Abstract
Microtubules have important functions ranging from maintenance of cell morphology to subcellular transport, cellular signaling, cell migration, and formation of cell polarity. At the organismal level, microtubules are crucial for various biological processes, such as viral entry, inflammation, immunity, learning and memory in mammals. Microtubules are subject to various covalent modifications. One such modification is tubulin acetylation, which is associated with stable microtubules and conserved from protists to humans. In the past three decades, this reversible modification has been studied extensively. In mammals, its level is mainly governed by opposing actions of α-tubulin acetyltransferase 1 (ATAT1) and histone deacetylase 6 (HDAC6). Knockout studies of the mouse enzymes have yielded new insights into biological functions of tubulin acetylation. Abnormal levels of this modification are linked to neurological disorders, cancer, heart diseases and other pathological conditions, thereby yielding important therapeutic implications. This review summarizes related studies and concludes that tubulin acetylation is important for regulating microtubule architecture and maintaining microtubule integrity. Together with detyrosination, glutamylation and other modifications, tubulin acetylation may form a unique ‘language’ to regulate microtubule structure and function.
Keywords: Tubulin code, Lysine acetylation, Mec17, HDAC5, SIRT2, Mechanosensing, Touch receptor neuron, Pillar cell, Axon regeneration, Inflammation, HDAC inhibitor
Introduction
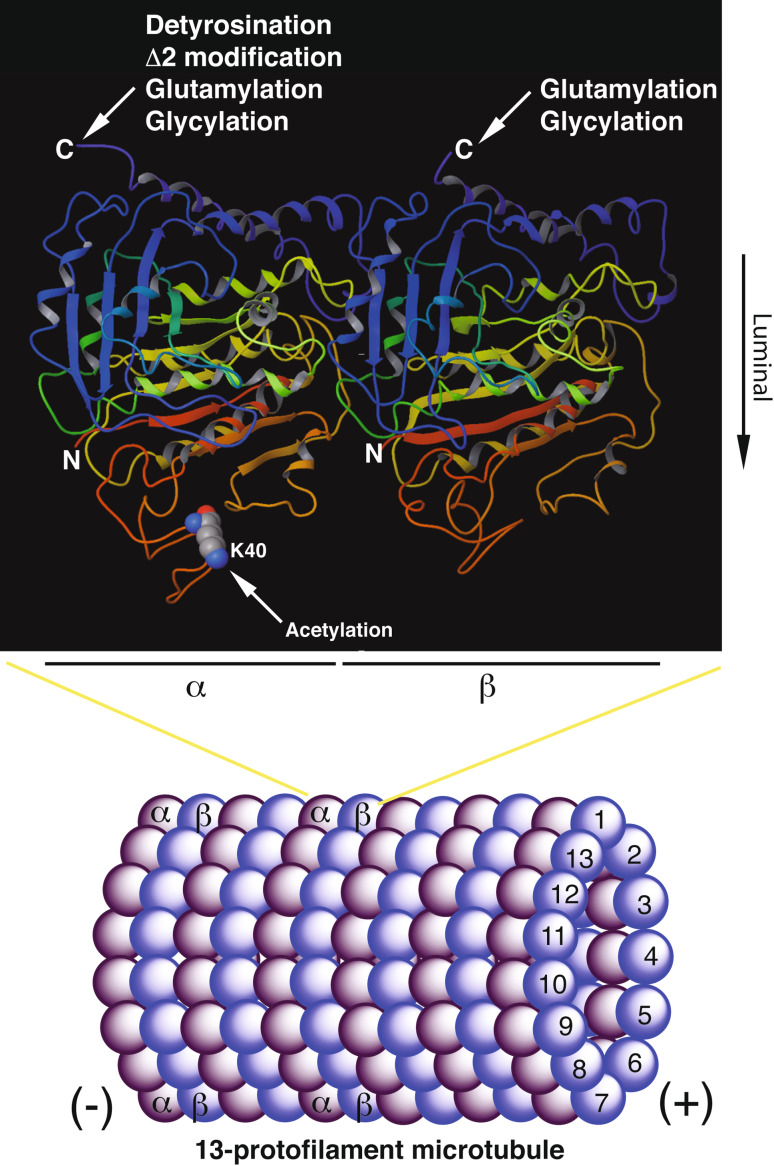
Microtubules are polar cylindrical polymers of α- and β-tubulin heterodimers (Fig. 1), and play important roles in maintaining cell morphology and regulating intracellular transport, mitosis, and cell migration [1–3]. Microtubules are an integral part of subcellular structures such as the cytoskeleton, mitotic spindles, centrioles, cilia and flagella [1–3]. To fulfill such diverse functions, at least three mechanisms are involved in the regulation: incorporation of different isoforms of α- and β-tubulin into microtubules, interaction with microtubule-associated proteins and plus-end-tracking proteins, and post-translational modifications of α- and β-tubulin [4–9]. The post-translational modifications include detyrosination (elimination of the C-terminal tyrosine residue from α-tubulin) [10], Δ2 modification (removal of the penultimate glutamate of α-tubulin after detyrosination) [11], acetylation [12, 13], phosphorylation [14], glutamylation [15] and glycylation [16]. In addition, new modifications, such as β-tubulin acetylation [17], polyamination [18] and succination [19], continue to be uncovered. Among all known tubulin modifications, detyrosination, Δ2 modification, acetylation, glutamylation and glycylation have been extensively characterized. While detyrosination, Δ2 modification and acetylation are specific to α-tubulin, glutamylation and glycylation target both α- and β-tubulin (Fig. 1). Among these five well-characterized modifications, detyrosination, Δ2 modification, glutamylation and glycylation are confined to the C-terminal tails of α- and β-tubulin (Fig. 1) [4–7]. These tails protrude out from the surface of microtubules [9, 20–22]. By contrast, α-tubulin acetylation is an exception because the modification site, Lys-40, is located on the luminal side of microtubules (Fig. 1) [9, 20–22]. The hollow structure of microtubules may impede the access of Lys-40 to other proteins, so acetylation may exert effects on microtubules through mechanisms different from those used by modifications of the C-terminal tails (Fig. 1).
Fig. 1.
Schematic cartoon illustrating microtubule structure and the location of α-tubulin acetylation and other post-translational modifications (PTMs). While lysine-40 (K40) of α-tubulin resides at the luminal side of microtubules, the C-terminal tails of α- and β-tubulin are located at the outer surface of microtubules [9, 20–22]. Thus, acetylation is in the lumen and PTMs at the C-terminal tails are outside from microtubules. Such PTMs include detyrosination, ∆2 modification, glutamylation and glycylation [4–7]. Detyrosination and ∆2 modification are present in α-tubulin, whereas glutamylation and glycylation modify both α- and β-tubulin. These two modifications occur at both mono- and poly-forms. Not illustrated here are other tubulin PTMs, including phosphorylation of β-tubulin at serine-172, polyamination of α- and β-tubulin, palmitoylation, ubiquitination and sumoylation [7, 8]. The α- and β-tubulin heterodimer structure shown in the upper panel is adapted from Ref. [199] and their flexible acidic C-terminals in different isoforms are not present in the structures [9, 21, 22]. For simplicity, the helical nature of microtubules is not reflected in the lower panel
How different tubulin modifications regulate the structure and function of microtubules is a fundamental question that has been extensively investigated [4–8]. Among the five well-characterized modifications, detyrosination, Δ2 modification and acetylation of α-tubulin are associated with stable microtubules [4–7]. Polyglutamylated tubulins are enriched in neurons, cilia and centrioles, whereas polyglycylation is restricted to cilia. Detyrosination and polyglutamylation are known to regulate interaction of microtubules with their associated proteins [4–8]. Importantly, inactivation of genes encoding the responsible enzymes has yielded direct evidence for the importance of tubulin modifications in vivo [7, 8]. Loss of mouse tubulin tyrosine ligase (TTL) leads to perinatal death (due to breathing difficulty and ataxia), elevated detyrosination and Δ2 modification, and disorganization of neuronal networks [23]. Excitingly, Purkinjie cell degeneration (pcd) mice carry a mutation in the gene for cytosolic carboxypeptidase 1 (CCP1), an enzyme that catalyzes deglutamylation [24, 25]. Although not essential for mouse survival, TTL1 (TTL-like 1), a major enzyme mediating neuronal polyglutamylation, is required for airway ciliary function [26]. Moreover, tubulin glycylases are important for stabilization and maintenance of ependymal cilia in the ventricular system of the mouse brain [27]. Thus, these tubulin modifications are important for mammalian development and proper tissue function. A relevant question is what roles tubulin acetylation plays in vivo.
As a unique luminal microtubule modification (Fig. 1), tubulin acetylation refers to the transfer of an acetyl moiety from acetyl-coenzyme A to the side chain of Lys-40 (Fig. 2). Lysine acetylation was first found on histones in the late 1960s [28]. In the 1970 and 1980s, several non-histone proteins including α-tubulin were found to possess acetyl-lysine [13, 29]. In the past decade, lysine acetylation has been recognized as a post-translational modification that rivals phosphorylation, with ~2000 acetylated proteins in the human proteome [30–32]. Since its identification 30 years ago [13, 29], tubulin acetylation has spurred wide research interests. In addition, the responsible acetyltransferases and deacetylases (Fig. 2) have been identified and analyzed at the molecular, structural and genetic levels. This review presents a brief summary of related studies, including discovery of tubulin acetylation, identification and genetic analysis of tubulin acetyltransferases and deacetylases, functions of tubulin acetylation, and its links to human diseases.
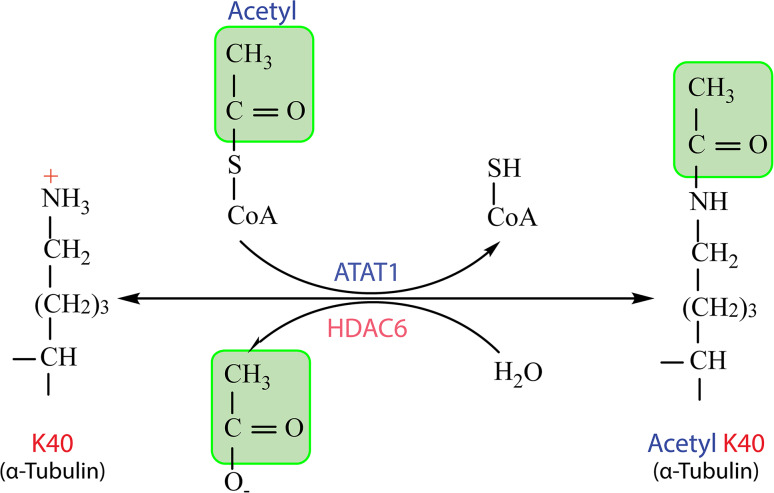
Fig. 2.
α-Tubulin acetylation refers to transfer of the acetyl group from acetyl-coenzyme A (CoA) to Lys-40. This modification is catalyzed by ATAT1 (alpha-tubulin N-acetyltransferase 1) in mammals or Mec-17 (mechanosensory abnormal 17) and its paralog, Atat-2, in C. elegans. The modification is reversible and its reverse reaction is catalyzed by histone deacetylase 6 (HDAC6)
Identification of Lys-40 as the α-tubulin acetylation site
The journey started in 1981 when it was found that the flagella of the single-cell green alga Chlamydomonas reinhardtii contained a tubulin isoform distinct from those from the cytoplasm, as judged from different isoelectric points [33]. Importantly, it was subsequently shown that the difference is due to a post-translational modification rather than expression from a different gene [34], because the cytoplasmic precursor could be converted to the flagellar form [12]. With tritiated acetate labelling, α-tubulin from the flagella was found to contain tritium, indicating that the protein contains an acetyl moiety [12]. Addition of hydrazine to acetylated microtubules produced acetyl-hydrazine, confirming the involvement of acetylation, and subsequent analysis of proteolytic digests by thin-layer chromatography revealed that the acetyl moiety is linked to the epsilon-amino group of a lysine residue [13]. In 1987, amino acid sequencing identified Lys-40 as the acetylation site [35]. Two years earlier, a monoclonal antibody specific for the acetylated form of α-tubulin was developed and found to recognize the acetylation in various organisms [29]. Amino acid sequence comparison revealed that the acetylation site is conserved from protists to mammals [29, 36] and is also present in flowering plants [37]. However, the acetylation site is not conserved in yeast [38]. Being commercially available for more than 20 years, the monoclonal antibody clone 6-11B-1, specific to acetylated α-tubulin [29], has been used as a routine marker for stable microtubules.
A recent large-scale acetylome study has identified other multiple acetylated sites on α- and β-tubulin [31], but these new sites need to be independently substantiated by other means. Although Lys-252 of β-tubulin is also acetylated, the modification only exists in soluble α- and β-tubulin heterodimers [17]. This acetylation is catalyzed by the acetyltransferase San and inhibits tubulin incorporation into microtubules [17]. Currently, Lys-40 is the only acetylation site known to occur on polymerized microtubules, so this modification has been widely referred to as tubulin or microtubule acetylation.
Tubulin acetyltransferases and deacetylases
Identification and analysis of tubulin acetyltransferases
In 1985, an acetyltransferase activity was detected in extracts isolated from Chlamydomonas flagella [39], but the identity of this enzyme remained obscure until recently. In vitro studies identified several candidates, including the transcriptional elongation regulator ELP3 (elongator protein 3) [40, 41], an N α-acetyltransferase complex [42] and the histone acetyltransferase GCN5 (homolog of yeast general control nonderepressible 5) [43]. Genetic screening for touch sensitivity defects in Caenorhabditis elegans identified various mutant alleles, one of which encodes Mec-17 (mechanosensory abnormality protein 17) [44, 45]. Interestingly, Mec-17 displays sequence similarity to members of the GCN5 superfamily of lysine acetyltransferases [46]. In 2010, Mec-17 and the mammalian ortholog ATAT1 (alpha-tubulin acetyltransferase 1) were identified as α-tubulin acetyltransferases [47, 48]. They possess intrinsic α-tubulin acetyltransferase activity [47, 48]. Moreover, ATAT1 functions as a bona-fide tubulin acetyltransferase in vivo, because deletion of the mouse gene leads to nearly complete loss of tubulin acetylation in embryos and various tissues [49–51]. Thus, ATAT1 is now considered as a major tubulin acetyltransferase in mammals. The other three candidates (i.e., ELP3, GNC5 and an N α-acetyltransferase) may be responsible for minor tubulin acetylation in vivo. Related to this, zebrafish embryos depleted of Mec-17 displayed loss of tubulin acetylation in neurons but not cilia [47], and in some tissues from Atat1 knockout mice, there are residual tubulin acetylation signals [49].
At the amino acid sequence level, the Mec-17/ATAT1 family is conserved from protists to humans. There are Mec-17 and a paralog (i.e., Atat-2) in C. elegans, but in higher organisms there is only one ortholog [47, 48]. Tubulin acetylation is present in flowering plants [37], but no Mec-17 related proteins are encoded in genomes of model plants such as Arabidopsis thaliana, indicating that other proteins may acetylate α-tubulin in angiosperms. Mec-17 or ATAT1 has sequence motifs characteristic of GCN5-related N-acetyltransferases and is thus a member to this superfamily [46]. In addition to catalyzing tubulin acetylation, ATAT1 interacts with microtubule-associated proteins such as doublecortin [49] and destabilizes microtubules independent of its acetyltransferase activity [50].
