Abstract
Research on the nanoscale membrane structures known as lipid rafts is relevant to the fields of cancer biology, inflammation and ischaemia. Lipid rafts recruit molecules critical to signalling and regulation of the invasion process in malignant cells, the leukocytes that provide immunity in inflammation and the endothelial cells that build blood and lymphatic vessels, as well as the patterning of neural networks. As angiogenesis is a common denominator, regulation of receptors and signalling molecules critical to angiogenesis is central to the design of new approaches aimed at reducing, promoting or normalizing the angiogenic process. The goal of this review is to highlight some of the key issues that indicate the involvement of endothelial cell lipid rafts at each step of so-called ‘sprouting angiogenesis’, from stimulation of the vascular endothelial growth factor to the choice of tip cells, activation of migratory and invasion pathways, recruitment of molecules that guide axons in vascular patterning and maturation of blood vessels. Finally, the review addresses opportunities for future studies to define how these lipid domains (and their constituents) may be manipulated to stimulate the so-called ‘normalization’ of vascular networks within tumors, and be identified as the main target, enabling the development of more efficient chemotherapeutics and cancer immunotherapies.
Keywords: Endothelial cell, Angiogenesis, Vascular guidance, Vessel maturation, Vessel normalization, Caveolin, Tip cell
Introduction
The vascular system, a complex and highly branched tubular network made up of endothelial cells (ECs), ensures the simultaneous and efficient transport of gases, liquids, nutrients, signalling molecules and circulating cells between tissues and organs. Insufficient blood vessel supply causes tissue ischaemia in cardiovascular diseases, whereas new vessel formation allows nutrients, oxygen and signalling molecules to be more available to inflamed tissues and tumors; it also facilitates cancer cell dissemination to distant organs in metastasis [1, 2]. Blood vessels can form anew after tubular organization of endothelial progenitor cells (EPCs; vasculogenesis) or by budding from pre-existing vessels (sprouting angiogenesis), the latter also being integrated by local recruitment of EPCs [3, 4]. Vessel growth is an example of coordinated proliferation, migration, matrix adhesion, guidance and differentiation that results in specialized tissues. During vessel formation, ECs degrade the underlying basement membrane and migrate in the interstitial extracellular matrix (ECM), before proliferating and undergoing cavitation to produce new tubular-like structures. In particular, a subset of cells located at the leading edge of the sprouting vessels, termed ‘tip cells’, acquires motile, invasive behavior and extends filopodia [2, 5, 6]. The tip and stalk cells of endothelial sprouts express a large array of pro-angiogenesis systems that regulate the temporal and spatial interaction of ECs and ECM. Such systems, regulated by the balance of pro- and anti-angiogenesis factors, include the vascular endothelial growth factor (VEGF) receptor [7], the receptor for the urokinase plasminogen activator [8], the ephrin and Eph-receptor tyrosine kinases [9], the Notch complex [10], integrins [11], cadherins [12, 13] and NADPH oxidase (Nox) [7], to cite only those most studied. The function of many of these systems is boosted by angiogenesis factor-dependent localization of the relevant molecules in specialized microdomains of the cell surface known as caveolar lipid rafts (LRs), signalling platforms that, in many cell types including ECs [14], recruit functionally important molecules involved in cell–matrix interactions and cell signalling. Such microdomains are enriched with cholesterol and glycosphingolipids; they are resistant to cold detergent extraction and show lower buoyant density than most of the plasma membrane [15]. These membrane domains are also enriched with membrane proteins such as caveolins, the Src family of kinases [16, 17] and glycosylphosphatidylinositol (GPI)-anchored proteins [18]. Only a limited number of membrane proteins are known to associate with LRs, but they are of paramount significance because many receptors for extracellular signals, including those involved in EC invasion and guidance, localize in LRs. In this review, we focus on the emerging role of LR microdomains in the regulation of the angiogenesis process.
Lipid rafts and caveolae
The membrane model of Singer and Nicholson [19] postulated a uniform lipid bilayer with randomly floating proteins, but it was soon realized that the membranes were not uniform and that there were clusters of lipids organized in a more ordered state within the generally disordered lipid milieu of the membrane. Such clusters are now referred to as LRs, which are defined as lipid-ordered phases of the cell membrane formed mainly from tightly packed sphingomyelin and cholesterol, rather than the other parts of the membrane that are mainly made up of phospholipids, forming the lipid-disordered phase. These specialized membrane microdomains compartmentalize cellular processes by serving as organizing centers for the assembly of signalling molecules, influencing membrane fluidity and membrane protein trafficking, and regulating neurotransmission and receptor trafficking [20, 21]. Although more ordered and tightly packed than the surrounding surface membrane, LRs float freely in the membrane bilayer [22]. Our concept of LRs has evolved with the realization that the association of raft components is dynamic and that their sizes range from small, short-lived, nanoscale ensembles (<50 nm in diameter) to more stable membrane domains, with sizes possibly reaching 500 nm in diameter [23]. However, as the average size of LRs is well below the resolution of light microscopy, until 15 years ago, there was a concern about visualizing LRs in cell membranes, because the existing microscopic approaches did not support the ‘raft concept’ [24]. A key issue was the method used to define an LR component. LRs were defined as the insoluble residue remaining after non-ionic detergent solubilization of cell membranes (hence the definition of LRs as detergent-resistant membranes or DRMs). If a protein became detergent soluble after treatment with methyl-β-cyclodextrin, which extracts cholesterol from the cell membrane, it was considered to be a raft component. If a biological process was inhibited by cyclodextrin treatment, it was considered to be raft dependent [25]. Although such criteria still hold true and have been adopted in most publications, advances in technology have permitted clarification of the ‘raft concept’. Visualizing rafts with new microscopic techniques (reviewed in Simons and Gerl [25]) has allowed the identification of LRs as a heterogeneous collection of domains differing both in protein and lipid composition and in temporal stability. This new concept is embodied in the consensus definition of an LR proposed at the 2006 Keystone Symposium: ‘Lipid rafts are small (10–200 nm), heterogeneous, highly dynamic, sterol- and sphingolipid-enriched domains that compartmentalize cellular processes. Small rafts can sometimes be stabilized to form larger platforms through protein–protein and protein–lipid interactions [26].
Nevertheless, there is still controversy over the existence of LRs [27], as well as their real composition. A recent study concluded that cholesterol does not play a direct role in LR organization [28]. In that review, the authors used high-resolution, secondary ion mass spectrometry to directly map the distributions of isotope-labeled cholesterol and sphingolipids in fibroblast plasma membranes. Although cholesterol depletion reduced the abundance of LRs, cholesterol was evenly distributed throughout the plasma membrane and not enriched within LRs. Thus, the authors ruled out favorable cholesterol–sphingolipid interactions dictating plasma membrane organization in fibroblasts. As LRs are disrupted by drugs that depolymerize the cell’s actin cytoskeleton, the authors suggested that cholesterol must affect sphingolipid organization via an indirect mechanism involving the cytoskeleton instead. Recent technological advances have allowed the building of artificial LR-like biomimetic membranes, mimicking physiological cell membranes, on solid supports (solid-supported bilayer lipid membranes), which provide excellent templates for studying in vitro LR interactions with several biological molecules. These biomimetic systems are formed by depositing ternary or binary mixtures of phospholipids, sphingolipids and cholesterol, enriched with LR-hosted receptors, on to a hydrophilic surface (typically mica or amorphous silica), via self-assembly or vesicle fusion. Thanks to their mobility on these surfaces, sphingolipids and cholesterol can freely come out of the lipid-disordered phase [29, 30] or assemble in a lipid-ordered macroarea, covering all the surfaces available [31, 32]. The ligand–receptor affinity can be easily demonstrated optically using surface plasmon resonance techniques [30–33].
It has now been firmly established that there are two major types of LRs: those that contain the cholesterol-binding protein caveolin-1 (Cav-1) in the inner leaflet and those that do not. Caveolin is an integral membrane protein that exists in three isoforms: Cav-1, Cav-2 and Cav-3. Although Cav-1 is widely expressed in many tissues, including endothelium, Cav-3 is muscle specific; Cav-2 is co-expressed with Cav-1 which it needs for stabilization and plasma-membrane localization (reviewed in Sotgia et al. [34]). Cav-1, highly expressed in ECs, is organized into specific domains [34]. Both the N- and C-termini of each Cav-1 monomer face the cytoplasm. Some 12–18 Cav-1/Cav-2 monomers form a polymerized filamentous structure [35]. The Golgi body is the subcellular location in which cholesterol, sphingolipids and Cav-1 polymers self-aggregate and generate vesicles that are transported to the plasma membrane to form caveolar LRs, giving the membrane characteristic, spherical, flask-like invaginations (caveolae or ‘little caves’) that can be detected with electron microscopy, can be widely represented at the external surface of ECs and was first described by GE Palade in 1953 [36]. The caveolar LRs of ECs, which occur in ordered linear arrays over the entire cell body [37], have been involved in many cellular functions such as endocytosis (transcytosis, pinocytosis, potocytosis), signal transduction, mechanotransduction and cholesterol trafficking [38]. However, as caveolin exists in cells that do not show morphological caveolae, efforts have been made to find other proteins producing these unique plasma membrane invaginations via a partnership with caveolin. Recent evidence has shown that caveolar LR formation requires the activity of the protein cavin (also called polymerase I and transcript release factor) [39]. Cavin is a peripheral membrane protein that binds to the phosphatidylserines within the caveolar LR [40]. Cavin and caveolin molecules exist in a stoichiometric ratio in caveolar LRs that are close together [41]. Therefore, cavin appears to be essential for the formation of morphological caveolar LRs. Another family of proteins described in LRs are the flotillin1–2/reggie1–2 complexes, originally described as neuronal proteins in retinal ganglion cells, and expressed during post-injury axon regeneration [42]. Flotillin/reggies oligomerize via their C-terminal domains and are involved in the endocytosis of GPI-anchored proteins [43]. Although caveolae have a diameter of 70–120 nm [44], planar LRs have one of 1–1,000 nm and are enriched in GPI-anchored proteins and flotillins [43, 44].
Although caveolin, flotillins/reggies and other proteins (reviewed in Lucero and Robbins [45]) can be permanently associated with LRs, other proteins are temporarily associated with them, mainly on the basis of protein post-translational modifications such as the addition of a GPI anchor, palmitoylation and myristoylation [45]. LRs may vary in their cholesterol and caveolin content, which, in turn, is related to LR enrichment in membrane and signalling receptors [18]. Caveolar and planar LRs may both undergo clustering according to alternative models proposing that either (1) non-LR receptors translocate into LRs after ligand binding, resulting in signal transduction, or (2) separate but close LRs may cluster only after ligand binding and receptor activation [46]. Whatever the case, clustering modifies LRs, thereby triggering new functions such as the creation of functional platforms that can be exploited for the signalling networks required in specific biological effects, such as angiogenesis [47, 48].
Involvement of LRs in specific steps of sprouting angiogenesis
The data that are available for the involvement of LRs in every step of sprouting angiogenesis show how LR clusters, which form on the surface of ECs subjected to an angiogenic challenge, provide an integrated platform that directs the formation, guidance, patterning and stabilization of the new vessels. The data that are reviewed here refer only to angiogenesis from pre-existing vessels, because this is only one of the features of tumor angiogenesis [49]. Tumor cells and the tumor microenvironment produce several angiogenic factors, including VEGF, that induce robust endothelial sprouting angiogenesis; however, they are also vasculogenic and actively participate in neovascularization through vascular mimicry [50], differentiation from tumor stem cells to tumor ECs [51] and co-option of vessels [52]. These tumor-specific features reflect the neoplastic transformation-related phenomenon: tumor cells express various embryonic genes, normally silent after birth and in adult life, that maintain the property of directing an ancestral vascular tube formation process [53].
The very beginning: LRs and VEGF stimulation
As a response to hypoxia, tissues overexpress pro-angiogenic growth factors [54]. The most important molecule controlling blood vessel morphogenesis is VEGFA, a member of a larger family of angiogenesis regulators including VEGFB, VEGFC and placental growth factor (PlGF) [55]. Alternative splicing of VEGFA can produce isoforms with anti-angiogenic properties [56]. The final balance of various isoforms controls angiogenesis. Binding of VEGFA to its receptor VEGFR2 (KDR or FLK1) promotes this, according to repetitive cycles that stop when excess anti-angiogenesis factors are present. Of VEGF isoforms and matrix proteins such as the thrombospondins [57], upregulation of VEGFR1/Flt1 is particularly important because it is a sort of VEGF buffer, based on its properties of weak signalling capacity and high VEGF affinity [58].
The main pro-angiogenic receptor VEGFR2 is present in EC caveolar LRs by association with Cav-1, which negatively regulates receptor activity in the basal state [59]. On binding, VEGFA promotes the release of VEGFR2 from caveolar LRs, which occurs together with tyrosine phosphorylation of Cav-1 and VEGFR2, and their co-localization at focal complexes on the edge of EC lamellipodia [60]. These events happen at the distal end of each sprout, which contains a specialized, highly motile and invasive EC termed a ‘tip cell’, provided with dynamic filopodia reminiscent of axonal growth cones [2, 6, 61].
Other functional links relate VEGFA signalling to caveolar LRs. Chronic exposure to reactive oxygen species (ROS) and the resulting oxidative stress, viewed as overproduction of ROS, the failure of antioxidant defence of the organism, or both, play a critical role in human pathology [62], and an excess of ROS production may heavily impair the pro-angiogenic activity of VEGF, despite the well-known redox regulation of VEGFA signalling in angiogenesis [63]. It is now recognized not only that ECs need physiological amounts of ROS to respond properly to a VEGFA challenge [64], but also that VEGF may signal through Nox-derived ROS [7]. This mechanism involves the activation and translocation of the small GTPase Rac1 to the plasma membrane, which stimulates the Nox2-based NADPH oxidase in EC caveolar LRs. ROS derived from this oxidase may reversibly oxidize and inactivate protein tyrosine phosphatases; these negatively regulate VEGFR2 autophosphorylation and the activation of downstream redox signalling events linked to EC proliferation and migration, which in turn contribute to angiogenesis [7, 65]. Therefore, in parallel with classic phosphorylation signalling (Fig. 1), VEGFR2 uses ROS as downstream signal mediators. In fact, several studies have demonstrated the involvement of the superoxide-producing enzyme Nox in VEGF signalling, and EC functions such as adhesion [66] and actin filament assembly [67].
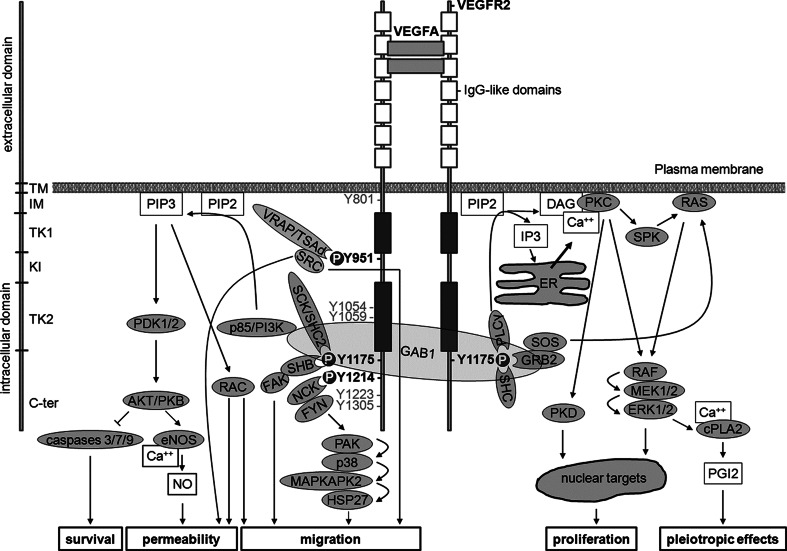
Fig. 1.
