Abstract
Symplekin is a dual location protein that has been localized to the cytoplasmic plaques of tight junctions but also occurs in the form of interchromatin particles in the karyoplasm. Here we report the identification of two novel and major symplekin-containing protein complexes in both the karyo- and the cytoplasm of Xenopus laevis oocytes. Buffer-extractable fractions from the karyoplasm of stage IV–VI oocytes contain an 11S particle, prepared by immunoselection and sucrose gradient centrifugation, in which symplekin is associated with the subunits of the cleavage and polyadenylation specificity factor (CPSF). Moreover, in immunofluorescence microscopy nuclear symplekin colocalizes with protein CPSF-100 in the “Cajal bodies.” However, symplekin is also found in cytoplasmic extracts of enucleated oocytes and egg extracts, where it occurs in 11S as well as in ca. 65S particles, again in association with CPSF-100. This suggests that, in X. laevis oocytes, symplekin is possibly involved in both processes, 3′-end processing of pre-mRNA in the nucleus and regulated polyadenylation in the cytoplasm. We discuss the possible occurrence of similar symplekin-containing particles involved in mRNA metabolism in the nucleus and cytoplasm of other kinds of cells, also in comparison with the nuclear forms of other dual location proteins in nuclei and cell junctions.
INTRODUCTION
Cell biologists had recently to recognize, much to their surprise, that certain proteins appear, often in the same cells, as “dual location proteins,” i.e., as general constituents of two rather distant and different structures: On the one hand, they occur as components of cytoskeletal plaques of a specific kind of intercellular junction, and on the other hand, they are located in karyoplasmic, interchromatinic granules, even in cells devoid of any junctions. Examples include the plakophilins PKP 1–3, typical of desmosomal plaques (Mertens et al., 1996; Schmidt et al., 1997, 1999; Bonnéet al., 1999), the adherens junction proteins, ARVCF (Borrmann, 2000; Borrmann et al., 2000; for cDNA transfection experiments see also Mariner et al., 2000), afadin (Mandai et al., 1997) and protein 4.1 (Krauss et al., 1997; Lallena et al., 1998), and the tight junction plaque proteins ZO-1 (Gottardi et al., 1996), symplekin (Keon et al., 1996), Ash-1 (Nakamura et al., 2000), and ZONAB (Balda and Matter, 2000). Obviously, this constitutively dual location at junctions and in nuclei has to be distinguished from observations of transient nuclear accumulations of certain other junctional plaque proteins in special stages of the cell cycle or differentiation or upon expression of certain transfected cDNAs or genes (for examples see, e.g., Funayama et al., 1995; Karnovsky and Klymkowsky, 1995; Behrens et al., 1996; Huber et al. 1996; Molenaar et al., 1996; Schneider et al., 1996; Yost et al., 1996; Daniel and Reynolds, 1999; for reviews see Behrens, 2000; Hübner et al., 2001).
Such a constitutively dual localization has also been reported in many diverse cultured cells and tissues for the tight junction-associated Mr 150,000 protein, symplekin, which occurs in mostly granular-looking karyoplasmic structures, and on mitotic telophase, rapidly reacumulates, in the nucleus, like a typical nuclear protein (Keon et al., 1996; for review see Stevenson and Keon, 1998). Analysis of the amino acid sequence of this protein, however, has not revealed homologies—or at least similarities—to any known nuclear protein (cf. reviews of Cáceres and Krainer, 1997; de la Cruz et al., 1999).
To elucidate the nuclear function(s) of symplekin we have applied biochemical methods for isolating and characterizing the protein, using methods that recently have been successful in studies of the nuclear forms of plakophilin PKP2, which has been shown to be part of RNA polymerase III complexes (Mertens et al., 2001). For the sake of clarity we have further decided to start with the exceptionally large nuclei of Xenopus laevis oocytes (“germinal vesicles”) allowing both mass and manual isolations with minimal cytoplasmic contamination as well as the preparation of enucleated “ooplasms,” and thus the parallel analysis of both karyoplasm and cytoplasm (cf. e.g., Bonner, 1975a, 1975b; De Robertis et al., 1978; Krohne and Franke, 1980a, 1980b, 1983; Kleinschmidt and Franke, 1982; Kleinschmidt et al., 1983; Peters et al., 1990, 1994). Here we report the identification of the nuclear form of Xenopus oocyte symplekin as a distinct particle of about 11S in association with subunits of the CPSF complex known to be part of the 3′-end pre-mRNA processing machinery. In addition, symplekin occurs, together with protein CPSF-100, coilin and many other proteins known to function in RNA synthesis and processing, in the so-called “Cajal bodies,” which are also very large in these oocytes (cf. Gall et al., 1999; Gall, 2000; Morgan et al., 2000). Surprisingly, however, we have also found that symplekin-containing particles are not restricted to the nucleus but can also be detected in enucleated oocytes and in eggs.
MATERIALS AND METHODS
Biological Materials
Clawed toads (Xenopus laevis) were purchased from the African Xenopus Facility C.C. (Knysna, Republic of South Africa). Tissue samples from X. laevis (skin, heart, ovaries) were snap-frozen in isopentane cooled by liquid nitrogen to about −140°C and stored at −80°C. For X. laevis blood smear preparations, blood was obtained from larger vessels of decapitated toads. The blood was directly smeared on glass slides and air-dried for 3 h. Subsequently the smears were fixed for 10 min with freshly prepared 2% formaldehyde in PBS with 1 mM MgCl2 and permeabilized for 3 min with 0.3% Triton X-100 in PBS with 1 mM MgCl2.
Cell culture cell lines used included X. laevis kidney epithelial (XLKE) line A6, human colon carcinoma line CaCo2, and human SV-40 transformed fibroblasts line SV80 (for sources see American Tissue Culture Collection, Manassas, VA, and Cordes et al., 1996).
Antibodies and Reagents
The monoclonal antibodies (mAbs) specific for symplekin used were mAb Sym-TJ-E150 (Keon et al., 1996) and mAb Sym-Nu (Becton Dickinson, Heidelberg, Germany). Guinea pig antibodies specific for symplekin (sym-CT) were obtained by immunization with a synthetic peptide representing the C-terminal sequence (AMKTPSPAAEDAREPEAKGNS, aa 1122–1144; cf. Keon et al., 1996; Ueki et al., 1997), coupled to keyhole limpet hemocyanin (Peptide Specialty Laboratories, Heidelberg, Germany). Rabbit sera specific for CPSF-100 (Jenny et al., 1994) or CPSF-73 (Jenny et al., 1996) have been described before. Rabbit antibodies specific for coilin (Bohmann et al., 1995) were generously provided by Dr. A. Lamond (University of Dundee, Scotland, United Kingdom). Mouse mAb H1 specific for coilin (Tuma et al., 1993) was obtained from Zytomed (Berlin, Germany), and mAb No-185 against a nucleolar protein (Schmidt-Zachmann et al., 1987) was kindly provided by M. Schmidt-Zachmann (German Cancer Research Center).
Secondary antibodies used for immunofluorescence microscopy were Texas Red-, Alexa 488-, Cy3-, Cy2-conjugated antibodies to immunoglobulins of mouse, guinea pig, or rabbit, respectively (Dianova, Hamburg, Germany).
Gel Electrophoresis and Immunoblotting
Proteins of total cells and cell fractions were separated by SDS-PAGE (Laemmli, 1970). After electroblotting of the proteins to PVDF membranes, the filters were blocked for 1 h in Tris-HCl–buffered saline (TBS) containing 0.05% Tween (TBST) and 5% nonfat dry milk. The specific antibodies were incubated with the membranes for 1 h in TBST. Bound antibodies were detected by chemiluminescence using the ECL-system (NEN, Cologne, Germany) after incubation with horseradish peroxidase-coupled secondary antibodies.
