Abstract
Extinction of fear memory is a particular form of cognitive function that is of special interest because of its involvement in the treatment of anxiety and mood disorders. Based on recent literature and our previous findings (EMBO J 30(19):4071–4083, 2011), we propose a new hypothesis that implies a tight relationship among IGF signaling, adult hippocampal neurogenesis and fear extinction. Our proposed model suggests that fear extinction-induced IGF2/IGFBP7 signaling promotes the survival of neurons at 2–4 weeks old that would participate in the discrimination between the original fear memory trace and the new safety memory generated during fear extinction. This is also called “pattern separation”, or the ability to distinguish similar but different cues (e.g., context). To understand the molecular mechanisms underlying fear extinction is therefore of great clinical importance.
Keywords: IGF2, IGFBP7, Neurogenesis, Fear extinction, Learning and memory, Epigenetics
Main features of fear extinction
Fear is a survival mechanism in animals and humans that is activated in the presence of a threat such as pain or danger. In rodents, fear is manifested as a cessation of all bodily movements except those required for respiration (i.e., freezing), changes in respiration, an increase in blood pressure, avoidance of the place where the shock occurred, or several other possible measures, in the presence of the conditioned stimulus (CS) [1, 2]. Freezing is widely used as an index of learned fear in rodents [3].
Excessive fear and anxiety are some of the main hallmarks of emotional disorders [4]. For example, patients with posttraumatic stress disorder (PTSD) show extreme levels of fear that they cannot cope with as a consequence of a terrifying or traumatic event [5–7]. Extinction training is crucial as a therapeutic approach to inhibit or at least to reduce the excessive fear. Fear inhibition is accomplished during extinction training by repeated re-exposure to the fear-eliciting stimulus (or CS) (e.g., a light, tone, or distinctive place “context”) in the absence of any aversive or unconditioned stimulus (US) (e.g., a footshock in rodents, a blast of air to the throat in humans). Here, extinction training leads to a gradual reduction of fear. In humans, this therapeutic approach is known as “exposure therapy”. Therefore, our understanding of the molecular mechanisms regulating the extinction of learned fear is essential and of great clinical relevance.
Proposed mechanisms for fear extinction
Many variations of the fear conditioning paradigm exist but the most studied is Pavlovian fear conditioning [8]. The most common theories suggest that fear extinction involves a combination of associative processes such as new learning and also nonassociative mechanisms involving decreased responsiveness to the fear-eliciting stimulus [1, 2]. The molecular processes that underlie fear extinction are only beginning to emerge, and it has been proposed that extinction is regulated by counteracting signaling pathways that either promote or prevent extinction of fear [2].
Current theories about the extinction of learned fear propose that extinction of fear involves unlearning, new learning and nonassociative mechanisms. Several studies have provided evidence to indicate that fear extinction is a new form of learning rather than the erasure of the original memory trace. The different mechanisms and theories about fear extinction have been extensively reviewed in several recent review articles [1, 2, 4, 9–12]. Although the molecular mechanisms of fear extinction are not well understood, a prominent view is that a new “safety memory” (i.e., a new learning) is generated during the extinction process that will compete with and inhibit the original fear memory [1, 2, 10, 13, 14]. When this mechanism is compromised as during pathological conditions, for example in PTSD [15], the safety memory will not be strong enough to inhibit the original fear memory, and the fear memory will be persistent [16].
Long-term potentiation (LTP) is one of the main cellular mechanisms that underlie learning and memory. LTP increases synaptic strength (or potentiation) that lasts a very long time. After short-term memory formation the new fear memories suffer what is called the “consolidation” process that involves new protein synthesis occurring during LTP and that convert the memories into fixed or long-term memories [17, 18]. Afterwards, this fear memory can undergo a process called “reconsolidation”, a form of memory consolidation that occurs when a memory formed previously is reactivated and is again susceptible to modification [1, 2, 18–20]. During this plastic state that persists for several hours after memory retrieval [21], the memory is open to enhancement or disruption [16, 22, 23]. The administration of an agent that impairs memory during this time of reactivation will induce amnesia in the animal (mouse or rat) [22]. During memory retrieval, fear memories will become labile again and be subjected to modification via the reconsolidation process leading to a “memory update” whose adaptive purpose might be to permit the integration of new information present at the time of retrieval into an updated memory representation [18, 19, 22, 24–26].
Extinction and reconsolidation are similar processes that can be distinguished via specific manipulations that alter extinction or reconsolidation (e.g., duration of retrieval), but there is as yet no definitive and clear distinction between the two processes [16]. The regulation of fear extinction critically depends on NMDA receptors that have been implicated in learning and memory processes [27]. d-cycloserine (DCS), a partial NMDA receptor agonist, facilitates fear extinction [28–30]. However, DCS also potentiates reconsolidation of the fear memory such that rats exhibit stronger fear memories when tested later [31]. Experimental data suggest that reconsolidation is promoted by short re-exposures, whereas extinction is preferably promoted by long re-exposures to the CS [32–34]. Furthermore, 14 days pre-exposure to either DCS or imipramine (a serotonin reuptake inhibitor) in rats disrupts the facilitating effect on extinction of a single injection of DCS immediately following extinction training [35]. This has implications for the clinical use of DCS. Thus, the duration of exposure therapy (the process of fear extinction in humans) [30] will favor memory reconsolidation (brief re-exposures) or extinction (longer re-exposures) [16]. We must bear in mind several factors that can influence fear extinction results such as the behavioral protocol, animal species, age, type of drug and timing of administration (before, during or after extinction), etc. Finally, we must note that DCS is not the only drug that facilitates extinction [10, 36, 37], and that further research is necessary to elucidate the molecular mechanisms underlying the processes of fear extinction and reconsolidation [16].
Primary brain regions involved in fear extinction
Numerous studies have suggested a major role for the amygdala, hippocampus and the medial prefrontal cortex (mPFC) in the acquisition, consolidation and retrieval of conditioned fear and its modulation by context [1, 2, 38–40]. Amygdala microcircuits involving coordinated excitatory glutamatergic and inhibitory GABAergic projections participate in the mediation of fear expression and fear extinction [39]. The mPFC, specifically the prelimbic and infralimbic subregions, has been implicated in the retention and expression of fear extinction and recent data indicate that the mPFC may regulate the amygdala output that controls the autonomic and emotional responses related to fear via the hypothalamic–pituitary–adrenal axis [41–43]. It is believed that the mPFC influences the central amygdala activity and output probably by exerting inhibitory control via the GABAergic intercalated cell masses that regulate the traffic between the basolateral and central amygdala [39, 43–45]. In addition, the hippocampal trisynaptic circuit involving the dentate gyrus (DG), CA3 and CA1 (DG–CA3–CA1) that receives information inflow from the entorhinal cortex plays an important role encoding contextual information during extinction of learned fear [46].
The hippocampus is involved in some but not all types of memory and works as a temporary storage for the new information that will be subsequently permanently stored in the cortical network [47–51]. Several studies have implicated the hippocampus with the regulation of spatial and contextual memory [1, 2, 52–58]. Besides, recent studies point to a region specificity in hippocampal function such that the dorsal hippocampus is mainly involved in cognitive functions while the ventral hippocampus participates more in stress, emotion and affect regulation [59–66]. Recent data suggest that this regional distribution in hippocampal function might be reflecting a similar disparity at the molecular level [13, 60, 67]. Furthermore, the hippocampus sends major projections to the amygdala [68] and the mPFC [69–71]. There are also reciprocal connections between the amygdala and the hippocampus reaching the ventral subiculum, CA1, CA2 and CA3 subfields of the hippocampus [68, 72]. The circuitry among these structures makes the regulation of fear extinction complicated to understand and a challenge to solve in fear extinction research.
Cellular and molecular mechanisms for fear extinction
Adult hippocampal neurogenesis
Main features of adult hippocampal neurogenesis
In the hippocampus, a region that plays an essential role in contextual fear extinction, newborn cells originate from proliferating neuronal progenitor cells in the subgranular zone (SGZ) of the DG [73–75]. The sensory information proceeding from the entorhinal cortex will reach the DG granular cells whose projecting mossy fibers will then impinge on the hippocampal CA3 pyramidal neurons. CA3 projections or Schaffer collaterals will then project to hippocampal CA1 pyramidal neurons and from here back again to the cortex. This circuit, the "hippocampal trisynaptic circuit" [46], is crucial for learning and memory formation.
After birth, the new hippocampal neurons must undergo a complex process of maturation and survival [46, 75–77]. There are several molecular mechanisms regulating adult neuronal stem cells and neurogenesis [78–80]. If the neuron survives this process that takes approximately 6 weeks, it will be incorporated into the DG to become part of this trisynaptic circuit. Once these adult mature neurons are integrated into the hippocampal circuit they may be involved in the learning and memory processes such as the extinction of learned fear. As we discuss in this review, adult hippocampal neurogenesis could be one of the main cellular processes underlying the extinction of learned fear.
The first experimental evidence for adult neurogenesis was reported during the 1960s [81, 82]. Adult neurogenesis is a complex process that consists of three main phases: proliferation of neuroprogenitor cells, differentiation into neurons and survival of the newly generated neurons [75, 83, 84]. There are two main regions in the brain with intrinsic neurogenesis: the subventricular zone and the hippocampal SGZ [75]. The different phases of adult neurogenesis are tightly regulated at the level of transcription [78] via epigenetic mechanisms [79, 85].
Many different neuronal markers (e.g., nestin, doublecortin, NeuN, calbindin, etc) are used to identify the different stages of neuronal differentiation and maturation [75]. In the young immature neurons, the response to γ-aminobutyric acid (GABA) switches from depolarization to hyperpolarization at 2–4 weeks after neuronal birth. This matches the growth of dendritic spines and the onset of glutamatergic responses [75]. Interestingly, newborn hippocampal neurons have already formed synapses with hilar and CA3 targets by 2 weeks after birth, though the complexity of these efferent synapses increases as neurons mature [86]. During this time window, the threshold for LTP is lower in these immature neurons [87, 88]. Within 1–4 weeks of birth, new neurons undergo a critical period of maturation in which they display greater plasticity than fully mature neurons [87, 89, 90]. At this time point, these immature neurons express the immediate early genes, c-fos and Zif268, that are used as indicators of neuronal activation [91]. Basically, during this period of maturation and differentiation the neurons must decide whether they survive to become fully mature adult neurons or undergo apoptosis and die.
During this sensitive period, the survival of these immature newborn cells may be affected by environmental stimuli such as environmental enrichment (EE) (e.g., voluntary exercise), stress or spatial learning [75, 83, 89, 92–96]. Hippocampal neurogenesis responds differently to the stress and learning in the different hippocampal subregions [64]. In addition, it has been recently shown that a traumatic event in mice such as a mild electric shock (2 s, 0.7 mA) during contextual fear conditioning is able to decrease survival of the immature newborn neuronal population (2–3 weeks after birth) without any effect on the adult mature neuronal population (>4 weeks after birth) [13]. At 4 weeks of age, hippocampal newborn neurons show typical features of mature granule cells, but they continue to evolve both physiologically and morphologically [88, 97, 98]. Thus, newborn neurons are very sensitive to intrinsic or external factors that affect their rate of proliferation and survival, which illustrates the complexity of the adult hippocampal neurogenesis process. Therefore, incorrect manipulations in space and in time may alter adult neurogenesis. This could lead to an alteration in the behavioral outcome and misinterpretation of results.
Neurogenesis and fear extinction
The contribution of adult neurogenesis to the process of fear extinction is not well understood. Adult neurogenesis has been implicated in various types of memory formation [75, 83, 99, 100]. It has been reported that, depending on the experimental paradigm, fear conditioning can impair hippocampal neurogenesis [101]. During aging, cognition is impaired and hippocampal neurogenesis is reduced. This affects the size of the hippocampi that is reduced [102]. Thus, patients with PTSD have a decreased hippocampal volume together with lower levels of hippocampal neurogenesis that contribute to the fear extinction impairment [15, 103]. As mentioned above, patients with PTSD can be treated with a form of fear extinction called exposure therapy. Moreover, chronic administration of fluoxetine, an antidepressant that increases neurogenesis and is used to treat anxiety and mood disorders including PTSD [103–106], also facilitates contextual fear extinction [107].
As fear extinction is a form of learning, it is possible to assume that adult hippocampal neurogenesis might have a role in extinction processes. Feng et al. [108] support the view that neurogenesis is not important for memory formation, but may play a role in the erasure of hippocampal memory traces. In the context of fear extinction, a first study by Ko et al. [109] showed that adult hippocampal neurogenesis does not affect the extinction of contextual fear memory in mice where neurogenesis is ablated on gamma irradiation or administration of an antimitotic agent. However, a recent and very elegant study has shown that conditional ablation of immature hippocampal neurons at 1–4 weeks old in Nestin-thymidine kinase transgenic mice impairs extinction of a contextual fear memory [110]. Similar results were found by Noonan et al. [111] for the extinction of cocaine-seeking behavior in rats following intravenous cocaine self-administration after disruption of hippocampal neurogenesis by irradiation. Furthermore, another study [13] has shown that fear extinction training specifically promotes the survival of hippocampal neurons at 17–19 days old while the proliferation of neuroprogenitor cells is not affected. A recent study has shown that a mixed polyunsaturated fatty acid diet regularizes hippocampal neurogenesis and reduces anxiety and normalizes the previous impaired fear extinction in serotonin transporter knockout rats [112]. Recently, it is been shown that ERK5 mitogen-activated protein kinase (MAPK) may also be a mediator in the regulation of adult neurogenesis during fear extinction [113]. Thus, the outcomes of studies discussed above clearly indicate the importance of adult hippocampal neurogenesis during the extinction of learned fear.
Neurogenesis and pattern separation
At first, it may appear counterintuitive that immature neurons contribute to extinction learning. Previous findings have indicated that such immature newborn neurons can participate in neuronal plasticity [13, 87, 89, 90, 110, 114]. This suggests that contextual fear extinction critically involves the survival of immature newborn hippocampal neurons that may play an immediate role in data processing within the hippocampal circuitry. In this context, one of the generally accepted functions of the DG is its role in pattern separation [75, 83, 115–117]. Pattern separation is the ability to distinguish similar but different cues, for example your mother’s face from her sister’s face. It has been proposed that a new experience activates a subset of DG neurons which affect a specific group of CA3 cells generating a specific CA3 pattern and that this process can be regulated by the recruitment of newborn neurons [83, 118–120]. Old dentate granular cells (≥4 weeks) in the mutant mouse (DG-TeTX) cannot excite their CA3 targets. Nakashiba et al. [121] have demonstrated the critical function of young newborn neurons (<4 weeks old) in pattern separation between similar environments (or contexts). Furthermore, suppression of adult hippocampal neurogenesis that affects the hippocampal DG–CA3 circuit impairs the coding of similar (but not dissimilar) contexts [122]. This means that when two experiences are very similar, we need younger granule cells in order to reach pattern separation, whereas the discrimination of a more distinct pair of experiences requires the participation of older granule cells.
Thus, we have proposed that extinction of learned fear recruits adult-generated neurons at 17–19 days old to produce a novel pattern that is, however, still connected to the original fear memory trace [13]. Under these circumstances, cFOS, a well-established marker of neuronal activity, is upregulated after exposure to extinction trial 1 (E1) and has been used to assay neuronal plasticity in immature newborn neurons [91, 96]. Newborn neurons at 17–19 days old exhibit augmented cFos expression upon exposure to fear extinction expanding the view that such neurons are able to participate in neuronal activity associated with extinction learning [13]. Thus, the specific recruitment of immature newly generated neurons into the original fear memory trace may affect the way this memory trace is retrieved. However, when the neurons become older than 4 weeks, they are not involved in pattern separation but in pattern completion, that is the process of retrieving more complete memories from partial cues [121]. Basically, recall of memories and formation of new memories involve different subgroups of granule cell populations with different ages in the hippocampal DG.
Fear conditioning and fear extinction do not affect the survival of neurons older than 4 weeks [13]. This supports the idea that the population of immature neurons between 2 and 4 weeks of age when they are highly active is crucial for the extinction of contextual fear memories (see Fig. 2). However, neurons older than 4 weeks become increasingly silent in their influence on pattern separation. We can then propose that neurons at 2–4 weeks old participate in the discrimination between the original fear memory trace and the new safety memory originating during fear extinction. This fits with the data obtained by Nakashiba et al. who found that granule cells at 3–4 weeks old are functionally integrated into the DG [121]. Without the constant new pool of immature neurons generated in the hippocampal SGZ, the ability to add new information and create the new safety memory would be impaired, and the ability for memory discrimination in a similar context would be affected.
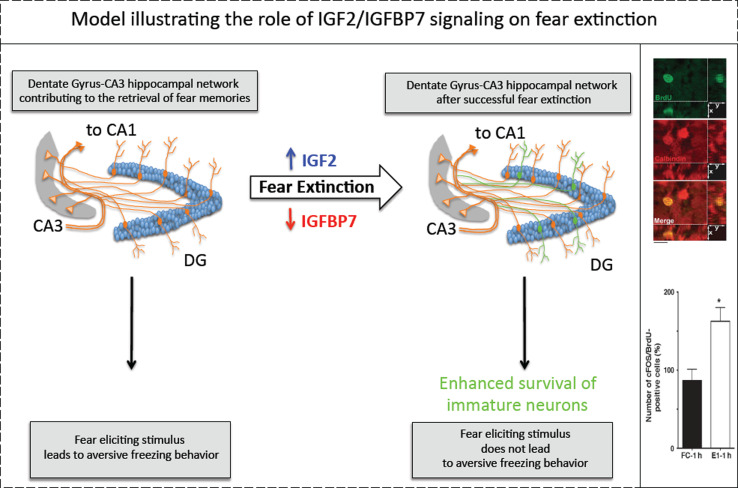
Fig. 2.
IGF2 facilitates fear extinction while IGFBP7 impairs fear extinction in an IGF2-dependent manner. Fear extinction initiates an upregulation of hippocampal IGF2 and a downregulation of IGFBP7 leading to survival enhancement of immature neurons as indicated by colabeling of BrdU and calbindin (top right inset), a marker of mature granule cells. In addition, fear extinction increases cFOS expression (a well-established marker of neuronal plasticity) in immature hippocampal neurons (bottom right inset) (modified from Agis-Balboa et al. [13])
Neurotransmitters and kinase signaling
Different neurotransmitter systems and kinase/phosphatase signaling pathways play a role in the regulation of fear extinction. More elaborate reviews of these topics are available [1, 2, 11, 123].
