Abstract
Over the past decade, we have begun to appreciate that the lymphatic vascular system does more than simply return plasma back into the circulatory system and, in fact, contributes to a wide variety of normal and disease states. For this reason, much research has been devoted to understanding how lymphatic vessels form and function, with a particular interest in which molecules contribute to lymphatic vessel growth and maintenance. In the following review, we focus on a potent lymphangiogenic factor, adrenomedullin, and its known roles in lymphangiogenesis, lymphatic function, and human lymphatic disease. As one of the first, pharmacologically tractable G protein-coupled receptor pathways characterized in lymphatic endothelial cells, the continued study of adrenomedullin effects on the lymphatic system may open new avenues for the modulation of lymphatic growth and function in a variety of lymphatic-related diseases that currently have few treatments.
Keywords: Lymphedema, Calcitonin receptor-like receptor (CLR = protein; Calcrl = gene), Receptor activity modifying protein (RAMP), CXCR7
Introduction
The lymphatic system is a vascular network in parallel with blood vessels that penetrates every tissue in the body, with the exception of bone marrow and the central nervous system [1]. Over the past dozen years, our understanding of how the lymphatic system develops, functions, and contributes to disease has markedly improved [2]. It has become apparent that this complex system is more than just a simple conduit for returning interstitial fluid to blood. The lymphatic system not only participates in maintaining fluid homeostasis, but also mediates fat absorption and provides a highway for immune cell trafficking to distant sites. In fact, the lymphatic system has now been recognized for its contribution to a wide variety of normal and pathophysiological states [3, 4].
Lymphedema, the most common and poorly treated lymphatic disease, affects 140–250 million people worldwide. Characterized by debilitating swelling of one or more limbs, lymphedema is a lifelong condition that can lead to inflammation, fibrosis, infection, subcutaneous fat accumulation, and decreased mobility and function [1, 5]. Despite its prevalence and morbidity, no pharmacological agents exist for the management of lymphedema.
A multitude of studies in genetic mouse models have also implicated lymphatics in other common diseases. For example, disruption of neolymphangiogenesis in the skin induces salt-sensitive hypertension in mice [6]. This model demonstrates that dermal lymphangiogenesis is required to maintain electrolyte balance and subsequently blood pressure homeostasis [6]. Further, haploinsufficiency of the essential transcription factor Prox1 leads to adult onset obesity due to abnormal fluid leakage from disrupted lymphatic vessels [7]. Examples like these suggest that novel therapeutics designed to modulate the lymphatic vasculature could offer treatment for prevalent diseases like lymphedema, essential hypertension, and obesity.
To this end, a great deal of effort has recently been devoted to identifying factors that participate in lymphangiogenesis and control lymphatic vascular function in adults. The following review will focus on one such factor, adrenomedullin (AM = peptide; Adm = gene), and will address what is currently known about its role in the lymphatic vascular system, and its potential as a novel therapeutic for the treatment of diseases linked to lymphatic dysfunction.
The adrenomedullin peptide family and its receptors
AM, a 52-amino acid peptide hormone, is classified as a member of the calcitonin gene-related peptide (CGRP) family due to its shared secondary structure and overlapping biological activity with other peptide family members [8, 9]. The four peptides of this family besides AM include amylin, calcitonin, intermedin, and CGRP. Each of these peptides has essential roles in normal physiology [10]. Similarities in their secondary structure allow for overlap in the pharmacology of the binding sites of the CGRP family and, consequently, cross-reactivity between their receptors [8, 11]. Therefore, discerning which biological activities are distinct to AM proved to be challenging until the discovery of a class of single-pass transmembrane proteins called receptor activity modifying proteins (RAMPs) [12]. These proteins bind to and confer ligand specificity for G protein-coupled receptors (GPCRs) and allow cells to distinguish between members of the CGRP family.
When bound to a GPCR, RAMPs dictate ligand specificity, downstream signaling, and receptor recycling [9, 12, 13]. The three identified RAMPs, RAMP1, -2, and -3 share 30 % sequence identity, with their nonhomologous domains modulating specificity [8]. For example, when calcitonin receptor-like receptor (CLR) associates with RAMP2 or RAMP3, the receptor binds AM with high affinity. When complexed with RAMP1, on the other hand, CLR performs as a high affinity CGRP receptor. Thus, specific tissue and temporal expression of the RAMPs determine whether a cell will respond to AM or CGRP [13]. As will be discussed below, recognition of the role of RAMPs in the responsiveness of AM allowed for the design of gene-targeted knockout mouse models that helped determine the physiological role of AM. The participation of RAMP2 with CLR was found to be absolutely necessary for AM response during development, as genetic deletion of Ramp2 phenocopied Adm knockout models [14]. This unusual signaling offers a unique opportunity for specific targeting without interrupting the activity of other, closely related ligand–receptor pathways.
Prior to the discovery of RAMPs, there were two additional putative AM receptors reported in the literature [15]. In 1995, Kapas and Clark suggested that two orphan GPCRs, RDC-1 and L1, served as AM receptors [16, 17]. An inability to reproduce these findings led to significant controversy regarding their ability to bind AM. While L1, now known as GPR182, remains an orphan receptor, RDC-1 has been identified as an atypical chemokine receptor, a promiscuous receptor that binds several ligands including SDF-1/CXCL12 [18], CXCL11 [19], and intermediate opioid peptide [20]. RDC-1 has since been renamed CXCR7 or atypical chemokine receptor 3 (ACKR3). The role of CXCR7 in AM signaling continues to evolve. Mounting evidence in the literature over the past decade suggests that RDC-1/CXCR7/ACKR3 does indeed associate with adrenomedullin [15, 21–24]. Although the discovery of RAMP-guided CLR responsiveness to AM overshadowed the role of these other receptors in AM biology, as will be discussed later in this review, recent studies demonstrate that AM-mediated downstream signal is indeed modulated through CXCR7 [25, 26]. The identification of a second AM receptor is especially exciting, as it offers another avenue for drug discovery for the treatment of AM-mediated pathologies.
AM has been shown to be involved in a wide variety of human diseases, including sepsis, myocardial infarction, and preeclampsia [13, 27, 28]. In particular, as a potent vasodilator, the AM signaling system has garnered interest as a potential biomarker and therapeutic target for cardiovascular diseases [29, 30]. Preliminary studies suggest that, when used as an adjunct therapy, intravenous AM can improve cardiovascular outcomes such as wall motion and infarct size [31]. Modulation of the AM signaling system, therefore, could prove to be a viable and safe treatment option not only for cardiovascular diseases, but also for other diseases in which AM plays a role. In particular, genetic models have uncovered an additional role for AM in the development and regulation of the lymphatic vascular system.
Adrenomedullin during lymphatic vascular development
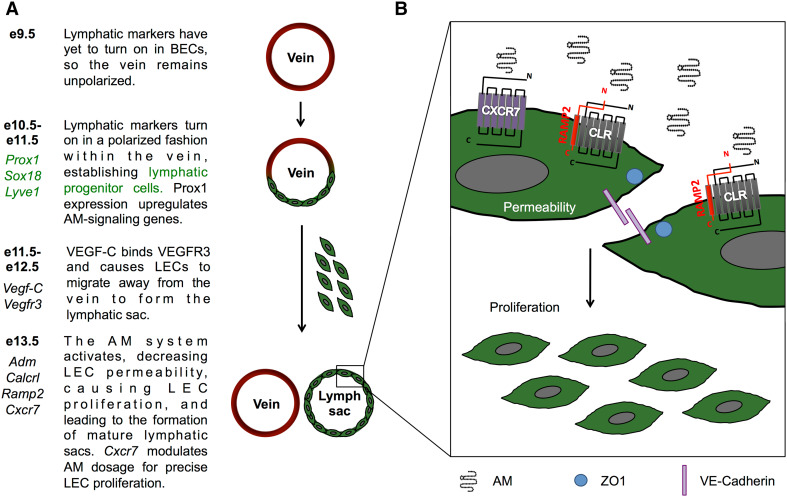
Initial lymphatic vessel formation begins between e9.5 and 10.5 with the commitment of endothelial cells of the cardinal vein (CV) to a lymphatic fate (Fig. 1a) [1]. After polarization of lymphatic progenitor cells in the jugular vein at e11.5–12.5, lymphatic endothelial cells (LECs) begin to migrate away and form a premature lymphatic vessel. Historically, this vessel has been referred to as the lymph sac. Recently, however, ultramicroscopy of whole mount mouse embryos has revealed that the lymph sac is comprised of two separate lymphatic structures, a peripheral longitudinal lymphatic vessel and a primordial thoracic duct [32]. These structures are formed by migrating LECs that originate from both the CV and additional venous sources [32]. Failure of these ECs to establish LEC fate, migrate, or proliferate results in severe edema and embryonic lethality. For example, genetic deletion of Prox1 prevents EC differentiation to lymphatic fate. Prox1-null animals are devoid of lymphatic vasculature and die by e14.5 [33–35]. Similarly, though ECs of VEGF-C-null mice can establish LEC fate, LECs fail to migrate away from the CV, lymphangiogenesis is arrested and embryonic edema ensues, demonstrating that VEGF-C is an essential lymphangiogenic factor [36–38]. The severity and timing of the edema and subsequent embryonic lethality in these mouse models provided an essential clue for determining the role of AM in lymphangiogenesis.
