Abstract
Interleukin (IL)-32 is known as a proinflammatory cytokine that is likely involved in several diseases, including infections, chronic inflammation, and cancer. Since the first report in 2005, IL-32 has been the subject of numerous studies to unravel the biological function of this molecule. For example, silencing of endogenous IL-32 in primary or cell lines of human origin consistently suppressed responses to Toll-like receptors. The protein folding structure of the six isoforms of IL-32 does not resemble that of any classical cytokine and as of this writing, a specific IL-32 receptor has not been identified. Instead, we propose a mechanism by which exposure to extracellular IL-32 or overexpression of the molecule results in binding to intracellular partners that influences functions such as gene expression, cell death, or survival. As such, this review offers insights into the role of IL-32 in several diseases, host defense, inflammation, immune function, and cancer. Finally, possibilities to target IL-32 in several diseases are proposed.
Keywords: Interleukin-32, Cytokines, Viral infections, Host defense, Inflammatory diseases, Cancer
The discovery of interleukin (IL)-32
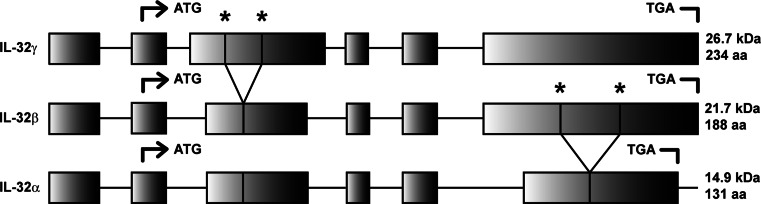
The first report on the molecule that is the topic of this review was in 1992. Dahl et al. [1] reported a protein that was highly expressed in activated T- and NK-cells and therefore it was called NK4. This NK4 protein was rapidly upregulated in human PBMCs after stimulation with PHA, a lectin, that primary activates T-cells. The expression could be blocked by cycloheximide, indicating that it was an early activated gene. The same report demonstrated that the NK4 gene was highly polymorphic. Sequence analysis revealed that the NK4-encoded protein had a predicted molecular mass of 27 kDa. In addition, it was suggested that the NK4 protein contained a RGD motif, which is important for adhesion of cells and therefore it was predicted that NK4 might play a role in the adhesive properties of cells. Nevertheless, at that time and for the next 13 years, the biological function of NK4 was not known. In 2005, using a microarray technology and an IL-18 responsive cell line, the NK4 gene was found as one of the most upregulated genes. Kim et al. [2] showed for the first time that the NK4 protein had biological function and a recombinant form of the protein induced several proinflammatory cytokines, including TNF-α and IL-8. Since the NK4 protein had proinflammatory properties, the name NK4 was renamed to interleukin (IL)-32. However, the structure of IL-32 did not match the sequence homology seen in most of the known cytokines. IL-32 mRNA was predominantly found in immune tissues and cells, but also in non-immune cells such as epithelial cells. IL-32 is located in the human chromosome 16p13.3. IL-32 occurs in four major splice variants isoforms of the mRNA, namely IL-32α, IL-32β, IL32γ, and IL-32δ [2]. Later, two new isoforms of IL-32, IL-32ε and IL-32ζ, were found within the IL-32 mRNA transcript, however IL-32β seems to be most abundant [3] (Fig. 1).
Fig. 1.
Frequently found isoforms of IL-32. IL-32α, IL-32β, and IL-32γ are the most observed isoforms of IL-32. IL-32γ is the most abundant transcript and by mRNA splicing, IL-32β and IL-32α mRNA transcripts are generated. Start (ATG) and stop (TGA) codons, mRNA splice sites (asterisk), molecular weight (MW), and number of amino acids (AA) are indicated
The different isoforms of IL-32 originate by splicing of pre-mRNA of the isoform IL-32γ. Several reports demonstrated that different transcripts of IL-32 occur both in vitro [3, 4] as well as in vivo [4]. It remains to be elucidated why the IL-32γ mRNA transcripts are spliced and if this phenomenon is similar in all cells. IL-32γ is the most potent isoform of IL-32, with respect to cell death and cell activation, and this may explain why IL-32γ is spliced to less harmful isoforms of IL-32, e.g., IL-32β and α [4, 5]. Of great interest was the finding that modulation of the splice-site in the IL-32γ mRNA resulted in a splice-resistant mutant. When this IL-32γ mutant was overexpressed in THP-1 cells, there was enhanced production of proinflammatory cytokines, such as IL-1β and IL-6 [4]. This observation contrasts to overexpression of spliceable IL-32γ that resulted in IL-32β and IL-32α isoforms in THP-1 cells, in which no differences were found on IL-1β and IL-6 production compared with the control group.
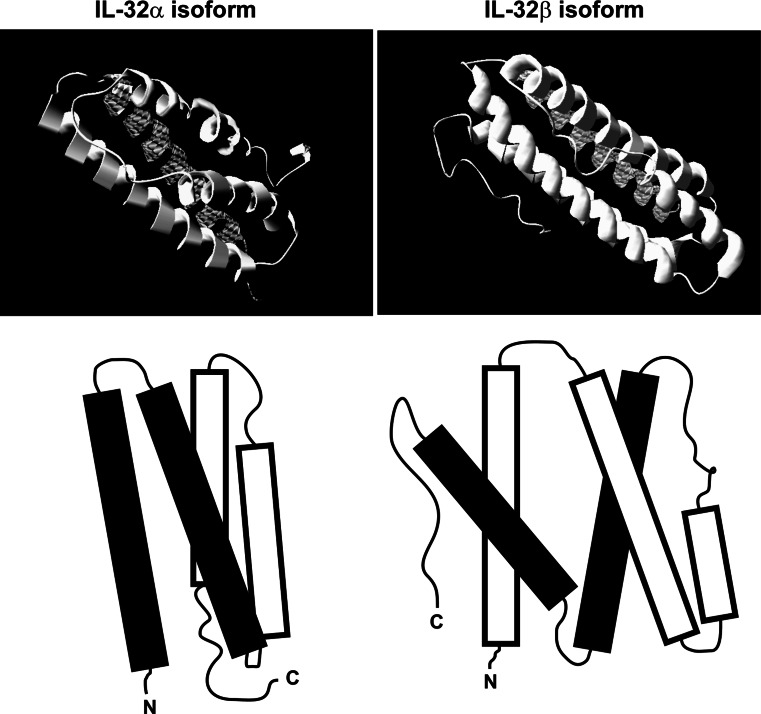
In addition to promoting cytokine production, overexpression of endogenous IL-32γ caused cell death, which in contrast did not occur with the IL-32α isoform [6]. The differential potency of the IL-32 isoforms was described in several reports [4–7], but the basis of these differences in potency between the isoforms remains unexplored. The difference in the size of the isoforms, ranging from 14.9 kDa (IL-32α) to 26.7 kDa (IL-32γ), and the tertiary structure of the isoforms may be part of the explanation for this phenomenon (Fig. 2).
Fig. 2.
Proposed structure of IL-32α and IL-32β. By using I-TASSER software, the structure of several IL-32 isoforms was predicted based on the amino acid sequence [6, 73]. Only the IL-32α and IL-32β models are shown since their modeling score was acceptable, whereas the modeling of IL-32γ was not sufficiently reliable [6]
The endogenous level of IL-32 can be modulated in immune cells by exposure to a plethora of stimuli. Pathogen-related agents, such as lipopolysaccharide (LPS), muramyl dipeptide (MDP), and double-stranded RNA (poly I:C), but also several cytokines such as TNF-α and IFN-γ induced IL-32 [8–12]. Exposure of monocytes, macrophages, or endothelial cells to these stimuli induced the expression of endogenous IL-32, both on mRNA and protein levels. Although the amount of endogenous IL-32 protein was low and difficult to determine, in most reports isoforms of IL-32 mRNA levels were examined using real-time PCR. However, the evaluation of IL-32 protein expression is still hampered by to the lack of validated quantification assays.
