Abstract
DLK1-DIO3 represents an imprinted cluster which genes are involved in physiological cell biology as early as the stem cell level and in the pathogenesis of several diseases. Transcription factor-mediated induced pluripotent cells (iPSCs) are considered an unlimited source of patient-specific hematopoietic stem cells for clinical application in patient-tailored regenerative medicine. However, to date there is no marker established able to distinguish embryonic stem cell-equivalent iPSCs or safe human iPSCs. Recent findings suggest that the DLK1-DIO3 locus possesses the potential to represent such a marker but there are also contradictory data. This review aims to report the current data on the topic describing both sides of the coin.
Keywords: DLK1-DIO3, MEG3, Pluripotency, Genomic imprinting, MicroRNAs, Stem cells, Reprogramming, Induced pluripotent stem cells
Genomic imprinting: an overview
Genomic imprinting is an epigenetic phenomenon that results in the allele-specific expression of several diploid genes, thus exhibiting a monoallelic gene expression pattern [33]. Although there are some single imprinted genes spread all over the entire genome, most of the imprinted genes are clustered in the genome spanning hundreds to thousands of kilobases, while they are conserved during vertebrate evolution. These clusters comprise both paternally and maternally expressed imprinted genes, some of them being non-coding RNAs, and also non-imprinted genes [6].
Genomic imprinting consists of three steps: establishment, maintenance, and erasure. Imprint establishment occurs in male and female gametogenesis, with DNA methylation being the critical mechanism of imprinting, through the action of DNA methyltransferases 3A/B (DNMT3A/B). Imprinting maintenance is important in the pre-implantation embryo protecting it from genome-wide epigenetic modifications. Maintenance is achieved through the action of DNMT1, the maternal proteins ZFP57 and PGC7, Polycomb repressive (PRC2) proteins, and the methyl-CpG-binding protein MBD3 [46, 62]. Imprinting is erased during early germ cell development and re-established upon full development of male and female gametes. Recent findings suggest that erasure is mediated by the 5-methylcytosine oxidizing Tet1 enzyme [45, 79]. Imprinted gene expression is regulated by a cis-acting imprinting control region (ICR). ICR is a germline-derived DNA sequence with differentially methylated regions (DMR) which controls the monoallelic expression of the gene cluster depending on their parental origin, and furthermore, controls the expression of all imprinted miRNAs in respective gene clusters [46]. Imprinted genes affect entire organisms’ developmental plasticity, which is the ability of a genotype to produce phenotypes in response to environmental stimuli, at the organ level and at the single cell level as well. In particular, at the single cell level imprinted genes are involved in early development, pluripotency, and neoplastic transformation [58]. Imprinted genes are also involved in hematopoiesis. For example, conditional deletion of the maternal but not paternal H19-DMR in mice results in reduced hematopoietic stem cell (HSCs) quiescence accompanied by increasing progenitor cell proliferation, and finally increasing total bone marrow cellularity [73]. It has also been suggested that imprinted genes play a role in processes other than growth, such as cognition and behavior, reactivity to novel environments, social dominance and memory consolidation [15]. The biallelic expression or on the contrary the complete silencing of the imprinted genes due to abnormal methylation pattern at the DMR leads to loss of imprinting (LOI), which might have a significant role in oncogenesis and cancer progression. Genomic imprinting is also involved in reprogramming, since LOI during the reprogramming process might affect the biological behavior of induced pluripotent stem cells (iPSCs) [61, 64].
Several theories for the evolution of genomic imprinting have been developed falling under the kinship theory and include evolution of the loci that are under sexually antagonistic selection, evolution under the selection for maternal-fetal co-evolution, and evolution under selection of parental resemblance [15]. A recently proposed model based on interactions of imprinted genes mediated by DNA, RNA and proteins, suggests that the evolution of imprinting at one locus can drive the imprinting at other loci that interact with that imprinted gene promoting a co-evolutionary pattern [76].
The DLK1-DIO3 imprinted cluster
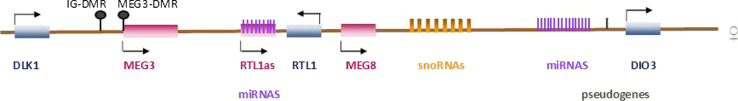
The DLK1-DIO3 (also called DLK1-MEG3) imprinted cluster is located on chromosomes 14q32 and 12qF1 in humans and mice, respectively. It contains protein-coding genes (the paternally expressed DLK1, RTL1, and DIO3), the maternally expressed long non-coding RNAs (lncRNAs) MEG3, MEG8, and the antisense RTL1 (RTL1as). It also contains a large cluster of microRNAs (miRNAs), considered probably the largest cluster throughout the genome, multiple C/D small nucleolar RNAs (snoRNAs), and several pseudogenes [11] (Fig. 1).
Fig. 1.
Schematic representation of the DLK1-DIO3 imprinted cluster in humans. In blue, the paternally expressed genes are shown, while the maternally expressed are shown in red. Paternal allele consists of the DLK1, RTL1, and DIO3 protein-coding genes, whereas the maternal alleles includes the MEG3, MEG8 and RTL1as long non-coding RNAs, pseudogenes, several microRNAs (miRNAs), and small nucleolar RNAs (snoRNAs). Differentially methylated regions (IG-DMR and MEG3-DMR) are shown as lollipops. MEG3-DMR is located within the MEG3 promoter
The 14q32 imprinted region includes two DMRs, the germline-derived primary DLK1-DIO3 inter-genic DMR (IG-DMR), which functions hierarchically and is an upstream regulator of the postfertilization-derived secondary MEG3-DMR which gains the appropriate methylation pattern during gestation, while, the existence of a third DMR located within the last exon of DLK1 has been proposed [6, 34].
Repressive histone marks are also found in MEG3-DMR and it is suggested that chromatin regulatory factors may play a role in MEG3 imprinting in embryonic stem cells (ESCs). However, it seems unlikely that repressive marks maintain MEG3 imprinting in mid-gestation embryos, probably because silencing of the other parental allele can be achieved through DNA methylation alone [54].
In an effort to study the in vivo effects of Hotair lncRNA deletion, Li et al. developed a mouse model in which Hotair-targeted deletion increased the expression of approximately 30 different genes from imprinted loci. These included Dlk1, Dio3, and Meg3 from the specific cluster. The results suggested that Hotair affects the Dlk1-Dio3 locus principally through control of histone methylation genome-wide in vivo, proposing moreover, that a lncRNA might affect the expression of other lncRNAs [43].
