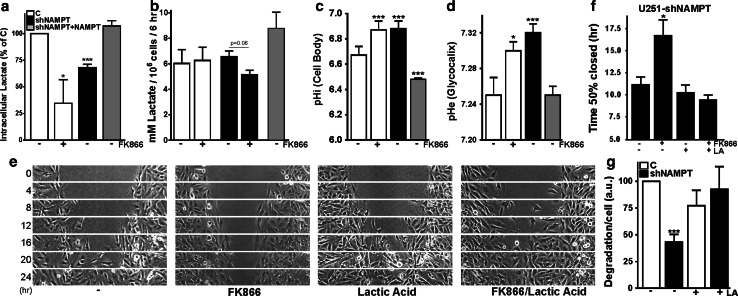
In an attempt to identify what could be the mechanistic link between [NAD(H)] and effects on motility, we decided to focus primarily on cytosolic reactions that need NAD(H) as co-factor. A cancer hallmark and quantitative major NAD+-dependent event is the conversion of pyruvate into lactate, which is catalyzed by LDH and uses NADH and one proton. Upon probing of lactate, we found that its intracellular concentration was significantly lower in U251-shNAMPT cells than in U251 controls, but that this decrease was fully rescued by NAMPT complementation (Fig. 6a). The rate of lactate secretion appeared only decreased for FK866 treated U251-shNAMPT cells, i.e., for cells with dramatically reduced NAD(H) levels. Accumulation of lactate in the extracellular medium was not different between U251-C and U251-shNAMPT cells (Fig. 6b). Apparently, only intracellular lactate, not extracellular lactate, can be involved in forming an associative coupling between lowering of NAD(H) and reducing of morphodynamic activity.
Fig. 6.
Lactic acid addition rescues the motility and matrix degradation phenotype induced by low [NAD]+. a Intracellular [lactate] in U251-shNAMPT and FK866-treated (10 nM, 24 h) control cells is lower than in U251-C or NAMPT-complemented cells. b Lactate secretion in U251-shNAMPT and FK866-treated (10 nM, 24 h) control cells is not different from U251-C or NAMPT-complemented cells. c, d Reduction of NAMPT activity in U251 cells results in intra- and pericellular pH (pHi and pHe) changes. The bar diagrams in c show the pHi, randomly determined, in cell bodies of U251-C, shNAMPT, and complemented cells. Knockdown or pharmacological inhibition of NAMPT resulted in higher pHi, whereas NAMPT complementation lowered pHi significantly, to even beyond normal values. Pericellular pH (pHe in the glycocalyx, d) varied comparably. e Gap closure assays of U251-shNAMPT cells untreated or treated with FK866 (10 nM) in the presence or absence of lactic acid (LA, 20 mM). LA completely rescued the FK866-effect on gap closure, see also Supplementary Movie 6 (as did NAD+ addition, see Fig. 4d). f Quantification of gap closure assays of e. Graph shows the time it takes to close 50 % of the initial gap. g Matrix degradation of U251-C and U251-shNAMPT cells in absence and presence of LA (20 mM). LA rescued the poor capacity to degrade extracellular matrix of U251-shNAMPT cells. All experiments were performed at least three times and shown as average ± SEM, *p < 0.05, **p < 0.01, ***p < 0.001
Because there is also involvement of protons in LDH-driven pyruvate-lactate conversion, we subsequently analyzed changes in intra- or pericellular pH (pHi for cytosolic pH and pHe for pericellular pH in the glycocalyx). Indeed, herein we found another correlation in that both pHi and pHe were more alkaline in U251-shNAMPT and FK866-treated U251-C cells (Fig. 6c, d). In complemented U251-shNAMPT cells, pHi and pHe were lowered again, confirming that variation in NAD+-salvage activity was linked to variation in pH values. To substantiate this picture and confirm that all substrates and products involved in the pyruvate-lactate conversion, including NAD(H), lactate and protons, act jointly (and non-discriminatory) in their control over motility, we performed gap closure assays in the absence or presence of lactic acid in the medium (Fig. 6e). We examined U251-shNAMPT cells with and without FK866 inhibition to set a broadest possible window for testing of rescue effects. Importantly, we observed that the effect of FK866 treatment was reversed but that lactic acid did not stimulate gap closure performance of U251-shNAMPT cells without FK866 (Fig. 6f; Supplementary Movie 7). In the matrix degradation assay, we could no longer discriminate between the degradation capacity of U251-shNAMPT and U251-C cells in the presence of lactic acid (Fig. 6g). Collectively, the results are consistent with the notion that NAD(H) modulates cell motility predominantly through the last step of glycolysis.
