Abstract
Segregating cells into compartments during embryonic development is essential for growth and pattern formation. In the developing hindbrain, boundaries separate molecularly, physically and neuroanatomically distinct segments called rhombomeres. After rhombomeric cells have acquired their identity, interhombomeric boundaries restrict cell intermingling between adjacent rhombomeres and act as signaling centers to pattern the surrounding tissue. Several works have stressed the relevance of Eph/ephrin signaling in rhombomeric cell sorting. Recent data have unveiled the role of this pathway in the assembly of actomyosin cables as an important mechanism for keeping cells from different rhombomeres segregated. In this Review, we will provide a short summary of recent evidences gathered in different systems suggesting that physical actomyosin barriers can be a general mechanism for tissue separation. We will discuss current evidences supporting a model where cell–cell signaling pathways, such as Eph/ephrin, govern compartmental cell sorting through modulation of the actomyosin cytoskeleton and cell adhesive properties to prevent cell intermingling.
Keywords: Hindbrain segmentation, Compartments, Boundaries, Tissue separation, Cell sorting, Cell segregation
Introduction: separating tissues
A fundamental feature of embryonic development is the separation of tissues that leads to the establishment of physically isolated embryonic cell populations. The early analysis of this feature led to the concept of compartments, which are subdivisions of embryonic tissues delimited by stable boundaries that prevent cell intermingling. Compartments were originally described in Drosophila, after the observation that the expansion of mosaic clones generated in the wing imaginal disc was restricted to one side of a straight line partitioning the tissue [1]. Later on, they were observed in other imaginal discs [2, 3] and embryonic epidermis [4]. The concept of compartments and boundaries explains how a tissue that undergoes intense cell proliferation and growth maintains its pattern during embryonic development. Importantly, these observations revealed that boundaries can be transitory and act to separate different tissues and organs, as well as to subdivide a single tissue.
Compartments and boundaries are also present in vertebrate embryos. Examples of these are the separation between germ layers—ectoderm and mesoderm—[5], and the isolation of the axial mesoderm from the adjacent presomitic mesoderm [6]. Later during embryonic development, important examples of segmented structures are: (i) the hindbrain, which is subdivided into seven compartments called rhombomeres that are highly conserved in vertebrates [7, 8]; and (ii) the somites, which arise as isolated blocks from the presomitic mesoderm in a head-to-tail succession [9].
Boundary formation
Separation between compartments is often foreshadowed by the presence of sharp gene expression borders before any sign of tissue differentiation or differences in morphology. This suggests an intimate link between patterning and restriction of cell movement (for review, see [10–12]). There are two classical questions that arise around embryonic boundaries: how position of boundaries is established, and how sharp boundaries are maintained during growth and morphogenesis. The first one relies on gene expression and cell identity. The second relates more to a process of cell separation/segregation. Recent studies have highlighted the importance of physical forces in maintaining boundaries, and the molecular control of these forces becomes a key issue (for review see [12, 13]).
The integrity of boundaries can be challenged by intercalation of dividing cells and by physical disruption and dispersal during morphogenesis. Cells challenging a boundary can be subjected either to cell plasticity or to cell sorting mechanisms. Upon cell plasticity, cells can move across gene expression boundaries and switch their identity and fate to that of its neighbors—non-lineage boundaries. Examples of this are the boundary between the wing pouch and the notum in the Drosophila wing disc [14], the somite boundaries [10, 15], and the boundary between the vertebrate foregut and hindgut [16]. On the other hand, cell sorting implies that cell fate is inherited and maintained on either side of the boundary. Therefore, mechanisms within the tissue need to be implemented to restrict cell intermingling and maintain a straight boundary while growing—lineage boundaries. In vertebrates, in vivo cell tracking experiments demonstrated that the MHB [17], as well as hindbrain boundaries [18], constitute cell lineage boundaries.
A growing body of data suggests that the local enrichment of barrier-like elements, such as actomyosin cables, is a conserved mechanism for restricting cell intermingling in different systems such as: the parasegmental boundaries in the embryonic epidermis [19] and the boundaries of distinct imaginal discs [20–24] in Drosophila; and in vertebrates the germ layers [5, 25, 26] and the hindbrain boundaries [18]. These data show how actomyosin cables restrict cell mixing between adjacent compartments conferring high tension, rigidity and stability to the boundary cell population. Nevertheless, the role of cell–cell adhesion/repulsion complexes needs to be considered in tissue separation. Recent studies on the role of Eph/ephrin signaling during germ-layer segregation support a model where tissue separation is controlled by cell surface cues, which upon cell–cell contact generate changes in cytoskeletal and adhesive properties to inhibit cell mixing [25]. Thus, the integration of multiple local cues may dictate both the global morphogenetic properties of a tissue and its separation from adjacent cell populations [12]. Now, one of the challenges is to understand two different but intimately related questions: how patterning is translated into the assembly of physical barriers to restrict cell mixing, and how disruption of boundary sharpness might affect segregation of different cell populations and the activity of these boundaries as patterning centers.
Boundaries within the central nervous system
The vertebrate embryonic neuroepithelium is characterized by a series of transient swellings that appear during the neural tube closure. Ultimately, the anterior territories give rise to the forebrain, midbrain and hindbrain, while the narrow posterior epithelium transforms into the spinal cord. These early morphological features are foreshadowed and positioned by localized expression of developmental genes establishing a Cartesian-like coordinate system of positional information along the AP and DV axes. This complex genetic network orchestrates the regional plan of the CNS and indicates future functional specializations [27, 28]. As development proceeds, neurogenesis further leads to the formation of distinct neuronal groups that will migrate to their final destination, where they will form synaptic connections and integrate into functional circuits. All these neurodevelopmental features are largely conserved across vertebrates.
During embryonic development, the CNS undergoes a patterning process along the AP axis that give rise to several boundaries, such as the Zona Limitans Intrathalamica (ZLI) between the telencephalon and diencephalon [29]; the Mid-Hindbrain Boundary (MHB) [30], which has been extensively studied due to the important role it plays as an organizing center during vertebrate brain development [31, 32]; and the hindbrain boundaries that will be explained below.
Hindbrain segmentation
We focus this review on the hindbrain, which is the most evolutionarily ancient region of the vertebrate brain [27, 33]. The hindbrain is fated to give rise to the medulla, the pons and the cerebellum. Hindbrain efferent nerves connect to different muscles and organs to control essential physiological processes, such as respiration, circulation, arousal and motor coordination. On the other hand, the cranial nerve afferent component conveys sensory stimuli from sensory organs and viscera to the hindbrain [34].
In all vertebrates, the hindbrain passes through a segmented phase shortly after the neural tube has formed. Hindbrain segmentation is a progressive process leading to the partition of the territory along the AP axis into seven segments called rhombomeres (r1–r7). Each rhombomere constitutes a developmental unit of gene expression and a cell lineage compartment [35, 36]. The segmental gene expression pattern is visible by the end of gastrulation and prefigures the presumptive rhombomeric territories. As cells segregate, the borders of gene expression refine positioning the rhombomeric boundaries precisely where morphological boundaries will appear (Fig. 1a, b) [8, 27]; this is dynamic process beautifully orchestracted, with a tight coordination between gene regulation and morphogenesis.
Fig. 1.
