Abstract
The migration of T cells and access to tumor antigens is of utmost importance for the induction of protective anti-tumor immunity. Once having entered a malignant site, T cells encounter a complex environment composed of non-tumor cells along with the extracellular matrix (ECM). It is now well accepted that a deregulated ECM favors tumor progression and metastasis. Recent progress in imaging technologies has also highlighted the impact of the matrix architecture found in solid tumor on immune cells and especially T cells. In this review, we argue that the ability of T cells to mount an antitumor response is dependent on the matrix structure, more precisely on the balance between pro-migratory reticular fiber networks and unfavorable migration zones composed of dense and aligned ECM structures. Thus, the matrix architecture, that has long been considered to merely provide the structural framework of connective tissues, can play a key role in facilitating or suppressing the antitumor immune surveillance. A new challenge in cancer therapy will be to develop approaches aimed at altering the architecture of the tumor stroma, rendering it more permissive to antitumor T cells.
Keywords: Tumor, T cells, Stroma, Extracellular matrix, Motility, Imaging
Introduction
A large majority of human solid tumors are of epithelial origin. However, carcinomas cannot be simply considered as a group of proliferating epithelial cells but rather as a complex ecosystem composed of a variety of cells organized in a specialized microenvironment, referred to as stroma. Indeed, the tumor stroma contains non-cancer cells, including endothelial cells and fibroblasts, along with the ECM. Although epithelial neoplastic cells and stromal cells are found in distinct areas (Fig. 1a), the growth of a tumor depends on dynamic and complex interactions between these cell populations.
Fig. 1.
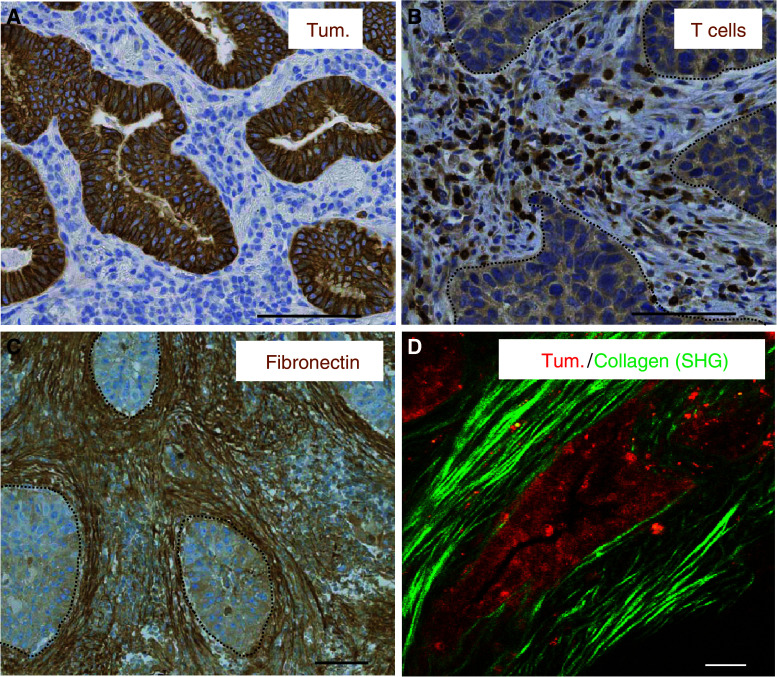
Spatial organization of tumor cells, T cells and ECM components in human lung and ovarian carcinomas. a In a human lung tumor, tumor cells (brown, stained for cytokeratin) form well-delineated tumor islets surrounded by a stroma (blue). b In a human lung tumor, T cells (brown, stained for CD3) are preferentially distributed within the tumor stroma and very rarely in contact with tumor cells. Dotted white lines denote tumor islets. c In a human lung tumor, thick and linear fibronectin fibers (brown, stained for fibronectin) surround tumor islets denoted by dotted white lines. d In a human ovarian tumor, tumor cells (red, stained for EpCAM) are surrounded by straight and parallel collagen fibers (green) detected by SHG on a two-photon microscope. Bar 100 μm
An increasing body of evidence suggests that several cells of the immune system are able to control the growth of a tumor. For instance, the presence of high numbers of memory Th1 T cells and CD8+ cytotoxic T cells has been reported as an indicator of good prognosis in many human cancers, such as melanoma or lung cancer [1]. However, to mount an effective antitumor response, T cells must pass through several distinct steps. First, T lymphocytes need to be fully activated by mature dendritic cells in the tumor-draining lymph node. Second, cancer-specific effector T cells must enter the tumor after they have left the blood vessels. Finally, tumor-infiltrating lymphocytes (TIL) need to accomplish their functional activities which eventually lead to tumor regression. However, experiments performed over the last decade indicate that the tumor environment has the ability to compromise antitumor immunity, and several escape mechanisms have been identified [2]. For example, the entry of lymphocytes into the tumor does not always occur normally, mostly due to abnormal vessel formation and reduced expression of adhesion molecules [3]. In addition, T cells found within a progressive tumor are usually unable to respond normally to antigen stimulation and show signs of anergy [4, 5]. Finally, T lymphocytes, as well as other immune cells, are not randomly distributed within tumors and are usually more concentrated in the stroma than in tumor islets [6–9] (Fig. 1b).
In recent years, functional alterations of lymphocytes in the tumor microenvironment have been accurately reviewed [10]. However, defects in T cell infiltration into tumor islets might well constitute an important obstacle for T cell-mediated antitumor activity, and understanding the mechanisms underlying the distribution of T cells would be of major interest. Given the pivotal role of chemokines in controlling the positioning of immune cells in various tissues, the paucity of T cells in carcinoma regions has been envisaged to result from a lack or an inactivation of chemoattractants within tumor islets [11, 12]. Another plausible mechanism by which T cells are prevented from entering tumor islets could be due to the expression of molecules by tumor cells that will act as a chemical barrier for T cells. These include proteins with chemorepulsive capacities like neuropilin-1, originally described in the nervous system and, more recently, in the immune system [13] but also chemokines that, under certain conditions, e.g., high doses or chronic exposure, can provoke active movements of lymphocytes away from chemokinetic agents [14].
Apart from features that depend on tumor cells, the recent development of improved imaging technologies, enabling the dynamic visualization of cells within intact tissues, has identified additional environmental cues that regulate the distribution and migration of T cells [15]. Indeed, evidence is accumulating that the ECM, by its physical and biochemical features, is essential for regulating T cell behavior. Along with signals transmitted by ECM proteins, leading to T cell activation and differentiation, the architecture of a tissue can both facilitate and create environmental obstacles to T cell traffic.
In this review, we will examine the diverse matrix structures found in normal, chronically inflamed, and tumor tissues, focusing our attention on the recent advances regarding the role of the ECM in the control of immune cell migration. We will also discuss some potential therapeutic approaches that, by modifying the ECM architecture, could favor T cell ability to contact and eliminate tumor cells.
Matrix architecture: from normal to tumor situation
Components and structures of the ECM
In this review, we will focus on the interstitial matrix that leukocytes encounter once they have entered a tissue. The structure and function of the basement membrane, which is a specialized form of sheet-like ECM interacting with the epithelium and endothelium, will not be addressed here.
The ECM is the non-cellular component of any tissue and organ [16]. It can be functionally divided into two main classes of macromolecules: the fibrillar fraction, that consists of arrays of fibrillar collagen bundles, elastin and fibronectin fibers, and the non-fibrillar fraction, which mainly consists of glycosaminoglycans and proteoglycans. These large and negatively charged sugars efficiently bind water and fill the space between the fibers. The ECM primarily fulfils a structural role by maintaining an insoluble scaffold, which ultimately defines the shape and stiffness of organs.
Most ECM components are produced by fibroblasts that also play a role in their assembly into fibers and their spatial disposition. Collagen cross-linking is almost exclusively mediated by an enzyme, the lysyl oxydase (LOX) [17]. The synthesis and cross-linking of ECM fibers is balanced by the action of metalloproteinases (MMPs) that degrade collagen and other ECM proteins [18].
Depending on the origin of the tissue and on the context (injury, inflammation, tumor), the three-dimensional (3D) matrix architecture can adopt a variety of conformations [19]. In some tissues, the ECM can be dense and stiff, while, in others, it is soft and more porous with gap size of different diameters. ECM fibers also display highly variable thickness, straightness, and spatial arrangements. They can be relaxed and non-oriented or, in contrast, linearized and oriented in a specific direction. These physical characteristics determine the architecture of the tissue but, as we will see later, they are also essential for regulating immune cell migration and behavior.
Our understanding of the matrix structure mainly comes from histological techniques, which include immunostaining, as well as the use of Masson’s trichrome and picrosirius red to stain collagens and examine their linearization and orientation. However, these standard procedures require tissue processing such as fixation, paraffin embedding, and cutting and therefore give only a static snapshot of the ECM and cannot document its complex dynamics. More specialized techniques, such as second harmonic generation (SHG) microscopy, have addressed these limitations [20]. SHG is an intrinsic signal highly sensitive to collagen that therefore rules out the use of external dyes. In addition, the non-linear excitation of a multi-photon microscope permits deep penetration in freshly biopsied tissues. Thus, a 4D spatio-temporal resolution of fibrillar collagen in thick samples, and even in intact organs, can be assessed with this non-invasive technique. However, SHG cannot be a routine technique designed to replace standard histological techniques, because of the expense of multiphoton microscopes and the amount of time required for the analysis, but it can still be envisaged as a complement to semi-quantitative histopathology of collagen deposition in tissues.
Matrix architecture in a normal epithelium
Epithelial tissues usually consist of a single layer of epithelial cells surrounded by a stroma composed primarily of non-activated fibroblasts, rare immune cells, and endothelial cells forming blood and lymph vessels. Even if each tissue has a unique matrix composition and structure, the stroma of a normal epithelium contains a relaxed meshwork of collagen fibers and ECM components, such as elastic fibers and fibronectin, embedded in a hydrogel of proteoglycans that fills most of the interstitial space. As an example, the density and physical organization of the collagen network found in normal mammary tissue have been relatively well characterized. By using SHG, several studies have shown the presence of collagen fibrils with a rod-like structure, measuring 67 nm in diameter in stromal regions surrounding epithelial cells [19, 21–24]. These works highlight the heterogeneous texture of the collagen networks in normal breast. Thereby, the presence of wavy, as well as straight, collagen fibers have been observed wrapping around the epithelial duct but also radiating away from the duct. Additionally, the connective tissue displays dense collagen bundles interspersed with loosely organized networks of variable widths, ranging from below 1 to 20 μm. As we will see later, these ECM determinants have profound impact on the way immune cells migrate within a tissue.
The structure of a tissue is tightly controlled by multiple regulatory mechanisms that ensure organ homeostasis, so that any deviation from the steady state induces a response aimed at rapidly restoring the integrity of the tissue. Notably, disruption of such control mechanisms must be transient in order to avoid organ dysfunction.
Alterations of matrix architecture in wound healing, fibrosis and chronic inflammation
Alterations of the matrix architecture are observed in several physiological and pathological conditions. Acute tissue injury triggers a reparation program during which the architecture of the stroma is strongly modified [25]. Multiple events are involved in repair, one of them being the recruitment to the wounded tissue of fibroblasts that become activated into myofibroblasts. These cells are actively involved in the tissue remodeling required for healing [26]. First, they exhibit increased capacities to produce and cross-link ECM proteins into fibers compared to non-activated fibroblasts [27]. Second, due to their contractile activity, myofibroblasts are able to organize matrix fibrils into linear and thick cables. This rich collagen network is beneficial to the host, as it provides a scaffold onto which other cells can migrate during tissue repair.
In normal conditions, this activation phase is transient and, once the tissue has regained its integrity, myofibroblasts die by apoptosis. However, under extreme conditions, such as prolonged tissue injury, myofibroblasts remain within the tissue leading to continuous ECM deposition, organ fibrosis, and, finally, organ dysfunction [26].
Chronic inflammation that accompanies an infection or an autoimmune disease gives rise to tissue modifications that can adopt different textures. The formation and development of fibrous or connective tissue (defined as desmoplasia) is a major pathological feature of many chronic inflammatory diseases, including autoimmune diseases such as scleroderma, rheumatoid arthritis, and Crohn’s disease [28].