Low NAD(H) levels and sensitivity towards glucose or pyruvate depletion
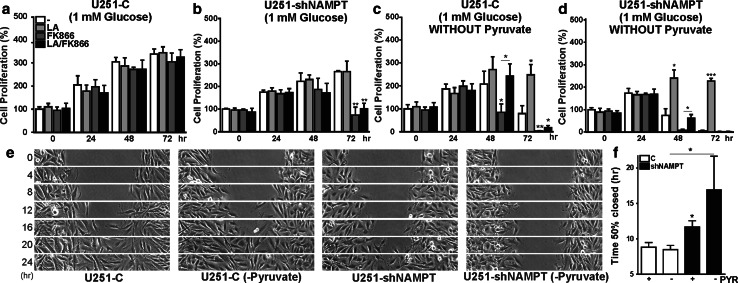
To confirm the mechanistic role of the pyruvate-lactate conversion reaction in yet another manner, we tested how NAMPT knockdown cells responded to lactic acid addition and/or pyruvate depletion under conditions of low glucose (1 mM) in the culture medium. As anticipated, growth of U251-C and U251-shNAMPT cells in low glucose (Fig. 7a, b) was generally slower than in medium containing high glucose (25 mM, Supplementary Fig. 7), the concentration used also in previous experiments. Both cell lines stopped proliferating after 48 h. Upon FK866 treatment, only growth of U251-shNAMPT cells was affected, resulting in cell death in both high and low glucose, recapitulating earlier findings described above. The addition of lactic acid prevented loss of cell mass after 72 h when cultivated in high glucose (black bars in Supplementary Fig. 7b), but did not prevent cell death under a low-glucose regime (Fig. 7b). Deprivation of pyruvate in low-glucose medium limited U251-C cell survival to only 48 h, but survival increased to up to 72 h when lactic acid was added. The absence of pyruvate rendered also U251-C cells FK866-sensitive (first noticeable after 48 h) but this effect could be reversed when lactic acid was co-added (Fig. 7c). For U251-shNAMPT cells, lactic acid addition rescued the effects on proliferation of low glucose and absence of pyruvate, or the combined effects of low glucose, pyruvate deficiency, and FK866 inhibition, for up to 72 and 48 h, respectively (Fig. 7d).
Fig. 7.
Lactic acid and pyruvate contribute to proliferation and motility differences in U251-C and U251-shNAMPT cells. Cell proliferation of U251-C (a, c) and U251-shNAMPT cells (b, d) in low glucose (a, b) and low glucose without pyruvate (c, d), without/with FK866, in the absence or presence of LA. Note that U251-C cells stop proliferating in low glucose after 48 h irrespective of treatment (a, compare with normal glucose, see Supplementary Fig. 6) and become sensitive to FK866 treatment when pyruvate is omitted. This effect could be rescued by the addition of LA for up to 48 h (c). U251-shNAMPT cells stop proliferating after 24 h (b). Without pyruvate, the U251-shNAMPT cells died 1 day earlier than U251-C cells and the effect of FK866 could be (partially) rescued by LA. Both U251-C and U251-shNAMPT cells survive on medium with LA, without pyruvate, and without FK866-treatment. e Gap closure assays in low-glucose medium with and without pyruvate. Migratory capacity of U251-shNAMPT—but not U251-C cells—was reduced by depletion of pyruvate, see also Supplementary Movie 7. f Quantification of gap closure assays of e. All experiments were performed at least three times and shown as average ± SEM, *p < 0.05 **p < 0.01, ***p < 0.001. PYR pyruvate
Under conditions of low glucose, in the 24-h intervals when U251-C and U251-shNAMPT cells were still proliferating, migration in gap closure was comparable to that under high glucose (Figs. 3e, 7f). Depletion of pyruvate from the low-glucose medium had no effect on performance of U251-C, but selectively affected cell motility of U251-shNAMPT cells (Fig. 7e, m; Supplementary Movie 8). Together, these findings demonstrate that pyruvate-lactate conversion is a pivotal point of regulation of growth and motility, confirming observations of others [18, 19] and our results above. Concomitantly, they also underscore the collective role of presence of both substrates, lactate and pyruvate, together with coenzyme NAD(H) and protons in the control over morphodynamics.
Knockdown of LDH-A recapitulates differential effects of NAD(H) depletion
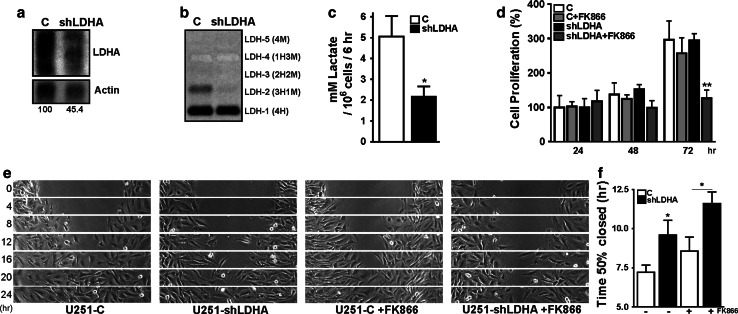
U251 cells have a LDH isoform profile different from that of other glioma cells like U87 (Supplementary Fig. 8). By knockdown of LDH-A gene products and change of this profile we sought to confirm that the rate of pyruvate-lactate conversion is critically linked, possibly with distinct thresholds, to both cell proliferation and motility. To test this idea, U251-shLDHA cells were established with 45 % residual mRNA levels of LDH-A (Fig. 8a), expressing undetectable low levels of M-subunit containing LDH tetramers (Fig. 8b). Cell proliferation of these cells was comparable to U251-C cells with or without FK866 treatment. Notably, U251-shLDHA cells were more sensitive to FK866 treatment (Fig. 8d), similar to the effects of NAMPT knockdown. The simplest interpretation of these findings is that the threshold for growth inhibition will be reached only if the flux through the LDH catalyzed reaction is sufficiently reduced, by superposition of effects of limitation of NAD+ substrate and/or reduction of LDH activity.
