Abstract
Accumulating evidence implicates mitochondrial and metabolic pathways in the establishment of pluripotency, as well as in the control of proliferation and differentiation programs. From classic studies in mouse embryos to the latest findings in adult stem cells, human embryonic and induced pluripotent stem cells, an increasing number of evidence suggests that mitochondrial and metabolic-related processes might intertwine with signaling networks and epigenetic rewiring, thereby modulating cell fate decisions. This review summarizes the progresses in this exciting field of research. Dissecting these complex mitochondrial and metabolic mechanisms may lead to a more comprehensive understanding of stemness biology and to potential improvements in stem cell applications for biomedicine, cell therapy, and disease modeling.
Keywords: iPSCs, Mitochondria, Metabolism, Stem cells, Reprogramming, Pluripotency
Introduction
Living entities, from individual cells to pluricellular organisms, need to obtain energy and to use it to perform their biological functions. Energy metabolism defines the highly coordinated mechanisms by which energy is used to produce and transform the molecular constituents in order to maintain cellular integrity and allow the generation of daughter cells and complex organisms. Nonetheless, these basic functional properties have only recently been started to be investigated in the context of stem cells and regenerative medicine.
Here, we review these recent developments with a main focus on the human system. We describe the properties of mitochondria and metabolism in relation to stemness, development, and differentiation, and discuss the implications of mitochondrial and metabolic restructuring occurring during the process of reprogramming somatic cells to pluripotency. Addressing how the manipulation of mitochondria and metabolism can influence the induction of pluripotency might shed light on the mechanisms regulating cell fate identity and conversion and possibly contribute to novel advances in stem cell-related biomedical applications.
Stem cell biology and biomedical applications
Properties and features of stem cells
Stem cells are the originating cells of all tissues in an organism, both during embryonic development and adult life, and are defined by two key properties: self-renewal capacity, indicative of the proliferating features, and potency, which refers to the ability to generate progressively differentiated progeny of cells through a hierarchical process. The differentiation and self-renewal programs are tightly regulated through (epi-)genetic control and environmental stimuli [1]. When committed toward a differentiation pathway, stem cells give rise to precursor cells, which proliferate before differentiation and are therefore also called transit-amplifying cells or progenitor cells [2].
With respect to potency, different types of mammalian stem cells can be distinguished. Totipotent stem cells are capable of giving rise to an entire organism, essentially fertilized eggs and cells in embryos until 4 days of development. Pluripotent stem cells (PSCs) have the potential to differentiate into any type of cell, but not to give rise to whole organisms, because they lack the capacity to generate the extra-embryonic tissues required for mammalian development. This is the case of embryonic stem cells (ESCs), derived from the inner mass cells of the blastocyst of mice [3] and humans [4], and induced pluripotent stem cells (iPSCs), which are differentiated cells forced back to a stem cell state through the process of “nuclear reprogramming” (see below). Finally, adult stem cells comprise the undifferentiated cells residing within adult differentiated tissues still retaining the ability to differentiate into a limited number of cell types of their own lineage. These multipotent stem cells include long-term hematopoietic stem cells (HSCs), mesenchymal stem cells (MSCs), and neural stem cells (NSCs) [2, 5].
The isolation of stem cells, both embryonic and adult, had a great impact on biomedical research, mainly due to their potential use for regenerative medicine. Given their recognized capability to give rise to virtually any cell type of the body [6], PSCs appear as the most promising candidates for cell-replacement therapies. However, ethical controversies hamper the use of human ESCs (hESCs). The discovery that adult somatic cells can be reprogrammed to an embryonic stem cell-like state, bypassing the need for human embryos, opened new avenues in stem cell research. iPSCs were first generated from mice in 2006 [7] and from humans in 2007 [8, 9]. This was originally achieved by the ectopic expression of stem cell inducing transcription factors, such as OCT4, SOX2, KLF4, and c-MYC (known as the Yamanaka factors) [7], or OCT4, SOX2, NANOG, and LIN28 (the Thomson factors) [8, 9].
Since then, vast progress has been made with respect to methodology. Today, iPSCs can be generated avoiding the integration of transgenes in the host genome and the associated risk of insertional mutagenesis and malignant transformations [9]. This can be accomplished using excisable vectors and non-integrative strategies, such as episomal plasmids, RNA-based viruses, minicircle vectors, or RNA, proteins, and microRNA delivery methods [10–14]. Several small molecules have also been shown to improve reprogramming efficiency and even reduce the reprogramming factors required for iPSC generation, mainly through the modulation of epigenetic mechanisms [15, 16], signaling pathways [17, 18] and cellular metabolism (see below) [19, 20]. Pure “chemical iPSCs” have also been obtained from mouse fibroblasts using solely a cocktail of small molecule compounds [21].
Finally, the highly anticipated derivation of somatic cell nuclear transfer (SCNT)-based human PSCs has been recently accomplished [22–24]. However, SNCT-PSCs are more cumbersome to derive and still requires the use of human eggs, and controversies exist whether they might bear lower levels of nuclear and epigenetic abnormalities compared to conventional iPSCs [25, 26].
Biomedical relevance of stem cell research
The generation of patient-derived PSCs has extremely interesting biomedical applications, as they can function as model systems for human diseases in which the cellular pathogenic mechanisms can be investigated at the molecular level [27]. The selection of one specific strategy or reprogramming approach will greatly depend on the purpose of the study and the starting cell material. For example, in vitro disease modeling or drug screening may not require as stringent quality controls as cell-based regenerative therapies in humans.
A growing number of disease-specific iPSCs have been successfully generated from patients affected by a wide variety of pathological conditions, including neurologic [28, 29], cardiac [30, 31], and metabolic diseases [32]. iPSC-derived cells have been found to exhibit disease-related phenotypes and therefore appear as promising model candidates for the discovery of novel therapeutic strategies. Furthermore, iPSC-derived hepatocytes or cardiomyocytes may be used for in vitro toxicology screenings, since unpredicted metabolism in human tissues is one of the main drawbacks in the current pharmacological pipeline [33].
Several stem cell-based therapies for regenerative purposes have been tested at subclinical and clinical levels. Within the website clinicaltrials.gov, the search for “stem cells” retrieves more than 4400 entries, indicating the current interest of stem cell application in biomedicine. Embryo-derived and PSC-derived NSCs have been shown to promote functional recovery when transplanted into rat, mouse and non-human primate models of spinal cord injury [34–37]. ESC-based therapy for spinal cord injury was brought to Phase I Clinical Trials in 2010 [38]. After its cancelation in 2011 [39], the trial will be resumed soon as announced by the California Stem Cell Report. MSCs are at present being tested in clinical trials for immune, neurodegenerative, cardiovascular, gastrointestinal, and blood disorders, and for the regeneration of bone and cartilage [40–43]. Finally, iPSC-derived cells are currently being employed in clinical trials of cell-replacement therapies in age-related macular degeneration [44, 45].
Mitochondria and metabolism during proliferation and development
Mitochondria and mtDNA
Mitochondria within eukaryotic cells are double-membrane organelles that generate energy in the form of ATP through the process of oxidative phosphorylation (OXPHOS). In addition, they exert crucial functions in various cellular processes, including programmed cell death (apoptosis), calcium homeostasis, reactive oxygen species (ROS) signaling, and detoxification.
Mitochondrial function is tightly regulated by quality control mechanisms [46]. The failure of this apparatus is implicated in the development of several neurological diseases [47]. The network of cellular mitochondria is rather dynamic, as the organelles undergo constant fusion and fission events that are balanced in a coordinated fashion in order to match the specific needs of the cells, such as control of cell cycle progression, differentiation, cell death, and metabolism [48]. Mitochondria and metabolism exhibit distinct features depending on the cell type, thus implying that cell-specific biochemistry might intimately be linked to cell function specifications.
Due to their endosymbiotic origin, mitochondria maintain a vestigial genome. The mitochondrial DNA (mtDNA) in humans is a circular molecule of 16.5 kb coding for 13 polypeptides, forming part of the complexes of the electron transport chain, for 22 tRNAs and 2 rRNAs, which are necessary for mtDNA translation, with only 600 non-coding nucleotides [49]. Even if the information encoded by mtDNA is much smaller than that present in the nuclear genome, mutations in mtDNA are the cause of many human pathologies [50, 51] and can also arise somatically upon aging and neurodegeneration [52, 53].
mtDNA is transmitted mainly by maternal inheritance. Although mitochondria present in the sperm cell can enter the cytoplasm of the oocyte during fertilization, there exist several mechanisms that eliminate paternal mtDNA from fertilized oocytes [54]. Given the existence of multiple copies of mtDNA per cell, a genomic variation may occur only in a portion of these genomes. This situation is known as heteroplasmy, which defines the presence of different mtDNA genotypes into the same cell. mtDNA sequence variants emerged in the female germline give rise to a transient heteroplasmic state that often segregate in a few generations in cattle [55, 56], with a more complex pattern in humans, where both slow and rapid segregation have been observed [57]. The mitochondrial bottleneck hypothesis seeks to explain the changes in heteroplasmy levels observed between mothers and their progeny [58]. In summary, it proposes that mitochondria carrying different genotypes are segregated due to a physical “bottleneck” in mitochondrial number both in primordial germ cell population and during development until after the blastocyst stage, although mechanisms as selective replication or degradation of particular mitochondrial genotypes, or the organization of mtDNA into homoplasmic segregating units, have also been proposed [59–61].
Energy metabolism and redox maintenance
Mitochondria and energy metabolism are central in supporting the specialized functions of cells. Through multiple and complex mechanisms, they enable the production of building blocks and energy, coordinate signaling pathways, and control regulation of gene expression [48]. Anabolic pathways branching out from glycolysis and the pentose phosphate pathway (PPP) provide essential intermediaries needed for the synthesis of macromolecules, such as amino acids, lipids, and nucleotides [62, 63]. Therefore, metabolic remodeling could have important downstream effects at the functional level.
Cellular metabolism is intertwined with redox homeostasis. ROS are indeed a common by-product of mitochondrial respiration [64]. When their production is increased, functional oxidative damage can take place, resulting into protein, lipid, or genomic aberrations and eventually apoptotic cell death [65]. To counteract these effects, cells possess fine-tuned machineries that balance radical species with reducing equivalents. The maintenance of this equilibrium is required for genome integrity and is thus critical for cells both in steady states and during adaptations to different conditions. In fact, decreased OXPHOS and tricarboxylic acid (TCA) cycle activity result into lower radical generation. At the same time, enhanced flux through the PPP can support the synthesis of the reducing equivalent NADPH, needed for antioxidant detoxification [63, 66].
ROS can also act as second messengers therefore exhibiting also a physiological role. They can modulate genetic and epigenetic modifications, by altering the expression of genes or their epigenetic control [67]. Therefore, ROS may be important in regulating not only cell death but also cellular proliferation and differentiation. A ROS rheostat has been therefore proposed to be at the center of stem cell function [68]. Among the important players of the ROS rheostat, the FOXO (insulin-forkhead box O transcription factors) family and FOXO3 in particular, may be central. They are activated, amongst other stimuli, by oxidative stress and starvation, and can coordinate the expression of genes involved in metabolism, autophagy, proteostasis, and response to oxidative damage, including the superoxide dismutase (SOD) and catalase [69, 70].
Metabolic adaptations of proliferative states
Regulation of energy metabolism represents a necessary mechanism for the adaptation to a different cellular state characterized by modified anabolic requirements. During proliferative conditions, such as malignant transformation or embryonic development, the necessity for anabolic growth increases and cells undergo metabolic transformation events culminating in an enhanced rate of glycolysis and reduced entry of pyruvate into mitochondria [71].
The benefits derived from this metabolic shift are diverse. Due to the expression of different enzyme isoforms, the carbon flux through glycolysis is accelerated and the entry of pyruvate into the mitochondria is reduced. This energy re-routing outside of the mitochondria avoids the production of free radicals and makes glucose-derived carbons available for entry into the anabolic pathways branching out from the glycolytic route and the PPP, thereby providing essential intermediates for cell growth. In this context, mitochondria acquire a role as anaplerotic sources of metabolic precursors for macromolecular biosynthesis [72].
Oxygen is a key regulator of metabolism. Under conditions of oxygen deprivation, cells rely less on OXPHOS and exhibit increased conversion of glucose to lactate, a phenomenon known as the “Pasteur effect” [73]. However, proliferative cells rewire their metabolic signature to respond to higher cellular demands and therefore shift to glycolysis-based metabolism even in the presence of high level of oxygen, a phenomenon known as aerobic glycolysis or “Warburg effect” [74]. This metabolic adaptation endows proliferative cells with the critical advantages of biomass growth and redox balance.
The pathways downstream of oxygen modulation include the hypoxia-inducible factors (HIF) 1 and 2 [75]. HIFs are heterodimers consisting of a constitutively expressed β subunit and an oxygen-regulated α subunit, which is physiologically degraded when oxygen is plenty. Under hypoxia, α proteins escape degradation and translocate into the nucleus, where they can set into motion a complex gene expression reconfiguration. HIF1α target genes include glucose transporters, which increase glucose uptake, and pyruvate dehydrogenase kinases (PDK1-3), which shunt pyruvate away from the mitochondria through the inhibition of pyruvate dehydrogenase [76–78]. HIF1α also interacts with the enzyme pyruvate kinase isoform M2 (PKM2), known to catalyze the conversion of phosphoenolpyruvate (PEP) into pyruvate in the last step of the glycolytic cascade. Upon oxidation, PKM2 may lose activity, thereby reducing pyruvate formation and diverting the glycolytic flux into the PPP [66, 79]. Therefore, oxygen-mediate modulation of energy metabolism has critical effects also at the level of redox homeostasis regulation.
Other critical metabolic checkpoints include AMPK (the AMP-activated protein kinase), which responds to reductions in the cellular energy state through switching off ATP-consuming anabolic biosynthetic pathways [80, 81], and the mammalian target of rapamycin (mTOR), a metabolic and stress sensor involved in the coordination of growth and metabolism [82]. mTOR can interact with members of nutrient-sensing signals such as the phosphatidylinositol-3,4,5-triphosphate kinase (PI3 K) and its activated kinase AKT [83]. This PI3 K/AKT/mTOR axis triggers a cascade of responses, from cell growth and proliferation and is thus instrumental during adaptations to different metabolic states.
