Abstract
Mediator is a large multisubunit complex that plays a central role in the regulation of RNA Pol II transcribed genes. Conserved in overall structure and function among eukaryotes, Mediator comprises 25–30 protein subunits that reside in four distinct modules, termed head, middle, tail, and CDK8/kinase. Different subunits of Mediator contact other transcriptional regulators including activators, co-activators, general transcription factors, subunits of RNA Pol II, and specifically modified histones, leading to the regulated expression of target genes. This review is focused on the interactions of specific Mediator subunits with diverse transcription regulators and how those interactions contribute to Mediator function in transcriptional activation.
Keywords: Transcription, Mediator, Co-activator, SAGA, Swi/Snf
Introduction
The transcriptional control of genes transcribed by RNA polymerase II (Pol II) depends on diverse transcriptional regulators that include transcriptional activators, a set of general transcription factors (GTFs), and transcription cofactors [1]. The initiation of transcription generally begins when gene-specific activators bind to their cognate sites, or when pre-bound activators are modified, leading to the recruitment of the general transcription machinery comprising a host of GTFs and RNA Pol II to form the pre-initiation complex (PIC). To accomplish this task, activators interact with a number of cofactors to communicate the activation signal to RNA Pol II and the GTFs. GTFs, comprising TFIIA, B, D, E, F, and H, are defined by their requirement for accurate and efficient transcription from all (or nearly all) Pol II transcribed genes [2, 3]. The transcription cofactors (coactivators and corepressors), which by definition do not directly bind to specific DNA sequences, act at specific subsets of genes and can be broadly categorized into two classes. The first class consists of complexes or proteins that modify the histones on promoter DNA or remodel the chromatin in an ATP dependent manner—for example, the Swi/Snf complex in yeast and CBP in mammalian cells [4, 5]. The other class includes general cofactors such as the Mediator complex that function principally to recruit or activate GTFs and other key components needed for transcriptional activation or repression. Some cofactors belong to both categories: the SAGA complex, for example, activates genes in yeast both by recruiting TBP via the Spt3/Spt8 sub-module and by acetylating histones, and the requirement for these two functions varies at different genes [6, 7].
Mediator plays a central role in the eukaryotic transcription program and the mechanisms of its action known so far seem to be conserved. Mediator is a large multiprotein complex comprising 25 subunits in yeast and 30 or more in higher organisms [8]. Considerable insight into the subunit composition of the Mediator complex has come from genetic and biochemical studies in yeast [9, 10]. Based on structural studies on the yeast Mediator complex, the subunits of Mediator reside in four distinct modules termed the head, middle, tail, and kinase. The head, middle, and tail modules form the core Mediator complex and, under normal conditions, are always found in association, while the kinase module associates less stably. Although many of the subunits of Mediator exhibit relatively low sequence conservation, structural studies indicate a conserved structural organization for Mediator [11–13]. The approximately 1.4 mDa Mediator complex is large enough to act as a scaffold in the PIC in which different Mediator subunits make contact with various transcription factors including activators, coactivators, GTFs, and subunits of Pol II. Several Mediator subunits have been shown to interact with various activators both in yeast and metazoans [14–18], whereas specific subunits of the head module interact with Pol II subunits and other GTFs [19–23]. These observations led to the classical model of Mediator function in which Mediator acts as an adaptor to convey transcription signals from activators to the general transcription machinery to help initiate transcription by Pol II [24]. Several recent reports, however, suggest Mediator function beyond initiation, for example in transcription elongation [25–27], termination [28], and mRNA processing [29], as well as in chromatin remodeling at the promoter [30–33]. These studies establish Mediator as a central regulator of Pol II transcription. Here, we discuss interactions of Mediator contributing to transcriptional activation, as well as its involvement in post-initiation events.
Mediator function upstream of PIC recruitment
Mediator interactions with transcriptional activators
Mediator was originally discovered on the basis of its ability to support enhancement of transcription by transcriptional activators [34, 35]. The interaction of Mediator with transcriptional activators results in the recruitment of the general transcription machinery to promoters, leading to transcriptional activation [24]. This function of Mediator is highly conserved among eukaryotes from yeast to human. Because Mediator functions downstream of activator binding, one might reasonably expect activator binding to occur independently of Mediator, and this has been observed for the activators Gal4 and Met4 in yeast [36, 37]. In contrast, mutations in the SAGA subunit Tra1 that specifically prevent SAGA recruitment by Gal4 also affect Gal4 binding, indicating a cooperative interaction between Gal4 and the SAGA complex [38]; thus, it is conceivable that cooperative binding between activators and Mediator occurs at some promoters. A recent study reports Mediator functioning upstream of Gal4, based on a role for Mediator in facilitating proteolytic degradation of the Gal4-binding repressor, Gal80; this upstream genetic influence, however, is independent of the binding of Gal4 to its upstream activating sequence (UAS) [39].
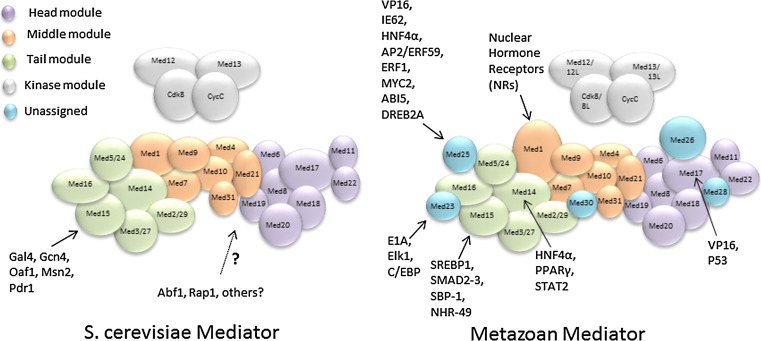
The mechanism by which activators contact Mediator varies (Fig. 1). The tail module subunits of the yeast Mediator complex were recognized early on as being important for transcriptional activation both in vitro and in vivo [24, 40, 41]. A discrete sub-module comprising Med2, Med3, and Med15 (originally named Gal11 in yeast; see Table 1) is a target of multiple activators in yeast. This sub-module binds to Gcn4 and the VP16 activation domain in vitro and can be recruited to active promoters even when association of the middle and head modules is reduced or eliminated in med16∆ or med17 ts yeast [40, 42]. Furthermore, Mediator recruitment to genes activated by Gcn4, Cha4, or Met4 is reduced or eliminated by single or (for Cha4) double mutants in subunits from this triad [37, 42, 43], and the effects on genome-wide expression in yeast deleted for any of these subunits are strongly similar [43, 44]. Taken together, these findings indicate that the tail module, and particularly the Med2/Med3/Med15 triad, is an important target by which activators recruit Mediator in yeast. Although these subunits display particularly low sequence conservation, the Med15 subunit from human interacts physically and functionally with the activators Smad2/3/4 and sterol regulatory element binding protein-1a (SREBP-1a) [45, 46]. Similarly, in Caenorhabditis elegans, the Med15 ortholog MDT-15 interacts with SBP-1, the C. elegans ortholog of the mammalian SREBPs, to regulate fatty acid and cholesterol metabolism [45]. Others have reported that MDT-15 integrates expression of certain metabolic genes in both a mNHR-49 (a nuclear hormone receptor) -dependent and -independent manner [47]. Evidence has been presented that Med27 and Med29 are metazoan counterparts of Med3 and Med2, but little is known regarding any role for these subunits as activator targets [8, 18, 48]. Furthermore, the dependence on individual Mediator subunits of Mediator recruitment to target genes has been examined for only a few cases in metazoans. Much remains to be learned in this area.
Fig. 1.
Interactions of transcriptional activators with Mediator complex. Organization of the yeast and metazoan Mediator complexes is adapted from reference [8]. Abf1 and Rap1 in yeast may interact directly or indirectly with middle or head module subunits, as indicated by the dotted line. Interactions of activators with subunits from metazoan Mediator are mostly derived from studies on mammalian Mediator, but also include some from C. elegans and A. thaliana. See text for more details
Table 1.
Mediator subunit nomenclature
| Module | Original name in S. cerevisiae | Universal name |
|---|---|---|
| Head | Srb4 | Med17 |
| Srb5 | Med18 | |
| Srb2 | Med20 | |
| Srb6 | Med22 | |
| Head/middle | Rox3 | Med19 |
| Middle | Cse2 | Med9 |
| Nut2 | Med10 | |
| Srb7 | Med21 | |
| Soh1 | Med31 | |
| Middle/tail | Rgr1 | Med14 |
| Tail | Pgd1/Hrs1 | Med3 |
| Nut1 | Med5 | |
| Gal11 | Med15 | |
| Sin4 | Med16 | |
| Cyclin/Cdk | Srb8 | Med12 |
| Srb9 | Med13 | |
| Srb10 | CDK8 | |
| Srb11 | CycC |
Mediator subunits having alternative names are indicated according to their original name(s) in S. cerevisiae and the revised universal nomenclature [163], and their location in the conserved domain architecture of Mediator is indicated. For the latter, we follow recent assignments of Lariviere et al. [65]
Close examination of the mechanism by which Med15 functions as an activator target has been illuminating. The extreme N-terminal region of metazoan Med15 contains a region similar to the activator binding ‘KIX’ domain of mammalian coactivator CREB binding protein/p300 [45]. Mammalian Med15 interacts through this KIX domain with SREBP-1a to activate its target genes. The KIX domain is also conserved in yeast and interacts with the activators Pdr1 and Oaf1; furthermore, transcriptional activation of Pdr1 target genes is compromised by the deletion of KIX domain. These findings indicate that the Med15 KIX domain is a conserved activator binding target [49, 50]. However, although transcriptional activation by Gcn4 in yeast depends on Med15, the KIX domain appears to be dispensable for this activation, although conflicting results have been reported [51, 52]. Instead, interaction of Med15 with Gcn4 depends on multiple conserved Gcn4 binding domains outside the KIX domain that bind Gcn4 with micromolar affinity [51, 52]; therefore, Gcn4 interacts with Med15 not by a single high affinity interaction but rather by multiple low affinity interactions [51]. These low affinity interactions are hydrophobic in nature, so that binding is best considered as occurring in a “fuzzy” complex that can adopt multiple conformations, as opposed to the crystallizable configuration characteristic of more classical high affinity interactions [14]. Somewhat similarly, NMR studies have demonstrated interactions having approximately micromolar affinity between the VP16 activation domain and an extended surface of the metazoan-specific Mediator subunit Med25 [15, 53]. Whether other activator–Mediator interactions may reflect similar “fuzzy” interactions is at present unknown. Nonetheless, from these studies, it is clear that different activators can employ one or more different activator binding regions in Mediator subunits to achieve specificity.