Fig. 8.
LDH knockdown phenocopies the effects of partial NAD+ depletion. a Northern blot from U251 cells stably transfected with a LDH-A shRNA producing vector. Knockdown to 55 % of the normal LDH-A RNA level in control cells is achieved. b LDH isoenzyme profile of U251-C and U251-shLDHA cells. LDH-A containing isoform LDH-2 is almost completely absent in U251-shLDHA cells. c Quantification of lactate secretion by U251-C and U251-shLDHA cells. Lactate accumulation was measured in conditioned medium collected from cells after a 24-h cultivation period. d Cell proliferation of U251-C and U251-shLDHA cells with and without FK866 treatment (10 nM). Note that U251-shLDHA and U251-C cells proliferated at comparable rates, but that only U251-shLDHA cells appear sensitive to FK866 treatment. e Gap closure assays of U251-C and U251-shLDHA cells with and without FK866 (10 nM). Both with and without FK866 treatment, U251-shLDHA cells migrated slower than U251-C cells. Images are derived from Supplementary Movie 8. f Quantification of gap closure assays shown in e. Experiments were repeated at least three times, *p < 0.05, **p < 0.01, ***p < 0.001
Interestingly, U251-shLDHA cells, which showed significantly reduced lactate production (Fig. 8c), also showed loss of migratory performance in gap closure assays (Fig. 8e, f; Supplementary Movie 9). Collectively, these observations corroborate the idea of discriminative thresholds in the LDH-catalyzed reaction. At moderately reduced rates, motility seems selectively affected, proliferation will be impaired only if flux through the reaction is largely blocked.
Discussion
Metabolic requirements for cancer cell proliferation and tumor growth have been relatively well characterized and the potential value of oncogenic adaptations in metabolism as entries for cancer therapy has now been convincingly demonstrated in several studies [20]. However, until now, surprisingly little is known about the coupling between cell metabolism and motility of tumor cells.
In the present study, we specifically uncovered a role in morphodynamics of NAD(H), which—together with NADP(H)—is the major determinant of cellular redox state. We demonstrated that [NAD(H)] homeostasis specifically and differentially couples to regulation of glioma cell motility and invasion, and proliferation. Under conditions where global NAD(H) levels varied within a range of 15–40 % of that of controls, cell proliferation proceeded normally, but cell motility was already markedly inhibited. These effects were proven to be uniquely and directly coupled to NAD+-salvage or -uptake capacity, as (partial) rescue was observed by both extracellular NAD+ supplementation and complementation with NAMPT. Somewhat to our surprise, we found that [NADP(H)] was consistently less affected by inhibition of NAD+-salvage than [NAD(H)]. Direct involvement of global [NADP(H)] in the highly sensitive regulation of morphodynamic activity appears therefore less likely, although we cannot rule out involvement of fluctuations in local [NADP(H)] [21]. Also, the NAD+/NADH redox couple did not vary noticeably when NAMPT activity was manipulated (Fig. 1, Supplementary Fig. 2), suggesting that our findings can be best explained as direct effects of absolute cellular levels of NAD(H) and hence the total cellular redox buffering capacity.
What could be the main molecular events involved in the effects of NAD+ on cell motility? Could NAD(H) molecules themselves be the drivers of cytoskeletal dynamics? Recent evidence suggests that direct redox regulation of F-actin assembly and disassembly is possible, but in this regulation NADP(H), not NAD(H) is involved [22]. Other evidence pointed out that a decrease in NADH may lead to slowing of cell migration via relief of repression of E-cadherin by the transcriptional co-repressor and redox-sensor CtBP [23]. NAD+ may also affect F-actin formation via influence on ADP-ribosylation [24]. Furthermore, activity regulation of tubulin via NAD+-dependent SIRT2 deacetylase [25] and/or SIRT1-promoted deacetylation of cortactin [26] may be involved. An alternative pathway for NAD+-salvage, i.e., phosphorylation of nicotinamide riboside via Nrk2b [27], has recently been functionally linked to cell morphodynamics. Clearly, further studies are necessary to discriminate between all these possible links and get further mechanistic insight in the role of NAD(H) as (in)direct driver of cytoskeletal alterations that result in cell movement.
Within the complex network of metabolic reactions that are NAD(H)-dependent, we identified LDH-catalyzed reversible pyruvate-lactate conversion, as one of the most dominant and sensitive candidate reactions for mechanistic control over cell motility. Indeed, knockdown of LDHA gene expression had a clear modulating influence as had deprivation of pyruvate, or supplementation of culture medium with lactic acid. Furthermore, effects of deprivation of pyruvate appeared reversible by lactic acid supplementation, confirming the involvement of the final step of glycolysis. Collectively, our data (summarized in Fig. 9) point to a combined effect and suggest that the molar concentration of all substrates and products including pyruvate, lactate, NAD+/NADH and protons, as well as the abundance of LDH isoforms, could in fact be all critical determinants of glioma cell motility.