Fig. 1.
Early development of the lymphatic system and the effects of AM on LECs. a Development of the lymphatic system begins between e10.5 and 11.5 when the master regulators of lymphatic fate, Prox1 and Sox18, turn on. By e13.5, the lymphatic sac is fully separated from the jugular vein and activation of the AM system allows LEC proliferation. b Additionally, AM acts to stabilize the lymphatic endothelial barrier by reorganizing the tight junction protein, ZO-1, and the adherens junction protein, VE-cadherin, thereby decreasing lymphatic vessel permeability. AM adrenomedullin, BEC blood endothelial cell, LEC lymphatic endothelial cell
In the early 2000s, an elegant series of gene-targeted knockout mouse models identified AM as an essential factor for proper lymphatic vascular development [13, 14, 39]. Several labs observed that mice globally lacking Adm exhibited profound edema, known as hydrops fetalis [40–42]. Similarly, knockouts of Calcrl, the gene for CLR, [39], or Ramp2 [14, 43, 44], which make up the canonical receptor for AM, have the same lethal phenotype. Furthermore, peptidylglycine alpha-amidating monooxygenase (PAM) knockout mice [45], an enzyme required for the amidation and function of AM, are also embryonic lethal due to extreme edema [27]. These models clearly demonstrate that AM is essential for life; however, the root cause of this edema was not understood.
Consistent with what was known about AM at the time and with the nascent lymphatic vascular field, early studies of these mouse models focused on AM in cardiac and blood vascular development rather than lymphangiogenesis. Indeed, AM was found to have a significant proliferative effect during cardiac development. Adm −/−, Calcrl −/−, and Ramp2 −/− mice share a phenotype of small, hypoplastic hearts [14, 39, 40]. All three genes were also found to be highly expressed in the heart and the vascular endothelium, confounding whether the observed edema could be attributed to cardiac defects or vascular dysfunction. To solve this problem, Fritz-Six et al. utilized mice expressing an endothelial cell-specific Cre recombinase via expression of a Tie2-Cre transgene [46]. Though Tie2-Cre expression has only been observed in restricted regions of adult lymphatic vessels, Tie2-Cre is expressed in the venous progenitors of lymphatic endothelial cells. Thus, prior to the development of lymphatic-specific Cre drivers, Tie2-Cre mice were widely used to excise genes from venous lymphatic progenitors during embryonic lymphangiogenesis [47–51].
Using this Tie2-Cre model, Fritz-Six et al. [14] found that specific deletion of Calcrl in the venous lymphatic progenitors results in extreme hydrops fetalis, suggesting that AM-mediated effects in endothelium, not the heart, were responsible for the edema. Because Tie2-Cre is expressed in progenitors that lead to both blood and lymphatic endothelial lineages [27], this model could not definitively distinguish whether blood or lymphatic vessel dysfunction led to the hydrops fetalis. However, as mentioned above, the onset of edema in the AM signaling-disrupted mice between e12.5 and e14.5 coincides with the separation of the lymphatic sac from the jugular vein and the beginning of lymphatic flow, suggesting that the edema could be lymphatic in origin.
Fritz-Six et al. [14] surveyed the AM system effects on developmental lymphangiogenesis by deleting Adm, Calcrl, and Ramp2. Each gene-targeted knockout model exhibited the same edematous phenotype, while no problems with blood vascular leakage were observed [14]. Quantitative PCR of both lymphatic and blood endothelial cells further revealed that CALCRL and RAMP2 were enriched in the lymphatic endothelium compared to the blood endothelium, a finding that has since been confirmed by other groups [14, 52]. Additionally, transfection of cultured LECs with a PROX1 expression plasmid led to a threefold increase in endogenous CALCRL expression, demonstrating that the genes required for AM signaling are inducible by this master lymphatic fate regulator [14]. Later studies have also shown that AM treatment of LECs causes an upregulation of PROX1 [53], demonstrating that not only are the genes required for AM signaling upregulated in LECs leading to preferential AM action, but that AM activity in LECs feeds back positively on PROX1 expression. Consistent with this notion, Fritz-Six et al. [14] found that prior to embryonic lethality, the lymphatic sacs of AM signaling-null mice were significantly smaller when compared to those of wild-type littermates. Careful study of proliferation in LECs revealed that loss of AM signaling results in hypoplasia of the lymphatic sacs. These hypoplastic lymphatic sacs failed to collect extravasated fluid and resulted in aberrant accumulation of interstitial fluid in mutant embryos. This finding was consistently observed in Adm −/− , Calcrl −/−, and Ramp2 −/− embryos.
Furthermore, the lymphatic vessels of AM signaling-null mice are hyperpermeable, thus perpetuating and contributing to edema formation. Previous work has shown that VEGF-A treatment of cultured blood endothelial cells (BECs) and LECs increases permeability to trypan blue-labeled albumin [54, 55]. However, co-treatment with AM and VEGF-A dose dependently prevents VEGF-A-induced permeability [54]. This reduction of permeability is caused by a reorganization of the cell–cell junctions. VEGF-A treatment of LECs disrupts the junctional proteins Zonulus Occludin-1 (ZO-1) and vascular endothelial cadherin (VE-Cadherin), thereby creating characteristic gaps and a zipper-like staining pattern [54]. AM treatment stabilizes ZO-1 and VE-cadherin (Fig. 1b) and abrogates VEGF-A-induced disruption, decreasing lymphatic permeability [54]. Fluorescent microlymphography of adult mice confirms this finding. AM-treated mice have significantly decreased uptake of FITC-dextran when compared to control mice, demonstrating that AM stabilizes the lymphatic endothelial barrier in vivo [54]. Therefore, it follows that mice lacking Adm, Calcrl, or Ramp2 may also have increased lymphatic permeability that exacerbates the edema. Taken together, the evidence strongly suggests that in the absence of AM signaling, edema is caused by aberrant lymph sac formation, failed lymphangiogenesis, and abnormally permeable lymphatic vessels.
Ichikawa-Shindo and colleagues observed similar edematous phenotypes in Ramp2 knockout mice [43]. However, while Fritz-Six et al. concluded the edema was predominantly lymphatic in origin, Ichikawa-Shindo et al. reported that Ramp2-deficient mice also exhibit significant vascular endothelial cell dysfunction and increased blood vascular permeability due to decreased expression of VE-Cadherin, claudin 5, and type-IV collagen [43, 56]. Disruption of these proteins, which make up tight and adherens junctions and the basement membrane of endothelial cells, results in increased pericellular leakage and edema [43]. While these studies did not examine lymphatic vascular development or function, the effects of loss of RAMP2 on the blood endothelium are consistent with the known role of AM to modulate the development and regulate the function of the blood vasculature [41, 57, 58]. Thus, leaky blood vessels coupled with arrested lymphangiogenesis and poor lymphatic uptake of interstitial fluid likely account for the severity of the edematous phenotype.
Moreover, the lymphatic and blood endothelium are interdependent. Lymphatic vessels develop directly from the venous vasculature and then parallel the blood vascular system throughout the body. These two vascular systems may only physically connect on either side of the body near the jugulo-subclavian junction where lymph is returned to the blood; however, these systems are developmentally, genetically, and molecularly connected in many complex ways [56]. The lymphatic vasculature has effects on the blood vasculature throughout development and beyond, and vice versa. Therefore, although aberrant lymphangiogenesis is the primary cause of AM-associated edema, the effects of AM on the blood vasculature should not be disregarded.
Likewise, it has become clear that spatial and temporal expression of receptors and their ligands in a microenvironment can affect development of surrounding tissue [59, 60]. Because of the close proximity of and complex connections between the lymphatic and blood vasculatures, it is possible that expression of Adm, Calcrl, and Ramp2 by both BECs and LECs has effects not only in their own microenvironments, but also in the adjacent vascular tissue. Recent studies from our lab have substantiated this hypothesis, definitively identifying CXCR7 as a potent modulator of AM signaling and AM-mediated lymphangiogenesis [25].