Isolation and Fractionation of Xenopus Oocytes and Egg Extract
Ovaries were surgically removed, and oocytes were defolliculated in 2 mg/ml collagenase (Sigma, Munich, Germany) in 87 mM NaCl, 2.5 mM KCl, 1 mM MgCl2, 1 mM Na2HPO4, 5 mM HEPES (pH 7.8) with constant agitation at 28°C for 2–3 h. For separation of nuclear content and nuclear envelope, nuclei were isolated in “5:1 buffer” supplemented with 10 mM MgCl2 (for review see Krohne and Franke, 1983). This allowed stripping of the nuclear envelope and yielded a fraction containing only the somewhat gelified nuclear content. Manual isolation of nuclei from stage VI oocytes (Dumont, 1972) was in “5:1 buffer” as described (Krohne and Franke, 1983), followed by fixation in ethanol. Total oocytes or enucleated ooplasms were transferred into a microcentrifuge tube and excess buffer was removed. The oocytes were resuspended in a small volume of “5:1 buffer” supplemented with 1 × “complete protease inhibitors” (Roche Diagnostics, Mannheim, Germany) and homogenized by pipetting up and down in a narrow bore pipette. Homogenates were centrifuged at 13,000 × g for 10 min at 4°C. To examine the efficiency of the removal of follicle epithelial cells from the oocytes, the total cytoskeletal material of collagenase-treated and untreated oocytes was separated by SDS-PAGE and probed in Western blots with the mAb, Vim 3B4, which specifically recognized vimentin, a marker protein for follicle cells (Herrmann et al., 1989). Large-scale isolation of nuclei from mature (stages IV–VI) X. laevis oocytes was carried out as described by Scalenghe et al. (1978) with the modifications of Kleinschmidt and Franke (1982). Subsequent fractionation of nuclear contents into low-speed pellet (LSP), high-speed pellet (HSP), and high-speed supernatant (HSS) was as described by Hügle et al. (1985). LSP fractions were cleared from yolk proteins by Freon extraction (Evans and Kay, 1991). Egg extracts were prepared as described by Cordes et al. (1993), and small scale preparations of nuclear extracts from A6 cells were done according to the method of Lee and Green (1990).
Sucrose Gradient Centrifugation
The supernatant fractions were directly loaded on top of a 5–30% linear sucrose gradient buffered with “extraction buffer” (80 mM KCl, 20 mM NaCl, 15 mM HEPES, pH 7.5, 5 mM EDTA, 1.0 mM DTT, 250 mM sucrose). Centrifugation was performed in a SW40 rotor (Beckman Instruments, Munich, Germany) at 35,000 rpm for 19 h at 4°C. Sixteen fractions of 0.8 ml each were collected from top to bottom of the gradient and tested by immunoblotting. Extracts were also layered on linear 10–40% sucrose gradients in 5:1 buffer (80 mM KCl, 20 mM NaCl, 10 mM Tris-HCl, pH 7.4, 1× Complete protease inhibitors). After centrifugation, fractions of 0.4 ml were collected from top to bottom. Marker proteins (bovine serum albumin [BSA], catalase, thyroglobulin; all from Sigma) or ribosomal subunits from X. laevis ovaries were used in parallel.
Immunoselection Experiments
Immunoselection was performed with Dynabeads (Dynal, Hamburg, Germany) coated with antibodies specific to mouse IgG. Samples were cleared by addition of Dynabeads for 2 h on a rotating wheel at 4°C. The beads were then separated, and the supernatants were transferred to a tube containing Dynabeads preloaded with symplekin-specific mAb Sym-Nu. After incubation overnight at 4°C on a rotating wheel, the Dynabeads were washed four times in ice-cold buffer (140 mM NaCl, 5 mM EDTA, 20 mM HEPES, pH 7.5, 1% Nonidet-P40), then boiled in sample buffer, processed by SDS-PAGE, and either stained with Commassie Brilliant Blue or blotted to PVDF membranes. As a control, the Dynabeads used for preclearing were processed in parallel. Protein bands were excised from the gel and cut into 1 × 1-mm pieces that were washed twice with deionized water, 50% acetonitrile/water 1:1, and acetonitrile. Proteins were digested with sequencing grade modified trypsin (Promega, Mannheim, Germany) in 40 mM ammonium bicarbonate at 37°C overnight. The reaction was stopped by freezing.
MALDI Mass Spectrometry
MALDI mass spectra were recorded in the positive ion reflector mode with delayed extraction on a Reflex II time-of-flight instrument (Bruker-Daltonik GmbH, Bremen, Germany) equipped with a SCOUT multiprobe inlet and a 337-nm nitrogen laser. Ion acceleration voltage was set to 20.0 kV, the reflector voltage was set to 21.5 kV, and the first extraction plate was set to 15.4 kV. Mass spectra were obtained by averaging 50 to 200 individual laser shots. Calibration of the spectra was performed internally by a two-point linear fit using the autolysis products of trypsin at m/z 842.50 and m/z 2211.10. For the mass spectrometric analysis of tryptic digests MALDI samples were prepared on thin film spots (Jensen et al., 1996).
Post-Source Decay Analysis
Post-source decay (PSD) analysis was performed in the positive ion reflector mode with delayed extraction by setting an ion gate width of 40 Da around the ion of interest. Data were acquired in 14 segments by decreasing the reflector voltage in a stepwise manner. For each segment 100–200 individual laser shots were accumulated. The fragment ion spectrum was obtained by pasting together all segments to a single spectrum using the FAST software provided by Bruker. Fragment ion calibration was performed externally with the fragment masses of the adrenocorticotropic hormone (ACTH) 18–39 clip. Sample preparation for PSD analysis was achieved by cocrystallization of matrix with ZipTip C18 (Millipore, Bedford, MA) concentrated samples (Regula et al., 2000).
Database Search
Singly charged monoisotopic peptide masses were used as inputs for database searching. Searches were performed against the NCBInr database using the ProFound search algorithm (http://129.85.19.192/prowl-cgi/ProFound.exe) and the Protein prospector software developed at the University of California, San Francisco, (http://prospector.ucsf.edu). Isoelectric points were allowed to range from 0 to 14, and the oxidation of methionine was included as possible modification. Up to one missed tryptic cleavage was considered, and the mass tolerance for the monoisotopic peptide masses was set to ±100 ppm or ±0.1 Da.
Searches with fragment masses from PSD experiments were performed against the NCBInr database using the MS-Tag search algorithm provided by the Protein prospector software package. Parent mass tolerance was set to ±0.1 Da and fragment ion tolerance was set to ±0.7 Da.
Immunofluorescence Microscopy
For immunofluorescence microscopy studies of cultured cells, cells grown on coverslips were fixed in methanol (5 min, −20°C), followed by acetone (30 s, −20°C), washed twice in PBS, and incubated with antibodies for 20 min at room temperature. After several PBS washes, cells were incubated for 20 min with the appropriate secondary antibodies, washed in PBS, dehydrated in ethanol, air-dried, and mounted in Fluoromount (Biozol, Eching, Germany).
Cryosections (5 μm) of frozen tissues were fixed either in acetone (−20°C) for 10 min or in PBS with 1 mM MgCl2 (PBS-MgCl2) containing 2% formaldehyde for 10 min at room temperature. Formaldehyde-fixed samples were washed in PBS-MgCl2 containing 50 mM NH4Cl for 5–10 min and twice for 5 min in PBS-MgCl2 before incubation with antibodies. In some experiments cells were stained with 4′,6-diaminidino-2-phenylindole (DAPI, 0.1 μg/ml; Serva, Heidelberg, Germany) for 5 min during incubation with the secondary antibodies. Micrographs were taken with an Axiophot microscope (Zeiss, Jena, Germany).
Confocal laser scanning immunofluorescence microscopy was done on a Zeiss LSM 410 UV instrument (Zeiss). For simultaneous double-label fluorescence, an argon ion laser operating at 488 nm and a helium-neon laser operating at 543 nm were used together with a band-pass filter combination of 510–525 nm and 590–610 nm for visualization of Cy-2 and Cy-3 fluorescence.
