Abstract
The melanocortin system is composed by the agonists adrenocorticotropic hormone and α, β and γ-melanocyte-stimulating hormone, and two naturally occurring antagonists, agouti and agouti-related protein. These ligands act by interaction with a family of five melanocortin receptors (MCRs), assisted by MCRs accessory proteins (MRAPs). MCRs stimulation activates different signaling pathways that mediate a diverse array of physiological processes, including pigmentation, energy metabolism, inflammation and exocrine secretion. This review focuses on the regulatory mechanisms of MCRs signaling, highlighting the differences among the five receptors. MCRs signal through G-dependent and independent mechanisms and their functional coupling to agonists at the cell surface is regulated by interacting proteins, namely MRAPs and β-arrestins. The knowledge of the distinct modulation pattern of MCRs signaling and function may be helpful for the future design of novel drugs able to combine specificity, safety and effectiveness in the course of their therapeutic use.
Keywords: Melanocortins, G-protein-coupled receptors (GPCR), Signaling pathways, β-Arrestins, ERK1/2
Introduction
The intracellular mechanisms triggered by an activated receptor at the plasma membrane are complex. They differ according to the type and duration of the stimulus, which defines the specificity of a given physiological response. It is self-evident that a solid knowledge of the signaling events activated by a diversity of receptors becomes an asset for the development of pathway modulators, aimed at specific targets. Eventually, they become a challenge to the pharmaceutical industry in the search for efficient and specific drugs. Regarding the melanocortin receptors (MCRs), members of the G-protein-coupled receptors (GPCRs) family, functional selectivity of signaling is particularly difficult to accomplish: melanocortins are able to cross-react with various receptors and more than one MCR is generally expressed in the same tissue. Nevertheless, in the last decade remarkable efforts helped to elucidate some of the molecular mechanisms behind melanocortin signaling, promising the possibility to modulate them and benefit human health.
The melanocortin system
The melanocortin system has an important role in the regulation of a variety of biological functions like pigmentation, inflammation, stress response, sexual behavior and energy homeostasis [1]. This multiplicity of actions of the melanocortin system is possible due to a highly regulated interaction of different biological molecules, namely different agonists, two naturally occurring antagonists, five different receptors and specific accessory proteins, which all integrate the melanocortin system.
Agonists: the melanocortins
The melanocortin peptides include the adrenocorticotropic hormone (ACTH) and α, β and γ-melanocyte-stimulating hormones (α, β, γ-MSH) (Table 1). They are generated upon successive cleavages of the polyprotein proopiomelanocortin (POMC), mainly expressed in the pituitary and arcuate nucleus of the hypothalamus. Melanocortins are then released into the blood stream or move through the sympathetic nervous system to the whole organism. POMC was also found in other regions of the central nervous system and in a variety of peripheral tissues like skin, lung, kidney, adrenal glands, gastrointestinal and genitourinary tract [2]. The ubiquitous expression and the tissue-specific proteolytic cleavage of POMC surely contribute to the extraordinary complexity of functions associated to melanocortins.
Table 1.
Melanocortin receptor family: agonist affinity, antagonists, tissue distribution and main functions
| MCR | Agonist affinity | Antagonists | Tissue expression | Main function |
|---|---|---|---|---|
| MC1R | α-MSH ≥ ACTH = β-MSH > γ-MSH | Agouti | Skin; immune cells; brain; pituitary; corpus luteum; placenta; testis; adipocytes | Pigmentation; anti-inflammatory |
| MC2R | ACTH | Agouti | Adrenal gland; skin; adipocytes | Adrenal steroidogenesis |
| MC3R | γ-MSH = α-MSH = ACTH > β-MSH | Agouti; AgRP | Brain; placenta; stomach; pancreas; duodenum; heart; testis; mammary gland; muscle cells; kidney; immune cells | Energy homeostasis; anti-inflammatory |
| MC4R | α-MSH ≥ ACTH > β-MSH > γ-MSH | Agouti; AgRP | Brain; sympathetic nervous system; muscles; adipocytes | Energy homeostasis |
| MC5R | α-MSH ≥ ACTH > β-MSH > γ-MSH | Agouti; AgRP | Exocrine glands; adrenal gland; pituitary; kidney; liver; lung; lymphatic system; testis; ovary; uterus; gastrointestinal tract; skin; skeletal muscle cells; adipocytes; B-lymphocytes; brain | Exocrine gland secretion; fatty acid β-oxidation; adipocyte lipid metabolism |
Antagonists: agouti and agouti-related protein (AgRP)
One of the most interesting aspects of the melanocortin system is the presence of two naturally occurring antagonists, the agouti and the agouti-related protein (AgRP) (Table 1). A great interest in agouti emerged when it was discovered that dominant mutations in the mice gene induce obesity, insulin resistance, yellow coat and predisposition for tumors [3]. In mice, the agouti protein is mainly expressed in the skin, but in those mutants, agouti is ectopically expressed in other tissues. Due to agouti affinity for different MCRs, pleiotropic phenotypes associated with the ectopic expression of agouti were indeed predictable (Table 1). In fact, the coat pigmentation arises from an antagonic effect on MC1R whereas the other effects may result from the antagonism of other MCRs in different tissues. For instance, the obesity syndrome may derive from the blockade of MC4R signaling in the central nervous system.
The agouti-signaling protein (ASIP) is the human homolog of the mouse agouti gene. It was detected in the skin, heart, ovary, testis, foreskin, adipose tissue, liver and kidney [4] and, although not so well characterized, it also appears to regulate human pigmentation [5].
The AgRP was later discovered based on its sequence homology to agouti. It is mainly expressed in the adrenal gland and hypothalamus, where it acts as a potent orexigenic factor by antagonizing MC3R and MC4R (Table 1). In fact, the effect of AgRP overexpression is similar to that described for agouti, excluding the pigmentation changes, because it does not antagonize MC1R [6]. Its function in adrenal glands remains to be elucidated since it has a lower antagonic effect on MC5R and no binding affinity for MC2R, the MCRs with major expression in this tissue [7].
The melanocortin receptors (MCRs): physiological role
The research in the melanocortin field has gained new insights with the identification of high levels of binding sites for α-MSH in human melanoma cells by Tatro and colleagues [8]. Later on, a cDNA library from these cells allowed cloning the first MCR, initially named α-MSH receptor [9, 10]. Concomitantly, a second receptor was cloned from human adrenal cells and termed ACTH receptor due to its high affinity for ACTH binding [10]. Afterwards, three more MCRs were discovered [11–15] and then all were numbered from 1 to 5 according to the order of their cloning (MC1R–MC5R). Structurally, all MCRs belong to the class of the GPCRs, which have an N-terminal extracellular domain, seven transmembrane regions and a C-terminal cytoplasmic domain [16].
The most exciting characteristics of MCRs regard the different binding affinities for the diverse melanocortins and antagonists and the absence of functional uniformity (Table 1). In fact, no other receptors have their activity regulated by both agonists and antagonists, making the MCRs unique among the superfamily of GPCRs. This type of modulation allows the increase on specificity and accuracy of melanocortin’s biological functions.
The MC1R is recognized as the classic receptor of melanocytes because it is particularly involved in the regulation of skin and hair pigmentation. The activation of MC1R promotes the synthesis of eumelanin (dark pigment) and decreases the production of pheomelanin (yellow pigment) which results in the darkness of skin and hair [17]. MC1R was also described in a large number of different immune cells where it exerts an anti-inflammatory effect [18, 19].
The main function of MC2R is the control of steroidogenesis in the adrenal gland and, consequently, mutations in MC2R gene accounts for 25 % of all cases of the familial glucocorticoid deficiency (FGD), a rare autosomal recessive disorder characterized by a severe glucocorticoid deficiency, associated with failure of adrenal responsiveness to ACTH [20–22]. This deficiency is also observed in the MC2R knockout mice [23]. Moreover, MC2R is present in human skin cells [24] and in mouse adipocytes [25–28], where it was suggested to regulate lipolysis [29].
MC3R and MC4R are expressed at the nervous system and are intimately related with the central regulation of energy homeostasis. Human mutations on MC3R gene are frequently related to obesity and type 2 diabetes [30] and some reports indicate that MC3R polymorphisms are associated with increased risk of childhood obesity [31–34]. Genetic disruption of the MC4R has been found to cause obesity in mice and mutations on MC4R human gene are responsible for up to 6 % of morbid obese individuals [35]. MC4R is also involved in the regulation of the sexual behavior and erectile function [36, 37] and pain [38, 39].
The MC5R was the last receptor of this family to be cloned and was found to be expressed in a variety of organs and tissues. The MC5R knockout mice revealed a deficient function of the sebaceous and other exocrine glands. Additional roles to MC5R have been attributed, namely the activation of regulatory T-lymphocytes during ocular immunity [40–43], immunomodulatory functions in B-lymphocytes [44] and stimulation of cytokine secretion in adipocytes [45]. In skeletal muscle cells, MC5R was specifically implicated on the regulation of fatty acid oxidation [46], while in adipocytes MC5R promotes lipolysis and impairs fatty acid re-esterification [26, 28, 47].
Accessory proteins for MCRs cell surface targeting
Two melanocortin receptor accessory proteins (MRAP1 and MRAP2) regulate the transport of the MCRs towards the cell membrane and also the ligand-induced signaling [48] (Table 2). The presence of interacting factors for MCRs function was first suggested by Noon et al. [49] who failed to obtain a correct trafficking of MC2R to cell surface when the receptor was expressed in cells that lack endogenous expression of the melanocortin system interveners. MRAP1 was further established as essential for MC2R trafficking and signaling [21, 50–53], a reason why 20 % of FGD type 2 patients have mutations in MRAP gene [1]. Later on, a closely related protein, named MRAP2, was found in adrenal gland and hypothalamus [48, 52]. Whereas MRAP1 is essential for MC2R-signaling activation, MRAP2 seems to be a competitive inhibitor of MRAP1, decreasing its ability to bind MC2R [52, 54] (Table 2). MRAPs assist MC2R targeting to cell membrane by facilitating ER export and further post-translational processing at Golgi apparatus, most probably glycosylation [55].
Table 2.
The role of MRAPs on MCRs cell surface expression and signaling capacity
| MRAP1 | MRAP2 | |||
|---|---|---|---|---|
| Cell surface expression | Cell signaling | Cell surface expression | Cell signaling | |
| MC1R | = [48, 55] | = [48] | = [48] | = [48] |
| MC2R | ↑ [48, 51–55] | ↑ [48, 51–55] | ↑ [48, 52, 54] | ↑ [48, 52, 54] |
| MC3R | = [48, 55] | = [48] | = [48] |
↓ [48] ↑ [59] |
| MC4R |
= [55] |
↓ [48] = [54] |
↓ [48, 54] |
↓ [48] = [54] ↑ [59] |
| MC5R | ↓ [48, 51, 55] | ↓ [48] | ↓ [48, 51] | ↓ [48] |
Although MRAPs are only essential for MC2R targeting to cell surface [48, 56], they are able to interact with all five MCRs regulating their trafficking and cell surface expression (Table 2) [57, 58]. When MRAP1 is present, it impairs the MC4R and MC5R trafficking to plasma membrane while having no effect on cell surface expression of MC1R and MC3R [48, 51]. Intriguingly, Chan et al. [48] demonstrated that both MRAPs act as negative regulators of MC1R, MC3R, MC4R and MC5R signaling, but recently, Asai et al. [59] showed that MRAP2 expression increases MC3R and MC4R signaling. The use of different agonists can explain the conflicting results: Chan et al. used NDP-MSH, a synthetic analogue of α-MSH, whereas Asai et al. used α-MSH [48, 59]. MRAP2 deletion causes obesity in mice and heterozygous variants of MRAP2 gene are associated with early onset obesity in humans [59]. Although not yet clear, MRAP2 seems to regulate body weight by facilitating MC4R function but may also act through other receptors expressed in peripheral tissues since MRAP2-null mice develop the obesity syndrome without hyperphagia [59, 60].