A common feature of activators known to interact with the Med2/Med3/Med15 tail module triad in yeast is that they are condition-specific inducers of activation. Mediator is also associated with promoters of constitutively active genes and is necessary for their activity, but it appears that the tail module triad is in most cases not required for Mediator recruitment to these genes [43, 54]. That Mediator is present at the promoters of such genes is shown by its decreased association in med17 ts yeast; furthermore, decreased association is also seen in abf1 ts and rap1 ts yeast at Abf1-dependent and Rap1-dependent constitutively active promoters [43, 54]. In general, yeast genes that depend on the SAGA complex and have consensus TATA elements in their promoters (about 15 % of all yeast genes) are enriched for dependence on the tail module triad, while the approximately 85 % of genes that lack consensus TATA elements and depend on TFIID are mostly not dependent on the tail module triad for activation [43]. Apparently, Mediator is recruited to these genes via middle or head module subunits. Such recruitment could occur via direct interactions with activators, and this possibility is supported by in vitro experiments indicating interactions between activators and subunits from the middle and head modules [42, 55]. Alternatively, recruitment could occur indirectly, for example via interactions with GTFs that are recruited by activator proteins (Fig. 1). Further studies are needed to resolve this issue.
In metazoans, it is clear that many activators recruit Mediator via subunits outside the Med2/Med3/Med15 triad. Med1 interacts with a large number of nuclear hormone receptors (NRs) and consequently has been heavily investigated in its role as a coactivator; in fact, mammalian Mediator was first identified from studies on the coactivator requirements of NRs [56, 57]. NRs are representative of transcriptional activators that function by binding to cognate DNA binding sites located upstream of the core promoter elements and nucleating the assembly of the general transcription machinery [58]. The physical and functional interaction of Med1 with diverse NRs such as vitamin D receptor, glucocorticoid receptor, estrogen receptor (ER), hepatic nuclear factor 4 (HNF4), liver X receptor, pregnane X receptor, and peroxisome proliferator-activated receptors often occurs through nuclear receptor recognition motifs (LxxLL) that bind to the conserved AF2 strong activation domain present in the NRs, and this interaction can lead to the recruitment of the entire Mediator complex to target promoters [59]. In concordance with the importance of this interaction, yeast Med1 lacks any LxxLL motif, and the AF2 domain on its own is inactive in yeast [60, 61]. However, in some cases, Mediator can be recruited by NRs and activate target genes in vivo independently of LxxLL motifs and, indeed, independently of Med1 [62, 63]. Further work indicated that the Med14 subunit tethers Mediator to the N-terminal domain of peroxisome proliferator-activated receptor-γ and is required for full transcriptional activity and adipogenesis [64]. Although Med14 is not a member of the Med2/Med3/Med15 triad, it is structurally close to this sub-module in yeast and has been variously assigned as a tail module subunit or as bridging the tail and middle module [10, 12, 41, 65]. Med14, along with Med1, has also been reported to interact with the glucocorticoid receptor and HNF4α [66, 67]. Med14 has also been shown to be involved in interferon (IFN)-induced signal transduction and transcription activation by the ISGF3 complex which comprises three proteins, STAT1, STAT2, and IRF9, interacting directly with STAT2 to activate ISGF3-mediated transcriptional activity [68].
Other Mediator subunits have also been identified as targets of transcriptional activators in metazoans [18, 48]. Med23, apparently absent from the yeast clade, was first identified based on its interaction with viral activator protein E1A [69], and also interacts with the Ets-like transcription factor Elk1 and with CCAAT/enhancer binding protein C/EBP. Another report shows that Med25, which is also absent from yeast, is required for the association of HNF4α with Mediator, its several cofactors, and RNA Pol II, thus regulating xenobiotic and lipid metabolism in human liver [70]. The interaction of Med25 has also been reported with the varicella-zoster virus major transactivator, IE62 [71], and this interaction appears to be fundamental for the transactivation by the IE62 activation domain [72].
Evidence for Mediator subunit interactions with activators has also been reported in plants. The recent purification of the Arabidopsis thaliana Mediator complex resulted in identification of 21 conserved and 6 plant-specific subunits [73]. Among the few genetically characterized Arabidopsis Mediator subunits [74–76] is Med25, which was found to play roles in regulating flowering [77], organ size [78], stress response [79], and, notably, jasmonate (JA)-signaling [80]. Med25 is a positive regulator of JA responsive gene expression in Arabidopsis where it was recently shown to interact with several key transcription regulators of the JA-signaling pathway, including the AP2/ERF transcription factors, octadecanoid-responsive Arabidopsis AP2/ERF59 (ORA59), and ethylene response factor 1 (ERF1), as well as the master regulator MYC2 [81]. Med25 was also reported to interact with ABI5 in Arabidopsis to regulate abscisic acid-induced gene expression [82]. A linear 130 aa long sequence in the Med25 subunit is a common target for the drought response element binding protein 2A, zinc finger homeodomain1, and myb-like transcription factors which are involved in different stress response pathways [79].
In sum, in eukaryotes from yeast to plants to mammals, diverse transcriptional activators interact with different Mediator subunits to elicit activation at a multitude of promoters. In yeast, the Mediator subunits that are direct targets of activators of inducible genes appear to most commonly belong to the tail module. However, Mediator is also present at constitutively active genes, at least in yeast, where its recruitment generally does not depend on the Med2/Med3/Med15 triad. The mechanism(s) by which Mediator is recruited in such instances remains to be examined. Furthermore, mutations in middle module subunits Med7 and Med31 cause defective activation of a subset of genes that is enriched for activation by specific transcription factors [83]; possibly, Med7/Med31 are direct targets of at least some of these transcription factors. In metazoans, mechanisms by which Mediator is recruited have been investigated for only a relatively small number of gene targets and activators (particularly NRs), but it is clear that at least several subunits in both tail and middle modules are activator targets.
Activators elicit conformational changes in Mediator
An early structural study from the Tjian laboratory [84] yielded two major findings. First, two distinct forms of mammalian Mediator were found, one including the kinase (or cyclin-CDK) module (comprising CDK8, cyclin C, Med12, and Med13), the other including Med26, with only the latter form being transcriptionally active in the in vitro assays employed. Second, distinct structures were observed depending on whether the complex was purified by affinity purification using a FLAG-tagged Mediator subunit, the VP16 activation domain, or the SREBP-1a activator. Furthermore, Mediator isolated in the absence of activator interactions could be converted to the activator-bound conformations by exposure to VP16 or SREBP. VP16 and SREBP-1a were shown to contact different regions of the Mediator surface, consistent with studies showing that VP16 targets Med17 in the head module and Med25, while SREBP-1a binds to Med15 [18, 84]. TR and vitamin D receptor, which interact with a different Mediator subunit than either VP16 or SREBP (Med1), gave rise to yet another distinct conformer of Mediator [85]. These findings have been further extended by a recent study showing that two domains of p53, the C terminus and the activation domain, interact with different Mediator subunits and differentially affect Mediator structure, with the conformation elicited by the activation domain being uniquely capable of activating stalled Pol II to a productively elongating state [86].
The discovery of distinct Mediator conformations induced by different activators immediately led to the idea that this could provide a mechanism by which promoter-specific functions, or dependencies on different co-activator complexes, could be conferred by specific activators [84, 87]. This hypothesis was supported by experiments in which proteins associated with the Cdk8-containing large Mediator complex, VP16-bound Mediator, and SREBP-1a bound Mediator were identified by mass spectrometry using the MudPIT approach [88]. These experiments resulted in identification of numerous proteins associated with SREBP–Mediator that were not found in VP16–Mediator or in CDK8–Mediator, as well as a few that were found associated with VP16–Mediator but not CDK8–Mediator. The proteins that were specific to SREBP–Mediator included several components of the SAGA complex and cohesin subunits, while three proteins involved in exchange of the histone variant H2A.Z were associated with both activator conformations but not with CDK8–Mediator. Although it is possible that some of these associations occur via cooperative interactions with Mediator and the specific activator(s), these findings suggest that downstream interactions of proteins with Mediator could be specified by conformations caused by distinct activator proteins. Notably, reports of distinct Mediator conformations have thus far been confined to mammalian Mediator; whether this phenomenon also pertains to Mediator in other metazoans or in yeast remains to be determined. In this regard, it is interesting to note that the yeast activators, Gal4, Gcn4, and Met4, show strong dependence on the Med2/Med3/Med15 triad, but show differential dependence on Med9, Med6, and Med18 from the middle and head modules [37, 89–91]. Perhaps these activators induce conformational differences in Mediator that cause distinct functional surfaces involving these subunits to become manifest. Consistent with the notion of yeast Mediator exhibiting activator-dependent conformational flexibility, recent structural analyses indicate flexibility within the head modules from Saccharomyces cerevisiae and Schizosaccharomyces pombe [11, 92].
Mediator interactions with chromatin
In addition to being recruited by activators, Mediator association with promoter sequences may in some cases be influenced by direct interactions with the histones of the associated chromatin. A direct interaction of Mediator with reconstituted mononucleosomes was reported in an early study [93], and specific Mediator subunit interactions with histone tails have been identified using a UV-crosslinking approach [32].Another recent study showed that purified Mediator forms stable complexes with the tails of histones H3 and H4, and the interaction of Mediator with histone H4 is specifically inhibited by H4K16 acetylation [33]. Genome-wide determination of Mediator localization revealed anti-correlation with H4K16ac at promoters, and Mediator association was decreased in yeast harboring the H4K8, 16Q mutation that mimicked the acetylated state. The extent to which Mediator interactions with chromatin influence transcriptional activation in yeast, and whether such interactions are important for Mediator function in metazoan cells, is currently unknown.
Mediator interactions with co-activators
The Mediator complex is large enough to provide a surface for interactions with multiple transcription regulators. In addition to activator interactions discussed above, Mediator also interacts with various cofactors (coactivators and corepressors) (Fig. 2). These cofactors can be broadly categorized into two classes—chromatin-modifying factors and general cofactors. Chromatin-modifying cofactors include a variety of histone-modifying and ATP-dependent nucleosome remodeling enzymes [94], and the general cofactors are those that, like TFIID and the Mediator complex itself, directly interact with basal TFs and RNA Pol II to stimulate and regulate the assembly of the transcription initiation complex [87, 95].
Fig. 2.
Interactions of Mediator with other co-activator complexes. a In Saccharomyces cerevisiae, activators make direct contacts to recruit both SAGA and Mediator, which may be independent or interdependent in associating with a particular promoter depending on both the activator and the promoter (dotted double arrow). Swi/Snf and Pol II recruitment generally depends on Mediator, with some possible exceptions. b In mammalian cells, Mediator participates in recruitment of a wide range of co-activators; see text for details. For clarity, interactions not involving Mediator are omitted
Among chromatin-remodeling complexes, Mediator interacts with both SAGA and Swi/Snf complexes in yeast. Activator-dependent recruitment of SAGA, Swi/Snf and Mediator has been investigated by ChIP analysis in several studies, with results varying for different activators and for different promoters, even when under control of the same activator (Fig. 2a). The Gcn4-dependent recruitment of Swi/Snf to the ARG1 and SNZ1 promoters strongly depends on SAGA and Mediator tail subunits Med15, Med2, and head module subunit Med19 [96]. At these same targets, the recruitment of Mediator and SAGA is independent of Swi/Snf [97], suggesting that Swi/Snf binds downstream of SAGA and Mediator. Recruitment of Swi/Snf to the GAL1 UAS also showed a requirement for Mediator [98]. This study reported that artificial recruitment of Mediator, by fusion of the Med15/Gal11 subunit to the Gal4 DNA binding domain, is sufficient to tether Swi/Snf to the GAL1 UAS, consistent with earlier results showing that activation by artificial recruitment of Mediator strongly depended on Swi/Snf at some promoters [98, 99]. Mediator was also found to be required for Swi/Snf-dependent remodeling of chromatin at the active RNR3 promoter, again consistent with Swi/Snf function downstream of Mediator [100]. Swi/Snf is also recruited to the CHA1 promoter upon induction [101]; surprisingly, this recruitment was observed both in wild-type and med17 ts yeast [102]. Since recruitment of middle and head domain subunits of Mediator was abrogated in med17 ts yeast at the restrictive temperature, but tail module association was still observed, this result indicates that Swi/Snf is either recruited to the CHA1 promoter by a mechanism independent of Mediator or through interactions with tail module subunits.
