Abstract
Regucalcin (RGN) is a calcium (Ca2+)-binding protein widely expressed in vertebrate and invertebrate species, which is also known as senescence marker protein 30, due to its molecular weight (33 kDa) and a characteristically diminished expression with the aging process. RGN regulates intracellular Ca2+ homeostasis and the activity of several proteins involved in intracellular signalling pathways, namely, kinases, phosphatases, phosphodiesterase, nitric oxide synthase and proteases, which highlights its importance in cell biology. In addition, RGN has cytoprotective effects reducing intracellular levels of oxidative stress, also playing a role in the control of cell survival and apoptosis. Multiple factors have been identified regulating the cell levels of RGN transcripts and protein, and an altered expression pattern of this interesting protein has been found in cases of reproductive disorders, neurodegenerative diseases and cancer. Moreover, RGN is a serum-secreted protein, and its levels have been correlated with the stage of disease, which strongly suggests the usefulness of this protein as a potential biomarker for monitoring disease onset and progression. The present review aims to discuss the available information concerning RGN expression and function in distinct cell types and tissues, integrating cellular and molecular mechanisms in the context of normal and pathological conditions. Insight into the cellular actions of RGN will be a key step towards deepening the knowledge of the biology of several human diseases.
Keywords: Regucalcin, SMP30, Calcium, Apoptosis, Oxidative stress, Cell proliferation
Introduction
Regucalcin (RGN) was initially discovered in 1978 by Yamaguchi [1] and, although classified as a calcium (Ca2+)-binding protein, it does not contain the typical EF-hand Ca2+-binding motif [2]. The overall structure of RGN protein contains 24 β-strands forming 6 β-sheets able to bind diverse divalent cations (Ca2+, Mg2+, Mn2+ and Zn2+) [3–6]. The RGN ability to bind Ca2+ was recently confirmed by X-ray diffraction studies which have allowed the resolving of the crystal structure of human RGN protein bound to Ca2+ or Zn2+ cations. Although Ca2+ and Zn2+ ions bind to the same amino acid residues forming a unique metal binding site in a nearly identical coordination, an very much higher level of dissociation constant is documented for Ca2+ which could be relevant under non-physiological conditions, whereas elevated Ca2+ levels can occur [4].
The RGN gene is localised in the p11.3-q11.2 and q11.1-12 segments of the human and rat X chromosome, respectively [7, 8]. In both cases, the gene consists of seven exons [9–11] encoding a protein of 299 amino acid residues with an approximate molecular weight of 33 kDa [2, 12]. For this reason, together with the diminished expression of RGN in tissues of aged animals, Fujita and co-authors [12–14] named it senescence marker protein 30 (SMP30).
RGN is highly expressed in the liver and kidney cortex [12, 15, 16], but it has been detected in several other tissues [16, 17] in a broad range of vertebrate and invertebrate species [18–20]. The transcription of RGN gene is enhanced by several regulatory transcription factors upstream of the 5′ flaking region, namely the AP1, NF1-A1, RGPR-p117 and β-catenin [21]. Ca2+ levels modulate RGN expression in a process involving, for example, calmodulin (CaM) or protein kinase C (PKC) [22–24]. Also, Ca2+-independent mechanisms [25], hormonal factors and others have been described as regulating the levels of RGN in cells [11, 26–30]. Moreover, altered expression patterns of RGN have been associated with several disease conditions in both human and animal models [11, 31–39], which highlights the importance of this protein in cell biology.
RGN has been localised to the cell nucleus and cytoplasm [26, 40, 41], as well as in the mitochondrial fraction [42], and multiple physiological functions have been assigned to this curious protein. Among them is the ability of RGN to influence Ca2+ homeostasis through the regulation of Ca2+-pumping activity in the cell membrane, nucleus, microsomes, endoplasmic reticulum and mitochondria of various cell types [43]. It has also been associated with intracellular signalling pathways, since it regulates several Ca2+-dependent enzymes such as protein kinases, tyrosine kinases, phosphatases, phosphodiesterase, nitric oxide synthase and proteases [43–48].
In addition, the antioxidant properties of RGN in reducing intracellular levels of oxidative stress have also been described. This effect is achieved through modulation of the activity of enzymes involved in generation of oxidative stress as well as in the antioxidant defence [49–52].
Several reports using gene-silencing and over-expression approaches have pointed out a role of RGN in regulating cell death and proliferation. Although the mechanisms implicated in this control are not completely understood, it has been demonstrated that RGN can regulate DNA synthesis and fragmentation [53–56], and modulate the expression of oncogenes, tumour suppressor genes and cell cycle regulators [53, 54, 57], influencing survival and apoptotic pathways [58–60].
This review discusses the current knowledge about the expression and function of RGN in several cell types and tissues, exploring concepts from the molecular biology point of view in signalling pathways and systems biology. The potential roles of RGN in pathological situations will also be discussed.
RGN in non-pathological and pathological tissues and cell lines
RGN has been identified in a wide range of species from invertebrates to mammalian and non-mammalian vertebrates, also including fungi and bacteria [10, 12, 18–20, 61–65]. Protein sequence alignment and determination of amino acid identities show that RGN is highly conserved throughout evolution (Table 1). Human RGN (NP_690608) is highly homologuous with other primate proteins showing 97 % identity with that of orang-utan (Pongo abelii, NP_001127502). Percentages of amino acid identity with other mammals range from 88 to 91 %: 88 % with pig (Sus scrofa, NP_001070688), rat (Rattus norvegicus, NP_113734) and mouse (Mus musculus, NP_033086), 89 % with rabbit (Oryctolagus cuniculus, NP_001075472), and 91 % with cow (Bos taurus, NP_776382.1). The overall identity decreases in comparison with non-mammalian vertebrates showing 77 % identity with chicken (Gallus gallus, NP_990060), 70 % with frog (Xenopus laevis, NP_001079124) and 62 % with fish species (catfish, Ictalurus punctatus, NP_001187297, and zebrafish, Danio rerio, NP_991309). Homology with disk abalone (Haliotis discus, ABO26616), fruit fly (Drosophila melanogaster, NP_727586) and louse (Acyrthosiphon pisum, NP_001155519) RGN proteins range from 41 to 30 %, what still is noticeable high since these are invertebrate species. Also with fungi (Aspergillus fumigates, XP_751966) and bacteria (Bacillus cereus, NP_978918 and Agrobacterium tumefaciens, NP_353727) the percentage of amino acid identities are very high, being 26, 32 and 22 %, respectively. This demonstrates that the RGN gene is highly conserved among various vertebrate and invertebrate species which corroborates the idea of its well-conserved basic biologic function throughout evolution.
Table 1.
Overall percentage of amino-acid identities of the RGN protein among vertebrate, invertebrate, bacteria and fungi species, determined by Genedoc softwarea after performing Clustalw alignmentb
| Pongo abelii | Homo sapiens | Bos taurus | Sus scrofa |
Oryctolagus
cuniculus |
Rattus
norvegicus |
Mus musculus | Gallus gallus | Xenopus laevis | Danio rerio |
Ictalurus
punctatus |
Haliotis discus |
Drosophila
melanogaster |
Acyrthosiphon
pisum |
Bacillus cereus |
Aspergillus
fumigatus |
Agrobacterium
tumefaciens |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pongo abelii c | 299 | ||||||||||||||||
| Homo sapiens | 97 % | 299 | |||||||||||||||
| Bos taurus | 90 % | 91 % | 299 | ||||||||||||||
| Sus scrofa | 88 % | 88 % | 93 % | 299 | |||||||||||||
| Oryctolagus cuniculus | 88 % | 89 % | 89 % | 89 % | 299 | ||||||||||||
| Rattus norvegicus | 87 % | 88 % | 87 % | 85 % | 85 % | 299 | |||||||||||
| Mus musculus | 87 % | 88 % | 87 % | 85 % | 85 % | 94 % | 299 | ||||||||||
| Gallus gallus | 77 % | 77 % | 77 % | 76 % | 76 % | 76 % | 75 % | 299 | |||||||||
| Xenopus laevis | 69 % | 70 % | 69 % | 68 % | 71 % | 71 % | 70 % | 73 % | 299 | ||||||||
| Danio rerio | 61 % | 62 % | 62 % | 61 % | 62 % | 61 % | 61 % | 61 % | 61 % | 295 | |||||||
| Ictalurus punctatus | 61 % | 62 % | 62 % | 59 % | 62 % | 62 % | 62 % | 64 % | 61 % | 74 % | 299 | ||||||
| Haliotis discus | 40 % | 41 % | 41 % | 42 % | 40 % | 43 % | 42 % | 43 % | 41 % | 43 % | 45 % | 305 | |||||
| Drosophila melanogaster | 32 % | 32 % | 32 % | 32 % | 33 % | 32 % | 33 % | 32 % | 31 % | 32 % | 31 % | 29 % | 303 | ||||
| Acyrthosiphon pisum | 30 % | 30 % | 31 % | 31 % | 31 % | 30 % | 31 % | 30 % | 31 % | 32 % | 32 % | 33 % | 41 % | 326 | |||
| Bacillus cereus | 32 % | 32 % | 32 % | 32 % | 32 % | 32 % | 32 % | 32 % | 33 % | 32 % | 33 % | 31 % | 28 % | 26 % | 300 | ||
| Aspergillus fumigatus | 26 % | 26 % | 26 % | 26 % | 26 % | 26 % | 25 % | 25 % | 27 % | 24 % | 25 % | 24 % | 20 % | 21 % | 24 % | 281 | |
| Agrobacterium tumefaciens | 22 % | 22 % | 23 % | 23 % | 23 % | 23 % | 23 % | 25 % | 25 % | 21 % | 22 % | 22 % | 19 % | 21 % | 26 % | 19 % | 295 |
aNicholas KB and HB Nicholas GeneDoc: a tool for editing and annotating multiple sequence alignments. EMBNEW.NEWS 1997 4:14
bThompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Research 1997 25:4876–4882
cCommon names and protein accession numbers are provided in the text
RGN was first identified in the liver where it is highly expressed [1, 2, 15], but it has also been found in a variety of pathological and non-pathological tissues and cell lines [10, 11, 18, 24, 26, 32, 66–68]. Table 2 summarises the distribution of RGN mRNA and/or protein in non-pathological tissues and body fluids of several species. It is present in a variety of reproductive [26, 60, 66] and non-reproductive tissues [12, 16, 17, 38, 60, 69–75], as well as in plasma [16, 35, 37, 76–78], seminiferous tubules fluid [66] and insect saliva [79].
Table 2.
Regucalcin expression in non-pathological tissues and body fluids of distinct species
| Tissue | Species | Biomolecule | References |
|---|---|---|---|
| Liver | h, r, m | mRNA/Protein | [1, 2, 12, 13, 15–18, 60, 71] |
| Kidney | h, r, m | mRNA/Protein | [12, 13, 15–18, 60, 71] |
| Adrenal gland | r | mRNA | [60] |
| Lung | r | mRNA/Protein | [16, 60] |
| Heart | h, r | mRNA/Protein | [16, 17, 69, 74] |
| Bone | r | mRNA/Protein | [70, 72] |
| Skeletal muscle | r | Protein | [16, 71] |
| Diaphragm muscle | m | Protein | [38] |
| Epidermis | r | mRNA | [60] |
| Brain | r | mRNA/Protein | [17, 60] |
| Cerebral cortex | r, m | Protein | [16, 18] |
| Hippocampus | r | Protein | [16] |
| Locus ceruleus | h | Protein | [78] |
| Stomach | r, m | mRNA/Protein | [60, 71] |
| Pancreas | h | ? | [69] |
| Duodenum | r | Protein | [16] |
| Submandibular gland | m | Protein | [73] |
| Spleen | r | Protein | [16] |
| Mammary gland | h, r | mRNA/Protein | [11, 26] |
| Uterus | r | mRNA | [60] |
| Ovary | r | mRNA | [60] |
| Prostate | h, r | mRNA/Protein | [11, 26, 66] |
| Testis | h, r | mRNA/Protein | [16, 60, 66] |
| Epididymis | r | mRNA/Protein | [66] |
| Seminal vesicles | r | mRNA/Protein | [66] |
| Seminiferous tubules fluid | r | Protein | [66] |
| Plasma | h, r | Protein | [16, 35, 37, 76–78] |
| Saliva | ap | Protein | [79] |
r rat, m mouse, h human, ap pea aphid Acyrthosiphon pisum
Moreover, RGN was identified in several non-pathological cell lines such as pig kidney cells (LLC-PK1) [80], rat kidney proximal tubular epithelial cells (NRK52E) [57, 67], rat astrocytes (CTX TNA2) [32] and rat liver cells (Ac2F) [68].
One distinctive characteristic of RGN expression pattern is the significant diminished expression in tissues of aged animals [13, 14]. Studies on the expression of RGN from embryonic to senescent stages of life revealed that, in rat liver and kidney, a maximum of expression is reached within the first month after birth. Substantial amounts of mRNA and protein are maintained up to 3 or 6.5 months, respectively, in kidney and liver, and a marked decrease of RGN expression is found in older animals [14]. In addition, it is interesting to note the existence of gender differences in RGN expression levels. Hepatic RGN mRNA expression is higher in male rats [81] and mice [82]. RGN protein levels are lower in female liver, kidney and serum, but no significant alteration was found in spleen or cerebral cortex [16, 71]. As an exception, stomach of females presents higher RGN levels [71]. In any case, in aged animals, where a down-regulation of RGN expression is expected to occur, female rat livers still present minor levels in comparison with males [81, 83].
Several reports have described an altered expression of RGN in distinct pathological conditions. Proteomic analysis studies identified RGN as a down-regulated gene in a muscular dystrophy mouse model [38] and in acute liver failure [35]. In contrast, RGN was up-regulated in human brain of Parkinson’s disease patients [75]. Also, human testicular tissues with defective phenotypes of spermatogenesis displayed an increased expression of RGN in comparison with normal cases [34].
Concerning tumoral conditions, RGN expression was analysed in hepatomas [84, 85], breast and prostate cancer tissues [11], as well as in cancer cell lines of these and other tissues (see Table 3) [86–92]. Under-expression of RGN mRNA was first reported in rat chemical-induced hepatomas [84]. More recently, RGN was found to be under-expressed in human hepatocellular carcinoma (HCC) [37] and breast and prostate cancers [11]. Moreover, the diminished expression of RGN was associated with histological grade of infiltrating ductal carcinoma of breast and cellular differentiation of prostate adenocarcinoma [11]. High RGN immunoreactivity was detected in 60 % of non-neoplastic prostate tissues, while only 40 and 12 % of well-differentiated and poorly differentiated adenocarcinomas, respectively, displayed this expression pattern. Likewise, 90 % of non-neoplastic tissues of human breast showed high RGN immunoreactivity contrasting with 12 and 0 % of grade I and grade III human breast infiltrating ductal carcinomas, respectively [11]. A gene expression profile study of rat liver by means of cDNA microarrays demonstrated that down-regulated expression of RGN starts occurring in pre-neoplastic lesions before acquisition of a tumoral phenotype [93]. Other report also established a correlation between detection of RGN in serum and cellular differentiation of HCC [37], with 52,6 % of positivity in well-differentiated tumours (grade I–II) as opposed to 19 % in poorly differentiated tumours (grades III–IV).