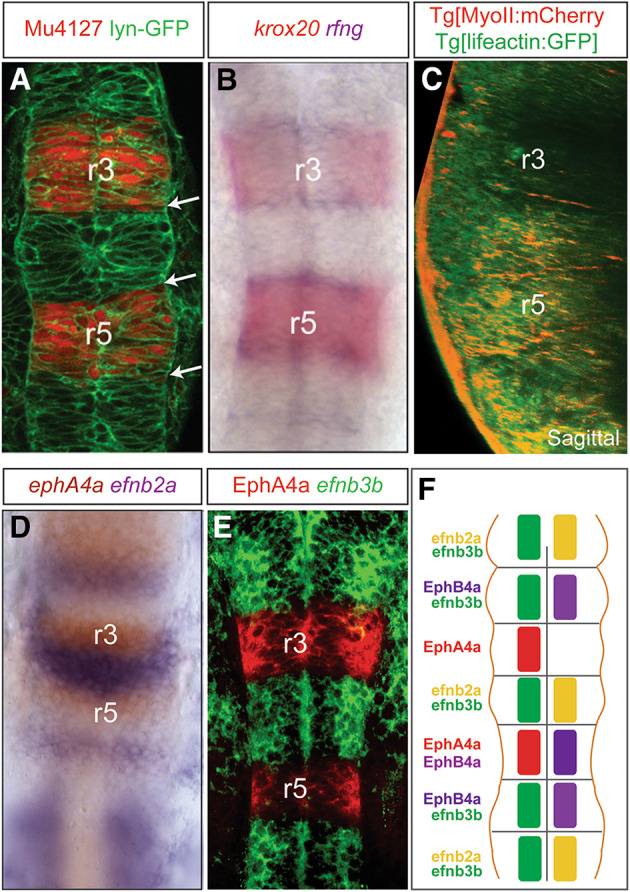
Borders of gene expression coincide with rhombomeric boundaries. a Morphological boundaries appear at the borders of rhombomeric gene expression (white arrows); Mu4127 embryos express mCherry in r3 and r5; lyn-GFP marks the plasma membranes; b double in situ hybridization (ISH) showing that segment-restricted gene expression (krox20) in the hindbrain prefigures the allocation of the boundary cell population (rfng-cells in blue). c Physical barriers appear at rhombomeric boundaries as shown by actomyosin cables in double transgenic embryos Tg[myosinII:mCherry]Tg[lifeactin:GFP]. d, e Double ISH with ephA4 and efnb2a or single efnb3b ISH followed by anti-EphA4 staining shows complementary borders of expression for ephrin ligands and Eph receptors; these borders are predicted sites for forward and reverse signaling. f Scheme summarizing the expression Eph receptors and ephrin ligands in specific rhombomeres. Note that although Eph receptors are generally expressed in odd- and ephrin ligands in even-rhombomeres, there are few exceptions: r2 and r6 display overlapping expression of Eph receptor and ephrin ligand. For simplicity, only data from zebrafish were plotted. Dorsal views with anterior to the top, except for C that is a sagittal view with dorsal to the left. r, rhombomere
Early expressed secreted molecules such as FGF and RA are important for establishing the crude AP patterning of the hindbrain through the genetic regulation of key transcriptional factors (for review see [28]). FGF8 is released from the MHB and controls the extension of r1 through the repression of Hox genes [37]. In addition, FGF3 and FGF8 are released from the r3–r5 region [38–42], where they are required for further refinement of r5 and r6 by acting together with vHnf1 to regulate expression of Krox20 in r5 and MafB/Kreisler in r5/r6 [41, 43–45]. On the other hand, treatment of mice embryos with RA demonstrated its role as posteriorizing factor in the hindbrain and in regulating Hoxb1 [46] and Hoxa1 transcription [47]. RA directly induces transcription of Hoxa1 and Hoxb1, which contain RA Response Elements (RARE) on their regulatory regions [48]. Hox expression domains are further defined in specific rhombomeres by local RA degradation regulated by Cyp26 enzymes expressed in the anterior hindbrain [49–51].
Gene expression experiments showed that the first transcription factors to be expressed partition the hindbrain in two regions according to the r4/r5 boundary; they are Irxs expressed in the anterior and vHnf1 in posterior hindbrain [41, 44, 49, 52]. These domains are further subdivided by the activity of HoxPG1 genes, Krox20 and MafB/Kreisler. Excellent functional work mainly in mice and zebrafish has provided insights into the code of Hox genes in hindbrain patterning. Posterior expression of Hoxa1 and Hoxb1 up to r3/r4 boundary is required for normal development of r4 and r5 [53–57]. Hoxa2 is required for maintaining the r2 properties and the size of r3, and Hoxb2 contributes to the maintenance or r4 [58]. Hox expression becomes further refined by direct transcriptional regulation through upstream factors, such as Krox20 and MafB/Kreisler, and through cross- and auto-regulatory interactions among the Hox genes themselves (for review, see [58]). Krox20 specifies r3 and r5 [59–61] and cooperates with Hoxa1 for the development of r3 as shown by the analysis of double-mutant embryos [62]. MafB/Kreisler has a conserved role in regulating the identity of r5 and r6 [7, 63] and, with vHnf1, is involved in a direct positive feedback loop in r5 and r6 [44, 50].
The gene patterning process confers specific rhombomeric identity and precedes hindbrain compartmentalization. Both events have an important impact on the neurogenesis pattern and the sequential organization of cranial branchimotor nerves. Moreover, neural crest migration streams are also dependent on hindbrain segmentation. These features stress the importance of segmentation in establishing functional specialization [64, 65].
Setting up rhombomeric boundaries
The initial establishment of the rhombomeric pattern does not follow a strict rostrocaudal order, but rather boundaries form in a non-stereotyped way [7, 40]. The first boundary to appear, r4/r5, is positioned by the complementary expression of Irx3 and vHnf1 genes [52, 66]. Then, the presumptive r4 territory acts as a local organizing center, signaling to adjacent territories and initiating a molecular cascade that leads to the further partitioning of the r3, r5, and r6 segments where FGF signals play an important role [40, 42].
As rhombomeres are formed, distinct physical boundaries appear to prevent intermingling of distinct rhombomeric cells [18, 35, 36]. The formation of sharp boundaries can be followed by the analysis of rhombomere gene expression patterns during embryonic development: molecular rhombomeric boundaries are fuzzy and jagged at early stages but eventually sharpen as development proceeds. Understanding how sharp borders form and how they are maintained, despite the extensive intermingling of cells occurring during growth and morphogenesis, is one of the current questions in the field. To ensure segregation of these adjacent cell populations requires a very robust system. Indeed, boundary sharpening implies that rhombomeric cells undergo cell sorting or/and cell plasticity [7]. An important body of evidence mainly from cell lineage analysis, in vivo cell tracking and grafting experiments, points to cell sorting as the major mechanism operating in the sharpening of gene expression in rhombomeric boundaries, independently of the cell identity and the position along the AP axis [18, 67–69]. However, two reports have suggested that an attenuation mechanism relying on intracellular noise induces cells to switch their identity—cell plasticity—during r4/r5 boundary sharpening [70, 71].
This segmentation process is followed by the induction of a specialized cell population at the interface of adjacent rhombomeres, named rhombomeric Boundary Cell Population (rBCP). The rBCP displays specific features that distinguish it from non-boundary regions, such as (i) boundary cells are wide at either the apical or basal end and long and narrow at the other end (triangle shaped) when compared with non-boundary cells that are spindle shaped [72], (ii) they display larger intercellular spaces [73], and (iii) they have enriched expression of foxb1.2, wnt1, rfng and semaphorins in zebrafish [68, 72, 74, 75] (Fig. 1b), or Fgf3 and Pax6 in chick [76, 77]. rBCPs also act as local signaling centers regulating hindbrain neurogenesis, and thus the segmental neuronal organization [75]. A number of signaling pathways have been reported upstream of boundary specification and maintenance: Eph/ephrin [68], Notch [78] and Wnt [74]. However, the question of how boundary cells are specified is still open; for example, ectopic activation of DN-Su(H) represses boundary specification, but activation of DA-Su(H) is not sufficient for re-specification of non-boundary cells to boundary cell fate, suggesting Notch is important for maintenance but not for specification of the rBCP [78].
