Abstract
This review is designed to provide an overview of the current literature concerning vascular endothelial growth factor signaling (VEGF) in acute myeloid leukemia (AML). Aberrant VEGF signaling operates in the bone marrow of AML patients and is related to a poor prognosis. The altered signaling pathway demonstrated to interfere in several autocrine and paracrine signaling pathways. VEGF signaling promotes autocrine AML blast cell proliferation, survival, and chemotherapy resistance. In addition, VEGF signaling can mediate paracrine vascular endothelial cell-controlled angiogenesis in AML. Both effects presumably explain the association of high VEGF levels and poor therapeutic outcome. More recently, researches focusing on bone marrow stem cell niches demonstrate a role for VEGF signaling in the preservation of several cell types within these niches. The bone marrow niches are proposed to be a protective microenvironment for AML cells that could be responsible for relapses in AML patients. This implies the need of sophisticated VEGF-targeted therapeutics in AML therapy strategies. This review highlights our current understanding of aberrant VEGF signaling in AML, appoints the interference of VEGF signaling in the AML-associated microenvironment, and reflects the novelty of current VEGF-targeted therapeutics used in clinical trails for the treatment of AML.
Keywords: AML, VEGF, VEGFR-2, KDR, VEGFR-3, FTL-4
Introduction
Acute myeloid leukemia (AML) plays an abundant role in modern society. Leukemia is the most common childhood malignancy. In adults, leukemia is included in the top 15 of the most common forms of cancer, according to the World Health Organization. Treatment options have evolved over the last 30 years and have improved the survival rates of AML patients considerably [1–5]. Overall survival rates predominantly improved in childhood AML (age 0–18) in 1973 from below 20 % up to 70 % in 2005, and in young adults (age 19–40) from 15 to 60 % [6]. Elderly AML patients (age >60) hardly showed any progress in survival rates. Therefore, AML remains a life-threatening disease in which new therapeutic approaches are desirable. Prognostic factors become more important in predicting patient outcome and monitor their drug responsiveness. Current prognostic factors include WBC counts, age, cytogenetic alterations; CBFβ-MYH11 (INV16), AML-ETO (t(8;21)), PML-RARα (t(15;17)), MLL (11q23), and molecular aberrations including mutations in; FLT3, NPM, CEBPα, and WT1, and overexpression of genes as EVI [7]. Many important newly identified genes arise from gene expression profiling and demonstrate to be relevant to AML. Vascular endothelial growth factors (VEGF) emerged from gene expression profiling and demonstrate to predict AML prognosis (VEGF-C) and can define AML subgroups (VEGF-A) [8, 9].
In this manuscript, we will review the role and relevance of VEGF-A and VEGF-C and its downstream signaling in acute myeloid leukemia. We point out the importance of the VEGF family members VEGF-A and VEGF-C for regulating AML blast proliferation and survival pathways, and reflect the interference of VEGF-A and VEGF-C between AML blasts and their associated microenvironment.
VEGF-A, VEGF-C, VEGFR-2, and VEGFR-3 are overexpressed in AML
The human VEGF family consists of VEGF-A, VEGF-B, VEGF-C, VEGF-D, and PlGF (placenta growth factor) [10]. Several isoforms of VEGF-A and VEGF-B can be formed through alternative splicing [11]. Different forms of VEGF-C and VEGF-D are the result of proteolytic processing. To date, there is no evidence that shows aberrant VEGF-B and/or VEGF-D expression levels in AML. The best-studied, and probably most important VEGF family members in AML, are VEGF-A and VEGF-C. VEGF ligands can bind to one or more VEGF receptors; VEGFR-1, VEGFR-2 (i.e., kinase insert domain receptor, KDR), and/or VEGFR-3 (i.e., fms-related tyrosine kinase-4, FLT-4). VEGF-A isoforms can bind to either VEGFR-1 or VEGFR-2. Proteolytic processing of VEGF-C regulates the receptor specificity [12]. VEGFR-2 preferentially binds the fully processed mature form of VEGF-C, while VEGFR-3 can bind the non-processed forms as well.
Approximately 85 % of the AML patient bone marrow biopsies displayed higher VEGF-A protein expression levels compared to normal bone marrow (NBM) [13, 14]. The highest VEGF-A mRNA expression levels are detected in t(8;21) and t(15;17) translocated AML patients [15, 16]. VEGF-C protein expression levels were significantly elevated in all AML bone marrow samples in comparison to NBM controls [17–20]. In addition to an elevated expression of VEGF ligands, the VEGF receptors are also highly expressed in multiple subgroups of AML patients. The overall VEGFR-1 protein expression levels were found to be similar between AML bone marrow biopsies and NBM controls [14]. Interestingly, comparing the t(15;17) translocated AML bone marrow samples to AML samples with other cytogenetic aberration revealed that this patient subgroup has significantly higher VEGFR-1 mRNA expression levels [15]. The overall VEGFR-2 protein levels are significantly elevated in the bone marrow of AML patients compared to NBM. In addition, the highest VEGFR-2 mRNA expression levels are observed in t(8;21) translocated AML patient samples compared to other cytogenetic AML subgroups [15]. We recently defined VEGFR-2 protein expression levels that are membrane-restricted, and observed that 88 % of the AML bone marrow samples expressed significantly higher membrane VEGFR-2 expression compared to NBM controls [20]. Remarkably, among all cytogenetic alterations, MLL-rearranged AML samples expressed the highest VEGFR-2 membrane expression levels [21]. The overall VEGFR-3 protein expression levels are also significantly higher in AML patient bone marrow biopsies, in contrast to NBM controls [19].
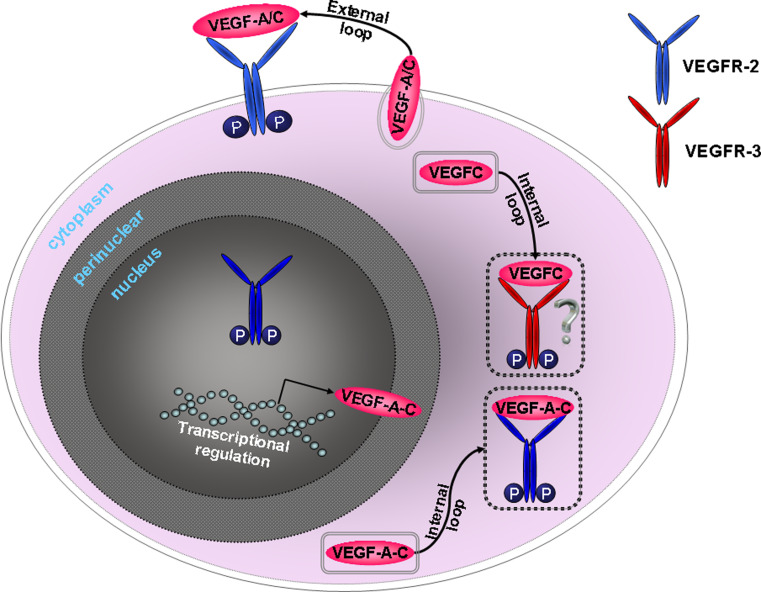
Taken together, VEGF-A and VEGF-C ligands are overexpressed in the bone marrow of AML patients in co-existence with an overexpression of VEGFR-2 and VEGFR-3. VEGF-A and VEGF-C protein expression is observed in the cytoplasm of AML blast cells and can also be excreted [13, 14, 20]. Total VEGFR-2 expression was found on the AML blast cell membrane, in the cytoplasm, and in the nucleus [23, 24]. The activated form of VEGFR-2 was located in the cytoplasm and within the nucleus of AML blast cells. VEGFR-3 was mainly expressed within the cytoplasm [19]. Activation of the VEGF receptors upon VEGF binding can occur both through internal and external signaling loops. AML cells secrete VEGF-A that they can bind to activate VEGFR-2 [23]. VEGF-C induces the phosphorylation of both VEGFR-2 and VEGFR-3 in AML cells [24]. The expression pattern of the VEGF receptors suggests that autocrine VEGF signaling in AML blasts might be regulated via internal VEGFR-2 and VEGFR-3 signaling loops (intrinsic signaling pathway), while the external loop (extrinsic signaling pathway) might be mainly VEGFR-2-restricted (Fig. 1).
