Abstract
The motif “SYDE”, incorporating the protein kinase CK2 consensus sequence (S-x-x-E) has been found to be phosphorylated at both its serine and tyrosine residues in several proteins. Of special interest is the case of cystic fibrosis Transmembrane-conductance Regulator (CFTR), where this motif is close to the residue (F508), whose deletion is the by far commonest cause of cystic fibrosis. Intriguingly, however, CFTR S511 cannot be phosphorylated by CK2 to any appreciable extent. Using a number of peptide substrates encompassing the CFTR “SYDE” site we have recently shown that: (1) failure of CK2 to phosphorylate the S511YDE motif is due to the presence of Y512; (2) CK2 readily phosphorylates S511 if Y512 is replaced by a phospho-tyrosine; (3) the Src family protein tyrosine kinase Lyn phosphorylates Y512 in a manner that is enhanced by the deletion of F508. These data, in conjunction with the recent observation that by inhibiting CK2 the degradation of F508delCFTR is reduced, lead us to hypothesize that the hierarchical phosphorylation of the motif SYDE by the concerted action of protein tyrosine kinases and CK2 is one of the mechanisms that cooperate to the premature degradation of F508delCFTR.
Keywords: CFTR, F508delCFTR, SYDE motif, CK2, Lyn, Hierarchical phosphorylation
Background
Protein kinase CK2 is a very pleiotropic enzyme responsible for the generation of a substantial proportion of the whole eukaryotic phospho-proteome [1]. CK2 is implicated in a wide spectrum of cellular functions [2] and its abnormally high level generates a cellular environment favorable to neoplastic transformation [3].
The consensus sequence recognized by CK2 is specified by a carboxylic or pre-phosphorylated side chain at position n + 3 with respect to the target residue (S/T-x-x-E/D/pS/pT/pY), and is generally accompanied by additional acidic/pre-phosphorylated residues at position(s) nearby [4].
In the first nucleotide binding domain (NBD1) of cystic fibrosis Transmembrane-conductance Regulator (CFTR), a site fulfilling the CK2 consensus (S 511 YD E) is located just down-stream from a phenylalanine (F508), whose deletion is the by far commonest cause of cystic fibrosis. The F508delCFTR mutant in fact fails to traffic to the plasma membrane and is proteolytically degraded via the proteasome [5–8]. Even the tiny amount (<1 %) that reaches the membrane is less stable than its wild-type counterpart [9], albeit it retains its functionality as a chloride channel [10]. Given the proximity of F508 to the CK2 consensus and the remarkable conservation of the whole sequence encompassing both these elements, many efforts have been made to see if S511 phosphorylation by CK2 is in some way related to the F508 deletion. However, no appreciable phosphorylation of S511 could be detected either in the presence or in the absence of F508 [11].
An explanation for this unexpected outcome could be provided by scrutinizing the PhosphoSitePlus® database (http://www.phosphosite.org) [12], where the same SYDE motif found in CFTR (recurrent 84 times in the whole human proteome) is often retrieved with its seryl residue phosphorylated (see Table 1). Intriguingly, however, in the majority of these cases (six out of ten) its phosphorylation is accompanied by the phosphorylation of the adjacent tyrosine, consistent with the idea that the two events are inter-dependent.
Table 1.
A repertoire of pSYDE motifs. The PhosphoSitePlus® database (http://www.phosphosite.org) was searched for the SYDE sequence

This observation prompted us to hypothesize that failure of CK2 to phosphorylate serine in the SYDE motif could be due to the presence of Y512 acting as a negative determinant unless it is previously phosphorylated. A priori, this would be consistent with the knowledge that phospho-tyrosine can successfully replace glutamic and aspartic acid residues as a positive determinant for CK2 site targeting [13] and with the observation that CFTR Y512 phosphorylation has been already reported to take place in cells [14].