One of the most noteworthy observations is that IL-32 is still not found in rodents, such as mice and rats. Many efforts have been undertaken to find proteins that resemble human IL-32 in mice. It appears that in mice the IL32 gene is located between the MMP25 and ZSCAN10 gene, however a large part of the IL32 gene is missing and it is still unknown whether a functional transcript is produced. In other species, such as pigs, cows, and horses, IL-32 homologs are present. In an attempt to find the IL-32 homolog in mice, we used systems biology to search for the gene coding for IL-32. However, no sequence was found in the murine genome that reveals any homology with the human IL32 gene (unpublished data LJ). It is remarkable that humans and other mammals express the IL32 gene in contrast to rodents because these latter animals evolved much later.
The role of IL-32 in host defense, inflammatory diseases, and cancer
Since the first description of functional IL-32 in 2005 by Kim et al., a large number of reports have been published in which IL-32 was associated with numerous diseases, ranging from infectious diseases, chronic inflammation, and cancer [9, 11, 13–23].
Viral infections
Circulating levels of IL-32 were measured in patients with H1N1 influenza infections and found to be significantly elevated [24]. The effect of recombinant IL-32γ was also assessed in the WISH cell line infected with vesicular stomatitis virus. Unlike the studies of Zepp et al. [23] who reported antiviral activity of recombinant IL-32γ, recombinant IL-32γ did not result in anti-viral activity in another laboratory [24]. However, supernatants from the human macrophage line THP-1 cells exposed to recombinant IL-32γ did possess antiviral activity in WISH cells infected with vesicular stomatitis virus [24]. Characterization of the anti-viral activity of the THP-1 supernatant revealed transferrin [24].
Influenza virus induced several genes in human epithelial cells: IFN-β, type III interferons as well as IL-1α, IL-1β, IL-6, IL-23, IL-12, and IL-32γ [25]. In another study, elevated levels of circulating IL-32 have been reported in 108 subjects with influenza infection compared to 115 healthy controls [16]. In human lung epithelial cells, IL-32 expression was shown to be dependent on cyclooxygenase-2 as the addition of inhibitors of PGE2 suppressed IL-32 expression. Silencing of endogenous IL-32 in these epithelial cells increased PGE2 production [16]. In another study, the authors concluded that although influenza viral infection induced IL-32, the cytokine IL-32 then serves to inhibit further viral replication [26].
Another study focused on the role of IL-32 in the suppression of HIV-1 infection using small interfering (si)RNA to silence endogenous IL-32 in freshly HIV-1 infected PBMC. When PBMC were pretreated with siRNA to IL-32, IL-6, IFN-γ, and TNF-α were reduced compared to scrambled siRNA [18]. Unexpectedly, HIV-1 production (as measured by p24) increased fourfold in these same PBMC when endogenous IL-32 was reduced [18]. Because IFN-γ was lower in siIL-32-treated PBMC, IFN-γ bioactivity was blocked, which enhanced the augmentation of p24 by siIL-32. Blockade of IFN-α/β bioactivity in IL-32-stimulated U1 macrophagic cells revealed that IFN-α conveys the anti-HIV-1 effect of recombinant IL-32γ [18]. In addition, silencing of endogenous IL-32 reduced the levels of Th1 and proinflammatory cytokines, which contribute to the anti-HIV-1 property of IL-32.
Mycobacterial infections
The first study that examined a possible role for IL-32 in mycobacterial infections revealed that Mycobacterium tuberculosis and Mycobacterium bovis induced IL-32 from human blood monocytes in vitro was dependent on IFN-γ [11]. Furthermore, the role for IFN-γ was actually a requirement for IL-18 production, which in turn was dependent on caspase-1. IL-32 expression was studied in patients with Mycobacterium avium intracellulare infections where a highly significant level of IL-32 was detected using immunohistochemistry compared with lung tissue from healthy subjects [27]. Expression was most prevalent in type I lung epithelial cells. IL-32 was also found in both type II alveolar cells and alveolar macrophages. In human monocyte-derived macrophages, recombinant IL-32γ significantly reduced the growth of intracellular M. avium [27]. The anti-mycobacterial effect of IL-32 may be due, in part, to increased apoptosis of infected cells.
However, a role for IL-32 in live mycobacterial infections was not addressed in these studies. Macrophagic cells are both a harbor for M. tuberculosis as well as a defense against the infection. Although M. tuberculosis lives in macrophages, macrophages can also kill the organism under conditions of apoptosis. To study a possible role for IL-32 in the survival of M. tuberculosis in human macrophages, endogenous IL-32 was silenced in the human macrophagic cell line, THP-1. Silencing of endogenous IL-32 in these cells resulted in decreased production of TNF-α, IL-1β, and IL-8 induced by M. tuberculosis [28]. Associated with the reduction in these cytokines was an increase in the number of organisms isolated from these cells [28]. In addition, there was an increase in the death in THP-1 cells infected with M. tuberculosis when exposed to recombinant IL-32γ. Consistent with this finding was the loss of apoptosis in THP-1 cell deprived of endogenous IL-32. Apoptosis in THP-1 cells exposed to recombinant IL-32 was due to an increase in caspase-3. The authors of that study concluded that IL-32 plays a beneficial host defense role against M. tuberculosis.
In further studies on a possible role for IL-32 in live mycobacterial infections, a strain of mice was generated that expressed IL-32γ under the surfactant protein C (SPC) promoter. This promoter restricts the expression of the gene to the type II pulmonary epithelial cell. Mice transgenic for lung IL-32γ were infected with live M. tuberculosis and studied for survival. Compared to wild-type mice, the IL-32γ transgenic mice exhibited greater survival from the infection compared to the wild type [29]. In addition, the number of M. tuberculosis organisms isolated from the lungs of the transgenic mice were significantly lower compared to the number isolated from wild-type mice. These studies are consistent with the in vitro data that IL-32 expression in human macrophages serves to protect the host by facilitating apoptosis of the host cell and thereby depriving M. tuberculosis of a protected survival as an intracellular microorganism.
Like M. tuberculosis infection, infection with M. leprae is a worldwide problem with considerable economic costs to developing countries. There are two forms of leprosy: the tuberculoid form of the disease is a successful containment of the organism in the granuloma, and the aggressive form of the disease is characterized by dissemination, and represents a failure of the host to contain the infection. The role of IL-32 was studied in the cells of subjects with M. leprae. Recombinant IL-32 induced human blood monocytes to differentiate into dendritic cells (DC). The presentation of antigen by IL-32 differentiated DC was greater than DC differentiated by granulocyte–macrophage colony-stimulating factor. Expression of both NOD2 and IL-32 by DC at the site of leprosy infection correlated with the type of leprosy. In patients with the restrictive tuberculoid form of leprosy, the expression of endogenous IL-32 and NOD2 was high; in contrast, the expression was low in cells from subjects with the progressive form of the disease [12]. The authors concluded that the leprosy antigen triggered NOD2 and that the form of the disease was dependent on IL-32-dependent differentiation of DC.