Table 3.
Regucalcin expression in human and murine cancer cell lines
| Cell line | Cell type | Biomolecule | Expression | References |
|---|---|---|---|---|
| HepG2 | Human hepatocarcinoma | mRNA/protein | ↓ | [51, 85, 88–91] |
| Transplantable Morris | Rat hepatocarcinoma | mRNA | – | [84, 85] |
| H4-II-E | mRNA/protein | ↓ | [24, 86, 87] | |
| MC3T3-E1 | Mouse osteoblast | mRNA/protein | – | [92] |
| MCF-7 | Human breast cancer | mRNA/protein | ↓ | [11] |
| LNCaP | Human prostate cancer | mRNA/protein | ↓ | [11] |
↓ Down-regulated, – data not available
Also, altered expression patterns of RGN were observed in non-tumoral liver diseases. Liver biopsies from patients with non-alcoholic fatty liver disease showed diminished RGN levels, which seems to be dependent of the stage of disease [39]. On the other hand, human patients with acute liver injury [35] or chronic liver failure presented high serum levels of RGN [94]. Induced liver failure in mice by administration of galactosamine [77, 78], carbon tetrachloride [76] or lipopolysaccharide (LPS) [78] is also accompanied by elevated plasma levels of RGN.
Collectively, available data raised much evidence supporting the idea that RGN may be a useful biomarker tracking the onset and/or progression of tumour and non-tumour pathologies.
Hormonal factors and others regulating RGN expression
Several cell-signalling factors have been shown to regulate RGN gene expression (Fig. 1) in a variety of tissues. The cell-response triggered by a specific signalling factor can be different from tissue to tissue, and several studies have shown that the regulation of RGN expression may be tissue-specific, thereby presenting different responses to the same signalling factor.
Fig. 1.
The myriad of factors regulating regucalcin (RGN) gene expression. Some exert up-regulation effects (solid arrows) while others up-regulated or down-regulated RGN expression (dashed arrows) depending on the cell type, doses and/or time of stimulation. Bar-headed arrow represents inhibition. T3 Triiodothyronine, DHT 5α-dihydrotestosterone, E 2 17β-estradiol, PTH parathyroid hormone, LPS lipopolysaccharide, CCl 4 carbon tetrachloride, CaM calmodulin, PKC protein kinase C, ER estrogen receptor, PTHR parathyroid hormone receptor, CTR calcitonin receptor, InsR insulin receptor, TrK tyrosine kinase, TR thyroid hormones receptor, AR androgen receptor, MR mineralocorticoid receptor, OS oxidative stress
Calcium
Ca2+, a second messenger triggering important cell signalling pathways, is one of the main factors involved in the regulation of RGN gene expression in liver and kidney. Several reports have shown that rats treated with Ca2+ chloride (CaCl2) present higher levels of RGN mRNA at 30, 60 and 120 min after administration [15, 22, 29, 82, 95, 96]. The role of Ca2+ in regulating RGN expression is also observed in H4-II-E hepatoma cells [24, 25].
Regarding the mechanisms underlying Ca2+ regulation of RGN expression, it was hypothesised that it could involve the Ca2+-binding protein, CaM. When Ca2+ and trifluoperazine (TFP), an antagonist of CaM, were simultaneously administrated, the effect of Ca2+ increasing RGN mRNA expression was blocked, which suggests that expression of RGN mRNA is mediated by CaM [22, 23]. A Ca2+/CaM complex regulates the activation of several enzymes involved in signal transduction, such as cyclic adenosine monophosphate (cAMP) phosphodiesterase or PKC. The effect of phorbol 12-myristate 13-acetate (PMA), an activator of PKC, was evaluated on the expression of RGN. Different doses of PMA did not produced any effect on RGN mRNA expression, suggesting that the downstream effect of CaM is not triggered by PKC [23]. Although the effect of Ca2+ in rat liver was not mediated by PKC, it was demonstrated in H4-II-E cells that it is mediated by CaM and involves PKC activation [24, 25].
Thyroid and parathyroid hormones
It is well known that calcitonin and parathyroid hormone (PTH) play an important role in maintenance of Ca2+ homeostasis [97]. M. Yamaguchi’s group have investigated the role of calcitonin regulating RGN expression. In rat liver, the effect of CaCl2 in RGN mRNA expression is completely abolished in thyroparathyroidectomised (TPTX) rats, but calcitonin administration to TPTX rats treated with CaCl2 induced an increase of RGN mRNA expression. These results suggested that the Ca2+ effect in RGN mRNA expression is dependent on calcitonin [29]. On the other hand, experiments using HepG2 cells did not find any effect on RGN mRNA expression triggered by calcitonin [90]. Regarding kidney, the administration of calcitonin or PTH to TPTX rats treated with CaCl2 did not cause any alteration in RGN mRNA levels, suggesting that RGN expression is not stimulated by hormones involved in Ca2+ metabolism [22, 96]. In normal rat kidney proximal tubular epithelial NRK52E cells, the RGN mRNA expression was stimulated by treatment with PTH, but no effect was detected using calcitonin [30, 67].
RGN seems to play an important role in maintaining bone homeostasis [98], since it has been described that bones of transgenic rats over-expressing RGN (RGN knock-in) are more fragile than that of wild-type animals [70]. This again raised the question of whether PTH may regulate RGN expression, and, in fact, treatment of osteoblastic MC3T3-E1 cells with PTH induced an increase in RGN mRNA transcripts [99]. On the other hand, both male and female RGN knock-in rats display significantly decreased Ca2+ levels in femoral diaphyseal and metaphyseal [70]. A recent report described that exogenous RGN stimulates osteoclastogenesis and suppresses osteoblastogenesis which occurs through the activation of the nuclear factor-kappa B (NF-kB) signalling transduction pathway [100]. Thus, the known effects of PTH in bone reabsorption may be mediated by increased expression of RGN.
Concerning T3 and T4 hormones, T3 treatment of female rats induced an increase in RGN mRNA and protein levels for up to 12 h of stimulation, which declined after 24 h and disappeared after 5 days [28]. No effect has been observed in response to T4 treatment [101], likely explained by the low biologic activity of this hormone. Recently, it was demonstrated that RGN mRNA is down-regulated by T3 in MCF-7 cells needing activated thyroid hormone receptors (TRs), but does not requiring high affinity between TR and thyroid-responsive elements on RGN gene promoter [102]. Down-regulation of RGN expression seems to be mediated through modification of histone acetylation triggered by T3 treatment [102].
Steroid hormones
RGN mRNA expression in rat kidney is suppressed by saline administration [103], and Ca2+-induced up-regulation of RGN mRNA expression is weakened by saline ingestion [96], suggesting the involvement of adrenal hormones on the regulation of RGN expression. The levels of RGN mRNA in the kidney were clearly diminished by administration of aldosterone. On the other hand, dexamethasone induced an increase in RGN mRNA levels, and hydrocortisone administration had no effect. The effect of dexamethasone is inhibited by administration of cycloheximide, suggesting that the effect of dexamethasone is dependent of newly synthesised proteins [27]. However, these effects are not clearly understood because adrenalectomy in rats caused a decrease in RGN mRNA levels, an effect not restored by dexamethasone administration [103]. On the contrary, treatment of kidney NRK52E cells with aldosterone stimulated RGN mRNA expression [30, 67]. These results suggested that other hormones synthesised by the adrenal gland may be involved, or a synergetic effect between them is required to restore or regulate the levels of RGN mRNA in cells. More studies are needed to clarify the role of adrenal hormones regulating RGN expression. Vitamin D has no effect on RGN expression in NRK52E cells [30, 67], while it seems to decrease its expression in MC3T3-E1 cells [99].
The effect of sex steroid hormones, androgens and estrogens on RGN expression has been evaluated in liver, kidney, bone, prostate, breast, and testis tissues or cell lines. In rat liver, the expression of RGN was not altered by orchidectomy or treatment with testosterone, suggesting that RGN expression in the liver is androgen-independent [13]. Also, in female rats, the ovariectomy did not cause a significant modification of RGN mRNA levels in the liver. In addition, the administration of 17β-estradiol (E2) to ovariecomised rats did not induced alterations in RGN mRNA expression [81]. However, other studies have shown that administration of E2 induced a remarkable increase of RGN mRNA levels both in rat and mice liver [101, 104]. This up-regulation in response to E2 has also been observed in MC3T3-E1 cells [99]. One report demonstrated that E2 decreases RGN mRNA levels in rat kidney [27]. The levels of RGN mRNA increased in the prostate of orchidectomised rats, an effect abrogated by E2 treatment for 7 days. The levels of RGN mRNA in the prostate of E2-treated rats are similar to those found in intact animals, suggesting that normal levels of E2 may down-regulate RGN mRNA expression [26]. However, it is possible that the levels of RGN mRNA in the prostate of intact animals could also be maintained by the paracrine effect of testosterone metabolite dihydrotestosterone (DHT). In fact, another study showed that DHT down-regulates RGN mRNA expression in human prostate cancer LNCaP cells by direct action of androgen receptor (AR), but requiring de novo protein synthesis [11].
RGN expression is higher in the mammary gland of ovariectomised rats in comparison with intact animals, but this effect is inhibited by treatment with E2 for 7 days [26]. In human breast cancer MCF-7 cells, E2 had a biphasic effect controlling RGN mRNA expression. Initially, E2 induced an increase in RGN mRNA levels at 6 and 12 h, but a down-regulation was observed after 24 and 48 h of stimulation. Moreover, the effects of E2 on RGN mRNA expression were not abrogated in the presence of ICI 182,780 [estrogen receptor (ER) antagonist], and E2-bound to BSA produced the same effect as E2, suggesting the involvement of a membrane-bound ER [11]. These results demonstrated that long exposure to E2 decrease the expression of RGN mRNA in both rat mammary gland and MCF-7 cells.
Also, in the testis, the effect of sex steroid hormones regulating RGN expression has been reported. DHT up-regulates RGN expression in rat seminiferous tubules cultured ex vivo, an effect blocked in the presence of flutamide (AR antagonist) suggesting the involvement of classical genomic mechanism of gene expression through AR [66].
Oxidative stress
Oxidative stress reduction trough calorie restriction (CR) is known to have anti-aging and antioxidative properties [105]. It has been shown that CR inverts the characteristic down-regulation of RGN expression in the liver and kidney of aged rats [106]. Rats fed ad libitum for 6, 12, 18 and 24 months showed a decrease of RGN expression when compared to animals under CR. Moreover, rats treated with LPS, which stimulates the production of reactive oxygen species (ROS), presented lower levels of RGN [106]. It has also been reported that treatment with carbon tetrachloride, an acute oxidative stress inducer, suppresses the RGN expression in rat liver during the necrotic phase [107]. These results suggest that the down-regulation of RGN expression in older animals is eventually due to the increased oxidative stress characteristic of the aging process.
Effects of RGN on calcium homeostasis
A tight regulation of intracellular Ca2+ concentrations is essential for maintenance of fundamental biological functions and oscillations between 50 and 150 nM promote activation of specific and diverse signalling pathways that are involved in both physiological and pathophysiological conditions [108–111]. Several studies have demonstrated that RGN plays a role regulating Ca2+ homeostasis through direct and/or indirect regulatory actions at plasma membrane Ca2+-ATPase (PMCA), sarco/endoplasmic reticulum Ca2+-ATPase (SERCA), nuclear outer membrane SERCA pumps, and increasing mitochondrial Ca2+ uptake by the mitochondrial Ca2+ uniporter (MCU) [43]. Although no effects have been described for RGN on Ca2+ channel activity, RGN over-expression in NRK52E normal rat kidney proximal tubular epithelial cells suppressed L-Type Ca2+ channels and Ca2+-sensing receptor mRNA expression [112].
RGN transfection in HepG2 cells [89] and addition of RGN to rat liver plasma membranes significantly increased PMCA activity [113, 114]. This effect was inhibited by N-ethylmaleimide (NEM) [115], a modifying reagent of sulfhydryl groups (SH), which suggests that PMCA activity induced by RGN implies the regulation of ATPase SH groups. Accordingly, NEM blocked the activator effect of dithiothreitol (DTT), which is a SH group protective compound [116]. In addition, it is thought that RGN regulates PMCA activity by direct binding to the plasma membrane [113], since the stimulatory effect is blocked by digitonin, a solubilising reagent of membrane lipids [116]. Elevation of Ca2+ levels in the liver induced by oral administration to rats also increases PMCA activity. This effect is abolished in the presence of anti-RGN antibody [117, 118], reflecting the role of endogenous RGN on the control of PMCA activity.
CaM activates PMCA through direct interaction with a specific CaM-binding domain located in the cytosolic tail of the pump [119]. Interestingly, some reports have pointed out that the RGN effect regulating PMCA activity may be CaM-dependent. The CaM inhibitor TFP has been shown to inhibit the RGN effect on PMCA activity in HepG2 cells over-expressing the protein [89]. Administration of carbon tetrachloride increased cytosolic Ca2+ levels in rat liver as a consequence of tissue injury and impairment of the RGN effect on PMCA activity [120].
The RGN role regulating PMCA activity seems to be Ca2+-dependent. RGN-induced Ca2+ uptake and increased PMCA activity in rat kidney cortex basolateral membranes are enhanced in the presence of Ca2+ in a dose-dependent manner [121].
Considering RGN influence on SERCA function, its effect has also been shown in enhancing pump activity [74, 121–123]. Moreover, increased mRNA and protein levels of SERCA were observed in COS-7 cells over-expressing RGN [124]. Thapsigargin, a specific microsomal ATPase inhibitor, and digitonin clearly decrease RGN-induced SERCA activity in rat liver microsomes [123], suggesting a membrane association. In opposition to this, A23187 increased RGN-induced ATPase activity [123]. RGN presumably acts on SERCA SH groups, since NEM and DTT, respectively, promote a decrease and an increase in RGN-induced SERCA activity [123]. Similar results were described for rat kidney cortex [121] and heart microsomes [74]. In addition, vanadate, a phosphorylation inhibitor, significantly decreased RGN-induced SERCA activity in kidney microsomes, suggesting a phosphorylation effect of RGN at enzyme sites [121]. Contrastingly with the previous observations, in rat brain microsomes, RGN decreased SERCA activity, an effect that was weakened with increasing age [125].
It has been reported that RGN can be found in the cell nucleus [26, 40, 41, 126], and SERCA pumps are also located in the nuclear outer membrane which is an extension of the endoplasmic reticulum [127]. RGN did not change Ca2+ uptake into rat liver nucleus [128] but reduced nuclear SERCA activity, while anti-RGN antibody caused the opposite effect [129]. Moreover, thapsigargin, NEM or vanadate prevented the effect of anti-RGN antibody increasing nuclear SERCA activity [129]. On the other hand, CaM enhanced the increased SERCA activity by the anti-RGN antibody, an effect that is reduced in the presence of TFP. Thus, RGN seems to modulate CaM effects on nuclear SERCA and to promote nuclear Ca2+ release in a way not so far clarified [128, 129].
