Abstract
Cell–cell adhesive processes are central to the physiology of multicellular organisms. A number of cell surface molecules contribute to cell–cell adhesion, and the dysfunction of adhesive processes underlies numerous developmental defects and inherited diseases. The nectins, a family of four immunoglobulin superfamily members (nectin-1 to -4), interact through their extracellular domains to support cell–cell adhesion. While both homophilic and heterophilic interactions among the nectins are implicated in cell–cell adhesion, cell-based and biochemical studies suggest heterophilic interactions are stronger than homophilic interactions and control a range of physiological processes. In addition to interactions within the nectin family, heterophilic associations with nectin-like molecules, immune receptors, and viral glycoproteins support a wide range of biological functions, including immune modulation, cancer progression, host-pathogen interactions and immune evasion. We review current structural and molecular knowledge of nectin recognition processes, with a focus on the biochemical and biophysical determinants of affinity and selectivity that drive distinct nectin associations. These proteins and interactions are discussed as potential targets for immunotherapy.
Keywords: Nectin, Nectin-like molecules, Immunoglobulin fold, Crystal structures, Nectin homodimers, Cadherin, Immune receptors, Viral entry receptors
Introduction
In multicellular organisms, the regulation and specificity of cell–cell adhesive processes are critical for the development and maintenance of tissues and organs. Individual cells are interconnected by macromolecular assemblies known as cell–cell junctions, which are formed by a wide range of cell-adhesion molecules. These interactions rely on the recognition of the ectodomains of cell-adhesion molecules with their cognate binding partners on neighboring cells [1–4]. Disruption of these molecular interactions leads to perturbation of cell–cell adhesion and the loss of tissue homeostasis, which has been implicated in various developmental abnormalities in human, as well as a range of diseases, including sensory and reproductive disorders and metastasis [5–7].
A large proportion of cell-adhesion molecules belongs to four protein families: integrins, selectins, immunoglobulin superfamily (IgSF), and cadherins. Integrins are a group of large heterodimeric transmembrane proteins that bind protein components of the extracellular matrix (e.g., fibronectin, collagen), while the selectins mediate cell–cell adhesion by recognizing carbohydrates presented on the cell surface (e.g., mucin) [8, 9]. Members of the IgSF and cadherin protein families mediate cell–cell adhesion via engagement of proteins, (typically) belonging to their respective families, on neighboring cells. The nectins and nectin-like (necl) molecules are two important classes of cell-adhesion molecules within the IgSF family. Both, the cadherins and nectins have been extensively investigated due to their significant role in the formation of adherens junctions that mediate cell–cell adhesion [1–3, 10].
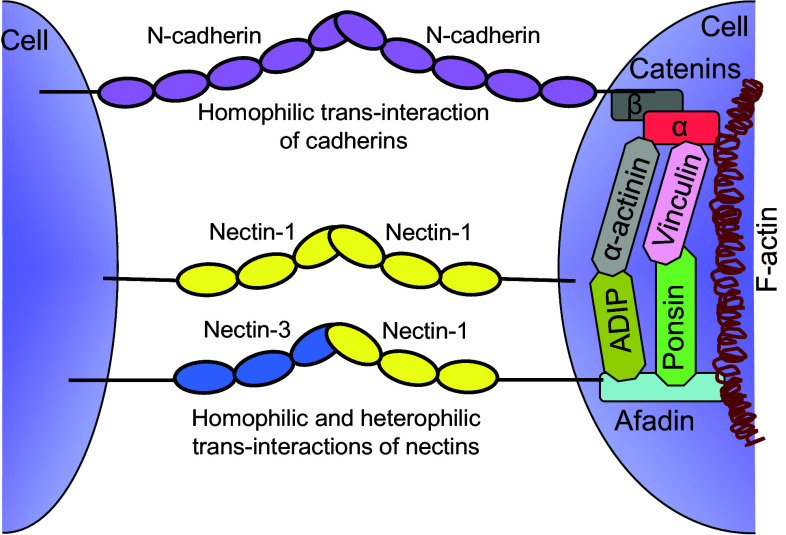
The Cadherin family includes >100 members and is subdivided into several groups, including the classical cadherins (E-cadherin found in epithelial tissue and N-cadherin found in neurons), protocadherins, and atypical cadherins [11, 12]. Both, E-cadherin and N-cadherin have an extracellular region containing five tandemly repeated β-fold domains, a single transmembrane region and a cytoplasmic region [10]. The nectin family members are characterized by an ectodomain composed of three immunoglobulin (Ig)-like domains, followed by a single transmembrane region and a cytoplasmic domain [13]. Central to adhesive processes are the cytoplasmic domains of cadherins and nectins, which recognize the β-catenin and afadin adaptor molecules, respectively [2, 14]. These adaptor molecules, as well as numerous others, are responsible for the subsequent binding and recruitment of filamentous actin assemblies (F-actin), which facilitate cell–cell adhesion [4, 7]. Nectins interact in a homophilic as well as heterophilic fashion between neighboring cells [2, 3, 13]. Cadherins participate in homophilic interactions to mediate cell–cell adhesion [10, 13], with several reports describing their ability to engage in heterophilic associations [12, 15–17]. A notable difference between these two groups of cell adhesion molecules is that interactions between cadherins are calcium-dependent, whereas interactions among nectins are calcium-independent [2, 3, 10, 13]. The members of nectin and cadherin protein families either function autonomously or in concert with each other at the site of cell–cell junctions. In humans, both nectin and cadherin-based cell–cell adhesions are prominent in adherens junctions of fibroblast and epithelial cells, and in synaptic junctions of neurons [2, 3, 18, 19].
The nectin family is composed of four members (nectin-1 to nectin-4), whose extracellular domains participate in a complex network of interactions through their membrane-distal IgV domains that include both homophilic and heterophilic adhesive associations between interacting cells (Fig. 1a). In addition to interactions within the family, nectins are also known to bind members of other protein families (necls and immune receptors), resulting in a wide range of biological functions, including immune modulation (Fig. 1) [20, 21]. Nectins also play important roles in host-pathogen interactions, as they are utilized by herpes and measles viruses as entry receptors [22–24]. Nectin-2 serves as an excellent example of the richness of the multiple synergistic and competing interactions and functions within this family. Nectin-2 interacts with nectin family members to mediate cell–cell adhesion, and interacts with immune receptors, CD226, and TIGIT on specialized immune cells, to modulate immune function, and also acts as an entry receptor for Herpes simplex virus by interacting with viral glycoprotein D (Fig. 1b).
Fig. 1.

Homophilic and heterophilic interactions of nectins. a Homophilic (black arrow) and heterophilic (red arrow) interactions among nectins are shown at the center. The homophilic affinities (equilibrium dissociation constant K d) of all the four nectins are stated. Nectins also participate in heterophilic interactions with a number of other proteins (shown in the periphery), including nectin-like molecules and some other immune receptors. b Recognition of nectin-2 by nectins, immune receptors (CD226, TIGIT) and viral glycoprotein D are important for cell–cell adhesion, immune modulation, and host-pathogen interactions, respectively
In this review, we summarize recent data on the mechanisms of nectin-mediated cell–cell adhesive interactions that control numerous physiological and pathological processes. Specifically, we focus on the molecular and structural features responsible for determining the affinities and selectivities of nectin-associated homophilic and heterophilic recognition events.
Nectins, nectin-like molecules and extended family members
The members of the nectin family are expressed as single-pass type-I membrane glycoproteins and are characterized by a shared domain organization, consisting of three Ig-like domains in the ectodomain (membrane-distal IgV domain followed by two IgC domains), followed by a transmembrane region and a cytoplasmic tail containing the afadin-binding motif (Fig. 2) [2, 25]. With the exception of three splice variants (nectin-1γ, which is secreted protein lacking transmembrane and cytoplasmic regions; and nectin-1β and nectin-3γ, which lack an afadin-binding motif in the cytoplasmic region) and nectin-4, the afadin-binding motif of all other nectins is a conserved sequence, Glu/Ala-X-Tyr-Val, which binds the PDZ domain of afadin. Although, nectin-4 lacks this conserved motif, it binds the adaptor molecule afadin through its C-terminal Gly-His-Leu-Val motif (Fig. 2). Five additional IgSF members, known as nectin-like molecules (necl-1 to necl-5) also share the nectin ectodomain architecture and play roles in cell–cell adhesion, although they lack afadin-binding motifs [2]. Thus, the broader nectin/necl family consists of nine members. In a recent bioinformatics study, Rubinstein and colleagues demonstrated that these molecules could be cleanly segregated into the nectin and necl subgroups solely on the basis of amino acid sequence [26]. This bioinformatic approach clustered necl-5 with the nectins rather than with the necl proteins, which is consistent with the fact that the ectodomain sequence and the gene structure of necl-5 are more similar with the ectodomains of nectins than the necl molecules [26, 27]. Although, necl-5 is composed of an ectodomain containing three Ig-like domains (membrane-distal IgV domain and two IgC domains) followed by a transmembrane region like other nectins, the cytoplasmic tail lacks the afadin-binding motif [2, 27]. Key functional properties of the nectin/necl family members are their ability to form homophilic and/or heterophilic associations, which are summarized in Table 1 and Fig. 1.