In brief, GABA is the main inhibitory neurotransmitter in the CNS and is essential for fear extinction. This has been shown by the administration of GABA agonists that impair extinction [124, 125] or GABA antagonists that facilitate fear extinction [126, 127]. Additionally, glutamate, the main excitatory neurotransmitter in the brain, has been associated with fear extinction. DCS, a partial NMDA receptor agonist, facilitates fear extinction [37]. Several other neurotransmitter systems are involved in fear extinction [1] such as the dopamine system [128] and the endogenous cannabinoid system [129]. Dopaminergic signaling facilitates learning but impairs extinction, while endogenous cannabinoids only affect extinction [1, 2, 12]. In conclusion, the action of several neurotransmitters is necessary for both the acquisition and extinction of fear memories.
Several studies have shown that the MAPK signaling pathway is important for the acquisition of fear memories [130]. However, the mechanism is not completely understood. Many pieces of evidence suggest that Erk1/2 could mediate learning and extinction, but with some differences between the two processes. It seems that the cytoskeletal Erk1/2 could be specific to early CS–US learning, whereas the nuclear action of pErk2 is needed for fear extinction. Several downstream pathways could be involved in fear extinction, but the upstream pathways that lead to Erk1/2 activation during learning and extinction are not well characterized (for review see Fischer and Tsai [2]). As discussed in the next sections of this review, one of these pathways could be the activation of insulin-like growth factor-1 receptor (IGF1R) via the IGFs. IGF signaling activates the ERKs followed by gene transcription and proliferative actions [13, 123, 131]. Several other kinases are involved in the extinction of fear memories such as calcium/calmodulin-dependent protein kinase II (CaMKII), protein kinase A, protein kinase C (PKC) and cyclin-dependent kinase 5 (Cdk5). A detailed description of the different molecular pathways involving the above-mentioned kinases is discussed in two recent reviews [1, 2]. In addition to the protein kinases, several protein phosphatases are also implicated in the extinction of learned fear. For example, calcineurin, which if inhibited impairs extinction [132], is a counterplayer of many kinases involved in learning such as Erk1/2 or CaMKII, and is associated with multiple synaptic pathways (e.g., synaptic vesicle endocytosis and synaptic depotentiation). Protein-phosphatase 1 is another phosphatase that could be involved in fear extinction because it plays an important role regulating the histone code (e.g., phosphorylation, acetylation and methylation) during memory formation [133].
In conclusion, it has recently been suggested that two counteracting molecular pathways regulate the extinction of fear, one that prevents and one that promotes extinction. Probably both associative and nonassociative mechanisms participate in those counteracting activities. During the process of fear extinction, the mechanisms preventing or inhibiting extinction will somehow be downregulated or suppressed so that the mechanisms facilitating fear extinction will eventually dominate and result in extinction. Thus, preventing and promoting mechanisms of fear extinction do not belong to the same downstream signaling pathways. Some of the molecular players that could form part of these counteracting mechanisms are the p21-activated kinase 1 (PAK-1) activity that facilitates extinction and the Rac-1/Cdk5 activity that prevents extinction [2]. The Rac-1/Cdk5/PAK-1 signaling pathway is a good example of pathways in which counteracting actions are mechanistically linked. It is important to mention that during fear extinction two memories are competing, the original memory trace that induces fear, and the new learned “safety memory” created with the extinction training that facilitates fear extinction.
Gene expression
An interesting question during the last few years was to understand how gene expression is regulated during the process of fear extinction [38]. Just recently and in a series of elegant experiments, Agis-Balboa et al. [13] completed a genome-wide analysis of the hippocampal transcriptome during fear extinction to study the longitudinal course of the extinction of learned fear. They performed hippocampal microarray analysis at two time points: (1) at the beginning of fear extinction when animals display high levels of freezing behavior, and (2) at the extinction trial on which the freezing behavior was significantly reduced after extinction training. This experimental approach clearly does not capture all critical time windows possibly implicated in contextual fear extinction, but it does identify genes that may have an important function in fear extinction and thus reveals new avenues for further experiments.
Agis-Balboa et al. [13] found that 137 genes, implicated in several biological processes such as neurogenesis, neuronal projection or cell proliferation, are differentially expressed during fear extinction. Consistent with their microarray results, they observed via quantitative PCR a strong upregulation of the expression of several immediate early genes such as Fos FBJ osteosarcoma oncogene (cFos), early growth response 2 (Egr2), activity-regulated cytoskeleton-associated protein (Arc) and chromodomain helicase DNA binding protein 3 (Chd3), 1 h after fear conditioning and E1. In particular, cFos induction is significantly reduced upon extinction low-freezing exposure, when compared to E1. For example, cFos and Zif268 are two immediate early genes that have been implicated in brain plasticity and are activated by the learning and memory processes [134, 135] not only in adult DG granular cells but also in immature hippocampal newborn neurons [91]. Moreover, previous data have shown that the immediate early gene cFos, a marker of neuronal activity, is transitorily upregulated at the beginning but towards the end of extinction training [136]. A more complete analysis of gene expression after different extinction trials and additional time points will indeed reveal further key candidate pathways. Nonetheless, among the identified genes that exhibit differential expression, those involved in insulin signaling are of particular interest such as insulin growth factor 2 (Igf2), insulin growth factor binding protein 7 (Igfbp7) and Klotho. However, this microarray did not allow the recognition of all possible transcriptome changes related to fear extinction, neither was it intended to dissect mechanisms of fear extinction from those linked to reconsolidation [18, 19, 26, 38, 137, 138].
The genomic anatomy of the hippocampal region has been characterized leading to the documentation of nine nonoverlapping gene expression domains with a very clear regionalized hippocampal expression that seems to be regulated independently and replicate underlying hippocampal circuitry [60, 65]. This is very interesting, since the hippocampus is crucial for memory formation and fear extinction [1, 2]. Another region that is directly involved in the regulation of fear extinction is the mPFC [43] as mentioned above. Thus, Bernard et al. [139] found that specializations in cortical cellular and functional architecture in rhesus macaque, a highly predictive nonhuman primate model system for human neocortical structure, are reflected by differential gene expression. This approach has been used to identify other gene clusters associated with neuronal cell types [140, 141] and subcellular compartments [141]. Moreover, recent findings have shown that extinction of contextual fear is enhanced if rats are exposed to novelty (e.g., open field), a type of learning, before or after extinction training. It is proposed that this is due to synaptic tagging by behavioral manipulation that involves new protein synthesis, which is termed behavioral tagging [142].
These recent studies illustrate how the application of modern technologies such as next-generation sequencing will help to quickly screen and identify possible candidate genes or clusters of genes, and relate them to specific brain areas and functions, even at tissue-specific, cell type-specific and even synapse-specific resolution [143, 144]. This, combined with the recently developed optogenetic tools [40, 65,145], is a new way to study the interaction among brain activity, gene expression and behavior. In addition, the current bioinformatic and genomic/proteomic databases allow cross-comparison of information obtained from different studies. This will dig into the potential molecular mechanisms involved in the regulation of brain processing during context of fear extinction under normal and pathological conditions. The outcomes of these experiments will lead to a better understanding of the connectivity among different neurons and their functional interdependence during the extinction of learned fear and the general processes of learning and memory formation.
IGF signaling and fear extinction
IGF (IGF1 and IGF2) signaling is crucial for many brain processes such as brain development, adult neurogenesis and organ growth. Most IGF actions are mediated through the insulin and IGF1 receptors and via the canonical kinase-phosphatase intracellular signaling pathways, RAS-MAPK and the phosphoinositide 3-kinase–v-akt murine thymoma viral oncogene homolog 1 (PI3K-AKT) cascades [123]. Recently, our group has demonstrated the specific role of IGF signaling in the regulation of fear extinction and neuronal survival [13]. In this section, we discuss the potential roles of IGF signaling in fear extinction and its regulation of adult neurogenesis.
IGF signaling in the brain
IGF signaling during development
In rodents, the levels of IGF1 and IGF2, their receptors and the IGF binding proteins (IGFBPs) are highest during development [146]. They affect proliferation, survival and differentiation of all types of brain cells [147]. In mammals, IGF1 and IGF2 regulate energy metabolism and are a class of growth factors that regulate prenatal growth and body size [123, 148–153]. Removal of the IGFs leads to smaller brains [150]. Although all tissues express IGFs, the main peripheral source of IGFs is hepatocytes. In adult brain, only low levels of IGF1 and IGF1R transcripts are expressed, and IGF2 is mainly produced by the choroid plexus and the meninges. Lack or deficiencies of IGFs in the brain induce altered circuit formation due to problems with cell proliferation and differentiation, synapse formation, axon guidance (for detailed reviews see Fernandez and Torres-Aleman [123] and Alberini and Chen [154]). This suggests, that the levels of IGFs must be tightly regulated spatially and temporally in order to have the correct brain circuitry formation and brain function.
There have been many studies of the function of IGF1 in the brain but only few about IGF2. As has been shown in mice, during early brain development IGF1 is produced by neuroepithelial cells [155], has autocrine proliferative [156] and prosurvival [157] actions, and participates in regionalization of the brain [158]. Later in development, IGF1 participates in the formation of the proper hippocampal, olfactory bulb and cortex circuitry [159–161] (three regions involved in learning and memory) via its contribution to processes such as the production of hippocampal neurons [161, 162], synaptogenesis [163] and formation of astrocytes [164, 165]. The highest levels of IGF2 are seen during brain development [166] and it is involved in brain growth and neuronal differentiation [167, 168]. Both IGF1 and IGF2, regulate oligodendrocyte differentiation during development, via AKT in the case of IGF1 [169, 170].
IGF1R and IGF2R signaling pathways
IGFs mediate their function through distinct receptors (insulin receptor, IGF1R, IGF2 receptor or “mannose-6-phosphate receptor”, and hybrid receptors), but many of the actions of IGF1 and IGF2 are mediated via the IGF1R, a member of the tyrosine kinase receptor family. After IGF1R activation, several intracellular kinase-phosphatase cascades are recruited that regulate diverse processes such as apoptosis, adult neurogenesis, gene transcription and protein translation [13, 114, 123, 131, 143, 154]. Mice lacking IGF1R die soon after birth, and show microcephaly or brain growth retardation [114, 171–173]. On the other hand, IGF1 overexpression accelerates cell survival leading to macrocephaly [156, 172–175]. IGFs are mainly produced in microglia and their targets are mainly astrocytes and neurons that generally overexpress IGF1R after brain injury [176]. Furthermore, IGF1R is expressed in newborn hippocampus neurons at 17–19 days old that participate in fear extinction [13]. In addition, IGF1R signaling is involved in insulin and IGF1 resistance in several brain diseases, such as Alzheimer’s disease (AD) [177].
It is believed that IGF2R acts as a regulatory sink, modulating the levels of IGF2 in different tissues [178]. IGF2 binds to the IGF2R with higher affinity than IGF1. IGF2 mainly works as a scavenger system targeting IGF2 to lysosomes [179]. It seems that IGF2R can recruit G proteins. This could lead to the activation of PKC and phospholipase C that are involved in different cell types with IGF2 actions such as regulation of calcium homeostasis [179–181]. Only a few studies, such as that of Chen et al., have related IGF2R with some specific brain functions such as memory consolidation and enhancement [154, 182].
IGFBPs
The bioavailability of IGF1 and IGF2 is regulated by IGFBPs, a family of proteins binding IGFs with high affinity, that are also used to transport IGFs, and are present in invertebrates and vertebrates [123, 183, 184]. The IGFBPs can sequester the IGFs away from their receptors inhibiting IGF-stimulated effects [123, 185]. The affinity of IGFBPs for IGFs is higher than for IGF receptors [185, 186]. There are at least seven high-affinity IGFBPs (IGFBP1 to IGFBP7), all expressed in the brain, and there are another ten IGFBP-related proteins with lower affinity [13, 187, 188]. The joint action of IGFs and IGFBPs helps to preserve neural tissue and homeostasis [176, 189]. IGFs and IGFBPs have been detected in cerebrospinal fluid (CSF) [187] and are produced in cells in the brain such as in the choroid plexus [187, 190–192]. Some data support the idea that CSF transports IGFs to deeper brain regions [193]. The IGFs produced by the CSF seem to be an important regulator of neuronal proliferation [114, 192]. Among all the IGFBPs, we are particularly interested in IGFBP7 as discussed in the following sections. Agis-Balboa et al. [13] have suggested that IGFBP7 is involved in the regulation of neuronal survival during the extinction of learned fear. Furthermore, IGFBP7 nanoparticles have recently been developed and could be used in the future for diagnosis and treatment of glioblastoma multiforme, the most common and difficult to treat brain tumor [194].
Local IGFs reach their targets in seconds or minutes, whereas systemic IGFs need minutes to hours [123]. The actions of IGFs are tissue-specific and source-specific depending on the origin of the IGF, systemic or local. This implies that brain cells must somehow distinguish between IGFs produced in the periphery or in situ in the brain, but the mechanism is unknown.
IGF signaling and cognition
IGF1 and IGF2 are present in neurons [13, 143, 146]. The underlying mechanism by which IGFs regulate learning and memory is still unclear, but some evidence shows that this action could be achieved by regulating some of the neurotransmitter systems, synaptic plasticity and hippocampal neurogenesis [123, 154, 181, 195, 196]. It has been shown in humans that treatment with insulin or growth hormone increases the levels of IGF1 in serum, improving cognitive function [197–199]. As has been shown in experiments using the conditioned eye-blink response, IGF1 is involved in associative conditioning in mammals [200]. Furthermore, it has been shown that insulin receptors, also used by insulin, IGF1 and IGF2 to exert their actions [123], are increased in the hippocampus after learning and even EE [201, 202]. It is well known that the hippocampus has intrinsic adult neurogenesis, and these newborn neurons participate in the processes of learning and memory including fear extinction.
During the last few years several reports have implicated IGF signaling in neurodegenerative diseases such as AD, Huntington’s disease and Parkinson’s disease [123, 177, 203, 204], and in mood disorders (e.g., depression) [205]. Recent studies have shown that IGF1 improves the cognitive ability in patients with AD [206], and seems to be neuroprotective during neurodegenerative processes [203, 207]. In patients with spinocerebellar ataxias (SCA3 type), IGF1 treatment for 8 months significantly improved the ataxia and stabilized disease progression [208]. Some of the IGF1 effects promoting neuronal survival seem to be mediated via the PI3K-AKT pathway [209]. In addition, IGF1 is involved in amyloid clearance [210] and protects against maladaptive inflammation [211]. However, only a few studies have shown that IGF2 is related to cognitive function and fear extinction [13, 154, 182, 212–214].
One of the underlying mechanisms for the effects of IGF1 and IGF2 in learning and memory is synaptic plasticity (for review see Fernandez and Torres-Aleman [123]). IGFs may play a functional role in neurotransmission [215]. For example, IGF1 is released in response to neuronal activity [215, 216]. Furthermore, IGF1 potentiates neurotransmitter release in hippocampal neurons via the MAPK pathway [217, 218] and the release of dopamine in chromaffin cells [219]. IGF1 also regulates the activity of L- and N-type calcium channels [220] and mechanoreceptor cation channels [221] via PI3K signaling. Moreover, IGF2 has recently been implicated in memory consolidation and enhancement, neurogenesis, fear extinction and antidepressant actions [13, 114, 143, 154, 182, 213, 214], but the molecular mechanisms involved in these actions are still unclear. These studies support a crucial role for IGF signaling in supporting circuitry properties in the brain [222].
IGF signaling and neurogenesis
IGF signaling is involved in adult hippocampal neurogenesis (e.g., cell morphology, neuronal proliferation and survival). This interaction between IGF signaling and neurogenesis could potentially contribute to the observed effects on fear extinction [13, 114, 123, 143, 223–230]. IGF1R signaling mediates most of the proliferation and survival actions of the IGFs [13, 123, 231].
One of the main targets of brain diseases is the hippocampus. Hippocampal function is usually impaired in anxiety or cognitive diseases and intrinsic adult neurogenesis also occurs in the hippocampus. IGF1R signaling mediates most of the proliferative actions of the IGFs (for reviews see Fernandez and Torres-Aleman [123], Arnaldez and Helman [131], Hixon et al. [232]) and it is essential for hippocampal development [173]. Adult hippocampal neurogenesis is a curious case of brain plasticity. Normally, during synaptic plasticity new neurites and synapses are formed or modified; however during neurogenesis whole newborn neurons are added to the network in an activity-dependent manner. Both physical and cognitive activities are the plasticity-inducing stimuli. Thus, adult neurogenesis can be regulated by environmental factors such as stress or physical activity [233–235]. Physical activity seems to potentiate the proliferation and maintenance of neuronal precursor cells. EE and learning promote survival of adult immature neurons [236]. EE also increases IGF signaling activity [201, 202] and some of the effects of EE due to increased physical activity are mediated by IGF1 [237, 238] via the AKT pathway [239]. In mice deficient in IGF1, IGF1 administration improves cognitive abilities and decreases anxiety correlating with increased neurogenesis [205]. It is known that stimulation of adult hippocampal neurogenesis, when combined with an intervention such as voluntary exercise, improves exploratory behavior and pattern separation [117]. In addition, IGF1 and IGF2 have been associated with antidepressant actions [213, 214, 240].
During the last three decades, an explosion in the neurogenesis field has demonstrated not only the existence of adult neurogenesis but also the importance of this process in learning and memory [83, 241]. New neurons in the hippocampal DG may play different roles in learning and memory, contributing to the formation of new memories [242] such as during the extinction of learned fear [13]. IGF1 [243, 244] and IGF2 can promote adult neurogenesis [13, 143]. However, IGF2 promotes stemness of neural/stem progenitor cells in a more effective manner than IGF1 [245]. IGF1, but not IGF2, function is critical for proper brain development [225, 229], suggesting that IGF2 mediates the fine-tuning regulation of the IGF system. It is thought that IGF2 levels in the CNS remain relatively stable during adulthood [246, 247]. In addition, it has been shown that IGFBP7 counteracts the effect of IGF2 during the extinction of learned fear by inhibiting neuronal survival [13]. IGF2 present in the CSF controls the growth and maintenance of neurospheres, an in vitro model of neural stem cells (NSCs) [114, 192, 248, 249]. Another study has demonstrated a direct role of the IGF2/IGF1R axis in human embryonic stem cell physiology [250]. Thus, a recent study involving gene expression profiling of NSCs and their progeny (or immature newborn neurons) revealed that IGF2 is a regulator of adult hippocampal neurogenesis [143]. This directly links IGF2 with the process of extinction of learned fear, during which contextual information is processed in the hippocampus.