RESULTS
Immunofluorescence Microscopy
Symplekin antibody mAb Sym-TJ-E150 reacted with cell–cell junctions of cultures of human CaCo2 cells, corresponding to tight junction markers such as occludin and protein ZO-1 (Keon et al., 1996) as well as throughout the karyoplasm (Figure 1A), whereas mAb Sym-Nu specifically recognized only the nuclear form of symplekin (Figure 1B). Gradual focusing through such nuclei allowed the resolution of individual granular structures, leaving the nucleoli negative. In dividing cells, symplekin staining with mAb Sym-TJ-E150 was still positive at the tight junction plaques (Figure 1A, inset), whereas the nuclear form was dispersed throughout the cytoplasm.
Figure 1.
Immunofluorescence microscopy of symplekin on cultured human and Xenopus laevis cell lines, frozen tissue sections, and blood smear preparations of Xenopus, demonstrating dual localization in nuclear and tight junctional forms. (A and B) Human colon carcinoma CaCo2 cells reacted with mAb Sym-TJ-E150 (A) or mAb Sym-Nu (B), showing dual (A) or exclusively nuclear (B) localization. The inset in A shows the distribution of symplekin during mitosis. (C and C′) Cultured X. laevis kidney epithelium XLKE cells, line A6 (C, epifluorescence with mAb Sym-Nu; C′ phase contrast). (D–D") Xenopus skin tissue, showing nuclear immunofluorescence in epidermal keratinocytes (D, epifluorecence with mAb Sym-Nu; D′, nuclear DAPI staining; D", phase contrast). (E and E′) Xenopus heart tissue, including numerous cardiomyocyte nuclei and a blood vessel with positive erythrocyte nuclei (E, epifluorescence with mAb Sym-Nu; E′, DAPI staining). (F) Blood smear preparation, showing the nuclear immunoreaction in the transcriptionally almost fully inactive erythrocyte nuclei. Bars, 20 μm; Inset, 10 μm.
A similarly intense karyoplasmic immunofluorescence was observed in cultured Xenopus kidney epithelial cells (Figure 1, C and C′). On frozen sections of Xenopus tissues, intense immunofluorescence was seen in nuclei of all the different cell types examined, including epidermal keratinocytes of all layers of the skin (Figure 1, D–D"), epithelial cells of glands and ducts, fibroblasts, and other dermal cells in the skin (our unpublished results), cardiomyocytes, endothelial cells, and erythrocytes of heart tissue (Figure 1, E and E′). Because of the unexpected reaction in Xenopus erythrocytes, in which transcriptional and replicational activities are notoriously low or absent, we further examined whole mount preparations of erythrocytes in blood smears. As shown in Figure 1F, the nuclei of erythrocytes were clearly positive for symplekin, indicating that this protein is a general nuclear constitutent and that its presence is not directly depending on ongoing nuclear RNA synthesis activities.
Symplekin in Xenopus Oocytes
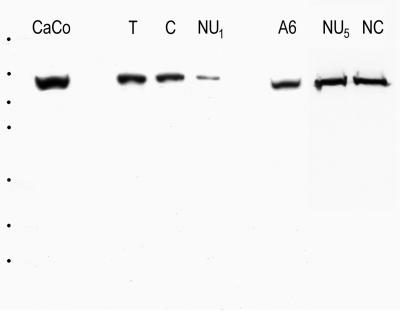
To study the nuclear structures containing symplekin, we decided to examine Xenopus oocytes as its large nucleus (“germinal vesicle”) can be isolated with minimal cytoplasmic contamination. We prepared extracts of total Xenopus oocytes, manually isolated their nuclei or ooplasmic material from enucleated oocytes, and analyzed the fractions obtained side-by-side by SDS-PAGE, followed by immunoblotting with symplekin-specific mAbs (Figure 2). A protein band of the typical size (Mr 150,000 as introduced for mammalian cells; cf. Keon et al., 1996, or slightly lower corresponding to ca. 140,000) was selectively reactive in total cell lysates from human CaCo2 and Xenopus A6 cells and in extracts from total Xenopus oocytes (Figure 2, compare CaCo, T, and A6), indicating that this band contains the Xenopus homologue to human symplekin. This band was also detected in samples from manually isolated nuclei and in extracts of enucleated oocytes. The protein reacting with the symplekin-specific antibodies seemed to be present in rather large amounts in Xenopus oocytes, as the material from one or two hand-isolated nuclei and one ooplasm was sufficient to yield a strong signal. Moreover, this protein was enriched in the nuclear content, as comparable amounts were detected in hand-isolated nuclei and in a fraction of nuclear contents from which the nuclear envelopes had been removed (compare Figure 2, NU5 and NC).
Figure 2.
Immunoblot detection of symplekin in Xenopus (X.l.) oocyte extracts. Total cell proteins of human CaCo2 cells (CaCo, lane 1) are compared with proteins of total X.l. oocyte extracts (T), cytoplasmic extracts from enucleated oocytes (“ooplasms,” C, equivalent to 0.5 ooplasm), total proteins from hand-isolated Xenopus oocyte nuclei (NU1, equivalent to one nucleus), total cell proteins from Xenopus XLKE-A6 cell culture (A6, total proteins), total proteins from five hand-isolated X.l. oocyte nuclei (NU5) side by side with the nuclear contents of five nuclei (nuclei devoid of nuclear envelopes, NC, last lane). Extracts were separated by SDS-PAGE and analyzed by immunoblotting with mAb Sym-Nu. Note the reaction of a single band at ca. 140 kDa. In lanes 2 and 6 no sample was loaded. The bars correspond to reference proteins with 212, 158, 116, 97, 66, 56, and 42 kDa (from top to bottom).
The fractionation of particles from isolated Xenopus oocyte nuclei by differential centrifugation leads to well-defined subnuclear fractions (Figure 3, left part; cf. Hügle et al., 1985; Schmidt-Zachmann et al., 1998). When the distribution of symplekin in such fractions from oocyte nuclei, egg extracts and somatic cells was analyzed by immunoblotting (Figure 3, A and B), symplekin was detected in total oocyte nuclei, in the HSP and HSS fractions and in egg extracts. For SDS-PAGE equal volumes of LSP, HSP, and HSS fractions were loaded. As the two pellets (LSP and HSP; Figure 3) were solubilized in small volumes their material was consequently concentrated, with respect to the HSS fraction. Thus, we concluded that the major proportion of symplekin was recovered in the HSS fraction containing nuclear proteins in soluble form or in small particles. In addition, some symplekin was bound to larger nucleoplasmic particles, as indicated by the reaction in the HSP fraction. The fractions were also characterized by immunoblotting with mAb No-185 against nucleolar protein NO38, known to be enriched in the LSP and HSP but absent from the HSS fraction (Schmidt-Zachmann et al., 1998), and by immunoblotting with mAb H1 against coilin. The protein coilin was enriched in the HSP fraction, minor amounts were also detected in the LSP and HSS fractions (our unpublished results).
Figure 3.
Identification of sym-plekin in different nuclear fractions from Xenopus oocytes. On the left a centrifugation scheme for the preparation of the LSP, HSP, and HSS fractions is shown. (A) Coomassie blue staining of various nuclear fractions from Xenopus oocytes and somatic cells separated by SDS-PAGE: Total mass-isolated nuclei (NU, lane 1), proteins of the LSP, HSP and HSS fractions from oocyte nuclei, total cellular proteins of Xenopus XLKE-A6 cells (A6) and of Xenopus egg extract (EGG). (B) Corresponding immunoblot probed with antibody Sym-TJ-E150. Note that symplekin is predominantly found in the HSP and HSS fraction. Reference (R) proteins are 212, 158, 116, 97, and 66 kDa (from top to bottom). Note also some symplekin degradation in lanes 3–6.