Fig. 1.
Autocrine VEGF signaling in AML cells. VEGF-A, VEGF-C, VEGFR-2, and VEGFR-3 are overexpressed by AML blasts. VEGF-A and VEGF-C proteins can pass the cell membrane through active transport using secretory vesicles. Studies in endothelial cells demonstrated that VEGFR-2 in the cytoplasm is captured within the endosomes, which is also likely for VEGFR-3 [82]. The cytoplasmic VEGFR-2 retains its signaling activity, even when trapped within the endosomes. Excretion of VEGF-A/C enables the AML cell to bind VEGF-A/C to the VEGFR-2, residing on the cell membrane (external loop). Moreover, VEGF-A/C can also signal internally through binding to VEGFR-2 and VEGFR-3 that reside in the cytoplasm (internal loop). The role of VEGFR-2 within the nucleus is unknown
VEGF-A and VEGF-C are adverse prognostic predictors in AML
VEGF-A and VEGF-C overexpression exerts prognostic relevance in AML. Enhanced VEGF-A plasma levels are associated with lower complete remission rates and a reduced survival in AML patients [25–27]. The variability in VEGF-A expression levels in AML patients might be dependent on differences in alternative exon splicing, single-nucleotide polymorphisms (SNPs), or gene mutations. VEGF-A can be alternatively spliced into several isoforms; VEGF121, VEGF145, VEGF148, VEGF165, VEGF183, VEGF189, and VEGF206. VEGF-A isoforms have distinct functions through their ability to bind the extracellular matrix (ECM) [10]. A significant co-expression of VEGF121, VEGF165, VEGF183, and VEGF189 was apparent in AML [28]. VEGF121 is a soluble factor, while VEGF165 occurs as soluble factor and can bind to the ECM. VEGF183 and VEGF189 are almost completely bound to the ECM. Despite the fact that these isoforms have distinct functions, their co-expression indicates that measuring a single isoform is representative for all VEGF isoforms in AML. No significant associations were found between specific VEGF-A isoforms in relation to prognostic parameters in AML. SNP arrays reveal that VEGF-A SNPs seem to have prognostic relevance in AML patients. A study in a Korean AML cohort demonstrated that the overall VEGF-A polymorphism score can predict outcome in AML patients [29]. In addition, Monzo et al. [30] found that a homozygous −634G > C SNP in the VEGF-A gene was significantly associated with the relapse rate in a Spanish group of AML patients. Some of these SNPs in the VEGF-A gene are described to correlate with the VEGF-A promoter activity and VEGF-A expression levels [31–33]. Nevertheless, reports on SNPs in the VEGF-A gene describe contradicting relations to the VEGF-A expression levels. Therefore, the exact contribution of VEGF-A SNPs in AML needs further evaluation with additional measurements of VEGF-A expression levels. Currently, there are no descriptions on mutations in the VEGF-A gene in AML patients.
High VEGF-C mRNA expression levels are associated with decreased in vitro drug responsiveness of AML patient samples [34]. Moreover, AML patients with high VEGFC mRNA expression levels at diagnose associate with poor biological responses: higher blast counts on day 15 in the bone marrow, and an elongated time to reach complete remission. We recently identified VEGF-C as an independent factor for the overall survival in pediatric and adult AML patients [8]. Polymorphisms in VEGF-C are a rare causal variant within a large cohort of three-generation pedigrees, which is suggested to possibly contribute to disease liability [35]. In addition, VEGF-C single-nucleotide polymorphisms have been described in a bone disorder. Two VEGF-C SNPs are associated with increased risk of osteonecrosis of the femoral head [36]. So far, no reports have focused on SNPs or mutations in the VEGF-C gene in association with AML.
In summary, both VEGF-A as well as VEGF-C can predict adverse outcome in AML. The relation between SNPs and mutations of VEGF-A and VEGF-C in association with AML outcome is still unclear. Several cohorts of AML patients are being sequenced at the moment using powerful high-throughput techniques including next-generation sequencing. The results of these studies will probably shed light on the genetic aberrations of VEGF family members in AML and their relation to outcome.
AML blast proliferation and survival is partially dependent on the VEGF/VEGFR signaling in subsets of AML patients
Important biological characteristics of a leukemic cell are their increased capacity to self-renew and proliferate, and in addition their survival advantage. The activated VEGF signaling pathway promotes AML cell proliferation and survival functions [23, 24]. In vitro VEGF-A stimulation was demonstrated to increase the AML blast proliferation in synergy with stem cell factor (SCF) [37]. VEGF-C stimulation of primary AML cells increases the number of viable cells in vitro, suggesting that VEGF-C promotes AML cell proliferation [24]. The VEGF ligand-induced proliferation of AML cells might rely on the availability of VEGF receptors. Enforced expression of VEGFR-2 in TF-1 AML cells demonstrated to induce leukemic growth upon VEGF stimulation [38]. This finding suggests that AML patient subgroups that demonstrate high expression of VEGF receptors might be more dependent on the VEGF/VEGFR signaling for AML cell proliferation. Both VEGF-A and VEGF-C can also promote the survival of AML cells in vitro [24, 39]. The pro-survival signaling mechanisms upon VEGF-A or VEGF-C stimulation protect AML cells from chemotherapy-induced apoptosis through the upregulation and activation of anti-apoptotic proteins [24, 39–41].
Studies that have focused on the aberrant VEGF signaling in AML cells demonstrate that many downstream pathways are activated upon ligand binding (Fig. 2). The group of Dias demonstrated that VEGF-A induces AML cell proliferation and survival through NF-κβ, Akt, Erk, HSP90 and Bcl-2 signaling proteins [23, 39, 42]. They also demonstrated that VEGF-C induces AML cell survival by promoting Bcl-2 expression [24]. Chien et al. [17] described the VEGF-C-induced activation of downstream signaling proteins in AML cells in more detail. VEGF-C could induce COX-2 transcription and protein expression via Erk and JNK signaling protein activation. Taking these results together demonstrates that VEGF ligand induced activation of Bcl-2, HSP, MAPK, and PI3 K proteins causes AML cell proliferation and survival. Blocking the VEGF signaling in AML cells also inhibits the activation of these downstream signaling proteins.
Fig. 2.