Besides MRAPs, other proteins were identified as potential accessory proteins for MCRs, namely attractin, attractin-like protein (ALP) and mahogunin ring finger 1 (MGRN1) [1, 61, 62]. MGRN1 contains a “really interesting new gene” (RING) finger domain characteristic of the E3 ubiquitin ligases. In fact, ubiquitination is a frequent modification of GPCRs important for their targeting for proteasome degradation, but also regulates GPCRs signaling, internalization and lysosomal degradation [63–65]. It was recently demonstrated that MGRN1 impairs MC1R and MC4R function by inhibiting receptor functional coupling to the cyclic adenosine monophosphate (cAMP) pathway [66]. These authors also suggested that signaling inhibition by MGRN1 occurs independently of receptor ubiquitination or internalization and might be specific for the MCR subfamily of GPCRs since it is not observed for the β2-adrenergic receptor (β2-AR) [66]. However, the role of MGRN1 on MC2R function was postulated to be related with trafficking and/or degradation rather than with signaling since MC2R became ubiquitinated in the presence of MGRN1 but did not exhibit differences in cAMP production [61].
Endoplasmic reticulum-resident chaperones, such as calnexin and calreticulin, the 70 kDa heat-shock protein (Hsp70) family, the receptor activity modifying proteins (RAMPs) and the GTPases family of Rab and Sar1/ARF all function broadly to facilitate folding and ER export of numerous GPCRs [57, 67]. It was recently shown that Hsc70, the cognate cytosolic Hsp70 protein, promotes cell surface expression and signaling of intracellular retained obesity-related MC4R mutants [68].
MCRs intracellular signaling
At the plasma membrane, MCRs are activated by ligand binding and undergo conformational changes, which trigger a complex intracellular network that translates extracellular signals into biological responses. Since MCRs belong to the GPCRs family, much of the research and progress on the field of MCRs signaling was inspired on GPCRs data.
G protein-dependent signaling
Classically, GPCRs signaling is primary mediated by the interaction with the heterotrimeric G-proteins, which consist of an α-subunit that binds GDP and a non-dissociable complex composed by β and γ-subunits. Ligand binding facilitates the exchange of GDP to GTP on α-subunit promoting the dissociation of G-protein from the receptor and the dissociation of the βγ heterodimer from GTP-bound α-subunit. Induction of signaling is terminated with the hydrolysis of GTP and subsequent re-association of inactive GDP-bound α-subunit with βγ complex, waiting for a new cycle of receptor activation.
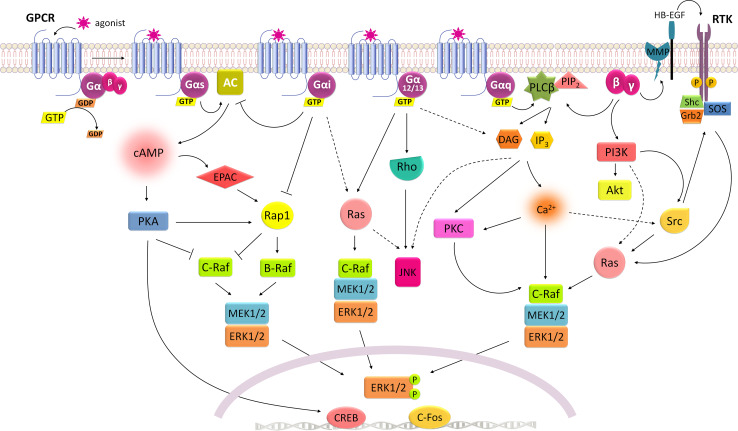
According to the sequence homology and functional similarities, G-protein α-subunits are divided into four classes: Gs, Gi/o, Gq/11 and G12/13 (Fig. 1) [69]. The signaling cascades generated by GPCRs activation are particularly dependent on the subtype of G-protein that couples to the receptor. For example, the protein Gs interacts with adenylyl cyclase (AC) thus mediating the increase in intracellular cAMP levels and subsequent activation of protein kinase A (PKA), whereas Gi protein inactivates AC and blocks PKA activation (Fig. 1) [69, 70]. Conversely, downstream effectors for Gq/11 comprise inositol 1,4,5 triphosphate (IP3) and diacylglycerol (DAG), both originated from the hydrolysis of phosphatidylinositol 4,5-bisphosphate (PIP2) by phospholipase C-β (PLCβ), and their signaling is conveyed mainly through the activation of protein kinase C (PKC) and Ca2+ pathways (Fig. 1) [69]. While G12/13 signaling is thought to be mediated by Rho-GTPases, it also modulates PKA and PKC activity [69, 71, 72].
Fig. 1.
Integrated mechanisms for G-protein-mediated signaling. Gs signaling is mainly conveyed through activation of AC/cAMP/PKA pathway, which inhibits C-Raf, but also leads to the activation of Rap1 that relays in B-Raf stimulation of ERK1/2 signaling. Gi inhibits AC activity blocking its inhibitory effect on C-Raf and concomitantly stimulates ERK1/2 signaling by a Ras-dependent mechanism. G12/13 strongly stimulates JNK activity but can also activate ERK1/2 through Ras or by DAG/PKC pathway. Gq can stimulate ERK1/2 via PLCβ/DAG/PKC as well as PLCβ/IP3/Ca2+-signaling mechanisms, either by direct phosphorylation of C-Raf by PKC or by a Ras-dependent manner, which may involve the recruitment of Src. Besides α-subunit, the complex βγ released from α subunit during GPCR activation is also able to promote ERK1/2 signaling through stimulation of PLCβ or PI3K. PI3K usually relays in Akt phosphorylation, but also leads to Src and/or Ras stimulation of ERK1/2. In addition, G-proteins can mediate the transactivation of RTK through its βγ-subunits. Activation of PI3K leads to Src-mediated receptor tyrosine kinase phosphorylation and subsequent recruitment of Shc, Grb2 proteins and SOS to stimulate Ras activity and ERK1/2 phosphorylation. RTK transactivation mediated by βγ-subunits may also occur through an inside-out model. This mechanism is well described for the epidermal growth factor (EGF) receptor, in which βγ activates matrix metalloprotease (MMP) proteins that cleave the ectodomains of membrane-bound growth factors (HB-EGF) to generate soluble EGF ligands that are released from the cell to activate its RTK. Whatever the mechanism of ERK1/2 activation is, these kinases are then able to phosphorylate a wide variety of cytoplasmic and nuclear targets. When translocating to the nucleus, ERK1/2 initiate gene transcription by phosphorylating several transcription factors like CREB and c-Fos
Notwithstanding, GPCRs frequently couple to more than one G-protein subtype, thereby initiating a complex intracellular signaling network rather than a simple cascade sequence [69, 72, 73]. For instance, β2-AR is able to couple to both Gs and Gi [74], orexin-2 receptor is functionally coupled to Gs, Gi and Gq [75] and the thrombin protease-activated receptor 1 couples to Gi/o, Gq/11 and G12/13 [76, 77]. For this, the quantification of specific early messengers like intracellular cAMP for Gs, or Ca2+ for Gq, is no longer sufficient to measure receptor activity, which requires the evaluation of multiple signaling pathways.
In addition to the α-subunits, the βγ dimer also has regulatory functions, although initially thought to have no relevance on GPCR signaling. It has been demonstrated that βγ-subunits modulate several intracellular pathways like phosphoinositide 3-kinase (PI3K), extracellular signal-regulated kinase 1/2 (ERK1/2), PKA and PKC (Fig. 1) [72, 78–80].
As part of the GPCRs family, all MCRs are functionally coupled to Gs and stimulate the cAMP/PKA pathway [81]. Additionally, MC3R, MC4R and MC5R were found to signal through Gi/o [82–84] and the Gq was also associated with MC4R activation [85]. MCRs also induce Ca2+ levels and promote ERK1/2 activation both in native and in overexpressing cell systems, although conveyed by different mechanisms [82, 86–94]. Indeed, many recent studies have highlighted a wide diversity of signaling partners downstream of MCRs (Figs. 2, 3, 4, 5, 6).
Fig. 2.
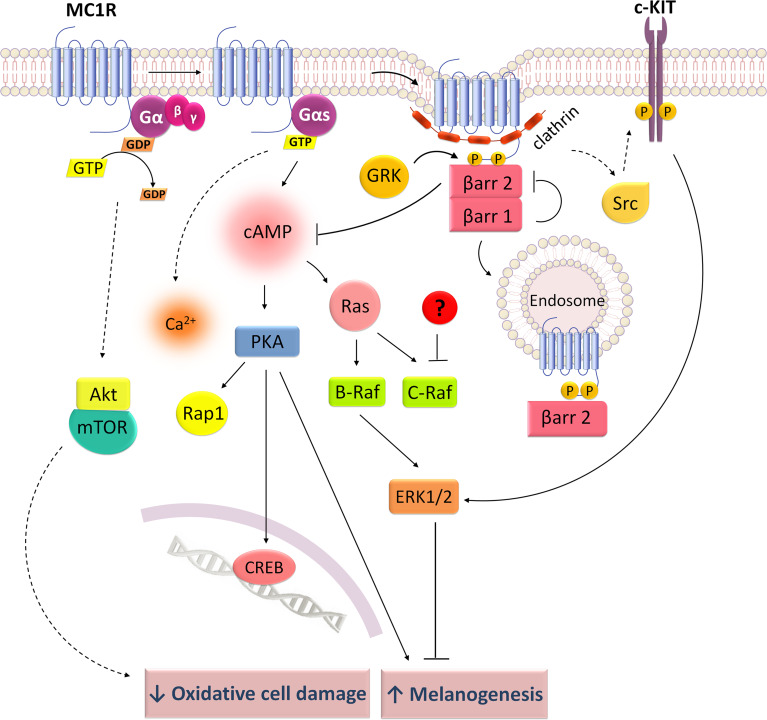
Signaling mechanisms of melanocortin 1 receptor (MC1R). MC1R couples to Gs stimulating cAMP/PKA and increasing intracellular Ca2+ levels. Ligand binding to MC1R promotes a cAMP-dependent activation of Rap1 and Ras, which leads to ERK1/2 phosphorylation through B-Raf signaling. By c-KIT transactivation, MC1R is also able to activate ERK1/2. β-arrestin 2 mediates MC1R internalization and desensitization, decreasing cAMP production but not affecting ERK1/2 activation. β-arrestin 1 competes with β-arrestin 2 for MC1R binding, which blocks β-arrestin 2-mediated cAMP inhibition. cAMP/PKA signaling increases synthesis of melanogenic pigments whereas ERK1/2 seems to have an inhibitory effect on this process. Akt/mTOR apparently increases cell survival during oxidative stress
Fig. 3.