Recruitment of SAGA and Mediator by Gal4 was found to be interdependent: SAGA association with active promoters was reduced by Mediator mutations and vice versa [37, 103, 104] (although one study reported Mediator recruitment to the GAL1-10 promoter to occur independently of SAGA [105]). Interdependent recruitment of SAGA and Mediator by Gcn4 was observed at the ARG4 and SNZ1 promoters, whereas recruitment of Mediator by the same activator showed little dependence on SAGA at the ARG1 locus [106]. In contrast to these results, the recruitment of Mediator and SAGA by the activator Met4 occurs independently [37]. Overall, these results indicate that complex interactions of specific activators with Mediator tail subunits, activators with Swi/Snf or SAGA subunits, and Mediator subunits with Swi/Snf and SAGA subunits provide transcriptional specificity at promoters.
Several studies in metazoans also report physical and functional interplay between Mediator and other coactivator complexes (Fig. 2b). Physical interactions between the human HAT complex STAGA (SPT3-TAF9-GCN5-acetylase) and a “core” form of the Mediator complex have been reported during transcriptional activation by the Myc oncoprotein in human cells. In this example, the STAF65γ component of STAGA is required for SPT3/STAGA interaction with core Mediator and for recruitment of SPT3, TAF9, and core Mediator components by Myc, resulting in transcriptional activation and cell proliferation [107]. In vitro studies have shown that Mediator acts synergistically with coactivator p300 in transcriptional activation by HNF4 [66]. Another study suggests functional interplay of Mediator with p300/CBP-SRC in the enhancement of ERα dependent transcription with chromatin templates [108]. An interaction of Mediator with p300 and TFIID has been reported in vitro in which Mediator was found to regulate Gal4-VP16 directed assembly of the PIC onto immobilized chromatin and naked DNA templates by binding to p300 and TFIID. In this study, an acetyl coA-dependent catalytic switch caused p300 to acetylate chromatin and then dissociate, allowing subsequent TFIID binding and active transcription [109]. Similarly, the formation of a stable subcomplex between Mediator and the ATAC histone acetyl transferase complex has been shown to regulate a subset of genes in mouse embryonic stem cells (ESCs) [110].
The involvement of Mediator in the recruitment of other cofactors and vice versa at specific genes suggests that Mediator aids in transcriptional activation, not only by interacting with activators but also by making contacts with coactivators involved in the remodeling of nucleosomes at the promoter. In support of this notion, a recent study demonstrated a direct interaction between murine Mediator and the chromodomain containing chromatin remodeling protein, Chd1. This interaction was disrupted by knockdown of Med1, which does not disrupt Mediator complex integrity, but not by knockdown of Med23, demonstrating specificity. Chd1 binds to nucleosomes trimethylated at H3K4 (H3K4me3) near the beginning of active genes, and this specificity was suggested to be due to its affinity for H3K4me3 together with its interaction with Mediator at the PIC at its target genes [111].
Finally, a novel interaction between Mediator and cohesin was recently described in ESCs [31]. Cohesin, whose prinicipal role has been understood as mediating sister chromatid cohesion, was shown to interact with Mediator and to be present with Mediator at thousands of promoter and enhancer sites in ESCs. Examination of a small number of specifically bound sites by chromosome conformation capture indicated that this association promoted the formation of enhancer–promoter loops. Thus, cohesin may be a novel kind of coactivator that contributes to the three-dimensional organization of active genes via its interactions with Mediator.
Mediator interactions with the general transcription machinery
Is Mediator a general transcription factor?
General transcription factors are defined operationally as those factors that are required to support in vitro transcription from a minimal promoter using only defined components. Mediator is not absolutely required for such “basal” transcription, as it was in fact first discovered based on its ability to increase basal transcription in the presence of activators [35], and therefore fails to satisfy this definition of a GTF as most strictly applied. However, a tacitly accepted corollary of the definition of GTFs is that they should be generally required for (almost) all mRNA transcription in vivo. In yeast, inactivation of temperature-sensitive mutants of Mediator results in loss of nearly all mRNA transcription with kinetics similar to those seen in a Pol II (rpb1-1) ts mutant, and this is accompanied by decreased Mediator occupancy of both induced and constitutively active genes [54, 112, 113], thus satisfying this criterion. Furthermore, although basal transcription can be observed without Mediator, it is substantially enhanced by addition of Mediator, but not by a Mediator ts mutant when assayed at the restrictive temperature [114]. This enhancement of basal transcription occurs at the stage of initiation and does not appear to reflect reversal of specific inhibitors that could be present in the in vitro reaction. Experiments using components derived from mammalian cells for in vitro transcription have also suggested a requirement for Mediator for basal transcription, as depletion of Mediator from crude extracts resulted in loss of transcription [115, 116]. Based on these findings, it has been argued that Mediator can be categorized as a GTF [17, 114].
Mediator structure and function are, not surprisingly, conserved between yeast and metazoans; however, whether Mediator is as universally required for mRNA transcription in metazoan cells as it is in yeast is not known. In fact, there are reports of Mediator-independent transcriptional activation occurring in mammalian systems [117, 118]. However, this may not be sufficient grounds to exclude Mediator as a GTF, as, for example, TBP is sometimes substituted for by variants [119] and is still considered as a GTF. Indeed, mammalian Mediator complexes can exist in multiple forms [87, 120–122]. It may be most productive to set aside concerns about Mediator’s status as a GTF and instead recognize it as occupying a unique position as a nearly universally required coactivator that contributes to transcriptional activation through a variety of mechanisms.
Mediator–RNA Pol II interactions
Early work showed that the yeast Mediator complex associates closely with RNA Pol II; this assemblage was termed the Pol II holoenzyme [123–125]. Direct Mediator–Pol II interaction was confirmed by copurification and co-immunoprecipitation experiments [123–125] and by in vivo formaldehyde crosslinking [126]. Electron microscopy (EM) studies suggest that several subunits of Pol II, including Rpb1, Rpb2, Rpb3, Rpb6, and Rpb11, contact the middle or head module of Mediator [127]. In S. pombe, the Med8 subunit of Mediator has been shown to interact with Rpb4 [128]. Structural, functional, and biochemical analyses have recently shown interactions of the head module with TBP and RNA Pol II subunits, Rpb4 and Rpb7 [21, 129]. Another recent study identified a direct contact between Rpb3 and Med17 by using an in vivo photo crosslinking approach complemented by genetic analysis, and showed that this interaction is essential for genome-wide Pol II recruitment in vivo [23]. Consistent with these findings, Mediator loss-of-function mutants have been found to compromise Pol II association with promoters of both induced and constitutively active genes in numerous studies [54, 70, 89, 130–134].
Mediator interactions with general transcription factors
In addition to causing reduced Pol II association with active promoters, Mediator mutations also result in decreased association of GTFs [19, 102, 131–135]. GTFs were found associated with Mediator in the Pol II “holoenzyme” in some early preparations, suggesting interactions between Mediator and the GTFs [124, 136]. Although later affinity purifications of Mediator showed these interactions to be less stable than those between Mediator and Pol II, and, among Mediator subunits themselves, numerous other reports showed genetic and physical interactions between Mediator and GTFs, particularly TBP, TFIIE, and TFIIH [20, 137–140]. These results suggest that Mediator, in addition to recruiting Pol II by direct interactions, also recruits or stabilizes promoter binding of GTFs through direct contacts.
In vitro studies point to Mediator–GTF interactions being important in Mediator function. As mentioned earlier, several studies have shown stimulation of basal transcription (in the absence of activators) by Mediator in vitro. In one such study, Mediator was found to be required for recruitment of TFIIB, with both Mediator and TFIIB being required for Pol II recruitment [141]. High levels of TFIIB in this in vitro system overcame the requirement for Mediator. This finding suggests that whether Mediator is required for TFIIB recruitment to activated genes in vivo could depend on whether other interactions (for instance, direct interactions with activators) increased local concentrations of TFIIB, thus providing a mechanism for gene-specific requirements for Mediator for GTF recruitment.
In an effort to identify how Mediator functions in the recruitment of GTFs to the PIC, Esnault and colleagues identified a direct interaction between yeast Mediator subunit Med11 and the Rad3 subunit of the GTF, TFIIH [20]. Specific mutants of Med11 that impaired interactions in vitro also affected recruitment of GTFs in vivo. Notable findings were that Mediator is important for recruitment of TFIIH and TFIIE; that differences were seen in effects on recruitment of TFIIK (Kin28) and the core TFIIH submodule (Rad3 and Ssl3) of TFIIH in specific mutants, indicating independent recruitment or stabilization; and that impaired recruitment of Pol II was observed with no effect on TFIIH recruitment in a specific med11 mutant. These findings, together with the well-documented ability of Mediator to dramatically enhance the phosphorylation of CTD by TFIIH [142–144], indicate a role for Mediator in influencing events in transcriptional activation occurring subsequent to Pol II recruitment.
Another means by which Mediator may impact GTF function in mammalian cells is by overcoming the repressive effect of Gdown1 on Pol II in the Pol II(G) complex [145, 146]. Gdown1 competes with TFIIF for binding to Pol II in vitro, thus inhibiting incorporation of TFIIF into a defined PIC that contains Pol II(G) rather than Pol II [145]. Mediator is able to recruit Pol II(G) in an activator-dependent manner in vitro; whether it allows Pol II(G) to function independently of TFIIF or Gdown1 is displaced to allow TFIIF incorporation is unclear. These two reports differ in some important aspects [147], but both agree that Gdown1 represses transcription by inhibiting TFIIF function and that Mediator recruitment by activators overcomes this effect. Interestingly, structural studies have reported that binding of human Mediator complex to Pol II is stabilized by TFIIF, suggesting a possible cooperative binding mechanism that could result in Gdown1 displacement and TFIIF incorporation [19, 22].
In sum, Mediator interacts intimately with GTFs to affect transcription in multiple ways. How these mechanisms are employed to differentially affect transcription occurring via different activators or at specific promoters remains to be elucidated.
