Abstract
As a peripheral tissue localized at the interface between internal and external environments, skin performs functions which are critical for the preservation of body homeostasis, in coordination with environmental changes. Some of these functions undergo daily variations, such as temperature or water loss, suggesting the presence of time-keeping mechanisms. Rhythmic functions are controlled by a network of circadian oscillators present virtually in every cell and coordinated by the central clock located in the suprachiasmatic nuclei. At the molecular level, circadian rhythms are generated by conserved transcriptional–translational feedback loops involving several clock genes, among which Per1 and Per2 play a central role. Here we characterize clock activity in skin of the transgenic Per1-luciferase rat during postnatal development and adulthood, by real-time recording of bioluminescence in explants and primary dermal fibroblasts, and report marked transformation in circadian properties, from early life to aging. Using primary dermal fibroblast cultures we provide evidence that melatonin treatment phase dependently increases the amplitude of circadian oscillations and that ambient temperature impacts on their period, with slight overcompensation. Together, these findings demonstrate that skin contains a self-sustained circadian clock undergoing age-dependent changes. Dermal fibroblasts, one of the major skin cell types, also exhibit robust, yet specific, circadian rhythmicity which can be fine-tuned by both internal (melatonin) and external (temperature) factors.
Keywords: Per1, Circadian rhythms, Development, Age, Bioluminescence
Introduction
In mammals, numerous physiological and behavioral processes exhibit circadian rhythms that are controlled by an orchestra of molecular pacemakers in which the master clock of the suprachiasmatic nuclei (SCN), synchronized to astronomical time, sets the phase coherence between and within the peripheral clocks. At the molecular level, rhythmic gene expression is controlled by interconnecting transcriptional/translational feedback loops involving clock genes such as Bmal1, Clock, Per1-3, Cry1-2, RevErbα, Rorα and Rorβ [1] and the newly discovered Chrono [2, 3]. The network of central and peripheral clocks undergoes dynamic changes during lifespan; temporal coordination is set during development ensuring early and efficient entrainment of the circadian system to environmental cues, while obvious decrease in the overall amplitude of circadian functions occurs during aging, likely reflecting a decline in signals triggered by the central clock or damped local oscillations [4–7].
Rhythmic clock gene expression has been described in human and murine skin as well as in derived cell types [8–11], indicating that skin consists of a network of distinct, cell-specific circadian oscillators synchronized by so far unknown signals. In line with cyclic expression of clock genes and as an adaptation to daily changes in light, temperature and humidity, skin displays marked rhythmic behavior. This is evidenced by the measure of biophysical parameters such as transepithelial water loss, temperature or pH, and by several physiological processes [12, 13]. In 1991, Brown [14] reported circadian rhythms in M-phase and S-phase of skin cell proliferation, with opposite phases between human and rodent epidermis. A more recent study showed that the temporal fate of both murine and human epidermal stem cells is controlled by the molecular clock [15, 16]. The circadian clock was described to also regulate the hair follicle cycle, a non-diurnal cyclic process [17, 18]. DNA repair rate in mouse skin exhibits daily rhythmicity, with a minimum in the morning and a maximum in the evening, indicating control of UVB-induced skin cancer by the circadian clock [19] and suggesting that humans may also exhibit a daily rhythm in the susceptibility to develop UVB-induced skin cancer [19, 20]. Finally, impairment of the molecular clockwork was reported to disrupt skin physiology with signs of premature aging [21, 22], indicating that integrity of the circadian clock is of crucial importance regarding skin health and resistance towards age-related decline.
Although their rhythmic properties within the skin have been barely characterized, fibroblasts of diverse origins have arisen as a widely used model for studying the molecular mechanisms of circadian clocks, including their resilience to temperature changes [23]. This temperature resilience, also known as temperature compensation, is commonly used as a criterion for defining circadian clocks. Contrary to all other biological processes that will be accelerated and slowed by high and low temperatures, respectively, circadian clocks exhibit the unusual propensity to keep a relatively constant periodicity within a wide range of temperatures [24]. Using Rat-1 fibroblasts, Balsalobre et al., showed for the first time that glucocorticoid treatment was able to reset the circadian clock, which was further confirmed for several peripheral tissues like cornea [25–27]. Melatonin, the night hormone released by the pineal gland, is another highly rhythmic output from the clock in which specific receptors are widely distributed in peripheral tissues including skin, indicating it may modulate temporal organization [28, 29]. Chronobiotic effects of exogenous melatonin on the central clock were observed in the rat when infusion was applied before the synthesis peak of endogenous melatonin, at day to night transition [30]. Moreover, acute melatonin injection was reported to increase the amplitude of its own release, likely through an action on the central clock [31, 32]. However, only preliminary attempts have been performed to assess the chronobiotic effects of melatonin on peripheral oscillations (see [33] for review).
In the present study we used in vitro real-time bioluminescence recordings, to get new insight into the properties of the skin clock. Our data show robust circadian oscillations in whole skin explants and in dermal fibroblast cultures from young adult Per1-luciferase rats, with age effect on the amplitude. Using dermal fibroblasts we report a marked effect of external temperature on the pattern of clock oscillations, with slight overcompensation regarding the period, and a phase-specific, positive effect of acute melatonin treatment on the amplitude of Per1 oscillations. This work provides a functional demonstration that the skin and derived dermal fibroblasts contain intrinsic circadian clocks able to generate rhythmic activity in vitro and sensitive to changes in both internal and external environments.
Materials and methods
Animals
All experiments in this study were performed on Per1-luciferase transgenic rats [34] provided by Dr M. Menaker. Males from 10 day to 2 year old, born and reared in the Chronobiotron animal facility UMS 3415, Strasbourg, were housed in standard cages under 12 h:12 h light–dark cycles (around 300 lux during the light period starting at 7:00 a.m.) with free access to food and water. Rats were handled according to the European Communities Council Directive of 24 November 1986 (86/609/EEC).
Skin explant cultures
Animals were euthanized with CO2 (20 %) in an air-tight box at variable time of the day, according to the total number of animals included in the study. The abdominal area was cleaned with 70 % ethanol, making sure that the fur is soaked with 70 % ethanol, and the fur was shaved. An abdominal skin sample (1–4 cm2) was dissected carefully using sterile scissors and forceps, to avoid cutting the fat layer, and immediately placed in ice-cold HBSS medium (Sigma Aldrich) supplemented with 40 µg/ml penicillin–40 U/ml streptomycin, 4.2 mM NaHCO3 and 10 mM HEPES until processing. After sampling, the skin biopsy was placed into a sterile 10 cm Petri dish with the epidermis side down and the hypodermis was removed from dermis using a sharp scalpel. Subsequently, the skin biopsy was rinsed 3 times with sterile HBSS medium. For the bioluminescence study, 3 × 3 mm skin explants were cut using a sharp scalpel and immediately placed with dermis side down onto a semipermeable PTFE membrane (Millipore) into a 35 mm plastic dish (Nunclon) containing 1 ml of pre-warmed 10 % FBS (Biowest)/bioluminescence recording medium [DMEM without phenol red (Sigma D2902) supplemented with 2 mM l-glutamine, 25 µg/ml penicillin–25 U/ml streptomycin, 4.2 mM NaHCO3, 10 mM HEPES, 25 mM d(+) glucose and 0.1 mM luciferin (Promega)]. Sample preparation was finished by the end of day time and bioluminescence recording started by 7–8 p.m. The remaining skin biopsy was placed into fresh ice-cold HBSS medium until further processing.