Summarized model for classical downstream VEGF/VEGFR signaling in AML cells. The VEGF receptor phosphorylates upon binding of either VEGF-A or VEGF-C. Phosphorylation of the VEGF receptor results in the activation of downstream signaling proteins involved in the PI3 kinase and MAPK signaling pathways. Chemotherapy-resistant mechanisms are regulated through VEGF receptor-mediated activation of Bcl-2 and HSPs. The MAPK route can induce transcription of COX-2. This protein is a pro-angiogenic factor, which in turn can induce paracrine VEGF signaling. The blue proteins evidently demonstrated to be important downstream proteins of the VEGF/VEGFR signaling for the proliferation, survival, and chemotherapy resistance of AML blast cells
As was previously introduced, VEGF signaling can occur both via internal and external loops in AML cells. Activation of internal and external loops might result in different downstream signaling in AML cells. Santos et al. [23] investigated the impact of blocking the VEGF-A/VEGFR-2 signaling pathway internally or externally in primary AML cells in vitro. The external loop was inhibited by blocking VEGF-A, while the internal loop was inhibited by an internal VEGFR-2 kinase inhibitor. Inhibition of both internal and external loops was sufficient to decrease nuclear VEGFR-2 protein expression. Both signaling loops show an overlap in downstream NF-κβ signaling, however, inhibition of the internal loop additionally affected Erk and Akt downstream proteins. Inhibition of the internal VEGFR-2 loop induced apoptosis in AML cell lines. Moreover, blocking VEGF-A externally did not induce apoptosis, but could enhance apoptosis in synergy with VEGFR-2 inhibition in AML cell lines. This could suggest that either the internal loop is more important for AML cell survival or that externally VEGF-C could substitute for the function of VEGF-A. These data imply that internal and external autocrine VEGF signaling is regulated via distinct signal transduction routes that are both important for AML cell survival.
The t(8;21) translocated AML and the MLL rearranged AML patient subgroups were demonstrated to express the highest levels of VEGFR-2, while the t(15;17) translocated AML subgroup expressed the highest levels of VEGFR-1. Imai et al. [43, 44] focused on the effect of VEGF receptor inhibition within different cytogenetic AML subgroups. Inhibiting the VEGFR-2 kinase activity in AML cells induced apoptosis of t(8;21) and MLL rearranged AML patient samples, while the t(15;17) AML samples did not respond to VEGFR-2 kinase inhibition. AML patient samples with other cytogenetic aberrations showed variable responses. In addition, they investigated the effects of anti-VEGFR-1 and anti-VEGFR-2 human monoclonal antibody therapy in AML patient samples. Again, patient samples with a t(8;21) translocation or a MLL rearrangement demonstrated to be more sensitive to anti-VEGFR-1, anti-VEGFR-2, and combined monoclonal antibody treatment compared to AML patient samples with other cytogenetics. Translocated t(15;17) AML patient samples solely responded to VEGFR-1 antibody therapy. These results clearly show that in vitro external and internal VEGF receptor targeted therapy is most effective in AML patient subgroups that demonstrate highest expression levels of VEGF receptors. These AML subgroups might be more dependent on the VEGF/VEGFR signaling for intrinsic regulation of AML cell proliferation and survival.
The role of VEGF in AML-associated angiogenesis
VEGF is one of the main mediators of angiogenesis. Angiogenesis is the process of new vessel formation from pre-existing vessels by vascular endothelial cells. VEGF controls angiogenic sprouting by guiding filopodial extension from endothelial tip cells, as a first step to form new vessels [45]. Vascular endothelial cells can express all VEGF receptors on their cell membrane during different stages of vascular development [46, 47]. VEGF can induce the proliferation, survival, and migration of vascular endothelial cells by binding to their VEGFR-2, thereby mediating the angiogenesis (Fig. 3) [48, 49].
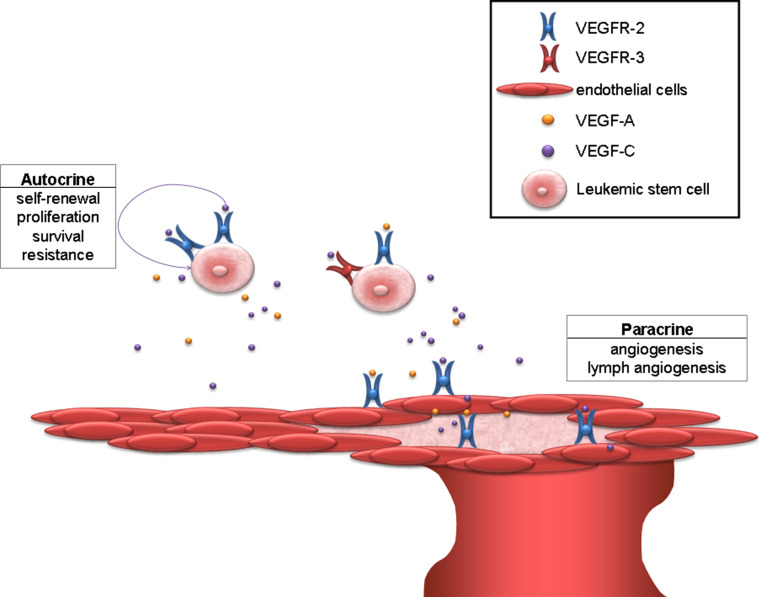
Fig. 3.
Autocrine and paracrine VEGF signaling in AML. VEGF signaling in AML patients demonstrates two separate signaling pathways that co-operate and promote the pathogenesis of AML. Via autocrine signaling, the AML cells activate the VEGF receptors to induce self-renewal, proliferation, survival, and chemotherapy resistance. Paracrine VEGF signaling promotes angiogenesis and lymph angiogenesis. Through these signaling pathways, the leukemic blasts can increase the vessel formation and their own stem cell maintenance. VEGF-A and VEGF-C can bind to activate AML cells as well as to endothelial cells
Increased angiogenesis is often observed in AML bone marrow biopsies [50]. The micro-vessel density was found to be higher in bone marrow biopsies of AML patients at diagnose compared to biopsies from patients that achieved complete remission, and NBM controls [51–53]. The increased micro-vessel density is associated with a poor prognosis in AML [52]. The vessels in AML patient bone marrow biopsies demonstrate variation in size and number. Three specific vascular morphology patterns in bone marrow biopsies of AML patients can be defined: a subgroup of AML patients with “low vessel count”, a subgroup demonstrating “angiogenic sprouting” (high number of small and immature vascular capillaries), and a subgroup with “vessel hyperplasia” (mature vessels with a large lumen) [54]. AML patients with “angiogenic sprouting” and “low vessel count” have a decreased event-free survival as compared to AML patients with “vessel hyperplasia” [55]. Rising evidence suggests that VEGF-A and VEGF-C promote the induced angiogenesis within the bone marrow of AML patients [17, 51, 52, 54]. The increased vessel formation in AML patients correlates significantly with VEGF-A expression levels [51, 52, 54]. A leukemic mice model demonstrates a role for VEGF-A in AML progression by inducing the formation of vascular capillaries [56]. Treatment with a VEGF-A antagonist was demonstrated to reduce angiogenesis and organ infiltration, and increased the survival of these leukemic mice. Moreover, VEGF-C was also shown to induce in vitro as well as in vivo AML-associated angiogenesis [17]. These results show that AML blasts secrete VEGF-A and VEGF-C to promote angiogenesis through activation of the paracrine VEGF signaling in vascular endothelial cells.
