Abstract
Intensive research in the last decade shows that the prototypic angiogenic factor vascular endothelial growth factor (VEGF) can have direct effects in neurons and modulate processes such as neuronal migration, axon outgrowth, axon guidance and neuronal survival. Depending on the neuronal cell type and the process, VEGF seems to exert these effects by signaling via different receptors. It is also becoming clear that other VEGF ligands such as VEGF-B, -C and -D can act in various neuronal cell types as well. Moreover, apart from playing a role in physiological conditions, VEGF and VEGF-B have been related to different neurological disorders. We give an update on how VEGF controls different processes during neurodevelopment as well as on its role in several neurodegenerative disorders. We also discuss recent findings demonstrating that other VEGF ligands influence processes such as neurogenesis and dendrite arborization and participate in neurodegeneration.
Keywords: Vascular endothelial growth factor, VEGF, VEGF ligands, VEGF receptors, Neurodevelopment, Neurodegeneration
Introduction
Research findings of the last decade have led to the concept of the neurovascular link describing the similarities between the vascular and the nervous system and how the two systems communicate and regulate each other. Unexpectedly, aside from exerting its angiogenic functions, VEGF also acts directly on different neural cell types. Since these recent discoveries, VEGF has been considered a multifunctional factor for the nervous system during development and adulthood and in disease conditions.
VEGF-A (in this review termed VEGF) is the founding member of a family of homodimeric disulfide-bound glycoproteins. In mammals, the family comprises the following members: VEGF, VEGF-B, VEGF-C, VEGF-D and placenta growth factor (PlGF). VEGF, VEGF-B and PlGF exist in different isoforms and VEGF-C and VEGF-D are proteolytically processed to a mature form [1]. Each of them has a highly conserved common cysteine knot motif involved in inter- and intramolecular disulfide bonds [2]. VEGF family members bind to different cell surface receptors with different affinities and selectivities. Three VEGF receptor tyrosine kinases have been identified and termed VEGFR-1, -2 and -3. VEGF family members also bind to nontyrosine kinase receptors of the neuropilin (NRP) family, NRP1 and NRP2 (also receptors for semaphorins), which are considered to function as coreceptors for the VEGFRs. VEGF binds to VEGFR-1, VEGFR-2, NRP1 and NRP2. Of the other members, VEGF-B binds to VEGFR-1 and NRP1, all PlGF isoforms bind to VEGFR-1 (PlGF-2 isoform also binds NRP2 and NRP1), while VEGF-C and VEGF-D interact with VEGFR-3, VEGFR-2, NRP1 and NRP2 [1]. All VEGF family members have been found to exert a direct function on different neural cells (in vitro or in vivo) upon binding to their different VEGF receptors (Table 1).
Table 1.
VEGF ligands and their functions in the nervous system
| Ligand | Function |
|---|---|
| VEGF | Neurogenesis, neuronal survival and proliferation, neuronal migration, axon growth and guidance, glia survival, glia migration, oligodendrocyte migration |
| VEGF-B | Neurogenesis, neuronal survival |
| VEGF-C | Neurogenesis, oligodendrocyte precursor proliferation |
| VEGF-D | Dendrite arborization |
| PlGF-2 | Neurite outgrowth |
In order to establish an adult neuronal network, neural cells in development need to differentiate, migrate, grow neurites and extend their axons to specific targets and establish precise synaptic contacts. Notably, VEGF ligands and receptors have been implicated in each of these processes. VEGF family members also regulate neurogenesis and serve as neuronal survival factors [3, 4]. Because of recent reviews reporting the role of VEGF in the nervous system [4, 5], we focus here on the most recent findings showing effects of VEGF family members in neurons and in glia, their signaling mechanisms and their importance during development and adulthood as well as in neurodegenerative diseases.
VEGF ligands and receptors in neurogenesis
Since there are two other reviews in this issue of Cellular and Molecular Life Sciences (Wittko-Schneider and Plate, and Licht and Keshet) which provide a more detailed overview of this topic, we will only briefly mention here some key findings indicating a role for VEGF in neurogenesis. The generation of neurons from neural stem and progenitor cells (neurogenesis) occurs both during development and in the adult. In adult mice, neural stem cells (NSCs) reside in vascular niches located in two defined central nervous system (CNS) regions, the subventricular zone (SVZ) and the subgranular zone (SGZ) of the hippocampal dentate gyrus. NSCs proliferate in these areas in close apposition to growing capillaries. Endothelial cells influence proliferation of NSCs and VEGF participates in the crosstalk between endothelial cells and NSCs [3]. Several in vivo studies have indicated that VEGF induces adult neurogenesis. First, in adult birds, testosterone-induced neurogenesis is mediated by upregulation of VEGF, which signals to endothelial cells to stimulate angiogenesis in the vocal cord. In response to VEGF, endothelial cells secrete brain-derived neurotrophic factor, which then stimulates neural cell proliferation [6]. Second, intracerebroventricular (ICV) delivery of VEGF, or neuronal specific overexpression of VEGF, stimulates neurogenesis in the SVZ and SGZ both under basal conditions and upon middle cerebral arterial occlusion [7–9]. Third, hippocampal expression of VEGF is increased in situations involving memory learning (i.e. increased environment and spatial maze) and overexpression of VEGF via viral gene transfer in the hippocampus of adult rats results in increased neurogenesis and improved cognition [10]. Overexpression of a dominant negative mutant form of VEGFR-2 reduces basal neurogenesis and impairs learning, as well as antagonizes VEGF-enhanced effects, without inhibition of endothelial cell proliferation [10]. And fourth, antidepressant therapy stimulates neurogenesis in part via VEGF upregulation in the hippocampus and VEGFR-2 signaling [11]. Despite these studies, it still remains unknown if VEGF signals in vivo directly to NSCs or indirectly through effects on endothelial cells or other cell types. Nevertheless, genetic studies in vitro support a direct role for VEGF in NSCs. For example, granulocyte colony-stimulating factor (G-CSF) stimulates neurogenesis by increasing the expression of VEGFR-2 and the release of VEGF in NSCs. Blockage of VEGFR-2 signaling inhibits neurogenesis induced by G-CSF [12]. The use of neurospheres from mice lacking VEGFR-2 in NSCs illustrates that VEGFR-2 signaling is essential for survival of cultured NSCs [13]. VEGFR-2 expression in NSCs seems to be controlled by bFGF signaling and to be required for VEGF-induced NSC proliferation [14].
VEGF-B also stimulates neurogenesis in the adult brain. BrdU labeling of immature neurons in the SVZ and SGZ is reduced in VEGF-B knockout mice and enhanced in VEGF-B-treated neuronal cultures and in rats given VEGF-B via ICV injection [15]. VEGF-C also regulates neurogenesis by signaling directly to VEGFR-3 expressing multipotent NSCs. VEGF-C, expressed by astroglial and other SVZ cells, activates VEGFR-3 in NSCs and induces neurogenesis in vitro. In mice, conditional inactivation of VEGFR-3 in NSCs and blocking of VEGFR-3 with antibodies reduces neurogenesis in the SVZ [16]. Consistently, overexpression of VEGF-C by unilateral injection of adeno-associated viruses into the striatum stimulates SVZ neurogenesis. In addition, VEGFR-3 is also expressed in oligodendrocyte precursor cells (OPCs) and its ligand VEGF-C is required for proper OPC proliferation [17, 18].
VEGF ligands and receptors in neuronal survival
VEGF has a survival effect on neural progenitors and different postmitotic neurons of the CNS and peripheral nervous system in vitro [3]. In primary motor neurons, VEGF stimulation enhances survival under basal conditions as well as under hypoxic/hypoglycemic conditions, serum deprivation or upon glutamate-induced neurotoxicity [19, 20]. The signaling mechanisms via which VEGF mediates neuronal survival seem to rely on the activation of the PI3K/Akt and MAPK pathways [3]. Interestingly, apart from activating the classical signaling pathways, VEGF also controls survival of the neuroblastoma cell line SH-SY5Y by modulating the phosphorylation of the voltage-gated ion channel Kv1.2 [21]. VEGF also protects primary hippocampal neurons against glutamate-induced cell death by activating VEGFR-2 and reducing the Ca2+ influx via the inhibitory activity of the HVA Ca2+ channels [22]. Likewise, VEGF is neuroprotective against AMPA receptor-mediated excitotoxicity in cultured motor neurons by upregulating the AMPA receptor subunit GluR2, which when present in AMPA channels renders those channels less permeable to Ca2+ [23].
Several other in vivo findings also implicate VEGF as a neuronal survival factor. In a transgenic mouse line that constitutively expresses VEGF in retinal ganglion cells but does not show prominent vascular abnormalities in the retina, VEGF protects axotomized retinal ganglion cells from degeneration via signaling through its receptor VEGFR-2 and downstream activation of ERK1/2 and Akt pathways in vivo [24]. VEGF can also act as a neuronal survival factor under physiological conditions. For instance, during development, gonadotropin releasing hormone (GnRH) neurons migrate from the nasal placode through the nose and forebrain to the hypothalamus, where they regulate sexual reproduction. Analysis of different VEGF and VEGF receptor transgenic mice (NRP1-null mice, neuronal specific NRP1 knockout mice, neuronal specific VEGFR-2 knockout mice and mice lacking the VEGF164 isoform) has shown that VEGF164 acts as a survival factor in migrating GnRH neurons through NRP1 binding and via activation of the PI3K/Akt and MAPK pathways. The examination of an endothelial cell-specific NRP1 knockout mice revealed that VEGF exerts its survival effect in GnRH neurons independently of its effects in the vascular system [25].
VEGF-B is also a survival factor and a potent apoptosis inhibitor for neurons. VEGF-B exists in two isoforms, a matrix-binding VEGF-B167 form and a diffusible VEGF-B186 isoform. VEGF-B167, through VEGFR-1 signaling, downregulates genes involved in apoptosis pathways such as the proapoptotic BH3-only proteins [26]. VEGF-B167 treatment rescues retinal neurons from apoptosis induced by N-methyl-d-aspartate (NMDA) in vitro. In mouse models of optic nerve crush injury or ischemic neuronal cell death, VEGF-B167 treatment prevents neuronal cell death [26]. Motoneurons also express VEGFR-1 and VEGF-B. VEGFR-1 signaling by VEGF-B186 protects motoneurons from degeneration [25]. However, VEGF-B186 treatment in motoneurons from mice lacking VEGFR-1 signaling (Flt1-TK−/− mice) fail to exert a neuroprotective effect, indicating that VEGF-B/VEGFR-1 signaling is essential for their survival.