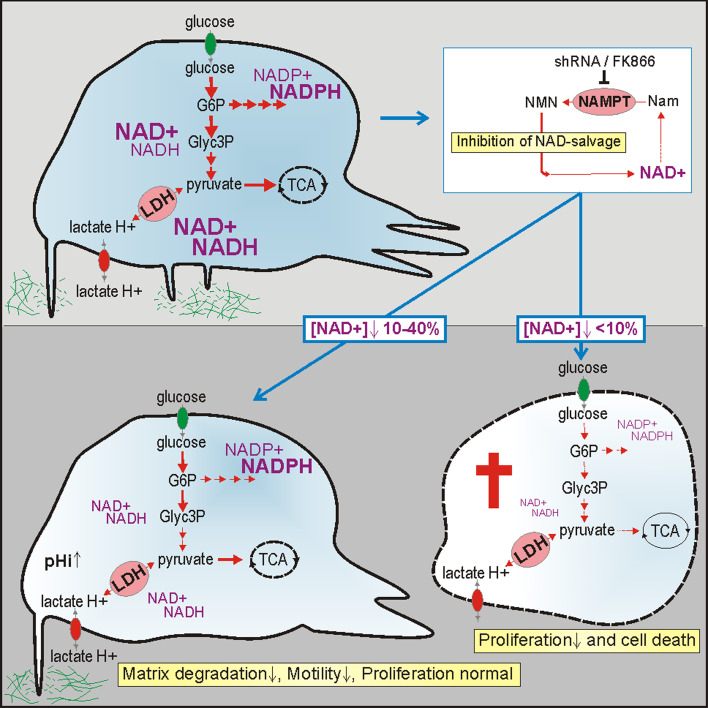
Fig. 9.
Scheme summarizing findings on differential effects of NAD+ levels on cell proliferation and morphodynamics. Mild NAD+ depletion through NAMPT inhibition reduces motility and invasive capacity of glioma cells, severe NAD+ depletion affects viability of glioma cells. The upper cell shows metabolites, enzymes, and processes studied in this paper. The white box indicates the NAD+-salvage pathway that was inhibited. Pivotal metabolic reaction steps involved in global or local steering of morphodynamic activity (lower cell to left) or coupled to viability and growth control (enzymes and pathways in dying cell to the right) are depicted in this scheme. Steady-state global NAD(H) concentrations are represented by font size and blue shading. G6P glucose-6-phosphate, Glyc3P glyceraldehyde-3-phosphate, TCA tricarboxylic acid cycle, LDH lactate dehydrogenase, NMN nicotinamide mononucleotide, Nam nicotinamide, pH i intracellular pH, pH e pericellular pH
To better interpret and integrate our evidence into existing knowledge, we need to reconcile that—together with the GAPDH reaction—LDH catalyzes the only metabolic reaction in the cell for which the cellular [NAD+] (0.1–1 mM), as a co-enzyme, may be “substrate limiting”. In the LDH reaction, stoichiometric conversion is involved and the intracellular concentrations of pyruvate (~0.1 mM) and lactate (~1 mM) fall within the same range as that of [NAD(H)] [28]. We feel that this could render pyruvate-to-lactate conversion generally and exquisitely sensitive to deviations in [NAD+] in a broad range of cancer cells. However, at this point in time we cannot over-generalize, because cellular concentrations of NAD(H), lactate, pyruvate, protons, and LDH activities differ considerably between cell types. It is of note here that U251 cells build up only a fraction (~10 %) of their total LDH activity by the expression of LDH-M subunits from the LDHA gene, while the LDHB gene is quantitatively more important.
Thus, to support our ideas on the role of LDH in morphodynamic responsiveness to NAD(H), we must assume that presence of at least one LDH-M subunit in the tetrameric enzyme is important for controlling the kinetics of intracellular lactate concentration or for intracellular partitioning and locating pyruvate-lactate conversion to cellular structures that are involved in motility and invasion [29]. Although the difference in LDH composition will not affect steady-state stoichiometric equilibrium of substrates and products, it will affect the speed at which the cell equilibrates the reaction, locally or globally. With regard to these latter points, it is important to note that our main findings could be reproduced in U87 cells (Supplementary Fig. 3), a cancer cell line with a different LDH1–5 isoenzyme profile (Supplementary Fig. 8). Different cells may use distinct LDH isoenzyme profiles and NAD(H) or pyruvate-lactate concentrations to optimize their sensitivity to motility and growth control, but further study is necessary to demonstrate that the basic principles of this regulation are conserved.