Signaling mechanisms of melanocortin 2 receptor (MC2R). MC2R couples to Gs and stimulates cAMP/PKA. Activation of ERK1/2 is dependent on PKA and MC2R internalization. MC2R also leads to the phosphorylation of p38 and PKC. MC2R promotes biphasic ERK1/2 activation, with a first transient wave, dependent from receptor internalization, but independent of β-arrestins. ERK1/2 seems to be related with proliferation and differentiation processes regulating the trophic and steroidogenic properties of ACTH in adrenal glands. PKA and PKC are also mediators of adrenal steroidogenesis
Fig. 4.
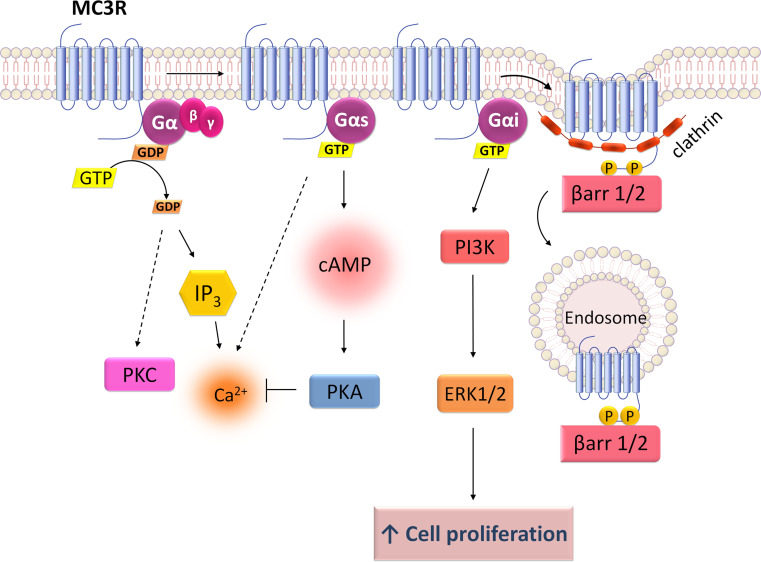
Signaling mechanisms of melanocortin 3 receptor (MC3R). MC3R couples to Gs stimulating cAMP/PKA but is also able to couple to Gi driving to ERK1/2 pathway through activation of PI3K. MC3R receptor is also known to increase intracellular Ca2+ levels and to interact with PKC and IP3. MC3R activation induces cell proliferation through a PI3K/ERK1/2-mediated mechanism that may be related to neuronal regeneration
Fig. 5.
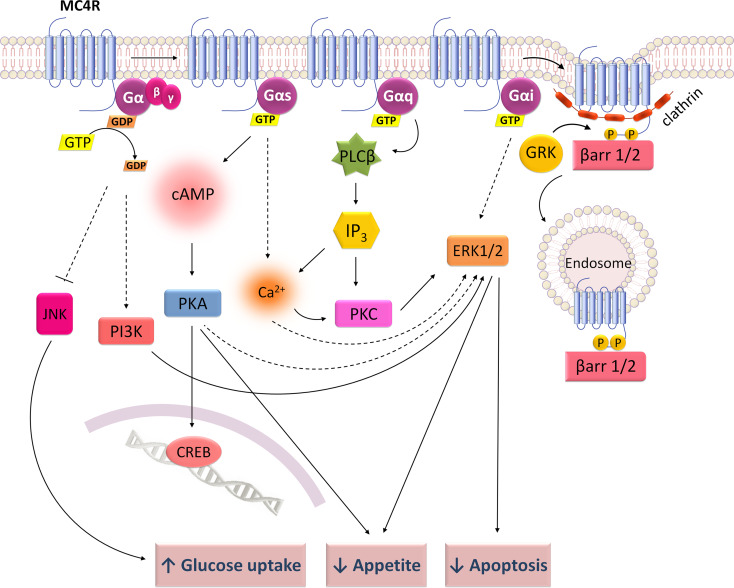
Signaling mechanisms of melanocortin 4 receptor (MC4R). MC4R couples to Gs stimulating cAMP/PKA and to Gq activating PKC dependently from PLCβ and IP3. It also activates ERK1/2 via Gi or through mechanisms dependent from Ca2+, PKA, PKC or PI3K. A PKA-dependent ERK1/2 signaling is associated with food intake suppression, whereas a PKA-independent ERK1/2 seems to decrease cell apoptosis upon neuronal damage. MC4R also inhibits JNK activity, possibly to increase insulin-stimulated glucose uptake
Fig. 6.
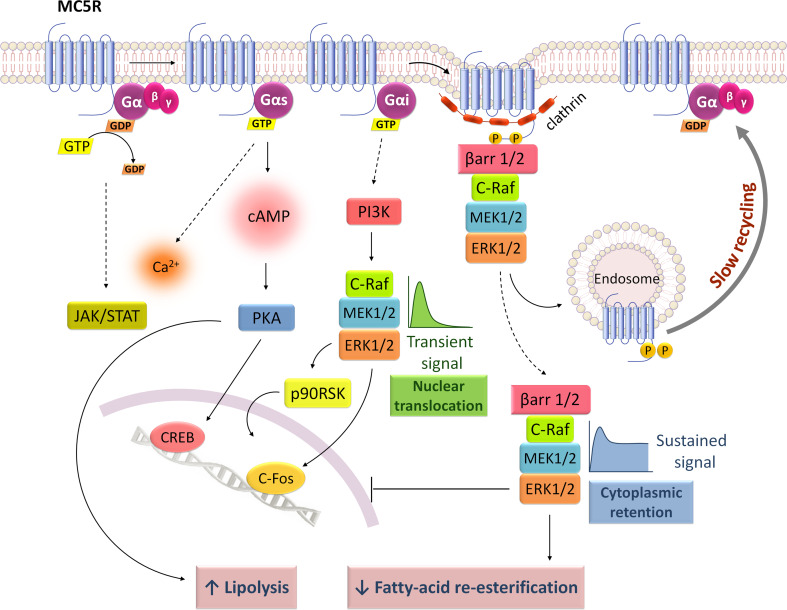
Signaling mechanisms of melanocortin 5 receptor (MC5R). MC5R activates two parallel signaling pathways, Gs/cAMP/PKA and Gi/PI3K/ERK1/2 that promote CREB phosphorylation and c-fos expression, respectively. MC5R is also known to increase intracellular Ca2+ levels and promote JAK/STAT activation. ERK1/2 signaling occurs through a biphasic fashion with an early transient peak dependent from Gi protein and a second one, more sustained in time, regulated by Gi and β-arrestins. MC5R internalizes independently from β-arrestins but these scaffold molecules seem to retain ERK1/2 signaling in the cytoplasm to mediate MC5R-dependent decrease of fatty acid re-esterification in adipocytes. MC5R-dependent increase of lipolysis rate is regulated by PKA activation
MC1R activation triggers the cAMP pathway, induces Ca2+ levels and stimulates ERK1/2 but it seems to have no effect on PKC pathway [86, 87, 95, 96] (Fig. 2). In HEK293 cells expressing MC1R and also in human melanoma cells, α-MSH-mediated PKA activation induces the phosphorylation of the transcription factor CREB [96]. In rodent melanocytes, the interaction of different pathways and specific interveners was described: cAMP activates RAP-1 through PKA and additionally induces RAS phosphorylation, which activates B-RAF, MEK and ERK1/2 [93] (Fig. 2). α-MSH-dependent cAMP activation mediates biosynthesis of photoprotective melanin pigments whereas ERK1/2 pathway seems to downregulate melanogenesis [93]. Human MC1R mutants expressed in heterologous systems present a reduced cAMP signaling in response to α-MSH but activate ERK1/2 with the same efficiency as MC1R wild type, suggesting no dependence between ERK1/2 and cAMP pathways [86, 87, 97]. This combined signaling might assume a protective role in skin cancers, where MC1R mutations are highly frequent and compromise cAMP signaling, but ERK1/2 pathway is still able to regulate cell survival by decreasing melanogenic activity. In retinal pigment epithelium cells, α-MSH activates ERK1/2 and AKT/mTOR signaling pathways, reducing the hydrogen peroxide induced cell damage [98] (Fig. 2), a function that might be crucial to promote cell survival under oxidative stress conditions.
Besides Gs/cAMP/PKA pathway, MC2R is also able to signal through Ca2+, PKC and ERK1/2 [88–90, 99–101], activating several transcriptional factors like CREB, c-Fos and c-Jun [102–105] (Fig. 3). In HEK293/FRT cells overexpressing MC2R and in human fasciculata cells, ACTH-induced ERK1/2 activation is PKA dependent [89]. By contrast, in Y1 and H295R adrenocortical cells, ERK1/2 signaling revealed to be independent from PKA [88, 90]. Additionally, PKC is not involved in ERK1/2 phosphorylation in both H295R and HEK293/FRT cells expressing MC2R [89, 90]. In H295R cells, Janes et al. [90] showed no interference of Ca2+ on ERK1/2 activation and did not observe any differences in p38 pathway after ACTH stimuli, however, Roy et al. [89] have demonstrated sustained p38 activation in HEK293/FRT cells expressing MC2R. The key factors that define which mechanisms might be activated under different situations seem to be the potency and duration of stimuli that may depend not only on the cell type and agonist concentration, but also on MC2R surface expression and occupancy that can be temporally regulated by the synchronization of different signaling pathways. In Y1 cells, for example, ACTH-stimulated ERK1/2 activation leads to a cyclic phosphorylation of SF-1, a transcription factor important for the transcriptional regulation of several steroidogenic enzymes and the MC2R itself, but, in parallel, ACTH activation of PKA is able to decrease ERK1/2 phosphorylation thereby reducing SF-1 function and MC2R expression [101]. ERK1/2 signaling has been closely associated with proliferation and differentiation processes, important to maintain adrenal structure and function. Using the Y1 cell line, Lotfi et al. [102] demonstrated that acute stimulations of ACTH induce cell cycle progression independently of cAMP, possibly mediated by ERK1/2, however, chronic exposure to ACTH had a cAMP-dependent inhibitory effect on cell proliferation. Together, the data demonstrate a dual effect of MC2R in adrenal function, coordinating both cAMP/PKA and ERK1/2 pathways through a compensatory mechanism to counterbalance the hypertrophic and steroidogenic capacity of adrenal glands (Fig. 3).
MC3R is also able to induce cell proliferation [82, 106] and to signal through both cAMP/PKA and ERK1/2 [82, 107] (Fig. 4). In HEK293 cells, NDP-MSH binding to overexpressed MC3R increases cell growth rate upon activation of ERK1/2 signaling by a PI3K- and Gi/o-dependent mechanism, but independently from PKA, Ca2+ and PKC [82] (Fig. 4). Similarly, in a neuronal cell line, the presence of MC3R is associated with an increase in cellular proliferation regulated by AKT [106]. In fact, an important neurotrophic role has been attributed to the melanocortins [108] and although the physiological significance of MC3R-mediated proliferation it still unsolved, it is possible to be related with neuronal regeneration. MC3R can also increase the intracellular Ca2+ levels [95, 107, 109] and interact with PKC pathway [109] (Fig. 4). Some authors report that the increased Ca2+ influx is dependent from IP3 [107] whereas others do not indicate the involvement of IP3 during calcium mobilization [95].