Mediator function after transcription initiation
Stimulation of activated transcription by Mediator by enhancing formation or stability of the PIC is well documented, but in recent years, Mediator has also been discovered to contribute to steps in transcription that occur subsequent to PIC formation. Some of the first strong evidence for a post-initiation role for Mediator came from Arnold Berk’s laboratory in a study examining Med23-dependent transcriptional activation of the Egr1 gene by the activator ELK1 in mouse ESCs [130]. Using a med23-/- murine ES cell line, it was shown that Med23 was important for recruitment of Mediator to the Egr1 promoter. However, although impaired recruitment of Mediator in med23-/- cells also resulted in decreased recruitment of Pol II and other GTFs, productive transcription was decreased by an amount greater than could be accounted for by this effect. Furthermore, the distribution of Pol II was affected in med23-/- cells, with less association at coding regions relative to the promoter, suggesting a defect in a post-recruitment step.
More recent work has provided evidence for a role of Mediator in transcription elongation. An in vitro study found that mammalian Mediator could stimulate transcription in a reconstituted system by overcoming inhibition caused by addition of DSIF, which comprises hSpt4 and hSpt5 subunits and normally functions in transcriptional elongation; however, no direct interaction between Mediator and DSIF was observed [148]. Evidence has also been reported for a similar role for yeast Mediator; mutants in Med7, Med14, Med19, and Med21 were shown to diminish activation of the HSP82 gene by heat-shock, with no change in Pol II recruitment but impaired transit through the coding region [25]. Another report showed that Med26 interacts with TFIID and complexes containing ELL/EAF and P-TEFb to facilitate the reactivation of paused Pol II, suggesting a model in which Mediator facilitates the transition from initiation to elongation by sequentially stimulating recruitment of TFIID and elongation complexes [26]. Paused Pol II can also be activated in vitro by the p53 activation domain; this domain also confers structural shifts in Mediator, specifically the formation of a large pocket domain at the Mediator-Pol II interaction site [86]. These results suggest a mechanism by which specific activators could enhance transcriptional elongation in an activator-specific fashion. Positive regulation of transcriptional elongation by Mediator was also observed in genes belonging to the serum response network, in this case by recruitment of P-TEFb and BRD4 that required Mediator containing the cyclin-CDK module [27]. Finally, as discussed in the preceding section, studies by the Price and Roeder laboratories show that Mediator can facilitate transcriptional activation by overcoming repression of TFIIF function by Gdown1. Cheng et al. report evidence that Gdown1 association is prevalent at sites having paused Pol II in vivo; the results of Jishage et al. are not entirely consistent with these findings, but both point to a possible regulation of elongation by Mediator [145–147].
In addition to playing roles in transcription elongation, Mediator has recently been reported to be required for the proper transcription termination of the induced INO1 and CHA1 genes in budding yeast [28]. This work shows that the non-essential Med18 subunit of Mediator is not required for the recruitment of TBP and TFIIB onto the promoters of INO1 and CHA1, but that its loss nonetheless causes a 3- to 4-fold reduction of transcript levels. Based on genetic and physical interactions between the Med18/Med20 submodule of Mediator and subunits of Pol II that are involved in transcription termination, the association of the cleavage–polyadenylation factors Rna5 and Pta1 with the 3′ end of these genes was assessed in wild-type and med18∆ yeast. Reduced levels of Rna5 and Pta1 were observed in med18∆ yeast, and a transcription run-on analysis confirmed a transcription readthrough phenotype in the absence of Med18. These results may also explain earlier observations of near-complete abrogation of CHA1 transcripts in med17 ts yeast in spite of only a 3- to 4-fold reduction in Pol II recruitment [102]. Finally, a recent study has shown a role for Mediator in mRNA processing [29].
Role of the kinase module in gene activation and repression
In addition to its many roles in activating transcription, Mediator also functions in transcriptional repression. Mutations in subunits from all three modules that cause increased non-stimulated expression of yeast heat shock genes were identified in one study [149], and transcriptome analyses have identified many genes that are up-regulated in Mediator mutants [43, 44, 83, 113, 150]. However, in most cases evidence for direct repression by Mediator is lacking, and in some cases indirect repression, for example by down-regulation of the activator MET28 in Mediator tail mutants, has been shown to be a likely mechanism [83].
Compelling evidence does exist for transcriptional repression by Mediator via the kinase module. This module, comprising four subunits (Med12, Med13, CDK8, and CycC), shows variable association with the Mediator complex and has long been thought to play a repressive role in transcription [9, 151]. Mammalian CDK8 has been found to be associated with inactive transcription complexes and was lost, with retention of core Mediator, upon gene activation, suggesting a direct repressive role [152]. Genome-wide epistasis experiments in S. cerevisiae showed that the kinase module represses a subset of genes by preventing activation via tail module subunits; at these targets, loss of critical tail module subunits suppresses up-regulation of genes by deletion of kinase module subunits [44]. A plausible mechanism for the repressive capability of the kinase module was provided by studies showing that its binding to the core Mediator complex from both yeast and human can prevent binding of Pol II [153, 154]. In addition, in S. cerevisiae, the kinase subunit, Cdk8, can phosphorylate Med2 at Ser208, and this phosphorylation contributes to reduced activation of a subset of genes [44]. Although one might have expected the kinase module to be present specifically at repressed target genes, a genome-wide location study in S. cerevisiae revealed that it associates with many promoters of both active and inactive genes, indicating that its gene-specific roles in repression and activation are dictated post-recruitment [155].
Direct positive roles for the kinase module have also been reported [27, 104, 156]. This module associates with the active GAL1 promoter in yeast where it facilitates TBP association [104], while in human cells, CDK8 was found to be essential for thyroid hormone-dependent transcription of and Pol II occupancy at the DioI promoter [156]. CDK8 recruitment has also been found to accompany Mediator-dependent gene activation in a murine system both in vitro and in vivo [130, 131]. In addition to its positive role in the initiation in these examples, the cyclin–CDK module is recruited to the active p21 locus, and CDK8 has been reported to play a positive role in transcriptional elongation of genes in the serum response network [27, 157]. Possible mechanistic insight into such positive roles for the kinase module was provided in one study that showed that CDK8 can phosphorylate Ser10 of histone H3, in turn stimulating H3K14 acetylation (normally associated with transcriptional activation) by GCN5L that is present in a complex with Mediator containing the kinase module [158]. However, the form of Mediator identified in this study (containing CDK8 and GCN5L, and termed T/G-Mediator) did not activate transcription in an in vitro assay using chromatin templates, thus leaving some uncertainty about its role in activation. In general, how the apparently opposing roles of the cyclin–CDK module at different promoters can be mechanistically reconciled is currently unclear and in need of additional study.
Summary and future perspectives
Mediator is a highly conserved, large multisubunit complex able to make extensive contacts with diverse transcription factors in the process of mRNA transcription. Mediator also contributes to transcription of microRNAs and some long noncoding RNAs in A. thaliana [159] and seems likely also to function in activation of such non-mRNA, Pol II-dependent genes in other organisms, but such a role has not yet been fully documented. By contacting both the activator proteins that initiate transcription in promoter-specific fashion and coactivators and components of the general transcription machinery, including Pol II, Mediator is able to act as a conduit between the initiating event and the machinery that controls the level of mRNA produced. Because Mediator can make diverse protein–protein contacts that may differ at distinct promoters, and can furthermore adopt activator-dependent conformations, it has been viewed as a potential modulator or integrative hub capable of generating diverse outputs in a promoter-specific fashion [17, 160]. Additional complexity in Mediator function is introduced by the tissue-specific role of individual Mediator subunits in higher organisms [18]. Outstanding questions regarding Mediator function include how activator contacts induce distinct conformational changes in Mediator; how promoter-specific functions of Mediator, including its dependence on co-activators such as the SAGA complex in yeast and its participation in distinct steps during transcriptional activation, are determined; what determines different dependence on individual Mediator subunits at specific promoters; and how the sum of interactions among Mediator and its partners contribute to the final transcriptional output at a given promoter. Studies addressing these issues, as well as how Mediator function varies in different physiological settings, will lead to a better understanding of the fundamental biology of this central complex as well as of the molecular basis of diseases which are linked to mutations in specific Mediator subunits or their dysregulation in different pathologic states [161, 162].
Acknowledgment
Supported by NSF grant MCB0949722.