Our conclusion that NAD(H)’s contribution to the production of lactate and protons is critical for adaptation of motile and invasive behavior is consistent with recent findings on a functional role of lactate and protons in cancer cell motility [30, 31]. Lactate efflux was earlier identified as a target for inhibition of glioma invasiveness [32] and in a breast cancer metastasis model, lactate stimulated migration and metastasis, without affecting primary tumor growth [33]. Lactate metabolism is involved in (local) pH regulation via its proton usage/production. Interestingly, this can regulate cytoskeleton dynamics via talin and cofilin [34]. Molecular mechanisms, whereby cofilin acts as a pH sensor to mediate pH-dependent actin filament dynamics, have been elucidated into detail [35] and also for cortactin a pH-dependent role in invasion was found [36]. In this regulation, global or local effects of lactate secretion (by MCTs) or of proton transport by the Na/H exchanger (NHE1) may be involved. Extracellular acidification of the peri-invadopodial space by NHE1 activity is necessary for invadopodial-dependent extracellular matrix degradation [37]. The changes that we measured in pHi and pHe support such a model.
Strikingly, we observed that in contrast to 2D motility and inverted invasion, matrix degradation and spheroid outgrowth could not be completely rescued by NAD+ addition, but were rescued by complementation with NAMPT (Figs. 3, 4, 5). Possibly, NAMPT activity controls [NAD(H)] and therewith pHi and pHe locally, and addition of NAD+ to cells may not sufficiently reach all cellular locales involved in invasive growth. Differential use of the actin machinery, with differences in NAD(H) coupling in 2D or 3D motility [38], may also explain our findings. Recently, we found evidence for the local presence of metabolic enzymes in invadopodia, including LDH-A [29]. Similar to the situation for ATP [39] localized presence of NAD(H) may thus be important for control of cell motility. A local role for NAD(H) is also implicated by the fact that its biosynthesis is localized and compartmentalized [7, 10]. Further studies with newly designed NAD(H) biosensors [15, 40] and pH-sensors [41] will be needed to reveal whether fluctuations in local [NAD(H)] are coupled to particular aspects of regional cell dynamics, like adhesion or filopodia, lamellipodia, or invadopodia formation.
The central role of NAD(H) in cancer biology in general and a specific function in the directionality of cell movements of smooth muscle cells have recently been reported [42, 43]. Identification of [NAD(H)] and metabolites involved in the LDH reaction as focal points for cell dynamics regulation is attractive because they have already been implicated in chemosensitivity of glioma and breast tumors [44, 45]. Moreover the relevance of manipulation of cancer metabolism has recently been demonstrated in studies on diet-based therapies in mice [46]. In vivo, the NAMPT reaction and the daily dietary intake of niacin (nicotinic acid plus nicotinamide) have key control over [NAD+] in cells of most tissues, including cancer cells, making it relatively easy to manipulate the NAD(H) levels. We and others observed that compared to normal tissue cells, NAMPT is strongly upregulated in transformed cells [8, 47], perhaps for serving a higher demand for NAD+ availability in cancers. Inhibitors like FK866 (or APO866) used here, or newer inhibitors like MPC-8640 and MPC-9528, target NAMPT with high specificity and are already in clinical trials. So far, studies on the use of NAD+-salvage reactions as therapeutic entries only dealt with the effects that long-term NAMPT inhibition and the resulting severe NAD+ depletion had on cell growth, while effects on motility and invasion were not examined [13, 48].
In summary, if our findings on differential dependency of motility and proliferation can be extrapolated to other tumor cell types, our results could have considerable implications for diet or clinical-therapeutic regulation of cell motility behavior. Tailored therapy with low doses of FK866 or other means of mild modulation of [NAD+], for example via formulation of dietary nutrient intake, could be used to manipulate migratory or invasive behavior of cancer cells. Further study is necessary to better understand the role of LDH isoenzymes and to know whether tuning or inhibition of NAMPT or LDH needs to be individualized for each invasive cancer type.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
We thank Dr. Joost van Schalkwijk and Dr. William Leenders for providing glioma cell lines, Dr. Frank van Leeuwen for NAMPT cDNA, and Dr. Wiljan Hendriks and Dr. Ad de Groof for helpful discussions. This study was supported by Grant KUN-2005-3333 from the Dutch Cancer Society (to BW), Grant Sto 654/3-2 from the Deutsche Forschungsgemeinschaft (to CS and AS), an EMBO Short Term Fellowship and a Vanderes Foundation Research Grant (number 225) (to RvH) and by grants from the Italian Association for Cancer Research (AIRC) and the European Union’s Seventh Framework Program (Grant No. 237946) (to RB).