VEGF signalling elicited by VEGFR2-phosphorylating events. Structure and domains of VEGFR2 are indicated on the left side. Dimerized VEGFA (or VEGFC or -D after proteolytic cleavage) binds to second and third IgG-like domains within the extracellular domain of VEGFR2, thus inducing VEGFR2 dimerization and autophosphorylation at several tyrosine residues within the intracellular domain. Signalling elicited by major tyrosine residues phosphorylated in human VEGFR2 951, 1175 and 1214 (corresponding to 949, 1173 and 1212, respectively, in mice) is described. Moreover, phosphorylation at Y1054 and Y1059 is required to achieve maximal kinase activity. Additional tyrosine phosphorylation may occur at Y1223, Y1305, Y1309 and Y1319 residues. However, the role of these events is still unknown. Autophosphorylation at Y801 may precede phosphorylation of Y1054 and Y1059. SH2 domain-containing proteins (grooved oval) interact with tyrosine phosphorylated (P) residues thus activating signalling pathways that elicit several biological effects including increased permeability, survival, migration and proliferation (lower boxes). TM transmembrane, IM intramembrane, TK tyrosine kinase, KI kinase insert, C-term C-terminal, VEGFA vascular endothelial growth factor A, VEGFR2 VEGF receptor 2, VRAP VEGFR-associated protein also known as TSAd, T cell-specific adaptor, SRC sarcoma, GAB1 GRB2- (growth factor receptor-bound protein 2) associated binder 1, GRB2 growth factor receptor-bound protein 2, PLC-γ phospholipase C γ, RAC Ras-related C3 botulinum toxin substrate, PIP3 phosphatidylinositol 3,4,5-trisphosphate, PI3K phosphatidylinositol 3 kinase, PDK phosphoinositide-dependent kinase, PKB protein kinase B also known as AKT, RAC-alpha serine/threonine-protein kinase, BAD BCL2 associated death promoter, BCL2 B-cell CLL/lymphoma 2, eNOS endothelial nitric oxide synthase, ER endoplasmic reticulum, PLC-γ phospholipase C γ, NO nitric oxide, FAK focal adhesion kinase, SHC src homology/collagen, SCK SHC-like protein also known as SHC2, (Src homology 2 domain containing) transforming protein), PIP2 phosphatidylinositol (4,5)-bisphosphate, IP3 inositol (1,4,5)-trisphosphate, DAG sn-1,2-diacylglycerol, PKC protein kinase C, ERK1/2 extracellular regulated kinases 1 and 2, MEK MAPK/Erk kinase, HSP27 heat-shock protein 27, MAPKAP 2/3 MAPK-activating protein kinases 2 and 3, PAK p21-activated protein kinase, p38 p38 mitogen-activated protein kinase, Ca ++ calcium, cPLA2 cytosolic phospholipase A2, SHB SH2 domain-containing adapter protein B. SPK sphingosine kinase, CDC42 cell division cycle 42
Nevertheless, ROS still have the potential to damage VEGFR2 pathways that need to be controlled to maintain a finely tuned, pro-angiogenic ROS concentration. In particular, the EC caveolar LR-localized VEGFR2 has an oxidation-sensitive cysteine residue with a functionally active reduced state that is preserved specifically by peroxiredoxin-2 [68]. Peroxiredoxins (Prxs) are a family of proteins with peroxidase activities that degrade H2O2 to H2O, thereby abolishing H2O2 toxicity. Prx-2-mediated VEGFR2 protection is achieved by association of the two proteins in the caveolar LRs of ECs [68]. In a recent study, based on proteomic identification of VEGFA-dependent protein enrichment of membrane caveolar LR microdomains in EPCs, we showed that not only Prx-2, but also Prx-6, is located on caveolar LRs, possibly pointing to a convergent role for the two enzymes in safeguarding the redox-sensitive VEGFR2 [69]. In that work, we have shown that the gene ontology (GO) term referred to as ‘H2O2 metabolic process’ (represented by Prx-2, Prx-6 and protein DJ-1) undergoes the highest enrichment (>70-fold) at the EPC caveolar LR level on VEGFA stimulation. Along with this, the GO terms related to the control of apoptosis/programmed cell death (including some heat shock proteins) are significantly overexpressed in caveolar LRs of VEGFA-challenged EPCs. The caveolar LR localization of such molecules further reinforces the need for EPCs to strictly control ROS production/activity, as well as eliminating and/or inactivating damaged molecules. Such molecules may result from the Nox generation of ROS that are spatially and temporally dependent on VEGFA signalling at caveolar LRs, leading edge/focal adhesion complexes and cell–cell junctions in ECs [7].
What happens at the caveolar LRs of ECs/EPCs upon VEGFA stimulation offers further insights into how changes in the organization of protein caveolar LR microdomains may affect antioxidant defence/pro-survival pathways, supporting the concept of caveolar LRs as ‘floating islands of death and survival’ [70].
A critical task: LRs and the choice of tip cells
If all ECs were to react indiscriminately with VEGFA, the relevant section of the vessel might disintegrate, thus compromising tissue perfusion [2]. In fact, only a fraction of ECs become tip cells and initiate the sprouting process, whereas others stay behind (‘stalk cells’) and maintain the vessel’s integrity (for model illustrations of the process, see the literature [2, 3, 71] ). The caveolar LR-located Notch system, which has key roles in many differentiation processes, regulates this tip–stalk decision [72, 73]. The Notch receptor is normally triggered by cell-to-cell contact, in which the transmembrane proteins of those cells in direct contact provide the ligands that bind the Notch receptor of adjacent cells. This kind of interaction induces the so-called ‘Notch cascade’, which consists of Notch ligands and intracellular proteins transmitting the Notch signal to the genome. Involvement of the Notch system in angiogenesis occurs according to this general model. VEGF upregulates expression of the Notch ligand Delta-like 4 (DLL4), which reaches very high levels in tip cells [74–76]. The Notch receptor protein spans the cell membrane of stalk ECs, and DLL4 binding to the extracellular domain induces the proteolytic cleavage and release of the intracellular domain, which enters the stalk EC’s nucleus to regulate gene expression [77]. Once Notch’s extracellular domain has interacted with the ligand, an ADAM family metalloprotease, known as TACE (tumor necrosis α-converting enzyme), cleaves the Notch receptor just outside the membrane [78], releasing the extracellular portion of Notch, which continues to interact with the ligand. After this first cleavage, an enzyme called γ-secretase cleaves the remaining moiety of the Notch protein just inside the inner leaflet of the cell membrane of the Notch-expressing cell. In addition, the γ-secretase complex, made up of several proteins including presenilin-1, nicastrin, anterior pharynx defective-1 and presenilin enhancer-2, has been shown to be localized to LRs in retinal ECs [79–81]. The activity of γ-secretase releases the intracellular domain of the Notch protein, which moves to the nucleus where it can regulate gene expression by activating the transcription factor CSL [82]. When the intracellular Notch domain binds to CSL, it represses the transcription of VEGFR2/3 in stalk cells, thereby blunting angiogenic sprouting [82]. In addition to DLL4, another transmembrane Notch ligand, Jagged 1, is involved in tip EC selection. Unlike DLL4, Jagged 1 is a positive regulator of angiogenesis and acts by inducing a sugar modification of the Notch receptor [76]. Therefore, caveolar LR-associated Notch signalling may be used to control the sprouting pattern of blood vessels during angiogenesis by selecting the tip ECs that will drive vascular patterning, and the stalk ECs that will be prevented from migrating into the developing sprout. The migratory behavior of connector stalk ECs must be limited to retain a patent connection with the original blood vessel [83, 84].
A further task of VEGF: LRs and activation of migratory and invasion pathways of ECs
In adults, quiescent blood vessels are surrounded on the abluminal surface by a basement membrane, which consists of laminins, collagen type IV, nidogens, and the heparan sulfate proteoglycan perlecan [85–87]. Under the influence of VEGF, ECs selected to become tip cells activate proteolysis-dependent invasion mechanisms within a few minutes to degrade the basement membrane and progress within a provisional ECM. It is, therefore, understandable that there is extensive literature on this issue. However, a number of studies, including gene deletions in mice, have pointed to the essential role of matrix metalloproteases (MMPs) and the urokinase-type plasminogen activator receptor (uPAR)-associated plasminogen activator/plasmin system in the onset of angiogenesis [88–93]. MMP2, MMP9 (also referred to as gelatinases) and MT1-MMP (membrane type-1 MMP) stimulate angiogenesis primarily by ECM degradation, but the activity of these proteases is complex and may include other effects such as the activation of growth factors and cytokines, recruitment of EPCs and the degradation of inhibitors [88, 89]. Comparison of the ability to enhance capillary-like tube formation in a collagen-rich matrix indicated that MT1-MMP enables ECs to form invading tubular structures, whereas MMP2 and MMP9, and their cognate cell-surface receptors β3-integrin and CD44, do not, although they are required to perform efficient angiogenesis [90, 94]. MT1-MMP belongs to a subfamily of MMPs that is anchored to the membrane rather than being secreted, and this fact makes such proteases prime candidates for coordinating extracellular cues with cellular responses. MT1-MMP proteolytic activity in ECs is closely linked to its regulated presence in specific domains of the plasma membrane, particularly in lamellipodia and filopodia, where MT1-MMP is associated with Cav-1 and integrin αvβ3 in caveolar LRs and is catalytically active [95, 96]. Caveolar LRs provide the main route for MT1-MMP internalization in ECs, but they may contribute to its mobilization to invadopodia, as reported for cancer cells [95, 97]. Once located in caveolar LRs of EC invadopodia, MT1-MMP efficiently degrades the basement membrane of the vessel, which becomes leaky and hyperpermeable to blood plasma proteins [98]. Such vascular hyperpermeability causes leakage from the blood of the ECM proteins fibrinogen, vitronectin and fibronectin [98]. Fibrinogen is subsequently converted into fibrin through the activation of coagulation and, together with other extravasated proteins and pre-existing collagen, forms a new provisional ECM that provides an optimal molecular bed for sprouting vessels. MT1-MMP exerts its influence on both vascular morphogenesis and endothelial tube formation within three-dimensional collagen matrices, where it creates ‘vascular guidance tunnels’ [90, 95, 99, 100] by proteolysis. Within such physical conduits, ECs are freely able to migrate in an MMP-independent manner, to organize each other and polarize against a fluid–ECM interface at the tunnel wall. Within such interconnected tubular structures, ECs can be induced to collapse by microtubule-depolymerizing agents [101], and the preformed conduits used to regrow tubes or remodel existing tube structures within the spaces [102]. Lumen formation within EC cords in both collagen and fibrin matrices is integrin- and Rho GTPase-dependent, and involves the formation and coalescence of pinocytic intracellular vacuoles together with MT1-dependent ECM proteolysis [90, 99, 100, 102–106]. Intracellular vacuoles coalesce to form intracellular lumina [91, 108].
Molecular mechanisms that coordinate proteolysis with the formation of the cytoskeleton and lumina have been extensively reviewed elsewhere [90]. The presence of fibrin and vitronectin in the angiogenesis provisional matrix is the reason why the caveolar LR-associated, urokinase plasminogen activator receptor (uPAR)-dependent, plasminogen activation system must have efficient angiogenesis. In fact, although several molecular interactions between the MMP and the plasminogen/plasmin (fibrinolytic) system may affect cellular fibrinolysis [107], the main cell-associated fibrinolytic system hinges on uPAR. Also known as CD87, uPAR is a glycoprotein organized into three domains and tethered to the cell membrane with a GPI anchor [108, 109], the presence of which determines the partitioning of uPAR in LRs in many cell types [110–112], including the caveolar LRs of ECs [113–116]. We have shown that LR partitioning of uPAR is also strictly related to its high affinity for the GM1 and GM3 gangliosides, identifying at least three uPAR compartments in human EPCs: the first associated with caveolar LRs, the second with GM1-rich LRs and the third with GM3-rich LRs [116]. Although the traditional role of uPAR is its cell-surface activation of uPA, leading to plasminogen activation that generates plasmin, and cascade activation of the plasmin-related MMPs, uPAR has been shown to contribute to many proteolysis-independent processes, as previously reviewed [117–121]. It can bind directly to vitronectin, which is abundant in the provisional matrix of sprouting vessels, with a domain distinct from its uPA-binding site [122]; even in the absence of a transmembrane and intracellular domain, uPAR serves as a ‘signalosome’ organizer that is triggered on uPA or vitronectin ligation and by simultaneous interactions with signalling-competent surface integrins and receptors, such as the epidermal growth factor receptor [123]. From a general point of view, the inactive precursor pro-uPA interacts with the receptor uPAR via its growth factor domain, allowing the conversion of uPAR-bound pro-uPA to active uPA [124]. Once activated, uPA cleaves the proenzyme plasminogen to yield active plasmin [125], which activates pro-MMPs to active MMPs, as well as pro-uPA to active uPA [117–121]. In the case of ECs, VEGF interaction with its receptor VEGFR2 rapidly induces prourokinase activation that is dependent on a phosphoinositide 3 (PI3)-kinase-mediated change in integrin affinity, MT1-MMP-mediated activation of MMP2 and subsequent uPAR-bound pro-uPA activation. This VEGF-induced, MMP2-mediated, pro-uPA activation of ECs is responsible for VEGF-dependent local fibrinolytic activity and might be one of the initial steps in the angiogenic process [126]. As a consequence, stimulation of ECs by VEGFA/VEGFR2 engagement leads to a redistribution of caveolar LR-associated MT1-MMP and uPAR to the leading edge of tip cells, thus focusing the proteolytic activity of the growing vessel on the invasive front of migrating ECs [97]. A possible reason for the concentration of tip-cell proteolytic activity in selected spots of the cell membrane could be the need for spatial and temporal control of proteolytic activities for efficient angiogenesis. In fact, excessive proteolysis can cause unwanted damage to the provisional ECM, by degrading and dissolving the three-dimensional structure required for anchoring the migrating cell, as we originally proposed in the ‘grip-and-go’ model of cell migration [117] on the basis of previous experiments performed in plasminogen activator inhibitor type 1-deficient mice with severely impaired angiogenesis [127, 128].
VEGF-challenged sprouting angiogenesis originates in mature vessels, where ECs are in a confluent state. The response of ECs to VEGF stimulation is reduced by cell density through the increased activity of the density-enhanced tyrosine phosphatase DEP1. High levels of DEP1 impair extracellular signal-regulated kinase (ERK) 1/2 activation, a downstream signalling event of the VEGF/VEGFR system, leading to a downregulation of uPAR synthesis that then blocks angiogenesis [129]. Sparsely growing cells overexpress uPAR as a consequence of DEP1 downregulation, so it is likely that overexpression of uPAR in invading tip ECs may be related to the absence of associated ECs at the leading edge of the sprouting vessel, with inhibition of DEP1 in the tip cell [130]. Other growth factors, such as fibroblast growth factor 2 (FGF2), epidermal growth factor (EGF) and hepatocyte growth factor (HGF), have been shown to induce overexpression of proteolytic activity and angiogenesis in ECs via the PI3-kinase pathway-dependent activation of uPAR-bound pro-uPA [131]. Through domains II and III, uPAR also interacts with α5β1-integrin in ECs, leading to integrin activation and the redistribution required for it to become available to ECM substrates, and providing further support to the ‘grip’ of invading ECs [132]. In addition, uPAR has been implicated in zymogen coagulation factor XII-dependent angiogenesis [133]. Due to VEGF-dependent vascular hyperpermeability, coagulation factor XII is present within the provisional ECM of the sprouting vessel. It binds to domain II of uPAR on the EC membrane. Factor XII engagement induces uPAR’s communication with the cell through a β1-integrin. Cell stimulation via uPAR and the integrin also includes the EGF receptor. These pathways lead to ERK1/2 and Akt phosphorylation, which stimulates EC growth, proliferation and angiogenesis [133].