Inflammatory diseases
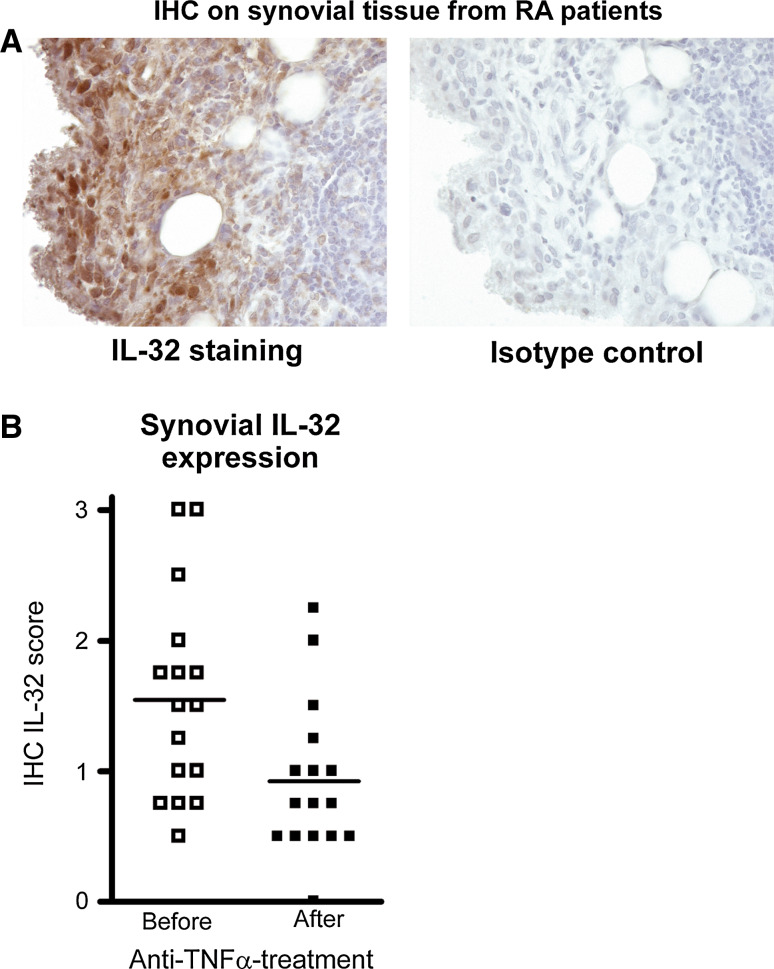
IL-32 is associated with several inflammatory diseases, including rheumatoid arthritis (RA), ankylosing spondylitis, chronic obstructive pulmonary disease (COPD), graft-versus-host disease (GVHD), chronic rhinosinusitis, and atherosclerosis [9, 13, 14, 30–34]. For example, IL-32 expression was elevated in tissue specimens obtained for these patients groups, both at the mRNA and protein level. In RA, synovial IL-32 expression correlated with inflammation, TNF-α, and IL-1β levels. In addition, the local IL-32 expression was associated with acute phase protein CRP and ESR [14]. Since TNF-α is one of the drivers of synovial inflammation and since IL-32 overexpression results in increased TNFs levels, it was suggested that IL-32 might be crucial for the autoinflammatory loop found in joints of RA patients. Heinhuis et al. [9] convincingly showed that indeed intracellular IL-32 in RA synovial fibroblasts was strongly upregulated after TNF-α exposure and the enhanced production of IL-6 and IL-8 induced by TNF-α was dependent on IL-32. Synovial tissue biopsies from RA patients that respond well to anti-TNF-α therapy exhibited less IL-32 expression, underscoring the inflammatory loop between TNF-α and IL-32 [9]. Also in COPD, it was reported that IL-32 protein expression in the lung specimens correlates with inflammatory markers, cell influx, and TNF-α levels. Moreover, IL-32 was linked to pathologic changes in the alveolar walls of the COPD patients [13]. Epithelial cells isolated from patients suffering from chronic rhinosinusitis produced more IL-32 when stimulated with TNF-α or IFN-γ. Together with the observation that tissue specimens from nasal polyps contain elevated IL-32 mRNA levels it was concluded that IL-32 might be crucial for the development of chronic rhinosinusitis [33] (Fig. 3).
Fig. 3.
IL-32 expression in RA synovial tissue and effect of anti-TNF-α treatment on local IL-32 expression. a IL-32 expression in RA synovial tissue specimen. Macrophage-like cells exhibit the highest level of IL-32, whereas the antibody (isotype) control was negative. b Synovial biopsies from 16 RA patients were analyzed for IL-32 expression by IHC before and after anti-TNF-α treatment
Cancer
In recent years, accumulative evidence has been published on the role of IL-32 in cell death and cancer-related diseases. One of the first reports indicating a potential role of IL-32 in cancer revealed that in chronic myelomonocytic leukemia, IL-32 expression was markedly reduced [35]. In contrast, Marcondes et al. demonstrated in the same report that in myelodysplastic syndrome (MDS), IL-32 expression was elevated and associated with enhanced apoptosis-mediated cell death of the bone marrow stem cells. These data suggested that endogenous IL-32 levels modulated cell survival. This concept was supported by the findings of several investigators who attempted to produce isoforms of the IL-32 proteins using transfection of IL-32 in HEK cells. HEK cells expressing high levels of IL-32β or IL-32γ died. In contrast, overexpression of IL-32α in human mammalian cell lines did not result in cell death [6].
In addition to MDS or leukemia, other cancers are associated with IL-32. IL-32 expression was noted in gastric cancer, hepatocellular carcinoma, lung cancer, and pancreatic cancer [15, 19, 21, 36]. Previously, it was shown that in the HeLa cancer cell line treated with the anti-cancer drug Cisplatin (Cadila Healthcare), there were markedly elevated levels of IL-32 [37]. In addition, it was demonstrated that caspase-3 was activated in Cisplatin-treated cells, suggesting an IL-32-caspase-3-mediated cell death pathway. This observation is consistent with the reports of Heinhuis et al. [6], showing that cell death, induced by endogenous overexpression of IL-32γ, was driven by caspase-3.
MicroRNAs (miRNAs), regulators of post-transcriptional gene expression and alterations in some miRNAs, is often linked to tumorigenesis. In fact, reduced expression of miRNA-205 was found in several types of cancer. MiRNA-205 expression has been shown to regulate IL-32 levels in cancer cells [38]. That study reported that miRNA-205 targets the IL-32 promoter (−631/−610 bp) and induced transcription and production of IL-32. Majid et al. [38] also demonstrated that in prostate cancer cells miRNA-205 was significantly downregulated and that overexpression of miRNA-205 resulted in impaired prostate cancer cell growth via apoptosis. These findings were corroborated in renal cell carcinoma, melanoma, and glioblastoma. Overexpression of miRNA-205 induced apoptosis, cell cycle arrest, impaired cell viability, clonability, and invasive properties of renal carcinoma, glioma- and melanoma cells [39–41]. Taken together, there is evolving evidence that IL-32 is a crucial mediator in cancer, and future research will be needed to investigate the precise role of endogenous IL-32 isoforms in the control of tumorigenesis. Indeed, targeting of the regulation of endogenous IL-32 may be a novel therapeutic approach for the treatment of cancer.
IL-32 signaling; extra- or intra-cellular pathways?