The miRNAs of the DLK1-DIO3 cluster
Several miRNAs of the cluster form a regulatory feedback loop by regulating the expression of all coding and non-coding genes within the cluster in fully pluripotent stem cells [48]. Recently, several miRNAs of the cluster have been implicated in the pathogenesis of acute promyelocytic leukemia, others have been used for early detection of specific solid cancers, while others have been involved in the pathogenesis of benign diseases such as diabetes [35, 50, 52].
DLK1
DLK1 encodes a transmembrane glycoprotein with six epidermal growth factor-like (EGF) motifs in the extracellular domain, similar to those present in the Delta/Notch/Serrate family of signaling molecules. DLK1 can interact with NOTCH through specific EGF-like repeats acting as a NOTCH antagonist, and can also affect the ERK/MAPK pathway and the FGF signaling pathway [20, 24, 69]. High DLK1 expression levels are observed in diverse embryonic tissues during early organogenesis, especially in placenta, liver, adipose tissue, skeletal muscle, lung, vertebrae, the pituitary and adrenal glands. Dlk1-deficient mice exhibit increased perinatal lethality, growth retardation, defects in postnatal neurogenesis, accelerated adiposity and other developmental defects [2, 3, 24, 25, 72].
In addition, DLK1 plays a role in hematopoiesis by inhibiting hematopoietic cell differentiation and is essential for fetal hematopoiesis in the liver at the time of HSCs generation and has a negative impact on HSPCs. Absence of Dlk1 causes a similar expansion of both HSCs and/or progenitors, while at the same time maintains their normal differentiation potential, giving rise to an expanded progenitor pool [37, 42, 55].
DLK1 and tumorigenesis
DLK1 is aberrantly expressed in several different types of solid and blood cancers conferring tumor cells a cancer stem cell-like phenotype. This effect is achieved via the prohibitin (PHB1/2) complex as observed in xenograft models, or through the constitutive activation of IκB kinase 2 (IKK2) promoting IKK2/NF-κB activation [7, 8, 74, 80]. Dlk1-expressing cells exhibit a potential of self-renewal and also exhibit chemoresistance similar to that reported for cancer stem and progenitor cells [40, 78].
DLK1 was aberrantly overexpressed characteristically in myelodysplastic syndrome (MDS) [41, 51, 82, 83], a subset of acute myeloid leukemia (AML) [39] cases, and might also contribute to primary myelofibrosis (PMF) pathogenesis [4, 28].
DIO3
DIO3 is the most distal gene of the imprinted cluster and encodes the enzyme D3 essential for thyroid hormone regulation since it degrades thyroid hormone in inactive metabolites. Similar to DLK1, DIO3 is highly expressed in fetal and placental tissues protecting the fetus and its developing tissues from the exposure to high maternal thyroid hormone levels. On the contrary, adult tissues have much lower expression levels of DIO3, which is however, re-expressed during proliferation and cell growth after tissue damage suggesting an involvement in tissue remodeling [23, 32]. The finding that Dio3 expression in rat brown preadipocytes is associated with cell proliferation rather than differentiation in an expression pattern similar to Dlk1, indicates that Dio3 expression correlates with cells that are in an undifferentiated status [31]. DIO3 promoter chromatin is characterized by high levels of histone 3 lysine 4 dimethylation (H3K4me2) in proliferating muscle cells that decrease promptly on differentiation, whereas DIO3 expression can be repressed by the demethylase LSD-1 suggesting an epigenetic regulatory mechanism of expression during different stages of differentiation [1].
DIO3 and tumorigenesis
Interestingly, DIO3 was found hypermethylated in a subset of human B cell, T-cell and myeloid malignancies as assessed by microarray-based methylation analysis, suggesting that aberrant epigenetic modifications may confer DIO3 tumor-associated properties [53].
RTL1
RTL1 is expressed mainly at the embryonic stages in some tissues and in the placenta where it is essential for the proper development in an extent similar to the other coding genes of the cluster [13]. It is essential for the maintenance of the fetal capillaries, and its abnormal expression might cause late-fetal and/or neonatal lethality in mice probably due to placental infarction [63]. Rtl1 expression promotes hepatocytes growth in vitro in the presence of extracellular matrix, while in vivo is able to promote liver tumorigenesis [59]. Rtl1-imprinting process seems to be under the control of the hosted miRNAs at least in cloned mouse embryos [19]. Concomitant over- and underexpression of Dlk1 and Rtl1 induced perinatal lethality, while restored expression failed to rescue lethality in IG-DMR deficient mice [70].
MEG3
MEG3 is a lncRNA gene with 12 cDNA isoforms identified to date, possessing tumor-suppressor properties. It is reciprocally imprinted with DLK1, regulating its expression in mouse ESCs (mESCs) by direct binding to PRC2 and recruitment of Ezh2 to Dlk1 promoter, and by increasing H3K27me3 in both DMRs [5, 84]. MEG3 is involved in normal cell biology and proper development as Meg3 deletion leads to perinatal death in mice, and it is also involved in the pathogenesis of a wide range of benign and malignant tumors. It interacts with fundamental key players in cell biology such as p53, MDM2, Rb and vascular endothelial growth factor (VEGF), thus affecting cell proliferation, differentiation, survival and angiogenesis [12]. Altered methylation pattern of MEG3-DMR observed in children born to obese mothers suggests that parental lifestyle may cause transgenerational epigenetic effects [66].
MEG3 and tumorigenesis
Aberrant DNA methylation of MEG3 has been detected in AML, MDS, multiple myeloma (MM) and adenomas, and has been associated with disease progression and clinical outcomes [9, 10, 86]. Variable MEG3 methylation levels were reported in AML without though being associated with the abnormally expressed DLK1 [39]. Methylation of MEG3 probably represents the fundamental silencing mechanism as no loss-of-function genetic mutations have been reported at least in non-functioning pituitary adenomas [85]. During megakaryopoietic differentiation of MDS, DLK1 and MEG3 were downregulated in developing megakaryocytes of both low- and high-risk MDS patients. The increased DLK1 expression in CD34+ cells and the concomitant decreased expression of DLK1 and MEG3 during megakaryocytic differentiation suggests that both genes are probably involved in abnormal megakaryocytic differentiation and proliferation in MDS [27].