Abbreviations
- ER
Estrogen receptor
- GTF
General transcription factor
- HNF4
Hepatic nuclear factor 4
- JA
Jasmonic acid
- NR
Nuclear receptor
- PIC
Pre-initiation complex
- Pol II
RNA polymerase II
Contributor Information
Suraiya A. Ansari, Phone: +1-518-4022357, Email: sansari@wadsworth.org
Randall H. Morse, Phone: +1-518-4863116, Email: randall.morse@wadsworth.org
References
- 1.Lemon B, Tjian R. Orchestrated response: a symphony of transcription factors for gene control. Genes Dev. 2000;14:2551–2569. doi: 10.1101/gad.831000. [DOI] [PubMed] [Google Scholar]
- 2.Butler JE, Kadonaga JT. The RNA polymerase II core promoter: a key component in the regulation of gene expression. Genes Dev. 2002;16:2583–2592. doi: 10.1101/gad.1026202. [DOI] [PubMed] [Google Scholar]
- 3.Thomas MC, Chiang CM. The general transcription machinery and general cofactors. Crit Rev Biochem Mol Biol. 2006;41:105–178. doi: 10.1080/10409230600648736. [DOI] [PubMed] [Google Scholar]
- 4.Belotserkovskaya R, Berger SL. Interplay between chromatin modifying and remodeling complexes in transcriptional regulation. Crit Rev Eukaryot Gene Expr. 1999;9:221–230. doi: 10.1615/CritRevEukarGeneExpr.v9.i3-4.70. [DOI] [PubMed] [Google Scholar]
- 5.Cosma MP, Tanaka T, Nasmyth K. Ordered recruitment of transcription and chromatin remodeling factors to a cell cycle—and developmentally regulated promoter. Cell. 1999;97:299–311. doi: 10.1016/S0092-8674(00)80740-0. [DOI] [PubMed] [Google Scholar]
- 6.Helmlinger D, Marguerat S, Villen J, Swaney DL, Gygi SP, Bahler J, Winston F. Tra1 has specific regulatory roles, rather than global functions, within the SAGA co-activator complex. EMBO J. 2011;30:2843–2852. doi: 10.1038/emboj.2011.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Timmers HT, Tora L. SAGA unveiled. Trends Biochem Sci. 2005;30:7–10. doi: 10.1016/j.tibs.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 8.Bourbon HM. Comparative genomics supports a deep evolutionary origin for the large, four-module transcriptional mediator complex. Nucleic Acids Res. 2008;36:3993–4008. doi: 10.1093/nar/gkn349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Myers LC, Kornberg RD. Mediator of transcriptional regulation. Annu Rev Biochem. 2000;69:729–749. doi: 10.1146/annurev.biochem.69.1.729. [DOI] [PubMed] [Google Scholar]
- 10.Guglielmi B, van Berkum NL, Klapholz B, Bijma T, Boube M, Boschiero C, Bourbon HM, Holstege FC, Werner M. A high resolution protein interaction map of the yeast mediator complex. Nucleic Acids Res. 2004;32:5379–5391. doi: 10.1093/nar/gkh878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lariviere L, Plaschka C, Seizl M, Wenzeck L, Kurth F, Cramer P. Structure of the mediator head module. Nature. 2012;492:448–451. doi: 10.1038/nature11670. [DOI] [PubMed] [Google Scholar]
- 12.Asturias FJ, Jiang YW, Myers LC, Gustafsson CM, Kornberg RD. Conserved structures of mediator and RNA polymerase II holoenzyme. Science. 1999;283:985–987. doi: 10.1126/science.283.5404.985. [DOI] [PubMed] [Google Scholar]
- 13.Dotson MR, Yuan CX, Roeder RG, Myers LC, Gustafsson CM, Jiang YW, Li Y, Kornberg RD, Asturias FJ. Structural organization of yeast and mammalian mediator complexes. Proc Natl Acad Sci USA. 2000;97:14307–14310. doi: 10.1073/pnas.260489497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brzovic PS, Heikaus CC, Kisselev L, Vernon R, Herbig E, Pacheco D, Warfield L, Littlefield P, Baker D, Klevit RE, Hahn S. The acidic transcription activator Gcn4 binds the mediator subunit Gal11/Med15 using a simple protein interface forming a fuzzy complex. Mol Cell. 2011;44:942–953. doi: 10.1016/j.molcel.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vojnic E, Mourao A, Seizl M, Simon B, Wenzeck L, Lariviere L, Baumli S, Baumgart K, Meisterernst M, Sattler M, Cramer P. Structure and VP16 binding of the mediator Med25 activator interaction domain. Nat Struct Mol Biol. 2011;18:404–409. doi: 10.1038/nsmb.1997. [DOI] [PubMed] [Google Scholar]
- 16.Lewis BA, Reinberg D. The mediator coactivator complex: functional and physical roles in transcriptional regulation. J Cell Sci. 2003;116:3667–3675. doi: 10.1242/jcs.00734. [DOI] [PubMed] [Google Scholar]
- 17.Malik S, Roeder RG. The metazoan mediator co-activator complex as an integrative hub for transcriptional regulation. Nat Rev Genet. 2010;11:761–772. doi: 10.1038/nrg2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Borggrefe T, Yue X. Interactions between subunits of the mediator complex with gene-specific transcription factors. Semin Cell Dev Biol. 2011;22:759–768. doi: 10.1016/j.semcdb.2011.07.022. [DOI] [PubMed] [Google Scholar]
- 19.Takagi Y, Calero G, Komori H, Brown JA, Ehrensberger AH, Hudmon A, Asturias F, Kornberg RD. Head module control of mediator interactions. Mol Cell. 2006;23:355–364. doi: 10.1016/j.molcel.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 20.Esnault C, Ghavi-Helm Y, Brun S, Soutourina J, Van Berkum N, Boschiero C, Holstege F, Werner M. Mediator-dependent recruitment of TFIIH modules in preinitiation complex. Mol Cell. 2008;31:337–346. doi: 10.1016/j.molcel.2008.06.021. [DOI] [PubMed] [Google Scholar]
- 21.Cai G, Imasaki T, Yamada K, Cardelli F, Takagi Y, Asturias FJ. Mediator head module structure and functional interactions. Nat Struct Mol Biol. 2010;17:273–279. doi: 10.1038/nsmb.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bernecky C, Grob P, Ebmeier CC, Nogales E, Taatjes DJ. Molecular architecture of the human mediator-RNA polymerase II-TFIIF assembly. PLoS Biol. 2011;9:e1000603. doi: 10.1371/journal.pbio.1000603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soutourina J, Wydau S, Ambroise Y, Boschiero C, Werner M. Direct interaction of RNA polymerase II and mediator required for transcription in vivo. Science. 2011;331:1451–1454. doi: 10.1126/science.1200188. [DOI] [PubMed] [Google Scholar]
- 24.Bjorklund S, Gustafsson CM. The yeast mediator complex and its regulation. Trends Biochem Sci. 2005;30:240–244. doi: 10.1016/j.tibs.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 25.Kremer SB, Kim S, Jeon JO, Moustafa YW, Chen A, Zhao J, Gross DS. Role of mediator in regulating Pol II elongation and nucleosome displacement in Saccharomyces cerevisiae . Genetics. 2012;191:95–106. doi: 10.1534/genetics.111.135806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takahashi H, Parmely TJ, Sato S, Tomomori-Sato C, Banks CA, Kong SE, Szutorisz H, Swanson SK, Martin-Brown S, Washburn MP, Florens L, Seidel CW, Lin C, Smith ER, Shilatifard A, Conaway RC, Conaway JW. Human mediator subunit MED26 functions as a docking site for transcription elongation factors. Cell. 2011;146:92–104. doi: 10.1016/j.cell.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Donner AJ, Ebmeier CC, Taatjes DJ, Espinosa JM. CDK8 is a positive regulator of transcriptional elongation within the serum response network. Nat Struct Mol Biol. 2010;17:194–201. doi: 10.1038/nsmb.1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mukundan B, Ansari A. Novel role for mediator complex subunit Srb5/Med18 in termination of transcription. J Biol Chem. 2011;286:37053–37057. doi: 10.1074/jbc.C111.295915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang Y, Li W, Yao X, Lin QJ, Yin JW, Liang Y, Heiner M, Tian B, Hui J, Wang G. Mediator complex regulates alternative mRNA processing via the MED23 subunit. Mol Cell. 2012;45:459–469. doi: 10.1016/j.molcel.2011.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khorosjutina O, Wanrooij PH, Walfridsson J, Szilagyi Z, Zhu X, Baraznenok V, Ekwall K, Gustafsson CM. A chromatin-remodeling protein is a component of fission yeast mediator. J Biol Chem. 2010;285:29729–29737. doi: 10.1074/jbc.M110.153858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kagey MH, Newman JJ, Bilodeau S, Zhan Y, Orlando DA, van Berkum NL, Ebmeier CC, Goossens J, Rahl PB, Levine SS, Taatjes DJ, Dekker J, Young RA. Mediator and cohesin connect gene expression and chromatin architecture. Nature. 2010;467:430–435. doi: 10.1038/nature09380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Z, Myers LC. Med5(Nut1) and Med17(Srb4) are direct targets of mediator histone H4 tail interactions. PLoS One. 2012;7:e38416. doi: 10.1371/journal.pone.0038416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu X, Zhang Y, Bjornsdottir G, Liu Z, Quan A, Costanzo M, Davila Lopez M, Westholm JO, Ronne H, Boone C, Gustafsson CM, Myers LC. Histone modifications influence mediator interactions with chromatin. Nucleic Acids Res. 2011;39:8342–8354. doi: 10.1093/nar/gkr551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Flanagan PM, RD Kelleher, Sayre MH, Tschochner H, Kornberg RD. A mediator required for activation of RNA polymerase II transcription in vitro. Nature. 1991;350:436–438. doi: 10.1038/350436a0. [DOI] [PubMed] [Google Scholar]
- 35.Kornberg RD. Mediator and the mechanism of transcriptional activation. Trends Biochem Sci. 2005;30:235–239. doi: 10.1016/j.tibs.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 36.Kuras L, Borggrefe T, Kornberg RD. Association of the mediator complex with enhancers of active genes. Proc Natl Acad Sci USA. 2003;100:13887–13891. doi: 10.1073/pnas.2036346100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leroy C, Cormier L, Kuras L. Independent recruitment of mediator and SAGA by the activator Met4. Mol Cell Biol. 2006;26:3149–3163. doi: 10.1128/MCB.26.8.3149-3163.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin L, Chamberlain L, Zhu LJ, Green MR. Analysis of Gal4-directed transcription activation using Tra1 mutants selectively defective for interaction with Gal4. Proc Natl Acad Sci USA. 2012;109:1997–2002. doi: 10.1073/pnas.1116340109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ang K, Ee G, Ang E, Koh E, Siew WL, Chan YM, Nur S, Tan YS, Lehming N. Mediator acts upstream of the transcriptional activator Gal4. PLoS Biol. 2012;10:e1001290. doi: 10.1371/journal.pbio.1001290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee YC, Park JM, Min S, Han SJ, Kim YJ. An activator binding module of yeast RNA polymerase II holoenzyme. Mol Cell Biol. 1999;19:2967–2976. doi: 10.1128/mcb.19.4.2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Myers LC, Gustafsson CM, Hayashibara KC, Brown PO, Kornberg RD. Mediator protein mutations that selectively abolish activated transcription [see comments] Proc Natl Acad Sci USA. 1999;96:67–72. doi: 10.1073/pnas.96.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang F, Sumibcay L, Hinnebusch AG, Swanson MJ. A triad of subunits from the Gal11/tail domain of Srb mediator is an in vivo target of transcriptional activator Gcn4p. Mol Cell Biol. 2004;24:6871–6886. doi: 10.1128/MCB.24.15.6871-6886.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ansari SA, Ganapathi M, Benschop JJ, Holstege FC, Wade JT, Morse RH. Distinct role of mediator tail module in regulation of SAGA-dependent, TATA-containing genes in yeast. EMBO J. 2012;31:44–57. doi: 10.1038/emboj.2011.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van de Peppel J, Kettelarij N, van Bakel H, Kockelkorn TT, van Leenen D, Holstege FC. Mediator expression profiling epistasis reveals a signal transduction pathway with antagonistic submodules and highly specific downstream targets. Mol Cell. 2005;19:511–522. doi: 10.1016/j.molcel.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 45.Yang F, Vought BW, Satterlee JS, Walker AK, Jim Sun ZY, Watts JL, DeBeaumont R, Saito RM, Hyberts SG, Yang S, Macol C, Iyer L, Tjian R, van den Heuvel S, Hart AC, Wagner G, Naar AM. An ARC/mediator subunit required for SREBP control of cholesterol and lipid homeostasis. Nature. 2006;442:700–704. doi: 10.1038/nature04942. [DOI] [PubMed] [Google Scholar]