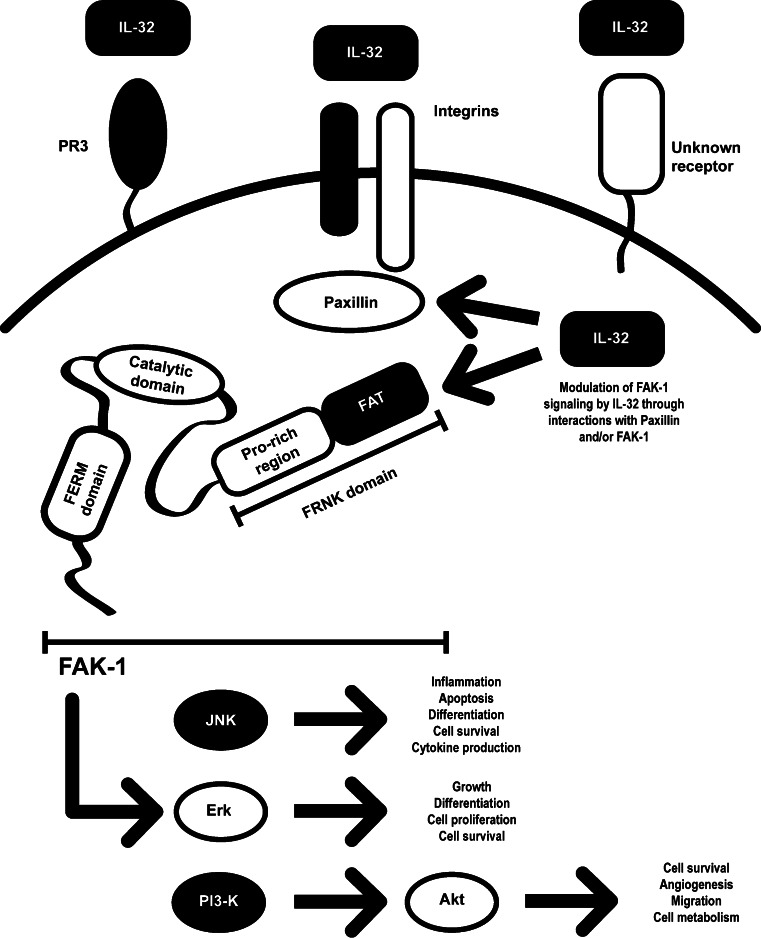
Since the first report on IL-32 by Kim et al., several studies have explored the IL-32 signaling cascade. Initially, it was thought that IL-32 signaling pathway was mediated via NF-κB and p38 MAPK pathways [2]. In another study, it was shown that IL-32 activates Erk1/2 and PI3 K/Akt pathways when osteoclasts precursors were exposed to IL-32 [42]. Furthermore, kinome analysis demonstrated that IL-32-exposed human macrophage-like THP-1 cells resulted in the phosphorylation of p300 and DAPK-1 [43]. It appeared that IL-32 can synergize with NOD1/NOD2 ligands that drives the production of IL-1β and IL-6 through a caspase-1-dependent mechanism [10]. Also, it has been reported that exposure of auditory cells of the inner ear to recombinant IL-32 resulted in a rise in intracellular Ca2+ followed by elevated IL-1β levels [44]. In contrast to previous reports, Oh et al. [45] demonstrated that in colon cancer cells, IL-32γ overexpression downregulated NF-κB and STAT3 signaling pathways. All the above-described intracellular signaling cascades have been linked to IL-32-mediated cell activation. However, since an unambiguous IL-32 receptor that would transmit a signal has not been isolated, differential uptake of IL-32 by cells may determine the downstream signaling pathway. Nevertheless, IL-32 can bind to membrane proteins such as integrins [6]. In an attempt to isolate an IL-32-binding protein in concentrated human urine as a soluble form of a cell bound IL-32 receptor, Novick et al. employed ligand-specific affinity chromatography using recombinant IL-32α co-valently immobilized on a matrix. Urinary proteinase-3 (PR3) bound specifically IL-32α with a high affinity (dissociation constant of 2.65 ± 0.4 nM) as well as to neutrophil-derived PR3 (dissociation constant of 1.2 ± 0.05) [46]. In addition, in some studies, the enzymatic action of PR3 increased the bioactivity of IL-32 supporting the concept that IL-32 is processed by PR3 [47]. It was also shown that IL-32 activity, induced by a mixed lymphocyte culture (MLC), could be modulated by the addition of alpha-1 antitrypsin (AAT), an inhibitor of PR3. These data are consistent with a role for PR3 in increasing the bioactivity of endogenous IL-32 [34].
Heinhuis et al. [6] demonstrated that IL-32 binds to cell surface integrins. Since IL-32 contains a RGD-motif and it is known that RGD-motifs binds to surface integrins, Heinhuis investigated the nature of IL-32 binding to integrins. The experiments revealed that IL-32 binds to the integrins αVβ3 and αVβ6, but not to αVβ8 [6]. Integrins are involved in cell signaling and are important for several cell functions, including cell adhesion, survival, and cytokine production [48, 49]. Hence, it has been suggested that αVβ3 and αVβ6 could be the receptors for extracellular IL-32 [6]. However, the majority of reports on IL-32 indicate that IL-32 is an intracellularly expressed protein. Taking this into account, extracellular IL-32 signaling is only possible when IL-32 is released after cell death. Since the structure of IL-32 does not resemble a classical cytokine, modeling software was used to examine the structure of IL-32. IL-32 has similarities with focal adhesion targeting region (FAT) of focal adhesion kinase (FAK-1) [6]. FAT targets FAK-1 to cluster with integrin via paxillin binding [50]. FAK and paxillin are two focal adhesion–associated proteins that have both a crucial function in mediating signals downstream of integrins [51]. These signals regulate important biological cell functions, such as migration, proliferation, and survival [52]. Since the structure of IL-32 partly resembles FAT, IL-32 might function as a regulator of FAK-1 activity. IL-32-mediated cell death could be inhibited by modulation of FAK-1 phosphorylation, using specific inhibitors [6]. In addition, IL-32 could bind to paxillin and FAK, indicating that intracellular IL-32 is an important mediator in the integrin-FAK signaling pathway (Fig. 4).
Fig. 4.
IL-32-integrin-FAK signaling pathway. IL-32 binds intracellularly to Paxillin and FAK, both components of the integrin signaling cascade. IL-32 resembles the FAT region of FAK and thereby interferes with the Paxillin–FAK binding. By interfering with the integrin pathway, IL-32 can modulate cell homeostasis through FAK and subsequent downstream pathways such as PI3 K, Erk, and JNK [5, 6]. Additionally, extracellular IL-32 can interact with integrins, PR3, or an unknown IL-32 receptor
The influence of IL-32 on cell function
The modulation of intracellular expression of IL-32 has been studied in different ways. Upregulation of IL-32 was explored by the addition of several exogenous stimuli to cells or IL-32 was overexpressed by using plasmids of adenoviral constructs approaches. Many studies have been published that reported IL-32 induction in a wide range of cells, including PBMCs, monocytes, NK-cells, T-cells, fibroblasts, keratinocytes, and endothelial cells by many stimuli [1, 9, 11, 53, 54]. Most reports demonstrated that IL-32 is predominantly expressed intracellularly, but membrane-bound expression has also been described [8]. Hasegawa et al. [8] showed that IL-32 was localized in lipid droplet structures that were associated with the membrane after exposure to IFN-γ and TNF-α. The exogenous stimuli that induce expression of IL-32 in various cell types range from cytokines to bacteria or bacterial products activating pattern recognition receptors, such as TLR and NLR [2, 9, 11, 12]. In addition, it has been shown that viruses or viral ligands are also potent inducers of endogenous IL-32 expression [22, 26, 55, 56]. Also, oxidative stress in combination with IFN-γ exposure was suggested as a potent inducer of IL-32 expression lung epithelial cells, suggesting a role for IL-32 in COPD [57]. The precise pathways that lead to elevated levels of endogenous or membrane-bound IL-32 have not been elucidated.
