Abstract
Influenza is a serious respiratory disease among immunocompromised individuals, such as the elderly, and its prevention is an urgent social issue. Influenza viruses rely on neuraminidase (NA) activity to release progeny viruses from infected cells and spreading the infection. NA is, therefore, an important target of anti-influenza drugs. A causal relationship between bacteria and influenza virus infection has not yet been established, however, a positive correlation between them has been reported. Thus, in this study, we examined the biological effects of oral mitis group streptococci, which are predominant constituents of human oral florae, on the release of influenza viruses. Among them, Streptococcus oralis ATCC 10557 and Streptococcus mitis ATCC 6249 were found to exhibit NA activity and their culture supernatants promoted the release of influenza virus and cell-to-cell spread of the infection. In addition, culture supernatants of these NA-producing oral bacteria increased viral M1 protein expression levels and cellular ERK activation. These effects were not observed with culture supernatants of Streptococcus sanguinis ATCC 10556 which lacks the ability to produce NA. Although the NA inhibitor zanamivir suppressed the release of progeny viruses from the infected cells, the viral release was restored upon the addition of culture supernatants of NA-producing S. oralis ATCC 10557 or S. mitis ATCC 6249. These findings suggest that an increase in the number of NA-producing oral bacteria could elevate the risk of and exacerbate the influenza infection, hampering the efficacy of viral NA inhibitor drugs.
Keywords: Influenza virus, Oral bacteria, Neuraminidase, Zanamivir
Introduction
Influenza virus infection, including seasonal and pandemic outbreaks, is a public health problem worldwide. Seasonal influenza outbreaks annually lead to 3–5 million cases of severe respiratory disease among immunocompromised individuals, such as the elderly and children, and they are annually responsible for up to 500,000 deaths worldwide [1]. More recently, an estimated 570,000 deaths were associated with the 2009 pandemic H1N1 [A(H1N1)pdm09] virus [2]. Thus, the development of effective methods to prevent seasonal and pandemic outbreaks is urgent.
Influenza type A and B viruses have only 2 of the 8 RNA segments of the genome encoding major surface proteins, hemagglutinins (HAs) and neuraminidases (NAs) [3–5]. At the initial steps in the infection, HAs play a key role, binding to sialic acid receptors on the cell surface and facilitating the entry of the influenza virus into host cells [4]. In contrast, NA is critical at the final stage of infection, and it thus represents an important target for structure-based anti-influenza drugs. After viral replication, NA is essential for further spreading the infection as it mediates the release of progeny viruses from the infected cells by removing terminally linked sialic acid, the major viral receptor determinant, from glycoconjugates [4]. NA inhibitors, such as oseltamivir and zanamivir, which are commercially available as Tamiflu and Relenza, respectively, and are currently licensed worldwide for therapeutic and preventive usage, are known to effectively prevent viral spread [5].
Influenza virus infection in humans usually causes only mild to moderate symptoms; however, clinical and epidemiological studies suggest that viral–bacterial co-infections can contribute to the progression and severity of influenza virus infections during seasonal and pandemic outbreaks [6–11]. It is known that viral–bacterial pneumonia and secondary bacterial pneumonia due to concomitant or subsequent infection with bacteria, respectively, influence the morbidity and mortality of influenza virus infections [7]. In fact, more than 90 % of those who died from infection caused by the 1918 Spanish influenza virus showed symptoms of severe bacterial pneumonia [8]. In addition, in fatal A(H1N1)pdm09 cases from the 2009 pandemic, an association between bacterial lung infections and increased mortality or developing complications was also reported [9, 10]. Empirical evidence suggests that proteases derived from Staphylococcus aureus strains influence the outcome of influenza virus infection by facilitating the cleavage of HA [11]. Thus, elucidating the molecular mechanisms involved in the microbial interaction between influenza virus and bacteria during the progression of influenza virus infection is crucial for developing novel therapeutic strategies and preventive measures to contain the infection.
By the way, although we have been focussing on the viral–bacterial co-infections during influenza virus infections, bacterial co-infection does not necessarily seem to a prerequisite for the viral–bacterial interaction. More than 700 bacterial species have been identified from the human oral cavity [12, 13]. The attached bacteria on the oral surfaces constitute indigenous biofilms, which are known as oral microflorae, such as dental plaque and tongue plaque. Two major endogenous oral infections, dental caries and periodontal diseases, are eventually related to dental plaque accumulation [13]. On the other hand, poor oral hygiene is often correlated with the occurrence of several systemic diseases such as respiratory infections, diabetes and cardiovascular diseases [14–16]. We recently reported that periodontopathic bacteria such as Porphyromonas gingivalis and Fusobacterium nucleatum could induce the reactivation of latent human immunodeficiency virus-1 and Epstein–Barr virus [17–19]. These observations suggest a possible role of oral bacteria in contributing to various systemic diseases.
A causal relationship between bacteria of oral florae and influenza virus infection has not yet been established, however, a positive correlation between them has been reported. In comparison with untreated mice, P. gingivalis-treated individuals reportedly show a reduced ability to clear influenza virus infection [20]. In addition, human oral species of Streptococcus were recently found in the lung of a patient infected with A(H1N1)pdm09 virus [21]. Moreover, although the underlying molecular mechanisms have not yet been understood, professional oral healthcare reduces oral bacteria, resulting in a reduction in the risk of infection with influenza virus [22].
Since previous studies have reported the presence of bacterial NA activity in dental plaque fluid and saliva [23, 24], we are interested in whether oral bacteria affect the release of influenza virus. Our results suggest that NA-producing oral bacteria can elevate the risk of influenza infection and exacerbate the influenza infection, which could be detrimental to viral NA inhibitor drugs.