Apart from regions harboring extensive collagen deposition, a variety of inflamed tissues, both in human and mice, are often heavily infiltrated with immune cells that form aggregates showing various degrees of internal organization [29–32]. In some, but not all, cases, these inflammatory infiltrates closely resemble secondary lymphoid organs (SLO), especially lymph nodes, with regard to cellular composition, organization, chemokines, and vasculature. For this reason, such organized structures induced at sites of chronic inflammation are usually referred to as tertiary lymphoid organs (TLO) [29]. According to pathologists, chronic infiltrates must fulfill a series of criteria to be named TLO, including distinct yet adjacent T and B cell compartments, the existence of functional germinal centers, and the presence, in the T cell area, of high endothelial venules (HEV), as well as a specialized subtype of fibroblasts. In both secondary and tertiary lymphoid organs, fibroblasts present within the T cell zone express contractile molecules that are normally restricted to smooth muscle cells (desmin, smooth muscle actin, etc.) and myofibroblasts of wounded and fibrotic tissues [33] (Table 1). These fibroblasts, named fibroblastic reticular cells (FRC), have been relatively well studied in secondary lymphoid organs, but less so in chronically inflamed tissues. These cells are responsible for building a 3D network that is highly interconnected via cell–cell contacts (Fig. 2, left panel). In SLO, FRC produce multiple ECM components that are specifically assembled around a core of collagen fibers and disposed in microvessels, called conduits, capable of transporting small molecules [34, 35]. Remarkably, unlike other tissues where the ECM produced by fibroblasts surrounds the cells, matrix fibers of the lymph nodes are ensheathed by FRC. As a result, most of the conduit system is shielded from lymphocytes within the T cell zone. By analogy, this 3D network, composed of FRC wrapping ECM fibers, structurally resembles the skeleton of a sponge, where large gaps between fibers, ranging between 15 and 20 μm, are completely filled with lymphocytes [36]. Although cells with the characteristic of FRC have been observed in TLO, their functions in chronically inflamed tissues are unclear. Additionally, the presence of conduits has been reported in only a few studies [30, 32], raising the question as to whether such structures are hallmarks of TLO.
Table 1.
Key features of reticular fiber and fibrotic networks
| Reticular fiber network | Fibrotic network | |
|---|---|---|
| Physical characteristics | ||
| Tissue gaps | 15–20 μm [7, 36] | <5 μm [7, 19] |
| Disposition of matrix fibers | In conduits, ensheathed by FRC [35] | Linear and parallel [7, 22, 40] |
| Phenotype of fibroblasts | Among many genes, FRC express αSMA, gp38, CCL19, CCL21, IL-7 [33] | Among many genes, fibroblasts found in fibrotic networks express αSMA, PDGF receptor-β, CXCR4, FAP, CXCL12, caveolin-1, LOX [46] |
| Presence of T cells | Numerous T cells filling gaps between fibers [36] | Few T cells, very elongated along ECM fibers [7] |
| Tissue localization | Secondary and tertiary lymphoid organs (chronically inflamed tissues, solid tumors) [30] | Wounded tissues, fibrosis, solid tumors [25] |
| Effects on T cells | Promotes chemokine-dependent migration, facilitating encounter with DC [100] | In tumors, prevents T cells from contacting malignant cells [7] |
Fig. 2.
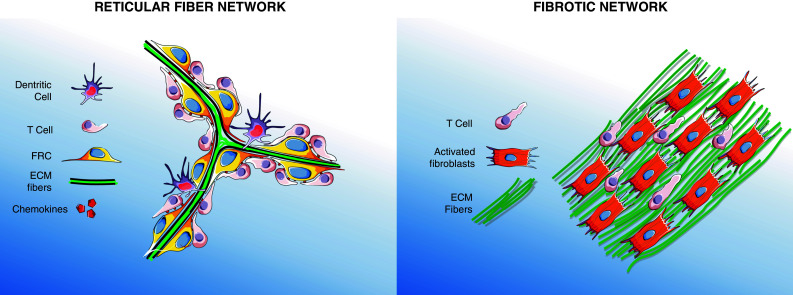
Characteristics of reticular fiber and fibrotic networks. The reticular fiber network of secondary and tertiary lymphoid organ is composed of light and spaced fibers made by FRC. This network provides a guidance path for T cells that migrate rapidly onto FRC coated with chemokines (left). Fibrotic networks are characterized by dense and parallel matrix fibers generated by activated fibroblasts. Fibrotic networks are unfavorable migration zone for T cells which hardly move between dense fibers (right). Features of both networks are described in Table 1
Matrix architecture of tumors
Tumors are known as “wounds that do not heal” [37]. Moreover, malignant sites are usually densely infiltrated by immune cells. Consistently, the structure of the stroma of most solid tumors shares similarities with that found in both healing tissues and chronically inflamed organs. Strong synthesis and deposition of the ECM is a hallmark of many solid tumors [38]. Besides these quantitative changes, qualitative modifications also take place. Indeed, matrix fibers are not as curvy and relaxed as they are in healthy tissues, but become thicker and linearized [39] (Fig. 2, right panel). Another peculiar feature of the matrix fibers found in the tumor stroma is their high order of alignment. Indeed, thick, linear and parallel matrix fibers are often observed close to neoplastic cells, around tumor islets (Fig. 1c, d). In human lung tumors, vessels are also surrounded by thick and linear ECM fibers [7].
Progressive modifications in the spatial alignment of collagen fibers around the tumor islets have been well studied in the spontaneous MMTV-Neu and MMTV-PyMT mammary tumor models and in human breast tumors [22, 40]. During tumor growth, straight collagen fibers are organized concentrically around the tumor nests to form ring structures. Similar disposition of matrix fibers has also been described in human lung tumors and in tumors from melanoma metastasis [7, 41]. In advanced breast tumors, collagen fibers are no longer parallel to the tumor cell regions, but instead lead directly into the tumor mass [22, 40].
Excessive ECM deposition and remodeling are not the only features that characterize the tumor stroma. Recent studies have unraveled the presence of lymphoid-like structures in several human tumors including lung, breast, colorectal, and melanoma cancers [42–44]. These structures share several features with those of secondary lymphoid organs, as they are characterized by the accumulation of B and T cells in adjacent areas, mature dendritic cells, and HEV. They also contain homeostatic chemokines, such as CCL19 and CXCL13, that are instrumental in controlling the positioning of naïve T and B cells [45]. In addition, the presence of germinal centers in some follicles suggests that adaptive immune responses take place in these structures [42]. Although the nature of the fibroblasts, the matrix composition, and the precise topography of these structures are still ill-defined, they are most likely composed of a reticular fiber network similar to that present in secondary lymphoid organs. The distribution of these well-delineated, rather small structures in relation to tumor cells is also a matter of debate. In human lung tumors, these lymphoid aggregates are often located far from carcinoma regions, at the boundary between the healthy tissue and the tumor stroma [45].
Regulation of tumor ECM architecture
Fibroblasts in tumors share several similarities with those found in fibrotic tissues, which is not surprising considering the progressive accumulation of ECM at malignant sites [46]. Both tumor-infiltrating and fibrotic fibroblasts are activated by mechanical and soluble factors. In vitro studies have highlighted the participation of a stiff environment, as well as of the interstitial pressure, in the maintenance of an activated fibroblast phenotype that leads to ECM production and parallel positioning of fibers, as observed in tumors [47, 48]. In addition, multiple soluble factors like TGF-β have been described to stimulate and maintain a myofibroblast phenotype [46]. Therefore, the dense and linear matrix fibers surrounding tumor islets most likely reflect tumor-induced fibroblast activation, mediated by a mechanical tension due to the outward growth of tumor cells, as well as by the soluble factors they produce. Moreover, it is becoming evident that several types of fibroblasts are present within the tumor stroma. The excessive deposition of the ECM most likely reflects a population of highly activated fibroblasts. On the other hand, fibroblasts distributed within lymphoid structures of the tumor stroma may share phenotypical and functional characteristics with lymph node FRC. Indeed, comparison of tumor fibroblasts and FRC transcripts reveals a high degree of homology between both cell types [49].
With regard to the cellular and molecular mechanisms that lead to intratumoral lymphoid structure formation, many questions have to be answered. In lymph nodes, it is established that lymphoid tissue inducer (LTi) cells contribute to the organogenesis by expressing lymphotoxin α, a member of the tumor necrosis factor family [31]. Whether LTi and lymphotoxin also participate in the formation of lymphoid aggregates within tumors is currently not known. However, it has been suggested that myeloid cells, in chronically inflamed tissues and tumors, could play the role of LTi for lymph nodes [49].
Besides myofibroblasts, the architecture of the tumor stroma also depends on several types of immune cells that release activating cytokines and growth factors. Among these cells, myeloid cells are often massively recruited to the tumor site and are associated with functional impairment of the adaptive immunity and structural alterations of the microenvironment, such as neoplastic invasion and vascular/lympho-angiogenesis. Myeloid cells at the tumor site mainly comprise tumor-associated macrophages and monocytes, like pro-angiogenic Tie-2-expressing monocytes, tumor-associated neutrophils, and myeloid-derived suppressor cells, that are in turn composed of both granulocytic and monocytic subsets, as already reviewed elsewhere [50–53].
Even if the role of these cells in the direct production and the structural organization of matrix fibers in tumors has not yet been clarified, experiments performed in fibrotic tissues have demonstrated the pivotal participation of myeloid cells in fibroblast activation and matrix deposition. Among these, several reports in mouse liver fibrosis attest the role of macrophages in the activation of fibroblasts during the injury phase, mainly through the release of TGF-β [54–57]. Macrophages have also been shown to be crucial for collagen deposition during pulmonary fibrosis, mainly through an IL-13-driven TGF-β production [58]. Moreover, in the developing mammary gland, macrophages are found in close association with collagen fibers, and their depletion leads to impaired organization of fibrillar collagen around terminal end buds, without affecting the global collagen production [59]. In support of the role of monocytes/macrophages in the development of fibrosis, CCR2 inhibitors or CCR2 deficiency in mice have proven effective in reducing monocytes/macrophages recruitment and renal interstitial fibrosis. This response was paralleled by a decrease in the expression of CCL2, TGF-β, and collagen I [60].
The impact of myeloid cells in matrix deposition in tumors is less clear, but evidence exists for the participation of macrophages to the epithelial–mesenchymal transition (EMT) of cancer cells, a TGF-β-dependent phenomenon. Indeed, in human lung tumors and in several mouse tumor models, a positive correlation has been noted between macrophage density and a mesenchymal phenotype of tumor cells; besides, macrophage-derived TGF-β was able to induce EMT in vitro in these tumor cells [61, 62]. Thus, by promoting the EMT and the development of ECM-producing cells, myeloid cells can presumably control tumor matrix architecture.
Matrix metalloproteinases produced by several cell types, including tumor cells and myeloid cells, can also modify the tumor stroma topography. These enzymes have long been described for their ability to degrade a number of substrates, including ECM components. By degrading the ECM, MMPs can also release pro- and anti-angiogenic molecules, thus affecting angiogenesis at the tumor site. Moreover, they also participate in the release and activation of growth factors anchored onto the ECM, including TGF-β [18]. TGF-β can, in turn, augment the activation of MMP-9, resulting in tumor invasion and a possible positive feedback loop for the production of this growth factor [63]. However, their role in facilitating invasion and metastatic spreading of tumor cells has been challenged by the failure of MMP inhibitors to increase survival rate in cancer patients [18]. It is now clear that these enzymes do more than degrade physical barriers, as they also affect multiple signaling pathways that may either suppress or promote tumorigenesis.
Tumor stroma architecture and prognosis
Along with the accumulation of activated fibroblasts [64–66], alterations in the production and deposition of ECM components in the tumor stroma have been frequently associated with a poor prognosis. Indeed, in different types of carcinomas, an increased amount of matrix molecules, such as fibronectin [67], hyaluronan [68–70], or collagen [71], is related to a worse prognosis. Of even more interest, some works have analyzed the spatial organization of these matrix fibers, showing that neoplastic transformation and progression can be linked to particular structures of the stroma. A study in murine mammary tumors demonstrates that the orientation of collagen fibers around tumor cells, defined as tumor-associated collagen signature (TACS), is related to cancer growth and spreading. In this report, TACS-1 is characterized by dense clusters of curly collagen around epithelial cells, TACS-2 by straight fibers with tangential orientation relative to the tumor edge, and TACS-3 by collagen fibers orientated perpendicular to tumor boundary regions. TACS-3 is associated with invasive tumors, and collagen fibers at this stage are aligned in the direction of cell invasion [22]. More importantly, TACS-3 has been shown to be an independent indicator of unfavorable prognosis in human breast cancer [40]. Similar findings have been obtained in human ovarian cancer, where the study of collagen by SHG reveals that tumor biopsies have higher collagen density and more organized structure, with more densely packed fibrils, compared to normal tissue [72]. Though the exact cause–effect relationship of this stromal reorganization around tumor cells has not been clarified, high collagen density seems to favor the proliferation and the invasive behavior of neoplastic cells, likely by altering Rho signaling that mediates epithelial cell contractility [23, 73]. A recent work has also highlighted how LOX-induced collagen cross-linking, and thus ECM stiffening, can enhance focal adhesions and PI3 K signaling, promoting ErbB2-dependent tumor invasion in mammary tumors [21].