Cell sorting within the hindbrain: the role of Eph/ephrin signaling
The segmental expression of Eph and their ligands ephrins in the hindbrain depends on rhombomeric genes; for instance, Krox20 transcriptionally activates EphA4 expression in rhombomeres 3 and 5 [79]. Several works have stressed the importance of EphA/ephrin signaling in rhombomere cell sorting due to the complementary expression of Eph receptors, and their membrane-bound ephrin ligands [80, 81] (Fig. 1d–f), and suggested that the activity of EphA4/ephrinB2a within the hindbrain segments regulates cell segregation. Two mechanisms were proposed to operate in parallel during cell sorting: first, repulsive interactions between cells from adjacent odd- (displaying Eph receptors) and even-rhombomeres (expressing ephrin ligands) at rhombomeric boundaries, based on the observation that disruption of Eph/ephrin signaling in zebrafish results in cell intermingling and fuzzy boundaries [67, 82]; and second, adhesive interactions between cells of the same rhombomeric identity; as example, EphA4MO and ephrinB2aMO cells segregate to the borders of the segments expressing EphA4 or ephrinB2 when grafted into wild-type embryos [68, 69]. Recently, we proposed an additional mechanism based on the presence of mechanical barriers [18]. A previous report showed the activity of myosin II in the hindbrain during the formation of the morphological bulges. They described myosin contractility as a major player in this morphogenetic change, since embryos with overactive myosin II—mypt1 mutants—displayed abnormal rhombomere morphogenesis, along with defects in neuroepithelial cell shape and expansion of the hindbrain ventricle [72]. These evidences supported the hypothesis that actomyosin generated tension occurred at hindbrain boundaries. We pursued this idea and showed the presence of actomyosin cables apically located at interhombomeric boundaries (Fig. 1c), and that their assembly could be modulated with pharmacological agents that either enhance or decrease the stability of actomyosin complexes, in a very precise time window. Interestingly, the disruption of actomyosin cables resulted in rhombomeric cell intermingling and jagged rhombomeric boundaries. Further on, abrogation of EphA/ephrin signaling disrupts actomyosin cable assembly, which as previously shown results in rhombomeric cell mixing [18]. This work helps to understand how the juxtaposition of different rhombomeric cells triggers actomyosin assembly at the interface of rhombomeric boundaries, and how mechanical barriers act downstream of EphA/ephrin signaling to segregate cells from different rhombomeres. However, our article does not rule out if differential cell adhesion or cell repulsion is also contributing mechanisms towards rhombomere cell sorting.
Lessons from other systems: cell repulsion, cell adhesion and physical barriers
The understanding of Eph/ephrin signaling pathway is a challenging issue for several reasons. Eph receptors constitute a large family of transmembrane tyrosine kinases divided into two subclasses according to their sequence and affinity to ephrin ligands: EphA receptors bind to ephrinA ligands (membrane GPI-anchor proteins), and EphB receptors binding to ephrinB (transmembrane proteins with tyrosine kinase motives) [83]. Specificity of Eph/ephrin pairs is rather constant. However, this rule is flexible, as exemplified by EphA4, which can bind to both ephrinA and B ligands [68, 84]. Additional features of Eph/ephrin signaling increase the level of complexity: (i) high number and promiscuity of Eph receptors and ephrin ligands often drive to genetic redundancy [85]; (ii) the same receptor/ligand pair might have different roles depending on the biological context [86]; (iii) possible forward- (from receptor), reverse- (from ligand) or bidirectional-signaling resulting in the activation of downstream targets in either or both neighboring cells [87]; and (iv) multiple intracellular effectors—including small GTPases—have been described for exerting Eph/ephrin cell functions [88] (Fig. 2).
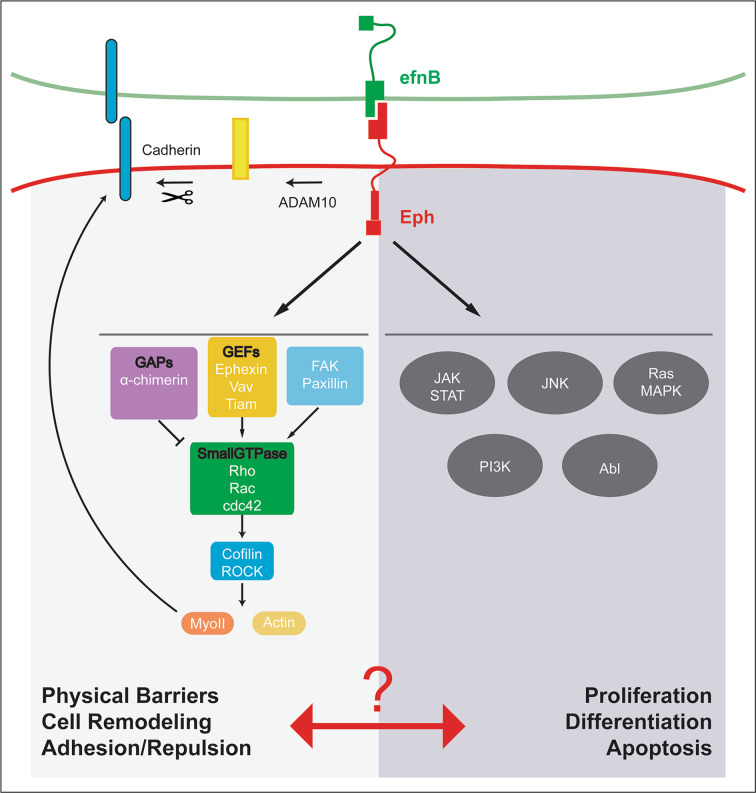
Fig. 2.
Intracellular events triggered by Eph/ephrin forward signaling. For the sake of simplicity, we describe only forward signaling events and a selection of published effectors. Upon ligand-receptor binding, Eph/ephrin forward signaling controls different cell responses; left panel physical barriers, cell remodeling, adhesion/repulsion are controlled by the activation of GEFs, GAPs and FAK/Paxillin, through the activation of small GTPases. Their downstream effectors, Rock and Cofilin, modulate the activity of myosin II and Actin (and their assembly) in processes of cell remodeling and generation of physical barriers. In addition, myosin II can control cadherin clustering, and ADAM10 that is activated by Eph/ephrin sheds cadherins. Right panel cell proliferation, cell differentiation and apoptosis are regulated by intracellular signaling complexes such as JAK/STAT, JNK, Ras/MAPK, PI3K and Abl. The nexus between cell responses and downstream molecules has not been unveiled yet
The main cell responses suggested to be triggered by Eph/ephrin signaling are contact-dependent cell repulsion, differential cell adhesion and cortical tension due to physical barriers [89]. However, their cooperation might define the final cell behavior.
Cell repulsion has a major role in the guidance of migrating cells and neuronal growth cones by Eph receptors and ephrins [87]. The hypothesis is that repulsion occurs at the border between receptor and ligand expression, where it prevents cells or neuronal growth cones from entering the inappropriate territory. An interesting example is found in embryonic germ layer separation in Xenopus using tissue explants; Fagotto’s group nicely described how cell segregation is restricted to tissue boundaries by a separation mechanism that involves repeated cycles of cell attachment and detachment at the boundary between two adjacent tissues with widespread expression of multiple ephrin ligands and Eph receptors. Their hypothesis is that the final output can be predicted by the relative Eph and ephrin concentrations and binding affinities. Thus, synergical repulsive signals would reach sufficient strength to trigger cell detachments along the boundary, and allow to explain how multiple Eph/ephrin cues integrate to generate discrete local repulsive outcomes [5, 25].