IGF1 is involved in brain development and neuronal stem cell behavior, stimulating the proliferation, survival and differentiation of neurons, oligodendrocytes and astrocytes. In addition, IGF1 plays a role in neuritic outgrowth and synaptogenesis [123, 172]. It is known that IGF2 reduces oxidative damage in the brain [251], acts together with Shh signaling to regulate proliferation of cerebellar progenitors [252], seems to be important for the proliferation of medulloblastoma cells [253] and also plays a crucial role in neurogenesis itself [13, 114, 143, 245]. IGF2 is expressed by the choroid plexus epithelium [187, 190] which is the probable source of IGF2 found in the CSF [114, 192]. The increase in hippocampal IGF2 (mRNA and protein) levels after treatment with galantamine, a drug used in AD, further supports the role of IGF2 in neurogenesis and as a neuroprotective agent [254].
The studies discussed above support the role of IGF signaling in adult neurogenesis, but most importantly indicate a clear interaction among learning (fear extinction), IGF signaling and neurogenesis. In the next section, we describe a new mechanism for fear extinction involving IGF signaling and adult hippocampal neurogenesis.
IGF2/IGFBP7 signaling: new pathway regulating fear extinction
IGF2/IGFBP7 signaling regulates fear extinction [13] (see Fig. 1) IGF2 and IGFBP7 are secreted proteins that act in an autocrine or paracrine manner [13, 143, 229]. Since IGFBPs attenuate the function of IGFs [255–258], such regulation would ultimately result in increased hippocampal IGF signaling. In addition, previous data have shown that IGF2 and IGFBP7 can be regulated in response to environmental stimuli [214, 259, 260] and have established that both proteins are present in hippocampal neurons [13, 143, 226, 261, 262].
Recently, Chen et al. [154, 182] have shown that IGF2 works as a cognitive enhancer and that IGF2 signaling plays a crucial role in memory consolidation in the CA1 hippocampal region. In addition, it has been demonstrated that fear extinction (a different type of learning) initiates upregulation of IGF2 and downregulation of IGFBP7 that is most prominent in the hippocampal DG where significant levels of IGF2 and IGFBP7 protein are produced [13, 143].The expression of other key molecules of the hippocampal IGF signaling pathway including IGF1, IGF1R, IGFBP4 and IGFBP5 is not altered during extinction training. In line with this observation, Agis-Balboa et al. [13] have demonstrated that IGF2 facilitates fear extinction, while IGFBP7 impairs fear extinction in an IGF2-dependent manner (see Fig. 1). These findings suggest a more general mechanism of IGF2 signaling in learning and memory processes.
Adult hippocampal neurogenesis is involved in the fear extinction process [13, 110]. In this framework, IGF2 administration increases neuronal survival and accelerates fear extinction, whereas IGFBP7, that binds IGF2 and inhibits IGF2 signaling, reduces neurogenesis and severely impairs fear extinction in an IGF2-dependent manner [13] (see Fig. 1). IGFBP7 also decreases the action of IGF1 and VEGF signaling [263] which promotes adult neurogenesis [264, 265]. Moreover, IGF1 has also been implicated in memory consolidation [205, 238, 244]. Patients with glioblastoma multiforme show elevated levels of IGF2 in the CSF that stimulates stem cell proliferation in an IGF2-dependent manner [114, 192]. A deficit in neurogenesis is observed in IGF2-deficient mice that affects the uppermost layers of the cortex [114]. Moreover, IGF2 regulates adult hippocampal neurogenesis via the IGF1R [13, 143]. The effect of IGFBP7 on fear extinction-dependent neuronal survival could be mediated via several targets. The recent studies clearly indicate the importance of IGF signaling and especially of IGF2/IGFBP7 signaling in the process of fear extinction by regulating adult hippocampal neurogenesis.
Fig. 1.
Hippocampal IGF2/IGFBP7 signaling regulates extinction of learned fear. a Microcannulae are implanted in the dorsal hippocampus and the position is visualized by methylene blue injection. b Fear extinction is enhanced after IGF2 injection in the dorsal hippocampus. c IGFBP7 injection in the dorsal hippocampus impairs fear extinction in an IGF2-dependent manner (adapted with permission from Agis-Balboa et al. [13])
The manipulation of IGF2/IGFBP7 signaling during fear extinction via intrahippocampal injections of recombinant IGF2, IGF2 antibody [13, 266] or IGFBP7 after implantation of microcannulae is in line with the paracrine action of these proteins [13, 225]. However, an autocrine action cannot be excluded [143]. This is in accordance with the fact that IGF2 and IGFBP7 are secreted proteins and the finding that hippocampal newborn neurons express IGF1R [13, 123]. The main receptor through which IGF2 mediates biological function is IGF1R, since IGF2R normally attenuates IGF2 signaling [267–270]. Several studies have shown that IGF1R signaling can be blocked using the IGF1R-specific blocking peptide (JB1) [131, 271] that affects adult hippocampal neurogenesis and fear extinction [13, 143]. However, IGF2R has recently been implicated in memory consolidation [154, 182]. Inhibition of IGF1R impairs extinction of learned fear while inhibition of IGF2R has no effect. In addition, IGF1 has no influence on fear extinction and also fails to rescue the effect of IGFBP7, suggesting that under physiological conditions the action of hippocampal IGF1 might be tightly regulated [13].
After binding the IGF1R, IGF1 and IGF2 trigger the downstream signaling mediated through the activation of the intracellular serine/threonine protein kinase B (also known as Akt) [123, 154]. Akt seems to be implicated in the regulation of cell survival and antiapoptotic actions throughout the brain [272, 273], and plays an important role during the precursor proliferation of adult NSCs [143, 175, 274, 275]. The molecular mechanisms downstream of Akt activation that mediate the regulation of neuronal survival during the extinction of learned fear in the context of IGF2 signaling remain unclear [123, 154]. Previous studies have shown that the insulin receptor substrate 1 (IRS1) becomes phosphorylated upon IGF receptor activation [276]. Furthermore, IGF2 acts via Akt signaling to regulate NSC proliferation [143, 275, 277]. In a series of elegant experiments, Bracko et al. [143] found that downregulation of IGF2 in DG NSCs via shRNAs leads to a reduction in IRS1 phosphorylation and a reduction in phosphorylated AKT levels. This impaired NSC proliferation in DG was rescued by overexpression of a dominant-active AKT. This study suggests that regulation of hippocampal NSC proliferation is mediated via IGF2 acting through AKT-dependent signaling. We could speculate that two counteracting mechanisms interact during the extinction of learned fear, one favoring prosurvival signals (such as IGF2) and another one inducing proapoptotic signals, such as BMP or PTEN signaling [143, 278–281]. It is clear that more studies are necessary to elucidate the interactions among the IGF signaling cascade, adult neurogenesis and the extinction of learned fear.
The findings discussed above clearly support a critical role of IGF2 in the regulation of adult hippocampal neurogenesis and also in the process of fear extinction. Hence, we can propose that a counteracting mechanism involving IGF2, that facilitates fear extinction, and IGFBP7, that inhibits fear extinction, must be spatially and temporally regulated during the process of fear extinction. This will lead to the production and survival of more newborn neurons that can be incorporated in the hippocampal learning circuitry to create the safety memory that will inhibit the original conditioned fear memory trace. As such, this could be part of the counteracting mechanism acting during fear extinction as proposed by Fischer and Tsai [2].
These data imply a scenario in which the fine tuning of hippocampal IGF2 signaling is necessary for successful fear extinction. Thus therapeutic approaches that potentiate IGF2 signaling and adult neurogenesis or inhibit IGFBP7 might be suitable for the treatment of diseases associated with excessive fear memory. Although we cannot exclude the possibility that IGF2 and IGFBP7 affect fear extinction via independent mechanisms, previous findings strongly support the view that a hippocampal IGF2/IGFBP7 signaling pathway mediates fear extinction. In summary, these findings strongly suggest a role of IGF2 and IGFBP7 in fear extinction that merits further investigation. Future studies investigating the function of IGF2/IGFBP7 signaling in memory reconsolidation should also be considered.
Epigenetic mechanisms and fear extinction
The precise molecular mechanisms that regulate memory function are still not well understood. Epigenetic regulation of gene expression occurs in all healthy individuals, but also contributes to many human diseases. In the context of fear extinction three major epigenetic events that occur at the molecular level are described in the following sections: histone acetylation, DNA methylation and non-coding RNAs.
Histone acetylation
Normally, intracellular signaling pathways often lead to altered gene expression. Chromatin modifications are essential for transcription [282]. DNA is wrapped around a protein complex of eight histones (dimers of H2A, H2B, H3, H4) to form the basic unit of chromatin structure. The basic amino-terminus tails protruding out of histones carry several posttranslational modifications such as acetylation, methylation and ubiquitination, which build up a pattern of chemical marks recognized and bound by other proteins (i.e., writers, erasers and readers) [283]. This is known as the histone code [284]. Transcription is precisely regulated by chromatin modifications such as the acetylation of histone proteins. Histone acetylation is an important epigenetic mechanism that is normally associated with gene expression, while histone deacetylation is related to gene silencing [285]. This contributes to proper genome–environment interactions. Recent studies have demonstrated dynamic regulation of neuronal histone acetylation in response to environmental stimuli that initiate memory formation [286–291]. Histone acetylation is implicated in several neuropsychiatric diseases such as AD, Huntington’s disease and schizophrenia [292]. In this context, Erk1/2 signaling that is involved in fear extinction regulation is known to affect transcription factors [293, 294]. Recently, it was shown that Erk1/2 activity affects chromatin remodeling, including histone acetylation [295].
Two of the main players regulating histone acetylation are the histone acetyltransferases (HATs) and the histone deacetylases (HDACs), which regulate histone–DNA interactions [296]. The use of HDAC inhibitors to target histone acetylation has received attention during the last few years mainly because of the neuroprotective and neuroregenerative actions in animal models of neurodegenerative and neuropsychiatric diseases [65, 286, 297–299]. A few studies have been done in the context of fear extinction. For example, intrahippocampal or systemic administration of trichostatin A, an nonselective HDAC inhibitor, facilitates extinction of contextual fear in mice [300]. A recent study using another HDAC inhibitor, sodium butyrate, showed that increasing histone acetylation in the dorsal hippocampus and infralimbic cortex enhances fear extinction and cFos expression [301]. Also, the nonselective HDAC inhibitor, valproate, has been shown to facilitate extinction of fear [302, 303]. It has also been shown that increased levels of histone 3 acetylation observed during extinction correlate with increased acetylation of the brain-derived neurotropic factor promoter [302].
Some of these HDACs have been shown to be involved in learning and memory processes. For example, HDAC2 negatively regulates fear memory formation [304], and its inhibition abolishes neurodegeneration-associated memory impairments [305]. HDAC3 also negatively regulates memory formation [306]. HDAC1 is elevated in post-mortem brain samples from schizophrenia patients [307, 308], a mouse model of Huntington’s disease [309] and in neurons under hypoxic conditions [310]. Lowering the levels of HDAC6 restored learning and memory deficits observed in a mouse model of AD [299]. HDAC5 loss impaired memory function in a mouse model of AD [297]. However, there are few studies illustrating the role of these enzymes during the process of fear extinction. For example, Bahari-Javan et al. [298] have shown in mouse hippocampus that HDAC1 is required for fear extinction learning via a mechanism that involves H3K9 deacetylation and trimethylation of target genes such as cFos. The use of HAT activators such as SVP106 also results in an enhancement of fear extinction and prevention of fear renewal [135]. Although not conclusive, these studies indicate that HDAC inhibitors could be a suitable therapeutic tool to treat anxiety diseases such as PTSD [16].
DNA methylation
DNA methylation is involved in gene expression regulation [311]. DNA methylation occurs mainly in CpG-rich regions known as CpG islands that can be located at annotated transcription start sites but also in intragenic and intergenic regions [312, 313]. It is known that the release of local cytokines and the methyl CpG binding protein 2 (MeCP2) participate in such regulation [314–316]. In addition, IGF1 increases spine density, restores LTP and increases PSD-95 in MeCP2 mutant mice, an animal model for the study of Rett syndrome [317]. Furthermore, IGF1R, which is present in neuronal nuclei, can directly affect gene transcription functioning as a transcription modulator [318, 319]. However, nothing is known about the influence of IGF2 at the level of gene transcription.
Among the IGFBPs currently characterized [123, 185, 188, 320], IGFBP7 is the only one that has been directly involved in the regulation of contextual fear extinction and the survival of adult hippocampal newborn neurons [13]. IGFBP7 is considered a human tumor suppressor (or natural inhibitor of cell proliferation) [261, 321, 322] regulated via p53 [258]. IGFBP7 is present and epigenetically inactivated via DNA methylation in several human cancers [260, 323–327].
The group of Sweatt and others [288, 328–331] has shown that DNA methylation and histone acetylation are crucial for learning and memory formation using the fear conditioning paradigm, but no similar studies have been performed during contextual fear extinction, a different type of learning. DNA demethylation is also involved in adult neurogenesis [332]. We suggest that DNA methylation and DNA demethylation are processes working dynamically together during fear extinction. Since IGFBP7 expression is downregulated during the course of fear extinction [13] and it is regulated via DNA methylation in several cancers, one possibility is the epigenetic regulation of the IGFBP7 gene via DNA methylation/demethylation during learning and memory processes. We cannot exclude the possibility that IGF2 (an imprinted gene) might also be regulated via DNA methylation/demethylation or some other epigenetic mechanism during contextual fear extinction.
Agis-Balboa et al. have shown that, during contextual fear extinction, IGFBP7 is downregulated when the extinction process is complete while expression of the other IGFBPs is unchanged. The fear extinction inhibition produced by IGFBP7 can be counteracted by injection of IGF2 but not by IGF1. This means that IGFBP7 regulation of fear extinction is IGF2-dependent. In addition, this is an IGF1R-mediated mechanism with unknown function on IGF2R. In the context of two counteracting mechanisms regulating fear extinction [2] and based on these findings, it seems that IGFBP7 regulates and potentiates the formation of the original fear memory trace, while IGF2 counteracts this mechanism by regulating and potentiating the formation of the new “safety memory” during the extinction process (see Fig. 2). An interesting question that arises from the work of Agis-Balboa et al.: Is IGFBP7 inhibiting contextual fear extinction or potentiating memory re-consolidation? Future studies in the field should consider clarifying this issue. This study by Agis-Balboa et al. was performed in male animals. It is known that several constituents of sexually dimorphic behaviors are managed by distinct genetic programs [333]. Social experience can modify sex-specific behaviors [334], and complex neuropsychiatric disorders may also be composed of discretely heritable traits [334, 335]. Future studies should investigate fear extinction behavior by comparing men and women in the context of PTSD.
Non-coding RNAs
Non-coding RNAs and their related proteins are involved in many human diseases such as cancer, and neurological, cardiovascular, developmental and other diseases [336]. Among all the non-coding RNAs, the miRNAs have the most functional relevance in human diseases, because of their cellular and synapse functions [67, 85, 336–339]. Different miRNAs are key regulators of tissue aging and cellular senescence [338]. During the aging process, the regulation of insulin and IGF1 signaling via miRNAs is important for lifespan [338]. However, little is known about the regulation of IGF2 and IGFBP7 via miRNAs [340–342]. Adult neurogenesis which is important in fear extinction [13] is also regulated via microRNAs [85] as are other cellular processes in the mammalian nervous system [339]. Only a few studies have investigated the role of non-coding RNAs such as miR-34c or miR-182 in learning and memory processes [67, 343]. Dicer1 is involved in miRNA processing. The deletion of the Dicer1 gene in adult mouse forebrain induces the loss of miRNAs enhancing learning and memory [344]. The formation of the new fear-extinction memory is facilitated by the miR-128b [345]. Thus, it is reasonable to assume that some miRNAs might regulate the expression of IGF signaling components (e.g., IGF2 and IGFBP7) in the context of fear extinction or in other forms of learning and memory functions. It is therefore reasonable to hypothesize that miRNAs might also participate in the regulation of the synthesis of new proteins that occurs during the process of behavioral tagging associated with fear extinction after novel exposure [142].
In conclusion, not much is known about the molecular mechanisms regulating the expression of IGF2/IGFBP7 signaling pathway components during fear extinction. Future studies oriented towards the study of epigenetic regulation of IGF2/IGFBP7 signaling via DNA methylation, histone acetylation and non-coding RNAs are necessary. The understanding of the epigenetic mechanisms underlying fear extinction will allow us in the future to develop pharmacogenomic therapies and more personalized treatments.
Conclusions and perspective
Fear extinction is used as a therapeutic strategy to inhibit excessive fear. Fear is normally a protective mechanism, but some fearful events push the system beyond its limits. This leads to excessive fear and mental illnesses such as anxiety and mood disorders. In this review, we describe some of the known molecular mechanisms involved in fear extinction such as kinase/phosphatase signaling pathways and epigenetic mechanisms. However, the core and main focus of this review is a new mechanism for the regulation of the extinction of learned fear. This new mechanism is based in the latest findings from several groups including ours, which have revealed that specifically IGF2 signaling throughout the IGF1R is an essential regulator of both adult hippocampal neurogenesis and fear extinction [13, 114, 143].
Our model suggests that the consolidation of fear memories recruits DG granular cells to establish a specific DG–CA3 pattern that participates in the retrieval of fear memories. Thus, during fear extinction training, enhanced IGF signaling, that is established via transiently increased expression of IGF2 and prolonged downregulation of IGFBP7, leads to increased survival of immature neurons (Fig. 2, green cells in right panel). We suggest that these cells are recruited into the original memory trace in order to modify the way a fear memory is retrieved. In another words, these newborn neurons are involved in the formation of the new safety memory and contribute to pattern separation. It is probable that the original fear memory trace is still intact but does not produce freezing behavior upon exposure to the fear-eliciting stimulus.
In conclusion, a better understanding of the molecular changes involved in the extinction of learned fear is needed. This will also help understand anxiety diseases such as PTSD. Thus, the enhancement of IGF2 signaling (and consequently adult hippocampal neurogenesis), possibly via inhibition of IGFBP7, might be an appropriate approach to the development of new therapeutic avenues seeking to stimulate fear extinction in the context of anxiety diseases.