As discussed in the previous section of this review, whether CXCR7, a known non-signaling decoy receptor, acts as an AM receptor has remained unclear for over a decade. The promiscuity of the decoy receptor has also made it challenging to discern which ligand is responsible for a given phenotype. This difficulty was particularly apparent in Cxcr7-null mice, which exhibited cardiac enlargement and hyperplasia, a phenotype that was not observed in mutant mice of the canonical ligands, SDF-1 and CXCL11 [21, 61, 62]. However, the cardiac phenotypes closely phenocopied a genetic model of AM overexpression (known as Adm hi/hi mice) which results in gross cardiac hyperplasia during embryogenesis [25, 63]. The similarity in cardiac phenotypes and the historical association of AM and CXCR7 sparked curiosity about whether CXCR7 could behave as a decoy receptor for AM. In this way, loss of the AM decoy receptor would result in a surplus of AM, excessive AM-mediated signal, and a phenocopy of the Adm hi/hi mice. Our lab hypothesized that if CXCR7 does behave as an AM decoy receptor, loss of CXCR7 would also lead to lymphatic vascular defects. Because loss of AM signaling results in small, hypoplastic hearts and lymphatic sacs [14, 39, 40], and AM overexpression results in enlarged, hyperplastic hearts, we predicted that loss of Cxcr7 would result in enlarged, hyperplastic lymphatic vessels.
Indeed, consistent with the notion that CXCR7 sequesters AM, Cxcr7 −/− mice exhibited enlarged, blood-filled lymphatic sacs. Careful examination of the number of proliferating LECs in the lymphatic sac demonstrated that the enlarged lymphatic sacs were hyperplastic (Fig. 2a). These data suggest that, in the absence of the decoy receptor, an excess of AM leads to hyperproliferation of LECs and subsequently enlarged, poorly formed lymphatic sacs [25]. Further examination of LECs in vitro and lymphatic beds in vivo confirmed the ability of CXCR7 to affect the lymphatic endothelium. When treated with AM, cultured CXCR7 knockdown LECs proliferated more than wild-type LECs [25]. Moreover, dermal and cardiac lymphatic beds were disrupted in Cxcr7-null mice and displayed dysmorphic vasculature consistent with hyperplasia [25].
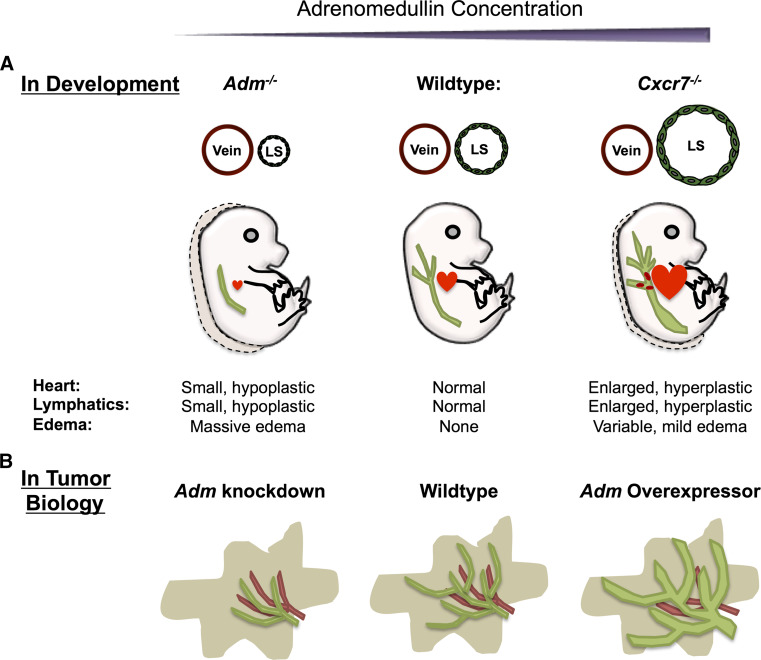
Fig. 2.
Proper AM dosage is required for normal lymphangiogenesis during development and in tumor biology. a Precise AM concentration is required during development to regulate LEC proliferation. Absent AM signaling results in small, hypoplastic lymphatic sacs. Conversely, loss of the decoy receptor, CXCR7, results in excessive AM signaling and enlarged, hyperplasic lymphatic sacs. b Similarly, overexpression of Adm in tumor models results in enhanced proliferation of LECs, dilated lymphatics, and increased tumor metastasis
To definitively show that these phenotypes were caused by excessive AM signaling, we genetically titrated AM ligand onto Cxcr7 −/− mice. Both genetic increase and reduction of AM peptide on the Cxcr7 null background resulted in striking effects on the lymphatic phenotypes. Genetic reduction of Adm in Cxcr7 −/− mice restored the lymphatic and cardiac hyperplasia to wild-type levels, indicating that the hyperplasia observed in Cxcr7 −/− mice resulted from excessive AM signaling [25]. Moreover, intercross of Cxcr7 mutant mice with Adm hi/hi mice resulted in an exacerbation of the lymphatic enlargement and increased embryonic lethality to the point that it was difficult to maintain a Cxcr7 +/− ; Adm hi/+ mouse colony due to embryonic and early post-natal loss [25]. Taken together, these data demonstrate that the dosage of AM is critical for maintaining proper cardiovascular development and that, without tight control of AM-mediated signaling, proliferation of both myocardial and lymphatic endothelial cells is disrupted (Fig. 2a).
Interestingly, while the lymphatic endothelium dynamically expresses Cxcr7, we observed that the blood endothelium directly adjacent to the lymphatic endothelium persistently expressed Cxcr7. Cxcr7 was infrequently observed in the dermal lymphatics where Cxcr7 −/− mice exhibited phenotypes consistent with dermal lymphatic hyperplasia but was regularly observed in the dermal blood vessels [25]. Consistent with previously published papers, therefore, we concluded that the presence of the scavenging receptor in the adjacent blood endothelium impacts development of the surrounding tissue [25]. These findings again highlight the importance that expression of the ligand and its receptors in proximity of (but not necessarily directly within) the lymphatic endothelium has the ability to impact lymphangiogenesis. Taken together, these data demonstrate that Cxcr7 modulates AM signal and identifies a new paradigm of 7-transmembrane decoy receptors as regulators of lymphatic vascular development.
AM signaling during adulthood
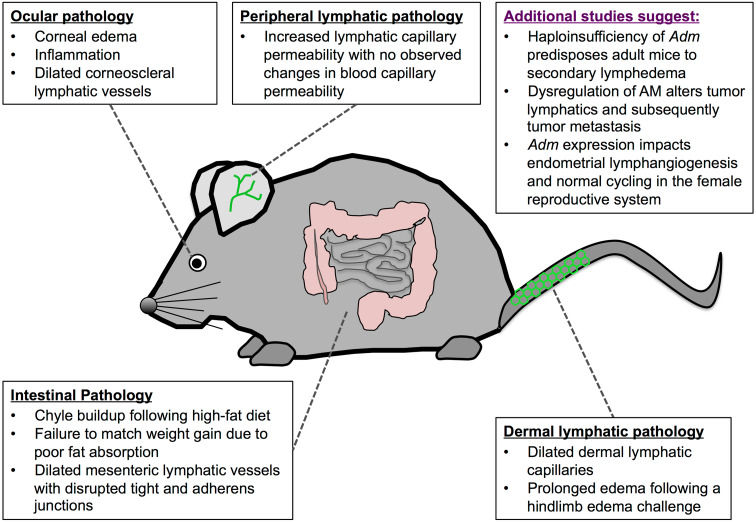
Mid-gestational embryonic lethality due to global knockout of the AM signaling system has made elucidation of the signaling effects of AM on the lymphatic system in adulthood difficult. The recent design of an inducible knockout model, however, has confirmed an important role for AM in lymphatic maintenance and function following normal lymphangiogenesis. Global reduction of Calcrl in Calcrl fl/fl animals using a ubiquitously expressed, tamoxifen-inducible Cre transgenic line (CAGGCre-ER™) resulted in acute and chronic lymphatic dysfunction, leading to ocular inflammation, disrupted fat absorption, and delayed wound healing due to persistent edema [64]. Seven to 10 days following tamoxifen induction of Cre, two thirds of Calcrl fl/fl CAGGCre-ER™ mice developed overt ocular opaqueness [64]. After ruling out glaucoma, Hoopes et al. reported that Calcrl-deficient mice exhibit corneal edema, inflammation, and dilated corneoscleral lymphatic vessels.
While healthy cornea is largely avascular, lymphangiogenesis occurs in response to inflammation and can ultimately lead to blindness [65]. Though no corneal lymphangiogenesis was reported in Calcrl fl/fl CAGGCre-ER™ mice, these mice were only evaluated after one week, which may not allow enough time for neolymphangiogenesis. Given more time, the inflammation in Calcrl fl/fl CAGGCre-ER™ may lead to corneal lymphangiogenesis. It is also possible that the absence of the AM receptor would prevent new lymphatic vessels from forming. Regardless, these findings highlight the importance of proper lymphatic function in maintenance of fluid homeostasis in and around the eye. Indeed, recent studies have demonstrated that the endothelial cells lining Schlemm’s canal express several lymphatic marker genes, are responsive to VEGF-C and thereby control the aqueous humor with the anterior chamber of the eye. [66–68]. Thus, modulation of these vessels may allow for either increased hydration or drainage of the eye, allowing for a novel treatment of dry eyes, ocular edema, and glaucoma.