The differential cell adhesion hypothesis aims to explain how cells bind preferentially among the ones of the same cohort, and sort from confronting cell populations according to their distinct cohesive affinity [90]. An interesting example is the segregation of Paneth cells within the intestine crypt, where the interplay between Eph/ephrin and E-cadherins promotes sorting of EphB-positive Paneth cells from ephrinB-positive Goblet cells [91]. Solanas et al. beautifully combined cell culture and genetic manipulation experiments in mice to describe how EphB receptors promote the activation of the metalloproteinase ADAM10 at the interface with ephrinB1-expressing cells. ADAM10 activation induces shedding of E-cadherin, which results in its asymmetric localization and, therefore, in differences in cell affinity between EphB- and ephrinB-positive cells. This model could well apply to the hindbrain, where Eph/ephrin signaling would control metalloproteinase activity at hindbrain boundaries to promote shedding of cadherins. In this regard, N-cadherin (cadh2 in zebrafish) is ubiquitously expressed in the hindbrain. However, cadh2 is not asymmetrically localized within the neuroepithelium ([92]; Calzolari and Pujades, unpublished results). Thus, if a similar mechanism applies most probably other cadherins, or cadherin-related mechanisms, should play this role. On this line, in the notochord-presomitic mesoderm boundary, EphB/ephrinB signaling-dependent myosin activity is responsible for the inhibition of cadherin clustering, which results as well in tissue separation [26]. Eph/ephrin promotes cadherin shedding in one context and inhibition of cadherin clustering in another. It is an exciting idea to further explore the contribution of these alternative mechanisms to hindbrain segmentation.
Regarding physical barriers, we previously explained the role of actomyosin cables in different compartment boundaries, including the rhombomeres. However, an alternative mechanism has been proposed in the embryonic intersomitic boundaries in zebrafish. Ephs and ephrins are expressed in juxtaposed regions that distinguish the anterior from the posterior portion of the same somite. The interplay between Eph/ephrin signaling and ligand-independent integrin clustering drives restriction of de novo extracellular matrix production to somite boundaries, generating a physical barrier made of extracellular matrix deposition between the posterior territory of one somite and the anterior domain of the following somite [93]. Although the complementary Eph/ephrin expression in the somites reminds their expression in rhombomeres, it is unlikely that fibronectin fences play a major role in keeping rhombomeric cells apart, since no contribution of fibronectin matrix deposition or α5-activated integrin clustering was observed in rhombomeric boundaries [18].
Eph/ephrin signaling cellular responses: searching the effectors
Eph/ephrin signaling promotes the outlined cellular responses through the activation of different molecular effectors, which number increases every year. As an example, to identify the phosphorylated molecules downstream of forward- and reverse-signaling, Jørgensen et al. confronted EphB2- to ephrinB1-positive cells and performed Quantitative Mass Spectrometry. They validated the relevance of the identified mediators by shRNA phenotypical analysis, and provided a list of putative candidates for mediating Eph/ephrin cellular responses [94]. These in vitro approaches are very useful for discovering novel mediators and confirming the previous ones, but the functional relevance of putative candidates or networks requires in vivo validation. In this respect, an extensive bibliography supports the upregulation of small GTPase family members such as RhoA, Rac1 or Cdc42 by Eph/ephrin signaling [83, 95]. Small GTPases regulate the phosphorylation of Cofilin [96] or Rock [97] to promote actin remodeling and actomyosin contraction (Fig. 2), which are essential mechanisms to regulate cell protrusion dynamics during contact-dependent cell repulsion. Likewise, either pharmacological inhibition or genetic disruption of RhoA phenocopies the defects observed by EphA4 downregulation in the actomyosin cable assembly and cell sorting in the hindbrain. This points to RhoA as a downstream effector of Eph/ephrin in this particular process [18].
It has been proposed that Eph/ephrin controls small GTPases activity through the phosphorylation of GEF proteins, such as Tiam1 [98], Vav [99] or Ephexin [100, 101]. In addition, other effectors are FAK and Paxillin, which control cell migration and dendritic morphogenesis downstream of Eph/ephrin [102–104]. However, α-chimerin GAP is activated by EphA4/ephrinB3 to downregulate Rac1 during motor circuit formation in mice [105]. These contradictory functions provide a nice example of how challenging is to dissect Eph/ephrin signaling, supporting the hypothesis that the cellular outcome is cell context dependent.
In addition to their role on cell remodeling, Eph/ephrin pathway is involved in regulating other cell behaviors such as cell proliferation, cell differentiation and apoptosis. How Eph/ephrin pathway signals to promote these additional cell behaviors is not fully understood, but some reports point to usual suspects such as the JAK/STAT [106], JNK [107], Ras/MAPK [108], or PI3K/Abl [109] signaling pathways. All these intracellular signaling complexes are indeed good candidates for mediating some of the Eph/ephrin signaling cellular outputs. It will be interesting to further explore how cell remodeling is coupled to cell proliferation/differentiation and the putative links between distinct downstream effectors (see Fig. 2 for a comprehensive scheme on selected Eph/ephrin cell responses and signaling effectors).
Revisiting the boundaries in the hindbrain: future perspectives
The key challenge to rhombomeric boundaries we have detected is cell division. Mitotic cells incurring into adjacent rhombomeres are pushed back to their rhombomere of origin, and functional experiments demonstrate that an elastic actomyosin barrier is involved in keeping different cell populations segregated. Actomyosin fibers are detected only at the edge of Eph/ephrin contacts, suggesting a very fine-tuning of these structures. As reported in other systems, in the hindbrain EphA4/ephrin may act through small GTPases to increase cortical tension at the boundaries, as it does for border sharpening [18]. It will be interesting to determine whether RhoA is activated, and tension regulated, downstream of forward- and/or reverse-signaling to better allocate the actomyosin cable, as well as the need for other downstream effectors. EphA4 is likely to interact with ephrinB3 in the hindbrain, since morphants for ephrinB3 display a closer phenotype to EphA4-morphants than ephrinB2-morphants do, in terms of boundary cell markers downregulation [68, 75]. However, since this phenotype is restricted to a subset of rhombomeric boundaries, unaffected boundaries remain sites of putative interactions for other Eph/ephrin members ([82]; Calzolari and Pujades, unpublished results); their putative role should be explored to have the full Eph/ephrin scenario within the hindbrain.
Regardless of the specific players, the picture that emerges is the existence of a conserved strategy between vertebrates and Drosophila to sort cells at compartment boundaries, which is based on actomyosin-driven mechanical forces. In the hindbrain, the assembly of actomyosin fibers is downstream of EphA/ephrin signaling, a crucial event to maintain rhombomere sharpening [18]. Accordingly, experiments affecting myosin II activity in chick [110] and zebrafish [18, 72] alter morphological constrictions in the hindbrain boundaries. To demonstrate whether myosin II activation is sufficient to account for cell segregation depending on Eph/ephrin signaling, the effect of tension should be properly investigated. For this purpose, new tools have to be generated to measure and manipulate, in a tight spatiotemporal frame, actomyosin cable integrity and cortical tension in a 3D-structure such as the hindbrain. Moreover, this sharpening mechanism might be a common strategy used for other boundaries where Eph/ephrin signaling is also involved during development, such as germ layers, gut or somites.
We hypothesize that mechanical barriers may act on top of the cell adhesion/repulsion mechanisms to maintain straight rhombomeric boundaries, given that sharpness is continuously challenged by cell division [18]. We favor a model in which the synergy between differential cell adhesion, which keeps cohesive rhombomeres, and tension within the boundary cell layer provided by actomyosin cables acting as an elastic mesh, impedes intermingling of cells from adjacent rhombomeres.