All of the data published to date about the role of the caveolar LR-associated proteolytic systems in ECs support the hypothesis that uPAR is the organizer and orchestrator of a spatially restricted cell interactome, which drives ECs through the initiation and termination of angiogenesis.
LR association of axon guidance molecule receptors in vascular patterning
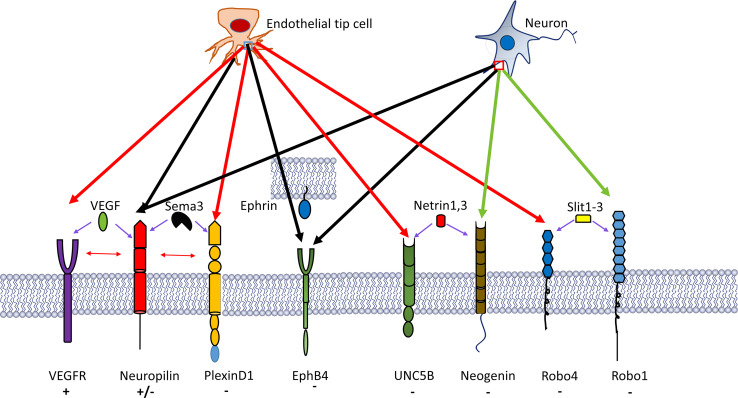
The vasculature develops in a way that is similar to the nervous system, forming a complex, highly branched, tree-like network [2, 134–136]. Surprisingly, the specialized tip ECs, which lead and drive endothelial sprouts, share several features with the guidance structure of the nervous system, the axonal growth cone [2, 135]. Tip cells are highly similar to the growth cones of developing axons, because they extend many filopodia that explore the environment, thereby sensing repulsive or attracting cues. Both structures use common signalling cues to regulate their guidance. Angiogenic ECs express receptors for axon guidance molecules (Fig. 2), including the roundabouts (Robos), UNC5B, neuropilins (Nrps), PlexinD1 and the Eph family receptor tyrosine kinases [136]. In particular, Robo4, UNC5B and PlexinD1 are mainly expressed in the vasculature, whereas Nrps and Eph-receptor expression are shared equally in the vasculature and nervous system.
Fig. 2.
A number of neural guidance molecular pathways have been recognized to participate in blood vessel branching morphogenesis. These pathways include semaphorin-3A (Sema3A)–plexinD1, ephrinB2–EphB4 and SLIT2–Robo4, which all elicit EC chemorepulsion, whereas SLIT2–Robo1 and VEGF–VEGFR2 (and possibly VEGF–NRP1) have been implicated in endothelial tip cell chemoattraction and elongation. Predominantly EC-expressed receptors are indicated by red arrows, receptors with shared expression in the nervous and the vascular system by black arrows, and molecules with uncertain expression in the vascular system by green arrows. Symbols plus and minus indicate chemoattraction and chemorepulsion, respectively
Slits and roundabouts
Robo4 (also referred to as ‘magic roundabout’) is a receptor for Slit1–3 glycoproteins, a family of ligands produced by many tissues, including tumors [137, 138]. In axonal growth, Slit proteins have an evolutionarily conserved role in the guidance of repulsion [139]. However, in angiogenesis, conflicting reports implicate them as both pro- and anti-angiogenic molecules. There are three vertebrate Slit proteins (Slit1–3), which are secreted ligands for the four members of the Robo family of receptors (Robo1–4). To date, it seems that any Slit protein can interact with any Robo receptor. Researchers interested in angiogenesis have focused on Slit2, because it is expressed in angiogenic tissues. Of the Robo receptors, Robo4 has received the most attention because it is expressed specifically in the vasculature and upregulated at sites of angiogenesis. Robo4/Slit2 interaction negatively regulates angiogenesis by inhibiting VEGF/VEGFR2 signalling [140]. However, other studies have identified Slit2 as a positive regulator of angiogenesis through interactions with either Robo4 or Robo1 [141, 142]. Conclusive demonstration of Robo1 function in ECs is lacking, because vascular phenotypes in Robo1 knockout mice have not been reported [135], even though small interfering RNA (siRNA) knocks out Robo1 but not Robo4 in ECs, so impairing migration to VEGF in vitro [143]. Whatever the case, the evidence relating Robo4 to LRs is mainly based on its interaction with other partner receptors. In ECs, Robo4 interacts with UNC5B, another EC guidance receptor that, because of its palmitoylation [144], selectively partitions in LRs; such co-localization is functionally important for vessel integrity and angiogenesis inhibition [145]. Furthermore, Robo4 is co-immunoprecipitated with soluble FLT1 (VEGFR1), which is an LR-associated molecule in podocytes [146].
Netrins and UNC5B
Netrins are a class of proteins involved in axon guidance; their structure resembles the ECM protein laminin. The netrin family is composed mostly of secreted proteins, which serve as bifunctional signals: attracting some neurons (chemotropic) but repelling others (chemorepellent) during brain development. Like Robo4, the netrin receptor UNC5B is vasculature specific, and expressed in tip and arterial ECs, and sprouting capillaries [135, 136]. UNC5B activation by Netrin-1/3 prevents filopodia extension in ECs and negatively regulates capillary branching in vessel patterning [147, 148]. Palmitoylation induces UNC5B partitioning in LRs of ECs [144], allowing inclusion of netrin–UNC5B repulsive activity among LR-dependent effects. Netrin-4, another netrin-negative regulator of angiogenesis, does not bind UNC5B directly, but it has been shown to bind to neogenin, an additional netrin receptor molecule that recruits UNC5B to mediate the anti-angiogenic activity of Netrin-4 [149]. The evidence clearly shows that UNC5B–neogenin interaction, which mediates the anti-angiogenic activity of Netrin-4, is an LR-driven process. Blocking neogenin-LR association influences axonal path finding and lowers axonal membrane cholesterol, a process that disrupts LRs and restores neuron locomotor function after spinal cord injury [150]. Another UNC5B ligand, namely FLRT3 (fibronectin and leucine-rich transmembrane protein 3), has been identified [151]. Overall, these data suggest that UNC5B has many ligands and interactors that trigger UNC5B activation and vascular patterning by LR recruitment. Netrins may also have bifunctional activities in the vasculature, because pro-angiogenic netrin activities have been reported [135].
Semaphorins, plexins and neuropilins
Semaphorins (Semas) are a large family (eight classes of molecules have been described so far) of secreted and membrane-bound proteins, characterized by the presence of a common Sema domain, originally described as axon guidance cues and later shown to be regulators of vascular patterning. Semas signal via two receptor families, plexins and neuropilins (Nrps). Membrane-bound Semas bind and signal directly through plexins, whereas most class 3 secreted Semas (Sema3A–3G) are known to bind to a holoreceptor complex that consists of Nrps as the ligand and plexins as the signal-transducing subunit [152, 153]. The exception to this rule is Sema3E, which binds to the vasculature-restricted plexinD1 receptor directly and independently of the Nrps [154]. Blood vessels deflect from chick embryo somites that overexpress Sema3E, indicating that this protein mediates EC repulsion, restricting blood vessel growth in mice [154]. In axonal growth, LRs mediate the inhibitory effects of Sema3A on growth cones in Xenopus spinal neurons [155]. Disruption of LRs by depletion of membrane cholesterol effectively blocks Sema3A-induced repulsion and the extension of growth cones in Xenopus spinal neuron cultures [156]. Furthermore, brief exposure to Sema3A increases the association of Nrp-1 with LRs, implying asymmetrical receptor–LR association and localized signalling in the growth cones during guidance responses. Activation of mitogen-activated protein kinases (MAPKs) on Sema3A treatment appears to depend on the integrity of LRs and is required for Sema3A-induced growth cone repulsion [157]. These data support a role for LRs in mediating growth cone guidance, by providing a molecular platform for the localized assembly of ligand–receptor complexes and their downstream effectors for cytoskeletal rearrangement and local protein synthesis, including Nrp-1, plexins, Src family kinases, Rho GPTases and MAPKs [156].
Besides Semas, VEGF family members also bind to the extracellular domain of Nrps by recognition of a different sequence [155]. In this case, the final result is attraction of vascular structures. In both vessels and axonal growth cones, the members of this system are located in LRs, as also shown by the LR-dependent endocytosis of Nrp1 induced by Sema3C in ECs [158] and by flotillin-mediated endocytic events that dictate cell type-specific responses to Sema3A in cortical neurons [159]. Although the guidance of Nrps in response to Semas in the nervous system is mainly repulsive and mediates growth cone collapse [160], Nrps are attractive in vessels and mediate tip EC extension and directional vessel sprouting in response to the VEGF family. Figure 3 shows a scheme summarizing the integrated activities of LR-associated molecules in tip-cell filopodia and at the tip-cell/stalk-cell interface.
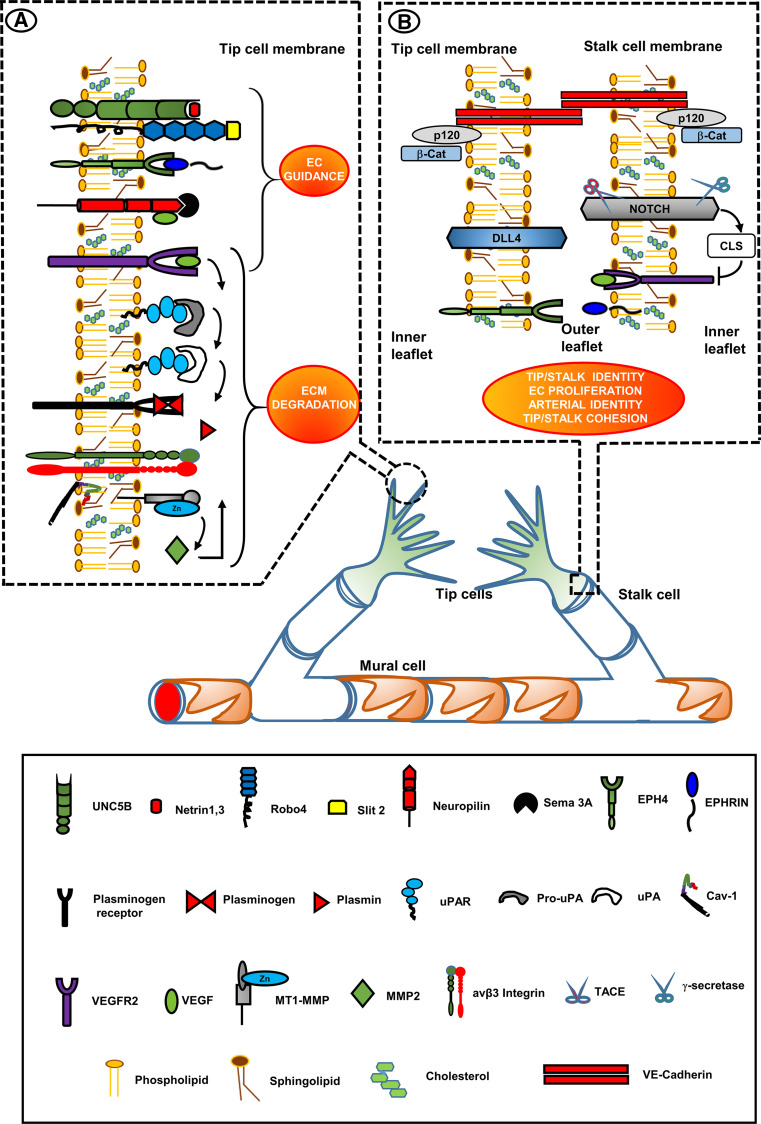
Fig. 3.
Vessel development: lipid-raft-localized molecules involved in vessel branching morphogenesis. Therapeutic targets. Insets show selected LRs of the tip-cell filopodia (inset a) and of the tip-cell/stalk-cell interface (inset b). Inset 1 VEGFR2 is located in filopodia [59, 60]. EC guidance receptors are in LRs and their cognate ligands generally induce EC repulsion [140, 144, 145, 147, 148]. VEGF family members also bind to a different extracellular sequence of Nrps, resulting in attraction of vascular structures [155] in a way similar to VEGFA-VEGFR2 interaction. Upon VEGFA/VEGFR2 interaction, MT1-MMP is associated with cav-1 and integrin αvβ3 in caveolar-LRs of filopodia, where it activates pro-MMP2 to MMP2 that is then bound to β3 integrins in LRs [95, 96, 126]. MMP2 activated uPAR-bound pro-uPA to uPA [124], which cleaves the proenzyme plasminogen to yield active plasmin [125] that in turn activates pro-MMPs to active MMPs, as well as pro-uPA to active uPA [117–121]. Inset 2 the Notch system is located in LRs of the stalk cell. VEGFR2 stimulation upregulates overexpression of the Notch ligand protein Dll4 in the rear moiety of the tip-cell, which leads to CSL-mediated silencing of VEGFR2 expression in stalk-cell [71]. The stalk cell becomes insensitive to VEGF stimulation. Tip-cell/stalk-cell connection is warranted by VE-cadherins (not associated to LRs), and by the LR-associated Eph-Ephrin system [2]. The red–orange ellipses identify the LR-directed processes, important in vessel development, that may be potentiated by forcing induction of LR formation to promote functional recovery and normalization of angiogenesis in tumors and other angiogenesis diseases
Ephrins and Eph receptors, a multi-purpose system involved in vascular guidance, EC–EC interaction and mural cell recruitment
The Eph-receptor tyrosine kinases and their membrane-bound ligands, the ephrins, mediate cell contact-dependent signalling that controls several aspects of nervous system development, as well as vascular differentiation, guidance, assembly and angiogenesis [135]. Ephrins are cell-surface proteins that are either attached via a GPI anchor (ephrin-A subclass) or a transmembrane sequence (ephrin-B subclass). Ephs have also been classified as EphA and EphB, based on their binding preference to ephrin-A or -B [161]. Besides activating the cognate Eph receptors (forward signalling), ephrins have receptor-like properties, being capable of transduction by themselves (reverse signalling) [161]. Several Ephs and ephrins are present in the vasculature. The ephrin-A1 ligand and its receptor EphA2 are expressed in developing vessels during tumor neovascularization [162], but also in tumor cells, and have been related to EC migration and VEGF expression [163]. Binding of ephrin-B2 to EphB4 modulates EC–EC interactions and is essential in angiogenesis, whereas ephrin-B2 expression in mural cells, such as pericytes and vascular smooth muscle cells (vSMCs), controls their motility, adhesion and recruitment in vessel wall assembly [164]. Among the class B ligands and receptors, ephrin-B2 and EphB4 selectively mark the endothelia of arteries and veins, respectively, and their expression in mice is essential for the angiogenic growth of embryonic vasculature [165]. In the adult, ephrin-B2 expression is upregulated in physiological and pathological angiogenesis [166, 167]. In line with the repulsive role of ephrin/Eph in the nervous system, EphB4 and reverse signalling by ephrin-B2 act as negative regulators of branching angiogenesis by promoting circumferential growth of blood vessels and suppressing EC sprouting [168]. Overall, these data indicate that the precise role of ephrin-B2 and EphB4 in the vasculature remains unclear [135].