Association of Symplekin with Cleavage and Polyadenylation Specificity Factors
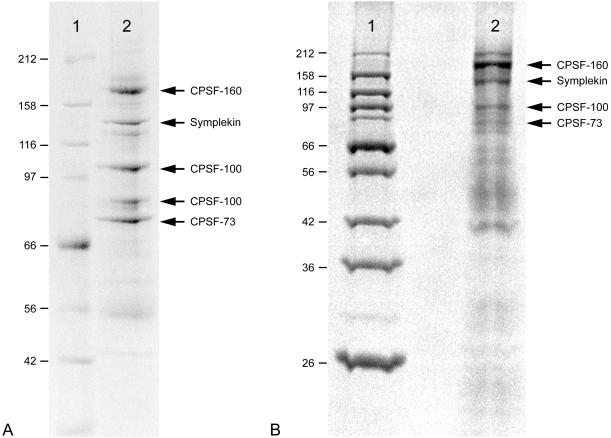
The HSS fraction obtained from Xenopus oocyte nuclei was used for immunoselection to obtain symplekin-containing protein complexes. Material bound to Dynabeads loaded with symplekin antibodies was solubilized with sample buffer and separated by 10% SDS-PAGE. After staining with Coomassie Brilliant Blue, five major protein bands, ranging from 70 to 160 kDa, were visible among the immunoselected proteins (Figure 4A), whereas no such enrichment was seen after preclearing of the lysate (unpublished results). By mass spectrometric sequencing using PSD analysis, two amino acid sequences were identified in the protein band of ca. 140 kDa: RRPEPIIPVTQGR and DPLLAHVR. From database search these sequences were identified as homologous to the tryptic peptides 521–533 and 1073–1080 of human symplekin (Keon et al., 1996; Ueki et al., 1997), with exchanges of amino acid P532 to G and I1073 to D, respectively. The other proteins present in these immunoprecipitates were identified as the Xenopus proteins CPSF-160, CPSF-100, and CPSF-73, respectively, and these identifications were verified by immunoblotting with specific antibodies. Interestingly, the two protein bands of ∼95 and 100 kDa were both identified as CPSF-100, corresponding to a recent report showing the existence of two forms of CPSF-100 in X. laevis oocytes (Dickson et al., 1999).
Figure 4.
Immunoselected proteins of symplekin-containing particles present in the HSS fraction from Xenopus oocyte nuclei (A) and from egg extracts (B). The precipitates were solubilized in sample buffer, separated by SDS-PAGE and stained with Coomassie Brilliant Blue. The prominent protein bands (lane 2) were analyzed by peptide mass fingerprinting. In addition to symplekin, 3 out of 4 proteins of the CPSF complex were identified as CPSF-160, CPSF-100, and CPSF-73. Lane 1, reference proteins (molecular mass values are indicated on the left).
Because all three proteins were known as constituents of the protein complex named CPSF (Bienroth et al., 1991; Murthy and Manley, 1992; for review see Wahle and Rüegsegger, 1999), we concluded that symplekin is also a component of this complex. A fourth polypeptide of 30 kDa that had also been described for this complex (Bienroth et al., 1991) was not identified in these immunoprecipitates, perhaps because it was obscured by the large amount of immunoglobulin light chains.
We also wondered to which proteins symplekin was associated in eggs, i.e., after the breakdown of the nuclear envelope, and examined egg extracts obtained by immunoselection with symplekin antibodies. After staining with Coomassie Brilliant Blue, four major polypeptide bands, ranging from 70–160 kDa, were visible (Figure 4B). By mass spectrometric analysis the proteins of 160, 100, and 70 kDa were identified as CPSF-160, CPSF-100, and CPSF-73, respectively. The protein band of −140 kDa showed a spectrometric pattern of fragments similar to that of the protein identified as symplekin. Moreover, by mass spectrometric sequencing using PSD analysis, the same amino acid sequence RRPEPIIPVTQGR was determined as mentioned for the tryptic fragment of nuclear symplekin (peptide 521–533; see above). The 160-kDa protein band showed an unexpectedly intense dye staining, indicative of either suprastochiometric amounts of the CPSF-160 subunit or the coincidence of an additional yet unidentified protein.
To analyze the physical state of symplekin-containing material in the HSS fraction of fractionated Xenopus oocyte nuclei, this fraction was further subjected to centrifugation in 5–30% sucrose gradients, and the resulting fractions were analyzed by SDS-PAGE and immunoblotting using PVDF membranes (Figure 5A). Symplekin was recovered in fractions 4–8, with a maximum in fractions 6 and 7, corresponding to −11S. When the PVDF membranes were reprobed with antibodies against protein CPSF-100, two protein bands of −100 and 95 kDa were decorated. The 100-kDa form of CPSF-100 was found in fractions 6–8, with a maximum between fractions 6 and 7, corresponding to the distribution of symplekin. The 95-kDa version of CPSF-100 was detected in fractions 7–9, with a maximum in fractions 7 and 8 (Figure 5A′ ). An identical distribution was found with CPSF-73 antibodies (unpublished results).
Figure 5.
Analysis of the native state of the particles containing proteins symplekin (A) and CPSF-100 (A′ ) by sucrose gradient centrifugation. The HSS fraction from Xenopus oocyte nuclei was analyzed by sucrose gradient centrifugation, fractions were collected from top to bottom, and the portions analyzed by immunoblotting either with mAb Sym-Nu (A) or CPSF-100-specific antibodies (A′ ). Reference proteins were BSA (4.3S, fraction 3), catalase (11.3S, fraction 6) and thyroglobulin (16.5S, fraction 9). The points correspond to SDS-PAGE reference proteins with 212, 158, 116, 97, 66, and 56 kDa (from top to bottom).
As a smaller proportion of the nuclear symplekin was recovered in the HSP fraction, the protein material of the pellet was resuspended and separated by sucrose gradient centrifugation. Some symplekin was again recovered in fractions corresponding to 10–14S, whereas the main portion was detected in the pellet, indicative of its association with relatively large structures (for Cajal bodies see below).
As the Xenopus oocyte is a highly specialized cell, we also used nuclear extracts from cultured Xenopus cells of line A6 (cf. Figure 1C) for sucrose gradient centrifugation. Here most of the symplekin present could also be detected in fractions corresponding to 10–11S (unpublished results).
Concentration of Symplekin in Cajal Bodies
To examine the intranuclear localization of symplekin, we performed immunocytochemistry on cryosections through Xenopus laevis ovaries. Using symplekin-specific antibodies, distinct intranuclear, nonnucleolar bodies of diameters of 5–10 μm were intensely stained (Figure 6A). Double-label experiments with CPSF-100 antibodies revealed a clear colocalization of both proteins (Figure 6C). Moreover, double-labeling with coilin-specific antibodies also showed colocalization, thus indicating that symplekin is concentrated in Cajal bodies, as has also been described for CPSF-100 and other factors of the 3′-end pre-mRNA processing complex by Gall and coworkers (Gall et al., 1999; Gall, 2000; Morgan et al., 2000). Using standard protocols, a weak immunofluorescence with symplekin antibodies was also seen throughout the karyoplasm. However, the intense immunolocalization of symplekin in the Cajal bodies seems to represent only a minor portion of the total nuclear symplekin, as indicated by our estimations from recovery experiments that only a small proportion of the nuclear symplekin is contained in HS pelletable structures (for general difficulties of demonstrating even most abundant extractable karyoplasmic proteins and particles such as actin, histones, nucleoplasmin, and their complexes see, e.g., Krohne and Franke, 1980b; Ankenbauer et al., 1989; Hofmann et al., 2001).
Figure 6.
Laser scanning confocal microscopy showing the result of a of double-labeling experiment, comparing here the localization of symplekin (A) with that of protein CPSF-100 (B) in a cryosection through a Xenopus laevis ovary. The corresponding merge picture is shown in (C), the corresponding phase contrast in (D). Note that the Cajal bodies in the nuclei are strongly positive for both symplekin (A) and CPSF-100 (B), as indicated by the yellow color in (C), whereas under the immunolocalization conditions applied here only a weak and diffuse reaction is seen in the karyo- and the cytoplasm. Bar, 100 μm.