Multiple structural studies have provided molecular insights into how human ATAT1 interacts with and acetylates α-tubulin (in a heterodimeric form with β-tubulin) and microtubules [52–56]. Consistent with the sequence homology [46], the overall 3D structure of ATAT1 is very similar to that of the well-studied acetyltransferase GCN5 [52–56]. Different from GCN5, ATAT1 contains a basic substrate-binding pocket for recognition of four acidic residues of α-tubulin, Asp-33, -39, -46 and -47 [53]. In support of this, mutagenesis analysis revealed that Asp-39 and -46 contribute to acetylation of Lys-40 [56]. Interestingly, Ser-38 is essential for the acetylation [56]. Thus, Lys-40 conforms to the consensus sequence xxxSDKxxxxxDxx, where S is essential, D is important, K is the acetylation site and x is any residue [56].
Tubulin acetylation is a hallmark for long-lived microtubules (Fig. 1) [4–7], so an interesting question is how tubulin acetyltransferase gains access to the luminal residue, Lys-40. It was shown that the acetylation of microtubules proceeds from both ends (Fig. 1), suggesting that enzymes diffuse through the microtubule lumen [47]. This model may be problematic as an antibody specific for an epitope inside microtubules was predicted to take years to diffuse through the lumen [57]. The acetylation reaction is not dependent on the length of microtubules [58] and the Km value of ATAT1 for free α-tubulin is almost identical to that for microtubules [48], indicating that ATAT1 binds freely to α-tubulin in microtubules. It was proposed that ATAT1 reaches the luminal acetylation site through transient holes in the microtubule lattice [48]. A new study indicates that ATAT1 acetylates microtubules without any preference for their ends and that the catalytic activity rather than substrate access is rate-limiting for microtubule acetylation [59].
To investigate whether there are other acetylation sites on α-tubulin in addition to Lys-40, Shida et al. engineered the point mutant K40R [48]. Wild-type α-tubulin but not the mutant is acetylated by ATAT1, confirming that Lys-40 is the only acetylation site [48]. In a related study, a pan-acetyl-lysine antibody was used to detect tubulin purified from the Atat1-deficient mouse brain and almost no positive signals were detected [50], supporting that ATAT1 is a major α-tubulin acetyltransferase and Lys-40 is the predominant acetylation site in vivo.
In genetic experiments, loss of Mec-17 and its paralog Atat-2 in C. elegans leads to disappearance of tubulin acetylation and decreases touch sensitivity of the worm [47]. While morpholino oligomers were used to treat zebrafish embryos, loss of tubulin acetylation was observed in neurons and the treated embryos displayed developmental defects, including curved body shape, shortened body axis, hydrocephalus, small head and eyes [47]. By staining for β-galactosidase activity in Atat1 LacZ mice, it was shown that Atat1 is expressed in a variety of tissues, with the strongest expression in the central nervous system and various other ciliated tissues, including the testis, lung, eye and inner ear (Li and Yang, unpublished data) [49]. In knockout mice, Atat1 deficiency leads to nearly total loss of tubulin acetylation but does not affect animal survival [49–51]. Except for some brain and sperm abnormalities, mutant mice develop rather normally [49–51]. The brain abnormalities include alteration of the dentate gyrus and dilation in the lateral ventricles (Li and Yang, unpublished data) [49]. In the mutant testis, apoptosis is activated [50]. As it is required for normal hippocampus development, ATAT1 may be important for advanced functions such as learning and memory [49].
Identification and analysis of tubulin deacetylases
In May 2002, Hubbert et al. first reported that histone deacetylase 6 (HDAC6) is an α-tubulin deacetylase co-localized with the microtubule network [60]. Inspired by an earlier report that HDAC6 is exclusively cytoplasmic [61], they considered whether HDAC6 has a function not related to histone metabolism and transcriptional regulation, subsequently leading to the unexpected discovery [60]. In agreement with this, Matsuyama et al. also showed that HDAC6 is an α-tubulin deacetylase [62]. They initially attempted to identify acetylated proteins in cells treated with the specific HDAC inhibitor trichostain A and subsequently found α-tubulin as a major acetylated protein [62]. That eventually led to the finding that HDAC6 possesses intrinsic deacetylase activity towards to acetylated α-tubulin [62]. This conclusion received support from another study [63]. In a yeast two-hybrid screen, Zhang et al. utilized an inactive HDAC6 mutant as bait to identify binding partners as potential substrates and found β-tubulin [63]. That link then led to the demonstration that HDAC6 reverses α-tubulin acetylation [63]. Moreover, inactivation of the Hdac6 gene in mouse embryonic stem (ES) cells caused α-tubulin hyperacetylation [63]. HDAC6 is conserved from C. elegans to humans and similar proteins are also present in plants [38]. Except for unicellular organisms such as Tetrahymena and green algae, this correlates well with the presence of α-tubulin acetylation in different organisms.
Sirtuin 2 (SIRT2), a human ortholog of yeast Sir2 (silent information regulator 2), was identified as an NAD+-dependent α-tubulin deacetylase in vitro [64], but deletion of the murine gene does not change the acetylation level in vivo [65, 66]. By contrast, deletion of the mouse Hdac6 gene leads to global α-tubulin hyperacetylation [67], indicating that HDAC6 is a major tubulin deacetylase in vivo. SIRT2 may function in special conditions. For example, the microtubule-associated protein Furry inhibits SIRT2 deacetylase activity and promotes α-tubulin acetylation in the mitotic spindle [68]. In murine macrophages, SIRT2 but not HDAC6 is the responsible α-tubulin deacetylase during inflammasome activation [69]. Interestingly, a recent study has identified HDAC5 as an injury-regulated tubulin deacetylase important for axon regeneration [70].
Human HDAC6 was initially considered to be exclusively cytoplasmic [71], but two recent studies have revealed that it is also present in the nucleus [72, 73]. Related to this, mouse HDAC6 is subject to active nucleocytoplasmic trafficking [61]. In addition to tubulin, HDAC6 deacetylates other proteins, including the molecular chaperone HSP90 (heat-shock protein 90 kDa) [74], the HSP70-interacting protein CHIP (carboxy terminus of HSP70-interacting protein) [75], the E3 ubiquitin ligase TRIM50 (tripartite motif containing protein 50) [76], the cytoskeletal protein cortactin (cortical actin binding protein) [77] and a DNA mismatch repair regulator [73]. HDAC6 also regulates degradation of misfolded and aggregated proteins [78, 79] and modulates transcriptional co-repression [80]. Moreover, HDAC6 is important for mitochondrial transport in hippocampal neurons [81] and has a role in neurodegenerative diseases [82]. The diverse substrates and multiple roles of HDAC6 in different cellular processes have complicated the efforts to dissect out its role as a tubulin deacetylase per se.
In Hdac6-null mice, α-tubulin is hyperacetylated in almost all tissues tested [67]. While the mutant mice are viable and fertile, they display some minor phenotypes such as moderately impaired immune responses [67], hyperactivity, decreased anxiety and lower depression tendency [83]. Tubulin deacetylation by HDAC6 is not required for platelet activation but influences platelet spreading [84]. Despite the lack of major phenotypes in the knockout mice, HDAC6 may fine-tune physiological and pathological processes. For example, HDAC6 is important for inflammatory response [85].
Functions of tubulin acetylation
Tubulin acetylation as a marker of stable microtubules
Tubulin acetylation was first identified in flagellar axonemes of Chlamydomonas reinhardtii [12]. Subsequent studies demonstrated that in addition to axonemes, this modification is also present in basal bodies and a subset of cytoplasmic microtubules [86]. Moreover, tubulin acetylation was found mainly on stable microtubules resistant to depolymerization induced by cold shock and other treatments, but not on dynamic microtubules such as those in neuronal growth cones [87–89]. In retina tissues, tubulin acetylation is more abundant in neurons than other cell types [90] and is particularly enriched in axons [91].
Notably, tubulin acetylation may be a consequence of, rather than a contributor to, microtubule stability. Tubulin acetylation and detyrosination exist in stable microtubules such as those in axons and ciliary axonemes [87, 91]. As other studies suggested that detyrosination alone does not promote microtubule stability per se [92–94], it was proposed tubulin acetylation might play a role in microtubule stabilization [88]. Some early reports supported this, but subsequent studies suggested that tubulin acetylation might be a consequence rather than a cause of enhanced microtubule stability [50, 59, 95, 96]. Related to this, detyrosination but not acetylation is important for chromosome congression (i.e., aligning chromosomes on the spindle) during mitosis [97].
HDAC6 overexpression significantly increases cell motility, perhaps due to decreased microtubule stability caused by reduced tubulin acetylation [60]. Pallazzo et al. challenged this conclusion and demonstrated that tubulin acetylation induced by HDAC6 inhibition does not promote resistance to nocodazole, a drug that induces microtubule depolymerization [95]. Another in vitro study demonstrated that highly acetylated microtubules exhibit a delay in drug-induced depolymerization and that HDAC6 overexpression promotes depolymerization [62]. However, it is unclear whether tubulin deacetylation or other functions of HDAC6 contribute to microtubule instability. Related to this, the stability of microtubules is determined by the level of HDAC6 binding but not tubulin acetylation [98]. Inhibition of HDAC6 by small-molecule inhibitors increases microtubule acetylation and significantly reduces microtubule growth and shrinkage, whereas siRNA knockdown increases microtubule acetylation but does not affect microtubule growth velocity [98].
ATAT1 overexpression destabilizes microtubules, but it is its interaction with microtubules rather than its enzymatic activity that regulates microtubule stability [50]. Interestingly, in C. elegans, Mec-17 loss leads to microtubule instability and axon degeneration, independent of its acetyltransferase activity [99]. This is not consistent with the observation that microtubules in Atat1 −/− mouse embryonic fibroblasts (MEFs) are more resistant to nocodazole treatment than those in wild-type MEFs [50]. Whether expression or loss of ATAT1 is beneficial for microtubule stability remains unclear, but both studies showed that microtubule instability is not caused by tubulin acetylation per se. Moreover, tubulin acetylation exerts no effects on microtubule structure in biochemical experiments [96], supporting that tubulin acetylation does not intrinsically affect microtubule stability. It was reported that ATAT1 has suboptimal enzymatic activity on microtubules and concluded that acetylation accumulates on stable microtubules because they exist for hours whereas dynamic microtubules have a much shorter half-life [59]. Thus, tubulin acetylation accumulates in stable microtubules, rather than directly contribute to enhanced microtubule stability [95], which also explains why tubulin acetylation is enriched in long-lived microtubules in axons [91].
Tubulin acetylation, microtubule architecture, and mechanosensation
Microtubules are cyclinders comprising linear polymers (termed protofilaments) of α- and β-tubulin heterodimers (Fig. 1). In many cells and organisms, microtubules are composed of 13 protofilaments (Fig. 1) [100, 101]. However, in some special situations, the protofilament number is different. Pillar and phalangeal cells in the mammalian inner ear contain mainly 15-protofilament microtubules [102]. Moreover, 12- and 16-protofilament microtubules have been found in lobster neurons and insect sperms, respectively [101, 103]. In C. elegans, most cells contain 11-protofilament microtubules, but sensory cilia possess 13-protofilament microtubules and neurites of touch receptor neurons are filled with 15-protofilament microtubules [104]. Thus, the protofilament number in microtubules varies from 11 to 16 and is cell type-specific. An important question is how this specificity is achieved.
Microtubule assembly experiments in vitro have shed some light on this question. While protofilaments are restricted to specific numbers in different cells in vivo, microtubule assembly in vitro yields heterogeneous populations [96, 105, 106]. For example, tubulin preparations from the crayfish nerve cord and porcine brain generate a mixture of 13- and 14-protofilament microtubules, with the ratio dependent on buffer conditions [96, 105]. Interestingly, even under the same buffer condition, tubulin from bovine brain forms mainly 13-protofilament microtubules with a small portion containing 12- and 14-protofilaments, but tubulin from C. elegans leads to formation of mixed microtubules with the protofilament number of 9, 10, 11 and 12, at 30, 22, 43 and 17 %, respectively [106]. The protofilament number difference between microtubules assembled from bovine and worm tubulins is in rough agreement with the observation that microtubules in bovine and worm tissues are mainly composed of 13- and 11-protofilaments, respectively [100, 101, 104]. Thus, in addition to buffer conditions, tubulin sequences and perhaps also post-translational modifications and microtubule-associated proteins affect the protofilament number of microtubules assembled in vitro. Interestingly, the microtubule-stabilizing anti-cancer drug Taxol (a.k.a. Paclitaxel) promotes formation of 12-protofilament microtubules [107]. Moreover, the microtubule stabilizing protein doublecortin stimulates assembly of 13-protofilament microtubules in vitro and the intracellular transport motor protein kinesin is also in favor of 13-protofilament microtubules [96, 108, 109], indicating that microtubule-association proteins directly affect the protofilament number of microtubules.
In addition to in vitro microtubule assembly experiments, genetic studies in C. elegans have yielded insights into the question why touch receptor neurons contain mainly 15-protofilament microtubules. Within the simple nematode nervous system, there are six touch receptor neurons to mediate touch sensitivity [45, 110]. Strategically positioned at different parts of the body, these neurons use long neurites (up to 0.5 mm) to detect touch signals of the entire body (~1 mm long in adults) [45, 110]. Strikingly, the long neurites are filled with staggered arrays of ~20-µm long 15-protofilament microtubules (as many as 50 per neurite section) [104, 111, 112]. As revealed by immunostaining with the monoclonal antibody (clone 6-11B-1 [29]) specific to acetylated α-tubulin, these microtubules are heavily acetylated [113, 114]. Moreover, Mec-17 is highly expressed in these neurons [48]. Thus, an interesting question is whether tubulin acetylation regulates the protofilament number of microtubules in C. elegans. Two studies have demonstrated that this is the case [112, 115]. In one study, Mec-17 loss was found to cause morphological defects in touch receptor neurons and disappearance of touch sensitivity [112]. In terms of microtubules, Mec-17 loss exerts dramatic impact on several aspects of touch receptor neurons: (1) decreased microtubule number per neurite section (from 46.5 in the wild-type to 8.8–14.7 in two mutant strains), (2) reduced microtubule diameter and decreased protofilament number (from uniform 15-protofilament microtubules in the wild-type to heterogeneous 11–15-protofilament microtubules in the two mutants, with 60 % of the mutant microtubules comprising 13-protofilaments), (3) loss of mysterious darkly stained material inside microtubules, and (4) appearance of microtubule hooks and bending [112]. As a result of microtubule defects, axonal processes display periodic swellings, where severe microtubule bending occurs [112]. Related to this, loss of mouse Atat1 leads to formation of similar swellings on sperm flagella [50]. Loss of the Mec-17 paralog Atat-2 in C. elegans has similar impact although it is not as dramatic as loss of Mec-17 itself [112]. The acetyltransferase activity of Mec-17 is essential for microtubule integrity but not worm touch sensitivity [112].
In another study, it was found that loss of either Mec-17 or Atat-2 reduces the number of microtubules per neurite section and shortens microtubule length in touch receptor neurons [115]. In addition, loss of Mec-17 but not Atat-2 alters the protofilament number (from >95 % 15-protofilament and <5 % 11-protofilament microtubules in the wild-type or Atat-2 mutant worms to heterogeneous 10–16-protofilament microtubules in Mec-17 mutants) [115]. In addition, microtubules in Mec-17 mutants display lattice openings (~150 nm wide, equivalent to ~20 tubulin heterodimers) that are not present in microtubules from wild-type or Atat-2 mutant worms [115]. According to molecular modeling, Lys-40 of α-tubulin forms a salt bridge with Glu-55 and prevents it from forming an intermolecular salt bridge with His-283 from a neighbouring α-tubulin monomer [115]. Lys-40 acetylation makes Glu-55 available for pairing with His-283, thereby facilitating assembly of 15-protofilament microtubules [115].