MEG8
MEG8 (Rian) is another lncRNA suggested to play functional roles in the development of diverse organs during embryonic development, and together with RTL1as are both involved in chromosome 14 uniparental disomy with characteristic phenotypes [13, 26].
DLK1-DIO3 imprinted genes in transcription factor induced pluripotency
Fully differentiated somatic cells reprogrammed to induced pluripotent stem cells (iPSCs) are pluripotent stem cells (PSCs) with molecular and functional properties similar to ESCs. However, genetic and epigenetic variations exist between iPSC lines, between iPSC and ESC lines, and different passages of the same iPSC line [47]. These variations prompted scientists to the continuous search for markers that could help to distinguish between iPSCs and ESCs bringing to the surface the importance of the DLK1-DIO3 imprinted cluster. Is there, however, a role for the DLK1-DIO3 imprinted domain in ESCs and iPSCs? Has the specific cluster the potential to be used as a marker of distinction between iPSCs and ESCs, and is such distinction important? Can the specific imprinted cluster be used to identify highly pluripotent and safe human iPSCs? The studies described below report very interesting findings that may partially answer these questions (Table 1).
Table 1.
Studies describing the current data on the DLK1-DIO3 members expression in iPSCs and ESCs, and the correlation with DNA methylation, and the transcription factors used in reprogramming
| DLK1-DIO3 member | Species | Gene expression levels | Comments | Reference |
|---|---|---|---|---|
| MEG3 | Human | Aberrantly expressed in iPSCs, no expression in ESCs | iPSCs should be examined for the expression of imprinted genes as a gold standard procedure | [57] |
| MEG3 | Mouse | Silenced | Kf4 and Oct4 bind to Meg3 promoter establishing methylation in iPSCs | [65] |
| MEG3 | Mouse | Silenced | Stoichiometry of the reprogramming TFs is more important than the DLK1-DIO3 LOI | [16] |
| MEG3 | Mouse | Silenced | Lower methylation levels in iPSCs than in the respective ESCs | [71] |
| MEG3 | Human | Silenced | MEG3 positives were indistinguishable from MEG3-negative iPSCs suggesting that aberrant MEG3-DMR occurs in early passaging | [56] |
| MEG3 | Human | Active in initial ESCs, silenced in early ESCs | Associated with reduced DNA damage-induced apoptosis in early ESCs associated with culture conditions | [77] |
| MEG3, RIAN microRNAS | Mouse | Silenced | Predictive of developmental potential | [58] |
| MEG3 | Mouse | Silenced | Hosted microRNAs control the expression of the cluster via PRC2 | [48] |
| microRNAS | Mouse | Downregulated | Increased microRNAs expression early in reprogramming reduces reprogramming efficiency | [30] |
| MEG3, DLK1, DIO3 | Porcine | MEG3 downregulation, DLK1 and DIO3 not significantly changed | MEG3 was irreversibly downregulated in early stage reprogramming | [17] |
| MEG3 | Human | Downregulated | MEG3-negative ESCs exhibit similar DNA methylation variation levels and gene expression as MEG3-positive ESCs | [14] |
iPSCs induced pluripotent stem cells, ESCs embryonic stem cells, TFs transcription factors, DMR differentially methylated region, LOI loss of imprinting, PRC2 polycomb repressive complex 2
Embryonic stem cells and iPSCs can be considered an unlimited source of patient-specific HSCs. Recent studies have provided proof-of-principle that genetically corrected iPSCs can be used to treat several different diseases including hematological disorders [60]. iPSCs have the potential to model hematological malignancies whereas, reciprocally hematological diseases may serve as an in vivo model for iPSCs biology [38]. However, at present there is no way to differentiate human ESCs/iPSCs into transplantable HSCs to generate engraftable hematopoietic stem progenitor cells (HSPCs) [21]. However, a system has been developed to respecify lineage-restricted CD34(+)CD45(+) myeloid precursors derived from hPSCs into multilineage progenitors that can be expanded in vitro and engrafted in vivo [22].
In mESCs, the Dlk1-Dio3 cluster expression seems to be epigenetically regulated in a reversible manner under the orchestrated action of H1 histone. H1 histone promotes recruitment of Dnmt1 and Dnmt3b to the ICR, and inhibits binding of SET7/9 methyltransferase H3K4me in chromatin. These data suggest that H1-linker histone promotes Dlk1-Dio3 silencing through the combined action of two epigenetic modifications [81]. However, the unstable genomic imprinting of MEG3 observed in human ESCs and human iPSCs has been attributed to DNA hypermethylation [36]. Furthermore, the PRC2 accessory component JARID2 binds to MEG3 in vivo, and probably represents the largest contribution to the affinity of MEG3 for PRC2, independently of EZH2 binding. MEG3 acts in trans on JARID2 and PRC2 by facilitating their recruitment on target genes in human iPSCs and mESCs. Lack of MEG3 in iPSCs alters finally the chromatin distribution of H3K27me3, PRC2, and JARID2. Hence, the PRC2–MEG3 interactions may play a role in induced pluripotency [36].
DLK1-DIO3 cluster exhibits different expression patterns during different number of passages, being active in initial ESCs (<10 passages) while it is silenced in early ESCs (20–30 passages). Such differential expression is inheritable during differentiation, although silencing does not compromise the multilineage differentiation of ESCs but is associated with reduced DNA damage-induced apoptosis in early ESCs. Probably, such silencing is carried over by differentiated progeny and may increase the anti-apoptotic ability in both ESCs and their differentiated counterparts. Surprisingly, a common pattern of gene expression change between initial ESCs and early ESCs was observed across different lines involving, MEG3, SNORD114–3 in the imprinted cluster which were however significantly downregulated in most early ESCs. The above results suggest that the DLK1-DIO3 cluster is normally imprinted in initial ESCs, but becomes completely silenced during early culture. Activation of the DLK1-DIO3 cluster in initial ESCs seems a normal status inherited from embryonic cells, and silencing of MEG3 expression in early ESCs probably represents an abnormal variation acquired through early culture. These observations of gene expression changes in the DLK1-DIO3 cluster provide new insight into the epigenetic instability during the early culture of ESCs, which may help to better characterize the genuine molecular profile of ESCs. Furthermore, DLK1-DIO3 silencing is probably an aberrant change resulting from inappropriate culture conditions, especially the use of atmospheric oxygen [77].
