Abstract
Chemotherapy and/or radiotherapy regular regimens used for conditioning of recipients of hematopoietic stem cell transplantation (SCT) induce a period of transient profound immunosuppression. The onset of a competent immunological response, such as the appearance of viral-specific T cells, is associated with a lower incidence of viral infections after haematopoietic transplantation. The rapid development of immunodominant peptide virus screening together with advances in the design of genetic and non-genetic viral- and tumoural-specific cellular selection strategies have opened new strategies for cellular immunotherapy in oncologic recipients who are highly sensitive to viral infections. However, the rapid development of cellular immunotherapy in SCT has disclosed the role of the T cell selection method in the modulation of functional cell activity and of in vivo secondary effects triggered following immunotherapy.
Electronic supplementary material
The online version of this article (doi:10.1007/s00018-013-1463-5) contains supplementary material, which is available to authorized users.
Keywords: Cellular immunotherapy, Antigen-specific cytotoxic T cells, Cellular functional modulation, In vitro cellular selection methods, Haematopoietic stem cell transplant
Introduction
Susceptibility to viral infections in allogeneic hematopoietic stem cell transplantation (HSCT) is the result of profoundly reduced innate and adaptive immunity caused by the immunoablative effect of the bone marrow conditioning regimens, host-versus-graft and graft-versus-host interactions which take place in the immediate post-transplant period [1, 2]. Administration of anti-viral drugs is nowadays the standard first line therapeutic treatment and it is even used as a prophylactic method in patients with high susceptibility to suffer viral primo-infection or reactivation. However, this treatment shows high regimen-related toxicity in different tissues, variability in efficacy and generation of viral-resistant variants after treatment. Therefore, many clinical trials alternate or combine anti-virals with prophylactic therapies based on infusion of virus-specific primed T cells [3]. Despite their success, there are many doubts about their application and efficacy after the reappearance of virus-associated clinical symptoms in some patients, correlating with the in vivo longevity of this therapeutic subpopulation and retention of virus reactivity. Interestingly, only a few researchers have analysed in depth if the T cell cytolytic effector function is in fact dependent on their selection method, which would alter the persistence of the adoptively transferred cells.
This review summarises the impact of classical and alternative protocols for purification of antigen-specific T cells on the establishment of immunological tolerance, the dynamics of in vivo T cell expansion and its clinical daily application. Our observations highlight the urgent need for standard technical approaches in immunotherapy facilities which would induce minor alterations in the T cells, guaranteeing a long-lived and optimum repopulating potential.
Adoptive immunotherapy precedents
The classical concept of adoptive immunotherapy is based on infusion of donor unmanipulated bulk lymphocytes (DLI) at variable intervals following bone marrow transplantation, enhancing, for example, the anti-leukaemic effect of the graft (GVL) [4]. This idea is supported from the cytogenetic remission that was observed in three patients receiving buffy coat infusion of their original marrow donors after chronic myelogenous leukemia diagnosis and conventional allograft transplant [5]. Moreover, five patients receiving nonirradiated DLI developed Epstein–Barr virus (EBV) lymphoma after transplantation. Clinical remissions were achieved within 30 days after cellular treatment [6]. Despite these promising clinical results, also corroborated by Heslop and colleagues [7], this type of cellular therapy presents a high risk for the patient’s health. Indeed, there is evidence that administration of total lymphocytes is associated with high morbidity and mortality rates, mainly due to severe graft-versus-host disease (GvHD) [8]. Alloreactive CD8+ T cells (CTLs) contained within the transferred bulk leukocyte population are directly responsible for the aggressive GvH syndrome. Consequently, different protocols have been developed to limit the presence of these self-reactive subpopulations to obtain a safer cellular product.
In immunocompetent subjects, the exposure to viral antigens and their recognition by T cells trigger T cell receptor (TCR)-signal dependent activation, which drives their expansion and differentiation and regulates the magnitude of the T cell response [9]. Virus-primed T cells are generated during this process [10]. This physiological process can be replicated ex vivo in lymphocyte cultures exposed to viral antigens. Thus, cell proliferation and differentiation into different T cell subsets can be achieved [11]. This is the basis of the incipient adoptive immunotherapy strategy suggested by Riddell and colleagues [12], and nowadays adopted by many researchers, proving that autologous CD3+ CD8+ CD4− cytomegalovirus (CMV)-specific clones can be expanded by co-culturing donor-derived peripheral-blood mononuclear cells with autologous virus-pulsed fibroblasts for 5–12 weeks. Successful adoptive immunotherapy was later performed with these virus-specific T cells. None of patients developed CMV-associated clinical disease or side effects usually associated with the treatment. The analyses of the rearrangements of the TCR β-chain variable region demonstrated in vivo clonal expansion from the transferred clones. These expanded T cells exhibited the same cytotoxicity by the parental cells from the immunocompetent donors for up to 4 weeks [13]. However, as this ex vivo expansion is undertaken in the presence of live virions, this methodology can jeopardise the patient’s safety, and, therefore, it has been limited to a restricted number of cases [14]. Nevertheless, the therapeutic potential of these findings, which have been corroborated in subsequent studies [15–49], represented a starting point for the designing of new cellular immunotherapy protocols [50, 51] (Table S1).
Improvement of virus-specific T cell production and cell enrichment methods
In the meantime, other similar approaches have also been used to generate anti-viral T cell responses. For example, dendritic cells (DCs), one of the most potent professional antigen-presenting cell (APCs) types, have been extensively used in immunotherapy protocols (Table S2). They may be used as raw biological material for in vitro T cell production of both protagonist cells of the cellular therapy products. The main advantage of using DCs is their high capacity for antigen processing and presentation of multiple epitopes in major histocompatibility complex (MHC) class I and II and their applicability to patients of all HLA types. It has also allowed the obtaining of polyclonal T cell subpopulations with the advantage that this characteristic provides synergism between different immunological subpopulations [14, 52]. Otherwise, the immunodominant T cell epitopes present in a particular given antigen have to be known to expand the patient’s T cells. In addition, it is possible to modulate the expression of their co-stimulatory molecules allowing the manipulation of the immunological synapsis that would enhance the activation or inhibition of the T cell response [53]. Similarly, EBV-transformed B-lymphoblastoid cell lines (BLCL), which were transduced with a retroviral vector encoding the immunodominant CMVpp65, have also been used as APCs to simultaneously expand EBV- and CMV-specific CTLs. These CTLs showed class I and II viral-bispecific restriction [54]. Furthermore, BLCL can be grown in large numbers, which would enhance the therapeutic protocol. Thus, Leen et al. [55] has perfected the system: BLCL were transformed with a recombinant adenovirus expressing CMVpp65, with the purpose of obtaining T cell preparations with trivirus-specific activity [EBV-, Adenovirus (AdV)-, and CMV-specific CTLs]. CD4 and CD8 T cell expansion was observed, leading to in vivo resolution of virus-associated clinical symptoms within the first month of the therapy.
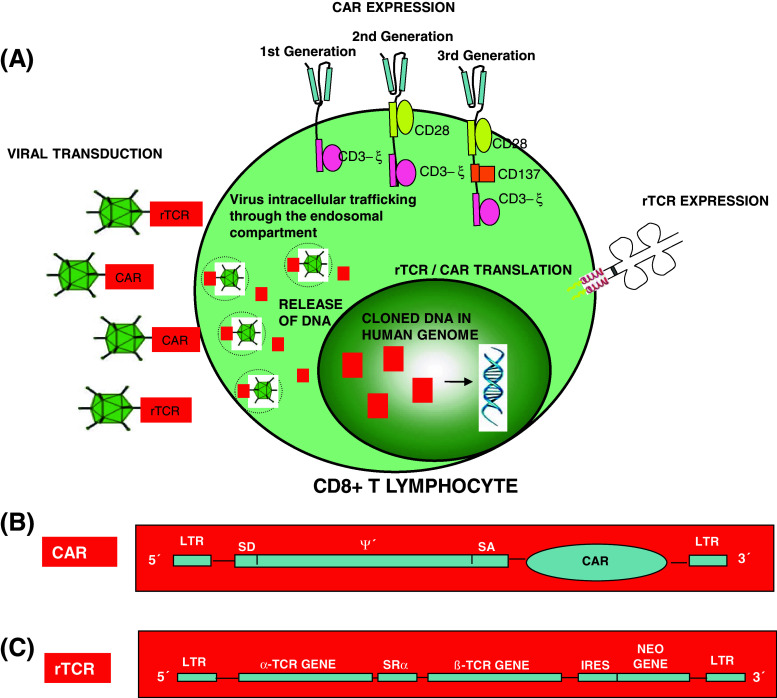
In fact, several clinical trials are being carried out to validate a new genetic engineering methodology consisting on the redirection of the T cell antigenic specificity by the introduction of TCR genes (variable α- and β-chains) or chimeric antigen receptors (CARs) (Fig. 1) followed by infusion in the patient (Table S3).
Fig. 1.
Genetic manipulation of the TCR, showing two different methods of genetic manipulation of the therapeutic cell: recombinant T-cell receptor (rTCR) transference and chimeric antigen receptors (CAR) cloning (a). This new genetic engineering methodology consists on the redirection of the T cell antigenic specificity by the introduction of CARs using viral vectors (b) or TCR genes (variable α- and β-chains) (c)
This strategy can be used to engineer fully functional virus-specific T cells, which would otherwise be “inactivated” after chronic viral antigen exposure [56]. For example, HBV-specific T cells are deleted or exhausted in chronic hepatitis B—and HBV-related hepatocarcinoma patients. Gehring and colleagues [57] and Wood [58] have obtained fully functional TCR re-directed HBV core (18–27)—and surface (183–191; 370–379)-specific effector T cells after epitope-specific TCR cloning by retroviral transduction. These T cells exhibited cytotoxic activity towards HBV-infected cells. Kessels and colleagues [59] demonstrated the expansion and maintenance of TCR-modified transferred T cells up to 81 days after inoculation. This clearly showed their in vivo proliferative capacities. At present, different kind of tumours and viral infections are treated with this type of cell transfer therapies, in some cases demonstrating cancer regression in 30 % of treated patients [60]. A potential problem of this methodology is the association of the recombinant α/β chains with the endogenously expressed TCR chains, generating T cells with unknown and undesired new autoantigen specificities [61] and with reduced TCR surface expression. In this way, sporadic autoreactive events could potentially appear in the recipients, something to be taken into consideration. The introduction of cysteines on each recombinant TCR chain to form an extra interchain disulfide bonds between both α- and β-structures could prevent this intracellular molecular event even significantly boost the effector activity [62]. Cohen et al. [63] designed a murinisation strategy based on the substitution of the human C regions with their murine counterparts. However, these xenogenic sequences might also potentially trigger immunogenicity against the murinised TCR. Bialer et al. [64] minimally engineered human C regions with selected murine residues mediating superior chimeric TCR expression and improved activity, which would result in a more efficient pairing of the murine Cα and Cβ1, decrease the formation of mixed TCR chain dimers and minimise autoimmune manifestations.
