Abstract
Cardiomyocytes continuously generate the contractile force to circulate blood through the body. Imbalances in contractile performance or energy supply cause adaptive responses of the heart resulting in adverse rearrangement of regular structures, which in turn might lead to heart failure. At the cellular level, cardiomyocyte remodeling includes (1) restructuring of the contractile apparatus; (2) rearrangement of the cytoskeleton; and (3) changes in energy metabolism. Dedifferentiation represents a key feature of cardiomyocyte remodeling. It is characterized by reciprocal changes in the expression pattern of “mature” and “immature” cardiomyocyte-specific genes. Dedifferentiation may enable cardiomyocytes to cope with hypoxic stress by disassembly of the energy demanding contractile machinery and by reduction of the cellular energy demand. Dedifferentiation during myocardial repair might provide cardiomyocytes with additional plasticity, enabling survival under hypoxic conditions and increasing the propensity to enter the cell cycle. Although dedifferentiation of cardiomyocytes has been described during tissue regeneration in zebrafish and newts, little is known about corresponding mechanisms and regulatory circuits in mammals. The recent finding that the cytokine oncostatin M (OSM) is pivotal for cardiomyocyte dedifferentiation and exerts strong protective effects during myocardial infarction highlights the role of cytokines as potent stimulators of cardiac remodeling. Here, we summarize the current knowledge about transient dedifferentiation of cardiomyocytes in the context of myocardial remodeling, and propose a model for the role of OSM in this process.
Keywords: Dilative cardiomyopathy, Hibernating myocardium, Hypertrophy, Hypoxia-inducible transcription factor, Myocardial infarction, Myocardial remodeling, Myoglobin, Oncostatin M
Introduction
In the developing embryo, the heart becomes the first functional organ, due to the increasing need for the distribution of nutrients and oxygen throughout the body [1]. The continuous contraction of the heart requires generation of high-energy phosphates in cardiomyocytes throughout their lifetime. To meet the steady increase in workload and energy demand, the heart switches from glycolysis to oxidative metabolism during the embryonic to early neonatal transition period [2, 3]. Cardiomyocytes reach a mature state during young adulthood concomitant with an increased rate of β-oxidation of fatty acids, which provide ca. 90 % of the total cardiac energy supply in adult hearts [1]. The close relationship between the energy consuming contraction machinery and the metabolic apparatus [4] is also evident during cardiac remodeling events, and will affect the contractile power of the myocardium.
The term cardiac remodeling was originally coined to describe “changes that result in rearrangement of normally existing structures” [5]. It occurs as a morphological transition from healthy to adapted tissue that is prone to destabilization and progression toward heart failure. At the cellular level, remodeling causes stress to the whole cardiomyocyte by altering energy metabolism, the structure of the contractile apparatus and the cytoskeleton. Remodeling is intimately connected to cellular dedifferentiation, indicated by a loss of characteristics of mature (differentiated) cardiomyocytes and re-expression of genes from embryonic or fetal developmental stages. Current concepts propose that cardiomyocytes gain resistance to altered metabolic and/or hypoxic stress by dedifferentiation or sarcomeric disassembly to survive and regenerate [6–9]. Relatively little is known about the corresponding mechanisms and regulatory circuits and their impact for myocardial regeneration in human beings or in mice, although several groups have addressed the importance of cardiomyocyte dedifferentiation for tissue regeneration in zebrafish and amphibians [10–13].
The classical view of the heart as a simple mechanical pump has led to the development of concepts in which it is regarded that heart failure is a hemodynamic disorder, thereby putting emphasis on approaches to improve contractile force [5, 14]. Such a mechanical view, however, does not adequately address the highly complex nature of cardiac adaptation to pathological conditions. Cardiomyocytes need to adjust metabolic and contractile activities to (patho-) physiological requirements. Clearly, if cardiomyocyte were locked in a differentiated state not allowing disassembly of the contractile apparatus and return to a more immature mode of energy production, such plasticity would be difficult to achieve. Indeed, dedifferentiation allows the myocardium to cope much better with hypoxia-induced or overload-induced cell death, preventing the progression from compensated hypertrophy to heart failure (HF) [15].
The discovery of cardiac stem cells and the potential for their use in therapeutic applications has led to novel attempts to reverse HF (reviewed in [16, 17]). However, repairing damaged adult hearts by administration of stem cells is much debated and has caused major scientific controversies [18], thereby questioning the validity and effectiveness of stem cell based therapies to treat heart failure. Stimulation of the endogenous repair potential of the myocardium, which might not necessarily rely on cardiac stem cells, seems to provide an alternative means to improve cardiac function. We hypothesize that the “on-board” resources of adult cardiomyocytes (i.e. the capability for dedifferentiation during remodeling) provide sufficient plasticity to deal with tissue damage, allowing functional recovery if damage is not excessive and temporarily restricted.
Remodeling of the diseased heart
The adult heart compensates for increased workload by physiologic growth to normalize myocardial wall stress while maintaining the differentiated status of cardiomyocytes. Classical examples are post-natal developmental hypertrophy [2, 3] and pregnancy-induced heart growth [19]. In athletes, physiologic adaptation can reach a remarkable extent, with up to 60 % increase of left ventricular mass without signs of cardiac failure [20, 21]. Importantly, structural cardiac adaptations during adolescence, pregnancy or endurance training must not be confused with compensatory remodeling in the stressed heart (reviewed by [22]), although in both cases, altered hemodynamics contribute to changes in the cardiac architecture. The term ‘increased workload’ is not well defined, and does not suffice to describe cardiac stress for both athletes and patients with valve defects or myocardial infarcts. More accurate, multi-parametric descriptions of (patho-) physiological tissue responses are needed to stratify therapeutic concepts (for review see [23]). Physiologic adaptation typically preserves the proportions of the heart, i.e. the ratio of wall thickness to lumen or the proportions of all four chambers, while compensatory responses to pathological stimuli usually start with concentric or dilative remodeling of the stressed chamber. Moreover, compensatory remodeling is accompanied by inflammatory infiltration, increase in interstitial connective tissue (increasing the oxygen diffusion distance and wall stiffness), reduction of cardiomyocyte cell contacts (myocytes slippage), and apoptotic cell death ultimately reducing the ventricular ejection capacity. Such maladaptive processes are absent in physiologic responses (reviewed in [23–25]). Here, we refer to the term ‘remodeling’ only to describe compensatory remodeling of the diseased heart, but not to structural adaptation of the growing healthy heart.