VEGF-A and VEGF-C; mediators of the endosteal and vascular stem cell niche
The stem cell niche is a supportive microenvironment of hematopoietic stem cells (HSC) that regulates cell fate and survival functions [57, 58]. Two types of HSC niches in vivo in mouse models are demonstrated within the bone marrow; a hypoxic endosteal bone marrow niche (within cancellous/trabecular bone) and a high oxygen vascular endothelium niche [59, 60]. The dormant HSC reside in the hypoxic endosteal niche to control stem cell quiescence [61, 62]. Active HSC are thought to reside within the vascular niche. Stem cell niches are composed of i.a. mesenchymal stem cells, osteoblasts, and osteoclasts (Fig. 4). Rising evidence indicates that VEGF-A and VEGF-C are supportive factors of the stem cell niches. The mesenchymal cells and osteoblasts produce VEGF-A [63–65]. VEGF-A is involved in osteoblast and osteoclast differentiation from their progeny [65–67]. The osteoclasts secrete VEGF-C to induce their bone resorption activity [68]. Osteoclasts play an important role in the stem cell niche, as inhibition of osteoclast resorption by calcitonin administration demonstrated to reduce the number of HSCs in mice [69]. These recent findings clearly support the hypothesis that VEGF is important for HSC niches and might as well be important for leukemic stem cell (LSC) niches. Moreover, it was shown in leukemic mice models that after high-dosage chemotherapy, the residual leukemic cells are localized to the perivascular endothelium and to the cancellous bone [70, 71]. Resistant LSCs that reside in protective endosteal and vascular niches are suggested to be the cause of AML relapse. Since VEGF supports the stem cell niche and is associated to AML relapse [26], it can be hypothesized that VEGF is involved in AML niche-associated relapses.
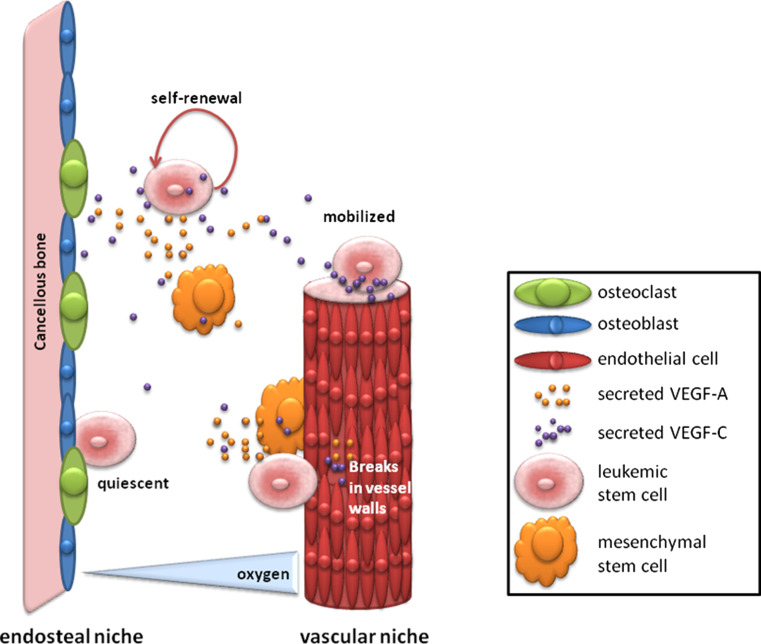
Fig. 4.
Stem cell niches. Leukemic stem cell niches are thought to protect the leukemic stem cells from therapeutic agents. Stem cells reside in a quiescent state in the endosteal bone marrow niche, which makes the AML cells unsusceptible for therapeutics that targets proliferating cells. Moreover, cells that reside in the vascular bone marrow niche can be mobilized by extrinsic factors to attract them into the bloodstream for organ infiltration, which is commonly observed in AML patients. Secreted VEGF-A and VEGF-C are essential for the supportive cells from which the stem cell niches are formed
VEGF-targeted therapy in AML
VEGF is a known target in AML, and several therapeutic strategies have been developed to inhibit the VEGF-induced signaling pathways. VEGF-targeted therapy in AML patients could inhibit the autocrine VEGF signaling in AML cells as well as the aberrant vessel formation by the vascular endothelial cells. Table 1 demonstrates the VEGF and VEGF receptor-targeted therapy strategies that have been tested in clinical trials for the treatment of AML. The VEGF receptor-targeted therapy strategies interfere with both the VEGF-A as well as the VEGF-C signaling pathway.
Table 1.
Overview of VEGF signaling-targeted therapeutics that are already used in clinical trials for monitoring their effectiveness in acute myeloid leukemia patients
| Therapeutics in clinical trials | Targets | Therapy | PR | CR | References |
|---|---|---|---|---|---|
| 1. Bevacizumab, i.e., Avastin | VEGF | Mono | 0/7 | 0/7 | Zahiragic et al. [72] |
| Combi | 7/48 | 16/48 | Karp et al. [73] | ||
| 2. PTK787/ZK222584, i.e., Vatalanib | VEGFRs, PDGFRs, SCFR, M-CSFR | Mono | 0/33 | 0/33 | Roboz et al. [74] |
| Combi | 2/15 | 4/15 | |||
| 3. SU5416, i.e., Semaxinib/Semaxanib | VEGFRs, SCFR, FLT3 | Mono | 8/42 | 0/42 | Fiedler et al. [76] |
| 4. SU11248, i.e., Sunitinib | VEGFRs, PDGFRs, SCFR, FLT3 | Mono | 6/15 | 0/15 | Fiedler et al. [77] |
| 5. AG-013736, i.e., Axitinib | VEGFR-2, SCFR, PDGFR-β | Mono | 1/6 | 0/6 | Giles et al. [78] |
| 6. BAY 43-9006, i.e., Sorafenib | FLT3, RAF-1 VEGFR-2, VEGFR-3, PDGFR-β, SCFR | Mono + allo-SCT | 5/6 | 1/6 | Metzelder et al. [79] |
| 7. PKC412, i.e., Midostaurin | FLT3, VEGFR-2, PDGFR, SCFR | Mono | 44/83 | 0/83 | Fischer et al. [80] |
| FLT3-WT | 22/51 | 0/51 | |||
| FLT3 mutant | 24/32 | 0/32 | |||
| 8. AZD2171, i.e., Cediranib | VEGFRs, SCFR, PDGFRs | Mono | 0/11 | 0/11 | Juckett et al. [81] |
| Combi | 0/15 | 0/15 |
FLT3 fms-like tyrosine kinase-3, M-CSFR macrophage colony-stimulating factor receptor, PDGFRs platelet-derived growth factor receptors, SCFR stem cell factor receptor, VEGFR vascular endothelial growth factor receptor, PR partial response including minor responses, and CR complete remission, Mono monotherapy, and combi monotherapy in combination with chemotherapy, Mono + allo-SCT monotherapy before or after allogenic stem cell transplantation
Bevacizumab (Avastin) is currently used in clinical trials for the treatment of AML. Bevacizumab is a monoclonal antibody directed against VEGF-A. A phase I clinical trial in refractory and relapsed AML patients demonstrated that bevacizumab monotherapy did not induce any clinical responses in these patients [72]. While no partial or complete responses were achieved, bevacizumab treatment did significantly decrease VEGF-A expression levels in bone marrow biopsies. A phase II clinical trial was set up to monitor the bevacizumab susceptibility in combination with chemotherapy. This clinical trial shows promising results in both relapsed and refractory adult AML patients [73]. Heterogeneity of the therapy responses might be explained by various factors including the expression level of VEGF and VEGF receptors, and/or the vascular morphology patterns in the bone marrow of the AML patients. Approximately 50 % of the AML patients demonstrated to be susceptible to bevacizumab in combination therapy [73].
PTK787/ZK222584/Vatalanib (PTK) is a multi-targeted inhibitor that was designed to target VEGFR-2 selectively, but was also demonstrated to inhibit VEGFR-1, VEGFR-3, stem cell factor receptor (SCFR; i.e., CD117, c-Kit), and macrophage colony-stimulating factor receptor (M-CSFR; i.e., c-fms, CSF-1). A phase I clinical trial monitored the effect of PTK in the treatment of AML and myelodysplastic syndrome (MDS) patients [74]. There were no significant responses to PTK treatment in refractory and relapsed AML patients upon monotherapy. Interestingly, a subgroup of patients (4/15) with secondary AML responded to PTK in combination with chemotherapy [74].