RGN also regulates cytosolic Ca2+ concentration by stimulation of Ca2+ uptake into the mitochondria matrix of rat liver [130, 131] and kidney cortex [132] cells, likely through MCU. In fact, it has been reported in liver [131], kidney cortex [132], heart [133] and brain [134] that MCU inhibitors, such as ruthenium red or lanthanum chloride, prevent Ca2+ uptake as well as RGN-induced mitochondrial ATPase activity. In the same way, increased mitochondrial ATPase activity was observed in heart and brain of RGN knock-in rats [133, 134]. In liver and kidney cortex of wild-type animals, digitonin and vanadate reduced mitochondrial ATPase activity even in the presence or absence of RGN, whereas CaM and DTT promoted an opposite effect [131, 132]. This means that RGN may regulate cytosolic Ca2+ homeostasis by acting on SH groups of mitochondrial ATPase and/or the MCU channel, depending on CaM, since ATPase activity is decreased by TFP in kidney cortex [132].
The described actions of RGN controlling Ca2+ pump activity and intracellular Ca2+ levels highlight the importance of this protein maintaining homeostasis and appropriate signalling for this ion, which may have profound implications in pathophysiologic conditions as a result of Ca2+ dysregulation. Nevertheless, the regulatory role of RGN in Ca2+ homeostasis and signalling needs to be further explored and extended to contemplate potential effects on Ca2+ channels or Ca2+-sensing receptor activities.
RGN and calcium-dependent intracellular signalling
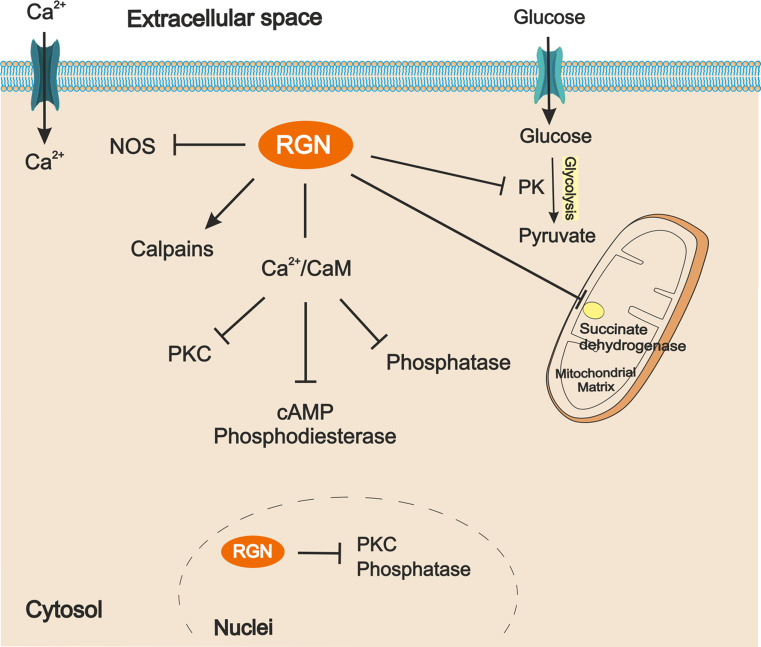
Beyond its capability to regulate cytosolic Ca2+ levels, RGN is also able to modify the activity of a wide range of Ca2+-dependent enzymes involved in intracellular signalling and cell metabolism (Fig. 2).
Fig. 2.
Schematic representation of regucalcin (RGN) actions on enzymes involved in intracellular signaling and metabolism. Arrows indicate activation by RGN and bar-headed arrows represent inhibition. RGN decreases NOS, PK and succinate dehydrogenase enzymes activity. RGN also inhibits Ca2+/CaM dependent activation of PKC, cAMP phosphodiesterase and phosphatases.NOS nitric oxide synthase, PK pyruvate kinase, CaM calmodulin, PKC protein kinase C
A Ca2+-dependent enzyme that is regulated by RGN is the 5′-nucleotidase. Ca2+ inhibits 5′-nucleotidase activity which is reverted by RGN [135]. At the metabolic level, mitochondrial succinate dehydrogenase activity is increased by Ca2+, whereas RGN induced an opposite effect [136]. So, mitochondrial Ca2+ regulation by RGN has an indirect effect on cell energy production. Also, enzymes such as glycogen phosphorylase a, an enzyme involved in glycogen hydrolysis in liver and muscle (glucogenolysis), pyruvate kinase (glycolysis) and microsomal glucose-6-phosphatase (gluconeogenesis), which are activated by Ca2+, have their activities reverted to control levels by RGN [137–139]. Moreover, ATP hydrolysis by adenosine 5′-triphosphatase in rat brain is increased by Ca2+, while RGN promoted an inhibitory effect as demonstrated by RGN-antibody administration [140]. This RGN action seems to be independent of CaM, since it is not inhibited by CaM or TFP [140]. In contrast, Ca2+-induced rat liver pyruvate kinase activity is reverted by RGN, and also by CaM [138]. Fructose-1.6-diphosphatase enzyme activity in rat and rabbit liver is found to be increased by Ca2+ and CaM, and diminished by the addition of RGN or CaM inhibitor, suggesting that effects may be mediated by Ca2+-CaM [141]. On the other hand, cytosolic deoxyuridine 5′-triphosphatase activity is decreased by Ca2+ and stimulated by RGN [142]. Altogether, available data indicate diverse regulatory roles of RGN on enzymes involved in different cellular energy production pathways, such as oxidative phosphorylation, glucogenolysis, gluconeogenesis and glycolysis, as well as in energy conversion enzymes. Moreover, it has also been suggested that RGN exerts its effects by direct actions on the regulation of CaM or CaM-dependent proteins.
cAMP, as well as Ca2+, is an ubiquitous second messenger essential to the control of cellular homeostasis [143]. The adenylyl cyclases (ACs) that synthesise cAMP are regulated by Ca2+ signalling pathways [143, 144] and activated by heterotrimeric G proteins [144]. In turn, cAMP phosphodiesterases are responsible for cAMP degradation. Thus, levels of cAMP are regulated by the activity balance of ACs and cAMP phosphodiesterases both activated by Ca2+/CaM [143, 145]. In rat liver and kidney, RGN inhibited Ca2+/CaM-dependent activation of cAMP phosphodiesterase [146, 147], an effect abolished by high Ca2+ levels and in the presence of TFP [146, 147]. Thus, RGN action on phosphodiesterase appears to be related to the capacity of Ca2+ binding, as it seems to be dependent on CaM.
Nitric oxide (NO) is a signalling agent produced by the nitric oxide synthase (NOS), which is regulated by free intracellular Ca2+ concentrations and CaM [148]. The addition of RGN to cytosol preparations from rat liver, kidney, heart and brain lead to a significant decrease of NOS activity [48, 149–151]. Furthermore, both Ca2+ and anti-RGN antibody stimulated NOS activity in rat liver, heart and brain cytosol, while it is blocked in RGN knock-in rats [150–152]. RGN over-expression in kidney proximal tubular epithelial NRK52E cells [153] also demonstrated the decrease in NOS activity even in the presence of Ca2+ and CaM, while, in MC3T3-E1 cells, anti-RGN antibody reverted this effect [92]. The mechanism by which RGN regulates NOS activity may be related with CaM, since, in liver and kidney, it is impaired in the presence of the CaM antagonist, TFP [48, 149, 150].
Calcineurin (CaN) is a CaM-dependent serine/threonine phosphatase widely distributed in mammalian tissues [154, 155]. It has been demonstrated that RGN significantly reduces cytosolic and nuclear phosphatase activities in the liver [156, 157], while anti-RGN antibody promotes the expected opposite effects [40, 156, 157]. Also, phosphotyrosine and other phosphatases activities in rat kidney cortex cytosol were significantly inhibited by RGN [158, 159]. Cytosolic and nuclear phosphotyrosine and phosphoserine activities were found to be diminished by vanadate, used as a tyrosine phosphatase inhibitor, and by cyclosporin A, a CaN inhibitor, even in the presence of anti-RGN antibody [159, 160]. Moreover, Ca2+ administration elevates cytosolic and nuclear phosphatase activity in rat kidney cortex, an effect abolished by the addition of RGN to the reaction mixture [161]. RGN suppressive effects on phosphatases activity were also demonstrated in rat heart cytosol [162]. RGN also presents a CaM-dependent inhibitory effect on tyrosine, serine and threonine phosphatases in rat brain cytosol [163] and in neuronal cells [164]. RGN suppressive role on phosphotyrosine activity in brain nucleus and microsomes has also been demonstrated, displaying attenuated effects with increasing age [165, 166].
RGN effect on Ca2+/CaM-dependent protein kinases has also been evaluated in several reports. In rat liver cytosol, an inhibitory role of RGN in protein kinase activity has been described, which is reverted with anti-RGN antibody [167, 168]. Moreover, RGN, which does not have kinase activity, decreased Ca2+ or phospholipid-stimulated cytosolic PKC activity [169]. Nuclear PKC activity in the liver was also inhibited by RGN, whereas the use of anti-RGN antibody led to the enhancement of PKC activity [46]. These findings demonstrated the regulatory role of RGN in cytosolic and nuclear Ca2+/CaM-dependent PKC activity. Similar results were obtained in rat renal cortex with increased PKC activity in response to Ca2+/CaM, phospholipids (phosphotidylserine or dioctanoygycerol), and PMA; RGN or TFP significantly inhibited enzyme activity [170, 171]. Also, in rat brain cytosol and neuronal cells, RGN exerted an inhibitor effect on protein kinase activity by preventing its activation by Ca2+/CaM or dioctanoyglycerol [172, 173]. This evidence is indicative of an effective regulatory function of RGN on PKC activity in rat liver, kidney and brain being tightly dependent of Ca2+/CaM pathway.
Calpains are a family of Ca2+-dependent activated neutral cysteine proteases that are ubiquitously expressed or tissue-specific [174]. The ubiquitous μ- and m-calpain isoforms are known to be activated in vitro by μM and mM Ca2+ concentrations [175]. Calpains have been described to have important roles in embryogenesis, cell cycle progression, apoptosis, necrosis, proliferation, differentiation, migration, meiosis and mitosis, besides being related to numerous diseases, such as muscular dystrophy, cardiac and cerebral ischemia, traumatic brain injury or rheumatoid arthritis [174, 175]. Calpain proteolytic activity is enhanced by RGN in rat liver and kidney cortex, even in the absence of Ca2+, and prevented by anti-RGN antibody and calpastatin, a calpain-specific inhibitor [45, 176–178]. RGN-induced proteolitic activity seems to be independent of serine proteases and metaloproteases [176, 178]. However, it may be associated with SH groups of cysteinyl-proteases, since it is increased by DTT and inhibited by NEM and leupeptin, an SH group inhibitor of proteases [176–178].
Cytoprotective effects of RGN
Alongside its well-recognised function in Ca2+ homeostasis and Ca2+-dependent intracellular signalling pathways, RGN has been identified as a gluconolactonase (GNL) [6]. In mammals, GNL activity is involved in the penultimate step of l-ascorbic acid (AA) synthesis in the liver. AA is a well-known antioxidant with free radical scavenger ability and a cofactor in metal-dependent oxygenases [179]. Genetic mutations in the gene, that codify the enzyme required for the last step of AA biosynthesis pathway, oblige human, non-human primates and guinea pigs to obtain it through diet [61, 179], while rodents maintain the ability to produce it endogenously. The establishment of RGN knock-out (RGN-KO) mice generated an animal model unable to synthesise vitamin C(VC). These animals develop scurvy symptoms [6] and pulmonary emphysema [180] when fed with a restrained VC diet. The RGN-KO model allowed the confirmation of an alternative AA synthesis pathway in vivo throughout D-glucono-γ-lactone [6] and demonstrated the antioxidant properties of RGN [31, 181, 182]. NADPH oxidase enzyme activity, an endogenous source of oxidative stress [183], and anion superoxide levels are increased in the brain of RGN-KO mice [181, 182]. Superoxide dismutase (SOD) and catalase activity remained unchanged, while glutathione peroxidase activity was reduced in animals without RGN [181, 183].
However, evidence of RGN protective role against oxidative stress is essentially reported in mice lungs. RGN-KO mice exposed to cigarette smoke showed elevated levels of protein carbonyls, an oxidative biomarker, in comparison with wild-type animals, and were the only group in which oxidase glutathione levels were sufficiently elevated to be measured [184].
RGN antioxidative capacity has also been established in other animal models and cell lines. RGN over-expression in the mouse embryonic carcinoma P19 cell line increased cell viability, protecting cells from oxidative stress-induced by tert-butyl hydroperoxide [49]. An intracellular favourable redox state has also been demonstrated in HepG2 cells transfected with RGN, which displayed diminished ROS levels both in mitochondria and post-mitochondrial fractions, as well as decreased lipid peroxidation levels and reduced protein levels and activity of glutathione and SOD, respectively [51]. In addition, SOD activity was enhanced in normal rat liver and heart in the presence of exogenous RGN, as well as in RGN knock-in rats [50, 52].
NO, produced by the activity of NOS, is involved in NO-dependent signal transduction pathways. However, it is a reactive species influencing cell redox state and being associated with modification of proteins, lipids, DNA and structure of organelles when present in cells at high levels [185]. In rat brain, NOS activity is increased by anti-RGN antibody, while enhancement of RGN in the brain cytosol of young and old female rats reduced the enzyme activity [48]. This suppressor role of RGN in NOS activity is also found in rat liver, kidney and heart cytosol, including in the presence of EGTA or TFP [149–151]. Similar results have been described in H4-II-E [56, 186], NRK52E [153] and MCT3-E1 [92] cells over-expressing RGN.
Neurodegenerative diseases, such as Alzheimer’s and Parkinson’s, are associated with oxidative stress deregulation. Kainate (KA) is an agonist for a subtype of ionotropic glutamate receptor that increases the ROS levels and disrupts Ca2+ homeostasis, leading to neuronal loss mainly in the hippocampus [187, 188], which has been used to generate models of neurodegenerative diseases. The levels of RGN protein in the rat hippocampus were significantly increased in response to KA treatment [32]. A similar effect on RGN expression has also been shown in rat astrocytes CTX TNA2 cells treated with KA, which is mediated by the ERK signalling pathway [32]. Accordingly, RGN-KO mice are more sensitive to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine, a neurotoxin used to induce Parkinson’s disease models, presenting significantly increased ROS levels in the striatum as well as microglial activation in comparison with wild-type counterparts. Moreover, RGN deficiency leads to astrocytes inactivation and decrease of brain-derived neurotrophic factor as result of blockage of ’sERK signalling pathway [31].
Overall, available studies, and particularly the information from RGN-KO mice, have demonstrated the influence of RGN maintaining physiological levels of oxidative stress and consequently its protective role against oxidative damage.