Symplekin Complexes in Cytoplasmic Extracts of Enucleated Oocytes
Unexpectedly, a large proportion of the oocyte's symplekin was found in extracts from “ooplasms,” i.e., enucleated oocytes (see Figure 2). To prove that hand-isolated, enucleated oocytes were not contaminated with follicle cells or remnants thereof, we performed immunoblotting with antibodies specific for vimentin as a marker of follicle cells (cf. Herrmann et al., 1989). These controls revealed that cytoskeletal preparations of collagenase-treated oocytes contained only traces if any vimentin (our unpublished results). Cytoplasmic extracts from enucleated oocytes were used for immunoselection (Figure 7). When the material bound to Dynabeads loaded with antibodies to symplekin was solubilized and separated by SDS-PAGE, enrichment of symplekin (Figure 7A) and protein CPSF-100 (Figure 7B) was detected by immunoblotting. This again showed that the immunoselected symplekin complexes also contained protein CPSF-100 (compare lane 3 in Figure 7, A and B).
Figure 7.
Immunoselection of symplekin-containing complexes present in cytoplasmic (ooplasmic) extracts of enucleated Xenopus oocyte. The precipitate has been solubilized in sample buffer, separated on SDS-PAGE and transferred to a PVDF-membrane. Lane 1, sample of cytoplasmic extract from five enucleated oocytes before immunoselection; lane 2, sample of cytoplasmic extract from five enucleated oocytes after immunoselection; lane 3, precipitate obtained with mAb Sym-Nu; lane 4, control reaction obtained with the extract using beads alone. Symplekin has been detected by Western blotting using mAb Sym-Nu (A), reaction with CPSF-100 specific antibodies is shown in (B). The dots correspond to reference proteins with 158, 116, 97 kDa (from top to bottom).
To determine the physical state of this symplekin-containing cytoplasmic material, sucrose gradient centrifugation was applied, and the resulting fractions were analyzed by SDS-PAGE, followed by immunoblotting for symplekin (Figure 8). Symplekin was distributed in three classes, one in fractions 5–8 with a peak in fraction 6, corresponding to ca. 11S, and the other in fractions 24 and 25, corresponding to particles larger than 65S, and some material accumulated in the last gradient fraction and the pellet.
Figure 8.
Characterization of symplekin-containing particles in cytoplasmic extracts of enucleated Xenopus oocytes by sucrose gradient centrifugation. Extracts from 40 ooplasms have been subjected to sucrose gradient centrifugation, as described in Figure 5, using catalase (11.3S, fraction 6), thyroglobulin (16.5S, fraction 9) and the 40S and 60S X.l. ribosomal subunits as reference. Dots on the right margin correspond to reference proteins with 158, 116, 97, 66, and 56 kDa (from top to bottom). Note that most of the symplekin is recovered in particles with a peak value of ∼11S, whereas minor portions appear with a mean peak corresponding to ∼65S or in the pellet.
DISCUSSION
In oocytes and eggs of X. laevis we have discovered and characterized distinct karyo- and cytoplasmic particles containing symplekin, a protein also described as a component of the tight junction plaque. A major result of our study is that a large part of the nuclear symplekin occurs in particles with an approximate mean peak value of 11S (“11S particles”), where it is complexed with proteins involved in mRNA biogenesis, notably 3′-end processing. Symplekin particles have also been found in egg extracts and, most surprisingly, in cytoplasmic particles of enucleated oocytes. In addition, a notable proportion of the nuclear symplekin is associated with much larger, i.e., readily pelletable structures, and this seems to include the Cajal body symplekin.
Clearly, the major part of the symplekin-containing nuclear particles can be precipitated together with CPSF subunits such as proteins CPSF-160, CPSF-100, and CPSF-73. Here, the identification of CPSF-73 presents further evidence that we have identified a nuclear protein complex, because this subunit has not been found in cytoplasmic CPSF complexes involved in regulated polyadenylation of mRNAs in Xenopus oocytes (Dickson et al., 1999, 2001). Furthermore, the relative staining intensities of the separated polypeptides of the immunoprecipitated particles indicate that symplekin is an iso-stoichiometric component with respect to the other CPSF proteins. The relative amount of total nuclear CPSF particle-bound symplekin may actually be even higher because we cannot exclude that pelleted material, including Cajal bodies, contains similar −11S particles, although in a state associated with—or integrated into—larger structures (Gall et al., 1999; Gall, 2000).
The finding of symplekin as a constituent of CPSF particles is not restricted to X. laevis oocytes and cultured A6 kidney-derived cells, as shown in the present study. In similar experiments using cell culture lines from various tissues and species, including human HeLa cells, we have also identified symplekin in association with such particles. Moreover, by immunodepletion the functional importance of symplekin for 3′-end cleavage and polyadenlation has recently been demonstrated in in vitro assays using extracts from HeLa cell nuclei (I. Hofmann, I. Kaufmann, W. Keller, and W.W. Franke, unpublished results). Therefore, we think it is a sensible working hypothesis that symplekin is a widespread, if not ubiquitous CPSF protein.
It is perhaps somewhat astonishing that in the numerous previous studies symplekin has been overlooked as a component involved in 3′-end processing of pre-mRNA. So far six transacting factors have been listed that are required for the in vitro reconstitution of mammalian 3′-end processing (reviewed by Wahle and Rüegsegger, 1999). Besides the symplekin-containing factor CPSF, composed of the five subunits CPSF-160, CPSF-100, CPSF-73, CPSF-30, and symplekin (cf. Bienroth et al., 1991; Murthy and Manley, 1992; this study), the trimeric cleavage stimulation factor (CstF), with the subunits CstF-77, CstF-64, and CstF-50, recognizes sequence elements on the pre-mRNA (for references see Wahle and Rüegsegger, 1999). In addition, cleavage factors I (CFI) and II (CFII), poly(A)-binding protein II and poly(A) polymerase are needed for 3′-end processing in vertebrates (Raabe et al., 1991; Wahle, 1991; Wahle et al., 1991; Bienroth et al., 1993; Rüegsegger et al., 1996; de Vries et al., 2000; Kim et al., 2001).
The concept of symplekin as a constituent of the larger 3′-end cleavage and polyadenylation complex is also supported by the observation of Takagaki and Manley (2000) who, in “far Western” screens with the protein CstF-64, have identified HeLa cell symplekin as a strongly interacting protein that in solid phase and pull down assays competes for binding to CstF-64. Moreover, using immuno-affinity column chromatography on CstF-64, these authors have copurified subunits of CstF, CPSF, and symplekin from nuclear extracts, indicating that CstF, CPSF, and symplekin are part of a large complex. That symplekin serves an important role in fundamental cellular processes is also suggested from the existence of homologues in the genomes of very distantly related species such as Caenorhabditis elegans (AF022973), Drosophila melanogaster (AE003601) and Arabidopsis thaliana (AL161746; these authors, unpublished results). The involvement of symplekin in 3′-end processing of premRNA is also supported by the notion of a homologue in Saccharomyces cerevisiae, protein PTA1p, described to be associated with the proteins of the yeast equivalent of CPSF (YHH1, YDH1, YSH1, YTH1) and to be important for cleavage and polyadenylation of pre-mRNA (Preker et al., 1997; Zhao et al., 1999).
Certainly, the most unexpected finding of our study is the discovery of ca. 11S complexes containing symplekin together with CPSF proteins, notably CPSF-100, in the cytoplasm. As these particles have also been obtained from manually enucleated ooplasms, a contamination by nuclear particles appears to be excluded. This finding indicates that reactions known to be involved in cytoplasmic polyadenylation (Hake and Richter, 1994; Dickson et al., 1999, 2001; for review see also Wickens et al., 2000) and in the regulation of translation (Stebbins-Boaz et al., 1999; Mendez et al., 2000; for reviews see Macdonald, 2001; Mendez and Richter, 2001) are located in symplekin-containing complexes.
Interestingly, all so far characterized nuclear forms of junctional plaque proteins have in common that they are somehow involved in processes of transcription, splicing, or 3′-end processing: Plakophilin 2 has been detected in RNA polymerase III complexes, p120ctn and β-catenin are involved in regulations of RNA polymerase II transcription, and protein 4.1 has been found in splicing factors (Behrens et al., 1996; Huber et al., 1996; Molenaar et al., 1996; Krauss et al., 1997; Lallena et al., 1998; Daniel and Reynolds, 1999; Mertens et al., 2001). Symplekin is the first protein associated with factors involved in 3′-end processing of premRNA in the nucleus as well as in cytoplasmic translational control. Future studies will help understanding the biological significance of the interactions between CPSF and their regulation and determining the functions of symplekin in oocytes as well as in somatic cells. In addition, it will be important to clarify whether the nuclear and the plaque-bound forms exist in a regulated exchange equilibrium.