Of importance, the level and structural organization of matrix fibers present within the tumor stroma can also influence the response to therapy by regulating drug delivery. A fibrous stroma around tumor islets has indeed been shown to act as a steric barrier for large molecular weight drugs [74]. Besides this “wall-like” barrier, a dense stroma also contributes to high interstitial fluid pressure within the tumor, compromising the penetration of anti-cancer drugs [75].
Impact of the ECM on immune cell behaviors
Besides the effect exerted on tumor cells, the physical and biochemical properties of the ECM are also able to modulate a number of processes in immune cells, especially lymphocytes, that can ultimately lead to inefficient tumor killing. ECM determinants can indeed promote tumor evasion from the immune system, both by inhibiting the anti-tumor effector activity of T cells, either directly or through the recruitment of immunosuppressive cells, and by limiting cell contact with tumor cells.
Along with collagen and fibronectin, some non-structural ECM proteins are often upregulated during tumor development and can modulate cell–cell and cell–ECM interactions, eventually restraining T cell activation [76, 77]. For instance, thrombospondin-1 has been recently shown to induce regulatory T cells and impair DC maturation in a melanoma model [78], while tenascin C seems to directly inhibit T cell proliferation and IFN-γ production in lung cancer [79]. The glycoprotein SPARC seems to influence the trafficking and the function of immune cells, as SPARC-knock-out mice display an increased number of macrophages and neutrophils in tumors and higher cytotoxicity in polymorphonuclear cells [80, 81]. In addition, several ECM fragments are able to recruit and activate macrophages and neutrophils to sites of inflammation, where they could regulate inflammation and adaptive responses [82, 83]. In two different models of pulmonary inflammation, elastin fragments and collagen-derived N-acetyl Pro-Gly-Pro (PGP) peptide were shown to recruit monocytes [84] and neutrophils [85], respectively, to the lungs of mice. Moreover, some of these molecules, such as tenascin C in rheumatoid arthritis [86], hyaluronan in acute lung injury [87], and biglycan in sepsis [88], can activate myeloid cells through TLR2/TLR4 binding. Even though only macrophage recruitment by hyaluronan has been studied in tumors (as described later), all these findings are likely relevant for cancer biology, given the importance of myeloid cells and TLR2/TLR4 activation in the suppression of the anti-tumor lymphocytes they mediate [89]. In summary, the biochemical determinants of the ECM affect many aspects of immune cell behaviors, but the role of its physical properties on the migration and function of T cells has been less explored.
Mode of T cell motility and influence of the matrix architecture
Like other immune cells, T cells harbor a specialized migratory behavior, which has been termed “amoeboid”, because it follows the paradigm of movements established for the amoeba Dictyostelium discoideum. Our current understanding of this mode of migration relies on studies initially performed in 3D collagen matrices [90]. There, T cells were found to migrate rapidly, with constant and vigorous shape changes characterized by the formation of a ruffling leading edge and a trailing uropod. This mode of migration contrasts with that of slow-moving cells such as fibroblasts. Studies performed by Peter Friedl and his colleagues have provided clear evidences for a non-proteolytic migration of T cells [91]. Accordingly, T cells have been shown to adapt their morphology and even deform their nucleus in order to flow through narrow gaps. However, prohibitive matrix density forces lymphocytes to change their direction towards paths of least resistance. Apart from shape adaptation, imaging experiments indicate that T cells migrate along pre-existing matrix structures, a process known as “contact guidance”. Strikingly, this guiding strategy is not, or only partially, dependent on adhesive interactions [92]. From these studies performed in culture systems, it was assumed that, in intact tissues, regions of loose ECM network could provide favorable migration zones for T cells, whereas dense networks of ECM could have detrimental effect on T cells, forcing them to migrate in regions of looser densities. These observations also suggest that the spatial alignment of the guiding fibers will have a profound effect on the way T cell localize and migrate within a tissue, either favoring or limiting their encounter with certain types of cells.
T cell migration in lymphoid organs
Since 2002, the ability to directly image immune cells in their native habitat has become possible using multi-photon microscopy [93]. Initial studies were aimed at imaging T cells in murine lymph nodes [94]. Once T cells enter a lymph node, they exhibit an intense and sustained migration that, although initially thought to be random, is strongly influenced by the FRC network within the T zone [95]. By analyzing the dynamic behavior of T cells in relation to stromal cells, Bajénoff et al. [36] have demonstrated that T cells actively follow the paths laid out by the FRC network, and that the frequent turns made by the lymphocytes are associated with corresponding turns of the supporting fibers. Besides this physical guidance, FRC provide chemical cues to T cells by secreting CCL19 and CCL21 chemokines, ligands of CCR7 expressed by naïve T cells and mature dendritic cells [33]. These chemokines are important since, in their absence, T cells migrate at significantly reduced speed [96–98]. Knowing the capacity of CCL21 to bind to the cell surface, our current understanding of T cell trafficking in lymph nodes is that the reticular fiber network provides a guidance path for lymphocytes, that migrate rapidly onto FRC coated with CCL21, presumably enabling T cells to contact multiple dendritic cells [99, 100]. Thus, by their highly organized architecture, the lymphoid organs provide a “road system” on which T cells can migrate and detect rarely expressed antigens, triggering their proliferation and differentiation into effector cells. It is hypothesized that such a guiding strategy has direct positive effects on the immune surveillance [101].
The importance of a functional FRC network for T cell migration and function is stressed by the fact that several infections mediate marked lymph node alterations. For instance, lymph nodes from HIV individuals show a loss of FRC network, resulting from chronic immune activation and excessive collagen deposition, that leads to reduced IL-7 secretion by FRC and apoptosis of naïve T cells [102, 103].
T cell migration in inflamed tissues
The use of intravital microscopy has recently revealed the behavior of T cells in inflamed tissues [93]. For example, brain infected with the protozoan parasite Toxoplasma gondii massively recruits effector memory T cells that exhibit an exploratory behavior comparable to that of naïve T cells within lymph nodes. Visualization of fluorescent T cells in relation to collagen structures assessed by SHG indicates that lymphocytes actively crawl onto a reticular network of fibers that present analogies with that of lymphoid organs [104]. Similarly, T cells in the immunized mouse ear migrate along collagen networks generated during the inflammation [105]. In this situation, it remains to be clarified whether T cells migrate directly onto the collagen fibers or onto the fibroblasts that enwrap the matrix fibers, as in lymph nodes. Thus, a chronic inflammation triggers an intense remodeling of the matrix architecture, leading to the formation of a reticular network of fibers that provides structural and presumably chemical support for lymphocyte migration.
T cell migration in tumors
Two studies published in 2006 and 2007 described for the first time the migratory behavior of T cells in murine transplantable tumors [106, 107]. At these sites, adoptively transferred fluorescent T cells actively migrated at the periphery of the tumor before making stable conjugates with tumor cells. Then, T cells regained motility and progressively infiltrated the tumor. During the phases of T cell migration, occasional T cell movements along collagen fibers or blood vessels have been reported. These studies are remarkable in that they detail the motility of T cells in live and intact tumors during their rejection. However, it is clearly recognized that transplanted tumors do not reproduce the structure of most human solid tumors, characterized by well-delineated tumor islets surrounded by a reactive stroma.
Recent studies using spontaneous tumor models that are more representative of naturally occurring human diseases have imaged immune cells in relation to tumor cells [108, 109]. In the MMTV-PyMT breast carcinoma model, T cells, DC, and other immune cells were found concentrated in the peri-tumoral region, but the importance of the matrix architecture in controlling the distribution and migration of these cells within the tumor was not investigated.
Likewise, in human solid tumors, T cells are rarely in contact with tumor cells but are greatly enriched in the stroma. For example, in lung tumors, 5–10 times more T cells were found in the stroma compared to tumor islets [7–9]. Different explanations can be envisaged to explain the paucity of T cells in tumor islets, including a reduced expression of T cell retention and migration factors by tumor cells. Indeed, keratinocytes from human skin tumors exhibit reduced expression of CCL27, a potent attractor of T cells toward the skin [12]. Intratumoral chemokines can also be modified by reactive nitrogen species, which leads to defective recruitment of T cells to tumor islets [11]. Additionally, molecules acting as chemorepulsive agents may also participate in limiting T cells from entering tumor islets.
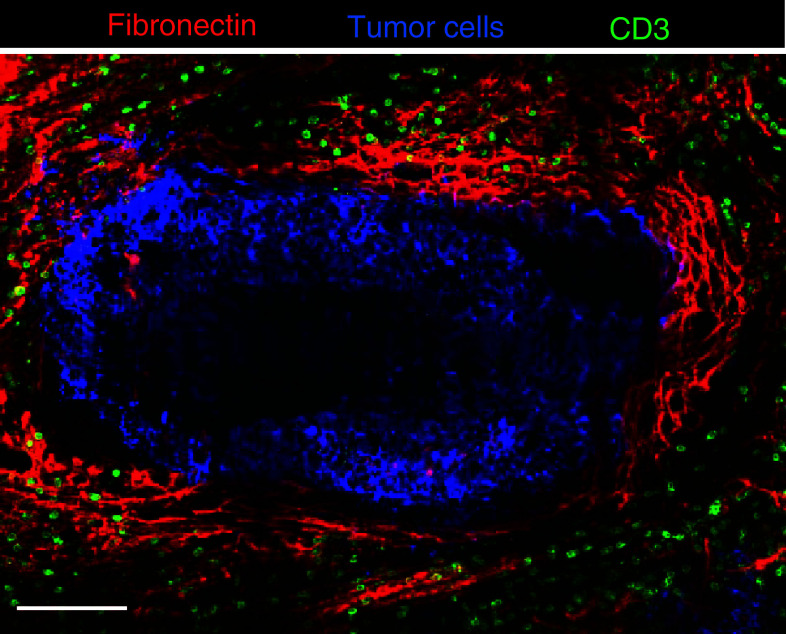
Apart from features that depend on tumor cells, the motile behavior of T cells and their capacity to infiltrate tumor cell regions are also controlled by stromal components, especially the density and the orientation of ECM fibers. By systematically imaging fluorescent T lymphocytes added to 400-μm-thick human lung tumor slices, we have recently reported that overlaid T cells behave like TIL already present in the resected tumor, as they migrate preferentially into the stroma and very rarely into tumor islets [7]. The visualization of T cells in relation to matrix fibers indicates that the stroma is heterogeneous, and composed of different territories in which T cell motility is either favored or restricted. Active T cell motility dependent on chemokines occurs in well-delineated stromal regions, characterized by a loosely organized network of matrix fibers and reminiscent of the reticular fiber networks of secondary lymphoid organs. Besides these favorable migration zones, other stromal zones are composed of dense matrix fibers containing very few T cells that exhibit limited and constrained displacement. Strikingly, dense and linear matrix fibers are frequently found around tumor islets with a majority of fibers being parallel to the tumor boundary (Fig. 3). In these peritumoral regions, T cells introduced into slices migrate mainly along fibers, therefore tangentially to the tumor/stroma border, and very rarely towards the neoplastic cells [7]. Thus, the dense and linear matrix fibers surrounding tumor islets can be seen as a cocoon shielding tumor cells from effector T cells. This obstacle furnishes an additional explanation for the tumor-promoting effect of a deregulated ECM.
Fig. 3.