Another multi-targeted inhibitor, semaxinib (SU5416), targets the VEGF receptors, SCFR, and fms-like tyrosine kinase-3 (FLT3). Semaxinib monotherapy led to a stable remission in a patient with a second AML relapse refractory towards conventional therapy [75]. However, a phase II clinical trial in patients with refractory AML demonstrated that none of the patients reached complete remission, while 19 % of the patients showed a partial response [76]. An association between the patients that responded to SU5416 treatment and high VEGF-A mRNA levels was found at onset of the treatment. This suggests that based on the VEGF-A mRNA levels of AML patients at diagnosis, it can be predicted which patient will respond to this specific therapy. Family member sunitinib (SU11248) did show a slight beneficial effect as a monotherapeutic in a phase I clinical trial [77]. None of the AML patients showed a complete remission. Nevertheless, the percentage of patients that demonstrated a partial response upon sunitinib treatment was doubled in comparison to semaxinib treatment. Patients with FLT3 mutations showed a higher response rate upon sunitinib treatment compared with FLT3 wild-type patients.
Axitinib (AG-013736) is a multi-targeted tyrosine kinase inhibitor that inhibits VEGFR-2, SCFR, and platelet-derived growth factor receptor β (PDGFR-β). Axitinib was used in a phase II clinical trial in the treatment of elderly AML patients with poor prognosis, without clinical responsiveness [78]. Nevertheless, as was mentioned previously, the elderly AML patients hardly showed any progress according to improved treatment approaches over the last 30 years. The effects that are observed in this patient group might underestimate the effect that could be achieved in younger AML patient groups.
Sorafenib (BAY 43-9006) is a dual-action inhibitor that targets the RAF/MEK/Erk pathway as well as the upstream tyrosine kinases, FLT3, VEGFR-2, VEGFR-3, PDGFR-β, and SCFR. Administration of sorafenib to treat AML patients was demonstrated effective before and after allogenic stem cell transplantations in AML patients that harbor a FLT3-ITD mutation [79]. All AML patients with a FLT3-ITD mutation (6/6) were shown to respond to sorafenib treatment. Since approximately 25 % of the AML patients demonstrate a FLT3-ITD mutation, this seems a promising therapeutic in a subset of AML patients.
Another multi-targeted therapeutic, midostaurin, which targets FLT3, VEGFR-2, PDGF receptors, and SCFR, demonstrates a high percentage of partial responses in AML patients in a monotherapy phase II clinical trial [80]. Randomly assigned AML patients with a FLT3 mutant (74 % ITD) demonstrated a higher partial response rate compared to FLT3 wild-type AML patients (75 and 43 %, respectively). None of the AML patients achieved a complete remission.
Cediranib (AZD2171) is a multi-targeted inhibitor of VEGF receptors, SCFR, and PDGF receptors. A phase II clinical study using cediranib showed disappointing results in elderly AML patients (age >60) and the study was stopped after the first-stage enrollment [81]. None of the patients responded to cediranib, not even after induction of chemotherapy.
To date, there are no studies performed in AML that focus on VEGF-C targeted therapy and how this affects the AML blast cells. Recently, we were able to show that in vitro VEGF-C-targeted therapy can be a potential new differentiation therapy in pediatric AML [20].
Bevacizumab in combination therapy demonstrated the most promising VEGF-targeted therapy responses in AML patients so far. Midostaurin demonstrated to be the most effective monotherapeutic in AML patients, yet clinical trials combining midostaurin with conventional therapy need to determine the suitability of midostaurin in AML patients to achieve complete remission. In some clinical trials, the elevated VEGF levels correlated to biological responses in AML patients. The role of VEGF in relation to AML responses upon VEGF-targeted therapy demands additional studies that include measurements of VEGF ligands and receptors before and after treatment. This clinical study approach might help to elucidate why some patients do respond to the VEGF-targeted therapeutics and will benefit from it, while other patients do not. Moreover, this may explain FLT3 mutation involvement in the VEGF-targeted therapy responses in AML patients.
Summary and future perspectives
In this review, we evaluated the role of VEGF signaling in the bone marrow of AML patients into detail. VEGF ligands and receptors are aberrantly expressed by AML blast cells. The increased VEGF ligand expression is significantly associated with a poor outcome in AML patients. AML blasts enhance autocrine VEGF signaling in order to increase their proliferation and to provide a survival advantage. This might explain why AML patients with VEGF ligand overexpression show a poorer outcome. In addition, VEGFR overexpressing AML subgroups with t(8;21) and t(15;17) translocations or MLL rearrangements seem to be most dependent on the VEGF/VEGFR signaling for their AML blast survival. Moreover, the altered VEGF signaling mediated by AML blast cells regulates paracrine vascular endothelial cell-induced angiogenesis, which contributes to AML progression. VEGF signaling is demonstrated to be important for cell types that make up bone marrow stem cell niches. This proposed ideal microenvironmental niche for leukemic stem cells can be the cause of AML progression and relapse. Might the aberrant VEGF signaling induce bone marrow niche formation to develop a protective microenvironment for AML blasts? Further studies are warranted. Current therapeutics developed to target the VEGF signaling demonstrated the best clinical responses in AML patients when combined with conventional chemotherapeutics. New sophisticated therapeutic approaches are necessary, which should consider all these AML-associated aberrant VEGF signaling hallmarks. Overall, there is still a lot of progress to achieve in AML patients concerning new therapy strategies in order to improve the survival of AML patients and their quality of life.
Acknowledgments
None.
Conflict of interest
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article. The authors declare that they have no conflicts of interest.