References
- 1.Myers KM, Davis M. Mechanisms of fear extinction. Mol Psychiatry. 2007;12:120–150. doi: 10.1038/sj.mp.4001939. [DOI] [PubMed] [Google Scholar]
- 2.Fischer A, Tsai LH. Counteracting molecular pathways regulating the reduction of fear: implications for the treatment of anxiety diseases. In: Shiromani PJ, Keane TM, LeDoux JE, editors. Post-traumatic stress disorder: basic science and clinical practice. Totowa, NJ: Humana; 2009. pp. 79–103. [Google Scholar]
- 3.Kandel ER. The molecular biology of memory storage: a dialog between genes and synapses. Biosci Rep. 2001;21:565–611. doi: 10.1023/a:1014775008533. [DOI] [PubMed] [Google Scholar]
- 4.Mahan AL, Ressler KJ. Fear conditioning, synaptic plasticity and the amygdala: implications for posttraumatic stress disorder. Trends Neurosci. 2012;35:24–35. doi: 10.1016/j.tins.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Friedman MJ, Schnurr PP, McDonagh-Coyle A. Post-traumatic stress disorder in the military veteran. Psychiatr Clin North Am. 1994;17:265–277. [PubMed] [Google Scholar]
- 6.Maren S, Chang CH. Recent fear is resistant to extinction. Proc Natl Acad Sci USA. 2006;103:18020–18025. doi: 10.1073/pnas.0608398103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feldner MT, Monson CM, Friedman MJ. A critical analysis of approaches to targeted PTSD prevention: current status and theoretically derived future directions. Behav Modif. 2007;31:80–116. doi: 10.1177/0145445506295057. [DOI] [PubMed] [Google Scholar]
- 8.Pavlov IP. Conditioned reflexes. London: Oxford University Press; 1927. [Google Scholar]
- 9.Maren S. Seeking a spotless mind: extinction, deconsolidation, and erasure of fear memory. Neuron. 2011;70:830–845. doi: 10.1016/j.neuron.2011.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Milad MR, Quirk GJ. Fear extinction as a model for translational neuroscience: ten years of progress. Annu Rev Psychol. 2012;63:129–151. doi: 10.1146/annurev.psych.121208.131631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Orsini CA, Maren S. Neural and cellular mechanisms of fear and extinction memory formation. Neurosci Biobehav Rev. 2012;36:1773–1802. doi: 10.1016/j.neubiorev.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tronson NC, Corcoran KA, Jovasevic V, Radulovic J. Fear conditioning and extinction: emotional states encoded by distinct signaling pathways. Trends Neurosci. 2012;35:145–155. doi: 10.1016/j.tins.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agis-Balboa RC, Arcos-Diaz D, Wittnam J, Govindarajan N, Blom K, Burkhardt S, Haladyniak U, Agbemenyah HY, Zovoilis A, Salinas-Riester G, Opitz L, Sananbenesi F, Fischer A. A hippocampal insulin-growth factor 2 pathway regulates the extinction of fear memories. EMBO J. 2011;30:4071–4083. doi: 10.1038/emboj.2011.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sananbenesi F, Fischer A, Wang X, Schrick C, Neve R, Radulovic J, Tsai LH. A hippocampal Cdk5 pathway regulates extinction of contextual fear. Nat Neurosci. 2007;10:1012–1019. doi: 10.1038/nn1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parsons RG, Ressler KJ. Implications of memory modulation for post-traumatic stress and fear disorders. Nat Neurosci. 2013;16:146–153. doi: 10.1038/nn.3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lattal KM, Wood MA. Epigenetics and persistent memory: implications for reconsolidation and silent extinction beyond the zero. Nat Neurosci. 2013;16:124–129. doi: 10.1038/nn.3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGaugh JL. Memory – a century of consolidation. Science. 2000;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- 18.McKenzie S, Eichenbaum H. Consolidation and reconsolidation: two lives of memories? Neuron. 2011;71:224–233. doi: 10.1016/j.neuron.2011.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Monfils MH, Cowansage KK, Klann E, LeDoux JE. Extinction-reconsolidation boundaries: key to persistent attenuation of fear memories. Science. 2009;324:951–955. doi: 10.1126/science.1167975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alberini CM. The role of reconsolidation and the dynamic process of long-term memory formation and storage. Front Behav Neurosci. 2011;5:12. doi: 10.3389/fnbeh.2011.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duvarci S, Nader K. Characterization of fear memory reconsolidation. J Neurosci. 2004;24:9269–9275. doi: 10.1523/JNEUROSCI.2971-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nader K, Schafe GE, Le Doux JE. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000;406:722–726. doi: 10.1038/35021052. [DOI] [PubMed] [Google Scholar]
- 23.Tronson NC, Wiseman SL, Olausson P, Taylor JR. Bidirectional behavioral plasticity of memory reconsolidation depends on amygdalar protein kinase A. Nat Neurosci. 2006;9:167–169. doi: 10.1038/nn1628. [DOI] [PubMed] [Google Scholar]
- 24.Alberini CM. Mechanisms of memory stabilization: are consolidation and reconsolidation similar or distinct processes? Trends Neurosci. 2005;28:51–56. doi: 10.1016/j.tins.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 25.Hupbach A, Gomez R, Hardt O, Nadel L. Reconsolidation of episodic memories: a subtle reminder triggers integration of new information. Learn Mem. 2007;14:47–53. doi: 10.1101/lm.365707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tronson NC, Taylor JR. Molecular mechanisms of memory reconsolidation. Nat Rev Neurosci. 2007;8:262–275. doi: 10.1038/nrn2090. [DOI] [PubMed] [Google Scholar]
- 27.Nicoll RA, Malenka RC. Expression mechanisms underlying NMDA receptor-dependent long-term potentiation. Ann N Y Acad Sci. 1999;868:515–525. doi: 10.1111/j.1749-6632.1999.tb11320.x. [DOI] [PubMed] [Google Scholar]
- 28.Sheinin A, Shavit S, Benveniste M. Subunit specificity and mechanism of action of NMDA partial agonist D-cycloserine. Neuropharmacology. 2001;41:151–158. doi: 10.1016/s0028-3908(01)00073-9. [DOI] [PubMed] [Google Scholar]
- 29.Walker DL, Ressler KJ, Lu KT, Davis M. Facilitation of conditioned fear extinction by systemic administration or intra-amygdala infusions of D-cycloserine as assessed with fear-potentiated startle in rats. J Neurosci. 2002;22:2343–2351. doi: 10.1523/JNEUROSCI.22-06-02343.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hofmann SG. Enhancing exposure-based therapy from a translational research perspective. Behav Res Ther. 2007;45:1987–2001. doi: 10.1016/j.brat.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee JL, Milton AL, Everitt BJ. Reconsolidation and extinction of conditioned fear: inhibition and potentiation. J Neurosci. 2006;26:10051–10056. doi: 10.1523/JNEUROSCI.2466-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eisenberg M, Kobilo T, Berman DE, Dudai Y. Stability of retrieved memory: inverse correlation with trace dominance. Science. 2003;301:1102–1104. doi: 10.1126/science.1086881. [DOI] [PubMed] [Google Scholar]
- 33.Pedreira ME, Maldonado H. Protein synthesis subserves reconsolidation or extinction depending on reminder duration. Neuron. 2003;38:863–869. doi: 10.1016/s0896-6273(03)00352-0. [DOI] [PubMed] [Google Scholar]
- 34.Suzuki A, Josselyn SA, Frankland PW, Masushige S, Silva AJ, Kida S. Memory reconsolidation and extinction have distinct temporal and biochemical signatures. J Neurosci. 2004;24:4787–4795. doi: 10.1523/JNEUROSCI.5491-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Werner-Seidler A, Richardson R. Effects of D-cycloserine on extinction: consequences of prior exposure to imipramine. Biol Psychiatry. 2007;62:1195–1197. doi: 10.1016/j.biopsych.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 36.Kaplan GB, Moore KA. The use of cognitive enhancers in animal models of fear extinction. Pharmacol Biochem Behav. 2011;99:217–228. doi: 10.1016/j.pbb.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 37.Myers KM, Carlezon WA, Jr, Davis M. Glutamate receptors in extinction and extinction-based therapies for psychiatric illness. Neuropsychopharmacology. 2011;36:274–293. doi: 10.1038/npp.2010.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Quirk GJ, Pare D, Richardson R, Herry C, Monfils MH, Schiller D, Vicentic A. Erasing fear memories with extinction training. J Neurosci. 2010;30:14993–14997. doi: 10.1523/JNEUROSCI.4268-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pare D, Duvarci S. Amygdala microcircuits mediating fear expression and extinction. Curr Opin Neurobiol. 2012;22:717–723. doi: 10.1016/j.conb.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu W, Südhof TC. A neural circuit for memory specificity and generalization. Science. 2013;339(6125):1290–1295. doi: 10.1126/science.1229534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- 42.Hugues S, Garcia R. Reorganization of learning-associated prefrontal synaptic plasticity between the recall of recent and remote fear extinction memory. Learn Mem. 2007;14:520–524. doi: 10.1101/lm.625407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Quirk GJ, Likhtik E, Pelletier JG, Pare D. Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. J Neurosci. 2003;23:8800–8807. doi: 10.1523/JNEUROSCI.23-25-08800.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Amano T, Duvarci S, Popa D, Pare D. The fear circuit revisited: contributions of the basal amygdala nuclei to conditioned fear. J Neurosci. 2011;31:15481–15489. doi: 10.1523/JNEUROSCI.3410-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wojtowicz JM. Adult neurogenesis. From circuits to models. Behav Brain Res. 2012;227:490–496. doi: 10.1016/j.bbr.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 47.Squire LR. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychol Rev. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- 48.McClelland JL, McNaughton BL, O’Reilly RC. Why there are complementary learning systems in the hippocampus and neocortex: insights from the successes and failures of connectionist models of learning and memory. Psychol Rev. 1995;102:419–457. doi: 10.1037/0033-295X.102.3.419. [DOI] [PubMed] [Google Scholar]
- 49.Squire LR, Alvarez P. Retrograde amnesia and memory consolidation: a neurobiological perspective. Curr Opin Neurobiol. 1995;5:169–177. doi: 10.1016/0959-4388(95)80023-9. [DOI] [PubMed] [Google Scholar]
- 50.Frankland PW, Bontempi B. The organization of recent and remote memories. Nat Rev Neurosci. 2005;6:119–130. doi: 10.1038/nrn1607. [DOI] [PubMed] [Google Scholar]
- 51.Wang SH, Teixeira CM, Wheeler AL, Frankland PW. The precision of remote context memories does not require the hippocampus. Nat Neurosci. 2009;12:253–255. doi: 10.1038/nn.2263. [DOI] [PubMed] [Google Scholar]
- 52.Morris RG, Garrud P, Rawlins JN, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- 53.Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256:675–677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- 54.Gale GD, Anagnostaras SG, Godsil BP, Mitchell S, Nozawa T, Sage JR, Wiltgen B, Fanselow MS. Role of the basolateral amygdala in the storage of fear memories across the adult lifetime of rats. J Neurosci. 2004;24:3810–3815. doi: 10.1523/JNEUROSCI.4100-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fischer A, Sananbenesi F, Schrick C, Spiess J, Radulovic J. Distinct roles of hippocampal de novo protein synthesis and actin rearrangement in extinction of contextual fear. J Neurosci. 2004;24:1962–1966. doi: 10.1523/JNEUROSCI.5112-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lattal KM, Radulovic J, Lukowiak K. Extinction: (corrected) does it or doesn’t it? The requirement of altered gene activity and new protein synthesis. Biol Psychiatry. 2006;60:344–351. doi: 10.1016/j.biopsych.2006.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ji J, Maren S. Hippocampal involvement in contextual modulation of fear extinction. Hippocampus. 2007;17:749–758. doi: 10.1002/hipo.20331. [DOI] [PubMed] [Google Scholar]
- 58.Zelikowsky M, Bissiere S, Fanselow MS. Contextual fear memories formed in the absence of the dorsal hippocampus decay across time. J Neurosci. 2012;32:3393–3397. doi: 10.1523/JNEUROSCI.4339-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bannerman DM, Rawlins JN, McHugh SB, Deacon RM, Yee BK, Bast T, Zhang WN, Pothuizen HH, Feldon J. Regional dissociations within the hippocampus – memory and anxiety. Neurosci Biobehav Rev. 2004;28:273–283. doi: 10.1016/j.neubiorev.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 60.Thompson CL, Pathak SD, Jeromin A, Ng LL, MacPherson CR, Mortrud MT, Cusick A, Riley ZL, Sunkin SM, Bernard A, Puchalski RB, Gage FH, Jones AR, Bajic VB, Hawrylycz MJ, Lein ES. Genomic anatomy of the hippocampus. Neuron. 2008;60:1010–1021. doi: 10.1016/j.neuron.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 61.van Strien NM, Cappaert NL, Witter MP. The anatomy of memory: an interactive overview of the parahippocampal–hippocampal network. Nat Rev Neurosci. 2009;10:272–282. doi: 10.1038/nrn2614. [DOI] [PubMed] [Google Scholar]
- 62.Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65:7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schmidt B, Marrone DF, Markus EJ. Disambiguating the similar: the dentate gyrus and pattern separation. Behav Brain Res. 2011;226:56–65. doi: 10.1016/j.bbr.2011.08.039. [DOI] [PubMed] [Google Scholar]
- 64.Hawley DF, Morch K, Christie BR, Leasure JL. Differential response of hippocampal subregions to stress and learning. PLoS One. 2012;7:e53126. doi: 10.1371/journal.pone.0053126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kheirbek MA, Drew LJ, Burghardt NS, Costantini DO, Tannenholz L, Ahmari SE, Zeng H, Fenton AA, Hen R. Differential control of learning and anxiety along the dorsoventral axis of the dentate gyrus. Neuron. 2013;77(5):955–68. doi: 10.1016/j.neuron.2012.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kerimoglu C, Agis-Balboa RC, Kranz A, Stilling RM, Bahari-Javan S, Benito-Garagorri E, Halder R, Burkhardt S, Stewart AF, Fischer A. Histone-methyltransferase MLL2 (KMT2B) is required for memory formation in mice. J Neurosci. 2013;33:3452–3464. doi: 10.1523/JNEUROSCI.3356-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zovoilis A, Agbemenyah HY, Agis-Balboa RC, Stilling RM, Edbauer D, Rao P, Farinelli L, Delalle I, Schmitt A, Falkai P, Bahari-Javan S, Burkhardt S, Sananbenesi F, Fischer A. MicroRNA-34c is a novel target to treat dementias. EMBO J. 2011;30:4299–4308. doi: 10.1038/emboj.2011.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pitkanen A, Pikkarainen M, Nurminen N, Ylinen A. Reciprocal connections between the amygdala and the hippocampal formation, perirhinal cortex, and postrhinal cortex in rat. A review. Ann N Y Acad Sci. 2000;911:369–391. doi: 10.1111/j.1749-6632.2000.tb06738.x. [DOI] [PubMed] [Google Scholar]
- 69.Jay TM, Witter MP. Distribution of hippocampal CA1 and subicular efferents in the prefrontal cortex of the rat studied by means of anterograde transport of Phaseolus vulgaris-leucoagglutinin. J Comp Neurol. 1991;313:574–586. doi: 10.1002/cne.903130404. [DOI] [PubMed] [Google Scholar]
- 70.Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- 71.Hoover WB, Vertes RP. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct Funct. 2007;212:149–179. doi: 10.1007/s00429-007-0150-4. [DOI] [PubMed] [Google Scholar]
- 72.Canteras NS, Swanson LW. Projections of the ventral subiculum to the amygdala, septum, and hypothalamus: a PHAL anterograde tract-tracing study in the rat. J Comp Neurol. 1992;324:180–194. doi: 10.1002/cne.903240204. [DOI] [PubMed] [Google Scholar]
- 73.Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- 74.Treves A, Tashiro A, Witter ME, Moser EI. What is the mammalian dentate gyrus good for? Neuroscience. 2008;154:1155–1172. doi: 10.1016/j.neuroscience.2008.04.073. [DOI] [PubMed] [Google Scholar]
- 75.Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 76.Sahay A, Wilson DA, Hen R. Pattern separation: a common function for new neurons in hippocampus and olfactory bulb. Neuron. 2011;70:582–588. doi: 10.1016/j.neuron.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Aimone JB, Deng W, Gage FH. Resolving new memories: a critical look at the dentate gyrus, adult neurogenesis, and pattern separation. Neuron. 2011;70:589–596. doi: 10.1016/j.neuron.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hsieh J. Orchestrating transcriptional control of adult neurogenesis. Genes Dev. 2012;26:1010–1021. doi: 10.1101/gad.187336.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jobe EM, McQuate AL, Zhao X. Crosstalk among epigenetic pathways regulates neurogenesis. Front Neurosci. 2012;6:59. doi: 10.3389/fnins.2012.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Faigle R, Song H. Signaling mechanisms regulating adult neural stem cells and neurogenesis. Biochim Biophys Acta. 2013;1830:2435–2448. doi: 10.1016/j.bbagen.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Altman J. Autoradiographic investigation of cell proliferation in the brains of rats and cats. Anat Rec. 1963;145:573–591. doi: 10.1002/ar.1091450409. [DOI] [PubMed] [Google Scholar]