In addition to the ophthalmic pathology, Calcrl fl/fl CAGGCre-ER™ mice also failed to match weight gain of their wild-type littermates. Examination of the mesenteric vessels of experimental mice showed dilated lymphatic vessels filled with chyle when fed a high-fat meal. These data suggest that AM signaling is critical for absorption of fat from mesenteric lymphatic vessels. Close examination of the mesenteric lymphatic vessels following high-fat diet revealed disrupted lymphatic junctions, a finding that was consistent with previously published work demonstrating that AM stabilizes the lymphatic endothelial barrier and increases vascular permeability [54]. Indeed, further examination of the lymphatic endothelium revealed disrupted lymphatic permeability. Intradermal injection of Evans blue dye into the ear revealed rapid lymphatic uptake in both experimental and control mice. At five min, however, mice lacking Calcrl showed increased leakage and diffuse spreading of the dye [64]. Importantly, no difference in blood vascular permeability was observed, reinforcing that AM loss affects primarily the lymphatic vasculature [64].
To induce edema, complete Freund’s adjuvant (CFA) was injected into the paw of the hind limb. In contrast to tamoxifen-injected controls, Calcrl fl/fl CAGGCre-ER™ mice had prolonged edema [64], suggesting that AM is required for proper reabsorption of interstitial fluid and resolution of edema. As discussed above, previous studies demonstrate that AM treatment stabilizes the lymphatic endothelial barrier (Fig. 1b) [54]. Therefore, similar to the mesenteric lymphatics, it follows that loss of Calcrl destabilizes the lymphatic endothelium in the peripheral lymphatics, increases vascular permeability, allows for lymphatic fluid leakage back into the interstitium, and prevents proper edema resolution.
These findings (summarized in Fig. 3) are particularly intriguing because they demonstrate that appropriate AM dosage following development is required for proper lymphatic maintenance and function. Loss of AM signaling results in leaky vessels, failure to resolve edema, and alterations in fat absorption. All of these data suggest that AM may be a reasonable target for the treatment of a variety of diseases for which we currently have limited therapies. Pharmaceuticals that modulate fat absorption could be a useful tool to help prevent or reduce the extent of obesity. Additionally, use of AM agonists could decrease lymphatic vessel leakiness and increase uptake of interstitial fluid, allowing for the partial or complete resolution of lymphatic-associated edema.
Fig. 3.
Loss of AM signaling in adulthood results in disruption of many lymphatic beds. Here, we provide a summary of the findings when AM signaling is disrupted following embryonic development. Conditional knockdown of Calcrl in adult mice results in significant alterations in the ocular, intestinal, dermal, and peripheral lymphatic vessels. Additional studies that suggest AM is critical for lymphangiogenesis during adulthood are highlighted in purple
Adrenomedullin and lymphedema
As mentioned above, lymphedema is the most common lymphatic disease, affecting hundreds of millions of people worldwide. Lymphedema is categorized as primary or secondary. Primary lymphedema often results from congenital abnormalities. Mutations in nineteen genes have been associated with primary lymphedema, which have been extensively reviewed recently by Brouillard and colleagues [69]. Many of these mutations disrupt genes critical for downstream signaling in LECs, including mutations in the genes encoding VEGFR3, VEGFC, and CCBE1 [69]. Additionally, disruption in several transcription factors required for LEC differentiation and specification (for example, GATA2, FOXC2, and SOX18) result in syndromic forms of primary lymphedema [1, 69]. Although no mutations in the AM signaling cascade have yet been identified in human primary lymphedema, as whole exome sequencing studies continue, it is possible that AM will be found to associate with primary lymphedema. Secondary lymphedema, on the other hand, stems from disruption of the normal lymphatic vasculature by infection or iatrogenic intervention [5]. Despite its prevalence and significant effect on quality of life, currently no cure exists for lymphedema. Additionally, it is not well understood why some individuals are predisposed to developing lymphedema.
Recently, AM was implicated as a molecule that might contribute to the onset of secondary lymphedema. Adm haploinsufficiency predisposed mice to developing secondary lymphedema following hind limb injury compared to wild-type mice [52]. Further, systemic injection of AM to Adm +/− mice restored proper wound healing, thereby preventing the onset of lymphedema [52]. Here, these data demonstrate the importance of AM dosage in the resolution of edema.
Though lymphedema has yet to be associated with mutations in the AM signaling system in humans, these studies suggest that AM administration has potential as a novel therapeutic for the treatment of secondary lymphedema. The primary cause of secondary lymphedema in western countries is radical axillary lymph node dissection, with 20–30 % of patients developing severe lymphedema following surgery [70–72]. The most promising treatment is the generation of new lymphatic vessels [70]. Jin and colleagues demonstrate that AM administration increases lymphatic flow, promotes angiogenesis and lymphangiogenesis, and ultimately decreases tail lymphedema in vivo [73]. The cause of this accelerated healing is likely due to a combination of new blood and lymph vessel formation, as both angiogenesis and lymphangiogenesis are required for wound healing [73–75]. However, the preferential effect of AM on lymphatic endothelial cells indicates that the primary cause for increased healing is neolymphangiogenesis. Therefore, AM administration following surgical resection of lymph nodes may not only promote wound healing, but also prevent the onset of lymphedema. While there are no current studies using AM infusion to treat lymphedema in humans, the potential of AM to act as a novel therapy for lymphedema is promising.
Adrenomedullin in cancer
Originally isolated from pheochromocytoma, a rare neuroendocrine tumor, elevated AM has been associated with neoplasms [76, 77]. Many cancer subtypes overexpress Adm [15], and plasma AM levels often correlate with metastasis, invasion, and poor survival [78, 79]. How AM modulates cancer progression is not well understood. Many experts have speculated that AM contributes to tumor survival and progression by increasing blood flow to a hypoxic tumor environment [15, 76, 80]. However, the importance of lymphangiogenesis and lymphatic vessel remodeling to cancer biology [81, 82] has also delineated a role for AM-mediated lymphangiogenesis in promoting distant metastasis.
Using a Lewis lung carcinoma (LLC) cell line, Karpinich et al. [83] generated a series of tumor cells that stably over- or under-expressed Adm and demonstrated a regulatory role for AM in tumor lymphangiogenesis. The LLC model was particularly attractive because LLCs do not express the canonical AM receptor, Calcrl and Ramp2. Consequently, alterations in Adm expression did not affect tumor cell proliferation [83]. Therefore, following injection into mice, AM-mediated increases in tumor size would not confound differences in tumor metastasis.
Interestingly, alterations in tumor Adm expression robustly impacted tumor lymphatic vessels. While Adm overexpression had little effect on blood vessel density or BEC proliferation, Adm overexpression resulted in a threefold elevation of LECs in both tumors and sentinel lymph nodes compared to tumors with reduced Adm expression (Fig. 2b) [83]. Lymphatic vessels of Adm-overexpressing tumors were also significantly dilated. Careful evaluation of tumor metastasis revealed that Adm overexpression increased tumor dissemination [83]. These data recapitulate what has been observed in human tumors and suggest that Adm expression can promote metastasis via lymphangiogenesis.
Importantly, these findings extend beyond the LLC model. Studies in cervical cancer have also found associations between AM-mediated lymphangiogenesis and severity of disease. Huang et al. [84] report that loss in the tumor stromal endothelium of miR-126, a microRNA that maintains vessel integrity during development, results in significant upregulation of Adm and strongly associates with invasive carcinomas. Upregulation of AM peptide also coupled with increases in CD31-positive endothelium (5.3 % in tumors with high levels of miR-126 expression vs. 71 % in invasive tumors with low miR-126 expression), suggesting that AM may promote disease progression through angiogenesis [84]. However, because lymphatic and blood vessels both stain CD31-positive, it is unclear whether this increase in vessel density is due AM-mediated effects on the blood or lymphatic endothelium. Taken together with previous in vivo and in vitro studies that demonstrate that AM has preferential effects on the lymphatic endothelium, it is expected that much of the increase in vessel density is due to AM-mediated lymphangiogenesis. Consistently, the authors found significant co-localization of AM peptide with LYVE1-positive vessels [84].
These findings make a compelling argument for an association between increased Adm expression and tumor invasion. As such, modulation of Adm expression via AM inhibitors may prove to be an exciting chemotherapeutic target. VEGF inhibitors, which target tumor angiogenesis, are being utilized in the clinic at present and have been found to increase patient survival in certain cancer types [85, 86]. However, the survival increase is modest, often measured only in months rather than years [86]. The benefit of VEGF inhibitors could be strengthened by co-administration with AM inhibitors [87]. This hypothesis has already been tested in mouse models of prostate cancer, where anti-AM antibodies were found to disrupt tumor vasculature, decrease lymphatic vessel density, increase LEC death, and suppress tumor growth [88]. Though this anti-AM antibody has not been fully characterized or utilized in other studies, this finding supports the hypothesis that AM inhibition may improve cancer-related outcomes.