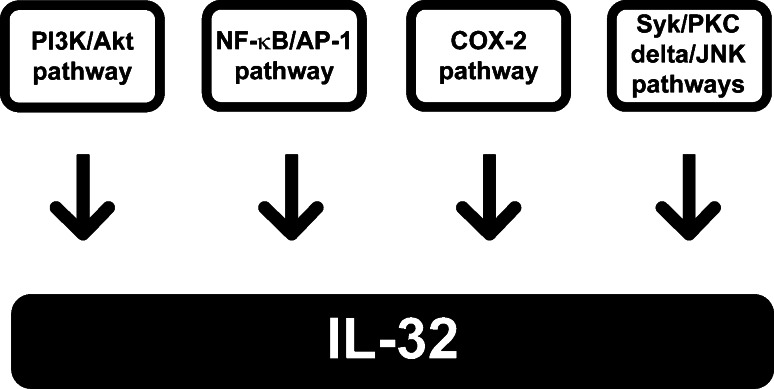
However, several pathways have been published including the PI3 K/Akt pathway and NF-κB/AP-1 pathway [58]. Also, the Syk/PKC delta/JNK pathways are reported to be important for the induction of IL-32 in RA synovial fibroblasts by TNF-α [59]. Viral-induced IL-32 expression seems to be mediated by cyclooxygenase (COX)-2 pathway [16]. Furthermore, downstream pathways are linked to upregulation of IL-32 expression. Netea et al. [10] demonstrated that Mycobacterium tuberculosis (MTB) induced IL-32 gene expression in PBMC was dependent on the caspase-1/IL-18/interferon-gamma pathway. The enhanced IL-32 expression was not due to recognition of MTB by TLR, but rather via intracellular NOD (Fig. 5).
Fig. 5.
Induction pathways of intracellular IL-32 expression. Several pathways have been reported to induce intracellular expression of IL-32, including PI3 k, NFκB/AP-1, COX-2, and Syk/PKC-delta/JNK pathways
The addition of recombinant IL-32 to primary human monocytes drives the differentiation into macrophages-type cells. IL-32 added to cultures of GM-CSF/IL-4-induced dendritic cells resulted in differentiation to macrophage-like cells. Moreover, macrophage-like cells generated following incubation with recombinant IL-32β produced more proinflammatory cytokines after exposure to bacterial ligands. This was mediated by the p38-MAPK pathway, whereas IL-32-driven differentiation into macrophage-like cells was dependent on caspase-3 [60]. Whether the IL-32-mediated differentiation of monocytes into macrophage-like cells was related to apoptosis was not studied. Since overexpression of IL-32β or IL-32γ, but not IL-32α, resulted in cell death via caspase-3- mediated processes [6], apoptotic bodies may determine the monocyte differentiation into macrophages [61]. Another study showed that IL-32 was involved in the differentiation of monocytes into osteoclasts. In combination with sRANKL, IL-32 promotes the generation of osteoclasts, but IL-32 was unable of inducing the maturation of the osteoclasts into bone-resorbing cells [62].
Overexpression of intracellular IL-32γ in human RA fibroblasts resulted in a more proinflammatory status of these RA synoviocytes, IL-6 and IL-8 production was strongly enhanced after stimulation with TLR ligands [9]. Another isoform of IL-32, namely IL-32β, resulted in enhanced proinflammatory cytokines and more severe sepsis in transgenic mice [63]. IL-32 expression in NK cells increased the cytotoxicity of the cells towards cancer cells, via death receptor (DR) 3 and caspase-3-mediated pathways [64]. Dendritic cells (DC) that had been engineered to overexpress IL-32β were effective therapeutically when injected directly in tumors [65]. In line with these results, overexpression of IL-32γ in DCs promoted T helper cell development into Th1 and Th17 signatures [66]. In general, exposure to or intracellular overexpression of IL-32 promotes the inflammatory activity of these particular cells. Mostly immune-related cells appear to be susceptible for IL-32-mediated phenotype change of these cells.
In considering the above-mentioned studies, recombinant IL-32 proteins produced in E. coli likely contain small amounts of microbial products, such as lipopolysaccharide (LPS) or peptidoglycans (PGN). In addition, plasmids or viral constructs used to overexpress intracellular IL-32 has an additional trigger, e.g., vector DNA. Thus, a response to exogenous IL-32 or overexpression using viral vectors provides two signals for NF-κB-mediated induction of cytokines, such as IL-1β and TNF-α. Nevertheless, when endogenous IL-32 is silenced, there is no significant production of these cytokines to agents that trigger NF-κB [67].
To eliminate the second signal of provided by microbial products present in recombinant IL-32γ preparations, a totally synthetic form of IL-32γ was added to PBMC. Although there was no induction of TNF-α or IL-1β over a large dose–response to the synthetic IL-32γ, the addition of a second signal such as a low concentration of LPS or MDP or pretreatment of cells with IFN-γ resulted in IL-1β and TNF-α [68]. The mechanism for the two-signal requirement of exogenous IL-32 induction of cytokines remains unclear at the present time. It is possible that the known interaction of IL-32 with surface integrins [6] serves as one of the signals. This concept is consistent with the known ability of surface integrins to signal.
Future perspectives
As discussed in this review, IL-32 is both a pro-inflammatory as well a pro-apoptotic mediator. Following exogenous stimulation with TLR, for example, IL-32 is synthesized but remains intracellularly; although IL-32 can be found in the cell membrane [8], the molecule is released through cell death or IL-32 does contain a potential transmembrane helix [6, 68], but most studies indicated that IL-32 is an intracellular protein. Since IL-32 is associated with several diseases, including inflammatory diseases, infectious diseases, and cancer, it would be of great importance to develop a targeting strategy for IL-32. For inflammatory disease, IL-32 should be down-modulated, whereas for infectious diseases and cancer, upregulation of IL-32 would comprise a therapeutic strategy. However, before these strategies can be developed, identification of the optimal isoform of IL-32 function for a particular disease will be necessary. It is clear from the splice mutation that IL-32γ is the most potent isoform of IL-32, but IL-32β is a possible candidate to target in diseases. Interestingly, IL-32α seems to have no effects on cells or even an anti-inflammatory activity [69]. Since a specific cell surface receptor for IL-32 is still not known, it will be difficult to target IL-32 signaling from the outside the cell because IL-32 is expressed mostly intracellular although binding to the integrins αVβ3 and αVβ6 might be crucial for IL-32 signaling [6]. In this case, antibodies direct against integrins would be an option to interfere with the extracellular IL-32 signaling cascade. Presently, several anti-integrin antibodies are in development for clinical application in several types of diseases, including autoimmune diseases [70]. It may be possible to interfere with the downstream signaling cascade of IL-32, but up to this moment the precise pathway is not yet known. Although several possible pathways have been suggested, the major pathway has not been elucidated.
Reductions in the expression of intracellular IL-32 using siRNA technology have been successfully used in in vitro systems [9, 18] but whether this approach can be used for the treatment of human disease is still an open question. For infectious diseases and cancer, IL-32 should be enhanced in the diseased tissues to control the pathogens or tumor growth. To achieve this, several options may be used, including adenoviral technology to overexpress IL-32 or specific TLR/NOD ligands that induced IL-32 expression. Since IL-32 binds to the integrin/FAK pathway, it may contribute to the progression of cancer. FAK phosphorylation seems to be crucial in tumor growth and FAK inhibitors are effective in models of cancer [71, 72].
In conclusion, the expression of IL-32 is modulated in several diseases, including inflammatory disorders, infectious diseases, and cancer. Understanding of the exact function of the different isoforms of IL-32 will lead to novel therapeutic options for indicated diseases.