Nowadays, human trials are in progress to evaluate the safety and feasibility of T cells transduced with CARs instead of αβTCR in adoptive cell therapy procedures [65]. Specifically, these constructs recognise tumour cell-surface molecules. They consist of the fusion of the antigen-recognition portion of a monoclonal antibody with an intracellular signalling domain capable of activating or enhancing T cell effector function by intensifying molecular signalling pathways in a MHC-dependent and -independent fashion [60, 66, 67]. Consequently, Micklethwaite et al. engineered virus-specific T cells stimulated with multispecific viral immunodominant antigens of CMV, EBV and AdV. These modified cells also exhibited anti-tumour activity that was conferred by their retroviral-mediated expression of CAR.CD19+, obtaining a bi-functional therapeutic harvest [68]. This approach allows the expansion of multivirus-specific CAR-modified CTLs (which retain their anti-viral activities), but with significantly increased ex vivo anti-tumoural activity against B-ALL blasts from patients with haematological disease. Recently, the monitoring of anti-CD19 CAR-modified T cells is possible through the use of an antibody consisting of human CD19 extracellular domains and human immunoglobulin domain. Similarly to MHC-multimer technology, the fluorescent labelling of this structure allows the direct visualisation of CAR-expressing T cells by flow cytometry, which makes this approach very attractive [69]. However, only a few tumour-specific antigens expressed exclusively by cancer cells and susceptible of being targeted have been identified. This problem could be solved by the CAR-target replacement by a fluorescein isothiocyanate (FITC) molecule. The use of cetuximab, trastuzumab, and rituximab monoclonal antibodies conjugated with FITC would expand the applicability of this tool allowing the simultaneous recognition of a variety of tumour-associated antigens by a single therapeutic product [70]. Using this molecular approach, Louis and colleagues [71] and Pule et al. [72] achieved effective anti-tumour responses in patients with advanced-stage neuroblastoma. The treated patients exhibited partial and complete tumour responses, respectively, and long-term persistence of modified T cells (beyond 96 weeks). These T cells were engineered from EBV-specific cytotoxic T cells expressing the CAR diasialoganglioside antigen-GD2. These encouraging clinical results have demonstrated the high viability of these therapeutic T cells in cancer patients. However, these results have not been reproduced by other authors. Kershaw and colleagues observed a high level of therapeutic T cells during the first few days after infusion of autologous anti-α folate receptor CAR-modified T cells in patients with metastatic ovarian cancer. These T cells were undetectable after a month of monitoring [73]. A poor choice of the pan-tumour antigens or a weak functional activity could explain these results.
Following this approach, three different CAR generations have been developed, which incorporate T cell co-stimulatory signalling molecules [CD28, CD3-zeta(ξ), OX40, 4-1BB (CD137)] in their structure, improving their signalling capacities in modified T cells [74, 75]. These molecular modifications have enhanced the in vivo expansion (>1,000-fold), have increased the long-term maintenance of the engineered CAR+ T cells into the patient (for at least 6 months) and have induced trafficking to the bone marrow or cerebrospinal fluid. The infusion of CAR+ T cells that targeted CD19 or ERBB2 (HER-2/neu) and contained costimulatory domains from CD28 or CD137 and the TCR ξ chain signalling element in patients with relapse or refractory chronic lymphocytic leukemia [76–78], acute lymphoblastic leukemia [79] and metastatic colon cancer [80] generated tumour regression by a potent anti-tumour effect in all patients treated (in some cases associated with morphologic and molecular remission). However, unexpected clinical adverse events were noted in these patients including the occurrence of delayed tumour lysis syndrome accompanied by a hemophagocytic syndrome, capillary leak syndrome, non-infectious fevers, hypotension, respiratory distress syndrome, grade 3/4 lymphopenia and loss of normal B cells. In most cases, the administration of glucocorticoids or anti-cytokine therapy resolved these reversible systemic effects, although hospitalisation in intensive care units was necessary depending on the case. A cytokine-release syndrome or “cytokine storm” has also been observed in a limited number of patients, either after intensive lymphodepletion and before immunotherapy treatment or after CAR-transduced T cells infusion itself [76, 77, 79]. Most patients responded well to anti-IL-6 (tocilizumab) and/or anti-TNF (etanercept), although, in a few medically fragile patients, cytokine blockade with drugs was not effective, resulting in multiple organ dysfunction and death [78, 80]. However, the precise aetiology of these patients’ deaths remains uncertain.
Although the administration of corticosteroids in doses used to treat GvHD or antibodies specific to T cells such as alemtuzumab (CAMPATH-1H) would deplete the majority of circulating transduced cells [81], alternative approaches are necessary. In this regard, the introduction of the herpes simplex thymidine-kinase (HSV-TK) suicide transgene in the viral construct has allowed the inducible apoptosis of transduced-cells through interference with DNA synthesis on exposure to ganciclovir administered in the event of GvHD after stem cell transplantation in several phase I–II clinical trials [82–84]. Acute and chronic GvHD were successfully controlled with this suicide HSV-TK approach in the context of allo-HSCT and haplo-HSCT. However, a number of drawbacks need to be improved, such as the immunogenicity of the TK protein, the restriction of killing to dividing cells and the elimination of transduced-cells when Ganciclovir is used for the treatment of CMV infection.
For this reason, other authors have investigated in a phase I–II clinical trial the suitability of an alternative suicide gene, inducible caspase 9 (iCasp9), showing >90 % apoptosis of the modified T cells within 30 min of the administration of a specific chemical dimeriser drug [85]. This iCasp9 cell-suicide system did not change the antigen-specific functionality [86, 87] even when combined with transgenic expression of IL-2 or IL-15 [88].
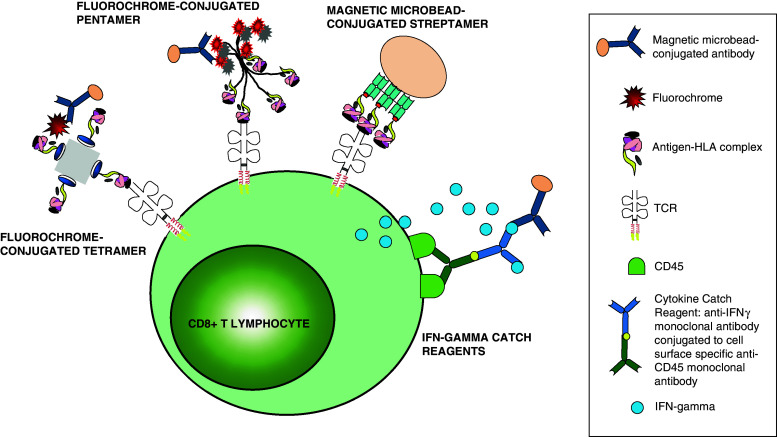
The development of multimer technology has provided an invaluable method for monitoring and purification of T cells with known antigenic specificities. The basis for multimer technology resides in the recognition of antigen-specific TCRs by a recombinant class I or II molecule complex to a certain immunodominant peptide. Identification of antigen-specific cytotoxic T cells regardless of their biological activity allows the preparation of an heterogeneous T cell population which circumvents the previous phenotypic characterisations required for the identification of primed subpopulations with long-term survival capacities. Consequently, this staining technology allows the isolation of T cells with a given antigen specificity from seropositive donors without any further manipulation [89]. Nowadays, there is a wide variety of available MHC multimer molecules such as dimers, tetramers, pentamers, streptamers, dextramers and octamers which are key for studies of adoptive immunotherapy. These multimer molecules have been extensively used to identify and select CMV-, EBV- and AdV-specific T cells from healthy donors, and their transfer to immunocompromised hosts has shown excellent results [90–92] (Table S4).
Other authors have demonstrated the value of IFNγ-secreted antigen-specific CD8+ T cells for the successful reconstitution of virus-specific immunity in allogeneic bone marrow transplant recipients [93]. Manz et al. [95] has developed a high-affinity physic matrix of cytokine-secreting cells that prevents cytokine spreading [94, 96]. A number of groups have successfully used this technique for the treatment of viral infections in immunocompromised patients, increasing viral clearance and avoiding the associated disease [97, 98]. However, several authors have questioned the homogeneity of the cellular product obtained with this capture method, following the identification of non-specific NK cells, B cells and monocytes in the harvest product [99–101]. The presence of some of these cell types can have important biosafety implications in immunocompromised patients. In fact, Mutis and colleagues [102] observed association between a high frequency of histocompatibility-minor antigen-specific mismatch and the development of grade II–IV GvHD. This pathological event after adoptive immunotherapy was explained by the presence of allo-reactive clones in the infused product [103].
The establishment of good manufacturing practices (GMP)-based facilities and methodologies to achieve homogenous functional T cell preparations would favour the development of these techniques to a clinical scale improving the standardisation of the process of cell selection methods for immunotherapy applications. Thus, the above-mentioned results would justify an increased investment of funds and resources to develop a safe approach to implementing these promising genetic and cellular therapies in medical practice. To identify funding sources to support the viral constructs and cellular products manufactured under GMP conditions, and to overcome the complex legal barriers, should be a priority for academia and industry, which we need to further develop these advanced technologies [104].
The therapeutic virus-primed T cell status determines its long-term persistence in the recipient
So far, it has been possible to reactivate the memory CTL pool through ex vivo antigen presentation by B cells-, fibroblasts-, DCs- and BLCL-based systems. This is possible because of the high prevalence of CMV, EBV and AdV infections in the human immunocompetent population. Theoretically, this implies that memory rather than naïve T cells would be the best candidate to be selectively expanded ex vivo. However, there is no doubt about the notable influence of the in vitro culture period to obtain differentiation status-specific phenotype T cell clones and their permanence ability in vivo [105]. A few years later, this natural phenomenon was demonstrated by Berger and colleagues [106], who have shown, in an experimental model of macaque with persistent CMV infection, that for its treatment the use of this ex vivo expansion system from effector memory T cell clones can hamper both correct homing to lymph nodes and bone marrow (BM) and their survival for a limited time in vivo. On the other hand, if T cells are derived from central memory clones, they retain their ability to respond to CMV, expand in vivo and undergo phenotypic conversion to both central- and effector-memory T cells. Therefore, selection and infusion of more incipient primed cells would ensure that, after the therapeutic procedure, virus-specific cytotoxic T cells would permanently re-establish the immune memory response [106–108]. The combination of this enrichment system with molecular engineering would have a high therapeutic potential. Thus, Wang et al. [109] isolated polyclonal T cells with a central memory-like phenotype (CD8+ CD45RA− CD62L+) which exhibited anti-tumour activity after CD19-specific CAR expression. Recently, the enrichment of CD8+ CD62L+ T cells using magnetic microbeads technology and priming with peptide-pulsed APCs before transduction with a lentivector encoding CD19-CAR has allowed the engineering of CMV- and EBV-specific modified T cells. These cells were further selected with reversible streptamers and showed equivalent T cell responses to tumours and endogenous viral-bispecific TCRs [110]. These results have shown that the previous selection of specifically primed T cells significantly increases the efficiency of the harvest. The studies conducted by Hinrichs and colleagues [111, 112] went further by showing, in a transgenic murine model of adoptive immunotherapy, that T cells from the naïve compartment resist terminal differentiation and possess the highest expansion potential and anti-tumour activities. This finding implies that the pre-priming of lymphocyte subpopulations improves gene modification, leading to a high transduction efficiency and transgene expression. The use of these T cells would require in vitro priming and extensive expansion from both seropositive and seronegative donors indistinctly. Following this line, Hanley et al. has achieved extensive ex vivo expansion of naïve T cells isolated from cord blood utilising EBV-infected B cells as APCs, after their modification with adenoviral vectors. In this way, they have generated large numbers of CMV-, EBV- and AdV-specific T cells [113]. Interestingly, expanded cytotoxic T cells from naïve precursors exhibited anti-leukemia activity after transplantation [114]. In addition, this methodology would bypass terminal differentiation, as demonstrated by Gattinoni and colleagues. Otherwise, terminally differentiated T cells would be less effective at triggering disease regression in vivo [105, 115, 116]. However, data published by several research groups indicate that naïve T cell populations contain alloreactive precursors. Thus, these authors have found high frequencies of GvHD in mice infused with naïve T cells in comparison with memory T cells [117, 118]. In agreement with this observation, Distler and colleagues [119] have demonstrated alloreactivity of sorted naïve T cells against single class I or II mismatched MHC alleles, questioning the validity of naive T cells as a substrate for adoptive immunotherapy in BM transplant recipients. Recently, Gattinoni and colleagues [120, 121] have identified in humans a lytic T cell memory subset with phenotypic (CD45RA+ CD62L+ CD95+ CCR7+ IL-7Rα+ IL-2Rβ+ CXCR3+) and functional (IFNγ+ IL-2+ TNFα+) characteristics shared by naïve T and stem cells [122]. Consequently, the use of T cells derived from naïve precursors requires an exhaustive analysis before its routine application in human therapy.