Adrenomedullin-induced lymphangiogenesis in the reproductive system
Perhaps the most exciting and novel aspect of AM-mediated lymphangiogenesis is its involvement in the lymphatic vasculature of the reproductive system. Our lab and others have shown that AM is critical in healthy pregnancy [9]. In a normal human pregnancy, AM increases three- to fivefold [89]. Dysregulation of this physiologic increase in both humans and mouse models has been associated with significant complications, most notably preeclampsia [90–92]. Additionally, haploinsufficiency of Adm in female mice results in reduced fertility due to implantation defects [93]. The essential role of AM in the normal reproductive system has, therefore, become increasingly apparent and clinically relevant over the past two decades. As such, there has been a rapidly growing interest in the role of AM-mediated angiogenesis and lymphangiogenesis in the reproductive system.
Consistent with the finding that AM promotes tumor progression, several studies report increased lymphangiogenesis and metastasis in pregnant women with melanoma when compared with non-pregnant melanoma patients [94, 95]. The direct cause of the increased lymphangiogenesis remains unknown. However, it stands to reason that the significant increase in Adm during pregnancy may be responsible. The physiologically higher levels of AM during pregnancy may interact with the tumor endothelium, thereby promoting LEC proliferation and lymphangiogenesis. This hypothesis differs from previous reports suggesting that AM originates from the tumor cell. Here, increased plasma concentration of AM originates from the host and impacts the tumor environment. It will be interesting to tease out whether levels of AM originating outside of the tumor impact cancer progression via lymphangiogenesis. Adm overexpression models may prove useful in determining host AM status on tumor progression [63].
Despite the focus on AM as a tumor-promoting peptide, increased Adm expression also plays important roles during the normal reproductive cycle. Recent studies have suggested that endometrial lymphangiogenesis is required for normal menstrual cycling and repair of damaged blood vessels during menstruation [96]. Examination of Adm during various stages of the menstrual cycle reveals that Adm expression is elevated during stages of endometrial repair [97]. Also, AM treatment results in increased lymphatic endometrial endothelial cell growth [97, 98]. Taken together, these findings suggest that AM-mediated lymphangiogenesis facilitates endometrial repair and allows progression through the menstrual cycle. Dysregulation of endometrial Adm expression may lead to improper repair of damaged blood vessels and prolonged or heavy menstrual bleeding [97]. These findings again suggest that modulation of the AM signaling system could be utilized clinically for the treatment of common diseases that significantly affect quality of life.
Concluding remarks
The essential role for AM in the development, maintenance, and function of the lymphatic vasculature has been clearly demonstrated over the past decade. Genetic models have greatly contributed to our understanding of how AM modulates lymphangiogenesis and underscored the importance of proper AM dosage: complete loss of AM is incompatible with life. Adm haploinsufficiency may predispose individuals to lymphedema, and overexpression of AM in cancer models correlates with severity of disease. Clearly, aberrant AM expression has the potential to lead to significant lymphatic-associated pathologies, and tight control of AM dosage from development through adulthood is critical for proper lymphatic function. Future studies will continue to elucidate the mechanisms of AM signaling. Whether the AM system interacts with other signaling cascades during lymphangiogenesis, such as Notch and VEGFR3, will be of particular interest, as previously published work has shown AM is capable of activating Notch and transactivating VEGFR2 [57, 99, 100]. These studies will expand our understanding of how AM impacts lymphatic physiology and offer the potential of identifying a G protein-coupled receptor target for the pharmacological modulation of the lymphatic vasculature in human disease.
Acknowledgments
Sources of funding: UNC-CH University Cancer Research Innovation Award and U.S. National Institutes of Health, grants # HD060860, DK099156 to K.M.C. HL118932 to KRK.
References
- 1.Tammela T, Alitalo K. Lymphangiogenesis: molecular mechanisms and future promise. Cell. 2010;140(4):460–476. doi: 10.1016/j.cell.2010.01.045. [DOI] [PubMed] [Google Scholar]
- 2.Zheng W, Aspelund A, Alitalo K. Lymphangiogenic factors, mechanisms, and applications. J Clin Invest. 2014;124(3):878–887. doi: 10.1172/JCI71603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kerjaschki D. The lymphatic vasculature revisited. J Clin Invest. 2014;124(3):874–877. doi: 10.1172/JCI74854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mortimer PS, Rockson SG. New developments in clinical aspects of lymphatic disease. J Clin Invest. 2014;124(3):915–921. doi: 10.1172/JCI71608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Warren AG, Brorson H, Borud LJ, Slavin SA. Lymphedema: a comprehensive review. Ann Plast Surg. 2007;59(4):464–472. doi: 10.1097/01.sap.0000257149.42922.7e. [DOI] [PubMed] [Google Scholar]
- 6.Wiig H, Schroder A, Neuhofer W, Jantsch J, Kopp C, Karlsen TV, Boschmann M, Goss J, Bry M, Rakova N, Dahlmann A, Brenner S, Tenstad O, Nurmi H, Mervaala E, Wagner H, Beck FX, Muller DN, Kerjaschki D, Luft FC, Harrison DG, Alitalo K, Titze J. Immune cells control skin lymphatic electrolyte homeostasis and blood pressure. J Clin Invest. 2013;123(7):2803–2815. doi: 10.1172/JCI60113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harvey NL, Srinivasan RS, Dillard ME, Johnson NC, Witte MH, Boyd K, Sleeman MW, Oliver G. Lymphatic vascular defects promoted by Prox1 haploinsufficiency cause adult-onset obesity. Nat Genet. 2005;37(10):1072–1081. doi: 10.1038/ng1642. [DOI] [PubMed] [Google Scholar]
- 8.Poyner DR, Sexton PM, Marshall I, Smith DM, Quirion R, Born W, Muff R, Fischer JA, Foord SM. International Union of Pharmacology. XXXII. The mammalian calcitonin gene-related peptides, adrenomedullin, amylin, and calcitonin receptors. Pharmacol Rev. 2002;54(2):233–246. doi: 10.1124/pr.54.2.233. [DOI] [PubMed] [Google Scholar]
- 9.Lenhart PM, Caron KM. Adrenomedullin and pregnancy: perspectives from animal models to humans. Trends Endocrinol Metab. 2012;23(10):524–532. doi: 10.1016/j.tem.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muff R, Born W, Lutz TA, Fischer JA. Biological importance of the peptides of the calcitonin family as revealed by disruption and transfer of corresponding genes. Peptides. 2004;25(11):2027–2038. doi: 10.1016/j.peptides.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 11.Smith DM, Coppock HA, Withers DJ, Owji AA, Hay DL, Choksi TP, Chakravarty P, Legon S, Poyner DR. Adrenomedullin: receptor and signal transduction. Biochem Soc Trans. 2002;30(4):432–437. doi: 10.1042/BST0300432. [DOI] [PubMed] [Google Scholar]
- 12.McLatchie LM, Fraser NJ, Main MJ, Wise A, Brown J, Thompson N, Solari R, Lee MG, Foord SM. RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature. 1998;393(6683):333–339. doi: 10.1038/30666. [DOI] [PubMed] [Google Scholar]
- 13.Gibbons C, Dackor R, Dunworth W, Fritz-Six K, Caron KM. Receptor activity-modifying proteins: RAMPing up adrenomedullin signaling. Mol Endocrinol. 2007;21(4):783–796. doi: 10.1210/me.2006-0156. [DOI] [PubMed] [Google Scholar]