- 46.Kato Y, Habas R, Katsuyama Y, Naar AM, He X. A component of the ARC/mediator complex required for TGF beta/Nodal signalling. Nature. 2002;418:641–646. doi: 10.1038/nature00969. [DOI] [PubMed] [Google Scholar]
- 47.Taubert S, Van Gilst MR, Hansen M, Yamamoto KR. A mediator subunit, MDT-15, integrates regulation of fatty acid metabolism by NHR-49-dependent and -independent pathways in C. elegans . Genes Dev. 2006;20:1137–1149. doi: 10.1101/gad.1395406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ries D, Meisterernst M. Control of gene transcription by mediator in chromatin. Semin Cell Dev Biol. 2011;22:735–740. doi: 10.1016/j.semcdb.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 49.Thakur JK, Arthanari H, Yang F, Pan SJ, Fan X, Breger J, Frueh DP, Gulshan K, Li DK, Mylonakis E, Struhl K, Moye-Rowley WS, Cormack BP, Wagner G, Naar AM. A nuclear receptor-like pathway regulating multidrug resistance in fungi. Nature. 2008;452:604–609. doi: 10.1038/nature06836. [DOI] [PubMed] [Google Scholar]
- 50.Thakur JK, Arthanari H, Yang F, Chau KH, Wagner G, Naar AM. Mediator subunit Gal11p/MED15 is required for fatty acid-dependent gene activation by yeast transcription factor Oaf1p. J Biol Chem. 2009;284:4422–4428. doi: 10.1074/jbc.M808263200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Herbig E, Warfield L, Fish L, Fishburn J, Knutson BA, Moorefield B, Pacheco D, Hahn S. Mechanism of mediator recruitment by tandem Gcn4 activation domains and three Gal11 activator-binding domains. Mol Cell Biol. 2010;30:2376–2390. doi: 10.1128/MCB.01046-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jedidi I, Zhang F, Qiu H, Stahl SJ, Palmer I, Kaufman JD, Nadaud PS, Mukherjee S, Wingfield PT, Jaroniec CP, Hinnebusch AG. Activator Gcn4 employs multiple segments of Med15/Gal11, including the KIX domain, to recruit mediator to target genes in vivo. J Biol Chem. 2010;285:2438–2455. doi: 10.1074/jbc.M109.071589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Milbradt AG, Kulkarni M, Yi T, Takeuchi K, Sun ZY, Luna RE, Selenko P, Naar AM, Wagner G. Structure of the VP16 transactivator target in the mediator. Nat Struct Mol Biol. 2011;18:410–415. doi: 10.1038/nsmb.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ansari SA, He Q, Morse RH. Mediator complex association with constitutively transcribed genes in yeast. Proc Natl Acad Sci USA. 2009;106:16734–16739. doi: 10.1073/pnas.0905103106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Koh SS, Ansari AZ, Ptashne M, Young RA. An activator target in the RNA polymerase II holoenzyme. Mol Cell. 1998;1:895–904. doi: 10.1016/S1097-2765(00)80088-X. [DOI] [PubMed] [Google Scholar]
- 56.Fondell JD, Ge H, Roeder RG. Ligand induction of a transcriptionally active thyroid hormone receptor coactivator complex. Proc Natl Acad Sci USA. 1996;93:8329–8333. doi: 10.1073/pnas.93.16.8329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rachez C, Suldan Z, Ward J, Chang CP, Burakov D, Erdjument-Bromage H, Tempst P, Freedman LP. A novel protein complex that interacts with the vitamin D3 receptor in a ligand-dependent manner and enhances VDR transactivation in a cell-free system. Genes Dev. 1998;12:1787–1800. doi: 10.1101/gad.12.12.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Roeder RG. The role of general initiation factors in transcription by RNA polymerase II. Trends Biochem Sci. 1996;21:327–335. [PubMed] [Google Scholar]
- 59.Malik S, Guermah M, Yuan CX, Wu W, Yamamura S, Roeder RG. Structural and functional organization of TRAP220, the TRAP/mediator subunit that is targeted by nuclear receptors. Mol Cell Biol. 2004;24:8244–8254. doi: 10.1128/MCB.24.18.8244-8254.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Louvion JF, Havaux-Copf B, Picard D. Fusion of GAL4-VP16 to a steroid-binding domain provides a tool for gratuitous induction of galactose-responsive genes in yeast. Gene. 1993;131:129–134. doi: 10.1016/0378-1119(93)90681-R. [DOI] [PubMed] [Google Scholar]
- 61.Stafford GA, Morse RH. Mutations in the AF-2/hormone-binding domain of the chimeric activator GAL4.estrogen receptor.VP16 inhibit hormone-dependent transcriptional activation and chromatin remodeling in yeast. J Biol Chem. 1998;273:34240–34246. doi: 10.1074/jbc.273.51.34240. [DOI] [PubMed] [Google Scholar]
- 62.Ge K, Cho YW, Guo H, Hong TB, Guermah M, Ito M, Yu H, Kalkum M, Roeder RG. Alternative mechanisms by which mediator subunit MED1/TRAP220 regulates peroxisome proliferator-activated receptor gamma-stimulated adipogenesis and target gene expression. Mol Cell Biol. 2008;28:1081–1091. doi: 10.1128/MCB.00967-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen W, Roeder RG. Mediator-dependent nuclear receptor function. Semin Cell Dev Biol. 2011;22:749–758. doi: 10.1016/j.semcdb.2011.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Grontved L, Madsen MS, Boergesen M, Roeder RG, Mandrup S. MED14 tethers mediator to the N-terminal domain of peroxisome proliferator-activated receptor gamma and is required for full transcriptional activity and adipogenesis. Mol Cell Biol. 2010;30:2155–2169. doi: 10.1128/MCB.01238-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lariviere L, Seizl M, Cramer P. A structural perspective on mediator function. Curr Opin Cell Biol. 2012;24:305–313. doi: 10.1016/j.ceb.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 66.Malik S, Wallberg AE, Kang YK, Roeder RG. TRAP/SMCC/mediator-dependent transcriptional activation from DNA and chromatin templates by orphan nuclear receptor hepatocyte nuclear factor 4. Mol Cell Biol. 2002;22:5626–5637. doi: 10.1128/MCB.22.15.5626-5637.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hittelman AB, Burakov D, Iniguez-Lluhi JA, Freedman LP, Garabedian MJ. Differential regulation of glucocorticoid receptor transcriptional activation via AF-1-associated proteins. EMBO J. 1999;18:5380–5388. doi: 10.1093/emboj/18.19.5380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lau JF, Nusinzon I, Burakov D, Freedman LP, Horvath CM. Role of metazoan mediator proteins in interferon-responsive transcription. Mol Cell Biol. 2003;23:620–628. doi: 10.1128/MCB.23.2.620-628.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Boyer TG, Martin ME, Lees E, Ricciardi RP, Berk AJ. Mammalian Srb/mediator complex is targeted by adenovirus E1A protein. Nature. 1999;399:276–279. doi: 10.1038/20466. [DOI] [PubMed] [Google Scholar]
- 70.Rana R, Surapureddi S, Kam W, Ferguson S, Goldstein JA. Med25 is required for RNA polymerase II recruitment to specific promoters, thus regulating xenobiotic and lipid metabolism in human liver. Mol Cell Biol. 2011;31:466–481. doi: 10.1128/MCB.00847-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yamamoto S, Eletsky A, Szyperski T, Hay J, Ruyechan WT. Analysis of the varicella-zoster virus IE62 N-terminal acidic transactivating domain and its interaction with the human mediator complex. J Virol. 2009;83:6300–6305. doi: 10.1128/JVI.00054-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang M, Hay J, Ruyechan WT. Varicella-zoster virus IE62 protein utilizes the human mediator complex in promoter activation. J Virol. 2008;82:12154–12163. doi: 10.1128/JVI.01693-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Backstrom S, Elfving N, Nilsson R, Wingsle G, Bjorklund S. Purification of a plant mediator from Arabidopsis thaliana identifies PFT1 as the Med25 subunit. Mol Cell. 2007;26:717–729. doi: 10.1016/j.molcel.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 74.Autran D, Jonak C, Belcram K, Beemster GT, Kronenberger J, Grandjean O, Inze D, Traas J. Cell numbers and leaf development in Arabidopsis: a functional analysis of the STRUWWELPETER gene. EMBO J. 2002;21:6036–6049. doi: 10.1093/emboj/cdf614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang W, Chen X. HUA ENHANCER3 reveals a role for a cyclin-dependent protein kinase in the specification of floral organ identity in Arabidopsis. Development. 2004;131:3147–3156. doi: 10.1242/dev.01187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kidd BN, Cahill DM, Manners JM, Schenk PM, Kazan K. Diverse roles of the mediator complex in plants. Semin Cell Dev Biol. 2011;22:741–748. doi: 10.1016/j.semcdb.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 77.Cerdan PD, Chory J. Regulation of flowering time by light quality. Nature. 2003;423:881–885. doi: 10.1038/nature01636. [DOI] [PubMed] [Google Scholar]
- 78.Xu R, Li Y. Control of final organ size by mediator complex subunit 25 in Arabidopsis thaliana . Development. 2011;138:4545–4554. doi: 10.1242/dev.071423. [DOI] [PubMed] [Google Scholar]
- 79.Elfving N, Davoine C, Benlloch R, Blomberg J, Brannstrom K, Muller D, Nilsson A, Ulfstedt M, Ronne H, Wingsle G, Nilsson O, Bjorklund S. The Arabidopsis thaliana Med25 mediator subunit integrates environmental cues to control plant development. Proc Natl Acad Sci USA. 2011;108:8245–8250. doi: 10.1073/pnas.1002981108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kidd BN, Edgar CI, Kumar KK, Aitken EA, Schenk PM, Manners JM, Kazan K. The mediator complex subunit PFT1 is a key regulator of jasmonate-dependent defense in Arabidopsis. Plant Cell. 2009;21:2237–2252. doi: 10.1105/tpc.109.066910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cevik V, Kidd BN, Zhang P, Hill C, Kiddle S, Denby KJ, Holub EB, Cahill DM, Manners JM, Schenk PM, Beynon J, Kazan K. MEDIATOR25 acts as an integrative hub for the regulation of jasmonate-responsive gene expression in Arabidopsis. Plant Physiol. 2012;160:541–555. doi: 10.1104/pp.112.202697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen R, Jiang H, Li L, Zhai Q, Qi L, Zhou W, Liu X, Li H, Zheng W, Sun J, Li C. The Arabidopsis mediator subunit MED25 differentially regulates jasmonate and abscisic acid signaling through interacting with the MYC2 and ABI5 transcription factors. Plant Cell. 2012;24:2898–2916. doi: 10.1105/tpc.112.098277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Koschubs T, Seizl M, Lariviere L, Kurth F, Baumli S, Martin DE, Cramer P. Identification, structure, and functional requirement of the mediator submodule Med7 N/31. EMBO J. 2009;28:69–80. doi: 10.1038/emboj.2008.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Taatjes DJ, Naar AM, Andel F, 3rd, Nogales E, Tjian R. Structure, function, and activator-induced conformations of the CRSP coactivator. Science. 2002;295:1058–1062. doi: 10.1126/science.1065249. [DOI] [PubMed] [Google Scholar]