Microenvironment of the recipient, a highlighted variable in the multivariate equation of biological immunotherapy
The host lymphoablative conditioning regimen essential for haematopoietic transplant carried out before adoptive T cell transfer-based immunotherapy may enhance anti-tumour responses by the modulation of the microenvironment through a range of mechanisms, including the inhibition of endogenous CD4+ CD25+ FOXP3+ regulatory T cells (Tregs), upregulation of MHC class I proteins, increase in the pool of peptides available for presentation, T cell trafficking, potentiation of innate immunity and increase in homeostatic cytokines (IL-2, IL-7, IL-15 and IL-21) [123–127]. This subject has been the central issue of different experimental studies for over a decade in radiated or pharmacologic-treated recipients.
Furthermore, Tregs suppress effector T cells by a number of mechanisms, including an increase of the activation threshold of effector T cells, expression of inhibitory costimulatory molecules, induction of anti-inflammatory biochemical pathways, direct or indirect killing, consumption of proinflammatory cytokines or production of immunoregulatory cytokines [128]. The downregulation of Tregs by exogenous immunostimulatory agents could therefore potentially improve the migratory properties, engraftment and cytolytic activity of the transferred T cells. Similar actions have been demonstrated through combined therapy of daclizumab (humanised anti-CD25 monoclonal antibody) with peptide vaccine, administering to breast cancer patients. In this clinical trial, Tregs eradication in situ and reprogramming induces robust augmented of physiological CTL and T helper response [129, 130]. Modulation of inducible Tregs as a consequence of the preparative regimen for transplant (immunosuppression) triggers inhibitory counterproductive cellular mechanisms that could limit the therapeutic potential of the infused cells.
Common γ-chain cytokines, including IL-7 and IL-15, have been reported to induce vital cellular activity such as proliferation of human T cells in the absence of TCR stimulation, furthermore avoiding apoptosis and maintaining cell metabolism after transfer into the lymphopenic host (homeostatic expansion). The absence of some of these homeostatic cytokines could result in the metabolic atrophy of infused T cells, which leads to delayed growth and proliferation following viral stimulation [125, 131]. Thus, it is known that expression of IL-7 and IL-15 receptors is key to the establishment of resting memory cells in cellular therapy procedures, as both cytokines synergistically drive T cells through this crucial checkpoint in their differentiation process [132]. Shen et al. demonstrated that downregulation of both IL-7R and IL-15R is likely to be a contributing factor for the poor survival of therapeutic influenza-specific memory CTLs in the respiratory tract. According to these authors, there could even exist molecular mechanisms that would condition their survival depending on the particular tissue [133]. On the other hand, this in vivo expansion is also thought to be driven by different factors such as self-peptides and other antigens. Furthermore, exposure to viral antigens during the period of profound lymphopenia results in a significant boost of cellular immunity [90, 134, 135], and it is a mandatory requirement for antigen-specific immunological recovery in the transplanted patient.
To date, the essential role of T helper cells for the maintenance of CD8s is a controversial issue, and different studies have sometimes shown inconsistent results. Cobbold and colleagues [90] observed no correlation between CMV-specific CD4+ T cells and the circulating level of CD8+ T cell. However, this is likely due to the low sample size (n = 5), so a declining trend in both subpopulations was observed in 4/5 patients 60 days from the time of infusion of the therapeutic product. Several studies confirmed this trend for a relatively long time. Riddell et al. [12] demonstrated that the adoptive transfer of donor CMV-specific CTLs in transplant patients results in a fast therapeutic activity, although their long-term maintenance is hampered by the absence of the appropriate T helper subpopulation. In line with these observations, Rosenberg and colleagues reported that HIV-1-infected subjects without acquired immunodeficiency syndrome development show a high virus-specific CD4+ proliferative response and an extremely vigorous CTL concomitant response [136]. The particular mechanisms whereby CD4+ T cells maintain effective anti-viral immunity are poorly understood, but they could be related to the orchestration of CTL precursor activity. According to this, there are already studies raising tumour-specific CTL responses in which a simultaneous activation of the T helper subpopulation is found after cellular vaccination with class I and II peptide-loaded DCs vaccines. With this strategy, bi-functional CD4+ activity is generated, resulting in increasing CTL proliferation and Treg inhibition [137]. In order to examine the relationship between both protective immunological populations and their role during the immunological recovery process in the post-transplant period, the development of MHC class II multimer complexes is being encouraged by biotechnology companies [138]. In fact, more studies are incorporating them as a research tool. As a matter of fact, the use of T helper cells as a therapeutic product has also been put in question, and the limited experience with these cells in adoptive cell therapy is insufficient to obtain objective conclusions. Quezada and colleagues transferred tyrosinase-related protein 1-specific TCR CD4+ cells into an irradiated RAG2−/− mouse model with advanced melanoma. Tumour eradication was mediated by cytotoxic CD4+ T cells, which appeared after acquisition of IFN-γ, TNF-α, IL-2, granzyme B and perforin expression. Other endogenous immunological subpopulations (helper- and cytotoxic-T, B and NK cells) did not play an essential role in the anti-tumour effect of these infused cells [139]. The lymphopenic microenvironment and the depletion of CD25+ FOXP3+ CTLA-4+ CD4+ Tregs apparently played a crucial role in the in vivo priming, expansion and activation of the exogenous naïve CD4+ cells and their cytotoxic-like phenotype conversion [140]. Furthermore, these conditions allowed the long-term establishment of a CD4+-memory subpopulation [141].
Finally, it is important to mention that many of these cytokines may play a role in manipulating the microenvironment in the context of allogeneic HSCT, and that there are ongoing clinical trials evaluating several modifications of the different conditioning regimens including non-chemotherapy-based conditioning regimens for transplantation [142, 143].
In vitro modulation of the functional ability of viral-specific CTLs by multimer complexes
In contrast to the other T cell selection techniques, multimer technology (Fig. 2) developed by Altman et al. [144] allows the identification and enrichment of viral-specific CTLs without altering their differentiation status. However, recent studies with soluble experimental MHC class I-tetramers have shown that continuous in vivo administration of an MHC multimer, induces unexpected outcomes in the antigen-reactive CD8+. An increase in the frequency of annexin V staining was observed, and could be attributed to the induction of cellular anergy or activation of induced cell death [145]. In the first case, anergy can be triggered by a strong “signal 1” provided by the binding of the experimental tetramer with its specific transgenic TCR, in the absence of “signals 2” (co-stimulation) and “signals 3” (cytokine priming) [53]. Instead, a limited expansion of these CD8+ T cells and their effector activities are compromised by the continuous presence of the soluble tetramer within the transgenic mice, leading to either clonal exhaustion or anergy, resulting in T cell dysfunction. In agreement with this, Neudorfer and colleagues [146] have observed in vitro that both peptide-specific activation-dependent cytotoxic activity and proliferation capacity of primed T cells were impaired and decreased following staining with conventional tetramers. Thus, gene transcription after tetramer complex–TCR interaction has previously been shown in experimental models. Other authors have confirmed these observations [147, 148], and some have even demonstrated the loss of protective capacity to Listeria monocytogenes in BALB/c mice after transference of CTLs pre-treated with MHC-tetramers [149]. However, administration of CMV-specific CTLs in stem cell transplant recipients that had been previously selected using tetramer complexes contributed efficiently to the control of virus dissemination [90]. In this study, the authors demonstrated persistence of CMV-specific CTLs at least 110 days after infusion, even in patients without CMV-primed CTLs before cell transfer, which suggested expansion of the infused cells. This would discard anergy in the infused T cells, at least for this period of study.
Fig. 2.
Multimer technology allows the identification and enrichment of viral-specific CTLs. A diagrammatic representation of the structure of three different multimers: tetramers, pentamers and streptamers together with the IFN-gamma (γ) catch reagent and their mechanism of action. The IFNγ-capture, pentamers and streptamers but not the tetramers are currently produced under GMP conditions
Therefore, experimental and physiological microenvironments show conflicting results about the long-term cellular effect induced by the multimer on the virus-specific CTL. However, it is currently unknown whether pentameric constructs would also induce anergy in these T cells. Pentamer and tetramer technologies are similar tools that use the same molecular approach, so it is theoretically possible that a similar impairment of T cell phenotype and function could occur in vitro using pentamers. However, Uhlin et al. demonstrated the presence of functional EBV-specific CTLs that induced regression of an EBV-driven lymphoma in vivo up to 189 days after infusion. It is worth mentioning that complete regression of EBV infection-associated lymphoma occurred in a patient diagnosed with post-transplant lymphoproliferative disease after therapeutic treatment [91].
One possible explanation to this mismatch between in vivo and in vitro results is the possibility that T cell hyporesponsiveness negatively modulated by multimer engagement can be reversed by a cytokine storm generated in the lymphopenic recipient. That assessment is proposed by Brown and colleagues [150], who demonstrated that anergic anti-tumour CD8+ T cells restore their function after transfer into RAG2−/− immunodeficient recipients promoting tumour rejection. Specifically, Teague et al. [151] has shown that exogenous addition of IL-15 rescued and expanded previously tolerised cytotoxic T cells in vitro. However, no data in the literature have been published concerning the association between cellular functional immunomodulation induced by the multimer complexes and the influence of the host microenvironment.
As discussed above, the influence of tetramer and pentamer staining on T cell functional status remains an important unresolved issue. This could substantially limit their clinical applicability. In order to address this problem, a reversible human MHC/peptide multimer, called streptamer, was constructed by Neudorfer and colleagues [146], using the molecular technology proposed by Knabel et al. [149]. “Naïve” antigen-specific T cells can be obtained after multimer complex staining. The reversible binding between both structures (streptamer–TCR) allows its easy disengagement through exposure to a competitor molecule. Following dissociation, the streptamer-treated CTLs are functionally indistinguishable from untreated T cells. Wang and colleagues [152] demonstrated that in vitro treatment of OT-I TCR-transgenic CTLs with peptide-loaded OT-I-streptamers markedly increased 3H-thymidine incorporation and upregulated early activation markers. The biochemical analysis of the signalling pathways in this assay identified several signalling molecules which were regulated after streptamer engagement. Sustained phosphorylation of Akt and ERK1/2 was observed, possibly increasing Bcl-xL expression, thus resulting in cell survival. According to these authors, streptamer engagement is not “silent”. However, this positive effect may be favourably used in adoptive immunotherapy protocols. The performance of cell enrichment methods using streptamer complexes has significantly improved in just a few years [103, 110, 153]. Recently, this methodology has even been combined with other genetic approaches, in which in vitro analysis has shown excellent T cell-dependent anti-viral and anti-tumoural activities, thus demonstrating the bi-specific system validity and the potential of this multimeric methodology [110]. However, it is unclear which signalling pathways may be activated by the streptamer–TCR interaction, or whether this would have synergistic effects with other signal transduction cascades. Whether these molecular changes in T cells would affect their therapeutic behaviour once administrated to the cancer patient is an open question. More detailed studies will be necessary to clarify this issue.