Distribution of T cells in relation to fibronectin fibers and tumor cells in a human lung tumor. Tumor islets (blue, stained for EpCAM) are surrounded by dense and tight fibronectin fibers (red, stained for fibronectin). T cells (green, stained for CD3) are sparse in regions immediately adjacent to the tumor mass but concentrated in light matrix fiber areas. Bar 100 μm
Apart from a physical hindrance effect exerted by collagen-rich tissue, an inappropriate guidance of T cells migrating along the fibers, organized concentrically around tumor islets, will also restrain lymphocytes from contacting tumor cells. A guiding strategy is supposed to control the migratory behavior of T cells in lymphoid organs and our current understanding is that the reticular fiber network provides a guidance path for lymphocytes that migrate rapidly onto FRC coated with CCL21. Thus, a contribution of this “road system” to the displacement of T cell around tumor islets can reasonably be hypothesized. Whereas in LN this guidance strategy favors the detection of antigens expressed at the surface of DC, in tumors this process would guide T cell away from cancer cells. At present, the nature of these T cell guidance molecules in the tumor stroma remains to be characterized. The relatively weak effect of pertussis toxin, which ablates chemokine signaling, on T cell motility measured in regions adjacent to tumor islets is suggestive of the participation of molecules other than chemokines to this process [7]. Candidate guidance molecules for T cells in these regions include cytokines and in particular IL-2, that, in vitro, is able to trigger cytoskeletal changes in activated, but not naïve T cells [110].
The notion that dense and linear matrix fibers surrounding tumor islets hinder T cells from contacting tumor cells has been put forward in other studies performed in animal and human models. By comparing the structure of the stroma and the distribution of T cells in different types of rat colorectal tumors, Lieubeau et al. [111] have demonstrated a correlation between the presence of activated fibroblasts surrounding tumor islets and the absence of T cells in these compartments. Notably, tumors with highly activated fibroblasts were not eliminated as efficiently as tumors with a poorly developed myofibroblastic reaction. Similar results were also obtained in murine breast tumors [112]. In human gastric carcinoma, a dense collagen stroma that predicts poor patient survival, was correlated with fewer CD8+ T cells in tumor islets [113]. Similarly, cervical carcinomas with high amount of versican—a large ECM proteoglycan—in the stroma contain statistically fewer CD8+ T cells in the tumor islets as compared to tumors with low versican expression [114].
Of note, these studies are correlative, and at the present moment we do not know whether the ECM determinants listed above are directly responsible for the poor capacity of T cells to contact and kill tumor cells. It is becoming evident that the distribution and migration of T cells within a tissue are controlled by a combination of structural and biochemical environmental factors [15]. Tumors have been linked to wounds that fail to heal, and indeed the tumor stroma exhibits changes usually found in unresolved wounds, including extensive ECM remodeling. Of importance, these stromal modifications result from cooperative mechanisms in which the ECM plays an important role. As stated previously, a number of ECM glycoproteins such as Tenascin C and versican, that are upregulated during tumor growth, participate in the recruitment of immune inflammatory cells that, through the release of MMP and fibrotic and immunosuppressive cytokines like TGF-β, can amplify the desmoplastic reaction. As a result, the stromal microenvironment of late stage tumors exhibits both structural and biochemical changes that negatively influence the anti-tumor immune response. Likewise, the migration of T cells and their capacity to interact with tumor cells are likely to be altered in advanced tumors, and, along with a deregulated ECM, several components could be involved in this dysfunction. This includes metabolic stressors such as low pH, amino acid deficiency, and hypoxia. As an example, a lack of oxygen is a common feature of growing tumors that could affect T cell migration, since this phenomenon has previously been shown to be strongly oxygen-dependent in lymph nodes [115]. Clearly, further experiments are required to establish the participation of these stromal components in controlling the distribution and migration of T cells in tumors.
With respect to the different ECM signatures found in tumors and their impact on T cells, a reorientation of matrix fibers has been noted in breast tumors. Whereas in progressive tumors collagen fibers are parallel to tumor islets, in late stage breast tumors, fibers are aligned perpendicular to the tumor/stroma boundary [40]. At present, no data is available on the behavior of T cells in advanced breast tumors, but, based on the guiding mechanisms described previously, one may hypothesize that perpendicular fibers, by helping T cells to contact tumor cells, promote tumor destruction. In fact, the findings of Conklin et al. [40] provide the evidence that breast cancer patients whose matrix fibers lead directly into the tumor mass show a poor survival rate. Even if perpendicular collagen fibers guide lymphocytes to contact tumor cells, we can presume that this positive effect will be counteracted by a hostile environment for T cells such as that of advanced tumors, which will ultimately limit their anti-tumor functions.
A cooperation between structural and biochemical determinants can also give rise to lymphoid-like structures that, unlike the fibrotic stroma of growing tumors, promotes efficient immune surveillance and anti-tumor immunity [42–44]. Homeostatic chemokines CCL19 and CCL21, instrumental in promoting T cell migration within lymph nodes, have been found in lymphoid aggregates of human lung tumors. Although the topography of the ECM in such areas remains to be determined, the active and chemokine-dependent T cell migration that has been observed in loose matrix areas of human lung tumors suggests the presence of a road systems composed of conduits and fibroblasts coated with guidance molecules.
In conclusion, the tumor stroma is a dynamic milieu in constant evolution, composed of multiple territories that greatly influences T cell migration and function. The ECM, with its physical and chemical properties, is an active player in the construction and function of these territories. Under fibrotic conditions, dense and linear collagen fibers act with other repair-related determinants to suppress immune function. Conversely, a reticular fiber network, together with T cell pro-migratory factors, favors immune surveillance and anti-tumor activities.
Matrix architecture and other immune cells
Immune cells other than T cells are also influenced in their migration by the topography and composition of the matrix architecture. For instance, studies performed in 3D collagen matrices have demonstrated that macrophages can adopt distinct migratory mechanisms according to the extracellular environment. In fibrillar collagen, macrophages employ a non-proteolytic amoeboid migration mode, but in dense ECM, such as gelled collagen I or Matrigel, they migrate in a protease-dependent mesenchymal mode [116]. Interestingly, the migratory behavior of cells of the mono-macrophage lineage seems to differ, at least in vitro, according to their differentiation and activation state; indeed, while monocytes are able to migrate along fibrillar collagen but not through Matrigel matrices, non-polarized and IL-4-stimulated M2 macrophages display both migration modes, whereas IFN-γ- or TNF-α-polarized M1 macrophages seem motionless in these contexts [117].
The notion that different subtypes of macrophages exhibit different migratory behavior according to their localization relative to the stroma has been confirmed in vivo using intravital microscopy in the MMTV-PyMT carcinoma model. Indeed, while phagocytic CD206+ macrophages and monocytes in the stroma and in peritumoral areas are mostly sessile cells, non-phagocytic Gr-1+ cells of the monocytic lineage in the same areas are motile; moreover, CD206− mono-macrophages inside the tumor mass are non-migratory [108]. In addition, intravital imaging experiments have provided evidence for a close association between macrophages and collagen fibers during both mammary gland development and murine mammary tumor invasion [59, 118]; in particular, in the former study, macrophages have been found to crawl on collagen matrix and between the fibers [59].
Apart from interacting with collagen, macrophages are also recruited by and can adhere to hyaluronan, an ECM proteoglycan associated with poor prognosis in several human cancers. Indeed, in a mammary tumor model, hyaluronan, together with versican, has been shown to mobilize macrophages to stromal regions. Depletion studies also suggest that macrophages are involved in the maintenance of hyaluronan-rich structures [119]. Moreover, in a model of lung viral infection, versican was shown to be crucial for monocyte binding to hyaluronan [120].
Similarly to T cells and monocytes, neutrophils—both blood-derived and thyoglicollate-induced—have been observed to migrate on fibrillar collagen but not in dense gelled collagen in vitro [117]. The amoeboid mode of displacement of neutrophils has been confirmed in vivo in lymph nodes of Toxoplasma gondii-infected mice, where these cells, recruited to subcapsular sinuses, have been observed to interact with collagen fibers and macrophages [121], but there are currently no studies addressing their migration in the tumor microenvironment. Given that tumor-infiltrating cells, especially those belonging to the myeloid lineage, greatly influence T cell-mediated anti-tumor immunity, it will thus be important to shed light on their migratory behavior in the tumor microenvironment.
Shifting the balance: strategies to improve antitumor surveillance of T cells
Abnormal ECM is known to promote cancer progression by various means, notably by facilitating cellular transformation and metastasis [18] and by compromising T cell infiltration into tumor islets [7]. In addition, anti-cancer drug penetration is also hindered by a dense connective tissue [74]. Therefore, targeting tumoral ECM is becoming extremely attractive, and several strategies are being developed. The two main approaches currently under evaluation attempt, on the one hand, to decrease the dense ECM network around the tumor and, on the other hand, to favor the formation of lymphoid structures that could facilitate T cell migration and functions (Fig. 4).
Fig. 4.
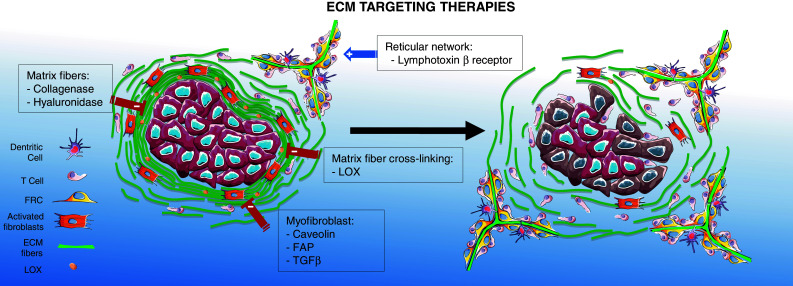
Promising targets for rendering the tumor stroma more permissive to T cells. Strategies to decrease the dense ECM network include the inhibition of collagen cross-linking enzymes such as LOX, the inhibition of fibroblast activation and the degradation of pre-existing matrix fibers. Strategies to promote the development of reticular fiber networks include the targeting of lymphotoxin β receptor
Recent studies performed in mouse tumor models have identified several targets that hold promises in the control of matrix fiber formation. Among these, the lysyl oxidase enzyme, that cross-links collagen fibers, and whose expression is upregulated in several human tumors, is particularly interesting [122]. Importantly, mammary cancer progression can be slowed, and metastasis suppressed, by inhibiting LOX activity using systemic injection of chemical inhibitors or soluble antibodies [21]. Targeting LOX activity might also prove beneficial in the treatment of fibrosis, and a humanized monoclonal antibody against LOX-Like-2, which belongs to the LOX family, is being explored as a therapy for cardiac and liver fibrosis [28].
Because myofibroblasts are key cells in the production and remodeling of the ECM, several attempts have also been made to reduce fibroblast recruitment, activation, and function. In this context, an interesting target, specifically expressed at the surface of tumor myofibroblasts, is caveolin-1. In caveolin-1 knock-out mice, the ECM of mammary carcinoma tumors is characterized by reduced stiffness and reduced linear ECM fibers [123]. As a result, mice deficient for caveolin-1 show a remarkable inhibition of tumor growth and metastasis. Fibroblast activation protein α (FAP) is another promising target selectively expressed in tumor stroma myofibroblasts [124]. Interestingly, selective elimination of FAP-expressing cells, using transgenic mice that express the diphtheria toxin receptor under the control of the FAP promoter, was indeed shown to reduce tumor growth by a process involving an antitumor immune response [125].
A number of cytokines and growth factors are associated with myofibroblasts activation and ECM deposition. Among them, TGF-β, which is often upregulated in cancer and linked to worse prognosis, plays a primary role in the desmoplastic response of the stroma, as previously described. Indeed, as recently reviewed in [126], many inhibitors of TGF-β (antisense oligonucleotides, antibodies, small molecule inhibitors) are currently being evaluated in clinical trials for the normalization of the matrix in both cancer and fibrosis. A very recent report describes the beneficial anti-fibrotic effect of an anti-TGF-β strategy in murine mammary carcinoma, highlighting the role of this cytokine in vessel and ECM alterations that lead to abnormal perfusion, high interstitial pressure, and inefficient drug delivery [127]. Therapeutic interventions aimed at regulating TGF-β signaling pathway are particularly interesting because this cytokine can contribute to tumor escape not only by altering the structural microenvironment but also by directly inhibiting innate and adaptive immunity. However, although promoting distal spreading and immunosuppression in the late phases of tumor growth, this cytokine can restrain neoplastic development in the initial phases of transformation (as reviewed in [128, 129]). Thus, given the pleiotropic effects of TGF-β in the context of tumor growth, these approaches have to be carefully evaluated in order to avoid undesired toxic, or even pro-tumoral, side effects.