Our hypothesis is further corroborated by the recent finding that a peptide encompassing the 500–523 sequence of F508delCFTR is not appreciably phosphorylated by CK2 unless Y512 is replaced by alanine or, even more effectively, by phospho-tyrosine [15]: this provides clear-cut evidence that Y512 plays the role of a negative determinant, becoming, however, a positive determinant once it is phosphorylated.
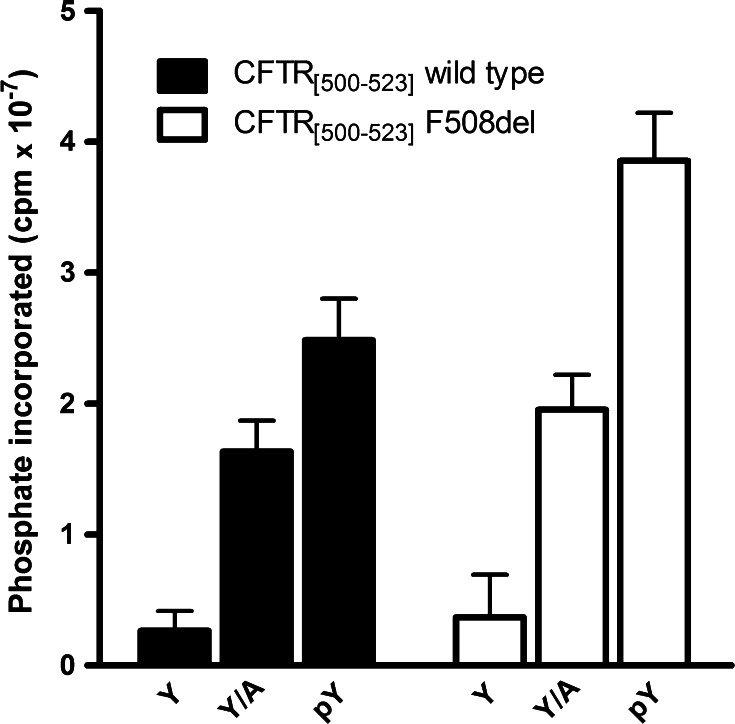
It should be noted in this respect that the phosphorylation of Y512 by the Src family protein tyrosine kinase Lyn is substantially enhanced by the F508 deletion, as it has been shown using either the 500–523 peptide or the whole NBD1 domain as a substrate [15]. Consistently, the F508delNBD1 domain previously phosphorylated by incubation with unlabeled ATP and Lyn is more susceptible to subsequent phospho-radiolabeling by CK2 than untreated F508delNBD1 [15]. On the other hand, the F508 deletion, which is more than doubling the phosphorylation of Y512 by Lyn [15], also increases, albeit to a lesser extent, the phosphorylation of the p-Y512 peptide by CK2 (see Fig. 1), suggesting that the SYDE motif of F508delCFTR is more susceptible to double phosphorylation than that of CFTR wild type.
Fig. 1.
Effect of the F508 deletion on the phosphorylation of peptides encompassing the CFTR 500-523 sequence either as such (Y) or with Y512 replaced by alanine (Y/A) or by phospho-tyrosine (pY). Solid bars wild-type peptides; open bars F508del peptides drown from [15]. Experimental conditions as in [15]
Outlook
Collectively taken, all available data support the working hypothesis schematically outlined in Fig. 2, according to which the F508 deletion makes Y512 more prone to phosphorylation by Lyn or by other tyrosine kinases with similar specificity. This in turn primes phosphorylation of S511 by CK2, an event which, by analogy with other similar situations [16–18], may commit the protein to accelerated degradation. Considering, moreover, that CFTR fragments encompassing the F508 deletion behave as allosteric activators of CK2 holoenzyme [11, 19], it is conceivable that the loop outlined in Fig. 2 through feedback activation of CK2, will also take place in F508delCFTR-expressing cells, resulting in accelerated degradation of the protein. A scenario like this would also account for a number of phenomenological observations already available in the literature, notably the defective maturation and reduced cell surface exposition of the CFTR mutant Y512A [20], which, unlike the wild type, is expected to be susceptible to phosphorylation by CK2 at S511 (see Fig. 1) and the increased accumulation of F508delCFTR upon cell treatment with a very specific CK2 inhibitor [21].