Concluding remarks: interrelationship between in vitro and ex vivo potential of the virus-specific primed T cells
The particular physiological characteristics of the recipient’s haematopoietic progenitors, the cellular composition and purity of the therapeutic product, and the selection method used to isolate or develop antigen-specific T cells will determine the long-term persistence of transferred lymphocyte populations. In some cases, the altered function and differentiation of these cells significantly affected the performance of adoptive immunotherapy used in SCT patients, making it difficult to predict clinical results. In future, multivariate analysis will be necessary to understand the interaction between all these physiological variables and to determine the optimal method of achieving anti-viral T cell immunity.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This work was supported by a research grant (PI10/00136) from the Fondo de Investigaciones Sanitarias (FIS) granted by the Instituto de Salud Carlos III (ISCIII).L.B. is a recipient of APPICS Predoctoral Fellowship from Departamento de Salud del Gobierno de Navarra. M.C. is a recipient of PFIS Predoctoral Fellowship from ISCIII. C.M. is a recipient of ANABASID Postdoctoral Fellowship from Departamento de Educación del Gobierno de Navarra.
Conflict of interest
The authors declare no conflict of interest regarding the topics discussed in this manuscript.
Contributor Information
Natalia Ramírez, Phone: +34-848-422865, FAX: +34-848-422200, Email: nramireh@cfnavarra.es.
Eduardo Olavarría, Phone: +34-848-428384, FAX: +34-848-422200, Email: CHNOlavarria@hotmail.com.
References
- 1.Zaia J, Baden L, Boeckh MJ, Chakrabarti S, Einsele H, Ljungman P, McDonald GB, Hirsch H. Viral disease prevention after hematopoietic cell transplantation. Bone Marrow Transplant. 2009;44(8):471–482. doi: 10.1038/bmt.2009.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Winston DJ, Ho WG, Bartoni K, Du Mond C, Ebeling DF, Buhles WC, Champlin RE. Ganciclovir prophylaxis of cytomegalovirus infection and disease in allogeneic bone marrow transplant recipients. Results of a placebo-controlled, double-blind trial. Ann Intern Med. 1993;118(3):179–184. doi: 10.7326/0003-4819-118-3-199302010-00004. [DOI] [PubMed] [Google Scholar]
- 3.Bollard CM, Kuehnle I, Leen A, Rooney CM, Heslop HE. Adoptive immunotherapy for posttransplantation viral infections. Biol Blood Marrow Transplant. 2004;10(3):143–155. doi: 10.1016/j.bbmt.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 4.Perry AR, Mackinnon S. Adoptive immunotherapy post bone-marrow transplantation. Blood Rev. 1996;10(4):237–241. doi: 10.1016/s0268-960x(96)90007-7. [DOI] [PubMed] [Google Scholar]
- 5.Kolb HJ, Mittermuller J, Clemm C, Holler E, Ledderose G, Brehm G, Heim M, Wilmanns W. Donor leukocyte transfusions for treatment of recurrent chronic myelogenous leukemia in marrow transplant patients. Blood. 1990;76(12):2462–2465. [PubMed] [Google Scholar]
- 6.Papadopoulos EB, Ladanyi M, Emanuel D, Mackinnon S, Boulad F, Carabasi MH, Castro-Malaspina H, Childs BH, Gillio AP, Small TN, et al. Infusions of donor leukocytes to treat Epstein–Barr virus-associated lymphoproliferative disorders after allogeneic bone marrow transplantation. N Engl J Med. 1994;330(17):1185–1191. doi: 10.1056/NEJM199404283301703. [DOI] [PubMed] [Google Scholar]
- 7.Heslop HE, Brenner MK, Rooney CM. Donor T cells to treat EBV-associated lymphoma. N Engl J Med. 1994;331(10):679–680. doi: 10.1056/NEJM199409083311017. [DOI] [PubMed] [Google Scholar]
- 8.Kolb HJ, Schattenberg A, Goldman JM, Hertenstein B, Jacobsen N, Arcese W, Ljungman P, Ferrant A, Verdonck L, Niederwieser D, van Rhee F, Mittermueller J, de Witte T, Holler E, Ansari H. Graft-versus-leukemia effect of donor lymphocyte transfusions in marrow grafted patients. Blood. 1995;86(5):2041–2050. [PubMed] [Google Scholar]
- 9.Lanzavecchia A, Sallusto F. Progressive differentiation and selection of the fittest in the immune response. Nat Rev Immunol. 2002;2(12):982–987. doi: 10.1038/nri959. [DOI] [PubMed] [Google Scholar]
- 10.Kaech SM, Wherry EJ, Ahmed R. Effector and memory T-cell differentiation: implications for vaccine development. Nat Rev Immunol. 2002;2(4):251–262. doi: 10.1038/nri778. [DOI] [PubMed] [Google Scholar]
- 11.Wherry EJ, Teichgraber V, Becker TC, Masopust D, Kaech SM, Antia R, von Andrian UH, Ahmed R. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol. 2003;4(3):225–234. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- 12.Riddell SR, Watanabe KS, Goodrich JM, Li CR, Agha ME, Greenberg PD. Restoration of viral immunity in immunodeficient humans by the adoptive transfer of T cell clones. Science. 1992;257(5067):238–241. doi: 10.1126/science.1352912. [DOI] [PubMed] [Google Scholar]
- 13.Riddell SR, Rabin M, Geballe AP, Britt WJ, Greenberg PD. Class I MHC-restricted cytotoxic T lymphocyte recognition of cells infected with human cytomegalovirus does not require endogenous viral gene expression. J Immunol. 1991;146(8):2795–2804. [PubMed] [Google Scholar]
- 14.Peggs K, Verfuerth S, Mackinnon S. Induction of cytomegalovirus (CMV)-specific T-cell responses using dendritic cells pulsed with CMV antigen: a novel culture system free of live CMV virions. Blood. 2001;97(4):994–1000. doi: 10.1182/blood.v97.4.994. [DOI] [PubMed] [Google Scholar]
- 15.Doubrovina E, Oflaz-Sozmen B, Prockop SE, Kernan NA, Abramson S, Teruya-Feldstein J, Hedvat C, Chou JF, Heller G, Barker JN, Boulad F, Castro-Malaspina H, George D, Jakubowski A, Koehne G, Papadopoulos EB, Scaradavou A, Small TN, Khalaf R, Young JW, O’Reilly RJ. Adoptive immunotherapy with unselected or EBV-specific T cells for biopsy-proven EBV+ lymphomas after allogeneic hematopoietic cell transplantation. Blood. 2012;119(11):2644–2656. doi: 10.1182/blood-2011-08-371971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peggs KS, Thomson K, Samuel E, Dyer G, Armoogum J, Chakraverty R, Pang K, Mackinnon S, Lowdell MW. Directly selected cytomegalovirus-reactive donor T cells confer rapid and safe systemic reconstitution of virus-specific immunity following stem cell transplantation. Clin Infect Dis. 2011;52(1):49–57. doi: 10.1093/cid/ciq042. [DOI] [PubMed] [Google Scholar]
- 17.Heslop HE, Slobod KS, Pule MA, Hale GA, Rousseau A, Smith CA, Bollard CM, Liu H, Wu MF, Rochester RJ, Amrolia PJ, Hurwitz JL, Brenner MK, Rooney CM. Long-term outcome of EBV-specific T-cell infusions to prevent or treat EBV-related lymphoproliferative disease in transplant recipients. Blood. 2010;115(5):925–935. doi: 10.1182/blood-2009-08-239186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Melenhorst JJ, Leen AM, Bollard CM, Quigley MF, Price DA, Rooney CM, Brenner MK, Barrett AJ, Heslop HE. Allogeneic virus-specific T cells with HLA alloreactivity do not produce GVHD in human subjects. Blood. 2010;116(22):4700–4702. doi: 10.1182/blood-2010-06-289991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Q, Liu H, Zhang X, Liu Q, Xing Y, Zhou X, Tong C, Zhu P. High doses of mother’s lymphocyte infusion to treat EBV-positive T-cell lymphoproliferative disorders in childhood. Blood. 2010;116(26):5941–5947. doi: 10.1182/blood-2010-01-262311. [DOI] [PubMed] [Google Scholar]
- 20.Jones K, Nourse JP, Morrison L, Nguyen-Van D, Moss DJ, Burrows SR, Gandhi MK. Expansion of EBNA1-specific effector T cells in posttransplantation lymphoproliferative disorders. Blood. 2010;116(13):2245–2252. doi: 10.1182/blood-2010-03-274076. [DOI] [PubMed] [Google Scholar]
- 21.Scheinberg P, Melenhorst JJ, Brenchley JM, Hill BJ, Hensel NF, Chattopadhyay PK, Roederer M, Picker LJ, Price DA, Barrett AJ, Douek DC. The transfer of adaptive immunity to CMV during hematopoietic stem cell transplantation is dependent on the specificity and phenotype of CMV-specific T cells in the donor. Blood. 2009;114(24):5071–5080. doi: 10.1182/blood-2009-04-214684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peggs KS, Verfuerth S, Pizzey A, Chow SL, Thomson K, Mackinnon S. Cytomegalovirus-specific T cell immunotherapy promotes restoration of durable functional antiviral immunity following allogeneic stem cell transplantation. Clin Infect Dis. 2009;49(12):1851–1860. doi: 10.1086/648422. [DOI] [PubMed] [Google Scholar]
- 23.Brestrich G, Zwinger S, Fischer A, Schmuck M, Rohmhild A, Hammer MH, Kurtz A, Uharek L, Knosalla C, Lehmkuhl H, Volk HD, Reinke P. Adoptive T-cell therapy of a lung transplanted patient with severe CMV disease and resistance to antiviral therapy. Am J Transplant. 2009;9(7):1679–1684. doi: 10.1111/j.1600-6143.2009.02672.x. [DOI] [PubMed] [Google Scholar]
- 24.Hill GR, Tey SK, Beagley L, Crough T, Morton JA, Clouston AD, Whiting P, Khanna R. Successful immunotherapy of HCMV disease using virus-specific T cells expanded from an allogeneic stem cell transplant recipient. Am J Transplant. 2009;10(1):173–179. doi: 10.1111/j.1600-6143.2009.02872.x. [DOI] [PubMed] [Google Scholar]
- 25.Ohira M, Ishiyama K, Tanaka Y, Doskali M, Igarashi Y, Tashiro H, Hiraga N, Imamura M, Sakamoto N, Asahara T, Chayama K, Ohdan H. Adoptive immunotherapy with liver allograft-derived lymphocytes induces anti-HCV activity after liver transplantation in humans and humanized mice. J Clin Invest. 2009;119(11):3226–3235. doi: 10.1172/JCI38374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leen AM, Christin A, Myers GD, Liu H, Cruz CR, Hanley PJ, Kennedy-Nasser AA, Leung KS, Gee AP, Krance RA, Brenner MK, Heslop HE, Rooney CM, Bollard CM. Cytotoxic T lymphocyte therapy with donor T cells prevents and treats adenovirus and Epstein–Barr virus infections after haploidentical and matched unrelated stem cell transplantation. Blood. 2009;114(19):4283–4292. doi: 10.1182/blood-2009-07-232454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Louis CU, Straathof K, Bollard CM, Gerken C, Huls MH, Gresik MV, Wu MF, Weiss HL, Gee AP, Brenner MK, Rooney CM, Heslop HE, Gottschalk S. Enhancing the in vivo expansion of adoptively transferred EBV-specific CTL with lymphodepleting CD45 monoclonal antibodies in NPC patients. Blood. 2009;113(11):2442–2450. doi: 10.1182/blood-2008-05-157222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Micklethwaite KP, Clancy L, Sandher U, Hansen AM, Blyth E, Antonenas V, Sartor MM, Bradstock KF, Gottlieb DJ. Prophylactic infusion of cytomegalovirus-specific cytotoxic T lymphocytes stimulated with Ad5f35pp 65 gene-modified dendritic cells after allogeneic hemopoietic stem cell transplantation. Blood. 2008;112(10):3974–3981. doi: 10.1182/blood-2008-06-161695. [DOI] [PubMed] [Google Scholar]