Materials and methods
Reagents
Zanamivir was purchased from Santa Cruz Biotechnology (Dallas, TX, USA), and 2′-(4-methylumbelliferyl)-α-d-N-acetylneuraminic acid (4-MUNANA) was purchased from Sigma (St. Louis, MO, USA).
Virus preparation and infection
Influenza A/Udorn/307/72 (H3N2) viral stocks were propagated in Madin-Darby canine kidney (MDCK) cells and grown at 34 °C and 5 % CO2 for 40 h in minimum essential medium (MEM; Life Technologies, Carlsbad, CA, USA) containing 2.5 µg/mL TPCK-trypsin (Worthington Biochemical, Lakewood, NJ, USA), penicillin and streptomycin (Life Technologies). Culture supernatants were collected and supplemented with 0.2 % bovine serum albumin (BSA), and then stored at −80 °C until use. To collect viral samples for analysis, MDCK cells were grown on 6-well plates and inoculated with the virus at multiplicity of infection (MOI) of 0.001 plaque-forming units (pfu) per cell, and incubated for 30 min at room temperature. Cells were then incubated at 34 °C and 5 % CO2 for 16 or 24 h in 1 mL MEM containing 2.5 µg/mL TPCK-trypsin along with bacterial culture supernatants. Supernatant fractions were used for both plaque assays and Western blotting. A fraction of the cells was lysed and was used for Western blotting.
Bacterial culture supernatant preparation
Actinomyces spp. (WVU 398A, ATCC 12104, ATCC 19039, LY7, ATCC 27044, 8A06, WVU627, T14V, ATCC 19246), Streptococcus oralis (JCM 12997, ATCC 9811, ATCC 10557), Streptococcus mitis (JCM 12971, ATCC 6249) and Streptococcus sanguinis (ATCC 10556) were grown in brain heart infusion (BHI) broth (BD, Franklin Lakes, NJ, USA) at 37 °C for 24 h. P. gingivalis (ATCC 33277, W83, FDC 381, W50, SU 63) were grown in BHI broth containing 5 µg/mL haemin and 0.5 µg/mL menadione in an anaerobic system (10 % CO2, 10 % H2 and 80 % N2; Model 1024, Forma Scientific, Marietta, OH, USA). Prior to downstream applications, turbidity was subsequently adjusted to an optical density at 600 nm of 1.0 using a Spectrophotometer (U-1100; HITACHI, Tokyo, Japan). Bacterial cultures were then centrifuged (at 7,000×g for 10 min at 4 °C) and filtered through sterile 0.22-µm-pore polyvinylidene fluoride syringe filters (Millipore, Billerica, MA, USA). The culture supernatants were then concentrated tenfold by ultrafiltration using Amicon Ultra concentrators (10-kDa molecular mass-cutoff; Millipore). To standardize the culture conditions, the same culture medium was used to cultivate each strain after 24 h incubation, and turbidity was adjusted before measuring NA activity.
Neuraminidase activity assay
NA activity of the supernatants was detected according to previously described techniques [25]. Briefly, samples were incubated at 37 °C for 2 h, and the reaction was stopped by adding 0.5 M Na2CO3. NA activity was determined using 0.1 mM of the fluorogenic substrate 4-MUNANA in 50 mM phosphate-buffered saline (PBS, pH 7.0), whereby the amount of 4-methylumbelliferone (MU) released was measured. The amount of 4-MU was determined using a fluorometer (TriStar LB941; Berthold Technologies, Bad Wildbad, Germany) with an excitation wavelength of 360 nm and an emission wavelength of 460 nm. One unit was defined as the enzyme activity required to release 1 µmol of 4-MU from 4-MUNANA per minute.
Plaque assay
Plaque assays were performed as previously described [26]. Briefly, tenfold serial dilutions of virus samples were prepared in Hanks balanced salt solution (HBSS; Life Technologies), and 0.1 mL of the dilutions were inoculated onto MDCK cell monolayers in 6-well plates. After viral adsorption onto the cells for 30 min at room temperature, 1.6 mL of L-15 medium (Life Technologies) containing 0.6 % SeaKem ME agarose (Lonza, Basel, Switzerland), 1.5 % gelatin (Nacalai Tesque, Kyoto, Japan), and 2.5 µg/mL TPCK-trypsin was added to each well and allowed to solidify. Plates were incubated for 3 days at 34 °C, and the number of plaques was then counted. Virus titers were expressed as pfu/mL.
Indirect immunofluorescence assay
MDCK cells grown on glass coverslips were inoculated with the virus at MOI of 0.01 and incubated for 1 h at 34 °C. The cells were then incubated for 12 h at 34 °C in MEM containing bacterial culture supernatants. The cells were subsequently fixed with 4 % paraformaldehyde for 5 min followed by cold methanol for 5 min at room temperature. Fixed cells were incubated for 1 h with rabbit polyclonal antibody against purified A/Udorn/72 virions [27] in PBS containing 1 % BSA, followed by incubation for 1 h with Alexa Fluor 555 goat anti-rabbit IgG (Life Technologies) in PBS containing 1 % BSA and Hoechst 33342 (Life Technologies). Coverslips were mounted on glass slides with ProLong Gold antifade reagent (Life Technologies) and examined using a fluorescence microscope (IX-FLA, Olympus, Tokyo, Japan). The images obtained were subsequently analyzed using InStudio (Pixera, Santa Clara, CA, USA).