Fig. 2.
Schematic representation of the proposed mechanism by which CK2 may contribute to accelerated degradation of CFTR. The F508 deletion makes Y512 more prone to phosphorylation by the Src family kinase Lyn [15]: this primes S511 phosphorylation by CK2, an event favoring CFTR degradation, which in turn generates fragments capable of stimulating CK2 activity [19] through a feed-back effect
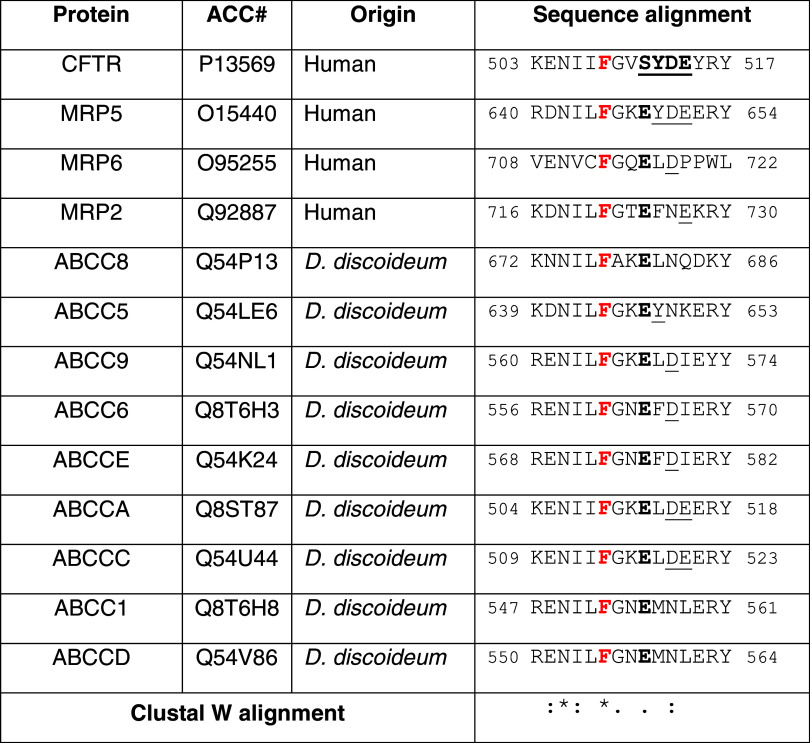
In conclusion, we propose that the motif SYDE, found in its bis-phosphorylated form in at least six phospho-proteins (Table 1), has evolved to provide a special case of hierarchical phosphorylation [22, 23], where under basal conditions the CK2 consensus SYDE is locked by the tyrosine, representing a negative determinant that can be removed upon activation of protein tyrosine kinases expectedly but not necessarily of the Src family. Phosphorylation of the tyrosine residue by PTKs converts it from a negative to a positive determinant (Fig. 1). Pertinent to this may be the observation that while the critical phenylalanine (F508), whose deletion is causative of cystic fibrosis, and the amino acids flanking it are highly conserved in the other members of the human ABC-C transporters sub-family [24], as well as in homologous proteins of lower eukaryotes, such as Dictyostelium discoideum [25], the SYDE motif just downstream from it is unique to CFTR (ABC-C7) (Table 2), suggesting that it has evolved to ensure a specialized regulatory function not shared with the other members of the ABC-C sub-family. Interestingly, moreover, the seryl residue of the SYDE motif in some members of the human ABC-C subfamily and in the majority of those of D. discoideum is replaced by a glutamic acid (see Table 2), suggestive of a constitutive role for a negatively charged side chain, which in CFTR alone has been replaced by a reversible regulatory mechanism relying on the intervention of two kinases. This device seems to be triggered by the basic defect, i.e., deletion of F508, which makes Y512, and to a lesser extent S511, more prone to phosphorylation by Lyn and CK2, respectively, ([15]; Fig. 1). This may provide a link between CK2 activity and premature degradation of F508delCFTR, thus including CK2 among molecules whose targeting may contribute to overcome/attenuate the consequences of the basic defect of cystic fibrosis.