DLK1-DIO3 cluster represents a specific genomic region that has been reported to possess the unique property of distinguishing murine iPSCs from ESCs, and identifies iPSCs clones with the full development potential of ESCs. Silencing of the DLK1-MEG3 cluster seems to be a unique event of reprogramming, and occurs in iPSCs derived from different cell types at various stages of differentiation [49, 67]. Moreover, the pluripotency levels of stable mouse iPS cells generated without c-Myc positively correlate with the activation of Dlk1-Dio3, indicating its potential as a marker to select fully pluripotent iPSCs lines [44].
Meg3, Rian, and several miRNAs within the cluster were repressed in murine iPSCs clones compared to ESCs indicating that the expression of a small number of transcripts of a single (Meg3) imprinted gene distinguishes most mouse iPSCs from ESCs and is predictive for their developmental potential [67]. Although this study was considered compromised because restoration of expression and rescue of the phenotype were not evaluated, it reopened the discussion of imprinted genes involvement in stemness [58]. Dlk1-Dio3 cluster was also found activated in tetraploid blastocyst complementated mouse embryos iPSCs (4n-iPSCs) compared to a repressed expression in 2n-iPSCs. The degree of activation of the cluster positively correlated with the pluripotency levels of iPSCs [48]. Dlk1-Dio3 might be the only genomic region with a clear on- and -off switch in fully pluripotent versus partial pluripotent cells.
With regard to miRNAs, Dlk1-Dio3 hosted miRNAs were downregulated at the earliest stages of reprogramming, and is assumed that such downregulation may improve reprogramming efficiency. That unique property of the cluster could represent a marker potential to identify fully iPSCs or ESCs [30, 48].
The notion of MEG3 as a single gene signature was contradicted by scientists who reported that MEG3 exhibited moderate specificity, since most of the ESCs lines were MEG3 negative and incorrectly classified as iPSCs in humans. It is not clear, however, if that difference is species specific [14, 17]. Similarly, Pick et al. reported that MEG3 showed aberrantly high expression levels in several iPSCs lines compared to human ESCs, and also showed a clone-specific aberrant MEG3 expression depending on the transcription factor (TF) used in reprogramming. Authors stated that analysis of imprinted genes will be crucial in iPSCs used in regenerative medicine to avoid tumor generation in recipients, because the aberrant expression of imprinted genes could cause problems with cell differentiation and promote even malignant transformation, while abnormal imprinting could confuse disease phenotype with imprinting disorders [57]. Klf4 was recruited to the Meg3 promoter during reprogramming whereas, iPSCs clones underwent Meg3 silencing after transgene excision, suggesting that the core reprogramming factors may be directly involved in the reprogramming process. In support of this idea, binding sites for Oct4 and Klf4 were identified in the Dlk1-Dio3 region, and further analysis revealed increased recruitment of Klf4 at Meg3 in transgene-carrying iPSCs. These findings support the notion of an active role of Klf4 (and possibly Oct4) in establishing the methylation status of Meg3 and suggest that, when present at abnormally high levels, Klf4 may protect this region from cytosine methylation [65]. Carey et al. in their elegant work showed that high expression levels of Oct4 and Klf4 combined with lower expression levels of c-Myc and Sox2 produced iPSCs cells that efficiently generated all-iPSC mice by tetraploid complementation, maintained normal imprinting at the Dlk1-Dio3 locus, and importantly did not create mice with tumors. Therefore, it was concluded that LOI at the Dlk1-Dio3 locus did not strictly correlate with reduced pluripotency. The efficiency however of generating all-iPSC mice was diminished, and hence, silencing of the specific imprinted cluster could not be used as an absolute marker of reduced pluripotency. It seems that the stoichiometry of the reprogramming factors influences the genetic and epigenetic status of the Dlk1-Dio3 locus as suggested by the expression levels of Oct4 and Klf4 during reprogramming, which can influence the epigenetic conformation of the IG-DMR. The findings of the study argued in favor of the TF stoichiometry rather than the absolute factor expression levels used to generate iPSCs, playing an important role in determining the epigenetic and pluripotent state of iPSCs [16].
The role of epigenetic modifications
Yet, it is not well understood how genomic imprinting is reprogrammed in iPSCs although it seems that DNA hypermethylation and histone methylation are the key players [36]. DNA hypermethylation of DLK1-DIO3 and its silencing might prevent cells from becoming fully pluripotent iPSCs. The loss of the parentally inherited imprint among iPSCs clones could be attributed to the variations in epigenetic reprogramming during the derivation of iPSCs. Dlk1-Dio3 methylation levels were maintained in most mouse iPSCs clones but were, however, lower than the respective levels in mESCs. For several other imprinted genes, DMR methylation was variably lost in iPSCs clones. These findings suggest that different imprinted regions exhibit different mechanisms in maintaining DNA methylation imprint. Probably, different epigenetic mechanisms may be present at different imprinted regions in the maintenance of imprint in iPSCs [71]. In fact, Nishino et al. studied the epigenetic and transcriptional status of human iPSCs deriving from five cell types of different origins during different passages. MEG3, among several other imprinted genes, showed aberrant MEG3-DMR methylation in human iPSCs with consequent MEG3 silencing. However, MEG3-negative iPSCs were almost indistinguishable from MEG3-positive iPSCs in DNA methylation profile and gene expression, suggesting that aberrant MEG3-DMR hypermethylation probably occurs at early passage and fixed at later stages of iPSCs passaging [56]. Such repression of Meg3 acts as a roadblock for the establishment of fully pluripotent iPSCs and for the achievement of high-grade chimerism in mice. That obstacle can be overridden through treatment with chemical compounds such as with the simple supplementation of vitamin C. Such vitamins prevent MEG3 silencing by preservation of active chromatin marks, which in turn inhibit Dnmt3a recruitment to the IG-DMR preserving a normal imprinting status [68]. Moreover, treatment of activated B-cells with the methyltransferase inhibitor 5-aza-2′-deoxycytidine before and at early stages of reprogramming attenuates hypermethylation of the Dlk1-Dio3 locus in resultant iPSCs and enables them to form high-grade chimerism in mice. Collectively, these findings highlight the necessity of developing reprogramming conditions free of Dlk-Dio3 locus hypermethylation during iPSC derivation [75]. However, aberrant DNA methylation within the Dlk1-Dio3 locus did not, in contrast to previous observations, preclude the generation of 4n-iPSCs although the efficiency of generating mice by tetraploid complementation decreased with aberrant methylation at this locus [16].