VEGF ligands and receptors in neuronal and glia migration
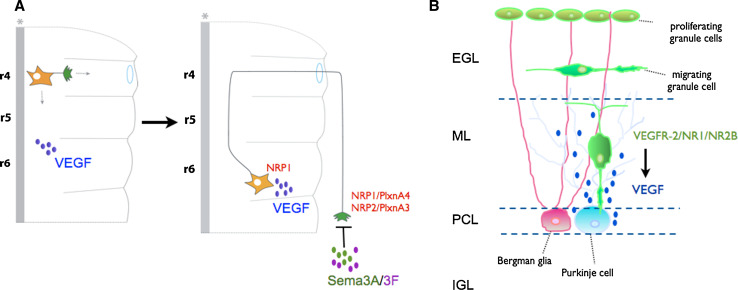
VEGF regulates neuronal migration in vivo, independently of its vascular effects, both during development and in adulthood. Mouse genetic studies, using transgenic lines expressing selective VEGF isoforms, or lacking semaphorins or NRP1 have revealed that VEGF is required for tangential migration of facial branchiomotor (FBM) neurons during hindbrain development, independently of its angiogenic properties [27]. In the developing mouse hindbrain, FBM neurons are born in rhombomere 4 and migrate to their final destination located in rhombomere 6. FBM neurons express the VEGF receptor NRP1. VEGF164 isoform (expressed in the migratory pathway that FBM follows) signals via NRP1 to control the caudal migration of these neurons in vivo [27]. Interestingly, VEGF164 is not required for the guidance of FBM axons in the peripheral nervous system, but instead, two other NRP ligands, semaphorin3A and semaphorin3F, control this process [27, 28] (Fig. 1a). The signaling mechanisms by which VEGF controls FBM neuronal migration via NRP1 remain unknown.
Fig. 1.
VEGF regulates neuronal migration. a VEGF controls migration of FBM neurons in the hindbrain. VEGF164 binds to NRP1 expressed in the FBM soma and controls their migration in the soma from rhombomere 4 (r4) to rhombomere 6 (r6). Axon guidance of these neurons is not controlled by VEGF164 but instead by semaphorin3A and semaphorin3F (SemaA/3F) which signal to receptor complexes formed by NRP1/PlxnA4 or NRP2/PlxnA3, respectively (adapted from Schwarz et al. [27]). b VEGF controls cerebellar granule cell migration during development. VEGF is expressed in Purkinje cells and controls granule cell migration from the external granule cell layer (EGL) towards the internal granule cell layer (IGL). Granule cells express VEGFR-2. VEGFR-2 forms a complex with NMDARs (NR1/NR2B) and VEGF signaling via VEGFR-2 induces the formation of VEGFR-2/NR1/NR2B receptor complexes and potentiates NMDAR-mediated currents and Ca2+ influx to control granule cell migration
VEGF also regulates granule cell (GC) migration during cerebellar development. GCs located in the external GC layer migrate across the molecular layer and Purkinje cell layer to reach their final destination at the internal GC layer. In the developing cerebellum, Purkinje cells express the VEGF188, VEGF164 and VEGF120 isoforms; these isoforms generate a VEGF gradient from the Purkinje cell layer towards the molecular layer. VEGFR-2 is expressed in migrating GCs [29]. Analysis of different transgenic mouse lines has revealed that reduced expression of VEGF or a disrupted VEGF gradient (caused by the absence of the VEGF164 and VEGF188 isoforms) impairs GC migration. Similarly, GC-specific elimination of VEGFR-2 leads to defects in GC migration in vivo [29] (Fig. 1b). Further analysis of the molecular mechanisms by which VEGF controls GC migration has shown that VEGF modulates NMDA receptor (NMDAR) channel activity in migrating GCs [30]. NMDARs are expressed in migrating GCs and their activation results in Ca2+ influx (required for proper GC migration) [31, 32]. In migrating GCs, VEGFR-2 associates in a complex with NMDARs (more precisely with NR1 and NR2B subunits; Fig. 1b). VEGF signaling in these neurons leads to a precise GC migration via the induction of clustering of VEGFR-2 and NMDARs, the activation of Src family kinases (SFKs) and the SFK-dependent phosphorylation of the NMDAR subunit NR2B, which results in the potentiation of NMDAR-mediated Ca2+ influx and NMDAR-mediated currents [30]. Based on these findings, it would be interesting to determine whether VEGFR-2 also associates with NMDARs at synapses and whether VEGF modulates NMDAR-mediated currents and Ca2+ influx.
VEGF has also been shown to control migration of neural progenitor cells (NPCs) along the rostral migratory stream (RMS), most likely by directly acting on NPCs independently of its effects in blood vessels. As this is the focus of another review in this issue, by Wittko-Schneider and Plate, we just briefly summarize below the main findings. NPCs express VEGFR-2, and VEGFR-2 is activated in NPCs migrating within the RMS. Glia cells within the RMS express VEGF and its receptor VEGFR-1 [33, 34]. Using mice lacking VEGFR-1 signaling (Flt1-TK−/− mice), it was shown that VEGFR-1 acts as a negative regulator of NPC migration in the RMS by controlling the amount of VEGF available for activating VEGFR-2 in VEGFR-2+ NPCs in the RMS [34]. Another in vitro study showed that NPCs isolated from the SVZ and cultured in the presence of FGF-2 express both VEGFR-1 and VEGFR-2 at the mRNA level [35]. Through VEGFR-2 activation and in the presence of FGF-2, VEGF exerts a chemoattractant effect on NPCs in SVZ explants [35].
Aside from regulating neuronal migration, other studies have indicated that VEGF also controls migration of neuroglia. In Drosophila, proper positioning of midline glia is a requirement for normal guidance of axons at the midline and consequently for maintaining the integrity of the glial sheet during axon enwrapment. The Drosophila VEGF homologs, PVFs ligands, are involved in this process of glia migration. PVF ligands are expressed in midline neurons (MP1 and VUM) while the PDGFR/VEGFR homolog, PVR, is expressed in midline glia. PVF/PVR signaling is required for midline glial survival and migration during axon guidance [36]. Vertebrate glia (oligodendrocyte) precursors (OPCs) migrate long distances to reach the axons in the CNS to ensure and sustain correct myelination. A role for VEGF in regulating the migration of OPCs in vitro has also been described. OPCs isolated from rat neonatal cortex express VEGFR-2 [18]. Stimulation of cultured OPCs with VEGF does not affect proliferation or differentiation but promotes OPC migration via VEGFR-2 and focal adhesion kinase-dependent mechanism [18, 37]. Further studies are needed to determine if VEGF also controls OPC migration in vivo. On the other hand, VEGFR-1 is upregulated in activated microglia and serves as a microglial chemotactic receptor in vitro and in vivo [38].
VEGF in CNS axon growth and guidance
Several reports indicate that VEGF and its receptors regulate axon growth and guidance in the CNS during development. Initial in vitro experiments showed that VEGF induces neurite outgrowth in ventral mesencephalic neuronal cultures [39], in primary cortical neurons and organotypic cortical explants [40, 41] and in retinal ganglion cells from retinal explants [42]. In all cases, VEGF-induced neurite outgrowth is mediated via VEGFR-2, expressed by these different neuronal types. The signaling pathways that VEGF activates to induce neurite outgrowth have also been investigated. In cortical neurons, one study showed that VEGF activates a signaling cascade comprising VEGFR-2 activation, Rho/Rho kinase (Rho/ROK) activation and phosphorylation of the actin dynamic regulator [41]. Another study using cortical explants showed that VEGF-induced neurite outgrowth requires the activation of VEGFR-2, MAPK (MEK1) and PI3K/Akt [40]. VEGF-B and PlGF do not stimulate neurite outgrowth in cultured cortical neurons in vitro, consistent with the absence of VEGFR-1 expression [41]. A study focused on the growth cone actin cytoskeleton of chicken dorsal root ganglia (DRG) neurons revealed that VEGF, via VEGFR-2 and NRP-1, induces a rapid effect (within a few minutes) on the actin cytoskeleton leading to enhanced growth cone area and increased motion [43].
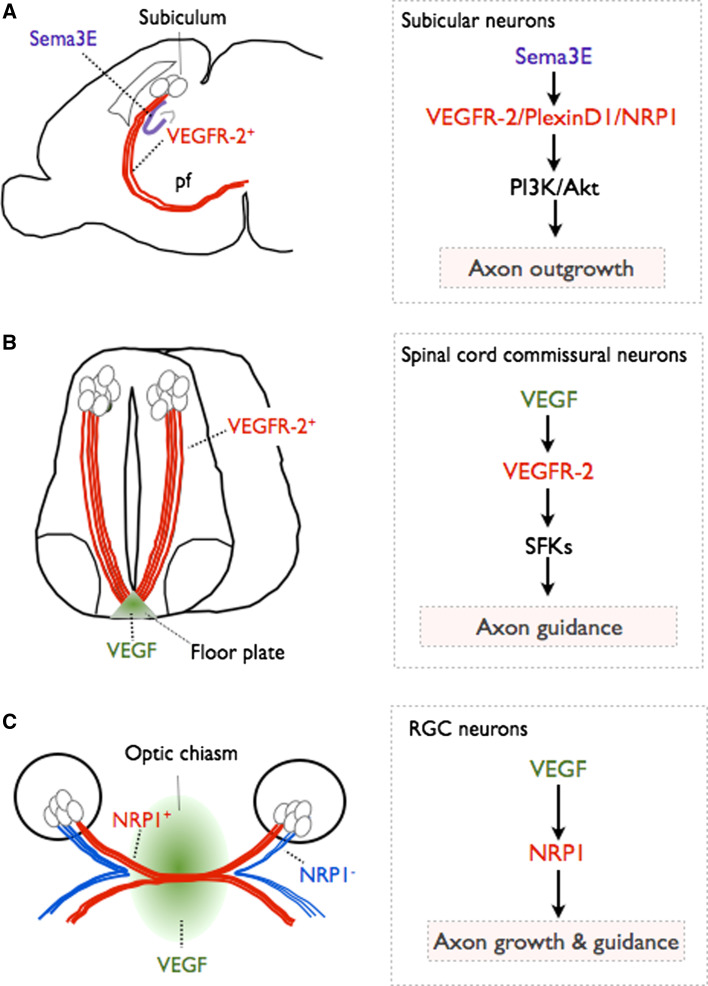
VEGFR-2 is also expressed in neurons of the subiculum (a subregion of the hippocampus formation) during embryonic development. Interestingly, analysis of a neuronal specific VEGFR-2 knockout mouse line shows that VEGFR-2 mediates axon elongation in a Sema3E-dependent, VEGF-independent manner [44]. Sema3E is expressed in the CA1 and CA3 fields of the hippocampus. In vitro experiments further show that VEGFR-2 associates with a PlexinD1/NRP1 complex and that Sema3E binds to the PlexinD1/NRP1/VEGFR-2 receptor complex and activates VEGFR-2 in a VEGF-independent manner. Sema3E-dependent VEGFR-2 activation and subsequent signaling through the PI3K/Akt pathway in subicular neurons are required for the stimulation of axon growth by Sema3E [44] (Fig. 2a). These results revealed a novel interplay between molecular players of neuronal and vascular development and showed for the first time that VEGFR-2 can be activated in neurons independently of VEGF binding.
Fig. 2.
