Abstract
The endoplasmic reticulum (ER) lumen is chemically complex and crowded with polypeptides in different stages of assembly. ER quality control monitors chaperone-assisted protein folding, stochastic errors and off-pathway intermediates. In acute conditions, potentially toxic polypeptides overflow the capacity of the chaperone system and lead to ER stress. Activation of the unfolded protein response (UPR) following ER stress buys time for non-native polypeptides to refold or be eliminated; otherwise cell death occurs. The clearance routes for deleterious proteins are endoplasmic reticulum-associated degradation (ERAD) and ER stress-activated autophagy. The ERAD pathway is a chaperone and proteasome-mediated polypeptide degradation, while autophagy applies to wider range of substances. ER stress signal transduction recruits diverse molecules and pathways upon UPR induction to compensate stress condition. NF-E2-related factor 1 (Nrf1) and Nrf2 are two transcription factors mostly known by their induction through an antioxidant response; they can also be activated by UPR machinery. Discovery of diverse molecules downstream of Nrf1 and Nrf2 has expanded our understanding of the biological impacts of these transcription factors beyond classic antioxidant activation. In this review, we summarize our current understanding of mutual relationships between Nrf1, Nrf2, and ER stress clearance mechanisms and highlight the crosstalk of specific molecules mediating these correlations.
Keywords: Nrf1, Nrf2, ER stress, UPR, Autophagy, ERAD
Introduction
The endoplasmic reticulum (ER) is a membranous network within the cytoplasm that is essential for the synthesis, folding, and modification of proteins destined to be secreted or embedded in the plasma membrane [1, 2]. This subcellular platform orchestrates major regulatory events in signal transduction of cellular development, differentiation, and stress. Since protein folding has a complex pathway, it is an error-prone process, resulting in unstable intermediates. Accumulation of unfolded/misfolded proteins in the lumen of ER can cause an imbalance between ER protein folding load and capacity, a condition referred to as “ER stress”, due to different physiological and pathological circumstances such as oxidative stress, glucose deprivation and excessive mutant proteins [3]. ER stress triggers an evolutionarily conserved response with an integrated signal transduction pathway termed the “unfolded protein response (UPR)”. Activation of the UPR facilitates protein folding and simultaneously attenuates aggregation of unfolded/misfolded protein. A transient shutdown in protein translation ameliorates the protein load in the lumen of ER, preventing subsequent damage [3]. Due to pathological processes associated with ER stress, some key regulatory molecules and subcellular signaling, acting as defensive responses, are destroyed, resulting in blockage of ER stress stimuli. Interestingly, in some of these cases, survival signaling can induce ER-dependent cell death. The correlations between neurodegenerative disorders such as Alzheimer’s disease (AD), amyotrophic lateral sclerosis (ALS), Parkinson’s diseases (PD), and ER stress are well documented [4–7].
During adverse conditions, UPR get activated, followed by upregulation of chaperones and components of ER-associated degradation (ERAD) machinery. ERAD degrades the misfolded proteins that are more than folding capacity of ER chaperones, by activation of the ubiquitin–proteasome pathway. To eliminate the toxic protein components outside the ER, autophagy has been shown as another major degradation system that is responsible for degrading a wider spectrum of substrates [8].
Interestingly, compelling evidence suggests that the ER can act as a sensor organelle in eukaryotic cells [1]. The ER can do this by sensing local stress through chaperones, Ca2+-binding proteins, Ca2+ release channels, and other stress response proteins such as nuclear factor-E2-related factor 1 (Nrf1) and Nrf2, and then the response will be directed to mitochondria, Golgi or nucleus [1, 2]. This leads the activation of a subset of molecules mainly those that are resident with high specificity in ER membrane, followed by various changes in physiological functions of the cell [3].
ER stress has been the subject of numerous studies and multiple models proposed to describe the molecular events and mechanisms of ER stress. Nrf2 as an effective bZIP Cap‘n’Collar transcription factor, is a target for PERK kinase activity and subsequent activation. Nrf1 is an ER membrane-settled transcription factor, sharing many similarities with Nrf2 in structure and activity. As a result of increasing evidence suggesting fundamental roles of Nrf1 and Nrf2 in oxidative and also non-oxidative stresses, this review will focus on the studies of Nrf2 and Nrf1 as regulatory transcription factors involved in ablation of stress in ER.
ER acting as local sensor organelle and leads to lunching UPR
UPR is a regulatory system that was first reported in the 1980s. Kozutsumi et al. [9] observed that the presence of misfolded proteins in the ER signals the induction of glucose-regulated protein (GRP). GRP78 is a chaperon with the ability to interact with both resident proteins and receptors. GRP78 is responsible for maintaining the inactive state of UPR signaling arms by preventing their dimerization. Elevation of misfolded/unfolded proteins occupies more GRP78 interacting sites and results in dissociation of GRP78 from signaling molecules and their subsequent dimerization.
Three components of UPR that are primarily initiated by ER transmembrane receptors are: inositol-requiring protein-1 (IRE1), activating transcription factor 6 (ATF6), and protein kinase RNA-like ER kinase (PERK) [3].
IREI, evolutionarily the oldest molecule in UPR pathway, has a dimerization domain and two effector sites, consisting of kinase and endoribonuclease (RNase) domains. X-box-binding protein 1(XBP1) mRNA is the main target of IRE1 RNase, resulting in spliced XBP1 (sXBP1) that encodes for an active leucine zipper (bZIP) transcription factor. XBP1 regulates the transcription of 380 target genes during the UPR activation process [10]. In IRE1 activation, the kinase arm of IRE1 is responsible for recruiting TNF-receptor-associated receptor 2 (TRAF2) in order to initiate the ASK1-MAP3 K-JNK/P38 signaling pathway [11]. Activation of mitogen-activated protein kinases (MAPKs), such as P38 and JNK, leads to activation of adaptive mechanisms or cell death via an apoptotic pathway. Pathway selection depends on cell status and other interfering factors [4].
Phosphorylation and inactivation of eukaryotic initiation factor-2α (eIF2α) causes a transient translational shutdown. PERK, a Ser/Thr protein kinase, is activated during ER stress by oligomerization and autophosphorylation and has been shown to phosphorylate and inactivate eIF2α [3]. However, certain mRNAs escape from this translational shut down, such as activating transcription factor 4 (ATF4), a member of the bZIP family of transcription factors, and the factors that contribute in autophagy.
ATF6α and ATF6β homologous to bZIP family transcription factors, similar to ATF4, are present in all cell lines. The mechanism of ATF6 activation is release from GRP78 and, following translocation into Golgi, it undergoes a protease cleavage at juxtamembrane site. Released ATF6α and ATF6β translocate to the nucleus and regulate gene expression such as XBP1 and GRP78 genes that are mainly related to enhancing ER chaperone activity and degradation of misfolded proteins [12, 13]. The summary of UPR pathway is illustrated in Fig. 1.
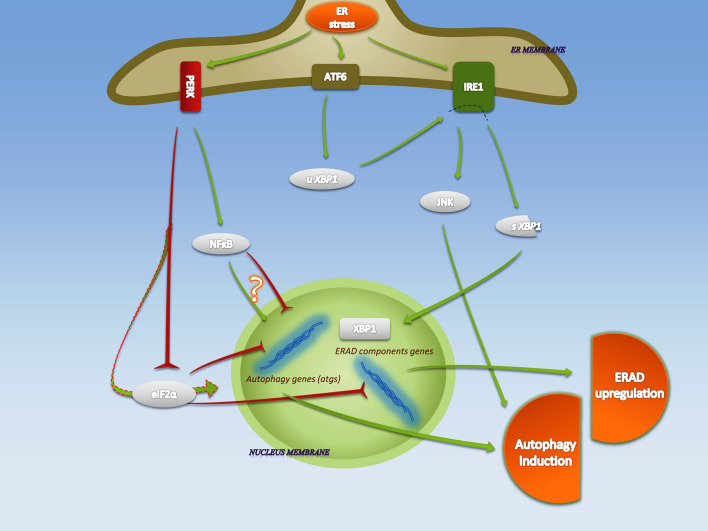
Fig. 1.
UPR-activated autophagy and ERAD in ER stress. Upon accumulation of misfolded proteins in the lumen of ER, the protein chaperone glucose-regulated protein, 78 KDa (GRP78) is released from the termini of IRE1α, PERK and ATF6, which causes their oligomerization and activation. ER stress associated clearance is mainly attributed to IRE1 and PERK activities. Endoribonuclease (RNase) activity of IRE1α on XBP1 results in formation of spliced XBP1 (sXBP1) which then translates to an active leucine zipper (bZIP) transcription factor and translocates to the nucleus, where it enhances the transcription of ERAD genes. Phosphorylation of JNK by kinase domain of IRE1α causes its activation and subsequent upregulation of autophagy components. Phosphorylation of eIF2α on Ser51 by PERK results in the rapid shutdown of general translation, while under these conditions selected mRNAs, including autophagy and ERAD components, are translated. Inhibiting the inhibitory impact of eIF2α on expression of certain genes results in upregulation of those gene products (arrow with dashed outline). PERK also phosphorylates and activates the NFκB, which regulates autophagy genes (atgs). ATF6 has been shown to assist IRE1 in its expressional signaling through upregulation of unspliced XBP1 (uXBP1). The green arrows and the red reverse arrows, represent activatory and inhibitory effects, respectively
An abstract of ER stress-associated substance deletion; different manifestations of ERAD and autophagy
Eukaryotic cells always face waste products, toxic substances, and nonfunctional structures that must be eliminated rapidly and efficiently. Autophagy and proteasomal degradation are in charge of active elimination of substances in the cell [14]. Interestingly, these major paths are responsible not only for homeostasis in healthy cells but also involved in stress cases with modified and enhanced function. This section attempts to make an overview of the main points of the ERAD and the ER stress-mediated autophagy, with attributed major molecules that are mentioned in the recent reviews.