In the context of malignant proliferation, the relative contribution of glycolysis and OXPHOS to energy production may depend on tumor type and microenvironment [84–86], but the metabolic reprogramming is believed to represent a key cellular adjustment supporting macromolecular synthesis, essential for cell growth and division, and redox balance [71, 87, 88]. A similar metabolic shift is in fact observed also in normal highly proliferating cells, such as enterocytes or lymphocytes [89].
Mitochondrial and metabolic reconfiguration during mammalian development
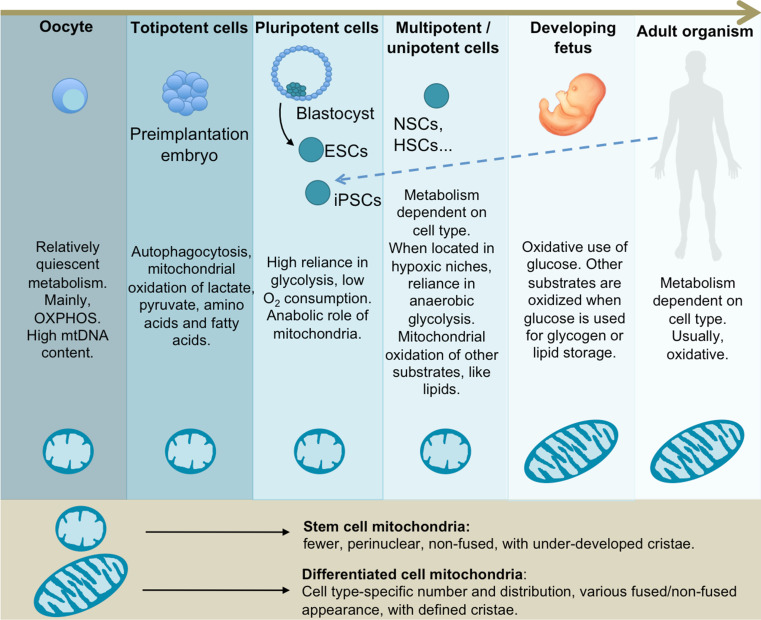
Mitochondria undergo distinct conformational and metabolic changes during development, where embryos evolve from the relatively metabolically inactive egg at ovulation to rapidly metabolizing tissues at and after implantation (Fig. 1).
Fig. 1.
Cell metabolic preferences and mitochondrial morphology during mammalian development. Mitochondria within unfertilized oocytes and pre-implantation stage embryos exhibit an immature structure. This is mirrored in vitro in adult stem cells and PSCs. Energy metabolism evolves from relatively quiescent in oocytes to highly active cells during embryonic development. Overall, stem cells share a preference for glycolysis, with variances in the case of adult stem cells. The fetus and the adult organism undergo a predominantly oxidative metabolism, with exceptions depending on the tissue type and physiological conditions
During the first week of development, the embryo increases in cell number, but not in size, and it seems that cells derive energy from protein catabolism by autophagocytosis, with a decrease in protein levels of around 26 % from the one cell stage to the morula formation [90]. In the pre-implantation embryo, the Krebs cycle and OXPHOS are used as the main energy source, with pyruvate as the more prominent energy substrate in most species during first cleavage (with some exceptions, such as porcine embryos), as shown under in vitro conditions [91]. Other major substrates used until the blastocyst stage includes lactate, amino acids, and triglyceride-derived fatty acids [91]. Until the morula stage, glucose uptake and usage is low, although it is necessary as a cell-signaling agent for the development up to the blastocyst stage [92, 93]. Indeed, high concentrations of glucose can inhibit early embryo development [94]. At the morula stage, glucose oxidation increases to rates similar to those of pyruvate [95].
In mice, from the fertilized egg up to the blastocyst stage (between 0 and 4.5 days postcoitum), the early embryo undergoes cell division without a concomitant net replication of mtDNA before implantation [61]. As a consequence, after every round of division, mtDNA is reduced in the daughter cells by around 50 % [96]. Consequently, ATP levels and ATP/ADP ratio decrease during this phase, while the NADH/NAD+ ratio remains relatively high [97], [98]. It has been suggested that this drop in ATP may play a role in the activation of glycolysis at the blastocyst stage, since ATP is an inhibitor of the rate-limiting glycolytic enzyme phosphofructokinase 1 (PFK1) [99].
In mouse embryos, from zygote to two-cell stage, mitochondria adopt a dumb-bell shape with concentrical cristae, while from the four-cell to the morula stage, mitochondria elongate, cristae relocate in a transverse manner, and a proportion of mitochondria seems to be vacuolated [100]. During these stages, cells exhibit structurally immature mitochondria, with spherical shape, few cristae and a matrix of high electron density. By the end of early embryogenesis, mitochondria elongate and develop cristae containing a matrix of low electron density, accompanied by an increase in inner mitochondrial membrane potential (ΔΨ m) [101]. In several mammals (such as hamster, mouse, human, and monkey), mitochondria have been observed to arrange around the cell nucleus in cleave-stage embryos [102–105]. Several benefits have been speculated to culminate form this peri-nuclear arrangement, such as more efficient transport of polypeptides into mitochondria, improved energy transfer for nuclear transport across nuclear pores, and buffering the nucleus from Ca2+ fluctuations in the cytoplasm [106] (Fig. 1).
This first differentiation event within the blastocyst is characterized by differential expression profiles in the inner cell mass and trophectoderm and associated key signaling pathways related to cell growth, proliferation, differentiation, and, interestingly, metabolic pathways [107]. In fact, blastocyst formation is accompanied by an increase in growth and metabolic activity. There is an initial burst in glucose uptake due to the expression of glucose transporters GLUT1 and 3 [108], followed by an increase of glycolysis and lactate production [95]. All these events are accompanied by an increase in oxygen consumption and OXPHOS, mainly due to the mitochondrial activity of cells within the trophectoderm [101].
It is important to note that the increase in glycolysis observed at this stage might be the result of an experimental artifact resulting from the removal of embryos from their natural environment for in vitro analyses [109]. However, while mitochondria of the trophectoderm are elongated and present both higher O2 consumption and membrane potential, the ICM mitochondria within the inner cell mass are spherical, depolarized and with low oxygen consumption, thereby supporting the hypothesis of reduced OXPHOS capacity of the cells of the inner cell mass, which are used for the derivation of embryonic stem cells [4, 100, 110].
During gastrulation, the mitochondria content is augmented to match the OXPHOS demands of differentiating cells [111]. Concurrently, there is decreased glycolysis coupled with an increase in mitochondrial oxidation of fatty acids and glucose-derived pyruvate [112]. Mitochondrial ultrastructure as well as distribution are also altered, as manifested by mitochondria enlargement and cristae enrichment [111] as well as by loss of peri-nuclear localization [113] (Fig. 1).
Mitochondrial and metabolic features of pluripotent stem cells
Mitochondrial ultrastructure in PSCs
Peri-nuclear distribution of mitochondria has been proposed as a “stemness” marker in stem cell populations [113]. Mitochondria within PSCs are few, with peri-nuclear distribution and round-shaped, non-fused morphology [114–116]. The cristae structure is considered as an indicator of OXPHOS function, as the electron transport chain components including the F1FO ATP synthase, crucial for OXPHOS activity and mitochondrial ATP synthesis, reside within the inner membrane of mitochondria [117]. Thus, the morphological features of PSC mitochondria suggest a potential metabolic preference of these cells for glycolysis [118]. Indeed, numerous studies have shown that both ESCs and iPSCs undergo glycolysis at higher rates when compared to their differentiated counterparts [19, 115, 118–126].
ΔΨ m is increased in mouse PSCs compared to somatic cells and this hyperpolarization seems to occur early in the reprogramming process [115]. Human PSCs also maintain an elevated membrane potential that diminishes during differentiation [127–129]. It has been proposed that this high membrane potential might support PSC in an energetically-primed state that could allow rapid responses to increments in energy demands associated with differentiation [130, 131]. Moreover, since mitochondrial fusion is dependent on mitochondrial depolarization [132], the high ΔΨ m might contribute to determine the fragmented non-fused morphological features of mitochondria within PSCs.
Metabolic switch upon reprogramming to pluripotency
Several lines of evidence demonstrate that a metabolic switch from OXPHOS to glycolysis occurs during the reprogramming of somatic cells to pluripotency [115, 118, 122, 125]. This metabolic reconfiguration may play an important role in the adjustments required by reprogrammed cells to meet the burden imposed by the increased demand for the synthesis of macromolecules, needed to support the enhanced proliferative capacity, and for the maintenance of redox equilibrium [71, 131, 133].
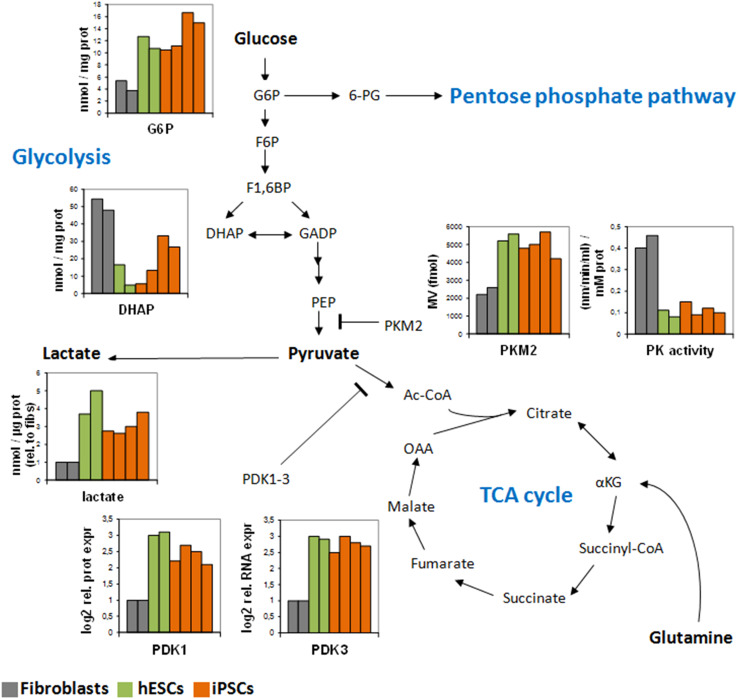
To this aim, the re-routing of energy flux toward the PPP may be of critical importance [63]. Accordingly, elevated expression of genes involved in the non-oxidative branch of the PPP have been detected in mouse iPSCs [115] and human PSCs [122, 134], together with the accumulation of the key PPP metabolite glucose-6-phosphate [134]. Additional key features of the iPSC-associated metabolic reconfiguration include increased expression of PDK1-3 and PKM2 [123, 134], indicative of reduced OXPHOS flux and enhanced glycolytic metabolism (Fig. 2).
Fig. 2.
Metabolic restructuring upon the induction of pluripotency. Key metabolic players are differentially expressed in hESCs (green) compared to somatic fibroblasts (gray) and undergo a drastic reconfiguration upon reprogramming to iPSCs (orange). This suggest that in order to adapt to a novel state, cellular metabolism needs to acquire a different profile characterized by reduced flux toward the mitochondria and enhanced glycolysis and PPP activity. The data were previously obtained in two human control fibroblasts (BJ and HFF1), two hESC lines (H1 and H9) and four iPSC lines (two from BJ fibroblasts and two from HFF1 fibroblasts) (see references [118, 123, 134])
The metabolic reconfiguration of reprogrammed cells resembles the Warburg effect associated with tumor formation. Indeed, many similarities can be seen with respect to the metabolic features of PSCs and cancer cells. Nonetheless, key mitochondrial differences exist. In particular, unlike tumor cells, PSCs are highly sensitive to apoptosis due to a mechanism called “mitochondrial priming”, which involves the maintenance of a high ratio of pro-apoptotic to anti-apoptotic proteins, close to the apoptotic threshold, making PSCs more sensitive to DNA damage [129, 135]. Accordingly, the maintenance of genome stability, which is not essential for cancer cells, is necessary for stem cells [136].
It is plausible that the initial forced expression of the reprogramming factors triggers the metabolic changes that, in turn, establish a positive feedback loop enhancing the expression of endogenous stemness-associated factors necessary to complete the reprogramming process. Indeed, several of the reprogramming-inducing factors may be able to potentiate the glycolytic shift. KLF4 has been shown to promote glycolysis through the induction of the platelet isoform of phosphofructokinase (PFKP) in breast cancer cells [137]. c-MYC is a well-known inducer of glycolysis and a driver of “glucose addiction” in cancer [138]. LIN28 mediates let-7 microRNA repression, hence regulates glucose metabolism in stem cells via the insulin-PI3 K-mTOR signaling pathway [139, 140]. Finally, OCT4 activity is regulated by HIF signaling and may interact for transcriptional regulation with PKM2 [141, 142].
There is an apparent controversy regarding the mitochondrial oxidative competence in pluripotent stem cells. Despite PSCs exhibiting a preference for glycolysis and being characterized by under-developed mitochondrial cristae, the cells consume O2 at their maximal capacity [122, 124, 125, 134]. Indeed, oxygen consumption normalized to mitochondrial mass appears equivalent in both PSCs and somatic cells [124]. The low respiratory capacity in PSCs may potentially be due to their overall reduction in mitochondrial copy number [118, 127, 143]. Importantly, the mitochondrial use of oxygen seems to be uncoupled from ATP synthesis (which occurs mainly through glycolysis) due to the expression of the uncoupler protein UCP2 [124]. Mitochondrial ATP synthase may thus function in a reverse manner, hydrolyzing the ATP produced in the cytoplasm through glycolysis [124]. The uncoupled function of the ETC would allow the conversion of NADH into NAD+, thereby facilitating the high glycolytic rate of these cells and maintaining an optimal membrane potential, which is essential for the flux of carbons through the TCA cycle needed for anaplerotic reactions. This would ultimately enable PSCs to feed biosynthetic growth through lipid synthesis from citrate and amino acid synthesis from oxalacetate and α-ketoglutarate (αKG) [144].
Modulation of reprogramming via metabolic manipulations
The Warburg-like metabolic shift may represent an early reprogramming event, preceding the expression of genes controlling pluripotency and self renewal in both mouse [115, 145] and human cells [123, 126]. Accordingly, it has been suggested that the induction of pluripotency occurs in two waves [146]. Metabolic changes can occur during the first wave, while the establishment of the pluripotency network takes place in the second wave. Thus, bioenergetic restructuring may not be a simple secondary consequence of the induction of pluripotency but it may exert an important modulatory role.