Fig. 2.
Domain organizations of the nectins and extended family members. Each nectin consists of three Ig-like domains (one IgV and two IgC) in their extracellular region, a single transmembrane (TM) region, and a cytoplasmic tail. The C-terminus of the cytoplasmic tail contains an afadin-binding motif
Table 1.
Interacting partners of nectin/necl proteins and their functions
| Proteins | Heterophilic partner | Function |
|---|---|---|
| Members of nectin family | ||
| Nectin-1 | Nectin-3, Nectin-4, Necl-1 | Cell adhesion |
| CD96 | Immune modulation | |
| Nectin-2 | Nectin-3 | Cell adhesion |
| CD226, TIGIT | Immune modulation | |
| Nectin-3 | Nectin-1, Nectin-2, Necl-1, 2, 5 | Cell adhesion |
| TIGIT | Immune modulation | |
| Nectin-4 | Nectin-1 | Cell adhesion |
| Members of necl family | ||
| Necl-1 | Nectin-1, Nectin-3, Necl-2, 3, 4 | Cell adhesion |
| Necl-2 | Nectin-3, Necl-1, Necl-3 | Cell adhesion |
| CRTAM | Immune modulation | |
| Necl-3 | Necl-1, Necl-2 | Cell adhesion |
| Necl-4a | Necl-1 | Cell adhesion |
| Necl-5a | Nectin-3 | Cell adhesion |
| CD96, CD226, TIGIT | Immune modulation | |
| Members of extended nectin/necl family | ||
| CD96a | Nectin-1, Necl-5 | Immune modulation |
| CD226a | Nectin-2, Necl-5 | Immune modulation |
| TIGIT | Nectin-2, Nectin-3, Necl-5 | Immune modulation |
| CRTAMa | Necl-2 | Immune modulation |
| CD200a | ? | ? |
?, unknown
arepresent members are not involved in homophilic interactions
Additional molecules are expressed in mammals that may significantly expand the nectin-related family and interaction network. Bioinformatics analysis suggested that five additional IgSF members, CD96, CD226, TIGIT, CRTAM, and CD200 are evolutionarily and functionally related to the nectin/necl proteins and may interact with members of this group [26]. To date, with the exception of CD200, these proteins have been reported to bind members of the nectin/necl family of proteins (Table 1; Fig. 1). The domain organizations of these proteins differ from that of the nectin/necl family members (Fig. 2); the extracellular region of CD96 contains three Ig-like domains, the ectodomains of CD200, CD226, and CRTAM contain 2 Ig-like domains, and TIGIT possesses a single IgV domain [21, 26]. In the subsequent section, we focus on the four nectins (nectin-1 to nectin-4) and their involvement in cell–cell adhesion, immune modulation and host-pathogen interactions at the molecular level.
Nectins in cell–cell adhesion
General perspective on nectin-mediated cell–cell adhesion
Nectins mediate cell–cell adhesion in a number of tissues, including epithelium, endothelium, and neural tissue during various stages of development [13, 28]. Although nectin family members are expressed in all tissues, individual cells may express one or multiple nectins in distinct but overlapping patterns [29]. Despite their importance in cell–cell adhesion in a wide variety of tissues, the individual knockouts of nectin-1, -2, and -3 in mice are not embryonic lethal, presumably due to the overlapping expression and functional redundancy of the nectin family members. Nevertheless, these knockouts suffer from a wide variety of abnormalities as they reach adulthood, reflecting the distinct functional contributions of specific nectins [29, 30].
All four nectins directly interact with afadin, an F-actin-binding protein, through their C-terminal cytoplasmic tail. In addition to the nectins, afadin associates with α-catenin through ponsin-vinculin and afadin DIL-domain-interacting protein (ADIP)-α-actinin units [2]. α-catenin also associates with β-catenin, which is recognized by the cytoplasmic domain of cadherins. As a result of these interactions, nectins and afadin recruit several proteins, including cadherins, to the nectin-associated cell–cell adhesion sites, resulting in the formation of adherens junctions. Core structural components of adherens junctions include the nectin–afadin and cadherin-catenin complexes, both of which associate with F-actin bundles (Fig. 3). Cell culture studies suggest that nectins initiate the formation of adherens junctions between two neighboring cells and subsequently recruit cadherin-catenin complexes through afadin [2, 31]. This view is consistent with the observation that a knockout of afadin in mice inhibits the formation of the cadherins-based adherens and tight junctions [32]. However, a recent report suggests that cadherins can be recruited to adherens junctions via an afadin-independent mechanism [33]. The interplay of nectins and cadherins with their respective adaptor molecules and subsequent association of these adaptor proteins with F-actin bundles serves to stabilize the adhesion sites between neighboring cells (Fig. 3).
Fig. 3.
The interplay of nectins and cadherins at the site of cell–cell adhesions. Both nectin–afadin and cadherin-catenin systems work together and interact with F-actin bundles
Physiological roles of nectin-mediated cell–cell adhesion
All four nectins participate in homophilic interactions that contribute to cell–cell adhesion and can drive cell aggregation when exogenously expressed in cells that are normally non-adherent [34, 35]. In addition to homophilic associations, nectins participate in selective heterophilic interactions: between nectin-1 and nectin-3, nectin-1 and nectin-4, and nectin-2 and nectin-3 (Fig. 1a). Nectins also selectively interact with the necl molecules to mediate cell–cell adhesion (Fig. 1a; Table 1).
While the homophilic trans-interaction of nectins is implicated in cell–cell adhesion processes, the heterophilic trans-interactions contribute to a range of important cellular functions in vivo [29, 36]. For example, nectin-2 and nectin-3 are expressed in Sertoli cells and spermatids, respectively, where their heterophilic trans-interaction regulates the organization of the Sertoli cell-spermatid junctions [36]. It has also been shown that the homozygous deletion of either nectin-2 or nectin-3 leads to male-specific infertility in mice [30, 37]. Another remarkable example is provided by the interaction of nectin-1 and nectin-3. These proteins are expressed in commissural axons and floor plate cells, respectively, during the early development of the vertebrate central nervous system and this heterophilic trans-interaction is critically involved in the control of axon guidance [28]. Nectin-1 and nectin-3 have been shown to play key roles in the formation of synapses between the mossy fiber terminals and dendrites of pyramidal cells in the CA3 area of the mouse hippocampus [18]. Nectin-1 and nectin-3 also make important contributions at the contact sites between pigmented and non-pigmented cell layers of the ciliary epithelium in the eye [38], between ameloblasts and stratum intermedium in the developing tooth [39], and between auditory hair cells and supporting cells in the auditory epithelium of the inner ear [29]. A recent study also reported that the heterophilic interaction between nectin-2 and N-cadherin through their extracellular domains regulates neural tube formation in Xenopus [40]. These examples highlight the prominent roles that nectins play in cell–cell adhesive processes that underlie the complex biology associated with multicellular organism.
Homophilic versus heterophilic interactions among nectin family members
All of the nectins exhibit a unique range of homophilic and heterophilic associations, with each interacting pair exhibiting distinct equilibrium dissociation constants (K d s) (Fig. 1a). The ectodomain of human nectin-1 and nectin-2 form relatively strong homophilic dimers (K d values of 17.5 and 0.4 μM, respectively), whereas the dimerization of nectin-3 and nectin-4 is relatively weak (K d values in the hundreds micromolar range) [41]. These relative affinities are also exhibited by the mouse nectins (which share 64–97 % sequence identity with the human orthologs in the IgV domains), with tighter homophilic binding for nectin-1 and nectin-2 than for nectin-3 and nectin-4 [41].
Although quantitative determinations of K d can be readily measured for homophilic interactions, similar characterization of heterophilic interactions remains challenging. Techniques such as surface plasmon resonance (SPR) and analytical ultracentrifugation (AUC), commonly used to determine the binding affinities between two proteins, are not fully amenable for analysis of heterophilic binding among the nectins. As all nectins exist as homodimers, difficulties arise from competing interactions between homophilic and heterophilic associations. For example, in SPR experiments, formation of nectin homodimers within the immobilized and mobile phases complicates the analysis of true heterophilic interactions. Nonetheless, semi-quantitative SPR data are in good agreement with previously reported cell-based binding assays showing that heterophilic interactions are generally stronger than homophilic interactions among the nectin family members [41]. Furthermore, AUC analysis suggests that the stoichiometry of heterophilic interactions in solution is 1:1 between nectin-1 and nectin-3, as well as nectin-1 and nectin-4 [41], consistent with the physiological roles proposed for these interactions.
In the subsequent section, we discuss the molecular determinants responsible for homophilic and heterophilic recognition and selectivity.