Western blotting
Culture supernatants and cell lysates were separated using sodium dodecyl sulfate-polyacrylamide gel electrophoresis before being transferred to polyvinylidene difluoride membranes (Millipore). Membranes were blocked with 2 % BSA and sequentially incubated with primary and secondary antibodies, followed by thorough washing. Primary antibodies included anti-M1 protein antibody (Takara Bio, Shiga, Japan), anti-ERK1/2 phospho-specific antibody, anti-ERK1/2 antibody (Cell Signaling Technology, Danvers, MA, USA), and anti-β-actin antibody (Santa Cruz Biotechnology). HRP-linked anti-rabbit IgG antibody and HRP-linked anti-mouse IgG antibody (GE Healthcare, Piscataway, NJ, USA) were used as secondary antibodies. All membranes were treated with ECL prime detection reagent (GE Healthcare) prior to examination. All bands were visualized using a ChemiDoc XRS System (Bio-Rad, Hercules, CA, USA).
Statistical analysis
All experiments were replicated three times. Analysis of variance (ANOVA) was performed to test for statistical significance between the experiments. Significance level was defined by P < 0.05. All data are expressed as mean ± standard deviation (SD).
Results
Neuraminidase activity in bacterial culture supernatants
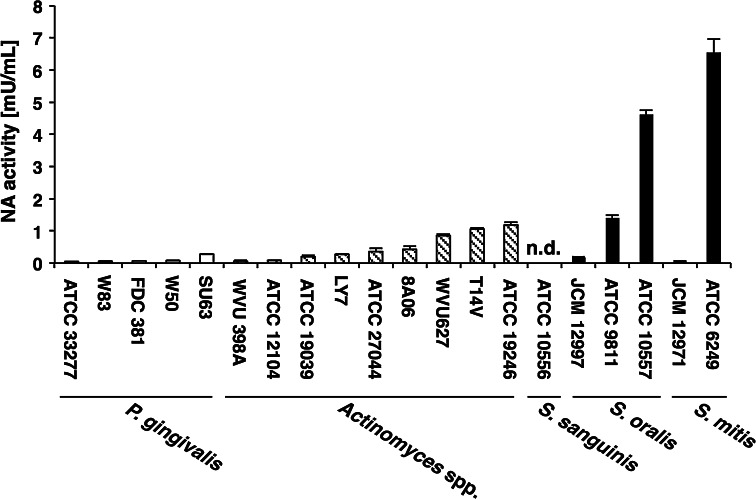
Previous studies have reported the presence of bacterial NA activity in dental plaque fluid and saliva [23, 24]. We, therefore, examined the NA activity in culture supernatants from 20 strains of 3 bacterial genera isolated from human oral cavity (Fig. 1).
Fig. 1.
Neuraminidase activity of oral bacteria. Neuraminidase activity in culture supernatants of oral bacteria expressed as arbitrary units of fluorescence signals. Values are presented as mean ± SD; n = 3. n.d. not detectable
Very low NA activity (0.04–0.27 mU/mL) was observed in bacterial culture supernatants of all P. gingivalis strains. NA activity of the supernatants of Actinomyces spp. was 0.05–1.18 mU/mL. Among the oral mitis group streptococci studied (i.e., S. mitis, S. oralis and S. sanguinis), S. oralis ATCC 10557 and S. mitis ATCC 6249 exhibited higher NA activity (4.61 ± 0.13 and 6.54 ± 0.42 mU/mL, respectively). However, NA activity was not detected in the supernatants of S. sanguinis ATCC 10556.
Effects of bacterial culture supernatants on progeny virus release
Considering S. oralis ATCC 10557 and S. mitis ATCC 6249 produced higher levels of NA, we examined, through in vitro infection models, whether culture supernatants of these bacteria could promote progeny virus release from the infected cells.
A/Udorn/72 (H3N2) virus, which is commonly used for in vitro analysis, was inoculated onto MDCK cells at MOI of 0.001 in the absence or presence of bacterial culture supernatants (1 or 5 %). After 24-h incubation, the culture media were collected, and virus titers were determined by plaque assays. We found that when the supernatants of S. oralis ATCC 10557 or S. mitis ATCC 6249 were added onto the infected cells, the number of progeny viruses released markedly increased (virus titers = 21.2- and 28.7-fold, respectively) (Fig. 2a). Progeny virus release from the infected cells was directly related, in a dose-dependent manner, to the bacterial NA activity. In contrast, such effects were not observed when the supernatants of S. sanguinis ATCC 10556, which lacks the ability to produce NA, were added onto the infected cells.
Fig. 2.
Effects of bacterial culture supernatants on the release of influenza virus. a Stimulation of influenza virus release by bacterial culture supernatants of oral mitis group streptococci. MDCK cells were infected with A/Udorn/72 influenza virus at an MOI of 0.001. After viral adsorption for 30 min, the cells were incubated with BHI (control) or bacterial culture supernatants (1 or 5 %, v/v). Following 24-h incubation, the culture media were harvested, and virus titers were determined by plaque assays (black bar). NA activities in bacterial culture supernatants were determined using 4-MUNANA (white bar). Values are presented as the mean ± SD, n = 3 (*P < 0.05; **P < 0.01). b Culture supernatants of S. oralis ATCC10557 and S. mitis ATCC6249 promoted the expression of viral M1 proteins. MDCK cells were infected with A/Udorn/72 influenza virus at an MOI of 0.001. After viral adsorption for 30 min, the cells were incubated with BHI (control) or bacterial culture supernatants (5 %, v/v). Following 16-h incubation, the culture supernatants were harvested, and the expression of viral M1 proteins was detected by Western blotting using a specific monoclonal antibody
Viral M1 proteins are the most abundant proteins in influenza virions, playing a critical role in both virus assembly and budding; thus, determining their expression levels is indicative of the amount of virus present in samples [28]. To investigate the effects of NA-producing oral bacteria on the expression of viral M1 proteins collected from a culture supernatant of infected cells, we performed Western blotting using the same conditions as those described in Fig. 2a. We observed that although the expression of viral M1 proteins remained unaltered upon the addition of supernatants of S. sanguinis ATCC 10556, the supernatants of S. oralis ATCC 10557 and S. mitis ATCC 6249 greatly induced the expression of these proteins (Fig. 2b). These results suggested that NA-producing oral bacteria promote the release of progeny viruses from the infected cells.