Primary fibroblast cultures
From the remaining cleaned skin biopsy, a number of 20–25 explants of 2 × 2 mm were cut and placed with epidermis down onto a sterile 10 cm plastic dish. They were allowed 5 min to attach to the bottom of the dish and subsequently incubated with 4 ml of pre-warmed DMEM medium (Gibco) supplemented with 20 % FBS, 2 mM l-glutamine, 100 µg/ml penicillin–100 U/ml streptomycin in a humidified 37 °C/5 % CO2 incubator. Medium was changed every third day to allow proliferation of fibroblasts from the explants. After 2 weeks, explants were removed from the dish. The fibroblasts were eventually incubated with 10 % FBS/DMEM medium until confluence and then split. For the bioluminescence study, 5 × 105 cells of the obtained cell suspension were seeded into a 35 mm Nunclon plastic dish in 10 % FBS/DMEM medium and allowed to grow until the confluent cell monolayer was formed. The remaining cell suspension was cryopreserved in DMEM medium containing 50 % FBS and 10 % DMSO until further experiment.
Bioluminescence recordings
Bioluminescence recordings were performed as described by Yamazaki and Takahashi [35]. Bioluminescence was monitored using a Lumicycle luminometer (Actimetrics) equipped with 4 photomultiplier tubes (PMT) which allows simultaneous assessment of 32 cultures. The photon counts were integrated every 15 min for 112 s. Skin explant dishes prepared as above were sealed with vacuum grease (Dow Corning) immediately after preparation of the culture, placed into the Lumicycle and bioluminescence was recorded over several days. Confluent primary fibroblast cultures were incubated with pre-warmed 10 % FBS/bioluminescence recording medium and sealed before being placed into the Lumicycle. To ensure a constant temperature during the recordings, the Lumicycle luminometer was placed in an incubator. All recordings were performed at 37 °C unless specified, as in the study of the temperature effect, at 32, 35, and 39 °C.
Bioluminescence data analysis
Using the Lumicycle analysis software, the 24 h running average was subtracted from the raw bioluminescence data to remove the baseline drift. Time was rescaled so that t = 0 corresponded to 24 h after the start of the culture (in order to get rid of the first peak) in explants and to the first trough in fibroblast cultures. Detrended data (3–4 cycles starting from the new t = 0) were analyzed by a non-linear least square regression with the help of SigmaPlot V12 (Systat Software Inc., San Jose, CA, USA) and software R (http://www.r-project.org) [9]. Data for each recording were fitted to a cosinor-derived sine wave function:
where a is the amplitude (counts/s), b is the period (h), c is an indicator of the phase (h) and d is the damping rate (days). Samples for which the R 2 value for the fit was below 50 % were systematically removed from the study (e.g. for 10 and 20 day explants). Regressions were validated when all of the following conditions were simultaneously fulfilled: non-significant Shapiro–Wilk’s test (homogeneity of residuals) and Bartlett’s test (homogeneity of variances), significant p value (F test) for the global regression, significant p values (t test) for each of the parameters.
Melatonin treatment of primary fibroblasts
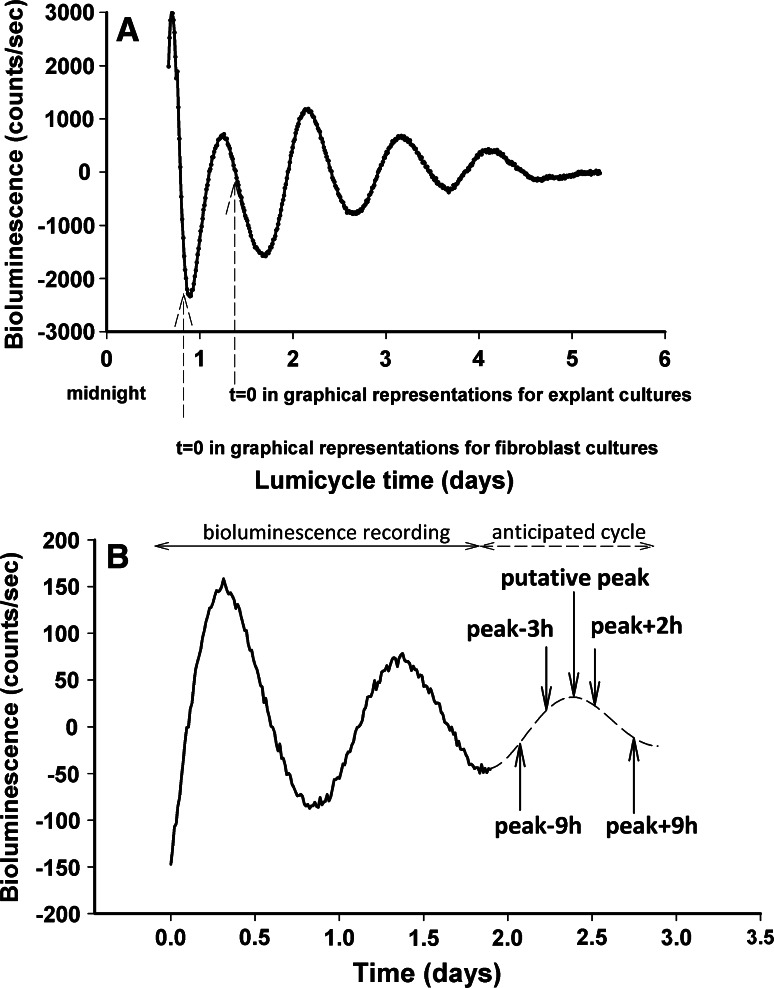
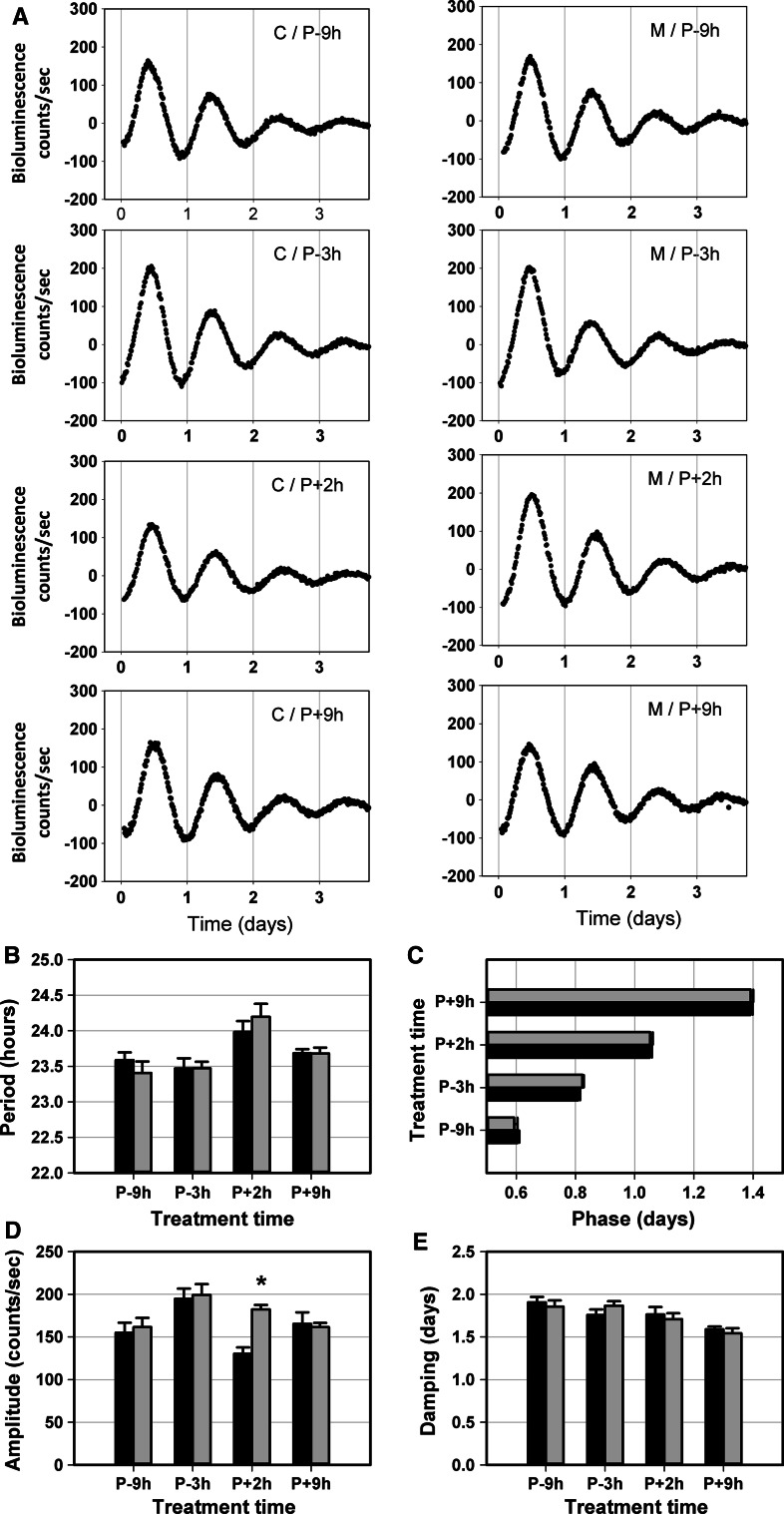
To assess the effect of melatonin treatment on skin clock properties, confluent fibroblast primary cultures derived from a 3 month donor were submitted to bioluminescence recording for several days prior to treatment, to define the precise phase for melatonin application. After two complete cycles, the period of the two initial oscillations was calculated using the Lumicycle analysis software (Actimetrics) to predict: (1) the peak of the third oscillation (P) and (2) the time of four melatonin treatments during the third cycle: 9 h before the putative peak (P − 9 h), 3 h before peak (P − 3 h), 2 h after peak (P + 2 h) and 9 h after peak (P + 9 h) (Fig. 1b). At the predicted treatment time, fibroblast cultures were removed from the Lumicycle and incubated at 37 °C with pre-warmed serum-free bioluminescence recording medium in the presence of 50 µM melatonin (Sigma Aldrich). After 30 min, the medium was changed to pre-warmed 10 % FBS/bioluminescence recording medium and the sealed culture dishes were transferred back to Lumicycle for further recording (at least 3–4 complete cycles). Control cultures (1/1,000× ethanol) were treated similarly for each melatonin-treated group. Cultures removed from the Lumicycle were carefully manipulated under dim red light.