The two C. elegans studies show that tubulin acetylation is necessary for maintaining microtubule structure in touch receptor neurons [112, 115], but the question whether tubulin acetylation is sufficient for the maintenance remains unaddressed. In a recent study, in vitro microtubule assembly was carried out with porcine brain tubulin to investigate whether acetylation per se affects microtubule structure [96]. Different from what was observed with Mec-17-deficient worms [112, 115], acetylation does not have a major effect on microtubule structure in vitro [96]. Under the conditions used, non-acetylated tubulin forms a mixture of 13- and 14-protofilament microtubules (Fig. 3a) [96]. Notably, there is a small fraction of 15-protofilament microtubules (~8 %) [96]. Upon acetylation, this fraction increases by ~twofold [96]. In the presence of kinesin, acetylation enhances the ratio of 13-protofilament microtubules [96]. Thus, acetylation exerts some minor effects on microtubule structure. However, it is clear that acetylation does not promote formation of uniform 15-protofilament microtubules in vitro [96], which is in stark contrast to what was observed in touch receptor neurons in vivo [112, 115].
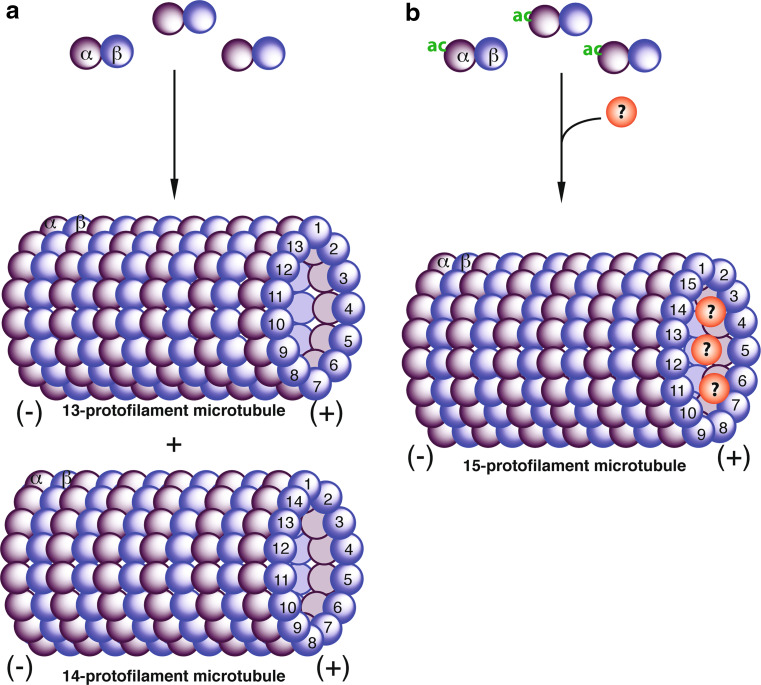
Fig. 3.
Hypothetical models on how α-tubulin acetylation may affect microtubule structure. a In the absence of acetylation, α- and β-tubulin heterodimers form 13- or 14-protofilament microtubules in vitro. However, in most cells, microtubules contain 13-protofilaments, so some cellular proteins may promote formation of such microtubules. b In the presence of acetylation (ac), α- and β-tubulin heterodimers are in favour of formation of 15-protofilament microtubules such as those in touch neuron receptors in C. elegans and pillar cells in the mammalian inner ear. However, acetylation is not sufficient for promoting formation of 15-protofilament microtubules. Acetylation may form a specific docking site for an unidentified protein(s), which may in turn promote formation of 15-protofilament microtubules. As 15-protofilament microtubules are not common, acetylation-specific binding partners may promote assembly of this particular type of microtubules. The question mark refers to an unidentified protein(s)
Different outcomes from structural studies of microtubules from C. elegans [112, 115] and in vitro assembly experiments [96] reiterate that acetylation is necessary but not sufficient for regulating microtubule architecture. As shown with the Mec-17 gene, mutation of the Mec-12 and Mec-7 genes (encoding α- and β-tubulin isoforms, respectively) also affect microtubule structure in touch receptor neurons [45, 114], supporting that acetylation is not sufficient for formation of 15-protofilament microtubules in touch receptor neurons. Based on this, it is tempting to propose that acetylation facilitates interaction of an unknown protein(s), which in turn regulates microtubule structure (Fig. 3b). Related to this, kinesin and doublecortin affect the protofilament number of microtubules assembled in vitro [96, 108, 109]. Moreover, deletion of the Mec-17 gene leads to loss of mysterious darkly stained material inside microtubules [112]. The mysterious material may represent the unidentified protein(s) that binds to acetylated tubulin (Fig. 3b). If so, identification of the mysterious protein(s) shall shed novel light on how acetylation regulates microtubule architecture in vivo.
Like touch receptor neurons in C. elegans, pillar and phalangeal cells in the mammalian inner ear contain densely packed 15-protofilament microtubules [102]. In addition, as touch receptor neurons mediate mechanosensing, pillar and phalangeal cells are integral parts of the organ of Corti in the inner ear. The stiffness of 15-protofilament microtubules in pillar cells may contribute to mechanosensation by the organ of Corti [116]. Moreover, similar to touch receptor neurons in C. elegans, 15-protofilament microtubules in pillar cells are heavily acetylated [117]. By analogy to touch receptor neurons [112, 115], it is reasonable to suggest that acetylation may regulate formation of 15-protofilament microtubules in pillar and phalangeal cells. If so, acetylation may also contribute to mammalian hearing sensation. Atat1 −/− mice [49, 50] should be valuable for investigating these two interesting issues.
Tubulin acetylation and intracellular transport
Tubulin acetylation has been proposed to participate in subcellular and intraflagellar transport. Loss of microtubule acetylation was reported to reduce the binding and motility of the molecular motor kinesin-1 in vitro [118]. Related to this, kinesin-1 and a kinesin-like protein preferably bind to stable microtubules marked by acetylation and detyrosination [119, 120]. Increased tubulin acetylation by HDAC6 inhibition causes recruitment of dynein and kinesin-1 to microtubules, thereby compensating intracellular transport deficits in Huntington’s disease [121]. While these studies support that tubulin acetylation recruits kinesin-1 and regulates intracellular transport in cell-based assays, enrichment or loss of acetylation on microtubules in a purified system does not result in significant changes in the kinesin-1 loading rate while detyrosination causes a moderate increase in the rate [122]. Similarly, increased tubulin acetylation alone without changing the status of other modifications does not alter the selectivity of kinesin-1 accumulation in polarized cells, while global enhancement of tubulin acetylation, detyrosination, and polyglutamylation by treatment with Taxol or inhibition of glycogen synthase kinase 3β (GSK3β) decreases the selectivity of kinesin-1 translocation and leads to formation of multiple axons [123]. Microtubule stabilization by Taxol promotes initial neuronal polarization [124]. To investigate whether this polarization is caused by tubulin acetylation, neurons were treated with the deacetylase inhibitors tubacin and trichostatin A. The treatment elevated microtubule acetylation but the polarity was not altered [124].
Tubulin acetylation in regulating cell motility and polarity
As discussed above, it was reported that HDAC6-mediated tubulin deacetylation enhances microtubule-dependent motility and was proposed that this enhanced motility is due to reduced microtubule stability caused by decreased tubulin acetylation [60]. Related to this, the ETS transcription factor ERG [E-twenty-six (ETS)-related gene product] directly regulates HDAC6 expression and tubulin acetylation during endothelial cell migration [125]. It was suggested that HDAC6-mediated enhancement of cellular motility is due to tubulin acetylation itself rather than microtubule stability as tubulin acetylation does not necessarily enhance microtubule stability [95]. Knockdown of Atat1 or Elp3 expression revealed that tubulin acetylation is important for neuronal migration [40, 126]. However, ELP3 is unlikely a major tubulin acetyltransferase in vivo [49–51], so impaired neuronal migration observed in Elp3-deficient mice may be due to other mechanisms [40]. Of relevance, ELP3 proteins from different organisms are able to modify the anticodon of tRNA [127].
Interestingly, the focal adhesion scaffold protein Paxillin interacts with HDAC6 and inhibits its deacetylase activity to upregulate microtubule acetylation during cell invasion and migration [128]. Moreover, ATAT1 interacts with the clathrin adaptor AP2, and clathrin-coated pits control microtubule acetylation [129]. This is one possible mechanism to ensure microtubule acetylation at the leading edge for promoting directional cell migration [129]. It was also reported that tubulin acetylation is required for cell–cell contact inhibition and cell adhesion [51]. Decreased dynamics of acetylated microtubules induced by HDAC6 inhibition affects turnover of cellular focal adhesion [130]. α-Tubulin acetylation was shown to regulate breast cancer invasion [131]. Because invasion inhibitory protein 45 (IIp45) and G protein-coupled receptor kinase 2 (GRK2) modulate cell motility by interacting with HDAC6 [132, 133], this deacetylase itself may also be involved in regulating cell migration.
Tubulin acetylation is also important for regulating cell polarity. As discussed above, for polarized epithelial cell migration, Paxillin binds to HDAC6 and stimulates microtubule acetylation [128]. Moreover, expression of two phosphorylation-defective mutants of NIMA (never in mitosis A)-related kinase 3 (NEK3) decreases tubulin acetylation, reduces neuronal polarity and alters cell morphology [134]. Pharmacological inhibition of HDAC6 reverses this effect [134], suggesting that through tubulin deacetylation, HDAC6 modulates neuronal polarity.
Tubulin acetylation and cilia
Cilia are subcellular structures differentiating from centrioles in non-proliferating cells and contain microtubule bundles constituting the axoneme. Cilia are evolutionarily related to flagella of lower eukaryotes like the green alga Chlamydomonas. Cilia were the first subcellular structures to be discovered to harbour tubulin acetylation [29]. Since then, this modification has been widely used as a marker for cilia and related structures. However, it still remains unclear whether tubulin acetylation plays a causal role in cilium formation (Fig. 4). Ciliary assembly and disassembly require intraflagellar transportation. Considering the fact that tubulin acetylation may be important for cargo transportation, an interesting question is whether this modification regulates dynamics of ciliary assembly and disassembly. Growth of human telomerase-expressing retinal pigment epithelial cells in the OptiMEM media stimulates cilia formation and addition of serum induces ciliary disassembly [135]. The pro-metastastic scaffolding protein HEF1 (human enhancer of filamentation 1; a.k.a. Cas-L and NEDD9) interacts with and activates Aurora kinase A, which then phosphorylates and activates HDAC6, leading to ciliary disassembly [135]. Knockdown or inhibition of Aurora kinase A, depletion of its activator HEF1, or inhibition of the downstream target HDAC6 prevents ciliary disassembly [135].
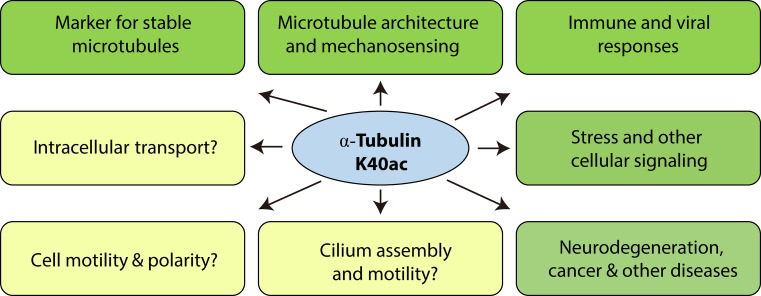
Fig. 4.
Known and potential roles of α-tubulin acetylation. This modification serves as a marker for stable microtubules, regulates microtubule architecture and controls stress and immune responses. In addition, the modification has potential roles in various other cellular processes, including intracellular transport, cilium assembly, cellular signaling, cell migration and neurodegeneration. Questions marks mark cellular processes where functions of α-tubulin acetylation remain to be firmly established. K40ac, Lys-40 acetylation
Inhibition of mouse HDAC6 by a specific inhibitor stimulates cilia formation in mouse embryos cultured in vitro [136]. Treatment of human KD diploid fibroblasts with 25–50 mM LiCl promotes α-tubulin acetylation and elongation of primary cilia [137]. siRNA-mediated depletion of ATAT1 expression showed that this enzyme is required for induced α-tubulin acetylation in these cells [137]. Similar depletion of ATAT1 expression in human telomerase-expressing retinal pigment epithelial cells slows down the kinetics of ciliary disassembly [48]. Primary cilium dysfunction affects the development and homeostasis of many organs in Bardet-Biedl syndrome and seven conserved Bardet-Biedl syndrome proteins form a stable complex that functions in primary cilia [138]. Interestingly, one small subunit of this complex promotes microtubule acetylation [138]. In addition, ceramide inhibits HDAC6 activation by Aurora kinase A and promotes tubulin acetylation in primary cilia in human neural stem cells in vitro [139].
The cylindromatosis (a.k.a. turban tumor syndrome) gene product CYLD is a tumor suppressor with intrinsic deubiquitinating enzyme activity [140]. Interestingly, it associates with microtubules and appears to stabilize microtubules. In agreement with this, it interacts with and inactivates HDAC6 [140]. Thus, in keratinocytes from Cyld −/− mice, tubulin acetylation is elevated [140]. A recent study indicates that CYLD is located at basal bodies [141]. Upon Cyld knockdown, tubulin acetylation is reduced and ciliary length decreases in mouse embryonic fibroblasts [142]. Moreover, treatment with selective HDAC6 inhibitors partially rescues ciliary defects in Cyld −/− mice [142]. Thus, CYLD interacts with HDAC6 and regulates ciliogenesis.
It is often assumed that HDAC6-mediated tubulin deacetylation destabilizes microtubules and leads to ciliary disassembly. As discussed above, tubulin acetylation does not contribute to microtubule stability and Hdac6 knockout mice do not display major phenotypes [67]. Cilia are essential for vertebrate development [143, 144], so tubulin acetylation may only play a fine-tuning rather than major role in cilia during animal development. In support of this, cilia are functional in Atat1 −/− mice although sperm flagella are slightly impaired [50]. Different from the Hdac6 or Atat1 knockout mice [49, 50, 67], a majority of studies that support a role of tubulin acetylation in ciliary assembly are based on cells or embryos cultured in vitro.
Tubulin acetylation in immune and viral responses
Multiple studies have linked tubulin acetylation to immunity. As mentioned above, Hdac6 knockout mice display a moderately impaired immune response [67]. Under unchallenged conditions, serum IgM and IgG levels of Hdac6 −/y mice (the murine Hdac6 gene is X-linked) are close to what was observed in wild-type littermates [67]. When immunized, IgG induction is 4-fold weaker in Hdac6 −/y mice when compared to the wild-type [67]. In addition, deletion of the Hdac6 gene or inhibition of its deacetylase activity enhances regulatory T cell functions in inflammation and autoimmunity, suggesting that HDAC6 inhibition is beneficial for treating colitis and suppressing allograft rejection [145]. Atat1 −/− mice display normal peripheral blood parameters [50], but roles of this acetyltransferase in the immune system remain to be examined.