MC4R can couple to Gi/o and Gq, besides Gs, and switches from second messengers such as cAMP and Ca2+ to trigger other downstream pathways than PKA, like PKC, PI3K and ERK1/2 [84, 85, 91, 92, 95, 110, 111] (Fig. 5). MC4R promotes Ca2+ formation through a mechanism mediated by PLCβ and IP3 [85]. NDP-α-MSH stimulation of MC4R endogenously expressed in hypothalamic cells promotes cAMP activation and transient ERK1/2 phosphorylation through Ca2+/PKC pathway but independently of PKA, PI3K and Gi protein [91] (Fig. 5). By contrast, in CHO cells stably transfected with MC4R, NDP-α-MSH induces a PKA-independent but PI3K-dependent phosphorylation of ERK1/2 [92] and in HEK293 cells expressing MC4R, Gi protein mediates NDP-α-MSH-induced ERK1/2 phosphorylation [91]. Structurally, the MC4R cytoplasmic tail was found to be necessary for the activation of ERK1/2 signaling [112]. Regarding the functional consequences of the different signaling mechanisms, a PKA-dependent increase of ERK1/2 phosphorylation was reported in solitary nucleus neurons after central administration of MTII in rats as a required mechanism for MC4R suppression of appetite, possibly mediated by CREB phosphorylation [111]. Notwithstanding, Chai et al. [91] showed a PKA-independent ERK1/2 signaling important to mediate the anti-apoptotic effects of melanocortins in hypothalamic cells. In fact, the neurotrophic role of melanocortin peptides has been widely associated to a neuroprotective effect, which reduces cell damage induced by neuronal injuries and increases recovery from nerve lesions [108]. Additionally, NDP-α-MSH inhibits c-Jun NH2-terminal kinases (JNK) activity and Insulin receptor substrate 1 phosphorylation in HEK293 cells expressing human MC4R. This interaction of the melanocortinergic system with the insulin signaling involves AKT phosphorylation and increases insulin-stimulated glucose uptake [113].
In an heterologous cell system stably expressing MC5R, the receptor activation promotes cAMP and Ca2+ increase, independently of IP3 [114]. In a similar cellular system, MC5R was found to elicit two parallel signals when activated by α-MSH: cAMP/PKA and ERK1/2 pathways (Fig. 6). In adipocytes, cAMP/PKA revealed to be important for lipolysis activation by α-MSH/MC5R whereas ERK1/2 pathway seems to be crucial for decreasing adipocyte fatty acid re-esterification [47]. ERK1/2 activation occurs independently of PKA, PKC and AKT but requires PI3K and leads to a downstream phosphorylation of 90-kDa ribosomal S6 kinases (p90RSK) and mitogen- and stress-activated protein kinase 1 (MSK1) [94] (Fig. 6). Moreover, only a small fraction (10 %) of activated p90RSK and ERK1/2 translocates to the cell nucleus inducing c-Fos expression. The cAMP/PKA activation by MC5R is dependent of Gs protein and induces nuclear CREB phosphorylation whereas ERK1/2 pathway is regulated through a biphasic mechanism with an early transient and a late sustained activation, both mediated by Gi protein [83] (Fig. 6).
G-protein-independent signaling
In the past few years, many GPCRs were found to signal independently from G-proteins, involving a broad set of binding molecules such as GPCR kinases (GRKs), β-arrestins, Src-family tyrosine kinases, Janus protein kinase/signal transducers and activators of transcription (JAK/STAT) and PDZ domain-containing proteins [115]. Additionally, it is now well established that GPCRs activation can lead to receptor tyrosine kinase (RTK) signaling, by a process termed transactivation. Briefly, ligand binding to GPCRs induces RTK activation, which dimerizes and autophosphorylates itself on cytoplasmic tyrosine residues, creating binding sites to assemble signaling partners at cell membrane, like the Src-homologous and collagen (Shc) protein.
Some G-independent mechanisms were described for MCRs, although the majority of signaling data involves G-protein-mediated mechanisms. For instance, in human melanoma cells, the binding of NDP-α-MSH to MC1R induces ERK1/2 signaling by a mechanism independent from cAMP, PKA, PKC or Ca2+ but involving Src phosphorylation and transactivation of the RTK stem cell factor receptor (c-KIT) [86] (Fig. 2). By contrast, in H295R adrenal cells, ACTH/MC2R-mediated ERK1/2 signaling does not involve transactivation of RTKs neither Src phosphorylation [90]. MC5R activates the JAK/STAT pathway in lymphocytes [44] (Fig. 6) which seems to be involved in cytokine-mediated inflammatory regulation. In fact, in adipocytes 3T3-L1, MC5R activation by α-MSH induces IL-6 expression and secretion [45].
β-Arrestins-driven-signaling mechanisms
Beyond G-protein-independent mechanisms, β-arrestins have been pointed out as one of the major molecules that control GPCR signaling [116]. Arrestins family is comprised of two visual arrestins expressed only in retina and, more importantly, two non-visual arrestins, β-arrestin 1 and β-arrestin 2, expressed in numerous tissues [117]. The recruitment of β-arrestins requires GPCRs phosphorylation on cytosolic serine and tyrosine residues by GRKs, an event that disrupts receptors signaling to G-proteins [118]. GPCRs are then internalized within endosomal vesicles and become dephosphorylated. Clathrin-coated pits (CCPs) constitute the main pathway for GPCR internalization: β-arrestins binding to GPCR facilitate the recruitment of clathrin complexes to the plasma membrane and other regulatory proteins that coordinate CCPs formation and budding leading to receptor internalization [119]. Afterwards, receptors return to the cell surface through recycling endosomes, or are addressed for lysosomal degradation.
It is clear for all the five MCRs that agonist binding induces the recruitment of β-arrestins and promotes receptor internalization by CCPs [83, 99, 106, 120–126] and not by a caveolae-dependent mechanism (Figs. 2, 3, 4, 5, 6) [99, 120, 127]. Moreover, GRKs were found to mediate internalization of MC1R [128] and MC4R [121]. Both MC2R [99] and MC4R [121, 122] internalize through PKA-dependent mechanisms, though Cai et al. [120] revealed that PKA is not required for β-arrestin recruitment by the MC1, 3, 4 and 5 receptors.
Additionally to the known role of β-arrestins in GPCR internalization, they are also recognized as central scaffold proteins for signal transduction of many GPCRs even after receptor internalization and desensitization. β-arrestins act as adapters for Src-family tyrosine kinases and have a major role as scaffolds for ERK1/2 signaling. In some cases, internalized GPCRs form complexes with β-arrestins and all the components of the ERK1/2 cascade that anchor signaling to intracellular endocytic vesicles for long periods of time [116, 117, 129, 130]. In this situation, β-arrestins interaction induces a slow ERK1/2 activation, more sustained in time and restricted to the cytoplasm [131]. Otherwise, other GPCRs dissociate from β-arrestins shortly after movement of the receptor into CCPs and do not colocalize with β-arrestin complexes in endosomes [130, 132, 133]. In this case, duration and distribution of ERK1/2 pathway activated by β-arrestins is quite similar to the G-protein-dependent mechanisms, which lead to a rapid and transient phosphorylation of ERK1/2 that, in general, translocate to nuclear compartments.
The β-arrestins-dependent ERK1/2 activation was firstly demonstrated for β2-AR by Daaka et al. [134] and Luttrell et al. [135]. β2-AR was shown to activate ERK1/2 through a biphasic mechanism, comprising a rapid and transient phase dependent from G-protein and a second one more sustained in time and driven by β-arrestins [132]. Intriguingly, the β2-AR rapidly dissociates from β-arrestins upon internalization and does not form stable receptor-β-arrestin complexes on endosomes, even though it is able to promote a sustained ERK1/2 activation [133].
The MC5R seems to behave similar to β2-AR [83]. Although β-arrestins are not involved in MC5R internalization, they appear to function as scaffolds to prolong ERK1/2 signaling in the cytoplasmic compartment and prevent their nuclear translocation [83]. A difference between MC5R and β2-AR arises in the type of G-protein involved: β2-AR activation induces an early Gs/Gi proteins dependent before the last β-arrestins-dependent ERK1/2 signaling [132], whereas MC5R promotes a transient and a sustained ERK1/2 activity, both dependent on Gi but not on Gs protein [83]. The earlier phase of ERK1/2 activation occurs independently of β-arrestins and seems to be important for nuclear translocation and c-Fos expression whereas the late sustained ERK1/2 activation, Gi and β-arrestins driven, may be associated with cytoplasmic functions [83] (Fig. 6). In fact, in adipocytes, a decrease in the cytoplasmic activity of phosphoenolpyruvate carboxykinase (PEPCK), important to glyceroneogenesis, was linked to the α-MSH-mediated ERK1/2 phosphorylation [47].
Similarly to MC5R, MC2R also promotes a biphasic ERK1/2 signaling [101] with the first transient activation being independent from β-arrestins [89]. It is still unknown whether the second wave of ERK1/2 phosphorylation requires β-arrestins, however, we can speculate a similar mechanism between MC2R and MC5R. Indeed, MC2R internalization is needed to promote a transient ERK1/2 activation [90] which occurs independently from β-arrestins [89], suggesting that β-arrestins recruitment might be a later event occurring after ERK1/2 activation. In this regard, it is still possible that β-arrestins binding to MC2R might form an intracellular complex retaining receptor signaling in cytoplasmic endosomes and thus being responsible for the second period of ERK1/2 phosphorylation. This mechanism could be an explanation for the lower nuclear translocation of ERK1/2 observed by Roy and colleagues [89].
Even though β-arrestin 1 and 2 have an apparent redundant function in MC5R signaling, recent data implicated β-arrestins in MC1R internalization and signaling in an isoform specific way: β-arrestin 2 inhibits MC1R agonist-dependent cAMP production, but not ERK activation, and colocalizes with the receptor in endocytic vesicles promoting internalization. β-arrestin 1, by contrast, functionally competes with β-arrestin 2 for binding to MC1R and consequently increases signaling upon displacement of β-arrestin 2 [136] (Fig. 2).
Conclusions
Given the physiological significance of the melanocortins, they are now recognized as a target for the pharmacological treatment of different disorders like obesity, diabetes and erectile dysfunction [37, 137]. Their therapeutic potential was, so far, only attained by designing analogues of MCRs agonists. However, MCRs present high homology between each other [16] and cross-reactions of their agonists, causing unwanted side-effects, are frequently observed. For instance, AstraZeneca in collaboration with Palatin technologies developed a melanocortin analogue for the treatment of obesity, AZD2820, but it was discontinued following serious adverse effects observed in phase I clinical trials. Moreover, bremelatonide (PT-141), initially developed for the treatment of obesity, promises efficacy for the treatment of sexual dysfunction and the phase III clinical trials are expected soon [37, 138]. MC4-NN2-0453 developed by Novo Nordisk, also failed to demonstrate effects in weight loss causing several skin-related adverse effects, headache and sexual disturbances [139].