Fig. 1.
Bioluminescence analysis of Per1-Luciferase recordings in skin and fibroblast cultures. a Detrended bioluminescence recording (24 h moving average). Dashed arrows indicate the start of analysis in explants (24 h after culture start) and fibroblast cultures (first trough of the detrended curve). b Detrended bioluminescence recording representative for melatonin-treated fibroblast cultures. Arrows indicate the predicted third peak and the time of melatonin administration
Data obtained after treatment (melatonin and control group) were subtracted (24 h running average) and analyzed by non-linear least square regression as described above.
Statistical analysis
Results are presented as mean ± SEM.
Differences in bioluminescence parameters between the groups were analyzed using one-or two-way analysis of variance (ANOVA) with or without repeated measures, and post hoc comparisons with the LSD Fisher test (SigmaPlot V12). Significant difference in dispersion of oscillation parameters between groups was measured by Bartlett’s test.
Results
Our study aimed at investigating the properties of the skin clock in vitro and characterizing its changes during development and aging. We performed in vitro bioluminescence recordings of Per1-luciferase reporter gene on rat skin explants and primary dermal fibroblast cultures. We also studied the effect of temperature and melatonin on Per1 expression in fibroblast cultures.
Ontogeny of Per1 circadian rhythms in rat skin explants
We established explant cultures to analyze the global Per1-luciferase expression at different ages in the rat skin at 37 °C in vitro. Males at three different ontogeny stages were used: (1) pups of 10 days (n = 8), 20 days (n = 16) and 1 month (immediately after weaning) (n = 9); (2) young adults of 2 months (n = 7), 3 months (n = 10) and 6 months (n = 9); and (3) aged animals at 12 months (n = 6), 18 months (n = 7) and 24 months (n = 4).
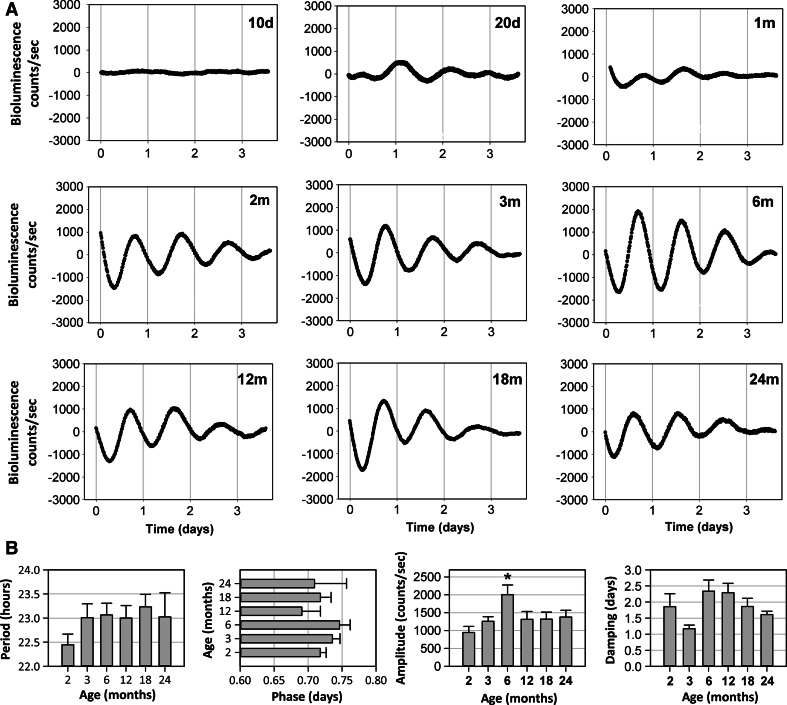
Recordings corresponding to the first stage of development did not show consistent oscillations. The fitted regressions at 10 days, 20 days and 1 month did not meet the criteria for further analysis (Fig. 2a, first line of plots). Starting with the age of 2 months until 24 months, sustained oscillations of Per1 were recorded during several days (Fig. 2a, second and third lines of plots).
Fig. 2.
Per1 bioluminescence recordings in rat skin explants. a Representative bioluminescence recordings over 4 days in vitro at different ages (d day, m month). b Variation of circadian parameters: period, phase, amplitude and damping during postnatal development and aging. Numbers of animals were as follows: 2 months (n = 7), 3 months (n = 10), 6 months (n = 9), 12 months (n = 6), 18 months (n = 7), 24 months (n = 4). Mean values ± SEM are shown (*p < 0.05)
We further determined the differences in parameters between the age groups (Fig. 2b). Calculated periods were in the circadian range, between 22.44 ± 0.59 h and 23 ± 0.69 h, with no significant difference between the groups (p = 0.52). Similarly, no significant difference was observed for the phase (time of the maximum of the first oscillation) (p = 0.37). However, phases were significantly more variable in the aged animals (24 m) than in the young ones (2 and 3 m) (p < 0.03). A significant age effect was determined in the case of the amplitude (p = 0.02). A highest value of 2,001.81 ± 275.25 counts/s was obtained for the 6 month group, 2.1 fold higher than 2 month group and 1.45–1.6 fold higher than the other age groups.