Nevertheless, is it all a matter of tension? Although evidences from other systems suggest contractility as the major parameter regulated during tissue separation, other physical principles could be involved. For example, Rohani et al. [25] propose that tissue separation is driven by Eph/ephrin signaling but resisted by cadherins. They found that the differential strength between these two opposing forces determined the outcome: increasing the expression of cadherins reduced the detachment, whereas decreasing their expression increased it. The detachment requires myosin II, given the inhibition of its function reduced tissue separation. Thus, cell segregation is the result of multiple Eph/ephrin interactions, as well as the strength of cadherins and the robustness of myosin II contraction. Can a similar system be operating in the hindbrain? As previously mentioned, the main actors of this play are there: Eph/ephrin activity, actomyosin cables and cadherins. The next challenge is to understand how several molecular pathways present at rhombomere boundaries, such as Eph/ephrin, Notch [59] or Wnt [74], are integrated to generate local responses. Hopefully, the coming years will bring us enough technological advancements to carefully address the intimate cross-talk between adhesion, tension and cell signaling in the hindbrain and other compartmentalized tissues.
Acknowledgments
We are grateful to members of Pujades lab for insightful discussions. JT was recipient of Beatriu de Pinos postdoctoral contract from AGAUR (Generalitat de Catalunya). This work was funded by BFU2012-31994 (Spanish Ministry of Economy and Competitiveness, MINECO) to CP.
Abbreviations
- ADAM10
A disintegrin and metalloproteinase domain-containing protein 10
- AP
Anteroposterior
- DV
Dorsoventral
- CNS
Central nervous system
- Cadh2
Cadherin 2
- Cyp26
Cytochrome p450 family 26 enzymes
- EphA4MO/ephrinB2Amo
EphA4/ephrinB2a-morphants
- FGF
Fibroblast growth factor
- GEFs
Guanine nucleotide exchange factors
- GAPs
GTPase-activating proteins
- HoxPG1
Hox paralogous group 1 genes
- MHB
Mid-hindbrain boundary
- RA
Retinoic acid
References
- 1.Garcia-Bellido A, Ripoll P, Morata G. Developmental compartmentalisation of the wing disk of Drosophila. Nature New Biol. 1973;245:251–253. doi: 10.1038/newbio245251a0. [DOI] [PubMed] [Google Scholar]
- 2.Morata G, Lawrence PA. Anterior and posterior compartments in the head of Drosophila. Nature. 1978;274:473–474. doi: 10.1038/274473a0. [DOI] [PubMed] [Google Scholar]
- 3.Lawrence PA, Struhl G, Morata G. Bristle patterns and compartment boundaries in the tarsi of Drosophila. J Embryol Exp Morphol. 1979;51:195–208. [PubMed] [Google Scholar]
- 4.Sanson B. Generating patterns from fields of cells. Examples from Drosophila segmentation. EMBO Rep. 2001;2:1083–1088. doi: 10.1093/embo-reports/kve255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rohani N, Canty L, Luu O, et al. EphrinB/EphB signaling controls embryonic germ layer separation by contact-induced cell detachment. PLoS Biol. 2011;9:e1000597. doi: 10.1371/journal.pbio.1000597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reintsch WE, Habring-Mueller A, Wang RW, et al. beta-Catenin controls cell sorting at the notochord-somite boundary independently of cadherin-mediated adhesion. J Cell Biol. 2005;170:675–686. doi: 10.1083/jcb.200503009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moens CB, Cordes SP, Giorgianni MW, et al. Equivalence in the genetic control of hindbrain segmentation in fish and mouse. Development. 1998;125:381–391. doi: 10.1242/dev.125.3.381. [DOI] [PubMed] [Google Scholar]
- 8.Tümpel S, Wiedemann LM, Krumlauf R. Hox genes and segmentation of the vertebrate hindbrain. Curr Top Dev Biol. 2009;88:103–137. doi: 10.1016/S0070-2153(09)88004-6. [DOI] [PubMed] [Google Scholar]
- 9.Stern CD, Keynes RJ. Interactions between somite cells: the formation and maintenance of segment boundaries in the chick embryo. Development. 1987;99:261–272. doi: 10.1242/dev.99.2.261. [DOI] [PubMed] [Google Scholar]
- 10.Dahmann C, Oates AC, Brand M. Boundary formation and maintenance in tissue development. Nat Rev Genet. 2011;12:43–55. doi: 10.1038/nrg2902. [DOI] [PubMed] [Google Scholar]
- 11.Xu Q, Wilkinson DG. Boundary formation in the development of the vertebrate hindbrain. Wiley Interdiscip Rev Dev Biol. 2013;2:735–745. doi: 10.1002/wdev.106. [DOI] [PubMed] [Google Scholar]
- 12.Fagotto F. The cellular basis of tissue separation. Development. 2014;141:3303–3318. doi: 10.1242/dev.090332. [DOI] [PubMed] [Google Scholar]
- 13.Munjal A, Lecuit T. Actomyosin networks and tissue morphogenesis. Development. 2014;141:1789–1793. doi: 10.1242/dev.091645. [DOI] [PubMed] [Google Scholar]
- 14.Zecca M, Struhl G. Subdivision of the Drosophila wing imaginal disc by EGFR-mediated signaling. Development. 2002;129:1357–1368. doi: 10.1242/dev.129.6.1357. [DOI] [PubMed] [Google Scholar]
- 15.Tepass U, Godt D, Winklbauer R. Cell sorting in animal development: signalling and adhesive mechanisms in the formation of tissue boundaries. Curr Opin Genet Dev. 2002;12:572–582. doi: 10.1016/S0959-437X(02)00342-8. [DOI] [PubMed] [Google Scholar]
- 16.Tremblay KD, Zaret KS. Distinct populations of endoderm cells converge to generate the embryonic liver bud and ventral foregut tissues. Dev Biol. 2005;280:87–99. doi: 10.1016/j.ydbio.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 17.Langenberg T, Dracz T, Oates AC, et al. Analysis and visualization of cell movement in the developing zebrafish brain. Dev Dyn. 2006;235:928–933. doi: 10.1002/dvdy.20692. [DOI] [PubMed] [Google Scholar]
- 18.Calzolari S, Terriente J, Pujades C. Cell segregation in the vertebrate hindbrain relies on actomyosin cables located at the interhombomeric boundaries. EMBO J. 2014;33:686–701. doi: 10.1002/embj.201386003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Monier B, Pélissier-Monier A, Brand AH, Sanson B (2010) An actomyosin-based barrier inhibits cell mixing at compartmental boundaries in Drosophila embryos. Nat Cell Biol 12:60–5–sup:1–9. doi:10.1038/ncb2005 [DOI] [PMC free article] [PubMed]
- 20.Major RJ, Irvine KD. Influence of Notch on dorsoventral compartmentalization and actin organization in the Drosophila wing. Development. 2005;132:3823–3833. doi: 10.1242/dev.01957. [DOI] [PubMed] [Google Scholar]
- 21.Major RJ, Irvine KD. Localization and requirement for Myosin II at the dorsal-ventral compartment boundary of the Drosophila wing. Dev Dyn. 2006;235:3051–3058. doi: 10.1002/dvdy.20966. [DOI] [PubMed] [Google Scholar]
- 22.Landsberg KP, Farhadifar R, Ranft J, et al. Increased cell bond tension governs cell sorting at the drosophila anteroposterior compartment boundary. Curr Biol. 2009;19:1950–1955. doi: 10.1016/j.cub.2009.10.021. [DOI] [PubMed] [Google Scholar]