VEGF regulates axon growth and guidance. a VEGFR-2 regulates axon outgrowth of subicular neurons. Subicular neurons extend their axons (red) from the subiculum through the postcommissural fornix tract (pf). Sema3E is expressed in the CA1 and CA3 fields of the hippocampus (blue). Subicular neurons express VEGR-2. In these neurons, VEGFR-2 forms a multiprotein complex with NRP1 and PlexinD1 and becomes activated in a Sema3E-dependent/VEGF-independent manner. Activation of VEGFR-2 leads to activation of PI3K/Akt and axon growth. b VEGF regulates commissural axon guidance at the spinal cord midline. During spinal cord development VEGF is expressed in the floor plate (green). Commissural neurons and their axons projecting towards the ventral midline express VEGFR-2 (red). Floor plate-derived VEGF signals to VEGFR-2 in commissural axons, activates SFKs at the growth cone and induces growth cone turning, thus controlling axon guidance. c VEGF controls retinal ganglion cell (RGC) axon growth and guidance at the optic chiasm. VEGF is expressed at the optic chiasm (green). Certain RGC axons express NRP1 (red). VEGF binds to NRP1 and guides the axons across the midline to project in the contralateral side. NRP1− RGC axons do not sense VEGF and are not attracted to the midline, and instead project ipsilaterally (blue)
Recent findings indicate that VEGF is also a physiological axon guidance molecule, independently of its effects in the vascular system. Commissural axon midline crossing occurs in different parts of the developing nervous system. In the developing mouse spinal cord, commissural neurons located in the dorsal part of the spinal cord send axons that project to the ventral side, chemoattracted by guidance factors secreted from the floor plate such as Netrin and sonic hedgehog (Shh). Once there, axons are repelled from the midline by guidance cues expressed at the floor plate, they cross the midline and project into the contralateral side of the spinal cord [45]. VEGF is expressed and secreted from the floor plate at the time of commissural axon guidance while VEGFR-2 is expressed by commissural neurons. Analysis of commissural axon guidance in transgenic mouse embryos expressing reduced levels of VEGF at the floor plate, or in mouse embryos where VEGFR-2 is inactivated in commissural neurons shows that VEGF and VEGR-2 act as a new ligand/receptor pair controlling midline axon guidance [46] (Fig. 2b). VEGF/VEGFR-2 signaling does not stimulate axon outgrowth from dorsal spinal cord explants or isolated commissural neurons. Instead, in vitro experiments have indicated that VEGF controls commissural axon growth cone turning via local activation of SFKs at the growth cone [46]. Thus, VEGF together with Netrin and Shh control commissural axon guidance and fasciculation at the midline.
Another example of commissural axon crossing occurs at the optic chiasm. Retinal ganglion cells (RGC) project axons from the retina towards the superior colliculus in mammals. Some of these axons will remain on the ipsilateral side and others will cross the midline to form the optic chiasm [45]. Expression studies have shown that VEGF is expressed in the diencephalon, where the optic chiasm will form, and that RGC axons express NRP1 when extending through the optic chiasm (Fig. 2b). Phenotyping of different VEGF isoform, NRP1 and NRP2 transgenic mice shows that VEGF164, via NRP1 binding, is essential for contralateral projection and fasciculation of RGC axons at the optic chiasm (Fig. 2b). In vitro experiments with retina explants or isolated RGCs have demonstrated that VEGF164 promotes RGC axon outgrowth and growth cone turning [47]. Hence, VEGF164 is a positive growth and guidance signal for RGC axons at the optic chiasm, which together with repulsive signals (Slits and Ephrins), cooperates to sort axons into their appropriate tracts.
Taken together, these two studies reveal a new role for VEGF as an axon growth and guidance signal, which, depending on the neuronal type and developmental process, acts via different receptors and different mechanisms to mediate its effects.
VEGF ligands and receptors in dendritogenesis and plasticity
In contrast to the increasing number of studies focusing on the role of VEGF ligands and receptors in neurogenesis, neuronal survival, neuronal migration or axon growth and guidance, so far fewer studies have been published suggesting a possible role for VEGF in neurite maturation, dendrite formation, and neuronal plasticity. As this is the focus of Licht and Keshet’s review in this same issue, we limit our description to the main findings in this topic. In primary cortical neurons, VEGF, via VEGFR-2 and activation of MAPK pathway, promotes the expression of microtubule-associated protein-2 (a protein enriched in dendrites) and neurite maturation [48], suggesting a role for VEGF in dendritogenesis. Several studies on primary hippocampal neurons have shown that VEGF enhances synaptic transmission and long-term potentiation [49, 50]. Interestingly, the exposure of mice to a short-term enriched environment leads to increased expression levels of VEGF in the hippocampus (via a HIF-1-mediated transcription) and to a significant increase in the number of dendritic spines in hippocampal CA1 pyramidal neurons [51]. Consistently, VEGF treatment increases the number of dendritic spines of primary hippocampal neurons in vitro and VEGFR-2 knockdown via shRNA abolishes this effect [51]. Further in vivo evidence for a role of VEGF in dendritogenesis and modulation of synaptic plasticity came from two other recent studies. In the first, analysis of a mouse transgenic system that allows conditionally knockdown of VEGF signaling at specific areas of the brain revealed that inactivated VEGF expression in the adult olfactory bulb caused a marked decrease in the density of dendritic spines of granule cells newly integrated into the olfactory bulb and in the number of dendrites of periglomerular neurons newly integrated into the olfactory bulb, with no obvious vascular changes [52]. In the second study, experiments using transgenic mouse models for conditional and reversible gain or loss of cerebral VEGF function demonstrated that VEGF plays a role in modulating plasticity of mature hippocampal neurons [53].
Interestingly, a role for VEGF-D and VEGFR-3 in maintaining dendrite arborization and memory formation was recently documented [54]. VEGF-D and VEGFR-3 are expressed in the hippocampus at different stages during development and adulthood. In primary hippocampal neurons, VEGF-D expression is controlled by basal neuronal activity through a mechanism initiated by NMDARs and L-type voltage-gated calcium channels and is dependent on nuclear calcium calmodulin-dependent protein kinase IV (CaMKIV) [54]. Blockage of nuclear calcium signaling leads to a decrease in total dendritic length and the complexity of dendrites. Interestingly, these defects are due to the downregulation of VEGF-D expression (upon blockage of nuclear calcium signaling) as re-addition of VEGF-D rescues the phenotype via VEGFR-3 signaling [54]. Consistent with the observed phenotype in cultured primary neurons, stereotaxic delivery of VEGF-D RNAi to the dorsal hippocampus in adult mice also leads to a reduction of the total length of dendrites and their complexity in vivo. Neuronal structural changes induced by RNAi-mediated knockdown of VEGF-D in vivo also cause cognitive deficits [54].
VEGF ligands and receptors in the peripheral nervous system
Several initial studies have shown expression of VEGF and its receptors in sensory neurons [3, 55, 56]. In vitro experiments using adult DRG in culture have shown that VEGF promotes neurite outgrowth, enhances cell survival and promotes Schwann cell proliferation [56]. In this case, VEGF-induced axonal outgrowth is mediated via VEGFR-2 signaling [57]. While these initial findings support a role for VEGF/VEGFR-2 in sensory neuron axon outgrowth, another study, using embryonic and neonatal DRGs, has shown that VEGFR-2 is expressed only in the vasculature, NRP1 is expressed in DRG neurons, and VEGF/VEGFR-2 signaling is only required for formation and maintenance of the DRG vasculature [58]. VEGF’s effect on DRG growth cone collapse has also been analyzed using DRGs from newborn rats. Newborn DRG neurons express high levels of NRP1 and much lower levels of VEGFR-2 [59]. In vitro functional studies have indicated that VEGF prevents sensory neuron growth cone collapse via NRP1. The presence of PlGF2 isoform (which binds NRP1 and VEGFR-1 but not VEGFR-2) also inhibits growth cone collapse, supporting NRP1 as the main VEGF receptor in newborn DRG neurons [59]. Whether the observed differences between the above-mentioned studies might be due to differences in the developmental stage analyzed (adult versus embryonic and newborn) and/or to the in vitro culture conditions needs further investigation. Sensory neuron-derived VEGF also acts on adjacent aligned blood vessels and promotes arterial differentiation via NRP1 signaling [60, 61].
Genetic evidence for a role of VEGF and VEGFR-2 in neuroprotection of adult sensory neurons was recently obtained using adult transgenic mice that had altered VEGFR-2 expression in postnatal neurons [62]. Treatment of isolated DRGs from wild-type mice with paclitaxel (a chemotherapeutic agent that causes neuropathy as a side effect) induces signs of neuronal stress, which are reduced upon treatment with VEGF. DRGs from transgenic mice overexpressing a wild-type form of VEGFR-2 under the neuron-specific Thy1.2 promoter (active in sensory neurons) are protected against paclitaxel-induced effects when pretreated with VEGF. Consistent with this, DRG sensory neurons from mice overexpressing a dominant-negative form of VEGFR-2 (lacking the intracellular domain) in Thy1.2+ neurons show aggravated sensory nerve defects [62]. In vivo studies have further shown that neuron-specific overexpression of VEGFR-2 protects against paclitaxel-induced neuropathy, whereas dominant-negative inhibition of neuronal VEGFR-2 induces a painful sensory neuropathy.
Recently, a role for VEGF-B and its receptor VEGFR-1 in sensory neurons has also been shown [63]. VEGF-B and VEGFR-1 are expressed in sensory neurons. Under healthy conditions, VEGF-B is not essential for survival of sensory neurons. However, DRG neurons isolated from adult VEGF-B knockout mice or from transgenic mice expressing a truncated form of VEGFR-1 (Flt1-TK−/− mice) exhibit increased neuronal stress under baseline culture conditions and are also more sensitive to stress stimuli. In vivo, when challenged with paclitaxel, VEGF-B-deficient mice, or Flt1-TK−/− mice, display increased signs of neuropathy and are more susceptible to retrograde degeneration of nerves. Consistent with a role for VEGF-B/VEGFR-1 signaling in protection of sensory neurons, transgenic mice overexpressing VEGFR-1 or VEGF-B specifically in neurons are protected against distal-induced neuropathy [63]. Interestingly, another study has shown that intramuscular PlGF-2 transfer ameliorates sensory neuropathy in diabetic mice without affecting the vascular system [64]. Whether PlGF-2 in diabetic adult mice signals directly to sensory neurons or whether it signals via VEGFR-1 and/or NRP1 remains unknown.
Apart from acting on the sensory/somatic system, several findings suggest that VEGF ligands and receptors also participate in different processes of the autonomic nervous system. The autonomic nervous system transmits impulses from the CNS to the peripheral organs in order to regulate their functions and homeostasis. It is subdivided into the sympathetic and parasympathetic nervous systems. Initial studies have shown that VEGF and VEGF receptors are expressed in sympathetic neurons and VEGF stimulates neurite outgrowth and enhanced cell survival of adult superior cervical ganglia neurons via VEGFR-2 signaling in vitro [56, 57].