In agreement with this concept, it has been demonstrated that conditions stimulating a glycolytic reconfiguration enhance reprogramming efficiency. Low oxygen (3–5 %) prevents premature differentiation of hESCs and improves the conversion of fibroblasts into iPSCs [147, 148]. Indeed, HIF1α might be essential for the early induction of the glycolytic shift during reprogramming, as somatic cells in which HIF1α is knocked-down are remarkably less efficiently reprogrammed into iPSCs [123, 126]. HIF2α has also been found to specifically induce the expression of OCT4 in mouse and human ESCs [141, 149]. However, within the context of iPSC derivation, HIF2α seems to be beneficial only during the early phase of reprogramming and not in later stages [126]. mTOR down-regulation by SOX2 may also represent an important factor for reprogramming initiation [150].
Small molecules regulating mitochondria and energy metabolism have been found to elicit significant effects on reprogramming. In particular, inhibition of glycolysis or HIF1α activity leads to impaired iPSC formation [19, 115, 124, 125, 148]. The same is true for AMPK activation, which may repress reprogramming through the transcriptional repression of OCT4 [151]. An inhibitor of mitochondrial fission also disrupts iPSC conversion, suggesting that the establishment of non-fused fragmented mitochondria might represent an important element of the induction of pluripotency [152].
On the other hand, small molecule stimulating glycolysis, HIF1α, or PDK activity has been found to enhance iPSC formation [19, 115, 123–126]. Accordingly, inhibition of OXPHOS may be beneficial for the maintenance of PSCs [153]. Chemical inhibition of mTOR can also lead to improved reprogramming efficiency [154], further highlighting the importance of metabolic sensors and regulators in the path toward pluripotency.
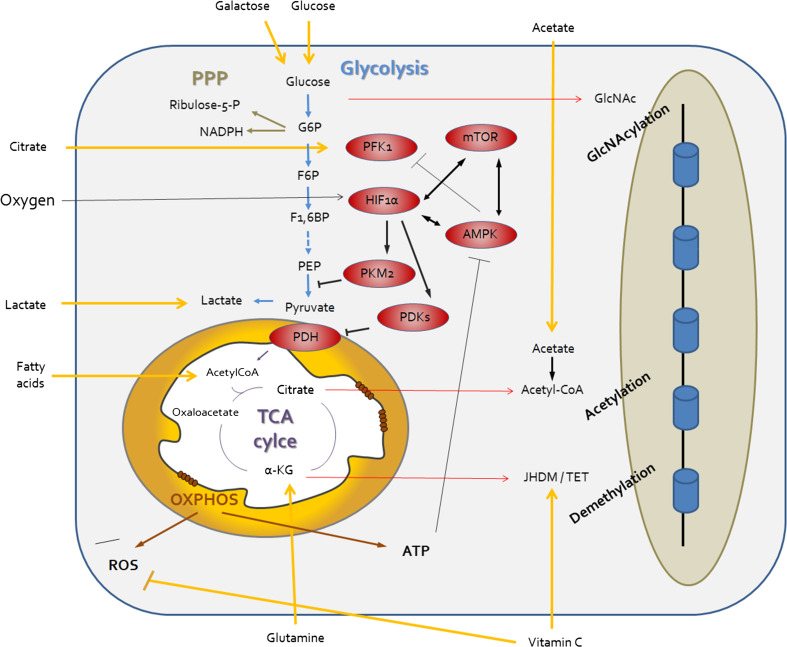
Finally, the addition of vitamin C has been shown to improve the reprogramming process and lead to better quality iPSCs through both its antioxidant role and its ability to modulate epigenetic processes by the control of DNA-modifiying dioxigenases [155]. Therefore, it is interesting to notice that procedures modulating mitochondrial-related pathways might influence both the efficiency and the quality of reprogrammed cells [156, 157] (Fig. 3).
Fig. 3.
Regulating the metabolic identity of pluripotent stem cells. The metabolic state of PSCs is drastically divergent from that of somatic cells and relies on enhanced flux through the glycolysis (in blue) and the PPP (in green). This energy re-routing bears the crucial advantage of maintaining low oxidative stress levels through reduced OXPHOS-mediated ROS production and increased PPP-derived NADPH. Consequently, mitochondria within PSCs exhibit unique morphological organization and sensitivity to apoptosis (also known as mitochondrial priming). In order to induce and sustain this metabolic configuration, specific mechanisms must therefore be in place. Among these, master metabolic regulators such as AMPK, mTOR, HIF1α, and PKM2 (in red) may influence the efficiency of reprogramming to pluripotency. The importance of bioenergetic metabolism for cell fate conversion is further highlighted by the evident crosstalk between central carbon metabolites and epigenetic regulation (red arrows). Finally, nutrients and external substrates including oxygen might function as further upstream modulators (yellow arrows). This effect may be at the level of metabolic route decisions (oxygen, glucose, galactose, fatty acid, lactate, and glutamine), antioxidant protection (vitamin C), or epigenetic control (vitamin C and acetate). Further studies are needed to clearly elucidate this complex interplay between cellular environment and specification of cell fate identity
Nutrients and lipids in PSC metabolism
Recent studies have highlighted the implication of nutrient-sensing signaling pathways in the establishment and maintenance of pluripotency [158]. Within the FOXO family, FOXO1 regulate the expression of OCT4 and SOX2 in hESCs, and its activity may be crucial for the maintenance of pluripotency in human and mouse ESCs [159]. Instead, FOXO4 regulates the proteasome activity in hESCs [160]. mTOR regulation also seems fundamental for mouse and human ESC self-renewal and pluripotency [161, 162], as its activity is augmented upon differentiation [163, 164] and negatively affects the generation of iPSCs [154, 165]. Overall, although more studies are warranted in this area, it appears that energy-sensing involved in the coordination of metabolic and proliferative responses might be central for the acquisition and maintenance of stem cell properties [158] (Fig. 3).
The relevance of lipid metabolism in the control of “stemness” and differentiation in multipotent NSCs and HSCs has been recently described [166, 167] (see below). However, the possible roles of lipids have not been extensively investigated in PSCs. It has been shown that culture medium supplemented with albumin-associated lipids promoted hESC self-renewal [168] and that addition of sphingosine-1-phosphate to hESC culture medium suppresses apoptosis and promotes proliferation of these cells, while decreasing pluripotency-associated gene expression [169, 170]. Chemical inhibition or siRNA-mediated down-regulation of stearoyl-CoA desaturase (SCD1), a key enzyme needed for the synthesis of monounsaturated fatty acids (MUFAs), has been found to selectively cause ER stress and cell death selectively in hESCs and iPSCs, and the supplementation with oleic acid, the product of the reaction catalyzed by SCD1, prevented these effects [171]. This demonstrates the importance of the MUFAs biosynthetic pathway for human PSCs. The enzymes acetyl-CoA carboxylase (ACC) and fatty acid synthase (FASN), both of them crucial players in fatty acid synthesis, are up-regulated in iPSCs when compared to differentiated cells, possibly due to the inactivation of AMPK [172]. In fact, impairment of de novo fatty acid synthesis during reprogramming greatly reduces the efficiency of iPSC formation [172]. These findings imply a relevant role of lipid synthesis in cell fate reprogramming. In light of these studies, it may be worth investigating in more detail the lipid-associated signaling and metabolism in the maintenance of pluripotency.
Metabolism-epigenetic crosstalk in PSCs
The fact that metabolic reconfiguration could precede the establishment of the pluripotency regulatory networks [115, 123, 126, 173, 174] suggests that modulating energy metabolism might have downstream effects at the epigenetic level (Fig. 3).
Several enzymatic players of epigenetic control need distinct metabolites as cofactors or substrates, thus making epigenetics sensitive to the metabolic status of the cell [175]. In particular, acetyl-CoA derived from the activity of ATP-citrate lyase (ACL, the enzyme responsible of the first step in fatty acid synthesis from citrate) is necessary for the activity of histone-acetyl transferases (HATs) in mammalian cells [176]. Sirtuins are NAD+-dependent class III histone deacetylases (HDACs). Both CoA/acetyl-CoA and NAD/NADH ratios are indicators of the cellular energetic status. Therefore, the lysine acetylation level of histones (and so their ability to bind DNA) is linked to the availability of acetyl-CoA and NAD+ in the cells [177].
Energy metabolism can exert epigenetic regulation in PSCs that may depend on the type of nutrient substrates. Indeed, glucose-dependent chromatin O-GlcNAcylation has been found to transcriptionally activate core components of the pluripotency network, as low glucose concentration in the media resulted in reduced reprogramming efficiency of mouse iPSCs [178]. Another example is the dependence on the threonine (Thr) metabolism in mouse ESCs. Thr -derived carbons are used to generate acetyl-CoA for the TCA cycle and diverse acetylation reactions, and 1-carbon equivalents for the folate pool [179]. The synthesis of 5-methyl-tetrahydrofolate modulates the metabolism of S-adenosylmethionine (SAM), the major methyl donor for DNA and histone methylation, linking the metabolism of Thr to the epigenetic control of growth and differentiation in mouse ESCs [145]. In accordance, Thr regulation significantly influences the efficiency of mouse somatic cell reprogramming [180]. In humans, however, threonine dehydrogenase, the enzyme responsible for the first step of Thr catabolism, is expressed as a non-functional pseudogene [181]. Interestingly, it has been recently shown that methionine (Met), and not Thr, is essential for SAM synthesis and cell viability in human ESCs and iPSCs [182]. Met is the substrate for SAM synthesis through the action of methionine adenosyltransferases (MATs). Met deprivation leads to a rapid decrease in SAM levels in human PSCs, which in turn results in changes of epigenetic patterns and modification of signaling pathways leading to cell differentiation, while prolonged Met deprivation results in pluripotent cell death [182].
Also the cellular redox status may be important for the epigenetic remodeling, as ROS can regulate the activation of methionine adenosyltransferases, the enzymes responsible for the synthesis of SAM, which is the methyl donor for DNA methylation [183]. Oxidation of cysteine residues in some methionine adenosyltransferases leads to their inhibition [184], consequently causing a decrease in SAM levels and changing epigenetic patterns in cancer cells [183].
Histones can also be modified by the addition of uridine diphosphate-N-acetylgluocosamine (UDP-GlcNAc), a product of the hexosamine biosynthetic pathway branching from glucose-related flux [185]. Finally, the TCA cycle intermediate αKG is a cofactor of dioxygenases like ten-eleven translocation (TET) DNA hydroxylases and Jumonji C histone demethylases (JHDM) [186] and its elevated concentration has been found beneficial for the epigenetic maintenance of naïve mouse ESCs [187].
Metabolic restructuring upon PSC differentiation
Multiple studies have shown a complex involvement of mitochondria during stem cell differentiation. During spontaneous differentiation of hESCs there is an increase in mitochondrial mass, oxygen consumption, mitochondrial proliferation, mtDNA transcription, ROS production, and synthesis of antioxidant molecules, such as SOD and peroxiredoxin [111, 188]. In accordance, the oxidation of unsaturated fatty acids by ROS and concomitant production of eicosanoids trigger the differentiation of mouse ESCs [189].
The conversion of hESCs and iPSCs into fibroblast-like cells is also accompanied by higher mitochondrial activity represented by increased mtDNA copy numbers, morphological maturation of mitochondria, and increased levels of ATP and oxidative damage [118]. The regulation of mitochondrial dynamics may indeed be involved in cell differentiation [48]. Mitochondrial fusion promotes proliferation allowing Cyclin E accumulation and entry in the S phase [190], while mitochondrial fission supports the exit from cell cycle and commitment toward differentiation [191].
The manipulation of mitochondria-related pathways may also be relevant for regulating differentiation along specific lineages. The promotion of mitochondrial biogenesis and OXPHOS can enhance the differentiation of hESCs into the mesendoderm lineage [192]. Moreover, inhibition of PPP may specifically trigger endodermal differentiation [193, 194]. Sustained expression of prohibitin 2 (PHB2), a protein-lipid scaffold at the mitochondrial inner membrane (MIM), inhibits the differentiation of mouse and human PSCs into the ectodermal and endodermal lineages, but not into the mesodermal lineage [195]. The inhibition of mitochondrial permeability transition pore (PTP) promotes the differentiation of mouse and human PSCs into cardiomyocytes due to an increase in mitochondrial oxidative metabolism, and this cardiomyogenyc effect is enhanced by the addition of antioxidants [196]. Taken together, these examples demonstrate that mitochondria exert a pivotal role in the process of differentiation, and further efforts are needed to clarify all the facets of this intricate influence.
Mitochondrial and metabolic features of multipotent stem cells
HSCs and MSCs
Most adult stem cells are quiescent cells, slowly cycling to prevent stem cell exhaustion (that would risk life-long capacity for tissue renewal), the accumulation of mutations during successive cell divisions (that would favor oncogenic transformation) [197], and cellular damage by OXPHOS-derived ROS [198]. Both hematopoietic stem cells (HSCs) and mesenchymal stem cells (MSCs) reside in hypoxic niches, and, consequently, seem to rely on glycolysis more than OXPHOS, when compared to more differentiated cells in their respective lineages [198, 199]. The hypoxic environments of adult stem cells could be a physiological adaptation to prevent oxidative stress. Numerous studies have shown the sensitivity of HSCs to ROS, which, at high levels, trigger responses such as differentiation, senescence, loss of stem cell function, or apoptosis [200–202].
In accordance with their preference for anaerobic metabolism, mitochondrial oxidative capacity in adult HSCs is down-regulated by the induction of the transcription factor HIF1α [203]. HIF1α leads to elevated expression of CRIPTO, that, in turn, non-cell autonomously activates its receptor GRP78, thereby inducing the expression of glycolytic genes, including PDKs [140]. The induction of PDKs has been postulated as a metabolic checkpoint able to modulate cell cycle progression and function of HSCs [204]. On the other hand, fatty acid ß-oxidation under the influence of PPARδ seems to play a crucial role in the control of the switch between asymmetric/symmetric cell division in HSCs [166], which indicates that mitochondrial oxidative metabolism is extremely relevant for these cells beyond the usage of glucose-derived substrates. In the case of MSCs, when expanded in normoxic conditions are able to efficiently use OXPHOS with high O2 consumption, and their proliferative and colony formation capacity is increased, but this is accompanied by induction of senescence [205, 206]. These results are in accordance with the notion that location into hypoxic environments may protect adult stem cells from oxidative stress and preserve their long-term self-renewal capacity.