Considering all these data, a promising approach for generating more effective and safer drugs may involve the management of a specific pathway step. Indeed, many GPCRs have been shown to elicit different responses when activated by distinct ligands, a phenomenon that was recently named as “biased agonism” [140, 141]. This concept arises from the discovery that the same receptor is able to activate both G-protein and β-arrestins-dependent-signaling pathways. Compared to a “normal ligand” that signals with similar efficacy through all the pathways available for the receptor, a “biased ligand” favors one pathway over another [142, 143]. This property has highlighted the therapeutic potential of “biased ligands” and the opportunity to design and produce selective drugs with improved efficacy and less unwanted effects [144]. A diversity of biased ligands for GPCRs has been identified so far [144]. In the setting of MCRs, biased agonism may constitute an encouraging challenge in drug development. An alternative approach considers the specific intracellular, instead of extracellular, delivery of melanocortin peptides. Innovative data revealed that α-MSH complexes with MC4R at the endoplasmic reticulum, a condition that induces maximal amplitude and constant signaling of cAMP production at the cell surface. In that complex, MC4R acquires a stable active conformation that does not become desensitized [145]. Development of agonists for intracellular targeting, instead of binding to MCRs at cell surface, is thus an attractive challenge aiming drug specificity.
All the data discussed in this review stresses out the strategical importance to study the molecular mechanisms and signaling pathways activated by receptors and agonists in the different tissues and cells. In fact, the intervention in a particular signaling cascade activated by a specific MCR seems to be the rational way to improve the efficacy and long-term safety of the pharmacological therapies.
Acknowledgments
Rodrigues A.R. was supported by POPH/FSE and “Fundação para a Ciência e Tecnologia” (SFRH/BPD/92868/2013).
Conflict of interest
The authors have no conflict of interest.
References
- 1.Cooray SN, Clark AJ. Melanocortin receptors and their accessory proteins. Mol Cell Endocrinol. 2011;331:215–221. doi: 10.1016/j.mce.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 2.Smith AI, Funder JW Proopiomelanocortin processing in the pituitary, central nervous system, and peripheral tissues. Endocr Rev. 1988;9:159–179. doi: 10.1210/edrv-9-1-159. [DOI] [PubMed] [Google Scholar]
- 3.Bultman SJ, Michaud EJ, Woychik RP. Molecular characterization of the mouse agouti locus. Cell. 1992;71:1195–1204. doi: 10.1016/s0092-8674(05)80067-4. [DOI] [PubMed] [Google Scholar]
- 4.Wilson BD, Ollmann MM, Kang L, Stoffel M, Bell GI, Barsh GS. Structure and function of ASP, the human homolog of the mouse agouti gene. Hum Mol Genet. 1995;4:223–230. doi: 10.1093/hmg/4.2.223. [DOI] [PubMed] [Google Scholar]
- 5.Voisey J, Kelly G, Van Daal A. Agouti signal protein regulation in human melanoma cells. Pigment Cell Res. 2003;16:65–71. doi: 10.1034/j.1600-0749.2003.00007.x. [DOI] [PubMed] [Google Scholar]
- 6.Ollmann MM, Wilson BD, Yang YK, Kerns JA, Chen Y, Gantz I, Barsh GS. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science. 1997;278:135–138. doi: 10.1126/science.278.5335.135. [DOI] [PubMed] [Google Scholar]
- 7.Bicknell AB, Lomthaisong K, Gladwell RT, Lowry PJ. Agouti related protein in the rat adrenal cortex: implications for novel autocrine mechanisms modulating the actions of pro-opiomelanocortin peptides. J Neuroendocrinol. 2000;12:977–982. doi: 10.1046/j.1365-2826.2000.00543.x. [DOI] [PubMed] [Google Scholar]
- 8.Tatro JB, Atkins M, Mier JW, Hardarson S, Wolfe H, Smith T, Entwistle ML, Reichlin S. Melanotropin receptors demonstrated in situ in human melanoma. J Clin Invest. 1990;85:1825–1832. doi: 10.1172/JCI114642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chhajlani V, Wikberg JE. Molecular cloning and expression of the human melanocyte stimulating hormone receptor cDNA. FEBS Lett. 1992;309:417–420. doi: 10.1016/0014-5793(92)80820-7. [DOI] [PubMed] [Google Scholar]
- 10.Mountjoy KG, Robbins LS, Mortrud MT, Cone RD. The cloning of a family of genes that encode the melanocortin receptors. Science. 1992;257:1248–1251. doi: 10.1126/science.1325670. [DOI] [PubMed] [Google Scholar]
- 11.Gantz I, Konda Y, Tashiro T, Shimoto Y, Miwa H, Munzert G, Watson SJ, DelValle J, Yamada T. Molecular cloning of a novel melanocortin receptor. J Biol Chem. 1993;268:8246–8250. [PubMed] [Google Scholar]
- 12.Gantz I, Miwa H, Konda Y, Shimoto Y, Tashiro T, Watson SJ, DelValle J, Yamada T. Molecular cloning, expression, and gene localization of a fourth melanocortin receptor. J Biol Chem. 1993;268:15174–15179. [PubMed] [Google Scholar]
- 13.Gantz I, Shimoto Y, Konda Y, Miwa H, Dickinson CJ, Yamada T. Molecular cloning, expression, and characterization of a fifth melanocortin receptor. Biochem Biophys Res Commun. 1994;200:1214–1220. doi: 10.1006/bbrc.1994.1580. [DOI] [PubMed] [Google Scholar]
- 14.Griffon N, Mignon V, Facchinetti P, Diaz J, Schwartz JC, Sokoloff P. Molecular cloning and characterization of the rat fifth melanocortin receptor. Biochem Biophys Res Commun. 1994;200:1007–1014. doi: 10.1006/bbrc.1994.1550. [DOI] [PubMed] [Google Scholar]
- 15.Labbé O, Desarnaud F, Eggerickx D, Vassart G, Parmentier M. Molecular cloning of a mouse melanocortin 5 receptor gene widely expressed in peripheral tissues. Biochemistry. 1994;33:4543–4549. doi: 10.1021/bi00181a015. [DOI] [PubMed] [Google Scholar]
- 16.Rodrigues AR, Sousa D, Almeida H, Gouveia AM. Structural determinants regulating cell surface targeting of melanocortin receptors. J Mol Endocrinol. 2013;51:R23–R32. doi: 10.1530/JME-13-0055. [DOI] [PubMed] [Google Scholar]
- 17.Cone RD. Studies on the physiological functions of the melanocortin system. Endocr Rev. 2006;27:736–749. doi: 10.1210/er.2006-0034. [DOI] [PubMed] [Google Scholar]
- 18.Catania A, Gatti S, Colombo G, Lipton JM. Targeting melanocortin receptors as a novel strategy to control inflammation. Pharmacol Rev. 2004;56:1–29. doi: 10.1124/pr.56.1.1. [DOI] [PubMed] [Google Scholar]
- 19.Catania A, Lonati C, Sordi A, Carlin A, Leonardi P, Gatti S. The melanocortin system in control of inflammation. Sci World J. 2010;10:1840–1853. doi: 10.1100/tsw.2010.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thistlethwaite D, Darling JA, Fraser R, Mason PA, Rees LH, Harkness RA. Familial glucocorticoid deficiency. Studies of diagnosis and pathogenesis. Arch Dis Child. 1975;50:291–297. doi: 10.1136/adc.50.4.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chung TT, Webb TR, Chan LF, Cooray SN, Metherell LA, King PJ, Chapple JP, Clark AJ. The majority of adrenocorticotropin receptor (melanocortin 2 receptor) mutations found in familial glucocorticoid deficiency type 1 lead to defective trafficking of the receptor to the cell surface. J Clin Endocrinol Metab. 2008;93:4948–4954. doi: 10.1210/jc.2008-1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clark AJ, Weber A. Adrenocorticotropin insensitivity syndromes. Endocr Rev. 1998;19:828–843. doi: 10.1210/edrv.19.6.0351. [DOI] [PubMed] [Google Scholar]
- 23.Chida D, Nakagawa S, Nagai S, Sagara H, Katsumata H, Imaki T, Suzuki H, Mitani F, Ogishima T, Shimizu C, et al. Melanocortin 2 receptor is required for adrenal gland development, steroidogenesis, and neonatal gluconeogenesis. Proc Natl Acad Sci USA. 2007;104:18205–18210. doi: 10.1073/pnas.0706953104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Slominski A, Ermak G, Mihm M. ACTH receptor, CYP11A1, CYP17 and CYP21A2 genes are expressed in skin. J Clin Endocrinol Metab. 1996;81:2746–2749. doi: 10.1210/jcem.81.7.8675607. [DOI] [PubMed] [Google Scholar]
- 25.Norman D, Isidori AM, Frajese V, Caprio M, Chew SL, Grossman AB, Clark AJ, Michael Besser G, Fabbri A. ACTH and alpha-MSH inhibit leptin expression and secretion in 3T3-L1 adipocytes: model for a central-peripheral melanocortin-leptin pathway. Mol Cell Endocrinol. 2003;200:99–109. doi: 10.1016/s0303-7207(02)00410-0. [DOI] [PubMed] [Google Scholar]
- 26.Boston BA, Cone RD. Characterization of melanocortin receptor subtype expression in murine adipose tissues and in the 3T3-L1 cell line. Endocrinology. 1996;137:2043–2050. doi: 10.1210/endo.137.5.8612546. [DOI] [PubMed] [Google Scholar]
- 27.Cho KJ, Shim JH, Cho MC, Choe YK, Hong JT, Moon DC, Kim JW, Yoon DY. Signaling pathways implicated in alpha-melanocyte stimulating hormone-induced lipolysis in 3T3-L1 adipocytes. J Cell Biochem. 2005;96:869–878. doi: 10.1002/jcb.20561. [DOI] [PubMed] [Google Scholar]
- 28.Møller CL, Raun K, Jacobsen ML, Pedersen T, Holst B, Conde-Frieboes KW, Wulff BS. Characterization of murine melanocortin receptors mediating adipocyte lipolysis and examination of signalling pathways involved. Mol Cell Endocrinol. 2011;341:9–17. doi: 10.1016/j.mce.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 29.Boston BA. The role of melanocortins in adipocyte function. Ann NY Acad Sci. 1999;885:75–84. doi: 10.1111/j.1749-6632.1999.tb08666.x. [DOI] [PubMed] [Google Scholar]
- 30.Mountjoy KG. Distribution and function of melanocortin receptors within the brain. Adv Exp Med Biol. 2010;681:29–48. doi: 10.1007/978-1-4419-6354-3_3. [DOI] [PubMed] [Google Scholar]
- 31.Feng N, Young SF, Aguilera G, Puricelli E, Adler-Wailes DC, Sebring NG, Yanovski JA. Co-occurrence of two partially inactivating polymorphisms of MC3R is associated with pediatric-onset obesity. Diabetes. 2005;54:2663–2667. doi: 10.2337/diabetes.54.9.2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mencarelli M, Dubern B, Alili R, Maestrini S, Benajiba L, Tagliaferri M, Galan P, Rinaldi M, Simon C, Tounian P, et al. Rare melanocortin-3 receptor mutations with in vitro functional consequences are associated with human obesity. Hum Mol Genet. 2011;20:392–399. doi: 10.1093/hmg/ddq472. [DOI] [PubMed] [Google Scholar]