Postganglionic sympathetic neurons innervate blood vessels and control blood pressure and flow. For example, by releasing neurotransmitters, sympathetic neurons control thermoregulation in the skin and redistribution of flow from internal organs to the brain in stress conditions. Within the last 3 or 4 years, several studies have shown a role for VEGF as a modulator of vascular sympathetic innervation. Postganglionic neurons and vascular smooth muscle cells covering innervated arteries express VEGF [65]. Postganglionic neurons also express VEGFR-1, VEGFR-2 and NRP1 [65–67]. In sympathetic neurons, VEGF and VEGFR-2 expression are regulated by NGF signaling [68, 69]. In vitro, VEGF induces growth cone enlargement via VEGFR-1 signaling and counteracts Sema3A-induced growth cone collapse of cultured sympathetic neurons [66]. The use of an in vitro culture system to evaluate sympathetic neurite outgrowth towards innervated vessels (femoral artery) or sparsely innervated vessels (carotid artery) show that vessel-derived VEGF and Sema3A drive selective sympathetic neurite outgrowth towards vessels. VEGF expression is similar in both types of vessels, but Sema3A expression is higher in carotid arteries, suggesting that the chemorepulsive cue Sema3A limits sympathetic outgrowth and innervation in carotid arteries. In this experimental setting, VEGF promotes vascular innervation via VEGFR-2 and NRP1 but not VEGFR-1 [67]. Whether VEGFR-1 and VEGFR-2 have different functions in sympathetic neurons, one controlling specifically growth cone morphology and the other controlling neurite outgrowth, remains unknown. In vivo evidence for a role of VEGF in vascular innervation has also been documented. First, treatment with a functional VEGF-blocking antibody reduces femoral arterial reinnervation after local nerve damage in wild-type mice [66]. Second, transgenic mice overexpressing VEGF show a denser femoral sympathetic innervation than wild-type littermates [67]. And third, mice with reduced levels of VEGF (VEGF∂/∂ mice, see below) have impaired vascular regulation of resistance arteries and as a consequence exhibit defects in thermoregulation [65]. The analysis of these mice shows that VEGF, which is expressed by smooth muscle cells (SMCs) and sympathetic neurons not only regulates innervation of resistance arteries but also controls the maintenance of the perivascular autonomic nerve plexus. It does so by regulating differentiation of vascular SMCs and maintaining them in a fully differentiated contractile state. SMCs express VEGFR-1, suggesting a possible autocrine mechanism [65]. In addition, VEGF also acts directly on sympathetic neurons, which express VEGFR-1 and VEGFR-2, and controls arterial neuroeffector junctions, the size of the varicosities and the width of the junctional cleft [65]. Short-term administration of a VEGF inhibitor to wild-type mice results in functional and structural defects of neuroeffector junctions with no defects in SMCs, supporting a direct effect of VEGF in sympathetic neurons.
VEGF in neurodegenerative diseases
Amyotrophic lateral sclerosis
Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disease characterized by progressive motor neuron degeneration in the brain and spinal cord leading to muscle atrophy, paralysis and death within 3–5 years of diagnosis. ALS exists in a familial inheritable form and a sporadic form. To date, mutations in several genes have been discovered that account for approximately 25–30 % of all familial ALS cases; these include the Cu/Zn superoxide dismutase gene (SOD1), TAR DNA-binding protein (TARDBP or TDP-43), the related RNA-binding protein fused in sarcoma (FUS), optineurion (OPTN) and valosin-containing protein (VCP) [64]. Recently, the expansion of a hexanucleotide repeat in a noncoding region of chromosome 9 (C90RF72) has been associated with 46 % of familial ALS and 21 % of sporadic ALS cases [70, 71], making it the most common genetic cause of ALS identified to date.
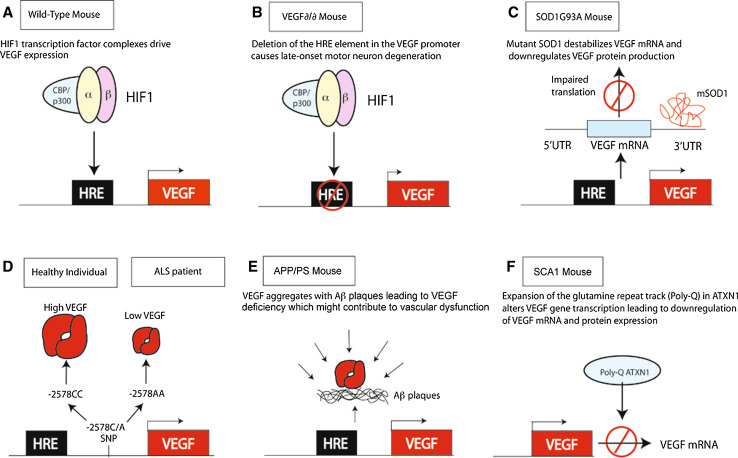
A role for VEGF in motor neuron degeneration was identified in VEGF∂/∂ mice, a transgenic mouse line in which the hypoxia response element in the VEGF promoter is deleted [72]. Due to this deletion, VEGF∂/∂ mice express about 25–40 % less VEGF in the CNS (Fig. 3a, b). VEGF∂/∂ mice develop adult-onset and progressive motor neuron degeneration, reminiscent of ALS in humans. When crossing VEGF∂/∂ with the SOD1G93A mouse model of ALS, VEGF∂/∂/SOD1G93A mice show increase severity of motor neuron degeneration and earlier onset of muscle weakness. Correlating with this finding, when SOD1G93A mice are crossed with mice overexpressing VEGF in neurons, double transgenic mice show delayed onset of paralysis and prolonged survival compared to single transgenic SOD1G93A mice [73]. From a therapeutic point of view, ICV administration of recombinant VEGF to ALS mice delays the onset of the disease and prolongs survival [74]. Similar results were observed in ALS mice in which the VEGF gene was transferred intramuscularly using viral gene transfer methods [75]. Gene variation studies have also suggested a link between VEGF and ALS in humans. A meta-analysis of different association studies on VEGF in ALS has shown that homozygous carriers of the −2578A haplotype (which results in reduced VEGF expression) exhibit a significantly increased risk of ALS [76]. Clinical trials to evaluate the safety of VEGF delivery in ALS patients are ongoing.
Fig. 3.
VEGF in neurodegenerative diseases. a Under normal conditions, HIF-1 is complexed with CBP/p300, binds to the HRE in the VEGF promoter and drives VEGF expression. b In VEGF∂/∂ mice, the HRE is mutated and as a consequence there is a reduced expression of VEGF leading to motor neuron cell death and ALS. c In mutant SOD1G93A mice, SOD1G93A destabilizes VEGF mRNA leading to less VEGF protein expression. d −22578C/A single nucleotide polymorphism. −2578A haplotype leads to reduced expression levels of VEGF and increases the risk of sporadic ALS in humans. e VEGF coaggregates with Aβ and is slowly released from the aggregates leading to reduced levels of available VEGF and neuronal and vascular dysfunction. f PolyQ-ATXN1 represses VEGF transcription leading to reduced levels of VEGF in the cerebellum and development of spinocerebellar ataxia type 1 (SCA1)
VEGF protects from motor neuron degeneration via VEGFR-2 signaling by at least two independent mechanisms. First, as described above, VEGF has a direct neuroprotective effect in motor neurons. Indeed, when mice overexpressing VEGFR-2 under the neuronal Thy1.2 promoter are intercrossed with SOD1G93A mice, the resultant double transgenic mice show reduced degeneration and improved motor performance when compared with single SOD1G93A mice [74]. Decreased VEGF expression is detected in spinal cords of SOD1G93A mice early in the course of the disease. Interestingly, SOD1G93A deregulates posttranscriptional processing of glia-derived VEGF by affecting the VEGF 3′-UTR region, leading to reduced VEGF mRNA and loss of protein expression [77] (Fig. 3c). Second, VEGF protects motor neurons indirectly by maintaining optimal levels of blood vessel perfusion and blood flow in the spinal cord and brain [72]. In addition, vascular structural and functional abnormalities were described in ALS mouse models even prior to motor neuron degeneration [22]. Among them, defects in the blood–brain barrier (BBB), due to reduced expression of tight junction proteins and of the glucose transported Glut1 in the endothelium, were observed [78, 79]. Evidence for a compromised BBB has also been found in ALS patients [48, 49].
VEGF-B and VEGFR-1 are also expressed in motor neurons and VEGFR-1 expression is upregulated in activated astrocytes of paralyzed SOD1G93A mutant mice [53]. As described above, VEGF-B treatment has a neuroprotective effect in isolated primary motor neurons via VEGFR-1 signaling. Mice lacking VEGF-B show normal behavior and do not suffer from ALS, indicating that VEGF-B is not required for the survival of motor neurons under normal conditions. However, when VEGF-B-deficient mice or Flt1-TK−/− mice are crossed with SOD1G93A mutant mice, the double transgenic mice develop a more severe form of motor neuron degeneration. ICV delivery of VEGF-B in SOD1G93A rats prolongs their survival without causing undesired angiogenesis or BBB leakiness [53]. These results suggest VEGF-B as an alternative therapeutic candidate for the treatment of ALS.
Multiple sclerosis
Multiple sclerosis (MS) is the most common autoimmune inflammatory disorder of the nervous system. It is characterized by dynamic inflammatory demyelinating lesions in the CNS. BBB permeability, which leads to edema, metabolic imbalance, excitotoxicity and ingression of inflammatory cells and factors into the CNS, is an early feature in MS patients [80, 81]. In MS, VEGF is expressed by reactive astrocytes and apart from exerting a neuroprotective effect, it could also signal to endothelial cells of the BBB to disrupt tight junctions and promote BBB breakdown [82]. Several studies have shown a correlation between VEGF expression levels and the magnitude of the lesion, suggesting that VEGF might aggravate disease progression [83, 84]. Interestingly, when mice with specific inactivation of VEGF in astrocytes (GfapCre:Vegffl/fl) are challenged with inflammatory or demyelinating lesions, reduced BBB breakdown, decreased lymphocyte infiltration and reduced neuropathology are observed, when compared to wild-type mice [80]. Similarly, GfapCre:Vegffl/fl mice subjected to a model of MS reveal a significant reduction in clinical disease, associated with decreased astrogliosis, BBB breakdown, inflammatory cell infiltration, multifocal demyelination and oligodendrocyte loss. Further analysis indicates that in CNS endothelial cells under inflammatory conditions, VEGF signals via VEGFR-2 to activate eNOS and subsequently leads to reduced expression of two tight-junctional molecules CLN-5 and OCLN [80]. Notably, inhibition of eNOS in MS mice during the acute clinical phase protects against paralysis and pathology [80]. Overall, this study suggests that blockage of brain endothelial cell VEGF signaling might be a protective avenue in patients with MS or other CNS inflammatory diseases.
Alzheimer’s disease
Alzheimer’s disease (AD) is a chronic neurodegenerative disease characterized by memory loss and cognitive decline. The main pathological features are neurofibrillary tangles and senile plaques caused by the progressive deposition of β-amyloid peptides (Aβ) in the brain, neuronal loss and inflammation. Similar to other neurodegenerative disorders, BBB dysfunction also contributes to neuronal degeneration in AD [77]. As the effect of the neurovascular unit and BBB dysfunction in AD have recently been reviewed [22, 77, 85], we focus here on findings describing a role for VEGF in AD.
Abnormal regulation of VEGF expression has been reported to occur in the pathogenesis of AD [3]. Although different association studies with distinct patient cohorts were performed, it still remains unclear whether functional polymorphisms within the VEGF promoter region that lower VEGF expression are associated with AD risk [3, 75]. VEGF immunoreactivity is enhanced in clusters of reactive astrocytes, in neurons and blood vessel walls, and also colocalizes with Aβ plaques [78, 79, 86] (Fig. 3e). Interestingly, in in vitro conditions, Aβ can bind the extracellular domain of VEGFR-2 and blocks VEGF-induced endothelial cell migration and capillary network formation [87]. Although VEGF might initially be activated in AD patients to counteract the insufficient perfusion and neurodegeneration, the coaggregation of VEGF with Aβ plaques and its slow release from the plaques might contribute to AD pathology by inhibiting VEGF from acting as a neurotrophic and neuronal survival factor as well as a vascular homeostatic factor [73] (Fig. 3e). Consistent with this idea, intraperitoneal injection of recombinant VEGF in AD mouse models ameliorates the cognitive impairment. In these mice, VEGF delivery leads to mobilization of CD34+ cells into the peripheral blood, homing of endothelial progenitor cells into the brain and enhanced hippocampal angiogenesis [88].