Other strategies, involving miRNAs, for achieving ideal reprogramming conditions have been developed. Recent data suggest that miR-29b enhances iPSCs generation by targeting Dnmts, which serve as a barrier to reprogramming. Sox2, in turn directly regulates miR-29b, and miR-29b-derived iPSCs lines maintain the active expression of Meg3, Rian and hosted miRNAs which are critical for development. These data indicate that Sox2-triggered miR-29b-DNMT signaling plays a crucial role in DNA methylation-related reprogramming events, including the regulation of Dlk1-Dio3 transcription silencing [29].
Conclusions
Imprinted genes are involved in several different biological and behavioral processes in different higher organisms and the DLK1-DIO3 cluster seems to represent one of the most important imprinted domains. Induced pluripotency and reprogramming is a promising field in regenerative medicine and a new tool to model genetic diseases. iPSCs are similar to ESCs but still not completely equivalent, nevertheless they complement each other [60]. Point mutations, copy number variations, and epigenetic changes differentiate iPSCs from ESCs. Undoubtedly, the role of iPSCs in personal regenerative medicine offers new perspectives, but problems regarding the differentiation or the possible malignant transformation of iPSCs should be solved first. Therefore, the quest for markers that would be able to distinguish ESCs-equivalent iPSCs has begun. Important findings nominated Dlk1-Dio3 cluster as an exclusive marker for all iPSCs. Silenced Dlk1-Dio3 cluster contributed poorly to chimeras and did not generate all iPSCs in mice, in contrast to iPSCs with normal Dlk1-Dio3 expression ***which generate high-grade chimeras. However, contradictory findings reported by others on the ability of the specific locus to represent a unique marker of iPSCs suggest that the Dlk1-Dio3 cluster has no significance in reprogramming, one can argue. Meg3 expression was increased in several iPSCs lines compared to the ESCs, whereas the majority of other imprinted genes presented normal monoallelic expression. Either repressed or enhanced expression of MEG3 in iPSCs lines has a distinct pattern of expression in reprogramming that confers MEG3 unique characteristics compared to other imprinted genes. Collectively, these results bring out the notion that iPSCs may behave differently based on variations of specific genomic regions, and that the Dlk1-Dio3 activation or silencing depends on the stage of reprogramming. The findings that specific TF stoichiometry used for reprogramming might affect genetic and epigenetic modifications in the DLK1-DIO3 domain might suggest that the imprinted cluster represents a marker only in the case of some exclusive factors used in reprogramming. Therefore, DLK1-DIO3 status should be evaluated in the case of reprogramming with other factors considering that the original Yamanaka’s factors (Oct4, Klf4, Sox2, and c-Myc) are all dispensable in reprogramming [18]. Perhaps, it is the methylation level of the cluster that should be taken into consideration as marker of pluripotency given that iPSCs exhibit constantly present but lower methylation levels than the respective ESCs. The fact that this particular cluster is self-regulated by the hosted miRNAs probably suggests that the cluster should be considered in its entirety including miRNAs, snoRNas and not only macroRNAs. Furthermore, another issue that should be considered in reprogramming is the gene dosage of the imprinted genes that are involved in embryonic development plasticity [20, 58].
It is not clear whether the identification of the DLK1-DIO3 profile as a marker in reprogramming has cleared or fogged the landscape, but it is sure that its influence on the pluripotency level of iPSCs merits further evaluation.
Acknowledgments
We would like to thank Nikolaos Benetatos MD for his helpful comments. We apologize to those authors whose work has not been cited.
Conflict of interest
None.
References
- 1.Ambrosio R, Damiano V, Sibilio A, et al. Epigenetic control of type 2 and 3 deiodinases in myogenesis: role of Lysine-specific Demethylase enzyme and FoxO3. Nucleic Acids Res. 2013;41:3551–3562. doi: 10.1093/nar/gkt065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersen DC, Laborda J, Baladron V, et al. Dual role of delta-like 1 homolog (DLK1) in skeletal muscle development and adult muscle regeneration. Development. 2013;140:3743–3753. doi: 10.1242/dev.095810. [DOI] [PubMed] [Google Scholar]
- 3.Andersen DC, Petersson SJ, Jorgensen LH, et al. Characterization of DLK1 + cells emerging during skeletal muscle remodeling in response to myositis, myopathies, and acute injury. Stem Cells. 2009;27:898–908. doi: 10.1634/stemcells.2008-0826. [DOI] [PubMed] [Google Scholar]
- 4.Argiropoulos B, Palmqvist L, Imren S, et al. Meis1 disrupts the genomic imprint of Dlk1 in a NUP98-HOXD13 leukemia model. Leukemia. 2010;24:1788–1791. doi: 10.1038/leu.2010.161. [DOI] [PubMed] [Google Scholar]
- 5.Balik V, Srovnal J, Sulla I, et al. MEG3: a novel long noncoding potentially tumor-suppressing RNA in meningiomas. J Neurooncol. 2013;112:1–8. doi: 10.1007/s11060-012-1038-6. [DOI] [PubMed] [Google Scholar]
- 6.Barlow DP, Bartolomei MS (2014) Genomic imprinting in mammals. Cold Spring Harb Perspect Biol 6(2). doi:10.1101/cshperspect.a018382 [DOI] [PMC free article] [PubMed]
- 7.Begum A, Kim Y, Lin Q, et al. DLK1, delta-like 1 homolog (Drosophila), regulates tumor cell differentiation in vivo. Cancer Lett. 2012;318:26–33. doi: 10.1016/j.canlet.2011.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Begum A, Lin Q, Yu C, et al. Interaction of delta-like 1 homolog (Drosophila) with prohibitins and its impact on tumor cell clonogenicity. Mol Cancer Res. 2014;12:155–164. doi: 10.1158/1541-7786.MCR-13-0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benetatos L, Dasoula A, Hatzimichael E, et al. Promoter hypermethylation of the MEG3 (DLK1/MEG3) imprinted gene in multiple myeloma. Clin Lymphoma Myeloma. 2008;8:171–175. doi: 10.3816/CLM.2008.n.021. [DOI] [PubMed] [Google Scholar]