Macrophages are major effector cells mediating innate immune responses. Activation of murine RAW264.7 macrophages by lipopolysaccharide (LPS) and IFN-γ in vitro enhances microtubule acetylation and secretion of matrix metalloproteinase-9, which then facilitates macrophage migration across the sub-endothelial basement membrane and the interstitial matrix composed mainly of collagen, to reach target tissues and initiate an inflammatory response [146]. Upon infection, pattern-recognition receptors in effector cells such as macrophages form protein complexes termed inflammasomes for maturation pro-inflammatory cytokines like interleukin 1β (IL-1β) to engage innate immune responses [147]. During screening for regulators of inflammasome activation, it was found that microtubule depolymerization suppresses IL-1β production in response to nigericin, an antibiotic derived from Streptomyces hygroscopicus and known to promote inflammasome activation [69]. Tubulin acetylation by ATAT1 is required for inflammasome activation [69], whereas the natural diphenol resveratrol inhibits tubulin acetylation and inflammasome activation [148]. Pharmacological inhibition revealed that mouse SIRT2 instead of HDAC6 is the responsible tubulin deacetylase [69], but it will be important to substantiate this with related knockout mice. In addition to production of pro-inflammatory molecules such as matrix metalloproteinase-9 and IL-1β, tubulin acetylation is also essential for production of an anti-inflammatory cytokine, IL-10, which may serve as a feedback mechanism to control inflammation. When challenged by LPS, RAW264.7 macrophages display extensive tubulin acetylation and induced production of IL-10 [85]. Knockdown of Atat1 expression suppresses LPS-induced tubulin acetylation and inhibits IL-10 induction, whereas in Hdac6-deficient macrophages and mice, LPS induces hyper-production of IL-10 [85]. Tubulin acetylation is required for LPS-induced activation of p38 kinases [85], but it remains unclear why this modification is required for p38 kinase activation. It should be noted that LPS inhibits tubulin acetylation exclusively in human pulmonary macrovascular endothelial cells [149], indicating that LPS effects on tubulin acetylation are cell type-specific.
Upon T cell stimulation, the transcription factor NF-AT (nuclear factor of activated T-cells) is dephosphorylated by calcineurin, promoting interaction with importin β and nuclear translocation [150]. Interestingly, α-tubulin binds to the N-terminal region of NFAT and stimulates complex formation with importin β for nuclear translocation, whereas α-tubulin acetylation inhibits this [150]. Acetate upregulates tubulin acetylation and hinders NF-AT nuclear translocation [150]. Of relevance, through increasing tubulin acetylation, acetate suppresses IL-8 production in epithelial cells induced by the bacterial flagellar protein flagellin. Tubulin hyperacetylation by treatment with an HDAC inhibitor also suppresses IL-8 production induced by flagellin, highlighting the role of tubulin acetylation in regulating IL-8 expression [151]. Thus, tubulin acetylation is important for both innate and acquired immunity.
In addition, this modification is important for viral responses. Human immunodeficiency virus (HIV) infection stabilizes microtubules and stimulates tubulin acetylation in host cells [152]. Overexpression of HDAC6 inhibits HIV infection [153]. Similar to HIV, influenza A virus induces microtubule acetylation [154]. In addition, HDAC6 inhibits release of this virus [155]. Tubulin acetylation is also important for infection by herpes viruses. Just 30 min after infection, Kaposi’s sarcoma-associated herpes virus enhances tubulin acetylation significantly [156]. About 4 h post infection, herpes simplex virus type 1 (HSV-1) increases tubulin acetylation, which in turn enhances HSP90 binding to microtubules and facilitates nuclear localization of viral capsid protein [157]. Notably, this effect is completely opposite from that on NF-AT nuclear localization [150], indicating that tubulin acetylation regulates nuclear import in a context-dependent manner.
Tubulin acetylation in stress and other signaling pathways
Chemical exposures, such as salt (e.g., NaCl), H2O2 and Taxol, as well as physical insults like UV irradiation, were shown to elevate tubulin acetylation [36, 158]. However, H2O2 was also reported to inhibit tubulin acetylation in human pulmonary macrovascular endothelial cells [149], indicating that the effects are cell-specific. Tubulin hyperacetylation is beneficial for cell survival after exposure to NaCl at 0.25 M, a high concentration that is detrimental to cultured cells [159]. KCl depolarization in neurons increases acetylation of α-tubulin in Cornu Ammonis 1 (CA1) of the hippocampus [160]. Moreover, expression of microtubule-associated proteins increases tubulin acetylation [161], and a pathological study on alcoholics revealed significant increase of tubulin acetylation in the prefrontal cortex [162].
The Hippo signaling pathway is crucial for organ size control, regulates cell–cell contact inhibition and plays an important role in cancer [163]. Merlin, encoded by the neurofibromatosis type 2 (NF2) gene and acting as an upstream component of the Hippo signaling pathway, is normally associated with acetylated microtubules, so loss of tubulin acetylation redistributes merlin to the cytosol, thereby impairing Hippo signaling [51]. Increased tubulin acetylation enhances sensitivity of microtubule disruption catalyzed by the microtubule-severing AAA protein katanin in neurons and fibroblasts [164]. Tubulin acetylation has been reported in regulating Na+ and K+-ATPase activity [165–169]. Expression of an acetylation-resistant α-tubulin mutant significantly inhibits adipogenesis [170], suggesting that adipocyte differentiation is dependent on tubulin acetylation. In Atat1-null mice, there are no significant adipocyte-related phenotypes [49, 50], so studies with high-fat diet and other dietary conditions are needed to investigate this issue more thoroughly. Tubulin acetylation is also involved in regulating autophagy. Tubulin acetylation is required for fusion of autophagosomes with lysosomes [171], and starvation-induced tubulin hyperacetylation is required for autophagy activation by nutrient deprivation [172]. Thus, tubulin acetylation plays a role in regulating stress and other signaling pathways.
Tubulin acetylation, human diseases and therapeutic implications
Pathologically, tubulin acetylation is associated with several neurological disorders. Charcot-Marie-Tooth disease affects approximately 1 in 2500 individuals and is the most common inherited disorder of the peripheral nervous system [173]. Mutations in the 27-kDa small heat-shock protein (HSPB1) gene cause axonal Charcot–Marie–Tooth disease or distal hereditary motor neuropathy [173]. Mice expressing HSPB1 exhibit axonal transport defects, axonal loss and decreased tubulin acetylation [173]. Importantly, HDAC6 inhibitors reverse these abnormalities [173]. Inflicting ~1 in 80,000 newborns, Joubert syndrome is a genetic disease mainly affecting the cerebellum. A recent study identified related mutations in the kinesin 7 gene and found that this kinesin is required for optimal tubulin acetylation [174]. Parkinson’s disease is a common neurodegenerative condition affecting the motor system in the central nervous system, and mutations of the LRRK2 (leucine-rich repeat kinase 2) gene are the most common genetic cause of this disease [175]. At the subcellular level, defective microtubule-based axonal transport is one possible cause. Related to this, LRRK2 interacts directly with β-tubulin and inhibits α-tubulin acetylation [176], indicating that in normal cellular contexts, this kinase acts as a negative regulator of microtubule acetylation. Unlike wild-type LRRK2, two mutants carrying Parkinson’s disease-associated mutations form filamentous subcellular structures [177]. Either expression of ATAT1 or inhibition of HDAC6 prevents formation of such structures [177]. Amyotrophic lateral sclerosis (a.k.a. Lou Gehrig’s disease) involves motor neuron degeneration in the motor cortex of the cerebrum, and transgenic mice expressing the G93A point mutant of superoxide dismutase 1 are known models of this devastating disease [66]. Loss of Hdac6 significantly extends survival of these mice and maintains motor axon integrity [66]. The protective effect of Hdac6 deletion is associated with increased tubulin acetylation [66].
Moreover, HDAC6 has been implicated in aging-related dementia such as Alzheimer’s disease (Table 1). Reduced expression of HDAC6 ameliorates cognitive deficits in a mouse model for this disease [178]. Inhibition of HDAC6 activity improves memory and reduces the level of the microtubule-associated protein Tau, a key marker for Alzheimer’s disease [179]. As HDAC6 is the major tubulin deacetylase in vivo, tubulin acetylation may be important in these processes. However, this can also be due to other HDAC6 functions, such as its ubiquitin-binding ability [38, 180, 181]. Importantly, inhibition of HDAC6 with selective inhibitors promotes tubulin acetylation and significantly improves learning-based performance in mice with β-amyloid-induced hippocampal lesions [182, 183].
Table 1.
Links of altered tubulin acetylation to human diseases
| Human diseases | Tubulin acetylation [References] |
Predicted therapeutic strategy |
|---|---|---|
| Neurologic disorders | ||
| Charcot-Marie-Tooth disease | Decrease [173] | HDAC6i |
| Joubert syndrome | Decrease [174] | HDAC6i |
| Parkinson’s disease | Decrease [121, 176, 177] | HDAC6i |
| Amyotrophic lateral sclerosis | Decrease [66] | HDAC6i |
| Alzheimer’s disease | Decrease? [178, 179] | HDAC6i |
| Axon injury | Decrease [70] | ATAT1i |
| Axon degeneration | Decrease? [99] | HDAC6i |
| Cancer | ||
| Head and neck squamous cell carcinoma | Increase [184] | ATAT1i |
| Breast cancer | Increase [185] | ATAT1i |
| Pancreatic cancer | Increase [186] | ATAT1i |
| Cylindromatosis | Decrease [140] | HDAC6i |
| Neurofibromatosis type 2 | Increase? [51] | ATAT1i |
| Multiple myeloma | Decrease? [51, 187] | HDAC6i |
| Heart diseases | ||
| Heart failure due to proteotoxicity | Increase and HDAC6 induction [188] | HDAC6i |
| Atrial fibrillation | Decrease [189] | HDAC6i |
| Lung disease | ||
| COPD | Decrease [190] | HDAC6i |
| Inflammation and immunity | ||
| Inflammasome activation | Increase [69] | HDAC6i |
| Colitis | Decrease [145, 191] | HDAC6i |
| Allograft rejection | Decrease [145] | HDAC6i |
| Virus entry | Increase [154] | ATAT1i |
Axon regeneration is crucial for neurological recovery following injury, and axon injury induces tubulin deacetylation in peripheral neurons in a mouse model [70]. Moreover, tubulin deacetylation is required for axonal growth and regeneration in the mouse model [70]. It is unclear, however, whether tubulin acetylation by ATAT1 is also required for the regeneration program. On the other hand, Mec-17 loss in C. elegans leads to axon degeneration [99]. These two studies suggest that while tubulin acetylation is important for axon maintenance, the deacetylation is required for axon regeneration.
In addition to neurological disorders, tubulin acetylation has been linked to cancer (Table 1). This modification is a prognostic marker for squamous cell carcinoma of the head and neck [184]. Elevated tubulin acetylation in primary breast tumors is linked to the basal-like subtype of breast cancer and an increased risk of disease progression and death [185]. Mechanistically, elevated tubulin acetylation promotes adhesion and invasion of breast cancer cells [185]. Ectopic ATAT1 expression in cultured cells increases tubulin acetylation and enhances formation of microtentacles, which are membrane protrusions in detached breast cancer cells [185]. ATAT1 is also associated with pancreatic cancer-initiating cells [186]. The turban tumor syndrome protein CYLD acts as a tumor suppressor and inhibits cell-cycle progression by inactivating HDAC6 and elevating tubulin acetylation [140]. In these cases, inhibition of tubulin acetylation may be beneficial (Table 1).
The neurofibromatosis type 2 (NF2) gene product merlin is a tumor suppressor upstream of the Hippo signaling pathway and is normally associated with acetylated microtubules [51]. Loss of tubulin acetylation impairs Hippo signaling and affects cell–cell contact inhibition [51]. Therefore, although the impact of tubulin acetylation is context-dependent, multiple lines of evidence suggest that it plays a role in cancer initiation and progression (Table 1). Moreover, in combination with the proteasome inhibitor bortezomib, low doses of a specific HDAC6 inhibitor displays anti-multiple myeloma activity [187], setting the stage for further clinical trials.
Proteotoxicity is an important mechanism in heart diseases. Transgenic expression of an αB-crystallin mutant in mouse cardiomyocytes causes cardiomyopathy due to aggregation of the mutant protein [188]. Tubulin hyperacetylation by pharmacological inhibition of mouse HDAC6 activity or knockdown of Hdac6 expression ameliorates the aggregation process [188]. Atrial fibrillation is a significant contributor to cardiovascular morbidity and mortality. Tachypacing (rapid pacing of the heart by an artificial electronic pacemaker) induces contractile dysfunction in cardiomyocytes and induces tubulin deacetylation [189]. HDAC6 activation contributes to tachypacing-induced contractile dysfunction in experimental and human atrial fibrillation [189]. These two studies suggest that human HDAC6 is a potential therapeutic target for cardiovascular diseases (Table 1).
Tubulin acetylation also plays a role in other pathological conditions (Table 1). Chronic obstructive pulmonary disease (COPD) involves abnormal airway inflammatory responses to cigarette smoke, which inhibits ciliary tubulin acetylation [190]. As discussed above, tubulin acetylation is important for viral entry, inflammation and other immune responses (Fig. 4). Moreover, the spore-forming bacterium Clostridium difficile is a causative pathogen of antibiotic-associated diarrhea and pseudomembranous colitis in humans [191]. Toxin A from the bacterium inhibits tubulin acetylation and activates acute inflammation, whereas inhibition of HDAC6 ameliorates colitis in a mouse model injected with toxin A [191]. Similarly, HDAC6 inhibition is beneficial for inhibiting murine colitis induced by dextran sodium sulfate [145]. Therefore, altered tubulin acetylation is linked to different human diseases and the responsible enzymes constitute two novel molecular targets for drug development and therapeutic intervention (Table 1).
Conclusion and future direction
Since its initial identification 30 years ago [13], tubulin acetylation has been extensively studied [4–8]. Different from many other microtubule modifications, tubulin acetylation occurs on the luminal side (Fig. 1). In mammals, the responsible enzymes have been identified and characterized as ATAT1 and HDAC6 (Fig. 2). Crystal structures of ATAT1 with and without acetyl coenzyme A binding have been determined [52–56] and form the basis for virtual screening of small-molecule inhibitors of these enzymes, which may be valuable for treating related diseases (Table 1). Neither mouse Atat1 nor Hdac6 is essential for survival. While ATAT1 plays a role in mouse brain development and spermatogenesis [49–51], HDAC6 regulates mouse immune responses, bone density and unusual behaviors such as hyperactivity, reduced anxiety and lower depression tendency [67, 83]. The minor phenotypes of Atat1 nor Hdac6 knockout mice may be promising for the development of specific inhibitors for treating related pathological conditions (Table 1), because of potentially low toxicity of these inhibitors.
Functions of tubulin acetylation have been investigated in numerous studies. It becomes clear that this modification serves as a marker of stable microtubules rather than playing an active role in stabilizing microtubules (Fig. 4). Genetic and structural studies using mutant C. elegans strains have demonstrated that tubulin acetylation is required for maintaining the microtubule integrity and protofilament number in touch receptor neurons [112, 115]. However, in vitro assembly reveals that this modification exerts only minor effects on microtubule architecture (Fig. 3a) [96]. Thus, tubulin acetylation is necessary but not sufficient for formation of 15-protofilament microtubules (Fig. 3b). This modification may cooperate with special tubulin isoforms. In support of this, loss of Mec-17 or two tubulin isoforms (Mec-7 and Mec-12) in C. elegans exerts similar impact on formation of 15-protofilament microtubules in touch receptor neurons [45]. Like these neurons, pillar and phalangeal cells in the mammalian inner ear contain mainly 15-protofilament microtubules [102]. It will be interesting to investigate whether such microtubules are altered and hearing is impaired in Atat1 −/− mice [49, 50].