In animal models, cardiac ischemia is used to study compensatory remodeling. It comprises response mechanisms caused by the lack of oxygen and includes secondary inflammatory infiltration. Importantly, remodeling affects both ischemic and non-ischemic regions of the ventricle with consequences for chamber size, shape and function. While smaller damages might only lead to hypertrophy of remaining cardiomyocytes in order to normalize wall stress, large infarcts cause global changes in heart architecture. One underlying mechanism for this global response is the so-called infarct expansion that contributes significantly to disproportionate ventricular dilatation and wall thinning [26]. Underlying mechanisms include myocardial cell death, as well as cardiomyocyte ‘slippage’ [27]. Myocyte slippage results from cardiomyocyte mobilization, which requires loosening of cell-to-cell contacts and subsequent dynamic rearrangement of cardiomyocytes. Failure to normalize contractile function in the ischemic heart results in dilative cardiomyopathy (DCM), which can also be caused by genetic predisposition or environmental factors, such as infections with cardiotropic viruses [28–30]. Indeed, studies in hearts of DCM patients revealed a high prevalence of viral genomes (67.4 %) [28] and a considerable amount of local cytokine concentrations [31, 32], highlighting the potential role of chronic cardiac inflammation in the development of contractile dysfunction.
The detection of elevated levels of monocyte chemotactic protein-1 (MCP-1), a potent attractor for macrophages, in the failing heart substantiates the importance of low-level chronic inflammation for the pathogenesis of DCM [33]. Macrophages attracted by MCP-1 contribute to local cytokine production after homing to sites of injury, further promoting adverse remodeling events if accumulation of inflammatory cells is not resolved [34, 35]. Several pathogenic features of DCM are recapitulated in transgenic animals expressing MCP-1 in the heart, thus providing a useful model to study DCM, especially as MCP-1 transgenic animals robustly succumb to cardiac failure at 6 months of age [36]. Cardiac remodeling is associated with multiple changes in the morphology of cardiomyocytes [15, 37]. In principle, the degree of cardiomyocyte degeneration and loss of differentiation markers can be correlated with the extent of inflammatory infiltration, which is prominent in the failing myocardium and in the border zone of myocardial infarcts [8, 38].
Cardiomyocyte dedifferentiation in the diseased heart
The disorganization of cytoskeletal proteins and loss of sarcomeric structures contribute to the pathogenesis of contractile dysfunction [15, 37]. The loss of contractile force is most evident in end-stage heart failure, and is associated with gene expression profiles typical for embryonic and fetal hearts [9]. Genes re-expressed in dedifferentiated cardiomyocytes include early cardiac transcription factors such as GATA4 and Nkx2.5 [39], but also markers normally found in stem cells such as c-kit, Runx1 and Dab2, which might contribute to the enhanced propensity of dedifferentiated cardiomyocytes to enter the cell cycle [8, 38]. Other characteristic markers of dedifferentiation include destrin, α-SM-actin, the natriuretic peptides ANP/BNP, smooth-muscle actinin (ACTN1), and moesin, which are all expressed in cardiomyocytes under pathological conditions, e.g. in the infarct zone, in hibernating myocardium or in cardiomyopathic tissue [8, 38].
Recently, we were able to show increased levels of the cytokine oncostatin M (OSM) in the myocardium after acute infarction, after ischemia/reperfusion, and in DCM patients at sites of extensive remodeling [8, 40]. Genetic inactivation of the OSM receptor reduces the degree of cardiomyocyte dedifferentiation in infarct areas and results in increased mortality [8]. Furthermore, inactivation of the OSM receptor prevents dedifferentiation of cardiomyocytes in an animal model of dilated cardiomyopathy [8]. Taken together, these data suggest that OSM is a critical player in cardiac remodeling by inducing cardiomyocyte dedifferentiation in ischemic and chronically inflamed myocardium. OSM might be also instrumental to reprogram cardiomyocytes in hibernating regions of the myocardium, allowing for survival of cardiomyocytes in conditions of reduced supply of nutrients and oxygen. Hibernation, which is characterized by regional dysfunction of myocardial tissue with persistently reduced contraction and decreased coronary blood flow, is reversible after revascularization [41]. Similarly, cardiomyocyte dedifferentiation induced by OSM is also partially reversible. OSM-induced, dedifferentiated adult cardiomyocytes respond to hypertrophic signals, e.g. insulin-like growth factor-1 (IGF-1), and are able to regain a functional, differentiated phenotype both in vivo and in vitro [38, 42]. In vivo, the recovery of contraction in hibernating myocardium is seen after revascularization, while stimulation of OSM-treated de-differentiated cardiomyocytes in vitro with IGF-1 induces formation of new sarcomeres, even in the absence of mechanical load (unpublished observation).
Of note, the actin depolymerizing protein destrin and α-SM actin (α-SM-actin) are markers of cytoskeletal reorganization of cardiomyocytes and are re-expressed in failing hearts [8, 38]. α-SM-actin re-expression has been observed in hibernating cardiomyocytes after chronic ischemia [43] and in the infarct border zones [44]. During early cardiogenesis, α-SM-actin is broadly expressed in the heart together with other smooth muscle cell genes, and precedes expression of α-cardiac actin and α-skeletal actin [45, 46]. The function of α-SM-actin during cardiomyogenesis, however, is unclear but has been associated with regulation of sarcomere formation [47]. In newborn rats, α-skeletal actin is expressed in cardiomyocytes, and is rapidly replaced by α-cardiac actin [48]. Since α-skeletal actin transcripts increase together with hemodynamic load, α-skeletal actin has been used as a biomarker similar to ANP/BNP. It is tempting to speculate that the switch in actin isoforms also has consequences for cardiac tissue architecture: a characteristic feature of actin filaments is their close interaction with plasma membranes, thereby linking the intracellular milieu to the extracellular matrix via integrins [25]. Re-expression of the cytoskeletal linker protein moesin in patients with dilative cardiomyopathy is indicative for ongoing cytoskeletal reorganization [38].
We propose that whole organ remodeling and cardiomyocyte dedifferentiation are intimately linked. Intracellular cardiomyocyte reorganization might enforce cardiac remodeling by contributing to the stability of the cardiomyocytes during pathophysiologic stressors, as described above. If the condition is not resolved rapidly, it ultimately paves the road to myocyte slippage and infarct expansion.