As mentioned above, chronic inflammation is a feature of AD pathology. One component of this inflammatory response is increased microglia mobility and chemotactic activity towards Aβ depositions. Interestingly, microglia obtained from human AD brain samples exhibit higher expression of VEGFR-1 and VEGF than microglia from control brains. Immunostaining of post-mortem AD brains or of APP23 mouse brains (a transgenic AD mouse model) has also confirmed that in AD VEGFR-1 and VEGF are expressed at higher levels and colocalize with microglia markers and Aβ depositions [38]. In vitro, blockage of VEGFR-1 signaling, using a VEGFR-1 functional blocking antibody, results in reduced Aβ-induced microglia chemotaxis. In vivo, intrahippocampal injection of Aβ leads to VEGFR-1+ microglia mobilization in the hippocampus. Notably, injection of an anti-VEGFR-1 functional blocking antibody into the hippocampus inhibits microglia migration towards Aβ depositions and confers partial neuroprotection [38]. Whether VEGF expressed in microglia activates VEGFR-1 to mediate the Aβ-induced effects, or whether VEGFR-1 is activated by other VEGF ligands remains to be determined. Moreover, it would be interesting to study whether vascular remodeling and BBB breakdown in AD is affected by VEGF-derived microglia, as occurs similarly in MS (see above).
Parkinson’s disease
Parkinson’s disease (PD) is the second most common neurodegenerative disorder after AD. It is characterized by the loss of dopamine-generating neurons in the substantia nigra and a consequent deficit of dopamine released into the striatum. Patients with PD suffer from rigidity, shaking, cognitive dementia, autonomic and psychiatric symptoms. As in the other neurodegenerative diseases discussed so far, a disrupted BBB and increased angiogenesis have been detected in PD patients [89]. So far, no association between VEGF polymorphisms or reduced serum levels of VEGF with PD has been found [90].
VEGF has been shown to have a neuroprotective effect in PD. When used at very low doses (1–10 ng/ml), VEGF protects primary dopaminergic neurons against cell death induced by 6-hydroxydopamine (6-OHDA; the most typical PD model) in vitro [91]. Likewise, transplantation of VEGF-secreting cells into the striatum of adult rats 1 week before induction of lesions by 6-OHDA also protects dopaminergic neurons against 6-OHDA-induced cell death with the highest neuroprotective effect observed with low doses of VEGF [91, 92]. Interestingly, VEGF protects dopaminergic neurons from cell death even when transplantation of VEGF-secreting cells is performed after 6-OHDA lesion induction [91]. The protective effects are most likely mediated by VEGF’s angiogenic and glial proliferative effects, as well as its direct effects on dopaminergic neurons [91]. VEGF gene transfer using adeno-associated viruses also improves rotational behavior in rat PD models and promotes survival of dopaminergic neurons and fibers [93]. Injection of VEGF/adeno-associated viruses significantly increases the number of reactive astrocytes and of glia-derived neurotrophic factor in the striatum but does not seem to induce angiogenesis or BBB leakiness when compared to control-injected mice [93]. Similar to MS and AD, VEGF overexpression has also been detected in reactive astrocytes within the substantia nigra of PD patients [13]. However, whether VEGF expression in reactive astrocytes contributes to the increased blood vessel density and BBB leakage observed in PD patients remains unknown. Studies with tissue-specific knockout mice for VEGF and its receptors are required to further elucidate the cellular and molecular mechanisms of the effect of VEGF.
Recently, a neuroprotective effect of VEGF-B in PD has also been reported. In vitro, rotenone treatment of midbrain neuronal cultures (another well-characterized PD model) leads to upregulation of VEGF-B, and exogenous VEGF-B stimulation protects midbrain neurons against rotenone-induced cell death [94]. Using a 6-OHDA rat in vivo PD model, Falk et al. [95] found that a single injection of recombinant VEGF-B186 into the striatum prior to 6-OHDA lesion induction improves rotational behavior and protects dopaminergic neurons in the caudal subregion of the substantia nigra. These reports suggest that VEGF-B may be a new protective factor for PD and open new avenues for further research. It would be interesting to determine whether the observed effects are due to a direct VEGF-B signaling to dopaminergic neurons or due to its effects in other brain cell types.
Spinocerebellar ataxia
Spinocerebellar ataxia (SCA) is a group of inherited progressive neurodegenerative diseases characterized by progressive incoordination of gait and motor deterioration [96]. Ataxia results from degeneration of neurons in the cerebellum, brain stem, spinocerebellar tracts and in their efferent and afferent connections. SCAs are dominantly inherited. Among the approximately 30 types of SCA identified so far, SCA1 is an adult-onset form of SCA caused by expansion of a glutamine repeat tract (PolyQ) in ataxin-1 (ATXN1). PolyQ ATXN1 alters gene transcription and leads to the selective death of mainly Purkinje cells in the cerebellum [96]. Interestingly, VEGF is expressed in Purkinje cells and its mRNA and protein levels are decreased in these cells in a SCA1 mouse model (Fig. 3f), before the appearance of any pathological signs [23]. Further molecular studies have shown that wild-type ATXN1 represses VEGF promoter activity and that the extent of the repression directly correlates with the expansion of the glutamine repeats of ATXN1 in vivo. In correlation with the proangiogenic function of VEGF, SCA1 mice show reduced microvascular density as well as higher levels of hypoxia in the cerebellum. Consistent with the neurotrophic effect of VEGF, inhibition of VEGF signaling via VEGFR-2 reduces growth and survival of cerebellar neurons in vitro. Pharmacological delivery of recombinant VEGF into the ventricle of SCA1 mice after disease onset restores cerebellar pathology and increases motor performance [23]. Whether VEGF expression in the adult cerebellum is also required for neuronal survival remains unknown. Nevertheless, these results open the door to exploring the use of VEGF in the treatment of SCA1.
Concluding remarks
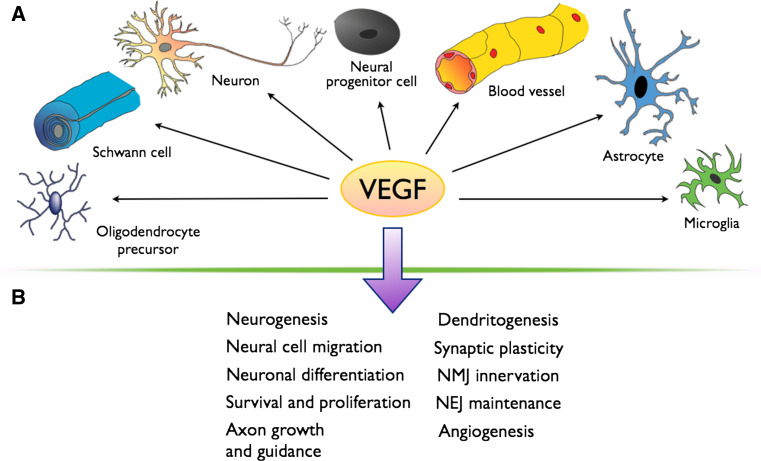
In the past decade great effort has resulted in the successful elucidation of the functional roles of VEGF and its receptors in neurons (Fig. 4). However, the signaling pathways that VEGF activates in neural cells in vivo are still not fully understood and it is still not known whether VEGF activates different signaling pathways depending on the VEGF-mediated effect. These recent findings also raise new questions for further research of the neurovascular link. For example, VEGF modulates NMDAR function in migrating GCs, but is there also a link between VEGF/VEGFR-2 and NMDARs in mature neurons? As NMDARs are crucial for synaptogenesis and synaptic plasticity, it would be interesting to determine whether VEGF modulates their function in dendritic spines and how this affects synaptic input and, for example, memory. VEGFR-2 can become activated in a Sema3E-dependent/VEGF-independent manner in subicular neurons, but does VEGF signaling via VEGFR-2, VEGFR-1 or NRP1 also activate other neuronal guidance receptors independently of their known ligands?
Fig. 4.
VEGF in the nervous system. a VEGF exerts its effects in many different cell types in the nervous system. b VEGF has a pleiotropic role in the nervous systems and plays a role in the indicated processes (NEJ neuroeffector junction, NMJ neuromuscular junction)
VEGF seems to play a double role in neurodegenerative diseases. On the one hand, it offers neuroprotection in neurons via a direct effect or indirectly via maintaining a stable CNS vascular system. On the other hand, excessive VEGF expression, for example by reactive astrocytes, seems to be deleterious, as activated astrocyte-derived VEGF seems to induce BBB breakdown and vascular leakage. Therefore, to design therapies based on VEGF ligands or receptors, a better understanding of the cellular types that participate in the disease, of VEGF expression and its effects, and of the dose needed for a therapeutic window where VEGF is beneficial, are required.
Moreover, other members of the VEGF family seem to play important roles in neurogenesis, neuronal survival and neuronal function. Further studies unraveling the signaling mechanisms of these VEGF ligands in neurons (and other neural cell types) and whether they could also have an effect in neurological disorders will help our understanding of the neurovascular link and perhaps in developing new avenues for therapeutic treatment.
Acknowledgments
The work of P.C. is supported by long-term structural funding (Methusalem funding from the Flemish Government). The work of C.R.A. is supported by a Marie Curie Career integration grant (FP7-PEOPLE-2011-CIG-304050), by the BZH (University of Heidelberg) and by the European Research Council (ERC-StG-2012; 311367).