The direct degradation of matrix fibers might also prove beneficial in the treatment of fibrotic tumors. By treating human lung slices with collagenase, we have shown that a reduction in collagen content enhances the number of plated T cells in contact with tumor cells [7]. Other investigators have used matrix modifiers, such as collagenase and MMP, to degrade the ECM in order to improve intratumoral penetration of drugs and oncolytic viruses. Hence, treatment with collagenase was found to increase the penetration of viral particles into mouse solid tumors, resulting in reduced tumor growth [130]. In human, the only approved therapeutic use of collagenase is for the treatment of a rare fibrotic proliferative disease, Dupuytren’s disease, in which locally administrated Clostridium collagenase is currently utilized [131, 132]. However, due to the ubiquitous presence of collagen in tissues, only a tumor-targeted approach could be envisaged for the treatment of cancer patients.
With respect to MMP, how degradation of the ECM by these proteases could affect lymphocyte migration at the tumor site, and thus tumor killing, has not been extensively studied. As stated previously, MMP function is more complex than initially thought, and, besides the degradation of putative physical barriers, these enzymes also affect, directly or indirectly, multiple signaling pathways that may modulate the function of T cells. Thus, in vitro and in vivo studies are needed to clearly establish the relevance of using MMP to reinstate a stromal structure favorable to T cell migration.
As previously discussed, hyaluronan is another interesting target in solid tumors, despite the complexity linked to the fact that fragments of different lengths can produce contrasting effects on immune cells including T cells [87, 133]. In a recent study, it was shown that the administration of a hyaluronan-targeting enzyme in mice bearing a spontaneous pancreatic tumor improved the penetration of a number of chemotherapeutic molecules, leading to a decrease in the metastatic tumor burden [134]. Intuitively, one can imagine that the degradation of dense matrix networks facilitates tumor cell migration and dissemination. Although recent evidence links tissue rigidity to alterations in cancer phenotype and tumor progression [38], more experiments are needed to establish the relationship between ECM density and tumor cells.
Secondary and tertiary lymphoid tissues provide a favorable environment for activating cellular immunity. Therefore, several attempts have been made to promote the development of such structures in tumors and to examine their consequences on tumor growth. In several mouse tumor models, the induction of lymphoid structures through the stimulation of lymphotoxin β receptor, a pathway engaged during lymph node organogenesis, results in the eradication of tumors, in agreement with the studies on human tumors linking the presence of such structures to good prognosis [135, 136].
However, the rationale of inducing lymphoid structures in tumors have been recently questioned by a controversial study showing that formation of lymphoid aggregates following CCL21 expression by mouse melanoma cells led to tumor progression instead of tumor regression [137]. Importantly, the structures formed under these conditions did not exhibit stereotyped characteristics of secondary and tertiary lymphoid tissues. Shields et al. [137] observed that lymphoid structures induced upon CCL21 expression were devoid of B cells, but contained regulatory T cells and myeloid-derived suppressor cells. Thus, a new challenge in the years to come will be to develop therapeutic approaches to improve the formation of structures facilitating the recruitment and migration of tumor-specific T cells, without recruiting immunosuppressive cells.
Conclusions and perspectives
Recent years have seen the emergence of novel cancer immunotherapies based on our increasing knowledge of molecules involved in the regulation of T cell responses. This has led to the development of several monoclonal antibody-based therapies, such as anti-CTLA-4 or anti-PD-1, that provide clinical benefit in several cancers [138]. Although some patients have shown an impressive survival response, response rates usually remain low, and it is now well accepted that multiple mechanisms suppressing antitumor immune functions take place in the tumor microenvironment. Abnormal ECM, as observed in solid tumors, can be considered another important player of the tumor stroma involved in blocking T cells in their antitumor activities. Here, we have reviewed the important advances that have been recently made in the study of T cell migratory behavior, highlighting the unfavorable effect of a dense cross-linked matrix on T cells. However, it is also becoming apparent that a light reticular fiber network, composed of thin and spaced fibers, may constitute an important territory that facilitates T cell migration and antitumor immune surveillance in the tumor stroma.
Despite considerable progress, many fundamental questions remain to be answered. For instance, what are the cells and factors that drive the differentiation of different subtypes of fibroblasts within a tumor, and consequently the formation of different stromal environments, which can either favor or compromise T cell antitumor activities?
Our knowledge of the physical properties of the tumor ECM mainly comes from studies performed in breast and lung tumors, where several stromal signatures have been identified. It will be important to investigate whether such matrix signatures are also present in other solid tumors and to determine their correlation with prognosis. In breast tumors, collagen fibers around tumor islets are re-oriented during tumor progression and become perpendicular to the tumor/stroma boundary. How these changes influence the distribution and migration of metastatic cells, T cells, and other immune cells still remain to be characterized.
In addition to these issues, several unanswered questions regard the influence of a stiff matrix on T cell activation and differentiation. ECM elasticity provides a major environmental cue that determines cell behavior. In this context, rigid matrices have been shown to trigger intracellular signaling pathways through mechanoreceptors, including integrin receptors [139]. How T cells sense and convert the elasticity of the tumor ECM needs to be precisely defined.
Another important point is the relationship between monocyte-derived cell populations and the matrix architecture. These cells are key regulators in various important fibrotic diseases, but we still ignore whether they control the matrix architecture in the tumor stroma. In addition, their precise localization and migratory behavior in relation to ECM components and T cells is still poorly understood. Are subtypes of myeloid cells able to migrate within dense stromal environments and eventually invade tumor islets?
Hopefully, the next few years will see deeper analysis of immune cells moving through diverse tumor environments. Relevant mouse and human tumor models, combined with powerful imaging technologies, should provide greater insights into how the matrix architecture controls the behavior of immune cells.
Acknowledgments
We thank Nadège Bercovici and Alain Trautmann for critical reading of the manuscript. We are grateful to Diane Damotte and Marie-Aude Le Frère-Belda for providing the human specimens shown in Fig. 1. This work was supported in part by grants from the Ligue Nationale Contre le Cancer and the Institut National Contre le Cancer. Ana Rivas-Caicedo is supported by an Association pour la recherche sur le Cancer postdoctoral fellowship. Elisa Peranzoni is a recipient of the Fondazione Italiana per la Ricerca sul Cancro.
Abbreviations
- 3D
Three-dimensional
- ECM
Extracellular matrix
- EMT
Epithelial-mesenchymal transition
- FRC
Fibroblastic reticular cells
- LOX
Lysyl oxidase
- LTi
Lymphoid tissue inducer
- MMP
Metalloproteinases
- SHG
Second-harmonic generation
- SLO
Secondary lymphoid organs
- TACS
Tumor-associated collagen signature
- TIL
Tumor-infiltrating lymphocytes
- TLO
Tertiary lymphoid organs
References
- 1.Pages F, Galon J, Dieu-Nosjean MC, Tartour E, Sautes-Fridman C, Fridman WH. Immune infiltration in human tumors: a prognostic factor that should not be ignored. Oncogene. 2009;29(8):1093–1102. doi: 10.1038/onc.2009.416. [DOI] [PubMed] [Google Scholar]
- 2.Kerkar SP, Restifo NP. Cellular constituents of immune escape within the tumor microenvironment. Cancer Res. 2012;72(13):3125–3130. doi: 10.1158/0008-5472.CAN-11-4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fisher DT, Chen Q, Appenheimer MM, Skitzki J, Wang WC, Odunsi K, Evans SS. Hurdles to lymphocyte trafficking in the tumor microenvironment: implications for effective immunotherapy. Immunol Invest. 2006;35(3–4):251–277. doi: 10.1080/08820130600745430. [DOI] [PubMed] [Google Scholar]
- 4.Vazquez-Cintron EJ, Monu NR, Frey AB. Tumor-induced disruption of proximal TCR-mediated signal transduction in tumor-infiltrating CD8+ lymphocytes inactivates antitumor effector phase. J Immunol. 2010;185(12):7133–7140. doi: 10.4049/jimmunol.1001157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang SF, Fouquet S, Chapon M, Salmon H, Regnier F, Labroquere K, Badoual C, Damotte D, Validire P, Maubec E, Delongchamps NB, Cazes A, Gibault L, Garcette M, Dieu-Nosjean MC, Zerbib M, Avril MF, Prevost-Blondel A, Randriamampita C, Trautmann A, Bercovici N. Early T cell signalling is reversibly altered in PD-1+ T lymphocytes infiltrating human tumors. PLoS One. 2011;6(3):e17621. doi: 10.1371/journal.pone.0017621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ohtani H. Focus on TILs: prognostic significance of tumor infiltrating lymphocytes in human colorectal cancer. Cancer Immun. 2007;7:4. [PMC free article] [PubMed] [Google Scholar]
- 7.Salmon H, Franciszkiewicz K, Damotte D, Dieu-Nosjean MC, Validire P, Trautmann A, Mami-Chouaib F, Donnadieu E. Matrix architecture defines the preferential localization and migration of T cells into the stroma of human lung tumors. J Clin Invest. 2012;122(3):899–910. doi: 10.1172/JCI45817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verdegaal EM, Hoogstraten C, Sandel MH, Kuppen PJ, Brink AA, Claas FH, Gorsira MC, Graadt van Roggen JF, Osanto S. Functional CD8+ T cells infiltrate into nonsmall cell lung carcinoma. Cancer Immunol Immunother. 2007;56(5):587–600. doi: 10.1007/s00262-006-0214-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wakabayashi O, Yamazaki K, Oizumi S, Hommura F, Kinoshita I, Ogura S, Dosaka-Akita H, Nishimura M. CD4+ T cells in cancer stroma, not CD8+ T cells in cancer cell nests, are associated with favorable prognosis in human non-small cell lung cancers. Cancer Sci. 2003;94(11):1003–1009. doi: 10.1111/j.1349-7006.2003.tb01392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frey AB, Monu N. Signaling defects in anti-tumor T cells. Immunol Rev. 2008;222:192–205. doi: 10.1111/j.1600-065X.2008.00606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Molon B, Ugel S, Del Pozzo F, Soldani C, Zilio S, Avella D, De Palma A, Mauri P, Monegal A, Rescigno M, Savino B, Colombo P, Jonjic N, Pecanic S, Lazzarato L, Fruttero R, Gasco A, Bronte V, Viola A. Chemokine nitration prevents intratumoral infiltration of antigen-specific T cells. J Exp Med. 2011;208(10):1949–1962. doi: 10.1084/jem.20101956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pivarcsi A, Muller A, Hippe A, Rieker J, van Lierop A, Steinhoff M, Seeliger S, Kubitza R, Pippirs U, Meller S, Gerber PA, Liersch R, Buenemann E, Sonkoly E, Wiesner U, Hoffmann TK, Schneider L, Piekorz R, Enderlein E, Reifenberger J, Rohr UP, Haas R, Boukamp P, Haase I, Nurnberg B, Ruzicka T, Zlotnik A, Homey B. Tumor immune escape by the loss of homeostatic chemokine expression. Proc Natl Acad Sci USA. 2007;104(48):19055–19060. doi: 10.1073/pnas.0705673104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lepelletier Y, Smaniotto S, Hadj-Slimane R, Villa-Verde DM, Nogueira AC, Dardenne M, Hermine O, Savino W. Control of human thymocyte migration by Neuropilin-1/Semaphorin-3A-mediated interactions. Proc Natl Acad Sci USA. 2007;104(13):5545–5550. doi: 10.1073/pnas.0700705104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vianello F, Olszak IT, Poznansky MC. Fugetaxis: active movement of leukocytes away from a chemokinetic agent. J Mol Med (Berl) 2005;83(10):752–763. doi: 10.1007/s00109-005-0675-z. [DOI] [PubMed] [Google Scholar]