- 82.Nottebohm F. The road we travelled: discovery, choreography, and significance of brain replaceable neurons. Ann N Y Acad Sci. 2004;1016:628–658. doi: 10.1196/annals.1298.027. [DOI] [PubMed] [Google Scholar]
- 83.Kempermann G, Wiskott L, Gage FH. Functional significance of adult neurogenesis. Curr Opin Neurobiol. 2004;14:186–191. doi: 10.1016/j.conb.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 84.Jessberger S, Toni N, Clemenson GD, Jr, Ray J, Gage FH. Directed differentiation of hippocampal stem/progenitor cells in the adult brain. Nat Neurosci. 2008;11:888–893. doi: 10.1038/nn.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lang MF, Shi Y. Dynamic roles of microRNAs in neurogenesis. Front Neurosci. 2012;6:71. doi: 10.3389/fnins.2012.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Toni N, Laplagne DA, Zhao C, Lombardi G, Ribak CE, Gage FH, Schinder AF. Neurons born in the adult dentate gyrus form functional synapses with target cells. Nat Neurosci. 2008;11:901–907. doi: 10.1038/nn.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schmidt-Hieber C, Jonas P, Bischofberger J. Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature. 2004;429:184–187. doi: 10.1038/nature02553. [DOI] [PubMed] [Google Scholar]
- 88.Ge S, Yang CH, Hsu KS, Ming GL, Song H. A critical period for enhanced synaptic plasticity in newly generated neurons of the adult brain. Neuron. 2007;54:559–566. doi: 10.1016/j.neuron.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tashiro A, Makino H, Gage FH. Experience-specific functional modification of the dentate gyrus through adult neurogenesis: a critical period during an immature stage. J Neurosci. 2007;27:3252–3259. doi: 10.1523/JNEUROSCI.4941-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Deng W, Aimone JB, Gage FH. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci. 2010;11:339–350. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jessberger S, Kempermann G. Adult-born hippocampal neurons mature into activity-dependent responsiveness. Eur J Neurosci. 2003;18:2707–2712. doi: 10.1111/j.1460-9568.2003.02986.x. [DOI] [PubMed] [Google Scholar]
- 92.Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- 93.van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- 94.Ge S, Goh EL, Sailor KA, Kitabatake Y, Ming GL, Song H. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature. 2006;439:589–593. doi: 10.1038/nature04404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lucassen PJ, Meerlo P, Naylor AS, van Dam AM, Dayer AG, Fuchs E, Oomen CA, Czeh B. Regulation of adult neurogenesis by stress, sleep disruption, exercise and inflammation: implications for depression and antidepressant action. Eur Neuropsychopharmacol. 2010;20:1–17. doi: 10.1016/j.euroneuro.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 96.Kee N, Teixeira CM, Wang AH, Frankland PW. Preferential incorporation of adult-generated granule cells into spatial memory networks in the dentate gyrus. Nat Neurosci. 2007;10:355–362. doi: 10.1038/nn1847. [DOI] [PubMed] [Google Scholar]
- 97.Toni N, Teng EM, Bushong EA, Aimone JB, Zhao C, Consiglio A, van Praag H, Martone ME, Ellisman MH, Gage FH. Synapse formation on neurons born in the adult hippocampus. Nat Neurosci. 2007;10:727–734. doi: 10.1038/nn1908. [DOI] [PubMed] [Google Scholar]
- 98.Zhao C, Teng EM, Summers RG, Jr, Ming GL, Gage FH. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J Neurosci. 2006;26:3–11. doi: 10.1523/JNEUROSCI.3648-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ. Learning enhances adult neurogenesis in the hippocampal formation. Nat Neurosci. 1999;2:260–265. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- 100.Saxe MD, Battaglia F, Wang JW, Malleret G, David DJ, Monckton JE, Garcia AD, Sofroniew MV, Kandel ER, Santarelli L, Hen R, Drew MR. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc Natl Acad Sci USA. 2006;103:17501–17506. doi: 10.1073/pnas.0607207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pham K, McEwen BS, Ledoux JE, Nader K. Fear learning transiently impairs hippocampal cell proliferation. Neuroscience. 2005;130:17–24. doi: 10.1016/j.neuroscience.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 102.Fotuhi M, Do D, Jack C. Modifiable factors that alter the size of the hippocampus with ageing. Nat Rev Neurol. 2012;8:189–202. doi: 10.1038/nrneurol.2012.27. [DOI] [PubMed] [Google Scholar]
- 103.Bremner JD, Elzinga B, Schmahl C, Vermetten E. Structural and functional plasticity of the human brain in posttraumatic stress disorder. Prog Brain Res. 2008;167:171–186. doi: 10.1016/S0079-6123(07)67012-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 105.Pollak DD, Monje FJ, Zuckerman L, Denny CA, Drew MR, Kandel ER. An animal model of a behavioral intervention for depression. Neuron. 2008;60:149–161. doi: 10.1016/j.neuron.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Takemura NU, Kato N. Adult neurogenesis and systemic adaptation: animal experiments and clinical perspectives for PTSD. Prog Brain Res. 2008;167:99–109. doi: 10.1016/S0079-6123(07)67007-1. [DOI] [PubMed] [Google Scholar]
- 107.Siegmund A, Wotjak CT. A mouse model of posttraumatic stress disorder that distinguishes between conditioned and sensitised fear. J Psychiatr Res. 2007;41:848–860. doi: 10.1016/j.jpsychires.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 108.Feng R, Rampon C, Tang YP, Shrom D, Jin J, Kyin M, Sopher B, Miller MW, Ware CB, Martin GM, Kim SH, Langdon RB, Sisodia SS, Tsien JZ. Deficient neurogenesis in forebrain-specific presenilin-1 knockout mice is associated with reduced clearance of hippocampal memory traces. Neuron. 2001;32:911–926. doi: 10.1016/s0896-6273(01)00523-2. [DOI] [PubMed] [Google Scholar]
- 109.Ko HG, Jang DJ, Son J, Kwak C, Choi JH, Ji YH, Lee YS, Son H, Kaang BK. Effect of ablated hippocampal neurogenesis on the formation and extinction of contextual fear memory. Mol Brain. 2009;2:1. doi: 10.1186/1756-6606-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Deng W, Saxe MD, Gallina IS, Gage FH. Adult-born hippocampal dentate granule cells undergoing maturation modulate learning and memory in the brain. J Neurosci. 2009;29:13532–13542. doi: 10.1523/JNEUROSCI.3362-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Noonan MA, Bulin SE, Fuller DC, Eisch AJ. Reduction of adult hippocampal neurogenesis confers vulnerability in an animal model of cocaine addiction. J Neurosci. 2010;30:304–315. doi: 10.1523/JNEUROSCI.4256-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Schipper P, Kiliaan AJ, Homberg JR. A mixed polyunsaturated fatty acid diet normalizes hippocampal neurogenesis and reduces anxiety in serotonin transporter knockout rats. Behav Pharmacol. 2011;22:324–334. doi: 10.1097/FBP.0b013e328347881b. [DOI] [PubMed] [Google Scholar]
- 113.Pan YW, Chan GC, Kuo CT, Storm DR, Xia Z. Inhibition of adult neurogenesis by inducible and targeted deletion of ERK5 mitogen-activated protein kinase specifically in adult neurogenic regions impairs contextual fear extinction and remote fear memory. J Neurosci. 2012;32:6444–6455. doi: 10.1523/JNEUROSCI.6076-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lehtinen MK, Zappaterra MW, Chen X, Yang YJ, Hill AD, Lun M, Maynard T, Gonzalez D, Kim S, Ye P, D’Ercole AJ, Wong ET, LaMantia AS, Walsh CA. The cerebrospinal fluid provides a proliferative niche for neural progenitor cells. Neuron. 2011;69:893–905. doi: 10.1016/j.neuron.2011.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.McHugh TJ, Jones MW, Quinn JJ, Balthasar N, Coppari R, Elmquist JK, Lowell BB, Fanselow MS, Wilson MA, Tonegawa S. Dentate gyrus NMDA receptors mediate rapid pattern separation in the hippocampal network. Science. 2007;317:94–99. doi: 10.1126/science.1140263. [DOI] [PubMed] [Google Scholar]
- 116.Clelland CD, Choi M, Romberg C, Clemenson GD, Jr, Fragniere A, Tyers P, Jessberger S, Saksida LM, Barker RA, Gage FH, Bussey TJ. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325:210–213. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sahay A, Scobie KN, Hill AS, O’Carroll CM, Kheirbek MA, Burghardt NS, Fenton AA, Dranovsky A, Hen R. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature. 2011;472:466–470. doi: 10.1038/nature09817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Aimone JB, Wiles J, Gage FH. Potential role for adult neurogenesis in the encoding of time in new memories. Nat Neurosci. 2006;9:723–727. doi: 10.1038/nn1707. [DOI] [PubMed] [Google Scholar]
- 119.Aimone JB, Wiles J, Gage FH. Computational influence of adult neurogenesis on memory encoding. Neuron. 2009;61:187–202. doi: 10.1016/j.neuron.2008.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tronel S, Belnoue L, Grosjean N, Revest JM, Piazza PV, Koehl M, Abrous DN. Adult-born neurons are necessary for extended contextual discrimination. Hippocampus. 2012;22:292–298. doi: 10.1002/hipo.20895. [DOI] [PubMed] [Google Scholar]
- 121.Nakashiba T, Cushman JD, Pelkey KA, Renaudineau S, Buhl DL, McHugh TJ, Barrera VR, Chittajallu R, Iwamoto KS, McBain CJ, Fanselow MS, Tonegawa S. Young dentate granule cells mediate pattern separation, whereas old granule cells facilitate pattern completion. Cell. 2012;149:188–201. doi: 10.1016/j.cell.2012.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Niibori Y, Yu TS, Epp JR, Akers KG, Josselyn SA, Frankland PW. Suppression of adult neurogenesis impairs population coding of similar contexts in hippocampal CA3 region. Nat Commun. 2012;3:1253. doi: 10.1038/ncomms2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Fernandez AM, Torres-Aleman I. The many faces of insulin-like peptide signalling in the brain. Nat Rev Neurosci. 2012;13:225–239. doi: 10.1038/nrn3209. [DOI] [PubMed] [Google Scholar]
- 124.Harris JA, Westbrook RF. Evidence that GABA transmission mediates context-specific extinction of learned fear. Psychopharmacology. 1998;140:105–115. doi: 10.1007/s002130050745. [DOI] [PubMed] [Google Scholar]
- 125.Corcoran KA, Desmond TJ, Frey KA, Maren S. Hippocampal inactivation disrupts the acquisition and contextual encoding of fear extinction. J Neurosci. 2005;25:8978–8987. doi: 10.1523/JNEUROSCI.2246-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.McGaugh JL, Castellano C, Brioni J. Picrotoxin enhances latent extinction of conditioned fear. Behav Neurosci. 1990;104:264–267. doi: 10.1037//0735-7044.104.2.264. [DOI] [PubMed] [Google Scholar]
- 127.Berlau DJ, McGaugh JL. Enhancement of extinction memory consolidation: the role of the noradrenergic and GABAergic systems within the basolateral amygdala. Neurobiol Learn Mem. 2006;86:123–132. doi: 10.1016/j.nlm.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 128.Pezze MA, Feldon J. Mesolimbic dopaminergic pathways in fear conditioning. Prog Neurobiol. 2004;74:301–320. doi: 10.1016/j.pneurobio.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 129.Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, Hermann H, Tang J, Hofmann C, Zieglgansberger W, Di Marzo V, Lutz B. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–534. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- 130.Sweatt JD. The neuronal MAP kinase cascade: a biochemical signal integration system subserving synaptic plasticity and memory. J Neurochem. 2001;76:1–10. doi: 10.1046/j.1471-4159.2001.00054.x. [DOI] [PubMed] [Google Scholar]
- 131.Arnaldez FI, Helman LJ. Targeting the insulin growth factor receptor 1. Hematol Oncol Clin North Am. 2012;26:527–542. doi: 10.1016/j.hoc.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yang YL, Lu KT. Facilitation of conditioned fear extinction by D-cycloserine is mediated by mitogen-activated protein kinase and phosphatidylinositol 3-kinase cascades and requires de novo protein synthesis in basolateral nucleus of amygdala. Neuroscience. 2005;134:247–260. doi: 10.1016/j.neuroscience.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 133.Koshibu K, Graff J, Beullens M, Heitz FD, Berchtold D, Russig H, Farinelli M, Bollen M, Mansuy IM. Protein phosphatase 1 regulates the histone code for long-term memory. J Neurosci. 2009;29:13079–13089. doi: 10.1523/JNEUROSCI.3610-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Filipkowski RK, Knapska E, Kaczmarek L. c-Fos and Zif268 in learning and memory – studies on expression and function. In: Pinaud R, Tremere LA, editors. Immediate early genes in sensory processing, cognitive performance and neurological disorders. New York: Springer; 2006. pp. 137–158. [Google Scholar]
- 135.Wei W, Coelho CM, Li X, Marek R, Yan S, Anderson S, Meyers D, Mukherjee C, Sbardella G, Castellano S, Milite C, Rotili D, Mai A, Cole PA, Sah P, Kobor MS, Bredy TW. p300/CBP-associated factor selectively regulates the extinction of conditioned fear. J Neurosci. 2012;32:11930–11941. doi: 10.1523/JNEUROSCI.0178-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Tronson NC, Schrick C, Guzman YF, Huh KH, Srivastava DP, Penzes P, Guedea AL, Gao C, Radulovic J. Segregated populations of hippocampal principal CA1 neurons mediating conditioning and extinction of contextual fear. J Neurosci. 2009;29:3387–3394. doi: 10.1523/JNEUROSCI.5619-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Fiorenza NG, Sartor D, Myskiw JC, Izquierdo I. Treatment of fear memories: interactions between extinction and reconsolidation. An Acad Bras Cienc. 2011;83:1363–1372. doi: 10.1590/s0001-37652011000400023. [DOI] [PubMed] [Google Scholar]
- 138.Soeter M, Kindt M. Disrupting reconsolidation: pharmacological and behavioral manipulations. Learn Mem. 2011;18:357–366. doi: 10.1101/lm.2148511. [DOI] [PubMed] [Google Scholar]
- 139.Bernard A, Lubbers LS, Tanis KQ, Luo R, Podtelezhnikov AA, Finney EM, McWhorter MM, Serikawa K, Lemon T, Morgan R, Copeland C, Smith K, Cullen V, Davis-Turak J, Lee CK, Sunkin SM, Loboda AP, Levine DM, Stone DJ, Hawrylycz MJ, Roberts CJ, Jones AR, Geschwind DH, Lein ES. Transcriptional architecture of the primate neocortex. Neuron. 2012;73:1083–1099. doi: 10.1016/j.neuron.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Oldham MC, Konopka G, Iwamoto K, Langfelder P, Kato T, Horvath S, Geschwind DH. Functional organization of the transcriptome in human brain. Nat Neurosci. 2008;11:1271–1282. doi: 10.1038/nn.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Winden KD, Oldham MC, Mirnics K, Ebert PJ, Swan CH, Levitt P, Rubenstein JL, Horvath S, Geschwind DH. The organization of the transcriptional network in specific neuronal classes. Mol Syst Biol. 2009;5:291. doi: 10.1038/msb.2009.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.de Carvalho Myskiw J, Benetti F, Izquierdo I. Behavioral tagging of extinction learning. Proc Natl Acad Sci USA. 2013;110:1071–1076. doi: 10.1073/pnas.1220875110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Bracko O, Singer T, Aigner S, Knobloch M, Winner B, Ray J, Clemenson GD, Jr, Suh H, Couillard-Despres S, Aigner L, Gage FH, Jessberger S. Gene expression profiling of neural stem cells and their neuronal progeny reveals IGF2 as a regulator of adult hippocampal neurogenesis. J Neurosci. 2012;32:3376–3387. doi: 10.1523/JNEUROSCI.4248-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bonn S, Zinzen RP, Girardot C, Gustafson EH, Perez-Gonzalez A, Delhomme N, Ghavi-Helm Y, Wilczynski B, Riddell A, Furlong EE. Tissue-specific analysis of chromatin state identifies temporal signatures of enhancer activity during embryonic development. Nat Genet. 2012;44:148–156. doi: 10.1038/ng.1064. [DOI] [PubMed] [Google Scholar]
- 145.Tye KM, Deisseroth K. Optogenetic investigation of neural circuits underlying brain disease in animal models. Nat Rev Neurosci. 2012;13:251–266. doi: 10.1038/nrn3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bondy CA, Lee WH. Patterns of insulin-like growth factor and IGF receptor gene expression in the brain. Functional implications. Ann N Y Acad Sci. 1993;692:33–43. doi: 10.1111/j.1749-6632.1993.tb26203.x. [DOI] [PubMed] [Google Scholar]
- 147.D’Ercole AJ, Ye P, Calikoglu AS, Gutierrez-Ospina G. The role of the insulin-like growth factors in the central nervous system. Mol Neurobiol. 1996;13:227–255. doi: 10.1007/BF02740625. [DOI] [PubMed] [Google Scholar]
- 148.Dechiara TM, Efstratiadis A, Robertson EJ. A growth-deficiency phenotype in heterozygous mice carrying an insulin-like growth factor-II gene disrupted by targeting. Nature. 1990;345:78–80. doi: 10.1038/345078a0. [DOI] [PubMed] [Google Scholar]
- 149.DeChiara TM, Robertson EJ, Efstratiadis A. Parental imprinting of the mouse insulin-like growth factor II gene. Cell. 1991;64:849–859. doi: 10.1016/0092-8674(91)90513-x. [DOI] [PubMed] [Google Scholar]
- 150.Baker J, Liu JP, Robertson EJ, Efstratiadis A. Role of insulin-like growth factors in embryonic and postnatal growth. Cell. 1993;75:73–82. [PubMed] [Google Scholar]
- 151.Sim FJ, Keyoung HM, Goldman JE, Kim DK, Jung HW, Roy NS, Goldman SA. Neurocytoma is a tumor of adult neuronal progenitor cells. J Neurosci. 2006;26:12544–12555. doi: 10.1523/JNEUROSCI.0829-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ye P, D’Ercole AJ. Insulin-like growth factor actions during development of neural stem cells and progenitors in the central nervous system. J Neurosci Res. 2006;83:1–6. doi: 10.1002/jnr.20688. [DOI] [PubMed] [Google Scholar]