- 33.Savastano DM, Tanofsky-Kraff M, Han JC, Ning C, Sorg RA, Roza CA, Wolkoff LE, Anandalingam K, Jefferson-George KS, Figueroa RE, et al. Energy intake and energy expenditure among children with polymorphisms of the melanocortin-3 receptor. Am J Clin Nutr. 2009;90:912–920. doi: 10.3945/ajcn.2009.27537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zegers D, Beckers S, de Freitas F, Peeters AV, Mertens IL, Verhulst SL, Rooman RP, Timmermans JP, Desager KN, Massa G, et al. Identification of three novel genetic variants in the melanocortin-3 receptor of obese children. Obesity (Silver Spring) 2011;19:152–159. doi: 10.1038/oby.2010.127. [DOI] [PubMed] [Google Scholar]
- 35.Loos RJ. The genetic epidemiology of melanocortin 4 receptor variants. Eur J Pharmacol. 2011;660:156–164. doi: 10.1016/j.ejphar.2011.01.033. [DOI] [PubMed] [Google Scholar]
- 36.Martin WJ, MacIntyre DE. Melanocortin receptors and erectile function. Eur Urol. 2004;45:706–713. doi: 10.1016/j.eururo.2003.03.001. [DOI] [PubMed] [Google Scholar]
- 37.Wikberg JE, Mutulis F. Targeting melanocortin receptors: an approach to treat weight disorders and sexual dysfunction. Nat Rev Drug Discov. 2008;7:307–323. doi: 10.1038/nrd2331. [DOI] [PubMed] [Google Scholar]
- 38.Starowicz K, Przewłocka B. The role of melanocortins and their receptors in inflammatory processes, nerve regeneration and nociception. Life Sci. 2003;73:823–847. doi: 10.1016/s0024-3205(03)00349-7. [DOI] [PubMed] [Google Scholar]
- 39.Starowicz K, Mousa SA, Obara I, Chocyk A, Przewłocki R, Wedzony K, Machelska H, Przewłocka B. Peripheral antinociceptive effects of MC4 receptor antagonists in a rat model of neuropathic pain—a biochemical and behavioral study. Pharmacol Rep. 2009;61:1086–1095. doi: 10.1016/s1734-1140(09)70171-9. [DOI] [PubMed] [Google Scholar]
- 40.Lee DJ, Taylor AW. Following EAU recovery there is an associated MC5r-dependent APC induction of regulatory immunity in the spleen. Invest Ophthalmol Vis Sci. 2011;52:8862–8867. doi: 10.1167/iovs.11-8153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taylor A, Namba K. In vitro induction of CD25+ CD4+ regulatory T cells by the neuropeptide alpha-melanocyte stimulating hormone (alpha-MSH) Immunol Cell Biol. 2001;79:358–367. doi: 10.1046/j.1440-1711.2001.01022.x. [DOI] [PubMed] [Google Scholar]
- 42.Taylor AW, Kitaichi N, Biros D. Melanocortin 5 receptor and ocular immunity. Cell Mol Biol (Noisy-le-grand) 2006;52:53–59. [PubMed] [Google Scholar]
- 43.Taylor AW, Lee D. Applications of the role of α-MSH in ocular immune privilege. Adv Exp Med Biol. 2010;681:143–149. doi: 10.1007/978-1-4419-6354-3_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Buggy JJ. Binding of alpha-melanocyte-stimulating hormone to its G-protein-coupled receptor on B-lymphocytes activates the Jak/STAT pathway. Biochem J. 1998;331(Pt 1):211–216. doi: 10.1042/bj3310211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jun DJ, Na KY, Kim W, Kwak D, Kwon EJ, Yoon JH, Yea K, Lee H, Kim J, Suh PG, et al. Melanocortins induce interleukin 6 gene expression and secretion through melanocortin receptors 2 and 5 in 3T3-L1 adipocytes. J Mol Endocrinol. 2010;44:225–236. doi: 10.1677/JME-09-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.An JJ, Rhee Y, Kim SH, Kim DM, Han DH, Hwang JH, Jin YJ, Cha BS, Baik JH, Lee WT, Lim SK. Peripheral effect of alpha-melanocyte-stimulating hormone on fatty acid oxidation in skeletal muscle. J Biol Chem. 2007;282:2862–2870. doi: 10.1074/jbc.M603454200. [DOI] [PubMed] [Google Scholar]
- 47.Rodrigues AR, Almeida H, Gouveia AM. Alpha-MSH signalling via melanocortin 5 receptor promotes lipolysis and impairs re-esterification in adipocytes. Biochim Biophys Acta. 2013;1831:1267–1275. doi: 10.1016/j.bbalip.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 48.Chan LF, Webb TR, Chung TT, Meimaridou E, Cooray SN, Guasti L, Chapple JP, Egertová M, Elphick MR, Cheetham ME, et al. MRAP and MRAP2 are bidirectional regulators of the melanocortin receptor family. Proc Natl Acad Sci USA. 2009;106:6146–6151. doi: 10.1073/pnas.0809918106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Noon LA, Franklin JM, King PJ, Goulding NJ, Hunyady L, Clark AJ. Failed export of the adrenocorticotrophin receptor from the endoplasmic reticulum in non-adrenal cells: evidence in support of a requirement for a specific adrenal accessory factor. J Endocrinol. 2002;174:17–25. doi: 10.1677/joe.0.1740017. [DOI] [PubMed] [Google Scholar]
- 50.Cooray SN, Almiro Do Vale I, Leung KY, Webb TR, Chapple JP, Egertová M, Cheetham ME, Elphick MR, Clark AJ. The melanocortin 2 receptor accessory protein exists as a homodimer and is essential for the function of the melanocortin 2 receptor in the mouse y1 cell line. Endocrinology. 2008;149:1935–1941. doi: 10.1210/en.2007-1463. [DOI] [PubMed] [Google Scholar]
- 51.Sebag JA, Hinkle PM. Opposite effects of the melanocortin-2 (MC2) receptor accessory protein MRAP on MC2 and MC5 receptor dimerization and trafficking. J Biol Chem. 2009;284:22641–22648. doi: 10.1074/jbc.M109.022400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sebag JA, Hinkle PM. Regions of melanocortin 2 (MC2) receptor accessory protein necessary for dual topology and MC2 receptor trafficking and signaling. J Biol Chem. 2009;284:610–618. doi: 10.1074/jbc.M804413200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roy S, Rached M, Gallo-Payet N. Differential regulation of the human adrenocorticotropin receptor [melanocortin-2 receptor (MC2R)] by human MC2R accessory protein isoforms alpha and beta in isogenic human embryonic kidney 293 cells. Mol Endocrinol. 2007;21:1656–1669. doi: 10.1210/me.2007-0041. [DOI] [PubMed] [Google Scholar]
- 54.Sebag JA, Hinkle PM. Regulation of G protein-coupled receptor signaling: specific dominant-negative effects of melanocortin 2 receptor accessory protein 2. Sci Signal. 2010;3:ra28. doi: 10.1126/scisignal.2000593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sebag JA, Hinkle PM. Melanocortin-2 receptor accessory protein MRAP forms antiparallel homodimers. Proc Natl Acad Sci USA. 2007;104:20244–20249. doi: 10.1073/pnas.0708916105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Webb TR, Clark AJ. Minireview: the melanocortin 2 receptor accessory proteins. Mol Endocrinol. 2010;24:475–484. doi: 10.1210/me.2009-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dong C, Filipeanu CM, Duvernay MT, Wu G. Regulation of G protein-coupled receptor export trafficking. Biochim Biophys Acta. 2007;1768:853–870. doi: 10.1016/j.bbamem.2006.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jean-Alphonse F, Hanyaloglu AC. Regulation of GPCR signal networks via membrane trafficking. Mol Cell Endocrinol. 2011;331:205–214. doi: 10.1016/j.mce.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 59.Asai M, Ramachandrappa S, Joachim M, Shen Y, Zhang R, Nuthalapati N, Ramanathan V, Strochlic DE, Ferket P, Linhart K, et al. Loss of function of the melanocortin 2 receptor accessory protein 2 is associated with mammalian obesity. Science. 2013;341:275–278. doi: 10.1126/science.1233000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sebag JA, Zhang C, Hinkle PM, Bradshaw AM, Cone RD. Developmental control of the melanocortin-4 receptor by MRAP2 proteins in zebrafish. Science. 2013;341:278–281. doi: 10.1126/science.1232995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cooray SN, Guasti L, Clark AJ. The E3 ubiquitin ligase Mahogunin ubiquitinates the melanocortin 2 receptor. Endocrinology. 2011;152:4224–4231. doi: 10.1210/en.2011-0147. [DOI] [PubMed] [Google Scholar]
- 62.He L, Eldridge AG, Jackson PK, Gunn TM, Barsh GS. Accessory proteins for melanocortin signaling: attractin and mahogunin. Ann N Y Acad Sci. 2003;994:288–298. doi: 10.1111/j.1749-6632.2003.tb03192.x. [DOI] [PubMed] [Google Scholar]
- 63.Shenoy SK. Seven-transmembrane receptors and ubiquitination. Circ Res. 2007;100:1142–1154. doi: 10.1161/01.RES.0000261939.88744.5a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Marchese A, Trejo J. Ubiquitin-dependent regulation of G protein-coupled receptor trafficking and signaling. Cell Signal. 2013;25:707–716. doi: 10.1016/j.cellsig.2012.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dores MR, Trejo J. Ubiquitination of G protein-coupled receptors: functional implications and drug discovery. Mol Pharmacol. 2012;82:563–570. doi: 10.1124/mol.112.079418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pérez-Oliva AB, Olivares C, Jiménez-Cervantes C, García-Borrón JC. Mahogunin ring finger-1 (MGRN1) E3 ubiquitin ligase inhibits signaling from melanocortin receptor by competition with Galphas. J Biol Chem. 2009;284:31714–31725. doi: 10.1074/jbc.M109.028100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang G, Wu G. Small GTPase regulation of GPCR anterograde trafficking. Trends Pharmacol Sci. 2012;33:28–34. doi: 10.1016/j.tips.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Meimaridou E, Gooljar SB, Ramnarace N, Anthonypillai L, Clark AJ, Chapple JP. The cytosolic chaperone Hsc70 promotes traffic to the cell surface of intracellular retained melanocortin-4 receptor mutants. Mol Endocrinol. 2011;25:1650–1660. doi: 10.1210/me.2011-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wettschureck N, Offermanns S. Mammalian G proteins and their cell type specific functions. Physiol Rev. 2005;85:1159–1204. doi: 10.1152/physrev.00003.2005. [DOI] [PubMed] [Google Scholar]
- 70.Taskén K, Aandahl EM. Localized effects of cAMP mediated by distinct routes of protein kinase A. Physiol Rev. 2004;84:137–167. doi: 10.1152/physrev.00021.2003. [DOI] [PubMed] [Google Scholar]