Accumulating evidence suggests that both ephrins and Eph receptors are loosely preclustered in LRs of the plasma membrane, forming low-affinity ephrin–ephrin and Eph–Eph dimers, and that ephrin docking may cause an Eph-receptor rearrangement that triggers stable aggregation into larger Eph–ephrin clusters, which may fuse together into larger signalling platforms on Eph receptor–ephrin binding [161, 169–173]. Moreover, it was found that receptor tyrosine kinases, including Eph family receptors, are concentrated and highly organized in caveolar LRs in the neuronal plasma membrane [174]. All the available evidence supports an important role for LR clustering in the initiation, propagation and maintenance of Eph signal transduction events. An interesting paper has highlighted the principle that Eph signalling may be different from outside and inside LR microdomains, which impart the stability of certain dimers of the Eph transmembrane domain, providing proper oligomerization of the receptor, and thus initiating the formation of bigger LRs with large ephrin–Eph signalling clusters within the plasma membrane. This kind of activation has been referred to as a ‘rotation-coupled activation mechanism’ which may take place during Eph-receptor signalling [175].
Lipid rafts and the maturation of blood vessels
The stepwise transition from a growing vasculature to a quiescent and functional vascular network has been referred to as ‘maturation’ [2]. This implies the suppression of EC proliferation, blocking of proteolytic degradation of the provisional matrix and of sprouting, stabilization of pre-formed vascular structures, differentiation of specific vascular structures such as valves, fenestrations and tight junctions, and juxtaposition of mural cells, such as pericytes and vSMCs. Mural cell recruitment is a particularly important event, which is fulfilled by pericytes around capillaries and vSMCs around larger diameter vessels. Their ontogeny from progenitor stem cells is still a matter of intensive research [176–178].
PDGF-B receptor and its partners
Expression of the platelet-derived growth factor receptor β (PDGFRβ) tyrosine kinase in pericytes and vSMCs is required for mural cell proliferation, chemotactic migration and incorporation into the vessel wall [179, 180]. PDGF-B depends on LRs for cell signalling, which implies the presence of PDGFRβ in LRs [181]. PDGFRβ requires the presence of partners for proper signalling in mural cells. The uPAR associates with PDGFRβ, which serves as a transmembrane adaptor for uPAR in vSMCs. Assembly of this complex is necessary for transduction of intracellular signalling and the initiation of functional changes in vSMCs. The tyrosine phosphatase SHP-2 mediates these processes, after co-localization with PDGFRβ and uPAR: such a multi-molecular complex is assembled on LRs, the disruption of which precludes SHP-2 phosphorylation, its association with PDGFRβ and vSMC functional responses [182, 183]. Another critical association of PDGFRβ is that with ephrin-B2: in vSMCs ephrin-B2 controls PDGFRβ distribution in the plasma membrane, endocytosis and signalling. In fact, an absence of ephrin-B2 leads to redistribution of PDGFRβ from caveolar LRs to clathrin-associated membrane fractions, impairing vSMC proliferation [184]. Accordingly, mutant mice lacking ephrin-B2 expression in vSMCs develop vessel wall defects and aortic aneurysms [184]. PDGFRβ function also involves cooperation with a further LR-associated family of G-protein-coupled receptors which bind sphingosine-1-phosphate (S1P), a sphingolipid secreted by ECs [185]. The four receptors for S1P, referred to as S1P1–4, are expressed in mural cells, and the number of pericytes and vSMCs recruited in S1P1-deficient mice is compromised [186]. S1P derives from sphingolipids that are marker constituents of LRs, so, in this case LRs not only harbor the relevant receptors, but are also likely to be the source of the cognate ligand [187]. The main role of S1P1 in ECs involves trafficking of the cell adhesion molecule N-cadherin to the EC-mural cell contact zone [188]. ECs express two different classic cadherins—vascular endothelial (VE) cadherin and neural (N) cadherin—with distinct functions in the vasculature. VE-cadherin is specific to EC-adherent junctions and necessary for vascular morphogenesis. It is associated with LRs. LR-associated VE-cadherin is, in turn, associated with p120-catenin, and this interaction is necessary for VE-cadherin recruitment in LRs [13]. N-cadherins show diffuse localization on the EC surface and interact with mural cells for vessel stabilization [13].
TGFβ1 and vascular maturation: a role for LRs?
The differentiation of progenitor cells into mural cells is controlled by transforming growth factor-β1 (TGFβ1) [189]. TGFβ1 is also involved in vascular formation through activin receptor-like kinase (ALK)-1 and ALK5. ALK5, which is expressed ubiquitously, phosphorylates Smad2 and Smad3, whereas EC-specific ALK1 activates Smad1 and Smad5 [190]. There is evidence that Smad2/3 signalling in ECs is indispensable for the maintenance of vascular integrity via the fine-tuning of N-cadherin, VE-cadherin and S1P1 expression in the vasculature [191]. It is well documented that TGFβ receptors are endocytosed via clathrin-coated vesicles. They may also enter cells via caveolar LRs [192]. Although receptor endocytosis is not essential for TGFβ signalling, clathrin-mediated endocytosis has been shown to promote TGFβ-induced Smad activation and transcriptional responses. Caveolar LRs are regarded as signalling centers for a wide variety of receptors, but are particularly involved in the facilitation of TGFβ-receptor degradation and therefore the turning off of TGFβ signalling [192]. Hence caveolar LRs have the role of controlling excess TGFβ activity.
TIE receptors and angiopoietins
Endothelial cells express TIE2, a tyrosine kinase receptor for angiopoietin (Ang) ligands [193]. Signalling by Ang1, expressed by mural cells, and by TIE2 of ECs promotes angiogenesis and mural-cell association with the endothelium, in addition to reducing vascular leakage [193, 194]. The major intracellular signalling systems activated by TIE2 in response to Ang1 include the Akt and ERK1/2 pathways. TIE2 is not detectable in the LR fraction of human umbilical vein endothelial cells (HUVECs) unless they are first stimulated with Ang1. After stimulation, a fraction of TIE2 associates tightly with the LRs. Treatment of HUVECs with the LR-disrupting agent methyl-β-cyclodextrin selectively inhibits Ang1-induced Akt phosphorylation [195]. Therefore, LRs serve as signalling platforms for TIE2 in vascular ECs, especially for the Akt pathway. Figure 4 shows a scheme summarizing the integrated activities of LR-associated molecules in EC-mural cells and EC–EC interactions for the vessel stabilization.
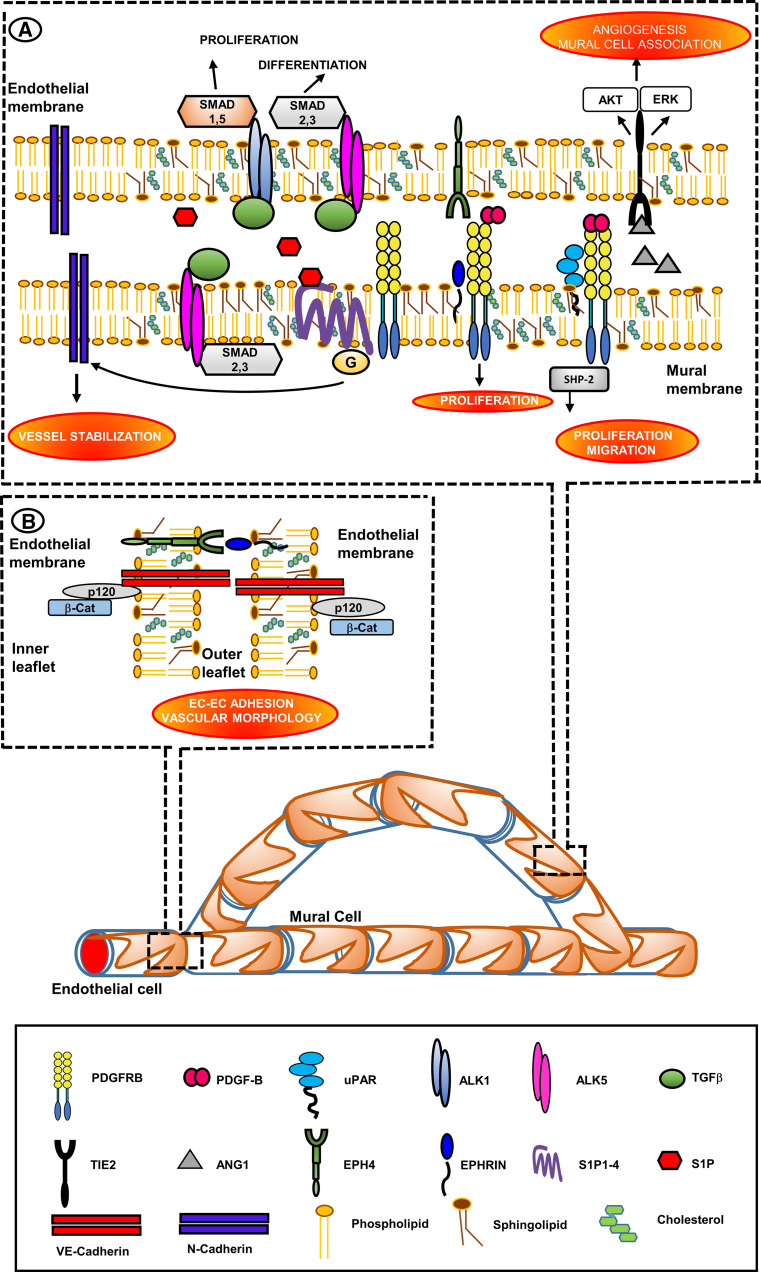
Fig. 4.
Vessel maturation: lipid-rafts as platforms of vessel stabilization by promotion of EC/mural cells and EC/EC interactions. Therapeutic targets. Insets show the LRs-located molecules that promote vessel stabilization and maturation by allowing mural cells (pericytes in capillaries and vSMS in larger vessels)/ECs (inset a) and EC/EC interactions (inset b). Inset a PDGFRβ is located in LRs of mural cells [181]. PDGFRβ requires the presence of partners for a proper signaling. uPAR associates with PDGFRβ in vSMCs. Assembly of this complex is necessary for signaling and initiation of functional changes in vSMCs mediated by the tyrosine phosphatase SHP-2. The complex is assembled on LRs [182, 183]. In vSMCs Ephrin-B2 interacts with PDGFRβ and controls its distribution and signaling, thereby promoting vSMCs proliferation [184]. PDGFRβ function also involves cooperation with LR-associated S1P receptors [185], thereby stimulating EC recruitment of pericytes and vSMCs [186]. S1P derives from EC sphingolipids that are marker constituents of LRs [187]. The main role of S1P1 receptor involves the trafficking of the cell adhesion molecule N-cadherin, which is not a LR-associated molecule, to the EC-mural-cell contact zone [188]. Tie2 associates with LRs following stimulation with Ang1 [193]. Caveolar-LRs facilitate the degradation of TGFβ receptors and therefore the turn-off of TGFβ signaling [190], thus controlling excess TGFβ activity. Inset b VE-cadherin is specific to EC adherent junctions and is necessary for vascular morphogenesis. VE-cadherin is associated to LRs. VE-cadherin is in turn associated with p120-catenin, and this interaction is necessary for VE-cadherin recruitment in LRs [13]. Ephrins and Eph receptors form clusters in LRs, providing low-affinity ephrin–ephrin and Eph–Eph dimers. Ephrin docking triggers stable aggregation into larger Eph-ephrin clusters which may fuse together into larger signaling platforms following Eph receptor-ephrin binding [161, 169–173]. The red–orange ellipses identify the LR-directed processes that have the chance to favor EC–EC interaction (to prevent endothelial fenestrations and vascular leakage), and vessel wall integrity upon EC-mural cells interaction. Even if N-cadherins are not associated to LRs, their overexpression is a LR-dependent event directed by S1P1 receptor. Potentiation of LR formation may therefore have the chance to promote functional recovery and normalization of angiogenesis in tumors and other angiogenesis diseases where endothelial fenestrations, vascular leakage and loss of mural cells compromise vessel wall integrity
Conclusions
LRs and vessel ‘normalization’, a therapeutic opportunity for tumors and other non-malignant angiogenic diseases
Given the importance and overall activity of LR platforms as organizers of ‘pro-angiogenic’ molecular assemblies in sprouting ECs, how should we exploit these LR properties for a possible therapeutic approach to control angiogenesis? This would include angiogenesis in pathological cancers and its lack in diseases of ischaemic origin [196]. Anti-angiogenic therapy has shown promise as a treatment for several cancers, opening a new avenue to anti-cancer therapy [50]. The anti-VEGF antibody bevacizumab has shown the best activity among the more than 20 anti-angiogenic drugs that have been investigated to date in clinical trials for several types of cancer. In combination with various chemotherapeutic agents, bevacizumab significantly prolongs progression-free survival for a period of time (3–6 months) and extends overall survival compared with chemotherapy alone [197, 198]. An in-depth analysis of the clinical data has shown that current angiogenic therapy effectively improves progression-free survival in some types of cancer, but that the anti-tumor effects are short-lived. Most patients who initially respond will eventually develop drug resistance, tumor recurrence and cancer metastases [199], indicating that anti-angiogenic therapy is a double-edged sword of tumor progression and metastasis. There are several reasons for this. First, current anti-angiogenic drugs reduce the oxygen supply in tumor tissue, inducing overexpression of hypoxia-inducible factor 1α (HIF-1α), which is a master transcription factor that promotes expression of a variety of tumor angiogenic and metastatic genes in tumor and stromal cells. Such tumor cells become more vasculogenic, aggressive and metastatic, resulting in tumor cell dissemination [200]. HIF-1α-dependent pro-invasive mechanisms of cancer cells involve overexpression of the HGF-receptor tyrosine kinase c-MET [201], a decrease of E-cadherin [202], and induction of MMPs [203, 204] and uPAR [205]. Second, current anti-angiogenic therapies mainly target VEGF signalling and vascular EC-mediated angiogenesis, but do not effectively affect tumor cell-mediated vascular mimicry and other tumor-specific angiogenic mechanisms, which are closely associated with tumor growth and cancer metastasis [44]. Third, the cancer vasculature and cancer microenvironment develop compensatory mechanisms when VEGF is blocked [206], including overexpression of the FGF family of ligands [207], inhibition of Notch signalling [208], increase of PlGF [209, 210], upregulation of Ang1 [211]. Work from the laboratory of Napoleone Ferrara has identified a specific myeloid cell population (CD11b+Gr1+) that migrates to tumors under the influence of granulocyte colony-stimulating factor secreted by tumors, interleukin 6 and stromal cell-derived factor 1, and mediates tumor angiogenesis and resistance to anti-VEGF therapy [212]. In the light of these limitations, researchers have explored new strategies for inhibiting angiogenesis. First, there have been many attempts to optimize combinations of anti-angiogenic drugs with chemotherapeutics. Second, researchers are investigating additional anti-angiogenic agents, beyond anti-VEGF and sprouting angiogenesis, to target all the alternative features of cancer angiogenesis. Third, the so-called ‘vascular normalization’ has emerged as a new option for the control of cancer angiogenesis. Vascular normalization aims to stabilize disorganized tumor vasculature and improve blood circulation in tumor tissue [213]. Preclinical and clinical data clearly show that tumor vascular normalization by antibodies, peptides, proteins, small molecules and pericytes decreases tumor size and tumor metastasis, even if such drugs target sprouting angiogenesis and display moderate anti-cancer efficiency, similar to anti-angiogenic therapeutics [214].
Beyond cancer angiogenesis, emerging evidence indicates that the amount and structure of vessels in many non-malignant diseases are also abnormal [196, 213]. Hallmarks of altered vessels are disruption of pericyte and EC contacts, thickening of basement membranes, vasodilatation, microaneurysms, vessel tortuosity, oedema, vascular fragility, hemorrhage and hypoxia [196, 213, 215]. Ocular macular degeneration, diabetic retinopathy, wound healing, neurological disorders, haemangiomas, psoriatic skin lesions, arthritic joint disease, atherosclerotic plaques, liver disease (192) and systemic sclerosis [216, 217] are all characterized by vessel tortuosity and abnormal vascularization. Moreover, blood vessel regeneration in peripheral arterial disease [218] and ischaemic heart disease [219] leads to lower capillary density matched against vasculature defects. Pharmacological approaches used to normalize vessels in cancer can also induce vessel normalization in other angiogenic disorders in animal models and patients [220–222].