Regarding the damping parameter, a significant difference (p = 0.015) was obtained only for the 3 month (1.17 ± 0.11 days) as compared to 6 (2.34 ± 0.34 days) and 12 month (2.29 ± 0.28 days) groups.
Per1 circadian rhythms in dermal fibroblasts
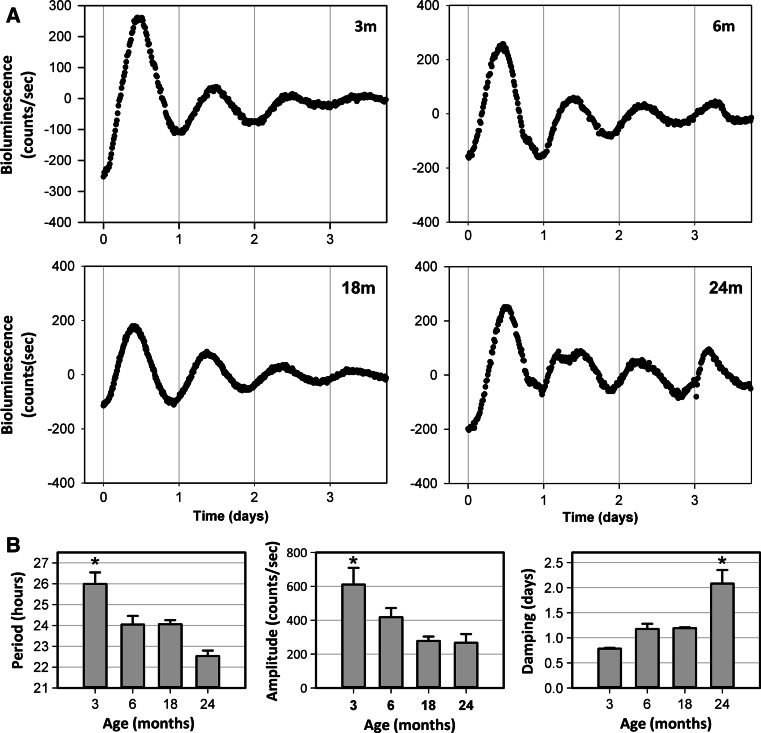
Rodent and human fibroblasts were shown to display rhythmic expression of clock genes and thus to harbor a circadian oscillator [36, 37]. We established primary fibroblast cultures derived from rat skin explants to analyze the Per1 expression during: (1) adult stage: 3 months (n = 7) and 6 months (n = 3); and (2) aged stage: 18 months (n = 10) and 24 months (n = 4). We obtained rhythmic bioluminescence recordings at 37 °C for all age groups (Fig. 3a), with significantly different circadian periods (p < 0.001) in which values were between 25.99 ± 0.55 h at 3 months and 22.5 ± 0.27 h at 24 months, with intermediate ~24 h periods at 6 and 18 months (Fig. 3b).
Fig. 3.
Per1 bioluminescence recordings in fibroblast cultures derived from rat abdominal dermis. a Representative bioluminescence recordings over 4 days in vitro at different ages (m month). b Variation of circadian parameters: period, amplitude and damping in dermal fibroblasts from adult and aged animals. Numbers of donors were as follows: 3 months (n = 7), 6 months (n = 3), 18 months (n = 10), 24 months (n = 4). Mean values ± SEM are shown (*p < 0.05)
Analysis of amplitude showed a highest level at 3 months, 610.63 ± 98.43 counts/s (p = 0.002), which declined progressively during aging, with a 2.3 fold reduction at 24 months (Fig. 3b). An opposite effect was observed for the damping parameter which significantly (p < 0.001) increased from 0.78 ± 0.01 days at 3 months to 2.08 ± 0.27 days at 24 months (Fig. 3b).
Temperature effect
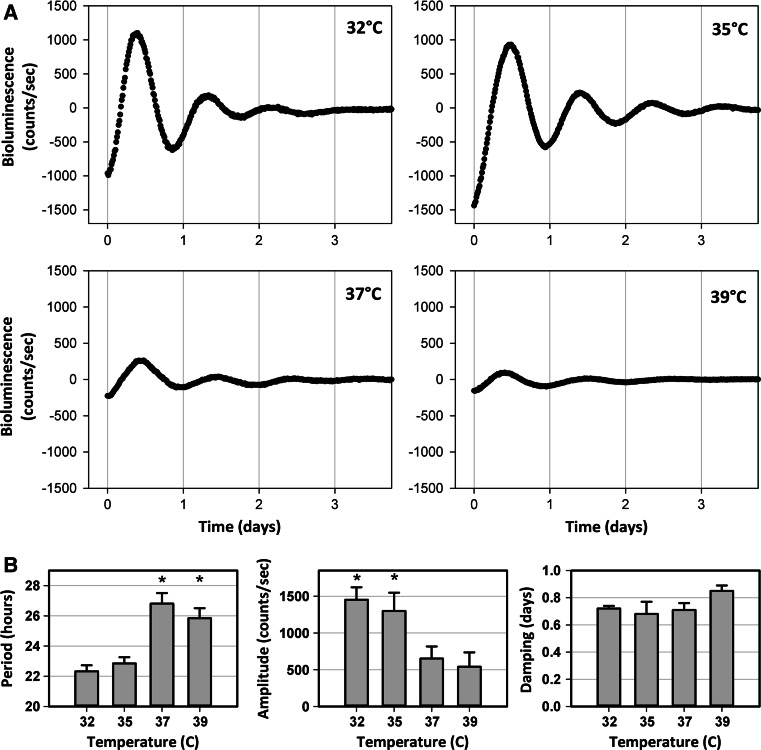
Fibroblasts obtained from the abdominal dermis of 3-month-old rats (n = 4) were submitted to real-time bioluminescence recording at four different temperatures (32, 35, 37 and 39 °C) to study the effect of environmental temperature on rhythmic expression of Per1-luciferase. Dermal fibroblasts exhibited circadian Per1-luciferase oscillations at all tested temperatures (Fig. 4a). A significant variation in the means of the calculated periods was observed between the groups (p < 0.001), with lower values at 32 °C (22.33 ± 0.40 h) and 35 °C (22.85 ± 0.41 h) and higher values at 37 °C (26.81 ± 0.70 h) and 39 °C (25.84 ± 0.67 h) (Fig. 4b). A statistically significant difference (p = 0.013) was observed in the case of the amplitude parameter, the amplitudes of the faster cycling groups (32 and 35 °C), being 2–2.7 fold higher than those of the slower cycling groups (37 and 39 °C) (Fig. 4b). There was no statistically significant difference between the temperature groups for the damping parameter (p = 0.35) (Fig. 4b).
Fig. 4.
Effect of temperature on Per1 bioluminescence recordings in fibroblast cultures derived from the dermis of 3-month-old rats. a Representative bioluminescence recordings over 4 days in vitro at 32, 35, 37 and 39 °C. b Variation of circadian parameters: period, amplitude and damping at different temperatures. Number of donors: n = 4. Mean values ± SEM are shown (*p < 0.05)
Melatonin effect on Per1 expression in primary fibroblast cultures
It was shown that melatonin can act via melatonin receptors in skin [28]. The chronobiotic effect of melatonin on the SCN was reported to be restricted to a specific time window corresponding to the subjective day to subjective night transition interval in diurnal and nocturnal rodents [30, 38]. We compared the effect on Per1-luciferase expression of melatonin application at four different time points expressed relatively to the peak (P) of Per1 expression, P − 9 h, P − 3 h, P + 2 h and P + 9 h, during the third cycle of real-time bioluminescence recording in dermal fibroblast cultures (n = 7–8 in treated and control groups) over the following 4 days in vitro (Fig. 5a). Melatonin treatment induced a 28 % increase of amplitude specifically at P + 2 h (p = 0.02) (Fig. 5d) and did not affect the other oscillation parameters (Fig. 5b, c, e).