ACKNOWLEDGMENTS
The authors are indebted to Dr. Walter Keller (Biozentrum, University of Basel, Switzerland) for continuous support and advice as well as to Drs. Reimer Stick (University of Bremen, Germany) and Marion Schmidt-Zachmann (German Cancer Research Center, Heidelberg, Germany) for stimulating discussions. They also thank Jutta Osterholt for preparing the photographs and Eva Ouis for arranging the manuscript. The technical assistance of Sonja Reidenbach and the expert help of Dr. Herbert Spring with the laser scanning confocal microscopy is also gratefully acknowledged. This study has been supported in part by the Deutsche Forschungsgemeinschaft.
Abbreviations used:
- CPSF
cleavage and polyadenylation specificity factor
- CstF
cleavage stimulation factor
- DAPI
4′,6-diaminidino-2-phenylindole
- HSP
high-speed pellet
- HSS
high-speed supernatant
- LSP
low-speed pellet
- PSD
post-source decay
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.01–12–0567. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.01–12–0567.
REFERENCES
- Ankenbauer T, Kleinschmidt JA, Walsh MJ, Weiner OH, Franke WW. Identification of a widespread nuclear actin binding protein. Nature. 1989;342:822–825. doi: 10.1038/342822a0. [DOI] [PubMed] [Google Scholar]
- Balda MS, Matter K. The tight junction protein ZO-1 and an interacting transcription factor regulate ErbB-2 expression. EMBO J. 2000;19:2024–2033. doi: 10.1093/emboj/19.9.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens J. Control of β-catenin signaling in tumor development. Ann NY Acad Sci. 2000;910:21–33. doi: 10.1111/j.1749-6632.2000.tb06698.x. [DOI] [PubMed] [Google Scholar]
- Behrens J, von Kries JP, Kühl M, Bruhn L, Wedlich D, Grosschedl R, Birchmeier W. Functional interaction of β-catenin with the transcription factor LEF-1. Nature. 1996;382:638–642. doi: 10.1038/382638a0. [DOI] [PubMed] [Google Scholar]
- Bienroth S, Wahle E, Suter-Crazzolara C, Keller W. Purification of the cleavage and polyadenylation factor involved in the 3′-processing of messenger RNA precursors. J Biol Chem. 1991;266:19768–19776. [PubMed] [Google Scholar]
- Bienroth S, Keller W, Wahle E. Assembly of a processive messenger RNA polyadenylation complex. EMBO J. 1993;12:585–594. doi: 10.1002/j.1460-2075.1993.tb05690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohmann K, Ferreira JA, Lamond AI. Mutational analysis of p80 coilin indicates a functional interaction between coiled bodies and the nucleolus. J Cell Biol. 1995;131:817–831. doi: 10.1083/jcb.131.4.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonné S, van Hengel J, Nollet F, Kools P, van Roy F. Plakophilin-3, a novel armadillo-like protein present in nuclei and desmosomes of epithelial cells. J Cell Sci. 1999;112:2265–2276. doi: 10.1242/jcs.112.14.2265. [DOI] [PubMed] [Google Scholar]
- Bonner WM. Protein migration into nuclei. I. Frog oocyte nuclei in vivo accumulate microinjected histones, allow entry to small proteins, and exclude large proteins. J Cell Biol. 1975a;64:421–30. doi: 10.1083/jcb.64.2.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner WM. Protein migration into nuclei. II. Frog oocyte nuclei accumulate a class of microinjected oocyte nuclear proteins and exclude a class of microinjected oocyte cytoplasmic proteins. J Cell Biol. 1975b;64:431–437. doi: 10.1083/jcb.64.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrmann C. Molekulare Charakterisierung der Adhärens-Zellverbindungen des Herzens. Identifizierung einer neuen Art, der Area composita. Ph.D. Thesis. Germany: Faculty of Biology, University of Heidelberg; 2000. [Google Scholar]
- Borrmann CM, Mertens C, Schmidt A, Langbein L, Kuhn C, Franke WW. Molecular diversity of plaques of epithelial adhering junctions. Ann NY Acad Sci. 2000;915:144–150. doi: 10.1111/j.1749-6632.2000.tb05237.x. [DOI] [PubMed] [Google Scholar]
- Cáceres JF, Krainer AR. Mammalian pre-mRNA splicing factors. In: Krainer AR, editor. Eukaryotic mRNA Processing. Oxford, UK: IRL Press at Oxford University Press; 1997. pp. 174–212. [Google Scholar]
- Cordes VC, Reidenbach S, Köhler A, Stuurman N, van Driel R, Franke WW. Intranuclear filaments containing a nuclear pore complex protein. J Cell Biol. 1993;123:1333–1344. doi: 10.1083/jcb.123.6.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordes VC, Reidenbach S, Franke WW. Cytoplasmic annulate lamellae in cultured cells: composition, distribution, and mitotic behavior. Cell Tissue Res. 1996;284:177–191. doi: 10.1007/s004410050578. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Reynolds AB. The catenin p120(ctn) interacts with Kaiso, a novel BTB/POZ domain zinc finger transcription factor. Mol Cell Biol. 1999;19:3614–3623. doi: 10.1128/mcb.19.5.3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Cruz J, Kressler D, Linder P. Unwinding RNA in Saccharomyces cerevisiae: DEAD-box proteins and related families. Trends Biochem Sci. 1999;24:192–198. doi: 10.1016/s0968-0004(99)01376-6. [DOI] [PubMed] [Google Scholar]
- De Robertis EM, Longthorne RF, Gurdon JB. Intracellular migration of nuclear proteins in Xenopus oocytes. Nature. 1978;272:254–256. doi: 10.1038/272254a0. [DOI] [PubMed] [Google Scholar]
- de Vries H, Rüegsegger U, Hübner W, Friedlein A, Langen H, Keller W. Human pre-mRNA cleavage factor IIm contains homologs of yeast proteins and bridges two other cleavage factors. EMBO J. 2000;19:5895–5904. doi: 10.1093/emboj/19.21.5895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson KS, Bilger A, Ballantyne S, Wickens MP. The cleavage and polyadenylation specificity factor in Xenopus laevis oocytes is a cytoplasmic factor involved in regulated polyadenylation. Mol Cell Biol. 1999;19:5707–5717. doi: 10.1128/mcb.19.8.5707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson KS, Thompson SR, Gray NK, Wickens M. Poly(A) polymerase and the regulation of cytoplasmic polyadenylation. J Biol Chem. 2001;276:41810–41816. doi: 10.1074/jbc.M103030200. [DOI] [PubMed] [Google Scholar]
- Dumont JN. Oogenesis in Xenopus laevis (Daudin). I. Stages of oocyte development in laboratory-maintained animals. J Morphol. 1972;136:153–164. doi: 10.1002/jmor.1051360203. [DOI] [PubMed] [Google Scholar]
- Evans JP, Kay BK. Biochemical fractionation of oocytes. Methods Cell Biol. 1991;26:133–146. doi: 10.1016/s0091-679x(08)60275-7. [DOI] [PubMed] [Google Scholar]
- Funayama N, Fagotto F, McCrea P, Gumbiner BM. Embryonic axis induction by the armadillo repeat domain of β-catenin: evidence for intracellular signaling. J Cell Biol. 1995;128:959–968. doi: 10.1083/jcb.128.5.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gall JG. Cajal bodies: the first 100 years. Annu Rev Cell Dev Biol. 2000;16:273–300. doi: 10.1146/annurev.cellbio.16.1.273. [DOI] [PubMed] [Google Scholar]