References
- 1.Dahl CA, Schall RP, He HL, Cairns JS. Identification of a novel gene expressed in activated natural killer cells and T cells. J Immunol. 1992;148:597–603. [PubMed] [Google Scholar]
- 2.Kim SH, Han SY, Azam T, Yoon DY, Dinarello CA. Interleukin-32: a cytokine and inducer of TNFalpha. Immunity. 2005;22:131–142. doi: 10.1016/j.immuni.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 3.Goda C, Kanaji T, Kanaji S, Tanaka G, Arima K, Ohno S, Izuhara K. Involvement of IL-32 in activation-induced cell death in T cells. Int Immunol. 2006;18:233–240. doi: 10.1093/intimm/dxh339. [DOI] [PubMed] [Google Scholar]
- 4.Heinhuis B, Koenders MI, van de Loo FA, Netea MG, van den Berg WB, Joosten LA. Inflammation-dependent secretion and splicing of IL-32{gamma} in rheumatoid arthritis. Proc Natl Acad Sci USA. 2011;108:4962–4967. doi: 10.1073/pnas.1016005108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heinhuis B, Netea MG, van den Berg WB, Dinarello CA, Joosten LA. Interleukin-32: a predominantly intracellular proinflammatory mediator that controls cell activation and cell death. Cytokine. 2012;60:321–327. doi: 10.1016/j.cyto.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 6.Heinhuis B, Koenders MI, van den Berg WB, Netea MG, Dinarello CA, Joosten LA. IL-32 contains a typical alpha-helix bundle structure that resembles the focal adhesion targeting region of focal adhesion kinase-1. J Biol Chem. 2012;287:5733–5743. doi: 10.1074/jbc.M111.288290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi JD, Bae SY, Hong JW, Azam T, Dinarello CA, Her E, Choi WS, Kim BK, Lee CK, Yoon DY, Kim SJ, Kim SH. Identification of the most active interleukin-32 isoform. Immunology. 2008;126:535–542. doi: 10.1111/j.1365-2567.2008.02917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hasegawa H, Thomas HJ, Schooley K, Born TL. Native IL-32 is released from intestinal epithelial cells via a non-classical secretory pathway as a membrane-associated protein. Cytokine. 2011;53:74–83. doi: 10.1016/j.cyto.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 9.Heinhuis B, Koenders MI, van Riel PL, van de Loo FA, Dinarello CA, Netea MG, van den Berg WB, Joosten LA. Tumour necrosis factor alpha-driven IL-32 expression in rheumatoid arthritis synovial tissue amplifies an inflammatory cascade. Ann Rheum Dis. 2010;70:660–667. doi: 10.1136/ard.2010.139196. [DOI] [PubMed] [Google Scholar]
- 10.Netea MG, Azam T, Ferwerda G, Girardin SE, Walsh M, Park JS, Abraham E, Kim JM, Yoon DY, Dinarello CA, Kim SH. IL-32 synergizes with nucleotide oligomerization domain (NOD) 1 and NOD2 ligands for IL-1beta and IL-6 production through a caspase 1-dependent mechanism. Proc Natl Acad Sci USA. 2005;102:16309–16314. doi: 10.1073/pnas.0508237102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Netea MG, Azam T, Lewis EC, Joosten LA, Wang M, Langenberg D, Meng X, Chan ED, Yoon DY, Ottenhoff T, Kim SH, Dinarello CA. Mycobacterium tuberculosis induces interleukin-32 production through a caspase- 1/IL-18/interferon-gamma-dependent mechanism. PLoS Med. 2006;3:e277. doi: 10.1371/journal.pmed.0030277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schenk M, Krutzik SR, Sieling PA, Lee DJ, Teles RM, Ochoa MT, Komisopoulou E, Sarno EN, Rea TH, Graeber TG, Kim S, Cheng G, Modlin RL. NOD2 triggers an interleukin-32-dependent human dendritic cell program in leprosy. Nat Med. 2012;18:555–563. doi: 10.1038/nm.2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calabrese F, Baraldo S, Bazzan E, Lunardi F, Rea F, Maestrelli P, Turato G, Lokar-Oliani K, Papi A, Zuin R, Sfriso P, Balestro E, Dinarello CA, Saetta M. IL-32, a novel proinflammatory cytokine in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;178:894–901. doi: 10.1164/rccm.200804-646OC. [DOI] [PubMed] [Google Scholar]
- 14.Joosten LA, Netea MG, Kim SH, Yoon DY, Oppers-Walgreen B, Radstake TR, Barrera P, van de Loo FA, Dinarello CA, van den Berg WB. IL-32, a proinflammatory cytokine in rheumatoid arthritis. Proc Natl Acad Sci USA. 2006;103:3298–3303. doi: 10.1073/pnas.0511233103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kang YH, Park MY, Yoon DY, Han SR, Lee CI, Ji NY, Myung PK, Lee HG, Kim JW, Yeom YI, Jang YJ, Ahn DK, Kim JW, Song EY. Dysregulation of overexpressed IL-32alpha in hepatocellular carcinoma suppresses cell growth and induces apoptosis through inactivation of NF-kappaB and Bcl-2. Cancer Lett. 2011;318:226–233. doi: 10.1016/j.canlet.2011.12.023. [DOI] [PubMed] [Google Scholar]
- 16.Li W, Liu Y, Mukhtar MM, Gong R, Pan Y, Rasool ST, Gao Y, Kang L, Hao Q, Peng G, Chen Y, Chen X, Wu J, Zhu Y. Activation of interleukin-32 pro-inflammatory pathway in response to influenza A virus infection. PLoS ONE. 2008;3:e1985. doi: 10.1371/journal.pone.0001985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moschen AR, Fritz T, Clouston AD, Rebhan I, Bauhofer O, Barrie HD, Powell EE, Kim SH, Dinarello CA, Bartenschlager R, Jonsson JR, Tilg H. Interleukin-32: a new proinflammatory cytokine involved in hepatitis C virus-related liver inflammation and fibrosis. Hepatology. 2011;53:1819–1829. doi: 10.1002/hep.24285. [DOI] [PubMed] [Google Scholar]
- 18.Nold MF, Nold-Petry CA, Pott GB, Zepp JA, Saavedra MT, Kim SH, Dinarello CA. Endogenous IL-32 controls cytokine and HIV-1 production. J Immunol. 2008;181:557–565. doi: 10.4049/jimmunol.181.1.557. [DOI] [PubMed] [Google Scholar]
- 19.Sakitani K, Hirata Y, Hayakawa Y, Serizawa T, Nakata W, Takahashi R, Kinoshita H, Sakamoto K, Nakagawa H, Akanuma M, Yoshida H, Maeda S, Koike K. Role of interleukin-32 in Helicobacter pylori-induced gastric inflammation. Infect Immun. 2012;80:3795–3803. doi: 10.1128/IAI.00637-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith AJ, Toledo CM, Wietgrefe SW, Duan L, Schacker TW, Reilly CS, Haase AT. The immunosuppressive role of IL-32 in lymphatic tissue during HIV-1 infection. J Immunol. 2011;186:6576–6584. doi: 10.4049/jimmunol.1100277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sorrentino C, Di CE. Expression of IL-32 in human lung cancer is related to the histotype and metastatic phenotype. Am J Respir Crit Care Med. 2009;180:769–779. doi: 10.1164/rccm.200903-0400OC. [DOI] [PubMed] [Google Scholar]
- 22.Xu Q, Pan X, Shu X, Cao H, Li X, Zhang K, Lu J, Zou Y, Li X, Liu H, Zhang Y, Yang D, Ning Q, Shen G, Li G. Increased interleukin-32 expression in chronic hepatitis B virus-infected liver. J Infect. 2012;65:336–342. doi: 10.1016/j.jinf.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 23.Zepp JA, Nold-Petry CA, Dinarello CA, Nold MF. Protection from RNA and DNA viruses by IL-32. J Immunol. 2011;186:4110–4118. doi: 10.4049/jimmunol.1000081. [DOI] [PubMed] [Google Scholar]