- 71.May LT, Hill SJ. ERK phosphorylation: spatial and temporal regulation by G protein-coupled receptors. Int J Biochem Cell Biol. 2008;40:2013–2017. doi: 10.1016/j.biocel.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 72.Woehler A, Ponimaskin EG. G protein—mediated signaling: same receptor, multiple effectors. Curr Mol Pharmacol. 2009;2:237–248. doi: 10.2174/1874467210902030237. [DOI] [PubMed] [Google Scholar]
- 73.Gudermann T, Kalkbrenner F, Schultz G. Diversity and selectivity of receptor-G protein interaction. Annu Rev Pharmacol Toxicol. 1996;36:429–459. doi: 10.1146/annurev.pa.36.040196.002241. [DOI] [PubMed] [Google Scholar]
- 74.Duarte T, Menezes-Rodrigues FS, Godinho RO. Contribution of the extracellular cAMP-adenosine pathway to dual coupling of β2-adrenoceptors to Gs and Gi proteins in mouse skeletal muscle. J Pharmacol Exp Ther. 2012;341:820–828. doi: 10.1124/jpet.112.192997. [DOI] [PubMed] [Google Scholar]
- 75.Tang J, Chen J, Ramanjaneya M, Punn A, Conner AC, Randeva HS. The signalling profile of recombinant human orexin-2 receptor. Cell Signal. 2008;20:1651–1661. doi: 10.1016/j.cellsig.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 76.Marinissen MJ, Servitja JM, Offermanns S, Simon MI, Gutkind JS. Thrombin protease-activated receptor-1 signals through Gq- and G13-initiated MAPK cascades regulating c-Jun expression to induce cell transformation. J Biol Chem. 2003;278:46814–46825. doi: 10.1074/jbc.M305709200. [DOI] [PubMed] [Google Scholar]
- 77.McLaughlin JN, Shen L, Holinstat M, Brooks JD, Dibenedetto E, Hamm HE. Functional selectivity of G protein signaling by agonist peptides and thrombin for the protease-activated receptor-1. J Biol Chem. 2005;280:25048–25059. doi: 10.1074/jbc.M414090200. [DOI] [PubMed] [Google Scholar]
- 78.Goldsmith ZG, Dhanasekaran DN. G protein regulation of MAPK networks. Oncogene. 2007;26:3122–3142. doi: 10.1038/sj.onc.1210407. [DOI] [PubMed] [Google Scholar]
- 79.Gutkind JS. Regulation of mitogen-activated protein kinase signaling networks by G protein-coupled receptors. Sci STKE. 2000;2000:re1. doi: 10.1126/stke.2000.40.re1. [DOI] [PubMed] [Google Scholar]
- 80.Luttrell LM. ‘Location, location, location’: activation and targeting of MAP kinases by G protein-coupled receptors. J Mol Endocrinol. 2003;30:117–126. doi: 10.1677/jme.0.0300117. [DOI] [PubMed] [Google Scholar]
- 81.Gantz I, Fong TM. The melanocortin system. Am J Physiol Endocrinol Metab. 2003;284:E468–E474. doi: 10.1152/ajpendo.00434.2002. [DOI] [PubMed] [Google Scholar]
- 82.Chai B, Li JY, Zhang W, Ammori JB, Mulholland MW. Melanocortin-3 receptor activates MAP kinase via PI3 kinase. Regul Pept. 2007;139:115–121. doi: 10.1016/j.regpep.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 83.Rodrigues AR, Almeida H, Gouveia AM. Melanocortin 5 receptor signaling and internalization: role of MAPK/ERK pathway and β-arrestins 1/2. Mol Cell Endocrinol. 2012;361:69–79. doi: 10.1016/j.mce.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 84.Büch TR, Heling D, Damm E, Gudermann T, Breit A. Pertussis toxin-sensitive signaling of melanocortin-4 receptors in hypothalamic GT1-7 cells defines agouti-related protein as a biased agonist. J Biol Chem. 2009;284:26411–26420. doi: 10.1074/jbc.M109.039339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Newman EA, Chai BX, Zhang W, Li JY, Ammori JB, Mulholland MW. Activation of the melanocortin-4 receptor mobilizes intracellular free calcium in immortalized hypothalamic neurons. J Surg Res. 2006;132:201–207. doi: 10.1016/j.jss.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 86.Herraiz C, Journé F, Abdel-Malek Z, Ghanem G, Jiménez-Cervantes C, García-Borrón JC. Signaling from the human melanocortin 1 receptor to ERK1 and ERK2 mitogen-activated protein kinases involves transactivation of cKIT. Mol Endocrinol. 2011;25:138–156. doi: 10.1210/me.2010-0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Herraiz C, Journé F, Ghanem G, Jiménez-Cervantes C, García-Borrón JC. Functional status and relationships of melanocortin 1 receptor signaling to the cAMP and extracellular signal-regulated protein kinases 1 and 2 pathways in human melanoma cells. Int J Biochem Cell Biol. 2012;44:2244–2252. doi: 10.1016/j.biocel.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 88.Le T, Schimmer BP. The regulation of MAPKs in Y1 mouse adrenocortical tumor cells. Endocrinology. 2001;142:4282–4287. doi: 10.1210/endo.142.10.8441. [DOI] [PubMed] [Google Scholar]
- 89.Roy S, Pinard S, Chouinard L, Gallo-Payet N. Adrenocorticotropin hormone (ACTH) effects on MAPK phosphorylation in human fasciculata cells and in embryonic kidney 293 cells expressing human melanocortin 2 receptor (MC2R) and MC2R accessory protein (MRAP)β. Mol Cell Endocrinol. 2011;336:31–40. doi: 10.1016/j.mce.2010.12.030. [DOI] [PubMed] [Google Scholar]
- 90.Janes ME, Chu KM, Clark AJ, King PJ. Mechanisms of adrenocorticotropin-induced activation of extracellularly regulated kinase 1/2 mitogen-activated protein kinase in the human H295R adrenal cell line. Endocrinology. 2008;149:1898–1905. doi: 10.1210/en.2007-0949. [DOI] [PubMed] [Google Scholar]
- 91.Chai B, Li JY, Zhang W, Newman E, Ammori J, Mulholland MW. Melanocortin-4 receptor-mediated inhibition of apoptosis in immortalized hypothalamic neurons via mitogen-activated protein kinase. Peptides. 2006;27:2846–2857. doi: 10.1016/j.peptides.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 92.Vongs A, Lynn NM, Rosenblum CI. Activation of MAP kinase by MC4-R through PI3 kinase. Regul Pept. 2004;120:113–118. doi: 10.1016/j.regpep.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 93.Buscà R, Abbe P, Mantoux F, Aberdam E, Peyssonnaux C, Eychène A, Ortonne JP, Ballotti R. Ras mediates the cAMP-dependent activation of extracellular signal-regulated kinases (ERKs) in melanocytes. EMBO J. 2000;19:2900–2910. doi: 10.1093/emboj/19.12.2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rodrigues AR, Pignatelli D, Almeida H, Gouveia AM. Melanocortin 5 receptor activates ERK1/2 through a PI3K-regulated signaling mechanism. Mol Cell Endocrinol. 2009;303:74–81. doi: 10.1016/j.mce.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 95.Mountjoy KG, Kong PL, Taylor JA, Willard DH, Wilkison WO. Melanocortin receptor-mediated mobilization of intracellular free calcium in HEK293 cells. Physiol Genomics. 2001;5:11–19. doi: 10.1152/physiolgenomics.2001.5.1.11. [DOI] [PubMed] [Google Scholar]
- 96.Newton RA, Smit SE, Barnes CC, Pedley J, Parsons PG, Sturm RA. Activation of the cAMP pathway by variant human MC1R alleles expressed in HEK and in melanoma cells. Peptides. 2005;26:1818–1824. doi: 10.1016/j.peptides.2004.11.031. [DOI] [PubMed] [Google Scholar]
- 97.Herraiz C, Jiménez-Cervantes C, Zanna P, García-Borrón JC. Melanocortin 1 receptor mutations impact differentially on signalling to the cAMP and the ERK mitogen-activated protein kinase pathways. FEBS Lett. 2009;583:3269–3274. doi: 10.1016/j.febslet.2009.09.023. [DOI] [PubMed] [Google Scholar]
- 98.Cheng LB, Cheng L, Bi HE, Zhang ZQ, Yao J, Zhou XZ, Jiang Q. Alpha-melanocyte stimulating hormone protects retinal pigment epithelium cells from oxidative stress through activation of melanocortin 1 receptor-Akt-mTOR signaling. Biochem Biophys Res Commun. 2014;443:447–452. doi: 10.1016/j.bbrc.2013.11.113. [DOI] [PubMed] [Google Scholar]
- 99.Kilianova Z, Basora N, Kilian P, Payet MD, Gallo-Payet N. Human melanocortin receptor 2 expression and functionality: effects of protein kinase A and protein kinase C on desensitization and internalization. Endocrinology. 2006;147:2325–2337. doi: 10.1210/en.2005-0991. [DOI] [PubMed] [Google Scholar]
- 100.Gallo-Payet N, Grazzini E, Côté M, Chouinard L, Chorvátová A, Bilodeau L, Payet MD, Guillon G. Role of Ca2+ in the action of adrenocorticotropin in cultured human adrenal glomerulosa cells. J Clin Invest. 1996;98:460–466. doi: 10.1172/JCI118812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Winnay JN, Hammer GD. Adrenocorticotropic hormone-mediated signaling cascades coordinate a cyclic pattern of steroidogenic factor 1-dependent transcriptional activation. Mol Endocrinol. 2006;20:147–166. doi: 10.1210/me.2005-0215. [DOI] [PubMed] [Google Scholar]
- 102.Lotfi CF, Todorovic Z, Armelin HA, Schimmer BP. Unmasking a growth-promoting effect of the adrenocorticotropic hormone in Y1 mouse adrenocortical tumor cells. J Biol Chem. 1997;272:29886–29891. doi: 10.1074/jbc.272.47.29886. [DOI] [PubMed] [Google Scholar]
- 103.Forti FL, Dias MH, Armelin HA. ACTH receptor: ectopic expression, activity and signaling. Mol Cell Biochem. 2006;293:147–160. doi: 10.1007/s11010-006-9237-0. [DOI] [PubMed] [Google Scholar]
- 104.Baccaro RB, Mendonça PO, Torres TE, Lotfi CF. Immunohistochemical Jun/Fos protein localization and DNA synthesis in rat adrenal cortex after treatment with ACTH or FGF2. Cell Tissue Res. 2007;328:7–18. doi: 10.1007/s00441-006-0352-8. [DOI] [PubMed] [Google Scholar]
- 105.Lefrancois-Martinez AM, Blondet-Trichard A, Binart N, Val P, Chambon C, Sahut-Barnola I, Pointud JC, Martinez A. Transcriptional control of adrenal steroidogenesis: novel connection between Janus kinase (JAK) 2 protein and protein kinase A (PKA) through stabilization of cAMP response element-binding protein (CREB) transcription factor. J Biol Chem. 2011;286:32976–32985. doi: 10.1074/jbc.M111.218016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nyan DC, Anbazhagan R, Hughes-Darden CA, Wachira SJ. Endosomal colocalization of melanocortin-3 receptor and beta-arrestins in CAD cells with altered modification of AKT/PKB. Neuropeptides. 2008;42:355–366. doi: 10.1016/j.npep.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 107.Konda Y, Gantz I, DelValle J, Shimoto Y, Miwa H, Yamada T. Interaction of dual intracellular signaling pathways activated by the melanocortin-3 receptor. J Biol Chem. 1994;269:13162–13166. [PubMed] [Google Scholar]