The major challenge of therapeutic approaches, based on vessel normalization, is that these strategies also stimulate the formation of immature, leaky and disorganized vessels that are poorly perfused and prone to regression once therapy has been halted [213]. We propose that an enhancement of LR activity within tumors and other diseases characterized by abnormal vascularization may promote vascular normalization; this would allow more efficient action of chemotherapeutics and cancer immunotherapy in tumors, and offer the advantage of delivering oxygen and nutrients more rapidly and efficiently to ischaemic tissue, thereby restoring tissue performance in diseases with the signature of dysfunctional angiogenesis. As discussed in this review, the presence of LRs in ECs allows proper organization of various forms of caveolin to form caveolar LRs, in which critical molecules are assembled that allow suitable angiogenesis to occur. Caveolins and caveolar LRs are therefore very relevant to maintaining EC membrane integrity in both structure and function [223, 224], indicating mutual liaison aimed at allowing normal vascular performance. The involvement of caveolae in different cardiovascular diseases makes caveolin-based therapeutic approaches an attractive possibility with which to combat myocardial ischaemia, heart failure and pulmonary hypertension [225]. All forms of caveolin (Cav-1, Cav-2 and Cav-3) are involved, according to their specific tissue distribution. In muscle cells, Cav-3 is associated with LR domains [226]. In line with these observations, the recent work of Roura and co-workers has studied the role of LRs in patients with idiopathic dilated cardiomyopathy (IDCM) [227], showing that the movement of the low-density lipoprotein receptor-related protein 1 (LRP1) to caveolar-LRs and the concomitant increase in non-LR-related ERK1/2/MMP9 activation may have crucial clinical implications in the progression of disease. Although not of ischaemic origin, progression of this disease also has the signature of marked vascular dysfunction, and myocardial LRs are conceivably new molecular actors and therapeutic targets.
Therefore, caveolins/caveolae are now considered to be therapeutic targets for a variety of diseases characterized by an excess of caveolar LR function, by targeting caveolar receptors with peptide antagonists (blockers) or agonists (activators), or with antibodies [228]. Significant alternative interventions to either increase or decrease caveolin expression are gene or cell therapy, anti-sense or siRNA approaches, the use of inhibitory peptides derived from caveolin scaffolding domains, or modulation of cellular cholesterol levels or caveolar lipid content [228].
It has been shown that dietary n-3 polyunsaturated fatty acids (n-3 PUFAs) alter the size and distribution of cell-surface LRs by forcing the segregation of cholesterol into LRs, thereby enhancing the extent of protein clustering within LRs and, subsequently, their function [229]. Moreover, n-3 PUFAs have an angiogenic impact [230] by stimulating cerebral angiogenesis after cerebral ischaemia [231] and reducing pathological retinal angiogenesis [232]. Overall, we believe that there is a theoretical and experimental background for further investigation of this fascinating and still unexplored field of angiogenesis control, with the aim of identifying possible new ways to support sustained angiogenesis normalization to help conventional anti-angiogenesis drugs and chemotherapeutics in tumors and angiogenic diseases characterized by the presence of dysfunctional vessels.
Figures 3 and 4 indicate LR-located molecules whose upregulation following forcing of LR cholesterol levels may promote vascular normalization.
Acknowledgments
This work was supported by grants of the Ente Cassa di Risparmio di Firenze to MDR, GF and GM; Istituto Toscano Tumori to MDR; Associazione Italiana Ricerca sul Cancro to MDR (AIRC Grant No. IG 2013 N. 14266); Brazilian FAPERJ to TDR. FM was supported by a post-doctoral joint fellowship of the European Union and Regione Toscana within the project UNIFI-4 MELOTAC.
Abbreviations
- ECs
Endothelial cells
- EPCs
Endothelial progenitor cells
- ECM
Extracellular matrix
- VEGF
Vascular endothelial growth factor
- LRs
Lipid rafts
- GPI
Glycosylphosphatidylinositol
- ssBLMs
Solid-supported bilayer lipid membranes
- Cav-1
Caveolin-1
- Cav
Caveolin
- PTRF
Polymerase I and transcript release factor
- VEGFR2
Vascular endothelial growth factor receptor 2
- KDR
Kinase insert domain receptor
- FLK1
Fetal liver kinase 1
- VEGFR1/Flt1
Vascular endothelial growth factor receptor 1/fms-related tyrosine kinase 1
- Prxs
Peroxirederoxins
- ROS
Reactive oxygen species
- MMP
Metalloprotease
- TACE
Tumor necrosis alpha converting enzyme
- MMPs
Matrix metalloproteinases
- uPAR
Urokinase-type-plasminogen activator receptor
- MT1-MMP
Membrane-type-1-MMP
- DEP1
Density-enhanced tyrosine phosphatase
- ERK1/2
Extracellular signal-regulated kinase 1/2
- Robos
Roundabouts
- UNC5B
Unc-5 homolog B
- Nrps
Neuropilins
- Slit1-3
Slit homolog 1-3
- RGMa
Repulsive guidance molecule a
- FLRT3
Fibronectin and leucine rich transmembrane protein 3
- Semas
Semaphorins
- vSMC
Vascular smooth muscle cell
- PDGFRβ
Platelet-derived growth factor receptor β
- S1P
Sphingosine-1-phosphate
- TGF β1
Transforming growth factor-β1 (TGFβ1)
- ALK1
Activin receptor-like kinase 1
- ALK5
Activin receptor-like kinase 5
- TIE2
TEK tyrosine kinase
- Ang
Angiopoietin
- n-3 PUFA
n-3 polyunsaturated fatty acids
Footnotes
A. Laurenzana and G. Fibbi contributed equally to this work.
Contributor Information
Mario Del Rosso, Email: delrosso@unifi.it.
Francesca Margheri, Email: fmargheri@unifi.it.
References
- 1.Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- 2.Adams RH, Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol. 2007;8:464–478. doi: 10.1038/nrm2183. [DOI] [PubMed] [Google Scholar]
- 3.Roura S, Gálvez-Montón C, Bayes-Genis A. The challenges for cardiac vascular precursor cell therapy: lessons from a very elusive precursor. J Vasc Res. 2013;50:304–323. doi: 10.1159/000353294. [DOI] [PubMed] [Google Scholar]
- 4.Logsdon EA, Finley SD, Popel AS, et al. A systems biology view of blood vessel growth and remodelling. J Cell Mol Med. 2014 doi: 10.1111/jcmm.12164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gerhardt H, Betsholtz C. How do endothelial cells orientate? Experientia. 2005;94:3–15. doi: 10.1007/3-7643-7311-3_1. [DOI] [PubMed] [Google Scholar]
- 6.Klagsbrun M, Eichmann A. A role for axon guidance receptors and ligands in blood vessel development and tumor angiogenesis. Cytokine Growth Factor Rev. 2005;16:535–548. doi: 10.1016/j.cytogfr.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 7.Ushio-Fukai M. VEGF signaling through NADPH oxidase-derived ROS. Antioxid Redox Signal. 2007;9:731–739. doi: 10.1089/ars.2007.1556. [DOI] [PubMed] [Google Scholar]
- 8.Montuori N, Ragno P. Role of uPA/uPAR in the modulation of angiogenesis. Chem Immunol Allergy. 2014;99:105–155. doi: 10.1159/000353310. [DOI] [PubMed] [Google Scholar]
- 9.Wang Y, Nakayama M, Pitulescu ME, et al. Ephrin-B2 controls VEGF-induced angiogenesis and lymphangiogenesis. Nature. 2010;465:483–486. doi: 10.1038/nature09002. [DOI] [PubMed] [Google Scholar]
- 10.Benedito R, Rocha SF, Woeste M, et al. Notch-dependent VEGFR3 upregulation allows angiogenesis without VEGF-VEGFR2 signalling. Nature. 2012;484:110–114. doi: 10.1038/nature10908. [DOI] [PubMed] [Google Scholar]
- 11.Urbinati C, Ravelli C, Tanghetti E, et al. Substrate-immobilized HIV-1 Tat drives VEGFR2/α(v)β(3)-integrin complex formation and polarization in endothelial cells. Arterioscler Thromb Vasc Biol. 2012;32:e25–e34. doi: 10.1161/ATVBAHA.111.242396. [DOI] [PubMed] [Google Scholar]
- 12.Deb M, Sengupta D, Patra SK. Integrin-epigenetics: a system with imperative impact on cancer. Cancer Metastasis Rev. 2012;31:221–234. doi: 10.1007/s10555-011-9341-9. [DOI] [PubMed] [Google Scholar]
- 13.Gentil-dit-Maurin A, Oun S, Almagro S, et al. Unraveling the distinct distributions of VE- and N-cadherins in endothelial cells: a key role for p120-catenin. Exp Cell Res. 2010;316:2587–2599. doi: 10.1016/j.yexcr.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 14.Pilarczyk M, Mateuszuk L, Rygula A, et al. Endothelium in spots: high-content imaging of lipid rafts clusters in db/db mice. PLoS ONE. 2014;9:e106065. doi: 10.1371/journal.pone.0106065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown DA, London E. Functions of lipid rafts in biological membranes. Annu Rev Cell Dev Biol. 1998;14:111–136. doi: 10.1146/annurev.cellbio.14.1.111. [DOI] [PubMed] [Google Scholar]
- 16.Anderson RG, Jacobson K. A role for lipid shells in targeting proteins to caveolae, rafts, and other lipid domains. Science. 2002;296:1821–1825. doi: 10.1126/science.1068886. [DOI] [PubMed] [Google Scholar]
- 17.Callera GE, Montezano AC, Yogi A, Tostes RC, Touyz RM. Vascular signaling through cholesterol-rich domains: implications in hypertension. Curr Opin Nephrol Hypertens. 2007;16:90–104. doi: 10.1097/MNH.0b013e328040bfbd. [DOI] [PubMed] [Google Scholar]
- 18.Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- 19.Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 20.Kenworthy AK. Lipid rafts, cholesterol, and the brain. Neuropharmacology. 2008;55:1265–1273. doi: 10.1016/j.neuropharm.2008.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pike LJ. The challenge of lipid rafts. J Lipid Res. 2008;50:S323. doi: 10.1194/jlr.R800040-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simons K, Ehehalt R. Cholesterol, lipid rafts, and disease. J Clin Invest. 2002;110:597–603. doi: 10.1172/JCI16390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carver LA, Schnitzer JE, Anderson RG, et al. Role of caveolae and lipid rafts in cancer: workshop summary and future needs. Cancer Res. 2003;63(20):6571–6574. [PubMed] [Google Scholar]
- 24.Jacobson K, Dietrich C. Looking at lipid rafts? Trends Cell Biol. 1999;9:87–91. doi: 10.1016/s0962-8924(98)01495-0. [DOI] [PubMed] [Google Scholar]
- 25.Simons K, Gerl MJ. Revitalizing membrane rafts: new tools and insights. Nat Rev Mol Cell Biol. 2010;11:688–699. doi: 10.1038/nrm2977. [DOI] [PubMed] [Google Scholar]
- 26.Pike LJ. Rafts defined: a report on the keystone symposium on lipid rafts and cell function. J Lipir Res. 2006;47:1597–1598. doi: 10.1194/jlr.E600002-JLR200. [DOI] [PubMed] [Google Scholar]
- 27.Leslie M. Mysteries of the cell. Do lipid rafts exist? Science. 2011;334:1046–1047. doi: 10.1126/science.334.6059.1046-b. [DOI] [PubMed] [Google Scholar]
- 28.Frisz JF, Klitzing HA, Lou K, et al. Sphingolipid domains in the plasma membranes of fibroblasts are not enriched with cholesterol. J Biol Chem. 2013;288:16855–16861. doi: 10.1074/jbc.M113.473207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Edwards PR, Lowe PA, Leatherbarrow RJ. Ligand loading at the surface of an optical biosensor and its effect upon the kinetics of protein–protein interactions. J Mol Recognit. 1997;10:128–134. doi: 10.1002/(SICI)1099-1352(199705/06)10:3<128::AID-JMR357>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 30.Mao Y, Tero R, Imai Y, et al. The morphology of GM1x/SM0.6–x/Chol0.4 planar bilayers supported on SiO2 surfaces. Chem Phys Lett. 2008;460:289–297. [Google Scholar]
- 31.Margheri G, D’Agostino R, Trigari S, et al. The β-subunit of cholera toxin has a high affinity for ganglioside GM1 embedded into solidsupported lipid membranes with a lipid raft-like composition. Lipids. 2014;49:203–206. doi: 10.1007/s11745-013-3845-8. [DOI] [PubMed] [Google Scholar]
- 32.Margheri G, D’Agostino R, Del Rosso M, et al. Fabrication of GM3-enriched sphingomyelin/cholesterol solid-supported lipid membranes on Au/SiO2 plasmoni substrates. Lipids. 2013;48:739–747. doi: 10.1007/s11745-013-3789-z. [DOI] [PubMed] [Google Scholar]
- 33.Margheri G, D’Agostino R, Becucci L, et al. Surface plasmon resonance as detection tool for lipids lateral mobility in biomimetic membranes. Biomed Opt Express. 2012;3:3119–3126. doi: 10.1364/BOE.3.003119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sotgia F, Martinez-Outschoorn UE, Howell A, et al. Caveolin-1 and cancer metabolism in the tumor microenvironment: markers, models, and mechanisms. Annu Rev Pathol Mech Dis. 2012;7:423–467. doi: 10.1146/annurev-pathol-011811-120856. [DOI] [PubMed] [Google Scholar]
- 35.Fernandez I, Ying Y, Albanesi J, et al. Mechanisms of caveolin filament assembly. Proc Natl Acad Sci USA. 2002;99:11193–11198. doi: 10.1073/pnas.172196599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Palade GE. Fine structure of blood capillaries. J Appl Phys. 1953;24:1424. [Google Scholar]
- 37.Simionescu M, Simionescu N, Palade E. Morphometric data on the endothelium of blood capillaries. J Cell Biol. 1974;60:128–152. doi: 10.1083/jcb.60.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stan RV. Structure and function of endothelial caveolae. Microsc Res Tech. 2002;57:350–364. doi: 10.1002/jemt.10089. [DOI] [PubMed] [Google Scholar]
- 39.Liu L, Pilch PF. A critical role of cavin (polymerase I and transcript release factor) in caveolae formation and organization. J Biol Chem. 2008;293:4314–4322. doi: 10.1074/jbc.M707890200. [DOI] [PubMed] [Google Scholar]
- 40.Aboulaich N, Vainonen JP, Stralfors P, et al. Vectorial proteomics reveal targeting, phosphorylation and specific fragmentation of polymerase I and transcript release factor (PTRF) at the surface of caveolae in human adipocytes. Biochem J. 2004;383:237–248. doi: 10.1042/BJ20040647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chadda R, Mayor S. PTRF triggers a cave in. Cell. 2008;132:23–24. doi: 10.1016/j.cell.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 42.Schulte T, Paschke KA, Laessing U, et al. Reggie-1 and reggie-2, two cell surface proteins expressed by retinal ganglion cells during axon regeneration. Development. 1997;124:577–587. doi: 10.1242/dev.124.2.577. [DOI] [PubMed] [Google Scholar]