NSCs
NSCs depend on low oxygen tensions to remain quiescent in their stem cell state. In the adult brain, NSC population within the thin subventricular zone (SVZ) give rise to immature progenitors of various lineages, which can produce migrating neuroblasts as well as glia. Low concentrations of oxygen (<1–8 %) in the SVZ are comparable to the hypoxic niches of HSCs (1–6 %) and MSCs (2–8 %) [207].
ROS levels are linked with NSC maintenance. Indeed, increased production of radicals, formed under normoxic conditions, induces differentiation of NSCs to a more proliferative progenitor state, contributing to a process designated as “stem cell priming” [208]. In addition to antioxidants, FOXO3 promotes quiescence in NSCs, as its deficiency results in loss of self-renewal and differentiation capacity [209]. Another important component of the NSC redox rheostat is the kinase ataxia telangiectasia mutated (ATM), which is activated in response to oxidative stress as well as DNA damage and is believed to be required by NSCs to avoid genomic aberrations [210].
Additional signaling events, especially nutrient-sensing, can also modulate NSC proliferation. As a phosphatase inhibiting PI3 K-AKT signaling, depletion of PTEN in NSCs aberrantly increases proliferation [211]. Overall, the hypoxic niche, deprived of ROS but also with low activation of nutrient-sensitive mTOR pathway, is essential in providing an appropriate environment for quiescent NSCs [140].
NSCs across several species were found to be glycolytic [212, 213], especially more glycolytic than their neuronal progeny. Nevertheless, there is uncertainty about the metabolic state of the progenitors resulting from NSCs upon differentiation. While specific granular progenitor types seem to up-regulate glycolysis via induction of Hexokinase-2 (HK2) [214], a key enzyme of glucose conversion, other cerebellar progenitors seem to heavily rely on ATP generated via OXPHOS [215] for proliferation and survival. The switch from glycolysis to OXPHOS has been shown to terminate the NSC state in Drosophila, thus leading to differentiation phase via Mediator [216]. The conserved mammalian system might fulfill a similar function. Hence, a specific metabolic signature of the NSC-derived progenitors may exist according to their subsequent cellular fate. More data, particularly in the human context, are needed to resolve this issue.
The mitochondrial ultrastructure in human PSC-derived NSCs is generally much more mature compared to PSCs displaying densely folded and compact cristae [212]. However, mitochondrial biogenesis and mitochondrial copy number remain low within PSC-derived NSCs and increase only upon terminal neuronal differentiation [212], potentially indicative of the more quiescent phenotype of NSCs [217]. Besides glucose and oxygen, fatty acid metabolism might meet the requirements of stem cells and their progeny. In particular, FASN has been recently found as a key catalyzer of lipogenesis in adult NSCs [167].The dependence on lipogenesis may be explained by their requirement of high amounts of membrane material, but a more detailed analysis of fatty acid metabolism is needed.
Summary and outlook
Bridging the gaps: the importance of metabolic restructuring for cell fate conversion
As described above, metabolic plasticity is the foundation for development in multicellular organisms and for tissue organization. The observation that only 40–57 % of the mitochondrial proteome is shared between different tissues [218] illustrates the enormous functional diversity of these organelles in fulfilling numerous environment-specific tasks. Most striking is the recently discovered reconfiguration of mitochondria and related metabolic signature during cellular reprogramming. Further knowledge is warranted to decipher the mechanisms underlying the restructuring of metabolism and redox balance, in order to improve strategies safeguarding the genome, given that genomic aberrations are an obvious hurdle for safe medical application of iPSCs [219].
The importance of master regulators of energy metabolism in cellular reprogramming still needs to be fully evaluated. Hypoxia-inducible factors are postulated as clear promising candidates, as a great proportion of the metabolic genes induced during reprogramming are related to hypoxia [123, 126]. Besides, several signaling pathways controlling proliferation and differentiation are also involved in the regulation of metabolic processes [220]. This orchestrated regulation may be operating to control the cell fate, and more studies are therefore needed to clarify the interplay between these various signaling networks.
The metabolic-driven epigenetic control of cellular identity also warrants further investigations. Given the potential impact that the metabolic switch experienced by cells during reprogramming can exert in epigenetic control of gene expression, further efforts are required to clarify the interplay between metabolism and epigenetic during cell fate conversion.
Another interesting aspect to address would be the analysis of the mitochondrial and metabolic changes occurring during direct reprogramming (also known as trans-differentiation). This phenomenon exists in vivo but can also be induced by over-expression of lineage-specific pioneer transcription factors [221, 222]. The influence of environmental metabolic factors in this process has not been clearly shown so far. Thus, a detailed assessment of the mitochondrial and metabolic changes occurring upon direct reprogramming might elucidate the necessary prerequisites for making cells amenable to be coaxed directly into other terminal fates.
Finally, the importance of cellular environment and substrate usage in the regulation and induction of stemness still have to be fully elucidated. The metabolic demands associated with the acquisition of pluripotency maintenance or differentiation result into changes in the preference for specific carbon sources (Fig. 2). This might be achieved by the control of metabolic flux through transcriptional and post-transcriptional regulation of rate-limiting enzymes and metabolite transporters and by spatiotemporal organization of metabolic pathways, including changes in mitochondrial morphology [158]. More information is needed in order to understand how to take advantage of these processes for the generation of iPSCs, the differentiation into specific cell lineages, and for the modulation of cell behavior in pathophysiological contexts.
In conclusion, growing evidence implicate mitochondria and energy metabolism in the modulation of cell fate decision making. The nuclear-centric view of stemness is no longer sufficient and we are moving toward the realization that epigenetic control of cell fate requires a complex integration of external and internal metabolic stimuli, whose modulation appears crucial for enabling cellular plasticity.
Acknowledgments
The authors declare no competing financial or commercial interests. M.V.R.P. is financed by CVI-06585 (Junta de Andalucía, Spain). R.B. receives a fellowship support from the MDC-PhD Program. A.P. acknowledges support from the Deutsche Forschungsgemeinschaft (DFG). J.A. acknowledges partial support from the European Union funding/FP7 (FP7/2007-2013)/Grant Agreement n° 305299 (AgedBrainSYSBIO), BMBF grant (10GN1005) and from the medical faculty of the Heinrich Heine University-Düsseldorf.
References
- 1.Clarke MF, Fuller M. Stem cells and cancer: two faces of eve. Cell. 2006;124:1111–1115. doi: 10.1016/j.cell.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 2.Raff M. Adult stem cell plasticity: fact or artifact? Annu Rev Cell Dev Biol. 2003;19:1–22. doi: 10.1146/annurev.cellbio.19.111301.143037. [DOI] [PubMed] [Google Scholar]
- 3.Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 4.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 5.Alvarez CV, Garcia-Lavandeira M, Garcia-Rendueles ME, Diaz-Rodriguez E, Garcia-Rendueles AR, Perez-Romero S, Vila TV, Rodrigues JS, Lear PV, Bravo SB. Defining stem cell types: understanding the therapeutic potential of ESCs, ASCs, and iPS cells. J Mol Endocrinol. 2012;49:R89–R111. doi: 10.1530/JME-12-0072. [DOI] [PubMed] [Google Scholar]
- 6.Bongso A, Richards M. History and perspective of stem cell research. Best Pract Res Clin Obstet Gynaecol. 2004;18:827–842. doi: 10.1016/j.bpobgyn.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 7.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 8.Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 9.Bayart E, Cohen-Haguenauer O. Technological overview of iPS induction from human adult somatic cells. Curr Gene Ther. 2013;13:73–92. doi: 10.2174/1566523211313020002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jia F, Wilson KD, Sun N, Gupta DM, Huang M, Li Z, Panetta NJ, Chen ZY, Robbins RC, Kay MA, Longaker MT, Wu JC. A nonviral minicircle vector for deriving human iPS cells. Nat Methods. 2010;7:197–199. doi: 10.1038/nmeth.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Warren L, Manos PD, Ahfeldt T, Loh YH, Li H, Lau F, Ebina W, Mandal PK, Smith ZD, Meissner A, Daley GQ, Brack AS, Collins JJ, Cowan C, Schlaeger TM, Rossi DJ. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell. 2010;7:618–630. doi: 10.1016/j.stem.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu K, Yu J, Suknuntha K, Tian S, Montgomery K, Choi KD, Stewart R, Thomson JA, Slukvin II. Efficient generation of transgene-free induced pluripotent stem cells from normal and neoplastic bone marrow and cord blood mononuclear cells. Blood. 2011;117:e109–e119. doi: 10.1182/blood-2010-07-298331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miyoshi N, Ishii H, Nagano H, Haraguchi N, Dewi DL, Kano Y, Nishikawa S, Tanemura M, Mimori K, Tanaka F, Saito T, Nishimura J, Takemasa I, Mizushima T, Ikeda M, Yamamoto H, Sekimoto M, Doki Y, Mori M. Reprogramming of mouse and human cells to pluripotency using mature microRNAs. Cell Stem Cell. 2011;8:633–638. doi: 10.1016/j.stem.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 14.Sommer CA, Mostoslavsky G. The evolving field of induced pluripotency: recent progress and future challenges. J Cell Physiol. 2013;228:267–275. doi: 10.1002/jcp.24155. [DOI] [PubMed] [Google Scholar]
- 15.Mali P, Chou BK, Yen J, Ye Z, Zou J, Dowey S, Brodsky RA, Ohm JE, Yu W, Baylin SB, Yusa K, Bradley A, Meyers DJ, Mukherjee C, Cole PA, Cheng L. Butyrate greatly enhances derivation of human induced pluripotent stem cells by promoting epigenetic remodeling and the expression of pluripotency-associated genes. Stem Cells. 2010;28:713–720. doi: 10.1002/stem.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huangfu D, Osafune K, Maehr R, Guo W, Eijkelenboom A, Chen S, Muhlestein W, Melton DA. Induction of pluripotent stem cells from primary human fibroblasts with only Oct4 and Sox2. Nat Biotechnol. 2008;26:1269–1275. doi: 10.1038/nbt.1502. [DOI] [PubMed] [Google Scholar]
- 17.Ichida JK, Blanchard J, Lam K, Son EY, Chung JE, Egli D, Loh KM, Carter AC, Di Giorgio FP, Koszka K, Huangfu D, Akutsu H, Liu DR, Rubin LL, Eggan K. A small-molecule inhibitor of tgf-Beta signaling replaces sox2 in reprogramming by inducing nanog. Cell Stem Cell. 2009;5:491–503. doi: 10.1016/j.stem.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin T, Ambasudhan R, Yuan X, Li W, Hilcove S, Abujarour R, Lin X, Hahm HS, Hao E, Hayek A, Ding S. A chemical platform for improved induction of human iPSCs. Nat Methods. 2009;6:805–808. doi: 10.1038/nmeth.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu S, Li W, Zhou H, Wei W, Ambasudhan R, Lin T, Kim J, Zhang K, Ding S. Reprogramming of human primary somatic cells by OCT4 and chemical compounds. Cell Stem Cell. 2010;7:651–655. doi: 10.1016/j.stem.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jung DW, Kim WH, Williams DR. Reprogram or reboot: small molecule approaches for the production of induced pluripotent stem cells and direct cell reprogramming. ACS Chem Biol. 2014;9:80–95. doi: 10.1021/cb400754f. [DOI] [PubMed] [Google Scholar]
- 21.Hou P, Li Y, Zhang X, Liu C, Guan J, Li H, Zhao T, Ye J, Yang W, Liu K, Ge J, Xu J, Zhang Q, Zhao Y, Deng H. Pluripotent stem cells induced from mouse somatic cells by small-molecule compounds. Science. 2013;341:651–654. doi: 10.1126/science.1239278. [DOI] [PubMed] [Google Scholar]
- 22.Tachibana M, Amato P, Sparman M, Gutierrez NM, Tippner-Hedges R, Ma H, Kang E, Fulati A, Lee HS, Sritanaudomchai H, Masterson K, Larson J, Eaton D, Sadler-Fredd K, Battaglia D, Lee D, Wu D, Jensen J, Patton P, Gokhale S, Stouffer RL, Wolf D, Mitalipov S. Human embryonic stem cells derived by somatic cell nuclear transfer. Cell. 2013;153:1228–1238. doi: 10.1016/j.cell.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chung YG, Eum JH, Lee JE, Shim SH, Sepilian V, Hong SW, Lee Y, Treff NR, Choi YH, Kimbrel EA, Dittman RE, Lanza R, Lee DR. Human somatic cell nuclear transfer using adult cells. Cell Stem Cell. 2014;14:777–780. doi: 10.1016/j.stem.2014.03.015. [DOI] [PubMed] [Google Scholar]