ERAD pathway and qualifications of ERAD substrates
One of the deleterious routes for ER resident proteins is known as ERAD, which is activated by UPR in severe conditions. IRE1 is predominantly liable for activation of ERAD genes through its ribonuclease action on XBP1 mRNA [3]. On the other hand, the ATF6 arm of UPR signaling is shown to cooperate with IRE1 by inducing XBP1 transcription [12, 13]. Inefficiency of IRE1 signal transduction in ERAD has been reported in many pathological indications, such as dysfunctional elimination of mutant rhodopsin polypeptides that leads to photoreceptor cell death and vision loss [15]. PERK as another arm in UPR signaling has the ability to induce ERAD genes through selective translational activation of eIF2α (Fig. 1).
Protein degradation, which is mediated by proteasome and activated by ER stress, has a multistage course that can be summarized as: substrate selection, targeting, cytosol retro-translocation, followed by ubiquitination. Finally, it leads to protein degradation in the protease active sites of proteasome [16, 17].
ER quality control (ERQC) is a chaperon-assisted process that verifies protein folding accuracy. ERQC regulates protein selection and allows the polypeptides to perform additional folding or tag them for cytosol retrotranslocation to undergo proteasomal cleavage [16]. Unstable proteins are not the only molecules to be picked up by ERQC, but wild-type proteins can also be selected and deleted by ERAD. Ubiquitin proteasome system (UPS) has been shown to be the primary and the main pathway devoted to elimination of misfolded/unfolded proteins in the ER [18]. Soluble proteins, conjugated with ubiquitin, are delivered to the proteasome where they are unfolded, deubiquitinated, and broken in oligopeptides in an ATPase-dependent manner [19]. At the end of the pathway, de-ubiquitination prior to proteasome degradation is essential and permits the protein to enter the protease complex.
Although the mechanisms through which the ER recognizes the misfolded and toxic substrate have not been completely understood, some key molecules are identified to be responsible for binding to aggregation-inducing/insoluble proteins. These couplings separate them from other resident proteins and also keep them soluble during cytosolic exportation. Heat shock protein 70 (HSP70) family members (such as GRP78) are well-studied samples of ER-luminal ATP dependent chaperones whose interactions with several ERAD substrates have been proved. Binding and dissociation of HSP70s are shown to maintain the solubility of ERAD polypeptides and to facilitate their selection and targeting [16, 18]. In this manner, HSP40s have been demonstrated to work as co-chaperones and to cooperate in ATPase activity of HSP70s. Since the main targets for HSP70s are the hydrophobic sites of polypeptides, HSP40s facilitate interactions that can be productive, withholding misled ones. During or after cytoplasmic retrotranslocation, ERAD substrates are ubiquitinated by means of enzymes, mainly those are resident in the cytoplasm, such as E3 ubiquitin ligases, Hrd1, Doa10. The ubiquitination occurs in different pathways, depending on their location (including ERAD-L, ERAD-C, and ERAD-M, referring to luminal, cytoplasmic, and membrane spanning pathways, respectively). Substrate recognition and targeting occur concomitantly in multiprotein complexes that result in undistinguishable intermediates. As an example, cell-division cycle-48 (Cdc48) is a cytoplasmic protein complex that plays a pivotal role in polypeptide retrotranslocation. It extracts substrates, using ATPase, and binds to some ER-resident components, such as GRP78 and Hrd1. Cdc48 mutations lead to complex pathological signs, including Paget’s disease and frontotemporal dementia with body myopathy manifestation [20].
ER-regulated autophagy and participant molecules
Autophagy, which includes macroautophagy, microautophagy and chaperone-mediated autophagy, is a highly conserved degradation pathway for bulk cellular components [14, 20]. Macroautophagy, generally known as autophagy, is different from microautophagy and chaperone-mediated autophagy, mainly in delivering substances to lysosome [21]. Autophagy is marked by dynamic formation of vesicles with double membranes, called autophagosomes. This newly developed autophagosome sequesters proteins, lipids, and organelles, which are destined to terminate by lysosomal enzymes. Atgs are the core regulators of autophagosome formation and its lysosomal conjugation. The products of atgs take part in all stages of autophagy, including autophagy induction, autophagosome nucleation, expansion, and eventually lysosomal conjugation with following content degradation. Regulatory and effector molecules affecting this pathway have been discovered extensively in recent years [21, 22].
As we observed in our recent study, ER can be a site for autophagy activation, mainly in stress, and a subject for neuroprotective substances [23]. In some specific cases, there are overlaps between ERAD and ER activated autophagy; however, protein half-lives seem to affect pathway selection [24], although interactions between specific substrates, or substrate overload, have been speculated to play significant roles in this manner [25]. P62-mediated selective autophagy can be described as a good example in connection between UPS and autophagy. Certain ubiquitinated substrates are subjected to delivery into autophagosome by simultaneous interaction of P62 with ubiquitin and Atg8 (LC3) [26, 27]. It seems, at least in certain circumstances, that autophagy and ERAD substrates are different. In this manner, knockout of autophagy-related genes 5 (atg5) or atg7 (key autophagic genes in neurons) leads to aggregation of polyubiquitinated polypeptides, where the UPS arm of degradation system is still working [28].
According to recent reports, IRE1 and PERK serve as a UPR platform for the induction of autophagy in context of ER stress. In a tunicamycin- or thapsigargin-induced ER stress, IRE1 component of UPR is necessary for autophagy induction [29]. JNK has been proposed and widely accepted as the main regulatory arm in controlling autophagy by IRE1 [21] (Fig. 1), while its RNase activity in mice indicates a XBP1-dependent autophagy regulation [30]. In this manner, JNK phosphorylation mediated by cytosolic adaptor TRAF2, leads to activation of Beclin-1 (Atg6). Becline-1 is an essential autophagy regulator, which then causes autophagy upregulation [8]. However, in an elegant study, Lee et al. [31] reported that the kinase activity of IRE1 negatively regulates autophagy induction. This indicates a complex contribution of kinase activity of IRE1, mainly on JNK, in controlling ER stress-associated autophagy. On the other hand, cell type-dependent or status-dependent regulation of downstream targets also plays a role. PERK signaling is considered to activate autophagy mainly through gene regulation [8]. Atg12 upregulation in a PERK-eIF2α-dependent manner acts in an ubiquitin-like protein conjugation complex, consisting of Atg12 and Atg5, that affects the expansion process of autophagosome formation. Although ATF4 plays a crucial role in Atg12 gene upregulation in a PERK-eIF2α-ATF4 axis, contribution of transcription factors p8 and C/EBP homologous protein (CHOP), essential mediators of the active component of marihuana (THC) in activating autophagy via ER stress, have been demonstrated in this pathway [8]. In another report, LC3 expression is suggested to be activated by ATF4 and it was also shown that CHOP could be responsible for upregulation of Atg5 [32].
Nuclear factor kappa B (NFκB) is one of the stress-sensing transcription factors and autophagy regulators (discussed below) whose activation has been indicated in PERK signaling, mainly through eIF2α phosphorylation [33]. PERK−/− and eIF2α−/− knockout cells show a decreased NFκB level, which suggests participation of PERK-eIF2α pathway in NFκB activation, importantly in stress conditions. Interestingly, IRE1 with its kinase activity is shown as another activation site for NFκB, which is considered as IRE1-TRAF2-mediated response [34] (Fig. 1). However, autophagy activated via UPR still has some unknown components and its exact mechanism remains to be unraveled.
Nrf1 and Nrf2’s overview: CNC bZIP family members
Cis-acting antioxidant responsive element (ARE, 5′-TGANNNNGC-3′), which is also called electrophile response element, is a target for a subset of transcription factors. These are in charge of adaptively and dynamically counteracting intrinsic and extrinsic oxidants, mainly generated by reactive oxygen and nitrogen species (ROS/RNS) [35, 36]. Nrf1 and Nrf2, as the members of Cap’n’Collar-basic leucine zipper (CNC-bZIP) transcription factors, bind preferentially to the ARE region. Subsequently, these launch an adaptive response aimed at reversing redox imbalance [37, 38]. Both Nrf1 and Nrf2 have the ability to regulate various genes in the antioxidant stress response. Target genes are comprised of a number of phase II of xenobiotic metabolism enzymes: GCLC (glutamate cysteine ligase catalytic subunit), glutathione peroxidase (GPX-1), heme oxygenase-1(HO-1), and catalase [37, 39–41]. Disequilibrium in oxidant production and antioxidant defense leads to an oxidative stress that can counteract cellular signaling pathways and physiological functions, where the major survival role of Nrf2 has been widely studied (reviewed by Qiang Ma) [42]. Since neuroprotective effects of Nrf1 and Nrf2 activation have been observed [43, 44], our investigations of Nrf2 inducers have also shown elevated neuronal antioxidant capacity, both in vitro and in vivo, along with reduced death signaling after various stress insults [45–48].
Since it has been reported that, in comparison to Nrf2, Nrf1 exhibits a lower transactivation activity, Nrf2 has been described as the crucial transcription factor in activation of ARE regulated genes, mainly in acute stress [49]. However, it should be kept in mind that basal expression in some ARE-containing genes has been covered by both Nrf1 and Nrf2 with an approximately equal affinity [50]. This suggests that the existence of a competitive occupation for each factor is possible. There is precedence for this, as specific Nrf1 knockout in mice has been reported to lead in hyper-activation of Nrf2 and downstream target genes [41]. Moreover, it seems transcriptional regulation of Nrf2 is affected by Nrf1 [51]. Interestingly, Wang et al. have independently discovered a 65-kDa isoform of Nrf1 that settles in the nucleus and is responsible for negative feedback regulation of ARE-containing gene expression. This inhibition arises from the ability of this p65 isoform to bind the ARE region with no further gene expression [52]. Distinct targets of Nrf1 and Nrf2 demonstrate their separate contribution in gene expression. Metallothionein-1 (MT1) and -2 (MT2) genes could be activated predominantly through Nrf1 transactivation, whereas Nrf2 persistent activation (by keap1 knockdown) has no significant modification in MT1 and MT2 expression level [41].
Microarray, transfection, and knockout studies have illustrated gene targets for Nrf1 and Nrf2 that are beyond classic oxidative response elements. Osteoblast and odontoblast development and inflammatory response are the examples of non-oxidative operative sites of Nrf1 transcriptional activity [53–55]. Furthermore, and consistently, Nrf2 microarray analysis has indicated that affected genes including both oxidative and non-oxidative agents (e.g., immunity protein and proteasome subunit genes) are major players in ER stress and autophagy [56].