- 85.Taatjes DJ, Schneider-Poetsch T, Tjian R. Distinct conformational states of nuclear receptor-bound CRSP-Med complexes. Nat Struct Mol Biol. 2004;11:664–671. doi: 10.1038/nsmb789. [DOI] [PubMed] [Google Scholar]
- 86.Meyer KD, Lin SC, Bernecky C, Gao Y, Taatjes DJ. p53 activates transcription by directing structural shifts in mediator. Nat Struct Mol Biol. 2010;17:753–760. doi: 10.1038/nsmb.1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Taatjes DJ, Marr MT, Tjian R. Regulatory diversity among metazoan co-activator complexes. Nat Rev Mol Cell Biol. 2004;5:403–410. doi: 10.1038/nrm1369. [DOI] [PubMed] [Google Scholar]
- 88.Ebmeier CC, Taatjes DJ. Activator–mediator binding regulates mediator-cofactor interactions. Proc Natl Acad Sci USA. 2010;107:11283–11288. doi: 10.1073/pnas.0914215107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Han SJ, Lee YC, Gim BS, Ryu GH, Park SJ, Lane WS, Kim YJ. Activator-specific requirement of yeast mediator proteins for RNA polymerase II transcriptional activation. Mol Cell Biol. 1999;19:979–988. doi: 10.1128/mcb.19.2.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lee YC, Min S, Gim BS, Kim YJ. A transcriptional mediator protein that is required for activation of many RNA polymerase II promoters and is conserved from yeast to humans. Mol Cell Biol. 1997;17:4622–4632. doi: 10.1128/mcb.17.8.4622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Swanson MJ, Qiu H, Sumibcay L, Krueger A, Kim SJ, Natarajan K, Yoon S, Hinnebusch AG. A multiplicity of coactivators is required by Gcn4p at individual promoters in vivo. Mol Cell Biol. 2003;23:2800–2820. doi: 10.1128/MCB.23.8.2800-2820.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Imasaki T, Calero G, Cai G, Tsai KL, Yamada K, Cardelli F, Erdjument-Bromage H, Tempst P, Berger I, Kornberg GL, Asturias FJ, Kornberg RD, Takagi Y. Architecture of the mediator head module. Nature. 2011;475:240–243. doi: 10.1038/nature10162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lorch Y, Beve J, Gustafsson CM, Myers LC, Kornberg RD. Mediator-nucleosome interaction. Mol Cell. 2000;6:197–201. doi: 10.1016/s1097-2765(00)00021-6. [DOI] [PubMed] [Google Scholar]
- 94.Li B, Carey M, Workman JL. The role of chromatin during transcription. Cell. 2007;128:707–719. doi: 10.1016/j.cell.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 95.Roeder RG. Transcriptional regulation and the role of diverse coactivators in animal cells. FEBS Lett. 2005;579:909–915. doi: 10.1016/j.febslet.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 96.Yoon S, Qiu H, Swanson MJ, Hinnebusch AG. Recruitment of SWI/SNF by Gcn4p does not require Snf2p or Gcn5p but depends strongly on SWI/SNF integrity, SRB mediator, and SAGA. Mol Cell Biol. 2003;23:8829–8845. doi: 10.1128/MCB.23.23.8829-9945.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Qiu H, Hu C, Zhang F, Hwang GJ, Swanson MJ, Boonchird C, Hinnebusch AG. Interdependent recruitment of SAGA and Srb mediator by transcriptional activator Gcn4p. Mol Cell Biol. 2005;25:3461–3474. doi: 10.1128/MCB.25.9.3461-3474.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lemieux K, Gaudreau L. Targeting of Swi/Snf to the yeast GAL1 UAS G requires the mediator, TAF IIs, and RNA polymerase II. EMBO J. 2004;23:4040–4050. doi: 10.1038/sj.emboj.7600416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ryan MP, Jones R, Morse RH. SWI-SNF complex participation in transcriptional activation at a step subsequent to activator binding. Mol Cell Biol. 1998;18:1774–1782. doi: 10.1128/mcb.18.4.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sharma VM, Li B, Reese JC. SWI/SNF-dependent chromatin remodeling of RNR3 requires TAF(II)s and the general transcription machinery. Genes Dev. 2003;17:502–515. doi: 10.1101/gad.1039503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Martens JA, Wu PY, Winston F. Regulation of an intergenic transcript controls adjacent gene transcription in Saccharomyces cerevisiae. Genes Dev. 2005;19:2695–2704. doi: 10.1101/gad.1367605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.He Q, Battistella L, Morse RH. Mediator requirement downstream of chromatin remodeling during transcriptional activation of CHA1 in yeast. J Biol Chem. 2008;283:5276–5286. doi: 10.1074/jbc.M708266200. [DOI] [PubMed] [Google Scholar]
- 103.Bhaumik SR, Green MR. SAGA is an essential in vivo target of the yeast acidic activator Gal4p. Genes Dev. 2001;15:1935–1945. doi: 10.1101/gad.911401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Larschan E, Winston F. The Saccharomyces cerevisiae Srb8-Srb11 complex functions with the SAGA complex during Gal4-activated transcription. Mol Cell Biol. 2005;25:114–123. doi: 10.1128/MCB.25.1.114-123.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bryant GO, Ptashne M. Independent recruitment in vivo by gal4 of two complexes required for transcription. Mol Cell. 2003;11:1301–1309. doi: 10.1016/S1097-2765(03)00144-8. [DOI] [PubMed] [Google Scholar]
- 106.Govind CK, Yoon S, Qiu H, Govind S, Hinnebusch AG. Simultaneous recruitment of coactivators by Gcn4p stimulates multiple steps of transcription in vivo. Mol Cell Biol. 2005;25:5626–5638. doi: 10.1128/MCB.25.13.5626-5638.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Liu X, Vorontchikhina M, Wang YL, Faiola F, Martinez E. STAGA recruits mediator to the MYC oncoprotein to stimulate transcription and cell proliferation. Mol Cell Biol. 2008;28:108–121. doi: 10.1128/MCB.01402-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Acevedo ML, Kraus WL. Mediator and p300/CBP-steroid receptor coactivator complexes have distinct roles, but function synergistically, during estrogen receptor alpha-dependent transcription with chromatin templates. Mol Cell Biol. 2003;23:335–348. doi: 10.1128/MCB.23.1.335-348.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Black JC, Choi JE, Lombardo SR, Carey M. A mechanism for coordinating chromatin modification and preinitiation complex assembly. Mol Cell. 2006;23:809–818. doi: 10.1016/j.molcel.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 110.Krebs AR, Demmers J, Karmodiya K, Chang NC, Chang AC, Tora L. ATAC and mediator coactivators form a stable complex and regulate a set of non-coding RNA genes. EMBO Rep. 2010;11:541–547. doi: 10.1038/embor.2010.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lin JJ, Lehmann LW, Bonora G, Sridharan R, Vashisht AA, Tran N, Plath K, Wohlschlegel JA, Carey M. Mediator coordinates PIC assembly with recruitment of CHD1. Genes Dev. 2011;25:2198–2209. doi: 10.1101/gad.17554711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Thompson CM, Young RA. General requirement for RNA polymerase II holoenzymes in vivo. Proc Natl Acad Sci USA. 1995;92:4587–4590. doi: 10.1073/pnas.92.10.4587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Holstege FC, Jennings EG, Wyrick JJ, Lee TI, Hengartner CJ, Green MR, Golub TR, Lander ES, Young RA. Dissecting the regulatory circuitry of a eukaryotic genome. Cell. 1998;95:717–728. doi: 10.1016/S0092-8674(00)81641-4. [DOI] [PubMed] [Google Scholar]
- 114.Takagi Y, Kornberg RD. Mediator as a general transcription factor. J Biol Chem. 2006;281:80–89. doi: 10.1074/jbc.M508253200. [DOI] [PubMed] [Google Scholar]
- 115.Mittler G, Kremmer E, Timmers HT, Meisterernst M. Novel critical role of a human mediator complex for basal RNA polymerase II transcription. EMBO Rep. 2001;2:808–813. doi: 10.1093/embo-reports/kve186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Baek HJ, Malik S, Qin J, Roeder RG. Requirement of TRAP/mediator for both activator-independent and activator-dependent transcription in conjunction with TFIID-associated TAF(II)s. Mol Cell Biol. 2002;22:2842–2852. doi: 10.1128/MCB.22.8.2842-2852.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Deato MD, Marr MT, Sottero T, Inouye C, Hu P, Tjian R. MyoD targets TAF3/TRF3 to activate myogenin transcription. Mol Cell. 2008;32:96–105. doi: 10.1016/j.molcel.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Thiaville MM, Dudenhausen EE, Awad KS, Gjymishka A, Zhong C, Kilberg MS. Activated transcription via mammalian amino acid response elements does not require enhanced recruitment of the mediator complex. Nucleic Acids Res. 2008;36:5571–5580. doi: 10.1093/nar/gkn538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Veenstra GJ, Wolffe AP. Gene-selective developmental roles of general transcription factors. Trends Biochem Sci. 2001;26:665–671. doi: 10.1016/S0968-0004(01)01970-3. [DOI] [PubMed] [Google Scholar]
- 120.Conaway RC, Conaway JW. Function and regulation of the mediator complex. Curr Opin Genet Dev. 2011;21:225–230. doi: 10.1016/j.gde.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mo X, Kowenz-Leutz E, Xu H, Leutz A. Ras induces mediator complex exchange on C/EBP beta. Mol Cell. 2004;13:241–250. doi: 10.1016/S1097-2765(03)00521-5. [DOI] [PubMed] [Google Scholar]
- 122.Uhlmann T, Boeing S, Lehmbacher M, Meisterernst M. The VP16 activation domain establishes an active mediator lacking CDK8 in vivo. J Biol Chem. 2007;282:2163–2173. doi: 10.1074/jbc.M608451200. [DOI] [PubMed] [Google Scholar]
- 123.Kim YJ, Bjorklund S, Li Y, Sayre MH, Kornberg RD. A multiprotein mediator of transcriptional activation and its interaction with the C-terminal repeat domain of RNA polymerase II. Cell. 1994;77:599–608. doi: 10.1016/0092-8674(94)90221-6. [DOI] [PubMed] [Google Scholar]
- 124.Koleske AJ, Young RA. An RNA polymerase II holoenzyme responsive to activators. Nature. 1994;368:466–469. doi: 10.1038/368466a0. [DOI] [PubMed] [Google Scholar]
- 125.Thompson CM, Koleske AJ, Chao DM, Young RA. A multisubunit complex associated with the RNA polymerase II CTD and TATA-binding protein in yeast. Cell. 1993;73:1361–1375. doi: 10.1016/0092-8674(93)90362-T. [DOI] [PubMed] [Google Scholar]