- 153.Sutter NB, Bustamante CD, Chase K, Gray MM, Zhao K, Zhu L, Padhukasahasram B, Karlins E, Davis S, Jones PG, Quignon P, Johnson GS, Parker HG, Fretwell N, Mosher DS, Lawler DF, Satyaraj E, Nordborg M, Lark KG, Wayne RK, Ostrander EA. A single IGF1 allele is a major determinant of small size in dogs. Science. 2007;316:112–115. doi: 10.1126/science.1137045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Alberini CM, Chen DY. Memory enhancement: consolidation, reconsolidation and insulin-like growth factor 2. Trends Neurosci. 2012;35:274–283. doi: 10.1016/j.tins.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Bondy C, Werner H, Roberts CT, Jr, LeRoith D. Cellular pattern of type-I insulin-like growth factor receptor gene expression during maturation of the rat brain: comparison with insulin-like growth factors I and II. Neuroscience. 1992;46:909–923. doi: 10.1016/0306-4522(92)90193-6. [DOI] [PubMed] [Google Scholar]
- 156.Hodge RD, D’Ercole AJ, O’Kusky JR. Insulin-like growth factor-I accelerates the cell cycle by decreasing G1 phase length and increases cell cycle reentry in the embryonic cerebral cortex. J Neurosci. 2004;24:10201–10210. doi: 10.1523/JNEUROSCI.3246-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Drago J, Murphy M, Carroll SM, Harvey RP, Bartlett PF. Fibroblast growth factor-mediated proliferation of central nervous system precursors depends on endogenous production of insulin-like growth factor I. Proc Natl Acad Sci USA. 1991;88:2199–2203. doi: 10.1073/pnas.88.6.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hodge RD, D’Ercole AJ, O’Kusky JR. Increased expression of insulin-like growth factor-I (IGF-I) during embryonic development produces neocortical overgrowth with differentially greater effects on specific cytoarchitectonic areas and cortical layers. Brain Res Dev Brain Res. 2005;154:227–237. doi: 10.1016/j.devbrainres.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 159.Ozdinler PH, Macklis JD. IGF-I specifically enhances axon outgrowth of corticospinal motor neurons. Nat Neurosci. 2006;9:1371–1381. doi: 10.1038/nn1789. [DOI] [PubMed] [Google Scholar]
- 160.Sosa L, Dupraz S, Laurino L, Bollati F, Bisbal M, Caceres A, Pfenninger KH, Quiroga S. IGF-1 receptor is essential for the establishment of hippocampal neuronal polarity. Nat Neurosci. 2006;9:993–995. doi: 10.1038/nn1742. [DOI] [PubMed] [Google Scholar]
- 161.Oishi K, Watatani K, Itoh Y, Okano H, Guillemot F, Nakajima K, Gotoh Y. Selective induction of neocortical GABAergic neurons by the PDK1-Akt pathway through activation of Mash1. Proc Natl Acad Sci USA. 2009;106:13064–13069. doi: 10.1073/pnas.0808400106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hurtado-Chong A, Yusta-Boyo MJ, Vergano-Vera E, Bulfone A, de Pablo F, Vicario-Abejon C. IGF-I promotes neuronal migration and positioning in the olfactory bulb and the exit of neuroblasts from the subventricular zone. Eur J Neurosci. 2009;30:742–755. doi: 10.1111/j.1460-9568.2009.06870.x. [DOI] [PubMed] [Google Scholar]
- 163.O’Kusky JR, Ye P, D’Ercole AJ. Insulin-like growth factor-I promotes neurogenesis and synaptogenesis in the hippocampal dentate gyrus during postnatal development. J Neurosci. 2000;20:8435–8442. doi: 10.1523/JNEUROSCI.20-22-08435.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Torres-Aleman I, Pons S, Arevalo MA. The insulin-like growth factor I system in the rat cerebellum: developmental regulation and role in neuronal survival and differentiation. J Neurosci Res. 1994;39:117–126. doi: 10.1002/jnr.490390202. [DOI] [PubMed] [Google Scholar]
- 165.Desai M, Li T, Ross MG. Fetal hypothalamic neuroprogenitor cell culture: preferential differentiation paths induced by leptin and insulin. Endocrinology. 2011;152:3192–3201. doi: 10.1210/en.2010-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ayer-le Lievre C, Stahlbom PA, Sara VR. Expression of IGF-I and -II mRNA in the brain and craniofacial region of the rat fetus. Development. 1991;111:105–115. doi: 10.1242/dev.111.1.105. [DOI] [PubMed] [Google Scholar]
- 167.Konishi Y, Takahashi K, Chui DH, Rosenfeld RG, Himeno M, Tabira T. Insulin-like growth factor II promotes in vitro cholinergic development of mouse septal neurons: comparison with the effects of insulin-like growth factor I. Brain Res. 1994;649:53–61. doi: 10.1016/0006-8993(94)91048-0. [DOI] [PubMed] [Google Scholar]
- 168.Hartnett L, Glynn C, Nolan CM, Grealy M, Byrnes L. Insulin-like growth factor-2 regulates early neural and cardiovascular system development in zebrafish embryos. Int J Dev Biol. 2010;54:573–583. doi: 10.1387/ijdb.092922lh. [DOI] [PubMed] [Google Scholar]
- 169.Barres BA, Hart IK, Coles HS, Burne JF, Voyvodic JT, Richardson WD, Raff MC. Cell death and control of cell survival in the oligodendrocyte lineage. Cell. 1992;70:31–46. doi: 10.1016/0092-8674(92)90531-g. [DOI] [PubMed] [Google Scholar]
- 170.Cui QL, Zheng WH, Quirion R, Almazan G. Inhibition of Src-like kinases reveals Akt-dependent and -independent pathways in insulin-like growth factor I-mediated oligodendrocyte progenitor survival. J Biol Chem. 2005;280:8918–8928. doi: 10.1074/jbc.M414267200. [DOI] [PubMed] [Google Scholar]
- 171.Kappeler L, De Magalhaes FC, Dupont J, Leneuve P, Cervera P, Perin L, Loudes C, Blaise A, Klein R, Epelbaum J, Le Bouc Y, Holzenberger M. Brain IGF-1 receptors control mammalian growth and lifespan through a neuroendocrine mechanism. PLoS Biol. 2008;6:e254. doi: 10.1371/journal.pbio.0060254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.D’ercole AJ, Ye P. Expanding the mind: insulin-like growth factor I and brain development. Endocrinology. 2008;149:5958–5962. doi: 10.1210/en.2008-0920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Liu W, Ye P, O’Kusky JR, D’Ercole AJ. Type 1 insulin-like growth factor receptor signaling is essential for the development of the hippocampal formation and dentate gyrus. J Neurosci Res. 2009;87:2821–2832. doi: 10.1002/jnr.22129. [DOI] [PubMed] [Google Scholar]
- 174.Ye P, Popken GJ, Kemper A, McCarthy K, Popko B, D’Ercole AJ. Astrocyte-specific overexpression of insulin-like growth factor-I promotes brain overgrowth and glial fibrillary acidic protein expression. J Neurosci Res. 2004;78:472–484. doi: 10.1002/jnr.20288. [DOI] [PubMed] [Google Scholar]
- 175.Mairet-Coello G, Tury A, DiCicco-Bloom E. Insulin-like growth factor-1 promotes G(1)/S cell cycle progression through bidirectional regulation of cyclins and cyclin-dependent kinase inhibitors via the phosphatidylinositol 3-kinase/Akt pathway in developing rat cerebral cortex. J Neurosci. 2009;29:775–788. doi: 10.1523/JNEUROSCI.1700-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Walter HJ, Berry M, Hill DJ, Logan A. Spatial and temporal changes in the insulin-like growth factor (IGF) axis indicate autocrine/paracrine actions of IGF-I within wounds of the rat brain. Endocrinology. 1997;138:3024–3034. doi: 10.1210/endo.138.7.5284. [DOI] [PubMed] [Google Scholar]
- 177.Carro E, Torres-Aleman I. The role of insulin and insulin-like growth factor I in the molecular and cellular mechanisms underlying the pathology of Alzheimer’s disease. Eur J Pharmacol. 2004;490:127–133. doi: 10.1016/j.ejphar.2004.02.050. [DOI] [PubMed] [Google Scholar]
- 178.Brown J, Jones EY, Forbes BE. Interactions of IGF-II with the IGF2R/cation-independent mannose-6-phosphate receptor mechanism and biological outcomes. Vitam Horm. 2009;80:699–719. doi: 10.1016/S0083-6729(08)00625-0. [DOI] [PubMed] [Google Scholar]
- 179.Hawkes C, Kar S. The insulin-like growth factor-II/mannose-6-phosphate receptor: structure, distribution and function in the central nervous system. Brain Res Brain Res Rev. 2004;44:117–140. doi: 10.1016/j.brainresrev.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 180.Poiraudeau S, Lieberherr M, Kergosie N, Corvol MT. Different mechanisms are involved in intracellular calcium increase by insulin-like growth factors 1 and 2 in articular chondrocytes: voltage-gated calcium channels, and/or phospholipase C coupled to a pertussis-sensitive G-protein. J Cell Biochem. 1997;64:414–422. [PubMed] [Google Scholar]
- 181.Hawkes C, Jhamandas JH, Harris KH, Fu W, MacDonald RG, Kar S. Single transmembrane domain insulin-like growth factor-II/mannose-6-phosphate receptor regulates central cholinergic function by activating a G-protein-sensitive, protein kinase C-dependent pathway. J Neurosci. 2006;26:585–596. doi: 10.1523/JNEUROSCI.2730-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Chen DY, Stern SA, Garcia-Osta A, Saunier-Rebori B, Pollonini G, Bambah-Mukku D, Blitzer RD, Alberini CM. A critical role for IGF-II in memory consolidation and enhancement. Nature. 2011;469:491–497. doi: 10.1038/nature09667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kim HS, Nagalla SR, Oh Y, Wilson E, Roberts CT, Jr, Rosenfeld RG. Identification of a family of low-affinity insulin-like growth factor binding proteins (IGFBPs): characterization of connective tissue growth factor as a member of the IGFBP superfamily. Proc Natl Acad Sci USA. 1997;94:12981–12986. doi: 10.1073/pnas.94.24.12981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Honegger B, Galic M, Kohler K, Wittwer F, Brogiolo W, Hafen E, Stocker H. Imp-L2, a putative homolog of vertebrate IGF-binding protein 7, counteracts insulin signaling in Drosophila and is essential for starvation resistance. J Biol. 2008;7:10. doi: 10.1186/jbiol72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Firth SM, Baxter RC. Cellular actions of the insulin-like growth factor binding proteins. Endocr Rev. 2002;23:824–854. doi: 10.1210/er.2001-0033. [DOI] [PubMed] [Google Scholar]
- 186.Evdokimova V, Tognon CE, Benatar T, Yang W, Krutikov K, Pollak M, Sorensen PH, Seth A. IGFBP7 binds to the IGF-1 receptor and blocks its activation by insulin-like growth factors. Sci Signal. 2012;5:ra92. doi: 10.1126/scisignal.2003184. [DOI] [PubMed] [Google Scholar]
- 187.Ocrant I, Fay CT, Parmelee JT. Characterization of insulin-like growth factor binding proteins produced in the rat central nervous system. Endocrinology. 1990;127:1260–1267. doi: 10.1210/endo-127-3-1260. [DOI] [PubMed] [Google Scholar]
- 188.Hwa V, Oh Y, Rosenfeld RG. The insulin-like growth factor-binding protein (IGFBP) superfamily. Endocr Rev. 1999;20:761–787. doi: 10.1210/edrv.20.6.0382. [DOI] [PubMed] [Google Scholar]
- 189.Lee WH, Wang GM, Seaman LB, Vannucci SJ. Coordinate IGF-I and IGFBP5 gene expression in perinatal rat brain after hypoxia-ischemia. J Cereb Blood Flow Metab. 1996;16:227–236. doi: 10.1097/00004647-199603000-00007. [DOI] [PubMed] [Google Scholar]
- 190.Hynes MA, Brooks PJ, Van Wyk JJ, Lund PK. Insulin-like growth factor II messenger ribonucleic acids are synthesized in the choroid plexus of the rat brain. Mol Endocrinol. 1988;2:47–54. doi: 10.1210/mend-2-1-47. [DOI] [PubMed] [Google Scholar]
- 191.Stenvers KL, Zimmermann EM, Gallagher M, Lund PK. Expression of insulin-like growth factor binding protein-4 and -5 mRNAs in adult rat forebrain. J Comp Neurol. 1994;339:91–105. doi: 10.1002/cne.903390109. [DOI] [PubMed] [Google Scholar]
- 192.Zappaterra MW, Lehtinen MK. The cerebrospinal fluid: regulator of neurogenesis, behavior, and beyond. Cell Mol Life Sci. 2012;69:2863–2878. doi: 10.1007/s00018-012-0957-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Carro E, Nunez A, Busiguina S, Torres-Aleman I. Circulating insulin-like growth factor I mediates effects of exercise on the brain. J Neurosci. 2000;20:2926–2933. doi: 10.1523/JNEUROSCI.20-08-02926.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Tomanek B, Iqbal U, Blasiak B, Abulrob A, Albaghdadi H, Matyas JR, Ponjevic D, Sutherland GR. Evaluation of brain tumor vessels specific contrast agents for glioblastoma imaging. Neurol Oncol. 2012;14:53–63. doi: 10.1093/neuonc/nor183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Anderson MF, Aberg MA, Nilsson M, Eriksson PS. Insulin-like growth factor-I and neurogenesis in the adult mammalian brain. Brain Res Dev Brain Res. 2002;134:115–122. doi: 10.1016/s0165-3806(02)00277-8. [DOI] [PubMed] [Google Scholar]
- 196.Ramsey MM, Adams MM, Ariwodola OJ, Sonntag WE, Weiner JL. Functional characterization of des-IGF-1 action at excitatory synapses in the CA1 region of rat hippocampus. J Neurophysiol. 2005;94:247–254. doi: 10.1152/jn.00768.2004. [DOI] [PubMed] [Google Scholar]
- 197.Deijen JB, de Boer H, van der Veen EA. Cognitive changes during growth hormone replacement in adult men. Psychoneuroendocrinology. 1998;23:45–55. doi: 10.1016/s0306-4530(97)00092-9. [DOI] [PubMed] [Google Scholar]
- 198.Dhamoon MS, Noble JM, Craft S. Intranasal insulin improves cognition and modulates beta-amyloid in early AD. Neurology. 2009;72:292–293. doi: 10.1212/01.wnl.0000344246.91081.2c. [DOI] [PubMed] [Google Scholar]
- 199.Gronbladh A, Johansson J, Nostl A, Nyberg F, Hallberg M. GH improves spatial memory and reverses certain anabolic androgenic steroid-induced effects in intact rats. J Endocrinol. 2013;216:31–41. doi: 10.1530/JOE-12-0315. [DOI] [PubMed] [Google Scholar]
- 200.Castro-Alamancos MA, Torres-Aleman I. Learning of the conditioned eye-blink response is impaired by an antisense insulin-like growth factor I oligonucleotide. Proc Natl Acad Sci USA. 1994;91:10203–10207. doi: 10.1073/pnas.91.21.10203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Zhao W, Chen H, Xu H, Moore E, Meiri N, Quon MJ, Alkon DL. Brain insulin receptors and spatial memory. Correlated changes in gene expression, tyrosine phosphorylation, and signaling molecules in the hippocampus of water maze trained rats. J Biol Chem. 1999;274:34893–34902. doi: 10.1074/jbc.274.49.34893. [DOI] [PubMed] [Google Scholar]
- 202.Nishijima T, Piriz J, Duflot S, Fernandez AM, Gaitan G, Gomez-Pinedo U, Verdugo JM, Leroy F, Soya H, Nunez A, Torres-Aleman I. Neuronal activity drives localized blood-brain-barrier transport of serum insulin-like growth factor-I into the CNS. Neuron. 2010;67:834–846. doi: 10.1016/j.neuron.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 203.Cohen E, Dillin A. The insulin paradox: aging, proteotoxicity and neurodegeneration. Nat Rev Neurosci. 2008;9:759–767. doi: 10.1038/nrn2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Piriz J, Muller A, Trejo JL, Torres-Aleman I. IGF-I and the aging mammalian brain. Exp Gerontol. 2011;46:96–99. doi: 10.1016/j.exger.2010.08.022. [DOI] [PubMed] [Google Scholar]
- 205.Trejo JL, Llorens-Martin MV, Torres-Aleman I. The effects of exercise on spatial learning and anxiety-like behavior are mediated by an IGF-I-dependent mechanism related to hippocampal neurogenesis. Mol Cell Neurosci. 2008;37:402–411. doi: 10.1016/j.mcn.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 206.Craft S, Baker LD, Montine TJ, Minoshima S, Watson GS, Claxton A, Arbuckle M, Callaghan M, Tsai E, Plymate SR, Green PS, Leverenz J, Cross D, Gerton B. Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: a pilot clinical trial. Arch Neurol. 2012;69:29–38. doi: 10.1001/archneurol.2011.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Endres M, Piriz J, Gertz K, Harms C, Meisel A, Kronenberg G, Torres-Aleman I. Serum insulin-like growth factor I and ischemic brain injury. Brain Res. 2007;1185:328–335. doi: 10.1016/j.brainres.2007.09.053. [DOI] [PubMed] [Google Scholar]
- 208.Arpa J, Sanz-Gallego I, Medina-Baez J, Portela LV, Jardim LB, Torres-Aleman I, Saute JA. Subcutaneous insulin-like growth factor-1 treatment in spinocerebellar ataxias: an open label clinical trial. Mov Disord. 2011;26:358–359. doi: 10.1002/mds.23423. [DOI] [PubMed] [Google Scholar]
- 209.Dudek H, Datta SR, Franke TF, Birnbaum MJ, Yao R, Cooper GM, Segal RA, Kaplan DR, Greenberg ME. Regulation of neuronal survival by the serine–threonine protein kinase Akt. Science. 1997;275:661–665. doi: 10.1126/science.275.5300.661. [DOI] [PubMed] [Google Scholar]
- 210.Carro E, Trejo JL, Gomez-Isla T, LeRoith D, Torres-Aleman I. Serum insulin-like growth factor I regulates brain amyloid-beta levels. Nat Med. 2002;8:1390–1397. doi: 10.1038/nm1202-793. [DOI] [PubMed] [Google Scholar]