- 24.Yamada M, Johannesson B, Sagi I, Burnett LC, Kort DH, Prosser RW, Paull D, Nestor MW, Freeby M, Greenberg E, Goland RS, Leibel RL, Solomon SL, Benvenisty N, Sauer MV, Egli D. Human oocytes reprogram adult somatic nuclei of a type 1 diabetic to diploid pluripotent stem cells. Nature. 2014;510:533–536. doi: 10.1038/nature13287. [DOI] [PubMed] [Google Scholar]
- 25.Ma H, Morey R, O’Neil RC, He Y, Daughtry B, Schultz MD, Hariharan M, Nery JR, Castanon R, Sabatini K, Thiagarajan RD, Tachibana M, Kang E, Tippner-Hedges R, Ahmed R, Gutierrez NM, Van Dyken C, Polat A, Sugawara A, Sparman M, Gokhale S, Amato P, Wolf DP, Ecker JR, Laurent LC, Mitalipov S. Abnormalities in human pluripotent cells due to reprogramming mechanisms. Nature. 2014;511:177–183. doi: 10.1038/nature13551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johannesson B, Sagi I, Gore A, Paull D, Yamada M, Golan-Lev T, Li Z, LeDuc C, Shen Y, Stern S, Xu N, Ma H, Kang E, Mitalipov S, Sauer MV, Zhang K, Benvenisty N, Egli D. Comparable frequencies of coding mutations and loss of imprinting in human pluripotent cells derived by nuclear transfer and defined factors. Cell Stem Cell. 2014;15:634–642. doi: 10.1016/j.stem.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 27.Onder TT, Daley GQ. New lessons learned from disease modeling with induced pluripotent stem cells. Curr Opin Genet Dev. 2012;22:500–508. doi: 10.1016/j.gde.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Byers B, Cord B, Nguyen HN, Schule B, Fenno L, Lee PC, Deisseroth K, Langston JW, Pera RR, Palmer TD. SNCA triplication Parkinson’s patient’s iPSC-derived DA neurons accumulate alpha-synuclein and are susceptible to oxidative stress. PLoS One. 2011;6:e26159. doi: 10.1371/journal.pone.0026159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koch P, Breuer P, Peitz M, Jungverdorben J, Kesavan J, Poppe D, Doerr J, Ladewig J, Mertens J, Tuting T, Hoffmann P, Klockgether T, Evert BO, Wullner U, Brustle O. Excitation-induced ataxin-3 aggregation in neurons from patients with Machado-Joseph disease. Nature. 2011;480:543–546. doi: 10.1038/nature10671. [DOI] [PubMed] [Google Scholar]
- 30.Itzhaki I, Maizels L, Huber I, Gepstein A, Arbel G, Caspi O, Miller L, Belhassen B, Nof E, Glikson M, Gepstein L. Modeling of catecholaminergic polymorphic ventricular tachycardia with patient-specific human-induced pluripotent stem cells. J Am Coll Cardiol. 2012;60:990–1000. doi: 10.1016/j.jacc.2012.02.066. [DOI] [PubMed] [Google Scholar]
- 31.Sun N, Yazawa M, Liu J, Han L, Sanchez-Freire V, Abilez OJ, Navarrete EG, Hu S, Wang L, Lee A, Pavlovic A, Lin S, Chen R, Hajjar RJ, Snyder MP, Dolmetsch RE, Butte MJ, Ashley EA, Longaker MT, Robbins RC, Wu JC. Patient-specific induced pluripotent stem cells as a model for familial dilated cardiomyopathy. Sci Transl Med. 2012;4:130–147. doi: 10.1126/scitranslmed.3003552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rashid ST, Corbineau S, Hannan N, Marciniak SJ, Miranda E, Alexander G, Huang-Doran I, Griffin J, Ahrlund-Richter L, Skepper J, Semple R, Weber A, Lomas DA, Vallier L. Modeling inherited metabolic disorders of the liver using human induced pluripotent stem cells. J Clin Invest. 2010;120:3127–3136. doi: 10.1172/JCI43122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Drews K, Jozefczuk J, Prigione A, Adjaye J. Human induced pluripotent stem cells–from mechanisms to clinical applications. J Mol Med (Berl) 2012;90:735–745. doi: 10.1007/s00109-012-0913-0. [DOI] [PubMed] [Google Scholar]
- 34.Ogawa Y, Sawamoto K, Miyata T, Miyao S, Watanabe M, Nakamura M, Bregman BS, Koike M, Uchiyama Y, Toyama Y, Okano H. Transplantation of in vitro-expanded fetal neural progenitor cells results in neurogenesis and functional recovery after spinal cord contusion injury in adult rats. J Neurosci Res. 2002;69:925–933. doi: 10.1002/jnr.10341. [DOI] [PubMed] [Google Scholar]
- 35.Iwanami A, Kaneko S, Nakamura M, Kanemura Y, Mori H, Kobayashi S, Yamasaki M, Momoshima S, Ishii H, Ando K, Tanioka Y, Tamaoki N, Nomura T, Toyama Y, Okano H. Transplantation of human neural stem cells for spinal cord injury in primates. J Neurosci Res. 2005;80:182–190. doi: 10.1002/jnr.20436. [DOI] [PubMed] [Google Scholar]
- 36.Kumagai G, Okada Y, Yamane J, Nagoshi N, Kitamura K, Mukaino M, Tsuji O, Fujiyoshi K, Katoh H, Okada S, Shibata S, Matsuzaki Y, Toh S, Toyama Y, Nakamura M, Okano H. Roles of ES cell-derived gliogenic neural stem/progenitor cells in functional recovery after spinal cord injury. PLoS ONE. 2009;4:e7706. doi: 10.1371/journal.pone.0007706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kobayashi Y, Okada Y, Itakura G, Iwai H, Nishimura S, Yasuda A, Nori S, Hikishima K, Konomi T, Fujiyoshi K, Tsuji O, Toyama Y, Yamanaka S, Nakamura M, Okano H. Pre-evaluated safe human iPSC-derived neural stem cells promote functional recovery after spinal cord injury in common marmoset without tumorigenicity. PLoS One. 2012;7:e52787. doi: 10.1371/journal.pone.0052787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bretzner F, Gilbert F, Baylis F, Brownstone RM. Target populations for first-in-human embryonic stem cell research in spinal cord injury. Cell Stem Cell. 2011;8:468–475. doi: 10.1016/j.stem.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 39.Frantz S. Embryonic stem cell pioneer Geron exits field, cuts losses. Nat Biotechnol. 2012;30:12–13. doi: 10.1038/nbt0112-12. [DOI] [PubMed] [Google Scholar]
- 40.Filardo G, Madry H, Jelic M, Roffi A, Cucchiarini M, Kon E. Mesenchymal stem cells for the treatment of cartilage lesions: from preclinical findings to clinical application in orthopaedics. Knee Surg Sports Traumatol Arthrosc. 2013;21:1717–1729. doi: 10.1007/s00167-012-2329-3. [DOI] [PubMed] [Google Scholar]
- 41.Voswinkel J, Francois S, Simon JM, Benderitter M, Gorin NC, Mohty M, Fouillard L, Chapel A. Use of mesenchymal stem cells (MSC) in chronic inflammatory fistulizing and fibrotic diseases: a comprehensive review. Clin Rev Allergy Immunol. 2013;45:180–192. doi: 10.1007/s12016-012-8347-6. [DOI] [PubMed] [Google Scholar]
- 42.Narita T, Suzuki K (2014) Bone marrow-derived mesenchymal stem cells for the treatment of heart failure. Heart Fail Rev (in press) [DOI] [PubMed]
- 43.Ng TK, Fortino VR, Pelaez D, Cheung HS. Progress of mesenchymal stem cell therapy for neural and retinal diseases. World J Stem Cells. 2014;6:111–119. doi: 10.4252/wjsc.v6.i2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reardon S, Cyranoski D. Japan stem-cell trial stirs envy. Nature. 2014;513:287–288. doi: 10.1038/513287a. [DOI] [PubMed] [Google Scholar]
- 45.Kamao H, Mandai M, Okamoto S, Sakai N, Suga A, Sugita S, Kiryu J, Takahashi M. Characterization of human induced pluripotent stem cell-derived retinal pigment epithelium cell sheets aiming for clinical application. Stem Cell Reports. 2014;2:205–218. doi: 10.1016/j.stemcr.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Campello S, Strappazzon F, Cecconi F. Mitochondrial dismissal in mammals, from protein degradation to mitophagy. Biochim Biophys Acta. 2014;1837:451–460. doi: 10.1016/j.bbabio.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 47.Martinelli P, Rugarli EI. Emerging roles of mitochondrial proteases in neurodegeneration. Biochim Biophys Acta. 2010;1797:1–10. doi: 10.1016/j.bbabio.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 48.Kasahara A, Scorrano L. Mitochondria: from cell death executioners to regulators of cell differentiation. Trends Cell Biol. 2014;24:761–770. doi: 10.1016/j.tcb.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 49.Andrews RM, Kubacka I, Chinnery PF, Lightowlers RN, Turnbull DM, Howell N. Reanalysis and revision of the Cambridge reference sequence for human mitochondrial DNA. Nat Genet. 1999;23:147. doi: 10.1038/13779. [DOI] [PubMed] [Google Scholar]
- 50.Farrar GJ, Chadderton N, Kenna PF, Millington-Ward S. Mitochondrial disorders: aetiologies, models systems, and candidate therapies. Trends Genet. 2013;29:488–497. doi: 10.1016/j.tig.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 51.Russell O, Turnbull D. Mitochondrial DNA disease-molecular insights and potential routes to a cure. Exp Cell Res. 2014;325:38–43. doi: 10.1016/j.yexcr.2014.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Coskun PE, Beal MF, Wallace DC. Alzheimer’s brains harbor somatic mtDNA control-region mutations that suppress mitochondrial transcription and replication. Proc Natl Acad Sci USA. 2004;101:10726–10731. doi: 10.1073/pnas.0403649101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sevini F, Giuliani C, Vianello D, Giampieri E, Santoro A, Biondi F, Garagnani P, Passarino G, Luiselli D, Capri M, Franceschi C, Salvioli S. mtDNA mutations in human aging and longevity: controversies and new perspectives opened by high-throughput technologies. Exp Gerontol. 2014;56:234–244. doi: 10.1016/j.exger.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 54.Sato M, Sato K. Maternal inheritance of mitochondrial DNA by diverse mechanisms to eliminate paternal mitochondrial DNA. Biochim Biophys Acta. 2013;1833:1979–1984. doi: 10.1016/j.bbamcr.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 55.Hauswirth WW, Laipis PJ. Mitochondrial DNA polymorphism in a maternal lineage of Holstein cows. Proc Natl Acad Sci USA. 1982;79:4686–4690. doi: 10.1073/pnas.79.15.4686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Olivo PD, Van de Walle MJ, Laipis PJ, Hauswirth WW. Nucleotide sequence evidence for rapid genotypic shifts in the bovine mitochondrial DNA D-loop. Nature. 1983;306:400–402. doi: 10.1038/306400a0. [DOI] [PubMed] [Google Scholar]
- 57.Howell N, Kubacka I, Mackey DA. How rapidly does the human mitochondrial genome evolve? Am J Hum Genet. 1996;59:501–509. [PMC free article] [PubMed] [Google Scholar]
- 58.Chinnery PF, Howell N, Lightowlers RN, Turnbull DM. Molecular pathology of MELAS and MERRF. The relationship between mutation load and clinical phenotypes. Brain. 1997;120(Pt 10):1713–1721. doi: 10.1093/brain/120.10.1713. [DOI] [PubMed] [Google Scholar]
- 59.Shoubridge EA. Mitochondrial DNA segregation in the developing embryo. Hum Reprod. 2000;15(Suppl 2):229–234. doi: 10.1093/humrep/15.suppl_2.229. [DOI] [PubMed] [Google Scholar]
- 60.Smith LC, Bordignon V, Couto MM, Garcia SM, Yamazaki W, Meirelles FV. Mitochondrial genotype segregation and the bottleneck. Reprod Biomed Online. 2002;4:248–255. doi: 10.1016/s1472-6483(10)61814-7. [DOI] [PubMed] [Google Scholar]
- 61.Carling PJ, Cree LM, Chinnery PF. The implications of mitochondrial DNA copy number regulation during embryogenesis. Mitochondrion. 2011;11:686–692. doi: 10.1016/j.mito.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 62.Ruiz-Perez MV, Sanchez-Jimenez F, Alonso FJ, Segura JA, Marquez J. Glutamine, Glucose and other Fuels for Cancer. Curr Pharm Des. 2013;20:2557–2579. doi: 10.2174/13816128113199990482. [DOI] [PubMed] [Google Scholar]
- 63.Stincone A, Prigione A, Cramer T, Wamelink MMC, Campbell K, Cheung E, Viridiana Olin-Sandoval M, Guening NM, Krueger A, Tauqeer Alam M, Keller MA, Breitenbach M, Brindle K, Rabinowitz J, Ralser M. The return of metabolism: biochemistry and physiology of the pentose phosphate pathway. Biol Rev Camb Philos Soc (in press) [DOI] [PMC free article] [PubMed]
- 64.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 65.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 66.Gruning NM, Rinnerthaler M, Bluemlein K, Mulleder M, Wamelink MM, Lehrach H, Jakobs C, Breitenbach M, Ralser M. Pyruvate kinase triggers a metabolic feedback loop that controls redox metabolism in respiring cells. Cell Metab. 2011;14:415–427. doi: 10.1016/j.cmet.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sarsour EH, Kumar MG, Chaudhuri L, Kalen AL, Goswami PC. Redox control of the cell cycle in health and disease. Antioxid Redox Signal. 2009;11:2985–3011. doi: 10.1089/ars.2009.2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Maryanovich M, Gross A. A ROS rheostat for cell fate regulation. Trends Cell Biol. 2013;23:129–134. doi: 10.1016/j.tcb.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 69.Eijkelenboom A, Burgering BM. FOXOs: signalling integrators for homeostasis maintenance. Nat Rev Mol Cell Biol. 2013;14:83–97. doi: 10.1038/nrm3507. [DOI] [PubMed] [Google Scholar]