- 43.Solis GP, Hoegg M, Munderloh C, et al. Reggie/flotillin proteins are organized into stable tetramers in membrane microdomains. Biochem J. 2007;403:313–322. doi: 10.1042/BJ20061686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Palade GE. Blood capillaries of the heart and other organs. Circulation. 1961;24:368–388. doi: 10.1161/01.cir.24.2.368. [DOI] [PubMed] [Google Scholar]
- 45.Lucero HA, Robbins PW. Lipid rafts–protein association and the regulation of protein activity. Arch Biochem Biophys. 2004;426:208–224. doi: 10.1016/j.abb.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 46.Sebastiao AM, Colino-Oliveira M, Assaife-Lopez A, et al. Lipid rafts, synaptic transmission and plasticity: impact in age-related neurodegenerative diseases. Neuropharmacology. 2013;64:97–107. doi: 10.1016/j.neuropharm.2012.06.053. [DOI] [PubMed] [Google Scholar]
- 47.Gonnord B, Blouin CM, Lamaze C. Membrane trafficking and signalling: two sides of the same coin. Semin Cell Dev Biol. 2012;23:154–164. doi: 10.1016/j.semcdb.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 48.Sowa G. Caveolae, cavins, and endothelial cell function: new insights. Front Physiol. 2012;2:120. doi: 10.3389/fphys.2011.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cao Z, Shang B, Zhang G, et al. Tumor cell-mediated neovascularization and lymphangiogenesis contrive tumor progression and cancer metastasis. Biochim Byophys Acta. 2013;1836:273–286. doi: 10.1016/j.bbcan.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 50.Hendrix MJ, Seftor EA, Hess AR, et al. Vasculogenic mimicry and tumor-cell plasticity: lessons from melanoma. Nat Rev Cancer. 2003;3:411–421. doi: 10.1038/nrc1092. [DOI] [PubMed] [Google Scholar]
- 51.Wang R, Chadalavada K, Wilshire J, et al. Glioblastoma stem-like cells give rise to tumor endothelium. Nature. 2010;468:829–833. doi: 10.1038/nature09624. [DOI] [PubMed] [Google Scholar]
- 52.Leenders WP, Kusters B, de Waal RM. Vessel co-option: how tumors obtain blood supply in the absence of sprouting angiogenesis. Endothelium. 2002;9:83–87. doi: 10.1080/10623320212006. [DOI] [PubMed] [Google Scholar]
- 53.Kucera T, Lammert E. Ancestral vascular tube formation and its adoption by tumors. Biol Chem. 2009;390:985–994. doi: 10.1515/BC.2009.115. [DOI] [PubMed] [Google Scholar]
- 54.Gardner LB, Corn PG. Hypoxic regulation of mRNA expression. Cell Cycle. 2008;7:1916–1924. doi: 10.4161/cc.7.13.6203. [DOI] [PubMed] [Google Scholar]
- 55.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nature Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 56.Ladomery MR, Harper SJ, Bates DO. Alternative splicing in angiogenesis: the vascular endothelial growth factor paradigm. Cancer Lett. 2006;249:133–142. doi: 10.1016/j.canlet.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 57.Tan K, Jack Lawler J. The interaction of Thrombospondins with extracellular matrix proteins. J Cell Commun Signal. 2009;3:177–187. doi: 10.1007/s12079-009-0074-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grant MA, Kalluri R. Structural basis for the function of endogenous angiogenesis inhibitors. Cold Spring Harb Symp Quant Biol. 2005;70:399–410. doi: 10.1101/sqb.2005.70.017. [DOI] [PubMed] [Google Scholar]
- 59.Labrecque L, Royal I, Surprenant DS, et al. Regulation of vascular endothelial growth factor receptor-2 activity by caveolin-1 and plasma membrane cholesterol. Mol Biol Cell. 2003;14:334–347. doi: 10.1091/mbc.E02-07-0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kaverina I, Krylyshkina O, Small JV. Regulation of substrate adhesion dynamics during cell motility. Int J Biochem Cell Biol. 2002;34:746–761. doi: 10.1016/s1357-2725(01)00171-6. [DOI] [PubMed] [Google Scholar]
- 61.Carmeliet P, Tessier-Lavigne M. Common mechanisms of nerve and blood vessel wiring. Nature. 2005;436:193–200. doi: 10.1038/nature03875. [DOI] [PubMed] [Google Scholar]
- 62.Galli F, Piroddi M, Annetti C, et al. Oxidative stress and the reactive oxygen species. Contrib Nephrol. 2005;149:240–260. doi: 10.1159/000085686. [DOI] [PubMed] [Google Scholar]
- 63.Shroder K. Isoform specific functions of Nox protein-derived reactive oxygen species in the vasculature. Curr Opin Pharmacol. 2010;10:122–126. doi: 10.1016/j.coph.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 64.Oshikawa J, Urao N, Won Kim H, et al. Extracellular SOD-derived H2O2 promotes VEGF signalling in caveolae/lipid rafts and post-ischemic angiogenesis in mice. PLoS ONE. 2010;5(4):e10189. doi: 10.1371/journal.pone.0010189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rhee GS, Kang SW, Jeong W, et al. Intracellular messenger function of hydrogen peroxide and its regulation by peroxiredoxins. Curr Opin Cell Biol. 2005;17:183–189. doi: 10.1016/j.ceb.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 66.Abid MR, Spokes KC, Shish SC, et al. NADPH oxidase activity selectively modulates vascular endothelial growth factor signalling pathways. J Biol Chem. 2007;282:35373–35385. doi: 10.1074/jbc.M702175200. [DOI] [PubMed] [Google Scholar]
- 67.Moldovan L, Mythreye K, GoldSchmidt-Clermont PJ, et al. Reactive oxygen species in vascular endothelial cell motility. Roles of NAD(P)H oxidase and Rac1. Cardiovasc Res. 2006;71:236–246. doi: 10.1016/j.cardiores.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 68.Kang DH, LeeDJ Lee KW, et al. Peroxiredoxin II is an essential anti-oxidant enzyme that prevents the oxidative inactivation of VEGF receptor-2 in vascular endothelial cells. Mol Cell. 2011;44:545–558. doi: 10.1016/j.molcel.2011.08.040. [DOI] [PubMed] [Google Scholar]
- 69.Chillà A, Magherini F, Margheri F, et al. Proteomic identification of VEGF-dependent protein enrichment to membrane caveolar-raft microdomains in endothelial progenitor cells. Mol Cell Proteomics. 2013;12:1926–1938. doi: 10.1074/mcp.M112.024638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gorge KS, Wu S. Lipid-raft: a floating island of death and survival. Toxicol Appl Pharmacol. 2012;259:311–319. doi: 10.1016/j.taap.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.De Spiegelaere W, Casteleyn C, Van den Broeck W, et al. Intussusceptive angiogenesis: a biologically relevant form of angiogenesis. J Vasc Res. 2012;49:390–404. doi: 10.1159/000338278. [DOI] [PubMed] [Google Scholar]
- 72.Thurston G, Kitajewski J. VEGF and delta-Notch: interacting signaling pathways in tumor angiogenesis. Br J Cancer. 2008;99:1204–1209. doi: 10.1038/sj.bjc.6604484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Phng LK, Gerhardt H. Angiogenesis: a team effort coordinated by notch. Dev Cell. 2009;16:196–208. doi: 10.1016/j.devcel.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 74.Hellstrom M, Phng LK, Hofmann JJ, et al. Dll4 signaling through Notch1 regulates formation of tip cells during angiogenesis. Nature. 2007;445:776–780. doi: 10.1038/nature05571. [DOI] [PubMed] [Google Scholar]
- 75.Lobov IB, Renard RA, Papadopoulos N, et al. Delta-like ligand 4 (Dll4) is induced by VEGF as a negative regulator of angiogenesis sprouting. Proc Natl Acad Sci USA. 2007;104:3219–3224. doi: 10.1073/pnas.0611206104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Benedito R, Roca C, Sörensen I. The notch ligands Dll4 and Jagged1 have opposing effects on angiogenesis. Cell. 2009;137:1124–1135. doi: 10.1016/j.cell.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 77.Oswald F, Täuber B, Dobner T, et al. p300 acts as a transcriptional coactivator for mammalian Notch-1. Mol Cell Biol. 2001;21:7761–7774. doi: 10.1128/MCB.21.22.7761-7774.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brou C, Logeat F, Gupta N, et al. A novel proteolytic cleavage involved in notch signaling: the role of the disintegrin-metalloprotease TACE. Mol Cell. 2000;5:207–216. doi: 10.1016/s1097-2765(00)80417-7. [DOI] [PubMed] [Google Scholar]
- 79.Vetrivel KS, Cheng H, Lin W, et al. Association of γ-secretase with lipid rafts in post-Golgi and endosome membranes. J Biol Chem. 2004;279:44945–44954. doi: 10.1074/jbc.M407986200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vetrivel KS, Thinakara G. Membrane rafts in Alzheimer’s disease beta-amyloid production. Biochim Biophys Acta. 2010;1801:860–867. doi: 10.1016/j.bbalip.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hu J, Popp R, Fromel T, et al. Muller glia cells regulate Notch signaling and retinal angiogenesis via the generation of 19,20-dihydroxydocosapentaenoic acid. J Exp Med. 2014;211:281–295. doi: 10.1084/jem.20131494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sacilotto N, Monteiro R, Fritzsche M, et al. Analysis of Dll4 regulation reveals a combinatorial role for Sox and Notch in arterial development. Proc Natl Acad Sci USA. 2013;110:11893–11898. doi: 10.1073/pnas.1300805110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Siekmann AF, Lawson ND. Notch signalling and the regulation of angiogenesis. Cell Adh Migr. 2007;1:104–106. doi: 10.4161/cam.1.2.4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Siekmann AF, Covassin L, Lawson ND. Modulation of VEGF signalling output by the Notch pathway. BioEssays. 2008;30:303–313. doi: 10.1002/bies.20736. [DOI] [PubMed] [Google Scholar]
- 85.Hayashi K, Madri JA, Yurchenko PD. Endothelial cells interact with the core protein of basement membrane perlecan through β1 and β3 integrin: an adhesion modulated by glycosaminoglycan. J Cell Biol. 1992;119:945–959. doi: 10.1083/jcb.119.4.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hallman R, Horn N, Segl M, et al. Expression and function of laminins in the embryonic and mature vasculature. Physiol Rev. 2005;85:979–1000. doi: 10.1152/physrev.00014.2004. [DOI] [PubMed] [Google Scholar]
- 87.Chang SH, Kanasaki K, Gocheva V, et al. VEGF-A induces angiogenesis by perturbing the cathepsin-cysteine protease inhibitor balance in venules, causing basement membrane degradation and mother vessel formation. Cancer Res. 2009;69:4537–4544. doi: 10.1158/0008-5472.CAN-08-4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pepper MS. Role of the matrix metalloproteinase and plasminogen activator-plasmin systems in angiogenesis. Arterioscler Thromb Vasc Biol. 2001;21:1104–1117. doi: 10.1161/hq0701.093685. [DOI] [PubMed] [Google Scholar]
- 89.Van Hinsbergh VWM, Engelse MA, Quax PHA. Pericellular proteases in angiogenesis and vasculogenesis. Arterioscler Thromb Vasc Biol. 2006;26:716–728. doi: 10.1161/01.ATV.0000209518.58252.17. [DOI] [PubMed] [Google Scholar]
- 90.Senger DR, Davis GE. Angiogenesis. Cold Spring Harb Perspect Biol. 2011;3:a005090. doi: 10.1101/cshperspect.a005090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Breuss JM, Uhrin P. VEGF-initiated angiogenesis and the uPA/uPAR system. Cell Adhes Migr. 2012;6:535–540. doi: 10.4161/cam.22243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Uhrin P, Breuss JM. uPAR: a modulator of VEGF-induced angiogenesis. Cell Adhes Migr. 2013;7:23–26. doi: 10.4161/cam.22124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Koziol A, Martín-Alonso M, Clemente C, et al. Site-specific cellular functions of MT1-MMP. Eur J Cell Biol. 2012;91:889–895. doi: 10.1016/j.ejcb.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 94.Chun TH, Sabeh F, Ota I, et al. MT1-MMP-dependent neovessel formation within the confines of the three-dimensional extracellular matrix. J Cell Biol. 2004;167:757–767. doi: 10.1083/jcb.200405001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gálvez BG, Matías-Román S, Yáñez-Mó M, et al. Caveolae are a novel pathway for membrane-type 1 matrix metalloproteinase traffic in human endothelial cells. Mol Biol Cell. 2004;15:678–687. doi: 10.1091/mbc.E03-07-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gálvez BG, Matías-Román S, Yáñez-Mó M, et al. ECM regulates MT1-MMP localization with beta1 or alphavbeta3 integrins at distinct cell compartments modulating its internalization and activity on human endothelial cells. J Cell Biol. 2002;159:509–521. doi: 10.1083/jcb.200205026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Frittoli E, Palamidessi A, Disanza A, et al. Secretory and endo/exocytic trafficking in invadopodia formation: the MT1-MMP paradigm. Eur J Cell Biol. 2011;90:108–114. doi: 10.1016/j.ejcb.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 98.Sundberg C, Nagy JA, Brown LF, et al. Glomeruloid microvascular proliferation follows adenoviral vascular permeability factor/vascular endothelial growth factor-164 gene delivery. Am J Pathol. 2001;158:1145–1160. doi: 10.1016/S0002-9440(10)64062-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Saunders WB, Bohnsack BL, Faske JB, et al. Coregulation of vascular tube stabilization by endothelial cell TIMP-2 and pericyte TIMP-3. J Cell Biol. 2006;175:179–191. doi: 10.1083/jcb.200603176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Stratman AN, Saunders WB, Sacharidou A, et al. Endothelial cell lumen and vascular guidance tunnel formation requires MT1-MMP-dependent proteolysis in 3-dimensional collagen matrices. Blood. 2009;114:237–247. doi: 10.1182/blood-2008-12-196451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bayless KJ, Davis GE. Microtubule depolymerization rapidly collapses capillary tube networks in vitro and angiogenic vessels in vivo through the small GTPase Rho. J Biol Chem. 2004;279:11686–11695. doi: 10.1074/jbc.M308373200. [DOI] [PubMed] [Google Scholar]
- 102.Davis GE, Camarillo CW. An alpha 2 beta 1 integrin-dependent pinocytic mechanism involving intracellular vacuole formation and coalescence regulates capillary lumen and tube formation in three-dimensional collagen matrix. Exp Cell Res. 1996;224:39–51. doi: 10.1006/excr.1996.0109. [DOI] [PubMed] [Google Scholar]
- 103.Bayless KJ, Davis GE. The Cdc42 and Rac1 GTPases are required for capillary lumen formation in three-dimensional extracellular matrices. J Cell Sci. 2002;115:1123–1136. doi: 10.1242/jcs.115.6.1123. [DOI] [PubMed] [Google Scholar]
- 104.Davis GE, Bayless KJ. An integrin and Rho GTPase-dependent pinocytic vacuole mechanism controls capillary lumen formation in collagen and fibrin matrices. Microcirculation. 2003;10:27–44. doi: 10.1038/sj.mn.7800175. [DOI] [PubMed] [Google Scholar]
- 105.Kamei M, Saunders WB, Bayless KJ, et al. Endothelial tubes assemble from intracellular vacuoles in vivo. Nature. 2006;442:453–456. doi: 10.1038/nature04923. [DOI] [PubMed] [Google Scholar]