References
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Friedl P, Alexander S. Cancer invasion and the microenvironment: plasticity and reciprocity. Cell. 2011;147(5):992–1009. doi: 10.1016/j.cell.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 3.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324(5930):1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 5.Deberardinis RJ, Sayed N, Ditsworth D, Thompson CB. Brick by brick: metabolism and tumor cell growth. Curr Opin Genet Dev. 2008;18(1):54–61. doi: 10.1016/j.gde.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Figueiredo LF, Gossmann TI, Ziegler M, Schuster S. Pathway analysis of NAD+ metabolism. Biochem J. 2011;439(2):341–348. doi: 10.1042/BJ20110320. [DOI] [PubMed] [Google Scholar]
- 7.Koch-Nolte F, Fischer S, Haag F, Ziegler M. Compartmentation of NAD+-dependent signalling. FEBS Lett. 2011;585(11):1651–1656. doi: 10.1016/j.febslet.2011.03.045. [DOI] [PubMed] [Google Scholar]
- 8.de Groof AJ, Te Lindert MM, van Dommelen MM, Wu M, Willemse M, Smift AL, Winer M, Oerlemans F, Pluk H, Fransen JA, Wieringa B. Increased OXPHOS activity precedes rise in glycolytic rate in H-RasV12/E1A transformed fibroblasts that develop a Warburg phenotype. Mol Cancer. 2009;8(1):54. doi: 10.1186/1476-4598-8-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garten A, Petzold S, Korner A, Imai S, Kiess W. Nampt: linking NAD biology, metabolism and cancer. Trends Endocrinol Metab. 2009;20(3):130–138. doi: 10.1016/j.tem.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nikiforov A, Dolle C, Niere M, Ziegler M. Pathways and subcellular compartmentation of NAD biosynthesis in human cells: from entry of extracellular precursors to mitochondrial NAD generation. J Biol Chem. 2011;286(24):21767–21778. doi: 10.1074/jbc.M110.213298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Houtkooper RH, Pirinen E, Auwerx J. Sirtuins as regulators of metabolism and healthspan. Nat Rev Mol Cell Biol. 2012;13(4):225–238. doi: 10.1038/nrm3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bowlby SC, Thomas MJ, D’Agostino RB, Jr, Kridel SJ. Nicotinamide phosphoribosyl transferase (Nampt) is required for de novo lipogenesis in tumor cells. PLoS ONE. 2012;7(6):e40195. doi: 10.1371/journal.pone.0040195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holen K, Saltz LB, Hollywood E, Burk K, Hanauske AR. The pharmacokinetics, toxicities, and biologic effects of FK866, a nicotinamide adenine dinucleotide biosynthesis inhibitor. Investig New Drugs. 2008;26(1):45–51. doi: 10.1007/s10637-007-9083-2. [DOI] [PubMed] [Google Scholar]
- 14.Cerna D, Li H, Flaherty S, Takebe N, Coleman CN, Yoo SS. Inhibition of nicotinamide phosphoribosyltransferase (NAMPT) activity by small molecule GMX1778 regulates reactive oxygen species (ROS)-mediated cytotoxicity in a p53- and nicotinic acid phosphoribosyltransferase1 (NAPRT1)-dependent manner. J Biol Chem. 2012;287(26):22408–22417. doi: 10.1074/jbc.M112.357301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao Y, Jin J, Hu Q, Zhou HM, Yi J, Yu Z, Xu L, Wang X, Yang Y, Loscalzo J. Genetically encoded fluorescent sensors for intracellular NADH detection. Cell Metab. 2011;14(4):555–566. doi: 10.1016/j.cmet.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Horssen R, Galjart N, Rens JA, Eggermont AM, ten Hagen TL. Differential effects of matrix and growth factors on endothelial and fibroblast motility: application of a modified cell migration assay. J Cell Biochem. 2006;99(6):1536–1552. doi: 10.1002/jcb.20994. [DOI] [PubMed] [Google Scholar]
- 17.Murphy DA, Courtneidge SA. The ‘ins’ and ‘outs’ of podosomes and invadopodia: characteristics, formation and function. Nat Rev Mol Cell Biol. 2011;12(7):413–426. doi: 10.1038/nrm3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fantin VR, St-Pierre J, Leder P. Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell. 2006;9(6):425–434. doi: 10.1016/j.ccr.2006.04.023. [DOI] [PubMed] [Google Scholar]
- 19.Le A, Cooper CR, Gouw AM, Dinavahi R, Maitra A, Deck LM, Royer RE, Vander Jagt DL, Semenza GL, Dang CV. Inhibition of lactate dehydrogenase A induces oxidative stress and inhibits tumor progression. Proc Natl Acad Sci USA. 2010;107(5):2037–2042. doi: 10.1073/pnas.0914433107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tennant DA, Duran RV, Gottlieb E. Targeting metabolic transformation for cancer therapy. Nat Rev Cancer. 2010;10(4):267–277. doi: 10.1038/nrc2817. [DOI] [PubMed] [Google Scholar]