Remodeling of cardiomyocytes in vitro and in vivo
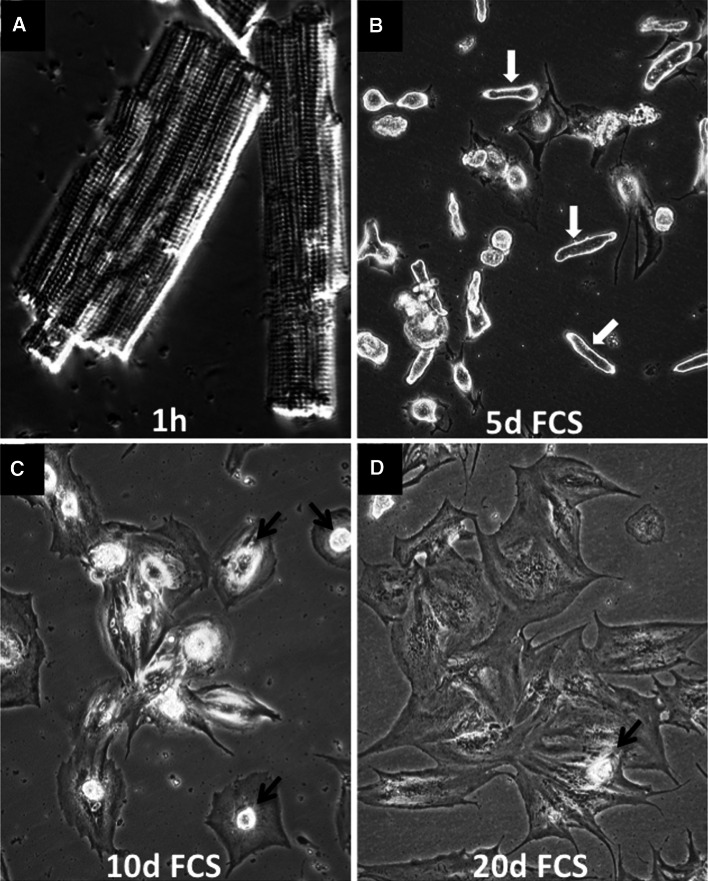
Although cardiac remodeling is a complex process characterized by the interplay of cardiomyocytes with non-cardiomyocytes such as fibroblasts, endothelial, and smooth muscle cells, in vitro experiments with isolated cardiomyocytes are needed to dissect the underlying molecular mechanisms. It is evident, however, that conclusions from in vitro experiments have to be evaluated carefully. Similar care is needed when selecting the experimental in vitro system. Due to their accessibility and ease of culturing, neonatal cardiomyocytes have been widely used to study processes of cardiomyocyte remodeling. However, neonatal cardiomyocytes differ substantially from terminally differentiated adult cardiomyocytes, both in respect to morphology and response to environmental cues [38, 49]. In contrast to neonatal cardiomyocytes, freshly isolated adult cardiomyocytes are characterized by a highly organized three-dimensional structure including sarcomeres, which can be easily identified within cardiomyocytes by phase contrast microscopy (Fig. 1a). Isolated adult cardiomyocytes are rather fragile, which is not fully explained by the collagenase perfusion required to release cardiomyocytes from the myocardium. Rather, the high susceptibility of adult cardiomyocytes to mechanical stress during the isolation procedure reflects the need of cardiomyocytes to adapt to changes in cardiac architecture in order to avoid unfavorable mechanical distortion. In vitro studies of long-term cultures of cardiomyocytes revealed that fully differentiated cardiomyocytes reestablish cell–cell contacts and return to synchronously contracting tissue-like sheets [31, 50], a process that requires complex cellular remodeling already starting 1 day after plating. Cardiomyocytes cultured in fetal calf serum round up and gradually lose their marked cross-striated appearance (white arrows in Fig. 1b), which allows spreading (black arrows in Fig. 1c, d). Existing cellular structures continuously disappear with the onset of remodeling. Since adult cardiomyocytes hardly migrate, cell-to-cell contacts are reestablished through cellular expansion (Fig. 1c, d). At high cell density, cardiomyocytes partially re-differentiate and accumulate sarcomeric proteins. After 2–3 weeks, re-differentiated cardiomyocytes begin to contract synchronously in a tissue-like sheet. These observations indicate that changes of the contractile and cytoskeletal apparatus are reversible features of cardiomyocytes, which enable cardiac remodeling during hypertrophy or after myocardial damage [14].
Fig. 1.
Morphology of cardiomyocytes in culture. Phase contrast micrographs of cultured adult rat cardiomyocytes freshly isolated (a) or after 5, 10 and 20 days in culture (b–d). Cultures were treated with 5 % fetal calf serum (FCS). White arrows cardiomyocytes round up apically and gradually loose cross-striated appearance. Black arrows rounded-up cardiomyocytes. Note the spreading of cardiomyocytes after FCS stimulation
Several factors have been identified to govern remodeling of cardiomyocytes in vivo. Most prominent are the Fibroblast (FGF) and Insulin-like growth factors (IGF), which are potent stimulators of adult cardiomyocyte growth and remodeling [42, 50]. FGF-2 (basic FGF) increases cell size, and induces cytoskeletal remodeling and re-expression of α-smooth muscle actin (α-SM actin), while myofibrillar growth is restrained [50]. In contrast, IGF-1 suppresses accumulation of α-SM actin, but promotes myofibrillar growth [42]. Only recently, it was found that OSM regulates reversion of cardiomyocytes to a more immature state, which might have an important effect on myocardial regeneration. In vitro, OSM favors elongation of cardiomyocytes towards neighboring cells, where cell-to-cell contacts are rapidly reestablished through filopodia-like extensions (Fig. 2). Extensions are continuously built and actin microfilaments are reconstructed while the main cell body remains stationary. The level of sarcomeric α-actinin protein dramatically decreases in OSM-treated cardiomyocytes [8], and cardiomyocytes show massive structural re-organization. Sarcomeric proteins such as α- and β-myosin heavy chain genes and the giant protein titin, needed for formation of new intact sarcomeres [51], are downregulated in OSM-treated cardiomyocytes. This observation correlates with clinical findings, where a lack of sarcomeres has been recognized as an important contribution to decreased compliance in failing hearts [15]. FGF-2 induces remodeling of adult cardiomyocytes and re-expression of α-SM-actin and ANP [52, 53], similar to OSM. However, FGF-2 treatment maintains a cross-striated pattern and contractile capacities of cardiomyocytes [50], while OSM causes an almost complete loss of sarcomeres. Furthermore, morphological changes in cardiomyocytes induced by FGF-2 in vitro depend on long-term stimulation with high serum concentrations and triiodothyronine [50], whereas OSM requires only short-term, low serum concentration during the initial attachment phase [8]. Inhibition of the OSM-receptor by siRNA inhibits cardiomyocyte dedifferentiation and re-expression of α-SM-actin, and prevents formation of cell-to-cell contacts (Fig. 2) [8], which are hallmarks of increased plasticity of cardiomyocytes in vivo. Surprisingly, inhibition of MEK/ERK signaling also prevented dedifferentiation of cardiomyocytes by OSM [8], although the Raf/MEK/ERK pathway has been described to promote hypertrophy of cardiomyocytes in vivo and in vitro [54]. It seems likely that context-dependent effects strongly affect the outcome of MEK/ERK pathway activation, which might explain such conflicting results.