- 29.Bollard CM, Gottschalk S, Leen AM, Weiss H, Straathof KC, Carrum G, Khalil M, Wu MF, Huls MH, Chang CC, Gresik MV, Gee AP, Brenner MK, Rooney CM, Heslop HE. Complete responses of relapsed lymphoma following genetic modification of tumor-antigen presenting cells and T-lymphocyte transfer. Blood. 2007;110(8):2838–2845. doi: 10.1182/blood-2007-05-091280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gandhi MK, Wilkie GM, Dua U, Mollee PN, Grimmett K, Williams T, Whitaker N, Gill D, Crawford DH. Immunity, homing and efficacy of allogeneic adoptive immunotherapy for posttransplant lymphoproliferative disorders. Am J Transplant. 2007;7(5):1293–1299. doi: 10.1111/j.1600-6143.2007.01796.x. [DOI] [PubMed] [Google Scholar]
- 31.Haque T, Wilkie GM, Jones MM, Higgins CD, Urquhart G, Wingate P, Burns D, McAulay K, Turner M, Bellamy C, Amlot PL, Kelly D, MacGilchrist A, Gandhi MK, Swerdlow AJ, Crawford DH. Allogeneic cytotoxic T-cell therapy for EBV-positive posttransplantation lymphoproliferative disease: results of a phase 2 multicenter clinical trial. Blood. 2007;110(4):1123–1131. doi: 10.1182/blood-2006-12-063008. [DOI] [PubMed] [Google Scholar]
- 32.Amrolia PJ, Muccioli-Casadei G, Huls H, Adams S, Durett A, Gee A, Yvon E, Weiss H, Cobbold M, Gaspar HB, Rooney C, Kuehnle I, Ghetie V, Schindler J, Krance R, Heslop HE, Veys P, Vitetta E, Brenner MK. Adoptive immunotherapy with allodepleted donor T-cells improves immune reconstitution after haploidentical stem cell transplantation. Blood. 2006;108(6):1797–1808. doi: 10.1182/blood-2006-02-001909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perruccio K, Tosti A, Burchielli E, Topini F, Ruggeri L, Carotti A, Capanni M, Urbani E, Mancusi A, Aversa F, Martelli MF, Romani L, Velardi A. Transferring functional immune responses to pathogens after haploidentical hematopoietic transplantation. Blood. 2005;106(13):4397–4406. doi: 10.1182/blood-2005-05-1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Comoli P, Pedrazzoli P, Maccario R, Basso S, Carminati O, Labirio M, Schiavo R, Secondino S, Frasson C, Perotti C, Moroni M, Locatelli F, Siena S. Cell therapy of stage IV nasopharyngeal carcinoma with autologous Epstein–Barr virus-targeted cytotoxic T lymphocytes. J Clin Oncol. 2005;23(35):8942–8949. doi: 10.1200/JCO.2005.02.6195. [DOI] [PubMed] [Google Scholar]
- 35.Bollard CM, Aguilar L, Straathof KC, Gahn B, Huls MH, Rousseau A, Sixbey J, Gresik MV, Carrum G, Hudson M, Dilloo D, Gee A, Brenner MK, Rooney CM, Heslop HE. Cytotoxic T lymphocyte therapy for Epstein–Barr virus+ Hodgkin’s disease. J Exp Med. 2004;200(12):1623–1633. doi: 10.1084/jem.20040890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Einsele H, Roosnek E, Rufer N, Sinzger C, Riegler S, Loffler J, Grigoleit U, Moris A, Rammensee HG, Kanz L, Kleihauer A, Frank F, Jahn G, Hebart H. Infusion of cytomegalovirus (CMV)-specific T cells for the treatment of CMV infection not responding to antiviral chemotherapy. Blood. 2002;99(11):3916–3922. doi: 10.1182/blood.v99.11.3916. [DOI] [PubMed] [Google Scholar]
- 37.Savoldo B, Huls MH, Liu Z, Okamura T, Volk HD, Reinke P, Sabat R, Babel N, Jones JF, Webster-Cyriaque J, Gee AP, Brenner MK, Heslop HE, Rooney CM. Autologous Epstein–Barr virus (EBV)-specific cytotoxic T cells for the treatment of persistent active EBV infection. Blood. 2002;100(12):4059–4066. doi: 10.1182/blood-2002-01-0039. [DOI] [PubMed] [Google Scholar]
- 38.Lin CL, Lo WF, Lee TH, Ren Y, Hwang SL, Cheng YF, Chen CL, Chang YS, Lee SP, Rickinson AB, Tam PK. Immunization with Epstein–Barr Virus (EBV) peptide-pulsed dendritic cells induces functional CD8+ T-cell immunity and may lead to tumor regression in patients with EBV-positive nasopharyngeal carcinoma. Cancer Res. 2002;62(23):6952–6958. [PubMed] [Google Scholar]
- 39.Haque T, Wilkie GM, Taylor C, Amlot PL, Murad P, Iley A, Dombagoda D, Britton KM, Swerdlow AJ, Crawford DH. Treatment of Epstein–Barr-virus-positive post-transplantation lymphoproliferative disease with partly HLA-matched allogeneic cytotoxic T cells. Lancet. 2002;360(9331):436–442. doi: 10.1016/S0140-6736(02)09672-1. [DOI] [PubMed] [Google Scholar]
- 40.Mitsuyasu RT, Anton PA, Deeks SG, Scadden DT, Connick E, Downs MT, Bakker A, Roberts MR, June CH, Jalali S, Lin AA, Pennathur-Das R, Hege KM. Prolonged survival and tissue trafficking following adoptive transfer of CD4zeta gene-modified autologous CD4(+) and CD8(+) T cells in human immunodeficiency virus-infected subjects. Blood. 2000;96(3):785–793. [PubMed] [Google Scholar]
- 41.Gustafsson A, Levitsky V, Zou JZ, Frisan T, Dalianis T, Ljungman P, Ringden O, Winiarski J, Ernberg I, Masucci MG. Epstein–Barr virus (EBV) load in bone marrow transplant recipients at risk to develop posttransplant lymphoproliferative disease: prophylactic infusion of EBV-specific cytotoxic T cells. Blood. 2000;95(3):807–814. [PubMed] [Google Scholar]
- 42.Small TN, Papadopoulos EB, Boulad F, Black P, Castro-Malaspina H, Childs BH, Collins N, Gillio A, George D, Jakubowski A, Heller G, Fazzari M, Kernan N, MacKinnon S, Szabolcs P, Young JW, O’Reilly RJ. Comparison of immune reconstitution after unrelated and related T-cell-depleted bone marrow transplantation: effect of patient age and donor leukocyte infusions. Blood. 1999;93(2):467–480. [PubMed] [Google Scholar]
- 43.Rooney CM, Smith CA, Ng CY, Loftin SK, Sixbey JW, Gan Y, Srivastava DK, Bowman LC, Krance RA, Brenner MK, Heslop HE. Infusion of cytotoxic T cells for the prevention and treatment of Epstein–Barr virus-induced lymphoma in allogeneic transplant recipients. Blood. 1998;92(5):1549–1555. [PubMed] [Google Scholar]
- 44.Rooney CM, Roskrow MA, Smith CA, Brenner MK, Heslop HE. Immunotherapy for Epstein–Barr virus-associated cancers. J Natl Cancer Inst Monogr. 1998;23:89–93. doi: 10.1093/oxfordjournals.jncimonographs.a024180. [DOI] [PubMed] [Google Scholar]
- 45.Roskrow MA, Suzuki N, Gan Y, Sixbey JW, Ng CY, Kimbrough S, Hudson M, Brenner MK, Heslop HE, Rooney CM. Epstein–Barr virus (EBV)-specific cytotoxic T lymphocytes for the treatment of patients with EBV-positive relapsed Hodgkin’s disease. Blood. 1998;91(8):2925–2934. [PubMed] [Google Scholar]
- 46.O’Reilly RJ, Small TN, Papadopoulos E, Lucas K, Lacerda J, Koulova L. Biology and adoptive cell therapy of Epstein–Barr virus-associated lymphoproliferative disorders in recipients of marrow allografts. Immunol Rev. 1997;157:195–216. doi: 10.1111/j.1600-065x.1997.tb00983.x. [DOI] [PubMed] [Google Scholar]
- 47.Rooney CM, Smith CA, Ng CY, Loftin S, Li C, Krance RA, Brenner MK, Heslop HE. Use of gene-modified virus-specific T lymphocytes to control Epstein–Barr-virus-related lymphoproliferation. Lancet. 1995;345(8941):9–13. doi: 10.1016/s0140-6736(95)91150-2. [DOI] [PubMed] [Google Scholar]
- 48.Bex F, Hermans P, Sprecher S, Achour A, Badjou R, Desgranges C, Cogniaux J, Franchioli P, Vanhulle C, Lachgar A, et al. Syngeneic adoptive transfer of anti-human immunodeficiency virus (HIV-1)-primed lymphocytes from a vaccinated HIV-seronegative individual to his HIV-1-infected identical twin. Blood. 1994;84(10):3317–3326. [PubMed] [Google Scholar]
- 49.Ho M, Armstrong J, McMahon D, Pazin G, Huang XL, Rinaldo C, Whiteside T, Tripoli C, Levine G, Moody D, et al. A phase 1 study of adoptive transfer of autologous CD8+ T lymphocytes in patients with acquired immunodeficiency syndrome (AIDS)-related complex or AIDS. Blood. 1993;81(8):2093–2101. [PubMed] [Google Scholar]
- 50.Walter EA, Greenberg PD, Gilbert MJ, Finch RJ, Watanabe KS, Thomas ED, Riddell SR. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. N Engl J Med. 1995;333(16):1038–1044. doi: 10.1056/NEJM199510193331603. [DOI] [PubMed] [Google Scholar]
- 51.Heslop HE, Ng CY, Li C, Smith CA, Loftin SK, Krance RA, Brenner MK, Rooney CM. Long-term restoration of immunity against Epstein–Barr virus infection by adoptive transfer of gene-modified virus-specific T lymphocytes. Nat Med. 1996;2(5):551–555. doi: 10.1038/nm0596-551. [DOI] [PubMed] [Google Scholar]
- 52.Peggs KS, Verfuerth S, Pizzey A, Khan N, Guiver M, Moss PA, Mackinnon S. Adoptive cellular therapy for early cytomegalovirus infection after allogeneic stem-cell transplantation with virus-specific T-cell lines. Lancet. 2003;362(9393):1375–1377. doi: 10.1016/S0140-6736(03)14634-X. [DOI] [PubMed] [Google Scholar]
- 53.Liechtenstein T, Dufait I, Lanna A, Breckpot K, Escors D. Modulating co-stimulation during antigen presentation to enhance cancer immunotherapy. Immunol Endocr Metab Agents Med Chem. 2013;12(3):224–235. doi: 10.2174/187152212802001875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sun Q, Pollok KE, Burton RL, Dai LJ, Britt W, Emanuel DJ, Lucas KG. Simultaneous ex vivo expansion of cytomegalovirus and Epstein–Barr virus-specific cytotoxic T lymphocytes using B-lymphoblastoid cell lines expressing cytomegalovirus pp 65. Blood. 1999;94(9):3242–3250. [PubMed] [Google Scholar]
- 55.Leen AM, Myers GD, Sili U, Huls MH, Weiss H, Leung KS, Carrum G, Krance RA, Chang CC, Molldrem JJ, Gee AP, Brenner MK, Heslop HE, Rooney CM, Bollard CM. Monoculture-derived T lymphocytes specific for multiple viruses expand and produce clinically relevant effects in immunocompromised individuals. Nat Med. 2006;12(10):1160–1166. doi: 10.1038/nm1475. [DOI] [PubMed] [Google Scholar]