- 10.Benetatos L, Hatzimichael E, Dasoula A, et al. CpG methylation analysis of the MEG3 and SNRPN imprinted genes in acute myeloid leukemia and myelodysplastic syndromes. Leuk Res. 2010;34:148–153. doi: 10.1016/j.leukres.2009.06.019. [DOI] [PubMed] [Google Scholar]
- 11.Benetatos L, Hatzimichael E, Londin E, et al. The microRNAs within the DLK1-DIO3 genomic region: involvement in disease pathogenesis. Cell Mol Life Sci. 2013;70:795–814. doi: 10.1007/s00018-012-1080-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benetatos L, Vartholomatos G, Hatzimichael E. MEG3 imprinted gene contribution in tumorigenesis. Int J Cancer. 2011;129:773–779. doi: 10.1002/ijc.26052. [DOI] [PubMed] [Google Scholar]
- 13.Benetatos L, Voulgaris E, Vartholomatos G. DLK1-MEG3 imprinted domain microRNAs in cancer biology. Crit Rev Eukaryot Gene Expr. 2012;22:1–15. doi: 10.1615/CritRevEukarGeneExpr.v22.i1.10. [DOI] [PubMed] [Google Scholar]
- 14.Bock C, Kiskinis E, Verstappen G, et al. Reference Maps of human ES and iPS cell variation enable high-throughput characterization of pluripotent cell lines. Cell. 2011;144:439–452. doi: 10.1016/j.cell.2010.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brandvain Y, Van Cleve J, Ubeda F, et al. Demography, kinship, and the evolving theory of genomic imprinting. Trends Genet. 2011;27:251–257. doi: 10.1016/j.tig.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 16.Carey BW, Markoulaki S, Hanna JH, et al. Reprogramming factor stoichiometry influences the epigenetic state and biological properties of induced pluripotent stem cells. Cell Stem Cell. 2011;9:588–598. doi: 10.1016/j.stem.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 17.Cheng D, Li Z, Liu Y, et al. Kinetic analysis of porcine fibroblast reprogramming toward pluripotency by defined factors. Cell Reprogram. 2012;14:312–323. doi: 10.1089/cell.2012.0025. [DOI] [PubMed] [Google Scholar]
- 18.Chou BK, Cheng L. And then there were none: no need for pluripotency factors to induce reprogramming. Cell Stem Cell. 2013;13:261–262. doi: 10.1016/j.stem.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cui XS, Zhang DX, Ko YG, et al. Aberrant epigenetic reprogramming of imprinted microRNA-127 and Rtl1 in cloned mouse embryos. Biochem Biophys Res Commun. 2009;379:390–394. doi: 10.1016/j.bbrc.2008.12.148. [DOI] [PubMed] [Google Scholar]
- 20.Da Rocha ST, Charalambous M, Lin SP, et al. Gene dosage effects of the imprinted delta-like homologue 1 (dlk1/pref1) in development: implications for the evolution of imprinting. PLoS Genet. 2009;5:e1000392. doi: 10.1371/journal.pgen.1000392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doulatov S, Notta F, Laurenti E, et al. Hematopoiesis: a human perspective. Cell Stem Cell. 2012;10:120–136. doi: 10.1016/j.stem.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 22.Doulatov S, Vo LT, Chou SS, et al. Induction of multipotential hematopoietic progenitors from human pluripotent stem cells via respecification of lineage-restricted precursors. Cell Stem Cell. 2013;13:459–470. doi: 10.1016/j.stem.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dudek KM, Suter L, Darras VM, et al. Decreased translation of Dio3 mRNA is associated with drug-induced hepatotoxicity. Biochem J. 2013;453:71–82. doi: 10.1042/BJ20130049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Falix FA, Aronson DC, Lamers WH, et al. Possible roles of DLK1 in the Notch pathway during development and disease. Biochim Biophys Acta. 2012;1822:988–995. doi: 10.1016/j.bbadis.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 25.Ferron SR, Charalambous M, Radford E, et al. Postnatal loss of Dlk1 imprinting in stem cells and niche astrocytes regulates neurogenesis. Nature. 2011;475:381–385. doi: 10.1038/nature10229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gu T, He H, Han Z, et al. Expression of macro non-coding RNAs Meg8 and Irm in mouse embryonic development. Acta Histochem. 2012;114:392–399. doi: 10.1016/j.acthis.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 27.Gueller S, Komor M, Nowak D, et al. Identification of defects in the transcriptional program during lineage-specific in vitro differentiation of CD34(+) cells selected from patients with both low- and high-risk myelodysplastic syndrome. Exp Hematol. 2010;38:718–732. doi: 10.1016/j.exphem.2010.04.018. [DOI] [PubMed] [Google Scholar]
- 28.Guglielmelli P, Zini R, Bogani C, et al. Molecular profiling of CD34+ cells in idiopathic myelofibrosis identifies a set of disease-associated genes and reveals the clinical significance of Wilms’ tumor gene 1 (WT1) Stem Cells. 2007;25:165–173. doi: 10.1634/stemcells.2006-0351. [DOI] [PubMed] [Google Scholar]
- 29.Guo X, Liu Q, Wang G, et al. MicroRNA-29b is a novel mediator of Sox2 function in the regulation of somatic cell reprogramming. Cell Res. 2013;23:142–156. doi: 10.1038/cr.2012.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Henzler CM, Li Z, Dang J, et al. Staged miRNA re-regulation patterns during reprogramming. Genome Biol. 2013;14:R149. doi: 10.1186/gb-2013-14-12-r149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hernandez A, Garcia B, Obregon MJ. Gene expression from the imprinted Dio3 locus is associated with cell proliferation of cultured brown adipocytes. Endocrinology. 2007;148:3968–3976. doi: 10.1210/en.2007-0029. [DOI] [PubMed] [Google Scholar]
- 32.Janssen R, Zuidwijk M, Muller A, et al. Cardiac expression of deiodinase type 3 (Dio3) following myocardial infarction is associated with the induction of a pluripotency microRNA signature from the Dlk1-Dio3 genomic region. Endocrinology. 2013;154:1973–1978. doi: 10.1210/en.2012-2017. [DOI] [PubMed] [Google Scholar]