- 24.Bae S, Kang D, Hong J, Chung B, Choi J, Jhun H, Hong K, Kim E, Jo S, Lee S, Kim SH, Kim S. Characterizing antiviral mechanism of interleukin-32 and a circulating soluble isoform in viral infection. Cytokine. 2012;58:79–86. doi: 10.1016/j.cyto.2011.12.024. [DOI] [PubMed] [Google Scholar]
- 25.Ioannidis I, McNally B, Willette M, Peeples ME, Chaussabel D, Durbin JE, Ramilo O, Mejias A, Flano E. Plasticity and virus specificity of the airway epithelial cell immune response during respiratory virus infection. J Virol. 2012;86:5422–5436. doi: 10.1128/JVI.06757-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li W, Sun W, Liu L, Yang F, Li Y, Chen Y, Fang J, Zhang W, Wu J, Zhu Y. IL-32: a host proinflammatory factor against influenza viral replication is upregulated by aberrant epigenetic modifications during influenza A virus infection. J Immunol. 2010;185:5056–5065. doi: 10.4049/jimmunol.0902667. [DOI] [PubMed] [Google Scholar]
- 27.Bai X, Ovrutsky AR, Kartalija M, Chmura K, Kamali A, Honda JR, Oberley-Deegan RE, Dinarello CA, Crapo JD, Chang LY, Chan ED. IL-32 expression in the airway epithelial cells of patients with Mycobacterium avium complex lung disease. Int Immunol. 2011;23:679–691. doi: 10.1093/intimm/dxr075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bai X, Kim SH, Azam T, McGibney MT, Huang H, Dinarello CA, Chan ED. IL-32 is a host protective cytokine against Mycobacterium tuberculosis in differentiated THP-1 human macrophages. J Immunol. 2010;184:3830–3840. doi: 10.4049/jimmunol.0901913. [DOI] [PubMed] [Google Scholar]
- 29.Bai X, Shange S, Orway D, Basaraba R, Orme IM, Ovrusky AR, Matsuda JL, Azam T, Kim S-H, Dinarello CA (2013) Mice expressing human interleukin-32 in the lungs are more resistant to Mycobacterium tuberculosis. J Am Thoras Soc (in press)
- 30.Ciccia F, Rizzo A, Ccardo-Palumbo A, Giardina A, Bombardieri M, Guggino G, Taverna S, Leo GD, Alessandro R, Triolo G. Increased expression of interleukin-32 in the inflamed ileum of ankylosing spondylitis patients. Rheumatology (Oxford) 2012;51:1966–1972. doi: 10.1093/rheumatology/kes170. [DOI] [PubMed] [Google Scholar]
- 31.Kobayashi H, Yazlovitskaya EM, Lin PC. Interleukin-32 positively regulates radiation-induced vascular inflammation. Int J Radiat Oncol Biol Phys. 2009;74:1573–1579. doi: 10.1016/j.ijrobp.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kobayashi H, Lin PC. Molecular characterization of IL-32 in human endothelial cells. Cytokine. 2009;46:351–358. doi: 10.1016/j.cyto.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soyka MB, Treis A, Eiwegger T, Menz G, Zhang S, Holzmann D, Akdis CA, Meyer N. Regulation and expression of IL-32 in chronic rhinosinusitis. Allergy. 2012;67:790–798. doi: 10.1111/j.1398-9995.2012.02820.x. [DOI] [PubMed] [Google Scholar]
- 34.Marcondes AM, Li X, Tabellini L, Bartenstein M, Kabacka J, Sale GE, Hansen JA, Dinarello CA, Deeg HJ. Inhibition of IL-32 activation by alpha-1 antitrypsin suppresses alloreactivity and increases survival in an allogeneic murine marrow transplantation model. Blood. 2011;118:5031–5039. doi: 10.1182/blood-2011-07-365247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marcondes AM, Mhyre AJ, Stirewalt DL, Kim SH, Dinarello CA, Deeg HJ. Dysregulation of IL-32 in myelodysplastic syndrome and chronic myelomonocytic leukemia modulates apoptosis and impairs NK function. Proc Natl Acad Sci USA. 2008;105:2865–2870. doi: 10.1073/pnas.0712391105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nishida A, Andoh A, Inatomi O, Fujiyama Y. Interleukin-32 expression in the pancreas. J Biol Chem. 2009;284:17868–17876. doi: 10.1074/jbc.M900368200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang F, Sha J, Wood TG, Galindo CL, Garner HR, Burkart MF, Suarez G, Sierra JC, Agar SL, Peterson JW, Chopra AK. Alteration in the activation state of new inflammation-associated targets by phospholipase A2-activating protein (PLAA) Cell Signal. 2008;20:844–861. doi: 10.1016/j.cellsig.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Majid S, Dar AA, Saini S, Yamamura S, Hirata H, Tanaka Y, Deng G, Dahiya R. MicroRNA-205-directed transcriptional activation of tumor suppressor genes in prostate cancer. Cancer. 2010;116:5637–5649. doi: 10.1002/cncr.25488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu S, Tetzlaff MT, Liu A, Liegl-Atzwanger B, Guo J, Xu X. Loss of microRNA-205 expression is associated with melanoma progression. Lab Invest. 2012;92:1084–1096. doi: 10.1038/labinvest.2012.62. [DOI] [PubMed] [Google Scholar]
- 40.Majid S, Saini S, Dar AA, Hirata H, Shahryari V, Tanaka Y, Yamamura S, Ueno K, Zaman MS, Singh K, Chang I, Deng G, Dahiya R. MicroRNA-205 inhibits Src-mediated oncogenic pathways in renal cancer. Cancer Res. 2011;71:2611–2621. doi: 10.1158/0008-5472.CAN-10-3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yue X, Wang P, Xu J, Zhu Y, Sun G, Pang Q, Tao R. MicroRNA-205 functions as a tumor suppressor in human glioblastoma cells by targeting VEGF-A. Oncol Rep. 2012;27:1200–1206. doi: 10.3892/or.2011.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mabilleau G, Sabokbar A. Interleukin-32 promotes osteoclast differentiation but not osteoclast activation. PLoS ONE. 2009;4:e4173. doi: 10.1371/journal.pone.0004173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Turner-Brannen E, Choi KY, Arsenault R, El-Gabalawy H, Napper S, Mookherjee N. Inflammatory cytokines IL-32 and IL-17 have common signaling intermediates despite differential dependence on TNF-receptor 1. J Immunol. 2011;186:7127–7135. doi: 10.4049/jimmunol.1002306. [DOI] [PubMed] [Google Scholar]
- 44.Jeong HJ, Han NR, Moon PD, Kim MH, Kim HM. Intracellular calcium level is upregulated by interleukin-32 in auditory cells. Cytokine. 2011;53:153–157. doi: 10.1016/j.cyto.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 45.Oh JH, Cho MC, Kim JH, Lee SY, Kim HJ, Park ES, Ban JO, Kang JW, Lee DH, Shim JH, Han SB, Moon DC, Park YH, Yu DY, Kim JM, Kim SH, Yoon DY, Hong JT. IL-32gamma inhibits cancer cell growth through inactivation of NF-kappaB and STAT3 signals. Oncogene. 2011;30:3345–3359. doi: 10.1038/onc.2011.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Novick D, Rubinstein M, Azam T, Rabinkov A, Dinarello CA, Kim SH. Proteinase 3 is an IL-32 binding protein. Proc Natl Acad Sci USA. 2006;103:3316–3321. doi: 10.1073/pnas.0511206103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim S, Lee S, Her E, Bae S, Choi J, Hong J, Jaekal J, Yoon D, Azam T, Dinarello CA, Kim S. Proteinase 3-processed form of the recombinant IL-32 separate domain. BMB Rep. 2008;41:814–819. doi: 10.5483/BMBRep.2008.41.11.814. [DOI] [PubMed] [Google Scholar]
- 48.Cabodi S, Di SP, Leal MP, Tinnirello A, Bisaro B, Morello V, Damiano L, Aramu S, Repetto D, Tornillo G, Defilippi P. Integrins and signal transduction. Adv Exp Med Biol. 2010;674:43–54. doi: 10.1007/978-1-4419-6066-5_5. [DOI] [PubMed] [Google Scholar]