- 56.Dembic Z, Haas W, Weiss S, McCubrey J, Kiefer H, von Boehmer H, Steinmetz M. Transfer of specificity by murine alpha and beta T-cell receptor genes. Nature. 1986;320(6059):232–238. doi: 10.1038/320232a0. [DOI] [PubMed] [Google Scholar]
- 57.Gehring AJ, Xue SA, Ho ZZ, Teoh D, Ruedl C, Chia A, Koh S, Lim SG, Maini MK, Stauss H, Bertoletti A. Engineering virus-specific T cells that target HBV infected hepatocytes and hepatocellular carcinoma cell lines. J Hepatol. 2011;55(1):103–110. doi: 10.1016/j.jhep.2010.10.025. [DOI] [PubMed] [Google Scholar]
- 58.Wood NJ. Immunotherapy: therapeutic potential of genetically modified HBV-specific T cells for chronic HBV infection and HBV-related HCC. Nat Rev. 2011;8(2):61. doi: 10.1038/nrgastro.2010.221. [DOI] [PubMed] [Google Scholar]
- 59.Kessels HW, Wolkers MC, van den Boom MD, van der Valk MA, Schumacher TN. Immunotherapy through TCR gene transfer. Nat Immunol. 2001;2(10):957–961. doi: 10.1038/ni1001-957. [DOI] [PubMed] [Google Scholar]
- 60.Johnson LA, Morgan RA, Dudley ME, Cassard L, Yang JC, Hughes MS, Kammula US, Royal RE, Sherry RM, Wunderlich JR, Lee CC, Restifo NP, Schwarz SL, Cogdill AP, Bishop RJ, Kim H, Brewer CC, Rudy SF, VanWaes C, Davis JL, Mathur A, Ripley RT, Nathan DA, Laurencot CM, Rosenberg SA. Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood. 2009;114(3):535–546. doi: 10.1182/blood-2009-03-211714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Heemskerk MH, Hoogeboom M, Hagedoorn R, Kester MG, Willemze R, Falkenburg JH. Reprogramming of virus-specific T cells into leukemia-reactive T cells using T cell receptor gene transfer. J Exp Med. 2004;199(7):885–894. doi: 10.1084/jem.20031110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cohen CJ, Li YF, El-Gamil M, Robbins PF, Rosenberg SA, Morgan RA. Enhanced antitumor activity of T cells engineered to express T-cell receptors with a second disulfide bond. Cancer Res. 2007;67(8):3898–3903. doi: 10.1158/0008-5472.CAN-06-3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cohen CJ, Zhao Y, Zheng Z, Rosenberg SA, Morgan RA. Enhanced antitumor activity of murine-human hybrid T-cell receptor (TCR) in human lymphocytes is associated with improved pairing and TCR/CD3 stability. Cancer Res. 2006;66(17):8878–8886. doi: 10.1158/0008-5472.CAN-06-1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bialer G, Horovitz-Fried M, Ya’acobi S, Morgan RA, Cohen CJ. Selected murine residues endow human TCR with enhanced tumor recognition. J Immunol. 2010;184(11):6232–6241. doi: 10.4049/jimmunol.0902047. [DOI] [PubMed] [Google Scholar]
- 65.Kandalaft LE, Powell DJ, Jr, Coukos G. A phase I clinical trial of adoptive transfer of folate receptor-alpha redirected autologous T cells for recurrent ovarian cancer. J Transl Med. 2012;10:157. doi: 10.1186/1479-5876-10-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brenner MK, Heslop HE. Adoptive T cell therapy of cancer. Curr Opin Immunol. 2010;22(2):251–257. doi: 10.1016/j.coi.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jena B, Dotti G, Cooper LJ. Redirecting T-cell specificity by introducing a tumor-specific chimeric antigen receptor. Blood. 2010;116(7):1035–1044. doi: 10.1182/blood-2010-01-043737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Micklethwaite KP, Savoldo B, Hanley PJ, Leen AM, Demmler-Harrison GJ, Cooper LJ, Liu H, Gee AP, Shpall EJ, Rooney CM, Heslop HE, Brenner MK, Bollard CM, Dotti G. Derivation of human T lymphocytes from cord blood and peripheral blood with antiviral and antileukemic specificity from a single culture as protection against infection and relapse after stem cell transplantation. Blood. 2010;115(13):2695–2703. doi: 10.1182/blood-2009-09-242263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.De Oliveira SN, Wang J, Ryan C, Morrison SL, Kohn DB, Hollis RP. A CD19/Fc fusion protein for detection of anti-CD19 chimeric antigen receptors. J Transl Med. 2013;11(1):23. doi: 10.1186/1479-5876-11-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tamada K, Geng D, Sakoda Y, Bansal N, Srivastava R, Li Z, Davila E. Redirecting gene-modified T cells toward various cancer types using tagged antibodies. Clin Cancer Res. 2012;18(23):6436–6445. doi: 10.1158/1078-0432.CCR-12-1449. [DOI] [PubMed] [Google Scholar]
- 71.Louis CU, Savoldo B, Dotti G, Pule M, Yvon E, Myers GD, Rossig C, Russell HV, Diouf O, Liu E, Liu H, Wu MF, Gee AP, Mei Z, Rooney CM, Heslop HE, Brenner MK. Anti-tumor activity and long-term fate of chimeric antigen receptor positive T-cells in patients with neuroblastoma. Blood. 2011;118(23):6050–6056. doi: 10.1182/blood-2011-05-354449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pule MA, Savoldo B, Myers GD, Rossig C, Russell HV, Dotti G, Huls MH, Liu E, Gee AP, Mei Z, Yvon E, Weiss HL, Liu H, Rooney CM, Heslop HE, Brenner MK. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat Med. 2008;14(11):1264–1270. doi: 10.1038/nm.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kershaw MH, Westwood JA, Parker LL, Wang G, Eshhar Z, Mavroukakis SA, White DE, Wunderlich JR, Canevari S, Rogers-Freezer L, Chen CC, Yang JC, Rosenberg SA, Hwu P. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin Cancer Res. 2006;12(20 Pt 1):6106–6115. doi: 10.1158/1078-0432.CCR-06-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shi H, Liu L, Wang Z. Improving the efficacy and safety of engineered T cell therapy for cancer. Cancer Lett. 2013;328(2):191–197. doi: 10.1016/j.canlet.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 75.Song DG, Ye Q, Carpenito C, Poussin M, Wang LP, Ji C, Figini M, June CH, Coukos G, Powell DJ., Jr In vivo persistence, tumor localization, and antitumor activity of CAR-engineered T cells is enhanced by costimulatory signalling through CD137 (4-1BB) Cancer Res. 2011;71(13):4617–4627. doi: 10.1158/0008-5472.CAN-11-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kalos M, Levine BL, Porter DL, Katz S, Grupp SA, Bagg A, June CH. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med. 2011;3(95):95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365(8):725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brentjens R, Yeh R, Bernal Y, Riviere I, Sadelain M. Treatment of chronic lymphocytic leukemia with genetically targeted autologous T cells: case report of an unforeseen adverse event in a phase I clinical trial. Mol Ther. 2010;18(4):666–668. doi: 10.1038/mt.2010.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, Teachey DT, Chew A, Hauck B, Wright JF, Milone MC, Levine BL, June CH. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368(16):1509–1518. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 2010;18(4):843–851. doi: 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Heslop HE. Safer CARS. Mol Ther. 2010;18(4):661–662. doi: 10.1038/mt.2010.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bonini C, Ferrari G, Verzeletti S, Servida P, Zappone E, Ruggieri L, Ponzoni M, Rossini S, Mavilio F, Traversari C, Bordignon C. HSV-TK gene transfer into donor lymphocytes for control of allogeneic graft-versus-leukemia. Science. 1997;276(5319):1719–1724. doi: 10.1126/science.276.5319.1719. [DOI] [PubMed] [Google Scholar]
- 83.Ciceri F, Bonini C, Marktel S, Zappone E, Servida P, Bernardi M, Pescarollo A, Bondanza A, Peccatori J, Rossini S, Magnani Z, Salomoni M, Benati C, Ponzoni M, Callegaro L, Corradini P, Bregni M, Traversari C, Bordignon C. Antitumor effects of HSV-TK-engineered donor lymphocytes after allogeneic stem-cell transplantation. Blood. 2007;109(11):4698–4707. doi: 10.1182/blood-2006-05-023416. [DOI] [PubMed] [Google Scholar]
- 84.Ciceri F, Bonini C, Stanghellini MT, Bondanza A, Traversari C, Salomoni M, Turchetto L, Colombi S, Bernardi M, Peccatori J, Pescarollo A, Servida P, Magnani Z, Perna SK, Valtolina V, Crippa F, Callegaro L, Spoldi E, Crocchiolo R, Fleischhauer K, Ponzoni M, Vago L, Rossini S, Santoro A, Todisco E, Apperley J, Olavarria E, Slavin S, Weissinger EM, Ganser A, Stadler M, Yannaki E, Fassas A, Anagnostopoulos A, Bregni M, Stampino CG, Bruzzi P, Bordignon C. Infusion of suicide-gene-engineered donor lymphocytes after family haploidentical haemopoietic stem-cell transplantation for leukaemia (the TK007 trial): a non-randomised phase I–II study. Lancet Oncol. 2009;10(5):489–500. doi: 10.1016/S1470-2045(09)70074-9. [DOI] [PubMed] [Google Scholar]
- 85.Di Stasi A, Tey SK, Dotti G, Fujita Y, Kennedy-Nasser A, Martinez C, Straathof K, Liu E, Durett AG, Grilley B, Liu H, Cruz CR, Savoldo B, Gee AP, Schindler J, Krance RA, Heslop HE, Spencer DM, Rooney CM, Brenner MK. Inducible apoptosis as a safety switch for adoptive cell therapy. N Engl J Med. 2011;365(18):1673–1683. doi: 10.1056/NEJMoa1106152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Straathof KC, Pule MA, Yotnda P, Dotti G, Vanin EF, Brenner MK, Heslop HE, Spencer DM, Rooney CM. An inducible caspase 9 safety switch for T-cell therapy. Blood. 2005;105(11):4247–4254. doi: 10.1182/blood-2004-11-4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tey SK, Dotti G, Rooney CM, Heslop HE, Brenner MK. Inducible caspase 9 suicide gene to improve the safety of allodepleted T cells after haploidentical stem cell transplantation. Biol Blood Marrow Transplant. 2007;13(8):913–924. doi: 10.1016/j.bbmt.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Quintarelli C, Vera JF, Savoldo B, Giordano Attianese GM, Pule M, Foster AE, Heslop HE, Rooney CM, Brenner MK, Dotti G. Co-expression of cytokine and suicide genes to enhance the activity and safety of tumor-specific cytotoxic T lymphocytes. Blood. 2007;110(8):2793–2802. doi: 10.1182/blood-2007-02-072843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ramirez N, Olavarria E (2013) Viral-specific adoptive immunotherapy after allo-SCT: the role of multimer-based selection strategies. Bone Marrow Transplant. doi:10.1038/bmt.2012.262 [Epub ahead of print] [DOI] [PubMed]