- 21.Gianni D, Diaz B, Taulet N, Fowler B, Courtneidge SA, Bokoch GM (2009) Novel p47(phox)-related organizers regulate localized NADPH oxidase 1 (Nox1) activity. Sci Signal 2(88):ra54. doi:10.1126/scisignal.2000370 [DOI] [PMC free article] [PubMed]
- 22.Hung RJ, Pak CW, Terman JR. Direct redox regulation of F-actin assembly and disassembly by Mical. Science. 2011;334(6063):1710–1713. doi: 10.1126/science.1211956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Q, Wang SY, Nottke AC, Rocheleau JV, Piston DW, Goodman RH. Redox sensor CtBP mediates hypoxia-induced tumor cell migration. Proc Natl Acad Sci USA. 2006;103(24):9029–9033. doi: 10.1073/pnas.0603269103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Terashima M, Shimoyama M, Tsuchiya M. Introduction of NAD decreases fMLP-induced actin polymerization in chicken polymorphonuclear leukocytes—the role of intracellular ADP-ribosylation of actin for cytoskeletal organization. Biochem Mol Biol Int. 1999;47(4):615–620. doi: 10.1080/15216549900201663. [DOI] [PubMed] [Google Scholar]
- 25.North BJ, Marshall BL, Borra MT, Denu JM, Verdin E. The human Sir2 ortholog, SIRT2, is an NAD+-dependent tubulin deacetylase. Mol Cell. 2003;11(2):437–444. doi: 10.1016/S1097-2765(03)00038-8. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y, Zhang M, Dong H, Yong S, Li X, Olashaw N, Kruk PA, Cheng JQ, Bai W, Chen J, Nicosia SV, Zhang X. Deacetylation of cortactin by SIRT1 promotes cell migration. Oncogene. 2009;28(3):445–460. doi: 10.1038/onc.2008.388. [DOI] [PubMed] [Google Scholar]
- 27.Goody MF, Kelly MW, Lessard KN, Khalil A, Henry CA. Nrk2b-mediated NAD+ production regulates cell adhesion and is required for muscle morphogenesis in vivo: Nrk2b and NAD+ in muscle morphogenesis. Dev Biol. 2010;344(2):809–826. doi: 10.1016/j.ydbio.2010.05.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quistorff B, Grunnet N. The isoenzyme pattern of LDH does not play a physiological role; except perhaps during fast transitions in energy metabolism. Aging (Albany NY) 2011;3(5):457–460. doi: 10.18632/aging.100329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Attanasio F, Caldieri G, Giacchetti G, van Horssen R, Wieringa B, Buccione R. Novel invadopodia components revealed by differential proteomic analysis. Eur J Cell Biol. 2011;90(2–3):115–127. doi: 10.1016/j.ejcb.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 30.Hirschhaeuser F, Sattler UG, Mueller-Klieser W. Lactate: a metabolic key player in cancer. Cancer Res. 2011;71(22):6921–6925. doi: 10.1158/0008-5472.CAN-11-1457. [DOI] [PubMed] [Google Scholar]
- 31.Goetze K, Walenta S, Ksiazkiewicz M, Kunz-Schughart LA, Mueller-Klieser W. Lactate enhances motility of tumor cells and inhibits monocyte migration and cytokine release. Int J Oncol. 2011;39(2):453–463. doi: 10.3892/ijo.2011.1055. [DOI] [PubMed] [Google Scholar]
- 32.Colen CB, Shen Y, Ghoddoussi F, Yu P, Francis TB, Koch BJ, Monterey MD, Galloway MP, Sloan AE, Mathupala SP. Metabolic targeting of lactate efflux by malignant glioma inhibits invasiveness and induces necrosis: an in vivo study. Neoplasia. 2011;13(7):620–632. doi: 10.1593/neo.11134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bonuccelli G, Tsirigos A, Whitaker-Menezes D, Pavlides S, Pestell RG, Chiavarina B, Frank PG, Flomenberg N, Howell A, Martinez-Outschoorn UE, Sotgia F, Lisanti MP. Ketones and lactate “fuel” tumor growth and metastasis: evidence that epithelial cancer cells use oxidative mitochondrial metabolism. Cell Cycle. 2010;9(17):3506–3514. doi: 10.4161/cc.9.17.12731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stock C, Schwab A. Protons make tumor cells move like clockwork. Pflugers Arch. 2009;458(5):981–992. doi: 10.1007/s00424-009-0677-8. [DOI] [PubMed] [Google Scholar]
- 35.Frantz C, Barreiro G, Dominguez L, Chen X, Eddy R, Condeelis J, Kelly MJ, Jacobson MP, Barber DL. Cofilin is a pH sensor for actin free barbed end formation: role of phosphoinositide binding. J Cell Biol. 2008;183(5):865–879. doi: 10.1083/jcb.200804161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Magalhaes MA, Larson DR, Mader CC, Bravo-Cordero JJ, Gil-Henn H, Oser M, Chen X, Koleske AJ, Condeelis J. Cortactin phosphorylation regulates cell invasion through a pH-dependent pathway. J Cell Biol. 2011;195(5):903–920. doi: 10.1083/jcb.201103045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Busco G, Cardone RA, Greco MR, Bellizzi A, Colella M, Antelmi E, Mancini MT, Dell’Aquila ME, Casavola V, Paradiso A, Reshkin SJ. NHE1 promotes invadopodial ECM proteolysis through acidification of the peri-invadopodial space. Faseb J. 2010;24(10):3903–3915. doi: 10.1096/fj.09-149518. [DOI] [PubMed] [Google Scholar]