Induction of Nrf2 includes its dissociation from Keap1 (Kelch-like ECH-associated protein1), primarily by electrophile attachment. Nrf2–Keap1 interconnection is not only responsible for preventing Nrf2 from nuclear translocation but it also facilitates its degradation in a ubiquitin–proteasome pathway by ubiquitination with E3 ligase action on Nrf2. In order to free Nrf2 from Keap1, cysteine residues in Keap1 with their sulfhydryl groups are good candidates as sensors mainly of oxidative inducers. Nonetheless, in another point of view, MAPKs have been shown to play a role in the activation of the Nrf2–Keap1 axis [57]. Contribution of MAPKs in activation of ARE, which is mediated by Nrf2, wee introduced primarily by Chen et al. [68]. Consistent with this conclusion, Keum et al. [59] reported the result of an elegant experiment that showed positive regulation of Nrf2 by JNK as an upstream activator. ERK and JNK, among the MAPKs, are suggested as more likely molecules to take part in Nrf2 activation, mainly by direct phosphorylation on serine and threonine residues of Keap1 and Nrf2 [57, 59]. Upon dissociation of Nrf2 from Keap1, it results in escaping of Nrf2 from further proteasome-mediated degradation; on the other hand, Nrf2 still being expressed. This means Nrf2 is stabilized within the cell and its total level is upregulated. Stabilized Nrf2 then translocates to the nucleus and upregulates its downstream components. Another important point is that Nrf2 stabilization through MAPKs phosphorylation seems not to be a general mechanism, and can be cell type/status dependent.
While ample reviews in recent years underscore Keap1 and its interrelation with both Neh2 [Nrf2–ECH (chicken Nrf2) homologous domain] domain of Nrf2 and cytoskeleton [57, 60], the localization and transactivation of Nrf1 remains unclear. Although Nrf1 contains a Neh2-like domain, susceptible to interrelate with Keap1, its N-terminal domain (NTD) has been widely discussed to control its topology and negative regulation, for the reason that Keap1 interaction seems not to play any important role in this mechanism [61, 62]. Since Nrf1 is subjected to localization mainly in ER-membrane by its NTD, the nuclear retrotranslocation is required in order to express its transcriptional duties on ARE-driven genes. Several post-translational conversions have been discussed for topological determination, predominantly in NTD (e.g., NHB2, SAS), in which protein–lipid interactions seem to play a pivotal role [63].
Linking Nrf2 to ER stress and subsequent degradation; our learned novel pathways
Destined to survive or die, the entire cell undergoes widespread modifications, physiologically or pathologically, during ER stress. In addition to the kinase cascades associated with direct and rapid activation/inactivation of substrates, the changes in expression profiles require transcriptional regulators [3]. Among transcription factors triggered or repressed via UPR, Nrf2 is proposed to be the one that may plays a pivotal role in upregulating the non-antioxidant response through UPR-gene regulation interrelation. This needs to be confirmed, however. Although much less is known about the interrelation between Nrf2 and ER stress-associated clearance, we endeavor to explain our collected evidences in order to represent the new outline in the way of interplay between Nrf2, ERAD, and ER stress-mediated autophagy.
Connecting UPR and Nrf2
Targets of Nrf2 are expanding, where they start from cell cycle regulation elements to protein degradation components. The most remarkable is Nrf2’s tendency for activation in diverse stress stimuli [56, 64]. ER stress is one of the conditions in which Nrf2 stabilization has been proven following UPR induction against oxidative stresses, such as cigarette smoke [65, 66]. In our recent in vivo study, we observed meaningful upregulation of Nrf2 level in Aβ-induced ER stress [46]. Participation of PERK as a transmembrane protein to regulate Nrf2 phosphorylation and dissociation from Keap1 is now well established [17, 58, 65] (Fig. 2). Although the PERK–Nrf2 pathway has been studied by several groups, and PERK has been discussed as a Nrf2 activator in the context of ER stress in many papers and reviews, the IRE1α-JNK–Nrf2 axis seems to take part in this phenomenon. Induction of apoptosis signal regulating kinase 1 (ASK1), a MAP3 K of the JNK MAPK pathway, through TRAF2-mediated kinase action of IRE1α, plays a leading role in this route [11, 67]. Jeon et al. [49] demonstrated that HO-1 as a downstream of Nrf2 abundantly upregulated in MAPKs-mediated Nrf2 stabilization. In that study, JNK1/2 inhibition significantly reduced ARE activity which was primarily elevated by genipin-induced phosphorylation and nuclear translocation of Nrf2. Although Jeon et al. reported JNK as a potential activator of Nrf2 pathway, they also showed PI3-kinase as a JNK upstream activator where MAP3 K failed to induce JNK phosphorylation.
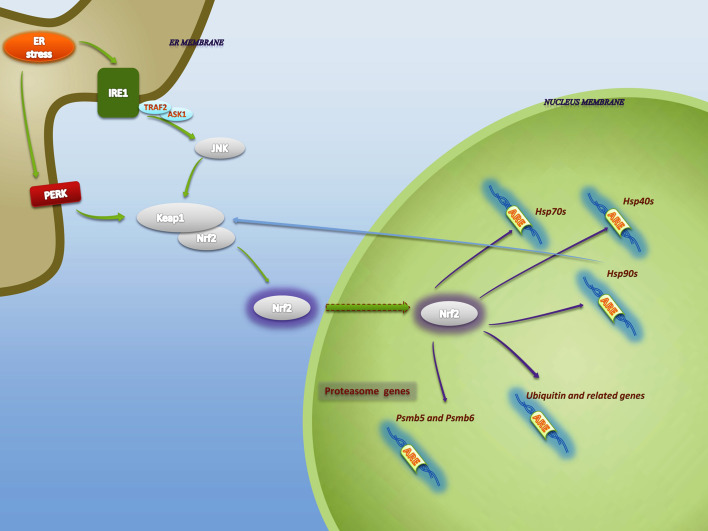
Fig. 2.
Connecting Nrf2 and ER stress-activated ERAD. IRE1 and PERK are the main pathways that activate Nrf2. PERK phosphorylates Nrf2 and disrupts its association with Keap1 resulting in its nuclear translocation. JNK, activated by kinase subunit of IRE1, induces Nrf2–Keap1 dissociation and Nrf2 stabilization. JNK activation requires recruitment of the adaptor protein TNF-receptor associated receptor 2 (TRAF2) which leads to the activation of apoptosis signal regulating kinase 1 (ASK1), a MAP3K of the JNK/p38 MAPK pathway. In the nucleus, Nrf2 upregulates genes associated with different components of ERAD pathway, including proteasomal subunits, ubiquitin-conjugational elements and molecular chaperones. Among chaperones upregulated by Nrf2, HSP90 shows a positive feedback on Nrf2 activation
Upon Nrf2 activation via UPR, an increase in a set of antioxidant genes as one of the major downstream groups of the Nrf2 signaling pathway has been demonstrated [69]. Furthermore, the upregulation of ATF4 and a decrease in CHOP expression, whose activities can be altered by Nrf2 transcription regulator activity, accompany the changes as the result of UPR [69, 70]. Altogether, Nrf2 has been suggested to be a PERK direct substrate and an indirect substrate for IRE1α kinase activity; therefore, an indispensable role can be proposed for Nrf2 activation during ER stress.
Nrf2 and ERAD; road to the proteasome
The hypothesis that Nrf2 is a master regulator of expressional regulation of ERAD components has been seriously tested, and interaction between Nrf2 and ubiquitin/proteasome genes assessed [71]. Although Nrf2 is a substrate for UPS [57], microarray-based studies showed that Nrf2 directly activates ubiquitin/proteasome genes [56, 72]. Studies by Kong’s and Kensler’s [56, 72] groups provided gene study support for distinct ARE/EpRE sequence(s) upstream of some proteasome subunit genes. In a recent study, the fundamental link between Nrf2-proteasome and ER stress has been tested. Tunicamycin-induced ER stress significantly upregulates proteasomal activity mainly due to Nrf2 activation via UPR [73] (Fig. 2). Furthermore, silencing Nrf2 using Nrf2-specific shRNA revealed increased tunicamycin sensitivity. It was followed by a considerable reduction in the transcriptional level of oxidative stress genes such as NQO1, GCLM, GSR, and also proteasomal genes such as PSMB5 and PSMB6. Interestingly, 3H-1,2-dithiole-3-thione (D3T) treatment, a Nrf2 activator, after tunicamycin insult resulted in a significant suppression of UPR genes. In support of this conclusion, Kwak et al. [72, 74] reported in their studies that, when both proteasomal 20S catalytic core and 19S regulatory core are subjected to enhancement by D3T, Nrf2 stabilization was induced. Sulforaphane, which is another compound from cruciferous vegetables (e.g., typical broccoli), has been widely used in order to induce phase II detoxifying enzymes primarily through Nrf2 [75]. In an in vivo study of Nrf2 knockout mice, elevation in proteasome subunits has been shown via microarray analysis in a prolonged (12 h) sulforaphane treatment [56]. As another consistent point of view, in a recent study, Pickering et al. [76] indicated that 20S proteasome and the Pa28αß regulator are affected downstream of Nrf2, and play a key role in oxidative stress response and adaptation by enhanced cellular proteolytic capacity. In spite of crucial role of Nrf2 in expression levels of proteasome subunits genes, noted in the several studies that we have described in this review, it appears that induced and adaptive increase in proteasomal activity, not basal, is under control of Nrf2, though it may depend on cell-type and/or other circumstances [74, 76]. Collectively, Nrf2–ARE binding not only reveals an upregulation in the cellular antioxidant pool but also facilitates elimination of oxidized proteins by enhancing proteasomal activity. Nevertheless, the precise and full function of Nrf2 in ER stress-induced proteasome upregulation remains to be determined.