In addition to regulating microtubule architecture, tubulin acetylation appears to be important in various cellular processes, including intracellular transport, ciliary assembly, cell migration and polarity (Fig. 4). Notably, tubulin acetylation regulates immune, stress, inflammation and viral responses [69, 85, 150, 152, 159], suggesting potentially important roles under adverse conditions. Of relevance, deletion of the mouse Atat1 or Hdac6 gene only yields minor phenotypes in unstressed mice [49, 50, 67], but when challenged with stressors, more dramatic phenotypes may become evident. As recently reported for another gene [192], it is worth investigating whether genetic compensation provides an explanation for the minor phenotypes of Atat1 or Hdac6 knockout mice. In comparison, RNAi knockdown and morpholino inhibition of Atat1 are less likely to trigger compensation [47, 126].
Tubulin acetylation may be important for advanced functions, such as learning and memory. Related to this, tubulin acetylation is highly abundant in the mouse brain and loss of Atat1 leads to abnormal mouse brain development [49, 50, 67]. Moreover, mutations of two tubulin genes are linked to genetic diseases with abnormal brain development and intellectual disability [193, 194], and the gene of the microtubule stabilizing protein doublecortin is mutated in X-linked lissencephaly (smooth brain) and ‘double cortex’, two genetic disorders characterized with brain abnormalities [195, 196].
In addition to tubulin acetylation, there are other microtubule modifications, including detyrosination, Δ2 modification, glutamylation and glycylation, which are enriched at the C-terminal tails of tubulin (Fig. 1). This is reminiscent of diverse histone modifications concentrated at the N-terminal tails of histones [197, 198]. By analogy, different modifications on microtubules may generate a post-translational modification language that differentiates microtubules from one another and allows association with distinct microtubule associated proteins. Within this conceptual framework, the impact of tubulin acetylation on cellular processes would be important to consider along with the other microtubule modifications, different tubulin isoforms and various microtubule-associated proteins found in the cell.
Acknowledgments
The research is supported by grants from Canadian Institutes of Health Research (CIHR) and Natural Sciences and Engineering Research Council of Canada (NSERC) (to X.J.Y.). L.L. received stipend support from China Scholarship Council and a Clifford C.F. Wong Scholarship.
References
- 1.Desai A, Mitchison TJ. Microtubule polymerization dynamics. Annu Rev Cell Dev Biol. 1997;13:83–117. doi: 10.1146/annurev.cellbio.13.1.83. [DOI] [PubMed] [Google Scholar]
- 2.Nogales E. Structural insights into microtubule function. Annu Rev Biochem. 2000;69:277–302. doi: 10.1146/annurev.biochem.69.1.277. [DOI] [PubMed] [Google Scholar]
- 3.Subramanian R, Kapoor TM. Building complexity: insights into self-organized assembly of microtubule-based architectures. Dev Cell. 2012;23(5):874–885. doi: 10.1016/j.devcel.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Westermann S, Weber K. Post-translational modifications regulate microtubule function. Nat Rev Mol Cell Biol. 2003;4(12):938–947. doi: 10.1038/nrm1260. [DOI] [PubMed] [Google Scholar]
- 5.Wloga D, Gaertig J. Post-translational modifications of microtubules. J Cell Sci. 2010;123(20):3447–3455. doi: 10.1242/jcs.063727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Janke C, Bulinski JC. Post-translational regulation of the microtubule cytoskeleton: mechanisms and functions. Nat Rev Mol Cell Biol. 2011;12(12):773–786. doi: 10.1038/nrm3227. [DOI] [PubMed] [Google Scholar]
- 7.Janke C. The tubulin code: molecular components, readout mechanisms, and functions. J Cell Biol. 2014;206(4):461–472. doi: 10.1083/jcb.201406055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song Y, Brady ST. Post-translational modifications of tubulin: pathways to functional diversity of microtubules. Trends Cell Biol. 2015;25(3):125–136. doi: 10.1016/j.tcb.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roll-Mecak A. Intrinsically disordered tubulin tails: complex tuners of microtubule functions? Semin Cell Dev Biol. 2015;37:11–19. doi: 10.1016/j.semcdb.2014.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barra HS, Rodrigue JA, Arce CA, Caputto R. Soluble preparation from rat-brain that incorporates into its own proteins [C-14]arginine by a ribonuclease-sensitive system and [C-14]tyrosine by a ribonuclease-insensitive system. J Neurochem. 1973;20(1):97–108. doi: 10.1111/j.1471-4159.1973.tb12108.x. [DOI] [PubMed] [Google Scholar]
- 11.Paturle-Lafanechere L, Edde B, Denoulet P, Van Dorsselaer A, Mazarguil H, Le Caer JP, Wehland J, Job D. Characterization of a major brain tubulin variant which cannot be tyrosinated. Biochemistry. 1991;30(43):10523–10528. doi: 10.1021/bi00107a022. [DOI] [PubMed] [Google Scholar]
- 12.Lhernault SW, Rosenbaum JL. Chlamydomonas alpha-tubulin is posttranslationally modified in the flagella during flagellar assembly. J Cell Biol. 1983;97(1):258–263. doi: 10.1083/jcb.97.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lhernault SW, Rosenbaum JL. Chlamydomonas alpha-tubulin is posttranslationally modified by acetylation on the epsilon-amino group of a lysine. Biochemistry. 1985;24(2):473–478. doi: 10.1021/bi00323a034. [DOI] [PubMed] [Google Scholar]
- 14.Eipper BA. Properties of rat-brain tubulin. J Biol Chem. 1974;249(5):1407–1416. [PubMed] [Google Scholar]
- 15.Edde B, Rossier J, Lecaer JP, Desbruyeres E, Gros F, Denoulet P. Posttranslational glutamylation of alpha-tubulin. Science. 1990;247(4938):83–85. doi: 10.1126/science.1967194. [DOI] [PubMed] [Google Scholar]
- 16.Redeker V, Levilliers N, Schmitter JM, Lecaer JP, Rossier J, Adoutte A, Bre MH. Polyglycylation of tubulin—a posttranslational modification in axonemal microtubules. Science. 1994;266(5191):1688–1691. doi: 10.1126/science.7992051. [DOI] [PubMed] [Google Scholar]
- 17.Chu CW, Hou FJ, Zhang JM, Phu L, Loktev AV, Kirkpatrick DS, Jackson PK, Zhao YM, Zou H. A novel acetylation of beta-tubulin by San modulates microtubule polymerization via down-regulating tubulin incorporation. Mol Biol Cell. 2011;22(4):448–456. doi: 10.1091/mbc.E10-03-0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song Y, Kirkpatrick LL, Schilling AB, Helseth DL, Chabot N, Keillor JW, Johnson GV, Brady ST. Transglutaminase and polyamination of tubulin: posttranslational modification for stabilizing axonal microtubules. Neuron. 2013;78(1):109–123. doi: 10.1016/j.neuron.2013.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Piroli GG, Manuel AM, Walla MD, Jepson MJ, Brock JW, Rajesh MP, Tanis RM, Cotham WE, Frizzell N. Identification of protein succination as a novel modification of tubulin. Biochem J. 2014;462(2):231–245. doi: 10.1042/BJ20131581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alushin GM, Lander GC, Kellogg EH, Zhang R, Baker D, Nogales E. High-resolution microtubule structures reveal the structural transitions in alphabeta-tubulin upon GTP hydrolysis. Cell. 2014;157(5):1117–1129. doi: 10.1016/j.cell.2014.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aiken J, Sept D, Costanzo M, Boone C, Cooper JA, Moore JK. Genome-wide analysis reveals novel and discrete functions for tubulin carboxy-terminal tails. Curr Biol. 2014;24(12):1295–1303. doi: 10.1016/j.cub.2014.03.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sirajuddin M, Rice LM, Vale RD. Regulation of microtubule motors by tubulin isotypes and post-translational modifications. Nat Cell Biol. 2014;16(4):335–344. doi: 10.1038/ncb2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Erck C, Peris L, Andrieux A, Meissirel C, Gruber AD, Vernet M, Schweitzer A, Saoudi Y, Pointu H, Bosc C, et al. A vital role of tubulin-tyrosine-ligase for neuronal organization. Proc Natl Acad Sci USA. 2005;102(22):7853–7858. doi: 10.1073/pnas.0409626102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fernandez-Gonzalez A, La Spada AR, Treadaway J, Higdon JC, Harris BS, Sidman RL, Morgan JI, Zuo J. Purkinje cell degeneration (pcd) phenotypes caused by mutations in the axotomy-induced gene, Nna1. Science. 2002;295(5561):1904–1906. doi: 10.1126/science.1068912. [DOI] [PubMed] [Google Scholar]
- 25.Rogowski K, van Dijk J, Magiera MM, Bosc C, Deloulme JC, Bosson A, Peris L, Gold ND, Lacroix B, Bosch Grau M, et al. A family of protein-deglutamylating enzymes associated with neurodegeneration. Cell. 2010;143(4):564–578. doi: 10.1016/j.cell.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 26.Ikegami K, Sato S, Nakamura K, Ostrowski LE, Setou M. Tubulin polyglutamylation is essential for airway ciliary function through the regulation of beating asymmetry. Proc Natl Acad Sci USA. 2010;107(23):10490–10495. doi: 10.1073/pnas.1002128107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bosch Grau M, Gonzalez Curto G, Rocha C, Magiera MM, Marques Sousa P, Giordano T, Spassky N, Janke C. Tubulin glycylases and glutamylases have distinct functions in stabilization and motility of ependymal cilia. J Cell Biol. 2013;202(3):441–451. doi: 10.1083/jcb.201305041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gershey EL, Vidali G, Allfrey VG. Chemical studies of histone acetylation. The occurrence of epsilon-N-acetyllysine in the f2a1 histone. J Biol Chem. 1968;243(19):5018–5022. [PubMed] [Google Scholar]
- 29.Piperno G, Fuller MT. Monoclonal-antibodies specific for an acetylated form of alpha-tubulin recognize the antigen in cilia and flagella from a variety of organisms. J Cell Biol. 1985;101(6):2085–2094. doi: 10.1083/jcb.101.6.2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim SC, Sprung R, Chen Y, Xu YD, Ball H, Pei JM, Cheng TL, Kho Y, Xiao H, Xiao L, et al. Substrate and functional diversity of lysine acetylation revealed by a proteomics survey. Mol Cell. 2006;23(4):607–618. doi: 10.1016/j.molcel.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 31.Choudhary C, Kumar C, Gnad F, Nielsen ML, Rehman M, Walther TC, Olsen JV, Mann M. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science. 2009;325(5942):834–840. doi: 10.1126/science.1175371. [DOI] [PubMed] [Google Scholar]
- 32.Kim GW, Yang XJ. Comprehensive lysine acetylomes emerging from bacteria to humans. Trends Biochem Sci. 2011;36(4):211–220. doi: 10.1016/j.tibs.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 33.Mckeithan TW, Rosenbaum JL. Multiple forms of tubulin in the cytoskeletal and flagellar microtubules of polytomella. J Cell Biol. 1981;91(2):352–360. doi: 10.1083/jcb.91.2.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mckeithan TW, Lefebvre PA, Silflow CD, Rosenbaum JL. Multiple forms of tubulin in polytomella and chlamydomonas—evidence for a precursor of flagellar alpha-tubulin. J Cell Biol. 1983;96(4):1056–1063. doi: 10.1083/jcb.96.4.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ledizet M, Piperno G. Identification of an acetylation site of Chlamydomonas alpha-tubulin. Proc Natl Acad Sci USA. 1987;84(16):5720–5724. doi: 10.1073/pnas.84.16.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Piperno G, Ledizet M, Chang XJ. Microtubules containing acetylated alpha-tubulin in mammalian-cells in culture. J Cell Biol. 1987;104(2):289–302. doi: 10.1083/jcb.104.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakagawa U, Suzuki D, Ishikawa M, Sato H, Kamemura K, Imamura A. Acetylation of alpha-tubulin on lys(40) Is a widespread post-translational modification in angiosperms. Biosci Biotechnol Biochem. 2013;77(7):1602–1605. doi: 10.1271/bbb.130261. [DOI] [PubMed] [Google Scholar]
- 38.Yang XJ, Grégoire S. Class II histone deacetylases: from sequence to function, regulation and clinical implication. Mol Cell Biol. 2005;25:2873–2884. doi: 10.1128/MCB.25.8.2873-2884.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Greer K, Maruta H, L’Hernault SW, Rosenbaum JL. Alpha-tubulin acetylase activity in isolated Chlamydomonas flagella. J Cell Biol. 1985;101(6):2081–2084. doi: 10.1083/jcb.101.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Creppe C, Malinouskaya L, Volvert ML, Gillard M, Close P, Malaise O, Laguesse S, Cornez I, Rahmouni S, Ormenese S, et al. Elongator controls the migration and differentiation of cortical neurons through acetylation of alpha-tubulin. Cell. 2009;136(3):551–564. doi: 10.1016/j.cell.2008.11.043. [DOI] [PubMed] [Google Scholar]
- 41.Solinger JA, Paolinelli R, Kloss H, Scorza FB, Marchesi S, Sauder U, Mitsushima D, Capuani F, Sturzenbaum SR, Cassata G (2010) The Caenorhabditis elegans elongator complex regulates neuronal alpha-tubulin acetylation. Plos Genet 6(1) [DOI] [PMC free article] [PubMed]
- 42.Ohkawa N, Sugisaki S, Tokunaga E, Fujitani K, Hayasaka T, Setou M, Inokuchi K. N-acetyltransferase ARD1-NAT1 regulates neuronal dendritic development. Genes Cells. 2008;13(11):1171–1183. doi: 10.1111/j.1365-2443.2008.01235.x. [DOI] [PubMed] [Google Scholar]
- 43.Conacci-Sorrell M, Ngouenet C, Eisenman RN. Myc-Nick: a cytoplasmic cleavage product of Myc that promotes alpha-tubulin acetylation and cell differentiation. Cell. 2010;142(3):480–493. doi: 10.1016/j.cell.2010.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang Y, Ma C, Delohery T, Nasipak B, Foat BC, Bounoutas A, Bussemaker HJ, Kim SK, Chalfie M. Identification of genes expressed in C. elegans touch receptor neurons. Nature. 2002;418(6895):331–335. doi: 10.1038/nature00891. [DOI] [PubMed] [Google Scholar]
- 45.Chalfie M. Neurosensory mechanotransduction. Nat Rev Mol Cell Biol. 2009;10(1):44–52. doi: 10.1038/nrm2595. [DOI] [PubMed] [Google Scholar]
- 46.Steczkiewicz K, Kinch L, Grishin NV, Rychlewski L, Ginalski K. Eukaryotic domain of unknown function DUF738 belongs to Gcn5-related N-acetyltransferase superfamily. Cell Cycle. 2006;5(24):2927–2930. doi: 10.4161/cc.5.24.3572. [DOI] [PubMed] [Google Scholar]