Molecular and structural bases of homophilic and heterophilic nectin interactions
Nectins homodimer structures
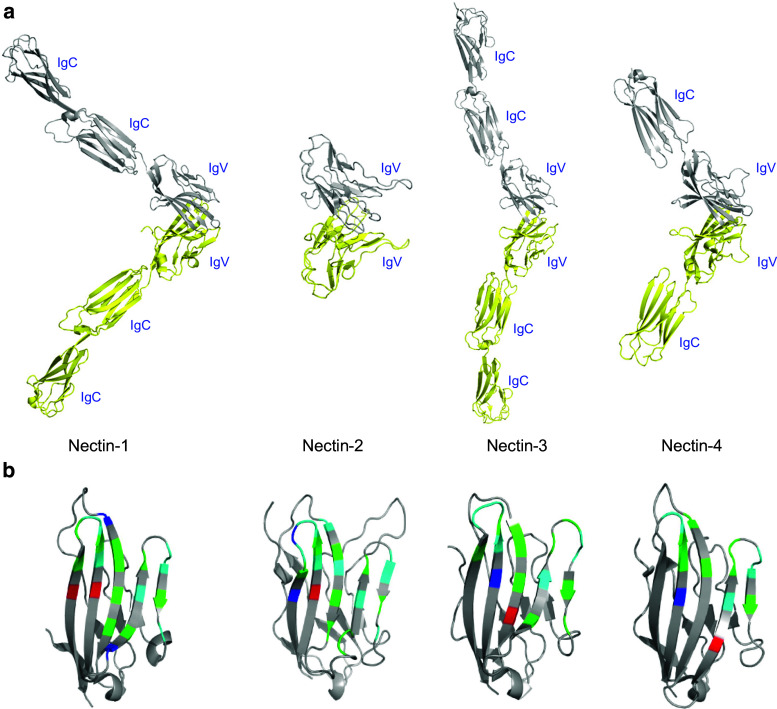
The structures of all the four nectins have been reported, with similar structures arising from non-glycosylated and glycosylated materials generated from bacterial and eukaryotic expression systems, respectively [41–43]. In each case, these molecules form a homodimer in the crystal lattice, which on the basis of mutagenesis experiments is consistent with solution behavior [41–43]. The membrane-distal IgV domain of all nectins exhibits the classic two-layer β-sandwich topology present in other IgV structures, with the front and back sheets being composed of the GFCC′C″ and ABED strands, respectively (Fig. 4a). The dimer interface is formed by nearly orthogonal association of C, C′, C″, F, and G strands from the front sheets of two engaging IgV domains (Fig. 4b). This organization results in a similar “kinked” dimer in all four nectins (Fig. 5a), which is similar to the quaternary structure observed in a number of other physiologically relevant dimers in the IgSF.
Fig. 4.

Structural features of the nectins. a Structure of the IgV domain of human nectin-2 (a representative member of the family) showing the classical two-layer β-sandwich topology. The front and back sheets of the domain are composed of the GFCC′C″ and ABED strands, respectively. b Two monomers (yellow and gray) interact in a nearly orthogonal fashion to form the dimer interface, involving the front β sheets with predominant contribution from the C, C′, C″ and F strands of each monomer. c Key residues of the dimer interface of nectin-2 are represented by ball-and-stick representation. d Structure-based sequence alignment of the human nectins. The secondary structure of the nectin-2 IgV domain is displayed on the top of the alignment. Residues with similar properties are marked by red color, whereas identical residues are colored in white with red background. Interfacial residues (green circles) and the cysteine residues involved in the formation of disulfide bond between B and F strand (blue circle) are marked. The long loop between the D and E strand of nectin-2, which is absent in other nectins, is also shown
Fig. 5.
Ribbon representations of human nectin homodimers showing the overall structural organizations and interfacial residues at the dimer interfaces. a Individual protomers are colored in yellow and grey. The presented structures of nectin-1 (3ALP) and nectin-3 (4FOM) contain all the three extracellular domains, while the structure of nectin-2 (3R0N) has only the IgV and the structure of nectin-4 (4FRW) has the IgV and only a single IgC. b Ribbon diagram of the IgV domains representing the interfacial residues involved in the homodimerization of each nectin. The residues in red/blue represent charged residues, green represents polar residues, and cyan represents hydrophobic residues
The homodimers of nectin-1 and nectin-2 bury a total surface area of 1,699 and 1,823 Å2, respectively, which is significantly larger than most IgV domain-mediated dimer interfaces. The homodimers of nectin-3 and nectin-4 bury a total surface area of about 1,350 Å2. In the crystalline state, necl-5 buries 1,254 Å2 of surface area, but a dimeric species could not be detected in solution [41]. The dimer interfaces are stabilized by a combination of hydrophobic contacts, hydrogen bonds, and salt bridges [41–43]. For example, ten residues (Gln-71, Gln-80, Asn-81, Ser-149, Arg-151, and their symmetry mates) are involved in eight potential hydrogen bonds at the interface of the nectin-2 homodimer (Fig. 4c). van der Waals contacts also make an important contributions to this homophilic binding interface, including Ser-66, Leu-67, His-86, Met-89, Gly-90, Ala-143, Thr-144, and Phe-145 (Fig. 4c), which are partially or completely buried at the dimer interface [42]. Site directed mutagenesis established the critical role of these interfacial residues, as point mutations at the dimer interface of nectin-2 results in destabilization of the homodimer [42].
Structure-based sequence alignment of the IgV domains of the human nectins reveals that most of the interface residues are not conserved in this family, particularly those involved in polar interactions; however, the sequence motif “TFPXG” in the FG loop is well conserved in the family (Fig. 4d). A detailed analysis of homodimer interface reveals that the intercalation of a phenylalanine in the FG loop (Phe129, Phe145, Phe153, and Phe132 in nectin-1, nectin-2, nectin-3, and nectin-4, respectively) into a hydrophobic pocket formed by the C′ and C″ strands of the partner molecule is well conserved. This phenylalanine plays a central role in stabilizing the dimer, as the F145A mutation severely affects the homophilic association of human nectin-2 [42]. Residues that stabilize each homodimer are illustrated in Fig. 5b.
Molecular basis for homophilic versus heterophilic interactions
While homophilic and heterophilic trans-interactions of nectins are implicated in cell–cell adhesion, several biochemical and cell-based studies suggest that heterophilic interactions are stronger than homophilic interactions [34, 41, 44]. Although, recent crystal structures of the four human nectins reveal the detailed view of homophilic adhesive interface, the heterophilic adhesive interfaces remain to be defined. In the absence of direct structural information, mutagenesis data support a model in which heterophilic associations utilize similar binding interfaces, resulting in an overall organization analogous to those observed for homophilic interactions [41, 43]. Based on these similarities, highly plausible and testable models can be proposed for the determinants that control heterophilic nectin assemblies.
One notable feature is the presence of a charged residue in the center of each homophilic interface (Glu125, Glu141, Lys149, and Arg128 in nectin-1, nectin-2, nectin-3 and nectin-4, respectively) facing its symmetry-related residue in the partner molecule [41, 42]. The close proximity of these charged residues at the homophilic interfaces is predicted to result in unfavorable electrostatic interactions that potentially destabilize the dimer (Fig. 6a, b). These charged residues are strictly conserved across species, being glutamic acid in nectin-1 and nectin-2, and lysine and arginine in nectin-3 and nectin-4 [42]. Mutagenesis studies demonstrate the importance of these interactions at the homodimer interface. The E141A mutation in nectin-2 results in a tighter dimer in solution as compared to the wild-type nectin-2, which is consistent with the idea that the close proximity of similarly charged residues at the dimer interface results in a reduction of the homophilic affinity in nectin family members [42]. In addition to this unfavorable electrostatic interaction, many additional interactions contribute to the homophilic interaction. Consistent with the larger buried surface areas, nectin-1 and nectin-2 form relatively strong homodimers due to a greater number of interacting residues at the homophilic adhesive interface relative to nectin-3 and nectin-4 (Fig. 5b).
Fig. 6.

Ribbon representations of homophilic and heterophilic interfaces of nectins. a Homodimer interface of human nectin-2 (PDB ID: 3R0N) showing the close proximity of two negatively charged side chains; Glu-141 is contributed from the F strand of each monomer (yellow and gray). b Dimeric interface of nectin-1 (PDB ID: 3ALP) showing the similar unfavorable repulsive electrostatics as depicted in case of nectin-2. c Molecular model showing the heterodimer interface of nectin-2 (yellow) and nectin-3 (brown). The modeling suggests that E141 of nectin-2 contacts K149 of nectin-3, forming a putative polar interaction at the center of the dimer interface which favors a strong heterophilic interaction
Strong heterophilic binding between cognate nectin pairs (nectin-1 and nectin-3, nectin-1 and nectin-4, and nectin-2 and nectin-3) may arise as the consequence of opposite and complementary charges at the positions analogous to Glu141 in nectin-2 [42], which afford favorable electrostatic contributions. For example, the heterophilic interaction between nectin-2 and nectin-3 is likely enhanced by the formation of ionic interactions between Glu141 (from nectin-2) and Lys149 (from nectin-3) as illustrated in Fig. 6c. Support for this proposal comes from the E141K mutant of nectin-2 which results in increased binding between the mutant and wild-type nectin-2 and mimics a heterophilic interaction (unpublished data). Furthermore, molecular modeling based on the nectin homodimers indicates that the heterophilic interaction is characterized by more hydrophobic contacts than the homophilic interaction [41]. Together, charge compatibility and buried hydrophobic surface area represent the major determinants responsible for stronger heterophilic binding (relative to homophilic binding) among the nectins. These observations highlight the critical concept that receptor-ligand interactions do not evolve to produce the highest possible affinity, but instead evolve to select the affinity that allows for the specificity, kinetics, and associated signaling properties that are biologically optimal.