Fig. 2.
Oncostatin M induces dedifferentiation of adult cardiomyocytes. Confocal images of adult rat cardiomyocytes after 7 days in culture stained with α-SM actin (red), sarcomeric α-actinin (green), and DAPI (blue). The upper panel represents merged images of all three channels (blue, red, green). Cultures were pretreated with 2 % FCS for 2 days and then kept in 2 % FCS (con) or stimulated with oncostatin M (OSM). Knock-down of the OSM receptor by siRNA (OSM + siOβ) prevents dedifferentiation. OSM treatment results in a massive loss of mature sarcomeres and morphological changes with typical cell extensions to re-establish cell-to-cell contacts. Scale bars left and middle rows 30 μm, right row 50 μm
Dedifferentiating cardiomyocytes undergo structural remodeling and changes in the gene expression profile. The concomitant induction of both processes by OSM argues for a tight linkage in the diseased and failing heart [8, 38], although further studies are necessary to investigate whether loss of sarcomeres can be uncoupled from re-expression of fetal genes. Decreased lethality of mice treated with OSM and increased lethality of mice lacking the OSM receptor after cardiac infarction suggest that transient dedifferentiation of cardiomyocytes improves survival of stressed cardiomyocytes. At present, however, it is unclear whether dedifferentiation of cardiomyocytes provides a basis for cardiac regeneration.
Dedifferentiation of cardiomyocytes might allow metabolic adaptation to reduced oxygen supply
Cardiac remodeling and metabolic adaptation are closely interconnected, as reflected by the characteristic switch from lipid to glucose utilization in stressed cardiomyocytes. Although changes in mitochondrial metabolism seem initially beneficial to sustain contractility and survival, increasing evidence suggests that metabolic reprogramming might eventually result in mitochondrial dysfunction and cardiac failure (for review see [55]). However, it is still debated whether mitochondrial adaptation is an epiphenomenon or a cause of heart failure. Clearly, further research is needed to answer the question as to whether a switch in substrate utilization is beneficial or detrimental for disease progression (for a review, see [56]).
The failing, mechanically dysfunctional heart suffers from an imbalance between oxygen consumption and contractile needs. As a consequence, the failing compared to the healthy heart requires more energy, and hence more oxygen. Structural abnormalities in late stage heart failure are independent from its etiology and generally restrict the physical activities of patients severely [57]. In this respect, it is noteworthy that the breakdown of the sarcomeric apparatus in the failing heart includes loss of myoglobin [58–60], which limits cardiac availability of oxygen to cardiomyocytes. Oxygen availability on the other hand, is a prerequisite for β-oxidation of fatty acids, the primary substrate of cardiomyocytes. Hence, dedifferentiation of cardiomyocytes, including loss of myoglobin, reduces oxygen-dependent generation of ATP, but maintains the ability of heart cells to generate energy by glycolysis. This phenomenon seems to support the conclusion that the metabolic switch in the remodeling myocardium is an epiphenomenon of the failing heart, and not a means to maintain basic cellular functions in an adverse hypoxic environment. In contrast, the fact that myoglobin-deficient adult mice are viable and maintain normal cardiac function illustrates that the myocardium is able to cope with reduced oxygen availability by activation of compensatory mechanisms [61, 62]. OSM-stimulated cardiomyocytes downregulate myoglobin together with myomesin 2, β-myosin heavy chain and cardiac myosin regulatory light chain 2 (MLC-2) [8], indicating profound changes in the metabolism of cardiomyocytes. We propose that OSM-mediated downregulation of myoglobin represents an adaptation to reduced oxidative phosphorylation in dedifferentiated cardiomyocytes. This assumption is supported by OSM-mediated stimulation of expression of hypoxia-inducible transcription factors 1α (HIF-1α) and 2 (Epas1, HIF-2α), and heat shock protein 27 (hsp27), which enable cardiomyocytes to survive reduced oxygen supply (unpublished observations). Without HIF-1α, such an adaption is not possible, as illustrated by the arrest of embryonic development, which proceeds at low oxygen concentrations, in the absence of HIF-1α [63].
It has been postulated that energy metabolism itself (β-oxidation of fatty acids versus glycolysis of carbohydrates) is critical for maintenance of the differentiated phenotype of cardiomyocytes [9]. Glucose-dependent energy metabolism is characterized by high levels of glucose transporters Glut1 and Glut4 (Slc2a1 and Slc2a4 in rodents) and muscle glycogen synthase (Gys1), whereas enzymes of the β-oxidation pathway are indicative for utilization of fatty acids and mitochondrial oxidative phosphorylation. Expression of enzymes of the β-oxidation pathway and mitochondrial biogenesis are controlled by the peroxisome proliferator-activated nuclear receptors (PPARs) and their co-activators (PPARGCs) [64]. OSM stimulation in vitro does not affect expression of proteins or genes regulating mitochondrial biogenesis, but causes upregulation of pyruvate dehydrogenase kinases (Pdk3 and 4), key negative regulators of the TCA cycle, indicating increased dependency on carbohydrates (unpublished observations). Interestingly, Pdk4 gene expression is upregulated in remodeling hypertrophic and atrophic rat hearts [65]. Moreover, dedifferentiated cardiomyocytes show reduced expression of mitochondrial complex I subunits, which corresponds to a decreased influx of NADH resulting from metabolic remodeling due to upregulation of Pdk. Despite the decline of complex I-associated respiration during early phases of heart failure [66] and in dedifferentiated cardiomyocytes, the extent of metabolic reprogramming in dedifferentiated cardiomyocytes does not seem to reach the level of metabolic remodeling and increased mitochondrial biogenesis during post-natal heart development [2, 3]. This might suggest that dedifferentiated cardiomyocytes remain in a metabolic “stand-by” mode, which allows them to return quickly to a fully differentiated state and to more effective oxidative phosphorylation of fatty acids. Taken together, the available data support the idea that the metabolic switch to carbohydrate-dependent energy production by OSM is protective for cardiomyocytes and represents a reversible state.