- 211.Fernandez AM, Fernandez S, Carrero P, Garcia–Garcia M, Torres-Aleman I. Calcineurin in reactive astrocytes plays a key role in the interplay between proinflammatory and anti-inflammatory signals. J Neurosci. 2007;27:8745–8756. doi: 10.1523/JNEUROSCI.1002-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Schmeisser MJ, Baumann B, Johannsen S, Vindedal GF, Jensen V, Hvalby OC, Sprengel R, Seither J, Maqbool A, Magnutzki A, Lattke M, Oswald F, Boeckers TM, Wirth T. IkappaB kinase/nuclear factor kappaB-dependent insulin-like growth factor 2 (Igf2) expression regulates synapse formation and spine maturation via Igf2 receptor signaling. J Neurosci. 2012;32:5688–5703. doi: 10.1523/JNEUROSCI.0111-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Cline BH, Steinbusch HW, Malin D, Revishchin AV, Pavlova GV, Cespuglio R, Strekalova T. The neuronal insulin sensitizer dicholine succinate reduces stress-induced depressive traits and memory deficit: possible role of insulin-like growth factor 2. BMC Neurosci. 2012;13:110. doi: 10.1186/1471-2202-13-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Jung S, Lee Y, Kim G, Son H, Lee DH, Roh GS, Kang SS, Cho GJ, Choi WS, Kim HJ. Decreased expression of extracellular matrix proteins and trophic factors in the amygdala complex of depressed mice after chronic immobilization stress. BMC Neurosci. 2012;13:58. doi: 10.1186/1471-2202-13-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Abbott MA, Wells DG, Fallon JR. The insulin receptor tyrosine kinase substrate p58/53 and the insulin receptor are components of CNS synapses. J Neurosci. 1999;19:7300–7308. doi: 10.1523/JNEUROSCI.19-17-07300.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Cao P, Maximov A, Sudhof TC. Activity-dependent IGF-1 exocytosis is controlled by the Ca(2+)-sensor synaptotagmin-10. Cell. 2011;145:300–311. doi: 10.1016/j.cell.2011.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Liou JC, Tsai FZ, Ho SY. Potentiation of quantal secretion by insulin-like growth factor-1 at developing motoneurons in xenopus cell culture. J Physiol. 2003;553:719–728. doi: 10.1113/jphysiol.2003.050955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Xing C, Yin Y, Chang R, Gong X, He X, Xie Z. Effects of insulin-like growth factor 1 on synaptic excitability in cultured rat hippocampal neurons. Exp Neurol. 2007;205:222–229. doi: 10.1016/j.expneurol.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 219.Hwang O, Choi HJ. Induction of gene expression of the catecholamine-synthesizing enzymes by insulin-like growth factor-I. J Neurochem. 1995;65:1988–1996. doi: 10.1046/j.1471-4159.1995.65051988.x. [DOI] [PubMed] [Google Scholar]
- 220.Blair LA, Marshall J. IGF-1 modulates N and L calcium channels in a PI 3-kinase-dependent manner. Neuron. 1997;19:421–429. doi: 10.1016/s0896-6273(00)80950-2. [DOI] [PubMed] [Google Scholar]
- 221.Ster J, Colomer C, Monzo C, Duvoid-Guillou A, Moos F, Alonso G, Hussy N. Insulin-like growth factor-1 inhibits adult supraoptic neurons via complementary modulation of mechanoreceptors and glycine receptors. J Neurosci. 2005;25:2267–2276. doi: 10.1523/JNEUROSCI.4053-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Turrigiano GG. The self-tuning neuron: synaptic scaling of excitatory synapses. Cell. 2008;135:422–435. doi: 10.1016/j.cell.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Leventhal PS, Randolph AE, Vesbit TE, Schenone A, Windebank A, Feldman EL. Insulin-like growth factor-II as a paracrine growth factor in human neuroblastoma cells. Exp Cell Res. 1995;221:179–186. doi: 10.1006/excr.1995.1365. [DOI] [PubMed] [Google Scholar]
- 224.Aberg MA, Aberg ND, Palmer TD, Alborn AM, Carlsson-Skwirut C, Bang P, Rosengren LE, Olsson T, Gage FH, Eriksson PS. IGF-I has a direct proliferative effect in adult hippocampal progenitor cells. Mol Cell Neurosci. 2003;24:23–40. doi: 10.1016/s1044-7431(03)00082-4. [DOI] [PubMed] [Google Scholar]
- 225.Bateman JM, McNeill H. Insulin/IGF signalling in neurogenesis. Cell Mol Life Sci. 2006;63:1701–1705. doi: 10.1007/s00018-006-6036-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Rodriguez S, Gaunt TR, Day IN. Molecular genetics of human growth hormone, insulin-like growth factors and their pathways in common disease. Hum Genet. 2007;122:1–21. doi: 10.1007/s00439-007-0378-3. [DOI] [PubMed] [Google Scholar]
- 227.Chao W, D’Amore PA. IGF2: epigenetic regulation and role in development and disease. Cytokine Growth Factor Rev. 2008;19:111–120. doi: 10.1016/j.cytogfr.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Scolnick JA, Cui K, Duggan CD, Xuan S, Yuan XB, Efstratiadis A, Ngai J. Role of IGF signaling in olfactory sensory map formation and axon guidance. Neuron. 2008;57:847–857. doi: 10.1016/j.neuron.2008.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Broughton S, Partridge L. Insulin/IGF-like signalling, the central nervous system and aging. Biochem J. 2009;418:1–12. doi: 10.1042/BJ20082102. [DOI] [PubMed] [Google Scholar]
- 230.Lee E, Son H. Adult hippocampal neurogenesis and related neurotrophic factors. BMB Rep. 2009;42:239–244. doi: 10.5483/bmbrep.2009.42.5.239. [DOI] [PubMed] [Google Scholar]
- 231.Weber MM, Melmed S, Rosenbloom J, Yamasaki H, Prager D. Rat somatotroph insulin-like growth factor-II (IGF-II) signaling: role of the IGF-I receptor. Endocrinology. 1992;131:2147–2153. doi: 10.1210/endo.131.5.1425415. [DOI] [PubMed] [Google Scholar]
- 232.Hixon ML, Paccagnella L, Millham R, Perez-Olle R, Gualberto A. Development of inhibitors of the IGF-IR/PI3K/Akt/mTOR pathway. Rev Recent Clin Trials. 2010;5:189–208. doi: 10.2174/157488710792007329. [DOI] [PubMed] [Google Scholar]
- 233.Duman RS. Depression: a case of neuronal life and death? Biol Psychiatry. 2004;56:140–145. doi: 10.1016/j.biopsych.2004.02.033. [DOI] [PubMed] [Google Scholar]
- 234.Ma DK, Marchetto MC, Guo JU, Ming GL, Gage FH, Song H. Epigenetic choreographers of neurogenesis in the adult mammalian brain. Nat Neurosci. 2010;13:1338–1344. doi: 10.1038/nn.2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Greenwood BN, Fleshner M. Exercise, learned helplessness, and the stress-resistant brain. Neuromol Med. 2008;10:81–98. doi: 10.1007/s12017-008-8029-y. [DOI] [PubMed] [Google Scholar]
- 236.Kempermann G, Fabel K, Ehninger D, Babu H, Leal-Galicia P, Garthe A, Wolf SA. Why and how physical activity promotes experience-induced brain plasticity. Front Neurosci. 2010;4:189. doi: 10.3389/fnins.2010.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Trejo JL, Carro E, Torres-Aleman I. Circulating insulin-like growth factor I mediates exercise-induced increases in the number of new neurons in the adult hippocampus. J Neurosci. 2001;21:1628–1634. doi: 10.1523/JNEUROSCI.21-05-01628.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Llorens-Martin M, Torres-Aleman I, Trejo JL. Exercise modulates insulin-like growth factor 1-dependent and -independent effects on adult hippocampal neurogenesis and behaviour. Mol Cell Neurosci. 2010;44:109–117. doi: 10.1016/j.mcn.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 239.Bruel-Jungerman E, Veyrac A, Dufour F, Horwood J, Laroche S, Davis S. Inhibition of PI3K-Akt signaling blocks exercise-mediated enhancement of adult neurogenesis and synaptic plasticity in the dentate gyrus. PLoS One. 2009;4:e7901. doi: 10.1371/journal.pone.0007901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Duman CH, Schlesinger L, Terwilliger R, Russell DS, Newton SS, Duman RS. Peripheral insulin-like growth factor-I produces antidepressant-like behavior and contributes to the effect of exercise. Behav Brain Res. 2009;198:366–371. doi: 10.1016/j.bbr.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Aimone JB, Deng W, Gage FH. Adult neurogenesis: integrating theories and separating functions. Trends Cogn Sci. 2010;14:325–337. doi: 10.1016/j.tics.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Kitamura T, Saitoh Y, Takashima N, Murayama A, Niibori Y, Ageta H, Sekiguchi M, Sugiyama H, Inokuchi K. Adult neurogenesis modulates the hippocampus-dependent period of associative fear memory. Cell. 2009;139:814–827. doi: 10.1016/j.cell.2009.10.020. [DOI] [PubMed] [Google Scholar]
- 243.Aberg ND, Brywe KG, Isgaard J. Aspects of growth hormone and insulin-like growth factor-I related to neuroprotection, regeneration, and functional plasticity in the adult brain. Sci World J. 2006;6:53–80. doi: 10.1100/tsw.2006.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Llorens-Martin M, Torres-Aleman I, Trejo JL. Mechanisms mediating brain plasticity: IGF1 and adult hippocampal neurogenesis. Neuroscientist. 2009;15:134–148. doi: 10.1177/1073858408331371. [DOI] [PubMed] [Google Scholar]
- 245.Ziegler AN, Schneider JS, Qin M, Tyler WA, Pintar JE, Fraidenraich D, Wood TL, Levison SW. Igf-II promotes stemness of neural restricted precursors. Stem Cells. 2012;30:1265–1276. doi: 10.1002/stem.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Rotwein P, Burgess SK, Milbrandt JD, Krause JE. Differential expression of insulin-like growth factor genes in rat central nervous system. Proc Natl Acad Sci USA. 1988;85:265–269. doi: 10.1073/pnas.85.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Valentino KL, Ocrant I, Rosenfeld RG. Developmental expression of insulin-like growth factor-II receptor immunoreactivity in the rat central nervous system. Endocrinology. 1990;126:914–920. doi: 10.1210/endo-126-2-914. [DOI] [PubMed] [Google Scholar]
- 248.Vescovi AL, Reynolds BA, Fraser DD, Weiss S. bFGF regulates the proliferative fate of unipotent (neuronal) and bipotent (neuronal/astroglial) EGF-generated CNS progenitor cells. Neuron. 1993;11:951–966. doi: 10.1016/0896-6273(93)90124-a. [DOI] [PubMed] [Google Scholar]
- 249.Pastrana E, Silva-Vargas V, Doetsch F. Eyes wide open: a critical review of sphere-formation as an assay for stem cells. Cell Stem Cell. 2011;8:486–498. doi: 10.1016/j.stem.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Bendall SC, Stewart MH, Menendez P, George D, Vijayaragavan K, Werbowetski-Ogilvie T, Ramos-Mejia V, Rouleau A, Yang J, Bosse M, Lajoie G, Bhatia M. IGF and FGF cooperatively establish the regulatory stem cell niche of pluripotent human cells in vitro. Nature. 2007;448:1015–1021. doi: 10.1038/nature06027. [DOI] [PubMed] [Google Scholar]
- 251.Castilla-Cortazar I, Garcia-Fernandez M, Delgado G, Puche JE, Sierra I, Barhoum R, Gonzalez-Baron S. Hepatoprotection and neuroprotection induced by low doses of IGF-II in aging rats. J Transl Med. 2011;9:103. doi: 10.1186/1479-5876-9-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Fernandez C, Tatard VM, Bertrand N, Dahmane N. Differential modulation of sonic-hedgehog-induced cerebellar granule cell precursor proliferation by the IGF signaling network. Dev Neurosci. 2010;32:59–70. doi: 10.1159/000274458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Corcoran RB, Raveh TB, Barakat MT, Lee EY, Scott MP. Insulin-like growth factor 2 is required for progression to advanced medulloblastoma in patched1 heterozygous mice. Cancer Res. 2008;68:8788–8795. doi: 10.1158/0008-5472.CAN-08-2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Kita Y, Ago Y, Takano E, Fukada A, Takuma K, Matsuda T. Galantamine increases hippocampal insulin-like growth factor 2 expression via alpha7 nicotinic acetylcholine receptors in mice. Psychopharmacology (Berl) 2013;225:543–551. doi: 10.1007/s00213-012-2841-7. [DOI] [PubMed] [Google Scholar]
- 255.Baxter RC. Insulin-like growth factor (IGF) binding proteins: the role of serum IGFBPs in regulating IGF availability. Acta Paediatr Scand Suppl. 1991;372:107–114. doi: 10.1111/j.1651-2227.1991.tb17983.x. [DOI] [PubMed] [Google Scholar]
- 256.Oh Y, Nagalla SR, Yamanaka Y, Kim HS, Wilson E, Rosenfeld RG. Synthesis and characterization of insulin-like growth factor-binding protein (IGFBP)-7. Recombinant human mac25 protein specifically binds IGF-I and -II. J Biol Chem. 1996;271:30322–30325. doi: 10.1074/jbc.271.48.30322. [DOI] [PubMed] [Google Scholar]
- 257.Yamanaka Y, Wilson EM, Rosenfeld RG, Oh Y. Inhibition of insulin receptor activation by insulin-like growth factor binding proteins. J Biol Chem. 1997;272:30729–30734. doi: 10.1074/jbc.272.49.30729. [DOI] [PubMed] [Google Scholar]
- 258.Suzuki H, Igarashi S, Nojima M, Maruyama R, Yamamoto E, Kai M, Akashi H, Watanabe Y, Yamamoto H, Sasaki Y, Itoh F, Imai K, Sugai T, Shen L, Issa JP, Shinomura Y, Tokino T, Toyota M. IGFBP7 is a p53-responsive gene specifically silenced in colorectal cancer with CpG island methylator phenotype. Carcinogenesis. 2010;31:342–349. doi: 10.1093/carcin/bgp179. [DOI] [PubMed] [Google Scholar]
- 259.Jiang B, Kumar SD, Loh WT, Manikandan J, Ling EA, Tay SS, Dheen ST. Global gene expression analysis of cranial neural tubes in embryos of diabetic mice. J Neurosci Res. 2008;86:3481–3493. doi: 10.1002/jnr.21800. [DOI] [PubMed] [Google Scholar]
- 260.Tomimaru Y, Eguchi H, Wada H, Noda T, Murakami M, Kobayashi S, Marubashi S, Takeda Y, Tanemura M, Umeshita K, Doki Y, Mori M, Nagano H. Insulin-like growth factor-binding protein 7 alters the sensitivity to interferon-based anticancer therapy in hepatocellular carcinoma cells. Br J Cancer. 2010;102:1483–1490. doi: 10.1038/sj.bjc.6605669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Wajapeyee N, Serra RW, Zhu X, Mahalingam M, Green MR. Oncogenic BRAF induces senescence and apoptosis through pathways mediated by the secreted protein IGFBP7. Cell. 2008;132:363–374. doi: 10.1016/j.cell.2007.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Csoregh L, Andersson E, Fried G. Transcriptional analysis of estrogen effects in human embryonic neurons and glial cells. Neuroendocrinology. 2009;89:171–186. doi: 10.1159/000153899. [DOI] [PubMed] [Google Scholar]
- 263.Tamura K, Hashimoto K, Suzuki K, Yoshie M, Kutsukake M, Sakurai T. Insulin-like growth factor binding protein-7 (IGFBP7) blocks vascular endothelial cell growth factor (VEGF)-induced angiogenesis in human vascular endothelial cells. Eur J Pharmacol. 2009;610:61–67. doi: 10.1016/j.ejphar.2009.01.045. [DOI] [PubMed] [Google Scholar]
- 264.Cao L, Jiao X, Zuzga DS, Liu Y, Fong DM, Young D, During MJ. VEGF links hippocampal activity with neurogenesis, learning and memory. Nat Genet. 2004;36:827–835. doi: 10.1038/ng1395. [DOI] [PubMed] [Google Scholar]
- 265.Heine VM, Zareno J, Maslam S, Joels M, Lucassen PJ. Chronic stress in the adult dentate gyrus reduces cell proliferation near the vasculature and VEGF and Flk-1 protein expression. Eur J Neurosci. 2005;21:1304–1314. doi: 10.1111/j.1460-9568.2005.03951.x. [DOI] [PubMed] [Google Scholar]
- 266.Ostrovsky O, Ahmed NT, Argon Y. The chaperone activity of GRP94 toward insulin-like growth factor II is necessary for the stress response to serum deprivation. Mol Biol Cell. 2009;20:1855–1864. doi: 10.1091/mbc.E08-04-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Gennigens C, Menetrier-Caux C, Droz JP. Insulin-like growth factor (IGF) family and prostate cancer. Crit Rev Oncol Hematol. 2006;58:124–145. doi: 10.1016/j.critrevonc.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 268.Brown J, Jones EY, Forbes BE. Keeping IGF-II under control: lessons from the IGF-II-IGF2R crystal structure. Trends Biochem Sci. 2009;34:612–619. doi: 10.1016/j.tibs.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 269.Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer. 2008;8:915–928. doi: 10.1038/nrc2536. [DOI] [PubMed] [Google Scholar]
- 270.Heidegger I, Pircher A, Klocker H, Massoner P. Targeting the insulin-like growth factor network in cancer therapy. Cancer Biol Ther. 2011;11:701–707. doi: 10.4161/cbt.11.8.14689. [DOI] [PubMed] [Google Scholar]
- 271.Pietrzkowski Z, Wernicke D, Porcu P, Jameson BA, Baserga R. Inhibition of cellular proliferation by peptide analogues of insulin-like growth factor 1. Cancer Res. 1992;52:6447–6451. [PubMed] [Google Scholar]
- 272.Fukunaga K, Kawano T. Akt is a molecular target for signal transduction therapy in brain ischemic insult. J Pharmacol Sci. 2003;92:317–327. doi: 10.1254/jphs.92.317. [DOI] [PubMed] [Google Scholar]
- 273.Brazil DP, Yang ZZ, Hemmings BA. Advances in protein kinase B signalling: AKTion on multiple fronts. Trends Biochem Sci. 2004;29:233–242. doi: 10.1016/j.tibs.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 274.Clemmons DR. Insulin-like growth factor binding proteins and their role in controlling IGF actions. Cytokine Growth Factor Rev. 1997;8:45–62. doi: 10.1016/s1359-6101(96)00053-6. [DOI] [PubMed] [Google Scholar]
- 275.Peltier J, O’Neill A, Schaffer DV. PI3K/Akt and CREB regulate adult neural hippocampal progenitor proliferation and differentiation. Dev Neurobiol. 2007;67:1348–1361. doi: 10.1002/dneu.20506. [DOI] [PubMed] [Google Scholar]
- 276.Sun XJ, Rothenberg P, Kahn CR, Backer JM, Araki E, Wilden PA, Cahill DA, Goldstein BJ, White MF. Structure of the insulin receptor substrate IRS-1 defines a unique signal transduction protein. Nature. 1991;352:73–77. doi: 10.1038/352073a0. [DOI] [PubMed] [Google Scholar]
- 277.Hartmann W, Koch A, Brune H, Waha A, Schuller U, Dani I, Denkhaus D, Langmann W, Bode U, Wiestler OD, Schilling K, Pietsch T. Insulin-like growth factor II is involved in the proliferation control of medulloblastoma and its cerebellar precursor cells. Am J Pathol. 2005;166:1153–1162. doi: 10.1016/S0002-9440(10)62335-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Groszer M, Erickson R, Scripture-Adams DD, Lesche R, Trumpp A, Zack JA, Kornblum HI, Liu X, Wu H. Negative regulation of neural stem/progenitor cell proliferation by the Pten tumor suppressor gene in vivo. Science. 2001;294:2186–2189. doi: 10.1126/science.1065518. [DOI] [PubMed] [Google Scholar]