References
- 1.Abrahamsson J, Forestier E, Heldrup J, et al. Response-guided induction therapy in pediatric acute myeloid leukemia with excellent remission rate. J Clin Oncol. 2011;29:310–315. doi: 10.1200/JCO.2010.30.6829. [DOI] [PubMed] [Google Scholar]
- 2.Faderl S, Ravandi F, Huang X, et al. Clofarabine plus low-dose cytarabine followed by clofarabine plus low-dose cytarabine alternating with decitabine in acute myeloid leukemia frontline therapy for older patients. Cancer. 2012 doi: 10.1002/cncr.27429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gallegos-Castorena S, Medina-Sanson A, Gonzalez-Ramella O, Sanchez-Zubieta F, Martinez-Avalos A. Improved treatment results in Mexican children with acute myeloid leukemia using a Medical Research Council (MRC)-acute myeloid leukemia 10 modified protocol. Leuk Lymphoma. 2009;50:1132–1137. doi: 10.1080/10428190902964768. [DOI] [PubMed] [Google Scholar]
- 4.Gerr H, Zimmermann M, Schrappe M, et al. Acute leukaemias of ambiguous lineage in children: characterization, prognosis and therapy recommendations. Br J Haematol. 2010;149:84–92. doi: 10.1111/j.1365-2141.2009.08058.x. [DOI] [PubMed] [Google Scholar]
- 5.Hann IM, Webb DK, Gibson BE, Harrison CJ. MRC trials in childhood acute myeloid leukaemia. Ann Hematol. 2004;83(Suppl 1):S108–S112. doi: 10.1007/s00277-004-0850-2. [DOI] [PubMed] [Google Scholar]
- 6.Derolf AR, Kristinsson SY, Andersson TM, Landgren O, Dickman PW, Bjorkholm M. Improved patient survival for acute myeloid leukemia: a population-based study of 9729 patients diagnosed in Sweden between 1973 and 2005. Blood. 2009;113:3666–3672. doi: 10.1182/blood-2008-09-179341. [DOI] [PubMed] [Google Scholar]
- 7.Grimwade D, Hills RK (2009) Independent prognostic factors for AML outcome. Hematology Am Soc Hematol Educ Program. 2009:385–395. doi:10.1182/asheducation-2009.1.385 [DOI] [PubMed]
- 8.de Jonge HJ, Valk PJ, Veeger NJ, et al. High VEGFC expression is associated with unique gene expression profiles and predicts adverse prognosis in pediatric and adult acute myeloid leukemia. Blood. 2010;116:1747–1754. doi: 10.1182/blood-2010-03-270991. [DOI] [PubMed] [Google Scholar]
- 9.Bullinger L, Dohner K, Bair E, et al. Use of gene-expression profiling to identify prognostic subclasses in adult acute myeloid leukemia. N Engl J Med. 2004;350:1605–1616. doi: 10.1056/NEJMoa031046. [DOI] [PubMed] [Google Scholar]
- 10.Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997;18:4–25. doi: 10.1210/er.18.1.4. [DOI] [PubMed] [Google Scholar]
- 11.Tammela T, Enholm B, Alitalo K, Paavonen K. The biology of vascular endothelial growth factors. Cardiovasc Res. 2005;65:550–563. doi: 10.1016/j.cardiores.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 12.Joukov V, Sorsa T, Kumar V, et al. Proteolytic processing regulates receptor specificity and activity of VEGF-C. EMBO J. 1997;16:3898–3911. doi: 10.1093/emboj/16.13.3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hou HA, Chou WC, Lin LI, et al. Expression of angiopoietins and vascular endothelial growth factors and their clinical significance in acute myeloid leukemia. Leuk Res. 2008;32:904–912. doi: 10.1016/j.leukres.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 14.Padro T, Bieker R, Ruiz S, et al. Overexpression of vascular endothelial growth factor (VEGF) and its cellular receptor KDR (VEGFR-2) in the bone marrow of patients with acute myeloid leukemia. Leukemia. 2002;16:1302–1310. doi: 10.1038/sj.leu.2402534. [DOI] [PubMed] [Google Scholar]
- 15.Hiramatsu A, Miwa H, Shikami M, et al. Disease-specific expression of VEGF and its receptors in AML cells: possible autocrine pathway of VEGF/type1 receptor of VEGF in t(1517) AML and VEGF/type2 receptor of VEGF in t(821) AML. Leuk Lymphoma. 2006;47:89–95. doi: 10.1080/10428190500270386. [DOI] [PubMed] [Google Scholar]
- 16.Ter Elst A, Ma B, Scherpen FJ, et al. Repression of vascular endothelial growth factor expression by the runt-related transcription factor 1 in acute myeloid leukemia. Cancer Res. 2011;71:2761–2771. doi: 10.1158/0008-5472.CAN-10-0402. [DOI] [PubMed] [Google Scholar]
- 17.Chien MH, Ku CC, Johansson G, et al. Vascular endothelial growth factor-C (VEGF-C) promotes angiogenesis by induction of COX-2 in leukemic cells via the VEGF-R3/JNK/AP-1 pathway. Carcinogenesis. 2009;30:2005–2013. doi: 10.1093/carcin/bgp244. [DOI] [PubMed] [Google Scholar]
- 18.Fielder W, Graeven U, Ergun S, et al. Expression of FLT4 and its ligand VEGF-C in acute myeloid leukemia. Leukemia. 1997;11:1234–1237. doi: 10.1038/sj.leu.2400722. [DOI] [PubMed] [Google Scholar]
- 19.Liersch R, Schliemann C, Bieker R, et al. Expression of VEGF-C and its receptor VEGFR-3 in the bone marrow of patients with acute myeloid leukaemia. Leuk Res. 2008;32:954–961. doi: 10.1016/j.leukres.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 20.Kampen KR, ter Elst A, Mulder AB et al (2011) Anti-VEGFC treatment reduces the leukemic outgrowth of primary CD34+ pediatric acute myeloid leukemia cells. Blood, Suppl ASH abstract 4319
- 21.Ter Elst A, Kampen KR, Diks SH et al (2010) Targeting multiple active kinase pathways in 11q23 translocated pediatric acute myeloid leukemia using a VEGFR2 antibody together with a MEK inhibitor. Blood, Suppl ASH abstract 3626
- 22.Blazquez C, Cook N, Micklem K, Harris AL, Gatter KC, Pezzella F. Phosphorylated KDR can be located in the nucleus of neoplastic cells. Cell Res. 2006;16:93–98. doi: 10.1038/sj.cr.7310012. [DOI] [PubMed] [Google Scholar]
- 23.Santos SC, Dias S. Internal and external autocrine VEGF/KDR loops regulate survival of subsets of acute leukemia through distinct signaling pathways. Blood. 2004;103:3883–3889. doi: 10.1182/blood-2003-05-1634. [DOI] [PubMed] [Google Scholar]
- 24.Dias S, Choy M, Alitalo K, Rafii S. Vascular endothelial growth factor (VEGF)-C signaling through FLT-4 (VEGFR-3) mediates leukemic cell proliferation, survival, and resistance to chemotherapy. Blood. 2002;99:2179–2184. doi: 10.1182/blood.V99.6.2179. [DOI] [PubMed] [Google Scholar]
- 25.Aguayo A, Kantarjian HM, Estey EH, et al. Plasma vascular endothelial growth factor levels have prognostic significance in patients with acute myeloid leukemia but not in patients with myelodysplastic syndromes. Cancer. 2002;95:1923–1930. doi: 10.1002/cncr.10900. [DOI] [PubMed] [Google Scholar]
- 26.De Bont ES, Fidler V, Meeuwsen T, Scherpen F, Hahlen K, Kamps WA. Vascular endothelial growth factor secretion is an independent prognostic factor for relapse-free survival in pediatric acute myeloid leukemia patients. Clin Cancer Res. 2002;8:2856–2861. [PubMed] [Google Scholar]
- 27.Wegiel B, Ekberg J, Talasila KM, Jalili S, Persson JL. The role of VEGF and a functional link between VEGF and p27Kip1 in acute myeloid leukemia. Leukemia. 2009;23:251–261. doi: 10.1038/leu.2008.300. [DOI] [PubMed] [Google Scholar]