Conclusion and future directions
The recent findings that cardiomyocytes display surprising cellular plasticity in response to stress emphasize the need for a better understanding of adaptive processes including cardiomyocyte dedifferentiation. Cardiomyocytes usually exhibit a stereotypical response to pathological stimuli, such as activation of the embryonic or fetal gene program, reduced oxidative metabolism and dedifferentiation, thereby facilitating survival and allowing organ regeneration. In the stressed heart, the co-existence of remodeling and dedifferentiation provides the necessary plasticity to adapt, survive and regain functionality (Fig. 3). We propose that cardiomyocytes in the heart maintain a dynamic balance between a mature working and a dedifferentiated, “regenerative” state, which resembles hibernation. Dedifferentiation and hypertrophic signals provide the extra time needed by the heart to remodel and adapt to hypoxia for reconstitution of functionality. Dedifferentiation allows cardiomyocytes to re-shape and re-build new cell-to-cell contacts, to execute a metabolic shift, and probably also to generate a certain (low) amount of new cardiomyocytes. Hypertrophic stimuli enable cardiomyocytes in the remodeling zone to grow in size to decrease transmural wall stress (Fig. 3). Most likely, the genes and proteins described so far in dedifferentiated cardiomyocytes [8, 38] represent only “the tip of the iceberg”. State-of-the-art profiling techniques (e.g. next generation sequencing) combined with sorting of dedifferentiated cardiomyocytes using appropriate markers should soon overcome this shortcoming, and lead to identification of additional molecules and a more comprehensive view of dedifferentiation. The identification and characterization of key regulatory cytokines such as OSM provides new options to develop therapeutic drugs and strategies. Clearly, careful cellular tracing of dedifferentiated cardiomyocytes in vivo and loss-of-function studies of potential regulators of cardiomyocyte dedifferentiation are needed to substantiate this concept.
Fig. 3.
Model of cardiomyocyte remodeling and dedifferentiation during cardiac regeneration and repair. Activation of oncostatin M (OSM) induces dedifferentiation and hibernation in surviving cardiomyocytes. Dedifferentiation might be reversed after revascularization and hypertrophic stimulation. Invading macrophages remove debris and release OSM in the damaged heart. Cardiomyocytes dedifferentiate in response to OSM and re-establish cell–cell contacts. Hypertrophic signals (IGF-1) induce re-differentiation and hypertrophy of cardiomyocytes. Extended presence of dedifferentiation signals compromises contractility and promotes adverse myocardial remodeling
Acknowledgments
The authors thank all members of the laboratory and specifically Sawa Kostin for continuous help, Jaakko Pohjoismäki (University of Eastern Finland, FIN) and Christopher Carroll (University of Helsinki, FIN) for critically reviewing this manuscript. Work in the laboratory was supported by the Max-Planck-Society, the DFG (Excellence Initiative “Cardiopulmonary System” and SFB/TRR 81), the University of Giessen-Marburg Lung Center (UGMLC) and the Cell and Gene Therapy Center (CGT) at the University of Frankfurt.
Conflict of interest
The authors declare that they have no conflicting commercial interests related to this work.
References
- 1.Lopaschuk GD, Jaswal JS. Energy metabolic phenotype of the cardiomyocyte during development, differentiation, and postnatal maturation. J Cardiovasc Pharmacol. 2010;56(2):130–140. doi: 10.1097/FJC.0b013e3181e74a14. [DOI] [PubMed] [Google Scholar]
- 2.Pohjoismaki JL, Boettger T, Liu Z, Goffart S, Szibor M, Braun T. Oxidative stress during mitochondrial biogenesis compromises mtDNA integrity in growing hearts and induces a global DNA repair response. Nucleic Acids Res. 2012;40(14):6595–6607. doi: 10.1093/nar/gks301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pohjoismaki JL, Kruger M, Al-Furoukh N, Lagerstedt A, Karhunen PJ, Braun T. Postnatal cardiomyocyte growth and mitochondrial reorganization cause multiple changes in the proteome of human cardiomyocytes. Mol BioSyst. 2013 doi: 10.1039/c3mb25556e. [DOI] [PubMed] [Google Scholar]
- 4.Rajabi M, Kassiotis C, Razeghi P, Taegtmeyer H. Return to the fetal gene program protects the stressed heart: a strong hypothesis. Heart Fail Rev. 2007;12(3–4):331–343. doi: 10.1007/s10741-007-9034-1. [DOI] [PubMed] [Google Scholar]
- 5.Cohn JN, Ferrari R, Sharpe N. Cardiac remodeling–concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. Behalf of an international forum on cardiac remodeling. J Am Coll Cardiol. 2000;35(3):569–582. doi: 10.1016/S0735-1097(99)00630-0. [DOI] [PubMed] [Google Scholar]
- 6.Bersell K, Arab S, Haring B, Kuhn B. Neuregulin1/ErbB4 signaling induces cardiomyocyte proliferation and repair of heart injury. Cell. 2009;138(2):257–270. doi: 10.1016/j.cell.2009.04.060. [DOI] [PubMed] [Google Scholar]