Role of RGN in cell death and proliferation
Since RGN is a protein involved in the regulation of intracellular Ca2+ levels, modulation of several cellular signalling pathways, and also with antioxidant properties, it is not surprising that its role in cell survival and proliferation has been questioned by many investigations.
It is well established that NO overproduction is a condition associated with many pathologies underlying deregulation of cell proliferation in cases of male infertility [189] and cancer [190]. In hypoxic conditions, ROS and Ca2+ levels are found to be decreased (~60 %) in cardiomyocytes over-expressing RGN, which presented lower cell death induced by H2O2 treatment [191]. Also, mouse embryonic carcinoma P19 RGN-transfected cells presented increased cell viability in response to butylhydroperoxide-induced oxidative stress in comparison with mock-transfected cells [49]. In H4-II-E cells, LPS treatment promoted a decrease of NOS activity and cell number, effects that were reverted in RGN over-expressing cells [192].
Several other reports have demonstrated the RGN suppressor effect on cell proliferation [54, 55, 57]. NRK52E and H4-II-E cells over-expressing RGN presented a lower index of proliferation than mock-transfected cells [53–55, 57], which was associated with a decrease of DNA synthesis activity [55, 193]. In addition, intracellular increase of RGN down-regulated mRNA expression of c-myc and H-ras, while up-regulating p53 and p21, which suggested that RGN suppresses cell proliferation by modulating the expression of proto-oncogenes and tumour suppressor genes [53, 54, 57]. Also, the expression of c-Jun and chk2 cell cycle regulators is decreased in RGN-transfected NRK52E cells [57].
However, and contrastingly with the previous information, it has also been described that cells over-expressing RGN do not undergo cell cycle arrest promoted by cell cycle inhibitors or other factors. The cell cycle inhibitors sulforaphane, butyrate and roscovitine diminish proliferation of wild-type cells, though this is not observed in RGN-transfected cells [54, 57]. Bay K 8644, genistein, wortmannin, an inhibitor of phosphatidylinositol 3-kinase, PD 98059, an ERK inhibitor, or dibucaine, an inhibitor of Ca2+-dependent protein kinase all hampered cell proliferation, an effect reverted by RGN over-expression [54].
There is also evidence of the involvement of RGN in the regulation of apoptosis. It has been reported that RGN affects rat liver nuclei function by suppressing Ca2+-induced DNA fragmentation in the presence or absence of CaM [194]. In fact, the enhancement of DNA fragmentation in NRK52E or H4-II-E cells, after incubation with Bay K 8644, thapsigargin, LPS, insulin or IGF-I, was suppressed by RGN over-expression in both cell lines [56, 195, 196]. Thus, accordingly, cell death of H4-II-E or NRK52E wild-type cells promoted by tumour necrosis factor-α (TNF-α) or thapsigargin was prevented in RGN-transfected cells [56, 196]. RGN over-expression in hepatocarcinoma HepG2 cells also rescues cell death induced by intracellular Ca2+ overload promoted by the Ca2+ ionophore A23187 [89]. In MCF-7 cells, the down-regulation of RGN expression, achieved by thyroid hormone treatment or silencing of the RGN gene, led to an increase of apoptosis [102].
RGN effects suppressing apoptosis may be related to the Akt survival signalling pathway. NRK52 RGN-transfected cells displayed increased levels of both Bcl-2 and Akt-1 mRNAs [196], while an activation of Akt was observed in HepG2 cells over-expressing RGN [58]. TFP attenuated apoptosis of HepG2 RGN-transfected cells and inhibited Akt activation [58]. Thus, enhancement of cell survival by RGN seems to depend on the interplay with CaM and the activation of the Akt pathway.
RGN anti-apoptotic effects are also evident on the basis of studies using knock-out animals. Primary cell cultures of hepatocytes from RGN-KO mice are highly susceptible to apoptosis induced by TNF-α and actinomycin D [60]. Accordingly, caspase 8 activity was two-fold greater in the hepatocytes of RGN-KO mice whereas no differences were observed in NF-kB activation [60].
Anti-Fas antibody administration to mice has been previously shown to induce severe damage of the liver by apoptosis [197]. RGN-KO mice presented a markedly increase of liver injury by anti-Fas antibody administration, while RGN +/− mice had an intermediate susceptibility between RGN−/− and wild-type animals [60]. Therefore, the RGN anti-apoptotic effect seems to be related to the Fas activation pathway and not with NF-kB activation. Inhibition of transforming growth factor-β (TGF-β) pathway through deletion of the Smad3 gene makes the hepatocytes of Smad3-KO mice more resistant to radiation-induced apoptosis than those of wild-type animals, which is concomitant with significantly increased levels of RGN [59].
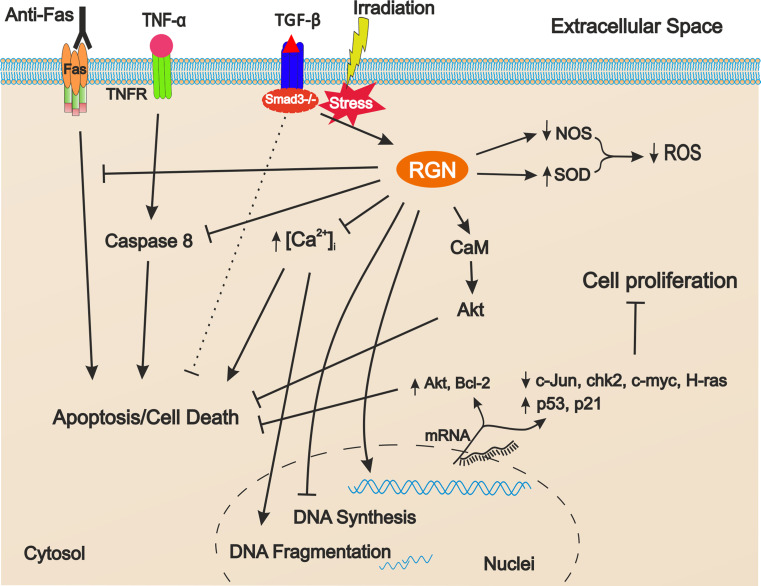
Altogether, the existing findings indicate that RGN, despite apparently having opposite functions, acting as a suppressor of both cell death and proliferation, may have a role in the control of the cell cycle, by modulation of the cell survival and death pathways (Fig. 3). Testis is one of the tissues where a tight balance between germ cell survival and apoptosis is required, which is the basis for a successful spermatogenesis and thus male fertility. Interestingly, in a recent report, it was shown that RGN expression is augmented in cases of hypospermatogenesis [34], but further research is needed to determine whether the increased RGN expression is the cause of insufficient production of spermatozoa by blockage in cell proliferation. It is also noteworthy that diminished expression of RGN is found in both rodent and human cancer tissues [11, 37, 93, 198, 199], which is also correlated with the degree of cellular differentiation of breast, prostate and liver carcinomas [11, 37]. In the near future, it will be essential to determine whether down-regulation of RGN is a selective event for malignant transformation or if it is a consequence of the cancer status. Nevertheless, dual distinctive roles over the control of cell proliferation and malignancy have also been reported for other proteins, for example the Ski-novel protein (SnoN). SnoN is a member of the Ski family proteins that is ubiquitously expressed in embryonic and adult tissues possessing. within tumorigenesis. both pro-oncogenic and anti-oncogenic activities [200]. SnoN over-expression in mice mammary gland leads to an increase of adenocarcinoma formation, although heterozygous mice that lack an extra copy of the gene are more susceptible to carcinogen-induced tumours [200]. At the same time, and as anti-oncogenic, SNO functions negatively regulated the TGF-β pathway while stabilising the p53 conformation and inducing premature senescence [200, 201]. There are also examples of proteins with a dual role controlling both apoptosis and the cell cycle. This is the case of Survivin which belongs to the inhibitor of the apoptosis protein family. It is localised both outside and inside the cell with pools at cytoplasmic, nuclear and mitochondrial compartments. When present at mitochondria, Survivin protect cells from apoptosis while its nuclear translocation facilitates cell cycle entry and progression [202].
Fig. 3.
Schematic representation of the mechanisms involved in the regucalcin (RGN) role controlling cell proliferation and apoptosis. Arrows indicate activation and bar-headed arrows represent inhibition. RGN diminishes the production of ROS, blocks increases of intracellular calcium, inhibits caspase 8 activity, enhances activity of Akt pathway and increases the expression of apoptosis inhibitors Akt-1 and Bcl-2 leading to inhibition of apoptosis. RGN also blocks apoptosis induced by Fas system. Dashed bar-headed arrow indicates the inhibition of apoptosis in Smad 3 knock-out animals concomitant with increased levels of RGN. In turn, RGN increases the expression of p53 and p21 proteins while repressing the expression of c-Jun, chk2, c-myc and H-ras genes, thus blocking cell proliferation. TNF-α tumor necrosis factor, TNFR TNF-α receptor, TGF-β tumor growth factor, NOS nitric oxide synthase, SOD superoxide dismutase, ROS reactive oxygen species, CaM calmodulin
In summary, it is likely that RGN plays an important role in cell physiology by maintaining a tight balance between cell proliferation and apoptosis (Fig. 3).
Final remarks
In recent years it has been demonstrated that RGN is a protein highly conserved throughout the evolutionary line, from vertebrates to invertebrate species, which indicates its relevant role in basic cell biologic processes. This particular and unique protein has a preponderant role in Ca2+ homeostasis, which is extensive in the control of cell signalling pathways, as well as the regulation of cell apoptosis and proliferation, and also of oxidative stress levels. The involvement of RGN in those processes has also been evaluated in pathological conditions, its association with several human diseases that range from muscular dystrophy and infertility to neurodegenerative diseases and carcinomas becoming evident. Moreover, RGN is a protein present in patients’ serum which has been correlated with stages of disease, highlighting its usefulness as a potential biomarker for monitoring disease onset and progression.
At the present moment, research efforts are needed to disclose the role of RGN over the control of the cell cycle and intracellular signalling mechanisms. Moreover, since the RGN protein can be detected in the nuclear compartment, the identification of putative partners for RGN actions in the nucleus is also clearly warranted. Thoroughly deciphering the RGN actions in cell physiology will be a research challenge in the coming years, which will also contribute to a better understanding of the biology of several human diseases.
Acknowledgments
This work was partially supported by Portuguese Foundation for Science and Technology (FCT) under Program COMPETE (PEst-C/SAU/UI0709/2011). Ricardo Marques, Cátia Vaz and Sara Correia were funded by FCT fellowships (SFRH/BD/66875/2009, SFRH/BD/70316/2010 and SFRH/BD/60945/2009, respectively).