Structurally characterized dimers represent trans-interaction of nectins
The overall organization of the four nectin homodimers is conserved and their adhesive interfaces are formed by the orthogonal association of membrane-distal IgV domains of two engaging molecules. Geometrically, the observed dimeric assembly of nectins could support either cis- or trans- interactions (i.e., on the same cell surface or between two interacting cell surfaces). Initial studies suggested that the nectin IgV domain is necessary to mediate trans-interactions [44] and the second Ig domain (IgC) contributed to the formation of cis-dimers [45, 46]; while a recent report suggests that nectin-1 forms a cis-dimer through the IgV domain [43]. These cis-dimers are proposed to form trans-interactions via “head-to-head” contacts involving the IgV domains of molecules on engaging cells [43]. However, this “head-to-head” trans-interaction of cis-dimers is likely to be sterically precluded because of the prior involvement of IgV domains to form the putative cis-dimer. For example, the phenylalanine residue in the FG loop of nectin-2, which is known to participate in trans-interaction [41, 47], is not accessible for further interactions because this residue is more than 80 % buried in the canonical dimer interface [42]. Notably, solution studies (size-exclusion chromatography and analytical ultracentrifugation) involving all nectins support the existence of dimers in solution, with no indication of higher-order oligomeric states as would be required by the proposed head-to-head trans-interaction of cis-dimers. However, it is important to appreciate that weak interactions could be missed by these solution approaches and more complex mechanisms cannot be fully ruled out.
In murine nectin-2, Phe-136 is buried at the dimer interface and is important for its trans-interaction but not for the cis-dimerization [41, 47]. The equivalent residue in human nectin-2 and in the other three human nectins is buried at the dimer interface as evidenced by crystal structures and the introduction of a point mutation at this position (for example F145A in human nectin-2) disrupts the dimer in solution [42]. In addition, a recent cross-linking study demonstrates that Phe-136 of mouse nectin-2 is required to mediate cell–cell adhesion in transfected cell lines [41]. All of these biophysical, structural, and functional studies strongly support the crystallographically observed association of nectins as an appropriate model for the trans-interaction that underlies their biological function.
Nectins in immune modulation and host-pathogen interaction
In addition to homophilic and heterophilic interactions within the nectin family, the nectins also bind other proteins that contribute to a range of biological processes, including immune modulation and viral entry.
Nectins as immune modulators
Some of the nectin family members specifically interact with receptors expressed on specialized immune cells (T cells and NK cells), resulting in the transmission of potent immune-modulatory signals [20, 21, 48]. For example, the engagement of nectin-1 by CD96, an immune receptor expressed on T cells, contributes to both adhesive and downstream signaling processes [20, 21, 49]. Of particular note is nectin-2, which plays major roles in immune modulation due to its recognition of CD226 and TIGIT (T cell immunoglobulin and ITIM domain) [50, 51]. Necl-5 [also known as polio virus receptor (PVR)] also recognizes the same CD226 and TIGIT immune receptors [50, 51]. Upon engagement of either nectin-2 or PVR, CD226 delivers stimulatory and TIGIT delivers inhibitory signals to T and NK cells [20, 21, 51–54] (Fig. 7). Nectin-2 is thus part of a complex signaling network consisting of CD226/TIGIT:nectin-2/PVR, which exhibits similarities to the well characterized CD28/CTLA-4:B7-1/B7-2 network (Fig. 7). CD28 and CTLA-4 are the two most important T cell costimulatory molecules, which upon ligation with their ligands B7-1/B7-2 (expressed on antigen presenting cells), deliver stimulatory and inhibitory signals, respectively [55, 56]. The signaling pathways associated with these receptor:ligand interactions make major contributions to the control of the adaptive immune responses in mammals. Like CD28/CTLA-4:B7-1/B7-2 network, the expanded nectin family network also regulates the functional outcome of T cell activation, and perturbation of the balance between activating and inhibitory signals results in increased susceptibility to infection and malignancies (reduced T cell activity) or the induction of autoimmunity (enhanced T cell activity) [57–59].
Fig. 7.
Schematic comparison of CD226/TIGIT:nectin-2/PVR and CD28/CTLA-4:B7-1/B7-2 networks. CD226 is a putative activating receptor-like CD28, whereas TIGIT is an inhibitory receptor-like CTLA-4. Both sets of molecules are engaged by sets of counter-ligands, TIGIT and CD226 by nectin-2 and PVR, and CTLA-4 and CD28 by B7-1 and B7-2. Activation is indicated by ‘+’ and inhibition is indicated by ‘–’ symbol
A number of recent studies suggest that the expression of nectins is up-regulated in different cancers. For example, nectin-4 is highly expressed in lung, breast, and ovarian cancers and is used as a histological and serological marker for breast cancer [60–62]. It was recently reported that the over expression of nectin-4 in cancer tissues promotes anchorage independent growth, which contributes to tumor progression and metastasis [61]. Several reports also suggest the up-regulation of nectin-2 in various cancers, including those of hematopoietic and non-hematopoietic origin [63, 64]. The recognition of nectin-2 on the surface of tumor cells by CD226 expressed on T or NK cells can result in activation and cytotoxicity against the tumor cell [64–66]. In contrast, when nectin-2 is recognized by TIGIT expressed on NK cells and T cells, it inhibits the cytotoxicity of NK and T cells towards the ligand expressing cells [51, 54]. Thus, nectin-2 over expression represents an effective mechanism exploited by malignancies to evade the host immune responses. Furthermore, it has been demonstrated that CD226 knockout mice exhibit enhanced tumorigenicity [67], while TIGIT knockout mice showed uncontrolled T cell proliferation and susceptibility to autoimmune diseases [57]. All of these reports establish the physiological and clinical importance of these receptors-ligand interactions in mediating NK and CD8+ T cell-mediated immune function [57, 67, 68]. Given the recent Food and Drug Administration (FDA) approval of a function blocking monoclonal CTLA-4 antibody (Ipilimumab, Yervoy) for the treatment of late stage melanoma [69–73], cancer immunotherapies targeting receptors that govern NK and T cell function are attracting enormous attention. Similarly, the targeting of CD226/TIGIT:nectin-2 signaling pathways represents valuable new therapeutic strategies for controlling tumors and autoimmune diseases.
While the biological contributions of the CD226/TIGIT:nectin-2/PVR signaling network are becoming increasingly clear, the molecular and structural details of these interactions are only beginning to be defined. A recent report reveals the binding interface between TIGIT and PVR [74]. While the overall recognition mode of TIGIT:PVR interaction is similar to the canonical homophilic interfaces of nectins, the details of the interactions of nectin-2 with CD226 and TIGIT are yet to be explored for the manipulation and subsequent targeting of these molecular interactions. A recent study reported a mutant nectin-2 (having 4 mutations, M89S, P94S, A143S, and F145S at the dimer interface), which is impaired in both homodimerization and CD226 binding [75]. The most conservative interpretation of these data suggests that CD226 recognizes the same nectin-2 surface involved in homodimer formation. Future efforts will need to be directed at expanding our structural understanding of the many biologically important heterophilic interactions formed with the expanded nectin family and the leveraging of this structural information to design mechanistically informative mutants with altered selectivities and affinities for in vitro and in vivo analyses.
Nectin as virus entry receptor
Many viruses exploit the same surfaces of IgSF proteins that mediate homophilic and heterophilic interactions to facilitate viral tropism, attachment and subsequent entry into target cells. Nectin-1 and nectin-2 (also known as poliovirus receptor-like 1 (PVRL1) and PVRL2, respectively) were originally isolated as entry receptors for herpes simplex viruses (HSV)-1 and HSV-2, as they directly interact with viral glycoprotein D (gD) [24, 76–78]. Recent studies also show that the nectin-4 recognizes the measles virus hemagglutinin (MV-H) and serves as an epithelial receptor for measles viral entry [79, 80].