- 106.Lubarsky B, Krasnow MA. Tube morphogenesis: making and shaping biological tubes. Cell. 2003;112:19–28. doi: 10.1016/s0092-8674(02)01283-7. [DOI] [PubMed] [Google Scholar]
- 107.Lijnen HR. Matrix metalloproteinases and cellular fibrinolytic activity. Biochemistry. 2002;67:107–115. doi: 10.1023/a:1013908332232. [DOI] [PubMed] [Google Scholar]
- 108.Høyer-Hansen G, Rønne E, Solberg H, et al. Urokinase plasminogen activator cleaves its cell surface receptor releasing the ligand-binding domain. J Biol Chem. 1992;267:18224–18229. [PubMed] [Google Scholar]
- 109.Ploug M, Rønne E, Behrendt N, et al. Cellular receptor for urokinase plasminogen activator. Carboxyl-terminal processing and membrane anchoring by glycosyl-phosphatidylinositol. J Biol Chem. 1991;266:1926–1933. [PubMed] [Google Scholar]
- 110.Sitrin RG, Johnson DR, Pan PM, et al. Lipid raft compartmentalization of urokinase receptor signaling in human neutrophils. Am J Respir Cell Mol Biol. 2004;30:233–241. doi: 10.1165/rcmb.2003-0079OC. [DOI] [PubMed] [Google Scholar]
- 111.Cunningham O, Andolfo A, Santovito ML, et al. Dimerization controls the lipid raft partitioning of uPAR/CD87 and regulates its biological functions. EMBO J. 2003;22:5994–6003. doi: 10.1093/emboj/cdg588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sahores M, Prinetti A, Chiabrando G, et al. uPA binding increases UPAR localization to lipid rafts and modifies the receptor microdomain composition. Biochim Biophys Acta. 2008;1778:250–259. doi: 10.1016/j.bbamem.2007.09.030. [DOI] [PubMed] [Google Scholar]
- 113.Rao JS, Gujrati M, Chetty C. Tumor-associated soluble uPAR-directed endothelial cell motility and tumor angiogenesis. Oncogenesis. 2013;2:e53. doi: 10.1038/oncsis.2013.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Raghu H, Sodadasu PK, Malla RR, et al. Localization of uPAR and MMP-9 in lipid rafts is critical for migration, invasion and angiogenesis in human breast cancer cells. BMC Cancer. 2010;10:647. doi: 10.1186/1471-2407-10-647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Margheri F, Chillà A, Laurenzana A, et al. Endothelial progenitor cell-dependent angiogenesis requires localization of the full-length form of uPAR in caveolae. Blood. 2011;118:3743–3755. doi: 10.1182/blood-2011-02-338681. [DOI] [PubMed] [Google Scholar]
- 116.Margheri F, Papucci L, Schiavone N, et al. Differential uPAR recruitment in caveolar-lipid rafts by GM1 and GM3 gangliosides regulates Endothelial progenitor cells angiogenesis. J Cell Mol Med. 2015;19:113–123. doi: 10.1111/jcmm.12410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Del Rosso M, Fibbi G, Pucci M, et al. Multiple pathways of cell invasion are regulated by multiple families of serine proteases. Clin Exp Metastasis. 2002;19:193–207. doi: 10.1023/a:1015531321445. [DOI] [PubMed] [Google Scholar]
- 118.Blasi F, Carmeliet P. uPAR: a versatile signalling orchestrator. Nat Rev Mol Cell Biol. 2002;3:932–943. doi: 10.1038/nrm977. [DOI] [PubMed] [Google Scholar]
- 119.Kugler MC, Wei Y, Chapman HA. Urokinase receptor and integrin interactions. Curr Pharm Des. 2003;9:1565–1574. doi: 10.2174/1381612033454658. [DOI] [PubMed] [Google Scholar]
- 120.D’Alessio S, Blasi F. The urokinase receptor as an entertainer of signal transduction. Front Biosci (Landmark Ed) 2009;14:4575–4587. doi: 10.2741/3550. [DOI] [PubMed] [Google Scholar]
- 121.Blasi F, Sidenius N. The urokinase receptor: focused cell surface proteolysis, cell adhesion and signaling. FEBS Lett. 2010;584:1923–1930. doi: 10.1016/j.febslet.2009.12.039. [DOI] [PubMed] [Google Scholar]
- 122.Sidenius N, Blasi F. Domain 1 of the urokinase receptor (uPAR) is required for uPAR-mediated cell binding to vitronectin. FEBS Lett. 2000;470:40–46. doi: 10.1016/s0014-5793(00)01282-5. [DOI] [PubMed] [Google Scholar]
- 123.Eden G, Archinti M, Furlan F, et al. The urokinase receptor interactome. Curr Pharm Des. 2011;17:1874–1889. doi: 10.2174/138161211796718215. [DOI] [PubMed] [Google Scholar]
- 124.Stoppelli MP, Tacchetti C, Cubellis MV, et al. Autocrine saturation of pro-urokinase receptors on human A431 cells. Cell. 1986;45:675–684. doi: 10.1016/0092-8674(86)90782-8. [DOI] [PubMed] [Google Scholar]
- 125.Stephens RW, Pöllänen J, Tapiovaara H, et al. Activation of pro-urokinase and plasminogen on human sarcoma cells: a proteolytic system with surface-bound reactants. J Cell Biol. 1989;108(5):1987–1995. doi: 10.1083/jcb.108.5.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Prager GW, Breuss JM, Steurer S, et al. Vascular endothelial growth factor (VEGF) induces rapid prourokinase (pro-uPA) activation on the surface of endothelial cells. Blood. 2004;103:955–962. doi: 10.1182/blood-2003-07-2214. [DOI] [PubMed] [Google Scholar]
- 127.Bajou K, Noël A, Gerard RD, et al. Absence of host plasminogen activator inhibitor 1 prevents cancer invasion and vascularization. Nat Med. 1998;4:923–928. doi: 10.1038/nm0898-923. [DOI] [PubMed] [Google Scholar]
- 128.Bajou K, Masson V, Gerard RD, et al. The plasminogen activator inhibitor PAI-1 controls in vivo tumor vascularization by interaction with proteases, not vitronectin. Implications for antiangiogenic strategies. J Cell Biol. 2001;152:777–784. doi: 10.1083/jcb.152.4.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Brunner PM, Heier PC, Mihaly-Bison J, et al. Density enhanced phosphatase-1 down-regulates urokinase receptor surface expression in confluent endothelial cells. Blood. 2011;117:4154–4161. doi: 10.1182/blood-2010-09-307694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Del Rosso M. uPAR in angiogenesis regulation. Blood. 2011;117:3941–3943. doi: 10.1182/blood-2011-02-337733. [DOI] [PubMed] [Google Scholar]
- 131.Poettler M, Unseld M, Mihaly-Bison J, et al. The urokinase receptor (CD87) represents a central mediator of growth factor-induced endothelial cell migration. Thromb Haemost. 2012;108:357–366. doi: 10.1160/TH11-12-0868. [DOI] [PubMed] [Google Scholar]
- 132.Alexander RA, Prager GW, Mihaly-Bison J, et al. VEGF-induced endothelial cell migration requires urokinase receptor (uPAR)-dependent integrin redistribution. Cardiovasc Res. 2012;94:125–135. doi: 10.1093/cvr/cvs017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Schmaier AH, Larusch G. Factor XII: new life for an old protein. Thromb Haemost. 2010;104:915–918. doi: 10.1160/TH10-03-0171. [DOI] [PubMed] [Google Scholar]
- 134.Chauvet S, Burk K, Mann F. Navigation rules for vessels and neurons: cooperative signaling between VEGF and neural guidance cues. Cell Mol Life Sci. 2013;70:1685–1703. doi: 10.1007/s00018-013-1278-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Adams RH, Eichmann A. Axon guidance molecules in vascular patterning. Cold Spring Harb Perspect Biol. 2010;2:a001875. doi: 10.1101/cshperspect.a001875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Larrivee B, Freitas C, Suchting S, et al. Guidance of vascular development: lessons from the nervous system. Circ Res. 2009;104:428–441. doi: 10.1161/CIRCRESAHA.108.188144. [DOI] [PubMed] [Google Scholar]
- 137.Wang B, XiaoY Ding B-B, et al. Induction of tumor angiogenesis by Slit-Robo signaling and inhibition of cancer growth by blocking Robo activity. Cancer Cell. 2003;4:19–29. doi: 10.1016/s1535-6108(03)00164-8. [DOI] [PubMed] [Google Scholar]
- 138.Yadav SS, Narayan G (2014) Role of ROBO4 signalling in developmental and pathological angiogenesis. Biomed Res Int (683025) [DOI] [PMC free article] [PubMed]
- 139.Brose K, Bland KS, Wang KH, et al. Slit proteins bind robo receptors and have an evolutionary conserved role in repulsive axon guidance. Cell. 1999;96:795–806. doi: 10.1016/s0092-8674(00)80590-5. [DOI] [PubMed] [Google Scholar]
- 140.Jones CA, London NR, Chen H, et al. Robo4 stabilizes the vascular network by inhibiting pathologic angiogenesis and endothelial hyperpermeability. Nat Med. 2008;14:448–453. doi: 10.1038/nm1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sheldon H, Andre M, Legg JA, et al. Active involvement of Robo1 and Robo4 in filopodia formation and endothelial cell motility mediated via WASP and other actin nucleation-promoting factors. FASEB J. 2009;23:513–522. doi: 10.1096/fj.07-098269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zhang B, Dietrich UM, Geng JG, et al. Repulsive axon guidance molecule Slit3 is a novel angiogenic factor. Blood. 2009;114:430–439. doi: 10.1182/blood-2008-12-193326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kaur S, Samant GV, Pramanik K, et al. Silencing of directional migration in roundabout4 knockdown endothelial cells. BMC Cell Biol. 2008;9:61. doi: 10.1186/1471-2121-9-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Maisse C, Rossin A, Cahuzac N, et al. Lipid raft localization and palmitoylation: identification of two requirements for cell death induction by the tumor suppressors UNC5H. Exp Cell Res. 2008;314:2544–2552. doi: 10.1016/j.yexcr.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 145.Koch AW, Mathivet T, Larrivée B, et al. Robo4 maintains vessel integrity and inhibits angiogenesis by interacting with UNC5B. Dev Cell. 2011;20:33–46. doi: 10.1016/j.devcel.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 146.Jin J, Sison K, Li C, et al. Soluble FLT1 binds lipid microdomains in podocytes to control cell morphology and glomerular barrier function. Cell. 2012;151:384–399. doi: 10.1016/j.cell.2012.08.037. [DOI] [PubMed] [Google Scholar]
- 147.Lu X, Le Noble F, Yuan L, et al. The netrin receptor UNC5B mediates guidance events controlling morphogenesis of the vascular system. Nature. 2004;432:179–186. doi: 10.1038/nature03080. [DOI] [PubMed] [Google Scholar]
- 148.Larrivée B, Freitas C, Trombe M, et al. Activation of the UNC5B receptor by Netrin-1 inhibits sprouting angiogenesis. Genes Dev. 2007;21:2433–2447. doi: 10.1101/gad.437807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lejmi E, Leconte L, Pédron-Mazoyer S, et al. Netrin-4 inhibits angiogenesis via binding to neogenin and recruitment of Unc5B. Proc Natl Acad Sci USA. 2008;105:2491–2496. doi: 10.1073/pnas.0804008105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Tassew NG, Mothe AJ, Shabanzadeh AP, et al. Modifying lipid rafts promotes regeneration and functional recovery. Cell Rep. 2014;8:1146–1159. doi: 10.1016/j.celrep.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 151.Karaulanov E, Böttcher RT, Stannek P, et al. Unc5B interacts with FLRT3 and Rnd1 to modulate cell adhesion in Xenopus embryos. PLoS ONE. 2009;4:e5742. doi: 10.1371/journal.pone.0005742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Pasterkamp RJ, Kolodkin AL. Semaphorin junction: making tracks toward neural connectivity. Curr Opin Neurobiol. 2003;13:79–89. doi: 10.1016/s0959-4388(03)00003-5. [DOI] [PubMed] [Google Scholar]
- 153.Gu C, Giraudo E. The role of semaphorins and their receptors in vascular development and cancer. Exp Cell Res. 2013;319:1306–1316. doi: 10.1016/j.yexcr.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Gu C, Yoshida Y, Livet JETAL. Smaphorin 3E and plexin-D1 control vascular pattern independently of neuropilins. Science. 2006;307:265–268. doi: 10.1126/science.1105416. [DOI] [PubMed] [Google Scholar]
- 155.Appleton BA, Wu P, Maloney J, et al. Structural studies of neuropilin/antibody complexes provide insights into semaphorin and VEGF binding. EMBO J. 2007;26:4902–4912. doi: 10.1038/sj.emboj.7601906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Guirland C, Suzuki S, Kojima METAL. Lipid rafts mediate chemotropic guidance of nerve growth cones. Neuron. 2004;42:51–62. doi: 10.1016/s0896-6273(04)00157-6. [DOI] [PubMed] [Google Scholar]
- 157.Campbell DS, Holt CE. Apoptotic pathway and MAPKs differentially regulate chemotropic responses of retinal growth cones. Neuron. 2003;37:939–952. doi: 10.1016/s0896-6273(03)00158-2. [DOI] [PubMed] [Google Scholar]
- 158.Salikhova A, Wang L, Lanahan AA, et al. Vascular endothelial growth factor and semaphorin induce neuropilin-1 endocytosis via separate pathways. Circ Res. 2008;103:e71–e79. doi: 10.1161/CIRCRESAHA.108.183327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Carcea I, Ma’ayan A, Mesias R, et al. Flotillin-mediated endocytic events dictate cell type-specific responses to semaphorin 3A. J Neurosci. 2010;30:15317–15329. doi: 10.1523/JNEUROSCI.1821-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Chen H, Bagri A, Zupicich JA, et al. Neuropilin-2 regulates the development of selective cranial and sensory nerves and hippocampal mossy fiber projections. Neuron. 2000;25:43–56. doi: 10.1016/s0896-6273(00)80870-3. [DOI] [PubMed] [Google Scholar]
- 161.Pasquale EB. Eph receptor signalling casts a wide net on cell behaviour. Nat Rev Mol Cell Biol. 2003;6:462–475. doi: 10.1038/nrm1662. [DOI] [PubMed] [Google Scholar]
- 162.Ogawa K, Pasqualini R, Lindberg RA, et al. The ephrin-A1 ligand and its receptor EphA2, are expressed during tumor neovascularization. Oncogene. 2000;19:6043–6052. doi: 10.1038/sj.onc.1204004. [DOI] [PubMed] [Google Scholar]
- 163.Brantley-Sieders DM, Fang WB, Hwang Y, et al. Ephrin-A1 facilitates mammary tumor metastasis through an angiogenesis-dependent mechanism mediated by EphA receptor and vascular endothelial growth factor in mice. Cancer Res. 2006;66:10315–10324. doi: 10.1158/0008-5472.CAN-06-1560. [DOI] [PubMed] [Google Scholar]
- 164.Foo SS, Turner CJ, Adams S, et al. Ephrin-B2 controls cell motility and adhesion during blood-vessel-wall assembly. Cell. 2006;124:161–173. doi: 10.1016/j.cell.2005.10.034. [DOI] [PubMed] [Google Scholar]
- 165.Wang HU, Chen ZF, Anderson DJ. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell. 1998;93:741–753. doi: 10.1016/s0092-8674(00)81436-1. [DOI] [PubMed] [Google Scholar]
- 166.Gale NW, Baluk P, Pan L, et al. Ephrin-B2 selectively marks arterial vessels and neovascularization sites in the adult, with expression in both endothelial and smooth-muscle cells. Dev Biol. 2001;230:151–160. doi: 10.1006/dbio.2000.0112. [DOI] [PubMed] [Google Scholar]
- 167.Noren NK, Foos G, Hauser CA, et al. The EphB4 receptor suppresses breast cancer cell tumorigenicity through an Abl-Crk pathway. Nat Cell Biol. 2006;8:815–825. doi: 10.1038/ncb1438. [DOI] [PubMed] [Google Scholar]