- 7.Engel FB, Schebesta M, Duong MT, Lu G, Ren S, Madwed JB, Jiang H, Wang Y, Keating MT. p38 MAP kinase inhibition enables proliferation of adult mammalian cardiomyocytes. Genes Dev. 2005;19(10):1175–1187. doi: 10.1101/gad.1306705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kubin T, Poling J, Kostin S, Gajawada P, Hein S, Rees W, Wietelmann A, Tanaka M, Lorchner H, Schimanski S, Szibor M, Warnecke H, Braun T. Oncostatin M is a major mediator of cardiomyocyte dedifferentiation and remodeling. Cell Stem Cell. 2011;9(5):420–432. doi: 10.1016/j.stem.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 9.Taegtmeyer H, Sen S, Vela D. Return to the fetal gene program: a suggested metabolic link to gene expression in the heart. Ann N Y Acad Sci. 2010;1188:191–198. doi: 10.1111/j.1749-6632.2009.05100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jopling C, Sleep E, Raya M, Marti M, Raya A, Izpisua Belmonte JC. Zebrafish heart regeneration occurs by cardiomyocyte dedifferentiation and proliferation. Nature. 2010;464(7288):606–609. doi: 10.1038/nature08899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laube F, Heister M, Scholz C, Borchardt T, Braun T. Re-programming of newt cardiomyocytes is induced by tissue regeneration. J Cell Sci. 2006;119(Pt 22):4719–4729. doi: 10.1242/jcs.03252. [DOI] [PubMed] [Google Scholar]
- 12.Zhang R, Han P, Yang H, Ouyang K, Lee D, Lin YF, Ocorr K, Kang G, Chen J, Stainier DY, Yelon D, Chi NC. In vivo cardiac reprogramming contributes to zebrafish heart regeneration. Nature. 2013;498(7455):497–501. doi: 10.1038/nature12322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kikuchi K, Holdway JE, Werdich AA, Anderson RM, Fang Y, Egnaczyk GF, Evans T, Macrae CA, Stainier DY, Poss KD. Primary contribution to zebrafish heart regeneration by gata4(+) cardiomyocytes. Nature. 2010;464(7288):601–605. doi: 10.1038/nature08804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fedak PW, Verma S, Weisel RD, Li RK. Cardiac remodeling and failure from molecules to man (Part II) Cardiovasc pathol: Off J Soc Cardiovasc Pathol. 2005;14(2):49–60. doi: 10.1016/j.carpath.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 15.Hein S, Arnon E, Kostin S, Schonburg M, Elsasser A, Polyakova V, Bauer EP, Klovekorn WP, Schaper J. Progression from compensated hypertrophy to failure in the pressure-overloaded human heart: structural deterioration and compensatory mechanisms. Circulation. 2003;107(7):984–991. doi: 10.1161/01.CIR.0000051865.66123.B7. [DOI] [PubMed] [Google Scholar]
- 16.Anversa P, Kajstura J, Rota M, Leri A. Regenerating new heart with stem cells. J Clin Investig. 2013;123(1):62–70. doi: 10.1172/JCI63068. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Mazhari R, Hare JM. Translational findings from cardiovascular stem cell research. Trends Cardiovasc Med. 2012;22(1):1–6. doi: 10.1016/j.tcm.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jesty SA, Steffey MA, Lee FK, Breitbach M, Hesse M, Reining S, Lee JC, Doran RM, Nikitin AY, Fleischmann BK, Kotlikoff MI. c-kit + precursors support postinfarction myogenesis in the neonatal, but not adult, heart. Proc Natl Acad Sci USA. 2012;109(33):13380–13385. doi: 10.1073/pnas.1208114109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mesa A, Jessurun C, Hernandez A, Adam K, Brown D, Vaughn WK, Wilansky S. Left ventricular diastolic function in normal human pregnancy. Circulation. 1999;99(4):511–517. doi: 10.1161/01.CIR.99.4.511. [DOI] [PubMed] [Google Scholar]
- 20.Fagard R. Athlete’s heart. Heart. 2003;89(12):1455–1461. doi: 10.1136/heart.89.12.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Milliken MC, Stray-Gundersen J, Peshock RM, Katz J, Mitchell JH. Left ventricular mass as determined by magnetic resonance imaging in male endurance athletes. Am J Cardiol. 1988;62(4):301–305. doi: 10.1016/0002-9149(88)90228-7. [DOI] [PubMed] [Google Scholar]
- 22.Weeks KL, McMullen JR. The athlete’s heart vs. the failing heart: can signaling explain the two distinct outcomes? Physiology. 2011;26(2):97–105. doi: 10.1152/physiol.00043.2010. [DOI] [PubMed] [Google Scholar]
- 23.Mann N, Rosenzweig A. Can exercise teach us how to treat heart disease? Circulation. 2012;126(22):2625–2635. doi: 10.1161/CIRCULATIONAHA.111.060376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hou J, Kang YJ. Regression of pathological cardiac hypertrophy: signaling pathways and therapeutic targets. Pharmacol Ther. 2012;135(3):337–354. doi: 10.1016/j.pharmthera.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kostin S, Hein S, Arnon E, Scholz D, Schaper J. The cytoskeleton and related proteins in the human failing heart. Heart Fail Rev. 2000;5(3):271–280. doi: 10.1023/A:1009813621103. [DOI] [PubMed] [Google Scholar]
- 26.Zardini P, Marino P, Golia G, Anselmi M, Castelli M. Ventricular remodeling and infarct expansion. Am J Cardiol. 1993;72(19):98G–106G. doi: 10.1016/0002-9149(93)90114-R. [DOI] [PubMed] [Google Scholar]
- 27.Olivetti G, Capasso JM, Sonnenblick EH, Anversa P. Side-to-side slippage of myocytes participates in ventricular wall remodeling acutely after myocardial infarction in rats. Circ Res. 1990;67(1):23–34. doi: 10.1161/01.RES.67.1.23. [DOI] [PubMed] [Google Scholar]
- 28.Kuhl U, Pauschinger M, Noutsias M, Seeberg B, Bock T, Lassner D, Poller W, Kandolf R, Schultheiss HP. High prevalence of viral genomes and multiple viral infections in the myocardium of adults with “idiopathic” left ventricular dysfunction. Circulation. 2005;111(7):887–893. doi: 10.1161/01.CIR.0000155616.07901.35. [DOI] [PubMed] [Google Scholar]