- 23.Becam I, Rafel N, Hong X, et al. Notch-mediated repression of bantam miRNA contributes to boundary formation in the Drosophila wing. Development. 2011;138:3781–3789. doi: 10.1242/dev.064774. [DOI] [PubMed] [Google Scholar]
- 24.Curt JR, de Navas LF, Sánchez-Herrero E. Differential activity of Drosophila Hox Genes induces myosin expression and can maintain compartment boundaries. PLoS One. 2013;8:e57159. doi: 10.1371/journal.pone.0057159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rohani N, Parmeggiani A, Winklbauer R, Fagotto F. Variable combinations of specific ephrin ligand/Eph receptor pairs control embryonic tissue separation. PLoS Biol. 2014;12:e1001955. doi: 10.1371/journal.pbio.1001955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fagotto F, Rohani N, Touret A-S, Li R. A molecular base for cell sorting at embryonic boundaries: contact inhibition of cadherin adhesion by ephrin/Eph-dependent contractility. Dev Cell. 2013 doi: 10.1016/j.devcel.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 27.Moens CB, Prince VE. Constructing the hindbrain: insights from the zebrafish. Dev Dyn. 2002;224:1–17. doi: 10.1002/dvdy.10086. [DOI] [PubMed] [Google Scholar]
- 28.Kiecker C, Lumsden A. Compartments and their boundaries in vertebrate brain development. Nat Rev Neurosci. 2005;6:553–564. doi: 10.1038/nrn1702. [DOI] [PubMed] [Google Scholar]
- 29.Bulfone A, Puelles L, Porteus MH, et al. Spatially restricted expression of Dlx-1, Dlx-2 (Tes-1), Gbx-2, and Wnt-3 in the embryonic day 12.5 mouse forebrain defines potential transverse and longitudinal segmental boundaries. J Neurosci. 1993;13:3155–3172. doi: 10.1523/JNEUROSCI.13-07-03155.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alvarado-Mallart RM, Martinez S, Lance-Jones CC. Pluripotentiality of the 2-day-old avian germinative neuroepithelium. Dev Biol. 1990;139:75–88. doi: 10.1016/0012-1606(90)90280-V. [DOI] [PubMed] [Google Scholar]
- 31.Liu A, Joyner AL. EN and GBX2 play essential roles downstream of FGF8 in patterning the mouse mid/hindbrain region. Development. 2001;128:181–191. doi: 10.1242/dev.128.2.181. [DOI] [PubMed] [Google Scholar]
- 32.Martinez S, Wassef M, Alvarado-Mallart RM. Induction of a mesencephalic phenotype in the 2-day-old chick prosencephalon is preceded by the early expression of the homeobox gene en. Neuron. 1991;6:971–981. doi: 10.1016/0896-6273(91)90237-T. [DOI] [PubMed] [Google Scholar]
- 33.Murakami Y, Uchida K, Rijli FM, Kuratani S. Evolution of the brain developmental plan: insights from agnathans. Developmental Biology. 2005;280:249–259. doi: 10.1016/j.ydbio.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 34.Guthrie S. Patterning and axon guidance of cranial motor neurons. Nat Rev Neurosci. 2007;8:859–871. doi: 10.1038/nrn2254. [DOI] [PubMed] [Google Scholar]
- 35.Fraser SE, Keynes R, Lumsden A. Segmentation in the chick embryo hindbrain is defined by cell lineage restrictions. Nature. 1990;344:431–434. doi: 10.1038/344431a0. [DOI] [PubMed] [Google Scholar]
- 36.Jimenez-Guri E, Udina F, Colas J-F, et al. Clonal analysis in mice underlines the importance of rhombomeric boundaries in cell movement restriction during hindbrain segmentation. PLoS One. 2010;5:e10112. doi: 10.1371/journal.pone.0010112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Irving C, Mason I. Signalling by FGF8 from the isthmus patterns anterior hindbrain and establishes the anterior limit of Hox gene expression. Development. 2000;127:177–186. doi: 10.1242/dev.127.1.177. [DOI] [PubMed] [Google Scholar]
- 38.McKay IJ, Muchamore I, Krumlauf R, et al. The kreisler mouse: a hindbrain segmentation mutant that lacks two rhombomeres. Development. 1994;120:2199–2211. doi: 10.1242/dev.120.8.2199. [DOI] [PubMed] [Google Scholar]
- 39.Walshe J, Maroon H, McGonnell IM, et al. Establishment of hindbrain segmental identity requires signaling by FGF3 and FGF8. Curr Biol. 2002;12:1117–1123. doi: 10.1016/S0960-9822(02)00899-0. [DOI] [PubMed] [Google Scholar]
- 40.Maves L, Jackman W, Kimmel CB. FGF3 and FGF8 mediate a rhombomere 4 signaling activity in the zebrafish hindbrain. Development. 2002;129:3825–3837. doi: 10.1242/dev.129.16.3825. [DOI] [PubMed] [Google Scholar]
- 41.Wiellette EL. vhnf1 and Fgf signals synergize to specify rhombomere identity in the zebrafish hindbrain. Development. 2003;130:3821–3829. doi: 10.1242/dev.00572. [DOI] [PubMed] [Google Scholar]
- 42.Aragon F, Pujades C. FGF signaling controls caudal hindbrain specification through Ras-ERK1/2 pathway. BMC Dev Biol. 2009;9:61. doi: 10.1186/1471-213X-9-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hernandez R, Rikhof HA, Bachmann R, Moens CB. vhnf1 integrates global RA patterning and local FGF signals to direct posterior hindbrain development in zebrafish. Development. 2004;131:4511–4520. doi: 10.1242/dev.01297. [DOI] [PubMed] [Google Scholar]
- 44.Aragon F, Vázquez-Echeverría C, Ulloa E, et al. vHnf1 regulates specification of caudal rhombomere identity in the chick hindbrain. Dev Dyn. 2005;234:567–576. doi: 10.1002/dvdy.20528. [DOI] [PubMed] [Google Scholar]
- 45.Labalette C, Bouchoucha YX, Wassef MA, et al. Hindbrain patterning requires fine-tuning of early krox20 transcription by Sprouty 4. Development. 2010;138:317–326. doi: 10.1242/dev.057299. [DOI] [PubMed] [Google Scholar]
- 46.Morriss-Kay GM, Murphy P, Hill RE, Davidson DR. Effects of retinoic acid excess on expression of Hox-2.9 and Krox-20 and on morphological segmentation in the hindbrain of mouse embryos. EMBO J. 1991;10:2985–2995. doi: 10.1002/j.1460-2075.1991.tb07849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pasqualetti M, Neun R, Davenne M, Rijli FM. Retinoic acid rescues inner ear defects in Hoxa1 deficient mice. Nat Genet. 2001;29:34–39. doi: 10.1038/ng702. [DOI] [PubMed] [Google Scholar]
- 48.Glover JC, Renaud J-S, Rijli FM. Retinoic acid and hindbrain patterning. J Neurobiol. 2006;66:705–725. doi: 10.1002/neu.20272. [DOI] [PubMed] [Google Scholar]
- 49.Sirbu IO, Gresh L, Barra J, Duester G. Shifting boundaries of retinoic acid activity control hindbrain segmental gene expression. Development. 2005;132:2611–2622. doi: 10.1242/dev.01845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hernandez RE, Putzke AP, Myers JP, et al. Cyp26 enzymes generate the retinoic acid response pattern necessary for hindbrain development. Development. 2007;134:177–187. doi: 10.1242/dev.02706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.White RJ, Nie Q, Lander AD, Schilling TF. Complex regulation of cyp26a1 creates a robust retinoic acid gradient in the Zebrafish embryo. PLoS Biol. 2007;5:e304. doi: 10.1371/journal.pbio.0050304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lecaudey V, Anselme I, Rosa F, Schneider-Maunoury S. The zebrafish Iroquois gene iro7 positions the r4/r5 boundary and controls neurogenesis in the rostral hindbrain. Development. 2004;131:3121–3131. doi: 10.1242/dev.01190. [DOI] [PubMed] [Google Scholar]