Neuraminidase-producing oral bacteria promoted cell-to-cell spread of virus infection
To examine other biological effects of NA-producing oral bacteria on influenza virus infection, we investigated whether oral bacteria affect the cell-to-cell spread of the infection by immunofluorescence analysis. A/Udorn/72 virus was inoculated at MOI of 0.01 onto MDCK cells grown on glass coverslips. The cells were then incubated for 12 h with bacterial culture supernatants, and viral antigens were stained with anti- A/Udorn/72 antibody. The number of antigen-positive cells increased after the addition of the supernatants of S. oralis ATCC 10557 or S. mitis ATCC 6249, which demonstrate NA activity (Fig. 3a, b). However, this effect was not observed with the supernatant of oral bacteria that do not produce NA. To further confirm the effects of these bacteria on the cell-to-cell spread of the infection, we detected the expression of viral M1 proteins in cell lysates.
Fig. 3.
Culture supernatants of S. mitis ATCC6249 and S. oralis ATCC10557 promoted the cell-to-cell spread of influenza viral infection. a, b NA-producing oral bacteria promoted the spread of influenza virus infection. For indirect immunofluorescence assay, MDCK cells were infected with A/Udorn/72 influenza virus at an MOI of 0.01. After viral adsorption for 1 h, the cells were incubated for 12 h with BHI or bacterial culture supernatants (5 %, v/v). The cells were then fixed and the antigens were stained with rabbit polyclonal antibody against purified A/Udorn/72 virions. Nuclei were counterstained by Hoechst 33342. Depicted is the merged image of virus staining (red) and nuclear DNA counterstaining (blue). The numbers of antigen-positive cells is shown in (b). Values are presented as mean ± SD; n = 3 (*P < 0.05; **P < 0.01). c Effects of bacterial culture supernatants on the expression of viral M1 proteins in the infected cells. Experiments were performed in a manner similar to that described in the legend for Fig. 2b. Cell lysates were harvested, and the expression of viral M1 proteins was detected
We found that the supernatants of S. oralis ATCC 10557 or S. mitis ATCC 6249 but not the supernatants of S. sanguinis ATCC 10556 increased viral M1 protein expression levels (Fig. 3c), suggesting that NA-producing oral bacterial can enhance the cell-to-cell spread of the infection.
Neuraminidase-producing oral bacteria increased ERK phosphorylation in the infected cells
Influenza viruses extensively manipulate signaling pathways within the host cell to support their replication. Four mitogen-activated protein kinase family members are reportedly activated after viral infection in vitro [29]. In particular, the Raf/MEK/ERK cascade is known to be essential for virus production and the formation of viral ribonucleoprotein complexes [30]. Using Western blot analysis, we examined the effects of the culture supernatants of NA-producing oral bacteria on ERK activation in the infected cells. ERK phosphorylation was observed in the infected cells (Fig. 4). When the cells were treated with the supernatants of S. oralis ATCC 10557 or S. mitis ATCC 6249, an increase in virus-stimulated ERK phosphorylation was observed (Fig. 4). In contrast, phosphorylation levels remained unaltered upon the addition of the supernatants of S. sanguinis ATCC 10556.
Fig. 4.
S. mitis ATCC6249 and S. oralis ATCC10557 culture supernatants enhanced ERK activation in the infected cells. MDCK cells were infected with the virus at an MOI of 0.001 in the presence or absence of BHI or bacterial culture supernatants (5 %). After 16-h incubation, cell lysates were harvested and phosphorylated ERK (p-ERK) or total ERK were detected by Western blotting using specific antibodies
Neuraminidase-producing oral bacteria restored the release of influenza virus suppressed by zanamivir
NA-specific inhibitors are commonly used both for prevention and treatment of influenza virus infection [5]. Zanamivir inhibits the enzyme activity of NA by binding to the active site pocket of NA, thereby preventing the spread of the infection [4]. Because the culture supernatants of S. oralis ATCC 10557 and S. mitis ATCC 6249 promoted the release of influenza virus, we were interested in understanding the effects of these NA-producing oral bacteria on influenza virus infection in the presence of zanamivir. Therefore, we first examined whether zanamivir inhibits oral bacterial NA activity. Zanamivir at a concentration of 250 nM is commonly used to treat viral infections in vitro; the same concentration almost completely inhibited NA activity of A/Udorn/72 virus (Fig. 5a). However, NA activity in the supernatants of S. oralis ATCC 10557 and S. mitis ATCC 6249 was unaffected upon treatment with zanamivir. We then tested whether these strains had any effects on influenza virus infection in the presence of zanamivir. We found that the inhibitory effects of zanamivir on influenza virus release were diminished in the presence of S. oralis ATCC 10557 or S. mitis ATCC 6249 culture supernatants (Fig. 5b). Zanamivir suppressed the release of progeny viruses from A/Udorn/72-infected cells to <2 % in comparison to those from untreated cells. It is notable that the number of progeny viruses released was restored upon further addition of the supernatants of S. oralis ATCC 10557 (by 57.1 %) or S. mitis ATCC 6249 (by 439.1 %) (Fig. 5b). In contrast, such an effect was not observed upon the addition of supernatants of S. sanguinis ATCC 10556. Figure 5c also shows that zanamivir inhibited the expression of viral M1 proteins in both culture supernatants and cell lysates of the infected cells, while the supernatants of S. oralis ATCC 10557 and S. mitis ATCC 6249 suppressed the inhibitory effects of zanamivir. Moreover, inhibitory effects of zanamivir on ERK phosphorylation in the infected cells were also re-established after the addition of the culture supernatants of NA-producing oral bacteria (Fig. 5d). These results suggest that NA produced by oral bacteria promotes release of the influenza viruses, even when viral NA activity has been inhibited.