- 29.Poller W, Kuhl U, Tschoepe C, Pauschinger M, Fechner H, Schultheiss HP. Genome-environment interactions in the molecular pathogenesis of dilated cardiomyopathy. J Mol Med. 2005;83(8):579–586. doi: 10.1007/s00109-005-0664-2. [DOI] [PubMed] [Google Scholar]
- 30.Shioi T, Matsumori A, Kihara Y, Inoko M, Ono K, Iwanaga Y, Yamada T, Iwasaki A, Matsushima K, Sasayama S. Increased expression of interleukin-1 beta and monocyte chemotactic and activating factor/monocyte chemoattractant protein-1 in the hypertrophied and failing heart with pressure overload. Circ Res. 1997;81(5):664–671. doi: 10.1161/01.RES.81.5.664. [DOI] [PubMed] [Google Scholar]
- 31.Horwitz MS, La Cava A, Fine C, Rodriguez E, Ilic A, Sarvetnick N. Pancreatic expression of interferon-gamma protects mice from lethal coxsackievirus B3 infection and subsequent myocarditis. Nat Med. 2000;6(6):693–697. doi: 10.1038/76277. [DOI] [PubMed] [Google Scholar]
- 32.Shioi T, Matsumori A, Sasayama S. Persistent expression of cytokine in the chronic stage of viral myocarditis in mice. Circulation. 1996;94(11):2930–2937. doi: 10.1161/01.CIR.94.11.2930. [DOI] [PubMed] [Google Scholar]
- 33.Seino Y, Ikeda U, Sekiguchi H, Morita M, Konishi K, Kasahara T, Shimada K. Expression of leukocyte chemotactic cytokines in myocardial tissue. Cytokine. 1995;7(3):301–304. doi: 10.1006/cyto.1995.0037. [DOI] [PubMed] [Google Scholar]
- 34.Apostolakis S, Lip GY, Shantsila E. Monocytes in heart failure: relationship to a deteriorating immune overreaction or a desperate attempt for tissue repair? Cardiovasc Res. 2010;85(4):649–660. doi: 10.1093/cvr/cvp327. [DOI] [PubMed] [Google Scholar]
- 35.van Amerongen MJ, Harmsen MC, van Rooijen N, Petersen AH, van Luyn MJ. Macrophage depletion impairs wound healing and increases left ventricular remodeling after myocardial injury in mice. Am J pathol. 2007;170(3):818–829. doi: 10.2353/ajpath.2007.060547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kolattukudy PE, Quach T, Bergese S, Breckenridge S, Hensley J, Altschuld R, Gordillo G, Klenotic S, Orosz C, Parker-Thornburg J. Myocarditis induced by targeted expression of the MCP-1 gene in murine cardiac muscle. Am J Pathol. 1998;152(1):101–111. [PMC free article] [PubMed] [Google Scholar]
- 37.Heling A, Zimmermann R, Kostin S, Maeno Y, Hein S, Devaux B, Bauer E, Klovekorn WP, Schlepper M, Schaper W, Schaper J. Increased expression of cytoskeletal, linkage, and extracellular proteins in failing human myocardium. Circ Res. 2000;86(8):846–853. doi: 10.1161/01.RES.86.8.846. [DOI] [PubMed] [Google Scholar]
- 38.Poling J, Gajawada P, Lorchner H, Polyakova V, Szibor M, Bottger T, Warnecke H, Kubin T, Braun T. The Janus face of OSM-mediated cardiomyocyte dedifferentiation during cardiac repair and disease. Cell Cycle. 2012;11(3):439–445. doi: 10.4161/cc.11.3.19024. [DOI] [PubMed] [Google Scholar]
- 39.Zhang Y, Li TS, Lee ST, Wawrowsky KA, Cheng K, Galang G, Malliaras K, Abraham MR, Wang C, Marban E. Dedifferentiation and proliferation of mammalian cardiomyocytes. PLOS One. 2010;5(9):e12559. doi: 10.1371/journal.pone.0012559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gwechenberger M, Moertl D, Pacher R, Huelsmann M. Oncostatin-M in myocardial ischemia/reperfusion injury may regulate tissue repair. Croat Med J. 2004;45(2):149–157. [PubMed] [Google Scholar]
- 41.Ross J., Jr Myocardial perfusion-contraction matching. Implications for coronary heart disease and hibernation. Circulation. 1991;83(3):1076–1083. doi: 10.1161/01.CIR.83.3.1076. [DOI] [PubMed] [Google Scholar]
- 42.Donath MY, Zapf J, Eppenberger-Eberhardt M, Froesch ER, Eppenberger HM. Insulin-like growth factor I stimulates myofibril development and decreases smooth muscle alpha-actin of adult cardiomyocytes. Proc Natl Acad Sci USA. 1994;91(5):1686–1690. doi: 10.1073/pnas.91.5.1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ausma J, Schaart G, Thoné F, Shivalkar B, Flameng W, Depre C, Vanoverschelde J-L, Ramaekers F, Borgers M (1995) Chronic ischemic viable myocardium in man: aspects of dedifferentiation. Cardiovasc Pathol: Off J Soc Cardiovasc Pathol 4(1):29–37. 10.1016/1054-8807(94)00028-P [DOI] [PubMed]
- 44.Dispersyn GD, Mesotten L, Meuris B, Maes A, Mortelmans L, Flameng W, Ramaekers F, Borgers M. Dissociation of cardiomyocyte apoptosis and dedifferentiation in infarct border zones. Eur Heart J. 2002;23(11):849–857. doi: 10.1053/euhj.2001.2963. [DOI] [PubMed] [Google Scholar]
- 45.Ruzicka DL, Schwartz RJ. Sequential activation of alpha-actin genes during avian cardiogenesis: vascular smooth muscle alpha-actin gene transcripts mark the onset of cardiomyocyte differentiation. J cell biol. 1988;107(6 Pt 2):2575–2586. doi: 10.1083/jcb.107.6.2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boettger T, Beetz N, Kostin S, Schneider J, Kruger M, Hein L, Braun T. Acquisition of the contractile phenotype by murine arterial smooth muscle cells depends on the Mir143/145 gene cluster. J Clin Investig. 2009;119(9):2634–2647. doi: 10.1172/JCI38864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harder BA, Hefti MA, Eppenberger HM, Schaub MC. Differential protein localization in sarcomeric and nonsarcomeric contractile structures of cultured cardiomyocytes. J Struct Biol. 1998;122(1–2):162–175. doi: 10.1006/jsbi.1998.3981. [DOI] [PubMed] [Google Scholar]