- 53.Rossel M, Capecchi MR. Mice mutant for both Hoxa1 and Hoxb1 show extensive remodeling of the hindbrain and defects in craniofacial development. Development. 1999;126:5027–5040. doi: 10.1242/dev.126.22.5027. [DOI] [PubMed] [Google Scholar]
- 54.Barrow JR, Stadler HS, Capecchi MR. Roles of Hoxa1 and Hoxa2 in patterning the early hindbrain of the mouse. Development. 2000;127:933–944. doi: 10.1242/dev.127.5.933. [DOI] [PubMed] [Google Scholar]
- 55.McNulty CL, Peres JN, Bardine N, et al. Knockdown of the complete Hox paralogous group 1 leads to dramatic hindbrain and neural crest defects. Development. 2005;132:2861–2871. doi: 10.1242/dev.01872. [DOI] [PubMed] [Google Scholar]
- 56.Wassef MA, Chomette D, Pouilhe M, et al. Rostral hindbrain patterning involves the direct activation of a Krox20 transcriptional enhancer by Hox/Pbx and Meis factors. Development. 2008;135:3369–3378. doi: 10.1242/dev.023614. [DOI] [PubMed] [Google Scholar]
- 57.Makki N, Capecchi MR. Hoxa1 lineage tracing indicates a direct role for Hoxa1 in the development of the inner ear, the heart, and the third rhombomere. Dev Biol. 2010;341:499–509. doi: 10.1016/j.ydbio.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Alexander T, Nolte C, Krumlauf R. Hox genes and segmentation of the hindbrain and axial skeleton. Annu Rev Cell Dev Biol. 2009;25:431–456. doi: 10.1146/annurev.cellbio.042308.113423. [DOI] [PubMed] [Google Scholar]
- 59.Schneider-Maunoury S, Topilko P, Seitandou T, et al. Disruption of Krox-20 results in alteration of rhombomeres 3 and 5 in the developing hindbrain. Cell. 1993;75:1199–1214. doi: 10.1016/0092-8674(93)90329-O. [DOI] [PubMed] [Google Scholar]
- 60.Schneider-Maunoury S, Seitanidou T, Charnay P, Lumsden A. Segmental and neuronal architecture of the hindbrain of Krox-20 mouse mutants. Development. 1997;124:1215–1226. doi: 10.1242/dev.124.6.1215. [DOI] [PubMed] [Google Scholar]
- 61.Voiculescu O, Taillebourg E, Pujades C, et al. Hindbrain patterning: Krox20 couples segmentation and specification of regional identity. Development. 2001;128:4967–4978. doi: 10.1242/dev.128.24.4967. [DOI] [PubMed] [Google Scholar]
- 62.Helmbacher F, Pujades C, Desmarquet C, et al. Hoxa1 and Krox-20 synergize to control the development of rhombomere 3. Development. 1998;125:4739–4748. doi: 10.1242/dev.125.23.4739. [DOI] [PubMed] [Google Scholar]
- 63.Moens CB, Yan YL, Appel B, et al. valentino: a zebrafish gene required for normal hindbrain segmentation. Development. 1996;122:3981–3990. doi: 10.1242/dev.122.12.3981. [DOI] [PubMed] [Google Scholar]
- 64.Lumsden A, Keynes R. Segmental patterns of neuronal development in the chick hindbrain. Nature. 1989;337:424–428. doi: 10.1038/337424a0. [DOI] [PubMed] [Google Scholar]
- 65.Lumsden A, Krumlauf R. Patterning the vertebrate neuraxis. Science. 1996;274:1109–1115. doi: 10.1126/science.274.5290.1109. [DOI] [PubMed] [Google Scholar]
- 66.Jimenez-Guri E, Pujades C. An ancient mechanism of hindbrain patterning has been conserved in vertebrate evolution. Evol Dev. 2011;13:38–46. doi: 10.1111/j.1525-142X.2010.00454.x. [DOI] [PubMed] [Google Scholar]
- 67.Xu Q, Mellitzer G, Robinson V, Wilkinson DG. In vivo cell sorting in complementary segmental domains mediated by Eph receptors and ephrins. Nature. 1999;399:267–271. doi: 10.1038/20452. [DOI] [PubMed] [Google Scholar]
- 68.Cooke JE, Kemp HA, Moens CB. EphA4 is required for cell adhesion and rhombomere-boundary formation in the Zebrafish. Curr Biol. 2005;15:536–542. doi: 10.1016/j.cub.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 69.Kemp HA, Cooke JE, Moens CB. EphA4 and EfnB2a maintain rhombomere coherence by independently regulating intercalation of progenitor cells in the zebrafish neural keel. Dev Biol. 2009;327:313–326. doi: 10.1016/j.ydbio.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schilling TF, Prince V, Ingham PW. Plasticity in zebrafish hox expression in the hindbrain and cranial neural crest. Dev Biol. 2001;231:201–216. doi: 10.1006/dbio.2000.9997. [DOI] [PubMed] [Google Scholar]
- 71.Zhang C, Frazier JM, Chen H, et al. Molecular and morphological changes in zebrafish following transient ethanol exposure during defined developmental stages. Neurotoxicol Teratol. 2014;44:1–11. doi: 10.1016/j.ntt.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gutzman JH, Sive H. Epithelial relaxation mediated by the myosin phosphatase regulator Mypt1 is required for brain ventricle lumen expansion and hindbrain morphogenesis. Development. 2010;137:795–804. doi: 10.1242/dev.042705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Guthrie S, Lumsden A. Formation and regeneration of rhombomere boundaries in the developing chick hindbrain. Development. 1991;112:221–229. doi: 10.1242/dev.112.1.221. [DOI] [PubMed] [Google Scholar]
- 74.Riley BB, Chiang M-Y, Storch EM, et al. Rhombomere boundaries are Wnt signaling centers that regulate metameric patterning in the zebrafish hindbrain. Dev Dyn. 2004;231:278–291. doi: 10.1002/dvdy.20133. [DOI] [PubMed] [Google Scholar]
- 75.Terriente J, Gerety SS, Watanabe-Asaka T, et al. Signalling from hindbrain boundaries regulates neuronal clustering that patterns neurogenesis. Development. 2012;139:2978–2987. doi: 10.1242/dev.080135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sela-Donenfeld D, Kayam G, Wilkinson DG. Boundary cells regulate a switch in the expression of FGF3 in hindbrain rhombomeres. BMC Dev Biol. 2009;9:16. doi: 10.1186/1471-213X-9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Prin F, Serpente P, Itasaki N, Gould AP. Hox proteins drive cell segregation and non-autonomous apical remodelling during hindbrain segmentation. Development. 2014 doi: 10.1242/dev.098954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cheng Y-C, Amoyel M, Qiu X, et al. Notch activation regulates the segregation and differentiation of rhombomere boundary cells in the zebrafish hindbrain. Dev Cell. 2004;6:539–550. doi: 10.1016/S1534-5807(04)00097-8. [DOI] [PubMed] [Google Scholar]
- 79.Theil T, Ariza-McNaughton L, Manzanares M, et al. Requirement for downregulation of kreisler during late patterning of the hindbrain. Development. 2002;129:1477–1485. doi: 10.1242/dev.129.6.1477. [DOI] [PubMed] [Google Scholar]
- 80.Nieto MA, Gilardi-Hebenstreit P, Charnay P, Wilkinson DG. A receptor protein tyrosine kinase implicated in the segmental patterning of the hindbrain and mesoderm. Development. 1992;116:1137–1150. doi: 10.1242/dev.116.4.1137. [DOI] [PubMed] [Google Scholar]