Fig. 5.
Effects of culture supernatants of S. mitis ATCC6249 and S. oralis ATCC10557 on the release of influenza virus in the presence of zanamivir. a Zanamivir did not affect bacterial NA activity. NA activities were measured in the absence or presence of zanamivir (250 nM) and are expressed as arbitrary units of fluorescence signals. Values are presented as mean ± SD; n = 3 (**P < 0.01). b NA-producing oral bacteria decreased the antiviral efficacy of zanamivir. MDCK cells were infected with A/Udorn/72 influenza virus at an MOI of 0.001. After viral adsorption for 30 min, the cells treated with zanamivir (250 nM) were incubated with BHI or bacterial culture supernatants (5 %, v/v) for 24 h. The culture media were harvested, and viral titers were determined by plaque assays. Values are presented as mean ± SD; n = 3 (*P < 0.05; **P < 0.01). c, d Culture supernatants of NA-producing oral bacteria reduced the inhibitory effects of zanamivir on the expression of viral M1 proteins and phosphorylation of ERK. Experiments were performed in a manner similar to that described in the legend for b. Culture supernatants (Sup) of viral M1 proteins and lysates of cells (Lysates) were used for detection of M1 proteins by Western blotting (c). The cell lysates were also used to analyze for phosphorylation of ERK (d)
Discussion
Influenza is a major health risk worldwide, and it can lead to fatal respiratory dysfunction in high-risk groups, such as the elderly and children. Bacterial respiratory infections can occur concomitant or secondary to influenza virus infection resulting in higher morbidity and mortality [7]; yet, the effects of bacterial exogenous and endogenous infections or bacterial parasitism on influenza virus release remain poorly understood. In this study, we examined the biological activity of the culture supernatant of S. oralis and S. mitis which is the Gram-positive facultatively anaerobic cocci and predominant constituents of human florae on all surfaces in the oral cavity and pharynx from birth and throughout life. These organisms are most frequently isolated from dental plaque and saliva and sometimes from the vegetations associated with subacute bacterial endocarditis [31]. We found that NA activity of oral mitis group streptococci efficiently promoted influenza virus release in vitro, suggesting that oral bacteria could contribute towards the clinical progression of influenza virus infection.
NA functions as enzyme that is required to release progeny viruses from infected cells [4]. In the absence of NA activity, progeny viruses are captured on the cell surface due to HA receptor-binding activity and fail to be released unless exogenous NA activity is provided [32, 33]. Here we found that S. oralis ATCC 10557 and S. mitis ATCC 6249 exhibits remarkable NA activity and its culture supernatants promoted the release of influenza virus and cell-to-cell spread of the infection. In addition, the culture supernatants of NA-producing oral bacteria also increased viral M1 protein expression and cellular ERK activation. Inoue et al. previously reported that NA activity of soil bacterium Clostridium perfringens improved the yield of NA-deficient virus from MDCK cells [34], suggesting that bacterial NA activity could promote the release of influenza viruses from the infected cells.
NA-specific inhibitors play a major role in controlling seasonal influenza outbreaks, and they may also provide preventive and therapeutic benefits during pandemic outbreaks [5]. Zanamivir, an inhaled drug, showed a higher in vitro antiviral activity compared to oseltamivir, an orally administered drug, against influenza A(H1N1), A(H1N1)pdm09 and B viruses, as indicated by lower mean IC50 values [35, 36]. We also observed that zanamivir almost completely suppressed the release of influenza viruses; however, the inhibitory effects of zanamivir were suppressed by the exogenous addition of the supernatant of S. oralis ATCC 10557 or S. mitis ATCC 6249. A recent report also demonstrated that the inhibitory effects of zanamivir on influenza virus release diminished after the addition of the supernatants of S. pneumoniae [37]. On the other hand, our present and past findings indicate that the NA-specific inhibitors do not repress bacterial NA activity [37, 38]. Taken together, these observations suggest that bacterial NA may supplement viral NA which reduces the antiviral effectiveness of the NA-specific inhibitors during influenza virus infection.
Respiratory infections, such as pneumonia and influenza, are a serious concern in hospitals and other healthcare facilities, such as nursing homes. There is growing evidence suggesting a relationship between poor oral hygiene and respiratory infections [39–41]. The salivary bacteria released from the oral biofilms can be aspirated into the respiratory tract, thereby causing bacterial infections such as aspiration pneumonia in immunocompromised hosts [39]. In addition, bacteria that colonize dentures also cause respiratory infections among the elderly [40]. Furthermore, clinical studies have demonstrated that improved oral hygiene and receiving professional oral healthcare reduce the number of oral bacteria, thereby resulting in a reduction in the risk of occurrence of respiratory diseases among the elderly [41]. These observations have been suggesting that oral microbial florae, including denture plaques, are a major reservoir of potentially respiratory pathogens and that professional oral healthcare is effective prevention and measures for the occurrence and progression of respiratory diseases.