- 15.Mrass P, Petravic J, Davenport MP, Weninger W (2010) Cell-autonomous and environmental contributions to the interstitial migration of T cells. Semin Immunopathol. doi:10.1007/s00281-010-0212-1 [DOI] [PMC free article] [PubMed]
- 16.Frantz C, Stewart KM, Weaver VM. The extracellular matrix at a glance. J Cell Sci. 2010;123(Pt 24):4195–4200. doi: 10.1242/jcs.023820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiao Q, Ge G. Lysyl oxidase, extracellular matrix remodeling and cancer metastasis. Cancer Microenviron. 2012;5(3):261–273. doi: 10.1007/s12307-012-0105-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141(1):52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wolf K, Alexander S, Schacht V, Coussens LM, von Andrian UH, van Rheenen J, Deryugina E, Friedl P. Collagen-based cell migration models in vitro and in vivo. Semin Cell Dev Biol. 2009;20(8):931–941. doi: 10.1016/j.semcdb.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown E, McKee T, diTomaso E, Pluen A, Seed B, Boucher Y, Jain RK. Dynamic imaging of collagen and its modulation in tumors in vivo using second-harmonic generation. Nat Med. 2003;9(6):796–800. doi: 10.1038/nm879. [DOI] [PubMed] [Google Scholar]
- 21.Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, Fong SF, Csiszar K, Giaccia A, Weninger W, Yamauchi M, Gasser DL, Weaver VM. Matrix cross-linking forces tumor progression by enhancing integrin signaling. Cell. 2009;139(5):891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Provenzano PP, Eliceiri KW, Campbell JM, Inman DR, White JG, Keely PJ. Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC Med. 2006;4(1):38. doi: 10.1186/1741-7015-4-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Provenzano PP, Inman DR, Eliceiri KW, Knittel JG, Yan L, Rueden CT, White JG, Keely PJ. Collagen density promotes mammary tumor initiation and progression. BMC Med. 2008;6:11. doi: 10.1186/1741-7015-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gritsenko PG, Ilina O, Friedl P. Interstitial guidance of cancer invasion. J Pathol. 2012;226(2):185–199. doi: 10.1002/path.3031. [DOI] [PubMed] [Google Scholar]
- 25.Duffield JS, Lupher M, Thannickal VJ, Wynn TA (2012) Host responses in tissue repair and fibrosis. Annu Rev Pathol. doi:10.1146/annurev-pathol-020712-163930 [DOI] [PMC free article] [PubMed]
- 26.Hinz B, Phan SH, Thannickal VJ, Prunotto M, Desmouliere A, Varga J, De Wever O, Mareel M, Gabbiani G. Recent developments in myofibroblast biology: paradigms for connective tissue remodeling. Am J Pathol. 2012;180(4):1340–1355. doi: 10.1016/j.ajpath.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hinz B. Formation and function of the myofibroblast during tissue repair. J Invest Dermatol. 2007;127(3):526–537. doi: 10.1038/sj.jid.5700613. [DOI] [PubMed] [Google Scholar]
- 28.Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med. 2012;18(7):1028–1040. doi: 10.1038/nm.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neyt K, Perros F, GeurtsvanKessel CH, Hammad H, Lambrecht BN. Tertiary lymphoid organs in infection and autoimmunity. Trends Immunol. 2012;33(6):297–305. doi: 10.1016/j.it.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Link A, Hardie DL, Favre S, Britschgi MR, Adams DH, Sixt M, Cyster JG, Buckley CD, Luther SA. Association of T-zone reticular networks and conduits with ectopic lymphoid tissues in mice and humans. Am J Pathol. 2011;178(4):1662–1675. doi: 10.1016/j.ajpath.2010.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aloisi F, Pujol-Borrell R. Lymphoid neogenesis in chronic inflammatory diseases. Nat Rev Immunol. 2006;6(3):205–217. doi: 10.1038/nri1786. [DOI] [PubMed] [Google Scholar]
- 32.Stranford S, Ruddle NH. Follicular dendritic cells, conduits, lymphatic vessels, and high endothelial venules in tertiary lymphoid organs: parallels with lymph node stroma. Front Immunol. 2012;3:350. doi: 10.3389/fimmu.2012.00350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Link A, Vogt TK, Favre S, Britschgi MR, Acha-Orbea H, Hinz B, Cyster JG, Luther SA. Fibroblastic reticular cells in lymph nodes regulate the homeostasis of naive T cells. Nat Immunol. 2007;8(11):1255–1265. doi: 10.1038/ni1513. [DOI] [PubMed] [Google Scholar]
- 34.Gretz JE, Anderson AO, Shaw S. Cords, channels, corridors and conduits: critical architectural elements facilitating cell interactions in the lymph node cortex. Immunol Rev. 1997;156:11–24. doi: 10.1111/j.1600-065x.1997.tb00955.x. [DOI] [PubMed] [Google Scholar]
- 35.Roozendaal R, Mebius RE, Kraal G. The conduit system of the lymph node. Int Immunol. 2008;20(12):1483–1487. doi: 10.1093/intimm/dxn110. [DOI] [PubMed] [Google Scholar]
- 36.Bajenoff M, Egen JG, Koo LY, Laugier JP, Brau F, Glaichenhaus N, Germain RN. Stromal cell networks regulate lymphocyte entry, migration, and territoriality in lymph nodes. Immunity. 2006;25(6):989–1001. doi: 10.1016/j.immuni.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315(26):1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 38.Lu P, Weaver VM, Werb Z. The extracellular matrix: a dynamic niche in cancer progression. J Cell Biol. 2012;196(4):395–406. doi: 10.1083/jcb.201102147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cukierman E, Bassi DE. Physico-mechanical aspects of extracellular matrix influences on tumorigenic behaviors. Semin Cancer Biol. 2010;20(3):139–145. doi: 10.1016/j.semcancer.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Conklin MW, Eickhoff JC, Riching KM, Pehlke CA, Eliceiri KW, Provenzano PP, Friedl A, Keely PJ. Aligned collagen is a prognostic signature for survival in human breast carcinoma. Am J Pathol. 2011;178(3):1221–1232. doi: 10.1016/j.ajpath.2010.11.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Soikkeli J, Podlasz P, Yin M, Nummela P, Jahkola T, Virolainen S, Krogerus L, Heikkila P, von Smitten K, Saksela O, Holtta E. Metastatic outgrowth encompasses COL-I, FN1, and POSTN upregulation and assembly to fibrillar networks regulating cell adhesion, migration, and growth. Am J Pathol. 2010;177(1):387–403. doi: 10.2353/ajpath.2010.090748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cipponi A, Mercier M, Seremet T, Baurain JF, Theate I, van den Oord J, Stas M, Boon T, Coulie PG, van Baren N. Neogenesis of lymphoid structures and antibody responses occur in human melanoma metastases. Cancer Res. 2012;72(16):3997–4007. doi: 10.1158/0008-5472.CAN-12-1377. [DOI] [PubMed] [Google Scholar]
- 43.Dieu-Nosjean MC, Antoine M, Danel C, Heudes D, Wislez M, Poulot V, Rabbe N, Laurans L, Tartour E, de Chaisemartin L, Lebecque S, Fridman WH, Cadranel J. Long-term survival for patients with non-small-cell lung cancer with intratumoral lymphoid structures. J Clin Oncol. 2008;26(27):4410–4417. doi: 10.1200/JCO.2007.15.0284. [DOI] [PubMed] [Google Scholar]
- 44.Martinet L, Garrido I, Filleron T, Le Guellec S, Bellard E, Fournie JJ, Rochaix P, Girard JP. Human solid tumors contain high endothelial venules: association with T- and B-lymphocyte infiltration and favorable prognosis in breast cancer. Cancer Res. 2011;71(17):5678–5687. doi: 10.1158/0008-5472.CAN-11-0431. [DOI] [PubMed] [Google Scholar]
- 45.de Chaisemartin L, Goc J, Damotte D, Validire P, Magdeleinat P, Alifano M, Cremer I, Fridman WH, Sautes-Fridman C, Dieu-Nosjean MC. Characterization of chemokines and adhesion molecules associated with T cell presence in tertiary lymphoid structures in human lung cancer. Cancer Res. 2011;71(20):6391–6399. doi: 10.1158/0008-5472.CAN-11-0952. [DOI] [PubMed] [Google Scholar]
- 46.Cirri P, Chiarugi P. Cancer associated fibroblasts: the dark side of the coin. Am J Cancer Res. 2011;1(4):482–497. [PMC free article] [PubMed] [Google Scholar]
- 47.Amatangelo MD, Bassi DE, Klein-Szanto AJ, Cukierman E. Stroma-derived three-dimensional matrices are necessary and sufficient to promote desmoplastic differentiation of normal fibroblasts. Am J Pathol. 2005;167(2):475–488. doi: 10.1016/S0002-9440(10)62991-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ng CP, Hinz B, Swartz MA. Interstitial fluid flow induces myofibroblast differentiation and collagen alignment in vitro. J Cell Sci. 2005;118(Pt 20):4731–4739. doi: 10.1242/jcs.02605. [DOI] [PubMed] [Google Scholar]
- 49.Peduto L, Dulauroy S, Lochner M, Spath GF, Morales MA, Cumano A, Eberl G. Inflammation recapitulates the ontogeny of lymphoid stromal cells. J Immunol. 2009;182(9):5789–5799. doi: 10.4049/jimmunol.0803974. [DOI] [PubMed] [Google Scholar]
- 50.Chioda M, Peranzoni E, Desantis G, Papalini F, Falisi E, Solito S, Mandruzzato S, Bronte V. Myeloid cell diversification and complexity: an old concept with new turns in oncology. Cancer Metastasis Rev. 2011;30(1):27–43. doi: 10.1007/s10555-011-9268-1. [DOI] [PubMed] [Google Scholar]
- 51.Pollard JW. Trophic macrophages in development and disease. Nat Rev Immunol. 2009;9(4):259–270. doi: 10.1038/nri2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schmid MC, Varner JA. Myeloid cells in the tumor microenvironment: modulation of tumor angiogenesis and tumor inflammation. J Oncol. 2010;2010:201026. doi: 10.1155/2010/201026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Murdoch C, Muthana M, Coffelt SB, Lewis CE. The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer. 2008;8(8):618–631. doi: 10.1038/nrc2444. [DOI] [PubMed] [Google Scholar]
- 54.Duffield JS, Forbes SJ, Constandinou CM, Clay S, Partolina M, Vuthoori S, Wu S, Lang R, Iredale JP. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Invest. 2005;115(1):56–65. doi: 10.1172/JCI22675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Karlmark KR, Weiskirchen R, Zimmermann HW, Gassler N, Ginhoux F, Weber C, Merad M, Luedde T, Trautwein C, Tacke F. Hepatic recruitment of the inflammatory Gr1 + monocyte subset upon liver injury promotes hepatic fibrosis. Hepatology. 2009;50(1):261–274. doi: 10.1002/hep.22950. [DOI] [PubMed] [Google Scholar]
- 56.Kisseleva T, Brenner DA. Mechanisms of fibrogenesis. Exp Biol Med (Maywood) 2008;233(2):109–122. doi: 10.3181/0707-MR-190. [DOI] [PubMed] [Google Scholar]
- 57.Ramachandran P, Iredale JP. Macrophages: central regulators of hepatic fibrogenesis and fibrosis resolution. J Hepatol. 2012;56(6):1417–1419. doi: 10.1016/j.jhep.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 58.Fichtner-Feigl S, Strober W, Kawakami K, Puri RK, Kitani A. IL-13 signaling through the IL-13alpha2 receptor is involved in induction of TGF-beta1 production and fibrosis. Nat Med. 2006;12(1):99–106. doi: 10.1038/nm1332. [DOI] [PubMed] [Google Scholar]