- 81.Becker N, Gilardi-Hebenstreit P, Seitanidou T, et al. Characterisation of the Sek-1 receptor tyrosine kinase. FEBS Lett. 1995;368:353–357. doi: 10.1016/0014-5793(95)00652-P. [DOI] [PubMed] [Google Scholar]
- 82.Cooke J, Moens C, Roth L, et al. Eph signalling functions downstream of Val to regulate cell sorting and boundary formation in the caudal hindbrain. Development. 2001;128:571–580. doi: 10.1242/dev.128.4.571. [DOI] [PubMed] [Google Scholar]
- 83.Pasquale EB. Eph-ephrin bidirectional signaling in physiology and disease. Cell. 2008;133:38–52. doi: 10.1016/j.cell.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 84.Himanen JP, Yermekbayeva L, Janes PW, et al. Architecture of Eph receptor clusters. Proc Natl Acad Sci. 2010;107:10860–10865. doi: 10.1073/pnas.1004148107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Poliakov A, Cotrina M, Wilkinson DG. Diverse roles of eph receptors and ephrins in the regulation of cell migration and tissue assembly. Dev Cell. 2004;7:465–480. doi: 10.1016/j.devcel.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 86.Noren NK, Pasquale EB. Paradoxes of the EphB4 receptor in cancer. Cancer Res. 2007;67:3994–3997. doi: 10.1158/0008-5472.CAN-07-0525. [DOI] [PubMed] [Google Scholar]
- 87.Egea J, Klein R. Bidirectional Eph-ephrin signaling during axon guidance. Trends Cell Biol. 2007;17:230–238. doi: 10.1016/j.tcb.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 88.Pitulescu ME, Adams RH. Eph/ephrin molecules–a hub for signaling and endocytosis. Genes Dev. 2010;24:2480–2492. doi: 10.1101/gad.1973910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cayuso J, Xu Q, Wilkinson DG (2014) Mechanisms of boundary formation by Eph receptor and ephrin signaling. Dev Biol 401:122–131. doi:10.1016/j.ydbio.2014.11.013 [DOI] [PubMed]
- 90.Martz E, Phillips HM, Steinberg MS. Contact inhibition of overlapping and differential cell adhesion: a sufficient model for the control of certain cell culture morphologies. J Cell Sci. 1974;16:401–419. doi: 10.1242/jcs.16.2.401. [DOI] [PubMed] [Google Scholar]
- 91.Solanas G, Cortina C, Sevillano M, Batlle E. Cleavage of E-cadherin by ADAM10 mediates epithelial cell sorting downstream of EphB signalling. Nat Cell Biol. 2011;13:1100–1107. doi: 10.1038/ncb2298. [DOI] [PubMed] [Google Scholar]
- 92.Stockinger P, Maitre JL, Heisenberg CP. Defective neuroepithelial cell cohesion affects tangential branchiomotor neuron migration in the zebrafish neural tube. Development. 2011;138:4673–4683. doi: 10.1242/dev.071233. [DOI] [PubMed] [Google Scholar]
- 93.Julich D, Mould AP, Koper E, Holley SA. Control of extracellular matrix assembly along tissue boundaries via Integrin and Eph/Ephrin signaling. Development. 2009;136:2913–2921. doi: 10.1242/dev.038935. [DOI] [PubMed] [Google Scholar]
- 94.Jørgensen C, Sherman A, Chen GI, et al. Cell-specific information processing in segregating populations of Eph receptor ephrin-expressing cells. Science. 2009;326:1502–1509. doi: 10.1126/science.1176615. [DOI] [PubMed] [Google Scholar]
- 95.Klein R. Eph/ephrin signalling during development. Development. 2012;139:4105–4109. doi: 10.1242/dev.074997. [DOI] [PubMed] [Google Scholar]
- 96.Defourny J, Poirrier A-L, Lallemend FCO et al. (1AD) Ephrin-A5/EphA4 signalling controls specific afferent targeting to cochlear hair cells. Nat Commun 4:1438. doi: 10.1038/ncomms2445 [DOI] [PubMed]
- 97.Yamazaki T, Masuda J, Omori T, et al. EphA1 interacts with integrin-linked kinase and regulates cell morphology and motility. J Cell Sci. 2009;122:243–255. doi: 10.1242/jcs.036467. [DOI] [PubMed] [Google Scholar]
- 98.Boissier P, Chen J, Huynh-Do U. EphA2 signaling following endocytosis: role of Tiam1. Traffic. 2013;14:1255–1271. doi: 10.1111/tra.12123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cowan CW, Shao YR, Sahin M, et al. Vav family GEFs link activated Ephs to endocytosis and axon guidance. Neuron. 2005;46:205–217. doi: 10.1016/j.neuron.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 100.Sahin M, Greer PL, Lin MZ, et al. Eph-dependent tyrosine phosphorylation of ephexin1 modulates growth cone collapse. Neuron. 2005;46:191–204. doi: 10.1016/j.neuron.2005.01.030. [DOI] [PubMed] [Google Scholar]
- 101.Hiramoto-Yamaki N, Takeuchi S, Ueda S, et al. Ephexin4 and EphA2 mediate cell migration through a RhoG-dependent mechanism. J Cell Biol. 2010;190:461–477. doi: 10.1083/jcb.201005141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Moeller ML, Shi Y, Reichardt LF, Ethell IM. EphB receptors regulate dendritic spine morphogenesis through the recruitment/phosphorylation of focal adhesion kinase and RhoA activation. J Biol Chem. 2006;281:1587–1598. doi: 10.1074/jbc.M511756200. [DOI] [PubMed] [Google Scholar]
- 103.Vindis C, Teli T, Cerretti DP, et al. EphB1-mediated cell migration requires the phosphorylation of paxillin at Tyr-31/Tyr-118. J Biol Chem. 2004;279:27965–27970. doi: 10.1074/jbc.M401295200. [DOI] [PubMed] [Google Scholar]
- 104.Carter N, Nakamoto T, Hirai H, Hunter T. EphrinA1-induced cytoskeletal re-organization requires FAK and p130(cas) Nat Cell Biol. 2002;4:565–573. doi: 10.1038/ncb823. [DOI] [PubMed] [Google Scholar]
- 105.Iwasato T, Katoh H, Nishimaru H, et al. Rac-GAP alpha-chimerin regulates motor-circuit formation as a key mediator of EphrinB3/EphA4 forward signaling. Cell. 2007;130:742–753. doi: 10.1016/j.cell.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 106.Bong Y-S, Lee H-S, Carim-Todd L, et al. ephrinB1 signals from the cell surface to the nucleus by recruitment of STAT3. Proc Natl Acad Sci USA. 2007;104:17305–17310. doi: 10.1073/pnas.0702337104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Becker E, Huynh-Do U, Holland S, et al. Nck-interacting Ste20 kinase couples Eph receptors to c-Jun N-terminal kinase and integrin activation. Mol Cell Biol. 2000;20:1537–1545. doi: 10.1128/MCB.20.5.1537-1545.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Miao H, Wei BR, Peehl DM, et al. Activation of EphA receptor tyrosine kinase inhibits the Ras/MAPK pathway. Nat Cell Biol. 2001;3:527–530. doi: 10.1038/35074604. [DOI] [PubMed] [Google Scholar]
- 109.Genander M, Halford MM, Xu N-J, et al. Dissociation of EphB2 signaling pathways mediating progenitor cell proliferation and tumor suppression. Cell. 2009;139:679–692. doi: 10.1016/j.cell.2009.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Filas BA, Oltean A, Majidi S, et al. Regional differences in actomyosin contraction shape the primary vesicles in the embryonic chicken brain. Phys Biol. 2012;9:066007. doi: 10.1088/1478-3975/9/6/066007. [DOI] [PMC free article] [PubMed] [Google Scholar]