S. oralis and S. mitis are frequently isolated from oral florae and bacterial NA activity has been usually detected in dental plaque fluid and saliva [23, 24, 42]. In addition, the bacteria are also recovered in considerable amounts from pharynx and other areas of upper respiratory tract [43]. These observations along with our findings suggest that an increase in the number of oral bacteria due to poor oral hygiene surely increase the activity of bacterial NA in the oral cavity as the upper respiratory tract; this may increase the risk of and exacerbate the influenza infection in infected individuals. Our results also suggest that when increase in the amount of NA-producing oral bacteria in the oral florae, the influenza virus infection and the release of virus may not be suppressed by the NA-specific inhibitors. In fact, previous studies have reported that receiving professional oral healthcare significantly decreases both the numbers of oral bacteria [22, 44] and NA activity in saliva, resulting in a reduction in the risk of influenza virus infection in the elderly [22].
Vaccination is generally recommended for individuals at a higher risk of influenza-associated complications [45]. However, vaccination alone is insufficient to control influenza virus infections, considering high mutational rates and virus reassembly [46]. This can lead to a temporal mismatch between the vaccine and circulating viral strains, resulting in a reduced effectiveness of the vaccine [4]. The efficacy of influenza vaccination is only 40 % in the elderly [47]. In addition, considering the emergence and spread of NA-specific inhibitor-resistant viruses [5], new research on effective prevention and measures for influenza virus infections are required.
Although additional basic and clinical studies are required, our findings suggest a causal relationship between oral bacterial parasitism and influenza virus infections. Furthermore, it provides the scientific basis to support the importance of keeping good oral hygiene and receiving professional oral healthcare as effective approaches in the prevention of occurrence or progression of influenza virus infection especially among high-risk groups.
Acknowledgments
This work was supported by Grants-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan; a grant from the Dental Research Center, Nihon University School of Dentistry, Tokyo; Nihon University President’s Grant for Specified Multidisciplinary Research; and the Strategic Research Base Development Program for Private Universities (S1001024) and Japan Initiative for Global Research Network on Infectious Diseases from the MEXT of Japan.
Contributor Information
Kenichi Imai, Phone: +81-3-3219-8125, Email: imai.kenichi@nihon-u.ac.jp.
Kuniyasu Ochiai, Phone: +81-3-3219-8125, Email: ochiai.kuniyasu@nihon-u.ac.jp.
References
- 1.Almond MH, McAuley DF, Wise MP, Griffiths MJ. Influenza-related pneumonia. Clin Med. 2012;12:67–70. doi: 10.7861/clinmedicine.12-1-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dawood FS, Iuliano AD, Reed C, Meltzer MI, Shay DK, Cheng PY, Bandaranayake D, Breiman RF, Brooks WA, Buchy P, Feikin DR, Fowler KB, Gordon A, Hien NT, Horby P, Huang QS, Katz MA, Krishnan A, Lal R, Montgomery JM, Molbak K, Pebody R, Presanis AM, Razuri H, Steens A, Tinoco YO, Wallinga J, Yu H, Vong S, Bresee J, Widdowson MA. Estimated global mortality associated with the first 12 months of 2009 pandemic influenza A H1N1 virus circulation: a modelling study. Lancet Infect Dis. 2012;12:687–695. doi: 10.1016/S1473-3099(12)70121-4. [DOI] [PubMed] [Google Scholar]
- 3.Webster RG, Bean WJ, Gorman OT, Chambers TM, Kawaoka Y. Evolution and ecology of influenza A viruses. Microbiol Rev. 1992;56:152–179. doi: 10.1128/mr.56.1.152-179.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luscher-Mattli M. Influenza chemotherapy: a review of the present state of art and of new drugs in development. Arch Virol. 2000;145:2233–2248. doi: 10.1007/s007050070017. [DOI] [PubMed] [Google Scholar]
- 5.Samson M, Pizzorno A, Abed Y, Boivin G. Influenza virus resistance to neuraminidase inhibitors. Antiviral Res. 2013;98:174–185. doi: 10.1016/j.antiviral.2013.03.014. [DOI] [PubMed] [Google Scholar]
- 6.Layne SP, Beugelsdijk TJ, Patel CK, Taubenberger JK, Cox NJ, Gust ID, Hay AJ, Tashiro M, Lavanchy D. A global lab against influenza. Science. 2001;293:1729. doi: 10.1126/science.293.5536.1729. [DOI] [PubMed] [Google Scholar]
- 7.McCullers JA. Insights into the interaction between influenza virus and pneumococcus. Clin Microbiol Rev. 2006;19:571–582. doi: 10.1128/CMR.00058-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taubenberger JK, Reid AH, Fanning TG. The 1918 influenza virus: a killer comes into view. Virology. 2000;274:241–245. doi: 10.1006/viro.2000.0495. [DOI] [PubMed] [Google Scholar]
- 9.Louie JK, Acosta M, Winter K, Jean C, Gavali S, Schechter R, Vugia D, Harriman K, Matyas B, Glaser CA, Samuel MC, Rosenberg J, Talarico J, Hatch D, California Pandemic Working G Factors associated with death or hospitalization due to pandemic 2009 influenza A(H1N1) infection in California. JAMA. 2009;302:1896–1902. doi: 10.1001/jama.2009.1583. [DOI] [PubMed] [Google Scholar]