- 28.Kruizinga RC, de Jonge HJ, Kampen KR, Walenkamp AM, De Bont ES. Vascular endothelial growth Factor A isoform mRNA expression in pediatric acute myeloid leukemia. Pediatr Blood Cancer. 2011;56:294–297. doi: 10.1002/pbc.22783. [DOI] [PubMed] [Google Scholar]
- 29.Kim DH, Lee NY, Lee MH, Sohn SK, Do YR, Park JY. Vascular endothelial growth factor (VEGF) gene (VEGFA) polymorphism can predict the prognosis in acute myeloid leukaemia patients. Br J Haematol. 2008;140:71–79. doi: 10.1111/j.1365-2141.2007.06893.x. [DOI] [PubMed] [Google Scholar]
- 30.Monzo M, Brunet S, Urbano-Ispizua A, et al. Genomic polymorphisms provide prognostic information in intermediate-risk acute myeloblastic leukemia. Blood. 2006;107:4871–4879. doi: 10.1182/blood-2005-08-3272. [DOI] [PubMed] [Google Scholar]
- 31.Debrah AY, Mand S, Toliat MR, et al. Plasma vascular endothelial growth Factor-A (VEGF-A) and VEGF-A gene polymorphism are associated with hydrocele development in lymphatic filariasis. Am J Trop Med Hyg. 2007;77:601–608. [PubMed] [Google Scholar]
- 32.Koukourakis MI, Papazoglou D, Giatromanolaki A, Bougioukas G, Maltezos E, Sivridis E. VEGF gene sequence variation defines VEGF gene expression status and angiogenic activity in non-small cell lung cancer. Lung Cancer. 2004;46:293–298. doi: 10.1016/j.lungcan.2004.04.037. [DOI] [PubMed] [Google Scholar]
- 33.Stevens A, Soden J, Brenchley PE, Ralph S, Ray DW. Haplotype analysis of the polymorphic human vascular endothelial growth factor gene promoter. Cancer Res. 2003;63:812–816. [PubMed] [Google Scholar]
- 34.de Jonge HJ, Weidenaar AC, Ter Elst A, et al. Endogenous vascular endothelial growth factor-C expression is associated with decreased drug responsiveness in childhood acute myeloid leukemia. Clin Cancer Res. 2008;14:924–930. doi: 10.1158/1078-0432.CCR-07-1821. [DOI] [PubMed] [Google Scholar]
- 35.Yip W, De G, Raby BA, et al. Identifying causal rare variants of disease through family-based analysis of Genetics Analysis Workshop 17 data set. BMC Proceedings. 2011;5(Suppl 9):S21. doi: 10.1186/1753-6561-5-S9-S21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hong JM, Kim TH, Kim HJ, Park EK, Yang EK, Kim SY. Genetic association of angiogenesis- and hypoxia-related gene polymorphisms with osteonecrosis of the femoral head. Exp Mol Med. 2010;42:376–385. doi: 10.3858/emm.2010.42.5.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Foss B, Mentzoni L, Bruserud O. Effects of vascular endothelial growth factor on acute myelogenous leukemia blasts. J Hematother Stem Cell Res. 2001;10:81–93. doi: 10.1089/152581601750098291. [DOI] [PubMed] [Google Scholar]
- 38.Coppola S, Narciso L, Feccia T, et al. Enforced expression of KDR receptor promotes proliferation, survival and megakaryocytic differentiation of TF1 progenitor cell line. Cell Death Differ. 2006;13:61–74. doi: 10.1038/sj.cdd.4401698. [DOI] [PubMed] [Google Scholar]
- 39.Dias S, Shmelkov SV, Lam G, Rafii S. VEGF(165) promotes survival of leukemic cells by Hsp90-mediated induction of Bcl-2 expression and apoptosis inhibition. Blood. 2002;99:2532–2540. doi: 10.1182/blood.V99.7.2532. [DOI] [PubMed] [Google Scholar]
- 40.Flandrin P, Guyotat D, Duval A, et al. Significance of heat-shock protein (HSP) 90 expression in acute myeloid leukemia cells. Cell Stress Chaperones. 2008;13:357–364. doi: 10.1007/s12192-008-0035-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schepers H, Geugien M, van der Toorn M, et al. HSP27 protects AML cells against VP-16-induced apoptosis through modulation of p38 and c-Jun. Exp Hematol. 2005;33:660–670. doi: 10.1016/j.exphem.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 42.Fragoso R, Elias AP, Dias S. Autocrine VEGF loops, signaling pathways, and acute leukemia regulation. Leuk Lymphoma. 2007;48:481–488. doi: 10.1080/10428190601064720. [DOI] [PubMed] [Google Scholar]
- 43.Imai N, Shikami M, Miwa H, et al. t(821) acute myeloid leukaemia cells are dependent on vascular endothelial growth factor (VEGF)/VEGF receptor type2 pathway and phosphorylation of Akt. Br J Haematol. 2006;135:673–682. doi: 10.1111/j.1365-2141.2006.06372.x. [DOI] [PubMed] [Google Scholar]
- 44.Imai N, Miwa H, Shikami M, et al. Growth inhibition of AML cells with specific chromosome abnormalities by monoclonal antibodies to receptors for vascular endothelial growth factor. Leuk Res. 2009;33:1650–1657. doi: 10.1016/j.leukres.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 45.Gerhardt H, Golding M, Fruttiger M, et al. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol. 2003;161:1163–1177. doi: 10.1083/jcb.200302047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hoeben A, Landuyt B, Highley MS, Wildiers H, van Oosterom AT, de Bruijn EA. Vascular endothelial growth factor and angiogenesis. Pharmacol Rev. 2004;56:549–580. doi: 10.1124/pr.56.4.3. [DOI] [PubMed] [Google Scholar]
- 47.Smith NR, Baker D, James NH, et al. Vascular endothelial growth factor receptors VEGFR-2 and VEGFR-3 are localized primarily to the vasculature in human primary solid cancers. Clin Cancer Res. 2010;16:3548–3561. doi: 10.1158/1078-0432.CCR-09-2797. [DOI] [PubMed] [Google Scholar]
- 48.Gerber HP, McMurtrey A, Kowalski J, et al. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3′-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J Biol Chem. 1998;273:30336–30343. doi: 10.1074/jbc.273.46.30336. [DOI] [PubMed] [Google Scholar]
- 49.Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling—in control of vascular function. Nat Rev Mol Cell Biol. 2006;7:359–371. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- 50.Hussong JW, Rodgers GM, Shami PJ. Evidence of increased angiogenesis in patients with acute myeloid leukemia. Blood. 2000;95:309–313. [PubMed] [Google Scholar]
- 51.De Bont ES, Rosati S, Jacobs S, Kamps WA, Vellenga E. Increased bone marrow vascularization in patients with acute myeloid leukaemia: a possible role for vascular endothelial growth factor. Br J Haematol. 2001;113:296–304. doi: 10.1046/j.1365-2141.2001.02722.x. [DOI] [PubMed] [Google Scholar]
- 52.Rabitsch W, Sperr WR, Lechner K, et al. Bone marrow microvessel density and its prognostic significance in AML. Leuk Lymphoma. 2004;45:1369–1373. doi: 10.1080/10428190410001663707. [DOI] [PubMed] [Google Scholar]
- 53.Padro T, Ruiz S, Bieker R, et al. Increased angiogenesis in the bone marrow of patients with acute myeloid leukemia. Blood. 2000;95:2637–2644. [PubMed] [Google Scholar]
- 54.Weidenaar AC, Ter Elst A, Koopmans-Klein G, et al. High acute myeloid leukemia-derived VEGFA levels are associated with a specific vascular morphology in the leukemic bone marrow. Cell Oncol (Dordr) 2011;34:289–296. doi: 10.1007/s13402-011-0017-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weidenaar AC, Ter Elst A, van Montfort CAGM et al (2011) Patterns of bone marrow micro vessel morphology in AML and high risk MDS predict treatment outcome following intensive chemotherapy and bevacizumab. Blood, Suppl ASH abstract 1555