- 49.Kinashi T. Overview of integrin signaling in the immune system. Methods Mol Biol. 2012;757:261–278. doi: 10.1007/978-1-61779-166-6_17. [DOI] [PubMed] [Google Scholar]
- 50.Schaller MD. FAK and paxillin: regulators of N-cadherin adhesion and inhibitors of cell migration? J Cell Biol. 2004;166:157–159. doi: 10.1083/jcb.200406151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yano H, Mazaki Y, Kurokawa K, Hanks SK, Matsuda M, Sabe H. Roles played by a subset of integrin signaling molecules in cadherin-based cell–cell adhesion. J Cell Biol. 2004;166:283–295. doi: 10.1083/jcb.200312013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol. 2005;6:56–68. doi: 10.1038/nrm1549. [DOI] [PubMed] [Google Scholar]
- 53.Meyer N, Zimmermann M, Burgler S, Bassin C, Woehrl S, Moritz K, Rhyner C, Indermitte P, Schmid-Grendelmeier P, Akdis M, Menz G, Akdis CA. IL-32 is expressed by human primary keratinocytes and modulates keratinocyte apoptosis in atopic dermatitis. J Allergy Clin Immunol. 2010;125:858–865. doi: 10.1016/j.jaci.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 54.Nold-Petry CA, Nold MF, Zepp JA, Kim SH, Voelkel NF, Dinarello CA. IL-32-dependent effects of IL-1beta on endothelial cell functions. Proc Natl Acad Sci USA. 2009;106:3883–3888. doi: 10.1073/pnas.0813334106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li W, Yang F, Liu Y, Gong R, Liu L, Feng Y, Hu P, Sun W, Hao Q, Kang L, Wu J, Zhu Y. Negative feedback regulation of IL-32 production by iNOS activation in response to dsRNA or influenza virus infection. Eur J Immunol. 2009;39:1019–1024. doi: 10.1002/eji.200838885. [DOI] [PubMed] [Google Scholar]
- 56.Rasool ST, Tang H, Wu J, Li W, Mukhtar MM, Zhang J, Mu Y, Xing HX, Wu J, Zhu Y. Increased level of IL-32 during human immunodeficiency virus infection suppresses HIV replication. Immunol Lett. 2008;117:161–167. doi: 10.1016/j.imlet.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 57.Kudo M, Ogawa E, Kinose D, Haruna A, Takahashi T, Tanabe N, Marumo S, Hoshino Y, Hirai T, Sakai H, Muro S, Date H, Mishima M. Oxidative stress induced interleukin-32 mRNA expression in human bronchial epithelial cells. Respir Res. 2012;13:19. doi: 10.1186/1465-9921-13-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nishida A, Andoh A, Shioya M, Kim-Mitsuyama S, Takayanagi A, Fujiyama Y. Phosphatidylinositol 3-kinase/Akt signaling mediates interleukin-32alpha induction in human pancreatic periacinar myofibroblasts. Am J Physiol Gastrointest Liver Physiol. 2008;294:G831–G838. doi: 10.1152/ajpgi.00535.2007. [DOI] [PubMed] [Google Scholar]
- 59.Mun SH, Kim JW, Nah SS, Ko NY, Lee JH, Kim JD, Kim DK, Kim HS, Choi JD, Kim SH, Lee CK, Park SH, Kim BK, Kim HS, Kim YM, Choi WS. Tumor necrosis factor alpha-induced interleukin-32 is positively regulated via the Syk/protein kinase Cdelta/JNK pathway in rheumatoid synovial fibroblasts. Arthritis Rheum. 2009;60:678–685. doi: 10.1002/art.24299. [DOI] [PubMed] [Google Scholar]
- 60.Netea MG, Lewis EC, Azam T, Joosten LA, Jaekal J, Bae SY, Dinarello CA, Kim SH. Interleukin-32 induces the differentiation of monocytes into macrophage-like cells. Proc Natl Acad Sci USA. 2008;105:3515–3520. doi: 10.1073/pnas.0712381105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ariel A, Serhan CN. New lives given by cell death: macrophage differentiation following their encounter with apoptotic leukocytes during the resolution of inflammation. Front Immunol. 2012;3:4. doi: 10.3389/fimmu.2012.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim YG, Lee CK, Oh JS, Kim SH, Kim KA, Yoo B. Effect of interleukin-32gamma on differentiation of osteoclasts from CD14 + monocytes. Arthritis Rheum. 2010;62:515–523. doi: 10.1002/art.27197. [DOI] [PubMed] [Google Scholar]
- 63.Kobayashi H, Huang J, Ye F, Shyr Y, Blackwell TS, Lin PC. Interleukin-32beta propagates vascular inflammation and exacerbates sepsis in a mouse model. PLoS ONE. 2010;5:e9458. doi: 10.1371/journal.pone.0009458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Park MH, Song MJ, Cho MC, Moon DC, Yoon DY, Han SB, Hong JT. Interleukin-32 enhances cytotoxic effect of natural killer cells to cancer cells via activation of death receptor 3. Immunology. 2012;135:63–72. doi: 10.1111/j.1365-2567.2011.03513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Qu Y, Taylor JL, Bose A, Storkus WJ. Therapeutic effectiveness of intratumorally delivered dendritic cells engineered to express the pro-inflammatory cytokine, interleukin (IL)-32. Cancer Gene Ther. 2011;18:663–673. doi: 10.1038/cgt.2011.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jung MY, Son MH, Kim SH, Cho D, Kim TS. IL-32{gamma} induces the maturation of dendritic cells with Th1- and Th17-polarizing ability through enhanced IL-12 and IL-6 production. J Immunol. 2011;186:6848–6859. doi: 10.4049/jimmunol.1003996. [DOI] [PubMed] [Google Scholar]
- 67.Hong J, Bae S, Kang Y, Yoon D, Bai X, Chan ED, Azam T, Dinarello CA, Lee S, Her E, Rho G, Kim S. Suppressing IL-32 in monocytes impairs the induction of the proinflammatory cytokines TNFalpha and IL-1beta. Cytokine. 2010;49:171–176. doi: 10.1016/j.cyto.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 68.Nold MF, Nold-Petry CA, Zepp JA, Dinkel H, Palmer BE, Farkas L, Cool CD, Taraseviciene-Stewart L, Kim S-H, Heinhuis B, Joosten LAB, Dinarello CA, Voelkel NF (2013) IL-32 promotes angiogenesis. J Immunol (in press) [DOI] [PMC free article] [PubMed]
- 69.Cheon S, Lee JH, Park S, Bang SI, Lee WJ, Yoon DY, Yoon SS, Kim T, Min H, Cho BJ, Lee HJ, Lee KW, Jeong SH, Park H, Cho D. Overexpression of IL-32{alpha} increases NK cell-mediated killing through up-regulation of Fas and ULBP2 expression in human chronic myeloid leukemia cells. J Biol Chem. 2011;286:12049–12055. doi: 10.1074/jbc.M110.159756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Byron A, Humphries JD, Askari JA, Craig SE, Mould AP, Humphries MJ. Anti-integrin monoclonal antibodies. J Cell Sci. 2009;122:4009–4011. doi: 10.1242/jcs.056770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hao HF, Takaoka M, Bao XH, Wang ZG, Tomono Y, Sakurama K, Ohara T, Fukazawa T, Yamatsuji T, Fujiwara T, Naomoto Y. Oral administration of FAK inhibitor TAE226 inhibits the progression of peritoneal dissemination of colorectal cancer. Biochem Biophys Res Commun. 2012;423:744–749. doi: 10.1016/j.bbrc.2012.06.030. [DOI] [PubMed] [Google Scholar]
- 72.Ma WW. Development of focal adhesion kinase inhibitors in cancer therapy. Anticancer Agents Med Chem. 2011;11:638–642. doi: 10.2174/187152011796817628. [DOI] [PubMed] [Google Scholar]
- 73.Zhang Y. I-TASSER server for protein 3D structure prediction. BMC Bioinforma. 2008;9:40. doi: 10.1186/1471-2105-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]