- 59.Ingman WV, Wyckoff J, Gouon-Evans V, Condeelis J, Pollard JW. Macrophages promote collagen fibrillogenesis around terminal end buds of the developing mammary gland. Dev Dyn. 2006;235(12):3222–3229. doi: 10.1002/dvdy.20972. [DOI] [PubMed] [Google Scholar]
- 60.Kitagawa K, Wada T, Furuichi K, Hashimoto H, Ishiwata Y, Asano M, Takeya M, Kuziel WA, Matsushima K, Mukaida N, Yokoyama H. Blockade of CCR2 ameliorates progressive fibrosis in kidney. Am J Pathol. 2004;165(1):237–246. doi: 10.1016/S0002-9440(10)63292-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Toh B, Wang X, Keeble J, Sim WJ, Khoo K, Wong WC, Kato M, Prevost-Blondel A, Thiery JP, Abastado JP. Mesenchymal transition and dissemination of cancer cells is driven by myeloid-derived suppressor cells infiltrating the primary tumor. PLoS Biol. 2011;9(9):e1001162. doi: 10.1371/journal.pbio.1001162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bonde AK, Tischler V, Kumar S, Soltermann A, Schwendener RA. Intratumoral macrophages contribute to epithelial-mesenchymal transition in solid tumors. BMC Cancer. 2012;12:35. doi: 10.1186/1471-2407-12-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ye XZ, Xu SL, Xin YH, Yu SC, Ping YF, Chen L, Xiao HL, Wang B, Yi L, Wang QL, Jiang XF, Yang L, Zhang P, Qian C, Cui YH, Zhang X, Bian XW. Tumor-associated microglia/macrophages enhance the invasion of glioma stem-like cells via TGF-beta1 signaling pathway. J Immunol. 2012;189(1):444–453. doi: 10.4049/jimmunol.1103248. [DOI] [PubMed] [Google Scholar]
- 64.Kawase A, Ishii G, Nagai K, Ito T, Nagano T, Murata Y, Hishida T, Nishimura M, Yoshida J, Suzuki K, Ochiai A. Podoplanin expression by cancer associated fibroblasts predicts poor prognosis of lung adenocarcinoma. Int J Cancer. 2008;123(5):1053–1059. doi: 10.1002/ijc.23611. [DOI] [PubMed] [Google Scholar]
- 65.Tsujino T, Seshimo I, Yamamoto H, Ngan CY, Ezumi K, Takemasa I, Ikeda M, Sekimoto M, Matsuura N, Monden M. Stromal myofibroblasts predict disease recurrence for colorectal cancer. Clin Cancer Res. 2007;13(7):2082–2090. doi: 10.1158/1078-0432.CCR-06-2191. [DOI] [PubMed] [Google Scholar]
- 66.Yamashita M, Ogawa T, Zhang X, Hanamura N, Kashikura Y, Takamura M, Yoneda M, Shiraishi T. Role of stromal myofibroblasts in invasive breast cancer: stromal expression of alpha-smooth muscle actin correlates with worse clinical outcome. Breast Cancer. 2012;19(2):170–176. doi: 10.1007/s12282-010-0234-5. [DOI] [PubMed] [Google Scholar]
- 67.Franke FE, Von Georgi R, Zygmunt M, Munstedt K. Association between fibronectin expression and prognosis in ovarian carcinoma. Anticancer Res. 2003;23(5b):4261–4267. [PubMed] [Google Scholar]
- 68.Anttila MA, Tammi RH, Tammi MI, Syrjanen KJ, Saarikoski SV, Kosma VM. High levels of stromal hyaluronan predict poor disease outcome in epithelial ovarian cancer. Cancer Res. 2000;60(1):150–155. [PubMed] [Google Scholar]
- 69.Auvinen P, Tammi R, Parkkinen J, Tammi M, Agren U, Johansson R, Hirvikoski P, Eskelinen M, Kosma VM. Hyaluronan in peritumoral stroma and malignant cells associates with breast cancer spreading and predicts survival. Am J Pathol. 2000;156(2):529–536. doi: 10.1016/S0002-9440(10)64757-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tammi RH, Kultti A, Kosma VM, Pirinen R, Auvinen P, Tammi MI. Hyaluronan in human tumors: pathobiological and prognostic messages from cell-associated and stromal hyaluronan. Semin Cancer Biol. 2008;18(4):288–295. doi: 10.1016/j.semcancer.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 71.Sis B, Sarioglu S, Sokmen S, Sakar M, Kupelioglu A, Fuzun M. Desmoplasia measured by computer assisted image analysis: an independent prognostic marker in colorectal carcinoma. J Clin Pathol. 2005;58(1):32–38. doi: 10.1136/jcp.2004.018705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nadiarnykh O, LaComb RB, Brewer MA, Campagnola PJ. Alterations of the extracellular matrix in ovarian cancer studied by second harmonic generation imaging microscopy. BMC Cancer. 2010;10:94. doi: 10.1186/1471-2407-10-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wozniak MA, Desai R, Solski PA, Der CJ, Keely PJ. ROCK-generated contractility regulates breast epithelial cell differentiation in response to the physical properties of a three-dimensional collagen matrix. J Cell Biol. 2003;163(3):583–595. doi: 10.1083/jcb.200305010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Smith E, Breznik J, Lichty BD. Strategies to enhance viral penetration of solid tumors. Hum Gene Ther. 2011;22(9):1053–1060. doi: 10.1089/hum.2010.227. [DOI] [PubMed] [Google Scholar]
- 75.Butcher DT, Alliston T, Weaver VM. A tense situation: forcing tumour progression. Nat Rev Cancer. 2009;9(2):108–122. doi: 10.1038/nrc2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sangaletti S, Colombo MP. Matricellular proteins at the crossroad of inflammation and cancer. Cancer Lett. 2008;267(2):245–253. doi: 10.1016/j.canlet.2008.03.027. [DOI] [PubMed] [Google Scholar]
- 77.Chong HC, Tan CK, Huang RL, Tan NS. Matricellular proteins: a sticky affair with cancers. J Oncol. 2012;2012:351089. doi: 10.1155/2012/351089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kudo-Saito C, Shirako H, Takeuchi T, Kawakami Y. Cancer metastasis is accelerated through immunosuppression during Snail-induced EMT of cancer cells. Cancer Cell. 2009;15(3):195–206. doi: 10.1016/j.ccr.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 79.Parekh K, Ramachandran S, Cooper J, Bigner D, Patterson A, Mohanakumar T. Tenascin-C, over expressed in lung cancer down regulates effector functions of tumor infiltrating lymphocytes. Lung Cancer. 2005;47(1):17–29. doi: 10.1016/j.lungcan.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 80.Alvarez MJ, Prada F, Salvatierra E, Bravo AI, Lutzky VP, Carbone C, Pitossi FJ, Chuluyan HE, Podhajcer OL. Secreted protein acidic and rich in cysteine produced by human melanoma cells modulates polymorphonuclear leukocyte recruitment and antitumor cytotoxic capacity. Cancer Res. 2005;65(12):5123–5132. doi: 10.1158/0008-5472.CAN-04-1102. [DOI] [PubMed] [Google Scholar]
- 81.Sangaletti S, Stoppacciaro A, Guiducci C, Torrisi MR, Colombo MP. Leukocyte, rather than tumor-produced SPARC, determines stroma and collagen type IV deposition in mammary carcinoma. J Exp Med. 2003;198(10):1475–1485. doi: 10.1084/jem.20030202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Adair-Kirk TL, Senior RM. Fragments of extracellular matrix as mediators of inflammation. Int J Biochem Cell Biol. 2008;40(6–7):1101–1110. doi: 10.1016/j.biocel.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sorokin L. The impact of the extracellular matrix on inflammation. Nat Rev Immunol. 2010;10(10):712–723. doi: 10.1038/nri2852. [DOI] [PubMed] [Google Scholar]
- 84.Houghton AM, Quintero PA, Perkins DL, Kobayashi DK, Kelley DG, Marconcini LA, Mecham RP, Senior RM, Shapiro SD. Elastin fragments drive disease progression in a murine model of emphysema. J Clin Invest. 2006;116(3):753–759. doi: 10.1172/JCI25617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Weathington NM, van Houwelingen AH, Noerager BD, Jackson PL, Kraneveld AD, Galin FS, Folkerts G, Nijkamp FP, Blalock JE. A novel peptide CXCR ligand derived from extracellular matrix degradation during airway inflammation. Nat Med. 2006;12(3):317–323. doi: 10.1038/nm1361. [DOI] [PubMed] [Google Scholar]
- 86.Midwood K, Sacre S, Piccinini AM, Inglis J, Trebaul A, Chan E, Drexler S, Sofat N, Kashiwagi M, Orend G, Brennan F, Foxwell B. Tenascin-C is an endogenous activator of Toll-like receptor 4 that is essential for maintaining inflammation in arthritic joint disease. Nat Med. 2009;15(7):774–780. doi: 10.1038/nm.1987. [DOI] [PubMed] [Google Scholar]
- 87.Jiang D, Liang J, Fan J, Yu S, Chen S, Luo Y, Prestwich GD, Mascarenhas MM, Garg HG, Quinn DA, Homer RJ, Goldstein DR, Bucala R, Lee PJ, Medzhitov R, Noble PW. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med. 2005;11(11):1173–1179. doi: 10.1038/nm1315. [DOI] [PubMed] [Google Scholar]
- 88.Schaefer L, Babelova A, Kiss E, Hausser HJ, Baliova M, Krzyzankova M, Marsche G, Young MF, Mihalik D, Gotte M, Malle E, Schaefer RM, Grone HJ. The matrix component biglycan is proinflammatory and signals through Toll-like receptors 4 and 2 in macrophages. J Clin Invest. 2005;115(8):2223–2233. doi: 10.1172/JCI23755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bunt SK, Clements VK, Hanson EM, Sinha P, Ostrand-Rosenberg S. Inflammation enhances myeloid-derived suppressor cell cross-talk by signaling through Toll-like receptor 4. J Leukoc Biol. 2009;85(6):996–1004. doi: 10.1189/jlb.0708446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Friedl P, Brocker EB. T cell migration in three-dimensional extracellular matrix: guidance by polarity and sensations. Dev Immunol. 2000;7(2–4):249–266. doi: 10.1155/2000/56473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wolf K, Muller R, Borgmann S, Brocker EB, Friedl P. Amoeboid shape change and contact guidance: T-lymphocyte crawling through fibrillar collagen is independent of matrix remodeling by MMPs and other proteases. Blood. 2003;102(9):3262–3269. doi: 10.1182/blood-2002-12-3791. [DOI] [PubMed] [Google Scholar]
- 92.Friedl P, Entschladen F, Conrad C, Niggemann B, Zanker KS. CD4+ T lymphocytes migrating in three-dimensional collagen lattices lack focal adhesions and utilize beta1 integrin-independent strategies for polarization, interaction with collagen fibers and locomotion. Eur J Immunol. 1998;28(8):2331–2343. doi: 10.1002/(SICI)1521-4141(199808)28:08<2331::AID-IMMU2331>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 93.Germain RN, Robey EA, Cahalan MD. A decade of imaging cellular motility and interaction dynamics in the immune system. Science. 2012;336(6089):1676–1681. doi: 10.1126/science.1221063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Miller MJ, Wei SH, Parker I, Cahalan MD. Two-photon imaging of lymphocyte motility and antigen response in intact lymph node. Science. 2002;296(5574):1869–1873. doi: 10.1126/science.1070051. [DOI] [PubMed] [Google Scholar]
- 95.Bajenoff M, Egen JG, Qi H, Huang AY, Castellino F, Germain RN. Highways, byways and breadcrumbs: directing lymphocyte traffic in the lymph node. Trends Immunol. 2007;28(8):346–352. doi: 10.1016/j.it.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 96.Asperti-Boursin F, Real E, Bismuth G, Trautmann A, Donnadieu E. CCR7 ligands control basal T cell motility within lymph node slices in a phosphoinositide 3-kinase-independent manner. J Exp Med. 2007;204(5):1167–1179. doi: 10.1084/jem.20062079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Okada T, Cyster JG. CC chemokine receptor 7 contributes to Gi-dependent T cell motility in the lymph node. J Immunol. 2007;178(5):2973–2978. doi: 10.4049/jimmunol.178.5.2973. [DOI] [PubMed] [Google Scholar]