- Gall JG, Bellini M, Wu Z, Murphy C. Assembly of the nuclear transcription and processing machinery: Cajal bodies (coiled bodies) and transcriptosomes. Mol Biol Cell. 1999;10:4385–4402. doi: 10.1091/mbc.10.12.4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottardi CJ, Arpin M, Fanning AS, Louvard D. The junction-associated protein, zonula occludens-1, localizes to the nucleus before the maturation and during the remodeling of cell-cell contacts. Proc Natl Acad Sci USA. 1996;93:10779–10784. doi: 10.1073/pnas.93.20.10779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hake LE, Richter JD. CPEB is a specificity factor that mediates cytoplasmic polyadenylation during Xenopus oocyte maturation. Cell. 1994;79:617–627. doi: 10.1016/0092-8674(94)90547-9. [DOI] [PubMed] [Google Scholar]
- Herrmann H, Fouquet B, Franke WW. Expression of intermediate filament proteins during development of Xenopus laevis. I. cDNA clones encoding different forms of vimentin. Development. 1989;105:279–298. doi: 10.1242/dev.105.2.279. [DOI] [PubMed] [Google Scholar]
- Hofmann W, Reichart B, Ewald A, Müller E, Schmitt I, Stauber RH, Lottspeich F, Jockusch BM, Scheer U, Hauber J, Dabauvalle M-C. Cofactor requirements for nuclear export of Rev response element (RRE)- and constitutive transport element (CTE)-containing retroviral RNAs: an unexpected role for actin. J Cell Biol. 2001;152:895–910. doi: 10.1083/jcb.152.5.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber O, Korn R, McLaughlin J, Ohsugi M, Herrmann BG, Kemler R. Nuclear localization of β-catenin by interaction with transcription factor LEF-1. Mech Dev. 1996;59:3–10. doi: 10.1016/0925-4773(96)00597-7. [DOI] [PubMed] [Google Scholar]
- Hübner S, Jans DA, Drenckhahn D. Roles of cytoskeletal and junctional plaque proteins in nuclear signaling. Int Rev Cytol. 2001;208:207–265. doi: 10.1016/s0074-7696(01)08005-6. [DOI] [PubMed] [Google Scholar]
- Hügle B, Scheer U, Franke WW. Ribocharin: a nuclear Mr 40,000 protein specific to precursor particles of the large ribosomal subunit. Cell. 1985;41:615–627. doi: 10.1016/s0092-8674(85)80034-9. [DOI] [PubMed] [Google Scholar]
- Jenny A, Hauri H-P, Keller W. Characterization of cleavage and polyadenylation specificity factor and cloning of its 100-kilodalton subunit. Mol Cell Biol. 1994;14:8183–8190. doi: 10.1128/mcb.14.12.8183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenny A, Minvielle-Sebastia L, Preker PJ, Keller W. Sequence similarity between the 73-kilodalton protein of mammalian CPSF and a subunit of yeast polyadenylation factor I. Science. 1996;274:1514–1517. doi: 10.1126/science.274.5292.1514. [DOI] [PubMed] [Google Scholar]
- Jensen ON, Podtelejnikov A, Mann M. Delayed extraction improves specificity in database searches by matrix-assisted laser desorption/ionization peptide maps. Rapid Commun Mass Spectrom. 1996;10:1371–1378. doi: 10.1002/(SICI)1097-0231(199608)10:11<1371::AID-RCM682>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Karnovsky A, Klymkowsky MW. Anterior axis duplication in Xenopus induced by the over-expression of the cadherin-binding protein plakoglobin. Proc Natl Acad Sci USA. 1995;92:4522–4526. doi: 10.1073/pnas.92.10.4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keon BH, Schäfer S, Kuhn C, Grund C, Franke WW. Symplekin, a novel type of tight junction plaque protein. J Cell Biol. 1996;134:1003–1018. doi: 10.1083/jcb.134.4.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Choi S-C, Chang J-Y, Han J-K. Poly(A) binding protein II in Xenopus laevis is expressed in developing brain and pancreas. Mech Dev. 2001;109:111–114. doi: 10.1016/s0925-4773(01)00514-7. [DOI] [PubMed] [Google Scholar]
- Kleinschmidt JA, Franke WW. Soluble acidic complexes containing histones H3 and H4 in nuclei of Xenopus laevis oocytes. Cell. 1982;29:799–809. doi: 10.1016/0092-8674(82)90442-1. [DOI] [PubMed] [Google Scholar]
- Kleinschmidt JA, Scheer U, Dabauvalle M-C, Bustin M, Franke WW. High mobility group proteins of amphibian oocytes: a large storage pool of a soluble high mobility group-1-like protein and involvement in transcriptional events. J Cell Biol. 1983;97:838–848. doi: 10.1083/jcb.97.3.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss SW, Larabell CA, Lockett S, Gascard P, Penman S, Mohandas N, Chasis JA. Structural protein 4.1 in the nucleus of human cells: dynamic rearrangements during cell division. J Cell Biol. 1997;137:275–289. doi: 10.1083/jcb.137.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krohne G, Franke WW. A major soluble acidic protein located in nuclei of diverse vertebrate species. Exp Cell Res. 1980a;129:167–189. doi: 10.1016/0014-4827(80)90341-9. [DOI] [PubMed] [Google Scholar]
- Krohne G, Franke WW. Immunological identification and localization of the predominant nuclear protein of the amphibian oocyte nucleus. Proc Natl Acad Sci USA. 1980b;77:1034–1038. doi: 10.1073/pnas.77.2.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krohne G, Franke WW. Proteins of pore complex-lamina structures from nuclei and nuclear membranes. Methods Enzymol. 1983;96:597–608. doi: 10.1016/s0076-6879(83)96052-4. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lallena MJ, Martinez C, Valcarcel J, Correas I. Functional association of nuclear protein 4.1 with pre-mRNA splicing factor. J Cell Sci. 1998;111:1963–1971. doi: 10.1242/jcs.111.14.1963. [DOI] [PubMed] [Google Scholar]
- Lee KAW, Green MR. Small-scale preparation of extracts from radiolabeled cells efficient in pre-mRNA splicing. Methods Enzymol. 1990;181:20–30. doi: 10.1016/0076-6879(90)81108-7. [DOI] [PubMed] [Google Scholar]
- Macdonald P. Diversity in translational regulation. Curr Opin Cell Biol. 2001;13:326–331. doi: 10.1016/s0955-0674(00)00215-5. [DOI] [PubMed] [Google Scholar]
- Mandai K, Nakanishi H, Satoh A, Obaishi H, Wada M, Nishioka H, Itoh M, Mizoguchi A, Aoki T, Fujimoto T, Matsuda Y, Tsukita S, Takai Y. Afadin: a novel actin filament-binding protein with one PDZ domain localized at cadherin-based cell-to-cell adherens junction. J Cell Biol. 1997;139:517–528. doi: 10.1083/jcb.139.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariner DJ, Wang J, Reynolds AB. ARVCF localizes to the nucleus and adherens junction and is mutually exclusive with p120ctn in E-cadherin complexes. J Cell Sci. 2000;113:1481–1490. doi: 10.1242/jcs.113.8.1481. [DOI] [PubMed] [Google Scholar]
- Mendez R, Richter JD. Translational control by CPEB: a means to the end. Mol Cell Biol. 2001;2:521–529. doi: 10.1038/35080081. [DOI] [PubMed] [Google Scholar]
- Mendez R, Murthy KGK, Ryan K, Manley JL, Richter JD. Phosphorylation of CPEB by Eg2 mediates the recruitment of CPSF into an active cytoplasmic polyadenylation complex. Mol Cell. 2000;6:1253–1259. doi: 10.1016/s1097-2765(00)00121-0. [DOI] [PubMed] [Google Scholar]