References
- 1.Koch S, Tugues S, Li X, Gualandi L, Claesson-Welsh L. Signal transduction by vascular endothelial growth factor receptors. Biochem J. 2011;437(2):169–183. doi: 10.1042/BJ20110301. [DOI] [PubMed] [Google Scholar]
- 2.Holmes DI, Zachary I. The vascular endothelial growth factor (VEGF) family: angiogenic factors in health and disease. Genome Biol. 2005;6(2):209. doi: 10.1186/gb-2005-6-2-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruiz de Almodovar C, Lambrechts D, Mazzone M, Carmeliet P. Role and therapeutic potential of VEGF in the nervous system. Physiol Rev. 2009;89(2):607–648. doi: 10.1152/physrev.00031.2008. [DOI] [PubMed] [Google Scholar]
- 4.Mackenzie F, Ruhrberg C. Diverse roles for VEGF-A in the nervous system. Development. 2012;139(8):1371–1380. doi: 10.1242/dev.072348. [DOI] [PubMed] [Google Scholar]
- 5.Rosenstein JM, Krum JM, Ruhrberg C. VEGF in the nervous system. Organogenesis. 2010;6(2):107–114. doi: 10.4161/org.6.2.11687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Louissaint A, Jr, Rao S, Leventhal C, Goldman SA. Coordinated interaction of neurogenesis and angiogenesis in the adult songbird brain. Neuron. 2002;34(6):945–960. doi: 10.1016/S0896-6273(02)00722-5. [DOI] [PubMed] [Google Scholar]
- 7.Jin K, Zhu Y, Sun Y, Mao XO, Xie L, Greenberg DA. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci U S A. 2002;99(18):11946–11950. doi: 10.1073/pnas.182296499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun Y, Jin K, Xie L, Childs J, Mao XO, Logvinova A, Greenberg DA. VEGF-induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia. J Clin Investig. 2003;111(12):1843–1851. doi: 10.1172/JCI17977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y, Jin K, Mao XO, Xie L, Banwait S, Marti HH, Greenberg DA. VEGF-overexpressing transgenic mice show enhanced post-ischemic neurogenesis and neuromigration. J Neurosci Res. 2007;85(4):740–747. doi: 10.1002/jnr.21169. [DOI] [PubMed] [Google Scholar]
- 10.Cao L, Jiao X, Zuzga DS, Liu Y, Fong DM, Young D, During MJ. VEGF links hippocampal activity with neurogenesis, learning and memory. Nat Genet. 2004;36(8):827–835. doi: 10.1038/ng1395. [DOI] [PubMed] [Google Scholar]
- 11.Warner-Schmidt JL, Duman RS. VEGF as a potential target for therapeutic intervention in depression. Curr Opin Pharmacol. 2008;8(1):14–19. doi: 10.1016/j.coph.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jung KH, Chu K, Lee ST, Kim SJ, Sinn DI, Kim SU, Kim M, Roh JK. Granulocyte colony-stimulating factor stimulates neurogenesis via vascular endothelial growth factor with STAT activation. Brain Res. 2006;1073-1074:190–201. doi: 10.1016/j.brainres.2005.12.037. [DOI] [PubMed] [Google Scholar]
- 13.Wada T, Haigh JJ, Ema M, Hitoshi S, Chaddah R, Rossant J, Nagy A, van der Kooy D. Vascular endothelial growth factor directly inhibits primitive neural stem cell survival but promotes definitive neural stem cell survival. J Neurosci. 2006;26(25):6803–6812. doi: 10.1523/JNEUROSCI.0526-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiao Z, Kong Y, Yang S, Li M, Wen J, Li L. Upregulation of Flk-1 by bFGF via the ERK pathway is essential for VEGF-mediated promotion of neural stem cell proliferation. Cell Res. 2007;17(1):73–79. doi: 10.1038/sj.cr.7310126. [DOI] [PubMed] [Google Scholar]
- 15.Sun Y, Jin K, Childs JT, Xie L, Mao XO, Greenberg DA. Vascular endothelial growth factor-B (VEGFB) stimulates neurogenesis: evidence from knockout mice and growth factor administration. Dev Biol. 2006;289(2):329–335. doi: 10.1016/j.ydbio.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 16.Calvo CF, Fontaine RH, Soueid J, Tammela T, Makinen T, Alfaro-Cervello C, Bonnaud F, Miguez A, Benhaim L, Xu Y, Barallobre MJ, Moutkine I, Lyytikka J, Tatlisumak T, Pytowski B, Zalc B, Richardson W, Kessaris N, Garcia-Verdugo JM, Alitalo K, Eichmann A, Thomas JL. Vascular endothelial growth factor receptor 3 directly regulates murine neurogenesis. Genes Dev. 2011;25(8):831–844. doi: 10.1101/gad.615311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Le Bras B, Barallobre MJ, Homman-Ludiye J, Ny A, Wyns S, Tammela T, Haiko P, Karkkainen MJ, Yuan L, Muriel MP, Chatzopoulou E, Breant C, Zalc B, Carmeliet P, Alitalo K, Eichmann A, Thomas JL. VEGF-C is a trophic factor for neural progenitors in the vertebrate embryonic brain. Nat Neurosci. 2006;9(3):340–348. doi: 10.1038/nn1646. [DOI] [PubMed] [Google Scholar]
- 18.Hayakawa K, Pham LD, Som AT, Lee BJ, Guo S, Lo EH, Arai K. Vascular endothelial growth factor regulates the migration of oligodendrocyte precursor cells. J Neurosci. 2011;31(29):10666–10670. doi: 10.1523/JNEUROSCI.1944-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Den Bosch L, Storkebaum E, Vleminckx V, Moons L, Vanopdenbosch L, Scheveneels W, Carmeliet P, Robberecht W. Effects of vascular endothelial growth factor (VEGF) on motor neuron degeneration. Neurobiol Dis. 2004;17(1):21–28. doi: 10.1016/j.nbd.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 20.Tolosa L, Mir M, Asensio VJ, Olmos G, Llado J. Vascular endothelial growth factor protects spinal cord motoneurons against glutamate-induced excitotoxicity via phosphatidylinositol 3-kinase. J Neurochem. 2008;105(4):1080–1090. doi: 10.1111/j.1471-4159.2007.05206.x. [DOI] [PubMed] [Google Scholar]
- 21.Qiu MH, Zhang R, Sun FY. Enhancement of ischemia-induced tyrosine phosphorylation of Kv1.2 by vascular endothelial growth factor via activation of phosphatidylinositol 3-kinase. J Neurochem. 2003;87(6):1509–1517. doi: 10.1046/j.1471-4159.2003.02110.x. [DOI] [PubMed] [Google Scholar]
- 22.Ma YY, Li KY, Wang JJ, Huang YL, Huang Y, Sun FY. Vascular endothelial growth factor acutely reduces calcium influx via inhibition of the Ca2+ channels in rat hippocampal neurons. J Neurosci Res. 2009;87(2):393–402. doi: 10.1002/jnr.21859. [DOI] [PubMed] [Google Scholar]
- 23.Cvetanovic M, Patel JM, Marti HH, Kini AR, Opal P. Vascular endothelial growth factor ameliorates the ataxic phenotype in a mouse model of spinocerebellar ataxia type 1. Nat Med. 2011;17(11):1445–1447. doi: 10.1038/nm.2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kilic U, Kilic E, Jarve A, Guo Z, Spudich A, Bieber K, Barzena U, Bassetti CL, Marti HH, Hermann DM. Human vascular endothelial growth factor protects axotomized retinal ganglion cells in vivo by activating ERK-1/2 and Akt pathways. J Neurosci. 2006;26(48):12439–12446. doi: 10.1523/JNEUROSCI.0434-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poesen K, Lambrechts D, Van Damme P, Dhondt J, Bender F, Frank N, Bogaert E, Claes B, Heylen L, Verheyen A, Raes K, Tjwa M, Eriksson U, Shibuya M, Nuydens R, Van Den Bosch L, Meert T, D’Hooge R, Sendtner M, Robberecht W, Carmeliet P. Novel role for vascular endothelial growth factor (VEGF) receptor-1 and its ligand VEGF-B in motor neuron degeneration. J Neurosci. 2008;28(42):10451–10459. doi: 10.1523/JNEUROSCI.1092-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Y, Zhang F, Nagai N, Tang Z, Zhang S, Scotney P, Lennartsson J, Zhu C, Qu Y, Fang C, Hua J, Matsuo O, Fong GH, Ding H, Cao Y, Becker KG, Nash A, Heldin CH, Li X. VEGF-B inhibits apoptosis via VEGFR-1-mediated suppression of the expression of BH3-only protein genes in mice and rats. J Clin Investig. 2008;118(3):913–923. doi: 10.1172/JCI33673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwarz Q, Gu C, Fujisawa H, Sabelko K, Gertsenstein M, Nagy A, Taniguchi M, Kolodkin AL, Ginty DD, Shima DT, Ruhrberg C. Vascular endothelial growth factor controls neuronal migration and cooperates with Sema3A to pattern distinct compartments of the facial nerve. Genes Dev. 2004;18(22):2822–2834. doi: 10.1101/gad.322904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwarz Q, Waimey KE, Golding M, Takamatsu H, Kumanogoh A, Fujisawa H, Cheng HJ, Ruhrberg C. Plexin A3 and plexin A4 convey semaphorin signals during facial nerve development. Dev Biol. 2008;324(1):1–9. doi: 10.1016/j.ydbio.2008.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruiz de Almodovar C, Coulon C, Salin PA, Knevels E, Chounlamountri N, Poesen K, Hermans K, Lambrechts D, Van Geyte K, Dhondt J, Dresselaers T, Renaud J, Aragones J, Zacchigna S, Geudens I, Gall D, Stroobants S, Mutin M, Dassonville K, Storkebaum E, Jordan BF, Eriksson U, Moons L, D’Hooge R, Haigh JJ, Belin MF, Schiffmann S, Van Hecke P, Gallez B, Vinckier S, Chedotal A, Honnorat J, Thomasset N, Carmeliet P, Meissirel C. Matrix-binding vascular endothelial growth factor (VEGF) isoforms guide granule cell migration in the cerebellum via VEGF receptor Flk1. J Neurosci. 2010;30(45):15052–15066. doi: 10.1523/JNEUROSCI.0477-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meissirel C, Ruiz de Almodovar C, Knevels E, Coulon C, Chounlamountri N, Segura I, de Rossi P, Vinckier S, Anthonis K, Deleglise B, de Mol M, Ali C, Dassonville K, Loyens E, Honnorat J, Michotte Y, Rogemond V, Smolders I, Voets T, Vivien D, Vanden Berghe P, Van Den Bosch L, Robberecht W, Chedotal A, Oliviero S, Dewerchin M, Schmucker D, Thomasset N, Salin P, Carmeliet P. VEGF modulates NMDA receptors activity in cerebellar granule cells through Src-family kinases before synapse formation. Proc Natl Acad Sci USA. 2011;108(33):13782–13787. doi: 10.1073/pnas.1100341108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Komuro H, Rakic P. Modulation of neuronal migration by NMDA receptors. Science. 1993;260(5104):95–97. doi: 10.1126/science.8096653. [DOI] [PubMed] [Google Scholar]
- 32.Mancini JD, Atchison WD. The NR2B subunit in NMDA receptors is functionally important during cerebellar granule cell migration. Neurosci Lett. 2007;429(2–3):87–90. doi: 10.1016/j.neulet.2007.09.079. [DOI] [PubMed] [Google Scholar]