- 98.Worbs T, Mempel TR, Bolter J, von Andrian UH, Forster R. CCR7 ligands stimulate the intranodal motility of T lymphocytes in vivo. J Exp Med. 2007;204(3):489–495. doi: 10.1084/jem.20061706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bajenoff M. Stromal cells control soluble material and cellular transport in lymph nodes. Front Immunol. 2012;3:304. doi: 10.3389/fimmu.2012.00304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mempel TR, Junt T, von Andrian UH. Rulers over randomness: stroma cells guide lymphocyte migration in lymph nodes. Immunity. 2006;25(6):867–869. doi: 10.1016/j.immuni.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 101.Lee M, Mandl JN, Germain RN, Yates AJ. The race for the prize: T-cell trafficking strategies for optimal surveillance. Blood. 2012;120(7):1432–1438. doi: 10.1182/blood-2012-04-424655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zeng M, Haase AT, Schacker TW. Lymphoid tissue structure and HIV-1 infection: life or death for T cells. Trends Immunol. 2012;33(6):306–314. doi: 10.1016/j.it.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 103.Zeng M, Southern PJ, Reilly CS, Beilman GJ, Chipman JG, Schacker TW, Haase AT. Lymphoid tissue damage in HIV-1 infection depletes naive T cells and limits T cell reconstitution after antiretroviral therapy. PLoS Pathog. 2012;8(1):e1002437. doi: 10.1371/journal.ppat.1002437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wilson EH, Harris TH, Mrass P, John B, Tait ED, Wu GF, Pepper M, Wherry EJ, Dzierzinski F, Roos D, Haydon PG, Laufer TM, Weninger W, Hunter CA. Behavior of parasite-specific effector CD8+ T cells in the brain and visualization of a kinesis-associated system of reticular fibers. Immunity. 2009;30(2):300–311. doi: 10.1016/j.immuni.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Matheu MP, Beeton C, Garcia A, Chi V, Rangaraju S, Safrina O, Monaghan K, Uemura MI, Li D, Pal S, De la Maza LM, Monuki E, Flugel A, Pennington MW, Parker I, Chandy KG, Cahalan MD. Imaging of effector memory T cells during a delayed-type hypersensitivity reaction and suppression by Kv1.3 channel block. Immunity. 2008;29(4):602–614. doi: 10.1016/j.immuni.2008.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Boissonnas A, Fetler L, Zeelenberg IS, Hugues S, Amigorena S. In vivo imaging of cytotoxic T cell infiltration and elimination of a solid tumor. J Exp Med. 2007;204(2):345–356. doi: 10.1084/jem.20061890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mrass P, Takano H, Ng LG, Daxini S, Lasaro MO, Iparraguirre A, Cavanagh LL, von Andrian UH, Ertl HC, Haydon PG, Weninger W. Random migration precedes stable target cell interactions of tumor-infiltrating T cells. J Exp Med. 2006;203(12):2749–2761. doi: 10.1084/jem.20060710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Egeblad M, Ewald AJ, Askautrud HA, Truitt ML, Welm BE, Bainbridge E, Peeters G, Krummel MF, Werb Z (2008) Visualizing stromal cell dynamics in different tumor microenvironments by spinning disk confocal microscopy. Dis Model Mech 1 (2–3):155–167; discussion 165. doi:10.1242/dmm.000596 [DOI] [PMC free article] [PubMed]
- 109.Engelhardt JJ, Boldajipour B, Beemiller P, Pandurangi P, Sorensen C, Werb Z, Egeblad M, Krummel MF. Marginating dendritic cells of the tumor microenvironment cross-present tumor antigens and stably engage tumor-specific T cells. Cancer Cell. 2012;21(3):402–417. doi: 10.1016/j.ccr.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Arrieumerlou C, Donnadieu E, Brennan P, Keryer G, Bismuth G, Cantrell D, Trautmann A. Involvement of phosphoinositide 3-kinase and Rac in membrane ruffling induced by IL-2 in T cells. Eur J Immunol. 1998;28(6):1877–1885. doi: 10.1002/(SICI)1521-4141(199806)28:06<1877::AID-IMMU1877>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 111.Lieubeau B, Heymann MF, Henry F, Barbieux I, Meflah K, Gregoire M. Immunomodulatory effects of tumor-associated fibroblasts in colorectal-tumor development. Int J Cancer. 1999;81(4):629–636. doi: 10.1002/(sici)1097-0215(19990517)81:4<629::aid-ijc20>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 112.Martin ML, Wall EM, Sandwith E, Girardin A, Milne K, Watson PH, Nelson BH. Density of tumour stroma is correlated to outcome after adoptive transfer of CD4+ and CD8+ T cells in a murine mammary carcinoma model. Breast Cancer Res Treat. 2010;121(3):753–763. doi: 10.1007/s10549-009-0559-y. [DOI] [PubMed] [Google Scholar]
- 113.Ohno S, Tachibana M, Fujii T, Ueda S, Kubota H, Nagasue N. Role of stromal collagen in immunomodulation and prognosis of advanced gastric carcinoma. Int J Cancer. 2002;97(6):770–774. doi: 10.1002/ijc.10144. [DOI] [PubMed] [Google Scholar]
- 114.Gorter A, Zijlmans HJ, van Gent H, Trimbos JB, Fleuren GJ, Jordanova ES. Versican expression is associated with tumor-infiltrating CD8-positive T cells and infiltration depth in cervical cancer. Mod Pathol. 2010;23(12):1605–1615. doi: 10.1038/modpathol.2010.154. [DOI] [PubMed] [Google Scholar]
- 115.Huang JH, Cardenas-Navia LI, Caldwell CC, Plumb TJ, Radu CG, Rocha PN, Wilder T, Bromberg JS, Cronstein BN, Sitkovsky M, Dewhirst MW, Dustin ML. Requirements for T lymphocyte migration in explanted lymph nodes. J Immunol. 2007;178(12):7747–7755. doi: 10.4049/jimmunol.178.12.7747. [DOI] [PubMed] [Google Scholar]
- 116.Van Goethem E, Poincloux R, Gauffre F, Maridonneau-Parini I, Le Cabec V. Matrix architecture dictates three-dimensional migration modes of human macrophages: differential involvement of proteases and podosome-like structures. J Immunol. 2010;184(2):1049–1061. doi: 10.4049/jimmunol.0902223. [DOI] [PubMed] [Google Scholar]
- 117.Cougoule C, Van Goethem E, Le Cabec V, Lafouresse F, Dupre L, Mehraj V, Mege JL, Lastrucci C, Maridonneau-Parini I. Blood leukocytes and macrophages of various phenotypes have distinct abilities to form podosomes and to migrate in 3D environments. Eur J Cell Biol. 2012;91(11–12):938–949. doi: 10.1016/j.ejcb.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 118.Wyckoff JB, Wang Y, Lin EY, Li JF, Goswami S, Stanley ER, Segall JE, Pollard JW, Condeelis J. Direct visualization of macrophage-assisted tumor cell intravasation in mammary tumors. Cancer Res. 2007;67(6):2649–2656. doi: 10.1158/0008-5472.CAN-06-1823. [DOI] [PubMed] [Google Scholar]
- 119.Kobayashi N, Miyoshi S, Mikami T, Koyama H, Kitazawa M, Takeoka M, Sano K, Amano J, Isogai Z, Niida S, Oguri K, Okayama M, McDonald JA, Kimata K, Taniguchi S, Itano N. Hyaluronan deficiency in tumor stroma impairs macrophage trafficking and tumor neovascularization. Cancer Res. 2010;70(18):7073–7083. doi: 10.1158/0008-5472.CAN-09-4687. [DOI] [PubMed] [Google Scholar]
- 120.Potter-Perigo S, Johnson PY, Evanko SP, Chan CK, Braun KR, Wilkinson TS, Altman LC, Wight TN. Polyinosine-polycytidylic acid stimulates versican accumulation in the extracellular matrix promoting monocyte adhesion. Am J Respir Cell Mol Biol. 2009;43(1):109–120. doi: 10.1165/rcmb.2009-0081OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Coombes JL, Han SJ, van Rooijen N, Raulet DH, Robey EA. Infection-induced regulation of natural killer cells by macrophages and collagen at the lymph node subcapsular sinus. Cell Rep. 2012;2(1):124–135. doi: 10.1016/j.celrep.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Barker HE, Cox TR, Erler JT. The rationale for targeting the LOX family in cancer. Nat Rev Cancer. 2012;12(8):540–552. doi: 10.1038/nrc3319. [DOI] [PubMed] [Google Scholar]
- 123.Goetz JG, Minguet S, Navarro-Lerida I, Lazcano JJ, Samaniego R, Calvo E, Tello M, Osteso-Ibanez T, Pellinen T, Echarri A, Cerezo A, Klein-Szanto AJ, Garcia R, Keely PJ, Sanchez-Mateos P, Cukierman E, Del Pozo MA. Biomechanical remodeling of the microenvironment by stromal caveolin-1 favors tumor invasion and metastasis. Cell. 2011;146(1):148–163. doi: 10.1016/j.cell.2011.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Brennen WN, Isaacs JT, Denmeade SR. Rationale behind targeting fibroblast activation protein-expressing carcinoma-associated fibroblasts as a novel chemotherapeutic strategy. Mol Cancer Ther. 2012;11(2):257–266. doi: 10.1158/1535-7163.MCT-11-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kraman M, Bambrough PJ, Arnold JN, Roberts EW, Magiera L, Jones JO, Gopinathan A, Tuveson DA, Fearon DT. Suppression of antitumor immunity by stromal cells expressing fibroblast activation protein-alpha. Science. 2010;330(6005):827–830. doi: 10.1126/science.1195300. [DOI] [PubMed] [Google Scholar]
- 126.Akhurst RJ, Hata A. Targeting the TGFbeta signalling pathway in disease. Nat Rev Drug Discov. 2012;11(10):790–811. doi: 10.1038/nrd3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Liu J, Liao S, Diop-Frimpong B, Chen W, Goel S, Naxerova K, Ancukiewicz M, Boucher Y, Jain RK, Xu L. TGF-beta blockade improves the distribution and efficacy of therapeutics in breast carcinoma by normalizing the tumor stroma. Proce Natl Acad Sci USA. 2012;109(41):16618–16623. doi: 10.1073/pnas.1117610109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bierie B, Moses HL. Tumour microenvironment: TGFbeta: the molecular Jekyll and Hyde of cancer. Nat Rev Cancer. 2006;6(7):506–520. doi: 10.1038/nrc1926. [DOI] [PubMed] [Google Scholar]
- 129.Flavell RA, Sanjabi S, Wrzesinski SH, Licona-Limon P. The polarization of immune cells in the tumour environment by TGFbeta. Nat Rev Immunol. 2010;10(8):554–567. doi: 10.1038/nri2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.McKee TD, Grandi P, Mok W, Alexandrakis G, Insin N, Zimmer JP, Bawendi MG, Boucher Y, Breakefield XO, Jain RK. Degradation of fibrillar collagen in a human melanoma xenograft improves the efficacy of an oncolytic herpes simplex virus vector. Cancer Res. 2006;66(5):2509–2513. doi: 10.1158/0008-5472.CAN-05-2242. [DOI] [PubMed] [Google Scholar]
- 131.Hurst LC, Badalamente MA, Hentz VR, Hotchkiss RN, Kaplan FT, Meals RA, Smith TM, Rodzvilla J. Injectable collagenase clostridium histolyticum for Dupuytren’s contracture. N Engl J Med. 2009;361(10):968–979. doi: 10.1056/NEJMoa0810866. [DOI] [PubMed] [Google Scholar]
- 132.Syed F, Thomas AN, Singh S, Kolluru V, Emeigh Hart SG, Bayat A. In vitro study of novel collagenase (XIAFLEX(R)) on Dupuytren’s disease fibroblasts displays unique drug related properties. PLoS One. 2012;7(2):e31430. doi: 10.1371/journal.pone.0031430. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 133.Bollyky PL, Wu RP, Falk BA, Lord JD, Long SA, Preisinger A, Teng B, Holt GE, Standifer NE, Braun KR, Xie CF, Samuels PL, Vernon RB, Gebe JA, Wight TN, Nepom GT. ECM components guide IL-10 producing regulatory T-cell (TR1) induction from effector memory T-cell precursors. Proc Natl Acad Sci USA. 2011;108(19):7938–7943. doi: 10.1073/pnas.1017360108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Provenzano PP, Cuevas C, Chang AE, Goel VK, Von Hoff DD, Hingorani SR. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;21(3):418–429. doi: 10.1016/j.ccr.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lukashev M, LePage D, Wilson C, Bailly V, Garber E, Lukashin A, Ngam-ek A, Zeng W, Allaire N, Perrin S, Xu X, Szeliga K, Wortham K, Kelly R, Bottiglio C, Ding J, Griffith L, Heaney G, Silverio E, Yang W, Jarpe M, Fawell S, Reff M, Carmillo A, Miatkowski K, Amatucci J, Crowell T, Prentice H, Meier W, Violette SM, Mackay F, Yang D, Hoffman R, Browning JL. Targeting the lymphotoxin-beta receptor with agonist antibodies as a potential cancer therapy. Cancer Res. 2006;66(19):9617–9624. doi: 10.1158/0008-5472.CAN-06-0217. [DOI] [PubMed] [Google Scholar]
- 136.Schrama D, Thor Straten P, Fischer WH, McLellan AD, Brocker EB, Reisfeld RA, Becker JC. Targeting of lymphotoxin-alpha to the tumor elicits an efficient immune response associated with induction of peripheral lymphoid-like tissue. Immunity. 2001;14(2):111–121. doi: 10.1016/s1074-7613(01)00094-2. [DOI] [PubMed] [Google Scholar]
- 137.Shields JD, Kourtis IC, Tomei AA, Roberts JM, Swartz MA. Induction of lymphoidlike stroma and immune escape by tumors that express the chemokine CCL21. Science. 2010;328(5979):749–752. doi: 10.1126/science.1185837. [DOI] [PubMed] [Google Scholar]
- 138.Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480(7378):480–489. doi: 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.DuFort CC, Paszek MJ, Weaver VM. Balancing forces: architectural control of mechanotransduction. Nat Rev Mol Cell Biol. 2011;12(5):308–319. doi: 10.1038/nrm3112. [DOI] [PMC free article] [PubMed] [Google Scholar]