Ubiquitin-conjugating and chaperone-protein trafficking systems should be considered as other parts of the ERAD clearance pathway as well as proteasomal function, whose expression modifications have been reported to be regulated by Nrf2 activation (Fig. 3). In one of our previous studies, we have demonstrated the concomitant activation of Nrf2 and chaperones in ER stress [45]. We used H2O2 as an ER stress inducer and observed that HSP70 and Nrf2 levels increased following H2O2 treatment. Chaperonin genes including HSP70, HSP90 chaperones, and HSP40 co-chaperones are upregulated through the Nrf2–ARE signaling system, followed by D3T, sulforaphane, or isothiocyanate treatments [56, 65, 72, 77]. Interestingly, oligonucleotide array analysis of sulforaphane-fed wild-type and Nrf2-deficient mice showed requirement of Nrf2 for basal expression of HSP40 (DnaJ) [65]. In another gene expression study, HSP90/HSP40 chaperone genes and components of ubiquitin pathways that are considered to have an ARE region, are capable of being activated by Nrf2 proper [72]. In this manner, in vivo D3T administration triggers the release of Nrf2 from Keap1 following upregulation of the mentioned genes. In the line of Nrf2’s impacts on chaperon level, a positive feedback, ascribed to HSP90, has been introduced [78]. It has been shown that HSP90 and its interaction with Keap1 mediate Nrf2 activation under stress conditions [78]. Inconsistently, in our trials, a negative impact of HSP90 on Nrf2 stabilization, in response to ER stress activation, has been detected [45]. However, it requires further investigations to establish what is the distinct position of chaperones and the ubiquitin system in the ER stress–Nrf2–ARE axis and what is the physiological significance of this expression modification in ER stress-mediated polypeptide degradation?
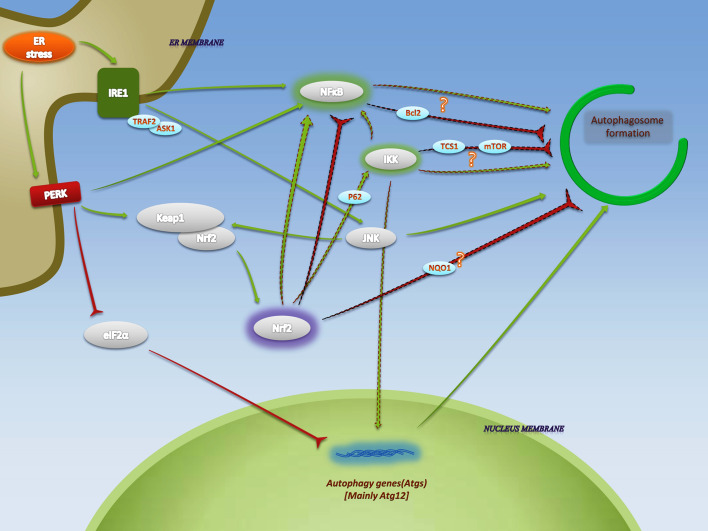
Fig. 3.
Complexity of the impact of Nrf2 on ER stress-mediated autophagy. IKK, NFκB, and JNK, which are activated via UPR, have been discussed in autophagy upregulation during ER stress. Inactivation of eIF2α also enhances autophagy. Following UPR-dependent Nrf2 stabilization, NFκB and IKK are supposed to be responsible for Nrf2’s effects on autophagy. Nrf2 activates IKK by enhancing the expression of P62. P62-dependent activation of IKK subsequently results in dissociation of IκB from NFκB and its activation. Complex interrelation of NFκB with autophagy regulating components as well as IKK-autophagy interactions indicate both inhibitory and induction effects, due to various cellular circumstances. In another point of view, NQO1 as a target for Nrf2 seems to be relevant to autophagosome formation. The arrows with dashed outline indicate pathways in Nrf2-activated autophagy
Nrf2 and Autophagy; complexity of correlations
As we described above, JNK and NFκB are the ER stress-activated intermediates, which are master regulators of autophagy induction. Along with molecule-to-molecule activation/inactivation of reaction cascades through JNK and NFκB, direct expressional modification is another UPR-controlled signaling in order to recruit autophagy for the elimination of oxidized, toxigenic, and aggregation-inducing proteins [8]. With the exception of JNK, acting downstream of UPR pathway, we and others demonstrated a distinctive role for JNK as an upstream activator of ER stress [79, 80]. In any case, in JNK-activated ER stress, our attempt to inhibit JNK leads to hyperactivation of Nrf2 [79].
As it appears to be, Nrf2 has been suggested to have interplay with autophagic pathways. Considerable effort has been invested in the autophagy-deficiency feedback in Nrf2 transactivation [81–84]. As an example, autophagy inhibition results in P62 upregulation and then P62 itself upregulates Nrf2 in a Keap1-dependent manner [84]. In spite of these efforts, there have been a few studies that directly investigated the Nrf2-autophagy in a feed-forward mechanism, in which Nrf2 regulates autophagy.
In a small proof-of-principle study, Rao et al. attempted to clarify the mechanism that is supposed to be responsible for negative regulation of autophagy by Nrf2. They have discovered that NAD(P)H dehydrogenase [quinone] 1 (NQO1) as a downstream target of Nrf2, may be responsible for this negative effect of Nrf2 on autophagosome formation [85] (Fig. 3). Very recently, Zhou et al. [86] reported that Nrf2 has the ability to inhibit autophagosome formation. In addition, they observed that silencing its mRNA in U251-Si-Nrf2 transfected cells enhanced basal and induced levels of autophagy. In another recent study, mitochondrial stress-related autophagy was downregulated when Nrf2 was activated against unilateral ureteral obstruction [85]. These data demonstrate a dominantly negative feedback following Nrf2 activation. However, it is not clear whether Nrf2 directly interrelated with autophagy-inducing agents, or whether there are intermediates whose interaction could play the main role in regulating autophagy. Interestingly, in striking contrast, it was observed that in ER stress conditions both Nrf2 and autophagy activated simultaneously, which cannot be consistent with the described dominant-negative regulation activity [8]. Consistently, deregulation of autophagy, with undigested/misdigested intermediates, has been reported in Nrf2 knockout of retinal pigment epithelium (RPE) cells [87].
In pursuit of further clues in functional linkage between Nrf2 and autophagy, Stępkowski and Kruszewski [88] have hypothesized that NFκB appears to be the pivotal platform downstream of Nrf2. They have described the Nrf2-P62-IKK pathway in order to explain NFκB-dependent autophagy activation (Fig. 3). Although indirect, this important proposal in parallel may support the observation of inconsistent impacts of Nrf2 on the autophagy. Primarily, a trial of clarifying the Nrf2 effect on P62 expression has revealed an ARE-containing region between nucleotides 1,305 and 1,295 on the P62 promoter that is responsible for P62 upregulation in an Nrf2-dependent manner [89]. The second step is referred to a mechanism in which TRAF6 oligomerization and self-polyubiquitination in a P62-dependent manner result in IκB kinase (IKK) activation [90]. IKK is composed of catalytic and regulatory subunits with a consensus role in NFκB activation [91]. Actually, the interaction between Nrf2 and NFκB is much more than the mentioned course. Many experiments have been conducted to clarify the direct and indirect interactions of Nrf2 and NFκB (reviewed by Wakabayashi et al. [92]). Surprisingly, negative impact of Nrf2 on NFκB is more accepted than positive regulation [92].
In spite of the fact that NFκB is a potent autophagy regulator, the manner of interrelation between NFκB and autophagy seems puzzling. Autophagy is activated in response to multiple distinct routes in the NFκB-autophagy signaling axis, such as heat-shock-activated autophagy and Beclin1 gene upregulation [93, 94]. Still, the contribution of NFκB in TNFα-induced autophagy and Bcl2-mediated autophagy inhibition shows inhibitory roles [95–97]. Bcl2 has been suggested as an NFκB target gene, whereas Bcl2 itself is responsible for autophagy downregulation through inhibiting Becline1.
Interestingly, to find distinct autophagy mediators in the NFκB activation pathway, it has been revealed that IKK, and not NFκB, is important for induction of autophagy [98] (Fig. 3). In vitro studies have implicated that both catalytic subunits of IKK (IKKα and IKKβ) are involved in IKK-dependent autophagy induction [98, 99]. However, in other independent studies, physical interactions between IKKα and IKKβ with mammalian target of rapamycin (mTOR) and tuberous sclerosis protein 1 (TCS1), respectively, have been proposed to activate the mTOR pathway and subsequently inhibit autophagosome formation [100, 101]. Aside from IKK and NFκB interference, it has been shown that P62 solely has the ability to inhibit autophagy by formation of a complex with Rag GTPases which recruits mTORC1 as an inhibitory agent [102]. In addition, LC3 and ubiquitin are p62-interacting partners that bind to P62 through the LC3-interacting region and ubiquitin-associated domain [26, 27]. This double linkage between LC3 and ubiquitin predisposes P62 as a selective autophagy mediator. Significantly, the presence of Keap1 in the LC3 and p62 protein complex has been detected; even so, the distinct role of Keap1 in P62-mediated autophagy still remains elusive [103].
Collectively, it is reasonable to speculate that there are intermediates in the pathway of autophagy regulation, more than assumed NQO1, with more complex interactions that we may not know yet. Although the ER stress is a potential activator of both Nrf2 and autophagy, a key challenge is to determine whether NFκB, as well as IKK, orchestrates the interrelation in these cases or whether there is an alternative mechanism in this path.
Evidence for and against Nrf1. Can it be regulated by ER stress?
The inactivated Nrf1, TCF11 with a mass of 120 kDa, anchors to ER membrane by its NTD, predominantly by its NHB1 domain (residues 11–30) [104]. Nrf1 undergoes fast degradation in non-stress and physiological conditions via HRD1-mediated ubiquitination and subsequently ERAD [105]. The NST domain is subjected to glycosylation since applying deglycosylating enzymes results in formation of 95-kDa activated protein and its translocation to the nucleus [104]. Moreover, not only does Nrf1 undergo deglycosylation but an intra-membrane/proteasomal cleavage has also been observed before nuclear translocation [104]. These processes provide posttranslational control of Nrf1; however, some co-translational modifications at ribosomal sites have been also displayed [63].