- 33.John RM, Lefebvre L. Developmental regulation of somatic imprints. Differentiation. 2011;81:270–280. doi: 10.1016/j.diff.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 34.Kagami M, O’sullivan MJ, Green AJ, et al. The IG-DMR and the MEG3-DMR at human chromosome 14q32.2: hierarchical interaction and distinct functional properties as imprinting control centers. PLoS Genet. 2010;6:e1000992. doi: 10.1371/journal.pgen.1000992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kameswaran V, Bramswig NC, Mckenna LB, et al. Epigenetic regulation of the DLK1-MEG3 microRNA cluster in human type 2 diabetic islets. Cell Metab. 2014;19:135–145. doi: 10.1016/j.cmet.2013.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaneko S, Bonasio R, Saldana-Meyer R, et al. Interactions between JARID2 and noncoding RNAs regulate PRC2 recruitment to chromatin. Mol Cell. 2014;53:290–300. doi: 10.1016/j.molcel.2013.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaneta M, Osawa M, Sudo K, et al. A role for pref-1 and HES-1 in thymocyte development. J Immunol. 2000;164:256–264. doi: 10.4049/jimmunol.164.1.256. [DOI] [PubMed] [Google Scholar]
- 38.Kelley JM, Daley GQ. Hematopoietic defects and iPSC disease modeling: lessons learned. Immunol Lett. 2013;155:18–20. doi: 10.1016/j.imlet.2013.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khoury H, Suarez-Saiz F, Wu S, et al. An upstream insulator regulates DLK1 imprinting in AML. Blood. 2010;115:2260–2263. doi: 10.1182/blood-2009-03-212746. [DOI] [PubMed] [Google Scholar]
- 40.Kim Y, Lin Q, Zelterman D, et al. Hypoxia-regulated delta-like 1 homologue enhances cancer cell stemness and tumorigenicity. Cancer Res. 2009;69:9271–9280. doi: 10.1158/0008-5472.CAN-09-1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Langer F, Stickel J, Tessema M, et al. Overexpression of delta-like (Dlk) in a subset of myelodysplastic syndrome bone marrow trephines. Leuk Res. 2004;28:1081–1083. doi: 10.1016/j.leukres.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 42.Li L, Forman SJ, Bhatia R. Expression of DLK1 in hematopoietic cells results in inhibition of differentiation and proliferation. Oncogene. 2005;24:4472–4476. doi: 10.1038/sj.onc.1208637. [DOI] [PubMed] [Google Scholar]
- 43.Li L, Liu B, Wapinski OL, et al. Targeted disruption of Hotair leads to homeotic transformation and gene derepression. Cell Rep. 2013;5:3–12. doi: 10.1016/j.celrep.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li W, Zhao XY, Wan HF, et al. iPS cells generated without c-Myc have active Dlk1-Dio3 region and are capable of producing full-term mice through tetraploid complementation. Cell Res. 2011;21:550–553. doi: 10.1038/cr.2011.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li X. Genomic imprinting is a parental effect established in mammalian germ cells. Curr Top Dev Biol. 2013;102:35–59. doi: 10.1016/B978-0-12-416024-8.00002-7. [DOI] [PubMed] [Google Scholar]
- 46.Li Y, Sasaki H. Genomic imprinting in mammals: its life cycle, molecular mechanisms and reprogramming. Cell Res. 2011;21:466–473. doi: 10.1038/cr.2011.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liang G, Zhang Y. Genetic and epigenetic variations in iPSCs: potential causes and implications for application. Cell Stem Cell. 2013;13:149–159. doi: 10.1016/j.stem.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu L, Luo GZ, Yang W, et al. Activation of the imprinted Dlk1-Dio3 region correlates with pluripotency levels of mouse stem cells. J Biol Chem. 2010;285:19483–19490. doi: 10.1074/jbc.M110.131995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lujan E, Wernig M. An imprinted signature helps isolate ESC-equivalent iPSCs. Cell Res. 2010;20:974–976. doi: 10.1038/cr.2010.117. [DOI] [PubMed] [Google Scholar]
- 50.Luk JM, Burchard J, Zhang C, et al. DLK1-DIO3 genomic imprinted microRNA cluster at 14q32.2 defines a stemlike subtype of hepatocellular carcinoma associated with poor survival. J Biol Chem. 2011;286:30706–30713. doi: 10.1074/jbc.M111.229831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ma X, Zhang Y, Yang L, et al. The effects of increased expression of DLK1 gene on the pathogenesis of myelodysplastic syndromes. Clin Lymphoma Myeloma Leuk. 2012;12:261–268. doi: 10.1016/j.clml.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 52.Manodoro F, Marzec J, Chaplin T, et al. Loss of imprinting at the 14q32 domain is associated with microRNA overexpression in acute promyelocytic leukemia. Blood. 2014;123:2066–2074. doi: 10.1182/blood-2012-12-469833. [DOI] [PubMed] [Google Scholar]
- 53.Martin-Subero JI, Ammerpohl O, Bibikova M, et al. A comprehensive microarray-based DNA methylation study of 367 hematological neoplasms. PLoS One. 2009;4:e6986. doi: 10.1371/journal.pone.0006986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mcmurray EN, Schmidt JV. Identification of imprinting regulators at the Meg3 differentially methylated region. Genomics. 2012;100:184–194. doi: 10.1016/j.ygeno.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mirshekar-Syahkal B, Haak E, Kimber GM, et al. Dlk1 is a negative regulator of emerging hematopoietic stem and progenitor cells. Haematologica. 2013;98:163–171. doi: 10.3324/haematol.2012.070789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nishino K, Toyoda M, Yamazaki-Inoue M, et al. DNA methylation dynamics in human induced pluripotent stem cells over time. PLoS Genet. 2011;7:e1002085. doi: 10.1371/journal.pgen.1002085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pick M, Stelzer Y, Bar-Nur O, et al. Clone- and gene-specific aberrations of parental imprinting in human induced pluripotent stem cells. Stem Cells. 2009;27:2686–2690. doi: 10.1002/stem.205. [DOI] [PubMed] [Google Scholar]
- 58.Radford EJ, Ferron SR, Ferguson-Smith AC. Genomic imprinting as an adaptative model of developmental plasticity. FEBS Lett. 2011;585:2059–2066. doi: 10.1016/j.febslet.2011.05.063. [DOI] [PubMed] [Google Scholar]