- 70.Webb AE, Brunet A. FOXO transcription factors: key regulators of cellular quality control. Trends Biochem Sci. 2014;39:159–169. doi: 10.1016/j.tibs.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Deberardinis RJ, Sayed N, Ditsworth D, Thompson CB. Brick by brick: metabolism and tumor cell growth. Curr Opin Genet Dev. 2008;18:54–61. doi: 10.1016/j.gde.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Icard P, Lincet H. A global view of the biochemical pathways involved in the regulation of the metabolism of cancer cells. Biochim Biophys Acta. 2012;1826:423–433. doi: 10.1016/j.bbcan.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 74.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 75.Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell. 2012;148:399–408. doi: 10.1016/j.cell.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kim JW, Tchernyshyov I, Semenza GL, Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006;3:177–185. doi: 10.1016/j.cmet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 77.Papandreou I, Cairns RA, Fontana L, Lim AL, Denko NC. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab. 2006;3:187–197. doi: 10.1016/j.cmet.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 78.Lu CW, Lin SC, Chen KF, Lai YY, Tsai SJ. Induction of pyruvate dehydrogenase kinase-3 by hypoxia-inducible factor-1 promotes metabolic switch and drug resistance. J Biol Chem. 2008;283:28106–28114. doi: 10.1074/jbc.M803508200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Anastasiou D, Poulogiannis G, Asara JM, Boxer MB, Jiang JK, Shen M, Bellinger G, Sasaki AT, Locasale JW, Auld DS, Thomas CJ, Vander Heiden MG, Cantley LC. Inhibition of pyruvate kinase M2 by reactive oxygen species contributes to cellular antioxidant responses. Science. 2011;334:1278–1283. doi: 10.1126/science.1211485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hardie DG. AMPK-Sensing Energy while Talking to Other Signaling Pathways. Cell Metab. 2014;20:939–952. doi: 10.1016/j.cmet.2014.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Prigione A, Cortopassi G. Mitochondrial DNA deletions induce the adenosine monophosphate-activated protein kinase energy stress pathway and result in decreased secretion of some proteins. Aging Cell. 2007;6:619–630. doi: 10.1111/j.1474-9726.2007.00323.x. [DOI] [PubMed] [Google Scholar]
- 82.Shimobayashi M, Hall MN. Making new contacts: the mTOR network in metabolism and signalling crosstalk. Nat Rev Mol Cell Biol. 2014;15:155–162. doi: 10.1038/nrm3757. [DOI] [PubMed] [Google Scholar]
- 83.Liu P, Begley M, Michowski W, Inuzuka H, Ginzberg M, Gao D, Tsou P, Gan W, Papa A, Kim BM, Wan L, Singh A, Zhai B, Yuan M, Wang Z, Gygi SP, Lee TH, Lu KP, Toker A, Pandolfi PP, Asara JM, Kirschner MW, Sicinski P, Cantley L, Wei W. Cell-cycle-regulated activation of Akt kinase by phosphorylation at its carboxyl terminus. Nature. 2014;508:541–545. doi: 10.1038/nature13079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zu XL, Guppy M. Cancer metabolism: facts, fantasy, and fiction. Biochem Biophys Res Commun. 2004;313:459–465. doi: 10.1016/j.bbrc.2003.11.136. [DOI] [PubMed] [Google Scholar]
- 85.Marin-Valencia I, Yang C, Mashimo T, Cho S, Baek H, Yang XL, Rajagopalan KN, Maddie M, Vemireddy V, Zhao Z, Cai L, Good L, Tu BP, Hatanpaa KJ, Mickey BE, Mates JM, Pascual JM, Maher EA, Malloy CR, Deberardinis RJ, Bachoo RM. Analysis of tumor metabolism reveals mitochondrial glucose oxidation in genetically diverse human glioblastomas in the mouse brain in vivo. Cell Metab. 2012;15:827–837. doi: 10.1016/j.cmet.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Salem AF, Whitaker-Menezes D, Lin Z, Martinez-Outschoorn UE, Tanowitz HB, Al-Zoubi MS, Howell A, Pestell RG, Sotgia F, Lisanti MP. Two-compartment tumor metabolism: autophagy in the tumor microenvironment and oxidative mitochondrial metabolism (OXPHOS) in cancer cells. Cell Cycle. 2012;11:2545–2556. doi: 10.4161/cc.20920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schulze A, Harris AL. How cancer metabolism is tuned for proliferation and vulnerable to disruption. Nature. 2012;491:364–373. doi: 10.1038/nature11706. [DOI] [PubMed] [Google Scholar]
- 88.Hsu PP, Sabatini DM. Cancer cell metabolism: Warburg and beyond. Cell. 2008;134:703–707. doi: 10.1016/j.cell.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 89.DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008;7:11–20. doi: 10.1016/j.cmet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 90.Brinster RL. Protein content of the mouse embryo during the first five days of development. J Reprod Fertil. 1967;13:413–420. doi: 10.1530/jrf.0.0130413. [DOI] [PubMed] [Google Scholar]
- 91.Leese HJ. Metabolism of the preimplantation embryo: 40 years on. Reproduction. 2012;143:417–427. doi: 10.1530/REP-11-0484. [DOI] [PubMed] [Google Scholar]
- 92.Martin KL, Leese HJ. Role of glucose in mouse preimplantation embryo development. Mol Reprod Dev. 1995;40:436–443. doi: 10.1002/mrd.1080400407. [DOI] [PubMed] [Google Scholar]
- 93.Jansen S, Pantaleon M, Kaye PL. Characterization and regulation of monocarboxylate cotransporters Slc16a7 and Slc16a3 in preimplantation mouse embryos. Biol Reprod. 2008;79:84–92. doi: 10.1095/biolreprod.107.066811. [DOI] [PubMed] [Google Scholar]
- 94.Brinster RL, Troike DE. Requirements for blastocyst development in vitro. J Anim Sci. 1979;49:26–34. doi: 10.1093/ansci/49.supplement_ii.26. [DOI] [PubMed] [Google Scholar]
- 95.Leese HJ, Barton AM. Pyruvate and glucose uptake by mouse ova and preimplantation embryos. J Reprod Fertil. 1984;72:9–13. doi: 10.1530/jrf.0.0720009. [DOI] [PubMed] [Google Scholar]
- 96.Cree LM, Samuels DC, de Sousa Lopes SC, Rajasimha HK, Wonnapinij P, Mann JR, Dahl HH, Chinnery PF. A reduction of mitochondrial DNA molecules during embryogenesis explains the rapid segregation of genotypes. Nat Genet. 2008;40:249–254. doi: 10.1038/ng.2007.63. [DOI] [PubMed] [Google Scholar]
- 97.Quinn P, Wales RG. Adenosine triphosphate content of preimplantation mouse embryos. J Reprod Fertil. 1971;25:133–135. doi: 10.1530/jrf.0.0250133. [DOI] [PubMed] [Google Scholar]
- 98.Wales RG. Maturation of the mammalian embryo: biochemical aspects. Biol Reprod. 1975;12:66–81. doi: 10.1095/biolreprod12.1.66. [DOI] [PubMed] [Google Scholar]
- 99.Johnson MT, Mahmood S, Patel MS. Intermediary metabolism and energetics during murine early embryogenesis. J Biol Chem. 2003;278:31457–31460. doi: 10.1074/jbc.R300002200. [DOI] [PubMed] [Google Scholar]
- 100.Stern S, Biggers JD, Anderson E. Mitochondria and early development of the mouse. J Exp Zool. 1971;176:179–191. doi: 10.1002/jez.1401760206. [DOI] [PubMed] [Google Scholar]
- 101.Van Blerkom J. Mitochondria in early mammalian development. Semin Cell Dev Biol. 2009;20:354–364. doi: 10.1016/j.semcdb.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 102.Batten BE, Albertini DF, Ducibella T. Patterns of organelle distribution in mouse embryos during preimplantation development. Am J Anat. 1987;178:204–213. doi: 10.1002/aja.1001780212. [DOI] [PubMed] [Google Scholar]
- 103.Barnett DK, Kimura J, Bavister BD. Translocation of active mitochondria during hamster preimplantation embryo development studied by confocal laser scanning microscopy. Dev Dyn. 1996;205:64–72. doi: 10.1002/(SICI)1097-0177(199601)205:1<64::AID-AJA6>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 104.Wilding M, Dale B, Marino M, di Matteo L, Alviggi C, Pisaturo ML, Lombardi L, De Placido G. Mitochondrial aggregation patterns and activity in human oocytes and preimplantation embryos. Hum Reprod. 2001;16:909–917. doi: 10.1093/humrep/16.5.909. [DOI] [PubMed] [Google Scholar]
- 105.Squirrell JM, Schramm RD, Paprocki AM, Wokosin DL, Bavister BD. Imaging mitochondrial organization in living primate oocytes and embryos using multiphoton microscopy. Microsc Microanal. 2003;9:190–201. doi: 10.1017/S1431927603030174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lonergan T, Bavister B, Brenner C. Mitochondria in stem cells. Mitochondrion. 2007;7:289–296. doi: 10.1016/j.mito.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Adjaye J, Huntriss J, Herwig R, BenKahla A, Brink TC, Wierling C, Hultschig C, Groth D, Yaspo ML, Picton HM, Gosden RG, Lehrach H. Primary differentiation in the human blastocyst: comparative molecular portraits of inner cell mass and trophectoderm cells. Stem Cells. 2005;23:1514–1525. doi: 10.1634/stemcells.2005-0113. [DOI] [PubMed] [Google Scholar]
- 108.Pantaleon M, Kaye PL. Glucose transporters in preimplantation development. Rev Reprod. 1998;3:77–81. doi: 10.1530/ror.0.0030077. [DOI] [PubMed] [Google Scholar]
- 109.Lane M, Gardner DK. Understanding cellular disruptions during early embryo development that perturb viability and fetal development. Reprod Fertil Dev. 2005;17:371–378. doi: 10.1071/rd04102. [DOI] [PubMed] [Google Scholar]
- 110.Houghton FD. Energy metabolism of the inner cell mass and trophectoderm of the mouse blastocyst. Differentiation. 2006;74:11–18. doi: 10.1111/j.1432-0436.2006.00052.x. [DOI] [PubMed] [Google Scholar]
- 111.St John JC, Ramalho-Santos J, Gray HL, Petrosko P, Rawe VY, Navara CS, Simerly CR, Schatten GP. The expression of mitochondrial DNA transcription factors during early cardiomyocyte in vitro differentiation from human embryonic stem cells. Cloning Stem Cells. 2005;7:141–153. doi: 10.1089/clo.2005.7.141. [DOI] [PubMed] [Google Scholar]
- 112.Shyh-Chang N, Daley GQ, Cantley LC. Stem cell metabolism in tissue development and aging. Development. 2013;140:2535–2547. doi: 10.1242/dev.091777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lonergan T, Brenner C, Bavister B. Differentiation-related changes in mitochondrial properties as indicators of stem cell competence. J Cell Physiol. 2006;208:149–153. doi: 10.1002/jcp.20641. [DOI] [PubMed] [Google Scholar]
- 114.Suhr ST, Chang EA, Tjong J, Alcasid N, Perkins GA, Goissis MD, Ellisman MH, Perez GI, Cibelli JB. Mitochondrial rejuvenation after induced pluripotency. PLoS One. 2010;5:e14095. doi: 10.1371/journal.pone.0014095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Folmes CD, Nelson TJ, Martinez-Fernandez A, Arrell DK, Lindor JZ, Dzeja PP, Ikeda Y, Perez-Terzic C, Terzic A. Somatic oxidative bioenergetics transitions into pluripotency-dependent glycolysis to facilitate nuclear reprogramming. Cell Metab. 2011;14:264–271. doi: 10.1016/j.cmet.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zeuschner D, Mildner K, Zaehres H, Scholer HR. Induced pluripotent stem cells at nanoscale. Stem Cells Dev. 2010;19:615–620. doi: 10.1089/scd.2009.0159. [DOI] [PubMed] [Google Scholar]
- 117.Strauss M, Hofhaus G, Schroder RR, Kuhlbrandt W. Dimer ribbons of ATP synthase shape the inner mitochondrial membrane. EMBO J. 2008;27:1154–1160. doi: 10.1038/emboj.2008.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Prigione A, Fauler B, Lurz R, Lehrach H, Adjaye J. The senescence-related mitochondrial/oxidative stress pathway is repressed in human induced pluripotent stem cells. Stem Cells. 2010;28:721–733. doi: 10.1002/stem.404. [DOI] [PubMed] [Google Scholar]
- 119.Kondoh H, Lleonart ME, Nakashima Y, Yokode M, Tanaka M, Bernard D, Gil J, Beach D. A high glycolytic flux supports the proliferative potential of murine embryonic stem cells. Antioxid Redox Signal. 2007;9:293–299. doi: 10.1089/ars.2006.1467. [DOI] [PubMed] [Google Scholar]
- 120.Chung S, Arrell DK, Faustino RS, Terzic A, Dzeja PP. Glycolytic network restructuring integral to the energetics of embryonic stem cell cardiac differentiation. J Mol Cell Cardiol. 2010;48:725–734. doi: 10.1016/j.yjmcc.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Prigione A, Adjaye J. Modulation of mitochondrial biogenesis and bioenergetic metabolism upon in vitro and in vivo differentiation of human ES and iPS cells. Int J Dev Biol. 2010;54:1729–1741. doi: 10.1387/ijdb.103198ap. [DOI] [PubMed] [Google Scholar]
- 122.Varum S, Rodrigues AS, Moura MB, Momcilovic O, Easley CA, 4th, Ramalho-Santos J, Van Houten B, Schatten G. Energy metabolism in human pluripotent stem cells and their differentiated counterparts. PLoS One. 2011;6:e20914. doi: 10.1371/journal.pone.0020914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Prigione A, Rohwer N, Hoffmann S, Mlody B, Drews K, Bukowiecki R, Blumlein K, Wanker EE, Ralser M, Cramer T, Adjaye J. HIF1alpha modulates cell fate reprogramming through early glycolytic shift and upregulation of PDK1-3 and PKM2. Stem Cells. 2014;32:364–376. doi: 10.1002/stem.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhang J, Khvorostov I, Hong JS, Oktay Y, Vergnes L, Nuebel E, Wahjudi PN, Setoguchi K, Wang G, Do A, Jung HJ, McCaffery JM, Kurland IJ, Reue K, Lee WN, Koehler CM, Teitell MA. UCP2 regulates energy metabolism and differentiation potential of human pluripotent stem cells. EMBO J. 2011;30:4860–4873. doi: 10.1038/emboj.2011.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Panopoulos AD, Yanes O, Ruiz S, Kida YS, Diep D, Tautenhahn R, Herrerias A, Batchelder EM, Plongthongkum N, Lutz M, Berggren WT, Zhang K, Evans RM, Siuzdak G, Izpisua Belmonte JC. The metabolome of induced pluripotent stem cells reveals metabolic changes occurring in somatic cell reprogramming. Cell Res. 2012;22:168–177. doi: 10.1038/cr.2011.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mathieu J, Zhou W, Xing Y, Sperber H, Ferreccio A, Agoston Z, Kuppusamy KT, Moon RT, Ruohola-Baker H. Hypoxia-inducible factors have distinct and stage-specific roles during reprogramming of human cells to pluripotency. Cell Stem Cell. 2014;14:592–605. doi: 10.1016/j.stem.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Armstrong L, Tilgner K, Saretzki G, Atkinson SP, Stojkovic M, Moreno R, Przyborski S, Lako M. Human induced pluripotent stem cell lines show stress defense mechanisms and mitochondrial regulation similar to those of human embryonic stem cells. Stem Cells. 2010;28:661–673. doi: 10.1002/stem.307. [DOI] [PubMed] [Google Scholar]