References
- 1.Yamaguchi M, Yamamoto T. Purification of calcium binding substance from soluble fraction of normal rat liver. Chem Pharm Bull (Tokyo) 1978;26(6):1915–1918. doi: 10.1248/cpb.26.1915. [DOI] [PubMed] [Google Scholar]
- 2.Shimokawa N, Yamaguchi M. Molecular cloning and sequencing of the cDNA coding for a calcium-binding protein regucalcin from rat liver. FEBS Lett. 1993;327(3):251–255. doi: 10.1016/0014-5793(93)80998-A. [DOI] [PubMed] [Google Scholar]
- 3.Kondo Y, Ishigami A, Kubo S, Handa S, Gomi K, Hirokawa K, Kajiyama N, Chiba T, Shimokado K, Maruyama N. Senescence marker protein-30 is a unique enzyme that hydrolyzes diisopropyl phosphorofluoridate in the liver. FEBS Lett. 2004;570(1–3):57–62. doi: 10.1016/j.febslet.2004.06.028. [DOI] [PubMed] [Google Scholar]
- 4.Chakraborti S, Bahnson BJ. Crystal structure of human senescence marker protein 30: insights linking structural, enzymatic, and physiological functions. Biochemistry. 2010;49(16):3436–3444. doi: 10.1021/bi9022297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamaguchi M. Role of regucalcin in calcium signaling. Life Sci. 2000;66(19):1769–1780. doi: 10.1016/S0024-3205(99)00602-5. [DOI] [PubMed] [Google Scholar]
- 6.Kondo Y, Inai Y, Sato Y, Handa S, Kubo S, Shimokado K, Goto S, Nishikimi M, Maruyama N, Ishigami A. Senescence marker protein 30 functions as gluconolactonase in l-ascorbic acid biosynthesis, and its knockout mice are prone to scurvy. Proc Natl Acad Sci USA. 2006;103(15):5723–5728. doi: 10.1073/pnas.0511225103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fujita T, Mandel JL, Shirasawa T, Hino O, Shirai T, Maruyama N. Isolation of cDNA clone encoding human homologue of senescence marker protein-30 (SMP30) and its location on the X chromosome. Biochim Biophys Acta. 1995;1263(3):249–252. doi: 10.1016/0167-4781(95)00120-6. [DOI] [PubMed] [Google Scholar]
- 8.Shimokawa N, Matsuda Y, Yamaguchi M. Genomic cloning and chromosomal assignment of rat regucalcin gene. Mol Cell Biochem. 1995;151(2):157–163. doi: 10.1007/BF01322338. [DOI] [PubMed] [Google Scholar]
- 9.Yamaguchi M, Makino R, Shimokawa N. The 5′ end sequences and exon organization in rat regucalcin gene. Mol Cell Biochem. 1996;165(2):145–150. doi: 10.1007/BF00229476. [DOI] [PubMed] [Google Scholar]
- 10.Fujita T, Shirasawa T, Maruyama N. Isolation and characterization of genomic and cDNA clones encoding mouse senescence marker protein-30 (SMP30) Biochim Biophys Acta. 1996;1308(1):49–57. doi: 10.1016/0167-4781(96)00064-4. [DOI] [PubMed] [Google Scholar]
- 11.Maia C, Santos C, Schmitt F, Socorro S. Regucalcin is under-expressed in human breast and prostate cancers: effect of sex steroid hormones. J Cell Biochem. 2009;107(4):667–676. doi: 10.1002/jcb.22158. [DOI] [PubMed] [Google Scholar]
- 12.Fujita T, Shirasawa T, Uchida K, Maruyama N. Isolation of cDNA clone encoding rat senescence marker protein-30 (SMP30) and its tissue distribution. Biochim Biophys Acta. 1992;1132(3):297–305. doi: 10.1016/0167-4781(92)90164-U. [DOI] [PubMed] [Google Scholar]
- 13.Fujita T, Uchida K, Maruyama N. Purification of senescence marker protein-30 (SMP30) and its androgen-independent decrease with age in the rat liver. Biochim Biophys Acta. 1992;1116(2):122–128. doi: 10.1016/0304-4165(92)90108-7. [DOI] [PubMed] [Google Scholar]
- 14.Fujita T, Shirasawa T, Uchida K, Maruyama N. Gene regulation of senescence marker protein-30 (SMP30): coordinated up-regulation with tissue maturation and gradual down-regulation with aging. Mech Ageing Dev. 1996;87(3):219–229. doi: 10.1016/0047-6374(96)01711-3. [DOI] [PubMed] [Google Scholar]
- 15.Shimokawa N, Yamaguchi M. Calcium administration stimulates the expression of calcium-binding protein regucalcin mRNA in rat liver. FEBS Lett. 1992;305(2):151–154. doi: 10.1016/0014-5793(92)80884-J. [DOI] [PubMed] [Google Scholar]
- 16.Yamaguchi M, Isogai M. Tissue concentration of calcium-binding protein regucalcin in rats by enzyme-linked immunoadsorbent assay. Mol Cell Biochem. 1993;122(1):65–68. doi: 10.1007/BF00925738. [DOI] [PubMed] [Google Scholar]
- 17.Yamaguchi M, Isogai M, Kato S, Mori S. Immunohistochemical demonstration of calcium-binding protein regucalcin in the tissues of rats: the protein localizes in liver and brain. Chem Pharm Bull (Tokyo) 1991;39(6):1601–1603. doi: 10.1248/cpb.39.1601. [DOI] [PubMed] [Google Scholar]
- 18.Shimokawa N, Isogai M, Yamaguchi M. Specific species and tissue differences for the gene expression of calcium-binding protein regucalcin. Mol Cell Biochem. 1995;143(1):67–71. doi: 10.1007/BF00925928. [DOI] [PubMed] [Google Scholar]
- 19.Misawa H, Yamaguchi M. The gene of Ca2 + -binding protein regucalcin is highly conserved in vertebrate species. Int J Mol Med. 2000;6(2):191–196. doi: 10.3892/ijmm.6.2.191. [DOI] [PubMed] [Google Scholar]
- 20.Goto SG. Expression of Drosophila homologue of senescence marker protein-30 during cold acclimation. J Insect Physiol. 2000;46(7):1111–1120. doi: 10.1016/S0022-1910(99)00221-8. [DOI] [PubMed] [Google Scholar]
- 21.Yamaguchi M. The transcriptional regulation of regucalcin gene expression. Mol Cell Biochem. 2011;346(1–2):147–171. doi: 10.1007/s11010-010-0601-8. [DOI] [PubMed] [Google Scholar]
- 22.Yamaguchi M, Kurota H. Expression of calcium-binding protein regucalcin mRNA in the kidney cortex of rats: the stimulation by calcium administration. Mol Cell Biochem. 1995;146(1):71–77. doi: 10.1007/BF00926884. [DOI] [PubMed] [Google Scholar]
- 23.Shimokawa N, Yamaguchi M. Expression of hepatic calcium-binding protein regucalcin mRNA is mediated through Ca2 +/calmodulin in rat liver. FEBS Lett. 1993;316(1):79–84. doi: 10.1016/0014-5793(93)81740-Q. [DOI] [PubMed] [Google Scholar]
- 24.Nakajima M, Murata T, Yamaguchi M. Expression of calcium-binding protein regucalcin mRNA in the cloned rat hepatoma cells (H4-II-E) is stimulated through Ca2 + signaling factors: involvement of protein kinase C. Mol Cell Biochem. 1999;198(1–2):101–107. doi: 10.1023/A:1006996506238. [DOI] [PubMed] [Google Scholar]
- 25.Yamaguchi M, Nakajima M. Involvement of intracellular signaling factors in the serum-enhanced Ca2 + -binding protein regucalcin mRNA expression in the cloned rat hepatoma cells (H4-II-E) J Cell Biochem. 1999;74(1):81–89. doi: 10.1002/(SICI)1097-4644(19990701)74:1<81::AID-JCB9>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 26.Maia CJ, Santos CR, Schmitt F, Socorro S. Regucalcin is expressed in rat mammary gland and prostate and down-regulated by 17beta-estradiol. Mol Cell Biochem. 2008;311(1–2):81–86. doi: 10.1007/s11010-007-9697-x. [DOI] [PubMed] [Google Scholar]
- 27.Kurota H, Yamaguchi M. Steroid hormonal regulation of calcium-binding protein regucalcin mRNA expression in the kidney cortex of rats. Mol Cell Biochem. 1996;155(2):105–111. doi: 10.1007/BF00229307. [DOI] [PubMed] [Google Scholar]
- 28.Sar P, Rath B, Subudhi U, Chainy GB, Supakar PC. Alterations in expression of senescence marker protein-30 gene by 3,3′,5-triiodo-L-thyronine (T3) Mol Cell Biochem. 2007;303(1–2):239–242. doi: 10.1007/s11010-007-9462-1. [DOI] [PubMed] [Google Scholar]
- 29.Yamaguchi M, Kanayama Y, Shimokawa N. Expression of calcium-binding protein regucalcin mRNA in rat liver is stimulated by calcitonin: the hormonal effect is mediated through calcium. Mol Cell Biochem. 1994;136(1):43–48. doi: 10.1007/BF00931603. [DOI] [PubMed] [Google Scholar]
- 30.Nakagawa T, Yamaguchi M. Nuclear localization of regucalcin is enhanced in culture with protein kinase C activation in cloned normal rat kidney proximal tubular epithelial NRK52E cells. Int J Mol Med. 2008;21(5):605–610. [PubMed] [Google Scholar]
- 31.Kim HS, Son TG, Park HR, Lee Y, Jung Y, Ishigami A, Lee J. Senescence marker protein 30 deficiency increases Parkinson’s pathology by impairing astrocyte activation. Neurobiol Aging. 2012 doi: 10.1016/j.neurobiolaging.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 32.Son TG, Park HR, Kim SJ, Kim K, Kim MS, Ishigami A, Handa S, Maruyama N, Chung HY, Lee J. Senescence marker protein 30 is up-regulated in kainate-induced hippocampal damage through ERK-mediated astrocytosis. J Neurosci Res. 2009;87(13):2890–2897. doi: 10.1002/jnr.22122. [DOI] [PubMed] [Google Scholar]
- 33.Sun L, Wang L, Sun Y, Tang SW, Hu Y. Protective effects of EUK4010 on beta-amyloid(1–42) induced degeneration of neuronal cells. Eur J Neurosci. 2006;24(4):1011–1019. doi: 10.1111/j.1460-9568.2006.04951.x. [DOI] [PubMed] [Google Scholar]
- 34.Laurentino SS, Correia S, Cavaco JE, Oliveira PF, de Sousa M, Barros A, Socorro S. Regucalcin, a calcium-binding protein with a role in male reproduction? Mol Hum Reprod. 2012;18(4):161–170. doi: 10.1093/molehr/gar075. [DOI] [PubMed] [Google Scholar]
- 35.Lv S, Wang JH, Liu F, Gao Y, Fei R, Du SC, Wei L. Senescence marker protein 30 in acute liver failure: validation of a mass spectrometry proteomics assay. BMC Gastroenterol. 2008;8:17. doi: 10.1186/1471-230X-8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamaguchi M, Tsurusaki Y, Misawa H, Inagaki S, Ma ZJ, Takahashi H. Potential role of regucalcin as a specific biochemical marker of chronic liver injury with carbon tetrachloride administration in rats. Mol Cell Biochem. 2002;241(1–2):61–67. doi: 10.1023/A:1020822610085. [DOI] [PubMed] [Google Scholar]
- 37.Zhou SF, Mo FR, Bin YH, Hou GQ, Xie XX, Luo GR. Serum immunoreactivity of SMP30 and its tissues expression in hepatocellular carcinoma. Clin Biochem. 2011;44(4):331–336. doi: 10.1016/j.clinbiochem.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 38.Doran P, Dowling P, Donoghue P, Buffini M, Ohlendieck K. Reduced expression of regucalcin in young and aged mdx diaphragm indicates abnormal cytosolic calcium handling in dystrophin-deficient muscle. Biochim Biophys Acta. 2006;1764(4):773–785. doi: 10.1016/j.bbapap.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 39.Park H, Ishigami A, Shima T, Mizuno M, Maruyama N, Yamaguchi K, Mitsuyoshi H, Minami M, Yasui K, Itoh Y, Yoshikawa T, Fukui M, Hasegawa G, Nakamura N, Ohta M, Obayashi H, Okanoue T. Hepatic senescence marker protein-30 is involved in the progression of nonalcoholic fatty liver disease. J Gastroenterol. 2009;45(4):426–434. doi: 10.1007/s00535-009-0154-3. [DOI] [PubMed] [Google Scholar]
- 40.Tsurusaki Y, Misawa H, Yamaguchi M. Translocation of regucalcin to rat liver nucleus: involvement of nuclear protein kinase and protein phosphatase regulation. Int J Mol Med. 2000;6(6):655–660. doi: 10.3892/ijmm.6.6.655. [DOI] [PubMed] [Google Scholar]
- 41.Ishigami A, Handa S, Maruyama N, Supakar PC. Nuclear localization of senescence marker protein-30, SMP30, in cultured mouse hepatocytes and its similarity to RNA polymerase. Biosci Biotechnol Biochem. 2003;67(1):158–160. doi: 10.1271/bbb.67.158. [DOI] [PubMed] [Google Scholar]
- 42.Arun P, Aleti V, Parikh K, Manne V, Chilukuri N. Senescence Marker Protein 30 (SMP30) Expression in Eukaryotic Cells: existence of Multiple Species and Membrane Localization. PLoS ONE. 2011;6(2):e16545. doi: 10.1371/journal.pone.0016545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yamaguchi M. Role of regucalcin in maintaining cell homeostasis and function (review) Int J Mol Med. 2005;15(3):371–389. [PubMed] [Google Scholar]
- 44.Yamaguchi M. Role of regucalcin in brain calcium signaling: involvement in aging. Integr Biol (Camb) 2012;4(8):825–837. doi: 10.1039/c2ib20042b. [DOI] [PubMed] [Google Scholar]
- 45.Baba T, Yamaguchi M. Stimulatory effect of regucalcin on proteolytic activity is impaired in the kidney cortex cytosol of rats with saline ingestion. Mol Cell Biochem. 2000;206(1–2):1–6. doi: 10.1023/A:1007007626813. [DOI] [PubMed] [Google Scholar]
- 46.Katsumata T, Yamaguchi M. Inhibitory effect of calcium-binding protein regucalcin on protein kinase activity in the nuclei of regenerating rat liver. J Cell Biochem. 1998;71(4):569–576. doi: 10.1002/(SICI)1097-4644(19981215)71:4<569::AID-JCB11>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 47.Fukaya Y, Yamaguchi M. Characterization of protein tyrosine phosphatase activity in rat liver microsomes: suppressive effect of endogenous regucalcin in transgenic rats. Int J Mol Med. 2004;14(3):427–432. [PubMed] [Google Scholar]
- 48.Tobisawa M, Yamaguchi M. Inhibitory role of regucalcin in the regulation of nitric oxide synthase activity in rat brain cytosol: involvement of aging. J Neurol Sci. 2003;209(1–2):47–54. doi: 10.1016/S0022-510X(02)00460-4. [DOI] [PubMed] [Google Scholar]
- 49.Son TG, Kim SJ, Kim K, Kim MS, Chung HY, Lee J. Cytoprotective roles of senescence marker protein 30 against intracellular calcium elevation and oxidative stress. Arch Pharm Res. 2008;31(7):872–877. doi: 10.1007/s12272-001-1240-3. [DOI] [PubMed] [Google Scholar]
- 50.Fukaya Y, Yamaguchi M. Regucalcin increases superoxide dismutase activity in rat liver cytosol. Biol Pharm Bull. 2004;27(9):1444–1446. doi: 10.1248/bpb.27.1444. [DOI] [PubMed] [Google Scholar]