Table 2.
Uniqueness of the human CFTR “SYDE” motif across proteins of the ABC–C subfamily
To note that in all ABC-C proteins the phenylalanine, F508 in CFTR, is conserved while the SYDE motif is not. Listed are ABC-C proteins, in which S511 of the CFTR SYDE motif is replaced by glutamic acid (see text)
Acknowledgments
We thank Prof. P.R. Fisher (La Trobe University, Australia) for helpful suggestions about Dictyostelium discoideum ABC proteins. Financial support from the Fondazione per la Ricerca sulla Fibrosi Cistica (grant #3/2011 adopted by Delegazione FFC della Valdadige and Associazione Trentina FC Fiaba “Il Villaggio di Natale” in ricordo di Massimiliano e Sebastiano) is gratefully acknowledged.
References
- 1.Salvi M, Sarno S, Cesaro L, et al. Extraordinary pleiotropy of protein kinase CK2 revealed by weblogo phosphoproteome analysis. Biochim Biophys Acta. 2009;1793:847–859. doi: 10.1016/j.bbamcr.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 2.Litchfield DW. Protein kinase CK2: structure, regulation and role in cellular decisions of life and death. Biochem J. 2003;369:1–15. doi: 10.1042/BJ20021469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pinna LA, Ruzzene M. How do protein kinases recognize their substrates? Biochim Biophys Acta. 1996;1314:191–225. doi: 10.1016/S0167-4889(96)00083-3. [DOI] [PubMed] [Google Scholar]
- 4.Meggio F, Pinna LA. One-thousand-and-one substrates of protein kinase CK2? FASEB J. 2003;17:349–368. doi: 10.1096/fj.02-0473rev. [DOI] [PubMed] [Google Scholar]
- 5.Kartner N, Augustinas O, Jensen TJ, et al. Mislocalization of ΔF508 CFTR in cystic fibrosis sweat gland. Nat Genet. 1992;1:321–327. doi: 10.1038/ng0892-321. [DOI] [PubMed] [Google Scholar]
- 6.Ward CL, Kopito RR. Intracellular turnover of cystic fibrosis transmembrane conductance regulator. Inefficient processing and rapid degradation of wild-type and mutant proteins. J Biol Chem. 1994;269:25710–25718. [PubMed] [Google Scholar]
- 7.Jensen TJ, Loo MA, Pind S, et al. Multiple proteolytic systems, including the proteasome, contribute to CFTR processing. Cell. 1995;83:129–135. doi: 10.1016/0092-8674(95)90241-4. [DOI] [PubMed] [Google Scholar]
- 8.Qu BH, Thomas PJ. Alteration of the cystic fibrosis transmembrane conductance regulator folding pathway. J Biol Chem. 1996;271:7261–7264. doi: 10.1074/jbc.271.13.7261. [DOI] [PubMed] [Google Scholar]
- 9.Heda GD, Tanwani M, Marino CR. The Delta F508 mutation shortens the biochemical half-life of plasma membrane CFTR in polarized epithelial cells. Am J Physiol Cell Physiol. 2001;280:C166–C174. doi: 10.1152/ajpcell.2001.280.1.C166. [DOI] [PubMed] [Google Scholar]
- 10.Dalemans W, Barbry P, Champigny G, et al. Altered chloride ion channel kinetics associated with the delta F508 cystic fibrosis mutation. Nature. 1991;354:526–528. doi: 10.1038/354526a0. [DOI] [PubMed] [Google Scholar]