- 10.Dhanoa A, Fang NC, Hassan SS, Kaniappan P, Rajasekaram G. Epidemiology and clinical characteristics of hospitalized patients with pandemic influenza A (H1N1) 2009 infections: the effects of bacterial co-infection. Virol J. 2011;8:501. doi: 10.1186/1743-422X-8-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tashiro M, Ciborowski P, Reinacher M, Pulverer G, Klenk HD, Rott R. Synergistic role of staphylococcal proteases in the induction of influenza virus pathogenicity. Virology. 1987;157:421–430. doi: 10.1016/0042-6822(87)90284-4. [DOI] [PubMed] [Google Scholar]
- 12.Kolenbrander PE. Oral microbial communities: biofilms, interactions, and genetic systems. Annu Rev Microbiol. 2000;54:413–437. doi: 10.1146/annurev.micro.54.1.413. [DOI] [PubMed] [Google Scholar]
- 13.Paster BJ, Olsen I, Aas JA, Dewhirst FE. The breadth of bacterial diversity in the human periodontal pocket and other oral sites. Periodontol 2000. 2006;42:80–87. doi: 10.1111/j.1600-0757.2006.00174.x. [DOI] [PubMed] [Google Scholar]
- 14.Gendron R, Grenier D, Maheu-Robert L. The oral cavity as a reservoir of bacterial pathogens for focal infections. Microbes Infect. 2000;2:897–906. doi: 10.1016/S1286-4579(00)00391-9. [DOI] [PubMed] [Google Scholar]
- 15.Azarpazhooh A, Leake JL. Systematic review of the association between respiratory diseases and oral health. J Periodontol. 2006;77:1465–1482. doi: 10.1902/jop.2006.060010. [DOI] [PubMed] [Google Scholar]
- 16.Pizzo G, Guiglia R, Lo Russo L, Campisi G. Dentistry and internal medicine: from the focal infection theory to the periodontal medicine concept. Eur J Intern Med. 2010;21:496–502. doi: 10.1016/j.ejim.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 17.Imai K, Ochiai K, Okamoto T. Reactivation of latent HIV-1 infection by the periodontopathic bacterium Porphyromonas gingivalis involves histone modification. J Immunol. 2009;182:3688–3695. doi: 10.4049/jimmunol.0802906. [DOI] [PubMed] [Google Scholar]
- 18.Imai K, Inoue H, Tamura M, Cueno ME, Inoue H, Takeichi O, Kusama K, Saito I, Ochiai K. The periodontal pathogen Porphyromonas gingivalis induces the Epstein–Barr virus lytic switch transactivator ZEBRA by histone modification. Biochimie. 2012;94:839–846. doi: 10.1016/j.biochi.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 19.Imai K, Yamada K, Tamura M, Ochiai K, Okamoto T. Reactivation of latent HIV-1 by a wide variety of butyric acid-producing bacteria. Cell Mol Life Sci. 2012;69:2583–2592. doi: 10.1007/s00018-012-0936-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bronstein-Sitton N, Cohen-Daniel L, Vaknin I, Ezernitchi AV, Leshem B, Halabi A, Houri-Hadad Y, Greenbaum E, Zakay-Rones Z, Shapira L, Baniyash M. Sustained exposure to bacterial antigen induces interferon-gamma-dependent T cell receptor zeta down-regulation and impaired T cell function. Nat Immunol. 2003;4:957–964. doi: 10.1038/ni975. [DOI] [PubMed] [Google Scholar]
- 21.Kuroda M, Katano H, Nakajima N, Tobiume M, Ainai A, Sekizuka T, Hasegawa H, Tashiro M, Sasaki Y, Arakawa Y, Hata S, Watanabe M, Sata T. Characterization of quasispecies of pandemic 2009 influenza A virus (A/H1N1/2009) by de novo sequencing using a next-generation DNA sequencer. PLoS One. 2010;5:e10256. doi: 10.1371/journal.pone.0010256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abe S, Ishihara K, Adachi M, Sasaki H, Tanaka K, Okuda K. Professional oral care reduces influenza infection in elderly. Arch Gerontol Geriatr. 2006;43:157–164. doi: 10.1016/j.archger.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 23.Rogers R, Newbrun E, Tatevossian A. Neuraminidase activity in human dental plaque fluid. Arch Oral Biol. 1979;24:703–705. doi: 10.1016/0003-9969(79)90121-3. [DOI] [PubMed] [Google Scholar]
- 24.Hannig C, Hannig M, Attin T. Enzymes in the acquired enamel pellicle. Eur J Oral Sci. 2005;113:2–13. doi: 10.1111/j.1600-0722.2004.00180.x. [DOI] [PubMed] [Google Scholar]
- 25.Takao A, Nagamune H, Maeda N. Sialidase of Streptococcus intermedius: a putative virulence factor modifying sugar chains. Microbiol Immunol. 2010;54:584–595. doi: 10.1111/j.1348-0421.2010.00257.x. [DOI] [PubMed] [Google Scholar]
- 26.Gopinath SC, Awazu K, Fujimaki M, Shimizu K. Evaluation of Anti-A/Udorn/307/1972 antibody specificity to influenza A/H3N2 viruses using an evanescent-field coupled waveguide-mode sensor. PLoS One. 2013;8:e81396. doi: 10.1371/journal.pone.0081396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shimizu K, Mukaigawa J, Oguro M, Ono Y, Nakajima K, Kida H. Inhibition of transcriptase activity of influenza A virus in vitro by anti-haemagglutinin antibodies. Vaccine. 1985;3:207–210. doi: 10.1016/0264-410X(85)90107-0. [DOI] [PubMed] [Google Scholar]