- 47.Akella JS, Wloga D, Kim J, Starostina NG, Lyons-Abbott S, Morrissette NS, Dougan ST, Kipreos ET, Gaertig J. MEC-17 is an alpha-tubulin acetyltransferase. Nature. 2010;467(7312):218–222. doi: 10.1038/nature09324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shida T, Cueva JG, Xu ZJ, Goodman MB, Nachury MV. The major alpha-tubulin K40 acetyltransferase alpha TAT1 promotes rapid ciliogenesis and efficient mechanosensation. Proc Natl Acad Sci USA. 2010;107(50):21517–21522. doi: 10.1073/pnas.1013728107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim GW, Li L, Gorbani M, You L, Yang XJ. Mice lacking alpha-tubulin acetyltransferase 1 are viable but display alpha-tubulin acetylation deficiency and dentate gyrus distortion. J Biol Chem. 2013;288(28):20334–20350. doi: 10.1074/jbc.M113.464792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kalebic N, Sorrentino S, Perlas E, Bolasco G, Martinez C, Heppenstall PA (2013) alpha TAT1 is the major alpha-tubulin acetyltransferase in mice. Nat Commun 4 [DOI] [PubMed]
- 51.Aguilar A, Becker L, Tedeschi T, Heller S, Iomini C, Nachury MV. alpha-Tubulin K40 acetylation is required for contact inhibition of proliferation and cell-substrate adhesion. Mol Biol Cell. 2014;25(12):1854–1866. doi: 10.1091/mbc.E13-10-0609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kormendi V, Szyk A, Piszczek G, Roll-Mecak A. Crystal structures of tubulin acetyltransferase reveal a conserved catalytic core and the plasticity of the essential N terminus. J Biol Chem. 2012;287(50):41569–41575. doi: 10.1074/jbc.C112.421222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Taschner M, Vetter M, Lorentzen E. Atomic resolution structure of human alpha-tubulin acetyltransferase bound to acetyl-CoA. Proc Natl Acad Sci USA. 2012;109(48):19649–19654. doi: 10.1073/pnas.1209343109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Friedmann DR, Aguilar A, Fan J, Nachury MV, Marmorstein R. Structure of the alpha-tubulin acetyltransferase, alphaTAT1, and implications for tubulin-specific acetylation. Proc Natl Acad Sci USA. 2012;109(48):19655–19660. doi: 10.1073/pnas.1209357109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Davenport AM, Collins LN, Chiu H, Minor PJ, Sternberg PW, Hoelz A. Structural and functional characterization of the alpha-tubulin acetyltransferase MEC-17. J Mol Biol. 2014;426(14):2605–2616. doi: 10.1016/j.jmb.2014.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yuzawa S, Kamakura S, Hayase J, Sumimoto H. Structural basis of cofactor-mediated stabilization and substrate recognition of the alpha-tubulin acetyltransferase alphaTAT1. Biochem J. 2015;467(1):103–113. doi: 10.1042/BJ20141193. [DOI] [PubMed] [Google Scholar]
- 57.Odde D. Diffusion inside microtubules. Eur Biophys J Biophys Lett. 1998;27(5):514–520. doi: 10.1007/s002490050161. [DOI] [PubMed] [Google Scholar]
- 58.Maruta H, Greer K, Rosenbaum JL. The acetylation of alpha-tubulin and its relationship to the assembly and disassembly of microtubules. J Cell Biol. 1986;103(2):571–579. doi: 10.1083/jcb.103.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Szyk A, Deaconescu AM, Spector J, Goodman B, Valenstein ML, Ziolkowska NE, Kormendi V, Grigorieff N, Roll-Mecak A. Molecular basis for age-dependent microtubule acetylation by tubulin acetyltransferase. Cell. 2014;157(6):1405–1415. doi: 10.1016/j.cell.2014.03.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hubbert C, Guardiola A, Shao R, Kawaguchi Y, Ito A, Nixon A, Yoshida M, Wang XF, Yao TP. HDAC6 is a microtubule-associated deacetylase. Nature. 2002;417(6887):455–458. doi: 10.1038/417455a. [DOI] [PubMed] [Google Scholar]
- 61.Verdel A, Curtet S, Brocard MP, Rousseaux S, Lemercier C, Yoshida M, Khochbin S. Active maintenance of mHDA2/mHDAC6 histone-deacetylase in the cytoplasm. Curr Biol. 2000;10(12):747–749. doi: 10.1016/S0960-9822(00)00542-X. [DOI] [PubMed] [Google Scholar]
- 62.Matsuyama A, Shimazu T, Sumida Y, Saito A, Yoshimatsu Y, Seigneurin-Berny D, Osada H, Komatsu Y, Nishino N, Khochbin S, et al. In vivo destabilization of dynamic microtubules by HDAC6-mediated deacetylation. EMBO J. 2002;21(24):6820–6831. doi: 10.1093/emboj/cdf682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang Y, Li N, Caron C, Matthias G, Hess D, Khochbin S, Matthias P. HDAC-6 interacts with and deacetylates tubulin and microtubules in vivo. EMBO J. 2003;22(5):1168–1179. doi: 10.1093/emboj/cdg115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.North BJ, Marshall BL, Borra MT, Denu JM, Verdin E. The human Sir2 ortholog, SIRT2, is an NAD(+)-dependent tubulin deacetylase. Mol Cell. 2003;11(2):437–444. doi: 10.1016/S1097-2765(03)00038-8. [DOI] [PubMed] [Google Scholar]
- 65.Bobrowska A, Donmez G, Weiss A, Guarente L, Bates G (2012) SIRT2 ablation has no effect on tubulin acetylation in brain, cholesterol biosynthesis or the progression of huntington’s disease phenotypes in vivo. Plos One 7(4) [DOI] [PMC free article] [PubMed]
- 66.Taes I, Timmers M, Hersmus N, Bento-Abreu A, Van den Bosch L, Van Damme P, Auwerx J, Robberecht W. Hdac6 deletion delays disease progression in the SOD1(G93A) mouse model of ALS. Hum Mol Genet. 2013;22(9):1783–1790. doi: 10.1093/hmg/ddt028. [DOI] [PubMed] [Google Scholar]
- 67.Zhang Y, Kwon S, Yamaguchi T, Cubizolles F, Rousseaux S, Kneissel M, Cao C, Li N, Cheng HL, Chua K, et al. Mice lacking histone deacetylase 6 have hyperacetylated tubulin but are viable and develop normally. Mol Cell Biol. 2008;28(5):1688–1701. doi: 10.1128/MCB.01154-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nagai T, Ikeda M, Chiba S, Kanno S, Mizuno K. Furry promotes acetylation of microtubules in the mitotic spindle by inhibition of SIRT2 tubulin deacetylase. J Cell Sci. 2013;126(19):4369–4380. doi: 10.1242/jcs.127209. [DOI] [PubMed] [Google Scholar]
- 69.Misawa T, Takahama M, Kozaki T, Lee H, Zou J, Saitoh T, Akira S. Microtubule-driven spatial arrangement of mitochondria promotes activation of the NLRP3 inflammasome. Nat Immunol. 2013;14(5):454–460. doi: 10.1038/ni.2550. [DOI] [PubMed] [Google Scholar]
- 70.Cho Y, Cavalli V. HDAC5 is a novel injury-regulated tubulin deacetylase controlling axon regeneration. EMBO J. 2012;31(14):3063–3078. doi: 10.1038/emboj.2012.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bertos NR, Gilquin B, Chan GK, Yen TJ, Khochbin S, Yang XJ. Role of the tetradecapeptide repeat domain of human histone deacetylase 6 in cytoplasmic retention. J Biol Chem. 2004;279(46):48246–48254. doi: 10.1074/jbc.M408583200. [DOI] [PubMed] [Google Scholar]
- 72.Liu YJ, Peng LR, Seto E, Huang SM, Qiu Y. Modulation of histone deacetylase 6 (HDAC6) nuclear import and tubulin deacetylase activity through acetylation. J Biol Chem. 2012;287(34):29168–29174. doi: 10.1074/jbc.M112.371120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang M, Xiang S, Joo HY, Wang L, Williams KA, Liu W, Hu C, Tong D, Haakenson J, Wang C, et al. HDAC6 deacetylates and ubiquitinates MSH2 to maintain proper levels of MutSalpha. Mol Cell. 2014;55(1):31–46. doi: 10.1016/j.molcel.2014.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kovacs JJ, Murphy PJM, Gaillard S, Zhao XA, Wu JT, Nicchitta CV, Yoshida M, Toft DO, Pratt WB, Yao TP. HDAC6 regulates Hsp90 acetylation and chaperone-dependent activation of glucocorticoid receptor. Mol Cell. 2005;18(5):601–607. doi: 10.1016/j.molcel.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 75.Cook C, Gendron TF, Scheffel K, Carlomagno Y, Dunmore J, DeTure M, Petrucelli L. Loss of HDAC6, a novel CHIP substrate, alleviates abnormal tau accumulation. Hum Mol Genet. 2012;21(13):2936–2945. doi: 10.1093/hmg/dds125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fusco C, Micale L, Augello B, Mandriani B, Pellico MT, De Nittis P, Calcagni A, Monti M, Cozzolino F, Pucci P, et al. HDAC6 mediates the acetylation of TRIM50. Cell Signal. 2014;26(2):363–369. doi: 10.1016/j.cellsig.2013.11.036. [DOI] [PubMed] [Google Scholar]
- 77.Zhang XH, Yuan ZG, Zhang YT, Yong S, Salas-Burgos A, Koomen J, Olashaw N, Parsons JT, Yang XJ, Dent SR, et al. HDAC6 modulates cell motility by altering the acetylation level of cortactin. Mol Cell. 2007;27(2):197–213. doi: 10.1016/j.molcel.2007.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Boyault C, Zhang Y, Fritah S, Caron C, Gilquin B, Kwon SH, Garrido C, Yao TP, Vourc’h C, Matthias P, et al. HDAC6 controls major cell response pathways to cytotoxic accumulation of protein aggregates. Genes Dev. 2007;21(17):2172–2181. doi: 10.1101/gad.436407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kawaguchi Y, Kovacs JJ, McLaurin A, Vance JM, Ito A, Yao TP. The deacetylase HDAC6 regulates aggresome formation and cell viability in response to misfolded protein stress. Cell. 2003;115(6):727–738. doi: 10.1016/S0092-8674(03)00939-5. [DOI] [PubMed] [Google Scholar]
- 80.Palijan A, Fernandes I, Bastien Y, Tang LQ, Verway M, Kourelis M, Tavera-Mendoza LE, Li Z, Bourdeau V, Mader S, et al. Function of histone deacetylase 6 as a cofactor of nuclear receptor coregulator LCoR. J Biol Chem. 2009;284(44):30264–30274. doi: 10.1074/jbc.M109.045526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen SG, Owens GC, Makarenkova H, Edelman DB (2010) HDAC6 regulates mitochondrial transport in hippocampal neurons. Plos One 5(5) [DOI] [PMC free article] [PubMed]
- 82.Pandey UB, Nie ZP, Batlevi Y, McCray BA, Ritson GP, Nedelsky NB, Schwartz SL, DiProspero NA, Knight MA, Schuldiner O, et al. HDAC6 rescues neurodegeneration and provides an essential link between autophagy and the UPS. Nature. 2007;447(7146):859–863. doi: 10.1038/nature05853. [DOI] [PubMed] [Google Scholar]
- 83.Fukada M, Hanai A, Nakayama A, Suzuki T, Miyata N, Rodriguiz RM, Wetsel WC, Yao TP, Kawaguchi Y (2012) Loss of deacetylation activity of hdac6 affects emotional behavior in mice. Plos One 7(2) [DOI] [PMC free article] [PubMed]
- 84.Sadoul K, Wang J, Diagouraga B, Vitte AL, Buchou T, Rossini T, Polack B, Xi XD, Matthias P, Khochbin S. HDAC6 controls the kinetics of platelet activation. Blood. 2012;120(20):4215–4218. doi: 10.1182/blood-2012-05-428011. [DOI] [PubMed] [Google Scholar]
- 85.Wang B, Rao YH, Inoue M, Hao R, Lai CH, Chen D, McDonald SL, Choi MC, Wang Q, Shinohara ML, et al. Microtubule acetylation amplifies p38 kinase signalling and anti-inflammatory IL-10 production. Nat Commun. 2014;5:3479. doi: 10.1038/ncomms4479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ledizet M, Piperno G. Cytoplasmic microtubules containing acetylated alpha-tubulin in Chlamydomonas-reinhardtii—spatial arrangement and properties. J Cell Biol. 1986;103(1):13–22. doi: 10.1083/jcb.103.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cambray-Deakin MA, Burgoyne RD. Acetylated and detyrosinated alpha-tubulins are co-localized in stable microtubules in rat meningeal fibroblasts. Cell Motil Cytoskelet. 1987;8(3):284–291. doi: 10.1002/cm.970080309. [DOI] [PubMed] [Google Scholar]
- 88.Webster DR, Borisy GG. Microtubules are acetylated in domains that turn over slowly. J Cell Sci. 1989;92:57–65. doi: 10.1242/jcs.92.1.57. [DOI] [PubMed] [Google Scholar]
- 89.Robson SJ, Burgoyne RD. Differential localization of tyrosinated, detyrosinated, and acetylated alpha-tubulins in neurites and growth cones of dorsal-root ganglion neurons. Cell Motil Cytoskelet. 1989;12(4):273–282. doi: 10.1002/cm.970120408. [DOI] [PubMed] [Google Scholar]
- 90.Sale WS, Besharse JC, Piperno G. Distribution of acetylated alpha-tubulin in retina and in vitro-assembled microtubules. Cell Motil Cytoskelet. 1988;9(3):243–253. doi: 10.1002/cm.970090306. [DOI] [PubMed] [Google Scholar]
- 91.Cambray-Deakin MA, Burgoyne RD. Posttranslational modifications of alpha-tubulin: acetylated and detyrosinated forms in axons of rat cerebellum. J Cell Biol. 1987;104(6):1569–1574. doi: 10.1083/jcb.104.6.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wehland J, Weber K. Turnover of the carboxy-terminal tyrosine of alpha-tubulin and means of reaching elevated levels of detyrosination in living cells. J Cell Sci. 1987;88:185–203. doi: 10.1242/jcs.88.2.185. [DOI] [PubMed] [Google Scholar]
- 93.Bre MH, Kreis TE, Karsenti E. Control of microtubule nucleation and stability in madin-darby canine kidney-cells—the occurrence of noncentrosomal, stable detyrosinated microtubules. J Cell Biol. 1987;105(3):1283–1296. doi: 10.1083/jcb.105.3.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Khawaja S, Gundersen GG, Bulinski JC. Enhanced stability of microtubules enriched in detyrosinated tubulin is not a direct function of detyrosination level. J Cell Biol. 1988;106(1):141–149. doi: 10.1083/jcb.106.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Palazzo A, Ackerman B, Gundersen GG. Cell biology—Tubulin acetylation and cell motility. Nature. 2003;421(6920):230. doi: 10.1038/421230a. [DOI] [PubMed] [Google Scholar]
- 96.Howes SC, Alushin GM, Shida T, Nachury MV, Nogales E. Effects of tubulin acetylation and tubulin acetyltransferase binding on microtubule structure. Mol Biol Cell. 2014;25(2):257–266. doi: 10.1091/mbc.E13-07-0387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Barisic M, Silva ESR, Tripathy SK, Magiera MM, Zaytsev AV, Pereira AL, Janke C, Grishchuk EL, Maiato H. Microtubule detyrosination guides chromosomes during mitosis. Science. 2015;348(6236):799–803. doi: 10.1126/science.aaa5175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zilberman Y, Ballestrem C, Carramusa L, Mazitschek R, Khochbin S, Bershadsky A. Regulation of microtubule dynamics by inhibition of the tubulin deacetylase HDAC6. J Cell Sci. 2009;122(19):3531–3541. doi: 10.1242/jcs.046813. [DOI] [PubMed] [Google Scholar]