- 11.Pagano MA, Arrigoni G, Marin O, et al. Modulation of protein kinase CK2 activity by fragments of CFTR encompassing F508 may reflect functional links with cystic fibrosis pathogenesis. Biochemistry. 2008;47:7925–7936. doi: 10.1021/bi800316z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hornbeck PV, Kornhauser JM, Tkachev S, et al. PhosphoSitePlus: a comprehensive resource for investigating the structure and function of experimentally determined post-translational modifications in man and mouse. Nucleic Acids Res. 2011;40:D261–D270. doi: 10.1093/nar/gkr1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meggio F, Perich JW, Reynolds EC, Pinna LA. Phosphotyrosine as a specificity determinant for casein kinase-2, a growth related Ser/Thr-specific protein kinase. FEBS Lett. 1991;279:307–309. doi: 10.1016/0014-5793(91)80174-2. [DOI] [PubMed] [Google Scholar]
- 14.Mendes AI, Matos P, Moniz S, et al. Antagonistic regulation of cystic fibrosis transmembrane conductance regulator cell surface expression by protein kinases WNK4 and spleen tyrosine kinase. Mol Cell Biol. 2011;31:4076–4086. doi: 10.1128/MCB.05152-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cesaro L, Marin O, Venerando A, et al. Phosphorylation of cystic fibrosis transmembrane conductance regulator (CFTR) serine-511 by the combined action of tyrosine kinases and CK2: the implication of tyrosine-512 and phenylalanine-508. Amino Acids. 2013;45:1423–1429. doi: 10.1007/s00726-013-1613-y. [DOI] [PubMed] [Google Scholar]
- 16.Torres J, Pulido R. The tumor suppressor PTEN is phosphorylated by the protein kinase CK2 at its C terminus. Implications for PTEN stability to proteasome-mediated degradation. J Biol Chem. 2001;276:993–998. doi: 10.1074/jbc.M009134200. [DOI] [PubMed] [Google Scholar]
- 17.Kato T, Jr, Delhase M, Hoffmann A, Karin M. CK2 Is a C-terminal ikappab kinase responsible for NF-kappaB activation during the UV response. Mol Cell. 2003;12:829–839. doi: 10.1016/S1097-2765(03)00358-7. [DOI] [PubMed] [Google Scholar]
- 18.Scaglioni PP, Yung TM, Cai LF, et al. A CK2-dependent mechanism for degradation of the PML tumor suppressor. Cell. 2006;126:269–283. doi: 10.1016/j.cell.2006.05.041. [DOI] [PubMed] [Google Scholar]
- 19.Pagano MA, Marin O, Cozza G, et al. Cystic fibrosis transmembrane regulator fragments with the Phe508 deletion exert a dual allosteric control over the master kinase CK2. Biochem J. 2010;426:19–29. doi: 10.1042/BJ20090813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luz S, Kongsuphol P, Mendes AI, et al. Contribution of casein kinase 2 and spleen tyrosine kinase to CFTR trafficking and protein kinase A-induced activity. Mol Cell Biol. 2011;31:4392–4404. doi: 10.1128/MCB.05517-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Venerando A, Franchin C, Cant N, et al. Detection of phospho-sites generated by protein kinase CK2 in CFTR: mechanistic aspects of Thr1471 phosphorylation. PLoS One. 2013 doi: 10.1371/journal.pone.0074232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roach PJ. Multisite and hierarchal protein phosphorylation. J Biol Chem. 1991;266:14139–14142. [PubMed] [Google Scholar]
- 23.Ubersax JA, Ferrell JE. Mechanisms of specificity in protein phosphorylation. Nat Rev Mol Cell Biol. 2007 doi: 10.1038/nrm2203. [DOI] [PubMed] [Google Scholar]
- 24.Vasiliou V, Vasiliou K, Nebert DW. Human ATP-binding cassette (ABC) transporter family. Hum Genomics. 2009;3:281–290. doi: 10.1186/1479-7364-3-3-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anjard C, Loomis WF. Evolutionary analyses of ABC transporters of Dictyostelium discoideum . Eukaryot Cell. 2002;1:643–652. doi: 10.1128/EC.1.4.643-652.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]