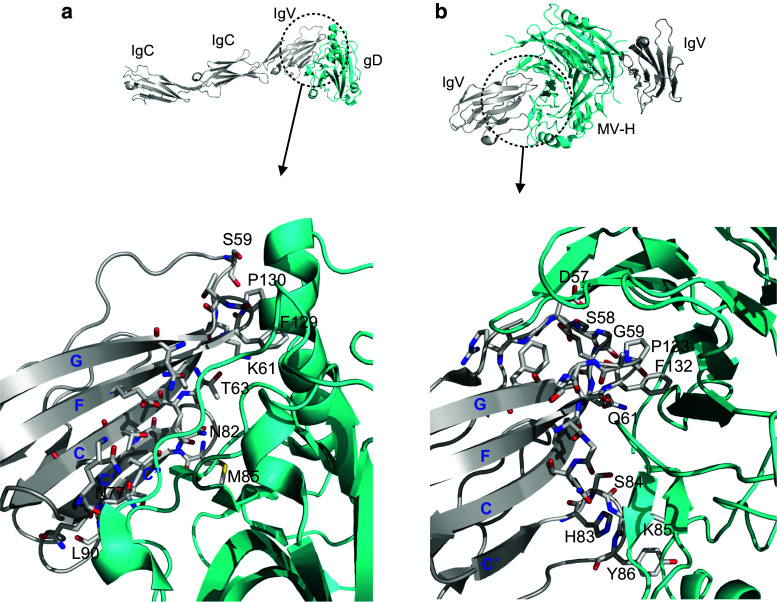
The structure of the complex formed by gD and nectin-1 reveals that gD binds the IgV domain of nectin-1 in a mode similar to that observed in the nectin homodimer and that the key residues responsible for nectin-1 homodimerization are the same ones recognized by HSV gD [81, 82] (Fig. 8a). These findings are consistent with previous reports that gD disrupts the homodimerization of nectin-1 [82]. A recent study also reported the structure of the membran-distal IgV domain of human nectin-4 in complex with MV-H [83]. This structure shows that nectin-4 binds the MV-H exclusively via its N-terminal IgV domain and the contact interface is largely composed of hydrophobic interactions involving the C′–C″, B–C, and F–G loops of nectin-4 (Fig. 8b) [83]. This manner of association represents a distinct recognition mode compared to the homophilic binding interface, highlighting that a range of different strategies have evolved for pathogens to co-opt host cell surface antigens.
Fig. 8.
Crystal structure of nectin-1 in complex with HSV-1 gD (3U82) and nectin-4 with MV-H (4GJT). a A ribbon representation of nectin-1/gD complex structure (nectin-1 in grey and gD in cyan) showing the interacting residues of nectin-1 that are distributed through out the front sheet (C, C′, C″, F, and G strands); most of them are identical to the residues required for nectin-1 homodimerization. b Ribbon representation of nectin-4 IgV/MV-H complex structure (nectin-4 in grey and MV-H in cyan) showing the interacting residues of nectin-4 that are localized in C′–C″, B–C and F–G loops, which are distinct from the residues involved in the homodimerization of nectin-4
Nectins and inheritable human diseases
The participation of nectins in a variety of cellular processes suggests that a number of human disorders may involve defects associated with these proteins. Several reports indicate that truncation mutants of human nectin-1 lacking the transmembrane region and the cytoplasmic tail interfere with its adhesive function, and may also impair the functions of other nectin-1-interacting proteins, including nectin-3 and nectin-4 [84, 85]. These mutations are implicated in cleft lip (also known as palate-ectodermal dysplasia) syndrome, which is clinically characterized by cleft lip/palate, dental anomalies, unusual facial appearance, thickening of palm skin, and in some cases, mental retardation [85–88]. Although the association between nectin-1 and palate-ectodermal dysplasia is identified in human patients, the same phenotype is not observed in nectin-1 knockout mice [39]. A point mutation (T185M) predicted to alter the expression and impair the function of human nectin-4 leads to ectodermal dysplasia-syndactyly syndrome. Affected individuals suffer from a combination of hair and tooth abnormalities, and cutaneous syndactyly (fingers or toes joined together by skin), which is consistent with the higher expression of nectin-4 in hair follicles and the separating digits [89, 90]. Recent reports also suggest a genetic association of single nucleotide polymorphism in human nectin-2 with the late onset of Alzheimer’s disease [91, 92]. Another recent report describes a translocation between chromosome 1 and 3 that is associated with lower expression of nectin-3, with the affected individual showing central nervous system abnormalities and ocular diseases [93]. This finding is consistent with observations that the homozygous deletion of nectin-3 in mice causes lens and other ocular defects [38, 93].
Conclusions and perspectives
We described the structural and biochemical features that control the molecular recognition processes involving the nectins. These determinants are responsible for the spectrum of affinities and interactions associated with the homophilic and heterophilic assemblies formed by the members of the nectin family. The relative homophilic and heterophilic binding affinities of nectins are important for understanding (and modulating) their respective roles in morphogenesis, development, normal physiology, and disease. Strong homophilic binding of nectin-1 and nectin-2 (in comparison to nectin-3 and nectin-4) suggests potential homophilic roles in vivo; however, the contributions to fitness have not been fully defined as a consequence of functional redundancy. The notably stronger heterophilic interactions, which have been observed in a number of cell-based studies, are due, in large part, to favorable and unfavorable electrostatic complementarity at the heterophilic and homophilic interfaces, respectively. Of particular interest will be the continued study of the synergy between the nectin and cadherin adhesive system. Especially notable is the provocative finding that nectin-2 and N-cadherin interact, suggesting a tight and regulated coupling between these two systems.
In addition to cell–cell adhesion, the nectins play important roles in immune modulation and viral entry. Involvement of CD226/TIGIT:nectin-2/PVR interactions in normal physiology and disease makes this signaling network a highly attractive target for therapeutic intervention. Although a recent study provides insights on the TIGIT:PVR interaction at the atomic level, the molecular mechanisms underlying the recognition of nectin-2 by CD226 and TIGIT, as well as other heterophilic associations, are yet to be explored. Recent advances in our structural understanding of the interactions between nectins and viral entry receptors (gD or MV-H) defined the mechanism and determinants underlying host-pathogen recognition, and afford new opportunities for the structure-guided design of inhibitor/therapeutics targeted against HSV-1 and measles. Going forward, additional structural and mechanistic studies of the expanded nectin family, and its numerous interactions, will be required to continue defining their roles in normal physiology and disease.
Acknowledgments
We thank Vladimir Vigdorovich for helpful comments and discussions. This work was supported by National Institutes of Health Grants GM094662 and GM094665 (to SCA). The Albert Einstein Cancer Center is supported by NIH P30CA013330.
Contributor Information
Dibyendu Samanta, Phone: (718) 430-2745, Email: dibyendu.samanta@einstein.yu.edu.
Steven C. Almo, Email: steve.almo@einstein.yu.edu
References
- 1.Yap AS, Brieher WM, Gumbiner BM. Molecular and functional analysis of cadherin-based adherens junctions. Annu Rev Cell Dev Biol. 1997;13:119–146. doi: 10.1146/annurev.cellbio.13.1.119. [DOI] [PubMed] [Google Scholar]
- 2.Takai Y, Ikeda W, Ogita H, Rikitake Y. The immunoglobulin-like cell adhesion molecule nectin and its associated protein afadin. Annu Rev Cell Dev Biol. 2008;24:309–342. doi: 10.1146/annurev.cellbio.24.110707.175339. [DOI] [PubMed] [Google Scholar]
- 3.Takai Y, Miyoshi J, Ikeda W, Ogita H. Nectins and nectin-like molecules: roles in contact inhibition of cell movement and proliferation. Nat Rev Mol Cell Biol. 2008;9(8):603–615. doi: 10.1038/nrm2457. [DOI] [PubMed] [Google Scholar]
- 4.Harris TJ, Tepass U. Adherens junctions: from molecules to morphogenesis. Nat Rev Mol Cell Biol. 2010;11(7):502–514. doi: 10.1038/nrm2927. [DOI] [PubMed] [Google Scholar]
- 5.Sakisaka T, Takai Y. Biology and pathology of nectins and nectin-like molecules. Curr Opin Cell Biol. 2004;16(5):513–521. doi: 10.1016/j.ceb.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 6.Rikitake Y, Mandai K, Takai Y. The role of nectins in different types of cell–cell adhesion. J Cell Sci. 2012;125:3713–3722. doi: 10.1242/jcs.099572. [DOI] [PubMed] [Google Scholar]
- 7.Shimono Y, Rikitake Y, Mandai K, Mori M, Takai Y. Immunoglobulin superfamily receptors and adherens junctions. Subcell Biochem. 2012;60:137–170. doi: 10.1007/978-94-007-4186-7_7. [DOI] [PubMed] [Google Scholar]
- 8.Chothia C, Jones EY. The molecular structure of cell adhesion molecules. Annu Rev Biochem. 1997;66:823–862. doi: 10.1146/annurev.biochem.66.1.823. [DOI] [PubMed] [Google Scholar]