Fig. 5.
Effect of melatonin treatment on Per1 bioluminescence recordings in fibroblast cultures derived from the dermis of a 3-month-old rat. a Representative bioluminescence recordings of control (C) and melatonin (M) samples at four treatment times over 4 days in vitro (P peak). Circadian parameters: period (b) phase (c), amplitude (d) and damping (e) of oscillations in both the control group (black) and melatonin group (grey) are presented for the four different treatment times (same donor, n = 7 or 8 cultures per treatment and time point). Mean values ± SEM are shown (*p < 0.05)
Discussion
Skin is a complex organ composed of a variety of structures and cell types and exposed to changing internal and external environments. Accordingly, it displays marked daily rhythms which coordination has not been understood so far. The present study demonstrates that skin explants from adult rats are able to display marked Per1-luciferase oscillations in vitro. We show that this capacity develops during the postnatal period and fades during old age, indicating the degree of synchrony between oscillators within the skin varies with age. We also assessed the concomitant evolution of circadian properties and the rhythmic behavior under varying temperatures in derived primary dermal fibroblasts. Finally, we report that melatonin treatment induces a phase-specific increase of amplitude in fibroblast cultures.
Ontogeny in skin and fibroblasts
No circadian oscillations that fulfilled our criteria based on mathematical analysis could be observed in vitro, in skin explants from rats at 10–20 days. Per1 expression profiles had globally arrhythmic patterns, indicating that, although Per1 gene is expressed at early ages, the molecular makeup of the clock is not fully developed or that the synchronization of individual oscillators is not yet achieved at the tissue level. In favor of the latter hypothesis, we observed a restoration of rhythmic expression of Per1 in 10 and 20 day explants upon medium change following 7–10 days of culture (data not shown). Thus, serum might contain strong synchronizing signals that are able to act on the immature molecular clock of skin explants adapted 1 week in culture. Our results are in agreement with the literature: while intrinsic rhythms may appear in the rat SCN at E19-E21 [6] and the master clock is fully developed at P10 [39], peripheral clocks (pineal, heart, liver, lung, adrenal, thyroid) may undergo maturation during postnatal development [7, 40–44] being entrained mainly by non-photic maternal cues until weaning [45]. Importantly, bioluminescence studies with rats expressing the Per2-dluc reporter described the capacity of peripheral organs to show oscillations in vitro as early as P5 [4], whereas we do not report any consistent oscillation in Per1-luc skin before 2 months. There are several possible explanations for this discrepancy: (1) the criteria used to evaluate rhythmicity differ between the two studies. Our study is more restrictive in this sense, since we based our conclusions on mathematical analysis designed to evidence and characterize sustained oscillations in samples. Indeed, we did see oscillations in skin explants from P20 rats (as shown in Fig. 2a), but these were irregular and did not fit satisfactorily to our mathematical tests. (2) The oscillating capacity of whole skin explants is effective at an age that is delayed with respect to other peripheral clocks. We presently do not know why but this might be related to the fact that skin is a rather complex organ, in which full synchronization of constituent oscillators might require more time than for other tissues. (3) The reporter construct, including the destabilized luciferase, carried by the Per2-dluc rat might be more appropriate (notably because of a more dynamic luciferase expression rate) to uncover circadian oscillations. We observed robust circadian oscillations in cultured skin explants starting at 2 month age, with a progressive increase in amplitude until the age of 6 months. These data likely reflect the sum of all functional oscillators present in the different skin cells types, including keratinocytes, fibroblasts and melanocytes [9] that act in a coordinated manner. We also recorded autonomous oscillations in skin explants from aged rats (12–24 months) but, importantly, these exhibited reduced amplitudes with respect to the skin from 6 month animals and, at least for the 24 month group, increased variability in the peak phase. Similar bioluminescence results were previously reported in other peripheral tissues of aged Per1-luciferase rats [46]. The decline of circadian rhythms was also reported in the central clock in aging mammals [47, 48] and, reciprocally, premature aging was promoted in clock gene deficient mice, such as Bmal1 −/− [22] and Clock −/− [21] animals ([49] for review). Decreased amplitude of Per1-luciferase oscillations in skin explants from aged rats could be due to desynchronization of local oscillators but we cannot exclude an effect of cell number, since skin from aged animals might contain reduced numbers of oscillating cells, and/or of hair cycling. Indeed, hair cycling is getting asynchronous with age in rodents, generating increasing complexity in patterns of hair growth [50, 51]. Since distinct hair cycle stages are related to differences in local cell numbers and in amplitudes of clock gene expression [17, 20], it is possible that the results in aged rats are affected by the asynchrony of hair cycling.
We further studied circadian expression of Per1-luciferase in primary dermal fibroblasts to understand their contribution to the temporal organization of rat skin. Our results indicate that intrinsic rhythmicity also occurs in primary fibroblasts in vitro at all studied ages but with characteristics distinct from those of whole skin explants. Rhythmic capacity of dermal fibroblasts derived from explants following 2 weeks of migration is likely to be modified by the isolation process and might not entirely reflect the circadian properties of these cells in vivo. Conversely, our data indicate that circadian behavior in the whole skin explants does not only reflect the clock properties of fibroblasts, as expected given the heterogeneous cellular content of the tissue [9]. While in skin explants Per1 oscillated with a period of around 23 h and did not show significant differences between groups, dissociated fibroblast cultures exhibited variable periods: approximately 26 h in the 3 month group and shorter periods (22.5–24 h) in the 6, 18 and 24 month animals. The 3 month fibroblasts showed also the highest amplitude and fastest damping rate. We do not know if these changes reflect the evolution of the fibroblast circadian clock upon aging or if they are linked to the isolation procedure. Fibroblasts from old donors took longer to reach confluence in our experiments, in accordance with the fact that they contain a lower proportion of cells able to proliferate [52]. Thus, even if the proliferative capacity of isolated cells was reported to be independent of age [52], it cannot be ruled out that differences linked to the isolation procedure itself contribute to the effects of donor age on circadian parameters in fibroblasts. Bioluminescence studies using mPer2 Luc mice [53] showed that rhythms in cultured fibroblasts become more coherent as they mature [54] and that dissociated fibroblasts display periods that range between 22.5 and 27 h [55]. It is not yet clear how robust rhythms are generated or why rhythms decline during aging. Noguchi et al. [56] showed that diffusible paracrine signals rather than direct contact with neighboring cells help to restore robust circadian rhythms in low density mPer2 Luc tail fibroblasts cultured in the presence of conditioned medium from high density cultures. Pagani et al. [57] demonstrated that the absence of yet unidentified thermolabile serum factors (distinct from cortisol and melatonin) rather than alteration of the molecular machinery may be responsible for the desynchronization of rhythms during aging. Taken together, our data from whole skin explants and derived fibroblasts however, indicate that the observed age-related decline in clock robustness might proceed from both a decrease in synchronizing signals as well as a local alteration in the skin recipient tissue.