- 128.Chung S, Dzeja PP, Faustino RS, Perez-Terzic C, Behfar A, Terzic A. Mitochondrial oxidative metabolism is required for the cardiac differentiation of stem cells. Nat Clin Pract Cardiovasc Med. 2007;4:S60–S67. doi: 10.1038/ncpcardio0766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Prigione A, Hossini AM, Lichtner B, Serin A, Fauler B, Megges M, Lurz R, Lehrach H, Makrantonaki E, Zouboulis CC, Adjaye J. Mitochondrial-associated cell death mechanisms are reset to an embryonic-like state in aged donor-derived iPS cells harboring chromosomal aberrations. PLoS ONE. 2011;6:e27352. doi: 10.1371/journal.pone.0027352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Folmes CD, Nelson TJ, Dzeja PP, Terzic A. Energy metabolism plasticity enables stemness programs. Ann N Y Acad Sci. 2012;1254:82–89. doi: 10.1111/j.1749-6632.2012.06487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Folmes CD, Dzeja PP, Nelson TJ, Terzic A. Metabolic plasticity in stem cell homeostasis and differentiation. Cell Stem Cell. 2012;11:596–606. doi: 10.1016/j.stem.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mattenberger Y, James DI, Martinou JC. Fusion of mitochondria in mammalian cells is dependent on the mitochondrial inner membrane potential and independent of microtubules or actin. FEBS Lett. 2003;538:53–59. doi: 10.1016/s0014-5793(03)00124-8. [DOI] [PubMed] [Google Scholar]
- 133.Bukowiecki R, Adjaye J, Prigione A. Mitochondrial function in pluripotent stem cells and cellular reprogramming. Gerontology. 2014;60:174–182. doi: 10.1159/000355050. [DOI] [PubMed] [Google Scholar]
- 134.Prigione A, Lichtner B, Kuhl H, Struys EA, Wamelink M, Lehrach H, Ralser M, Timmermann B, Adjaye J. Human induced pluripotent stem cells harbor homoplasmic and heteroplasmic mitochondrial DNA mutations while maintaining human embryonic stem cell-like metabolic reprogramming. Stem Cells. 2011;29:1338–1348. doi: 10.1002/stem.683. [DOI] [PubMed] [Google Scholar]
- 135.Liu JC, Lerou PH, Lahav G. Stem cells: balancing resistance and sensitivity to DNA damage. Trends Cell Biol. 2014;24:268–274. doi: 10.1016/j.tcb.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Park Y, Gerson SL. DNA repair defects in stem cell function and aging. Annu Rev Med. 2005;56:495–508. doi: 10.1146/annurev.med.56.082103.104546. [DOI] [PubMed] [Google Scholar]
- 137.Moon JS, Kim HE, Koh E, Park SH, Jin WJ, Park BW, Park SW, Kim KS. Kruppel-like factor 4 (KLF4) activates the transcription of the gene for the platelet isoform of phosphofructokinase (PFKP) in breast cancer. J Biol Chem. 2011;286:23808–23816. doi: 10.1074/jbc.M111.236737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Dang CV. MYC on the path to cancer. Cell. 2012;149:22–35. doi: 10.1016/j.cell.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Shyh-Chang N, Daley GQ. Lin28: primal regulator of growth and metabolism in stem cells. Cell Stem Cell. 2013;12:395–406. doi: 10.1016/j.stem.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ito K, Suda T. Metabolic requirements for the maintenance of self-renewing stem cells. Nat Rev Mol Cell Biol. 2014;15:243–256. doi: 10.1038/nrm3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Covello KL, Kehler J, Yu H, Gordan JD, Arsham AM, Hu CJ, Labosky PA, Simon MC, Keith B. HIF-2alpha regulates Oct-4: effects of hypoxia on stem cell function, embryonic development, and tumor growth. Genes Dev. 2006;20:557–570. doi: 10.1101/gad.1399906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lee J, Kim HK, Han YM, Kim J. Pyruvate kinase isozyme type M2 (PKM2) interacts and cooperates with Oct-4 in regulating transcription. Int J Biochem Cell Biol. 2008;40:1043–1054. doi: 10.1016/j.biocel.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 143.Kelly RD, Sumer H, McKenzie M, Facucho-Oliveira J, Trounce IA, Verma PJ, St John JC. The effects of nuclear reprogramming on mitochondrial DNA replication. Stem Cell Rev. 2013;9:1–15. doi: 10.1007/s12015-011-9318-7. [DOI] [PubMed] [Google Scholar]
- 144.Shyh-Chang N, Zheng Y, Locasale JW, Cantley LC. Human pluripotent stem cells decouple respiration from energy production. EMBO J. 2011;30:4851–4852. doi: 10.1038/emboj.2011.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Shyh-Chang N, Locasale JW, Lyssiotis CA, Zheng Y, Teo RY, Ratanasirintrawoot S, Zhang J, Onder T, Unternaehrer JJ, Zhu H, Asara JM, Daley GQ, Cantley LC. Influence of threonine metabolism on S-adenosylmethionine and histone methylation. Science. 2013;339:222–226. doi: 10.1126/science.1226603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Polo JM, Anderssen E, Walsh RM, Schwarz BA, Nefzger CM, Lim SM, Borkent M, Apostolou E, Alaei S, Cloutier J, Bar-Nur O, Cheloufi S, Stadtfeld M, Figueroa ME, Robinton D, Natesan S, Melnick A, Zhu J, Ramaswamy S, Hochedlinger K. A molecular roadmap of reprogramming somatic cells into iPS cells. Cell. 2012;151:1617–1632. doi: 10.1016/j.cell.2012.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ezashi T, Das P, Roberts RM. Low O2 tensions and the prevention of differentiation of hES cells. Proc Natl Acad Sci USA. 2005;102:4783–4788. doi: 10.1073/pnas.0501283102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Yoshida Y, Takahashi K, Okita K, Ichisaka T, Yamanaka S. Hypoxia enhances the generation of induced pluripotent stem cells. Cell Stem Cell. 2009;5:237–241. doi: 10.1016/j.stem.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 149.Forristal CE, Wright KL, Hanley NA, Oreffo RO, Houghton FD. Hypoxia inducible factors regulate pluripotency and proliferation in human embryonic stem cells cultured at reduced oxygen tensions. Reproduction. 2010;139:85–97. doi: 10.1530/REP-09-0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wang S, Xia P, Ye B, Huang G, Liu J, Fan Z. Transient activation of autophagy via Sox2-mediated suppression of mTOR is an important early step in reprogramming to pluripotency. Cell Stem Cell. 2013;13:617–625. doi: 10.1016/j.stem.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 151.Vazquez-Martin A, Vellon L, Quiros PM, Cufi S, Ruiz de Galarreta E, Oliveras-Ferraros C, Martin AG, Martin-Castillo B, Lopez-Otin C, Menendez JA. Activation of AMP-activated protein kinase (AMPK) provides a metabolic barrier to reprogramming somatic cells into stem cells. Cell Cycle. 2012;11:974–989. doi: 10.4161/cc.11.5.19450. [DOI] [PubMed] [Google Scholar]
- 152.Vazquez-Martin A, Cufi S, Corominas-Faja B, Oliveras-Ferraros C, Vellon L, Menendez JA. Mitochondrial fusion by pharmacological manipulation impedes somatic cell reprogramming to pluripotency: new insight into the role of mitophagy in cell stemness. Aging. 2012;4:393–401. doi: 10.18632/aging.100465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Varum S, Momcilovic O, Castro C, Ben-Yehudah A, Ramalho-Santos J, Navara CS. Enhancement of human embryonic stem cell pluripotency through inhibition of the mitochondrial respiratory chain. Stem Cell Res. 2009;3:142–156. doi: 10.1016/j.scr.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Chen T, Shen L, Yu J, Wan H, Guo A, Chen J, Long Y, Zhao J, Pei G. Rapamycin and other longevity-promoting compounds enhance the generation of mouse induced pluripotent stem cells. Aging Cell. 2011;10:908–911. doi: 10.1111/j.1474-9726.2011.00722.x. [DOI] [PubMed] [Google Scholar]
- 155.Chen J, Guo L, Zhang L, Wu H, Yang J, Liu H, Wang X, Hu X, Gu T, Zhou Z, Liu J, Liu J, Wu H, Mao SQ, Mo K, Li Y, Lai K, Qi J, Yao H, Pan G, Xu GL, Pei D. Vitamin C modulates TET1 function during somatic cell reprogramming. Nat Genet. 2013;45:1504–1509. doi: 10.1038/ng.2807. [DOI] [PubMed] [Google Scholar]
- 156.Ji J, Sharma V, Qi S, Guarch ME, Zhao P, Luo Z, Fan W, Wang Y, Mbabaali F, Neculai D, Esteban MA, McPherson JD, Batada NN. Antioxidant supplementation reduces genomic aberrations in human induced pluripotent stem cells. Stem Cell Reports. 2014;2:44–51. doi: 10.1016/j.stemcr.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Prigione A, Adjaye J. A mitochondrial strategy for safeguarding the reprogrammed genome. Cell Regen. 2014;3:5. doi: 10.1186/2045-9769-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Stanley IA, Ribeiro SM, Gimenez-Cassina A, Norberg E, Danial NN. Changing appetites: the adaptive advantages of fuel choice. Trends Cell Biol. 2014;24:118–127. doi: 10.1016/j.tcb.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zhang X, Yalcin S, Lee DF, Yeh TY, Lee SM, Su J, Mungamuri SK, Rimmele P, Kennedy M, Sellers R, Landthaler M, Tuschl T, Chi NW, Lemischka I, Keller G, Ghaffari S. FOXO1 is an essential regulator of pluripotency in human embryonic stem cells. Nat Cell Biol. 2011;13:1092–1099. doi: 10.1038/ncb2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Vilchez D, Boyer L, Morantte I, Lutz M, Merkwirth C, Joyce D, Spencer B, Page L, Masliah E, Berggren WT, Gage FH, Dillin A. Increased proteasome activity in human embryonic stem cells is regulated by PSMD11. Nature. 2012;489:304–308. doi: 10.1038/nature11468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Schieke SM, Ma M, Cao L, McCoy JP, Jr, Liu C, Hensel NF, Barrett AJ, Boehm M, Finkel T. Mitochondrial metabolism modulates differentiation and teratoma formation capacity in mouse embryonic stem cells. J Biol Chem. 2008;283:28506–28512. doi: 10.1074/jbc.M802763200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zhou J, Su P, Wang L, Chen J, Zimmermann M, Genbacev O, Afonja O, Horne MC, Tanaka T, Duan E, Fisher SJ, Liao J, Chen J, Wang F. mTOR supports long-term self-renewal and suppresses mesoderm and endoderm activities of human embryonic stem cells. Proc Natl Acad Sci USA. 2009;106:7840–7845. doi: 10.1073/pnas.0901854106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Easley CA, 4th, Ben-Yehudah A, Redinger CJ, Oliver SL, Varum ST, Eisinger VM, Carlisle DL, Donovan PJ, Schatten GP. mTOR-mediated activation of p70 S6K induces differentiation of pluripotent human embryonic stem cells. Cell Reprogram. 2010;12:263–273. doi: 10.1089/cell.2010.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sampath P, Pritchard DK, Pabon L, Reinecke H, Schwartz SM, Morris DR, Murry CE. A hierarchical network controls protein translation during murine embryonic stem cell self-renewal and differentiation. Cell Stem Cell. 2008;2:448–460. doi: 10.1016/j.stem.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 165.He J, Kang L, Wu T, Zhang J, Wang H, Gao H, Zhang Y, Huang B, Liu W, Kou Z, Zhang H, Gao S. An elaborate regulation of Mammalian target of rapamycin activity is required for somatic cell reprogramming induced by defined transcription factors. Stem Cells Dev. 2012;21:2630–2641. doi: 10.1089/scd.2012.0015. [DOI] [PubMed] [Google Scholar]
- 166.Ito K, Carracedo A, Weiss D, Arai F, Ala U, Avigan DE, Schafer ZT, Evans RM, Suda T, Lee CH, Pandolfi PP. A PML-PPAR-delta pathway for fatty acid oxidation regulates hematopoietic stem cell maintenance. Nat Med. 2012;18:1350–1358. doi: 10.1038/nm.2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Knobloch M, Braun SM, Zurkirchen L, von Schoultz C, Zamboni N, Arauzo-Bravo MJ, Kovacs WJ, Karalay O, Suter U, Machado RA, Roccio M, Lutolf MP, Semenkovich CF, Jessberger S. Metabolic control of adult neural stem cell activity by Fasn-dependent lipogenesis. Nature. 2013;493:226–230. doi: 10.1038/nature11689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Garcia-Gonzalo FR, Izpisua Belmonte JC. Albumin-associated lipids regulate human embryonic stem cell self-renewal. PLoS One. 2008;3:e1384. doi: 10.1371/journal.pone.0001384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Inniss K, Moore H. Mediation of apoptosis and proliferation of human embryonic stem cells by sphingosine-1-phosphate. Stem Cells Dev. 2006;15:789–796. doi: 10.1089/scd.2006.15.789. [DOI] [PubMed] [Google Scholar]
- 170.Avery K, Avery S, Shepherd J, Heath PR, Moore H. Sphingosine-1-phosphate mediates transcriptional regulation of key targets associated with survival, proliferation, and pluripotency in human embryonic stem cells. Stem Cells Dev. 2008;17:1195–1205. doi: 10.1089/scd.2008.0063. [DOI] [PubMed] [Google Scholar]
- 171.Ben-David U, Gan QF, Golan-Lev T, Arora P, Yanuka O, Oren YS, Leikin-Frenkel A, Graf M, Garippa R, Boehringer M, Gromo G, Benvenisty N. Selective elimination of human pluripotent stem cells by an oleate synthesis inhibitor discovered in a high-throughput screen. Cell Stem Cell. 2013;12:167–179. doi: 10.1016/j.stem.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 172.Vazquez-Martin A, Corominas-Faja B, Cufi S, Vellon L, Oliveras-Ferraros C, Menendez OJ, Joven J, Lupu R, Menendez JA. The mitochondrial H(+)-ATP synthase and the lipogenic switch: new core components of metabolic reprogramming in induced pluripotent stem (iPS) cells. Cell Cycle. 2013;12:207–218. doi: 10.4161/cc.23352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hansson J, Rafiee MR, Reiland S, Polo JM, Gehring J, Okawa S, Huber W, Hochedlinger K, Krijgsveld J. Highly coordinated proteome dynamics during reprogramming of somatic cells to pluripotency. Cell Rep. 2012;2:1579–1592. doi: 10.1016/j.celrep.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Buganim Y, Faddah DA, Jaenisch R. Mechanisms and models of somatic cell reprogramming. Nat Rev Genet. 2013;14:427–439. doi: 10.1038/nrg3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Yun J, Johnson JL, Hanigan CL, Locasale JW. Interactions between epigenetics and metabolism in cancers. Front Oncol. 2012;2:163. doi: 10.3389/fonc.2012.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wellen KE, Hatzivassiliou G, Sachdeva UM, Bui TV, Cross JR, Thompson CB. ATP-citrate lyase links cellular metabolism to histone acetylation. Science. 2009;324:1076–1080. doi: 10.1126/science.1164097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kim SC, Sprung R, Chen Y, Xu Y, Ball H, Pei J, Cheng T, Kho Y, Xiao H, Xiao L, Grishin NV, White M, Yang XJ, Zhao Y. Substrate and functional diversity of lysine acetylation revealed by a proteomics survey. Mol Cell. 2006;23:607–618. doi: 10.1016/j.molcel.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 178.Jang H, Kim TW, Yoon S, Choi SY, Kang TW, Kim SY, Kwon YW, Cho EJ, Youn HD. O-GlcNAc regulates pluripotency and reprogramming by directly acting on core components of the pluripotency network. Cell Stem Cell. 2012;11:62–74. doi: 10.1016/j.stem.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 179.Wang J, Alexander P, Wu L, Hammer R, Cleaver O, McKnight SL. Dependence of mouse embryonic stem cells on threonine catabolism. Science. 2009;325:435–439. doi: 10.1126/science.1173288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Han C, Gu H, Wang J, Lu W, Mei Y, Wu M. Regulation of l-threonine dehydrogenase in somatic cell reprogramming. Stem Cells. 2013;31:953–965. doi: 10.1002/stem.1335. [DOI] [PubMed] [Google Scholar]