- 9.Taylor ME, Drickamer K. Paradigms for glycan-binding receptors in cell adhesion. Curr Opin Cell Biol. 2007;19(5):572–577. doi: 10.1016/j.ceb.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 10.Brasch J, Harrison OJ, Honig B, Shapiro L. Thinking outside the cell: how cadherins drive adhesion. Trends Cell Biol. 2012;22(6):299–310. doi: 10.1016/j.tcb.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel SD, Chen CP, Bahna F, Honig B, Shapiro L. Cadherin-mediated cell–cell adhesion: sticking together as a family. Curr Opin Struct Biol. 2003;13(6):690–698. doi: 10.1016/j.sbi.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 12.Halbleib JM, Nelson WJ. Cadherins in development: cell adhesion, sorting, and tissue morphogenesis. Genes Dev. 2006;20(23):3199–3214. doi: 10.1101/gad.1486806. [DOI] [PubMed] [Google Scholar]
- 13.Takai Y, Nakanishi H. Nectin and afadin: novel organizers of intercellular junctions. J Cell Sci. 2003;116:17–27. doi: 10.1242/jcs.00167. [DOI] [PubMed] [Google Scholar]
- 14.Shapiro L, Weis WI. Structure and biochemistry of cadherins and catenins. Cold Spring Harb Perspect Biol. 2009;1(3):a003053. doi: 10.1101/cshperspect.a003053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shimoyama Y, Tsujimoto G, Kitajima M, Natori M. Identification of three human type-II classic cadherins and frequent heterophilic interactions between different subclasses of type-II classic cadherins. Biochem J. 2000;349:159–167. doi: 10.1042/0264-6021:3490159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Katsamba P, Carroll K, Ahlsen G, Bahna F, Vendome J, Posy S, Rajebhosale M, Price S, Jessell TM, Ben-Shaul A, et al. Linking molecular affinity and cellular specificity in cadherin-mediated adhesion. Proc Natl Acad Sci USA. 2009;106(28):11594–11599. doi: 10.1073/pnas.0905349106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Straub BK, Rickelt S, Zimbelmann R, Grund C, Kuhn C, Iken M, Ott M, Schirmacher P, Franke WW. E-N-cadherin heterodimers define novel adherens junctions connecting endoderm-derived cells. J Cell Biol. 2011;195(5):873–887. doi: 10.1083/jcb.201106023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mizoguchi A, Nakanishi H, Kimura K, Matsubara K, Ozaki-Kuroda K, Katata T, Honda T, Kiyohara Y, Heo K, Higashi M, et al. Nectin: an adhesion molecule involved in formation of synapses. J Cell Biol. 2002;156(3):555–565. doi: 10.1083/jcb.200103113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yagi T, Takeichi M. Cadherin superfamily genes: functions, genomic organization, and neurologic diversity. Genes Dev. 2000;14(10):1169–1180. [PubMed] [Google Scholar]
- 20.Chan CJ, Andrews DM, Smyth MJ. Receptors that interact with nectin and nectin-like proteins in the immunosurveillance and immunotherapy of cancer. Curr Opin Immunol. 2012;24(2):246–251. doi: 10.1016/j.coi.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 21.Fuchs A, Colonna M. The role of NK cell recognition of nectin and nectin-like proteins in tumor immunosurveillance. Semin Cancer Biol. 2006;16(5):359–366. doi: 10.1016/j.semcancer.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 22.Delpeut S, Noyce RS, Siu RW, Richardson CD. Host factors and measles virus replication. Curr Opin Virol. 2012;2(6):773–783. doi: 10.1016/j.coviro.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 23.Noyce RS, Richardson CD. Nectin 4 is the epithelial cell receptor for measles virus. Trends Microbiol. 2012;20(9):429–439. doi: 10.1016/j.tim.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 24.Geraghty RJ, Krummenacher C, Cohen GH, Eisenberg RJ, Spear PG. Entry of alphaherpesviruses mediated by poliovirus receptor-related protein 1 and poliovirus receptor. Science. 1998;280(5369):1618–1620. doi: 10.1126/science.280.5369.1618. [DOI] [PubMed] [Google Scholar]
- 25.Mandai K, Nakanishi H, Satoh A, Obaishi H, Wada M, Nishioka H, Itoh M, Mizoguchi A, Aoki T, Fujimoto T, et al. Afadin: a novel actin filament-binding protein with one PDZ domain localized at cadherin-based cell-to-cell adherens junction. J Cell Biol. 1997;139(2):517–528. doi: 10.1083/jcb.139.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rubinstein R, Ramagopal UA, Nathenson SG, Almo SC, Fiser A. Functional classification of immune regulatory proteins. Structure. 2013;21(5):766–776. doi: 10.1016/j.str.2013.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Biederer T. Bioinformatic characterization of the SynCAM family of immunoglobulin-like domain-containing adhesion molecules. Genomics. 2006;87(1):139–150. doi: 10.1016/j.ygeno.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 28.Okabe N, Shimizu K, Ozaki-Kuroda K, Nakanishi H, Morimoto K, Takeuchi M, Katsumaru H, Murakami F, Takai Y. Contacts between the commissural axons and the floor plate cells are mediated by nectins. Dev Biol. 2004;273(2):244–256. doi: 10.1016/j.ydbio.2004.05.034. [DOI] [PubMed] [Google Scholar]
- 29.Togashi H, Kominami K, Waseda M, Komura H, Miyoshi J, Takeichi M, Takai Y. Nectins establish a checkerboard-like cellular pattern in the auditory epithelium. Science. 2011;333(6046):1144–1147. doi: 10.1126/science.1208467. [DOI] [PubMed] [Google Scholar]
- 30.Inagaki M, Irie K, Ishizaki H, Tanaka-Okamoto M, Miyoshi J, Takai Y. Role of cell adhesion molecule nectin-3 in spermatid development. Genes Cells. 2006;11(9):1125–1132. doi: 10.1111/j.1365-2443.2006.01006.x. [DOI] [PubMed] [Google Scholar]
- 31.Tachibana K, Nakanishi H, Mandai K, Ozaki K, Ikeda W, Yamamoto Y, Nagafuchi A, Tsukita S, Takai Y. Two cell adhesion molecules, nectin and cadherin, interact through their cytoplasmic domain-associated proteins. J Cell Biol. 2000;150(5):1161–1176. doi: 10.1083/jcb.150.5.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ikeda W, Nakanishi H, Miyoshi J, Mandai K, Ishizaki H, Tanaka M, Togawa A, Takahashi K, Nishioka H, Yoshida H, et al. Afadin: a key molecule essential for structural organization of cell–cell junctions of polarized epithelia during embryogenesis. J Cell Biol. 1999;146(5):1117–1132. doi: 10.1083/jcb.146.5.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tanaka-Okamoto M, Hori K, Ishizaki H, Itoh Y, Onishi S, Yonemura S, Takai Y, Miyoshi J. Involvement of afadin in barrier function and homeostasis of mouse intestinal epithelia. J Cell Sci. 2011;124:2231–2240. doi: 10.1242/jcs.081000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Satoh-Horikawa K, Nakanishi H, Takahashi K, Miyahara M, Nishimura M, Tachibana K, Mizoguchi A, Takai Y. Nectin-3, a new member of immunoglobulin-like cell adhesion molecules that shows homophilic and heterophilic cell–cell adhesion activities. J Biol Chem. 2000;275(14):10291–10299. doi: 10.1074/jbc.275.14.10291. [DOI] [PubMed] [Google Scholar]
- 35.Aoki J, Koike S, Asou H, Ise I, Suwa H, Tanaka T, Miyasaka M, Nomoto A. Mouse homolog of poliovirus receptor-related gene 2 product, mPRR2, mediates homophilic cell aggregation. Exp Cell Res. 1997;235(2):374–384. doi: 10.1006/excr.1997.3685. [DOI] [PubMed] [Google Scholar]
- 36.Ozaki-Kuroda K, Nakanishi H, Ohta H, Tanaka H, Kurihara H, Mueller S, Irie K, Ikeda W, Sakai T, Wimmer E, et al. Nectin couples cell–cell adhesion and the actin scaffold at heterotypic testicular junctions. Curr Biol. 2002;12(13):1145–1150. doi: 10.1016/S0960-9822(02)00922-3. [DOI] [PubMed] [Google Scholar]
- 37.Bouchard MJ, Dong Y, McDermott BM, Jr, Lam DH, Brown KR, Shelanski M, Bellvé AR, Racaniello VR. Defects in nuclear and cytoskeletal morphology and mitochondrial localization in spermatozoa of mice lacking nectin-2, a component of cell–cell adherens junctions. Mol Cell Biol. 2000;20(8):2865–2873. doi: 10.1128/MCB.20.8.2865-2873.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Inagaki M, Irie K, Ishizaki H, Tanaka-Okamoto M, Morimoto K, Inoue E, Ohtsuka T, Miyoshi J, Takai Y. Roles of cell-adhesion molecules nectin 1 and nectin 3 in ciliary body development. Development. 2005;132(7):1525–1537. doi: 10.1242/dev.01697. [DOI] [PubMed] [Google Scholar]