Effect of temperature
One important feature of circadian rhythms is temperature compensation, the ability to maintain a relatively stable period of oscillation over a wide range of temperatures. This property indicates also the robustness of a circadian clock in varying environmental conditions. We studied the effect of temperature in primary dermal fibroblast cultures maintained at 32, 35, 37 and 39 °C and sustained oscillations were characterized at all tested temperatures. However, despite the fact that calculated periods were in the circadian range in all temperature groups, we observed significantly longer periods at higher temperatures, 37 and 39 °C, compared to low temperatures, 32 and 35 °C, which suggests that synthesis and degradation rates of core clock components might be different at some temperatures and that primary adult skin fibroblasts do not behave exactly like NIH3T3 or tail fibroblasts in this respect [23, 58, 59]. We also found that, while the damping rates were similar between groups, the amplitude of the first oscillation at low temperatures was significantly higher, in agreement with the overcompensation-like property observed in the case of periods. However, we cannot exclude the possibility that the amplitude reduction at 37 and 39 °C also reflects the effect of ambient temperature on luciferase enzymatic activity, which is likely to decrease between 32 and 39 °C [60]. Altogether, our data indicate that dermal fibroblasts show a particular response to temperature in vitro, which might reflect specific properties of the skin clock regarding the strong temperature fluctuations to which it is exposed.
Effect of melatonin
Fibroblasts were the first non-neuronal cells shown to exhibit circadian rhythms when synchronized in vitro by a serum shock or dexamethasone [25, 36] or by forskolin [58], hence demonstrating the extensive presence of circadian clocks throughout the body. The melatonin hormone, synthesized and secreted by pineal gland during the night both in diurnal and nocturnal species, is a strong output of the central clock. Plasma melatonin profile is thus a valuable marker of circadian phase and melatonin was reported to affect, in vitro, rhythmic parameters of peripheral oscillators including adrenal gland [61] and primary adipocytes [62]. In mammals, melatonin acts via MT1 and MT2 receptors which are expressed in various peripheral tissues including skin [28], and represents a potential synchronizer of local rhythms in skin cells. Here we investigated the effect of melatonin on Per1 expression in dermal fibroblast cultures when administered at four different phases of a complete cycle. The reference time point in the experimental design was the putative peak of the third Per1 cycle. In vivo, the maximum of Per1 transcript expression in the mouse skin coincides with the day-to-night transition [11]. We found a significant 28 % increase in Per1 amplitude when fibroblasts were treated with melatonin 2 h after the peak of Per1. This is in agreement with previous finding showing the chronobiotic effect of melatonin on the master clock, when administered at dusk [30]. Melatonin could thus modulate the amplitude of circadian rhythms in cultured fibroblasts. Two hypotheses might explain such effect. Either melatonin increases the amplitude of the clock at the cellular level or it slightly resets individual oscillators, increasing synchrony at the culture level. These alternatives can be hardly distinguished in the present study. At the tissue level, amplitude usually reflects the degree of synchronization of constitutive oscillators; this is an important finding that points out the potential chronomodulatory property of melatonin on the skin clock. Furthermore, it suggests that oral or topical, timed melatonin administration might be used as a skin clock synchronizer in various pathologies, including those potentially associated with abnormal circadian rhythms of melatonin, such as atopic eczema and psoriasis [63, 64].
In conclusion, our results with skin explants and cultured fibroblasts demonstrate that rat skin harbors an autonomous circadian clock that is fully functional starting at 2 months until 24 months and which period can be modulated by temperature. We show also for the first time that the amplitude of Per1 rhythm can be potentiated by melatonin. Taken together, our data highlight that the skin is a valuable model to understand the mechanisms of internal as well as external synchronization of peripheral clocks.
Acknowledgments
We thank Dr. Sophie Reibel, Dr. Dominique Sage, Aurore Senser and Nicolas Lethenet from Chronobiotron UMS 3415 (Strasbourg, France) for animal care. We are grateful to Dr. Michael Menaker for kindly providing the Per1-luciferase rats. TL was supported by a fellowship from the Chinese CSC scholarship program.
References
- 1.Ko CH, Takahashi JS. Molecular components of the mammalian circadian clock. Hum Mol Genet. 2006;15 Spec No 2:271–277. doi: 10.1093/hmg/ddl207. [DOI] [PubMed] [Google Scholar]
- 2.Anafi RC, Lee Y, Sato TK, Venkataraman A, Ramanathan C, Kavakli IH, Hughes ME, Baggs JE, Growe J, Liu AC, Kim J, Hogenesch JB. Machine learning helps identify CHRONO as a circadian clock component. PLoS Biol. 2014;12(4):e1001840. doi: 10.1371/journal.pbio.1001840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goriki A, Hatanaka F, Myung J, Kim JK, Yoritaka T, Tanoue S, Abe T, Kiyonari H, Fujimoto K, Kato Y, Todo T, Matsubara A, Forger D, Takumi T. A novel protein, CHRONO, functions as a core component of the mammalian circadian clock. PLoS Biol. 2014;12(4):e1001839. doi: 10.1371/journal.pbio.1001839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nishide SY, Hashimoto K, Nishio T, Honma K, Honma S. Organ-specific development characterizes circadian clock gene Per2 expression in rats. Am J Physiol Regul Integr Comp Physiol. 2014;306(1):R67–R74. doi: 10.1152/ajpregu.00063.2013. [DOI] [PubMed] [Google Scholar]
- 5.Ohta H, Honma S, Abe H, Honma K. Effects of nursing mothers on rPer1 and rPer2 circadian expressions in the neonatal rat suprachiasmatic nuclei vary with developmental stage. Eur J Neurosci. 2002;15(12):1953–1960. doi: 10.1046/j.1460-9568.2002.02016.x. [DOI] [PubMed] [Google Scholar]
- 6.Reppert SM, Schwartz WJ. The suprachiasmatic nuclei of the fetal rat: characterization of a functional circadian clock using 14C-labeled deoxyglucose. J Neurosci. 1984;4(7):1677–1682. doi: 10.1523/JNEUROSCI.04-07-01677.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamazaki S, Yoshikawa T, Biscoe EW, Numano R, Gallaspy LM, Soulsby S, Papadimas E, Pezuk P, Doyle SE, Tei H, Sakaki Y, Block GD, Menaker M. Ontogeny of circadian organization in the rat. J Biol Rhythms. 2009;24(1):55–63. doi: 10.1177/0748730408328438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bjarnason GA, Jordan RC, Wood PA, Li Q, Lincoln DW, Sothern RB, Hrushesky WJ, Ben-David Y. Circadian expression of clock genes in human oral mucosa and skin: association with specific cell-cycle phases. Am J Pathol. 2001;158(5):1793–1801. doi: 10.1016/S0002-9440(10)64135-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sandu C, Dumas M, Malan A, Sambakhe D, Marteau C, Nizard C, Schnebert S, Perrier E, Challet E, Pevet P, Felder-Schmittbuhl MP. Human skin keratinocytes, melanocytes, and fibroblasts contain distinct circadian clock machineries. Cell Mol Life Sci. 2012;69(19):3329–3339. doi: 10.1007/s00018-012-1026-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sporl F, Korge S, Jurchott K, Wunderskirchner M, Schellenberg K, Heins S, Specht A, Stoll C, Klemz R, Maier B, Wenck H, Schrader A, Kunz D, Blatt T, Kramer A. Kruppel-like factor 9 is a circadian transcription factor in human epidermis that controls proliferation of keratinocytes. Proc Natl Acad Sci USA. 2012;109(27):10903–10908. doi: 10.1073/pnas.1118641109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tanioka M, Yamada H, Doi M, Bando H, Yamaguchi Y, Nishigori C, Okamura H. Molecular clocks in mouse skin. J Invest Dermatol. 2009;129(5):1225–1231. doi: 10.1038/jid.2008.345. [DOI] [PubMed] [Google Scholar]