- 99.Neumann B, Hilliard MA. Loss of MEC-17 leads to microtubule instability and axonal degeneration. Cell Reports. 2014;6(1):93–103. doi: 10.1016/j.celrep.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tilney LG, Bryan J, Bush DJ, Fujiwara K, Mooseker MS, Murphy DB, Snyder DH. Microtubules: evidence for 13 protofilaments. J Cell Biol. 1973;59(2 Pt 1):267–275. doi: 10.1083/jcb.59.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Burton PR, Hinkley RE, Pierson GB. Tannic acid-stained microtubules with 12, 13, and 15 protofilaments. J Cell Biol. 1975;65(1):227–233. doi: 10.1083/jcb.65.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Saito K, Hama K. Structural diversity of microtubules in the supporting cells of the sensory epithelium of guinea pig organ of Corti. J Electron Microsc (Tokyo) 1982;31(3):278–281. [PubMed] [Google Scholar]
- 103.Nagano T, Suzuki F. Microtubules with 15 subunits in cockroach epidermal cells. J Cell Biol. 1975;64(1):242–245. doi: 10.1083/jcb.64.1.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chalfie M, Thomson JN. Organization of neuronal microtubules in the nematode Caenorhabditis elegans . J Cell Biol. 1979;82(1):278–289. doi: 10.1083/jcb.82.1.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pierson GB, Burton PR, Himes RH. Wall substructure of microtubules polymerized in vitro from tubulin of crayfish nerve cord and fixed with tannic acid. J Cell Sci. 1979;39:89–99. doi: 10.1242/jcs.39.1.89. [DOI] [PubMed] [Google Scholar]
- 106.Aamodt EJ, Culotti JG. Microtubules and microtubule-associated proteins from the nematode Caenorhabditis elegans: periodic cross-links connect microtubules in vitro. J Cell Biol. 1986;103(1):23–31. doi: 10.1083/jcb.103.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Andreu JM, Bordas J, Diaz JF, Garcia de Ancos J, Gil R, Medrano FJ, Nogales E, Pantos E, Towns-Andrews E. Low resolution structure of microtubules in solution. synchrotron X-ray scattering and electron microscopy of taxol-induced microtubules assembled from purified tubulin in comparison with glycerol and MAP-induced microtubules. J Mol Biol. 1992;226(1):169–184. doi: 10.1016/0022-2836(92)90132-4. [DOI] [PubMed] [Google Scholar]
- 108.Moores CA, Perderiset M, Francis F, Chelly J, Houdusse A, Milligan RA. Mechanism of microtubule stabilization by doublecortin. Mol Cell. 2004;14(6):833–839. doi: 10.1016/j.molcel.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 109.Bechstedt S, Brouhard GJ. Doublecortin recognizes the 13-protofilament microtubule cooperatively and tracks microtubule ends. Dev Cell. 2012;23(1):181–192. doi: 10.1016/j.devcel.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.White JG, Southgate E, Thomson JN, Brenner S. The structure of the nervous system of the nematode Caenorhabditis elegans . Philos Trans R Soc Lond B Biol Sci. 1986;314(1165):1–340. doi: 10.1098/rstb.1986.0056. [DOI] [PubMed] [Google Scholar]
- 111.Cueva JG, Mulholland A, Goodman MB. Nanoscale organization of the MEC-4 DEG/ENaC sensory mechanotransduction channel in Caenorhabditis elegans touch receptor neurons. J Neurosci. 2007;27(51):14089–14098. doi: 10.1523/JNEUROSCI.4179-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Topalidou I, Keller C, Kalebic N, Nguyen KCQ, Somhegyi H, Politi KA, Heppenstall P, Hall DH, Chalfie M. Genetically separable functions of the MEC-17 tubulin acetyltransferase affect microtubule organization. Curr Biol. 2012;22(12):1057–1065. doi: 10.1016/j.cub.2012.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Siddiqui SS, Aamodt E, Rastinejad F, Culotti J. Anti-tubulin monoclonal antibodies that bind to specific neurons in Caenorhabditis elegans . J Neurosci. 1989;9(8):2963–2972. doi: 10.1523/JNEUROSCI.09-08-02963.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fukushige T, Siddiqui ZK, Chou M, Culotti JG, Gogonea CB, Siddiqui SS, Hamelin M. MEC-12, an alpha-tubulin required for touch sensitivity in C. elegans . J Cell Sci. 1999;112(Pt 3):395–403. doi: 10.1242/jcs.112.3.395. [DOI] [PubMed] [Google Scholar]
- 115.Cueva JG, Hsin J, Huang KC, Goodman MB. Posttranslational acetylation of alpha-tubulin constrains protofilament number in native microtubules. Curr Biol. 2012;22(12):1066–1074. doi: 10.1016/j.cub.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tolomeo JA, Holley MC. Mechanics of microtubule bundles in pillar cells from the inner ear. Biophys J. 1997;73(4):2241–2247. doi: 10.1016/S0006-3495(97)78255-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tannenbaum J, Slepecky NB. Localization of microtubules containing posttranslationally modified tubulin in cochlear epithelial cells during development. Cell Motil Cytoskelet. 1997;38(2):146–162. doi: 10.1002/(SICI)1097-0169(1997)38:2<146::AID-CM4>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 118.Reed NA, Cai DW, Blasius TL, Jih GT, Meyhofer E, Gaertig J, Verhey KJ. Microtubule acetylation promotes kinesin-1 binding and transport. Curr Biol. 2006;16(21):2166–2172. doi: 10.1016/j.cub.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 119.Cai DW, McEwen DP, Martens JR, Meyhofer E, Verhey KJ (2009) Single molecule imaging reveals differences in microtubule track selection between kinesin motors. Plos Biol 7(10) [DOI] [PMC free article] [PubMed]
- 120.Bhuwania R, Castro-Castro A, Linder S. Microtubule acetylation regulates dynamics of KIF1C-powered vesicles and contact of microtubule plus ends with podosomes. Eur J Cell Biol. 2014;93(10–12):424–437. doi: 10.1016/j.ejcb.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 121.Dompierre JP, Godin JD, Charrin BC, Cordelieres FP, King SJ, Humbert S, Saudou F. Histone deacetylase 6 inhibition compensates for the transport deficit in Huntington’s disease by increasing tubulin acetylation. J Neurosci. 2007;27(13):3571–3583. doi: 10.1523/JNEUROSCI.0037-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kaul N, Soppina V, Verhey KJ. Effects of alpha-tubulin K40 acetylation and detyrosination on kinesin-1 motility in a purified system. Biophys J. 2014;106(12):2636–2643. doi: 10.1016/j.bpj.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hammond JW, Huang CF, Kaech S, Jacobson C, Banker G, Verhey KJ. Posttranslational modifications of tubulin and the polarized transport of kinesin-1 in neurons. Mol Biol Cell. 2010;21(4):572–583. doi: 10.1091/mbc.E09-01-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Witte H, Neukirchen D, Bradke F. Microtubule stabilization specifies initial neuronal polarization. J Cell Biol. 2008;180(3):619–632. doi: 10.1083/jcb.200707042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Birdsey GM, Dryden NH, Shah AV, Hannah R, Hall MD, Haskard DO, Parsons M, Mason JC, Zvelebil M, Gottgens B, et al. The transcription factor Erg regulates expression of histone deacetylase 6 and multiple pathways involved in endothelial cell migration and angiogenesis. Blood. 2012;119(3):894–903. doi: 10.1182/blood-2011-04-350025. [DOI] [PubMed] [Google Scholar]
- 126.Li L, Wei D, Wang Q, Pan J, Liu R, Zhang X, Bao L. MEC-17 deficiency leads to reduced alpha-tubulin acetylation and impaired migration of cortical neurons. J Neurosci. 2012;32(37):12673–12683. doi: 10.1523/JNEUROSCI.0016-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Selvadurai K, Wang P, Seimetz J, Huang RH. Archaeal Elp3 catalyzes tRNA wobble uridine modification at C5 via a radical mechanism. Nat Chem Biol. 2014;10(10):810–812. doi: 10.1038/nchembio.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Deakin NO, Turner CE. Paxillin inhibits HDAC6 to regulate microtubule acetylation, Golgi structure, and polarized migration. J Cell Biol. 2014;206(3):395–413. doi: 10.1083/jcb.201403039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Montagnac G, Meas-Yedid V, Irondelle M, Castro-Castro A, Franco M, Shida T, Nachury MV, Benmerah A, Olivo-Marin JC, Chavrier P. alphaTAT1 catalyses microtubule acetylation at clathrin-coated pits. Nature. 2013;502(7472):567–570. doi: 10.1038/nature12571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tran ADA, Marmo TP, Salam AA, Che S, Finkelstein E, Kabarriti R, Xenias HS, Mazitschek R, Hubbert C, Kawaguchi Y, et al. HDAC6 deacetylation of tubulin modulates dynamics of cellular adhesions. J Cell Sci. 2007;120(8):1469–1479. doi: 10.1242/jcs.03431. [DOI] [PubMed] [Google Scholar]
- 131.Castro-Castro A, Janke C, Montagnac G, Paul-Gilloteaux P, Chavrier P. ATAT1/MEC-17 acetyltransferase and HDAC6 deacetylase control a balance of acetylation of alpha-tubulin and cortactin and regulate MT1-MMP trafficking and breast tumor cell invasion. Eur J Cell Biol. 2012;91(11–12):950–960. doi: 10.1016/j.ejcb.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 132.Wu Y, Song SW, Sun JY, Bruner JM, Fuller GN, Zhang W. IIp45 inhibits cell migration through inhibition of HDAC6. J Biol Chem. 2010;285(6):3554–3560. doi: 10.1074/jbc.M109.063354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Penela P, Lafarga V, Tapia O, Rivas V, Nogues L, Lucas E, Vila-Bedmar R, Murga C, Mayor F (2012) Roles of GRK2 in cell signaling beyond GPCR desensitization: GRK2-HDAC6 interaction modulates cell spreading and motility. Sci Signal 5(224) [DOI] [PubMed]
- 134.Chang JF, Baloh RH, Milbrandt J. The NIMA-family kinase Nek3 regulates microtubule acetylation in neurons. J Cell Sci. 2009;122(13):2274–2282. doi: 10.1242/jcs.048975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Pugacheva EN, Jablonski SA, Hartman TR, Henske EP, Golemis EA. HEF1-dependent aurora a activation induces disassembly of the primary cilium. Cell. 2007;129(7):1351–1363. doi: 10.1016/j.cell.2007.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bangs FK, Schrode N, Hadjantonakis AK, Anderson KV. Lineage specificity of primary cilia in the mouse embryo. Nat Cell Biol. 2015;17(2):113–122. doi: 10.1038/ncb3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Nakakura T, Asano-Hoshino A, Suzuki T, Arisawa K, Tanaka H, Sekino Y, Kiuchi Y, Kawai K, Hagiwara H. The elongation of primary cilia via the acetylation of alpha-tubulin by the treatment with lithium chloride in human fibroblast KD cells. Med Mol Morphol. 2015;48(1):44–53. doi: 10.1007/s00795-014-0076-x. [DOI] [PubMed] [Google Scholar]
- 138.Loktev AV, Zhang Q, Beck JS, Searby CC, Scheetz TE, Bazan JF, Slusarski DC, Sheffield VC, Jackson PK, Nachury MV. A BBSome subunit links ciliogenesis, microtubule stability, and acetylation. Dev Cell. 2008;15(6):854–865. doi: 10.1016/j.devcel.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 139.He Q, Wang G, Wakade S, Dasgupta S, Dinkins M, Kong JN, Spassieva SD, Bieberich E. Primary cilia in stem cells and neural progenitors are regulated by neutral sphingomyelinase 2 and ceramide. Mol Biol Cell. 2014;25(11):1715–1729. doi: 10.1091/mbc.E13-12-0730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wickstrom SA, Masoumi KC, Khochbin S, Fassler R, Massoumi R. CYLD negatively regulates cell-cycle progression by inactivating HDAC6 and increasing the levels of acetylated tubulin. EMBO J. 2010;29(1):131–144. doi: 10.1038/emboj.2009.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Eguether T, Ermolaeva MA, Zhao Y, Bonnet MC, Jain A, Pasparakis M, Courtois G, Tassin AM. The deubiquitinating enzyme CYLD controls apical docking of basal bodies in ciliated epithelial cells. Nat Commun. 2014;5:4585. doi: 10.1038/ncomms5585. [DOI] [PubMed] [Google Scholar]
- 142.Yang Y, Ran J, Liu M, Li D, Li Y, Shi X, Meng D, Pan J, Ou G, Aneja R, et al. CYLD mediates ciliogenesis in multiple organs by deubiquitinating Cep70 and inactivating HDAC6. Cell Res. 2014;24(11):1342–1353. doi: 10.1038/cr.2014.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Oh EC, Katsanis N. Cilia in vertebrate development and disease. Development. 2012;139(3):443–448. doi: 10.1242/dev.050054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Goetz SC, Anderson KV. The primary cilium: a signalling centre during vertebrate development. Nat Rev Genet. 2010;11(5):331–344. doi: 10.1038/nrg2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.de Zoeten EF, Wang L, Butler K, Beier UH, Akimova T, Sai H, Bradner JE, Mazitschek R, Kozikowski AP, Matthias P, et al. Histone deacetylase 6 and heat shock protein 90 control the functions of Foxp3(+) T-regulatory cells. Mol Cell Biol. 2011;31(10):2066–2078. doi: 10.1128/MCB.05155-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hanania R, Sun HS, Xu K, Pustylnik S, Jeganathan S, Harrison RE. Classically activated macrophages use stable microtubules for matrix metalloproteinase-9 (MMP-9) secretion. J Biol Chem. 2012;287(11):8468–8483. doi: 10.1074/jbc.M111.290676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140(6):821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 148.Misawa T, Takahama M, Kozaki T, Park S, Saitoh T, Akira S (2015) Resveratrol inhibits the acetylated alpha-tubulin-mediated assembly of the NLRP3-inflammasome. Int Immunol [Epub ahead of print] [DOI] [PubMed]
- 149.Kratzer E, Tian Y, Sarich N, Wu T, Meliton A, Leff A, Birukova AA. Oxidative stress contributes to lung injury and barrier dysfunction via microtubule destabilization. Am J Respir Cell Mol Biol. 2012;47(5):688–697. doi: 10.1165/rcmb.2012-0161OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ishiguro K, Ando T, Maeda O, Watanabe O, Goto H. Cutting edge: tubulin alpha functions as an adaptor in NFAT-importin beta interaction. J Immunol. 2011;186(5):2710–2713. doi: 10.4049/jimmunol.1003322. [DOI] [PubMed] [Google Scholar]
- 151.Ishiguro K, Ando T, Maeda O, Watanabe O, Goto H. Suppressive action of acetate on interleukin-8 production via tubulin-α acetylation. Immunol Cell Biol. 2014;92:624–630. doi: 10.1038/icb.2014.31. [DOI] [PubMed] [Google Scholar]
- 152.Sabo Y, Walsh D, Barry DS, Tinaztepe S, de Los Santos K, Goff SP, Gundersen GG, Naghavi MH. HIV-1 induces the formation of stable microtubules to enhance early infection. Cell Host Microbe. 2013;14(5):535–546. doi: 10.1016/j.chom.2013.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Valenzuela-Fernandez A, Alvarez S, Gordon-Alonso M, Barrero M, Ursa A, Cabrero JR, Fernandez G, Naranjo-Suarez S, Yanez-Mo M, Serrador JM, et al. Histone deacetylase 6 regulates human immunodeficiency virus type 1 infection. Mol Biol Cell. 2005;16(11):5445–5454. doi: 10.1091/mbc.E05-04-0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Husain M, Harrod KS. Enhanced acetylation of alpha-tubulin in influenza A virus infected epithelial cells. FEBS Lett. 2011;585(1):128–132. doi: 10.1016/j.febslet.2010.11.023. [DOI] [PubMed] [Google Scholar]