- 56.Schuch G, Machluf M, Bartsch G, Jr, et al. In vivo administration of vascular endothelial growth factor (VEGF) and its antagonist, soluble neuropilin-1, predicts a role of VEGF in the progression of acute myeloid leukemia in vivo. Blood. 2002;100:4622–4628. doi: 10.1182/blood.V100.13.4622. [DOI] [PubMed] [Google Scholar]
- 57.Spradling A, Drummond-Barbosa D, Kai T. Stem cells find their niche. Nature. 2001;414:98–104. doi: 10.1038/35102160. [DOI] [PubMed] [Google Scholar]
- 58.Wilson A, Trumpp A. Bone-marrow haematopoietic-stem-cell niches. Nat Rev Immunol. 2006;6:93–106. doi: 10.1038/nri1779. [DOI] [PubMed] [Google Scholar]
- 59.Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 60.Lo CC, Fleming HE, Wu JW, et al. Live-animal tracking of individual haematopoietic stem/progenitor cells in their niche. Nature. 2009;457:92–96. doi: 10.1038/nature07434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kubota Y, Takubo K, Suda T. Bone marrow long label-retaining cells reside in the sinusoidal hypoxic niche. Biochem Biophys Res Commun. 2008;366:335–339. doi: 10.1016/j.bbrc.2007.11.086. [DOI] [PubMed] [Google Scholar]
- 62.Parmar K, Mauch P, Vergilio JA, Sackstein R, Down JD. Distribution of hematopoietic stem cells in the bone marrow according to regional hypoxia. Proc Natl Acad Sci USA. 2007;104:5431–5436. doi: 10.1073/pnas.0701152104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kagiwada H, Yashiki T, Ohshima A, Tadokoro M, Nagaya N, Ohgushi H. Human mesenchymal stem cells as a stable source of VEGF-producing cells. J Tissue Eng Regen Med. 2008;2:184–189. doi: 10.1002/term.79. [DOI] [PubMed] [Google Scholar]
- 64.Wang M, Zhang W, Crisostomo P, et al. STAT3 mediates bone marrow mesenchymal stem cell VEGF production. J Mol Cell Cardiol. 2007;42:1009–1015. doi: 10.1016/j.yjmcc.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Deckers MM, van Bezooijen RL, van der Horst G, et al. Bone morphogenetic proteins stimulate angiogenesis through osteoblast-derived vascular endothelial growth factor A. Endocrinology. 2002;143:1545–1553. doi: 10.1210/en.143.4.1545. [DOI] [PubMed] [Google Scholar]
- 66.Kodama I, Niida S, Sanada M, et al. Estrogen regulates the production of VEGF for osteoclast formation and activity in op/op mice. J Bone Miner Res. 2004;19:200–206. doi: 10.1359/JBMR.0301229. [DOI] [PubMed] [Google Scholar]
- 67.Knowles HJ, Athanasou NA. Hypoxia-inducible factor is expressed in giant cell tumour of bone and mediates paracrine effects of hypoxia on monocyte-osteoclast differentiation via induction of VEGF. J Pathol. 2008;215:56–66. doi: 10.1002/path.2319. [DOI] [PubMed] [Google Scholar]
- 68.Zhang Q, Guo R, Lu Y, et al. VEGF-C, a lymphatic growth factor, is a RANKL target gene in osteoclasts that enhances osteoclastic bone resorption through an autocrine mechanism. J Biol Chem. 2008;283:13491–13499. doi: 10.1074/jbc.M708055200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lymperi S, Ersek A, Ferraro F, Dazzi F, Horwood NJ. Inhibition of osteoclast function reduces hematopoietic stem cell numbers in vivo. Blood. 2011;117:1540–1549. doi: 10.1182/blood-2010-05-282855. [DOI] [PubMed] [Google Scholar]
- 70.Ishikawa F, Yoshida S, Saito Y, et al. Chemotherapy-resistant human AML stem cells home to and engraft within the bone-marrow endosteal region. Nat Biotechnol. 2007;25:1315–1321. doi: 10.1038/nbt1350. [DOI] [PubMed] [Google Scholar]
- 71.Ninomiya M, Abe A, Katsumi A, et al. Homing, proliferation and survival sites of human leukemia cells in vivo in immunodeficient mice. Leukemia. 2007;21:136–142. doi: 10.1038/sj.leu.2404432. [DOI] [PubMed] [Google Scholar]
- 72.Zahiragic L, Schliemann C, Bieker R, et al. Bevacizumab reduces VEGF expression in patients with relapsed and refractory acute myeloid leukemia without clinical antileukemic activity. Leukemia. 2007;21:1310–1312. doi: 10.1038/sj.leu.2404632. [DOI] [PubMed] [Google Scholar]
- 73.Karp JE, Gojo I, Pili R, et al. Targeting vascular endothelial growth factor for relapsed and refractory adult acute myelogenous leukemias: therapy with sequential 1-beta-d-arabinofuranosylcytosine, mitoxantrone, and bevacizumab. Clin Cancer Res. 2004;10:3577–3585. doi: 10.1158/1078-0432.CCR-03-0627. [DOI] [PubMed] [Google Scholar]
- 74.Roboz GJ, Giles FJ, List AF, et al. Phase 1 study of PTK787/ZK 222584, a small molecule tyrosine kinase receptor inhibitor, for the treatment of acute myeloid leukemia and myelodysplastic syndrome. Leukemia. 2006;20:952–957. doi: 10.1038/sj.leu.2404213. [DOI] [PubMed] [Google Scholar]
- 75.Mesters RM, Padro T, Bieker R, et al. Stable remission after administration of the receptor tyrosine kinase inhibitor SU5416 in a patient with refractory acute myeloid leukemia. Blood. 2001;98:241–243. doi: 10.1182/blood.V98.1.241. [DOI] [PubMed] [Google Scholar]
- 76.Fiedler W, Mesters R, Tinnefeld H, et al. A phase 2 clinical study of SU5416 in patients with refractory acute myeloid leukemia. Blood. 2003;102:2763–2767. doi: 10.1182/blood-2002-10-2998. [DOI] [PubMed] [Google Scholar]
- 77.Fiedler W, Serve H, Dohner H, et al. A phase 1 study of SU11248 in the treatment of patients with refractory or resistant acute myeloid leukemia (AML) or not amenable to conventional therapy for the disease. Blood. 2005;105:986–993. doi: 10.1182/blood-2004-05-1846. [DOI] [PubMed] [Google Scholar]
- 78.Giles FJ, Bellamy WT, Estrov Z, et al. The anti-angiogenesis agent, AG-013736, has minimal activity in elderly patients with poor prognosis acute myeloid leukemia (AML) or myelodysplastic syndrome (MDS) Leuk Res. 2006;30:801–811. doi: 10.1016/j.leukres.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 79.Metzelder S, Wang Y, Wollmer E, et al. Compassionate use of sorafenib in FLT3-ITD-positive acute myeloid leukemia: sustained regression before and after allogeneic stem cell transplantation. Blood. 2009;113:6567–6571. doi: 10.1182/blood-2009-03-208298. [DOI] [PubMed] [Google Scholar]
- 80.Fischer T, Stone RM, Deangelo DJ, et al. Phase IIB trial of oral Midostaurin (PKC412), the FMS-like tyrosine kinase 3 receptor (FLT3) and multi-targeted kinase inhibitor, in patients with acute myeloid leukemia and high-risk myelodysplastic syndrome with either wild-type or mutated FLT3. J Clin Oncol. 2010;28:4339–4345. doi: 10.1200/JCO.2010.28.9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Juckett M, LaPlant B, Flynn PJ et al (2011) Phase II study of AZD2171 for the treatment of patients with acute myelogenous leukemia. J Clin Oncol 29, ASCO, Suppl abstract 6574
- 82.Bruns AF, Herbert SP, Odell AF, et al. Ligand-stimulated VEGFR2 signaling is regulated by co-ordinated trafficking and proteolysis. Traffic. 2010;11:161–174. doi: 10.1111/j.1600-0854.2009.01001.x. [DOI] [PubMed] [Google Scholar]