- 14.Fritz-Six KL, Dunworth WP, Li M, Caron KM. Adrenomedullin signaling is necessary for murine lymphatic vascular development. J Clin Invest. 2008;118(1):40–50. doi: 10.1172/JCI33302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hay DL, Walker CS, Poyner DR. Adrenomedullin and calcitonin gene-related peptide receptors in endocrine-related cancers: opportunities and challenges. Endocr Relat Cancer. 2011;18(1):C1–14. doi: 10.1677/ERC-10-0244. [DOI] [PubMed] [Google Scholar]
- 16.Kapas S, Catt KJ, Clark AJ. Cloning and expression of cDNA encoding a rat adrenomedullin receptor. J Biol Chem. 1995;270(43):25344–25347. doi: 10.1074/jbc.270.43.25344. [DOI] [PubMed] [Google Scholar]
- 17.Kapas S, Clark AJ. Identification of an orphan receptor gene as a type 1 calcitonin gene-related peptide receptor. Biochem Biophys Res Commun. 1995;217(3):832–838. doi: 10.1006/bbrc.1995.2847. [DOI] [PubMed] [Google Scholar]
- 18.Balabanian K, Lagane B, Infantino S, Chow KY, Harriague J, Moepps B, Arenzana-Seisdedos F, Thelen M, Bachelerie F. The chemokine SDF-1/CXCL12 binds to and signals through the orphan receptor RDC1 in T lymphocytes. J Biol Chem. 2005;280(42):35760–35766. doi: 10.1074/jbc.M508234200. [DOI] [PubMed] [Google Scholar]
- 19.Burns JM, Summers BC, Wang Y, Melikian A, Berahovich R, Miao Z, Penfold ME, Sunshine MJ, Littman DR, Kuo CJ, Wei K, McMaster BE, Wright K, Howard MC, Schall TJ. A novel chemokine receptor for SDF-1 and I-TAC involved in cell survival, cell adhesion, and tumor development. J Exp Med. 2006;203(9):2201–2213. doi: 10.1084/jem.20052144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ikeda Y, Kumagai H, Skach A, Sato M, Yanagisawa M. Modulation of circadian glucocorticoid oscillation via adrenal opioid-CXCR7 signaling alters emotional behavior. Cell. 2013;155(6):1323–1336. doi: 10.1016/j.cell.2013.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sierro F, Biben C, Martinez-Munoz L, Mellado M, Ransohoff RM, Li M, Woehl B, Leung H, Groom J, Batten M, Harvey RP, Martinez AC, Mackay CR, Mackay F. Disrupted cardiac development but normal hematopoiesis in mice deficient in the second CXCL12/SDF-1 receptor, CXCR7. Proc Natl Acad Sci USA. 2007;104(37):14759–14764. doi: 10.1073/pnas.0702229104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Autelitano DJ, Tang F. Co-expression of prepro-adrenomedullin with a putative adrenomedullin receptor gene in vascular smooth muscle. Clin Sci (Lond) 1999;96(5):493–498. doi: 10.1042/CS19980311. [DOI] [PubMed] [Google Scholar]
- 23.Chakravarty P, Suthar TP, Coppock HA, Nicholl CG, Bloom SR, Legon S, Smith DM. CGRP and adrenomedullin binding correlates with transcript levels for calcitonin receptor-like receptor (CRLR) and receptor activity modifying proteins (RAMPs) in rat tissues. Br J Pharmacol. 2000;130(1):189–195. doi: 10.1038/sj.bjp.0702975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ladoux A, Frelin C. Coordinated Up-regulation by hypoxia of adrenomedullin and one of its putative receptors (RDC-1) in cells of the rat blood-brain barrier. J Biol Chem. 2000;275(51):39914–39919. doi: 10.1074/jbc.M006512200. [DOI] [PubMed] [Google Scholar]
- 25.Klein KR, Karpinich NO, Espenschied ST, Willcockson HH, Dunworth WP, Hoopes SL, Kushner EJ, Bautch VL, Caron KM. Decoy receptor CXCR7 modulates adrenomedullin-mediated cardiac and lymphatic vascular development. Dev Cell. 2014;30(5):528–540. doi: 10.1016/j.devcel.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Betterman KL, Harvey NL. Decoys and cardiovascular development: CXCR7 and regulation of adrenomedullin signaling. dev cell. 2014;30(5):490–491. doi: 10.1016/j.devcel.2014.08.021. [DOI] [PubMed] [Google Scholar]
- 27.Karpinich NO, Hoopes SL, Kechele DO, Lenhart PM, Caron KM. Adrenomedullin function in vascular endothelial cells: insights from genetic mouse models. Curr Hypertens Rev. 2011;7(4):228–239. doi: 10.2174/157340211799304761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Di Iorio R, Marinoni E, Letizia C, Alo P, Villaccio B, Cosmi EV. Adrenomedullin, a new vasoactive peptide, is increased in preeclampsia. Hypertension. 1998;32(4):758–763. doi: 10.1161/01.HYP.32.4.758. [DOI] [PubMed] [Google Scholar]
- 29.Nuki C, Kawasaki H, Kitamura K, Takenaga M, Kangawa K, Eto T, Wada A. Vasodilator effect of adrenomedullin and calcitonin gene-related peptide receptors in rat mesenteric vascular beds. Biochem Biophys Res Commun. 1993;196(1):245–251. doi: 10.1006/bbrc.1993.2241. [DOI] [PubMed] [Google Scholar]
- 30.Hinson JP, Kapas S, Smith DM. Adrenomedullin, a multifunctional regulatory peptide. Endocr Rev. 2000;21(2):138–167. doi: 10.1210/edrv.21.2.0396. [DOI] [PubMed] [Google Scholar]
- 31.Kataoka Y, Miyazaki S, Yasuda S, Nagaya N, Noguchi T, Yamada N, Morii I, Kawamura A, Doi K, Miyatake K, Tomoike H, Kangawa K (2010) The first clinical pilot study of intravenous adrenomedullin administration in patients with acute myocardial infarction. J Cardiovasc Pharmacol 56(4):413–419. doi:10.1097/FJC.0b013e3181f15b45 [DOI] [PubMed]
- 32.Hagerling R, Pollmann C, Andreas M, Schmidt C, Nurmi H, Adams RH, Alitalo K, Andresen V, Schulte-Merker S, Kiefer F. A novel multistep mechanism for initial lymphangiogenesis in mouse embryos based on ultramicroscopy. EMBO J. 2013;32(5):629–644. doi: 10.1038/emboj.2012.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang Y, Oliver G. Development of the mammalian lymphatic vasculature. J Clin Invest. 2014;124(3):888–897. doi: 10.1172/JCI71609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wigle JT, Harvey N, Detmar M, Lagutina I, Grosveld G, Gunn MD, Jackson DG, Oliver G. An essential role for Prox1 in the induction of the lymphatic endothelial cell phenotype. EMBO J. 2002;21(7):1505–1513. doi: 10.1093/emboj/21.7.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wigle JT, Oliver G. Prox1 function is required for the development of the murine lymphatic system. Cell. 1999;98(6):769–778. doi: 10.1016/S0092-8674(00)81511-1. [DOI] [PubMed] [Google Scholar]
- 36.Alitalo K, Tammela T, Petrova TV. Lymphangiogenesis in development and human disease. Nature. 2005;438(7070):946–953. doi: 10.1038/nature04480. [DOI] [PubMed] [Google Scholar]
- 37.Karkkainen MJ, Haiko P, Sainio K, Partanen J, Taipale J, Petrova TV, Jeltsch M, Jackson DG, Talikka M, Rauvala H, Betsholtz C, Alitalo K. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat Immunol. 2004;5(1):74–80. doi: 10.1038/ni1013. [DOI] [PubMed] [Google Scholar]
- 38.Makinen T, Jussila L, Veikkola T, Karpanen T, Kettunen MI, Pulkkanen KJ, Kauppinen R, Jackson DG, Kubo H, Nishikawa S, Yla-Herttuala S, Alitalo K. Inhibition of lymphangiogenesis with resulting lymphedema in transgenic mice expressing soluble VEGF receptor-3. Nat Med. 2001;7(2):199–205. doi: 10.1038/84651. [DOI] [PubMed] [Google Scholar]
- 39.Dackor RT, Fritz-Six K, Dunworth WP, Gibbons CL, Smithies O, Caron KM. Hydrops fetalis, cardiovascular defects, and embryonic lethality in mice lacking the calcitonin receptor-like receptor gene. Mol Cell Biol. 2006;26(7):2511–2518. doi: 10.1128/MCB.26.7.2511-2518.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Caron KM, Smithies O. Extreme hydrops fetalis and cardiovascular abnormalities in mice lacking a functional Adrenomedullin gene. Proc Natl Acad Sci USA. 2001;98(2):615–619. doi: 10.1073/pnas.98.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shindo T, Kurihara Y, Nishimatsu H, Moriyama N, Kakoki M, Wang Y, Imai Y, Ebihara A, Kuwaki T, Ju KH, Minamino N, Kangawa K, Ishikawa T, Fukuda M, Akimoto Y, Kawakami H, Imai T, Morita H, Yazaki Y, Nagai R, Hirata Y, Kurihara H. Vascular abnormalities and elevated blood pressure in mice lacking adrenomedullin gene. Circulation. 2001;104(16):1964–1971. doi: 10.1161/hc4101.097111. [DOI] [PubMed] [Google Scholar]
- 42.Shimosawa T, Shibagaki Y, Ishibashi K, Kitamura K, Kangawa K, Kato S, Ando K, Fujita T. Adrenomedullin, an endogenous peptide, counteracts cardiovascular damage. Circulation. 2002;105(1):106–111. doi: 10.1161/hc0102.101399. [DOI] [PubMed] [Google Scholar]