- 155.Husain M, Cheung CY. Histone deacetylase 6 inhibits influenza A virus release by downregulating the trafficking of viral components to the plasma membrane via its substrate, acetylated microtubules. J Virol. 2014;88(19):11229–11239. doi: 10.1128/JVI.00727-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Naranatt PP, Krishnan HH, Smith MS, Chandran B. Kaposi’s sarcoma-associated herpesvirus modulates microtubule dynamics via RhoA-GTP-diaphanous 2 signaling and utilizes the dynein motors to deliver its DNA to the nucleus. J Virol. 2005;79(2):1191–1206. doi: 10.1128/JVI.79.2.1191-1206.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zhong M, Zheng K, Chen M, Xiang Y, Jin F, Ma K, Qiu X, Wang Q, Peng T, Kitazato K, et al. Heat-shock protein 90 promotes nuclear transport of herpes simplex virus 1 capsid protein by interacting with acetylated tubulin. PLoS One. 2014;9(6):e99425. doi: 10.1371/journal.pone.0099425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Li W, Zhao YZ, Chou IN. Nickel (Ni2+) enhancement of alpha-tubulin acetylation in cultured 3T3 cells. Toxicol Appl Pharmacol. 1996;140(2):461–470. doi: 10.1006/taap.1996.0243. [DOI] [PubMed] [Google Scholar]
- 159.Mackeh R, Lorin S, Ratier A, Mejdoubi-Charef N, Baillet A, Bruneel A, Hamai A, Codogno P, Pous C, Perdiz D. Reactive oxygen species, AMP-activated protein kinase, and the transcription cofactor p300 regulate alpha- Tubulin acetyltransferase-1 (alpha TAT-1/MEC-17)- dependent microtubule hyperacetylation during cell stress. J Biol Chem. 2014;289(17):11816–11828. doi: 10.1074/jbc.M113.507400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Pandey K, Sharma SK. Activity-dependent acetylation of alpha tubulin in the hippocampus. J Mol Neurosci. 2011;45(1):1–4. doi: 10.1007/s12031-011-9506-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Takemura R, Okabe S, Umeyama T, Kanai Y, Cowan NJ, Hirokawa N. Increased microtubule stability and alpha-tubulin acetylation in cells transfected with microtubule-associated proteins Map1b, Map2 or Tau. J Cell Sci. 1992;103:953–964. doi: 10.1242/jcs.103.4.953. [DOI] [PubMed] [Google Scholar]
- 162.Erdozain AM, Morentin B, Bedford L, King E, Tooth D, Brewer C, Wayne D, Johnson L, Gerdes HK, Wigmore P, et al. Alcohol-related brain damage in humans. PLoS One. 2014;9(4):e93586. doi: 10.1371/journal.pone.0093586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Pan D. The hippo signaling pathway in development and cancer. Dev Cell. 2010;19(4):491–505. doi: 10.1016/j.devcel.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sudo H, Baas PW. Acetylation of microtubules influences their sensitivity to severing by katanin in neurons and fibroblasts. J Neurosci. 2010;30(21):7215–7226. doi: 10.1523/JNEUROSCI.0048-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Casale CH, Previtali G, Barra HS. Involvement of acetylated tubulin in the regulation of Na+, K+ -ATPase activity in cultured astrocytes. FEBS Lett. 2003;534(1–3):115–118. doi: 10.1016/S0014-5793(02)03802-4. [DOI] [PubMed] [Google Scholar]
- 166.Santander VS, Bisig CG, Purro SA, Casale CH, Arce CA, Barra HS. Tubulin must be acetylated in order to form a complex with membrane Na+, K+ -ATPase and to inhibit its enzyme activity. Mol Cell Biochem. 2006;291(1–2):167–174. doi: 10.1007/s11010-006-9212-9. [DOI] [PubMed] [Google Scholar]
- 167.Casale CH, Previtali G, Serafino JJ, Arce CA, Barra HS. Regulation of acetylated tubulin/Na+, K+-ATPase interaction by l-glutamate in non-neural cells: involvement of microtubules. Biochim Biophys Acta. 2005;1721(1–3):185–192. doi: 10.1016/j.bbagen.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 168.Casale CH, Alonso AD, Barra HS. Brain plasmamembrane NA(+), K+ -ATPase is inhibited by acetylated tubulin. Mol Cell Biochem. 2001;216(1–2):85–92. doi: 10.1023/A:1011029125228. [DOI] [PubMed] [Google Scholar]
- 169.Arce CA, Casale CH, Barra HS. Submembraneous microtubule cytoskeleton: regulation of ATPases by interaction with acetylated tubulin. FEBS J. 2008;275(19):4664–4674. doi: 10.1111/j.1742-4658.2008.06615.x. [DOI] [PubMed] [Google Scholar]
- 170.Yang WL, Guo XX, Thein S, Xu F, Sugii S, Baas PW, Radda GK, Han WP. Regulation of adipogenesis by cytoskeleton remodelling is facilitated by acetyltransferase MEC-17-dependent acetylation of alpha-tubulin. Biochem J. 2013;449:605–612. doi: 10.1042/BJ20121121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Xie R, Nguyen S, McKeehan WL, Liu LY. Acetylated microtubules are required for fusion of autophagosomes with lysosomes. Bmc Cell Biol. 2010;11:89. doi: 10.1186/1471-2121-11-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Geeraert C, Ratier A, Pfisterer SG, Perdiz D, Cantaloube I, Rouault A, Pattingre S, Proikas-Cezanne T, Codogno P, Pous C. Starvation-induced hyperacetylation of tubulin is required for the stimulation of autophagy by nutrient deprivation. J Biol Chem. 2010;285(31):24184–24194. doi: 10.1074/jbc.M109.091553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.d’Ydewalle C, Krishnan J, Chiheb DM, Van Damme P, Irobi J, Kozikowski AP, Vanden Berghe P, Timmerman V, Robberecht W, Van Den Bosch L. HDAC6 inhibitors reverse axonal loss in a mouse model of mutant HSPB1-induced Charcot-Marie-Tooth disease. Nat Med. 2011;17(8):968–974. doi: 10.1038/nm.2396. [DOI] [PubMed] [Google Scholar]
- 174.Dafinger C, Liebau MC, Elsayed SM, Hellenbroich Y, Boltshauser E, Korenke GC, Fabretti F, Janecke AR, Ebermann I, Nurnberg G, et al. Mutations in KIF7 link Joubert syndrome with Sonic Hedgehog signaling and microtubule dynamics. J Clin Invest. 2011;121(7):2662–2667. doi: 10.1172/JCI43639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Gilks WP, Abou-Sleiman PM, Gandhi S, Jain S, Singleton A, Lees AJ, Shaw K, Bhatia KP, Bonifati V, Quinn NP, et al. A common LRRK2 mutation in idiopathic Parkinson’s disease. Lancet. 2005;365(9457):415–416. doi: 10.1016/S0140-6736(05)17830-1. [DOI] [PubMed] [Google Scholar]
- 176.Law BM, Spain VA, Leinster VH, Chia R, Beilina A, Cho HJ, Taymans JM, Urban MK, Sancho RM, Blanca Ramirez M, et al. A direct interaction between leucine-rich repeat kinase 2 and specific beta-tubulin isoforms regulates tubulin acetylation. J Biol Chem. 2014;289(2):895–908. doi: 10.1074/jbc.M113.507913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Godena VK, Brookes-Hocking N, Moller A, Shaw G, Oswald M, Sancho RM, Miller CC, Whitworth AJ, De Vos KJ. Increasing microtubule acetylation rescues axonal transport and locomotor deficits caused by LRRK2 Roc-COR domain mutations. Nat Commun. 2014;5:5245. doi: 10.1038/ncomms6245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Govindarajan N, Rao P, Burkhardt S, Sananbenesi F, Schluter OM, Bradke F, Lu J, Fischer A. Reducing HDAC6 ameliorates cognitive deficits in a mouse model for Alzheimer’s disease. EMBO Mol Med. 2013;5(1):52–63. doi: 10.1002/emmm.201201923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Selenica ML, Benner L, Housley SB, Manchec B, Lee DC, Nash KR, Kalin J, Bergman JA, Kozikowski A, Gordon MN, et al. Histone deacetylase 6 inhibition improves memory and reduces total tau levels in a mouse model of tau deposition. Alzheimers Res Ther. 2014;6(1):12. doi: 10.1186/alzrt241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Seigneurin-Berny D, Verdel A, Curtet S, Lemercier C, Garin J, Rousseaux S, Khochbin S. Identification of components of the murine histone deacetylase 6 complex: link between acetylation and ubiquitination signaling pathways. Mol Cell Biol. 2001;21(23):8035–8044. doi: 10.1128/MCB.21.23.8035-8044.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Hook SS, Orian A, Cowley SM, Eisenman RN. Histone deacetylase 6 binds polyubiquitin through its zinc finger (PAZ domain) and copurifies with deubiquitinating enzymes. Proc Natl Acad Sci USA. 2002;99(21):13425–13430. doi: 10.1073/pnas.172511699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Yu CW, Chang PT, Hsin LW, Chern JW. Quinazolin-4-one derivatives as selective histone deacetylase-6 inhibitors for the treatment of Alzheimer’s disease. J Med Chem. 2013;56(17):6775–6791. doi: 10.1021/jm400564j. [DOI] [PubMed] [Google Scholar]
- 183.Zhang L, Liu C, Wu J, Tao JJ, Sui XL, Yao ZG, Xu YF, Huang L, Zhu H, Sheng SL, et al. Tubastatin A/ACY-1215 Improves Cognition in Alzheimer’s Disease Transgenic Mice. J Alzheimers Dis. 2014;41(4):1193–1205. doi: 10.3233/JAD-140066. [DOI] [PubMed] [Google Scholar]
- 184.Saba NF, Magliocca KR, Kim S, Muller S, Chen Z, Owonikoko TK, Sarlis NJ, Eggers C, Phelan V, Grist WJ, et al. Acetylated tubulin (AT) as a prognostic marker in squamous cell carcinoma of the head and neck. Head Neck Pathol. 2014;8(1):66–72. doi: 10.1007/s12105-013-0476-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Boggs AE, Vitolo MI, Whipple RA, Charpentier MS, Goloubeva OG, Ioffe OB, Tuttle KC, Slovic J, Lu Y, Mills GB, et al. alpha-Tubulin acetylation elevated in metastatic and basal-like breast cancer cells promotes microtentacle formation, adhesion, and invasive migration. Cancer Res. 2015;75(1):203–215. doi: 10.1158/0008-5472.CAN-13-3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Bailey JM, Alsina J, Rasheed ZA, McAllister FM, Fu YY, Plentz R, Zhang H, Pasricha PJ, Bardeesy N, Matsui W, et al. DCLK1 marks a morphologically distinct subpopulation of cells with stem cell properties in preinvasive pancreatic cancer. Gastroenterology. 2014;146(1):245–256. doi: 10.1053/j.gastro.2013.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Santo L, Hideshima T, Kung AL, Tseng JC, Tamang D, Yang M, Jarpe M, van Duzer JH, Mazitschek R, Ogier WC, et al. Preclinical activity, pharmacodynamic, and pharmacokinetic properties of a selective HDAC6 inhibitor, ACY-1215, in combination with bortezomib in multiple myeloma. Blood. 2012;119(11):2579–2589. doi: 10.1182/blood-2011-10-387365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.McLendon PM, Ferguson BS, Osinska H, Bhuiyan MS, James J, McKinsey TA, Robbins J. Tubulin hyperacetylation is adaptive in cardiac proteotoxicity by promoting autophagy. Proc Natl Acad Sci USA. 2014;111(48):E5178–E5186. doi: 10.1073/pnas.1415589111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Zhang D, Wu CT, Qi X, Meijering RA, Hoogstra-Berends F, Tadevosyan A, Deniz GC, Durdu S, Akar AR, Sibon OC, et al. Activation of histone deacetylase-6 induces contractile dysfunction through derailment of alpha-tubulin proteostasis in experimental and human atrial fibrillation. Circulation. 2014;129(3):346–358. doi: 10.1161/CIRCULATIONAHA.113.005300. [DOI] [PubMed] [Google Scholar]
- 190.Lam HC, Cloonan SM, Bhashyam AR, Haspel JA, Singh A, Sathirapongsasuti JF, Cervo M, Yao H, Chung AL, Mizumura K, et al. Histone deacetylase 6-mediated selective autophagy regulates COPD-associated cilia dysfunction. J Clin Invest. 2013;123(12):5212–5230. doi: 10.1172/JCI69636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Nam HJ, Kang JK, Kim SK, Ahn KJ, Seok H, Park SJ, Chang JS, Pothoulakis C, Lamont JT, Kim H. Clostridium difficile toxin A decreases acetylation of tubulin, leading to microtubule depolymerization through activation of histone deacetylase 6, and this mediates acute inflammation. J Biol Chem. 2010;285(43):32888–32896. doi: 10.1074/jbc.M110.162743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Rossi A, Kontarakis Z, Gerri C, Nolte H, Holper S, Kruger M, Stainier DY. Genetic compensation induced by deleterious mutations but not gene knockdowns. Nature. 2015 doi: 10.1038/nature14580. [DOI] [PubMed] [Google Scholar]
- 193.Keays DA, Tian G, Poirier K, Huang GJ, Siebold C, Cleak J, Oliver PL, Fray M, Harvey RJ, Molnar Z, et al. Mutations in alpha-tubulin cause abnormal neuronal migration in mice and lissencephaly in humans. Cell. 2007;128(1):45–57. doi: 10.1016/j.cell.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Tischfield MA, Baris HN, Wu C, Rudolph G, Van Maldergem L, He W, Chan WM, Andrews C, Demer JL, Robertson RL, et al. Human TUBB3 mutations perturb microtubule dynamics, kinesin interactions, and axon guidance. Cell. 2010;140(1):74–87. doi: 10.1016/j.cell.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.des Portes V, Pinard JM, Billuart P, Vinet MC, Koulakoff A, Carrie A, Gelot A, Dupuis E, Motte J, Berwald-Netter Y, et al. A novel CNS gene required for neuronal migration and involved in X-linked subcortical laminar heterotopia and lissencephaly syndrome. Cell. 1998;92(1):51–61. doi: 10.1016/S0092-8674(00)80898-3. [DOI] [PubMed] [Google Scholar]
- 196.Gleeson JG, Allen KM, Fox JW, Lamperti ED, Berkovic S, Scheffer I, Cooper EC, Dobyns WB, Minnerath SR, Ross ME, et al. Doublecortin, a brain-specific gene mutated in human X-linked lissencephaly and double cortex syndrome, encodes a putative signaling protein. Cell. 1998;92(1):63–72. doi: 10.1016/S0092-8674(00)80899-5. [DOI] [PubMed] [Google Scholar]
- 197.Strahl BD, Allis CD. The language of covalent histone modifications. Nature. 2000;403(6765):41–45. doi: 10.1038/47412. [DOI] [PubMed] [Google Scholar]
- 198.Berger SL. The complex language of chromatin regulation during transcription. Nature. 2007;447(7143):407–412. doi: 10.1038/nature05915. [DOI] [PubMed] [Google Scholar]
- 199.Yang XJ, Seto E. Lysine acetylation: codified crosstalk with other posttranslational modifications. Mol Cell. 2008;31(4):449–461. doi: 10.1016/j.molcel.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Butler KV, Kalin J, Brochier C, Vistoli G, Langley B, Kozikowski AP. Rational design and simple chemistry yield a superior, neuroprotective HDAC6 inhibitor, tubastatin A. J Am Chem Soc. 2010;132(31):10842–10846. doi: 10.1021/ja102758v. [DOI] [PMC free article] [PubMed] [Google Scholar]