There is now a large body of evidence indicating that Nrf1 is a potent enhancer in expression of proteasomal subunits, with even more affinity compared to Nrf2 (Fig. 4). In support of this, Krüger et al. [105] reported that proteasomal inhibitors trigger Nrf1 nuclear translocation following de novo synthetizing 20S proteasomal subunits through binding to ARE targets on proteasome genes. Another independent report showed that, in mouse embryonic fibroblasts Nrf1, but not Nrf2, is necessary for de novo expression of proteasomal subunits upon applying proteasomal inhibitors [106] (Fig. 4). Interestingly, proteasomal inhibition could be one of the ER stress inducers due to the failed ERAD pathway and subsequent misfolded/unfolded protein accumulation [107, 108]. Apart from proteasomal inhibition, H2O2 preconditioning induces Nrf1 via deglycosylation and nuclear localization. When the cells were transfected with Nrf1-siRNA, Nrf1 activity following H2O2 preconditioning was abolished by downregulation of its phase II detoxifying enzyme targets [109]. Also, in a recent study, Nrf1 phosphorylation is discussed as an alternative pathway, although their parallel experiments show that hypoxia and not proteasome inhibition is involved in Nrf1 transactivation [110]. They have discussed that, since proteasome is one of major platforms for Nrf1 proteolysis and activation, its inhibition is unfavorable to transactivate Nrf1. In fact, the mechanism through which proteasome inhibition and other stressors trigger Nrf1 is not well recognized. Still, it is reasonable to speculate that, according to ER membrane localization of the inactive form of Nrf1 and its proteasome upregulatory function as an ERAD pathway component, Nrf1 has a strong capacity of being induced through ER stress as well as by other ER stressors. The idea of ER stress-mediated activation of Nrf1 was put in test primarily by treating with tunicamycin as an ER stress inducer in the cells transfected with Myc-tagged Nrf1 expression vectors [111]. A study by Wang and Chan showed that Myc-tagged Nrf1 migrates in two independent bands with 110 and 120 kDa in both tunicamycin-treated and untreated cells. Interestingly, higher levels of p110 Nrf1 were found in ER stress-induced cells, particularly in nuclear fractions, with noticeable Nrf1 localization in the nucleus. These findings indicate a possible membranous cleavage of 120 kDa Nrf1 and nuclear translocation following ER stress initiation. In contrast to Wang and Chan, Zhang’s group later reported that, although ER stressors trigger formation of 110-kDa Nrf1, this isoform of Nrf1 does not have the ability to transactivate downstream ARE-containing genes. Furthermore, even the subcellular localization of Nrf1 remains unchanged next to ER stressors administration, such as tunicamycin and thapsigargin [62].
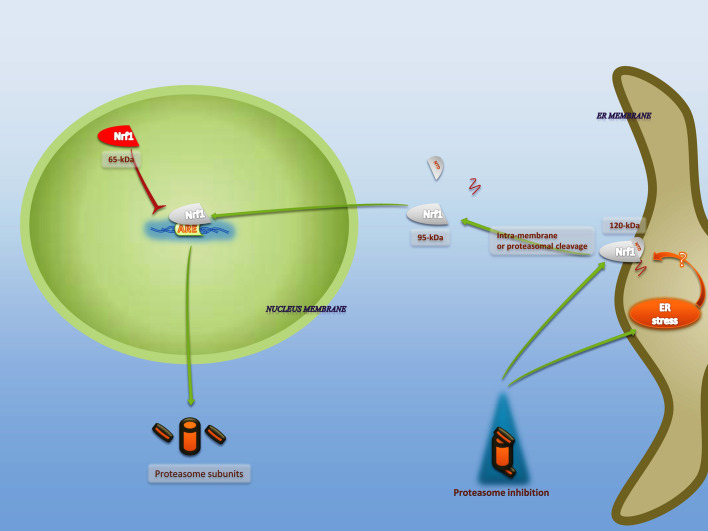
Fig. 4.
Upregulation of ERAD components through Nrf1 activation. Proteasomal inhibition and ER stress are likely activators of Nrf1. Although proteasomal inhibition induces ER stress, the activating impact of ER stress on Nrf1 activation is doubtful. Interamembrane or proteasomal cleavage is required for Nrf1 activation and nuclear translocation. Since Nrf1 is glycosylated near its N-terminal, deglycosylation is expected during nuclear translocation. Upon nuclear accumulation of cleaved Nrf1, it binds to ARE-containing proteasomal genes and potentially enhances their transcription as ERAD components. A dominant inhibitory p65 isoform of Nrf1, a product of alternative translation, translocates to the nucleus and inhibits the expression of ARE-containing genes
The interrelation between ER stress and Nrf1 requires further investigation. To determine the precise role of Nrf1 in ER stress, transactivation, posttranslational/co-translational modification, nuclear translocation, and downstream gene induction as targets of Nrf1, must also be studied.
Conclusion
The UPR is a stress response that is activated upon the accumulation of misfolded or unfolded proteins in the ER. The degradation of aberrant ER proteins is mediated by two processes: ERAD and autophagy. In this review, we summarized the molecular events occurring during ERAD and ER stress-associated autophagy and their crosstalk with Nrf2 and Nrf1 as two stress-sensing transcription factors. Compelling evidence suggests that Nrf2 can upregulate ERAD components in ER stress conditions. To date, only a limited number of studies have specifically examined direct impact of Nrf2 on the autophagy. As another deleterious route, Nrf2 has been supposed to induce autophagosome formation via P62-IKK-NFκB pathway that can be seen in ER stress conditions. In contrast, Nrf2 has also been shown to activate some autophagy inhibitory pathways. Eventually, it seems that cell type/status, stress severity and duration are the determinant factors in this pathway selection. Moreover, P62 upregulation in autophagy-deficiency feedback results in Nrf2 activation in a Keap1-dependent manner. Nrf1 also have shown to induce proteasome subunits as ERAD components; however, the impact of ER stress on Nrf1 activation is still unknown. Further studies are required to elucidate detailed mechanisms of the mentioned pathways.
Acknowledgments
Authors acknowledge the funding support by Shahid Beheshti University of Medical Science to H.D. and F.K.H. and National Elite Fund, Iran, for the award of Young Scientist Research Fellowship to F.K.H. and funds by the University of Arkansas (Startup fund) and Center for Translational Neuroscience (UAMS-COBRE), to M.K.
References
- 1.Berridge MJ. The endoplasmic reticulum: a multifunctional signaling organelle. Cell Calcium. 2002;32:235–249. doi: 10.1016/s0143416002001823. [DOI] [PubMed] [Google Scholar]
- 2.Görlach A, Klappa P, Kietzmann T. The endoplasmic reticulum: folding, calcium homeostasis, signaling, and redox control. Antioxid Redox Signal. 2006;8:1391–1418. doi: 10.1089/ars.2006.8.1391. [DOI] [PubMed] [Google Scholar]
- 3.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 4.Kim I, Xu W, Reed JC. Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. Nat Rev Drug Discov. 2008;7:1013–1030. doi: 10.1038/nrd2755. [DOI] [PubMed] [Google Scholar]
- 5.Shim S, Lee W, Chung H, Jung YK. Amyloid β-induced FOXRED2 mediates neuronal cell death via inhibition of proteasome activity. Cell Mol Life Sci. 2011;68:2115–2127. doi: 10.1007/s00018-010-0561-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bernard-Marissal N, Moumen A, Sunyach C, Pellegrino C, Dudley K, Henderson CE, Raoul C, Pettmann B. Reduced calreticulin levels link endoplasmic reticulum stress and Fas-triggered cell death in motoneurons vulnerable to ALS. J Neurosci. 2012;32:4901–4912. doi: 10.1523/JNEUROSCI.5431-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ansari N, Khodagholi F. Molecular mechanism aspect of ER stress in Alzheimer’s disease: current approaches and future strategies. Curr Drug Targ. 2012;14:114–122. doi: 10.2174/138945013804806532. [DOI] [PubMed] [Google Scholar]
- 8.Verfaillie T, Salazar M, Velasco G, Agostinis P. Linking ER stress to autophagy: potential implications for cancer therapy. Int J Cell Biol. 2010;2010:930509. doi: 10.1155/2010/930509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kozutsumi Y, Segal M, Normington K, Gething MJ, Sambrook J. The presence of malfolded proteins in the endoplasmic reticulum signals the induction of glucose-regulated proteins. Nature. 1988;332:462–464. doi: 10.1038/332462a0. [DOI] [PubMed] [Google Scholar]
- 10.Travers KJ, Patil CK, Wodicka L, Lockhart DJ, Weissman JS, Walter P. Functional and genomic analyses reveal an essential coordination between the unfolded protein response and ER-associated degradation. Cell. 2000;101:249–258. doi: 10.1016/s0092-8674(00)80835-1. [DOI] [PubMed] [Google Scholar]
- 11.Urano F, Wang X, Bertolotti A, Zhang Y, Chung P, Harding HP, Ron D. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287:664–666. doi: 10.1126/science.287.5453.664. [DOI] [PubMed] [Google Scholar]
- 12.Yamamoto K, Sato T, Matsui T, Sato M, Okada T, Yoshida H, Harada A, Mori K. Transcriptional induction of mammalian ER quality control proteins is mediated by single or combined action of ATF6alpha and XBP1. Dev Cell. 2007;13:365–376. doi: 10.1016/j.devcel.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 13.Wu J, Rutkowski DT, Dubois M, Swathirajan J, Saunders T, Wang J, Song B, Yau GD, Kaufman RJ. ATF6alpha optimizes long-term endoplasmic reticulum function to protect cells from chronic stress. Dev Cell. 2007;13:351–364. doi: 10.1016/j.devcel.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 14.Wong E, Cuervo AM. Integration of clearance mechanisms: the proteasome and autophagy. Cold Spring Harb Perspect Biol. 2010;2:a006734. doi: 10.1101/cshperspect.a006734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kroeger H, Chiang WC, Lin JH. Endoplasmic reticulum-associated degradation (ERAD) of misfolded glycoproteins and mutant P23H rhodopsin in photoreceptor cells. Adv Exp Med Biol. 2012;723:559–565. doi: 10.1007/978-1-4614-0631-0_71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vembar SS, Brodsky JL. One step at a time: endoplasmic reticulum-associated degradation. Nat Rev Mol Cell Biol. 2008;9:944–957. doi: 10.1038/nrm2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schröder M, Kaufman RJ. The mammalian unfolded protein response. Annu Rev Biochem. 2005;74:739–789. doi: 10.1146/annurev.biochem.73.011303.074134. [DOI] [PubMed] [Google Scholar]