- 43.Ichikawa-Shindo Y, Sakurai T, Kamiyoshi A, Kawate H, Iinuma N, Yoshizawa T, Koyama T, Fukuchi J, Iimuro S, Moriyama N, Kawakami H, Murata T, Kangawa K, Nagai R, Shindo T. The GPCR modulator protein RAMP2 is essential for angiogenesis and vascular integrity. J Clin Invest. 2008;118(1):29–39. doi: 10.1172/JCI33022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dackor R, Fritz-Six K, Smithies O, Caron K. Receptor activity-modifying proteins 2 and 3 have distinct physiological functions from embryogenesis to old age. J Biol Chem. 2007;282(25):18094–18099. doi: 10.1074/jbc.M703544200. [DOI] [PubMed] [Google Scholar]
- 45.Czyzyk TA, Ning Y, Hsu MS, Peng B, Mains RE, Eipper BA, Pintar JE. Deletion of peptide amidation enzymatic activity leads to edema and embryonic lethality in the mouse. Dev Biol. 2005;287(2):301–313. doi: 10.1016/j.ydbio.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 46.Kisanuki YY, Hammer RE, Miyazaki J, Williams SC, Richardson JA, Yanagisawa M. Tie2-Cre transgenic mice: a new model for endothelial cell-lineage analysis in vivo. Dev Biol. 2001;230(2):230–242. doi: 10.1006/dbio.2000.0106. [DOI] [PubMed] [Google Scholar]
- 47.Eklund L, Bry M, Alitalo K. Mouse models for studying angiogenesis and lymphangiogenesis in cancer. Mol Oncol. 2013;7(2):259–282. doi: 10.1016/j.molonc.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tammela T, Saaristo A, Lohela M, Morisada T, Tornberg J, Norrmen C, Oike Y, Pajusola K, Thurston G, Suda T, Yla-Herttuala S, Alitalo K. Angiopoietin-1 promotes lymphatic sprouting and hyperplasia. Blood. 2005;105(12):4642–4648. doi: 10.1182/blood-2004-08-3327. [DOI] [PubMed] [Google Scholar]
- 49.Morisada T, Oike Y, Yamada Y, Urano T, Akao M, Kubota Y, Maekawa H, Kimura Y, Ohmura M, Miyamoto T, Nozawa S, Koh GY, Alitalo K, Suda T. Angiopoietin-1 promotes LYVE-1-positive lymphatic vessel formation. Blood. 2005;105(12):4649–4656. doi: 10.1182/blood-2004-08-3382. [DOI] [PubMed] [Google Scholar]
- 50.Srinivasan RS, Dillard ME, Lagutin OV, Lin FJ, Tsai S, Tsai MJ, Samokhvalov IM, Oliver G. Lineage tracing demonstrates the venous origin of the mammalian lymphatic vasculature. Genes Dev. 2007;21(19):2422–2432. doi: 10.1101/gad.1588407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen L, Mupo A, Huynh T, Cioffi S, Woods M, Jin C, McKeehan W, Thompson-Snipes L, Baldini A, Illingworth E. Tbx1 regulates Vegfr3 and is required for lymphatic vessel development. J Cell Biol. 2010;189(3):417–424. doi: 10.1083/jcb.200912037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nikitenko LL, Shimosawa T, Henderson S, Makinen T, Shimosawa H, Qureshi U, Pedley B, Rees MC, Fujita T, Boshoff C. Adrenomedullin haploinsufficiency predisposes to secondary lymphedema. J Invest Dermatol. 2013 doi: 10.1038/jid.2013.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jin D, Otani K, Yamahara K, Ikeda T, Nagaya N, Kangawa K. Adrenomedullin reduces expression of adhesion molecules on lymphatic endothelial cells. Regul Pept. 2011;166(1–3):21–27. doi: 10.1016/j.regpep.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 54.Dunworth WP, Fritz-Six KL, Caron KM. Adrenomedullin stabilizes the lymphatic endothelial barrier in vitro and in vivo. Peptides. 2008;29(12):2243–2249. doi: 10.1016/j.peptides.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cullen VC, Mackarel AJ, Hislip SJ, O’Connor CM, Keenan AK. Investigation of vascular endothelial growth factor effects on pulmonary endothelial monolayer permeability and neutrophil transmigration. Gen Pharmacol. 2000;35(3):149–157. doi: 10.1016/S0306-3623(01)00102-1. [DOI] [PubMed] [Google Scholar]
- 56.Kahn ML. Blood is thicker than lymph. J Clin Invest. 2008;118(1):23–26. doi: 10.1172/JCI34485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nicoli S, Tobia C, Gualandi L, De Sena G, Presta M. Calcitonin receptor-like receptor guides arterial differentiation in zebrafish. Blood. 2008;111(10):4965–4972. doi: 10.1182/blood-2007-10-118166. [DOI] [PubMed] [Google Scholar]
- 58.Ribatti D, Nico B, Spinazzi R, Vacca A, Nussdorfer GG. The role of adrenomedullin in angiogenesis. Peptides. 2005;26(9):1670–1675. doi: 10.1016/j.peptides.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 59.Moissoglu K, Majumdar R, Parent CA. Cell migration: sinking in a gradient. Curr Biol. 2014;24(1):R23–R25. doi: 10.1016/j.cub.2013.10.075. [DOI] [PubMed] [Google Scholar]
- 60.Venkiteswaran G, Lewellis SW, Wang J, Reynolds E, Nicholson C, Knaut H. Generation and dynamics of an endogenous, self-generated signaling gradient across a migrating tissue. Cell. 2013;155(3):674–687. doi: 10.1016/j.cell.2013.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yu S, Crawford D, Tsuchihashi T, Behrens TW, Srivastava D. The chemokine receptor CXCR7 functions to regulate cardiac valve remodeling. Dev Dyn. 2011;240(2):384–393. doi: 10.1002/dvdy.22549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gerrits H, van Ingen Schenau DS, Bakker NE, van Disseldorp AJ, Strik A, Hermens LS, Koenen TB, Krajnc-Franken MA, Gossen JA. Early postnatal lethality and cardiovascular defects in CXCR7-deficient mice. Genesis. 2008;46(5):235–245. doi: 10.1002/dvg.20387. [DOI] [PubMed] [Google Scholar]
- 63.Wetzel-Strong SE, Li M, Klein KR, Nishikimi T, Caron KM. Epicardial-derived adrenomedullin drives cardiac hyperplasia during embryogenesis. Dev Dyn. 2013;243(2):243–256. doi: 10.1002/dvdy.24065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hoopes SL, Willcockson HH, Caron KM. Characteristics of multi-organ lymphangiectasia resulting from temporal deletion of calcitonin receptor-like receptor in adult mice. PLoS One. 2012;7(9):e45261. doi: 10.1371/journal.pone.0045261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Regenfuss B, Bock F, Parthasarathy A, Cursiefen C. Corneal (lymph)angiogenesis—from bedside to bench and back: a tribute to Judah Folkman. Lymphat Res Biol. 2008;6(3–4):191–201. doi: 10.1089/lrb.2008.6348. [DOI] [PubMed] [Google Scholar]
- 66.Park DY, Lee J, Park I, Choi D, Lee S, Song S, Hwang Y, Hong KY, Nakaoka Y, Makinen T, Kim P, Alitalo K, Hong YK, Koh GY. Lymphatic regulator PROX1 determines Schlemm’s canal integrity and identity. J Clin Invest. 2014;124(9):3960–3974. doi: 10.1172/JCI75392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Aspelund A, Tammela T, Antila S, Nurmi H, Leppanen VM, Zarkada G, Stanczuk L, Francois M, Makinen T, Saharinen P, Immonen I, Alitalo K. The Schlemm’s canal is a VEGF-C/VEGFR-3-responsive lymphatic-like vessel. J Clin Invest. 2014;124(9):3975–3986. doi: 10.1172/JCI75395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Karpinich NO, Caron KM. Schlemm’s canal: more than meets the eye, lymphatics in disguise. J Clin Invest. 2014;124(9):3701–3703. doi: 10.1172/JCI77507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brouillard P, Boon L, Vikkula M. Genetics of lymphatic anomalies. J Clin Invest. 2014;124(3):898–904. doi: 10.1172/JCI71614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Alitalo K. The lymphatic vasculature in disease. Nat Med. 2011;17(11):1371–1380. doi: 10.1038/nm.2545. [DOI] [PubMed] [Google Scholar]