- 48.Suurmeijer AJ, Clement S, Francesconi A, Bocchi L, Angelini A, Van Veldhuisen DJ, Spagnoli LG, Gabbiani G, Orlandi A. Alpha-actin isoform distribution in normal and failing human heart: a morphological, morphometric, and biochemical study. J Pathol. 2003;199(3):387–397. doi: 10.1002/path.1311. [DOI] [PubMed] [Google Scholar]
- 49.Kubin T, Vogel S, Wetzel J, Hein S, Pipp F, Herold J, Heil M, Kampmann A, Hehlgans S, von der Ahe D, Schaper W, Zimmermann R. Porcine aortic endothelial cells show little effects on smooth muscle cells but are potent stimulators of cardiomyocyte growth. Mol Cell Biochem. 2003;242(1–2):39–45. doi: 10.1023/A:1021177326151. [DOI] [PubMed] [Google Scholar]
- 50.Gosteli-Peter MA, Harder BA, Eppenberger HM, Zapf J, Schaub MC. Triiodothyronine induces over-expression of alpha-smooth muscle actin, restricts myofibrillar expansion and is permissive for the action of basic fibroblast growth factor and insulin-like growth factor I in adult rat cardiomyocytes. J Clin Investig. 1996;98(8):1737–1744. doi: 10.1172/JCI118972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Person V, Kostin S, Suzuki K, Labeit S, Schaper J. Antisense oligonucleotide experiments elucidate the essential role of titin in sarcomerogenesis in adult rat cardiomyocytes in long-term culture. J Cell Sci. 2000;113(Pt 21):3851–3859. doi: 10.1242/jcs.113.21.3851. [DOI] [PubMed] [Google Scholar]
- 52.Kubin T, Ando H, Scholz D, Bramlage P, Kostin S, van Veen A, Heling A, Hein S, Fischer S, Breier A, Schaper J, Schaper W. Microvascular endothelial cells remodel cultured adult cardiomyocytes and increase their survival. Am J Physiol. 1999;276(6 Pt 2):H2179–H2187. doi: 10.1152/ajpheart.1999.276.6.H2179. [DOI] [PubMed] [Google Scholar]
- 53.Kubin T, Yanagida M, Mori S, Hayashi Y, Gohda E, Yamamoto I. Inhibition of DNA synthesis of adult rat hepatocytes in primary culture by dibutyrylcytidine 3’, 5’-cyclic monophosphate. Cell Biol Int Rep. 1989;13(11):907–917. doi: 10.1016/0309-1651(89)90073-8. [DOI] [PubMed] [Google Scholar]
- 54.Wang Y. Mitogen-activated protein kinases in heart development and diseases. Circulation. 2007;116(12):1413–1423. doi: 10.1161/CIRCULATIONAHA.106.679589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Verdejo HE, del Campo A, Troncoso R, Gutierrez T, Toro B, Quiroga C, Pedrozo Z, Munoz JP, Garcia L, Castro PF, Lavandero S. Mitochondria, myocardial remodeling, and cardiovascular disease. Curr Hypertens Rep. 2012;14(6):532–539. doi: 10.1007/s11906-012-0305-4. [DOI] [PubMed] [Google Scholar]
- 56.van Bilsen M, van Nieuwenhoven FA, van der Vusse GJ. Metabolic remodelling of the failing heart: beneficial or detrimental? Cardiovasc Res. 2009;81(3):420–428. doi: 10.1093/cvr/cvn282. [DOI] [PubMed] [Google Scholar]
- 57.Katz AM. Future perspectives in basic science understanding of congestive heart failure. Am J Cardiol. 1990;66(4):468–471. doi: 10.1016/0002-9149(90)90707-8. [DOI] [PubMed] [Google Scholar]
- 58.O’Brien PJ, Gwathmey JK. Myocardial Ca(2 +)- and ATP-cycling imbalances in end-stage dilated and ischemic cardiomyopathies. Cardiovasc Res. 1995;30(3):394–404. doi: 10.1016/S0008-6363(95)00059-3. [DOI] [PubMed] [Google Scholar]
- 59.O’Brien PJ, O’Grady M, McCutcheon LJ, Shen H, Nowack L, Horne RD, Mirsalimi SM, Julian RJ, Grima EA, Moe GW, et al. Myocardial myoglobin deficiency in various animal models of congestive heart failure. J Mol Cell Cardiol. 1992;24(7):721–730. doi: 10.1016/0022-2828(92)93386-X. [DOI] [PubMed] [Google Scholar]
- 60.Weil J, Eschenhagen T, Magnussen O, Mittmann C, Orthey E, Scholz H, Schafer H, Scholtysik G. Reduction of myocardial myoglobin in bovine dilated cardiomyopathy. J Mol Cell Cardiol. 1997;29(2):743–751. doi: 10.1006/jmcc.1996.0318. [DOI] [PubMed] [Google Scholar]
- 61.Meeson AP, Radford N, Shelton JM, Mammen PP, DiMaio JM, Hutcheson K, Kong Y, Elterman J, Williams RS, Garry DJ. Adaptive mechanisms that preserve cardiac function in mice without myoglobin. Circ Res. 2001;88(7):713–720. doi: 10.1161/hh0701.089753. [DOI] [PubMed] [Google Scholar]
- 62.Molojavyi A, Lindecke A, Raupach A, Moellendorf S, Kohrer K, Godecke A. Myoglobin-deficient mice activate a distinct cardiac gene expression program in response to isoproterenol-induced hypertrophy. Physiol Genomics. 2010;41(2):137–145. doi: 10.1152/physiolgenomics.90297.2008. [DOI] [PubMed] [Google Scholar]
- 63.Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell. 2012;148(3):399–408. doi: 10.1016/j.cell.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Scarpulla RC. Metabolic control of mitochondrial biogenesis through the PGC-1 family regulatory network. Biochim Biophys Acta. 2011;1813(7):1269–1278. doi: 10.1016/j.bbamcr.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Taegtmeyer H, Razeghi P, Young ME. Mitochondrial proteins in hypertrophy and atrophy: a transcript analysis in rat heart. Clin Exp Pharmacol Physiol. 2002;29(4):346–350. doi: 10.1046/j.1440-1681.2002.03656.x. [DOI] [PubMed] [Google Scholar]
- 66.Lemieux H, Semsroth S, Antretter H, Hofer D, Gnaiger E. Mitochondrial respiratory control and early defects of oxidative phosphorylation in the failing human heart. Int J Biochem Cell Biol. 2011;43(12):1729–1738. doi: 10.1016/j.biocel.2011.08.008. [DOI] [PubMed] [Google Scholar]