- 18.Brodsky JL, McCracken AA. ER protein quality control and proteasome-mediated protein degradation. Semin Cell Dev Biol. 1999;10:507–513. doi: 10.1006/scdb.1999.0321. [DOI] [PubMed] [Google Scholar]
- 19.Glickman MH, Ciechanover A. The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol Rev. 2002;82:373–428. doi: 10.1152/physrev.00027.2001. [DOI] [PubMed] [Google Scholar]
- 20.Watts GD, Wymer J, Kovach MJ, Mehta SG, Mumm S, Darvish D, Pestronk A, Whyte MP, Kimonis VE. Inclusion body myopathy associated with Paget disease of bone and frontotemporal dementia is caused by mutant valosin-containing protein. Nat Genet. 2004;36:377–381. doi: 10.1038/ng1332. [DOI] [PubMed] [Google Scholar]
- 21.Kroemer G, Mariño G, Levine B. Autophagy and the integrated stress response. Mol Cell. 2010;40:280–293. doi: 10.1016/j.molcel.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pyo JO, Nah J, Jung YK. Molecules and their functions in autophagy. Exp Mol Med. 2012;44:73–80. doi: 10.3858/emm.2012.44.2.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rafatian G, Khodagholi F, Farimani MM, Abraki SB, Gardaneh M. Increase of autophagy and attenuation of apoptosis by Salvigenin promote survival of SH-SY5Y cells following treatment with H2O2 . Mol Cell Biochem. 2012;371:9–22. doi: 10.1007/s11010-012-1416-6. [DOI] [PubMed] [Google Scholar]
- 24.Ding WX, Yin XM. Sorting, recognition and activation of the misfolded protein degradation pathways through macroautophagy and the proteasome. Autophagy. 2008;4:141–150. doi: 10.4161/auto.5190. [DOI] [PubMed] [Google Scholar]
- 25.Fujita E, Kouroku Y, Isoai A, Kumagai H, Misutani A, Matsuda C, Hayashi YK, Momoi T. Two endoplasmic reticulum-associated degradation (ERAD) systems for the novel variant of the mutant dysferlin: ubiquitin/proteasome ERAD(I) and autophagy/lysosome ERAD(II) Hum Mol Genet. 2007;16:618–629. doi: 10.1093/hmg/ddm002. [DOI] [PubMed] [Google Scholar]
- 26.Bjørkøy G, Lamark T, Brech A, Outzen H, Perander M, Overvatn A, Stenmark H, Johansen T. p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. J Cell Biol. 2005;171:603–614. doi: 10.1083/jcb.200507002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Komatsu M, Waguri S, Koike M, Sou YS, Ueno T, Hara T, Mizushima N, Iwata J, Ezaki J, Murata S, Hamazaki J, Nishito Y, Iemura S, Natsume T, Yanagawa T, Uwayama J, Warabi E, Yoshida H, Ishii T, Kobayashi A, Yamamoto M, Yue Z, Uchiyama Y, Kominami E, Tanaka K. Homeostatic levels of p62 control cytoplasmic inclusion body formation in autophagy-deficient mice. Cell. 2007;131:1149–1163. doi: 10.1016/j.cell.2007.10.035. [DOI] [PubMed] [Google Scholar]
- 28.Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, Yokoyama M, Mishima K, Saito I, Okano H, Mizushima N. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441:885–889. doi: 10.1038/nature04724. [DOI] [PubMed] [Google Scholar]
- 29.Ogata M, Hino S, Saito A, Morikawa K, Kondo S, Kanemoto S, Murakami T, Taniguchi M, Tanii I, Yoshinaga K, Shiosaka S, Hammarback JA, Urano F, Imaizumi K. Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol Cell Biol. 2006;26:9220–9231. doi: 10.1128/MCB.01453-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hetz C, Thielen P, Matus S, Nassif M, Court F, Kiffin R, Martinez G, Cuervo AM, Brown RH, Glimcher LH. XBP-1 deficiency in the nervous system protects against amyotrophic lateral sclerosis by increasing autophagy. Genes Dev. 2009;23:2294–2306. doi: 10.1101/gad.1830709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee H, Noh JY, Oh Y, Kim Y, Chang JW, Chung CW, Lee ST, Kim M, Ryu H, Jung YK. IRE1 plays an essential role in ER stress-mediated aggregation of mutant huntingtin via the inhibition of autophagy flux. Hum Mol Genet. 2012;21:101–114. doi: 10.1093/hmg/ddr445. [DOI] [PubMed] [Google Scholar]
- 32.Kouroku Y, Fujita E, Tanida I, Ueno T, Isoai A, Kumagai H, Ogawa S, Kaufman RJ, Kominami E, Momoi T. ER stress (PERK/eIF2alpha phosphorylation) mediates the polyglutamine-induced LC3 conversion, an essential step for autophagy formation. Cell Death Differ. 2007;14:230–239. doi: 10.1038/sj.cdd.4401984. [DOI] [PubMed] [Google Scholar]
- 33.Deng J, Lu PD, Zhang Y, Scheuner D, Kaufman RJ, Sonenberg N, Harding HP, Ron D. Translational repression mediates activation of nuclear factor kappa B by phosphorylated translation initiation factor 2. Mol Cell Biol. 2004;24:10161–10168. doi: 10.1128/MCB.24.23.10161-10168.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaneko M, Niinuma Y, Nomura Y. Activation signal of nuclear factor-kappa B in response to endoplasmic reticulum stress is transduced via IRE1 and tumor necrosis factor receptor-associated factor 2. Biol Pharm Bull. 2003;26:931–935. doi: 10.1248/bpb.26.931. [DOI] [PubMed] [Google Scholar]
- 35.Sykiotis GP, Bohmann D. Stress-activated cap’n’collar transcription factors in aging and human disease. Sci Signal. 2010;3:re3. doi: 10.1126/scisignal.3112re3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nguyen T, Sherratt PJ, Pickett CB. Regulatory mechanisms controlling gene expression mediated by the antioxidant response element. Annu Rev Pharmacol Toxicol. 2003;43:233–260. doi: 10.1146/annurev.pharmtox.43.100901.140229. [DOI] [PubMed] [Google Scholar]
- 37.Venugopal R, Jaiswal AK. Nrf2 and Nrf1 in association with Jun proteins regulate antioxidant response element-mediated expression and coordinated induction of genes encoding detoxifying enzymes. Oncogene. 1998;17:3145–3156. doi: 10.1038/sj.onc.1202237. [DOI] [PubMed] [Google Scholar]
- 38.Derjuga A, Gourley TS, Holm TM, Heng HH, Shivdasani RA, Ahmed R, Andrews NC, Blank V. Complexity of CNC transcription factors as revealed by gene targeting of the Nrf3 locus. Mol Cell Biol. 2004;24:3286–3294. doi: 10.1128/MCB.24.8.3286-3294.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Motohashi H, O’Connor T, Katsuoka F, Engel JD, Yamamoto M. Integration and diversity of the regulatory network composed of Maf and CNC families of transcription factors. Gene. 2002;294:1–12. doi: 10.1016/s0378-1119(02)00788-6. [DOI] [PubMed] [Google Scholar]
- 40.Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, Yamamoto M, Nabeshima Y. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun. 1997;236:313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- 41.Ohtsuji M, Katsuoka F, Kobayashi A, Aburatani H, Hayes JD, Yamamoto M. Nrf1 and Nrf2 play distinct roles in activation of antioxidant response element-dependent genes. J Biol Chem. 2008;283:33554–33562. doi: 10.1074/jbc.M804597200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ma Q. Role of nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol. 2013;53:401–426. doi: 10.1146/annurev-pharmtox-011112-140320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hertel M, Braun S, Durka S, Alzheimer C, Werner S. Upregulation and activation of the Nrf-1 transcription factor in the lesioned hippocampus. Eur J Neurosci. 2002;15:1707–1711. doi: 10.1046/j.1460-9568.2002.01992.x. [DOI] [PubMed] [Google Scholar]
- 44.Cuadrado A, Moreno-Murciano P, Pedraza-Chaverri J. The transcription factor Nrf2 as a new therapeutic target in Parkinson’s disease. Expert Opin Ther Targ. 2009;13:319–329. doi: 10.1517/13543780802716501. [DOI] [PubMed] [Google Scholar]
- 45.Tusi SK, Khalaj L, Ashabi G, Kiaei M, Khodagholi F. Alginate oligosaccharide protects against endoplasmic reticulum- and mitochondrial-mediated apoptotic cell death and oxidative stress. Biomaterials. 2011;32:5438–5458. doi: 10.1016/j.biomaterials.2011.04.024. [DOI] [PubMed] [Google Scholar]
- 46.Ashabi G, Alamdary SZ, Ramin M, Khodagholi F. Reduction of hippocampal apoptosis by intracerebroventricular administration of extracellular signal-regulated protein kinase and/or p38 inhibitors in amyloid beta rat model of Alzheimer’s disease: involvement of nuclear-related factor-2 and nuclear factor-κB. Basic Clin Pharmacol Toxicol. 2013;112:145–155. doi: 10.1111/bcpt.12000. [DOI] [PubMed] [Google Scholar]