- 108.Bertolini A, Tacchi R, Vergoni AV. Brain effects of melanocortins. Pharmacol Res. 2009;59:13–47. doi: 10.1016/j.phrs.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 109.Wachira SJ, Hughes-Darden CA, Taylor CV, Ochillo R, Robinson TJ. Evidence for the interaction of protein kinase C and melanocortin 3-receptor signaling pathways. Neuropeptides. 2003;37:201–210. doi: 10.1016/s0143-4179(03)00026-x. [DOI] [PubMed] [Google Scholar]
- 110.Daniels D, Patten CS, Roth JD, Yee DK, Fluharty SJ. Melanocortin receptor signaling through mitogen-activated protein kinase in vitro and in rat hypothalamus. Brain Res. 2003;986:1–11. doi: 10.1016/s0006-8993(03)03162-7. [DOI] [PubMed] [Google Scholar]
- 111.Sutton GM, Duos B, Patterson LM, Berthoud HR. Melanocortinergic modulation of cholecystokinin-induced suppression of feeding through extracellular signal-regulated kinase signaling in rat solitary nucleus. Endocrinology. 2005;146:3739–3747. doi: 10.1210/en.2005-0562. [DOI] [PubMed] [Google Scholar]
- 112.Patten CS, Daniels D, Suzuki A, Fluharty SJ, Yee DK. Structural and signaling requirements of the human melanocortin 4 receptor for MAP kinase activation. Regul Pept. 2007;142:111–122. doi: 10.1016/j.regpep.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 113.Chai B, Li JY, Zhang W, Wang H, Mulholland MW. Melanocortin-4 receptor activation inhibits c-Jun N-terminal kinase activity and promotes insulin signaling. Peptides. 2009;30:1098–1104. doi: 10.1016/j.peptides.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hoogduijn MJ, McGurk S, Smit NP, Nibbering PH, Ancans J, van der Laarse A, Thody AJ. Ligand-dependent activation of the melanocortin 5 receptor: cAMP production and ryanodine receptor-dependent elevations of [Ca(2+)](I) Biochem Biophys Res Commun. 2002;290:844–850. doi: 10.1006/bbrc.2001.6283. [DOI] [PubMed] [Google Scholar]
- 115.Sun Y, McGarrigle D, Huang XY. When a G protein-coupled receptor does not couple to a G protein. Mol BioSyst. 2007;3:849–854. doi: 10.1039/b706343a. [DOI] [PubMed] [Google Scholar]
- 116.Defea K. Beta-arrestins and heterotrimeric G-proteins: collaborators and competitors in signal transduction. Br J Pharmacol. 2008;153(Suppl 1):S298–S309. doi: 10.1038/sj.bjp.0707508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Luttrell LM, Lefkowitz RJ. The role of beta-arrestins in the termination and transduction of G-protein-coupled receptor signals. J Cell Sci. 2002;115:455–465. doi: 10.1242/jcs.115.3.455. [DOI] [PubMed] [Google Scholar]
- 118.Calebiro D, Nikolaev VO, Persani L, Lohse MJ. Signaling by internalized G-protein-coupled receptors. Trends Pharmacol Sci. 2010;31:221–228. doi: 10.1016/j.tips.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 119.Marchese A, Paing MM, Temple BR, Trejo J. G protein-coupled receptor sorting to endosomes and lysosomes. Annu Rev Pharmacol Toxicol. 2008;48:601–629. doi: 10.1146/annurev.pharmtox.48.113006.094646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cai M, Varga EV, Stankova M, Mayorov A, Perry JW, Yamamura HI, Trivedi D, Hruby VJ. Cell signaling and trafficking of human melanocortin receptors in real time using two-photon fluorescence and confocal laser microscopy: differentiation of agonists and antagonists. Chem Biol Drug Des. 2006;68:183–193. doi: 10.1111/j.1747-0285.2006.00432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Shinyama H, Masuzaki H, Fang H, Flier JS. Regulation of melanocortin-4 receptor signaling: agonist-mediated desensitization and internalization. Endocrinology. 2003;144:1301–1314. doi: 10.1210/en.2002-220931. [DOI] [PubMed] [Google Scholar]
- 122.Gao Z, Lei D, Welch J, Le K, Lin J, Leng S, Duhl D. Agonist-dependent internalization of the human melanocortin-4 receptors in human embryonic kidney 293 cells. J Pharmacol Exp Ther. 2003;307:870–877. doi: 10.1124/jpet.103.055525. [DOI] [PubMed] [Google Scholar]
- 123.Mohammad S, Baldini G, Granell S, Narducci P, Martelli AM. Constitutive traffic of melanocortin-4 receptor in Neuro2A cells and immortalized hypothalamic neurons. J Biol Chem. 2007;282:4963–4974. doi: 10.1074/jbc.M608283200. [DOI] [PubMed] [Google Scholar]
- 124.Benned-Jensen T, Mokrosinski J, Rosenkilde MM. The E92K melanocortin 1 receptor mutant induces cAMP production and arrestin recruitment but not ERK activity indicating biased constitutive signaling. PLoS One. 2011;6:e24644. doi: 10.1371/journal.pone.0024644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Roy S, Roy SJ, Pinard S, Taillefer LD, Rached M, Parent JL, Gallo-Payet N. Mechanisms of melanocortin-2 receptor (MC2R) internalization and recycling in human embryonic kidney (hek) cells: identification of Key Ser/Thr (S/T) amino acids. Mol Endocrinol. 2011;25:1961–1977. doi: 10.1210/me.2011-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Breit A, Wolff K, Kalwa H, Jarry H, Büch T, Gudermann T. The natural inverse agonist agouti-related protein induces arrestin-mediated endocytosis of melanocortin-3 and -4 receptors. J Biol Chem. 2006;281:37447–37456. doi: 10.1074/jbc.M605982200. [DOI] [PubMed] [Google Scholar]
- 127.Baig AH, Swords FM, Szaszák M, King PJ, Hunyady L, Clark AJ. Agonist activated adrenocorticotropin receptor internalizes via a clathrin-mediated G protein receptor kinase dependent mechanism. Endocr Res. 2002;28:281–289. doi: 10.1081/erc-120016798. [DOI] [PubMed] [Google Scholar]
- 128.Sánchez-Laorden BL, Jiménez-Cervantes C, García-Borrón JC. Regulation of human melanocortin 1 receptor signaling and trafficking by Thr-308 and Ser-316 and its alteration in variant alleles associated with red hair and skin cancer. J Biol Chem. 2007;282:3241–3251. doi: 10.1074/jbc.M606865200. [DOI] [PubMed] [Google Scholar]
- 129.Shenoy SK, Lefkowitz RJ. Multifaceted roles of beta-arrestins in the regulation of seven-membrane-spanning receptor trafficking and signalling. Biochem J. 2003;375:503–515. doi: 10.1042/BJ20031076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lefkowitz RJ, Shenoy SK. Transduction of receptor signals by beta-arrestins. Science. 2005;308:512–517. doi: 10.1126/science.1109237. [DOI] [PubMed] [Google Scholar]
- 131.Caunt CJ, Finch AR, Sedgley KR, McArdle CA. Seven-transmembrane receptor signalling and ERK compartmentalization. Trends Endocrinol Metab. 2006;17:276–283. doi: 10.1016/j.tem.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 132.Shenoy SK, Drake MT, Nelson CD, Houtz DA, Xiao K, Madabushi S, Reiter E, Premont RT, Lichtarge O, Lefkowitz RJ. Beta-arrestin-dependent, G protein-independent ERK1/2 activation by the beta2 adrenergic receptor. J Biol Chem. 2006;281:1261–1273. doi: 10.1074/jbc.M506576200. [DOI] [PubMed] [Google Scholar]
- 133.Zhang J, Barak LS, Anborgh PH, Laporte SA, Caron MG, Ferguson SS. Cellular trafficking of G protein-coupled receptor/beta-arrestin endocytic complexes. J Biol Chem. 1999;274:10999–11006. doi: 10.1074/jbc.274.16.10999. [DOI] [PubMed] [Google Scholar]
- 134.Daaka Y, Luttrell LM, Ahn S, Della Rocca GJ, Ferguson SS, Caron MG, Lefkowitz RJ. Essential role for G protein-coupled receptor endocytosis in the activation of mitogen-activated protein kinase. J Biol Chem. 1998;273:685–688. doi: 10.1074/jbc.273.2.685. [DOI] [PubMed] [Google Scholar]
- 135.Luttrell LM, Ferguson SS, Daaka Y, Miller WE, Maudsley S, Della Rocca GJ, Lin F, Kawakatsu H, Owada K, Luttrell DK, et al. Beta-arrestin-dependent formation of beta2 adrenergic receptor-Src protein kinase complexes. Science. 1999;283:655–661. doi: 10.1126/science.283.5402.655. [DOI] [PubMed] [Google Scholar]
- 136.Abrisqueta M, Herraiz C, Pérez Oliva AB, Sanchez-Laorden BL, Olivares C, Jiménez-Cervantes C, García-Borrón JC. Differential and competitive regulation of human melanocortin 1 receptor signaling by β-arrestin isoforms. J Cell Sci. 2013;126:3724–3737. doi: 10.1242/jcs.128322. [DOI] [PubMed] [Google Scholar]
- 137.Mountjoy KG, Wong J. Obesity, diabetes and functions for proopiomelanocortin-derived peptides. Mol Cell Endocrinol. 1997;128:171–177. doi: 10.1016/s0303-7207(96)04017-8. [DOI] [PubMed] [Google Scholar]
- 138.Hadley ME, Dorr RT. Melanocortin peptide therapeutics: historical milestones, clinical studies and commercialization. Peptides. 2006;27:921–930. doi: 10.1016/j.peptides.2005.01.029. [DOI] [PubMed] [Google Scholar]
- 139.Royalty JE, Konradsen G, Eskerod O, Wulff BS, Hansen BS. Investigation of safety, tolerability, pharmacokinetics, and pharmacodynamics of single and multiple doses of a long-acting α-MSH analog in healthy overweight and obese subjects. J Clin Pharmacol. 2014;54:394–404. doi: 10.1002/jcph.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Violin JD, Lefkowitz RJ. Beta-arrestin-biased ligands at seven-transmembrane receptors. Trends Pharmacol Sci. 2007;28:416–422. doi: 10.1016/j.tips.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 141.Rajagopal S, Ahn S, Rominger DH, Gowen-MacDonald W, Lam CM, Dewire SM, Violin JD, Lefkowitz RJ. Quantifying ligand bias at seven-transmembrane receptors. Mol Pharmacol. 2011;80:367–377. doi: 10.1124/mol.111.072801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rajagopal S, Rajagopal K, Lefkowitz RJ. Teaching old receptors new tricks: biasing seven-transmembrane receptors. Nat Rev Drug Discov. 2010;9:373–386. doi: 10.1038/nrd3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Latek D, Modzelewska A, Trzaskowski B, Palczewski K, Filipek S. G protein-coupled receptors—recent advances. Acta Biochim Pol. 2012;59:515–529. [PMC free article] [PubMed] [Google Scholar]
- 144.Whalen EJ, Rajagopal S, Lefkowitz RJ. Therapeutic potential of β-arrestin- and G protein-biased agonists. Trends Mol Med. 2011;17:126–139. doi: 10.1016/j.molmed.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Granell S, Molden BM, Baldini G. Exposure of MC4R to agonist in the endoplasmic reticulum stabilizes an active conformation of the receptor that does not desensitize. Proc Natl Acad Sci USA. 2013;110:E4733–E4742. doi: 10.1073/pnas.1219808110. [DOI] [PMC free article] [PubMed] [Google Scholar]