- 90.Cobbold M, Khan N, Pourgheysari B, Tauro S, McDonald D, Osman H, Assenmacher M, Billingham L, Steward C, Crawley C, Olavarria E, Goldman J, Chakraverty R, Mahendra P, Craddock C, Moss PA. Adoptive transfer of cytomegalovirus-specific CTL to stem cell transplant patients after selection by HLA-peptide tetramers. J Exp Med. 2005;202(3):379–386. doi: 10.1084/jem.20040613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Uhlin M, Okas M, Gertow J, Uzunel M, Brismar TB, Mattsson J. A novel haplo-identical adoptive CTL therapy as a treatment for EBV-associated lymphoma after stem cell transplantation. Cancer Immunol Immunother. 2009;59(3):473–477. doi: 10.1007/s00262-009-0789-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Casalegno-Garduno R, Schmitt A, Yao J, Wang X, Xu X, Freund M, Schmitt M. Multimer technologies for detection and adoptive transfer of antigen-specific T cells. Cancer Immunol Immunother. 2010;59(2):195–202. doi: 10.1007/s00262-009-0778-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Morita-Hoshi Y, Heike Y, Kawakami M, Sugita T, Miura O, Kim SW, Mori SI, Fukuda T, Tanosaki R, Tobinai K, Takaue Y. Functional analysis of cytomegalovirus-specific T lymphocytes compared to tetramer assay in patients undergoing hematopoietic stem cell transplantation. Bone Marrow Transplant. 2008;41(6):515–521. doi: 10.1038/sj.bmt.1705932. [DOI] [PubMed] [Google Scholar]
- 94.Assenmacher M, Scheffold A, Schmitz J, Segura Checa JA, Miltenyi S, Radbruch A. Specific expression of surface interferon-gamma on interferon-gamma producing T cells from mouse and man. Eur J Immunol. 1996;26(1):263–267. doi: 10.1002/eji.1830260141. [DOI] [PubMed] [Google Scholar]
- 95.Manz R, Assenmacher M, Pfluger E, Miltenyi S, Radbruch A. Analysis and sorting of live cells according to secreted molecules, relocated to a cell-surface affinity matrix. Proc Natl Acad Sci USA. 1995;92(6):1921–1925. doi: 10.1073/pnas.92.6.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Assenmacher M, Lohning M, Scheffold A, Manz RA, Schmitz J, Radbruch A. Sequential production of IL-2, IFN-gamma and IL-10 by individual staphylococcal enterotoxin B-activated T helper lymphocytes. Eur J Immunol. 1998;28(5):1534–1543. doi: 10.1002/(SICI)1521-4141(199805)28:05<1534::AID-IMMU1534>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 97.Moosmann A, Bigalke I, Tischer J, Schirrmann L, Kasten J, Tippmer S, Leeping M, Prevalsek D, Jaeger G, Ledderose G, Mautner J, Hammerschmidt W, Schendel DJ, Kolb HJ. Effective and long-term control of EBV PTLD after transfer of peptide-selected T cells. Blood. 2010;115(14):2960–2970. doi: 10.1182/blood-2009-08-236356. [DOI] [PubMed] [Google Scholar]
- 98.Feuchtinger T, Opherk K, Bethge WA, Topp MS, Schuster FR, Weissinger EM, Mohty M, Or R, Maschan M, Schumm M, Hamprecht K, Handgretinger R, Lang P, Einsele H. Adoptive transfer of pp 65-specific T cells for the treatment of chemorefractory cytomegalovirus disease or reactivation after haploidentical and matched unrelated stem cell transplantation. Blood. 2010;116(20):4360–4367. doi: 10.1182/blood-2010-01-262089. [DOI] [PubMed] [Google Scholar]
- 99.Brosterhus H, Brings S, Leyendeckers H, Manz RA, Miltenyi S, Radbruch A, Assenmacher M, Schmitz J. Enrichment and detection of live antigen-specific CD4(+) and CD8(+) T cells based on cytokine secretion. Eur J Immunol. 1999;29(12):4053–4059. doi: 10.1002/(SICI)1521-4141(199912)29:12<4053::AID-IMMU4053>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 100.Desombere I, Meuleman P, Rigole H, Willems A, Irsch J, Leroux-Roels G. The interferon gamma secretion assay: a reliable tool to study interferon gamma production at the single cell level. J Immunol Methods. 2004;286(1–2):167–185. doi: 10.1016/j.jim.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 101.Jedema I, Meij P, Steeneveld E, Hoogendoorn M, Nijmeijer BA, van de Meent M, van Luxemburg-Heijs SA, Willemze R, Falkenburg JH. Early detection and rapid isolation of leukemia-reactive donor T cells for adoptive transfer using the IFN-gamma secretion assay. Clin Cancer Res. 2007;13(2 Pt 1):636–643. doi: 10.1158/1078-0432.CCR-06-2093. [DOI] [PubMed] [Google Scholar]
- 102.Mutis T, Gillespie G, Schrama E, Falkenburg JH, Moss P, Goulmy E. Tetrameric HLA class I-minor histocompatibility antigen peptide complexes demonstrate minor histocompatibility antigen-specific cytotoxic T lymphocytes in patients with graft-versus-host disease. Nat Med. 1999;5(7):839–842. doi: 10.1038/10563. [DOI] [PubMed] [Google Scholar]
- 103.Wang X, Schmitt A, Chen B, Xu X, Mani J, Linnebacher M, Freund M, Schmitt M. Streptamer-based selection of WT1-specific CD8+ T cells for specific donor lymphocyte infusions. Exp Hematol. 2010;38(11):1066–1073. doi: 10.1016/j.exphem.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 104.Kohn DB, Dotti G, Brentjens R, Savoldo B, Jensen M, Cooper LJ, June CH, Rosenberg S, Sadelain M, Heslop HE. CARs on track in the clinic. Mol Ther. 2011;19(3):432–438. doi: 10.1038/mt.2011.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gattinoni L, Klebanoff CA, Palmer DC, Wrzesinski C, Kerstann K, Yu Z, Finkelstein SE, Theoret MR, Rosenberg SA, Restifo NP. Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8+ T cells. J Clin Invest. 2005;115(6):1616–1626. doi: 10.1172/JCI24480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Berger C, Jensen MC, Lansdorp PM, Gough M, Elliott C, Riddell SR. Adoptive transfer of effector CD8+ T cells derived from central memory cells establishes persistent T cell memory in primates. J Clin Invest. 2008;118(1):294–305. doi: 10.1172/JCI32103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 108.Fiorenza S, Kenna TJ, Comerford I, McColl S, Steptoe RJ, Leggatt GR, Frazer IH. A combination of local inflammation and central memory T cells potentiates immunotherapy in the skin. J Immunol. 2012;189(12):5622–5631. doi: 10.4049/jimmunol.1200709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang X, Naranjo A, Brown CE, Bautista C, Wong CW, Chang WC, Aguilar B, Ostberg JR, Riddell SR, Forman SJ, Jensen MC. Phenotypic and functional attributes of lentivirus-modified CD19-specific human CD8+ central memory T cells manufactured at clinical scale. J Immunother. 2012;35(9):689–701. doi: 10.1097/CJI.0b013e318270dec7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Terakura S, Yamamoto TN, Gardner RA, Turtle CJ, Jensen MC, Riddell SR. Generation of CD19-chimeric antigen receptor modified CD8+ T cells derived from virus-specific central memory T cells. Blood. 2012;119(1):72–82. doi: 10.1182/blood-2011-07-366419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hinrichs CS, Borman ZA, Cassard L, Gattinoni L, Spolski R, Yu Z, Sanchez-Perez L, Muranski P, Kern SJ, Logun C, Palmer DC, Ji Y, Reger RN, Leonard WJ, Danner RL, Rosenberg SA, Restifo NP. Adoptively transferred effector cells derived from naive rather than central memory CD8+ T cells mediate superior antitumor immunity. Proc Natl Acad Sci USA. 2009;106(41):17469–17474. doi: 10.1073/pnas.0907448106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hinrichs CS, Borman ZA, Gattinoni L, Yu Z, Burns WR, Huang J, Klebanoff CA, Johnson LA, Kerkar SP, Yang S, Muranski P, Palmer DC, Scott CD, Morgan RA, Robbins PF, Rosenberg SA, Restifo NP. Human effector CD8+ T cells derived from naive rather than memory subsets possess superior traits for adoptive immunotherapy. Blood. 2011;117(3):808–814. doi: 10.1182/blood-2010-05-286286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hanley PJ, Cruz CR, Savoldo B, Leen AM, Stanojevic M, Khalil M, Decker W, Molldrem JJ, Liu H, Gee AP, Rooney CM, Heslop HE, Dotti G, Brenner MK, Shpall EJ, Bollard CM. Functionally active virus-specific T cells that target CMV, adenovirus, and EBV can be expanded from naive T-cell populations in cord blood and will target a range of viral epitopes. Blood. 2009;114(9):1958–1967. doi: 10.1182/blood-2009-03-213256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Distler E, Wolfel C, Kohler S, Nonn M, Kaus N, Schnurer E, Meyer RG, Wehler TC, Huber C, Wolfel T, Hartwig UF, Herr W. Acute myeloid leukemia (AML)-reactive cytotoxic T lymphocyte clones rapidly expanded from CD8(+) CD62L((high)+) T cells of healthy donors prevent AML engraftment in NOD/SCID IL2Rgamma(null) mice. Exp Hematol. 2008;36(4):451–463. doi: 10.1016/j.exphem.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 115.Gattinoni L, Powell DJ, Jr, Rosenberg SA, Restifo NP. Adoptive immunotherapy for cancer: building on success. Nat Rev Immunol. 2006;6(5):383–393. doi: 10.1038/nri1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Speiser DE, Romero P. Toward improved immunocompetence of adoptively transferred CD8+ T cells. J Clin Invest. 2005;115(6):1467–1469. doi: 10.1172/JCI25427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Anderson BE, McNiff J, Yan J, Doyle H, Mamula M, Shlomchik MJ, Shlomchik WD. Memory CD4+ T cells do not induce graft-versus-host disease. J Clin Invest. 2003;112(1):101–108. doi: 10.1172/JCI17601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Beilhack A, Schulz S, Baker J, Beilhack GF, Wieland CB, Herman EI, Baker EM, Cao YA, Contag CH, Negrin RS. In vivo analyses of early events in acute graft-versus-host disease reveal sequential infiltration of T-cell subsets. Blood. 2005;106(3):1113–1122. doi: 10.1182/blood-2005-02-0509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Distler E, Bloetz A, Albrecht J, Asdufan S, Hohberger A, Frey M, Schnurer E, Thomas S, Theobald M, Hartwig UF, Herr W. Alloreactive and leukemia-reactive T cells are preferentially derived from naive precursors in healthy donors: implications for immunotherapy with memory T cells. Haematologica. 2011;96(7):1024–1032. doi: 10.3324/haematol.2010.037481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gattinoni L, Zhong XS, Palmer DC, Ji Y, Hinrichs CS, Yu Z, Wrzesinski C, Boni A, Cassard L, Garvin LM, Paulos CM, Muranski P, Restifo NP. Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells. Nat Med. 2009;15(7):808–813. doi: 10.1038/nm.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gattinoni L, Lugli E, Ji Y, Pos Z, Paulos CM, Quigley MF, Almeida JR, Gostick E, Yu Z, Carpenito C, Wang E, Douek DC, Price DA, June CH, Marincola FM, Roederer M, Restifo NP. A human memory T cell subset with stem cell-like properties. Nat Med. 2011;17(10):1290–1297. doi: 10.1038/nm.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sallusto F, Lanzavecchia A. Memory in disguise. Nat Med. 2011;17(10):1182–1183. doi: 10.1038/nm.2502. [DOI] [PubMed] [Google Scholar]