- 28.Gorai T, Goto H, Noda T, Watanabe T, Kozuka-Hata H, Oyama M, Takano R, Neumann G, Watanabe S, Kawaoka Y. F1Fo-ATPase, F-type proton-translocating ATPase, at the plasma membrane is critical for efficient influenza virus budding. Proc Natl Acad Sci USA. 2012;109:4615–4620. doi: 10.1073/pnas.1114728109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ehrhardt C, Seyer R, Hrincius ER, Eierhoff T, Wolff T, Ludwig S. Interplay between influenza A virus and the innate immune signaling. Microbes Infect. 2010;12:81–87. doi: 10.1016/j.micinf.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 30.Pleschka S, Wolff T, Ehrhardt C, Hobom G, Planz O, Rapp UR, Ludwig S. Influenza virus propagation is impaired by inhibition of the Raf/MEK/ERK signalling cascade. Nat Cell Biol. 2001;3:301–305. doi: 10.1038/35060098. [DOI] [PubMed] [Google Scholar]
- 31.Aas JA, Paster BJ, Stokes LN, Olsen I, Dewhirst FE. Defining the normal bacterial flora of the oral cavity. J Clin Microbiol. 2005;43:5721–5732. doi: 10.1128/JCM.43.11.5721-5732.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palese P, Tobita K, Ueda M, Compans RW. Characterization of temperature sensitive influenza virus mutants defective in neuraminidase. Virology. 1974;61:397–410. doi: 10.1016/0042-6822(74)90276-1. [DOI] [PubMed] [Google Scholar]
- 33.Shibata S, Yamamoto-Goshima F, Maeno K, Hanaichi T, Fujita Y, Nakajima K, Imai M, Komatsu T, Sugiura S. Characterization of a temperature-sensitive influenza B virus mutant defective in neuraminidase. J Virol. 1993;67:3264–3273. doi: 10.1128/jvi.67.6.3264-3273.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Inoue E, Ieko M, Takahashi N, Osawa Y, Okazaki K. An NA-deficient 2009 pandemic H1N1 influenza virus mutant can efficiently replicate in cultured cells. Arch Virol. 2013 doi: 10.1007/s00705-013-1887-0. [DOI] [PubMed] [Google Scholar]
- 35.Sheu TG, Deyde VM, Okomo-Adhiambo M, Garten RJ, Xu X, Bright RA, Butler EN, Wallis TR, Klimov AI, Gubareva LV. Surveillance for neuraminidase inhibitor resistance among human influenza A and B viruses circulating worldwide from 2004 to 2008. Antimicrob Agents Chemother. 2008;52:3284–3292. doi: 10.1128/AAC.00555-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Centers for Disease C, Prevention Oseltamivir-resistant 2009 pandemic influenza A (H1N1) virus infection in two summer campers receiving prophylaxis—North Carolina, 2009. MMWR Morb Mortal Wkly Rep. 2009;58:969–972. [PubMed] [Google Scholar]
- 37.Nishikawa T, Shimizu K, Tanaka T, Kuroda K, Takayama T, Yamamoto T, Hanada N, Hamada Y. Bacterial neuraminidase rescues influenza virus replication from inhibition by a neuraminidase inhibitor. PLoS One. 2012;7:e45371. doi: 10.1371/journal.pone.0045371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mendel DB, Tai CY, Escarpe PA, Li W, Sidwell RW, Huffman JH, Sweet C, Jakeman KJ, Merson J, Lacy SA, Lew W, Williams MA, Zhang L, Chen MS, Bischofberger N, Kim CU. Oral administration of a prodrug of the influenza virus neuraminidase inhibitor GS 4071 protects mice and ferrets against influenza infection. Antimicrob Agents Chemother. 1998;42:640–646. doi: 10.1128/aac.42.3.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scannapieco FA. Role of oral bacteria in respiratory infection. J Periodontol. 1999;70:793–802. doi: 10.1902/jop.1999.70.7.793. [DOI] [PubMed] [Google Scholar]
- 40.Russell SL, Boylan RJ, Kaslick RS, Scannapieco FA, Katz RV. Respiratory pathogen colonization of the dental plaque of institutionalized elders. Spec Care Dentist. 1999;19:128–134. doi: 10.1111/j.1754-4505.1999.tb01413.x. [DOI] [PubMed] [Google Scholar]
- 41.Adachi M, Ishihara K, Abe S, Okuda K. Professional oral health care by dental hygienists reduced respiratory infections in elderly persons requiring nursing care. Int J Dent Hyg. 2007;5:69–74. doi: 10.1111/j.1601-5037.2007.00233.x. [DOI] [PubMed] [Google Scholar]
- 42.Simon-Soro A, Tomas I, Cabrera-Rubio R, Catalan MD, Nyvad B, Mira A. Microbial geography of the oral cavity. J Dent Res. 2013;92:616–621. doi: 10.1177/0022034513488119. [DOI] [PubMed] [Google Scholar]
- 43.Frandsen EV, Pedrazzoli V, Kilian M. Ecology of viridans streptococci in the oral cavity and pharynx. Oral Microbiol Immunol. 1991;6:129–133. doi: 10.1111/j.1399-302X.1991.tb00466.x. [DOI] [PubMed] [Google Scholar]
- 44.Ishikawa A, Yoneyama T, Hirota K, Miyake Y, Miyatake K. Professional oral health care reduces the number of oropharyngeal bacteria. J Dent Res. 2008;87:594–598. doi: 10.1177/154405910808700602. [DOI] [PubMed] [Google Scholar]
- 45.Nicholson KG, Wood JM, Zambon M. Influenza. Lancet. 2003;362:1733–1745. doi: 10.1016/S0140-6736(03)14854-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hedlund M, Larson JL, Fang F. Antiviral strategies for pandemic and seasonal influenza. Viruses. 2010;2:1766–1781. doi: 10.3390/v2081766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Michiels B, Govaerts F, Remmen R, Vermeire E, Coenen S. A systematic review of the evidence on the effectiveness and risks of inactivated influenza vaccines in different target groups. Vaccine. 2011;29:9159–9170. doi: 10.1016/j.vaccine.2011.08.008. [DOI] [PubMed] [Google Scholar]