- 126.Tardiff DF, Abruzzi KC, Rosbash M. Protein characterization of Saccharomyces cerevisiae RNA polymerase II after in vivo cross-linking. Proc Natl Acad Sci USA. 2007;104:19948–19953. doi: 10.1073/pnas.0710179104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Davis JA, Takagi Y, Kornberg RD, Asturias FA. Structure of the yeast RNA polymerase II holoenzyme: mediator conformation and polymerase interaction. Mol Cell. 2002;10:409–415. doi: 10.1016/S1097-2765(02)00598-1. [DOI] [PubMed] [Google Scholar]
- 128.Mehta S, Miklos I, Sipiczki M, Sengupta S, Sharma N. The Med8 mediator subunit interacts with the Rpb4 subunit of RNA polymerase II and Ace2 transcriptional activator in Schizosaccharomyces pombe . FEBS Lett. 2009;583:3115–3120. doi: 10.1016/j.febslet.2009.08.036. [DOI] [PubMed] [Google Scholar]
- 129.Cai G, Imasaki T, Takagi Y, Asturias FJ. Mediator structural conservation and implications for the regulation mechanism. Structure. 2009;17:559–567. doi: 10.1016/j.str.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang G, Balamotis MA, Stevens JL, Yamaguchi Y, Handa H, Berk AJ. Mediator requirement for both recruitment and postrecruitment steps in transcription initiation. Mol Cell. 2005;17:683–694. doi: 10.1016/j.molcel.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 131.Cantin GT, Stevens JL, Berk AJ. Activation domain-mediator interactions promote transcription preinitiation complex assembly on promoter DNA. Proc Natl Acad Sci USA. 2003;100:12003–12008. doi: 10.1073/pnas.2035253100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kuras L, Struhl K. Binding of TBP to promoters in vivo is stimulated by activators and requires Pol II holoenzyme. Nature. 1999;399:609–613. doi: 10.1038/21239. [DOI] [PubMed] [Google Scholar]
- 133.Li XY, Virbasius A, Zhu X, Green MR. Enhancement of TBP binding by activators and general transcription factors. Nature. 1999;399:605–609. doi: 10.1038/21232. [DOI] [PubMed] [Google Scholar]
- 134.Qiu H, Hu C, Yoon S, Natarajan K, Swanson MJ, Hinnebusch AG. An array of coactivators is required for optimal recruitment of TATA binding protein and RNA polymerase II by promoter-bound Gcn4p. Mol Cell Biol. 2004;24:4104–4117. doi: 10.1128/MCB.24.10.4104-4117.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bhaumik SR, Raha T, Aiello DP, Green MR. In vivo target of a transcriptional activator revealed by fluorescence resonance energy transfer. Genes Dev. 2004;18:333–343. doi: 10.1101/gad.1148404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Koleske AJ, Buratowski S, Nonet M, Young RA. A novel transcription factor reveals a functional link between the RNA polymerase II CTD and TFIID. Cell. 1992;69:883–894. doi: 10.1016/0092-8674(92)90298-Q. [DOI] [PubMed] [Google Scholar]
- 137.Lariviere L, Geiger S, Hoeppner S, Rother S, Strasser K, Cramer P. Structure and TBP binding of the mediator head subcomplex Med8-Med18-Med20. Nat Struct Mol Biol. 2006;13:895–901. doi: 10.1038/nsmb1143. [DOI] [PubMed] [Google Scholar]
- 138.Seizl M, Lariviere L, Pfaffeneder T, Wenzeck L, Cramer P. Mediator head subcomplex Med11/22 contains a common helix bundle building block with a specific function in transcription initiation complex stabilization. Nucleic Acids Res. 2011;39:6291–6304. doi: 10.1093/nar/gkr229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sakurai H, Fukasawa T. Artificial recruitment of certain mediator components affects requirement of basal transcription factor IIE. Genes Cells. 2003;8:41–50. doi: 10.1046/j.1365-2443.2003.00613.x. [DOI] [PubMed] [Google Scholar]
- 140.Sakurai H, Fukasawa T. Functional correlation among Gal11, transcription factor (TF) IIE, and TFIIH in Saccharomyces cerevisiae. Gal11 and TFIIE cooperatively enhance TFIIH-mediated phosphorylation of RNA polymerase II carboxyl-terminal domain sequences. J Biol Chem. 1998;273:9534–9538. doi: 10.1074/jbc.273.16.9534. [DOI] [PubMed] [Google Scholar]
- 141.Baek HJ, Kang YK, Roeder RG. Human mediator enhances basal transcription by facilitating recruitment of transcription factor IIB during preinitiation complex assembly. J Biol Chem. 2006;281:15172–15181. doi: 10.1074/jbc.M601983200. [DOI] [PubMed] [Google Scholar]
- 142.Guidi BW, Bjornsdottir G, Hopkins DC, Lacomis L, Erdjument-Bromage H, Tempst P, Myers LC. Mutual targeting of mediator and the TFIIH kinase Kin28. J Biol Chem. 2004;279:29114–29120. doi: 10.1074/jbc.M404426200. [DOI] [PubMed] [Google Scholar]
- 143.Sogaard TM, Svejstrup JQ. Hyperphosphorylation of the C-terminal repeat domain of RNA polymerase II facilitates dissociation of its complex with mediator. J Biol Chem. 2007;282:14113–14120. doi: 10.1074/jbc.M701345200. [DOI] [PubMed] [Google Scholar]
- 144.Akoulitchev S, Chuikov S, Reinberg D. TFIIH is negatively regulated by cdk8-containing mediator complexes. Nature. 2000;407:102–106. doi: 10.1038/35024111. [DOI] [PubMed] [Google Scholar]
- 145.Jishage M, Malik S, Wagner U, Uberheide B, Ishihama Y, Hu X, Chait BT, Gnatt A, Ren B, Roeder RG. Transcriptional regulation by Pol II(G) involving mediator and competitive interactions of Gdown1 and TFIIF with Pol II. Mol Cell. 2012;45:51–63. doi: 10.1016/j.molcel.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Cheng B, Li T, Rahl PB, Adamson TE, Loudas NB, Guo J, Varzavand K, Cooper JJ, Hu X, Gnatt A, Young RA, Price DH. Functional association of Gdown1 with RNA polymerase II poised on human genes. Mol Cell. 2012;45:38–50. doi: 10.1016/j.molcel.2011.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Espinosa JM. Get back TFIIF, don’t let me Gdown1. Mol Cell. 2012;45:3–5. doi: 10.1016/j.molcel.2011.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Malik S, Barrero MJ, Jones T. Identification of a regulator of transcription elongation as an accessory factor for the human mediator coactivator. Proc Natl Acad Sci USA. 2007;104:6182–6187. doi: 10.1073/pnas.0608717104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Singh H, Erkine AM, Kremer SB, Duttweiler HM, Davis DA, Iqbal J, Gross RR, Gross DS. A functional module of yeast mediator that governs the dynamic range of heat-shock gene expression. Genetics. 2006;172:2169–2184. doi: 10.1534/genetics.105.052738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Young ET, Yen K, Dombek KM, Law GL, Chang E, Arms E. Snf1-independent, glucose-resistant transcription of Adr1-dependent genes in a mediator mutant of Saccharomyces cerevisiae . Mol Microbiol. 2009;74:364–383. doi: 10.1111/j.1365-2958.2009.06866.x. [DOI] [PubMed] [Google Scholar]
- 151.Hampsey M. Molecular genetics of the RNA polymerase II general transcriptional machinery. Microbiol Mol Biol Rev. 1998;62:465–503. doi: 10.1128/mmbr.62.2.465-503.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Pavri R, Lewis B, Kim TK, Dilworth FJ, Erdjument-Bromage H, Tempst P, de Murcia G, Evans R, Chambon P, Reinberg D. PARP-1 determines specificity in a retinoid signaling pathway via direct modulation of mediator. Mol Cell. 2005;18:83–96. doi: 10.1016/j.molcel.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 153.Elmlund H, Baraznenok V, Lindahl M, Samuelsen CO, Koeck PJ, Holmberg S, Hebert H, Gustafsson CM. The cyclin-dependent kinase 8 module sterically blocks mediator interactions with RNA polymerase II. Proc Natl Acad Sci USA. 2006;103:15788–15793. doi: 10.1073/pnas.0607483103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Knuesel MT, Meyer KD, Bernecky C, Taatjes DJ. The human CDK8 subcomplex is a molecular switch that controls mediator coactivator function. Genes Dev. 2009;23:439–451. doi: 10.1101/gad.1767009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Andrau JC, van de Pasch L, Lijnzaad P, Bijma T, Koerkamp MG, van de Peppel J, Werner M, Holstege FC. Genome-wide location of the coactivator mediator: binding without activation and transient Cdk8 interaction on DNA. Mol Cell. 2006;22:179–192. doi: 10.1016/j.molcel.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 156.Belakavadi M, Fondell JD. Cyclin-dependent kinase 8 positively cooperates with mediator to promote thyroid hormone receptor-dependent transcriptional activation. Mol Cell Biol. 2010;30:2437–2448. doi: 10.1128/MCB.01541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Donner AJ, Szostek S, Hoover JM, Espinosa JM. CDK8 is a stimulus-specific positive coregulator of p53 target genes. Mol Cell. 2007;27:121–133. doi: 10.1016/j.molcel.2007.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Meyer KD, Donner AJ, Knuesel MT, York AG, Espinosa JM, Taatjes DJ. Cooperative activity of cdk8 and GCN5L within mediator directs tandem phosphoacetylation of histone H3. EMBO J. 2008;27:1447–1457. doi: 10.1038/emboj.2008.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kim YJ, Zheng B, Yu Y, Won SY, Mo B, Chen X. The role of mediator in small and long noncoding RNA production in Arabidopsis thaliana . EMBO J. 2011;30:814–822. doi: 10.1038/emboj.2011.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Taatjes DJ. The human mediator complex: a versatile, genome-wide regulator of transcription. Trends Biochem Sci. 2010;35:315–322. doi: 10.1016/j.tibs.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hashimoto S, Boissel S, Zarhrate M, Rio M, Munnich A, Egly JM, Colleaux L. MED23 mutation links intellectual disability to dysregulation of immediate early gene expression. Science. 2011;333:1161–1163. doi: 10.1126/science.1206638. [DOI] [PubMed] [Google Scholar]
- 162.Makinen N, Mehine M, Tolvanen J, Kaasinen E, Li Y, Lehtonen HJ, Gentile M, Yan J, Enge M, Taipale M, Aavikko M, Katainen R, Virolainen E, Bohling T, Koski TA, Launonen V, Sjoberg J, Taipale J, Vahteristo P, Aaltonen LA. MED12, the mediator complex subunit 12 gene, is mutated at high frequency in uterine leiomyomas. Science. 2011;334:252–255. doi: 10.1126/science.1208930. [DOI] [PubMed] [Google Scholar]
- 163.Bourbon HM, Aguilera A, Ansari AZ, Asturias FJ, Berk AJ, Bjorklund S, Blackwell TK, Borggrefe T, Carey M, Carlson M, Conaway JW, Conaway RC, Emmons SW, Fondell JD, Freedman LP, Fukasawa T, Gustafsson CM, Han M, He X, Herman PK, Hinnebusch AG, Holmberg S, Holstege FC, Jaehning JA, Kim YJ, Kuras L, Leutz A, Lis JT, Meisterernest M, Naar AM, Nasmyth K, Parvin JD, Ptashne M, Reinberg D, Ronne H, Sadowski I, Sakurai H, Sipiczki M, Sternberg PW, Stillman DJ, Strich R, Struhl K, Svejstrup JQ, Tuck S, Winston F, Roeder RG, Kornberg RD. A unified nomenclature for protein subunits of mediator complexes linking transcriptional regulators to RNA polymerase II. Mol Cell. 2004;14:553–557. doi: 10.1016/j.molcel.2004.05.011. [DOI] [PubMed] [Google Scholar]