- 123.Gattinoni L, Finkelstein SE, Klebanoff CA, Antony PA, Palmer DC, Spiess PJ, Hwang LN, Yu Z, Wrzesinski C, Heimann DM, Surh CD, Rosenberg SA, Restifo NP. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J Exp Med. 2005;202(7):907–912. doi: 10.1084/jem.20050732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, Topalian SL, Sherry R, Restifo NP, Hubicki AM, Robinson MR, Raffeld M, Duray P, Seipp CA, Rogers-Freezer L, Morton KE, Mavroukakis SA, White DE, Rosenberg SA. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298(5594):850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jacobs SR, Michalek RD, Rathmell JC. IL-7 is essential for homeostatic control of T cell metabolism in vivo. J Immunol. 2010;184(7):3461–3469. doi: 10.4049/jimmunol.0902593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dudley ME, Yang JC, Sherry R, Hughes MS, Royal R, Kammula U, Robbins PF, Huang J, Citrin DE, Leitman SF, Wunderlich J, Restifo NP, Thomasian A, Downey SG, Smith FO, Klapper J, Morton K, Laurencot C, White DE, Rosenberg SA. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008;26(32):5233–5239. doi: 10.1200/JCO.2008.16.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Muranski P, Boni A, Wrzesinski C, Citrin DE, Rosenberg SA, Childs R, Restifo NP. Increased intensity lymphodepletion and adoptive immunotherapy—how far can we go? Nat Clin Pract Oncol. 2006;3(12):668–681. doi: 10.1038/ncponc0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Brusko TM, Putnam AL, Bluestone JA. Human regulatory T cells: role in autoimmune disease and therapeutic opportunities. Immunol Rev. 2008;223:371–390. doi: 10.1111/j.1600-065X.2008.00637.x. [DOI] [PubMed] [Google Scholar]
- 129.Morita R, Hirohashi Y, Sato N. Depletion of Tregs in vivo: a promising approach to enhance antitumor immunity without autoimmunity. Immunotherapy. 2012;4(11):1103–1105. doi: 10.2217/imt.12.116. [DOI] [PubMed] [Google Scholar]
- 130.Rech AJ, Mick R, Martin S, Recio A, Aqui NA, Powell DJ, Jr, Colligon TA, Trosko JA, Leinbach LI, Pletcher CH, Tweed CK, DeMichele A, Fox KR, Domchek SM, Riley JL, Vonderheide RH. CD25 blockade depletes and selectively reprograms regulatory T cells in concert with immunotherapy in cancer patients. Sci Transl Med. 2012;4(134):134ra162. doi: 10.1126/scitranslmed.3003330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Unutmaz D, Pileri P, Abrignani S. Antigen-independent activation of naive and memory resting T cells by a cytokine combination. J Exp Med. 1994;180(3):1159–1164. doi: 10.1084/jem.180.3.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Buentke E, Mathiot A, Tolaini M, Di Santo J, Zamoyska R, Seddon B. Do CD8 effector cells need IL-7R expression to become resting memory cells? Blood. 2006;108(6):1949–1956. doi: 10.1182/blood-2006-04-016857. [DOI] [PubMed] [Google Scholar]
- 133.Shen CH, Ge Q, Talay O, Eisen HN, Garcia-Sastre A, Chen J. Loss of IL-7R and IL-15R expression is associated with disappearance of memory T cells in respiratory tract following influenza infection. J Immunol. 2008;180(1):171–178. doi: 10.4049/jimmunol.180.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mackall C, Fry T, Gress R, Peggs K, Storek J, Toubert A. Background to hematopoietic cell transplantation, including post transplant immune recovery. Bone Marrow Transplant. 2009;44(8):457–462. doi: 10.1038/bmt.2009.255. [DOI] [PubMed] [Google Scholar]
- 135.Hakki M, Riddell SR, Storek J, Carter RA, Stevens-Ayers T, Sudour P, White K, Corey L, Boeckh M. Immune reconstitution to cytomegalovirus after allogeneic hematopoietic stem cell transplantation: impact of host factors, drug therapy, and subclinical reactivation. Blood. 2003;102(8):3060–3067. doi: 10.1182/blood-2002-11-3472. [DOI] [PubMed] [Google Scholar]
- 136.Rosenberg ES, Billingsley JM, Caliendo AM, Boswell SL, Sax PE, Kalams SA, Walker BD. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science. 1997;278(5342):1447–1450. doi: 10.1126/science.278.5342.1447. [DOI] [PubMed] [Google Scholar]
- 137.Teramoto K, Ohshio Y, Fujita T, Hanaoka J, Kontani K. Simultaneous activation of T helper function can augment the potency of dendritic cell-based cancer immunotherapy. J Cancer Res Clin Oncol. 2013;139(5):861–870. doi: 10.1007/s00432-013-1394-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ayyoub M, Dojcinovic D, Pignon P, Raimbaud I, Schmidt J, Luescher I, Valmori D. Monitoring of NY-ESO-1 specific CD4+ T cells using molecularly defined MHC class II/His-tag-peptide tetramers. Proc Natl Acad Sci USA. 2010;107(16):7437–7442. doi: 10.1073/pnas.1001322107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Quezada SA, Simpson TR, Peggs KS, Merghoub T, Vider J, Fan X, Blasberg R, Yagita H, Muranski P, Antony PA, Restifo NP, Allison JP. Tumor-reactive CD4(+) T cells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopenic hosts. J Exp Med. 2010;207(3):637–650. doi: 10.1084/jem.20091918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Vanasek TL, Nandiwada SL, Jenkins MK, Mueller DL. CD25+ Foxp3+ regulatory T cells facilitate CD4+ T cell clonal anergy induction during the recovery from lymphopenia. J Immunol. 2006;176(10):5880–5889. doi: 10.4049/jimmunol.176.10.5880. [DOI] [PubMed] [Google Scholar]
- 141.Tanchot C, Le Campion A, Leaument S, Dautigny N, Lucas B. Naive CD4(+) lymphocytes convert to anergic or memory-like cells in T cell-deprived recipients. Eur J Immunol. 2001;31(8):2256–2265. doi: 10.1002/1521-4141(200108)31:8<2256::aid-immu2256>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 142.Reagan JL, Fast LD, Safran H, Nevola M, Winer ES, Castillo JJ, Butera JN, Quesenberry MI, Young CT, Quesenberry PJ. Cellular immunotherapy for refractory hematological malignancies. J Transl Med. 2013;11:150. doi: 10.1186/1479-5876-11-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sato T, Ikeda M, Yotsumoto S, Shimada Y, Higuchi T, Kobayashi H, Fukuda T, Ohashi T, Suda T, Ohteki T. Novel interferon-based pre-transplantation conditioning in the treatment of a congenital metabolic disorder. Blood. 2013;121(16):3267–3273. doi: 10.1182/blood-2012-07-443713. [DOI] [PubMed] [Google Scholar]
- 144.Altman JD, Moss PA, Goulder PJ, Barouch DH, McHeyzer-Williams MG, Bell JI, McMichael AJ, Davis MM. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274(5284):94–96. doi: 10.1126/science.274.5284.94. [DOI] [PubMed] [Google Scholar]
- 145.Maile R, Wang B, Schooler W, Meyer A, Collins EJ, Frelinger JA. Antigen-specific modulation of an immune response by in vivo administration of soluble MHC class I tetramers. J Immunol. 2001;167(7):3708–3714. doi: 10.4049/jimmunol.167.7.3708. [DOI] [PubMed] [Google Scholar]
- 146.Neudorfer J, Schmidt B, Huster KM, Anderl F, Schiemann M, Holzapfel G, Schmidt T, Germeroth L, Wagner H, Peschel C, Busch DH, Bernhard H. Reversible HLA multimers (Streptamers) for the isolation of human cytotoxic T lymphocytes functionally active against tumor- and virus-derived antigens. J Immunol Methods. 2007;320(1–2):119–131. doi: 10.1016/j.jim.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 147.Daniels MA, Jameson SC. Critical role for CD8 in T cell receptor binding and activation by peptide/major histocompatibility complex multimers. J Exp Med. 2000;191(2):335–346. doi: 10.1084/jem.191.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.O’Herrin SM, Slansky JE, Tang Q, Markiewicz MA, Gajewski TF, Pardoll DM, Schneck JP, Bluestone JA. Antigen-specific blockade of T cells in vivo using dimeric MHC peptide. J Immunol. 2001;167(5):2555–2560. doi: 10.4049/jimmunol.167.5.2555. [DOI] [PubMed] [Google Scholar]
- 149.Knabel M, Franz TJ, Schiemann M, Wulf A, Villmow B, Schmidt B, Bernhard H, Wagner H, Busch DH. Reversible MHC multimer staining for functional isolation of T-cell populations and effective adoptive transfer. Nat Med. 2002;8(6):631–637. doi: 10.1038/nm0602-631. [DOI] [PubMed] [Google Scholar]
- 150.Brown IE, Blank C, Kline J, Kacha AK, Gajewski TF. Homeostatic proliferation as an isolated variable reverses CD8+ T cell anergy and promotes tumor rejection. J Immunol. 2006;177(7):4521–4529. doi: 10.4049/jimmunol.177.7.4521. [DOI] [PubMed] [Google Scholar]
- 151.Teague RM, Sather BD, Sacks JA, Huang MZ, Dossett ML, Morimoto J, Tan X, Sutton SE, Cooke MP, Ohlen C, Greenberg PD. Interleukin-15 rescues tolerant CD8+ T cells for use in adoptive immunotherapy of established tumors. Nat Med. 2006;12(3):335–341. doi: 10.1038/nm1359. [DOI] [PubMed] [Google Scholar]
- 152.Wang X, Simeoni L, Lindquist JA, Saez-Rodriguez J, Ambach A, Gilles ED, Kliche S, Schraven B. Dynamics of proximal signaling events after TCR/CD8-mediated induction of proliferation or apoptosis in mature CD8+ T cells. J Immunol. 2008;180(10):6703–6712. doi: 10.4049/jimmunol.180.10.6703. [DOI] [PubMed] [Google Scholar]
- 153.Schmitt A, Tonn T, Busch DH, Grigoleit GU, Einsele H, Odendahl M, Germeroth L, Ringhoffer M, Ringhoffer S, Wiesneth M, Greiner J, Michel D, Mertens T, Rojewski M, Marx M, von Harsdorf S, Dohner H, Seifried E, Bunjes D, Schmitt M. Adoptive transfer and selective reconstitution of streptamer-selected cytomegalovirus-specific CD8+ T cells leads to virus clearance in patients after allogeneic peripheral blood stem cell transplantation. Transfusion. 2010;51(3):591–599. doi: 10.1111/j.1537-2995.2010.02940.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.