- 71.Donker M, van Tienhoven G, Straver ME, Meijnen P, van de Velde CJ, Mansel RE, Cataliotti L, Westenberg AH, Klinkenbijl JH, Orzalesi L, Bouma WH, van der Mijle HC, Nieuwenhuijzen GA, Veltkamp SC, Slaets L, Duez NJ, de Graaf PW, van Dalen T, Marinelli A, Rijna H, Snoj M, Bundred NJ, Merkus JW, Belkacemi Y, Petignat P, Schinagl DA, Coens C, Messina CG, Bogaerts J, Rutgers EJ. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981-22023 AMAROS): a randomised, multicentre, open-label, phase 3 non-inferiority trial. Lancet Oncol. 2014;15(12):1303–1310. doi: 10.1016/S1470-2045(14)70460-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McLaughlin SA, Wright MJ, Morris KT, Giron GL, Sampson MR, Brockway JP, Hurley KE, Riedel ER, Van Zee KJ. Prevalence of lymphedema in women with breast cancer 5 years after sentinel lymph node biopsy or axillary dissection: objective measurements. J Clin Oncol. 2008;26(32):5213–5219. doi: 10.1200/JCO.2008.16.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jin D, Harada K, Ohnishi S, Yamahara K, Kangawa K, Nagaya N. Adrenomedullin induces lymphangiogenesis and ameliorates secondary lymphoedema. Cardiovasc Res. 2008;80(3):339–345. doi: 10.1093/cvr/cvn228. [DOI] [PubMed] [Google Scholar]
- 74.Hirakawa S, Detmar M. New insights into the biology and pathology of the cutaneous lymphatic system. J Dermatol Sci. 2004;35(1):1–8. doi: 10.1016/j.jdermsci.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 75.Tonnesen MG, Feng X, Clark RA. Angiogenesis in wound healing. J Investig Dermatol Symp Proc. 2000;5(1):40–46. doi: 10.1046/j.1087-0024.2000.00014.x. [DOI] [PubMed] [Google Scholar]
- 76.Zudaire E, Martinez A, Cuttitta F. Adrenomedullin and cancer. Regul Pept. 2003;112(1–3):175–183. doi: 10.1016/S0167-0115(03)00037-5. [DOI] [PubMed] [Google Scholar]
- 77.Kitamura K, Kangawa K, Kawamoto M, Ichiki Y, Nakamura S, Matsuo H, Eto T. Adrenomedullin: a novel hypotensive peptide isolated from human pheochromocytoma. Biochem Biophys Res Commun. 1993;192(2):553–560. doi: 10.1006/bbrc.1993.1451. [DOI] [PubMed] [Google Scholar]
- 78.Hu Z, Fan C, Livasy C, He X, Oh DS, Ewend MG, Carey LA, Subramanian S, West R, Ikpatt F, Olopade OI, van de Rijn M, Perou CM. A compact VEGF signature associated with distant metastases and poor outcomes. BMC Med. 2009;7:9. doi: 10.1186/1741-7015-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Park HC, Seong J, An JH, Kim J, Kim UJ, Lee BW. Alteration of cancer pain-related signals by radiation: proteomic analysis in an animal model with cancer bone invasion. Int J Radiat Oncol Biol Phys. 2005;61(5):1523–1534. doi: 10.1016/j.ijrobp.2004.12.070. [DOI] [PubMed] [Google Scholar]
- 80.Nikitenko LL, Fox SB, Kehoe S, Rees MC, Bicknell R. Adrenomedullin and tumour angiogenesis. Br J Cancer. 2006;94(1):1–7. doi: 10.1038/sj.bjc.6602832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stacker SA, Williams SP, Karnezis T, Shayan R, Fox SB, Achen MG. Lymphangiogenesis and lymphatic vessel remodelling in cancer. Nat Rev Cancer. 2014;14(3):159–172. doi: 10.1038/nrc3677. [DOI] [PubMed] [Google Scholar]
- 82.Ji RC. Hypoxia and lymphangiogenesis in tumor microenvironment and metastasis. Cancer Lett. 2014;346(1):6–16. doi: 10.1016/j.canlet.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 83.Karpinich NO, Kechele DO, Espenschied ST, Willcockson HH, Fedoriw Y, Caron KM. Adrenomedullin gene dosage correlates with tumor and lymph node lymphangiogenesis. FASEB J. 2013;27(2):590–600. doi: 10.1096/fj.12-214080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Huang TH, Chu TY. Repression of miR-126 and upregulation of adrenomedullin in the stromal endothelium by cancer-stromal cross talks confers angiogenesis of cervical cancer. Oncogene. 2013 doi: 10.1038/onc.2013.335. [DOI] [PubMed] [Google Scholar]
- 85.Tewari KS, Sill MW, Long HJ, 3rd, Penson RT, Huang H, Ramondetta LM, Landrum LM, Oaknin A, Reid TJ, Leitao MM, Michael HE, Monk BJ. Improved survival with bevacizumab in advanced cervical cancer. N Engl J Med. 2014;370(8):734–743. doi: 10.1056/NEJMoa1309748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer. 2008;8(8):592–603. doi: 10.1038/nrc2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bzowska M, Mezyk-Kopec R, Prochnicki T, Kulesza M, Klaus T, Bereta J. Antibody-based antiangiogenic and antilymphangiogenic therapies to prevent tumor growth and progression. Acta Biochim Pol. 2013;60(3):263–275. [PubMed] [Google Scholar]
- 88.Berenguer-Daize C, Boudouresque F, Bastide C, Tounsi A, Benyahia Z, Acunzo J, Dussault N, Delfino C, Baeza N, Daniel L, Cayol M, Rossi D, El Battari A, Bertin D, Mabrouk K, Martin PM, Ouafik L. Adrenomedullin blockade suppresses growth of human hormone-independent prostate tumor xenograft in mice. Clin Cancer Res. 2013;19(22):6138–6150. doi: 10.1158/1078-0432.CCR-13-0691. [DOI] [PubMed] [Google Scholar]
- 89.Di Iorio R, Marinoni E, Scavo D, Letizia C, Cosmi EV. Adrenomedullin in pregnancy. Lancet. 1997;349(9048):328. doi: 10.1016/S0140-6736(05)62827-9. [DOI] [PubMed] [Google Scholar]
- 90.Lenhart PM, Nguyen T, Wise A, Caron KM, Herring AH, Stuebe AM. Adrenomedullin signaling pathway polymorphisms and adverse pregnancy outcomes. Am J Perinatol. 2014;31(4):327–334. doi: 10.1055/s-0033-1349345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li M, Schwerbrock NM, Lenhart PM, Fritz-Six KL, Kadmiel M, Christine KS, Kraus DM, Espenschied ST, Willcockson HH, Mack CP, Caron KM. Fetal-derived adrenomedullin mediates the innate immune milieu of the placenta. J Clin Invest. 2013;123(6):2408–2420. doi: 10.1172/JCI67039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Matson BC, Corty RW, Karpinich NO, Murtha AP, Valdar W, Grotegut CA, Caron KM. Midregional pro-adrenomedullin plasma concentrations are blunted in severe preeclampsia. Placenta. 2014;35(9):780–783. doi: 10.1016/j.placenta.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Li M, Yee D, Magnuson TR, Smithies O, Caron KM. Reduced maternal expression of adrenomedullin disrupts fertility, placentation, and fetal growth in mice. J Clin Invest. 2006;116(10):2653–2662. doi: 10.1172/JCI28462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rodero MP, Prignon A, Avril MF, Boitier F, Aractingi S, Khosrotehrani K. Increase lymphangiogenesis in melanoma during pregnancy: correlation with the prolactin signalling pathway. J Eur Acad Dermatol Venereol. 2013;27(1):e144–e145. doi: 10.1111/j.1468-3083.2012.04550.x. [DOI] [PubMed] [Google Scholar]
- 95.Khosrotehrani K, Nguyen Huu S, Prignon A, Avril MF, Boitier F, Oster M, Mortier L, Richard MA, Maubec E, Kerob D, Mansard S, Merheb C, Moguelet P, Nassar D, Guegan S, Aractingi S. Pregnancy promotes melanoma metastasis through enhanced lymphangiogenesis. Am J Pathol. 2011;178(4):1870–1880. doi: 10.1016/j.ajpath.2010.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rogers PA, Donoghue JF, Girling JE (2008) Endometrial lymphangiogenesis. Placenta 29(Suppl A):S48–S54. doi:10.1016/j.placenta.2007.09.009 [DOI] [PubMed]
- 97.Maybin JA, Battersby S, Hirani N, Nikitenko LL, Critchley HO, Jabbour HN. The expression and regulation of adrenomedullin in the human endometrium: a candidate for endometrial repair. Endocrinology. 2011;152(7):2845–2856. doi: 10.1210/en.2010-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nikitenko LL, MacKenzie IZ, Rees MC, Bicknell R. Adrenomedullin is an autocrine regulator of endothelial growth in human endometrium. Mol Hum Reprod. 2000;6(9):811–819. doi: 10.1093/molehr/6.9.811. [DOI] [PubMed] [Google Scholar]
- 99.Yurugi-Kobayashi T, Itoh H, Schroeder T, Nakano A, Narazaki G, Kita F, Yanagi K, Hiraoka-Kanie M, Inoue E, Ara T, Nagasawa T, Just U, Nakao K, Nishikawa S, Yamashita JK. Adrenomedullin/cyclic AMP pathway induces Notch activation and differentiation of arterial endothelial cells from vascular progenitors. Arterioscler Thromb Vasc Biol. 2006;26(9):1977–1984. doi: 10.1161/01.ATV.0000234978.10658.41. [DOI] [PubMed] [Google Scholar]
- 100.Guidolin D, Albertin G, Spinazzi R, Sorato E, Mascarin A, Cavallo D, Antonello M, Ribatti D. Adrenomedullin stimulates angiogenic response in cultured human vascular endothelial cells: involvement of the vascular endothelial growth factor receptor 2. Peptides. 2008;29(11):2013–2023. doi: 10.1016/j.peptides.2008.07.009. [DOI] [PubMed] [Google Scholar]