- 33.Balenci L, Saoudi Y, Grunwald D, Deloulme JC, Bouron A, Bernards A, Baudier J. IQGAP1 regulates adult neural progenitors in vivo and vascular endothelial growth factor-triggered neural progenitor migration in vitro. J Neurosci. 2007;27(17):4716–4724. doi: 10.1523/JNEUROSCI.0830-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wittko IM, Schanzer A, Kuzmichev A, Schneider FT, Shibuya M, Raab S, Plate KH. VEGFR-1 regulates adult olfactory bulb neurogenesis and migration of neural progenitors in the rostral migratory stream in vivo. J Neurosci. 2009;29(27):8704–8714. doi: 10.1523/JNEUROSCI.5527-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang H, Vutskits L, Pepper MS, Kiss JZ. VEGF is a chemoattractant for FGF-2-stimulated neural progenitors. J Cell Biol. 2003;163(6):1375–1384. doi: 10.1083/jcb.200308040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Learte AR, Forero MG, Hidalgo A. Gliatrophic and gliatropic roles of PVF/PVR signaling during axon guidance. Glia. 2008;56(2):164–176. doi: 10.1002/glia.20601. [DOI] [PubMed] [Google Scholar]
- 37.Hayakawa K, Seo JH, Pham LD, Miyamoto N, Som AT, Guo S, Kim KW, Lo EH, Arai K. Cerebral endothelial derived vascular endothelial growth factor promotes the migration but not the proliferation of oligodendrocyte precursor cells in vitro. Neurosci Lett. 2012;513(1):42–46. doi: 10.1016/j.neulet.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ryu JK, Cho T, Choi HB, Wang YT, McLarnon JG. Microglial VEGF receptor response is an integral chemotactic component in Alzheimer’s disease pathology. J Neurosci. 2009;29(1):3–13. doi: 10.1523/JNEUROSCI.2888-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pitzer MR, Sortwell CE, Daley BF, McGuire SO, Marchionini D, Fleming M, Collier TJ. Angiogenic and neurotrophic effects of vascular endothelial growth factor (VEGF165): studies of grafted and cultured embryonic ventral mesencephalic cells. Exp Neurol. 2003;182(2):435–445. doi: 10.1016/S0014-4886(03)00100-6. [DOI] [PubMed] [Google Scholar]
- 40.Rosenstein JM, Mani N, Khaibullina A, Krum JM. Neurotrophic effects of vascular endothelial growth factor on organotypic cortical explants and primary cortical neurons. J Neurosci. 2003;23(35):11036–11044. doi: 10.1523/JNEUROSCI.23-35-11036.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jin K, Mao XO, Greenberg DA. Vascular endothelial growth factor stimulates neurite outgrowth from cerebral cortical neurons via Rho kinase signaling. J Neurobiol. 2006;66(3):236–242. doi: 10.1002/neu.20215. [DOI] [PubMed] [Google Scholar]
- 42.Bocker-Meffert S, Rosenstiel P, Rohl C, Warneke N, Held-Feindt J, Sievers J, Lucius R. Erythropoietin and VEGF promote neural outgrowth from retinal explants in postnatal rats. Invest Ophthalmol Vis Sci. 2002;43(6):2021–2026. [PubMed] [Google Scholar]
- 43.Olbrich L, Foehring D, Happel P, Brand-Saberi B, Theiss C. Fast rearrangement of the neuronal growth cone’s actin cytoskeleton following VEGF stimulation. Histochem Cell Biol. 2012 doi: 10.1007/s00418-012-1036-y. [DOI] [PubMed] [Google Scholar]
- 44.Bellon A, Luchino J, Haigh K, Rougon G, Haigh J, Chauvet S, Mann F. VEGFR2 (KDR/Flk1) signaling mediates axon growth in response to semaphorin 3E in the developing brain. Neuron. 2010;66(2):205–219. doi: 10.1016/j.neuron.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 45.Nawabi H, Castellani V. Axonal commissures in the central nervous system: how to cross the midline? Cell Mol Life Sci. 2011;68(15):2539–2553. doi: 10.1007/s00018-011-0691-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ruiz de Almodovar C, Fabre PJ, Knevels E, Coulon C, Segura I, Haddick PC, Aerts L, Delattin N, Strasser G, Oh WJ, Lange C, Vinckier S, Haigh J, Fouquet C, Gu C, Alitalo K, Castellani V, Tessier-Lavigne M, Chedotal A, Charron F, Carmeliet P. VEGF mediates commissural axon chemoattraction through its receptor Flk1. Neuron. 2011;70(5):966–978. doi: 10.1016/j.neuron.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Erskine L, Reijntjes S, Pratt T, Denti L, Schwarz Q, Vieira JM, Alakakone B, Shewan D, Ruhrberg C. VEGF signaling through neuropilin 1 guides commissural axon crossing at the optic chiasm. Neuron. 2011;70(5):951–965. doi: 10.1016/j.neuron.2011.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Khaibullina AA, Rosenstein JM, Krum JM. Vascular endothelial growth factor promotes neurite maturation in primary CNS neuronal cultures. Brain Res Dev Brain Res. 2004;148(1):59–68. doi: 10.1016/j.devbrainres.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 49.Huang YF, Yang CH, Huang CC, Tai MH, Hsu KS. Pharmacological and genetic accumulation of hypoxia-inducible factor-1alpha enhances excitatory synaptic transmission in hippocampal neurons through the production of vascular endothelial growth factor. J Neurosci. 2010;30(17):6080–6093. doi: 10.1523/JNEUROSCI.5493-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim BW, Choi M, Kim YS, Park H, Lee HR, Yun CO, Kim EJ, Choi JS, Kim S, Rhim H, Kaang BK, Son H. Vascular endothelial growth factor (VEGF) signaling regulates hippocampal neurons by elevation of intracellular calcium and activation of calcium/calmodulin protein kinase II and mammalian target of rapamycin. Cell Signal. 2008;20(4):714–725. doi: 10.1016/j.cellsig.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 51.Huang YF, Yang CH, Huang CC, Hsu KS. Vascular endothelial growth factor-dependent spinogenesis underlies antidepressant-like effects of enriched environment. J Biol Chem. 2012;287(49):40938–40955. doi: 10.1074/jbc.M112.392076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Licht T, Eavri R, Goshen I, Shlomai Y, Mizrahi A, Keshet E. VEGF is required for dendritogenesis of newly born olfactory bulb interneurons. Development. 2010;137(2):261–271. doi: 10.1242/dev.039636. [DOI] [PubMed] [Google Scholar]
- 53.Licht T, Goshen I, Avital A, Kreisel T, Zubedat S, Eavri R, Segal M, Yirmiya R, Keshet E. Reversible modulations of neuronal plasticity by VEGF. Proc Natl Acad Sci U S A. 2011;108(12):5081–5086. doi: 10.1073/pnas.1007640108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mauceri D, Freitag HE, Oliveira AM, Bengtson CP, Bading H. Nuclear calcium-VEGFD signaling controls maintenance of dendrite arborization necessary for memory formation. Neuron. 2011;71(1):117–130. doi: 10.1016/j.neuron.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 55.Sondell M, Kanje M. Postnatal expression of VEGF and its receptor flk-1 in peripheral ganglia. Neuroreport. 2001;12(1):105–108. doi: 10.1097/00001756-200101220-00028. [DOI] [PubMed] [Google Scholar]
- 56.Sondell M, Lundborg G, Kanje M. Vascular endothelial growth factor has neurotrophic activity and stimulates axonal outgrowth, enhancing cell survival and Schwann cell proliferation in the peripheral nervous system. J Neurosci. 1999;19(14):5731–5740. doi: 10.1523/JNEUROSCI.19-14-05731.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sondell M, Sundler F, Kanje M. Vascular endothelial growth factor is a neurotrophic factor which stimulates axonal outgrowth through the flk-1 receptor. Eur J Neurosci. 2000;12(12):4243–4254. doi: 10.1046/j.0953-816X.2000.01326.x. [DOI] [PubMed] [Google Scholar]
- 58.Kutcher ME, Klagsbrun M, Mamluk R. VEGF is required for the maintenance of dorsal root ganglia blood vessels but not neurons during development. FASEB J. 2004;18(15):1952–1954. doi: 10.1096/fj.04-2320fje. [DOI] [PubMed] [Google Scholar]
- 59.Cheng L, Jia H, Lohr M, Bagherzadeh A, Holmes DI, Selwood D, Zachary I. Anti-chemorepulsive effects of vascular endothelial growth factor and placental growth factor-2 in dorsal root ganglion neurons are mediated via neuropilin-1 and cyclooxygenase-derived prostanoid production. J Biol Chem. 2004;279(29):30654–30661. doi: 10.1074/jbc.M402488200. [DOI] [PubMed] [Google Scholar]
- 60.Mukouyama YS, Shin D, Britsch S, Taniguchi M, Anderson DJ. Sensory nerves determine the pattern of arterial differentiation and blood vessel branching in the skin. Cell. 2002;109(6):693–705. doi: 10.1016/S0092-8674(02)00757-2. [DOI] [PubMed] [Google Scholar]
- 61.Mukouyama YS, Gerber HP, Ferrara N, Gu C, Anderson DJ. Peripheral nerve-derived VEGF promotes arterial differentiation via neuropilin 1-mediated positive feedback. Development. 2005;132(5):941–952. doi: 10.1242/dev.01675. [DOI] [PubMed] [Google Scholar]
- 62.Verheyen A, Peeraer E, Nuydens R, Dhondt J, Poesen K, Pintelon I, Daniels A, Timmermans JP, Meert T, Carmeliet P, Lambrechts D. Systemic anti-vascular endothelial growth factor therapies induce a painful sensory neuropathy. Brain. 2012;135(Pt 9):2629–2641. doi: 10.1093/brain/aws145. [DOI] [PubMed] [Google Scholar]
- 63.Dhondt J, Peeraer E, Verheyen A, Nuydens R, Buysschaert I, Poesen K, Van Geyte K, Beerens M, Shibuya M, Haigh JJ, Meert T, Carmeliet P, Lambrechts D. Neuronal FLT1 receptor and its selective ligand VEGF-B protect against retrograde degeneration of sensory neurons. FASEB J. 2011;25(5):1461–1473. doi: 10.1096/fj.10-170944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Orr HT. FTD and ALS: genetic ties that bind. Neuron. 2011;72(2):189–190. doi: 10.1016/j.neuron.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 65.Storkebaum E, Ruiz de Almodovar C, Meens M, Zacchigna S, Mazzone M, Vanhoutte G, Vinckier S, Mizskiewicz K, Poesen K, Lambrechts D, Janssen GM, Fazzi GE, Verstreken P, Haigh J, Schiffers PM, Rohrer H, Van der Linden A, De Mey JG, Carmeliet P. Impaired autonomic regulation of resistance arteries in mice with low vascular endothelial growth factor or upon vascular endothelial growth factor trap delivery. Circulation. 2010;122(3):273–281. doi: 10.1161/CIRCULATIONAHA.109.929364. [DOI] [PubMed] [Google Scholar]
- 66.Marko SB, Damon DH. VEGF promotes vascular sympathetic innervation. Am J Physiol Heart Circ Physiol. 2008;294(6):H2646–H2652. doi: 10.1152/ajpheart.00291.2008. [DOI] [PubMed] [Google Scholar]