- 51.Handa S, Maruyama N, Ishigami A. Over-expression of Senescence Marker Protein-30 decreases reactive oxygen species in human hepatic carcinoma Hep G2 cells. Biol Pharm Bull. 2009;32(10):1645–1648. doi: 10.1248/bpb.32.1645. [DOI] [PubMed] [Google Scholar]
- 52.Ichikawa E, Yamaguchi M. Regucalcin increases superoxide dismutase activity in the heart cytosol of normal and regucalcin transgenic rats. Int J Mol Med. 2004;14(4):691–695. [PubMed] [Google Scholar]
- 53.Tsurusaki Y, Yamaguchi M. Overexpression of regucalcin modulates tumor-related gene expression in cloned rat hepatoma H4-II-E cells. J Cell Biochem. 2003;90(3):619–626. doi: 10.1002/jcb.10652. [DOI] [PubMed] [Google Scholar]
- 54.Yamaguchi M, Daimon Y. Overexpression of regucalcin suppresses cell proliferation in cloned rat hepatoma H4-II-E cells: involvement of intracellular signaling factors and cell cycle-related genes. J Cell Biochem. 2005;95(6):1169–1177. doi: 10.1002/jcb.20490. [DOI] [PubMed] [Google Scholar]
- 55.Misawa H, Inagaki S, Yamaguchi M. Suppression of cell proliferation and deoxyribonucleic acid synthesis in the cloned rat hepatoma H4-II-E cells overexpressing regucalcin. J Cell Biochem. 2002;84(1):143–149. doi: 10.1002/jcb.1274. [DOI] [PubMed] [Google Scholar]
- 56.Izumi T, Yamaguchi M. Overexpression of regucalcin suppresses cell death in cloned rat hepatoma H4-II-E cells induced by tumor necrosis factor-alpha or thapsigargin. J Cell Biochem. 2004;92(2):296–306. doi: 10.1002/jcb.20056. [DOI] [PubMed] [Google Scholar]
- 57.Nakagawa T, Sawada N, Yamaguchi M. Overexpression of regucalcin suppresses cell proliferation of cloned normal rat kidney proximal tubular epithelial NRK52E cells. Int J Mol Med. 2005;16(4):637–643. [PubMed] [Google Scholar]
- 58.Matsuyama S, Kitamura T, Enomoto N, Fujita T, Ishigami A, Handa S, Maruyama N, Zheng D, Ikejima K, Takei Y, Sato N. Senescence marker protein-30 regulates Akt activity and contributes to cell survival in Hep G2 cells. Biochem Biophys Res Commun. 2004;321(2):386–390. doi: 10.1016/j.bbrc.2004.06.161. [DOI] [PubMed] [Google Scholar]
- 59.Jeong DH, Goo MJ, Hong IH, Yang HJ, Ki MR, Do SH, Ha JH, Lee SS, Park JK, Jeong KS. Inhibition of radiation-induced apoptosis via overexpression of SMP30 in Smad3-knockout mice liver. J Radiat Res (Tokyo) 2008;49(6):653–660. doi: 10.1269/jrr.08042. [DOI] [PubMed] [Google Scholar]
- 60.Ishigami A, Fujita T, Handa S, Shirasawa T, Koseki H, Kitamura T, Enomoto N, Sato N, Shimosawa T, Maruyama N. Senescence marker protein-30 knockout mouse liver is highly susceptible to tumor necrosis factor-alpha- and Fas-mediated apoptosis. Am J Pathol. 2002;161(4):1273–1281. doi: 10.1016/S0002-9440(10)64404-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Maruyama N, Ishigami A, Kondo Y. Pathophysiological significance of senescence marker protein-30. Geriatr Gerontol Int. 2010;10(Suppl 1):S88–S98. doi: 10.1111/j.1447-0594.2010.00586.x. [DOI] [PubMed] [Google Scholar]
- 62.Nikapitiya C, De Zoysa M, Kang HS, Oh C, Whang I, Lee J. Molecular characterization and expression analysis of regucalcin in disk abalone (Haliotis discus discus): intramuscular calcium administration stimulates the regucalcin mRNA expression. Comp Biochem Physiol B. 2008;150(1):117–124. doi: 10.1016/j.cbpb.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 63.Wu YD, Jiang L, Zhou Z, Zheng MH, Zhang J, Liang Y. CYP1A/regucalcin gene expression and edema formation in zebrafish embryos exposed to 2,3,7,8-Tetrachlorodibenzo-p-dioxin. Bull Environ Contam Toxicol. 2008;80(6):482–486. doi: 10.1007/s00128-008-9395-8. [DOI] [PubMed] [Google Scholar]
- 64.Nakajima Y, Natori S. Identification and characterization of an anterior fat body protein in an insect. J Biochem. 2000;127(5):901–908. doi: 10.1093/oxfordjournals.jbchem.a022685. [DOI] [PubMed] [Google Scholar]
- 65.Gomi K, Hirokawa K, Kajiyama N. Molecular cloning and expression of the cDNAs encoding luciferin-regenerating enzyme from Luciola cruciata and Luciola lateralis. Gene. 2002;294(1–2):157–166. doi: 10.1016/S0378-1119(02)00764-3. [DOI] [PubMed] [Google Scholar]
- 66.Laurentino SS, Correia S, Cavaco JE, Oliveira PF, Rato L, Sousa M, Barros A, Socorro S. Regucalcin is broadly expressed in male reproductive tissues and is a new androgen-target gene in mammalian testis. Reproduction. 2011;142(3):447–456. doi: 10.1530/REP-11-0085. [DOI] [PubMed] [Google Scholar]
- 67.Nakagawa T, Yamaguchi M. Hormonal regulation on regucalcin mRNA expression in cloned normal rat kidney proximal tubular epithelial NRK52E cells. J Cell Biochem. 2005;95(3):589–597. doi: 10.1002/jcb.20422. [DOI] [PubMed] [Google Scholar]
- 68.Jung KJ, Maruyama N, Ishigami A, Yu BP, Chung HY. The redox-sensitive DNA binding sites responsible for age-related downregulation of SMP30 by ERK pathway and reversal by calorie restriction. Antioxid Redox Signal. 2006;8(3–4):671–680. doi: 10.1089/ars.2006.8.671. [DOI] [PubMed] [Google Scholar]
- 69.Fujita T, Maruyama N. Expression and structure of senescence marker protein-30 (SMP30) and its physiological function. Nippon Ronen Igakkai Zasshi. 1998;35(9):654–657. doi: 10.3143/geriatrics.35.654. [DOI] [PubMed] [Google Scholar]
- 70.Yamaguchi M, Misawa H, Uchiyama S, Morooka Y, Tsurusaki Y. Role of endogenous regucalcin in bone metabolism: bone loss is induced in regucalcin transgenic rats. Int J Mol Med. 2002;10(4):377–383. [PubMed] [Google Scholar]
- 71.Yamaguchi M, Morooka Y, Misawa H, Tsurusaki Y, Nakajima R. Role of endogenous regucalcin in transgenic rats: suppression of kidney cortex cytosolic protein phosphatase activity and enhancement of heart muscle microsomal Ca2 + -ATPase activity. J Cell Biochem. 2002;86(3):520–529. doi: 10.1002/jcb.10249. [DOI] [PubMed] [Google Scholar]
- 72.Yamaguchi M, Sawada N, Uchiyama S, Misawa H, Ma ZJ. Expression of regucalcin in rat bone marrow cells: involvement of osteoclastic bone resorption in regucalcin transgenic rats. Int J Mol Med. 2004;13(3):437–443. [PubMed] [Google Scholar]
- 73.Ishii K, Tsubaki T, Fujita K, Ishigami A, Maruyama N, Akita M. Immunohistochemical localization of senescence marker protein-30 (SMP30) in the submandibular gland and ultrastructural changes of the granular duct cells in SMP30 knockout mice. Histol Histopathol. 2005;20(3):761–768. doi: 10.14670/HH-20.761. [DOI] [PubMed] [Google Scholar]
- 74.Yamaguchi M, Nakajima R. Role of regucalcin as an activator of sarcoplasmic reticulum Ca2 + -ATPase activity in rat heart muscle. J Cell Biochem. 2002;86(1):184–193. doi: 10.1002/jcb.10209. [DOI] [PubMed] [Google Scholar]
- 75.van Dijk KD, Berendse HW, Drukarch B, Fratantoni SA, Pham TV, Piersma SR, Huisman E, Breve JJ, Groenewegen HJ, Jimenez CR, van de Berg WD. The proteome of the locus ceruleus in Parkinson’s disease: relevance to pathogenesis. Brain Pathol. 2012;22(4):485–498. doi: 10.1111/j.1750-3639.2011.00540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Isogai M, Shimokawa N, Yamaguchi M. Hepatic calcium-binding protein regucalcin in released into the serum of rats administered orally carbon tetrachloride. Mol Cell Biochem. 1994;131(2):173–179. doi: 10.1007/BF00925954. [DOI] [PubMed] [Google Scholar]
- 77.Isogai M, Oishi K, Yamaguchi M. Serum release of hepatic calcium-binding protein regucalcin by liver injury with galactosamine administration in rats. Mol Cell Biochem. 1994;136(1):85–90. doi: 10.1007/BF00931609. [DOI] [PubMed] [Google Scholar]
- 78.Lv S, Wei L, Wang JH, Wang JY, Liu F. Identification of novel molecular candidates for acute liver failure in plasma of BALB/c murine model. J Proteome Res. 2007;6(7):2746–2752. doi: 10.1021/pr0701759. [DOI] [PubMed] [Google Scholar]
- 79.Carolan JC, Fitzroy CI, Ashton PD, Douglas AE, Wilkinson TL. The secreted salivary proteome of the pea aphid Acyrthosiphon pisum characterised by mass spectrometry. Proteomics. 2009;9(9):2457–2467. doi: 10.1002/pmic.200800692. [DOI] [PubMed] [Google Scholar]
- 80.Inoue H, Fujita T, Kitamura T, Shimosawa T, Nagasawa R, Inoue R, Maruyama N, Nagasawa T. Senescence marker protein-30 (SMP30) enhances the calcium efflux from renal tubular epithelial cells. Clin Exp Nephrol. 1999;3(4):261–267. doi: 10.1007/s101570050045. [DOI] [Google Scholar]
- 81.Ueoka S, Yamaguchi M. Sexual difference of hepatic calcium-binding protein regucalcin mRNA expression in rats with different ages: effect of ovarian hormone. Biol Pharm Bull. 1998;21(4):405–407. doi: 10.1248/bpb.21.405. [DOI] [PubMed] [Google Scholar]
- 82.Murata T, Yamaguchi M. Molecular cloning of the cDNA coding for regucalcin and its mRNA expression in mouse liver: the expression is stimulated by calcium administration. Mol Cell Biochem. 1997;173(1–2):127–133. doi: 10.1023/A:1006887929369. [DOI] [PubMed] [Google Scholar]
- 83.Fujita T, Shirasawa T, Maruyama N. Expression and structure of senescence marker protein-30 (SMP30) and its biological significance. Mech Ageing Dev. 1999;107(3):271–280. doi: 10.1016/S0047-6374(98)00136-5. [DOI] [PubMed] [Google Scholar]
- 84.Makino R, Yamaguchi M. Expression of calcium-binding protein regucalcin mRNA in hepatoma cells. Mol Cell Biochem. 1996;155(1):85–90. doi: 10.1007/BF00714337. [DOI] [PubMed] [Google Scholar]
- 85.Yamaguchi M. Role of calcium-binding protein regucalcin in regenerating rat liver. J Gastroenterol Hepatol. 1998;13(Suppl):S106–S112. doi: 10.1111/jgh.1998.13.s1.106. [DOI] [PubMed] [Google Scholar]
- 86.Murata T, Yamaguchi M. Ca2 + administration stimulates the binding of AP-1 factor to the 5′-flanking region of the rat gene for the Ca2 + -binding protein regucalcin. Biochem J. 1998;329(Pt 1):157–163. doi: 10.1042/bj3290157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Inagaki S, Misawa H, Yamaguchi M. Role of endogenous regucalcin in protein tyrosine phosphatase regulation in the cloned rat hepatoma cells (H4-II-E) Mol Cell Biochem. 2000;213(1–2):43–50. doi: 10.1023/A:1007100631753. [DOI] [PubMed] [Google Scholar]
- 88.Ishigami A, Fujita T, Inoue H, Handa S, Kubo S, Kondo Y, Maruyama N. Senescence marker protein-30 (SMP30) induces formation of microvilli and bile canaliculi in Hep G2 cells. Cell Tissue Res. 2005;320(2):243–249. doi: 10.1007/s00441-004-1073-5. [DOI] [PubMed] [Google Scholar]
- 89.Fujita T, Inoue H, Kitamura T, Sato N, Shimosawa T, Maruyama N. Senescence marker protein-30 (SMP30) rescues cell death by enhancing plasma membrane Ca(2 +)-pumping activity in Hep G2 cells. Biochem Biophys Res Commun. 1998;250(2):374–380. doi: 10.1006/bbrc.1998.9327. [DOI] [PubMed] [Google Scholar]
- 90.Murata T, Shinya N, Yamaguchi M. Expression of calcium-binding protein regucalcin mRNA in the cloned human hepatoma cells (HepG2): stimulation by insulin. Mol Cell Biochem. 1997;175(1–2):163–168. doi: 10.1023/A:1006844815743. [DOI] [PubMed] [Google Scholar]
- 91.Misawa H, Yamaguchi M. Transcript heterogeneity of the human gene for Ca2 + -binding protein regucalcin. Int J Mol Med. 2000;5(3):283–287. [PubMed] [Google Scholar]
- 92.Yamaguchi M, Kobayashi M, Uchiyama S. Suppressive effect of regucalcin on cell differentiation and mineralization in osteoblastic MC3T3-E1 cells. J Cell Biochem. 2005;96(3):543–554. doi: 10.1002/jcb.20524. [DOI] [PubMed] [Google Scholar]
- 93.Suzuki S, Asamoto M, Tsujimura K, Shirai T. Specific differences in gene expression profile revealed by cDNA microarray analysis of glutathione S-transferase placental form (GST-P) immunohistochemically positive rat liver foci and surrounding tissue. Carcinogenesis. 2004;25(3):439–443. doi: 10.1093/carcin/bgh030. [DOI] [PubMed] [Google Scholar]
- 94.Yamaguchi M, Isogai M, Shimada N. Potential sensitivity of hepatic specific protein regucalcin as a marker of chronic liver injury. Mol Cell Biochem. 1997;167(1–2):187–190. doi: 10.1023/A:1006859121897. [DOI] [PubMed] [Google Scholar]
- 95.Yamaguchi M, Oishi K, Isogai M. Expression of hepatic calcium-binding protein regucalcin mRNA is elevated by refeeding of fasted rats: involvement of glucose, insulin and calcium as stimulating factors. Mol Cell Biochem. 1995;142(1):35–41. doi: 10.1007/BF00928911. [DOI] [PubMed] [Google Scholar]
- 96.Shinya N, Yamaguchi M. Stimulatory effect of calcium administration on regucalcin mRNA expression is attenuated in the kidney cortex of rats ingested with saline. Mol Cell Biochem. 1998;178(1–2):275–281. doi: 10.1023/A:1006898910955. [DOI] [PubMed] [Google Scholar]
- 97.Ramasamy I. Recent advances in physiological calcium homeostasis. Clin Chem Lab Med. 2006;44(3):237–273. doi: 10.1515/CCLM.2006.046. [DOI] [PubMed] [Google Scholar]
- 98.Yamaguchi M. Regucalcin and metabolic disorders: osteoporosis and hyperlipidemia are induced in regucalcin transgenic rats. Mol Cell Biochem. 2010;341(1–2):119–133. doi: 10.1007/s11010-010-0443-4. [DOI] [PubMed] [Google Scholar]
- 99.Yamaguchi M, Otomo Y, Uchiyama S, Nakagawa T. Hormonal regulation of regucalcin mRNA expression in osteoblastic MC3T3-E1 cells. Int J Mol Med. 2008;21(6):771–775. [PubMed] [Google Scholar]
- 100.Yamaguchi M, Weitzmann MN, Murata T. Exogenous regucalcin stimulates osteoclastogenesis and suppresses osteoblastogenesis through NF-kappaB activation. Mol Cell Biochem. 2012;359(1–2):193–203. doi: 10.1007/s11010-011-1014-z. [DOI] [PubMed] [Google Scholar]