- 38.Olson MF, Sahai E. The actin cytoskeleton in cancer cell motility. Clin Exp Metastasis. 2009;26(4):273–287. doi: 10.1007/s10585-008-9174-2. [DOI] [PubMed] [Google Scholar]
- 39.Van Horssen R, Janssen E, Peters W, van de Pasch L, Te Lindert MM, van Dommelen MM, Linssen PC, ten Hagen TL, Fransen JA, Wieringa B. Modulation of cell motility by spatial repositioning of enzymatic ATP/ADP exchange capacity. J Biol Chem. 2009;284(3):1620–1627. doi: 10.1074/jbc.M806974200. [DOI] [PubMed] [Google Scholar]
- 40.Hung YP, Albeck JG, Tantama M, Yellen G. Imaging cytosolic NADH-NAD(+) redox state with a genetically encoded fluorescent biosensor. Cell Metab. 2011;14(4):545–554. doi: 10.1016/j.cmet.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koivusalo M, Welch C, Hayashi H, Scott CC, Kim M, Alexander T, Touret N, Hahn KM, Grinstein S. Amiloride inhibits macropinocytosis by lowering submembranous pH and preventing Rac1 and Cdc42 signaling. J Cell Biol. 2010;188(4):547–563. doi: 10.1083/jcb.200908086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chiarugi A, Dolle C, Felici R, Ziegler M. The NAD metabolome—a key determinant of cancer cell biology. Nat Rev Cancer. 2012;12(11):741–752. doi: 10.1038/nrc3340. [DOI] [PubMed] [Google Scholar]
- 43.Yin H, Veer EV, Frontini MJ, Thibert V, O’Neil C, Watson A, Szasz P, Chu MW, Pickering JG. Intrinsic directionality of migrating vascular smooth muscle cells is regulated by NAD+ biosynthesis. J Cell Sci. 2012 doi: 10.1242/jcs.110262. [DOI] [PubMed] [Google Scholar]
- 44.Zhou M, Zhao Y, Ding Y, Liu H, Liu Z, Fodstad O, Riker AI, Kamarajugadda S, Lu J, Owen LB, Ledoux SP, Tan M. Warburg effect in chemosensitivity: targeting lactate dehydrogenase-A re-sensitizes taxol-resistant cancer cells to taxol. Mol Cancer. 2010;9:33. doi: 10.1186/1476-4598-9-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goellner EM, Grimme B, Brown AR, Lin YC, Wang XH, Sugrue KF, Mitchell L, Trivedi RN, Tang JB, Sobol RW. Overcoming temozolomide resistance in glioblastoma via dual inhibition of NAD+ biosynthesis and base excision repair. Cancer Res. 2011;71(6):2308–2317. doi: 10.1158/0008-5472.CAN-10-3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ho VW, Leung K, Hsu A, Luk B, Lai J, Shen SY, Minchinton AI, Waterhouse D, Bally MB, Lin W, Nelson BH, Sly LM, Krystal G. A low carbohydrate, high protein diet slows tumor growth and prevents cancer initiation. Cancer Res. 2011;71(13):4484–4493. doi: 10.1158/0008-5472.CAN-10-3973. [DOI] [PubMed] [Google Scholar]
- 47.Menssen A, Hydbring P, Kapelle K, Vervoorts J, Diebold J, Luscher B, Larsson LG, Hermeking H. The c-MYC oncoprotein, the NAMPT enzyme, the SIRT1-inhibitor DBC1, and the SIRT1 deacetylase form a positive feedback loop. Proc Natl Acad Sci USA. 2012;109(4):E187–E196. doi: 10.1073/pnas.1105304109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang B, Hasan MK, Alvarado E, Yuan H, Wu H, Chen WY. NAMPT overexpression in prostate cancer and its contribution to tumor cell survival and stress response. Oncogene. 2011;30(8):907–921. doi: 10.1038/onc.2010.468. [DOI] [PubMed] [Google Scholar]
- 49.Wosikowski K, Mattern K, Schemainda I, Hasmann M, Rattel B, Loser R. WK175, a novel antitumor agent, decreases the intracellular nicotinamide adenine dinucleotide concentration and induces the apoptotic cascade in human leukemia cells. Cancer Res. 2002;62(4):1057–1062. [PubMed] [Google Scholar]
- 50.Dordick RS, Brierley GP, Garlid KD. On the mechanism of A23187-induced potassium efflux in rat liver mitochondria. J Biol Chem. 1980;255(21):10299–10305. [PubMed] [Google Scholar]
- 51.Krahling H, Mally S, Eble JA, Noel J, Schwab A, Stock C. The glycocalyx maintains a cell surface pH nanoenvironment crucial for integrin-mediated migration of human melanoma cells. Pflugers Arch. 2009;458(6):1069–1083. doi: 10.1007/s00424-009-0694-7. [DOI] [PubMed] [Google Scholar]
- 52.Stock C, Mueller M, Kraehling H, Mally S, Noel J, Eder C, Schwab A. pH nanoenvironment at the surface of single melanoma cells. Cell Physiol Biochem. 2007;20(5):679–686. doi: 10.1159/000107550. [DOI] [PubMed] [Google Scholar]
- 53.Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, Warren JT, Bokesch H, Kenney S, Boyd MR. New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst. 1990;82(13):1107 –1112. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.