- 59.Riordan JD, Keng VW, Tschida BR, et al. Identification of rtl1, a retrotransposon-derived imprinted gene, as a novel driver of hepatocarcinogenesis. PLoS Genet. 2013;9:e1003441. doi: 10.1371/journal.pgen.1003441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Robinton DA, Daley GQ. The promise of induced pluripotent stem cells in research and therapy. Nature. 2012;481:295–305. doi: 10.1038/nature10761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schneider G, Bowser MJ, Shin DM, et al. The paternally imprinted DLK1-GTL2 locus is differentially methylated in embryonal and alveolar rhabdomyosarcomas. Int J Oncol. 2014;44:295–300. doi: 10.3892/ijo.2013.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Seisenberger S, Peat JR, Reik W. Conceptual links between DNA methylation reprogramming in the early embryo and primordial germ cells. Curr Opin Cell Biol. 2013;25:281–288. doi: 10.1016/j.ceb.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 63.Sekita Y, Wagatsuma H, Nakamura K, et al. Role of retrotransposon-derived imprinted gene, Rtl1, in the feto-maternal interface of mouse placenta. Nat Genet. 2008;40:243–248. doi: 10.1038/ng.2007.51. [DOI] [PubMed] [Google Scholar]
- 64.Semi K, Matsuda Y, Ohnishi K, et al. Cellular reprogramming and cancer development. Int J Cancer. 2013;132:1240–1248. doi: 10.1002/ijc.27963. [DOI] [PubMed] [Google Scholar]
- 65.Sommer CA, Christodoulou C, Gianotti-Sommer A, et al. Residual expression of reprogramming factors affects the transcriptional program and epigenetic signatures of induced pluripotent stem cells. PLoS One. 2012;7:e51711. doi: 10.1371/journal.pone.0051711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Soubry A, Murphy SK, Wang F et al (2013) Newborns of obese parents have altered DNA methylation patterns at imprinted genes. Int J Obes (Lond). doi:10.1038/ijo.2013.193 [DOI] [PMC free article] [PubMed]
- 67.Stadtfeld M, Apostolou E, Akutsu H, et al. Aberrant silencing of imprinted genes on chromosome 12qF1 in mouse induced pluripotent stem cells. Nature. 2010;465:175–181. doi: 10.1038/nature09017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stadtfeld M, Apostolou E, Ferrari F, et al. Ascorbic acid prevents loss of Dlk1-Dio3 imprinting and facilitates generation of all-iPS cell mice from terminally differentiated B cells. Nat Genet. 2012;44(398–405):S391–S392. doi: 10.1038/ng.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sul HS. Minireview: Pref-1: role in adipogenesis and mesenchymal cell fate. Mol Endocrinol. 2009;23:1717–1725. doi: 10.1210/me.2009-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Takahashi N, Kobayashi R, Kono T. Restoration of Dlk1 and Rtl1 is necessary but insufficient to rescue lethality in intergenic differentially methylated region (IG-DMR)-deficient mice. J Biol Chem. 2010;285:26121–26125. doi: 10.1074/jbc.M109.075325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Takikawa S, Ray C, Wang X, et al. Genomic imprinting is variably lost during reprogramming of mouse iPS cells. Stem Cell Res. 2013;11:861–873. doi: 10.1016/j.scr.2013.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Traustadottir GA, Kosmina R, Sheikh SP, et al. Preadipocytes proliferate and differentiate under the guidance of Delta-like 1 homolog (DLK1) Adipocyte. 2013;2:272–275. doi: 10.4161/adip.24994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Venkatraman A, He XC, Thorvaldsen JL, et al. Maternal imprinting at the H19-Igf2 locus maintains adult haematopoietic stem cell quiescence. Nature. 2013;500:345–349. doi: 10.1038/nature12303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vlantis K, Wullaert A, Sasaki Y, et al. Constitutive IKK2 activation in intestinal epithelial cells induces intestinal tumors in mice. J Clin Invest. 2011;121:2781–2793. doi: 10.1172/JCI45349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wesemann DR, Portuguese AJ, Magee JM, et al. Reprogramming IgH isotype-switched B cells to functional-grade induced pluripotent stem cells. Proc Natl Acad Sci USA. 2012;109:13745–13750. doi: 10.1073/pnas.1210286109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wolf JB. Evolution of genomic imprinting as a coordinator of coadapted gene expression. Proc Natl Acad Sci USA. 2013;110:5085–5090. doi: 10.1073/pnas.1205686110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xie P, Sun Y, Ouyang Q, et al. Physiological oxygen prevents frequent silencing of the DLK1-DIO3 cluster during human embryonic stem cells culture. Stem Cells. 2014;32:391–401. doi: 10.1002/stem.1558. [DOI] [PubMed] [Google Scholar]
- 78.Xu X, Liu RF, Zhang X, et al. DLK1 as a potential target against cancer stem/progenitor cells of hepatocellular carcinoma. Mol Cancer Ther. 2012;11:629–638. doi: 10.1158/1535-7163.MCT-11-0531. [DOI] [PubMed] [Google Scholar]
- 79.Yamaguchi S, Shen L, Liu Y, et al. Role of Tet1 in erasure of genomic imprinting. Nature. 2013;504:460–464. doi: 10.1038/nature12805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yanai H, Nakamura K, Hijioka S, et al. Dlk-1, a cell surface antigen on foetal hepatic stem/progenitor cells, is expressed in hepatocellular, colon, pancreas and breast carcinomas at a high frequency. J Biochem. 2010;148:85–92. doi: 10.1093/jb/mvq034. [DOI] [PubMed] [Google Scholar]
- 81.Yang SM, Kim BJ, Norwood Toro L, et al. H1 linker histone promotes epigenetic silencing by regulating both DNA methylation and histone H3 methylation. Proc Natl Acad Sci USA. 2013;110:1708–1713. doi: 10.1073/pnas.1213266110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yue LZ, Fu R, Wang HQ, et al. Expression of DLK1 Gene in the Bone Marrow Cells of Patients with Myelodysplastic Syndromes and Its Clinical Significance. Cancer Biol Med. 2012;9:188–191. doi: 10.7497/j.issn.2095-3941.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang W, Shao Z, Fu R, et al. Effect of DLK1 on tumorigenesis in CD34CD38 bone marrow cells in myelodysplastic syndromes. Oncol Lett. 2013;6:203–206. doi: 10.3892/ol.2013.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhao J, Ohsumi TK, Kung JT, et al. Genome-wide identification of polycomb-associated RNAs by RIP-seq. Mol Cell. 2010;40:939–953. doi: 10.1016/j.molcel.2010.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhou Y, Zhang X, Klibanski A. Genetic and epigenetic mutations of tumor suppressive genes in sporadic pituitary adenoma. Mol Cell Endocrinol. 2014;386:16–33. doi: 10.1016/j.mce.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhou Y, Zhang X, Klibanski A. MEG3 noncoding RNA: a tumor suppressor. J Mol Endocrinol. 2012;48:R45–R53. doi: 10.1530/JME-12-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]