- 279.Vincent AM, Feldman EL. Control of cell survival by IGF signaling pathways. Growth Horm IGF Res. 2002;12:193–197. doi: 10.1016/s1096-6374(02)00017-5. [DOI] [PubMed] [Google Scholar]
- 280.Mathieu C, Sii-Felice K, Fouchet P, Etienne O, Haton C, Mabondzo A, Boussin FD, Mouthon MA. Endothelial cell-derived bone morphogenetic proteins control proliferation of neural stem/progenitor cells. Mol Cell Neurosci. 2008;38:569–577. doi: 10.1016/j.mcn.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 281.Segu L, Lecomte MJ, Wolff M, Santamaria J, Hen R, Dumuis A, Berrard S, Bockaert J, Buhot MC, Compan V. Hyperfunction of muscarinic receptor maintains long-term memory in 5-HT4 receptor knock-out mice. PLoS One. 2010;5:e9529. doi: 10.1371/journal.pone.0009529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Wang Z, Zang C, Rosenfeld JA, Schones DE, Barski A, Cuddapah S, Cui K, Roh TY, Peng W, Zhang MQ, Zhao K. Combinatorial patterns of histone acetylations and methylations in the human genome. Nat Genet. 2008;40:897–903. doi: 10.1038/ng.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Arrowsmith CH, Bountra C, Fish PV, Lee K, Schapira M. Epigenetic protein families: a new frontier for drug discovery. Nat Rev Drug Discov. 2012;11:384–400. doi: 10.1038/nrd3674. [DOI] [PubMed] [Google Scholar]
- 284.Strahl BD, Allis CD. The language of covalent histone modifications. Nature. 2000;403:41–45. doi: 10.1038/47412. [DOI] [PubMed] [Google Scholar]
- 285.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 286.Fischer A, Sananbenesi F, Mungenast A, Tsai LH. Targeting the correct HDAC(s) to treat cognitive disorders. Trends Pharmacol Sci. 2010;31:605–617. doi: 10.1016/j.tips.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 287.Houston I, Peter CJ, Mitchell A, Straubhaar J, Rogaev E, Akbarian S. Epigenetics in the human brain. Neuropsychopharmacology. 2013;38:183–197. doi: 10.1038/npp.2012.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Levenson JM, O’Riordan KJ, Brown KD, Trinh MA, Molfese DL, Sweatt JD. Regulation of histone acetylation during memory formation in the hippocampus. J Biol Chem. 2004;279:40545–40559. doi: 10.1074/jbc.M402229200. [DOI] [PubMed] [Google Scholar]
- 289.Fontan-Lozano A, Romero-Granados R, Troncoso J, Munera A, Delgado-Garcia JM, Carrion AM. Histone deacetylase inhibitors improve learning consolidation in young and in KA-induced-neurodegeneration and SAMP-8-mutant mice. Mol Cell Neurosci. 2008;39:193–201. doi: 10.1016/j.mcn.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 290.Peleg S, Sananbenesi F, Zovoilis A, Burkhardt S, Bahari-Javan S, Agis-Balboa RC, Cota P, Wittnam JL, Gogol-Doering A, Opitz L, Salinas-Riester G, Dettenhofer M, Kang H, Farinelli L, Chen W, Fischer A. Altered histone acetylation is associated with age-dependent memory impairment in mice. Science. 2010;328:753–756. doi: 10.1126/science.1186088. [DOI] [PubMed] [Google Scholar]
- 291.Bousiges O, Vasconcelos AP, Neidl R, Cosquer B, Herbeaux K, Panteleeva I, Loeffler JP, Cassel JC, Boutillier AL. Spatial memory consolidation is associated with induction of several lysine-acetyltransferase (histone acetyltransferase) expression levels and H2B/H4 acetylation-dependent transcriptional events in the rat hippocampus. Neuropsychopharmacology. 2010;35:2521–2537. doi: 10.1038/npp.2010.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Sananbenesi F, Fischer A. The epigenetic bottleneck of neurodegenerative and psychiatric diseases. Biol Chem. 2009;390:1145–1153. doi: 10.1515/BC.2009.131. [DOI] [PubMed] [Google Scholar]
- 293.Sananbenesi F, Fischer A, Schrick C, Spiess J, Radulovic J. Phosphorylation of hippocampal Erk-1/2, Elk-1, and p90-Rsk-1 during contextual fear conditioning: interactions between Erk-1/2 and Elk-1. Mol Cell Neurosci. 2002;21:463–476. doi: 10.1006/mcne.2002.1188. [DOI] [PubMed] [Google Scholar]
- 294.Kelleher RJ, 3rd, Govindarajan A, Jung HY, Kang H, Tonegawa S. Translational control by MAPK signaling in long-term synaptic plasticity and memory. Cell. 2004;116:467–479. doi: 10.1016/s0092-8674(04)00115-1. [DOI] [PubMed] [Google Scholar]
- 295.Chwang WB, O’Riordan KJ, Levenson JM, Sweatt JD. ERK/MAPK regulates hippocampal histone phosphorylation following contextual fear conditioning. Learn Mem. 2006;13:322–328. doi: 10.1101/lm.152906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Kimura A, Matsubara K, Horikoshi M. A decade of histone acetylation: marking eukaryotic chromosomes with specific codes. J Biochem. 2005;138:647–662. doi: 10.1093/jb/mvi184. [DOI] [PubMed] [Google Scholar]
- 297.Agis-Balboa RC, Pavelka Z, Kerimoglu C, Fischer A. Loss of HDAC5 impairs memory function: implications for Alzheimer’s disease. J Alzheimers Dis. 2012;33:35–44. doi: 10.3233/JAD-2012-121009. [DOI] [PubMed] [Google Scholar]
- 298.Bahari-Javan S, Maddalena A, Kerimoglu C, Wittnam J, Held T, Bahr M, Burkhardt S, Delalle I, Kugler S, Fischer A, Sananbenesi F. HDAC1 regulates fear extinction in mice. J Neurosci. 2012;32:5062–5073. doi: 10.1523/JNEUROSCI.0079-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Govindarajan N, Rao P, Burkhardt S, Sananbenesi F, Schluter OM, Bradke F, Lu J, Fischer A. Reducing HDAC6 ameliorates cognitive deficits in a mouse model for Alzheimer’s disease. EMBO Mol Med. 2013;5:52–63. doi: 10.1002/emmm.201201923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Lattal KM, Barrett RM, Wood MA. Systemic or intrahippocampal delivery of histone deacetylase inhibitors facilitates fear extinction. Behav Neurosci. 2007;121:1125–1131. doi: 10.1037/0735-7044.121.5.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Stafford JM, Raybuck JD, Ryabinin AE, Lattal KM. Increasing histone acetylation in the hippocampus-infralimbic network enhances fear extinction. Biol Psychiatry. 2012;72:25–33. doi: 10.1016/j.biopsych.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Bredy TW, Wu H, Crego C, Zellhoefer J, Sun YE, Barad M. Histone modifications around individual BDNF gene promoters in prefrontal cortex are associated with extinction of conditioned fear. Learn Mem. 2007;14:268–276. doi: 10.1101/lm.500907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Bredy TW, Barad M. The histone deacetylase inhibitor valproic acid enhances acquisition, extinction, and reconsolidation of conditioned fear. Learn Mem. 2008;15:39–45. doi: 10.1101/lm.801108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Guan JS, Haggarty SJ, Giacometti E, Dannenberg JH, Joseph N, Gao J, Nieland TJ, Zhou Y, Wang X, Mazitschek R, Bradner JE, DePinho RA, Jaenisch R, Tsai LH. HDAC2 negatively regulates memory formation and synaptic plasticity. Nature. 2009;459:55–60. doi: 10.1038/nature07925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Gräff J, Rei D, Guan JS, Wang WY, Seo J, Hennig KM, Nieland TJ, Fass DM, Kao PF, Kahn M, Su SC, Samiei A, Joseph N, Haggarty SJ, Delalle I, Tsai LH. An epigenetic blockade of cognitive functions in the neurodegenerating brain. Nature. 2012;483:222–226. doi: 10.1038/nature10849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.McQuown SC, Barrett RM, Matheos DP, Post RJ, Rogge GA, Alenghat T, Mullican SE, Jones S, Rusche JR, Lazar MA, Wood MA. HDAC3 is a critical negative regulator of long-term memory formation. J Neurosci. 2011;31:764–774. doi: 10.1523/JNEUROSCI.5052-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Benes FM, Lim B, Matzilevich D, Walsh JP, Subburaju S, Minns M. Regulation of the GABA cell phenotype in hippocampus of schizophrenics and bipolars. Proc Natl Acad Sci USA. 2007;104:10164–10169. doi: 10.1073/pnas.0703806104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Sharma RP, Grayson DR, Gavin DP. Histone deactylase 1 expression is increased in the prefrontal cortex of schizophrenia subjects: analysis of the national brain databank microarray collection. Schizophr Res. 2008;98:111–117. doi: 10.1016/j.schres.2007.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Quinti L, Chopra V, Rotili D, Valente S, Amore A, Franci G, Meade S, Valenza M, Altucci L, Maxwell MM, Cattaneo E, Hersch S, Mai A, Kazantsev A (2010) Evaluation of histone deacetylases as drug targets in Huntington’s disease models. Study of HDACs in brain tissues from R6/2 and CAG140 knock-in HD mouse models and human patients and in a neuronal HD cell model. PLoS Curr 2 pii: rrn1172 [DOI] [PMC free article] [PubMed]
- 310.Wang Z, Yang D, Zhang X, Li T, Li J, Tang Y, Le W. Hypoxia-induced down-regulation of neprilysin by histone modification in mouse primary cortical and hippocampal neurons. PLoS One. 2011;6:e19229. doi: 10.1371/journal.pone.0019229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Holliday R, Pugh JE. DNA modification mechanisms and gene activity during development. Science. 1975;187:226–232. [PubMed] [Google Scholar]
- 312.Deaton AM, Bird A. CpG islands and the regulation of transcription. Genes Dev. 2011;25:1010–1022. doi: 10.1101/gad.2037511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Jones PA. Functions of DNA methylation: islands, start sites, gene bodies and beyond. Nat Rev Genet. 2012;13:484–492. doi: 10.1038/nrg3230. [DOI] [PubMed] [Google Scholar]
- 314.Wood TL, O’Donnell SL, Levison SW. Cytokines regulate IGF binding proteins in the CNS. Prog Growth Factor Res. 1995;6:181–187. doi: 10.1016/0955-2235(95)00035-6. [DOI] [PubMed] [Google Scholar]
- 315.Holmin S, Mathiesen T, Langmoen IA, Sandberg Nordqvist AC. Depolarization induces insulin-like growth factor binding protein-2 expression in vivo via NMDA receptor stimulation. Growth Horm IGF Res. 2001;11:399–406. doi: 10.1054/ghir.2001.0252. [DOI] [PubMed] [Google Scholar]
- 316.Itoh M, Ide S, Takashima S, Kudo S, Nomura Y, Segawa M, Kubota T, Mori H, Tanaka S, Horie H, Tanabe Y, Goto Y. Methyl CpG-binding protein 2 (a mutation of which causes Rett syndrome) directly regulates insulin-like growth factor binding protein 3 in mouse and human brains. J Neuropathol Exp Neurol. 2007;66:117–123. doi: 10.1097/nen.0b013e3180302078. [DOI] [PubMed] [Google Scholar]
- 317.Tropea D, Giacometti E, Wilson NR, Beard C, McCurry C, Fu DD, Flannery R, Jaenisch R, Sur M. Partial reversal of Rett syndrome-like symptoms in MeCP2 mutant mice. Proc Natl Acad Sci USA. 2009;106:2029–2034. doi: 10.1073/pnas.0812394106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Garcia-Segura LM, Rodriguez JR, Torres-Aleman I. Localization of the insulin-like growth factor I receptor in the cerebellum and hypothalamus of adult rats: an electron microscopic study. J Neurocytol. 1997;26:479–490. doi: 10.1023/a:1018581407804. [DOI] [PubMed] [Google Scholar]
- 319.Sehat B, Tofigh A, Lin Y, Trocme E, Liljedahl U, Lagergren J, Larsson O. SUMOylation mediates the nuclear translocation and signaling of the IGF-1 receptor. Sci Signal. 2010;3:ra10. doi: 10.1126/scisignal.2000628. [DOI] [PubMed] [Google Scholar]
- 320.Kelley KM, Oh Y, Gargosky SE, Gucev Z, Matsumoto T, Hwa V, Ng L, Simpson DM, Rosenfeld RG. Insulin-like growth factor-binding proteins (IGFBPs) and their regulatory dynamics. Int J Biochem Cell Biol. 1996;28:619–637. doi: 10.1016/1357-2725(96)00005-2. [DOI] [PubMed] [Google Scholar]
- 321.Ruan W, Xu E, Xu F, Ma Y, Deng H, Huang Q, Lv B, Hu H, Lin J, Cui J, Di M, Dong J, Lai M. IGFBP7 plays a potential tumor suppressor role in colorectal carcinogenesis. Cancer Biol Ther. 2007;6:354–359. doi: 10.4161/cbt.6.3.3702. [DOI] [PubMed] [Google Scholar]
- 322.Alic N, Hoddinott MP, Vinti G, Partridge L. Lifespan extension by increased expression of the Drosophila homologue of the IGFBP7 tumour suppressor. Aging Cell. 2011;10:137–147. doi: 10.1111/j.1474-9726.2010.00653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Lin J, Lai M, Huang Q, Ma Y, Cui J, Ruan W. Methylation patterns of IGFBP7 in colon cancer cell lines are associated with levels of gene expression. J Pathol. 2007;212:83–90. doi: 10.1002/path.2144. [DOI] [PubMed] [Google Scholar]
- 324.Lin J, Lai M, Huang Q, Ruan W, Ma Y, Cui J. Reactivation of IGFBP7 by DNA demethylation inhibits human colon cancer cell growth in vitro. Cancer Biol Ther. 2008;7:1896–1900. doi: 10.4161/cbt.7.12.6937. [DOI] [PubMed] [Google Scholar]
- 325.Chen Y, Cui T, Knosel T, Yang L, Zoller K, Petersen I. IGFBP7 is a p53 target gene inactivated in human lung cancer by DNA hypermethylation. Lung Cancer. 2011;73:38–44. doi: 10.1016/j.lungcan.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 326.Heesch S, Bartram I, Neumann M, Reins J, Mossner M, Schlee C, Stroux A, Haferlach T, Goekbuget N, Hoelzer D, Hofmann WK, Thiel E, Baldus CD. Expression of IGFBP7 in acute leukemia is regulated by DNA methylation. Cancer Sci. 2011;102:253–259. doi: 10.1111/j.1349-7006.2010.01760.x. [DOI] [PubMed] [Google Scholar]
- 327.Scurr LL, Pupo GM, Becker TM, Lai K, Schrama D, Haferkamp S, Irvine M, Scolyer RA, Mann GJ, Becker JC, Kefford RF, Rizos H. IGFBP7 is not required for B-RAF-induced melanocyte senescence. Cell. 2010;141:717–727. doi: 10.1016/j.cell.2010.04.021. [DOI] [PubMed] [Google Scholar]
- 328.Miller CA, Sweatt JD. Covalent modification of DNA regulates memory formation. Neuron. 2007;53:857–869. doi: 10.1016/j.neuron.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 329.Miller CA, Gavin CF, White JA, Parrish RR, Honasoge A, Yancey CR, Rivera IM, Rubio MD, Rumbaugh G, Sweatt JD. Cortical DNA methylation maintains remote memory. Nat Neurosci. 2010;13:664–666. doi: 10.1038/nn.2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Zovkic IB, Sweatt JD. Epigenetic mechanisms in learned fear: implications for PTSD. Neuropsychopharmacology. 2013;38:77–93. doi: 10.1038/npp.2012.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Leach PT, Poplawski SG, Kenney JW, Hoffman B, Liebermann DA, Abel T, Gould TJ. Gadd45b knockout mice exhibit selective deficits in hippocampus-dependent long-term memory. Learn Mem. 2012;19:319–324. doi: 10.1101/lm.024984.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Ma DK, Jang M-H, Guo JU, Kitabatake Y, Chang M-l, Pow-anpongkul N, Flavell RA, Lu B, Ming G-l, Song H. Neuronal activity-induced Gadd45b promotes epigenetic DNA demethylation and adult neurogenesis. Science. 2009;323:1074–1077. doi: 10.1126/science.1166859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Xu X, Coats JK, Yang CF, Wang A, Ahmed OM, Alvarado M, Izumi T, Shah NM. Modular genetic control of sexually dimorphic behaviors. Cell. 2012;148:596–607. doi: 10.1016/j.cell.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Insel TR, Fernald RD. How the brain processes social information: searching for the social brain. Annu Rev Neurosci. 2004;27:697–722. doi: 10.1146/annurev.neuro.27.070203.144148. [DOI] [PubMed] [Google Scholar]
- 335.Kellendonk C, Simpson EH, Kandel ER. Modeling cognitive endophenotypes of schizophrenia in mice. Trends Neurosci. 2009;32:347–358. doi: 10.1016/j.tins.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Esteller M. Non-coding RNAs in human disease. Nat Rev Genet. 2011;12:861–874. doi: 10.1038/nrg3074. [DOI] [PubMed] [Google Scholar]
- 337.Schratt G. MicroRNAs at the synapse. Nat Rev Neurosci. 2009;10:842–849. doi: 10.1038/nrn2763. [DOI] [PubMed] [Google Scholar]
- 338.Smith-Vikos T, Slack FJ. MicroRNAs and their roles in aging. J Cell Sci. 2012;125:7–17. doi: 10.1242/jcs.099200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.O’Carroll D, Schaefer A. General principals of miRNA biogenesis and regulation in the brain. Neuropsychopharmacology. 2013;38:39–54. doi: 10.1038/npp.2012.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Balzer E, Heine C, Jiang Q, Lee VM, Moss EG. LIN28 alters cell fate succession and acts independently of the let-7 microRNA during neurogliogenesis in vitro. Development. 2010;137:891–900. doi: 10.1242/dev.042895. [DOI] [PubMed] [Google Scholar]
- 341.Wilting SM, van Boerdonk RA, Henken FE, Meijer CJ, Diosdado B, Meijer GA, le Sage C, Agami R, Snijders PJ, Steenbergen RD. Methylation-mediated silencing and tumour suppressive function of hsa-miR-124 in cervical cancer. Mol Cancer. 2010;9:167. doi: 10.1186/1476-4598-9-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Ge Y, Sun Y, Chen J. IGF-II is regulated by microRNA-125b in skeletal myogenesis. J Cell Biol. 2011;192:69–81. doi: 10.1083/jcb.201007165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Griggs EM, Young EJ, Rumbaugh G, Miller CA. MicroRNA-182 regulates amygdala-dependent memory formation. J Neurosci. 2013;33:1734–1740. doi: 10.1523/JNEUROSCI.2873-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Konopka W, Kiryk A, Novak M, Herwerth M, Parkitna JR, Wawrzyniak M, Kowarsch A, Michaluk P, Dzwonek J, Arnsperger T, Wilczynski G, Merkenschlager M, Theis FJ, Kohr G, Kaczmarek L, Schutz G. MicroRNA loss enhances learning and memory in mice. J Neurosci. 2010;30:14835–14842. doi: 10.1523/JNEUROSCI.3030-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Lin Q, Wei W, Coelho CM, Li X, Baker-Andresen D, Dudley K, Ratnu VS, Boskovic Z, Kobor MS, Sun YE, Bredy TW. The brain-specific microRNA miR-128b regulates the formation of fear-extinction memory. Nat Neurosci. 2011;14:1115–1117. doi: 10.1038/nn.2891. [DOI] [PubMed] [Google Scholar]