- 101.Yamaguchi M, Oishi K. 17 beta-Estradiol stimulates the expression of hepatic calcium-binding protein regucalcin mRNA in rats. Mol Cell Biochem. 1995;143(2):137–141. doi: 10.1007/BF01816947. [DOI] [PubMed] [Google Scholar]
- 102.Sar P, Peter R, Rath B, Mohapatra AD, Mishra SK. 3, 3′5 Triiodo L Thyronine Induces Apoptosis in Human Breast Cancer MCF-7cells, Repressing SMP30 Expression through Negative Thyroid Response Elements. PLoS ONE. 2011;6(6):e20861. doi: 10.1371/journal.pone.0020861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shinya N, Kurota H, Yamaguchi M. Calcium-binding protein regucalcin mRNA expression in the kidney cortex is suppressed by saline ingestion in rats. Mol Cell Biochem. 1996;162(2):139–144. doi: 10.1007/BF00227541. [DOI] [PubMed] [Google Scholar]
- 104.Fukui M, Senmaru T, Hasegawa G, Yamazaki M, Asano M, Kagami Y, Ishigami A, Maruyama N, Iwasa K, Kitawaki J, Itoh Y, Okanoue T, Ohta M, Obayashi H, Nakamura N. 17beta-Estradiol attenuates saturated fatty acid diet-induced liver injury in ovariectomized mice by up-regulating hepatic senescence marker protein-30. Biochem Biophys Res Commun. 2011;415(2):252–257. doi: 10.1016/j.bbrc.2011.10.025. [DOI] [PubMed] [Google Scholar]
- 105.Speakman JR, Mitchell SE. Caloric restriction. Mol Aspects Med. 2011;32(3):159–221. doi: 10.1016/j.mam.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 106.Jung KJ, Ishigami A, Maruyama N, Takahashi R, Goto S, Yu BP, Chung HY. Modulation of gene expression of SMP-30 by LPS and calorie restriction during aging process. Exp Gerontol. 2004;39(8):1169–1177. doi: 10.1016/j.exger.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 107.Ishigami T, Fujita T, Simbula G, Columbano A, Kikuchi K, Ishigami A, Shimosawa T, Arakawa Y, Maruyama N. Regulatory effects of senescence marker protein 30 on the proliferation of hepatocytes. Pathol Int. 2001;51(7):491–497. doi: 10.1046/j.1440-1827.2001.01238.x. [DOI] [PubMed] [Google Scholar]
- 108.Lee WJ, Monteith GR, Roberts-Thomson SJ. Calcium transport and signaling in the mammary gland: targets for breast cancer. BBA Rev Cancer. 2006;1765(2):235–255. doi: 10.1016/j.bbcan.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 109.Cheng HP, Wei S, Wei LP, Verkhratsky A. Calcium signaling in physiology and pathophysiology. Acta Pharmacol Sin. 2006;27(7):767–772. doi: 10.1111/j.1745-7254.2006.00399.x. [DOI] [PubMed] [Google Scholar]
- 110.Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4(7):517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 111.Birnbaumer L. The TRPC class of ion channels: a critical review of their roles in slow, sustained increases in intracellular Ca(2 +) concentrations. Annu Rev Pharmacol Toxicol. 2009;49:395–426. doi: 10.1146/annurev.pharmtox.48.113006.094928. [DOI] [PubMed] [Google Scholar]
- 112.Nakagawa T, Yamaguchi M. Overexpression of regucalcin enhances its nuclear localization and suppresses L-type Ca2 + channel and calcium-sensing receptor mRNA expressions in cloned normal rat kidney proximal tubular epithelial NRK52E cells. J Cell Biochem. 2006;99(4):1064–1077. doi: 10.1002/jcb.20863. [DOI] [PubMed] [Google Scholar]
- 113.Yamaguchi M, Mori S, Kato S. Calcium-binding protein regucalcin is an activator of (Ca2 + -Mg2 +)-adenosine triphosphatase in the plasma membranes of rat liver. Chem Pharm Bull (Tokyo) 1988;36(9):3532–3539. doi: 10.1248/cpb.36.3532. [DOI] [PubMed] [Google Scholar]
- 114.Takahashi H, Yamaguchi M. Regucalcin modulates hormonal effect on (Ca(2 +)-Mg2 +)-ATPase activity in rat liver plasma membranes. Mol Cell Biochem. 1993;125(2):171–177. doi: 10.1007/BF00936446. [DOI] [PubMed] [Google Scholar]
- 115.Takahashi H, Yamaguchi M. Stimulatory effect of regucalcin on ATP-dependent calcium transport in rat liver plasma membranes. Mol Cell Biochem. 1997;168(1–2):149–153. doi: 10.1023/A:1006811222806. [DOI] [PubMed] [Google Scholar]
- 116.Takahashi H, Yamaguchi M. Activating effect of regucalcin on (Ca(2 +)-Mg2 +)-ATPase in rat liver plasma membranes: relation to sulfhydryl group. Mol Cell Biochem. 1994;136(1):71–76. doi: 10.1007/BF00931607. [DOI] [PubMed] [Google Scholar]
- 117.Takahasi H, Yamaguchi M. Enhancement of plasma membrane (Ca(2 +)-Mg2 +)-ATPase activity in regenerating rat liver: involvement of endogenous activating protein regucalcin. Mol Cell Biochem. 1996;162(2):133–138. doi: 10.1007/BF00227540. [DOI] [PubMed] [Google Scholar]
- 118.Takahashi H, Yamaguchi M. Increase of (Ca(2 +)-Mg2 +)-ATPase activity in hepatic plasma membranes of rats administered orally calcium: the endogenous role of regucalcin. Mol Cell Biochem. 1995;144(1):1–6. doi: 10.1007/BF00926733. [DOI] [PubMed] [Google Scholar]
- 119.Di Leva F, Domi T, Fedrizzi L, Lim D, Carafoli E. The plasma membrane Ca2 + ATPase of animal cells: structure, function and regulation. Arch Biochem Biophys. 2008;476(1):65–74. doi: 10.1016/j.abb.2008.02.026. [DOI] [PubMed] [Google Scholar]
- 120.Takahashi H, Yamaguchi M. Activatory effect of regucalcin on hepatic plasma membrane (Ca(2 +)-Mg2 +)-ATPase is impaired by liver injury with carbon tetrachloride administration in rats. Mol Cell Biochem. 1996;158(1):9–16. doi: 10.1007/BF00225877. [DOI] [PubMed] [Google Scholar]
- 121.Kurota H, Yamaguchi M. Activatory effect of calcium-binding protein regucalcin on ATP-dependent calcium transport in the basolateral membranes of rat kidney cortex. Mol Cell Biochem. 1997;169(1–2):149–156. doi: 10.1023/A:1006894332337. [DOI] [PubMed] [Google Scholar]
- 122.Yamaguchi M, Mori S. Activation of hepatic microsomal Ca2 + -adenosine triphosphatase by calcium-binding protein regucalcin. Chem Pharm Bull (Tokyo) 1989;37(4):1031–1034. doi: 10.1248/cpb.37.1031. [DOI] [PubMed] [Google Scholar]
- 123.Takahashi H, Yamaguchi M. Role of regucalcin as an activator of Ca(2 +)-ATPase activity in rat liver microsomes. J Cell Biochem. 1999;74(4):663–669. doi: 10.1002/(SICI)1097-4644(19990915)74:4<663::AID-JCB15>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 124.Lai P, Yip NC, Michelangeli F. Regucalcin (RGN/SMP30) alters agonist- and thapsigargin-induced cytosolic [Ca(2 +)] transients in cells by increasing SERCA Ca(2 +)ATPase levels. FEBS Lett. 2011;585(14):2291–2294. doi: 10.1016/j.febslet.2011.05.058. [DOI] [PubMed] [Google Scholar]
- 125.Yamaguchi M, Hanahisa Y, Murata T. Expression of calcium-binding protein regucalcin and microsomal Ca2 + -ATPase regulation in rat brain: attenuation with increasing age. Mol Cell Biochem. 1999;200(1–2):43–49. doi: 10.1023/A:1006928402184. [DOI] [PubMed] [Google Scholar]
- 126.Omura M, Yamaguchi M. Regulation of protein phosphatase activity by regucalcin localization in rat liver nuclei. J Cell Biochem. 1999;75(3):437–445. doi: 10.1002/(SICI)1097-4644(19991201)75:3<437::AID-JCB9>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 127.Brini M, Carafoli E. Calcium pumps in health and disease. Physiol Rev. 2009;89(4):1341–1378. doi: 10.1152/physrev.00032.2008. [DOI] [PubMed] [Google Scholar]
- 128.Yamaguchi M. Effect of calcium-binding protein regucalcin on Ca2 + transport system in rat liver nuclei: stimulation of Ca2 + release. Mol Cell Biochem. 1992;113(1):63–70. doi: 10.1007/BF00230886. [DOI] [PubMed] [Google Scholar]
- 129.Tsurusaki Y, Yamaguchi M. Role of endogenous regucalcin in the regulation of Ca(2 +)-ATPase activity in rat liver nuclei. J Cell Biochem. 2000;78(4):541–549. doi: 10.1002/1097-4644(20000915)78:4<541::AID-JCB3>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 130.Mori S, Yamaguchi M. Calcium-binding protein regucalcin stimulates the uptake of Ca2 + by rat liver mitochondria. Chem Pharm Bull (Tokyo) 1991;39(1):224–226. doi: 10.1248/cpb.39.224. [DOI] [PubMed] [Google Scholar]
- 131.Takahashi H, Yamaguchi M. Stimulatory effect of regucalcin on ATP-dependent Ca(2 +) uptake activity in rat liver mitochondria. J Cell Biochem. 2000;78(1):121–130. doi: 10.1002/(SICI)1097-4644(20000701)78:1<121::AID-JCB11>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 132.Xue JH, Takahashi H, Yamaguchi M. Stimulatory effect of regucalcin on mitochondrial ATP-dependent calcium uptake activity in rat kidney cortex. J Cell Biochem. 2000;80(2):285–292. doi: 10.1002/1097-4644(20010201)80:2<285::AID-JCB180>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 133.Akhter T, Sawada N, Yamaguchi M. Regucalcin increases Ca2 + -ATPase activity in the heart mitochondria of normal and regucalcin transgenic rats. Int J Mol Med. 2006;18(1):171–176. [PubMed] [Google Scholar]
- 134.Yamaguchi M, Takakura Y, Nakagawa T. Regucalcin increases Ca2 + -ATPase activity in the mitochondria of brain tissues of normal and transgenic rats. J Cell Biochem. 2008;104(3):795–804. doi: 10.1002/jcb.21664. [DOI] [PubMed] [Google Scholar]
- 135.Yamaguchi M, Mori S. Effect of Ca2 + and Zn2 + on 5′-nucleotidase activity in rat liver plasma membranes: hepatic calcium-binding protein (regucalcin) reverses the Ca2 + effect. Chem Pharm Bull (Tokyo) 1988;36(1):321–325. doi: 10.1248/cpb.36.321. [DOI] [PubMed] [Google Scholar]
- 136.Yamaguchi M, Shibano H. Reversible effect of calcium-binding protein on the Ca2 + -induced activation of succinate dehydrogenase in rat liver mitochondria. Chem Pharm Bull (Tokyo) 1987;35(9):3700–3766. doi: 10.1248/cpb.35.3766. [DOI] [PubMed] [Google Scholar]
- 137.Yamaguchi M, Shibano H. Effect of calcium-binding protein on the activation of phosphorylase a in rat hepatic particulate glycogen by Ca2+ Chem Pharm Bull (Tokyo) 1987;35(6):2581–2584. doi: 10.1248/cpb.35.2581. [DOI] [PubMed] [Google Scholar]
- 138.Yamaguchi M, Shibano H. Calcium-binding protein isolated from rat liver cytosol reverses activation of pyruvate kinase by Ca2+ Chem Pharm Bull (Tokyo) 1987;35(5):2025–2029. doi: 10.1248/cpb.35.2025. [DOI] [PubMed] [Google Scholar]
- 139.Yamaguchi M, Mori S, Suketa Y. Effects of Ca2 + and V5 + on glucose-6-phosphatase activity in rat liver microsomes: the Ca2 + effect is reversed by regucalcin. Chem Pharm Bull (Tokyo) 1989;37(2):388–390. doi: 10.1248/cpb.37.388. [DOI] [PubMed] [Google Scholar]
- 140.Hanahisa Y, Yamaguchi M. Effect of calcium-binding protein on adenosine 5′-triphosphatase activity in the brain cytosol of rats of different ages: the inhibitory role of regucalcin. Biol Pharm Bull. 1999;22(3):313–316. doi: 10.1248/bpb.22.313. [DOI] [PubMed] [Google Scholar]
- 141.Yamaguchi M, Yoshida H. Regulatory effect of calcium-binding protein isolated from rat liver cytosol on activation of fructose 1,6-diphosphatase by Ca2 + -calmodulin. Chem Pharm Bull (Tokyo) 1985;33(10):4489–4493. doi: 10.1248/cpb.33.4489. [DOI] [PubMed] [Google Scholar]
- 142.Yamaguchi M, Sakurai T. Reversible effect of calcium-binding protein regucalcin on the Ca(2 +)-induced inhibition of deoxyuridine 5′-triphosphatase activity in rat liver cytosol. Mol Cell Biochem. 1992;110(1):25–29. doi: 10.1007/BF02385002. [DOI] [PubMed] [Google Scholar]
- 143.Halls ML, Cooper DM. Regulation by Ca2 + -signaling pathways of adenylyl cyclases. Cold Spring Harb Perspect Biol. 2011;3(1):a004143. doi: 10.1101/cshperspect.a004143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tresguerres M, Levin LR, Buck J. Intracellular cAMP signaling by soluble adenylyl cyclase. Kidney Int. 2011;79(12):1277–1288. doi: 10.1038/ki.2011.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Levy I, Horvath A, Azevedo M, de Alexandre RB, Stratakis CA. Phosphodiesterase function and endocrine cells: links to human disease and roles in tumor development and treatment. Curr Opin Pharmacol. 2011;11(6):689–697. doi: 10.1016/j.coph.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Yamaguchi M, Tai H. Inhibitory effect of calcium-binding protein regucalcin on Ca2 +/calmodulin-dependent cyclic nucleotide phosphodiesterase activity in rat liver cytosol. Mol Cell Biochem. 1991;106(1):25–30. doi: 10.1007/BF00231185. [DOI] [PubMed] [Google Scholar]
- 147.Yamaguchi M, Kurota H. Inhibitory effect of regucalcin on Ca2 +/calmodulin-dependent cyclic AMP phosphodiesterase activity in rat kidney cytosol. Mol Cell Biochem. 1997;177(1–2):209–214. doi: 10.1023/A:1006829926590. [DOI] [PubMed] [Google Scholar]
- 148.Daff S. NO synthase: structures and mechanisms. Nitric Oxide. 2010;23(1):1–11. doi: 10.1016/j.niox.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 149.Ma ZJ, Yamaguchi M. Regulatory effect of regucalcin on nitric oxide synthase activity in rat kidney cortex cytosol: role of endogenous regucalcin in transgenic rats. Int J Mol Med. 2003;12(2):201–206. [PubMed] [Google Scholar]
- 150.Yamaguchi M, Takahashi H, Tsurusaki Y. Suppressive role of endogenous regucalcin in the enhancement of nitric oxide synthase activity in liver cytosol of normal and regucalcin transgenic rats. J Cell Biochem. 2003;88(6):1226–1234. doi: 10.1002/jcb.10452. [DOI] [PubMed] [Google Scholar]
- 151.Ma ZJ, Yamaguchi M. Suppressive role of endogenous regucalcin in the regulation of nitric oxide synthase activity in heart muscle cytosol of normal and regucalcin transgenic rats. Int J Mol Med. 2002;10(6):761–766. [PubMed] [Google Scholar]