- 12.Le Fur I, Reinberg A, Lopez S, Morizot F, Mechkouri M, Tschachler E. Analysis of circadian and ultradian rhythms of skin surface properties of face and forearm of healthy women. J Invest Dermatol. 2001;117(3):718–724. doi: 10.1046/j.0022-202x.2001.01433.x. [DOI] [PubMed] [Google Scholar]
- 13.Yosipovitch G, Xiong GL, Haus E, Sackett-Lundeen L, Ashkenazi I, Maibach HI. Time-dependent variations of the skin barrier function in humans: transepidermal water loss, stratum corneum hydration, skin surface pH, and skin temperature. J Invest Dermatol. 1998;110(1):20–23. doi: 10.1046/j.1523-1747.1998.00069.x. [DOI] [PubMed] [Google Scholar]
- 14.Brown WR. A review and mathematical analysis of circadian rhythms in cell proliferation in mouse, rat, and human epidermis. J Invest Dermatol. 1991;97(2):273–280. doi: 10.1111/1523-1747.ep12480379. [DOI] [PubMed] [Google Scholar]
- 15.Janich P, Pascual G, Merlos-Suarez A, Batlle E, Ripperger J, Albrecht U, Cheng HY, Obrietan K, Di Croce L, Benitah SA. The circadian molecular clock creates epidermal stem cell heterogeneity. Nature. 2011;480(7376):209–214. doi: 10.1038/nature10649. [DOI] [PubMed] [Google Scholar]
- 16.Janich P, Toufighi K, Solanas G, Luis NM, Minkwitz S, Serrano L, Lehner B, Benitah SA. Human epidermal stem cell function is regulated by circadian oscillations. Cell Stem Cell. 2013;13(6):745–753. doi: 10.1016/j.stem.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 17.Lin KK, Kumar V, Geyfman M, Chudova D, Ihler AT, Smyth P, Paus R, Takahashi JS, Andersen B. Circadian clock genes contribute to the regulation of hair follicle cycling. PLoS Genet. 2009;5(7):e1000573. doi: 10.1371/journal.pgen.1000573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Plikus MV, Vollmers C, de la Cruz D, Chaix A, Ramos R, Panda S, Chuong CM. Local circadian clock gates cell cycle progression of transient amplifying cells during regenerative hair cycling. Proc Natl Acad Sci USA. 2013;110(23):E2106–E2115. doi: 10.1073/pnas.1215935110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaddameedhi S, Selby CP, Kaufmann WK, Smart RC, Sancar A. Control of skin cancer by the circadian rhythm. Proc Natl Acad Sci USA. 2011;108(46):18790–18795. doi: 10.1073/pnas.1115249108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geyfman M, Kumar V, Liu Q, Ruiz R, Gordon W, Espitia F, Cam E, Millar SE, Smyth P, Ihler A, Takahashi JS, Andersen B. Brain and muscle Arnt-like protein-1 (BMAL1) controls circadian cell proliferation and susceptibility to UVB-induced DNA damage in the epidermis. Proc Natl Acad Sci USA. 2012;109(29):11758–11763. doi: 10.1073/pnas.1209592109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dubrovsky YV, Samsa WE, Kondratov RV. Deficiency of circadian protein CLOCK reduces lifespan and increases age-related cataract development in mice. Aging (Albany NY) 2010;2(12):936–944. doi: 10.18632/aging.100241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kondratov RV, Kondratova AA, Gorbacheva VY, Vykhovanets OV, Antoch MP. Early aging and age-related pathologies in mice deficient in BMAL1, the core component of the circadian clock. Genes Dev. 2006;20(14):1868–1873. doi: 10.1101/gad.1432206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsuchiya Y, Akashi M, Nishida E. Temperature compensation and temperature resetting of circadian rhythms in mammalian cultured fibroblasts. Genes Cells. 2003;8(8):713–720. doi: 10.1046/j.1365-2443.2003.00669.x. [DOI] [PubMed] [Google Scholar]
- 24.Pittendrigh CS. Temporal organization: reflections of a Darwinian clock-watcher. Annu Rev Physiol. 1993;55:16–54. doi: 10.1146/annurev.ph.55.030193.000313. [DOI] [PubMed] [Google Scholar]
- 25.Balsalobre A, Brown SA, Marcacci L, Tronche F, Kellendonk C, Reichardt HM, Schutz G, Schibler U. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science. 2000;289(5488):2344–2347. doi: 10.1126/science.289.5488.2344. [DOI] [PubMed] [Google Scholar]
- 26.Dickmeis T. Glucocorticoids and the circadian clock. J Endocrinol. 2009;200(1):3–22. doi: 10.1677/JOE-08-0415. [DOI] [PubMed] [Google Scholar]
- 27.Pezuk P, Mohawk JA, Wang LA, Menaker M. Glucocorticoids as entraining signals for peripheral circadian oscillators. Endocrinology. 2012;153(10):4775–4783. doi: 10.1210/en.2012-1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Slominski A, Wortsman J, Tobin DJ. The cutaneous serotoninergic/melatoninergic system: securing a place under the sun. FASEB J. 2005;19(2):176–194. doi: 10.1096/fj.04-2079rev. [DOI] [PubMed] [Google Scholar]
- 29.Slominski RM, Reiter RJ, Schlabritz-Loutsevitch N, Ostrom RS, Slominski AT. Melatonin membrane receptors in peripheral tissues: distribution and functions. Mol Cell Endocrinol. 2012;351(2):152–166. doi: 10.1016/j.mce.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pitrosky B, Kirsch R, Malan A, Mocaer E, Pevet P. Organization of rat circadian rhythms during daily infusion of melatonin or S20098, a melatonin agonist. Am J Physiol. 1999;277(3 Pt 2):R812–R828. doi: 10.1152/ajpregu.1999.277.3.R812. [DOI] [PubMed] [Google Scholar]
- 31.Bothorel B, Barassin S, Saboureau M, Perreau S, Vivien-Roels B, Malan A, Pevet P. In the rat, exogenous melatonin increases the amplitude of pineal melatonin secretion by a direct action on the circadian clock. Eur J Neurosci. 2002;16(6):1090–1098. doi: 10.1046/j.1460-9568.2002.02176.x. [DOI] [PubMed] [Google Scholar]
- 32.Castanho A, Bothorel B, Seguin L, Mocaer E, Pevet P. Like melatonin, agomelatine (S20098) increases the amplitude of oscillations of two clock outputs: melatonin and temperature rhythms. Chronobiol Int. 2013;31(3):371–381. doi: 10.3109/07420528.2013.860457. [DOI] [PubMed] [Google Scholar]
- 33.Pevet P, Challet E. Melatonin: both master clock output and internal time-giver in the circadian clocks network. J Physiol Paris. 2011;105(4–6):170–182. doi: 10.1016/j.jphysparis.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 34.Yamazaki S, Numano R, Abe M, Hida A, Takahashi R, Ueda M, Block GD, Sakaki Y, Menaker M, Tei H. Resetting central and peripheral circadian oscillators in transgenic rats. Science. 2000;288(5466):682–685. doi: 10.1126/science.288.5466.682. [DOI] [PubMed] [Google Scholar]