- 47.Yang L, Calingasan NY, Thomas B, Chaturvedi RK, Kiaei M, Wille EJ, Liby KT, Williams C, Royce D, Risingsong R, Musiek ES, Morrow JD, Sporn M, Beal MF. Neuroprotective effects of the triterpenoid, CDDO methyl amide, a potent inducer of Nrf2 mediated transcription. PLoS ONE. 2009;4:e5757. doi: 10.1371/journal.pone.0005757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Neymotin A, Calingasan NY, Wille E, Naseri N, Petri S, Damiano M, Liby KT, Risingsong R, Sporn M, Beal MF, Kiaei M. Neuroprotective effect of Nrf2/ARE activators, CDDO ethylamide and CDDO trifluoroethylamide, in a mouse model of amyotrophic lateral sclerosis. Free Radic Biol Med. 2011;51:88–96. doi: 10.1016/j.freeradbiomed.2011.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jeon WK, Hong HY, Kim BC. Genipin up-regulates heme oxygenase-1 via PI3-kinase-JNK1/2-Nrf2 signaling pathway to enhance the anti-inflammatory capacity in RAW264.7 macrophages. Arch Biochem Biophys. 2011;512:119–125. doi: 10.1016/j.abb.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 50.Leung L, Kwong M, Hou S, Lee C, Chan JY. Deficiency of the Nrf1 and Nrf2 transcription factors results in early embryonic lethality and severe oxidative stress. J Biol Chem. 2003;278:48021–48029. doi: 10.1074/jbc.M308439200. [DOI] [PubMed] [Google Scholar]
- 51.Kwak MK, Itoh K, Yamamoto M, Kensler TW. Enhanced expression of the transcription factor Nrf2 by cancer chemopreventive agents: role of antioxidant response element-like sequences in the nrf2 promoter. Mol Cell Biol. 2002;22:2883–2892. doi: 10.1128/MCB.22.9.2883-2892.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang W, Kwok AM, Chan JY. The p65 isoform of Nrf1 is a dominant negative inhibitor of ARE-mediated transcription. J Biol Chem. 2007;282:24670–24678. doi: 10.1074/jbc.M700159200. [DOI] [PubMed] [Google Scholar]
- 53.Narayanan K, Ramachandran A, Peterson MC, Hao J, Kolstø AB, Friedman AD, George A. The CCAAT enhancer-binding protein (C/EBP)beta and Nrf1 interact to regulate dentin sialophosphoprotein (DSPP) gene expression during odontoblast differentiation. J Biol Chem. 2004;279:45423–45432. doi: 10.1074/jbc.M405031200. [DOI] [PubMed] [Google Scholar]
- 54.Xing W, Singgih A, Kapoor A, Alarcon CM, Baylink DJ, Mohan S. Nuclear factor-E2-related factor-1 mediates ascorbic acid induction of osterix expression via interaction with antioxidant-responsive element in bone cells. J Biol Chem. 2007;282:22052–22061. doi: 10.1074/jbc.M702614200. [DOI] [PubMed] [Google Scholar]
- 55.Biswas M, Chan JY. Role of Nrf1 in antioxidant response element-mediated gene expression and beyond. Toxicol Appl Pharmacol. 2010;244:16–20. doi: 10.1016/j.taap.2009.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hu R, Xu C, Shen G, Jain MR, Khor TO, Gopalkrishnan A, Lin W, Reddy B, Chan JY, Kong AN. Gene expression profiles induced by cancer chemopreventive isothiocyanate sulforaphane in the liver of C57BL/6 J mice and C57BL/6 J/Nrf2 (−/−) mice. Cancer Lett. 2006;243:170–192. doi: 10.1016/j.canlet.2005.11.050. [DOI] [PubMed] [Google Scholar]
- 57.Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- 58.Cullinan SB, Zhang D, Hannink M, Arvisais E, Kaufman RJ, Diehl JA. Nrf2 is a direct PERK substrate and effector of PERK-dependent cell survival. Mol Cell Biol. 2003;23:7198–7209. doi: 10.1128/MCB.23.20.7198-7209.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Keum YS, Owuor ED, Kim BR, Hu R, Kong AN. Involvement of Nrf2 and JNK1 in the activation of antioxidant responsive element (ARE) by chemopreventive agent phenethyl isothiocyanate (PEITC) Pharm Res. 2003;20:1351–1356. doi: 10.1023/a:1025737622815. [DOI] [PubMed] [Google Scholar]
- 60.Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, Yamamoto M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999;13:76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang Y, Crouch DH, Yamamoto M, Hayes JD. Negative regulation of the Nrf1 transcription factor by its N-terminal domain is independent of Keap1: Nrf1, but not Nrf2, is targeted to the endoplasmic reticulum. Biochem J. 2006;399:373–385. doi: 10.1042/BJ20060725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang Y, Lucocq JM, Hayes JD. The Nrf1 CNC/bZIP protein is a nuclear envelope-bound transcription factor that is activated by t-butyl hydroquinone but not by endoplasmic reticulum stressors. Biochem J. 2009;418:293–310. doi: 10.1042/BJ20081575. [DOI] [PubMed] [Google Scholar]
- 63.Zhang Y, Hayes JD. Identification of topological determinants in the N-terminal domain of transcription factor Nrf1 that control its orientation in the endoplasmic reticulum membrane. Biochem J. 2010;430:497–510. doi: 10.1042/BJ20100471. [DOI] [PubMed] [Google Scholar]
- 64.Thimmulappa RK, Mai KH, Srisuma S, Kensler TW, Yamamoto M, Biswal S. Identification of Nrf2-regulated genes induced by the chemopreventive agent sulforaphane by oligonucleotide microarray. Cancer Res. 2002;62:5196–5203. [PubMed] [Google Scholar]
- 65.Cullinan SB, Diehl JA. Coordination of ER and oxidative stress signaling: the PERK/Nrf2 signaling pathway. Int J Biochem Cell Biol. 2006;38:317–332. doi: 10.1016/j.biocel.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 66.Kelsen SG, Duan X, Ji R, Perez O, Liu C, Merali S. Cigarette smoke induces an unfolded protein response in the human lung: a proteomic approach. Am J Respir Cell Mol Biol. 2008;38:541–550. doi: 10.1165/rcmb.2007-0221OC. [DOI] [PubMed] [Google Scholar]
- 67.Nishitoh H, Matsuzawa A, Tobiume K, Saegusa K, Takeda K, Inoue K, Hori S, Kakizuka A, Ichijo H. ASK1 is essential for endoplasmic reticulum stress-induced neuronal cell death triggered by expanded polyglutamine repeats. Genes Dev. 2002;16:1345–1355. doi: 10.1101/gad.992302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen C, Yu R, Owuor ED, Kong AN. Activation of antioxidant-response element (ARE), mitogen-activated protein kinases (MAPKs) and caspases by major green tea polyphenol components during cell survival and death. Arch Pharm Res. 2000;23:605–612. doi: 10.1007/BF02975249. [DOI] [PubMed] [Google Scholar]
- 69.Cullinan SB, Diehl JA. PERK-dependent activation of Nrf2 contributes to redox homeostasis and cell survival following endoplasmic reticulum stress. J Biol Chem. 2004;279:20108–20117. doi: 10.1074/jbc.M314219200. [DOI] [PubMed] [Google Scholar]
- 70.He CH, Gong P, Hu B, Stewart D, Choi ME, Choi AM, Alam J. Identification of activating transcription factor 4 (ATF4) as an Nrf2-interacting protein. Implication for heme oxygenase-1 gene regulation. J Biol Chem. 2001;276:20858–20865. doi: 10.1074/jbc.M101198200. [DOI] [PubMed] [Google Scholar]
- 71.Chapple SJ, Siow RC, Mann GE. Crosstalk between Nrf2 and the proteasome: therapeutic potential of Nrf2 inducers in vascular disease and aging. Int J Biochem Cell Biol. 2012;44:1315–1320. doi: 10.1016/j.biocel.2012.04.021. [DOI] [PubMed] [Google Scholar]
- 72.Kwak MK, Wakabayashi N, Itoh K, Motohashi H, Yamamoto M, Kensler TW. Modulation of gene expression by cancer chemopreventive dithiolethiones through the Keap1-Nrf2 pathway. Identification of novel gene clusters for cell survival. J Biol Chem. 2003;278(10):8135–8145. doi: 10.1074/jbc.M211898200. [DOI] [PubMed] [Google Scholar]
- 73.Lee S, Hur EG, Ryoo IG, Jung KA, Kwak J, Kwak MK. Involvement of the Nrf2-proteasome pathway in the endoplasmic reticulum stress response in pancreatic β-cells. Toxicol Appl Pharmacol. 2012;264:431–438. doi: 10.1016/j.taap.2012.08.021. [DOI] [PubMed] [Google Scholar]
- 74.Kwak MK, Wakabayashi N, Greenlaw JL, Yamamoto M, Kensler TW. Antioxidants enhance mammalian proteasome expression through the Keap1-Nrf2 signaling pathway. Mol Cell Biol. 2003;23:8786–8794. doi: 10.1128/MCB.23.23.8786-8794.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shinkai Y, Sumi D, Fukami I, Ishii T, Kumagai Y. Sulforaphane, an activator of Nrf2, suppresses cellular accumulation of arsenic and its cytotoxicity in primary mouse hepatocytes. FEBS Lett. 2006;580:1771–1774. doi: 10.1016/j.febslet.2006.02.031. [DOI] [PubMed] [Google Scholar]
- 76.Pickering AM, Linder RA, Zhang H, Forman HJ, Davies KJ. Nrf2-dependent induction of proteasome and Pa28αβ regulator are required for adaptation to oxidative stress. J Biol Chem. 2012;287:10021–10031. doi: 10.1074/jbc.M111.277145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hu R, Xu C, Shen G, Jain MR, Khor TO, Gopalkrishnan A, Lin W, Reddy B, Chan JY, Kong AN. Identification of Nrf2-regulated genes induced by chemopreventive isothiocyanate PEITC by oligonucleotide microarray. Life Sci. 2006;79:1944–1955. doi: 10.1016/j.lfs.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 78.Niture SK, Jaiswal AK. Hsp90 interaction with INrf2(Keap1) mediates stress-induced Nrf2 activation. J Biol Chem. 2010;285:36865–36875. doi: 10.1074/jbc.M110.175802. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 79.Yenki P, Khodagholi F, Shaerzadeh F. Inhibition of phosphorylation of JNK suppresses Aβ-induced ER stress and upregulates prosurvival mitochondrial proteins in rat hippocampus. J Mol Neurosci. 2013;49:262–269. doi: 10.1007/s12031-012-9837-y. [DOI] [PubMed] [Google Scholar]