- 152.Tobisawa M, Yamaguchi M. Role of endogenous regucalcin in brain function: suppression of cytosolic nitric oxide synthase and nuclear protein tyrosine phosphatase activities in brain tissue of transgenic rats. Int J Mol Med. 2003;12(4):581–585. [PubMed] [Google Scholar]
- 153.Nakagawa T, Yamaguchi M. Overexpression of regucalcin suppresses cell response for tumor necrosis factor-alpha or transforming growth factor-beta1 in cloned normal rat kidney proximal tubular epithelial NRK52E cells. J Cell Biochem. 2007;100(5):1178–1190. doi: 10.1002/jcb.21105. [DOI] [PubMed] [Google Scholar]
- 154.Sugiura R, Sio SO, Shuntoh H, Kuno T. Calcineurin phosphatase in signal transduction: lessons from fission yeast. Genes Cells. 2002;7(7):619–627. doi: 10.1046/j.1365-2443.2002.00557.x. [DOI] [PubMed] [Google Scholar]
- 155.Rusnak F, Mertz P. Calcineurin: form and function. Physiol Rev. 2000;80(4):1483–1521. doi: 10.1152/physrev.2000.80.4.1483. [DOI] [PubMed] [Google Scholar]
- 156.Omura M, Yamaguchi M. Inhibition of Ca2 +/calmodulin-dependent phosphatase activity by regucalcin in rat liver cytosol: involvement of calmodulin binding. J Cell Biochem. 1998;71(1):140–148. doi: 10.1002/(SICI)1097-4644(19981001)71:1<140::AID-JCB14>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 157.Omura M, Yamaguchi M. Effect of anti-regucalcin antibody on neutral phosphatase activity in rat liver cytosol: involvement of endogenous regucalcin. Mol Cell Biochem. 1999;197(1–2):25–29. doi: 10.1023/A:1006979606225. [DOI] [PubMed] [Google Scholar]
- 158.Omura M, Kurota H, Yamaguchi M. Inhibitory effect of regucalcin on Ca2 +/calmodulin-dependent phosphatase activity in rat renal cortex cytosol. Biol Pharm Bull. 1998;21(5):440–443. doi: 10.1248/bpb.21.440. [DOI] [PubMed] [Google Scholar]
- 159.Morooka Y, Yamaguchi M. Suppressive role of endogenous regucalcin in the regulation of protein phosphatase activity in rat renal cortex cytosol. J Cell Biochem. 2001;81(4):639–646. doi: 10.1002/jcb.1098. [DOI] [PubMed] [Google Scholar]
- 160.Morooka Y, Yamaguchi M. Inhibitory effect of regucalcin on protein phosphatase activity in the nuclei of rat kidney cortex. J Cell Biochem. 2001;83(1):111–120. doi: 10.1002/jcb.1214. [DOI] [PubMed] [Google Scholar]
- 161.Morooka Y, Yamaguchi M. Endogenous regucalcin suppresses the enhancement of protein phosphatase activity in the cytosol and nucleus of kidney cortex in calcium-administered rats. J Cell Biochem. 2002;85(3):553–560. doi: 10.1002/jcb.10157. [DOI] [PubMed] [Google Scholar]
- 162.Ichikawa E, Tsurusaki Y, Yamaguchi M. Suppressive effect of regucalcin on protein phosphatase activity in the heart cytosol of normal and regucalcin transgenic rats. Int J Mol Med. 2004;13(2):289–293. [PubMed] [Google Scholar]
- 163.Hamano T, Yamaguchi M. Inhibitory effect of regucalcin on Ca2 +/calmodulin-dependent protein phosphatase activity in rat brain cytosol. Int J Mol Med. 1999;3(6):615–619. doi: 10.3892/ijmm.3.6.615. [DOI] [PubMed] [Google Scholar]
- 164.Yamaguchi M, Hamano T, Misawa H. Expression of Ca(2 +)-binding protein regucalcin in rat brain neurons: inhibitory effect on protein phosphatase activity. Brain Res Bull. 2000;52(5):343–348. doi: 10.1016/S0361-9230(00)00270-7. [DOI] [PubMed] [Google Scholar]
- 165.Tobisawa M, Yamaguchi M. Suppressive effect of endogenous regucalcin on protein tyrosine phosphatase activity in the nucleus of rat brain: attenuation with increasing age. Int J Mol Med. 2003;11(2):205–210. [PubMed] [Google Scholar]
- 166.Tobisawa M, Tsurusaki Y, Yamaguchi M. Decrease in regucalcin level and enhancement of protein tyrosine phosphatase activity in rat brain microsomes with increasing age. Int J Mol Med. 2003;12(4):577–580. [PubMed] [Google Scholar]
- 167.Yamaguchi M, Katsumata T. Enhancement of protein kinase activity in the cytosol of regenerating rat liver: regulatory role of endogenous regucalcin. Int J Mol Med. 1999;3(5):505–510. doi: 10.3892/ijmm.3.5.505. [DOI] [PubMed] [Google Scholar]
- 168.Mori S, Yamaguchi M. Hepatic calcium-binding protein regucalcin decreases Ca2 +/calmodulin-dependent protein kinase activity in rat liver cytosol. Chem Pharm Bull (Tokyo) 1990;38(8):2216–2218. doi: 10.1248/cpb.38.2216. [DOI] [PubMed] [Google Scholar]
- 169.Yamaguchi M, Mori S. Inhibitory effect of calcium-binding protein regucalcin on protein kinase C activity in rat liver cytosol. Biochem Med Metab Biol. 1990;43(2):140–146. doi: 10.1016/0885-4505(90)90019-W. [DOI] [PubMed] [Google Scholar]
- 170.Kurota H, Yamaguchi M. Inhibitory effect of calcium-binding protein regucalcin on protein kinase C activity in rat renal cortex cytosol. Biol Pharm Bull. 1998;21(4):315–318. doi: 10.1248/bpb.21.315. [DOI] [PubMed] [Google Scholar]
- 171.Kurota H, Yamaguchi M. Inhibitory effect of regucalcin on Ca2 +/calmodulin-dependent protein kinase activity in rat renal cortex cytosol. Mol Cell Biochem. 1997;177(1–2):239–243. doi: 10.1023/A:1006819507433. [DOI] [PubMed] [Google Scholar]
- 172.Hamano T, Yamaguchi M. Inhibitory role of regucalcin in the regulation of Ca2 + dependent protein kinases activity in rat brain neurons. J Neurol Sci. 2001;183(1):33–38. doi: 10.1016/S0022-510X(00)00476-7. [DOI] [PubMed] [Google Scholar]
- 173.Hamano T, Hanahisa Y, Yamaguchi M. Inhibitory effect of regucalcin on Ca(2 +)-dependent protein kinase activity in rat brain cytosol: involvement of endogenous regucalcin. Brain Res Bull. 1999;50(3):187–192. doi: 10.1016/S0361-9230(99)00184-7. [DOI] [PubMed] [Google Scholar]
- 174.Wu HY, Tomizawa K, Matsui H. Calpain-calcineurin signaling in the pathogenesis of calcium-dependent disorder. Acta Med Okayama. 2007;61(3):123–137. doi: 10.18926/AMO/32905. [DOI] [PubMed] [Google Scholar]
- 175.Liu X, Van Vleet T, Schnellmann RG. The role of calpain in oncotic cell death. Annu Rev Pharmacol Toxicol. 2004;44:349–370. doi: 10.1146/annurev.pharmtox.44.101802.121804. [DOI] [PubMed] [Google Scholar]
- 176.Baba T, Yamaguchi M. Stimulatory effect of regucalcin on proteolytic activity in rat renal cortex cytosol: involvement of thiol proteases. Mol Cell Biochem. 1999;195(1–2):87–92. doi: 10.1023/A:1006964114831. [DOI] [PubMed] [Google Scholar]
- 177.Yamaguchi M, Tai H. Calcium-binding protein regucalcin increases calcium-independent proteolytic activity in rat liver cytosol. Mol Cell Biochem. 1992;112(1):89–95. doi: 10.1007/BF00229647. [DOI] [PubMed] [Google Scholar]
- 178.Yamaguchi M, Nishina N. Characterization of regucalcin effect on proteolytic activity in rat liver cytosol: relation to cysteinyl-proteases. Mol Cell Biochem. 1995;148(1):67–72. doi: 10.1007/BF00929504. [DOI] [PubMed] [Google Scholar]
- 179.Linster CL, Van Schaftingen E. Vitamin C. Biosynthesis, recycling and degradation in mammals. FEBS J. 2007;274(1):1–22. doi: 10.1111/j.1742-4658.2006.05607.x. [DOI] [PubMed] [Google Scholar]
- 180.Koike K, Kondo Y, Sekiya M, Sato Y, Tobino K, Iwakami SI, Goto S, Takahashi K, Maruyama N, Seyama K, Ishigami A. Complete lack of vitamin C intake generates pulmonary emphysema in senescence marker protein-30 knockout mice. Am J Physiol Lung Cell Mol Physiol. 2010;298(6):L784–L792. doi: 10.1152/ajplung.00256.2009. [DOI] [PubMed] [Google Scholar]
- 181.Kondo Y, Sasaki T, Sato Y, Amano A, Aizawa S, Iwama M, Handa S, Shimada N, Fukuda M, Akita M, Lee J, Jeong KS, Maruyama N, Ishigami A. Vitamin C depletion increases superoxide generation in brains of SMP30/GNL knockout mice. Biochem Biophys Res Commun. 2008;377(1):291–296. doi: 10.1016/j.bbrc.2008.09.132. [DOI] [PubMed] [Google Scholar]
- 182.Sato Y, Kajiyama S, Amano A, Kondo Y, Sasaki T, Handa S, Takahashi R, Fukui M, Hasegawa G, Nakamura N, Fujinawa H, Mori T, Ohta M, Obayashi H, Maruyama N, Ishigami A. Hydrogen-rich pure water prevents superoxide formation in brain slices of vitamin C-depleted SMP30/GNL knockout mice. Biochem Biophys Res Commun. 2008;375(3):346–350. doi: 10.1016/j.bbrc.2008.08.020. [DOI] [PubMed] [Google Scholar]
- 183.Son TG, Zou Y, Jung KJ, Yu BP, Ishigami A, Maruyama N, Lee J. SMP30 deficiency causes increased oxidative stress in brain. Mech Ageing Dev. 2006;127(5):451–457. doi: 10.1016/j.mad.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 184.Sato T, Seyama K, Sato Y, Mori H, Souma S, Akiyoshi T, Kodama Y, Mori T, Goto S, Takahashi K, Fukuchi Y, Maruyama N, Ishigami A. Senescence marker protein-30 protects mice lungs from oxidative stress, aging, and smoking. Am J Respir Crit Care Med. 2006;174(5):530–537. doi: 10.1164/rccm.200511-1816OC. [DOI] [PubMed] [Google Scholar]
- 185.Ambs S, Glynn SA. Candidate pathways linking inducible nitric oxide synthase to a basal-like transcription pattern and tumor progression in human breast cancer. Cell Cycle. 2011;10(4):619–624. doi: 10.4161/cc.10.4.14864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Izumi T, Tsurusaki Y, Yamaguchi M. Suppressive effect of endogenous regucalcin on nitric oxide synthase activity in cloned rat hepatoma H4-II-E cells overexpressing regucalcin. J Cell Biochem. 2003;89(4):800–807. doi: 10.1002/jcb.10544. [DOI] [PubMed] [Google Scholar]
- 187.Ogita K, Takagi R, Oyama N, Okuda H, Ito F, Okui M, Shimizu N, Yoneda Y. Decrease in level of APG-2, a member of the heat shock protein 110 family, in murine brain following systemic administration of kainic acid. Neuropharmacology. 2001;41(3):285–293. doi: 10.1016/S0028-3908(01)00081-8. [DOI] [PubMed] [Google Scholar]
- 188.Zheng XY, Zhang HL, Luo Q, Zhu J. Kainic acid-induced neurodegenerative model: potentials and limitations. J Biomed Biotechnol. 2011;2011:457079. doi: 10.1155/2011/457079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Makker K, Agarwal A, Sharma R. Oxidative stress & male infertility. Indian J Med Res. 2009;129(4):357–367. [PubMed] [Google Scholar]
- 190.Crowell JA, Steele VE, Sigman CC, Fay JR. Is inducible nitric oxide synthase a target for chemoprevention? Mol Cancer Ther. 2003;2(8):815–823. [PubMed] [Google Scholar]
- 191.Lim S, Song BW, Cha MJ, Choi EJ, Ham O, Lee CY, Choi SY, Lee SY, Jang Y, Hwang KC. Differential expression of regucalcin (SMP30) and its function in hypoxic cardiomyocytes. Tissue Eng Regen Med. 2009;6(13):1273–1281. [Google Scholar]
- 192.Izumi T, Yamaguchi M. Overexpression of regucalcin suppresses cell death and apoptosis in cloned rat hepatoma H4-II-E cells induced by lipopolysaccharide, PD 98059, dibucaine, or Bay K 8644. J Cell Biochem. 2004;93(3):598–608. doi: 10.1002/jcb.20214. [DOI] [PubMed] [Google Scholar]
- 193.Tsurusaki Y, Yamaguchi M. Suppressive role of endogenous regucalcin in the enhancement of deoxyribonucleic acid synthesis activity in the nucleus of regenerating rat liver. J Cell Biochem. 2002;85(3):516–522. doi: 10.1002/jcb.10153. [DOI] [PubMed] [Google Scholar]
- 194.Yamaguchi M, Sakurai T. Inhibitory effect of calcium-binding protein regucalcin on Ca2(+)-activated DNA fragmentation in rat liver nuclei. FEBS Lett. 1991;279(2):281–284. doi: 10.1016/0014-5793(91)80168-3. [DOI] [PubMed] [Google Scholar]
- 195.Fukaya Y, Yamaguchi M. Overexpression of regucalcin suppresses cell death and apoptosis in cloned rat hepatoma H4-II-E cells induced by insulin or insulin-like growth factor-I. J Cell Biochem. 2005;96(1):145–154. doi: 10.1002/jcb.20485. [DOI] [PubMed] [Google Scholar]
- 196.Nakagawa T, Yamaguchi M. Overexpression of regucalcin suppresses apoptotic cell death in cloned normal rat kidney proximal tubular epithelial NRK52E cells: change in apoptosis-related gene expression. J Cell Biochem. 2005;96(6):1274–1285. doi: 10.1002/jcb.20617. [DOI] [PubMed] [Google Scholar]
- 197.Ogasawara J, Watanabe-Fukunaga R, Adachi M, Matsuzawa A, Kasugai T, Kitamura Y, Itoh N, Suda T, Nagata S. Lethal effect of the anti-Fas antibody in mice. Nature. 1993;364(6440):806–809. doi: 10.1038/364806a0. [DOI] [PubMed] [Google Scholar]
- 198.Elchuri S, Naeemuddin M, Sharpe O, Robinson WH, Huang TT. Identification of biomarkers associated with the development of hepatocellular carcinoma in CuZn superoxide dismutase deficient mice. Proteomics. 2007;7(12):2121–2129. doi: 10.1002/pmic.200601011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Kim W, Oe Lim S, Kim JS, Ryu YH, Byeon JY, Kim HJ, Kim YI, Heo JS, Park YM, Jung G. Comparison of proteome between hepatitis B virus- and hepatitis C virus-associated hepatocellular carcinoma. Clin Cancer Res. 2003;9(15):5493–5500. [PubMed] [Google Scholar]
- 200.Jahchan NS, Luo K. SnoN in mammalian development, function and diseases. Curr Opin Pharmacol. 2010;10(6):670–675. doi: 10.1016/j.coph.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Lamouille S, Derynck R. Oncogene and tumour suppressor: the two faces of SnoN. EMBO J. 2009;28(22):3459–3460. doi: 10.1038/emboj.2009.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Dallaglio K, Marconi A, Pincelli C. Survivin: a dual player in healthy and diseased skin. J Invest Dermatol. 2012;132(1):18–27. doi: 10.1038/jid.2011.279. [DOI] [PubMed] [Google Scholar]