- 168.Erber R, Eichelsbacher U, Powajbo V, et al. EphB4 controls blood vascular morphogenesis during postnatal angiogenesis. EMBO J. 2006;25:628–641. doi: 10.1038/sj.emboj.7600949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Blits-Huizinga CT, Nelersa CM, Malhotra A, et al. Ephrins and their receptors: binding versus biology. IUBMB Life. 2004;56:257–265. doi: 10.1080/15216540412331270076. [DOI] [PubMed] [Google Scholar]
- 170.Wimmer-Kleikamp SH, Janes PW, Squire A, et al. Recruitment of Eph receptors into signaling clusters does not require ephrin contact. J Cell Biol. 2004;164:661–666. doi: 10.1083/jcb.200312001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Gauthier LR, Robbins SM. Ephrin signaling: One raft to rule them all? One raft to sort them? One raft to spread their call and in signaling bind them? Life Sci. 2003;74:207–216. doi: 10.1016/j.lfs.2003.09.029. [DOI] [PubMed] [Google Scholar]
- 172.Vearing CJ, Lackmann M. Eph receptor signalling; dimerisation just isn’t enough. Growth Factors. 2005;23:67–76. doi: 10.1080/08977190500055869. [DOI] [PubMed] [Google Scholar]
- 173.Himanen JP, Saha N, Nikolov DB. Cell-cell signaling via Eph receptors and ephrins. Curr Opin Cell Biol. 2007;19:534–542. doi: 10.1016/j.ceb.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wu C, Butz S, Ying Y, et al. Tyrosine kinase receptors concentrated in caveolae-like domains from neuronal plasma membrane. J Biol Chem. 1997;272:3554–3559. doi: 10.1074/jbc.272.6.3554. [DOI] [PubMed] [Google Scholar]
- 175.Bocharov EV, Mayzel ML, Volynsky PE, et al. Left-handed dimer of EphA2 transmembrane domain: helix packing diversity among receptor tyrosine kinases. Biophys J. 2010;98:881–889. doi: 10.1016/j.bpj.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ahn GO, Brown JM. Role of endothelial progenitors and other bone marrow-derived cells in the development of the tumor vasculature. Angiogenesis. 2009;12:159–164. doi: 10.1007/s10456-009-9135-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Psaltis PJ, Harbuzariu A, Delacroix S, et al. Resident vascular progenitor cells–diverse origins, phenotype, and function. J Cardiovasc Transl Res. 2011;4:161–176. doi: 10.1007/s12265-010-9248-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Marcelo KL, Goldie LC, Hirschi KK. Regulation of endothelial cell differentiation and specification. Circ Res. 2013;112:1272–1287. doi: 10.1161/CIRCRESAHA.113.300506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circ Res. 2005;97:512–523. doi: 10.1161/01.RES.0000182903.16652.d7. [DOI] [PubMed] [Google Scholar]
- 180.Betsholtz C, Lindblom P, Gerhardt H. Role of pericytes in vascular morphogenesis. EXS. 2005;94:115–125. doi: 10.1007/3-7643-7311-3_8. [DOI] [PubMed] [Google Scholar]
- 181.Stehr M, Adam RM, Khoury J, et al. Platelet derived growth factor-BB is a potent mitogen for rat ureteral and human bladder smooth muscle cells: dependence on lipid rafts for cell signaling. J Urol. 2003;169:1165–1170. doi: 10.1097/01.ju.0000041501.01323.b9. [DOI] [PubMed] [Google Scholar]
- 182.Kiyan J, Haller H, Dumler I. The tyrosine phosphatase SHP-2 controls urokinase-dependent signaling and functions in human vascular smooth muscle cells. Exp Cell Res. 2009;315:1029–1039. doi: 10.1016/j.yexcr.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 183.Kiyan J, Smith G, Haller H, et al. Urokinase-receptor-mediated phenotypic changes in vascular smooth muscle cells require the involvement of membrane rafts. Biochem J. 2009;423:343–351. doi: 10.1042/BJ20090447. [DOI] [PubMed] [Google Scholar]
- 184.Nakayama A, Nakayama M, Turner CJ, et al. Ephrin-B2 controls PDGFRβ internalization and signaling. Genes Dev. 2013;27:2576–2589. doi: 10.1101/gad.224089.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Spiegel S, Milstien S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat Rev Mol Cell Biol. 2003;4:397–407. doi: 10.1038/nrm1103. [DOI] [PubMed] [Google Scholar]
- 186.Kono M, Mi Y, Liu Y, et al. The sphingosine-1-phosphate receptors S1P1, S1P2, and S1P3 function coordinately during embryonic angiogenesis. J Biol Chem. 2004;279:29367–29373. doi: 10.1074/jbc.M403937200. [DOI] [PubMed] [Google Scholar]
- 187.Oskouian B, Saba J. Sphingosine-1-phosphate metabolism and intestinal tumorigenesis: lipid signaling strikes again. Cell Cycle. 2007;6:522–527. doi: 10.4161/cc.6.5.3903. [DOI] [PubMed] [Google Scholar]
- 188.Paik JH, Skoura A, Chae SS, et al. Sphingosine 1-phosphate receptor regulation of N-cadherin mediates vascular stabilization. Genes Dev. 2004;18:2392–2403. doi: 10.1101/gad.1227804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Kokudo T, Suzuki Y, Yoshimatsu Y, et al. Snail is required for TGFbeta-induced endothelial-mesenchymal transition of embryonic stem cell-derived endothelial cells. J Cell Sci. 2008;121:3317–3324. doi: 10.1242/jcs.028282. [DOI] [PubMed] [Google Scholar]
- 190.Goumans MJ, Lebrin F, Valdimarsdottir G. Controlling the angiogenic switch: a balance between two distinct TGF-b receptor signaling pathways. Trends Cardiovasc Med. 2003;13:301–307. doi: 10.1016/s1050-1738(03)00142-7. [DOI] [PubMed] [Google Scholar]
- 191.Itoh F, Itoh S, Adachi T, et al. Smad2/Smad3 in endothelium is indispensable for vascular stability via S1PR1 and N-cadherin expressions. Blood. 2012;119:5320–5328. doi: 10.1182/blood-2011-12-395772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Chen YG. Endocytic regulation of TGF-beta signaling. Cell Res. 2009;19:58–70. doi: 10.1038/cr.2008.315. [DOI] [PubMed] [Google Scholar]
- 193.Thurston G. Role of Angiopoietins and Tie receptor tyrosine kinases in angiogenesis and lymphangiogenesis. Cell Tissue Res. 2003;314:61–68. doi: 10.1007/s00441-003-0749-6. [DOI] [PubMed] [Google Scholar]
- 194.Eklund L, Olsen BR. Tie receptors and their angiopoietin ligands are context-dependent regulators of vascular remodeling. Exp Cell Res. 2006;312:630–641. doi: 10.1016/j.yexcr.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 195.Katoh SY, Kamimoto T, Yamakawa D, et al. Lipid rafts serve as signaling platforms for Tie2 receptor tyrosine kinase in vascular endothelial cells. Exp Cell Res. 2009;315:2818–2823. doi: 10.1016/j.yexcr.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 196.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 197.Al-Husein B, Abdalla M, Trepte M, et al. Antiangiogenic therapy for cancer: an update. Pharmacotherapy. 2012;32:1095–1111. doi: 10.1002/phar.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Grothey A, Allegra C. Antiangiogenesis therapy in the treatment of metastatic colorectal cancer. Ther Adv Med Oncol. 2012;4:301–319. doi: 10.1177/1758834012454464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Wu JM, Staton CA. Anti-angiogenic drug discovery: lessons from the past and thoughts for the future. Expert Opin Drug Discov. 2012;7:723–743. doi: 10.1517/17460441.2012.695774. [DOI] [PubMed] [Google Scholar]
- 200.Osinsky S, Zavelevich M, Vaupel P. Tumor hypoxia and malignant progression. Exp Oncol. 2009;31:80–86. [PubMed] [Google Scholar]
- 201.Kong DS, Song SY, Kim DH, et al. Prognostic significance of c-Met expression in glioblastomas. Cancer. 2009;115:140–148. doi: 10.1002/cncr.23972. [DOI] [PubMed] [Google Scholar]
- 202.Takeda T, Okuyama H, Nishizaka Y, Tomita S, Inoue M. Hypoxia-inducible factor-1alpha is necessary for invasive phenotype in VEGF-deleted islet cell tumors. Sci Rep. 2012;2:494. doi: 10.1038/srep00494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Deryugina EI, Quigley JP. Pleiotropic roles of matrox metalloproteinases in tumor angiogenesis: contrasting, overlapping and compensatory functions. Biochim Biophys Acta. 2010;1803:103–120. doi: 10.1016/j.bbamcr.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Lu KV, Bergers G. Mechanisms of evasive resistance to anti-VEGF therapy in glioblastoma. CNS Oncol. 2013;2:49–65. doi: 10.2217/cns.12.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Lester RD, Jo M, Montel V, Takimoto S, Gonias SL. uPAR induces epithelial-mesenchymal transition in hypoxic breast cancer cells. J Cell Biol. 2007;30(178):425–436. doi: 10.1083/jcb.200701092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Ellis LM, Hicklin DJ. Pathways mediating resistance to vascular endothelial growth factor-targeted therapy. Clin Cancer Res. 2008;14:6371–6375. doi: 10.1158/1078-0432.CCR-07-5287. [DOI] [PubMed] [Google Scholar]
- 207.Casanovas O, Hicklin DJ, Bergers G, Hanahan D. Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell. 2005;8:299–309. doi: 10.1016/j.ccr.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 208.Yan M, Plowman GD. Delta-like 4/Notch signaling and its therapeutic implications. Clin Cancer Res. 2007;13:7243–7246. doi: 10.1158/1078-0432.CCR-07-1393. [DOI] [PubMed] [Google Scholar]
- 209.Batchelor TT, Sorensen AG, di Tomaso E, et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007;11:83–95. doi: 10.1016/j.ccr.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Fischer C, Jonckx B, Mazzone M, et al. Anti-PlGF inhibits growth of VEGF(R)-inhibitor-resistant tumors without affecting healthy vessels. Cell. 2007;131:463–475. doi: 10.1016/j.cell.2007.08.038. [DOI] [PubMed] [Google Scholar]
- 211.Winkler F, Kozin SV, Tong RT, et al. Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell. 2004;6:553–563. doi: 10.1016/j.ccr.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 212.Shojaei F, Wu X, Malik AK, et al. Tumor refractoriness to anti-VEGF treatment is mediated by CD11b+ Gr1+ myeloid cells. Nat Biotechnol. 2007;25:911–920. doi: 10.1038/nbt1323. [DOI] [PubMed] [Google Scholar]
- 213.Carmeliet P, Jain RK. Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat Rev Drug Discov. 2011;10:417–427. doi: 10.1038/nrd3455. [DOI] [PubMed] [Google Scholar]
- 214.Shang B, Cao Z, Zhou Q. Progress in tumor vascular normalization for anticancer therapy: challenge and perspectives. Front Med. 2012;6:67–78. doi: 10.1007/s11684-012-0176-8. [DOI] [PubMed] [Google Scholar]
- 215.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;2011(473):298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Trojanowska M. Cellular and molecular aspects of vascular dysfunction in systemic sclerosis. Nat Rev Rheumatol. 2010;6:453–460. doi: 10.1038/nrrheum.2010.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Murdaca G, Colombo BM, Cagnati P, Gulli R, Spanò F, Puppo F. Endothelial dysfunction in rheumatic autoimmune diseases. Atherosclerosis. 2012;224:309–317. doi: 10.1016/j.atherosclerosis.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 218.Hannex BH. Therapeutic angiogenesis for critical limb ischemia. Nat Rev Cardiol. 2013;10:387–396. doi: 10.1038/nrcardio.2013.70. [DOI] [PubMed] [Google Scholar]
- 219.Zhang H, van Olden C, Sweeney D, Martin-Rendon E. Blood vessel repair and regeneration in the ischaemic heart. Open Heart. 2014;1:e000016. doi: 10.1136/openhrt-2013-000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Van de Veire S, Stalmans I, Heindryckx F, et al. Further pharmacological and genetic evidence for the efficacy of PlGF inhibition in cancer and eye disease. Cell. 2010;141:178–190. doi: 10.1016/j.cell.2010.02.039. [DOI] [PubMed] [Google Scholar]
- 221.Van Steenkiste C, Geerts A, Vanheule E, et al. Role of placental growth factor in mesenteric neoangiogenesis in a mouse model of portal hypertension. Gastroenterology. 2009;137:2112–2124. doi: 10.1053/j.gastro.2009.08.068. [DOI] [PubMed] [Google Scholar]
- 222.Plotkin SR, Stemmer-Rachamimov AO, Barker FG, 2nd, et al. Hearing improvement after bevacizumab in patients with neurofibromatosis type 2. N Engl J Med. 2009;361:358–367. doi: 10.1056/NEJMoa0902579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Williams TM, Lisanti MP. The caveolin genes: from cell biology to medicine. Ann Med. 2004;36:584–595. doi: 10.1080/07853890410018899. [DOI] [PubMed] [Google Scholar]
- 224.Navarro G, Borroto-Escuela DO, Fuxe K, Franco R. Potential of caveolae in the therapy of cardiovascular and neurological diseases. Front Physiol. 2014;5:370. doi: 10.3389/fphys.2014.00370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Fridolfsson HN, Patel HH. Caveolin and caveolae in age-associated cardiovascular disease. J Geriatr Cardiol. 2013;10:66–74. doi: 10.3969/j.issn.1671-5411.2013.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Galbiati F, Engelman JA, Volonte D, et al. Caveolin-3 null mice show a loss of caveolae, changes in the microdomain distribution of the dystrophin-glycoprotein complex, and t-tubule abnormalities. J Biol Chem. 2001;276(24):21425–21433. doi: 10.1074/jbc.M100828200. [DOI] [PubMed] [Google Scholar]
- 227.Roura S, Cal R, Gálvez-Montón C, et al. Inverse relationship between raft LRP1 localization and non-raft ERK1,2/MMP9 activation in idiopathic dilated cardiomyopathy: potential impact in ventricular remodeling. Int J Cardiol. 2014;176:805–814. doi: 10.1016/j.ijcard.2014.07.270. [DOI] [PubMed] [Google Scholar]
- 228.Gumbleton M, Hollins AJ, Omidi Y, Campbell L, Taylor G. Targeting caveolae for vesicular drug transport. J Control Release. 2003;87:139–151. doi: 10.1016/s0168-3659(02)00358-9. [DOI] [PubMed] [Google Scholar]
- 229.Chapkin RS, Wang N, Fan Y-Y, et al. Docosahexaenoic acid alters the size and distribution of cell surface microdomains. Biochem Biophys Acta. 2008;1778:466–471. doi: 10.1016/j.bbamem.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Szymczak M, Murray M, Petrovic N. Modulation of angiogenesis by omega-3 polyunsaturated fatty acids is mediated by cyclooxygenases. Blood. 2008;111:3514–3521. doi: 10.1182/blood-2007-08-109934. [DOI] [PubMed] [Google Scholar]
- 231.Wang J, Shi Y, Zhang L, et al. Omega-3 polyunsaturated fatty acids enhance cerebral angiogenesis and provide long-term protection after stroke. Neurobiol Dis. 2014;68:91–103. doi: 10.1016/j.nbd.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Connor KM, SanGiovanni JP, Lofqvist C, et al. Increased dietary intake of omega-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nat Med. 2007;13:868–873. doi: 10.1038/nm1591. [DOI] [PMC free article] [PubMed] [Google Scholar]