- 80.Verma G, Datta M. IL-1beta induces ER stress in a JNK dependent manner that determines cell death in human pancreatic epithelial MIA PaCa-2 cells. Apoptosis. 2010;15:864–876. doi: 10.1007/s10495-010-0498-4. [DOI] [PubMed] [Google Scholar]
- 81.Riley BE, Kaiser SE, Kopito RR. Autophagy inhibition engages Nrf2-p62 Ub-associated signaling. Autophagy. 2011;7:338–340. doi: 10.4161/auto.7.3.14780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Riley BE, Kaiser SE, Shaler TA, Ng AC, Hara T, Hipp MS, Lage K, Xavier RJ, Ryu KY, Taguchi K, Yamamoto M, Tanaka K, Mizushima N, Komatsu M, Kopito RR. Ubiquitin accumulation in autophagy-deficient mice is dependent on the Nrf2-mediated stress response pathway: a potential role for protein aggregation in autophagic substrate selection. J Cell Biol. 2010;191(3):537–552. doi: 10.1083/jcb.201005012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Komatsu M, Kurokawa H, Waguri S, Taguchi K, Kobayashi A, Ichimura Y, Sou YS, Ueno I, Sakamoto A, Tong KI, Kim M, Nishito Y, Iemura S, Natsume T, Ueno T, Kominami E, Motohashi H, Tanaka K, Yamamoto M. The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat Cell Biol. 2010;12:213–223. doi: 10.1038/ncb2021. [DOI] [PubMed] [Google Scholar]
- 84.Lau A, Wang XJ, Zhao F, Villeneuve NF, Wu T, Jiang T, Sun Z, White E, Zhang DD. A noncanonical mechanism of Nrf2 activation by autophagy deficiency: direct interaction between Keap1 and p62. Mol Cell Biol. 2010;30:3275–3285. doi: 10.1128/MCB.00248-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rao VA, Klein SR, Bonar SJ, Zielonka J, Mizuno N, Dickey JS, Keller PW, Joseph J, Kalyanaraman B, Shacter E. The antioxidant transcription factor Nrf2 negatively regulates autophagy and growth arrest induced by the anticancer redox agent mitoquinone. J Biol Chem. 2010;285:34447–34459. doi: 10.1074/jbc.M110.133579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhou Y, Wang HD, Zhu L, Cong ZX, Li N, Ji XJ, Pan H, Wang JW, Li WC. Knockdown of Nrf2 enhances autophagy induced by temozolomide in U251 human glioma cell line. Oncol Rep. 2013;29:394–400. doi: 10.3892/or.2012.2115. [DOI] [PubMed] [Google Scholar]
- 87.Zhao Z, Chen Y, Wang J, Sternberg P, Freeman ML, Grossniklaus HE, Cai J. Age-related retinopathy in NRF2-deficient mice. PLoS ONE. 2011;6:e19456. doi: 10.1371/journal.pone.0019456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Stępkowski TM, Kruszewski MK. Molecular cross-talk between the NRF2/KEAP1 signaling pathway, autophagy, and apoptosis. Free Radic Biol Med. 2011;50:1186–1195. doi: 10.1016/j.freeradbiomed.2011.01.033. [DOI] [PubMed] [Google Scholar]
- 89.Jain A, Lamark T, Sjøttem E, Larsen KB, Awuh JA, Øvervatn A, McMahon M, Hayes JD, Johansen T. p62/SQSTM1 is a target gene for transcription factor NRF2 and creates a positive feedback loop by inducing antioxidant response element-driven gene transcription. J Biol Chem. 2010;285:22576–22591. doi: 10.1074/jbc.M110.118976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Duran A, Linares JF, Galvez AS, Wikenheiser K, Flores JM, Diaz-Meco MT, Moscat J. The signaling adaptor p62 is an important NF-kappaB mediator in tumorigenesis. Cancer Cell. 2008;13:343–354. doi: 10.1016/j.ccr.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 91.Israël A. The IKK complex, a central regulator of NF-kappaB activation. Cold Spring Harb Perspect Biol. 2010;2:a000158. doi: 10.1101/cshperspect.a000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wakabayashi N, Slocum SL, Skoko JJ, Shin S, Kensler TW. When NRF2 talks, who’s listening? Antioxid Redox Signal. 2010;13:1649–1663. doi: 10.1089/ars.2010.3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Copetti T, Bertoli C, Dalla E, Demarchi F, Schneider C. p65/RelA modulates BECN1 transcription and autophagy. Mol Cell Biol. 2009;29:2594–2608. doi: 10.1128/MCB.01396-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nivon M, Richet E, Codogno P, Arrigo AP, Kretz-Remy C. Autophagy activation by NFkappaB is essential for cell survival after heat shock. Autophagy. 2009;5:766–783. doi: 10.4161/auto.8788. [DOI] [PubMed] [Google Scholar]
- 95.Djavaheri-Mergny M, Amelotti M, Mathieu J, Besançon F, Bauvy C, Souquère S, Pierron G, Codogno P. NF-kappaB activation represses tumor necrosis factor-alpha-induced autophagy. J Biol Chem. 2006;281:30373–30382. doi: 10.1074/jbc.M602097200. [DOI] [PubMed] [Google Scholar]
- 96.Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, Packer M, Schneider MD, Levine B. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122:927–939. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 97.Mattson MP, Meffert MK. Roles for NF-kappaB in nerve cell survival, plasticity, and disease. Cell Death Differ. 2006;13:852–860. doi: 10.1038/sj.cdd.4401837. [DOI] [PubMed] [Google Scholar]
- 98.Comb WC, Cogswell P, Sitcheran R, Baldwin AS. IKK-dependent, NF-κB-independent control of autophagic gene expression. Oncogene. 2011;30(14):1727–1732. doi: 10.1038/onc.2010.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Criollo A, Senovilla L, Authier H, Maiuri MC, Morselli E, Vitale I, Kepp O, Tasdemir E, Galluzzi L, Shen S, Tailler M, Delahaye N, Tesniere A, De Stefano D, Younes AB, Harper F, Pierron G, Lavandero S, Zitvogel L, Israel A, Baud V, Kroemer G. The IKK complex contributes to the induction of autophagy. EMBO J. 2010;29:619–631. doi: 10.1038/emboj.2009.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dan HC, Baldwin AS. Differential involvement of IkappaB kinases alpha and beta in cytokine- and insulin-induced mammalian target of rapamycin activation determined by Akt. J Immunol. 2008;180:7582–7589. doi: 10.4049/jimmunol.180.11.7582. [DOI] [PubMed] [Google Scholar]
- 101.Lee DF, Kuo HP, Chen CT, Hsu JM, Chou CK, Wei Y, Sun HL, Li LY, Ping B, Huang WC, He X, Hung JY, Lai CC, Ding Q, Su JL, Yang JY, Sahin AA, Hortobagyi GN, Tsai FJ, Tsai CH, Hung MC. IKK beta suppression of TSC1 links inflammation and tumor angiogenesis via the mTOR pathway. Cell. 2007;130:440–455. doi: 10.1016/j.cell.2007.05.058. [DOI] [PubMed] [Google Scholar]
- 102.Duran A, Amanchy R, Linares JF, Joshi J, Abu-Baker S, Porollo A, Hansen M, Moscat J, Diaz-Meco MT. p62 is a key regulator of nutrient sensing in the mTORC1 pathway. Mol Cell. 2011;44(1):134–146. doi: 10.1016/j.molcel.2011.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fan W, Tang Z, Chen D, Moughon D, Ding X, Chen S, Zhu M, Zhong Q. Keap1 facilitates p62-mediated ubiquitin aggregate clearance via autophagy. Autophagy. 2010;6:614–621. doi: 10.4161/auto.6.5.12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang Y, Lucocq JM, Yamamoto M, Hayes JD. The NHB1 (N-terminal homology box 1) sequence in transcription factor Nrf1 is required to anchor it to the endoplasmic reticulum and also to enable its asparagine-glycosylation. Biochem J. 2007;408:161–172. doi: 10.1042/BJ20070761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Steffen J, Seeger M, Koch A, Krüger E. Proteasomal degradation is transcriptionally controlled by TCF11 via an ERAD-dependent feedback loop. Mol Cell. 2010;40:147–158. doi: 10.1016/j.molcel.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 106.Radhakrishnan SK, Lee CS, Young P, Beskow A, Chan JY, Deshaies RJ. Transcription factor Nrf1 mediates the proteasome recovery pathway after proteasome inhibition in mammalian cells. Mol Cell. 2010;38:17–28. doi: 10.1016/j.molcel.2010.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang Q, Mora-Jensen H, Weniger MA, Perez-Galan P, Wolford C, Hai T, Ron D, Chen W, Trenkle W, Wiestner A, Ye Y. ERAD inhibitors integrate ER stress with an epigenetic mechanism to activate BH3-only protein NOXA in cancer cells. Proc Natl Acad Sci USA. 2009;106:2200–2205. doi: 10.1073/pnas.0807611106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Meister S, Schubert U, Neubert K, Herrmann K, Burger R, Gramatzki M, Hahn S, Schreiber S, Wilhelm S, Herrmann M, Jäck HM, Voll RE. Extensive immunoglobulin production sensitizes myeloma cells for proteasome inhibition. Cancer Res. 2007;67:1783–1792. doi: 10.1158/0008-5472.CAN-06-2258. [DOI] [PubMed] [Google Scholar]
- 109.Angeloni C, Motori E, Fabbri D, Malaguti M, Leoncini E, Lorenzini A, Hrelia S. H2O2 preconditioning modulates phase II enzymes through p38 MAPK and PI3 K/Akt activation. Am J Physiol Heart Circ Physiol. 2011;300:H2196–H2205. doi: 10.1152/ajpheart.00934.2010. [DOI] [PubMed] [Google Scholar]
- 110.Chepelev NL, Bennitz JD, Huang T, McBride S, Willmore WG. The Nrf1 CNC-bZIP protein is regulated by the proteasome and activated by hypoxia. PLoS ONE. 2011;6:e29167. doi: 10.1371/journal.pone.0029167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wang W, Chan JY. Nrf1 is targeted to the endoplasmic reticulum membrane by an N-terminal transmembrane domain. Inhibition of nuclear translocation and transacting function. J Biol Chem. 2006;281:19676–19687. doi: 10.1074/jbc.M602802200. [DOI] [PubMed] [Google Scholar]