- 181.Edgar AJ. The human l-threonine 3-dehydrogenase gene is an expressed pseudogene. BMC Genet. 2002;3:18. doi: 10.1186/1471-2156-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Shiraki N, Shiraki Y, Tsuyama T, Obata F, Miura M, Nagae G, Aburatani H, Kume K, Endo F, Kume S. Methionine Metabolism Regulates Maintenance and Differentiation of Human Pluripotent Stem Cells. Cell Metab. 2014;19:780–794. doi: 10.1016/j.cmet.2014.03.017. [DOI] [PubMed] [Google Scholar]
- 183.Hitchler MJ, Domann FE. Redox regulation of the epigenetic landscape in cancer: a role for metabolic reprogramming in remodeling the epigenome. Free Radic Biol Med. 2012;53:2178–2187. doi: 10.1016/j.freeradbiomed.2012.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Pajares MA, Duran C, Corrales F, Pliego MM, Mato JM. Modulation of rat liver S-adenosylmethionine synthetase activity by glutathione. J Biol Chem. 1992;267:17598–17605. [PubMed] [Google Scholar]
- 185.Hanover JA, Krause MW, Love DC. Bittersweet memories: linking metabolism to epigenetics through O-GlcNAcylation. Nat Rev Mol Cell Biol. 2012;13:312–321. doi: 10.1038/nrm3334. [DOI] [PubMed] [Google Scholar]
- 186.Yuan HX, Xiong Y, Guan KL. Nutrient sensing, metabolism, and cell growth control. Mol Cell. 2013;49:379–387. doi: 10.1016/j.molcel.2013.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Carey BW, Finley LW, Cross JR, Allis CD and Thompson CB (2014) Intracellular alpha-ketoglutarate maintains the pluripotency of embryonic stem cells. Nature (in press) [DOI] [PMC free article] [PubMed]
- 188.Cho YM, Kwon S, Pak YK, Seol HW, Choi YM, Park do J, Park KS, Lee HK. Dynamic changes in mitochondrial biogenesis and antioxidant enzymes during the spontaneous differentiation of human embryonic stem cells. Biochem Biophys Res Commun. 2006;348:1472–1478. doi: 10.1016/j.bbrc.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 189.Yanes O, Clark J, Wong DM, Patti GJ, Sanchez-Ruiz A, Benton HP, Trauger SA, Desponts C, Ding S, Siuzdak G. Metabolic oxidation regulates embryonic stem cell differentiation. Nat Chem Biol. 2010;6:411–417. doi: 10.1038/nchembio.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Mitra K, Wunder C, Roysam B, Lin G, Lippincott-Schwartz J. A hyperfused mitochondrial state achieved at G1-S regulates cyclin E buildup and entry into S phase. Proc Natl Acad Sci USA. 2009;106:11960–11965. doi: 10.1073/pnas.0904875106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Mitra K. Mitochondrial fission-fusion as an emerging key regulator of cell proliferation and differentiation. BioEssays. 2013;35:955–964. doi: 10.1002/bies.201300011. [DOI] [PubMed] [Google Scholar]
- 192.Prowse AB, Chong F, Elliott DA, Elefanty AG, Stanley EG, Gray PP, Munro TP, Osborne GW. Analysis of mitochondrial function and localisation during human embryonic stem cell differentiation in vitro. PLoS One. 2012;7:e52214. doi: 10.1371/journal.pone.0052214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Filosa S, Fico A, Paglialunga F, Balestrieri M, Crooke A, Verde P, Abrescia P, Bautista JM, Martini G. Failure to increase glucose consumption through the pentose-phosphate pathway results in the death of glucose-6-phosphate dehydrogenase gene-deleted mouse embryonic stem cells subjected to oxidative stress. Biochem J. 2003;370:935–943. doi: 10.1042/BJ20021614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Manganelli G, Fico A, Masullo U, Pizzolongo F, Cimmino A, Filosa S. Modulation of the pentose phosphate pathway induces endodermal differentiation in embryonic stem cells. PLoS One. 2012;7:e29321. doi: 10.1371/journal.pone.0029321. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 195.Kowno M, Watanabe-Susaki K, Ishimine H, Komazaki S, Enomoto K, Seki Y, Wang YY, Ishigaki Y, Ninomiya N, Noguchi TA, Kokubu Y, Ohnishi K, Nakajima Y, Kato K, Intoh A, Takada H, Yamakawa N, Wang PC, Asashima M, Kurisaki A. Prohibitin 2 regulates the proliferation and lineage-specific differentiation of mouse embryonic stem cells in mitochondria. PLoS One. 2014;9:e81552. doi: 10.1371/journal.pone.0081552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Cho SW, Park JS, Heo HJ, Park SW, Song S, Kim I, Han YM, Yamashita JK, Youm JB, Han J, Koh GY. Dual modulation of the mitochondrial permeability transition pore and redox signaling synergistically promotes cardiomyocyte differentiation from pluripotent stem cells. J Am Heart Assoc. 2014;3:e000693. doi: 10.1161/JAHA.113.000693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Greco V, Guo S. Compartmentalized organization: a common and required feature of stem cell niches? Development. 2010;137:1586–1594. doi: 10.1242/dev.041103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Suda T, Takubo K, Semenza GL. Metabolic regulation of hematopoietic stem cells in the hypoxic niche. Cell Stem Cell. 2011;9:298–310. doi: 10.1016/j.stem.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 199.Chen CT, Shih YR, Kuo TK, Lee OK, Wei YH. Coordinated changes of mitochondrial biogenesis and antioxidant enzymes during osteogenic differentiation of human mesenchymal stem cells. Stem Cells. 2008;26:960–968. doi: 10.1634/stemcells.2007-0509. [DOI] [PubMed] [Google Scholar]
- 200.Tothova Z, Kollipara R, Huntly BJ, Lee BH, Castrillon DH, Cullen DE, McDowell EP, Lazo-Kallanian S, Williams IR, Sears C, Armstrong SA, Passegue E, DePinho RA, Gilliland DG. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell. 2007;128:325–339. doi: 10.1016/j.cell.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 201.Yahata T, Takanashi T, Muguruma Y, Ibrahim AA, Matsuzawa H, Uno T, Sheng Y, Onizuka M, Ito M, Kato S, Ando K. Accumulation of oxidative DNA damage restricts the self-renewal capacity of human hematopoietic stem cells. Blood. 2011;118:2941–2950. doi: 10.1182/blood-2011-01-330050. [DOI] [PubMed] [Google Scholar]
- 202.Taniguchi Ishikawa E, Gonzalez-Nieto D, Ghiaur G, Dunn SK, Ficker AM, Murali B, Madhu M, Gutstein DE, Fishman GI, Barrio LC, Cancelas JA. Connexin-43 prevents hematopoietic stem cell senescence through transfer of reactive oxygen species to bone marrow stromal cells. Proc Natl Acad Sci USA. 2012;109:9071–9076. doi: 10.1073/pnas.1120358109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Simsek T, Kocabas F, Zheng J, Deberardinis RJ, Mahmoud AI, Olson EN, Schneider JW, Zhang CC, Sadek HA. The distinct metabolic profile of hematopoietic stem cells reflects their location in a hypoxic niche. Cell Stem Cell. 2010;7:380–390. doi: 10.1016/j.stem.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Takubo K, Nagamatsu G, Kobayashi CI, Nakamura-Ishizu A, Kobayashi H, Ikeda E, Goda N, Rahimi Y, Johnson RS, Soga T, Hirao A, Suematsu M, Suda T. Regulation of glycolysis by Pdk functions as a metabolic checkpoint for cell cycle quiescence in hematopoietic stem cells. Cell Stem Cell. 2013;12:49–61. doi: 10.1016/j.stem.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Pattappa G, Thorpe SD, Jegard NC, Heywood HK, de Bruijn JD, Lee DA. Continuous and uninterrupted oxygen tension influences the colony formation and oxidative metabolism of human mesenchymal stem cells. Tissue Eng Part C Methods. 2013;19:68–79. doi: 10.1089/ten.TEC.2011.0734. [DOI] [PubMed] [Google Scholar]
- 206.Geissler S, Textor M, Kuhnisch J, Konnig D, Klein O, Ode A, Pfitzner T, Adjaye J, Kasper G, Duda GN. Functional comparison of chronological and in vitro aging: differential role of the cytoskeleton and mitochondria in mesenchymal stromal cells. PLoS One. 2012;7:e52700. doi: 10.1371/journal.pone.0052700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Mohyeldin A, Garzon-Muvdi T, Quinones-Hinojosa A. Oxygen in stem cell biology: a critical component of the stem cell niche. Cell Stem Cell. 2010;7:150–161. doi: 10.1016/j.stem.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 208.Noble M, Mayer-Proschel M, Proschel C. Redox regulation of precursor cell function: insights and paradoxes. Antioxid Redox Signal. 2005;7:1456–1467. doi: 10.1089/ars.2005.7.1456. [DOI] [PubMed] [Google Scholar]
- 209.Renault VM, Rafalski VA, Morgan AA, Salih DA, Brett JO, Webb AE, Villeda SA, Thekkat PU, Guillerey C, Denko NC, Palmer TD, Butte AJ, Brunet A. FoxO3 regulates neural stem cell homeostasis. Cell Stem Cell. 2009;5:527–539. doi: 10.1016/j.stem.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Allen DM, van Praag H, Ray J, Weaver Z, Winrow CJ, Carter TA, Braquet R, Harrington E, Ried T, Brown KD, Gage FH, Barlow C. Ataxia telangiectasia mutated is essential during adult neurogenesis. Genes Dev. 2001;15:554–566. doi: 10.1101/gad.869001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Groszer M, Erickson R, Scripture-Adams DD, Lesche R, Trumpp A, Zack JA, Kornblum HI, Liu X, Wu H. Negative regulation of neural stem/progenitor cell proliferation by the Pten tumor suppressor gene in vivo. Science. 2001;294:2186–2189. doi: 10.1126/science.1065518. [DOI] [PubMed] [Google Scholar]
- 212.Birket MJ, Orr AL, Gerencser AA, Madden DT, Vitelli C, Swistowski A, Brand MD, Zeng X. A reduction in ATP demand and mitochondrial activity with neural differentiation of human embryonic stem cells. J Cell Sci. 2011;124:348–358. doi: 10.1242/jcs.072272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Candelario KM, Shuttleworth CW, Cunningham LA. Neural stem/progenitor cells display a low requirement for oxidative metabolism independent of hypoxia inducible factor-1alpha expression. J Neurochem. 2013;125:420–429. doi: 10.1111/jnc.12204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Gershon TR, Crowther AJ, Tikunov A, Garcia I, Annis R, Yuan H, Miller CR, Macdonald J, Olson J, Deshmukh M. Hexokinase-2-mediated aerobic glycolysis is integral to cerebellar neurogenesis and pathogenesis of medulloblastoma. Cancer Metab. 2013;23:2. doi: 10.1186/2049-3002-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Lee Y, Oh SB, Park HR, Kim HS, Kim MS, Lee J. Selective impairment on the proliferation of neural progenitor cells by oxidative phosphorylation disruption. Neurosci Lett. 2013;535:134–139. doi: 10.1016/j.neulet.2012.12.050. [DOI] [PubMed] [Google Scholar]
- 216.Homem CC, Steinmann V, Burkard TR, Jais A, Esterbauer H, Knoblich JA. Ecdysone and mediator change energy metabolism to terminate proliferation in Drosophila neural stem cells. Cell. 2014;158:874–888. doi: 10.1016/j.cell.2014.06.024. [DOI] [PubMed] [Google Scholar]
- 217.Sola S, Morgado AL, Rodrigues CM. Death receptors and mitochondria: two prime triggers of neural apoptosis and differentiation. Biochim Biophys Acta. 2013;1830:2160–2166. doi: 10.1016/j.bbagen.2012.09.021. [DOI] [PubMed] [Google Scholar]
- 218.Mootha VK, Bunkenborg J, Olsen JV, Hjerrild M, Wisniewski JR, Stahl E, Bolouri MS, Ray HN, Sihag S, Kamal M, Patterson N, Lander ES, Mann M. Integrated analysis of protein composition, tissue diversity, and gene regulation in mouse mitochondria. Cell. 2003;115:629–640. doi: 10.1016/s0092-8674(03)00926-7. [DOI] [PubMed] [Google Scholar]
- 219.Pera MF. Stem cells: The dark side of induced pluripotency. Nature. 2011;471:46–47. doi: 10.1038/471046a. [DOI] [PubMed] [Google Scholar]
- 220.Agathocleous M, Harris WA. Metabolism in physiological cell proliferation and differentiation. Trends Cell Biol. 2013;23:484–492. doi: 10.1016/j.tcb.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 221.Wapinski OL, Vierbuchen T, Qu K, Lee QY, Chanda S, Fuentes DR, Giresi PG, Ng YH, Marro S, Neff NF, Drechsel D, Martynoga B, Castro DS, Webb AE, Sudhof TC, Brunet A, Guillemot F, Chang HY, Wernig M. Hierarchical mechanisms for direct reprogramming of fibroblasts to neurons. Cell. 2013;155:621–635. doi: 10.1016/j.cell.2013.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Graf T. Historical origins of transdifferentiation and reprogramming. Cell Stem Cell. 2011;9:504–516. doi: 10.1016/j.stem.2011.11.012. [DOI] [PubMed] [Google Scholar]