- 35.Yamazaki S, Takahashi JS. Real-time luminescence reporting of circadian gene expression in mammals. Methods Enzymol. 2005;393:288–301. doi: 10.1016/S0076-6879(05)93012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Balsalobre A, Damiola F, Schibler U. A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell. 1998;93(6):929–937. doi: 10.1016/S0092-8674(00)81199-X. [DOI] [PubMed] [Google Scholar]
- 37.Brown SA, Fleury-Olela F, Nagoshi E, Hauser C, Juge C, Meier CA, Chicheportiche R, Dayer JM, Albrecht U, Schibler U. The period length of fibroblast circadian gene expression varies widely among human individuals. PLoS Biol. 2005;3(10):e338. doi: 10.1371/journal.pbio.0030338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Slotten HA, Krekling S, Sicard B, Pevet P. Daily infusion of melatonin entrains circadian activity rhythms in the diurnal rodent Arvicanthis ansorgei . Behav Brain Res. 2002;133(1):11–19. doi: 10.1016/S0166-4328(01)00411-9. [DOI] [PubMed] [Google Scholar]
- 39.Weinert D. Ontogenetic development of the mammalian circadian system. Chronobiol Int. 2005;22(2):179–205. doi: 10.1081/CBI-200053473. [DOI] [PubMed] [Google Scholar]
- 40.Ansari N, Agathagelidis M, Lee C, Korf HW, von Gall C. Differential maturation of circadian rhythms in clock gene proteins in the suprachiasmatic nucleus and the pars tuberalis during mouse ontogeny. Eur J Neurosci. 2009;29(3):477–489. doi: 10.1111/j.1460-9568.2008.06605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Christ E, Korf HW, von Gall C. When does it start ticking? Ontogenetic development of the mammalian circadian system. Prog Brain Res. 2012;199:105–118. doi: 10.1016/B978-0-444-59427-3.00006-X. [DOI] [PubMed] [Google Scholar]
- 42.Polidarova L, Olejnikova L, Pauslyova L, Sladek M, Sotak M, Pacha J, Sumova A. Development and entrainment of the colonic circadian clock during ontogenesis. Am J Physiol Gastrointest Liver Physiol. 2014;306(4):G346–G356. doi: 10.1152/ajpgi.00340.2013. [DOI] [PubMed] [Google Scholar]
- 43.Sakamoto K, Oishi K, Ishida N. Ontogeny of circadian expression of serotonin N-acetyltransferase mRNA in the rat retina. Neurosci Lett. 2002;317(1):53–55. doi: 10.1016/S0304-3940(01)02407-7. [DOI] [PubMed] [Google Scholar]
- 44.Sladek M, Jindrakova Z, Bendova Z, Sumova A. Postnatal ontogenesis of the circadian clock within the rat liver. Am J Physiol Regul Integr Comp Physiol. 2007;292(3):R1224–R1229. doi: 10.1152/ajpregu.00184.2006. [DOI] [PubMed] [Google Scholar]
- 45.Seron-Ferre M, Valenzuela GJ, Torres-Farfan C. Circadian clocks during embryonic and fetal development. Birth Defects Res C Embryo Today. 2007;81(3):204–214. doi: 10.1002/bdrc.20101. [DOI] [PubMed] [Google Scholar]
- 46.Yamazaki S, Straume M, Tei H, Sakaki Y, Menaker M, Block GD. Effects of aging on central and peripheral mammalian clocks. Proc Natl Acad Sci USA. 2002;99(16):10801–10806. doi: 10.1073/pnas.152318499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aujard F, Herzog ED, Block GD. Circadian rhythms in firing rate of individual suprachiasmatic nucleus neurons from adult and middle-aged mice. Neuroscience. 2001;106(2):255–261. doi: 10.1016/S0306-4522(01)00285-8. [DOI] [PubMed] [Google Scholar]
- 48.Nakamura TJ, Nakamura W, Yamazaki S, Kudo T, Cutler T, Colwell CS, Block GD. Age-related decline in circadian output. J Neurosci. 2011;31(28):10201–10205. doi: 10.1523/JNEUROSCI.0451-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yu EA, Weaver DR. Disrupting the circadian clock: gene-specific effects on aging, cancer, and other phenotypes. Aging (Albany NY) 2011;3(5):479–493. doi: 10.18632/aging.100323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Durward A, Rudall KM. Studies on hair growth in the rat. J Anat. 1949;83(4):325–335, 324 pl. [PMC free article] [PubMed] [Google Scholar]
- 51.Plikus MV, Chuong CM. Complex hair cycle domain patterns and regenerative hair waves in living rodents. J Invest Dermatol. 2008;128(5):1071–1080. doi: 10.1038/sj.jid.5701180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bayreuther K, Francz PI, Rodemann HP. Fibroblasts in normal and pathological terminal differentiation, aging, apoptosis and transformation. Arch Gerontol Geriatr. 1992;15(Suppl 1):47–74. doi: 10.1016/S0167-4943(05)80006-8. [DOI] [PubMed] [Google Scholar]
- 53.Yoo SH, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, Siepka SM, Hong HK, Oh WJ, Yoo OJ, Menaker M, Takahashi JS. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci USA. 2004;101(15):5339–5346. doi: 10.1073/pnas.0308709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.O’Neill JS, Hastings MH. Increased coherence of circadian rhythms in mature fibroblast cultures. J Biol Rhythms. 2008;23(6):483–488. doi: 10.1177/0748730408326682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Leise TL, Wang CW, Gitis PJ, Welsh DK. Persistent cell-autonomous circadian oscillations in fibroblasts revealed by six-week single-cell imaging of PER2::LUC bioluminescence. PLoS ONE. 2012;7(3):e33334. doi: 10.1371/journal.pone.0033334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Noguchi T, Wang LL, Welsh DK. Fibroblast PER2 circadian rhythmicity depends on cell density. J Biol Rhythms. 2013;28(3):183–192. doi: 10.1177/0748730413487494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pagani L, Schmitt K, Meier F, Izakovic J, Roemer K, Viola A, Cajochen C, Wirz-Justice A, Brown SA, Eckert A. Serum factors in older individuals change cellular clock properties. Proc Natl Acad Sci USA. 2011;108(17):7218–7223. doi: 10.1073/pnas.1008882108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Izumo M, Johnson CH, Yamazaki S. Circadian gene expression in mammalian fibroblasts revealed by real-time luminescence reporting: temperature compensation and damping. Proc Natl Acad Sci USA. 2003;100(26):16089–16094. doi: 10.1073/pnas.2536313100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Saini C, Morf J, Stratmann M, Gos P, Schibler U. Simulated body temperature rhythms reveal the phase-shifting behavior and plasticity of mammalian circadian oscillators. Genes Dev. 2012;26(6):567–580. doi: 10.1101/gad.183251.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Koksharov MI, Ugarova NN. Thermostabilization of firefly luciferase by in vivo directed evolution. Protein Eng Des Sel. 2011;24(11):835–844. doi: 10.1093/protein/gzr044. [DOI] [PubMed] [Google Scholar]
- 61.Torres-Farfan C, Mendez N, Abarzua-Catalan L, Vilches N, Valenzuela GJ, Seron-Ferre M. A circadian clock entrained by melatonin is ticking in the rat fetal adrenal. Endocrinology. 2011;152(5):1891–1900. doi: 10.1210/en.2010-1260. [DOI] [PubMed] [Google Scholar]
- 62.Alonso-Vale MI, Andreotti S, Mukai PY, Borges-Silva C, Peres SB, Cipolla-Neto J, Lima FB. Melatonin and the circadian entrainment of metabolic and hormonal activities in primary isolated adipocytes. J Pineal Res. 2008;45(4):422–429. doi: 10.1111/j.1600-079X.2008.00610.x. [DOI] [PubMed] [Google Scholar]
- 63.Mozzanica N, Tadini G, Radaelli A, Negri M, Pigatto P, Morelli M, Frigerio U, Finzi A, Esposti G, Rossi D, et al. Plasma melatonin levels in psoriasis. Acta Derm Venereol. 1988;68(4):312–316. [PubMed] [Google Scholar]
- 64.Schwarz W, Birau N, Hornstein OP, Heubeck B, Schonberger A, Meyer C, Gottschalk J. Alterations of melatonin secretion in atopic eczema. Acta Derm Venereol. 1988;68(3):224–229. [PubMed] [Google Scholar]