- 67.Long JB, Jay SM, Segal SS, Madri JA. VEGF-A and Semaphorin3A: modulators of vascular sympathetic innervation. Dev Biol. 2009;334(1):119–132. doi: 10.1016/j.ydbio.2009.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Saygili E, Pekassa M, Rackauskas G, Hommes D, Noor-Ebad F, Gemein C, Zink MD, Schwinger RH, Weis J, Marx N, Schauerte P, Rana OR. Mechanical stretch of sympathetic neurons induces VEGF expression via a NGF and CNTF signaling pathway. Biochem Biophys Res Commun. 2011;410(1):62–67. doi: 10.1016/j.bbrc.2011.05.105. [DOI] [PubMed] [Google Scholar]
- 69.Calza L, Giardino L, Giuliani A, Aloe L, Levi-Montalcini R. Nerve growth factor control of neuronal expression of angiogenetic and vasoactive factors. Proc Natl Acad Sci U S A. 2001;98(7):4160–4165. doi: 10.1073/pnas.051626998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.DeJesus-Hernandez M, Mackenzie IR, Boeve BF, Boxer AL, Baker M, Rutherford NJ, Nicholson AM, Finch NA, Flynn H, Adamson J, Kouri N, Wojtas A, Sengdy P, Hsiung GY, Karydas A, Seeley WW, Josephs KA, Coppola G, Geschwind DH, Wszolek ZK, Feldman H, Knopman DS, Petersen RC, Miller BL, Dickson DW, Boylan KB, Graff-Radford NR, Rademakers R. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron. 2011;72(2):245–256. doi: 10.1016/j.neuron.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Renton AE, Majounie E, Waite A, Simon-Sanchez J, Rollinson S, Gibbs JR, Schymick JC, Laaksovirta H, van Swieten JC, Myllykangas L, Kalimo H, Paetau A, Abramzon Y, Remes AM, Kaganovich A, Scholz SW, Duckworth J, Ding J, Harmer DW, Hernandez DG, Johnson JO, Mok K, Ryten M, Trabzuni D, Guerreiro RJ, Orrell RW, Neal J, Murray A, Pearson J, Jansen IE, Sondervan D, Seelaar H, Blake D, Young K, Halliwell N, Callister JB, Toulson G, Richardson A, Gerhard A, Snowden J, Mann D, Neary D, Nalls MA, Peuralinna T, Jansson L, Isoviita VM, Kaivorinne AL, Holtta-Vuori M, Ikonen E, Sulkava R, Benatar M, Wuu J, Chio A, Restagno G, Borghero G, Sabatelli M, Heckerman D, Rogaeva E, Zinman L, Rothstein JD, Sendtner M, Drepper C, Eichler EE, Alkan C, Abdullaev Z, Pack SD, Dutra A, Pak E, Hardy J, Singleton A, Williams NM, Heutink P, Pickering-Brown S, Morris HR, Tienari PJ, Traynor BJ. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron. 2011;72(2):257–268. doi: 10.1016/j.neuron.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Oosthuyse B, Moons L, Storkebaum E, Beck H, Nuyens D, Brusselmans K, Van Dorpe J, Hellings P, Gorselink M, Heymans S, Theilmeier G, Dewerchin M, Laudenbach V, Vermylen P, Raat H, Acker T, Vleminckx V, Van Den Bosch L, Cashman N, Fujisawa H, Drost MR, Sciot R, Bruyninckx F, Hicklin DJ, Ince C, Gressens P, Lupu F, Plate KH, Robberecht W, Herbert JM, Collen D, Carmeliet P. Deletion of the hypoxia-response element in the vascular endothelial growth factor promoter causes motor neuron degeneration. Nat Genet. 2001;28(2):131–138. doi: 10.1038/88842. [DOI] [PubMed] [Google Scholar]
- 73.Wang Y, Mao XO, Xie L, Banwait S, Marti HH, Greenberg DA, Jin K. Vascular endothelial growth factor overexpression delays neurodegeneration and prolongs survival in amyotrophic lateral sclerosis mice. J Neurosci. 2007;27(2):304–307. doi: 10.1523/JNEUROSCI.4433-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Storkebaum E, Lambrechts D, Dewerchin M, Moreno-Murciano MP, Appelmans S, Oh H, Van Damme P, Rutten B, Man WY, De Mol M, Wyns S, Manka D, Vermeulen K, Van Den Bosch L, Mertens N, Schmitz C, Robberecht W, Conway EM, Collen D, Moons L, Carmeliet P. Treatment of motoneuron degeneration by intracerebroventricular delivery of VEGF in a rat model of ALS. Nat Neurosci. 2005;8(1):85–92. doi: 10.1038/nn1360. [DOI] [PubMed] [Google Scholar]
- 75.Azzouz M, Ralph GS, Storkebaum E, Walmsley LE, Mitrophanous KA, Kingsman SM, Carmeliet P, Mazarakis ND. VEGF delivery with retrogradely transported lentivector prolongs survival in a mouse ALS model. Nature. 2004;429(6990):413–417. doi: 10.1038/nature02544. [DOI] [PubMed] [Google Scholar]
- 76.Lambrechts D, Poesen K, Fernandez-Santiago R, Al-Chalabi A, Del Bo R, Van Vught PW, Khan S, Marklund SL, Brockington A, van Marion I, Anneser J, Shaw C, Ludolph AC, Leigh NP, Comi GP, Gasser T, Shaw PJ, Morrison KE, Andersen PM, Van den Berg LH, Thijs V, Siddique T, Robberecht W, Carmeliet P. Meta-analysis of vascular endothelial growth factor variations in amyotrophic lateral sclerosis: increased susceptibility in male carriers of the -2578AA genotype. J Med Genet. 2009;46(12):840–846. doi: 10.1136/jmg.2008.058222. [DOI] [PubMed] [Google Scholar]
- 77.Lu L, Zheng L, Viera L, Suswam E, Li Y, Li X, Estevez AG, King PH. Mutant Cu/Zn-superoxide dismutase associated with amyotrophic lateral sclerosis destabilizes vascular endothelial growth factor mRNA and downregulates its expression. J Neurosci. 2007;27(30):7929–7938. doi: 10.1523/JNEUROSCI.1877-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Garbuzova-Davis S, Saporta S, Haller E, Kolomey I, Bennett SP, Potter H, Sanberg PR. Evidence of compromised blood-spinal cord barrier in early and late symptomatic SOD1 mice modeling ALS. PLoS One. 2007;2(11):e1205. doi: 10.1371/journal.pone.0001205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Henkel JS, Beers DR, Wen S, Bowser R, Appel SH. Decreased mRNA expression of tight junction proteins in lumbar spinal cords of patients with ALS. Neurology. 2009;72(18):1614–1616. doi: 10.1212/WNL.0b013e3181a41228. [DOI] [PubMed] [Google Scholar]
- 80.Argaw AT, Asp L, Zhang J, Navrazhina K, Pham T, Mariani JN, Mahase S, Dutta DJ, Seto J, Kramer EG, Ferrara N, Sofroniew MV, John GR. Astrocyte-derived VEGF-A drives blood-brain barrier disruption in CNS inflammatory disease. J Clin Investig. 2012;122(7):2454–2468. doi: 10.1172/JCI60842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bruck W, Bitsch A, Kolenda H, Bruck Y, Stiefel M, Lassmann H. Inflammatory central nervous system demyelination: correlation of magnetic resonance imaging findings with lesion pathology. Ann Neurol. 1997;42(5):783–793. doi: 10.1002/ana.410420515. [DOI] [PubMed] [Google Scholar]
- 82.Argaw AT, Gurfein BT, Zhang Y, Zameer A, John GR. VEGF-mediated disruption of endothelial CLN-5 promotes blood-brain barrier breakdown. Proc Natl Acad Sci U S A. 2009;106(6):1977–1982. doi: 10.1073/pnas.0808698106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dobrogowska DH, Lossinsky AS, Tarnawski M, Vorbrodt AW. Increased blood-brain barrier permeability and endothelial abnormalities induced by vascular endothelial growth factor. J Neurocytol. 1998;27(3):163–173. doi: 10.1023/A:1006907608230. [DOI] [PubMed] [Google Scholar]
- 84.Proescholdt MA, Jacobson S, Tresser N, Oldfield EH, Merrill MJ. Vascular endothelial growth factor is expressed in multiple sclerosis plaques and can induce inflammatory lesions in experimental allergic encephalomyelitis rats. J Neuropathol Exp Neurol. 2002;61(10):914–925. doi: 10.1093/jnen/61.10.914. [DOI] [PubMed] [Google Scholar]
- 85.Sun A, Binay KR, Xiang F, Zhao J, Wang Y, Xu L, Ma H, Wang K, Zou Y, Huang W, Ge J. CTSS promoter -25G/A: not a risk factor for CHD in Chinese. Acta Cardiol. 2009;64(3):393–396. doi: 10.2143/AC.64.3.2038027. [DOI] [PubMed] [Google Scholar]
- 86.Storkebaum E, Quaegebeur A, Vikkula M, Carmeliet P. Cerebrovascular disorders: molecular insights and therapeutic opportunities. Nat Neurosci. 2011;14(11):1390–1397. doi: 10.1038/nn.2947. [DOI] [PubMed] [Google Scholar]
- 87.Patel NS, Mathura VS, Bachmeier C, Beaulieu-Abdelahad D, Laporte V, Weeks O, Mullan M, Paris D. Alzheimer’s beta-amyloid peptide blocks vascular endothelial growth factor mediated signaling via direct interaction with VEGFR-2. J Neurochem. 2010;112(1):66–76. doi: 10.1111/j.1471-4159.2009.06426.x. [DOI] [PubMed] [Google Scholar]
- 88.Wang P, Xie ZH, Guo YJ, Zhao CP, Jiang H, Song Y, Zhu ZY, Lai C, Xu SL, Bi JZ. VEGF-induced angiogenesis ameliorates the memory impairment in APP transgenic mouse model of Alzheimer’s disease. Biochem Biophys Res Commun. 2011;411(3):620–626. doi: 10.1016/j.bbrc.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 89.Faucheux BA, Bonnet AM, Agid Y, Hirsch EC. Blood vessels change in the mesencephalon of patients with Parkinson’s disease. Lancet. 1999;353(9157):981–982. doi: 10.1016/S0140-6736(99)00641-8. [DOI] [PubMed] [Google Scholar]
- 90.Mihci E, Ozkaynak SS, Sallakci N, Kizilay F, Yavuzer U. VEGF polymorphisms and serum VEGF levels in Parkinson’s disease. Neurosci Lett. 2011;494(1):1–5. doi: 10.1016/j.neulet.2011.02.027. [DOI] [PubMed] [Google Scholar]
- 91.Yasuhara T, Shingo T, Muraoka K, Kameda M, Agari T, Wen Ji Y, Hayase H, Hamada H, Borlongan CV, Date I. Neurorescue effects of VEGF on a rat model of Parkinson’s disease. Brain Res. 2005;1053(1-2):10–18. doi: 10.1016/j.brainres.2005.05.027. [DOI] [PubMed] [Google Scholar]
- 92.Yasuhara T, Shingo T, Kobayashi K, Takeuchi A, Yano A, Muraoka K, Matsui T, Miyoshi Y, Hamada H, Date I. Neuroprotective effects of vascular endothelial growth factor (VEGF) upon dopaminergic neurons in a rat model of Parkinson’s disease. Eur J Neurosci. 2004;19(6):1494–1504. doi: 10.1111/j.1460-9568.2004.03254.x. [DOI] [PubMed] [Google Scholar]
- 93.Tian YY, Tang CJ, Wang JN, Feng Y, Chen XW, Wang L, Qiao X, Sun SG. Favorable effects of VEGF gene transfer on a rat model of Parkinson disease using adeno-associated viral vectors. Neurosci Lett. 2007;421(3):239–244. doi: 10.1016/j.neulet.2007.05.033. [DOI] [PubMed] [Google Scholar]
- 94.Falk T, Zhang S, Sherman SJ. Vascular endothelial growth factor B (VEGF-B) is up-regulated and exogenous VEGF-B is neuroprotective in a culture model of Parkinson’s disease. Mol Neurodegener. 2009;4:49. doi: 10.1186/1750-1326-4-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Falk T, Yue X, Zhang S, McCourt AD, Yee BJ, Gonzalez RT, Sherman SJ. Vascular endothelial growth factor-B is neuroprotective in an in vivo rat model of Parkinson’s disease. Neurosci Lett. 2011;496(1):43–47. doi: 10.1016/j.neulet.2011.03.088. [DOI] [PubMed] [Google Scholar]
- 96.Orr HT. Cell biology of spinocerebellar ataxia. J Cell Biol. 2012;197(2):167–177. doi: 10.1083/jcb.201105092. [DOI] [PMC free article] [PubMed] [Google Scholar]