- Mertens C, Kuhn C, Franke WW. Plakophilins 2a and 2b: constitutive proteins of dual location in the karyoplasm and the desmosomal plaque. J Cell Biol. 1996;135:1009–1025. doi: 10.1083/jcb.135.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertens C, Hofmann I, Wang Z, Teichmann M, Sepehri Chong S, Schnölzer M, Franke WW. Nuclear particles containing RNA polymerase III complexes associated with the junctional plaque protein plakophilin 2. Proc Natl Acad Sci USA. 2001;98:7795–7800. doi: 10.1073/pnas.141219498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molenaar M, van de Wetering M, Oosterwegel M, Peterson-Maduro J, Godsave S, Korinek V, Roose J, Destrée O, Clevers H. XTcf-3 transcription factor mediates β-catenin-induced axis formation in Xenopus embryos. Cell. 1996;86:391–399. doi: 10.1016/s0092-8674(00)80112-9. [DOI] [PubMed] [Google Scholar]
- Morgan GT, Doyle O, Murphy C, Gall JG. RNA polymerase II in Cajal bodies of amphibian oocytes. J Struct Biol. 2000;129:258–268. doi: 10.1006/jsbi.2000.4231. [DOI] [PubMed] [Google Scholar]
- Murthy KGK, Manley JL. Characterization of the multisubunit cleavage-polyadenylation specificity factor from calf thymus. J Biol Chem. 1992;267:14804–14811. [PubMed] [Google Scholar]
- Nakamura T, Blechman J, Tada S, Rozovskaia T, Itoyama T, Bullrich F, Mazo A, Croce CM, Geiger B, Canaani E. huASH1 protein, a putative transcription factor encoded by a human homologue of the Drosophila ash1 gene, localizes to both nuclei, and cell-cell tight junctions. Proc Natl Acad Sci USA. 2000;97:7284–7289. doi: 10.1073/pnas.97.13.7284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J-M, Walsh MJ, Franke WW. An abundant and ubiquitous homo-oligomeric ring-shaped ATPase particle related to the putative vesicle fusion proteins Sec18p and NSF. EMBO J. 1990;9:1757–1767. doi: 10.1002/j.1460-2075.1990.tb08300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J-M, Franke WW, Kleinschmidt JA. Distinct 19 S and 20 S subcomplexes of the 26 S proteasome and their distribution in the nucleus and the cytoplasm. J Biol Chem. 1994;269:7709–7718. [PubMed] [Google Scholar]
- Preker PJ, Ohnacker M, Minvielle-Sebastia L, Keller W. A multisubunit 3′ end processing factor from yeast containing poly(A) polymerase and homologues of the subunits of mammalian cleavage and polyadenylation specificity factor. EMBO J. 1997;16:4727–4737. doi: 10.1093/emboj/16.15.4727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raabe T, Bollum FJ, Manley JL. Primary structure and expression of bovine poly(A) polymerase. Nature. 1991;353:229–234. doi: 10.1038/353229a0. [DOI] [PubMed] [Google Scholar]
- Regula T, Ueberle B, Boguth G, Görg A, Schnölzer M, Herrmann R, Frank R. Towards a two-dimensional proteome map of Mycoplasma pneumoniae. Electrophoresis. 2000;21:3765–3780. doi: 10.1002/1522-2683(200011)21:17<3765::AID-ELPS3765>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Rüegsegger U, Beyer K, Keller W. Purification and characterization of human cleavage factor Im involved in the 3′ end processing of messenger RNA precursors. J Biol Chem. 1996;271:6107–6113. doi: 10.1074/jbc.271.11.6107. [DOI] [PubMed] [Google Scholar]
- Scalenghe F, Buscaglia M, Steinheil C, Crippa M. Large scale isolation of nuclei and nucleoli from vitellogenic oocytes in Xenopus laevis. Chromosoma. 1978;66:299–308. doi: 10.1007/BF00328531. [DOI] [PubMed] [Google Scholar]
- Schmidt A, Langbein L, Rode M, Prätzel S, Zimbelmann R, Franke WW. Plakophilins 1a and 1b: widespread nuclear proteins recruited in specific epithelial cells as desmosomal plaque components. Cell Tissue Res. 1997;290:481–499. doi: 10.1007/s004410050956. [DOI] [PubMed] [Google Scholar]
- Schmidt A, Langbein L, Prätzel S, Rode M, Rackwitz H-R, Franke WW. Plakophilin 3—a novel cell-type-specific desmosomal plaque protein. Differentiation. 1999;64:291–306. doi: 10.1046/j.1432-0436.1999.6450291.x. [DOI] [PubMed] [Google Scholar]
- Schmidt-Zachmann MS, Hügle-Dörr B, Franke WW. A constitutive nucleolar protein identified as a member of the nucleoplasmin family. EMBO J. 1987;6:1881–1890. doi: 10.1002/j.1460-2075.1987.tb02447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Zachmann MS, Knecht S, Krämer A. Molecular characterization of a novel, widespread nuclear protein that colocalizes with spliceosome components. Mol Biol Cell. 1998;9:143–160. doi: 10.1091/mbc.9.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider S, Steinbeisser H, Warga RM, Hausen P. β-Catenin translocation into nuclei demarcates the dorsalizing centers in frog and fish embryos. Mech Dev. 1996;57:191–198. doi: 10.1016/0925-4773(96)00546-1. [DOI] [PubMed] [Google Scholar]
- Stebbins-Boaz B, Cao Q, de Moor CH, Mendez R, Richter JD. Maskin is a CPEB-associated factor that transiently interacts with eIF-4E. Mol Cell. 1999;4:1017–1027. doi: 10.1016/s1097-2765(00)80230-0. [DOI] [PubMed] [Google Scholar]
- Stevenson BR, Keon BH. The tight junction: morphology to molecules. Annu Rev Cell Dev Biol. 1998;14:89–109. doi: 10.1146/annurev.cellbio.14.1.89. [DOI] [PubMed] [Google Scholar]
- Takagaki Y, Manley JL. Complex protein interactions within the human polyadenylation machinery identify a novel component. Mol Cell Biol. 2000;20:1515–1525. doi: 10.1128/mcb.20.5.1515-1525.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuma RS, Stolk JA, Roth MB. Identification and characterization of a sphere organelle protein. J Cell Biol. 1993;122:767–773. doi: 10.1083/jcb.122.4.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueki K, Ramaswamy S, Billings SJ, Mohrenweiser HW, Louis DN. Chromosomal localization to 19q13.3., partial genomic structure and 5′ cDNA sequence of the human symplekin gene. Somatic Cell Mol Gen. 1997;23:229–231. doi: 10.1007/BF02721375. [DOI] [PubMed] [Google Scholar]
- Wahle E. A novel poly(A)-binding protein acts as a specificity factor in the second phase of messenger RNA polyadenylation. Cell. 1991;66:759–768. doi: 10.1016/0092-8674(91)90119-j. [DOI] [PubMed] [Google Scholar]
- Wahle E, Rüegsegger U. 3′-End processing of pre-mRNA in eukaryotes. FEMS Microbiol Rev. 1999;23:277–295. doi: 10.1111/j.1574-6976.1999.tb00400.x. [DOI] [PubMed] [Google Scholar]
- Wahle E, Martin G, Schiltz E, Keller W. Isolation and expression of cDNA clones encoding mammalian poly(A) polymerase. EMBO J. 1991;10:4251–4257. doi: 10.1002/j.1460-2075.1991.tb05003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickens M, Goodwin EB, Kimble J, Strickland S, Hentze M. Translational control of developmental decisions. In: Sonenberg N, Hershey JWB, Mathews MB, editors. Translational Control of Gene Expression. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press; 2000. pp. 295–370. [Google Scholar]
- Yost C, Torres M, Miller JR, Huang E, Kimelman D, Moon RT. The axis-inducing activity, stability, and subcellular distribution of β-catenin is regulated in Xenopus embryos by glycogen synthase kinase 3. Genes Dev. 1996;10:1443–1454. doi: 10.1101/gad.10.12.1443. [DOI] [PubMed] [Google Scholar]
- Zhao J, Kessler M, Helmling S, O'Connor JP, Moore C. Pta1, a component of yeast CF II, is required for both cleavage and poly(A) addition of mRNA precursor. Mol Cell Biol. 1999;19:7733–7740. doi: 10.1128/mcb.19.11.7733. [DOI] [PMC free article] [PubMed] [Google Scholar]