- 39.Yoshida T, Miyoshi J, Takai Y, Thesleff I. Cooperation of nectin-1 and nectin-3 is required for normal ameloblast function and crown shape development in mouse teeth. Dev Dyn. 2010;239(10):2558–2569. doi: 10.1002/dvdy.22395. [DOI] [PubMed] [Google Scholar]
- 40.Morita H, Nandadasa S, Yamamoto TS, Terasaka-Iioka C, Wylie C, Ueno N. Nectin-2 and N-cadherin interact through extracellular domains and induce apical accumulation of F-actin in apical constriction of Xenopus neural tube morphogenesis. Development. 2010;137(8):1315–1325. doi: 10.1242/dev.043190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harrison OJ, Vendome J, Brasch J, Jin X, Hong S, Katsamba PS, Ahlsen G, Troyanovsky RB, Troyanovsky SM, Honig B, Shapiro L. Nectin ectodomain structures reveal a canonical adhesive interface. Nat Struct Mol Biol. 2012;19(9):906–915. doi: 10.1038/nsmb.2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Samanta D, Ramagopal UA, Rubinstein R, Vigdorovich V, Nathenson SG, Almo SC. Structure of Nectin-2 reveals determinants of homophilic and heterophilic interactions that control cell–cell adhesion. Proc Natl Acad Sci USA. 2012;109(37):14836–14840. doi: 10.1073/pnas.1212912109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Narita H, Yamamoto Y, Suzuki M, Miyazaki N, Yoshida A, Kawai K, Iwasaki K, Nakagawa A, Takai Y, et al. Crystal Structure of the cis-Dimer of Nectin-1: implications for the architecture of cell–cell junctions. J Biol Chem. 2011;286(14):12659–12669. doi: 10.1074/jbc.M110.197368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reymond N, Fabre S, Lecocq E, Adelaïde J, Dubreuil P, Lopez M. Nectin4/PRR4, a new afadin-associated member of the nectin family that trans-interacts with nectin1/PRR1 through V domain interaction. J Biol Chem. 2001;276(46):43205–43215. doi: 10.1074/jbc.M103810200. [DOI] [PubMed] [Google Scholar]
- 45.Momose Y, Honda T, Inagaki M, Shimizu K, Irie K, Nakanishi H, Takai Y. Role of the second immunoglobulin-like loop of nectin in cell–cell adhesion. Biochem Biophys Res Commun. 2002;293(1):45–49. doi: 10.1016/S0006-291X(02)00183-3. [DOI] [PubMed] [Google Scholar]
- 46.Yasumi M, Shimizu K, Honda T, Takeuchi M, Takai Y. Role of each immunoglobulin-like loop of nectin for its cell–cell adhesion activity. Biochem Biophys Res Commun. 2003;302(1):61–66. doi: 10.1016/S0006-291X(03)00106-2. [DOI] [PubMed] [Google Scholar]
- 47.Miyahara M, Nakanishi H, Takahashi K, Satoh-Horikawa K, Tachibana K, Takai Y. Interaction of nectin with afadin is necessary for its clustering at cell–cell contact sites but not for its cis dimerization or trans interaction. J Biol Chem. 2000;275(1):613–618. doi: 10.1074/jbc.275.1.613. [DOI] [PubMed] [Google Scholar]
- 48.Chan CJ, Martinet L, Gilfillan S, Souza-Fonseca-Guimaraes F, Chow MT, Town L, Ritchie DS, Colonna M, Andrews DM, Smyth MJ. The receptors CD96 and CD226 oppose each other in the regulation of natural killer cell functions. Nat Immunol. 2014;15(5):431–438. doi: 10.1038/ni.2850. [DOI] [PubMed] [Google Scholar]
- 49.Seth S, Maier MK, Qiu Q, Ravens I, Kremmer E, Förster R, Bernhardt G. The murine pan T cell marker CD96 is an adhesion receptor for CD155 and nectin-1. Biochem Biophys Res Commun. 2007;364(4):959–965. doi: 10.1016/j.bbrc.2007.10.102. [DOI] [PubMed] [Google Scholar]
- 50.Bottino C, Castriconi R, Pende D, Rivera P, Nanni M, Carnemolla B, Cantoni C, Grassi J, Marcenaro S, Reymond N, et al. Identification of PVR (CD155) and Nectin-2 (CD112) as cell surface ligands for the human DNAM-1 (CD226) activating molecule. J Exp Med. 2003;198(4):557–567. doi: 10.1084/jem.20030788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yu X, Harden K, Gonzalez LC, Francesco M, Chiang E, Irving B, Tom I, Ivelja S, Refino CJ, Clark H, et al. The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nat Immunol. 2009;10(1):48–57. doi: 10.1038/ni.1674. [DOI] [PubMed] [Google Scholar]
- 52.Pende D, Bottino C, Castriconi R, Cantoni C, Marcenaro S, Rivera P, Spaggiari GM, Dondero A, Carnemolla B, Reymond N, et al. PVR (CD155) and Nectin-2 (CD112) as ligands of the human DNAM-1 (CD226) activating receptor: involvement in tumor cell lysis. Mol Immunol. 2005;42(4):463–469. doi: 10.1016/j.molimm.2004.07.028. [DOI] [PubMed] [Google Scholar]
- 53.Tahara-Hanaoka S, Shibuya K, Onoda Y, Zhang H, Yamazaki S, Miyamoto A, Honda S, Lanier LL, Shibuya A. Functional characterization of DNAM-1 (CD226) interaction with its ligands PVR (CD155) and nectin-2 (PRR-2/CD112) Int Immunol. 2004;16(4):533–538. doi: 10.1093/intimm/dxh059. [DOI] [PubMed] [Google Scholar]
- 54.Stanietsky N, Simic H, Arapovic J, Toporik A, Levy O, Novik A, Levine Z, Beiman M, Dassa L, Achdout H, et al. The interaction of TIGIT with PVR and PVRL2 inhibits human NK cell cytotoxicity. Proc Natl Acad Sci USA. 2009;106(42):17858–17863. doi: 10.1073/pnas.0903474106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lenschow DJ, Walunas TL, Bluestone JA. CD28/B7 system of T cell costimulation. Annu Rev Immunol. 1996;14:233–258. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- 56.Chen L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol. 2004;4(5):336–347. doi: 10.1038/nri1349. [DOI] [PubMed] [Google Scholar]
- 57.Joller N, Hafler JP, Brynedal B, Kassam N, Spoerl S, Levin SD, Sharpe AH, Kuchroo VK. Cutting edge: TIGIT has T cell-intrinsic inhibitory functions. J Immunol. 2011;186(3):1338–1342. doi: 10.4049/jimmunol.1003081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Levin SD, Taft DW, Brandt CS, Bucher C, Howard ED, Chadwick EM, Johnston J, Hammond A, Bontadelli K, Ardourel D, et al. Vstm3 is a member of the CD28 family and an important modulator of T-cell function. Eur J Immunol. 2011;41(4):902–915. doi: 10.1002/eji.201041136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Joller N, Lozano E, Burkett PR, Patel B, Xiao S, Zhu C, Xia J, Tan TG, Sefik E, Yajnik V, et al. Treg cells expressing the coinhibitory molecule TIGIT selectively inhibit proinflammatory Th1 and Th17 cell responses. Immunity. 2014;40(4):569–581. doi: 10.1016/j.immuni.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Takano A, Ishikawa N, Nishino R, Masuda K, Yasui W, Inai K, Nishimura H, Ito H, Nakayama H, Miyagi Y, et al. Identification of nectin-4 oncoprotein as a diagnostic and therapeutic target for lung cancer. Cancer Res. 2009;69(16):6694–6703. doi: 10.1158/0008-5472.CAN-09-0016. [DOI] [PubMed] [Google Scholar]
- 61.Pavlova NN, Pallasch C, Elia AE, Braun CJ, Westbrook TF, Hemann M, Elledge SJ. A role for PVRL4-driven cell–cellinteractions in tumorigenesis. Elife. 2013;2:e00358. doi: 10.7554/eLife.00358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fabre-Lafay S, Monville F, Garrido-Urbani S, Berruyer-Pouyet C, Ginestier C, Reymond N, Finetti P, Sauvan R, Adélaïde J, Geneix J, et al. Nectin-4 is a new histological and serological tumor associated marker for breast cancer. BMC Cancer. 2007;7:73. doi: 10.1186/1471-2407-7-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Oshima T, Sato S, Kato J, Ito Y, Watanabe T, Tsuji I, Hori A, Kurokawa T, Kokubo T. Nectin-2 is a potential target for antibody therapy of breast and ovarian cancers. Mol Cancer. 2013;12:60. doi: 10.1186/1476-4598-12-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lakshmikanth T, Burke S, Ali TH, Kimpfler S, Ursini F, Ruggeri L, Capanni M, Umansky V, Paschen A, Sucker A, et al. NCRs and DNAM-1 mediate NK cell recognition and lysis of human and mouse melanoma cell lines in vitro and in vivo. J Clin Invest. 2009;119(5):1251–1263. doi: 10.1172/JCI36022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shibuya A, Campbell D, Hannum C, Yssel H, Franz-Bacon K, McClanahan T, Kitamura T, Nicholl J, Sutherland GR, Lanier LL, Phillips JH. DNAM-1, a novel adhesion molecule involved in the cytolytic function of T lymphocytes. Immunity. 1996;4(6):573–581. doi: 10.1016/S1074-7613(00)70060-4. [DOI] [PubMed] [Google Scholar]
- 66.Carlsten M, Björkström NK, Norell H, Bryceson Y, van Hall T, Baumann BC, Hanson M, Schedvins K, Kiessling R, Ljunggren HG, Malmberg KJ. DNAX accessory molecule-1 mediated recognition of freshly isolated ovarian carcinoma by resting natural killer cells. Cancer Res. 2007;67(3):1317–1325. doi: 10.1158/0008-5472.CAN-06-2264. [DOI] [PubMed] [Google Scholar]
- 67.Iguchi-Manaka A, Kai H, Yamashita Y, Shibata K, Tahara-Hanaoka S, Honda S, Yasui T, Kikutani H, Shibuya K, Shibuya A. Accelerated tumor growth in mice deficient in DNAM-1 receptor. J Exp Med. 2008;205(13):2959–2964. doi: 10.1084/jem.20081611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hafler JP, Maier LM, Cooper JD, Plagnol V, Hinks A, Simmonds MJ, Stevens HE, Walker NM, Healy B, Howson JM. CD226 Gly307Ser association with multiple autoimmune diseases. Genes Immun. 2009;10(1):5–10. doi: 10.1038/gene.2008.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lipson EJ, Drake CG. Ipilimumab: an anti-CTLA-4 antibody for metastatic melanoma. Clin Cancer Res. 2011;17(22):6958–6962. doi: 10.1158/1078-0432.CCR-11-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sharma P, Wagner K, Wolchok JD, Allison JP. Novel cancer immunotherapy agents with survival benefit: recent successes and next steps. Nat Rev Cancer. 2011;11(11):805–812. doi: 10.1038/nrc3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sarnaik AA, Weber JS. Recent advances using anti-CTLA-4 for the treatment of melanoma. Cancer J. 2009;15(3):169–173. doi: 10.1097/PPO.0b013e3181a7450f. [DOI] [PubMed] [Google Scholar]
- 72.Callahan MK, Wolchok JD, Allison JP. Anti-CTLA-4 antibody therapy: immune monitoring during clinical development of a novel immunotherapy. Semin Oncol. 2010;37(5):473–484. doi: 10.1053/j.seminoncol.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lipson EJ, Bodell MA, Kraus ES, Sharfman WH (2014) Successful administration of Ipilimumab to two kidney transplantation patients with metastatic melanoma. J Clin Oncol (Epub ahead of print) [DOI] [PMC free article] [PubMed]
- 74.Stengel KF, Harden-Bowles K, Yu X, Rouge L, Yin J, Comps-Agrar L, Wiesmann C, Bazan JF, Eaton DL, Grogan JL. Structure of TIGIT immunoreceptor bound to poliovirus receptor reveals a cell–celladhesion and signaling mechanism that requires cis-trans receptor clustering. Proc Natl Acad Sci USA. 2012;109(14):5399–5404. doi: 10.1073/pnas.1120606109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu J, Qian X, Chen Z, Xu X, Gao F, Zhang S, Zhang R, Qi J, Gao GF, Yan J. Crystal structure of cell adhesion molecule nectin-2/CD112 and its binding to immune receptor DNAM-1/CD226. J Immunol. 2012;188(11):5511–5520. doi: 10.4049/jimmunol.1200324. [DOI] [PubMed] [Google Scholar]
- 76.Lopez M, Cocchi F, Menotti L, Avitabile E, Dubreuil P, Campadelli-Fiume G. Nectin2alpha (PRR2alpha or HveB) and nectin2delta are low-efficiency mediators for entry of herpes simplex virus mutants carrying the Leu25Pro substitution in glycoprotein D. J Virol. 2000;74(3):1267–1274. doi: 10.1128/JVI.74.3.1267-1274.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Manoj S, Jogger CR, Myscofski D, Yoon M, Spear PG. Mutations in herpes simplex virus glycoprotein D that prevent cell entry via nectins and alter cell tropism. Proc Natl Acad Sci USA. 2004;101(34):12414–12421. doi: 10.1073/pnas.0404211101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Spear PG, Manoj S, Yoon M, Jogger CR, Zago A, Myscofski D. Different receptors binding to distinct interfaces on herpes simplex virus gD can trigger events leading to cell fusion and viral entry. Virology. 2006;344(1):17–24. doi: 10.1016/j.virol.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 79.Noyce RS, Bondre DG, Ha MN, Lin LT, Sisson G, Tsao MS, Richardson CD. Tumor cell marker PVRL4 (nectin 4) is an epithelial cell receptor for measles virus. PLoS Pathog. 2011;7(8):e1002240. doi: 10.1371/journal.ppat.1002240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mühlebach MD, Mateo M, Sinn PL, Prüfer S, Uhlig KM, Leonard VH, Navaratnarajah CK, Frenzke M, Wong XX, Sawatsky B, et al. Adherens junction protein nectin-4 is the epithelial receptor for measles virus. Nature. 2011;480(7378):530–533. doi: 10.1038/nature10639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang N, Yan J, Lu G, Guo Z, Fan Z, Wang J, Shi Y, Qi J, Gao GF. Binding of herpes simplex virus glycoprotein D to nectin-1 exploits host cell adhesion. Nat Commun. 2011;2:577. doi: 10.1038/ncomms1571. [DOI] [PubMed] [Google Scholar]
- 82.Di Giovine P, Settembre EC, Bhargava AK, Luftig MA, Lou H, Cohen GH, Eisenberg RJ, Krummenacher C, Carfi A. Structure of herpes simplex virus glycoprotein D bound to the human receptor nectin-1. PLoS Pathog. 2011;7(9):e1002277. doi: 10.1371/journal.ppat.1002277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang X, Lu G, Qi J, Li Y, He Y, Xu X, Shi J, Zhang CW, Yan J, Gao GF. Structure of measles virus hemagglutinin bound to its epithelial receptor nectin-4. Nat Struct Mol Biol. 2013;20(1):67–72. doi: 10.1038/nsmb.2432. [DOI] [PubMed] [Google Scholar]
- 84.Sözen MA, Suzuki K, Tolarova MM, Bustos T, Fernández Iglesias JE, Spritz RA. Mutation of PVRL1 is associated with sporadic, non-syndromic cleft lip/palate in northern Venezuela. Nat Genet. 2001;29(2):141–142. doi: 10.1038/ng740. [DOI] [PubMed] [Google Scholar]
- 85.Suzuki K, Hu D, Bustos T, Zlotogora J, Richieri-Costa A, Helms JA, Spritz RA. Mutations of PVRL1, encoding a cell–celladhesion molecule/herpesvirus receptor, in cleft lip/palate-ectodermal dysplasia. Nat Genet. 2000;25(4):427–430. doi: 10.1038/78119. [DOI] [PubMed] [Google Scholar]
- 86.Bustos T, Simosa V, Pinto-Cisternas J, Abramovits W, Jolay L, Rodriguez L, Fernandez L, Ramela M. Autosomal recessive ectodermal dysplasia: I. An undescribed dysplasia/malformation syndrome. Am J Med Genet. 1991;41(4):398–404. doi: 10.1002/ajmg.1320410403. [DOI] [PubMed] [Google Scholar]
- 87.Zlotogora J. On the inheritance of the split hand/split foot malformation. Am J Med Genet. 1994;53(1):29–32. doi: 10.1002/ajmg.1320530107. [DOI] [PubMed] [Google Scholar]
- 88.Zlotogora J, Zilberman Y, Tenenbaum A, Wexler MR. Cleft lip and palate, pili torti, malformed ears, partial syndactyly of fingers and toes, and mental retardation: a new syndrome? J Med Genet. 1987;24(5):291–293. doi: 10.1136/jmg.24.5.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Brancati F, Fortugno P, Bottillo I, Lopez M, Josselin E, Boudghene-Stambouli O, Agolini E, Bernardini L, Bellacchio E, Iannicelli M, et al. Mutations in PVRL4, encoding cell adhesion molecule nectin-4, cause ectodermal dysplasia-syndactyly syndrome. Am J Hum Genet. 2010;87(2):265–273. doi: 10.1016/j.ajhg.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Brancati F, Agolini E, Fortugno P. Nectinopathies: an emerging group of ectodermal dysplasia syndromes. G Ital Dermatol Venereol. 2013;148(1):59–64. [PubMed] [Google Scholar]
- 91.Logue MW, Schu M, Vardarajan BN, Buros J, Green RC, Go RC, Griffith P, Obisesan TO, Shatz R, Borenstein A, et al. A comprehensive genetic association study of Alzheimer disease in African Americans. Arch Neurol. 2011;68(12):1569–1579. doi: 10.1001/archneurol.2011.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Takei N, Miyashita A, Tsukie T, Arai H, Asada T, Imagawa M, Shoji M, Higuchi S, Urakami K, Kimura H, et al. Genetic association study on in and around the APOE in late-onset Alzheimer disease in Japanese. Genomics. 2009;93(5):441–448. doi: 10.1016/j.ygeno.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 93.Lachke SA, Higgins AW, Inagaki M, Saadi I, Xi Q, Long M, Quade BJ, Talkowski ME, Gusella JF, Fujimoto A, et al. The cell adhesion gene PVRL3 is associated with congenital ocular defects. Hum Genet. 2012;131(2):235–250. doi: 10.1007/s00439-011-1064-z. [DOI] [PMC free article] [PubMed] [Google Scholar]